Abstract
Racial disparities in health outcomes between African Americans and European Americans have been well-documented, but not fully understood. Chronic inflammation contributes to several of the diseases showing racial disparities (e.g., Human Immunodeficiency Virus [HIV]), and racial differences in stress exposure (e.g., experiences of racial discrimination) that stimulate pro-inflammatory processes that may contribute to differential health outcomes.
We performed a cross-sectional bioinformatic analyses relating perceived discrimination (as measured by the Perceived Ethnic Discrimination Questionnaire [PED-Q]) to the activity of pro-inflammatory, neuroendocrine, and antiviral transcription control pathways relevant to the conserved transcriptional response to adversity (CTRA) in peripheral blood leukocytes. Subjects were 71 individuals (37 HIV-seropositive (HIV+); 34 HIV-seronegative (HIV-)) (mean age = 53 years, range 27–63), who self-identified either as African American/Black (n=48) or European American/White (n=23). This provided the opportunity to examine the independent effects of race and HIV, as well as the modifying role of perceived discrimination on pathways involved in CTRA. Exploratory analysis examined the interactive effects of HIV and race on pathways involved in CTRA. Relative to European Americans, African Americans showed increased activity of two key pro-inflammatory transcription control pathways (NF-кB and AP-1) and two stress-responsive signaling pathways (CREB and glucocorticoid receptor); these effects did not differ significantly as a function of HIV infection (HIV x Race interaction, all p > .10). Results suggested that differences in experiences of racial discrimination could potentially account for more than 50% of the total race-related difference in pro-inflammatory transcription factor activity. In sum, differential exposure to racial discrimination may contribute to racial disparities in health outcomes in part by activating threat-related molecular programs that stimulate inflammation and contribute to increased risk of chronic illnesses.
Keywords: Racial discrimination, gene expression, HIV, African Americans
1. Introduction
Racism and racial discrimination has permeated the lives of many African Americans for decades, and is conceptualized as a chronic social stressor (Thoits, 2010). In a 2015 survey by the Kaiser Family Foundation, 35 percent of African Americans reported discriminatory experiences such as being denied a job or housing, or being prevented from voting, compared to about 11 percent of European Americans (see www.kff.org). Persistent racial inequality in areas of employment, housing, and other domains is a sobering reminder that racial discriminatory practices have yet to subside. As with other types of chronic stressors, racial discrimination may serve as a social pathway that contributes to Black-White disparities in a number of documented health and disease outcomes (Geronimus et al. 1996; Kochanek et al. 2004; Levine et al.2001; MMWR 2005; Smith et al. 1998; Williams & Jackson 2005).
Experiences with racial discrimination have been linked to several psychiatric and medical risk factors (e.g., depression, anxiety, cardiovascular disease, hypertension; Barnes et al., 2004; Brondolo et al., 2008; Lewis et al., 2006; Mozzaffarian et al., 2015; Williams & Mohammed, 2009; Sims et al., 2012; Kessler, Mickelson, & Williams, 1999; Mays, Cochran, & Barnes, 2007), mortality rates (Clark et al., 1999; Barnes et al., 2008), and cognitive compromise (Barnes et al., 2012; Thames et al., 2013). While perceived racial discrimination has been linked to socioeconomic status (SES) (Forman, Williams, & Jackson, 1997), it continues to be associated with race-related differences in health after SES is controlled (Williams, Neighbors, & Jackson, 2003), suggesting that poverty alone cannot explain these differences (Mays et al., 2007).
More recent evidence suggests that perceived racial discrimination is linked to premature aging (Diez Roux et al., 2009; Rewak et al., 2014; Chae et al., 2014, Lee et al., 2017; Liu & Kawachi, 2017), which may partly explain why certain groups have worse medical (e.g., cardiovascular disease, diabetes, hypertension) and psychiatric outcomes (e.g., depression, anxiety). In studies of premature aging, perceived racial discrimination was associated with shorter leukocyte telomere length (LTL) in African American men who demonstrated an implicit bias within their own group (Chae et al., 2014) and lower levels of depression (Chae et al., 2016), but the precise mechanisms of this effect remain unclear. This is particularly interesting since LTL has been found to be longer among African Americans when compared to European Americans/Whites (Lynch et al., 2016). The emerging field of social genomics may provide additional insights into racial disparities in disease risk by identifying specific types of human genes that are activated in association with racial discrimination (Cole, 2013; Cole, 2014). A number of studies have identified a common pattern of transcriptional alterations that is activated by chronic low-grade activation of the sympathetic nervous system (SNS) during experiences of socioeconomic disadvantage, social rejection, or threat. This “conserved transcriptional response to adversity” (CTRA) profile is characterized by increased expression of genes involved in inflammation, and decreased expression of genes involved in innate antiviral responses (e.g., Type I interferons) and genes encoding specific isotypes of antibodies (IgG in particular) (Cole, 2013; Cole, 2014). Experimental studies have shown that CTRA gene expression is mediated in part by activation of the sympathetic nervous system (SNS; Powell et al., 2013). Specifically, perceptions of social threat activate the SNS, leading to release of norepinephrine at SNS nerve terminals, activation of β-adrenergic receptors on adjacent cells, and stimulation or repression of specific transcription factors in response to the cyclic 3′−5′ adenosine monophosphate/protein kinase A (cAMP/PKA) signaling pathway. β-adrenergic-responsive transcription factors stimulate transcription of genes that encode proinflammatory cytokines and suppressing transcription of genes encoding Type I interferons and IgG antibodies, which contribute to CTRA gene expression (Cole, 2014).
CTRA activation has been observed in a wide range of adverse life circumstances including chronic social isolation (Cole et al., 2007; 2011; 2015), imminent bereavement (Miller et al., 2008; Miller et al., 2014), low SES (Chen et al., 2009; Miller et al., 2009; Chen et al., 2011), and of relevance to the current study, animal models of low social rank and repeated social defeat (Cole et al., 2010; Powell et al., 2013; Tung et al., 2012; Irwin & Cole, 2011). Experiences of racial discrimination have not yet been examined as potential triggers for CTRA gene expression, but the key role of perceived threat in triggering CTRA responses in other contexts suggests that similar dynamics may occur in response to experiences of racial discrimination. If so, CTRA-related increases in pro-inflammatory gene expression could contribute to racial disparities in rates of inflammation-related chronic illnesses such as cardiovascular, neoplastic, and neurodegenerative diseases. Moreover, CTRA-related decreases in antiviral responses may contribute to racial disparities in infectious diseases such as HIV.
African Americans represent over 40% of the HIV US population, yet only account for 12% of the total US population, which reflects a substantial health disparity (www.kff.org/hivaids/fact-sheet). Innate immune responses are key determinants of HIV outcomes, which include critical events in the earliest stages of infection such HIV transmission, establishment of initial foci of infection and local virus replication/spread as well as virus dissemination, and subsequent level of ongoing viral replication and rate of disease progression (Borrow, Shattock, Vyakarnam et al., 2010; Yamamoto, Barre-Sinoussi, Pedersen et al., 1986). Therefore, African Americans may be, in part, vulnerable to HIV acquisition and poor disease outcomes as a function of CTRA responses.
It is unknown if the presence of HIV infection increases vulnerability to CTRA activation as experiences of social adversity increase. HIV-infection results in a marked increase in immune activation, which involves both the adaptive and innate immune systems (Borrow, Shattock, and Vyakarnam et al., 2010). Myeloid lineage immune cells and their effector molecules are implicated in both HIV-infection and exposure to adverse socioenvironmental conditions (MacGillivray & Kollmann, 2014; Nicholson, 2016; Irwin & Cole, 2013). Thus, it is plausible that the presence of HIV may place individuals in adverse social circumstances at even higher risk for poor immune health.
In a recent study from our group, we found the effects of psychosocial stress on depression (Williamson et al., 2017) and cognitive/brain outcomes (Thames et al., 2017) to be greater among individuals with HIV. Previous research has also found that chronic activation of the SNS can promote the pathogenesis of HIV-1 infection (e.g., accelerating viral replication and inhibiting antiviral immune responses (Cole, 2008; Cole et al., 2003; Cole et al., 2001; Cole et al., 1998; Collado-Hidalgo et al., 2006), and experimental studies in animal models have linked CTRA activation to impaired antiviral response to the simian immunodeficiency virus (Cole, Capitanio, Chun et al., 2015).
The current study examined the effects of race in general, and perceived racial discrimination in particular, on the activity of key pro-inflammatory and antiviral transcription control pathways involved in generating CTRA responses, in a sample of HIV+ and HIV- African American and European Americans with similar income and employment status as well as perceived stress (other than racial discrimination). We hypothesized: 1) increased activity of pro-inflammatory and neuroendocrine-related transcription control pathways, and decreased activity of antiviral pathways, in African Americans relative to European Americans; 2) increased activity of pro-inflammatory and neuroendocrine-related transcription control pathways, and decreased activity of antiviral pathways in HIV+ relative to HIV- participants; and 3) differential experiences of racial discrimination might account, at least in part, for race- associated differences in CTRA-related transcription factor activity. As an exploratory analysis, we examined the interactive effects of race and HIV, and hypothesized that African Americans who were HIV+ would demonstrate the greatest activity of pro-inflammatory and neuroendocrine-related transcription control pathways, and decreased activity of antiviral pathways.
2. Methods
2.1. Participants
Participants were a total of 71 individuals, including 37 HIV+ persons and 34 HIV-persons, who either self-identified as African American (n = 48; 26 HIV+) or European Americans (n = 23; 11 HIV+), who were a subset of subjects enrolled in a larger study examining the effects of HIV on neurocognitive functioning and brain imaging among African American and European American individuals. Participants were recruited for the substudy if they had completed all procedures from the larger parent project and provided informed consent to be contacted by the research team about future studies. For this substudy, 71 subjects were randomly identified out of 180 total eligible participants in the parent study. There were no differences between participants in this substudy sample and those in the larger parent project sample with respect to a number of demographic and clinical characteristics such as age, ethnicity/race, gender, and education (all p’s >.20). Participants were recruited from various local community clinics and HIV service agencies in the Greater Los Angeles area. All procedures received approval by the University of California, Los Angeles.
Participants were included in the parent study if they were over the age of 18 years (range 27–63), reported English as their primary language, scored ≥ 26 on the Mini-Mental Status Exam (MMSE; Folstein et al. 1975), self-identified as African-American or European American, and were able to provide informed consent, as indicated by the participant communicating their understanding of the consent form. Participants were excluded if they reported either current abuse/dependence of cocaine or amphetamines, past stimulant abuse/dependence, or current/past diagnosis of a psychotic-spectrum disorder, as assessed by the Structured Clinical Interview for DSM-IV (Spitzer et al. 1995). Recent illicit drug use was assessed via urine toxicology in addition to a self-report drug screen (i.e., Brief Drug History Questionnaire). Participants were excluded if they screened positive for stimulants or hallucinogens. Participants were surveyed about alcohol, tobacco and other substances using the Drug Use History Form (DHQ) created by the University of California, Los Angeles’ Center for Advanced Longitudinal Drug Abuse Research. Data was used as covariates in statistical models.
Participants with central nervous system (CNS) confounds (e.g., HIV-associated opportunistic infections or neurosyphilis), Hepatitis C coinfection, (confirmed by serology), major head injury (loss of consciousness >30 min), or contraindication to the safe use of magnetic resonance imaging, were also excluded.
The HIV+ participants in the current substudy were a chronically-infected (average years with known HIV infection = 18.5, range = 12 to 20), and clinically-stable population, with self- reported continuous anti-retroviral therapy (ART) for at least 3 months, plasma HIV RNA viral loads below virologic failure (< 5000 copies/mL) (Alvarez-Uria et al., 2012; WHO 2010) or undetectable (< 20 copies/mL), and current CD4+ T cell counts averaging above 600 cells/mm. All participants provided informed consent prior to study procedures (see Table 1 for demographic and clinical characteristics of substudy sample).
Table 1.
Participant demographics (overall and by race) and group statistics for African American compared to European American participants
| Overall sample (n = 71) Mean (SD) or % |
African American (n = 48) Mean (SD) or % |
European American (n = 23) Mean (SD) or % |
Effect Size | Racial difference p-value |
|
|---|---|---|---|---|---|
| Age, years | 52.9 (9.9) | 52.8 (8.5) | 53.2 (12.7) | η2 = 0.00 | .96 |
| Education, years | 13.8 (2.3) | 13.3 (1.9) | 15.0 (2.5) | η2 =.13 | .02 |
| Gender | φ =0.34 | .08 | |||
| Male | 68% | 63% | 82% | - | - |
| Female | 29% | 33% | 18% | ||
| Trans (Male-to-Female) | 3% | 4% | 0 | ||
| HIV Status | |||||
| % positive | 52% | 54% | 48% | φ =0.08 | .50 |
| *Nadir CD4 count, cells/mm3 | 266 (159) | 282 (173) | 226 (117) | η2 = .02 | .44 |
| *Current CD4 count, cells/mm3 | 645 (271) | 608 (285) | 737 (220) | η2 = .05 | .20 |
| *Viral load, % undetectable | 66% | 64% | 80% | φ =0.15 | .65 |
| *Self-reported length of HIV infection, years | 14.27 (6.80) | 21.20 (8.77) | η2 = .16 | .01 | |
| BMI, kg/M2 | 27.5 (6.4) | 28.5 (7.15) | 25.4 (3.5) | η2 =0.05 | .07 |
| Tobacco use | |||||
| % endorsed use (past 12 months) | 29% | 35% | 14% | φ = 0.27 | .07 |
| Alcohol use | . | ||||
| % endorsed use (past 12 months) | 62% | 58% | 71% | φ =0.12 | .30 |
| Socioeconomic Status | |||||
| Hollingshead Index Score | 2.8 (1.8) | 2.1 (1.0) | 2.4 (1.2) | η2 = 0.03 | .45 |
| Perceived Stress Score | 23.3 (6.7) | 23.9 (6.9) | 22.0 (6.2) | η2 = 0.02 | .29 |
| Perceived Discrimination Score (PED-Q) | 32.2 (29.0) | 34.3 (13.2) | 27.6 (10.3) | η2 = 0.06 | .04 |
HIV+ group only
2.2. Materials and Methods
2.2.1. Perceived Discrimination
The Brief Perceived Ethnic Discrimination Questionnaire – Community Version PED-Q- CV (Brondolo et al., 2005) is a 17-item questionnaire assessing the frequency and intensity of instances when the participant felt discriminated against based upon his or her race/ethnicity. The respondent is instructed to rate on a Likert scale (1–5) how often the instances have occurred. Examples of PED-Q-CV items include: (1) “Have others thought that you couldn’t do things or handle a job?” (2) “Have others threatened to hurt you?”. The questionnaire includes four subscales: social exclusion, stigmatization, workplace discrimination, and threat/harassment. The PED-Q-CV has demonstrated high reliability (Cronbach’s α = 0.87) and is considered a valid measure of perceived discrimination (Brondolo et al., 2005). For statistical analyses, we used the PED-Q-CV total score, which is a sum of all items as well as the subscales. Higher scores represent greater experiences with discrimination.
2.2.2. Socioeconomic Status
The Hollingshead Index of Social Status (i.e., a weighted average of years of education, current or longest held occupation, and total household income of the participant) was used to assess current personal socioeconomic status. Years of education were based upon self-report from the participant and occupation was coded using the Hollingshead Index (Hollingshead, 1975). For married participants, the spouse’s education and occupation were also used to compute the score. The Hollingshead Index is a reliable and valid measure of social status (Cirino et al., 2002), and it has been used in a racially diverse sample of HIV+ adults (Arentoft et al., 2015). Hollingshead scores range from 0 to 4, with higher scores representing higher levels of SES
2.2.3. Perceived Stress
Participants’ levels of stress were measured using a shortened version of the Perceived Stress Scale (Cohen, Kamarck, and Mermelstein, 1983). The shortened version of the Perceived Stress scale (a = .82) consists of 12 items that ask how often in the last month the participants experienced symptoms of stress. Sample items include: “felt stressed,” “felt in control” (reverse coded), and “had problems dealing with responsibilities.” Participants used a 5-point response scale (1 = never to 5 = very often). Higher scores indicate greater stress levels over the past month.
2.2.4. Gene expression profiling and analysis
Total leukocyte RNA was extracted from whole blood samples drawn into PAXgene RNA tubes (Qiagen RNeasy) and checked for suitable mass (> 500 ng by NanoDrop 1000) and integrity (RNA integrity number > 8 by Agilent TapeStation capillary electrophoresis). All samples meeting quality criteria were assayed by RNA sequencing (RNAseq) in the UCLA Neuroscience Genomics Core Laboratory using Illumina TruSeq cDNA library synthesis and multiplex DNA sequencing on an Illumina HiSeq 4000 instrument with single strand 65 nt sequence reads (all following the manufacturer’s standard protocol). Samples yielded >30 million sequence reads, each of which was mapped to the RefSeq human genome sequence using HISAT2 [Kim, Langmead, & Salzberg, SL, 2015] and quantified as transcript counts per million total transcripts (TPM) using StringTie [Pertea et al., 2016]. The following steps were performed to test study hypotheses:
TPM values were floored at .1 and log2-transformed for analysis by a standard linear statistical models to estimate the magnitude of difference in transcript abundance for African Americans vs. European Americans while controlling for age, gender, body mass index (kg/m2), smoking history, alcohol consumption history, SES, years of education, (log10) HIV-1 viral load (0 for HIV- and floored at the 19 copy/mL lower limit of assay detection for HIV+ individuals with undetectable viral load), and duration of HIV infection (0 for HIV-).
The next set of analyses additionally controlled for individual differences in experienced discrimination to determine the extent to which they contribute to the overall differences observed in African Americans vs. European Americans.
In each analysis, all genes showing a point estimate of differential expression > 1.5- fold served as input into higher-order bioinformatics analyses using TELiS promoter sequence analysis (Cole, Yan, Galic, et al., 2005). This analysis tests a priori- specified hypotheses regarding activity of transcription control pathways relevant to CTRA biology, including sympathetic neurotransmitter signaling (CREB, measured with TRANSFAC position-specific weight matrix V$CREB_01); the positive CTRA component of inflammation (NF-кB, V$NFKAPPAB_01; AP-1, V$AP1_C); the inverse CTRA component of innate antiviral responses (interferon response factors, IRFs, V$ISRE_01); and the glucocorticoid receptor (GR, V$GRE_C), which is involved in cortisol signaling from the hypothalamus-pituitary-adrenal axis and is sometimes desensitized in association with the CTRA.
Each TELiS analysis was conducted using 9 different parametric combinations of promoter DNA sequence length (−300, −600, and −1000 to +200 nucleotides surrounding the RefSeq-designated transcription start site) and transcription factor- binding motif (TFBM) detection stringency (TRANSFAC mat_sim values of .80, .90, and .95) (Cole et al., 2005). Log2-transformed TFBM ratios (comparing prevalence in promoters of up- vs. down-regulated genes) were averaged across the 9 parametric combinations and tested for statistical significance using standard errors derived from bootstrap resampling of linear model residual vectors (controlling for potential correlation across genes).
Individual genes were not tested for statistically significant difference in expression because the goal of this study was solely to assess differences in average expression of sets of genes that reflect common transcription factor influences; for this application statistical significance screening at the individual gene level results in prevalent false negative errors and biases results from the subsequent set-based statistical analyses of transcription factor activity (see analytic results presented in Supporting Information for (Fredrickson et al., 2013)).
3. Results
3.1. Demographic comparisons
Results of demographic and clinical comparisons between African Americans and European Americans are reported in Table 1. African Americans reported fewer years of education than European Americans (p = .02), and greater incidences of racial discrimination (higher PED-Q scores) than European Americans (p = .04). There was a trend towards higher body mass index among African Americans relative to European Americans (p = .07) as well as a trend towards greater prevalence of tobacco use among African Americans compared to European Americans (p =.07). Among HIV+ participants, European Americans had a longer duration of known HIV infection than African Americans (p = .01).
3.2. Race-related differences in gene regulation (Hypothesis 1: Supported)
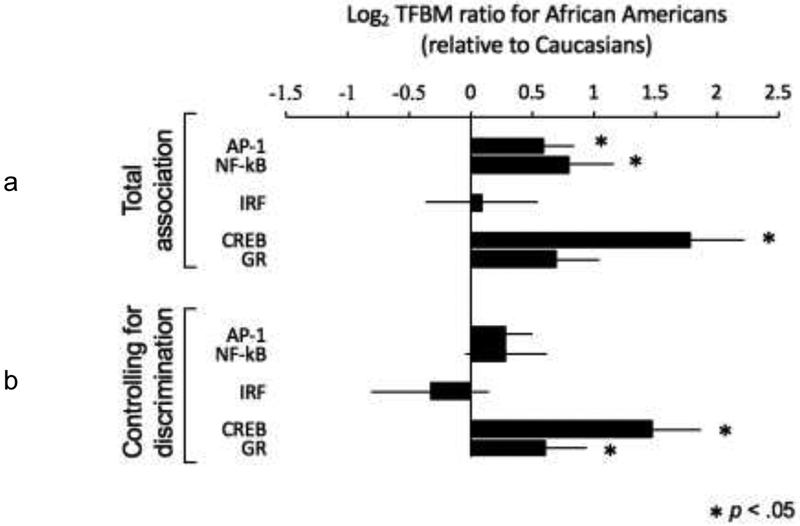
To determine whether African Americans showed differential activity of CTRA-related transcription control pathways relative to European Americans, we conducted RNA-sequencing- based transcriptome profiling of circulating white blood cell samples to serve as input into TELiS promoter-based bioinformatics analyses. CTRA-related analyses assess the relative activity of pro-inflammatory transcription factors (NF-кB, AP-1), innate antiviral response transcription factors (IRFs), and transcription factors involved in stress-related neuroendocrine signaling pathways (CREB, which mediates beta-adrenergic signaling from sympathetic nervous system catecholamines, and the GR, which mediates cortisol signaling from the hypothalamic- pituitary-adrenal axis). Analyses of differential gene expression controlled for age, gender, body mass index, smoking history, alcohol consumption history, and HIV-1 viral load. Results of these analyses (Figure 1a) indicated significantly greater activity of both pro-inflammatory transcription control pathways in African Americans relative to European Americans (NF-кB: mean +0.63 ± 0.21 log2 TFBM ratio in promoters of up- vs. down-regulated genes, p = .003; AP-1: +0.43 ± 0.22, p = .049). Results also indicated greater activity of IRF factors involved in Type I interferon antiviral signaling (+0.97 ± 0.47, p = .041). Analyses of the two neuroendocrine-related pathways indicated greater activity of GR (+0.84 ± 0.35, p = .018) but no significant difference in CREB activity (−0.09 ± 0.42, p = .813) in circulating leukocytes from African Americans compared to European Americans.
Figure 1.
Relationships between CTRA-related transcription factor activity and race. a: Total association using Log2 transcription factor-binding motifs ratio. b: Association between CTRA-related transcription factor activity and race/ethnicity after controlling for discrimination.
3.3. Effect of HIV infection (Hypothesis 2: Supported)
TELiS analyses were also performed comparing subjects by HIV status. Two transcription control pathways showed increased activity in HIV+ individuals relative to HIV-: NF- к B (+0.61 ± 0.22, p = .006) and IRF factors (+1.25 ± 0.43, p = .004).
3.4. Role of racial discrimination (Hypothesis 3: Partially supported)
As noted above, African Americans reported experiencing greater levels of racial discrimination than did European Americans (PED-Q-CV mean = 34.3 ± standard deviation 13.2 for African Americans vs. 27.6 ± 10.0 for European Americans; difference, p = .03). To determine whether these differences might contribute to race-related difference in CTRA-related transcription factor activity, we conducted a second set of analyses that additionally controlled for PED-Q-CV scores. Results (Figure 1b) indicated that a substantial portion of the total race- related difference in pro-inflammatory signaling may potentially stem from correlated variations in experienced discrimination. Control for PED-Q-CV scores attenuated race-related differences in NF- к B activity by 34% (+0.42 ± 0.21, p = .042), race-related differences in AP-1 activity by 58% (+0.18 ± 0.24, p = .45), and race-related differences in IRF activity by 16% (+0.82 ± 0.49, p = .099). In analyses of the two neuroendocrine-related pathways, control for PED-Q-CV scores attenuated race-related differences in GR activity by 31% (+0.58 ± 0.39, p = .140), whereas CREB continued to show no significant race-related difference in activity (+0.23 ± 0.39, p = .561).
3.5. Interactive effects of HIV and race (Exploratory Hypothesis; Not supported)
Among the transcription factors that showed significant race-related differences in activity (NF- к B, AP-1, and GR), the magnitude of those effects did not differ significantly as a function of HIV infection (HIV x Race interaction terms all p > .10).
4. Discussion
In the present analyses of leukocyte transcriptome profiles, 1.) African Americans showed higher levels of inflammatory signaling and higher levels of stress-related neuroendocrine signaling than did European Americans, and, 2.) a substantial fraction of the elevated inflammatory signaling activity in African Americans may be associated with increased experiences of racial discrimination. These effects were not modified by HIV status. The profile of transcriptional/gene expression differences observed here is consistent with patterns previously observed in studies exploring the relationship between perceptions of the social environment and CTRA gene expression (Fredrickson et al. 2013; Miller et al. 2008, 2009, 2014; Powell et al. 2013). These results are also consistent with previous data linking chronic exposure to environmental stressors, such as perceived racism, to physiological processes such as chronic inflammation (Cunningham et al., 2012; Lewis et al, 2010; Moody et al., 2014). Considering that chronic SNS activation is a major driver of CTRA gene expression, and that SNS activation can result from perceptions of social threat, our findings are consistent with the idea that discrimination is a form of chronic stress. Given that the proportion of gene regulatory variation accounted for by discrimination was substantial in magnitude, it is important to think comprehensively about the various psychosocial factors that track with perceived discrimination such as lack of economic opportunity, personal safety concerns, and maladaptive forms of coping (e.g., drug/alcohol use) that may explain why discrimination effects on health outcomes are robust throughout the literature.
Contrary to our exploratory hypothesis, we did not find that racial differences in transcriptional/gene expression of NF- к B, AP-1, CREB, and GR was greater in our HIV+ group. These results may be due, in part, to differences in transcription response pathways involved in threat/stress vs. infection, or the shorter duration of known HIV-infection in African Americans relative to European Americans, but they may also stem from a lack of statistical power to determine interactive effects of race and HIV infection given the limited sample size available in this study. Such interactions may exist but simply not be detectable in the present study, which is why the primary focus of this study was not on the interactive effects. Larger samples of HIV+ and HIV- individuals would be necessary for a well-powered analysis of the effects of HIV infection on gene expression overall, and the extent to which infection modifies the impact of race and discrimination on gene expression.
The biological profile of the current findings differs somewhat from another recent transcriptome profiling study that compared leukocyte transcriptome profiles from African Americans and European Americans (McDade et al., 2016). That study of healthy community- dwelling adults from the Chicago metropolitan area found elevated levels of Type I interferon signaling in leukocytes from African Americans but no difference in inflammatory signaling activity. Similar observations emerged from another ancillary analysis of racial differences in leukocyte gene expression profiles (Kohrt et al. 2016). The contrasting pattern observed here may stem from differences in infectious disease background (this study containing a substantial number of clinically-stable but HIV-seropositive individuals) or differences in socio-economic conditions or other environmental conditions that remain to be identified in future research. This report also differs from those analyses in identifying one mediating psychosocial pathway (i.e., the stress of experienced discrimination) for such racial differences in gene expression, whereas the previous studies were solely descriptive of race differences and did not seek to identify their etiology.
Limitations of this study include the cross-sectional study design (which precludes drawing causal conclusions), and the limited sample size, which was sufficient to test this study’s a priori hypotheses regarding gene set-based bioinformatic assessments of CTRA-related transcription factor activity but was not powered for hypothesis-free discovery of statistically significant race-related differences at the level of individual genes, nor for extensive examination of HIV/race interactions. Future research in larger samples would be required to discover reliable associations of specific gene transcripts with race, and will also be required to evaluate the generalizability of the present findings in other settings. Further, larger samples are needed to examine the role of how intersecting stigmatized identities play a role in discrimination experiences. Discrimination in healthcare settings (particularly in the context of HIV) is not only potentially stressful, but may impact the quality of healthcare that patients receive. It is possible that discrimination due to HIV-status may be more salient for our HIV+ participants. We did not ask about discrimination as a function of HIV status, therefore, this is an area that needs further exploration. Finally, no direct measures of health outcomes are available in this study, so the health significance of the observed transcriptomic differences also remain to be determined in future research. The magnitude of the race-related TFBM ratio signals observed here is relatively large in comparison to other types of psychosocial risk factors (corresponding to 30% or greater asymmetries in TF binding-site prevalence in up- vs. down-regulated genes), but it remains unclear how these biological signaling metrics would translate quantitatively into an increased risk of disease. This study recruited a targeted sub-population of community-dwelling adults and the generalizability of these results to other socio-demographic contexts also remains to be determined in future studies.
These limitations aside, our study findings contribute to the extant literature on racial discrimination and health by demonstrating a potential biological mechanism (i.e., leukocyte gene profiles) through which racial discrimination influences health outcomes, and may partially explain racial disparities in health. With this knowledge, efforts can be directed towards better understanding racial disparities in health, particularly in diseases such as hypertension and cardiovascular disease, which have demonstrated clear racial disparities in prevalence and progression. If racial discrimination is perceived by the larger society as a social “health risk factor” similar to that of smoking, obesity, high blood pressure, and substance abuse, this may promote greater interest in reducing behaviors that may unintentionally disadvantage or discriminate against racial and ethnic minority groups.
Highlights.
We examined race and HIV differences in transcription activity in pathways relevant to the conserved transcriptional response to adversity
Relative to European Americans, African Americans showed greater activity of pro-inflammatory transcription control and stress-signaling pathways
Experienced discrimination accounted for more than 50% of total race-related difference in pro-inflammatory transcription factor activity
HIV group differences were found in pro-inflammatory and antiviral transcription pathways
Funding and Acknowledgments
This study was supported by the following grants: K23 MH095661; NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881;Inflammatory Biology Core of the UCLA Older Americans Independence Center (5P30AG028748); Social Genomics Core (P30 AG017265) of USC/UCLA Center on Biodemography and Population Health.
Support for this project was also provided by UCLA Cousins Center for Psychoneuroimmunology
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Appay V & Sauce D (2008), Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol, 214: 231–241. doi: 10.1002/path.2276 [DOI] [PubMed] [Google Scholar]
- Barnes LL, Mendes de Leon CF, Lewis TT, Bienias JL, Wilson RS, & Evans DA (2008). Perceived Discrimination and Mortality in a Population-based Study of Older Adults. American Journal of Public Health, 98(7), 1241–1247. 10.2105/AJPH.2007.114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Shattock RJ, Vyakarnam A (2010). Innate immunity against HIV: a priority target for HIV prevention research. Retrovirology. ;7:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W, Jamieson BD, Hultin LE, Hultin PM, Effros RB, & Detels R (2009). Premature Aging of T cells Is Associated With Faster HIV-1 Disease Progression. Journal of Acquired Immune Deficiency Syndromes. 50(2), 137–147. 10.1097/QAI.0b013e3181926c28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Epel ES, Nuru-Jeter AM, Lincoln KD, Taylor RJ, Lin J, Blackburn EH, & Thomas SB (2016). Discrimination, Mental Health, and Leukocyte Telomere Length Among African American Men. Psychoneuroendocrinology, 63, 10–16. 10.1016/j.psyneuen.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae DH, Nuru-Jeter AM, Adler NE, Brody GH, Lin J, Blackburn EH, & Epel ES (2014). Discrimination, Racial Bias, and Telomere Length in African-American Men. American Journal of Preventive Medicine, 46(2), 103–111. 10.1016/j.amepre.2013.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Anderson NB, Clark VR, & Williams DR (1999). Racism as a stressor for African Americans: A biopsychosocial model. American Psychologist, 54(10), 805–816. 10.1037/0003-066X.54.10.805 [DOI] [PubMed] [Google Scholar]
- Cole SW (2008) Psychosocial influences on HIV-1 disease progression: neural, endocrine, and virologic mechanisms. Psychosomatic Medicine, 70, 562–568. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Sung C, and Cole SW (2006) Adrenergic inhibition of innate antiviral response: PKA blockade of Type I interferon gene transcription mediates catecholamine support for HIV-1 replication. Brain, Behavior, and Immunity, 20, 552–563. [DOI] [PubMed] [Google Scholar]
- Cole SW (2014) Human Social Genomics. PLoS Genet 10(8): e1004601 10.1371/journal.pgen.1004601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Capitanio JP, Chun K, Arevalo JMG, Ma J, & Cacioppo JT (2015). Myeloid differentiation architecture of leukocyte transcriptome dynamics in perceived social isolation. Proceedings of the National Academy of Sciences of the USA, 112, 15142–15147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Kemeny ME, Fahey JL, Zack JA & Naliboff BD (2003). Psychological risk factors for HIV pathogenesis: Mediation by the autonomic nervous system. Biological Psychiatry, 54, 1444–1456. [DOI] [PubMed] [Google Scholar]
- Cole SW, Korin YD, Fahey JL, & Zack JA (1998). Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. Journal of Immunology, 161, 610–616. [PubMed] [Google Scholar]
- Cole SW, Naliboff BD, Kemeny ME, Griswold M, Fahey JL, & Zack JA (2001). Impaired response to HAART in patients with high autonomic nervous system activity. Proceedings of the National Academy of Sciences of the USA, 98, 12695–12670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW, Yan W, Galic Z, Arevalo J, Zack JA (2005). Expression-based monitoring of transcription factor activity: the TELiS database. Bioinformatics, 21(6):803–810. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe CI, & Berkman LF (2012). Racial/ethnic and gender differences in the association between self- reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Social Science and Medicine, 75(5), 922–931. doi: 10.1016/j.socscimed.2012.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Phillips AN (2009). HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 338:a3172. [DOI] [PubMed] [Google Scholar]
- Desai S, & Landay A (2010). Early Immune Senescence in HIV Disease. Current HIV/AIDS Reports,7(1), 4–10. 10.1007/s11904-009-0038-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, & Seeman T (2009). Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell, 8(3), 251–257. 10.1111/j.1474-9726.2009.00470.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douek DC, McFarland RD Keiser PH, Gage EA, Massey JM, Haynes BF, Polis MA,Haase AT, Feinberg MB, Sullivan JL, Jamieson BD, Zack JA, Picker LJ, Koup RA(1998). Changes in thymic function with age and during the treatment of HIV infection. Nature.396:690–695 [DOI] [PubMed] [Google Scholar]
- Effros RB, Allsopp R, Chiu CP, Hausner MA, Hirji K, Wang L, Harley CB, Villeponteau B, West MD, Giorgi JV (1996) Shortened telomeres in the expanded CD28-CD8+ cell subset in HIV disease implicate replicative senescence in HIV pathogenesis. AIDS. (8):F17–F22 [DOI] [PubMed] [Google Scholar]
- Effros RB, Dagarag M, Spaulding C and Man J (2005), The role of CD8+ T-cell replicative senescence in human aging. Immunological Reviews, 205: 147–157. doi: 10.1111/j.0105-2896.2005.00259.x [DOI] [PubMed] [Google Scholar]
- Effros RB, Fletcher CV, Gebo K, Halter JB, Hazzard WR, Horne FM, Huebner RE,Janoff EN, Justice AC, Kuritzkes D, Nayfield SG, Plaeger SF, Schmader KE, Ashworth JR, Campanelli C, Clayton CP, Rada B, Woolard NF & High KP (2008). Workshop on HIV Infection and Aging: What Is Known and Future Research Directions. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America, 47(4), 542–553. 10.1086/590150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson BL, Grewen KM, Coffey KA, Algoe SB, Firestine AM, Arevalo JMG, Ma J, & Cole SW (2013). A functional genomic perspective on human well-being. Proceedings of the National Academy of Sciences of the USA, 110, 13684–13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BA, Chalan P, Koopman G, Boots AMH (2013). Chronic autoimmune-mediated inflammation: a senescent immune response to injury, Drug Discovery Today, 18(7), 372–379. 10.1016/j.drudis.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Kohrt BA, Worthman CM, Adhikari RP, Luitel NP, Arevalo JM, Ma J, McCreath H, Seeman TE, Crimmins E, & Cole SW (2016). Psychological resilience and the gene regulatory impact of posttraumatic stress in Nepali child soldiers. Proceedings of the National Academy of Sciences, 113 (29): 8156–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Langmead B, & Salzberg SL (2015). HISAT: a fast spliced aligner with low memory requirements. Nature Methods, 12(4), 357–360. 10.1038/nmeth.3317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger N (1999). Embodying Inequality: A review of concepts, measures, and methods for studying health consequences of discrimination. International Journal of Health Services, 29(2):295–352. doi: 10.2190/M11W-VWXE-KQM9-G97Q [DOI] [PubMed] [Google Scholar]
- Lee D, Kim E, & Neblett E (2017). The link between discrimination and telomere length in African American adults. Health Psychology, 36(5), 458–467. doi: 10.1037/hea0000450 [DOI] [PubMed] [Google Scholar]
- Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL (2010). Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain, behavior, and immunity, 24:438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, & Kawachi I (2017). Discrimination and telomere length among older adults in the united states: Does the association vary by race and type of discrimination? Public Health Reports, 132(2), 220–230. doi: 10.1177/0033354916689613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SM, Peek MK, Mitra N, Ravichandran K, Branas C, Spangler E, Zhou W, Paskett ED, Gehlert S, DeGraffinreid C, Rebbeck TR & Riethman H (2016). Race, Ethnicity, Psychosocial Factors, and Telomere Length in a Multicenter Setting. PLoS ONE, 11(1), e0146723 10.1371/journal.pone.0146723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MA (2012). Family Structure and the Intergenerational Transmission of Educational Advantage. Social Science Research, 41(1), 33–47. 10.1016/j.ssresearch.2011.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito S, & Viding E (2010). Research review: The neurobiology and genetics of maltreatment and adversity. Journal of Child Psychology and Psychiatry, 51(10), 1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x [DOI] [PubMed] [Google Scholar]
- McDade TW, Ross K, Fried R, Arevalo JMG, Ma J, Miller GE, & Cole SW (2016).Genome-Wide Profiling of RNA from Dried Blood Spots: Convergence with Bioinformatic Results Derived from Whole Venous Blood and Peripheral Blood MononuclearCells. Biodemography and Social Biology, 62(2), 182–197. 10.1080/19485565.2016.1185600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen EE, Fok AA, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS (2009). Low early-life social class leaves a biological residue manifest by decreased glucocorticoid and increased pro-inflammatory signaling. Proc Natl Acad Sci USA, 106:14716–14721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, Ma R, Cole SW (2008). A genomic fingerprint of chronic stress in humans: Blunted glucocorticoid and increased NF-κB signaling. Biol Psychiatry, 64:266–272.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Murphy MLM, Cashman R, Ma R, Ma J, Arevalo JMG, Kobor MS, & Cole SW (2014) Greater inflammatory activity and blunted glucocorticoid signaling in monocytes of chronically stressed caregivers. Brain, Behavior, and Immunity, 41, 191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JMG, Doll R, Cole SW (2008). A Functional Genomic Fingerprint of Chronic Stress in Humans: Blunted Glucocorticoid and Increased NF-κB Signaling. Biological Psychiatry, 64(4), 266–272. 10.1016/j.biopsych.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody D, Brown C, Matthews K, & Bromberger J (2014). Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: Study of women’s health across the nation. Journal of Social Issues, 70(2), 298–314. doi: 10.1111/josi.12061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RD, Gebo KA, Lucas GM, & Keruly JC (2008). Rate of comorbidities not related to HIV infection or AIDS among HIV-infected patients, by CD4 cell count and HAART use status. Clinical Infectious Diseases, 47(8), 1102–1104. doi: 10.1086/592115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradies Y (2006). A systematic review of empirical research on self-reported racism and health. International Journal of Epidemiology, 35(4):888–901. doi: 10.1093/ije/dyl056. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Effros RB, Caruso C, Remarque E & Barnett Y (1999). T cells and aging. Front Biosci;4:D216–269 [DOI] [PubMed] [Google Scholar]
- Pertea M, Kim D, Pertea G, Leek JT, & Salzberg SL (2016). Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie, and Ballgown. Nature Protocols, 11(9), 1650–1667. 10.1038/nprot.2016.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell ND, Sloan EK, Bailey MT, Arevalo JMG, Miller GE, Chen E, Kobor MS, Reader BF, Sheridan JF, & Cole SW (2013) Social stress up-regulates inflammatory gene expression in the leukocyte transcriptome via beta-adrenergic induction of myelopoiesis. Proceedings of the National Academy of Sciences of the USA, 110, 16574–16579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewak M, Buka S, Prescott J, De Vivo I, Loucks EB, Kawachi I, Non AL & Kubzansky LD (2014). Race-related health disparities and biological aging: does rate of telomere shortening differ across blacks and whites? Biol. Psychol, 99, 92–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer MH, Wilkinson HR & Ferraro KF (2013). Childhood (mis)fortune, educational attainment, and adult health: Contingent benefits of a college degree? Soc Forces, 91(84),1007–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SS, McGowan JP, Smith C, Blum S & Klein RS (2002). Comorbid conditions, treatment, and health maintenance in older persons with human immunodeficiency virus infection in New York City. Clin Infect Dis, 35:1238–43 [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Berkman LF, & Reuben DB (2004). Cumulative biological risk and socio-economic differences in mortality: MacArthur studies of successful aging. Social Science & Medicine, 58:1985–1997. doi: 10.1016/S0277-9536(03)00402-7 [DOI] [PubMed] [Google Scholar]
- Thames AD, Kuhn TP, Mahmood Z, Bilder RM, Williamson TJ, Singer EJ, & Arentoft A (2017). Effects of social adversity and HIV on subcortical shape and neurocognitive function. Brain Imaging and Behavior, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Mugavero M, Willig J, Raper JL, & Saag MS (2010) Aging with HIV: a cross- sectional study of comorbidity prevalence and clinical characteristics across decades of life. Journal of the Association of Nurses in AIDS Care, 22(1),17–25 [DOI] [PubMed] [Google Scholar]
- Veldman K, Bultmann U, Almansa J, & Reijneveld SA (2015). Childhood adversities and educational attainment in young adulthood: The role of mental health problems in adolescence. Journal of Adolescent Health, 57(5), 462–467. doi: 10.1016/j.jadohealth.2015.08.004 [DOI] [PubMed] [Google Scholar]
- Vines AI, Baird DD, McNeilly M, Hertz-Picciotto I, Light KC, & Stevens J (2006). Social Correlates of the Chronic Stress of Perceived Racism Among Black Women. Ethnicity & Disease, 16(1), 101–107. [PMC free article] [PubMed] [Google Scholar]
- Wickrama KAS, Conger RD, Lorenz FO, & Jung T (2008). Family Antecedents and Consequences of Trajectories of Depressive Symptoms from Adolescence to Young Adulthood: A Life Course Investigation. Journal of Health and Social Behavior, 49(4), 468–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson TJ, Mahmood Z, Kuhn TP, & Thames AD (2017). Differential relationships between social adversity and depressive symptoms by HIV status and racial/ethnic identity. Health Psychology, 36(2), 133–142. 10.1037/hea0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto JK, Barre-Sinoussi F, Bolton V, Pedersen NC, Gardner MB. Human alpha- and beta- interferon but not gamma- suppress the in vitro replication of LAV, HTLV-III and ARV-2 (1986). J Interferon Res;6:143–152. [DOI] [PubMed] [Google Scholar]