Abstract
Infectious diseases represent a major public health challenge worldwide. There are various modes for the transmission of these diseases, with surface and airborne transmission being two of the most important ones. The inefficiencies of current intervention methods have resulted in the emergence of nosocomial infections. Here, we report the use of a nanotechnology based antimicrobial platform using Engineered Water Nanostructures (EWNS) generated using a combined electrospray and ionization of an aqueous suspension of various active ingredients (AIs). These EWNS based nano-sanitizers were tested in terms of their ability to efficiently deliver AI and inactivate Acinetobacter baumannii and Influenza H1N1/PR/8 on both surfaces and air. Results indicate a significant reduction in the concertation of the pathogens, while the delivered to pathogen AI doses required for inactivation were miniscule (nanogram level), indicating the viability of such nano-carrier platform as an intervention technology against infectious microorganisms.
Keywords: Nano-carrier, Engineered Water Nanostructures, Airborne Influenza, Nosocomial Infections
Introduction
Infectious disease transmission occurs primarily through direct contact, indirect contact, and airborne routes1–3. Pathogens have been known to survive on inanimate surfaces for days to weeks4, making them extremely difficult to eradicate. In particular, hospitals environments have an increased risk due to susceptible populations and large numbers of infected patients in close proximity, as sources of infection. Each year, nearly two million Americans develop hospital-acquired infections (HAI), resulting in 99,000 deaths, ranking death due to HAIs among the top 5 leading causes of death in the United States5. Tackling these infections poses a great challenge to the healthcare industry, not only in terms of patient morbidity and mortality, but also with an ever-increasing cost of treatment. The average cost of treating HAI can be as high as $45000 per case6. Overall, CDC reports estimate the total financial burden of HAIs to be $35 to $45 billion annually7.
The major routes of nosocomial transmission are either through surfaces such as hospital beddings, tools and medical equipment, from patient to patient, or, for some pathogens such as Mycobacterium tuberculosis, through air1,8,9. Microorganisms such as Klebsiella pneumonia10, Pseudomonas aeruginosa and more recently Acinetobacter baumannii11 have been identified as some of the major species involved. These species are often also multi-drug resistant, increasing the difficulty of treatment12.
In addition to these bacterial infections, viral infections also pose a major challenge to the healthcare environment. Viral infections constitute 60% of all infections13, and unlike bacterial diseases, viral illness cannot be tackled through the use of antibiotics. There is evidence that contaminated surfaces play a key role in the spread of nosocomial viral infections13, with Influenza being one of the major viruses responsible14.
Currently, there are various traditional methodologies that are being employed for tackling this issue of nosocomial infections in the healthcare setting, beyond the basic measures such as proper hand hygiene to prevent cross contamination15. Some of these methods utilize chemical gases/vapors as disinfectants for surfaces (i.e. hydrogen peroxide mist), as well as the use of antiseptics (i.e. chlorhexidine and iodine)16. UV-C installations and gas plasma sterilizers are also emerging as hospital surface disinfection options17,18. More recently nanotechnology based antimicrobial materials (i.e. silver nanoparticles) are also being used with silver dressings, silver coated medicinal devices and surfaces, among other uses19,20. There are however, many limitations associated with these approaches. Chlorhexidine, for example, has been shown to produce chemical toxicity on the skin and retard wound healing21, UV and other technologies such as e-beam ionizers have high investment costs and require bulky specialized systems for exposure22. Silver and other engineered nanoparticles have been shown to have cytotoxic effects when inhaled or ingested23–28. Clinical options are often ineffectual due to the high incidence of antibiotic resistance29.
Apart from surface transmission, airborne transmission of influenza viruses from infected individuals to uninfected individuals through aerosolized droplets expelled during exhalation, such as during coughing, sneezing, and tidal breathing requires attention30,31. This is an especially dangerous mode of transmission as the recent emergence of zoonotic pathogens such as avian influenza A viruses (e.g. H5N1 and H7N9) has raised the risk of future influenza pandemics with unprecedented health and economic impacts if these pathogens can be transmitted through the aerial route32.
There are a few technologies currently being employed, such as HEPA air filtration, UV radiation, and other newer methods such as air ionizer and electrical discharges33–35 currently employed for air disinfection. However, these technologies have their inherent limitations and drawbacks, as HEPA filtration requires high clean air delivery rates that cannot be achieved in many cases, UV relies on the well-mixed room air concept and air ionizers and electrical discharges require high energy consumption36. Other disinfection methods based on the use of biocidal gases, such ethylene oxide, vaporized hydrogen peroxide, ozone, and chlorine dioxide, also have serious limitations associated with inhalation toxicity37,38.
Recently, our group has developed a novel nanotechnology based method for the inactivation of microorganisms on surfaces and in air using Engineered Water Nanostructures (EWNS). These EWNS are synthesized using a novel method consisting of the electrospray and ionization of deionized (DI) water. The resulting nanostructures have unique properties such as high surface electric charge, nanoscale size and are loaded with reactive oxygen species (ROS) generated from ionization of water39–44. The EWNS platform was further expanded recently as a carrier platform to incorporate antimicrobial active ingredients (AI) into EWNS chemical structure. A number of AIs were used to produce ‘iEWNS’ nano-sanitizers with “i” denoting the incorporation of “i” active ingredient. This EWNS based nano-carrier platform can be utilized for the precise and targeted delivery of miniscule, yet effective, quantities of AIs, in order to produce efficient inactivation on surfaces of food related pathogens such as Listeria innocua, with no chemical residues and byproducts39,45.
In this study, the efficacy of iEWNS nano-sanitizers for inactivation of nosocomial infection related viruses and bacteria on both surfaces and air was evaluated. Three distinct iEWNS nano-sanitizers were synthesized using DI water (termed EWNS), 1% w/v Hydrogen peroxide solution (h1EWNS) and Electrolyzed water (rEWNS). Their ability to inactivate Influenza H1N1/PR/8 virus on surfaces was assessed. Furthermore, the h1EWNS nano-sanitizer was chosen, due to its higher efficacy, to inactivate the gram-negative Acinetobacter baumannii bacterium on surfaces and H1N1/PR/8 in air was also assessed.
Methods
Synthesis of iEWNS nano-sanitizers
iEWNS Generation: Figure 1 illustrates the concept for the generation of the iEWNS nano-aerosol. The details of their synthesis have been detailed in our recent publication45. In short, the generation module consists of a stainless-steel capillary (termed iEWNS emitter) (0.3-mm diameter, Hamilton Robotics, Reno, NV) held vertically and a grounded funnel shaped electrode assembly placed directly underneath. The top of the iEWNS generating capillary is connected to the container containing the A.I. solution. The reservoirs are connected to an air compressor, which is used to push the liquid flow through each individual capillary. A high voltage power supply (Spraybase, Cambridge, MA) is utilized to produce the −6.8 kV to be delivered to the metal capillary. The capillary is held at a distance of 4 cm from the ground electrode. The grounded electrode is an aluminum disk with a 0.72 cm opening at the center. The disk sits atop a funnel that was connected to a sampling apparatus that can be used for iEWNS characterization as outlined in our previous publications39,40. During the process of iEWNS generation, the applied electric field causes, at the tip of the individual capillary, the formation of the so-called Taylor cone, and, from the tip of this cone, highly charged particles, of the aqueous suspension of various AIs, are emitted. The formation and stability of this Taylor cone is observed visually using a camera (Point Grey Cameleon, FLIR Integrated Imaging Solutions Inc., Richmond, Canada). Each capillary is monitored individually for the formation and stability of the Taylor cone.
Figure 1:
Concept of the iEWNS generation. The Active Ingredient (A.I.) solution is added to a container held under pressure. The iEWNS emitter (needle) is connected to a high voltage source. The iEWNS particles emitted are collected through a funnel placed underneath the emitter. The iEWNS particles are loaded with ROS and A.I. utilized to produce them.
Active Ingredients utilized: Three distinct solutions were utilized for the production of iEWNS based nano-sanitizers. For the production of baseline EWNS, only DI water (Nanopure, Barnstead Water, Van Nuys, CA) was utilized. The AI solution for rEWNS was prepared through the electrolysis of DI water, in order to enhance the ROS levels. The electrolysis was performed with a fixed current of 0.2 A for a duration of 1 hour. This process is further explained in earlier work39. For the h1EWNS generation, the AI solution was prepared by diluting 30% hydrogen peroxide (w/v) solution (Sigma Aldrich, St. Louis, MO) in DI water to a 1% (w/v) concentration.
Microorganism inactivation on surface
Exposure setup: Figure 2 shows the three-emitter setup utilized in the study. The three emitters can work independently of each other as described above. Each iEWNS emitter is connected to an AI solution containing bottle. The flow of the A.I. solution was adjusted by the air pressure and was fine tuned to optimal condition by adjusting the relative height of the bottle to the tip of the emitter. Each emitter was visually inspected for proper functioning during the experiments with a digital camera that ensure that the Taylor cone was present and in the right state.
Figure 2:
Experimental setup for the inactivation of microorganisms on surfaces. The three emitter setup for exposure is shown. The exposure chamber contains three iEWNS emitters placed laterally as shown.
Exposure protocol: Stainless steel coupons inoculated with the pathogens, as described below, were placed under each emitter for a predetermined amount of time depending on the iEWNS and the pathogen. Once the exposure was completed, the coupons were removed and were processed, in order to recover the pathogens. The recovery protocol depended on the pathogen and is described below.
Controls were executed with inoculated stainless steel coupons that were kept in the same environmental conditions away from the iEWNS for equal timepoints as the treatment. During the experiments, the environmental conditions such as Relative Humidity and Temperature were closely monitored and were kept the same between control and exposure.
Bacterial exposure on surface protocols
Bacterial culture: Acinetobacter baumannii (ATCC #19606) was obtained from ATCC (Manassas, VA). A culture was grown overnight in Tryptic Soy Broth (TSB) (Hardy Diagnostics, Santa Maria, CA) inside a shaker incubator at 37°C, centrifuged at 3000 rpm for five minutes and the pellet was re-suspended in DI water. The final concentration of the inoculum was adjusted to 108 cfu/ml through O.D.600 measurement.
Inoculation methodology: 10 μl the bacterial inoculum was distributed on stainless steel coupons (diameter 1.82 cm, stainless steel 304, Stainless Supply, Monroe NC), by adding ten 1-μl droplets in a concentric manner near the center of the coupon. Thus, the effective concentration of bacteria on the coupons was 106 cfu/coupon. The inoculated coupons were then placed inside a petri dish and allowed to dry, placed inside a biosafety cabinet. The exposure coupons were then removed from the petri dishes and utilized for experimentation in the exposure chamber, whilst the control coupons were placed in the same exposure chamber, but isolated from the iEWNS, as previously described.
Recovery and enumeration: Post-exposure, the control and exposed coupons were each added to a 50-ml micro-centrifuge tube containing 5 ml of 1x phosphate-buffered saline (VWR International, Radnor PA). The coupons were vortexed for 30 seconds and the resulting rinsate was utilized for a dilution plate counting assay.
Viral inactivation on surface protocols
Viral culture: A suspension of influenza virus (A/PR/8/34 H1N1) (Virasource Inc., Durham NC) was used. A stock of 107 Plaque Forming Units/ml (pfu/ml) of the virus was utilized for these experiments. The stock was thawed, divided into single-use portions, and stored at −80°C until needed.
Inoculation methodology: The inoculation methodology for the Influenza/H1N1/PR/8 was as previously described for the bacterial inoculation, with the following changes: 10 μl of 107 pfu/ml viral stock solution was utilized for inoculation, with the effective concentration of virus on the coupons being 105 pfu. The exposure protocol was identical to the one described above for the bacterial exposure.
Inoculum recovery and enumeration: Post exposure, the coupons were removed and washed in 2 ml of influenza virus growth media containing DMEM with L-Glutamine (Gibco, Life Technologies, Carlsbad CA) supplemented with 25mM HEPES (Lonza, Basel Switzerland), 1% 100X Pen/Strep (Fisher Scientific, Waltham MA), 0.2% BSA (Sigma Aldrich, St.Louis MO) and 2 ug/ml L-1-Tosylamide-2-phenylethyl chloromethyl ketone (TPCK)-treated trypsin. The viral inoculum was further diluted to 5ml with influenza virus growth media to a final concentration of 2 ×104 pfu/ml (assuming complete recovery of virus without decay). Serial dilutions were prepared at 1:2 inoculum/viral growth media. For each dilution 100ul of viral suspension was then added to 10 wells of a 96 well plate containing washed MDCK cells. The plates were incubated for 2 hours. The viral suspension was then removed by gentle aspiration and washed once. A final 200ul of virus infection media was added to the cells and further incubated for 48–72 hours. The cytopathic effect (CPE) was observed in each well under an inverted microscope and noted. The plates were then fixed with 4% formaldehyde for one hour, decanted and stained with 0.5% Crystal Violet in 40% ethanol for approximately 30 minutes. The stain was decanted and the plates were washed with PBS and allowed to dry in a biosafety hood. The plates were then scored as live/dead. The 50% Tissue culture Infective Dose (TCID50) score was obtained as the dilution at which 50% of the wells of the assay showed CPE. This value was then converted into the Infectious Unite (IU)/ml value by utilizing the Spearman Kerber Method46.
Inactivation of Influenza H1N1/PR/8 virus in air
Figure 3 shows the experimental setup for the airborne Influenza inactivation experiments. The setup consisted of four components: 1) an environmental chamber; 2) bioaerosol generation setup; 3) iEWNS generation system; and 4) bio-aerosol sampling apparatus. The methodology of exposure is described below.
Figure 3:
Schematic for the inactivation of Influenza H1N1/PR/8 in air, utilizing the environmental chamber.
Environmental chamber: The environmental chamber consisted of a stainless-steel box of 150-liter volume. The chamber was manufactured with a removable stainless steel top (Minitec Framing, Farmington, NY). The chamber contained 1/2 inch diameter ports on each lateral face, for use as injection/sampling ports. A pressure gauge was connected at one of the ports to check for pressure inside chamber. A temperature and RH probe was inserted into the chamber through one of the ports. All surfaces of the chamber were grounded with external grounding. All exhaust points from the chamber were equipped with HEPA filters and contained within the biological safety cabinet. The total airflow through the environmental chamber during experiments was maintained at 8.1 lpm corresponding to an Air Changes per Hour (ACH) rate of 3.24 (Figure 3 summarizes all air flow conditions).
Viral bioaerosol generation: Briefly, A single jet Collison nebulizer (CH Technologies, NJ) containing 5×107 pfu/ml H1N1/PR/8 in PBSA (PBS + 0.1% Bovine Serum Albumin), was operated at 40 psi input pressure. The output of the nebulizer, producing viral bioaerosols at 3.3 lpm (liters per minute) was connected to a silicone drum (20-liter volume), used as a drying chamber. Additional dry air (3.3 lpm) was also pumped in. This was done in order to avoid large PBSA droplets with suspended viruses and clusters. The drum output was injected into the environmental chamber.
iEWNS generation setup: The iEWNS generation setup utilized consisted of the exact same three emitter setup described for inactivation of microorganisms on surfaces (Figure 2). The three iEWNS emitter assembly, with each of the emitters working independently, was placed inside a polypropylene chamber. The iEWNS particles were collected through funnels placed underneath each of the three emitters. The iEWNS nano-sanitizer aerosol thus collected was injected into the environmental chamber through brass tubing.
Bioaerosol exposure methodology: Control experiments were performed with no iEWNS presence in the environmental chamber using HEPA filtered air of same air flow rate, in lieu of iEWNS aerosol. Whereas, during h1EWNS treatment, the h1EWNS aerosol was mixed in environmental chamber at predetermined airflow with bio aerosol generated as shown in Figure 3. Air from the environmental chamber was sampled and concentration of Influenza H1N1/PR/8 virus at distinct time-points was measured as described below.
The protocol of the control experiments was as follows: at t=0, the nebulizer and the drying air inputs were initiated. A HEPA filter was connected in lieu of the iEWNS aerosol input with the total system airflow maintained the same. The system was exhausted through the auxiliary exhaust pump for 18 minutes, equivalent to 1 air exchange through the system. At t=18, the first sample was taken.
During the bioaerosol sampling period, the auxiliary exhaust pump was turned OFF and the entire airflow was sampled with the biosampler. The chamber was sampled for 10 minutes. After the end of sampling period, the sampling pumps were turned OFF and system was switched to the exhaust mode. At t=36, the second sample was taken with the same methodology as the first. At the end of the second sample, all airflows were turned OFF.
The protocol for the h1EWNS exposure is as follows: at t=0, the nebulizer and the drying air inputs were initiated. The iEWNS generator was operated with iEWNS airflow maintained at 1.5 lpm into the chamber. The system was exhausted through the auxiliary exhaust pump for 18 minutes, equivalent to 1 air exchange through the system. The viral and iEWNS aerosols were allowed to interact inside the system and at t=18, the first sample was taken. The sampling protocol was identical as described above, with 10 minutes of sampling. At t=36, the second sample was taken with the same methodology as the first. At the end of the second sample, all airflows were turned OFF.
Bioaerosol sampling and enumeration: 8.1 lpm of air was sampled from environmental chamber using an SKC Biosampler (SKC Inc, Eighty Four, PA). An additional 4.4 lpm of HEPA filtered air was supplied to the Biosampler using a supplementary air pump to meet the sampler’s operating volumetric flow of 12.5 lpm (Figure 3). A 5-ml aliquot of the infection media described above, was added to the sampler as the collection medium. This sample was then used for analysis using the TCID50 assay47. In summary, the collected sample was added to the wells of a 96 well plate in serial dilutions. The plates were incubated for 2 hours. The virus suspension was aspirated and replaced with 200μl of fresh infection media. The plates were further incubated for a minimum of two days. The cytopathic effect (CPE) was observed after three days. The TCID50 score was obtained from the wells showing CPE. This value was then converted into the IU/ml value by utilizing the Spearman Kerber Method46.
Monitoring of hydrogen peroxide gas levels, temperature and relative humidity in environmental chamber: The concentration of hydrogen peroxide inside the environmental chamber was monitored in real time using the Porta Sense II hydrogen peroxide sensor (Gas Sensing, Hull IA). This hydrogen peroxide sensing probe was connected at the exhaust port of the chamber and a continuous measurement of the hydrogen peroxide levels inside the chamber was performed for the entire duration of the experiment. Temperature and relative humidity levels were also monitored in real time during both the control and h1EWNS exposure trials; and the results are detailed in the supplementary data (Figure S1).
h1EWNS particle concentration measurement: The iEWNS particle number concentration as a function of size inside the environmental chamber under the same airflow conditions was also monitored in separate experiments using a Scanning Mobility Particle sizer (SMPS, TSI, Shoreview, MN). The data are presented in Figure S2 of supplemental data.
Data interpretation and statistical analysis
Each inactivation experiment was repeated in triplicate. Each data point represents the arithmetic mean of three replicates. The standard deviation of the three trials was used as the error bar. A linear fit analysis with was performed on the data points, to determine the rate of inactivation.
Results
iEWNS Characterization:
The physico-chemical characterization of the EWNS, rEWNS and h1EWNS nanosanitizers has been carried out and reported in previous publications39,40,45. In summary, the baseline EWNS had a diameter of 12.1 (± 0.1) nm and a charge of 13 (±0) e− charge. The rEWNS particles were larger in diameter with 13.2 (±0.2) nm size and 13 (± 2) e− charge. The h1EWNS are slightly smaller than the baseline EWNS at 11.9 (± 0.3) nm size and have slightly less charge per particle at 11 (±0) e−. The summary of these properties is given in supplementary table 1.
Pathogen inactivation on surfaces:
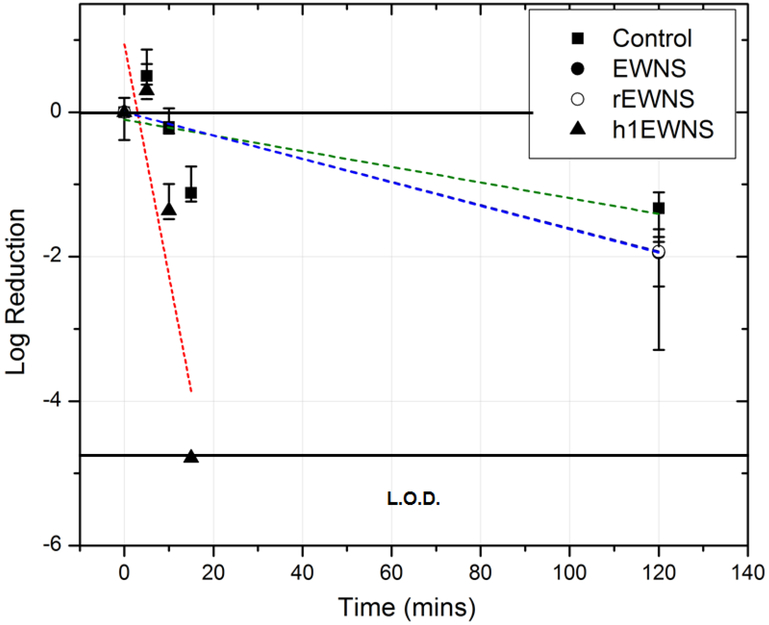
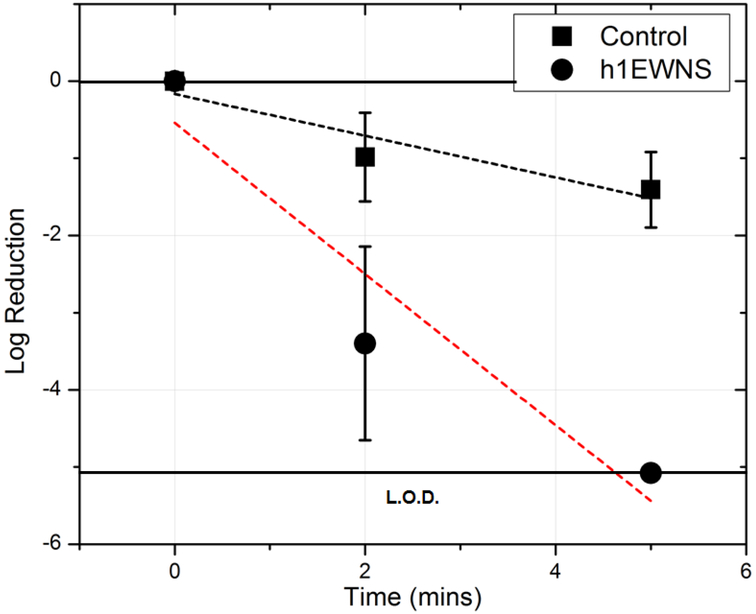
Influenza H1N1/PR/8: The inactivation of Influenza H1N1/PR/8 on surface, as a function of exposure time is shown in Figure 4. The control decay was assessed to be 1.32 logs at 2 hours’ post inoculation, at a decay rate of 0.011 (± 0.006) logs/min (R2 = 0.34). For the baseline EWNS treatment, there was 1.94 logs reduction in the concentration of H1N1/PR/8 after 2 hours of exposure, a rate of 0.016 logs/min. The rEWNS exposure, produced similar 1.92 logs reduction at 2 hours, a rate of 0.016 logs/min. For the case of h1EWNS exposure, a complete eradication took place at 15 minutes of exposure with 4.78 log reduction in the concentration, at a rate of 0.32 (±0.12) log10/mins. No viruses were recovered at this data point. The iEWNS dose delivered to virus on the surface at the highest treatment timepoint was also calculated at 0.924 (±0.03) ng, 0.672 (±0.24) ng and 0.083 (±0.009) ng for EWNS, rEWNS and h1EWNS respectively Acinetobacter baumannii: For the inactivation of Acinetobacter baumannii on surface (Figure 5), h1EWNS exposure resulted in a 3.3 log and 5 log reduction in the concentration recovered from the exposure surface for 2 and 5 minute exposure respectively. No culturable bacteria were recovered from the coupons at this 5 minute timepoint, with the rate of inactivation being 1 log/min. The delivered to bacteria dosage of the Hydrogen peroxide was calculated to be miniscule (0.0278 ±0.003 ng) after 5 minutes of exposure.
Figure 4:
Inactivation of H1N1/PR/8 on surface. The various treatment conditions are shown. Linear fit was performed on each set and the trendlines are shown as dotted lines.
Figure 5:
Inactivation of Acinetobacter baumannii on surface. The various treatment conditions are shown. Linear fit was performed on each set and the trendlines are shown as dotted lines.
Inactivation of Influenza/H1N1/PR/8 in air
The concentration levels of Influenza H1N1/PR/8 bioaerosol in the environmental chamber for the control (no h1EWNS nano-aerosol in the environmental chamber) and with the presence of h1EWNS aerosol in the environmental chamber are shown in Figure 6. For the control scenario, at 18 minutes (1 air exchange equivalent time) the airborne Influenza H1N1/PR/8 concentration level of 114.04 IU/Liter of air was measured in the chamber. At 36-minute time point, (after 2 air exchanges) the airborne Influenza H1N1/PR/8 concentration level was found to be 167.81 IU/Liter of air.
Figure 6:
Inactivation of Influenza H1N1/PR/8 in air. The results shown here are for the 3.24 ACH scenario. The control trials (w HEPA filtered air) and treatment trials (w h1EWNS) are shown.
In the presence of h1EWNS nanoparticles in the environmental chamber, the concentration of airborne Influenza H1N1/PR/8 was observed to be 7.45 IU/Liter of air and 11.63 IU/Liter of air for the 18 and 36-minute time points respectively. This indicates a 94% reduction in the viral concentration compared to the controls.
It is worth noting that the h1EWNS particle number concentration in the environmental chamber was measured and found at 80,000 particles/cc of air for this ACH scenario. It is worth noting that the levels of H2O2 gas inside the chamber was found to be lower than the acceptable OSHA personal exposure limit (PEL) of 1 ppm48 and also lower than the detection limit of the sensor utilized (<0.1 ppm).
Discussion
The study details the utilization of the novel iEWNS antimicrobial platform for surface and airborne disinfection of microorganisms relevant to the healthcare setting.
It has been shown in our previous studies41,42,45 that the observed microbicidal action of the baseline EWNS synthesized using only DI water is primarily through the action of ROS. As shown in Figure 4, the incorporation of hydrogen peroxide in the chemical structure of h1EWNS enhanced dramatically the inactivation potency to 4.78 log reduction due to synergistic effects from ROS and AI utilized in this case. It is worth noting that the dose delivered to the microorganisms inoculated on surfaces is miniscule and in the pico to nanogram range.
There have not been many studies that detail the effects of very low (<100 ppm) concentration of H O 49 2 2 on surface microorganism disinfection. Heckert et al. reported similar inactivation levels of surface deposited influenza, as reported here, although it should be stated that the vapor phase hydrogen peroxide concentration used in that study was at extremely high levels (about 1200 ppm) and with an exposure time close to 30 minutes50. Similarly, in another study by Rudnick et al., 90 ppm of vapor phase H2O2 was required in order to achieve 5-log reductions in similar to 30 minute time of exposure used in this study49. It is worth noting that using a gas phase H2O2 approach for both air and surface disinfection at such high concentrations means that the rooms need to be vacated from humans for hours, resulting in significant down time which is problematic in hospital settings.
While in the case of h1EWNS here, an exposure time of 5 mins resulted in 4.78 log reduction. The inactivation rates observed at much lower doses (gas phase H2O2 levels in ppb levels) in this study can be attributed to the delivery using a nanoparticle delivery approach. This approach results in a more precise and targeted delivery of the AI to the surface of interest, and the high mobility and surface per volume of the generated h1EWNS nanoparticles contribute to this effect.
It is also worth noting that the increased potency of h1EWNS over EWNS and rEWNS for the Influenza H1N1/PR/8 surface disinfection in this study is also congruous with earlier results with food related pathogens such as the gram negative E. coli39,45. This is especially important, since the viral solution utilize to contaminate the surface contains PBS as well albumin. Hence, the result demonstrates not only the effectiveness of the iEWNS technology against surface contamination, but also shows the ability of the iEWNS particles to penetrate through the layer of protein and salts that are possibly present in biological environments
Acinetobacter baumannii has been identified as one of the most significant nosocomial pathogens. Originally found primarily outside the United States, mainly in Europe and Asia11, it has been increasing found in hospitals in the United States12. It’s ability to develop ntimicrobial resistance and cause nosocomial outbreaks of infection makes it particularly dangerous51. This organism frequently causes infections in medical devices, e.g., vascular catheters, cerebrospinal fluid shunts or Foley catheters52. It is also quite often implicated in wound related infections53. Lemmen et al. reported 4-log reductions in the concentration of Acinetobacter on surfaces using vaporized hydrogen peroxide (VHP), although the peak hydrogen peroxide concentration in those cases was considerable (500–600 ppm)54. As shown in this study, the 5 log reductions in the concentration of A. baumannii on surfaces can be obtained using with the h1EWNS platform with dosage lower than 1 ng.
The 94% reduction in the concentration of airborne levels of Influenza H1N1/PR/8 at very low levels of H2O2 (less than 0.1 ppm) in the environmental chamber in the presence of iEWNS nano-sanitizers is potentially game changing and provides an alternative intervention strategy for air disinfection in high risk microenvironments.
It is worth noting that inactivation of airborne influenza has generally been limited to the use of UVC (254nm) light. Jensen at al. showed 254nm (UVC) yielded 3 log reductions when influenza aerosols were passed through an exposure cell55. Although upper room germicidal UV has been proven to be an excellent intervention technology for airborne infection, the use of such approach is limited to upper room, due to exposure concerns when used in real time in a patient ward. This study is one of the first to demonstrate an intervention technology, other than UVC light, for inactivating influenza virus.
The toxicological implications of inhaled baseline EWNS (using DI water only, no active ingredient) was assessed in an acute rat study44 and no lung injury and inflammation was found. It is also worth noting that while the H2O2 levels in the environmental chamber were found to be less than permissible levels by OSHA of 1 ppm which is encouraging in terms of potential toxicological implications, further toxicological assessment is required to ensure the safe use of this technology in the presence of human occupancy. We plan to repeat in near future the same acute inhalation toxicological study using the h1EWNS nanoparticles to assess potential lung injury and inflammation.
Finally, in the future the use of iEWNS platform in wound healing management and inhibition of biofilm formation will also be assessed. Medical facilities have a high risk of transmission from patients with non-healing wounds, such as surgical site wounds56. Wounds represent an attractive surface environment for the colonization of pathogens, leading to the evolution of difficult to treat wound infections that are often consisting of multiple species of microbes57. The results presented here would make the iEWNS an effective technology for the prevention of wound infections by controlling the microbial growth. The rapid inactivation rate produced by h1EWNS indicates the strength of this iEWNS technology to deliver various AI using a nanoparticle approach to produce inactivation while delivering only nano-scale dosage. The miniscule dosage delivered reduces the risk of chemical toxicity and makes this a cost effective technology for prevention of nosocomial infections on both surfaces and in the air. Other AI will be explored in the future and used as EWNS nano-sanitizers to be used in future infectious control studies. Examples of such AIs include: nano-clusters, qauntum dots, etc58.
Supplementary Material
In this study, aqueous suspensions of various active ingredients (A.I.s) were utilized to generate Engineered Water Nanostructures (EWNS) nano-sanitizers, containing the A.I. utilized to produced them (termed iEWNS). These iEWNS had nanoscale size, were loaded with ROS and contained the A.I. utilized to produce them. They were challenged with Influenza H1N1/PR/8 and Acinetobacter baumannii on surfaces and in air. The results indicate that a nanogram dose of A.I. is effective in producing significant inactivation in Influenza H1N1/PR/8 and Acinetobacter baumannii.
Acknowledgements
This work was supported by NIH [Grant #1R21AI119481–01]. The Harvard-Brazilian Program at HSPH provided financial support for LM and AM. The Fulbright Visiting Scholar Program provided support for ME.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Weber DJ, Rutala WA, Miller MB, Huslage K & Sickbert-Bennett E Role of hospital surfaces in the transmission of emerging health care-associated pathogens: Norovirus, Clostridium difficile, and Acinetobacter species. Am. J. Infect. Control 38, S25–S33 (2010). [DOI] [PubMed] [Google Scholar]
- 2.Zhu S, Srebric J, Spengler JD & Demokritou P An advanced numerical model for the assessment of airborne transmission of influenza in bus microenvironments. Build. Environ 47, 67–75 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu S, Demokritou P & Spengler J Experimental and numerical investigation of micro-environmental conditions in public transportation buses. Build. Environ 45, 2077–2088 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramer A, Schwebke I & Kampf G How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infectious Diseases 6, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ventola CL The antibiotic resistance crisis: part 1: causes and threats. P T 40, 277–83 (2015). [PMC free article] [PubMed] [Google Scholar]
- 6.Zimlichman E et al. Health care-associated infections: A Meta-analysis of costs and financial impact on the US health care system. JAMA Intern. Med 173, 2039–2046 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Scott RD The direct medical costs of healthcare-associated infections in U.S. hospitals and the benefits of prevention Cdc; (2009). doi:http://www.cdc.gov/hai/pdfs/hai/scott_costpaper.pdf [Google Scholar]
- 8.Aitken C & Jeffries DJ Nosocomial spread of viral disease. Clinical Microbiology Reviews 14, 528–546 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otter JA & French GL Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J. Clin. Microbiol 47, 205–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaze ND, Emery CL, Hamilton RJ, Brooks AD & Joshi SG Patient Demographics and Characteristics of Infection with Carbapenem-Resistant Acinetobacter baumannii in a Teaching Hospital from the United States *. Adv. Infect. Dis 3, 10–16 (2013). [Google Scholar]
- 11.Shanthi M & Sekar U Multi-drug resistant Pseudomonas aeruginosa and Acinetobacter baumannii infections among hospitalized patients: risk factors and outcomes. J. Assoc. Physicians India 57, 636, 638–40, 645 (2009). [PubMed] [Google Scholar]
- 12.Wisplinghoff H et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect 64, 282–90 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Barker J, Stevens D & Bloomfield SF Spread and prevention of some common viral infections in community facilities and domestic homes. Journal of Applied Microbiology 91, 7–21 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perry KA et al. Persistence of Influenza A (H1N1) Virus on Stainless Steel Surfaces doi: 10.1128/AEM.04046-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allegranzi B & Pittet D Role of hand hygiene in healthcare-associated infection prevention. J. Hosp. Infect 73, 305–15 (2009). [DOI] [PubMed] [Google Scholar]
- 16.Koburger T, Hubner N-O, Braun M, Siebert J & Kramer A Standardized comparison of antiseptic efficacy of triclosan, PVP-iodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother 65, 1712–1719 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Andersen BM, Bånrud H, Bøe E, Bjordal O & Drangsholt F Comparison of UV C Light and Chemicals for Disinfection of Surfaces in Hospital Isolation Units. Infect. Control Hosp. Epidemiol 27, 729–734 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Rutala WA & Weber DJ New disinfection and sterilization methods. in Emerging Infectious Diseases 7, 348–353 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai M, Yadav A & Gade A Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances 27, 76–83 (2009). [DOI] [PubMed] [Google Scholar]
- 20.Eleftheriadou M, Pyrgiotakis G & Demokritou P Nanotechnology to the rescue: using nano-enabled approaches in microbiological food safety and quality. Curr. Opin. Biotechnol 44, 87–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atiyeh BS, Costagliola M, Hayek SN & Dibo SA Effect of silver on burn wound infection control and healing: Review of the literature. Burns 33, 139–148 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Tata A & Beone F Hospital waste sterilization: A technical and economic comparison between radiation and microwaves treatments. Radiat. Phys. Chem 46, 1153–1157 (1995). [Google Scholar]
- 23.Pirela SV et al. Effects of intratracheally instilled laser printer-emitted engineered nanoparticles in a mouse model: A case study of toxicological implications from nanomaterials released during consumer use. NanoImpact 1, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu X et al. In vivo epigenetic effects induced by engineered nanomaterials: A case study of copper oxide and laser printer-emitted engineered nanoparticles. Nanotoxicology 10, 629–639 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sohal IS, O’Fallon KS, Gaines P, Demokritou P & Bello D Ingested engineered nanomaterials: state of science in nanotoxicity testing and future research needs. Part. Fibre Toxicol 15, 29 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLoid G et al. Effects of engineered nanomaterial exposure on macrophage innate immune function. NanoImpact 2, 70–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLoid GM et al. An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part. Fibre Toxicol 14, 40 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demokritou P et al. An in vivo and in vitro toxicological characterisation of realistic nanoscale CeO2 inhalation exposures. Nanotoxicology 7, 1338–1350 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaze ND et al. Inactivation of bacteria in flight by direct exposure to nonthermal plasma. IEEE Trans. Plasma Sci 38, 3234–3240 (2010). [Google Scholar]
- 30.Morawska L Droplet fate in indoor environments, or can we prevent the spread of infection? in Indoor Air 16, 335–347 (2006). [DOI] [PubMed] [Google Scholar]
- 31.McDevitt J, Rudnick S, First M & Spengler J Role of absolute humidity in the inactivation of influenza viruses on stainless steel surfaces at elevated temperatures. Appl. Environ. Microbiol 76, 3943–3947 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herfst S et al. Drivers of airborne human-to-human pathogen transmission. Current Opinion in Virology 22, 22–29 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai KM, Burge HA & First MW Size and UV germicidal irradiation susceptibility of Serratia marcescens when aerosolized from different suspending media. Appl. Environ. Microbiol 70, 2021–7 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mead K & Johnson DL An evaluation of portable high-efficiency particulate air filtration for expedient patient isolation in epidemic and emergency response. Ann. Emerg. Med 44, 635–645 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaze N, Park S, Brooks AD, Fridman A & Joshi SG Involvement of multiple stressors induced by non-thermal plasma-charged aerosols during inactivation of airborne bacteria. PLoS One 12, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed NG The history of ultraviolet germicidal irradiation for air disinfection. Public Health Reports 125, 15–27 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor RH, Falkinham JO, Norton CD & LeChevallier MW Chlorine, chloramine, chlorine dioxide, and ozone susceptibility of Mycobacterium avium. Appl. Environ. Microbiol 66, 1702–5 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed K et al. Radiant panel and air heating performance in large industrial buildings. Build. Simul 11, 293–303 (2018). [Google Scholar]
- 39.Vaze N et al. An integrated electrolysis – electrospray – ionization antimicrobial platform using Engineered Water Nanostructures (EWNS) for food safety applications. Food Control 85, 151–160 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pyrgiotakis G et al. Optimization of a nanotechnology based antimicrobial platform for food safety applications using Engineered Water Nanostructures (EWNS). Sci. Rep 6, 21073 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pyrgiotakis G et al. Inactivation of Foodborne Microorganisms Using Engineered Water Nanostructures (EWNS). Environ. Sci. Technol 49, 3737–3745 (2015). [DOI] [PubMed] [Google Scholar]
- 42.Pyrgiotakis G et al. Mycobacteria inactivation using Engineered Water Nanostructures (EWNS). Nanomedicine 10, 1175–83 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pyrgiotakis G, McDevitt J, Yamauchi T & Demokritou P A novel method for bacterial inactivation using electrosprayed water nanostructures. J. Nanoparticle Res 14, 1027 (2012). [Google Scholar]
- 44.Pyrgiotakis G et al. A chemical free, nanotechnology-based method for airborne bacterial inactivation using engineered water nanostructures. Environ. Sci. Nano 1, 15–26 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaze N et al. A nano-carrier platform for the targeted delivery of nature-inspired antimicrobials using Engineered Water Nanostructures for food safety applications. Food Control 96, 365–374 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramakrishnan MA Determination of 50% endpoint titer using a simple formula. World J. Virol 5, 85 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LaBarre DD & Lowy RJ Improvements in methods for calculating virus titer estimates from TCID50 and plaque assays. J. Virol. Methods 96, 107–126 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Center for Disease Control and Prevention. CDC - NIOSH Pocket Guide to Chemical Hazards - Hydrogen peroxide NIOSH Pocket Guide to Chemical Hazards; (2018). doi: 10.1016/S1870-5766(13)71794-X [DOI] [Google Scholar]
- 49.Rudnick SN, McDevitt JJ, First MW & Spengler JD Inactivating influenza viruses on surfaces using hydrogen peroxide or triethylene glycol at low vapor concentrations. Am. J. Infect. Control 37, 813–819 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heckert RA et al. Efficacy of Vaporized Hydrogen Peroxide against Exotic Animal Viruses. Appl. Environ. Microbiol 63, 3916–3918 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richet H & Fournier PE Nosocomial Infections Caused by Acinetobacter baumannii A Major Threat Worldwide. Infect. Control Hosp. Epidemiol 27, 645–646 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Rodríguez-Baño J et al. Clinical Features and Epidemiology of Acinetobacter baumannii Colonization and Infection in Spanish Hospitals. Infect. Control Hosp. Epidemiol 25, 819–824 (2004). [DOI] [PubMed] [Google Scholar]
- 53.Rezaei E, Safari H, Naderinasab M & Aliakbarian H Common pathogens in burn wound and changes in their drug sensitivity. Burns 37, 804–806 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Lemmen S et al. Evaluation of hydrogen peroxide vapor for the inactivation of nosocomial pathogens on porous and nonporous surfaces. Am. J. Infect. Control 43, 82–85 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Jensen MM Inactivation of Airborne Viruses by Ultraviolet Irradiation. Appl. Microbiol 12, 418–420 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo S & DiPietro LA Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res 89, 219–229 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colsky AS, Kirsner RS & Kerdel FA Analysis of antibiotic susceptibilities of skin wound flora in hospitalized dermatology patients. Arch. Dermatol 134, 1006–9 (1998). [DOI] [PubMed] [Google Scholar]
- 58.Ding X et al. Defect engineered bioactive transition metals dichalcogenides quantum dots. Nat. Commun 10, 41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.