Abstract
Double-stranded (ds) RNA, both synthetic and produced during virus replication, rapidly stimulates MAPK and NF-κB signaling that results in expression of the inflammatory genes inducible nitric oxide synthase, cyclooxygenase 2, and IL-1β by macrophages. Using biochemical and genetic approaches, we have identified the chemokine ligand-binding C-C chemokine receptor type 5 (CCR5) as a cell surface signaling receptor required for macrophage expression of inflammatory genes in response to dsRNA. Activation of macrophages by synthetic dsRNA does not require known dsRNA receptors, as poly(inosinic:cytidylic) acid [poly(I:C)] activates signaling pathways leading to expression of inflammatory genes to similar levels in wild-type and Toll-like receptor 3- or melanoma differentiation antigen 5-deficient macrophages. In contrast, macrophage activation in response to poly(I:C) is attenuated in macrophages isolated from mice lacking CCR5. These findings support a role for CCR5 as a cell surface signaling receptor that participates in activation of inflammatory genes in macrophages in response to the viral dsRNA mimetic poly(inosinic:cytidylic) acid by pathways that are distinct from classical dsRNA receptor-mediated responses.
Keywords: C-C chemokine receptor type 5, cyclooxygenase, double-stranded RNA, inflammatory genes, interleukin 1, macrophage, nitric oxide synthase, poly(I:C)
INTRODUCTION
Macrophages are differentiated monocytic cells that express genetically inherited pattern recognition receptors, which allow these cells to identify invading pathogens by recognition of conserved pathogen-associated molecular patterns (PAMPs) (60). Double-stranded (ds) RNA, produced during the replication of most viruses, functions as a PAMP that is recognized by the innate immune system and participates in the cellular production of antiviral effectors, including type I interferons (IFN-α and IFN-β) (23, 49, 58). Primary dsRNA sensors are located in the cytosol (to recognize dsRNA produced during replication of a virus) and in the endosome (to recognize viral dsRNA released from virally lysed cells) (47, 60). After binding to dsRNA, the dsRNA-dependent protein kinase R (PKR) is activated by autophosphorylation and then phosphorylates eukaryotic initiation factor-2α. This prevents guanine nucleotide exchange and results in inhibition of protein synthesis, including synthesis of viral proteins (14, 65). Retinoic acid-inducible gene-I (RIG-I) and melanoma differentiation antigen-5 (mda-5) are cytosolic dsRNA sensors that use the NH2-terminal caspase recruitment domains adaptor and mitochondrial antiviral signaling protein to stimulate expression of antiviral genes, including type I IFNs, following dsRNA binding to their COOH-terminal helicase domain (25, 59, 67). Toll-like receptor 3 (TLR3) is an endosomally localized dsRNA sensor that stimulates production of type I IFNs in response to dsRNA (1, 38, 44, 58). Rather than recognize dsRNA that accumulates within cells during virus infection, TLR3 senses dsRNA released from neighboring virally infected cells (52, 58).
Nuclear factor (NF)-κB is a transcription factor that is a shared target of each of these dsRNA sensors (1, 4, 67). NF-κB is normally held in the cytoplasm in an inactive dimer with the inhibitory (I) κBα. After phosphorylation by IκB kinase, IκBα is degraded in a proteasomal-dependent manner, allowing release of NF-κB and translocation to the nucleus, where it functions as a transcription factor activating the expression of genes (5, 32). We have shown that dsRNA stimulates expression of the inflammatory genes inducible nitric oxide (NO) synthase (iNOS), cyclooxygenase-2 (COX-2), and interleukin-1β (IL-1β) in a NF-κB-dependent manner (18, 56). In addition to NF-κB, a secondary signaling cascade that is selective for the target gene of interest is also required for dsRNA-induced inflammatory gene expression. These secondary cascades include Ca2+-independent PLA2, PKA, and cAMP response element-binding protein (CREB) for iNOS (36, 37, 41); Jun NH2-terminal kinase (JNK) and p38 for COX-2 (55); and extracellular signal-regulated kinase (ERK) for IL-1β expression (35). The rapid activation (>15 min after stimulation) of each of these signaling cascades in macrophages (42, 55) suggests that poly(inosinic:cytidylic) acid [poly(I:C)] may function by activating a cell surface receptor.
C-C chemokine receptor type 5 (CCR5) is a 41-kDa cell surface G protein-coupled receptor expressed on T cells, macrophages, and dendritic cells (45). CCR5 interacts with multiple ligands, notably the chemokines CCL3 (macrophage inflammatory protein-1α), CCL4 (macrophage inflammatory protein-1β), and CCL5 [regulated on activation, normal T cell expressed and secreted (RANTES)] (31, 45, 48, 51). CCR5 also functions as a co-receptor for macrophage-tropic human immunodeficiency virus (HIV) (2, 12), where it interacts with gp120 HIV envelope protein (64). CCR5 promotes macrophage activation and survival during parainfluenza and influenza infection in mice, at least in part, through CCR5-dependent activation of MAPKs and phosphatidylinositol 3-kinase (PI3K) signaling (62). Bacterial heat shock protein (Hsp) 70 has also been shown to stimulate the activation of dendritic cells in a CCR5-dependent manner (13). Recently, we identified a requirement for CCR5 in the activation of macrophages and the expression of inflammatory genes in response to the capsid protein of encephalomyocarditis virus (EMCV) (11). EMCV is a single-stranded (ss) RNA virus of the Picornaviridae family that produces dsRNA during replication (9). Macrophage infection by EMCV results in activation of MAPKs, PI3K, and NF-κB in a CCR5-dependent manner (11, 53). The diversity of known ligands that activate CCR5 and similarities in the pathways activated by poly(I:C) and EMCV led us to examine the possibility that CCR5 may function as a cell surface signaling receptor for poly(I:C). Employing a blocking antibody to CCR5 and using peritoneal exudate cells isolated from CCR5−/− mice, we present evidence supporting a role for CCR5 as a cell surface receptor responsible for dsRNA-stimulated inflammatory gene expression by macrophages.
EXPERIMENTAL PROCDURES
Materials and animals.
C57BL/6J mice were purchased from Harlan, and wild-type C57BL/6J, CCR5−/−, and TLR3–/– mice were purchased from Jackson Laboratories (Bar Harbor, ME). Also, a breeding pair of CCR5–/– (B6/129) mice was provided by Dr. Michael Holtzman (Washington University School of Medicine, St. Louis, MO). Macrophages, harvested from wild-type and mda-5−/− mice, as described previously (16), were provided by Dr. Marco Colonna (Washington University). All animal use was approved by and in accordance with Saint Louis University and Medical College of Wisconsin guidelines. DMEM, CMRL-1066 tissue culture medium, l-glutamine, penicillin, and streptomycin were obtained from Invitrogen Life Technologies (Grand Island, NY). Fetal calf serum was purchased from Biowest (Kansas City, MO). Poly(I:C) was purchased from Sigma-Aldrich and prepared for use as previously described (34). Primary antibodies and their sources are as follows: rabbit anti-phosphorylated p38, rabbit anti-phosphorylated JNK, and rabbit anti-phosphorylated ERK from Promega (Madison, WI); rabbit anti-IκBα (catalog no. C-21) and rabbit anti-p38 (catalog no. C-20) from Santa Cruz Biotechnology (Santa Cruz, CA); rabbit anti-Akt, rabbit anti-phosphorylated (Ser473) Akt, and rabbit anti-phosphorylated (Thr389) p70 ribosomal protein S6 kinase (p70S6K) from Cell Signaling Technology (Beverly, MA); mouse anti-GAPDH from Life Technologies (Grand Island, NY); and rabbit anti-COX-2 and rabbit anti-iNOS from Cayman Chemical (Ann Arbor, MI). Mouse anti-pro-IL-1β 3ZD was obtained from the National Institutes of Health. Horseradish-peroxidase (HRP)-conjugated donkey anti-rabbit and HRP-conjugated donkey anti-mouse were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All other reagents were obtained from commercially available sources.
Peritoneal macrophage isolation and cell culture.
Primary peritoneal exudate cells (PECs) were harvested from ≥8-wk-old mice by lavage, as previously described (7). After isolation, 2 × 105 cells/500 µl of complete CMRL-1066 or 5 × 105 cells/ml of complete CMRL-1066 were incubated at 37°C under an atmosphere of 5% CO2 for ≥12 h before the initiation of experiments. Cells were washed three times with complete CMRL-1066 to remove nonadherent cells before treatment with poly(I:C). RAW264.7 macrophage cells were removed from growth flasks by treatment with 0.05% trypsin and 0.02% EDTA for 5 min at 37°C, washed with DMEM, and plated at 2 × 105 cells/500 µl of DMEM. Experiments were initiated 2 h after plating or following an overnight culture, and no differences in the response to poly(I:C) were observed between the two approaches. In some experiments, poly(I:C) was administered to mice by intraperitoneal injection [100 µg poly(I:C) (Sigma-Aldrich) in 100 µl of sterile saline vehicle or 100 µl of saline alone]. After 6 h, PECs were harvested as described above and then lysed for real-time PCR analysis. mRNA levels were normalized to GAPDH mRNA and are presented as fold increase compared with the pooled threshold cycle (ΔCt) values of macrophages isolated from vehicle-injected mice.
Interferon release.
The VeriKine mouse IFN-β or mouse IFN-α ELISA kit (PBL Assay Science) was used according to the manufacturer’s instructions to measure IFNs released from poly(I:C)-stimulated macrophages. Briefly, culture supernatant was diluted in sample buffer and incubated with primary antibody solution followed by HRP-conjugated secondary antibody solution, and absorbance was measured at 540 nm using the BioTek SynergyMx plate reader following the addition of tetramethylbenzidine substrate. IFN concentration was calculated using an IFN standard curve run in triplicate.
Nitrite determination.
Nitrite production was determined by addition of 50 μl of the Griess reagent to 50 μl of culture supernatant (17). Absorbance was measured at 540 nm using the BioTek SynergyMx plate reader, and nitrite concentrations were calculated from a sodium nitrite standard curve.
Western blot analysis.
Cells were washed with phosphate-buffered saline and lysed with Laemmli lysis buffer. Protein samples were separated by SDS-PAGE, transferred to nitrocellulose membranes (Amersham Life Sciences, Pittsburgh, PA) under semidry transfer conditions, and blocked in either 3% BSA in Tris-buffered saline-Tween 20 (TBST) or 5% milk in TBST for 1 h. Membranes were incubated overnight at 4°C with 1:20,000 dilution of mouse anti-GAPDH, 1:5,000 dilutions of rabbit anti-phosphorylated JNK and rabbit anti-phosphorylated ERK, 1:2,000 dilution of rabbit anti-phosphorylated p38, and 1:1,000 dilutions of all other primary antibodies. After three washes in TBST, membranes were incubated with 1:10,000 dilutions of HRP-conjugated secondary antibodies for 1 h. Antigen was detected by chemiluminescence, as previously described (29).
Real-time PCR analysis.
After lysis, total cellular RNA was isolated using the RNeasy RNA isolation kit according to the manufacturer’s instructions (Qiagen). TURBO DNA-free (Applied Biosystems) was used for DNase digestion. First-strand cDNA synthesis was performed using oligo(dT) and the reverse transcriptase SuperScript preamplification system (Invitrogen). cDNA samples were subjected to semiquantitative real-time PCR analysis using QuantiTect SYBR green reagent (Qiagen) and the MJ Research DNA Engine Opticon system or the SsoFast Evagreen supermix (Bio-Rad) and the Bio-Rad CFX96 real-time detection system according to the manufacturers’ instructions. Each sample was normalized to GAPDH (ΔCt), and poly(I:C)-treated conditions are expressed as fold increase compared with each corresponding untreated control (2−ΔΔCt). cDNAs were amplified using the following primers purchased from Integrated DNA Technologies (Coralville, IA): 5′-CGA GAC TTC TGT GAC ACA CAG C−3′ and 5′-CAT CTC CTG GTG GAA CAC AGG G-3′ for iNOS, 5′-TTT GTT GAG TCA TTC ACC AGA CAG AT-3′ and 5′-CAG TAT TGA GGA GAA CAG ATG GGA TT-3′ for COX-2, 5′-CCT GTG GCC TTG GGC CTC AA-3′ and 5′-GGT GCT GAT GTA CCA GTT GGG-3′ for pro-IL-1β, 5′-AGG TGA GAC ATC CGT TCC C-3′ and 5′-AGG AAG ACC ATC ATG TTA CCC AC-3′ for CCR5, 5′-TCC GGA GAG GAG ACT TCA CA-3′ and 5′-GAC TCT GGC TTT GCT TTT CTT GT-3′ for IL-6, 5′-CCA CCA CGC TCT TCT GTC TAC-3′ and 5′-GAG TGT GAG GGT CTG GGC-3′ for TNF-α, 5′-GGC TTC ATC TGC TGC TTG GAA TAC-3′ and 5′-TCC TTC TCT TCA CTC AGT CTT GGC-3′ for IFN-α, 5′-GCA CTG GGT GGA ATG AGA CTA TTG-3′ and 5′-TCT GAG GCA TCA ACT GAC AGG TC-3′ for IFN-β, and 5′-GAC ATC AAG AAG GTG GTG AAG C-3′ and 5′-TCC AGG GTT TCT TAC TCC TTG G-3′ for GAPDH.
Statistics.
Student’s t-test was used for statistical comparisons between two groups, and one-way analysis of variance was used for statistical comparisons between three or more independent conditions. Significant differences between groups (P < 0.05) were determined using the Tukey-Kramer post hoc test.
RESULTS
Poly(I:C) stimulates inflammatory gene expression in TLR3−/− and mda-5−/− macrophages.
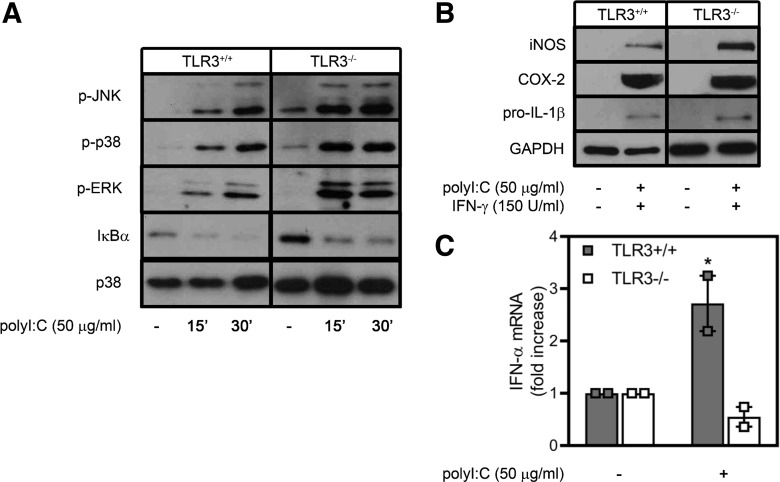
To investigate the role of the dsRNA receptor TLR3 in the antiviral responses activated by poly(I:C), naïve primary macrophages were isolated from the peritoneum of wild-type and TLR3−/− mice. Treatment with poly(I:C) results in rapid activation of MAPK signaling (increased JNK, p38, and ERK phosphorylation; Fig. 1A) and NF-κB activation, as evidenced by degradation of IκBα (Fig. 1A) following 15–30 min of incubation. Notably, the absence of TLR3 has little effect on poly(I:C)-stimulated MAPK or NF-κB activation. Naïve PECs require two proinflammatory signals for the expression of a number of inflammatory genes, including iNOS (18, 43). Treatment of primary macrophages with poly(I:C) in combination with IFN-γ for 24 h results in iNOS, COX-2, and pro-IL-1β expression to similar levels in macrophages isolated from wild-type or TLR3−/− mice (Fig. 1B). Consistent with our previous observation that TLR3 is not required for poly(I:C)-stimulated NO formation (55), these findings suggest that TLR3 is not required for dsRNA-stimulated inflammatory gene expression. In contrast to inflammatory genes, TLR3−/− macrophages fail to express IFN-α in response to poly(I:C) (Fig. 1C), consistent with TLR3’s established role in regulating the induction of type I IFNs in response to recognition of extracellular viral dsRNA (1).
Fig. 1.
Poly(inosinic:cytidylic) acid [poly(I:C)] activates proinflammatory signaling and inflammatory gene expression in the absence of Toll-like receptor (TLR) 3. A: naïve peritoneal exudate cells, isolated from wild-type or TLR3−/− mice, were treated for 15 and 30 min with poly(I:C) and harvested, and ERK, JNK, and p38 phosphorylation (p-) and IκBα degradation were detected by Western blot analysis. Total p38 is shown as a loading control. B: macrophages were treated for 24 h with poly(I:C) and IFN-γ and harvested, and inducible nitric oxide synthase (iNOS) and pro-IL-1β were detected by Western blot analysis. GAPDH is shown as a loading control. C: macrophages were isolated from wild-type or TLR3−/− mice and treated for 16 h with poly(I:C). RNA was isolated, and IFN-α mRNA accumulation was quantified by real-time PCR and normalized to actin mRNA. Results are representative (A and B) or means ± SE (C) of 3 independent experiments. *P < 0.05 vs. TLR−/−. COX-2, cyclooxygenase-2.
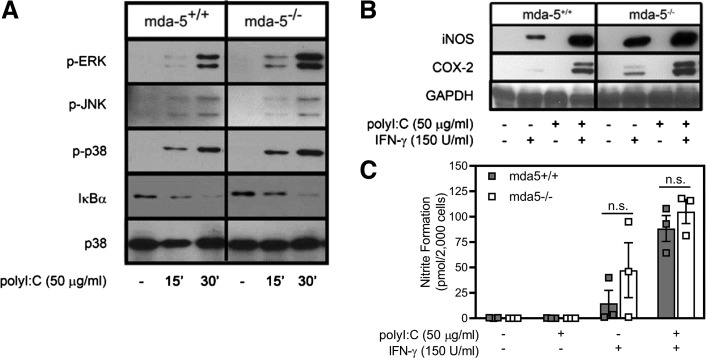
The dsRNA receptors PKR, mda-5, and RIG-I respond to dsRNA in the cytoplasm (40, 50, 60). Neither PKR nor RIG-I is believed to participate in macrophage activation in response to poly(I:C). Previous studies have shown that poly(I:C) stimulates NF-κB and MAPK activation and expression of inflammatory genes in macrophages harvested from PKR−/− mice (34, 35, 36, 42, 56). In addition, RIG-I does not efficiently recognize long dsRNA [multiple kilobase-length dsRNAs, such as the EMCV genome, and poly(I:C)]. Instead, RIG-I recognizes short dsRNA or uncapped ssRNA in the cytosol (26, 27). Since mda-5 is required for cytosolic recognition of the EMCV genome or poly(I:C) (16, 26, 27, 46), the role of mda-5 in the regulation of inflammatory gene expression following poly(I:C) stimulation was examined. Similar to TLR3−/− macrophages, MAPK is activated, IκBα is degraded, IL-1β, iNOS, and COX-2 are expressed, and NO is produced to similar levels by PECs isolated from wild-type and mda-5−/− mice following treatment with poly(I:C) or poly(I:C) + IFN-γ (Fig. 2, A–C). As previously reported in the case of PKR (22, 35, 36, 42, 56), these findings indicate that neither TLR3 nor mda-5 is required for poly(I:C)-stimulated MAPK signaling, NF-κB activation, or inflammatory gene expression by macrophages. The rapid kinetics (5–15 min) of cell signaling activated by poly(I:C) suggest that cell surface receptors, in addition to the known intracellular dsRNA receptors, may participate in the macrophage response to dsRNA.
Fig. 2.
Poly(inosinic:cytidylic) acid [poly(I:C)] activates proinflammatory signaling and inflammatory gene expression in the absence of melanoma differentiation antigen 5 (mda-5). A: peritoneal exudate cells, isolated from wild-type or mda-5−/− mice, were treated for 15 and 30 min with poly(I:C) and harvested, and ERK, JNK, and p38 phosphorylation (p-) and IκBα degradation were detected by Western blot analysis. Total p38 is shown as a loading control. B: macrophage expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 was determined by Western blot analysis following 24 h of treatment with poly(I:C) and IFN-γ. C: supernatants (from B) were collected, and nitrite formation was determined by the Griess assay. Results are representative (A and B) or means ± SE (C) of 3 independent experiments. ns, Not significant.
Antibody neutralization of CCR5 attenuates poly(I:C)-activated signaling and inflammatory gene expression in macrophages.
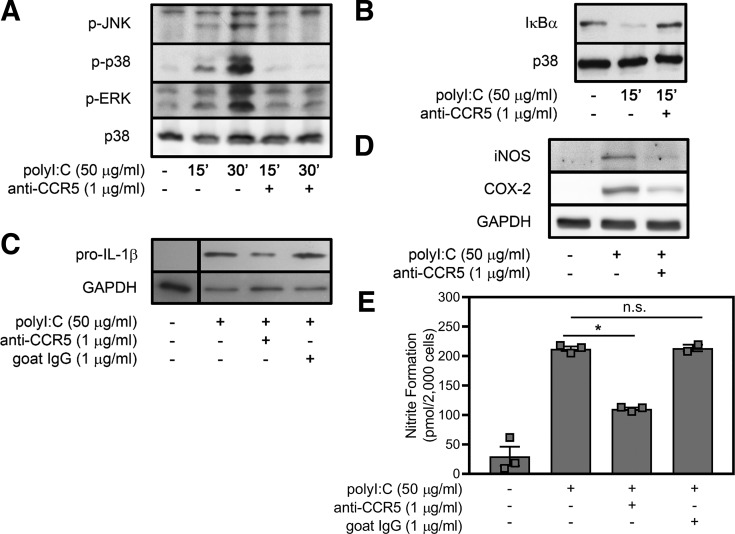
CCR5 signaling can be activated by structurally diverse PAMPs, such as bacterial Hsp70 (13), HIV envelope protein (2, 12), and a wide range of chemokines (31, 45, 48, 51). The diversity of known ligands for CCR5, together with our observations that CCR5 is necessary for EMCV-stimulated inflammatory gene expression (11) and that EMCV and poly(I:C) each stimulate the activation of similar inflammatory signaling pathways within minutes of treatment (42, 55), led us to hypothesize that CCR5 may serve as the cell surface signaling receptor for poly(I:C). To test this hypothesis, RAW264.7 macrophages were pretreated with a neutralizing antibody specific to a 19-amino-acid sequence in the NH2 terminus of CCR5 (62) for 4 h before stimulation with poly(I:C). Phosphorylation of ERK, JNK, and p38 and degradation of IκBα in response to poly(I:C) are attenuated by antibody neutralization of CCR5 (Fig. 3, A and B). This CCR5-blocking antibody also attenuates the stimulatory effects of poly(I:C) on IL-1β, iNOS, and COX-2 expression (Fig. 3, C and D) and NO production following 24 h of incubation (Fig. 3E). Species-specific control goat serum does not affect the stimulatory actions of poly(I:C) on pro-IL-1β expression (Fig. 3C) or NO production (Fig. 3E). In previous studies we showed that control goat serum also does not modify EMCV-stimulated JNK, ERK, p38, and NF-κB signaling or iNOS and COX-2 expression in macrophages (11). The ability of a CCR5-neutralizing antibody to attenuate poly(I:C)-induced signaling and inflammatory gene expression suggests that CCR5 may function as a signaling receptor that is activated by poly(I:C).
Fig. 3.
Antibody neutralization of C-C chemokine receptor type 5 (CCR5) attenuates macrophage activation in response to poly(inosinic:cytidylic) acid [poly(I:C)]. RAW264.7 macrophages were pretreated with neutralizing goat anti-CCR5 antibody for 4 h before incubation with poly(I:C). A and B: phosphorylation (p-) of ERK, JNK, and p38 and degradation of IκBα were determined by Western blot analysis following 15 and 30 min of treatment with poly(I:C). C–E: expression of IL-1β, inducible nitric oxide synthase (iNOS), and cyclooxygenase (COX)-2 was determined by Western blot analysis, and nitrite formation was determined by Griess assay following 24 h of treatment with poly(I:C). Pretreatment with goat serum was used as a neutralizing antibody control. Total p38 and GAPDH levels are shown as loading controls. Results are representative (A–D) or means ± SE (E) of 3 independent experiments. *P < 0.05; ns, not significant.
Effects of poly(I:C) on activation of CCR5−/− macrophages.
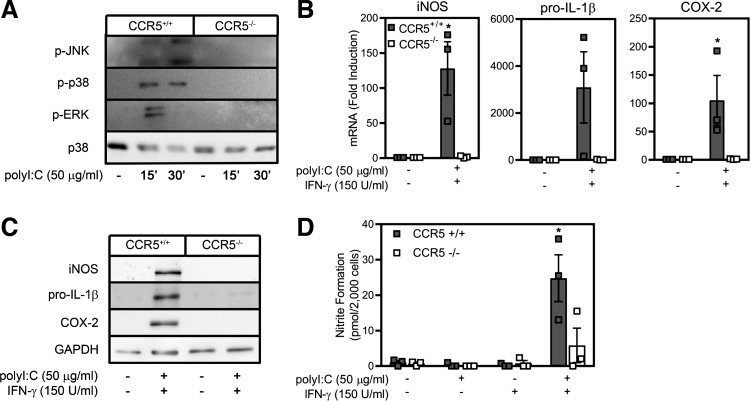
To confirm that CCR5 participates in the regulation of inflammatory gene expression, the effects of poly(I:C) on activation of peritoneal macrophages isolated from wild-type and CCR5−/− mice were examined. Poly(I:C) stimulates JNK, ERK, and p38 phosphorylation in macrophages isolated from wild-type, but not CCR5−/−, mice (Fig. 4A). In combination with IFN-γ, poly(I:C)-induced iNOS, COX-2, and IL-1β mRNA accumulation following 4 h of incubation is nearly completely prevented in macrophages isolated from CCR5−/− mice compared with the >100-fold increase in the accumulation of each mRNA in macrophages isolated from wild-type mice (Fig. 4B). Consistent with mRNA accumulation, poly(I:C) + IFN-γ fails to stimulate iNOS, IL-1β, and COX-2 expression and NO production in macrophages isolated from CCR5−/− mice compared with macrophages isolated from wild-type mice (Fig. 4, C and D).
Fig. 4.
C-C chemokine receptor type 5 (CCR5) is required for poly(inosinic:cytidylic) acid [poly(I:C)]-stimulated inflammatory gene expression by mouse macrophages. A: peritoneal exudate cells, isolated from wild-type or CCR5−/− mice, were treated for 15 or 30 min with poly(I:C) (50 µg/ml), and ERK, JNK, and p38 phosphorylation (p-) was examined. p38 levels are shown as loading control. B: after 4 h of treatment with poly(I:C) + IFN-γ, total mRNA was harvested, and inducible nitric oxide synthase (iNOS), pro-IL-1β, and cyclooxygenase (COX)-2 mRNA accumulation in wild-type and CCR5−/− macrophages was quantified by real-time PCR. mRNA accumulation was normalized to GAPDH mRNA levels. C and D: expression of iNOS, COX-2, and pro-IL-1β, as determined by Western blot analysis, and nitrite production was examined in wild-type and CCR5−/− macrophages treated for 24 h with or without poly(I:C) + IFN-γ. GAPDH levels are shown as loading control. Results are representative (A and C) or means ± SE (B and D) of 3 independent experiments. *P < 0.05.
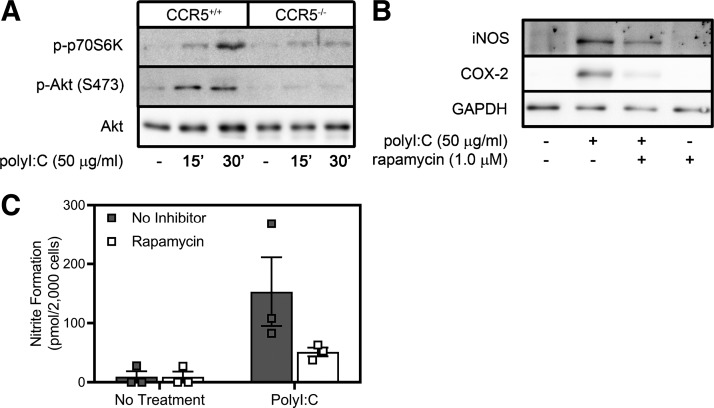
In addition to the regulation of signaling pathways that control transcription of inflammatory genes in response to EMCV infection, CCR5 also controls translation of inflammatory genes by a PI3K- and mammalian target of rapamycin complex 1 (mTORC1)-dependent mechanism (53). We have shown that poly(I:C) stimulates PI3K and mTORC1 activation (assessed by phosphorylation of Akt and p70S6K, respectively) within minutes of treatment and that this effect is attenuated in macrophages isolated from CCR5−/− mice (Fig. 5A). Rapamycin, a pharmacological inhibitor of mTORC1, attenuates expression of iNOS and COX-2 and production of NO in response to 24 h of treatment with poly(I:C) (Fig. 5, B and C). These findings provide evidence that CCR5 is required for poly(I:C) to activate MAPKs, NF-κB, PI3K, and mTORC1 signaling and the transcription and translation of inflammatory genes.
Fig. 5.
Mammalian target of rapamycin complex 1 (mTORC1) controls poly(inosinic:cytidylic) acid [poly(I:C)]-stimulated translation of inflammatory genes in a C-C chemokine receptor type 5 (CCR5)-dependent manner. A: peritoneal exudate cells, isolated from wild-type or CCR5−/− mice, were treated for 15 or 30 min with poly(I:C) (50 µg/ml), and phosphorylation (p-) of phosphatidylinositol 3-kinase (PI3K) and mTORC1 effectors Akt and p70 ribosomal protein S6 kinase (p70S6K) was examined. Akt levels are shown as a loading control. B and C: the mTORC1 inhibitor rapamycin (1 µM) was used to examine the role of mTORC1 in regulation of inducible nitric oxide synthase (iNOS) and COX-2 expression following poly(I:C) treatment as determined by Western blot analysis and nitrite accumulation in RAW264.7 macrophages. Results are representative (A and B) or means ± SE (C) of 3 independent experiments.
CCR5 does not participate in regulation of type I IFN production in poly(I:C)-treated macrophages.
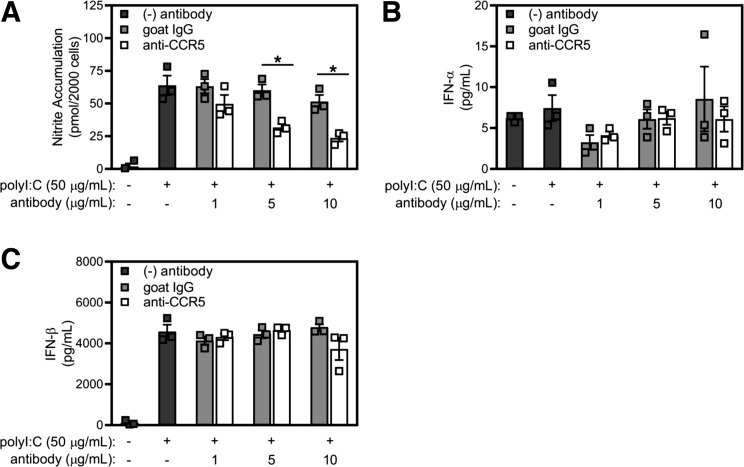
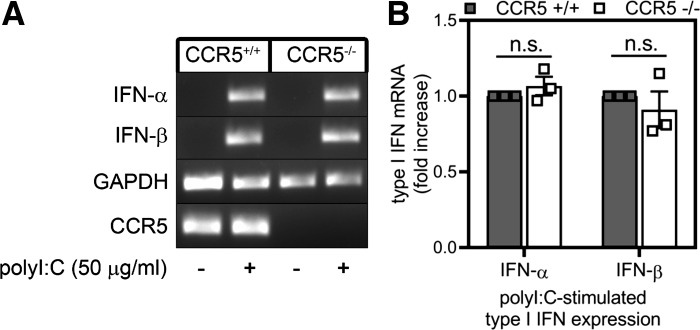
The results of Figs. 1–5 suggest that at least two independent pathways regulate the macrophage response to poly(I:C): 1) a CCR5-dependent pathway, which is responsible for the expression of inflammatory genes, and 2) a dsRNA sensor pathway (TLR3 and mda-5), which controls the production of type I IFNs in response to viral dsRNA and poly(I:C) (Fig. 1D) (1, 11, 25, 58). Consistent with this view, in a concentration-related manner CCR5-neutralizing antibody (but not goat IgG control) attenuates the stimulatory effects of poly(I:C) on nitrite formation by RAW264.7 macrophages (Fig. 6A). While poly(I:C) does not stimulate the release of IFN-α (assessed by ELISA; Fig. 6B), it does induce the release of IFN-β from RAW264.7 macrophages, and neither CCR5 neutralization antibody nor goat IgG control modifies this response (Fig. 6C). Additionally, macrophages isolated from wild-type and CCR5−/− mice accumulate IFN-α and IFN-β mRNA to similar levels in response to poly(I:C) treatment (Fig. 7), supporting the presence of two independent pathways for the macrophage response to poly(I:C): 1) a CCR5-dependent pathway, which controls inflammatory gene expression, and 2) a dsRNA sensor-dependent pathway, which is responsible for induction of type I IFN production.
Fig. 6.
Effects of antibody neutralization of C-C chemokine receptor type 5 (CCR5) on macrophage release of type I IFNs. A–C: RAW264.7 macrophages were pretreated with varying concentrations of neutralizing goat anti-CCR5 or control IgG antibody for 4 h before addition of poly(inosinic:cytidylic) acid [poly(I:C)]. After 24 h of incubation, nitrite formation was determined by Griess assay, and release of IFN-α and IFN-β was evaluated by ELISA. Results are means ± SE of 3 independent experiments. *P < 0.05.
Fig. 7.
C-C chemokine receptor type 5 (CCR5) is not required for macrophage expression of type I interferons in response to poly(inosinic:cytidylic) acid [poly(I:C)]. A and B: wild-type and CCR5−/− peritoneal exudate cells were treated with poly(I:C) for 6 h, total RNA was isolated, and accumulation of type I IFN mRNA was determined by agarose gel electrophoresis and quantified by real-time PCR. mRNA accumulation of target genes was normalized to GAPDH mRNA. Results are representative (A) or means ± SE (B) of 3 independent experiments. ns, Not significant.
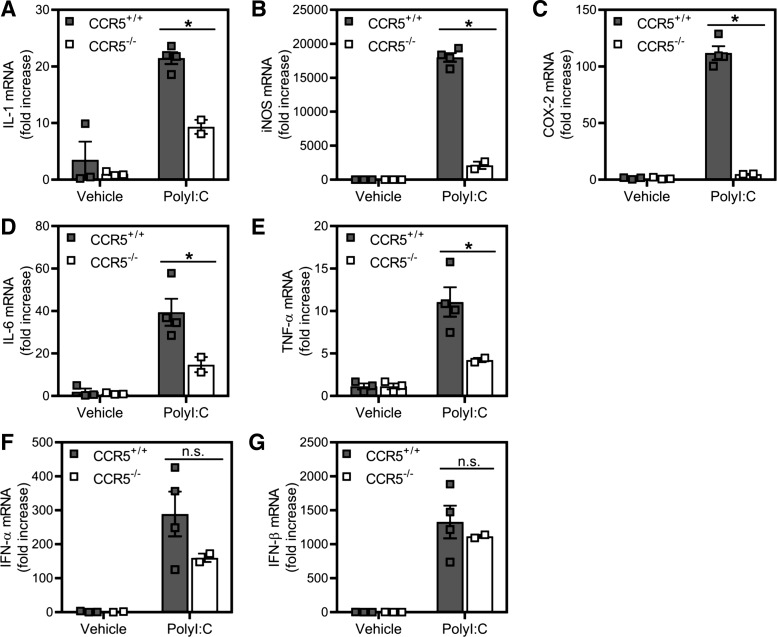
Role of CCR5 in regulation of macrophage responses to poly(I:C) in vivo.
To confirm our in vitro findings, wild-type and CCR5−/− mice were injected intraperitoneally with poly(I:C). At 6 h postinjection, peritoneal macrophages were isolated, and accumulation of antiviral gene mRNA was quantified by real-time PCR (Fig. 8). In CCR5−/− mice, accumulation of mRNA of the inflammatory genes IL-1β, iNOS, COX-2, IL-6, and TNF-α occurs to levels that are significantly lower than those measured in macrophages harvested from wild-type CCR5+/+ mice (Fig. 8, A–E). In contrast, type I IFN mRNA accumulates to similar levels in wild-type and CCR5−/− mice injected with poly(I:C) (Fig. 8, F and G). These findings provide in vivo evidence that CCR5 is required for inflammatory gene expression by macrophages in response to poly(I:C) and that CCR5 is not required for dsRNA-stimulated expression of type I IFNs.
Fig. 8.
Effects of poly(inosinic:cytidylic) acid [poly(I:C)] administration on inflammatory gene expression in C-C chemokine receptor type 5 (CCR5)-deficient (CCR5−/−) and CCR5+/+ mice. A–G: macrophages, harvested from CCR5+/+ and CCR5−/− mice 6 h after intraperitoneal administration of poly(I:C) (100 µg/mouse) or saline, were lysed, and total RNA was isolated for determination of inflammatory (A–E) and type I IFN mRNA (F and G) gene accumulation by real-time PCR. COX-2, cyclooxygenase 2; iNOS, nitric oxide synthase. Results are means ± SE of 2–4 individual mice per condition. *P < 0.05.
DISCUSSION
In this study we present evidence supporting a role of CCR5 as a signaling receptor responsible for induction of inflammatory gene expression by macrophages in response to dsRNA. Antibody neutralization of CCR5 attenuates ERK, JNK, and p38 phosphorylation, NF-κB activation, and iNOS, COX-2, and IL-1β expression in mouse macrophages treated with poly(I:C). In macrophages from CCR5−/− mice, poly(I:C) fails to activate MAPKs or to stimulate expression of inflammatory genes. The role of CCR5 in macrophage activation was confirmed in vivo in studies showing that macrophage iNOS, IL-1, IL-6, TNF, and COX-2 mRNA accumulation in response to intraperitoneal administration of poly(I:C) is attenuated in the absence of CCR5 compared with accumulation in wild-type control mice. In contrast, induction of type I IFNs in response to poly(I:C) is not modified by the presence or absence of CCR5. These findings provide biochemical and genetic evidence that CCR5 functions to regulate inflammatory gene expression in macrophages following treatment with dsRNA.
A number of antiviral responses are initiated when dsRNA is sensed by the cytosolic kinase PKR (14, 47, 65) or by the cytoplasmic proteins mda-5 and RIG-I (25, 67) and endosomally localized TLR3 (1, 38, 44) during a virus infection. PKR classically phosphorylates eukaryotic initiation factor-2α upon activation, which inhibits protein synthesis (6, 14, 39, 65). Additionally, PKR also can stimulate apoptosis of infected cells upon recognition of intracellular dsRNA (28, 66). TLR3, mda-5, and RIG-I are dsRNA sensors, each of which, upon activation, can stimulate the type I IFN response. However, there does not appear to be redundancy or compensation, as each dsRNA sensor is selective for different viral dsRNA ligands and/or localized to specific cellular compartments. RIG-I and mda-5 are cytosolic dsRNA sensors that recognize dsRNA that accumulates within virally infected cells. However, mda-5 selectively recognizes multiple-kilobase-long dsRNA [poly(I:C) and viral genome of picornaviruses, such as EMCV and coxsackievirus, and reoviruses], while RIG-I selectively recognizes short (<1-kilobase-long) dsRNA and ssRNA structures (paramyxoviruses, influenza virus, flaviviruses, and vesicular stomatitis virus) (16, 26, 27, 46, 50). Mda-5 is also required for type I IFN expression in response to transfected poly(I:C) (16). Localized to endosomes, TLR3 senses extracellular dsRNA that is taken up by macrophages and dendritic cells during endocytosis (1, 38, 44). Our findings are in agreement with previous studies showing that mda-5−/− macrophages fail to produce type I IFN in response to EMCV infection (11) and TLR3−/− macrophages fail to produce type I IFN in response to poly(I:C) (Fig. 1) (11).
Most studies focusing on these dsRNA sensors have examined the activation of pathways that participate in regulation of type I IFN production (19, 58), leaving the potential role of dsRNA sensors in regulation of inflammatory gene expression in response to virus infection or synthetic dsRNA relatively unknown (40, 58). In recent years, we have shown that poly(I:C) stimulates expression of iNOS, COX-2, and IL-1β and have identified the signaling pathways responsible for the regulation of each gene. NF-κB is required for expression of all three genes (18, 56), and at least one additional signaling cascade that is selective for each of the target genes is required for poly(I:C)-induced expression. Ca2+-independent PLA2, PKA, and CREB are required for iNOS expression (36, 41), JNK and p38 control COX-2 expression (55), and ERK regulates IL-1β expression (35). While pathways regulating expression of these inflammatory genes have been identified, the signaling receptor(s) required for the activation of each of these signaling cascades in response to poly(I:C) has remained elusive.
Macrophages and mouse embryonic fibroblasts derived from PKR−/− mice have been used to rule out a role for this dsRNA-activated protein kinase as a regulator of poly(I:C)-stimulated NF-κB activation, MAPK signaling, and inflammatory and cytokine gene expression (21, 22, 35, 36, 42, 56). While labeling studies have shown that dsRNA rapidly co-localizes with TLR3 and late endosomal markers as early as 30 min after its addition to cell culture (24), which temporally correlates with poly(I:C)-stimulated inflammatory signaling, the proinflammatory response of macrophages to poly(I:C) treatment is similar in wild-type and TLR3−/− macrophages (Fig. 1) (55). A third known pathway that responds to dsRNA comprises the cytoplasm-localized receptors RIG-I and mda-5. Both of these receptors are believed to recognize viral dsRNA produced during replication in infected cells (25, 67), and mda-5, rather than RIG-I, appears to be selective for long dsRNA produced by Picornaviridae viruses, such as EMCV, as well as poly(I:C) (16, 26, 27, 46, 50). In this study we show that the absence of mda-5 does not modulate MAPK activation, IκB degradation, or inflammatory gene expression resulting from macrophage stimulation with poly(I:C) (Fig. 2).
The chemokine ligand-binding CCR5 is a promiscuous receptor that can either directly bind to or be activated by multiple ligands of diverse structure. CCR5 is activated by endogenous ligands, including the chemokines macrophage inflammatory proteins-1α and -1β, RANTES, monocyte chemoattractant protein, eotaxin, and hemofiltrate CC chemokines-1 and -4 (31, 45, 48, 51). CCR5 directly interacts with gp120 envelope protein of HIV (64), which facilitates HIV entry into macrophages (2, 12). Gp120 has been shown to stimulate expression of iNOS, COX-2, and IL-1β in macrophages (8, 10, 33, 61), and gp120-stimulated IL-1β expression is dependent on CCR5, Src-family kinases, and PI3K (10, 61). CCR5 has also been shown to regulate signaling in response to bacterial Hsp70, leading to IL-6 expression by dendritic cells (13), and direct binding between CCR5 and Hsp70 has been demonstrated using surface plasmon resonance (63). Furthermore, we recently observed that CCR5 is required for macrophage signaling and expression of inflammatory genes in response to EMCV capsid protein (11). In this study we provide evidence that dsRNA [poly(I:C)] also stimulates CCR5-dependent signaling in macrophages. Activation of MAPKs, NF-κB, PI3K, and mTORC1 occurs within minutes of poly(I:C) treatment in a manner dependent on CCR5 (Figs. 1–5). While poly(I:C) can stimulate chemokine expression (20), it is unlikely that chemokines (such as RANTES) are expressed and released within minutes of dsRNA treatment to mediate, in a paracrine or autocrine manner, the actions of poly(I:C) on CCR5-dependent signaling in macrophages. While direct binding of EMCV capsid protein and poly(I:C) to CCR5 has not been demonstrated, it is unlikely that all these structurally diverse ligands directly bind to CCR5. Chemokine receptors and other members of the G protein-coupled receptor family have been shown to oligomerize to facilitate signaling (3, 15, 54, 57) and can also facilitate “cross talk,” or interaction, with components of other signaling pathways, including TLRs (30). These findings suggest that CCR5 is a cell surface signaling receptor that could coordinate the rapid activation of an inflammatory response after direct binding to ligands or heterodimerization with other pattern recognition receptors.
Studies characterizing the role of TLR3 in response to extracellular poly(I:C) treatment (1–100 µg/ml) showed an attenuation in accumulation of IL-6 and TNF-α mRNA in bone marrow-derived macrophages or splenocytes harvested from TLR3−/− mice compared with wild-type controls (7). Few studies have examined the role of TLR3 and mda5 in the regulation of iNOS, IL-1β, and COX-2 expression in response to dsRNA (2, 7, 12). We now show that poly(I:C)-induced IL-6 and TNF-α expression is attenuated in macrophages isolated from CCR5−/− mice (Fig. 8, D and E). While the mechanisms by which CCR5 and TLR3 (or signaling components directed by each receptor) coordinate to regulate IL-6 and TNF-α expression are unclear, future studies directed at the potential cross talk between these pathways will help elucidate the mechanisms by which dsRNA activates a rapid and robust inflammatory response in macrophages.
Perspectives and Significance
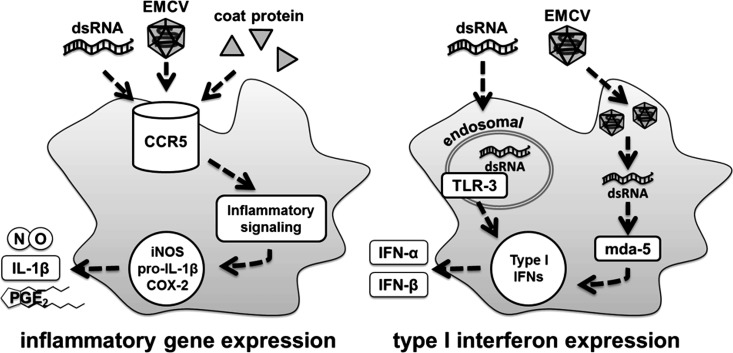
Results presented in this study suggest that the pathways regulating inflammatory gene expression are separate and distinct from the pathways regulating expression of type I IFNs in response to dsRNA (Fig. 9). We show that proinflammatory gene expression is attenuated in poly(I:C)-treated macrophages harvested from CCR5−/− mice compared with wild-type controls (Figs. 4 and 8); however, the absence of CCR5 does not modify the production or expression of type I IFNs, as they are produced to similar levels by macrophages harvested from CCR5+/+ and CCR5−/− mice treated with poly(I:C) (Fig. 8, F and G). Consistent with two independent pathways controlling macrophage responses to dsRNA, PECs harvested from TLR3−/− mice fail to accumulate IFN-α mRNA (Fig. 1D). This finding is consistent with the established role of TLR3 in the production of type I IFNs (1, 50, 58). Our findings support at least two independent pathways that contribute to the response of macrophages to virus infection and dsRNA. One pathway includes activation of a CCR5-dependent signaling cascade that is triggered by dsRNA, either directly or via potential interacting partners, to stimulate expression of proinflammatory genes such as iNOS, COX-2, and IL-1β. Similar to poly(I:C), EMCV capsid protein also triggers a CCR5-dependent signaling cascade resulting in activation of macrophages (11). This response is early and rapid and can be characterized as a general inflammatory cascade that functions to limit virus replication (11). The response is limited to cells expressing CCR5, or cells of hematopoietic origin, as cells lacking CCR5 (such as pancreatic β-cells) fail to express inflammatory genes in response to poly(I:C) (18). In macrophages that accumulate dsRNA during virus replication or following endocytosis of dsRNA from extracellular compartments, the classical dsRNA receptors are activated and induce a more specific innate immune response that includes production of antiviral type I IFNs. The antiviral dsRNA sensor-regulated responses are not limited to cells of hematopoietic origin, as dsRNA receptors are ubiquitously expressed. These findings support the induction of general inflammation as an early response to an infection that may function to limit growth/replication of the invading pathogen until a more selective innate response can be activated. In the case of virus infection, activation of a CCR5-dependent early inflammatory response functions to limit virus replication (11, 53) while allowing time for induction of a more selective antiviral dsRNA-dependent type I IFN response to combat the virus.
Fig. 9.
Schematic of pathways activated by double-stranded RNA (dsRNA) in macrophages. Either encephalomyocarditis virus (EMCV) capsid protein or genome (dsRNA) can stimulate activation of proinflammatory signaling [MAP kinases (MAPKs), phosphatidylinositol 3-kinase (PI3K), and NF-κB] within minutes of treatment, and these pathways regulate transcription and translation of the proinflammatory genes inducible nitric oxide synthase (iNOS), pro-IL-1β, and cyclooxygenase (COX)-2. Macrophage proinflammatory response is dependent on C-C chemokine receptor type 5 (CCR5) and does not require dsRNA sensors or type I IFN expression. The endosomal dsRNA sensor Toll-like receptor (TLR)-3 recognizes extracellular dsRNA, while the cytosolic dsRNA sensor melanoma differentiation antigen 5 (mda-5) recognizes dsRNA that accumulates during EMCV replication within the cell. Each of these receptors, upon activation, can stimulate expression of type I IFNs.
GRANTS
This work was supported by National Institutes of Health Grants DK-052194 and AI-44458 (to J. A. Corbett); Fellowship F30 DK-102363-01A1 and National Institute of General Medical Sciences Training Grant T32-GM080202 (to Z. R. Shaheen); and American Heart Association Fellowship 17PRE3253000 (to J. D. Stafford).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Z.R.S., B.S.C., J.M.M., R.M.L.B., and J.A.C. conceived and designed research; Z.R.S., B.S.C., J.D.S., and J.M.M. performed experiments; Z.R.S., B.S.C., J.D.S., J.M.M., R.M.L.B., and J.A.C. analyzed data; Z.R.S., B.S.C., J.M.M., R.M.L.B., and J.A.C. interpreted results of experiments; Z.R.S., B.S.C., and J.D.S. prepared figures; Z.R.S. and J.A.C. drafted manuscript; Z.R.S., B.S.C., J.D.S., and J.A.C. edited and revised manuscript; Z.R.S., B.S.C., J.D.S., J.M.M., and J.A.C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Colleen Kelly Bratcher, Aaron Naatz, and Jennifer McGraw for expert technical assistance and Dr. Marco Colonna (Washington University) for providing wild-type and mda-5−/− macrophages. We thank the late Dr. Mark Buller for his long-standing collaboration and assistance with this research program.
REFERENCES
- 1.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 413: 732–738, 2001. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272: 1955–1958, 1996. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol 25: 787–820, 2007. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 4.Andrejeva J, Childs KS, Young DF, Carlos TS, Stock N, Goodbourn S, Randall RE. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, mda-5, and inhibit its activation of the IFN-β promoter. Proc Natl Acad Sci USA 101: 17264–17269, 2004. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baeuerle PA, Baltimore D. IκB: a specific inhibitor of the NF-κB transcription factor. Science 242: 540–546, 1988. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 6.Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN. Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13: 129–141, 2000. doi: 10.1016/S1074-7613(00)00014-5. [DOI] [PubMed] [Google Scholar]
- 7.Beckerman KP, Rogers HW, Corbett JA, Schreiber RD, McDaniel ML, Unanue ER. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol 150: 888–895, 1993. [PubMed] [Google Scholar]
- 8.Bukrinsky MI, Nottet HS, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med 181: 735–745, 1995. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carocci M, Bakkali-Kassimi L. The encephalomyocarditis virus. Virulence 3: 351–367, 2012. doi: 10.4161/viru.20573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG. Signaling mechanism of HIV-1 gp120 and virion-induced IL-1β release in primary human macrophages. J Immunol 180: 6675–6684, 2008. doi: 10.4049/jimmunol.180.10.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christmann BS, Moran JM, McGraw JA, Buller RM, Corbett JA. Ccr5 regulates inflammatory gene expression in response to encephalomyocarditis virus infection. Am J Pathol 179: 2941–2951, 2011. doi: 10.1016/j.ajpath.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton RE, Hill CM, Davis CB, Peiper SC, Schall TJ, Littman DR, Landau NR. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381: 661–666, 1996. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 13.Floto RA, MacAry PA, Boname JM, Mien TS, Kampmann B, Hair JR, Huey OS, Houben EN, Pieters J, Day C, Oehlmann W, Singh M, Smith KG, Lehner PJ. Dendritic cell stimulation by mycobacterial Hsp70 is mediated through CCR5. Science 314: 454–458, 2006. doi: 10.1126/science.1133515. [DOI] [PubMed] [Google Scholar]
- 14.Gale M Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther 78: 29–46, 1998. doi: 10.1016/S0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- 15.George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov 1: 808–820, 2002. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- 16.Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA 103: 8459–8464, 2006. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126: 131–138, 1982. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 18.Heitmeier MR, Scarim AL, Corbett JA. Double-stranded RNA-induced inducible nitric-oxide synthase expression and interleukin-1 release by murine macrophages requires NF-κB activation. J Biol Chem 273: 15301–15307, 1998. doi: 10.1074/jbc.273.24.15301. [DOI] [PubMed] [Google Scholar]
- 19.Honda K, Takaoka A, Taniguchi T. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25: 349–360, 2006. doi: 10.1016/j.immuni.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Huang CC, Duffy KE, San Mateo LR, Amegadzie BY, Sarisky RT, Mbow ML. A pathway analysis of poly(I:C)-induced global gene expression change in human peripheral blood mononuclear cells. Physiol Genomics 26: 125–133, 2006. doi: 10.1152/physiolgenomics.00002.2006. [DOI] [PubMed] [Google Scholar]
- 21.Iordanov MS, Paranjape JM, Zhou A, Wong J, Williams BR, Meurs EF, Silverman RH, Magun BE. Activation of p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase by double-stranded RNA and encephalomyocarditis virus: involvement of RNase L, protein kinase R, and alternative pathways. Mol Cell Biol 20: 617–627, 2000. doi: 10.1128/MCB.20.2.617-627.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iordanov MS, Wong J, Bell JC, Magun BE. Activation of NF-κB by double-stranded RNA (dsRNA) in the absence of protein kinase R and RNase L demonstrates the existence of two separate dsRNA-triggered antiviral programs. Mol Cell Biol 21: 61–72, 2001. doi: 10.1128/MCB.21.1.61-72.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacobs BL, Langland JO. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219: 339–349, 1996. doi: 10.1006/viro.1996.0259. [DOI] [PubMed] [Google Scholar]
- 24.Johnsen IB, Nguyen TT, Ringdal M, Tryggestad AM, Bakke O, Lien E, Espevik T, Anthonsen MW. Toll-like receptor 3 associates with c-Src tyrosine kinase on endosomes to initiate antiviral signaling. EMBO J 25: 3335–3346, 2006. doi: 10.1038/sj.emboj.7601222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang DC, Gopalkrishnan RV, Wu Q, Jankowsky E, Pyle AM, Fisher PB. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc Natl Acad Sci USA 99: 637–642, 2002. doi: 10.1073/pnas.022637199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody TS, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med 205: 1601–1610, 2008. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441: 101–105, 2006. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman RJ. Double-stranded RNA-activated protein kinase mediates virus-induced apoptosis: a new role for an old actor. Proc Natl Acad Sci USA 96: 11693–11695, 1999. doi: 10.1073/pnas.96.21.11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan P, Idrees D, Moxley MA, Corbett JA, Ahmad F, von Figura G, Sly WS, Waheed A, Hassan MI. Luminol-based chemiluminescent signals: clinical and non-clinical application and future uses. Appl Biochem Biotechnol 173: 333–355, 2014. doi: 10.1007/s12010-014-0850-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lattin J, Zidar DA, Schroder K, Kellie S, Hume DA, Sweet MJ. G-protein-coupled receptor expression, function, and signaling in macrophages. J Leukoc Biol 82: 16–32, 2007. doi: 10.1189/jlb.0107051. [DOI] [PubMed] [Google Scholar]
- 31.Lederman MM, Penn-Nicholson A, Cho M, Mosier D. Biology of CCR5 and its role in HIV infection and treatment. JAMA 296: 815–826, 2006. doi: 10.1001/jama.296.7.815. [DOI] [PubMed] [Google Scholar]
- 32.Liou HC, Baltimore D. Regulation of the NF-ηB/rel transcription factor and IκB inhibitor system. Curr Opin Cell Biol 5: 477–487, 1993. doi: 10.1016/0955-0674(93)90014-H. [DOI] [PubMed] [Google Scholar]
- 33.Lisi L, Tramutola A, De Luca A, Navarra P, Dello Russo C. Modulatory effects of the CCR5 antagonist maraviroc on microglial pro-inflammatory activation elicited by gp120. J Neurochem 120: 106–114, 2012. doi: 10.1111/j.1471-4159.2011.07549.x. [DOI] [PubMed] [Google Scholar]
- 34.Maggi LB Jr, Heitmeier MR, Scheuner D, Kaufman RJ, Buller RM, Corbett JA. Potential role of PKR in double-stranded RNA-induced macrophage activation. EMBO J 19: 3630–3638, 2000. doi: 10.1093/emboj/19.14.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maggi LB Jr, Moran JM, Buller RM, Corbett JA. ERK activation is required for double-stranded RNA- and virus-induced interleukin-1 expression by macrophages. J Biol Chem 278: 16683–16689, 2003. doi: 10.1074/jbc.M211744200. [DOI] [PubMed] [Google Scholar]
- 36.Maggi LB Jr, Moran JM, Scarim AL, Ford DA, Yoon JW, McHowat J, Buller RM, Corbett JA. Novel role for calcium-independent phospholipase A2 in the macrophage antiviral response of inducible nitric-oxide synthase expression. J Biol Chem 277: 38449–38455, 2002. doi: 10.1074/jbc.M206247200. [DOI] [PubMed] [Google Scholar]
- 37.Martinson BD, Albert CJ, Corbett JA, Wysolmerski RB, Ford DA. Calcium-independent phospholipase A2 mediates CREB phosphorylation in double-stranded RNA-stimulated endothelial cells. J Lipid Res 44: 1686–1691, 2003. doi: 10.1194/jlr.M300018-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto M, Funami K, Tanabe M, Oshiumi H, Shingai M, Seto Y, Yamamoto A, Seya T. Subcellular localization of Toll-like receptor 3 in human dendritic cells. J Immunol 171: 3154–3162, 2003. doi: 10.4049/jimmunol.171.6.3154. [DOI] [PubMed] [Google Scholar]
- 39.Meurs E, Chong K, Galabru J, Thomas NS, Kerr IM, Williams BR, Hovanessian AG. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell 62: 379–390, 1990. doi: 10.1016/0092-8674(90)90374-N. [DOI] [PubMed] [Google Scholar]
- 40.Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell 22: 561–569, 2006. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Moran JM, Buller RM, McHowat J, Turk J, Wohltmann M, Gross RW, Corbett JA. Genetic and pharmacologic evidence that calcium-independent phospholipase A2β regulates virus-induced inducible nitric-oxide synthase expression by macrophages. J Biol Chem 280: 28162–28168, 2005. doi: 10.1074/jbc.M500013200. [DOI] [PubMed] [Google Scholar]
- 42.Moran JM, Moxley MA, Buller RM, Corbett JA. Encephalomyocarditis virus induces PKR-independent mitogen-activated protein kinase activation in macrophages. J Virol 79: 10226–10236, 2005. doi: 10.1128/JVI.79.16.10226-10236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nathan C, Xie QW. Regulation of biosynthesis of nitric oxide. J Biol Chem 269: 13725–13728, 1994. [PubMed] [Google Scholar]
- 44.Nishiya T, DeFranco AL. Ligand-regulated chimeric receptor approach reveals distinctive subcellular localization and signaling properties of the Toll-like receptors. J Biol Chem 279: 19008–19017, 2004. doi: 10.1074/jbc.M311618200. [DOI] [PubMed] [Google Scholar]
- 45.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell Signal 16: 1201–1210, 2004. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Pichlmair A, Schulz O, Tan CP, Rehwinkel J, Kato H, Takeuchi O, Akira S, Way M, Schiavo G, Reis e Sousa C. Activation of MDA5 requires higher-order RNA structures generated during virus infection. J Virol 83: 10761–10769, 2009. doi: 10.1128/JVI.00770-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pindel A, Sadler A. The role of protein kinase R in the interferon response. J Interferon Cytokine Res 31: 59–70, 2011. doi: 10.1089/jir.2010.0099. [DOI] [PubMed] [Google Scholar]
- 48.Raport CJ, Gosling J, Schweickart VL, Gray PW, Charo IF. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1β, and MIP-1α. J Biol Chem 271: 17161–17166, 1996. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 49.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol 8: 559–568, 2008. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saito T, Gale M Jr. Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J Exp Med 205: 1523–1527, 2008. doi: 10.1084/jem.20081210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry 35: 3362–3367, 1996. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 52.Schulz O, Diebold SS, Chen M, Näslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljeström P, Reis e Sousa C. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433: 887–892, 2005. doi: 10.1038/nature03326. [DOI] [PubMed] [Google Scholar]
- 53.Shaheen ZR, Naatz A, Corbett JA. CCR5-dependent activation of mTORC1 regulates translation of inducible NO aynthase and COX-2 during encephalomyocarditis virus infection. J Immunol 195: 4406–4414, 2015. doi: 10.4049/jimmunol.1500704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Steen A, Larsen O, Thiele S, Rosenkilde MM. Biased and G protein-independent signaling of chemokine receptors. Front Immunol 5: 277, 2014. doi: 10.3389/fimmu.2014.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steer SA, Moran JM, Christmann BS, Maggi LB Jr, Corbett JA. Role of MAPK in the regulation of double-stranded RNA- and encephalomyocarditis virus-induced cyclooxygenase-2 expression by macrophages. J Immunol 177: 3413–3420, 2006. doi: 10.4049/jimmunol.177.5.3413. [DOI] [PubMed] [Google Scholar]
- 56.Steer SA, Moran JM, Maggi LB Jr, Buller RM, Perlman H, Corbett JA. Regulation of cyclooxygenase-2 expression by macrophages in response to double-stranded RNA and viral infection. J Immunol 170: 1070–1076, 2003. doi: 10.4049/jimmunol.170.2.1070. [DOI] [PubMed] [Google Scholar]
- 57.Stephens B, Handel TM. Chemokine receptor oligomerization and allostery. Prog Mol Biol Transl Sci 115: 375–420, 2013. doi: 10.1016/B978-0-12-394587-7.00009-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stetson DB, Medzhitov R. Type I interferons in host defense. Immunity 25: 373–381, 2006. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 59.Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity 24: 633–642, 2006. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell 140: 805–820, 2010. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 61.Tomkowicz B, Lee C, Ravyn V, Cheung R, Ptasznik A, Collman RG. The Src kinase Lyn is required for CCR5 signaling in response to MIP-1β and R5 HIV-1 gp120 in human macrophages. Blood 108: 1145–1150, 2006. doi: 10.1182/blood-2005-12-012815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’Sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med 11: 1180–1187, 2005. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whittall T, Wang Y, Younson J, Kelly C, Bergmeier L, Peters B, Singh M, Lehner T. Interaction between the CCR5 chemokine receptors and microbial HSP70. Eur J Immunol 36: 2304–2314, 2006. doi: 10.1002/eji.200635953. [DOI] [PubMed] [Google Scholar]
- 64.Wu L, Gerard NP, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso AA, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature 384: 179–183, 1996. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]
- 65.Wu S, Kaufman RJ. A model for the double-stranded RNA (dsRNA)-dependent dimerization and activation of the dsRNA-activated protein kinase PKR. J Biol Chem 272: 1291–1296, 1997. doi: 10.1074/jbc.272.2.1291. [DOI] [PubMed] [Google Scholar]
- 66.Yeung MC, Chang DL, Camantigue RE, Lau AS. Inhibitory role of the host apoptogenic gene PKR in the establishment of persistent infection by encephalomyocarditis virus in U937 cells. Proc Natl Acad Sci USA 96: 11860–11865, 1999. doi: 10.1073/pnas.96.21.11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5: 730–737, 2004. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]