Hypervirulent K. pneumoniae (hvKp) is an evolving pathotype that is more virulent than classical K. pneumoniae (cKp). hvKp usually infects individuals from the community, who are often healthy.
KEYWORDS: Friedlander’s bacillus, Klebsiella pneumoniae, abscess, aerobactin, colonization, hypervirulent, infection control, metastatic spread, virulence determinants, virulence plasmid
SUMMARY
Hypervirulent K. pneumoniae (hvKp) is an evolving pathotype that is more virulent than classical K. pneumoniae (cKp). hvKp usually infects individuals from the community, who are often healthy. Infections are more common in the Asian Pacific Rim but are occurring globally. hvKp infection frequently presents at multiple sites or subsequently metastatically spreads, often requiring source control. hvKp has an increased ability to cause central nervous system infection and endophthalmitis, which require rapid recognition and site-specific treatment. The genetic factors that confer hvKp’s hypervirulent phenotype are present on a large virulence plasmid and perhaps integrative conjugal elements. Increased capsule production and aerobactin production are established hvKp-specific virulence factors. Similar to cKp, hvKp strains are becoming increasingly resistant to antimicrobials via acquisition of mobile elements carrying resistance determinants, and new hvKp strains emerge when extensively drug-resistant cKp strains acquire hvKp-specific virulence determinants, resulting in nosocomial infection. Presently, clinical laboratories are unable to differentiate cKp from hvKp, but recently, several biomarkers and quantitative siderophore production have been shown to accurately predict hvKp strains, which could lead to the development of a diagnostic test for use by clinical laboratories for optimal patient care and for use in epidemiologic surveillance and research studies.
INTRODUCTION
Klebsiella pneumoniae is an increasingly important bacterial pathogen that is capable of causing severe organ and life-threatening disease. A critical trait of K. pneumoniae that has enabled its ongoing evolution is the ability to acquire new genetic material. As a result, two pathotypes termed classical K. pneumoniae (cKp) and hypervirulent K. pneumoniae (hvKp) are presently circulating, each of which presents unique challenges for the clinician (1, 2). Both pathotypes are global pathogens, but the incidence of infections due to hvKp has been steadily increasing over the last 3 decades in countries that comprise the Asian Pacific Rim (3–7). By contrast, to date, cKp has been the dominant offending agent in Western countries, but infections due to hvKp are being increasingly recognized outside Asia (8, 9).
Clinicians are all too familiar with cKp, which most commonly is an opportunistic pathogen causing infections primarily in the health care setting in hosts with comorbidities, who are immunocompromised, or who have existing barrier breakdown (e.g., intravascular devices, endotracheal tube, or surgical wound). This pathotype has demonstrated the ability to acquire an increasing number of elements that confer antimicrobial resistance, which has earned it a place among the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) pathogens (10). The most problematic are genes that encode extended-spectrum β-lactamases (ESBLs) (e.g., CTX-M, SHV, and TEM) that hydrolyze third-generation cephalosporins, aztreonam, and (in some instances) fourth-generation cephalosporins, and genes that encode carbapenemases (11). It is logical that extensively drug-resistant (XDR) cKp strains are able to thrive in the health care setting where significant antimicrobial use gives them a selective advantage. A major challenge with infections due to XDR cKp involves difficulties with treatment. XDR cKp has been responsible for lethal hospital outbreaks (12), and a woman infected with a pan-drug-resistant (PDR) cKp strain died from a lack of treatment options (13), a harbinger of the feared postantibiotic era.
The characteristics of hvKp and its differences from cKp are less well appreciated (Table 1). hvKp is best described as a virulent pathogen (14). The majority of reported infections due to hvKp have been acquired in the community. Features that are highly suggestive of hvKp infection are its ability to infect healthy individuals of any age and the propensity of infected patients to present with multiple sites of infection and/or develop subsequent metastatic spread, an unusual occurrence for other members of the family Enterobacteriaceae. The hallmark clinical syndrome is a hepatic abscess in the absence of biliary tract disease. However, hvKp can infect nearly every site of the body. A few examples of these infectious syndromes include nonhepatic abscesses, pneumonia, necrotizing fasciitis, endophthalmitis, and meningitis. A trait that was initially believed to be sensitive and specific for hvKp strains was a hypermucoviscous phenotype, which is defined by a positive string test (15). This has since been shown not be the case; not all hvKp strains are hypermucoviscous, and some cKp strains possess this characteristic (16, 17). This misperception has created some confusion in the literature when this phenotype was used alone to define an hvKp strain. Likewise, chromosomal genes that encode the aerobactin (iutA) receptor or iroE is present in a number of cKp strains and alone cannot be used to define an isolate as being hvKp, nor can the presence of the K1 or K2 capsule type. However, hvKp has acquired a number of virulence genes present on large virulence plasmids (e.g., pK2044 and pLVPK) and within integrated chromosomal elements (ICE) that confer its hypervirulent phenotype. Biomarkers present on the virulence plasmid have been shown to most accurately differentiate hvKp from cKp strains (17).
TABLE 1.
Demographic and clinical features that can assist in differentiating infection due to hypervirulent and classical K. pneumoniae strainsa
| Parameter | Finding for pathotype |
|
|---|---|---|
| hvKp | cKp | |
| Location for the development of infection | More commonly the communityb | More commonly a health care setting |
| Host | All ages; often otherwise healthy | Older, with some form of compromise |
| Ethnic background | Often Asian, Pacific Islander, Hispanic | No ethnic predilection |
| Hepatic abscess | Usually occurs in the absence of biliary disease | Usually occurs in the presence of biliary disease |
| Number of sites of infection | Often multiple | Usually single |
| Unusual infectious syndromes for K. pneumoniae | Endophthalmitis, meningitis,c brain abscess, necrotizing fasciitis, splenic abscess, epidural abscess | None |
| Copathogens at the site of infection | Rare, usually monomicrobial | Not uncommon, especially with abdominal, soft tissue, or urinary catheter infection |
These are general features; exceptions occur. Definitive diagnosis requires identification of specific biomarkers, but assays for these markers are not presently FDA approved or routinely performed by clinical microbiology laboratories.
With the advent of XDR cKp strains acquiring the hvKp virulence plasmid and thereby the hypervirulent phenotype, an increasing number of hvKp infections are developing in the health care setting; to date, this has been primarily reported from China.
hvKp meningitis occurs in patients with a competent meningeal barrier (as opposed to those with an incompetent meningeal barrier, e.g., neonates or those who have undergone neurosurgery or trauma).
Although initial isolates of hvKp were antimicrobial sensitive, management challenges included rapid initiation of therapy to prevent subsequent spread, detection of occult abscess to enable source control, and appropriate site-specific therapy (e.g., meningitis, endophthalmitis, and prostatic abscess). Most recently, clinicians have been faced with an even greater challenge, the confluence of antimicrobial resistance determinants possessed by cKp and the virulence factors possessed by hvKp on the same or coexisting plasmids. The result is the evolution of multidrug-resistant (MDR) and XDR hvKp. This has occurred by two mechanisms. The first was via hvKp strains gaining antimicrobial resistance genes by acquisition of resistance plasmids (18, 19) or by the insertion of resistance elements into hvKp’s virulence plasmid (20, 21). The converse has also occurred; XDR cKp has acquired a modified hvKp virulence plasmid (22). This scenario caused a lethal intensive care unit (ICU) outbreak. A hypervirulent XDR strain is approaching the worst-case scenario. To date, such strains have been described only in China; however, the prospect of XDR hvKp undergoing wider dissemination is concerning.
The need to increase awareness of hvKp and its evolutionary trajectory is paramount. Significant knowledge gaps exist on its epidemiology, pathogenesis, host susceptibility, optimal treatment, and appropriate infection control measures (Table 2). Further, the existence of hvKp strains that evolved when XDR cKp strains acquired the hvKp virulence plasmid has blurred the traditional epidemiologic differences between these pathotypes, namely, hvKp infection being community acquired and antimicrobial sensitive and cKp infection being health care associated and commonly more antimicrobial resistant. This recently recognized development will create new challenges for the clinician until the time when the clinical microbiology laboratories have the capability to routinely identify hvKp isolates. The goal of this report is to summarize our present understanding of this dangerous and evolving pathogen. For the purposes of this review, we focus on the literature in which hvKp strains were defined by the presence of more predictive biomarkers (e.g., rmpA and iucA to -D) (17), the presence of an hvKp virulence plasmid, the demonstration of in vivo virulence in an appropriate model, or a highly suggestive clinical scenario (e.g., invasive infection in an otherwise healthly host from the community, especially with multiple sites of infection or metastatic spread).
TABLE 2.
Major knowledge gaps that exist for hypervirulent K. pneumoniae
| Area of interest | Knowledge gap |
|---|---|
| Epidemiology | Incidence of infection in various countries |
| Prevalence of antimicrobial resistance | |
| Incidence of health care-associated infections | |
| Mechanism of acquisition | |
| Pathogenesis | Mechanism of entry |
| Delineation of hvKp-specific virulence genes and mechanism of action | |
| Mechanism of metastatic spread | |
| Factors responsible for tissue damage | |
| Carcinogenic potential | |
| Host susceptibility | Ethnic/genetic predisposition |
| Treatment | Optimal approach for source control |
| Treatment duration | |
| Management of endophthamitis, especially for XDR strains | |
| Role of adjunct therapy | |
| Infection control | Is there a benefit for implementing infection control measures when a hospitalized patient is infected with an hvKp antimicrobial-sensitive strain on a ward or an ICU? |
| Is there a benefit for implementing infection control measures when a hospitalized patient is infected with an hvKp antimicrobial-sensitive strain to protect selected patient groups (e.g., those of certain ethnic backgrounds, such as Asian, or immunocompromised hosts)? | |
| Is there a benefit of prophylaxis for close contacts? |
HISTORY AND EVOLUTION
A genomic analysis of 328 K. pneumoniae isolates supports the division of K. pneumoniae into three distinct species, K. pneumoniae, K. quasipneumoniae, and K. variicola (23, 24). Human infection has been reported for all of these species, and K. quasipneumoniae and K. variicola are frequently misidentified as K. pneumoniae by clinical microbiology laboratories (25, 26). K. pneumoniae is responsible for the majority of human infections (24, 25, 27), and hvKp strains belong to K. pneumoniae (28). Although hypermucoviscous strains of K. quasipneumoniae and K. variicola have been described (29, 30), these isolates do not have the genomic content that predicts a hypervirulent phenotype; however, it seems likely that this event will occur at some point or has already occurred and is unrecognized due to the difficulties for clinical microbiology laboratories to identify K. quasipneumoniae and K. variicola. Nonetheless, the focus of this review is on hvKp; therefore, K. quasipneumoniae and K. variicola will not be further discussed.
The Emergence of Present-Day hvKp
The first clinical report that brought hvKp to the forefront was a 1986 publication by Liu et al., who reported 7 cases of invasive K. pneumoniae infection in individuals from the community who presented with hepatic abscess in the absence of biliary tract disease and septic endophthalmitis (31). Some individuals had additional infectious syndromes, such as meningitis, pneumonia, and prostatic abscess. Several features of these patients were distinctive and characteristic for hvKp. First, those infected were healthy members of the community, although 4/7 were diabetic. Second at presentation, patients either had multiple sites of infection or had experienced subsequent metastatic spread. At that time most infections due to K. pneumoniae were occurring the health care environment, and unlike the case for selected Gram-positive pathogens (e.g., Staphylococcus aureus), it was unusual for infections due to Enterobacteriaceae to involve multiple sites or undergo metastatic spread. However, the moniker of hvKp was not yet assigned to these strains.
Interestingly, a 1986 nonclinical report by Nassif and Sansonetti described seven strains of K. pneumoniae (K1 and K2 serotypes) that were highly virulent in mice as demonstrated by a 50% lethal dose (LD50) of <103 CFU (32). A more detailed analysis of 4 of these strains demonstrated the presence of a large (180-kb) plasmid that contained genes for the production of aerobactin and its cognate receptor. This plasmid was absent in avirulent strains as defined by an LD50 of >106 CFU. Subsequent studies demonstrated that this plasmid also contained genes that conferred a hypermucoid phenotype, which proved to be mediated by the capsular polysaccharide regulator RmpA (33, 34). Details on the clinical syndromes caused by these strains were not reported. However, based on our present understanding of genes and phenotypes that define hvKp (17), these isolates would be predicted to be hvKp.
In 2004, Fang et al. reported that K. pneumoniae strains that caused hepatic abscesses in patients from Taiwan were more likely to possess a hypermucoviscous phenotype than noninvasive strains (15). Hypermucoviscosity was defined by the formation of viscous strings >5 mm in length when a loop was used to stretch the colony on agar plate, also known as a positive string test (15). A subsequent report further supported this association (35). As a result, for a period hvKp strains were sometimes designated in the literature as hypermucoviscous. However, eventually the designation hypervirulent K. pneumoniae was more commonly utilized (36–39). The report by Pomakova et al. also designated the pathotype responsible for the majority of health care associated infections as classical K. pneumoniae (39). This distinction between cKp and hvKp frames the genotypic and phenotypic differences between these pathotypes. As discussed, the use of the term hypermucoviscous has proven to be problematic, since this phenotype is not optimally sensitive or specific for hvKp strains: not all hvKp strains are hypermucoviscous, and some cKp demonstrate this phenotype (16, 17). Some studies used solely a positive string test to define hvKp strains, which has resulted in some strains of K. pneumoniae being misclassified as hvKp and consequently created some confusion in the literature.
Friedlander’s Bacillus: Likely an hvKp Pathotype or Variant
The clinical syndrome Friedlander’s pneumonia was eventually recognized to be due to K. pneumoniae (40–42). This entity and the offending agent were first described in 1882 by Friedlander (43), hence the initial designation as Friedlander’s bacillus (Bacillus friedlanderi). A subsequent and now antiquated designation was Bacillus mucosus capsulatus (44). The acute syndrome has a number of distinctive clinical features which are consistent with some, if not all, of the offending strains being either hvKp or at least K. pneumoniae isolates that had acquired a portion of the hvKp virulence factor repertoire.
In the preantibiotic era, Friedlander’s pneumonia had a mortality rate of approximately 80%, which was 3- to 4-fold greater than pneumonia caused by Streptococcus pneumoniae (45, 46). Presentations were usually acute, and death could ensue within 24 to 48 h and on average occurred 5.5 days after presentation, compared to 9 days for pneumonia due to S. pneumoniae (46, 47). Bacteremia was noted, on average, in 60% of cases (45, 46). Radiographically, findings were indistinguishable from pneumococcal pneumonia, with bronchopneumonic, lobular, and lobar manifestations observed, which were often multifocal. However, in contrast to pneumococcal pneumonia, tissue destruction leading to overt cavitation and/or necrosis observed on histology was far more likely to develop (48). Although not diagnostic, a bulging fissure and/or cavitation increased the likelihood that the pneumonia was due to Friedlander’s bacillus (40). After cavitation, empyema was the next most common pulmonary complication; purulent pericarditis also could develop (48, 49). If the patient survived the acute episode, progression to chronic cavitary disease that mimicked tuberculosis and persisted for months could develop (50). Nearly all cases of Friedlander’s pneumonia occurred in ambulatory patients. Although chronic alcoholism was touted as a critical risk factor, many patients were healthy hosts (45, 48, 49, 51). Perhaps the increased risk of infection in alcoholics was due not solely to compromised host defense factors but also to the increased likelihood of macroaspiration. Males were more commonly infected, and although infections were reported in all age groups, the fifth and sixth decades of life were most common (47–49, 52). Likewise, in most contemporaneous studies of hvKp, men are more commonly infected than women (9, 53, 54). Mercifully, Friedlander’s pneumonia accounted for only 0.5 to 5% of community-acquired pneumonias (42, 48, 49, 52).
Friedlander’s bacillus has also been implicated in a variety of extrapulmonary infections in the presence or absence of pneumonia. These include renal abscess, hepatic abscess, osteomyelitis, cavernous sinus thrombosis, abscess of the jugular bulb, meningitis, brain abscess, splenic infection, spontaneous bacterial peritonitis, and soft tissue abscesses in the neck and arm (47–49, 55–61). Similar to the case with individuals infected with hvKp, multiple sites of infection were noted in a number of patients. Extrapulmonary sites of infection were undoubtedly underestimated in an era in which advanced imaging modalities were nonexistent. Interestingly, septic endophthalmitis was not noted, which could be easily diagnosed clinically.
Additional features suggested that Friedlander’s bacillus was most consistent with hvKp isolates. A phenotypic feature of many hvKp strains is hypermucoviscosity, i.e., an inoculation loop can generate a viscous string >5 mm in length from the bacterial colony; this trait is due to increased capsular polysaccharide production mediated by RmpA and/or RmpA2 (62). Although hypermucoviscosity is not pathognomonic for hvKp since this phenotype also can be observed in cKp strains (17), it is suggestive. Numerous reports remark on Friedlander’s bacillus possessing this characteristic. In cases of meningitis the spinal fluid is often referred to being “gelatinous” and “the drawing out of a filament from the stylet of the spinal needle” (56). Likewise, “[o]n agar plates the colonies appear…as round, raised, slimy, gray colonies, which string out when drawn up with a wire loop” (48). Sputum was commonly described as “tenacious” or gelatinous (41, 49), and cut lung sections were described as “covered by a characteristic viscid, abundant, mucinous exudate which sticks to the knife” (48). Another paper states that “[t]he name Bacillus mucosus capsulatus (Friedlander’s bacillus) draws attention to the two most prominent and distinctive features of the organism, namely, the marked degree of capsular development and its power of producing large amounts of mucoid material in its growth both on artificial media and in the human body” (44). A serotyping schema was developed for Friedlander’s bacillus with types A (equates to K1), B (equates to K2), and C (equates to K. pneumoniae rhinoscleroma), and group X (other) (63, 64). The majority of strains were types A and B (45, 46, 48, 49, 57), with type A being most common, similar to the observation for hvKp strains (2, 17, 65), although cKp strains also can produce K1 and K2 capsules (66).
In one series that reviewed cases of meningitis due to K. pneumoniae, patients were more likely to have diabetes mellitus (56), also similar to what has been observed in most studies on hvKp-infected individuals (67–75). Additionally, in a recent study authored by Lam et al., hvKp sequence type 23 (ST23) was calculated to have evolved around 1878, lending further credibility to Friedlander’s bacillus being the first description of an hvKp strain (76).
Lastly, in experimental reports studying Friedlander’s bacillus, “[s]ubcutaneous or intraperitoneal injections of 1:1 million or 1: 1 billion dilutions of young cultures often kill mice in 1–3 days” (49); likewise, another report presented data that 10−5, 10−6, and 10−7 dilutions of a culture grown “4-6 h” resulted in 100% mortality of mice challenged intraperitoneally with 0.5 ml of diluted bacteria over 15 to 48 h (63). Although some guesswork is required, if one assumes a “young culture” and a “4-6 h” culture maximally consist of 1 × 109 CFU/ml, then lethal doses would be in the range of 1.0 to 5,000 CFU. Another study reported that 9.0 × 104 CFU killed mice within 18 h, “a result not obtainable with coliform organisms or A. aerogenes” (59). A lethal effect from these low challenge inocula would clearly identify such strains as hvKp and not cKp (17).
Clinical challenges with Friedlander’s pneumonia included early recognition and appropriate treatment. S. pneumoniae was responsible for the overwhelming majority of cases of pneumonia, and at that time treatment with penicillin was efficacious but was ineffective for Friedlander’s bacillus, for which tetracyclines and/or streptomycin were the preferred antimicrobials. Given the fulminant course and high mortality seen with untreated Friedlander’s pneumonia, a lack of recognition was problematic. A similar scenario occurred with meningitis due to Neisseria meningitidis versus Friedlander’s bacillus (58). This scenario echoes a different form of diagnostic issues that occur with hvKp today: K. pneumoniae can be readily identified, but differentiating cKp from kvKp is more challenging. As discussed below, hvKp presents different management challenges and if this pathotype is unrecognized, the consequences could be significant, especially for XDR hvKp strains (22).
Taken together, the described features of infection due to Friedlander’s bacillus, namely, the ability to cause life-threatening disease in healthy patients from the community, multiple sites of infection or subsequent metastatic spread (including meningitis and brain abscess), hypermucoviscosity, and capsule type, as well as experimental mouse data are consistent with at least some of these strains being hvKp. Of course, it would be interesting and informative if properly stored isolates of Friedlander’s bacillus were available for sequencing and in vivo assessment in appropriate infection models. These data would also generate insights into the evolution of present-day hvKp strains. For example, was a virulence factor(s) which enables present-day hvKp to cause endophthalmitis absent from the Friedlander’s bacillus? Did Friedlander’s bacillus have a greater tropism for the lung than hvKp, or were nonpulmonary sites underrecognized due to the lack of modern-day imaging technologies? Further, it is intriguing to speculate that cKp evolved from hvKp by loss of the virulence plasmid and perhaps other genetic material once introduced into the health care environment.
hvKp Viewed through the Genomic Lens
Origins of hvKp.
Recent molecular epidemiologic studies have shed additional light on the origins of hvKp. Lam et al. performed a comparative analysis of 97 genomes from clonal group 23 (CG23) strains of both human and equine origin (76). CG23 is strongly associated with the K1 capsule and severe, invasive clinical disease that occurs with hvKp infection (38, 66, 77, 78). Ninety-four of the 97 genomes contained virulence plasmid sequence and the plasmid-associated gene loci iro (salmochelin biosynthesis), iuc (aerobactin synthesis), rmpA (regulator of mucoid phenotype), and rmpA2, all of which are highly predictive of an hvKp strain (17, 76). These data identified several sublineages, with CG23-I being dominant, accounting for 81 of 97 isolates. Equine isolates were nested within CG23-I. Estimates of the dates for the most recent common ancestors for the entire CG23 population, the CG23-I sublineage, and the equine strains were 1878, 1928, and 1972, respectively. Therefore, these data, which are representative of 37 to 64% of hvKP isolates (17, 79–82), support the concept that hvKp strains have been circulating as long as the 1800s. They also support the notion that Friedlander’s bacillus could have been an hvKp strain or perhaps an evolutionary variant.
hvKp virulence plasmid.
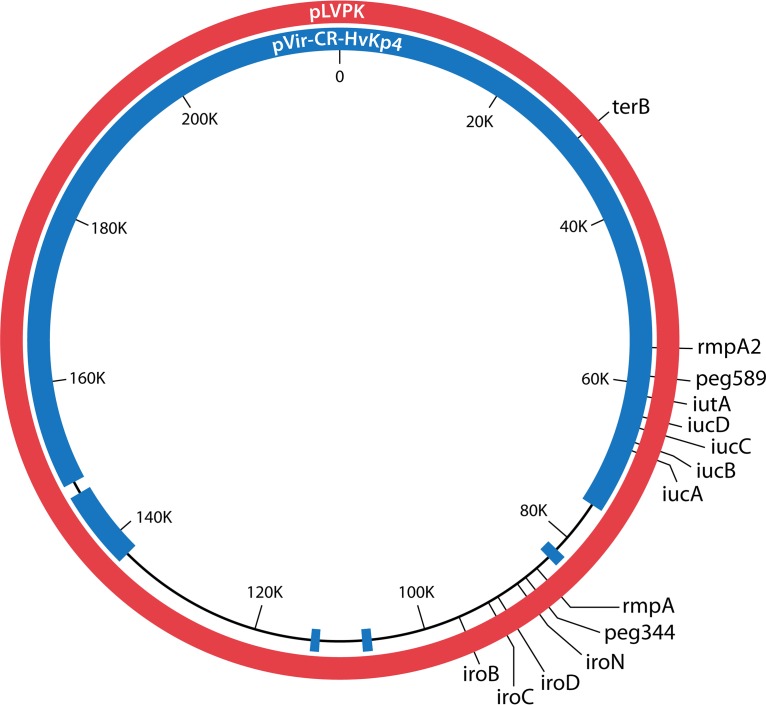
Initial sequencing of hvKp strains identified the presence of the large, highly similar virulence plasmids pK2044 (224,152 bp) and pLVPK (219,385 bp) (83, 84) (Fig. 1). The loss of this plasmid significantly decreased virulence (32, 34, 85). Further, the best-characterized virulence factors with experimental support for conferring the hypervirulent phenotype are encoded by genes present on these plasmids, which include iuc (biosynthetic genes for the siderophore aerobactin), peg-344 (a metabolic transporter of unknown function), and rmpA and rmpA2 (regulators that increase capsule production) (32, 34, 62, 85–88) (Fig. 1). Lery et al. described the presence of two virulence plasmids in the hvKp strain Kp52.145 (also known as B5055) (89). A 121-kb plasmid contained iuc, iro, and rmpA, whereas the second, 90-kb plasmid contained rmpA2. Ye et al. studied 40 hvKp strains isolated from patients with community-acquired hepatic abscess, all of which possessed iuc, iro, rmpA, and rmpA2 (78). Plasmid profiles of these 40 strains demonstrated that 35 of 40 strains possessed at least one plasmid. Nineteen strains contained a single plasmid similar in size to pK2044 (approximately 220 kb). Twelve strains possessed a single plasmid with sizes ranging from 140 to 250 kb, and four strains retained 2 or 3 plasmids. Interestingly, five strains had no detectable plasmid, but iuc, iro, rmpA, and rmpA2 were detectable by PCR, suggesting chromosomal integration. Struve et al. determined that all 30 hvKp strains isolated from cases of liver abscess or community-acquired pneumonia harbored pLVPK-like plasmids that contained iuc, iro, rmpA, and rmpA2, but some plasmids had undergone deletions in other regions (90). Likewise, in the CG23 genomes analyzed by Lam et al., pK2044-like virulence plasmids were detected in 94 strains (which included 27 CG23 strains sequenced and analyzed by Struve (76, 90). iro was present in all 94 plasmids, and iuc, rmpA, and rmpA2 were variably present in 92 plasmids.
FIG 1.
Schematic representation of the hvKp virulence plasmid pLVPK (red circle, 219,385 bp) (83) and pVir-CR-HvKp4 (blue circle, 178,154 bp) (22). The locations of various virulence genes and/or biomarkers are marked.
More recently, cKp strains have been described that have acquired hvKp virulence plasmids and thus have a hypervirulent phenotype. The pLVPK-like virulence plasmid pVir-CR-hvKP4 (178,154 bp) was acquired by the ST11 cKp strain K. pneumoniae 4, which showed an enhanced virulence phenotype (22). However, pVir-CR-hvKP4 had a 41,231-bp deletion compared to pLVPK (Fig. 1), which included the virulence genes rmpA and iro; the iuc genes and rmpA2 were retained, and the presence of rmpA2 appeared to confer a hypermucoviscous phenotype. Presently, the effect, if any, of this deletion on the hypervirulent phenotype is unclear. Unrecognized virulence genes may have been lost. But, the loss of iro, which encodes salmochelin, may be inconsequential for systemic infection based on data from a mouse subcutaneous-challenge model that demonstrated that salmochelin did not contribute to virulence (87). Further, a redundancy may exist for RmpA and RmpA2 since each can enhance capsule production (22, 62, 87). Aerobactin production appears to be less dispensable (86, 87). Therefore, minimally the ability to produce aerobactin and either RmpA or RmpA2 is likely needed to confer some degree of hypervirulence. Another report from Taiwan described the ST11 cKp strain TVGHCRE225, which harbored pVir (297,984 bp), a hybrid hvKp virulence plasmid (91). Approximately 38% of pVir possessed 49% and 47% coverage with pK2044 and pLVPK at 99% identity; the remaining portion of pVir possessed 61% coverage with pPMK-NDM, a resistance plasmid present in an NDM-producing K. pneumoniae strain, at 99% identity. Interestingly, although iroBCDN, iucABCDiutA, rmpA, and rmpA2 were present in pVir, TVGHCRE225 pVir was not maximally virulent in a mouse systemic infection model, suggesting that the absent portions of pK2044 and pLVPK contained important and unrecognized virulence determinants.
Integrative and conjugative elements.
Not surprisingly given K. pneumoniae’s receptivity to horizontal gene transfer and recombination, ICE (also known as genomic or pathogenicity islands or mobile genetic elements) are commonly observed in both cKp and hvKp strains. Integration usually occurs at tRNA sites. In one study, 73% of the K. pneumoniae strains had an ICE inserted into one or more of the four asparagine tRNA genes (92). The best-characterized and -studied ICE family, first described by Lin et al., was defined by the presence of biosynthetic genes for the siderophore yersiniabactin (93). This 76-kb ICE (ICEKp1) in the hvKp strain NTUH-K2044 was more prevalent in hvKp strains (38/42) than cKp strains (5/32), suggesting a role in hvKp pathogenesis. In addition to a region homologous to the high-pathogenicity island of Yersinia that contained yersiniabactin biosynthetic genes, another region was homologous to the virulence plasmid pK2044 and contained homologues to genes that encoded the synthesis of the siderophore salmochelin (iro) and the capsular polysaccharide regulator RmpA. However, subsequent studies demonstrated that ICEKp1 was not representative of ICEKp homologues present in the majority of other hvKp strains. Lai et al. described a more widely conserved ICEKp (KPHPI208-GM1 also designated ICEKp10) that consisted of 8 genomic modules (94). The yersiniabactin encoding genes were retained, but the rmpA homologue and the iro genes were absent (94). In addition, a 50-kb region that encoded the genotoxin colibactin and the bacteriocin microcin E492 was present (94). Struve et al. reported that ICEKp10 homologues were present in all 27 hvKp CG23 strains studied, although deletions of genes that encoded colibactin and E492 were present in 4 strains and genes that encoded yersiniabactin were deleted in 3 of those 4 strains (90). In the 3 non-CG23 hvKp strains studied, ICEKp10 was poorly conserved, with 2 of 3 strains possessing only genes that encoded yersiniabactin (90). This body of work has been extended by Lam et al. (76, 95). Their comparative analysis of 97 CG23 genomes demonstrated that the 81 members of sublineage CG23-I had acquired ICEKp10, which contained genes that encode yersiniabactin and colibactin. This event was estimated to occur in 1928, which was followed by global population expansion of CG23-I.
With the availability and analysis of an increasing body of sequence data, it is clear that ICEKp acquisition within the general K. pneumoniae population of both cKp and hvKp strains is robust and that many variants exist; 14 have been reported to date (95). Further, the acquisition of other ICE or genomic islands is the rule (89, 92). However, the acquisition and/or maintenance of these elements may not always be beneficial, depending on the strain and environment, as evidenced by the presence of various gene disruptions or module deletions in ICEKp variants (95). Selective pressures are likely site and strain specific. A pathogenic role for yersiniabactin in cKp has experimental support (96). But presently, a role for yersiniabactin in hvKp infection is less clear (87). It is proposed that acquisition of colibactin was the critical event for the increase in hvKp strains within the CG23-I clade (76). Potential mechanisms include some combination of colibactin mediating enhanced colonization, mucosal invasion, and/or dissemination (97). Further, additional factors present on ICEKp or other genomic islands may prove to be important in various settings. ICEKp does appear to be K. pneumoniae specific, which suggests an important species-specific evolutionary role (95). Further, the acquisition of ICEKp10 and subsequent expansion of CG23-I support an important role for factors encoded by this element in the biology of hvKp CG23-I. Future studies will hopefully further clarify the relative importance of specific ICE-encoded factors for hvKp compared to cKp and their role for survival in environmental niches, mucosal colonization, and infection.
Molecular definition of hvKp.
Analyses of CG and ST, which is based on core genes (98), and capsule types have been used to define hvKp (99). However, the utilization of these typing modalities to differentiate hvKp from cKp strains is imperfect. The genes used to identify ST and capsule type are present in both hvKp and cKp strains (66). Although selected STs (e.g., ST23, ST65, and ST86) and capsule types (e.g., K1 and K2) are commonly associated with hvKp strains, genes that enable hypervirulence are more broadly distributed across a number of STs and capsule types (24, 65, 66, 79, 80, 100). Further, ST and capsule types commonly associated with hvKp strains may not possess the requisite virulence genes that confer hypervirulence. For example, K1, K2, and K54 capsule types are also expressed by cKp strains (65, 66, 100, 101). In addition, CG23 strains may not possess virulence plasmid sequences or the associated virulence genes (e.g., iuc, rmpA, and rmpA2) (76). Lastly, as described above (hvKp virulence plasmid), an extensively drug-resistant ST11 cKp strain that was endemic in China acquired a 170-kb pLVPK-like virulence plasmid. The hypervirulent phenotype conferred by this genetic event resulted in a lethal outbreak (22). These variations are likely due to the ability of K. pneumoniae to undergo a significant degree of horizontal gene transfer and recombination, including genes that encode capsule types (24, 66, 76, 90, 100–102). Taken together, these data support the concept that hvKp is best defined by its virulence gene repertoire (24, 35, 66, 101).
The delineation of hvKp virulence genes remains incomplete, and it remains unknown which combination of genes are needed for maximal virulence. Genes present on the virulence plasmids (e.g., pK2044 and pLVPK) (83, 84, 90) and within ICE (76, 92, 94, 103) have been implicated by molecular epidemiologic associations (24, 35, 65, 66, 76, 90). To date, virulence genes present in hvKp strains, but not in cKp strains, that have been shown to contribute to virulence in vitro, ex vivo, and in vivo are present on the virulence plasmids (62, 86–88). Factors encoded on ICE are less accurate for defining hvKP since these genomic elements can be present in cKp strains as well (e.g., yersiniabactin) (17) or are present in only a subset of hvKp strains (e.g., colibactin) (94, 103). A recent study demonstrated that iroB, iucA, peg-344, rmpA, and rmpA2 were the most accurate molecular markers for defining hvKp (17), all of which have been shown to present on virulence plasmids. K. pneumoniae’s proven propensity for undergoing recombination or deletion of genes under selective pressure supports the concept that the best markers should be critical factors in conferring the hypervirulent phenotype. If such markers are lost, then the phenotype will no longer be hypervirulent. To date, based on experimental data, iuc, rmpA, and rmpA2 best fit this role. It appears that the functions of rmpA and rmpA2 may be redundant. If so, iuc and/or either rmpA or rmpA2 would be predicted to be the best markers. The study by Gu et al. in which an XDR cKp strain became hypervirulent supports this concept since iro, peg-344, and rmpA were deleted in the relevant plasmid (22). Of course, as more hvKp-specific genes are identified, additional markers may join this list or prove to be even more accurate.
K. pneumoniae and zoonotic infection.
K. pneumoniae is capable of causing infection in a variety of nonhuman hosts. Lethal outbreaks have been described to occur in sea lion pups from New Zealand (104), in which meningitis was a prominent clinical manifestation, and in juvenile sea lions from California, in which pneumonia, lung abscess, and empyema were the predominant manifestations (105). In the pups, the responsible isolates were hypermucoviscous, ST86, and possessed the K2 capsule type and rmpA (104); subsequent sequence data supported 7/9 of these strains as being hvKp based on the presence of rmpA and iucD (106). In the juveniles, 21/21 strains were hypermucoviscous and possessed the K2 capsule type and rmpA. A lethal outbreak was reported in which 7 African green monkeys from a research facility developed multiple abscesses (107). The one strain studied possessed the K2 capsule type and rmpA. Nine strains were studied from buffalo and cows that developed mastitis (108). All strains were lethal when BALB/cByl mice were intraperitoneally challenged with 102 to 106 CFU and 6/9 strains had rmpA identified by PCR, again consistent with at least some of these strains being hvKp. Two of 33 K. pneumoniae strains isolated from nasal swabs of sick cattle suffering from respiratory disease in China were rmpA positive (109). Lam et al. performed a genomic analysis of 15 K. pneumoniae K1 strains isolated from horses (76). Compared to human isolates, these strains appeared to be hvKp by virtue of possessing the pK2044 virulence plasmid. Entry of this type of strain into the horse population was inferred to occur via a single event, and the type of strain since has circulated within equine hosts via sexual transmission. There was no evidence of human-to-horse transmission in this study. Other than the equine isolates studied by Lam (76), the evolutionary relationship between zoonotic isolates that appear to be hvKp strains and human hvKp strains awaits further study. Since humans have the potential to interact with these animals directly or indirectly via waste products deposited into the environment, it would not be surprising if at least some of the genetic elements that define hvKp originated from an animal host.
EPIDEMIOLOGY
Acquisition and Colonization May Lead to Infection
K. pneumoniae organisms can be members of normal animal and human microbiotas and/or the microbiotas of various environmental habitats (110, 111). Acquisition of and colonization with K. pneumoniae appear to be requisite for, but do not necessarily lead to, infection (112–117). Otherwise healthy individuals from the community are at risk for developing hvKp infection, whereas it is uncommon for cKp infection to develop in this population. Healthy people can be colonized with cKp, but in the absence of some form of host compromise, infection rarely occurs. By contrast, healthy individuals colonized with hvKp are at much greater risk for developing infection. However, the frequency with which infection develops after colonization with hvKp and the factors that modulate this risk are not well understood. The relative importance of colonization versus that of the quantity of the colonizing hvKp strain, host factors, and degrees of hvKp virulence is an important issue that requires active investigation.
Colonization with Undefined Pathotypes of K. pneumoniae
In healthy humans from the community in Western countries, the prevalences of K. pneumoniae colonic colonization ranged from 5 to 35% (112, 117, 118). In Asian countries, K. pneumoniae colonization rates in stool from health adults were 87.7%, 61.1%, 75%, 58.8%, 57.9%, 18.8%, 52.9%, and 41.3% for Malaysia, Singapore, Taiwan, Hong Kong, China, Japan, Thailand, and Vietnam, respectively (115). Another study from Korea reported a K. pneumoniae colonization rate in stool of 21.1% (248/1,175) (113).
In Western countries, 1 to 5% of healthy humans from the community are nasopharyngeally colonized with K. pneumoniae. In children <10 years of age from Brazil and Vietnam, 1.4% (17/1,192) and 1.6% had nasopharyngeal colonization with K. pneumoniae, respectively (119, 120). By contrast, 7% of Indonesian children (16/243) were colonized with K. pneumoniae (121). Nasopharyngeal colonization rates increase with age; in Indonesia 15% of adults (38/253) were found to be colonized with K. pneumoniae in the nasopharynx (121) and in Vietnam the overall colonization rate was 14.7% but exceeded 20% in those >40 years of age (120). Increased nasopharyngeal colonization rates were associated with poorer states of sanitation and increased contamination of food and water (121), age, smoking, alcohol use, and living in a rural community (120). In Malaysia, 32% of samples from street food were contaminated with K. pneumoniae (122). Interestingly, in a study of community-acquired pneumonia from Indonesia, K. pneumoniae was the most common bacterial agent identified, causing 14% of 148 cases; by contrast, S. pneumoniae caused 13% of cases (123). Data such as these support the concept that increased colonization has the potential for increasing the incidence of infection.
The dogma is that skin colonization with K. pneumoniae is uncommon and transient. However, in one study from the United States, axillary colonization was relatively common, occurring in up to 50% of individuals, and colonization of other dermal sites, albeit uncommon, increases in warmer months (124).
Colonization with hvKp
Obtaining accurate data on hvKp colonization rates of individuals from the community is more challenging since hvKp-specific markers have not always been used to determine their relative proportions compared to cKp. A colonic colonization study of healthy Koreans demonstrated a colonization rate of 4.6% for hvKp (based on less sensitive K1 capsule and ST23 sequence typing) (113). Another report demonstrated colonization rates of 14.1%, 14.9%, 11.3%, 12%, 11.7%, 16.7%, 2.7%, and 0% for healthy individuals from Malaysia, Singapore, Taiwan, Hong Kong, China, Japan, Thailand, and Vietnam, respectively, for putative hvKp strains (based on K1 and K2 capsule types, phenotypes not optimally sensitive or specific) (115). For Australia, 1.3% (1/80) of K. pneumoniae isolates from rectal swabs were hvKp strains (28). In a study that performed nasopharygeal cultures on adults seen at an outpatient otorhinolaryngology clinic for sinusitis and rhinitis in Taiwan, 11.5% (39/340) of isolates were K. pneumoniae and 77.5% of K. pneumoniae isolates tested were predicted to be hvKp based on being rmpA positive (125). We were unable to identify data on dermal colonization with hvKp. Despite the lack of optimal data, it is clear that a significant minority of Asians are colonized with hvKp. More data on hvKp colonization rates from Western countries, in which there is a lower incidence of hvKp infection, would be welcomed. These data may generate insights into the relative risk of acquisition versus genetic factors (e.g., ethnic background) for subsequent infection. Likewise, data on skin colonization could be insightful, since this represents a potential source of entry.
A point of concern is that a variety of Gram-negative bacilli, including cKp, emerge as the dominant colonizers of both mucosal and skin surfaces in the health care setting, particularly in association with antimicrobial use, indwelling devices, severe illness, and extended length of stay (117, 126, 127). Hospitals and long-term-care facilities have been identified as important reservoirs for XDR cKp (128, 129). In these settings, transmission from health care workers to patients, especially with lax hand hygiene, and transmission via instrumentation are important mechanisms that could be minimized via appropriate infection control measures (130, 131). Although this venue was once the realm of cKp, that reality is changing as of late. In part, this is due to XDR cKp strains that acquired the hvKp virulence plasmid, thereby evolving into XDR hvKp strains (22). Additional cases of health care acquisition of hvKp also have been described (132, 133). If hvKp even partially replaces cKp as a colonizer in the health care setting, which will undoubtedly lead to infection in a proportion of these patients (134), then the incidence of hvKp infections, morbidity, mortality, and costs are predicted to increase, given the vulnerability of this patient cohort and the virulence of hvKp, particularly if XDR hvKp is the offending pathogen (135, 136).
Settings for Acquisition and Subsequent Development of Infection
One of the initial defining features of hvKp infection is acquisition in the community (31, 35, 70, 75, 137). The mechanism for acquisition of hvKp within the community is presently undefined, but based on data from cKp and other Enterobacteriaceae, some combination of contaminated food or water, person-to-person transmission (e.g., close contacts such as family members or sexual partners), and perhaps zoonotic transmission is possible. Support for food as a possible source comes from a report that identified two probable hvKp strains (ST23, K1, positive string test) that harbored blaKPC-2 from cucumber (138).
Although the majority of hvKp infections are community acquired, there are increasing numbers of reports that describe infections developing in health care settings (22, 132, 133). This is due to a combination of increasing recognition of antimicrobial-sensitive hvKp strains causing nosocomial infection, an increasing prevalence of hvKp strains that have acquired antimicrobial resistance determinants and therefore are more likely to survive and be transmitted in this setting, and lastly, the fact that XDR cKp strains that were entrenched in the health care environment have evolved into hvKp strains by virtue of acquiring the hvKp virulence plasmid (22).
Geographic Distribution of hvKp Infection
The predominance of infections to date has been reported from the Asian Pacific Rim. However, infections are increasingly being reported worldwide (8, 9, 139). With an increasing awareness and the identification of validated biomarkers (140), hopefully an accurate assessment of the incidence of hvKp infection across the globe will be achieved. Table 3 summarizes some of the data presently available.
TABLE 3.
Estimated proportion of hvKpa organisms among K. pneumoniae infections in various geographic locales
| Site | Time frame | Isolate source/characteristic | No./total (%) of K. pneumoniae infections due to hvKpb | Reference |
|---|---|---|---|---|
| Australia | 2001–2014 | Urine | 3/193 (1.6) | 28 |
| Australia | 2001–2014 | Mixed clinical (minus urine) | 19/141 (13.5) | 28 |
| Canada (Alberta) | 2001–2007 | Community-acquired blood isolates | 9/134 (6.7) | 354 |
| Canada (Quebec) | 2009–2013 | Blood isolates | 1/110 (0.9) | 17 |
| China | 2015 | ST11, carbapenem resistant | 11/387 (3) | 22 |
| China | 2008–2012 | Blood isolates | 32/70 (46) | 355 |
| China | 2014–2016 | Carbapenem-resistant isolates | 32/66 (48.5) | 311 |
| China | 2014–2016 | Carbapenem-sensitive isolates | 31/45 (68.9) | 311 |
| India | 2014–2015 | Urine, respiratory, and blood isolates | 3/370 (0.8) | 356 |
| India | 2014–2015 | Carbapenem-resistant blood isolates | 6/86 (7) | 357 |
| Japan | 2011–2012 | Sputum and urine isolates | 22/130 (16.9) | 133 |
| Nepal | 2008–2012 | Mixed clinical | 1/131 (0.76) | |
| Spain (Barcelona) | 2007–2013 | Blood isolates | 37/878 (4.2) | 139 |
| UK (Oxford) | 2008–2011 | Blood isolates | 4/69 (5.8) | 17 |
| USA (Texas) | 2009–2010 | Clinical isolates | 4/64 (6.3) | 358 |
| USA | 2013 | Carbapenem-resistant blood isolates | 0/97 (0) | 311 |
| USA | 2007–2013 | Urine isolates | 1/191 (0.5) | 28 |
| USA | 1937–2014 | Mixed clinical isolates (minus urine) | 26/490 (5.3) | 28 |
| Vietnam | 2003–2009 | Mixed clinical isolates | 16/41 (39) | 28 |
Defined by the presence of iuc or rmpA or rmpA2.
Collection bias cannot be excluded.
STRUCTURE AND FUNCTION
hvKp, similar to other Enterobacteriaceae, possesses an extracytoplasmic outer membrane, which consists of a lipid bilayer with associated proteins, lipoproteins, and lipopolysaccharide (LPS). The capsular polysaccharide is situated outside the outer membrane. Although also produced by cKp strains, the most common hvKp capsule types are K1, K2, K5, K20, K54, and K57, with K1 and K2 accounting for approximately 70% of hvKp isolates (2, 17, 65, 99). However, if the recently recognized trend of cKp isolates acquiring the hvKp virulence plasmid, which confers the hypervirulent phenotype, continues, then an expanding number of capsule types (e.g., K47 and K64) is predicted to be observed for hvKp strains (141, 142). hvKp strains also possess the O-antigen moiety of LPS. The K1 and K2 capsule types are usually associated with the O1 O-antigen type; therefore, this is the most common O-antigen type observed for hvKp strains (143). The capsule and outer membrane interface with the external environment, including the human host. These components are critical determinants in pathogenesis (e.g., capsule) and antimicrobial resistance (e.g., permeability barrier and efflux pumps). Additionally, secreted products play an important role for both host infection (e.g., iron acquisition molecules [siderophores]) and environmental niche survival and colonization (type VI secretion systems) (144).
A relatively unique structural feature of hvKp strains compared to cKp and other Enterobcteriaceae is the RmpA- and/or RmpA2-mediated overproduction of capsular polysaccharide, which is capsule type independent. This phenotype (15) is not necessarily synonymous with a mucoid colonial morphology and has been shown to contribute to systemic virulence (62). Its roles in other aspects of the infectious process, such as colonization, metastatic spread, and transmission, have been less well studied.
PATHOGENESIS
The hypervirulent phenotype of hvKp strains is built on the foundation of virulence factors possessed by cKp strains. These factors have been reported and reviewed elsewhere (1, 145–151). In this section, we focus on factors that are primarily hvKp specific.
Colonization
The epidemiology of acquisition and colonization by hvKp has been discussed. A number of bacterial factors are required to overcome the combination of host factors and competing microbes, which may be site specific. It is important to delineate the factors and define the mechanisms that enable hvKp to successfully colonize various epidermal and mucosal surfaces since this represents a potential point of intervention for decreasing the incidence of infection. To date, the bulk of studies have focused on gastrointestinal colonization, and the majority of genes identified are also variably present in cKp strains.
Colibactin is a peptide-polyketide that is produced by nonribosomal synthesis. Its biosynthetic genes (pks) are located within a mobile genetic element (ICEKp10) in hvKp strains which also usually contains genes for yersiniabactin and microcin E492 synthesis (76, 90). This element is present in most CG23 (K1 capsule type) hvKp strains, but less commonly in other hvKp strains (76, 94, 103). The acquisition of ICEKp10 by the sublineage CG23-I was calculated to have occurred in 1928, with subsequent global dissemination (76). This suggests that colibactin may have been an evolutionary asset. The pks gene cluster also can be present in cKp strains (90), and these genes are highly homologous to those reported for Escherichia coli (152) and other Enterobacteriaecae (153). Colibactin has been shown to promote colonization in E. coli (154) and in hvKp strain 1084 (97). Microcin E492 is an 8-kDa bacteriocin that is active against Enterobacteriaceae (155). Activity requires attachment of salmochelin, which enables the uptake of microcin by the target bacteria (156). Therefore, hvKp strains that produce the combination of colibactin, microcin E492, and salmochelin would be predicted to have a significant colonization advantage in the competitive colonic environment.
Several genes have been identified by signature-tagged mutagenesis that appear to play a role in some combination of intestinal colonization and/or invasion across the mucosal barrier after intragastric (i.g.) challenge in mice (157). hvKp strain CG43 (ST86, K2 serotype) was used in this study. After i.g. instillation, the recovery of 28 mutant derivatives was less from liver and spleen homogenates than that of their wild-type parent. Gastric challenge with mutant derivatives with single disruptions of genes that encoded a LuxR family transcriptional regulator (kva15), a putative type III fimbrial usher protein (mrkC), a monamine regulon positive regulator (moaR), a two-component regulator system (kvgA-kvgS, which has been shown to contribute to capsule production) (158), a uracil permease (kva28), or 2 hypothetical proteins (kva7 and kva21) resulted in 0% mortality, compared to 100% mortality observed for their parent, CG43. However, after intraperitoneal (i.p.) challenge, these mutants were as lethal as CG43. These data are consistent with a role for these factors in intestinal colonization and/or invasion across the mucosa. However, all of these genes are also present in cKp strains and therefore are not hvKp specific.
A mediator of ferric iron uptake, Kfu, is more prevalent in hvKp stains than in cKp strains. Kfu was shown to contribute to virulence after i.g., but not i.p., challenge in mice (159, 160). These data support a role for intestinal colonization and/or invasion. However, given its known role in free iron acquisition, which is available in the gastrointestinal tract, a contribution to colonization seems more likely. Likewise, genes that metabolize allantoin are more prevalent in hvKp strains with a K1 capsule type than in cKp strains, but not in hvKp strains with non-K1 capsule type serotypes (65, 66, 161). Similarly, these genes were requisite for maximal virulence after i.g., but not i.p., challenge in mice, thereby supporting a role for intestinal colonization and/or invasion (161). The products of these genes enable nitrogen assimilation from either exogenous allantoin or the catabolism of purines, substrates present in the gastrointestinal tract. Therefore, although a role in mucosal invasion cannot be excluded, it is biologically more plausible that the products of these genes play a role in colonization. It should be noted that the absence of these genes in non-K1 hvKp strains suggests that the ability to metabolize allantoin is not requisite for the hvKp hypervirulent phenotype but may increase the pathogenic potential of K1 strains by increasing their ability to colonize the gastrointestinal tract.
The sensitivity to antimicrobial peptides (SAP) transporter was shown to increase colonic colonization of the undefined K. pneumoniae strain Ca0437 in a mouse i.g. challenge model (162). The SAP transporter also enhanced adherence to intestinal epithelial cells in vitro. Interestingly, and unpredictably, the SAP transporter affected transcriptional levels of other genes, including those that encoded type I fimbriae. Therefore, it is unclear whether the effect was direct or indirect.
Lastly, in the hvKp strain NTUH-K2044, the disruption of treC, whose product enables trehalose utilization, resulted in decreased intestinal colonization in mice when the strain was in competition with its wild-type parent. Additional effects observed were decreased capsule production and biofilm formation, suggesting potential mechanisms (163). Similarly, the result of the loss of celB, whose product is needed for the transport of cellobiose into the cytoplasm, led to decreased biofilm formation, intestinal colonization, and lethality in mice challenged i.g. (164). Although the experimental design is not discriminatory for which step(s) in the pathogenic process was affected, these data are consistent with capsule and/or biofilm formation as important factors in mediating intestinal colonization, a first and requisite step in Klebsiella pathogenesis (163, 164). However, it has also been shown that capsule promotes colonic colonization for cKp strains as well (165). It is unclear whether the ability of NTUH-K2044 and other hvKp strains to produce more capsule and biofilm than cKp strains (163) enhances their ability to colonize compared to that of cKp and perhaps other Enterobacteriaceae strains.
Entry
For some hvKp infections, the route of entry seems straightforward. Oropharyngeal colonization could lead to pneumonia via micro- or macroaspiration. Colonic/perineal colonization could lead to urinary tract infection via the ascending route, although this appears to be uncommon for hvKp (2). For some patients, cryptogenic liver abscesses due to K. pneumoniae have been associated with colonic carcinoma (166); although the K. pneumoniae strains were undefined, it is likely that a number of them were hvKp. In the health care setting, disruptions of mucosal or epithelial barriers (e.g., endotracheal tubes, surgical incisions, and catheters) may enable entry (22).
However, for most patients that develop hvKp infection, the initial site of entry is unclear. To date, most hvKp infections are community acquired, and they often occur in otherwise healthy hosts for whom there is no overt mucosal or epithelial barrier disruption. Since hepatic abscess is the most commonly reported infectious syndrome, it seems logical that hvKp is able to cross the intestinal mucosa and seed the liver through a portal bacteremia. A gut-vascular barrier exists which protects the host from indiscriminate entry of pathogens from the intestinal microbiota (167). Certain pathogens, such as Salmonella enterica serovar Typhi, have developed the ability to translocate across the normal intestinal mucosa, mediated in part by type III secretion systems (168). But for hvKp strains in humans, this does not routinely appear to be the case, since colonization does not necessarily result in infection. The mechanism by which hvKp is able to cross mucosal and/or epithelial barriers in humans is unclear. Considerations include entry through occult disruptions in the skin, with resultant bacteremia and subsequent spread to distant sites, a mechanism employed by Staphylococcus aureus. Another possibility is that the quantity of hvKp organisms colonizing the intestinal tract is a critical factor for entry. In support of this hypothesis, oral ampicillin or amoxicillin treatment, agents inactive against Klebsiella, was associated with an increased risk of subsequent hvKp infection (169). If the magnitude of the intestinal colonizing hvKp titer proves to be an important factor for facilitating entry, then as hvKp becomes increasingly antimicrobial resistant, it is predicted that the risk of infection will increase with antimicrobial use, especially in the health care setting. Of course, yet-to-be-defined host genetic differences that enable increased intestinal binding and/or translocation may be contributory. Needless to say, an improved understanding of the mechanism that enables hvKp to enter the host would facilitate preventative strategies.
Experimentally, hvKp strains have been shown to be capable of translocating across human intestinal epithelial cell lines and mouse colonic epithelial cells (170). A number of bacterial factors have been identified that could facilitate entry through the intestinal barrier. Based on experimental design, the factors identified by Tu et al. could contribute to entry and/or colonization (please see above for details) (157). The SAP transporter was shown to increase translocation of Ca0437 across intestinal epithelial cell monolayers in vitro in a mouse i.g. challenge model (162). But, the models used to identify these factors may not optimally translate. The identified factors are not hvKp specific and are present also in cKp and, in some instances, other Enterobacteriaceae. In contrast to hvKp, these pathogens can colonize the intestinal tract but require an overt mucosal disruption for entry. Therefore, it would appear that these factors alone cannot be responsible for hvKp entry leading to infection.
Paradoxically, studies using hvKp strain 52145 demonstrated that capsule impeded entry into A549 epithelial cells (171), and those using undefined K. pneumoniae strains demonstrated that capsule impeded invasion of an ileocecal epithelial cell line, at least in part by decreasing adherence (172). Further studies on the effect of increased capsule production that occurs with hvKp strains on adherence and cellular invasion would be of interest.
Growth and Survival
hvKp strains have been shown to be more resistant to phagocytosis, neutrophil- and complement-mediated activity, and neutrophil extracellular traps (NETs) than cKp strains (15, 39, 99, 173). hvKp strains also demonstrate enhanced growth in human body fluids ex vivo and increased virulence in a variety of infection models compared to cKp strains (39, 174). The hvKp-specific factors identified to date that mediate these phenotypes and clinical manifestations are discussed in the following sections. However, undoubtedly additional factors will be defined in the future.
RmpA, RmpA2, and capsule production.
Capsule is an established virulence factor for cKp (175–177). However, a critical feature that contributes to the hvKp phenotype is the ability to produce increased amounts of capsular polysaccharide. This is mediated at least in part by RmpA and/or RmpA2, which are hvKp-specific factors located on the hvKp virulence plasmid (17, 83, 178). The loss of RmpA and/or RmpA2 decreases capsule production and virulence (33, 62, 178, 179). Glucose is an environmental signal that has been shown to increase capsule production (178, 180). As a result, it has been speculated that the association of diabetes mellitus and hvKp infection may be due in part to increased serum glucose levels in this patient group. A number of other regulators have been shown to modulate capsule production and/or the transcription of capsular polysaccharide biosynthetic genes (149, 158, 180–182). However, these factors also are present in cKp strains. This implies that RmpA and RmpA2 are critical factors for contributing to the increased virulence of hvKp strains relative to that of cKp strains. The regulator Fur (ferric uptake regulator) has been shown to repress capsule production in hvKp strain CG43 via repressing the expression of rmpA and rmpA2 (62, 181). Therefore, hvKp capsule production is predicted to be increased in iron-limiting environments, such as within the human host.
In studies using hvKp strains, the capsular polysaccharide has been shown to protect against phagocytosis (149, 183, 184) and human defensin-mediated bactericidal activity (185), and it attenuated the production of human defensins in vitro (185). It also enhanced infection in mouse pneumonia models (171, 183, 184). It is logical to assume that increased capsule production may increase these biologic functions relative to smaller amounts of capsule produced by cKp strains, but experimental data are limited (62).
Capsule type.
Several investigations have examined whether the K1 and/or K2 capsule types enhanced virulence compared to non-K1/K2 types (99, 186). These studies have variously reported that metastatic spread was more common in the K1/K2 groups and that diabetes mellitus was less common. However, it is clear that non-K1/K2 hvKp strains are capable of causing multisite infection, metastatic spread, and lethal disease (17, 187). Further, in the absence of some combination of increased capsule production and/or the presence of additional hvKp virulence factors, K1/K2 capsule type cKp strains do not possess the hypervirulent phenotype. Therefore, although it is possible that capsule type could modulate the overall virulence capability of hvKp, hypervirulence is by no means unique to these capsule types.
Iron acquisition and aerobactin.
The primary mechanism of iron acquisition in K. pneumoniae is through the production of small molecules called siderophores that are secreted, bind iron, and reenter the bacterial cell through specific receptors (188, 189). hvKP strains variably have the capability to produce 4 different siderophores: enterobactin, salmochelin, yersiniabactin, and aerobactin. Molecular epidemiologic studies have shown that salmochelin, yersiniabactin, and aerobactin are more commonly present in hvKp strains than cKp strains (95, 159, 174). Yersiniabactin is also present in cKp strains, whereas salmochelin and aerobactin are hvKp specific (17). Enterobactin is ubiquitous in K. pneumoniae strains but is inactivated by the host protein lipocalin 2 (190); therefore, enterobactin is unlikely to play a role in systemic infection.
Surprisingly, quantitative siderophore assays with hvKP strains have demonstrated that hvKp strains produce quantitatively more siderophores than cKp strains (17, 86, 191). A total siderophore concentration of >30 µg/ml was strongly predictive of an isolate being hvKp and increased lethality in a mouse systemic infection model (17). Further, despite the ability to produce 4 different siderophores, aerobactin accounts for >80 to 90% of total production (86). Ex vivo and in vivo studies using isogenic constructs that variably express enterobactin, salmochelin, yersiniabactin, and aerobactin demonstrated that only aerobactin significantly enhances survival in human ascites, serum, and outbred mouse systemic and pulmonary infection models (86). These data are consistent with aerobactin being the primary virulence determinant among hvKp’s siderophores that enables systemic infection. It remains unclear whether the total amount of siderophores produced by hvKp or specific characteristics of aerobactin are the critical feature responsible for mediating increased virulence. Yersiniabactin has been shown to be an important factor for cKp in mouse infection models (96). It is unclear why this is not the case for hvKp strains. Perhaps the quantitatively dominant amount of aerobactin produced by hvKp strains obscures a potential contribution by yersiniabactin. Interestingly to date, data do not support a role for salmochelin in systemic infection (86). However, genes that produce salmochelin are hvKp specific (17), suggesting that salmochelin plays a yet-to-be-defined role in the pathogenesis of hvKp infection. One possibility is that salmochelin, in conjunction with the microcin E492, is a critical mediator of hvKp colonization as discussed above (see “Colonization”). It is interesting that in the XDR cKp strains K. pneumoniae 1 to 5 that acquired a 178-kb pLVPK-like virulence plasmid and were subsequently responsible for a lethal ICU outbreak, the salmochelin biosynthetic genes were deleted from this plasmid (22). Perhaps, the XDR phenotype combined with barrier disruption in these patients made salmochelin production dispensable.
PEG344.
peg-344 is hvKp specific and is located on the hvKp virulence plasmid (17). The function of PEG344 is unknown, but homology modeling suggests a possible role as a transporter located in the inner membrane. When hvKp was grown in human ascites, the RNA abundance of peg-344 was increased (88). A potential role in virulence was assessed in outbred CD1 mice. PEG344 was required for full virulence in a pneumonia model, as measured by survival and competition experiments, but did not appear contribute to the pathogenesis of systemic infection that developed after subcutaneous challenge (88). The mechanism by which PEG344 contributes to survival in pulmonary infection is unknown.
Colibactin.
Besides a potential role in colonization, colibactin also has been shown to contribute to survival in the bloodstream of mice infected intranasally or intravenously and, to a lesser extent, after orogastric challenge (97). The responsible mechanism is unclear.
LPS.
Both cKp and hvKp strains possess a complete LPS. The role of LPS has been studied in hvKp strains. In one study LPS did not appear to play a role in pneumonia in a mouse infection model (183). However, in others, it has been shown to protect against phagocytosis, complement-mediated bactericidal activity, antimicrobial peptides, and enhanced virulence in mouse pneumonia, systemic infection, and orogastric challenge models (148, 149, 184, 192–194). Presently, however, studies have not identified unique structural features in LPS produced by hvKp strains (e.g., lipid A substitutions) that would suggest these findings are specific for hvKp. Similar results have been obtained when cKp strains were studied (195).
Tellurite and silver resistance.
Genes for tellurite (terZA to -E and terWXY) and silver resistance (silS) are often present on the hvKp virulence plasmid and therefore have been hypothesized to be important for systemic infection (85, 196). But these genes are not hvKp specific (17, 150), and the loss of tellurite resistance did not affect virulence in a mouse pneumonia model (150).
cAMP receptor protein.
cAMP receptor protein (CRP) is a transcriptional regulator with pleiotropic effects. An hvKp strain (NTUH-K2044) with in which crp was disrupted was less virulent than its wild-type parent when mice were challenged via the IP route (197). CRP was also shown to positively regulate allS (allantoin metabolism) (197), but allantoin metabolism genes do not appear to be important for systemic infection (161). Therefore, these data support a role for an unrecognized gene(s) regulated by CRP in systemic infection, which may or may not be hvKp specific (198).
Metastatic Spread
In humans, multiple sites of infection and/or metastatic spread are more common with hvKp than cKp strains (17, 174). cKp and other members of the Enterobacteriaceae family rarely establish infection in secondary sites as a result of bacteremia, except in the setting of an immunocompromised host (e.g., neutropenia). The primary means by which hvKp strains infect multiple sites is via the bloodstream; whether this occurs at the time of entry and/or when a primary site of infection (e.g., hepatic abscess) is established (which, in turn, serves as the source for subsequent bacteremias and distant spread) is unclear. Likely both mechanisms are operational. Regardless, the ability to invade the bloodstream and survive the resident host defense factors is the first requisite step. Resistance of hvKp strains to the bactericidal activity of complement, which is mediated in part by capsule, is needed to accomplish this and contributes to this step in the process (99). However, most pathogenic members of the Enterobacteriaceae family are also capable of causing bacteremia and are resistant to the bactericidal activity of complement as well as the other host defense factors within the bloodstream. This suggests that hvKp is more efficient in invading tissue from the bloodstream. The mechanism by which this occurs is incompletely defined. Although the animal models used presently to study hvKp can measure bacteremia and subsequent spread to various organs or sites, models that directly measure hvKp tissue invasion from the bloodstream at the cellular level could assist in identifying potential factors that enable systemic tissue invasion.
Thrombophlebitis of the hepatic and portal veins has been described in association with hepatic abscess (199–201), but this complication could only account for spread to the lungs unless a patent ductus arteriosus also was present. Further, thrombophlebitis of the hepatic and portal veins and metastatic spread has been described in association with hepatic abscess due to other bacterial pathogens, albeit less frequently (201).
A “Trojan horse” mechanism has been postulated with neutrophils implicated as a possible vehicle (202). hvKp strains were shown to be able to survive within neutrophils (202, 203) and delay apoptosis up to 24 h (203), and i.p. injection of infected neutrophils resulted in disseminated infection; however, it was unclear if the integrity of the injected neutrophils was maintained postinjection (202). However, a cKp strain was also shown to be able to survive within macrophages and trigger apoptosis (176). Further, this concept, at least for professional phagocytes such as neutrophils or macrophages, seems counterintuitive, since these cells are attracted to the site of infection and would not be expected depart this inflammatory environment after acquiring bacterial cargo.
In most infections complicated by bacteremia, titers of 1 to 102 CFU/ml commonly are seen (204). Titers of >102 CFU/ml for S. aureus, H. influenzae, S. pneumoniae, and N. meningitidis have been associated with more severe disease and metastatic spread (204–207). Therefore, a quantitatively high titer of hvKp during bacteremia could be responsible or contributory. There is some limited support for this hypothesis. Albeit in subcutaneously infected outbred CD1 mice, 104 to 105 CFU/ml were measured in blood 48 h after bacterial challenge (191) and titers of 105 CFU/ml were measured after intranasal or intravenous challenge (97). Another possibility is that spread could be driven hvKp’s increased capsule production. Although speculative, perhaps this phenotype results in greater in vivo clumping of bacterial cells, which, in turn, enhances survival with hematogenous dissemination.
Colibactin has been suggested to contribute to meningeal spread (97). Whether this is directly mediated by colibactin or due to the magnitude of bacteremia is unclear. It should be noted that the hvKp strain NTUH-K2044 was isolated from a patient with meningitis, but this isolate does not produce colibactin (93).
Tissue Damage
hvKp infection often results in abscess formation. However, to date there is limited insight into the responsible bacterial or host factors. The best-defined factor is colibactin, which has been shown to be genotoxic, causing DNA damage and cell death (94, 97). However, non-colibactin-producing hvKp strains (e.g., non-CG23-K1 capsule type) also have caused abscesses in multiple sites. This suggests the possibility that unrecognized hvKp factors also may be contributory. Undoubtedly an unregulated host response resulting in collateral damage is contributory to some degree as well.
Association with Malignancy
The incidence of colonic cancer increased over the first decade in the new millennium in Taiwan. Several studies have identified an increased risk of colonic cancer in patients with hepatic abscess due to K. pneumoniae compared to patients without a hepatic abscess or with hepatic abscesses due to non-K. pneumoniae strains (208, 209). Since some K. pneumoniae strains, including hvKp, can produce the genotoxin colibactin, it has been hypothesized that such strains are the causative agent. An alternative hypothesis would be that hvKp-mediated hepatic abscess is a marker for cryptogenic colonic carcinoma, similar to Streptococcus bovis bacteremia/endocarditis (210) or infection due to Clostridium septicum (211). Further, in another epidemiologic study from Taiwan, an increased risk of hepatocellular carcinoma was observed in patients with a history of liver abscess (212). Given the well-established risk of hepatocellular carcinoma due to hepatitis B and C virus infection, although those infections are more chronic, there is a biologic plausibility for this association. More data are clearly needed to define cause and effect and the relative risks. However, presently it is reasonable to consider a screening colonoscopy for patients diagnosed with a hepatic abscess due to hvKp, especially for those not recently studied or with risk factors (e.g., age or family history). Whether long-term follow-up should be performed for the possible development of hepatocellular carcinoma in patients who have suffered a hepatic abscess due to hvKp is less clear.
HOST SUSCEPTIBILITY RISK FACTORS
Ethnic Background
Although hvKp infections occur in all ethnic groups, even those acquired in Western countries commonly involve Asians (37, 39, 213–221) and (to a lesser degree) Pacific Islanders and Hispanics (9, 215, 216). One explanation could be that hvKp infection occurs more frequently in Asians from geographically defined pathogen exposure, acquisition, and increased colonization (113), a requisite step for subsequent infection. In some cases, there is a history of Asians infected in the West that travelled to or were exposed to individuals who had recently been in the Asian Pacific Rim (39). In fact, a case in a Caucasian from Denmark who traveled to Shanghai, China, suggests this very scenario (220). Alternatively, an underlying genetic abnormality that is more commonly, but not exclusively, present in Asians, Pacific Islanders, and Hispanics could also be contributory. This concept of genetic susceptibility is consistent with a case report involving a Japanese father and son who developed infection due to hvKp, but the mother was only colonized (114). Further, another report from Singapore suggests that genetic background may contribute to the development of hvKp infection. In 70 patients with liver abscess, among whom 67 cases were probably caused by hvKp, only 1.4% of these infections were in Indians, despite Indians comprising 7.4% of the population and being more likely to be diabetic. However, differences in diet and colonization were potential factors that were not accounted for (81). Conversely, in a study from France, of 14 patients with hepatic abscess due to hvKp, 5 were Caucasian, 6 were African, and only 3 were Asian (8).
Data are needed to address whether a genetic variation is contributory to hvKp infection. These data would have important implications for the development of preventative strategies and/or infection control processes (see “Infection Control and Prevention” below). Potential mechanisms remain speculative. However, considerations include genetic variants that enhance of binding of hvKp within the gastrointestinal tract or enable increased survival within the host. A nongenetic mechanism that negatively modulates host defenses is the development of autoantibodies directed against a host factor; although this has not been described for hvKp, it has been implicated in infections mediated by other pathogens (222, 223).
Diabetes Mellitus
Diabetes has been implicated as a risk factor for a variety of infections, including those due to hvKp. The majority of studies support this contention (67–75), although some reports concluded that diabetes mellitus (DM) was not predictive for developing hvKp infection (99, 132, 174). Poor glycemic control has been associated with an increase in metastatic complications (224). For the syndromes of endophthalmitis and necrotizing fasciitis due to hvKp the association is stronger in most (54, 225–229) but not all (230) studies. An unsubstantiated but biologically plausible mechanism for this observation is metastatic seeding by hvKp during bacteremia as a result of DM-related loss of vascular patency or integrity. Equally important as the association of hvKp infection with DM is the fact the hvKp infection is frequently seen in younger, otherwise healthy individuals. The clinician needs to be cognizant of the fact that this potentially devastating disease can develop in the absence of DM or any other comorbidities (99).
Sex
Males might be slightly more likely to be infected than females. Siu et al. reviewed several studies of hepatic abscess due to hvKp (53). Overall, males were more likely to be infected than females (55% [483/871]); however, geographic differences were noted. Males were infected in 68% (26/38), 42% (136/321), and 63% (321/512) of cases in the United States, South Korea, and Taiwan, respectively. Among 61 patients with hvKp infection complicated by endophthalmitis, 80.3% (49/61) were male (231). By contrast, in another study, men and women were similarly infected by hvKp strains causing liver abscesses: 52.5% (21/40) were male and 47.5% (19/40) were female (78).
Immunoglobulin Deficiencies
Recurrent hvKp bacteremia and low levels of IgG2 have been reported (232). Additional studies that examined immunoglobulin levels and hvKp infection would be of interest.
Treatment with Selected Medications
A retrospective population study reported that individuals who used ampicillin or amoxicillin in the prior 30 days had an increased risk (odds ratio [OR], 3.5) of developing a cryptogenic hepatic abscess due to K. pneumoniae (it is predicted that most of these cases would be due to hvKp) (169). Mice challenged orally with hvKp and then treated orally with ampicillin for 5 days starting 2 days after bacterial challenge had increased mortality compared to mice treated with water alone (169). Similarly, individuals that used proton pump inhibitors had an increased risk (OR, 4.7) of developing a cryptogenic hepatic abscess due to K. pneumoniae compared to controls (233). Although these are single reports, both are biologically plausible. Proton pump inhibitors could lead to increased acquisition as has been described for enteric pathogens (234). Treatment with ampicillin or amoxicillin could increase the titer of colonizing hvKp (given the intrinsic resistance of K. pneumoniae to these agents) by killing bacteria that mediate colonization resistance, which, in turn, could lead to increased entry. But, this remains speculative since the mechanism for entry by hvKp is presently unknown.
Treatment of Esophageal Varices
There have been several reports from Taiwan in which various treatments for esophageal varices were complicated by central nervous system (CNS) infection or endophthalmitis due to K. pneumoniae (235–237). Although no studies were performed to confirm that these infections were due to hvKp, the clinical sites of infection and geographic location are highly suggestive.
INFECTIOUS SYNDROMES
Sites of Infection
Abdominal disease.
The defining clinical syndrome of hvKp infection that first led to its recognition in the 1980s is cryptogenic pyogenic liver abscess (PLA) (31, 67, 69) (Fig. 2). Prior to the 1980s, Escherichia coli was the most commonly isolated enteric Gram-negative bacillus isolated from PLA in the Asian Pacific Rim (238). Since then, the predominant pathogen of PLA in this region has been steadily shifting to K. pneumoniae, mirroring the increasing prevalence of hvKp over the same period. By 2004, approximately 80% of all cases of PLA in Taiwan were caused by K. pneumoniae (239–242).
FIG 2.
An image from an abdominal CT scan of a previously healthy 24-year-old Vietnamese man shows a primary liver abscess (red arrow) with metastatic spread to the spleen (black arrow). (Courtesy of Chiu-Bin Hsaio and Diana Pomakova; reprinted with permission from McGraw-Hill Education [353].)
Beyond triggering epidemiologic shifts in the etiologies of PLA, hvKp is driving an increasing overall incidence of PLA in regions where it is endemic. In Taiwan, PLA cases increased from 11.15 to 17.59 per 100,000 between 1996 and 2004 (5). In comparison, incidence of PLA in North America over the same time frame was 3.6 per 100,000, with pathotype-undefined K. pneumoniae isolated from only 9.2% of cultured liver abscesses (243). More recent data seem to suggest that the epidemiologic trends observed in the Asian Pacific Rim have begun diffusing across the West. Some regions of Europe and North America still report low but increasing rates of this infection (214, 219). For example, a review of 158 pyogenic liver abscesses in Paris, France, revealed that the majority were due to mixed enteric flora and associated with biliary disease or instrumentation (8). However, within the subset of cryptogenic PLA cases, K. pneumoniae is now the most commonly isolated pathogen, with 8.7% of all PLA cases due to hvKp (8). Regions of the United States with substantial Asian populations have reported even higher rates than these. Two small series out of New York City reported that K. pneumoniae was the responsible pathogen in 41% and 36% of cases, respectively (216, 244), and a tertiary hospital in California has reported that K. pneumoniae has superseded all other causes of PLA regionally (215). The K. pneumoniae in these series was not specifically identified as hvKp. Nonetheless, an increasing number of cases of PLA due to hvKp are being reported in various Western countries (39, 213–215, 219, 221, 245, 246), supporting the inference that the epidemiologic trends observed in East Asia are likewise occurring to various degrees worldwide.
The clinical and laboratory presentation of hvKp-mediated PLA is superficially like that of other PLA; presenting signs and symptoms typically include abdominal pain, fever, chills, and leukocytosis regardless of causative pathogen (247). However, several specific features of hvKp PLA set it apart as a unique syndrome. hvKp-mediated PLA is almost always monomicrobial, while non-hvKp PLA is almost always polymicrobial (67, 248). hvKp PLA is also associated with significantly more metastatic complications, which are detailed below (53, 67, 249). Unlike other liver abscesses, which are often consequences of biliary pathology or preceding interventional procedures, hvKp PLA typically occurs in individuals with normal biliary and hepatic function (249). Though not established definitively as a distinguishing feature of hvKp, a number of reports describe late reinfection or relapses of hvKp infection at the same or different sites months after initial therapy had been completed (69, 114, 245, 250). Further investigations will be required to substantiate if this is a characteristic of hvKp, to understand the mechanism by which it may occur, and, if so, optimal treatment. Hypothesized mechanisms include the survival of the hvKp infecting strain at the site of initial infection with subsequent relapse due to inadequate therapy or persistent colonization with subsequent reinfection, perhaps facilitated by the host having an increased susceptibility to hvKp infection.
Regional thrombophlebitis has been reported in up to one-third of cases of PLA due to K. pneumoniae, compared to approximately 5% with other causes of PLA (251). Affected vasculature may be the hepatic vein or, less commonly, the portal vein (199, 200). Although this complication may be responsible for septic emboli to the lungs, resolution occurs with the management of the infection. There are no controlled data, but anticoagulation does not appear to be beneficial or necessary for recannulation (199, 252).
Multiple other foci of intra-abdominal infection are possible in invasive hvKp syndromes, including splenic abscess. Specific data regarding the incidence of splenic abscess due to hvKp are lacking. However, regions where hvKp is endemic, such as Taiwan and Korea, have reported K. pneumoniae as a leading cause of splenic abscess (253–255). High rates of concurrent PLA were noted in K. pneumoniae splenic abscesses (44%) but not with splenic abscesses of other etiologies (0%) (256). Historically, K. pneumoniae has been a relatively rare cause of splenic abscess, isolated alone or in polymicrobial growth in only 5% of cases (257). It is reasonable to infer that hvKp produces these epidemiologic changes.
In contrast to splenic abscess, preliminary data would suggest that the microbiology of spontaneous bacterial peritonitis is not changing substantially with the emergence of hvKp. E. coli remains the most common cause of monomicrobial SBP in Taiwan, with monomicrobial K. pneumoniae comprising only 3 to 7% of all cases (70, 258). Though phenotypically hypermucoviscous strains of K. pneumoniae have been reported to cause SBP (70), the invasive syndrome characteristic of hvKp is significantly less likely to occur in cirrhotic patients than noncirrhotic (132).
Thoracic disease.
Data from the 1990s suggest that K. pneumoniae accounted for less than 1% of cases of community-acquired pneumonia (CAP) requiring hospitalization in North America, Argentina, Europe, and Australia (70, 259–261). Pneumonia due to cKp does occur but almost always in health care settings in patients with comorbid conditions. By contrast, Klebsiella has been reported as an important cause of severe, bacteremic CAP in South Africa and Taiwan over the same time frame (70). A more recent study from Taiwan assessing cases of bacteremic CAP from 2001 to 2008 reported that hvKp now has superseded S. pneumoniae as the most common etiology regionally (6). Importantly, patients with bacteremic CAP due to hvKp had significantly higher rates of respiratory failure, bilateral lobar involvement, septic shock, and mortality than patients with bacteremic S. pneumoniae CAP. Mortality in the above-referenced study was 55.1% for those infected with hvKp (6). Cases of hvKp CAP outside the Asian Pacific Rim are still relatively rare but are being reported in increasing frequency. A recent French study found that 24% of patients admitted from the community to the ICU with K. pneumoniae infection had community-acquired hvKp pneumonia (262). These patients had significantly higher rates of multiorgan failure than other patients admitted to the ICU with other causes of CAP, as has been observed in countries where hvKp is endemic (262).
Though the characteristic features of hvKp are community onset infections, hospital-acquired ventilator-associated pneumonia due to hvKp has been reported from China (263, 264). Disturbingly, multiple nosocomial outbreaks of ventilator-associated pneumonia due to carbapenemase-producing hvKp have now been reported across Asia, with uniformly negative outcomes. An outbreak of 5 cases of VIM-2-positive hvKp, harbored on an IncN plasmid, caused 80% mortality in an Iranian ICU (265), and similarly, five XDR ST11 cKp K. pneumoniae strains, harboring blaKPC-2, blaCTX-M-65, and blaTEM-1, that acquired an hvKp virulence plasmid caused a uniformly fatal outbreak of ventilator-associated pneumonia in China (22).
In additional to lobar pneumonia, hvKp may cause a variety of other pulmonary infections, including empyema, lung abscess, and septic pulmonary emboli. hvKp has become the most common cause of community-acquired empyema in Taiwan and is associated with high mortality rates (32.4%) (266), significantly higher than those of non-Klebsiella empyema (267). Although most pleural space infections are due to an adjoining pneumonia, spread may also occur via transdiaphragmatic rupture of pyogenic liver abscess (267). K. pneumoniae has now also become the most common single cause of lung abscesses in Taiwan, though it has not become more common than mixed anaerobic lung abscesses considered collectively (3). More patients with K. pneumoniae lung abscess have concomitant bacteremia and multiple cavities observed radiographically than those with anaerobic bacterial lung abscess (3).
Septic pulmonary emboli often occur as a complication of hvKp-mediated PLA (268) and nonhepatic sites infected by hvKp (67, 269–272). Characteristic radiographic findings of septic pulmonary emboli due to hvKp are similar to those for pulmonary emboli due to infection by other pathogens and include pulmonary nodules with or without cavitation, adjacent pleural effusions, peripheral wedge-shaped opacities, and ground-glass opacities (268).
Endophthalmitis.
Endophthalmitis, a devastating complication of hvKp infection, was first described in 1986 and was one of the first clinical manifestations recognized that distinguished hvKp from cKP infection (31) (Fig. 3). Endogenous endophthalmitis (EE) due to enteric Gram-negative bacilli was extraordinarily uncommon in ambulatory, healthy hosts until the advent of hvKp. Now it has become a frequent complication: approximately 5% of individuals with hvKp bacteremia ultimately develop EE (132). By contrast, exogenous endophthalmitis, which is associated with trauma or surgery rather than hematogenous spread, has rarely or never been attributed to hvKp.
FIG 3.
A previously healthy 33-year-old Chinese male presented with endophthalmitis. (Courtesy of Chiu-Bin Hsaio; reprinted with permission from McGraw-Hill Education [353].)
The most typical presentation of hvKp EE is painful ocular swelling, redness, and sudden onset of blurred vision (229). Bilateral involvement occurs in 13% to 25% of patients (226, 229, 231, 273). Due to its rapid onset, some patients may sustain irreversible blindness even before a systemic infectious process is recognized (226). Onset also may occur as late as 30 days after hvKp’s initial presentation (231). Even with intravenous and intravitreal antibiotic treatment, outcomes of EE are poor; posttreatment visual acuity is light perception or worse in 89% of cases (229). Forty-one percent of affected eyes eventually required evisceration or enucleation (229). An instructive case report that underscores the aggressive nature of this infection describes a patient undergoing treatment for hvKp bacteremia and PLA who reported a vague “veil-like” visual disturbance while in hospital. Because the patient retained perfect visual acuity and was without hypopyon or vitritis on exam, treatment was deferred. The patient’s vision declined to 20/400 (6/120) overnight, with interval development of vitritis on exam. Recovery of visual acuity, despite aggressive treatment, was suboptimal. Diagnosis of a small peripheral retinal abscess was made in retrospect (274). Though most cases of hvKp EE are reported in Southeast Asia, cases are now being documented worldwide in increasing incidence (275–278). Prompt recognition and treatment of hvKp EE may improve outcomes and may—in cases of unilateral initial symptoms—prevent a worse outcome from occult infection in the contralateral eye (31, 227, 229, 231).
Central nervous system disease.
As with other hvKp-mediated infectious syndromes, the face of community-acquired meningitis has changed in Southeast Asia because of the emergence of hvKp (279). Alarmingly, K. pneumoniae has become a major cause of community-acquired meningitis in Asia in the absence of neurosurgery or head trauma (7, 279–281). Shifts also are being observed beyond the Asian Pacific Rim as hvKp spreads across the globe. Gram-negative meningitis in community-dwelling, healthy adults was almost unheard of in the West up until recently (282), but this has begun to change (283).
In Taiwan, 50% of cases of community-acquired meningitis requiring ICU admission are due to K. pneumoniae (284). Outcomes of K. pneumoniae community-acquired meningitis are uniformly worse than for S. pneumoniae community-acquired meningitis, with higher rates of septic shock, extrameningeal infectious foci, in-hospital mortality, and 28-day mortality (285). Although not all the strains in the above-referenced studies have been genetically characterized as hvKp, several studies have confirmed that the syndrome of community-acquired K. pneumoniae meningitis was attributed to the hvKp pathotype (132, 174, 219). Hypervirulent K. pneumoniae meningitis may be the presenting primary infection or secondary to metastatic spread (286, 287).
Characteristic imaging findings are not yet well characterized for hvKp meningitis. One case report described the presence of multiple, irregular cord-like structures that bridged the dura and pia mater in the subarachnoid space and were hyperintense relative to cerebrospinal fluid on fluid-attenuated inversion-recovery magnetic resonance imaging (288). Another report described initial MRI findings of patchy leptomeningeal enhancement, followed by a focal nodular lesion in the subacute phase that subsequently resolved with treatment (289).
Some cases of hvKp meningitis have been diagnosed concurrently with brain abscesses, which tend to be diffuse and multiple (290, 291). Ventriculitis may also occur simultaneously (291). Other CNS manifestations of invasive hvKp disease reported to date include subdural empyema and epidural abscess (290, 292–295).
Musculoskeletal and soft tissue infection.
hvKp has become a common cause of necrotizing fasciitis in Taiwan, causing a similar number of cases as group A streptococcus but having a higher mortality (47% versus 19%) (296). Though most common in Southeast Asia, cases have been reported worldwide (220, 297). Intramuscular abscesses may occur, with or without concurrent necrotizing fasciitis (67, 298). Of note is hvKp’s propensity to cause psoas abscess. In Taiwan, where hvKp is endemic, K. pneumoniae is second only to S. aureus as causative agent of nontuberculous psoas abscess (250). Pyomyositis, especially of the lower extremities, has also been reported, both alone and in association with contiguous septic arthritis (299). Osteomyelitis may occur and may involve multiple distant sites. In fact, the multifocal, lytic nature of hvKp osteomyelitis has been mistaken for malignancy, with the correct diagnosis made only after progression to contiguous necrotizing fasciitis (300). Bone pain due to osteomyelitis may be the primary presenting complaint in a patient later discovered to have disseminated hvKp infection (301).
Deep neck infections have been described as the primary presenting complaint of systemic hvKp infections, often in the setting of other systemic metastatic complications (37, 217). Neck abscesses may be complicated by septic thrombophlebitis (i.e., Lemierre syndrome) and descending mediastinitis (213). In addition to the above deep foci of infection, superficial skin and soft tissue infection has been reported, with cultures of cutaneous septic emboli eventually providing the underlying diagnosis of disseminated hvKp infection (302).
Genitourinary tract.
Most urinary tract infections occur via the ascending route, as fecal flora ascends through the urethra to infect the bladder, occasionally reaching the kidneys and the systemic circulation. cKp causes infections in this manner. This pathogenesis mechanism is not as clearly established for hvKp, though biologically plausible. The urinary tract is cited as a source of hvKp bacteremia (132), but the primary mechanism by which hvKp establishes bacteremic infection appears to be independent of the urinary tract. To the contrary, most genitourinary infections reported due to hvKp result from hematogenous seeding from preceding bacteremia. Bacteremic spread to the kidneys, perinephric region, and prostate resulting in local abscess formation are well described (31, 67, 303). Although additional data are needed, hvKp isolated from the urine may serve more reliably as a marker for hvKp bacteremia than a source for it, similar to S. aureus bacteriuria (304).
Bacteremia/endovascular infection.
Bacteremia is an extremely common complication of hvKp site-specific infection. The most common underlying infection in cases of hvKp bacteremia is pyogenic liver abscess, though many other primary sources are possible (132). A unique feature of hvKp bacteremia is the high proportion of cases that occur with no infectious source immediately apparent. Individuals with hvKp bacteremia are more likely to have blood cultures turn positive before the primary site of infection is identified or cultured, compared with those infected with cKp (305). Despite the range of aggressive clinical manifestations hvKp is capable of, endocarditis is an extremely rare presentation of hvKp disease. A case of native valve endocarditis due to hvKp has been reported (306), but purulent pericarditis has been reported more frequently, either as a direct extension from PLA (307) or via hematogenous spread (290). Septic thromboembolism associated with hvKp infection occurs commonly, both regionally in the areas of primary infection and as distant embolic sequela. Details regarding hepatic, pulmonary, and internal jugular septic thromboemboli due to hvKp are discussed in their respective clinical syndrome discussions above.
Miscellaneous.
Infections of nearly every body site have been reported with hvKp. A few less common manifestations of hvKp infection include orbital cellulitis (308), epididymitis (270), and Bartholin’s abscess (309). It is likely, given the tissue invasive nature of hvKp, that more unusual manifestations of invasive hvKp infection will continue to be reported.
DIAGNOSIS
Microbiologic Identification
A significant issue that is presently impeding optimal care of hvKp-infected patients is the inability of clinical microbiology laboratories to distinguish cKp from hvKp. To resolve this shortcoming, the availability of a commercial test that has been approved by appropriate regulatory bodies is needed. Identification of hvKp as the infecting agent would be important. Specifically, if infection due to hvKp was not previously considered, this would suggest to the treating physician the need to obtain additional imaging (computed tomography [CT] or magnetic resonance imaging [MRI]) in search of additional sites of infection, which may be unrecognized and could require source control (e.g., drainage) (295). Further, the identification of certain occult sites of infection, such as in cases of meningitis (which may be mistaken for sepsis-related changes in mental status), brain or prostatic abscesses, or endophthalmitis, would be important since site-specific antimicrobial regimens that achieve adequate drug concentrations are needed for an optimal outcome (31). Of particular note is endophthalmitis, a devastating complication of hvKp infection. Ocular infection may not be present or apparent at presentation. Due to the rapidity of tissue damage at this site, immediate treatment is required to maximize the odds of maintaining visual acuity. Therefore, established or suspected hvKp infection dictates involvement of an ophthalmologist, who has the capability of performing an appropriate evaluation and immediately initiating treatment specific for endophthalmitis, such as vitrectomy and intravitreal antibiotics if needed (229, 231). The hypermucoviscous phenotype of hvKp can be problematic in the management of abscesses. Its increased viscosity can impede percutaneous drainage (PD) and increase the likelihood of the catheter becoming clogged (39, 310). If it is known at the time of catheter placement that hvKp is the infecting strain, then the clinician can consider the use of the largest bore practical. Once the drainage catheter is placed, it would be equally important to perform more frequent irrigations than usual to try to maintain drainage, which, in turn, will decrease the need for subsequent open surgical drainage (SD) if percutaneous drainage fails. Anecdotally, hvKp infection has been associated with relapse (69, 114, 245, 250). In the absence of controlled data, when hvKp is identified as the infecting agent, a more prolonged treatment course may be needed to maximize cure rates and minimize relapse.
Presently, the suspicion of an astute clinician is the only means by which hvKp would be considered (Table 1). However, clinical criteria are becoming increasingly problematic. The clinical definition that requires the occurrence of a tissue-invasive, community-acquired infection in a healthy host precludes recognition of hvKp infection in patients who have comorbidities, are immunocompromised, or are in a health care setting; the last scenario is becoming increasingly common.
Fortunately, peg-344, iroB, iucA, prmpA, prmpA2, and siderophore production greater than 30 μg/ml have been shown to accurately differentiate hvKp from cKp strains (17). These biomarkers could enable the development of a diagnostic test which, if FDA approved, could be used by clinical laboratories for the identification of hvKp strains. A sensitive and specific test is needed not only for patient care but also for use in surveillance and research studies (140). This study and others have demonstrated that the accuracy of the string test for defining hvKp was not optimal, especially in low-prevalence regions, and that this diagnostic modality should no longer be used as the sole means for identifying hvKp (16, 17). However, it is important to note that genetic variation is commonplace with K. pneumoniae. Therefore, it would therefore seem logical that the best marker for hvKp strains would be one that clearly contributes to the hypervirulence phenotype. Total siderophore production has been shown to strongly correlate with in vivo virulence (17, 86, 87), and aerobactin has been shown to be the dominant siderophore produced by hvKp strains and the critical siderophore that enhances virulence ex vivo and in vivo (87). Further, increased production of capsule, which is mediated by rmpA or rmpA2, also has been shown to contribute to the hypervirulent phenotype (33, 62, 178, 179). Therefore, total siderophore production, or iuc and/or either rmpA or rmpA2, would be predicted to be the most accurate and durable marker. This concept is supported by the report by Gu et al. in which an XDR cKp strain became hypervirulent despite iro, peg-344, and rmpA, but not iuc and rmpA2, being deleted in the acquired hvKp plasmid (22). Of course, as more hvKp-specific genes are identified, additional markers may prove to be viable alternatives or even more accurate.
A facile assay to measure quantitative siderophore production would require development for routine use in clinical laboratories. However, identification of genetic biomarkers could be easily achieved via a PCR assay (311). Identification of hvKp via matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (MS) has been explored but requires further refinement (312–314).
Radiographic Considerations
hvKp infection is characterized by often having multisite involvement upon presentation or subsequent metastatic spread. Infected sites may declare themselves by some combination of signs or symptoms or may be occult (292, 295). Defining the full extent of hvKp infection is critical since selected sites may require a modification of the antimicrobial regimen (e.g., CNS, prostate) and/or dictate the need for source control. Therefore, the clinician should have a low threshold for performing appropriate radiographic studies with the goal of defining the full extent of infection.
TREATMENT
Source Control
hvKp infection is often manifested by abscess development. The liver is the most common location, but nonhepatic abscesses have been described for virtually every organ and anatomic location. There is a limited body of data that addresses the management of hvKp mediated abscess. Further, extrapolation of data from non-hvKp strains may not be applicable due to important differences between hvKp and other pathogens, namely, its hypermucoviscous phenotype, which can result in an extremely viscous fluid content within the abscess. Lastly, management may be driven by the location of the abscess and acuity of illness.
A retrospective study from Singapore compared percutaneous drainiage with open surgical drainage (SD) for the management of hepatic abscess larger than 5 cm; 80% were multiloculated (310). K. pneumoniae was the offending pathogen in 67% (24/36) and 61% (27/44) of the PD and SD groups, respectively. Although the pathotypes were undefined, it is likely that a high proportion of these K. pneumoniae strains were hvKp given the location of the study and that the majority of abscesses were cryptogenic (8). Overall, there was no difference in mortality between the groups, but the SD group had a shorter length of stay, were less likely to fail therapy, and required fewer subsequent procedures; no subgroup analysis for the K. pneumoniae infections was performed. A retrospective study from Taiwan compared PD alone (n = 46), PD followed by hepatic resection (PD-HR) for patients that failed PD (n = 19), and HR as the initial treatment for patients (n = 16) with liver abscess and an APACHE II score of ≥15 (315). The mortality rate was lower in the HR group than in the PD group, but the rates were similar between the HR and PD-HR groups. The HR group was reported to consist of patients with peritonitis, biliary tract infection, or suspected malignancy. No microbiologic data were reported, and the majority of abscesses were described as cryptogenic. Another study from Singapore reported that hepatic abscesses due to K. pneumoniae (unclear what proportion of organisms were hvKp) that were >5 cm (71/109) were more likely to have a delayed response to therapy but not an increased risk of death or readmission; the presence of loculations was not predictive of a delayed response. Of these 109 patients, 7 were treated with open or laparoscopic surgical drainage, 65 with percutaneous drainage, and 37 with antimicrobials alone (26 of which had abscess <5 cm) (316).
Given the limited published data set, which primarily addresses hepatic abscesses, a reasonable approach for hvKp-mediated abscesses in any location should be an individualized assessment of risk and benefit. Abscesses in critical sites (e.g., brain and epidural space) or ruptured abscesses would warrant consideration for more immediate surgical intervention. Large abscesses in noncritical sites may be best managed by percutaneous drainage pending accessibility, with a surgical intervention reserved for failures. Since abscess fluid with hvKp infection is commonly viscous, the largest-bore drainage catheter feasible should be used, with frequent flushing to try to prevent catheter blockage, a reported complication (310). Smaller abscesses (<5 cm) have the potential to be cured with antimicrobial therapy alone. Imaging can be used to assess the response to therapy and define duration of therapy. With the availability of biomarkers to accurately identify hvKp (17), controlled trials that address various issues related to source control would be welcomed.
hvKp infection also can result in necrotizing fasciitis (296). Although there are no controlled data that addresses the management of this manifestation of hvKp infection, the standard of care for this potentially lethal entity is rapid surgical intervention (fasciotomy, debridement, and amputation) in addition to medical treatment (228, 296).
Antimicrobial Resistance
Antimicrobial resistance can be mediated by several mechanisms (317), all of which are variably occurring. The first is acquisition by an hvKp strain of a conjugal plasmid(s) that contains antimicrobial resistance determinants (19). Since hvKp strains have not acquired antimicrobial resistance determinants as rapidly as cKp strains, there has been speculation that plasmid incompatibilities, a physical barrier due to overexpression of capsule, and CRISPR systems could be contributory (76). The second mechanism consists of acquisition and integration of an ICE containing antimicrobial resistance determinants into either the chromosome or virulence plasmid of an hvKp strain (20, 318). The third mechanism is disruption or mutations in chromosomal genes (e.g., genes for outer membrane proteins [OMPs]) (319). The fourth is acquisition of the hvKp virulence plasmid by MDR or XDR cKp strains (22, 319). The hvKp virulence plasmid appears to be nonconjugal. However, a potential mechanism for mediating transfer has been postulated. Dong et al. demonstrated that there is a common 11.2-kb region between at least some hvKp virulence plasmids and a conjugal plasmid that encodes the KPC carbapenemase. This suggests the possibility of initial integration of these extrachromosomal elements, subsequent transfer, and resolution. However, this hypothesis requires experimental confirmation (319).
Broad-spectrum β-lactamases.
All K. pneumoniae strains, including hvKp, are intrinsically resistant to ampicillin and ticarcillin and are inconsistently susceptible to nitrofurantoin.
Aminoglycoside, trimethoprim-sulfamethoxazole, tetracycline, and fluoroquinolone resistance genes.
The determinants for resistance to aminoglycosides, trimethoprim-sulfamethoxazole, tetracycline, and fluoroquinolones are frequently linked on conjugal or transferable plasmids containing genes for extended-spectrum β-lactamases (ESBLs) and carbapenemase.
ESBLs.
ESBLs (e.g., CTX-M, SHV, and TEM) are modified broad-spectrum β-lactamases that hydrolyze third-generation cephalosporins, aztreonam, and (in some instances) fourth-generation cephalosporins, in addition to the drugs hydrolyzed by broad-spectrum β-lactamases. Gram-negative bacteria that express ESBLs may also possess porin mutations that result in decreased uptake of cephalosporins, β-lactam–β-lactamase inhibitor combinations, and carbapenems, thereby further reducing susceptibility to these agents. hvKp strains possessing ESBLs have been described in a number of studies (320–322). In one investigation from China in which 230 clinical K. pneumoniae isolates from 2013 were studied, 85/230 (37%) were predicted to be hvKp based on the presence of rmpA, and of these, 11 (13%) produced an ESBL (249).
AmpC β-lactamases.
High levels of expression confer resistance to the same substrates as do ESBLs, plus to the cephamycins (e.g., cefoxitin and cefotetan). Some strains of K. pneumoniae, including hvKp isolates, have acquired plasmids containing AmpC β-lactamase genes (322, 323).
Carbapenemases.
Carbapenemases (e.g., KPC [class A], NDM, VIM, and IMP [class B], and OXA [class D]) confer resistance to the same drugs as do ESBLs, plus to cephamycins and carbapenems. Transposon-mediated spread (e.g., Tn4401 for KPC) is also important. An increasing number of reports, primarily but not exclusively from Asia (324–327), have described hvKp strains that have acquired carbapenemases. Acquisition of a KPC is most common (18–20, 22, 91, 324, 328–331), but NDM-1- and OXA-producing isolates also have been described (326, 327, 332–336). It is not uncommon for carbapenemase-producing strains to possess ESBLs as well as multiple additional linked antimicrobial resistance determinants. In addition, carbapenem resistance may be conferred by the combination of an ESBL and porin mutations (e.g., OMPK35/36) (142, 328, 336).
Polymyxin resistance.
Several mechanisms have been identified that confer resistance to polymyins. The recent emergence of the polymyxin resistance gene mcr-1 on a stable, transferable plasmid is extremely concerning since polymyxins (polymyxins B and E [colistin]) currently are a last line of defense against strains that produce metallocarbapenemases (e.g., NDM-1). Unfortunately, this resistance mechanism has been described for hvKp (337). Another mechanism that mediates polymyxin resistance is increased expression of the PhoP-PhoQ-Arn pathway. Activation of PhoP-PhoQ results in overexpression of the arn operon, which results in the addition of cationic groups to the phosphate moieties of lipid A. This, in turn, leads to a decrease in negative charge and decreased activity in polymyxins. Insertion into mgrB, which encodes the PhoP-PhoQ regulatory pathway suppressor MgrB, results in polymyxin resistance (338). This mechanism also has been described for hvKp strains (91, 319).
Tigecycline resistance.
Tigecycline resistance has been reported for an cKp strain that evolved into an hvKP strain via acquisition of a portion of an hvKp virulence plasmid (91). Overexpression of the efflux pump gene acrR and its regulatory gene ramA, which has been associated with tigecycline resistance, was demonstrated.
Effect of Antimicrobial Resistance Genes on hvKp Biofitness
The literature is conflicting on the effect of antimicrobial resistance genes on hvKp biofitness. This may due to the mechanism (autonomous resistance plasmid versus integrated resistance determinants) and the nature (e.g., ESBL versus carbapenemase) of resistance. Reports in the clinical literature of lethal outbreaks due to XDR hvKp strains are highly concerning but uncontrolled (22). Some studies in which hvKp strains were unable to maintain antimicrobial resistance plasmids interpreted this finding as a potential negative effect on biofitness (336). By contrast, an hvKp strain that contained a conjugal plasmid that produced an AmpC β-lactamase demonstrated plasmid stability in the absence of selection and high lethality, similar to the case with the prototypical strain NTUH-K2044 in a mouse infection model (323). A 2014 report described the introduction of a KPC-containing plasmid into an hvKp strain without a loss in resistance to complement-mediated bactericidal activity or lethality in mice (339). A clinical hvKp isolate (strain 3) that was carbapenem resistant due to the combination of ESBL production and decreased OMPK35/36 expression was highly virulent in a mouse lethality assay, but two other hvKp strains that expressed KPC were not (328).
Treatment Options
Antimicrobials.
There are no controlled trials that have assessed the efficacy of various antimicrobials against hvKp infection. This is due, in part, to the inability of clinical microbiology laboratories to differentiate cKp from hvKp strains. The recent identification of biomarkers that can accurately identify hvKp strains should enable future clinical trials (140). This issue has become increasingly important with the ever-increasing incidence of XDR hvKp infections.
Given the potential severity of hvKp infection and its propensity for metastatic spread, the empirical regimen chosen should be predicted to be active pending susceptibility results. This would be predicated to minimize subsequent spread (e.g., endophthalmitis). hvKp strains that are carbapenem resistant are most problematic. Ceftazidime-avibactam, colistin, and tigecycline were active against all 65 carbapenem-resistant hvKp strains tested in vitro, but none possessed a metallocarbapenemase (e.g., NDM) (142). Although colistin and tigecycline have been active in vitro, these agents were not curative in a lethal ICU outbreak due to a KPC2-producing XDR hvKp strain (22), although these patients were immunocompromised and active therapy was not optimally timely in all cases. Once susceptibility results are available, antimicrobial therapy should be deescalated appropriately.
It is important to be cognizant that a number of sites infected by hvKp present increased therapeutic challenges due to the poor penetration of selected antimicrobials. If active based on susceptibility data, for CNS infection, ceftriaxone and meropenem are reliable agents; for prostatic infection fluoroquinolones, trimethoprim-sulfamethoxazole or fosfomycin achieves therapeutic concentrations in this site; for ocular infection, a combination of systemic and intravitreal therapies (e.g., cefazolin, ceftazidime, aminoglycosides, and imipenem) is appropriate. Intraocular steroid treatment has been used (322), but its role, if any, is unclear. Given the rapidly of ocular damage and subsequent loss of vision with hvKp infection, clinical trials are sorely needed to assess various agents and therapeutic approaches, especially with the advent of XDR hvKp strains that severely limit treatment options.
Passive immunization.
The confluence of the expanding development and use of monoclonal antibodies (MAbs) for a variety of medical conditions and the increasing prevalence of XDR and PDR strains have increased interest in antibody-based therapy. Surface exposure and conservation are desirable target characteristics. OMPs are tempting targets due to many having a high degree of conservation. But OMPs may be problematic targets due to shielding by surface polysaccharides (340, 341). Surface polysaccharides are ideally situated and form the basis for the pneumococcal, meningococcal, and Haemophilus influenzae type b vaccines. Experimentally, this approach has been successfully used to treat and prevent hvKp infection with MAbs directed against the K1 capsule (342) and the O-antigen moiety of LPS (192). The challenge for using passive immunization for the treatment of hvKp infection will be overcoming antigenic diversity of the surface polysaccharides (143). Capsule types are more diverse than O-antigen types (143). Successful passive immunization for the treatment of hvKp infection likely will require the availability of multiple capsule or perhaps O-antigen MAbs and a point-of-care test that can rapidly identify the capsule or O-antigen type from the infecting strain.
Phage therapy.
Treatment of bacterial infections with bacteriophage has been used in certain countries in Eastern Europe and the former Soviet Union (343). Although this approach has potential, a variety of scientific and regulatory concerns exist (344). Nonetheless, the increasing incidence of infections due to XDR and PDR bacteria has led to a resurgence of research in this arena. Lin et al. identified a bacteriophage that recognized the K1 capsule (but not other capsule types), was bactericidal against the hvKp strain NTUH-K2044, and was efficacious in a mouse infection model (345). Similar results were obtained for a bacteriophage that recognized the K5 capsule (346). It is unknown whether a bacteriophage can be identified that would recognize all hvKp strains. If not, similar to passive immunization, bacteriophage therapy of hvKp infection will require point-of-care testing for the presence of the bacteriophage receptor in the infecting strain. This area is intriguing but is still in development.
INFECTION CONTROL AND PREVENTION
Reservoirs and Mechanism of Spread
There is a lack of data to guide hvKp infection prevention and control practices beyond standard precautions. The reservoirs and mechanism of spread for hvKp strains have not been established. Pending the generation of hvKp-specific data, utilizing cKp-based data seems reasonable. Potential reservoirs include both the environment and colonized patients. Neonatal ICU incubator water (347) and waste disposal hoppers (348) were recently identified as a potential environmental sources for NDM-1- and KPC-producing K. pneumoniae, respectively. However, it is not known whether these findings are applicable to hvKp. Further, it remains unclear which of these reservoirs, if either, is the most important source for transmission. Data for cKp from a multicenter ICU study demonstrated that cephalosporin-resistant K. pneumoniae appeared to be more easily transmissible than E. coli; this finding heightens concern for spread regardless of the source (349).
Regardless of the mechanism, it is clear that hvKp strains can undergo nosocomial spread. Gu et al. described a lethal outbreak in an ICU due to an XDR hvKP strain (22). Likewise, additional studies are suggestive of nosocomial dissemination (331, 332). In addition, data published by Harada et al. (114) and unpublished data from our group have demonstrated that healthy, close contacts of hvKp-infected patients may be colonized and/or infected with the index hvKp strain. However, presently the relative roles of environmental and person-to-person acquisition are unclear.
Is Enhanced Infection Control Beneficial for Antimicrobial-Sensitive hvKp?
Whether enhanced infection control is beneficial for antimicrobial-resistant hvKp is a critical question that is presently unanswered. It is further complicated by the fact that many hvKp infections may not be recognized, which would preclude institution of appropriate infection control measures if deemed appropriate. Until clinical microbiology laboratories implement testing to identify hvKp, a presumptive or potential diagnosis of hvKp infection relies on an astute physician (Table 1). This task is feasible when a patient from the community presents with typical epidemiologic and clinical features, but it presently is difficult to impossible with health care-associated hvKp infection.
Nonetheless, if a presumptive diagnosis of hvKp infection is made, it seems reasonable to assume that hospitalized patients would be at a greater risk for developing infection once colonized. The frequency remains undefined. Data on which patient groups (e.g., those of particular ethnic backgrounds) or settings (e.g., ICUs), if any, are at greater risk for developing or fostering infection also would be informative, and perhaps these data could be used for targeted interventions. Regardless, when considered from a risk-benefit perspective, the consequences of hvKp infection due to antimicrobial-sensitive strains can be severe even in an otherwise healthy host. It is predicted that consequences would be more significant in patients with comorbidities or various degrees of immunocompromise. Therefore, contact precautions are worthy of consideration. At this time, there is a lack of data to determine if healthy contacts or hospitalized patients exposed to hvKp-infected persons should be empirically treated to decrease the chances of subsequent infection or whether these individuals should be screened for hvKp colonization and, if colonized, preemptively treated.
Infection Control Measures for MDR (ESBL-Producing) and XDR (Carbapenemase-Producing) hvKp
The need for point-of-care testing to diagnose infection due to XDR hvKp would appear to be less pressing since infection control measures for patients infected with antimicrobial-resistant hvKp strains should be equivalent to those employed for other highly drug-resistant pathogens independent of virulence potential. For patients infected with carbapenemase-producing hvKp strains, contact precautions, informing receiving facilities that the patient being transferred is colonized or infected with a carbapenem-resistant isolate, and cleaning of areas that the patient is in contact with on a daily basis are recommended (350). Consideration should also be given to active surveillance for extensively drug-resistant strains and contact screening.
There is an ongoing debate as to whether ESBL-producing Enterobacteriaceae warrant contact precautions, at least in the nonoutbreak setting (351, 352). Similar to considerations for antimicrobial sensitive hvKP, the consequences of infection with MDR-hvKp is increased relative to other Enterobacteriaceae and likely antimicrobial-sensitive hvKp due to decreased treatment options. Therefore, until data become available, it seems reasonable to strongly consider contact precautions and, if feasible, screening of contacts and active surveillance for MDR hvKp, particularly in an outbreak setting.
ACKNOWLEDGMENTS
This work was supported by NIH grants 1R21AI088318 and 1R21AI123558 (Thomas A. Russo) and the Department of Veterans Affairs VA Merit Review (1I01BX000984) (Thomas A. Russo).
We thank the University at Buffalo Health Sciences library staff for their assistance in procuring copies of older publications.
Biographies

Thomas A. Russo (M.D., C.M.) is a Professor of Medicine, Chief of the Division of Infectious Diseases, and a Vice Chair in the Department of Medicine in the Jacobs School of Medicine & Biomedical Sciences at the University at Buffalo. Dr. Russo received his medical degree from McGill University, completed his internship and residency at Harvard-New England Deaconess Hospital, and completed a clinical and research fellowship in infectious diseases at Harvard Medical School and Tufts-New England Medical Center. Following his fellowship, Dr. Russo was a senior staff fellow at the National Institute of Allergy and Infectious Disease’s Laboratory of Clinical Investigation for 5 years before being appointed at the University at Buffalo in 1994. Dr. Russo’s research focus has been on pathogenesis and vaccine and drug development against extraintestinal pathogenic Escherichia coli, Acinetobacter baumannii, and hypervirulent Klebsiella pneumoniae.

Candace M. Marr (D.O.) is a Clinical Associate Professor in the Department of Medicine and the Division of Infectious Diseases in the Jacobs School of Medicine & Biomedical Sciences. She received her medical degree from Lake Erie College of Osteopathic Medicine, summa cum laude. She completed internal medicine residency and infectious disease fellowship at the University at Buffalo, where she was inducted into Alpha Omega Alpha. She is a 2014 recipient of the IDSA Kass grant and a 2013 recipient of the John Latorolla and Marie Mazzio Memorial Award for academic excellence. Her current research efforts pertain to clinical management of biofilm-associated infections, especially of orthopedic hardware. She has presented original research both regionally and nationally and has published multiple articles on Clostridium difficile and hypervirulent Klebsiella pneumoniae.
REFERENCES
- 1.Podschun R, Ullmann U. 1998. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev 11:589–603. doi: 10.1128/CMR.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shon AS, Bajwa RP, Russo TA. 2013. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence 4:107–118. doi: 10.4161/viru.22718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang JL, Chen KY, Fang CT, Hsueh PR, Yang PC, Chang SC. 2005. Changing bacteriology of adult community-acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis 40:915–922. doi: 10.1086/428574. [DOI] [PubMed] [Google Scholar]
- 4.Chung DR, Lee SS, Lee HR, Kim HB, Choi HJ, Eom JS, Kim JS, Choi YH, Lee JS, Chung MH, Kim YS, Lee H, Lee MS, Park CK. 2007. Emerging invasive liver abscess caused by K1 serotype Klebsiella pneumoniae in Korea. J Infect 54:578–583. doi: 10.1016/j.jinf.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Tsai FC, Huang YT, Chang LY, Wang JT. 2008. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis 14:1592–1600. doi: 10.3201/eid1410.071254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin YT, Jeng YY, Chen TL, Fung CP. 2010. Bacteremic community-acquired pneumonia due to Klebsiella pneumoniae: clinical and microbiological characteristics in Taiwan, 2001–2008. BMC Infect Dis 10:307. doi: 10.1186/1471-2334-10-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang WN, Huang CR, Lu CH, Chien CC. 2012. Adult Klebsiella pneumoniae meningitis in Taiwan: an overview. Acta Neurol Taiwan 21:87–96. [PubMed] [Google Scholar]
- 8.Rossi B, Gasperini ML, Leflon-Guibout V, Gioanni A, de Lastours V, Rossi G, Dokmak S, Ronot M, Roux O, Nicolas-Chanoine MH, Fantin B, Lefort A. 2018. Hypervirulent Klebsiella pneumoniae in cryptogenic liver abscesses, Paris, France. Emerg Infect Dis 24:221–229. doi: 10.3201/eid2402.170957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fazili T, Sharngoe C, Endy T, Kiska D, Javaid W, Polhemus M. 2016. Klebsiella pneumoniae liver abscess: an emerging disease. Am J Med Sci 351:297–304. doi: 10.1016/j.amjms.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2013. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR Morb Mortal Wkly Rep 62:165–170. [PMC free article] [PubMed] [Google Scholar]
- 12.Snitkin ES, Zelazny AM, Thomas PJ, Stock F, Henderson DK, Palmore TN, Segre JA. 2012. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 4:148ra116. doi: 10.1126/scitranslmed.3004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A. 2017. Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae—Washoe County, Nevada, 2016. MMWR Morb Mortal Wkly Rep 66:33. doi: 10.15585/mmwr.mm6601a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nataro JP. 2015. Pathogenesis—thoughts from the front line. Microbiol Spectr 3:MBP-0012-2014. doi: 10.1128/microbiolspec.MBP-0012-2014. [DOI] [PubMed] [Google Scholar]
- 15.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. 2004. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med 199:697–705. doi: 10.1084/jem.20030857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catalan-Najera JC, Garza-Ramos U, Barrios-Camacho H. 2017. Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence 8:1111–1123. doi: 10.1080/21505594.2017.1317412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo TA, Olson R, Fang CT, Stoesser N, Miller M, MacDonald U, Hutson A, Barker JH, La Hoz RM, Johnson JR. 2018. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J Clin Microbiol 56:e00776-18. doi: 10.1128/JCM.00776-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei DD, Wan LG, Deng Q, Liu Y. 2016. Emergence of KPC-producing Klebsiella pneumoniae hypervirulent clone of capsular serotype K1 that belongs to sequence type 11 in mainland China. Diagn Microbiol Infect Dis 85:192–194. doi: 10.1016/j.diagmicrobio.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Lu Y, Yao Z, Zong Z. 2018. Carbapenem-resistant hypervirulent Klebsiella pneumoniae of sequence type 36. Antimicrob Agents Chemother 62:e02644-17. doi: 10.1128/AAC.02644-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang R, Lin D, Chan EW, Gu D, Chen GX, Chen S. 2016. Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother 60:709–711. doi: 10.1128/AAC.02173-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L, Tang L, Wang S, Liu Q, Liu Y, Zhang Z, Zhang L, Li Y, Chen W, Wang G, Zhou Y. 2018. Co-location of the blaKPC-2, blaCTX-M-65, rmtB and virulence relevant factors in an IncFII plasmid from a hypermucoviscous Klebsiella pneumoniae isolate. Microb Pathog 124:301–304. doi: 10.1016/j.micpath.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 22.Gu D, Dong N, Zheng Z, Lin D, Huang M, Wang L, Chan EW, Shu L, Yu J, Zhang R, Chen S. 2017. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis 18:37–46. doi: 10.1016/s1473-3099(17)30489-9. [DOI] [PubMed] [Google Scholar]
- 23.Brisse S, Verhoef J. 2001. Phylogenetic diversity of Klebsiella pneumoniae and Klebsiella oxytoca clinical isolates revealed by randomly amplified polymorphic DNA, gyrA and parC genes sequencing and automated ribotyping. Int J Syst Evol Microbiol 51:915–924. doi: 10.1099/00207713-51-3-915. [DOI] [PubMed] [Google Scholar]
- 24.Holt KE, Wertheim H, Zadoks RN, Baker S, Whitehouse CA, Dance D, Jenney A, Connor TR, Hsu LY, Severin J, Brisse S, Cao H, Wilksch J, Gorrie C, Schultz MB, Edwards DJ, Nguyen KV, Nguyen TV, Dao TT, Mensink M, Minh VL, Nhu NT, Schultsz C, Kuntaman K, Newton PN, Moore CE, Strugnell RA, Thomson NR. 2015. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A 112:E3574–E3581. doi: 10.1073/pnas.1501049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long SW, Linson SE, Ojeda Saavedra M, Cantu C, Davis JJ, Brettin T, Olsen RJ. 2017. Whole-genome sequencing of human clinical Klebsiella pneumoniae isolates reveals misidentification and misunderstandings of Klebsiella pneumoniae, Klebsiella variicola, and Klebsiella quasipneumoniae. mSphere 2:e00290-17. doi: 10.1128/mSphereDirect.00290-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maatallah M, Vading M, Kabir MH, Bakhrouf A, Kalin M, Naucler P, Brisse S, Giske CG. 2014. Klebsiella variicola is a frequent cause of bloodstream infection in the Stockholm area, and associated with higher mortality compared to K. pneumoniae. PLoS One 9:e113539. doi: 10.1371/journal.pone.0113539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brisse S, van Himbergen T, Kusters K, Verhoef J. 2004. Development of a rapid identification method for Klebsiella pneumoniae phylogenetic groups and analysis of 420 clinical isolates. Clin Microbiol Infect 10:942–945. doi: 10.1111/j.1469-0691.2004.00973.x. [DOI] [PubMed] [Google Scholar]
- 28.Lam MMC, Wyres KL, Judd LM, Wick RR, Jenney A, Brisse S, Holt KE. 2018. Tracking key virulence loci encoding aerobactin and salmochelin siderophore synthesis in Klebsiella pneumoniae. Genome Med 10:77. doi: 10.1186/s13073-018-0587-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arena F, Henrici De Angelis L, Pieralli F, Di Pilato V, Giani T, Torricelli F, D’Andrea MM, Rossolini GM. 2015. Draft genome sequence of the first hypermucoviscous Klebsiella quasipneumoniae subsp. quasipneumoniae isolate from a bloodstream infection. Genome Announc 3:e00952-15. doi: 10.1128/genomeA.00952-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garza-Ramos U, Silva-Sanchez J, Barrios H, Rodriguez-Medina N, Martinez-Barnetche J, Andrade V. 2015. Draft genome sequence of the first hypermucoviscous Klebsiella variicola clinical isolate. Genome Announc 3:e01352-14. doi: 10.1128/genomeA.01352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu YC, Cheng DL, Lin CL. 1986. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med 146:1913–1916. doi: 10.1001/archinte.1986.00360220057011. [DOI] [PubMed] [Google Scholar]
- 32.Nassif X, Sansonetti PJ. 1986. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun 54:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nassif X, Fournier JM, Arondel J, Sansonetti PJ. 1989. Mucoid phenotype of Klebsiella pneumoniae is a plasmid-encoded virulence factor. Infect Immun 57:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nassif X, Honore N, Vasselon T, Cole ST, Sansonetti PJ. 1989. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence-plasmid genes of Klebsiella pneumoniae. Mol Microbiol 3:1349–1359. doi: 10.1111/j.1365-2958.1989.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 35.Yu WL, Ko WC, Cheng KC, Lee HC, Ke DS, Lee CC, Fung CP, Chuang YC. 2006. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis 42:1351–1358. doi: 10.1086/503420. [DOI] [PubMed] [Google Scholar]
- 36.Fang FC, Sandler N, Libby SJ. 2005. Liver abscess caused by magA+ Klebsiella pneumoniae in North America. J Clin Microbiol 43:991–992. doi: 10.1128/JCM.43.2.991-992.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nadasy KA, Domiati-Saad R, Tribble MA. 2007. Invasive Klebsiella pneumoniae syndrome in North America. Clin Infect Dis 45:e25–e28. doi: 10.1086/519424. [DOI] [PubMed] [Google Scholar]
- 38.Siu LK, Fung CP, Chang FY, Lee N, Yeh KM, Koh TH, Ip M. 2011. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol 49:3761–3765. doi: 10.1128/JCM.00977-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomakova DK, Hsiao CB, Beanan JM, Olson R, Macdonald U, Keynan Y, Russo TA. 2012. Clinical and phenotypic differences between classic and hypervirulent Klebsiella pneumonia [sic]: an emerging and under-recognized pathogenic variant. Eur J Clin Microbiol Infect Dis 31:981–989. doi: 10.1007/s10096-011-1396-6. [DOI] [PubMed] [Google Scholar]
- 40.Holmes RB. 1956. Friedlander’s pneumonia. Am J Roentgenol Radium Ther Nucl Med 75:728–745. [PubMed] [Google Scholar]
- 41.Oseasohn R. 1962. Friedlander’s pneumonia. Med Sci 11:1000–1008. [PubMed] [Google Scholar]
- 42.Lampe WT., II. 1964. Klebsiella pneumonia: a review of forty-five cases and re-evaluation of the incidence and antibiotic sensitivities. Dis Chest 46:599–606. doi: 10.1378/chest.46.5.599. [DOI] [PubMed] [Google Scholar]
- 43.Friedlander C. 1882. Ueber die Schizomyceten bei der acuten fibrosen Pneumonie. Virchows Arch Pathol Anat Physiol Klin Med 87:319–324. [Google Scholar]
- 44.Bensley EH. 1932. A case of Friedlander’s pneumonia. Can Med Assoc J 26:681–684. [PMC free article] [PubMed] [Google Scholar]
- 45.Julianelle LA. 1941. The pneumonia of Friedlander’s bacillus. Ann Intern Med 15:190–206. [Google Scholar]
- 46.Jervey LP., Jr 1957. The treatment of acute Friedlaender’s bacillus pneumonia; a continuing problem. AMA Arch Intern Med 99:1–7. doi: 10.1001/archinte.1957.00260010003001. [DOI] [PubMed] [Google Scholar]
- 47.Bullowa JGM, Chess J, Friedman NB. 1937. Pneumonia due to bacillus Friedlanderi. A report on forty-one patients with consideration of specific serum therapy. Arch Intern Med (Chic) 60:735–752. doi: 10.1001/archinte.1937.00180050002001. [DOI] [Google Scholar]
- 48.Solomon S. 1937. Primary Friedlander pneumonia. JAMA 108:937–947. doi: 10.1001/jama.1937.02780120007002. [DOI] [Google Scholar]
- 49.Hyde L, Hyde B. 1943. Primary Friedlander pneumonia. Am J Med Sci 205:660–675. doi: 10.1097/00000441-194305000-00004. [DOI] [Google Scholar]
- 50.Solomon S. 1940. Chronic Friedlander infections of the lung. JAMA 115:1527–1536. doi: 10.1001/jama.1940.02810440019005. [DOI] [Google Scholar]
- 51.Erasmus LD. 1956. Friedlander bacillus infection of the lung. Q J Med 100:507–521. doi: 10.1093/oxfordjournals.qjmed.a066764. [DOI] [PubMed] [Google Scholar]
- 52.Perlman E, Bullowa J. 1941. Primary bacillus Friedlander (Klebsiella pneumoniae) pneumonia. Arch Intern Med (Chic) 67:907–920. doi: 10.1001/archinte.1941.00200050015002. [DOI] [Google Scholar]
- 53.Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. 2012. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis 12:881–887. doi: 10.1016/S1473-3099(12)70205-0. [DOI] [PubMed] [Google Scholar]
- 54.Fung CP, Chang FY, Lee SC, Hu BS, Kuo BI, Liu CY, Ho M, Siu LK. 2002. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut 50:420–424. doi: 10.1136/gut.50.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baerh G, Shwartzman G, Greenspan EB. 1933. The role of Bacillus Frielander in infection. Trans Assoc Am Phys 18:353–354. [Google Scholar]
- 56.Thompson AJ, Williams EB, Williams ED, Anderson JM. 1952. Klebsiella pneumoniae meningitis; review of the literature and report of a case with bacteremia and pneumonia, with recovery. AMA Arch Intern Med 89:405–420. doi: 10.1001/archinte.1952.00240030054006. [DOI] [PubMed] [Google Scholar]
- 57.Julianelle LA. 1930. The distribution of Friedlander’s bacilli of different types. J Exp Med 52:539–545. doi: 10.1084/jem.52.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ransmeier JC, Major JW. 1943. Friedlander’s bacillus septicemia and meningitis: report of a case and autopsy, with an analysis of twenty-nine cases collected from the literature. Arch Intern Med 72:319–328. doi: 10.1001/archinte.1943.00210090020002. [DOI] [Google Scholar]
- 59.Anderson G, Yount E. 1953. Friedlander’s meningitis: report of a case complicating Friedlander’s pneumonia with recovery. N C Med J 14:578–581. [PubMed] [Google Scholar]
- 60.Findlay M, Skapinker S. 1953. Further observations on Friedlander’s osteomyelitis of long bones. Br J Radiol 26:358–361. doi: 10.1259/0007-1285-26-307-358. [DOI] [PubMed] [Google Scholar]
- 61.Muskat DA, Findlay M. 1951. Osteomyelitis due to Friedlander’s bacillus. Lancet 1:1154–1156. [DOI] [PubMed] [Google Scholar]
- 62.Cheng HY, Chen YS, Wu CY, Chang HY, Lai YC, Peng HL. 2010. RmpA regulation of capsular polysaccharide biosynthesis in Klebsiella pneumoniae CG43. J Bacteriol 192:3144–3158. doi: 10.1128/JB.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Julianelle LA. 1926. A Biological classification of encapsulatus pneumoniae (Friedlander’s bacillus). J Exp Med 44:113–128. doi: 10.1084/jem.44.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards PR, Fife MA. 1952. Capsule types of Klebsiella. J Infect Dis 91:92–104. doi: 10.1093/infdis/91.1.92. [DOI] [PubMed] [Google Scholar]
- 65.Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. 2008. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non-K1/K2 serotypes. Diagn Microbiol Infect Dis 62:1–6. doi: 10.1016/j.diagmicrobio.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 66.Brisse S, Fevre C, Passet V, Issenhuth-Jeanjean S, Tournebize R, Diancourt L, Grimont P. 2009. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One 4:e4982. doi: 10.1371/journal.pone.0004982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. 1991. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med 151:1557–1559. doi: 10.1001/archinte.1991.00400080059010. [DOI] [PubMed] [Google Scholar]
- 68.Han SH. 1995. Review of hepatic abscess from Klebsiella pneumoniae. An association with diabetes mellitus and septic endophthalmitis. West J Med 162:220–224. [PMC free article] [PubMed] [Google Scholar]
- 69.Wang JH, Liu YC, Lee SS, Yen MY, Chen YS, Wang JH, Wann SR, Lin HH. 1998. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin Infect Dis 26:1434–1438. doi: 10.1086/516369. [DOI] [PubMed] [Google Scholar]
- 70.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, Casellas JM, Goossens H, Mulazimoglu L, Trenholme G, Klugman KP, McCormack JG, Yu VL. 2002. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis 8:160–166. doi: 10.3201/eid0802.010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsay RW, Siu LK, Fung CP, Chang FY. 2002. Characteristics of bacteremia between community-acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community-acquired infection. Arch Intern Med 162:1021–1027. doi: 10.1001/archinte.162.9.1021. [DOI] [PubMed] [Google Scholar]
- 72.Huang CR, Lu CH, Chang HW, Lee PY, Lin MW, Chang WN. 2002. Community-acquired spontaneous bacterial meningitis in adult diabetic patients: an analysis of clinical characteristics and prognostic factors. Infection 30:346–350. doi: 10.1007/s15010-002-3010-4. [DOI] [PubMed] [Google Scholar]
- 73.Chen SC, Yen CH, Tsao SM, Huang CC, Chen CC, Lee MC, Bell WR. 2005. Comparison of pyogenic liver abscesses of biliary and cryptogenic origin. An eight-year analysis in a university hospital. Swiss Med Wkly 135:344–351. [DOI] [PubMed] [Google Scholar]
- 74.Kim JK, Chung DR, Wie SH, Yoo JH, Park SW. 2009. Risk factor analysis of invasive liver abscess caused by the K1 serotype Klebsiella pneumoniae. Eur J Clin Microbiol Infect Dis 28:109–111. doi: 10.1007/s10096-008-0595-2. [DOI] [PubMed] [Google Scholar]
- 75.Yang CC, Yen CH, Ho MW, Wang JH. 2004. Comparison of pyogenic liver abscess caused by non-Klebsiella pneumoniae and Klebsiella pneumoniae. J Microbiol Immunol Infect 37:176–184. [PubMed] [Google Scholar]
- 76.Lam MMC, Wyres KL, Duchene S, Wick RR, Judd LM, Gan YH, Hoh CH, Archuleta S, Molton JS, Kalimuddin S, Koh TH, Passet V, Brisse S, Holt KE. 2018. Population genomics of hypervirulent Klebsiella pneumoniae clonal-group 23 reveals early emergence and rapid global dissemination. Nat Commun 9:2703. doi: 10.1038/s41467-018-05114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turton JF, Englender H, Gabriel SN, Turton SE, Kaufmann ME, Pitt TL. 2007. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J Med Microbiol 56:593–597. doi: 10.1099/jmm.0.46964-0. [DOI] [PubMed] [Google Scholar]
- 78.Ye M, Tu J, Jiang J, Bi Y, You W, Zhang Y, Ren J, Zhu T, Cao Z, Yu Z, Shao C, Shen Z, Ding B, Yuan J, Zhao X, Guo Q, Xu X, Huang J, Wang M. 2016. Clinical and genomic analysis of liver abscess-causing Klebsiella pneumoniae identifies new liver abscess-associated virulence genes. Front Cell Infect Microbiol 6:165. doi: 10.3389/fcimb.2016.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Luo Y, Wang Y, Ye L, Yang J. 2014. Molecular epidemiology and virulence factors of pyogenic liver abscess causing Klebsiella pneumoniae in China. Clin Microbiol Infect 20:O818–O824. doi: 10.1111/1469-0691.12664. [DOI] [PubMed] [Google Scholar]
- 80.Liao CH, Huang YT, Chang CY, Hsu HS, Hsueh PR. 2014. Capsular serotypes and multilocus sequence types of bacteremic Klebsiella pneumoniae isolates associated with different types of infections. Eur J Clin Microbiol Infect Dis 33:365–369. doi: 10.1007/s10096-013-1964-z. [DOI] [PubMed] [Google Scholar]
- 81.Lee IR, Molton JS, Wyres KL, Gorrie C, Wong J, Hoh CH, Teo J, Kalimuddin S, Lye DC, Archuleta S, Holt KE, Gan YH. 2016. Differential host susceptibility and bacterial virulence factors driving Klebsiella liver abscess in an ethnically diverse population. Sci Rep 6:29316. doi: 10.1038/srep29316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qu TT, Zhou JC, Jiang Y, Shi KR, Li B, Shen P, Wei ZQ, Yu YS. 2015. Clinical and microbiological characteristics of Klebsiella pneumoniae liver abscess in East China. BMC Infect Dis 15:161. doi: 10.1186/s12879-015-0899-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL. 2004. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene 337:189–198. doi: 10.1016/j.gene.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 84.Wu KM, Li LH, Yan JJ, Tsao N, Liao TL, Tsai HC, Fung CP, Chen HJ, Liu YM, Wang JT, Fang CT, Chang SC, Shu HY, Liu TT, Chen YT, Shiau YR, Lauderdale TL, Su IJ, Kirby R, Tsai SF. 2009. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH-K2044, a strain causing liver abscess and meningitis. J Bacteriol 191:4492–4501. doi: 10.1128/JB.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tang HL, Chiang MK, Liou WJ, Chen YT, Peng HL, Chiou CS, Liu KS, Lu MC, Tung KC, Lai YC. 2010. Correlation between Klebsiella pneumoniae carrying pLVPK-derived loci and abscess formation. Eur J Clin Microbiol Infect Dis 29:689–698. doi: 10.1007/s10096-010-0915-1. [DOI] [PubMed] [Google Scholar]
- 86.Russo TA, Olson R, Macdonald U, Metzger D, Maltese LM, Drake EJ, Gulick AM. 2014. Aerobactin mediates virulence and accounts for increased siderophore production under iron-limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae. Infect Immun 82:2356–2367. doi: 10.1128/IAI.01667-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA. 2015. Aerobactin, but not yersiniabactin, salmochelin and enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun 83:3325–3333. doi: 10.1128/IAI.00430-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bulger J, MacDonald U, Olson R, Beanan J, Russo TA. 2017. Metabolite transporter PEG344 is required for full virulence of hypervirulent Klebsiella pneumoniae strain hvKP1 after pulmonary but not subcutaneous challenge. Infect Immun 85:e00093-17. doi: 10.1128/IAI.00093-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lery LM, Frangeul L, Tomas A, Passet V, Almeida AS, Bialek-Davenet S, Barbe V, Bengoechea JA, Sansonetti P, Brisse S, Tournebize R. 2014. Comparative analysis of Klebsiella pneumoniae genomes identifies a phospholipase D family protein as a novel virulence factor. BMC Biol 12:41. doi: 10.1186/1741-7007-12-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Struve C, Roe CC, Stegger M, Stahlhut SG, Hansen DS, Engelthaler DM, Andersen PS, Driebe EM, Keim P, Krogfelt KA. 2015. Mapping the evolution of hypervirulent Klebsiella pneumoniae. mBio 6:e00630. doi: 10.1128/mBio.00630-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang YH, Chou SH, Liang SW, Ni CE, Lin YT, Huang YW, Yang TC. 2018. Emergence of an XDR and carbapenemase-producing hypervirulent Klebsiella pneumoniae strain in Taiwan. J Antimicrob Chemother 73:2039–2046. doi: 10.1093/jac/dky164. [DOI] [PubMed] [Google Scholar]
- 92.Marcoleta AE, Berrios-Pasten C, Nunez G, Monasterio O, Lagos R. 2016. Klebsiella pneumoniae asparagine tDNAs are integration hotspots for different genomic islands encoding microcin E492 production determinants and other putative virulence factors present in hypervirulent strains. Front Microbiol 7:849. doi: 10.3389/fmicb.2016.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin TL, Lee CZ, Hsieh PF, Tsai SF, Wang JT. 2008. Characterization of integrative and conjugative element ICEKp1-associated genomic heterogeneity in a Klebsiella pneumoniae strain isolated from a primary liver abscess. J Bacteriol 190:515–526. doi: 10.1128/JB.01219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lai YC, Lin AC, Chiang MK, Dai YH, Hsu CC, Lu MC, Liau CY, Chen YT. 2014. Genotoxic Klebsiella pneumoniae in Taiwan. PLoS One 9:e96292. doi: 10.1371/journal.pone.0096292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lam MMC, Wick RR, Wyres KL, Gorrie CL, Judd LM, Jenney AWJ, Brisse S, Holt KE. 9 September 2018. Genetic diversity, mobilisation and spread of the yersiniabactin-encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microb Genom doi: 10.1099/mgen.0.000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bachman MA, Oyler JE, Burns SH, Caza M, Lepine F, Dozois CM, Weiser JN. 2011. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun 79:3309–3316. doi: 10.1128/IAI.05114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu MC, Chen YT, Chiang MK, Wang YC, Hsiao PY, Huang YJ, Lin CT, Cheng CC, Liang CL, Lai YC. 2017. Colibactin contributes to the hypervirulence of pks(+) K1 CC23 Klebsiella pneumoniae in mouse meningitis infections. Front Cell Infect Microbiol 7:103. doi: 10.3389/fcimb.2017.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bialek-Davenet S, Criscuolo A, Ailloud F, Passet V, Jones L, Delannoy-Vieillard A-S, Garin B, Le Hello S, Arlet G, Nicolas-Chanoine M-H, Decré D, Brisse S. 2014. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis 20:1812–1820. doi: 10.3201/eid2011.140206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL, Chang SC. 2007. Klebsiella pneumoniae genotype K1: an emerging pathogen that causes septic ocular or central nervous system complications from pyogenic liver abscess. Clin Infect Dis 45:284–293. doi: 10.1086/519262. [DOI] [PubMed] [Google Scholar]
- 100.Guo C, Yang X, Wu Y, Yang H, Han Y, Yang R, Hu L, Cui Y, Zhou D. 2015. MLST-based inference of genetic diversity and population structure of clinical Klebsiella pneumoniae, China. Sci Rep 5:7612. doi: 10.1038/srep07612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Turton JF, Payne Z, Micah K, Turton JA. 2018. Capsular type K54, clonal group 29 and virulence plasmids: an analysis of K54 and non-K54 closely related isolates of Klebsiella pneumoniae. Epidemiol Infect 146:1813–1823. doi: 10.1017/S0950268818001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen L, Mathema B, Pitout JD, DeLeo FR, Kreiswirth BN. 2014. Epidemic Klebsiella pneumoniae ST258 is a hybrid strain. mBio 5:e01355-14. doi: 10.1128/mBio.01355-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen YT, Lai YC, Tan MC, Hsieh LY, Wang JT, Shiau YR, Wang HY, Lin AC, Lai JF, Huang IW, Lauderdale TL. 2017. Prevalence and characteristics of pks genotoxin gene cluster-positive clinical Klebsiella pneumoniae isolates in Taiwan. Sci Rep 7:43120. doi: 10.1038/srep43120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roe WD, Rogers L, Pinpimai K, Dittmer K, Marshall J, Chilvers BL. 2015. Septicaemia and meningitis caused by infection of New Zealand sea lion pups with a hypermucoviscous strain of Klebsiella pneumoniae. Vet Microbiol 176:301–308. doi: 10.1016/j.vetmic.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 105.Jang S, Wheeler L, Carey RB, Jensen B, Crandall CM, Schrader KN, Jessup D, Colegrove K, Gulland FM. 2010. Pleuritis and suppurative pneumonia associated with a hypermucoviscosity phenotype of Klebsiella pneumoniae in California sea lions (Zalophus californianus). Vet Microbiol 141:174–177. doi: 10.1016/j.vetmic.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 106.Pinpimai K, Roe WD, Biggs PJ, Dittmer KE. 2018. Draft whole-genome sequences of seven isolates of Klebsiella pneumoniae from New Zealand sea lions. Microbiol Resour Announc 7:e01270-18. doi: 10.1128/MRA.01270-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Twenhafel NA, Whitehouse CA, Stevens EL, Hottel HE, Foster CD, Gamble S, Abbott S, Janda JM, Kreiselmeier N, Steele KE. 2008. Multisystemic abscesses in African green monkeys (Chlorocebus aethiops) with invasive Klebsiella pneumoniae—identification of the hypermucoviscosity phenotype. Vet Pathol 45:226–231. doi: 10.1354/vp.45-2-226. [DOI] [PubMed] [Google Scholar]
- 108.Osman KM, Hassan HM, Orabi A, Abdelhafez AS. 2014. Phenotypic, antimicrobial susceptibility profile and virulence factors of Klebsiella pneumoniae isolated from buffalo and cow mastitic milk. Pathog Glob Health 108:191–199. doi: 10.1179/2047773214Y.0000000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cheng F, Li Z, Lan S, Liu W, Li X, Zhou Z, Song Z, Wu J, Zhang M, Shan W. 2018. Characterization of Klebsiella pneumoniae associated with cattle infections in southwest China using multi-locus sequence typing (MLST), antibiotic resistance and virulence-associated gene profile analysis. Braz J Microbiol 49(Suppl 1):93–100. doi: 10.1016/j.bjm.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bagley ST. 1985. Habitat association of Klebsiella species. Infect Control 6:52–58. doi: 10.1017/S0195941700062603. [DOI] [PubMed] [Google Scholar]
- 111.Podschun R, Pietsch S, Holler C, Ullmann U. 2001. Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl Environ Microbiol 67:3325–3327. doi: 10.1128/AEM.67.7.3325-3327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin RM, Cao J, Brisse S, Passet V, Wu W, Zhao L, Malani PN, Rao K, Bachman MA. 2016. Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 1:e00261-16. doi: 10.1128/mSphere.00261-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chung DR, Lee H, Park MH, Jung SI, Chang HH, Kim YS, Son JS, Moon C, Kwon KT, Ryu SY, Shin SY, Ko KS, Kang CI, Peck KR, Song JH. 2012. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis 31:481–486. doi: 10.1007/s10096-011-1334-7. [DOI] [PubMed] [Google Scholar]
- 114.Harada S, Tateda K, Mitsui H, Hattori Y, Okubo M, Kimura S, Sekigawa K, Kobayashi K, Hashimoto N, Itoyama S, Nakai T, Suzuki T, Ishii Y, Yamaguchi K. 2011. Familial spread of a virulent clone of Klebsiella pneumoniae causing primary liver abscess. J Clin Microbiol 49:2354–2356. doi: 10.1128/JCM.00034-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lin YT, Siu LK, Lin JC, Chen TL, Tseng CP, Yeh KM, Chang FY, Fung CP. 2012. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol 12:13. doi: 10.1186/1471-2180-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fung CP, Lin YT, Lin JC, Chen TL, Yeh KM, Chang FY, Chuang HC, Wu HS, Tseng CP, Siu LK. 2012. Klebsiella pneumoniae in gastrointestinal tract and pyogenic liver abscess. Emerg Infect Dis 18:1322–1325. doi: 10.3201/eid1808.111053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gorrie CL, Mirceta M, Wick RR, Edwards DJ, Thomson NR, Strugnell RA, Pratt NF, Garlick JS, Watson KM, Pilcher DV, McGloughlin SA, Spelman DW, Jenney AWJ, Holt KE. 2017. Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin Infect Dis 65:208–215. doi: 10.1093/cid/cix270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Thom BT. 1970. Klebsiella in faeces. Lancet ii:1033. [DOI] [PubMed] [Google Scholar]
- 119.Lima AB, de Oliveira Leao LS, Oliveira LS, Pimenta FC. 2010. Nasopharyngeal Gram-negative bacilli colonization in Brazilian children attending day-care centers. Braz J Microbiol 41:24–27. doi: 10.1590/S1517-83822010000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dao TT, Liebenthal D, Tran TK, Ngoc Thi Vu B, Ngoc Thi Nguyen D, Thi Tran HK, Thi Nguyen CK, Thi Vu HL, Fox A, Horby P, Van Nguyen K, Wertheim HF. 2014. Klebsiella pneumoniae oropharyngeal carriage in rural and urban Vietnam and the effect of alcohol consumption. PLoS One 9:e91999. doi: 10.1371/journal.pone.0091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Farida H, Severin JA, Gasem MH, Keuter M, van den Broek P, Hermans PW, Wahyono H, Verbrugh HA. 2013. Nasopharyngeal carriage of Klebsiella pneumoniae and other Gram-negative bacilli in pneumonia-prone age groups in Semarang, Indonesia. J Clin Microbiol 51:1614–1616. doi: 10.1128/JCM.00589-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Haryani Y, Noorzaleha AS, Fatimah AB, Noorjahan BA, Patrick GB, Shamsinar AT, Laila RAS, Son R. 2007. Incidence of Klebsiella pneumoniae in street foods sold in Malaysia and their characterization by antibiotic resistance, plasmid profiling, and RAPD-PCR analysis. Food Control 18:847–853. doi: 10.1016/j.foodcont.2006.04.009. [DOI] [Google Scholar]
- 123.Farida H, Gasem MH, Suryanto A, Keuter M, Zulkarnain N, Satoto B, van der Eijk AA, Djokomoeljanto R, Wahyono H, Verbrugh HA, Severin JA, van den Broek PJ. 2015. Viruses and Gram-negative bacilli dominate the etiology of community-acquired pneumonia in Indonesia, a cohort study. Int J Infect Dis 38:101–107. doi: 10.1016/j.ijid.2015.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kloos WE, Musselwhite MS. 1975. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol 30:381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lin YT, Wang YP, Wang FD, Fung CP. 2015. Community-onset Klebsiella pneumoniae pneumonia in Taiwan: clinical features of the disease and associated microbiological characteristics of isolates from pneumonia and nasopharynx. Front Microbiol 9:122. doi: 10.3389/fmicb.2015.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Filius PM, Gyssens IC, Kershof IM, Roovers PJ, Ott A, Vulto AG, Verbrugh HA, Endtz HP. 2005. Colonization and resistance dynamics of gram-negative bacteria in patients during and after hospitalization. Antimicrob Agents Chemother 49:2879–2886. doi: 10.1128/AAC.49.7.2879-2886.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rose HD, Babcock JB. 1975. Colonization of intensive care unit patients with gram-negative bacilli. Am J Epidemiol 101:495–501. doi: 10.1093/oxfordjournals.aje.a112120. [DOI] [PubMed] [Google Scholar]
- 128.Hayden MK, Lin MY, Lolans K, Weiner S, Blom D, Moore NM, Fogg L, Henry D, Lyles R, Thurlow C, Sikka M, Hines D, Weinstein RA. 2015. Prevention of colonization and infection by Klebsiella pneumoniae carbapenemase-producing Enterobacteriaceae in long-term acute-care hospitals. Clin Infect Dis 60:1153–1161. doi: 10.1093/cid/ciu1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Snitkin ES, Won S, Pirani A, Lapp Z, Weinstein RA, Lolans K, Hayden MK. 2017. Integrated genomic and interfacility patient-transfer data reveal the transmission pathways of multidrug-resistant Klebsiella pneumoniae in a regional outbreak. Sci Transl Med 9:eaan0093. doi: 10.1126/scitranslmed.aan0093. [DOI] [PubMed] [Google Scholar]
- 130.Skally M, Duffy F, Burns K, Doyle D, Foley S, Thomas T, Collins C, Smyth E, Turton J, Humphreys H. 2014. What may be lurking in the hospital undergrowth? Inapparent cross-transmission of extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. J Hosp Infect 88:156–161. doi: 10.1016/j.jhin.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 131.Gastmeier P, Vonberg RP. 2014. Klebsiella spp. in endoscopy-associated infections: we may only be seeing the tip of the iceberg. Infection 42:15–21. doi: 10.1007/s15010-013-0544-6. [DOI] [PubMed] [Google Scholar]
- 132.Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. 2006. Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia. J Intern Med 259:606–614. doi: 10.1111/j.1365-2796.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 133.Ikeda M, Mizoguchi M, Oshida Y, Tatsuno K, Saito R, Okazaki M, Okugawa S, Moriya K. 2018. Clinical and microbiological characteristics and occurrence of Klebsiella pneumoniae infection in Japan. Int J Gen Med 11:293–299. doi: 10.2147/IJGM.S166940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Selden R, Lee S, Wang WL, Bennett JV, Eickhoff TC. 1971. Nosocomial Klebsiella infections: intestinal colonization as a reservoir. Ann Intern Med 74:657–664. doi: 10.7326/0003-4819-74-5-657. [DOI] [PubMed] [Google Scholar]
- 135.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. 2009. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 136.Maragakis LL, Perencevich EN, Cosgrove SE. 2008. Clinical and economic burden of antimicrobial resistance. Expert Rev Anti Infect Ther 6:751–763. doi: 10.1586/14787210.6.5.751. [DOI] [PubMed] [Google Scholar]
- 137.Lee SS, Chen YS, Tsai HC, Wann SR, Lin HH, Huang CK, Liu YC. 2008. Predictors of septic metastatic infection and mortality among patients with Klebsiella pneumoniae liver abscess. Clin Infect Dis 47:642–650. doi: 10.1086/590932. [DOI] [PubMed] [Google Scholar]
- 138.Liu BT, Zhang XY, Wan SW, Hao JJ, Jiang RD, Song FJ. 2018. Characteristics of carbapenem-resistant Enterobacteriaceae in ready-to-eat vegetables in China. Front Microbiol 9:1147. doi: 10.3389/fmicb.2018.01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cubero M, Grau I, Tubau F, Pallares R, Dominguez MA, Linares J, Ardanuy C. 2016. Hypervirulent Klebsiella pneumoniae clones causing bacteraemia in adults in a teaching hospital in Barcelona, Spain (2007–2013). Clin Microbiol Infect 22:154–160. doi: 10.1016/j.cmi.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 140.Harada S, Doi Y. 2018. Hypervirulent Klebsiella pneumoniae: a call for consensus definition and international collaboration. J Clin Microbiol 56:e00959-18. doi: 10.1128/JCM.00959-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Liu Y, Liu PP, Wang LH, Wei DD, Wan LG, Zhang W. 2017. Capsular polysaccharide types and virulence-related traits of epidemic KPC-producing Klebsiella pneumoniae isolates in a Chinese university hospital. Microb Drug Resist 23:901–907. doi: 10.1089/mdr.2016.0222. [DOI] [PubMed] [Google Scholar]
- 142.Yu F, Lv J, Niu S, Du H, Tang YW, Bonomo RA, Kreiswirth BN, Chen L. 2018. In vitro activity of ceftazidime-avibactam against carbapenem-resistant and hypervirulent Klebsiella pneumoniae isolates. Antimicrob Agents Chemother 62:e01031-18. doi: 10.1128/AAC.01031-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Follador R, Heinz E, Wyres KL, Ellington MJ, Kowarik M, Holt KE, Thomson NR. 2016. The diversity of Klebsiella pneumoniae surface polysaccharides. Microb Genom 2:e000073. doi: 10.1099/mgen.0.000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sarris PF, Zoumadakis C, Panopoulos NJ, Scoulica EV. 2011. Distribution of the putative type VI secretion system core genes in Klebsiella spp. Infect Genet Evol 11:157–166. doi: 10.1016/j.meegid.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 145.Hsieh PF, Lin HH, Lin TL, Wang JT. 2010. CadC regulates cad and tdc operons in response to gastrointestinal stresses and enhances intestinal colonization of Klebsiella pneumoniae. J Infect Dis 202:52–64. doi: 10.1086/653079. [DOI] [PubMed] [Google Scholar]
- 146.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Martin RM, Bachman MA. 2018. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol 8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mills G, Dumigan A, Kidd T, Hobley L, Bengoechea JA. 2017. Identification and characterization of two Klebsiella pneumoniae lpxL lipid A late acyltransferases and their role in virulence. Infect Immun 85:e00068-17. doi: 10.1128/IAI.00068-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pan YJ, Lin TL, Hsu CR, Wang JT. 2011. Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae. Infect Immun 79:997–1006. doi: 10.1128/IAI.00906-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Martin RM, Cao J, Wu W, Zhao L, Manthei DM, Pirani A, Snitkin E, Malani PN, Rao K, Bachman MA. 2018. Identification of pathogenicity-associated loci in Klebsiella pneumoniae from hospitalized patients. mSystems 3:e00015-18. doi: 10.1128/mSystems.00015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bachman MA, Breen P, Deornellas V, Mu Q, Zhao L, Wu W, Cavalcoli JD, Mobley HL. 2015. Genome-wide identification of Klebsiella pneumoniae fitness genes during lung infection. mBio 6:e00775. doi: 10.1128/mBio.00775-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nougayrede JP, Homburg S, Taieb F, Boury M, Brzuszkiewicz E, Gottschalk G, Buchrieser C, Hacker J, Dobrindt U, Oswald E. 2006. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313:848–851. doi: 10.1126/science.1127059. [DOI] [PubMed] [Google Scholar]
- 153.Putze J, Hennequin C, Nougayrede JP, Zhang W, Homburg S, Karch H, Bringer MA, Fayolle C, Carniel E, Rabsch W, Oelschlaeger TA, Oswald E, Forestier C, Hacker J, Dobrindt U. 2009. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect Immun 77:4696–4703. doi: 10.1128/IAI.00522-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Raisch J, Buc E, Bonnet M, Sauvanet P, Vazeille E, de Vallee A, Dechelotte P, Darcha C, Pezet D, Bonnet R, Bringer MA, Darfeuille-Michaud A. 2014. Colon cancer-associated B2 Escherichia coli colonize gut mucosa and promote cell proliferation. World J Gastroenterol 20:6560–6572. doi: 10.3748/wjg.v20.i21.6560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.de Lorenzo V. 1984. Isolation and characterization of microcin E492 from Klebsiella pneumoniae. Arch Microbiol 139:72–75. doi: 10.1007/BF00692715. [DOI] [PubMed] [Google Scholar]
- 156.Lagos R, Baeza M, Corsini G, Hetz C, Strahsburger E, Castillo JA, Vergara C, Monasterio O. 2001. Structure, organization and characterization of the gene cluster involved in the production of microcin E492, a channel-forming bacteriocin. Mol Microbiol 42:229–243. [DOI] [PubMed] [Google Scholar]
- 157.Tu YC, Lu MC, Chiang MK, Huang SP, Peng HL, Chang HY, Jan MS, Lai YC. 2009. Genetic requirements for Klebsiella pneumoniae-induced liver abscess in an oral infection model. Infect Immun 77:2657–2671. doi: 10.1128/IAI.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lin CT, Huang TY, Liang WC, Peng HL. 2006. Homologous response regulators KvgA, KvhA and KvhR regulate the synthesis of capsular polysaccharide in Klebsiella pneumoniae CG43 in a coordinated manner. J Biochem 140:429–438. doi: 10.1093/jb/mvj168. [DOI] [PubMed] [Google Scholar]
- 159.Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT. 2008. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis 197:1717–1727. doi: 10.1086/588383. [DOI] [PubMed] [Google Scholar]
- 160.Ma LC, Fang CT, Lee CZ, Shun CT, Wang JT. 2005. Genomic heterogeneity in Klebsiella pneumoniae strains is associated with primary pyogenic liver abscess and metastatic infection. J Infect Dis 192:117–128. doi: 10.1086/430619. [DOI] [PubMed] [Google Scholar]
- 161.Chou HC, Lee CZ, Ma LC, Fang CT, Chang SC, Wang JT. 2004. Isolation of a chromosomal region of Klebsiella pneumoniae associated with allantoin metabolism and liver infection. Infect Immun 72:3783–3792. doi: 10.1128/IAI.72.7.3783-3792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hsu CR, Chang IW, Hsieh PF, Lin TL, Liu PY, Huang CH, Li KT, Wang JT. 2019. A novel role for the Klebsiella pneumoniae Sap (sensitivity to antimicrobial peptides) transporter in intestinal cell interactions, innate immune responses, liver abscess, and virulence. J Infect Dis 219:1294–1306. doi: 10.1093/infdis/jiy615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu MC, Lin TL, Hsieh PF, Yang HC, Wang JT. 2011. Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS One 6:e23500. doi: 10.1371/journal.pone.0023500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wu MC, Chen YC, Lin TL, Hsieh PF, Wang JT. 2012. Cellobiose-specific phosphotransferase system of Klebsiella pneumoniae and its importance in biofilm formation and virulence. Infect Immun 80:2464–2472. doi: 10.1128/IAI.06247-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Favre-Bonte S, Licht TR, Forestier C, Krogfelt KA. 1999. Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice. Infect Immun 67:6152–6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Qu K, Liu C, Wang ZX, Tian F, Wei JC, Tai MH, Zhou L, Meng FD, Wang RT, Xu XS. 2012. Pyogenic liver abscesses associated with nonmetastatic colorectal cancers: an increasing problem in Eastern Asia. World J Gastroenterol 18:2948–2955. doi: 10.3748/wjg.v18.i23.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Spadoni I, Zagato E, Bertocchi A, Paolinelli R, Hot E, Di Sabatino A, Caprioli F, Bottiglieri L, Oldani A, Viale G, Penna G, Dejana E, Rescigno M. 2015. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 168.Johnson R, Mylona E, Frankel G. 2018. Typhoidal Salmonella: distinctive virulence factors and pathogenesis. Cell Microbiol 20:e12939. doi: 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- 169.Lin YT, Liu CJ, Yeh YC, Chen TJ, Fung CP. 2013. Ampicillin and amoxicillin use and the risk of Klebsiella pneumoniae liver abscess in Taiwan. J Infect Dis 208:211–217. doi: 10.1093/infdis/jit157. [DOI] [PubMed] [Google Scholar]
- 170.Hsu CR, Pan YJ, Liu JY, Chen CT, Lin TL, Wang JT. 2015. Klebsiella pneumoniae translocates across the intestinal epithelium via Rho GTPase- and phosphatidylinositol 3-kinase/Akt-dependent cell invasion. Infect Immun 83:769–779. doi: 10.1128/IAI.02345-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.de Astorza B, Cortes G, Crespi C, Saus C, Rojo JM, Alberti S. 2004. C3 promotes clearance of Klebsiella pneumoniae by A549 epithelial cells. Infect Immun 72:1767–1774. doi: 10.1128/IAI.72.3.1767-1774.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sahly H, Podschun R, Oelschlaeger TA, Greiwe M, Parolis H, Hasty D, Kekow J, Ullmann U, Ofek I, Sela S. 2000. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect Immun 68:6744–6749. doi: 10.1128/IAI.68.12.6744-6749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang L, Shen D, Wu H, Ma Y. 2017. Resistance of hypervirulent Klebsiella pneumoniae to both intracellular and extracellular killing of neutrophils. PLoS One 12:e0173638. doi: 10.1371/journal.pone.0173638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu VL, Hansen DS, Ko WC, Sagnimeni A, Klugman KP, von Gottberg A, Goossens H, Wagener MM, Benedi VJ. 2007. Virulence characteristics of Klebsiella and clinical manifestations of K. pneumoniae bloodstream infections. Emerg Infect Dis 13:986–993. doi: 10.3201/eid1307.070187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lawlor MS, Hsu J, Rick PD, Miller VL. 2005. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol 58:1054–1073. doi: 10.1111/j.1365-2958.2005.04918.x. [DOI] [PubMed] [Google Scholar]
- 176.Cano V, March C, Insua JL, Aguilo N, Llobet E, Moranta D, Regueiro V, Brennan GP, Millan-Lou MI, Martin C, Garmendia J, Bengoechea JA. 2015. Klebsiella pneumoniae survives within macrophages by avoiding delivery to lysosomes. Cell Microbiol 17:1537–1560. doi: 10.1111/cmi.12466. [DOI] [PubMed] [Google Scholar]
- 177.Alvarez D, Merino S, Tomas JM, Benedi VJ, Alberti S. 2000. Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain-deficient Klebsiella pneumoniae clinical isolates. Infect Immun 68:953–955. doi: 10.1128/IAI.68.2.953-955.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lai YC, Peng HL, Chang HY. 2003. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol 185:788–800. doi: 10.1128/JB.185.3.788-800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hsu CR, Lin TL, Chen YC, Chou HC, Wang JT. 2011. The role of Klebsiella pneumoniae rmpA in capsular polysaccharide synthesis and virulence revisited. Microbiology 157:3446–3457. doi: 10.1099/mic.0.050336-0. [DOI] [PubMed] [Google Scholar]
- 180.Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. 2013. Role of the cAMP-dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae. PLoS One 8:e54430. doi: 10.1371/journal.pone.0054430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lin CT, Wu CC, Chen YS, Lai YC, Chi C, Lin JC, Chen Y, Peng HL. 2011. Fur regulation of the capsular polysaccharide biosynthesis and iron-acquisition systems in Klebsiella pneumoniae CG43. Microbiology 157:419–429. doi: 10.1099/mic.0.044065-0. [DOI] [PubMed] [Google Scholar]
- 182.Dorman MJ, Feltwell T, Goulding DA, Parkhill J, Short FL. 2018. The capsule regulatory network of Klebsiella pneumoniae defined by density-TraDISort. mBio 9:e01863-18. doi: 10.1128/mBio.01863-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Cortes G, Borrell N, de Astorza B, Gomez C, Sauleda J, Alberti S. 2002. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun 70:2583–2590. doi: 10.1128/IAI.70.5.2583-2590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.March C, Cano V, Moranta D, Llobet E, Perez-Gutierrez C, Tomas JM, Suarez T, Garmendia J, Bengoechea JA. 2013. Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS One 8:e56847. doi: 10.1371/journal.pone.0056847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Moranta D, Regueiro V, March C, Llobet E, Margareto J, Larrarte E, Larrate E, Garmendia J, Bengoechea JA. 2010. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta-defensins by airway epithelial cells. Infect Immun 78:1135–1146. doi: 10.1128/IAI.00940-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yu WL, Chan KS, Ko WC, Lee CC, Chuang YC. 2007. Lower prevalence of diabetes mellitus in patients with Klebsiella pneumoniae primary liver abscess caused by isolates of K1/K2 than with non-K1/K2 capsular serotypes. Clin Infect Dis 45:1529–1530. doi: 10.1086/523006. [DOI] [PubMed] [Google Scholar]
- 187.Gu DX, Huang YL, Ma JH, Zhou HW, Fang Y, Cai JC, Hu YY, Zhang R. 2016. Detection of colistin resistance gene mcr-1 in hypervirulent Klebsiella pneumoniae and Escherichia coli isolates from an infant with diarrhea in China. Antimicrob Agents Chemother 60:5099–5100. doi: 10.1128/AAC.00476-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Miethke M, Marahiel MA. 2007. Siderophore-based iron acquisition and pathogen control. Microbiol Mol Biol Rev 71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Neilands JB. 1981. Microbial iron compounds. Annu Rev Biochem 50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 190.Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. 2004. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature 432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 191.Russo TA, Shon AS, Beanan JM, Olson R, Macdonald U, Pomakov AO, Visitacion MP. 2011. Hypervirulent K. pneumoniae secretes more and more active iron-acquisition molecules than “classical” K. pneumoniae thereby enhancing its virulence. PLoS One 6:e26734. doi: 10.1371/journal.pone.0026734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hsieh PF, Lin TL, Yang FL, Wu MC, Pan YJ, Wu SH, Wang JT. 2012. Lipopolysaccharide O1 antigen contributes to the virulence in Klebsiella pneumoniae causing pyogenic liver abscess. PLoS One 7:e33155. doi: 10.1371/journal.pone.0033155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Llobet E, Martinez-Moliner V, Moranta D, Dahlstrom KM, Regueiro V, Tomas A, Cano V, Perez-Gutierrez C, Frank CG, Fernandez-Carrasco H, Insua JL, Salminen TA, Garmendia J, Bengoechea JA. 2015. Deciphering tissue-induced Klebsiella pneumoniae lipid A structure. Proc Natl Acad Sci U S A 112:E6369–E6378. doi: 10.1073/pnas.1508820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kidd TJ, Mills G, Sa-Pessoa J, Dumigan A, Frank CG, Insua JL, Ingram R, Hobley L, Bengoechea JA. 2017. A Klebsiella pneumoniae antibiotic resistance mechanism that subdues host defences and promotes virulence. EMBO Mol Med 9:430–447. doi: 10.15252/emmm.201607336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shankar-Sinha S, Valencia GA, Janes BK, Rosenberg JK, Whitfield C, Bender RA, Standiford TJ, Younger JG. 2004. The Klebsiella pneumoniae O antigen contributes to bacteremia and lethality during murine pneumonia. Infect Immun 72:1423–1430. doi: 10.1128/IAI.72.3.1423-1430.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Passet V, Brisse S. 2015. Association of tellurite resistance with hypervirulent clonal groups of Klebsiella pneumoniae. J Clin Microbiol 53:1380–1382. doi: 10.1128/JCM.03053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xue J, Tan B, Yang S, Luo M, Xia H, Zhang X, Zhou X, Yang X, Yang R, Li Y, Qiu J. 2016. Influence of cAMP receptor protein (CRP) on bacterial virulence and transcriptional regulation of allS by CRP in Klebsiella pneumoniae. Gene 593:28–33. doi: 10.1016/j.gene.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 198.Meyer M, Dimroth P, Bott M. 2001. Catabolite repression of the citrate fermentation genes in Klebsiella pneumoniae: evidence for involvement of the cyclic AMP receptor protein. J Bacteriol 183:5248–5256. doi: 10.1128/JB.183.18.5248-5256.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Molton JS, Chee YL, Hennedige TP, Venkatesh SK, Archuleta S. 2015. Impact of regional vein thrombosis in patients with Klebsiella pneumoniae liver abscess. PLoS One 10:e0140129. doi: 10.1371/journal.pone.0140129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Maffiolo C, Novellas S, Chevallier P, Brunner P, Mourou MY, Bruneton JN. 2006. Thrombophlebitis of the hepatic veins: complication of a Klebsiella liver abscess. Clin Imaging 30:63–65. doi: 10.1016/j.clinimag.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 201.Alsaif HS, Venkatesh SK, Chan DS, Archuleta S. 2011. CT appearance of pyogenic liver abscesses caused by Klebsiella pneumoniae. Radiology 260:129–138. doi: 10.1148/radiol.11101876. [DOI] [PubMed] [Google Scholar]
- 202.Lin JC, Chang FY, Fung CP, Yeh KM, Chen CT, Tsai YK, Siu LK. 2010. Do neutrophils play a role in establishing liver abscesses and distant metastases caused by Klebsiella pneumoniae? PLoS One 5:e15005. doi: 10.1371/journal.pone.0015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lee CH, Chuah SK, Tai WC, Chang CC, Chen FJ. 2017. Delay in human neutrophil constitutive apoptosis after infection with Klebsiella pneumoniae serotype K1. Front Cell Infect Microbiol 7:87. doi: 10.3389/fcimb.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Yagupsky P, Nolte FS. 1990. Quantitative aspects of septicemia. Clin Microbiol Rev 3:269–279. doi: 10.1128/CMR.3.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Whimbey E, Kiehn TE, Brannon P, Benezra D, Armstrong D. 1987. Clinical significance of colony counts in immunocompromised patients with Staphylococcus aureus bacteremia. J Infect Dis 155:1328–1330. doi: 10.1093/infdis/155.6.1328. [DOI] [PubMed] [Google Scholar]
- 206.Sullivan TD, LaScolea LJ Jr, Neter E. 1982. Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics 69:699–702. [PubMed] [Google Scholar]
- 207.Sullivan TD, LaScolea LJ Jr. 1987. Neisseria meningitidis bacteremia in children: quantitation of bacteremia and spontaneous clinical recovery without antibiotic therapy. Pediatrics 80:63–67. [PubMed] [Google Scholar]
- 208.Kao WY, Hwang CY, Chang YT, Su CW, Hou MC, Lin HC, Lee FY, Lee SD, Wu JC. 2012. Cancer risk in patients with pyogenic liver abscess: a nationwide cohort study. Aliment Pharmacol Ther 36:467–476. doi: 10.1111/j.1365-2036.2012.05212.x. [DOI] [PubMed] [Google Scholar]
- 209.Huang WK, Chang JW, See LC, Tu HT, Chen JS, Liaw CC, Lin YC, Yang TS. 2012. Higher rate of colorectal cancer among patients with pyogenic liver abscess with Klebsiella pneumoniae than those without: an 11-year follow-up study. Colorectal Dis 14:e794–e801. doi: 10.1111/j.1463-1318.2012.03174.x. [DOI] [PubMed] [Google Scholar]
- 210.Boltin D, Goldberg E, Bugaevsky O, Kelner E, Birkenfeld S, Gingold-Belfer R, Keller N, Niv Y, Dickman R. 2015. Colonic carriage of Streptococcus bovis and colorectal neoplasia: a prospective 17-year longitudinal case-control study. Eur J Gastroenterol Hepatol 27:1449–1453. doi: 10.1097/MEG.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 211.Srivastava I, Aldape MJ, Bryant AE, Stevens DL. 2017. Spontaneous C. septicum gas gangrene: a literature review. Anaerobe 48:165–171. doi: 10.1016/j.anaerobe.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 212.Chu CS, Lin CC, Peng CY, Chuang PH, Su WP, Lai SW, Chen HJ, Chung CJ, Lai HC. 2017. Does pyogenic liver abscess increase the risk of delayed-onset primary liver cancer? Evidence from a nationwide cohort study. Medicine (Baltimore) 96:e7785. doi: 10.1097/MD.0000000000007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.McCabe R, Lambert L, Frazee B. 2010. Invasive Klebsiella pneumoniae infections, California, USA. Emerg Infect Dis 16:1490–1491. doi: 10.3201/eid1609.100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Keynan Y, Karlowsky JA, Walus T, Rubinstein E. 2007. Pyogenic liver abscess caused by hypermucoviscous Klebsiella pneumoniae. Scand J Infect Dis 39:828–830. doi: 10.1080/00365540701266763. [DOI] [PubMed] [Google Scholar]
- 215.Lederman ER, Crum NF. 2005. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol 100:322–331. doi: 10.1111/j.1572-0241.2005.40310.x. [DOI] [PubMed] [Google Scholar]
- 216.Pastagia M, Arumugam V. 2008. Klebsiella pneumoniae liver abscesses in a public hospital in Queens, New York. Travel Med Infect Dis 6:228–233. doi: 10.1016/j.tmaid.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 217.Frazee BW, Hansen S, Lambert L. 2009. Invasive infection with hypermucoviscous Klebsiella pneumoniae: multiple cases presenting to a single emergency department in the United States. Ann Emerg Med 53:639–642. doi: 10.1016/j.annemergmed.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 218.Vila A, Cassata A, Pagella H, Amadio C, Yeh KM, Chang FY, Siu LK. 2011. Appearance of Klebsiella pneumoniae liver abscess syndrome in Argentina: case report and review of molecular mechanisms of pathogenesis. Open Microbiol J 5:107–113. doi: 10.2174/1874285801105010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Decre D, Verdet C, Emirian A, Le Gourrierec T, Petit JC, Offenstadt G, Maury E, Brisse S, Arlet G. 2011. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol 49:3012–3014. doi: 10.1128/JCM.00676-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gunnarsson GL, Brandt PB, Gad D, Struve C, Justesen US. 2009. Monomicrobial necrotizing fasciitis in a white male caused by hypermucoviscous Klebsiella pneumoniae. J Med Microbiol 58:1519–1521. doi: 10.1099/jmm.0.011064-0. [DOI] [PubMed] [Google Scholar]
- 221.Sobirk SK, Struve C, Jacobsson SG. 2010. Primary Klebsiella pneumoniae liver abscess with metastatic spread to lung and eye, a North-European case report of an emerging syndrome. Open Microbiol J 4:5–7. doi: 10.2174/1874285801004010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Crum-Cianflone NF, Lam PV, Ross-Walker S, Rosen LB, Holland SM. 2017. Autoantibodies to granulocyte-macrophage colony-stimulating factor associated with severe and unusual manifestations of Cryptococcus gattii infections. Open Forum Infect Dis 4:ofx211. doi: 10.1093/ofid/ofx211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Rosen LB, Rocha Pereira N, Figueiredo C, Fiske LC, Ressner RA, Hong JC, Gregg KS, Henry TL, Pak KJ, Baumgarten KL, Seoane L, Garcia-Diaz J, Olivier KN, Zelazny AM, Holland SM, Browne SK. 2015. Nocardia-induced granulocyte macrophage colony-stimulating factor is neutralized by autoantibodies in disseminated/extrapulmonary nocardiosis. Clin Infect Dis 60:1017–1025. doi: 10.1093/cid/ciu968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lin YT, Wang FD, Wu PF, Fung CP. 2013. Klebsiella pneumoniae liver abscess in diabetic patients: association of glycemic control with the clinical characteristics. BMC Infect Dis 13:56. doi: 10.1186/1471-2334-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sheu SJ, Kung YH, Wu TT, Chang FP, Horng YH. 2011. Risk factors for endogenous endophthalmitis secondary to klebsiella pneumoniae liver abscess: 20-year experience in Southern Taiwan. Retina 31:2026–2031. doi: 10.1097/IAE.0b013e31820d3f9e. [DOI] [PubMed] [Google Scholar]
- 226.Margo CE, Mames RN, Guy JR. 1994. Endogenous Klebsiella endophthalmitis. Report of two cases and review of the literature. Ophthalmology 101:1298–1301. doi: 10.1016/S0161-6420(94)31176-6. [DOI] [PubMed] [Google Scholar]
- 227.Liao HR, Lee HW, Leu HS, Lin BJ, Juang CJ. 1992. Endogenous Klebsiella pneumoniae endophthalmitis in diabetic patients. Can J Ophthalmol 27:143–147. [PubMed] [Google Scholar]
- 228.Liu YM, Chi CY, Ho MW, Chen CM, Liao WC, Ho CM, Lin PC, Wang JH. 2005. Microbiology and factors affecting mortality in necrotizing fasciitis. J Microbiol Immunol Infect 38:430–435. [PubMed] [Google Scholar]
- 229.Yang CS, Tsai HY, Sung CS, Lin KH, Lee FL, Hsu WM. 2007. Endogenous Klebsiella endophthalmitis associated with pyogenic liver abscess. Ophthalmology 114:876–880. doi: 10.1016/j.ophtha.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 230.Hu CC, Ho JD, Lou HY, Keller JJ, Lin HC. 2012. A one-year follow-up study on the incidence and risk of endophthalmitis after pyogenic liver abscess. Ophthalmology 119:2358–2363. doi: 10.1016/j.ophtha.2012.05.022. [DOI] [PubMed] [Google Scholar]
- 231.Ang M, Jap A, Chee SP. 2011. Prognostic factors and outcomes in endogenous Klebsiella pneumoniae endophthalmitis. Am J Ophthalmol 151:338–344.e2. doi: 10.1016/j.ajo.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 232.Alsaedi A, Janower A, Wang JT, Nichol K, Karlowsky J, Orr P, Keynan Y. 2014. Hypermucoviscous Klebsiella syndrome without liver abscess in a patient with immunoglobulin g2 immune deficiency. Open Forum Infect Dis 1:ofu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang YP, Liu CJ, Chen TJ, Lin YT, Fung CP. 2015. Proton pump inhibitor use significantly increases the risk of cryptogenic liver abscess: a population-based study. Aliment Pharmacol Ther 41:1175–1181. doi: 10.1111/apt.13203. [DOI] [PubMed] [Google Scholar]
- 234.Bavishi C, Dupont HL. 2011. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther 34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 235.Hung HC, Chen WC, Chao Y, Hou MC, Lin HC, Chang FY, Lee SD. 1998. Klebsiella pneumoniae panophthalmitis: a possible complication of endoscopic variceal injection sclerotherapy. Am J Gastroenterol 93:2603–2604. doi: 10.1111/j.1572-0241.1998.00490.x. [DOI] [PubMed] [Google Scholar]
- 236.Wang WM, Chen CY, Jan CM, Chen LT, Wu DC. 1990. Central nervous system infection after endoscopic injection sclerotherapy. Am J Gastroenterol 85:865–867. [PubMed] [Google Scholar]
- 237.Shih HI, Lee HC, Chuang CH, Ko WC. 2006. Fatal Klebsiella pneumoniae meningitis and emphysematous brain abscess after endoscopic variceal ligation in a patient with liver cirrhosis and diabetes mellitus. J Formos Med Assoc 105:857–860. doi: 10.1016/S0929-6646(09)60275-8. [DOI] [PubMed] [Google Scholar]
- 238.Chang WY, Cn CY. 1970. Clinical observation of liver abscess. Taiwan Yi Xue Hui Za Zhi 69:670–708. [PubMed] [Google Scholar]
- 239.Chiu CT, Lin DY, Wu CS, Chang-Chien CS, Sheen IS, Liaw YF. 1987. A clinical study on pyogenic liver abscess. Taiwan Yi Xue Hui Za Zhi 86:405–412. (In Chinese.) [PubMed] [Google Scholar]
- 240.Cheng DL, Liu YC, Yen MY, Liu CY, Shi FW, Wang LS. 1989. Causal bacteria of pyogenic liver abscess. Taiwan Yi Xue Hui Za Zhi 88:1008–1011. [PubMed] [Google Scholar]
- 241.Chang FY, Chou MY, Fan RL, Shaio MF. 1988. A clinical study of Klebsiella liver abscess. Taiwan Yi Xue Hui Za Zhi 87:282–287. [PubMed] [Google Scholar]
- 242.Lee TY, Wan YL, Tsai CC. 1994. Gas-containing liver abscess: radiological findings and clinical significance. Abdom Imaging 19:47–52. doi: 10.1007/BF02165861. [DOI] [PubMed] [Google Scholar]
- 243.Meddings L, Myers RP, Hubbard J, Shaheen AA, Laupland KB, Dixon E, Coffin C, Kaplan GG. 2010. A population-based study of pyogenic liver abscesses in the United States: incidence, mortality, and temporal trends. Am J Gastroenterol 105:117–124. doi: 10.1038/ajg.2009.614. [DOI] [PubMed] [Google Scholar]
- 244.Rahimian J, Wilson T, Oram V, Holzman RS. 2004. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis 39:1654–1659. doi: 10.1086/425616. [DOI] [PubMed] [Google Scholar]
- 245.Fierer J, Walls L, Chu P. 2011. Recurring Klebsiella pneumoniae pyogenic liver abscesses in a resident of San Diego, California, due to a K1 strain carrying the virulence plasmid. J Clin Microbiol 49:4371–4373. doi: 10.1128/JCM.05658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Pope JV, Teich DL, Clardy P, McGillicuddy DC. 2011. Klebsiella pneumoniae liver abscess: an emerging problem in North America. J Emerg Med 41:e103–e105. doi: 10.1016/j.jemermed.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 247.Kong H, Yu F, Zhang W, Li X. 2017. Clinical and microbiological characteristics of pyogenic liver abscess in a tertiary hospital in East China. Medicine (Baltimore) 96:e8050. doi: 10.1097/MD.0000000000008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Oikonomou KG, Aye M. 2017. Klebsiella pneumoniae liver abscess: a case series of six Asian patients. Am J Case Rep 18:1028–1033. doi: 10.12659/AJCR.905191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Wang R, Wang H. 2016. High prevalence of hypervirulent Klebsiella pneumoniae infection in China: geographic distribution, clinical characteristics, and antimicrobial resistance. Antimicrob Agents Chemother 60:6115–6120. doi: 10.1128/AAC.01127-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Chang CM, Ko WC, Lee HC, Chen YM, Chuang YC. 2001. Klebsiella pneumoniae psoas abscess: predominance in diabetic patients and grave prognosis in gas-forming cases. J Microbiol Immunol Infect 34:201–206. [PubMed] [Google Scholar]
- 251.Kim SB, Je BK, Lee KY, Lee SH, Chung HH, Cha SH. 2007. Computed tomographic differences of pyogenic liver abscesses caused by Klebsiella pneumoniae and non-Klebsiella pneumoniae. J Comput Assist Tomogr 31:59–65. doi: 10.1097/01.rct.0000224629.48068.69. [DOI] [PubMed] [Google Scholar]
- 252.Wang YF, Chang CC, Lee TC, Shih IL, Lien WC, Chen SJ, Wang HP, Liu KL. 2013. Recent trend of pylephlebitis in Taiwan: Klebsiella pneumoniae liver abscess as an emerging etiology. Infection 41:1137–1143. doi: 10.1007/s15010-013-0497-9. [DOI] [PubMed] [Google Scholar]
- 253.Lee WS, Choi ST, Kim KK. 2011. Splenic abscess: a single institution study and review of the literature. Yonsei Med J 52:288–292. doi: 10.3349/ymj.2011.52.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chang KC, Chuah SK, Changchien CS, Tsai TL, Lu SN, Chiu YC, Chen YS, Wang CC, Lin JW, Lee CM, Hu TH. 2006. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol 12:460–464. doi: 10.3748/wjg.v12.i3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Tung CC, Chen FC, Lo CJ. 2006. Splenic abscess: an easily overlooked disease? Am Surg 72:322–325. [PubMed] [Google Scholar]
- 256.Lee CH, Hu TH, Liu JW. 2005. Splenic abscess caused by Klebsiella pneumoniae and non-Klebsiella pneumoniae in Taiwan: emphasizing risk factors for acquisition of Klebsiella pneumoniae splenic abscess. Scand J Infect Dis 37:905–909. doi: 10.1080/00365540500333624. [DOI] [PubMed] [Google Scholar]
- 257.Brook I, Frazier EH. 1998. Microbiology of liver and spleen abscesses. J Med Microbiol 47:1075–1080. doi: 10.1099/00222615-47-12-1075. [DOI] [PubMed] [Google Scholar]
- 258.Wu TL, Lin WT, Chao CM, Lai CC. 2013. Spontaneous bacterial peritonitis caused by Klebsiella pneumoniae. OA Case Rep 2:1. [Google Scholar]
- 259.Marrie TJ, Durant H, Yates L. 1989. Community-acquired pneumonia requiring hospitalization: 5-year prospective study. Rev Infect Dis 11:586–599. doi: 10.1093/clinids/11.4.586. [DOI] [PubMed] [Google Scholar]
- 260.Carpenter JL. 1990. Klebsiella pulmonary infections: occurrence at one medical center and review. Rev Infect Dis 12:672–682. doi: 10.1093/clinids/12.4.672. [DOI] [PubMed] [Google Scholar]
- 261.Vergis EN, Indorf A, File TM Jr, Phillips J, Bates J, Tan J, Sarosi GA, Grayston JT, Summersgill J, Yu VL. 2000. Azithromycin vs cefuroxime plus erythromycin for empirical treatment of community-acquired pneumonia in hospitalized patients: a prospective, randomized, multicenter trial. Arch Intern Med 160:1294–1300. doi: 10.1001/archinte.160.9.1294. [DOI] [PubMed] [Google Scholar]
- 262.Rafat C, Messika J, Barnaud G, Dufour N, Magdoud F, Billard-Pomarès T, Gaudry S, Dreyfuss D, Branger C, Decré D, Ricard J-D. 2018. Hypervirulent Klebsiella pneumoniae, a 5-year study in a French ICU. J Med Microbiol 67:1083–1089. doi: 10.1099/jmm.0.000788. [DOI] [PubMed] [Google Scholar]
- 263.Liu C, Guo J. 2018. Characteristics of ventilator-associated pneumonia due to hypervirulent Klebsiella pneumoniae genotype in genetic background for the elderly in two tertiary hospitals in China. Antimicrob Resist Infect Control 7:95. doi: 10.1186/s13756-018-0371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Yan Q, Zhou M, Zou M, Liu WE. 2016. Hypervirulent Klebsiella pneumoniae induced ventilator-associated pneumonia in mechanically ventilated patients in China. Eur J Clin Microbiol Infect Dis 35:387–396. doi: 10.1007/s10096-015-2551-2. [DOI] [PubMed] [Google Scholar]
- 265.Mohammad Ali Tabrizi A, Badmasti F, Shahcheraghi F, Azizi O. 2018. Outbreak of hypervirulent Klebsiella pneumoniae harbouring blaVIM-2 among mechanically-ventilated drug-poisoning patients with high mortality rate in Iran. J Glob Antimicrob Resist 15:93–98. doi: 10.1016/j.jgar.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 266.Lin YT, Chen TL, Siu LK, Hsu SF, Fung CP. 2010. Clinical and microbiological characteristics of community-acquired thoracic empyema or complicated parapneumonic effusion caused by Klebsiella pneumoniae in Taiwan. Eur J Clin Microbiol Infect Dis 29:1003–1010. doi: 10.1007/s10096-010-0961-8. [DOI] [PubMed] [Google Scholar]
- 267.Cho E, Park SW, Jun CH, Shin SS, Park EK, Lee KS, Park SY, Park CH, Kim HS, Choi SK, Rew JS. 2018. A rare case of pericarditis and pleural empyema secondary to transdiaphragmatic extension of pyogenic liver abscess. BMC Infect Dis 18:40. doi: 10.1186/s12879-018-2953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chou DW, Wu SL, Chung KM, Han SC. 2015. Septic pulmonary embolism caused by a Klebsiella pneumoniae liver abscess: clinical characteristics, imaging findings, and clinical courses. Clinics (Sao Paulo) 70:400–407. doi: 10.6061/clinics/2015(06)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Akasaka K, Tokita S, Koyama A, Ikegami G, Koyama K, Fujihara H, Ichiwata T, Nagao K. 2008. A case of septic pulmonary embolism with endogenous endophthalmitis in a healthy adult. Nihon Kokyuki Gakkai Zasshi 46:291–296. (In Japanese.) [PubMed] [Google Scholar]
- 270.Cheng FY, Su YJ. 2016. Septic pulmonary emboli associated with Klebsiella pneumoniae epididymitis. J Emerg Med 50:e23–e24. doi: 10.1016/j.jemermed.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 271.Korsten P, Vasko R, Gross O, Schrempf LE, Zimmermann O, Schulze MH, Muller GA. 2014. Endophthalmitis, liver abscess, and cerebral and pulmonary emboli in a 48-year-old Vietnamese man. Internist (Berl) 55:722–725. (In German.) doi: 10.1007/s00108-014-3484-z. [DOI] [PubMed] [Google Scholar]
- 272.Liu JW, Lin TC, Chang YT, Tsai CA, Hu SY. 2017. Prostatic abscess of Klebsiella pneumonia complicating septic pulmonary emboli and meningitis: a case report and brief review. Asian Pac J Trop Med 10:102–105. doi: 10.1016/j.apjtm.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 273.Wong JS, Chan TK, Lee HM, Chee SP. 2000. Endogenous bacterial endophthalmitis: an East Asian experience and a reappraisal of a severe ocular affliction. Ophthalmology 107:1483–1491. doi: 10.1016/S0161-6420(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 274.Siu GD, Lo EC, Young A. 2015. Endogenous endophthalmitis with a visual acuity of 6/6. BMJ Case Rep 2015:bcr2014205048. doi: 10.1136/bcr-2014-205048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sharma M, Chow DR, Muller MP. 2009. Endogenous Klebsiella endophthalmitis in a Vietnamese immigrant. CMAJ 181:495–497. doi: 10.1503/cmaj.090060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Shields RA, Smith SJ, Pan CK, Do DV. 2019. Endogenous Klebsiella pneumoniae endophthalmitis in Northern California. Retina 39:614–620. doi: 10.1097/IAE.0000000000001994. [DOI] [PubMed] [Google Scholar]
- 277.Odouard C, Ong D, Shah PR, Gin T, Allen PJ, Downie J, Lim LL, McCluskey P. 2017. Rising trends of endogenous Klebsiella pneumoniae endophthalmitis in Australia. Clin Exp Ophthalmol 45:135–142. doi: 10.1111/ceo.12827. [DOI] [PubMed] [Google Scholar]
- 278.Dehghani AR, Masjedi A, Fazel F, Ghanbari H, Akhlaghi M, Karbasi N. 2011. Endogenous Klebsiella endophthalmitis associated with liver abscess: first case report from Iran. Case Rep Ophthalmol 2:10–14. doi: 10.1159/000323449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tang LM, Chen ST, Hsu WC, Chen CM. 1997. Klebsiella meningitis in Taiwan: an overview. Epidemiol Infect 119:135–142. doi: 10.1017/S0950268897007930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Jang TN, Wang FD, Wang LS, Yu KW, Liu CY. 1993. Gram-negative bacillary meningitis in adults: a recent six-year experience. J Formos Med Assoc 92:540–546. [PubMed] [Google Scholar]
- 281.Fang CT, Chang SC, Hsueh PR, Chen YC, Sau WY, Luh KT. 2000. Microbiologic features of adult community-acquired bacterial meningitis in Taiwan. J Formos Med Assoc 99:300–304. [PubMed] [Google Scholar]
- 282.Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr, Swartz MN. 1993. Acute bacterial meningitis in adults. A review of 493 episodes. N Engl J Med 328:21–28. doi: 10.1056/NEJM199301073280104. [DOI] [PubMed] [Google Scholar]
- 283.Melot B, Brisse S, Breurec S, Passet V, Malpote E, Lamaury I, Thiery G, Hoen B. 2016. Community-acquired meningitis caused by a CG86 hypervirulent Klebsiella pneumoniae strain: first case report in the Caribbean. BMC Infect Dis 16:736. doi: 10.1186/s12879-016-2065-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Hsu CL, Chang CH, Wong KN, Chen KY, Yu CJ, Yang PC. 2009. Management of severe community-acquired septic meningitis in adults: from emergency department to intensive care unit. J Formos Med Assoc 108:112–118. doi: 10.1016/S0929-6646(09)60041-3. [DOI] [PubMed] [Google Scholar]
- 285.Jung J, Park KH, Park SY, Song EH, Lee EJ, Choi SH, Choo EJ, Kwak YG, Sung H, Kim SH, Lee SO, Kim MN, Kim YS, Woo JH, Choi SH. 2015. Comparison of the clinical characteristics and outcomes of Klebsiella pneumoniae and Streptococcus pneumoniae meningitis. Diagn Microbiol Infect Dis 82:87–91. doi: 10.1016/j.diagmicrobio.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 286.Lin YT, Liu CJ, Chen TJ, Fung CP. 2012. Long-term mortality of patients with septic ocular or central nervous system complications from pyogenic liver abscess: a population-based study. PLoS One 7:e33978. doi: 10.1371/journal.pone.0033978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Fang CT, Chen YC, Chang SC, Sau WY, Luh KT. 2000. Klebsiella pneumoniae meningitis: timing of antimicrobial therapy and prognosis. QJM 93:45–53. doi: 10.1093/qjmed/93.1.45. [DOI] [PubMed] [Google Scholar]
- 288.Takahashi K, Miura A, Yamaguchi T, Kanematsu M. 2015. Novel cord-like structures on MRI in a case of hypervirulent Klebsiella pneumoniae. Intern Med 54:355–356. doi: 10.2169/internalmedicine.54.3485. [DOI] [PubMed] [Google Scholar]
- 289.Iwasaki Y, Inokuchi R, Harada S, Aoki K, Ishii Y, Shinohara K. 2017. Bacterial meningitis caused by hypervirulent Klebsiella pneumoniae capsular genotype K54 with development of granuloma-like nodal enhancement in the brain during the subacute phase. Intern Med 56:373–376. doi: 10.2169/internalmedicine.56.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hentzien M, Rosman J, Decre D, Brenkle K, Mendes-Martins L, Mateu P. 2017. Seven hypervirulent ST380 Klebsiella pneumoniae septic localizations. Med Mal Infect 47:171–173. doi: 10.1016/j.medmal.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 291.Yoon W. 2013. A rare presentation of brain abscess and ventriculitis due to Klebsiella pneumoniae. J Neurol Sci 333:1.23735777 [Google Scholar]
- 292.Hsieh MJ, Lu TC, Ma MH, Wang HP, Chen SC. 2009. Unrecognized cervical spinal epidural abscess associated with metastatic Klebsiella pneumoniae bacteremia and liver abscess in nondiabetic patients. Diagn Microbiol Infect Dis 65:65–68. doi: 10.1016/j.diagmicrobio.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 293.Kuramochi G, Takei SI, Sato M, Isokawa O, Takemae T, Takahashi A. 2005. Klebsiella pneumoniae liver abscess associated with septic spinal epidural abscess. Hepatol Res 31:48–52. doi: 10.1016/j.hepres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 294.Doud MS, Grimes-Zeppegno R, Molina E, Miller N, Balachandar D, Schneper L, Poppiti R, Mathee K. 2009. A k2A-positive Klebsiella pneumoniae causes liver and brain abscess in a Saint Kitt’s man. Int J Med Sci 6:301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Patel PK, Russo TA, Karchmer AW. 2014. Brief report on hypervirulent Klebsiella pneumoniae. Open Forum Infect Dis 1:ofu028. doi: 10.1093/ofid/ofu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cheng NC, Yu YC, Tai HC, Hsueh PR, Chang SC, Lai SY, Yi WC, Fang CT. 2012. Recent trend of necrotizing fasciitis in Taiwan: focus on monomicrobial Klebsiella pneumoniae necrotizing fasciitis. Clin Infect Dis 55:930–939. doi: 10.1093/cid/cis565. [DOI] [PubMed] [Google Scholar]
- 297.Ng D, Frazee B. 2015. Necrotizing fasciitis caused by hypermucoviscous Klebsiella pneumoniae in a Filipino female in North America. West J Emerg Med 16:165–168. doi: 10.5811/westjem.2014.11.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Mgbemena O, Serota DP, Kumar S, Wozniak JE, Weiss DS, Kempker RR. 2017. Peculiar purulence: hypervirulent Klebsiella pneumoniae causing pyomyositis. Int J Infect Dis 65:90–92. doi: 10.1016/j.ijid.2017.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Kishibe S, Okubo Y, Morino S, Hirotaki S, Tame T, Aoki K, Ishii Y, Ota N, Shimomura S, Sakakibara H, Terakawa T, Horikoshi Y. 2016. Pediatric hypervirulent Klebsiella pneumoniae septic arthritis. Pediatr Int 58:382–385. doi: 10.1111/ped.12806. [DOI] [PubMed] [Google Scholar]
- 300.Prokesch BC, TeKippe M, Kim J, Raj P, TeKippe EM, Greenberg DE. 2016. Primary osteomyelitis caused by hypervirulent Klebsiella pneumoniae. Lancet Infect Dis 16:e190–e195. doi: 10.1016/S1473-3099(16)30021-4. [DOI] [PubMed] [Google Scholar]
- 301.Sturm E, Tai A, Lin B, Kwong J, Athan E, Howden BP, Angliss RD, Asaid R, Pollard J. 2018. Bilateral osteomyelitis and liver abscess caused by hypervirulent Klebsiella pneumoniae—a rare clinical manifestation (case report). BMC Infect Dis 18:380. doi: 10.1186/s12879-018-3277-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Paraschiv F, Popescu GA, Borcan AM. 2018. Septic cutaneous emboli revealing a severe case of Klebsiella pneumoniae liver abscess syndrome. JMM Case Rep 5:e005148. doi: 10.1099/jmmcr.0.005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Vandevelde A, Stepanovic B. 2014. On a boat: a case in Australia of endophthalmitis and pyogenic liver, prostatic, and lung abscesses in a previously well patient due to Klebsiella pneumoniae. Case Rep Infect Dis 2014:137248. doi: 10.1155/2014/137248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Asgeirsson H, Kristjansson M, Kristinsson KG, Gudlaugsson O. 2012. Clinical significance of Staphylococcus aureus bacteriuria in a nationwide study of adults with S. aureus bacteraemia. J Infect 64:41–46. doi: 10.1016/j.jinf.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 305.Wu H, Li D, Zhou H, Sun Y, Guo L, Shen D. 2017. Bacteremia and other body site infection caused by hypervirulent and classic Klebsiella pneumoniae. Microb Pathog 104:254–262. doi: 10.1016/j.micpath.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 306.Rivero A, Gomez E, Alland D, Huang DB, Chiang T. 2010. K2 serotype Klebsiella pneumoniae causing a liver abscess associated with infective endocarditis. J Clin Microbiol 48:639–641. doi: 10.1128/JCM.01779-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Balestrino D, Ghigo JM, Charbonnel N, Haagensen JA, Forestier C. 2008. The characterization of functions involved in the establishment and maturation of Klebsiella pneumoniae in vitro biofilm reveals dual roles for surface exopolysaccharides. Environ Microbiol 10:685–701. doi: 10.1111/j.1462-2920.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 308.Yang SJ, Park SY, Lee YJ, Kim HY, Seo JA, Kim SG, Choi DS. 2010. Klebsiella pneumoniae orbital cellulitis with extensive vascular occlusions in a patient with type 2 diabetes. Korean J Intern Med 25:114–117. doi: 10.3904/kjim.2010.25.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Pinsky BA, Baron EJ, Janda JM, Banaei N. 2009. Bartholin's abscess caused by hypermucoviscous Klebsiella pneumoniae. J Med Microbiol 58:671–673. doi: 10.1099/jmm.0.006734-0. [DOI] [PubMed] [Google Scholar]
- 310.Tan YM, Chung AY, Chow PK, Cheow PC, Wong WK, Ooi LL, Soo KC. 2005. An appraisal of surgical and percutaneous drainage for pyogenic liver abscesses larger than 5 cm. Ann Surg 241:485–490. doi: 10.1097/01.sla.0000154265.14006.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yu F, Lv J, Niu S, Du H, Tang YW, Pitout JDD, Bonomo RA, Kreiswirth BN, Chen L. 2018. Multiplex PCR analysis for rapid detection of Klebsiella pneumoniae carbapenem-resistant (sequence type 258 [ST258] and ST11) and hypervirulent (ST23, ST65, ST86, and ST375) strains. J Clin Microbiol 56:e00731-18. doi: 10.1128/JCM.00731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Rodrigues C, Novais A, Sousa C, Ramos H, Coque TM, Canton R, Lopes JA, Peixe L. 2017. Elucidating constraints for differentiation of major human Klebsiella pneumoniae clones using MALDI-TOF MS. Eur J Clin Microbiol Infect Dis 36:379–386. doi: 10.1007/s10096-016-2812-8. [DOI] [PubMed] [Google Scholar]
- 313.Rodrigues C, Passet V, Rakotondrasoa A, Brisse S. 2018. Identification of Klebsiella pneumoniae, Klebsiella quasipneumoniae, Klebsiella variicola and related phylogroups by MALDI-TOF mass spectrometry. Front Microbiol 9:3000. doi: 10.3389/fmicb.2018.03000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Huang Y, Li J, Gu D, Fang Y, Chan EW, Chen S, Zhang R. 2015. Rapid detection of K1 hypervirulent Klebsiella pneumoniae by MALDI-TOF MS. Front Microbiol 6:1435. doi: 10.3389/fmicb.2015.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Hsieh HF, Chen TW, Yu CY, Wang NC, Chu HC, Shih ML, Yu JC, Hsieh CB. 2008. Aggressive hepatic resection for patients with pyogenic liver abscess and APACHE II score ≥15. Am J Surg 196:346–350. doi: 10.1016/j.amjsurg.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 316.Chan DS, Archuleta S, Llorin RM, Lye DC, Fisher D. 2013. Standardized outpatient management of Klebsiella pneumoniae liver abscesses. Int J Infect Dis 17:e185–e188. doi: 10.1016/j.ijid.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 317.Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, Jeong BC, Lee SH. 2017. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence-associated determinants, and resistance mechanisms. Front Cell Infect Microbiol 7:483. doi: 10.3389/fcimb.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Turton JF, Payne Z, Coward A, Hopkins KL, Turton JA, Doumith M, Woodford N. 2018. Virulence genes in isolates of Klebsiella pneumoniae from the UK during 2016, including among carbapenemase gene-positive hypervirulent K1-ST23 and ‘non-hypervirulent’ types ST147, ST15 and ST383. J Med Microbiol 67:118–128. doi: 10.1099/jmm.0.000653. [DOI] [PubMed] [Google Scholar]
- 319.Dong N, Yang X, Zhang R, Chan EW, Chen S. 2018. Tracking microevolution events among ST11 carbapenemase-producing hypervirulent Klebsiella pneumoniae outbreak strains. Emerg Microbes Infect 7:146. doi: 10.1038/s41426-018-0146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Yu WL, Lee MF, Chen CC, Tang HJ, Ho CH, Chuang YC. 2017. Impacts of hypervirulence determinants on clinical features and outcomes of bacteremia caused by extended-spectrum beta-lactamase-producing Klebsiella pneumoniae. Microb Drug Resist 23:376–383. doi: 10.1089/mdr.2016.0018. [DOI] [PubMed] [Google Scholar]
- 321.Surgers L, Boyd A, Girard PM, Arlet G, Decre D. 2016. ESBL-producing strain of hypervirulent Klebsiella pneumoniae K2, France. Emerg Infect Dis 22:1687–1688. doi: 10.3201/eid2209.160681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Xu M, Li A, Kong H, Zhang W, Chen H, Fu Y, Fu Y. 2018. Endogenous endophthalmitis caused by a multidrug-resistant hypervirulent Klebsiella pneumoniae strain belonging to a novel single locus variant of ST23: first case report in China. BMC Infect Dis 18:669. doi: 10.1186/s12879-018-3543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Xie Y, Tian L, Li G, Qu H, Sun J, Liang W, Li X, Wang X, Deng Z, Liu J, Ou HY. 2018. Emergence of the third-generation cephalosporin-resistant hypervirulent Klebsiella pneumoniae due to the acquisition of a self-transferable blaDHA-1-carrying plasmid by an ST23 strain. Virulence 9:838–844. doi: 10.1080/21505594.2018.1456229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Cejas D, Fernandez Canigia L, Rincon Cruz G, Elena AX, Maldonado I, Gutkind GO, Radice MA. 2014. First isolate of KPC-2-producing Klebsiella pneumonaie sequence type 23 from the Americas. J Clin Microbiol 52:3483–3485. doi: 10.1128/JCM.00726-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Compain F, Vandenberghe A, Gominet M, Genel N, Lebeaux D, Ramahefasolo A, Podglajen I, Decre D. 2017. Primary osteomyelitis caused by an NDM-1-producing K. pneumoniae strain of the highly virulent sequence type 23. Emerg Microbes Infect 6:e57. doi: 10.1038/emi.2017.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Becker L, Kaase M, Pfeifer Y, Fuchs S, Reuss A, von Laer A, Sin MA, Korte-Berwanger M, Gatermann S, Werner G. 2018. Genome-based analysis of carbapenemase-producing Klebsiella pneumoniae isolates from German hospital patients, 2008–2014. Antimicrob Resist Infect Control 7:62. doi: 10.1186/s13756-018-0352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Roulston KJ, Bharucha T, Turton JF, Hopkins KL, Mack D. 2018. A case of NDM-carbapenemase-producing hypervirulent Klebsiella pneumoniae sequence type 23 from the UK. JMM Case Rep 5:e005130. doi: 10.1099/jmmcr.0.005130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Zhang Y, Zeng J, Liu W, Zhao F, Hu Z, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Cao B, Wang H. 2015. Emergence of a hypervirulent carbapenem-resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect 71:553–560. doi: 10.1016/j.jinf.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 329.Du P, Zhang Y, Chen C. 2018. Emergence of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Lancet Infect Dis 18:23–24. doi: 10.1016/S1473-3099(17)30625-4. [DOI] [PubMed] [Google Scholar]
- 330.Yao B, Xiao X, Wang F, Zhou L, Zhang X, Zhang J. 2015. Clinical and molecular characteristics of multi-clone carbapenem-resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in a tertiary hospital in Beijing, China. Int J Infect Dis 37:107–112. doi: 10.1016/j.ijid.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 331.Zhan L, Wang S, Guo Y, Jin Y, Duan J, Hao Z, Lv J, Qi X, Hu L, Chen L, Kreiswirth BN, Zhang R, Pan J, Wang L, Yu F. 2017. Outbreak by hypermucoviscous Klebsiella pneumoniae ST11 isolates with carbapenem resistance in a tertiary hospital in China. Front Cell Infect Microbiol 7:182. doi: 10.3389/fcimb.2017.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Liu Z, Gu Y, Li X, Liu Y, Ye Y, Guan S, Li J. 2019. Identification and characterization of NDM-1-producing hypervirulent (hypermucoviscous) Klebsiella pneumoniae in China. Ann Lab Med 39:167–175. doi: 10.3343/alm.2019.39.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Shankar C, Nabarro LE, Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Daniel JL, Doss CG, Veeraraghavan B. 2016. Draft genome sequences of three hypervirulent carbapenem-resistant Klebsiella pneumoniae isolates from bacteremia. Genome Announc 4:e01081-16. doi: 10.1128/genomeA.01081-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Wei DD, Wan LG, Liu Y. 2018. Draft genome sequence of an NDM-1- and KPC-2-coproducing hypervirulent carbapenem-resistant Klebsiella pneumoniae strain isolated from burn wound infections. Genome Announc 6:e00192-18. doi: 10.1128/genomeA.00192-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Mei YF, Liu PP, Wan LG, Liu Y, Wang LH, Wei DD, Deng Q, Cao XW. 2017. Virulence and genomic feature of a virulent Klebsiella pneumoniae sequence type 14 strain of serotype K2 harboring blaNDM-5 in China. Front Microbiol 8:335. doi: 10.3389/fmicb.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Simner PJ, Antar AAR, Hao S, Gurtowski J, Tamma PD, Rock C, Opene BNA, Tekle T, Carroll KC, Schatz MC, Timp W. 2018. Antibiotic pressure on the acquisition and loss of antibiotic resistance genes in Klebsiella pneumoniae. J Antimicrob Chemother 73:1796–1803. doi: 10.1093/jac/dky121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Lu Y, Feng Y, McNally A, Zong Z. 2018. The occurence of colistin-resistant hypervirulent Klebsiella pneumoniae in China. Front Microbiol 9:2568. doi: 10.3389/fmicb.2018.02568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Cannatelli A, Giani T, D’Andrea MM, Di Pilato V, Arena F, Conte V, Tryfinopoulou K, Vatopoulos A, Rossolini GM. 2014. MgrB inactivation is a common mechanism of colistin resistance in KPC-producing Klebsiella pneumoniae of clinical origin. Antimicrob Agents Chemother 58:5696–5703. doi: 10.1128/AAC.03110-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Siu LK, Huang DB, Chiang T. 2014. Plasmid transferability of KPC into a virulent K2 serotype Klebsiella pneumoniae. BMC Infect Dis 14:176. doi: 10.1186/1471-2334-14-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Wang-Lin SX, Olson R, Beanan JM, MacDonald U, Balthasar JP, Russo TA. 2017. The capsular polysaccharide of Acinetobacter baumannii is an obstacle for therapeutic passive immunization strategies. Infect Immun 85:e00591-17. doi: 10.1128/IAI.00591-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Russo TA, Beanan JM, Olson R, MacDonald U, Cope JJ. 2009. Capsular polysaccharide and the O-specific antigen impede antibody binding: a potential obstacle for the successful development of an extraintestinal pathogenic Escherichia coli vaccine. Vaccine 27:388–395. doi: 10.1016/j.vaccine.2008.10.082. [DOI] [PubMed] [Google Scholar]
- 342.Diago-Navarro E, Calatayud-Baselga I, Sun D, Khairallah C, Mann I, Ulacia-Hernando A, Sheridan B, Shi M, Fries BC. 2017. Antibody-based immunotherapy to treat and prevent infection with hypervirulent Klebsiella pneumoniae. Clin Vaccine Immunol 24:e00456-16. doi: 10.1128/CVI.00456-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Wittebole X, De Roock S, Opal SM. 2014. A historical overview of bacteriophage therapy as an alternative to antibiotics for the treatment of bacterial pathogens. Virulence 5:226–235. doi: 10.4161/viru.25991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Pelfrene E, Willebrand E, Cavaleiro Sanches A, Sebris Z, Cavaleri M. 2016. Bacteriophage therapy: a regulatory perspective. J Antimicrob Chemother 71:2071–2074. doi: 10.1093/jac/dkw083. [DOI] [PubMed] [Google Scholar]
- 345.Lin TL, Hsieh PF, Huang YT, Lee WC, Tsai YT, Su PA, Pan YJ, Hsu CR, Wu MC, Wang JT. 2014. Isolation of a bacteriophage and its depolymerase specific for K1 capsule of Klebsiella pneumoniae: implication in typing and treatment. J Infect Dis 210:1734–1744. doi: 10.1093/infdis/jiu332. [DOI] [PubMed] [Google Scholar]
- 346.Hsieh PF, Lin HH, Lin TL, Chen YY, Wang JT. 2017. Two T7-like bacteriophages, K5-2 and K5-4, each encodes two capsule depolymerases: isolation and functional characterization. Sci Rep 7:4624. doi: 10.1038/s41598-017-04644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Zheng R, Zhang Q, Guo Y, Feng Y, Liu L, Zhang A, Zhao Y, Yang X, Xia X. 2016. Outbreak of plasmid-mediated NDM-1-producing Klebsiella pneumoniae ST105 among neonatal patients in Yunnan, China. Ann Clin Microbiol Antimicrob 15:10. doi: 10.1186/s12941-016-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Mathers AJ, Vegesana K, German Mesner I, Barry KE, Pannone A, Baumann J, Crook DW, Stoesser N, Kotay S, Carroll J, Sifri CD. 2018. Intensive care unit wastewater interventions to prevent transmission of multi-species Klebsiella pneumoniae carbapenemase (KPC) producing organisms. Clin Infect Dis 67:171–178. doi: 10.1093/cid/ciy052. [DOI] [PubMed] [Google Scholar]
- 349.Gurieva T, Dautzenberg MJD, Gniadkowski M, Derde LPG, Bonten MJM, Bootsma M. 2018. The transmissibility of antibiotic-resistant Enterobacteriaceae in intensive care units. Clin Infect Dis 66:489–493. doi: 10.1093/cid/cix825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.National Center for Emerging and Zoonotic Infectious Diseases, CDC. 2015. Facility guidance for control of carbapenem-resistant Enterobacteriaceae (CRE). November 2015 update—CRE toolkit. CDC, Atlanta, GA. [Google Scholar]
- 351.Tschudin-Sutter S, Frei R, Dangel M, Stranden A, Widmer AF. 2012. Rate of transmission of extended-spectrum beta-lactamase-producing Enterobacteriaceae without contact isolation. Clin Infect Dis 55:1505–1511. doi: 10.1093/cid/cis770. [DOI] [PubMed] [Google Scholar]
- 352.Tschudin-Sutter S, Lucet JC, Mutters NT, Tacconelli E, Zahar JR, Harbarth S. 2017. Contact precautions for preventing nosocomial transmission of extended-spectrum beta lactamase-producing Escherichia coli: a point/counterpoint review. Clin Infect Dis 65:342–347. doi: 10.1093/cid/cix258. [DOI] [PubMed] [Google Scholar]
- 353.Russo TA, Johnson JR. 2018. Diseases caused by gram-negative enteric bacilli, p 1146–1160. In Jameson JL, Fauci AS, Kasper DL, Hauser SL, Longo DL, Loscalzo J (ed), Harrison’s principles of internal medicine, 20th ed McGraw-Hill Education, New York, NY. [Google Scholar]
- 354.Peirano G, Pitout JD, Laupland KB, Meatherall B, Gregson DB. 2013. Population-based surveillance for hypermucoviscosity Klebsiella pneumoniae causing community-acquired bacteremia in Calgary, Alberta. Can J Infect Dis Med Microbiol 24:e61–e64. doi: 10.1155/2013/828741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Liu YM, Li BB, Zhang YY, Zhang W, Shen H, Li H, Cao B. 2014. Clinical and molecular characteristics of emerging hypervirulent Klebsiella pneumoniae bloodstream infections in mainland China. Antimicrob Agents Chemother 58:5379–5385. doi: 10.1128/AAC.02523-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Remya P, Shanthi M, Sekar U. 2018. Occurrence and characterization of hyperviscous K1 and K2 serotype in Klebsiella pneumoniae. J Lab Physicians 10:283–288. doi: 10.4103/JLP.JLP_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Shankar C, Nabarro LE, Anandan S, Ravi R, Babu P, Munusamy E, Jeyaseelan V, Rupali P, Verghese VP, Veeraraghavan B. 2018. Extremely high mortality rates in patients with carbapenem-resistant, hypermucoviscous Klebsiella pneumoniae blood stream infections. J Assoc Phys India 66:13–16. [PubMed] [Google Scholar]
- 358.Chou A, Nuila RE, Franco LM, Stager CE, Atmar RL, Zechiedrich L. 2016. Prevalence of hypervirulent Klebsiella pneumoniae-associated genes rmpA and magA in two tertiary hospitals in Houston, TX, USA. J Med Microbiol 65:1047–1048. doi: 10.1099/jmm.0.000309. [DOI] [PMC free article] [PubMed] [Google Scholar]