Abstract
Malnutrition is a common complication of cirrhosis, increases in frequency with Child-Turcotte-Pugh (CTP) score, and is associated with an increased morbidity and mortality. Although malnutrition is easily recognized in chronically ill patients with CTP class C cirrhosis, it is present but often unrecognized in up to 50% of patients with CTP class A cirrhosis; thus, all patients with cirrhosis, regardless of etiology or severity, should be screened for malnutrition. A nutritional screening should be incorporated into the routine clinical care of patients with cirrhosis, with a more extensive nutritional assessment that includes a detailed history, dietary recall, baseline nutrition laboratory tests, and evaluation of sarcopenia using imaging modalities or strength testing to determine the degree of frailty. A thorough assessment will allow for a personalized treatment plan that provides the patient with total daily caloric intake goals with an emphasis on quality protein, education on timing of oral intake with a reduction in periods of fasting, identification and treatment of micronutrient deficiencies, and recommendation of safe and realistic exercise programs to help prevent and/or reduce sarcopenia and improve frailty.
Keywords: Cirrhosis, malnutrition, sarcopenia, frailty, nutritional assessment
Malnutrition is a common complication of cirrhosis, although the definition of malnutrition is quite variable in adults and is even more nebulous in patients with hepatic dysfunction. In adults, malnutrition in cirrhosis is typically defined as loss of skeletal muscle mass and strength (sarcopenia) in addition to diminished subcutaneous and visceral fat mass (adipopenia) from reduced protein and energy consumption. Hepatic cachexia is another term used to describe the loss of skeletal muscle in patients with cirrhosis. Lack of consensus in regard to the precise definition of malnutrition in this patient population makes outcome-based studies difficult to perform and interpret.1 Protein-calorie malnutrition is associated with low body mass index (BMI), sarcopenia, and immune incompetence.2 Sarcopenia is one of the major components of frailty; however, frailty not only involves loss of skeletal mass but also requires loss of performance.3 The prevalence of malnutrition in patients with cirrhosis ranges from 50% to 90%, increases in patients with higher Child-Turcotte-Pugh (CTP) scores, and is associated with an increased morbidity and mortality.4,5 Although malnutrition is obvious in chronically ill patients with CTP class C cirrhosis, it is present in approximately 50% of patients with CTP class A cirrhosis and is often underrecognized.5 Malnutrition has been most commonly associated with chronic viral and/or alcoholic liver disease attributed to inadequate protein-calorie consumption; however, with the increasing prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis (NASH), there is now a distinct phenotype of overweight and obese patients with cirrhosis who also meet criteria for malnutrition and/or sarcopenia.6
Pathophysiology of Malnutrition in Chronic Liver Disease
The pathogenesis of malnutrition in cirrhosis is multi-factorial. Factors include decreased oral intake and both maldigestion and malabsorption, particularly in patients with cholestasis.4,7 Decreased oral intake occurs for several reasons, including anorexia, dysgeusia owing to zinc deficiency, and/or unpalatable diets due to sodium restriction and inappropriate protein restriction for patients who have hepatic encephalopathy or chronic renal insufficiency. Additionally, patients with decompensated cirrhosis and ascites experience early satiety because of extrinsic compression of the gastrointestinal tract from peritoneal fluid.7 Poor oral intake also occurs frequently during hospitalization because of procedures and/or hepatic encephalopathy.8 Cirrhotic patients also experience fat malabsorption because of diminished luminal bile acids resulting from decreased synthesis and portosystemic shunting as well as coexisting chronic pancreatitis in patients with chronic alcohol consumption.4 Malabsorption may also occur in patients with portal hypertensive gastropathy and/or enteropathy, intestinal dysbiosis, and chronic lactulose use.7 In addition to decreased oral intake and malabsorption, patients with cirrhosis have alterations in metabolism because of decreased hepatocyte mass, which results in a shift from glycogenolysis to gluconeogenesis as a source of energy. Gluconeogenesis subsequently leads to lipopenia and sarcopenia.4 Hypermetabolism is also seen in 15% to 34% of patients with cirrhosis and may be related to sympathetic overactivity, gastrointestinal bacterial translocation, and a proinflammatory phenotype.9,10
Sarcopenia is a major consequence of malnutrition and correlates with frailty.7 Sarcopenia occurs as a consequence of increased proteolysis and a reduction in protein synthesis. Glycogen store depletion in cirrhosis leads to an increased reliance on gluconeogenesis as a source of glucose. Gluconeogenesis primarily utilizes aromatic amino acids (AAAs) and branched-chain amino acids (BCAAs), which are released from skeletal muscle via proteolysis. BCAAs are catabolized in skeletal muscle, which leads to low serum levels. Conversely, AAAs are catabolized in the liver, and serum levels are elevated because of decreased hepatic uptake due to portosystemic shunting and hepatocellular dysfunction. A decrease in circulating BCAAs, particularly leucine, subsequently causes decreased protein synthesis and increased protein catabolism.11,12 Other disturbances that promote proteolysis and protein synthesis inhibition include increased skeletal muscle ammonia production, endotoxemia, and low testosterone levels.8,11,12
Impact of Malnutrition on Clinical Outcomes
Malnutrition is prevalent in the cirrhotic population and is associated with increased morbidity and mortality. One systematic review reported that sarcopenia is associated with a hazard ratio (HR) of 1.84 (95% CI, 1.11-3.05; P=.02) for posttransplantation mortality and an HR of 1.72 (95% CI, 0.99-3.00; P=.05) for patients on the transplant waiting list.13 Sarcopenia has also been associated with increased risk for bacterial infections both before and after transplantation, as well as a reduced quality of life.12 In regard to loss of performance, research has demonstrated that frailty leads to worse outcomes independent of the Model for End-Stage Liver Disease (MELD) score. In one study, waitlist mortality or delisting because of critical illness was 9% for nonfrail patients with MELD scores less than 18, and increased to 16% for patients with MELD scores of at least 18. However, in frail patients, this rate was 23% irrespective of the MELD score.14
Nutritional Evaluation
Obtaining an accurate and reliable nutritional assessment in cirrhotic patients presents unique challenges. Typical nutrition biomarkers are skewed in cirrhosis because of decreased protein synthesis (albumin) and volume overload leading to alterations in body weight. Currently, there are sparse validated screening tools as well as a lack of consensus of the definition of malnutrition in this patient population. An assessment typically starts with a nutritional screening questionnaire to identify patients at risk of malnutrition. If this initial screening raises concern, it should be followed by a more extensive nutritional assessment by a registered dietitian with expertise in liver disease.7
Any patient with cirrhosis should undergo a nutritional assessment; however, particular attention should be made to patients with a BMI of less than 18.5 or with CTP class C cirrhosis, as these patients are at highest risk of malnutrition, frailty, and sarcopenia.7,15 Two screening tools have been developed for patients with liver disease, the Royal Free Hospital–Nutritional Prioritizing Tool (RFH-NPT) and the Liver Disease Undernutrition Screening Tool (LDUST).
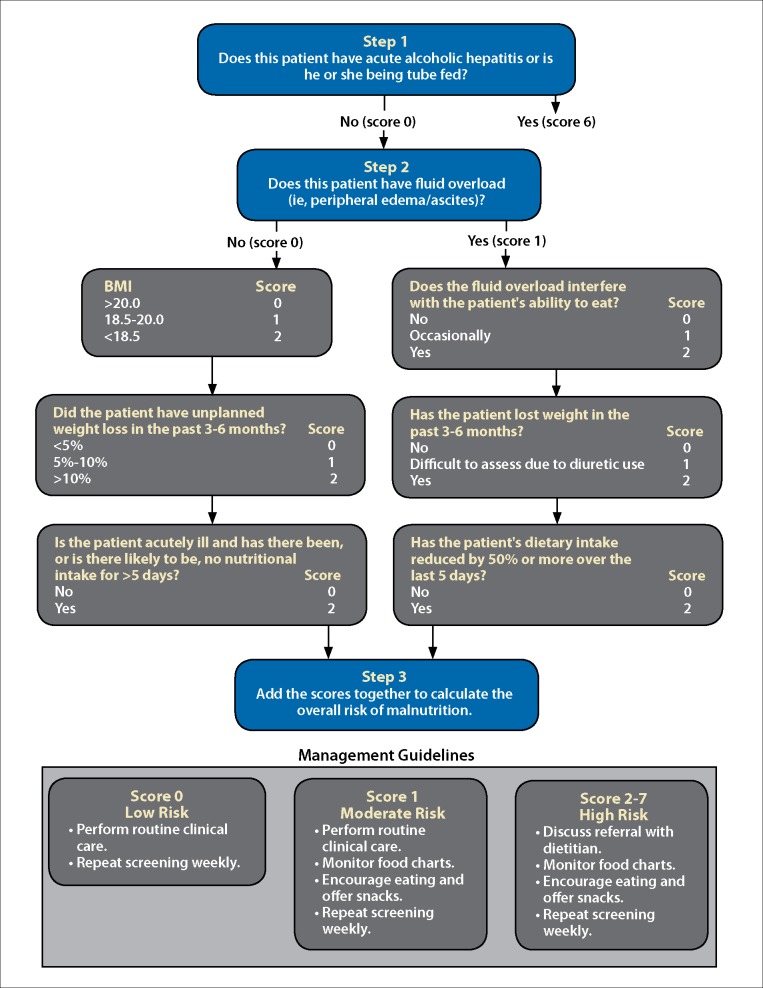
The RFH-NPT helps estimate a patient’s risk of malnutrition and is an independent predictor of hepatic decompensation and transplant-free survival (Figure).16 Patients who demonstrate an improvement in their RFH-NPT score have an improved survival. The score is based on several factors, including fluid overload, BMI, recent weight loss, and decreased oral intake. Patients are then categorized as being at low, moderate, or high risk for malnutrition. Of note, patients with acute alcoholic hepatitis or who are receiving enteral tube feeding are automatically considered high risk.17
Figure.
A flow chart showing the Royal Free Hospital–Nutritional Prioritizing Tool to determine a patient’s risk of malnutrition.
BMI, body mass index.
Reproduced from Amodio et al16 with permission by Wiley.
The LDUST is a 6-item questionnaire that incorporates oral intake, weight loss, loss of subcutaneous fat or muscle mass, fluid retention, and functional status.18,19 The questionnaire has a positive predictive value of 93.0% for malnutrition and a negative predictive value of 37.5%, indicating that a negative test does not reliably exclude malnutrition.18
Nutritional Assessment
A comprehensive nutritional assessment should include a detailed evaluation of a patient’s dietary intake, body composition, and functional assessment as well as evaluation for micronutrient deficiencies.7,20
Assessment of dietary intake should include the composition and timing of food and liquid consumption, with particular attention to periods of fasting, which may be especially detrimental in cirrhosis. Twenty-four– hour dietary recalls, food frequency questionnaires, and calorie counts should be utilized to determine whether or not patients are meeting their daily caloric needs.20 Barriers to adequate oral intake, including anorexia, dysgeusia, nausea, ascites, hepatic encephalopathy, and dietary restrictions, are also important to identify and address.7,20
Assessment of body composition includes calculation of BMI and identification of volume overload, sarcopenia, and lipopenia. There is no validated method to adjust the BMI calculation in cirrhotic patients, which is often inaccurate in volume overload. Prior research has utilized postparacentesis weight or calculated dry weight empirically based on the severity of ascites.7 One such method estimates dry weight by subtracting 5%, 10%, and 15% of the actual weight in the presence of mild, moderate, or severe ascites, respectively, with an additional 5% subtracted for pedal edema.21,22
Sarcopenia and lipopenia may be underestimated in patients with volume overload. The presence of sarcopenia is easily overlooked in obese patients, particularly in those with NASH cirrhosis.7 Specific definitions for sarcopenia in cirrhosis have recently been proposed. The skeletal muscle area (SMA) is calculated (in cm2) as the cross-sectional area of the abdominal muscles on computed tomography at the top of the L3 vertebral level (notably including the psoas, paraspinal, and abdominal wall muscles). The skeletal muscle index (SMI) is calculated by dividing the SMA by height squared (in m2). An SMI less than 50 cm2/m2 for men or less than 39 cm2/m2 for women suggests sarcopenia and is associated with an increased mortality risk in patients with end-stage liver disease.23 Dual-energy x-ray absorptiometry scans can also be used to assess sarcopenia and have the capability to measure bone, fat, and lean muscle mass content.24 Patients with cirrhosis may also develop myosteatosis, an increased accumulation of intramuscular and intermuscular fat. Importantly, sarcopenia, sarcopenic obesity, and myosteatosis correlate with increased mortality.25
In addition to sarcopenia, assessment of muscle function correlates with mortality and can be easily evaluated by measuring handgrip strength (HS).14,26,27 Overall functional status and frailty may also be assessed with the Short Physical Performance Battery (SPPB), the 6-minute walk test, or the Clinical Frailty Scale (CFS).14,27-29 HS can be assessed in less than 1 minute with a Jamar dynamometer with the mean of 3 readings taken with the dominant hand. In men, HS of less than 29 kg (BMI ≤24), less than 30 kg (BMI 24.1-28), and less than 32 kg (BMI >28) is considered weak, whereas in women, HS of less than 17 kg (BMI ≤23), less than 17.3 kg (BMI 23.1-26), less than 18 kg (BMI 26.1-29), and less than 21 kg (BMI >29) is considered weak and is associated with increased waitlist mortality. The SPPB consists of timed repeated chair stands, balance testing, and a timed 13-foot walk. The SPPB takes up to 3 minutes to complete, and patients are given up to 4 points for each task with a maximum score of 12. Scores of 9 or lower were associated with increased waitlist mortality.14,27 A 6-minute walking distance of less than 250 m was also associated with an increased mortality risk.28 The CFS is a 10-point descriptive scale that assesses overall performance status. A CFS score of 5 or greater was associated with an increased risk of hospitalization and death.29
Micronutrient Deficiencies
Deficiencies in fat-soluble vitamins are common in patients with advanced cirrhosis due to malabsorption, decreased intake, and reduced production of carrier proteins, and are especially prevalent in patients with cholestatic liver disease.20,30 Vitamin A deficiency can be assessed with serum retinol levels (normal, 32.5-78.0 µg/dL) and is associated with nyctalopia (night blindness). Replacement with 25,000 units daily of vitamin A for 4 to 8 weeks is recommended.20,31 Vitamin D deficiency is also common and may lead to osteopenia, osteoporosis, and osteomalacia. A daily intake of 2000 IU of vitamin D2 or D3 and 1200 to 1500 mg of calcium is recommended. In patients with vitamin D deficiency (as defined by a 25-hydroxyvitamin D level <20 ng/mL), intake of 50,000 IU of vitamin D weekly for 8 to 12 weeks is recommended, with a target 25-hydroxyvitamin D level of at least 30 ng/mL.8,32 Vitamin E deficiency has been linked to hemolytic anemia, neuropathy, and creatinuria.20 Vitamin K deficiency results in prothrombin time prolongation and increased risk of bleeding. The risk of bleeding is often difficult to predict solely based on the degree of prolongation because of decreased synthesis of clotting factors in the setting of hepatic dysfunction as well as prothrombotic factors that occur in this state. Vitamin K deficiency can be treated either orally, intramuscularly, or intravenously, often depending on the clinical scenario (eg, patients with active hemorrhage require an intramuscular or intravenous route of administration).8,20
Water-soluble vitamin deficiencies are also prevalent in patients with cirrhosis. Thiamine (vitamin B1) deficiency may be present in both alcohol- and non– alcohol-related liver diseases. Its manifestations include neurologic dysfunction (Wernicke encephalopathy and Korsakoff psychosis) as well as high-output heart failure (wet beriberi).8,20 Deficiencies in pyridoxine (vitamin B6), folate (vitamin B9), and cobalamin (vitamin B12) are less common, although patients with cirrhosis are at risk due to reduced hepatic storage.8 In contrast, serum vitamin B12 levels are elevated in patients with cirrhosis due to the leakage of inactive cobalamin analogues into the circulation despite decreased tissue levels.33
Zinc deficiency is common in patients with cirrhosis and has been linked to dysgeusia, which may contribute to food aversion. Other manifestations include acrodermatitis, glossitis, hypogonadism, and impaired wound healing. Serum zinc levels do not accurately reflect total body stores and may be misleading. If deficiency is suspected, a once-daily dose of 50 mg of elemental zinc (220 mg of zinc sulfate) may be used and does not interfere with copper absorption.20,34 Magnesium deficiency has been associated with muscle cramps; however, serum magnesium levels are poorly reflective of total body stores and do not predict cramping. Supplementation with 400 mg of magnesium oxide is commonly performed in clinical practice.35,36 Manganese and copper are excreted in bile and may be elevated in patients with chronic liver disease and should not be included in total parenteral nutrition in patients with cholestasis.34,37
Management of Malnutrition and Sarcopenia
In regard to macronutrient requirements, patients with compensated cirrhosis need at least 35 kcal/kg/day using body weight corrected for ascites, and at least 1.2 to 1.5 g/kg/day of protein in order to prevent or reverse sarcopenia. Patients unable to achieve these goals may benefit from enteral nutrition for supplementation. In patients with obesity, a hypocaloric diet (500-800 kcal below daily requirements) in combination with a high-protein diet (>1.5 g/kg/day) is recommended to promote weight loss while preventing concomitant muscle loss.8 The nutritional requirements for patients with hepatic encephalopathy are similar to those for patients with compensated cirrhosis (35-40 kcal/kg/day and 1.2-1.5 g/kg/day of protein).16
Not only are total caloric intake and macronutrient composition important, but the timing of feeding is also a critical component in the reduction of sarcopenia and malnutrition. After an overnight fast, patients with cirrhosis have increased fat oxidation, increased gluconeogenesis, and decreased glycogenolysis, all of which mimic what occurs in healthy subjects after 2 to 3 days of fasting.38 In one study, the use of a nighttime nutritional supplement led to an increase in total body protein stores when compared to the use of an isocaloric daytime supplement. Importantly, this benefit was only statistically significant in the subgroup with CTP class A cirrhosis, although the subgroups with CTP classes B and C may have been underpowered. However, this emphasizes the need to target all patients with cirrhosis, not only those with more advanced disease.39 Another study demonstrated improved cognitive function in patients with minimal hepatic encephalopathy after eating breakfast compared to patients who had fasted.40
The use of oral BCAAs deserves special consideration because of conflicting data. Several randomized, controlled trials (RCTs) have demonstrated an increase in albumin synthesis and protein synthesis in skeletal muscle as well as a decreased risk of hepatic decompensation, although smaller studies have not shown a significant effect.41-46 The recommended daily dose of oral BCAAs is 4 g per day, although optimal timing, route of administration, and preparation are still not entirely clear.47
In addition to meeting the required protein-calorie energy goals and having adequate vitamin and mineral supplementation, increasing physical activity has been shown to be important in preventing and/or reversing sarcopenia.8 Patients with cirrhosis typically have poor baseline exercise tolerance with sedentary lifestyles that exaggerate muscle wasting.48-50 Studies suggest that physical activity training programs can improve exercise capacity in as little as 1 month.50,51 The ideal exercise program has not yet been determined and can vary significantly depending on the particular patient and the presence of underlying portal hypertension and other comorbidities that limit exercise intensity.52 Studies in cirrhotic patients demonstrate improvement in skeletal muscle volume and 6-minute walk test results, as well as improved health-related quality of life in patients who walked 5000 or more steps per day or engaged in 3 hours of walking or cycling per week in conjunction with adequate caloric intake.50,53 Overall, based on a recent systematic review, the evidence suggests that exercise, nutritional interventions, and transjugular intrahepatic portosystemic shunts may improve sarcopenia in cirrhotic patients, although high-quality RCTs are still needed.54
Summary
Malnutrition is highly prevalent among patients with cirrhosis, including otherwise well-compensated patients with CTP class A cirrhosis. It is important to screen all patients with cirrhosis, regardless of etiology or severity, and to identify patients with malnutrition because of its marked impact on morbidity, mortality, and quality of life. In patients who fail to meet their nutritional goals, a period of supplemental enteral feeding may be considered. A thorough nutritional and exercise assessment will allow for a personalized treatment plan that includes both dietary and exercise recommendations. The dietary portion should provide total daily calorie goals with an emphasis on quality protein as well as address any vitamin and micronutrient deficiencies with nutrient-rich foods and/or supplementation. The exercise recommendations should be tailored for each individual patient. Activity goals should take into account any activity limitations as well as the degree of portal hypertension in order to reduce skeletal muscle loss and frailty.
References
- Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16(1):95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roongpisuthipong C, Sobhonslidsuk A, Nantiruj K, Songchitsomboon S. Nutritional assessment in various stages of liver cirrhosis. Nutrition. 2001;17(9):761–765. doi: 10.1016/s0899-9007(01)00626-8. [DOI] [PubMed] [Google Scholar]
- Fried LP, Tangen CM, Walston J. et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype J Gerontol A Biol Sci Med Sci. 2001. 56 3M146-M156. [DOI] [PubMed] [Google Scholar]
- Cheung K, Lee SS, Raman M. Prevalence and mechanisms of malnutrition in patients with advanced liver disease, and nutrition management strategies. Clin Gastroenterol Hepatol. 2012;10(2):117–125. doi: 10.1016/j.cgh.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Huisman EJ, Trip EJ, Siersema PD, van Hoek B, van Erpecum KJ. Protein energy malnutrition predicts complications in liver cirrhosis. Eur J Gastroenterol Hepatol. 2011;23(11):982–989. doi: 10.1097/MEG.0b013e32834aa4bb. [DOI] [PubMed] [Google Scholar]
- Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014;18(1):179–190. doi: 10.1016/j.cld.2013.09.004. [DOI] [PubMed] [Google Scholar]
- Tandon P, Raman M, Mourtzakis M, Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65(3):1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J Hepatol. 2019;70(1):172–193. doi: 10.1016/j.jhep.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85(5):1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Böttcher J, Selberg O et al. Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr. 1999;69(6):1194–1201. doi: 10.1093/ajcn/69.6.1194. [DOI] [PubMed] [Google Scholar]
- Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair M, Gow PJ, Grossmann M, Angus PW. Review article: sarcopenia in cirrhosis—aetiology, implications and potential therapeutic interventions. Aliment Pharmacol Ther. 2016;43(7):765–777. doi: 10.1111/apt.13549. [DOI] [PubMed] [Google Scholar]
- van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16(8):2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14(8):1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederholm T, Bosaeus I, Barazzoni R et al. Diagnostic criteria for malnutrition—an ESPEN Consensus Statement. Clin Nutr. 2015;34(3):335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Amodio P, Bemeur C, Butterworth R et al. The nutritional management of hepatic encephalopathy in patients with cirrhosis: International Society for Hepatic Encephalopathy and Nitrogen Metabolism Consensus. Hepatology. 2013;58(1):325–336. doi: 10.1002/hep.26370. [DOI] [PubMed] [Google Scholar]
- Borhofen SM, Gerner C, Lehmann J et al. The Royal Free Hospital–Nutritional Prioritizing Tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61(6):1735–1743. doi: 10.1007/s10620-015-4015-z. [DOI] [PubMed] [Google Scholar]
- Booi AN, Menendez J, Norton HJ, Anderson WE, Ellis AC. Validation of a screening tool to identify undernutrition in ambulatory patients with liver cirrhosis. Nutr Clin Pract. 2015;30(5):683–689. doi: 10.1177/0884533615587537. [DOI] [PubMed] [Google Scholar]
- McFarlane M, Hammond C, Roper T. et al. Comparing assessment tools for detecting undernutrition in patients with liver cirrhosis Clin Nutr ESPEN. 2018. 23:156 161 [DOI] [PubMed] [Google Scholar]
- Johnson TM, Overgard EB, Cohen AE, DiBaise JK. Nutrition assessment and management in advanced liver disease. Nutr Clin Pract. 2013;28(1):15–29. doi: 10.1177/0884533612469027. [DOI] [PubMed] [Google Scholar]
- Tandon P, Low G, Mourtzakis M et al. A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol. 2016;14(10):1473–1480e3. doi: 10.1016/j.cgh.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Tandon P, Ney M, Irwin I et al. Severe muscle depletion in patients on the liver transplant wait list: its prevalence and independent prognostic value. Liver Transpl. 2012;18(10):1209–1216. doi: 10.1002/lt.23495. [DOI] [PubMed] [Google Scholar]
- Carey EJ, Lai JC, Wang CW et al. Fitness, Life Enhancement, and Exercise in Liver Transplantation Consortium. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transpl. 2017;23(5):625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Visser M, De Meersman RE et al. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol (1985). 1997;83(1):229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- Montano-Loza AJ, Angulo P, Meza-Junco J et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvares-da-Silva MR, Reverbel da Silveira T. Comparison between handgrip strength, subjective global assessment, and prognostic nutritional index in assessing malnutrition and predicting clinical outcome in cirrhotic outpatients. Nutrition. 2005;21(2):113–117. doi: 10.1016/j.nut.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Wang CW, Feng S, Covinsky KE et al. A comparison of muscle function, mass, and quality in liver transplant candidates: results from the functional assessment in liver transplantation study. Transplantation. 2016;100(8):1692–1698. doi: 10.1097/TP.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A, Chang YH, Carpenter S et al. Relationship between sarcopenia, six-minute walk distance and health-related quality of life in liver transplant candidates. Clin Transplant. 2015;29(2):134–141. doi: 10.1111/ctr.12493. [DOI] [PubMed] [Google Scholar]
- Tandon P, Tangri N, Thomas L et al. A rapid bedside screen to predict unplanned hospitalization and death in outpatients with cirrhosis: a prospective evaluation of the Clinical Frailty Scale. Am J Gastroenterol. 2016;111(12):1759–1767. doi: 10.1038/ajg.2016.303. [DOI] [PubMed] [Google Scholar]
- O’Brien A, Williams R. Nutrition in end-stage liver disease: principles and practice. Gastroenterology. 2008;134(6):1729–1740. doi: 10.1053/j.gastro.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Newsome PN, Beldon I, Moussa Y et al. Low serum retinol levels are associated with hepatocellular carcinoma in patients with chronic liver disease. Aliment Pharmacol Ther. 2000;14(10):1295–1301. doi: 10.1046/j.1365-2036.2000.00849.x. [DOI] [PubMed] [Google Scholar]
- Stokes CS, Volmer DA, Grünhage F, Lammert F. Vitamin D in chronic liver disease. Liver Int. 2013;33(3):338–352. doi: 10.1111/liv.12106. [DOI] [PubMed] [Google Scholar]
- Baker H, Leevy CB, DeAngelis B, Frank O, Baker ER. Cobalamin (vitamin B12) and holotranscobalamin changes in plasma and liver tissue in alcoholics with liver disease. J Am Coll Nutr. 1998;17(3):235–238. doi: 10.1080/07315724.1998.10718752. [DOI] [PubMed] [Google Scholar]
- Rahelić D, Kujundzić M, Romić Z, Brkić K, Petrovecki M. Serum concentration of zinc, copper, manganese and magnesium in patients with liver cirrhosis. Coll Antropol. 2006;30(3):523–528. [PubMed] [Google Scholar]
- Vidot H, Carey S, Allman-Farinelli M, Shackel N. Systematic review: the treatment of muscle cramps in patients with cirrhosis. Aliment Pharmacol Ther. 2014;40(3):221–232. doi: 10.1111/apt.12827. [DOI] [PubMed] [Google Scholar]
- Baskol M, Ozbakir O, Coşkun R, Baskol G, Saraymen R, Yucesoy M. The role of serum zinc and other factors on the prevalence of muscle cramps in non-alcoholic cirrhotic patients. J Clin Gastroenterol. 2004;38(6):524–529. doi: 10.1097/01.mcg.0000129059.69524.d9. [DOI] [PubMed] [Google Scholar]
- Plauth M, Cabré E, Campillo B et al. ESPEN. ESPEN Guidelines on Parenteral Nutrition: hepatology. Clin Nutr. 2009;28(4):436–444. doi: 10.1016/j.clnu.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Owen OE, Trapp VE, Reichard GA, Jr et al. Nature and quantity of fuels consumed in patients with alcoholic cirrhosis. J Clin Invest. 1983;72(5):1821–1832. doi: 10.1172/JCI111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank LD, Gane EJ, Peng S et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48(2):557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- Vaisman N, Katzman H, Carmiel-Haggai M, Lusthaus M, Niv E. Breakfast improves cognitive function in cirrhotic patients with cognitive impairment. Am J Clin Nutr. 2010;92(1):137–140. doi: 10.3945/ajcn.2010.29211. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Okita K, Suzuki K et al. Hepatic Nutritional Therapy (HNT) Study Group. BCAA-enriched snack improves nutritional state of cirrhosis. Nutrition. 2007;23(2):113–120. doi: 10.1016/j.nut.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Muto Y, Sato S, Watanabe A et al. Long-Term Survival Study Group. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3(7):705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Bianchi G, Merli M et al. Italian BCAA Study Group. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124(7):1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Nishiguchi S, Habu D. Effect of oral supplementation with branched-chain amino acid granules in the early stage of cirrhosis. Hepatol Res. 2004;30S:36–41. doi: 10.1016/j.hepres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Jefferson LS. Signaling pathways and molecular mechanisms through which branched-chain amino acids mediate translational control of protein synthesis. J Nutr. 2006;136(1) suppl:227S–231S. doi: 10.1093/jn/136.1.227S. [DOI] [PubMed] [Google Scholar]
- Moriwaki H, Miwa Y, Tajika M, Kato M, Fukushima H, Shiraki M. Branched-chain amino acids as a protein- and energy-source in liver cirrhosis. Biochem Biophys Res Commun. 2004;313(2):405–409. doi: 10.1016/j.bbrc.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Toshikuni N, Arisawa T, Tsutsumi M. Nutrition and exercise in the management of liver cirrhosis. World J Gastroenterol. 2014;20(23):7286–7297. doi: 10.3748/wjg.v20.i23.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Momoki C, Yuikawa M et al. Nutritional status in relation to lifestyle in patients with compensated viral cirrhosis. World J Gastroenterol. 2012;18(40):5759–5770. doi: 10.3748/wjg.v18.i40.5759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Matsumoto Y, Momoki C et al. Physical inactivity and insufficient dietary intake are associated with the frequency of sarcopenia in patients with compensated viral liver cirrhosis. Hepatol Res. 2013;43(12):1264–1275. doi: 10.1111/hepr.12085. [DOI] [PubMed] [Google Scholar]
- Campillo B, Fouet P, Bonnet JC, Atlan G. Submaximal oxygen consumption in liver cirrhosis. Evidence of severe functional aerobic impairment. J Hepatol. 1990;10(2):163–167. doi: 10.1016/0168-8278(90)90046-t. [DOI] [PubMed] [Google Scholar]
- García-Pagàn JC, Santos C, Barberá JA et al. Physical exercise increases portal pressure in patients with cirrhosis and portal hypertension. Gastroenterology. 1996;111(5):1300–1306. doi: 10.1053/gast.1996.v111.pm8898644. [DOI] [PubMed] [Google Scholar]
- Román E, Torrades MT, Nadal MJ et al. Randomized pilot study: effects of an exercise programme and leucine supplementation in patients with cirrhosis. Dig Dis Sci. 2014;59(8):1966–1975. doi: 10.1007/s10620-014-3086-6. [DOI] [PubMed] [Google Scholar]
- Naseer M, Turse EP, Syed A, Dailey FE, Zatreh M, Tahan V. Interventions to improve sarcopenia in cirrhosis: a systematic review. World J Clin Cases. 2019;7(2):156–170. doi: 10.12998/wjcc.v7.i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]