Abstract
Neuroinflammatory activation of glia is considered a pathological hallmark of Parkinson’s disease (PD) and is seen in both human PD patients and in animal models of PD; however, the relative contributions of these cell types, especially astrocytes, to the progression of disease is not fully understood. The transcription factor, nuclear factor kappa B (NFκB), is an important regulator of inflammatory gene expression in glia and is activated by multiple cellular stress signals through the kinase complex, IKK2. We sought to determine the role of NFκB in modulating inflammatory activation of astrocytes in a model of PD by generating a conditional knockout mouse (hGfapcre/Ikbk2F/F) in which IKK2 is specifically deleted in astrocytes. Measurements of IKK2 revealed a 70% deletion rate of IKK2 within astrocytes, as compared to littermate controls (Ikbk2F/F). Use of this mouse in a subacute, progressive model of PD through exposure to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp) revealed significant protection in exposed mice to direct and progressive loss of dopaminergic neurons in the substantia nigra (SN). hGfapcre/Ikbk2F/F mice were also protected against MPTPp-induced loss in motor activity, loss of striatal proteins, and genomic alterations in nigral NFκB gene expression, but were not protected from loss of striatal catecholamines. Neuroprotection in hGfapcre/Ikbk2F/F mice was associated with inhibition of MPTPp-induced astrocytic expression of inflammatory genes and protection against nitrosative stress and apoptosis in neurons. These data indicate that deletion of IKK2 within astrocytes is neuroprotective in the MPTPp model of PD and suggests that reactive astrocytes directly contribute the potentiation of dopaminergic pathology.
Keywords: Neuroinflammation, NFκB, astrocytes, MPTP, IKK2, transgenic models
Introduction
Activation of glial cells is implicated in the progression of neuronal loss in neurodegenerative disorders such as Parkinson’s disease (PD) through the upregulation and release of pro-inflammatory and pro-oxidant factors that exert toxic effects on surrounding neurons (Hirsch and Hunot, 2009). Neuroinflammation is seen in both human patients and in animal models of PD, characterized by the presence of reactive astrocytes and microglia in affected brain regions that closely associate with protein aggregates (Damier et al., 1993; Nagatsu and Sawada, 2005; Ouchi et al., 2009). Several lines of evidence point to reactive glia being important participants in disease pathology, because upregulation of glial-derived factors such as tumor necrosis factor alpha (TNF) and inducible nitric oxide synthase 2 (NOS2) occurs prior to loss of dopaminergic neurons (Sugama et al., 2003; Hirsch and Hunot, 2009; Saijo et al., 2009; Miller et al., 2011) and alterations in familial PD genes can affect production of inflammatory cytokines (Dzamko et al., 2015). Experimental models in which these and other inflammatory factors are genetically or pharmacologically inhibited markedly protect against neurodegeneration (Dehmer et al., 2000; 2003; McCarty, 2006; Mondal et al., 2012). Yet, these studies have focused primarily on limiting microglia responses due to their early activation in the disease (Hirsch and Hunot, 2009), yielding hopeful targets for drug development that ultimately fail in clinical trials (Cheng et al., 2015). Additionally, although gliosis is associated with neuronal injury in PD (McCarty, 2006; Chen et al., 2009), glial responses can also be neuroprotective. In astrocytes this is linked with an A1 (neurotoxic) versus A2 (neuroprotective) phenotype (Frank-Cannon et al., 2009; Neal and Richardson, 2018; Liddelow et al., 2017), revealing time- and phenotype-dependent mechanisms in modulating neuronal function and survival.
Inflammatory signaling through the canonical (NFκB) pathway involves activation of inhibitory kappa alpha kinase beta (IKKβ/IKK2) and subsequent translocation of p65/p50 dimers to the nucleus that stimulate inflammatory gene expression through binding to cis-acting promoter elements (Karin, 1999; Bonizzi and Karin, 2004; Karin, 2005), which is critical for production of inflammatory mediators in glial cells (Glass et al., 2010). Activation of NFκB is directly associated with PD, as noted studies describe upregulation and nuclear translocation of NFκB/p65 in both neurons (Hunot et al., 1997) and glia of the PD brain (Ghosh et al., 2007) and in experimental animal models (Saijo et al., 2009). Studies have demonstrated that globally suppressing NFκB can be protective in neurotoxin-based models of PD (Dehmer et al., 2003; Ghosh et al., 2007; Saijo et al., 2009; Mondal et al., 2012; De Miranda et al., 2013) but these studies do not ascertain the specific cellular protective mechanisms and are difficult to translate to clinical applications due to detrimental effects from complete functional loss of NFκB (Grilli and Memo, 1999; Herrmann et al., 2005). Additionally, neuronal NFκB activation appears to protect neurons against degeneration (Mettang et al., 2018), whereas excess activation NFκB in glia promotes neurodegeneration (Mattson and Camandola, 2001; Brambilla et al., 2009; Zhang et al., 2017).
Previous studies in our laboratory utilizing a transgenic reporter mouse expressing green fluorescent protein under the control of NFκB enhancer elements reported activation of the pathway in astrocytes prior to an overt loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc; Miller et al., 2011). Other neurodegenerative models including multiple sclerosis, traumatic spinal cord injury, and Huntington’s disease have also shown that selective deletion of the NFκB pathway to be neuroprotective (Brambilla, 2005; van Loo et al., 2006; Brambilla et al., 2009) while constitutive activation to lead to high levels of basal inflammation (Oeckl et al., 2012;, Lian et al., 2012). Based on these previous studies, we postulated that cell-specific inhibition of inflammatory NFκB signaling in astrocytes would suppress a reactive phenotype and protect dopaminergic neurons in the MPTPp model of PD. As the phosphorylation of the IκB inhibitor complex by IKKβ/IKK2 is an important regulatory step in the classical inflammatory pathway of NFκB (Karin, 1999), we sought to test this hypothesis by utilizing Cre-loxP technology to delete IKKβ/IKK2 selectively in astrocytes to determine the role of this signaling factor in injury to dopaminergic neurons.
Materials and Methods
Animals and Genotyping
Astrocyte-specific Ikbk2-deficient mice (hGfapcre/Ikbk2F/F) were generated by breeding Ikbk2-floxed mice (Li et al., 2003); C57Bl/6 background; provided by Dr. Michael Karin at University of California San Diego) with hGfapcre transgenic mice (Zhuo et al., 2001); FVB-Tg(GFAP-CRE)25Mes/J; FVB background; Jackson Laboratories, Bar Harbor, ME) expressing Cre under the control of the human glial fibrillary acidic protein (GFAP) promoter. Mice were bred to homozygosity for the floxed-Ikbk2 allele and both male and female littermates from the 4th generation aged to 5 months were utilized in expression and treatment studies. The majority of animals were used in studies by 5 months of age to avoid the development of spontaneous inflammatory and neoplastic skin lesions associated with this genotype during aging, as we recently reported (Kirkley et al., 2017). Littermates lacking hGfapcre (known as Ikbk2F/F) were utilized as controls.
PCR genotyping on ear tags was performed using the primers 5’-GTC ATT TCC ACA GCC CTG TGA-3’ and 5’-CCT TGT CCT ATA GAA GCA CAA C-3’, that amplifies both the Ikbk2+ (220-bp) and Ikbk2F (310-bp) alleles and primers 5’-ACT CCT TCA TAA AGC CCT CG-3’ and 5’-ATC ACT CGT TGC ATC GAC CG-3’, to amplify the hGfapcre allele (190-bp). Animals were housed in microisolator cages (2–3 animals per cage), kept on a 12-h light/dark cycle, and had access to both chow and water ad libitum. All procedures were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals and with the approval by the Institutional Animal Care and Use Committee (IACUC) of Colorado State University.
Primary Glial and Neuronal Cultures
Mixed glial cultures from whole brain (excluding cerebellum and brain stem) of postnatal day 1 (neonatal) and 3-month old (adult) Ikbk2F/F and hGfapcre/Ikbk2F/F mice were isolated using a modification of a previously described method (Aschner and Kimelberg, 1991; Carbone et al., 2008; Moreno et al., 2008). Briefly, mice were euthanized by decapitation under isoflurane anesthesia and whole brains were rapidly dissected out and placed into ice-cold minimum essential medium with L-glutamine (MEM; Gibco). Meninges were removed, and tissues completely digested with dispase (1.5U/ml) with each animal extracted separately. Dissociated cells from individual animals were plated onto 100-mm tissue culture plates and kept in MEM supplemented with 10% heat-inactivated FBS (Sigma) and penicillin (0.002 mg/ml), streptomycin (0.002 mg/ml), and neomycin (0.001 mg/ml) antibiotic mixture (PSN). Media was changed every 4–5 days and cells were maintained at 37°C and 5% CO2 in humidified chambers until confluency was reached (~14–18 days). Microglia were purified from astrocytes via column-free magnetic separation using the EasySep mouse CD11b positive selection kit (Stemcell Technologies, Vancouver, Canada) according to manufacturer instructions and as described (Gordon et al., 2011; Kirkley et al., 2017) and determined to be 97% pure via flow cytometry.
Primary striatal neurons were extracted in a similar fashion as the mixed glial cultures except performed in neurobasal medium. Primary neuronal cultures were seeded onto poly(L-lysine)-coated 22mm glass coverslips at 4 × 105 cells/well and maintained in neurobasal media supplemented with 2mM L-glutamine, B27 supplement, and PSN antibiotic mixture (Gibco, Waltham, MA). Neuronal culture media was changed every 2 days with purity ascertained via cell morphology and immunolabeling with the neuron-specific marker microtubule-associated factor 2 (MAP2).
Flow Cytometry
The percent of glia in astrocyte cultures were determined by immunophenotyping using direct labeling with anti-GLAST-PE (Miltenyi Biotec, San Diego, CA), anti-Cd11b-FITC (BD Biosciences, San Jose, CA) followed by flow cytometric analysis as described (Kirkley et al., 2017). Briefly, purified cells were counted using a Bio-Rad TC10 automated cell counter, and 1 × 106 cells/mL were resuspended in 100 μL of incubation buffer (PBS with 0.05% bovine serum albumin). Purified cells were labeled using the mouse anti-GLAST-PE (20 μg/mL) and mouse anti-CD11b-FITC (10 μg/mL) at room temperature for 1 h. After labeling, the cells were washed twice in incubation buffer and resuspended at a final volume of 500 μL of PBS and stored at 37 °C until analysis. Flow cytometry was performed on a Beckman Coulter CyAn ADP flow cytometer operated with Summit software for data collection at Colorado State University’s Flow Cytometry Core Facility. All further data analysis was done utilizing FlowJo software (version 10.1; FlowJo, Ashland, OR).
Evaluation of Genomic Deletion of Ikbk2F/F via qPCR
Genomic DNA was isolated from neonatal and adult astrocyte cultures and neonatal microglia and striatal neuronal cultures utilizing a DNeasy kit (Qiagen, Valencia, CA) with purity and concentration confirmed using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE). 100 ng of DNA was mixed with Sybrgreen (Bio-Rad, Hercules, CA) and primers (10μM) 5’-AAG ATG GGC AAA CTG TGA TGT G-3’ and 5’-CAT ACA GGC ATC CTG CAG AAC A-3’ to amplify the Ikbk2F allele or 5’-ATG GCC TTG CAT GAG GAT ACA CCA-3’ and 5’-GAG TCT CAG TCT TCA ACT CCC TGT-3’ to amplify the Nos2 promoter which was utilized as a control. Percent expression of Ikbk2F in astrocytes, microglia, and neurons from hGfapcre/Ikbk2F/F mice was determined based on a comparison of Ikbk2F signal from Ikbk2F/F littermate controls, defined at 100%, after normalization to Nos2 promoter signal.
Measurement of IKK2 via Western Blot
Purified neonatal astrocyte cultures from Ikbk2F/F and hGfapcre/Ikbk2F/F and mice were lysed using a RIPA lysis buffer supplemented with complete protease inhibitor (Roche, Indianapolis, IN). Protein was quantified using a BCA Assay (Pierce, Rockford, IL) and 50μg of protein was separated using gel electrophoresis in a 10% SDS polyacrylamide gel followed by a wet transfer to a polyvinylidene fluoride membrane (Pall Corporation, Pensacola, FL). Membranes were blocked in 5% non-fat dry milk in Tris-buffered saline containing 0.2% Tween-20 then incubated with primary rabbit anti-IKK2 (1:500; Cell Signaling, Danvers, MA) overnight followed by incubation in horseradish peroxidase-conjugated secondary antibody (1:5000, Santa Cruz, Dallas, TX) for one hour. Chemiluminescent detection was performed and analyzed using the ChemiDoc XRX imaging system (Bio-Rad). Membranes were reprobed with β-actin as a control with all densitometric analysis normalized to β-actin signal using Fiji software.
Immunofluorescence of IKK2
Purified astrocytes, microglia, and striatal neurons from Ikbk2F/F and hGfapcre/Ikbk2F/F mice were plated at a density of 1 × 104 cells on 12mm poly-D-lysine coated glass coverslips and allowed to adhere for 48 hours. Cells were fixed using methanol, washed in PBS, and then blocked in 1% bovine serum albumin (w/v) in PBS for one hour. Cells were incubated overnight at 4°C in primary antibodies for IKK2 (1:50; Imgenex, San Diego, CA) and for cell-specific markers GFAP (1:500; Sigma, St. Louis, MO), ionized binding adaptor protein-1 (IBA1; 1:250; Wako, Osaka, Japan), or MAP2 (1:100; Abcam, Cambridge, MA). After rinsing in PBS, cells were incubated in for one hour at room temperature in AlexaFluor-488 (IKK2) and AlexaFluor-647 (cell markers) conjugated secondary antibodies (1:500; Invitrogen, Carlsbad, CA) and then mounted in medium containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) to detect cell nuclei.
In vivo assessment of IKK2 was performed on free-floating 40μm brain sections obtained from the SN of Ikbk2F/F and hGfapcre/Ikbk2F/F mice (procedure detailed below) using primary antibodies for IKK2 (1:50; Imgenex) and MAP2 (1:100; Abcam) and Alexafluor-488 and Alexa-Fluor-647 conjugated secondary antibodies (1:500; Invitrogen). Sections were mounted in medium containing DAPI. Images were acquired using a 40× air plan apochromatic objectives on a Zeiss Axiovert 200M inverted fluorescence microscope (Carl Zeiss, Inc., Thornwood, NY) equipped with a Hamamatsu ORCA-ER-cooled charge-coupled device camera (Hamamatsu Photonics, Hamamatsu City, Japan). Mean fluorescence intensity of IKK2, reported with a subtraction of mean background fluorescence, was determined by utilizing Slidebook software (Intelligent Imaging Innovations Inc., Denver, CO) with 10–15 individual fields examined per animal with at least 3 animals utilized per genotype.
MPTP Treatment Protocol
Ikbk2F/F and hGfapcre/Ikbk2F/F male and female littermates aged to 5 months were divided between treatment groups and then exposed to a subacute PD lesioning model utilizing 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid (MPTPp) as described previously (Miller et al., 2011; De Miranda et al., 2013). In brief, mice were injected every other day with probenecid (250 mg/kg i.p. prepared in 5% sodium bicarbonate; Sigma) and with saline or MPTP (20 mg/kg s.c. prepared in saline as a free base, Sigma) for 7 days receiving a total of 4 injections. Mice were euthanized under deep isoflurane anesthesia at either 7 days (MPTPp7d) or 14 days (MPTPp14d) after their initial injection (Figure 3A). For all groups, a minimum of 7 mice was utilized per parameter (stereology versus neurochemistry) with a minimum of 10 mice used for neurobehavioral data. All procedures were performed under an approved IACUC protocol in accordance with NIH policy for the care and use of laboratory species to minimize pain and discomfort.
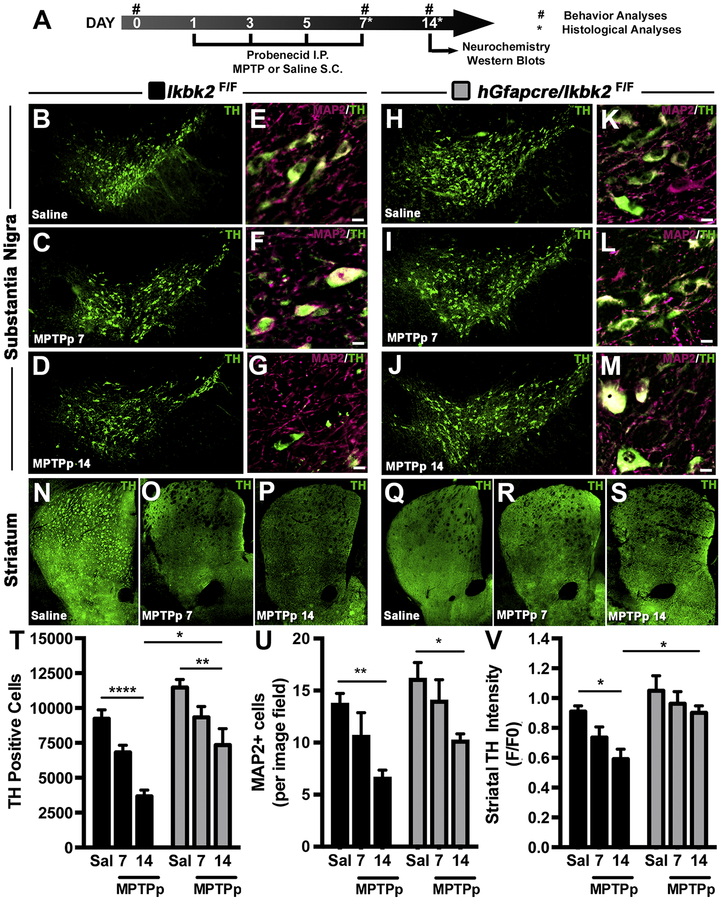
Figure 3.
MPTP-induced loss of dopaminergic neurons and neuronal projections is decreased in hGfapcre/Ikbk2F/F mice. A. Schematic of treatment and experimental regimen for the study. B-M. The number of TH+ neurons (green) in the substantia nigra pars compacta (SNpc) was assessed via immunofluorescence-based stereology and presented as representative montages for Ikbk2F/F (B-D) and hGFAP-Cre/IkkβF/F mice (H-J). Total neuronal loss was determined via counts of MAP2+ neurons within the SNpc through immunofluorescence and presented as representative images showing MAP2 (magenta) and TH (green) for Ikbk2F/F (E-G) and hGFAP-Cre/IkkβF/F mice (K-M). N-S. Loss of dopaminergic nerve terminals was assessed via quantification of immunofluorescence intensity of TH in the striatum (ST) as shown in representative montages for Ikbk2F/F (N-P) and hGFAP-Cre/IkkβF/F mice (Q-S). T. Quantitative stereological counts estimating the total number of TH+ neurons presented as an average number of TH+ cells ± SEM. U. Quantitative counts of MAP2+ neurons to estimate total neuronal loss presented as an average number of MAP2+ cells per image field ± SEM. V. Loss of striatal TH intensity was quantified via measuring total striatal TH intensity and normalized to saline Ikbk2F/F mice. Data is presented as average TH intensity ± SEM. Data was analyzed by two-way ANOVA with Tukey-Kramer post hoc test (* p < 0.05, ** p < 0.01, and **** p < 0.0001).
Tissue Processing and Sectioning
At day 7 and 14, mice were euthanized, and tissues obtained as reported previously (Miller et al., 2011). Briefly, animals were terminated under isoflurane anesthesia and transcardially perfused with 20 mM cacodylate-phosphate buffered saline (cPBS) containing 10U/ml heparin, followed by 4% paraformaldehyde in cPBS. Brains were carefully removed from the skull and placed within 4% formaldehyde in cPBS overnight then cryoprotected in 15% sucrose (w/v cPBS) then 30% sucrose (w/v cPBS). Brains were stored in 30% sucrose at 4°C until sectioning. Coronal 40μm sections through the entire length of the striatum (ST) and substantia nigra (SN) were collected using a freezing sliding microtome (Microm HM450; Thermoscientific, Waltham, MA). Sections were stored free floating at −20°C in cryoprotectant (30% w/v sucrose, 30% v/v ethylene glycol; 0.05M phosphate buffer) until staining.
Stereological Counts of Tyrosine Hydroxylase (TH) Positive Neurons
Stereological assessment of TH-positive dopaminergic neurons within the SN were done perfomed in “Miller et al., 2011.” In brief, free-floating serial sections were obtained by systematic sampling of every third tissue from sections encompassing the entire length of the SNpc and immunolabeled using primary rabbit anti-TH antibody (1:500 overnight, Chemicon, Temecula, CA) and AlexaFluor-555 conjugated secondary antibody (1:500 for 3 hours; Invitrogen). Slides were mounted in DAPI containing medium and stored at 4°C until imaging.
Slides were imaged using a 40× air plan apochromatic objective on a Zeiss Axiovert 200M inverted fluorescence microscope (Carl Zeiss) with stereological counts of TH-positive cells performed using Slidebook software (v5.0, Intelligent Imaging Innovations, Denver, CO). The boundary of the SNpc was determined using 10× magnification montaging and numbers of TH-positive cells determined via assessment of uniform (40×) randomly placed counting frames (100μm × 100μm) using an optical dissector of 30μm with 5μm upper and lower guard zones. Representative montage images were generated for each treatment group with use of BX51 microscope (Olympus, Center Valley, PA, USA) equipped with a Hamamatsu ORCA-Flash4.0 digital CMOS camera, ProScan III stage controller (Prior, Rockland, MA USA) and CellSens Dimension software (version 1.12, Olympus, Center Valley, PA, USA). Representative images were processed using Fiji (National Institutes of Health freeware) with shade correction, standard background subtraction, and contrast enhancement. Original hues were altered when indicated to limit the use of red-green combinations and for consistency.
Behavioral Assessment
Open field activity parameters were assessed using the Versamax behavior chambers with an infrared beam grid detection array (Accuscan Instruments, Inc., Columbus, OH). Mice were monitored for 10 minutes under low ambient light in the presence of white noise. Stride length was assessed via video recording mice freely walking across a plexiglass track (5 cm × 1m) with 3 recordings obtained per mouse per assessment. Animals were pre-conditioned one day prior to their first treatment and then assessed at day 0 (first day of treatment) to establish a baseline, day 7, and day 14. Several behavioral parameters were collected and analyzed using Versadat Software (Accuscan Instruments, Inc.) including total distance traveled, number of movements, time spent moving, time spent in the margin, and the number of rearing movements. Stride length was calculated using the Tracker Video Modeling software (Tracker v.4.85 for MacOS X). These parameters have been previously shown to assess basal ganglia function (Liu et al., 2006; Moreno et al., 2009; Miller et al., 2011; Streifel et al., 2012) and were reported as a change from baseline (day 0) assessment.
HPLC Analysis of Striatal Dopamine and Metabolites
Mice were terminated at day 7 and day 14 under deep isoflurane anesthesia and quickly decapitated followed by rapid removal of the ST using a brain matrix block for reference. The tissue was flash-frozen in liquid nitrogen and stored at −80°C until analysis. Samples were coded for unbiased analysis and sent to the Neurochemistry Core Laboratory at Vanderbilt University’s Center for Molecular Neuroscience Research (Nashville, TN). High-Performance Liquid Chromatography with electrochemical detection was used to determine the concentrations of dopamine (DA) and dopamine-related metabolites 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) in the striatum of control and MPTPp treated mice as detailed in (Perez and Palmiter, 2005).
Western Blot Analysis of Striatal Proteins
Flash-frozen striatal tissue from Ikbk2F/F and hGfapcre/Ikbk2F/F mice was collected at day 7 and day 14, flash frozen in liquid nitrogen and stored at −80°C until homogenized using a glass pestle and grinder followed by low power sonication in RIPA lysis buffer supplemented with a complete protease inhibitor. Protein was quantified using a BCA Assay (Pierce, Rockford, IL) and 20μg of protein was separated using gel electrophoresis in a 10% SDS polyacrylamide gel followed by semi-dry transfer to a polyvinylidene fluoride membrane. Membranes were blocked in 5% non-fat dry milk in Tris-buffered saline containing 0.2% Tween-20 then incubated with rabbit anti-TH (1:500; Millipore, Burlington, MA), rabbit anti-VMAT2 (1:500, Millipore), or rabbit anti-DAT (1:100, Santa Cruz) overnight followed by incubation in horseradish peroxidaseconjugated secondary antibody (1:5000, Santa Cruz) for one hour. Chemiluminescent detection was performed and analyzed using the ChemiDoc XRX imaging system. Membranes were reprobed with β-actin as a control with all densitometric analysis normalized to β-actin signal using Fiji.
In Vivo Immunofluorescence
Free-floating 40 μM coronal sections of SN or ST described above were mounted on positively charged microscope slides and immunolabeled for TH (1:500), MAP2 (1:100, Abcam), 3-nitrotyrosine (3-NT; 1:100, Abcam), GFAP (1:500, Dako), IBA1 (1:100, Wako), TNF (1:100, Cell signaling), and NOS2 (1:100, BD Bioscience) with Alexa Fluor 488, 555, and 647 secondary antibodies (1:500; Invitrogen,). Three serial sections from each brain region per animal were immunolabeled for quantitative studies of glial phenotype. Slides were imaged using a 10× or 40× air plan apochromat objective on a Zeiss Axiovert 200M inverted fluorescence microscope (Carl Zeiss) for quantitative analysis and BX51 microscope (Olympus) equipped with a Hamamatsu ORCA-Flash4.0 digital CMOS camera, ProScan III stage controller (Prior) and CellSens Dimension software (version 1.12, Olympus) for representative images. All representative images were processed using Fiji with standard background subtraction and contrast enhancement. Montages had further processing of shade correction to account for stitched images. Original hues were altered when indicated to limit the use of red-green combinations and for consistency.
Quantitative analysis of TH intensity in the ST was performed on 10× magnification montages. Randomized images were segmented manually to highlight the ST and an average TH fluorescence intensity generated. Average TH fluorescence intensity was normalized via division by the mean of the average TH intensity of the saline Ikbk2F/F processed on the same day to account for inconsistencies in staining or imaging during batch processing.
Generation of total neuronal counts in the SNpc was performed on sections immunolabeled for TH and MAP2. As used for TH cell counts described above, the boundary of the SNpc was determined via 10× magnification montaging and then quantified on random 40× counting frames (100μM × 100μM) using Slidebook software. For assessment of gliosis and gliosis expression of TNF and NOS2, slides were imaged using a 40× air plan apochromat objective with analysis determined using Slidebook software. The development of random 40× counting frames (150μM × 150μM) was similar to methods used for stereological counting for TH described above. Images were randomized, and images obtained per tissue segmented for the protein of interest. Once segmented, Slidebook generated an object count based on parameters of objects greater than 300 μM (GFAP) or 200μM (IBA1). These object counts were summed for a total count per tissue in gliosis analysis and per frame for assessment of TNF and NOS2 intensity. Furthermore, in TNF and NOS2 analysis in GFAP+ cells, the total sum intensity in only GFAP+ cells was calculated and normalized to the total GFAP+ cell count for that image.
qPCR Array Analysis
RNA from the SN of Ikbk2F/F and hGfapcre/Ikbk2F/F mice collected at euthanasia, flash frozen in liquid nitrogen, and stored at −80 °C until homogenized and lysed using a Qiashredder (Qiagen) and then purified using the RNeasy kit (Qiagen). RNA was quantified and converted to cDNA as described above with 250 ng per sample amplified using RT2 profiler PCR array for NFkB signaling pathway genes (Qiagen PAMM-025z). Gene expression fold change was analyzed using SAbiosciences software with genes divided for biological gene ontology using DAVID Bioinformatics Resources 6.8 (Da Wei Huang et al., 2009; Huang et al., 2009). Calculation of false discovery rate (FDR) was performed using significance analysis of microarray (SAM) version 5.0 from Stanford University (Tusher et al., 2001).
Neuronal Viability
Primary astrocytes from Ikbk2F/F and hGfapcre/Ikbk2F/F mice and cortical neurons from wild-type C57/Bl6J mice were cultured in appropriate medium as described in detail above. Neurons were seeded directly onto poly(L-lysine) coated 12-mm glass coverslips at 1 × 105 cells/well. At confluency, astrocytes were treated with 10 μM MPTP and 10 pg/mL TNF and 1 ng/mL interferon-gamma (IFNγ) for 8 hours; an established protocol known to elicit neuroinflammatory activation in astrocytes (Carbone et al., 2008). Medium was removed, and astrocytes washed 3 times with PBS to prevent carryover of treatment to neurons and then placed in neurobasal media supplemented with 2mM L-glutamine, B27 supplement, and PSN antibiotic mixture for 24 hours. After 24 hours, the medium was removed, spun down to remove any cellular debris and placed on cultured neurons for an additional 24 hours. After 24 hours, neurons were assessed for cellular death via live-cell fluorescence imaging. Caspase activity was determined using CellEvent caspase-3/7 green detection reagent (Thermofisher) according to manufacturer’s instructions, overall cell death with 3 μM propidium iodide (PI; Sigma), and nuclei using 2μM Hoechst 33342 (Thermofisher). Using a 20× Plan apochromatic air objective, 10–12 fields per treatment were blindly captured and assessed via blind cell counts using Slidebook software. For each genotype and treatment, there was a minimum of 3 biological replicates with 3–4 repetitions of the experiment.
P65 translocation
Primary astrocytes from Ikbk2F/F and hGfapcre/Ikbk2F/F mice were seeded directly onto 12mm glass coverslips at 5 × 104 cells/well and treated with saline or 10 μM MPTP and 10 pg/mL TNF and 1 ng/mL IFNγ for 1 hour. Cells were rinsed with PBS and fixed using cold-methanol as described above. Cells were immunolabeled for p65 (polyclonal 1:100, Cell Signaling) and GFAP (monoclonal 1:500, Cell Signaling) with Alexa Fluor 568 and 488 secondary antibodies (Invitrogen), respectively. Using a 40× apochromatic air objective, 10–12 fields per treatment were blindly captured and assessed for positive nuclear p65 per GFAP+ cell per field using Slidebook software. For each genotype and treatment, there was a minimum of 3 biological replicates with 3–4 repetitions of the experiment.
Inflammatory Gene Expression in Cultured Astrocytes
Confluent primary astrocytes cultures from Ikbk2F/F and hGfapcre/Ikbk2F/F mice were treated with saline or 10 μM MPTP and 10 pg/mL TNF and 1 ng/mL IFNγ for 8 hours. Cells were then rinsed with cold PBS and RNA was isolated from glia utilizing the RNeasy Mini Kit (QIAGEN, Valencia, CA) with purity and concentration confirmed using a NanoDrop ND-1000 spectrophotometer. Five hundred ng of RNA was used as a template for reverse transcriptase reactions using the iScript RT kit (Bio-Rad) cDNA was mixed with SYBR Green (Bio-Rad) with primer pairs for Tnf, Nos2, interleukin 1-beta (Il-1β), interleukin 6 (Il-6), chemokine-like ligand 2 and 5 (Ccl2 and Ccl5) as published previously (Kirkley et al., 2017).
Statistical Analyses
All statistical analyses were performed using Prism software (version 6.0; Graphpad Software, Inc., San Diego, CA) with a Student’s t-test utilized for comparison of two means, whereas a two-way analysis of variance (ANOVA) followed by a Tukey-Kramer multiple comparison post-hoc test was used for comparison of three or more means. Independent variables for two-way ANOVA were defined as genotype (versus Ikbk2F/F versus hGfapcre/Ikbk2F/F) and treatment (saline versus MPTPp). Statistical significance was defined as a p-value less than 0.05 and indicated by asterisks.
Results
Conditional Deletion of IKK2 in Astrocytes
To study the role of reactive astrogliosis in the onset and progression of PD, we generated a mouse with a conditional deletion of IKK2, an essential kinase involved in the initiation of inflammation in the NFkβ pathway (Bonizzi and Karin, 2004). This was accomplished through breeding Ikbk2-floxed mice (Li et al., 2003) with hGfapcre transgenic mice expressing Cre under the control of the human Gfap promoter (Zhuo et al., 2001; Fig. 1A). Three generations of pairing were required to generate mice that had both the hGfapcre allele and were homozygous for floxed-Ikbk2 (hGfapcre/Ikbk2F/F) and thus for all experiments littermates homozygous for floxed-Ikbk2, but lacking hGfapcre, known as Ikbk2F/F, were utilized as controls. Genotype assessment for the presence of hGfapcre and Ikbk2F (Fig. 1B) was achieved through PCR on ear tags in adults and tail biopsies from neonatal mice.
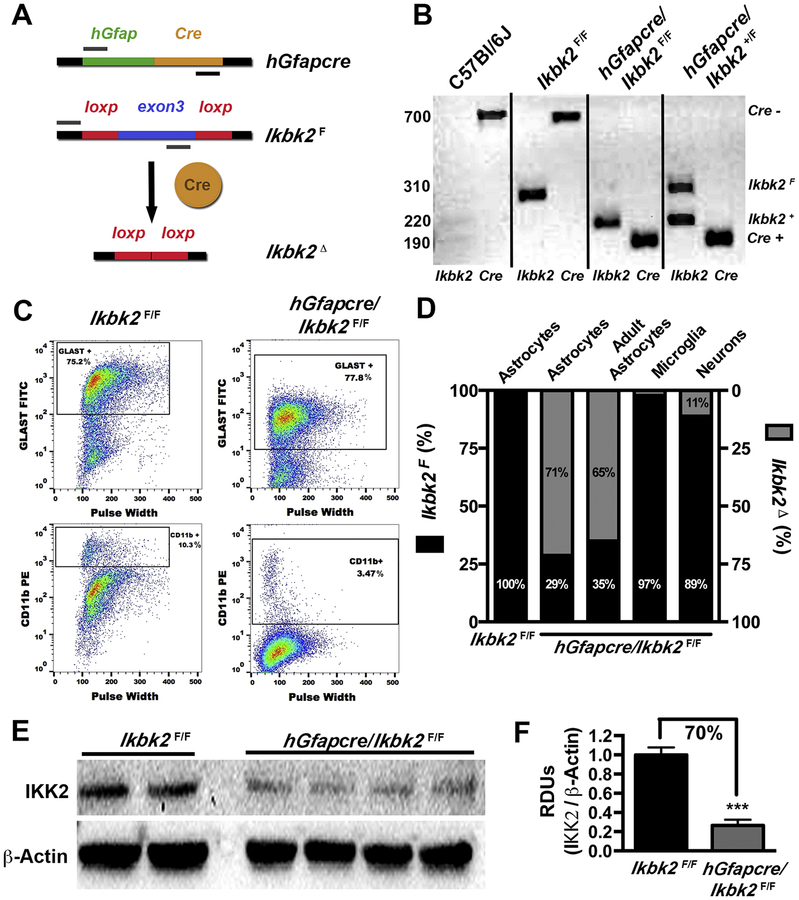
Figure 1.
Astrocyte-specific Ikbk2 gene deletion in hGfapcre/Ikbk2F/F mice. A. Conditional knockout of Ikbk2 gene in astrocytes was achieved through breeding mice with cyclic recombinase in control of the human glial fibrillary acidic promoter (hGfapcre) to mice that were homozygous for a floxed-Ikbk2 (Ikbk2F) gene resulting in deletion of the Ikbk2 (Ikbk2Δ). B. Ikbk2F/F and hGfapcre/Ikbk2F/F mice were genotyped via PCR for the presence of hGfapcre allele and presence for floxed (F) or wild-type (+) Ikbk2 allele. Genotyping of a C57/Bl6/J mouse is provided for reference. C. Primary cultures of astrocytes cultured from Ikbk2F/F and hGfapcre/Ikbk2F/F mice were assessed for culture purity through flow cytometric analysis for percent GLAST (astrocyte) and CD11b (microglia) expression. D. Genomic DNA from primary astrocytes, microglia, and neurons cultured from neonatal (day 1) and astrocytes cultured from adult (20-week) Ikbk2F/F and hGfapcre/Ikbk2F/F mice were analyzed via qPCR to assess the rate of Ikbk2 deletion. Percentages of Ikbk2F and Ikbk2Δ are shown in black and gray, respectively. E. Protein isolated from hGfapcre/Ikbk2F/F and Ikbk2F/F cultured astrocytes was analyzed for the amount of IKK2 via western blot. F. IKK2 protein levels were quantified using densitometric analysis with normalization to levels of β-Actin. Data are presented as mean ± SEM with Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F (grey bars; Student t-test. *** p < 0.001).
The use of hGfap-Cre mice to target specific deletion in astrocytes has shown conflicting results, with studies reporting non-targeted recombination events in neurons (Zhuo et al., 2001; Malatesta et al., 2003; Casper and McCarthy, 2006). To test the efficiency and specificity of Cre induced recombination in the brain, we cultured primary astrocytes, microglia, and striatal neurons from Ikbk2F/F and hGfapcre/Ikbk2F/F mice. The purity of cultured astrocytes and microglia was determined using flow cytometry against GLAST for astrocytes and CD11b for possible microglial contamination (Fig. 1C). Both Ikbk2F/F and hGfapcre/Ikbk2F/F mice had between a 75–78% GLAST positive population and less than 10% presence of CD11b+ cells. Consistently, hGfapcre/Ikbk2F/F astrocyte cultures had less microglia presence than their Ikbk2F/F counterparts. Microglia purity was determined to be around 90% (data not shown) for both genotypes.
The deletion of Ikbk2 at the genomic level in cultured cells was determined utilizing qPCR (Fig. 1D). Cultured astrocytes from hGfapcre/Ikbk2F/F neonates showed a deletion rate of 71% for Ikbk2F while cultured astrocytes from hGfapcre/Ikbk2F/F adults (20 weeks) was slightly reduced to 65% loss of Ikbk2F. Cultured microglia and striatal neurons from hGfapcre/Ikbk2F/F mice had minimal loss of genomic Ikbk2 with only 3% and 11% loss, respectively. Astrocytic genomic loss of Ikbk2 corresponded to a similar loss of IKK2 protein (~70%) measured by western blotting (Fig. 1E & 1F).
We further examined expression of IKK2 via immunofluorescence in primary astrocytes (Fig. 2A & 2B), microglia (Fig. 2C & 2D) and striatal neurons (Fig. 2E & 2F) cultured from Ikbk2F/F and hGfapcre/Ikbk2F/F neonates and in tissue from the SN (Fig. 2G & 2H) to evaluate mid-brain neurons. Both striatal neurons (p = 0.88, N between 5–12) and microglia (p = 0.1204, N between 9–12) from hGfapcre/Ikbk2F/F mice showed no significant loss of IKK2 immunolabeling in comparison to cells obtained from Ikbk2F/F littermates (Fig. 2I & 2J). This is in sharp contrast to the significant decrease in IKK2 fluorescence seen in astrocytes (Fig. 2I; p = 0.0023, N = 6). Furthermore, IKK2 was intact in neurons of the SNpc in adult hGfapcre/Ikbk2F/F mice with frequency and intensity of levels of IKK2 not significantly different than Ikbk2F/F littermates (Fig. 2J; p = 0.45, N between 10–12). These data indicate a specific reduction of IKK2 in hGfapcre/Ikbk2F/F to astroglial cells within the brain of both neonatal and adult mice.
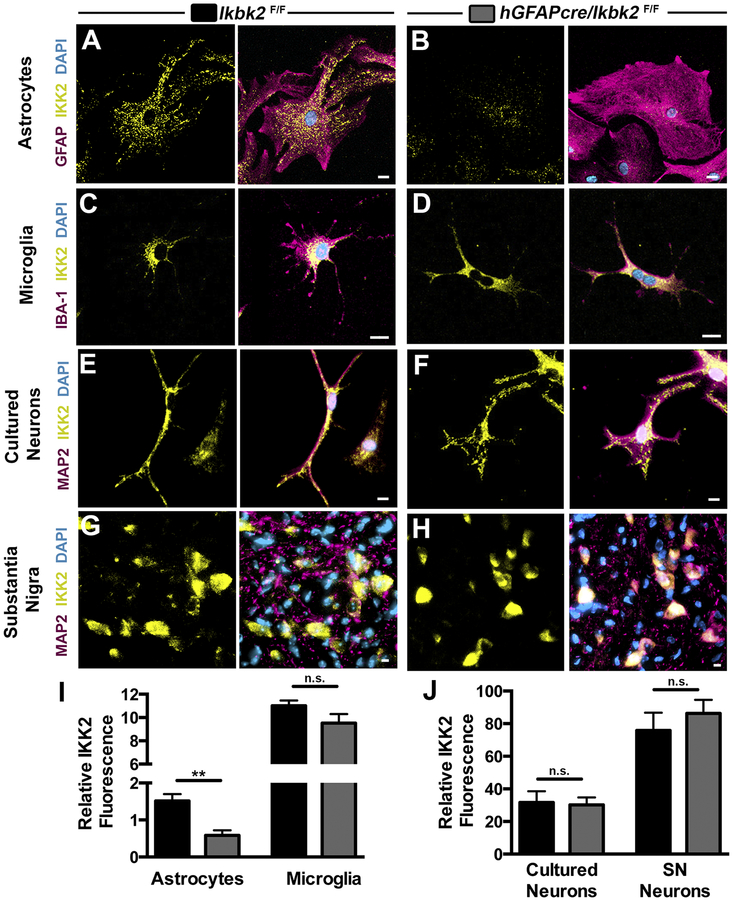
Figure 2.
Astrocyte-specific deletion of IKK2 in hGfapcre/Ikbk2F/F mice. Cell-specific IKK2 expression in astrocytes, microglia, and neurons cultured from Ikbk2F/F (A, C, E) and hGfapcre/Ikbk2F/F (B, D, F) mice were immunolabeled for IKK2 (yellow), cell-specific markers (magenta) for astrocytes (GFAP; A and B), microglia (IBA1; C and D), or neurons (MAP2, E and F) and counterstained with DAPI (cyan) to visualize cell nuclei. Scale bar = 10μm. G-H. Representative 40× images of colocalization of MAP2+ neurons (magenta) and IKK2 (yellow) in the substantia nigra (SN) of adult Ikbk2F/F (G) and hGFAP-Cre/Ikbk2F/F(H) mice. Cyan = DAPI. Q - R. The mean IKK2 fluorescence intensity (with background subtraction) between Ikbk2F/F(black bars) and hGfapcre/Ikbk2F/F(grey bars) was measured in astrocytes and microglia (Q) and in cultured neurons versus SN neurons (R). Data are presented as mean fluorescence intensity ± SEM. (Student t-test; ** p < 0.01 and n.s. = not significant).
Conditional Deletion of IKK2 in Astrocytes is Neuroprotective
After establishing specificity of IKK2 loss in astrocytes, Ikbk2F/F and hGfapcre/Ikbk2F/F mice were used in an established subacute dosing model of PD whereby the mice were treated over the course of 7 days with probenecid and MPTP (MPTPp) and assessed at the end of dosing (7 days) or a week following cessation of dosing (14 days; Fig. 3A; Miller et al., 2011). To determine the extent of MPTPp induced dopamine neurodegeneration, unbiased, systematic stereological counts of TH+ neurons in the SNpc were performed (Fig. 3). MPTPp-treated Ikbk2F/F mice had a 45% reduction in the number of TH+ cells at day 7 as compared to saline-treated mice (Fig. 3B–D), with a 60% loss in the number of TH+ cells by day 14, even after cessation of MPTPp treatment at day 7. In contrast, hGfapcre/Ikbk2F/F mice largely significantly protected from both direct lesioning effects of MPTPp treatment as well as from progressive loss of TH+ neurons from day 7 to day 14, as indicated by preservation of dopaminergic soma in the SNpc (Fig. 3H–J). Quantitative stereological cell counts (Fig. 3T) indicated wild-type Ikbk2F/F had a 45% loss of TH+ cells by day 7 and 60% loss by day 14 (p < 0.0001, N between 5–7), whereas there was no significant loss of TH+ neurons in hGfapcre/Ikbk2F/F KO mice at day 7 and significant 36% loss by day 14 (p = 0.0056, N between 5–7). Analysis by two-way ANOVA revealed an effect of MPTPp treatment on SNpc levels of TH+ neurons (F(2,25) = 23.29, p < 0.0001) that differed depending on genotype (F(1,25) = 23.10, p < 0.0001) with the most marked and statistically significant difference observed during the post-lesioning progression phase at days 7–14 in wildtype mice.
In addition to TH+ cell counts, total neuronal counts via MAP2 immunofluorescence of the SNpc and TH intensity of the ST were assessed (Fig. 3). Following similar trends to TH counts, MPTPp-treated Ikbk2F/F mice had marked reductions in the number of MAP2+ cells at day 7 with the number of TH+ cells continuing to decline from day 7–14 (Fig. 3E–G; p = 0.024, N between 5–7) while MPTP-treated hGfapcre/Ikbk2F/F mice had a more minimal, yet significant loss of MAP2+ cells (Fig. 3K–M; p = 0.03, N between 5–7). Analysis via a two-way ANOVA revealed a substantial effect on MPTPp treatment (F(2,24) = 13.24, p = 0.0001) and genotype (F(1,24) = 9.06, p = 0.0061) of MAP2 levels in the SNpc (Fig. 3U). Loss of TH intensity in the ST after MPTPp treatment also revealed marked loss in MPTPp-treated Ikbk2F/F mice (Fig. 3N–P; p = 0.027, N = 6) that was greatly diminished in hGfapcre/Ikbk2F/F mice (Fig. 3Q–S). Analysis of fluorescence intensity of striatal TH by two-way ANOVA indicated a significant loss of striatal TH in the post-lesioning phase in Ikbk2F/F mice, but not in hGfapcre/Ikbk2F/F mice (Fig. 3V; p = 0.033, N=6).
Conditional Deletion of IKK2 in Astrocytes Protects Against MPTPp Induced Changes in Locomotor Function and Striatal Protein Loss but not Striatal Catecholamines
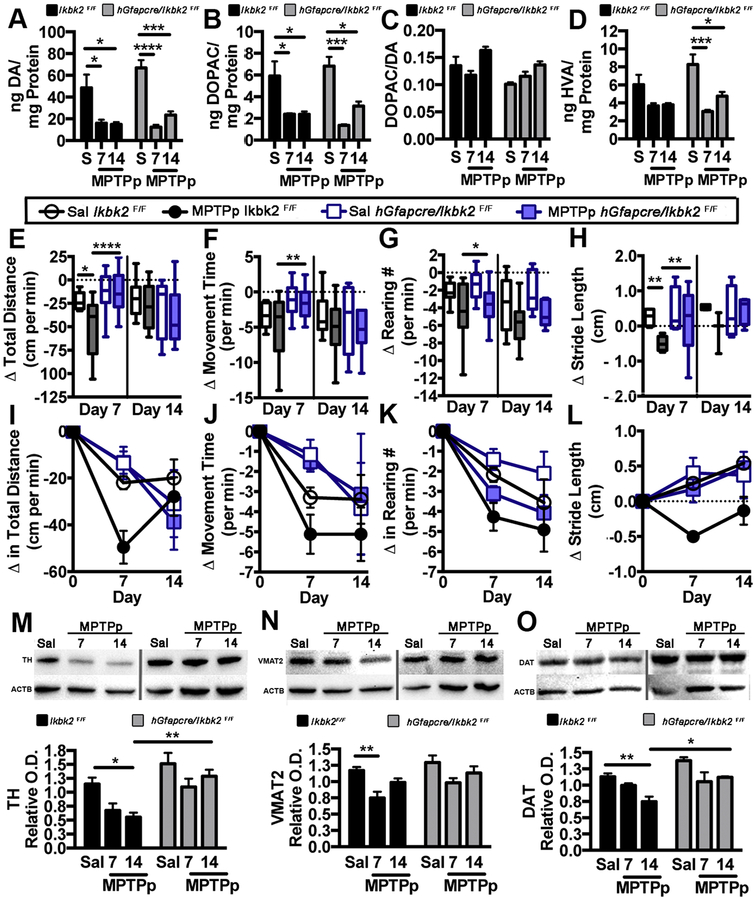
MPTP treatment in rodents is known to severely damage dopaminergic terminals in the striatum producing losses in catecholamines and DA-associated proteins that are linked to acute changes in locomotor behavior (Fredriksson et al., 1990). Striatal levels of DA and its metabolites, DOPAC and HVA, were measured in Ikbk2F/F and hGfapcre/Ikbk2F/F mice via HPLC following lesioning with MPTPp (Fig. 4A–D). Levels of DA were significantly decreased at day 7 in Ikbk2F/F (p = 0.027, N = 5) and hGfapcre/Ikbk2F/F mice (p < 0.0001, N = 5), with continued, but no further reduction, at day 14 (Fig. 4A; p < 0.017 and p < 0.0001, respectively). There were no significant differences in DA loss between genotypes; however, MPTPp-treated hGfapcre/Ikbk2F/F mice trended toward higher recovery at 14 days than their wild-type Ikbk2F/F littermate genotype controls. Similar patterns were observed for the dopamine metabolites DOPAC (Fig. 4B) and HVA (Fig. 4D). Measurements of the DOPAC to DA ratio were unchanged by treatment and were not different between genotypes (Fig. 4C).
Figure 4.
hGfapcre/Ikbk2F/F mice are protected from MPTP-induced locomotor changes and striatal protein losses but not from loss of striatal dopamine (DA) and its metabolites. A-D. Striatal levels of DA (A), its metabolites DOPAC (B) and HVA (D) were analyzed via HPLC. The rate of dopamine metabolism was determined by measuring the DOPAC/DA (C). Data are presented as mean ± SEM. E-L. Changes in open field locomotion and stride length were monitored in Ikbk2F/F and hGfapcre/Ikbk2F/F mice at days 0, 7, and 14. Changes in total distance moved (E, I), time spent moving (F, J), number of rearing movements (G, K) and stride length (H, L) are expressed between groups (E-H) and over time (I-L) and represented as the average change from baseline (day 0) ± SEM. M-O. Levels of TH (M), VMAT2 (N), and DAT (O) from the striatum of Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F mice (grey bars) were assessed via western blot and quantified using densitometric analysis with normalization to levels of β-Actin (ACTB). Data was analyzed by two-way ANOVA with Tukey-Kramer post hoc test (* p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001).
The effects of MPTPp on neurobehavioral function in Ikbk2F/F versus hGfapcre/Ikbk2F/F mice were determined by open-field activity measurements and video recordings of stride length (Fig 4E–H). In comparison to baseline measurements recorded at day 0, only Ikbk2F/F mice exposed to MPTPp had significant depression of spontaneous locomotor activity observed at day 7 (N between 10–21) compared with their saline controls, whereas MPTPp-treated hGfapcre/Ikbk2F/F mice displayed no changes in locomotion. Analysis via two-way ANOVA showed significant effects with treatment for total distance traveled (Fig 4E; F(1.57) = 4.63, p = 0.036), rearing number (Fig. 4G; F(1.57) = 12.13, p = 0.001), and stride length (Fig. 4H; F(1.57) = 9.31, p = 0.004) but not for movement time (Fig. 4F; F(1.57) = 0.84, p = 0.36) and margin time (data not shown). Changes in these parameters over time are depicted in Fig.4 I–L. Genotype had a significant effect with total distance traveled (F(1.57) = 12.30, p = 0.0009), movement time (F(1.57) = 12.89, p = 0.0007), margin time (F(1.57) = 7.31, p = 0.009) and stride length (F(1.57) = 6.47, p = 0.014), but not in rearing number (F(1.57) = 3.35, p = 0.07). Only total distance traveled showed an interaction between treatment and genotype (F(1.57) = 4.63, p = 0.033). Post-hoc analysis revealed that decreases in all neurobehavioral parameters analyzed in MPTPp-treated Ikbk2F/F mice were statistically different from MPTPp-treated hGfapcre/Ikbk2F/F mice at day 7 (p < 0.05). However, there was not a significant effect on these parameters by day 14 for treatment or genotype with post-hoc analysis revealing no differences in the behavioral scores of Ikbk2F/F and hGfapcre/Ikbk2F/F mice.
Levels of DA associated striatal proteins were determined through western blotting of flash-frozen striatal tissue from Ikbk2F/F and hGfapcre/Ikbk2F/F mice collected at day 7 and day 14 post-treatment (Fig. 4M–O). Wild type Ikbk2F/F mice treated with MPTPp showed significant reduction in protein levels of TH (Fig. 4M; day 14 p = 0.025, N = 7), VMAT2 (Fig. 4N; day 7 p = 0.0094, N = 7), and DAT (Fig. 4O; day 14 p = 0.0071, N = 7) compared to saline controls whereas hGfapcre/Ikbk2F/F mice treated with MPTPp had no significant loss. Analysis of densitometry via two-way ANOVA indicated that treatment (TH F(2, 33) = 7.0, p = 0.0029; VMAT F(2,33) = 9.56, p = 0.0005); and DAT F(2,33) = 69.87, p = 0.0004) and genotype (TH F(1, 33) = 22.07, p < 0.0001; VMAT F(1,33) = 5.85, p = 0.021; and DAT F(1,33) = 14.10, p = 0.0007) had significant effects on all proteins with post-hoc analysis revealing significant differences in TH (p = 0.005) and DAT (p = 0.013) in day 14 of MPTPp-treated Ikbk2F/F and hGfapcre/Ikbk2F/F mice.
Conditional deletion of IKK2 in astrocytes results in attentuated glial activation in the substantia nigra and striatum after treatment with MPTP
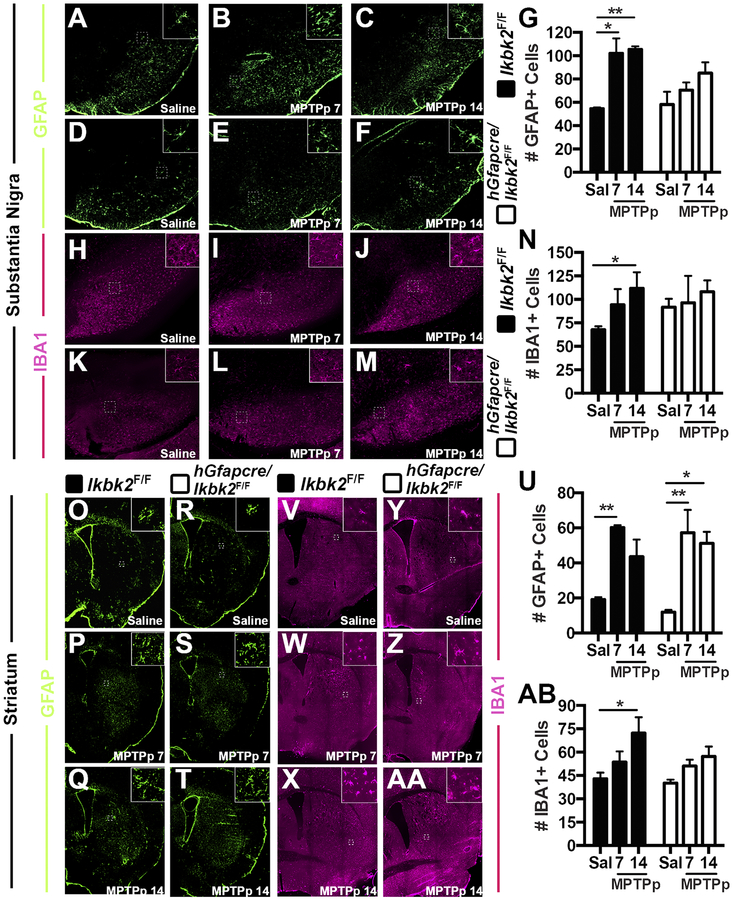
To assess glial activation in response to neuronal injury, cryo-preserved frozen serial sections of SN and ST from Ikbk2F/F and hGfapcre/Ikbk2F/F mice treated in the MPTPp dosing model were assessed for the number of astrocytes and microglia utilizing immunolabeling for GFAP and IBA1, respectively (Fig. 5, N between 5–8). Within the SN of Ikbk2F/F mice, representative montages for GFAP reveal a visible increase in the number of GFAP+ cells in response to MPTPp treatment as compared to saline (Fig. 5A), that was maximal by day 7 (Fig. 5B) and did not further increase by day 14 (Fig. 5C). The SN of hGfapcre/Ikbk2F/F mice showed similar basal levels of GFAP+ cells in saline-treated mice (Fig. 5D) that showed a trend toward increase following MPTPp treatment from day 7 (Fig. 5E) to day 14 (Fig. 5F), although this increase was not statistically significant, in contrast to their wild-type littermate counterparts. Analysis of quantitative GFAP+ counts by two-way ANOVA (Fig. 5G) indicate both a statistical effect of treatment (F(2, 26) = 8.94, p = 0.0011) and genotype (F(1, 26) = 4.23, p = 0.05), with post-hoc analysis revealing increases in numbers of GFAP+ cells from saline in day 7 (p = 0.012) and day 14 (p = 0.006) MPTPp treated Ikbk2F/F mice. These data indicate that deletion of astrocyte IKK2 is protective against astrocytic activation and that MPTPp did not cause any further increase in GFAP+ cells within a week of treatment in Ikbk2F/F mice.
Figure 5.
MPTP-induced gliosis is partially reduced in hGfapcre/Ikbk2F/F mice. Gliosis following saline or MPTPp treatment was quantified via counts of positively immunolabeled cells for GFAP (astrocytes) and IBA1 (microglia) in the SN and ST of Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F (grey bars). A-F. Representative montages of GFAP+ cells (green) in the SN of Ikbk2F/F (A-C) and hGfapcre/Ikbk2F/F (D-F) mice. G. Quantitative counts of GFAP+ cells in the SN presented as mean counts ± SEM. H-M. Representative montages of IBA1+ cells (magenta) in the SN of Ikbk2F/F (H-J) and hGfapcre/Ikbk2F/F (K-M) mice. N. Quantitative counts of IBA1+ cells in the SN presented as mean counts ± SEM. O-T. Representative montages of GFAP+ cells (green) in the ST of Ikbk2F/F (O-Q) and hGfapcre/Ikbk2F/F (R-T) mice. U. Quantitative counts of GFAP+ cells in the ST presented as mean counts ± SEM. V-AA. Representative montages of IBA1+ cells (magenta) in the ST of Ikbk2F/F (V-X) and hGfapcre/Ikbk2F/F (Y-AA) mice. AB. Quantitative counts of IBA1+ cells in the ST presented as mean counts ± SEM. Data was analyzed by two-way ANOVA with Tukey-Kramer post hoc test (* p < 0.05, ** p < 0.01 and ***p < 0.001).
The microglial response in the SN was determined by counting cells positively immunolabeled for IBA1 (Fig. 5H–N; N between 5–8). Within the SN of Ikbk2F/F mice, representative montages for IBA1 reveal a visible increase in the number of IBA1+ cells in response to MPTPp treatment as compared to saline (Fig. 5H) with progressive increases in IBA1 immunolabeling from day 7 (Fig. 5I) to day 14 (Fig. 5J). In contrast, the SN of hGfapcre/Ikbk2F/F mice showed slightly higher basal levels of IBA1+ cells in saline-treated mice (Fig. 5K) that did not change with MPTPp treatment at either day 7 (Fig. 5L) or day 14 (Fig. 5M). Analysis of quantitative IBA1+ counts by two-way ANOVA with post-hoc analysis (Fig. 5N) revealing the only significant increases in IBA1 from saline occurred in MPTPp-treated Ikbk2F/F mice at day 14 (p = 0.047). These data indicate that deletion of IKK2 in astrocytes prevents microglial activation upon MPTPp treatment and may also have a role in limiting microglial numbers in basal conditions.
Astrogliosis was also assessed within the ST of Ikbk2F/F and hGfapcre/Ikbk2F/F mice (Fig. 5O–AB, N between 5–6). Within the ST of Ikbk2F/F mice, representative montages for GFAP reveal a visible increase in the number of GFAP+ cells in response to MPTPp treatment as compared to saline (Fig. 5O), with loss of GFAP immunolabeling from day 7 (Fig. 5P) to day 14 (Fig. 5Q). The ST of hGfapcre/Ikbk2F/F mice showed similar basal levels of GFAP+ cells in saline-treated mice (Fig. 5R) that were expanded upon MPTPp treatment with no progression from day 7 (Fig. 5S) to day 14 (Fig. 5T) and at similar levels as their Ikbk2F/F genotype control counterparts. Analysis of quantitative GFAP+ counts by two-way ANOVA (Fig. 5U) indicate a statistical effect of only treatment (F(2,25) = 17.75, p < 0.0001) with post-hoc analysis revealing significant increases in GFAP numbers from saline in MPTPp treated Ikbk2F/F mice at day 7 (p = 0.0092) and at both day 7 (p = 0.0036) and day 14 (p = 0.014) of MPTPp-treated hGfapcre/Ikbk2F/F mice. These data indicate that deletion of astrocyte IKK2 is not protective against astrogliosis in the ST in response to MPTPp treatment.
The microglial response in the ST was also determined through counts of positively immunolabeled IBA1 cells (Fig. 5V–AB, N = 6). Within the ST of Ikbk2F/F mice, representative montages for IBA1 reveal a visible increase in the number of IBA1+ cells in response to MPTPp treatment as compared to saline (Fig. 5V) with progressive increases in IBA1 immunolabeling from day 7 (Fig. 5W) to day 14 (Fig. 5X). In the ST of hGfapcre/Ikbk2F/F mice, basal levels of IBA1+ cells in saline-treated mice (Fig. 5Y) were similar to their wild-type counterparts that increased minimally with MPTPp treatment at either day 7 (Fig. 5Z) to day 14 (Fig. 5AA). Analysis of quantitative IBA1+ counts by two-way ANOVA (Fig. 5AB) indicate a statistical effect of treatment (F(2,30) = 7.109, p = .003) with post-hoc analysis revealing significant increases in IBA1+ cells from saline in MPTPp treated Ikbk2F/F mice at day 14 (p = 0.023). These data indicate that deletion of astrocyte IKK2 is protective against microgliosis in the ST upon MPTPp treatment.
Glial activation itself does not necessarily indicate neuroinflammatory damage, because different states of glial activation can alter the dynamics of tissue repair and response to damage (Burda and Sofroniew, 2014). Thus, to determine if MPTPp-induced expression of inflammatory genes in astrocytes was suppressed with deletion of IKK2, we examined astrocyte-specific expression of NOS2/iNOS (Supplemental Figure 1; N = 4) and TNF (Supplemental Figure 2; N = 4) using co-immunofluorescence in the SN and ST of Ikbk2F/F and hGfapcre/Ikbk2F/F. Levels of NOS2 in GFAP+ cells of the SN of Ikbk2F/F mice were minimal in saline-treated mice (Supp. Fig. 1A) but highly increased upon exposure to MPTPp with progressive increases from day 7 (Supp. Fig. 1B) to day 14 (Supp. Fig. 1C). In contrast, NOS2 levels in GFAP+ cells of the SN of hGfapcre/Ikbk2F/F mice were not detectable in either saline (Supp. Fig. 1D) or in day 7 (Supp. Fig. 1E) or day 14 (Supp. Fig. 1F) of MPTPp-treated mice. Similar trends were seen in the ST of Ikbk2F/F (Supp. Fig. 1G–I) and hGfapcre/Ikbk2F/F (Supp. Fig. 1J–L) mice. Levels of TNF in GFAP+ cells of the SN of Ikbk2F/F mice were minimal in saline-treated mice (Supp. Fig. 2A) and at day 7 in MPTPp-treated mice (Supp. Fig. 2B) but were greatly increased by day 14 after MPTPp treatment (Supp. Fig. 2C). In contrast, expression of TNF in the SN in GFAP+ cells of hGfapcre/Ikbk2F/F mice was not detectable in either the saline group (Supp. Fig. 2D) or at day 7 (Supp. Fig. 2E) or day 14 (Supp. Fig. 2F) in MPTPp-treated mice. Similar trends were seen in the ST of Ikbk2F/F mice (Supp. Fig. 2G–I) and hGfapcre/Ikbk2F/F mice (Supp. Fig. 2J–L) but with expression of TNF only trending upward in MPTPp-treated Ikbk2F/F mice. Quantitative analysis of NOS2 and TNF immunofluorescence indicated that the large increases in expression of NOS2 and TNF in WT mice by day 14 were completely inhibited in hGfapcre/Ikbk2F/F mice (Supp. Fig. 1 Mn; Supp. Fig. 2 M,N).
Conditional Deletion of IKK2 in Astrocytes Reverses MPTPp-induced changes in NFκB gene expression
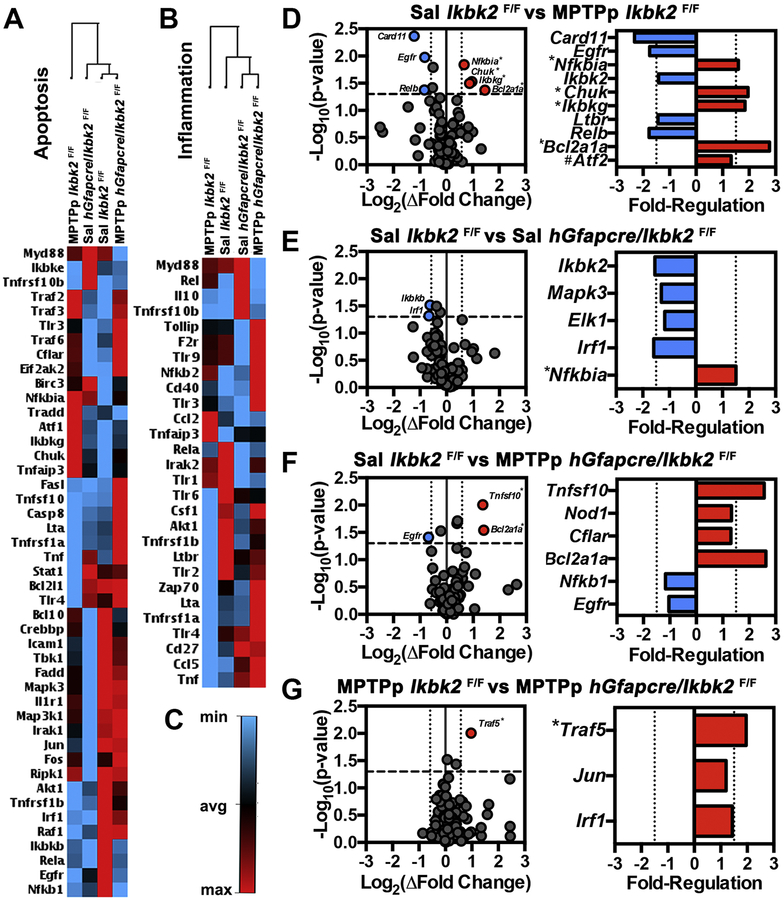
NFκB is involved in regulating numerous biological pathways, including apoptosis and inflammation, which are important pathological mechanisms underlying neurodegeneration in PD (Mattson and Camandola, 2001). To assess transcriptional changes in NFκB-regulated genes involved in apoptosis and inflammation at day 14 following MPTPp treatment, RNA in SN tissue from Ikbk2F/F and hGfapcre/Ikbk2F/F mice was purified and assessed by qPCR microarray analysis (Fig. 6; N = 4). Heat map analysis of apoptotic genes expressed in the NFκB signaling pathway (Fig. 6A) revealed 1st order clustering of saline-treated Ikbk2F/F and MPTPp-treated hGfapcre/Ikbk2F/F mice and 2nd order clustering with saline-treated hGfapcre/Ikbk2F/F mice with MPTPp-treated Ikbk2F/F mice clustering outside all groups. Heat map analysis of inflammatory gene expression regulated by NFκB (Fig. 6B) revealed 1st order clustering of saline-treated hGfapcre/Ikbk2F/F and MPTPp-treated hGfapcre/Ikbk2F/F mice and 2nd order clustering with saline-treated Ikbk2F/F mice with MPTPp-treated Ikbk2F/F mice clustering outside all groups. This indicates that in both gene arrays, MPTP-treated Ikk2 knockout mice clustered with the control group. Interestingly, MPTPp-treated Ikbk2F/F and MPTPp-treated hGfapcre/Ikbk2F/F mice clustered on opposing ends of the apoptotic and inflammatory heat maps with almost reversed fold-regulation of assessed genes, indicating that IKK2 knockout mice treated with MPTPp had patterns of gene expression more similar to saline-treated controls. Analysis of fold-expression changes in genes expressed in the NFκB signaling pathway were deemed significant if p-values were less than 0.05 and had a greater than 1.5-fold change in expression as represented by colored red (up) or blue (down) in volcano plots in Figure 6D–6G, with genes with an FDR less than 0.05 are indicated with an asterisk (*). Fold-regulation of all genes with p-values less than 0.05 are shown in bar graphs. In wild-type Ikbk2F/F mice, treatment with MPTPp resulted in upregulation of 4 genes NFκB inhibitor alpha (Nfkbia), IKK1/IKKalpha (Chuk), IKKgamma (Ikbkg) and BCL-2 related protein A1 (Bcl2a1a) and down-regulation of 3 genes including the caspase recruitment domain-containing protein 1 (Card11), epidermal growth factor receptor (Egfr) and transcription factor Relb (Relb). Control hGfapcre/Ikbk2F/F mice only had significant down-regulation of 2 genes compared to saline Ikbk2F/F mice, Ikbk2 and interferon regulatory factor (Irf1) (Fig. 6E). With MPTPp treatment, hGfapcre/Ikbk2F/F mice had 2 genes upregulated, TNF superfamily member 10 (Tnfsf10) and Bcl2a1a and down-regulation of 1 gene, Egfr, as compared to saline Ikbk2F/F mice (Fig. 6F). Interestingly, comparison of gene expression patterns between MPTPp-treated Ikbk2F/F and hGfapcre/Ikbk2F/F mice revealed upregulation of only 1 gene, TNF receptor association factor 5 (Traf5) in hGfapcre/Ikbk2F/F mice as compared to Ikbk2F/F mice (Fig. 6G).
Figure 6.
Microarray analysis of NFκB signaling pathway genes from the SN following MPTPp treatment. Following saline or MPTPp treatment, mRNA from SN’s from Ikbk2F/F and hGfapcre/Ikbk2F/F mice were analyzed in an NFκB signaling microarray for alterations in inflammatory and apoptotic genes. A. Heat map representing fold regulation changes in NFκB signaling genes involved in apoptosis B. Heat map representing fold regulation changes in NFκB signaling genes involved in inflammation C. Color guide to heat maps with blue representing down-regulation and red indicating up-regulation D-E. Volcano plots (right) and fold regulation graphs (left) for changes in NFκB signaling genes for MPTPp treated Ikbk2F/F mice (D), saline-treated hGfapcre/Ikbk2F/F mice (E), and MPTPp treated hGfapcre/Ikbk2F/F mice (F) in comparison to saline-treated Ikbk2F/F mice. G. Volcano plots (right) and fold regulation graphics (left) for changes in NFκB signaling genes in MPTPp treated hGfapcre/Ikbk2F/F mice in comparison to MPTPp treated Ikbk2F/F mice. Volcano plots represent all genes evaluated in the microarray with colored dots meeting criteria of a p-value < 0.05 on (y-axis) and fold change above 1.5 (x-axis) while fold regulation is only genes whose p-value was < 0.05. All data was analyzed using SA biosciences software. * indicates that the calculated FDR was also < 0.05. # indicates a FDR < 0.05 but a p above 0.05.
Neurons are protected against MPTPp induced 3-NT and Caspase Activation with Loss of Astrocytic IKK2
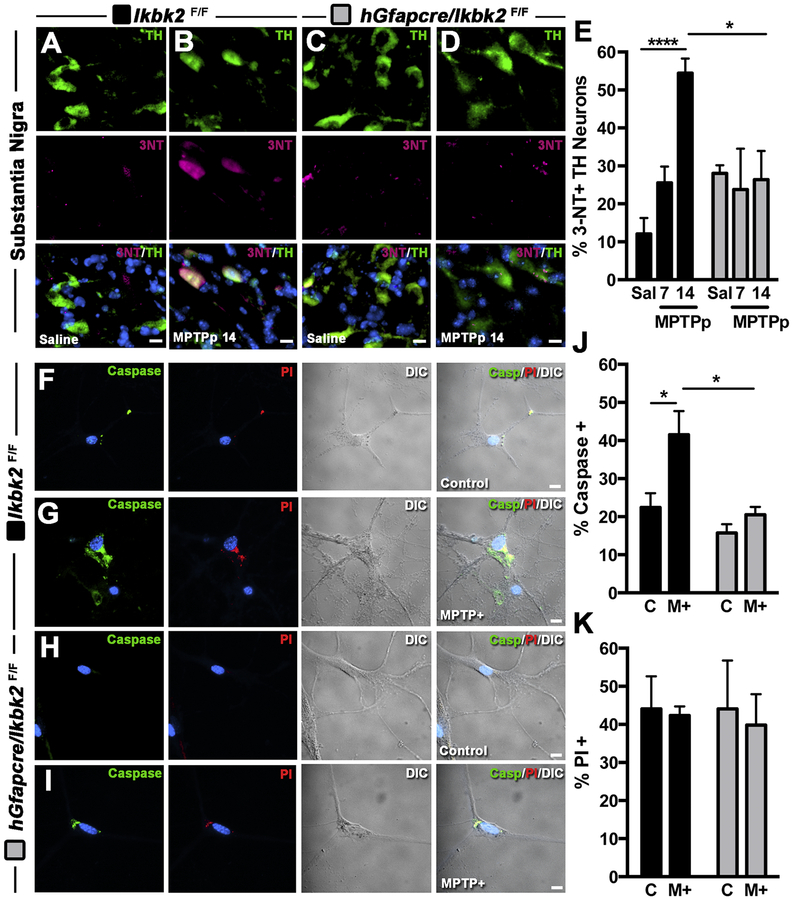
Given that deletion of IKK2 in astrocytes suppressed both glial activation and changes in expression of genes regulating inflammation and neuronal apoptosis in vivo, we wanted to determine whether attenuation of innate immune responses in astrocytes would directly modulate neuronal viability during treatment with MPTPp both in vivo and in vitro. The number of TH+ neurons containing 3-nitrotyrosine as a measure of oxidative/nitrosative stress in the SNpc was measured by co-immunofluorescence in Ikbk2F/F and hGfapcre/Ikbk2F/F mice following exposure to MPTPp (Fig. 7A–E; N between 4–7). As shown in representative 40× images, MPTPp treatment in Ikbk2F/F mice drastically elevated 3-NT production in TH+ neurons at day 14 (Fig. 7B) compared to saline-treated Ikbk2F/F mice (Fig. 7A). In contrast, hGfapcre/Ikbk2F/F mice showed minimal 3-NT protein adducts in TH+ neurons in either saline (Fig. 7C) or MPTPp-treated mice at day 14 (Fig. 7D). Two-way ANOVA analysis of percent TH+ neurons expressing 3-NT reveal an effect of treatment (F(2,19) = 7.44, p = 0.0041) with an interaction between treatment and genotype (F(2,19) = 7.99, p = 0.003; Fig. 7E). Post-hoc analysis revealed progressive increases in 3-NT in wild-type Ikbk2F/F mice following MPTPp treatment with significance from saline at day 14 (p < 0.0001), whereas hGfapcre/Ikbk2F/F mice showed no increases in the percent of TH+ neurons with 3-NT adducts following MPTPp treatment.
Figure 7.
Treatment with MPTPp results in the formation of 3-nitrotyrosine (3NT) adducts and activation of caspase 3 in dopaminergic neurons in Ikbk2F/F but not hGfapcre/Ikbk2F/F mice. A-E. Percent of 3NT expression in dopaminergic neurons was determined via immunofluorescence in the SN of Ikbk2F/F and hGfapcre/Ikbk2F/F mice treated with saline or MPTPp. Representative images of 3NT (magenta) and TH (green) co-localization counterstained with DAPI (cyan) in the SN of Ikbk2F/F (A,B) and hGfapcre/Ikbk2F/F (C, D) mice. E. Quantitative measurement of the percent of TH+ neurons within the SN of Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F (grey bars) mice positive for 3NT presented as mean percent ± SEM. F-K. Percent expression of caspase 3/7 and propidium iodide (PI) in cultured neurons co-cultured with astrocytes from Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F (grey bars) mice were determined via immunofluorescence. Representative images of caspase 3/7 (green) and PI (red) counterstained with DAPI (cyan) in cultured neurons co-cultured with astrocytes from Ikbk2F/F (F, G) and hGfapcre/Ikbk2F/F (H, I) mice exposed to vehicle or MPTP+. J. Quantitative measurement of the percent of caspase 3/7+ neurons presented as mean percent ± SEM. K. Quantitative measurement of the percent of PI+ neurons presented as mean percent ± SEM. All data were analyzed by two-way ANOVA with Tukey-Kramer post hoc test (* p < 0.05 and **** p < 0.0001).
The function of IKK2 in astrocytes in modulating neuronal injury and apoptosis in the absence of direct MPP+ toxicity was investigated using live-cell fluorescence imaging of primary neurons. Neurons were exposed to glial conditioned medium (GCM) from Ikbk2F/F or hGfapcre/Ikbk2F/F astrocytes following treatment with MPTP and inflammatory cytokines (MPTP+/M+), as shown in representative fluorescence images of caspase 3/7, PI and differential interference contrast images (Fig. 7F–J; N = 6). Activation of caspase 3/7 as a measure of apoptosis, was rarely detected in control neurons exposed to GCM from saline-treated astrocytes from either Ikbk2F/F (Fig. 7F) or hGfapcre/Ikbk2F/F (Fig.7H) mice. However, there were marked increase in caspase activation in neurons exposed to GCM from MPTP+-treated Ikbk2F/F astrocytes (Fig. 7G) but not MPTP+-treated hGfapcre/Ikbk2F/F astrocytes (Fig.7I). Two-way ANOVA analysis of the percent of neurons expressing active caspase 3/7 showed an effect of treatment (F(1,8) = 9.14, p = 0.017) and genotype (F(1,8) = 12.24, p = 0.0081; Fig. 7J). Post-hoc analysis revealed significant increases in caspase 3/7 only in neurons exposed to MPTP+ treated Ikbk2F/F astrocytes (p = 0.037) and that this increase was significantly different than the neuronal caspase 3/7 percent expression in neurons exposed to MPTP+-treated hGfapcre/Ikbk2F/F astrocytes (p = 0.023). Levels of PI were unchanged with treatment (Fig. 7K). These results indicate that treatment with MPTP leads to nitrosative stress and increased apoptosis in neurons that can be prevented by deletion of IKK2 in astrocytes.
Loss of IKK2 Prevents MPTP-Induced p65 Translocation and Activation of Inflammatory Genes in Astrocytes
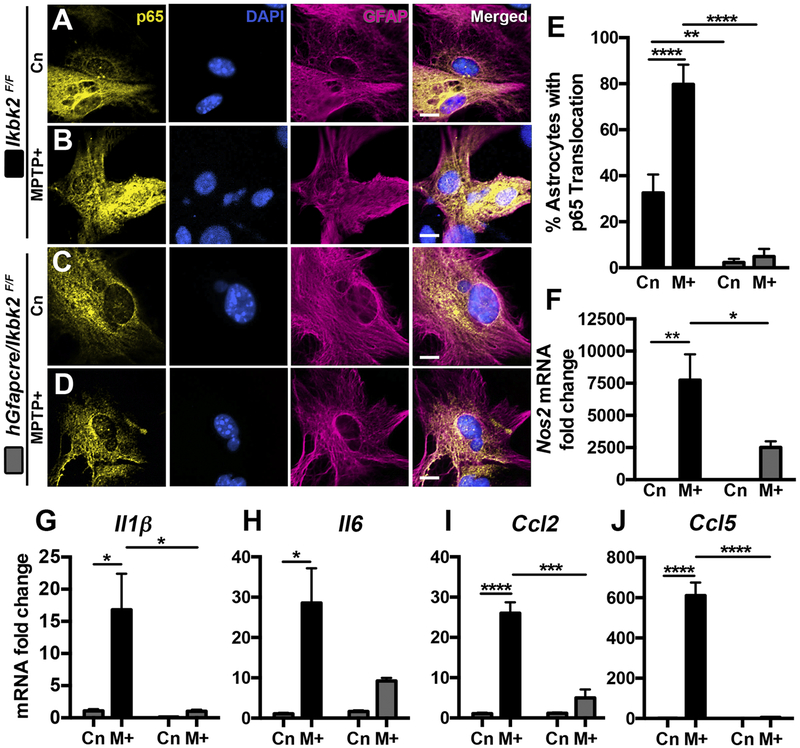
The previous data support that loss of IKK2 in astrocytes is neuroprotective both in vivo and in vitro, due at least in part to reductions in nitrosative stress and apoptosis in neurons. To identify specific factors within astrocytes regulating neuronal injury and apoptosis, we investigated translocation of p65 (Fig. 8A–E; N = 15–19) and pro-inflammatory gene expression (Fig. 8F–J; N = 3) in primary cultured astrocytes from Ikbk2F/F and hGfapcre/Ikbk2F/F mice exposed to MPTP+/M+ using immunofluorescence and qPCR, respectively. The number of astrocytes with p65 translocation to the nucleus, as shown in representative images, showed low nuclear expression in control Ikbk2F/F astrocytes (Fig. 8A) that markedly increased following 1-hour exposure to MPTP+ (Fig. 8B). In contrast, astrocytes from hGfapcre/Ikbk2F/F mice had minimal nuclear p65 in control (Fig. 8C) that remained unchanged with MPTP+ treatment (Fig. 8D). Analysis of the percent of astrocytes with p65 translocation revealed an interaction of treatment and genotype (F(1,63) = 11.69; p = 0.0011; Fig. 8E). Post-hoc analysis revealed significant differences in p65 expression in control Ikbk2F/F and hGfapcre/Ikbk2F/F astrocytes (p = 0.0071), control Ikbk2F/F and MPTP+-treated Ikbk2F/F astrocytes (p < 0.0001), and MPTP+-treated Ikbk2F/F and hGfapcre/Ikbk2F/F astrocytes (p < 0.0001). Taken together, these data demonstrate that loss of IKK2 prevents even basal levels of p65 translocation to the nucleus and inhibits p65 translocation in response to an inflammatory stimulus.
Figure 8.
Conditional deletion of Ikbk2 in astrocytes prevents p65 translocation and suppresses activation of inflammatory genes in response to MPTP treatment. Primary cultures of astrocytes from Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F (grey bars) mice were treated with vehicle or MPTP and cytokines (M+) and assessed for p65 translocation via immunofluorescence or activation of inflammatory genes by qPCR. A-D. Representative images showing p65 (yellow) translocation in GFAP+ (magenta) control or M+ treated astrocytes from Ikbk2F/F (A-B) and hGfapcre/Ikbk2F/F (C-D) mice counterstained with DAPI (cyan). E. Quantitative assessment of the % of astrocytes per image field with p65 translocation to the nucleus presented as mean percent ± SEM. F-J. mRNA fold change in inflammatory genes Nos2 (F), Il1β (G), Il6 (H), Ccl2 (I), and Ccl5 (J) in control or MPTP+ astrocytes from Ikbk2F/F (black bars) and hGfapcre/Ikbk2F/F (grey bars) mice presented as mean mRNA fold change ± SEM. All data was analyzed by two-way ANOVA with Tukey-Kramer post hoc test (* p < 0.05. ** p < 0.01, *** p < 0.001, and **** p < 0.0001).
Because nuclear translocation of p65 is necessary for NFκB-dependent transactivation of inflammatory genes, we examined the expression of multiple proinflammatory genes via qPCR in Ikbk2F/F astrocytes and in hGfapcre/Ikbk2F/F astrocytes (Fig. 8F–J). In wild type Ikbk2F/F astrocytes, exposure to MPTP+ resulted in significant increases in expression of Nos2 (p = 0.0032; Fig. 8F), Il1β (p = 0.018; Fig. 8G), Il6 (p = 0.016; Fig. 8H), Ccl2 (p < 0.0001; Fig. 8I) and Ccl5 (p < 0.0001; Fig. 8J) that was minimal to absent in MPTP+ exposed hGfapcre/Ikbk2F/F astrocytes. Analysis via two-way ANOVA revealed a significant interaction between treatment and genotype for all genes (Nos2 F(1,8) = 6.43, p = 0.035; Il1β F(1,8) = 6.95, p = 0.03; Ccl2 F(1,7) = 38.24, p = 0.0005; Ccl5 F(1,7) = 67.26, p < 0.0001) except Il6, which only showed a significant effect of genotype (F(1,7) = 12.85, p = 0.009). This indicates that deletion of IKK2 in astrocytes inhibits the ability of astrocytes to express proinflammatory genes in response to treatment with MPTP and inflammatory cytokines.
Discussion
To understand the role of inflammatory activation of astrocytes in neurodegenerative disease, we generated an astrocyte-specific IKK2 knockout mouse (hGfapcre/Ikbk2F/F), postulating that deletion of astrocytic IKK2 would protect mice against progressive neuronal loss when treated with MPTPp. To assess the extent and specificity of loss, the rate of IKK2 deletion was measured in primary astrocytes, microglia, and neurons from hGfapcre/Ikbk2F/F and littermate controls (Ikbk2F/F). The genomic frequency of recombination in astrocytes was ~ 70% (Fig. 1), which correlated with decreased protein levels as assessed by both western blotting and immunofluorescence (Figures 1 and 2). Complete deletion of IKK2 was not observed, which is commonly seen with the use of Cre/loxP systems and often attributed to epigenetic modification of Cre expression (Kaufman et al., 2008). Other conditional Cre/loxP systems have reported even lower recombination rates, including 36% in a microglial-specific IKK2 knockout (Cho et al., 2008) and only 30 to 70% seen in other Gfap-Cre mouse models (Casper et al., 2007; Chow et al., 2008). Other factors that may have influenced the frequency of deletion could be the presence of low levels of microglia contamination in cell preparations, although studies from our laboratory often show 97% astrocyte purity in culture (Carbone et al., 2008). Another possibility is a clonal expansion of cells with incomplete deletion, which could account for the robust but partial deletion of IKK2 measured in immunopurified astrocytes, which could indicate a higher deletion rate in situ.
Cre-mediated deletion of IKK2 was found to be specific to astrocytes with no apparent loss measured in cultured microglia or striatal neurons (Fig. 2). Furthermore, this preservation of IKK2 was confirmed in vivo revealing that MAP2 positive neurons of the SNpc retained IKK2 (Fig 2F–G). In vivo expression of IKK2 was not detectable in astrocytes or microglia in adult animals even in Ikbk2F/F littermates (data not shown), consistent with other studies citing the same inability to detect glial expression of IKK2 in situ (Cho et al., 2008) and is most likely due to low constitutive expression Kaltschmidt et al., 2009). We were careful to confirm the specificity of deletion given the problems of the hGFAP-cre mouse showing expression in radial glial-derived neuronal populations prior to 3 months of age (Zhou et al., 2001). We further confirmed this specificity by showing retention of IKK2 in the ST and SN neurons even though these neuronal populations are shown to be neuroepithelial derived and unlikely to express GFAP (Malatesta et al., 2003). Taken together, these data argue that we were successful in creating a conditional astrocyte-specific Ikbk2 mouse and that hGfapcre/Ikbk2F/F mice are a useful tool for elucidating the in vivo contribution of innate immune responses in astrocytes to neuroinflammation.
Other studies show that inhibition of NFκB is neuroprotective (Ghosh et al., 2007; Mondal et al., 2012, Hammond et al., 2018). However, these models employ the use of pharmacological inhibitors of NFκB and thus effects and mechanisms specific to neurons versus glial cells cannot be differentiated. Models employing specific deletion of IKK2 in astrocytes have shown to be neuroprotective (Cho et al., 2008; Brambilla et al., 2009; Dvoriantchikova et al., 2009) and evidence in cultured cells indicates that NFκB activation in astrocytes is important in MPTP-induced neuronal death (Carbone et al., 2008; Miller et al., 2011), but whether this translates to astrocytes in vivo in PD has not been proven. In this regard, our study clearly demonstrates an important role of for NFκB/IKK2-mediated astrocyte inflammation in neuronal injury in the MPTPp model of PD.
Astrocyte-specific IKK2 knockout mice (hGfapcre/Ikbk2F/F) were treated with MPTP and probenecid to induce both loss of dopaminergic neurons and progressive inflammatory activation of glia. This dosing regimen has been previously published in our lab to produce more modest and progressive lesioning with MPTP that better recapitulates the slow, continuous loss of neurons seen in PD (De Miranda et al., 2013). hGfapcre/Ikbk2F/F mice exposed to MPTP this model showed significant protection from both direct neurotoxicant induced SN dopaminergic neuronal loss as well as progressive inflammatory neuronal loss (Fig. 3). No significant loss of TH+ neurons was detected in hGfapcre/Ikbk2F/F mice at day 7 and only 36% loss at day 14, compared to 45% and 60% loss in WT mice at day 7 and 14, respectively. This indicates that NFκB-dependent neuroinflammation in astrocytes has a vital role in not only progressive neuronal degeneration, but also in the initiation of MPTP neurotoxicity. This is consistent with experimental evidence where use of anti-inflammatory approaches prior to induction of neurotoxic injury to dopamine neurons was shown to be neuroprotective (Sugama et al., 2003; Sastre, 2010) but is in contrast to a study published by (Oeckl et al., 2012) where overexpression of astrocytic IKK2 did not enhance sensitivity to MPTP. However, Oeckl et al., 2012 employed an acute MPTP dosing strategy and showed enhanced neuroinflammation and loss of neurons even prior to dosing with high levels of constitutive neuroinflammation. Our data suggest that limiting innate immune inflammatory responses in astrocytes through the NFκB signaling pathway is directly neuroprotective.
In accordance with preservation of dopaminergic neurons, measures of neurobehavioral function in MPTPp-exposed mice indicated significant protection of hGfapcre/Ikbk2F/F mice from reductions in locomotor activity (Fig. 4). This difference from littermate controls was most evident on day 7 of the treatment course. There appeared to be some functional recovery from these motor disturbances in all mice because, by day 14, hGfapcre/Ikbk2F/F mice treated with MPTPp showed observable downward trends in both aforementioned parameters while treated Ikbk2F/F mice showed partial recovery. The reversible nature of MPTP effects on hypokinesia is often observed after cessation of MPTP treatments (Sedelis et al., 2001, Miller et al., 2011), although other studies utilizing pharmacological inhibition of NFkB (Ghosh et al., 2007, Mondal et al., 2012) showed more persistent protection against changes in neurobehavior than measured in this study. These differences could be a factor that microglial and neuronal expression of NFκB remain unaltered in this model and thus may have important influences on MPTPp-induced alterations in locomotion.
The observed differences in neurobehavioral parameters between Ikbk2F/F and hGfapcre/Ikbk2F/F mice were not explainable by alterations in striatal catecholamines. Measurements of DA, DOPAC, and HVA were sharply reduced in striatal tissue all mice at both day 7 and day 14 even in the face of improved neurobehavior measures (Fig. 4). Other MPTP models have shown a similar disconnect between neurochemistry and neurobehavioral parameters (Dehmer et al., 2003; Miller et al., 2011; Mondal et al., 2012) that most likely reflects the insensitivity of catecholamine measurements to reflect subtle changes in brain environment and the ability of other systems to compensate for the changes in dopamine (Przedborski et al., 2000).
Despite lack of protection against MPTPp-induced loss of striatal catecholamines, hGfapcre/Ikbk2F/F mice showed marked protection against dopaminergic nerve terminal loss (Fig. 3), striatal protein loss (Figs. 3 and 4) and MPTPp-induced peroxynitrite formation (Fig.7) as compared to their wild-type Ikbk2F/F littermates. VMAT and DAT are important in regulating intracellular and extracellular concentrations of DA with levels but functionally decline in PD due to protein loss and nitrosylation (German et al., 2015; Hammond et al., 2018). Additionally, peroxynitrite formed from NOS2 and NAPDH oxidase upregulation in glia impairs neuronal function further contributing to neurobehavioral alterations (McCarty, 2006). Thus, preservation of these synaptic proteins likely contributes to preservation of locomotor reduction in Ikk2 KO mice.
Loss of dopaminergic neurons is mediated by numerous factors including excitotoxicity, oxidant stress, mitochondrial dysregulation and direct cytotoxicity by glia-derived cytokines (Tansey and Goldberg, 2010; Durrenberger et al., 2014) that lead to neuronal apoptosis (Venderova and Park, 2012). In this model, we established that MPTPp treatment resulted in increased apoptotic gene expression in vivo (Fig. 6) that was reduced by deletion of IKK2 in astrocytes. These protections were unlikely caused by alterations in MPTP metabolism as alterations in MPTP metabolism are not shown when astrocyte IKK2 expression is altered (Oeckl et al., 2012). Because microarray of brain tissue is limited in power and specificity (Nisenbaum, 2002; Lewis and Cookson, 2012), we further confirmed protection of neuronal apoptosis through cell culture experiments (Fig. 7), confirming IKK2 deletion in astrocytes prevented caspase activation in neurons.
The decline in apoptotic gene expression was correlated with decreased neuronal nitrosative stress (Fig. 7), levels of gliosis (Fig. 5) and inflammatory gene expression (Figs. 6, 8 and Supp. Fig. 1, 2). IKK2 deletion in astrocytes only partly limited MPTPp-induced increases in astrocyte and microglia counts in the SN and was only fully protective against microglia increases in the ST. However, there was significant protection against astrocyte expression of inflammatory genes/proteins such as TNF, NOS2 and other cytokines and chemokines, most likely through suppression of p65 translocation (Fig. 8). Other studies of IKK2 suppression in astrocytes report similar results whereby astrocytosis remains unaltered while other parameters of glial inflammation including microgliosis are suppressed (More et al., 2013; Saggu et al., 2016; Douglass et al., 2017; Zhang et al., 2017), suggesting that IKK2 induces a reactive A1 inflammatory phenotype in astrocytes that subsequently enhances the reactivity of microglia (Liddelow et al., 2017). This finding may also be more broadly applicable to the role of reactive astrocytes in neurodegeneration, highlighted by studies demonstrating that an adeno-associated virus-based approach for inhibition of the Ca2+/calmodulin-dependent phosphatase calcineurin (CN) and its target transcription factor, nuclear factor of activated T cells (NFAT4), in astrocytes prevents neuronal injury and Alzheimer’s pathology in a mouse model of Alzheimer’s disease (Sompol et al., 2017). The role of glial-glial communication in amplifying neuroinflammation (Saijo et al., 2009; Liddelow et al., 2017) underscores that targeting signaling pathways in astrocytes that regulate innate immune inflammatory responses can both reduce the severity of microgliosis and increase neuronal survival. We recently reported on this phenomenon, demonstrating that IKK2 in astrocytes directly regulates microglial reactivity through release of the chemokine, CCL2, which is required for neuronal injury in response to the neurotoxic metal, manganese (Popichak et al., 2018).
These data demonstrate that we were successful in generating mice with an astrocyte-specific deficiency in NFκB signaling. Utilizing this animal in a neurotoxin-based model of PD showed that inhibition of neuroinflammatory activation of astrocytes via NFκB protected against loss of dopaminergic neurons in the substantia nigra. The high degree of neuroprotection we observed in this model was associated with reductions in reactive gliosis and decreased production of glial-derived inflammatory mediators both in vivo and in vitro. This indicates that NFκB signaling in astrocytes is an important regulator of neuronal pathology in the MPTPp model of PD and also suggests that this and related pathways regulating neuroinflammation and glial reactivity could be important therapeutic targets for disease modification.
Supplementary Material
Highlights.
Inflammatory activation of astrocytes is a pathological feature of neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD)
The transcription factor, nuclear factor kappa B (NFκB), is an key regulator of inflammatory gene expression in glia
Cell-specific knockout mice were constructed to delete IKK2, the upstream activating kinase for NFκB, in astrocytes
IKK2 knockout mice had reduced neuronal injury and glial activation following treatment with MPTP than wildtype control mice
IKK2 is an important modulator of astrocyte phenotype and regulates the production of neurotoxic inflammatory genes in the MPTP model of PD
Acknowledgments:
The authors would like to thank Michael Karin for providing the floxed-Ikbk2 mouse and other members of the Tjalkens lab (Jim Miller and Briana De Miranda) who supported completion of this study. This work was funded by National Institutes of Health grant 5R01ES024183 (RBT).
Glossary
- DAT
Dopamine Transporter
- GFAP
Glial Fibrillary Acidic Protein
- IBA-1
Ionized Binding Adaptor protein-1
- iNOS/NOS2
Inducible Nitric Oxide Synthase
- IKK2
Inhibitory kappa alpha kinase beta
- NFκB
Nuclear Factor kappa Beta
- PD
Parkinson’s Disease
- ST
Striatum
- SN
Substantia Nigra
- TNF
Tumor Necrosis Factor alpha
- TH
Tyrosine Hydroxylase
- VMAT
Vesicular Monoamine Transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschner M, Kimelberg HK (1991) The use of astrocytes in culture as model systems for evaluating neurotoxic-induced-injury. Neurotoxicology 12:505–517. [PubMed] [Google Scholar]
- Bonizzi G, Karin M (2004) The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends in Immunology 25:280–288. [DOI] [PubMed] [Google Scholar]
- Brambilla R (2005) Inhibition of astroglial nuclear factor B reduces inflammation and improves functional recovery after spinal cord injury. Journal of Experimental Medicine 202:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR (2009) Transgenic Inhibition of Astroglial NF-B Improves Functional Outcome in Experimental Autoimmune Encephalomyelitis by Suppressing Chronic Central Nervous System Inflammation. The Journal of Immunology 182:2628–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone DL, Popichak KA, Moreno JA, Safe S, Tjalkens RB (2008) Suppression of 1 - Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-Induced Nitric-Oxide Synthase 2 Expression in Astrocytes by a Novel Diindolylmethane Analog Protects Striatal Neurons against Apoptosis. Molecular Pharmacology 75:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper KB, Jones K, McCarthy KD (2007) Characterization of astrocyte-specific conditional knockouts. Genesis 45:292–299. [DOI] [PubMed] [Google Scholar]
- Casper KB, McCarthy KD (2006) GFAP-positive progenitor cells produce neurons and oligodendrocytes throughout the CNS. Molecular and Cellular Neuroscience 31:676–684. [DOI] [PubMed] [Google Scholar]
- Chen PC, Vargas MR, Pani AK, Smeyne RJ, Johnson DA, Kan YW, Johnson JA (2009) Nrf2-mediated neuroprotection in the MPTP mouse model of Parkinson’s disease: Critical role for the astrocyte. Proc Natl Acad of Sci USA 106:2933–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S, Hou J, Zhang C, Xu C, Wang L, Zou X, Yu H, Shi Y, Yin Z, Chen G (2015). MInocycline reduces neuroinflammation but does not ameliorate neuronal loss in a mouse model of neurodegeneration. Scientific Reports 5:10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IH, Hong J, Suh EC, Kim JH, Lee H, Lee JE, Lee S, Kim CH, Kim DW, Jo EK, Lee KE, Karin M, Lee SJ (2008) Role of microglial IKK in kainic acid-induced hippocampal neuronal cell death. Brain 131:3019–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LML, Zhang J, Baker SJ (2008) Inducible Cre recombinase activity in mouse mature astrocytes and adult neural precursor cells. Transgenic Res 17:919–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Wei Huang, Sherman BT, Lempicki RA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature Protocols 4:44–57. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 52:1–6. [DOI] [PubMed] [Google Scholar]
- De Miranda BR, Miller JA, Hansen RJ, Lunghofer PJ, Safe S, Gustafson DL, Colagiovanni D, Tjalkens RB (2013) Neuroprotective Efficacy and Pharmacokinetic Behavior of Novel Anti-Inflammatory Para-Phenyl Substituted Diindolylmethanes in a Mouse Model of Parkinson’s Disease. Journal of Pharmacology and Experimental Therapeutics 345:125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmer T, Heneka MT, Sastre M, Dichgans J, Schulz JB (2003) Protection by pioglitazone in the MPTP model of Parkinson’s disease correlates with IκBα induction and block of NFκB and iNOS activation. J Neurochem 88:494–501. [DOI] [PubMed] [Google Scholar]
- Dehmer T, Lindenau J, Haid S, Dichgans J, Schulz JB (2000) Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J Neurochem 74:2213–2216. [DOI] [PubMed] [Google Scholar]
- Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP (2017) Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Molecular Metabolism 6:366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrenberger PF, Fernando FS, Kashefi SN, Bonnert TP, Seilhean D, Nait-Oumesmar B, Schmitt A, Gebicke-Haerter PJ, Falkai P, Grünblatt E, Palkovits M, Arzberger T, Kretzschmar H, Dexter DT, Reynolds R (2014) Common mechanisms in neurodegeneration and neuroinflammation: a BrainNet Europe gene expression microarray study. J Neural Transm 122:1055–1068. [DOI] [PubMed] [Google Scholar]
- Dvoriantchikova G, Barakat D, Brambilla R, Agudelo C, Hernandez E, Bethea JR, Shestopalov VI, Ivanov D (2009) Inactivation of astroglial NF-κB promotes survival of retinal neurons following ischemic injury. European Journal of Neuroscience 30:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, Geczy CL, Halliday GM (2015) Inflammation is genetically implicated in Parkinson’s disease. Neuroscience 302:89–102. [DOI] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegeneration 4:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson A, Plaznik A, Sundstrom E, Jonsson G, Archer T (1990) MPTP-induced hypoactivity in mice: reversal by L-dopa. Pharmacol Toxicol 67:295–301. [DOI] [PubMed] [Google Scholar]
- German CL, Baladi MG, McFadden LM, Hanson GR, Fleckenstein AE, Daws LC (2015) Regulation of the Dopamine and Vesicular Monoamine Transporters: Pharmacological Targets and Implications for Disease. Pharmacol Rev 67:1005–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K (2007) Selective inhibition of NF-kB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc Natl Acad Sci USA 104:18754–18759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms Underlying Inflammation in Neurodegeneration. Cell 140:918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RR, Hogan CEC, Neal MLM, Anantharam VV, Kanthasamy AGA, Kanthasamy AA (2011) A simple magnetic separation method for high-yield isolation of pure primary microglia. J Neurosci Methods 194:287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilli M, Memo M (1999) Nuclear factor-κB/Rel proteins. Biochemical Pharmacology 57:1–7. [DOI] [PubMed] [Google Scholar]
- Hammond SL, Popichak KA, Li X, Hunt LG, Richman EH, Damale PU, Chong EKP, Backos DS, Safe S, Tjalkens RB (2018) The Nurr1 Ligand, 1, 1-bis(3’-Indolyl)-1-(p-Chlorophenyl)Methane, Modulates Glial Reactivity and Is Neuroprotective in MPTP-Induced Parkinsonism. Journal of Pharmacology and Experimental Therapeutics 365:636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann O, Baumann B, de Lorenzi R, Muhammad S, Zhang W, Kleesiek J, Malfertheiner M, Köhrmann M, Potrovita I, Maegele I, Beyer C, Burke JR, Hasan MT, Bujard H, Wirth T, Pasparakis M, Schwaninger M (2005) IKK mediates ischemia-induced neuronal death. Nat Med 11:1322–1329. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Hunot S (2009) Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol 8:382–397. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acid Research 37: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S, Brugg B, Ricard D, Michel PP, Muriel M-P, Ruberg M, Faucheux BA, Agid Y, Hirsch EC (1997) Nuclear translocation of NF-κB is increased in dopaminergic neurons of patients with Parkinson disease. Proc Natl Acad Sci USA 95: 7531–7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M (1999) the role of the IkB kinase (IKK) complex. Oncogene:1–8. [DOI] [PubMed] [Google Scholar]
- Karin M (2005) Inflammation-activated Protein Kinases as Targets for Drug Development. Proceedings of the American Thoracic Society 2:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman WL, Kocman I, Agrawal V, Rahn HP, Besser D, Gossen M (2008) Homogeneity and persistence of transgene expression by omitting antibiotic selection in cell line isolation. Nucleic Acids Research 36:e111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley KS, Walton KD, Duncan C, and Tjalkens RB. 2017. Spontaneous Development of Cutaneous Squamous Cell Carcinoma in Mice with Cell-specific Deletion of Inhibitor of kappaB Kinase 2. Comp Med. 67:407–415. [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier A, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, and Barres BA. 2017. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 541:481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popichak KA, Afzali MF, Kirkley KS, and Tjalkens RB. 2018. Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese. J Neuroinflammation. 15:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompol P, Furman JL, Pleiss MM, Kraner SD, Artiushin IA, Batten SR, Quintero JE, Simmerman LA, Beckett TL, Lovell MA, Murphy MP, Gerhardt GA, and Norris CM. 2017. Calcineurin/NFAT Signaling in Activated Astrocytes Drives Network Hyperexcitability in Abeta-Bearing Mice. J Neurosci. 37:6132–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley KS, Popichak KA, Afzali MF, Legare ME, Tjalkens RB (2017) Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. Journal of Neuroinflammation 14:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis PA, Cookson MR (2012) Gene expression in the Parkinson’s disease brain. Brain Res Bull 88:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA (2017). Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 46(6):957–967 [DOI] [PubMed] [Google Scholar]
- Li Z-W, Omori SA, Labuda T, Karin M, Rickert RC (2003) IKK beta is required for peripheral B cell survival and proliferation. J Immunol 170:4630–4637. [DOI] [PubMed] [Google Scholar]
- Liu XX, Sullivan KAK, Madl JEJ, Legare MM, Tjalkens RBR (2006) Manganese-induced neurotoxicity: the role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol Sci 91:521–531. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, Kettenmann H, Klinkert W, Kirchhoff F, Götz M (2003) Neuronal or glial progeny: regional differences in radial glia fate. Neuron 37:751–764. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Camandola S (2001) NF-κB in neuronal plasticity and neurodegenerative disorders. J Clin Invest 107:247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF (2006) Down-regulation of microglial activation may represent a practical strategy for combating neurodegenerative disorders. Medical Hypotheses 67:251–269. [DOI] [PubMed] [Google Scholar]
- Mettang M, Reichel SN, Lattke M, Palmer A, Abaei A, Rasche V, Huber-Lang M, Baumann B, Wirth T (2018) IKK2/NF-κB signaling protects neurons after traumatic brain injury. The FASEB Journal 32:1916–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Trout BR, Sullivan KA, Bialecki RA, Roberts RA, Tjalkens RB (2011) Low-dose 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine causes inflammatory activation of astrocytes in nuclear factor-κB reporter mice prior to loss of dopaminergic neurons. J Neurosci Res 89:406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S, Roy A, Jana A, Ghosh S, Kordower JH, Pahan K (2012) Testing NF-κB-based Therapy in Hemiparkinsonian Monkeys. Jrnl NeuroImmune Pharm 7:544–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More SV, Kumar H, Kim IS, Song S-Y, Choi D-K (2013) Cellular and molecular mediators of neuroinflammation in the pathogenesis of Parkinson’s disease. Mediators of Inflammation 2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, Tjalkens RB (2008) Manganese potentiates nuclear factor-kappaB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. J Neurosci Res 86:2028–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Yeomans EC, Streifel KM, Brattin BL, Taylor RJ, Tjalkens RB (2009) Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicological Sciences 112:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M (2005) Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des 11:999–1016. [DOI] [PubMed] [Google Scholar]
- Neal M, Richardson JR (2018) Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration. Biochim Biophys Acta 1864:432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum LK (2002) The ultimate chip shot: can microarray technology deliver for neuroscience? Genes Brain Behav 1:27–34. [DOI] [PubMed] [Google Scholar]
- Oeckl P, Lattke M, Wirth T, Baumann B, Ferger B (2012) Astrocyte-specific IKK2 activation in mice is sufficient to induce neuroinflammation but does not increase susceptibility to MPTP. Neurobiology of Disease 48:481–487. [DOI] [PubMed] [Google Scholar]
- Ouchi Y, Yagi S, Yokokura M, Sakamoto M (2009) Neuroinflammation in the living brain of Parkinson’s disease. Parkinsonism Relat Disord 15 Suppl 3:S200–S204. [DOI] [PubMed] [Google Scholar]
- Perez FA, Palmiter RD (2005) Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci USA 102:2174–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Djaldetti R, Liberatore G, Vila M, Vukosavic S, Almer G (2000) The parkinsonian toxin MPTP: action and mechanism. Restor Neurol Neurosci 16:135–142. [PubMed] [Google Scholar]
- Saggu R, Schumacher T, Gerich F, Rakers C, Tai K, Delekate A, Petzold GC (2016) Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathologica Communications 2016 4:1–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK (2009) A Nurr1/CoREST Pathway in Microglia and Astrocytes Protects Dopaminergic Neurons from Inflammation-Induced Death. Cell 137:47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M (2010) NSAIDs: how they work and their prospects as therapeutics in Alzheimer’s disease. Front Ag Neurosci 2:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RKW, Huston JP (2001) Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behavioural Brain Research 125:109–125. [DOI] [PubMed] [Google Scholar]
- Streifel KM, Moreno JA, Hanneman WH, Legare ME, Tjalkens RB (2012) Gene deletion of nos2 protects against manganese-induced neurological dysfunction in juvenile mice. Toxicological Sciences 126:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Yang L, Cho BP, DeGiorgio LA, Lorenzl S, Albers DS, Beal MF, Volpe BT, Joh TH (2003) Age-related microglial activation in 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Research 964:288–294. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS (2010) Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiology of Disease 37:510–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher V, Tibshirani R, Chu G (2001) SAM Significance Analysis of Microarraysapplied to transcriptional responses to ionizing radiation. Proc Natl Acad Sci USA 98:5116–5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loo G, de Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, Pasparakis M (2006) Inhibition of transcription factor NF-kB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol 7:954–961. [DOI] [PubMed] [Google Scholar]
- Venderova K, Park DS (2012) Programmed Cell Death in Parkinson’s Disease. Cold Spring Harb Perspect Med 2:a009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Reichel JM, Han C, Zuniga-Hertz JP, Cai D (2017) Astrocytic Process Plasticity and IKKbeta;/NF-&kappaB in Central Control of Blood Glucose, Blood Pressure, and Body Weight. Cell Metabolism 25:1091–1102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo L, Theis M, Alvarez-Maya I, Brenner M, Willecke K, Messing A (2001) hGFAP-cre transgenic mice for manipulation of glial and neuronal function in vivo. Genesis 31:85–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.