Abstract
Background.
Kaposiform lymphangiomatosis (KLA) is a rare lymphatic anomaly with significant morbidity and mortality. KLA is characterized by diffuse multifocal lesions comprised of focal areas of “kaposiform” spindled cells accompanying malformed lymphatic channels. The goal of this study was to identify activated signaling pathways in cells isolated from three KLA patients for the purpose of testing new therapies.
Procedure.
Cells were obtained from the lungs of one patient isolated at autopsy and the spleen of two patients removed in surgery due to disease complications. A protein kinase array was performed on the KLA cell lysates and normal lymphatic endothelial cells.
Results.
Higher activation of key signaling pathways in the KLA cells, including PRAS40, AKT1/2/3 and ERK-1/2, was identified by protein kinase array and confirmed by western blot analysis. This indicated a role for highly activated PI3 Kinase (PI3K) - AKT and mitogen activated protein kinase (MAPK)/ERK-1/2 signaling pathways in KLA cells. Cell proliferation studies assessed PI3K inhibitors (LY294002; BYL719), AKT inhibitor ARQ092, mTOR inhibitor rapamycin and MAPK inhibitor U0126. These studies demonstrated that PI3K-AKT-mTOR and MAPK signaling are important mediators of KLA cell proliferation. BYL719 and rapamycin were more effective at inhibiting KLA cell proliferation than U0126.
Conclusions.
Our studies using cells from KLA patient lesions demonstrate that these cells are highly proliferative and the PI3K-AKT-mTOR and MAPK pathways are promising therapeutic targets. Development and clinical trials of PI3K, AKT, and MAPK inhibitors for cancer treatment and the data in this study lend support for early clinical trials assessing the efficacy of these inhibitors in KLA patients.
Keywords: Vascular Malformations; All, molecular diagnosis & therapy; Pharmacology
INTRODUCTION
Lymphatic malformations (LM) are vascular anomalies that affect infants and children of any age and gender. Kaposiform lymphangiomatosis (KLA) is a recently identified1 rare LM that may be a subtype and/or more severe form of generalized lymphatic anomaly (GLA). The pathology of KLA is characterized by multifocal areas of spindle-shaped “endothelial-like” cells in sheets/clusters that are associated with abnormal diffuse lymphatic channels1. Patients with KLA suffer from high morbidity and mortality with 51% survival after 5 years2 and overall survival rate of 34%1. This poor prognosis is due to a constellation of life-threatening symptoms including pleural and/pericardial effusions and ascites, consumptive coagulopathy, and hemorrhage1,3. Pulmonary and abdominal involvement is especially associated with poor outcome. Diagnosis and therefore treatment of KLA is often delayed as the disease is rare, usually misdiagnosed, and thus needs highly specialized expertise in imaging and pathology, with biopsy carrying considerable risk for bleeding. A number of different treatments have been used in KLA patients, although the most widely used to date is the mTOR inhibitor sirolimus (rapamycin)2,4. Unfortunately not every KLA patient responds5 to sirolimus and some experience rebound disease if therapy is discontinued. Hence there is a significant need for additional therapeutic options which will require a better understanding of the etiology of KLA.
The aim of this study was to determine key activated signaling pathways in cells from KLA patients and so aid identification of new therapeutic targets. Cells isolated from the lung and spleens of KLA patients were utilized6. The ability to study these cells, even though they only represent three patients, is a valuable opportunity as KLA is rare and cells have only been isolated from a few patients since this disease was first identified. A protein kinase array of KLA cell lysates was performed and this identified a number of activated kinases and related pathways including phosphoinositide 3-kinase (PI3K) and mitogen activated protein kinase (MAPK). The role of these pathways in KLA cell proliferation was tested using inhibitors of PI3K/AKT, mTOR and MAPK/ERK pathways. We used some standard inhibitors of PI3K (LY290042), mTOR (rapamycin) and ERK (U0126), as well as a new PI3Kα-selective inhibitor BYL719 (Alpelisib)7 and a pan-AKT (protein kinase B) inhibitor ARQ092 (Miransertib)8. BYL719 and ARQ092, have shown some early promise in pre-clinical studies and early phase of clinical trials 7,9–11.
METHODS
Patient Characteristics
All diagnoses were confirmed by radiologic and histopathologic examination. Table 1 lists information on the KLA patients. Patient samples were obtained with written informed consent in accordance with Institutional Review Board guidelines and approval. De-identified tissue was obtained from patients undergoing surgical procedures or postmortem. Tissues from external centers was shipped on ice to Cincinnati Children’s Hospital Medical Center by overnight carrier and then processed for cell isolation immediately upon arrival.
TABLE 1.
KLA Patient Information
| Patient Number | Tissue Used to Isolate Cells | Center | Age | Sex |
|---|---|---|---|---|
| 1607 | Lung | Texas Children’s | 14 years | Female |
| 1528 | Spleen | Cincinnati Children’s | 5 years | Male |
| 2360 | Spleen | UCSF* | 10 months | Female |
University of California San Francisco
KLA Cell Isolation and Characterization
For cell isolation minced tissue was homogenized enzymatically with 0.2% collagenase type I (Worthington Biochemical Corp.) in Dulbecco Modified Eagle Medium and passed through a 100 µM nylon sieve6. Cells were collected by centrifugation and expanded on fibronectin-coated plastic. Normal human neonatal dermal lymphatic endothelial cells (LEC) were obtained from Lonza (catalog number CC-2812). LEC and KLA cells were grown in endothelial basal medium (EBM™-2) (Lonza; catalog number CC-3156) plus endothelial growth factors kit (Lonza; EGM™-2 MV SingleQuots™ supplements CC-4147) and fetal bovine serum (FBS) at 20% (v/v). This media is hereafter referred to as endothelial growth medium (EGM-2MV). Cells were used between passages 7–20.
Protein Kinase Array and Western Blot Analysis
Protein kinase activity was analyzed using a human phospho-kinase array (R&D systems; catalog number ARY003B) to identify signaling pathways that were especially active in the KLA cells compared to LEC. Cell lysates (375μg protein) were prepared from confluent cells cultured in EGM-2MV media. Array assays were performed according to manufacturer’s protocol and imaged using Kodak X-ray film. Films were imaged on a scanner and signals quantitated by densitometry. Results from the array were confirmed by performing western blot analysis on cell lysates with antibodies for phospho-AKT (Cell Signaling Technology; Ser473 catalog number 4060 and Thr308 catalog number 2965), phospho-Proline-Rich AKT Substrate of 40 kDa (Cell Signaling Technology; p-PRAS40 Thr246; catalog number 22997), phospho-p44/42 MAPK (ERK1/2)(Cell Signaling Technology; catalog number 9101). Total AKT (Cell Signaling Technology; catalog number 9272), PRAS40 (Cell Signaling Technology; catalog number 2691), ERK-1/2 (Cell Signaling Technology; Cell Signaling Technology; catalog number 9102), and C4 actin (Chemicon; catalog number MAB1501) were also assessed.
Cell proliferation and PI3K/AKT/mTOR and MAPK/ERK Inhibitors
Proliferation studies were conducted comparing KLA cells to LEC. Cells were plated in 96 well plates (6×105 cells per well) coated with fibronectin in EGM-2MV media. Medium was replaced 12 hours after plating the cells and this was designated time zero (T=0). Proliferation was measured using the Sulforhodamine B (SRB) method12 at 0, 24, 48, and 72 hours. For the inhibitor studies cells were plated in 96 well plates (8×105 cells per well) coated in fibronectin in EGM-2MV media. The media was replaced 5 hours after plating (Time 0) with EGM-2MV media containing inhibitors or the vehicle control (dimethyl sulfoxide; DMSO). Inhibitors to PI3K (LY294002 10μM; BYL719 10μM), AKT/protein kinase B (ARQ092 5 & 10μM), MAPK (U0126 10μM), rapamycin (sirolimus)(15nM) dissolved in DMSO were used. Inhibitors were purchased from Selleckchem (LY294002 catalog number 15447–36-6; BYL719 catalog number 1217486–61-7; ARQ092 catalog number 1313883–00-9; U0126 catalog number 1173097–76-1) or LC Laboratories (Rapamycin; catalog number R-5000). DMSO was added to control cell wells to match the volume used for inhibitors (0.2% vol/vol). Cell numbers were measured 72 hours after plating using the SRB method12. Western blot analysis was also performed on cell lysates 1 hour after treatment with inhibitors to assess inhibitory effects on p-AKT and p-ERK1/2.
DNA sequencing.
The presence of common PIK3CA mutations in the KLA cells was assessed by DNA Sanger sequencing as previously described13. Briefly, amplified DNA product was generated by PCR using primers for PIK3CA exons 7, 9, and 20 (Integrated DNA Technologies). PCR products were sequenced at the CCHMC DNA Sequencing and Genotyping Core. The NRAS gene was analyzed for mutations using molecular inversion probe sequencing14.
Data and Statistical Analysis
Graphing and statistical analysis were performed using Prism 7 software. Cell proliferation time course data was converted to show fold changes from baseline and then analyzed in log-scale to counter for skewness. Two-way Analysis of Variance was performed with cell group and time point as factors, along with their interaction. For the inhibitor studies, mean percent inhibition of cell proliferation was calculated relative to vehicle control. Confidence intervals (95%) for the means were calculated to show the magnitude and certainty of the inhibition. One-way ANOVA and Tukey’s multiple comparison method was used to compare the effects of the different pharmacologic inhibitors on KLA cell proliferation.
RESULTS
Patient Characteristics and Cell Isolation
Table 1 shows summary data for 3 KLA patients seen at different US Children’s Hospitals. All patients had been treated with prednisone and sirolimus. In addition, patient 1607 had also been treated with vincristine. Cells were isolated at post-mortem from the lungs of a 14-year-old female patient (1607) with KLA involvement of multiple organs. Cells were derived from the spleens of a 5-year-old male (1528) and 10 month-old female (2360) at surgery.
KLA Cell Characteristics
Cells from the three KLA patients are shown at near confluence along with normal lymphatic endothelial cells (LEC)(Fig. 1). Immunostaining of fixed KLA cells and LEC for D2–40 (podoplanin) is shown in Fig. 1. KLA cells were spindle-shaped under phase contrast and stained positive for D2–40 (podoplanin), as previously reported 6.
Fig. 1.
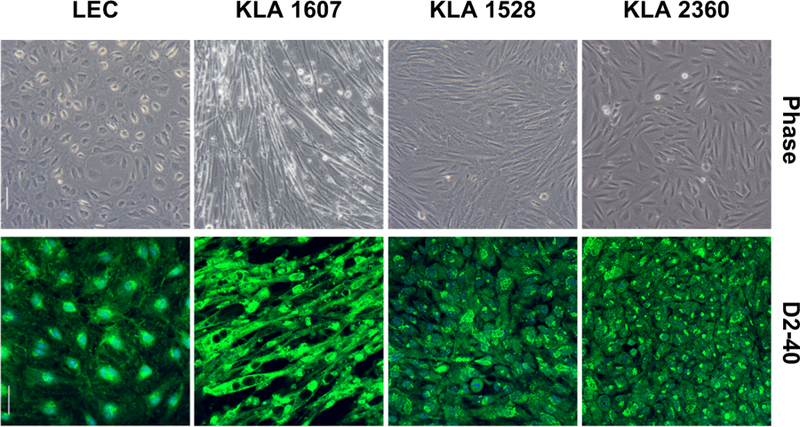
Cells from KLA patients and neonatal human lymphatic endothelial cells (LEC) cultured on fibronectin-coated plates. Phase contrast pictures were taken using a 10X objective, scale bar is 200 μM. Pictures of immunostaining for D2–40 (podoplanin) immunofluorescence pictures were taken using a 40X objective, scale bar is 50 μM.
Protein Kinase Array and Western Blot Verification
Supplemental Fig. 1A shows a representative kinase array blot using cell lysates from LEC and KLA 1607 cells (unstimulated). Examples of protein targets that were elevated in KLA patient cells compared to LEC are shown. After densitometry, histograms of the data (Supplemental Fig. 1B) showed higher levels of phosphorylated proteins in KLA cells relative to LEC that included PRAS40 (Thr246), AKT1/2/3 (Ser473) and ERK1/2 (Thr202/Tyr182, Thr185/Tyr187). For p-HSP60 only one KLA cell type (1607) showed elevated levels and so this was not pursued further. Western blot analysis was used to confirm that activation levels of PRAS40, AKT and ERK1/2 were higher in KLA cells than LEC (Fig. 2). Greater signal for p-AKT (Ser473 and Thr308), p-PRAS40 (Thr246), p-ERK1/2 were confirmed in all 3 KLA cells. Total PRAS40 levels also were higher in KLA cells. While p-AKT Thr308 was not elevated in the kinase array, on western blot analysis it was higher than LEC although more variable between the KLA patients than p-AKT Ser473.
Fig. 2.
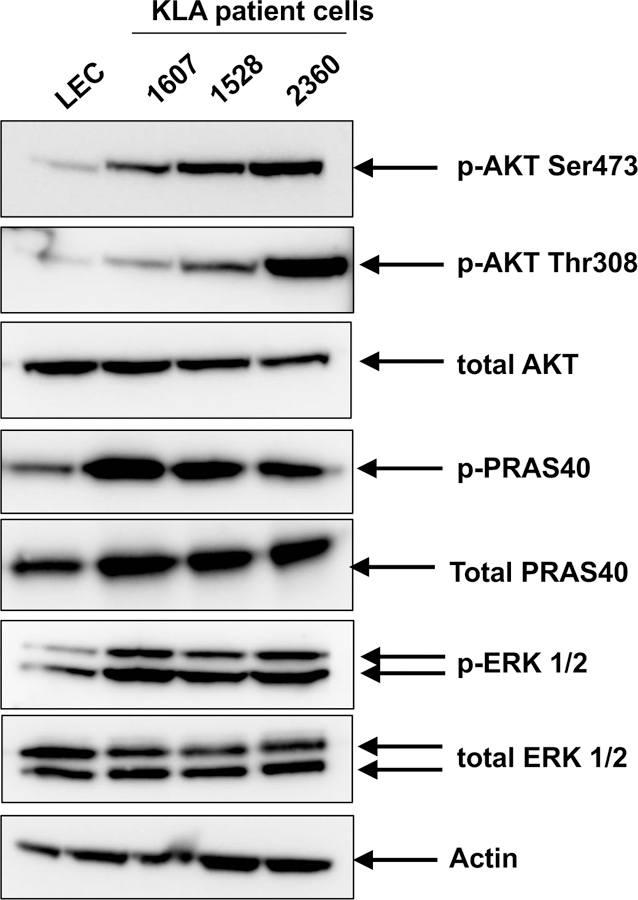
AKT and ERK phosphorylation in cells from KLA patients and neonatal human lymphatic endothelial cells (LEC). Western blot analysis was performed on cell lysates (20μg total protein) from KLA cells and LEC. Blots were probed for phosphorylated-AKT (p-Ser473 and p-Thr308), total AKT, p-PRAS40 (downstream target of AKT), total PRAS40, p-ERK1/2, total ERK1/2 and actin.
Cell Proliferation and Signaling Pathway Inhibitors
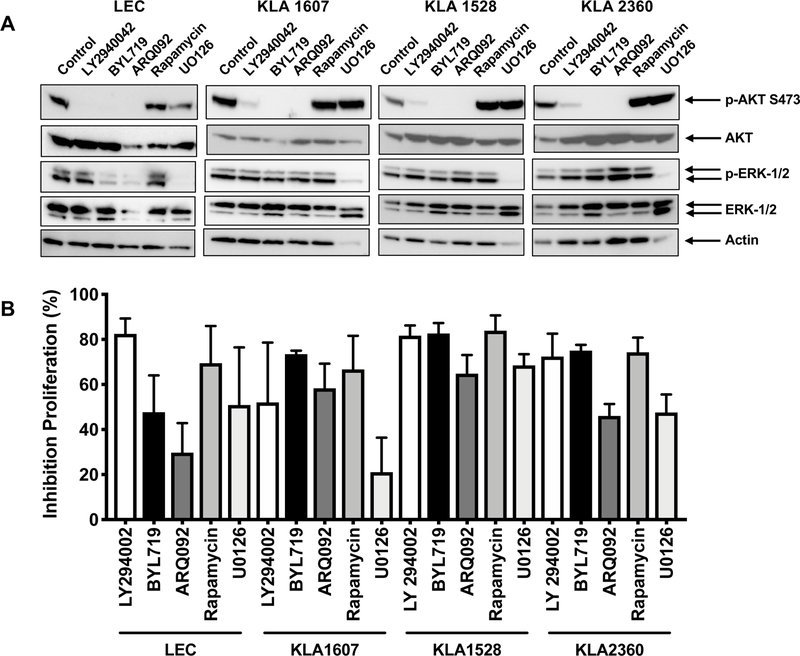
Cell proliferation studies were used to assess the proliferation rates of the KLA cells in 20% FBS (Fig. 3). The KLA cell group had a significantly higher proliferation rate than LEC cells (p=0.0003). There was also a significant increase in proliferation with time (p=0.0063). Next, we assessed the effects of PI3K, AKT, mTOR and MAPK inhibitors on the activated pathways and cell proliferation. Fig. 4A shows western blot analysis 1 hour after treatment to assess the inhibition of p-AKT and p-ERK levels by the inhibitors. The PI3K/AKT inhibitors LY290042, BYL719, ARQ092 ablated AKT phosphorylation while U0126 decreased ERK-1/2 activation in all the KLA cells analyzed and LEC. Furthermore, LY290042, BYL719, ARQ092, rapamycin and the MAPK inhibitor U0126 prevented KLA and LEC cell proliferation (Fig. 4B). Mean inhibition (percentage) and confidence intervals indicate that there was significant inhibition of proliferation with the inhibitors used to treat the KLA cells (Table 2). In our preliminary studies 10μM ARQ092 completely blocked cell proliferation and so 5μM was used for all studies reported here. By Tukey’s multiple comparison method, we found that inhibition of proliferation of KLA cells with BYL719 and rapamycin treatments were significantly different from U0126 (p=0.0163 and p=0.0289, respectively). There was no evidence of significance among the other groups. In summary, all the inhibitors tested efficiently inhibited proliferation of KLA cells, with BYL719 and rapamycin more effective than U0126.
Fig. 3.
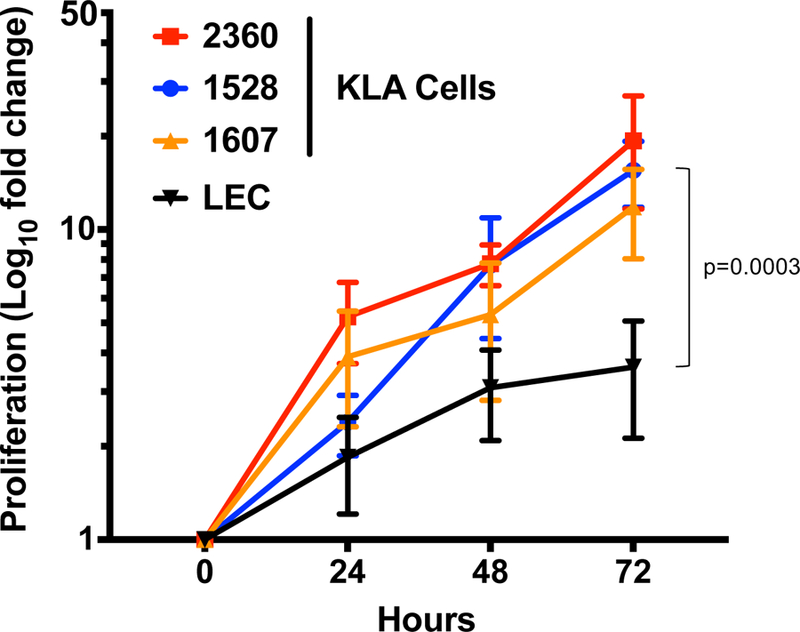
Proliferation of cells from KLA patients and human lymphatic endothelial cells (LEC). Cells were cultured in 20% FBS in EGM-2MV media on fibronectin-coated 96 well plates. Cell number was assessed at 0, 24, 48 and 72 hours using SRB assay. Cell numbers are expressed as Log10 of fold change in cell number compared to time 0. Data represents the results from 3 independent experiments.
Fig. 4.
A. Inhibition of AKT and ERK1/2 phosphorylation in KLA cells and human lymphatic endothelial cells (LEC) treated with inhibitors. Western blot analysis was performed on cell lysates (10μg total protein) from KLA cells (1607, 1528, 2360) and LEC, after treatment (1 hour) with either DMSO vehicle (control), PI3K inhibitors LY294002 (10μM) and BYL719 (10μM), AKT inhibitor ARQ092 (5μM), rapamycin (15nM) or MAPK inhibitor U0126 (10μM). Blots were probed with antibodies to phosphorylated-AKT (pSer473), total AKT, p-ERK1/2, total ERK and actin. B. KLA cells and LEC were plated on fibronectin-coated plates in EGM-2MV media. After plating (5 hours) the media was changed to media containing either DMSO vehicle (control), PI3K inhibitors LY294002 (10μM) and BYL719 (5μM), AKT inhibitor ARQ092 (5μM), rapamycin (15nM) or MAPK inhibitor U0126 (10μM). Proliferation was measured using SRB assay 72 hours after treatment with inhibitors. Data represents the results from 3 independent experiments and show percent inhibition compared to vehicle control.
TABLE 2.
Inhibition of Cell Proliferation
| KLA Cells | LY294002 10μM | BYL719 10μM | ARQ092 5μM | Rapamycin 15nM | U0126 10μM |
|---|---|---|---|---|---|
| Mean Inhibition (%) | 69 | 77* | 56 | 75** | 46 |
| Std. Deviation | 28 | 6 | 15 | 17 | 26 |
| 95% CI of mean | 47–90 | 72–82 | 45–68 | 62–88 | 26–66 |
BYL719 is significantly different from U0126 p=0.0163 (Tukey’s method)
Rapamycin is significantly different from U0126 p=0.0289 (Tukey’s method)
Mutation Analysis
No hotspot mutations in PIK3CA (C420 mutation in exon 7, E542, E545, E546 mutations in exon 9, and H1047 mutations in exon 20) were found in the KLA cells. All samples were sequenced to a depth across the entire NRAS coding sequence of >1000X and no missense mutations affecting residues p.G12, pG13, or pQ61 were observed at greater than the background rate for this technique.
DISCUSSION
The cellular basis for KLA are not well understood although both abnormal LEC channels and “spindle cells” that express LEC markers are characteristic of the histopathology of patient lesions. In a study by Glaser et al6, cells derived from KLA patients were found to be highly proliferative compared to adipose-derived mesenchymal stem cells (ADSCs). In our study we utilized KLA cells from two of the patients in the Glaser study6, (KLA 1 and KLA2) (1528 cells from spleen and for patient 1607 cells were from the lung), as well as cells derived from the spleen of an additional KLA patient (2360). We compared KLA cells to LEC since they have some similarities to the spindle cells in KLA lesions. While the choice of a normal cell type to compare the KLA cells to is a limitation, the main goal of our study was to identify key signaling pathways mediating proliferation of cells from KLA patient lesions to identify potential new therapeutic targets. Our kinase array study and verification with western blot analysis showed high AKT activation as well as MAPK. Hyperactive PI3K-AKT-mTOR signaling has also been found in a number of vascular malformations14–19.
We did not find any of the common PIK3CA mutations in the KLA cells that have been found in vascular anomalies associated with tissue overgrowth including Klippel-Trenaunay syndrome (KTS) and congenital lipomatous overgrowth vascular malformations epidermal nevi and scoliosis/skeletal/spinal anomalies (CLOVES)15–19. In the Glaser study6, whole exome sequencing of KLA-derived cells identified a potential pathogenic variant in one KLA patient (which is patient 1607 in our study), a 3-base pair deletion resulting in loss of Serine 1043 in tuberous sclerosis complex 1 (TSC1). TSC1 is an inhibitor of mTOR an important signaling mediator downstream of PI3K/AKT a major growth/survival pathway in angiogenesis. While the exact significance of the deletion in TSC1 that Glaser et al6 found is unclear, our studies indicate that the PI3K/AKT/mTOR pathway is very active in KLA cells. We also found high phosphorylation of PRAS40 in KLA cells. PRAS40 is downstream target of AKT, a component of the mTOR complex 1 (mTORC1) that regulates mTOR activity, and when phosphorylated dissociates from mTORC1 so that it can function20. PRAS40 is increasingly used as a biomarker of AKT activation in tumors21. The mTOR inhibitor sirolimus (rapamycin) has been used to treat KLA patients with a partial response in KLA patients2. Of the KLA patients who responded to therapy, all continued sirolimus treatment without any disease progression although long-term efficacy in these patients is still unclear. While sirolimus is now commonly used to treat KLA patients, disease symptoms can rebound if patients discontinue treatment. Recently Barclay et al22 identified an NRAS activating mutation p.Q61R in tissue from KLA patients. This NRAS mutation can increase MAPK and PI3K signaling. While the NRAS variant was present in the tissue of the majority of patients analyzed in Barclay’s study22, the allelic frequency ranged from 1% to 28% and so not all cells in the lesions had the mutation. It remains unclear what cell type(s) in the KLA lesions harbor the NRAS mutation and also which cell type actually drives the disease process although spindle cells and abnormal LEC are potential contributors1,3. We did not find the NRAS gene mutation (p.Q61R) in the cells from the KLA patients in our study. However, the PI3K and MAPK signaling pathways were contributing to cell proliferation suggesting a common mechanism.
A number of PI3K inhibitors have been developed and are in clinical trials for cancer therapeutics23. These include pan-PI3K and PI3K isoform-selective inhibitors; PI3K inhibitor that is selective for the δ isoform, Idelalisib, is now approved for treatment of chronic lymphocytic leukemia, relapsed follicular B-cell non-Hodgkin’s lymphoma and relapsed small lymphocytic lymphoma. BYL719 (Alpelisib) is a specific inhibitor of the PI3K isoform α (encoded by PIK3CA gene) that has shown some early promise in PI3K-altered solid tumors7 and in patients with CLOVES11. ARQ092 (Miransertib) is a new allosteric inhibitor of AKT that is orally bioavailable and has a manageable safety profile in patients with advanced solid tumors. Studies using fibroblasts from PROS patients showed that ARQ092 had good antiproliferative activity 24 and has now been granted “Fast Track” designation by the Food and Drug Administration for treatment of PROS as well as Rare Pediatric Disease designation for treatment of Proteus syndrome25. Based on this promising background we thought that it might be beneficial to evaluate this new drug in cells from KLA patients to generate data of pre-clinical interest since these cells have hyperactive AKT. We found that both BLY719 and ARQ092 inhibitors were effective at inhibiting growth of KLA cells at similar doses to LY294002, that is an older generation PI3K inhibitor. BYL719 and rapamycin were also significantly better at inhibiting KLA cell proliferation than the MAPK inhibitor U0126.
In conclusion, our studies using cells from KLA patient lesions demonstrate that they are highly proliferative and that inhibition of PI3K and AKT may represent potential therapeutic targets. Novel findings are the identification of the signaling pathways that mediate KLA cell proliferation and the use of inhibitors that are in clinical trials for other cancer therapy, as well as vascular anomalies and overgrowth syndromes. The data from this study may lend support for the pre-clinical testing of these novel PI3K and AKT inhibitors in KLA patients. Future studies may also need to include dual inhibitor agents if the toxicity profile is adequate.
Supplementary Material
ACKNOWLEDGEMENT
Funding for this project was provided by the Lymphatic Malformation Institute (LMI)(T.D.L.). Research reported in this manuscript was also supported by the National Heart, Lung, and Blood Institute, under Award Number R01 HL117952 (E.B.), part of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Paula Mobberley-Schuman and Megan Metcalf for help consenting patients to the study and Lin Fei for advice with statistical analysis and the CCHMC DNA Sequencing and Genotyping Core. We thank Drs. M.L. Warman, J.A. Goss, and A.Y. Huang at Boston Children’s Hospital for performing molecular inversion probe sequencing of NRAS.
ABBREVIATIONS KEY
- KLA
Kaposiform lymphangiomatosis
- PI3K
phosphoinositide 3-kinase
- MAPK
mitogen activated protein kinase
- GLA
generalized lymphatic anomaly
- FBS
fetal bovine serum
- EGM-2MV
endothelial growth medium
- SRB
Sulforhodamine B
- DMSO
dimethyl sulfoxide
- α–SMA
α–smooth muscle actin
- ADSCs
adipose-derived mesenchymal stem cells
- KTS
Klippel-Trenaunay syndrome
- CLOVES
congenital lipomatous overgrowth, vascular malformations, epidermal nevi and scoliosis/skeletal/spinal anomalies
- TSC1
tuberous sclerosis complex 1
- PRAS40
phospho-Proline-Rich AKT Substrate of 40 kDa
- mTORC1
mTOR complex 1
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have nothing to declare.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Croteau SE, Kozakewich HP, Perez-Atayde AR, et al. Kaposiform lymphangiomatosis: a distinct aggressive lymphatic anomaly. J Pediatr 2014;164:383–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams DM, Trenor CC 3rd, Hammill AM, et al. Efficacy and Safety of Sirolimus in the Treatment of Complicated Vascular Anomalies. Pediatrics 2016;137:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fernandes VM, Fargo JH, Saini S, et al. Kaposiform lymphangiomatosis: unifying features of a heterogeneous disorder. Pediatr Blood Cancer 2015;62:901–4. [DOI] [PubMed] [Google Scholar]
- 4.Wang Z, Li K, Yao W, Dong K, Xiao X, Zheng S. Successful treatment of kaposiform lymphangiomatosis with sirolimus. Pediatric blood & cancer 2015;62:1291–3. [DOI] [PubMed] [Google Scholar]
- 5.Triana P, Dore M, Cerezo VN, et al. Sirolimus in the Treatment of Vascular Anomalies. Eur J Pediatr Surg 2017;27:86–90. [DOI] [PubMed] [Google Scholar]
- 6.Glaser K, Dickie P, Dickie BH. Proliferative Cells From Kaposiform Lymphangiomatosis Lesions Resemble Mesenchyme Stem Cell-like Pericytes Defective in Vessel Formation. Journal of pediatric hematology/oncology 2018;40:e495–e504. [DOI] [PubMed] [Google Scholar]
- 7.Juric D, Rodon J, Tabernero J, et al. Phosphatidylinositol 3-Kinase alpha-Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J Clin Oncol 2018;36:1291–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapierre JM, Eathiraj S, Vensel D, et al. Discovery of 3-(3-(4-(1-Aminocyclobutyl)phenyl)-5-phenyl-3H-imidazo[4,5-b]pyridin-2-yl)pyridin −2-amine (ARQ 092): An Orally Bioavailable, Selective, and Potent Allosteric AKT Inhibitor. J Med Chem 2016;59:6455–69. [DOI] [PubMed] [Google Scholar]
- 9.Lindhurst MJ, Yourick MR, Yu Y, Savage RE, Ferrari D, Biesecker LG. Repression of AKT signaling by ARQ 092 in cells and tissues from patients with Proteus syndrome. Scientific reports 2015;5:17162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain S, Shah AN, Santa-Maria CA, et al. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast cancer research and treatment 2018;171:371–81. [DOI] [PubMed] [Google Scholar]
- 11.Venot Q, Blanc T, Rabia SH, et al. Targeted therapy in patients with PIK3CA-related overgrowth syndrome. Nature 2018;558:540–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orellana EA, Kasinski AL. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio Protoc 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goines J, Li X, Cai Y, et al. A xenograft model for venous malformation. Angiogenesis 2018;21:725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luks VL, Kamitaki N, Vivero MP, et al. Lymphatic and other vascular malformative/overgrowth disorders are caused by somatic mutations in PIK3CA. J Pediatr 2015;166:1048–54 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keppler-Noreuil KM, Parker VE, Darling TN, Martinez-Agosto JA. Somatic overgrowth disorders of the PI3K/AKT/mTOR pathway & therapeutic strategies. Am J Med Genet C Semin Med Genet 2016;172:402–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keppler-Noreuil KM, Rios JJ, Parker VE, et al. PIK3CA-related overgrowth spectrum (PROS): diagnostic and testing eligibility criteria, differential diagnosis, and evaluation. Am J Med Genet A 2015;167A:287–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Lopez A, Blasco-Morente G, Perez-Lopez I, et al. CLOVES syndrome: review of a PIK3CA-related overgrowth spectrum (PROS). Clin Genet 2017;91:14–21. [DOI] [PubMed] [Google Scholar]
- 18.Vahidnezhad H, Youssefian L, Uitto J. Klippel-Trenaunay syndrome belongs to the PIK3CA-related overgrowth spectrum (PROS). Exp Dermatol 2016;25:17–9. [DOI] [PubMed] [Google Scholar]
- 19.Yeung KS, Ip JJ, Chow CP, et al. Somatic PIK3CA mutations in seven patients with PIK3CA-related overgrowth spectrum. Am J Med Genet A 2017;173:978–84. [DOI] [PubMed] [Google Scholar]
- 20.Wiza C, Nascimento EB, Ouwens DM. Role of PRAS40 in Akt and mTOR signaling in health and disease. Am J Physiol Endocrinol Metab 2012;302:E1453–60. [DOI] [PubMed] [Google Scholar]
- 21.Lv D, Guo L, Zhang T, Huang L. PRAS40 signaling in tumor. Oncotarget 2017;8:69076–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barclay SF, Inman KW, Luks VL, et al. A somatic activating NRAS variant associated with kaposiform lymphangiomatosis. Genet Med 2018. [DOI] [PMC free article] [PubMed]
- 23.Massacesi C, Di Tomaso E, Urban P, et al. PI3K inhibitors as new cancer therapeutics: implications for clinical trial design. Onco Targets Ther 2016;9:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ranieri C, Di Tommaso S, Loconte DC, et al. In vitro efficacy of ARQ 092, an allosteric AKT inhibitor, on primary fibroblast cells derived from patients with PIK3CA-related overgrowth spectrum (PROS). Neurogenetics 2018;19:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosa K Miransertib Granted Fast Track Designation for Treatment of PIK3CA-Related Overgrowth. RareDiseaseReport2018:https://www.raredr.com/news/miransertib-granted-fast-track-designation-for-treatment-of-pik3ca-related-overgrowth.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.