Abstract
Background:
People living with HIV/AIDS (PLWH) smoke tobacco at higher rates and have more difficulty quitting than the general population, which contributes to significant life-years lost. The effectiveness of varenicline, one of the most effective tobacco dependence treatments, is understudied in HIV. We evaluated the safety and efficacy of varenicline for smoking cessation among PLWH.
Methods:
This was a single-site randomized, double-blind, placebo-controlled, phase 3 clinical trial ( NCT01710137). PLWH on antiretroviral therapy (ART) who were treatment-seeking daily smokers were randomized (1:1) to 12 weeks of varenicline (n=89) or placebo (n=90). All participants were offered six smoking cessation behavioral counseling sessions. The primary outcome was 7-day point prevalence abstinence, confirmed with breath carbon monoxide, at Weeks 12 and 24. Continuous abstinence and time to relapse were secondary outcomes. Safety measures were treatment-related side effects, adverse events, blood pressure, viral load, and ART adherence.
Results:
Of the 179 smokers, 81% were African American, and 68% were male. Varenicline increased cessation at Week 12 (28.1% vs. 12.1%; OR=4.54, 95% CI:1.83–11.25, P=.001). Continuous abstinence from Week 9 to 12 was higher for varenicline vs. placebo (23.6% vs. 10%; OR=4.65, 95% CI:1.71–12.67, P=.003); at Week 24, there was no effect of varenicline for point prevalence (14.6% vs. 10%), continuous abstinence (10.1% vs. 6.7%), or time to relapse (Ps>.05). There were no differences between varenicline and placebo on safety measures (Ps>.05).
Conclusions:
Varenicline is safe and efficacious for short-term smoking cessation among PLWH and should be used to reduce tobacco-related life-years lost in this population.
Keywords: HIV, AIDS, Tobacco Use, Smoking Cessation, Varenicline, Nicotine Dependence
1. Introduction
Antiretroviral therapy (ART) for people living with HIV/AIDS (PLWH) substantially improved life expectancy (Wandeler et al., 2016) but has led to the need to address modifiable risk factors associated with the leading causes of death among PLWH, including cardiovascular disease and cancer, such as tobacco smoking (Althoff, 2016; Petoumenos and Law, 2016). HIV-infected smokers lose more life-years due to tobacco use than to HIV (Helleberg et al., 2013) and exhibit greater immune activation versus HIV-infected non-smokers (Valiathan et al., 2014). Tobacco cessation among PLWH could save 265,000 life-years, would yield greater life-years saved than hepatitis C treatment or ART for those with higher CD4+ T-cell counts, and can yield health benefits up to 10 years after diagnosis (Reddy et al., 2016).
Unfortunately, the prevalence of tobacco use among PLWH greatly exceeds that found in the general population (Raposeiras-Roubin et al., 2017). Current smoking among PLWH in the US surpasses 40% (Mdodo et al., 2015) [vs. 14% in the general population (Norris et al., 2017)] and exceeds 30% in low- and middle- income countries (Akhtar-Khaleel et al., 2016; Mdege et al., 2017). PLWH may also experience unique barriers to smoking cessation. For instance, depression and deficits in cognitive function are risk factors for relapse (Hitsman et al., 2013; Loughead et al., 2015) and are more prevalent among PLWH than HIV-uninfected individuals (Heaton et al., 2015; Nanni et al., 2015). Moreover, PLWH report that nicotine dependence, concerns about cravings, weight gain, and the ability to manage stress, as well as having a social network of smokers are barriers to smoking cessation (Cioe et al., 2018; Weinberger et al., 2018). Thus, there is a clear need to evaluate the efficacy of nicotine dependence treatments in this population.
Varenicline, a nicotine acetylcholine α4β2 receptor partial agonist, when paired with counseling, is one of the most efficacious medications for tobacco dependence (Cahill et al., 2016). Clinicians who care for PLWH are uniquely positioned to provide evidence-based treatment for tobacco dependence, including varenicline. Although ~10% of smokers in the general population report using varenicline (Shah et al., 2017), less than 4% of PLWH report using varenicline, and 1 in 5 clinicians caring for PLWH recommend varenicline to their patients who smoke (Pacek et al., 2017). The paucity of evidence from rigorous studies establishing the efficacy of varenicline for PLWH, coupled with concerns about the psychiatric (Wu et al., 2016) and cardiovascular (Sterling et al., 2016) side effects of varenicline, may limit patient and physician use of this evidence-based treatment.
There have been remarkably few tobacco dependence treatment studies for PLWH relative to the general population, and three reviews show that there are insufficient data to conclude that tobacco dependence interventions that are efficacious in the general population are efficacious for PLWH (Ledgerwood and Yskes, 2016; Pacek and Cioe, 2015; Pool et al., 2016). Further, many studies have methodological weaknesses, including the lack of randomization and a control group, infrequent use of biological verification of tobacco abstinence, and lack of post-treatment follow-up (Ferketich et al., 2013; Ledgerwood and Yskes, 2016). The safety and efficacy of varenicline in PLWH has been evaluated in only one randomized, placebo-controlled trial (Mercie et al., 2018). While this trial, conducted in France, showed that varenicline was safe and efficacious for HIV- infected smokers, additional studies are needed to generalize these results and to facilitate the pooling of data for meta-analytic evaluations. The current study tested the efficacy and safety of varenicline among smokers with HIV. We expected higher abstinence rates among the varenicline group, compared to placebo, but did not expect differences in safety measures between treatment arms.
2. Methods
2.1. Study Design
This was a randomized, placebo-controlled, phase 3 clinical trial evaluating the safety and efficacy of 12 weeks of varenicline vs. 12 weeks of placebo for HIV-infected smokers. The trial was implemented at the University of Pennsylvania and recruited smokers through the university’s Infectious Diseases Division, a community-based HIV medical clinic, and advertisements. The trial was registered with ClinicalTrials.gov ( NCT01710137), approved by the University of Pennsylvania IRB, and conformed to US Federal Policy for the Protection of Human Subjects. Written informed consent was obtained from participants.
2.2. Participants
Prospective participants were screened by telephone and, if initially eligible, were scheduled for an in-person visit. To be eligible, individuals had to be age >18, have a confirmed HIV diagnosis, be treated with ART with HIV viral loads <1000 copies/ml and CD4+ counts >200 cells/mm3, report daily smoking, ALT and AST <2 times upper limit of normal, and creatinine clearance >50 mL/min. Exclusion criteria included: self-reported history of psychosis or a suicide attempt, self-reported current or planned pregnancy, self-reported current use of smoking cessation medications, unstable or untreated alcohol/substance abuse [those with current alcohol/substance use disorder were considered stable if they were currently receiving treatment (e.g., medication, group therapy, etc.) and had beenin treatment and/or not using for more than 30 days], and uncontrolled hypertension (systolic>160 or diastolic>100).
2.3. Randomization and Masking
Eligible participants were randomized 1:1 by a computer-generated protocol provided by the study statistician to the University of Pennsylvania’s Investigational Drug Service (IDS), who maintained the supply of varenicline and placebo. As part of this investigator-initiated project, Pfizer provided varenicline and placebo directly to IDS who repackaged the pills into blister packs. All participants and study personnel, aside from IDS, were blinded from treatment arm allocation throughout the trial. The study statistician was permitted to be unblinded if a Serious Adverse Event (SAE) occurred.
2.4. Procedures
During the eligibility visit, trained personnel administered self-report questionnaires, ascertained laboratory results, and completed a medical history, the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) to assess for DSM-IV psychiatric disorders, and the Columbia Suicide Severity Rating Scale (CSSRS) (Posner et al., 2011). Study physicians and a clinical psychologist confirmed eligibility. Eligible participants were randomized to treatment arm and scheduled for a pre-quit session (Week 0). Subjects were considered ITT once the pre-quit session was completed and medication was assigned. Varenicline was provided at Week 0 based on U.S. Food and Drug Administration labeling: Day 1-Day 3 (0.5 mg once daily), Day 4–7 (0.5 mg twice daily), and Day 8-Day 84 (1.0 mg twice daily). Placebo pills were identical in appearance and dosing regimen.
All participants were offered six standardized, PHS guideline-based smoking cessation counseling sessions at Weeks 0, 1, 3, 5, 7, and 9, in-person or by telephone (Fiore et al., 2008). All sessions were one-on-one and delivered by trained counselors who were supervised by a clinical psychologist. Sessions were designed to help participants understand the risks associated with smoking, prepare for a target quit-date at Week 1, and develop skills to manage nicotine withdrawal and avoid relapse (Lerman et al., 2015; Schnoll et al., 2010; Schnoll et al., 2015). One HIV-specific module was included to educate participants about the unique health risks associated with smoking among PLWH. Thirty percent of sessions were recorded and assessed for fidelity by senior study personnel.
At the eligibility visit, demographic information (e.g., age, race), mode of HIV acquisition and current ART medications and adherence were ascertained from self-report. Disease-related characteristics, including HIV viral load and CD4+ count (within the past 6 months), were collected from medical records. Smoking-related data included current smoking rate, number of previous quit attempts, years smoked, measures of nicotine dependence including time-to-first- cigarette (TTFC) (Transdisciplinary Tobacco Use Research Center Tobacco Dependence et al., 2007) and the heaviness of smoking index (HSI) (Kozlowski et al., 1994), and a breath carbon monoxide (CO) assessment.
Safety assessments occurred at Weeks 0, 1, 3, 5, 7, 9, and 12. Varenicline-related side effects (e.g., nausea, sleep problems, and hostility) (Lerman et al., 2015; Price et al., 2017) were rated from 0 (none) to 3 (severe), and ratings were summed to create a side-effects index (these are referred to as “side effects”). Participants were instructed to contact study personnel if they experienced any problems between assessments. Using a previously developed algorithm that considered severity and expectedness (Lerman et al., 2015), side effects were classified as Code 1–4, with Code 3 reports possibly representing adverse events (AEs) and Code 4 events representing Serious Adverse Events (SAEs) based on whether the event was considered by the participant to be debilitating or if they required hospitalization. The study physicians and psychologist were consulted to determine if an event was an AE and an SAE and what, if any, treatment or follow-up was required. Blood pressure and self-reported ART adherence were assessed at each time-point (Holmes et al., 2007). Viral load was reassessed via chart review at Week 12.
Pill and counseling adherence were assessed at Weeks 0, 1, 3, 5, 7, 9, and 12 (Crawford et al., 2018; Lerman et al., 2015). Counseling adherence was defined as completing at least 5/6 sessions. Pill adherence was tracked using the timeline follow-back method (Brown et al., 1998) and blister-pack collection. The total pills taken out of the total pills prescribed (i.e., 165) was computed for an overall proportion of medication adherence. Pill adherence was defined by taking >80% of prescribed medication (Pacek et al., 2018).
Smoking behavior was assessed at Weeks 0, 1, 3, 5, 7, 9, 12, 18 and 24 using the timeline follow-back procedure (Lerman et al., 2015; Schnoll et al., 2015). The primary smoking cessation outcomes for this trial were 7-day point-prevalence abstinence at Weeks 12 and 24, based on no self-reported tobacco use (not even a puff) during the 7 days preceding the assessment and a CO ≤8ppm (Benowitz et al., 2002; Hughes et al., 2003). Secondary outcomes included point-prevalence abstinence at Week 18, continuous abstinence rates (with CO) from Weeks 9–12, 9–18, and 9–24, defined as no self-reported tobacco use for the duration of the timeframe (based on previous varenicline trials (Gonzales et al., 2006; Mercie et al., 2018)), and time to relapse across the 24-week trial. An ITT approach was taken such that participants who completed the pre-quit session (Week 0) and were randomized to treatment arm but were lost to follow-up or failed to provide the CO measure were considered not abstinent.
2.5. Data Analysis
The sample size was based on our expectation of a 14–16% difference in quit rates between the varenicline and placebo groups at Week 12 (Gonzales et al., 2006) (80% power, α=.05). Specifically, the sample size of 179 provided 80% power to detect an OR of 2.2 given a 12% quit rate in the placebo group at Week 12. For Week 24, we had 80% power to detect an OR of 2.3 given a 10% quit rate in the placebo group. Sample characteristics were examined for clinically meaningful baseline differences between groups. Variables associated with treatment arm (P<.10) were included as covariates in subsequent models. Varenicline and counseling adherence and Weeks 12, 18, and 24 assessment completion rates were characterized as proportions, and differences between treatment arms were determined using chi-square. Lastly, we (Schnoll et al., 2018) and others (Tanner and Tyndale, 2017) have found the antiretroviral treatment efavirenz to be positively associated with nicotine metabolism rate, which is a strong predictor of smoking abstinence and response to varenicline (Lerman et al., 2015). Because 18% of the current sample reported taking efavirenz, efavirenz use was a covariate in all models.
The primary analyses utilized multiple logistic regression models to test the relationship between treatment arm and 7-day point-prevalence abstinence, CO-confirmed. Separate models were conducted for point-prevalence abstinence outcomes at Weeks 12 and 24. A secondary outcome was 7-day point-prevalence abstinence at Week 18. The results were characterized by odds ratios and 95% confidence intervals. We then used longitudinal logistic regression (General Estimating Equations, GEE) to account for within-subject correlations among repeated measures at each follow-up time-point. The GEE models included a variable for time-point as a fixed effect and subject as a random effect. An interaction term between time-point and treatment arm was included to determine whether the treatment effect varied across time. This approach was also used for continuous abstinence. For all models, missing outcomes were considered non-abstinent. Lastly, we evaluated time to relapse through Week 24 using a Cox regression model excluding individuals who failed to quit for at least 24 hours. All models included covariates (see below).
Safety was assessed by examining treatment arm effects on mean (average of severity rating for all side effects at a given time point) and total count (number of symptoms with responses of 1, 2, or 3) on the side effects checklist from Weeks 0–12. Because nausea is a common side effect of varenicline (Cahill et al., 2016), we also examined this item separately. Repeated-measures ANOVA was used with mean or count side effects from Weeks 0, 3, 7, and 12 across treatment arms. Chi-square compared the cumulative frequencies of AEs and SAEs from Weeks 0–12 across treatment arms and high blood pressure rates at Weeks 0, 3, 7, and 12. Mean changes in viral load and ART adherence from Weeks 0–12 across treatment arms were assessed using ANOVA. No adjustments were made for multiple comparisons. Analyses were conducted using SPSS (IBM Corporation, Armonk, NY) or STATA (StataCorp, College Station, TX).
3. Results
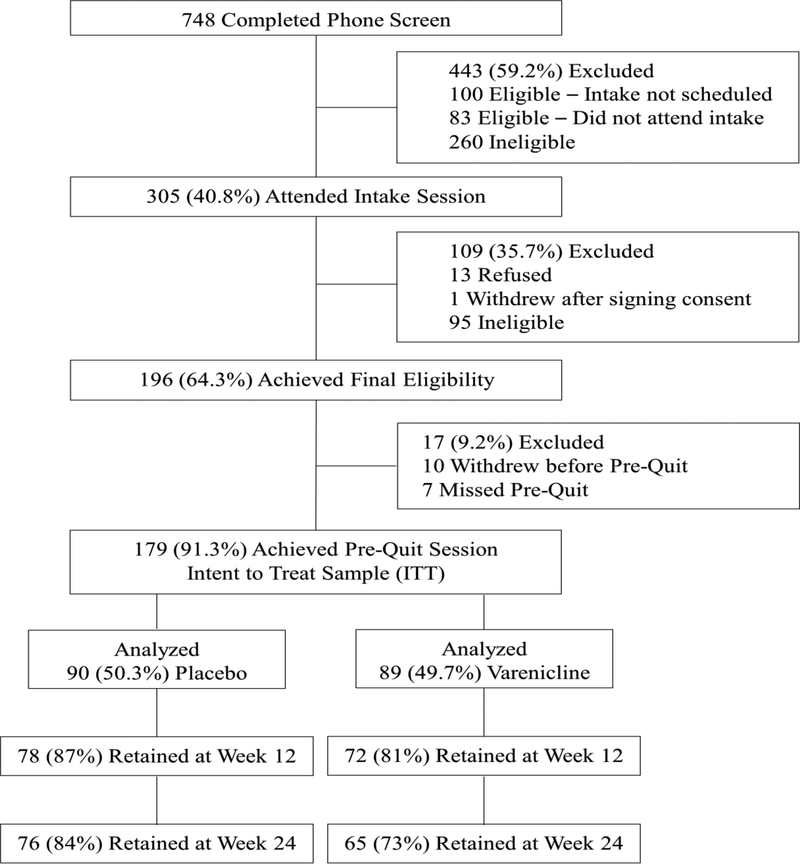
The study was conducted from October 2012 to June 2018. As shown in the CONSORT diagram (Figure 1), 748 individuals were screened, of which 305 (40.7%) were eligible and attended the in-person eligibility evaluation; 179 individuals were eligible, willing to enroll, completed the pre-quit session, and randomized to varenicline (n=89) or placebo (n=90). Overall, only 7/179 participants (3.9%) withdrew from the trial. Visit completion rates in the varenicline arm were 81%, 75%, and 73% at Weeks 12, 18, and 24, respectively, and were 87%, 80%, and 84% at Weeks 12, 18, and 24 for placebo participants, respectively (P’s>.05).
Figure 1.
CONSORT Diagram.
3.1. Sample Characteristics
Demographic, smoking-related, and HIV-related characteristics are shown in Table 1. The placebo arm had more very low-income participants than the varenicline arm. Study medication adherence was greater in the placebo arm [138.0 pills (SD=40.3)] than varenicline arm [124.0 pills (SD=51.5), P=.05]; 75.6% of placebo participants reported taking >80% of pills, compared to 58.4% of varenicline participants (P=.02). The average number of counseling sessions completed was 5.4 out of 6 (SD=1.3); 90% of placebo participants completed >5 counseling sessions, compared to 82% of varenicline participants (χ2[1]=2.40, P=.14). Lastly, the prevalence of any past or current diagnosis of alcohol or substance use disorder was similar between the placebo (n=16, 18%) and varenicline groups (n=21, 24%). Income, TTFC, varenicline adherence (number of pills), and efavirenz use were included as covariates in all models (Supplementary Table 1* contains all ARTs reported).
Table 1.
Baseline demographic, smoking-related, and disease-related characteristics across treatment arms for the ITT sample
| Placebo (N=90) |
Varenicline (N=89) |
Total (N=179) |
|
|---|---|---|---|
| Variable | N (%) or M (SD) |
N (%) or M (SD) |
N (%) or M (SD) |
| Demographic variables | |||
| Race (% African American) | 72 (83.7) | 69 (79.3) | 141 (81.5) |
| Sex (% Male) | 58 (64.4) | 64 (71.9) | 122 (68.2) |
| Education (% High School Grad or less) | 55 (61.1) | 46 (51.7) | 101 (56.4) |
| Annual Household Income (<20K) | 71 (79.8) | 55 (61.8) | 126 (70.8) |
| Age (Range: 21–70) | 48.5 (9.7) | 48.7 (10.1) | 48.6 (9.9) |
| BMI (Range: 15.3–58.2) | 27.1 (6.3) | 27.5 (7.2) | 27.3 (6.7) |
| Smoking-related variables | |||
| HSI (% High HSI) | 60 (66.7) | 60 (67.4) | 120 (67.0) |
| More than 60 minutes | 37 (41.1) | 37 (41.6) | 74 (41.3) |
| Cigarettes/Day in Past 7 Days (Range: 1–60) | 11.8 (9.0) | 11.2 (6.6) | 11.5 (7.9) |
| Breath CO, ppm (Range: 1–60) | 13.1 (7.9) | 15.0 (10.4) | 14.1 (9.2) |
| Number of Years Smoking (Range: 4–56) | 31.1 (11.0) | 31.8 (10.8) | 31.5 (10.9) |
| Number of Times Quit Smoking for>24 Hours (Range: 0–500) | 4.9 (12.3) | 8.8 (52.9) | 6.9 (38.2) |
| Disease-related characteristics | |||
| % of ART Prescribed in Past 2 Weeks Taken (Range: 79–100) | 98 (1.0) | 99 (3.0) | 98 (4.0) |
| % Undetectable Viral Load (<50 copies/ml) | 69 (77) | 75 (84) | 144 (80) |
| CD4+ cells/mm3 (Range: 214–1932) | 688.8 (322.7) | 737.2 (329.5) | 712.8 (326.1) |
| % Acquired HIV via Sex | 71 (78.9) | 73 (82.0) | 144 (80.4) |
| % ART regimen containing efavirenz | 18 (20.9) | 14 (15.9) | 32 (18.4) |
| Estimated creatinine clearance (mL/min) | 107.7 (39.2) | 103.5 (38.9) | 105.6(39.0) |
| Drug and Alcohol Use History | |||
| # Alcohol Drinks in Past 7 Days (Range: 0–27) | 4.9 (4.2) | 5.1 (6.3) | 5.0 (5.2) |
| DSM-IV Alcohol Abuse (Current) | 3 (3) | 2 (2) | 5 (3) |
| DSM-IV Alcohol Abuse (Past) | 1 (1) | 4 (4) | 5 (3) |
| DSM-IV Substance Abuse (Current)a | 3 (3) | 6 (7) | 9 (5) |
| DSM-IV Substance Abuse (Past)a | 11 (12) | 12 (13) | 23 (13) |
| History Injection Drug Use | 4 (4) | 3 (3) | 7 (4) |
| History/Current Alcohol or Substance Abuse | 16 (18) | 21 (24) | 37 (21) |
Note. BMI=Body Mass Index; CO=Carbon Monoxide; HSI=Heaviness of Smoking Index; TTFC=Time to First Cigarette.
Other than nicotine
3.2. Varenicline Efficacy
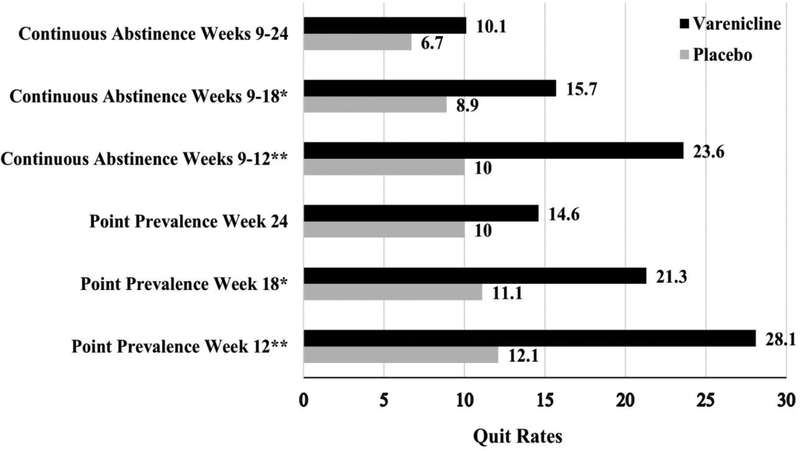
The 7-day point prevalence abstinence rates are shown in Figure 2. As shown in Table 2, at Week 12, varenicline participants reported significantly higher point prevalence abstinence rates (OR=4.5 [95% CI: 1.83–11.2], P=.001), but this effect was no longer significant at Week 24 (OR=1.9 [95% CI: 0.71–5.1], P=.20). Point prevalence abstinence at Week 18 was evaluated as a secondary outcome. Similar to Week 12, those in the varenicline group were more likely to be abstinent than those in the placebo group (P=.02). The overall GEE model for point prevalence abstinence, including all three time-points, indicated a significant effect of varenicline (OR=3.2 [95% CI: 1.4–7.3], P=.006). The effect of time-point (OR=0.72 [95% CI: 0.60–0.88], P=.001) and the treatment arm by time-point interaction (OR=0.67 [95% CI: 0.45–1.0], P=.05) indicated that varenicline’s efficacy declined over time. Varenicline participants reported significantly higher continuous abstinence rates between Weeks 9–12 (OR=4.65 [95% CI: 1.71–12.67], P=.003), but this effect was no longer significant between Weeks 9–24 (OR=1.92 [95% CI: .57–6.47, P=.29], see Figure 2 and Table 2). Similar to point prevalence abstinence, those in the varenicline group reported higher continuous abstinence rates between Weeks 9–18 than the placebo group (P=.05). The overall GEE model for continuous abstinence, including all three time-points, revealed a significant effect of varenicline (OR=2.3 [95% CI: 1.1–4.6], P=.02), although neither the time-point or treatment arm by time-point interaction was significant (P’s>.05). The effect of varenicline was not significant in the survival analysis (HR=0.75, [95% CI: 0.38–1.5], P=.40).
Figure 2.
Rates of 7-day point prevalence and continuous abstinence at weeks 12, 18, and 24 across treatment arm.
Intent to treat sample (N=179); point prevalence quit rates are 7-day self-reported, carbon monoxide confirmed; continuous abstinence are self-reported cessation from week 9 to the follow-up time-point, confirmed with carbon monoxide at the follow-up assessment time-point. All values are % abstinent. Weeks 12 and 24 were primary outcomes and Week 18 was a secondary end point. ** P<0.001; * P <0.05
Table 2.
Multiple logistic regression models predicting 7-day point prevalence and continuous abstinence at Weeks 12, 18, and 24
| Dependent Variable: Point Prevalence Abstinence Week 12 | OR | 95% CI | P |
|---|---|---|---|
| Income (Reference=20K or less) | 1.23 | 0.78, 1.93 | 0.37 |
| Medication Adherence | 1.01 | 1.00, 1.02 | 0.12 |
| Efavirenz Use (Reference=Yes) | 2.83 | 1.04, 7.73 | 0.04 |
| TTFC (Reference=Within 30 minutes) | 0.59 | 0.22, 1.57 | 0.29 |
| Treatment Arm (Reference=Placebo) | 4.54 | 1.83, 11.25 | 0.001 |
| Dependent Variable: Point Prevalence Abstinence Week 18 | OR | 95% CI | p |
| Income (Reference=20K or less) | 1.16 | 0.75, 1.86 | 0.56 |
| Medication Adherence | 1.01 | 0.99, 1.02 | 0.31 |
| Efavirenz Use (Reference=Yes) | 1.84 | 0.62, 5.42 | 0.27 |
| TTFC (Reference=Within 30 minutes) | 0.52 | 0.18, 1.39 | 0.19 |
| Treatment Arm (Reference=Placebo) | 3.12 | 1.22, 7.97 | 0.02 |
| Dependent Variable: Point Prevalence Abstinence Week 24 | OR | 95% CI | p |
| Income (Reference=20K or less) | 1.10 | 0.64, 1.89 | 0.72 |
| Medication Adherence | 1.01 | 0.99, 1.02 | 0.30 |
| Efavirenz Use (Reference=Yes) | 1.84 | 0.58, 5.77 | 0.30 |
| TTFC (ReferencêWithin 30 minutes) | 0.60 | 0.19, 1.85 | 0.37 |
| Treatment Arm (Reference=Placebo) | 1.90 | 0.71, 5.10 | 0.20 |
| Dependent Variable: Continuous Abstinence Weeks 9–12 | OR | 95% CI | p |
| Income (Reference=20K or less) | 1.37 | 0.86, 2.18 | 0.19 |
| Medication Adherence | 1.01 | 1.00, 1.02 | 0.18 |
| Efavirenz Use (Reference=Yes) | 3.16 | 1.1, 9.1 | 0.03 |
| TTFC (Reference=Within 30 minutes) | 0.54 | 0.19, 1.52 | 0.24 |
| Treatment Arm (Reference=Placebo) | 4.65 | 1.71, 12.67 | 0.003 |
| Dependent Variable: Continuous Abstinence Weeks 9–18 | OR | 95% CI | p |
| Income (Reference=20K or less) | 1.28 | 0.76, 2.16 | 0.36 |
| Medication Adherence | 1.01 | 0.99, 1.02 | 0.27 |
| Efavirenz Use (Reference=Yes) | 2.11 | 0.65, 6.83 | 0.21 |
| TTFC (Reference=Within 30 minutes) | 0.56 | 0.18, 1.78 | 0.33 |
| Treatment Arm (Reference=Placebo) | 2.90 | 1.00, 8.43 | 0.05 |
| Dependent Variable: Continuous Abstinence Weeks 9–24 | OR | 95% CI | p |
| Income (Reference=20K or less) | 1.36 | 0.76, 2.43 | 0.31 |
| Medication Adherence | 1.01 | 0.99, 1.02 | 0.51 |
| Efavirenz Use (Reference=Yes) | 2.43 | 0.66, 8.94 | 0.18 |
| TTFC (Reference=Within 30 minutes) | 0.72 | 0.18, 2.88 | 0.64 |
| Treatment Arm (Reference=Placebo) | 1.92 | 0.57, 6.47 | 0.29 |
Note. Medication adherence was continuous measure of number of pills taken
3.3. Varenicline Safety
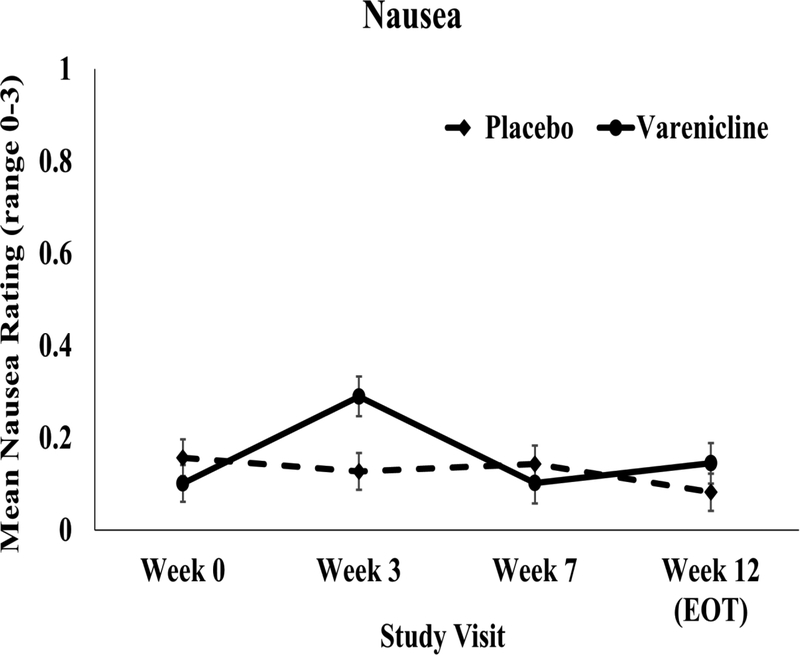
There was no significant time by treatment arm effects on mean side effect severity or side effect counts from Week 0 through Weeks 3, 7, and 12 (Ps>0.05; Supplementary Table 2*). As shown in Figure 3, when nausea was evaluated separately, the varenicline group reported a greater increase from Week 0 to Week 3 vs. the placebo group (P=.002), but there were no treatment arm effects at any other timepoint (Ps>.1). There were no ratings of severe nausea, and across all visits there were 11 counts of moderate nausea (4 placebo, 7 varenicline). There were no significant differences between treatment arms in the number of participants with AEs or SAEs coded as either Code 3 or Code 4 (Ps>0.05; see Table 3) or in the rate of hypertension at any time-point (P’s>.05). Examination of changes in ART adherence or the proportion of subjects with detectable viral load from Week 0 to Week 12 indicated no time × treatment arm interaction (Ps>.05).
Figure 3.
Nausea severity by treatment arm at Weeks 0, 3, 7, and 12.
Mean ratings of nausea severity (range: 0=none at all to 3=severe) throughout the trial. Except for a greater increase in nausea between Weeks 0 and 3 in the varenicline group, there were no treatment arm effects at any other timepoint.
Table 3.
Side effects, adverse events, and serious adverse events across treatment arm
| Variable | Placebo (N=90) |
Varenicline (N=89) |
Total (N=179) |
|---|---|---|---|
| Participants with an Adverse Event | 28 (31.1%) | 19 (21.3%) | 47 (26.3%) |
| Participants with a Serious Adverse Event | 3 (3.3%) | 5 (5.6%) | 8 (4.5%) |
| Total Number of Adverse Events | 70 | 43 | 113 |
| Skin swelling | 28 | 19 | 47 |
| Depression | 16 | 7 | 23 |
| Agitation | 16 | 8 | 24 |
| Weakness | 0 | 1 | 1 |
| Hostility | 5 | 2 | 7 |
| Irritability | 2 | 0 | 2 |
| Skin redness | 1 | 1 | 2 |
| Dizziness | 0 | 1 | 1 |
| Headache | 1 | 3 | 4 |
| Abdominal pain | 1 | 1 | 2 |
| ER visit | 2 | 0 | 2 |
| Total Number of Serious Adverse Events | 8 | 8 | 16 |
| Suicidality | 1 | 1 | 2 |
| Cancer diagnosis | 1 | 1 | 2 |
| Cancer metastasis | 0 | 1 | 1 |
| Hospitalization | 3 | 5 | 8 |
| Death | 1 | 0 | 1 |
| Number of High Blood Pressure Recordingsa | 30 | 17 | 47 |
Note. Adverse events and serious adverse events were determined by study physicians to be coded either Code 3 or Code 4.
A total of 10 recordings documented at baseline (5 per treatment arm).
4. Discussion
Tobacco use is a major health concern among PLWH, with prevalence rates that far exceed those seen in the general population and substantial adverse health consequences. Yet, there have been remarkably few studies rigorously testing smoking cessation interventions in this population and only one randomized, placebo-controlled trial of varenicline among PLWH, one of the most efficacious treatments available for tobacco dependence. The present study demonstrates that varenicline is safe and effective in the short-term and should be widely considered for treating tobacco dependence among PLWH.
The efficacy data showed that varenicline-treated participants showed a significant increase in smoking cessation, measured as CO-confirmed 7-day point prevalence or continuous abstinence, at the end of treatment, and this effect was maintained for 6 weeks following treatment cessation. While the quit rates are lower than in the general population (Gonzales et al., 2006) and when compared to an open-label study of varenicline with PLWH (Cui et al., 2012), varenicline more than doubled abstinence rates at Week 12. Although this effect decreased over time, with only about a 10% difference between the placebo and varenicline groups at Week 18, even small increases in abstinence can have meaningful effects. A recent US study estimated that a 10% reduction in smoking prevalence in each state could yield a $63 billion reduction in healthcare costs the following year (Lightwood and Glantz, 2016). However, there is limited support for the long-term effects of tobacco cessation interventions for PLWH (Pool et al., 2016). Indeed, although the treatment effect was no longer significant at Week 24, there was a ~5% difference in abstinence rates between the varenicline and placebo groups. The present results are also remarkably similar to the only other randomized placebo-controlled varenicline trial for smokers with HIV that was conducted in France (Mercie et al., 2018). Taken together, varenicline is efficacious for promoting tobacco cessation among PLWH in the short-term.
In contrast to studies with the general population (Gonzales et al., 2006), we found that the efficacy of varenicline decreased over time, and quit rates at Week 24 did not differ between groups. Thus, to maintain the benefits of varenicline, future studies should pair effective relapse prevention interventions with varenicline. One option could be the continued use of varenicline after 12 weeks. In the general population, 12 additional weeks of varenicline after confirmed abstinence following an initial 12 weeks of treatment increased the abstinence rate at 24 weeks (Tonstad et al., 2006), and retreatment after failure from 12 weeks of treatment increased cessation in the long-term (Gonzales et al., 2014). The FDA labeling allows for extended duration use, which may be particularly important for smokers with HIV. Importantly, recent evidence suggests that the effectiveness of varenicline, including extended duration use, is highly dependent upon adherence to the treatment regimen (Pacek et al., 2018; Schnoll et al., 2019). Thus, future studies should examine the added benefit of maintenance treatment, perhaps combined with approaches to improve medication adherence, as a strategy for improving quit rates in this population.
Analyses of the safety data showed no indication that varenicline use among PLWH is associated with adverse outcomes. While we were not powered to detect small differences, the varenicline- and placebo-treated participants did not differ in terms of the severity or total number of side effects, the total number of AEs or SAEs, or the number of participants who reported an AE or SAE. Neither psychiatric events, such as depression or suicidality, nor the rate of hypertension were significantly different across treatment arms, indicating that varenicline is not associated with adverse events that may underlie the reluctance to use and prescribe varenicline. Furthermore, varenicline was not associated with adverse HIV-related outcomes, including change in viral load or ART adherence. These results are similar to previous varenicline trials with HIV-infected smokers (Mercie et al., 2018) and smokers with serious mental illness (Anthenelli et al., 2016).
Thus, in most cases, concerns about side effects or AEs associated with varenicline should not serve as a barrier to use among PLWH.
These results should be interpreted within the context of study limitations. First, when recruitment began in 2012, safety concerns regarding varenicline required implementation of strict inclusion/exclusion criteria which reduced interest in the study and the number of eligible subjects. Despite the removal of several restrictions in 2016, we did not meet our proposed target sample of 310, and the final sample represented 24% of the overall screened sample, with 47% of those screened considered ineligible and 29% of those screened refusing to enroll. Nevertheless, our final sample of 179 was adequately powered for our primary outcome. Future studies should collect data regarding decisions not to enroll in clinical trials. Given the inclusion/exclusion criteria to control for confounding variables and increase safety, and the need for participants to be motivated to quit smoking, the present study may be generalizable to a smaller proportion of PLWH who is relatively healthy and motivated to quit smoking. Second, the rate of varenicline adherence was 58%. While this proportion compares to the general population (Peng et al., 2017) and another study with HIV-infected smokers (Shelley et al., 2015), the strong association between adherence and cessation (Catz et al., 2011) indicates the need to evaluate novel behavioral approaches to increase medication adherence to maximize the benefits of varenicline (Tseng et al., 2016). Moreover, low adherence may have resulted in underestimating the potential efficacy of varenicline and may have contributed to the lack of a statistically significant effect at Week 24. Lastly, while the sample was representative of the population of PLWH and smokers in Philadelphia, findings may not be generalizable to the broader U.S. population.
4.1. Conclusions
Nevertheless, there are several notable strengths to this trial. Most noteworthily, this is only the second placebo-controlled randomized clinical trial of varenicline for HIV-infected smokers and the first conducted in the US. Methodological strengths include follow-up of smoking cessation outcomes until 24 weeks, biochemical verification of self-reported abstinence, inclusion of standard behavioral smoking cessation counseling, examination of a broad range of potential adverse outcomes, and excellent retention across the 24 weeks. The overall findings indicate that use of varenicline combined with behavioral counseling is safe and effective in the short-term for HIV-infected smokers compared with behavioral counseling alone. Varenicline was not associated with adverse outcomes, including cardiovascular and psychiatric events, and significantly increased quit rates at the end-of-treatment and through 6 weeks following end-of-treatment. These results provide important information for patients and clinicians as they engage in efforts to address smoking among PLWH and improve their quality of life.
Supplementary Material
Highlights.
Varenicline is safe for smokers with HIV to use as a smoking cessation aid
Varenicline improved smoking cessation in the short-term among smokers with HIV
At 6 months, there was no difference between varenicline and placebo in quit rates
Acknowledgments
We would like to thank Sue Ware and Paul Sanborn for their assistance with data collection and management.
Role of Funding Source
This research was supported by grants from the National Institute on Drug Abuse (R01 DA033681 and K24 DA045244) and through core services and support from the Penn Center for AIDS Research (P30 AI045008) and the Penn Mental Health AIDS Research Center (P30 MH097488). Pfizer provided medication and placebo free of charge. The United States National Institutes of Health and Pfizer had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. The study Principal Investigator and members of the research team and co-authors had full access to all study data and final responsibility for the decision to submit this paper for publication. Pfizer reviewed this manuscript prior to submission but has no influence over the decision to submit for review.
De-identified data and the full study protocol are available upon request from the corresponding author.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
Dr. Schnoll received medication and placebo free of charge from Pfizer for clinical trials and has provided consultation to Pfizer, GlaxoSmithKline, and Curaleaf. Dr. Gross serves on a Pfizer Data and Safety Monitoring Board for a drug unrelated to smoking or HIV. Dr. Ashare has an investigator-initiated grant from Novo Nordisk for a drug unrelated to the current study.
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:…
References
- U.S. Department of Health and Human Services. AIDS.gov: HIV and smoking U.S. Department of Health and Human Services, Washington, D.C. [Google Scholar]
- Akhtar-Khaleel WZ, Cook RL, Shoptaw S, Surkan PJ, Teplin LA, Stall R, Beyth RJ, Manini TM, Plankey M, 2016. Long-term cigarette smoking trajectories among HIV-seropositive and seronegative MSM in the multicenter AIDS cohort study. AIDS Behav 20, 1713–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althoff KN, 2016. The shifting paradigm of care for adults living with HIV:Smoking cessation for longer life. J. Infect. Dis 214, 1618–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthenelli RM, Benowitz NL, West R, St Aubin L, McRae T, Lawrence D, Ascher J, Russ C, Krishen A, Evins AE, 2016. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): A double-blind, randomised, placebo-controlled clinical trial. Lancet 387, 2507–2520. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Hansson A, Lichtenstein E, Henningfield J, Tsoh J, Hurt RD, Velicer W, 2002. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res 4, 149–159.12028847 [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW, 1998. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav 12, 101–112. [Google Scholar]
- Cahill K, Lindson-Hawley N, Thomas KH, Fanshawe TR, Lancaster T, 2016. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catz SL, Jack LM, McClure JB, Javitz HS, Deprey M, Zbikowski SM, McAfee T, Richards J, Swan GE, 2011. Adherence to varenicline in the COMPASS smoking cessation intervention trial. Nicotine Tob. Res 13, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioe PA, Gordon REF, Guthrie KM, Freiberg MS, Kahler CW, 2018. Perceived barriers to smoking cessation and perceptions of electronic cigarettes among persons living with HIV. AIDS Care 30, 1469–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford G, Weisbrot J, Bastian J, Flitter A, Jao NC, Carroll A, Kalhan R, Leone F, Hitsman B, Schnoll R, 2018. Predictors of varenicline adherence among cancer patients treated for tobacco dependence and its association with smoking cessation. Nicotine Tob. Res Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Q, Robinson L, Elston D, Smaill F, Cohen J, Quan C, McFarland N, Thabane L, McIvor A, Zeidler J, Smieja M, 2012. Safety and tolerability of varenicline tartrate (Champix®/Chantix®) for smoking cessation in HIV-infected subjects: A pilot open-label study. AIDS Patient Care STDS 26, 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferketich AK, Diaz P, Browning KK, Lu B, Koletar SL, Reynolds NR, Wewers ME, 2013. Safety of varenicline among smokers enrolled in the lung HIV study. Nicotine Tob. Res 15, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF, Froelicher ES, Goldstein MG, Healton CG, Henderson PN, Heyman RB, Koh HK, Kottke TE, Lando HA, Mecklenburg RE, Mermelstein RJ, Mullen PD, Orleans CT, Robinson L, Stitzer ML, Tommasello AC, Villejo L, Wewers ME, Murray EW, Bennett G, Heishman S, Husten C, Morgan G, Williams C, Christiansen BA, Piper ME, Hasselblad V, Fraser D, Theobald W, Connell M, Leitzke C, 2008. Treating tobacco use and dependence: 2008 update U.S. Public Health Service Clinical Practice Guideline executive summary. Respir. Care 53, 1217–1222. [PubMed] [Google Scholar]
- Gonzales D, Hajek P, Pliamm L, Nackaerts K, Tseng LJ, McRae TD, Treadow J, 2014. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: A randomized, placebo-controlled trial. Clin. Pharmacol. Ther 96, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB,Watsky EJ, Gong J, Williams KE, Reeves KR, Varenicline Phase 3 Study, G., 2006. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: A randomized controlled trial. JAMA 296, 47–55. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR Jr., Deutsch R, Letendre S, Ellis RJ, Casaletto K, Marquine MJ, Woods SP, Vaida F, Atkinson JH, Marcotte TD, McCutchan JA, Collier AC, Marra CM, Clifford DB, Gelman BB, Sacktor N, Morgello S, Simpson DM, Abramson I, Gamst AC, Fennema-Notestine C, Smith DM, Grant I, 2015. Neurocognitive change in the era of HIV combination antiretroviral therapy: The longitudinal CHARTER study. Clin. Infect. Dis 60, 473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N, 2013. Mortality attributable to smoking among HIV-1-infected individuals: A nationwide, population-based cohort study. Clin. Infect. Dis 56, 727–734. [DOI] [PubMed] [Google Scholar]
- Hitsman B, Papandonatos GD, McChargue DE, DeMott A, Herrera MJ, Spring B, Borrelli B, Niaura R, 2013. Past major depression and smoking cessation outcome: A systematic review and meta-analysis update. Addiction 108, 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes WC, Bilker WB, Wang H, Chapman J, Gross R, 2007. HIV/AIDS-specific quality of life and adherence to antiretroviral therapy over time. J. Acquir. Immune Defic. Syndr 46, 323–327. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE, 2003. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob. Res 5, 13–25. [PubMed] [Google Scholar]
- Kozlowski LT, Porter CQ, Orleans CT, Pope MA, Heatherton T, 1994. Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend 34, 211–216. [DOI] [PubMed] [Google Scholar]
- Ledgerwood DM, Yskes R, 2016. Smoking cessation for people living with HIV/AIDS: A literature review and synthesis. Nicotine Tob. Res 18, 2177–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Schnoll RA, Hawk LW Jr., Cinciripini P, George TP, Wileyto EP, Swan GE, Benowitz NL, Heitjan DF, Tyndale RF, Group PPR, 2015. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: A randomised, double-blind placebo-controlled trial. Lancet Respir. Med 3, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightwood J, Glantz SA, 2016. Smoking behavior and healthcare expenditure in the United States, 1992–2009: Panel data estimates. PLoS Med 13, e1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J, Wileyto EP, Ruparel K, Falcone M, Hopson R, Gur R, Lerman C, 2015. Working memory-related neural activity predicts future smoking relapse. Neuropsychopharmacology 40, 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K, 2017. Tobacco use among people living with HIV: Analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob. Health 5, e578–e592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J, 2015. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann. Intern Med 162, 335–344. [DOI] [PubMed] [Google Scholar]
- Mercie P, Arsandaux J, Katlama C, Ferret S, Beuscart A, Spadone C, Duvivier C, Reynes J, Wirth N, Moinot L, Benard A, Zucman D, Duval X, Molina JM, Spire B, Fagard C, Chene G, ANRS 144-InterACTIV Study Group, 2018. Efficacy and safety of varenicline for smoking cessation in people living with HIV in France (ANRS 144 Inter-ACTIV): A randomised controlled phase 3 clinical trial. Lancet HIV 5, e126–e135. [DOI] [PubMed] [Google Scholar]
- Nanni MG, Caruso R, Mitchell AJ, Meggiolaro E, Grassi L, 2015. Depression in HIV infected patients: A review. Curr. Psychiatry Rep 17, 530. [DOI] [PubMed] [Google Scholar]
- Norris T, Schiller JS, Clarke TC, 2017. Early release of selected estimates based on data from the National Health Interview Survey National Center for Health Statistics, Hyattsville, MD. [Google Scholar]
- Pacek LR, Cioe PA, 2015. Tobacco use, use disorders, and smoking cessation interventions in persons living with HIV. Curr. HIV/AIDS Rep 12, 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, McClernon FJ, Bosworth HB, 2018. Adherence to pharmacological smoking cessation interventions: A literature review and synthesis of correlates and barriers. Nicotine Tob. Res 20, 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacek LR, Rass O, Johnson MW, 2017. Positive smoking cessation-related interactions with HIV care providers increase the likelihood of interest in cessation among HIV-positive cigarette smokers. AIDS Care 29, 1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AR, Morales M, Wileyto EP, Hawk LW Jr., Cinciripini P, George TP, Benowitz NL, Nollen NL, Lerman C, Tyndale RF, Schnoll R, 2017. Measures and predictors of varenicline adherence in the treatment of nicotine dependence. Addict. Behav 75, 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petoumenos K, Law MG, 2016. Smoking, alcohol and illicit drug use effects on survival in HIV-positive persons. Curr. Opin. HIV AIDS 11, 514–520. [DOI] [PubMed] [Google Scholar]
- Pool ER, Dogar O, Lindsay RP, Weatherburn P, Siddiqi K, 2016. Interventions for tobacco use cessation in people living with HIV and AIDS. Cochrane Database Syst. Rev CD011120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner K, Brown GK, Stanley B, Brent DA, Yershova KV, Oquendo MA, Currier GW, Melvin GA, Greenhill L, Shen S, Mann JJ, 2011. The Columbia-Suicide Severity Rating Scale: Initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am. J. Psychiatry 168, 1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S, Hitsman B, Veluz-Wilkins A, Blazekovic S, Brubaker TR, Leone F, Hole A, Wileyto EP, Langer C, Kalhan R, Patel J, Schnoll R, 2017. The use of varenicline to treat nicotine dependence among patients with cancer. Psychooncology 26, 1526–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposeiras-Roubin S, Abu-Assi E, Iniguez-Romo A, 2017. Tobacco, illicit drugs use and risk of cardiovascular disease in patients living with HIV. Curr. Opin. HIV AIDS 12, 523–527. [DOI] [PubMed] [Google Scholar]
- Reddy KP, Parker RA, Losina E, Baggett TP, Paltiel AD, Rigotti NA, Weinstein MC, Freedberg KA, Walensky RP, 2016. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: A US-based modeling study. J. Infect. Dis 214, 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll R, Leone F, Veluz-Wilkins A, Miele A, Hole A, Jao NC, Paul Wileyto E, Carroll AJ, Kalhan R, Patel J, Langer C, Lubitz SF, Hitsman B, 2019. A randomized controlled trial of 24 weeks of varenicline for tobacco use among cancer patients: Efficacy, safety, and adherence. Psycho-Oncology Epub, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Goelz PM, Veluz-Wilkins A, Blazekovic S, Powers L, Leone FT, Gariti P, Wileyto EP, Hitsman B, 2015. Long-term nicotine replacement therapy: A randomized clinical trial. JAMA Intern. Med 175, 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, Lerman C, 2010. Effectiveness of extended-duration transdermal nicotine therapy: A randomized trial. Ann. Intern. Med 152, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Thompson M, Serrano K, Leone F, Metzger D, Frank I, Gross R, Mounzer K, Tyndale RF, Weisbrot J, Meline M, Collman RG, Ashare RL, 2018. Rate of nicotine metabolism and tobacco use among persons with HIV: Implications for treatment and research. J. Acquir. Immune Defic. Syndr Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah D, Shah A, Tan X, Sambamoorthi U, 2017. Trends in utilization of smoking cessation agents before and after the passage of FDA boxed warning in the United States. Drug Alcohol Depend 177, 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC, 1998. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59 Suppl 20, 22–57. [PubMed] [Google Scholar]
- Shelley D, Tseng TY, Gonzalez M, Krebs P, Wong S, Furberg R, Sherman S, Schoenthaler A, Urbina A, Cleland CM, 2015. Correlates of adherence to varenicline among HIV+ smokers. Nicotine Tob. Res 17, 968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling LH, Windle SB, Filion KB, Touma L, Eisenberg MJ, 2016. Varenicline and adverse cardiovascular events: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner JA, Tyndale RF, 2017. Variation in CYP2A6 activity and personalized medicine. J. Pers. Med 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR, Varenicline Phase 3 Study Group, 2006. Effect of maintenance therapy with varenicline on smoking cessation: A randomized controlled trial. JAMA 296, 64–71. [DOI] [PubMed] [Google Scholar]
- Transdisciplinary Tobacco Use Research Center Tobacco Dependence, Baker TB, Piper ME, McCarthy DE, Bolt DM, Smith SS, Kim SY, Colby S, Conti D, Giovino GA, Hatsukami D, Hyland A, Krishnan-Sarin S, Niaura R, Perkins KA, Toll BA, 2007. Time to first cigarette in the morning as an index of ability to quit smoking: Implications for nicotine dependence. Nicotine Tob. Res 9, S555–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TY, Krebs P, Schoenthaler A, Wong S, Sherman S, Gonzalez M, Urbina A, Cleland CM, Shelley D, 2016. Combining text messaging and telephone counseling to increase varenicline adherence and smoking abstinence among cigarette smokers living with HIV: A randomized controlled study. AIDS Behav 21, 1964–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiathan R, Miguez MJ, Patel B, Arheart KL, Asthana D, 2014. Tobacco smoking increases immune activation and impairs T-cell function in HIV infected patients on antiretrovirals: A cross-sectional pilot study. PLoS One 9, e97698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandeler G, Johnson LF, Egger M, 2016. Trends in life expectancy of HIV-positive adults on antiretroviral therapy across the globe: Comparisons with general population. Curr. Opin. HIV AIDS 11, 492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger AH, Seng EK, Esan H, Shuter J, 2018. Perceived risks and benefits of quitting smoking in a sample of adults living with HIV/AIDS. AIDS Care 30, 564–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Gilbody S, Peckham E, Brabyn S, Parrott S, 2016. Varenicline for smoking cessation and reduction in people with severe mental illnesses: Systematic review and meta-analysis. Addiction 111, 1554–1567. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.