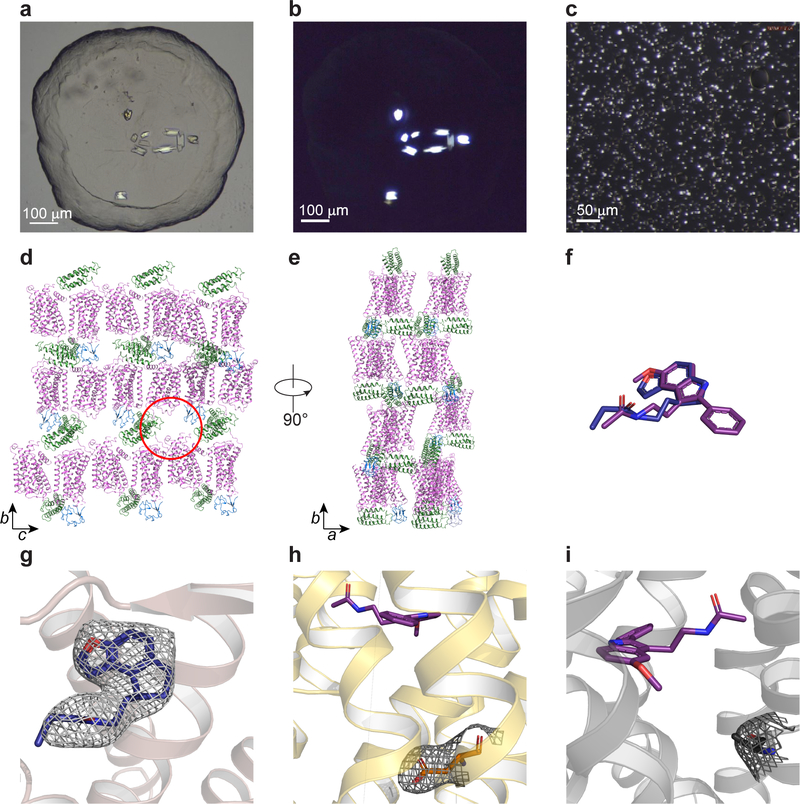
Abstract
The human MT11 and MT22 melatonin receptors are G protein-coupled receptors (GPCRs) involved in the regulation of circadian rhythm and sleep patterns3. Drug development efforts target both receptors for treatment of insomnia, circadian rhythm and mood disorders, and cancer3, while MT2 has also been implicated in type 2 diabetes (T2D)4,5. Here we report the X-ray Free Electron Laser (XFEL) structures of the human MT2 receptor in complex with agonists 2-phenylmelatonin (2-pmt) and ramelteon6 at resolutions of 2.8 Å and 3.3 Å, respectively, along with two structures of function-related mutants, H2085.46A (superscripts represent the Ballesteros-Weinstein residue numbering nomenclature7) and N862.50D, obtained in complex with 2-pmt. Comparison of the MT2 structures with MT18 reveals that, despite the fact that the orthosteric ligand-binding site residues are conserved, there are notable conformational variations as well as differences in [3H]-melatonin dissociation kinetics that provide new insights into the selectivity between melatonin receptor subtypes. In addition to the membrane-buried lateral ligand entry channel that is also observed in MT1, the MT2 structures reveal a narrow opening towards the solvent in the extracellular part of the receptor. We provide functional and kinetic data supporting a prominent role for the intramembrane ligand entry in both receptors, while simultaneously suggesting the possibility of an extracellular entry path in MT2. Our findings contribute to a molecular understanding of melatonin receptor subtype selectivity and ligand access modes, which are essential for the design of highly selective melatonin tool compounds and therapeutic agents.
To enhance low surface expression and stability of the wild-type receptor, eight point mutations were introduced based on homology to other class A receptors: D862.50N9, L108ECL1F, F1293.41W10, N1373.49D, C1403.52L, W2646.48F, A3057.50P, and N3128.47D, which were essential for high-resolution structure determination of MT2 as well as MT18. To promote crystal contacts, we used a double-fusion approach, with rubredoxin11 in the intracellular loop 3 (ICL3) and thermostabilised apocytochrome b562RIL (BRIL)11, attached to the receptor N-terminus. Radioligand binding assays revealed a 120-fold reduction of melatonin binding affinity (~30-fold reduction at physiological concentration of NaCl), likely due to the stabilisation of the crystallised construct in an inactive “low agonist affinity” state deficient of G-protein coupling and signaling9,12 (Extended Data Table 1). All four MT2 structures were obtained using lipidic cubic phase (LCP)13 crystallisation (Extended Data Fig. 1, Extended Data Table 2). The overall receptor conformation was found to be similar in all four structures (Cα r.m.s.d. < 0.3 Å), therefore the highest resolution MT2-2-pmt structure is used in the analysis below unless otherwise noted.
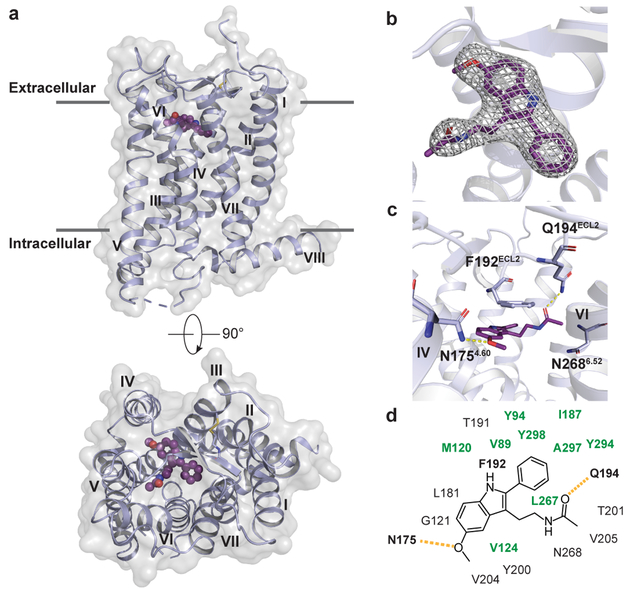
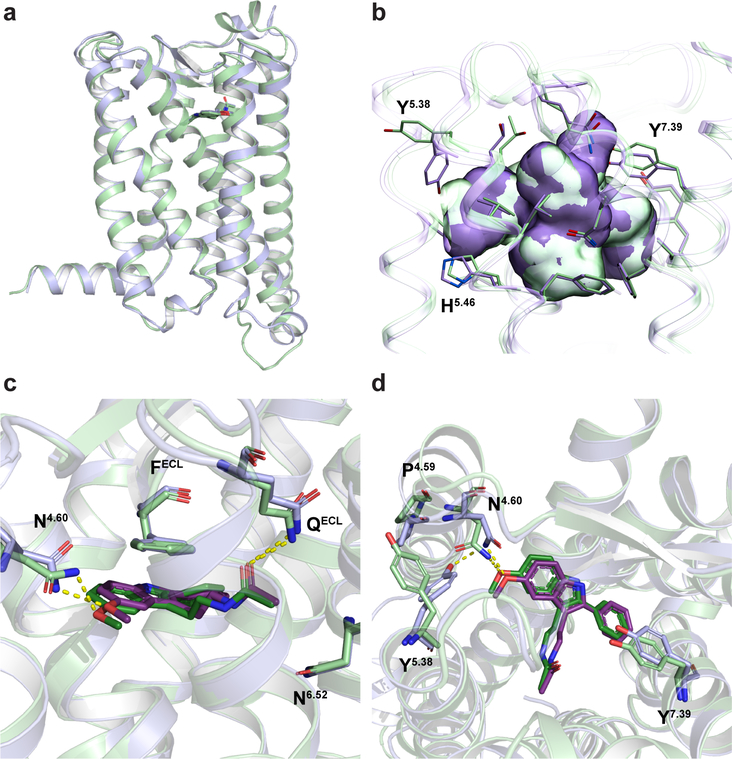
MT2 adopts the canonical 7TM-fold of class A receptors, with the short amphipathic helix VIII parallel to the membrane on the intracellular side (Fig. 1a). Like in MT18, the 7TM bundle of MT2 is found in inactive conformation. Restoring the function-impairing D862.50N mutation (Extended Data Table 3) allowed us to solve the MT2-N86D-2-pmt structure at lower resolution, revealing no significant effect of this mutation on the overall receptor conformation, as also supported by molecular dynamics (MD) simulations (Supplementary Fig. 1). Structural comparison of MT2 vs. MT1, which share 68% sequence identity, reveals a remarkable overall similarity (Cα r.m.s.d. < 0.6 Å), with all ligand-interacting residues conserved8 (Fig. 1d, Extended Data Fig. 2c). We observe a common pharmacophore between receptor subtypes that consists of aromatic stacking of the ligand core with F192ECL2, as well as hydrogen bonds between the methoxy group of 2-pmt and N1754.60 and between the ligand alkylamide tail and Q194ECL2 (Fig. 1c, d). Stability of these ligand-anchoring interactions is confirmed by MD simulations (Extended Data Fig. 3). Further, mutating F192ECL2 to isoleucine or alanine causes loss of ligand binding and signaling (Extended Data Tables 1, 4), as also observed for MT18. In contrast to MT1, however, mutating N1754.60 to alanine retains receptor function, pointing to a different role of this residue in the activation of the two receptor subtypes. While mutating either Q194ECL2 or N2686.52 to alanine only has minor effects on receptor ligand affinity, receptor activation, or stability (Extended Data Tables 1, 4, 5), the double mutant Q194ECL2A/N2686.52A results in a dramatic loss of receptor activity (Extended Data Tables 4, 5), suggesting a functional redundancy of these residues in MT2. Intriguingly, despite the binding site residues being conserved between the two receptors, we observe subtle conformational differences, such as in the side chains of Y2005.38, Y2947.39 and the backbone region surrounding P1744.59 (Extended Data Fig. 2d). Furthermore, the MT2 binding pocket is about 50 Å3 (7 %) larger than that of MT1, with most of the volume difference attributed to the region around the alkylamide tail and the hydrophobic sub-pocket that accommodates substituents of melatonin analogues in our structures (Fig. 1d, Extended Data Fig. 2b) and plays a key role in MT2 selectivity as further discussed.
Fig. 1 |. Overview of the MT2 structure.
a, Overview of MT2 (violet) shows the canonical 7TM topology, with the ligand 2-pmt (purple) in the binding pocket. A 90° view shows the receptor from the extracellular side. Approximate membrane boundaries are shown as grey lines. b, 2mFo-DFc density (grey mesh) of 2-pmt contoured at 1 σ. c, Binding pocket with key ligand interaction residues. d, Schematic diagram of ligand-interacting residues. Residues in the hydrophobic sub-pocket are coloured green. Hydrogen bonds are shown as dashed yellow lines in c and d.
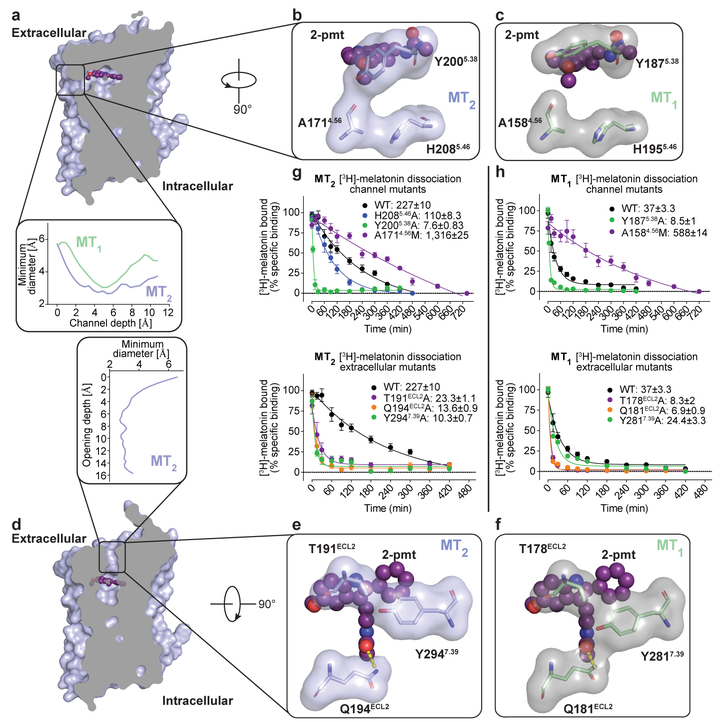
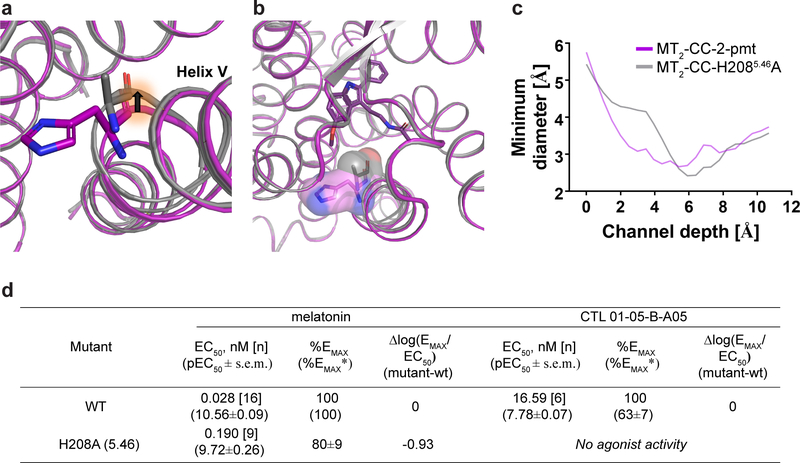
Structural analysis of MT2 reveals an opening between helices IV and V from the orthosteric ligand binding site to the membrane (Fig. 2a). This channel is similar to the one observed in MT1, but more constricted (~2.6 Å in diameter at the narrowest part). A comparison between the MT2 and MT1 structures reveals that Y2005.38 in MT2 makes a hydrogen bond to N1754.60, constricting the channel, while in MT1 it adopts a different conformation pointing towards the lipid interface (Fig 2a–c, Extended Data Fig. 2c, d). Close to the entrance is H2085.46, which in our MT2-H208A-2-pmt structure further closes off the opening by an ~0.9 Å inward shift of helix V (Extended Data Fig. 4), suggesting that this residue plays a role in controlling the channel entrance, albeit only moderately influencing ligand affinity and receptor function in MT2 (Extended Data Table 1, 4). Further analysis of the MT2 structures reveals a potential secondary access route to the orthosteric binding site from the solvent-exposed extracellular (ECL) region (Fig. 2d). This second opening has a slightly larger diameter (~2.5–3 Å) and is lined by aromatic Y2947.39 and hydrophilic T191ELC2, Q194ECL2 residues (Fig. 2e). In MT1, the corresponding residues Q181ECL2 and Y2817.39 adopt different conformations, completely sealing off this entrance (Fig. 2f).
Fig. 2 |. Two possible ligand entries in MT2.
a, View of the membrane-buried channel in MT2. Insert shows the channel diameter profile across its length for MT1 and MT2. b, A 90° view of the channel in MT2, highlighting three residues discussed in the text. c, The same as in b view of MT1 (green) showing a different conformation of Y1875.38 that widens the channel compared to MT2. d, View of the ECL opening found in MT2 (violet) with 2-pmt (purple). Insert shows the ECL opening profile across the length. e, A 90° view through the ECL opening in MT2, highlighting three residues discussed in the text. f, The same as in e view of MT1 (green), showing a different conformation of Y2817.39 that seals the ECL opening. g, [3H]-melatonin dissociation kinetics for MT2 membrane channel mutants (top) and ECL opening mutants (bottom). h, same as in g for MT1. Residence time (1/koff) in g and h is given in minutes. Data are shown as mean±s.e.m. for n=3 independent experiments.
To test the relative importance of these two putative binding site access routes we performed kinetic ligand dissociation studies on both receptors using [3H]-melatonin as a tracer. The ligand residence time (koff−1) in wild-type MT2 is substantially longer than that in MT1, suggesting that the narrower membrane entry channel indeed restricts ligand access (Fig. 2g, h). Mutation of the membrane channel-lining residue Y5.38A, designed to widen the access channel, shows a drastic 30-fold decrease in residence time for MT2 (with a similar ligand affinity), while the corresponding mutation in MT1 displays a more modest decrease in residence time, in agreement with the wider channel and a different conformation of Y5.38 in MT1. To constrict the channel, we mutated A4.56, a critical residue at the interface of helices IV and V in both receptors, into a bulkier methionine. Strikingly, this mutation dramatically increases residence time for both receptors (Fig. 2g, h), reaching up to 20 hours in MT2, suggesting a prominent role of this channel for ligand access in both receptors.
For mutants designed to widen the ECL opening in both receptors, ligand residence time was reduced more than 10-fold at MT2 mutants T191ECL2A and Q194ECL2A, and roughly 5-fold at equivalent ECL2 mutants in MT1 (Fig. 2g, h). Mutating Y2947.39A in MT2 showed even greater decrease in ligand residence time (22-fold) relative to wild-type, while the equivalent mutant in MT1 showed similar residence time to wild-type. These differences can be reconciled by a higher importance of the ECL ligand site access in MT2 compared to MT1 in agreement with the crystal structures, where residue Y2947.39 adopts a different conformation in MT2, allowing for easier ligand egress through the ECL opening.
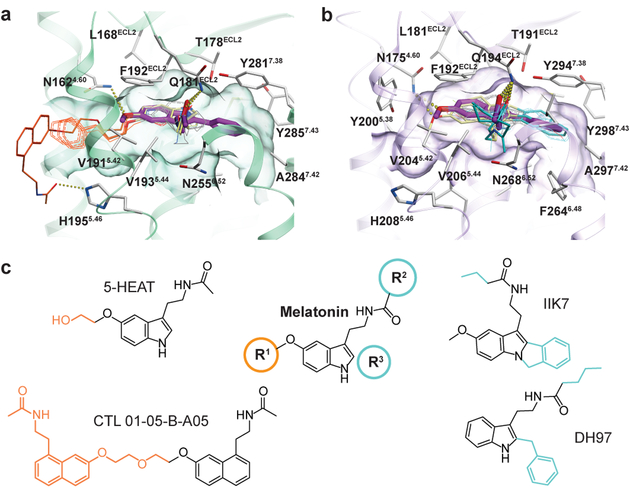
The elucidation of high-resolution structures of both melatonin receptor subtypes and published ligand structure-activity relationship (SAR) data14,15 allowed us to establish a model of receptor subtype ligand selectivity. To this end, we utilised molecular docking of several available selective ligands to both receptors. Docking of the moderately MT1-selective compound 5-HEAT16 and bitopic ligand CTL 01–05-B-A058 suggests that although an extension or substitution of the R1 position by a linear alkyl chain can be accommodated by the membrane access channel in both MT1 and MT2 (Fig. 3a, c), the narrower MT2 channel renders binding of the extended portion of the bitopic ligands suboptimal due to potential steric clashes. Accordingly, an H2085.46A mutation in MT2 abolished Gi-agonist efficacy of the bitopic ligand CTL 01–05-B-A05 (Extended Data Fig. 4d), likely by further restricting the channel and/or eliminating the hydrogen bond between H2085.46 and CTL 01–05-B-A05, observed in docking to MT18. This mutation had negligible effect on monotopic ligand binding and function (Extended Data Tables 1, 4, 5), suggesting that a sufficiently wide membrane channel (as in MT1) is critical for accommodation of bitopic ligands.
Fig. 3 |. Selectivity determinants of ligands at MT1 and MT2.
a, Docking of selective ligands into MT1 (green), with 2-pmt (purple) from the crystal structure shown as reference. Ligands selective for MT1 (compounds 63, 64, 65a, and 65b)22 are shown in grey. Two representative ligands, 5-HEAT16 and CTL 01–05-B-A058 are coloured pale yellow, with their selectivity-conferring substituents (R1 position) shown in orange. b, Docking of ligands into MT2 (violet), with 2-pmt (purple) shown as reference. Non-selective (tasimelteon, TIK30122) and selective (UCM1014, K185, and 4P-PDOT)22 ligands are shown in grey. Two representative ligands, DH9717 and IIK717 are coloured pale yellow, with selectivity-conferring substituents (R2 and R3 positions) shown in cyan. Predicted hydrogen bonds are shown as dotted lines in a and b. c, Melatonin SAR, where R1 substituents confer MT1 selectivity (orange), and substituents in R2 and R3 positions confer MT2 selectivity (cyan). See Supplementary Table 1 for a list of all docked ligands.
The MT2-selective ligands IIK7 and DH97 (both ~90-fold selective)17,18 adopt “tail up” binding modes similar to that of 2-pmt with their alkylamide tails (R2 position in Fig. 3b, c) interacting with Q194ECL2. In contrast, in MT1 the longer alkylamide tails of these ligands avoid such upward tail position due to steric clashes and can only adopt suboptimal “tail down” conformations. Bulky substituents in the R3 position confer MT2 selectivity by utilising the larger hydrophobic sub-pocket of the receptor (Fig. 3b, c). In summary, our analysis suggests that R1 substituents are important for MT1 selectivity, while R2 and R3 mostly convey selectivity towards MT2 (Fig. 3c). The slightly larger binding site in MT2 also helps to achieve selectivity, as reflected by the larger number of compounds moderately selective for MT2 (Extended Data Fig. 5).
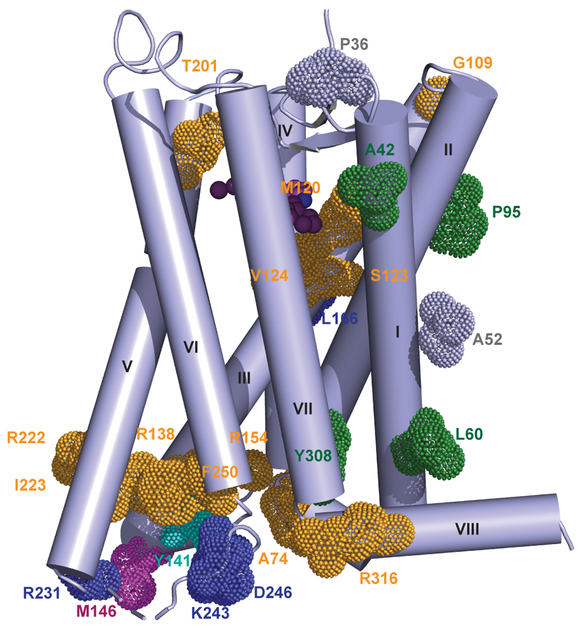
Subtype-selective compounds are desirable due to the involvement of MT2 in T2D, where a number of single nucleotide polymorphisms (SNPs) have been reported4,5. Mapping these sites onto our MT2 structure, we observed clustering of residues in the vicinity of the ligand binding pocket and on the receptor surface, along the membrane interface of helices I and II and the intracellular G protein and β-arrestin binding regions (Fig. 4). The exposed positions of these residues could point to their involvement in interactions with intracellular and membrane partners. Other instances of T2D SNPs include P952.59L of the YPYP motif, which was found to play a role in receptor stability and function in MT18, and mutations in known microswitches such as R1383.50H/L/C of the E/DRY motif19 and Y3087.53S of the NPxxY motif12. While none of the analysed SNPs is involved in direct interactions with melatonin, the M120I and V124I variants are located in the hydrophobic sub-pocket of the receptor, which could influence ligand binding and affect subsequent signaling pathways4,5.
Fig. 4 |. MT2 mutations implicated in type 2 diabetes.
Mapping of residues implicated in T2D as described in Refs.4,5 on the MT2 crystal structure. Residues, mutations of which lead to defects in two or more pathways, are coloured gold, G protein-specific defects - cyan, β-arrestin 2-specific - blue, ERK-specific – magenta, mutations abolishing melatonin-binding are shown in green, and those similar to WT shown in grey. T2D mutations in residues, not observed in the crystal structure, are not shown.
The structural basis for melatonin receptor subtype selectivity revealed here has the potential to inspire a new generation of highly selective pharmacological tools that will help to further dissect the melatonin system. We also provide insights into differences in ligand entry between the two receptors by demonstrating the potential of MT2 to support extracellular ligand access to the binding pocket. This difference in ligand entry can be exploited to facilitate melatonin receptor subtype selectivity, as the ECL route in MT2 could accommodate more polar compounds compared to the membrane-buried channel. We therefore expect that our results will lead to new therapies involving these pleiotropic receptors, aimed at but not limited to T2D, cancer, and sleep disorders.
Methods
Design and expression of MT2-CC
The DNA sequence of human MT2 receptor (UniProt20 identifier P49286) was synthesised by GenScript with optimisation for expression in insect cells. The crystallised construct (MT2-CC) has truncations of N-terminal residues 1–30 and C-terminal residues 341–362. The thermostabilised apocytochrome b562RIL (BRIL, UniProt P0ABE7) from Escherichia coli with mutations M7W, H102I, and R106L was fused to the truncated N-terminus of MT2 with a six-residue linker (amino acid sequence GDGARP). Another fusion protein, rubredoxin (Rub, Uniprot P00268), was fused in the ICL3, replacing receptor residues 232–240. For construct optimisation (to increase monodispersity, thermostability, and crystallisability), the following point mutations were added: D862.50N9, L108ECL1F, F1293.41W10, N1373.49D, C1403.52L, W2646.48F, A3057.50P, and N3128.47D (see the accompanying paper for details8). The MT2-CC coding sequence was subcloned into a modified pFastBac1 (Invitrogen) vector, with a haemagglutinin (HA) signal sequence and a Flag tag on the N-terminus and a PreScission protease cleavage site followed by a 10×His tag on the C-terminus. The receptor was expressed in Spodoptera frugiperda cells (Sf9, purchased from ATCC, CRL-1711, authenticated by supplier using morphology and growth characteristics, certified mycoplasma-free), which were harvested and stored as described in the accompanying paper8.
Purification of MT2-CC
Insect cell membranes were prepared by thawing frozen cell pellets in a hypotonic buffer containing 10 mM HEPES (pH 7.5), 10 mM MgCl2, 20 mM KCl, and homemade protease inhibitor cocktail. Extensive washing of the raw membranes was performed by repeated Dounce homogenisation and centrifugation in hypotonic buffer (once), followed by high osmotic buffer containing 1.0 M NaCl, 10 mM HEPES (pH 7.5), 10 mM MgCl2, 20 mM KCl, and homemade protease inhibitor cocktail (two or three times), thereby separating soluble and membrane associated proteins from integral membrane proteins. Stocks (100 mM) of 2-pmt (Tocris) and ramelteon (Apex Biosciences) were dissolved in DMSO. Washed membranes were resuspended into a buffer containing 50 μM 2-pmt or ramelteon, 2 mg ml−1 iodoacetamide, and homemade protease inhibitor cocktail, and incubated at 4 °C for 30 min before solubilisation. The membranes were then solubilised in 50 mM HEPES (pH 7.5), 150 mM NaCl, 1% (wt/vol) n-dodecyl-β-D-maltopyranoside (DDM, Anatrace), 0.2% (wt/vol) cholesteryl hemisuccinate (CHS, Sigma-Aldrich) at 4 °C for 3 h. The supernatant was isolated by centrifugation at 60,000×g for 50 min, and incubated in 20 mM HEPES (pH 7.5), 800 mM NaCl with Talon (immobilized metal affinity chromatography IMAC) resin (Clontech) overnight at 4 °C. After binding, the resin was washed with twenty column volumes of wash buffer 1 (50 mM HEPES (pH 7.5), 50 μM 2-pmt or ramelteon, 800 mM NaCl, 10% (vol/vol) glycerol, 0.1% (wt/vol) DDM, 0.02% (wt/vol) CHS, 10 mM imidazole), followed by ten column volumes of wash buffer 2 (50 mM HEPES (pH 7.5), 50 μM 2-pmt or ramelteon, 150 mM NaCl, 10% (vol/vol) glycerol, 0.05% (wt/vol) DDM, 0.01% (wt/vol) CHS, 50 mM imidazole). The protein was then eluted in minimal volumes of elution buffer (50 mM HEPES (pH 7.5), 50 μM 2-pmt or ramelteon, 150 mM NaCl, 10% (vol/vol) glycerol, 0.02% (wt/vol) DDM, 0.01% (wt/vol) CHS, 220 mM imidazole). PD MiniTrap G-25 columns (GE Healthcare) were used to remove imidazole. The protein was then treated overnight with His-tagged PreScission protease (Genscript) to cleave the C-terminal His-tag. PreScission protease and the cleaved C-terminal fragment were removed by binding to Talon IMAC resin for 1.5 h at 4 °C. The protein was collected as the TALON IMAC column flow-through. The ligand concentration was increased to 100 μM, and the protein was concentrated to 30–40 mg ml−1 with a 100 kDa molecular mass cut-off Vivaspin centrifuge concentrator (Sartorius).
Protein stability assays
The stability of purified MT2-CC was analysed by the microscale thermostability assay21 using Rotorgene (QIAGEN). Briefly, 1–5 μg of protein was mixed with 1.5 μM 7-diethylamino-3-(4′-maleimidylphenyl)-4-methylcoumarin (CPM) dye (2.5 mM stock in DMSO) in 25 mM HEPES pH 7.5, 150 mM NaCl, 0.02% DDM (wt/vol), 0.004% CHS (wt/vol), 10% glycerol (vol/vol), and indicated concentrations of compounds to a final volume of 100 μl. Samples were incubated for 15 min at 20 °C and then heated gradually from 25 °C to 95 °C at a rate of 2 °C min−1, monitoring CPM fluorescence (excitation 365 nm, emission 460 nm). The melting temperature (Tm) was determined using the derivative of the resulting melting temperature curve after background subtraction using Prism 5 (GraphPad, San Diego, California, USA).
Crystallisation
Purified MT2-CC in complex with 2-pmt or ramelteon was reconstituted into LCP by mixing it with molten lipid using a mechanical syringe mixer13. The protein–LCP mixture contained 40% (wt/wt) receptor solution, 54% (wt/wt) monoolein, and 6% (wt/wt) cholesterol. Crystallisation trials were performed in 96-well glass sandwich plates (Marienfeld) using an NT8-LCP robot (Formulatrix) by dispensing 40 nl of protein-laden LCP and 800 nl of precipitant solution per well. Plates were incubated and imaged at 20 °C using an automatic incubator/imager (RockImager 1000, Formulatrix). Initial crystal hits were identified in a condition containing 100 mM HEPES, pH 6.8, 30% (vol/vol) PEG 400, 100 mM NH₄CH₃CO₂. These crystals, approximately 30×30×70 μm3, were harvested using micromounts (MiTeGen) and flash frozen in liquid nitrogen for data collection at a microfocus synchrotron source. After extensive optimisation, the best crystals diffracted to about 3.0 Å resolution, but suffered from radiation damage, resulting in a 3.5 Å complete dataset. Additives had no effect on diffraction quality. Microcrystals for SFX data collection were prepared in gas-tight syringes (Hamilton) as previously described22. After optimisation, diffraction-quality crystals were obtained from 100 mM ADA pH 5.8–6.5, 24–28% (vol/vol) PEG 400, 10–200 mM NH₄CH₃CO₂, 50 μM 2-pmt or ramelteon, by injecting 5 μl of protein-laden LCP into 50 μl precipitant in syringes. Before loading the microcrystals into the LCP injector, excess precipitant was removed and 7.9 MAG lipid was added to the LCP to absorb any residual precipitant solution and to prevent crystalline phase formation upon rapid cooling when injecting LCP into vacuum23.
Crystallographic data collection
Data collection was performed at the Coherent X-ray Imaging (CXI)24 end station of the Linac Coherent Light Source (LCLS), which operated at a wavelength of 1.3 Å (9.83 keV) delivering individual X-ray pulses of 30 and 42 fs pulse duration and approximately 1011 photons per pulse focused into a spot size of approximately 1.5 μm in diameter using a pair of Kirkpatrick–Baez mirrors. Microcrystals (Extended Data Fig. 1b) of MT2 (approximately 5×5×5 μm3) were delivered in the LCP media using an LCP microextrusion injector23 with 50 μm nozzle running at a flow rate of approximately 300 nl min−1. Diffraction images were recorded at a rate of 7,200 patterns per minute (120 Hz) with the 2.3 Megapixel Cornell-SLAC Pixel Array Detector (CSPAD)25. Initial diffraction frames were corrected and filtered using the software package Cheetah26. A crystal “hit” was defined as an image containing a minimum of 20 diffraction peaks with a signal to noise ratio above 4 and a number of pixels above 3. After further refinement of parameters (peak detection, prediction, and integration), images were indexed using MOSFLM27, DirAx28, and XDS29 and integrated and merged into a final dataset by CrystFEL v.0.6.3 software suite30. Integration radii of 3, 5, and 6 pixels with per pattern resolution cut-offs 1.0 nm−1 above the conservative resolution estimates for each crystal were applied (push-res option), otherwise default values were used. The total numbers of collected images/hits/indexed images are as follows: 2,154,963/84,928/31,677 (MT2-CC-2-pmt), 476,863/59,071/28,130 (MT2-CC-H208A-2-pmt), 293,060/22.267/20,704 (MT2-CC-N86D-2-pmt), 727,004/60,005/28,834 (MT2-CC-ramelteon). As resolution cutoff, the criterion31 of CC∗>0.5 was employed for all datasets (see Extended Data Table 2 for data statistics). The space group was determined to be P21, with two molecules per asymmetric unit.
Structure determination
To solve the 2.8 Å resolution MT2-CC-2-pmt structure, a search model was generated as follows: the MT2 receptor sequence was sent to the HHpred server32, and the output models were reduced by removing all low resolution (< 3.0 Å) and NMR structures. The PDB files of the top ten hits were downloaded and prepared with Sculptor33. The models were structurally superimposed, and the side chains were pruned, yielding the conserved receptor core model. The model that produced a successful molecular replacement (MR) solution with Phaser34 (TFZ score of 14.9 and LLG of 320) was based on the C-C chemokine receptor 2 structure (PDB ID: 5T1A)35. This solution containing two receptor molecules was fixed as a partial solution, and the search continued with rubredoxin (PDB ID: 1IRO), where one molecule was placed in the asymmetric unit. The resulting three-component solution was subjected to several rounds of refining with phenix.refine36 and model building with phenix.autobuild37 followed by manual refinement in Coot38. BRIL (PDB ID: 1M6T) was then used independently as a search model for remaining fusion partners in the asymmetric unit. The second BRIL was manually modelled into the electron density; however, no density could be found for the second rubredoxin molecule, which, therefore, was not modelled in the final structure. This rubredoxin fusion partner is most likely disordered and does not participate in crystal contacts, however, there is space for it in the crystal lattice (Extended Data Fig. 1d). A zinc ion was modelled in rubredoxin as previously described39. Refinement and model completion were performed by repetitive cycling between Refmac540 or autoBUSTER41 2.10.2 and manual rebuilding in Coot38 using both 2mFo−DFc and mFo−DFc maps. Ligand restraints for refinement of 2-pmt and ramelteon coordinates were generated by Prodrg42. For the other three datasets, the MT2-CC-2-pmt structure was used as a search model for MR, and the refinement procedure was repeated as described above. The Ramachandran plot obtained by MolProbity43 shows that with exception of Y92 from the YPYP motif all residues are in the favoured/allowed regions: 95.4/4.4% of residues (MT2-CC-2-pmt), 93/6.8% of residues (MT2-CC-H208A), 94.8/5.0% of residues (MT2-CC-N86D), 95.3/4.5% of residues (MT2-CC-ramelteon). Data collection and refinement statistics are summarised for each structure in Extended Data Table 2. Figures containing electron density and molecular structures were generated using PyMol44.
Channel profile calculations
The channel diameter profile along its length was calculated with CAVER analyst v.2.045 using default parameters. Further details can be found in the accompanying paper8.
Molecular docking
MT2 receptor ligands obtained from the ChEMBL database15 were docked into the 2-pmt-bound crystal structures using an energy based docking in ICM-Pro v3.8–646 as described in the accompanying paper8.
Molecular dynamics simulations
The experimental structure of MT2 was prepared and subjected to molecular dynamics simulations as described in the accompanying paper8. The simulation periodic box had dimensions (x, y, z) of 75.5 Å, 75.5 Å, 105.4 Å, and contained lipids (129 POPC molecules), 10,281 water molecules, 26 sodium, and 36 chloride ions.
Radioligand binding assays
Equilibrium binding assays were performed and analysed as described in the MT1 paper8. HEK293T cells (purchased from ATCC, CRL-11268, authenticated by supplier using morphology, growth characteristics and STR profiling, certified mycoplasma-free). For kinetic studies, to initiate dissociation of [3H]-melatonin, 10 μL of cold excess melatonin (10 μM) was added per well at specific time points ranging from 2 minutes to 10 hours, and immediately at time = 0 min plates were harvested. Dissociation experiments were performed at 25 °C for MT1 and 37 °C for MT2 because of the slow kinetics in MT2. For all assays, non-specific activity was defined by the addition of 5 μM 2-pmt. Ligand dissociation data were analysed using “Dissociation-One phase exponential decay” to yield estimates of koff using GraphPad Prism 5.0.
MT2 Gi/o-mediated cAMP inhibition assay
MT2 Gi/o-mediated cAMP inhibition assays were performed in HEK293T cells as described in the accompanying paper8.
Extended Data
Extended Data Fig. 1 |. Crystallisation of MT2: crystals, crystal packing, and electron density.
a, Bright field and b, cross-polarised images of representative MT2-2-pmt crystals optimized for synchrotron data collection (representing three independent crystallisation setups). c, cross-polarised image of representative MT2-N86D-2-pmt crystals used for XFEL data collection (representing three independent crystallisation setups). See Extended Data Table 2 for data collection statistics. d, e, Crystal packing (receptor - purple, BRIL – green, and rubredoxin - blue). Space for missing rubredoxin in molecule B of the asymmetric unit is indicated with a red circle. Lattice rotated 90° is shown in e. f, Overlay of 2-pmt (purple) and ramelteon (blue) ligands of MT2. g-e, 2mFo-DFc density (grey) contoured at 1 σ of ramelteon (g), N862.50D mutation (h), and H2085.46A mutation (i). 2-pmt is shown in purple.
Extended Data Fig. 2 |. Structural differences between MT1 and MT2.
a, Overlay between MT1-2-pmt (green) and MT2-2-pmt (violet) structures (Cα r.m.s.d = 0.6 Å). b, Comparison of MT1 (green) and MT2 (violet) binding pockets. Overall, the binding pocket is about 50 Å3 larger for MT2. c, Comparison of 2-pmt ligand conformations in MT1 (green) and MT2 (violet). Hydrogen bonds are shown as yellow dashed lines. d, Overlay of MT1 and MT2, showing residues that display different conformations in the vicinity of the binding pocket. N4.60 makes a hydrogen bond with Y5.38 in MT2 but not in MT1.
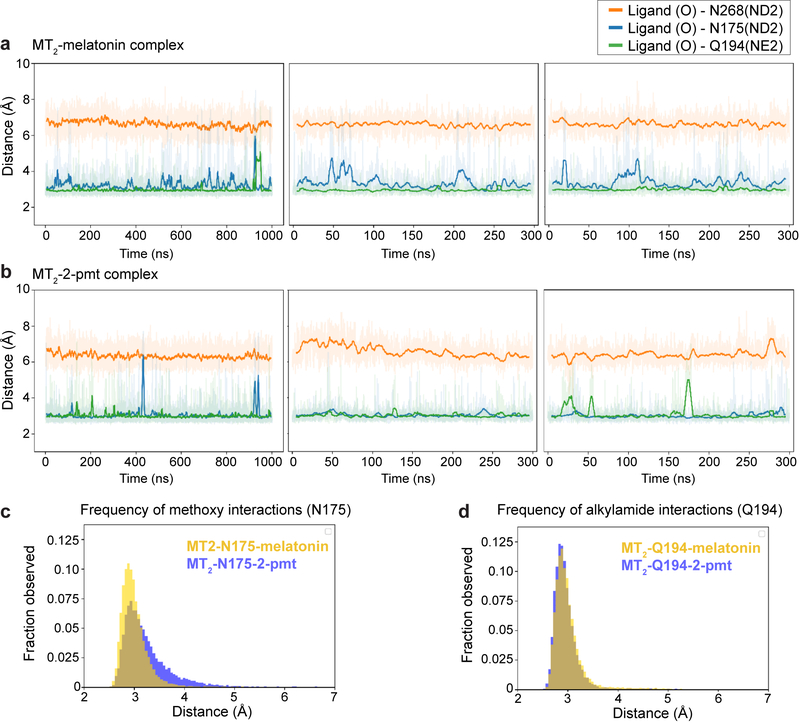
Extended Data Fig. 3 |. Molecular dynamics simulations.
a, b, Distance plots for interactions between residues in MT2 (N1754.60, atom type ND2 (Nδ); Q194ECL2, atom NE2 (Nε); N2686.52, atom ND2), and closest oxygen atoms of the ligand methoxy and acetyl groups, respectively, in complexes with melatonin (a) and 2-pmt (b) from three independent simulations runs. c, Distance histograms for interactions of methoxy with N1754.60 with melatonin (yellow) and 2-pmt (violet). d, Distance histograms for interactions of methoxy with and Q194ECL2 with ligand alkylamide tail with melatonin (yellow) and 2-pmt (violet).
Extended Data Fig. 4 |. Structural and functional differences between MT2-pmt and MT2-H208A5.46-2-pmt.
a, Overlay of the MT2-2-pmt (purple) structure with MT2-H2085.46A-2-pmt (grey) reveals an inward shift of helix V of ~0.9 Å due to the H2085.46A mutation (as shown by black arrow). b, Surface representation of the H2085.46 and H2085.46A residues. Rotation of helix V renders the binding pocket volume ~50 Å3 smaller for the H2085.46A structure (binding site volume for MT2-2-pmt: 766 Å3 compared to 716 Å3 for the MT2-H2085.46A structure). c, Comparison of the channel profiles (from the outside of the protein towards the ligand) for MT2-2-pmt (purple) and MT2-H2085.46A-2-pmt (grey) reveals a narrowing of the MT2-H2085.46A-2-pmt channel around 6 Å as a consequence of the mutation and subsequent inward rotation of helix V. d, Functional data for WT and the H2085.46A mutant expressed in HEK293T cells by using GloSensor to measure Gi/o-mediated cAMP inhibition. Data represent mean ± s.e.m. for n independent experiments as indicated in square brackets. %EMAX is relative to wild-type receptor (in columns), and (%EMAX*) is relative to melatonin activity (in rows). See Methods for further information and Supplementary Figure 6 for dose response curves.
Extended Data Fig. 5 |. Selectivity analysis of melatonergic compounds.
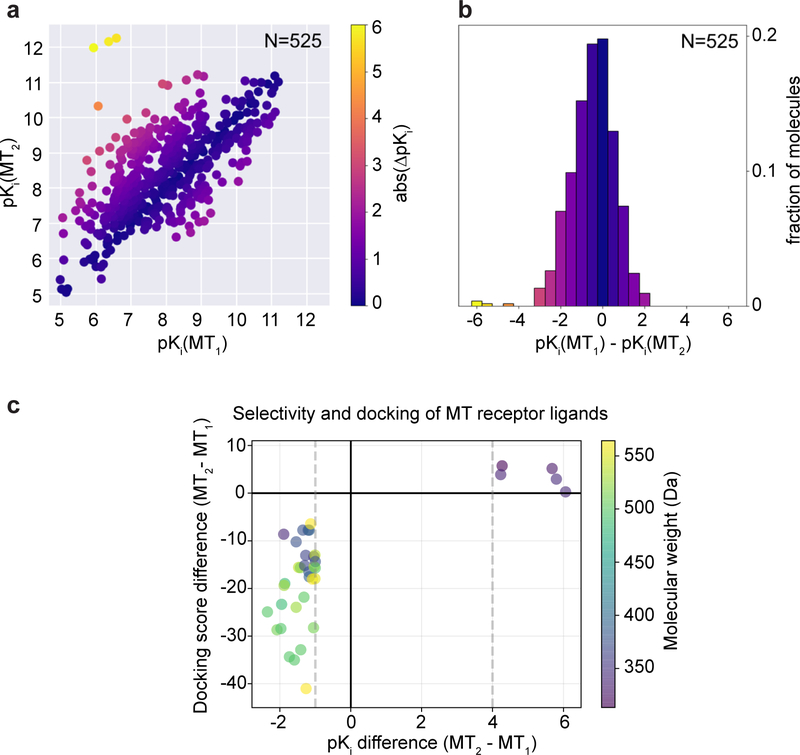
a, Binding affinities of ligands for MT1 (ChEMBL target identifier CHEMBL1945) and MT2 (CHEMBL1946) were retrieved from the ChEMBL database19 (v. 24) of experimental literature values. Of these ligands, 525 have affinities reported for both receptor subtypes. For ligands with multiple reported affinity values for a given receptor, pKi values were averaged. MT1-selective ligands are in the lower right quadrant; MT2-selective ligands are in the upper left quadrant. Data points are coloured by absolute pKi difference between subtypes, i.e. selectivity. b, Histogram of observed ligand selectivities. MT2 selective ligands are on the left of the panel, MT1 selective ligands are on the right. c, Plot of the docking score difference of select ligands that were docked between MT2 and MT1 versus their pKi difference (MT2-MT1). Dashed lines indicate pKi selectivity cutoff criteria (MT1: 1 and MT2: −4). Data points are colored by molecular weight (Da). See Supplementary Table 1 for details of docked ligands.
Extended Data Table 1 |. Ligand affinity data for MT2 mutants.
Data were acquired with MT2 wild-type (WT) and mutants expressed in HEK293T cells by radioligand competition binding using [3H]-melatonin (0.2–0.8 nM, unless otherwise indicated) to yield Kd or Ki affinity estimates. Data represent mean ± s.e.m. for n independent experiments as indicated in square brackets. Crystal constructs (CC) were expressed in Sf9 cells. ND, not determined. Binding isotherms are shown in Supplementary Figure 2. For determining the effect of NaCl, binding assays were performed in the presence of 147 nM NaCl (binding isotherms in Supplementary Figure 3).
| Mutant | melatonin | 2-pmt | ramelteon | agomelatine |
|---|---|---|---|---|
| Kd, nM [n] (pKd ±s.e.m.) |
Ki, nM [n] (pKi ±s.e.m.) |
Ki, nM [n] (pKi ±s.e.m.) |
Ki, nM [n] (pKi ±s.e.m.) |
|
| WT | 0.54
[10] (9.27±0.12) |
0.17
[10] (9.78±0.11) |
0.23
[4] (9.66±0.10) |
0.24
[4] (9.63±0.08) |
| WT + NaCI | 1.56 [6] (8.81 ± 0.18) |
ND | ND | ND |
| MT2-CC (Sf9) | 63.10
[3] (7.20±0.06) |
3.14
[4] (8.50±0.06) |
2.60
[4] (8.59±0.02) |
6.88 [4] (8.16± 0.05) |
| MT2-CC (sf9) + NaCI | 48.23
[3] (7.32±0.03) |
ND | ND | ND |
| MT2-CC-N862.50D (Sf9) | 29.40
[6] (7.53±0.34) |
6.46
[4] (8.19±0.15) |
7.37
[4] (8.13±0.09) |
26.76
[4] (7.57±0.05) |
| MT2-CC-H2085.46A (Sf9) | 10.81
[6] (7.97±0.16) |
3.57
[6] (8.45±0.03) |
2.03
[6] (8.69±0.13) |
4.98
[6] (8.30±0.04) |
| D86N (2.50) | 5.80
[3] (8.24±0.04) |
0.33
[3] (9.48±0.12) |
ND | ND |
| D86N (2.50) + NaCI | 3.26
[3] (8.49±0.09) |
ND | ND | ND |
| L108F (ECL1) | 0.94 [3] (9.03±0.11) |
0.13 [3] (9.87±0.15) |
ND | ND |
| F129W (3.41) | 2.84 [3] (8.55±0.04) |
0.22 [3] (9.65±0.08) |
ND | ND |
| N137D (3.49) | 1.24 [3] (8.91±0.13) |
0.12 [3] (9.92±0.00) |
ND | ND |
| C140L (3.52) | 0.21
[3] (9.68±0.05) |
0.03
[3] (10.50±0.02) |
ND | ND |
| W264F (6.48) | 0.88
[3] (9.06±0.14) |
0.06
[3] (10.25±0.26) |
ND | ND |
| A305P (7.50) | 3.94
[3] (8.40±0.19) |
0.47
[3] (9.32±0.06) |
ND | ND |
| N312D (7.57) | 2.85
[3] (8.54±0.07) |
0.36
[3] (9.44±0.04) |
ND | ND |
| P95A (2.59) | No specific binding up to 7 nM [3H]-melatonin | |||
| M120A(3.32) | 0.44
[3] (9.42±0.16) |
0.028
[3] (10.7±0.3) |
0.055
[3] (10.28±0.09) |
0.052
[3] (10.35±0.18) |
| N175A(4.60) | 0.86 [3] (9.2±0.3) |
0.09
[3] (10.06±0.06) |
0.08
[3] (10.12±0.11) |
0.25
[3] (9.60±0.04) |
| F192A (ECL2) | Low expression, no specific binding up to 7 nM [3H]-melatonin | |||
| F192I (ECL2) | No specific binding up to 7 nM [3H]-melatonin | |||
| Q194A (ECL2) | 0.62 [3] (9.4±0.3) |
0.043
[3] (10.38±0.07) |
0.051
[3] (10.4±0.2) |
0.12
[3] (9.94±0.09) |
| Y200A (5.38) | 0.63 [3] (9.3±0.3) |
0.14
[3] (9.86±0.01) |
0.19
[3] (9.73±0.03) |
0.67
[3] (9.18±0.02) |
| A203F(5.41) | 0.82
[5] (9.09±0.01) |
0.12
[5] (9.94±0.06) |
0.19
[5] (9.47±0.19) |
0.42
[5] (9.37±0.19) |
| H208A (5.46) | 1.24
[3] (8.94±0.13) |
0.17
[3] (9.79±0.09) |
0.18
[3] (9.77±0.11) |
0.22
[3] (9.68±0.11) |
| N268A (6.52) | 0.96 [3] (9.3±0.4) |
0.09
[3] (10.08±0.09) |
0.12
[3] (9.92±0.05) |
0.20
[3] (9.69±0.03) |
| Y294A (7.39) | 1.07
[3] (8.99±0.09) |
0.042
[3] (10.38±0.04) |
0.049
[3] (10.33±0.09) |
0.10
[3] (10.04±0.10) |
| Y308S (7.53) | No specific binding up to 7 nM [3H]-melatonin | |||
Extended Data Table 2 |. MT2 Crystallographic data collection and refinement statistics.
| MT2-CC-2-pmta | MT2-CC-H2085.46A-2-pmtb | MT2-CC-N862.50D-2-pmtc | MT2-CC-ramelteond | |||||
|---|---|---|---|---|---|---|---|---|
| Data collection | ||||||||
| Space group | P21 | P21 | P21 | P21 | ||||
| Cell dimensions | ||||||||
| a, b, c (Å) | 69.5, 146.2, 77.3 | 69.2, 146.2, 77.3 | 68.7, 145.8, 77.0 | 69.4, 145.7, 77.2 | ||||
| α, β, γ (°) | 90, 111.7, 90 | 90, 105.2, 90 | 90, 107.4, 90 | 90, 106.2, 90 | ||||
| Resolution (Å) | 21.99–2.80 (2.88–2.80) | 21.99–3.20 (3.34–3.20) | 22.0–3.10 (3.23–3.10) | 22.0–3.30 (3.46–3.30) | ||||
| Rsplit | 0.146 (4.31) | 0.181 (3.26) | 0.189 (4.70) | 0.201 (2.90) | ||||
| I/σI | 3.07 (0.46) | 4.02 (0.39) | 3.87 (0.2) | 3.67 (0.42) | ||||
| CC* | 0.999 (0.52) | 0.997 (0.54) | 0.997 (0.60) | 0.997 (0.54) | ||||
| Completeness (%) | 100 (100) | 100 (100) | 100 (100) | 100 (100) | ||||
| Redundancy | 571.2 (141.3) | 196.6 (39) | 133 (38.2) | 221.1 (84.6) | ||||
| Refinement | ||||||||
| Resolution (Å) | 21.99–2.80 | 21.99–3.20 | 22.0–3.10 | 22.0–3.30 | ||||
| No. reflections | 35,193 | 24,439 | 26,179 | 22,122 | ||||
| Rwork/Rfree | 0.219/0.249 | 0.224/0.250 | 0.234/0.262 | 0.248/0.270 | ||||
| No. atoms | A | B | A | B | A | B | A | B |
| Protein | 3,333 | 2,852 | 3,343 | 2,786 | 3,293 | 2,752 | 3,227 | 2,738 |
| Ligand/Zn+2 | 23/1 | 23/0 | 23/1 | 23/0 | 23/1 | 23/0 | 19/1 | 19/0 |
| Lipid and other | 0 | 11 | 0 | 12 | 0 | 0 | 0 | 0 |
| B-factors (Å2) | ||||||||
| Receptor | 116.4 | 121.0 | 95.0 | 99.6 | 114.2 | 117.2 | 114.1 | 118.2 |
| BRIL | 162.3 | 188.9 | 143.2 | 176.4 | 167.6 | 208.8 | 185.3 | 248.1 |
| Rubredoxin | 114.7 | n/a | 100.3 | n/a | 116.2 | n/a | 118.6 | n/a |
| Ligand/Zn+2 | 101.6/114.7 | 106.1/n/a | 73.3/91.2 | 88.7/n/a | 96.9/112.3 | 102.1/n/a | 94.9/117.7 | 105.6/n/a |
| Lipids and other | n/a | 138.8 | n/a | 111.0 | n/a | n/a | n/a | n/a |
| R.m.s. deviations | ||||||||
| Bond lengths (Å) | 0.009 | 0.010 | 0.009 | 0.009 | ||||
| Bond angles (°) | 0.97 | 1.06 | 0.96 | 0.95 | ||||
Footnote: Number of crystals used for structure determination:
31,677,
28,130,
20,704, and
28,834.
Values in parentheses are for highest-resolution shell.
Extended Data Table 3 |. Functional data (Gi/o GloSensor) for MT2crystal construct mutants.
Data were acquired with MT2 wild-type (WT) and mutants expressed in HEK293T cells by using GloSensor to measure Gi/o-mediated cAMP inhibition via isoproterenol stimulation. Data represent mean ± s.e.m. for n independent experiments as indicated in square brackets. %EMAX is relative to wild-type receptor (in columns), and (%EMAX*) is relative to melatonin (in rows). Mutant effects were calculated by the change in relative activity or log(Emax/EC50) subtracting WT from mutant. Dose-response curves are shown in Supplementary Figure 4.
| Mutant | melatonin | 2-pmt | ||||
|---|---|---|---|---|---|---|
| EC50, nM
[n] (pEC50 ±s.e.m.) |
%EMAX (%EMAX*) |
Δlog(EMAX /
EC50) (mutant-WT) |
EC50, nM
[n] (pEC50±s.e.m.) |
%
EMAX (%EMAX*) |
Δlog(EMAX /
EC50) (mutant-WT) |
|
| WT | 0.028
[16] (10.56±0.09) |
100 (100) |
0 | 0.018
[14] (10.75±0.11) |
100 (100±5) |
0 |
| MT2-CC (Sf9) | No activity | |||||
| MT2-Rub | No activity | |||||
| D86N (2.50) | 3.951
[3] (8.40±0.34) |
80±18 (100) |
−2.25 | 1.995
[3] (8.70±0.11) |
101±17 (126±21) |
−2.04 |
| L108F (ECL1) | 0.029
[5] (10.54±0.10) |
72±4 (100) |
−0.16 | 0.011
[6] (10.95±0.19) |
80±8 (110±11) |
0.11 |
| F129W (3.41) | 0.011
[6] (10.95±0.17) |
128±9 (100) |
+0.50 | 0.007 [6] (11.16+0.20) |
128±7 (99±6) |
0.52 |
| N137D (3.49) | 0.019
[6] (10.72±0.12) |
88±6 (100) |
−0.11 | 0.016
[6] (10.81±0.10) |
90±5 (103±5) |
0.19 |
| C140L (3.52) | 0.072
[7] (10.15±0.16) |
90±8 (100) |
−0.46 | 0.035
[6] (10.45±0.16) |
89±6 (101 ±4) |
−0.34 |
| W264F (6.48) | 0.044
[7] (10.36±0.09) |
117±6 (100) |
−0.13 | 0.020
[5] (10.70±0.40) |
118±8 (101±6) |
−0.03 |
| A305P (7.50) | 0.141
[7] (9.85±0.16) |
129±5 (100) |
−0.60 | 0.073
[5] (10.14±0.26) |
143±4 (91 ±6) |
−0.45 |
| N312D (7.57) | 0.069
[5] (10.16±0.15) |
135±6 (100) |
−0.26 | 0.041
[3] (10.39±0.18) |
138±10 (102±8) |
−0.21 |
Extended Data Table 4 |. Functional data (Gi/o GloSensor) for MT2 mutants.
Data were acquired with MT2 mutants by using GloSensor to measure Gi/o-mediated cAMP inhibition via isoproterenol stimulation. Data represent mean ± s.e.m. for n independent experiments as indicated in square brackets. %EMAX is relative to wild-type receptor (in columns), and (%EMAX*) is relative to melatonin (in rows). Mutant effects were calculated by the change in relative activity, or log(EMAX /EC50) subtracting wild-type from mutant. ND, not determined. Dose-response curves are shown in Supplementary Figure 5.
| Mutant | melatonin | 2-pmt | ramelteon | agomelatine | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EC50, nM
[n] (pEC50±s.e.m.) |
%
EMAX (%EMAX*) |
Δlog(EMAX / EC50) (mutant-wt) | EC50, nM [n] (pEC50±s.e.m.) | % EMAX (%EMAX*) | Δlog(EMAX / EC50) (mutant-wt) | EC50, nM [n] (pEC50±s.e.m.) | % EMAX (%EMAX*) | Δlog(EMAX / EC50) (mutant-wt) | EC50, nM [n] (pEC50±s.e.m.) | % EMAX (%MAX*) | Δlog(EMAX / EC50) (mutant-wt) | |
| WT | 0.028
[16] (10.56±0.09) |
100 (100) |
0 | 0.018
[12] (10.75±0.11) |
100 (100±4) |
0 | 0.016
[12] (10.81±0.14) |
100 (108±3) |
0 | 0.018
[10] (10.75±0.12) |
100 (105±4) |
0 |
| P95A (2.59) |
No activity | |||||||||||
| A171M (4.56) |
0.075
[9] 10.13±0.14 |
66±9 (100) |
−0.61 | 0.032
[8] 10.49±0.11 |
62±9 (94±14) |
−0.46 | 0.031
[7] 10.51±0.19 |
67±9 (90±12) |
−0.47 | 0.025
[6] 10.60±0.16 |
75±10 (107±15) |
−0.27 |
| N175A (4.60) |
0.070
[9] (10.16±0.15) |
74±10 (100) |
−0.53 | 0.0192
[8] (10.72±0.12) |
67±14 91±19 |
−0.20 | 0.010
[7] (11.00±0.21) |
72±12 (87±14) |
+0.05 | 0.015(7] (10.82±0.13) |
71 ±7 (91±10) | 0.08 |
| F192A (ECL2) |
99.235
[6] (7.00±0.26) |
122±7 (100) |
−3.46 | 4.808
[10] 8.32±0.09 |
139±3 (114±6) |
−2.28 | 4.799
[9] 8.32±0.04 |
150±4 (109±3) |
−2.31 | 5.316
[9] 8.27±0.05 |
145±2 (111±2) |
−2.31 |
| F192I (ECL2) |
3.00
[3] (8.52±0.30) |
159±4 (100) |
−1.83 | 0.211
[3] (9.68±0.07) |
159±3 (100±1) |
−0.87 | 0.571
[3] (9.24±0.16) |
169±5 (94±3) |
−1.34 | 2.754
[3] (8.56±0.05) |
160±8 (94±5) |
−1.98 |
| Q194A (ECL2) |
0.025
[3] (10.60±0.22) |
131±4 (100) |
+0.16 | 0.011
[3] (10.96±0.08) |
130±3 (99±3) |
+0.33 | 0.006
[3] (11.23±0.10) |
130±6 (88±4) |
+0.54 | 0.005
[3] (11.30±0.13) |
110±15 (78±10) |
+0.59 |
| Y200A (5.38) |
0.517
[3] (9.29±0.32) |
161±10 (100) |
−1.06 | 0.009
[3] (11.07±0.08) |
158±16 (98±10) |
+0.53 | 0.014
[3] (10.86±0.07) |
164±21 (90±10) |
0.26 | 0.314
[3] (9.50±0.31) |
149±14 (87±8) |
−1.07 |
| H208A (5.46) |
0.190
[9] (9.72±0.26) |
80±9 (100) |
−0.93 | 0.101
[10] (10.00±0.15) |
75±9 (93±11) |
−0.88 | 0.035
[8] (10.45±0.28) |
79±11 (88±12) |
−0.46 | 0.083
[8] (10.08±0.26) |
76±2 (89±3) |
−0.79 |
| N268A (6.52) Q194A |
0.046
[3] (10.33±0.27) |
141±10 (100) |
−0.08 | 0.013
[3] (10.87±0.10) |
140±8 (99±6) |
+0.28 | 0.009
[3] (11.05±0.10) |
132±9 (83±6) |
+0.36 | 0.007
[3] (11.16±0.10) |
112±4 (75±3) |
+0.46 |
| (ECL2)/ N268A (6.52) |
2.405
[9] 8.62±0.21 |
116±7 (100) |
−1.88 | 0.033
[10] 10.49±0.18 |
112±6 (96±5) |
−0.21 | 0.136
[9] 9.87±0.15 |
121±7 (93±5) |
−0.86 | 0.759
[8] 9.12±0.14 |
116±4 (94±4) |
−1.56 |
| Y294A (7.39) |
0.460
[4] (9.34±0.15) |
148±6 (100) |
−1.05 | 0.008 [4] (11.12+0.11) |
153+7 (94+9) |
+0.56 | 0.008 [3] (11.11+0.09) |
153±11 (114+11) |
+0.48 | 0.015
[3] (10.83±0.20) |
118±11 (143±12) |
−0.15 |
| Y308S (7.53) |
No activity | |||||||||||
Extended Data Table 5 |. Thermostability data for MT2 mutants.
Melting temperature Tm determined using the CPM assay27 (mean ± s.d. for n=3 independent experiments) for the crystallised construct (MT2-CC), and indicated mutants (in the MT2-CC background), purified in absence (apo) and presence (100 μM) of ligand (mlt, melatonin and 2-pmt, 2-phenylmelatonin). ND, not determined. W129F refers to MT2-CC but without the F1293.41W mutation. Melting curves are shown in Supplementary Figure 7.
| Mutant | apo Tm, °C |
mlt Tm, °C |
2-pmt Tm, °C |
mlt ΔTm, °C |
2-pmt ΔTm, °C |
|---|---|---|---|---|---|
| MT2-CC | 63.6±0.3 | 73.4±0.1 | 79.9±0.4 | +9.8 | +16.3 |
| W129F (3.41) | 59.0±0.2 | 72.4±0.1 | 78.9±0.3 | +13.4 | +19.9 |
| N175A (4.60) | 64.6±0.3 | 70.6±0.1 | 78.1±0.2 | +6 | +13.5 |
| F192A (ECL2) | 57.1±0.5 | 66.5±0.1 | 75.4±0.1 | +9.4 | +18.3 |
| Q194A (ECL2) | 65.5±0.3 | 69.9±0.3 | 77.3±0.2 | +4.4 | +11.8 |
| H208A (5.46) | 58.7±0.6 | 72.6±0.4 | 78.9±0.3 | +13.9 | +20.2 |
| N268A (6.52) | 63.3±0.3 | 66.1 ±0.2 | 74.6±0.3 | +2.8 | +11.3 |
| Q194A (ECL2)/N268A (6.52) | 67.9±0.3 | 67.9±0.1 | 71.4±0.3 | 0 | +3.5 |
| Y308S (7.53) | ND | 65.5±0.2 | 75.8±0.3 | ND | ND |
Supplementary Material
Acknowledgements
We thank M. Chu, C. Hanson, K. Villers, and J. Velasquez for help with cloning and expression, T. Grant for XFEL data processing, and H. Shaye for technical support. This work was supported by the National Institutes of Health grants R35 GM127086 (V.C.), R21 DA042298 (W.L.), R01 GM124152 (W.L.), U24DK116195 (B.L.R.), R01MH112205 (B.L.R.), the NIMH Psychoactive Drug Screening Program and the Michael Hooker Distinguished Professorship to B.L.R., F31-NS093917 (R.H.J.O.), the STC Program of the National Science Foundation (NSF) through BioXFEL (No. 1231306) (U.W., W.L., N.A.Z., V.C.), NSF ABI grant 1565180 (C.L, N.Z., U.W.), HFSP long-term fellowship LT000046/2014-L (L.C.J.), postdoctoral fellowship from the Swedish Research Council (L.C.J.); EMBO ALTF 677–2014 (B.S.). Parts of this research were carried out at the LCLS, a National User Facility operated by Stanford University on behalf of the U.S. Department of Energy and is supported by the U.S. Department of Energy Office of Science, Office of Basic Energy Sciences under Contract No. DE-AC02–76SF00515. This research benefited from the use of credits from the National Institutes of Health (NIH) Cloud Credits Model Pilot, a component of the NIH Big Data to Knowledge (BD2K) program.
Footnotes
Data availability Structure factors and coordinates were deposited in the Protein Data Bank under the following accession codes: 6ME6 (MT2-CC-2-pmt), 6ME7 (MT2-CC-H208A-2-pmt), 6ME8 (MT2-CC-N86D-2-pmt), 6ME9 (MT2-CC-ramelteon).
The authors declare no competing interests.
References
- 1.Ebisawa T, Karne S, Lerner MR & Reppert SM Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc Natl Acad Sci USA 91, 6133–6137 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reppert SM et al. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA 92, 8734–8738 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu J et al. MT1 and MT2 Melatonin Receptors: A Therapeutic Perspective. Annu Rev Pharmacol Toxicol 56, 361–383 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonnefond A et al. Rare MTNR1B variants impairing melatonin receptor 1B function contribute to type 2 diabetes. Nat Genet 44, 297–301 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karamitri A et al. Type 2 diabetes-associated variants of the MT2 melatonin receptor affect distinct modes of signaling. Sci Signal 11, eaan6622 (2018). [DOI] [PubMed] [Google Scholar]
- 6.Kato K et al. Neurochemical properties of ramelteon (TAK-375), a selective MT1/MT2 receptor agonist. Neuropharmacology 48, 301–310 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Ballesteros JA, Weinstein H Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. 25, 366–428 (1995). [Google Scholar]
- 8.Stauch B, et al. Structural basis for ligand recognition at the human MT1 melatonin receptor. Nature accompanying manuscript (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White KL et al. Structural Connection between Activation Microswitch and Allosteric Sodium Site in GPCR Signaling. Structure 26, 259–269 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth CB, Hanson MA & Stevens RC Stabilization of the human beta2-adrenergic receptor TM4-TM3-TM5 helix interface by mutagenesis of Glu122(3.41), a critical residue in GPCR structure. J Mol Biol 376, 1305–1319 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun E et al. Fusion partner toolchest for the stabilization and crystallization of G protein-coupled receptors. Structure 20, 967–976 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Audet M & Bouvier M Restructuring G-protein- coupled receptor activation. Cell 151, 14–23 (2012). [DOI] [PubMed] [Google Scholar]
- 13.Caffrey M & Cherezov V Crystallizing membrane proteins using lipidic mesophases. Nat Protoc 4, 706–731 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivara S, Mor M, Bedini A, Spadoni G & Tarzia G Melatonin receptor agonists: SAR and applications to the treatment of sleep-wake disorders. Curr Top Med Chem 8, 954–968 (2008). [DOI] [PubMed] [Google Scholar]
- 15.Bento AP et al. The ChEMBL bioactivity database: an update. Nucleic Acids Res 42, D1083–1090 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nonno R et al. A new melatonin receptor ligand with mt1-agonist and MT2-antagonist properties. J Pineal Res 29, 234–240 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Zlotos DP, Jockers R, Cecon E, Rivara S & Witt-Enderby PA MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J Med Chem 57, 3161–3185 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Teh MT & Sugden D Comparison of the structure-activity relationships of melatonin receptor agonists and antagonists: lengthening the N-acyl side-chain has differing effects on potency on Xenopus melanophores. Naunyn Schmiedebergs Arch Pharmacol 358, 522–528 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Valentin-Hansen L et al. The arginine of the DRY motif in transmembrane segment III functions as a balancing micro-switch in the activation of the beta2-adrenergic receptor. J Biol Chem 287, 31973–31982 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Consortium UniProt. UniProt: the universal protein knowledgebase. Nucleic Acids Res 46, 2699 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexandrov AI, Mileni M, Chien EY, Hanson MA & Stevens RC Microscale fluorescent thermal stability assay for membrane proteins. Structure 16, 351–359 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Liu W et al. Serial femtosecond crystallography of G protein-coupled receptors. Science 342, 1521–1524 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weierstall U et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat Commun 5, 3309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boutet SW, The GJ Coherent X-ray Imaging (CXI) instrument at the Linac Coherent Light Source (LCLS). New Journal of Physics 12, 035024 (2010). [Google Scholar]
- 25.Hart P, Boutet S, Carini G, Dubrovin M, Duda B, Fritz D, et al. The CSPAD megapixel x-ray camera at LCLS. Moeller SP, Yabashi M, & Hau-Riege SP (Eds.), 8504, 85040C–85012 (2012). [Google Scholar]
- 26.Barty A et al. Cheetah: software for high-throughput reduction and analysis of serial femtosecond X-ray diffraction data. J Appl Crystallogr 47, 1118–1131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Battye TG, Kontogiannis L, Johnson O, Powell HR & Leslie AG iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr 67, 271–281 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duisenberg AJM Indexing in Single-Crystal Diffractometry with an Obstinate List of Reflections J. Appl. Cryst 25, 92–96 (1992). [Google Scholar]
- 29.Kabsch W Xds. Acta Crystallogr D Biol Crystallogr 66, 125–132 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.White TA et al. Recent developments in CrystFEL. J Appl Crystallogr 49, 680–689 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karplus PA & Diederichs K Linking crystallographic model and data quality. Science 336, 1030–1033 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zimmermann L et al. A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core. J Mol Biol 430, 2237–2243 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Bunkoczi G & Read RJ Improvement of molecular-replacement models with Sculptor. Acta Crystallogr D Biol Crystallogr 67, 303–312 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCoy AJ et al. Phaser crystallographic software. J Appl Crystallogr 40, 658–674 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng Y et al. Structure of CC chemokine receptor 2 with orthosteric and allosteric antagonists. Nature 540, 458–461 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Afonine PV et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 68, 352–367 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adams PD et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emsley P, Lohkamp B, Scott WG & Cowtan K Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66, 486–501 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan Q et al. Structure of the CCR5 chemokine receptor-HIV entry inhibitor maraviroc complex. Science 341, 1387–1390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murshudov GN, Vagin AA & Dodson EJ Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53, 240–255 (1997). [DOI] [PubMed] [Google Scholar]
- 41.BUSTER v. 2.10.2. [Google Scholar]
- 42.Schuttelkopf AW & van Aalten DM PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr D Biol Crystallogr 60, 1355–1363 (2004). [DOI] [PubMed] [Google Scholar]
- 43.Chen VB et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66, 12–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The PyMOL Molecular Graphics System.
- 45.Jurcik A et al. CAVER Analyst 2.0: Analysis and Visualization of Channels and Tunnels in Protein Structures and Molecular Dynamics Trajectories. Bioinformatics (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abagyan RA, Totrov MM, Kuznetsov DA ICM: A New Method For Protein Modeling and Design: Applications To Docking and Structure Prediction From The Distorted Native Conformation. J. Comp. Chem 15, 488–506. (1994). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.