Abstract
Dietary restriction (DR) remains the most reproducible and consistent laboratory intervention to extend lifespan and improve health in mammals. DR has been primarily characterized in males due to issues of cost, perceived heightened variability amongst females, and the misconception that the reproductive system is the only important difference between sexes in mammals. In reality, existing data point to clear sex differences in mammalian responses to DR. Here we discuss recent advances in our understanding of sex differences in the responses to DR in rodent models.
Introduction
Dietary restriction (DR) is an all-encompassing term describing interventions that improve health by restricting some aspect of nutrition. Such interventions include: calorie restriction (CR), involving reduced food intake without malnutrition (usually by 20–40% relative to ad libitum fed animals); intermittent fasting (IF), involving enforced periods of fasting such as every other day (EOD) feeding, periodic fasting (PF) or time restricted feeding (TRF); and dietary dilution of specific macronutrients such as protein or essential amino acids such as methionine without enforced food restriction. Classically, the success of a given DR intervention has been based upon its ability to increase lifespan. However, DR has a range of health benefits in preclinical models that are of equal or perhaps greater importance, including extension of healthspan, or time spent in good health in the last stages of life [1].
CR was the first intervention shown to increase lifespan in the early 20th century and remains the most consistent non-genetic intervention to delay aging in a variety of species today [2]. CR increases maximum and/or median lifespan in fruit flies, nematode worms, rodents, fish and non-human primates, and extends healthspan by delaying onset or reducing severity of a range of age-related diseases [2,3]. Because mammals on CR eat their allotments quickly and spend an extended period of time between meals in the fasted state, CR can also be considered a form of IF. Other IF paradigms including EOD fasting and TRF result in similar longevity and/or health benefits even if overall calorie intake is not reduced [1,4]. In humans, DR studies tend to focus on improvements in health outcomes rather than lifespan extension due to the obvious limitations in assessing this metric. As with preclinical models, studies of DR in humans either find no sex differences, do not include sex differences as an experimental outcome [5,6], or include only one sex seeking an answer to a specific experimental question [7]. A recent study in non-obese humans suggests that a two-year 25% CR regimen is feasible in non-obese humans; the only sex differences observed were a slightly lower BMI in the cohort that was predominantly women (69.7%) [6]. Notably, there are sex specific differences in brown and beige markers in adipose tissue of women, which are maintained after 8 weeks of CR [8]. It is encouraging to witness the inclusion of females in these very important clinical trials, but also to observe investigators reporting stratification of results by sex. This will invariably lead to discoveries on how sex affects the pathways which modulate the benefits of CR.
The molecular basis of the benefits of CR is thought to lie in the activation of nutrient sensing and stress response pathways resulting in increased metabolic efficiency, reduced inflammation, increased repair, and ultimately extended longevity (Figure 1) as reviewed in depth elsewhere [1,3]. Interestingly, pathways implicated in longevity have been shown to exhibit sexual dimorphism such as the nutrient-responsive mTOR [9], the Sirtuins (particularly SIRT-6) [10], IGF-1 [11], and growth hormone [12,13].
Figure 1.
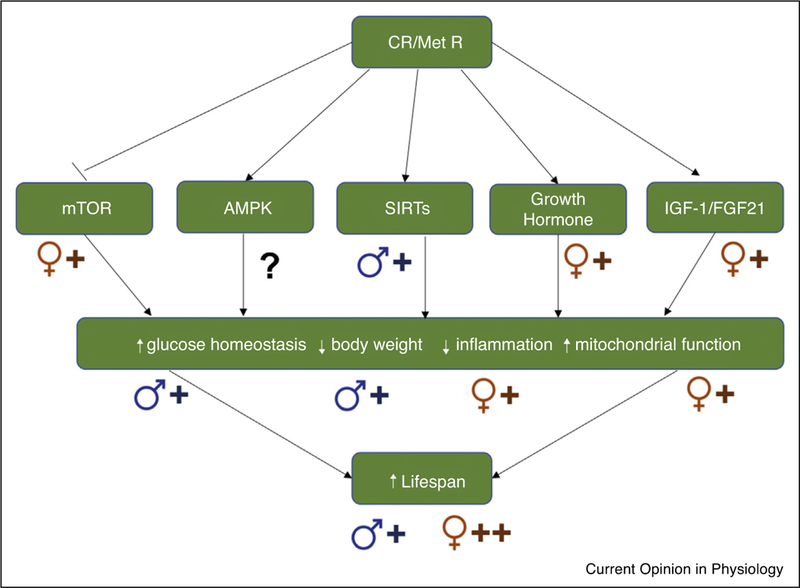
Possible sex effects on aspects of the CR/Met R mechanistic pathways. CR and MetR exert their beneficial health and lifespan effects, at least in part, by modulating the nutrient sensing and aging pathways of mammalian target of rapamycin (mTOR), AMP-activated protein kinase (AMPK), sirtuins (SIRTs) and insulin-like growth factor 1 (IGF-1) or fibroblast growth factor 21 (FGF21). Activation (AMPK, SIRT, IGF-1) or inhibition (mTOR) of these pathways results in potential health benefits including increased glucose homeostasis, decreased body weight, decreased inflammation and increased mitochondrial function. There are possible differential modulations of these aging pathways and their associated health responses in males and females. Studies suggest that overall females may have a greater response to CR, with females having an increased response to mTOR inhibition or IGF-1/FGF21 activation, and greater inflammatory and mitochondrial health responses. Studies suggest that males may have an increased response to SIRT activation, and a greater decrease in bodyweight and increase in glucose homeostasis in response to CR. More studies are needed to clarify these potential sex differences. The male (♂) or female (♀) symbols indicate if there is a reported sexual dimorphism in response to the pathway or outcome, with the plus symbols (+) indicating the degree of the effect. The combination of the male (♂) or female (♀) symbol with the plus (+) symbols indicates the degree of the sex effect on the pathway or outcome, with ++ indicating a larger effect than +.
Dilution of dietary macronutrient content without enforced food restriction can also have beneficial health and longevity effects. For example, restriction of sulfur amino acids methionine and cysteine, also known as methionine restriction (MetR) which was first shown to extend lifespan in rats, also has health effects overlapping those of CR in multiple organisms [14]. Large-scale studies using the Geometric Framework to investigate optimal ratios of dietary macronutrients, reveal that low protein, high carbohydrate diets have the greatest effect on increasing lifespan [2] and that there are optimal macronutrient compositions to regulate fertility [15]
As with most biomedical preclinical research, females have been historically under-represented in DR research. Thus, while it is established that different rodent strains show sexual dimorphism with respect to DR-mediated longevity [16,17], sex differences in DR-mediated healthspan and other responses, as well as underlying mechanisms of such differences, are poorly understood. Recently, a number of studies have begun to shed light on such differences. This review will limit its scope to CR and MetR in the last two years (2015–2017), as these are two of the most thoroughly studied examples of DR in the literature. These studies are summarized in Table 1 and discussed below.
Table 1.
Recent preclinical studies of sex differences in dietary restriction responses (2015–2017) in rodents.
| Type of dietary restriction | Preclinical model | Sexes included | Effect of sex | Reference |
|---|---|---|---|---|
| 30% CR (2 months) | C57BL/6 mice | M and F | The effect of CR on expression of some circadian clock genes, and IGF-1 signaling, was sex dependent | [25••] |
| 30% CR (lifelong) | C57BL/6 mice | M and F | CR resulted in an increase in lifespans in both males and females | [22•] |
| 20 and 40% CR (lifelong) | C57BL/6 and DBA/2J mice | M and F | 20% CR increased lifespan in both strains and sexes. 40% CR increased lifespan in C57BL/6 and DBA/2J males, but C57BL/6 females had no increased lifespan and DBA/2J females had no increase above that seen with 20% CR. | [21••] |
| 30% CR (lifelong) | Ercc1 Δ/− mice on C57BL/6 background | M and F | CR increased median lifespan 2-fold in females and 2.5-fold in males | [23] |
| 40% CR (lifelong) | Sprague Dawley Rats | M and F | Vitamin K concentrations decreased in both sexes with CR. PT was decreased in male CR rats, but not females | [26] |
| 40% CR (lifelong) | C57BL/6 and DBA/2J mice | M and F | CR reduced frailty index scores in C57BL/6 males, but not females. No significant effect of CR on frailty in DBA/2J mice | [27] |
| 3mo of a MetR diet in young (2mo) and old (9mo) mice | C57BL/6 mice | M and F | Males gain more weight compared to females that is attenuated with MetR; bone morphology is altered in an age-specific and sex-specific manner potentially under hormonal regulation. No impairment of bone biomechanical properties | [37•] |
| 10mo old mice fed MetR for 8 weeks | C57BL/6J mice | M and F | Sex independent improvement of glucose tolerance, and lower fasting blood glucose levels with MetR diet. Sex specific regulation of metabolic pathways with MetR: upregulation of FGF21 mRNA in MetR-fed females, while SIRT1 mRNA was upregulated in males | [35] |
| 14 week old mice fed a methionine deficient diet for 1 week | C57BL/6J mice | M and F | Sex independent energy expenditure in MetR mice; but a sex difference in the FGF21-UPC-1 axis, which is upregulated in males, but not females | [38] |
| TRF of a high fat diet | C57BL/6J mice | F only | Improvements in metabolic health in model of postmenopausal obesity | [43] |
| 4–5 week old mice on TRF for up to 26 days | C57BL/6NCrl mice | M only | TRF shifts the phase and alters the amplitude of the skin circadian clock, also altering ~10% of the skin transcription. They show that daytime-restricted feeding affects the sensitivity to DNA damage in the skin of mice, and dampens the expression of a key DNA repair factor, Xpa. The authors should consider replicating this study using both sexes and adult mice (16 weeks age) | [42] |
| 8 week old mice subjected to TRF at night | ICR mice | M and F | No difference in caloric intake, body weight, and locomotor activity rhythm in these young mice. The authors report that immobility in the forced swim test was higher in TRF male mice, but do not show convincing evidence to support this claim | [44] |
| 4 week old mice on IF (every other day feeding) for 9mo | C57BL/6J mice | M and F | Sex specific effects of IF on bodyweight (% bodyweight gain in males is more than females); decreased area and lower levels of heterogeneity in hepatocytes of IF animals that was sex independent; significant decreases in the expression of mRNA for caspase 3 and Bax in females but not males; this suggests IF promotes cellular maintenance in the liver which is more pronounced in females | [41] |
| EOD initiated in 5mo mice for 5 months | APP/PS1 double-transgenic mice [B6C3-Tg(APPswe, PS1dE) 85Dbo/J] and WT littermates | M and F | EOD feeding was protective in mice by protecting against Aβ deposition in the brains of AD mice and ameliorating some of the learning deficits. Sex was not considered as an independent variable by the authors when they performed their analysis of effects | [48] |
| 8–12 weeks old mice on IF for up to 40 days | C57BL/6J mice | F only | IF is protective when initiated in the early phases of autoimmune encephalitis but no mention of a sex specific effect | [47] |
CR, calorie restriction; MetR, methionine restriction; IGF-1, insulin-like growth factor 1; PT, prothrombin time; FGF21, fibroblast growth factor 21; M, male; F, female; AD, Alzheimer’s disease; WT, wild type; EOD, every other day; IF, intermittent fasting.
Sex differences in response to CR in preclinical models
Based on large-scale rodent CR studies in both sexes using multiple hybrid, inbred and recombinant inbred strains showing considerable sex and strain variation in CR-mediated longevity [16,17], it has been proposed that in mice, females respond better to CR [18–20] whilst in rats the opposite is true [20]. However, there are many reported exceptions, making these generalizations unhelpful. Rather, sexual dimorphism in CR-mediated longevity appears to be far more complex, with potential interaction between additional factors including strain, dose, dietary composition and timing [21••].
Two recent mouse CR studies in strains with engineered defects in circadian clock (Bmal1-KO) or DNA repair (Ercc1 hypomorph) components included males and females. CR-mediated lifespan extension was observed in controls of both studies as well as in mice lacking DNA repair function, but not those lacking circadian rhythmicity, with similar effects in both sexes [22•,23]. In rhesus monkeys, one recent study found lifespan extension upon CR in both sexes, while another found healthspan extension without lifespan extension upon CR in both sexes, but a reduction in bodyweight and increase in insulin sensitivity upon CR only in males [24]. This implies a sex differences in mechanisms underlying health effects of CR in non-human primates.
Mitchell and colleagues recently reported sexual dimorphism in health and longevity benefits of 20% versus 40% CR in a large-scale study in two strains of mice [21••]. In the C57BL/6 mice, 20% CR increased lifespan in both males and females, whereas 40% CR had no effect on lifespan in females and increased lifespan in males but to a lesser extent than 20% CR. In DBA/2J mice, CR increased lifespan in both sexes, but in a dose-dependent manner only in males, with females already maximal at 20% restriction. In terms of health-related outcomes, 20% CR reduced body temperatures in C57BL/6 males but elevated temperature in females, while males had a greater reduction in major urinary proteins (a marker of reproductive capacity) than females. Interestingly they also report an uncoupling of health and lifespan benefits. In C57BL/6J females, fasting glucose and insulin show the same fold reduction with 20 or 40% CR, however only 20% CR extends lifespan while 40% CR in females is detrimental [21••]. Clearly there is a complex interplay between sex and amount of CR. The authors also observed sex-specific differences in protein ubiquitination, glucose homeostasis and IGFBP-1 levels [21••].
Sexual dimorphism is also apparent in studies of healthrelated outcomes of CR. One study exploring sex-dependent gene expression upon CR (30% for 2 months) in young C57BL/6 mice found differential signaling of circadian clock genes and IGF-1 in females compared to males [25••]. Another study in rats exploring the effect of CR on blood clotting in the context of cardiovascular disease found similar reductions in vitamin K concentrations upon CR in both sexes, but a reduced prothrombin time in males but not females, implying a more robust response to CR in males than females [26]. Finally, a study exploring the effects of lifelong CR on frailty in mice found that frailty was significantly reduced with CR in male C57BL/6 mice, but not females [27].
The mechanisms underlying sexual dimorphism in the response to lifespan and healthspan benefits of CR are not well understood. Proposed mechanisms include better mitochondrial control in females leading to increased sensitivity to CR [17]; increased sensitivity of females to manipulation of the nutrient sensing pathways such as IGF-1 [19,20]; differences in sex steroids [28]; and/or differences in adiponectin/leptin levels, immune function or body fat distribution [20] (Figure 1). There have even been suggestions that CR ‘feminizes’ the gene expression profile in males [29], however this study does not actually include female mice so while the results are interesting, clearly more research is needed to understand the mechanisms underlying sexual dimorphism of CR responses.
Sex differences in response to MetR in preclinical models
An inherent difficulty in translating DR to humans is quite simply that most humans would find it incredibly difficult to reduce daily caloric intake by 20–40%. Thus interventions allowing for DR benefits without actual food restriction represent an attractive alternative. More than 20 years ago, Orentreich and colleagues reported that a reduction in the concentration of a single dietary essential amino acid, methionine (from 0.86% to 0.17% w/w), resulted in a 30% longer lifespan of male Fisher-344 rats [30]. While perhaps more aptly referred to as sulfur amino acid restriction (SAAR) due to the fact that the nonessential sulfur amino acid cysteine is absent in MetR diets, the phenomenon has been replicated in different rodent models [31]. Additional benefits of MetR that overlap with CR despite ad libitum access to food include reductions in bodyweight, fat mass and oxidative stress coupled with improvements in insulin sensitivity as well as changes in circulating insulin, glucose, leptin, adiponectin, IGF-1 and FGF-21 [31,32].
While the seminal work showing lifespan extension by MetR (0.1–0.15%) in mice from 6 weeks of age used only females [33] a subsequent study showing lifespan extension by MetR initiated at 12mo of age only tested males for longevity and females for changes in hepatic gene expression [34]. A recent study of 10mo old male and female C57BL/6J wild-type mice demonstrated sex specific differences in kidney gene expression as a function of an 8-week MetR diet (0.172%). Females significantly upregulated FGF-21 and its co-receptor, b-Klotho, which controls energy expenditure, while male mice significantly upregulated SIRT-1 with potential renoprotective effects on lipid and glucose metabolism [35]. These sex differences in response to MetR diet may help explain the sexual dimorphism in the acute-to-chronic kidney disease transition [36].
A recent study of young and old male and female mice noted that bone morphology is altered in an age and gender specific manner. They demonstrated that although MetR mice may show reduced bone mass, when corrected for body size, there is no impairment in bio-mechanical properties [37•]. This points to a hormonal regulation of bone morphology in response to MetR and highlights the importance of considering how sex steroids can alter experimental outcomes. Sexual dimorphism of hormonal responses was also observed in the response to short-term (up to 5 weeks) methionine deficiency in young mice [38]. While Met-deficient diets increase energy expenditure independent of sex, this was clearly linked to an increase in hepatic FGF21 expression and WAT Ucp1 only in males. Furthermore, while activation of hepatic FGF21 expression was intact in males with constitutive mTORC1 activation, in females this alone was sufficient to activate FGF21 expression independent of diet [38]. While the molecular mechanism underlying these sex differences is not known, it is plausible to attribute these differences at least in part to sex hormones. Indeed, estrogen removal in animals or menopause in women is associated with metabolic disturbances including hepatic triglyceride accumulation and decreased insulin sensitivity [39].
Sex differences in response to timing of food intake in preclinical models
In recent years, the idea of focusing on the timing of food intake, rather than total calorie intake or diet composition, has gained significant momentum for its potential clinical applicability. Such IF paradigms, including EOD feeding, PF and TRF, are based on the notion that while few people can abide long-term food restriction or altered composition, many may be able to do so for short periods of time. EOD-type feeding regimens usually involve a water-only or very low calorie period lasting ~24 h, followed by a normal feeding period for 1–2 days. PF lasts 2 or more days and is separated from the next cycle by at least 1 week [4]. The benefits and limitations of these fasting protocols have been reviewed elegantly in two recent publications [4,40]. In contrast, TRF allows free access to food of any caloric content within a predefined window, for example 8 h feeding/16 h fasting per day, but with limited studies comparing sex differences.
Sex differences in response to IF have been noted with respect to weight loss and changes in levels of apoptotic markers in the liver with potential implications for cellular maintenance with age [41]. TRF improves metabolic fitness [4], and protects against diurnal sensitivity to UVB-induced DNA damage [42] in young adult male mice, as well as a model of postmenopausal obesity using ovariectomized female mice [43]. A recent study in nighttime fed mice found no sexual dimorphism in the forced swimming test [44], but a study of male mice subjected to TRF or CR found a compensatory increase in behavior with TRF/CR using an automated feeder system [45]. Clearly this disparity between sexes and types of behavior and how it relates to metabolic outcomes and aging among others requires further study. Gonadal sex hormones have been proposed to mediate the sex differences in food anticipatory activity (FAA) in rodents [46]. Recent studies have observed sex differences in FAA, but in a follow-up there were no differences between intact and gonadectomized mice in the onset or magnitude of FAA [46]. Clearly more research is warranted into this highly complex area.
Two additional recent IF studies that included females further illustrate the need to carefully consider both sexes. In the first study, IF initiated in the early phases of autoimmune encephalitis showed protective effects in female mice [47]. However, because only female mice were used in this study, sex-specific responses to DR [21•] and/or the etiology and progression of diseases [18] prevent generalization of the finding. In the second study, EOD feeding ameliorated some of the detrimental learning effects and protected against Ab deposition in brains of Alzheimer’s disease (AD) model mice [48]. However, because males and females were grouped together and sex was not considered as an independent variable, we learn nothing of the potential effect of sex on DR/AD interaction despite previous reports that female AD mice exhibit significantly greater Aβ burden and larger behavioral deficits than age-matched males (https://www.jax.org/strain/005864) [49]. Thus, both sexes should be studied, and sex treated as an independent variable in DR studies.
Concluding remarks
More than 20 years ago, the National Institutes of Health (NIH) established the Office of Research on Women’s Health with the idea that excluding women from clinical research was bad for women and bad for science [50]. In recent years, the NIH has mandated that any NIH-funded pre-clinical research must include females [50]. A common theme we observed in the rodent DR literature was not the absence of female models per se, but rather the use of females to address specific experimental questions. Despite the recent increase in inclusion of both sexes in preclinical studies, we still have further to go, including reporting of sex as an independent variable. Understanding inherent biological differences between males and females is of the utmost importance due to the sexual dimorphisms in disease and aging processes. Only by understanding these differences can we develop appropriate therapeutics — be they dietary, genetic or pharmacological — for everyone.
Acknowledgements
We apologize to the authors who we were unable to include their work in this review due to journal space limitations. AEK is supported by an NHMRC CJ Martin biomedical fellowship (GNT1122542).
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of interest statement
DS is a consultant and/or inventor on patents licensed to Ovascience, Jumpstart Fertility, Liberty Biosecurity, EdenRoc Sciences, and Metro International Biotech. The remaining authors declare no conflicts. No funding sources were involved in the preparation of this article.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lee C, Longo V: Dietary restriction with and without caloric restriction for healthy aging. F1000Res 2016, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Couteur DG et al. : The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci 2016, 73:1237–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez-Lluch G, Navas P: Calorie restriction as an intervention in ageing. J Physiol 2016, 594:2043–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longo VD, Panda S: Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab 2016, 23:1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fontana L et al. : Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell 2016, 15:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravussin E et al. : A 2-year randomized controlled trial of human caloric restriction: feasibility and effects on predictors of health span and longevity. J Gerontol: Ser A 2015, 70:1097–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Utami FA et al. : Effects of calorie restriction plus fish oil supplementation on abnormal metabolic characteristics and the iron status of middle-aged obese women. Food Funct 2018, 9:1152–1162. [DOI] [PubMed] [Google Scholar]
- 8.Barquissau V et al. : Caloric restriction and diet-induced weight loss do not induce browning of human subcutaneous white adipose tissue in women and men with obesity. Cell Rep 2018, 22:1079–1089. [DOI] [PubMed] [Google Scholar]
- 9.Baar EL et al. : Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 2016, 15:155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharples AP et al. : Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 2015, 14:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashpole NM et al. : IGF-1 has sexually dimorphic, pleiotropic, and time-dependent effects on healthspan, pathology, and lifespan. Geroscience 2017, 39:129–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Junnila RK et al. : Disruption of the GH receptor gene in adult mice increases maximal lifespan in females. Endocrinology 2016, 157:4502–4513. [DOI] [PubMed] [Google Scholar]
- 13.Sun LY et al. : Longevity is impacted by growth hormone action during early postnatal period. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hine C, Mitchell JR: Calorie restriction and methionine restriction in control of endogenous hydrogen sulfide production by the transsulfuration pathway. Exp Gerontol 2015, 68:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solon-Biet SM et al. : Macronutrient balance, reproductive function, and lifespan in aging mice. Proc Natl Acad Sci U S A 2015, 112:3481–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turturro A et al. : Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 1999, 54:B492–B501. [DOI] [PubMed] [Google Scholar]
- 17.Liao CY et al. : Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 2010, 9:92–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tower J: Sex-specific gene expression and life span regulation. Trends Endocrinol Metab 2017, 28:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garratt M, Nakagawa S, Simons MJP: Life-span extension with reduced somatotrophic signaling: moderation of aging effect by signal type, sex, and experimental cohort. J Gerontol A Biol Sci Med Sci 2017, 72:1620–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austad SN, Bartke A: Sex differences in longevity and in responses to anti-aging interventions: a mini-review. Gerontology 2015, 62:40–46. [DOI] [PubMed] [Google Scholar]
- 21. ••.Mitchell SJ et al. : Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 2016, 23:1093–1112.In this study Mitchell and colleagues perform a large-scale lifespan study using three diets (ad libitum, 20% CR and 40% CR lifelong), two strains of mice (C57BL/6J and DBA/2J) and both sexes to determine the sex and strain specific responses to CR. This is one of the first studies of its kind that systematically investigate these variables within the same study. They highlight the importance of considering sex when performing life-span intervention studies, and show an uncoupling of healthspan and lifespan benefits. They link metabolomic, gene expression and physiological data to describe how sex/genetic background and amount of CR modulate pathways proposed to play a role in lifespan but do not delve further into underlying mechanisms.
- 22. •.Patel SA et al. : Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J 2016, 30:1634–1642.Here the authors investigate the role of BMAL1 in the lifespan increasing effect of calorie restriction. They show that BMAL1 knockout mice do not resond to CR in terms of extending lifespan, and that the sexual dimorphism present in wildtype mice in response to CR is not present in BMAL1 knockout mice. The authors conclude that BMAL1 is an important mediator of CR, and activation of BMAL1 might link CR mechanisms with biologic clocks. Interestingly, the authors show that global knockout of BMAL1 results in a shortened lifespan of mice that is sex independent and that addition of CR to BMAL1 knockout mice is detrimental to mean lifespan. However, the authors do not distinguish between peripheral and central circadian clocks and investigate clock targets in the liver only.
- 23.Vermeij WP et al. : Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 2016, 537:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mattison JA et al. : Caloric restriction improves health and survival of rhesus monkeys. Nat Commun 2017, 8:14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. ••.Astafev AA, Patel SA, Kondratov RV: Calorie restriction effects on circadian rhythms in gene expression are sex dependent. Sci Rep 2017, 7:9716.In this study the authors show that short term (2mo) 30% CR in male and female mice induces a sex-independent modulation of some circadian clock genes in the livers of these mice. There was no sexual dimorphism in the circadian clock genes Bmal1, Per1, Per2 and Per3. However, the expression of several clock genes: Cry1, Cry2, Rev-Erb α and Ror γγ was significantly different between males and females in both ad libitum and 30% CR fed mice. In addition, the effect of CR on the expression of Cry1, Rev-Erb α and Ror γ was sex-dependent. This highlights and important role for considering how sex and genes interact when interpreting or implementing CR regimens in mice. While the authors discuss the central clock in the discussion, all results focus on the peripheral liver clock.
- 26.Ferland G, Doucet I, Mainville D: Phylloquinone and menaquinone-4 tissue distribution at different life stages in male and female sprague-dawley rats fed different VK levels since weaning or subjected to a 40% calorie restriction since adulthood. Nutrients 2016, 8:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kane AE et al. : Impact of longevity interventions on a validated mouse clinical frailty index. J Gerontol A Biol Sci Med Sci 2016, 71:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vasconcelos AR et al. : The role of steroid hormones in the modulation of neuroinflammation by dietary interventions. Front Endocrinol (Lausanne) 2016, 7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu ZD, Klaassen CD: Short-term calorie restriction feminizes the mRNA profiles of drug metabolizing enzymes and transporters in livers of mice. Toxicol Appl Pharmacol 2014, 274:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orentreich N et al. : Low methionine ingestion by rats extends life span. J Nutr 1993, 123:269–274. [DOI] [PubMed] [Google Scholar]
- 31.Dong Z, Sinha R, Richie JP Jr: Disease prevention and delayed aging by dietary sulfur amino acid restriction: translational implications. Ann N Y Acad Sci 2018. [DOI] [PubMed]
- 32.Ables GP, Johnson JE: Pleiotropic responses to methionine restriction. Exp Gerontol 2017, 94:83–88. [DOI] [PubMed] [Google Scholar]
- 33.Miller RA et al. : Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 2005, 4:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun L et al. : Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol Ser A: Biol Sci Med Sci 2009, 64A:711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grant L et al. : Methionine restriction improves renal insulin signalling in aged kidneys. Mech Ageing Dev 2016, 157:35–43. [DOI] [PubMed] [Google Scholar]
- 36.Lima-Posada I et al. : Gender differences in the acute kidney injury to chronic kidney disease transition. Sci Rep 2017, 7:12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. •.Ouattara A et al. : Methionine restriction alters bone morphology and affects osteoblast differentiation. Bone Rep 2016, 5:33–42.In this study, authors report on the effect of age, sex and methionine restriction on bone parameters in mice. They find both age and sex dependent effects on bone morphology in methionine restricted mice. Importantly, they show that while MetR is associated with a reduction in bone mass, when normalized to body size this effect is no longer present and does not impair the material level biomechanical properties. It is important to note that 9 months of age was considered ‘old’, and that retired breeders were used for this group, but virgin mice in the young group (age 2 months).
- 38.Yu D et al. : Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J 2018. fj201701211R. [DOI] [PMC free article] [PubMed]
- 39.Garcia-Carrizo F et al. : Sexual dimorphism in the age-induced insulin resistance, liver steatosis, and adipose tissue function in rats. Front Physiol 2017, 8:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattson MP, Longo VD, Harvie M: Impact of intermittent fasting on health and disease processes. Ageing Res Rev 2017, 39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piotrowska K et al. : Gender differences in response to prolonged every-other-day feeding on the proliferation and apoptosis of hepatocytes in mice. Nutrients 2016, 8:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang H et al. : Time-restricted feeding shifts the skin circadian clock and alters UVB-induced DNA damage. Cell Rep 2017, 20:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung H et al. : Time-restricted feeding improves insulin resistance and hepatic steatosis in a mouse model of postmenopausal obesity. Metabolism 2016, 65:1743–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haraguchi A et al. : Night eating model shows time-specific depression-like behavior in the forced swimming test. Sci Rep 2018, 8:1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acosta-Rodrıģuez VA et al. : Mice under caloric restriction self-impose a temporal restriction of food intake as revealed by an automated feeder system. Cell Metab 2017, 26 267–277.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguayo A et al. : Sex differences in circadian food anticipatory activity are not altered by individual manipulations of sex hormones or sex chromosome copy number in mice. PLOS ONE 2018, 13:e0191373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Razeghi Jahromi S et al. : Effects of intermittent fasting on experimental autoimune encephalomyelitis in C57BL/6 mice. Iran J Allergy Asthma Immunol 2016, 15:212–219. [PubMed] [Google Scholar]
- 48.Zhang J et al. : Intermittent fasting protects against Alzheimer’s disease possible through restoring aquaporin-4 polarity. Front Mol Neurosci 2017, 10:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carroll JC et al. : Sex differences in b-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res 2010, 1366:233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clayton JA, Collins FS: Policy: NIH to balance sex in cell and animal studies. Nature 2014, 509:282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]


