Abstract
Aging is associated with complex biological changes that can be accelerated, slowed, or even temporarily reversed by biological and non-biological factors. This article focuses on the link between biological aging, psychological stressors, and mental illness. Rather than comprehensively reviewing this rapidly expanding field, we highlight challenges in this area of research and propose potential strategies to accelerate progress in this field. This effort requires the interaction of scientists across disciplines - including biology, psychiatry, psychology, and epidemiology; and across levels of analysis that emphasize different outcome measures - functional capacity, physiological, cellular, and molecular. Dialogues across disciplines and levels of analysis naturally lead to new opportunities for discovery but also to stimulating challenges. Some important challenges consist of 1) establishing the best objective and predictive biological age indicators or combinations of indicators, 2) identifying the basis for inter-individual differences in the rate of biological aging, and 3) examining to what extent interventions can delay, halt or temporarily reverse aging trajectories. Discovering how psychological states influence biological aging, and vice versa, has the potential to create novel and exciting opportunities for healthcare and possibly yield insights into the fundamental mechanisms that drive human aging.
Keywords: biological age, psychopathology, DNA methylation, brain, mitochondria, telomere length
Graphical abstract
INTRODUCTION
Aging is the strongest risk factor for many chronic illnesses, loss of functional capacity, and mortality (Fernandes et al., 2016). It is associated with complex biological changes, but there is no consensus on the very definition of aging, nor on the best methods to quantify it biologically (Xia et al., 2017). Chronological age is based on the passage of time and is invariable. But biological age may fall behind or else outpace chronological age – it is modifiable. Based on specific molecular and other measures discussed below, the rate of biological aging has been reported to vary substantially between individuals (Cole et al., 2018; Xia et al., 2017), although the causes of such inter-individual differences are mostly unclear. In particular, a major gap in knowledge is reflected in our ignorance of the mechanisms for the transduction of psychological states, and of psychopathology, into changes in biological aging (Figure 1). How do “mind” states influence biological aging and vice versa?
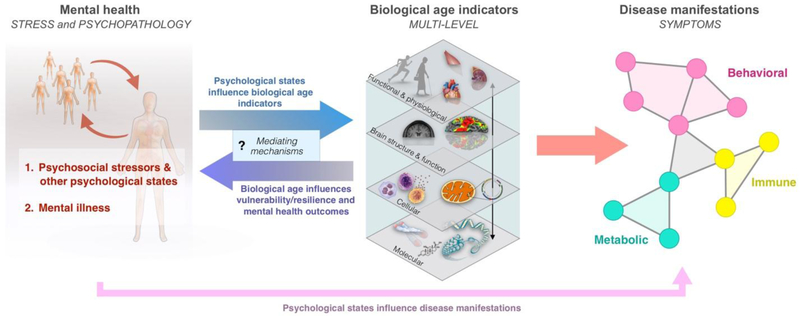
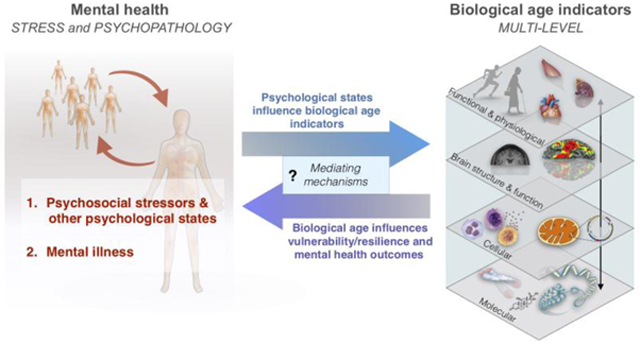
Figure 1. Integrative model for the transduction of mental health into biological aging and downstream disease manifestations.
(Left) Two main domains of mental health are considered: 1. Acute and chronic psychosocial stressors, which include distress and other subjective experiences; 2. Mental illness and clinical psychopathology (e.g., depression, anxiety, schizophrenia, bipolar disorder, etc). (Middle) These factors are transduced into biological age indicators, which span functional and physiological, brain structure and function, cellular, and molecular levels of analysis. In turn, the reverse association may transduce increased biological age into increased vulnerability and resilience to life stressors. The mechanisms responsible for the bi-directional flow of information between psychological states, psychopathology, and biological age indicators largely remain to be defined. (Right) Increased biological aging reflected in individual or combinations of biological age indicators manifest in symptoms across multiple interconnected systems, represented here as a functional network. Mental health domains can also directly contribute to disease manifestations (bottom arrow). Molecular indicators refer to components that are inert when in isolation (e.g. DNA, proteins) whereas cellular indicators refer to animated “living” components (e.g. breathing mitochondria, dividing/secreting cells).
This psycho-biological problem is a unique opportunity to make scientific progress on two main fronts: First, it is an opportunity to develop new measurements and technical approaches to capture meaningful, valid, and reproducible measures of biological aging. Second, this interdisciplinary problem requires dialogue across research and clinical domains. We see the intersection of experiential, psychological, and biological aging processes as a platform for the development of new (and possibly radically different) concepts and measures that will most faithfully capture human health and the aging process. Currently, although we have some quantifiable measures of biological aging in humans - biological age indicators - we still know little about their causal role in the aging process, and about their modifiability by psychological states and psychopathology.
One important shared goal towards enhancing well-being across the lifespan is to understand aging as dynamic trajectories determined by a variety of factors. Some determinants of biological aging are pre-programmed (“intrinsic”; e.g., genetic), while others are affected by the environment (“extrinsic”; e.g., diet, adversity) (de Magalhães, 2012). Most definitions of biological aging include loss of function,increased propensity to certain diseases, and closer proximity to death (de Magalhães and Passos,2018). Certain objective biological measures (or “clocks”) may also track biological aging. Development and validation of biological age indicators and clarification of their mediators and moderators are high priorities, since they may lead to a better understanding of the underpinnings of healthy and unhealthy aging trajectories. These indicators may also present proximal outcomes, or “early warning signs” that portend disease development and may provide a more sensitive platform to detect – and intervene upon – meaningful interactions between psychological, social, and bio-behavioral factors that influence aging trajectories and health outcomes.
Biological age indicators currently being investigated include telomere length (TL), epigenetic changes, alterations of mitochondrial function and mitochondrial DNA (mtDNA), age-related brain structure and function, and transcriptomic, metabolomic, and proteomic changes, among others (see,Cole et al., 2018; Jylhava et al., 2017; Xia et al., 2017 for recent reviews). Current topics of investigation include the nature of the inter-relationship of these biological age indicators, whether they measure the same or different aspects of biological aging, whether they are causally involved in the aging process, whether they have a causal role in disease and disorders, and the best ways to assess them. The possibility that the aging process is accelerated by chronic psychological stress and that it plays a role in the pathophysiology of some mental illnesses has been supported by observations that chronically stressed or psychiatrically ill individuals are at increased risk of acquiring specific age-related diseases and have a reduced life expectancy (Epel and Prather, 2018; Lindqvist et al., 2015; Penninx et al., 2013; Verhoeven et al., 2014; Walker et al., 2015). But certain conceptual and methodological obstacles are impeding growth in this field and hinder replication of findings across laboratories.
Rather than comprehensively reviewing this rapidly expanding field, here we focus on highlighting various challenges and arising opportunities for this interdisciplinary endeavor. We conclude by proposing strategies to accelerate the progress of this field towards a predictive science that can enhance our understanding of the psychobiological factors that influence the aging process and lifespan.
1. The concept of biological aging: Definitions and obstacles
Chronological age is strictly quantitative and requires no more than a calendar to measure. Biological age is more elusive as it reflects the functional and biological condition of an individual. The difference between biological age and chronological age can indicate whether the individual’s biological state is “older” or “younger” than would be expected for a given chronological age. This is often referred to as “accelerated” or “slowed” aging, respectively. However, cross-sectional assessments of biological age do not allow to determine aging rates, or to distinguish between “accelerated” and “premature” or “advanced” aging (Figure 2). The rate of increase in biological aging over time may also exhibit nonlinear behavior, particularly in early life where the measured rate of aging may be more rapid than across adult life (Cole et al., 2018; Lohr et al., 2015).
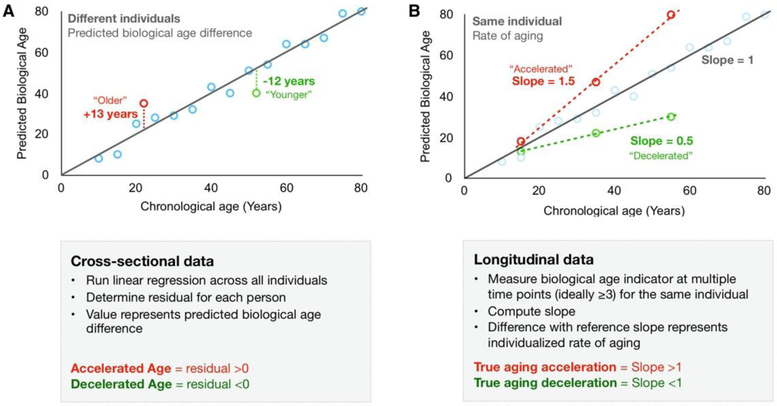
Figure 2. Computing age acceleration using cross-sectional and longitudinal data.
(A) From cross-sectional data, accelerated aging is established when biological age is over-predicted relative to the chronological age reference (group regression line). (B) In longitudinal data, the rate of aging is directly determined from multiple measurements in the same person (also same tissue and cell type). The slope for each individual can be compared to the theoretical slope of 1 to ascertain true aging acceleration or deceleration.
Since it is not possible to directly assess the total biological state of a person, biological age indicators serve as proxies. Biological age indicators are functional, anatomical, biochemical, cellular or molecular measures that are correlated with age and that may reflect the health status of specific cell types and/or organ systems. The term biomarker, in contrast to indicators as defined here, is best used in the context of specific disease or health outcomes. By definition, biomarkers must exhibit both sensitivity and specificity in relation to the outcome we design them to predict (Abi-Dargham and Horga, 2016). For aging, a still broadly defined process compared to a disease that can be ascertained with certainty, the term indicator is rendered more appropriate. Although some biological age indicators have undoubtedly established their sensitivity to chronological age, few have convincingly demonstrated their specificity - that changes in their value occurs specifically in response to the aging process and not in response to other pathophysiological process. Some biological age indicators are indeed modified by disease states independent of aging, and some as discussed below may notably be sensitive to psychological states, namely stress and psychopathology.
2. Biological age indicators
Aging is a multifaceted and complex process that manifests across multiple levels. In recent decades, measurements spanning each of these levels have been developed, reflecting our prevailing reductionist scientific approach to biomedical sciences. Here, rather than providing an exhaustive overview that can be found in recent reviews (Engelfriet et al., 2013; Horvath and Raj, 2018; Jylhava et al., 2017; López-Otín et al., 2013; Wagner et al., 2016; Xia et al., 2017), we provide a selective overview of aging indicators commonly studied in relation to mental health (Table 1). This table, which is illustrative rather than comprehensive, also includes some emerging biological age indicators that reflect the development of omics technologies and of computational approaches to integrate multiple metrics into composite indices, as also discussed in Section 5. Due to space limitation, we do not cover self-reported age (Stephan et al., 2018), self-reported perception of aging (Levy et al., 2002), more specific brain measures (Cole, 2018), or inflammatory markers (Franceschi et al., 2000), which have also been associated with lifespan. We then discuss the practical limitations and conceptual challenges commonly associated with these measurements.
Table 1.
Selective list of biological age indicators.
| Category | Biological age indicator |
Method | Correlation with chronological age |
Evidence for mental health association | Representative survival or method-related references |
|---|---|---|---|---|---|
| Functional & physiological measures |
Walking speed/gait | Timed distance walked Self-report |
Inverse | Impaired in depression (Lemke et al., 2000; Michalak et al.,2009) | (Cooper et al., 2010†; Graham et al., 2008†; Yates et al., 2017) |
| Hand-grip strength | Dynamometer | Inverse | Lower in depression (Lee et al., 2018; Lever-van Milligen et al., 2017) | (Bohannon, 2008†; Ortega et al., 2012; Sasaki et al., 2007; Sayer and Kirkwood, 2015) | |
| Lung function | Spirometer | Inverse | Impaired in depression (Lever-van Milligen et al., 2017) | (Stavem et al., 2005) | |
| Brain measures | Brain ageΔ | T1-MRI | Positive/high | Higher in SCZ (Schnack et al., 2016), MDD, BPD, and psychosis (risk) (Kolenic et al., 2018; Koutsouleris et al.,2014) Inconsistent in BD (Hajek et al., 2017; Nenadic et al., 2017) Higher with more negative fateful life events (Hatton et al.,2018) |
(Cole et al., 2018, 2017) |
| Cellular measures | Respiratory capacity | Mitochondrial enzymatic activities Respiratory capacity in fresh cells |
Inverse | Enzymatic activities: correlated with previous day positive mood (Picard et al., 2018) Cellular respiration: associations with early life adversity (Boeck et al., 2018a) and MDD (Boeck et al., 2018d; Karabatsiakis et al., 2014) |
(Gonzalez-Freire et al., 2018;Greco et al., 2003; Tyrrell et al., 2015) |
| Molecular measures |
Telomere length | qPCR Southern blot Q-FISH |
Inverse | Shorter in MDD (Schutte and Malouff, 2015), anxiety disorders (Malouff and Schutte, 2017), SCZ (Polho et al.,2015), PTSD (Li et al., 2017) N.S. in BD |
(Aubert et al., 2012; Aviv et al.,2011; Cawthon, 2002; Wang et al., 2018†) |
| Epigenetic ageΔ | Microarray and sequencing | Positive/high | Increased in response to traumatic stress (Wolf et al., 2018), life stress (Zannas et al., 2015), BD (Fries et al., 2017), MDD (Han et al., 2018; Whalley et al., 2017) N.S. in SCZ (McKinney et al., 2017a) |
(Chen et al., 2016†; Christiansen et al., 2016; Hannum et al., 2013; Horvath, 2013; Marioni et al., 2015; Vetter et al., 2018) | |
| Transcriptomic ageΔ | RNA-seq | Positive/moderate to high | NA | (Huan et al., 2018; Peters et al., 2015) | |
| Proteomic ageΔ | Mass spectrometry | Positive/high | NA | (Menni et al., 2015; Tanaka et al., 2018) | |
| Metabolomic ageΔ | Mass spectrometry | Positive/high | NA | (Chaleckis et al., 2016; Hertel et al., 2016) | |
| GlycomicsΔ | Microarray and mass spectrometry | Positive/moderate to high | Altered in MDD (Boeck et al., 2018c; Park et al., 2018) GlycoAge Test was higher in PTSD (Moreno-Villanueva et al., 2013) |
(Krištić et al., 2014; Peng et al., 2018; Vanhooren et al., 2008; Yu et al., 2016) | |
| ccf-mtDNA | qPCR | Positive | Higher in plasma of suicidal patients (Lindqvist et al., 2016) and MDD (Lindqvist et al., 2018) Acutely elevated with induced psychological stress in plasma (Hummel et al., 2018) and serum (Trumpff et al., 2019) |
(Pinti et al., 2014) | |
| mtDNAcn | qPCR (whole blood) |
Inverse | Higher in BD (Fries et al., 2017) No evidence for an association with depression Increased in mixed psychiatric disorders |
(Mengel-From et al., 2014; Verhoeven et al., 2018; Zhang et al., 2017) |
Abbreviations: BD, bipolar disorder; BPD, borderline personality disorder; ccf-mtDNA, circulating cell-free mitochondrial DNA; MDD, major depressive disorder; MRI, magnetic resonance imaging; mtDNAcn, mitochondrial DNA copy number; NA, not applicable; N.S., not significant; PTSD, post-traumatic stress disorder; qPCR, quantitative polymerase chain reaction; Q-FISH, quantitative fluorescent in situ hybridization; SCZ, schizophrenia. Note that correlation coefficients (r) for biological age indicators and chronological age is dependent on the age range within each dataset and should not be use as sole metric of accuracy. Criteria used for size of r: High: 0.7-0.9 (−0.7 to −0.9); Moderate: 0.5-0.7 (−0.5 to −0.7); Low: 0.3– 0.5 (−0.3 to −0.5); Negligible: 0 - 0.3 (−0.3 to 0). Effect size reflects correlation coefficient reported in the original publications of the multivariate composites, indicated byΔ. Systematic reviews and meta-analytic studies are indicated by†
2.1. General limitations common to biological age indicators
There are limitations inherent to existing biological age indicators: they rely on specific organs or tissue types, can be confounded by cell type heterogeneity, and represent static measures of dynamic states (Figure 3). These limitations apply to most molecular biological age indicators and should represent the foundation from which we design research projects, and interpret findings. However, these limitations are often not well understood and, instead, only considered post-hoc once data is collected and is being analyzed. Here, to be consistent with the logic whereby limitations inform research design, method development, and data interpretation, we discuss these challenges prior to the literature review. Recommendations to overcome some of these limitations are also discussed in Section 5.
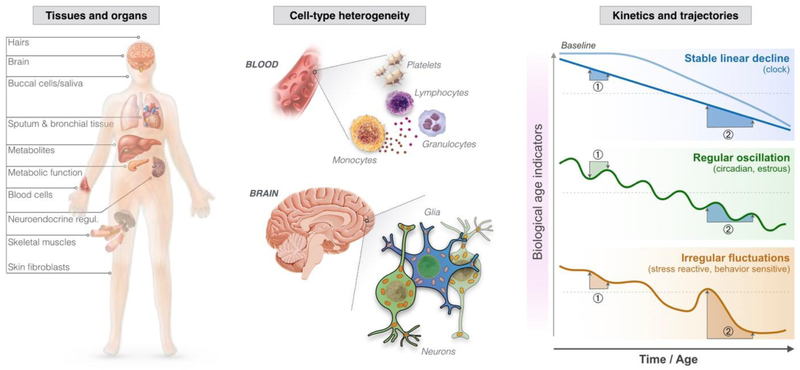
Figure 3. Major limitations of biological age indicators.
(Left) Biological age indicators are measured in samples from different tissues and organs of the body. (Middle) Individual tissues such as blood and the cortical regions of the brain also exhibit substantial heterogeneity marked by relative abundance of different cell-types. (Right) Three hypothetical kinetics for biological age indicators are shown: The first shows a stable decline, which is typically assumed, but not necessarily accurate for most biological age indicators. The second illustrates an indicator subject to either circadian regulation or monthly estrous cycle, thus exhibiting regular oscillations. One such example is cortisol. Knowledge of this oscillatory pattern can be used to adequately schedule time of sampling (e.g., morning or evening) and derive useful parameters (e.g., cortisol awakening response). The third indicator shows irregular fluctuations, which could arise from sensitivity to acute stress mediators or to behavior (exercise, sleep, or other). Note the two hypothetical pairs of assessments on each trajectory. Mis-timing of measurement ① for the regularly oscillating measure leads to pseudo-reversal of the biological aging indicator. Assessment ② shows an exaggerated decline in the irregular fluctuation.
2.1.1. Organs and tissue types
A common limitation of biological age indicators is that they are generally measured in one particular tissue and then used as a general age estimator for the person from whom the sample was obtained. However, it is unlikely that every tissue - peripheral and central - are entirely synchronized and that one tissue accurately reflects the biological age of all other tissues. Tissues from multiple organs have been found to age at different rates in terms of epigenetic age (Horvath et al., 2015). With age, mtDNA mutations also accumulate differently between brain regions (Bender et al., 2006; Corral-Debrinski et al., 1992; Picard and Hirano, 2016) and even between cells of a given organ (Vincent et al., 2018). Within the brain, TL also varies between different cortical areas (Mamdani et al., 2015). This limitation may also apply to measures of functional capacity. For example, there is relatively poor agreement between muscle strength measured from handgrip or knee extension, suggesting that the most commonly used metric of muscle strength - handgrip strength - is not a proxy for overall muscle strength (Yeung et al., 2018). Developing approaches to measure and perhaps capitalize on the heterogeneous nature of aging dynamics across tissues requires further research.
2.1.2. Cellular composition and heterogeneity
Tissues such as the brain, heart, muscles, and the blood are composed of multiple different cell types. Although all cell types have the same genome, they show unique epigenetic, morphological, functional, and molecular differences relevant to biological age indicators. For example, different blood cell types have different epigenomes (Reinius et al., 2012), telomerase activity and TL (Boeck et al., 2018b, 2018d; Lin et al., 2010), and mitochondrial respiratory capacity (Chacko et al., 2013). These intrinsic differences are inevitable and may introduce bias and confound findings when assessed individually. Similar limitations of cellular heterogeneity also apply to saliva, skin, and any tissue such as placenta, brain, and others. However, in many cases, the relative cellular composition of these tissues is poorly characterized or methods may not be available to effectively disentangle cellular composition effects, relative to the true biological aging signal.
2.1.3. Static indicators of dynamic processes
Most biological age indicators reflect the current state of the organism, and the biological age of the sampled tissue and cells, at the moment of collection. In many cases, it is unknown how dynamic these markers are. In other words, how much they change from day-to-day, across the day (i.e., diurnal variation), or sometimes even within minutes, as is the case for neuroendocrine mediators and blood-based metabolites. It is generally assumed that most biological age indicators (and the specific measures that compose some of them) are largely stable, changing slowly over the course of years, but that assumption has gone untested or proven false for most indicators listed in Table 1. Unrecognized, unmeasured, or uncontrolled variability of biological age indicators due to regular or irregular changes over time has two undesirable effects: it introduces noise that cannot be accounted for, and possibly limits our interpretation of the downstream result. Biological age indicators may follow different trajectories over time and may theoretically be differentially sensitive to behaviors such as sleep, exercise, diet, meditation, and others. Studies with frequently – over hours, days, months, and years – repeated measures of biological age indicators will be necessary to establish the temporal kinetics for existing and new biological age indicators.
2.2. Specific limitations to measurements of biological aging
2.2.1. Limitations of functional capacity and physiological measures
A major advantage of functional capacity and physiological measures is the high efficiency in terms of costs and collection as well as their integrative informativeness, specifically compared to blood-based, molecular and DNA-based measurements. However, the predictive value of objective functional and physiological measures on mortality has mostly been reported in older populations (Cooper et al. 2010) and in middle-aged populations (Rantanen et al., 2000). Except for some data reporting associations between handgrip strength and mortality in male adolescents (Ortega et al., 2012), whether functional capacity measures such as walking speed are sensitive to aging and predictive of morbidity and mortality in younger populations largely remain to be established.
2.2.2. Limitations of brain function- and structure-based measures
Normal aging is accompanied by brain atrophy and loss of brain tissue volume, which can be quantified non-invasively with magnetic resonance imaging (MRI). Voxel-based morphometry and surface-based analysis are two commonly used image preprocessing techniques, which may yield divergent results (Chung et al., 2017; Clarkson et al., 2011). Moreover, the macroscopic volumetric changes observed in T1- and T2-weighted MR imaging reflect microscopic changes at the tissue and cellular levels, and in many circumstances possibly represent an aggregate of multiple cellular mechanisms related to synapses, neurons, and glial cells (Tardif et al., 2016). Thus, what changes in brain volume represent is not fully understood.
Age-related differences in brain function can also be detected with functional connectivity (e.g., Geerligs et al., 2015) and novel analytics on brain response are also available. For example, Garrett and colleagues (2010) showed that the age-predictive power of the brain’s signal variability was five times higher than that of the conventional method of assessing the average signal across time. But functional MRI data has low signal-to-noise ratio, and movement artifacts are one source of such noise. Because individuals of different ages might move differently in response to assessments, movement artifacts are a possible confounder in many study designs. Implementing methods to systematically review individual participants’ images and manually separating noise from signal (Griffanti et al., 2017) could be a useful technique to minimize artifacts. There is evidence that manually cleaned BOLD-fMRI data, compared to data preprocessed with conventional automatic methods, better predicts chronological age (Garrett et al.,2010), emphasizing the importance of data quality and pre-processing procedures in conclusions derived from brain-based age indicators.
A popular approach in neuroscience is to use statistical approaches to translate complex whole-brain multivariate patterns of aging into a single outcome (Luders et al., 2016), the so-called “brain age” (see Cole and Franke, 2017 for details). Brain age algorithms (Aycheh et al., 2018; Ball et al., 2017; Cole et al., 2016; Gaser et al., 2013; Liem, 2016; Schnack et al., 2016) generate accurate individual age predictions in healthy controls, but show greater prediction errors when applied to patient groups (Cole et al., 2018). Within this framework, neuropathology may be reflected by the trajectory of aberrant normal aging, rather than a different deteriorating pattern of pathology. Gutierrez Becker and colleagues (2018) show that Gaussian Process uncertainty in age estimation may yield a better separation between cases and healthy individuals than the prediction error. Nevertheless, brain age models have high reliability in terms of test-retest performance at both same and different scanners (Cole et al., 2016; Franke and Gaser, 2012), and have shown biologically meaningful associations with health, clinical, and neuropsychiatric phenotypes (Cole et al., 2018).
2.2.3. Limitations of cellular measures
Although cellular measures of aging have been used widely in the laboratory setting, they are seldom applied to human (clinical, epidemiological) research. For instance, replicative senescence, a cellular measure of aging, involves monitoring cells grown in culture, and counting the number of cells over time (e.g., Lawless et al., 2010). This enables the investigator to count the number of times cells divide (i.e., total population doublings or “hayflick limit”), and determine the time required per cell division (i.e., population doubling time), which increases as cells age and divide more slowly. It should be noted that although senescence - defined as the loss of the ability of a cell to grow or divide - is associated with aging, it is not equivalent and can be dissociated from chronological age. Indeed, other factors such as irradiation can specifically induce senescence, even in chronologically young cells, thus reflecting biological aging.
The assessment of cellular bioenergetics, particularly mitochondrial content and functions, represents another domain of cellular aging measures. These indicators reflect the ability of cells to generate energy through oxygen-dependent mechanisms (for a review, see Picard and McEwen, 2018a). Respiration can be measured in whole cells (Boeck et al., 2018d) where it mostly reflects cellular energy demand, or in permeabilized cells (Ehinger et al., 2015) and isolated mitochondria where the intrinsic function of the organelle can be directly assessed independent of cellular contributions (Tyrrell et al., 2015). A major limitation of functional measures on intact cells and mitochondria is that measurements must be performed rapidly after blood sampling (within minutes to hours), which limits throughput and increases technical variability between samples, requiring exceptional standardization of procedures.
Other approaches relying on lysates (homogenized cells or mitochondria) from frozen samples allow measurement of enzymatic activity (e.g., telomerase, mitochondrial respiratory chain complexes) for multiple samples at once or in large batches (Picard et al., 2018). An ubiquitous limitation to all measures of biological activity (as opposed to inert molecules) is the degradation of cellular and enzymatic activities over time when samples are stored under suboptimal conditions. An important unknown in this field is the degree to which storage conditions, and especially the length of time a sample resides in a freezer, contributes to changes in assay results. This issue is worthy of applied investigation, since there is commonly a trade-off between freezing samples for shorter periods of time vs. freezing samples for longer periods of time to minimize inter-assay variability in assaying sequential frozen batches. In contrast to molecular analytes that are mostly or fully preserved at −80°C, samples destined for functional measurements should be stored in liquid nitrogen (< −150°C).
2.2.4. Limitations of molecular measures
A large fraction of the most widely used biological age indicators are molecular in nature. They include DNA methylation (DNAm), metabolites, proteins, TL, mtDNAcn, circulating cell-free mtDNA (ccf-mtDNA), mtDNA damage, and others. One important consideration to all molecular measures is that inadequate handling of fresh samples can alter the concentration of various analytes, particularly metabolites. For example, whereas DNA markers are believed to be quite stable over minute to days, blood glucose concentration decreases within minutes when the blood is left at room temperature (Chan et al., 1989), owing to metabolic activities of white and red blood cells. The same must also apply to other metabolites that are detected by metabolomics. Gene expression assessed from messenger RNA transcript levels is also subject to rapid degradation and special care must be applied to blood destined to transcriptomic analyses (Kågedal et al., 2005). These effects are minimized by rapid separation of the liquid and cellular components of whole blood by centrifugation immediately after blood draw, refrigeration (immediate storage of samples on wet ice, 4°C), and subsequently freezing biological samples in a timely fashion.
Below we discuss specific molecular biological age indicators that have been subject of considerable research in relation to stress and psychopathology. Although exciting new findings from proteomic (Menni et al., 2015; Tanaka et al., 2018) and metabolomic (Chaleckis et al., 2016; Hertel et al.,signatures of aging are beginning to arise, they have not been examined in relation to psychological factors. In this section, we focus our discussion on DNAm, TL, and mtDNAcn.
2.2.4.1. DNA methylation and epigenetic age
To date, several epigenetic age estimators have been developed from e.g. whole blood (Hannum et al., 2013; Levine et al., 2018; Weidner et al., 2014), neonatal cord blood and blood spots (Knight et al., 2017, and skin and blood cells (Horvath et al., 2018). Many potential confounders may cause technical variation in DNAm studies, of which population stratification and genetic ancestry are major contributors (Heijmans and Mill, 2012; Ratanatharathorn et al., 2017; Susser et al., 2016). Therefore, there is also reason to assume that genetic variation impacts epigenetic age estimates, particularly considering recent studies that report strong genetic links (Lu et al., 2018). Other potential confounders include smoking(Elliott et al., 2014), sex, and prenatal factors (Simpkin et al., 2016). Technically, DNAm arrays can show large variations between individual arrays and batches, and methods have been designed to statistically correct for these prior to analyses (Akulenko et al., 2016; Price and Robinson, 2018).
While the validity of different methylation-based predictors is questioned, applications of the original pan-tissue Horvath clock (Horvath, 2013) have been successful across tissue types and proven accurate even in embryonic brain samples (Spiers et al., 2015). The latest developed skin and blood predictor seems to be even more robust across tissue types (Horvath et al., 2018). More recently, methods using as little as 3-10 CpG sites from blood samples also accurately predict age (Li et al., 2018; Weidner et al., 2014) and mortality risk scores (Gao et al., 2018).
Whether statistical adjustment for cell type composition should be uniformly applied to whole blood-derived DNA to achieve optimal age prediction is the subject of ongoing debate. Whereas it has been argued that the Horvath method incorporates the estimation of cell type composition from blood and may not require cell type adjustment, some have shown that intrinsic (without adjustment for cell type composition) and extrinsic (with adjustment for cell type composition) age estimates may have a different biological meaning (Chen et al., 2017). Also important to mention here is that other DNAm-based indicators of aging have been developed that are trained on phenotypic markers of age (DNAm PhenoAge) rather than chronological age, leading to improved predicted risk of mortality (Levine et al., 2018). Briefly, phenotypic age is a combination of chronological age and nine disease-related biomarkers selected based on their association with mortality. The sensitivity of epigenetic predictors to psychosocial stress and psychopathology remains a gap of knowledge.
2.2.4.2. Telomere length
Issues related to TL assessments have been presented elsewhere (Aubert et al., 2012) and will not be discussed in detail here. In general, we will note that multiple different assays exist, which vary in their cost, required volume, applicability on frozen samples, and throughput. Technically, these are important considerations that impact the feasibility of clinical and epidemiological studies. Moreover, because of the relatively inexpensive and high-throughput capacity of qPCR-based methods, TL has frequently been measured on total DNA extracted from cell mixtures, which can be derived from a variety of sources such as buccal swabs (which include both epithelial cells and leukocytes) and whole blood (which include a variety of leukocytes). For these measurements, and as for mtDNA measurements below, the limitations presented in Section 2.1 are particularly important.
2.2.4.3. mtDNA copy number and circulating cell-free mtDNA
Counting the number of mtDNA molecules per cell, or mtDNAcn, can indirectly provide an indication of the bioenergetic state of the cell. The mtDNAcn measurements are based on either qPCR (e.g., Tyrka et al., 2016) or derived from whole exome or genome sequencing data (e.g., Cai et al., 2015) where “counts” of both the mitochondrial and nuclear genomes are estimated. The ratio of mtDNA and nuclear DNA (nDNA) is then multiplied by 2 to account for the diploid nature of the nuclear genome and taken as mtDNAcn (Malik et al., 2011). Here, given that cells with different metabolic demand can differ by as much as an order of magnitude in their content of mtDNA, cell type differences may have a particularly profound effect on this measure. When applied to a homogenous cell population, mtDNAcn can provide valuable information. However, most reported studies with mtDNAcn have relied on whole blood DNA, which is confounded by cell type heterogeneity, and by the presence of platelets. Platelets do not have nDNA, but have mtDNA, which artificially inflates mtDNA copy number in whole blood preparations (Hurtado-Roca et al., 2016; Urata et al., 2008). In tissues with less heterogeneity than blood, such as skeletal muscle, some have observed no difference in mtDNAcn between young and old individuals (Miller et al., 2003). The rate of decline in mtDNAcn per year also varies widely between studies, possibly as a result of differences in methodology and tissue source.
Similarly, measures of ccf-mtDNA are sensitive to cellular contamination and biological sample used. Serum (post-coagulation fraction of whole blood) may contain substantially more ccf-mtDNA than plasma (liquid fraction collected with an anticoagulant) (Xia et al., 2009). Sufficient centrifugation speed and time are required to successfully eliminate cells, particularly platelets, that could artificially inflate serum or plasma ccf-mtDNA (Nakahira et al., 2013). In studies where blood samples were not centrifuged at sufficient speeds, ccf-mtDNA levels are reportedly higher, making platelet contamination the most likely contributor to measured mtDNA levels and thus complicating interpretation of these results.
2.3. Associations among biological age indicators
While there is preliminary evidence for some cross-correlations among the different biological aging indicators, few have been examined in relation to other indicators. This highlights the need for examining multiple markers in an integrative study, as e.g. Belsky et al. (2018) recently showed low agreement between eleven quantifications of biological aging, with only modest associations to e.g. physical functioning and cognitive decline. Other studies suggest that TL is correlated to mtDNAcn (Tyrka et al., 2015), but the direction of stress and psychopathology effects with mtDNAcn or mitochondrial content (citrate synthase) and TL may vary (Boeck et al., 2018d; Cai et al., 2015; Picard et al., 2018; Tyrka et al., 2016). TL is not correlated with epigenetic age (Breitling et al., 2016; Han et al., 2018; Marioni et al., 2016), although cell type composition adjustments may reveal a modest association (Chen et al., 2017). Both epigenetic age and TL seem uncorrelated to brain age, and no associations were found between brain predicted age difference (brain-PAD) and epigenetic predicted age difference (Cole et al., 2017). The correlations between the Hannum and Horvath clocks vary from relatively strong (r=0.76) to low (r=0.37) in independent studies (Belsky et al., 2018; Chen et al., 2016), and both clocks showed modest correlations (0.10-0.33) to the transcriptomic age indicator by Peters et al. (2015). The microRNA age indicator of Huan et al. (2018) was modestly correlated to epigenetic age (r=0.3) and microRNA expression (r=0.2). Cross-correlations between metabolomic/proteomic aging and other biological aging indicators remain to be explored.
3. Do psychological stress and psychopathology influence biological aging?
Nearly 15 years have elapsed since Epel and colleagues first described the association of psychosocial stress with short leukocyte telomeres in a sample of healthy premenopausal mothers of a chronically ill child and mothers of healthy children (Epel et al., 2004). The association of shortened telomeres with stress exposure has since been replicated in a wide variety of studies, and this observation stimulated several related lines of research examining the relationship between various forms of stress exposure or perceived stress and TL across the lifespan. Several excellent qualitative reviews describe and critically review this literature (Entringer et al., 2018; Epel and Prather, 2018; Price et al., 2013; Shalev et al., 2013; Shields and Slavich, 2017) and meta-analyses now quantify the magnitude of these associations and identify potential moderators of effects (e.g., Hanssen et al., 2017; Mathur et al., 2016; Ridout et al., 2018, 2016; Schutte and Malouff, 2015, 2014). More recently, an appreciation of the role of mitochondria in the acute stress response and chronic allostatic load (Picard et al., 2014; Picard and McEwen, 2018a, 2018b) has led to investigations of the association of psychological states, stress exposure in relation to mitochondrial functions and mtDNA (Boeck et al., 2016; Cai et al., 2015; Picard et al., 2018; Tyrka et al., 2016). Rather than being a comprehensive review, this section provides a brief overview of this field, emphasizing recent developments.
3.1. What is (psychological) stress?
Broadly defined, “stress” is the condition of being subjected to a stimulus (i.e., stressor) that invokes a response requiring the use of resources to adapt or cope (Monroe, 2008; Shields and Slavich,2017). “Stress” may refer to particular life events (e.g., job loss, death of a loved one, assault), contexts that are experienced as stressful or contain numerous stressors (e.g., poverty, neighborhood violence, famine) or the psychological or biological response to such an event or exposure (i.e., stress response, perceived stress) (Epel et al., 2018). Characteristics of the stressor(s) and the stress response may be important determinants of the biological response or adaptation and account for heterogeneity in the literature on stress and aging.
While some stressors occur in isolation, it is important to recognize that stress-inducing contexts, exposures, and perceptions of stress often covary, for example when families living in poverty experience neighborhood violence and feel unsafe. In addition, the level of perceived stress may vary substantially within a group exposed to the same stressor. Determinants of psychological and biological stress responses include the nature of the stressor in terms of type, scope, severity, chronicity, and how predictable and controllable the stressor is. Individual and social characteristics influencing the level of perceived stress include social, financial, cognitive, emotional, and behavioral resources for coping with, controlling, avoiding, and compensating for stressors (Epel et al., 2018).
3.2. Stress and biological aging: Evidence for a stress-aging axis involving telomeres and mitochondria
The literature on the association of stressors and perceived stress on TL is now sufficiently large that a number of meta-analyses have been conducted on the topic. Meta-analyses of the association between TL and childhood psychosocial stressors document significant effects that vary from small to medium in size (Hanssen et al., 2017; Li et al., 2017; Ridout et al., 2018). Moderator analyses suggest larger effects for studies that examine more severe exposures (Hanssen et al., 2017) and those that include wide range of adversity types (Ridout et al., 2018). In addition to cross-sectional investigations, a longitudinal study (Shalev et al., 2012) found that exposure to violence over a 5-year period in childhood predicted greater TL attrition, suggesting the possibility of a causal relationship. The biological mechanisms whereby adverse or positive experiences exert their lasting health effects remain mostly unknown. Effects on the germline and stem cells reserves, metabolic reprogramming, and rewiring of neural networks and brain circuitry are among many areas that deserve further research.
Turning to stressors that occur in adulthood, a significant association between perceived stress and shorter TL has been documented in meta-analyses, though this effect ranged from very small (Mathur et al., 2016) to modest (Schutte and Malouff, 2016) in size. Several studies have also shown associations of shorter TL with measures of severe or cumulative stress exposure in adulthood (for reviews see Shalev et al, 2013; Epel & Prather, 2018; Oliveira et al., 2016). Although some studies suggest that childhood adversity may account for associations between TL and adult stressors (Puterman et al., 2016; Revesz et al., 2016), there is also evidence that stressors experienced in adulthood prospectively predict telomere attrition (Puterman et al., 2015; Van Ockenburg et al., 2015).
Given the growing literature demonstrating the central role that mitochondria play in the stress response and the aging process, recent studies have examined the association of early life stress with measures of mitochondrial function or mtDNAcn. Although mtDNAcn is not a measure of mitochondrial function and is impossible to interpret on its own, it is easily measured from stored DNA and has been measured in different studies. For example, childhood trauma or adversity, as well as adult psychopathology have been linked to higher mtDNAcn (Cai et al., 2015; Tyrka et al., 2016). In a small study of postpartum women, early life adversity was associated with greater cellular respiration reflecting increased cellular energy demand, which in turn was positively correlated with levels of pro-inflammatory cytokines and childhood maltreatment (Boeck et al., 2016). In a study of caregiving stress, caregivers were found to have reductions in a functional index of mitochondrial health (MHI) in blood leukocytes. Mitochondrial health was operationalized as a multivariate index designed to reflect functional capacity on a “per mitochondrion” basis. In this first study of MHI in mixed human leukocytes, the index included biochemical enzymatic activities for three mitochondrial enzymes and mtDNAcn. Using this composite index as an outcome, this study found that positive mood was associated with higher MHI and was a mediator of the association between caregiving and MHI (Picard et al., 2018). Another study found that suicide attempters have significantly higher plasma levels of ccf-mtDNA (Lindqvist et al., 2016) and another study found elevated ccf-mtDNA levels in individuals with major depressive disorder (MDD) (Lindqvist et al., 2018).
A limitation of this body of research is that certain behavioral, psychiatric and medical conditions frequently co-occur with stress exposures and covary with TL and other biological processes central to aging, and thus may have confounding effects. These include smoking, obesity, dietary influences, anxiety, depressed mood, post-traumatic stress disorder (PTSD), medications, and cardiometabolic conditions, among others. These influences are not uniformly assessed, excluded, or statistically controlled. A meta-analysis on the association of early adversity and TL identified that the magnitude of the effect was smaller in studies that included participants with medical or psychiatric conditions, and participants on medications (Ridout et al., 2018). This finding suggests that the relationship between these conditions and shortened telomeres might obscure the effect of stress exposure, or, alternatively, that the psychiatric and medical conditions may be primarily responsible for some of the telomere effect (Epel & Prather, 2018). Thus, the complex inter-relationships among these exposures, behavioral factors, and health conditions should be carefully considered when designing studies and analyzing and interpreting results.
3.3. Psychopathology and biological aging
Psychiatric disorders are associated with increased risk of aging-related medical conditions, including cardiovascular disease, stroke, dementia, diabetes, and obesity (Penninx et al., 2013; Viron and Stern, 2010), and early mortality (Walker et al., 2015). While part of the association may be explained by differences in health behaviors, because individuals with psychiatric disorders are more likely to smoke, drink alcohol, eat poorly, and exercise less than others (van Gool et al., 2007), associations between psychiatric disorder status and medical morbidity remain significant after adjusting for these factors. This has led to the hypothesis that psychiatric conditions may induce or result from accelerated or premature biological aging. As reviewed in this review, there are multiple biological age indicators, including a range of cellular and molecular measures such as TL, mitochondrial dysfunction, oxidative stress, gene expression, and others (López-Otín et al., 2013). Notably, inflammation is also a widely-used indicator of biological aging (Baylis et al., 2013), coined as “inflammaging” by Franceschi et al. (2000). However, because of its elaborate discussion elsewhere (Franceschi et al., 2018b; Fulop et al., 2018), as well as in respect to mental health (Diniz and Vieira, 2018), we do not discuss it here. Overall, the following section will review the most frequently studied biological age indicators in epidemiological psychiatric research, including TL, epigenetic age, brain age, and to a lesser extent pro-inflammatory cytokines.
3.3.1. Associations of psychiatric disorders and biological age indicators
Simon and colleagues (Simon et al., 2006) were the first to report a relationship between psychiatric disorders and shorter telomeres in a sample that included MDD, bipolar disorder (BD) and anxiety disorder patients. Since then, a large number of studies have been conducted in an assortment of psychiatric disorders. MDD is among the most frequently studied disorders in this context, possibly as a consequence of its relatively well-documented associations with dysregulated physical health (Penninx et al., 2013). Several meta-analyses, the largest one containing >34,000 subjects from 38 studies, summarized the results and provided consistent evidence of an inverse association between TL and depression, generally with small to medium effect sizes (Darrow et al., 2016; Ridout et al., 2016; Schutte and Malouff, 2015). Similar meta-analytic results were found for anxiety disorders (N>19,000) (Malouff and Schutte, 2017), and PTSD (N>3,800) (Li et al., 2017). BD, schizophrenia and other psychotic disorders have been less extensively examined. A meta-analysis including 1,100 subjects from 7 studies found no difference in TL between BD cases and controls (Colpo et al., 2015). Two meta-analyses on schizophrenia of 1,200 and 1,600 subjects, respectively, found small effects for TL differences (Polho et al., 2015; Rao et al., 2016).
The epigenetic age indicator is most frequently examined in individuals with PTSD. A metaanalysis using data from 9 cohorts (combined N=2,186) found significant, albeit small, associations of greater epigenetic age with traumatic stress, but not with PTSD diagnosis (Wolf et al., 2018). Other studies also found such relations using the Horvath predictor (Boks et al., 2015; Mehta et al., 2018; Zannas et al., 2015), consistent with enrichment for glucocorticoid response elements (Zannas et al., 2015). Two recent studies for the first time showed “older” epigenetic age in MDD patients versus controls (Han et al., 2018; Whalley et al., 2017), while this is not seen in schizophrenia (McKinney et al., 2017b; Voisey et al., 2017).
Furthermore, chronic, low-grade inflammation that increases on average with age is captured in the term “inflammaging” - a pro-inflammatory state proposed to contribute to the pathogenesis of age- related diseases (Franceschi et al., 2018a). An increase in the inflammatory response, together with microglial activation, in turn, can contribute to psychiatric diseases, such as MDD, schizophrenia, BD, and autism (Khandaker et al., 2015; Martiníez-Cengotitabengoa et al., 2016; Réus et al., 2015). The mechanisms responsible for increased inflammation in mood disorders remains poorly understood.Recent evidence suggests that ccf-mtDNA could contribute to this pro-inflammatory state, although evidence is mixed (Kageyama et al., 2018; Lindqvist et al., 2018, 2016). Related to the bacterial origin of mitochondria, the mtDNA is immunogenic. Released mtDNA molecules thereby act as damage associated molecular patterns (DAMPs) recognized by toll-like receptors on immune cells and trigger immune cell activation (West and Shadel, 2017). Inflammaging could in part be due to increased ccf-mtDNA in older individuals (Pinti et al., 2014).
Increased plasma levels of ccf-mtDNA have been reported in suicidal and depressed patients (Lindqvist et al., 2016). Worse response to an antidepressant was associated with increasing ccf-mtDNA levels over the treatment course, and ccf-mtDNA was correlated with antioxidant enzyme glutathione peroxidase, possibly as a result of a compensatory response to cellular oxidative stress (Lindqvist et al., 2018). An experimental study using psychological stress induction in healthy middle-aged individuals also demonstrated that an acute bout of psychological stress may be sufficient to elicit a 2-3 fold increase in serum ccf-mtDNA within 30 minutes, suggesting that ccf-mtDNA is dynamically regulated (Trumpff et al., 2019). Consistent with previous findings linking ccf-mtDNA levels to cortisol levels following a dexamethasone suppression test (Lindqvist et al., 2016), glucocorticoid stimulation of human cells (fibroblasts) induced the release of mtDNA by mitochondria within minutes (Trumpff et al., 2019). Thus, the causes of elevated ccf-mtDNA in certain psychiatric conditions remain unknown, although clinical and cellular studies suggest that canonical neuroendocrine stress mediators, including but not limited to glucocorticoids, may be implicated.
In addition, associations have been reported between “older” brain age and psychiatric disorders such as borderline personality disorder (Nenadic et al., 2017), schizophrenia (Koutsouleris et al., 2014; Nenadic et al., 2017; Schnack et al., 2016), and first-episode psychosis (Kolenic et al., 2018), as compared to younger in BD (Nenadic et al., 2017). One relatively small study by Koutsouleris et al. (2014) showed a higher brain-PAD of +4.0 years in MDD (N=104). However, a preliminary study by Kaufmann et al. (2018) finds increased brain age in schizophrenia (Cohen’s d=0.55) and BD (d=0.30), but not MDD (N=211, d=0.10). In addition, these authors suggest that the brain age gap is a genetically modulated trait that is heritable and overlaps with polygenic architecture observed in common brain disorders. There is also preliminary evidence of an association between psychiatric pathology and glycomic-based biological age indicators, which represent sugar-based modifications of proteins, RNA, and DNA molecules. Two studies have shown altered protein N-glycosylation profiles in female patients with MDD (Boeck et al., 2018c) and in PTSD (Moreno-Villanueva et al., 2013), indicative of advanced aging at the glycomic level. Associations with other omics-based indicators (transcriptomics, proteomics, metabolomics) and their interrelation remain to be explored.
3.3.2. Biological aging and psychopathology: The chicken or the egg?
While robust cross-sectional associations between psychiatric disorders and biological aging have been documented - at least for TL - the nature and direction of these associations remain unclear. Longitudinal studies have found mixed effects (see, e.g. Boks et al., 2015; Maniates et al., 2018; Shalev et al., 2014; Vance et al., 2018; Verhoeven et al., 2016). It is currently unknown whether 1) psychopathology-associated physiological disturbances accelerate biological aging, 2) premature biological aging antedates and is a vulnerability factor that causes psychopathology, or alternatively, 3) psychopathology and biological aging processes share underlying etiological roots, such as shared genetic risks, and happen to be correlated without a causal link between them. Recent studies using genomic and causal inference tools are being developed to overcome this limitation of observational studies (e.g., Wium-Andersen et al., 2017). This challenge, among others, as well as recommendations to move the field towards a predictive science are discussed in details in Section 5.
4. Clinical implications
4.1. Disorder-specific or transdiagnostic phenomenon?
In a large meta-analysis considering multiple psychiatric disorders and TL, including depressive and anxiety disorders, PTSD, bipolar and psychotic disorder, no difference in effect sizes between disorders was found (Darrow et al., 2016). This indicates that different DSM-based diagnoses may not be associated with meaningful differences in biological aging (Lindqvist et al., 2015). Furthermore, several studies showed that short TL is associated with the same physiological dysregulations that are found in some but not all persons with psychiatric disorders. These include increased inflammation, oxidative stress markers, dysregulated HPA-axis, and metabolic dysregulations (Lee et al., 2011; Monickaraj et al., 2012; Wikgren et al., 2012; Zhang et al., 2016). While the degree to which telomeres are causally related to these mechanisms is unknown, this is suggestive of pathways through which telomere shortening and psychiatric disorders are interrelated, that are not limited to one diagnostic category. The current evidence suggests that short TL may be a non-specific biological marker for conditions in which people experience chronic psychological or physiological stress, rather than being a marker of a specific psychiatric condition. Similarly, the downstream biological ramifications of different disorders also overlap, with alterations at the organs and systems level (e.g. brain aging patterns, Cole, 2018). This evidence leaves us to consider that these indicators are not disease-specific nor suitable as diagnostic tools, but rather general indicators of psychopathology or abnormal mental states.
4.2. Biological age indicators as predictors of treatment outcome
Only a small number of studies have investigated whether biological aging indicators predict antidepressant treatment response. The first study suggesting such a link showed in a small sample of previously unmedicated MDD subjects that low baseline telomerase activity, and a greater increase in telomerase activity during eight weeks of selective serotonin reuptake inhibitors (SSRI) treatment were associated to superior clinical outcome (Wolkowitz et al., 2012). However, this study lacked a control condition, leaving open the possibility that naturally different clinical trajectories contributed to these effects. Nevertheless, these findings suggested that depressed patients with relatively low baseline telomerase activity may most benefit from therapies that may secondarily induce telomerase activation, and that telomerase activation may represent a mechanism of antidepressant action, consistent with several animal studies (reviewed in Bersani et al., 2015).
Subsequently, one human study found that shorter leukocyte TL predicted worse antidepressant response to an SSRI (Hough et al., 2016). To the extent that accelerated biological aging is associated with antidepressant response, this effect does not seem likely to be confined to a specific treatment modality or drug. Shorter TL may also predict worse antidepressant response to pioglitazone, which also has antidiabetic effects (Rasgon et al., 2016). Turning to other disorders, in a group of bipolar and schizophrenia/psychosis patients, lithium non-responders had shorter telomeres than responders (Martinsson et al., 2013), and similar associations were found linking short TL to poor treatment response (Li et al., 2015; Yu et al., 2008). No studies have investigated the association between TL and response to psychotherapy (e.g., cognitive behavioral therapy), although preliminary evidence suggests a positive correlation between mindfulness/meditation practices and telomere biology, including increased telomerase activity (Conklin et al., 2018; Schutte and Malouff, 2014). Furthermore, an increased inflammatory response may hamper the responsiveness to mood disorder treatments, and higher baseline inflammation may lead to treatment resistance (Réus et al., 2015; Strawbridge et al., 2015). Changes in ccf-mtDNA levels were also found to be associated with SSRI treatment response, with the non-responders showing an increase in ccf-mtDNA and responders not changing (Lindqvist et al., 2018). A shared genetic disposition for inflammation, psychopathology, and treatment responsiveness has also been suggested (Zwicker et al., 2018). Furthermore, inflammatory indicators may potentially offer personalized antidepressant recommendations, and could eventually guide the development of novel antidepressant treatments (Jha and Trivedi, 2018). Overall, the link between biological age and treatment responsiveness is a young field requiring further research.
4.3. Can biological aging be reversed with treatment?
One of the most relevant clinical questions in the field of biological aging and psychiatry is whether accelerated biological aging is a permanent imprint or a reversible process. While this may differ between indicators of biological aging, reversibility is at least possible to some extent for some indicators. For TL, the main mechanism of restoration is likely telomerase activation. Animal and in-vitro research provided evidence that telomerase-associated recovery of TL is to some extent possible (Batista et al., 2011; Jaskelioff et al., 2011). Several intervention studies have attempted to influence TL in humans (see Verhoeven et al., 2014 for an overview). For example, recent small controlled studies have shown elongation of telomeres in response to highly controlled aerobic exercise verified with actigraphy (Puterman et al., 2018), losing and maintaining a weight loss of 10% or greater (Mason et al., 2018), and meditation-based interventions (Conklin et al., 2018). As mentioned above, for the field of psychiatry telomerase activation may be a mechanism of antidepressant action. Although no strong conclusions can be drawn due to a lack of well-powered clinical studies, there are several potential mechanisms by which psychiatric medications might modulate telomerase activity or TERT expression, including via increased brain-derived neurotrophic factor (BDNF) expression (Bersani et al., 2015). Increased telomerase activity may, in turn, induce clinical effects by promoting cellular survival and/or functioning. It should however be noted that increased telomerase activity can prevent cell senescence, an anti-cancer mechanism, and thus excessive telomerase activity is also associated with increased risk of cancer. Thus, while telomerase activation may hold the promise of reducing risk of aging-related disease, the risk of less common but very serious cancer outcomes must be carefully weighed (Blackburn et al., 2015).
Cross-sectional studies show that certain behaviors may provide some protection against brain aging. Higher physical activity levels are associated with lower brain age (Steffener et al., 2016), and “younger brains” are seen in those that learn to play an instrument (Rogenmoser et al., 2018) and those who have practiced meditation for long periods (Luders et al., 2016). It remains to be elucidated whether brain age is responsive to intervention, but a randomized controlled trial (RCT) showed that ibuprofen temporarily reduced brain-PAD by 1.1 years in healthy individuals, likely due to its acute anti-inflammatory effects (Le et al., 2018). Physical activity may slow age-related DNAm changes in humans (Ren et al., 2012; Voisin et al., 2015). Longitudinal data shows that increasing BMI is associated with increasing epigenetic age (Quach et al., 2017), but in another report, epigenetic age from the liver was not “decelerated” after successful weight loss over a 9-month period through bariatric surgery (Horvath et al., 2014). Quach et al. (2017) also found that consumption of fish, fruits, and vegetables, as well as effects of moderate alcohol, education, and income and exercise induced anti-aging effects based on epigenetic age (Hannum clock). However, these findings are cross-sectional observations rather than longitudinal effects from RCTs. Nonetheless, a recent RCT suggests that vitamin D supplementation may decrease epigenetic aging based on the Horvath, but not Hannum epigenetic clock (Chen et al., 2018).
More controlled intervention studies are needed to determine whether biological aging indicators are truly modifiable in response to exercise, nutritional and/or pharmacological interventions. A common problem to observational studies is that behaviors tend to correlate, making it difficult to evaluate the specific influence of a given intervention or behavior in isolation. Individuals who exercise more tend to practice meditation more frequently, eat more plant-based and vegetarian diets, consume less illicit substances, etc. An additional problem with observational studies is that it is impossible to establish the direction of effects; while exercise may attenuate indicators of aging, biologically younger individuals may be more able and inclined to exercise.
5. Key challenges and priorities for future research
There are key challenges to accurately measure and interpret biological age indicators and to further our understanding of stress and biological aging. A partial list of six major challenges related to priority areas for the field, as well as recommendations to overcome them is presented in this section. We also summarize essential steps and propose a minimum standard for the design, collection, processing, analysis, and reporting of data involving biological age indicators (Figure 4).
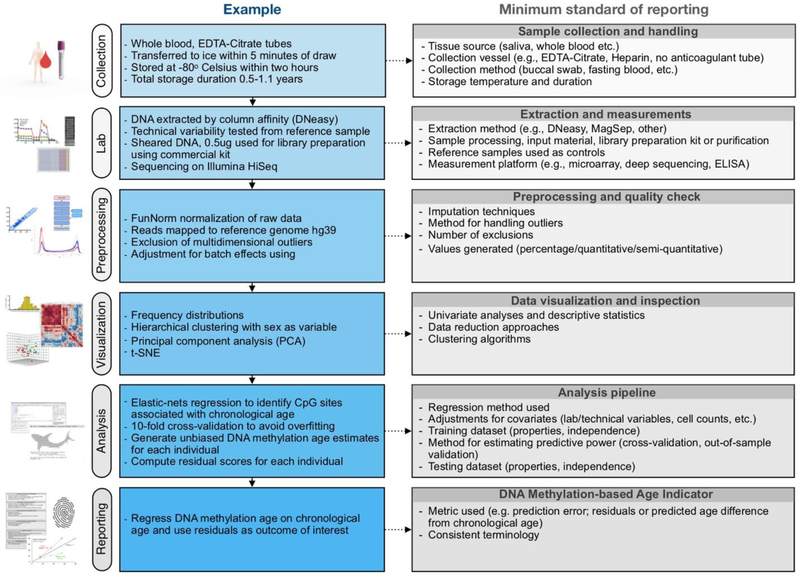
Figure 4. Overview of the biological age indicator prediction process and recommended minimum reporting guidelines.
(Left) Example workflow for calculating a epigenetic age indicator and age acceleration. (Right) Recommended workflow that can inform study design, execution, analysis, and preparation of methods section in the resulting reports. Adherence to such standards for reporting results would facilitate harmonization of datasets across laboratories and cohort studies.
Challenge 1. Correlation is not causation.
It is hazardous to infer causality from cross-sectional correlational data (Simons, 2015). For example, in the case of TL, it is possible that telomere shortening reflects states of stress, or responds to somatic or psychiatric illness, or at least to biochemical abnormalities associated with these states. It is also possible, however, that telomere shortening precedes somatic or psychiatric illness (Gotlib et al., 2014) or even underlies biological changes that causes these conditions. Biological age indicators could be entirely independent from directly assessing biological age mechanisms that drive the aging process. Changes in biological age indicators could be the “canary in the coal mine” (Effros, 2009), representing factors associated with aging rather than aging itself.
Recommendation 1: Collect data longitudinally, consider experimentation, and choose prediction over explanation.
This is a three-part recommendation. First, future studies should include longitudinal designs to increase the reliability and accuracy of measuring aging (Moffitt et al., 2017). Longitudinal measurements will also be important in determining which variables are critical for the maintenance of successful aging throughout the lifespan (e.g. absolute levels, change, variability), the “recipe” of which may vary between individuals (Sanders et al., 2012). Moreover, it will be critical to determine the optimal intervals of time between repeated assessments that are needed to detect meaningful changes in specific biological age indicators (see Figure 3). This information about the timing and spacing of repeated measures, and real-life constraints, should then be used to inform the design and choice of outcome measures when evaluating the effectiveness of interventions aiming to influence biological aging.
Second, experimental approaches utilizing cellular (or animal) models allow the direct manipulation of a specific (set of) variable(s). Thus, if we assume that a given stressor or predictor can be modeled accurately in vitro, experimental designs can provide direct causal evidence that a given factor is necessary and sufficient to produce a given outcome of interest. For this approach to empirically support a biological interaction between stressors and biological aging, the biological age indicator also needs to be detectable and meaningful in vitro, such as epigenetic age (e.g., Horvath et al., 2018). In cases where experimental demonstration is not possible, statistical methods such as causal inference (Bind et al., 2017 and genomic methods including Mendelian randomization (Burgess et al., 2012; Wium-Andersen et al., 2017) can substantially reinforce our confidence regarding the direction of effects.
Third, in some cases, the number of predictors one wishes to consider is very large, either because there is no prior knowledge of their relative importance, or because the problem is truly complex – such as human biological aging. In such cases, the number of predictors can be large relative to the number of individuals, providing insufficient power for traditional inference-based statistics. In such cases, machine learning-based predictive modeling may be advisable to discover and validate predictive relationships between variables. Whereas statistics draw population inferences from a sample, machine learning finds generalizable predictive patterns (Bzdok et al., 2018). Using predictive modeling approaches that identify and validate combinations of predictors in relation to a particular health outcome can increase the likelihood that the identified predictors of biological age are robust, specific, and generalizable. An excellent article on the value of prediction over explanation in the psychological sciences is Yarkoni and Westfall (2017). Regardless of the analytical approach taken, we should emphasize the value of converging evidence collected using different methods, measuring multiple (related and unrelated) predictors in parallel, and assessing multiple outcomes (Munafò and Davey Smith, 2018)
Challenge 2. Single biological age indicators are not correlated and may be better integrated.
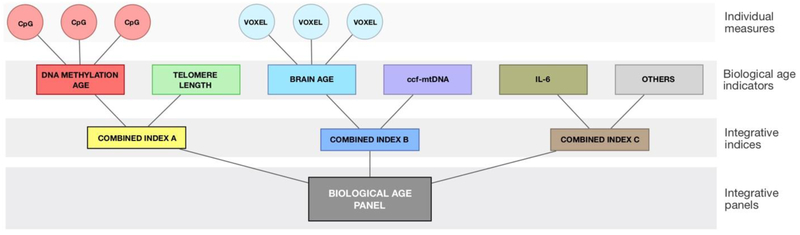
Not all measurements of biological aging are equally useful or inter-related, and it remains to be elucidated if and how different indicators relate to one another and which biological determinants are consistent across measures. As previously noted (Cole et al., 2018), no single biological age indicator can currently fully capture the complexity of the aging process, nor predict future health outcomes or lifespan with sufficient accuracy. There is therefore a need for combined indices that logically integrate multiple indicators (Figure 5), hopefully resulting in accurate integrative panels that outperform single measurements of biological aging, also previously suggested byXia et al. (2017). Nevertheless, integration of indicators cannot compensate for inadequately powered studies, which require large sample sizes to ensure high generalizability.
Figure 5. Topology of biological age indicators, upstream measures, and downstream integrative indices and panels.
Biologically-informed and functionally relevant composite indices integrating two or more indicators can be derived from individual indicators. Their added predictive power should be validated by either in-sample cross-validation or preferably out-of-sample validation. Integrative biological age panels may eventually outperform single biological age indicators and indices due to the strength of their association, their construct stability, and/or their greater generalizability across individuals and independent samples.
Recommendation 2: Combine machine learning and other artificial intelligence techniques to create composite indices and panels of biological age indicators relevant to mental health.
To date, several efforts to develop combined indices have been presented. For example, Marioni et al. (2016) showed an additive effect of combining TL and epigenetic age in explaining the proportion of age variance of their model. Similarly, Cole et al. (2017) explained significantly more variance in the prediction of mortality by combining brain-PAD and the Horvath epigenetic predicted age difference, than either indicator alone. Other examples include multivariate indicators of aging incorporating multiple physiological and functional measures (Belsky et al., 2015) and indices integrating multiple enzymatic and molecular measures of mitochondrial content and function in blood leukocytes as the MHI in association to psychological states (i.e., positive mood) (Picard et al., 2018). These constitute early attempts to reverse our reductionist inclinations and to move towards integrative metrics that will hopefully lead to improved prediction.
Challenge 3. Biological aging may be tissue- and cell-type specific.
Related to the above, biological age indicators derived from specific cell or tissue types may not generalize to other cells or tissues. Biomarkers assessed in blood, for example, represents the average of multiple heterogeneous cell types. But cellular or organismal health may be more closely related to or reflected by indicators within individual cell types or those with the most extreme values (e.g., Flow-FISH, Aubert et al., 2012). Furthermore, TL differs across leukocyte cell types, such as naïve vs mature T cells (Chou and Effros, 2013; Lin et al., 2016), and differs in different regions of the brain (Mamdani et al., 2015). Moreover, TL of particular cell types can be differentially vulnerable to attrition or affected by stress-related pathology (Boeck et al., 2018b).
Recommendation 3: Purify cell types using established molecular markers.
Purification of cell types can be accomplished by a variety of methods (flow cytometry, magnetic-activated cell sorting), yielding living cells amenable to downstream molecular and cellular analyses (Lin et al., 2010). Under certain conditions where it is not possible to isolate specific cell subtypes, it may be difficult to interpret certain indicators that exhibit large cell type-specific values (such as mtDNAcn in blood). In some limited cases where a lot of information is available for adjustment, such as for DNAm measured on bead chips (100,000’s of data points), it is possible to use statistical approaches, i.e. reference-based (Hattab et al., 2017) or reference-free (Houseman et al., 2014), to infer underlying cell type proportions (Titus et al., 2017) and adjust results accordingly. Another interesting application is demonstrated in a preliminary study by Chan et al. (2018) that uses deconvolution approaches to show novel MDD-methylation associations in individual sub-populations of neurons/glia from bulk brain, as well as in granulocytes/T-cells/B-cells/monocytes from bulk blood data. In addition, creative ways to harvest other cell types from tissues other than blood could yield increasingly meaningful biological age indicators. For example, the DNAm-based skin & blood clock by (Horvath et al., 2018) is robust across tissues (e.g. fibroblasts, buccal, endothelial, saliva samples) and can, therefore, be applied to many organs, as well as ex vivo. Thus, this approach provides extended information on synchronized biological aging, independent of sampling source.
Challenge 4. Within-group variance may be larger than between-group variance.
Although group differences in biological age indicators have been reported in several psychiatric illnesses (Darrow et al., 2016; Lindqvist et al., 2015), these reflect average group differences. However, there is often considerable within-groups variability and considerable overlap between groups, making it very difficult to use most biological age indicators as diagnostic aids. In addition, even specific psychopathological diagnoses (e.g., MDD or schizophrenia) often include individuals that vary widely in their symptoms and presentation, making the search for predictors of mental illnesses defined diagnostically somewhat elusive (Cuthbert and Insel, 2013). As Wolfers et al. (2018) suggest, the complex, highly polygenic and multifaceted causes of severe mental disorders may only be fully understood by mapping patients’ individual signatures, rather than studying the average patient. Population-based normal ranges have yet to be reliably determined, so biological age indicators may be more useful in detecting within-subject changes over time rather than in comparing individuals or in establishing actuarial norms. Nonetheless, a recent study utilizing flow-FISH assay techniques reported reproducible and definable upper and lower normal boundaries for TL in a hospital population (Alder et al., 2018), indicating that standardization of these measures may be achievable. Within-group variance is often due to measurable individual differences in behavior, health condition, and lifestyle.
Recommendation 4: Visualize your data, carefully assess known influences, move beyond group-based analyses, and use within-person modeling approaches.
To visualize data where there are multiple measures over time per individual, we advocate for spaghetti plots (example with epigenetic age and TL can be found in Marioni et al. (2016), and with cortisol trajectories in Ram and Grimm (2009). Standard measures of variance can also be used to model population variability at different timepoints and to compare sub-groups, and more sophisticated mathematical approaches can be useful to generate individualized phenotypes (Hertel et al., 2018). Data reduction approaches such as principal component analysis (PCA), partial least square discriminant analysis (PLS-DA), and t-distributed stochastic neighbor embedding (t-SNE) are also useful to visualize high-dimensional data in two or three dimensions and to assess whether subsets of individuals in the sample naturally cluster together (Maaten and Hinton, 2008; Xia et al., 2015). This kind of approach can provide evidence of shared phenotypes or trajectories that would otherwise remain undetectable by standard uni- or multivariate analyses. Other statistical approaches to identify different subgroups exhibiting different trajectories in biological aging or in clinical course include growth mixture modeling (Ram and Grimm, 2009), and random coefficients linear regression models to examine within-person changes over time (Diaz et al., 2018).
Challenge 5. Large sample sizes are needed to detect small effect sizes.
Related to the point above, group differences between individuals with certain psychiatric illnesses vs. healthy individuals, even if statistically significant, often have small effect sizes that require large sample sizes to be demonstrated (Darrow et al., 2016; Han et al., 2018; Verhoeven et al., 2014; Whalley et al., 2017). Similarly, even using predictive modeling and machine learning approaches, small samples sizes are more susceptible to overfitting (Yarkoni and Westfall, 2017). It would be informative to pool databases across studies and to check for consistency and predictive accuracy across studies, albeit at the cost of increasing heterogeneity of the samples studied and of the laboratory methods used. (Schnack and Kahn, 2016) argue that larger sample sizes will have more generalization power. This is important for creating robust “canonical” publicly available prediction models (e.g. Horvath’s epigenetic clock) that can be readily applied to smaller studies that cannot permit partitioning their data into a training and validation set.
Recommendation 5: Collaborate and harmonize data collection and analysis protocols to facilitate data pooling worldwide.
Certain types of data may be available through crowdsourcing, a data collection process with remarkable scalability that may enable identification of robust small effect size effects (Mohammadi, 2015). Large-scale collaborative initiatives like the Enhancing Neuroimaging Genetics through Meta-Analysis consortium (ENIGMA) for imaging and genetics data may be useful examples (Thompson et al., 2014). When not possible to replicate findings on sufficiently large independent datasets, use cross-validation methods to avoid overfitting (Yarkoni and Westfall, 2017).
Challenge 6. Non-uniform laboratory assays and storage conditions.
Different assay methodologies can yield relatively different results (e.g. qPCR vs. Southern blot for TL assessment, Aviv et al., 2011), and most assays such as Flow-FISH assays and qPCR ascertain relative TL rather than absolute length. These issues have been discussed in detail elsewhere (Aubert et al., 2012), and the relative merits of the different assays have been compared. A more mundane but important and under-appreciated caveat is that methodological differences can yield spurious results (e.g., length of time a specimen was kept in a freezer, freezer temperature, whether whole blood or intact cells vs. cellular lysate were frozen, specific batch of reagents used, assay technique, method of DNA extraction [for TL] (Dagnall et al., 2017; Khincha et al., 2017; Shiels, 2010). An important issue that has not yet been experimentally assessed in multi-year human studies is the relative merit of assaying batches of samples at short intervals (e.g., every year) to minimize freezer time, compared to keeping all samples frozen until the end of the entire multi-year study so that all samples can be assayed simultaneously using identical procedures and reagents.
Recommendation 6: Harmonize measurements and storage conditions - colder is better.
Follow guidelines, where available, to ensure that samples are collected and measured with the highest standards. Efforts are currently underway to systematically compare methods available to measure TL and will hopefully produce specific guidelines that can be implemented at a large scale. Figure 4 summarizes some measures that can be considered in the design, collection, pre-processing, analysis, and reporting of data to give researchers the ability to critically evaluate published results and hopefully harmonize methods and datasets.
6. Summary
Much progress has been made in the last decade towards developing objective biological age indicators but several key challenges and opportunities remain. Multiple new indicators spanning physiological and functional capacity, brain function and structure, and cellular and molecular levels of analysis have recently emerged, particularly under the force of ‘omics’ technologies. Most indicators show good to excellent correlations with chronological age, and some have the ability to predict age-related outcomes such as mortality with moderate accuracy (see Table 1). However, some have not yet been prospectively studied in large cohorts so their predictive power in relation to health outcomes remains unknown. In fact, many proposed biological age indicators still require replication and validation in larger independent datasets. As a whole, the development of multi-systemic biological age indicators has demonstrated that aging occurs not at a single level in a specific cell type, but rather manifests somewhat differently in various organs and tissues, cell types, and across a number of levels (see Figure 1). Rapidly developing methods show that biological age indicators gain in precision and prediction accuracy by leveraging biologically-informed approaches to integrate individual indicators into more powerful indices and panels of measures.
In relation to psychological stress and mental health, much work also remains to establish to what extent and how psychological states influence the aging process. Equally important to mapping these psycho-biological processes is to understand the “reverse” causal link whereby accelerated biological aging may impact physiological vulnerability and resilience to life stressors and psychopathology. A shared objective for our field is to disentangle these associations and identify the causal pathways that drive aging and mental health trajectories. To do so, we need to address number of key challenges that, if adequately met, will yield exciting opportunities to advance our understanding of human health.
To achieve this objective, it will also be essential for clinicians, psychologists, epidemiologists, and behavioral scientists to enter in a dialogue with experimental biologists. Such dialogue immediately opens new questions that would not otherwise arise within the silos of individual disciplines, departments, and laboratories. For example, the joining of ideas around psychosocial stress and cellular aging (Epel et al. 2004), age-related elevation in circulating DNA (Teo et al. 2018), and of psychoneuroimmunology and the pro-inflammatory effects of psychological stress (Marsland et al. 2017) has led to the idea that acute psychological stress may rapidly trigger immunogenic mtDNA release into the circulation (Trumpff et al. 2019). If met with sufficient enthusiasm and resources, these jointly-created interdisciplinary questions can subsequently push the development of new laboratory methods, statistical and analytical tools, and new theoretical models. The interdisciplinary field of human psychobiology is replete with opportunities for innovation and discoveries.
Overall, we believe that developing, validating, and studying predictive biological age indicators – and defining how psychological states or psychiatric illnesses influence them – will fill important knowledge gaps linking stress and mental illness to aging. Achieving this goal will have at least three main positive consequences for the biomedical sciences generally. First, it will enable the stratification of individuals (and group of individuals) based on their current health state and future disease risk, including symptoms, disorders. This notion converges with the precision medicine agenda that aims to identify individualized predictors of future mental health and disease states (Insel and Cuthbert, 2015; Abi-Dargham and Horga, 2016). Achieving this goal could benefit clinical practice by providing clear and objective guidelines to direct health-promoting interventions and treatment delivery in a personalized way, specifically one that is most aligned with the individual’s needs (Picard et al., 2013). Second, achieving this goal will provide objective and sensitive methods to evaluate the effectiveness of health-promoting interventions. The efficacy of interventions aimed at decreasing or even reversing advanced or premature aging, as well as interventions aimed at enhancing well-being and other health outcomes would be more effectively assessed using precise and sensitive biological age indicators. Finally, mapping stress-sensitive biological age indicators will also generate knowledge about human aging that can be taken back to the laboratory bench to orient basic biological science. Specifically, understanding the basis of normal and abnormal aging processes may identify new physiological targets for therapeutic intervention. Thus, an unintended consequence of improving existing biological age indicators and developing new end points for clinical and epidemiological human research may, in the end, create leaps in understanding about the fundamental biological mechanisms that explain why we age in the first place.
Highlights.
Stress and mental illness are prospectively associated with biological aging
Technical limitations can compromise the interpretation of biological age indicators
Integrating biological age indicators into composite indices improves their usefulness
Human psychobiology is an opportunity to elucidate basic aging processes
We highlight key challenges and suggest recommendations to overcome them
Acknowledgements
This paper was inspired by a focus group meeting hosted by J.E. Verhoeven and B.W.J.H. Penninx held at Amsterdam UMC, Vrije Universiteit, Department of Psychiatry in Amsterdam in June 2018. The authors are grateful to investigators and patients whose work has energized this review, and to the anonymous reviewers for their constructive criticisms. Work of the authors is supported by the Wharton Fund, NIH grants GM119793 and MH113011 (MP), HD086487 and MH101107 (ART), MH083784 (OMW).
LIST OF ABBREVIATIONS
- BD
Bipolar Disorde
- BDNF
Brain-derived Neurotrophic Factor
- Brain-PAD
Brain-predicted age difference
- ccf-mtDNA
Circulating cell-free mitochondrial DNA
- DNAm
DNA methylation
- MDD
Major Depressive Disorder
- MHI
Mitochondrial Health Index
- mtDNA
Mitochondrial DNA
- mtDNAcn
Mitochondrial DNA copy number
- nDNA
Nuclear DNA
- PTSD
Post-traumatic Stress Disorder
- SCZ
Schizophrenia
- SSRI
Selective Serotonin Reuptake Inhibitor
- TL
Telomere length
Footnotes
Conflict of interest
The authors have no conflict of interest to report
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abi-Dargham A, Horga G, 2016. The search for imaging biomarkers in psychiatric disorders. Nat. Med. 22,1248–1255. [DOI] [PubMed] [Google Scholar]
- Akulenko R, Merl M, Helms V, 2016. BEclear: Batch Effect Detection and Adjustment in DNA Methylation Data. PLoS One 11, e0159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alder JK, Hanumanthu VS, Strong MA, DeZern AE, Stanley SE, Takemoto CM, Danilova L, Applegate CD, Bolton SG, Mohr DW, Brodsky RA, Casella JF, Greider CW, Jackson JB, Armanios M, 2018. Diagnostic utility of telomere length testing in a hospital-based setting. Proc. Natl. Acad. Sci. U. S. A. 115, E2358–E2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Hills M, Lansdorp PM, 2012. Telomere length measurement-caveats and a critical assessment of the available technologies and tools. Mutat. Res. 730, 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E, 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 39, e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aycheh HM, Seong J-K, Shin J-H, Na DL, Kang B, Seo SW, Sohn K-A, 2018. Biological Brain Age Prediction Using Cortical Thickness Data: A Large Scale Cohort Study. Front. Aging Neurosci. 10, 1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Adamson C, Beare R, Seal ML, 2017. Modelling neuroanatomical variation during childhood and adolescence with neighbourhood-preserving embedding. Sci. Rep. 7, 17796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista LFZ, Pech MF, Zhong FL, Nguyen HN, Xie KT, Zaug AJ, Crary SM, Choi J, Sebastiano V, Cherry A, Giri N, Wernig M, Alter BP, Cech TR, Savage SA, Reijo Pera RA, Artandi SE, 2011. Telomere shortening and loss of self-renewal in dyskeratosis congenita induced pluripotent stem cells. Nature 474, 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis D, Bartlett DB, Patel HP, Roberts HC, 2013. Understanding how we age: insights into inflammaging. Longev Healthspan 2, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE, 2015. Quantification of biological aging in young adults. Proc. Natl. Acad. Sci. U. S. A. 112, E4104–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA, Schaefer J, Sugden K, Williams B, Poulton R, Caspi A, 2018. Eleven Telomere, Epigenetic Clock, and Biomarker- Composite Quantifications of Biological Aging: Do They Measure the Same Thing? Am. J.Epidemiol. 187, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM, 2006. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 38, 515–517. [DOI] [PubMed] [Google Scholar]
- Bersani FS, Lindqvist D, Mellon SH, Penninx BWJH, Verhoeven JE, Revesz D, Reus VI, Wolkowitz OM, 2015. Telomerase activation as a possible mechanism of action of psychopharmacological interventions. Drug Discov. Today. 20, 1305–1309. [DOI] [PubMed] [Google Scholar]
- Bind M-A, VanderWeele TJ, Schwartz JD, Coull BA, 2017. Quantile causal mediation analysis allowing longitudinal data. Stat. Med. 36, 4182–4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J, 2015. Human telomere biology: A contributory and interactive factor in aging, disease risks, and protection. Science 350, 1193–1198. [DOI] [PubMed] [Google Scholar]
- Boeck C, Gumpp AM, Calzia E, Radermacher P, Waller C, Karabatsiakis A, Kolassa I-T, 2018a. The association between cortisol, oxytocin, and immune cell mitochondrial oxygen consumption in postpartum women with childhood maltreatment. Psychoneuroendocrinology 96, 69–77. [DOI] [PubMed] [Google Scholar]
- Boeck C, Koenig AM, Schury K, Geiger ML, Karabatsiakis A, Wilker S, Waller C, Gündel H, Fegert JM, Calzia E, Kolassa I-T, 2016. Inflammation in adult women with a history of child maltreatment: The involvement of mitochondrial alterations and oxidative stress. Mitochondrion 30, 197–207. [DOI] [PubMed] [Google Scholar]
- Boeck C, Krause S, Karabatsiakis A, Schury K, Gündel H, Waller C, Kolassa l.-T., 2018b. History of child maltreatment and telomere length in immune cell subsets: Associations with stress- and attachment-related hormones. Dev. Psychopathol. 30, 539–551. [DOI] [PubMed] [Google Scholar]
- Boeck C, Pfister S, Bürkle A, Vanhooren V, Libert C, Salinas-Manrique J, Dietrich DE, Kolassa I-T, Karabatsiakis A, 2018c. Alterations of the serum N-glycan profile in female patients with Major Depressive Disorder. J. Affect. Disord. 234, 139–147. [DOI] [PubMed] [Google Scholar]
- Boeck C, Salinas-Manrique J, Calzia E, Radermacher P, von Arnim CAF, Dietrich DE, Kolassa I-T, Karabatsiakis A, 2018d. Targeting the association between telomere length and immuno-cellular bioenergetics in female patients with Major Depressive Disorder. Sci. Rep. 8, 9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohannon RW, 2008. Hand-grip dynamometry predicts future outcomes in aging adults. J. Geriatr.Phys. Ther. 31,3–10. [DOI] [PubMed] [Google Scholar]
- Boks MP, van Mierlo HC , Rutten BPF, Radstake TRDJ, De Witte L, Geuze E, Horvath S, Schalkwyk LC, Vinkers CH, Broen JCA, Vermetten E, 2015. Longitudinal changes of telomere length and epigenetic age related to traumatic stress and post-traumatic stress disorder. Psychoneuroendocrinology 51,506–512. [DOI] [PubMed] [Google Scholar]
- Breitling LP, Saum K-U, Perna L, Schöttker B, Holleczek B, Brenner H, 2016. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin. Epigenetics 8, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess S, Butterworth A, Malarstig A, Thompson SG, 2012. Use of Mendelian randomisation to assess potential benefit of clinical intervention. BMJ 345, e7325. [DOI] [PubMed] [Google Scholar]
- Bzdok D, Altman N, Krzywinski M, 2018. Points of significance: statistics versus machine learning. Nat. Methods 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, Song L, Kretzschmar W, Gan X, Nicod J, Rivera M, Deng H, Du B, Li K, Sang W, Gao J, Gao S, Ha B, Ho H-Y, Hu C, Hu J, Hu Z, Huang G, Jiang G, Jiang T, Jin W, Li G, Li K, Li Y, Li Y, Li Y, Lin Y-T, Liu L, Liu T, Liu Y, Liu Y, Lu Y, Lv L, Meng H, Qian P, Sang H, Shen J, Shi J, Sun J, Tao M, Wang G, Wang G, Wang J, Wang L, Wang X, Wang X, Yang H, Yang L, Yin Y, Zhang J, Zhang K, Sun N, Zhang W, Zhang X, Zhang Z, Zhong H, Breen G, Wang J, Marchini J, Chen Y, Xu Q, Xu X, Mott R, Huang G-J, Kendler K, Flint J, 2015. Molecular signatures of major depression. Curr. Biol. 25, 1146–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, 2002. Telomere measurement by quantitative PCR 30, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko BK, Kramer PA, Ravi S, Johnson MS, Hardy RW, Ballinger SW, Darley-Usmar VM, 2013. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab. Invest. 93, 690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleckis R, Murakami I, Takada J, Kondoh H, Yanagida M, 2016. Individual variability in human blood metabolites identifies age-related differences. Proc. Natl. Acad. Sci. U. S. A. 113, 4252–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AY, Swaminathan R, Cockram CS, 1989. Effectiveness of sodium fluoride as a preservative of glucose in blood. Clin. Chem. 35, 315–317. [PubMed] [Google Scholar]
- Chan RF, Turecki G, Shabalin AA, Guintivano J, Zhao M, Xie LY, van Grootheest G, Kaminsky ZA, Dean B, Penninx BWJH, Aberg KA, van den Oord EJCG, 2018. Cell-type-specific methylome-wide association studies implicate neurodegenerative processes and neuroimmune communication in major depressive disorder. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Carty CL, Kimura M, Kark JD, Chen W, Li S, Zhang T, Kooperberg C, Levy D, Assimes T, Absher D, Horvath S, Reiner AP, Aviv A, 2017. Leukocyte telomere length, T cell composition and DNA methylation age. Aging 9, 1983–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BH, Marioni RE, Colicino E, Peters MJ, Ward CK, Tsai PC, Roetker NS, Just AC, Demerath EW, Bressler J, Fornage M, Studenski S, Vandiver AR, Tanaka T, Kiel DP, Liang L, Vokonas P, Lunetta KL, Murabito JM, Bandinelli S, Dena G, Melzer D, Nalls M, Pilling LC, Price TR, Andrew B, Gieger C, Holle R, Kretschmer A, Kronenberg F, Visscher PM, Shah S, Wray NR, Mcrae AF, Levine ME, Lu AT, Tsao PS, Hou L, Manson JE, Levy D, Baccarelli A, Meurs JV, Bell JT, 2016. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging 8, 1844–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Dong Y, Bhagatwala J, Raed A, Huang Y, Zhu H, 2018. Effects of Vitamin D3 supplementation on epigenetic aging in overweight and obese African Americans with suboptimal vitamin D status: a randomized clinical trial. J. Gerontol. A Biol. Sci. Med. Sci. https://doi.Org/10.1093/gerona/gly223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou JP, Effros RB, 2013. T cell replicative senescence in human aging. Curr. Pharm. Des. 19, 1680–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen L, Lenart A, Tan Q, Vaupel JW, Aviv A, Mcgue M, Christensen K, 2016. DNA methylation age is associated with mortality in a longitudinal Danish twin study. Aging Cell 15, 149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Wang X, Lui YW, 2017. Influence of T1-Weighted Signal Intensity on FSL Voxel-Based Morphometry and FreeSurfer Cortical Thickness. AJNR Am. J. Neuroradiol. 38, 726–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson MJ, Cardoso MJ, Ridgway GR, Modat M, Leung KK, Rohrer JD, Fox NC, Ourselin S, 2011. A comparison of voxel and surface based cortical thickness estimation methods. Neuroimage 57, 856–865. [DOI] [PubMed] [Google Scholar]
- Cole JH, 2018. Neuroimaging Studies Illustrate the Commonalities Between Ageing and Brain Diseases. Bioessays 1700221, 1700221. [DOI] [PubMed] [Google Scholar]
- Cole JH, Franke K, 2017. Predicting Age Using Neuroimaging: Innovative Brain Ageing Biomarkers. Trends Neurosci. 40, 681–690. [DOI] [PubMed] [Google Scholar]
- Cole JH, Marioni RE, Harris SE, Deary IJ, Cole JH, 2018. Brain age and other bodily “ ages ”: implications for neuropsychiatry. Mol. Psychiatry, 10.1038/s41380-018-0098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Poudel RPK, Tsagkrasoulis D, Caan MWA, Steves C, Spector TD, Montana G, 2016. Predicting brain age with deep learning from raw imaging data results in a reliable and heritable biomarker. [DOI] [PubMed] [Google Scholar]
- Cole JH, Ritchie SJ, Bastin ME, Valdés Hernández MC, Muñoz Maniega S, Royle N, Corley J, Pattie A, Harris SE, Zhang Q, Wray NR, Redmond P, Marioni RE, Starr JM, Cox SR, Wardlaw JM, Sharp DJ, Deary IJ, 2017. Brain age predicts mortality. Mol. Psychiatry 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpo GD, Leffa DD, Kohler CA, Kapczinski F, Quevedo J, Carvalho AF, 2015. Is bipolar disorder associated with accelerating aging? A meta-analysis of telomere length studies. J. Affect. Disord. 186, 241–248. [DOI] [PubMed] [Google Scholar]
- Conklin QA, Crosswell AD, Saron CD, Epel ES, 2018. Meditation, Stress Processes, and Telomere Biology. Current Opinion in Psychology. https://doi.Org/10.1016/j.copsyc.2018.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R, Kuh D, Hardy R, Mortality Review Group, FALCon and HALCyon Study Teams, 2010. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ 341, C4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral-Debrinski M, Horton T, Lott MT, Shoffner JM, Beal MF, Wallace DC, 1992. Mitochondrial DNA deletions in human brain: regional variability and increase with advanced age. Nat. Genet. 2, 324–329. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR, 2013. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 11, 126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagnall CL, Hicks B, Teshome K, Hutchinson AA, Gadalla SM, Khincha PP, Yeager M, Savage SA, 2017. Effect of pre-analytic variables on the reproducibility of qPCR relative telomere length measurement. PLoS One 12, e0184098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BWJH, Delucchi KL, Wolkowitz OM, Mathews CA, 2016. The Association Between Psychiatric Disorders and Telomere Length. Psychosom. Med. 78, 776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, 2012. Programmatic features of aging originating in development: aging mechanisms beyond molecular damage? FASEB J. 26, 4821–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Magalhães JP, Passos JF, 2018. Stress, cell senescence and organismal ageing. Mech. Ageing Dev. 170, 2–9. [DOI] [PubMed] [Google Scholar]
- Diaz KM, Thanataveerat A, Parsons FE, Yoon S, Cheung YK, Alcántara C, Duran AT, Ensari I, Krupka DJ, Schwartz JE, Burg MM, Davidson KW, 2018. The Influence of Daily Stress on Sedentary Behavior: Group and Person (N of 1) Level Results of a 1-Year Observational Study. Psychosom. Med. 80, 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Vieira EM, 2018. Stress, Inflammation, and Aging: An Association Beyond Chance. Am. J. Geriatr. Psychiatry 26, 964–965. [DOI] [PubMed] [Google Scholar]
- Effros RB, 2009. Kleemeier Award Lecture 2008—The Canary in the Coal Mine: Telomeres and Human Healthspan. J. Gerontol. A Biol. Sci. Med. Sci. 64A, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger JK, Morota S, Hansson MJ, Paul G, Elmér E, 2015. Mitochondrial dysfunction in blood cells from amyotrophic lateral sclerosis patients. J. Neurol. 262, 1493–1503. [DOI] [PubMed] [Google Scholar]
- Elliott HR, Tillin T, McArdle WL, Ho K, Duggirala A, Frayling TM, Davey Smith G, Hughes D, Chaturvedi N, Relton CL, 2014. Differences in smoking associated DNA methylation patterns in South Asians and Europeans. Clin. Epigenetics 6, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelfriet PM, Jansen EHJM, Picavet HSJ, Dollé MET, 2013. Biochemical markers of aging for longitudinal studies in humans. Epidemiol. Rev. 35, 132–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD, 2018. The fetal programming of telomere biology hypothesis: an update. Philos. Trans. R. Soc. Lond. B Biol. Sci. 373 https://doi.Org/10.1098/rstb.2017.0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM, 2004. Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U. S. A. 101, 17312–17315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Crosswell AD, Mayer SE, Prather AA, Slavich GM, Puterman E, Mendes WB, 2018. More than a feeling: A unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Prather AA, 2018. Stress, Telomeres, and Psychopathology: Toward a Deeper Understanding of a Triad of Early Aging. Annu. Rev. Clin. Psychol. 14, 371–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes M, Wan C, Tacutu R, Barardo D, Rajput A, Wang J, Thoppil H, Thornton D, Yang C, Freitas A, de Magalhães JP, 2016. Systematic analysis of the gerontome reveals links between aging and age-related diseases. Hum. Mol. Genet. 25, 4804–4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, 2000. Inflamm-aging: an evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 908, 244–254. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A, 2018a. Inflammaging: a new immune- metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol, 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- Franceschi C, Zaikin A, Gordleeva S, Ivanchenko M, Bonifazi F, Storci G, Bonafè M, 2018b. Inflammaging 2018: An update and a model. Semin. Immunol https://doi.Org/10.1016/j.smim.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Franke K, Gaser C, 2012. Longitudinal Changes in Individual BrainAGE in Healthy Aging, Mild Cognitive Impairment, and Alzheimer’s Disease 1Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni. GeroPsych 25, 235–245. [Google Scholar]
- Fries GR, Bauer IE, Scaini G, Wu MJ, Kazimi IF, Valvassori SS, Zunta-Soares G, Walss- Bass C, Soares JC, Quevedo J, 2017. Accelerated epigenetic aging and mitochondrial DNA copy number in bipolar disorder. Transl. Psychiatry 7 10.1038/s41398-017-0048-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C, 2018. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8, 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Zhang Y, Mons U, Brenner H, 2018. Leukocyte telomere length and epigenetic-based mortality risk score: associations with all-cause mortality among older adults. Epigenetics. https://doi.Org/10.1080/15592294.2018.1514853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett DD, Kovacevic N, McIntosh AR, Grady CL, 2010. Blood oxygen level-dependent signal variability is more than just noise. J. Neurosci. 30, 4914–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaser C, Franke K, Klöppel S, Koutsouleris N, Sauer H, 2013. BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS One 8 10.1371/journal.pone.0067346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerligs L, Renken RJ, Saliasi E, Maurits NM, Lorist MM, 2015. A Brain-Wide Study of Age- Related Changes in Functional Connectivity. Cereb. Cortex 25, 1987–1999. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Freire M, Scalzo P, D’Agostino J, Moore ZA, Diaz-Ruiz A, Fabbri E, Zane A, Chen B, Becker KG, Lehrmann E, Zukley L, Chia CW, Tanaka T, Coen PM, Bernier M, de Cabo R, Ferrucci L, 2018. Skeletal muscle ex vivo mitochondrial respiration parallels decline in vivo oxidative capacity, cardiorespiratory fitness, and muscle strength: The Baltimore Longitudinal Study of Aging. Aging Cell 17 10.1111/acel.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, Lin J, Wolkowitz OM, 2014. Telomere length and cortisol reactivity in children of depressed mothers. Mol. Psychiatry 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco M, Villani G, Mazzucchelli F, Bresolin N, Papa S, Attardi G, 2003. Marked aging-related decline in efficiency of oxidative phosphorylation in human skin fibroblasts. FASEB J. 17, 1706–1708. [DOI] [PubMed] [Google Scholar]
- Griffanti L, Douaud G, Bijsterbosch J, Evangelisti S, Alfaro-Almagro F, Glasser MF, Duff EP, Fitzgibbon S, Westphal R, Carone D, Beckmann CF, Smith SM, 2017. Hand classification of fMRI ICA noise components. Neuroimage 154, 188–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez Becker B, Klein T, Wachinger C, 2018. Gaussian process uncertainty in age estimation as a measure of brain abnormality. Neuroimage 175, 246–258. [DOI] [PubMed] [Google Scholar]
- Hajek T, Franke K, Kolenic M, Capkova J, Matejka M, Propper L, Uher R, Stopkova P, Novak T, Paus T, Kopecek M, Spaniel F, Alda M, 2017. Brain Age in Early Stages of Bipolar Disorders or Schizophrenia. Schizophr. Bull, 10.1093/schbul/sbx172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LKM, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, Zhao M, Kumar G, Xie LY, Jansen R, Milaneschi Y, Dean B, Aberg KA, van den Oord EJCG, Penninx WJH, 2018. Epigenetic Aging in Major Depressive Disorder. Am. J. Psychiatry appi.ajp.2018.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, Klotzle B, Bibikova M, Fan JB, Gao Y, Deconde R, Chen M, Rajapakse I, Friend S, Ideker T, Zhang K, 2013. Genome wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol. Cell 49, 359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen LM, Schutte NS, Malouff JM, Epel ES, 2017. The Relationship Between Childhood Psychosocial Stressor Level and Telomere Length: A Meta-Analysis. Health Psychol Res 5, 6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattab MW, Shabalin AA, Clark SL, Zhao M, Kumar G, Chan RF, Xie LY, Jansen R, Han KM, Magnusson PKE, van Grootheest G, Hultman CM, Penninx BWJH, Aberg KA, van den Oord EJCG, 2017. Correcting for cell-type effects in DNA methylation studies: reference-based method outperforms latent variable approaches in empirical studies. Genome Biol. 18 https://doi.Org/10.1186/si3059-017-1149-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton SN, Franz CE, Elman JA, Panizzon MS, Hagler DJ Jr, Fennema-Notestine C, Eyler T, McEvoy LK, Lyons MJ, Dale AM, Kremen WS, 2018. Negative fateful life events in midlife and advanced predicted brain aging. Neurobiol. Aging 67, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heijmans BT, Mill J, 2012. Commentary: The seven plagues of epigenetic epidemiology. Int. J. Epidemiol. 41,74–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel J, Frenzel S, König J, Wittfeld K, Fuellen G, Holtfreter B, Pietzner M, Friedrich N, Nauck M , Völzke H, Kocher T, Grabe HJ, 2018. The informative error: A framework for the construction of individualized phenotypes. Stat. Methods Med. Res. 962280218759138. [DOI] [PubMed] [Google Scholar]
- Hertel J, Friedrich N, Wittfeld K, Pietzner M, Budde K, Van der Auwera S, Lohmann T, Teumer A, Volzke H, Nauck M, Grabe HJ, 2016. Measuring Biological Age via Metabonomics: The Metabolic Age Score. J. Proteome Res. 15, 400–410. [DOI] [PubMed] [Google Scholar]
- Horvath S, 2013. DNA methylation age of human tissues and cell types. Genome Biol. 14, R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Erhart W, Brosch M, Ammerpohl O, Schönfels W von, Ahrens M, Heits N, Bell JT, Tsai P-C, Spector TD, Deloukas P, Siebert R, Sipos B, Becker T, Röcken C, Schafmayer C, Hampe J, 2014. Obesity accelerates epigenetic aging of human liver. Proceedings of the National Academy of Sciences 201412759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Mah V, Lu AT, Woo JS, Choi O-W, Jasinska AJ, Riancho JA, Tung S, Coles NS, Braun J, Vinters HV, Coles LS, 2015. The cerebellum ages slowly according to the epigenetic clock. Aging 7, 294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Oshima J, Martin GM, Lu AT, Quach A, Cohen H, Felton S, Matsuyama M, Lowe D, Kabacik S, Wilson JG, Reiner AP, Maierhofer A, Flunked J, Aviv A, Hou L, Baccarelli AA, Li Y, Stewart JD, Whitsel EA, Ferrucci L, Matsuyama S, Raj K, 2018. Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging 10, 1758–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, Raj K, 2018. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 19, 371–384. [DOI] [PubMed] [Google Scholar]
- Hough CM, Bersani FS, Mellon SH, Epel ES, Reus VI, Lindqvist D, Lin J, Mahan L, Rosser R, Burke H, Coetzee J, Nelson JC, Blackburn EH, Wolkowitz OM, 2016. Leukocyte telomere length predicts SSRI response in major depressive disorder: A preliminary report. Mol Neuropsychiatry 2, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houseman EA, Molitor J, Marsit CJ, 2014. Reference-free cell mixture adjustments in analysis of DNA methylation data. Bioinformatics 30, 1431–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huan T, Chen G, Liu C, Bhattacharya A, Rong J, Chen BH, Seshadri S, Tanriverdi K, Freedman JE, Larson MG, Others, 2018. Age-associated micro RNA expression in human peripheral blood is associated with all-cause mortality and age-related traits. Aging Cell 17, e12687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel EM, Hessas E, Müller S, Beiter T, Fisch M, Eibl A, Wolf OT, Giebel B, Platen P, Kumsta R, Moser DA, 2018. Cell-free DNA release under psychosocial and physical stress conditions. Transl. Psychiatry 8, 236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado-Roca Y, Ledesma M, Gonzalez-Lazaro M, Moreno-Loshuertos R, Fernandez-Silva P, Enriquez JA, Laclaustra M, 2016. Adjusting MtDNA Quantification in Whole Blood for Peripheral Blood Platelet and Leukocyte Counts. PLoS One 11, e0163770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN, 2015. Brain disorders? Precisely. Science 348, 499–500. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik J-HH, Thomas E, Jiang S, Adams AC, Sahin E, Kost-Alimova M, Protopopov A, Cadinanos J, Horner JW, Maratos-Flier E, DePinho R. a., Cadinanos J, Horner JW, Maratos-Flier E, DePinho R. a., 2011. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Trivedi MH, 2018. Personalized Antidepressant Selection and Pathway to Novel Treatments: Clinical Utility of Targeting Inflammation. Int. J. Mol. Sci 19 10.3390/ijms19010233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jylhava J, Pedersen NL, Hagg S, 2017. Biological Age Predictors. EBioMedicine 21,29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kågedal B, Lindqvist M, Farnebäck M, Lenner L, Peterson C, 2005. Failure of the PAXgene Blood RNA System to maintain mRNA stability in whole blood. Clin. Chem. Lab. Med. 43, 1190–1192. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Kasahara T, Kato M, Sakai S, Deguchi Y, Tani M, Kuroda K, Hattori K, Yoshida S, Goto Y, Kinoshita T, Inoue K, Kato T, 2018. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J. Affect. Disord. 233, 15–20. [DOI] [PubMed] [Google Scholar]
- Karabatsiakis A, Böck C, Salinas-Manrique J, Kolassa S, Calzia E, Dietrich DE, Kolassa I-T, 2014. Mitochondrial respiration in peripheral blood mononuclear cells correlates with depressive subsymptoms and severity of major depression. Transl. Psychiatry 4, e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann T, Meer DVD, Doan NT, Schwarz E, Martina J, Alnæs D, Barch DM, 2018. Genetics of brain age suggest an overlap with common brain disorders. [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB, 2015. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry 2, 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khincha PP, Dagnall CL, Hicks B, Jones K, Aviv A, Kimura M, Katki H, Aubert G, Giri N, Alter BP, Savage SA, Gadalla SM, 2017. Correlation of Leukocyte Telomere Length Measurement Methods in Patients with Dyskeratosis Congenita and in Their Unaffected Relatives. Int. J. Mol. Sci 18 10.3390/ijms18081765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight AK, Conneely KN, Smith AK, 2017. Gestational age predicted by DNA methylation: potential clinical and research utility. Epigenomics. 10.2217/epi-2016-0157 [DOI] [PubMed] [Google Scholar]
- Kolenic M, Franke K, Hlinka J, Matejka M, Capkova J, Pausova Z, Uher R, Alda M, Spaniel F, Hajek T, 2018. Obesity, dyslipidemia and brain age in first-episode psychosis. J. Psychiatr. Res. 99, 151–158. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, Falkai P, Riecher- Rössler A, Moller HJ, Reiser M, Pantelis C, Meisenzahl E, 2014. Accelerated brain aging in schizophrenia and beyond: A neuroanatomical marker of psychiatric disorders. Schizophr. Bull. 40, 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krištić J, Vučković F, Menni C, Klarić L, Keser T, Beceheli I, Pučić-Baković M, Novokmet M, Mangino M, Thaqi K, Rudan P, Novokmet N, Sarac J, Missoni S, Kolčić I, Polašek O, Rudan I, Campbell H, Hayward C, Aulchenko Y, Valdes A, Wilson JF, Gornik O, Primorac D, Zoldoš V, Spector T, Lauc G, 2014. Glycans are a novel biomarker of chronological and biological ages. J. Gerontol. A Biol. Sci. Med. Sci. 69, 779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawless C, Wang C, Jurk D, Merz A, von Zglinicki T, Passos JF, 2010. Quantitative assessment of markers for cell senescence. Exp. Gerontol. 45, 772–778. [DOI] [PubMed] [Google Scholar]
- Lee M, Martin H, Firpo MA, Demerath EW, 2011. Inverse association between adiposity and telomere length: The Fels Longitudinal Study. Am. J. Hum. Biol. 23, 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-R, Jung SM, Bang H, Kim HS, Kim YB, 2018. The association between muscular strength and depression in Korean adults: a cross-sectional analysis of the sixth Korea National Health and Nutrition Examination Survey (KNHANES VI) 2014. BMC Public Health 18, 1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke MR, Wendorff T, Mieth B, Buhl K, Linnemann M, 2000. Spatiotemporal gait patterns during overground locomotion in major depression compared with healthy controls. J. Psychiatr. Res. 34, 277–283. [DOI] [PubMed] [Google Scholar]
- Le TT, Kuplicki R, Yeh HW, Aupperle RL, Khalsa SS, Simmons WK, Paulus MP, 2018. Effect of Ibuprofen on BrainAGE: A Randomized, Placebo-Controlled, Dose-Response Exploratory Study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever-van Milligen BA, Lamers F, Smit JH, Penninx BW, 2017. Six-year trajectory of objective physical function in persons with depressive and anxiety disorders. Depress. Anxiety 34, 188–197. [DOI] [PubMed] [Google Scholar]
- Levine ME, Lu AT, Quach A, Chen BH, Assimes TL, Bandinelli S, Hou L, Baccarelli AA, Stewart JD, Li Y, Whitsel EA, Wilson JG, Reiner AP, Aviv A, Lohman K, Liu Y, Ferrucci L, Horvath S, 2018. An epigenetic biomarker of aging for lifespan and healthspan. Aging 10, 573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy BR, Slade MD, Kunkel SR, Kasl SV, 2002. Longevity increased by positive self-perceptions of aging. J. Pers. Soc. Psychol. 83, 261–270. [DOI] [PubMed] [Google Scholar]
- Liem F, 2016. Predicting brain-age from multimodal imaging data captures cognitive impairment. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, Penninx BW, Revesz D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM, 2015. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev. 55, 333–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Fernström J, Grudet C, Ljunggren L, Träskman-Bendz L, Ohlsson L, Westrin Å, 2016. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl. Psychiatry 6, e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernström J, Westrin Å, Hough M, Lin J, Reus VI, Epel ES, Mellon SH, 2018. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 43, 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Cheon J, Brown R, Coccia M, Puterman E, Aschbacher K, Sinclair E, Epel E, Blackburn H, 2016. Systematic and Cell Type-Specific Telomere Length Changes in Subsets of Lymphocytes. J Immunol Res 2016, 5371050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitz O, Mellon S, Blackburn E, 2010. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J. Immunol. Methods 352, 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Li W, Xu Y, 2018. Human Age Prediction Based on DNA Methylation Using a Gradient Boosting Regressor. Genes 9 10.3390/genes9090424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wang J, Zhou J, Huang P, Li J, 2017. The association between post-traumatic stress disorder and shorter telomere length: A systematic review and meta-analysis. J. Affect. Disord. 218, 322–326. [DOI] [PubMed] [Google Scholar]
- Li Z, He Y, Wang D, Tang J, Chen X, 2017. Association between childhood trauma and accelerated telomere erosion in adulthood: A meta-analytic study. J. Psychiatr. Res. 93, 64–71. [DOI] [PubMed] [Google Scholar]
- Li Z, Hu M, Zong X, He Y, Wang D, Dai L, Dong M, Zhou J, Cao H, Lv L, Chen X, Tang J, 2015. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naïve schizophrenia. Sci. Rep. 5, 18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, Thorp SR, Jeste DV, 2015. Is Post-Traumatic Stress Disorder Associated with Premature Senescence? A Review of the Literature. Am. J. Geriatr. Psychiatry 23, 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu AT, Xue L, Salfati EL, Chen BH, Ferrucci L, Levy D, Joehanes R, Murabito JM, Kiel DP, Tsai P-C, Yet I, Bell JT, Mangino M, Tanaka T, McRae AF, Marioni RE, Visscher PM, Wray NR, Deary IJ, Levine ME, Quach A, Assimes T, Tsao PS, Absher D, Stewart JD, Li Y, Reiner AP, Hou L, Baccarelli AA, Whitsel EA, Aviv A, Cardona A, Day FR, Wareham NJ, Perry JRB, Ong KK, Raj K, Lunetta KL, Horvath S, 2018. GWAS of epigenetic aging rates in blood reveals a critical role forTERT. Nat. Commun. 9, 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Cherbuin N, Gaser C, 2016. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. Neuroimage 134, 508–513. [DOI] [PubMed] [Google Scholar]
- van der Maaten L, Hinton G, 2008. Visualizing Data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605. [Google Scholar]
- Malik AN, Shahni R, Rodriguez-de-Ledesma A, Laftah A, Cunningham P, 2011. Mitochondrial DNA as a non-invasive biomarker: accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem. Biophys. Res. Commun. 412, 1–7. [DOI] [PubMed] [Google Scholar]
- Malouff JM, Schutte NS, 2017. A meta-analysis of the relationship between anxiety and telomere length. Anxiety Stress Coping 30, 264–272. [DOI] [PubMed] [Google Scholar]
- Mamdani F, Rollins B, Morgan L, Myers RM, Barchas JD, Schatzberg a. F., Watson SJ, Akil H, Potkin SG, Bunney WE, Vawter MP, Sequeira P. a., 2015. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl. Psychiatry 5, e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniates H, Stoop TB, Miller MW, Halberstadt L, Wolf EJ, 2018. Stress-Generative Effects of Posttraumatic Stress Disorder: Transactional Associations Between Posttraumatic Stress Disorder and Stressful Life Events in a Longitudinal Sample. J. Trauma. Stress 31, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Harris SE, Shah S, Mcrae AF, Zglinicki TV, Martin-ruiz C, Wray NR, Visscher PM, Deary IJ, 2016. The epigenetic clock and telomere length are independently associated with chronological age and mortality. Int. J. Epidemiol. 1–9.27433568 [Google Scholar]
- Marioni RE, Shah S, McRae AF, Chen BH, Colicino E, Harris SE, Gibson J, Henders AK, Redmond P, Cox SR, Pattie A, Corley J, Murphy L, Martin NG, Montgomery GW, Feinberg AP, Fallin MD, Multhaup ML, Jaffe AE, Joehanes R, Schwartz J, Just AC, Lunetta KL, Murabito JM, Starr JM, Horvath S, Baccarelli AA, Levy D, Visscher PM, Wray NR, Deary IJ, 2015. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 16, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA, 2017. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain, Behavior, and Immunity, 64, 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Cengotitabengoa M, Carrascón L, O’Brien JT, Díaz-Gutiérrez M-J, Bermúdez-Ampudia C, Sanada K, Arrasate M, González-Pinto A, 2016. Peripheral Inflammatory Parameters in Late-Life Depression: A Systematic Review. Int. J. Mol. Sci. 17 10.3390/ijms17122022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinsson L, Wei Y, Xu D, Melas PA, Mathé AA, Schalling M, Lavebratt C, Backlund L, 2013. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl. Psychiatry 3, e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason AE, Hecht FM, Daubenmier JJ, Sbarra DA, Lin J, Moran PJ, Schleicher SG, Acree M, Prather AA, Epel ES, 2018. Weight Loss Maintenance and Cellular Aging in the Supporting Health Through Nutrition and Exercise Study. Psychosom. Med. 80, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, Khazeni N, 2016. Perceivedstress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immun. 54, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA, 2017a. DNA methylation age is not accelerated in brain or blood of subjects with schizophrenia. Schizophr. Res https://doi.Org/10.1016/j.schres.2017.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney BC, Lin H, Ding Y, Lewis DA, Sweet RA, 2017b. DNA methylation evidence against the accelerated aging hypothesis of schizophrenia, npj Schizophrenia 3, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Bruenig D, Lawford B, Harvey W, Carrillo-Roa T, Morris CP, Jovanovic T, Young RM, Binder EB, Voisey J, 2018. Accelerated DNA methylation aging and increased resilience in veterans: The biological cost for soldiering on. Neurobiol Stress 8, 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengel-From J, Thinggaard M, Dalgård C, Kyvik KO, Christensen K, Christiansen L, 2014. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 133, 1149–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C, Kiddle SJ, Mangino M, Viñuela A, Psatha M, Steves C, Sattlecker M, Buil A, Newhouse S, Nelson S, Williams S, Voyle N, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Spector TD, Dobson R, Valdes AM, 2015. Circulating Proteomic Signatures of Chronological Age. J. Gerontol. A Biol. Sci. Med. Sci. 70, 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak J, Troje NF, Fischer J, Vollmar P, Heidenreich T, Schulte D, 2009. Embodiment of Sadness and Depression—Gait Patterns Associated With Dysphoric Mood. Psychosom. Med. 71, 580. [DOI] [PubMed] [Google Scholar]
- Miller FJ, Rosenfeldt FL, Zhang C, Linnane AW, Nagley P, 2003. Precise determination ofmitochondrial DNA copy number in human skeletal and cardiac muscle by a PCR-based assay: lack of change of copy number with age. Nucleic Acids Res. 31, e61–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A, 2017. The Longitudinal Study of Aging in Human Young Adults: Knowledge Gaps and Research Agenda. J. Gerontol. A Biol. Sci. Med. Sci. 72, 210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi D, 2015. ENIGMA: crowdsourcing meets neuroscience. Lancet Neurol. 14, 462–463. [DOI] [PubMed] [Google Scholar]
- Monickaraj F, Gokulakrishnan K, Prabu P, Sathishkumar C, Anjana RM, Rajkumar JS, Mohan V, Balasubramanyam M, 2012. Convergence of adipocyte hypertrophy, telomere shortening and hypoadiponectinemia in obese subjects and in patients with type 2 diabetes. Clin. Biochem. 45, 1432–1438. [DOI] [PubMed] [Google Scholar]
- Monroe SM, 2008. Modern approaches to conceptualizing and measuring human life stress. Annu. Rev. Clin. Psychol. 4, 33–52. [DOI] [PubMed] [Google Scholar]
- Moreno-Villanueva M, Morath J, Vanhooren V, Elbert T, Kolassa S, Libert C, Biirkle A, Kolassa I-T, 2013. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl. Psychiatry 3, e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Davey Smith G, 2018. Robust research needs many lines of evidence. Nature 553, 399–401. [DOI] [PubMed] [Google Scholar]
- Nakahira K, Kyung S-Y, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, Lawler LA, Christie JD, Meyer NJ, Me Causland FR, Waikar SS, Waxman AB, Chung RT, Bueno R, Rosas IO, Fredenburgh LE, Baron RM, Christiani C, Hunninghake GM, Choi AMK, 2013. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 10, e1001577; discussion e1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nenadic I, Dietzek M, Langbein K, Sauer H, Gaser C, 2017. BrainAGE score indicates accelerated brain aging in schizophrenia, but not bipolar disorder. Psychiatry Research: Neuroimaging. https://doi.Org/10.1016/j.pscychresns.2017.05.006 [DOI] [PubMed] [Google Scholar]
- Ortega FB, Silventoinen K, Tynelius P, Rasmussen F, 2012. Muscular strength in male adolescents and premature death: cohort study of one million participants. BMJ 345, e7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DI, Štambuk J, Razdorov G, Pučić-Baković M, Martins-de-Souza D, Lauc G, Turck CW, 2018. Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response. Sci. Rep. 8, 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Zhao J, Dong X, Banazadeh A, Huang Y, Hussien A, Mechref Y, 2018. Clinical application of quantitative glycomics. Expert Rev. Proteomics 15, 1007–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx BWJH, Milaneschi Y, Lamers F, Vogelzangs N, 2013. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 11, 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters MJ, Joehanes R, Pilling LC, Schurmann C, Conneely KN, Powell J, Reinmaa E, Sutphin GL, Zhernakova A, Schramm K, Wilson YA, Kobes S, Tukiainen T, NABEC/UKBEC Consortium, Ramos YF, Goring HHH, Fornage M, Liu Y, Gharib SA, Stranger BE, De Jager PL, Aviv A, Levy D, Murabito JM, Munson PJ, Huan T, Hofman A, Uitterlinden G, Rivadeneira F, van Rooij J, Stolk L, Broer L, Verbiest MMPJ, Jhamai M, Arp P, Metspalu A, Tserel L, Milani L, Samani NJ, Peterson P, Kasela S, Codd V, Peters A, Ward-Caviness CK, Herder C, Waldenberger M, Roden M, Singmann P, Zeilinger S, Illig T, Homuth G, Grabe H-J, Volzke H, Steil L, Kocher T, Murray A, Melzer D, Yaghootkar H, Bandinelli S, Moses EK, Kent JW, Curran JE, Johnson MP, Williams-Blangero S, Westra H-J, McRae AF, Smith JA, Kardia SLR, Hovatta I, Perola M, Ripatti S, Salomaa V, Henders AK, Martin NG, Smith AK, Mehta D, Binder EB, Nylocks KM, Kennedy EM, Klengel T, Ding J, Suchy-Dicey AM, Enquobahrie DA, Brody J, Rotter JI, Chen Y.-D.l., Houwing-Duistermaat J, Kloppenburg M, Slagboom PE, Helmer Q, den Hollander W, Bean S , Raj T, Bakhshi N, Wang QP, Oyston LJ, Psaty BM, Tracy RP, Montgomery GW, Turner ST, Blangero J, Meulenbelt I, Ressler KJ, Yang J, Franke L, Kettunen J, Visscher PM, Neely GG, Korstanje R, Hanson RL, Prokisch H, Ferrucci L, Esko T, Teumer A, van Meurs JBJ, Johnson AD, 2015. The transcriptional landscape of age in human peripheral blood. Nat. Commun. 6, 8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Hirano M, 2016. Disentangling (Epi) Genetic and Environmental Contributions to theMitochondrial 3243A> G Mutation Phenotype: Phenotypic Destiny in Mitochondrial Disease? JAMA Neurol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Juster R-P, McEwen BS, 2014. Mitochondrial allostatic load puts the “glue” back in glucocorticoids. Nat. Rev. Endocrinol. 10, 303–310. [DOI] [PubMed] [Google Scholar]
- Picard M, Juster R-P, Sabiston CM, 2013. Is the whole greater than the sum of the parts? Self-rated health and transdisciplinarity. Health 5, 24. [Google Scholar]
- Picard M, McEwen BS, 2018a. Psychological Stress and Mitochondria: A Systematic Review. Psychosom. Med. 80, 141–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, McEwen BS, 2018b. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 80, 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard M, Prather AA, Puterman E, Cuillerier A, Coccia M, Aschbacher K, Burelle Y, Epel ES, 2018. A Mitochondrial Health Index Sensitive to Mood and Caregiving Stress. Biol. Psychiatry 84, 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinti M, Cevenini E, Nasi M, De Biasi S, Salvioli S, Monti D, Benatti S, Gibellini L, Cotichini R, Stazi MA, Trenti T, Franceschi C, Cossarizza A, 2014. Circulating mitochondrial DNA increases with age and is a familiar trait: Implications for “inflamm-aging.” Eur. J. Immunol. 44, 1552–1562. [DOI] [PubMed] [Google Scholar]
- Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS, 2015. Leukocyte telomere length in patients with schizophrenia: A meta-analysis. Schizophr. Res. 165, 195–200. [DOI] [PubMed] [Google Scholar]
- Price EM, Robinson WP, 2018. Adjusting for Batch Effects in DNA Methylation Microarray Data, a Lesson Learned. Front. Genet 9, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Kao H-T, Burgers DE, Carpenter LL, Tyrka AR, 2013. Telomeres and early-life stress: an overview. Biol. Psychiatry 73, 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Weiss J, Lin J, Schilf S, Slusher AL, Johansen KL, Epel ES, 2018. Aerobicexercise lengthens telomeres and reduces stress in family caregivers: A randomized controlled trial - Curt Richter Award Paper 2018. Psychoneuroendocrinology 98, 245–252. [DOI] [PubMed] [Google Scholar]
- Quach A, Levine ME, Tanaka T, Lu AT, Chen BH, Ferrucci L, Ritz B, Bandinelli S, Neuhouser ML, Beasley JM, Snetselaar L, Wallace RB, Tsao PS, Absher D, Assimes TL, Stewart JD, Li Y, Hou L, Baccarelli AA, Whitsel EA, Horvath S, 2017. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging 9, 419–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram N, Grimm KJ, 2009. Growth Mixture Modeling: A Method for Identifying Differences inLongitudinal Change Among Unobserved Groups. Int. J. Behav. Dev. 33, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM, 2000. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J.Gerontol. A Biol. Sci. Med. Sci. 55, M168–73. [DOI] [PubMed] [Google Scholar]
- Rao S, Kota LN, Li Z, Yao Y, Tang J, Mao C, Jain S, Xu Y, Xu Q, 2016. Acceleratedleukocyte telomere erosion in schizophrenia: Evidence from the present study and a meta-analysis. J. Psychiatr. Res. 79, 50–56. [DOI] [PubMed] [Google Scholar]
- Rasgon N, Lin KW, Lin J, Epel E, Blackburn E, 2016. Telomere length as a predictor of response to Pioglitazone in patients with unremitted depression: a preliminary study. Transl. Psychiatry 6, e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratanatharathorn A, Boks MP, Maihofer AX, Aiello AE, Amstadter AB, Ashley-Koch AE, Baker G, Beckham JC, Bromet E, Dennis M, Garrett ME, Geuze E, Guffanti G, Hauser MA, Kilaru V, Kimbrel NA, Koenen KC, Kuan P-F, Logue MW, Luft BJ, Miller MW, Mitchell C, Nugent NR, Ressler KJ, Rutten BPF, Stein MB, Vermetten E, Vinkers CH, Youssef NA, VA Mid-Atlantic MIRECC Workgroup, PGC PTSD Epigenetics Workgroup, Uddin M, Nievergelt CM, Smith AK, 2017. Epigenome-wide association of PTSD from heterogeneous cohorts with a common multi-site analysis pipeline. Am. J. Med. Genet. B Neuropsychiatr. Genet 174, 619–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlén S-E, Greco D, Soderhall C, Scheynius A, Kere J, 2012. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 7, e41361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Collins V, Clarke SJ, Han J-S, Lam P, Clay F, Williamson LM, Andy Choo KH, 2012. Epigenetic changes in response to tai chi practice: a pilot investigation of DNA methylation marks. Evid. Based. Complement. Alternat. Med. 2012, 841810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réeus GZ, Fries GR, Stertz L, Badawy M, Passos IC, Barichello T, Kapczinski F, Quevedo J, 2015. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300, 141–154. [DOI] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR, 2018. Early life adversity and telomere length: a meta-analysis. Mol. Psychiatry 23, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR, 2016. Depression and telomere length: A meta-analysis. J. Affect. Disord. 191,237–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogenmoser L, Kernbach J, Schlaug G, Gaser C, 2018. Keeping brains young with making music. Brain Struct. Funct. 223, 297–305. [DOI] [PubMed] [Google Scholar]
- Sanders JL, Boudreau RM, Newman AB, Newman AB, Newman AB, 2012. Understanding the Aging Process Using Epidemiologic Approaches, in: Newman AB, Cauley JA (Eds.), The Epidemiology of Aging. Springer; Netherlands, Dordrecht, pp. 187–214. [Google Scholar]
- Sasaki H, Kasagi F, Yamada M, Fujita S, 2007. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am. J. Med. 120, 337–342. [DOI] [PubMed] [Google Scholar]
- Sayer AA, Kirkwood TBL, 2015. Grip strength and mortality: a biomarker of ageing? Lancet 386, 226–227. [DOI] [PubMed] [Google Scholar]
- Schnack HG, Kahn RS, 2016. Detecting neuroimaging biomarkers for psychiatric disorders: Sample size matters. Front. Psychiatry 7 10.3389/fpsyt.2016.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnack HG, van Haren NEM, Nieuwenhuis M, Hulshoff Pol HE, Cahn W, Kahn RS, 2016. Accelerated Brain Aging in Schizophrenia: A Longitudinal Pattern Recognition Study. Am. J. Psychiatry 173, 607–616. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2016. The relationship between perceived stress and telomere length: A meta-analysis. Stress Health 32, 313–319. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2015. The association between depression and leukocyte telomere length: A meta-analysis. Depress. Anxiety 32, 229–238. [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2014. A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrinology 42, 45–48. [DOI] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES, 2013. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A, 2014. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol. Psychiatry 19, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Mill J, Arseneault L, Caspi A, 2012. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age : a longitudinal study. Mol. Psychiatry 18, 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields GS, Slavich GM, 2017. Lifetime Stress Exposure and Health: A Review of Contemporary Assessment Methods and Biological Mechanisms. Soc. Personal. Psychol. Compass 11 https://doi.Org/10.1111/spc3.12335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, 2010. Improving precision in investigating aging: why telomeres can cause problems. J. Gerontol. A Biol. Sci. Med. Sci. 65, 789–791. [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Nierenberg AA, Fava M, Wong KK, 2006. Telomere shortening and mood disorders: preliminary support for a chronic stress model of accelerated aging. Biol. Psychiatry 60, 432–435. [DOI] [PubMed] [Google Scholar]
- Simons MJP, 2015. Questioning causal involvement of telomeres in aging. Ageing Res. Rev. 24, 191–196. [DOI] [PubMed] [Google Scholar]
- Simpkin AJ, Hemani G, Suderman M, Gaunt TR, Lyttleton O, Mcardle WL, Ring SM, Sharp GC, Tilling K, Horvath S, Kunze S, Peters A, Waldenberger M, Ward-Caviness C, Nohr A, Sørensen TIA, Relton CL, Smith GD, 2016. Prenatal and early life influences on epigenetic age in children: a study of mother-offspring pairs from two cohort studies. Hum. Mol. Genet. 25, 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiers H, Hannon E, Schalkwyk LC, Smith R, Wong CCY, O’Donovan MC, Bray NJ, Mill J, 2015. Methylomic trajectories across human fetal brain development. Genome Res. 25, 338–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavem K, Aaser E, Sandvik L, Bjørnholt JV, Erikssen G, Thaulow E, Erikssen J, 2005. Lung function, smoking and mortality in a 26-year follow-up of healthy middle-aged males. Eur. Respir. J. 25, 618–625. [DOI] [PubMed] [Google Scholar]
- Steffener J, Habeck C, O’Shea D, Razlighi Q, Bherer L, Stern Y, 2016. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol. Aging 40, 138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan Y, Sutin AR, Terracciano A, 2018. Subjective Age and Mortality in Three Longitudinal Samples. Psychosom. Med. 80, 659–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur. Neuropsychopharmacol. 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Susser E, Verhulst S, Kark JD, Factor-Litvak PR, Keyes K, Magnus P, Aviv A, 2016. Non-Dynamic Association of Depressive and Anxiety Disorders With Leukocyte Telomere Length? Am. J. Psychiatry 173, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Biancotto A, Moaddel R, Moore AZ, Gonzalez-Freire M, Aon MA, Candia J, Zhang P, Cheung F, Fantoni G, CHI consortium, Semba RD, Ferrucci L, 2018. Plasma proteomic signature of age in healthy humans. Aging Cell e12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif CL, Gauthier CJ, Steele CJ, Bazin P-L, Schäfer A, Schaefer A, Turner R, Villringer A, 2016. Advanced MRI techniques to improve our understanding of experience-induced neuroplasticity. Neuroimage 131,55–72. [DOI] [PubMed] [Google Scholar]
- Teo YV, Capri M, Morsiani C, Pizza G, Faria AMC, Franceschi C, Neretti N, 2018. Cell-free DNA as a biomarker of aging. Aging Cell, e12890 Doi: 10.1111/acel.12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, Toro R, Jahanshad N, Schumann G, Franke B, Wright MJ, Martin NG, Agartz I, Alda M, Alhusaini S , Almasy L, Almeida J, Alpert K, Andreasen NC, Andreassen OA, Apostolova LG, Appel K, Armstrong NJ, Aribisala B, Bastin ME, Bauer M, Bearden CE, Bergmann O, Binder B, Blangero J, Bockholt HJ, Bøen E, Bois C, Boomsma DI, Booth T, Bowman IJ, Bralten J, Brouwer RM, Brunner HG, Brohawn DG, Buckner RL, Buitelaar J, Bulayeva K, Bustillo JR, Calhoun VD, Cannon DM, Cantor RM, Carless MA, Caseras X, Cavalleri GL, Chakravarty MM, Chang KD, Ching CRK, Christoforou A, Cichon S, Clark VP, Conrod P, Coppola G, Crespo-Facorro B, Curran JE, Czisch M, Deary IJ, de Geus EJC, den Braber A, Delvecchio G, Depondt C, de Haan L, de Zubicaray GI, Dima D, Dimitrova R, Djurovic S, Dong H, Donohoe G, Duggirala R, Dyer TD, Ehrlich S, Ekman CJ, Elvsåshagen T, Emsell L, Erk S, Espeseth T, Fagerness J, Fears S, Fedko I, Fernández G, Fisher SE, Foroud T, Fox PT, Francks C, Frangou S, Frey EM, Frodl T, Frouin V, Garavan H, Giddaluru S, Glahn DC, Godlewska B, Goldstein RZ, Gollub RL, Grabe HJ, Grimm O, Gruber O, Guadalupe T, Gur RE, Gur RC, Göring HHH, Hagenaars S, Hajek T , Hall GB, Hall J, Hardy J, Hartman CA, Hass J, Hatton SN, Haukvik UK, Hegenscheid K, Heinz A, Hickie IB, Ho B-C, Hoehn D, Hoekstra PJ, Hollinshead M, Holmes AJ, Homuth G, Hoogman M, Hong LE, Hosten N, Hottenga J-J, Hulshoff Pol HE, Hwang KS, Jack CR jr, Jenkinson M, Johnston C, Jönsson EG, Kahn RS, Kasperaviciute D, Kelly S, Kim S, Kochunov P, Koenders L, Krämer B, Kwok JBJ, Lagopoulos J, Laje G, Landen M, Landman BA, Lauriello J, Lawrie SM, Lee PH, Le Hellard S, Lemaitre H, Leonardo CD, Li C-S, Liberg B, Liewald DC, Liu X, Lopez LM, Loth E, Lourdusamy A, Luciano M, Macciardi F, Machielsen MWJ, Macqueen GM, Malt UF, Mandl R, Manoach DS, Martinot J-L, Matarin M, Mather KA, Mattheisen M, Mattingsdal M, Meyer-Lindenberg A, McDonald C, McIntosh AM, McMahon FJ, McMahon KL, Meisenzahl E, Melle I, Milaneschi Y, Mohnke S, Montgomery GW, Morris DW, Moses EK, Mueller BA, Muñoz Maniega S, Mühleisen TW, Müller-Myhsok B, Mwangi B, Nauck M, Nho K, Nichols TE, Nilsson L-G, Nugent AC, Nyberg L, Olvera RL, Oosterlaan J, Ophoff RA, Pandolfo M, Papalampropoulou-Tsiridou M, Papmeyer M, Paus T, Pausova Z, Pearlson GD, Penninx W, Peterson CP, Pfennig A, Phillips M, Pike GB, Poline J-B, Potkin SG, Pütz B, Ramasamy A, Rasmussen J, Rietschel M, Rijpkema M, Risacher SL, Roffman JL, Roiz-Saãtianez R, Romanczuk-Seiferth N, Rose EJ, Royle NA, Rujescu D, Ryten M, Sachdev PS, Salami A, Satterthwaite TD, Savitz J, Saykin AJ, Scanlon C, Schmaal L, Schnack G, Schork AJ, Schulz SC, Schür R, Seidman L, Shen L, Shoemaker JM, Simmons A, Sisodiya SM, Smith C, Smoller JW, Soares JC, Sponheim SR, Sprooten E, Starr JM, Steen VM, Strakowski S, Strike L, Sussmann J, Sämann PG, Teumer A, Toga AW, Tordesillas-Gutierrez D, Trabzuni D, Trost S, Turner J, Van den Heuvel M, van der Wee NJ, van Eijk K, van Erp TGM, van Haren NEM, van Eijt D, van Tol M-J, Valdés Hernández MC, Veltman DJ, Versace A, Völzke H, Walker R, Walter H, Wang L, Wardlaw JM, Weale ME, Weiner MW, Wen W, Westlye LT, Whalley HC, Whelan CD, White T„ Winkler AM, Wittfeld K, Woldehawariat G, Wolf C, Zilles D, Zwiers MP, Thalamuthu A, Schofield PR, Freimer NB, Lawrence NS, Drevets W, Alzheimer’s Disease Neuroimaging Initiative, EPIGEN Consortium, IMAGEN Consortium, Saguenay Youth Study (SYS) Group, 2014. The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain Imaging Behav. 8, 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus AJ, Gallimore RM, Salas LA, Christensen BC, 2017. Cell-type deconvolution from DNA methylation: a review of recent applications. Hum. Mol. Genet. 26, R216–R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumpff C, Marsland L, Basualto-Alarcon C, Martin JL, Carroll JE, Sturm G, Vincent AE, Mosharov EV, Gu Z, Kaufman BA, Picard M., 2019. Acute Psychological Stress Triggers Circulating Cell-FreeMitochondrial DNA. Psychoneuroendocrinology (under revision). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Carpenter LL, Kao H-T, Porton B, Philip NS, Ridout SJ, Ridout KK, Price LH, 2015. Association of telomere length and mitochondrial DNA copy number in a community sample of healthy adults. Exp. Gerontol. 66, 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Parade SH, Price LH, Kao H-T, Porton B, Philip NS, Welch ES, Carpenter LL, 2016. Alterations of Mitochondrial DNA Copy Number and Telomere Length With Early Adversity and Psychopathology. Biol. Psychiatry 79, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell DJ, Bharadwaj MS, Van Horn CG, Kritchevsky SB, Nicklas BJ, Molina AJA, 2015. Respirometric Profiling of Muscle Mitochondria and Blood Cells Are Associated With Differences in Gait Speed Among Community-Dwelling Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 70, 1394–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urata M, Koga-Wada Y, Kayamori Y, Kang D, 2008. Platelet contamination causes large variation as well as overestimation of mitochondrial DNA content of peripheral blood mononuclear cells. Ann.Clin. Biochem. 45, 513–514. [DOI] [PubMed] [Google Scholar]
- Vance MC, Bui E, Hoeppner SS, Kovachy B, Prescott J, Mischoulon D, Walton ZE, Dong M, Nadal MF, Worthington JJ, Hoge EA, Cassano P, Orr EH, Fava M, de Vivo I, Wong K-K, Simon NM, 2018. Prospective association between major depressive disorder and leukocyte telomere length over two years. Psychoneuroendocrinology 90, 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool CH, Kempen GIJM, Bosma H, van Boxtel MPJ, Jolles J, van Eijk JTM, 2007. Associations between lifestyle and depressed mood: longitudinal results from the Maastricht Aging Study. Am. J. Public Health 97, 887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhooren V, Laroy W, Libert C, Chen C, 2008. N-glycan profiling in the study of human aging. Biogerontology 9, 351–356. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BWJH, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BWJH, 2014. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mo I. Psychiatry 19, 895–901. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Picard M, Epel EE, Wolkowitz OM, Matthews KA, Penninx BWJH, Puterman E, 2018. Depression, telomeres and mitochondrial DNA: Between- and within-person associations from a 10-year longitudinal study. Mol. Psychiatry 23, 850–857. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Wolkowitz OM, Penninx BWJH, 2014. Cellular aging in depression: Permanent imprint or reversible process?: An overview of the current evidence, mechanistic pathways, and targets for interventions. Bioessays 1–11. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, van Oppen P, Revesz D, Wolkowitz OM, Penninx BWJH, 2016. Depressive and Anxiety Disorders Showing Robust, but Non-Dynamic, 6-Year Longitudinal Association With Short Leukocyte Telomere Length. Am. J. Psychiatry 173, 617–624. [DOI] [PubMed] [Google Scholar]
- Vetter VM, Antje M, Karbasiyan M, Steinhagen-Thiessen E, Hopfenmiiller W, Demuth I, 2018. Epigenetic clock and relative telomere length represent largely different aspects of aging in the Berlin Aging Study II (BASE-II). J. Gerontol. A Biol. Sci. Med. Sci 10.1093/gerona/gly184 [DOI] [PubMed] [Google Scholar]
- Vincent AE, Rosa HS, Pabis K, Lawless C, Chen C, Griinewald A, Rygiel KA, Rocha MC, Reeve AK, Falkous G, Perissi V, White K, Davey T, Petrof BJ, Sayer AA, Cooper C, Deehan D, Taylor RW, Turnbull DM, Picard M, 2018. Subcellular origin of mitochondrial DNA deletions in human skeletal muscle. Ann. Neurol, 10.1002/ana.25288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viron MJ, Stern TA, 2010. The impact of serious mental illness on health and healthcare. Psychosomatics 51,458–465. [DOI] [PubMed] [Google Scholar]
- Voisey J, Lawford BR, Morris CP, Wockner LF, Noble EP, Young RM, Mehta D, 2017. Epigenetic analysis confirms no accelerated brain aging in schizophrenia, npj Schizophrenia 3, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin S, Eynon N, Yan X, Bishop DJ, 2015. Exercise training and DNA methylation in humans. Acta Physiol. 213, 39–59. [DOI] [PubMed] [Google Scholar]
- Wagner KH, Cameron-Smith D, Wessner B, Franzke B, 2016. Biomarkers of aging: From function to molecular biology. Nutrients 8, 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker ER, McGee RE, Druss BG, 2015. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry 72, 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Zhan Y, Pedersen NL, Fang F, Hägg S, 2018. Telomere Length and All-Cause Mortality: A Meta-analysis. Ageing Res. Rev. 48, 11–20. [DOI] [PubMed] [Google Scholar]
- Weidner CI, Lin Q, Koch CM, Eisele L, Beier F, Ziegler P, Bauerschlag DO, Jöckel K-H, Erbel R, Mühleisen TW, Zenke M, Brümmendorf TH, Wagner W, 2014. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 15, R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Shadel GS, 2017. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat. Rev. Immunol. 17, 363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalley HC, Gibson J, Marioni R, Walker RM, Clarke T-K, Howard DM, Adams MJ, Hall L, 2017. Accelerated epigenetic ageing in depression. [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjll K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF, Nordfjall K, Bergdahl J, Hultdin J, Del-Favero J, Roos G, Nilsson LG, Adolfsson R, Norrback KF, 2012. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol.Psychiatry 71,294–300. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Rode L, Bojesen SE, Nordestgaard BG, 2017. Telomere length and depression: prospective cohort study and Mendelian randomisation study in 67 306 individuals. Br. J. Psychiatry 210, 31–38. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, Ashley-Koch AE, Garrett M, Kimbrel NA, Lori A, Va Mid-Atlantic Mirecc Workgroup, Aiello AE, Baker DG, Beckham JC, Boks MP, Galea S, Geuze E, Hauser MA, Kessler RC, Koenen KC, Miller MW, Ressler KJ, Risbrough V, Rutten BPF, Stein MB, Ursano RJ, Vermetten E, Vinkers H, Uddin M, Smith AK, Nievergelt CM, Logue MW, 2018. Traumatic stress and accelerated DNA methylation age: A meta-analysis. Psychoneuroendocrinology 92, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfers T, Doan NT, Kaufmann T, Alnæss D, Moberget T, Agartz I, Buitelaar JK, Ueland T, Melle I, Franke B, Andreassen OA, Beckmann CF, Westlye LT, Marquand AF, 2018. Mapping the Heterogeneous Phenotype of Schizophrenia and Bipolar Disorder Using Normative Models. JAMA Psychiatry, 10.1001/jamapsychiatry.2018.2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, Rosser R, Burke H, Compagnone M, Nelson JC, Dhabhar FS, Blackburn EH, 2012. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol. Psychiatry 17, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS, 2015. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia P, Radpour R, Zachariah R, Fan AXC, Kohler C, Hahn S, Holzgreve W, Zhong XY, 2009. Simultaneous quantitative assessment of circulating cell-free mitochondrial and nuclear DNA by multiplex real-time PCR. Genet. Mol. Biol. 32, 20–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, Chen W, McDermott J, Han J-DJ, 2017. Molecular and phenotypic biomarkers of aging. F1000Res. 6, 860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Westfall J, 2017. Choosing Prediction Over Explanation in Psychology: Lessons From Machine Learning. Perspect. Psychol. Sci. 12, 1100–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates T, Zaccardi F, Dhalwani NN, Davies MJ, Bakrania K, Celis-Morales CA, Gill JMR, Franks PW, Khunti K, 2017. Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: a UK Biobank observational study. Eur. Heart J. 38, 3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung SSY, Reijnierse EM, Trappenburg MC, Hogrel J-Y, McPhee JS, Piasecki M, Sipila S, Salpakoski A, Butler-Browne G, Pääsuke M, Gapeyeva H, Narici MV, Meskers CGM, Maier AB, 2018. Handgrip Strength Cannot Be Assumed a Proxy for Overall Muscle Strength. J. Am. Med. Dir. Assoc 19, 703–709. [DOI] [PubMed] [Google Scholar]
- Yu W-Y, Chang H-W, Lin C-H, Cho C-L, 2008. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J. Psychiatry Neurosci. 33, 244–247. [PMC free article] [PubMed] [Google Scholar]
- Yu X, Wang Y, Kristie J, Dong J, Chu X, Ge S, Wang H, Fang H, Gao Q, Liu D, Zhao Z, Peng H, Pucic Bakovic M, Wu L, Song M, Rudan I, Campbell H, Lauc G, Wang W, 2016. Profiling IgG N-glycans as potential biomarker of chronological and biological ages: A community- based study in a Han Chinese population. Medicine 95, e4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas AS, Arloth J, Carrillo-Roa T, lurato S, Röh S, Ressler KJ, Nemeroff CB, Smith AK, Bradley B, Heim C, Menke A, Lange JF, Brückl T, Ising M, Wray NR, Erhardt A, Binder EB, Mehta D, 2015. Lifetime stress accelerates epigenetic aging in an urban, African American cohort: relevance of glucocorticoid signaling. Genome Biol. 16, 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Rane G, Dai X, Shanmugam MK, Arfuso F, Sarny RP, Lai MKP, Kappei D, Kumar AP, Sethi G, 2016. Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res. Rev. 25, 55–69. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Ye K, Picard M, Gu Z, 2017. Independent impacts of aging on mitochondrial DNA quantity and quality in humans. BMC Genomics 18, 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwicker A, Fabbri C, Rietschel M, Hauser J, Mors O, Maier W, Zobel A, Farmer A, Aitchison KJ, McGuffin P, Lewis CM, Uher R, 2018. Genetic disposition to inflammation and response to antidepressants in major depressive disorder. J. Psychiatr. Res. 105, 17–22. [DOI] [PubMed] [Google Scholar]