Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide. Despite broad literature including basic and translational scientific studies, many gaps in our understanding of host-pathogen interactions remain.
KEYWORDS: inflammation, influenza, lung, staphylococcus, streptococcus, superinfection
SUMMARY
Community-acquired pneumonia (CAP) is a leading cause of morbidity and mortality worldwide. Despite broad literature including basic and translational scientific studies, many gaps in our understanding of host-pathogen interactions remain. In this review, pathogen virulence factors that drive lung infection and injury are discussed in relation to their associated host immune pathways. CAP epidemiology is considered, with a focus on Staphylococcus aureus and Streptococcus pneumoniae as primary pathogens. Bacterial factors involved in nasal colonization and subsequent virulence are illuminated. A particular emphasis is placed on bacterial pore-forming toxins, host cell death, and inflammasome activation. Identified host-pathogen interactions are then examined by linking pathogen factors to aberrant host response pathways in the context of acute lung injury in both primary and secondary infection. While much is known regarding bacterial virulence and host immune responses, CAP management is still limited to mostly supportive care. It is likely that improvements in therapy will be derived from combinatorial targeting of both pathogen virulence factors and host immunomodulation.
INTRODUCTION
Pneumonia has been a significant cause of morbidity and mortality throughout human history. Excess mortality is associated with community-acquired pneumonia (CAP), rather than hospital-acquired pneumonia. In children, CAP is the leading cause of death worldwide, resulting in 900,000 deaths in 2015 (1). In the preantibiotic era, 95% of cases were due to Streptococcus pneumoniae (2). In recent years, the advent of pneumococcal vaccines in children and adults has reduced the incidence of S. pneumoniae to 10% to 15% of CAP cases in the United States, which is a 2- to 4-fold reduction in incidence (1). Overall, pneumonia deaths in children have decreased by as much as half since 2000 (3). While progress has been made, CAP is still commonplace. CAP incidence in adults between 2010 and 2012 was 24.8 cases per 10,000 individuals, with the incidence 6-fold higher in those over 80 years of age (4). Detection of CAP etiology remains a significant clinical problem, as 62% of cases have no pathogen detected. However, in the last decade, molecular diagnostics utilizing mass spectrometry and PCR have drastically increased clinicians’ ability to detect pathogens in patient sputum or endotracheal aspirate, with molecular testing boasting an 87% detection rate versus 39% for culture-based methods (5). These advances will pave the way for clinicians to use pathogen-specific therapies, many of which are currently in development.
While CAP was traditionally characterized by bacterial pathogen etiology, viral pathogens are also predominant. Viral pathogens such as rhinovirus, respiratory syncytial virus, human metapneumovirus, and influenza virus are now common causes of CAP (1). In the period from 2010 to 2012, influenza virus has become the second leading cause of CAP (behind rhinovirus) (4). In fatal cases of influenza in children between 2010 and 2014, approximately 47% of deaths were observed in children with no preexisting high-risk conditions (6). As far back as the 1918 Spanish influenza pandemic, 94% of fatalities were associated with secondary bacterial pathogens, predominantly S. pneumoniae (7). These findings illuminate the changing nature of CAP in the last century.
In recent years, Staphylococcus aureus has become an emerging cause of CAP. The rise of methicillin-resistant Staphylococcus aureus (MRSA) prevalence has increased the threat of this pathogen. CAP caused by S. aureus is often severe, with 81% of cases requiring intensive care therapy and 29% mortality, in one study (8). In a meta-analysis of S. aureus CAP, leukopenia and preceding influenza-like symptoms were shown to be significant risk factors for mortality (9). The 2009 influenza pandemic resulted in approximately 60 million cases in the United States with 12,000 deaths (10). Pandemic modeling predicted up to 200,000 deaths worldwide (11). Infection rates were 24% overall and as high as 47% in children (12). During the 2009 pandemic, 8.5% of children admitted to a pediatric intensive care unit tested positive for S. aureus, which was a significant risk factor for mortality (13). In a study of 683 critically ill patients, 207 (30%) were superinfected with bacteria, with S. aureus (45%) the most common (14). In that study, the mean time from onset of influenza symptoms to hospitalization with superinfection was 5.2 days. In more recent years, S. aureus has continued to be the most prevalent bacterial species associated with influenza virus infection. During the 2013-2014 season, 23.2% of adult and 17.5% of child influenza patients were superinfected with bacterial pathogens (15). Of those superinfected, 36% were infected with S. aureus, compared to 5.4% with S. pneumoniae. Bacterial superinfection is often associated with severe illness and acute respiratory distress syndrome. In a study of influenza-associated pediatric deaths between 2004 and 2012, 35% of patients died before hospital admission (16). In that study, 40% of fatalities were associated with bacterial superinfection, with S. aureus being most prevalent in 49% of cases compared to 14% for S. pneumoniae. Bacterial superinfection is not isolated to H1N1 influenza virus infection. During the 2017-2018 influenza season, H3N2 virus accounted for 86% of influenza A hospitalizations (17). A total of 165 pediatric deaths were reported to the Centers for Disease Control and Prevention; of these, half were associated with secondary bacterial infection, with S. aureus as the leading cause in over one-third of cases (18). These data demonstrate the current relevance of S. aureus-associated CAP in the context of primary influenza virus infection.
Humans likely develop CAP and secondary bacterial infections of the lung by translocation or aspiration of nasal colonizing bacteria. These bacteria usually act as commensals in the nares but can infect the lung upon the expression of a wide array of virulence factors that differ between the many bacterial strains. Lung infection is often potentiated by damage and alterations in pulmonary antibacterial immunity induced by the preceding viral infection. In the context of CAP, S. aureus and S. pneumoniae are most commonly associated with human disease, but CAP can be caused by a wide range of bacteria, including both Gram-positive and -negative organisms. Herein, pathogen and host factors associated with colonization and infection will be discussed in the context of primary or secondary CAP.
PNEUMONIA-CAUSING BACTERIAL COLONIZATION
While pneumonia pathogens are infectious and can spread in the environment, colonization can increase the risk of developing an infection. Despite its invasive infectious potential, S. aureus can also form part of the microbiome (19). The primary reservoir for S. aureus in humans is the anterior nares, with approximately 30% of individuals colonized and ranging from 104 to 105 CFU/ml in persistent colonizers (20, 21). Nasal carriage is a significant risk for staphylococcal infection, with >80% of infecting isolates originating from the nose (22). Studies in the early 2000s found an increasing rate of nasal colonization with MRSA, ranging from 2% to 8% from 2001 to 2004 (23, 24). A retrospective cohort study found that 17% of patients in the intensive care unit (ICU) had a positive nasal swab for MRSA, and 28.6% of those patients who tested positive for MRSA nasal colonization went on to develop pneumonia (25). Another study found that patients colonized with S. aureus at ICU admission had up to a 15-fold-increased risk for developing staphylococcal pneumonia (26).
Streptococcus pneumoniae is another major colonizer of the upper respiratory tract, typically found in the nasopharynx. Individuals become colonized with S. pneumoniae within the first few months of life, although the age varies and may be influenced by environmental factors (27, 28). Carriage is more common in children, with a prevalence of 20% to 40% and peaking around the age of 1 to 2 years (29). Nasopharyngeal colonization is a major predisposing factor for S. pneumoniae infections, especially acute otitis media, but contributes to more-invasive diseases such as pneumonia (30). The polysaccharide capsule of S. pneumoniae, which defines serotypes, is thought to modulate the degree of colonization, with more-common serotypes having longer carriage (31). There are over 90 serotypes of pneumococcus, and children typically acquire new serotypes before developing disease (32). Prior to the implementation of the 7-valent pneumococcal vaccine (PCV7), the majority of invasive diseases was caused by seven of the pneumococcal serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) (33). The introduction of PCV7 in the early 2000s led to a decrease of the 7 vaccine serotypes, but these were replaced in the population by nonvaccine serotypes (34). This led to the creation of the 13-valent vaccine to cover an additional 6 serotypes (1, 3, 5, 6A, 7F, and 19A), although this has also spurred serotype replacement with non-PCV13 serotypes (35, 36). PCV implementation has had effects on cocarriage and disease caused by other bacteria, most commonly nontypeable (NT) Haemophilus influenzae and S. aureus (37, 38). S. pneumoniae and H. influenzae can compete against each other for dominance of the niche, and colonization with S. pneumoniae is associated with decreased colonization of H. influenzae. In areas where vaccine serotypes have been eradicated, nontypeable H. influenzae otitis media infections have increased (37). There is also a negative association between carriage of S. pneumoniae and S. aureus, and some PCV7 studies have found changes to S. aureus carriage, although reproducibility has varied based on the study setup (39). S. pneumoniae nasal colonization has been reviewed in detail elsewhere (30, 40, 41).
Colonization Factors
S. aureus, like all bacteria, expresses myriad virulence factors upon entering the host environment that aid it in adhering to host tissues, proliferating inside the host, and evading the immune system. This has been modeled by introducing S. aureus into the nasopharynxes of cotton rats, revealing upregulated expression of a variety of virulence factors after 4 days (42). The highly virulent MRSA strain USA300 upregulated adhesion genes (sdrC, sdrD, tarK, sasG, and clfB), and a methicillin-susceptible S. aureus (MSSA) strain had additional upregulation of the related adhesion gene clfA. ClfB, SdrD, SdrC, and SasG have been proposed to play a role in nasal colonization (42, 43). The metal cation transporter genes isdA, isdB, isdH, and fhuD were upregulated in MRSA upon exposure to the nasal environment, while isdA, fhuD, and sstD were upregulated in MSSA. These are all involved in iron acquisition and play a role in virulence, which is expected as the nares are a low-iron environment (44, 45). Immune evasion genes sbi and spa, both encoding antibody binding proteins, were upregulated in both MRSA and MSSA, while the genes encoding pore-forming toxins alpha-hemolysin (hla) and a subunit of Panton-Valentine leukocidin (lukF-PV) were decreased. While there was upregulation of the factors mentioned above, expression levels of adhesion and metal acquisition genes sdrC, fhuD, and sstD increased further in bacteremia and heart infection models, suggesting that these genes might play a role in the transition from colonization to infection (42). Similar results were found in swabs from persistently colonized healthy individuals analyzed for S. aureus gene expression (20). Expression of genes for adhesion and iron binding molecules (fnbA, clfB, and isdA) was increased compared to that during in vitro growth, while genes for pore-forming toxins (hla, psm, and blhB) were poorly expressed. This suggests that adhesion and metabolic genes can be stably expressed during colonization while pore-forming toxins are necessary only during invasive disease and that S. aureus controls these gene expression programs separately. The wall teichoic acid (WTA) has a known role in colonization adherence, and enzymes involved in its production, as well as other cell-remodeling enzymes, were detected at high transcriptional levels compared to those during in vitro growth. Expression of spa was increased, as was that of other immune evasion genes, sak and chp. Evaluation of stress response and metabolic regulators indicates that the nose does not induce an SOS response (upregulation of RecA) or amino acid insufficiency (upregulation of stringent response proteins RelA and CodY), as proteins involved in these processes were at or below in vitro expression levels. This suggests that during colonization, S. aureus is not under environmental stress compared to growth in culture, potentially due to lack of nutrient limitation. Four of the five prominent regulators to environmental stimuli (Agr, SaeRS, SigB, and GraRS) were inactive during nose colonization, while the two-component system WalKR was highly transcribed in some individuals, with expression similar to in vitro post-exponential-phase expression, suggesting that this regulatory system plays a role during colonization (20).
S. pneumoniae colonization has been extensively reviewed elsewhere (41, 46, 47). Herein, we will highlight some of the most important colonization factors. The capsular polysaccharide (CPS) is a major virulence factor of pneumococcus. CPSs are primarily negatively charged, allowing repulsion from the negatively charged sialic acid-rich mucopolysaccharides. This prevents mucus entrapment and allows the bacterium to attach to the epithelial surface. Regulation of CPS expression is important, as modification of the core promoter leads to attenuation of virulence (48). While CPS expression is important for pathogenesis, some pneumococcal isolates do not express a capsule and are referred to as nontypeable (NT) pneumococcal isolates. In a subset of NT pneumococci, a novel open reading frame (ORF) encoding a protein called pneumococcal surface protein Korea (pspK) was found in the cps locus. PspK is a peptidoglycan-attached surface protein that appears to be essential for nasal colonization in NT strains and contains a YPT motif that is known to bind to the polymeric immunoglobulin receptor (pIgR) (49). At the cell surface, S. pneumoniae uses adhesion molecules PavA, PavB, and Eno, which bind to the extracellular matrix proteins fibronectin and plasminogen (41). ChoP (choline phosphate) can bind to the platelet-activating factor receptor (PAFR), while CbpA (choline binding protein A) can bind to the secretory component of pIgR, leading to uptake of the bacteria within nasopharyngeal cells (41, 46). The enzymes PrsA and SlrA also contribute to adherence to epithelial cells by promoting biofilm formation (41). The choline binding protein, CbpL facilitates migration from the nasopharynx to the lung and blood. Pneumococcus also encodes extracellular glycosidases, and some have been shown to enhance adherence by modifying host glycoconjugates to reveal glycan receptors. Neuraminidase A (NanA)-mediated cleavage of sialic acid has been shown to promote biofilm growth as well as to increase carbon availability during nasal colonization (50). NanA, β-galactosidase (BgaA), and β-N-glucosaminidase (SrtH) can also enhance bacterial adhesion independently of the their enzymatic activity (46). Interestingly, the presence of an ahemolytic pneumolysin (PLYa) increases the level of colonization in mice irrespective of capsule type, which may stem from these strains being less immunogenic (51). While the factors expressed by S. aureus and S. pneumoniae differ, upon entering the host both upregulate expression of adhesion molecules, proteins for nutrient acquisition and immune evasion. Both bacteria stably colonize the nasopharynx using a gene program distinct from that involved in infection, where they upregulate their expression of pore-forming toxins and factors specifically involved in active infection.
BACTERIAL VIRULENCE IN PNEUMONIA
Pore-Forming Toxins
Many bacteria secrete pore-forming toxins, which not only can cause cytolysis of host cells but also can modulate host intracellular signaling (Fig. 1). As S. pneumoniae and its toxins have already been extensively studied, we will highlight some newer findings (52–54). The S. pneumoniae pore-forming toxin, pneumolysin (PLY) is a member of the cholesterol-dependent cytolysin (CDC) family, which form large pores in eukaryotic cells with cholesterol-containing membranes and some immune cells with varying susceptibility. PLY is unique in that it is not actively secreted by the bacterium and is expressed at higher levels in clinical versus laboratory strains (52, 55). PLY has been shown to induce inflammatory cytokine release in both nasal and bronchial epithelial lines (56). PLY has also been implicated in exacerbating fibrosis in murine pulmonary fibrosis models as well as leading to alveolar type II cell death within the lung (57). It is important to note that there are sequence variants of the toxin, and this can alter its biological function.
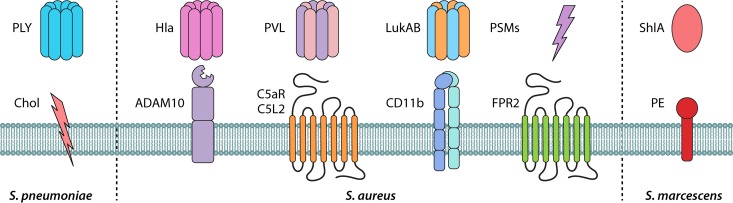
FIG 1.
Pore-forming toxins and their receptors in pneumonia. Staphylococcus aureus and other bacterial toxins involved in bacterial pneumonia are shown. S. aureus alpha-toxin (Hla), Panton-Valentine leukocidin (PVL), leukotoxin AB (LukAB), and phenol-soluble modulins (PSMs) bind to their corresponding membrane receptors to mediate damage and inflammation. C5aR and C5L2, complement component 5a receptors; CD11b, subunit that forms the integrin αMβ2, also known as macrophage-1 antigen (Mac-1) or complement receptor 3 (CR3); ADAM10, disintegrin and metalloproteinase domain-containing protein 10; FPR2, formyl peptide receptor 2. PSMs and Hla can target both human and mouse cells, while PVL and LukAB are human-specific toxins. Streptococcus pneumoniae cytolysin pneumolysin (PLY) and Serratia marcescens toxin ShlA both bind to components of the membrane, i.e., cholesterol (Chol) or phosphatidylethanolamine (PE), respectively, to induce damage and inflammation.
In staphylococcal pneumonia, alpha-hemolysin (Hla) plays a critical role in virulence in murine pneumonia models (58–62). Hla assembles into heptamers at the cell membrane by binding to its receptor disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) and creates a small pore sufficient for the movement of ions across the membrane (Fig. 1) (63). This results in loss of ion regulation, leading to internal signaling that can lead to cytokine release or cell death. In the airway, Hla induces calcium fluxes, proinflammatory signaling, and alterations of ciliary beat frequency. Deletion of ADAM10 in the alveolar epithelium compartment results in reduced morbidity and mortality (64, 65).
While it is known that Hla induces epithelial damage during pneumonia, the mechanism of how this occurs is unknown. Recently, Hook et al. discovered that S. aureus forms microaggregates (MAs) that interact with the alveolar epithelium to induce Hla-mediated membrane damage (66). These MAs form within 1 h via intranasal installation or within minutes via micropipette microinstillation into alveoli, and they form at alveolar epithelium niches, the curved regions of the alveolar wall at septal junctions. This is dependent on the alveolar microanatomy, as flat alveolar septa have only small clusters of bacteria that are easily washed away, and MAs are wash resistant. MA formation is dependent on expression of the phosphonate transporter PhnD at the bacterial cell surface but is independent of host factors, suggesting a role in biofilm formation. Interestingly, alveoli that do not contain MAs have membrane damage which is mediated by intercellular cytoplasmic Ca2+ signaling through connexin 43-containing gap junctions. This leads to loss of mitochondrial potential, inhibition of surfactant secretion, and loss of alveolar barrier integrity. Thus, S. aureus MAs can damage epithelial cells without having direct contact. The authors also found that Hla is secreted only at the MA-alveolar epithelium contact site and can reach concentrations of more than 100 μg/ml, which accounts for the rapid loss of membrane integrity in MA-containing alveoli and lung edema leading to increased mortality. Interestingly, PhnD also reduces the ability of vancomycin-sized solutes to enter MAs, potentially explaining why treatment is not effective in S. aureus pneumonia. Mice infected with wild-type but not ΔphnD S. aureus did not show decreased mortality with vancomycin treatment, while ΔphnD mutant-treated mice had limited mortality and restored small-molecule penetration of MAs (66).
NLRP3 Inflammasome Activation
It was shown in 2009 that Hla can activate the NLR family pyrin domain-containing 3 (NLRP3) inflammasome in human and mouse monocytes, and recently these interactions have been actively studied (Fig. 2) (58–62, 67, 68). The NLRP3 inflammasome consists of the sensor molecule NLRP3, the adapter protein ASC, and pro-caspase-1. Activation of the inflammasome leads to cleavage of pro-caspase-1 to its active form and subsequent cleavage and activation of interleukin-1β (IL-1β) and IL-18, promoting an inflammatory response (69). Hla activates the NLRP3 inflammasome during staphylococcal pneumonia and leads to necrotic pulmonary injury independent of IL-1β signaling (58). Mice lacking the NLRP3 inflammasome had increased survival, less morbidity, and lower bronchoalveolar lavage (BAL) fluid IL-1β and IL-18 levels (58, 61, 67). As mice lacking the IL-1 receptor (IL-1R−/−) fared as poorly as wild-type animals, IL-1β appears to be a by-product of the effect rather than directing it. However, neutralizing IL-1β or IL-18 increases survival, most likely due to decreased lung inflammation (58, 60, 61, 66, 67). This effect is due to Hla changing the localization of the NLRP3 inflammasome and mitochondria away from the phagosome in murine bone marrow derived-monocytes (BMDMs) and human monocytes (61). This is presumably due to the efflux of K+ through pores formed by Hla, leading to activation of the NLRP3 inflammasome before mitochondria can localize to the phagosome (59, 69).
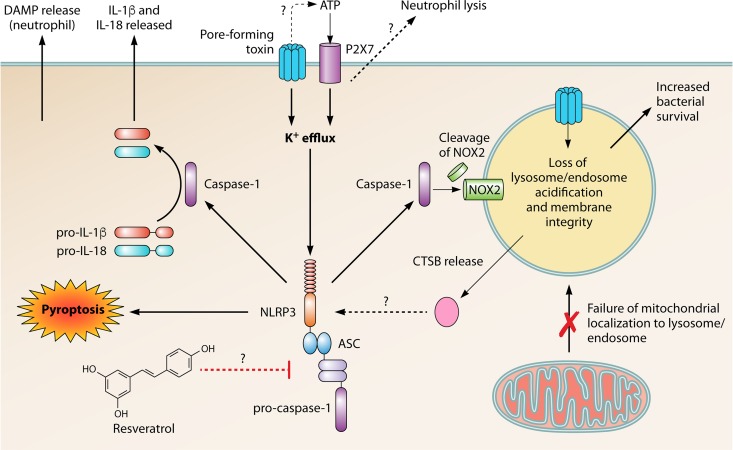
FIG 2.
Pore-forming toxins induce the NLRP3 inflammasome in phagocytes. Bacteria that encode pore-forming toxins can induce the NLRP3 inflammasome by forming pores within the membrane and facilitating potassium efflux. ATP, potentially leaving the cell via bacterial pores, binds to the purinoceptor P2X7 and induces K+ efflux that contributes to NLRP3 inflammasome activation and induces lysis in neutrophils through an unknown mechanism. Potassium efflux leads to oligomerization and activation of the NLRP3 inflammasome, consisting of the sensor protein NLRP3, adapter protein ASC, and pro-caspase-1, leading to cleavage and activation of effector caspase-1. Activation of caspase-1 leads to cleavage of pro-IL-1β and pro-IL-18 and subsequent release from the cell. Inflammasome activation can also lead execution of necrotic cell death via pyroptosis as well as DAMP release in neutrophils. Caspase-1 can also cleave components of the phagocyte NADPH oxidase, NOX2, which can lead to loss of endosomal acidification. Bacterial pores on the endosome also contribute to lack of acidification due to loss of membrane permeability as well as preventing the mitochondria from localizing with the endosome due to the strong activation of NLRP3. Loss of acidification leads to increased survival of bacteria within the phagosome/endosome. Cathepsin B (CTSB) can be released from damaged lysosomes and can also induce NLRP3 inflammasome activation, via an unknown mechanism. The antioxidant resveratrol can inhibit the expression and activation of the NLRP3 inflammasome, leading to a decrease in bacterial survival.
It has been appreciated that activation of the NLRP3 inflammasome by Hla may be a protective mechanism for S. aureus to avoid internalized killing (59–61). Hla decreases the acidification of phagosomes/lysosomes in macrophages but not neutrophils (39, 59, 61), which may be due to the difference in expression of ADAM10 between cell types (63). Failure to acidify the phagosome increases the S. aureus burden, leading to high rates of mortality (59). Inhibition of acidification is dependent on the NLRP3 inflammasome and subsequent activation of caspase-1, phagocytosis, and the pore-forming ability of Hla, while it is independent of nuclear factor κB (NF-κB), Toll-like receptor 2 (TLR2), myeloid differentiation primary response 88 (MyD88), and TIR domain-containing adapter-inducing interferon beta (TRIF) (39, 59). The mechanism of how caspase-1 inhibits acidification of the phagosome appears to be due to cleavage and inactivation of components of the phagocyte NADP (NADPH) oxidase, NADPH oxidase (NOX2), as caspase-1 can cleave both the GTPase regulator Rac family small GTPase (RAC1) and the gp91phox subunit. Inhibiting caspase-1 decreases the acidity of endosomes as seen by an increase in fluorescein isothiocyanate (FITC) mean fluorescence intensity (MFI) (61). This decrease in phagosomal acidification can also facilitate survival of coinfecting Gram-negative pathogens such as Pseudomonas aeruginosa (70).
In the context of human staphylococcal pneumonia, it has been shown that several human-specific S. aureus pore-forming toxins can activate the NLRP3 inflammasome (Fig. 1) (71, 72). Panton-Valentine leukocidin (PVL), a bicomponent leukotoxin, recognizes the human complement 5a (C5a) receptors C5aR and C5L2, specifically targeting phagocytes (71, 73). PVL positivity among MRSA strains varies across the world, with studies in the United States showing rates ranging from 36% to 98% (74, 75). PVL+ strains have been associated with more severe staphylococcal disease, such as necrotizing hemorrhagic pneumonia (76). PVL treatment produces high levels of IL-1β in human monocytes and macrophages while inducing release of large amounts of the danger-associated molecular patterns (DAMPs) calprotectin (MRP8/14, S100A8/9) and S100A12 in neutrophils. PVL-mediated IL-1β release is dependent on K+ efflux, leading to cathepsin B (CTSB)-mediated NLRP3 inflammasome activation. The toxin LukAB (also known as LukGH) is the most recently identified S. aureus leukotoxin, leading to death of human neutrophils, monocytes, macrophages, and dendritic cells (DCs) through its receptor cluster of differentiation 11b (CD11b) (77). S. aureus-induced IL-1β and IL-18 release is dependent on capsase-1 and apoptosis-associated speck-like protein containing a CARD (ASC) and occurs after a 5-min lag time after LukAB treatment (72). Strains of S. aureus lacking LukAB have a reduced ability to activate the NLRP3 inflammasome and kill human monocytes. Interestingly, intracellular S. aureus can induce cell death by LukAB binding to CD11b independently of the NLRP3 inflammasome, but this requires ASC and NLRP3 when the bacteria are located extracellularly. The difference in cell death based on infection location may be due to cellular localization-dependent changes in CD11b signaling (72).
A recent humanized mouse MRSA pneumonia model has recapitulated many of the aspects of human infections (78). Immunodeficient NOD scid gamma (NSG) mice reconstituted with human stem cells (hNSG) have higher bacterial burdens in the lung and BAL fluid than murine-reconstituted NSG mice (mNSG). The CFU burden is further increased in the BAL fluid and lung in human IL-3/granulocyte-monocyte colony-stimulating factor (GM-CSF) knock-in mice, which increases human myeloid reconstitution (79). These knock-in mice have increased myeloid cell recruitment and trend toward increased IL-1β and IL-8. Expression of the PVL receptor, hC5aR, increases on human macrophages and neutrophils following S. aureus infection and is decreased after treatment with anti-PVL or using Δpvl S. aureus. When PVL is blocked, the macrophage number is increased, most likely due to better survival. Interestingly, while some groups have found an importance of LukAB in the death of human monocytes (72), this study found no difference in lung or BAL fluid CFU between wild-type and ΔlukAB S. aureus strains (78).
S. pneumoniae pneumolysin (PLY) can also induce inflammasome activation in macrophages and neutrophils and, like for S. aureus, has been shown to require the cytolytic activity of the toxin (21, 53, 54). However, the cytolytic activity does not seem to be as important for an immune response against the pathogen, although this appears to be strain specific (80). PLY has been extensively studied and reviewed (see reference 53 for more information regarding PLY-mediated inflammasome activation).
Host Cell Death
Pore-forming toxins can also induce cell death in epithelial and immune cells via necroptosis. Necroptosis is an inflammatory programmed cell death pathway that is independent of caspase activation. Upstream death receptor signals lead to the formation of the necrosome, consisting of receptor-interacting protein kinase 1 (RIP1) and RIP3, which form large amyloid-like structures. RIP3 phosphorylates downstream targets, including mixed-lineage kinase domain-like (MLKL), and pMLKL associates with the membrane, promoting ion flux and cell lysis (81). Tumor necrosis factor alpha (TNF-α) can enhance necroptosis, as TNFR1 signaling recruits RIP1, which undergoes multiple protein modifications before recruiting RIP3 (82). In the human alveolar epithelial cell line A549, the addition of TNF-α to cells treated with S. aureus increased lactate dehydrogenase (LDH) release and the percentage of necroptotic cells (83). TNF-α, in addition to S. aureus, induced expression of RIP3 in A549 cells but did not lead to activation of caspase-1 or -8, while necroptosis was dependent on RIP3 (83). This is presumably through the action of Hla, as another study using mice lacking a disintegrin and metalloproteinase domain-containing protein (ADAM10) in myeloid and alveolar epithelial cells had complete abrogation of mortality and decreased morbidity (64). S. pneumoniae PLY as well as the Gram-negative Serratia marcescens pore-forming toxin ShlA have also been shown to induce necroptosis in the lung in both mice and nonhuman primates. Induction of necroptosis is independent of death receptor and TLR signaling but instead is activated by membrane permeabilization leading to calcium and potassium dysregulation (84).
Cell death in macrophages.
The staphylococcal pore-forming toxin Hla can induce necroptosis in macrophages, causing a decrease in number of alveolar macrophages (AMs), but not neutrophils, in the lungs at 24 h postinfection through the formation of cytoplasmic pores (Fig. 2) (62). S. aureus infection of both primary human macrophages and the human monocytic cell line THP-1 increases active phosphorylated mixed-lineage kinase domain-like protein (pMLKL) levels. This is reduced by the necroptosis inhibitors necrostatin-1 (Nec-1) and necrosulfonamide, which inhibit RIP1 and MLKL, respectively. THP-1 cells also display reduced IL-1β release upon necroptosis inhibition. Mice lacking RIP3 or wild-type mice treated with Nec-1 have less AM death, a lower bacterial burden, and decreased lung leak and inflammation. However, clodronate depletion of lung macrophages increased the bacterial burden and lung leak in wild-type but not RIP3 knockout mice, which suggests that necroptosis of other cell types also contributes to lung pathology (62).
In addition to staphylococcal Hla, pore-forming toxins from a variety of Gram-positive and negative bacteria can induce necroptosis in macrophages (85). S. marcescens, S. aureus, S. pneumoniae, Listeria monocytogenes, and uropathogenic Escherichia coli (UPEC) all initiate the necrosome. The S. marcescens pore-forming toxin ShlA depletes macrophages during pneumonia, and mice lacking necroptosis components have increased AM numbers in the BAL fluid (Fig. 1). ShlA induces pMLKL plasma membrane colocalization in immortalized murine AMs, resulting in necroptosis; inhibiting pMLKL prevents this aggregation. In that study, macrophage necroptosis signals included loss of iron homeostasis at the plasma membrane, cytochrome c release via direct or indirect mitochondrial damage, ATP depletion, and generation of reactive oxygen species (ROS). The RIP1 inhibitor necrostatin-5, along with coenzyme Q10, enhances ATP production and reduces the severity of S. marcescens pneumonia. Resveratrol, which can increase mitochondrial numbers (86), was protective against cell death. In S. marcescens pneumonia, unlike S. aureus pneumonia (85), clodronate-depleted mice had a decreased burden, suggesting that AM death is detrimental to the host, potentially through nutrient availability to pathogens or alarmin release. Interestingly, ASC-, NLRP3-, and MyD88-deficient BMDMs have some protection from necroptosis for the bacteria mentioned above, suggesting that the inflammasome and TLR signaling components also participate in toxin-mediated death independent of caspase-1 activation. IL-1β release is observed, but this could be due to cell lysis, as pro-IL-1β and active IL-1β were not distinguished (85).
In contrast to other pore-forming toxins which overwhelmingly induce inflammatory cell death, PLY can induce apoptosis in macrophages by two independent mechanisms, both independent of pore formation. Apoptosis is a noninflammatory form of cell death, where the cellular contents are still contained within a membrane. Loss of lysosomal and phagosomal membrane permeabilization (LMP) can increase susceptibility to programmed cell death. PLY is necessary for apoptosis induction extracellularly by inducing LMP but requires other microbial signals through MyD88 or TLR2 and TLR4 and is independent of pore-forming ability, NLRP3 and ASC inflammasomes, and macrophage phagocytosis. PLY-induced LMP promotes apoptosis, as Δply strains result in macrophage necrosis. Once S. pneumoniae is internalized, PLY induces apoptosis by activation of lysosomal protease cathepsin D, leading to loss of mitochondrial function, caspase-3 activation, and apoptosis. Similar results were found in the related species Streptococcus mitis, which encodes a PLY-related cytolysin, mitilysin, suggesting that this is a conserved response (80).
Cell death in neutrophils.
PLY can also induce death in neutrophils, resulting in release of neutrophil elastase (NE), which inhibits phagocytosis by macrophages and causes epithelial damage (87). S. pneumoniae culture supernatant can induce lysis in neutrophils, causing dose-dependent LDH release, but not in macrophages or epithelial cells. Recombinant PLY (rPLY) treatment causes neutrophils to release NE through cell lysis dependent on the upregulation of the extracellular ATP purinoceptor P2X7. While this was typically not expressed on unstimulated neutrophils, it was highly upregulated on dead neutrophils (87). NE- or PLY-treated neutrophil supernatants induce dose-dependent cell rounding and detachment in both A549 and RAW264.7 cells and can be inhibited by the NE inhibitor sivelestat. RAW264.7 cells also have decreased phagocytosis when exposed to NE. How lysis occurs is still unclear, but ligation of P2X7 with ATP leads to opening of the ligand gated channel, causing a rapid increase in cytosolic Ca2+ and K+ efflux, which leads to NLRP3 inflammasome activation and IL-1β release in human and murine neutrophils (21). This suggests that PLY may mediate neutrophil lysis by inducing ATP release via its pores, allowing ATP to bind to P2X7, although the loss of ion regulation and the subsequent NLRP3 inflammasome may play a role (21, 87).
Staphylococcal toxins can also cause cell death in neutrophils. Staphylococcal species produce phenol-soluble modulins (PSMs), which are pore-forming toxins consisting of 7 amphipathic α-helical peptides, PSMα1 to -4, PSMβ1 and -2, and the delta-toxin (88). PSMs are regulated by the virulence regulator Agr, and cytolysis of host cells most likely occurs through nonspecific receptor-independent mechanisms, although the cellular receptor formyl peptide receptor 2 (FPR2) induces inflammatory effects. A large portion of the highly pathogenic potential of community-associated MRSA (CA-MRSA) is due to lysis of human neutrophils (88). PSMs can be inhibited indirectly by inhibiting the Agr system through the staphylococcal heptapeptide RNAIII-inhibiting peptide (89). Mice treated with RNAIII-inhibiting peptide had dose-dependent decreased expression of PSMs in the lung and had reduced weight loss, bacterial burden, and mortality (90). Necroptosis inhibitors increased survival and decreased bacterial burden and pathology in mice. Neutrophils, but not macrophages, are essential for the protective effect of RNAIII-inhibiting peptide in vivo and are protected by necrostatin-1 (Nec-1) or necrosulfonamide (NSA) treatment. Necroptosis is dependent on FPR2 as well as TNF-α, as blocking by WRW4 or anti-TNF-α decreased necroptosis (90).
PSMs are not the only mechanism by which S. aureus kills the neutrophils responding to infection. Two-component toxins, including PVL and LukAB, are also able to lyse neutrophils, which can in turn neutralize PVL with antimicrobial peptides such as alpha-defensins (91). S. aureus also has defenses against complement, which, along with immunoglobulins, opsonizes the bacteria, thus making it more easily phagocytosed by neutrophils and other phagocytes (92). Such defenses include staphylokinase, which complexes with plasminogen to degrade complement protein C3 (93) as well as antimicrobial peptides (94), and the CHIPS protein, which binds the complement receptor C5aR (95). S. aureus can also subvert neutrophil killing by reactive oxygen species (ROS) by establishing a hypoxic environment through creation of bacterial biofilms (96), reducing ROS production through lack of molecular oxygen and independent of NADPH oxidase expression (97). S. aureus uses the highly oxygenated environment of blood capillaries to its advantage, recruiting neutrophils to these capillaries, which deform to fit the shape of the capillaries and inhibit blood perfusion, thus increasing tissue damage (98).
S. aureus secretes nucleases, specifically Nuc1 (99), to degrade neutrophil extracellular traps (NETs). NETs are formed rapidly upon S. aureus interaction, approximately 3 h after initial contact (100). “NETosis” begins with the unraveling of the neutrophil’s chromatin and nuclear membrane, which allows the DNA and histones to mix with granule proteins such as antimicrobial peptides and proteases in the cytoplasm. These NETs are then released during the lysis of the neutrophil and extend extracellularly with a diameter of approximately 15 to 17 nm in order to entrap bacteria and degrade them using captured neutrophil granule enzymes (101). Paradoxically, S. aureus biofilms have been shown to induce NETs through various leukocidins regulated by the Agr/SaeRS network, including LukAB (102), PVL, and gamma-hemolysin AB. While NETs have been shown to be effective in clearing S. aureus, they are inefficient at clearing biofilms both in vitro and in vivo (103).
Neutrophil efferocytosis.
Efferocytosis is a highly regulated uptake and degradation of apoptotic bodies in macrophages and is important in pathogen clearance. When a cell is dying, it produces “eat me” signals, such as phosphatidylserine, on the extracellular membrane leaflet, and macrophages express specific receptors, such as the scavenger receptor CD36, that bind to ligands on the apoptotic cell. The macrophage will engulf the apoptotic body and degrade its contents, including pathogens, via the lysosome (104). S. aureus can prevent efferocytosis of human neutrophils by macrophages. Coculture of macrophages with apoptotic neutrophils leads to transient binding and engulfment in a time-dependent manner. In comparison, neutrophils that have ingested S. aureus (PMN-SA) had increased binding but were not engulfed by macrophages. However, S. aureus does not utilize neutrophils as a “Trojan horse,” as the majority of S. aureus was bound to the surface of macrophages after neutrophil uptake (105).
Upon phagocytosis of S. aureus, neutrophils begin to die due to loss of mitochondrial membrane potential. As up to 50% of bacteria remain viable 3 h after phagocytosis, it is necessary for control of infection that dying neutrophils are disposed of by macrophages. While PMN-SA display increased exposed surface phosphatidylserine, they continue to express high levels of the “don’t eat me” signal molecule CD47, which must be downregulated for proper efferocytosis (105). This suggests that S. aureus is able to derail efferocytosis by sustaining surface expression of CD47 on neutrophils. Moreover, the cell cycle protein proliferating cell nuclear antigen (PCNA), which promotes neutrophil survival and is decreased during apoptosis, has sustained cytoplasmic levels in PMN-SA for up to 24 h (105, 106). Macrophages that ingest PMN-SA have decreased IL-1RA, basal levels of inflammatory cytokines and NLRP3 activation, and increased IL-10 expression, although inflammatory cytokines were increased with higher PMN-SA-to-macrophage ratios (105).
In mice, the staphylococcal toxin Hla decreases efferocytosis of neutrophils by macrophages through multiple pathways (60). Alveolar macrophages play a role in clearance of neutrophils in the lung and when treated with Hla ex vivo internalize fewer neutrophils. Treatment with the anti-Hla antibody MEDI4983 or use of Δhla S. aureus abrogates the toxin’s effect but does not alter the expression of CD47 or its receptor CD172 on neutrophils and macrophages, respectively. This process is dependent on d-alanyl-alanine ligase (DD1α) expression on host cells, which has been shown to play a role in efferocytosis in cancer (107), and using an anti-DD1α antibody decreases neutrophil uptake without changing the expression level of the receptor. Cellular communication network factor 1 (CCN1) expression, which is also involved in neutrophil clearance (108), was increased in MEDI4983-treated mice as well as in A549 cells treated with H35L compared to wild-type Hla (60).
IMMUNITY TO BACTERIAL PNEUMONIA
The lung has robust immunity to pathogenic bacteria, which are introduced by every breath into the lungs. Since immune mechanisms have been extensively reviewed (109), a short summary is provided here. The first layer of defense against infection is barrier function; mucus and cilia work together to clear the airway of debris and pathogens, and surfactant proteins bind bacteria to improve clearance. Alveolar macrophages patrol alveoli, eliminating extracellular bacteria and displaying antigen to T cells. When pathogenic bacteria enter the lung, recognition by pattern recognition receptors occurs in cells at barrier sites. Epithelial cells produce antimicrobial peptides, which can directly lyse bacteria. Alveolar macrophages make interferons (IFNs) and inflammatory cytokines, resulting in neutrophil and monocyte activation and homing to the lung. When unimpaired, this influx of phagocytes provides bacterial phagocytosis and killing to control the infection, including generation of reactive oxygen species. Bacteria induce variable chemokine responses, but overall cytokine responses are often conserved. The influx of cells into the lung brings fluid, which can lead to acute respiratory distress syndrome (ARDS) and significant mortality if unresolved. Herein, the focus is on host-pathogen interactions that mediate immune dysregulation.
Impairment of Host Defense
A preceding viral infection is known to increase susceptibility to secondary bacterial pneumonia (110, 111). The influenza virus burden peaks at 3 to 4 days following infection and is cleared between days 10 and 14 (111, 112). Inflammation peaks at around 7 days after viral infection (113–115), coinciding with the period of susceptibility to secondary bacterial infection. This strongly suggests that underlying immune dysfunction is crucial to the pathogenesis of superinfection. Studies have shown a plethora of antibacterial immune defenses that are reduced by preceding influenza. In the case of Staphylococcus aureus superinfection, these include type 17 immunity, IL-1 cytokine/inflammasome activation, and antimicrobial peptide production (116–119).
The innate immune response to influenza virus has been well characterized (120). The first cells involved in influenza virus infection are alveolar epithelial cells, which are preferentially infected by the influenza virus. Influenza virus strains have distinct tropisms but mostly infect epithelial cells and alveolar macrophages (121). Influenza virus replicates inside these cells and buds off virions. Infected cells downregulate protein synthesis to control the infection (122), leading to death by apoptosis (123). By days six to seven of influenza virus infection, cytotoxic CD8+ T cells arrive in the lung and contribute to epithelial death and sloughing (124). Alveolar macrophages phagocytose dying epithelial cells (125), sensing foreign nucleic acids and producing inflammatory chemokines to recruit immune cells to the infection. Production of type I and III interferons (IFNs) by epithelial cells, macrophages, and dendritic cells leads to interferon regulatory factor (IRF)-mediated induction of interferon-stimulated genes (ISGs), promoting an antiviral state in the lung (126–128).
A week into influenza virus infection, the inflammatory response is maximal and there is significant fluid in the lung due to immune cell influx. The epithelial barrier has eroded, leading to disrupted gas exchange and poor blood oxygenation. The lung is now “primed” by influenza for bacterial superinfection. Extracellular bacteria, both Gram positive and Gram negative, now take advantage of exposed adherence sites (129) and begin secondary infection in a nutrient-rich environment (130). When these pathogens enter the influenza virus-infected lung, antibacterial immune responses are blunted. Antimicrobial peptides, a broad swath of proteins, including cathelicidins and defensins, are made by epithelial cells and neutrophils as direct killing mechanisms against bacteria (131). These peptides can also serve to recruit neutrophils and act as chemokines (132). Prior influenza virus infection suppresses this antimicrobial peptide response, especially through inhibiting lipocalin 2 production. Restoration of antibacterial immunity can be achieved when lipocalin 2 is given exogenously (119).
Macrophages and dendritic cells (DCs) in the lung produce type I IFNs in response to bacterial infection (133, 134). In the case of S. pneumoniae infection, bacterial DNA enters macrophages via PLY, leading to production of IFN-β. IFN-β acts in an autocrine manner on macrophages to promote production of the granulocyte chemokine C-C motif chemokine ligand 5 (CCL5), as well as in a paracrine manner to activate ISGs and CCL5 production in epithelial cells (135). Similarly, S. aureus infection also leads to IFN-β production via stimulator of interferon gene (STING) protein and the cytosolic DNA sensor cyclic GMP-AMP synthase (cGAS). Importantly, this production of IFN-β is pathogenic to the host, as mice lacking STING effectively clear cutaneous S. aureus infection (136). Influenza virus induces production of type I and significantly more type III IFN (137, 138), with expression peaking at 3 to 5 days after infection (139). While wild-type mice show an increase in bacterial burden when influenza precedes pulmonary S. aureus infection, mice lacking the type I IFN receptor (IFNAR−/−) show no such increase (116). This effect is not specific to S. aureus and has been shown for S. pneumoniae (140) as well as Gram-negative bacteria, including Escherichia coli and Pseudomonas aeruginosa (141). Mice lacking the IFN-induced transcription factor signal transducer and activator of transcription 1 (STAT1) or STAT2 (142) also exhibit reduced susceptibility to secondary bacterial infection (143). These findings were recapitulated in mice lacking the type III IFN receptor, suggesting that both type I and III IFNs promote bacterial superinfection (144). Interestingly, wild-type mice are surprisingly less susceptible than IFNAR−/− mice to bacterial superinfection early during influenza virus infection. This resistance to bacterial superinfection tracks with an increase in the type I IFN family member IFN-β, whereas later susceptibility correlates with increased IFN-α. Importantly, antibody inhibition of IFN-β at 1 day after viral infection increased the bacterial burden in the lung, while antibody inhibition of IFN-α at 5 days after viral infection reduced the lung bacterial burden. Mice treated with recombinant IFN-α at 1 day before lung infection with MRSA had an increased bacterial burden in the lung, suggesting that increased IFN-α is critical to enhancing susceptibility to superinfection (145).
Type I IFN has been shown to inhibit myriad antibacterial defenses in the lung. Type 17 cytokines are crucial to antibacterial defense and are inhibited by the type I IFN response to prior influenza. Mice lacking the receptor for the type 17 cytokine IL-17 or IL-22 had a significantly increased lung bacterial burden during primary S. aureus infection. In mice that received influenza virus at 6 days prior to S. aureus challenge, type I IFNs inhibited the induction of IL-17, IL-22, and IL-23, a potent inducer of type 17 cytokines. Exogenous IL-23 rescued the production of both IL-17 and IL-22 and increased bacterial clearance during S. aureus superinfection (116). Type 17 cytokines are produced predominantly by gamma-delta T cells (146), which are present in or quickly recruited to tissue during bacterial infection, in contrast to conventional CD4+ T helper 17 (Th17) cells.
Besides IFN production, influenza leads to recruitment of neutrophils to the airspace. These cells produce inflammatory chemokines, recruiting granulocytes to the lung. Neutrophils recruited to fight pulmonary bacteria during influenza have reduced bactericidal capacity yet retain their inflammatory functions, leading to increased immunopathology. It has been shown that neutrophils from influenza virus-infected mouse lungs display reduced bacterial phagocytosis and intracellular ROS production upon bacterial challenge (147). Macrophages also display decreased bacterial phagocytosis when infected with influenza virus (148). This suppression of neutrophils is recapitulated in humans, where influenza virus infection leads to a defect in lysozyme secretion by sputum neutrophils (149). However, the presence of these neutrophils in the lung is still required, as antibody depletion of Ly6G+ cells resulted in increased bacterial burden during MRSA challenge at 7 days after influenza virus infection (145). In fact, increased neutrophil numbers can aid bacterial clearance when combined with an increase in function (150).
Strains of S. aureus that lack the PVL have a significantly blunted ability to induce IL-1β production by human macrophages (151). Indeed, S. aureus protects itself by inducing shedding of the type II IL-1 receptor from monocytes and neutrophils, dampening the host IL-1β response (152). IL-1β is activated by caspase cleavage through inflammasomes, the best studied of which is the NLRP3 inflammasome. Interestingly, the NLRP3 inflammasome has been shown to help S. aureus evade macrophage killing. Specific inhibition of the NLRP3 inflammasome with the small molecule MCC950 decreased the bacterial burden during S. aureus lung infection by increasing mitochondrial colocalization with bacteria. Treatment with the specific mitochondrial ROS scavenger MitoTEMPO reduced monocyte killing of alpha-toxin-deficient S. aureus, which more often localized with mitochondria than wild-type S. aureus, suggesting that alpha-toxin-triggered NLRP3 inflammasome formation helps S. aureus evade killing by mitochondrial ROS (61).
While IL-1 family cytokine activation may be beneficial to bacteria during primary pulmonary infection, they aid the host defense during influenza virus superinfection. Influenza has been shown to inhibit production of two IL-1 family members, IL-1β and IL-33, during bacterial superinfection. Exogenous administration of either of these cytokines increases bacterial clearance during influenza virus and S. aureus superinfection (117, 150). Moreover, IL-1R−/− mice have increased mortality and lung bacterial burden during superinfection with S. aureus (117) or S. pneumoniae (153). However, MCC950 inhibition of the NLRP3 inflammasome reduces the bacterial burden during superinfection, and mice lacking the inflammasome adapter protein ASC have reduced mortality and bacterial burden during bacterial superinfection, both of which are concurrent with a reduction in IL-1β (118). Supporting this, the use of resveratrol, a natural antioxidant and NLRP3 inhibitor, lowers mortality and IL-1β, IL-18, and TNF-α release in the BAL fluid and lung (67). These data are a microcosm of the overall investigation into immunity during superinfection, demonstrating the need to balance antibacterial immunity and overall inflammation to promote the best outcomes during superinfection. Perturbations in host antibacterial immunity due to influenza are summarized in Fig. 3. Although significant strides have been made in understanding the complex interactions between influenza and its superinfecting bacteria, more investigation is clearly warranted to better define this relationship and inform clinical treatment of associated CAP.
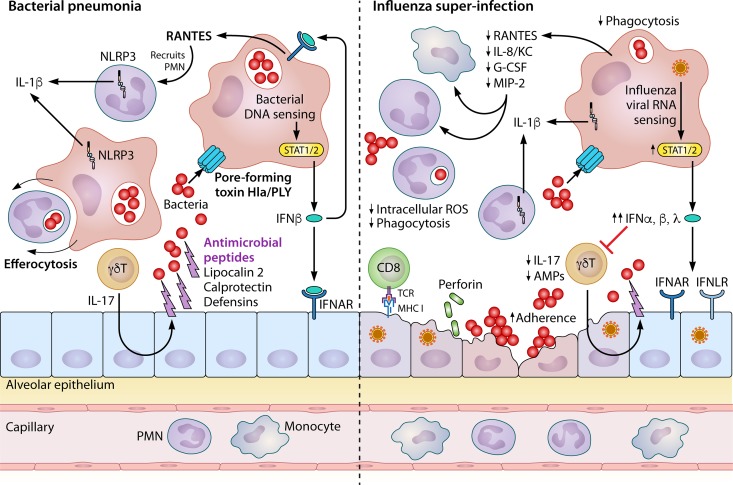
FIG 3.
Preceding influenza inhibits pulmonary immunity to bacterial pneumonia. Pulmonary innate immunity to bacteria (left) is orchestrated mainly by epithelial cells, macrophages, neutrophils, and gamma-delta T cells. The epithelium provides a physical barrier to infection and expresses antimicrobial peptides to kill extracellular bacteria. This expression is augmented by type 17 cytokines from gamma-delta T cells (γδT). Alveolar macrophages (AMs) patrolling the airspaces engulf bacteria through phagocytosis, eventually leading to killing in acidified phagosomes. Bacterial pore-forming toxins (notably Staphylococcus aureus Hla and Streptococcus pneumoniae PLY) allow the entry of bacterial DNA into the cytoplasm of alveolar macrophages, leading to interferon beta (IFN-β) production via STAT1/2 signaling. This IFN-β can bind receptors on epithelial cells but also signals in an autocrine fashion on these macrophages to induce production of RANTES and other chemokines. These chemokines recruit mainly neutrophils from the bloodstream, which can also phagocytose bacteria. Both neutrophils and macrophages contain the NLRP3 inflammasome, a scaffold of proteins serving to activate caspase-1 and other enzymes that cleave IL-1 cytokine family members (mainly IL-1β). Finally, macrophages prevent excess inflammation by engulfing dead or dying cells through a phagocytic process known as efferocytosis. Influenza induces susceptibility to bacterial infection through inhibiting antibacterial immune defenses (right). Influenza virus preferentially infects epithelial cells, leading to destruction of the epithelial barrier from viral infection and later cytotoxic CD8+ T cell activation. This increases the ability of bacteria to adhere to the epithelium. Production of both type I (α and β) and III (λ) IFNs is highly increased during the antiviral immune response, leading to inhibition of type 17 cytokines and antimicrobial peptide production. IL-1β production is also reduced by preceding influenza. Overall, chemokine production is reduced, while airspace cellularity is increased due to the immunopathologic neutrophil recruitment in response to influenza virus infection. Phagocytosis of bacteria by both macrophages and neutrophils is blunted, concomitant with a decrease in intracellular reactive oxygen species important for bacterial killing in the phagosome.
ANTIBACTERIAL THERAPIES
As lung function is critical to life, development of new antimicrobial therapies to fight bacterial pneumonias is necessary. Pneumococcal vaccination has drastically reduced streptococcal pneumonia since the initial licensing of the vaccine in 1977 (154). However, S. pneumoniae disease is still prevalent. Molecular targets for antibody neutralization have been proposed, including PLY (155), as well as newer protein vaccine candidates such as the choline binding protein PspA (156, 157). Vaccines against S. aureus have not yet cleared phase III clinical trials, but many are in development, as summarized in a recent review (158). Neutralizing antibodies against staphylococcal toxins, namely, alpha-toxin, have been successful in a variety of trials (159, 160). Many other therapies against bacterial pore-forming toxins have been developed over the past few decades and have recently been comprehensively reviewed (161).
One path toward creating effective therapies is examining factors that affect patient survival, as mice and humans differ significantly in their immunity to S. aureus infection, as demonstrated by the fact that humanized mice show increased susceptibility to S. aureus skin infection (162). Children displayed increased antibody titers to the bicomponent pore-forming toxin LukAB upon seroconversion from acute phase to convalescent phase during invasive S. aureus infection, and serum containing anti-LukAB antibodies was able to neutralize the cytotoxicity of S. aureus isolates (163). Thomsen et al. were able to generate three hybridomas from a pediatric patient with S. aureus osteomyelitis that produce monoclonal antibodies with anti-LukAB activity. These antibodies were effective against cytotoxicity and together were able to reduce colony counts in a murine model of S. aureus sepsis (164). LukAB has now been shown to kill not only neutrophils and macrophages but also human dendritic cells (165), suggesting that this toxin is a prime candidate for therapeutic targeting. The possibility remains that neutralizing only one toxin may not be effective, as others become more highly expressed to take its place in the phenomenon of “counterinhibition” (166). To address this, a single human monoclonal antibody with the ability to neutralize alpha-hemolysin as well as four other bicomponent leukocidins has been developed, and more recently a combination therapy with two human monoclonal antibodies which together can neutralize six S. aureus cytotoxins has been developed (167, 168).
Finally, the intriguing concept of dominant negative toxins as therapy against S. aureus has recently been introduced. Deletion of glycine-rich stem domains from the staphylococcal bicomponent leukocidin LukED resulted in the creation of single-toxin mutants, which were able to protect human neutrophils against lysis by wild-type LukED as well as PVL, LukAB, and gamma-hemolysins AB and CB (169). Gamma-hemolysin AB targets human neutrophils by binding chemokine receptors C-X-C motif chemokine receptor 1 (CXCR1), CXCR2, and C-C chemokine receptor 2 (CCR2), while gamma-hemolysin CB binds the complement receptors C5aR and C5L2 (170). While conventional therapies (i.e., vaccines and neutralizing antibodies) are being developed and will much sooner be ready for use in the clinic, these highly novel strategies are exciting and may prove more effective as they are further investigated in preclinical and clinical trials.
CONCLUSIONS
CAP is characterized by complex host-pathogen interactions, which determine the extent of infection and tissue damage. In this review, the focus is on bacterial pneumonia and pathogenesis driven by virulence factor interaction with the host immune system. Both S. aureus and S. pneumoniae produce numerous toxins that may prove to be clinically relevant targets for therapy, and may also prove effective in developing therapeutics against other bacteria with similar strategies against the host. For example, other lung pathogens such as Klebsiella pneumoniae and Aspergillus fumigatus induce NETs from neutrophils, and an even broader group of pathogens has specific NET evasion strategies (171). Development of strategies to prevent binding of host receptors by toxins, such as blocking antibodies to ADAM10 and complement receptors, may also prove useful against a wide variety of pulmonary pathogens (172).
However, a critical challenge faced by clinicians is a lack of effective interventions to limit lung pathology during CAP. Broad-spectrum antibiotics can limit bacterial outgrowth and dissemination; however, these are often ineffective at preventing acute lung injury. Further, the rise of antibiotic-resistant bacteria relevant to lung infection clouds the future of this approach. Existing antiviral compounds are constrained by delivery timing (early after infection). In addition, CAP can be complicated by pathogen-pathogen interactions in coinfections and superinfection, and little is currently known. Beyond the pathogen, host-mediated immune activation drives lung pathology during CAP. A critical balance exists between host defense and tissue integrity, which is often not maintained, leading to the requirement for supportive critical care. Anti-inflammatory glucocorticoids have limited efficacy in CAP. Immunomodulatory therapies present an attractive method to limit lung injury. This therapeutic area is a focus for many inflammatory disease states, as well as cancer. The overarching goal is to eradicate the pathogen, while limiting collateral damage to the delicate lung architecture. It is possible that limiting deleterious inflammation by targeting host factors (cytokines, signaling cascades, etc.) will yield significant breakthroughs in CAP therapy. When coupled with a thorough understanding of pathogen virulence factors, novel therapeutics are likely to improve patient care. A greater understanding of host-mediated immunopathology is necessary to differentiate which factors are indeed protective and necessary from those that are detrimental and superfluous to pathogen clearance. The majority of what we understand about immunopathology has been determined in animal models. Additional translational studies are needed to fully define the pathogenesis of CAP. As illustrated in this review, host and pathogen factors interact in many ways. It is likely that the most effective therapeutic strategies will combine targeting of both host and pathogen factors.
FUTURE DIRECTIONS
In the last century, mortality associated with CAP has declined significantly, with further progress in the last decade due to improvements in supportive care. Beyond the critical need for novel therapeutics, CAP presents many global challenges. Identification of CAP etiology remains a challenge today even in the age of sophisticated protein and nucleic acid detection. This is especially problematic in the developing world where pathogen identification is limited. The CAP research community faces a challenge to identify effective biomarkers of etiology to guide appropriate care. As immunomodulatory therapies advance in the coming years, disease etiology identification will be critical to tailored care. At the minimum, differentiating viral versus bacterial CAP is critical to limit inappropriate antibiotic use and indicate which pathogen-targeted therapies would be appropriate. CAP care would also benefit greatly from improved biomarkers of disease severity. An improved molecular understanding of immunopathology during CAP could lead to clinically detectable markers that guide patient care. In order to prevent severe CAP outcomes, early identification of patients who are most likely to progress would present a critical opportunity for immunotherapy or targeting of pathogen factors. The molecular picture of lung injury is likely to be complex, involving many factors. The concept of singular biomarkers is unlikely to prove useful. Advances in machine learning and disease computational modeling provide hope that in coming years the field will be able to define pathways and critical disease-driving nodes in CAP. A recent trend in infectious disease has been the coupling of immunologists and microbiologists into integrated research teams. This has also been occurring at the graduate training level with an increase in programs focused on host-pathogen interactions. It is important that researchers in these disciplines continue to work together to understand the complex pathogenic mechanisms involved in CAP. In the next few years, the understanding of pathogen-pathogen and pathogen-microbiome interactions will continue to emerge, opening up new avenues for therapeutic discovery. Integration of pathogen-targeted therapy with host immunomodulation is likely to be the pathway to significant discovery in the treatment of CAP. A consensus statement on the needs for future CAP research has been recently published (173).
ACKNOWLEDGMENTS
This work was supported by NIH grants R01HL107380 (J.F.A.), T32AI060525 (J.A.G.), and T32AI089443 (H.E.R.).
We have no relevant conflict of interest to report.
Biographies

Jennifer A. Grousd is currently in her third year of Ph.D. training at the University of Pittsburgh School of Medicine. She graduated from Grand Valley State University with a B.S. with high honors in cell and molecular biology and biomedical sciences. Her research interests broadly focus on host-pathogen interactions, more specifically on how pathogens influence the host immune response. For her thesis work, she is looking at how Staphylococcus aureus cell wall-anchored proteins influence innate immune responses in the lung during pneumonia and influenza pneumonia superinfection.

Helen E. Rich is currently completing a Ph.D. after five years at the University of Pittsburgh School of Medicine, and she previously received a B.A. with high honors in biology from Oberlin College. Her research interests fall under the umbrella of host-pathogen immunology at mucosal surfaces, focusing on the role of type III interferon during dual pulmonary infection with influenza virus and Staphylococcus aureus for her thesis work. She is interested broadly in the host response to infection at mucosal surfaces and more specifically in cytokine and other cellular interactions at these barriers.

John F. Alcorn, Ph.D. is an Associate Professor with tenure in the Departments of Pediatrics and Immunology at the University of Pittsburgh. His laboratory is located at UPMC Children’s Hospital of Pittsburgh. Dr. Alcorn completed his doctoral training at Duke University and postdoctoral training at the University of Vermont. His research team is focused on pulmonary immunity to infection and allergy. Dr. Alcorn’s laboratory developed and has characterized a preclinical mouse model of influenza virus and bacterial superinfection and has indicated a role for attenuated innate and T cell function in disease pathogenesis.
REFERENCES
- 1.Katz SE, Williams DJ. 2018. Pediatric community-acquired pneumonia in the United States: changing epidemiology, diagnostic and therapeutic challenges, and areas for future research. Infect Dis Clin North Am 32:47–63. doi: 10.1016/j.idc.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Musher DM, Thorner AR. 2014. Community-acquired pneumonia. N Engl J Med 371:1619–1628. doi: 10.1056/NEJMra1312885. [DOI] [PubMed] [Google Scholar]
- 3.Le Roux DM, Zar HJ. 2017. Community-acquired pneumonia in children—a changing spectrum of disease. Pediatr Radiol 47:1392–1398. doi: 10.1007/s00247-017-3827-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, Reed C, Grijalva CG, Anderson EJ, Courtney DM, Chappell JD, Qi C, Hart EM, Carroll F, Trabue C, Donnelly HK, Williams DJ, Zhu Y, Arnold SR, Ampofo K, Waterer GW, Levine M, Lindstrom S, Winchell JM, Katz JM, Erdman D, Schneider E, Hicks LA, McCullers JA, Pavia AT, Edwards KM, Finelli L, CDC EPIC Study Team. 2015. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 373:415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadsby NJ, Russell CD, McHugh MP, Mark H, Conway Morris A, Laurenson IF, Hill AT, Templeton KE. 2016. Comprehensive molecular testing for respiratory pathogens in community-acquired pneumonia. Clin Infect Dis 62:817–823. doi: 10.1093/cid/civ1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flannery B, Reynolds SB, Blanton L, Santibanez TA, O'Halloran A, Lu PJ, Chen J, Foppa IM, Gargiullo P, Bresee J, Singleton JA, Fry AM. 2017. Influenza vaccine effectiveness against pediatric deaths: 2010–2014. Pediatrics 139:e20164244. doi: 10.1542/peds.2016-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng ZM, Chertow DS, Ambroggio X, McCall S, Przygodzki RM, Cunningham RE, Maximova OA, Kash JC, Morens DM, Taubenberger JK. 2011. Autopsy series of 68 cases dying before and during the 1918 influenza pandemic peak. Proc Natl Acad Sci U S A 108:16416–16421. doi: 10.1073/pnas.1111179108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hageman JC, Uyeki TM, Francis JS, Jernigan DB, Wheeler JG, Bridges CB, Barenkamp SJ, Sievert DM, Srinivasan A, Doherty MC, McDougal LK, Killgore GE, Lopatin UA, Coffman R, MacDonald JK, McAllister SK, Fosheim GE, Patel JB, McDonald LC. 2006. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003-04 influenza season. Emerg Infect Dis 12:894–899. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vardakas KZ, Matthaiou DK, Falagas ME. 2009. Incidence, characteristics and outcomes of patients with severe community acquired-MRSA pneumonia. Eur Respir J 34:1148–1158. doi: 10.1183/09031936.00041009. [DOI] [PubMed] [Google Scholar]
- 10.Shrestha SS, Swerdlow DL, Borse RH, Prabhu VS, Finelli L, Atkins CY, Owusu-Edusei K, Bell B, Mead PS, Biggerstaff M, Brammer L, Davidson H, Jernigan D, Jhung MA, Kamimoto LA, Merlin TL, Nowell M, Redd SC, Reed C, Schuchat A, Meltzer MI. 2011. Estimating the burden of 2009 pandemic influenza A (H1N1) in the United States (April 2009-April 2010). Clin Infect Dis 52(Suppl 1):S75–S82. doi: 10.1093/cid/ciq012. [DOI] [PubMed] [Google Scholar]
- 11.Simonsen L, Spreeuwenberg P, Lustig R, Taylor RJ, Fleming DM, Kroneman M, Van Kerkhove MD, Mounts AW, Paget WJ. 2013. Global mortality estimates for the 2009 influenza pandemic from the GLaMOR project: a modeling study. PLoS Med 10:e1001558. doi: 10.1371/journal.pmed.1001558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Kerkhove MD, Hirve S, Koukounari A, Mounts AW, group HNpsw. 2013. Estimating age-specific cumulative incidence for the 2009 influenza pandemic: a meta-analysis of A(H1N1)pdm09 serological studies from 19 countries. Influenza Other Respir Viruses 7:872–886. doi: 10.1111/irv.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Randolph AG, Vaughn F, Sullivan R, Rubinson L, Thompson BT, Yoon G, Smoot E, Rice TW, Loftis LL, Helfaer M, Doctor A, Paden M, Flori H, Babbitt C, Graciano AL, Gedeit R, Sanders RC, Giuliano JS, Zimmerman J, Uyeki TM. 2011. Critically ill children during the 2009–2010 influenza pandemic in the United States. Pediatrics 128:e1450–e1458. doi: 10.1542/peds.2011-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chertow DS, Memoli MJ. 2013. Bacterial coinfection in influenza: a grand rounds review. JAMA 309:275–282. doi: 10.1001/jama.2012.194139. [DOI] [PubMed] [Google Scholar]
- 15.Shah NS, Greenberg JA, McNulty MC, Gregg KS, Riddell J, Mangino JE, Weber DM, Hebert CL, Marzec NS, Barron MA, Chaparro-Rojas F, Restrepo A, Hemmige V, Prasidthrathsint K, Cobb S, Herwaldt L, Raabe V, Cannavino CR, Hines AG, Bares SH, Antiporta PB, Scardina T, Patel U, Reid G, Mohazabnia P, Kachhdiya S, Le BM, Park CJ, Ostrowsky B, Robicsek A, Smith BA, Schied J, Bhatti MM, Mayer S, Sikka M, Murphy-Aguilu I, Patwari P, Abeles SR, Torriani FJ, Abbas Z, Toya S, Doktor K, Chakrabarti A, Doblecki-Lewis S, Looney DJ, David MZ. 2016. Bacterial and viral co-infections complicating severe influenza: incidence and impact among 507 U.S. patients, 2013-14. J Clin Virol 80:12–19. doi: 10.1016/j.jcv.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong KK, Jain S, Blanton L, Dhara R, Brammer L, Fry AM, Finelli L. 2013. Influenza-associated pediatric deaths in the United States, 2004-2012. Pediatrics 132:796–804. doi: 10.1542/peds.2013-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Budd AP, Wentworth DE, Blanton L, Elal AIA, Alabi N, Barnes J, Brammer L, Burns E, Cummings CN, Davis T, Flannery B, Fry AM, Garg S, Garten R, Gubareva L, Jang Y, Kniss K, Kramer N, Lindstrom S, Mustaquim D, O’Halloran A, Olsen SJ, Sessions W, Taylor C, Xu X, Dugan VG, Katz J, Jernigan D. 2018. Update: influenza activity—United States, October 1, 2017-February 3, 2018. MMWR Morb Mortal Wkly Rep 67:169–179. doi: 10.15585/mmwr.mm6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. 2018. Influenza-associated pediatric mortality. https://gis.cdc.gov/GRASP/Fluview/PedFluDeath.html.
- 19.van Belkum A, Verkaik NJ, de Vogel CP, Boelens HA, Verveer J, Nouwen JL, Verbrugh HA, Wertheim HF. 2009. Reclassification of Staphylococcus aureus nasal carriage types. J Infect Dis 199:1820–1826. doi: 10.1086/599119. [DOI] [PubMed] [Google Scholar]
- 20.Burian M, Wolz C, Goerke C. 2010. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG, Camilli A, Kadioglu A, Dubyak GR, Pearlman E. 2015. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. J Immunol 194:1763–1775. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wertheim HF, Vos MC, Ott A, van Belkum A, Voss A, Kluytmans JA, van Keulen PH, Vandenbroucke-Grauls CM, Meester MHM, Verbrugh HA. 2004. Risk and outcome of nosocomial Staphylococcus aureus bacteraemia in nasal carriers versus non-carriers. Lancet 364:703–705. doi: 10.1016/S0140-6736(04)16897-9. [DOI] [PubMed] [Google Scholar]
- 23.Creech CB, Kernodle DS, Alsentzer A, Wilson C, Edwards KM. 2005. Increasing rates of nasal carriage of methicillin-resistant Staphylococcus aureus in healthy children. Pediatr Infect Dis J 24:617–621. doi: 10.1097/01.inf.0000168746.62226.a4. [DOI] [PubMed] [Google Scholar]
- 24.Gorwitz RJ, Kruszon-Moran D, McAllister SK, McQuillan G, McDougal LK, Fosheim GE, Jensen BJ, Killgore G, Tenover FC, Kuehnert MJ. 2008. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001-2004. J Infect Dis 197:1226–1234. doi: 10.1086/533494. [DOI] [PubMed] [Google Scholar]
- 25.Tilahun B, Faust AC, McCorstin P, Ortegon A. 2015. Nasal colonization and lower respiratory tract infections with methicillin-resistant Staphylococcus aureus. Am J Crit Care 24:8–12. doi: 10.4037/ajcc2015102. [DOI] [PubMed] [Google Scholar]
- 26.Paling FP, Wolkewitz M, Bode LGM, Klein Klouwenberg PMC, Ong DSY, Depuydt P, de Bus L, Sifakis F, Bonten MJM, Kluytmans J. 2017. Staphylococcus aureus colonization at ICU admission as a risk factor for developing S. aureus ICU pneumonia. Clin Microbiol Infect 23:49.e9–49.e14. doi: 10.1016/j.cmi.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Aniansson G, Aim B, Andersson B, Larsson P, Nylen O, Peterson H, Rigner P, Svanborg M, Svanborg C. 1992. Nasopharyngeal colonization during the first year of life. J Infect Dis 165:S38–S42. doi: 10.1093/infdis/165-Supplement_1-S38. [DOI] [PubMed] [Google Scholar]
- 28.Principi N, Marchisio P, Schito GC, Mannelli S. 1999. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Pediatr Infect Dis J 18:517–523. doi: 10.1097/00006454-199906000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Hussain M, Melegaro A, Pebody RG, George R, Edmunds WJ, Talukdar R, Martin SA, Efstratiou A, Miller E. 2005. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect 133:891–898. doi: 10.1017/S0950268805004012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simell B, Auranen K, Käyhty H, Goldblatt D, Dagan R, O’Brien KL, Pneumococcal Carriage Group. 2012. The fundamental link between pneumococcal carriage and disease. Expert Rev Vaccines 11:841–855. doi: 10.1586/erv.12.53. [DOI] [PubMed] [Google Scholar]
- 31.Lipsitch M, Abdullahi O, Dʼamour A, Xie W, Weinberger DM, Tchetgen Tchetgen E, Scott J. 2012. Estimating rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kenya with a Markov transition model. Epidemiology 23:510–519. doi: 10.1097/EDE.0b013e31824f2f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syrjanen RK, Auranen KJ, Leino TM, Kilpi TM, Mäkelä PH. 2005. Pneumococcal acute otitis media in relation to pneumococcal nasopharyngeal carriage. Pediatr Infect Dis J 24:801–806. doi: 10.1097/01.inf.0000178072.83531.4f. [DOI] [PubMed] [Google Scholar]
- 33.Johnson HL, Deloria-Knoll M, Levine OS, Stoszek SK, Freimanis Hance L, Reithinger R, Muenz LR, O'Brien KL. 2010. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med 7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spratt BG, Greenwood BM. 2000. Prevention of pneumococcal disease by vaccination: does serotype replacement matter? Lancet 356:1210–1211. doi: 10.1016/S0140-6736(00)02779-3. [DOI] [PubMed] [Google Scholar]
- 35.Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, Sherwood L, Zmitrovich A, Jerris R, Satola SW, Beall B, Moore MR, Jain S, Farley MM. 2015. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J 34:1168–1174. doi: 10.1097/INF.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 36.Cohen R, Varon E, Doit C, Schlemmer C, Romain O, Thollot F, Bechet S, Bonacorsi S, Levy C. 2015. A 13-year survey of pneumococcal nasopharyngeal carriage in children with acute otitis media following PCV7 and PCV13 implementation. Vaccine 33:5118–5126. doi: 10.1016/j.vaccine.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 37.Devine VT, Jefferies JM, Clarke SC, Faust SN. 2015. Nasopharyngeal bacterial carriage in the conjugate vaccine era with a focus on pneumococci. J Immunol Res 2015:394368. doi: 10.1155/2015/394368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biesbroek G, Wang X, Keijser BJF, Eijkemans RMJ, Trzciński K, Rots NY, Veenhoven RH, Sanders EAM, Bogaert D. 2014. Seven-valent pneumococcal conjugate vaccine and nasopharyngeal microbiota in healthy children. Emerg Infect Dis 20:201–210. doi: 10.3201/eid2002.131220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiss-Mandel A, Regev-Yochay G. 2016. Staphylococcus aureus and Streptococcus pneumoniae interaction and response to pneumococcal vaccination: myth or reality?. Hum Vaccin Immunother 12:351–357. doi: 10.1080/21645515.2015.1081321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donkor ES. 2013. Understanding the pneumococcus: transmission and evolution. Front Cell Infect Microbiol 3:7. doi: 10.3389/fcimb.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henriques-Normark B, Tuomanen EI. 2013. The pneumococcus: epidemiology, microbiology, and pathogenesis. Cold Spring Harb Perspect Med 3:a010215. doi: 10.1101/cshperspect.a010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins A, Diep BA, Mai TT, Vo NH, Warrener P, Suzich J, Stover CK, Sellman BR. 2015. Differential expression and roles of Staphylococcus aureus virulence determinants during colonization and disease. mBio 6:e02272-14. doi: 10.1128/mBio.02272-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weidenmaier C, Goerke C, Wolz C. 2012. Staphylococcus aureus determinants for nasal colonization. Trends Microbiol 20:243–250. doi: 10.1016/j.tim.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 44.Beasley FC, Marolda CL, Cheung J, Buac S, Heinrichs DE. 2011. Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by staphyloferrin A, staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect Immun 79:2345–2355. doi: 10.1128/IAI.00117-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haley KP, Skaar EP. 2012. A battle for iron: host sequestration and Staphylococcus aureus acquisition. Microbes Infect 14:217–227. doi: 10.1016/j.micinf.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weiser JN, Ferreira DM, Paton JC. 2018. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol 16:355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao Y, Marks LR, Pettigrew MM, Hakansson AP. 2014. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front Cell Infect Microbiol 4:194. doi: 10.3389/fcimb.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shainheit MG, Mule M, Camilli A. 2014. The core promoter of the capsule operon of Streptococcus pneumoniae is necessary for colonization and invasive disease. Infect Immun 82:694–705. doi: 10.1128/IAI.01289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park IH, Kim K-H, Andrade AL, Briles DE, McDaniel LS, Nahm MH. 2012. Nontypeable pneumococci can be divided into multiple cps types, including one type expressing the novel gene pspK. mBio 3:e00035-12. doi: 10.1128/mBio.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanchette KA, Shenoy AT, Milner J II, Gilley RP, McClure E, Hinojosa CA, Kumar N, Daugherty SC, Tallon LJ, Ott S, King SJ, Ferreira DM, Gordon SB, Tettelin H, Orihuela CJ. 2016. Neuraminidase A-exposed galactose promotes Streptococcus pneumoniae biofilm formation during colonization. Infect Immun 84:2922–2932. doi: 10.1128/IAI.00277-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khan MN, Coleman JR, Vernatter J, Varshney AK, Dufaud C, Pirofski LA. 2014. An ahemolytic pneumolysin of Streptococcus pneumoniae manipulates human innate and CD4(+) T-cell responses and reduces resistance to colonization in mice in a serotype-independent manner. J Infect Dis 210:1658–1669. doi: 10.1093/infdis/jiu321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell TJ, Dalziel CE. 2014. The biology of pneumolysin. Subcell Biochem 80:145–160. doi: 10.1007/978-94-017-8881-6_8. [DOI] [PubMed] [Google Scholar]
- 53.Rabes A, Suttorp N, Opitz B. 2016. Inflammasomes in pneumococcal infection: innate immune sensing and bacterial evasion strategies. Curr Top Microbiol Immunol 397:215–227. doi: 10.1007/978-3-319-41171-2_11. [DOI] [PubMed] [Google Scholar]
- 54.Feldman C, Anderson R. 2016. The role of Streptococcus pneumoniae in community-acquired pneumonia. Semin Respir Crit Care Med 37:806–818. doi: 10.1055/s-0036-1592074. [DOI] [PubMed] [Google Scholar]
- 55.Hu D-K, Liu Y, Li X-Y, Qu Y. 2015. In vitro expression of Streptococcus pneumoniae ply gene in human monocytes and pneumocytes. Eur J Med Res 20:52. doi: 10.1186/s40001-015-0142-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Küng E, Coward WR, Neill DR, Malak HA, Mühlemann K, Kadioglu A, Hilty M, Hathaway LJ. 2014. The pneumococcal polysaccharide capsule and pneumolysin differentially affect CXCL8 and IL-6 release from cells of the upper and lower respiratory tract. PLoS One 9:e92355. doi: 10.1371/journal.pone.0092355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Knippenberg S, Ueberberg B, Maus R, Bohling J, Ding N, Tort Tarres M, Hoymann H-G, Jonigk D, Izykowski N, Paton JC, Ogunniyi AD, Lindig S, Bauer M, Welte T, Seeger W, Guenther A, Sisson TH, Gauldie J, Kolb M, Maus UA. 2015. Streptococcus pneumoniae; triggers progression of pulmonary fibrosis through pneumolysin. Thorax 70:636. doi: 10.1136/thoraxjnl-2014-206420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205:807–817. doi: 10.1093/infdis/jir846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokolovska A, Becker CE, Ip WK, Rathinam VA, Brudner M, Paquette N, Tanne A, Vanaja SK, Moore KJ, Fitzgerald KA, Lacy-Hulbert A, Stuart LM. 2013. Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol 14:543–553. doi: 10.1038/ni.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen TS, Jones-Nelson O, Hotz M, Cheng L, Miller LS, Suzich J, Stover CK, Sellman BR. 2016. S. aureus blocks efferocytosis of neutrophils by macrophages through the activity of its virulence factor alpha toxin. Sci Rep 6:35466. doi: 10.1038/srep35466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen TS, Boland ML, Boland BB, Takahashi V, Tovchigrechko A, Lee Y, Wilde AD, Mazaitis MJ, Jones-Nelson O, Tkaczyk C, Raja R, Stover CK, Sellman BR. 2018. S. aureus evades macrophage killing through NLRP3-dependent effects on mitochondrial trafficking. Cell Rep 22:2431–2441. doi: 10.1016/j.celrep.2018.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitur K, Parker D, Nieto P, Ahn DS, Cohen TS, Chung S, Wachtel S, Bueno S, Prince A. 2015. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog 11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilke GA, Bubeck Wardenburg J. 2010. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc Natl Acad Sci U S A 107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Becker RE, Berube BJ, Sampedro GR, DeDent AC, Bubeck Wardenburg J. 2014. Tissue-specific patterning of host innate immune responses by Staphylococcus aureus alpha-toxin. J Innate Immun 6:619–631. doi: 10.1159/000360006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parker D, Prince A. 2012. Immunopathogenesis of Staphylococcus aureus pulmonary infection. Semin Immunopathol 34:281–297. doi: 10.1007/s00281-011-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hook JL, Islam MN, Parker D, Prince AS, Bhattacharya S, Bhattacharya J. 2018. Disruption of staphylococcal aggregation protects against lethal lung injury. J Clin Invest 128:1074–1086. doi: 10.1172/JCI95823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu S, Huang J. 2017. Resveratrol alleviates Staphylococcus aureus pneumonia by inhibition of the NLRP3 inflammasome. Exp Ther Med 14:6099–6104. doi: 10.3892/etm.2017.5337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gong T, Yang Y, Jin T, Jiang W, Zhou R. 2018. Orchestration of NLRP3 inflammasome activation by ion fluxes. Trends Immunol 39:393–406. doi: 10.1016/j.it.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 70.Cohen TS, Hilliard JJ, Jones-Nelson O, Keller AE, O’Day T, Tkaczyk C, DiGiandomenico A, Hamilton M, Pelletier M, Wang Q, Diep BA, Le VTM, Cheng L, Suzich J, Stover CK, Sellman BR. 2016. Staphylococcus aureus alpha toxin potentiates opportunistic bacterial lung infections. Sci Transl Med 8:329ra31. doi: 10.1126/scitranslmed.aad9922. [DOI] [PubMed] [Google Scholar]
- 71.Holzinger D, Gieldon L, Mysore V, Nippe N, Taxman DJ, Duncan JA, Broglie PM, Marketon K, Austermann J, Vogl T, Foell D, Niemann S, Peters G, Roth J, Loffler B. 2012. Staphylococcus aureus Panton-Valentine leukocidin induces an inflammatory response in human phagocytes via the NLRP3 inflammasome. J Leukoc Biol 92:1069–1081. doi: 10.1189/jlb.0112014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Melehani JH, James DB, DuMont AL, Torres VJ, Duncan JA. 2015. Staphylococcus aureus leukocidin A/B (LukAB) kills human monocytes via host NLRP3 and ASC when extracellular, but not intracellular. PLoS Pathog 11:e1004970. doi: 10.1371/journal.ppat.1004970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spaan AN, Henry T, van Rooijen WJM, Perret M, Badiou C, Aerts PC, Kemmink J, de Haas CJC, van Kessel KPM, Vandenesch F, Lina G, van Strijp J. 2013. The staphylococcal toxin Panton-Valentine leukocidin targets human C5a receptors. Cell Host Microbe 13:584–594. doi: 10.1016/j.chom.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. 2006. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med 355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 75.Brown ML, O'Hara FP, Close NM, Mera RM, Miller LA, Suaya JA, Amrine-Madsen H. 2012. Prevalence and sequence variation of panton-valentine leukocidin in methicillin-resistant and methicillin-susceptible staphylococcus aureus strains in the United States. J Clin Microbiol 50:86–90. doi: 10.1128/JCM.05564-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gillet Y, Issartel B, Vanhems P, Fournet J-C, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 77.DuMont AL, Yoong P, Day CJ, Alonzo F III, McDonald WH, Jennings MP, Torres VJ. 2013. Staphylococcus aureus LukAB cytotoxin kills human neutrophils by targeting the CD11b subunit of the integrin Mac-1. Proc Natl Acad Sci U S A 110:10794–10799. doi: 10.1073/pnas.1305121110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Prince A, Wang H, Kitur K, Parker D. 2017. Humanized mice exhibit increased susceptibility to Staphylococcus aureus pneumonia. J Infect Dis 215:1386–1395. doi: 10.1093/infdis/jiw425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Auerbach W, Eynon EE, Stevens S, Manz MG, Flavell RA. 2011. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci U S A 108:2390–2395. doi: 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bewley MA, Naughton M, Preston J, Mitchell A, Holmes A, Marriott HM, Read RC, Mitchell TJ, Whyte MK, Dockrell DH. 2014. Pneumolysin activates macrophage lysosomal membrane permeabilization and executes apoptosis by distinct mechanisms without membrane pore formation. mBio 5:e01710-14. doi: 10.1128/mBio.01710-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sridharan H, Upton JW. 2014. Programmed necrosis in microbial pathogenesis. Trends Microbiol 22:199–207. doi: 10.1016/j.tim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 82.Linkermann A, Green DR. 2014. Necroptosis. N Engl J Med 370:455–465. doi: 10.1056/NEJMra1310050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wen SH, Lin LN, Wu HJ, Yu L, Lin L, Zhu LL, Li HY, Zhang HL, Li CC. 2018. TNF-alpha increases Staphylococcus aureus-induced death of human alveolar epithelial cell line A549 associated with RIP3-mediated necroptosis. Life Sci 195:81–86. doi: 10.1016/j.lfs.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 84.González-Juarbe N, Bradley KM, Shenoy AT, Gilley RP, Reyes LF, Hinojosa CA, Restrepo MI, Dube PH, Bergman MA, Orihuela CJ. 2017. Pore-forming toxin-mediated ion dysregulation leads to death receptor-independent necroptosis of lung epithelial cells during bacterial pneumonia. Cell Death Differ 24:917–928. doi: 10.1038/cdd.2017.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonzalez-Juarbe N, Gilley RP, Hinojosa CA, Bradley KM, Kamei A, Gao G, Dube PH, Bergman MA, Orihuela CJ. 2015. Pore-forming toxins induce macrophage necroptosis during acute bacterial pneumonia. PLoS Pathog 11:e1005337. doi: 10.1371/journal.ppat.1005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. 2009. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol 297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Domon H, Oda M, Maekawa T, Nagai K, Takeda W, Terao Y. 2016. Streptococcus pneumoniae disrupts pulmonary immune defence via elastase release following pneumolysin-dependent neutrophil lysis. Sci Rep 6:38013. doi: 10.1038/srep38013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Otto M. 2014. Phenol-soluble modulins. Int J Med Microbiol 304:164–169. doi: 10.1016/j.ijmm.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gov Y, Bitler A, Dell’Acqua G, Torres VJ, Balaban N. 2001. RNAIII inhibiting peptide (RIP), a global inhibitor of Staphylococcus aureus pathogenesis: structure and function analysis. Peptides 22:1609–1620. doi: 10.1016/S0196-9781(01)00496-X. [DOI] [PubMed] [Google Scholar]
- 90.Zhou Y, Niu C, Ma B, Xue X, Li Z, Chen Z, Li F, Zhou S, Luo X, Hou Z. 2018. Inhibiting PSMalpha-induced neutrophil necroptosis protects mice with MRSA pneumonia by blocking the agr system. Cell Death Dis 9:362. doi: 10.1038/s41419-018-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cardot-Martin E, Casalegno JS, Badiou C, Dauwalder O, Keller D, Prevost G, Rieg S, Kern WV, Cuerq C, Etienne J, Vandenesch F, Lina G, Dumitrescu O. 2015. α-Defensins partially protect human neutrophils against Panton-Valentine leukocidin produced by Staphylococcus aureus. Lett Appl Microbiol 61:158–164. doi: 10.1111/lam.12438. [DOI] [PubMed] [Google Scholar]
- 92.van Kessel KP, Bestebroer J, van Strijp JA. 2014. Neutrophil-mediated phagocytosis of Staphylococcus aureus. Front Immunol 5:467. doi: 10.3389/fimmu.2014.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Molkanen T, Tyynela J, Helin J, Kalkkinen N, Kuusela P. 2002. Enhanced activation of bound plasminogen on Staphylococcus aureus by staphylokinase. FEBS Lett 517:72–78. doi: 10.1016/S0014-5793(02)02580-2. [DOI] [PubMed] [Google Scholar]
- 94.Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. 2004. Staphylococcus aureus resists human defensins by production of staphylokinase, a novel bacterial evasion mechanism. J Immunol 172:1169–1176. doi: 10.4049/jimmunol.172.2.1169. [DOI] [PubMed] [Google Scholar]
- 95.Tawk MY, Zimmermann-Meisse G, Bossu J-L, Potrich C, Bourcier T, Dalla Serra M, Poulain B, Prévost G, Jover E. 2015. Internalization of staphylococcal leukotoxins that bind and divert the C5a receptor is required for intracellular Ca(2+) mobilization by human neutrophils. Cell Microbiol 17:1241–1257. doi: 10.1111/cmi.12434. [DOI] [PubMed] [Google Scholar]
- 96.Lodge KM, Thompson AA, Chilvers ER, Condliffe AM. 2017. Hypoxic regulation of neutrophil function and consequences for Staphylococcus aureus infection. Microbes Infect 19:166–176. doi: 10.1016/j.micinf.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 97.McGovern NN, Cowburn AS, Porter L, Walmsley SR, Summers C, Thompson AAR, Anwar S, Willcocks LC, Whyte MKB, Condliffe AM, Chilvers ER. 2011. Hypoxia selectively inhibits respiratory burst activity and killing of Staphylococcus aureus in human neutrophils. J Immunol 186:453–463. doi: 10.4049/jimmunol.1002213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Harding MG, Zhang K, Conly J, Kubes P. 2014. Neutrophil crawling in capillaries; a novel immune response to Staphylococcus aureus. PLoS Pathog 10:e1004379. doi: 10.1371/journal.ppat.1004379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schilcher K, Andreoni F, Uchiyama S, Ogawa T, Schuepbach RA, Zinkernagel AS. 2014. Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin. J Infect Dis 210:473–482. doi: 10.1093/infdis/jiu091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pleskova SN, Gorshkova EN, Kriukov RN. 2018. Dynamics of formation and morphological features of neutrophil extracellular traps formed under the influence of opsonized Staphylococcus aureus. J Mol Recognit 31:e2707. doi: 10.1002/jmr.2707. [DOI] [PubMed] [Google Scholar]
- 101.von Kockritz-Blickwede M, Blodkamp S, Nizet V. 2016. Interaction of bacterial exotoxins with neutrophil extracellular traps: impact for the infected host. Front Microbiol 7:402. doi: 10.3389/fmicb.2016.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Malachowa N, Kobayashi SD, Freedman B, Dorward DW, DeLeo FR. 2013. Staphylococcus aureus leukotoxin GH promotes formation of neutrophil extracellular traps. J Immunol 191:6022–6029. doi: 10.4049/jimmunol.1301821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bhattacharya M, Berends ETM, Chan R, Schwab E, Roy S, Sen CK, Torres VJ, Wozniak DJ. 2018. Staphylococcus aureus biofilms release leukocidins to elicit extracellular trap formation and evade neutrophil-mediated killing. Proc Natl Acad Sci U S A 115:7416–7421. doi: 10.1073/pnas.1721949115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Martin CJ, Peters KN, Behar SM. 2014. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol 17:17–23. doi: 10.1016/j.mib.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Greenlee-Wacker MC, Rigby KM, Kobayashi SD, Porter AR, DeLeo FR, Nauseef WM. 2014. Phagocytosis of Staphylococcus aureus by human neutrophils prevents macrophage efferocytosis and induces programmed necrosis. J Immunol 192:4709–4717. doi: 10.4049/jimmunol.1302692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Witko-Sarsat V, Mocek J, Bouayad D, Tamassia N, Ribeil JA, Candalh C, Davezac N, Reuter N, Mouthon L, Hermine O, Pederzoli-Ribeil M, Cassatella MA. 2010. Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival. J Exp Med 207:2631–2645. doi: 10.1084/jem.20092241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yoon KW, Byun S, Kwon E, Hwang S, Chu K, Hiraki M, Jo S, Weins A, Hakroush S, Cebulla A, Sykes DB, Greka A, Mundel P, Fisher DE, Mandinova A, Lee SW. 2015. Control of signaling-mediated clearance of apoptotic cells by the tumor suppressor p53. Science 349:1261669. doi: 10.1126/science.1261669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jun JI, Kim KH, Lau LF. 2015. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun 6:7386. doi: 10.1038/ncomms8386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Prince A. (ed). 2013. Mucosal immunology of acute bacterial pneumonia. Springer, New York, NY. [Google Scholar]
- 110.Rynda-Apple A, Robinson KM, Alcorn JF. 2015. Influenza and bacterial superinfection: illuminating the immunologic mechanisms of disease. Infect Immun 83:3764–3770. doi: 10.1128/IAI.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McCullers JA. 2014. The co-pathogenesis of influenza viruses with bacteria in the lung. Nat Rev Microbiol 12:252–262. doi: 10.1038/nrmicro3231. [DOI] [PubMed] [Google Scholar]
- 112.Swain SL, Dutton RW, Woodland DL. 2004. T cell responses to influenza virus infection: effector and memory cells. Viral Immunol 17:197–209. doi: 10.1089/0882824041310577. [DOI] [PubMed] [Google Scholar]
- 113.Buchweitz JP, Harkema JR, Kaminski NE. 2007. Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol Pathol 35:424–435. doi: 10.1080/01926230701302558. [DOI] [PubMed] [Google Scholar]
- 114.Gotts JE, Abbott J, Matthay MA. 2014. Influenza causes prolonged disruption of the alveolar-capillary barrier in mice unresponsive to mesenchymal stem cell therapy. Am J Physiol Lung Cell Mol Physiol 307:L395–L406. doi: 10.1152/ajplung.00110.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumar Y, Liang C, Limmon GV, Liang L, Engelward BP, Ooi EE, Chen J, Tannenbaum SR. 2014. Molecular analysis of serum and bronchoalveolar lavage in a mouse model of influenza reveals markers of disease severity that can be clinically useful in humans. PLoS One 9:e86912. doi: 10.1371/journal.pone.0086912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kudva A, Scheller EV, Robinson KM, Crowe CR, Choi SM, Slight SR, Khader SA, Dubin PJ, Enelow RI, Kolls JK, Alcorn JF. 2011. Influenza A inhibits Th17-mediated host defense against bacterial pneumonia in mice. J Immunol 186:1666–1674. doi: 10.4049/jimmunol.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robinson KM, Choi SM, McHugh KJ, Mandalapu S, Enelow RI, Kolls JK, Alcorn JF. 2013. Influenza A exacerbates Staphylococcus aureus pneumonia by attenuating IL-1beta production in mice. J Immunol 191:5153–5159. doi: 10.4049/jimmunol.1301237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Robinson KM, Ramanan K, Clay ME, McHugh KJ, Pilewski MJ, Nickolich KL, Corey C, Shiva S, Wang J, Alcorn JF. 2018. The inflammasome potentiates influenza/Staphylococcus aureus superinfection in mice. JCI Insight 3:97470. doi: 10.1172/jci.insight.97470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, Enelow RI, Chan YR, Kolls JK, Alcorn JF. 2014. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J Infect Dis 209:865–875. doi: 10.1093/infdis/jit527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tripathi S, White MR, Hartshorn KL. 2015. The amazing innate immune response to influenza A virus infection. Innate Immun 21:73–98. doi: 10.1177/1753425913508992. [DOI] [PubMed] [Google Scholar]
- 121.Travanty E, Zhou B, Zhang H, Di YP, Alcorn JF, Wentworth DE, Mason R, Wang J. 2015. Differential susceptibilities of human lung primary cells to H1N1 influenza viruses. J Virol 89:11935–11944. doi: 10.1128/JVI.01792-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Vreede FT, Fodor E. 2010. The role of the influenza virus RNA polymerase in host shut-off. Virulence 1:436–439. doi: 10.4161/viru.1.5.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Herold S, Ludwig S, Pleschka S, Wolff T. 2012. Apoptosis signaling in influenza virus propagation, innate host defense, and lung injury. J Leukoc Biol 92:75–82. doi: 10.1189/jlb.1011530. [DOI] [PubMed] [Google Scholar]
- 124.Hufford MM, Kim TS, Sun J, Braciale TJ. 2015. The effector T cell response to influenza infection. Curr Top Microbiol Immunol 386:423–455. doi: 10.1007/82_2014_397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tumpey TM, García-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solórzano A, Van Rooijen N, Katz JM, Basler CF. 2005. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol 79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. 2009. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol 182:1296–1304. doi: 10.4049/jimmunol.182.3.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang J, Nikrad MP, Travanty EA, Zhou B, Phang T, Gao B, Alford T, Ito Y, Nahreini P, Hartshorn K, Wentworth D, Dinarello CA, Mason RJ. 2012. Innate immune response of human alveolar macrophages during influenza A infection. PLoS One 7:e29879. doi: 10.1371/journal.pone.0029879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang J, Nikrad MP, Phang T, Gao B, Alford T, Ito Y, Edeen K, Travanty EA, Kosmider B, Hartshorn K, Mason RJ. 2011. Innate immune response to influenza A virus in differentiated human alveolar type II cells. Am J Respir Cell Mol Biol 45:582–591. doi: 10.1165/rcmb.2010-0108OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fainstein V, Musher DM, Cate TR. 1980. Bacterial adherence to pharyngeal cells during viral infection. J Infect Dis 141:172–176. doi: 10.1093/infdis/141.2.172. [DOI] [PubMed] [Google Scholar]
- 130.Siegel SJ, Roche AM, Weiser JN. 2014. Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe 16:55–67. doi: 10.1016/j.chom.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Scharf S, Zahlten J, Szymanski K, Hippenstiel S, Suttorp N, N'Guessan PD. 2012. Streptococcus pneumoniae induces human beta-defensin-2 and -3 in human lung epithelium. Exp Lung Res 38:100–110. doi: 10.3109/01902148.2011.652802. [DOI] [PubMed] [Google Scholar]
- 132.De Filippo K, Neill DR, Mathies M, Bangert M, McNeill E, Kadioglu A, Hogg N. 2014. A new protective role for S100A9 in regulation of neutrophil recruitment during invasive pneumococcal pneumonia. FASEB J 28:3600–3608. doi: 10.1096/fj.13-247460. [DOI] [PubMed] [Google Scholar]
- 133.Parker D, Martin FJ, Soong G, Harfenist BS, Aguilar JL, Ratner AJ, Fitzgerald KA, Schindler C, Prince A. 2011. Streptococcus pneumoniae DNA initiates type I interferon signaling in the respiratory tract. mBio 2:e00016-11. doi: 10.1128/mBio.00016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Parker D, Cohen TS, Alhede M, Harfenist BS, Martin FJ, Prince A. 2012. Induction of type I interferon signaling by Pseudomonas aeruginosa is diminished in cystic fibrosis epithelial cells. Am J Respir Cell Mol Biol 46:6–13. doi: 10.1165/rcmb.2011-0080OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Koppe U, Hogner K, Doehn JM, Muller HC, Witzenrath M, Gutbier B, Bauer S, Pribyl T, Hammerschmidt S, Lohmeyer J, Suttorp N, Herold S, Opitz B. 2012. Streptococcus pneumoniae stimulates a STING- and IFN regulatory factor 3-dependent type I IFN production in macrophages, which regulates RANTES production in macrophages, cocultured alveolar epithelial cells, and mouse lungs. J Immunol 188:811–817. doi: 10.4049/jimmunol.1004143. [DOI] [PubMed] [Google Scholar]
- 136.Scumpia PO, Botten GA, Norman JS, Kelly-Scumpia KM, Spreafico R, Ruccia AR, Purbey PK, Thomas BJ, Modlin RL, Smale ST. 2017. Opposing roles of Toll-like receptor and cytosolic DNA-STING signaling pathways for Staphylococcus aureus cutaneous host defense. PLoS Pathog 13:e1006496. doi: 10.1371/journal.ppat.1006496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jewell NA, Cline T, Mertz SE, Smirnov SV, Flano E, Schindler C, Grieves JL, Durbin RK, Kotenko SV, Durbin JE. 2010. Lambda interferon is the predominant interferon induced by influenza A virus infection in vivo. J Virol 84:11515–11522. doi: 10.1128/JVI.01703-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wei H, Wang S, Chen Q, Chen Y, Chi X, Zhang L, Huang S, Gao GF, Chen JL. 2014. Suppression of interferon lambda signaling by SOCS-1 results in their excessive production during influenza virus infection. PLoS Pathog 10:e1003845. doi: 10.1371/journal.ppat.1003845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galani IE, Triantafyllia V, Eleminiadou EE, Koltsida O, Stavropoulos A, Manioudaki M, Thanos D, Doyle SE, Kotenko SV, Thanopoulou K, Andreakos E. 2017. Interferon-lambda mediates non-redundant front-line antiviral protection against influenza virus infection without compromising host fitness. Immunity 46:875–890. doi: 10.1016/j.immuni.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 140.Shahangian A, Chow EK, Tian X, Kang JR, Ghaffari A, Liu SY, Belperio JA, Cheng G, Deng JC. 2009. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J Clin Invest 119:1910–1920. doi: 10.1172/JCI35412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee B, Robinson KM, McHugh KJ, Scheller EV, Mandalapu S, Chen C, Di YP, Clay ME, Enelow RI, Dubin PJ, Alcorn JF. 2015. Influenza-induced type I interferon enhances susceptibility to gram-negative and gram-positive bacterial pneumonia in mice. Am J Physiol Lung Cell Mol Physiol 309:L158–L167. doi: 10.1152/ajplung.00338.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gopal R, Lee B, McHugh KJ, Rich HE, Ramanan K, Mandalapu S, Clay ME, Seger PJ, Enelow RI, Manni ML, Robinson KM, Rangel-Moreno J, Alcorn JF. 2018. STAT2 signaling regulates macrophage phenotype during influenza and bacterial super-infection. Front Immunol 9:2151. doi: 10.3389/fimmu.2018.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee B, Gopal R, Manni ML, McHugh KJ, Mandalapu S, Robinson KM, Alcorn JF. 2017. STAT1 is required for suppression of type 17 immunity during influenza and bacterial superinfection. Immunohorizons 1:81–91. doi: 10.4049/immunohorizons.1700030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Planet PJ, Parker D, Cohen TS, Smith H, Leon JD, Ryan C, Hammer TJ, Fierer N, Chen EI, Prince AS. 2016. Lambda interferon restructures the nasal microbiome and increases susceptibility to Staphylococcus aureus superinfection. mBio 7:e01939-15. doi: 10.1128/mBio.01939-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shepardson KM, Larson K, Morton RV, Prigge JR, Schmidt EE, Huber VC, Rynda-Apple A. 2016. Differential type I interferon signaling is a master regulator of susceptibility to postinfluenza bacterial superinfection. mBio 7:e00506-16. doi: 10.1128/mBio.00506-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li W, Moltedo B, Moran TM. 2012. Type I interferon induction during influenza virus infection increases susceptibility to secondary Streptococcus pneumoniae infection by negative regulation of gammadelta T cells. J Virol 86:12304–12312. doi: 10.1128/JVI.01269-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McNamee LA, Harmsen AG. 2006. Both influenza-induced neutrophil dysfunction and neutrophil-independent mechanisms contribute to increased susceptibility to a secondary Streptococcus pneumoniae infection. Infect Immun 74:6707–6721. doi: 10.1128/IAI.00789-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cooper GE, Pounce ZC, Wallington JC, Bastidas-Legarda LY, Nicholas B, Chidomere C, Robinson EC, Martin K, Tocheva AS, Christodoulides M, Djukanovic R, Wilkinson TM, Staples KJ. 2016. Viral inhibition of bacterial phagocytosis by human macrophages: redundant role of CD36. PLoS One 11:e0163889. doi: 10.1371/journal.pone.0163889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pang G, Clancy R, Cong M, Ortega M, Zhigang R, Reeves G. 2000. Influenza virus inhibits lysozyme secretion by sputum neutrophils in subjects with chronic bronchial sepsis. Am J Respir Crit Care Med 161:718–722. doi: 10.1164/ajrccm.161.3.9812047. [DOI] [PubMed] [Google Scholar]
- 150.Robinson KM, Ramanan K, Clay ME, McHugh KJ, Rich HE, Alcorn JF. 2018. Novel protective mechanism for interleukin-33 at the mucosal barrier during influenza-associated bacterial superinfection. Mucosal Immunol 11:199–208. doi: 10.1038/mi.2017.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Perret M, Badiou C, Lina G, Burbaud S, Benito Y, Bes M, Cottin V, Couzon F, Juruj C, Dauwalder O, Goutagny N, Diep BA, Vandenesch F, Henry T. 2012. Cross-talk between Staphylococcus aureus leukocidins-intoxicated macrophages and lung epithelial cells triggers chemokine secretion in an inflammasome-dependent manner. Cell Microbiol 14:1019–1036. doi: 10.1111/j.1462-5822.2012.01772.x. [DOI] [PubMed] [Google Scholar]
- 152.Giai C, Gonzalez CD, Sabbione F, Garofalo A, Ojeda D, Sordelli DO, Trevani AS, Gomez MI. 2016. Staphylococcus aureus induces shedding of IL-1RII in monocytes and neutrophils. J Innate Immun 8:284–298. doi: 10.1159/000443663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bansal S, Yajjala VK, Bauer C, Sun K. 2018. IL-1 signaling prevents alveolar macrophage depletion during influenza and Streptococcus pneumoniae coinfection. J Immunol 200:1425–1433. doi: 10.4049/jimmunol.1700210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berical AC, Harris D, Dela Cruz CS, Possick JD. 2016. Pneumococcal vaccination strategies. An update and perspective. Ann Am Thorac Soc 13:933–944. doi: 10.1513/AnnalsATS.201511-778FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Anderson R, Feldman C. 2017. Pneumolysin as a potential therapeutic target in severe pneumococcal disease. J Infect 74:527–544. doi: 10.1016/j.jinf.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 156.Kaur R, Surendran N, Ochs M, Pichichero ME. 2014. Human antibodies to PhtD, PcpA, and Ply reduce adherence to human lung epithelial cells and murine nasopharyngeal colonization by Streptococcus pneumoniae. Infect Immun 82:5069–5075. doi: 10.1128/IAI.02124-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Darrieux M, Goulart C, Briles D, Leite LC. 2015. Current status and perspectives on protein-based pneumococcal vaccines. Crit Rev Microbiol 41:190–200. doi: 10.3109/1040841X.2013.813902. [DOI] [PubMed] [Google Scholar]
- 158.Redi D, Raffaelli CS, Rossetti B, De Luca A, Montagnani F. 2018. Staphylococcus aureus vaccine preclinical and clinical development: current state of the art. New Microbiol 41:208–213. [PubMed] [Google Scholar]
- 159.Ragle BE, Bubeck Wardenburg J. 2009. Anti-alpha-hemolysin monoclonal antibodies mediate protection against Staphylococcus aureus pneumonia. Infect Immun 77:2712–2718. doi: 10.1128/IAI.00115-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sause WE, Buckley PT, Strohl WR, Lynch AS, Torres VJ. 2016. Antibody-based biologics and their promise to combat Staphylococcus aureus infections. Trends Pharmacol Sci 37:231–241. doi: 10.1016/j.tips.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Escajadillo T, Nizet V. 2018. Pharmacological targeting of pore-forming toxins as adjunctive therapy for invasive bacterial infection. Toxins (Basel) 10:E542. doi: 10.3390/toxins10120542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tseng CW, Biancotti JC, Berg BL, Gate D, Kolar SL, Muller S, Rodriguez MD, Rezai-Zadeh K, Fan X, Beenhouwer DO, Town T, Liu GY. 2015. Increased susceptibility of humanized NSG mice to Panton-Valentine leukocidin and Staphylococcus aureus skin infection. PLoS Pathog 11:e1005292. doi: 10.1371/journal.ppat.1005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thomsen IP, Dumont AL, James DB, Yoong P, Saville BR, Soper N, Torres VJ, Creech CB. 2014. Children with invasive Staphylococcus aureus disease exhibit a potently neutralizing antibody response to the cytotoxin LukAB. Infect Immun 82:1234–1242. doi: 10.1128/IAI.01558-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thomsen IP, Sapparapu G, James DBA, Cassat JE, Nagarsheth M, Kose N, Putnam N, Boguslawski KM, Jones LS, Wood JB, Creech CB, Torres VJ, Crowe JE. Jr, 2017. Monoclonal antibodies against the Staphylococcus aureus bicomponent leukotoxin AB isolated following invasive human infection reveal diverse binding and modes of action. J Infect Dis 215:1124–1131. doi: 10.1093/infdis/jix071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Berends ETM, Zheng X, Zwack EE, Menager MM, Cammer M, Shopsin B, Torres VJ. 2019. Staphylococcus aureus impairs the function of and kills human dendritic cells via the LukAB toxin. mBio 10:e01918-18. doi: 10.1128/mBio.01918-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Yoong P, Torres VJ. 2015. Counter inhibition between leukotoxins attenuates Staphylococcus aureus virulence. Nat Commun 6:8125. doi: 10.1038/ncomms9125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Diep BA, Le VT, Visram ZC, Rouha H, Stulik L, Dip EC, Nagy G, Nagy E. 2016. Improved protection in a rabbit model of community-associated methicillin-resistant Staphylococcus aureus necrotizing pneumonia upon neutralization of leukocidins in addition to alpha-hemolysin. Antimicrob Agents Chemother 60:6333–6340. doi: 10.1128/AAC.01213-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rouha H, Weber S, Janesch P, Maierhofer B, Gross K, Dolezilkova I, Mirkina I, Visram ZC, Malafa S, Stulik L, Badarau A, Nagy E. 2018. Disarming Staphylococcus aureus from destroying human cells by simultaneously neutralizing six cytotoxins with two human monoclonal antibodies. Virulence 9:231–247. doi: 10.1080/21505594.2017.1391447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reyes-Robles T, Lubkin A, Alonzo F 3rd, Lacy DB, Torres VJ. 2016. Exploiting dominant-negative toxins to combat Staphylococcus aureus pathogenesis. EMBO Rep 17:428–440. doi: 10.15252/embr.201540994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Spaan AN, Vrieling M, Wallet P, Badiou C, Reyes-Robles T, Ohneck EA, Benito Y, de Haas CJ, Day CJ, Jennings MP, Lina G, Vandenesch F, van Kessel KP, Torres VJ, van Strijp JA, Henry T. 2014. The staphylococcal toxins gamma-haemolysin AB and CB differentially target phagocytes by employing specific chemokine receptors. Nat Commun 5:5438. doi: 10.1038/ncomms6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Storisteanu DM, Pocock JM, Cowburn AS, Juss JK, Nadesalingam A, Nizet V, Chilvers ER. 2017. Evasion of neutrophil extracellular traps by respiratory pathogens. Am J Respir Cell Mol Biol 56:423–431. doi: 10.1165/rcmb.2016-0193PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Seilie ES, Bubeck Wardenburg J. 2017. Staphylococcus aureus pore-forming toxins: the interface of pathogen and host complexity. Semin Cell Dev Biol 72:101–116. doi: 10.1016/j.semcdb.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dela Cruz CS, Wunderink RG, Christiani DC, Cormier SA, Crothers K, Doerschuk CM, Evans SE, Goldstein DR, Khatri P, Kobzik L, Kolls JK, Levy BD, Metersky ML, Niederman MS, Nusrat R, Orihuela CJ, Peyrani P, Prince AS, Ramirez JA, Ridge KM, Sethi S, Suratt BT, Sznajder JI, Tsalik EL, Walkey AJ, Yende S, Aggarwal NR, Caler EV, Mizgerd JP. 2018. Future research directions in pneumonia: NHLBI working group report. Am J Respir Crit Care Med 198:256–263. doi: 10.1164/rccm.201801-0139WS. [DOI] [PMC free article] [PubMed] [Google Scholar]