Abstract
Objective:
We sought to identify optimal strategies for integrating HIV- and opioid use disorder-(OUD) screening and treatment in diverse settings.
Design:
Systematic review.
Methods:
We searched Ovid MEDLINE, PubMed, Embase, and PsycINFO and pre-identified websites. Studies were included if they were published in English on or after 2002 through May 2017, and evaluated interventions that integrated, at an organizational level, screening and/or treatment for HIV and OUD in any care setting in any country.
Results:
Twenty-nine articles met criteria for inclusion, including 23 unique studies: six took place in HIV care settings, 12 in opioid treatment settings, and five elsewhere. Eight involved screening strategies, 22 involved treatment strategies, and seven involved strategies that encompassed screening and treatment. Randomized controlled studies demonstrated low to moderate risk of bias and observational studies demonstrated fair to good quality. Studies in HIV care settings (n=6) identified HIV- and OUD-related clinical benefits with the use of buprenorphine/naloxone for OUD. No studies in HIV care settings focused on screening for OUD. Studies in opioid treatment settings (n=12) identified improving HIV screening uptake and clinical benefits with antiretroviral therapy when provided on-site. Counseling intensity for OUD medication adherence or HIV-related risk reduction was not associated with clinical benefits.
Conclusion:
Screening for HIV can be effectively delivered in opioid treatment settings, yet there is a need to identify optimal OUD screening strategies in HIV care settings. Strategies integrating the provision of medications for HIV and for OUD should be expanded and should not be contingent on resources available for behavioral interventions.
Registration:
A protocol for record eligibility was developed a priori and was registered in the PROSPERO database of systematic reviews (registration number CRD42017069314).
Keywords: HIV, opioid-related disorders, integrated delivery system, systematic review
Introduction
HIV and opioid use disorder (OUD) are substantial global risk factors for disability and mortality, and they manifest as intersecting epidemics worldwide [1, 2]. Particularly prevalent among persons living with HIV (PLWH) [3, 4], OUD is associated with worse clinical outcomes, ongoing HIV risk behaviors [5], and ongoing HIV transmission [6, 7]. The growing burden of OUD and related HIV outbreaks highlight a need for novel strategies for screening and treatment of both disorders [8, 9]. These public health trends have garnered unprecedented motivation for the adoption of unified models for OUD management and primary care, including HIV primary care [10].
Although care for people with or at risk for HIV and OUD can be complex [11], integrating HIV- and OUD-related care is feasible in HIV care settings, opioid treatment settings, and other care sites like primary care or public health clinics [12–21]. Integrated care is valued by PLWH with OUD [22, 23], a patient population that has demonstrated limited engagement with routine health services [24] and that is subject to overlapping forms of stigma that may obstruct care and worsen biologic and/or social outcomes [25–27]. International organizations, including the Joint United Nations Programme on HIV/AIDS and the World Health Organization [28, 29] as well as US health reform laws [30] support the integration of substance use disorder services with primary care (including HIV primary care). Yet integration often does not occur [31, 32], how to integrate effectively in different care settings remains poorly understood, and quality metrics for integrated care are lacking [33].
Previous systematic reviews have demonstrated the feasibility and impact of integrating HIV care with other chronic conditions, including cardiovascular disease, diabetes [34], and mental health disorders [35]. One systematic review examined strategies for integrating HIV- with substance use-related care including 51 articles published through October 2015, but it did not use opioid-derived search terms nor compare between integrated and non-integrated models or between different integration strategies [36]. Therefore, we performed a systematic review to identify interventions wherein HIV and OUD-related care are integrated, across the spectrum of care from screening to treatment and across diverse care settings. This review will generate a comprehensive understanding of the evidence for integrated care to inform practice and policy for a large and vulnerable population.
Materials and methods
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards of quality for reporting systematic reviews [37]. This is part of a larger systematic review on the integration of OUD care with the care of infectious complications including hepatitis C and HIV. We developed a protocol for study eligibility a priori and registered it in the PROSPERO database of systematic reviews (registration number CRD42017069314) [38]. Our data synthesis process drew from realist synthesis, an analytic approach that considers the interaction between context, mechanism, and outcome in evaluating an intervention [39–41]. This study was not considered human subjects research by the Yale School of Medicine Human Investigation Committee.
Search strategy
Guided by the PICOS framework, we defined the populations, interventions, comparisons, outcomes, and study designs of interest a priori [42] (see Text Box). We defined integrated interventions as screening and/or treatment (including medications—such as antiretroviral therapy [ART], methadone, buprenorphine/naloxone, or naltrexone—or behavioral interventions) for HIV and OUD, employed at an organizational level [43].
Text box.
Inclusion criteria (PICOS1 framework).
| Population | Adults with or at risk for (as deemed by study authors) HIV and/or opioid use disorder; n ≥ 10 |
| Intervention | Screening or treatment for both HIV and opioid use disorder occurring within the same treatment setting or across settings that are integrated at the organizational level |
| Comparison | Screening or treatment for HIV and opioid use disorder occurring not in integrated settings, which may include a historical control, or a comparison between two integrated care strategies |
| Outcome | HIV or opioid use disorder screening uptake, HIV or opioid use disorder quality of care measures, HIV or opioid use disorder biochemical outcomes, or general health outcomes |
| Study design | Randomized trials, prospective cohort studies with controls, historically controlled trials |
PICOS = Population, Intervention, Comparison, Outcome, Study Design
We searched Ovid MEDLINE, PubMed, Embase, and PsycINFO on May 24, 2017. We identified additional studies by scanning other systematic reviews and bibliographies. We also searched the websites of the following pre-identified organizations: Society for General Internal Medicine, Substance Abuse and Mental Health Services Organization, the Association for Medical Education and Research in Substance Abuse, and the U.S. Health and Human Services’ HIV.gov website. We limited our search to studies with human subjects and those published in the English language. We also limited our search to those studies published in or after 2002 for three reasons. First, buprenorphine/naloxone was approved by the US Food and Drug Administration as the first schedule III medication for the treatment of OUD in 2002, heralding a new era for the outpatient management of OUD [44], including in PLWH [45]. Second, in late 2001 the US Centers for Disease Control and Prevention published new recommendations for screening for HIV that focused on the expansion of screening in all medical settings, including those that provide services related to substance use disorders [46]. Third, this time point corresponds approximately with the advent of the late ART era (2000 and later), during which HIV treatments were associated with greater efficacy than previous eras [47].
To produce relevant vocabulary terms, we analyzed five previously identified key articles using the Yale MeSH Analyzer (http://mesh.med.yale.edu/). In each database, we ran scoping searches and used an iterative process to translate and refine the search strategies. We used the previously identified articles to validate the success of our searches (eSearch 1 in Supplement).
Study selection
Two authors (BO and NM) independently screened titles to remove clearly irrelevant citations, then these two authors independently screened abstracts using an algorithm developed a priori, resolving conflicts by consensus. We used Covidence, a systematic review software, to facilitate screeners’ independent organization, retrieval, and assessment of articles [48].
Quality assessment
Two reviewers independently completed the quality assessment of each study using the Cochrane Risk of Bias Tool for randomized trials [49] and the Newcastle-Ottawa Scales for observational studies [50] (BO reviewed all articles; NM, JT, and EJE shared the role of second reviewer).
Data extraction
For each screened article, two authors independently abstracted information about the context (HIV care settings, opioid treatment settings, or other), participants, intervention type (screening or treatment), and outcomes (including uptake of services, behavioral or biochemical outcomes, and general health outcomes along the care continuum [51]) in a standardized form. Extraction occurred concurrently with quality assessment by the same two authors (BO and either NM, JT, or EJE).
Intervention and outcome heterogeneity precluded meta-analyses. Instead, because we were interested in how integration strategies differed in different care settings, we drew from a realist synthesis strategy in which reviewers delineated the contextual influences that contributed to the outcomes of interest [40, 41] based on the following typology.
Intervention typology
Similar to previous systematic reviews on the integration of HIV care with the management of other disorders [35, 36], we classified interventions according to the entry point at which patients receive care: HIV care settings, opioid treatment settings, and other settings. HIV care settings included Infectious Disease clinics, specialized HIV clinics, and community-based HIV or AIDS services organizations (which focus on case management and other supportive services, and may provide clinical services). Opioid treatment settings included opioid treatment programs or settings licensed to provide medications for OUD. Other clinical and community-based settings included those that could not be classified into the previous categories. In addition to corresponding with other reviews, this typology may facilitate the development of best-practices in different care settings.
Results
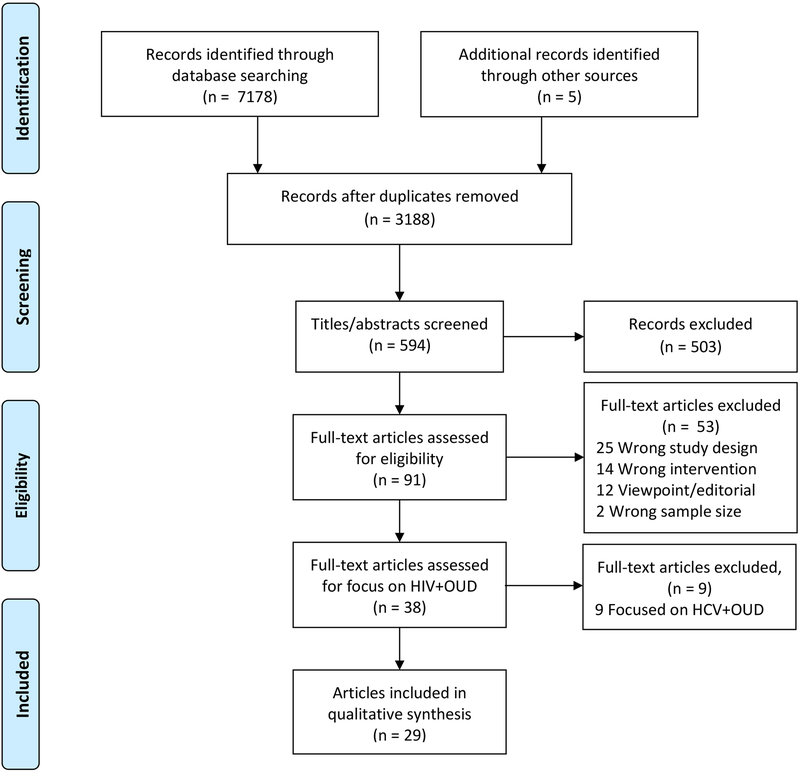
Our search yielded 7,178 articles and 3,188 remained after the removal of duplicates (Figure 1). After screening titles, we screened 594 abstracts and identified 29 articles that met criteria for inclusion [12, 15, 17–20, 52–74]. On three occasions, multiple articles presented data from one study ([12, 17–19, 57, 62], [65, 66], and [69, 75]) yielding a total of 23 unique studies. These included eight randomized trials [20, 55, 58, 61, 63, 64, 69, 73, 75], 13 cohort studies [12, 15, 17–19, 52, 53, 56, 57, 59, 60, 62, 65–68, 70–72], and two cross-sectional studies [54, 74].
Figure 1.
PRISMA flow chart of study selection.(OUD: opioid use disorder; HCV: hepatitis C virus)
Description of studies
We identified six studies that occurred in HIV care settings [12, 17–20, 57, 58, 61–63, 67, 73], 12 studies that occurred in opioid treatment settings [52–56, 60, 64–66, 68–70, 74, 75], and five studies that occurred in other settings, including correctional settings [71, 72], primary care clinics [58], mobile syringe-exchange units [15] or public health clinics [59]. The added element that defined interventions as integrated care was most often either OUD care or HIV care alone to a facility already providing care for the other disorder; in two studies both OUD and HIV care were introduced simultaneously and correctional facilities were the points-of-entry for both [71, 72]. With respect to outcomes measured, 19 studies examined clinical and laboratory outcomes, four examined health-related quality of life [54, 58, 62, 72], two examined quality of care indicators [18, 54], one examined cost [19], and one examined patient satisfaction [74].
While most studies took place in North America (n=15 in the United States and n=1 in Canada), four took place in Europe [53, 54, 60, 68] and three took place in Asia [52, 59, 74]. Half of studies had a follow-up period of 12 months or longer (Table 1).
Table 1.
Characteristics of studies (n=23).
| Characteristic | n (%) |
|---|---|
| Study design Randomized trial Cohort study Cross-sectional study |
8 (35) 13 (57) 2 (7) |
| Point of entry into care HIV care setting OUD care setting Other facility |
6 (26) 12 (52) 5 (22) |
| Added intervention OUD screening only OUD treatment only OUD screening and treatment HIV screening only HIV treatment only HIV screening and treatment Both HIV care and OUD care |
0 (0) 5 (22) 1 (4) 1 (4) 8 (35) 6 (26) 2 (9) |
| Length of follow-up 0 months (cross-sectional) <6 months 6–12 months >12 months |
2 (9) 6 (26) 4 (17) 11 (48) |
| Location of study North America Europe Asia |
16 (70) 4 (17) 3 (13) |
Quality assessment
The risk of bias among randomized studies was low to moderate (eTable 1 in Supplement), although no randomized studies blinded participants and personnel of the treatment phase and few described blinding of outcome assessment [55, 73]. Cohort studies were of fair to good quality (eTable 2 in Supplement). The most common reason for low quality assessment was the low quality of the non-exposed cohort (9 of 13), which was either drawn from a different source as the exposed cohort or no comparator cohort was generated other than a pre-intervention baseline [12, 15, 17–19, 52, 56, 57, 59, 60, 62, 68, 70, 71].
Interventions in HIV care settings
Among studies in HIV care settings (n=6), five examined interventions involving medications for OUD [12, 17–20, 57, 61–63, 73] and one in which motivational interviewing, social work, and peer groups were offered [67] (Table 2). Four of the five medication-based interventions included buprenorphine/naloxone and recruited an aggregate of 414 patients [12, 17–20, 57, 62, 63, 73], while one offered injectable naltrexone [61] and assigned 25 patients to the intervention and 26 to treatment-as-usual. Studies that centered on the provision of buprenorphine/naloxone demonstrated that administering these medications in HIV clinics correlates with clinical benefits such as initiation ART [12], decreased needle sharing [57], decreased opioid use [17],; operational sequelae included increased labor, overhead, and urine toxicology costs [19] as well as more primary care visits to the HIV specialist [63]. Health-related quality of life and HIV quality of care indicators were favorably associated with buprenorphine/naloxone administration if it was continued for one year [62]. Injectable naltrexone offered in HIV care settings demonstrated feasibility and safety and suggested that those offered injectable naltrexone were more likely to initiate and be retained on treatment than those offered treatment-as-usual, but the study was not powered to detect clinical HIV or OUD outcomes [61]. The study whose intervention did not provide on-site treatment with medications for OUD, but instead on-site motivational interviewing with referral for medications, did not show improvements in HIV clinical outcomes [67].
Table 2.
Characteristics of interventions based in HIV care settings (n=6).
| Added intervention | First author(s) (or study name) and country | Study design | Intervention description | Number of patients in intervention | Key findings |
|---|---|---|---|---|---|
| OUD1 care | BHIVES2 Collaborative 2011 USA [13, 18–20, 55, 60] |
Cohort | PLWH3 with OUD initiated buprenorphine/naloxone in HIV clinics | 303 | Initiating buprenorphine/naloxone in HIV clinical settings correlates with:
|
| Korthuis 2017 USA [59] |
Randomized trial | PLWH with OUD initiated extended-release naltrexone in HIV clinics | 25 (26 control: treatment as usual) |
Compared with treatment as usual, extended-release naltrexone is feasible and safe for treatment of OUD in HIV clinics; study not powered to detect secondary HIV or OUD outcomes | |
| Lucas 2010 USA [61] |
Randomized trial | PLWH with OUD initiated buprenorphine/naloxone in an HIV clinic | 48 (48 control: referral to OUD treatment |
Compared with referred treatment, HIV clinic-based buprenorphine/naloxone led to:
|
|
| Sullivan 2006 USA [71] |
Randomized trial | PLWH with OUD initiated buprenorphine/naloxone in an HIV clinic and brief physician management with or without nurse-led counseling and adherence management | 16 | No significant differences based on counseling intervention were detected in CD4 cell count, HIV-1 RNA levels, or opioid-positive weekly urine tests. | |
| Tetrault 2012 USA [21] |
Randomized trial | PLWH with OUD initiated buprenorphine/naloxone in an HIV clinic with 15-min physician management with or without 45-min nurse-led enhanced medical management | 25 physician management only; 22 physician management with enhanced medical management | No significant differences based on counseling intervention were detected in percentage of opioid-negative urine tests, duration of abstinence, HIV-1 RNA levels or CD4 cell counts. | |
| OUD screening and OUD care | Pisu 2010 USA [65] |
Cohort | PLWH received OUD-targeted weekly motivational interviewing, social work, peer groups in HIV clinics, and referrals to psychiatry for medications and psychology for psychotherapy | 128 | Compared to those who declined the program, the intervention was associated with
|
OUD = opioid use disorder
BHIVES = Buprenorphine-HIV Evaluation and Support
PLWH = persons living with HIV
Two randomized controlled trials examined different intensities of adjunctive counseling in addition to buprenorphine/naloxone [20, 73].Neither the addition of nurse-led counseling and adherence management [73], nor nurse-led enhanced medical management improved HIV- or OUD-related outcomes [20] compared favorably to physician-delivered medical management of buprenorphine/naloxone.
Interventions in opioid treatment settings
Among studies in opioid treatment settings (n=12), all occurred in outpatient settings and one included hospital-based detoxification programs [55], two examined HIV testing and counseling [55, 69, 75], six examined HIV medication management with antiretroviral therapy (ART) [53, 56, 60, 64–66, 68, 70], and three examined interventions that involved both HIV testing and counseling as well as ART management [52, 54, 74] (Table 3). In two randomized trials, HIV testing and counseling alone in opioid treatment settings led to increased HIV testing performed and feedback of results [55, 75], but the addition of sexual risk-reduction counseling did not lead to significant changes in sexual risk behaviors [69]. ART management in opioid treatment settings involved the prescription of ART in methadone clinics [68] or directly-administered ART (DAART) [53, 56, 60, 64–66, 70]. DAART in residential opioid treatment settings [53] and methadone clinics was safe and feasible [56, 60, 64–66, 70], but a randomized trial demonstrated that DAART compared with self-administered ART in methadone clinics did not lead to significant differences in clinical outcomes [63].
Table 3.
Characteristics of interventions based in opioid treatment settings (n=12).
| Added intervention | First author(s) (or study name) and country | Study design | Intervention description | Number of patients in intervention | Key findings |
|---|---|---|---|---|---|
| HIV screening | Bartholow 2005 USA [53] |
Randomized trial | People who inject drugs randomized to receive home HIV testing kits or traditional counseling and testing in three settings: methadone clinics, hospital-based detox, and syringe exchange | 239 home testing; 249 traditional counseling and testing | Compared with traditional counseling and testing, home testing led to:
|
| Metsch 2012 & Schwartz 2013 USA [67, 73] |
Randomized trial | People already enrolled in drug treatment programs cluster-randomized to referral for off-site HIV testing, on-site testing, or on-site testing with counseling | 1,281 | Compared with off-site referral for HIV testing, on-site HIV testing led to:
|
|
| HIV Care | Babudieri 2011 Italy [51] |
Cohort | PLWH with OUD offered directly-administered medications for HIV in residential drug treatment facilities | 106 directly-observed; 106 self-administered | Compared with self-administering medications, directly-observed HIV medication management was associated with:
|
| Conway 2004 Canada [54] |
Cohort | PLWH with OUD offered directly-observed medications for HIV in methadone clinics | 54 | Directly-observed medications for HIV can be administered in a methadone clinic:
|
|
| Lucas 2004 & 2006 USA [63, 64] |
Cohort | PLWH with OUD offered directly-observed medications for HIV in methadone clinics | 38 [64] + 82 [63] | Compared with self-administered medications for HIV, directly-observed medications were associated with:
|
|
| Kinahan 2016 Ireland [58] |
Cohort | PLWH with OUD offered medication management and directly-observed medications for HIV in a methadone clinic | 19 | After engaging with HIV care in the methadone clinic, there was:
|
|
| Lucas 2013 USA [62] |
Randomized trial | PLWH with OUD randomized to directly-observed medications for HIV in methadone clinics or self-administered medications | 55 directly-observed, 52 self-administered | Compared with self-administered therapy, directly-observed therapy led to:
|
|
| Sánchez 2012 Spain [66] |
Cohort | PLWH with OUD offered HIV medication management with psychosocial support in a methadone clinic | 71 | Compared to PLWH presumed to have acquired HIV through sexual transmission, those with OUD participating in this study:
|
|
| Sorensen 2012 USA [68] |
Cohort | PLWH with OUD offered directly-observed medications for HIV in a methadone clinic | 24 | Directly-observed medications were associated with:
|
|
| HIV screening and HIV care | Achmad 2009 Indonesia [50] |
Cohort | People who inject drugs offered HIV testing and HIV medication management in methadone clinics | 35 patients starting HIV medication management in methadone clinics compared with 175 starting elsewhere | Compared with those starting HIV medications elsewhere, patients starting HIV medications in methadone clinics:
|
| Bachireddy 2014 Ukraine [52] |
Cross-sectional | PLWH with OUD receive either co-located (OUD and HIV care at the same location), non-co-located (OUD and HIV care at different locations) or harm reduction only (syringe exchange and case management but no medications) | 97 (co-located) 104 (non-co-located) 95 (harm reduction only) |
Compared with non-co-located care and harm reduction-only care, co-located care was associated with
|
|
| Tran 2015 Vietnam [72] |
Cross-sectional | Patients with OUD received methadone at clinics with and without HIV medication management | 1,016 | Compared with patients receiving methadone at clinics without HIV health care services availably on-site, those at sites with HIV care on-site were more likely to report patient satisfaction |
Interventions in opioid treatment settings that involved both HIV counseling and testing as well as ART management demonstrated high patient satisfaction [74], achievement of HIV and addiction quality indicators and higher likelihood of ART receipt [54], but no significant differences in clinical outcomes or health-related quality of life [52, 54].
Interventions in other settings
Other interventions (n=5) occurred in primary care clinics [58], mobile syringe-exchange units [15], government-run public health clinics [59], or used the criminal justice system as a point-of-entry [71, 72]. An intervention in primary care settings demonstrated that those receiving buprenorphine/naloxone, when randomized to brief sexual risk management or enhanced sexual risk management, exhibited similar process (retention in OUD treatment) and clinical outcomes (health-related quality of life or sexual risk) [58]. When methadone and HIV testing and treatment were integrated into public health clinics, more clients seek testing and start ART [59].
One small cohort (n=13 patients) demonstrated that HIV medication management in a syringe-exchange mobile unit led to improvements in HIV-1 RNA levels and CD4 cell counts in a pre-post design [15].
Two studies enrolled participants upon release from the criminal justice system. These demonstrated that, compared to those who did not opt for buprenorphine/naloxone, those who did along with ART provided in a community-based setting demonstrated improved viral suppression [72] and fewer opioid cravings [71].
Implementing integrated care
Many studies did not explicitly describe specifics of program implementation, such as the additional staff or training needed [12, 15, 17, 18, 52–55, 57–60, 69, 74, 76]. When a need for additional staff was described in the studies, these involved study personnel such as study clinicians [61] or research assistants [64, 70], nurses [19, 20, 63], peers or counselors [67, 71, 72], addiction clinicians [19, 73] or HIV clinicians [56, 68].
Discussion
Summary of the evidence
This systematic review identified 23 studies that investigated the integration of care for HIV and OUD across a range of clinical settings, including HIV care settings, opioid treatment settings, primary care or public health clinics, mobile syringe-exchange units, and the US criminal justice system. These data suggest three principles to guide further research, clinical practice, and policy. First, HIV testing can be successfully integrated into a range of settings where OUD care occurs, including primary care clinics, hospital-based detoxification programs, mobile syringe-exchange vans, and methadone clinics. However, there is a lack of studies focused on OUD screening in HIV care settings. Second, medications for the treatment of HIV and OUD can be integrated into a range of HIV, opioid treatment, and other settings, but it is unclear which clinicians should be optimally involved (infectious diseases specialists, addiction specialists, primary care physicians or other providers). Third, while high-quality, randomized trials support the integration of HIV- and OUD-related care in specialty settings (HIV clinics and methadone clinics), high-quality evidence is needed to identify integration strategies in other settings such as primary care clinics, mobile syringe-exchange units, and community-based AIDS services organizations.
While previous systematic reviews have demonstrated that HIV care can be integrated with care for other disorders [34, 35] including substance use disorders [36], this is the first systematic review to focus on OUD specifically and draw comparisons between integration strategies. By focusing specifically on OUD and not all substance use disorders, this review facilitates comparison across models and strategies (for example, medications versus behavioral interventions). This review is timely given the evolving burden of OUD in the US, the interplay between the OUD epidemic and HIV transmission globally [8, 9, 77, 78], and the need for integrated care for these two disorders [10, 23, 33].
Medications are the cornerstone of treatment for HIV [79] and for OUD [80]. Integrated care strategies identified in this review focused on administering medications for both disorders in single organizations. While behavioral interventions may be important adjunctive treatments for PLWH who have OUD, the strategies identified in our review did not demonstrate added benefit to nurse-led adherence management nor to sexual risk-reduction counseling. Therefore, a lack of ability to provide psychosocial and behavioral interventions should not be a barrier to the development of integrated care strategies. This is concordant with other systematic reviews that have not demonstrated benefit of behavioral interventions when combined with medications in the management of OUD [81].
Of the FDA-approved medications for the management of OUD, buprenorphine/naloxone and naltrexone are two practical options for HIV and/or primary care providers in the US given the regulatory restrictions on methadone dispensing [80, 82]. The evidence appraised here supports the use of buprenorphine/naloxone in HIV care settings. When enough prescribers are available to ensure sufficient coverage and a buprenorphine/naloxone coordinator (such as a nurse or counselor) is available, this integrated practice is feasible and can be highly satisfying to providers [83]. Only one study examined the use of naltrexone in HIV care settings and demonstrated feasibility and safety only [61]. This identifies a need for clinicians who care for PLWH to be proficient and certified to prescribe buprenorphine/naloxone given a low prevalence of certified providers [84] and as prioritization among HIV providers often localizes OUD treatment lower than other health domains [85]. Furthermore, in countries where there are regulations on the prescription of buprenorphine/naloxone or methadone by primary care providers, such as in the United States, policies that decrease these barriers should be considered [86, 87] to increase access.
However, no studies directly compared methadone and buprenorphine/naloxone in integration strategies that were otherwise identical. Methadone remains an integral and effective treatment for OUD and is associated with HIV risk reduction [88].
The evidence appraised here supports the expansion of HIV medication management in opioid treatment settings, including methadone clinics, settings that provide buprenorphine/naloxone, and other settings that offer evidence-based strategies for harm reduction among people with OUD such as mobile needle-exchange vans. Directly-observed ART therapy is feasible in these settings but not necessary, as data to date do not suggest that DAART is superior to self-administration [64]. As HIV medical management has become simpler [89], it is feasible that primary care clinicians including advanced practice nurses and non-specialists can provide this care in opioid treatment settings [90].
Gaps in the literature persist regarding optimal strategies for screening for OUD in HIV treatment settings as well as the optimal arrangements of providers who should be involved in integrated care provision. Minimizing barriers that primary care providers face in providing evidence-based treatments for OUD and/or HIV is needed. The development of best practices will depend upon filling these gaps, as well as ongoing feedback from front-line providers and patients who are engaging in novel integrated models [91]. Effectiveness-implementation hybrid study designs—which evaluate clinical interventions while collecting data on implementation [92]—may allow researchers to identify ideal models regarding staffing, training, and infrastructure, and such efforts are currently underway to address implementation of addiction treatment in HIV care settings [93]. Implementing co-located services may be a non-trivial investment in many settings and so future research should identify those implementation strategies that are associated with improved outcomes, cost-effectiveness, and feasibility.
Limitations
This systematic review was limited to only English-language publications, so studies from low- and middle-income countries may have not been included. However, a recent systematic review of articles that focus on integration of HIV with substance use disorder care included studies from other languages and only identified one, and this was published before our time period of interest [36]. By including only studies published in or after 2002, we may have missed articles from earlier periods. However, treatment for OUD and screening recommendations for HIV changed considerably in 2001 and 2002 [45–47], suggesting opportunities for integrated care not applicable to previous time periods. The varying nature of the clinical sites and interventions prohibited meta-analyses, and so we employed a narrative synthesis based on a realist framework to identify strategies that work in different clinical contexts [41]. Because we focused on interventions wherein care was integrated within one organization, we may have missed effective strategies that promote coordinated care across organizations [94]. Finally, because we included only quantitative studies, we did not include qualitative studies that may offer valuable insights into complex care processes and patient experiences [95].
Conclusion
Integrating HIV and OUD-related care can be effective across a range of settings and can be associated with improved provision of evidence-based treatments and better patient outcomes. Moderate to high-quality evidence supports the use of medications for HIV and OUD when integrated in single sites, including methadone clinics, HIV clinics, other opioid treatment settings, primary care clinics, or sites that offer evidence-based harm reduction strategies such as mobile syringe-exchange units. A lack of ability to provide behavioral interventions should not prohibit organizations from offering buprenorphine/naloxone to PLWH with OUD, and a lack of infrastructure for DAART should not prohibit organizations from offering HIV medication management. Policies that reduce the barriers for primary care providers to offer buprenorphine/naloxone or methadone to PLWH with OUD should be considered so that access to evidence-based medications can increase and strategies for care integration in primary care sites can be evaluated.
Supplementary Material
Table 4.
Characteristics of interventions based in other settings (n=5).
| Added intervention | First author(s) (or study name) and country | Study design | Intervention description | Number of patients in intervention | Key findings |
|---|---|---|---|---|---|
| HIV screening | Edelman 2013 USA [56] |
Randomized trial | People with OUD already receiving buprenorphine/naloxone randomized to HIV testing with brief sexual risk management (2 sessions) or enhanced sexual risk management (4 sessions) in a primary care clinic | 15 brief sexual risk management; 15 enhanced sexual risk management | Compared with brief sexual risk management, enhanced sexual risk management did not lead to differences in process (retention in OUD treatment) or clinical outcomes (health-related quality of life, sexual risk) |
| HIV care | Altice 2003 USA [16] |
Cohort | PLWH with OUD offered HIV medication management in a syringe exchange van | 13 | From before intervention to 12-months after:
|
| OUD care | Springer 2010 USA [69] |
Cohort | PLWH with OUD were offered medications for OUD following release from prison out of a group already engaging in directly-observed medications for HIV | 30 | Compared with baseline, participants:
|
| Springer 2012 USA [70] |
Cohort | PLWH with OUD offered medications or OUD after prison release out of a group already receiving directly-observed medications for HIV | 50 | Retention on buprenorphine/naloxone for 24 months correlated with maximum viral suppression, while the receipt of directly-observed medications for HIV or methadone did not. | |
| HIV care and OUD care | Hung 2016 Vietnam [57] |
Cohort | PLWH with OUD offered HIV testing, HIV treatment, and methadone maintenance at outpatient clinics | 7,395 | In sites that integrated methadone, HIV testing, and HIV treatment:
|
Acknowledgements
Dr. Oldfield was supported by the Peter M. Muehrer Award of the Yale National Clinician Scholars Program and the Office of Academic Affiliations of the Department of Veterans’ Affairs. Dr. Edelman was supported by the National Institutes on Drug Abuse (R01DA041067) during the conduct of this work.
Sources of support:
Dr. Oldfield was supported by the Peter M. Muehrer Award of the Yale National Clinician Scholars Program and the Office of Academic Affiliations of the Department of Veterans’ Affairs. Dr. Edelman was supported by the National Institutes on Drug Abuse (R01DA041067).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Larney S, Peacock A, Leung J, Colledge S, Hickman M, Vickerman P, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health 2017,5:e1208–e1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peacock A, Leung J, Larney S, Colledge S, Hickman M, Rehm J, et al. Global statistics on alcohol, tobacco and illicit drug use: 2017 status report. Addiction 2018. [DOI] [PubMed] [Google Scholar]
- 3.Hansen L, Penko J, Guzman D, Bangsberg DR, Miaskowski C, Kushel MB. Aberrant behaviors with prescription opioids and problem drug use history in a community-based cohort of HIV-infected individuals. J Pain Symptom Manage 2011,42:893–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesko CR, Keil AP, Moore RD, Chander G, Fojo AT, Lau B. Measurement of Current Substance Use in a Cohort of HIV-infected Persons in Continuity HIV Care, 2007–2015. Am J Epidemiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tetrault JM, Kozal MJ, Chiarella J, Sullivan LE, Dinh AT, Fiellin DA. Association between risk behaviors and antiretroviral resistance in HIV-infected patients receiving opioid agonist treatment. J Addict Med 2013,7:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azar MM, Springer SA, Meyer JP, Altice FL. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend 2010,112:178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction 2004,99:361–368. [DOI] [PubMed] [Google Scholar]
- 8.Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, et al. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N Engl J Med 2016,375:229–239. [DOI] [PubMed] [Google Scholar]
- 9.Strathdee SA, Beyrer C. Threading the Needle--How to Stop the HIV Outbreak in Rural Indiana. N Engl J Med 2015,373:397–399. [DOI] [PubMed] [Google Scholar]
- 10.McGovern M, Dent K, Kessler R. A Unified Model of Behavioral Health Integration in Primary Care. Acad Psychiatry 2018. [DOI] [PubMed] [Google Scholar]
- 11.Bruce RD, Kresina TF, McCance-Katz EF. Medication-assisted treatment and HIV/AIDS: aspects in treating HIV-infected drug users. AIDS 2010,24:331–340. [DOI] [PubMed] [Google Scholar]
- 12.Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, et al. HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: results from a multisite study. J Acquir Immune Defic Syndr 2011,56 Suppl 1:S22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altice FL, Kamarulzaman A, Soriano VV, Schechter M, Friedland GH. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet 2010,376:367–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altice FL, Maru DS, Bruce RD, Springer SA, Friedland GH. Superiority of directly administered antiretroviral therapy over self-administered therapy among HIV-infected drug users: a prospective, randomized, controlled trial. Clin Infect Dis 2007,45:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Altice FL, Springer S, Buitrago M, Hunt DP, Friedland GH. Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. J Urban Health 2003,80:416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham CO, Sohler NL, Cooperman NA, Berg KM, Litwin AH, Arnsten JH. Strategies to improve access to and utilization of health care services and adherence to antiretroviral therapy among HIV-infected drug users. Subst Use Misuse 2011,46:218–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiellin DA, Weiss L, Botsko M, Egan JE, Altice FL, Bazerman LB, et al. Drug treatment outcomes among HIV-infected opioid-dependent patients receiving buprenorphine/naloxone. J Acquir Immune Defic Syndr 2011,56 Suppl 1:S33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korthuis PT, Fiellin DA, Fu R, Lum PJ, Altice FL, Sohler N, et al. Improving adherence to HIV quality of care indicators in persons with opioid dependence: the role of buprenorphine. J Acquir Immune Defic Syndr 2011,56 Suppl 1:S83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schackman BR, Leff JA, Botsko M, Fiellin DA, Altice FL, Korthuis PT, et al. The cost of integrated HIV care and buprenorphine/naloxone treatment: results of a cross-site evaluation. J Acquir Immune Defic Syndr 2011,56 Suppl 1:S76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tetrault JM, Moore BA, Barry DT, O’Connor PG, Schottenfeld R, Fiellin DA, et al. Brief versus extended counseling along with buprenorphine/naloxone for HIV-infected opioid dependent patients. J Subst Abuse Treat 2012,43:433–439. [DOI] [PubMed] [Google Scholar]
- 21.Walley AY, Palmisano J, Sorensen-Alawad A, Chaisson C, Raj A, Samet JH, et al. Engagement and Substance Dependence in a Primary Care-Based Addiction Treatment Program for People Infected with HIV and People at High-Risk for HIV Infection. J Subst Abuse Treat 2015,59:59–66. [DOI] [PubMed] [Google Scholar]
- 22.Drainoni ML, Farrell C, Sorensen-Alawad A, Palmisano JN, Chaisson C, Walley AY. Patient perspectives of an integrated program of medical care and substance use treatment. AIDS Patient Care STDS 2014,28:71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oldfield BJ, Munoz N, Boshnack N, Leavitt R, McGovern MP, Villanueva M, et al. “No more falling through the cracks”: a qualitative study to inform measurement of integration of care of HIV and opioid use disorder. J Subst Abuse Treat 2019,97:28–40. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham WE, Sohler NL, Tobias C, Drainoni ML, Bradford J, Davis C, et al. Health services utilization for people with HIV infection: comparison of a population targeted for outreach with the U.S. population in care. Med Care 2006,44:1038–1047. [DOI] [PubMed] [Google Scholar]
- 25.Farmer P, Connors M, Simmons J. Women, poverty, and AIDS : sex, drugs, and structural violence. Cambridge, MA: Common Courage Press; 1996. [Google Scholar]
- 26.Metzl JM, Hansen H. Structural competency: theorizing a new medical engagement with stigma and inequality. Soc Sci Med 2014,103:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker R, Aggleton P. HIV and AIDS-related stigma and discrimination: a conceptual framework and implications for action. Soc Sci Med 2003,57:13–24. [DOI] [PubMed] [Google Scholar]
- 28.Joint United Nations Programme on HIV/AIDS. HIV care and support. In. Geneva; 2016. [Google Scholar]
- 29.World Health Organization. Framework on integrated, people-centered health services. In: Sixty-Ninth World Health Assembly Provisional Agenda Item 16.1 Geneva; 2016. [Google Scholar]
- 30.Humphreys K, McLellan AT. A policy-oriented review of strategies for improving the outcomes of services for substance use disorder patients. Addiction 2011,106:2058–2066. [DOI] [PubMed] [Google Scholar]
- 31.Edelman EJ, Dinh AT, Moore BA, Schottenfeld RS, Fiellin DA, Sullivan LE. Human immunodeficiency virus testing practices among buprenorphine-prescribing physicians. J Addict Med 2012,6:159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knudsen HK, Cook J, Lofwall MR, Walsh SL, Studts JL, Havens JR. A mixed methods study of HIV-related services in buprenorphine treatment. Subst Abuse Treat Prev Policy 2017,12:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldman ML, Spaeth-Rublee B, Pincus HA. Quality Indicators for Physical and Behavioral Health Care Integration. JAMA 2015,314:769–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haldane V, Legido-Quigley H, Chuah FLH, Sigfrid L, Murphy G, Ong SE, et al. Integrating cardiovascular diseases, hypertension, and diabetes with HIV services: a systematic review. AIDS Care 2018,30:103–115. [DOI] [PubMed] [Google Scholar]
- 35.Chuah FLH, Haldane VE, Cervero-Liceras F, Ong SE, Sigfrid LA, Murphy G, et al. Interventions and approaches to integrating HIV and mental health services: a systematic review. Health Policy Plan 2017,32:iv27–iv47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldane V, Cervero-Liceras F, Chuah FL, Ong SE, Murphy G, Sigfrid L, et al. Integrating HIV and substance use services: a systematic review. J Int AIDS Soc 2017,20:21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009,151:264–269, W264. [DOI] [PubMed] [Google Scholar]
- 38.National Institute for Health Research. PROSPERO: International prospective register of systematic reviews. In: NHS; 2018. [Google Scholar]
- 39.Pawson R The science of evaluation : a realist manifesto. London; Thousand Oaks, Calif.: SAGE Publications; 2013. [Google Scholar]
- 40.Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med 2013,11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greenhalgh T, Wong G, Westhorp G, Pawson R. Protocol--realist and meta-narrative evidence synthesis: evolving standards (RAMESES). BMC Med Res Methodol 2011,11:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2006. [Google Scholar]
- 43.Atun R, de Jongh T, Secci F, Ohiri K, Adeyi O. Integration of targeted health interventions into health systems: a conceptual framework for analysis. Health Policy Plan 2010,25:104–111. [DOI] [PubMed] [Google Scholar]
- 44.Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, et al. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med 2006,355:365–374. [DOI] [PubMed] [Google Scholar]
- 45.Khalsa J, Vocci F, Altice F, Fiellin D, Miller V. Buprenorphine and HIV primary care: new opportunities for integrated treatment. Clin Infect Dis 2006,43 Suppl 4:S169–172. [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention. HIV Testing. 2018; https://www.cdc.gov/hiv/guidelines/testing.html. Accessed November 4, 2018. [Google Scholar]
- 47.Lohse N, Hansen AB, Pedersen G, Kronborg G, Gerstoft J, Sorensen HT, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med 2007,146:87–95. [DOI] [PubMed] [Google Scholar]
- 48.Veritas Health Information. Covidence systematic review software. In. Melbourne, Australia; 2018. [Google Scholar]
- 49.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011,343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessment the quality of nonrandomised studies in meta-analyses. Ottawa, Ontario: Ottawa Hospital Research Institute; 2008. [Google Scholar]
- 51.U.S. Department of Health and Human Services. HIV Care Continuum. In. Washington, DC: HIV.gov; 2016. [Google Scholar]
- 52.Achmad YM, Istiqomah AN, Iskandar S, Wisaksana R, van Crevel R, Hidayat T. Integration of methadone maintenance treatment and HIV care for injecting drug users: a cohort study in Bandung, Indonesia. Acta Med Indones 2009,41 Suppl 1:23–27. [PubMed] [Google Scholar]
- 53.Babudieri S, Dorrucci M, Boschini A, Carbonara S, Longo B, Monarca R, et al. Targeting candidates for directly administered highly active antiretroviral therapy: benefits observed in HIV-infected injecting drug users in residential drug-rehabilitation facilities. AIDS Patient Care STDS 2011,25:359–364. [DOI] [PubMed] [Google Scholar]
- 54.Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, Altice FL. Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug Alcohol Depend 2014,134:106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bartholow BN. A comparison of consumer-controlled and traditional HIV counseling and testing: implications for screening and outreach among injection drug users. Atlanta, GA: Georgia State University; 2005:92. [Google Scholar]
- 56.Conway B, Prasad J, Reynolds R, Farley J, Jones M, Jutha S, et al. Directly observed therapy for the management of HIV-infected patients in a methadone program. Clin Infect Dis 2004,38 Suppl 5:S402–408. [DOI] [PubMed] [Google Scholar]
- 57.Edelman EJ, Chantarat T, Caffrey S, Chaudhry A, O’Connor PG, Weiss L, et al. The impact of buprenorphine/naloxone treatment on HIV risk behaviors among HIV-infected, opioid-dependent patients. Drug Alcohol Depend 2014,139:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Edelman EJ, Moore BA, Caffrey S, Sikkema KJ, Jones ES, Schottenfeld RS, et al. HIV testing and sexual risk reduction counseling in office-based buprenorphine/naloxone treatment. J Addict Med 2013,7:410–416. [DOI] [PubMed] [Google Scholar]
- 59.Hung V, Nguyen ST, Tieu VT, Nguyen TT, Duong TH, Lyss S, et al. Evaluation of the integrated clinic model for HIV/AIDS services in Ho Chi Minh City, Viet Nam, 2013–2014. Public Health Action 2016,6:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kinahan JC, Surah S, Keating S, Bergin C, Mulcahy F, Lyons F, et al. Effect of integrating HIV and addiction care for non-engaging HIV-infected opiate-dependent patients. Ir J Med Sci 2016,185:623–628. [DOI] [PubMed] [Google Scholar]
- 61.Korthuis PT, Lum PJ, Vergara-Rodriguez P, Ahamad K, Wood E, Kunkel LE, et al. Feasibility and safety of extended-release naltrexone treatment of opioid and alcohol use disorder in HIV clinics: a pilot/feasibility randomized trial. Addiction 2017,112:1036–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korthuis PT, Tozzi MJ, Nandi V, Fiellin DA, Weiss L, Egan JE, et al. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J Acquir Immune Defic Syndr 2011,56 Suppl 1:S39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Ann Intern Med 2010,152:704–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lucas GM, Mullen BA, Galai N, Moore RD, Cook K, McCaul ME, et al. Directly administered antiretroviral therapy for HIV-infected individuals in opioid treatment programs: results from a randomized clinical trial. PLoS One 2013,8:e68286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, Moore RD. Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clin Infect Dis 2006,42:1628–1635. [DOI] [PubMed] [Google Scholar]
- 66.Lucas GM, Weidle PJ, Hader S, Moore RD. Directly administered antiretroviral therapy in an urban methadone maintenance clinic: a nonrandomized comparative study. Clin Infect Dis 2004,38 Suppl 5:S409–413. [DOI] [PubMed] [Google Scholar]
- 67.Pisu M, Cloud G, Austin S, Raper JL, Stewart KE, Schumacher JE. Substance abuse treatment in an urban HIV clinic: who enrolls and what are the benefits? AIDS Care 2010,22:348–354. [DOI] [PubMed] [Google Scholar]
- 68.Sanchez GV, Llibre JM, Torrens M, Sanvisens A, Mateu G, Knobel H, et al. Effectiveness of antiretroviral therapy in HIV-1-infected active drug users attended in a drug abuse outpatient treatment facility providing a multidisciplinary care strategy. Curr HIV Res 2012,10:356–363. [DOI] [PubMed] [Google Scholar]
- 69.Schwartz RP, Stitzer ML, Feaster DJ, Korthuis PT, Alvanzo AA, Winhusen TM, et al. HIV rapid testing in drug treatment: comparison across treatment modalities. J Subst Abuse Treat 2013,44:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sorensen JL, Haug NA, Larios S, Gruber VA, Tulsky J, Powelson E, et al. Directly administered antiretroviral therapy: pilot study of a structural intervention in methadone maintenance. J Subst Abuse Treat 2012,43:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Springer SA, Chen S, Altice FL. Improved HIV and substance abuse treatment outcomes for released HIV-infected prisoners: the impact of buprenorphine treatment. J Urban Health 2010,87:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Springer SA, Qiu J, Saber-Tehrani AS, Altice FL. Retention on buprenorphine is associated with high levels of maximal viral suppression among HIV-infected opioid dependent released prisoners. PLoS One 2012,7:e38335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sullivan LE, Barry D, Moore BA, Chawarski MC, Tetrault JM, Pantalon MV, et al. A trial of integrated buprenorphine/naloxone and HIV clinical care. Clin Infect Dis 2006,43 Suppl 4:S184–190. [DOI] [PubMed] [Google Scholar]
- 74.Tran BX, Nguyen LH, Phan HT, Latkin CA. Patient Satisfaction with Methadone Maintenance Treatment in Vietnam: A Comparison of Different Integrative-Service Delivery Models. PLoS One 2015,10:e0142644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Metsch LR, Feaster DJ, Gooden L, Matheson T, Mandler RN, Haynes L, et al. Implementing rapid HIV testing with or without risk-reduction counseling in drug treatment centers: results of a randomized trial. Am J Public Health 2012,102:1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCance-Katz EF, Gourevitch MN, Arnsten J, Sarlo J, Rainey P, Jatlow P. Modified directly observed therapy (MDOT) for injection drug users with HIV disease. Am J Addict 2002,11:271–278. [DOI] [PubMed] [Google Scholar]
- 77.Reece R, Dugdale C, Touzard-Romo F, Noska A, Flanigan T, Rich JD. Care at the Crossroads: Navigating the HIV, HCV, and Substance Abuse Syndemic. Fed Pract 2014,31:37S–40S. [PMC free article] [PubMed] [Google Scholar]
- 78.Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths--United States, 2010–2015. MMWR Morb Mortal Wkly Rep 2016,65:1445–1452. [DOI] [PubMed] [Google Scholar]
- 79.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. In: Department of Health and Human Services; 2018. [Google Scholar]
- 80.Edelman EJ, Oldfield BJ, Tetrault JM. Office-Based Addiction Treatment in Primary Care: Approaches That Work. Med Clin North Am 2018,102:635–652. [DOI] [PubMed] [Google Scholar]
- 81.Amato L, Minozzi S, Davoli M, Vecchi S. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev 2011:CD004147. [DOI] [PubMed] [Google Scholar]
- 82.Substance Abuse and Mental Health Services Administration. Federal Guidelines for Opioid Treatment Programs. In. Edited by Services USDoHaH; 2015. [Google Scholar]
- 83.Weiss L, Netherland J, Egan JE, Flanigan TP, Fiellin DA, Finkelstein R, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr 2011,56 Suppl 1:S68–75. [DOI] [PubMed] [Google Scholar]
- 84.Cunningham CO, Kunins HV, Roose RJ, Elam RT, Sohler NL. Barriers to obtaining waivers to prescribe buprenorphine for opioid addiction treatment among HIV physicians. J Gen Intern Med 2007,22:1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fredericksen RJ, Edwards TC, Merlin JS, Gibbons LE, Rao D, Batey DS, et al. Patient and provider priorities for self-reported domains of HIV clinical care. AIDS Care 2015,27:1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samet JH, Botticelli M, Bharel M. Methadone in Primary Care - One Small Step for Congress, One Giant Leap for Addiction Treatment. N Engl J Med 2018,379:7–8. [DOI] [PubMed] [Google Scholar]
- 87.Wakeman SE, Barnett ML. Primary Care and the Opioid-Overdose Crisis - Buprenorphine Myths and Realities. N Engl J Med 2018,379:1–4. [DOI] [PubMed] [Google Scholar]
- 88.MacArthur GJ, Minozzi S, Martin N, Vickerman P, Deren S, Bruneau J, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ 2012,345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saag MS, Benson CA, Gandhi RT, Hoy JF, Landovitz RJ, Mugavero MJ, et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2018 Recommendations of the International Antiviral Society-USA Panel. JAMA 2018,320:379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kimmel AD, Martin EG, Galadima H, Bono RS, Tehrani AB, Cyrus JW, et al. Clinical outcomes of HIV care delivery models in the US: a systematic review. AIDS Care 2016,28:1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Burstin H, Agrawal S, Qaseem A. Moving to Measures That Matter and Motivate Change. Ann Intern Med 2017,167:442–443. [DOI] [PubMed] [Google Scholar]
- 92.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care 2012,50:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.U.S. National Library of Medicine. Working With HIV Clinics to Adopt Addiction Treatments Using Implementation Facilitation (WHAT IF?). In. Edited by ClinicalTrials.gov; 2018. [Google Scholar]
- 94.Claborn K, Becker S, Ramsey S, Rich J, Friedmann PD. Mobile technology intervention to improve care coordination between HIV and substance use treatment providers: development, training, and evaluation protocol. Addict Sci Clin Pract 2017,12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ 1995,311:42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.