Abstract
Obstructive sleep apnoea (OSA) is a global health problem of increasing prevalence. Effective treatments are available with continuous positive airway pressure (CPAP) therapy and mandibular advancement devices (MAD). However, there is limited long-term adherence to therapy, as CPAP and MAD require permanent usage to avoid recurrence of the symptoms and adverse ill health. Alternative treatments would aid in the treatment cascade to manage OSA effectively whenever standard therapy has been trialled and failed. Hypoglossal nerve stimulation (HNS), an invasive approach to stimulate the pharyngeal dilator muscles of the upper airway during sleep, has been approved for the treatment of OSA by several healthcare systems in recent years. In parallel to the development of HNS, a non-invasive approach has been developed to deliver electrical stimulation. Transcutaneous electrical stimulation in obstructive sleep apnoea (TESLA) uses non-invasive electrical stimulation to increase neuromuscular tone of the upper airway dilator muscles of patients with OSA during sleep. Data from previous feasibility studies and randomised controlled trials have helped to identify a subgroup of patients who are “responders” to this treatment. However, further investigations are required to assess usability, functionality and task accomplishment of this novel treatment. Consideration of these factors in the study design of future clinical trials will strengthen research methodology and protocols, improve patient related outcome measures and assessments, to optimise this emerging therapeutical option. In this review, we will introduce a conceptual framework for the TESLA home programme highlighting qualitative aspects and outcomes.
Keywords: Hypoglossal nerve stimulation (HNS), neural stimulation, continuous positive airway pressure (CPAP), sleepiness, compliance
Introduction
Obstructive sleep apnoea (OSA) is a global health problem associated with increased morbidity and mortality (1-4). Patients with OSA experience repetitive upper airway closure during sleep which results in complete or partial cessation of airflow and causes oxygen desaturations; the airway closure leads to arousal from sleep, causing sleep fragmentation and daytime symptoms (5). OSA puts patients and others at risk of injury due to road traffic accidents (6,7) caused by drowsiness and results in decreased neurocognitive function (8); OSA is associated with increased cardiovascular risk (3), hypertension (9,10) and endothelial dysfunction (11), potentially contributing to myocardial infarction (12), stroke (13) and congestive heart failure (14). In addition to hypertension, risk factors associated with OSA include the male gender, age, obesity and smoking (15-18).
Various factors contribute to upper airway obstruction during sleep (19,20), including an abnormal anatomy (e.g., a narrow upper airway, enlarged tonsils, adenoids, retrognathia, obesity) and decreased neuromuscular tone (5,21-24). Different anatomical levels of upper airway obstruction, the severity of OSA, posture and sleep state can all influence the efficacy of treatment (24-28). The best way to assess OSA severity is overnight polysomnography (“sleep study”), although symptom questionnaires and risk scores are used in the clinical setting and in population-based studies, and are essential to identify patients and define the syndrome (21,29).
Currently available treatments for OSA include continuous positive airway pressure (CPAP), mandibular advancement devices (MAD) and dental devices (30-33), lifestyle advice, weight loss and positional therapy (34), maxillo-mandibular surgery and ENT interventions (e.g., uvulopalatopharyngoplasty) (35) and, less frequently, non-invasive ventilation (5). CPAP remains the Gold-standard treatment (36,37), however, there is limited long-term adherence to CPAP and only about half of the patients will use it for the recommended four hours a night during at least five days a week after one year (5,38,39). The clear association between obesity and sleep apnoea (5,23,40-43) underlines the importance of developing effective treatments for weight loss to complement CPAP therapy.
Hypoglossal nerve stimulation (HNS) has been recently developed as a novel treatment for OSA (44,45). It requires the implantation of a stimulator cuff that is in contact with the hypoglossal nerve; it works by increasing the neuromuscular tone of the upper airway dilator muscles and thereby maintaining a patent upper airway during sleep (46-48). However, delivery of electrical stimulation is not exclusively an invasive option. A similar effect on the neuromuscular tone of the upper airway dilator muscles (49,50) can be achieved non-invasively by the use of transcutaneous electrical stimulation (46,47). Patient-and-public-involvement (PPI) surveys have shown that patients with OSA, even those who are adherent to CPAP therapy, are interested in the development and the testing of novel and particularly non-invasive treatments (51). Participation in and recruitment to future clinical trials will benefit from this involvement.
In 2014, HNS was approved by the United States Food and Drug Administration (FDA) (52), and there are now post-market research registries and studies (e.g., Germany). In the UK, the National Institute for Health and Care Excellence (NICE) has published its interventional procedure guidance on HNS (IPG598) (53). The transcutaneous approach (TESLA) is currently undergoing a domiciliary feasibility study (TESLA home; NCT03160456, for the protocol see online: http://fp.amegroups.cn/cms/jtd.2019.05.04-1.pdf), involving three-months treatment and assessment. The TESLA home methodology delivers electrical current via transcutaneous patches in the submental area, targeting the genioglossus muscle to maintain airway patency. This randomised controlled trial recruits patients with OSA (apnoea-hypopnoea index 5–35/hour) who have failed to use CPAP effectively (usage <4 hours/night). Slim, overweight and mildly obese subjects (body-mass index, BMI<32 kg/m2) with an antero-posterior wall collapse and a slim neckline are known to represent the phenotype of “responders” to this therapy. In particular women with OSA have been identified to benefit from this method (50). Similar to the selection criteria utilised for HNS, morbidly obese subjects with more severe OSA, multi-level or concentric upper airway collapse do not seem to sufficiently benefit and are excluded from upper airway stimulation trials. In addition to objectively measuring the effectiveness of electrical stimulation on preventing upper airway collapse, qualitative assessments are important to test the feasibility of such a novel treatment. Patients’ feedback will help to determine the feasibility of domiciliary transcutaneous electrical stimulation, design future clinical trials and inform further modification of the technology and the device (54). In this review, we will introduce a conceptual framework for the TESLA home programme to highlight qualitative aspects and outcomes.
Conceptual framework
A framework was developed based on the conceptual idea to capture relevant aspects of the research undertaken in the TESLA programme, analyse and address health-regulatory body requirements (Medicines & Healthcare Products Regulatory Agency, MHRA) and produce qualitative data from clinical trials that are not directly linked to primary or secondary outcomes of the clinical trial (54). The published literature of electrical stimulation for sleep apnoea was screened and databases were searched (PubMed, Web of Science and Google Scholar) using specific criteria (“electrical stimulation”, “transcutaneous electrical stimulation”, “hypoglossal nerve stimulation”, “Obstructive Sleep Apnoea”, “treatment”, “usability”, “medical devices”, “MHRA”); the found results were assessed by two independent reviewers (R Tas, M Beach) and included for discussion with two other reviewers (M Nido, J Steier) to generate and refine a model of a conceptual framework for the TESLA programme. The described conceptual framework was created with the intention to better assess the interaction between the three domains “medical device” (TESLA), “user” (patient) and “task” (OSA treatment) (54) which are defined as (I) “usability”, (II) “functionality” and (III) “task accomplishment” (Figure 1).
Figure 1.
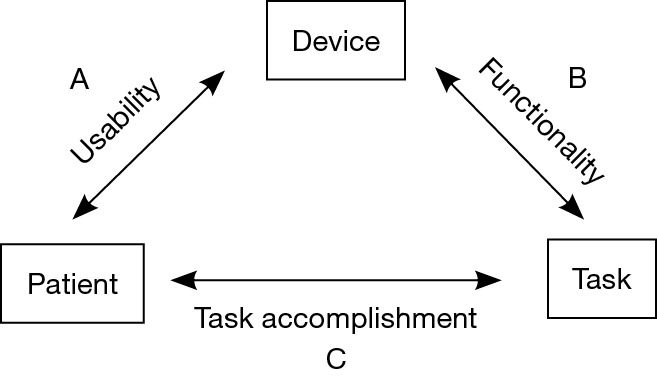
Schematic proposal of a conceptual framework TESLA. A: refers to “usability” to reflect the interaction between patient and device; B: refers to “functionality” to describe the link between device and task; C: refers to “task accomplishment” to assess the link between patient and task.
“Usability”
The “usability” describes the degree to which the patient can make use of a medical device, to achieve quantifiable objectives efficiently, effectively and satisfactorily (55) (Figure 1). The user experience is complex and can, in part, be explained by a model that incorporates seven aspects of a treatment from the perspective of the user: whether it is usable, valuable, useful, desirable, accessible, credible and findable (56). “Usability” testing is considered a cornerstone in user-centred design, as it provides information about the machine-user interaction (57).
The perceived “usability” of a treatment can affect adherence, which is the limiting factor for other sleep apnoea treatments like CPAP therapy and MAD. It is essential to understand this feature to better treat non-adherent patients. Long-term adherence depends on how individuals judge their personal need for a treatment relative to their concerns about its potential adverse effects. Adherence is a primary determinant of the effectiveness of treatment, good long-term adherence improves the effectiveness of therapeutic interventions (58).
To asses usability quantitative and qualitative approaches and methods can be used (59). Questionnaires (quantitative method) can rate the “ease of use”, “instructions for use”, and the “clarity of the design”, as well as ergonomics on given rating scales. With qualitative methods, such as (semi-)structured interviews, additional information can be gathered assessing patients’ “adherence to intended use”, or obvious design errors. The collected quantitative and qualitative data can then be used to address issues of “usability” and improve the machine-user interaction. Patients’ diaries to record practicability and potential adverse events can further measure “usability” of transcutaneous electrical stimulation for OSA (60).
Psychological and personal aspects of a patient are essential characteristic when discussing “usability”, as they will affect any interaction with medical devices (61-63). Additionally, the attitude to treatment can be positive, negative or neutral based on previous experiences and expectations, as it is set out in motivational theories, such as the Valence-Instrumentality-Expectancy (V-I-E) model (64). Motivation to use a device can therefore be explained as a multiplied connection of expectancy of a certain outcome (“the device will help to improve my health”) and the value of the result (“better health is of high relevance to the patient”).
The following assessment can assist to address usability of the method:
❖ Patient comfort and device acceptance: Semiquantitative visual analogue scales can be used to assess the comfort of a new treatment (‘very much/very good’/“0” points, to ‘not at all/terrible’/“10”points) (54). In the context of TESLA, this can be used to identify adverse effects that can arise from the treatment, such as ‘skin discomfort’.
❖ Qualitative and descriptive parameters and feedback: Patient feedback about usage, acceptance, problems and perceived benefits, may assess acceptability of a novel treatment. Difficulties with the device, such as reliability, faults, ease of use, can be useful to modify the feasibility of the machine and, in the case of the TESLA programme, also the skin interface (hydrogel/patches).
In the context of TESLA-home the described features play an important role (Figure 2; online: http://fp.amegroups.cn/cms/jtd.2019.05.04-1.pdf) and patient-and-public-involvement (PPI) (51) was undertaken at an early stage to understand the user, the task and physiological requirements, encourage early and active involvement, incorporate user-centred evaluations, address the entire user experience, encourage a multi-disciplinary design, and continuously engage with the users during the process (ISO 9241-210) (65).
Figure 2.
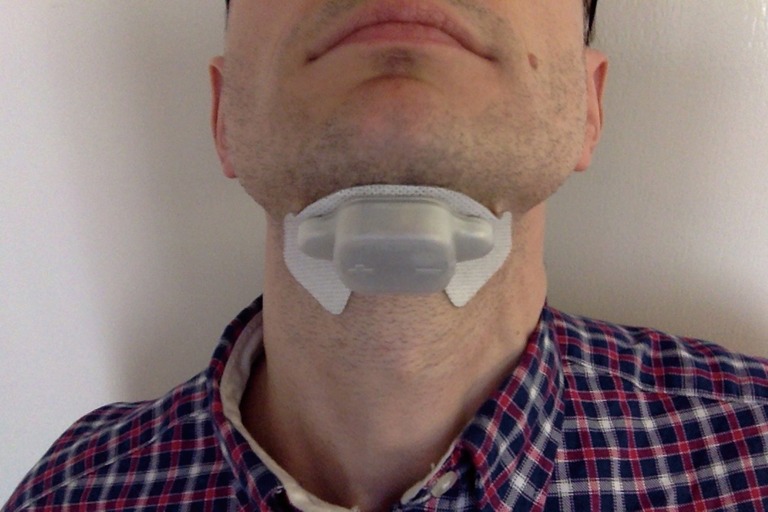
Picture of one of the prototypes of the TESLA programme attached to the submental area.
“Functionality”
The “functionality” tests the interaction between the ‘device’ and the ‘task’, it could also be described as efficacy in achieving a sufficient contraction of the dilator muscles of the upper airway to avoid upper airway obstruction (Figure 1). It assesses whether the muscles are sufficiently stimulated, and is further characterised by the suitability of the method, accuracy, interoperability, security, and functionality compliance; these features aim to satisfy the stated or implied needs (66). To maximise the effect and increase the neuromuscular tone of the genioglossus, improve upper airway patency and, thereby, treat OSA, some parameters including several basic properties of the medical device such as current, pulse width, stimulation frequency, wave form and pad shapes (size, location, uni- vs. bilateral stimulation, material), and stimulation timing (triggered, intermittent, continuous) could be varied to further refine the treatment. These variables impact on the generated force, the fatiguability on the neuromuscular junction, the skin sensation and the tolerability of the method.
Relevant MHRA guidelines (67)
The MHRA have issued relevant guidance on device resilience. To be resilient, the medical device’s functioning and performance should not be affected adversely through normal conditions of use over time. Furthermore, the device measurements and outputs must be accurate and remain so for the duration of the devices use with any accuracy limits clearly specified. Any measurements made should be expressed in the appropriate units as described in the Council Directive 80/181/EEC, and functions involving measuring and monitoring should be developed according to ergonomic principles. Devices involving electronic systems must be designed to function according to their intended use with ensured reliability and repeatability of performance.
Specific to the TESLA methodology, it is important to measure a number of quantitative variables that help the understanding of the effect on the targeted muscles, describe physiological changes on the airway structure and the skin sensation of the electrical activity:
❖ Imaging to observe muscle contraction and improvement in upper airway patency:
⬥ Upper airway magnetic resonance imaging (MRI) or computer tomography (CT) can be used to accurately visualize and calculate the diameter (mm2) of the upper airway awake and during sleep, as well as with the stimulation turned ‘on’ or ‘off’.
⬥ Ultrasound is ubiquitous available in hospitals and can be used to identify and localise upper airway obstruction (68). The genioglossus can be visualised in different planes (49,68) and the contraction of this muscle during stimulation can be tested at the bedside. Ultrasound measurements (frequency 5 to 13 MHz, Figure 3) can track significant contraction in the genioglossus during stimulation.
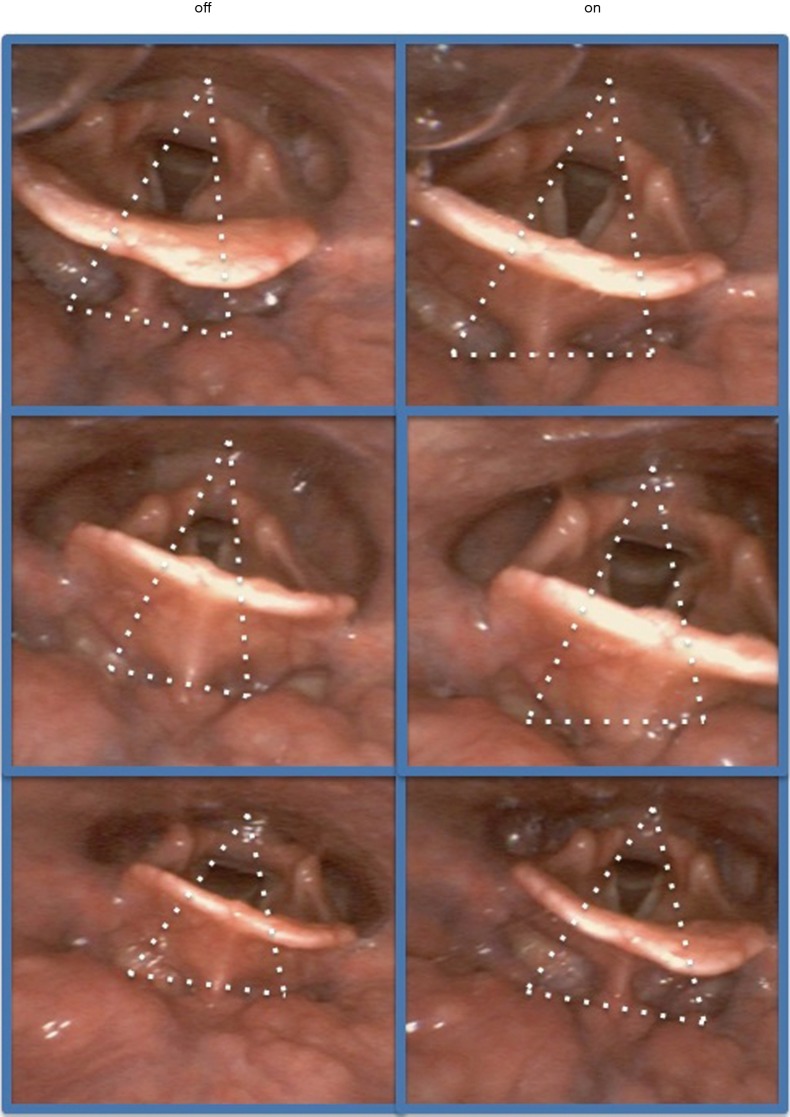
⬥ Endoscopy of the upper airway is an option to evaluate the severity of upper airway obstruction (69,70) and offers the chance to assess upper airway patency during stimulation (Figure 4).
❖ Electromyography of the submental muscles can record targeted muscle activity (49,71-73).
Figure 3.
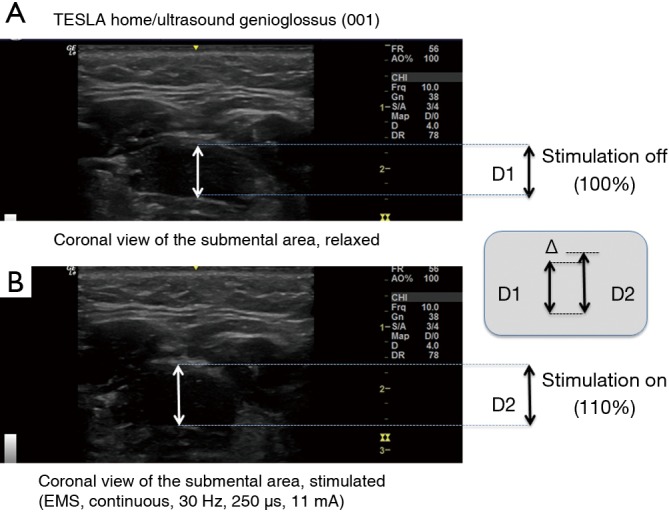
Ultrasound images of the genioglossus muscle, (A) it shows the relaxed muscle (without stimulation) in a coronal view, (B) it shows the muscle during electrical stimulation and the resulting change in the diameter caused by contraction. As the muscle contracts, it shortens, pulling the pharyngeal wall towards the anterior direction. An increase in the radius by +10% results in an approximate increase in the cross-sectional area (CSA) of the muscle of +21%, assuming a round model (CSA= π × r2).
Figure 4.
Endoscopic images of the upper airway. The left panels show the upper airway with the vocal cords and the epiglottis while electrical stimulation is turned “off”, the right panel shows the same area when stimulation is turned “on”. The anterior-posterior diameter increases with stimulation, the tonsilla lingualis becomes visible just underneath the epiglottis when electrical stimulation is turned on (right lower panel).
For the TESLA home trial, physiological measurements focus on upper airway morphology and muscle contractions during electrical stimulation using ultrasound, upper airway endoscopy in the awake participants and functional assessments (see online: http://fp.amegroups.cn/cms/jtd.2019.05.04-1.pdf); these measurements are available at the bedside in most clinical settings.
“Task accomplishment”
The interaction between the user and the task/goal can be further described to determine “task accomplishment”, which can be assessed subjectively as well as objectively. Patients’ preconception of what they believe resembles a successful treatment compared with the treating teams’ point of view may not be entirely compatible, as they can prioritise different criteria [e.g., sleepiness vs. apnoea-hypopnoea index (AHI)].
Objectively, treatment data can be measured using tools that provide quantitative results related to the assessment of sleep, restoration of sleep architecture and improved symptom control, quality of life and mastery. The main outcome parameter for clinical trials in OSA is typically the AHI that is used to define severity of the disease. However, the following parameters can be used to further address objective assessments:
❖ Nocturnal polysomnography results are the Gold standard to assess OSA severity and treatment efficacy (74). Certain indices derived from the polysomnography are of importance when describing upper airway patency and functional assessment during sleep:
⬥ Apnoea-hypopnoea index (AHI): the AHI is the number of apnoea’s (airflow absence for ≥10 seconds) and hypopnoeas (reduction in respiratory effort by ≥30%, associated with oxygen desaturation of ≥3% and/or arousal) per hour of recorded sleep. Excessive daytime sleepiness and an AHI greater than 5 are key features of OSA. The AHI is a standardised method that evaluates both severity and treatment outcome for OSA (mild OSA: AHI 5–14/hour, moderate OSA: AHI 15–30/hour, and severe OSA: AHI >30/hour) (75). The AHI does not assess the time spent in respiratory events or allow the differentiation of hypopnoeas or apnoeas (76). An incomplete reopening of the upper airway using an insufficient treatment may convert apnoeas into hypopnoeas, which would not be reflected accurately in the AHI. However, the AHI is a widely accepted tool for assessing OSA (52).
⬥ Oxygen Desaturation Index (ODI): the ODI can be recorded according to the desaturation threshold, most commonly the 3% or the 4% ODI are reported. The ODI is used to assess OSA in clinical settings and when tracking treatment of OSA (77-81).
⬥ Sleep Architecture: this term refers to the cyclical pattern of sleep cycles and the preserved features of a hypnogram, including non-rapid eye movement (N1-3) and rapid eye movement (REM) sleep. OSA causes disruption of the natural sleep architecture, with frequent arousals leading to numerous sleep stage shifts and abnormal cycling of sleep (82). On CPAP therapy, patients with OSA have an improved sleep quality (83). Similarly, the recording of the hypnogram can supplement the assessment of treatment and “task accomplishment”.
⬥ Arousal Index (AI): the AI is defined as the number of awakenings per hour during a recorded sleep study period, it is used in parallel to the hypnogram to understand sleep fragmentation and architecture (84).
⬥ Snoring: snoring is a symptom associated with OSA that predominantly affects partners and others. However, the percentage of the night that patients snore may not reflect the severity of OSA (85), although there is a positive correlation between louder snoring and severe OSA (86).
In addition, subjective assessments of “task accomplishment” are important, as patients need to be offered the opportunity to report on usage, sensation, expectation and efficacy of the treatment. The following tools may provide guidance for these assessments:
❖ Epworth Sleepiness Scale (ESS): this is a commonly used questionnaire including eight questions about patient’s perception of sleepiness, scoring form “0” (not at all) to “3” (highly likely to doze). The minimal total score is “0” and the maximum “24” points (87). A score higher than 8 points has a 76% specificity for OSA (88) and treatments for OSA are considered effective if the ESS score improves. Accuracy can be improved through the use of a patient-partner consensus score (89,90); pictorial (91) and online ESS (92) are used for screening. However, the ESS remains a subjective report and is subject to relevant sources of bias and inaccuracy (93). Despite its limitations, the minimal clinically important difference (MCID) for the ESS has been described as an improvement of more than two points (94).
❖ Berlin Questionnaire (BQ): this questionnaire is typically used to screen for OSA. The BQ includes questions about snoring, daytime somnolence, body mass index (BMI), and hypertension (95). It is a brief and validated screening tool that identifies people in the community who are at risk of OSA (96). It has a high sensitivity but low specificity (97,98).
❖ Stanford Sleepiness Scale (SSS): the SSS is a self-reported questionnaire to assess how awake the patient feels throughout the day (99). It can be used to compare sleepiness in hourly intervals of the day, for example prior to new treatment and to assess success thereafter. The SSS can be a useful measure to observe individual progress and to compare results with other studies (99).
❖ Hospital Anxiety and Depression Scale (HADS): the HADS is a tool used for assessing anxiety and depression (100,101). It is widely used in clinical practice and research. It contains fourteen straightforward questions (102). The link between depression/anxiety and chronic conditions like OSA is well reported (103). Hence, this tool is important in the assessment of how OSA affects the mental health of an individual. In addition, mental health disorders often co-exist with OSA. These conditions can include depression, anxiety, schizophrenia, bipolar disorder, post-traumatic stress disorder (PTSD) and substance use disorders (104). Used as a screening tool, the HADS can be added prior to commencing on new treatments, and repeatedly measured to follow up on the treatment effect (67).
❖ Functional Outcomes of Sleep Questionnaire (FOSQ): this is a validated questionnaire to assess the impact of sleepiness on a patient’s ability to perform activities of daily living (ADLs) (105). The questionnaire contains 30 questions, covering 5 subscales (General Productivity, Social Outcome, Activity Level, Vigilance, Intimate Relationships and Sexual Activity) (106).
❖ European Quality of Life five dimensions scale (EQ-5D): the EQ-5D is used for the standardised measurement of health outcomes (107,108) and is available in 130 languages (109); the results can be used for reference-case analyses and for health-economics (110).
❖ Other Questionnaires: according to a systematic review of outcome measures for OSA, the most suitable additional assessments include the Maugeri OSA syndrome (MOSAS) questionnaire (quality of life), the sleep apnoea quality of life index (SAQLI), the OSA patient-orientated severity index (OSAPOSI) and the Quebec sleep questionnaire (QSQ) (100,111).
The discussion about suitable outcome parameters in OSA remains contentious, partially due to the conflict of subjective vs. objective disease burden (subjective symptoms vs. objective disease severity). However, for any clinical trial it is important to capture enough data to describe the ‘syndrome’ (symptoms and sleep apnoea pathophysiology) and, thus, the TESLA home trial includes all of the above objective markers, including full polysomnography, and many of the symptom questionnaires plus some semi-quantitative interviews during the follow up period (see online: http://fp.amegroups.cn/cms/jtd.2019.05.04-1.pdf).
Conclusions
In order to assess a novel treatment method, as used in the TESLA home trial, it is important to understand additional components other than the primary outcome parameters of change in disease severity in response to treatment; these features define treatment uptake by addressing “usability”, “functionality” and “task accomplishment”. The conceptual framework for future studies using TESLA methodology acknowledges these key elements to address relevant guidelines, including those required by the NICE and the MHRA.
The TESLA home programme incorporates the following points:
❖ Development of a human factors engineering (HFE) programme within the existing product development process that satisfies regulations and standards.
❖ Design of user interfaces that not only enable safe and effective user interactions, but are also perceived as usable and appealing by early and continuous involvement of the user (PPI).
❖ Suitable labels and information for users that enable and enhance the user’s ability to engage with the product effectively and safely (112) and disseminate information from clinical trials and device performance.
The proposed patient related outcome measures (PROMS) have been designed for future studies and could be used to test the efficacy of the treatment; accurate recording of PROMS will provide invaluable information from clinical trials to refine the method, optimise future treatment performance and design study protocols.
Acknowledgments
The TESLA-investigator group includes Professor John Moxham, Professor Michael I Polkey, Professor Yuanming Luo, Professor Adrian Williams, Dr Gerard Rafferty, Dr Deeban Ratneswaran, Dr Esther I Schwarz, Dr Michael Cheng, Dr Miral Al-Sherif, Dr Baiting He, Dr Brian Kent, Ms Gill Arbane, Ms Jennifer Owusu-Afriyie, Mr Paul Eze-John, Mr Athanasius Ishak, Dr Kai Lee, Dr Nimish Shah, Professor Nicholas Hart, Dr Martino Pengo and Professor Joerg Steier.
We gratefully acknowledge the support of the clinical team at the Lane Fox Unit and the Sleep Disorders Centre at Guy’s and St Thomas’ NHS Foundation Trust, London, the Channel Scheme of the Egyptian Embassy and the University of Minia, Egypt, and Guangzhou Medical College, China. The TESLA-home trial (ClinicalTrials.gov Identifier: NCT03160456) is supported by a grant of the British Lung Foundation (BLF) and the NIHR CLRN South London. Professor Steier’s contributions were partially supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London, UK. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Conflicts of Interest: J Steier is named inventor on patent WO 2016/124739 Al (‘Apparatus for treatment of snoring and sleep apnoea’) on behalf of King’s College London and Guy’s & St Thomas’ NHS Foundation Trust. Other authors have no conflicts of interest to declare.
References
- 1.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med 2009;6:e1000132. 10.1371/journal.pmed.1000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieto FJ, Peppard PE, Young T, et al. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2012;186:190-4. 10.1164/rccm.201201-0130OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 2005;365:1046-53. 10.1016/S0140-6736(05)74229-X [DOI] [PubMed] [Google Scholar]
- 4.Lavie P, Lavie L, Herer P. All-cause mortality in males with sleep apnoea syndrome: declining mortality rates with age. Eur Respir J 2005;25:514-20. 10.1183/09031936.05.00051504 [DOI] [PubMed] [Google Scholar]
- 5.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet 2014;383:736-47. 10.1016/S0140-6736(13)60734-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horstmann S, Hess CW, Bassetti C, et al. Sleepiness-related accidents in sleep apnea patients. Sleep 2000;23:383-9. 10.1093/sleep/23.3.1e [DOI] [PubMed] [Google Scholar]
- 7.Tregear S, Reston J, Schoelles K, et al. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med 2009;5:573-81. [PMC free article] [PubMed] [Google Scholar]
- 8.Goel N, Rao H, Durmer JS, et al. Neurocognitive consequences of sleep deprivation. Semin Neurol 2009;29:320-39. 10.1055/s-0029-1237117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geiger SD, Shankar A. The Relationship between Sleep-Disordered Breathing and Hypertension in a Nationally Representative Sample. Sleep Disord 2015;2015:769798. 10.1155/2015/769798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peppard PE, Young T, Palta M, et al. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N Engl J Med 2000;342:1378-84. 10.1056/NEJM200005113421901 [DOI] [PubMed] [Google Scholar]
- 11.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med 2007;3:409-15. [PMC free article] [PubMed] [Google Scholar]
- 12.Roubille F, Lairez O, Mewton N, et al. Cardioprotection by clopidogrel in acute ST-elevated myocardial infarction patients: a retrospective analysis. Basic Res Cardiol 2012;107:275. 10.1007/s00395-012-0275-3 [DOI] [PubMed] [Google Scholar]
- 13.Redline S, Yenokyan G, Gottlieb DJ, et al. Obstructive sleep apnea-hypopnea and incident stroke: the sleep heart health study. Am J Respir Crit Care Med 2010;182:269-77. 10.1164/rccm.200911-1746OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001;163:19-25. 10.1164/ajrccm.163.1.2001008 [DOI] [PubMed] [Google Scholar]
- 15.Deng X, Gu W, Li Y, et al. Age-group-specific associations between the severity of obstructive sleep apnea and relevant risk factors in male and female patients. PloS One 2014;9:e107380. 10.1371/journal.pone.0107380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hedner J, Bengtsson-Boström K, Peker Y, et al. Hypertension prevalence in obstructive sleep apnoea and sex: a population-based case–control study. Eur Respir J 2006;27:564-70. 10.1183/09031936.06.00042105 [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Xu H, Chen R, et al. Smoking, obstructive sleep apnea syndrome and their combined effects on metabolic parameters: Evidence from a large cross-sectional study. Sci Rep 2017;7:8851. 10.1038/s41598-017-08930-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romero-Corral A, Caples SM, Lopez-Jimenez F, et al. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest 2010;137:711-9. 10.1378/chest.09-0360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Badr MS. Pathophysiology of upper airway obstruction during sleep. Clin Chest Med 1998;19:21-32. 10.1016/S0272-5231(05)70429-9 [DOI] [PubMed] [Google Scholar]
- 20.Kuna ST, Sant'Ambrogio G. Pathophysiology of Upper Airway Closure During Sleep. JAMA 1991;266:1384-9. 10.1001/jama.1991.03470100076036 [DOI] [PubMed] [Google Scholar]
- 21.Malhotra A, White DP. Obstructive sleep apnoea. Lancet 2002;360:237-45. 10.1016/S0140-6736(02)09464-3 [DOI] [PubMed] [Google Scholar]
- 22.Framnes SN, Arble DM. The Bidirectional Relationship Between Obstructive Sleep Apnea and Metabolic Disease. Front Endocrinol (Lausanne) 2018;9:440. 10.3389/fendo.2018.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim AM, Keenan BT, Jackson N, et al. Tongue fat and its relationship to obstructive sleep apnea. Sleep 2014;37:1639-48. 10.5665/sleep.4072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman M, Ibrahim H, Joseph NJ. Staging of obstructive sleep apnea/hypopnea syndrome: a guide to appropriate treatment. Laryngoscope 2004;114:454-9. 10.1097/00005537-200403000-00013 [DOI] [PubMed] [Google Scholar]
- 25.Oksenberg A, Silverberg DS. Avoiding the supine posture during sleep for patients with mild obstructive sleep apnea. Am J Respir Crit Care Med 2009;180:101; author reply 101-2. 10.1164/ajrccm.180.1.101 [DOI] [PubMed] [Google Scholar]
- 26.Joosten SA, O'Driscoll DM, Berger PJ, et al. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev 2014;18:7-17. 10.1016/j.smrv.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 27.Herrero Á, Sedano J, Baruque B, et al. 10th International Conference on Soft Computing Models in Industrial and Environmental Applications. Springer International Publishing 2015. [Google Scholar]
- 28.Oksenberg A, Silverberg D, Offenbach D, et al. Positional therapy for obstructive sleep apnea patients: A 6-month follow-up study. Laryngoscope 2006;116:1995-2000. 10.1097/01.mlg.0000237674.66716.a7 [DOI] [PubMed] [Google Scholar]
- 29.Calleja JM, Esnaola S, Rubio R, et al. Comparison of a cardiorespiratory device versus polysomnography for diagnosis of sleep apnoea. Eur Respir J 2002;20:1505-10. 10.1183/09031936.02.00297402 [DOI] [PubMed] [Google Scholar]
- 30.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med 2014;10:215-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jat KR, Mathew JL. Continuous positive airway pressure (CPAP) for acute bronchiolitis in children. Cochrane Database Syst Rev 2015;1:CD010473. [DOI] [PubMed] [Google Scholar]
- 32.Chaves CM, Junior, Dal Fabbro C, Machado MAC, et al. Use of Mandibular Advancement Devices For Obstructive Sleep Apnoea Treatment In Adults. Int Arch Med 2017;10. doi: https://doi.org/. 10.3823/2502 [DOI] [Google Scholar]
- 33.Kuhn E, Schwarz EI, Bratton DJ, et al. Effects of CPAP and Mandibular Advancement Devices on Health-Related Quality of Life in OSA: A Systematic Review and Meta-analysis. Chest 2017;151:786-94. 10.1016/j.chest.2017.01.020 [DOI] [PubMed] [Google Scholar]
- 34.de Vries GE, Hoekema A, Doff MHJ, et al. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med 2015;11:131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaghi S, Holty J-EC, Certal V, et al. Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea: A Meta-analysisMaxillomandibular Advancement for Treatment of Obstructive Sleep ApneaMaxillomandibular Advancement for Treatment of Obstructive Sleep Apnea. JAMA Otolaryngol Head Neck Surg 2016;142:58-66. 10.1001/jamaoto.2015.2678 [DOI] [PubMed] [Google Scholar]
- 36.Spicuzza L, Caruso D, Di Maria G. Obstructive sleep apnoea syndrome and its management. Ther Adv Chronic Dis 2015;6:273-85. 10.1177/2040622315590318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao MT, Sternbach JM, Guilleminault C. Continuous positive airway pressure therapy in obstuctive sleep apnea: benefits and alternatives. Expert Rev Respir Med 2017;11:259-72. 10.1080/17476348.2017.1305893 [DOI] [PubMed] [Google Scholar]
- 38.Richard W, Venker J, den Herder C, et al. Acceptance and long-term compliance of nCPAP in obstructive sleep apnea. Eur Arch Otorhinolaryngol 2007;264:1081-6. 10.1007/s00405-007-0311-3 [DOI] [PubMed] [Google Scholar]
- 39.Pengo MF, Czaban M, Berry MP, et al. The effect of positive and negative message framing on short term continuous positive airway pressure compliance in patients with obstructive sleep apnea. J Thorac Dis 2018;10:S160-9. 10.21037/jtd.2017.07.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230-5. 10.1056/NEJM199304293281704 [DOI] [PubMed] [Google Scholar]
- 41.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc 2008;5:136-43. 10.1513/pats.200709-155MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steier J, Martin A, Harris J, et al. Predicted relative prevalence estimates for obstructive sleep apnoea and the associated healthcare provision across the UK. Thorax 2014;69:390-2. 10.1136/thoraxjnl-2013-203887 [DOI] [PubMed] [Google Scholar]
- 43.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013;177:1006-14. 10.1093/aje/kws342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strollo PJ, Soose RJ, Maurer JT, et al. Upper-Airway Stimulation for Obstructive Sleep Apnea. N Engl J Med 2014;370:139-49. 10.1056/NEJMoa1308659 [DOI] [PubMed] [Google Scholar]
- 45.Heiser C, Thaler E, Boon M, et al. Updates of operative techniques for upper airway stimulation. Laryngoscope 2016;126 Suppl 7:S12-6. 10.1002/lary.26158 [DOI] [PubMed] [Google Scholar]
- 46.Pengo MF, Steier J. Emerging technology: electrical stimulation in obstructive sleep apnoea. J Thorac Dis 2015;7:1286-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bisogni V, Pengo MF, De Vito A, et al. Electrical stimulation for the treatment of obstructive sleep apnoea: a review of the evidence. Expert Rev Respir Med 2017;11:711-20. 10.1080/17476348.2017.1358619 [DOI] [PubMed] [Google Scholar]
- 48.Heiser C, Hofauer B. Stimulation for sleep apnea: Targeting the hypoglossal nerve in the treatment of patients with OSA. HNO 2018;66:705-16. 10.1007/s00106-018-0534-1 [DOI] [PubMed] [Google Scholar]
- 49.Steier J, Seymour J, Rafferty GF, et al. Continuous transcutaneous submental electrical stimulation in obstructive sleep apnea: a feasibility study. Chest 2011;140:998-1007. 10.1378/chest.10-2614 [DOI] [PubMed] [Google Scholar]
- 50.Pengo MF, Xiao S, Ratneswaran C, et al. Randomised sham-controlled trial of transcutaneous electrical stimulation in obstructive sleep apnoea. Thorax 2016;71:923-31. 10.1136/thoraxjnl-2016-208691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campbell T, Pengo MF, Steier J. Patients' preference of established and emerging treatment options for obstructive sleep apnoea. J Thorac Dis 2015;7:938-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.US FDA approved devices. 2014. Available online: http://wayback.archive-it.org/7993/20170111091458/http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm381097.htm.
- 53.National Institute for Health and Care Excellence (NICE). Hypoglossal nerve stimulation for moderate to severe obstructive sleep apnoea - Guidance and guidelines. 2017. Available online: https://www.nice.org.uk/guidance/ipg598
- 54.ClinicalTrials.gov. Domiciliary Transcutaneous Electrical Stimulation in Obstructive Sleep Apnoea (TESLA-Home) 2018. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03160456?term=Steier&rank=1.
- 55.Ergonomic Requirements for Office Work with Visual Display Terminals, ISO 9241-11, ISO, Geneva, 1998. Available online: https://en.wikipedia.org/wiki/Usability.
- 56.Morville P. User Experience Design. 2004. Available online: http://semanticstudios.com/user_experience_design/.
- 57.Schmettow M, Schnittker R, Schraagen JM. An extended protocol for usability validation of medical devices: Research design and reference model. J Biomed Inform 2017;69:99-114. 10.1016/j.jbi.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 58.Sabaté E. Adherence to long-term therapies: evidence for action / Geneva: World Health Organization. . 2003. Available online: http://www.who.int/iris/handle/10665/42682. [Google Scholar]
- 59.Hass C, Berlin D, editors. Usability Testing Medical Devices: A Practical Guide to Minimizing Risk and Maximizing Success. Design, User Experience, and Usability. Health, Learning, Playing, Cultural, and Cross-Cultural User Experience; 2013 2013//; Berlin, Heidelberg: Springer Berlin Heidelberg. [Google Scholar]
- 60.Verse T, Schwalb J, Hormann K, et al. Submental transcutaneous electrical stimulation for obstructive sleep apnea. HNO 2003;51:966-70. 10.1007/s00106-003-0842-x [DOI] [PubMed] [Google Scholar]
- 61.Bohm J. Two-factor theory - at the intersection of health care management and patient satisfaction. Clinicoecon Outcomes Res 2012;4:277-85. 10.2147/CEOR.S29347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tuch AN, Hornbæk K. Does Herzberg's Notion of Hygienes and Motivators Apply to User Experience? ACM Transactions on Computer-Human Interaction (TOCHI) 2015;22:16 10.1145/2724710 [DOI] [Google Scholar]
- 63.Money AG, Barnett J, Kuljis J, et al. The role of the user within the medical device design and development process: medical device manufacturers' perspectives. BMC Med Inform Decis Mak 2011;11:15. 10.1186/1472-6947-11-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vroom VH. Work and motivation. Work and motivation. Oxford, England: Wiley; 1964. [Google Scholar]
- 65.Kübler A, Holz EM, Riccio A, et al. The user-centered design as novel perspective for evaluating the usability of BCI-controlled applications. PLoS One 2014;9:e112392. 10.1371/journal.pone.0112392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.ISO/IEC 9126. Available online: https://en.wikipedia.org/wiki/ISO/IEC_9126.
- 67.Communities OJotE. COUNCIL DIRECTIVE 93/42/EEC of concerning medical devices. 1993. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CONSLEG:1993L0042:20071011:en:PDF.
- 68.Isaiah A, Mezrich R, Wolf J. Ultrasonographic Detection of Airway Obstruction in a Model of Obstructive Sleep Apnea. Ultrasound Int Open 2017;3:E34-E42. 10.1055/s-0042-124503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harvin G, Ali E, Raina A, et al. Airway observations during upper endoscopy predicting obstructive sleep apnea. Ann Gastroenterol 2016;29:481-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DE Corso E , Fiorita A, Rizzotto G, et al. The role of drug-induced sleep endoscopy in the diagnosis and management of obstructive sleep apnoea syndrome: our personal experience. Acta Otorhinolaryngol Ital 2013;33:405-13. [PMC free article] [PubMed] [Google Scholar]
- 71.Steier J, Jolley CJ, Polkey MI, et al. Nocturnal asthma monitoring by chest wall electromyography. Thorax 2011;66:609-14. 10.1136/thx.2010.152462 [DOI] [PubMed] [Google Scholar]
- 72.Dotan Y, Pillar G, Tov N, et al. Dissociation of electromyogram and mechanical response in sleep apnoea during propofol anaesthesia. Eur Respir J 2013;41:74-84. 10.1183/09031936.00159611 [DOI] [PubMed] [Google Scholar]
- 73.Steier J, Jolley CJ, Seymour J, et al. Increased load on the respiratory muscles in obstructive sleep apnea. Respir Physiol Neurobiol 2010;171:54-60. 10.1016/j.resp.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 74.Medical Advisory Secretariat Polysomnography in patients with obstructive sleep apnea: an evidence-based analysis. Ont Health Technol Assess Ser 2006;6:1-38. [PMC free article] [PubMed] [Google Scholar]
- 75.Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical Practice Guideline for the Pharmacologic Treatment of Chronic Insomnia in Adults: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:307-49. 10.5664/jcsm.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Asghari A, Mohammadi F. Is Apnea-Hypopnea Index a proper measure for Obstructive Sleep Apnea severity? Med J Islam Repub Iran 2013;27:161-2. [PMC free article] [PubMed] [Google Scholar]
- 77.Basheer H, Sharma S, Patel M. Can we use the oxygen desaturation index alone to reliably diagnose obstructive sleep apnoea in obese patients? Eur Respir J 2016;48:PA2315. [Google Scholar]
- 78.Chung F, Liao P, Elsaid H, et al. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg 2012;114:993-1000. 10.1213/ANE.0b013e318248f4f5 [DOI] [PubMed] [Google Scholar]
- 79.Fabius TM, Benistant JR, Bekkedam L, et al. Validation of the oxygen desaturation index to exclude sleep apnea. Eur Respir J 2016;48:PA2318. [DOI] [PubMed] [Google Scholar]
- 80.Ernst G, Bosio M, Salvado A, et al. Difference between apnea-hypopnea index (AHI) and oxygen desaturation index (ODI): proportional increase associated with degree of obesity. Sleep Breath 2016;20:1175-83. 10.1007/s11325-016-1330-3 [DOI] [PubMed] [Google Scholar]
- 81.Fawzi A, Basheer H, Patel M, et al. P62 Oxygen desaturation index for diagnosing obstructive sleep apnoea in patients with morbid obesity. Thorax 2017;72:A115. [Google Scholar]
- 82.Basunia M, Fahmy SA, Schmidt F, et al. Relationship of symptoms with sleep-stage abnormalities in obstructive sleep apnea-hypopnea syndrome. J Community Hosp Intern Med Perspect 2016;6:32170. 10.3402/jchimp.v6.32170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McArdle N, Douglas NJ. Effect of continuous positive airway pressure on sleep architecture in the sleep apnea-hypopnea syndrome: a randomized controlled trial. Am J Respir Crit Care Med 2001;164:1459-63. 10.1164/ajrccm.164.8.2008146 [DOI] [PubMed] [Google Scholar]
- 84.Malhotra A, Jordan A. The importance of arousal in obstructive sleep apnea-updates from the American Thoracic Society 2016. J Thorac Dis 2016;8:S542-4. 10.21037/jtd.2016.06.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bearpark H, Elliott L, Grunstein R, et al. Snoring and sleep apnea. A population study in Australian men. Am J Respir Crit Care Med 1995;151:1459-65. 10.1164/ajrccm.151.5.7735600 [DOI] [PubMed] [Google Scholar]
- 86.Maimon N, Hanly PJ. Does snoring intensity correlate with the severity of obstructive sleep apnea? J Clin Sleep Med 2010;6:475-8. [PMC free article] [PubMed] [Google Scholar]
- 87.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540-5. 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 88.Rosenthal LD, Dolan DC. The Epworth sleepiness scale in the identification of obstructive sleep apnea. J Nerv Ment Dis 2008;196:429-31. 10.1097/NMD.0b013e31816ff3bf [DOI] [PubMed] [Google Scholar]
- 89.Doneh B. Epworth Sleepiness Scale. Occup Med (Lond). 2015;65:508. 10.1093/occmed/kqv042 [DOI] [PubMed] [Google Scholar]
- 90.Bonzelaar LB, Salapatas AM, Yang J, et al. Validity of the epworth sleepiness scale as a screening tool for obstructive sleep apnea. Laryngoscope 2017;127:525-31. 10.1002/lary.26206 [DOI] [PubMed] [Google Scholar]
- 91.Karim A, Arora VK, Gupta MB. Emerging applications: Screening OSA by Modified Pictorial Epworth Sleepiness Scale in Indian subjects. Indian J Tuberc 2015;62:222-5. 10.1016/j.ijtb.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 92.Boyes J, Drakatos P, Jarrold I, et al. The use of an online Epworth Sleepiness Scale to assess excessive daytime sleepiness. Sleep Breath 2017;21:333-40. 10.1007/s11325-016-1417-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johns M. Rethinking the assessment of sleepiness. Sleep Med Rev 1998;2:3-15. 10.1016/S1087-0792(98)90050-8 [DOI] [PubMed] [Google Scholar]
- 94.Crook S, Sievi NA, Bloch KE, et al. Minimum important difference of the Epworth Sleepiness Scale in obstructive sleep apnoea: estimation from three randomised controlled trials. Thorax 2019;74:390-6. [DOI] [PubMed] [Google Scholar]
- 95.Netzer NC, Stoohs RA, Netzer CM, et al. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999;131:485-91. 10.7326/0003-4819-131-7-199910050-00002 [DOI] [PubMed] [Google Scholar]
- 96.Thurtell MJ, Bruce BB, Rye DB, et al. The Berlin questionnaire screens for obstructive sleep apnea in idiopathic intracranial hypertension. J Neuroophthalmol 2011;31:316-9. 10.1097/WNO.0b013e31821a4d54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kiciński P, Przybylska-Kuc SM, Tatara K, et al. Reliability of the Epworth Sleepiness Scale and the Berlin Questionnaire for screening obstructive sleep apnea syndrome in the context of the examination of candidates for drivers. Med Pr 2016;67:721-8. 10.13075/mp.5893.00494 [DOI] [PubMed] [Google Scholar]
- 98.Chiu HY, Chen PY, Chuang LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med Rev 2017;36:57-70. 10.1016/j.smrv.2016.10.004 [DOI] [PubMed] [Google Scholar]
- 99.Hoddes E, Zarcone V, Smythe H, et al. Quantification of sleepiness: a new approach. Psychophysiology 1973;10:431-6. 10.1111/j.1469-8986.1973.tb00801.x [DOI] [PubMed] [Google Scholar]
- 100.Daabis R, Gharraf H. Predictors of anxiety and depression in patients with obstructive sleep apnea. Egypt J Chest Dis Tuberc 2012;61:171-7. 10.1016/j.ejcdt.2012.10.032 [DOI] [Google Scholar]
- 101.Maslow AH. A theory of human motivation. Psychol Rev 1943;50:370-96. 10.1037/h0054346 [DOI] [Google Scholar]
- 102.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. 10.1111/j.1600-0447.1983.tb09716.x [DOI] [PubMed] [Google Scholar]
- 103.Schröder CM, O'Hara R. Depression and Obstructive Sleep Apnea (OSA). Ann Gen Psychiatry 2005;4:13. 10.1186/1744-859X-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Viswanath A, Ramamurthy J, Dinesh SP, et al. Obstructive sleep apnea: awakening the hidden truth. Niger J Clin Pract 2015;18:1-7. [DOI] [PubMed] [Google Scholar]
- 105.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep 2009;32:915-9. 10.1093/sleep/32.7.915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weaver TE. Outcome measurement in sleep medicine practice and research. Part 1: assessment of symptoms, subjective and objective daytime sleepiness, health-related quality of life and functional status. Sleep Med Rev 2001;5:103-28. 10.1053/smrv.2001.0152 [DOI] [PubMed] [Google Scholar]
- 107.Gülbay BE, Acican T, Onen ZP, et al. Health-related quality of life in patients with sleep-related breathing disorders: relationship with nocturnal parameters, daytime symptoms and comorbid diseases. Respiration 2008;75:393-401. 10.1159/000104865 [DOI] [PubMed] [Google Scholar]
- 108.EQ5D. Available online: https://euroqol.org/
- 109.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011;20:1727-36. 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Position statement on use of the EQ-5D-5L valuation set for England (updated November 2018). Available online: https://www.nice.org.uk/about/what-we-do/our-programmes/nice-guidance/technology-appraisal-guidance/eq-5d-5l
- 111.Abma IL, van der Wees PJ, Veer V, et al. Measurement properties of patient-reported outcome measures (PROMs) in adults with obstructive sleep apnea (OSA): A systematic review. Sleep Med Rev 2016;28:18-31. 10.1016/j.smrv.2015.07.006 [DOI] [PubMed] [Google Scholar]
- 112.Human Factors Engineering (HFE) and Usability Testing for Medical Devices Available online: https://www.emergobyul.com/services/united-states/human-factors-engineering-hfe-and-usability-testing-medical-devices.