Abstract
Hypertension affects an estimated 103 million Americans yet gaps in knowledge continue to limit its successful management. Rapidly emerging evidence is linking gut dysbiosis to many disorders and diseases including hypertension. The evolution of the –omics techniques has allowed determination of the abundance and potential function of gut bacterial species by next generation bacterial sequencing, while metabolomics techniques report shifts in bacterial metabolites in the systemic circulation of hypertensive patients and rodent models of hypertension. The gut microbiome and host have evolved to exist in balance and cooperation, and there is extensive crosstalk between the two to maintain this balance, including during regulation of blood pressure. However, an understanding of the mechanisms of dysfunctional host-microbiome interactions in hypertension is still lacking. Here, we synthesize some of our recent data with published reports and present concepts and a rationale for our emerging hypothesis of a dysfunctional gut-brain axis in hypertension. Hopefully, this new information will improve the understanding of hypertension and help to address some of these knowledge gaps.
Keywords: Hypertension, Microbiome, Metabolomics, Autonomic Nervous System
Introduction
The 2017 Hypertension Guidelines reported that almost half of the US adult population currently has high blood pressure or hypertension, substantially higher than stated in the previous Guideline. Additionally, approximately 15% of adult Americans are diagnosed with treatment resistant hypertension since they do not achieve controlled blood pressure despite lifestyle modifications and 3–4 antihypertensive medications1–3. This presents a tremendous socio-economic and health care resource burden, further complicated by racial and sex-related disparities and the increasingly recognized problem of “masked hypertension”4. Moreover, the new Guidelines indicate a lowered threshold for pharmacologic treatment of >130/80 mmHg, for persons with or without known cardiovascular disease1. Despite these huge problems, the mechanisms that are responsible for this disease are not well understood. This is especially relevant for treatment resistant hypertension, where a dysfunctional autonomic nervous system, immune system, and more recently gut dysbiosis appear to greatly contribute to the hypertensive phenotype5–7. Indeed, imbalance and dysregulation of the autonomic nervous system precede and are coincident with the development of hypertension8–12. Evidence from animal models describes a change in gut microbiota secondary to the dysfunction of the autonomic nervous system6. Gut dysbiosis has been found in some pre-hypertensive populations13, a condition also characterized by dysfunctional autonomic nervous system8–11 Despite the breadth of emerging knowledge, current control and treatment of hypertension is not targeting these dysfunctional mechanisms.
The gut microbiome and host have evolved to exist in balance and cooperation, and there is extensive crosstalk between the two systems to maintain this homeostasis, including during regulation of blood pressure. Problems within one system are reflected in the other as the crosstalk is disrupted by the ensuing gut dysbiosis and raised blood pressure. The overarching objective of this review is to summarize and update the involvement of the gut and its microbiota in the control of blood pressure. This review will also specifically address the interaction of the gastrointestinal tract with the autonomic nervous system and the gut microbiota as it pertains to blood pressure regulation. Finally, we discuss mechanisms of host-microbiome crosstalk dysregulation within these systems in hypertension. The call for more extensive research in this area is the major focus of this review.
Gut dysbiosis in hypertension: evidence for and against
The gastrointestinal tract presents a vast interface between the external environment and symbiotic and/or pathogenic factors such as food and microbes that interact with the human host. It is the initial point of entry for many deleterious environmental risk factors for hypertension. Moreover, endogenous factors in the gastrointestinal tract, such as its epithelium, metabolism, immune-, endocrine- and nervous systems14–17 have the potential to play a pivotal role in hypertension. Epidemiological studies have long linked the gut with regulation of blood pressure and hypertension. Early studies suggested environmental factors that affect the gut such as diet and alcohol intake are risk factors for hypertension18,19. More recently evidence has been presented indicating that probiotics, antibiotics and dietary supplements can rebalance gut dysbiosis and improve overall gut homeostasis20,21, as well as decrease high blood pressure7, 22–28. One such supplementation is with the short chain fatty acids, which are the end products of bacterial metabolism generally considered to be beneficial to the host. Despite a plethora of epidemiological studies, the interest in gut dysbiosis and host-microbiota interactions in hypertension only began to rise in the last decade with the evolution of next generation bacterial genome sequencing and metabolomics. There is now persuasive evidence of gut dysbiosis in several forms of hypertension, from hypertension associated with metabolic syndrome, pulmonary hypertension, hypertension in obese pregnancies as well as treatment resistant hypertension and pre-hypertension13, 29–32.
Recent findings from a cohort study question the enthusiasm for a role of gut dysbiosis in hypertension33. However, as pointed out by the Marques group34, the considerable overlap between diseases, medications, and microbes could account for the lack of strong correlation between the gut and hypertension in that study. Thus, it is important to characterize the microbiome of different phenotypes of hypertension as well as include other confounding factors in microbiome analyses. These may be broken down into three categories: (i) patient choices, which include medication compliance, exercise, alcohol consumption, diet including salt intake and lifestyle; (ii) patient characteristics, such as age, gender, and race; and (iii) patient conditions like hyperlipidemia, diabetes and circulating hormone levels. In addition, employment of metagenomic vs 16S sequencing would elucidate the function of the bacteria in the gut microbiome. Shotgun metagenomics sequencing can reveal the presence of other organisms such as archaea, viruses and fungi in the microbiome and suggest their relevance in various forms of hypertension. This information coupled with metabolomics analyses is beginning to yield a much more complete and rather complex picture of the role of gut dysbiosis in hypertension35–37.
Most if not all studies to date, however, remain associative and explorative rather than addressing the causal mechanisms, which undoubtedly presents the biggest challenge in clinical research. An increasing body of work in animal models of hypertension offers indications of mechanism, however vague they may be. Many questions remain unanswered, including: (i) is gut dysbiosis a cause or a consequence of hypertension? (ii) what are the mechanisms that lead to development of gut dysbiosis in hypertension? (iii) what is the role of gut epithelium in altered host-microbiome communication in hypertension? The potential role of hypertensive risk factors such as salt, nutrients, hormones, and obesity, that initiate epithelial epigenetic mechanisms must be considered in this regard; (iv) what effect does gut dysbiosis have on development and/or maintenance of hypertension? Several studies report imbalances in short chain fatty acid levels in rodent models of hypertension and conversely beneficial effects of supplementation of these gut microbiota metabolites in reducing blood pressure in hypertensive animals7, 25–28. A clearer mechanistic link is presented in animal fecal matter transplant studies, where significant increases in blood pressure have been reported in rodents receiving fecal matter transplant from their hypertensive counterparts38, 39. Conversely, fecal matter transplant from normotensive donor rats to hypertensive recipients significantly decreased blood pressure39. The mechanisms for these changes are not yet determined.
Our group has recently linked hypothalamic neuroinflammation and increased sympathetic drive with changes in gut physiology and microbiota associated with angiotensin II-induced hypertension. This suggests a role for a dysfunctional autonomic nervous system in gut dysbiosis, although we were not able to discount a direct effect of hypertension and/or angiotensin II on the gut40. This, along with our evidence of decreased proteins that mediate cell-cell connections in gut epithelium of prehypertensive spontaneously hypertensive rats, as well as of gut dysbiosis in prehypertensive and hypertensive populations13, 24, 28, 35, 38, 41–45), led us to propose a dysfunctional autonomic nervous system-gut hypothesis that contributes to development and maintenance of hypertension (Figure 2). Potential mechanisms utilized by the gut-brain axis for blood pressure control and their failure in hypertension, are discussed below.
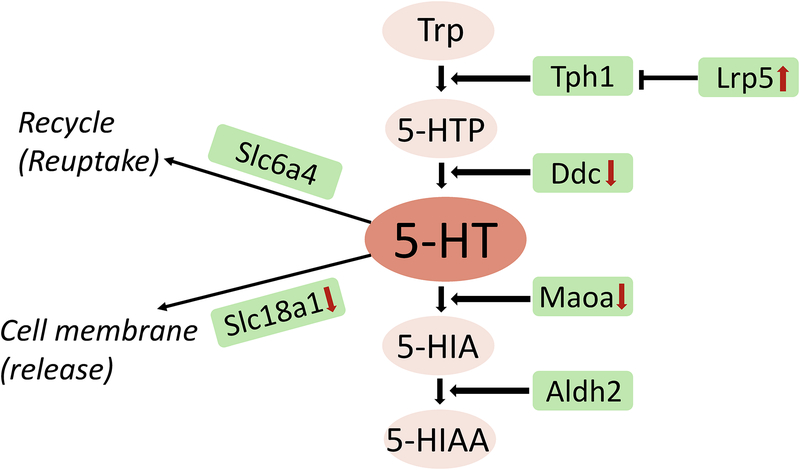
Figure 2:
Relative expression levels of genes associated with gut epithelial serotonergic synthesis, metabolism, release and uptake in spontaneously hypertensive rats (SHR) and their normotensive controls (WKY) from RNAseq analysis. Genes are shown in green boxes, tryptophan metabolites in pink circles. Red arrows indicate significant changes (upwards arrow for increase, downwards arrow for decrease) in SHR compared with WKY.
Gut as a cardio-regulatory organ: role of the sympathetic nervous system
Sympathetic innervation of the gastrointestinal tract has been extensively studied and characterized. The noradrenergic fibers within the gut wall originate in the prevertebral sympathetic ganglia (celiac-mesenteric ganglia, inferior mesenteric ganglia, and pelvic ganglia) and innervate the stomach, small intestine, large intestine, and rectum. Tyrosine hydroxylase-positive fibers innervate the myenteric and submucosal plexi and gastrointestinal blood vessels. These sympathetic fibers affect gastrointestinal functions such as peristalsis, secretion, absorption, fluid and electrolyte transport across the epithelium, as well as vessel smooth muscle contraction and hence the blood flow to the gastrointestinal tract46, 47. Indeed, our recent study highlighted a drastic difference in both the gastrointestinal blood flow and gut stiffness in hypertensive rats, perhaps owing to the increased gastrointestinal sympathetic nerve activity in these rats6, 48. Moreover, increased sympathetic nerve activity has been linked with elevated inflammation both systemically and in the gut5, 49–51, which could contribute to the vascular gastrointestinal leakage52. Lastly activation of adrenergic receptors on enteroendocrine cells modifies release of gastrointestinal hormones such as serotonin53. Considering the close proximity of gastrointestinal vagal innervation to the same cells49, 54, this may be the point of convergence of the two arms of the autonomic nervous system (see below paragraphs for relevance of the vagal innervation).
Early investigations highlighted the importance of the splanchnic vascular bed and sympathetic nerve activity in blood pressure regulation in humans and animals55. Splanchnic sympathetic nerve activity is essential in the development of high blood pressure in the deoxycorticosterone acetate-salt and angiotensin II rodent models of hypertension56–58; conversely, experimental splanchnic denervation lowers blood pressure in hypertensive animals59, 60. Splanchnic sympathetic nerve activity regulates the capacitance of the vast mesenteric blood vessel network such that any changes in mesenteric vascular capacitance result in significant modulation of blood pressure58. A study also demonstrated that the antihypertensive effects of bariatric surgery are related to reduced sympathetic nerve activity in both hypertensive patients and rats50. This is similar to the reduction of renal sympathetic nerve activity by percutaneous catheter-based renal nerve ablation, a novel surgical approach to the treatment of resistant hypertension. In another study in mice, decreases in blood pressure following vertical sleeve gastrectomy are associated with body mass-independent decreases in hypothalamic endoplasmic reticulum stress, hypothalamic inflammation, sympathetic nerve activity, and significant shifts in the gut microbiota61. Thus, even though the effects of bariatric surgery may be multifactorial, part of the antihypertensive mechanism appears related to changes in gut microbiota as well as sympathetic tone in the gut.
Our group recently demonstrated links between enhanced hypothalamus-driven gut sympathetic tone, gut pathology, dysbiosis, and inflammation that play key roles in rodent models of hypertension6, 40, 48. The elevated splanchnic sympathetic nerve activity and mild gut pathology in juvenile pre-hypertensive rodents precede hypertension-related gut dysbiosis6, suggesting that elevated gut sympathetic nerve activity modulates the gastrointestinal environment prior to the development of hypertension6,8. Elevated sympathetic tone to the gut in hypertension increases stiffness and hypertrophy of the muscle layer. The increased sympathetic tone promotes gut leakiness by direct effects on epithelial cells, affects the activity of the immune system and specific gut bacteria6, 62–64. This elevated sympathetic tone to the gut may contribute to gut dysbiosis. Conversely, diminished beta adrenergic signaling, indicative of reduced overall sympathetic tone, is associated with beneficial effects in the gastrointestinal tract. These effects include increased abundance of the largely beneficial bacteria, Bacilli Lactobacillales, increased colonic production of the short chain fatty acids, butyrate, acetate and propionate, and dampened systemic and gut immune responses65.
More studies are required to directly confirm these ideas, but the currently available data from animal models of hypertension as well as the human prehypertensive population suggest two possible scenarios. One is that gut dysbiosis may be a consequence of the progressively hypertensive gut environment resulting from elevated sympathetic drive to the gut6,13. Alternatively, altered gut-microbiota crosstalk elicited by prolonged hypertensive stimuli results in elevated sympathetic drive to the gut (Figure 1)6,13. Support for this latter scenario is evidenced by studies demonstrating that fecal matter transplant from spontaneously hypertensive rats into normotensive Wistar Kyoto rats enhances sympathetic activity, neuroinflammation and increases blood pressure39. Consistent with this are studies that demonstrate that fecal matter transplant from normotensive animals to animal models of hypertension reduce blood pressure38,39. To resolve this a study needs to be performed determining whether gut dysbiosis precedes increased sympathetic nervous system activity to the gut or vice versa. Thus, it appears that our understanding of the complex and interconnected nature of microbial-host interactions is still incomplete and more effort is needed to fully understand the mechanisms and role of gut dysbiosis in hypertension.
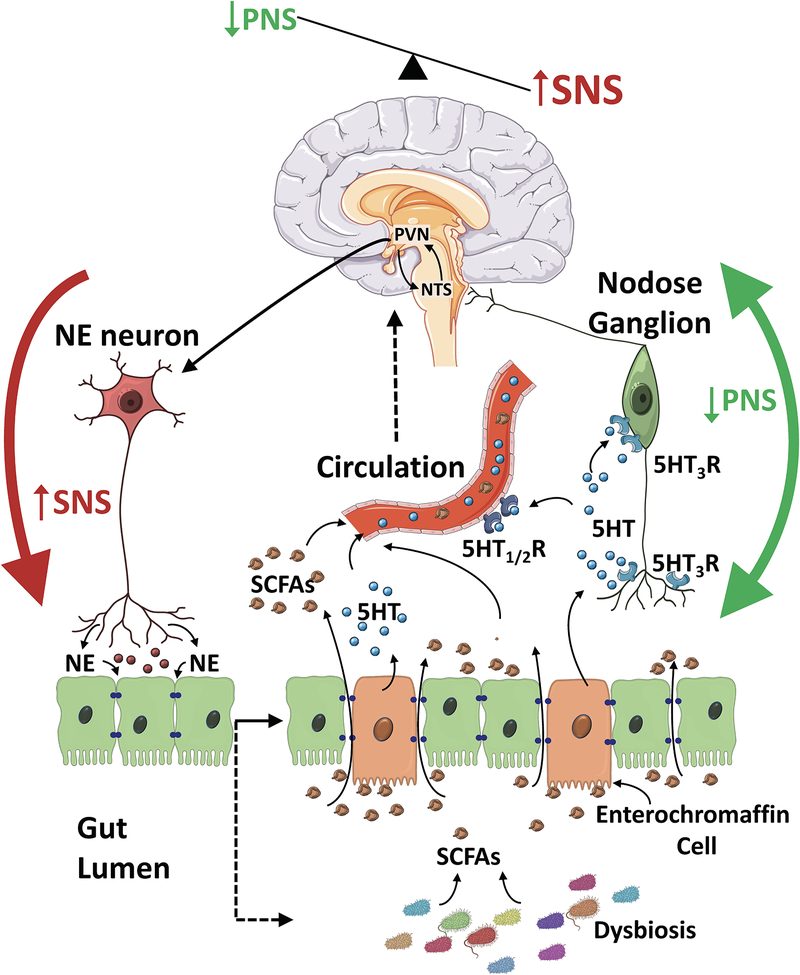
Figure 1:
A hypothetical representation of host-microbiota interactions in hypertension. Increased sympathetic nervous system activity (SNS) to the gut contributes to epithelial dysfunction leading to gut dysbiosis. The dysbiosis-related bacterial metabolite imbalance including shifts in short chain fatty acid (SCFA) production and their accumulation in the gut lumen increases production of serotonin (5HT) by the enterochromaffin cells in the gut. The local 5HT is able to modulate the activity of gut vagal afferents via 5HT3 receptors (5HT3R) potentially dampening the vagal gut-brain neural axis, while 5HT released into circulation can affect the vasculature and cause vasoconstriction. Moreover, circulating 5HT can reach the brain cardioregulatory regions via the circulation and across the leaky blood brain barrier that occurs in hypertension. Thus, the combination of increased SNS to the gut and dampened vagal afferent parasympathetic nervous system (PNS) drive from the gut to the brain cardioregulatory regions such as the nucleus of the solitary tract (NTS) contributes to perpetuation of the hypertensive phenotype.
Role of the vagus nerve: is the vagal afferent feedback from the gut to the brain cardio-regulatory regions perturbed in hypertension?
Role of vagal afferent input from the gut
The vagal nerve is part of the pivotal neural circuitry connecting the heart, lungs, and structures in the neck and abdomen to the brain. In the gastrointestinal tract, a rich network of vagal connections extends from the brain and nodose ganglia to interpenetrate layers of the gastrointestinal tract. This circuitry welds the central nervous system and gastrointestinal tract together in a bidirectional neural network in which outflow from the central nervous system has pervasive effects on gastrointestinal function, and the gastrointestinal tract influences the central nervous system via extensive vagal afferent nerves. Unmyelinated vagal afferent nerves with cell bodies in the nodose ganglion innervate the mucosa or the muscularis layer of the gastrointestinal tract. The nerves innervating the mucosa predominately express neurotensin receptor 1 and G-protein coupled receptor 35 (Gpr35). The vagal afferent nerves in the muscularis layer are a more mixed population but predominantly express glucagon-like peptide 1 receptor and neuropeptide Y receptor Y266. Vagal regulation of gastrointestinal physiology involves sensory stimulation of these afferent vagal fibers that receive information about the internal milieu of the gastrointestinal tract. This information may be sensed directly by the afferent nerve fibers that have receptors for gut microbiota metabolites such as short chain fatty acids (FFAR3 or Gpr41), aromatic acidic metabolites, the tryptophan metabolite kynurenic acid (Gpr35), many lipid receptors, e.g. Lpar3, and peptide hormones released from enteroendocrine cells such as cholecystokinin and NPY. They may receive information mechanically, for example by distension (stretch) of the gastrointestinal wall, and immunologic stimulation (i.e. proinflammatory cytokines).
The afferent fibers also receive input from the gut enterochromaffin cells via serotonin type 3 receptors in “synapse like”67 or very close connections66. Enterochromaffin cells have receptors for various gut microbiota metabolites, e.g. Gpr41, and Gpbar1 for secondary bile acids, etc. and for glucagon-like peptide 1. This peptide is released from a subtype of enteroendocrine cells which have many receptors that respond to substances in food, for example, proteins and lipids, reviewed in68, and acts in a paracrine fashion on enterochromaffin cells. Upon adequate stimulation of these receptors, enterochromaffin cells release serotonin in a calcium dependent fashion. The released serotonin increases activity in the vagal afferents. There is also a newly described enteroendocrine cell function in the small intestine and colon. The new function lead to the suggestion of neuropod as an alternative name for the cell. Neuropods form glutamate synapses with vagal afferents54. These cells responded to glucose to increase vagal afferent firing rates. It will be interesting to discover if there are other neuropod responses to substances such as alcohol and salt, ingestion of which are known risk factors for hypertension, to alter gut afferent input to the brain and autonomic system regulation.
This sensory information is transmitted to the nucleus of the solitary tract to initiate an appropriate efferent response through autonomic nerves from the dorsal motor nucleus of the vagus. These efferent pathways innervate the enteric nervous system, the gut immune system, the enteroendocrine cells and the gut epithelium, regulating secretion of neuroendocrine hormones, variations in gastrointestinal motility, barrier function, and the intestinal immune response. Thus, perturbations within any or all of these variables may lead to dysfunction of the gut-brain axis, as documented in several disease states. Most notably, work by Cryan et al highlights the role of the gut vagal projections in mental and neurological disorders such as anxiety, depression, and some neurodevelopmental and neurodegenerative disorders69–71. Interestingly, both anxiety and depression are comorbid with cardiovascular disease72–75 suggesting that some of the etiology may overlap. Moreover, electrical stimulation of the vagus nerve has been proposed recently as a treatment for inflammatory disorders of the gastrointestinal tract such as inflammatory bowel disease (IBD, e.g. Crohn’s disease, ulcerative colitis, etc.)76,77, while potentially producing changes in the gut microbiota via decreases in intestinal permeability78–80. Again, considering the presence of inflammation and increased intestinal permeability in rodent models of hypertension as well as in human hypertension6, 7, 28, 40, 48, 81, the presence of reduced vagal influence on the gut in hypertension is highly probable.
The role of aberrant vagal afferent inputs from the gut to the brain in hypertension becomes increasingly plausible when considering the overlap in the regions of the nucleus of the solitary tract receiving input from cardiac and gut sensory afferents82. Eighty to ninety percent of the vagus nerve consists of afferent sensory fibers83, 84, and those distributed in the gastrointestinal tract are responsible for sensing changes in mechanical, chemical, endocrine and immune factors. These gut afferents report to a region of the nucleus of the solitary tract that overlaps with the area receiving cardiorespiratory afferent inputs from both baro- and chemoreceptors. Moreover, there appears to be very little viscerotopography of putative second order neurons controlling the cardiorespiratory and gastrointestinal tract85,86. Thus, one sub-region of the nucleus of the solitary tract can control multiple functions, and effects of activation of cardiorespiratory reflexes may be indistinguishable from effects of activation of gastrointestinal reflexes - in terms of reflex specificity82. Both baroreceptor- and chemoreceptor-mediated afferent feedback to the nucleus of the solitary tract as well as the processing of the cardiorespiratory reflexes by second order neurons in the nucleus, are altered in and contribute to hypertension86–90. There is indirect evidence suggesting alterations in afferent vagal feedback from the gut in hypertension. There is decreased vagal afferent excitability in diet-induced experimental obesity91, a condition often accompanied by hypertension. Considering that gut vagal afferents sense at a minimum, proinflammatory signals, bacterial metabolites, and endocrine factors78 all of which are dysregulated in the hypertensive gut6, 7, 28, 37, 40, 48, 51, 92, it is reasonable to propose that aberrant vagal afferent function also exists in hypertension. Activation of the gastrointestinal vagal afferents results in immediate bradycardia and reduction in blood pressure93. This confirms that gastrointestinal vagal afferents contribute to regulation of blood pressure. The reflex can become dysregulated to cause both blood pressure and heart rate to decline in vasovagal syncope (the most common cause of fainting). Thus, in hypertension, dampening of the vago-vagal reflexes originating in the gastrointestinal tract, similar to those regulating cardiac and respiratory function, may contribute, in part, to the hypertensive phenotype. Further studies are warranted to address this knowledge gap.
The potential role of vagal efferents on the gut in hypertension
Reduced cardiac vagal tone, in addition to increased sympathetic tone, is associated with development and maintenance of high blood pressure in both animal and human hypertension94–100. Impaired vagal influence on the heart has been detected from early hypertensive phases throughout established hypertension In humans101–107. A reduction in cardiac vagal tone has also been observed in comorbid conditions of cardiovascular disease such as obstructive sleep apnea and diabetes, as well as heart failure108–112. To our knowledge, disturbances in vagal efferent modulation of the gastrointestinal tract are yet to be reported in hypertension. Considering the presence of gut pathology and inflammation in rodent and human hypertension6, 28, 48, and the reported decrease in vagal tone to the gut in diabetes113 and inflammatory gastrointestinal disorders such as IBD114, 115, it is interesting to hypothesize that similar to the heart, decreased vagal efferent tone to the gut is also present in hypertension.
Functional aspects of the gut microbiome in hypertension
Several studies have investigated the functional potential of the gut microbiome in human hypertension13,28,37,116–122. This was made possible by newer -omics techniques and made necessary by increased understanding of the importance of functional characterization of microbiota beyond mere sequencing of bacterial content. Not only may metabolic products of the gut bacteria have direct cardio- and neuro-regulatory properties, but gut bacteria can also affect production of hormones and neurotransmitters by the host that are essential for physiological homeostasis. We focus on three pivotal factors to illustrate their importance in hypertension below.
Short chain fatty acids in hypertension
Short chain fatty acids are the products of gut microbial fermentation of complex polysaccharides such as the fiber found in vegetables and fruits. They are almost completely absent in animals lacking gut microbiota, so the host relies on the gut microbiota to supply them123. Short chain fatty acids, commonly acetate, butyrate and propionate, are important energy sources for the gut epithelium, and are also absorbed across the epithelium into the circulation. One advance in understanding the host-microbiota interactions in hypertension is confirmation of the presence of specific short chain fatty acid-sensing receptors in the kidney and vasculature27, 124. These receptors are traditionally known as odorant receptors since they are expressed on the cell membranes of olfactory receptor neurons to give rise to the sense of smell. The short chain fatty acids act as ligands for Gpr41 and OlfR78, as well as for other receptors, of the host. Stimulation of these two receptors modulates renin secretion and vascular tone, and so these products of gut bacterial metabolism have the ability to regulate blood pressure27,124. For example, propionate caused the release of renin from the kidney via Olfr78, while Olfr78 knockout mice had reduced basal blood pressure124 On the other hand, the vasodilatory response to propionate is mediated via Gpr41125. Indeed, the abundance of specific short chain fatty acid-producing gut bacteria as well as circulating short chain fatty acids such as butyrate are reduced in hypertensive rodents and patients28,30,126–131. Conversely, supplementation with the short chain fatty acids butyrate and acetate, can alleviate hypertension25–28, 124, presumably not only via actions on their receptors in the vascular system and kidney126.
We have recently demonstrated reduced circulating short chain fatty acids in the spontaneously hypertensive rat and human hypertension28, 128. Lack of short chain fatty acids in hypertension can potentially affect the nervous system. Indeed, acetate has effects via the central nervous system126, 127 and alleviates hypertension26. Central effects of short chain fatty acids are further supported by our recent data demonstrating reduced short chain fatty acid receptors in the paraventricular nucleus of the hypothalamus, the hub of sympathetic regulation in the forebrain. Intracerebroventricular administration of butyrate leads to a decrease in blood pressure in normotensive Wistar Kyoto rats. This effect is dampened in the spontaneously hypertensive rat, and is associated with reduced butyrate-sensing receptors. As a consequence there are dampened effects on neural activation in the paraventricular nucleus of the hypothalamus and other cardioregulatory brain regions128. Fecal matter transplantation from normotensive animals to hypertensive animals lowers blood pressure. The fecal matter of normotensive animals is rich in short chain fatty acid-producing bacteria and this process also increases expression of the short chain fatty acid receptors, Gpr41 and Gpr43 in the paraventricular nucleus. Activation of these receptors is associated with lowering of blood pressure39. Moreover, short chain fatty acids can directly regulate the sympathetic nervous system via receptors expressed on sympathetic ganglia and potentially affect the neural feedback from the gut via the receptors expressed on vagal afferents78, 132, 133. Thus, we propose that gut bacterial metabolites such as short chain fatty acids may be involved in neural regulation of blood pressure via their receptors expressed in cardioregulatory brain regions (Figure 2) and sympathetic and vagal ganglia. Therefore, reduction in both the availability of short chain fatty acids in the circulation and short chain fatty acid-sensing receptors may contribute to the pathophysiology of hypertension.
Gut bacterial metabolites promote host health by exerting local protective functions regulating mucosal barriers134 and immune mechanisms135. Short chain fatty acids such as butyrate have potent immunosuppressant activity, both systemically and centrally127, 128, 136–139. Centrally, they can produce anti-inflammatory and metabolic effects in microglia and astrocytes137,128, activation of which has been associated with central neuro-modulating/neuroinflammatory effects in hypertension140, 141. This view is supported by our finding that butyrate normalizes expression of angiotensin II type1a receptors in astrocytes of the spontaneously hypertensive rat in vitro142. This is consistent with the role of butyrate in epigenetic modification127, 128, 136–139, 143, since heightened angiotensin type 1a receptor expression in the spontaneously hypertensive rat is linked to hypomethylation of the promotor region of the gene144. Most of the short chain fatty acids are absorbed and utilized by the gut epithelial cells as a crucial source of energy or are transported into the systemic circulation largely via specialized trans-epithelial short chain fatty acid transporters in the colon128, 145, 146. Our data indicate that low butyrate in the systemic circulation of hypertensive rodents may be partly due to reduced trans-epithelial transport of this short chain fatty acid across the colon. This would cause accumulation of butyrate in the colon128 despite presumably lower production due to decreased abundance of butyrate-producing bacteria in the spontaneously hypertensive rat gut microbiota7. Thus, butyrate accumulates in the colon to be excreted in the feces, depriving the host of its anti-hypertensive effects. Moreover, accumulation of butyrate in the gut could also directly or indirectly have deleterious effects on the local gut bacterial, epithelial, immune and neural environment, including via epigenetic influences in the spontaneously hypertensive rat gut128. Considering the low circulating butyrate in the systemic circulation of hypertensive patients28, further studies are needed to ascertain whether these mechanisms observed in rodent models of hypertension are translational. Thus, several short chain fatty acid-dependent mechanisms are currently being investigated to test whether using nutritional and bacterial supplements would alleviate gut dysbiosis and attenuate hypertension.
Increased circulating metabolites of hypertensive patients that may contribute to enhanced sympathetic drive.
We have recently undertaken a study of the metabolome of hypertensive patients compared with reference subjects. Our unpublished findings show several metabolites that support increased sympathetic drive in hypertension. There was increased 3 methoxy-4-hydroxymandelate, a byproduct of catabolism of neuronal norepinephrine, in the systemic circulation of hypertensive patients compared to normotensive reference subjects (by 1.5-fold, adjusted p value=0.007). That 3 methoxy-4-hydroxymandelate is increased in the circulation of hypertensive patients aligns with increased sympathetic drive. Also, the circulating concentration of gamma amino butyric acid (GABA), a major inhibitory neurotransmitter, was depleted in hypertensive patients compared to normotensive reference subjects (by 0.7 fold, adjusted p value=0.01). Quinolinic acid, a neurotoxic and gliotoxic metabolite of tryptophan, likely increases sympathetic drive in hypertension by increasing neuroinflammation, although this has not been experimentally proven. Quinolinic acid was increased in the circulation of African Americans with hypertension compared with non-Hispanic Caucasian Americans with hypertension.37. African Americans also have a higher prevalence of treatment resistant hypertension, are more salt-sensitive, and develop hypertension earlier and with more severe end organ damage than other American races and ethnic populations. Thus, insights into mechanisms of this hypertension issue in African Americans may advance our understanding and lead to improved control methods to address these high and damaging rates.
Tryptophan metabolism in hypertension
Quinolinic acid is not the only metabolite of tryptophan whose metabolism is altered during hypertension. Our unpublished data from a small cohort of hypertensive patients suggest shifts in serotonin metabolism in hypertension. Tryptophan is a dietary precursor to serotonin, which is synthesized predominantly in gut enterochromaffin cells (80–90% of total body content of serotonin) by tryptophan hydroxylase 1. Tryptophan can be metabolized towards kynurenine and indole pathways. The indole pathway is specifically a gut microbiota-dependent pathway, but the kynurenine pathway is both host and microbiota mediated, albeit via different enzymes. Since serotonin synthesis is substrate dependent, as tryptophan hydroxylase is never fully saturated, its synthesis is dependent upon the supply of tryptophan and the amount of tryptophan directed to the other pathways. Kynurenine can be metabolized to either kynurenic acid, that is considered to be beneficial, or to quinolinic acid, which is neurotoxic and gliotoxic. Interestingly, kynurenic acid is a ligand for Gpr35, a receptor that is activated by aromatic acidic metabolites. Gpr35 is found on enterochromaffin cells in the gut, the cells that produce serotonin, and their stimulation results in release of serotonin. The released serotonin binds to serotonin type-3 receptors on unmyelinated vagal afferents in the gut mucosa to alter vagal afferent input to the nucleus of the solitary tract. The cell bodies of these afferents in the nodose ganglion also express Gpr35, and their activation inhibits N-type calcium channels, reducing neurotransmitter release147. This would tend to decrease vagal afferent input to the brain. Clearly, it would be very useful if we understood how kynurenic acid is involved in modulating sympathetic outflow, if at all, and whether the increased kynurenine in hypertensive people affects this. In these patients we found that circulating kynurenine is increased compared with normotensive reference subjects (by 1.2-fold, adjusted p value=0.04, unpublished).
The altered kynurenine in hypertension may also affect serotonin synthesis. Increased kynurenine has been suggested to predict a decrease in serotonin synthesis in the hypertensive gut because of a shift towards synthesis of kynurenine from tryptophan, at the expense of serotonin148. Serotonin synthesis may be further compromised in hypertension because tryptophanase A, the gene for the bacterial enzyme that converts tryptophan to indole, is enriched in the gut microbiome of hypertensive patients; nonetheless, tryptophanase A expression can be regulated by the amount of tryptophan present in the gut149. However, the major stable breakdown product of serotonin in the host, 5-hydroxyindole acetic acid, suggests an increase in the circulation of hypertensive subjects (unpublished data) and increased serotonin bioavailability in hypertension. In addition, most circulating serotonin is taken up by platelets, unless they are activated150, such as is the case in hypertension as well as in patients with atherothrombosis151, 152. Moreover, short chain fatty acids such as butyrate stimulate production of serotonin in the enterochromaffin cells of the gut153, 154. This may be important because despite decreased abundance of butyrate producing bacteria in the gut of hypertensive rodents, there is more butyrate in the proximal colon of these rats than in their normotensive controls. This is due to decreased transport of butyrate across the gut epithelium128. The result is low circulating butyrate, but potentially increased serotonin production in the enterochromaffin cells of the gut due to the stimulation by butyrate trapped in the gut. Since we also observed reduced circulating butyrate and decreased butyrate producing bacteria in our hypertensive patient cohort28, a similar mechanism could be present in human hypertension. These apparently conflicting results deserve further research efforts for resolution.
Gut serotonergic signaling in blood pressure control
The putative link between gut dysbiosis and serotonergic metabolism in hypertension is intriguing, considering the comorbidity of high blood pressure with anxiety and depression, where irregular serotonin neurotransmission is an established part of the pathophysiology81, 155–157. As mentioned above, most serotonin is synthesized by the specialized enterochromaffin cells in the gastrointestinal tract. While gut bacteria can also produce serotonin158, most importantly they exert a powerful influence on the production of serotonin by the host gut154, 159. To probe for this possibility in hypertension-induced gut dysbiosis, we compared relative gene expression levels in gut epithelial cells isolated from spontaneously hypertensive rats and their normotensive controls, Wistar Kyoto rats, using RNAseq methods. We observed remarkable differences in the expression of genes associated with the synthesis, metabolism and reuptake of serotonin in the gut epithelium of the hypertensive rats compared to the normotensive rats (Figure 2). More studies are needed to delineate the functional changes associated with these alterations in serotonergic pathways in hypertension-induced gut dysbiosis, especially in humans.
Enterochromaffin cell-derived serotonin has both local gastrointestinal, as well as more widespread systemic effects, on endothelial cells, vascular smooth muscle cells, enteric neurons, vagal afferents, enterocytes, and immune cells150, 160–165. Thus, serotonin has the ability to dramatically change the functions of neural, immune, gut and cardiovascular cells and disrupt homeostasis, including blood pressure regulation150, 160, 161. In the systemic circulation, serotonin regulates blood pressure via its action on blood mononuclear cells, the vasculature, heart, adrenal glands, kidney, and brain circumventricular organs150, 160, 161. Increased circulating serotonin is known to heighten vascular constriction in hypertension162–165. Systemically, it reduces sodium excretion by the kidney which can cause renal failure166,167 while it can also promote cardiac hypertrophy in the mouse via a direct effect on cardiomyocytes166, 168, 169. The effects of serotonin on the heart can also be exerted via actions on both the vagus and sympathetic nerves controlling the heart169, which are both receptor subtype- and species-dependent. This suggests that serotonin can directly modulate the autonomic nervous system. Indeed, serotonin directly affects the sympathetic ganglia170 and inhibition of serotonin receptors decreases blood pressure in obese Zucker rats171. Serotonin also prevents hypertension caused by psychosocial stress in rats172. Thus, long-term activation of neurons in the sympathetic ganglia by serotonin may increase the sympathetic drive in hypertension. Centrally, serotonin influences on blood pressure arise from stimulation of serotonin receptors on neurons within the neural circuits that determine sympathetic and vagal outflows173–176. Considering that there are a multitude of central cardioregulatory brain regions as well as serotonin receptor subtypes, this indoleamine could have divergent effects determining the sympathetic and vagal outputs controlling blood pressure. These could potentially be imbalanced in during hypertension.
Serotonin afferent feedback in blood pressure control
As mentioned before, the nucleus of the solitary tract, as a major cardio-regulatory site, is the site of termination of primary sensory neurons, including those from the baroreceptors, chemoreceptors, and cardiopulmonary receptors that participate in reflex regulation of blood pressure177, 178. In addition, the nucleus of the solitary tract receives peripheral inputs from the vagal afferent nerve terminals in the gut with cell bodies in the nodose ganglia86, 179. The activity of these are modulated by serotonin via the its type 3 receptor150, 180. Indeed, activation of serotonin type 3 receptors on the vagal afferents in the gut is involved in several physiological and pathophysiological conditions, including distention- and chemical-evoked vagal reflexes, nausea, and vomiting, as well as visceral hypersensitivity180. Moreover, stimulation of serotonin type 3 receptors on the cardiac vagal afferents accounts for the bradycardia elicited by activation of the Bezold-Jarisch reflex150, potentially by affecting the processing of the arterial chemoreceptor reflex within the nucleus of the solitary tract181. In this way, gut serotonin signaling could be converging with or modulating other cardio-regulatory feedback mechanisms within the NTS, which are deregulated in hypertension89, 182–184. Thus, we propose that changes in the availability of serotonin in the gut and systemic circulation, as a result of gut dysbiosis, will modulate vagal sensory afferent feedback to the NTS to promote hypertension (Figure 2). Both the sensory and synaptic terminals of primary afferents in the nucleus of the solitary tract have serotonin receptors. This suggests that blood pressure control by serotonin at this site could be influenced by the levels of circulating serotonin (largely from gut and platelets) and by serotonin released within the nucleus of the solitary tract from the terminals of raphe neurons. Considering the data suggesting changes in serotonergic metabolism and the presence of gut dysbiosis in hypertension that affects synthesis and transport of serotonin in the gut, further studies are needed to elucidate the potential role of gut microbial dysbiosis on the serotonergic gut-brain axis in hypertension.
Conclusions and Future Directions
Despite the myriad of proof that associates gut dysbiosis with hypertension, evidence about the mechanistic role of gut dysbiosis in hypertension is lacking. We propose that the focus of hypertension research should be shifted to address functional aspects of host-microbiome interactions in order to better understand and address the clinical problem of hypertension. This was recently highlighted by a special NIH report calling for mechanistic research into the role of microbiota in blood pressure regulation185. Here, we propose a role for gut dysbiosis-associated neuromodulating factors such as short chain fatty acids and serotonin in promoting autonomic nervous system dysfunction. Thus, this would contribute to the hypertensive phenotype, an effect that may be exerted both locally via modulation of gut vagal afferents feedback to the brain cardioregulatory regions, and centrally, via modulation of efferent sympatho-vagal balance (Figure 2). In this review, we have presented the concepts and rationale behind our hypothesis. Our approach has been somewhat provocative in order to stimulate critical thinking and further investigation aimed at determining the mechanisms of host gut-microbiome communication in blood pressure control and hypertension.
However, many questions remain unanswered. Some are presented as follows:
Do hypertensive signals, such as high salt intake, directly influence gut epithelial cells epigenetically to initiate host-microbiome cross-talk? There is a plethora of evidence of microbiome-mediated changes in the gut epithelium186–188. However, the epithelium-initiated signaling concept is rapidly evolving and appears to be extremely relevant for hypertension, and its comorbid conditions like atherosclerosis and heart failure.
How do sympathetic and vagal pathways interact with the gut and coordinate their influences in hypertension? Elucidation of effects of gut-derived serotonin on vagal afferent function in dysbiosis would be valuable for delineating the involvement of the central nervous system.
Is the central nervous system the site receiving hypertensive signals that initiate hypertensive mechanisms, and which neuronal and glial phenotypes are influenced?
Topics related to gut-brain axis dysfunction in human hypertension should be addressed in parallel: Can unique metabolomic and microbiome signatures be validated? Do they respond to hypertensive stimuli and are there racial and gender differences in metabolic and microbiome signatures in hypertension? Initial studies have provided tantalizing data. Therefore, a large cohort study should be undertaken.
Are there metabolomic and microbiome signatures associated with the pre-hypertensive state and can they potentially serve as guides for development of biomarkers for hypertension?
In conclusion, the host-microbiome field in hypertension holds much potential for the development of novel and personalized strategies for environmental, racial and sex-based management of hypertension.
Sources of Funding:
HL33610 and HL132448
Abbreviations:
- Metabolites Trp
tryptophan
- 5-HTP
5 hydroxytryptophan
- 5-HT
serotonin or 5-hydroxytryptamine
- 5-HIA
5-hydroxyindole acetate, 5-HIAA, 5-hydroxyindole acetate Genes: Tph1, tryptophan hydroxylase 1
- Ddc
Dopa decarboxylase
- Moa
Monoaminoxidase a
- Aldh2
Aldehyde dehydrogenase 2
- Lrp5
LDL receptor related protein 5
- Slc6a4
solute carrier family 6 member 4 or serotonin transporter
- Slc18a
solute carrier family 18 member A1 or vesicular monoamine transporter
Non-Standard Abbreviations and Acronyms:
- Gpr35
G protein-coupled receptor 35
- Gpr41
G protein-coupled receptor 41
Footnotes
Conflicts of Interest: None.
References
- 1.Whelton P, Carey R, Aronow W, Casey DJ, Collins K, Dennison Himmelfarb C, DePalma S, Gidding S, Jamerson K, Jones D, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: A report of the american college of Cardiology/American heart association task force on clinical practice guidelines.. J Am Coll Cardiol 2018;71(19):e127–248. [DOI] [PubMed] [Google Scholar]
- 2.Muntner P, Carey R, Gidding S, Jones D, Taler S, Wright JJ, Whelton P. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018;71(2):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muntner P, Carey R, Gidding S, Jones D, Taler S, Wright JJ, Whelton P. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018;137(2):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddiqui M, Judd E, Jaeger B, Bhatt H, Dudenbostel T, Zhang B, Edwards L, Oparil S, Calhoun D. Out-of-clinic sympathetic activity is increased in patients with masked uncontrolled hypertension. Hypertension 2019;73(1):132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Santisteban MM, Ahmari N, Carvajal JM, Zingler MB, Qi Y, Kim S, Joseph J, Garcia-Pereira F, Johnson RD, Shenoy V, et al. Involvement of bone marrow cells and neuroinflammation in hypertension. Circ Res 2015;117(2):178–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, et al. Hypertension-linked pathophysiological alterations in the gut. Circ Res 2017;120(2):312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M,., Gong M, Qi Y, Zubcevic J, et al. Gut dysbiosis is linked to hypertension. Hypertension 2015;65(6):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simms A, Paton J, Pickering A, Allen A. Amplified respiratory-sympathetic coupling in the spontaneously hypertensive rat: Does it contribute to hypertension? J Physiol 2009;587(3):597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancia G, Grassi G. The autonomic nervous system and hypertension. Circ Res 2014;114(11):1804–14. [DOI] [PubMed] [Google Scholar]
- 10.Seravalle G, Lonati L, Buzzi S, Cairo M, Quarti Trevano F, Dell’Oro R, Facchetti R, Mancia G, Grassi G. Sympathetic nerve traffic and baroreflex function in optimal, normal, and high-normal blood pressure states. J Hypertens 2015;33(7):1411–7. [DOI] [PubMed] [Google Scholar]
- 11.Hering D, Kara T, Kucharska W, Somers V, Narkiewicz K. Longitudinal tracking of muscle sympathetic nerve activity and its relationship with blood pressure in subjects with prehypertension. Blood Press 2016;25(3):184–92. [DOI] [PubMed] [Google Scholar]
- 12.Cabassi A, Vinci S, Calzolari M, Bruschi G, Borghetti A. Regional sympathetic activity in pre-hypertensive phase of spontaneously hypertensive rats. Life Sci 1998;62(12):1111–8. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang B, Zhong J, Lin H, Zhao Z, Yan Z, He H, Ni Y, Liu D, Zhu Z. Blood pressure-lowering effects of glp-1 receptor agonists exenatide and liraglutide: A meta-analysis of clinical trials. Diabetes Obes Metab 2013;15(8):737–49. [DOI] [PubMed] [Google Scholar]
- 15.Mayer E Gut feelings: The emerging biology of gut-brain communication. Nat Rev Neurosci 2011;12(8):453–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux C, Bloom S, Darzi A, Athanasiou T, Marchesi J, et al. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 2011;60(9):1214–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandoval D, Seeley R. The microbes made me eat it. Science 2010;328(5975):179–80. [DOI] [PubMed] [Google Scholar]
- 18.Klatsky AL, Friedman GD, Siegelaub AB, Gérard MJ. Alcohol consumption and blood pressure Kaiser-Permanente multiphasic health examination data. N Engl J Med 1977;296(21):1194–200. [DOI] [PubMed] [Google Scholar]
- 19.Wright A, Burstyn PG, Gibney MJ. Dietary fibre and blood pressure. Br Med J 1979;2(6204):1541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Danneskiold-Samsøe N, Dias de Freitas Queiroz Barros H, Santos R, Bicas J, Cazarin C, Madsen L, Kristiansen K, Pastore G, Brix S, Maróstica M Júnior. Interplay between food and gut microbiota in health and disease. Food Res Int 2019;115(23):31. [DOI] [PubMed] [Google Scholar]
- 21.Schiattarella G, Sannino A, Esposito G, Perrino C. Diagnostics and therapeutic implications of gut microbiota alterations in cardiometabolic diseases. Trends Cardiovasc Med 2018;pii: S1050–1738(18)30155–5. [DOI] [PubMed] [Google Scholar]
- 22.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Hypertension 2014;64(4):897–903. [DOI] [PubMed] [Google Scholar]
- 23.Jauhiainen T, Vapaatalo H, Poussa T, Kyrönpalo S, Rasmussen M, Korpela R. Lactobacillus helveticus fermented milk lowers blood pressure in hypertensive subjects in 24-h ambulatory blood pressure measurement. Am J Hypertens 2005;18(12 Pt1):1600–5. [DOI] [PubMed] [Google Scholar]
- 24.Wilck N, Matus M, Kearney S, Olesen S, Forslund K, Bartolomaeus H, Haase S, Mähler A, Balogh A, Markó L, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature 2017;551(7682):585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang L, Zhu Q, Lu A, Liu X, Zhang L, Xu C, Liu X, Li H, Yang T. Sodium butyrate suppresses angiotensin II-induced hypertension by inhibition of renal (pro)reninreceptor and intrarenal renin-angiotensin system. J Hypertens 2017;35(9):1899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marques F, Nelson E, Chu P, Horlock D, Fiedler A, Ziemann M, Tan J, Kuruppu S, Rajapakse N, El-Osta A, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017;135(10):964–77. [DOI] [PubMed] [Google Scholar]
- 27.Pluznick J Microbial short-chain fatty acids and blood pressure regulation. Curr Hypertens Rep 2017;19(4):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, Mohammed M, Handberg E, Richards E, Pepine C, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci (Lond) 2018. March 30;132(6):701–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Z, Xiong S, Liu D. The gastrointestinal tract: An initial organ of metabolic hypertension? Cell Physiol Biochem 2016;38(5):1681–94. [DOI] [PubMed] [Google Scholar]
- 30.Gomez-Arango LF, Barrett HL, McIntyre HD, Callaway LK, Morrison M, Dekker Nitert M, SPRING Trial Group. Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension 2016;68(4):974–81. [DOI] [PubMed] [Google Scholar]
- 31.Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao J, Petrosino JF, Correa A, He J. Gut microbiome associates with lifetime cardiovascular disease risk profile among Bogalusa heart study participants. Circ Res 2016;119(8):956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callejo M, Mondejar-Parreño G, Barreira B, Izquierdo-Garcia J, Morales-Cano D, Esquivel-Ruiz S, Moreno L, Cogolludo Á, Duarte J, Perez-Vizcaino F. Pulmonary arterial hypertension affects the rat gut microbiome. Sci Rep 2018;8(1):9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson M, Verdi S, Maxan M, Shin C, Zierer J, Bowyer R, Martin T, Williams F, Menni C, Bell J, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun 2018; 9:2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jama H, Kaye D, Marques F. Population-based gut microbiome associations with hypertension. Circ Res 2018;123(11):1185–7. [DOI] [PubMed] [Google Scholar]
- 35.Marques F, Mackay C, Kaye D. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat Rev Cardiol 2018; 15:20–32. [DOI] [PubMed] [Google Scholar]
- 36.Marques F Missing heritability of hypertension and our microbiome. Circulation 2018;138(14):1381–3. [DOI] [PubMed] [Google Scholar]
- 37.Walejko J, Kim S, Goel R, Handberg E, Richards E, Pepine C, Raizada M. Gut microbiota and serum metabolite differences in African Americans and White Americans with high blood pressure. Int J Cardiol 2018; 271:336–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Adnan S, Nelson J, Ajami N, Venna V, Petrosino J, Bryan RJ, Durgan D. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics 2017. 23;49(2):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toral M, Robles-Vera I, de la Visitación N, Romero M, Yang T, Sánchez M, Gómez-Guzmán M, Jiménez R, Raizada M, Duarte J. Critical role of the interaction gut microbiota - sympathetic nervous system in the regulation of blood pressure. Front Physiol 2019; 10:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma R, Yang T, Oliveira A, Lobaton G, Aquino V, Kim S, Richards E, Pepine C, Sumners C, Raizada M. Microglial cells impact gut microbiota and gut pathology in angiotensin II-induced hypertension. Circ Res 2019; 124:727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mell B, Jala V, Mathew A, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S, Joe B. Evidence for a link between gut microbiota and hypertension in the dahl rat. Physiol Genomics 2015;47(6):187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karbach S, Schönfelder T, Brandão I, Wilms E, Hörmann N, Jäckel S, Schüler R, Finger S, Knorr M, Lagrange J, et al. Gut microbiota promote angiotensin II-induced arterial hypertension and vascular dysfunction. J Am Heart Assoc 2016;5(9):pii: e003698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marques F, Nelson E, Chu P, Horlock D, Fiedler A, Ziemann M, Tan J, Kuruppu S, Rajapakse N, El-Osta A, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017;135(10):964–77. [DOI] [PubMed] [Google Scholar]
- 44.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C, Han X, Huang Y, Zhao L, Li P, et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol 2017; 7:381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos-Romero S, Hereu M, Atienza L, Casas J, Jáuregui O, Amézqueta S, Dasilva G, Medina I, Nogués M, Romeu M, et al. Mechanistically different effects of fat and sugar on insulin resistance, hypertension, and gut microbiota in rats. Am J Phsiol Endocrinol Metab 2018;314(6): E552–63. [DOI] [PubMed] [Google Scholar]
- 46.Furness J, Costa M. The adrenergic innervation of the gastrointestinal tract. Ergeb Physiol 1974;69(0):2–51. [PubMed] [Google Scholar]
- 47.Phillips R, Rhodes B, Powley T. Effects of age on sympathetic innervation of the myenteric plexus and gastrointestinal smooth muscle of fischer 344 rats. Anat Embryol (Berl) 2006;211(6):673–83. [DOI] [PubMed] [Google Scholar]
- 48.Stewart D, Rubiano A, Santisteban M, Shenoy V, Qi Y, Pepine C, Raizada M, Simmons C. Hypertension-linked mechanical changes of rat gut. Acta Biomater 2016; 45:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Straub R, Wiest R, Strauch U, Harle P, Schölmerich J. The role of the sympathetic nervous system in intestinal inflammation. Gut 2006;55(11):1640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Pu Y, Chen J, Tong W, Cui Y, Sun F, Zheng Z, Li Q, Yang T, Meng C, et al. gastrointestinal intervention ameliorates high blood pressure through antagonizing overdrive of the sympathetic nerve in hypertensive patients and rats. J Am Heart Assoc 2014;3(5): e000929.doi: 10.1161/JAHA.114.000929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zubcevic J, Jun J, Kim S, Perez P, Afzal A, Shan Z, Li W, Santisteban M, Yuan W, Febo M, et al. Altered inflammatory response is associated with an impaired autonomic input to the bone marrow in the spontaneously hypertensive rat. Hypertension 2014;63(3):542–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeong J, Kim K, Lim D, Kim K, Kim H, Lee S, Song J, Moon B, Choy H, Park S. Microvasculature remodeling inthe mouse lower gut during inflammaging. Sci Rep 2017;7:39848, doi: 10.1038/srep39848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Racké K, Reimann A, Schwörer H, Kilbinger H. Regulation of 5-HT release from enterochromaffin cells. Behav Brain Res 1996;73(1–2):83–7. [DOI] [PubMed] [Google Scholar]
- 54.Kaelberer M, Buchanan K, Klein M, Barth B, Montoya M, Shen X, Bohórquez D. a gut-brain neural circuit for nutrient sensory transduction. Science 2018; 361:6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowell L, Detry J, Blackmon J, Wyss C. Importance of the splanchnic vascular bed in human blood pressure regulation. J Appl Physiol 1972;32(2):213–20. [DOI] [PubMed] [Google Scholar]
- 56.Kandlikar S, Fink G. Splanchnic sympathetic nerves in the development of mild DOCA-salt hypertension. Am J Physiol Heart Circ Physiol 2011;301(5):H1965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.King A, Osborn J, Fink G. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 2007;50(3):547–56. [DOI] [PubMed] [Google Scholar]
- 58.Osborn J, Fink G, Kuroki M. Neural mechanisms of angiotensin II-salt hypertension: Implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep 2011;13(3):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kremer M, Wright S. The effects on blood-pressure of section of the splanchnic nerves. Exp Physiol 1932;21(4):319–35. [Google Scholar]
- 60.Li M, Galligan J, Wang D, Fink G. The effects of celiac ganglionectomy on sympathetic innervation to the splanchnic organs in the rat. Auton Neurosci 2010; 154:66–73. [DOI] [PubMed] [Google Scholar]
- 61.McGavigan A, Henseler Z, Garibay D, Butler S, Jayasinghe S, Ley R, Davisson R, Cummings B. Vertical sleeve gastrectomy reduces blood pressure and hypothalamic endoplasmic reticulum stress in mice. Dis Model Mech 2017;10(3):235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cogan T, Thomas A, Rees L, Taylor A, Jepson M, Williams P, Ketley J, Humphrey T. Norepinephrine increases the pathogenic potential of campylobacter jejuni. Gut 2007;56(8):1060–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mittal R, Debs L, Patel A, Nguyen D, Patel K, O’Connor G, Grati M, Mittal J, Yan D, Eshraghi A, et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J Cell Physiol 2017;232(9):2359–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Jonge W The gut’s little brain in control of intestinal immunity. ISRN Gastroenterol 2013; 630159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartley A, Yang T, Arocha R, Malphurs W, Larkin R, Magee K, Vickroy T, Zubcevic J. Increased abundance of lactobacillales in the colon of beta-adrenergic receptor knock out mouse is associated with increased gut bacterial production of short chain fatty acids and reduced IL17 expression in circulating CD4+ immune cells. Front Physiol 2018; 9(1593). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Egerod K, Petersen N, Timshel P, Rekling J, Wang Y, Liu Q, Schwartz T, Gautron L. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metab 2018;12:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bellono N, Bayrer J, Leitch D, Castro J, Zhang C, O’Donnell T, Brierley S, Ingraham H, Julius D. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways Cell 2017;170(1):185, 198 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Psichas A, Reimann F, Gribble F. Gut chemosensing mechanisms. J Clin Invest 2015;125(3):908–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lach G, Schellekens H, Dinan T, Cryan J. Anxiety, depression, and the microbiome: A role for gut peptides. Neurotherapeutics 2018;15(1):36–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bravo J, Forsythe P, Chew M, Escaravage E, Savignac H, Dinan T, Bienenstock J, Cryan J. Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci U S A 2011;108(38):16050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.O’Leary O, Ogbonnaya E, Felice D, Levone B, C Conroy L, Fitzgerald P, Bravo J, Forsythe P, Bienenstock J, Dinan T, et al. The vagus nerve modulates BDNF expression and neurogenesis in the hippocampus. Eur Neuropsychopharmacol 2018;28(2):307–16. [DOI] [PubMed] [Google Scholar]
- 72.Dhar A, Barton D. Depression and the link with cardiovascular disease. Front Psychiatry 2016; 7:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hare D, Toukhsati S, Johansson P, Jaarsma T. Depression and cardiovascular disease: A clinical review. Eur Heart J 2014;35(21):1365–72. [DOI] [PubMed] [Google Scholar]
- 74.Celano C, Daunis D, Lokko H, Campbell K, Huffman J. Anxiety disorders and cardiovascular disease. Curr Psychiatry Rep 2016;18(11):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ouakinin S Anxiety as a risk factor for cardiovascular diseases. Front Psychiatry 2016;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonaz B, Sinniger V, Pellissier S. Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. J Intern Med 2017;282(1):46–63. [DOI] [PubMed] [Google Scholar]
- 77.Marshall R, Taylor I, Lahr C, Abell T, Espinoza I, Gupta N, Gomez C. Bioelectrical stimulation for the reduction of inflammation in inflammatory bowel disease. Clin Med Insights Gastroenterol 2015; 8:55–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci 2018; 12:49. doi: 10.3389/fnins.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costantini T, Bansal V, Peterson C, Loomis W, Putnam J, Rankin F, Wolf P, Eliceiri B, Baird A, Coimbra R. Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J Trauma 2010;68(6):1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Krzyzaniak M, Peterson C, Loomis W, Hageny A, Wolf P, Reys L, Putnam J, Eliceiri B, Baird A, Bansal V, et al. Postinjury vagal nerve stimulation protects against intestinal epithelial barrier breakdown. J Trauma 2011;70(5):1168–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stevens B, Goel R, Seungbum K, Richards E, Holbert R, Pepine C, Raizada M. Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut 2018;67(8):1555–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paton J The Sharpey-Schafer prize lecture: Nucleus tractus solitarii: integrating structures. Exp Physiol 1999;84(5):815–33. [PubMed] [Google Scholar]
- 83.Agostoni E, Chinnock JE, De Daly MB, and Murray JG. Functional and histological studies of the vagus nerve and its branches to the heart, lungs and abdominal viscera in the cat. J Physiol 1957; 135:182–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berthoud HR, and Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85:1–17. [DOI] [PubMed] [Google Scholar]
- 85.Deuchars J, Li Y-, Kasparov S, Paton J. Morphology of neurones in the nucleus tractus solitarius (NTS) of the working heart—brainstem preparation (WHBP) of the rat receiving afferent input from baroreceptors. Journal of Physiology 1998; 513.P, 83P.9706000 [Google Scholar]
- 86.Paton J, Deuchars J, Li Y, Kasparov S. Properties of solitary tract neurones receiving sub-diaphragmatic vagus nerve inputs. Journal of Physiology 1999; 518:176–7. [Google Scholar]
- 87.Moraes D, Machado B, Paton J. Carotid body overactivity induces respiratory neurone channelopathy contributing to neurogenic hypertension. J Physiol 2015;593(14):3055–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zubcevic J, Potts J. Role of GABAergic neurones in the nucleus tractus solitarii in modulation of cardiovascular activity. Exp Physiol 2010;95(9):909–18. [DOI] [PubMed] [Google Scholar]
- 89.Gordin D, Fadl Elmula F, Andersson B, Gottsäter A, Elf J, Kahan T, Christensen K, Vikatmaa P, Vikatmaa L, Bastholm Olesen T, et al. The effects of baroreflex activation therapy on blood pressure and sympathetic function in patients with refractory hypertension: The rationale and design of the nordic BAT study. Blood Press 2017;26(5):294–302. [DOI] [PubMed] [Google Scholar]
- 90.Head G Cardiac baroreflexes and hypertension. Clin Exp Pharmacol Physiol 1994;21(10):791–802. [DOI] [PubMed] [Google Scholar]
- 91.Daly D, Park S, Valinsky W, Beyak M. Impaired intestinal afferent nerve satiety signaling and vagal afferent excitability in diet-induced obesity in the mouse. J Physiol 2011;589(11):2857–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jun J, Zubcevic J, Qi Y, Afzal A, Carvajal J, Thinschmidt J, Grant M, Mocco J, Raizada M. Brain-mediated dysregulation of the bone marrow activity in angiotensin II-induced hypertension. Hypertension 2012;60(5):1316–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grundy D, Davison J. Cardiovascular changes elicited by vagal gastric afferents in the rat. Q J Exp Physiol 1981; 66:307–10. [DOI] [PubMed] [Google Scholar]
- 94.Folkow B Physiological aspects of primary hypertension. Physiol Rev 1982; 62:347–504. [DOI] [PubMed] [Google Scholar]
- 95.Oparil S The sympathetic nervous system in clinical and experimental hypertension. Kidney Int 1986;30(437):452. [DOI] [PubMed] [Google Scholar]
- 96.Mark A The sympathetic nervous system in hypertension: A potential long-term regulator of arterial pressure. J Hypertens Suppl 1996; 14:S159–65. [PubMed] [Google Scholar]
- 97.Mancia G, Grassi G, Giannattasio C, Seravalle G. Sympathetic activation in the pathogenesis of hypertension and progression of organ damage. Hypertension 1999; 34:724–8. [DOI] [PubMed] [Google Scholar]
- 98.Palatini P, Julius S. The role of cardiac autonomic function in hypertension and cardiovascular disease. Curr Hypertens Rep 2009; 11:199–205. [DOI] [PubMed] [Google Scholar]
- 99.Grassi G Assessment of sympathetic cardiovascular drive in human hypertension: Achievements and perspectives. Hypertension 2009; 54:690–7. [DOI] [PubMed] [Google Scholar]
- 100.Esler M Sympathetic nervous system moves toward center stage in cardiovascular medicine: From thomas willis to resistant hypertension. Hypertension 2014;63: e25–32. [DOI] [PubMed] [Google Scholar]
- 101.Piccirillo G, Viola E, Nocco M, Durante M, Tarantini S, Marigliano V. Autonomic modulation of heart rate and blood pressure in normotensive offspring of hypertensive subjects. J Lab Clin Med 2000; 135:145–52. [DOI] [PubMed] [Google Scholar]
- 102.Maver J, Struci M, Accetto R. Autonomic nervous system in normotensive subjects with a family history of hypertension. Clin Auton Res 2004; 14:369–75. [DOI] [PubMed] [Google Scholar]
- 103.Hedman A, Hartikainen J, Tahvanainen K, Hakumäki M. The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic ‘tone’. Acta Physiol Scand 1995; 155:267–73. [DOI] [PubMed] [Google Scholar]
- 104.Julius S, Krause L, Schork N, Mejia A, Jones K, van de Ven C, Johnson E, Sekkarie M, Kjeldsen S, Petrin J. Hyperkinetic borderline hypertension in Tecumseh, Michigan. J Hypertens 1991; 9:77–84. [DOI] [PubMed] [Google Scholar]
- 105.Julius S, Pascual A, London R. Role of parasympathetic inhibition in the hyperkinetic type of borderline hypertension. Circulation 1971; 44:413–8. [DOI] [PubMed] [Google Scholar]
- 106.Julius S, Randall O, Esler M, Kashima T, Ellis C, Bennett J. Altered cardiac responsiveness and regulation in the normal cardiac output type of borderline hypertension. Circ Res 1975; 36:199–207. [DOI] [PubMed] [Google Scholar]
- 107.Böhm R, van Baak M, van Hooff M, Moy J, Rahn K. Salivary flow in borderline hypertension. Klin Wochenschr 1985;63(suppl 3):154–6. [PubMed] [Google Scholar]
- 108.Gu H, Lin M, Liu J, Gozal D, Scrogin K, Wurster R, et al. Selective impairment of central mediation of baroreflex in anesthetized young adult fischer 344 rats after chronic intermittent hypoxia. Am J Physiol Heart Circ Physiol 2007;293(5):H2809–18. [DOI] [PubMed] [Google Scholar]
- 109.Lin M, Liu R, Gozal D, Wead W, Chapleau M, Wurster R, et al. Chronic intermittent hypoxia impairs baroreflex control of heart rate but enhances heart rate responses to vagal efferent stimulation in anesthetized mice. Am J Physiol Heart Circ Physiol 2007;293(2):H997–1006. [DOI] [PubMed] [Google Scholar]
- 110.Yan B, Soukhova-O’Hare G, Li L, Lin Y, Gozal D, Wead W, et al. Attenuation of heart rate control and neural degeneration in nucleus ambiguus following chronic intermittent hypoxia in young adult fischer 344 rats. Neuroscience 2008;153(3):709–20. [DOI] [PubMed] [Google Scholar]
- 111.Dergacheva O, Dyavanapalli J, Pinol R, Mendelowitz D. Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem. Hypertension 2014;64(3):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Desai M, Watanabe M, Laddu A, Hauptman P. Pharmacologic modulation of parasympathetic activity in heart failure. Heart Fail Rev 2011;16(2):179–93. [DOI] [PubMed] [Google Scholar]
- 113.Undeland K, Hausken T, Svebak S, Aanderud S, Berstad A. Wide gastric antrum and low vagal tone in patients with diabetes mellitus type 1 compared to patients with functional dyspepsia and healthy individuals. Dig Dis Sci 1996;41(1):9–16. [DOI] [PubMed] [Google Scholar]
- 114.Smart H, Atkinson M. Abnormal vagal function in irritable bowel syndrome. Lancet 1987;2(8557):475–8. [DOI] [PubMed] [Google Scholar]
- 115.Lindgren S, Lilja B, Rosén I, Sundkvist G. Disturbed autonomic nerve function in patients with Crohn’s disease. Scand J Gastroenterol 1991;26(4):361–6. [DOI] [PubMed] [Google Scholar]
- 116.Ameta K, Gupta A, Kumar S, Sethi R, Kumar D, Mahdi A. Essential hypertension: A filtered serum-based metabolomics study. Sci Rep 2017;7(1):2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rhodes C, Ghataorhe P, Wharton J, Rue-Albrecht K, Hadinnapola C, Watson G, Bleda M, Haimel M, Coghlan G, Corris P, et al. Plasma metabolomics implicates modified transfer RNAs and altered bioenergetics in the outcomes of pulmonary arterial hypertension. Circulation 2017;135(5):460–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang M, Yu Z, Deng S, Chen X, Chen L, Guo Z, Zheng H, Chen L, Cai D, Wen B, et al. A targeted metabolomics MRM-MS study on identifying potential hypertension biomarkers in human plasma and evaluating acupuncture effects. Sci Rep 2016; 6:25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Martin-Lorenzo M, Martinez P, Baldan-Martin M, Ruiz-Hurtado G, Prado J, Segura J, de la Cuesta F, Barderas M, Vivanco F, Ruilope L, et al. Citric acid metabolism in resistant hypertension: Underlying mechanisms and metabolic prediction of treatment response. Hypertension 2017;70(5):1049–1056. [DOI] [PubMed] [Google Scholar]
- 120.Chakraborty S, Galla S, Cheng X, Yeo J, Mell B, Singh V, Yeoh B, Saha P, Mathew A, Vijay-Kumar M, et al. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep 2018;25(3):677–689.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Derkach A, Sampson J, Joseph J, Playdon M, Stolzenberg-Solomon R. Effects of dietary sodium on metabolites: The dietary approaches to stop hypertension (DASH)-sodium feeding study. Am J Clin Nutr 2017;106(4):1131–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Loo R, Zou X, Appel L, Nicholson J, Holmes E. Characterization of metabolic responses to healthy diets and association with blood pressure: Application to the optimal macronutrient intake trial for heart health (OmniHeart), a randomized controlled study. Am J Clin Nutr 2018;107(3):323–34. [DOI] [PubMed] [Google Scholar]
- 123.Sekirov I, Russell S, Antunes L, Finlay B. Gut microbiota in health and disease. Physiol Rev 2010;90(3):859–904. [DOI] [PubMed] [Google Scholar]
- 124.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci U S A 2013;110(11):4410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Natarajan N, Hori D, Flavahan S, Steppan J, Flavahan N, Berkowitz D, Pluznick J. Microbial short chain fatty acid metabolites lower blood pressure via endothelial g protein-coupled receptor 41. Physiol Genomics 2016;48(11):826–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Soliman M, Rosenberger T. Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem 2011;352(1–2):173–80. [DOI] [PubMed] [Google Scholar]
- 127.Soliman M, Smith M, Houdek H, Rosenberger T. Acetate supplementation modulates brain histone acetylation and decreases interleukin-1β expression in a rat model of neuroinflammation. J Neuroinflammation 2012; 9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang T, Magee K, Colon-Perez L, Larkin R, Liao Y, Balazic E, Cowart J, Arocha R, Redler T, Febo M, et al. Impaired butyrate absorption in the proximal colon, low serum butyrate and diminished central effects of butyrate on blood pressure in spontaneously hypertensive rats. Acta Physiol (Oxf) 2019. January 18; e13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Febo M, Akbarian S, Schroeder F, Ferris C. Cocaine-induced metabolic activation in cortico-limbic circuitry is increased after exposure to the histone deacetylase inhibitor, sodium butyrate. Neurosci Lett 2009;465(3):267–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Won Y, Lu V, Puhl H 3, Ikeda S. β-Hydroxybutyrate modulates N-type calcium channels in rat sympathetic neurons by acting as an agonist for the G-protein-coupled receptor FFA3.. J Neurosci 2013;33(49):19314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dinan T, Cryan J. The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 2017;46(1):77–89. [DOI] [PubMed] [Google Scholar]
- 132.Goswami C, Iwasaki Y, Yada T. Short-chain fatty acids suppress food intake by activating vagal afferent neurons. J Nutr Biochem 2018; 57:130–5. [DOI] [PubMed] [Google Scholar]
- 133.Cuche G, Blat S, Malbert C. Desensitization of ileal vagal receptors by short-chain fatty acids in pigs. Am J Physiol Gastrointest Liver Physiol 2001;280(5): G1013–21. [DOI] [PubMed] [Google Scholar]
- 134.Bäckhed F, Ley R, Sonnenburg J, Peterson D, Gordon J. Host-bacterial mutualism in the human intestine. Science 2005;307(5717):1915–20. [DOI] [PubMed] [Google Scholar]
- 135.Mazmanian S, Liu C, Tzianabos A, Kasper D. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005;122(1):107–18. [DOI] [PubMed] [Google Scholar]
- 136.Corrêa-Oliveira R, Fachi J, Vieira A, Sato F, Vinolo M. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology 2016;5(4): e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short-chain fatty acids. Br J Pharmacol 2004;141(5):874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Matt S, Allen J, Lawson M, Mailing L, Woods J, Johnson R. Butyrate and dietary soluble fiber improve neuroinflammation associated with aging in mice. Front Immunol 2018; 9:1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Reisenauer C, Bhatt D, Mitteness D, Slanczka E, Gienger H, Watt J, Rosenberger T. Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J Neurochem 2011;117(2):264–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stern J, Filosa J. Bidirectional neuro-glial signaling modalities in the hypothalamus: role in neurohumoral regulation. Auton Neurosci 2013;175(1–2):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Stern J, Son S, Biancardi V, Zheng H, Sharma N, Patel K. Astrocytes contribute to angiotensin II stimulation of hypothalamic neuronal activity and sympathetic outflow. Hypertension 2016;68(6):1483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yang T, Rodriguez V, Malphurs W, Schmidt J, Ahmari N, Sumners C, Martyniuk C, Zubcevic J. Butyrate regulates inflammatory cytokine expression without affecting oxidative respiration in primary astrocytes from spontaneously hypertensive rats. Physiol Rep 2018;6(14): e13732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Davie J Inhibition of histone deacetylase activity by butyrate. J Nutr 2003;133(7 Suppl):2485S–93S. [DOI] [PubMed] [Google Scholar]
- 144.Pei F, Wang X, Yue R, Chen C, Huang J, Huang J, Li X, Zeng C. Differential expression and DNA methylation of angiotensin type 1a receptors in vascular tissues during genetic hypertension development. Mol Cell Biochem 2015; 402:1–8. [DOI] [PubMed] [Google Scholar]
- 145.Nedjadi T, Moran A, Al-Rammahi M, Shirazi-Beechey S. Characterization of butyrate transport across the luminal membranes of equine large intestine. Exp Physiol 2014;99(10):1335–47. [DOI] [PubMed] [Google Scholar]
- 146.Thibault R, Blachier F, Darcy-Vrillon B, de Coppet P, Bourreille A, Segain J. Butyrate utilization by the colonic mucosa in inflammatory bowel diseases: A transport deficiency. Inflamm Bowel Dis 2010;16(4):684–95. [DOI] [PubMed] [Google Scholar]
- 147.Guo J, Williams D, Puhl H 3, Ikeda S. Inhibition of N-type calcium channels by activation of GPR35, an orphan receptor, heterologously expressed in rat sympathetic neurons. J Pharmacol Exp Ther 2008;324(1):342–51. [DOI] [PubMed] [Google Scholar]
- 148.Agus A, Planchais J, Sokol H. Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 2018;23(6):716–24. [DOI] [PubMed] [Google Scholar]
- 149.Li G, Young K. Indole production by the tryptophanase tnaA in Escherichia coli is determined by the amount of exogenous tryptophan. Microbiology 2013; 159:402–10. [DOI] [PubMed] [Google Scholar]
- 150.Watts S, Morrison S, Davis R, Barman S. Serotonin and blood pressure regulation. Pharmacol Rev 2012;64(2):359–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alexandru N, Popov D, Dragan E, Andrei E, Georgescu A. Platelet activation in hypertension associated with hypercholesterolemia: Effects of irbesartan. J Thromb Haemost 2011;9(1):173–84. [DOI] [PubMed] [Google Scholar]
- 152.Gkaliagkousi E, Passacquale G, Douma S, Zamboulis C, Ferro A. Platelet activation in essential hypertension: Implications for antiplatelet treatment. Am J Hypertens 2010;23(3):229–36. [DOI] [PubMed] [Google Scholar]
- 153.Canani R, Costanzo M, Leone L, Pedata M, Meli R, Calignano A. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol 2011;17(12):1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reigstad C, Salmonson C, Rainey J 3, Szurszewski J, Linden D, Sonnenburg J, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. Faseb j 2015;29(4):1395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang L, de Kloet AD, Pati D, Hiller H, Smith JA, Pioquinto DJ, Ludin JA, Oh SP, Katovich MJ, Frazier CJ, et al. Increasing brain angiotensin converting enzyme 2 activity decreases anxiety-like behavior in male mice by activating central mas receptors. Neuropharmacology 2016;105(114):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wang L, de Kloet A, Smeltzer M, Cahill K, Hiller H, Bruce E, Pioquinto D, Ludin J, Katovich M, Raizada M, et al. Coupling corticotropin-releasing-hormone and angiotensin converting enzyme 2 dampens stress responsiveness in male mice. Neuropharmacology 2018; 133:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Martins Lima A, Xavier C, Ferreira A, Raizada M, Wallukat G, Velloso E, dos Santos R, Fontes M. Activation of angiotensin-converting enzyme 2/angiotensin-(1–7)/Mas axis attenuates the cardiac reactivity to acute emotional stress. Am J Physiol Heart Circ Physiol 2013;305(7):H1057–67. [DOI] [PubMed] [Google Scholar]
- 158.Tsavkelova E, Klimova S, Cherdyntseva T, Netrusov A. [Hormones and hormone-like substances of microorganisms: A review]. Prikl Biokhim Mikrobiol 2006;42(3):261–8. [PubMed] [Google Scholar]
- 159.Yano J, Yu K, Donaldson G, Shastri G, Ann P, Ma L, Nagler C, Ismagilov R, Mazmanian S, Hsiao E. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 2015;161(2):264–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takeuchi Y, and Sano Y. Serotonin distribution in the circumventricular organs of the rat. an immunohistochemical study. Anat Embryol 1983; 167:311–9. [DOI] [PubMed] [Google Scholar]
- 161.Scrogin K, Johnson A, and Schmid H. Multiple receptor subtypes mediate the effects of serotonin on rat subfornical organ neurons. Am J Physiol 1998;275: R2035–42. [DOI] [PubMed] [Google Scholar]
- 162.Cummings S, Groszmann R, Kaumann A. Hypersensitivity of mesenteric veins to 5-hydroxytryptamine- and ketanserin-induced reduction of portal pressure in portal hypertensive rats. Br J Pharmacol 1986;89(3):501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huzoor-Akbar C NY, Fossen D, Wallace D. Increased vascular contractile sensitivity to serotonin in spontaneously hypertensive rats is linked with increased turnover of phosphoinositide. Life Sci 1989;45(7):577–83. [DOI] [PubMed] [Google Scholar]
- 164.Dohi Y, Lüscher T. Altered intra- and extraluminal effects of 5-hydroxytryptamine in hypertensive mesenteric resistance arteries: Contribution of the endothelium and smooth muscle. J Cardiovasc Pharmacol 1991;18(2):278–84. [DOI] [PubMed] [Google Scholar]
- 165.Watts S, Baez M, Webb R. The 5-hydroxytryptamine2B receptor and 5-HT receptor signal transduction in mesenteric arteries from deoxycorticosterone acetate-salt hypertensive rats. J Pharmacol Exp Ther 1996;277(2):1103–13. [PubMed] [Google Scholar]
- 166.Nebigil C, Jaffré F, Messaddeq N, Hickel P, Monassier L, Launay J, Maroteaux L. Overexpression of the serotonin 5-HT2B receptor in heart leads to abnormal mitochondrial function and cardiac hypertrophy. Circulation 2003;107(25):3223–9. [DOI] [PubMed] [Google Scholar]
- 167.Blackmore W Effect of serotonin on renal hemodynamics and sodium excretion in the dog. Am J Physiol 1958;193(3):639–43. [DOI] [PubMed] [Google Scholar]
- 168.Gustafsson B, Tømmerås K, Nordrum I, Loennechen J, Brunsvik A, Solligård E, Fossmark R, Bakke I, Syversen U, Waldum H. Long-term serotonin administration induces heart valve disease in rats. Circulation 2005;111(12):1517–22. [DOI] [PubMed] [Google Scholar]
- 169.Nebigil C, Maroteaux L. A novel role for serotonin in heart. Trends Cardiovasc Med 2001;11(8):329–35. [DOI] [PubMed] [Google Scholar]
- 170.Pierce P, Xie G, Levine J, Peroutka S. 5-hydroxytryptamine receptor subtype messenger RNAs in rat peripheral sensory and sympathetic ganglia: A polymerase chain reaction study. Neuroscience 1996;70(2):553–9. [DOI] [PubMed] [Google Scholar]
- 171.Gerges N, Aleisa A, Alhaider A, Alkadhi K. 1. Reduction of elevated arterial blood pressure in obese zucker rats by inhibition of ganglionic long-term potentiation. Neuropharmacology 2002; 43:1070–6. [DOI] [PubMed] [Google Scholar]
- 172.Alkadhi K, Alzoubi K, Aleisa A, Tanner F, Nimer A. Psychosocial stress-induced hypertension results from in vivo expression of long-term potentiation in rat sympathetic ganglia. Neurobiol Dis 2005; 20:849–57. [DOI] [PubMed] [Google Scholar]
- 173.Callera J, Bonagamba L, Sévoz C, Laguzzi R, Machado B. Cardiovascular effects of microinjection of low doses of serotonin into the NTS of unanesthetized rats. Am J Physiol 1997;272(4):R1135–42. [DOI] [PubMed] [Google Scholar]
- 174.Sévoz C, Nosjean A, Callera J, Machado B, Hamon M, Laguzzi R. Stimulation of 5-HT3 receptors in the NTS inhibits the cardiac Bezold-Jarisch reflex response. Am J Physiol 1996;271(1):H80–7. [DOI] [PubMed] [Google Scholar]
- 175.Jordan D Vagal control of the heart: central serotonergic (5-HT) mechanisms. Exp Physiol 2005;90(2):175–81. [DOI] [PubMed] [Google Scholar]
- 176.Lin L, Lin M. Hypothalamic serotonin release and raised blood pressure after raphe nuclei stimulation in rats. Brain Res Bull 1996;39(5):305–9. [DOI] [PubMed] [Google Scholar]
- 177.Boscan P, Pickering A, Paton J. The nucleus of the solitary tract: An integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol 2002;87(2):259–66. [DOI] [PubMed] [Google Scholar]
- 178.Accorsi-Mendonça D, Machado B. Synaptic transmission of baro- and chemoreceptors afferents in the NTS second order neurons. Auton Neurosci 2013;175(1–2):3–8. [DOI] [PubMed] [Google Scholar]
- 179.Laguzzi R Serotonin2 receptors in the nucleus tractus solitarius: Characterization and role in the baroreceptor reflex arc. Cell Mol Neurobiol 2003; 23:709–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Browning K Role of central vagal 5-HT3 receptors in gastrointestinal physiology and pathophysiology. Front Neurosci 2015; 9:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Moreira T, Takakura A, Colombari E, and Guyenet P. Activation of 5-hydroxytryptamine type 3 receptor-expressing C-fiber vagal afferents inhibits retrotrapezoid nucleus chemoreceptors in rats. J Neurophysiol 2007;98:3627–37. [DOI] [PubMed] [Google Scholar]
- 182.Vasquez E, Meyrelles S, Mauad H, Cabral A. Neural reflex regulation of arterial pressure in pathophysiological conditions: Interplay among the baroreflex, the cardiopulmonary reflexes and the chemoreflex. Braz J Med Biol Res 1997;30(4):521–32. [DOI] [PubMed] [Google Scholar]
- 183.Pijacka W, McBryde F, Marvar P, Lincevicius G, Abdala A, Woodward L, Li D, Paterson D, Paton J. Carotid sinus denervation ameliorates renovascular hypertension in adult Wistar rats. J Physiol 2016;594(21):6255–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Prabhakar N Carotid body chemoreflex: A driver of autonomic abnormalities in sleep apnoea. Exp Physiol 2016;101(8):975–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Raizada M, Joe B, Bryan N, Chang E, Dewhirst F, Borisy G, Galis Z, Henderson W, Jose P, Ketchum C, et al. Report of the national heart, lung, and blood institute working group on the role of microbiota in blood pressure regulation: Current status and future directions. Hypertension 2017. July 13; pii: HYPERTENSIONAHA.117.09699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Krautkramer KA, Kreznar JH, Romano KA, Vivas EI, Barrett-Wilt GA, Rabaglia ME, Keller MP, Attie Alan D., Rey Federico E., Denu JM. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Molecular Cell 2016; 64:982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The host shapes the gut microbiota via fecal MicroRNA. Cell Host Microbe 2016;19(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qin Y, Wade P. Crosstalk between the microbiome and epigenome: Messages from bugs. J Biochem 2018;163(2):105–12. [DOI] [PMC free article] [PubMed] [Google Scholar]