Abstract
Humoral and cellular host defense mechanisms including diverse phagocytes, leukocytes, and immune cells have evolved over millions of years to protect the body from microbes and other external and internal threats. These policing forces recognize engineered sub-micron drug delivery systems (DDS) as such a threat, and react accordingly. This leads to impediment of the therapeutic action, extensively studied and discussed in the literature. Here, we focus on side effects of DDS interactions with host defenses. We argue that for nanomedicine to reach its clinical potential, the field must redouble its efforts in understanding the interaction between drug delivery systems and the host defenses, so that we can engineer safer interventions with the greatest potential for clinical success.
Keywords: Drug delivery system, nanoparticle, nanotoxicology, biocompatibility, immunogenicity, inflammation
Graphical abstract
1. Introduction
Drug delivery systems (DDSs) designed to improve effect of pharmacological agents inevitably elicit unintended effects in the body. Some of these effects are fairly benign or at least tolerable in the context of the medical application. Some may cause serious problems, adversities, and toxicities, which may preclude use of the DDS [1–3].
Practically every component of a DDS, including drug cargoes, may exert actions leading to undesirable effects within and outside the target. Many of these effects are distinct from those of a free drug, due to different pharmacokinetic, biodistribution, metabolism, and excretion. The carrier’s interactions with body lead to additional, sometimes quite challenging safety issues [1,2,4].
Theoretically, every organ, tissue, cell, and molecule may represent a site of non-therapeutic activities of a DDS. In many cases, the intended target is the main site of side effects, which are relatively specific for each DDS. In the case of tumor eradication, such effects are beneficial, whereas in many other medical situations unintended interference with target molecule or cell has negative consequences [5,6].
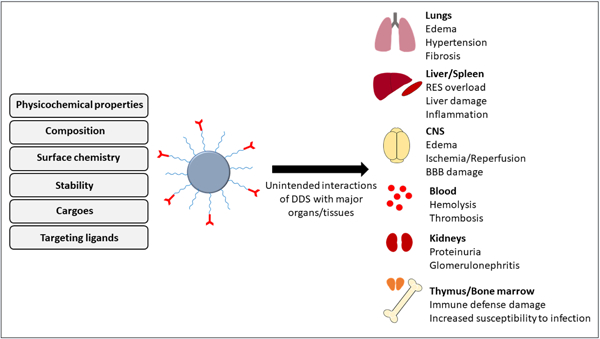
There are components in the body, which are commonly involved in and/or affected by DDS-induced side effects. They include tissue components at the administration site, components of blood and vascular walls (specifically, endothelium lining the lumen), as well as main clearing organs (liver, kidneys, lymphatics, and spleen) and host defense systems (Fig. 1).
Fig.1.
Examples of unintended interactions of drug delivery systems with major organs/tissues and their potential subsequent side effects. RES: reticuloendothelial system, BBB: the blood-brain barrier.
The multifaceted reactive systems of host defense are “professionally trained” to deal with natural invaders, which share many features of DDSs. Their interactions with DDS may lead to diverse potentially harmful consequences including elimination of DDS, activation of complement, white blood cells and resident macrophages. For the sake of focus and generalizability, this review will be focused on the unintended interactions of nanoscale DDS with host defense.
DDS’s key physicochemical characteristics, including size, shape; surface properties such as morphology, rigidity, chemistry, and charge; materials’ degradability; presence of impurities; as well as drug release kinetics can control the extent and nature of adverse effects. Several aspects of a nanoscale DDS that may contribute to its biological outcomes and unintended consequences are summarized in Fig. 2. In most cases, combination of these factors determines the fate of nanoparticle (NP) in the body. In this respect, while trying to provide the most probable NP feature connected to specific observed consequences, we understand the complex nature of such adverse effects which are generally orchestrated by multiple factors rather than a singular NP characteristic.
Fig. 2.
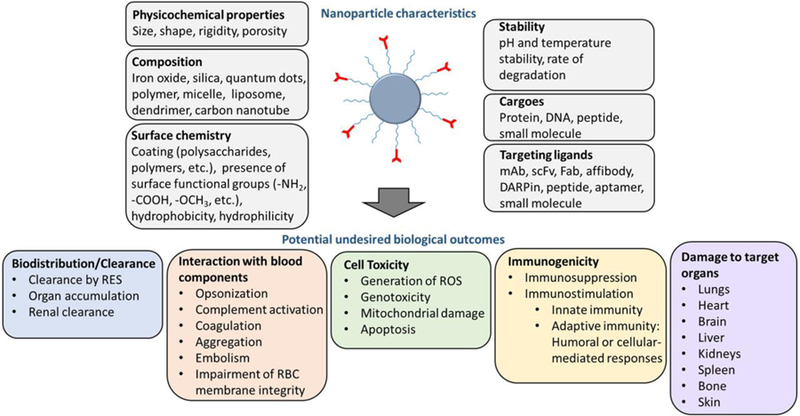
Nanoparticle characteristics and their influence on biological outcomes. Several features of drug delivery systems can impact their outcome in vivo. These include nanoparticle physicochemical properties, its composition, surface coating, type of cargo, and the particular targeting ligand. All of which determine the biocompatibility and the efficacy of the targeted nanoparticles in vivo.
2. General aspects of DDS toxicology
2.1. Materials nature, excipients, and impurities
2.1.1. Biodegradable versus non-biodegradable materials
Biocompatibility of the materials is not equivalent to their biodegradation. Some non-destructible materials such as metals and ceramics are fully biocompatible in form of implants and prosthetics, while some degradable materials can exert adverse toxic effects. Biocompatibility should be considered in the context of the patient’s condition, off-target interactions, route of administration, and characteristics of the materials.
Development of biodegradable and biocompatible DDSs is essential for prevention of any material-related harmful side effects. Biodegradable nanoparticles include those based on proteins, polysaccharides, and natural or synthetic polymers. Alginate, chitosan, agarose, and gelatin are examples of natural biodegradable polymers [7–10]. These natural polymers have been extensively used in development of DDSs as well as scaffolds in tissue engineering. Synthetic biodegradable polymers include poly(D,L-lactide-co-glycolide) (PLGA), polylactic acid (PLA), and poly-ε-caprolactone (PCL) [11–14]. Although biodegradable, synthetic polymeric materials have been reported to degrade into acidic byproducts (e.g. glycolic and lactic acids), which can reduce the local pH and lead to an inflammatory reaction [15,16].
Examples of non-biodegradable nanoparticles include ceramics, metal colloids, and polymers, such as (polyethylenimine) PEI and poly(dimethylaminoethyl methacrylate) (PDMAEMA) [17,18]. Non-biodegradable nanoparticles are cleared by the mononuclear phagocytic system and accumulate in the liver and spleen. This can further lead to potential irreversible toxic side effects [11]. Non-biodegradable rigid nanoparticles have been reported to induce reactive oxygen species (ROS) and autophagy. Polystyrene nanospheres, silica nanospheres, single-walled carbon nanotubes, and elongated iron oxide nanorods are among those NPs that induce ROS generation. Rigid, non-biodegradable nanoparticles, whether elongated or spherical have been found to trigger similar cellular effects, with the generation of ROS and autophagy being more severe in the elongated nanoparticles [19]. Although the precise mechanism for such enhanced ROS generation is not clear, elongated nanoparticles are believed to be able to escape endosome/lysosome [19]. In contrast to non-biodegradable NPs, reports of ROS generation as a result of biodegradable NPs has been minimal, exhibiting mainly in cases of biodegradable inorganic nanoparticles such as calcium carbonate nanocrystals and zinc oxide nanoparticles [20,21].
Polycationic polymers, such as PEI and PDMAEM are well-known for their efficiency in nucleic acid delivery [22], however they exhibit high cytotoxicity and are non-biodegradable. This can result in further complications, particularly when used as a long-term delivery system, where the non-biodegradable polymeric material continues to accumulate in the cytoplasm or the nucleus of the transfected cells [17,18]. High molecular weight (HMW) PEI (PEI 25k)-based nucleic acid delivery systems are non-biodegradable, and therefore changes have been made to these polycationic polymer to allow them to maintain high transfection efficiency, but in the same time reduce toxicity and enhance their degradability [22,23]. Some have tried to develop an efficient biodegradable PEI delivery system by combining low molecular (LMW) PEI polymers together using degradable cross-linkers to mimic HMW constructs. These modified PEIs were reported to be biodegradable and very efficient in gene delivery. Others have developed co-polymers of PEI with biocompatible and biodegradable polymers such as poly(β-amino esters) (PBAEs) [24]. PBAEs were developed using combinatorial synthesis and screening methods, with the lead candidates demonstrating high gene delivery efficiency, excellent biodegradability, and low cytotoxicity [25,26].
The therapeutic application and target organ of the nanoparticles must be considered when fine-tuning the biodegradability of the nanoparticle. There are instances where biodegradable nanoparticles are non-biocompatible and where non-biodegradable nanoparticles are biocompatible. These can occur when the nanoparticle degradation byproducts induce inflammatory or toxic effects [1,15,27], or when a non-biodegradable nanoparticles induces anti-oxidant or cytoprotective effects [28–30].
2.1.2. Excipients and impurities
Different types of non-APIs (non-active pharmaceutical ingredients) may be used in nanomedicine formulations, similar to any other type of pharmaceutical formulation. These could encompass a variety of excipients such as solvents/vehicles, surfactants, preservatives, stabilizers, etc. Excipients in nanomedicines should be chosen carefully to avoid any side effects originated from these components of formulation. Excipient stability after nanosizing, freeze-drying, and sterilization techniques should also be clearly monitored [31]. As reviewed by Center for Drug Evaluation and Research, FDA, the required specific safety data for excipients will depend on clinical factors including route of administration, treatment duration, existing reproductive and genetic toxicity, carcinogenicity, and hypersensitivity data [32]. For example, when applied intravenously, propylene glycol can be cardiotoxic and provoke thrombophlebitis [32].
Cremophor-EL is a nanosized micellar excipient often used to solubilize hydrophobic drugs such as paclitaxel. However, it can activate the complement system and induce mononuclear cells to produce pro-inflammatory chemokine IL-8 [33,34]. In this regard, protein-bound paclitaxel, also known as nanoparticle albumin–bound paclitaxel or nab-paclitaxel (Abraxane) is a good example of an improved nanoparticle formulation design. Nab-paclitaxel does not require any toxic solvent unlike its conventional counterparts, thus avoiding solvent-related toxicity issues such as hypersensitivity and neuropathy, which was among the side effects of conventional formulations of paclitaxel [35]. A common problem with solvent-based paclitaxel is the micelle entrapment of drug resulting in non-linear pharmacokinetics (PK). This issue does not happen with nab-paclitaxel [36]. Moreover, patients taking nab-paclitaxel do not require any premedication such as antihistamines and corticosteroids to prevent hypersensitivity reactions related to the solvents [37].
Contamination with impurities such as bacterial lipopolysaccharide (LPS), or endotoxin should be also cautiously monitored in NP preparations. Due to their high surface area, small particles can easily adsorb high amount of endotoxin if available. Undetected presence of endotoxin when formulating NPs could cause misinterpretation of NP-associated toxicological and inflammatory responses [38–41]. Monocytes and dendritic cells are particularly sensitive to endotoxin and exhibit inflammatory responses to minute amounts of LPS, usually considered as “endotoxin-free” in standard commercial products [38,42]. LPS not only interacts with well-known cell receptors such as TLR4 [43], but also stimulate intracellular LPS sensors [44,45]. It is now believed that LPS can be recognized within the cytoplasm by caspases 4 and 5 in humans and by caspase 11 in mice. Caspase-mediated LPS signal may potentially trigger inflammasome activation [46]. Therefore, it is plausible that an endotoxin contaminant transported into a cell via NP preparation could interact with caspases, inducing inflammasome stimulation in monocytes and DCs. These observations and potential unintended events make it necessary to recognize and remove even trace levels of LPS or any other similar contaminant containing pathogen-associated molecular patterns (PAMPs) when preparing NP formulations.
Several sterilization techniques such as filtration, thermal sterilization, irradiation, and other methods have been reviewed for nanoparticle formulation sterilization [47]. As far as they do not affect the stability of NP and biological activity of active ingredients, these approaches are pretty effective on microbial decontamination [40,48]. However, common sterilization procedures are usually not efficient in removing endotoxin contamination in NP samples. Techniques developed to remove endotoxin from samples are usually called ‘depyrogenation’ techniques [40,49].
Ultrafiltration was proposed as one such method. For example, endotoxin is shown to be retained by a 0.025 μm filter, as endotoxin can adsorb to the filter membrane [40,50]. A drawback of applying this procedure for depyrogenation of nanoparticle formulations is that particles may trap on membrane as well. Moreover, the contaminated particles could still escape through the filter if endotoxin is already adsorbed on the particle surface [40].
Another example is two-phase extraction technique [51], in which endotoxin can be removed with the added detergent to sample via the nonpolar interaction of lipid A. Gold nanoparticles were reported to become nonpyrogenic after treating with this two-phase method [52]. However, usually traces of detergent remain in the sample, which requires further purification steps such as adsorption or gel-filtration to remove the detergent [40].
The plasma discharge method can be given as another example of effective strategy for sterilization and depyrogenation. Active species (e.g., atomic oxygen, OH radicals) produced by the plasma discharge are very potent in killing spores and microorganisms, as well as destroying other contaminants such as pyrogens/endotoxin [53]. Although demonstrated to be effective in removing endotoxin from nanoparticle formulations, this method should be validated for each type of nanoparticle as it can significantly alter the surface characteristics of NPs [40,48].
Pyrogen inactivation techniques involving acid-base hydrolysis, oxidation, treatment with sodium hydroxide and heating, are other effective strategies to remove endotoxin [40]. These procedures have been successfully applied for microparticles such as titanium [54] or cobalt chrome particles [55]. Due to the aggressive nature of these techniques, their use for nanomaterials is usually challenging. Among these, the incineration method seems to be feasible for nanoparticles in solid form (powder) including carbon nanotubes or titanium dioxide NPs, since they can tolerate the high temperature. For example, silicon oxide, titanium oxide, zirconium oxide, and cobalt nanoparticles were efficiently depyrogenated at 180°C for 4 h [56].
Other than strategies mentioned above, there are less-investigated methods that have been suggested for endotoxin separation. These include ion-exchange chromatography, affinity adsorption chromatography, and gel filtration chromatography [40]. Overall, it is obvious that endotoxin removal is not an easy task and its applicability is highly dependent on the nature of NP formulation that needs to be decontaminated. Therefore, preventing contamination by using endotoxin-free reagents and glassware during the NP synthesis seems to be the best way for obtaining endotoxin-free particles [40,57].
2.2. Size
Unlike large drug delivery systems that are usually restricted to limited body areas, nanoparticles typically travel throughout the body quickly and face several cellular and non-cellular systemic components. Therefore, sub-micron DDS adversities may include mechanisms that are not ignited by large delivery systems of the same material. Similarly, nanoparticle is also different from bulk materials. A common diameter size range for NPs injected systemically is 50–300 nm [58]. Enhanced surface/mass (S/M) ratio in NPs compared to larger counterparts exerts significant impact on their circulation, degradation, and intracellular delivery (Table 1). For instance, highly increased S/M may enable degradation via burst release instead of slower, safer surface erosion. S/M ratio also enhances the ability of NP to absorb, activate, and deplete biomolecules [59,60]. Extravasation, tissue penetration, and localization may also differ from larger delivery systems.
Table 1.
| Features | Large vs. small DDS | |
|---|---|---|
| Size | Large (millimeters) | Small (sub-micron) |
| Surface/mass ratio | Low | High |
| Release kinetics | Surface erosion | Burst release |
| Blood circulation | No | Yes |
| Cellular uptake | No | Yes |
| Capacity of interactions with biomolecules | Modest | Extensive |
Due to the small size and high surface area in nanoparticles, chemically reactive groups are highly exposed and could play a role in the adverse biological effects. For instance, the enhanced surface reactivity may lead to protein unfolding, membrane impairment, DNA damage, immune reactivity, and inflammatory responses [1]. It has been demonstrated that titanium dioxide (TiO2) NP size modulate the affinity of plasma proteins such as fibrinogen towards the TiO2 surfaces [61,62]. NP aggregates with extensive surface area and correspondingly increased number of contact points with fibrinogen [61] triggered elevated inflammatory responses [62].
Smaller particles bind to cells and may internalize via vacuolar uptake mechanisms unavailable to larger objects. Particles in the size range of 10–500 nm and even up to micron size were reported to get internalized into cells via vesicular pathways [63–65]. The large particles can be engulfed via macropinocytosis [64], whereas particles with sizes ranging 10–300 nm were reported to enter through clathrin-mediated mechanism [63,66]. Caveolae-mediated pathway may facilitate the entry of nanoparticles of 60–80 nm [64,67] and in some cases up to 100 nm in diameter [68]. It should be noted that modulation of the uptake and trafficking by size of particles is also cell-type specific. For example, professional phagocytes consume relatively larger particles in the micron range faster, whereas endothelial cells prefer an order of magnitude smaller particles [69].
Some adverse effects of internalizable DDS may relate to their interactions with sub-cellular organelles. Endoplasmic reticulum (ER) is a vital sub-cellular compartment mediating protein synthesis, protein folding, Ca2+ storage, and lipid biosynthesis [70]. The effect of NP exposure on ER stress has been reviewed elsewhere [1,71,72]. As depicted by Hussain et al. [72], NPs are capable of inducing ROS production via their inherent effect on catalysis of oxidation reduction reactions through their surfaces. They may also interact with cellular components and/or normal ROS production mechanisms such as mitochondria and NADPH oxidase system. Moreover, NPs can decrease the cellular anti-oxidant defense mechanisms through savaging/inactivation or decreased production of anti-oxidants [72]. A hierarchical oxidative stress model was proposed by Nel et al. [1] as 1) inducing anti-oxidant enzymes at low levels, 2) activating pro-inflammatory responses at intermediate levels and 3) triggering cell death at very high oxidative stress levels. For instance, ER stress may evolve into mitochondrial-dependent apoptosis due to the surge in cytosolic Ca2+ and ROS levels [73,74]. ER stress may also trigger autophagy to enable the clearance of accumulated proteins [70]. Another subcellular compartment, the lysosome, functions as a cellular digestive organelle. If the function of a lysosome is impaired by the accrual of nanomaterials, autophagy flux can be blocked by subsequent autophagosome accumulation. Furthermore, the release of cathepsins and other associated lysosomal hydrolases into the cytoplasm may trigger inflammation responses and mitochondrial depolarization [70,73,75].
NP size may also affect changes in cell phenotype. Particle size along with other characteristics play a role in inducing M1 polarization in macrophages [2]. Ma et al. [76] reported a size-dependent interaction between graphene oxide (GO) nanoparticles and plasma membrane. They demonstrated that larger (750–1300 nm) GO could drastically stimulate TLRs (e.g., TLR4) leading to macrophage M1 polarization through canonical NF-κB signaling, which could promote pro-inflammatory responses. The authors correlated this effect to a robust adsorption of larger GOs onto the plasma membrane rather than massive phagocytosis that mainly occurs with smaller (50–350 nm) GO sheets. A slightly reversed phenomenon was reported by Yen et al. [77] for gold nanoparticles (AuNPs), in which the macrophages treated with smaller AuNPs showed giant spread morphology representative of macrophage activation. Moreover, AuNPs at 1 ppm led to up-regulation of pro-inflammatory cytokines such as IL-1, IL-6, and TNFα in macrophages shortly (3 hrs) after the treatment. Here, the inflammatory response was described as being activated by NP uptake rather than spread over macrophages. Serum adsorption onto negatively-charged AuNPs could possibly result in internalization via complicated endocytic pathways that led to the pro-inflammatory reaction. Size, therefore, can be used as a property of NP to target specific cells. For example, particle size range of 20–40 nm has been suggested to be ideal for NPs uptake into dendritic cells (DCs) [78,79].
Nanoscaled DDS usually consists of different entities including carrier components, excipients, and therapeutic content; each may hold toxicity induction capabilities. Hence, any study evaluating the adverse behavior of DDS demands careful investigation of each entity individually, and in combination with other parts as a whole nanoparticulate system. Nanomaterials may induce complex cellular signaling mechanisms or modify existing signaling pathways resulting in adverse/unexpected consequences [72]. Mechanistic studies specifically focused on the fate of nanoparticles in vivo, such as the effect of each individual NP building block/feature on interaction with plasma components as well as cellular entities and the subsequent potential side effects will allow us to design safer DDS moving forward.
2.3. Shape
Shape of NP can be considered as even more of a determining factor in cellular uptake than size. The rate by which phagocytic cells engulf nanoDDS changes with the geometrical properties. Champion et al. [80] demonstrated that the local shape of polystyrene beads at the point of initial contact with macrophages, and not their size, determine whether cells will proceed with phagocytosis or simply spread over the particle. For instance, elongated prolate ellipsoidal shaped polystyrene particles attach to phagocytic cells better than spheres, but are phagocytosed less efficiently [81]. Therefore, it was suggested that elongated particles deliver drugs to various types of cells more efficiently by eluding internalization into phagocytic cells [80,82,83]. Similarly, disk-shaped polystyrene beads presented longer half-lives in circulation and greater targeting specificity in mice compared to analogous spherical particles [84]. In rodents, long worm-shaped PEG-polyethylethylene filomicelles, block copolymer micelles that form into filaments, exhibited prolonged circulation time, escaped macrophage internalization, and accumulated highly in tumors [85,86].
Although non-spherical particles seem to evade the clearance mechanism to some extent, one can imagine that the higher contact area of elongated shaped NPs with the cells may lead to more damage to cell membrane [87]. In this context, rod-shaped silver NPs were found to be toxic whereas spherical NPs with the same mass concentration were shown to be safe on the human lung epithelial cell (A549 cells). The direct contact and accumulation of long wires onto the cells was proposed to cause toxicity by inducing small pores in the cell membrane. A similar trend was observed by comparing the toxic behavior of rod-shaped zinc oxide (ZnO) NPs versus spherical counterparts on A549 [88], although it should be noted that A549 cells are not highly phagocytic in nature. Using different types of cells, similar conclusions were made in the work of Wang et al. [89], in which long anodic alumina nanotubes (AANTs-L) triggered significant alternations in cell morphology, TNF-α release, lysosomal membrane permeabilization (LMP), and ER stress leading to cell death and inflammation to a greater extent than shorter nanotubes. This study was performed on both RAW 264.7 mouse macrophage cells and MDA-MB 231-TXSA human breast cancer cells. A combination of NP properties including size, shape, and surface chemistry may direct the particles into sub-cellular compartments including lysosomes and orchestrate their subsequent mechanical and chemical vacuole rupture. The resulting release of danger signals into the cytoplasm will potentially induce toxicity, although this effect has been exploited to enhance cancer vaccine efficiency [90,91].
Particle shape has also been linked to the genotoxicity observed by some NPs [92]. For example, tubular-shaped particles such as carbon nanotubes (CNTs) induced greater DNA damage than ZnO particles. It should be noted that since ZnO particles create a higher level of oxidative stress, the superior DNA damage detected by CNTs likely comes from mechanical injury and not oxidative effect. The fact that there is evidence on CNTs crossing the nuclear membrane and reaching the nucleus justifies this physical contact-based theory [93,94].
The impact of particle shape on the biological outcome of NPs was also reported in large animals. Wibroe et al. [95] showed that spherical particles were more rapidly cleared from the blood as compared to non-spherical particles (rods and disks) when injected directly in pigs (Fig. 3a–d). Although initially, spheres, rods, and disks did not stimulate the complement system five minutes post I.V. injection, delayed complement activation was induced more robustly by rods and disks, rather than spheres (Fig. 3e and f). In the same study, rods and disks did not induce any notable cardiopulmonary distress when compared to spheres (Fig. 3g). It was implied that immediate and robust particle phagocytosis by resident pulmonary intravascular macrophages (PIMs) may lead to cardiopulmonary responses [95]. In a different context, wire-shaped aluminum oxide nanoparticles exhibited pro-inflammatory effects such as NLRP3 inflammasome activation and enhanced TGF-β level on primary splenocytes more significantly than spherical counterparts. Nanowires led to more profound inflammation and enhanced degree of lung metastasis in a syngeneic mouse tumor model relative to spheres [4].
Fig. 3.
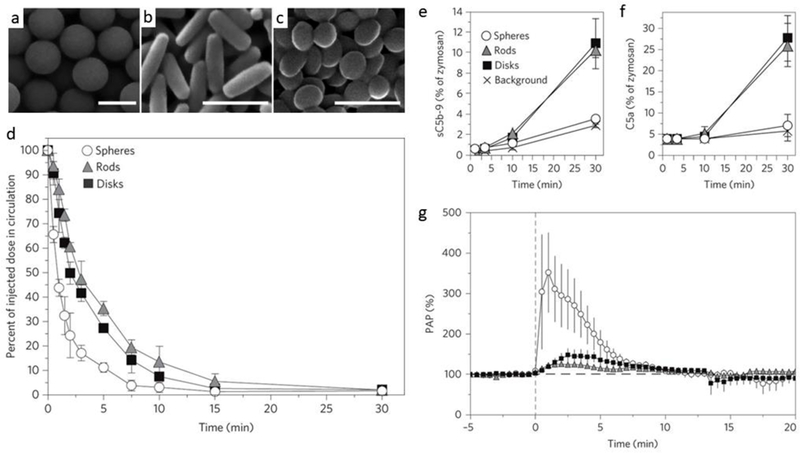
The effect of nanoparticles’ shape on biological outcome in pigs. (a) Scanning electron microscopy (SEM) of spheroids, rods (prolate spheroids), and disks (oblate spheroids). (b) Circulation profile of particles in pigs following intravenous injection at 1.5×1011 particles per 20 kg body weight. Complement activation in blood (c), and pulmonary arterial pressure (PAP) (d) after injection of spheres (circles), rods (triangles) and disks (squares) in pigs was compared with background level. Reprinted with permission from ref [95].
2.4. Elasticity
Careful control of the mechanical properties of nanomaterials has emerged as a design consideration in nanomedicine [58,96]. The mechanical flexibility of a nanoparticle can tune biomedical outcomes including pharmacokinetics and in vivo targeting, which in turn modulate clearance mechanisms and off-target behaviors.
Most notably, flexible nanoparticles have delayed clearance relative to stiff counterparts. Red blood cells represent a natural blueprint for engineering of materials that are benign during prolonged circulation in the blood. Among their salient characteristics, red cells are capable of avoiding splenic filtration and undergoing repeated extrusion through capillaries of ~1/10 their diameter [97]. Highly flexible hydrogel nanoparticles designed to mimic the shape and size of red cells have circulation times prolonged relative to similar particles with higher crosslinker density [98,99]. However, even without mimicry of the size and shape of red cells, more flexible nanoparticles have reduced RES interactions. PEG diacrylate nanogels with elastic moduli better resembling those of cells (~10 kPa) circulated longer and more effectively targeted the lungs as compared to harder (~3000 kPa) PEG diacrylate particles [100]. Filomicelles manifest a combination of high aspect ratio elongated shape and low rigidity [85,101,102]. Their properties uniquely allow alignment with blood flow and, all told, the filament particles circulate ~10 times longer than similar spherical particles [85,101]
Beyond behavior in flowing blood, minimized RES clearance of flexible filomicelles may also be explained by the minimal interactions of the particles with cells in vitro in the absence of antibody-directed adhesion to cell surfaces [101]. Indeed, the mechanical properties of nanoparticles may generally represent a variable affecting nanoparticle interaction with and uptake by a variety of cell types. Noting that mechanical flexibility of the microenvironment or substrate hosting a cell can dramatically affect the behaviors of the cell [103], it is perhaps unsurprising that the mechanical flexibility of nanoparticles interacting with cells can impact the tendencies of the cell-particles interaction. It was confirmed in vitro that rigid disks were consumed up to 3-fold more efficiently by mouse bone marrow-derived macrophages and macrophage-like cell lines in comparison to softer counterpart particles [104,105]. During the phagocytic process, soft particles can be easily deformed, which makes their internalization less energetically favorable than rigid particles [106]. Polyallylamine particles escaped phagocytosis when they were made softer by reduction of crosslinking density, with soft polyacrylamide particles avoiding Fc-mediated uptake in vitro [107,108]. Similarly, the PEG diacrylate particles described above more effectively avoided non-specific uptake in tumor cells, endothelial cells, and macrophages when they were manufactured with ~10 kPa modulus, as opposed to ~3000 kPa [100].
While there is evidence that nanoparticle flexibility may reduce non-specific uptake in immune cells and avoid RES clearance, lower elastic moduli may conversely enhance nanoparticle targeting in certain cases. Soft platelet-mimetic polyallylamine hydrochloride-BSA particles exceeded counterpart rigid particles with identical size, shape, and surface chemistry in targeting fibro-collagenous surfaces [109]. Increased membrane fluidity of antibody-functionalized lipid particles has been shown to enhance selectivity of targeting to endothelial markers [110]. Beyond direct effects on nanoparticle affinity interactions, the synergy of affinity targeting and prolonged circulation due to mechanical flexibility has yielded enhanced targeting to intercellular adhesion molecule (ICAM) in the case of PEG diacrylate nanogels [100]. There is also evidence of a role for nanoparticle mechanical flexibility in non-affinity targeting. Mammary epithelial cells and breast cancer cells more avidly took up lipid-alginate particles with lower Young’s moduli (as controlled by alginate crosslinking density). In vivo, the highly flexible lipid-alginate particles successfully targeted tumors in an orthotopic model, while concurrently manifesting reduced liver uptake, relative to rigid counterparts [111].
Of note, precise tuning and characterization of the mechanical flexibility of biocompatible nanomaterials is an area of ongoing research [85,112,113]. Further consideration given to the role of physical structural properties for injectable nanomaterials may identify additional effects and side effects of mechanical properties. For instance, recent studies have explored nanomaterials that either mimic or interact with blood components. A critical role for materials flexibility has been identified for nanoparticles incorporating in the coagulation process, where nanogels targeted to fibrin have been shown to affect clot structure during the process of clot contraction. Inflexible particles disrupt the process of clot contraction and solidification, while flexible counterparts participate in and enhance that process [114].
Conversely, modifying the mechanical properties of nanomaterials may come with new unintended side effects. Liposomes, hydrogels, and polymer-lipid composites can be modified via chemical structure of molecular components or features of the supramolecular assembly [96,110,111,115]. A standard and direct approach to modification of the flexibility of polymeric nanomaterials is the inclusion of crosslinkers [107,115]. However, inclusion of crosslinking (either intrinsic to the polymer or via introduction of exogenous bifunctional linkers) entails variation of polymerization conditions and possible modifications to the size distribution and surface chemistry of the resultant nanomaterials. Therefore, interpretation of flexibility effects on biomedical behavior of nanomaterials may be subject to assessment of the interplay of mechanical properties with chemistry, size, and shape. Likewise, outcomes regarding side effects and pharmacokinetics, including those enumerated here, may require further investigation exploring a more thorough space of physical design parameters [96].
2.5. Surface chemistry and charge
Multiple surface properties such as charge, hydrophobicity, and specific functional groups can contribute significantly to nanoparticle-associated toxicity. Even slight changes in surface chemistry may modulate extent of NPs interaction with cells. As an example, galactose- and mannose-modified silver nanoparticles showed considerably less toxicity than glucose- and citrate-modified NPs on both a neuronal-like cell line (Neuro-2A) and a hepatocyte cell line (HepG2) [116]. Pre-coating of particles with serum proteins also appears to reduce NPs harmful impact [117]. Serum protein coat on TiO2 particles prevented photo-generated radical production, suggestive of the barrier role of serum proteins [118].
The net charge on the particle surface is a major factor controlling not only the interaction with plasma components or cells, but also with subsequent cargo release. Nucleic acid carriers such as polyethylenimine (PEI), poly(propylene imine) (PPIs) dendrimers, polyamidoamine (PAMAM) dendrimers, etc. take advantage of their highly positive charged surface to interact with cell membranes effectively [22]. Polycationic vehicles have been shown to destabilize endosomal membranes, buffer the acidic endosomal pH, and mediate lysosomal rupture, leading to high cargo release into cytoplasm and enhancing transfection efficiency [119]. The same effective interaction with several layers of membranes may also lead to membrane perturbation and toxicity, especially when used at high doses.
Both linear and branched PEIs induced rapid plasma membrane disturbance in three clinically relevant human cell lines (Jurkat T cells, umbilical vein endothelial cells, and THLE3 hepatocyte-like cells) within 30 min of exposure. These early necrotic-like changes include substantial lactate dehydrogenase release and phosphatidylserine translocation from the inner plasma membrane to the outer cell surface. Later toxic events (24 hrs post-treatment) are characterized by activation of a mitochondrially-driven apoptotic program [120]. In a similar way, highly positively charged amidine functionalized polystyrene (PS) particles impaired lysosome function. Possibly PS particles displayed their disruptive effect on lysosomes through a similar lysosomal rupture mechanism as explained for PEI [121]. Compared to linear PEI, branched PEI architecture led to an enhanced intracellular ROS levels. The branched PEI structure also induced a concentration-dependent collapse in glycolytic flux reducing glucose flux through the pentose phosphate pathway (PPP). As mentioned above, leakiness to key player enzymes of glycolysis such as LDH could probably disturb the rate of glycolytic flux [122].
The presence of dense clusters of surface cationic charge in polycations and polyplexes enhances complement activation [123]. Cardiopulmonary distress observed upon PEI injection in the porcine model [124] can be explained in part by complement activation. Moreover, a strong polycation and polyplex clearance by intravascular pulmonary macrophages in pigs independently of complement stimulation leads to a surged release of thromboxane A2, prostaglandin, and prostacyclin molecules which could subsequently mediate periods of peak vasoconstriction, bronchoconstriction, and pulmonary hypertension [125].
Although not extensively discussed, anionic NPs can also initiate adverse effects. Positively charged AuNPs were reported to adsorb proteins with a pI <5.5 such as albumin, whereas negatively charged AuNPs were covered with a protein corona mainly composed of proteins with a pI >5.5 such as apolipoprotein [126]. In another report, negatively charged poly(acrylic acid) (PAA)-conjugated AuNPs were shown to adsorb fibrinogen on their surface. Upon attachment to the surface of NPs, fibrinogen becomes unfolded and induces inflammatory cytokine release via the integrin receptor, Mac-1, leading to subsequent NF-kB signaling [62]. Negatively-charged NPs have been also found to induce the classical complement cascade [127].
It is generally believed that scavenger receptors, a family of cell surface glycoproteins, recognize some anionic surfaces [128]. Different types of scavenger receptors on macrophages are reported to remove a variety of negatively charged particles such as liposomes containing negatively charged phospholipids [129], and negatively charged polystyrene nanospheres [130]. Nagayama et al. [130] demonstrated that a serum protein called fetuin, associated on the surface of negatively charged polystyrene nanospheres, directed its uptake by Kupffer cells via scavenger receptors. It was reported that scavenger receptor SR-B1, which is involved in the uptake of AgNPs, could activate immune system by inducing pro-inflammatory cytokines and up-regulation of co-stimulatory molecules [131,132]. Furthermore, macrophages from SR-B1 deficient mice internalized lower number of AgNPs and showed a reduced inflammatory response as measured by neutrophilic influx and IL-6 mRNA expression [131,132].
2.6. In vitro-in vivo correlation in nanotoxicology
Numerous types of in vitro toxicity assays (summarized in Table 2) exist to evaluate the toxic behavior of NPs, however, any extrapolation from in vitro data to in vivo behavior should be made with caution. Additionally, caution should also be taken when making comparison among different in vitro studies. For example, the type of cells used for an experiment impacts the observed toxicity pattern, explained by differences in phagocytic ability, cell proliferation capability, and functional status of cell in body [133,134]. The RAW 264.7 cell line is an extensively studied macrophage cell line for nanotoxicology, largely due to its defense roles in the immune system. Cancer cell lines have also been widely used for toxicity evaluations; however, their common apoptosis resistance characteristics may counteract the accuracy of the toxicity analysis. Hence, it is suggested to complement in vitro toxicity studies in cancer cell lines with primary cells to help finding adverse trends more accurately [135].
Table 2.
In vitro assays to evaluate toxicity of NP preparations.
| Assay | Mechanism | Advantages/use | Limitations | References |
|---|---|---|---|---|
| Chromium-51 release assay | Detection of irreversible damage to cell plasma membrane. | High sensitivity, accuracy, and simplicity. | Requires handling of hazardous radioisotopes, and needs pre-labeling of cells. | [136–138] |
| LDH release assay | Detection of damage to cell membrane by measuring the release of intracellular LDH enzyme. | No need in cell labeling and use of isotopes. | Less sensitive, takes more time and less high-throughput than above. | [139,140] |
| Metabolic activity assay (MTT, MTS, etc.) | Colorimetric assay measuring cellular metabolic activity, such as ability to reduce tetrazolium dye. | Simple, Cost-effective, Indicates total metabolic status. | Numerous intracellular factors can influence reduction of dye leading to inaccurate results. | [141–144] |
| Protease activity assays (CytoTox-Glo) | Luminescent assay measuring activity of intracellular protease released from cells, which have lost their membrane integrity. | Different protease activities can be measured. | Costly, Signal interference from fluorescent nanoparticles. | [145–147] |
| Calcein AM assay | Calcein AM is a permeable molecule that enters cells and is hydrolyzed by intracellular estrases to Calcein which is fluorescent and is retained inside cells. | Simple, Indicative of membrane damage. | Signal interference from fluorescent nanoparticles. | [148–150] |
| Oxidative stress assays (DCFH assay) | Measures the release of reactive oxygen species by detecting conversion of substrates into fluorescent or colorimetric outputs. | Simple, Direct measure of cellular redox state. | Not a direct measure of H2O2.Several other oxidative species can oxidize DCFH. Released cytochrome C can oxidize DCFH. | [151–153] |
| Apoptosis assays (Annexin V staining, caspase activity assays) | Detects presence of phosphatidyl serine in apoptosing cells by annexin V staining; or measurement of caspases such as caspase 3 activated during apoptosis. | Simple, Distinguishes apoptosis from necrosis. | Annexin V staining requires live cells, and for adherent cells must use detachment agents that do not damage the cell membrane. | [154–156] |
| Genotoxicity assays (comet assay) | Single cell gel electrophoresis assay that measure DNA strand breaks. | Rapid, Indicates DNA damage. | Requires an internal reference to avoid variations. Inability to detect specific mutations generated. | [157–160] |
| Hemocompati bility assays (Hemolysis assay) | Spectrophotometric measurement of the amount of hemoglobin released. | Simple, Indicates RBC damage. | Nanoparticle binding to complement proteins can alter the hemolytic activity. | [161–164] |
| Macrophage cytokine profiling | Measures cytokines released from macrophages, using systems such as ELISA or Luminex bead array assays. | Simple, extensively used, High throughput assay for several cytokine measurement in one assay. | Endotoxin contamination can produce false positive results. | [165–167] |
| Leukocyte proliferation assay | Leukocytes are incubated with different concentration of nanoparticles, followed by measurement of effect on leukocyte proliferation, such as by 3H-thymidine incorporation. | Quantitative analysis of lymphocyte activation and proliferation. | Some assays require radioisotopes, which are biological hazards. | [168–170] |
Particle size combined with particle number and surface area will ultimately determine the actual dose of exposure to nanoparticles. NP properties such as size and shape may change markedly after injection due to agglomeration and adsorption of biomolecules [58]. Notably, in vivo parameters such as diet, body temperature, health status, dynamic and fluidic variations could possibly challenge all assumptions based on in vitro assays. Therefore, it would be ideal to study both therapeutic and side effects of NPs in a relevant environment. A list of in vivo assays evaluating toxicity behavior of NPs is provided in Table 3.
Table 3.
In vivo assays for evaluation of NP preparations.
| Assay | Mechanism | Advantages/use | Limitations | References |
|---|---|---|---|---|
| Acute, subacute, subchronic, and chronic toxicity assays (Clinical chemistry tests, Hematological tests, Coagulation tests, Weight change assessment, Carcinogenicity tests, and Histopathology) | To evaluate systemic response by testing different routes of administration and durations of exposure. | Thorough assessment of biological changes as a result of substance administration. | Time-consuming, and expensive. | [171–175] |
| Reproductive toxicity tests (Mammalian germ cell cytogenetic assay, Heritable translocation assay, Mouse spot test, Micronucleus test, Chromosomal analysis) | To evaluate effects on fertility of the host anddevelopment of offspring by looking for changes at the genomic or embryonic level. | Accurate detection of mutations, deletions, and chromosomal aberrations. | Skill-demanding, time-consuming, and expensive. | [176–179] |
| Ocular- and skin-irritation tests (Eye irritation draize test, and Trans-epithelial water loss (TEWL) test) | Measurement of skin and eye irritancy by dripping the test substance on eye or skin of the host and looking for irritations such as inflammation, bleeding, ulceration, swelling, or permanent damage. | Accurate assessment of harmfulness of substances to the eye or skin. | Ethical concern over animal welfare. | [180–184] |
| Hypersensitivity tests (Skin prick test, Intradermal test, Patch test) | Treatment of skin with various concentration of test substance and observing for any immediate skin reactions, itching edema, erythema, urticaria, angioedema, signs of anaphylaxis; as well as testing for release of histamine, IgE, IgG, and Histopathological analysis. | Accurate detection of immediate contact reactions. | Difficult to attain sensitivity and specificity values, variations in symptoms. | [185–187] |
3. Unintended interactions of DDSs with host defenses
Formation of superstructures from primary NPs can occur during formulation, storage, and/or administration. Size, surface chemistry, and charge can control the agglomeration/aggregation propensity of particles. Agglomerate/aggregates may have significantly different biodistribution and organ accumulation relative to their primary building blocks. Unstable nanoparticles may form large micron-sized aggregates, which can be trapped in the capillary bed of the lungs and pose danger to patients [74]. Below, we will discuss the interactions with host defense largely occurring at nanoparticle level rather than micron-sized aggregates.
3.1. Protein corona formation and subsequent events
Immediately upon NP exposure to biofluids, principally plasma, protein components start coating NP surface forming a protein corona (PC). PC can be categorized as “hard corona” and “soft corona”. Hard corona is usually composed of proteins with higher affinity to the NP surface that may permanently bind to NPs. Soft corona consists of lower affinity proteins, which are reversibly bound to NPs; the content of soft corona can be changed over time due to the loose interactions. Nguyen and Lee [188] reviewed the parameters affecting protein corona formation on nanoparticles such as media composition, protein concentrations, exposure time, temperature, and pH, and the effect of different nanoparticle characteristics on the protein corona composition. Evidently, PC content and conformation depends not only on size, but also on curvature, flexibility, surface chemistry, charge, functional groups, and hydrophobicity [117].
It is now believed that protein composition within the PC strongly impacts the NPs fate. If the PC is rich in dysopsonins, namely albumin or apolipoproteins (Apos), PC-coated NPs have longer circulation times. In contrast, if complement factors, fibrinogen, or IgG are abundant in protein corona, these may enhance rapid clearance of NPs by superior uptake into macrophages [189–192]. Close proximity of PC proteins to NPs may disturb their biological functions. For example, fibrinogen has been reported to bind several types of nanomaterials in plasma, and plays a critical role in leukocyte activation [193] and blood coagulation [194]. NPs interference with coagulation factors via binding to plasma fibrinogen may potentially dismantle clotting events. For instance, when cationic 7th generation PAMAM interacted with blood, fibrinogen aggregation was induced in a thrombin-independent manner [195]. Binding of coagulation proteins onto NP surface could also inactivate those proteins or others of the coagulation cascade, thus leading to deficiency in coagulation reactions [194]. Furthermore, unfolded proteins may lead to enhanced immune responses. It has been shown that negatively charged poly(acrylic acid) (PAA)-conjugated AuNPs induced unfolding of fibrinogen and triggered release of inflammatory cytokines via the integrin receptor, Mac-1, and NF-kB signaling [62]. Similarly, unmodified silica NPs interact with intrinsic coagulation factors such as factor XII leading to their damage on coagulation pathway [196]. However, the amine-modified silica NPs prevented abnormal activation of the coagulation cascade after systemic administration in mice, probably due to lower affinity to factor XII [197].
Multiple IV injections of NPs with selective binding to particular proteins may deplete those proteins, preventing their effective role in several biological events. Moreover, recruitment of immune cells such as macrophages for NP clearance may hinder them from their foremost function of combating disease or infection [198,199]. This may make the individual more susceptible to real threats such as infectious particles. Additionally, engagement of both innate and adaptive immunity may induce harmful effects on immune cells, as well as all other tissues and cells in the body affected by the aggravated immune system.
3.2. Innate immunity and nanoparticles
3.2.1. Complement activation
The complement system, acting through a network of over thirty proteins both in circulation and membrane bound [200], plays a key role innate immunity, acting to identify and eliminate particulate matter and pathogens, driving interactions with these nanoscale foreign objects [201]. Fig. 4 illustrates the interdependent nature of NP interactions within the complement system, and its impact on therapeutic efficacy and viability. The complement cascade is proteolysis driven, acting through three main pathways, which converge as the third complement protein (C3) is cleaved generating C3 [2]. Classical pathway activation involves antibodies binding nanoparticles, activation of the C1 complex, C2, C4 and C3 leading to the C3 and C5 convertases.
Fig.4.
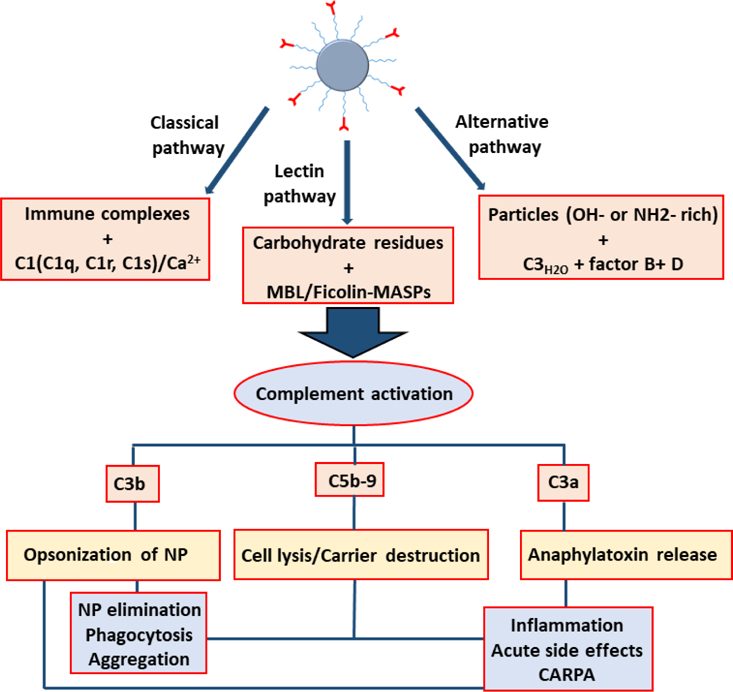
Interdependence of effects of complement activation on nanotherapuetics. Partially adapted from [200,202,203]. CARPA: C activation-related pseudoallergy, MBL: mannose-binding lectin, MASP: mannose-associated serine protease.
Generally speaking, activation of the alternative pathway of the complement system by nanoparticles is initiated through the spontaneous hydrolysis of the thioester in C3 to form C3(H2O), which subsequently bind complement factor B to form C3(H2O)B. Furthermore, factor B is cleaved and activated by complement factor D which forms C3(H2O)Bb, the fluid phase convertase. This converts C3 into the anaphylatoxin C3a and the opsonic molecule C3b, which binds covalently to hydroxyl and amino groups presented on surfaces of nanoparticles [204]. Once bound on the surface, C3b is converted to C3bBb through binding of factor B and subsequent cleavage by factor D, whose complex product amplifies the alternative pathway, and through interactions with protein factors cleaves C3 into C3a and C3b, thereby feeding a positive feedback loop generating more C3b deposition on the nanoparticle surface [205].
The earliest mention of a nanoparticle interaction with complement proteins is of liposomes, the earliest synthesized nanoparticles. In 1969, only a few years after liposomes were described by Bangham and Horne [206], Haxby et al. [207] described the utility of bilayer ‘model’ membrane interactions with complement proteins and antibodies as a tool to study ‘the complement lytic mechanism’. Haptenized liposomes were used as a tool to study interactions between complement proteins and biological membranes, leading to the understanding that all charged phospholipid/cholesterol bilayers intrinsically activate complement proteins [208], although the consequences of the phenomena vary by the species, the individual, and the vesicle properties. Further studies revealed the impact of these interactions results in the increased clearance of the opsonized particles and the release of complement proteins C3a and C5a [208].
Particle physicochemical characteristics obviously matter here. Particles larger than a few microns present markedly reduced complement activation [209]. As discussed earlier, spheres are less prone to activate complement than rod- and disk-shaped NPs [95]. Deposition of complement protein fragments on the surface of NPs such as liposome significantly alters blood level kinetics and particle integrity [200]. Studies have shown across a diversity of particles that complement activation is sensitive to surface coatings varying by charge, thickness, surface density, and accessibility to reactive groups. In terms of surface charge, negatively-charged NPs mainly activate the classical complement cascade, while their positively-charged counterparts induce the alternative pathway [127,204]. Modulation of complement activation through modification of surface chemistry, coatings, and charge has been studied in a wide array of nanoparticle species [202,205,210–215]. As an example, the addition of PEG or poloxamine 908 surface coating reduced nanoparticle activation of complement, and dextran coatings increase it. In another example, complement activation was prevented with >90% efficiency by coating NPs with negative regulatory complement factor H [216]. Similarly, the lower level of complement activation observed by galactose polymer modified NPs compared with the glucose modified NPs, was attributed to adsorbing the complement H protein on their surface [217].
After more than forty years of data since the first clinical availability of the PEGylated liposomal doxorubicin drug (Doxil™), some patients have demonstrated a complement activation-related hypersensitivity syndrome called C activation-related pseudoallergy (CARPA). This has highlighted the need, as Doxil’s patent expired and alternatives emerge on the market, that generic formulations be carefully evaluated for bioequivalence since modifications of formulation and processing may produce variable immunity and toxicity profiles [203]. The drawback from the standpoint of formulating and producing potentially useful drug delivery vehicles is striking; the particle may not only trigger damaging biological reactions, it also may not be retained long enough to produce any therapeutic benefit, or slight variability in production may elicit unforeseen consequences. Existing literature on these important issues is extensive [2,202,208,210,212–214,218–222] and distinctions of nanoparticle species, e.g. inorganic engineered nanoparticles (gold, silica, superparamagnetic iron oxide) versus liposomes, solid lipid nanoparticles, polymer particles (either solid or vesicular) or protein-based nanoparticles dictate the different interactions that particulate matter experiences via phase transformations, particle aggregation, surface reconstruction and dissolution. These processes then influence the nanoparticles functional interactions, reactivity, bioavailability, pharmacokinetics, and potential for immunotoxicity [223]. Our necessarily limited discussions herein relate mainly to medical and diagnostic nanoparticles that contact the bloodstream.
3.2.2. Resident intravascular leukocytes patrol the blood, apprehending NPs and activating inflammation
The blood is full of vigilant patrolmen, leukocytes, which surveil for pathogens and particulate matter, subsequently engulfing them and setting off a cascade of immunological responses. Many of these leukocytes are freely moving within the blood. However, many leukocytes sit in the vasculature of one organ, which can give them an outsize role in NP effects and distribution, as described below. Therefore, here we define an oft-overlooked grouping of these patrolling leukocytes which we give the term Solid-organ-Associated Intravascular Leukocytes (SAILs). We define SAILs as leukocytes that reside permanently or for prolonged periods in one organ, but reside inside the blood vessel lumen of that organ’s vasculature, not in the tissue parenchyma. This latter feature distinguishes SAILs from the common term of “tissue resident leukocytes”. For example, the Kupffer cells of the liver can be considered SAILs, as they sit in the intravascular space, where they grab pathogens and nanoparticles (NPs) from circulation. Because of their unique position, SAILs have been shown to play important roles in NP biodistribution, effects, and toxicities.
The term SAILs may at first seem redundant with the more common terms of the reticulo-endothelial system (RES) or monocyte-macrophage system (MPS). The capture of NPs by intravascular leukocytes is widely recognized by NP engineers in the form of the RES macrophages (Kupffer cells) that take up large fractions of NPs into the liver [224,225]. The RES is thus portrayed as a single cell type in one primary organ (liver), removing NPs from the circulation without consequence beyond the loss of NPs for the target organ. However, as we outline below, this simplified model leaves out numerous other key features of SAILs’ interactions with NPs: the liver is not the only organ with patrolling SAILs; these SAILs are not just macrophages or monocytes; SAILs often appear only in a diseased organ; and the uptake of NPs into these SAILs is not just a loss of NPs (as often portrayed by nanomedicine engineers), but rather can have major impacts on the immune system and even survival. In this section, we explore the diversity of SAILs that can take up intravascular NPs and the consequences of such interactions. Fig. 5 shows the different classes of leukocytes able to interact with nanoparticles (NPs), many of which at times act as SAILs.
Fig.5.
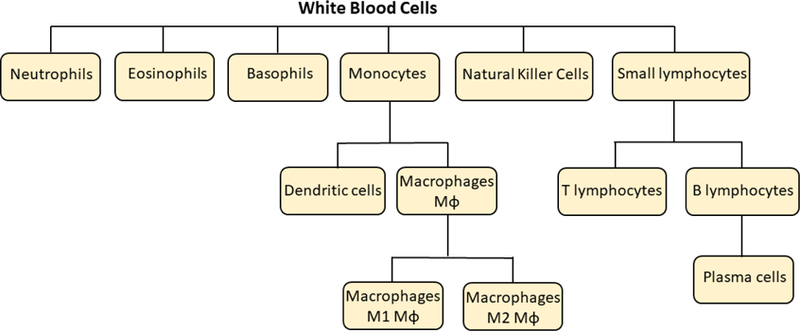
Different classes of leukocytes that participate in recognition of, interaction with and destruction/elimination of nanoparticles.
Before exploring the diversity of SAILs, we will first focus on the most well-known SAILs and RES organ in nanomedicine: the Kuppfer cells of the liver. In most studies of intravascular NPs, the liver does indeed have the highest NP uptake, usually attributed to its RES function, via intravascular macrophages. Indeed, a recent meta-analysis showed that < 0.7% of anti-cancer NPs localize to solid tumors, with the vast majority going to the liver [226]. Most of that liver uptake is in Kupffer cells, the resident tissue macrophages that sit in the liver sinusoids, where they screen the passing blood for pathogens and then engulf the pathogens [227]. The role of these Kupffer cells in liver uptake was recently quantified by killing the Kupffer cells using clodronate liposomes (CL), followed by measuring the distribution of a variety of NPs. For example, 50 nm gold NPs had ~80% of the injected dose (%ID) go to the liver at 24 hours post-injection in naive mice, while CL treated mice had ~20%ID in the liver [228]. Thus, intravascular macrophages in the liver account for a very large proportion of total NP uptake.
Beyond the liver, there are multiple other organs that have classically RES-type functions. The most well known of these is the spleen, where red pulp macrophages contribute to splenic uptake in the 5–10%ID range in the studies cited above for Kupffer quantification [228]. Additionally, the bone marrow has resident macrophages exposed to the blood and thus likely acts in an RES fashion for NPs, but this has been explored very little. Thus, these cells would be considered SAILs.
The most overlooked RES organ is the lung, where at least two major SAILs have been defined. Nanomedicine may have overlooked the lung as an RES organ largely due to high species variability and a minimal role in healthy mice. The large inter-species variability is attributed to the presence of pulmonary intravascular macrophages (PIMs). PIMs are resident macrophages which are adhered to the capillary endothelium of the alveoli (air sacs) of the lungs (Fig. 6), thus exposed to the bloodstream in the same orientation as Kupffer cells [229]. Of many animals tested, a great number, including pigs, sheep, and cats, have large numbers of PIMs constitutively present even in healthy animals. However, a few species, notably including rodents and humans, do not have such “constitutive” PIMs, but instead only manifest PIMs in pathological states (“induced PIMs”). These interspecies differences in PIMs cause enormous differences in the localization of nanoparticles in the lungs versus liver. For example, IV-injected 20 nm gold NPs injected into sheep (which have very high PIM numbers [230]) had 60%ID (injected dose) in the lungs at 30 min, compared to 0% for mice [231]. Notably, eliminating PIMs with CL greatly reduces lung uptake of NPs in pigs [95]. Since mice and humans do not have constitutive PIMs, it might be assumed that PIMs are not important to NP engineering. However, in numerous important pathological states, induced PIMs develop in great numbers in the lungs of rodents and humans [229]. For example, rat models of sepsis and cirrhosis both develop PIMs, and these rat PIMs efficiently take up adenovirus, which is the same size as many NPs [232,233]. Thus, PIMs may become major patrolmen for the RES in major diseases, though significantly more research is needed to determine the extent and resulting effects. The study of PIMs can thus serve as a prototype for a type of SAIL involved in nanomedicine other than the oft-studied Kupffer cells.
Fig. 6.
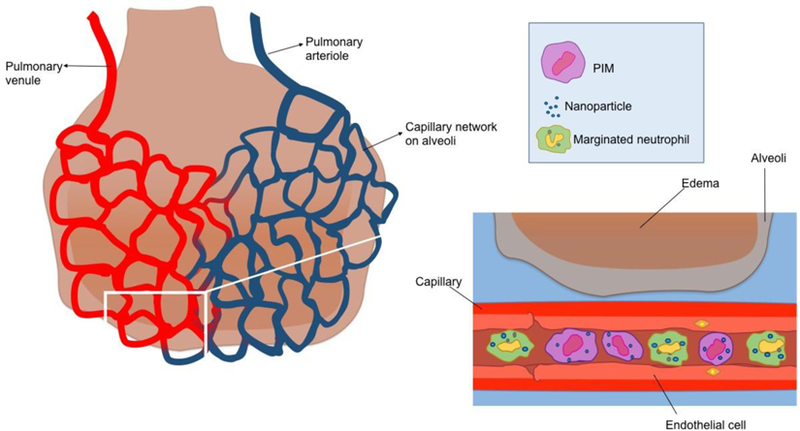
Illustration of pulmonary intravascular macrophages (PIMs) and marginated neutrophils that can phagocytose a significant amount of IV-injected nanoparticles are presented in capillaries adjacent to alveoli [229–237].
With respect to the relatively hidden revelation that there are key RES organs besides the liver, there are also SAILs besides macrophages within the RES. There are multiple types of leukocytes in the blood, including in descending order of numbers neutrophils, lymphocytes, monocytes, eosinophils, and basophils. Most of these have not been examined in detail or at all for their interactions with NPs, especially in the intravascular space [2]. However, their free circulation in the blood gives them direct access to systemically delivered NPs, and thus a very real chance of having a role in the disposition and side effects of NPs.
A glimpse into the possibility of other ILs regulating NP disposition was garnered by the recent finding that ~100 nm NPs made of denatured albumin are taken up by intravascular neutrophils in mice [238,239]. The neutrophils recognized and phagocytosed the NPs via the Fc-gamma receptor, and then were able to cross the endothelial barrier of inflamed tissues carrying therapeutic drugs. Neutrophils are not only the most abundant ILs (60% of ILs), they also have a unique physical position within the vasculature to allow them to capture NPs. A large fraction of neutrophils are “marginated”, meaning that they adhere to the capillary lumen with much slower transit through the capillaries than red blood (Fig. 6) [234,235]. The marginated pool is largest in the lungs; e.g., 70% of neutrophils in rabbits are in the pulmonary marginated pool [236]. This marginated pool of pulmonary intravascular neutrophils has been shown to form a defensive barrier, capturing pathogens such as bacteria very rapidly after their introduction into the blood [237]. Future studies are needed to determine whether the marginated pool of neutrophils also takes up NPs, how pathology plays a role, and whether such NP uptake leads to either positive or negative consequences.
The consequences of NP uptake into SAILs are important to understand in the development of nanomedicines. SAILs evolved in part to detect intravascular pathogens, and such detection usually leads to inflammation, and adverse health effects. Numerous in vitro studies have shown that phagocytes such as macrophages and monocytes can release pro-inflammatory cytokines when exposed to various NPs [240–242]. For example, one such inflammatory pathway is the inflammasome NLRP3, which responds to a wide array of NPs by upregulating IL-1β and IL-18 [243,244]. However, far fewer studies have demonstrated the in vivo consequences of NP-IL interactions. One recent exception found that in pigs, PIMs rapidly take up polystyrene NPs, causing a sudden spike in pulmonary artery pressures [95]. Because of the paucity of in vivo studies, for new NPs moving towards clinical translation, it will be crucial to understand which ILs take up the NPs in animal models of pathology, and determine the consequences, by measuring IL responses, cytokine levels, and physiological endpoints with clinical relevance.
In summary, the term SAILs is a convenient descriptor for the numerous types of leukocytes that reside within the blood vessels of multiple organs and greatly affect NP biodistribution, efficacy, and toxicity. It is now important to more thoroughly investigate other SAIL types (e.g., neutrophils) in organs beyond just the liver.
3.3. Adaptive immunity and nanoparticles
Upon administration, nanoparticles encounter not only a range of plasma proteins and first cellular line of defense (leukocytes), but several classes of immune cells that influence their immunogenicity. Following uptake by antigen-presenting cells, the fate of nanoparticles depends on their composition and physicochemical properties. This can consist of simple clearance of the particles or multilevel activation of the immune system involving innate and/or adaptive immune responses [245]. Of interest to the drug delivery field is how to design nanomedicine that evade the immune system or are recognized as self.
3.3.1. Mechanism of adaptive immune response
Adaptive immunity (also called acquired immunity) consists of T and B cells that are activated in response to specific antigens. There are two types of adaptive immune responses: cellular immunity involving activation of cytotoxic T cells and humoral immunity response generated by activated B cells producing antigen-specific antibodies. Helper T cells are essential cells in the adaptive immune system that control the direction of the immune response. Upon uptake of antigens by APCs, the antigens are processed and presented to naive T helper cells that can differentiate into either a Th1 or a Th2 effector cell. Th1 cells secrete cytokines such as TNFα and IFNγ that lead to activation of antigen-specific cytotoxic T cells. Th2 cells secrete cytokines such as IL-4, −5, −6, and −10, which can stimulate B cells to produce antigen-specific antibodies [246,247].
Antigen presenting cells play a major role in priming the adaptive immune system. A three-signal model has been proposed to describe T cell activation (Fig. 7). Signal 1 is the engagement of peptide/MHC (pMHC) on APCs with T cell receptor (TCR). Insufficient to activate the T cells alone, signal 1 has been reported to induce T cell anergy and silencing. Signal 2 activates T cells through co-stimulatory receptors expressed by APCs. On the other hand, stimulation by co-inhibitory receptors on APCs can also lead to T cell anergy or formation of regulatory T cells (Tregs). Signals 1 and 2 may be sufficient to induce T cell activation, however a third signal is generally required for T cell activation. Signal 3 is the secretion of cytokines by APCs which activate the T cells and polarize by differentiation into various types of effector T cells [247–249]
Fig. 7.
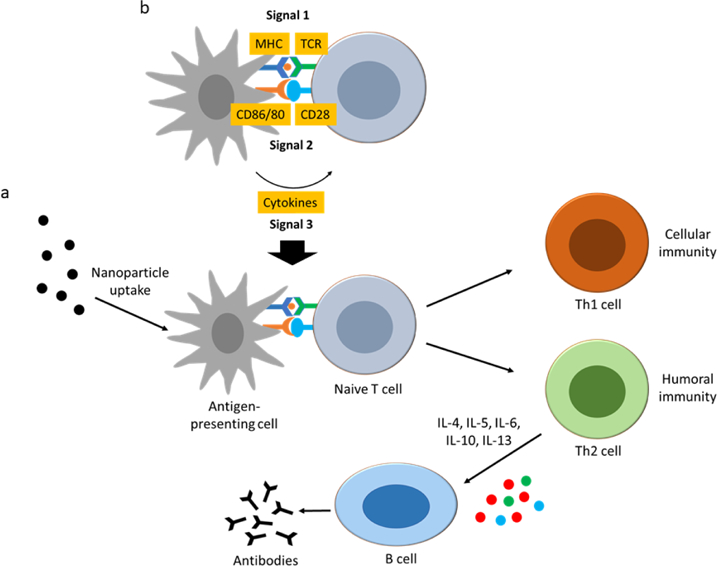
Illustration of adaptive immune system interactions with nanoparticles. (a) Uptake of nanoparticles by antigen-presenting cells leads to processing and presentation of the antigens to naive T cells that can differentiate into T helper cells Th1 or Th2 effector cells, leading to activation of cytotoxic T cells or activation of B cells to produce antigen-specific antibodies. (b) Three-signal model proposed for activation of naive T cells. Signal 1 is the engagement of peptide/MHC (pMHC) on APCs with T cell receptor (TCR). Signal 2 is activation by co-stimulatory receptors expressed by APCs. Signal 3 is the secretion of cytokines by APCs to stimulate T cells. MHC: major histocompatibility complex.
3.3.2. Interaction of nanoparticles and adaptive immunity components
Nanoparticles have been reported to activate the adaptive immune response. Several factors can influence the immunogenicity of nanoparticles such as size, charge, hydrophobicity, surface characteristics, solubility, and its composition [245]. Some nanoparticles can serve as adjuvants and enhance the immunogenicity of weakly antigenic cargoes as in the case of both lecithin- and polymethylmethacrylate-based nanoparticles [250,251]. Lipid-coated polysaccharide particles have been used as adjuvants for rabies vaccine. The mixed nanoparticles/rabies antigens generated a much higher antibody response than immunization with Alum adjuvants [252]. Fifth generation PAMAM dendrimers were also found to have immunopotentiating effect when used as adjuvants, generating both Th1 and Th2 type immunity [253]. Correlation of nanoparticle size with the resulting immune response has been rather controversial, with varied responses being reported. Large nanoparticles (>1um) have been found to be generally associated with inducing a Th1 response, compared to smaller nanoparticles (<500nm) that induced more Th2 responses. However, there have been exceptions, with smaller nanoparticles such as PLGA, dendrosome, nanoemulsions, and PEG-PHDA nanoparticles inducing Th1 responses. Polystyrene nanoparticles (<100nm) were found to induce both higher cellular and humoral response than larger polystyrene particles (>500nm) [254,255]. Nanoparticle surface charge has been reported to play a significant role in its immunogenicity. Charged nanoparticles (cationic or anionic) are phagocytosed at a higher rate than neutral nanoparticles. Cationic nanoparticles have been found to show greater immunogenicity with increased production of pro-inflammatory cytokines, whereas anionic nanoparticles had lower immunogenicity [256–258].
The association of cargoes with the nanoparticles can result in their conformational changes leading to their immunogenicity. C60 fullerene nanoparticles have been reported to have anti-inflammatory and anti-oxidative properties and induce immunosuppressive response, however conjugation of C60 fullerene derivative to bovine serum albumin, resulted in immunostimulation and generation of antibodies toward the nanoparticle [259]. Conversion to an immunostimulatory response was also observed with conjugation of polyaminoamine dendrimers to BSA, resulting in the generation of dendrimer-specific antibodies. Polyamidoamine (PAMAM) dendrimer conjugated to cytokine human interleukin-3 (hIL-3) induced dendrimer-specific antibody response, whereas the unmodified PAMAM dendrimer did not [260]. Conjugation of targeting ligands such as antibodies to nanoparticles has been one of the main approaches in development of targeted drug delivery system. However, the addition of antibodies to nanoparticles may also increase their immunogenicity resulting in poor pharmacokinetics, as has been shown with immunoliposome studies in mice [261,262].
It is very important to maintain a controlled nanoparticle manufacturing process. The immunogenicity of nanoparticles could vary from batch to batch as result of small differences in composition or conformation changes that can be introduced during the manufacturing process. For example, the liposome manufacturing process could affect its immunogenicity. Liposomes produced by high shear extrusion technique can lead to denaturation of encapsulated proteins. Another source for induction of immunogenicity of nanoparticles is contamination by endotoxins which can be introduced during the manufacturing process and has been discussed earlier in this review [245,256].
4. Approaches to alleviate the NP-associated adverse effects
4.1. Stealthiness, the easiest strategy to avoid unintended uptake
As we have described, the physicochemical properties of nanoparticles can influence how they are recognized by the immune system. Many different hydrophilic agents including polyethylene glycol (PEG), chitosan, hyaluronic acid, poloxamer, polyvinylpyrrolidone (PVP), and dextran have been used to introduce stealthiness on the nanomaterials preventing recognition by the immune system [188,263–266]. Among all polymers mentioned, PEG is the most commonly used for NP surface modification. In liposomes, the addition of as much as 10% surface coating of PEG resulted in ‘stealth’ liposomes and increasing the blood circulation half-life of the vesicles by orders of magnitude [210]. The addition of PEG in polymer and lipid nanoparticles has been shown to reduce complement activation [211]. To improve the “stealth effect” observed by PEG, many studies focused on increasing the PEG density on the surface of NP by using branched PEGs [267], using a hydrophobic layer as spacer [268], or by incubating NPs in an excess amount of PEG [269].
Although very promising at first glance and extensively used, PEG is a non-biodegradable polyether and its accumulation in the body may cause adverse effects especially if PEG-coated NPs are going to be administered over a long period. Indeed, there have been reports that PEG-specific antibodies are generated following administration of PEG-coated liposomes, which then resulted an accelerated blood clearance (ABC) phenomenon of PEG-coated liposomes (Fig. 8). Interestingly, the anti-PEG antibody response was not found to be induced by the commonly known T helper-based activation of the humoral immune response, but rather by direct B cell activation via a T independent antigen 2 (TI-2) based activation of B cells inducing a strong anti-PEG IgM antibody response. It was suggested that the PEGylated liposomes activated the B cells in splenic marginal zone, which is the splenic compartment central in clearance of blood-borne pathogens [270–273]. Notably, a significant number of the normal population have pre-existing anti-PEG antibodies in their blood without any treatment with PEGylated therapeutics [274]. As discussed earlier, PEG has been also found to activate complement cascade inducing anaphylactic reactions in sensitive individuals [275].
Fig. 8.
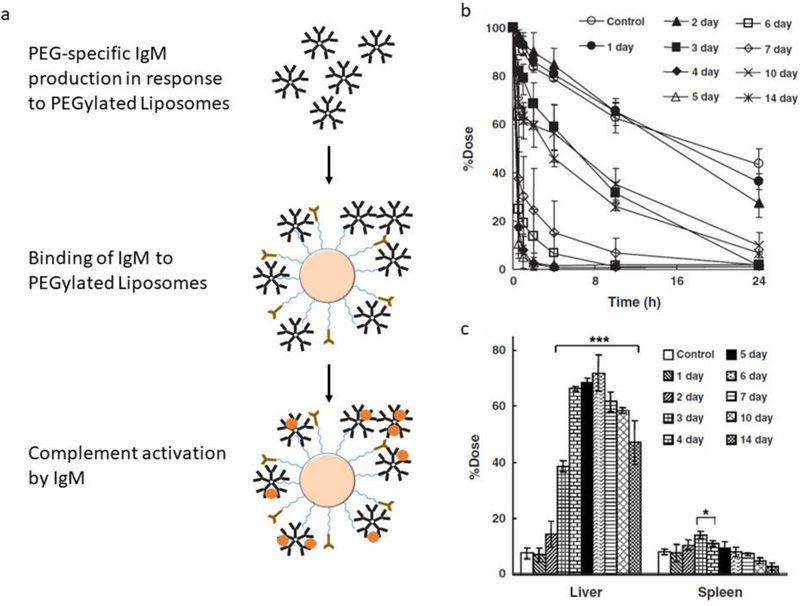
Accelerated blood clearance (ABC) phenomenon of PEGylated liposomes. (a) Illustration of anti-PEG IgM in response to PEGylated liposomes. (b) Blood clearance levels of PEGylated liposomes following injection at different days after post-first injection. (c) Accumulation of PEGylated liposomes in liver and spleen 24 hrs after injection. Reprinted with permission from ref. [281].
Degradable polymers including hydroxyethyl starch (HES), polysialic acid, dextrin, and poly(phosphoester)s (PPEs) have been proposed as PEG alternatives [276,277]. As a biological alternative to PEG, NP surfaces have been coated with cell membrane components [278,279]. Hu et al. [280] reported platelet membrane-cloaked NPs (PNPs) prepared by fusing human platelet membrane with 100-nm PLGA nanoparticles. PNPs internalized into human THP-1 macrophage-like cells less than uncoated counterparts and did not induce complement activation in autologous human plasma. Moreover, Docetaxel- and Vancomycin-loaded PNPs had elevated therapeutic efficacy in a rat model of coronary restenosis and a mouse model of systemic bacterial infection, respectively. Parodi et al. [278] reported a similar technology with a different membrane source and different particle core. They coated the surface of nanoporous silicon particles (NPS) with cellular membranes isolated from freshly harvested leukocytes, and named them LeukoLike Vectors (LLVs). The membrane coating protected particles from protein opsonization and markedly decreased cellular uptake. Furthermore, unlike NPS, LLV did not induce any significant change in the cell membranes or any vesicle formation when internalized. The same group showed that the membrane coat improved particle interaction with tumor blood vessels, providing enhanced targeting and strong adhesion at the tumor site [279]. Besides plasma membrane fragments, there have been some attempts on coating the NPs using individual membrane proteins such as CD47 to reduce their phagocytic uptake. Although all these strategies proved to extend the circulating time of NPs, there is still not enough quantitative data on the biodistribution of such engineered particles in major organs such as liver, spleen, and lung.
4.2. Replacement of antibodies by safer fragments that do not elicit Fc-mediated side effects
Myriad targeting molecules have been developed for use in targeted drug delivery. These include monoclonal antibodies, single-chain variable fragments (scFvs), antigen-binding fragments (Fabs), single domain antibodies (sdAb), DARPins, peptides, aptamers, and small molecules (Fig. 9) [282–284]. Monoclonal antibodies consist of two immunoglobulin (Ig) heavy chains and two Ig light chains, each with constant and variable domain regions. Fragments of the parental antibody (scFv and Fab) have been developed in order to increase tissue penetration, reduce immunogenicity, and by lacking the Fc region, prevent interaction with Fc receptors. ScFvs have been developed by fusion of immunoglobulin variable heavy (VH) and light chains (VL) regions. Fabs consist of a single constant and variable domain form each heavy and light chains. Compared to conventional IgG antibodies (~150kDa), single-chain variable fragment (scFv) antibodies (~27kDa) and antigen-binding (Fab) fragments (~57kDa) are smaller in molecular size and weight [282,285,286].
Fig. 9.
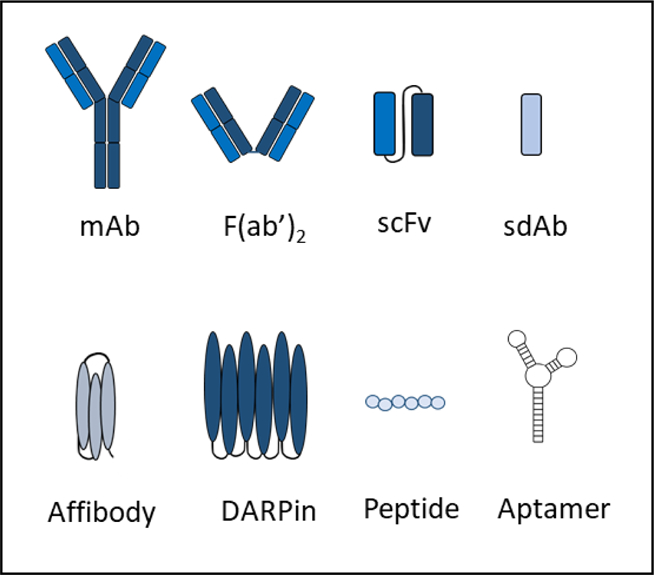
Different types of targeting molecules that have been used in targeted drug delivery systems. mAb: monoclonal antibody, scFv: single-chain variable fragment, F(ab')2: antigen-binding fragment, sdAb: single domain antibody, DARPin: designed ankyrin repeat protein.
Single domain antibodies (sdAbs), which may be man-made or natural, have attracted a lot of attention from the pharmaceutical industry due to their small size, accessibility to cryptic structures, high affinity, and deep penetration into tissue. Man-made sdAbs have been developed using phage display technology. Phage display immune libraries have been used to display antibody fragments on bacteriophages to generate highly diverse populations. A biopanning technique is then used to select the candidates with the highest affinity and specificity. Non-conventional immunoglobulins have been discovered in nature, and used to develop nanobodies. Camelids (camels and llamas) and cartilaginous fish (wobbegong and nurse sharks) have single variable domains with a constant domain framework. The single domain regions of camelids (VhH) and shark antibodies (V-NAR) have long surface loops that are able to reach protein cavities better than conventional antibodies. These nanobodies are around 12–15 kDa and have very small dimensions of 2.5nm by 4nm [285,287]. DARPins (designed ankyrin repeat proteins) are another interesting non-immunoglobulin targeting molecules derived from natural ankyrin repeat proteins, that can vary in molecular weight from 14 to 21 kDa. DARPins are around one-tenth the size of conventional IgG molecules and have high thermal stability. Another type of single domain targeting molecules are affibodies [282,288]. Affibodies are very small (−6.5kDa) targeting moieties developed by combinatorial protein engineering of the staphylococcal protein A-derived Z-domain scaffold. As compared to the above mentioned targeting moieties, the affibodies can be produced by peptide synthesis because of their small size and rapid folding [289,290]. Other molecules that have also been used as targeting ligands include peptides, aptamers, and small molecules [282,291].
Smaller antibody fragments or moieties have several advantages over larger intact antibodies for nanoparticle-mediated targeting such as, lack of binding to Fc receptors or complement activation, lower chance of immunogenicity due to lack of Fc region, and increased ligand multivalency because of their smaller size. The constant fragment (Fc) of the antibodies binds the Fc receptors on macrophages and other antigen-presenting cells. Conjugation of intact antibodies to nanoparticles could increase their immunogenicity by increasing their uptake and presentation by the reticuloendothelial system (RES), whereas smaller fragments could potentially avoid direct interaction with the RES [292]. In one study, the immunogenicity of doxorubicin-loaded nanoparticles was diminished potentially by the destruction of the antigen-presenting cells [292,293]. Others have attempted to utilize ‘don’t eat me’ signals such as CD47 to evade uptake by the RES [294–296]. There are currently no FDA-approved antibody targeted-nanoparticle therapeutics on the market, however with the advances in antibody engineering and development of more novel biocompatible antibody fragments and single domain antibodies, and with possible incorporation of immunoevasion strategies, targeted nanomedicine have a very promising future [297,298].
4.3. Innovative approaches for immune tolerance
Tolerance-inducing approaches function by either suppressing antigen-specific effector T cells or inducing generation of regulatory T cells. These include interventions at the Signal 1 and Signal 2 such as through modulation of co-stimulatory and co-inhibitory molecules to induce T cell anergy or death. Other strategies have included the use of T cell depleting antibodies, immunotoxins, and modulation of cytokines. One approach that has shown a lot of promise for induction of antigen-specific immune tolerance has been through the use of tolerogenic nanoparticles.
There have been three primary strategies using tolerogenic nanoparticles for induction of tolerance to foreign material, which include exploitation of the natural tolerogenic environments or processes, targeting of tolerance-inducing receptors, or use of nanoparticles carrying tolerance-inducing payload. These innovative approaches could potentially be used to induce tolerance to nanomedicine. The first strategy is utilizing the natural tolerogenic processes such as targeting the liver to take advantage of the hepatic tolerogenic environment, targeting the GI tract for induction of oral tolerance, or apoptotic cell mimicry [256]. In addition, it is possible to use the natural tolerogenic processes such as apoptotic cell death, which largely induce a tolerogenic response. One example is targeting of aged RBCs that clear in a tolerogenic fashion. Antigen-specific tolerance has also been reported by attachment of immunogenic proteins on surface of RBCs. Lorentz K.M. et al. [299] attached e. coli L-asparaginase enzyme on surface of RBCs which led to antigen-specific reduction in antibody titer by more than 1000-fold. This was achieved by chemical conjugation of glycophorin-A binding peptides (ERY1) on asparaginase enzyme. This approach was used to take advantage of the natural apoptotic signaling mechanism of aged RBCs to elicit humoral immune tolerance. Second approach for tolerance induction is the development of tolerogenic nanoparticles targeting tolerogenic receptors such as CD22 inhibitory receptor, FAS receptor, and aryl hydrocarbon receptor [256]. One example is induction of antigen-specific immune tolerance by sialic acid–binding Ig-like lectin (SIGLEC)-engaging tolerance-inducing antigenic liposomes (STALs). STALs display on their surface, the antigens and the B cell co-inhibitory receptor CD22 ligands, resulting in selective binding and suppression of antigen-specific B cells [300]. Third is development of tolerogenic nanoparticles loaded with tolerance inducing payload (such as NF-κB inhibitors or mTOR inhibitors) to induce formation of tolerogenic antigen presenting cells [256].
Targeted modulation of APCs has been one of the key strategies used to induce antigen-specific immune tolerance. Nanoparticles in particular have made excellent vehicles for APC modulation. Tolerogenic nanoparticles can be in the form of nanoparticle encapsulated with antigens along with tolerogenic pharmacologic agents, or nanoparticles encapsulating only the tolerogenic pharmacologic agents that is admixed with the free antigen. Selecta Biosciences has been one of the main leaders in developing a platform to prevent the formation of anti-drug antibodies (ADAs) against biologic therapeutics. The tolerance induction platform consists of biodegradable polymeric nanoparticle encapsulated with immunomodulator agents such as the mTOR inhibitor Rapamycin. SEL-212 is an example of tolerance-inducing nanoparticle technology currently in Phase II clinical trials for treatment of gout. It is a combination therapy that consists of co-administration of PEGylated uricase (pegsiticase) with rapamycin-containing tolerogenic nanoparticles. Pegsiticase has been reported to induce immunogenicity in 90% of patients. In Phase I clinical trial, SEL-212 prevents ADA formation and allowed sustained control of serum uric acid levels for 30 days following a single dose administration. This type of tolerance induction approach holds great promise for a range of biologic therapeutics, including nanomedicine. Nanoparticle therapeutics could be admixed with tolerance-inducing nanoparticles to prevent ADA formation and aid in their immune evasion. Another example is the SEL-403, an admixture of anti-mesothelin antibody fragment/pseudomonas exotoxin A recombinant fusion immunotoxin with SVP-Rapamycin as combination therapy for mesothelioma and pancreatic cancer patients. This has been designed to prevent ADA formation against the fusion protein, and in particular the immunotoxin which is derived from a bacterial host and is highly immunogenic. This platform is also being investigated for viral gene therapy treatments (SEL-302 and SEL-313) to induce tolerance to the viral vector and the transgene. Work is also being carried out along with Spark Therapeutics for developing a non-immunogenic hemophilia gene therapy medicine [301].
Development of a non-immunogenic targeted drug delivery system can be challenging. Factors that can influence immunogenicity of targeted nanomedicine include their physicochemical properties, modifications made to attach targeting ligands or immunoevasive coatings, cargoes or ligands that can be structurally perturbed during the manufacturing process, aggregation, and so on. Targeted nanomedicine face many obstacles in achieving complete immune evasion, nevertheless, there are many tolerance-induction strategies available that offer solutions which could be applied to nanomedicine.
5. Biomedical and translational aspects
5.1. Role of health (disease) status of the recipient
The health and physiological condition of the individual receiving a nanoparticle therapeutic may impact the extent of DDS’s toxicity. Pre-existing pulmonary, allergic, inflammatory, thrombotic, or metabolic conditions, malignancies, infection, smoking, and pregnancy may further complicate the nanoparticle administration consequences. The term “personalized protein corona (PPC)” was introduced by Hajipour et al. [302] to emphasize that small alterations among patients/individuals (protein source) could affect the composition of the hard corona at the nano-biointerface. In fact, their data demonstrate that the composition of the hard corona differs among healthy individuals, as well as among patients with diverse diseases/medical conditions. Even temperature change in body could possibly facilitate the NP interaction with proteins in blood leading to changes in biodistribution and bioavailability [188,303]. One can imagine that such variations may result in diverse scenarios in terms of biological and toxicological fate of nanoparticles in vivo.
To evaluate how sensitized and non-sensitized mice may react differently to nanoparticles exposure, Gustafsson et al. [304] compared the responses in sensitized mice with an established allergic airway disease to healthy mice after exposure to iron oxide (hematite) nanoparticles intratracheally. Exposure to hematite NPs induced a significant increase in number of inflammatory cells such as neutrophils and eosinophils in the airways and lymphocytes in the draining lymph nodes of healthy mice. In contrast, same particles led to unspecific cell reduction in the alveolar space and the lung-draining lymph nodes in mice with established eosinophilic inflammation. The authors suggested that ionic irons released from hematite NPs in the acidic eosinophilic environment may potentially amplify generation of ROS and cell toxicity. Surprisingly, in another study, competing pro-inflammatory response elicited by titanium dioxide particles could potentially be a key factor in their significant downregulation impact on Th2 type inflammation in allergic mice [305].
Tracking specific nanoparticles interactions in vivo will help pinpoint potential harmful effects in susceptible subjects. As mentioned earlier, negatively charged nanoparticles can unfold fibrinogen and promote activation of the Mac-1 receptor pathway [62]. Considering the role of the fibrinogen-Mac-1 pathway in inflammatory responses observed in the pathogenesis of disorders such as Alzheimer’s [306] and arthritis [193], the risk of exacerbating these conditions by nanoparticles known to interfere with that specific pathway should be carefully examined [62]. In another example, aberrant NLRP3 activation was shown to contribute to the pathology of various infectious and sterile inflammatory diseases, such as gout, obesity, atherosclerosis, alzheimer’s disease, and type 2 diabetes mellitus [307–309]. Several types of nanoparticles including silica, asbestos and alum, TiO2, SiO2, and carbon nanotubes have been proved to activate NLRP3 inflammasome, leading to inflammation through IL-1β release [310–312]. Whether administration of such active particles in susceptible subjects can potentiate the existing inflammatory conditions remains an open question.
While NPs with immune stimulation properties may develop into promising immune adjuvants, their application in patients with elevated immune activity/inflammatory responses should be cautiously investigated. Manshian et al. [4] demonstrated that administration of aluminum oxide NPs amplified inflammatory responses and enhanced lung metastasis in syngeneic tumor mouse model. To demonstrate the response in vitro, they showed that spherical and wire-shaped aluminum oxide nanoparticles could trigger a clear activation of NLRP3 inflammasome and TGF-β secretion in primary splenocytes. Cancer cells exposed to these cytokines exhibited an augmented level of epithelial-to-mesenchymal transition, indicating cancer metastasis. Exposure to the nanoparticles themselves did not manifest such characteristics.
Pregnant women are a highly vulnerable population, since NPs can transport to developing embryos/fetuses through the placenta [313–315]. Any perturbation to placenta’s regular performance will have possibly permanent and even life-threatening prenatal and postnatal effects on fetus. Polystyrene particles (50–500 nm) were taken up by the human placenta and crossed the placental barrier in an ex vivo study [316]. Similarly, quantum dots passed the placental barrier in mice [314]. Upon systemic administration in mice, silica and titanium dioxide NPs were found in the placenta, fetal liver, and fetal brain [317]. A few studies also investigated the effect of NPs on fetus-baring animal rather than the fetus itself. For example, silica nanoparticles with diameters of 70 nm could induce pregnancy complications when injected intravenously into pregnant mice, whereas larger (300 and 1,000 nm) silica particles did not cause such complications.
5.2. Auxiliary drugs to manage inflammation and other side effects
Given the outsize role macrophages play in the early side effects of NPs, the future of nanomedicine may depend on strategies to reduce macrophage-related toxicities. We have considered in previous sections ways to reduce macrophage toxicities by modifying the properties of the NPs themselves. However, an additional approach is to deliver an auxiliary agent concurrent with the NP delivery that would modulate the macrophages’ response. Such auxiliary agents have been proposed in two main classes: 1) agents that decrease the macrophage’s uptake of NPs; 2) agents that decrease macrophage inflammatory responses.
Auxiliary agents that decrease macrophage uptake of NPs include both small molecule drug therapy and NPs themselves. The best example of the former is chloroquine. Pre-treating mice with cholorquine (a small molecule drug that is FDA-approved for malaria) for 2 days decreased Kupffer cell phagocytosis of IV-injected NPs, thereby slightly improving their delivery to tumors [318]. NPs themselves can decrease the uptake of other NPs, possibly by saturating the RES. For example, injection of very high doses liposomes increased the circulation time of inorganic NPs, thus very slightly improving uptake in tumors of the inorganic NPs [319]. These methods are thus proof-of-principle, but need significant optimization to create an effect of significant magnitude.
Auxiliary agents that decrease macrophage inflammatory responses also include small molecule drugs and NPs themselves. Small molecule drugs used to limit NP-induced inflammation have seen few if any studies for non-biological NPs, but have been well studied for one of nature’s NPs, viruses. In the field of viral gene therapy, it was found that intravascular leukocyte-mediated inflammation can be reduced by pre-treating with corticosteroids (CS). This prophylaxis of NP-induced inflammation can be modified by packaging the prophylactic drugs into NPs themselves. An example of modulating macrophage inflammatory responses with NPs comes from the study of a mouse model of multiple sclerosis [320]. In that study, it was shown that while free CS strongly affected T cells, liposomal CS instead accumulated most in macrophages, altering the macrophages from a proinflammatory state to an anti-inflammatory state similar to the classic M2 phenotype. The ability of CS-loaded liposomes to modulate macrophage inflammatory status has even been demonstrated in isolated human macrophages, though the picture was more complex than that found in mice, with CS-liposomes downregulating most inflammatory markers but upregulating some [321]. Thus, while inflammation-modifying drugs should in theory be able to modulate the negative consequences of engineered NPs, studies are needed to prove this is true and devise clinically practical strategies to do so.
5.3. Species-specific aspects of DDS toxicology
Despite all attempts on moving novel NP formulations to clinic, the field is still suffering from poor clinical translation. One reason could be due to significant differences between animal models and humans in biodistribution, pharmacokinetics, and even adverse reactions towards NP administration. For example, protein corona composition is different among species and even among individuals at different health conditions. Using lipid-based NP formulations, Pozzi et al. [322] demonstrated that the protein corona in human plasma was much less dense in proteins compared with that formed in mouse plasma. In a similar study, incubation of PEGylated lipid NPs with mouse and human plasma resulted in a significantly different composition of formed protein corona. Notably, only half of the 25 most abundant corona proteins identified on these NPs, were in common between mouse and human coronas. Compared to human plasma-based corona, mouse plasma-based corona was more enriched in apolipoproteins and less enriched in opsonins [323]. These different coronas may modulate a different fate for the NPs governed by specific body environment in each species.
Structure and function of the immune system varies among different species. For example, the human pulmonary alveolar macrophages (PAMs) are larger and have superior phagocytic capability as compared to rodents, dogs, or non-human primate species. Although pigs and sheep are often used as predictive models of nanomedicine-mediated reactions in humans, significant differences have been observed between these species and human. For instance, pulmonary intravascular macrophages (PIMs) are more abundant in pig and sheep lungs than in humans. PIMs are giant (20 to 80 um) phagocytic cells which are capable of clearing NPs a few minutes after the injection [324,325]. This extensive phagocytosis of NPs may also trigger the release of thromboxane A2, prostaglandin, and prostacyclin molecules leading to vasoconstriction, bronchoconstriction, and pulmonary hypertension [324–326]. As mentioned above, the human lung lacks PIMs [229,324,325], instead hepatic Kupffer cells are the predominant phagocytic system in direct contact with the blood and therefore, responsible for principle clearance of particles from the blood.
Conclusion
Host defenses can attack and react to nano-scaled DDS the same way they face real enemies such as viruses and bacteria. This reaction could possibly trigger a variety of toxic/inflammatory events. While these challenges are numerous and powerful, engineered DDSs can certainly overcome them. The clinical success of engineered nanoparticles such as Doxil and Abraxane shows that therapeutic benefit can be realized even in the face of host-defense-mediated toxicities. The key lies in studying the host defense interactions with DDSs, so that the DDSs can be modified, co-administered with auxillary therapies, or in some cases, given up for more promising options. If nanomedicine continues to focus disproportionately on therapeutic effects and not on off-target side effects, clinical success will remain largely out of reach. Since host defenses are one of the main causes of nanomedicine side effects, future research in nanomedicine must pay more attention to this area to ensure that nanomedicine can reach its full potential to help patients.
Acknowledgments
This work is supported by National Heart, Lung and Blood Institute (NHLBI) grants HL125462, HL128398 and HL126874 to VRM, and T32 HL007915 to HP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Nel A, Xia T, Mädler L, Li N, Toxic potential of materials at the nanolevel, Science (80-. ). 311 (2006) 622–627. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- [2].Boraschi D, Italiani P, Palomba R, Decuzzi P, Duschl A, Fadeel B, Moghimi SM, Nanoparticles and innate immunity: new perspectives on host defence, Semin. Immunol 34 (2017) 33–51. doi: 10.1016/j.smim.2017.08.013. [DOI] [PubMed] [Google Scholar]
- [3].Sharma A, V Madhunapantula S, Robertson GP, Toxicological considerations when creating nanoparticle based drugs and drug delivery systems, Expert Opin. Drug Metab. Toxicol 8 (2012) 47–69. doi: 10.1517/17425255.2012.637916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Manshian BB, Poelmans J, Saini S, Pokhrel S, Grez JJ, Himmelreich U, Mädler L, Soenen SJ, Nanoparticle-induced inflammation can increase tumor malignancy, Acta Biomater (2017). doi: 10.1016/j.actbio.2017.12.020. [DOI] [PubMed] [Google Scholar]
- [5].Christofidou-Solomidou M, Kennel S, Scherpereel A, Wiewrodt R, Solomides CC, Pietra GG, Murciano J-C, Shah SA, Ischiropoulos H, Albelda SM, Muzykantov VR, Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury, Am. J. Pathol 160 (2002) 1155–1169. doi: 10.1016/S0002-9440(10)64935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Howard M, Zern BJ, Anselmo AC, V Shuvaev V, Mitragotri S, Muzykantov V, Vascular Targeting of Nanocarriers: Perplexing Aspects of the Seemingly Straightforward Paradigm, ACS Nano 8 (2014) 4100–4132. doi: 10.1021/nn500136z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mahapatro A, Singh DK, Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines, J. Nanobiotechnology 9 (2011) 55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang F, Yang S, Yuan J, Gao Q, Huang C, Effective method of chitosan-coated alginate nanoparticles for target drug delivery applications, J. Biomater. Appl 31 (2016) 3–12. doi: 10.1177/0885328216648478. [DOI] [PubMed] [Google Scholar]
- [9].Xue M, Hu S, Lu Y, Zhang Y, Jiang X, An S, Guo Y, Zhou X, Hou H, Jiang C, Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor, Int. J. Pharm 495 (2015) 771–782. doi: 10.1016/j.ijpharm.2015.08.056. [DOI] [PubMed] [Google Scholar]
- [10].Sabet S, George MA, El-Shorbagy HM, Bassiony H, Farroh KY, Youssef T, Salaheldin TA, Gelatin nanoparticles enhance delivery of hepatitis C virus recombinant NS2 gene, PLoS One 12 (2017) 1–15. doi: 10.1371/journal.pone.0181723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Naahidi S, Jafari M, Edalat F, Raymond K, Khademhosseini A, Chen P, Biocompatibility of engineered nanoparticles for drug delivery, J. Control. Release 166 (2013) 182–194. doi: 10.1016/j.jconrel.2012.12.013. [DOI] [PubMed] [Google Scholar]
- [12].Makadia HK, Siegel SJ, Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier, Polymers (Basel) 3 (2011) 1377–1397. doi: 10.3390/polym3031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Roussaki M, Gaitanarou A, Diamanti PC, Vouyiouka S, Papaspyrides C, Kefalas P, Detsi A, Encapsulation of the natural antioxidant aureusidin in biodegradable PLA nanoparticles, Polym. Degrad. Stab 108 (2014) 182–187. doi: 10.1016/j.polymdegradstab.2014.08.004. [DOI] [Google Scholar]
- [14].Jia WJ, Gu YC, Gou M, Dai M, Li X, Kan B, Yang JL, Song QF, Wei YQ, Qian ZY, Preparation of biodegradable polycaprolactone/poly (ethylene glycol)/polycaprolactone (PCEC) nanoparticles, Drug Deliv 15 (2008) 409–416. doi: 10.1080/10717540802321727. [DOI] [PubMed] [Google Scholar]
- [15].Liu H, Slamovich EB, Webster TJ, Less harmful acidic degradation of poly(lactic-co-glycolic acid) bone tissue engineering scaffolds through titania nanoparticle addition, Int. J. Nanomedicine 1 (2006) 541–545. doi: 10.2147/nano.2006.1.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gentile P, Chiono V, Carmagnola I, Hatton PV, An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering, Int. J. Mol. Sci 15 (2014) 3640–3659. doi: 10.3390/ijms15033640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sue Anne Chew A, Hacker Michael C., Saraf Anita, Raphael Robert M., Kasper F. Kurtis, Mikos AG, Biodegradable branched polycationic polymers with varying hydrophilic spacers for non-viral gene delivery, Biomacromolecules 10 (2009) 2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Agarwal S, Zhang Y, Maji S, Greiner A, PDMAEMA based gene delivery materials, Mater. Today 15 (2012) 388–393. doi: 10.1016/S1369-7021(12)70165-7. [DOI] [Google Scholar]
- [19].Anozie UC, Dalhaimer P, Molecular links among non-biodegradable nanoparticles, reactive oxygen species, and autophagy, Adv. Drug Deliv. Rev 122 (2017) 65–73. doi: 10.1016/j.addr.2017.01.001. [DOI] [PubMed] [Google Scholar]
- [20].Kamba AS, Ismail M, Ibrahim TAT, Zakaria ZAB, Biocompatibility of Bio Based Calcium Carbonate Nanocrystals Aragonite Polymorph on NIH 3T3 Fibroblast Cell Line, African J. Tradit. Complement. Altern. Med 11 (2014) 31–38. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202393/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang Y, Nayak TR, Hong H, Cai W, Biomedical Applications of Zinc Oxide Nanomaterials, Curr. Mol. Med 13 (2013) 1633–1645. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3838497/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Parhiz H, Shier WT, Ramezani M, From rationally designed polymeric and peptidic systems to sophisticated gene delivery nano-vectors, Int. J. Pharm 457 (2013) 237–259. doi: 10.1016/j.ijpharm.2013.09.014. [DOI] [PubMed] [Google Scholar]
- [23].Parhiz H, Hashemi M, Hatefi A, Shier WT, Amel Farzad S, Ramezani M, Arginine-rich hydrophobic polyethylenimine: Potent agent with simple components for nucleic acid delivery, Int. J. Biol. Macromol 60 (2013) 18–27. doi: 10.1016/j.ijbiomac.2013.05.001. [DOI] [PubMed] [Google Scholar]
- [24].Wen Y, Pan S, Luo X, Zhang X, Zhang W, Feng M, A biodegradable low molecular weight polyethylenimine derivative as low toxicity and efficient gene vector, Bioconjug. Chem 20 (2009) 322–332. doi: 10.1021/bc800428y. [DOI] [PubMed] [Google Scholar]
- [25].Green JJ, Langer R, Anderson DG, A combinatorial polymer library approach yields insight into nonviral gene delivery, Acc. Chem. Res 41 (2008) 749–759. doi: 10.1021/ar7002336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eltoukhy AA, Siegwart DJ, Alabi CA, Rajan JS, Langer R, Anderson DG, Effect of molecular weight of amine end-modified poly(β-amino ester)s on gene delivery efficiency and toxicity, Biomaterials 33 (2012) 3594–3603. doi: 10.1016/j.biomaterials.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Fattal E, Grabowski N, Mura S, Vergnaud J, Tsapis N, Hillaireau H, Lung toxicity of biodegradable nanoparticles, J. Biomed. Nanotechnol 10 (2014) 2852–2864. doi: 10.1166/jbn.2014.1939. [DOI] [PubMed] [Google Scholar]
- [28].Yuan B, Webster TJ, Roy AK, Cytoprotective effects of cerium and selenium nanoparticles on heat-shocked human dermal fibroblasts: An in vitro evaluation, Int. J. Nanomedicine 11 (2016) 1427–1433. doi: 10.2147/IJN.S104082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nelson B, Johnson M, Walker M, Riley K, Sims C, Antioxidant Cerium Oxide Nanoparticles in Biology and Medicine, Antioxidants 5 (2016) 15. doi: 10.3390/antiox5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Manne NDPK, Arvapalli R, Graffeo VA, Bandarupalli VVK, Shokuhfar T, Patel S, Rice KM, Ginjupalli GK, Blough ER, Prophylactic Treatment with Cerium Oxide Nanoparticles Attenuate Hepatic Ischemia Reperfusion Injury in Sprague Dawley Rats, Cell. Physiol. Biochem 42 (2017). doi: 10.1159/000479540. [DOI] [PubMed] [Google Scholar]
- [31].Lungu M, Neculae A, Bunoiu M, Biris C, Nanoparticles’ promises and risks: Characterization, manipulation, and potential hazards to humanity and the environment, 2015. doi: 10.1007/978-3-319-11728-7. [DOI] [Google Scholar]
- [32].Osterberg RE, See NA, Toxicity of Excipients—A Food and Drug Administration Perspective, Int. J. Toxicol 22 (2003) 377–380. doi: 10.1177/109158180302200507. [DOI] [PubMed] [Google Scholar]
- [33].Szebeni J, Alving CR, Savay S, Barenholz Y, Priev A, Danino D, Talmon Y, Formation of complement-activating particles in aqueous solutions of Taxol: possible role in hypersensitivity reactions, Int. Immunopharmacol 1 (2001) 721–735. doi: 10.1016/S1567-5769(01)00006-6. [DOI] [PubMed] [Google Scholar]
- [34].Ilinskaya AN, Clogston JD, McNeil SE, Dobrovolskaia MA, Induction of oxidative stress by Taxol® vehicle Cremophor-EL triggers production of interleukin-8 by peripheral blood mononuclear cells through the mechanism not requiring de novo synthesis of mRNA, Nanomedicine 11 (2015) 1925–1938. doi: 10.1016/j.nano.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, Rha SY, Lee MY, Ro J, Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer, Breast Cancer Res. Treat 108 (2008) 241–250. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- [36].Hawkins MJ, Soon-Shiong P, Desai N, Protein nanoparticles as drug carriers in clinical medicine, Adv. Drug Deliv. Rev 60 (2008) 876–885. doi: 10.1016/j.addr.2007.08.044. [DOI] [PubMed] [Google Scholar]
- [37].Kloover JS, Den Bakker MA, Gelderblom H, Van Meerbeeck JP, Fatal outcome of a hypersensitivity reaction to paclitaxel: A critical review of premedication regimens, Br. J. Cancer 90 (2004) 304–305. doi: 10.1038/sj.bjc.6601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Oostingh GJ, Casals E, Italiani P, Colognato R, Stritzinger R, Ponti J, Pfaller T, Kohl Y, Ooms D, Favilli F, Leppens H, Lucchesi D, Rossi F, Nelissen I, Thielecke H, Puntes VF, Duschl A, Boraschi D, Problems and challenges in the development and validation of human cell-based assays to determine nanoparticle-induced immunomodulatory effects, Part. Fibre Toxicol 8 (2011). doi: 10.1186/1743-8977-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Krug HF, Nanosafety research-are we on the right track?, Angew. Chemie - Int. Ed 53 (2014) 12304–12319. doi: 10.1002/anie.201403367. [DOI] [PubMed] [Google Scholar]
- [40].Li Y, Boraschi D, Endotoxin contamination: a key element in the interpretation of nanosafety studies, Nanomedicine 11 (2016) 269–287. doi: 10.2217/nnm.15.196. [DOI] [PubMed] [Google Scholar]
- [41].Li Y, Fujita M, Boraschi D, Endotoxin contamination in nanomaterials leads to the misinterpretation of immunosafety results, Front. Immunol 8 (2017). doi: 10.3389/fimmu.2017.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Schwarz H, Schmittner M, Duschl A, Horejs-Hoeck J, Residual endotoxin contaminations in recombinant proteins are sufficient to activate human CD1c+ dendritic cells, PLoS One 9 (2014). doi: 10.1371/journal.pone.0113840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Triantafilou M, Triantafilou K, Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster, Trends Immunol 23 (2002) 301–304. doi: 10.1016/S1471-4906(02)02233-0. [DOI] [PubMed] [Google Scholar]
- [44].Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynśki A, Forsberg LS, Carlson RW, Dixit VM, Noncanonical inflammasome activation by intracellular LPS independent of TLR4, Science (80-. ). 341 (2013) 1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- [45].Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA, Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock, Science (80-. ). 341 (2013) 1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yang J, Zhao Y, Shao F, Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity, Curr. Opin. Immunol 32 (2015) 78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- [47].Vetten MA, Yah CS, Singh T, Gulumian M, Challenges facing sterilization and depyrogenation of nanoparticles: Effects on structural stability and biomedical applications, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 1391–1399. doi: 10.1016/j.nano.2014.03.017. [DOI] [PubMed] [Google Scholar]
- [48].Ángela F, Beatriz P, María M, Christian S, Andrea H, Cristina F, Valeria G, de la FJM, Isabel P, L.L. M, África G, Sterilization Matters: Consequences of Different Sterilization Techniques on Gold Nanoparticles, Small 6 (2009) 89–95. doi: 10.1002/smll.200901006. [DOI] [PubMed] [Google Scholar]
- [49].Anne SL, Brendan K, T.W. R, Kasey M, Endotoxin removal and prevention for pre‐clinical biologics production, Biotechnol. J 7 (2012) 1509–1516. doi: 10.1002/biot.201200220. [DOI] [PubMed] [Google Scholar]
- [50].Madaeni SS, The application of membrane technology for water disinfection, Water Res 33 (1999) 301–308. doi: 10.1016/S0043-1354(98)00212-7. [DOI] [Google Scholar]
- [51].Aida Y, Pabst MJ, Removal of endotoxin from protein solutions by phase separation using triton X-114, J. Immunol. Methods (1990). doi: 10.1016/0022-1759(90)90029-U. [DOI] [PubMed] [Google Scholar]
- [52].Dobrovolskaia MA, Neun BW, Clogston JD, Ding H, Ljubimova J, McNeil SE, Ambiguities in applying traditional Limulus Amoebocyte Lysate tests to quantify endotoxin in nanoparticle formulations, Nanomedicine (Lond) 5 (2010) 555–562. doi: 10.2217/nnm.10.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lerouge S, Wertheimer MR, Yahia L, Plasma Sterilization: A Review of Parameters, Mechanisms, and Limitations, Plasmas Polym 6 (2001) 175–188. doi: 10.1023/A:1013196629791. [DOI] [Google Scholar]
- [54].Ragab a a, Van De Motter R, Lavish S. a, Goldberg VM, Ninomiya JT, Carlin CR, Greenfield EM, Measurement and removal of adherent endotoxin from titanium particles and implant surfaces., J. Orthop. Res (1999). doi: 10.1002/jor.1100170603. [DOI] [PubMed] [Google Scholar]
- [55].Papageorgiou I, Brown C, Schins R, Singh S, Newson R, Davis S, Fisher J, Ingham E, Case CP, The effect of nano- and micron-sized particles of cobalt–chromium alloy on human fibroblasts in vitro, Biomaterials 28 (2007) 2946–2958. doi: 10.1016/j.biomaterials.2007.02.034. [DOI] [PubMed] [Google Scholar]
- [56].Lucarelli M, Gatti AM, Savarino G, Quattroni P, Martinelli L, Monari E, Boraschi D, Innate defence functions of macrophages can be biased by nano-sized ceramic and metallic particles, Eur. Cytokine Netw (2004). [PubMed]
- [57].Vallhov H, Qin J, Johansson SM, Ahlborg N, Muhammed MA, Scheynius A, Gabrielsson S, The Importance of an Endotoxin-Free Environment during the Production of Nanoparticles Used in Medical Applications, Nano Lett 6 (2006) 1682–1686. doi: 10.1021/nl060860z. [DOI] [PubMed] [Google Scholar]
- [58].Myerson JW, Anselmo AC, Liu Y, Mitragotri S, Eckmann DM, Muzykantov VR, Non-affinity factors modulating vascular targeting of nano- and microcarriers, Adv. Drug Deliv. Rev 99 (2016) 97–112. doi: 10.1016/j.addr.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Fenoglio I, Fubini B, Ghibaudi EM, Turci F, Multiple aspects of the interaction of biomacromolecules with inorganic surfaces, Adv. Drug Deliv. Rev 63 (2011) 1186–1209. doi: 10.1016/j.addr.2011.08.001. [DOI] [PubMed] [Google Scholar]
- [60].Khalili Fard J, Jafari S, Eghbal MA, A Review of Molecular Mechanisms Involved in Toxicity of Nanoparticles, Adv. Pharm. Bull 5 (2015) 447–454. doi: 10.15171/apb.2015.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Marucco A, Fenoglio I, Turci F, Fubini B, Interaction of fibrinogen and albumin with titanium dioxide nanoparticles of different crystalline phases, in: J. Phys. Conf. Ser, 2013. doi: 10.1088/1742-6596/429/1/012014. [DOI] [Google Scholar]
- [62].Deng ZJ, Liang M, Monteiro M, Toth I, Minchin RF, Nanoparticle-induced unfolding of fibrinogen promotes Mac-1 receptor activation and inflammation, Nat. Nanotechnol 6 (2011) 39–44. doi: 10.1038/nnano.2010.250. [DOI] [PubMed] [Google Scholar]
- [63].Akinc A, Battaglia G, Exploiting endocytosis for nanomedicines, Cold Spring Harb. Perspect. Biol (2013). doi: 10.1101/cshperspect.a016980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kou L, Sun J, Zhai Y, He Z, The endocytosis and intracellular fate of nanomedicines: Implication for rational design, Asian J. Pharm. Sci 8 (2013) 1–10. doi: 10.1016/j.ajps.2013.07.001. [DOI] [Google Scholar]
- [65].Beddoes CM, Case CP, Briscoe WH, Understanding nanoparticle cellular entry: A physicochemical perspective, Adv. Colloid Interface Sci 218 (2015) 48–68. doi: 10.1016/j.cis.2015.01.007. [DOI] [PubMed] [Google Scholar]
- [66].Ehrlich M, Boll W, Van Oijen A, Hariharan R, Chandran K, Nibert ML, Kirchhausen T, Endocytosis by random initiation and stabilization of clathrin-coated pits, Cell (2004). doi: 10.1016/j.cell.2004.08.017. [DOI] [PubMed] [Google Scholar]
- [67].Alexandre B, Christophe L, Clathrin‐Coated Pits: Vive La Différence?, Traffic 8 (2007) 970–982. doi: 10.1111/j.1600-0854.2007.00585.x. [DOI] [PubMed] [Google Scholar]
- [68].Wang Z, Tiruppathi C, Minshall RD, Malik AB, Size and Dynamics of Caveolae Studied Using Nanoparticles in Living Endothelial Cells, ACS Nano 3 (2009) 4110–4116. doi: 10.1021/nn9012274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Muro S, Koval M, Muzykantov V, Endothelial endocytic pathways: gates for vascular drug delivery., Curr. Vasc. Pharmacol (2004). doi: 10.2174/1570161043385736. [DOI] [PubMed] [Google Scholar]
- [70].Kim I, Xu W, Reed JC, Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities, Nat. Rev. Drug Discov 7 (2008) 1013 10.1038/nrd2755. [DOI] [PubMed] [Google Scholar]
- [71].Cao Y, Long J, Liu L, He T, Jiang L, Zhao C, Li Z, A review of endoplasmic reticulum (ER) stress and nanoparticle (NP) exposure, Life Sci 186 (2017) 33–42. doi: 10.1016/j.lfs.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [72].Hussain S, Garantziotis S, Rodrigues-Lima F, Dupret J-M, Baeza-Squiban A, Boland S, Intracellular Signal Modulation by Nanomaterials, Adv. Exp. Med. Biol 811 (2014) 111–134. doi: 10.1007/978-94-017-8739-0_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Belmont PJ, Tadimalla A, Chen WJ, Martindale JJ, Thuerauf DJ, Marcinko M, Gude N, Sussman MA, Glembotski CC, Coordination of Growth and Endoplasmic Reticulum Stress Signaling by Regulator of Calcineurin 1 (RCAN1), a Novel ATF6-inducible Gene, J. Biol. Chem 283 (2008) 14012–14021. doi: 10.1074/jbc.M709776200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC, Effects of the Isoform-specific Characteristics of ATF6α and ATF6β on Endoplasmic Reticulum Stress Response Gene Expression and Cell Viability, J. Biol. Chem . 282 (2007) 22865–22878. doi: 10.1074/jbc.M701213200. [DOI] [PubMed] [Google Scholar]
- [75].Rao RV, Bredesen DE, Misfolded proteins, endoplasmic reticulum stress and neurodegeneration, Curr. Opin. Cell Biol 16 (2004) 653–662. doi: 10.1016/j.ceb.2004.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ma J, Liu R, Wang X, Liu Q, Chen Y, Valle RP, Zuo YY, Xia T, Liu S, Crucial Role of Lateral Size for Graphene Oxide in Activating Macrophages and Stimulating Pro-inflammatory Responses in Cells and Animals, ACS Nano 9 (2015) 10498–10515. doi: 10.1021/acsnano.5b04751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Yen H-J, Hsu S, Tsai C-L, Cytotoxicity and Immunological Response of Gold and Silver Nanoparticles of Different Sizes, Small 5 (2009) 1553–1561. doi: 10.1002/smll.200900126. [DOI] [PubMed] [Google Scholar]
- [78].Reddy ST, Swartz MA, Hubbell JA, Targeting dendritic cells with biomaterials: developing the next generation of vaccines, Trends Immunol 27 (2006) 573–579. doi: 10.1016/j.it.2006.10.005. [DOI] [PubMed] [Google Scholar]
- [79].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Nanoparticles target distinct dendritic cell populations according to their size, Eur. J. Immunol 38 (2008) 1404–1413. doi: 10.1002/eji.200737984. [DOI] [PubMed] [Google Scholar]
- [80].Champion JA, Mitragotri S, Role of target geometry in phagocytosis, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 4930 LP-4934. http://www.pnas.org/content/103/1¾930.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Sharma G, Valenta DT, Altman Y, Harvey S, Xie H, Mitragotri S, Smith JW, Polymer particle shape independently influences binding and internalization by macrophages, J. Control. Release 147 (2010) 408–412. doi: 10.1016/j.jconrel.2010.07.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Gratton SEA, Ropp PA, Pohlhaus PD, Luft JC, Madden VJ, Napier ME, DeSimone JM, The effect of particle design on cellular internalization pathways, Proc. Natl. Acad. Sci 105 (2008) 11613–11618. doi: 10.1073/pnas.0801763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Champion JA, Mitragotri S, Shape induced inhibition of phagocytosis of polymer particles, Pharm. Res 26 (2009) 244–249. doi: 10.1007/s11095-008-9626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Muro S, Garnacho C, Champion JA, Leferovich J, Gajewski C, Schuchman EH, Mitragotri S, Muzykantov VR, Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers, Mol. Ther 16 (2008) 1450–1458. doi: 10.1038/mt.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, Discher DE, Shape effects of filaments versus spherical particles in flow and drug delivery, Nat. Nanotechnol 2 (2007) 249–255. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Liu Y, Tan J, Thomas A, Ou-Yang D, Muzykantov VR, The shape of things to come: importance of design in nanotechnology for drug delivery., Ther. Deliv 3 (2012) 181–94. doi: 10.4155/tde.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Stoehr LC, Gonzalez E, Stampfl A, Casals E, Duschl A, Puntes V, Oostingh GJ, Shape matters: Effects of silver nanospheres and wires on human alveolar epithelial cells, Part. Fibre Toxicol 8 (2011). doi: 10.1186/1743-8977-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Heng BC, Zhao X, Tan EC, Khamis N, Assodani A, Xiong S, Ruedl C, Ng KW, Loo JSC, Evaluation of the cytotoxic and inflammatory potential of differentially shaped zinc oxide nanoparticles, Arch. Toxicol 85 (2011) 1517–1528. doi: 10.1007/s00204-011-0722-1. [DOI] [PubMed] [Google Scholar]
- [89].Wang Y, Kaur G, Zysk A, Liapis V, Hay S, Santos A, Losic D, Evdokiou A, Systematic invitro nanotoxicity study on anodic alumina nanotubes with engineered aspect ratio: Understanding nanotoxicity by a nanomaterial model, Biomaterials 46 (2015) 117–130. doi: 10.1016/j.biomaterials.2014.12.008. [DOI] [PubMed] [Google Scholar]
- [90].Demento SL, Eisenbarth SC, Foellmer HG, Platt C, Caplan MJ, Mark Saltzman W, Mellman I, Ledizet M, Fikrig E, Flavell RA, Fahmy TM, Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy, Vaccine 27 (2009) 3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wang C, Li P, Liu L, Pan H, Li H, Cai L, Ma Y, Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation, Biomaterials 79 (2016) 88–100. doi: 10.1016/j.biomaterials.2015.11.040. [DOI] [PubMed] [Google Scholar]
- [92].Yang H, Liu C, Yang D, Zhang H, Xi Z, Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: The role of particle size, shape and composition, J. Appl. Toxicol 29 (2009) 69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- [93].Pantarotto D, Briand J-P, Prato M, Bianco A, Translocation of bioactive peptides across cell membranes by carbon nanotubes, Chem. Commun (2004) 16–17. doi: 10.1039/B311254C. [DOI] [PubMed] [Google Scholar]
- [94].Mu Q, Broughton DL, Yan B, Endosomal Leakage and Nuclear Translocation of Multiwalled Carbon Nanotubes: Developing a Model for Cell Uptake, Nano Lett 9 (2009) 4370–4375. doi: 10.1021/nl902647x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Wibroe PP, Anselmo AC, Nilsson PH, Sarode A, Gupta V, Urbanics R, Szebeni J, Hunter AC, Mitragotri S, Mollnes TE, Moghimi SM, Bypassing adverse injection reactions to nanoparticles through shape modification and attachment to erythrocytes, Nat. Nanotechnol 12 (2017) 589–594. doi: 10.1038/nnano.2017.47. [DOI] [PubMed] [Google Scholar]
- [96].Mitragotri S, Lahann J, Physical approaches to biomaterial design, Nat. Mater 8 (2009) 15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Muzykantov VR, Drug delivery by red blood cells: vascular carriers designed by Mother Nature, Expert Opin. Drug Deliv 7 (2011) 403–427. doi: 10.1517/17425241003610633.Drug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Merkel TJ, Chen K, Jones SW, Pandya AA, Tian S, Napier ME, Zamboni WE, Desimone JM, The effect of particle size on the biodistribution of low-modulus hydrogel PRINT particles, J. Control. Release 162 (2012) 37–44. doi: 10.1016/j.jconrel.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Doshi N, Zahr AS, Bhaskar S, Lahann J, Mitragotri S, Red blood cell-mimicking synthetic biomaterial particles, Proc. Natl. Acad. Sci 106 (2009) 21495–21499. doi: 10.1073/pnas.0907127106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Anselmo AC, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S, Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis, and targeting, ACS Nano 9 (2015) 3169–3177. doi: 10.1021/acsnano.5b00147. [DOI] [PubMed] [Google Scholar]
- [101].Dalhaimer P, Engler AJ, Parthasarathy R, Discher DE, Targeted worm micelles, Biomacromolecules 5 (2004) 1714–1719. doi: 10.1021/bm049884v. [DOI] [PubMed] [Google Scholar]
- [102].Simone EA, Dziubla TD, Colon-Gonzalez F, Discher DE, Muzykantov VR, Effect of polymer amphiphilicity on loading of a therapeutic enzyme into protective filamentous and spherical polymer nanocarriers, Biomacromolecules 8 (2007) 3914–3921. doi: 10.1021/bm700888h. [DOI] [PubMed] [Google Scholar]
- [103].Rehfeldt F, Engler AJ, Eckhardt A, Ahmed F, Discher DE, Cell responses to the mechanochemical microenvironment-Implications for regenerative medicine and drug delivery, Adv. Drug Deliv. Rev 59 (2007) 1329–1339. doi: 10.1016/j.addr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Key J, Palange AL, Gentile F, Aryal S, Stigliano C, Di Mascolo D, De Rosa E, Cho M, Lee Y, Singh J, Decuzzi P, Soft Discoidal Polymeric Nanoconstructs Resist Macrophage Uptake and Enhance Vascular Targeting in Tumors, ACS Nano 9 (2015) 11628–11641. doi: 10.1021/acsnano.5b04866. [DOI] [PubMed] [Google Scholar]
- [105].Palange AL, Palomba R, Rizzuti IF, Ferreira M, Decuzzi P, Deformable Discoidal Polymeric Nanoconstructs for the Precise Delivery of Therapeutic and Imaging Agents, Mol. Ther 25 (2017) 1514–1521. doi: 10.1016/j.ymthe.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Anselmo AC, Mitragotri S, Impact of particle elasticity on particle-based drug delivery systems, Adv. Drug Deliv. Rev 108 (2017) 51–67. doi: 10.1016/j.addr.2016.01.007. [DOI] [PubMed] [Google Scholar]
- [107].a Beningo K, Wang Y, Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target., J. Cell Sci 115 (2002) 849–856. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- [108].Banquy X, Suarez F, Argaw A, Rabanel J-M, Grutter P, Bouchard J-F, Hildgen P, Giasson S, Effect of mechanical properties of hydrogel nanoparticles on macrophage cell uptake, Soft Matter 5 (2009) 3984. doi: 10.1039/b821583a. [DOI] [Google Scholar]
- [109].Anselmo AC, Lynn Modery-Pawlowski C, Menegatti S, Kumar S, Vogus DR, Tian LL, Chen M, Squires TM, Sen Gupta A, Mitragotri S, Platelet-like nanoparticles: Mimicking shape, flexibility, and surface biology of platelets to target vascular injuries, ACS Nano 8 (2014) 11243–11253. doi: 10.1021/nn503732m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gunawan RC, Auguste DT, The role of antibody synergy and membrane fluidity in the vascular targeting of immunoliposomes, Biomaterials 31 (2010) 900–907. doi: 10.1016/j.biomaterials.2009.09.107. [DOI] [PubMed] [Google Scholar]
- [111].Guo P, Liu D, Subramanyam K, Wang B, Yang J, Huang J, Auguste DT, Moses MA, Nanoparticle elasticity directs tumor uptake, Nat. Commun 9 (2018). doi: 10.1038/s41467-017-02588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Guo D, Xie G, Luo J, Mechanical properties of nanoparticles: Basics and applications, J. Phys. D. Appl. Phys 47 (2014). doi: 10.1088/0022-3727/47/1/013001. [DOI] [Google Scholar]
- [113].Euliss LE, DuPont JA, Gratton S, DeSimone J, Imparting size, shape, and composition control of materials for nanomedicine, Chem. Soc. Rev 35 (2006) 1095. doi: 10.1039/b600913c. [DOI] [PubMed] [Google Scholar]
- [114].Brown AC, Stabenfeldt SE, Ahn B, Hannan RT, Dhada KS, Herman ES, Stefanelli V, Guzzetta N, Alexeev A, Lam WA, Lyon LA, Barker TH, Ultrasoft microgels displaying emergent platelet-like behaviours, Nat. Mater 13 (2014) 1108–1114. doi: 10.1038/nmat4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Eckmann DM, Composto RJ, Tsourkas A, Muzykantov VR, Nanogel carrier design for targeted drug delivery, J. Mater. Chem. B 2 (2014) 8085–8097. doi: 10.1039/C4TB01141D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Kennedy DC, Orts-Gil G, Lai CH, Müller L, Haase A, Luch A, Seeberger PH, Carbohydrate functionalization of silver nanoparticles modulates cytotoxicity and cellular uptake, J. Nanobiotechnology 12 (2014). doi: 10.1186/s12951-014-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Jain P, Pawar RS, Pandey RS, Madan J, Pawar S, Lakshmi PK, Sudheesh MS, In-vitro in-vivo correlation (IVIVC) in nanomedicine: Is protein corona the missing link?, Biotechnol. Adv 35 (2017) 889–904. doi: 10.1016/j.biotechadv.2017.08.003. [DOI] [PubMed] [Google Scholar]
- [118].Garvas M, Testen A, Umek P, Gloter A, Koklic T, Strancar J, Protein Corona Prevents TiO2 Phototoxicity., PLoS One 10 (2015) e0129577. doi: 10.1371/journal.pone.0129577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP, A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine., Proc. Natl. Acad. Sci 92 (1995) 7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Moghimi SM, Symonds P, Murray JC, Hunter AC, Debska G, Szewczyk A, A two-stage poly(ethylenimine)-mediated cytotoxicity: implications for gene transfer/therapy, Mol. Ther 11 (2005) 990–995. doi: 10.1016/j.ymthe.2005.02.010. [DOI] [PubMed] [Google Scholar]
- [121].Meindl C, Kueznik T, Bösch M, Roblegg E, Fröhlich E, Intracellular calcium levels as screening tool for nanoparticle toxicity, J. Appl. Toxicol 35 (2015) 1150–1159. doi: 10.1002/jat.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hall A, Parhamifar L, Lange MK, Meyle KD, Sanderhoff M, Andersen H, Roursgaard M, Larsen AK, Jensen PB, Christensen C, Bartek J, Moghimi SM, Polyethylenimine architecture-dependent metabolic imprints and perturbation of cellular redox homeostasis, Biochim. Biophys. Acta - Bioenerg 1847 (2015) 328–342. doi: 10.1016/j.bbabio.2014.12.002. [DOI] [PubMed] [Google Scholar]
- [123].Hall A, Lächelt U, Bartek J, Wagner E, Moghimi SM, Polyplex Evolution: Understanding Biology, Optimizing Performance, Mol. Ther 25 (2017) 1476–1490. doi: 10.1016/j.ymthe.2017.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Merkel OM, Urbanics R, Bedocs P, Rozsnyay Z, Rosivall L, Toth M, Kissel T, Szebeni J, In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers, Biomaterials 32 (2011) 4936–4942. doi: 10.1016/j.biomaterials.2011.03.035. [DOI] [PubMed] [Google Scholar]
- [125].Moghimi SM, Complement Propriety and Conspiracy in Nanomedicine: Perspective and a Hypothesis, Nucleic Acid Ther 26 (2016) 67–72. doi: 10.1089/nat.2015.0587. [DOI] [PubMed] [Google Scholar]
- [126].Wang J-Y, Chen J, Yang J, Wang H, Shen X, Sun Y-M, Guo M, Zhang X-D, Effects of surface charges of gold nanoclusters on long-term in vivo biodistribution, toxicity, and cancer radiation therapy, Int. J. Nanomedicine 11 (2016) 3475–3485. doi: 10.2147/IJN.S106073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chonn A, Cullis PR, V Devine D, The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes., J. Immunol 146 (1991) 4234 LP-4241. [PubMed] [Google Scholar]
- [128].Nishikawa K, Arai H, Inoue K, Scavenger receptor-mediated uptake and metabolism of lipid vesicles containing acidic phospholipids by mouse peritoneal macrophages, J Biol Chem (1990). [PubMed]
- [129].Rigotti A, Acton SL, Krieger M, The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids, J. Biol. Chem (1995). doi: 10.1074/jbc.270.27.16221. [DOI] [PubMed] [Google Scholar]
- [130].Nagayama S, Ogawara K, Minato K, Fukuoka Y, Takakura Y, Hashida M, Higaki K, Kimura T, Fetuin mediates hepatic uptake of negatively charged nanoparticles via scavenger receptor, Int. J. Pharm 329 (2007) 192–198. doi: 10.1016/j.ijpharm.2006.08.025. [DOI] [PubMed] [Google Scholar]
- [131].Shannahan JH, Bai W, Brown JM, Implications of scavenger receptors in the safe development of nanotherapeutics, Recept. Clin. Investig 2 (2015) e811. doi: 10.14800/rci.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Aldossari AA, Shannahan JH, Podila R, Brown JM, Scavenger receptor B1 facilitates macrophage uptake of silver nanoparticles and cellular activation, J. Nanoparticle Res (2015). doi: 10.1007/s11051-015-3116-0. [DOI] [Google Scholar]
- [133].Sahu D, Kannan GM, Tailang M, Vijayaraghavan R, In Vitro Cytotoxicity of Nanoparticles: A Comparison between Particle Size and Cell Type, J. Nanosci 2016 (2016) 1–9. doi: 10.1155/2016/4023852. [DOI] [Google Scholar]
- [134].Lanone S, Rogerieux F, Geys J, Dupont A, Maillot-Marechal E, Boczkowski J, Lacroix G, Hoet P, Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines, Part. Fibre Toxicol 6 (2009). doi: 10.1186/1743-8977-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Torres F, Fadeel B, Programmed Cell Death: Molecular Mechanisms and Implications for Safety Assessment of Nanomaterials, Accounts Chem. Res ‘ 733 Receiv January 46 (2012) 733–742. doi: 10.1021/ar300020b. [DOI] [PubMed] [Google Scholar]
- [136].Gow AJ, Branco F, Christofidou-Solomidou M, Black-Schultz L, Albelda SM, Muzykantov VR, Immunotargeting of glucose oxidase: intracellular production of H(2)O(2) and endothelial oxidative stress, Am. J. Physiol 277 (1999) L271–81. [DOI] [PubMed] [Google Scholar]
- [137].Dziubla TD, V Shuvaev V, Hong NK, Hawkins B, Muniswamy M, Takano H, Simone E, Nakada MT, Fisher A, Albelda SM, Muzykantov VR, Endothelial Targeting of Semi-permeable Polymer Nanocarriers for Enzyme Therapies, Biomaterials 29 (2008) 215–227. doi: 10.1016/j.biomaterials.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Muro S, Cui X, Gajewski C, Murciano J-C, Muzykantov VR, Koval M, Slow intracellular trafficking of catalase nanoparticles targeted to ICAM-1 protects endothelial cells from oxidative stress., Am. J. Physiol. Cell Physiol 285 (2003) C1339–47. doi: 10.1152/ajpcell.00099.2003. [DOI] [PubMed] [Google Scholar]
- [139].Sweitzer TD, Thomas AP, Wiewrodt R, Nakada MT, Branco F, Muzykantov VR, Pecam-directed immunotargeting of catalase: Specific, rapid and transient protection against hydrogen peroxide, Free Radic. Biol. Med 34 (2003) 1035–1046. doi: 10.1016/S0891-5849(03)00029-7. [DOI] [PubMed] [Google Scholar]
- [140].Wiewrodt R, Thomas AP, Cipelletti L, Christofidou-Solomidou M, Weitz DA, Feinstein SI, Schaffer D, Albelda SM, Koval M, Muzykantov VR, Size-dependent intracellular immunotargeting of therapeutic cargoes into endothelial cells, Blood 99 (2002) 912–922. doi: 10.1182/blood.V99.3.912. [DOI] [PubMed] [Google Scholar]
- [141].Chen J, Zhu J, Cho HH, Cui K, Li F, Zhou X, Rogers JT, Wong STC, Huang X, Differential cytotoxicity of metal oxide nanoparticles, J. Exp. Nanosci 3 (2008) 321–328. doi: 10.1080/17458080802235765. [DOI] [Google Scholar]
- [142].Marcela Gonzales A, Mitsumori Lee M., Kushleika John V., Rosenfeld Michael E., Krishnan KM, Cytotoxicity of iron oxide nanoparticles made from the thermal decomposition of organometallics and aqueous phase transfer with Pluronic F127, 5 (2011) 286–293. doi: 10.1002/cmmi.391.Cytotoxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Kong B, Seog JH, Graham LM, Lee SB, Experimental considerations on the cytotoxicity of nanoparticles, Nanomedicine 6 (2011) 929–941. doi: 10.2217/nnm.11.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Vijayakumar S, Ganesan S, In vitro cytotoxicity assay on gold nanoparticles with different stabilizing agents, J. Nanomater 2012 (2012). doi: 10.1155/2012/734398. [DOI] [Google Scholar]
- [145].Yuan Y-G, Peng Q-L, Gurunathan S, Silver nanoparticles enhance the apoptotic potential of gemcitabine in human ovarian cancer cells: combination therapy for effective cancer treatment., Int. J. Nanomedicine 12 (2017) 6487–6502. doi: 10.2147/IJN.S135482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kosmides AK, Sidhom JW, Fraser A, Bessell CA, Schneck JP, Dual Targeting Nanoparticle Stimulates the Immune System to Inhibit Tumor Growth, ACS Nano 11 (2017) 5417–5429. doi: 10.1021/acsnano.6b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Li H, Sun Y, Chen D, Zhao H, Zhao M, Zhu X, Ke C, Zhang G, Jiang C, Zhang L, Zhang F, Wei H, Li W, Synergistic anti-tumor therapy by a comb-like multifunctional antibody nanoarray with exceptionally potent activity, Sci. Rep 5 (2015) 1–13. doi: 10.1038/srep15712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Wang Xiu Ming, Terasaki PI, Rankin GW, Chia D, Zhong Hui Ping, Hardy S, A new microcellular cytotoxicity test based on calcein AM release, Hum. Immunol 37 (1993) 264–270. doi: 10.1016/0198-8859(93)90510-8. [DOI] [PubMed] [Google Scholar]
- [149].Monteiro-Riviere NA, Inman AO, Zhang LW, Limitations and relative utility of screening assays to assess engineered nanoparticle toxicity in a human cell line, Toxicol. Appl. Pharmacol 234 (2009) 222–235. doi: 10.1016/j.taap.2008.09.030. [DOI] [PubMed] [Google Scholar]
- [150].Wang ZQ, Liu K, Huo ZJ, Li XC, Wang M, Liu P, Pang B, Wang SJ, A cell-targeted chemotherapeutic nanomedicine strategy for oral squamous cell carcinoma therapy, J. Nanobiotechnology 13 (2015) 1–10. doi: 10.1186/s12951-015-0116-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Aranda A, Sequedo L, Tolosa L, Quintas G, Burello E, Castell JV, Gombau L, Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells, Toxicol. Vitr 27 (2013) 954–963. doi: 10.1016/j.tiv.2013.01.016. [DOI] [PubMed] [Google Scholar]
- [152].Pal AK, Bello D, Budhlall B, Rogers E, Milton DK, Screening for oxidative stress elicited by engineered nanomaterials: Evaluation of acellular DCFH assay, Dose-Response 10 (2012) 308–330. doi: 10.2203/dose-response.10-036.Pal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Pal AK, Hsieh SF, Khatri M, Isaacs JA, Demokritou P, Gaines P, Schmidt DF, Rogers EJ, Bello D, Screening for oxidative damage by engineered nanomaterials: A comparative evaluation of FRAS and DCFH, J. Nanoparticle Res 16 (2014). doi: 10.1007/s11051-013-2167-3. [DOI] [Google Scholar]
- [154].Lu X, Qian J, Zhou H, Gan Q, Tang W, Lu J, Yuan Y, Liu C, In vitro cytotoxicity and induction of apoptosis by silica nanoparticles in human HepG2 hepatoma cells., Int. J. Nanomedicine 6 (2011) 1889–901. doi: 10.2147/IJN.S24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bendale Y, Bendale V, Paul S, Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis, Integr. Med. Res 6 (2017) 141–148. doi: 10.1016/j.imr.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Azizi M, Ghourchian H, Yazdian F, Bagherifam S, Bekhradnia S, Nyström B, Anti-cancerous effect of albumin coated silver nanoparticles on MDA-MB 231 human breast cancer cell line, Sci. Rep 7 (2017) 1–18. doi: 10.1038/s41598-017-05461-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Catalán J, Suhonen S, Huk A, Dusinska M, Analysis of Nanoparticle-Induced DNA Damage by the Comet Assay, in: Genotoxicity DNA Repair, 2014: pp. 241–268. doi: 10.1007/978-1-4939-1068-7. [DOI] [Google Scholar]
- [158].Karlsson HL, Di Bucchianico S, Collins AR, Dusinska M, Can the comet assay be used reliably to detect nanoparticle-induced genotoxicity?, Environ. Mol. Mutagen 56 (2015) 82–96. doi: 10.1002/em.21933. [DOI] [PubMed] [Google Scholar]
- [159].Klaude M, Eriksson S, Nygren J, Ahnström G, The comet assay: Mechanisms and technical considerations, Mutat. Res. - DNA Repair 363 (1996) 89–96. doi: 10.1016/0921-8777(95)00063-1. [DOI] [PubMed] [Google Scholar]
- [160].Barnes CA, Elsaesser A, Arkusz J, Smok A, Palus J, Leśniak A, Salvati A, Hanrahan JP, de Jong WH, Dziubałtowska E, Stepnik M, Rydzyński K, McKerr G, Lynch I, Dawson KA, Howard CV, Reproducible comet assay of amorphous silica nanoparticles detects no genotoxicity., Nano Lett 8 (2008) 3069–3074. doi: 10.1021/nl801661w. [DOI] [PubMed] [Google Scholar]
- [161].Choi J, Reipa V, Hitchins VM, Goering PL, Malinauskas RA, Physicochemical Characterization and in vitro hemolysis evaluation of silver nanoparticles, Toxicol. Sci 123 (2011) 133–143. doi: 10.1093/toxsci/kfr149. [DOI] [PubMed] [Google Scholar]
- [162].Pham CTN, Thomas DG, Beiser J, Mitchell LM, Huang JL, Senpan A, Hu G, Gordon M, Baker NA, Pan D, Lanza GM, Hourcade DE, Application of a hemolysis assay for analysis of complement activation by perfluorocarbon nanoparticles, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 651–660. doi: 10.1016/j.nano.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Neun BW, Ilinskaya AN, Dobrovolskaia MA, Updated method for in vitro analysis of nanoparticle hemolytic properties, in: Methods Mol. Biol, 2018: pp. 91–102. doi: 10.1007/978-1-4939-7352-1_9. [DOI] [PubMed] [Google Scholar]
- [164].Yu T, Malugin A, Ghandehari H, Impact of silica nanoparticle design on cellular toxicity and hemolytic activity, in: ACS Nano, 2011: pp. 5717–5728. doi: 10.1021/nn2013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].MacParland SA, Tsoi KM, Ouyang B, Ma XZ, Manuel J, Fawaz A, Ostrowski MA, Alman BA, Zilman A, Chan WCW, McGilvray ID, Phenotype Determines Nanoparticle Uptake by Human Macrophages from Liver and Blood, ACS Nano 11 (2017) 2428–2443. doi: 10.1021/acsnano.6b06245. [DOI] [PubMed] [Google Scholar]
- [166].Nicolete R, Dos Santos DF, Faccioli LH, The uptake of PLGA micro or nanoparticles by macrophages provokes distinct in vitro inflammatory response, Int. Immunopharmacol 11 (2011) 1557–1563. doi: 10.1016/j.intimp.2011.05.014. [DOI] [PubMed] [Google Scholar]
- [167].Elsabahy M, Wooley KL, Cytokines as biomarkers of nanoparticle immunotoxicity, Chem. Soc. Rev 42 (2013) 5552. doi: 10.1039/c3cs60064e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Devanabanda M, Latheef SA, Madduri R, Immunotoxic effects of gold and silver nanoparticles: Inhibition of mitogen-induced proliferative responses and viability of human and murine lymphocytes in vitro, J. Immunotoxicol 13 (2016) 897–902. doi: 10.1080/1547691X.2016.1234522. [DOI] [PubMed] [Google Scholar]
- [169].Huang H, Lai W, Cui M, Liang L, Lin Y, Fang Q, Liu Y, Xie L, An Evaluation of Blood Compatibility of Silver Nanoparticles, Sci. Rep 6 (2016). doi: 10.1038/srep25518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Brzicová T, Lochman I, Danihelka P, Lochmanová A, Lach K, Mička V, Immunological assays as an opportunity of assessment of health risks of airborne particle mixture including nanoparticles, in: J. Phys. Conf. Ser, 2013. doi: 10.1088/1742-6596/429/1/012032. [DOI] [Google Scholar]
- [171].Venkatasubbu GD, Ramasamy S, Gaddam PR, Kumar J, Acute and subchronic toxicity analysis of surface modified paclitaxel attached hydroxyapatite and titanium dioxide nanoparticles, Int. J. Nanomedicine 10 (2015) 137–148. doi: 10.2147/IJN.S79991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Park HS, Kim SJ, Lee TJ, Kim GY, Meang E, Hong JS, Kim SH, Koh SB, Hong SG, Sun YS, Kang JS, Kim YR, Kim MK, Jeong J, Lee JK, Son WC, Park JH, A 90-day study of sub-chronic oral toxicity of 20 nm positively charged zinc oxide nanoparticles in Sprague Dawley rats, Int. J. Nanomedicine 9 (2014). doi: 10.2147/IJN.S57927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Bin Zhai Z, Meng AM, Liu PX, Zhang LA, Fan FY, Toxicologic effects of gold nanoparticles in vivo by different administration routes, Int. J. Nanomedicine 5 (2010) 771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Qu X, Xu K, Zhao C, Song X, Li J, Li L, Nie W, Bao H, Wang J, Niu F, Li J, Genotoxicity and acute and subchronic toxicity studies of a bioactive polyoxometalate in Wistar rats, BMC Pharmacol. Toxicol 18 (2017). doi: 10.1186/s40360-017-0133-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Strickland J, Clippinger AJ, Brown J, Allen D, Jacobs A, Matheson J, Lowit A, Reinke EN, Johnson MS, Quinn MJ, Mattie D, Fitzpatrick SC, Ahir S, Kleinstreuer N, Casey W, Status of acute systemic toxicity testing requirements and data uses by U.S. regulatory agencies, Regul. Toxicol. Pharmacol 94 (2018) 183–196. doi: 10.1016/j.yrtph.2018.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Russo A, In vivo cytogenetics: Mammalian germ cells, Mutat. Res. - Fundam. Mol. Mech. Mutagen 455 (2000) 167–189. doi: 10.1016/S0027-5107(00)00115-9. [DOI] [PubMed] [Google Scholar]
- [177].Adler ID, Reitmeir P, Schmöller R, Schriever-Schwemmer G, Dose response for heritable translocations induced by acrylamide in spermatids of mice, Mutat. Res. Regul. Pap 309 (1994) 285–291. doi: 10.1016/0027-5107(94)90103-1. [DOI] [PubMed] [Google Scholar]
- [178].Gocke E, Wild D, Eckhardt K, King MT, Mutagenicity studies with the mouse spot test, Mutat. Res. Toxicol 117 (1983) 201–212. doi: 10.1016/0165-1218(83)90168-4. [DOI] [PubMed] [Google Scholar]
- [179].Hayashi M, The micronucleus test-most widely used in vivo genotoxicity test, Genes Environ 38 (2016). doi: 10.1186/s41021-016-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Wilhelmus KR, The Draize eye test., Surv. Ophthalmol 45 (2001) 493–515. doi: 10.1016/S0039-6257(01)00211-9. [DOI] [PubMed] [Google Scholar]
- [181].Kalam MA, Alshamsan A, Poly (D, L-lactide-co-glycolide) nanoparticles for sustained release of tacrolimus in rabbit eyes, Biomed. Pharmacother 94 (2017) 402–411. doi: 10.1016/j.biopha.2017.07.110. [DOI] [PubMed] [Google Scholar]
- [182].Gökçe EH, Sandri G, Eǧrilmez S, Bonferoni MC, Güneri T, Caramella C, Cyclosporine a-loaded solid lipid nanoparticles: Ocular tolerance and in vivo drug release in rabbit eyes, Curr. Eye Res 34 (2009) 996–1003. doi: 10.3109/02713680903261405. [DOI] [PubMed] [Google Scholar]
- [183].Şenyiğit T, Sonvico F, Rossi A, Tekmen I, Santi P, Colombo P, Nicoli S, Özer Ö, In vivo assessment of clobetasol propionate-loaded lecithin-chitosan nanoparticles for skin delivery, Int. J. Mol. Sci 18 (2017). doi: 10.3390/ijms18010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Leite-Silva VR, De Almeida MM, Fradin A, Grice JE, Roberts MS, Delivery of drugs applied topically to the skin, Expert Rev. Dermatol 7 (2012) 383–397. doi: 10.1586/edm.12.32. [DOI] [Google Scholar]
- [185].Corominas M, Gastaminza G, Lobera T, Hypersensitivity reactions to biological drugs, J. Investig. Allergol. Clin. Immunol 24 (2014) 212–225. doi: 10.1016/j.soncn.2007.05.009. [DOI] [PubMed] [Google Scholar]
- [186].Montañez MI, Ruiz-Sanchez AJ, Perez-Inestrosa E, A perspective of nanotechnology in hypersensitivity reactions including drug allergy, Curr. Opin. Allergy Clin. Immunol 10 (2010) 297–302. doi: 10.1097/ACI.0b013e32833b1f17. [DOI] [PubMed] [Google Scholar]
- [187].Szebeni J, Alving CR, Rosivall L, Bünger R, Baranyi L, Bedöcs P, Tóth M, Barenholz Y, Animal models of complement-mediated hypersensitivity reactions to liposomes and other lipid-based nanoparticles, J. Liposome Res 17 (2007) 107–117. doi: 10.1080/08982100701375118. [DOI] [PubMed] [Google Scholar]
- [188].Nguyen VH, Lee BJ, Protein corona: A new approach for nanomedicine design, Int. J. Nanomedicine 12 (2017) 3137–3151. doi: 10.2147/IJN.S129300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Thiele L, Diederichs JE, Reszka R, Merkle HP, Walter E, Competitive adsorption of serum proteins at microparticles affects phagocytosis by dendritic cells, Biomaterials 24 (2003) 1409–1418. doi: 10.1016/S0142-9612(02)00525-2. [DOI] [PubMed] [Google Scholar]
- [190].Ogawara KI, Furumoto K, Nagayama S, Minato K, Higaki K, Kai T, Kimura T, Pre-coating with serum albumin reduces receptor-mediated hepatic disposition of polystyrene nanosphere: Implications for rational design of nanoparticles, J. Control. Release 100 (2004) 451–455. doi: 10.1016/j.jconrel.2004.07.028. [DOI] [PubMed] [Google Scholar]
- [191].Peng Q, Zhang S, Yang Q, Zhang T, Wei XQ, Jiang L, Zhang CL, Chen QM, Zhang ZR, Lin YF, Preformed albumin corona, a protective coating for nanoparticles based drug delivery system, Biomaterials 34 (2013) 8521–8530. doi: 10.1016/j.biomaterials.2013.07.102. [DOI] [PubMed] [Google Scholar]
- [192].Göppert TM, Müller RH, Adsorption kinetics of plasma proteins on solid lipid nanoparticles for drug targeting, Int. J. Pharm 302 (2005) 172–186. doi: 10.1016/j.ijpharm.2005.06.025. [DOI] [PubMed] [Google Scholar]
- [193].Flick MJ, Du X, Witte DP, Jiroušková M, Soloviev DA, Busuttil SJ, Plow EF, Degen JL, Leukocyte engagement of fibrin(ogen) via the integrin receptor α(M)β(2)/Mac-1 is critical for host inflammatory response in vivo, J. Clin. Invest 113 (2004) 1596–1606. doi: 10.1172/JCI200420741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Ilinskaya AN, Dobrovolskaia MA, Nanoparticles and the blood coagulation system. Part II: safety concerns, Nanomedicine 8 (2013) 969–981. doi: 10.2217/nnm.13.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Jones CF, Campbell RA, Brooks AE, Assemi S, Tadjiki S, Thiagarajan G, Mulcock C, Weyrich AS, Brooks BD, Ghandehari H, Grainger DW, Cationic PAMAM dendrimers aggressively initiate blood clot formation, ACS Nano 6 (2012) 9900–9910. doi: 10.1021/nn303472r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Nabeshi H, Yoshikawa T, Matsuyama K, Nakazato Y, Arimori A, Isobe M, Tochigi S, Kondoh S, Hirai T, Akase T, Yamashita T, Yamashita K, Yoshida T, Nagano K, Abe Y, Yoshioka Y, Kamada H, Imazawa T, Itoh N, Kondoh M, Yagi K, Mayumi T, Tsunoda SI, Tsutsumi Y, Amorphous nanosilicas induce consumptive coagulopathy after systemic exposure, Nanotechnology 23 (2012). doi:. [DOI] [PubMed] [Google Scholar]
- [197].Yoshida T, Yoshioka Y, Morishita Y, Aoyama M, Tochigi S, Hirai T, Tanaka K, Nagano K, Kamada H, Tsunoda SI, Nabeshi H, Yoshikawa T, Higashisaka K, Tsutsumi Y, Protein corona changes mediated by surface modification of amorphous silica nanoparticles suppress acute toxicity and activation of intrinsic coagulation cascade in mice, Nanotechnology 26 (2015). doi: 10.1088/0957-4484/26/24/245101. [DOI] [PubMed] [Google Scholar]
- [198].Allen TM, Toxicity of drug carriers to the mononuclear phagocyte system, Adv. Drug Deliv. Rev 2 (1988) 55–67. doi: 10.1016/0169-409X(88)90005-1. [DOI] [Google Scholar]
- [199].Chen D, Ganesh S, Wang W, Amiji M, Plasma protein adsorption and biological identity of systemically administered nanoparticles, Nanomedicine 12 (2017) 2113–2135. doi: 10.2217/nnm-2017-0178. [DOI] [PubMed] [Google Scholar]
- [200].Moghimi SM, Hamad I, Liposome-mediated triggering of complement cascade, J. Liposome Res 18 (2008) 195–209. doi: 10.1080/08982100802309552. [DOI] [PubMed] [Google Scholar]
- [201].Holers VM, Complement and Its Receptors: New Insights into Human Disease, Annu. Rev. Immunol 32 (2014) 433–459. doi: 10.1146/annurev-immunol-032713-120154. [DOI] [PubMed] [Google Scholar]
- [202].Szebeni J, Baranyi L, Savay S, Milosevits J, Bodo M, Bunger R, Alving CR, The interaction of liposomes with the complement system: In vitro and in vivo assays, Methods Enzymol 373 (2003) 136–154. doi: 10.1016/S0076-6879(03)73010-9. [DOI] [PubMed] [Google Scholar]
- [203].Szebeni J, Storm G, Complement activation as a bioequivalence issue relevant to the development of generic liposomes and other nanoparticulate drugs, Biochem. Biophys. Res. Commun 468 (2015) 490–497. doi: 10.1016/j.bbrc.2015.06.177. [DOI] [PubMed] [Google Scholar]
- [204].Moghimi SM, Andersen AJ, Ahmadvand D, Wibroe PP, Andresen TL, Hunter AC, Material properties in complement activation, Adv. Drug Deliv. Rev 63 (2011) 1000–1007. doi: 10.1016/j.addr.2011.06.002. [DOI] [PubMed] [Google Scholar]
- [205].Moghimi SM, Simberg D, Complement activation turnover on surfaces of nanoparticles, Nano Today 15 (2017) 8–10. doi: 10.1016/j.nantod.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Bangham AD, Horne RW, Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope, J. Mol. Biol 8 (1964) IN2–IN10. doi: 10.1016/S0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- [207].Haxby JA, Kinsky CB, Kinsky SC, Immune response of a liposomal model membrane., Proc. Natl. Acad. Sci. U. S. A 61 (1968) 300–307. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC285936/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Szebeni J, The interaction of liposomes with the complement system, Crit Rev Ther Drug Carr. Syst 15 (1998) 57–88. http://www.ncbi.nlm.nih.gov/pubmed/9523086. [PubMed] [Google Scholar]
- [209].PACHECO PM, LE B, WHITE D, SULCHEK T, Tunable complement activation by particles with variable size and fc density, Nano Life (2013). doi: 10.1142/S1793984413410018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Owens DE, Peppas NA, Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles, Int. J. Pharm 307 (2006) 93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- [211].Dobrovolskaia MA, Aggarwal P, Hall JB, McNeil SE, Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution, in: Mol. Pharm, 2008: pp. 487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Allen TM, Cullis PR, Drug Delivery Systems: Entering the Mainstream, Science (80-. ). 303 (2004) 1818–1822. doi: 10.1126/science.1095833. [DOI] [PubMed] [Google Scholar]
- [213].Benasutti H, Wang G, Vu VP, Scheinman R, Groman E, Saba L, Simberg D, Variability of Complement Response toward Preclinical and Clinical Nanocarriers in the General Population, Bioconjug. Chem 28 (2017) 2747–2755. doi: 10.1021/acs.bioconjchem.7b00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Wang G, Chen F, Banda NK, Holers VM, Wu LP, Moghimi SM, Simberg D, Activation of human complement system by dextran-coated iron oxide nanoparticles is not affected by dextran/Fe ratio, hydroxyl modifications, and crosslinking, Front. Immunol 7 (2016). doi: 10.3389/fimmu.2016.00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Moghimi SM, Hunter AC, Complement monitoring of nanomedicines and implants, Adv. Drug Deliv. Rev 63 (2011) 963–964. doi: 10.1016/j.addr.2011.06.008. [DOI] [PubMed] [Google Scholar]
- [216].Belling JN, Jackman JA, Yorulmaz Avsar S, Park JH, Wang Y, Potroz MG, Ferhan AR, Weiss PS, Cho N-J, Stealth Immune Properties of Graphene Oxide Enabled by Surface-Bound Complement Factor H, ACS Nano 10 (2016) 10161–10172. doi: 10.1021/acsnano.6b05409. [DOI] [PubMed] [Google Scholar]
- [217].Yu K, Lai BFL, Foley JH, Krisinger MJ, Conway EM, Kizhakkedathu JN, Modulation of Complement Activation and Amplification on Nanoparticle Surfaces by Glycopolymer Conformation and Chemistry, ACS Nano 8 (2014) 7687–7703. doi: 10.1021/nn504186b. [DOI] [PubMed] [Google Scholar]
- [218].Vonarbourg A, Passirani C, Saulnier P, Simard P, Leroux JC, Benoit JP, Evaluation of pegylated lipid nanocapsules versus complement system activation and macrophage uptake, J. Biomed. Mater. Res. - Part A 78 (2006) 620–628. doi: 10.1002/jbm.a.30711. [DOI] [PubMed] [Google Scholar]
- [219].Zhang XQ, Xu X, Bertrand N, Pridgen E, Swami A, Farokhzad OC, Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine, Adv. Drug Deliv. Rev 64 (2012) 1363–1384. doi: 10.1016/j.addr.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Ishida T, Kojima H, Harashima H, Kiwada H, Biodistribution of liposomes and C3 fragments associated with liposomes: Evaluation of their relationship, Int. J. Pharm 205 (2000) 183–193. doi: 10.1016/S0378-5173(00)00511-1. [DOI] [PubMed] [Google Scholar]
- [221].Jackman JA, Mészáros T, Fülöp T, Urbanics R, Szebeni J, Cho NJ, Comparison of complement activation-related pseudoallergy in miniature and domestic pigs: Foundation of a validatable immune toxicity model, Nanomedicine Nanotechnology, Biol. Med 12 (2016) 933–943. doi: 10.1016/j.nano.2015.12.377. [DOI] [PubMed] [Google Scholar]
- [222].Molino NM, Bilotkach K, Fraser DA, Ren D, Wang S-W, Complement Activation and Cell Uptake Responses Toward Polymer-Functionalized Protein Nanocapsules, Biomacromolecules 13 (2012) 974–981. doi: 10.1021/bm300083e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Barbero F, Russo L, Vitali M, Piella J, Salvo I, Borrajo ML, Busquets-Fité M, Grandori R, Bastús NG, Casals E, Puntes V, Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System, Semin. Immunol 34 (2017) 52–60. doi: 10.1016/j.smim.2017.10.001. [DOI] [PubMed] [Google Scholar]
- [224].Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J, The big picture on small medicine: the state of nanomedicine products approved for use or in clinical trials, Nanomedicine 9 (2013) 1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Caron WP, Song G, Kumar P, Rawal S, Zamboni WC, Interpatient pharmacokinetic and pharmacodynamic variability of carrier-mediated anticancer agents, Clin. Pharmacol. Ther 91 (2012) 802–812. doi: 10.1038/clpt.2012.12. [DOI] [PubMed] [Google Scholar]
- [226].Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW, Analysis of nanoparticle delivery to tumours, Nat. Rev. Mater 1 (2016). doi: 10.1038/natrevmats.2016.14. [DOI] [Google Scholar]
- [227].Zhang YN, Poon W, Tavares AJ, McGilvray ID, Chan WCW, Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination, J. Control. Release 240 (2016) 332–348. doi: 10.1016/j.jconrel.2016.01.020. [DOI] [PubMed] [Google Scholar]
- [228].Tavares AJ, Poon W, Zhang Y-N, Dai Q, Besla R, Ding D, Ouyang B, Li A, Chen J, Zheng G, Robbins C, Chan WCW, Effect of removing Kupffer cells on nanoparticle tumor delivery, Proc. Natl. Acad. Sci (2017) 201713390. doi: 10.1073/pnas.1713390114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Schneberger D, Aharonson-Raz K, Singh B, Pulmonary intravascular macrophages and lung health: What are we missing?, AJP Lung Cell. Mol. Physiol 302 (2012) L498–L503. doi: 10.1152/ajplung.00322.2011. [DOI] [PubMed] [Google Scholar]
- [230].Wheeldon EB, Hansen-Flaschen JH, Intravascular macrophages in the sheep lung, J Leukoc Biol 40 (1986) 657–661. http://www.jleukbio.org/content/40/5/657.full.pdf. [DOI] [PubMed] [Google Scholar]
- [231].Brain JD, Molina RM, DeCamp MM, Warner AE, Pulmonary intravascular macrophages: their contribution to the mononuclear phagocyte system in 13 species., Am. J. Physiol 276 (1999) L146–54. http://www.ncbi.nlm.nih.gov/pubmed/9887067. [DOI] [PubMed] [Google Scholar]
- [232].Gill SS, Suri SS, Janardhan KS, Caldwell S, Duke T, Singh B, Role of pulmonary intravascular macrophages in endotoxin-induced lung inflammation and mortality in a rat model, Respir. Res 9 (2008) 69. doi: 10.1186/1465-9921-9-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Smith JS, Tian J, Lozier JN, Byrnes AP, Severe pulmonary pathology after intravenous administration of vectors in cirrhotic rats, Mol. Ther 9 (2004) 932–941. doi: 10.1016/j.ymthe.2004.03.010. [DOI] [PubMed] [Google Scholar]
- [234].Kuebler WM, Goetz AE, The marginated pool, Eur. Surg. Res 34 (2002) 92–100. doi: 10.1159/000048894. [DOI] [PubMed] [Google Scholar]
- [235].Doerschuk CM, Mechanisms of leukocyte sequestration in inflamed lungs, Microcirculation 8 (2001) 71–88. doi: 10.1038/sj.mn.7300151. [DOI] [PubMed] [Google Scholar]
- [236].Doerschuk CM, Allard MF, Martin B. a, MacKenzie a, Autor a P., Hogg JC, Marginated pool of neutrophils in rabbit lungs., J. Appl. Physiol 63 (1987) 1806–1815. [DOI] [PubMed] [Google Scholar]
- [237].Yipp BG, Kim JH, Lima R, Zbytnuik LD, Petri B, Swanlund N, Ho M, Szeto VG, Tak T, Koenderman L, Pickkers P, Tool ATJ, Kuijpers TW, van den Berg TK, Looney MR, Krummel MF, Kubes P, The lung is a host defense niche for immediate neutrophil-mediated vascular protection, Sci. Immunol 2 (2017) eaam8929. doi: 10.1126/sciimmunol.aam8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Wang Z, Li J, Cho J, Malik AB, Prevention of vascular inflammation by nanoparticle targeting of adherent neutrophils, Nat. Nanotechnol 9 (2014) 204–210. doi: 10.1038/nnano.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Chu D, Gao J, Wang Z, Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection, ACS Nano 9 (2015) 11800–11811. doi: 10.1021/acsnano.5b05583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Nishanth RP, Jyotsna RG, Schlager JJ, Hussain SM, Reddanna P, Inflammatory responses of RAW 264.7 macrophages upon exposure to nanoparticles: Role of ROS-NFκB signaling pathway, Nanotoxicology 5 (2011) 502–516. doi: 10.3109/17435390.2010.541604. [DOI] [PubMed] [Google Scholar]
- [241].Park J, Lim D-H, Lim H-J, Kwon T, Choi J, Jeong S, Choi I-H, Cheon J, Size dependent macrophage responses and toxicological effects of Ag nanoparticles., Chem. Commun. (Camb) 47 (2011) 4382–4384. doi: 10.1039/c1cc10357a. [DOI] [PubMed] [Google Scholar]
- [242].Chen X, Gao C, Influences of size and surface coating of gold nanoparticles on inflammatory activation of macrophages, Colloids Surfaces B Biointerfaces 160 (2017) 372–380. doi: 10.1016/j.colsurfb.2017.09.046. [DOI] [PubMed] [Google Scholar]
- [243].Meunier E, Coste A, Olagnier D, Authier H, Lefèvre L, Dardenne C, Bernad J, Béraud M, Flahaut E, Pipy B, Double-walled carbon nanotubes trigger IL-1β release in human monocytes through Nlrp3 inflammasome activation, Nanomedicine Nanotechnology, Biol. Med 8 (2012) 987–995. doi: 10.1016/j.nano.2011.11.004. [DOI] [PubMed] [Google Scholar]
- [244].Marzaioli V, Groß CJ, Weichenmeier I, Schmidt-Weber CB, Gutermuth J, Groß O, Alessandrini F, Specific surface modifications of silica nanoparticles diminish inflammasome activation and in vivo expression of selected inflammatory genes, Nanomaterials 7 (2017). doi: 10.3390/nano7110355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Desai N, Challenges in Development of Nanoparticle-Based Therapeutics, AAPS J 14 (2012) 282–295. doi: 10.1208/s12248-012-9339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Alberts B LJ, Johnson A, Helper T Cells and Lymphocyte Activation, 2002.
- [247].Chaplin DD, Overview of the Immune Response, J Allergy Clin Immunol 125 (2010) S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Corthay A, A three-cell model for activation of naïve T helper cells, Scand. J. Immunol 64 (2006) 93–96. doi: 10.1111/j.1365-3083.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- [249].Liechtenstein T, Dufait I, Lanna A, Breckpot K, Escors D, Modulating Co-Stimulation During Antigen Presentation To Enhance Cancer Immunotherapy., Immunol. Endocr. Metab. Agents Med. Chem 12 (2012) 224–235. doi: 10.2174/187152212802001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Sloat BR CZ, Sandoval MA, Hau AM, He Y, Strong Antibody Responses Induced by Protein Antigens Conjugated onto the Surface of Lecithin-Based Nanoparticles, J Control Release 141 (2010) 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].K. J, Nanoparticles as adjuvants for vaccines, Pharm Biotechnol 6 (1995) 463–72. [DOI] [PubMed] [Google Scholar]
- [252].Castignolles N PP, Morgeaux S, Gontier-Jallet C, Samain D, Betbeder D, A new family of carriers (biovectors) enhances the immunogenicity of rabies antigens., Vaccine 14 (1996) 1353–60. [DOI] [PubMed] [Google Scholar]
- [253].Rajananthanan P MW, Attard GS, Sheikh NA, Evaluation of novel aggregate structures as adjuvants: composition, toxicity studies and humoral responses., Vaccine 17 (1999) 715–30. [DOI] [PubMed] [Google Scholar]
- [254].Dobrovolskaia MA, McNeil SE, Immunological properties of engineered nanomaterials, Handb. Immunol. Prop. Eng. Nanomater Volume 1 (2012) 1–23. doi: 10.1142/9789814390262_0001. [DOI] [Google Scholar]
- [255].Xiang SD PM, Scholzen A, Minigo G, David C, Apostolopoulos V, Mottram PL, Pathogen recognition and development of particulate vaccines: does size matter?, Methods 40 (2006) 1–9. [DOI] [PubMed] [Google Scholar]
- [256].Kishimoto TK, Maldonado RA, Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance, Front. Immunol 9 (2018). doi: 10.3389/fimmu.2018.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Hunter Z MS, McCarthy DP, Yap WT, Harp CT, Getts DR, Shea LD, A biodegradable nanoparticle platform for the induction of antigen-specific immune tolerance for treatment of autoimmune disease, ACS Nano 8 (2014) 2148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Bryant J LX, Hlavaty KA, Zhang X, Yap WT, Zhang L, Shea LD, Nanoparticle delivery of donor antigens for transplant tolerance in allogeneic islet transplantation, Biomaterials 35 (2014) 8887–8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Chen B-X, Wilson SR, Das M, Coughlin DJ, Erlanger BF, Antigenicity of fullerenes: Antibodies specific for fullerenes and their characteristics, Proc. Natl. Acad. Sci. USA 95 (1998) 10809–10813. doi: 10.1073/pnas.95.18.10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Lee SC, Parthasarathy R, Botwin K, Kunneman D, Rowold E, Lange G, Klover J, Abegg A, Zobel J, Beck T, Miller T, Hood W, Monahan J, McKearn JP, Jansson R, Voliva CF, Biochemical and immunological properties of cytokines conjugated to dendritic polymers, Biomed. Microdevices 6 (2004) 191–202. doi: 10.1023/B:BMMD.0000042048.18186.ff. [DOI] [PubMed] [Google Scholar]
- [261].Aragnol D, Leserman LD, Immune clearance of liposomes inhibited by an anti-Fc receptor antibody in vivo., Proc. Natl. Acad. Sci. U. S. A 83 (1986) 2699–703. doi: 10.1073/pnas.83.8.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Phillips NC DJ, Immunogenicity of immunoliposomes: reactivity against species-specific IgG and liposomal phospholipids., Immunol Lett 45 (1995) 149–52. [DOI] [PubMed] [Google Scholar]
- [263].Alconcel SNS, Baas AS, Maynard HD, FDA-approved poly(ethylene glycol)–protein conjugate drugs, Polym. Chem 2 (2011) 1442. doi: 10.1039/c1py00034a. [DOI] [Google Scholar]
- [264].Baier G, Baumann D, Siebert JM, Musyanovych A, Mailänder V, Landfester K, Suppressing unspecific cell uptake for targeted delivery using hydroxyethyl starch nanocapsules, Biomacromolecules 13 (2012) 2704–2715. doi: 10.1021/bm300653v. [DOI] [PubMed] [Google Scholar]
- [265].Wörz A, Berchtold B, Moosmann K, Prucker O, Rühe J, Protein-resistant polymer surfaces, J. Mater. Chem 22 (2012) 19547. doi: 10.1039/c2jm30820g. [DOI] [Google Scholar]
- [266].Shubhra QTH, Tóth J, Gyenis J, Feczkó T, Surface modification of HSA containing magnetic PLGA nanoparticles by poloxamer to decrease plasma protein adsorption, Colloids Surfaces B Biointerfaces 122 (2014) 529–536. doi: 10.1016/j.colsurfb.2014.07.025. [DOI] [PubMed] [Google Scholar]
- [267].Prencipe G, Tabakman SM, Welsher K, Liu Z, Goodwin AP, Zhang L, Henry J, Dai H, PEG branched polymer for functionalization of nanomaterials with ultralong blood circulation, J. Am. Chem. Soc 131 (2009) 4783–4787. doi: 10.1021/ja809086q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Larson TA, Joshi PP, Sokolov K, Preventing Protein Adsorption and Macrophage Uptake of Gold Nanoparticles via a Hydrophobic Shield, ACS Nano 6 (2012) 9182–9190. doi: 10.1021/nn3035155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [269].Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW, Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake, J. Am. Chem. Soc 134 (2012) 2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- [270].Ishida T, Wang X, Shimizu T, Nawata K, Kiwada H, PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner, J. Control. Release 122 (2007) 349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- [271].Verhoef JJF, Carpenter JF, Anchordoquy TJ, Schellekens H, Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics, Drug Discov. Today 19 (2014) 1945–1952. doi: 10.1016/j.drudis.2014.08.015. [DOI] [PubMed] [Google Scholar]
- [272].Shimizu T, Ichihara M, Yoshioka Y, Ishida T, Nakagawa S, Kiwada H, Intravenous Administration of Polyethylene Glycol-Coated (PEGylated) Proteins and PEGylated Adenovirus Elicits an Anti-PEG Immunoglobulin M Response, Biol. Pharm. Bull 35 (2012) 1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- [273].Verhoef JJF, Anchordoquy TJ, Questioning the use of PEGylation for drug delivery, Drug Deliv. Transl. Res 3 (2013) 499–503. doi: 10.1007/s13346-013-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Armstrong JK, Leger R, Wenby RB, Meiselman HJ, Garratty G, Fisher TC, Occurrence of an antibody to poly(ethylene glycol) in normal donors., Blood 102 (2003) 556A. [Google Scholar]
- [275].Hamad I, Hunter AC, Szebeni J, Moghimi SM, Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process, Mol. Immunol 46 (2008) 225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- [276].Pelegri-Oday EM, Lin EW, Maynard HD, Therapeutic protein-polymer conjugates: Advancing beyond pegylation, J. Am. Chem. Soc 136 (2014) 14323–14332. doi: 10.1021/ja504390x. [DOI] [PubMed] [Google Scholar]
- [277].Schöttler S, Becker G, Winzen S, Steinbach T, Mohr K, Landfester K, Mailänder V, Wurm FR, Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers, Nat. Nanotechnol 11 (2016) 372 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- [278].Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E, Biomimetic functionalization with leukocyte membranes imparts cell like functions to synthetic particles, Nat. Nanotechnol 8 (2013) 61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Palomba R, Parodi A, Evangelopoulos M, Acciardo S, Corbo C, De Rosa E, Yazdi IK, Scaria S, Molinaro R, Furman NET, You J, Ferrari M, Salvatore F, Tasciotti E, Biomimetic carriers mimicking leukocyte plasma membrane to increase tumor vasculature permeability, Sci. Rep 6 (2016). doi: 10.1038/srep34422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Hu CMJ, Fang RH, Wang KC, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L, Nanoparticle biointerfacing by platelet membrane cloaking, Nature 526 (2015) 118–121. doi: 10.1038/nature15373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Ishida T, Ichihara M, Wang XY, Yamamoto K, Kimura J, Majima E, Kiwada H, Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes, J. Control. Release 112 (2006) 15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [282].Toporkiewicz M, Meissner J, Matusewicz L, Czogalla A, Sikorski AF, Toward a magic or imaginary bullet? Ligands for drug targeting to cancer cells: Principles, hopes, and challenges, Int. J. Nanomedicine 10 (2015) 1399–1414. doi: 10.2147/IJN.S74514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Iezzi ME, Policastro L, Werbajh S, Podhajcer O, Canziani GA, Single-Domain Antibodies and the Promise of Modular Targeting in Cancer Imaging and Treatment, Front. Immunol 9 (2018) 1–11. doi: 10.3389/fimmu.2018.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Hudson PJ, Souriau C, Engineered antibodies, Nat. Med 9 (2003) 129–134. doi: 10.1038/nm0103-129. [DOI] [PubMed] [Google Scholar]
- [285].Wang Y, Fan Z, Shao L, Kong X, Hou X, Tian D, Sun Y, Xiao Y, Yu L, Nanobody-derived nanobiotechnology tool kits for diverse biomedical and biotechnology applications, Int. J. Nanomedicine 11 (2016) 3287–3303. doi: 10.2147/IJN.S107194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Richards DA, Maruani A, Chudasama V, Antibody fragments as nanoparticle targeting ligands: a step in the right direction, Chem. Sci 8 (2017) 63–77. doi: 10.1039/C6SC02403C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Holliger P, Hudson PJ, Engineered antibody fragments and the rise of single domains, Nat. Biotechnol 23 (2005) 1126–1136. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- [288].Stumpp MT, Binz HK, Amstutz P, DARPins: A new generation of protein therapeutics, Drug Discov. Today 13 (2008) 695–701. doi: 10.1016/j.drudis.2008.04.013. [DOI] [PubMed] [Google Scholar]
- [289].Vazquez-Lombardi R, Phan TG, Zimmermann C, Lowe D, Jermutus L, Christ D, Challenges and opportunities for non-antibody scaffold drugs, Drug Discov. Today 20 (2015) 1271–1283. doi: 10.1016/j.drudis.2015.09.004. [DOI] [PubMed] [Google Scholar]
- [290].Löfblom J, Feldwisch J, Tolmachev V, Carlsson J, Ståhl S, Frejd FY, Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications, FEBS Lett 584 (2010) 2670–2680. doi: 10.1016/j.febslet.2010.04.014. [DOI] [PubMed] [Google Scholar]
- [291].Wu X, Chen J, Wu M, Zhao JX, Aptamers: Active targeting ligands for cancer diagnosis and therapy, Theranostics 5 (2015) 322–344. doi: 10.7150/thno.10257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Pietersz GA, Wang X, Yap ML, Lim B, Peter K, Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies, Nanomedicine 12 (2017) 1873–1889. doi: 10.2217/nnm-2017-0043. [DOI] [PubMed] [Google Scholar]
- [293].Yang E, Qian W, Cao Z, Wang L, Bozeman EN, Ward C, Yang B, Selvaraj P, Lipowska M, Wang YA, Mao H, Yang L, Theranostic nanoparticles carrying doxorubicin attenuate targeting ligand specific antibody responses following systemic delivery, Theranostics 5 (2015) 43–61. doi: 10.7150/thno.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Rodriguez PL, Harada T, Christian DA, Pantano DA, Richard K, Discher DE, Minimal “Self” Peptides That Inhibit Phagocytic Clearance and Enhance Delivery of Nanoparticles, Science (80-. ). 339 (2013) 971–975. doi: 10.1126/science.1229568.Minimal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Qie Y, Yuan H, Von Roemeling CA, Chen Y, Liu X, Shih KD, Knight JA, Tun HW, Wharen RE, Jiang W, Kim BYS, Surface modification of nanoparticles enables selective evasion of phagocytic clearance by distinct macrophage phenotypes, Sci. Rep 6 (2016) 1–10. doi: 10.1038/srep26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Sosale NG, Spinler KR, Alvey C, Discher DE, Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific “Marker of Self” CD47, and target physical properties, Curr. Opin. Immunol 35 (2015) 107–112. doi: 10.1016/j.coi.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].van G.E. van der Meel R, Vehmeijer LJ, Kok RJ, Storm G, Ligand-targeted particulate nanomedicines undergoing clinical evaluation: current status., Adv Drug Deliv Rev 65 (2013) 1284–98. [DOI] [PubMed] [Google Scholar]
- [298].Anselmo AC, Mitragotri S, Nanoparticles in the clinic, Bioeng. Transl. Med 1 (2016) 10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Lorentz KM, Kontos S, Diaceri G, Henry H, Hubbell JA, Engineered binding to erythrocytes induces immunological tolerance to E. coli asparaginase, Sci. Adv 1 (2015) e1500112. doi: 10.1126/sciadv.1500112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Macauley MS, Pfrengle F, Rademacher C, Nycholat CM, Gale AJ, Von Drygalski A, Paulson JC, Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis, J. Clin. Invest 123 (2013) 3074–3083. doi: 10.1172/JCI69187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Developing a Range of SVP-Enabled Candidates for Rare and Serious Diseases, (n.d.). http://selectabio.com/pipeline/developing-range-svp-enabled-candidates-rare-serious-diseases/ (accessed April 10, 2018).
- [302].Hajipour MJ, Laurent S, Aghaie A, Rezaee F, Mahmoudi M, Personalized protein coronas: a “key” factor at the nanobiointerface, Biomater. Sci 2 (2014) 1210. doi: 10.1039/C4BM00131A. [DOI] [PubMed] [Google Scholar]
- [303].Mahmoudi M, Abdelmonem AM, Behzadi S, Clement JH, Dutz S, Ejtehadi MR, Hartmann R, Kantner K, Linne U, Maffre P, Metzler S, Moghadam MK, Pfeiffer C, Rezaei M, Ruiz-Lozano P, Serpooshan V, Shokrgozar MA, Nienhaus GU, Parak WJ, Temperature: The “ignored” Factor at the NanoBio interface, ACS Nano 7 (2013) 6555–6562. doi: 10.1021/nn305337c. [DOI] [PubMed] [Google Scholar]
- [304].Gustafsson Å, Bergström U, Ågren L, Österlund L, Sandström T, Bucht A, Differential cellular responses in healthy mice and in mice with established airway inflammation when exposed to hematite nanoparticles, Toxicol. Appl. Pharmacol 288 (2015) 1–11. doi: 10.1016/j.taap.2015.07.001. [DOI] [PubMed] [Google Scholar]
- [305].Rossi EM, Pylkkänen L, Koivisto AJ, Nykäsenoja H, Wolff H, Savolainen K, Alenius H, Inhalation exposure to nanosized and fine TiO2particles inhibits features of allergic asthma in a murine model, Part. Fibre Toxicol 7 (2010). doi: 10.1186/1743-8977-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Ryu JK, McLarnon JG, A leaky blood-brain barrier, fibrinogen infiltration and microglial reactivity in inflamed Alzheimer’s disease brain, J. Cell. Mol. Med 13 (2009) 2911–2925. doi: 10.1111/j.1582-4934.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Strowig T, Henao-Mejia J, Elinav E, Flavell R, Inflammasomes in health and disease, Nature 481 (2012) 278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- [308].Abderrazak A, Syrovets T, Couchie D, El Hadri K, Friguet B, Simmet T, Rouis M, NLRP3 inflammasome: From a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases, Redox Biol 4 (2015) 296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Ozaki E, Campbell M, Doyle SL, Targeting the NLRP3 inflammasome in chronic inflammatory diseases: Current perspectives, J. Inflamm. Res 8 (2015) 15–27. doi: 10.2147/JIR.S51250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Yazdi AS, Guarda G, Riteau N, Drexler SK, Tardivel A, Couillin I, Tschopp J, Nanoparticles activate the NLR pyrin domain containing 3 (Nlrp3) inflammasome and cause pulmonary inflammation through release of IL-1α and IL-1β, Proc. Natl. Acad. Sci 107 (2010) 19449 LP-19454. http://www.pnas.org/content/107/45/19449.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [311].Baron L, Gombault A, Fanny M, Villeret B, Savigny F, Guillou N, Panek C, Le Bert M, Lagente V, Rassendren F, Riteau N, Couillin I, The NLRP3 inflammasome is activated by nanoparticles through ATP, ADP and adenosine, Cell Death &Amp; Dis 6 (2015) e1629 10.1038/cddis.2014.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Sun B, Wang X, Ji Z, Wang M, Liao YP, Chang CH, Li R, Zhang H, Nel AE, Xia T, NADPH Oxidase-Dependent NLRP3 Inflammasome Activation and its Important Role in Lung Fibrosis by Multiwalled Carbon Nanotubes, Small 11 (2015) 2087–2097. doi: 10.1002/smll.201402859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Carney EW, Scialli AR, Watson RE, DeSesso JM, Mechanisms regulating toxicant disposition to the embryo during early pregnancy: An interspecies comparison, Birth Defects Res. Part C - Embryo Today Rev 72 (2004) 345–360. doi: 10.1002/bdrc.20027. [DOI] [PubMed] [Google Scholar]
- [314].Chu M, Wu Q, Yang H, Yuan R, Hou S, Yang Y, Zou Y, Xu S, Xu K, Ji A, Sheng L, Transfer of quantum dots from pregnant mice to pups across the placental barrier, Small 6 (2010) 670–678. doi: 10.1002/smll.200902049. [DOI] [PubMed] [Google Scholar]
- [315].Grafmueller S, Manser P, Diener L, Diener PA, Maeder-Althaus X, Maurizi L, Jochum W, Krug HF, Buerki-Thurnherr T, von Mandach U, Wick P, Bidirectional transfer study of polystyrene nanoparticles across the placental barrier in an ex vivo human placental perfusion model, Environ. Health Perspect 123 (2015) 1280–1286. doi: 10.1289/ehp.1409271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Wick P, Malek A, Manser P, Meili D, Maeder-Althaus X, Diener L, Diener PA, Zisch A, Krug HF, Von Mandach U, Barrier capacity of human placenta for nanosized materials, Environ. Health Perspect 118 (2010) 432–436. doi: 10.1289/ehp.0901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Yamashita K, Yoshioka Y, Higashisaka K, Mimura K, Morishita Y, Nozaki M, Yoshida T, Ogura T, Nabeshi H, Nagano K, Abe Y, Kamada H, Monobe Y, Imazawa T, Aoshima H, Shishido K, Kawai Y, Mayumi T, Tsunoda SI, Itoh N, Yoshikawa T, Yanagihara I, Saito S, Tsutsumi Y, Silica and titanium dioxide nanoparticles cause pregnancy complications in mice, Nat. Nanotechnol 6 (2011) 321–328. doi: 10.1038/nnano.2011.41. [DOI] [PubMed] [Google Scholar]
- [318].Wolfram J, Nizzero S, Liu H, Li F, Zhang G, Li Z, Shen H, Blanco E, Ferrari M, A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery, Sci. Rep 7 (2017). doi: 10.1038/s41598-017-14221-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [319].Liu T, Choi H, Zhou R, Chen IW, RES blockade: A strategy for boosting efficiency of nanoparticle drug, Nano Today 10 (2015) 11–21. doi: 10.1016/j.nantod.2014.12.003. [DOI] [Google Scholar]
- [320].Schweingruber N, Haine A, Tiede K, Karabinskaya A, van den Brandt J, Wüst S, Metselaar JM, Gold R, Tuckermann JP, Reichardt HM, Lühder F, Liposomal encapsulation of glucocorticoids alters their mode of action in the treatment of experimental autoimmune encephalomyelitis., J. Immunol 187 (2011) 4310–8. doi: 10.4049/jimmunol.1101604. [DOI] [PubMed] [Google Scholar]
- [321].Bartneck M, Peters FM, Warzecha KT, Bienert M, van Bloois L, Trautwein C, Lammers T, Tacke F, Liposomal encapsulation of dexamethasone modulates cytotoxicity, inflammatory cytokine response, and migratory properties of primary human macrophages, Nanomedicine Nanotechnology, Biol. Med 10 (2014) 1209–1220. doi: 10.1016/j.nano.2014.02.011. [DOI] [PubMed] [Google Scholar]
- [322].Pozzi D, Caracciolo G, Capriotti AL, Cavaliere C, La Barbera G, Anchordoquy TJ, Laganà A, Surface chemistry and serum type both determine the nanoparticle-protein corona, J. Proteomics 119 (2015) 209–217. doi: 10.1016/j.jprot.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Caracciolo G, Pozzi D, Capriotti AL, Cavaliere C, Piovesana S, La Barbera G, Amici A, Laganà A, The liposome–protein corona in mice and humans and its implications for in vivo delivery, J. Mater. Chem. B 2 (2014) 7419–7428. doi: 10.1039/C4TB01316F. [DOI] [PubMed] [Google Scholar]
- [324].Chitko-McKown CG, Blecha F, Pulmonary intravascular macrophages: a review of immune properties and functions., Ann. Rech. Vet 23 (1992) 201–214. [PubMed] [Google Scholar]
- [325].Warner AE, Pulmonary intravascular macrophages: Role in acute lung injury, Clin. Chest Med 17 (1996) 125–135. doi: 10.1016/S0272-5231(05)70303-8. [DOI] [PubMed] [Google Scholar]
- [326].Miyamoto K, Schultz E, Heath T, Mitchell MD, Albertine KH, Staub NC, Pulmonary intravascular macrophages and hemodynamic effects of liposomes in sheep, J. Appl. Physiol. (Bethesda, Md. 1985) 64 (1988) 1143–1152. [DOI] [PubMed] [Google Scholar]


