Abstract
Objective
The aim of this study was to examine long-term trends in the receipt of medicines information (MI) among adult medicine users from 1999 to 2014.
Design
Repeated cross-sectional postal survey from the years 1999, 2002, 2005 and 2008–2014.
Setting
Each study year, a new nationally representative sample of 5000 Finns aged 15–64 years was drawn from the Population Register Centre of Finland.
Participants
The range of annual respondents varied from 2545 to 3371 and response rates from 53% to 67%. Of the total responses (n=29 465), 64% were from medicine users (n=18 862, ranging by year from 58% to 68%).
Outcome measures
Receipt of information on medicines in use within 12 months prior to the survey from a given list of consumer MI sources available in Finland.
Results
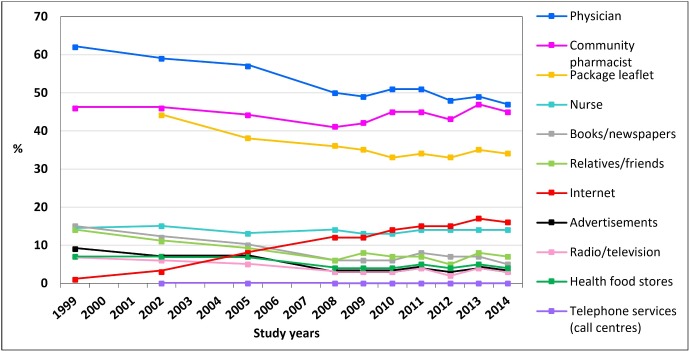
Physicians, community pharmacists and package leaflets were the most common MI sources throughout the study period. Receipt of MI increased most from the Internet (from 1% in 1999 to 16% in 2014), while decreased most from physicians (62% to 47%) and package leaflets (44% to 34%), and remained stable from community pharmacists (46% to 45%) and nurses (14% to 14%). In 1999, of the medicine users 4% did not report receipt of MI from any of the sources listed in the survey, while this proportion had remarkably increased to 28% in 2014.
Conclusions
Healthcare professionals and package leaflets had still a dominating importance in 2014 despite the growing number of MI sources over time, but still a minority of adult medicine users reported receiving MI via the Internet in 2014. Worrying is that the proportion of adult medicine users who did not receive MI from any of the sources became seven fold during the study period.
Keywords: medicines information, medicines information sources, population study, repeated cross-sectional survey, finland
Strengths and limitations of this study.
The key strength of this national population study is that it examines trends in the receipt of medicines information (MI) among adult medicine users within a 15-year time period by using representative random population samples with high enough response rates for generalisable results.
Repeated surveys are necessary to indicate population level changes in the utilisation of available MI sources and reveal needs to develop MI practices and policies at the national level.
Due to the cross-sectional method without cohorts, it is not possible to follow-up changes in the receipt of MI over time at the individual level.
The data did not provide any information about the quality, validity or amount of the MI received.
Factors contributing to a growing number of adult medicine users not receiving MI from any sources should be better understood and focused on in future research.
Introduction
Consumer access to medicines information (MI) has dramatically improved during the last decades.1–7 Driving forces for more open access to MI have been drug safety issues, patients’ right to know about medicinal interventions that they are exposed to and tendency to empower people in taking more responsibility for self-management of their diseases.2 4 7 These changes have led to improved availability of MI, first on paper and later via the Internet and electronic applications in smartphones and other electronic devices. The applications are evolving fast towards systems enabling customised MI, interactive communications and following up treatments.1 3 4 8–10 Improved communication on medications has also been a strategic priority in national and international medicines policies, for example, within the European Union.2 4 11–16
Consumers’ health information seeking, including MI seeking from various information sources, has been widely researched.17–20 Previous research on the receipt of MI among the adult population have either (1) focused on particular patient groups such as asthmatics,21 people with cancer,22 cardiovascular diseases,23 HIV/AIDS,24 mental disorders25 or vasculitis26 and pregnant women,27 28 or (2) focused on certain medicine user groups such as users of hormone replacement therapies,29–31 analgesics,32 33 antidepressants,34 antihypertensives,35 cardiovascular medicines36 or psychotropics.37 Previous studies have mostly applied single cross-sectional study designs.38–46 We found only one study that compared results from 2 years, covering a 7-year period.47 The consistent findings from the previous studies are that physicians, pharmacists and package leaflets are the most common sources of MI regardless of the research method, the study year, the country and the research population.21–47 Consumers usually sought MI from only one or two information sources.28 34 39 43 44 The use of the Internet as a source of MI has become more common over time, but it is not yet as commonly used source of MI for consumers as healthcare professionals.22 24–26 30 31 33–35 38–40 42 44–46 However, there is a lack of long-term population-based studies describing trends in the receipt of MI among adult medicine users. Repeated surveys are necessary to indicate population level changes in the utilisation of available MI sources and to reveal needs to develop MI practices and policies at the national level. In Finland, improving the accessibility and quality of MI have been among the key strategic medicines policy goals over the last decades.2 15 The long-term comparative information in the receipt of MI and the proportion of people receiving MI are important measures to indicate whether the desired outcomes are met. Therefore, this study examined long-term trends in the receipt of MI among Finnish adult medicine users in 1999–2014.
Methods
Context
Similar to many developed countries, availability of consumer MI has dramatically improved in Finland during the last decades.2 4 7 Until 1983, patients and medicine users received information about their medicines exclusively from their physicians.7 48 The remarkable landmarks towards more open access to MI have been pharmacists’ duty to counsel on prescription and non-prescription medicines in 1983, followed in 1986 by the launch of the first computerised database providing leaflets for consumers in community pharmacies. Package leaflets became mandatory across the European Union in 1999.49 About the same time, the Internet and mobile phones became more common and eventually revolutionised access to health and MI. ‘From paper the cyber’ shift has improved access to statutory MI, for example, by making package leaflets available online in written and audio format.50 51 A wide range of stakeholders from the drug industry to non-profit professional and patient organisations have been developing new databases and modes for communicating on medicines to consumers. To coordinate MI practices and enhance public–private partnerships, the European Union has recommended its member states to establish national MI programmes and strategies.13 Such a strategy was established for the first time in Finland in 2012 by the Finnish Medicines Agency.2 The ultimate goal of the strategy is to improve adherence to long-term therapies by enhanced MI by 2020.
Study design
The study was conducted as a repeated cross-sectional postal survey using each year a new nationally representative sample (n=5000) of the Finnish adult population aged 15–64 years.52 The national health behaviour survey used in this study has its origins in the North Karelia Project, started in 1972, which has been instrumental in improving public health in Finland.53 The annual ‘Health Behaviour and Health among the Finnish Adult Population’ survey was established in 1978 to perform as an indicator for changes in the population health and related risk factors, such as smoking, food and alcohol consumption and physical activity.52 The survey has been targeted to the adult working age population of 15–64 years old. The survey has been repeated every year in the same way to yield comparable results. In addition to the original standard set of structured questions, some other questions have been added to the survey instrument over the years. One of the added questions was the one used in our study concerning receipt of MI from different sources available for consumers/medicine users in Finland (added to the survey instrument in 1999).
The sample has been derived from the Population Register Centre of Finland which is a government-based register where all Finnish citizens and permanent residents are obliged to be registered.52 54 The survey has been conducted every year (1978–2014) as a postal survey.52 The distribution of the questionnaires by mail has assured better coverage of the entire study population than for example, using online surveys. This is because the Population Register Centre has the current address available for all Finns and permanent residents. In order to maintain response rate high enough for generalisable results, three reminders were sent during the study period covered in our study (1999–2014).55 Data from the years 1999, 2002, 2005 and 2008–2014 were compared as these are the years when the survey instrument included the question on the receipt of MI.
Survey instrument and measures
The main outcome measure was the receipt of MI on medicines in use. The survey instrument contained the question ‘In the past year (12 months), from which sources did you receive information on the medicines you have been using?’ The question was followed by a list of MI sources available for consumers in Finland at the time of the study (figure 1). Respondents could indicate from the list as many information sources as applicable. It was not possible to report other sources than those mentioned in the survey. In 2002, package leaflets and telephone services were added to the list of MI sources.
Figure 1.
Trends in the receipt of medicines information among adult medicine users (n=18 862) in 1999–2014 (% of the respondents who reported use of at least one prescription or non-prescription medicine within 7 days prior to the survey). The survey was not conducted in 2000, 2001, 2003, 2004, 2006 and 2007.
Sociodemographic variables used in this study were gender, age and education. Health-related variables were respondents’ medicine use and diagnosed diseases. Use of medicines was assessed by the question ‘Have you used any tablets, powders or other medicines within the past week (7 days)?’ This question was followed by a list of commonly used prescription and non-prescription medicines for common chronic and acute conditions (table 1). Respondents could indicate from the list as many medicines as they had been using within 7 days prior to the survey. It was not possible to report any other medicines other than those mentioned in the list. The use of medication within the past 7 days was used as a measure in order to control recall bias.56 Diagnosed diseases were asked by a question ‘Within the past year (12 months), have you had any of the following diagnosed diseases or diseases treated by the physicians?’ This question was followed by a list of chronic and acute diseases common in Finland (table 1). Respondents could indicate from the list as many diseases as they had been suffering from within the year prior to the survey. It was not possible to report any other diseases than those mentioned in the list.
Table 1.
Characteristics of the respondents (n=29 465) according to study year. The percentages are calculated from the total number of the respondents each year or of the respondents reporting use of at least one prescription or non-prescription medicine within 7 days prior to survey (n=18 862)*
| Characteristics | Study years | ||||||||||
| Total† n (%) |
1999 n (%) |
2002 n (%) |
2005 n (%) |
2008 n (%) |
2009 n (%) |
2010 n (%) |
2011 n (%) |
2012 n (%) |
2013 n (%) |
2014 n (%) |
|
| Number of respondents (response rate) | 29 465 (59) | 3371 (67) | 3259 (65) | 3287 (66) | 3216 (64) | 2943 (59) | 2826 (57) | 2787 (56) | 2601 (52) | 2545 (51) | 2630 (53) |
| Respondents using medicines‡ | 18 862 (64) | 1944 (58) | 2000 (61) | 2038 (62) | 2101 (65) | 1957 (66) | 1871 (66) | 1844 (66) | 1759 (68) | 1677 (66) | 1671 (64) |
| Gender§ | |||||||||||
| Female | 11 859 (63) | 1217 (63) | 1278 (64) | 1258 (62) | 1332 (63) | 1235 (63) | 1181 (63) | 1128 (61) | 1101 (63) | 1061 (63) | 1068 (64) |
| Male | 7003 (37) | 727 (37) | 722 (36) | 780 (38) | 769 (37) | 722 (37) | 690 (37) | 716 (39) | 658 (37) | 616 (37) | 603 (36) |
| Age (years)§ | |||||||||||
| 15–24 | 2535 (13) | 314 (16) | 308 (15) | 269 (13) | 270 (13) | 263 (13) | 255 (14) | 231 (13) | 211 (12) | 199 (12) | 215 (13) |
| 25–34 | 2798 (15) | 331 (17) | 308 (15) | 302 (15) | 305 (15) | 287 (15) | 258 (14) | 277 (15) | 251 (14) | 240 (14) | 239 (14) |
| 35–44 | 3409 (18) | 419 (22) | 411 (21) | 394 (19) | 376 (18) | 335 (17) | 304 (16) | 318 (17) | 292 (17) | 275 (16) | 285 (17) |
| 45–54 | 4558 (24) | 481 (25) | 486 (24) | 491 (24) | 490 (23) | 460 (24) | 484 (26) | 438 (24) | 430 (24) | 411 (25) | 387 (23) |
| 55–64 | 5562 (29) | 399 (21) | 487 (24) | 582 (29) | 660 (31) | 612 (31) | 570 (31) | 580 (32) | 575 (32) | 552 (33) | 545 (33) |
| Mean age (SD) | 43.8 (14.2) | 41.2 (13.9) | 42.1 (14.0) | 43.6 (14.0) | 44.3 (14.1) | 44.2 (14.3) | 44.3 (14.3) | 44.4 (14.1) | 44.9 (14.1) | 45.0 (14.1) | 44.6 (14.3) |
| Education§ | |||||||||||
| Primary school or lower (≤9 years) | 3048 (16) | 499 (26) | 444 (23) | 402 (20) | 351 (17) | 306 (16) | 253 (14) | 226 (12) | 222 (13) | 170 (10) | 175 (11) |
| Higher than primary school (>9 years) | 15 495 (84) | 1420 (74) | 1499 (77) | 1608 (80) | 1705 (83) | 1621 (84) | 1590 (86) | 1591 (88) | 1517 (87) | 1482 (90) | 1462 (89) |
| Respondents using medicines for§ | |||||||||||
| Headache | 9806 (52) | 1039 (53) | 1032 (52) | 1026 (50) | 1073 (51) | 1024 (52) | 980 (52) | 981 (53) | 901 (51) | 866 (52) | 884 (53) |
| Ache, pain (other than headache) | 5467 (29) | 556 (29) | 549 (28) | 592 (29) | 604 (29) | 541 (28) | 531 (29) | 550 (30) | 544 (31) | 508 (30) | 492 (29) |
| High blood pressure | 4077 (22) | 291 (15) | 367 (18) | 429 (21) | 465 (22) | 450 (23) | 434 (23) | 446 (24) | 409 (23) | 395 (24) | 391 (23) |
| Contraception (oral) | 2310 (12) | 264 (14) | 296 (15) | 247 (12) | 258 (12) | 238 (12) | 223 (12) | 193 (11) | 188 (11) | 199 (12) | 204 (12) |
| High blood cholesterol | 2196 (12) | 98 (5) | 143 (7) | 217 (11) | 263 (13) | 296 (15) | 290 (16) | 235 (13) | 217 (12) | 211 (13) | 226 (14) |
| Women’s hormone replacement therapy | 2036 (11) | 233 (12) | 266 (13) | 233 (11) | 222 (11) | 188 (10) | 188 (10) | 166 (9) | 198 (11) | 174 (10) | 168 (10) |
| Cough | 1316 (7) | 220 (11) | 186 (9) | 168 (8) | 160 (8) | 113 (6) | 77 (4) | 111 (6) | 101 (6) | 103 (6) | 77 (5) |
| Insomnia | 1497 (8) | 136 (7) | 153 (8) | 153 (8) | 179 (9) | 177 (9) | 140 (8) | 139 (8) | 134 (8) | 146 (9) | 140 (8) |
| Sedation | 915 (5) | 133 (7) | 93 (5) | 121 (6) | 102 (5) | 94 (5) | 70 (4) | 85 (5) | 76 (4) | 69 (4) | 72 (4) |
| Men’s sexual potency dysfunction | 252 (1) | 13 (1) | 20 (1) | 28 (1) | 26 (1) | 21 (1) | 26 (1) | 37 (2) | 34 (2) | 19 (1) | 28 (2) |
| Depression¶ | 1314 (7) | – | 111 (6) | 143 (7) | 148 (7) | 176 (9) | 136 (5) | 147 (8) | 173 (10) | 134 (8) | 146 (9) |
| Diabetes (other than insulin)** | 705 (4) | – | – | 72 (4) | 82 (4) | 88 (4) | 88 (5) | 80 (4) | 98 (6) | 91 (5) | 106 (6) |
| Diabetes (insulin)** | 361 (2) | – | – | 46 (2) | 49 (2) | 46 (2) | 47 (3) | 51 (3) | 36 (2) | 50 (3) | 36 (2) |
| Number of medicines in use/person§ | |||||||||||
| 1 | 10 558 (56) | 1215 (63) | 1183 (59) | 1143 (56) | 1154 (55) | 1060 (54) | 1029 (55) | 1011 (55) | 946 (54) | 907 (54) | 910 (54) |
| 2 | 5116 (27) | 505 (26) | 559 (28) | 566 (28) | 584 (28) | 536 (27) | 512 (27) | 491 (27) | 492 (28) | 442 (26) | 429 (26) |
| 3 | 1984 (11) | 161 (8) | 166 (8) | 198 (10) | 221 (11) | 220 (11) | 210 (11) | 210 (11) | 191 (11) | 208 (12) | 199 (12) |
| >3 | 1204 (6) | 63 (3) | 92 (5) | 131 (6) | 142 (7) | 141 (7) | 120 (6) | 132 (7) | 130 (7) | 120 (7) | 133 (8) |
| Respondents with diagnosed diseases§,†† | |||||||||||
| High blood pressure, hypertension | 4297 (23) | 345 (18) | 426 (21) | 465 (23) | 496 (24) | 478 (24) | 443 (24) | 468 (25) | 421 (24) | 379 (22) | 376 (23) |
| Degenerative disc disease, other back illness | 2712 (14) | 262 (14) | 284 (14) | 314 (15) | 321 (15) | 263 (13) | 260 (14) | 272 (15) | 260 (15) | 227 (14) | 249 (15) |
| Asthma | 1163 (6) | 113 (6) | 114 (6) | 112 (6) | 120 (6) | 132 (7) | 125 (7) | 113 (6) | 120 (7) | 99 (6) | 115 (7) |
| Digestive illness (gastric catarrh, gastritis, ulcer) | 904 (5) | 96 (5) | 97 (5) | 108 (5) | 110 (5) | 93 (5) | 91 (5) | 70 (4) | 85 (5) | 89 (5) | 65 (4) |
| Coronary disease, angina pectoris (=chest pain during exercise) |
414 (2) | 66 (3) | 48 (2) | 52 (3) | 47 (2) | 35 (2) | 41 (2) | 34 (2) | 31 (2) | 26 (2) | 30 (2) |
| Diabetes | 1092 (6) | 50 (2) | 78 (4) | 113 (6) | 121 (6) | 129 (7) | 121 (7) | 110 (6) | 124 (7) | 108 (6) | 138 (8) |
| Rheumatoid arthritis | 396 (2) | 46 (2) | 36 (2) | 37 (2) | 46 (2) | 37 (2) | 40 (2) | 41 (2) | 44 (3) | 37 (2) | 32 (2) |
| Chronic bronchitis, pulmonary emphysema | 322 (2) | 34 (2) | 30 (2) | 35 (2) | 38 (2) | 52 (3) | 28 (2) | 36 (2) | 20 (1) | 22 (1) | 27 (2) |
| Coronary thrombosis, myocardial infarction | 118 (1) | 9 (1) | 13 (1) | 13 (1) | 13 (1) | 8 (<1) | 11 (1) | 17 (1) | 10 (1) | 9 (1) | 15 (1) |
| High blood cholesterol¶ | 2913 (17) | – | 267 (13) | 338 (17) | 356 (17) | 409 (21) | 368 (20) | 319 (17) | 282 (16) | 287 (16) | 287 (17) |
| Depression** | 1242 (8) | – | – | 173 (9) | 175 (8) | 178 (9) | 134 (7) | 150 (8) | 161 (9) | 124 (7) | 147 (9) |
| Other mental health disorder** | 534 (4) | – | – | 64 (3) | 73 (4) | 66 (3) | 47 (3) | 82 (4) | 65 (4) | 73 (4) | 64 (4) |
| Cancer** | 206 (1) | – | – | 29 (1) | 26 (1) | 26 (1) | 28 (2) | 23 (1) | 34 (2) | 23 (1) | 17 (1) |
| Hay or allergic rhinitis‡‡ | 2044 (16) | – | – | – | 340 (16) | 292 (15) | 275 (15) | 272 (15) | 283 (16) | 275 (16) | 307 (18) |
| Food allergy‡‡ | 580 (5) | – | – | – | 85 (4) | 84 (4) | 92 (4) | 78 (4) | 85 (5) | 72 (4) | 84 (5) |
| Number of diagnosed diseases/person§,†† | |||||||||||
| 0 | 8590 (46) | 1228 (63) | 1121 (56) | 971 (48) | 850 (40) | 772 (39) | 796 (43) | 746 (40) | 705 (42) | 721 (42) | 689 (41) |
| 1 | 5176 (27) | 502 (26) | 527 (26) | 593 (29) | 605 (29) | 546 (28) | 482 (26) | 517 (28) | 499 (28) | 455 (27) | 450 (27) |
| 2 | 2924 (16) | 151 (8) | 232 (12) | 270 (13) | 367 (17) | 358 (22) | 335 (18) | 345 (19) | 302 (17) | 279 (17) | 285 (17) |
| >2 | 2172 (12) | 63 (3) | 120 (6) | 204 (10) | 279 (13) | 281 (14) | 258 (14) | 236 (13) | 253 (14) | 231 (14) | 247 (15) |
*Discrepancies in totals are due to rounding errors.
†Calculated from study years available.
‡Percentages have been calculated from the respondents of each year.
§Percentages have been calculated from the respondents of each year who reported using medicines, including prescription and non-prescription medicines (list in the survey) during the last week (7 days).
¶Added to the survey instrument in 2002.
**Added to the survey instrument in 2005.
††Respondents who had a disease (list in the survey) diagnosed by a physician during the last year (12 months).
‡‡Added to the survey instrument in 2008.
Analysis
Statistical analyses were conducted with the Statistical Package for Social Sciences software (IBM SPSS Statistics for Windows V.24.0). Only respondents who reported using at least one prescription or non-prescription medicine during the 7 days’ time frame prior to the survey were included in the analysis as medicine users.
Age calculation was based on the year of birth, and the respondents’ age was divided into five groups. Education was measured as the total number of self-reported school years and were divided into two educational levels. The number of medicines in use was counted for each respondent, and respondents were divided into following groups: people using one, two, three and four or more medicines. Also, the number of diagnosed diseases was counted for each respondent, and respondents were divided into following groups: no diseases, one, two, three or more diseases. The receipt of MI was presented by all these medicine user groups and diagnosed diseases. The number of MI sources from which the respondents had received information on the medicines they used was divided into following groups: no sources, one, two, three, four, five and six or more sources.
Trends in the receipt of MI from different information sources and the number of MI sources used by the respondent were counted for each study year 1999, 2002, 2005 and 2008–2014. The significance of the change in the receipt of MI between the study years was analysed with logistic regression. Analyses were adjusted for potential confounding factors (ie, age, gender, educational, number of medicines in use and number of diagnosed diseases). The receipt of MI from different sources was calculated by gender, age, number of medicines in use and number of diagnosed diseases for each study year.
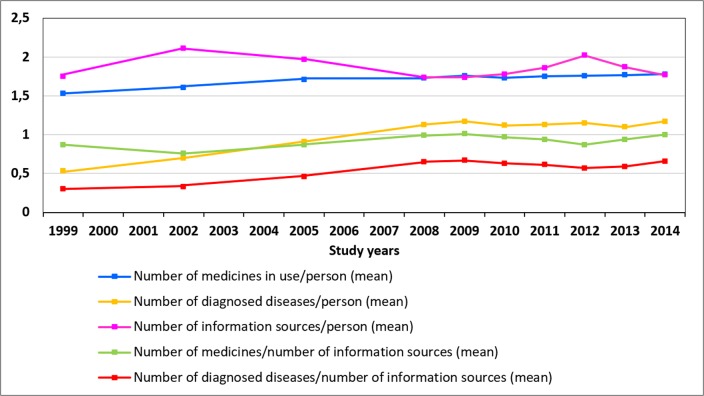
Finally, a ratio between the mean number of medicines in use and the mean number of diagnosed diseases compared with the mean number of MI sources from which MI was received was calculated to indicate whether any remarkable changes were seen over time in the number of MI sources used in relation to morbidity and medicine use.
Patient and public involvement
Patient perspective was taken into account in designing the research question on MI by reviewing previous international and national research on the topic.6 57–59 The question as it appears in the survey instrument is a result of extensive work by senior researchers in public health and medicines information. The question was piloted in several formats with the target group (5–10 individuals from the target group recruited as a convenience sample) and the current version was found to be most valid for the primary purpose of the survey that was to indicate long-term trends. The results of the study have not been sent to the study participants for comments, but the annual reports of the ‘Health Behaviour and Health among the Finnish Adult Population’ surveys are available online.52 As this study was a secondary analysis using routinely collected and fully anonymized data, ethics approval was not applicable.60
Results
The number of respondents varied by year from 2545 to 3371, and the response rate decreased from 67% in 1999 to 53% in 2014 (table 1). Of the total responses (n=29 465), 64% were from medicine users (n=18 862, ranging by year from 58% to 68%). The gender distribution of the respondents who reported using medicines remained the same throughout the study period, 61%–64% being female (table 1). The annual mean age varied between 41 and 45 years. The respondents used most commonly one medicine, ranging from 63% in 1999 to 54% in 2014 (included prescription and non-prescription medicines). The respondents reported using medicines most commonly for headaches (range 50%–53%), other aches or pains (28%–31%) and high blood pressure (15%–23%). More than a third of the medicine users reported having at least one diagnosed disease of the diseases listed in the survey, increasing from 37% in 1999 to 59% in 2014. The most common diseases reported were high blood pressure or hypertension (range 18%–25%), high blood cholesterol (13%–21%), hay or allergic rhinitis (15%–18%) and degenerative disk disease or other back illness (13%–15%).
MI sources among adult medicine users
The most commonly reported MI sources were physicians, community pharmacists and package leaflets throughout the study period among adult medicine users (figure 1 and table 2). These information sources were most common despite gender, age, number of medicines in use or diagnosed diseases (online supplementary appendices A and B). Receipt of MI from physicians (62% to 47%) and package leaflets (44% to 34%) decreased most during the study period, while remained stable from community pharmacists (46% to 45%) and nurses (14% to 14%) (figure 1). In 1999, of the medicine users 17% (n=335/1944) did not report any healthcare professionals (physicians, community pharmacists or nurses) as their source of MI, and by 2014 the proportion had grown to 38% (n=639/1671). The use of the Internet as MI source increased rather steadily being 1% in 1999 and 16% in 2014.
Table 2.
Trends in the receipt of medicines information among adult medicine users. Results of age, gender, educational, number of medicines in use and number of diagnosed diseases adjusted logistic regression
| Characteristics | Study years* | ||||||||||
| 1999 n (%) |
2002 n (%) |
2005 n (%) |
2008 n (%) |
2009 n (%) |
2010 n (%) |
2011 n (%) |
2012 n (%) |
2013 n (%) |
2014 n (%) |
P value† | |
| Respondents using medicines | 1944 | 2000 | 2038 | 2101 | 1957 | 1871 | 1844 | 1759 | 1677 | 1671 | |
| Physician | 1206 (62) | 1186 (59) | 1153 (57) | 1050 (50) | 950 (49) | 953 (51) | 933 (51) | 839 (48) | 816 (49) | 785 (47) | |
| OR (95% CI) | 1 | 0.80 (0.70 to 0.91) | 0.63 (0.55 to 0.72) | 0.43 (0.37 to 0.49) | 0.39 (0.34 to 0.45) | 0.45 (0.39 to 0.52) | 0.43 (0.37 to 0.49) | 0.37 (0.32 to 0.43) | 0.40 (0.34 to 0.46) | 0.36 (0.31 to 0.41) | <0.0001 |
| Community pharmacist | 892 (46) | 912 (46) | 901 (44) | 861 (41) | 812 (42) | 842 (45) | 825 (45) | 760 (43) | 793 (47) | 759 (46) | |
| OR (95% CI) | 1 | 0.92 (0.81 to 1.04) | 0.82 (0.72 to 0.93) | 0.68 (0.59 to 0.77) | 0.67 (0.59 to 0.77) | 0.79 (0.70 to 0.91) | 0.78 (0.68 to 0.89) | 0.72 (0.63 to 0.82) | 0.86 (0.75 to 0.99) | 0.78 (0.68 to 0.90) | <0.0001 |
| Package leaflet‡ | – | 876 (44) | 782 (38) | 745 (36) | 685 (35) | 620 (33) | 618 (34) | 572 (33) | 580 (35) | 566 (34) | |
| OR (95% CI) | – | 1 | 0.76 (0.66 to 0.86) | 0.65 (0.57 to 0.74) | 0.62 (0.54 to 0.71) | 0.57 (0.50 to 0.65) | 0.58 (0.51 to 0.67) | 0.55 (0.47 to 0.63) | 0.61 (0.53 to 0.70) | 0.58 (0.50 to 0.67) | <0.0001 |
| Nurse | 274 (14) | 307 (15) | 254 (13) | 297 (14) | 257 (13) | 243 (13) | 258 (14) | 247 (14) | 232 (14) | 228 (14) | |
| OR (95% CI) | 1 | 1.06 (0.89 to 1.28) | 0.78 (0.65 to 0.94) | 0.84 (0.70 to 1.01) | 0.75 (0.62 to 0.91) | 0.76 (0.62 to 0.92) | 0.84 (0.69 to 1.02) | 0.85 (0.70 to 1.03) | 0.83 (0.68 to 1.01) | 0.78 (0.64 to 0.96) | 0.003 |
| Books/newspapers | 292 (15) | 247 (12) | 203 (10) | 125 (6) | 112 (6) | 104 (6) | 137 (7) | 94 (5) | 111 (7) | 91 (5) | |
| OR (95% CI) | 1 | 0.75 (0.62 to 0.90) | 0.56 (0.46 to 0.68) | 0.31 (0.25 to 0.39) | 0.29 (0.23 to 0.37) | 0.28 (0.22 to 0.36) | 0.39 (0.31 to 0.49) | 0.27 (0.21 to 0.35) | 0.34 (0.27 to 0.43) | 0.27 (0.21 to 0.35) | <0.0001 |
| Relatives/friends | 269 (14) | 213 (11) | 184 (9) | 127 (6) | 160 (8) | 130 (7) | 145 (8) | 120 (7) | 125 (8) | 112 (7) | |
| OR (95% CI) | 1 | 0.73 (0.60 to 0.89) | 0.61 (0.50 to 0.76) | 0.40 (0.32 to 0.50) | 0.54 (0.44 to 0.67) | 0.46 (0.36 to 0.58) | 0.51 (0.41 to 0.64) | 0.46 (0.37 to 0.58) | 0.51 (0.41 to 0.65) | 0.44 (0.34 to 0.56) | <0.0001 |
| Internet | 26 (1) | 68 (3) | 157 (8) | 243 (12) | 243 (12) | 261 (14) | 276 (15) | 267 (15) | 283 (17) | 262 (16) | |
| OR (95% CI) | 1 | 2.46 (1.56 to 3.90) | 5.57 (3.65 to 8.49) | 8.44 (5.59 to 12.8) | 8.84 (5.85 to 13.4) | 10.2 (6.79 to 15.5) | 11.0 (7.26 to 16.5) | 11.3 (7.52 to 17.1) | 12.9 (8.53 to 19.4) | 11.5 (7.60 to 17.3) | <0.0001 |
| Advertisements | 180 (9) | 135 (7) | 137)7) | 66 (3) | 57 (3) | 51 (3) | 69 (4) | 50 (3) | 60 (4) | 41 (3) | |
| OR (95% CI) | 1 | 0.71 (0.56 to 0.90) | 0.74 (0.58 to 0.93) | 0.33 (0.25 to 0.45) | 0.31 (0.23 to 0.42) | 0.29 (0.21 to 0.40) | 0.39 (0.29 to 0.52) | 0.30 (0.22 to 0.42) | 0.38 (0.28 to 0.51) | 0.25 (0.18 to 0.36) | <0.0001 |
| Radio/television | 130 (7) | 127 (6) | 101 (5) | 55 (3) | 50 (3) | 57 (3) | 81 (4) | 43 (2) | 60 (4) | 43 (3) | |
| OR (95% CI) | 1 | 0.90 (0.69 to 1.16) | 0.68 (0.51 to 0.89) | 0.34 (0.25 to 0.48) | 0.33 (0.24 to 0.47) | 0.40 (0.29 to 0.56) | 0.57 (0.43 to 0.77) | 0.32 (0.22 to 0.45) | 0.47 (0.34 to 0.65) | 0.33 (0.23 to 0.47) | <0.0001 |
| Health food stores | 141 (7) | 141 (7) | 136 (7) | 88 (4) | 79 (4) | 77 (4) | 90 (5) | 66 (4) | 77 (5) | 66 (4) | |
| OR (95% CI) | 1 | 0.94 (0.73 to 1.20) | 0.88 (0.68 to 1.13) | 0.52 (0.39 to 0.69) | 0.50 (0.37 to 0.66) | 0.51 (0.38 to 0.61) | 0.59 (0.51 to 0.79) | 0.46 (0.36 to 0.62) | 0.57 (0.42 to 0.76) | 0.48 (0.35 to 0.65) | <0.0001 |
| Telephone services‡ | – | 4 (<1) | 5 (<1) | 6 (<1) | 7 (<1) | 1 (<1) | 4 (<1) | 3 (<1) | 3 (<1) | 5 (<1) | |
| OR (95% CI) | – | 1 | 1.28 (0.34 to 4.79) | 1.22 (0.32 to 4.60) | 1.81 (0.52 to 6.30) | 0.28 (0.03 to 2.53) | 1.15 (0.28 to 4.69) | 0.91 (0.20 to 4.11) | 0.64 (0.12 to 3.52) | 1.52 (0.40 to 5.77) | 0.784 |
| MI received from at least one source§ | 1867 (96) | 1804 (90) | 1762 (87) | 1571 (75) | 1404 (72) | 1392 (74) | 1393 (76) | 1236 (70) | 1233 (74) | 1204 (72) | |
| OR (95% CI) | 1 | 0.33 (0.25 to 0.44) | 0.21 (0.16 to 0.28) | 0.09 (0.07 to 0.11) | 0.07 (0.06 to 0.09) | 0.09 (0.07 to 0.11) | 0.09 (0.07 to 0.12) | 0.07 (0.05 to 0.09) | 0.08 (0.06 to 0.10) | 0.07 (0.06 to 0.10) | <0.0001 |
*Calculated from study years available.
†P-value for the difference in the receipt of medicines information (MI) between the study years.
‡Added to the survey instrument in 2002.
§MI sources listed in the survey.
CI, 95% CI of logistic regression; OR, age, gender, educational, number of medicines in use and number of diagnosed diseases adjusted OR.
bmjopen-2018-026377supp001.pdf (375.7KB, pdf)
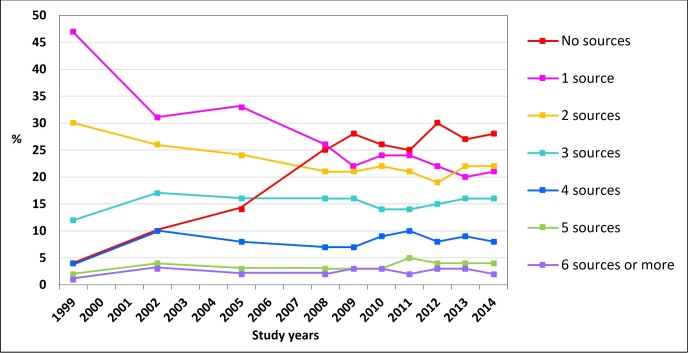
The number of MI sources from which medicine users reported receipt of MI changed over the study period 1999–2014 (figure 2). The most noticeable decreases occurred in those who reported receipt of MI from one (47% to 21%) or two (30% to 22%) sources. The number of medicine users receiving MI from more than two sources increased moderately. In 1999, of the medicine users, 4% (n=77/1944) did not report receipt of MI from any of the information sources listed in the survey, while this proportion had increased to 28% (n=467/1671) in 2014.
Figure 2.
Number of medicines information sources from which the adult medicine users (n=18 862) had received information on the medicines they used. The survey was not conducted in 2000, 2001, 2003, 2004, 2006 and 2007.
Receipt of MI among subgroups
Women reported receiving MI from all information sources listed in the survey more commonly than men during the study period (online supplementary appendix A). Receipt of MI from physicians decreased most among women (66% to 48%) and among medicine users aged over 45 years (75%–52%). Receipt of MI from package leaflets decreased both in women (48% to 38%) and men (36% to 26%), while remained nearly unchanged from community pharmacists (51% to 47% in women vs 37% to 42% in men). The receipt of MI from community pharmacists increased most among medicine users aged 55–64 years (34% to 46%), and decreased most among medicine users aged 33–44 years (55% to 43%). Package leaflets, relatives and friends were reported to be most common MI sources for medicine users under 25 years, although receipt of MI from package leaflets (59% to 37%) and from relatives and friends (35% to 16%) decreased most in this age group. Receipt of MI from the Internet increased in both genders, slightly more in women than in men (2% to 18% vs 1% to 12%, respectively), and in all age groups, most among medicine users aged 25–34 years (2% to 21%) and 15–24 years (2% to 20%). More male (6% to 33%) than female (3% to 25%) and more medicine users under 45 years (5% to 33%) than medicine users 45 years or older (3% to 25%) did not report receipt of MI from any of the information sources listed in the survey during the study period.
As the number of medicines in use or the number of diagnosed diseases increased, the number of different MI sources increased (online supplementary appendix B). However, the opposite changes occurred in the receipt of MI from physicians, the proportion of medicine users receiving MI from physicians decreased 14%–26% depending on the number of medicines in use or the number of diagnosed diseases, the highest decline occurring for medicine users with two medicines (76% to 52%) and for medicine users with two diagnosed diseases (83% to 57%). The number of medicines and the number of diagnosed diseases had the opposite influence on the receipt of MI from community pharmacists. The receipt of MI from community pharmacists increased most in medicine users with three medicines (47% to 59%) and in those with three or more diagnosed diseases (49% to 63%), whereas the receipt of MI decreased most in medicine users with one medicine (44% to 38%) and in those medicine users without any diagnosed diseases (45% to 34%). Receipt of MI package leaflets decreased mainly in all medicine users, most in those with one (40% to 28%) or two (48% to 35%) medicines in use and in medicine users without any diagnosed diseases (44% to 30%) or in those with one diagnosed disease (43% to 31%). Receipt of MI from the Internet increased in all medicine users regardless of the number of medicines in use or the number of diagnosed diseases, most among respondents with two (1% to 19%) and four or more medicines (5% to 22%), and respondents with three or more diagnosed diseases (3% to 23%). Respondents using one (6% to 35%) or two (1% to 24%) medicines and medicine users without any diagnosed disease listed in the survey (5% to 38%) or with one disease (2% to 25%) most commonly did not report receipt of MI from any of the information sources listed in the survey during the study period.
Overall, the mean number of medicines in use and the mean number of diagnosed diseases increased slightly among medicine users, while the mean number of MI sources from which MI was received remained relatively stable during the study period 1999–2014 (figure 3). The ratio between the mean number of medicines in use and the mean number of MI sources from which MI was received remained relatively stable, but the ratio between the mean number of diagnosed diseases and the mean number of MI sources increased.
Figure 3.
Ratio between mean number of medicines in use and mean number of diagnosed diseases compared with the mean number of medicines information sources from which the medicine users (n=18 862) received medicines information. The survey was not conducted in 2000, 2001, 2003, 2004, 2006 and 2007.
Discussion
To our knowledge, this is the first study analysing long-term national trends in the receipt of MI among adult population. The 15-year period covered in this study (1999–2014) provides unique insights into how improved consumer access to MI and the shift from paper to cyber has influenced receipt of MI from various sources. It seems that the key MI sources (physicians, community pharmacists and package leaflets) have remained similar which is in line with previous studies.21–47 Surprising was that even though the availability and the use of MI sources has diversified among the adult population, an increasing number of medicine users did not report receipt of MI from any of the sources.
The proportion of medicine users who did not report receiving MI from any of the listed sources became sevenfold during the study period (4% to 28%). Furthermore, the proportion of those who did not report receiving MI on medicines they used from any of the healthcare professionals more than doubled from 17% in 1999 to 38% in 2014. Particularly, MI received from the physicians declined over time. The decline was similar (22%–26%) in respondents using two or more medicines or having or not having diagnosed diseases. According to age, the decline was most evident among medicine users 45 years and older. These findings may indicate that physicians are becoming less involved in actual patient care as the healthcare has become more fragmented. Thus, time allocated for physician office visits has shortened, leading to a situation that physicians do not have time to concentrate on their patients’ medications.61–64 Consequently, those medicine users who were dependent on MI received from their physicians do not have that source available anymore. It also seems that community pharmacists have become more common sources of MI for people with multiple medications instead of physicians, but nurses have not replaced physicians as a MI source. In the future, special attention should be paid to the receipt of MI among people with multiple diseases and medications and the ageing populations whose proportion is growing.
Our findings indicate that MI is not evenly distributed among medicine users, it may have become more unevenly distributed over time. During the study period, women, people aged 45 years or older, people with three or more medicines in use and people with three or more diagnosed diseases received MI more commonly on their medicines than other adult medicine users. These findings are in line with previous cross-sectional studies.23 25 26 40 41 Other previous studies have shown that MI seeking behaviour and the use of MI sources is usually influenced by gender and age, but also education, ethnic background, income, employment, health status and medical history.27 34 39 Potential reasons and system-based root causes for differences in the receipt of MI among medicine users need to be addressed in future research. Our example from Finland demonstrates that availability of a wide range of MI sources does not necessarily guarantee their actual and evenly distributed use among medicine users.
This study indicates that the receipt of MI from the Internet was quite rare as more than 90% of the Finns aged 16–64 years were Internet users in 2014.65 There are no similar population-based long-term trend studies from other countries to compare our results. According to previous studies, use of the Internet as a source of MI has varied between 4% and 29% in the 2000s.22 25 31 33 34 39 40 42 44–46 It is also known that some patient and medicine user groups use the Internet considerably more (59%–68%) than the adult population in general, for example, patients with chronic conditions and pregnant women.27 28 41 Thus, if we want to reach the majority of the adult population, we could not solely count on the Internet-based MI sources and services. Further population-based research is needed to get a more comprehensive understanding of the importance and usage patterns of the Internet as a MI source, also the opportunities it provides for improving MI for various medication user segments.
Strengths and limitations of this study
As a repeated national population survey, this study allows for examination of trends over time at the population level. Although the response rate decreased from 67% to 53% during the study period, reflecting that the representativeness of the results to the entire population is getting weaker, it is still adequate for generalisable results.66 67 The non-respondents more often tended to be young men, unmarried or single and with a lower level of education.55 Due to the cross-sectional method without cohorts, it is not possible to follow-up changes in the receipt of MI over time at the individual level. The respondents did not have the opportunity to report MI from other sources than those listed in the survey, to report separately MI sources on prescription and non-prescription medicines and to distinguish between active MI seeking or passive receipt of MI. This should be taken account when interpreting results and potential implications. For example, the gender difference in the use of MI sources may differ depending on whether these are discrepancies in the information being provided or gender differences in information seeking-behaviours.23 25 26 33 40 41 45 Furthermore, people using prescription versus non-prescription medicines may differ in the amount and use of different MI sources. However, in Finland all medicine users should receive MI from their healthcare providers while prescribing and dispensing both prescription and non-prescription medicines.68 69 The data did not provide any information about the quality, validity or amount of the MI received.
Implications and future research
The strategic development of MI will continue both nationally and internationally to ensure the availability and access to reliable, up-to-date and high-quality MI and MI sources.2 4 13 70 As part of this work, it is necessary to continue research on trends in the receipt of MI at the population level and to identify population groups needing special attention, such as older adults. Consumers’ MI literacy should be further investigated and considered in the development of MI for different patient and medicine user groups, for example, by including the question related to MI literacy in population surveys. The present study provides a foundation for further analysis that could go deeper in understanding receipt of MI in various population groups, changes over time and factors influencing it. Further studies are needed also on factors contributing to a growing number of medicine users not receiving MI from any sources.
Conclusions
Healthcare professionals and package leaflets had still a dominating importance in 2014 despite the growing number of MI sources over time, but still a minority of adult medicine users reported receiving MI via the Internet in 2014. Worrying is that the proportion of adult medicine users who did not receive MI from any of the sources became sevenfold during the study period.
Supplementary Material
Acknowledgments
We thank the National Institute for Health and Welfare for providing their incidence data and participants of the national surveys. We gratefully acknowledge the Elli Turunen Fund of the Finnish Cultural Foundation for providing funding. We warmly thank Tero Vahlberg for statistical support and Richard Stevenson for proofreading the manuscript.
Footnotes
Contributors: SH has been involved in designing the survey. SH and MSAA have been involved in developing the survey instrument concerning the receipt of medicines information. NM, MSAA, KH-A and MP-M planned the analysis and reported this particular study. These data were applied from the National Institute for Health and Welfare. NM performed the data analysis, and MSAA, KH-A, SH and MP-M contributed in the interpretation of the data. NM prepared the initial draft of the manuscript. MSAA, KH-A, SH and MP-M critically reviewed and revised the manuscript. All authors read and gave the final approval of the version to be published.
Funding: This research was supported by the Elli Turunen Fund of the Finnish Cultural Foundation.
Competing interests: None declared.
Ethics approval: As this study was a secondary analysis using routinely collected and fully anonymised data, ethics approval was not applicable. Answering the survey was considered as giving informed consent. The study followed the national ethical guidelines for researchers. All study procedures were conducted in accordance with good research practice.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
Patient consent for publication: Not required.
References
- 1. Bailey SC, Belter LT, Pandit AU, et al. The availability, functionality, and quality of mobile applications supporting medication self-management. J Am Med Inform Assoc 2014;21:542–6. 10.1136/amiajnl-2013-002232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Finnish Medicines Agency Fimea. Rational use of medicines through information and guidance. Medicines information services: Current state and strategy for 2020. 2012. Serial Publication Fimea Develops, Assesses and Informs 1/2012.
- 3. Ho I, Nielsen L, Jacobsgaard H, et al. Chat-based telepharmacy in Denmark: design and early results. Int J Pharm Pract 2015;23:61–6. 10.1111/ijpp.12109 [DOI] [PubMed] [Google Scholar]
- 4. International Pharmaceutical Federation. Medicines information: Strategic development. The Hague: International Pharmaceutical Federation, 2017. https://fip.org/files/fip/publications/2017-01-Medicines-information-strategic-development.pdf (Accessed 21 Jun 2018). [Google Scholar]
- 5. Lee K, Hoti K, Hughes JD, et al. Dr Google and the consumer: a qualitative study exploring the navigational needs and online health information-seeking behaviors of consumers with chronic health conditions. J Med Internet Res 2014;16:e262 10.2196/jmir.3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mononen N, Järvinen R, Hämeen-Anttila K, et al. A national approach to medicines information research: A systematic review. Res Soc Adm Pharm 2018;14:1106–24. 10.1016/j.sapharm.2018.01.011 [DOI] [PubMed] [Google Scholar]
- 7. Pohjanoksa-Mäntylä M. Medicines information sources and services for consumers: A special focus on the Internet and people with depression [dissertation]. 2010. University of Helsinki http://urn.fi/URN:ISBN:978-952-10-6128-8.
- 8. Benetoli A, Chen TF, Spagnardi S, et al. Provision of a medicines information service to consumers on facebook: An australian case study. J Med Internet Res 2015;17:e265 10.2196/jmir.4161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eysenbach G, Powell J, Englesakis M, et al. Health related virtual communities and electronic support groups: systematic review of the effects of online peer to peer interactions. BMJ 2004;328:1166 10.1136/bmj.328.7449.1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanlon P, Daines L, Campbell C, et al. Telehealth interventions to support self-management of long-term conditions: A systematic metareview of diabetes, heart failure, asthma, chronic obstructive pulmonary disease, and cancer. J Med Internet Res 2017;19:e172 10.2196/jmir.6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Department of Health. Better information, better choices, better health: Putting information at the centre of health. 2004. http://www.formazione.eu.com/_documents/consenso/documenti/better%20information.pdf (Accessed 21 Jun 2018).
- 12. Department of Health. The power of information: Putting all of us in control of the health and care information we need. 2012. https://data.gov.uk/sites/default/files/DH%20Open%20Data%20Strategy_10.pdf (Accessed 21 Jun 2018).
- 13. European Commission. High Level Pharmaceutical Forum 2005-2008: Conclusions and recommendations. European Communities. 2008.
- 14. Ministry of Health and Social Affairs. The national pharmaceutical strategy 2016–2018: Government Offices of Sweden, 2015. https://lakemedelsverket.se/upload/om-lakemedelsverket/NLS/The-National-Pharmaceutical-Strategy-2016-2018.pdf (Accessed 21 Jun 2018). [Google Scholar]
- 15. Ministry of Social Affairs and Health. Medicines Policy 2020: Towards efficient, safe, rational and cost effective use of medicines: Ministry of Social Affairs and Health, 2011. http://urn.fi/URN:ISBN:978-952-00-3101-5. [Google Scholar]
- 16. Pharmaceutical Society of Ireland. Future pharmacy practice in Ireland: Meeting patients’ needs 2016. https://www.thepsi.ie/Libraries/Pharmacy_Practice/PSI_Future_Pharmacy_Practice_in_Ireland.sflb.ashx (Accessed 21 Jun 2018).
- 17. Finney Rutten LJ, Agunwamba AA, Wilson P, et al. Cancer-related information seeking among cancer survivors: Trends over a decade (2003-2013). J Cancer Educ 2016;31:348–57. 10.1007/s13187-015-0802-7 [DOI] [PubMed] [Google Scholar]
- 18. Kalantzi S, Kostagiolas P, Kechagias G, et al. Information seeking behavior of patients with diabetes mellitus: a cross-sectional study in an outpatient clinic of a university-affiliated hospital in Athens, Greece. BMC Res Notes 2015;8:48 10.1186/s13104-015-1005-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Somera LP, Lee HR, Badowski G, et al. Health information seeking, source trust, and culture: A comparative analysis of health information trends and needs between guam and the united states. J Health Commun 2016;21:469–78. 10.1080/10810730.2015.1095822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Volkman JE, Luger TM, Harvey KL, et al. The National Cancer Institute’s Health Information National Trends Survey [HINTS]: a national cross-sectional analysis of talking to your doctor and other healthcare providers for health information. BMC Fam Pract 2014;15:111 10.1186/1471-2296-15-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raynor DK, Savage I, Knapp P, et al. We are the experts: people with asthma talk about their medicine information needs. Patient Educ Couns 2004;53:167–74. 10.1016/S0738-3991(03)00126-5 [DOI] [PubMed] [Google Scholar]
- 22. Chen X, Siu LL. Impact of the media and the internet on oncology: survey of cancer patients and oncologists in Canada. J Clin Oncol 2001;19:4291–7. 10.1200/JCO.2001.19.23.4291 [DOI] [PubMed] [Google Scholar]
- 23. Bults M, Beaujean DJ, Wijkmans CJ, et al. Why did patients with cardiovascular disease in the Netherlands accept Q fever vaccination? Vaccine 2012;30:3369–75. 10.1016/j.vaccine.2012.03.056 [DOI] [PubMed] [Google Scholar]
- 24. Zukoski AP, Thorburn S, Stroud J. Seeking information about HIV/AIDS: a qualitative study of health literacy among people living with HIV/AIDS in a low prevalence context. AIDS Care 2011;23:1505–8. 10.1080/09540121.2011.582077 [DOI] [PubMed] [Google Scholar]
- 25. Pohjanoksa-Mäntylä M, Bell JS, Helakorpi S, et al. Is the Internet replacing health professionals? A population survey on sources of medicines information among people with mental disorders. Soc Psychiatry Psychiatr Epidemiol 2011;46:373–9. 10.1007/s00127-010-0201-7 [DOI] [PubMed] [Google Scholar]
- 26. Carpenter DM, DeVellis RF, Hogan SL, et al. Use and perceived credibility of medication information sources for patients with a rare illness: differences by gender. J Health Commun 2011;16:629–42. 10.1080/10810730.2011.551995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hämeen-Anttila K, Jyrkkä J, Enlund H, et al. Medicines information needs during pregnancy: a multinational comparison. BMJ Open 2013;3:e002594 10.1136/bmjopen-2013-002594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hämeen-Anttila K, Nordeng H, Kokki E, et al. Multiple information sources and consequences of conflicting information about medicine use during pregnancy: a multinational Internet-based survey. J Med Internet Res 2014;16:e60 10.2196/jmir.2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tiihonen MJ, Heikkinen AM, Ahonen RS. Do Finnish women using hormone replacement therapy need more information about risks. Pharm World Sci 2007;29:635–40. 10.1007/s11096-007-9115-1 [DOI] [PubMed] [Google Scholar]
- 30. Tiihonen M, Saarela M, Saarinen S, et al. Menopausal hormone therapy--benefits, adverse reactions, concerns and information sources in 2009. Maturitas 2011;70:69–73. 10.1016/j.maturitas.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 31. Wathen CN. Health information seeking in context: how women make decisions regarding hormone replacement therapy. J Health Commun 2006;11:477–93. 10.1080/10810730600751979 [DOI] [PubMed] [Google Scholar]
- 32. Ngo SN, Stupans I, Leong WS, et al. Appropriate use of non-prescription ibuprofen: a survey of patients' perceptions and understanding. Int J Pharm Pract 2010;18:63–5. [DOI] [PubMed] [Google Scholar]
- 33. Närhi U, Helakorpi S. Analgesic users' sources of medicine information. Int J Pharm Pract 2007;15:251–3. 10.1211/ijpp.15.3.0013 [DOI] [Google Scholar]
- 34. Sleath B, Wurst K, Lowery T. Drug information sources and antidepressant adherence. Community Ment Health J 2003;39:359–68. 10.1023/A:1024080410284 [DOI] [PubMed] [Google Scholar]
- 35. Baldwin AS, Cvengros JA, Christensen AJ, et al. Preferences for a patient-centered role orientation: association with patient-information-seeking behavior and clinical markers of health. Ann Behav Med 2008;35:80–6. 10.1007/s12160-007-9011-x [DOI] [PubMed] [Google Scholar]
- 36. Gordon K, Smith F, Dhillon S. Effective chronic disease management: patients' perspectives on medication-related problems. Patient Educ Couns 2007;65:407–15. 10.1016/j.pec.2006.09.012 [DOI] [PubMed] [Google Scholar]
- 37. Black E, Murphy AL, Gardner DM. Community pharmacist services for people with mental illnesses: preferences, satisfaction, and stigma. Psychiatr Serv 2009. 60:1123–7. 10.1176/ps.2009.60.8.1123 [DOI] [PubMed] [Google Scholar]
- 38. Carter SR, Moles R, White L, et al. Medication information seeking behavior of patients who use multiple medicines: how does it affect adherence? Patient Educ Couns 2013;92:74–80. 10.1016/j.pec.2013.01.019 [DOI] [PubMed] [Google Scholar]
- 39. DeLorme DE, Huh J, Reid LN. Source selection in prescription drug information seeking and influencing factors: applying the comprehensive model of information seeking in an American context. J Health Commun 2011;16:766–87. 10.1080/10810730.2011.561914 [DOI] [PubMed] [Google Scholar]
- 40. Ho CH, Ko Y, Tan ML. Patient needs and sources of drug information in Singapore: is the Internet replacing former sources? Ann Pharmacother 2009;43:732–9. 10.1345/aph.1L580 [DOI] [PubMed] [Google Scholar]
- 41. Hämeen-Anttila K, Pietilä K, Pylkkänen L, et al. Internet as a source of medicines information (MI) among frequent internet users. Res Soc Adm Pharm 2018;14:758–64. 10.1016/j.sapharm.2017.09.007 [DOI] [PubMed] [Google Scholar]
- 42. Krska J, Morecroft CW. Patients' use of information about medicine side effects in relation to experiences of suspected adverse drug reactions: a cross-sectional survey in medical in-patients. Drug Saf 2013;36:673–80. 10.1007/s40264-013-0065-3 [DOI] [PubMed] [Google Scholar]
- 43. Newby DA, Hill SR, Barker BJ, et al. Drug information for consumers: should it be disease or medication specific? Results of a community survey. Aust N Z J Public Health 2001;25:564–70. 10.1111/j.1467-842X.2001.tb00327.x [DOI] [PubMed] [Google Scholar]
- 44. Närhi U. Sources of medicine information and their reliability evaluated by medicine users. Pharm World Sci 2007;29:688–94. 10.1007/s11096-007-9131-1 [DOI] [PubMed] [Google Scholar]
- 45. Närhi U, Helakorpi S. Sources of medicine information in Finland. Health Policy 2007;84:51–7. 10.1016/j.healthpol.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 46. Tio J, LaCaze A, Cottrell WN. Ascertaining consumer perspectives of medication information sources using a modified repertory grid technique. Pharm World Sci 2007;29:73–80. 10.1007/s11096-006-9076-9 [DOI] [PubMed] [Google Scholar]
- 47. Tiihonen M, Heikkinen AM, Leppänen HM, et al. Information sources used by women in Finland who use hormonal contraceptives. Pharm World Sci 2010;32:66–72. 10.1007/s11096-009-9344-6 [DOI] [PubMed] [Google Scholar]
- 48. Puumalainen I. Development of instruments to measure the quality of patient counselling [dissertation]. University of Kuopio;2005 http://urn.fi/URN:ISBN:951-27-0053-0. [Google Scholar]
- 49. Council Directive 92/27/EEC of 31 March 1992 on the labelling of medicinal products for human use and on package leaflets. Council of the European Communities. 1992. http://data.europa.eu/eli/dir/1992/27/oj (Accessed 23 Aug 2018).
- 50. Finnish Medicine Agency Fimea. SmPCs and PLs www.fimea.fi/web/en/databases_and_registeries/spcs (Accessed 21 Jun 2018). [Google Scholar]
- 51. Pharmaceutical Information Centre. Package leaflets of medicinal products. Finnish https://laakeinfo.fi/Frontpage.aspx (Accessed 21 Jun 2018).
- 52. : Helldán A, Helakorpi S, Health behaviour and health among the Finnish adult population, Spring 2014: National Institute for Health and Welfare, 2015. http://urn.fi/URN:ISBN:978-952-302-447-2. [Google Scholar]
- 53. Puska P, Vartiainen E, Nissinen A, et al. Background, principles, implementation, and general experiences of the North Karelia Project. Glob Heart 2016;11:173–8. 10.1016/j.gheart.2016.04.010 [DOI] [PubMed] [Google Scholar]
- 54. Population Register Centre. https://vrk.fi/en/population-register-centre (Accessed 20 Dec 2018).
- 55. Tolonen H, Helakorpi S, Talala K, et al. 25-year Trends and Socio-demographic Differences in Response Rates: Finnish Adult Health Behaviour Survey. Eur J Epidemiol 2006;21:409–15. 10.1007/s10654-006-9019-8 [DOI] [PubMed] [Google Scholar]
- 56. Gama H, Correia S, Lunet N. Questionnaire design and the recall of pharmacological treatments: a systematic review. Pharmacoepidemiol Drug Saf 2009;18:175–87. 10.1002/pds.1703 [DOI] [PubMed] [Google Scholar]
- 57. Airaksinen M. Customer feedback as a tool for improving pharmacy services in Finland [dissertation]. University of Kuopio 1996. [Google Scholar]
- 58. Airaksinen M, Ahonen R, Enlund H. The “questions to ask about your medicines” campaign. An evaluation of pharmacists’ and the public’s response. Med Care 1998;36:422–7. [DOI] [PubMed] [Google Scholar]
- 59. Vainio K, Airaksinen M, Väisänen T, et al. Assessing the importance of community pharmacists as providers of drug information. J Appl Ther Res 2004;5:24–9. [Google Scholar]
- 60. Finnish National Advisory Board on Research Ethics. Ethical principles of research in the humanities and social and behavioural sciences and proposals for ethical review. 2009. www.tenk.fi/sites/tenk.fi/files/ethicalprinciples.pdf (Accessed 21 Jun 2018).
- 61. Guy Jr GP, Richardson LC. Visit duration for outpatient physician office visits among patients with cancer. J Oncol Pract 2012;8:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kallio S, Kumpusalo-Vauhkonen A, Järvensivu T, et al. Towards interprofessional networking in medication management of the aged: current challenges and potential solutions in Finland. Scand J Prim Health Care 2016;34:368–76. 10.1080/02813432.2016.1249055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kangaspunta V, Koskela T, Soini E, et al. Factors impacting the perceived usefulness of a patient’s health centre visit. Suom Laakaril 2014;69:1654–9. [Google Scholar]
- 64. Shaw MK, Davis SA, Fleischer AB, et al. The duration of office visits in the United States, 1993 to 2010. Am J Manag Care 2014;20:820–6. [PubMed] [Google Scholar]
- 65. Statistics Finland. One half of Finnish residents participate in social network services. https://www.stat.fi/til/sutivi/2014/sutivi_2014_2014-11-06_tie_001_en.html (Accessed 30 Nov 2018).
- 66. Baruch Y, Holtom BC. Survey response rate levels and trends in organizational research. Hum Relat 2008;61:1139–60. 10.1177/0018726708094863 [DOI] [Google Scholar]
- 67. Tolonen H, Helakorpi S, Talala K, et al. 25-year trends and socio-demographic differences in response rates: Finnish adult health behaviour survey. Eur J Epidemiol 2006;21:409–15. 10.1007/s10654-006-9019-8 [DOI] [PubMed] [Google Scholar]
- 68. Decree on the prescription of medicines 1088/2010. https://www.finlex.fi/fi/laki/alkup/2010/20101088 (Accessed 1 Dec 2018).
- 69. Medicines Act 395/1987. https://www.finlex.fi/fi/laki/ajantasa/1987/19870395 (Accessed 1 Dec 2018).
- 70. European Commission. High Level Group on innovation and provision of medicines in the European Union: Recommendations for action. G10 Medicines report, European Communities 2002. http://ec.europa.eu/health/ph_overview/Documents/key08_en.pdf (Accessed 21 Jun 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026377supp001.pdf (375.7KB, pdf)