Abstract
Aging affects numerous physiological processes, as well as behavior. A large number of these processes are regulated, at least partially, by hypothalamic orexin neurons, and orexin tone may decrease with normal aging. In this study, we hypothesized that designer receptors exclusively activated by designer drugs (DREADD) stimulation of orexin neuronal activity will ameliorate the effect of aging on behavioral and metabolic alterations in young and middle-aged mice. DREADD targeting was achieved by stereotaxic injection of AAV vectors (AAV2-hSyn-DIO-hM3D(Gq)-mCherry) into the lateral hypothalamus of 5- and 12-mo old orexin-cre female mice and was confirmed by immunohistochemistry (IHC) analysis of orexin A and mCherry expression. After recovery, animals were subjected to a behavioral test battery consisting of the elevated plus maze (EPM), open field (OFT), and novel object recognition tests (NORT) to assess effects of aging on anxiety-like behavior, general locomotion, and working memory. A comprehensive laboratory animal monitoring system (CLAMS) was used to measure spontaneous physical activity (SPA) and energy expenditure (EE). The results indicate that activation of orexin neurons mitigates aging-induced reductions in anxiety-like behavior in middle-aged mice (P < 0.005) and increases locomotion in both young and middle-aged mice (P < 0.05). Activation of orexin neurons increases SPA (P < 0.01) and EE (P < 0.005) in middle-aged mice, restoring the levels to that observed in young animals. Results from this study identify orexin neurons as potential therapeutic targets for age-related impairments in cognitive and anxiety-related behavior, and energy balance.
Keywords: aging, DREADD, energy expenditure, Orexin, spontaneous physical activity
INTRODUCTION
Aging is a complex, multifactorial, intrinsic, and cumulative process that, with time, leads to the decline of multiple physiological functions and, ultimately, death. Functional impairments due to aging reduces the capability of organisms to cope with external stressors and increases the incidence of metabolic, inflammatory, cardiovascular, and neurodegenerative diseases and cancer, which significantly reduces life span and overall quality of life (4, 29, 59). Advancements in science and technology have considerably improved life expectancy, but these improvements have not provided a comparable reduction or delay in degenerative deficits associated with aging. Aging is accompanied by reduced physical activity and energy expenditure (EE) and declines in physiological functional capacity (4, 29). Further, aging-induced metabolic impairments are linked to obesity, diabetes, and cognitive decline (6, 54, 58).
The hypothalamus plays a critical role in aging through integrating endocrine and nervous system function and regulating a wide range of physiological functions. Mechanisms underlying hypothalamus-mediated aging include declines in nutrient sensing and cell metabolism, epigenetic alterations, proteostasis imbalances, and impairments in stem cell exosomal miRNA production (16, 38, 45, 49, 91). Furthermore, functional changes in specific hypothalamic neuronal populations promote age-associated physiological impairments (38).
Orexin (hypocretin, Orx) is a hypothalamic neurotransmitter produced in a specific subpopulation of neurons located in the lateral hypothalamus (LH). As described in original studies from Sakurai et al. (67) and Yoshida et al. (90), these neurons show a complex projection pattern throughout the brain. Initially, this neuronal population was recognized for its involvement in the regulation of hypothalamic regulated physiological functions, such as eating behavior, sleep, and spontaneous physical activity (SPA) (13, 84a, 33, 41, 62, 83). Orexin neurons also play a crucial role in many diverse states and processes that include mood, cognition, response to stress, anxiety, and pain (18, 35, 36, 51, 56, 65, 89). In aging, orexin system impairments have been shown in both humans and animal models. In humans, orexin neuronal loss and/or dysregulation in orexin function was detected in normal aging (31, 60), as well as in neurodegenerative disorders, such as Parkinson’s (23, 81) and Alzheimer’s (22, 43) disease. In aged rodents, a decrease in orexin peptide levels in addition to orexin neuronal loss is well documented (37, 71a, 77). Orexin neuronal loss not only affects processes regulated by orexin-signaling pathways, but it also disrupts normal communication between different brain regions due to diminished brain circuitry function. Altogether, current scientific data strongly suggest that compromised orexin function is an important mediator of age-related metabolic and behavioral disturbances.
In this study, we hypothesized that chemogenetic activation of orexin neurons will ameliorate middle-aged-associated changes in physical activity, EE, as well as cognitive and anxiety-related behavior. To test our hypothesis, we used the targeted expression of genetically modified designer receptors exclusively activated by designer drugs (DREADD) approach. A virus containing an excitatory DREADD construct encoded in an inverted open reading frame and flanked by lox-P recombination sites was stereotaxically injected into the LH of Orx/Cre mice. In these mice, Cre-recombinase expression is driven by the Orx promoter, which is exclusive for orexin neurons. Stimulation of the inserted DREADDs using clozapine-N-oxide (CNO) restored metabolic and behavioral impairments in middle-aged mice and influenced these processes in young animals. Orexin stimulation also increased general locomotion, as well as anxiety-like behavior in both young and middle-aged animals. These findings strongly suggest orexin neurons as potential therapeutic targets for approaches to mitigate age-related impairments.
MATERIALS AND METHODS
Animals and Ethics Statement
All experimental procedures in this study were approved by the University of Minnesota Animal Care and Use Committee. Mice were maintained on a 12:12-h light-dark cycle with chow (3.1 kcal/g; 18.6% protein; 6.2% fat; 44% carbohydrates; Teklad Global 18% protein rodent diet; Envigo, Hillcrest, UK) and water ad libitum. Adult, female C57BL/6J wild-type (WT) and orexin-cre (Orx/Cre) female animals were used for this study. Orx/Cre mice were initially obtained from Prof. Takeshi Sakurai (Kanazawa University) and bred on C57BL/6J background mice in our colony. Generation and initial phenotyping of Orx/Cre and WT heterozygous mice were conducted and have been described previously (50, 92). The mice were not monitored for estrous stage, so as not to introduce any behavioral stressors, which could confound the behavioral and physiological data.
Body Mass and Composition
Individual body weights were taken on all mice using an electronic balance (Ohaus, Parsippany, NJ). Body composition was estimated by measuring lean and fat mass using magnetic resonance imaging (Echo MRI 3-in-1; Echo Medical System).
Behavioral Test Battery
The behavioral test battery consisted of three assays performed in the following order: elevated plus maze (EPM), open field test (OFT), and novel object recognition test (NORT) (Fig. 1).
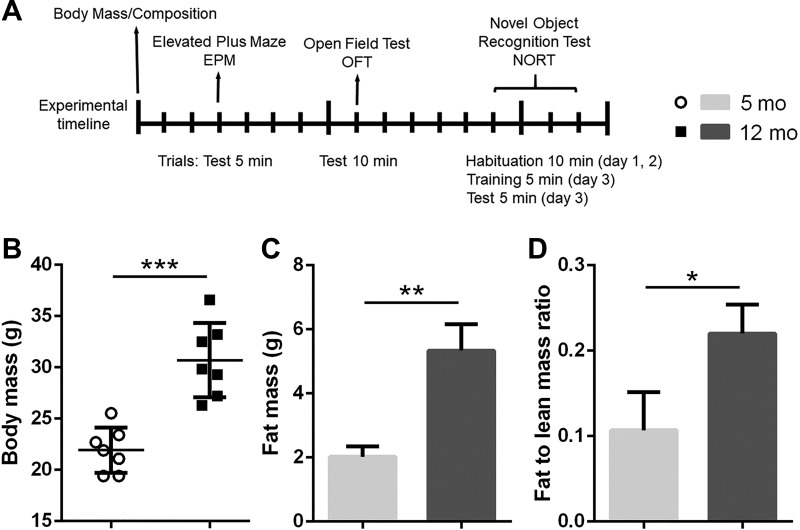
Fig. 1.
Differences in body composition between young (5 mo old) and middle-aged (12 mo old) female mice. A: timeline of the experimental procedures. Body mass and composition measurements were followed by a behavioral test battery. The elevated plus maze (EPM), open field test (OFT), and novel object recognition test (NORT) were conducted 5 days apart. Middle-aged mice had increased body (B) and fat (C) mass, as well as the ratio of fat to lean mass (D) relative to young mice (n = 7/group; *P < 0.05, **P < 0.01, ***P < 0.005).
The EPM and OFT are two of the most widely used tests for measuring anxiety-like behavior (39, 71, 85). These tests also provide measures of general locomotion. NORT is a commonly used behavioral assay for the investigation of various aspects of learning and memory in mice (2, 46). These assays were conducted in an order that minimized behavioral effects from testing in one assay to influence those in the next assay. The mice had at least 48 h rest time between tests to decrease carryover effects from prior tests, as well as to enable the washout of CNO physiological effects. The order of tests in which mice were tested was the same for each mouse, and each mouse was tested once per assay. Visual cues were placed on the walls around the testing area for all assays except the novel object recognition trials. All test trials were video-recorded, tracked, and analyzed with ANY-maze software (San Diego Instruments, San Diego, CA). The maze or arena was cleaned thoroughly with 70% ethanol between each session. All tests in the battery were conducted by the same experimenter in the University of Minnesota Behavioral Core in the early light cycle phase between 8 AM and 12 PM.
Elevated Plus Maze
A backlit elevated plus maze apparatus (Med Associates, Fairfax, VT) was used for this experiment. The maze was placed in the center of a room with its stage 95 cm above the floor level, and all the arms were at least 70 cm away from any object in the room. The light intensity was set to 50 lux measured at the maze level. A video camera was installed 60 cm above the center of the maze. The video camera was connected to a computer and ANY-maze software (San Diego Instruments) was used to track and analyze the movement in real-time mode. Animals were intraperitoneally injected either with saline or 3 mg/kg of CNO dissolved in saline 30 min before the test. Subjects were placed in the center of the maze facing toward one of the open arms and allowed to freely explore the maze for 5 min. The total distance traveled, distance traveled in open and closed arms, as well as of time spent in closed and opened arms of the maze were recorded and analyzed. Time spent in open arms (%) was calculated as (time in open arms × 100)/(total time).
Open Field Test
An opaque, white acrylic arena (50 × 50 × 25 cm) was used for this experiment. A video camera was installed 40 cm above the center of the maze. The camera was connected to a computer, and ANY-maze software (San Diego Instruments) was used to track and analyze the movement in real-time mode. The light intensity was set to 250 lux measured at the arena level. Animals were intraperitoneally injected either with saline or 3 mg/kg of CNO dissolved in saline 30 min before the test. Mice were placed in the middle of the arena and allowed to freely explore the arena for 10 min. Total distance traveled, distance traveled in open and closed areas, as well as time spent in closed and opened area of the arena were recorded and analyzed. Time spent in open area (%) was calculated as (time in open area × 100)/(total time).
Novel Object Recognition Test
An opaque, white acrylic arena (50 × 50 × 25 cm) was used for this experiment. A video camera was installed 40 cm above the center of the maze. The camera was connected with a computer, and ANY-maze software (San Diego Instruments) was used to track and analyze the movement in real-time mode. The light intensity was set to 40 lux measured at the arena level. Before the experiments, both objects, as well as object positions, were tested with a separate cohort of mice to demonstrate that the mice showed a preference for either object or object position (data not shown). A habituation trial was performed for 2 days before the experiment by allowing animals to explore the empty box for 10 min. Animals were injected intraperitoneally either with saline or 3 mg/kg of CNO dissolved in saline 30 min before the test. Mice were placed in the arena containing two objects for a 5-min training session and then returned to their home cages. After 1 h, mice were placed back into the same arena with one of the previous objects and a novel object for a 5-min testing session. The time spent interacting with the familiar, as well as novel objects, was recorded and analyzed. Novel object interaction (%) was calculated as (novel object interaction × 100)/(sample object interaction + novel object interaction).
Viral Injections and Drug Administration
Animals were anesthetized with isoflurane (1–4%) and placed in a stereotactic apparatus (Kopf Instruments, Tujunga, CA). DREADD targeting was achieved by stereotaxic injection of a Cre-dependent AAV vector expressing a double-floxed inverted open reading frame (DIO) around the DREADD transcript and a fluorescent tag (mCherry). Vectors (Addgene, Watertown, MA) were injected into the LH (AP, −1.8/DV, −5.5/ML ±0.9 mm from bregma; 333 nl/5 min) of Orx/Cre mice. Excitatory neuromodulation was achieved via Gq-coupled pAAV-hSyn-DIO-hM3D(Gq)-mCherry (2.5 × 1013 GC/ml). Animals recovered from the surgery for 2 wk, after which they were randomly assigned to appropriate experimental groups before testing.
Water-soluble CNO dihydrochloride was obtained from HelloBio. For behavioral tests, 3 mg/kg of CNO in saline or saline was injected via a small-gauge (32) syringe once 30 min before testing. Acute tests were followed by a 5-day long washout period. For indirect calorimetry and SPA studies, animals were given CNO dissolved in drinking water (0.25 mg/ml).
Comprehensive Laboratory Animal Monitoring System
The Comprehensive Laboratory Animal Monitoring System (CLAMS, Columbus Instruments) is a set of live-in cages for automated, noninvasive calorimetry assessment. Body mass and composition were measured, and animals were placed in CLAMS cages, while oxygen consumption and CO2 production were recorded for 48 h (the first 24 h were considered the habituation period, while the last 24 h were used in the analysis). Respiratory exchange ratio (RER) was calculated as the volume of CO2 expired versus volume of oxygen consumed (V̇co2/V̇o2) and used to calculate EE using a formula provided by the manufacturer. Energy expenditure was expressed as kcal/h and analyzed with lean body mass as a covariate in an ANCOVA model. SPA was assessed by continuous measurement of ambulatory activity and total movement detected by infrared beam breaks in the x and y axes.
Immunohistochemistry
Mice were perfused intracardially with ice-cold saline, followed by 20 ml of 4% paraformaldehyde (PFA) in PBS. Brains were harvested and postfixed in 4% PFA/PBS overnight at 4°C, followed by 30% (wt/vol) sucrose in PBS solution at 4°C until the brains sank. The brains were imbedded in OCT (optimal cutting temperature compound), frozen in dry ice-cooled ethanol, and then immediately cut. Forty-micrometer-thick coronal brain sections were collected and stored in cryoprotectant (30% (wt/vol) sucrose, 30% (vol/vol) ethylene glycol, 1% (wt/vol) PVP-40 in PB). Brain sections were washed six times for 5 min with 0.1 M PBS, pH 7.4. Antigen retrieval was performed using antigen unmasking solution (Vector Laboratories, Burlingame, CA). After initial washing (0.1 M PBS, pH 7.4, three times for 5 min) was completed, sections were transferred in antigen unmasking solution and incubated 30 min on 90°C. The brain slices were then washed three times for 5 min with PBS and incubated with 5% normal horse serum in PBST for 2 h at room temperature. After being washed three times in PBST, the sections were incubated with primary antibodies (mouse monoclonal orexin A, rabbit polyclonal c-Fos 1:500 Santa Cruz, CA) overnight at RT on a platform shaker. Brain sections were washed in PBST four times for 10 min after primary antibody incubation and incubated with secondary antibodies conjugated with Alexa Fluor dyes (donkey anti-mouse, donkey anti-rabbit 1:500, Invitrogen, Carlsbad, CA). Brain sections were then washed four times for 10 min in PBST and then mounted with ProLong Gold mounting media (Invitrogen).
Orexin A Cell Counts and Densitometry
For quantification of orexin A-positive cells and orexin A densitometry analysis, every sixth coronal section from −0.94 to −2.18 bregma (20) (five in total) was stained for orexin A and analyzed. Orexin A-positive cell density in the LH orexin field was determined by counting orexin A-positive cells (ImageJ software, National Institutes of Health, Bethesda, MD) and dividing by region of interest (ROI) area. Optical density was determined by measurement of the mean gray value of the orexin field and division by the R01 area. Images were captured using ×20 magnification.
Imaging
Immunofluorescence images were captured using the Nikon Eclipse NI-E microscope (Nikon), with a monochrome Nikon black and white camera DS-QiMc (Nikon). Each fluorochrome is represented as a pseudocolor in the images.
Statistical Analysis
All data were analyzed using either Prism 6.0 (GraphPad Software, San Diego, CA) or SPSS (IBM). Statistical analyses of behavioral data, body mass, and composition were performed using a Student’s t-test. For the DREADD study, the analyses of behavioral data, body mass and composition, and total SPA were performed using two-way ANOVA followed by a Tukey post hoc test. For the hourly SPA data, two-way ANOVA followed by Tukey’s post hoc test was used to detect group differences. Indirect calorimetry data were analyzed with lean body mass as a covariate in an ANCOVA model followed by the Sidak post hoc test (82).
Experimental Design and Exclusion Criteria
The initial phenotyping study was performed on 5- and 12-mo-old C57BL/6J female mice (5 mo: n = 7; 12 mo: n = 7). Before evaluation in the behavioral test battery, animal mass and body composition were measured. The behavioral test battery consisted of EPM, OFT, and NORT. Tests were performed 5 days apart to prevent behavioral effects from testing in one assay to influence those in the next assay (Fig. 1A).
The DREADD study was performed on 5- and 12-mo-old-old Orx/Cre animals subjected to viral intracranial injections (pAAV2-hSyn-DIO-hM3D(Gq)-mCherry). After a 2-wk recovery period, animals were introduced to the behavioral test battery (EPM, OFT, and NORT) (5 mo: qDREADD and saline; n = 8; 12 mo: qDREADD and saline; n = 8; 5 mo: qDREADD and CNO; n = 9; 12 mo: qDREADD and CNO; n = 10). Behavioral tests were followed by indirect calorimetry and SPA measurements (5 mo: qDREADD and water; n = 6; 12 mo: qDREADD and water; n = 6; 5 mo: qDREADD and CNO; n = 5; 12 mo: qDREADD and CNO; n = 5; Fig. 3A). All animals were perfused, and brains were collected for injection placement confirmation. Animals were excluded from the experiment if post hoc histological analyses showed inaccurate viral injection placement. Mice were observed for neurological deficits and underperformance on behavioral tests, although none were observed. For DREADD expression and c-Fos analyses, subjects were injected with either saline or CNO (5 mg/kg) 90 min before perfusion to confirm functional activation of the DREADD in orexin neurons by c-Fos (immediate early gene product) labeling. Every sixth section containing LH was stained for orexin A and c-Fos.
Fig. 3.
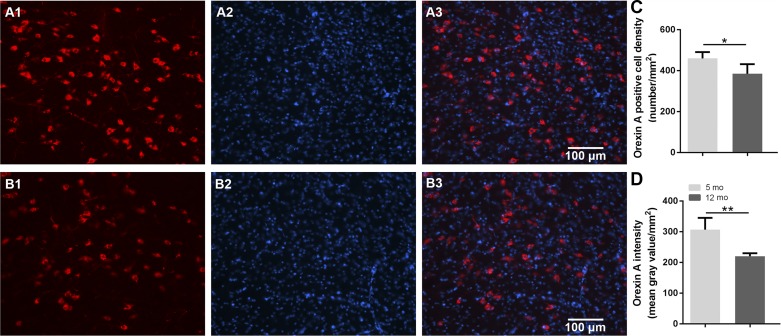
Age effects on orexin neuron density and orexin A expression. A: representative images displaying orexin A neurons in lateral hypothalamus: orexin A-positive neurons in red (A1: 5 mo old; B1: 12 mo old), DAPI in blue (A2: 5 mo old; B2: 12 mo old), and merged images (A3: 5 mo old; B2: 12 mo old). C: middle-aged female mice showed less orexin A-positive cell density compared with that in 5-mo-old female mice. D: reduced intensity of orexin A staining, indicating reduced expression of orexin A in 12-mo-old female mice compared with 5-mo-old female controls (n = 5/group; *P < 0.05, **P < 0.01).
RESULTS
Body Weight, Adiposity, Locomotion, and Anxiety-Like Behavior
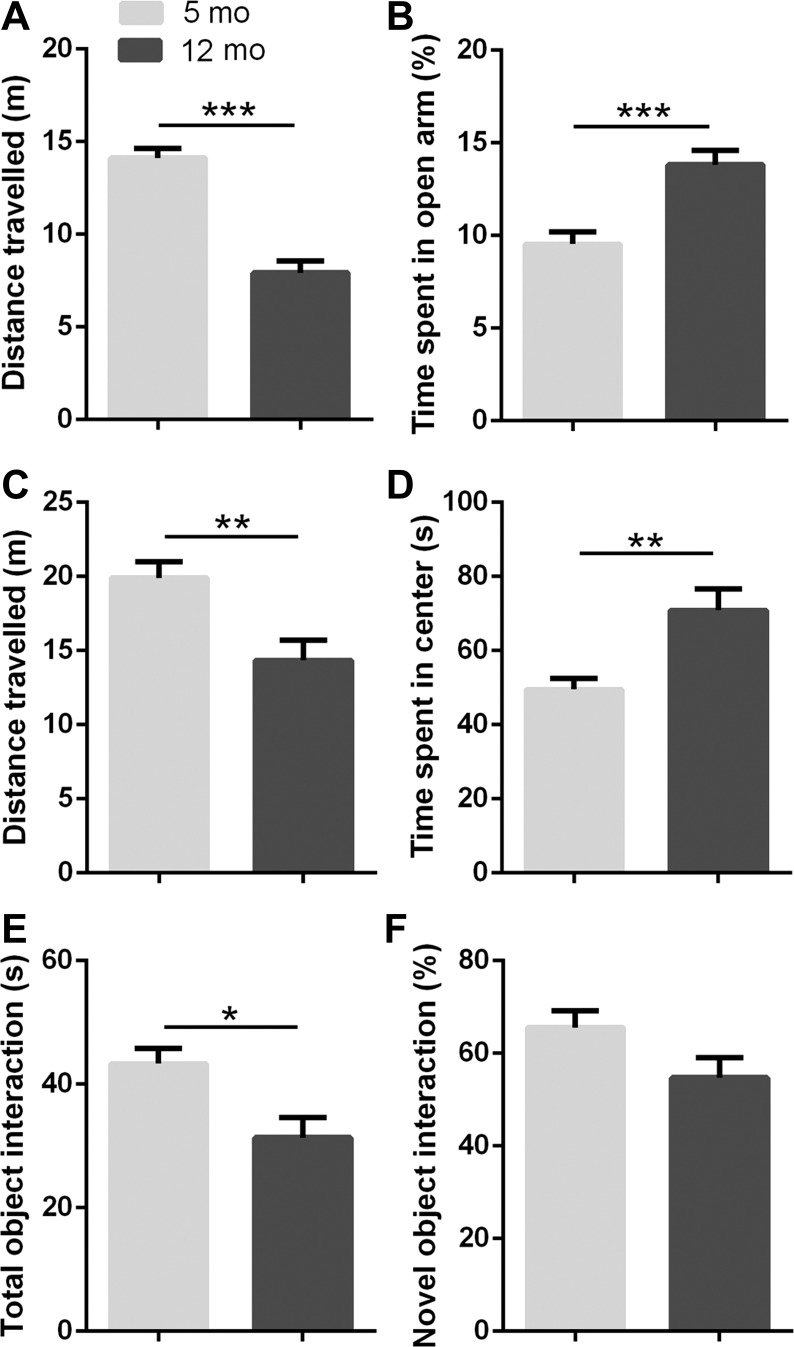
To characterize middle-aged-induced changes in body mass, composition, and behavior, we used 5- and 12-mo-old old female WT mice. There is a large body of evidence emphasizing aging-associated changes in metabolism and behavior. Consistent with previous studies, middle-aged animals had increased body mass (P < 0.05; Fig. 1B), fat mass (P < 0.01; Fig. 1C), and the fat-to-lean mass ratio (P < 0.05; Fig. 1D). Furthermore, middle-aged animals covered less distance in both the EPM (P < 0.005) and OFT (P < 0.01; Fig. 2, A and C), while showing less exploratory activity, which is represented as the total object interaction in the NORT (P < 0.05; Fig. 2E). The reduction in general locomotion and exploratory activity corresponds with results from previous studies (8, 75). Interestingly, middle-aged animals showed reduced anxiety-like behavior in both the EPM and OFT. The time spent in open arms in the EPM was significantly higher in the middle-aged group (P < 0.005; Fig. 2B), as was the time spent in the center in the OFT (P < 0.01; Fig. 2D). Finally, no differences in working memory were observed between young and middle-aged animals (Fig. 2F).
Fig. 2.
Aging-induced behavioral differences between young (5 mo old) and middle-aged (12 mo old) female mice. A: younger female mice covered more distance in the elevated plus maze (EPM) assay compared with middle-aged female mice. B: time spent in open arms was significantly higher in middle-aged compared with younger female mice in the EPM. C: aging reduced general locomotor activity and exploratory behavior. D: middle-aged female mice spent more time in the center of the maze, as compared with younger female mice (**P < 0.01). E: middle-aged female mice showed less interest in exploring objects and lower exploratory activity. F: there was no significant difference in novel object interaction time (n = 7/group; *P < 0.05, **P < 0.01, ***P < 0.005).
Orexin Neuron Density and Orexin Expression in the Lateral Hypothalamus
Immunohistochemistry was performed to determine whether middle-aged mice have reduced numbers of orexin neurons and reduced expression of orexin, as suggested in previous studies (10, 29, 35, 62, 68). Middle-aged, 12-mo-old mice showed lower orexin A-positive cell density compared with younger, 5-mo-old mice (P < 0.05; Fig. 3C). Furthermore, expression levels of orexin A were significantly lower in middle-aged female mice compared with young female controls (P < 0.01; Fig. 3D).
Chemogenetic Activation of Orexin Neurons and Behavior
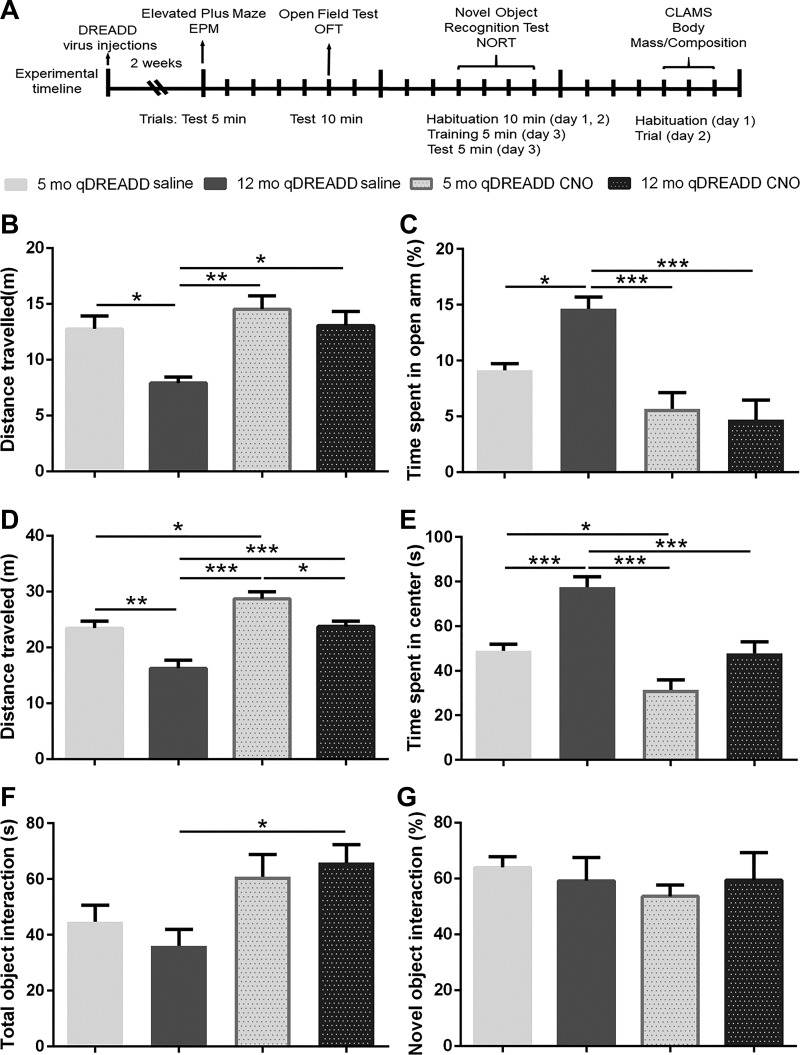
To test whether increased orexin neuronal activation can mitigate age-related behavioral changes, we used a behavioral test battery consisting of EPM, OFT, and NORT. EPM and OFT were used to assess anxiety-like behavior, as well as general locomotion. Compared with young animals, middle-aged animals spent more time in the open arm (5 mo: qDREADD saline vs. 12 mo: qDREADD saline, P < 0.05; Fig. 4C). The chemogenetic intervention did not affect anxiety-like behavior in young animals, but it reduced time spent in the open arms in middle-aged animals (12 mo: qDREADD and saline vs. 12 mo: qDREADD and CNO, P < 0.005; Fig. 4C). Furthermore, we observed changes between middle-aged naïve and young CNO-treated animals (12 mo: qDREADD and saline vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 4C). Middle-aged saline-treated animals covered less distance compared with young animals (5 mo: qDREADD and saline vs. 12 mo: qDREADD and saline, P < 0.05; Fig. 4B), while qDREADD activation increased distance traveled (12 mo: qDREADD saline vs. 12 mo: qDREADD CNO, P < 0.05; Fig. 4B; 5 mo: qDREADD CNO vs. 12 mo: qDREADD saline, P < 0.05; Fig. 4B).
Fig. 4.
Chemogenetic modulation of behavior in young (5 mo old) and middle-aged (12 mo old) female mice. A: timeline of the experimental procedures. Orx/Cre female mice received intracranial injections of virus containing the excitatory designer receptors exclusively activated by designer drugs (DREADD) construct. After 2 wk of recovery time, a behavioral test battery consisting of the elevated plus maze (EPM), open field test (OFT), and novel object recognition test (NORT) was performed. Tests were conducted 5 days apart. Body composition was measured 5 days after the NORT test, and the female mice were taken to the comprehensive laboratory animal monitoring system (CLAMS) cages and habituated for 1 day. Spontaneous physical activity and energy expenditure were then measured for 24 consecutive hours. B: middle-aged female mice covered less distance than young female mice in the EPM. Chemogenetic activation of orexin neurons did not affect the young mice, while it increased locomotor activity in the middle-aged female mice, restoring it to the level observed in young mice. C: aging reduces anxiety-like behavior in the EPM in control female mice. Orexin neuronal stimulation increases time spent in the closed arms in middle-aged mice, restoring it to the control values. D: aging reduces locomotion in the OFT. Clozapine-N-oxide (CNO) treatment increased locomotion in both young and middle-aged mice. Effects of orexin neuronal activation in middle-aged female mice could not match the effects observed in young mice. E: middle-aged female mice had reduced anxiety-like behavior. Activation of orexin neurons increased time spent in the center for both young and middle-aged mice. F: while there were no detected differences in total object interaction between 5-mo-old and 12-mo-old mice, CNO treatment induced changes between the control and CNO-treated middle-aged female mice. G: stimulation of orexin neurons did not affect novel object interaction in young or middle-aged female mice in both groups (5 mo: DREADD saline, n = 8; 12 mo: DREADD saline, n = 8; 5 mo: DREADD CNO, n = 9; 12 mo: DREADD CNO, n = 10; *P < 0.05, **P < 0.01, ***P < 0.005).
In the OFT, middle-aged, saline-treated animals spent more time in the center compared with young animals (5 mo: qDREADD and saline vs. 12 mo: qDREADD and saline, P < 0.005) (Fig. 4E). CNO treatment increased anxiety-like behavior in both young and middle-aged animals (5 mo: qDREADD and saline vs. 5 mo: qDREADD and CNO, P < 0.05; 12 mo: qDREADD and saline vs. 12 mo: qDREADD and CNO, P < 0.005; and 12 mo: qDREADD and saline vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 4E). Aging affected total distance traveled in OFT (5 mo: qDREADD and saline vs. 12 mo: qDREADD and saline, P < 0.01; Fig. 4E). CNO treatment increased locomotion in both young and middle-aged mice (5 mo: qDREADD and saline vs. 5 mo: qDREADD and CNO, P < 0.05; Fig. 4D; 12 mo: qDREADD and saline vs. 12 mo: qDREADD and CNO, P < 0.005; Fig. 4D), and also in younger CNO-treated mice compared with middle-aged, saline-treated mice (12 mo: qDREADD and saline vs. 5 mo: qDREADD and CNO; P < 0.005; Fig. 4D). Finally, differences in locomotor activity were observed between young and middle-aged CNO groups (5 mo: qDREADD and CNO vs. 12 mo: qDREADD and CNO; P < 0.05).
To assess whether orexin neuronal specific qDREADD activation affects working memory, animals were subjected to the NORT assay. Working memory was not affected by age or by CNO treatment (Fig. 4G), but activation of orexin neurons increased total object interaction time in middle-aged mice (12 mo: qDREADD and saline vs. 5 mo: qDREADD and CNO; P < 0.05; Fig. 4F), suggesting that chemogenetic activation of orexin neurons promotes exploratory activity.
Effect of Chemogenetic Activation of Orexin Neurons on SPA and EE
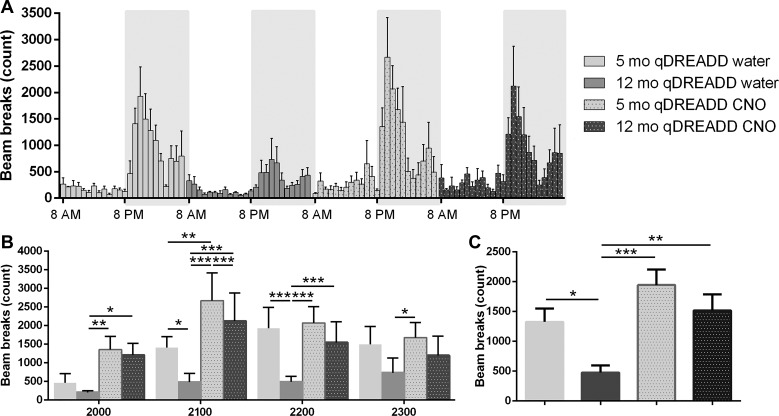
Chemogenetic activation of orexin neurons increased SPA. Two-way ANOVA identified statistically significant effects of both aging and the DREADD intervention on SPA. A post hoc analysis showed that differences in SPA occurred exclusively during the early dark (active) phase from 2000 to 2300 h. In the first hour (2000), DREADD activation increased SPA in middle-aged animals (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO, P < 0.05; Fig. 5B). Moreover, we observed differences between 12 mo: control and 5 mo: CNO-treated mice (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO; P < 0.001; Fig. 5B). During the second hour (2100), aging-induced reductions in SPA were observed (5 mo: qDREADD and water vs. 12 mo with qDREADD and water, P < 0.05; Fig. 5B). Furthermore, DREADD intervention increased SPA in young animals (5 mo: qDREADD and water vs. 5 mo: qDREADD and CNO, P < 0.01), as well as in middle-aged animals (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO, P < 0.005). Differences between middle-aged control animals and young CNO-treated animals were also observed (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 5B). In the third hour (2200), aging-induced reductions in SPA were detected (5 mo: qDREADD and water vs. 12 mo: qDREADD and water, P < 0.005; Fig. 5B). CNO treatment increased SPA in middle-aged animals (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO, P < 0.005; Fig. 5B). Differences between middle-aged control animals and young CNO-treated animals were also detected (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 5B). During the final hour (2300), the only observable differences were between middle-aged control and young CNO-treated mice (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO; P < 0.005) (Fig. 5B).
Fig. 5.
Activation of orexin neurons increases spontaneous physical activity (SPA) in middle-aged female mice. A: SPA measured per hour during the 24-h period shown by the number of beam breaks. The white area represents the light phase, while the shaded area represents the dark phase. B: SPA during the 4-h period of highest activity at the beginning of the active (dark) phase. Aging-induced reductions in SPA were observable in the second (2100 h) and third hour (2200 h). Stimulation of orexin neurons increased SPA in young (2100 h) and middle-aged female mice (2000, 2100, 2200 h). C: aging decreased SPA in the control female mice. Stimulation of orexin neurons increased SPA in middle-aged female mice [5-mo-old designer receptors exclusively activated by designer drugs (DREADD) saline, n = 6; 12-mo-old DREADD saline, n = 6; 5-mo-old DREADD clozapine-N-oxide (CNO), n = 5; 12-mo-old DREADD CNO, n = 5; *P < 0.05, **P < 0.01, ***P < 0.005].
Our analyses of total SPA during the 4-h high-activity period showed a significant effect of aging on SPA (5 mo: qDREADD and water vs. 12 mo: qDREADD and water, P < 0.05; Fig. 5B), as well as a significant effect of CNO, but only in middle-aged animals (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO, P < 0.01; Fig. 5B). Finally, SPA differences between middle-aged control animals and younger CNO-treated animals (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 5C) were observed.
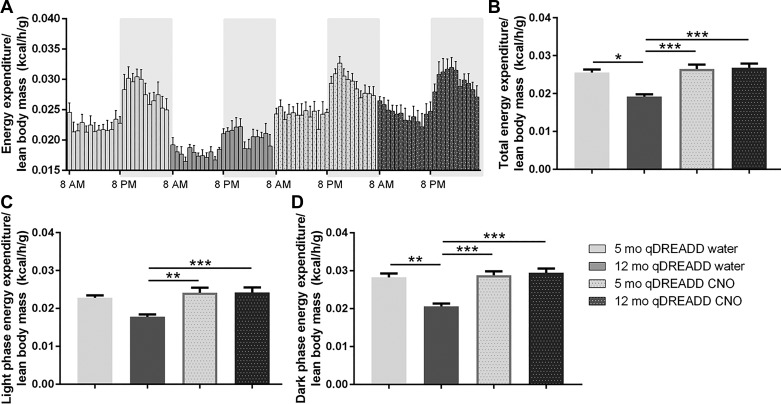
To test whether orexin neuronal activation influences SPA and EE in young and middle-aged animals, Orx/Cre animals injected with a Cre-dependent qDREADD virus into the LH. Excitation of orexin neurons was induced by CNO dissolved in their drinking water (0.25 g/ml). ANCOVA analysis showed significant differences in total [F(3, 17) = 14.28, P < 0.005; Fig. 6B], light [F(3, 17) = 7.98, P < 0.005; Fig. 6C], and dark phase EE [F(3, 17) = 16.488, P < 0.005; Fig. 6D]. The post hoc analysis identified a reduction in total EE in middle-aged animals (5 mo: qDREADD and water vs. 12 mo: qDREADD and water, P < 0.05; Fig. 6B). Furthermore, CNO treatment increased total EE in the middle-aged group (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO; P < 0.005; Fig. 6B). Finally, we observed a difference between the aged control group compared with the young CNO-treated group (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 6B).
Fig. 6.
Chemogenetic activation of orexin neurons elevated energy expenditure (EE) in middle-aged female mice. A: EE measured per hour during the 24-h period. White area represents the light phase of the day, while the shaded area represents the dark phase of the day. B: EE was reduced in middle-aged mice. Treatment with clozapine-N-oxide (CNO) did not affect EE in young female mice, but it increased EE in middle-aged mice. C: designer receptors exclusively activated by designer drugs (DREADD) stimulation affected EE during the light phase in middle-aged mice. D: stimulation of orexin neurons increased EE only in middle-aged female mice (5-mo-old DREADD saline, n = 6; 12-mo-old DREADD saline, n = 6; 5-mo-old DREADD CNO, n = 5; 12-mo-old DREADD CNO, n = 5; *P < 0.05, **P < 0.01, ***P < 0.005).
Similar changes in EE were observed in the dark phase. Aging reduced EE (5 mo: qDREADD and water vs. 12 mo: qDREADD and water; P < 0.01; Fig. 6D), while CNO treatment restored EE values to that observed in the young animals (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO; P < 0.005; Fig. 6D). Additionally, significant differences were observed between middle-aged CNO-treated mice and young control mice (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO, P < 0.005; Fig. 6D). During the light phase, EE was not affected by aging (Fig. 6C), but CNO treatment increased EE in middle-aged mice (12 mo: qDREADD and water vs. 12 mo: qDREADD and CNO, P < 0.005; Fig. 6C). There was also a difference between the middle-aged control group relative to the young DREADD-treated group (12 mo: qDREADD and water vs. 5 mo: qDREADD and CNO; P < 0.001; Fig. 6C).
Confirmation of Injection Placement and Chemogenetic Activation of Orexin Neurons
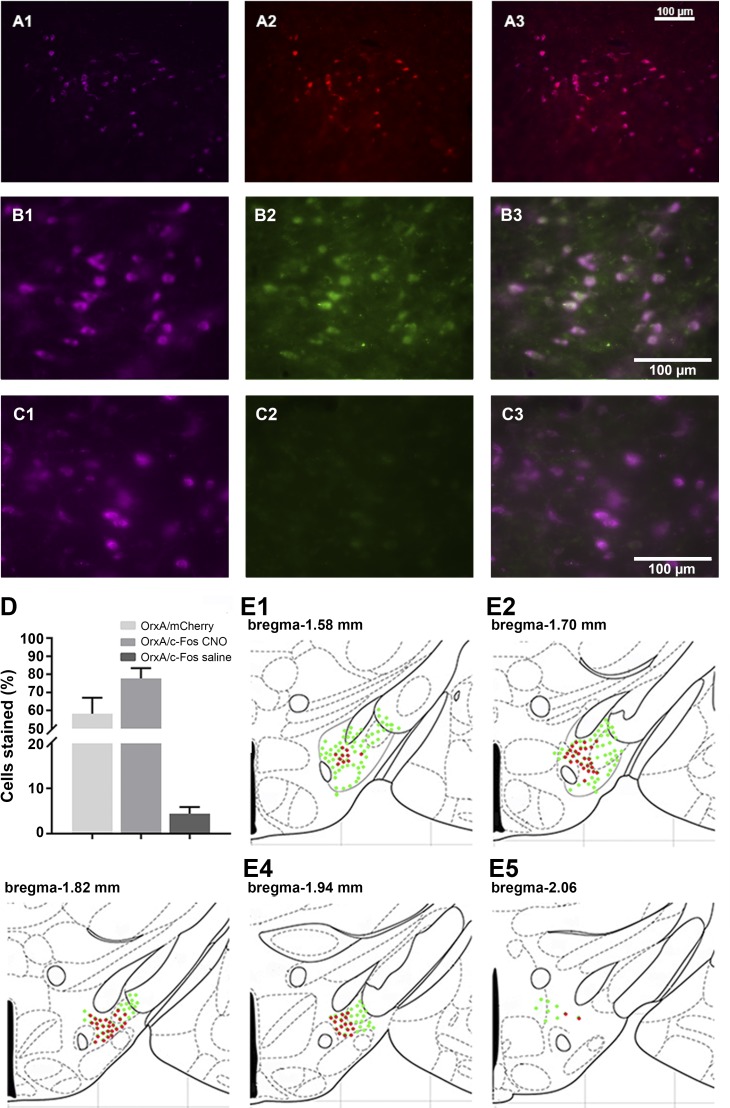
Orexin-cre animals used in the DREADD study received bilateral DREADD viral injections. Immunohistological analyses confirmed the selective expression of hM3Dq-mCherry in orexin neurons. Clear colocalization of orexin-stained cells and hM3Dq-mCherry (OrxA/mCherry total means ± SE, 58.5 ± 7.2; Fig. 7, A and D) were observed. Higher-magnification images were used to estimate orexin neuron-specific expression of the immediate early gene product, c-Fos. We observed that a majority of orexin neurons responded to CNO stimulation by c-Fos expression (OrxA/c-Fos CNO means ± SE: 77.2 ± 5.2) (Fig. 7, B and D). The group of animals that received saline had minimal coexpression of orexin and c-Fos (OrxA/c-Fos saline means ± SE: 4.21 ± 2.17) (Fig. 7, C and D).
Fig. 7.
Expression of the designer receptors exclusively activated by designer drugs (DREADD) and functionality confirmation. A: representative images displaying viral expression of DREADDs in the lateral hypothalamus (LH): orexin A-positive neuron in purple (A1), mCherry-positive neurons in red (A2), and merged images (A3). B and C: representative images displaying c-Fos (early gene product) expression. The LH of mice with the excitatory DREADD and treated with clozapine-N-oxide (CNO) 90 min before perfusion (B) and mice with the excitatory DREADD treated with saline 90 min before perfusion. Orexin A in purple (B1, C1), c-Fos in green (B2, C2), and then the merged images (B3, C3). D: number of OrxA/mCherry and OrxA/c-Fos colocalized cells. E1–E5: schematic drawings displaying the spread of viral expression along the LH; green orexin-expressing cells, red mCherry-expressing cells.
DISCUSSION
Aging-associated disability reduces quality of life and represents a tremendous burden on society. It is estimated that by 2050, the worldwide population will consist of 2 billion (21.1%) people older than 60 yr of age, which is almost double that in 2014 (42, 68, 74). The process of aging has deleterious effects on many physiological processes, including general activity and energy metabolism. These changes occur in the early stages of aging, and their cumulative effects can lead to more serious outcomes, such as obesity, diabetes, and cardiovascular impairments, as well as cognitive decline and neurodegenerative diseases (5, 12, 54, 73).
Orexin neurons have an integrative and regulatory function in receiving both external and internal signals for physiological phenomena (33). In addition to being strongly involved in the regulation of hypothalamic and prefrontal cortex function, orexin neuron sends regulatory signals to the hippocampus and amygdala and can impact cognition and emotion. Aging reduces orexin production in the hypothalamus and alters orexin receptor expression throughout the brain (10, 64, 70, 77, 79), suggesting that loss of orexin function with age may be one of the factors in aging-related sleep and, metabolic disturbances, as well as cognitive decline (55, 60, 77).
The overall goal of this study was to identify early aging-related changes in SPA, general locomotion, EE, and behavior, as well as to determine whether the targeted in vivo activation of orexin neurons could mitigate middle-aged-related behavioral, metabolic, and cognitive decline. To address this goal, we first established early aging effects on general behavior, body mass, and composition by performing basic body mass and composition measurements, as well as a battery of behavioral tests (EPM, OFT, and NORT). As described in earlier studies, the middle-aged (12-mo-old) animals have greater body and fat mass. These changes may be related to many factors, such as glucose intolerance, insulin resistance, reduced fatty acid oxidation, reduced ketogenesis, decreased mitochondrial biogenesis, oxidative phosphorylation, expression of stress defense genes, and apoptotic regulation (4, 9, 53). Adult body and fat mass is typically retained until mice reach ~18 mo of age, at which point, they begin to exhibit declines in body and fat mass (28).
Aging considerably affects behavior as well. The current studies indicate a reduction in general locomotion, exploratory activity, as well as anxiety-like behavior consistent with previously reported data (8, 17, 19, 75, 76). Interestingly, changes in working memory were not detected. Some studies suggest that the working memory component of cognition is more resistant to aging than other components such as memory consolidation or long-term memory (17, 86). The current data suggest that the capability of distinguishing a novel object from a familiar one within the working memory time frame is not diminished in 12-mo-old mice; it is possible that testing in an older age group could reveal differences. Similar to the observations in the present study, other studies have shown loss of orexin production and orexin neurons with age (10, 62, 68) (31, 37). Tabuchi et al. (78) proposed that minor reductions in orexin neuron numbers may not induce significant physiological changes, and it is important to note that orexin-mediated functional losses are just one of many processes affected by age. Yet orexin is important for normal functions of many of the behaviors affected by age and, thus, represents a strong candidate target for chemogenetic manipulation to improve functional losses with age.
There is a large amount of evidence emphasizing the relationship between aging and declines in general metabolism and activity (4, 15, 29, 44, 75). In the current studies, middle-aged animals had reduced SPA and EE. Interestingly, the detected changes were only in the dark, active phase of the day. Aging did not induce changes in SPA and EE during the light phase, suggesting an absence of major sleep disturbances in these middle-aged female mice. While sleep impairments and disruption of the circadian rhythm are commonly observed in the later stages of aging (57, 87), a recent study showed that fine alterations in sleep architecture can be observed in middle-aged animals if more sophisticated sleep/wake measures are implemented (52). Sasaki et al. (69) reported that chemogenetic activation of orexin neurons alters sleep/wake states. In the current study, we did not measure sleep/wake states by EEG/EMG. Rather, we measured SPA, which can be considered a gross correlate of sleep and wake states, and we did not observe changes in SPA during the light (inactive) phase. This suggests that in contrast to the Sasaki study, the chemogenetic stimulation of orexin neurons did not affect sleep/wake states. It is possible that if we had used sensitive sleep/wake measurements [similar to that of Sasaki et al. (69)], differences would have been observed. Another important contrast between the two studies is that in the present study CNO was administrated in the drinking water, whereas in the Sasaki study, the CNO was given intraperitoneally. The intraperitoneal injection approach can create more robust responses and may explain the differences between the two studies.
As mentioned above, an important aspect of the current study is that the DREADD ligand, CNO, was administered in the drinking water, and as such, if there are differences between groups in water intake, this could affect the dose of CNO and, therefore, influence result interpretation. A previous study had shown that DREADD activation of orexin neurons induces changes in water intake when CNO is administrated intraperitoneally (32), and thus, we tested whether CNO administered by drinking water affects water consumption. As shown in Supplemental Fig. S1 (see online supplemental figures at the Journal website), we observed no differences in water consumption between groups, and thus, we are confident that differential water intake did not affect interpretability of the data. Furthermore, Gomez et al. (27) showed that CNO does not readily cross the blood-brain barrier in vivo, converts to clozapine, and activates DREADDs. Clozapine is known for antipsychotic effects that could potentially affect mouse behavior, and thus, additional tests were performed to ensure that there were no effects of CNO in behavioral assays in the non-DREADD mice. As shown in Supplemental Fig. S2, clozapine had no effect on behavior in the non-DREADD mice.
The next goal was to determine whether stimulation of orexin neuronal activity could ameliorate aging-induced effects on behavior, as well as energy metabolism and SPA. Activation of orexin neurons using the DREADD approach significantly increased locomotion, which was observable only in the OFT for young animals, and in both OFT and EPM in middle-aged mice. This is consistent with the literature, as orexin treatment restores the locomotor impairment in orexin knockout mice and increases locomotion in wild-type animals (11, 47).
Under stress-inducing conditions, orexin is a strong modulator of the stress response, as well as anxiety and depression (20, 21, 67), but it is not when under nonstressful conditions (1, 30, 84). One study from Arendt et al. (3) showed anxiolytic effects of orexin 2 receptor in the basolateral amygdala. In the current study, activation of orexin neurons increased anxiety-like behavior in the OFT but not in the EPM. Furthermore, locomotor activity, which is usually associated with increased anxiety (21, 72), was not altered. More studies are needed to determine whether chemogenetic activation of orexin neurons induces anxiogenic or anxiolytic effects, and if so, which regional populations of orexin neurons are implicated.
Activation of orexin neurons with CNO did not affect working memory performance but simultaneously increased exploration in middle-aged animals. Several studies have shown that orexin is important for memory and cognition. Studies with orexin/ataxin-3 mice (a genetic model of selective orexin neuronal neurodegeneration) identified an impairment in cognition that can be restored by orexin treatment (51, 88). Furthermore, a study from Jaeger et al. (34) showed that aging-associated cognitive impairment is ameliorated by orexin treatment. These studies addressed memory consolidation and/or retention but did not address working memory. It is possible that orexins are mainly involved in memory consolidation and retention processes and not working memory, an idea that is supported by earlier studies, suggesting direct effects of orexin on memory consolidation (14, 66).
A large number of studies support the notion that orexin is crucial for SPA and EE regulation. Pharmacological orexin intervention increases SPA and EE, while selective orexin receptor antagonist treatment (40, 41, 61, 93), as well as orexin neuronal loss reduces SPA and EE (7, 80). Being a non-goal-directed activity, such as fidgeting, and nonspecific ambulatory behavior, SPA is a major contributor to EE. Energy expenditure associated with SPA is known as nonexercise activity thermogenesis (NEAT). In sedentary humans, NEAT can represent up to 28% of total EE (24) and is an interesting target for weight loss therapy. With aging, orexin neurons and orexin-stimulated SPA is reduced, and we posited that orexin stimulation in middle-aged animals would ameliorate the observed reductions in SPA. This hypothesis was confirmed as stimulation of orexin neurons using DREADD neuromodulation increased SPA in the middle-aged animals, suggesting that age-reduced EE and SPA can be restored by enhancement of orexin neuronal activity.
The current study provides several interesting findings. First, it demonstrates that early aging (i.e., vs. late aging, or that beyond 18 mo old in mice) induces changes in both behavior and energy metabolism. We showed that middle-aged animals show reduced EE, SPA, general locomotion, and anxiety-like behavior, in addition to increases in body and fat mass. Furthermore, we identified that the primary EE and SPA differences between young and middle-aged animals occur during the dark, active phase of the day. Second, for the first time to our knowledge, we showed that age-related reductions in locomotion, anxiety-like behavior, SPA, and EE can be influenced by in vivo activation of orexin neurons using DREADDs. Moreover, the effects of orexin activation were more pronounced during the active phase of the day, suggesting that circadian rhythm plays an important role in orexin neuron functions. Finally, DREADD activation of orexin neurons had a greater effect in middle-aged animals, confirming the presence of orexin function disturbances in middle-aged animals and showing that aged orexin neurons have neuromodulation potential. The multiple observed effects of orexin neuronal stimulation can be explained by the complex patterns of orexin neurons projections (48, 63, 67, 90). Together, the present results confirm that orexin neurons can be considered potential and therapeutic targets for approaches to reverse age-related impairments, such as weight gain, reduced activity, and reduced cognition.
Perspectives and Significance
Aging affects a variety of orexin-regulated processes, and functioning of the orexin system diminishes with aging. In the current study, we showed that orexin system impairment occurs in middle-aged female mice and that changes in locomotion, spontaneous physical activity, energy expenditure, and anxiety-like behavior can be ameliorated by chemogenetic activation of orexin neurons. Results from this study imply that the orexin system contributes significantly to energy metabolism and behavior, particularly in the context of aging. This study also identifies orexin neurons as potential targets for treatment of aging-associated cognitive and metabolic impairments.
GRANTS
This work was supported by the Department of Veterans Affairs Grant 5I01RX000441-04 (to C. M. Kotz) and the National Institutes of Health Grant 5R01DK100281-03.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.S., J.P.P.Y., and C.M.K. conceived and designed research; M.S., J.P.P.Y., and V.M. performed experiments; M.S. and J.P.P.Y. analyzed data; M.S., J.P.P.Y., and C.M.K. interpreted results of experiments; M.S. and J.P.P.Y. prepared figures; M.S. drafted manuscript; C.M.K. edited and revised manuscript; C.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the Department of Neuroscience Mouse Behavior Core, as well as the Integrative Biology and Physiology Phenotyping Core at the University of Minnesota for support.
REFERENCES
- 1.Alexandre C, Andermann ML, Scammell TE. Control of arousal by the orexin neurons. Curr Opin Neurobiol 23: 752–759, 2013. doi: 10.1016/j.conb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process 13: 93–110, 2012. doi: 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, Ronan PJ, Summers CH. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology 40: 17–26, 2014. doi: 10.1016/j.psyneuen.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azzu V, Valencak TG. Energy metabolism and ageing in the mouse: a mini-review. Gerontology 63: 327–336, 2017. doi: 10.1159/000454924. [DOI] [PubMed] [Google Scholar]
- 5.Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes 61: 1315–1322, 2012. doi: 10.2337/db11-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bischof GN, Park DC. Obesity and aging: consequences for cognition, brain structure and brain function. Psychosom Med 77: 697–709, 2015. doi: 10.1097/PSY.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blais A, Drouin G, Chaumontet C, Voisin T, Couvelard A, Even PC, Couvineau A. Impact of orexin-a treatment on food intake, energy metabolism and body weight in mice. PLoS One 12: e0169908, 2017. doi: 10.1371/journal.pone.0169908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordner KA, Kitchen RR, Carlyle B, George ED, Mahajan MC, Mane SM, Taylor JR, Simen AA. Parallel declines in cognition, motivation, and locomotion in aging mice: association with immune gene upregulation in the medial prefrontal cortex. Exp Gerontol 46: 643–659, 2011. doi: 10.1016/j.exger.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brayton CF, Treuting PM, Ward JM. Pathobiology of aging mice and GEM: background strains and experimental design. Vet Pathol 49: 85–105, 2012. doi: 10.1177/0300985811430696. [DOI] [PubMed] [Google Scholar]
- 10.Brownell SE, Conti B. Age- and gender-specific changes of hypocretin immunopositive neurons in C57Bl/6 mice. Neurosci Lett 472: 29–32, 2010. doi: 10.1016/j.neulet.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgess CR. Histamine and orexin in the control of arousal, locomotion, and motivation. J Neurosci 30: 2810–2811, 2010. doi: 10.1523/JNEUROSCI.0045-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costantino S, Paneni F, Cosentino F. Ageing, metabolism and cardiovascular disease. J Physiol 594: 2061–2073, 2016. doi: 10.1113/JP270538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lecea L, Huerta R. Hypocretin (orexin) regulation of sleep-to-wake transitions. Front Pharmacol 5: 16, 2014. doi: 10.3389/fphar.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci 27: 14239–14247, 2007. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duivenvoorde LPM, van Schothorst EM, Swarts HJ, Keijer J. Assessment of metabolic flexibility of old and adult mice using three noninvasive, indirect calorimetry-based treatments. J Gerontol A Biol Sci Med Sci 70: 282–293, 2015. doi: 10.1093/gerona/glu027. [DOI] [PubMed] [Google Scholar]
- 16.Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 517: 302–310, 2015. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahlström A, Yu Q, Ulfhake B. Behavioral changes in aging female C57BL/6 mice. Neurobiol Aging 32: 1868–1880, 2011. doi: 10.1016/j.neurobiolaging.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Flores Á, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology 39: 2732–2741, 2014. doi: 10.1038/npp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E. Spatial memory deficits in middle-aged mice correlate with lower exploratory activity and a subordinate status: role of hippocampal neurotrophins. Eur J Neurosci 23: 711–728, 2006. doi: 10.1111/j.1460-9568.2006.04585.x. [DOI] [PubMed] [Google Scholar]
- 20.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Cambridge, MA: Academic, 2008. [Google Scholar]
- 21.Fraser LM, Brown RE, Hussin A, Fontana M, Whittaker A, O’Leary TP, Lederle L, Holmes A, Ramos A. Measuring anxiety- and locomotion-related behaviours in mice: a new way of using old tests. Psychopharmacology (Berl) 211: 99–112, 2010. doi: 10.1007/s00213-010-1873-0. [DOI] [PubMed] [Google Scholar]
- 22.Fronczek R, van Geest S, Frölich M, Overeem S, Roelandse FWC, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol Aging 33: 1642–1650, 2012. doi: 10.1016/j.neurobiolaging.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Fronczek R, Overeem S, Lee SYY, Hegeman IM, van Pelt J, van Duinen SG, Lammers GJ, Swaab DF. Hypocretin (orexin) loss in Parkinson’s disease. Brain 130: 1577–1585, 2007. doi: 10.1093/brain/awm090. [DOI] [PubMed] [Google Scholar]
- 24.Garland T Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol 214: 206–229, 2011. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girault EM, Yi C-X, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog Brain Res 198: 47–64, 2012. doi: 10.1016/B978-0-444-59489-1.00005-7. [DOI] [PubMed] [Google Scholar]
- 27.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507, 2017. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamrick MW, Ding K-H, Pennington C, Chao YJ, Wu Y-D, Howard B, Immel D, Borlongan C, McNeil PL, Bollag WB, Curl WW, Yu J, Isales CM. Age-related loss of muscle mass and bone strength in mice is associated with a decline in physical activity and serum leptin. Bone 39: 845–853, 2006. doi: 10.1016/j.bone.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Houtkooper RH, Argmann C, Houten SM, Cantó C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J. The metabolic footprint of aging in mice. Sci Rep 1: 134, 2011. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Z-L, Qu W-M, Li W-D, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA 98: 9965–9970, 2001. [Erratum in Proc Natl Acad Sci USA 99: 1098, 2002]. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunt NJ, Rodriguez ML, Waters KA, Machaalani R. Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol Aging 36: 292–300, 2015. doi: 10.1016/j.neurobiolaging.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A. Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85: 451–460, 2014. doi: 10.1016/j.neuropharm.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Inutsuka A, Yamanaka A. The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne) 4: 18, 2013. doi: 10.3389/fendo.2013.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jaeger LB, Farr SA, Banks WA, Morley JE. Effects of orexin-A on memory processing. Peptides 23: 1683–1688, 2002. doi: 10.1016/S0196-9781(02)00110-9. [DOI] [PubMed] [Google Scholar]
- 35.James MH, Campbell EJ, Dayas CV. Role of the orexin/hypocretin system in stress-related psychiatric disorders. Curr Top Behav Neurosci 33: 197–219, 2017. doi: 10.1007/7854_2016_56. [DOI] [PubMed] [Google Scholar]
- 36.Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res 198: 133–161, 2012. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kessler BA, Stanley EM, Frederick-Duus D, Fadel J. Age-related loss of orexin/hypocretin neurons. Neuroscience 178: 82–88, 2011. doi: 10.1016/j.neuroscience.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim K, Choe HK. Role of hypothalamus in aging and its underlying cellular mechanisms. Mech Ageing Dev 177: 74–79, 2019. doi: 10.1016/j.mad.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Komada M, Takao K, Miyakawa T. Elevated plus maze for mice. J Vis Exp 22: 1088, 2008. doi: 10.3791/1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Ann NY Acad Sci 1264: 72–86, 2012. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kotz CM. Integration of feeding and spontaneous physical activity: role for orexin. Physiol Behav 88: 294–301, 2006. doi: 10.1016/j.physbeh.2006.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Kudo S, Mutisya E, Nagao M. Population aging: an emerging research agenda for sustainable development. Soc Sci 4: 940–966, 2015. doi: 10.3390/socsci4040940. [DOI] [Google Scholar]
- 43.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, Albanese M, Mercuri NB, Izzi F, Bernardini S, Nitti A, Sancesario GM, Sica F, Marciani MG, Placidi F. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurol 71: 1498–1505, 2014. doi: 10.1001/jamaneurol.2014.2510. [DOI] [PubMed] [Google Scholar]
- 44.Logan S, Owen D, Chen S, Chen W-J, Ungvari Z, Farley J, Csiszar A, Sharpe A, Loos M, Koopmans B, Richardson A, Sonntag WE. Simultaneous assessment of cognitive function, circadian rhythm, and spontaneous activity in aging mice. Geroscience 40: 123–137, 2018. doi: 10.1007/s11357-018-0019-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu AT, Hannon E, Levine ME, Crimmins EM, Lunnon K, Mill J, Geschwind DH, Horvath S. Genetic architecture of epigenetic and neuronal ageing rates in human brain regions. Nat Commun 8: 15353, 2017. doi: 10.1038/ncomms15353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp 126: 55718, 2017. doi: 10.3791/55718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mang GM, Dürst T, Bürki H, Imobersteg S, Abramowski D, Schuepbach E, Hoyer D, Fendt M, Gee CE. The dual orexin receptor antagonist almorexant induces sleep and decreases orexin-induced locomotion by blocking orexin 2 receptors. Sleep (Basel) 35: 1625–1635, 2012. doi: 10.5665/sleep.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marcus JN, Elmquist JK. Orexin projections and localization of orexin receptors. In: The Orexin/Hypocretin System, edited by Nishino S and Sakurai T. New York: Humana, 2006, p. 21–43. [Google Scholar]
- 49.Martínez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell 16: 615–623, 2017. doi: 10.1111/acel.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsuki T, Nomiyama M, Takahira H, Hirashima N, Kunita S, Takahashi S, Yagami K, Kilduff TS, Bettler B, Yanagisawa M, Sakurai T. Selective loss of GABA(B) receptors in orexin-producing neurons results in disrupted sleep/wakefulness architecture. Proc Natl Acad Sci USA 106: 4459–4464, 2009. [Erratum in Proc Natl Acad Sci USA 106: 8790, 2009.] doi: 10.1073/pnas.0811126106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mavanji V, Butterick TA, Duffy CM, Nixon JP, Billington CJ, Kotz CM. Orexin/hypocretin treatment restores hippocampal-dependent memory in orexin-deficient mice. Neurobiol Learn Mem 146: 21–30, 2017. doi: 10.1016/j.nlm.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McKillop LE, Fisher SP, Cui N, Peirson SN, Foster RG, Wafford KA, Vyazovskiy VV. Effects of aging on cortical neural dynamics and local sleep homeostasis in mice. J Neurosci 38: 3911–3928, 2018. doi: 10.1523/JNEUROSCI.2513-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes JA, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, Wahl D, Ali A, Calvo-Rubio M, Burón MI, Guiterrez V, Ward TM, Palacios HH, Cai H, Frederick DW, Hine C, Broeskamp F, Habering L, Dawson J, Beasley TM, Wan J, Ikeno Y, Hubbard G, Becker KG, Zhang Y, Bohr VA, Longo DL, Navas P, Ferrucci L, Sinclair DA, Cohen P, Egan JM, Mitchell JR, Baur JA, Allison DB, Anson RM, Villalba JM, Madeo F, Cuervo AM, Pearson KJ, Ingram DK, Bernier M, de Cabo R. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab 23: 1093–1112, 2016. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mobbs CV, Moreno CL, Poplawski M. Metabolic mystery: aging, obesity, diabetes, and the ventromedial hypothalamus. Trends Endocrinol Metab 24: 488–494, 2013. doi: 10.1016/j.tem.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morairty SR, Wisor J, Silveira K, Sinko W, Kilduff TS. The wake-promoting effects of hypocretin-1 are attenuated in old rats. Neurobiol Aging 32: 1514–1527, 2011. doi: 10.1016/j.neurobiolaging.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA Jr. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA 111: E1648–E1655, 2014. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura TJ, Nakamura W, Yamazaki S, Kudo T, Cutler T, Colwell CS, Block GD. Age-related decline in circadian output. J Neurosci 31: 10201–10205, 2011. doi: 10.1523/JNEUROSCI.0451-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen JCD, Killcross AS, Jenkins TA. Obesity and cognitive decline: role of inflammation and vascular changes. Front Neurosci 8: 375, 2014. doi: 10.3389/fnins.2014.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Niccoli T, Partridge L. Ageing as a risk factor for disease. Curr Biol 22: R741–R752, 2012. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 60.Nixon JP, Mavanji V, Butterick TA, Billington CJ, Kotz CM, Teske JA. Sleep disorders, obesity, and aging: the role of orexin. Ageing Res Rev 20: 63–73, 2015. doi: 10.1016/j.arr.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Perez-Leighton CE, Boland K, Teske JA, Billington C, Kotz CM. Behavioral responses to orexin, orexin receptor gene expression, and spontaneous physical activity contribute to individual sensitivity to obesity. Am J Physiol Endocrinol Metab 303: E865–E874, 2012. doi: 10.1152/ajpendo.00119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Leighton C, Little MR, Grace M, Billington C, Kotz CM. Orexin signaling in rostral lateral hypothalamus and nucleus accumbens shell in the control of spontaneous physical activity in high- and low-activity rats. Am J Physiol Regul Integr Comp Physiol 312: R338–R346, 2017. doi: 10.1152/ajpregu.00339.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peyron C, Kilduff TS. Mapping the hypocretin/orexin neuronal system: an unexpectedly productive journey. J Neurosci 37: 2268–2272, 2017. doi: 10.1523/JNEUROSCI.1708-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Porkka-Heiskanen T, Kalinchuk A, Alanko L, Huhtaniemi I, Stenberg D. Orexin A and B levels in the hypothalamus of female rats: the effects of the estrous cycle and age. Eur J Endocrinol 150: 737–742, 2004. doi: 10.1530/eje.0.1500737. [DOI] [PubMed] [Google Scholar]
- 65.Razavi BM, Hosseinzadeh H. A review of the role of orexin system in pain modulation. Biomed Pharmacother 90: 187–193, 2017. doi: 10.1016/j.biopha.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 66.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA 108: 13305–13310, 2011. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron 46: 297–308, 2005. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 68.Sander M, Oxlund B, Jespersen A, Krasnik A, Mortensen EL, Westendorp RGJ, Rasmussen LJ. The challenges of human population ageing. Age Ageing 44: 185–187, 2015. doi: 10.1093/ageing/afu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki K, Suzuki M, Mieda M, Tsujino N, Roth B, Sakurai T. Pharmacogenetic modulation of orexin neurons alters sleep/wakefulness states in mice. PLoS One 6: e20360, 2011. doi: 10.1371/journal.pone.0020360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sawai N, Ueta Y, Nakazato M, Ozawa H. Developmental and aging change of orexin-A and -B immunoreactive neurons in the male rat hypothalamus. Neurosci Lett 468: 51–55, 2010. doi: 10.1016/j.neulet.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 71.Seibenhener ML, Wooten MC. Use of the open field maze to measure locomotor and anxiety-like behavior in mice. J Vis Exp 96: e52434, 2015. doi: 10.3791/52434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71a.Sellayah D, Sikder D. Food for thought: understanding the multifaceted nature of orexins (Online). https://academic.oup.com/endo/article/154/11/3990/2422573 [30 Aug. 2018]. [DOI] [PubMed]
- 72.Sestakova N, Puzserova A, Kluknavsky M, Bernatova I. Determination of motor activity and anxiety-related behaviour in rodents: methodological aspects and role of nitric oxide. Interdiscip Toxicol 6: 126–135, 2013. doi: 10.2478/intox-2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shalev D, Arbuckle MR. Metabolism and memory: obesity, diabetes, and dementia. Biol Psychiatry 82: e81–e83, 2017. doi: 10.1016/j.biopsych.2017.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shetty P. Grey matter: ageing in developing countries. Lancet 379: 1285–1287, 2012. doi: 10.1016/S0140-6736(12)60541-8. [DOI] [PubMed] [Google Scholar]
- 75.Shoji H, Takao K, Hattori S, Miyakawa T. Age-related changes in behavior in C57BL/6J mice from young adulthood to middle age. Mol Brain 9: 11, 2016. doi: 10.1186/s13041-016-0191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Souza LC, Jesse CR, Del Fabbro L, de Gomes MG, Gomes NS, Filho CB, Goes ATR, Wilhelm EA, Luchese C, Roman SS, Boeira SP. Aging exacerbates cognitive and anxiety alterations induced by an intracerebroventricular injection of amyloid-β1-42 peptide in mice. Mol Cell Neurosci 88: 93–106, 2018. doi: 10.1016/j.mcn.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 77.Stanley EM, Fadel J. Aging-related deficits in orexin/hypocretin modulation of the septohippocampal cholinergic system. Synapse 66: 445–452, 2012. doi: 10.1002/syn.21533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tabuchi S, Tsunematsu T, Black SW, Tominaga M, Maruyama M, Takagi K, Minokoshi Y, Sakurai T, Kilduff TS, Yamanaka A. Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function. J Neurosci 34: 6495–6509, 2014. doi: 10.1523/JNEUROSCI.0073-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Takano S, Kanai S, Hosoya H, Ohta M, Uematsu H, Miyasaka K. Orexin-A does not stimulate food intake in old rats. Am J Physiol Gastrointest Liver Physiol 287: G1182–G1187, 2004. doi: 10.1152/ajpgi.00218.2004. [DOI] [PubMed] [Google Scholar]
- 80.Teske JA, Mavanji V. Energy expenditure: role of orexin. Vitam Horm 89: 91–109, 2012. doi: 10.1016/B978-0-12-394623-2.00006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thannickal TC, Lai Y-Y, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain 130: 1586–1595, 2007. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tschöp MH, Speakman JR, Arch JRS, Auwerx J, Brüning JC, Chan L, Eckel RH, Farese RV Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Müller TD, Münzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nat Methods 9: 57–63, 2011. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsujino N, Sakurai T. Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev 61: 162–176, 2009. doi: 10.1124/pr.109.001321. [DOI] [PubMed] [Google Scholar]
- 84.Tsujino N, Sakurai T. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci 7: 28, 2013. doi: 10.3389/fnbeh.2013.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84a.von der Goltz C, Koopmann A, Dinter C, Richter A, Grosshans M, Fink T, Wiedemann K, Kiefer F. Involvement of orexin in the regulation of stress, depression and reward in alcohol dependence. Horm Behav 60: 644–650, 2011. doi: 10.1016/j.yhbeh.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 85.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322–328, 2007. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wimmer ME, Hernandez PJ, Blackwell J, Abel T. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol Aging 33: 2220–2224, 2012. doi: 10.1016/j.neurobiolaging.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wimmer ME, Rising J, Galante RJ, Wyner A, Pack AI, Abel T. Aging in mice reduces the ability to sustain sleep/wake states. PLoS One 8: e81880, 2013. doi: 10.1371/journal.pone.0081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang L, Zou B, Xiong X, Pascual C, Xie J, Malik A, Xie J, Sakurai T, Xie XS. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J Neurosci 33: 5275–5284, 2013. doi: 10.1523/JNEUROSCI.3200-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci 8: 36, 2014. doi: 10.3389/fnins.2014.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol 494: 845–861, 2006. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Kim MS, Jia B, Yan J, Zuniga-Hertz JP, Han C, Cai D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548: 52–57, 2017. doi: 10.1038/nature23282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zink AN, Bunney PE, Holm AA, Billington CJ, Kotz CM. Neuromodulation of orexin neurons reduces diet-induced adiposity. Int J Obes 42: 737–745, 2018. doi: 10.1038/ijo.2017.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zink AN, Perez-Leighton CE, Kotz CM. The orexin neuropeptide system: physical activity and hypothalamic function throughout the aging process. Front Syst Neurosci 8: 211, 2014. doi: 10.3389/fnsys.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]