Abstract
The introduction of more relevant cell models in early preclinical drug discovery, combined with high-content imaging and automated analysis, is expected to increase the quality of compounds progressing to preclinical stages in the drug development pipeline. In this review we discuss the current switch to more relevant 3D cell culture models and associated challenges for high-throughput screening and high-content analysis. We propose that overcoming these challenges will enable front-loading the drug discovery pipeline with better biology, extracting the most from that biology, and, in general, improving translation between in vitro and in vivo models. This is expected to reduce the proportion of compounds that fail in vivo testing due to a lack of efficacy or to toxicity.
Keywords: high-content screening, 3D culture, imaging, image analysis
Abstract
創薬の前臨床早期において、ハイコンテントイメージング自動アナリシスを併用しての適性度の高い細胞モデルの導入は、新薬開発パイプラインの前臨床段階に進む化合物の質を高めるものと期待される。本レビューでは、ハイスループットスクリーニングおよびハイコンテントアナリシスにおける、適性度の高い三次元細胞培養モデルへの現行の切り替えとそれに伴う課題について検討する。それらの課題を克服することで、適切なバイオロジーによる創薬パイプラインのフロントローディングが可能になり、そのバイオロジーから最大限のものを引き出し、概してin vivo外挿(translation between in vitro and in vivo models)を向上させることを我々は提案する。これにより、有効性欠如または毒性が原因でin vivo試験に不合格となる化合物の割合を低下できるものと期待される。
Abstract
대용량 이미징과 자동화 분석과 함께 초기 임상 전 신약 개발에서 관련성이 더 높은 세포 모형을 도입하면 신약 개발에서 임상 전 단계로 넘어가는 물질의 품질을 높여줄 것으로 기대된다. 본 고찰에서는 관련성이 더 높은 3D 세포 배양 모형으로 넘어가고 있는 현재의 변화와 이에 따른 초고속 스크리닝 및 대용량 분석의 문제점을 논하고자 한다. 이러한 문제점을 극복하면 신약 개발의 초반 부분에 더 나은 세포 작용을 제공하고, 이러한 세포 작용에서 최대한 추출을 하고, 세포 및 동물 모형 사이의 중개를 개선시킬 것이라 제안한다. 이는 효능과 독성 때문에 동물 시험에서 실패하는 물질의 비율을 감소시킬 것으로 기대된다.
Abstract
在早期临床前药物研发中引入相关性更好的细胞模型,结合高内涵成像和自动化分析,有望提高药物开发过程中进展至临床前阶段的化合物的质量。在本综述中,我们讨论了目前相关性更好的三维细胞培养模型的应用情况,以及相应高通量筛选和高内涵分析种的挑战。我们认为,克服这些挑战可以为药物研发流程提供更好的前期生物学基础,最大化的利用这些生物学基础,并在总体上改善从体外模型到体内模型的转换。预计这将减少因药效缺乏或毒性而在体内测试中失败的化合物的比例。
Introduction
Declining drug success rates and increasing costs suggest that alternative strategies are required in early drug discovery.1 Traditional drug discovery has favored a target-based approach where drugs are selected to manipulate a single molecular target. However, for many diseases targets are either poorly defined or unknown and inhibition of a single target is often not sufficient for effective therapy. As an alternative strategy, phenotypic screening for drug effects on disease-relevant phenotypic parameters has proven successful.2–4 Yet, approaches in phenotypic screening are still largely limited to combinations of suspension or two-dimensional (2D) monolayer cultures of a given cell type with a given endpoint measurement, such as cell viability or cell proliferation. The pleiotropic nature of such endpoints limits their sensitivity and selectivity for the most promising drugs.5 Furthermore, cells cultured as a monolayer often respond differently to drugs compared with native tissues.6 Also, primary cells may rapidly change compared with native tissue when cultured in a 2D environment.7,8 There are many likely reasons underlying the aberrant responses of 2D-cultured cell lines compared with tissues, but one dominant artifact is the grossly distorted architecture of cells stretched on rigid plastic. The impact on drug selection is considerable. For example, cancer cells grown as a monolayer have a deregulated cell cycle, often doubling every 24 h, while tumors in vivo typically show only a few percent of actively cycling cells and only have a marginally higher rate of proliferation compared with healthy tissue. As a result, cancer drugs selected on the basis of arresting proliferation in culture often do little in vivo, or, if they do, will also show adverse effects in healthy tissues.9 Taken together, front-loading the early in vitro stages of drug discovery with more disease-relevant biological models will inevitably increase the quality of molecules entering the pipeline. For example, image-based profiling of drug responses on ex vivo biobanked patient biopsies could lead to improved patient treatment.10 A more faithful in vitro representation of the pathways and processes in disease in vivo will improve drug testing even with simple endpoint measurements.11 However, the maximum potential of more disease-relevant biological models such as 3D-cultured tissues can only be realized by exploiting the phenotypic complexity with high-content endpoints. Developments in this area are ongoing, and while being highly promising, they also have identified a number of challenges that still limit successful implementation in large-scale drug screening pipelines.
Opportunities and Challenges in 3D Cell Culture Models for High-Throughput Screening
3D Tissue Culture Models Simulate Aberrant Tissue Organization in Pathology
Over the last three decades or so, three-dimensional (3D) cell culture techniques have been developed that have resulted in models that more accurately mimic physiological and diseased states than their 2D counterparts.12–17 These have the potential to provide a more physiologically relevant context for drug screening. 3D cultures can vary in complexity from spheroids derived from a single cell line to more complex multicellular structures derived from combinations of multiple cell types, or organoids derived from stem cells that develop into multicellular organ-like structures through self-renewal and differentiation capacities.18–25
The resulting biological complexity of 3D cell cultures makes them particularly well suited for phenotypic drug discovery. Traditional endpoints, such as proliferation and viability, can be combined with 3D assays—using either biochemical assays or specific fluorescent labels.26 But just as modern histopathology relies on a diverse range of cell and tissue architectural characteristics of patient material for decision making, maximum leverage of the more complex biology of 3D-cultured tissues can also be gained from the analysis of diverse morphological characteristics. This can be of particular value when aberrant tissue organization is directly associated with pathology, for example, with neurodegenerative disorders,27,28 tissue fibrosis,29 cancer,30–33 and ciliopathies such as polycystic kidney disease (PKD).34,35 In the context of these diseases, 2D-cultured cell lines fail profoundly to capture properties critically associated with the pathophysiology. The modeling of cystopathies is a particularly clear example since cysts, such as those formed in the kidneys of PKD patients, are 3D structures that cannot be recapitulated in 2D cell cultures. Therefore, mechanistic studies and compound efficacy testing can only effectively be studied in a 3D environment or in vivo. Similarly, to evaluate tumor dysplasia and invasion, 2D cell cultures lack the required physical environment. Aspects such as tumor cell plasticity are not observed in 2D but play a critical role in behavior in a 3D environment.36 These and many other examples underscore the need for more disease-relevant 3D cell culture models ( Fig. 1 ).
Figure 1.
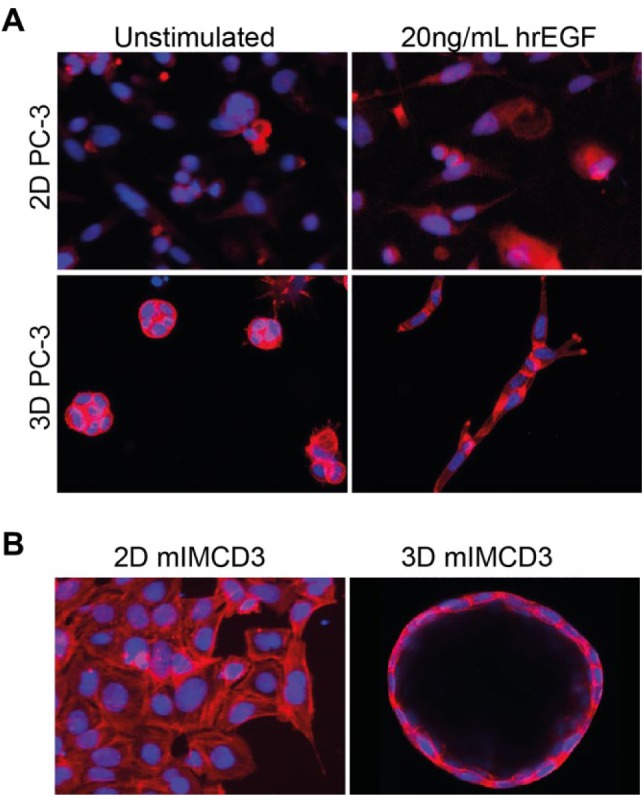
3D cell cultures provide a more physiologically relevant context for drug screening. (A) Prostate carcinoma (PC-3) cells cultured as 2D monolayer (top) show negligible morphological changes in response to growth factor (20 ng/mL hrEGF) stimulation but become invasive if embedded in 3D hydrogels (bottom) after growth factor stimulation. These invasive characteristics can be used to investigate the efficacy of inhibitors of receptor tyrosine kinases.96 Images in the top panel were obtained using a wide-field BD pathway 855 with a 10× objective, and images in the bottom panel were obtained using a Nikon Ti Eclipse confocal microscope with a 20× objective. (B) mIMCD3 cells transduced with a short-hairpin targeting Pkd1 form a monolayer in 2D culture (left panel, BD pathway 855 with 10× objective), but form cysts in 3D hydrogels, representing a more pathophysiologically relevant model of PKD (right panel, Nikon Ti Eclipse confocal microscope with 20× objective).108 F-actin (rhodamine-phalloidin), red; nuclei (Hoechst 33258), blue.
Variations in 3D Tissue Culture Models
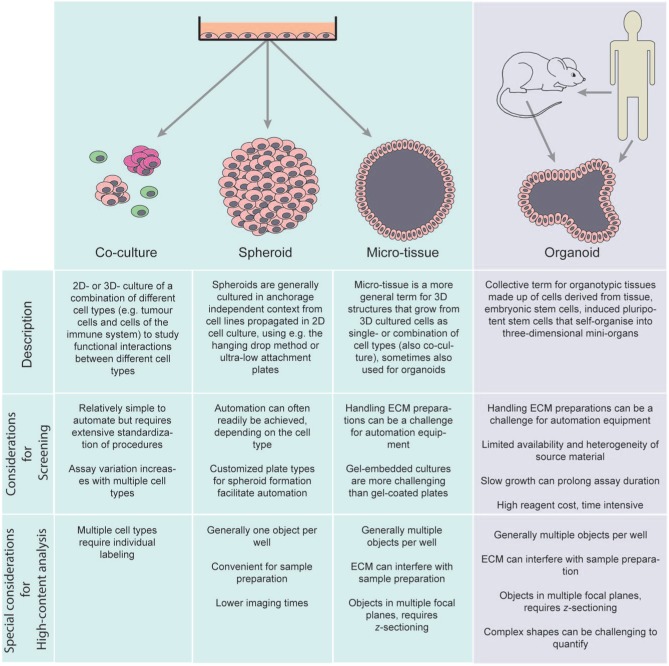
Many different options to culture cells in 3D have emerged, each with specific limitations and advantages for the evaluation of compound effects.1,37–41 Due to the enormous popularity of 3D cell culture assays and rapid developments in the field, terminology is often used in an inconsistent manner. In Figure 2 we provide an overview of the similarities and differences of popular 3D cell culture terminology and their implications for screening. 3D culture techniques often make use of immortalized cell lines due to the ease of culturing and relative lack of heterogeneity, and while convenient for high-throughput screens, these cells do not accurately represent tissues, since these generally require the interaction of multiple cell types for normal function. This problem may be ameliorated by the introduction of co-cultures,42 as has been shown for different co-culture systems.43–45 However, co-culture systems also introduce an increased level of complexity to the culture system, which can be undesirable for high-throughput screens. For example, cell ratios and cell culture media require optimization to support the growth of both co-cultured cell types to obtain functional tissues.42,44 In addition, the growth rate of the co-cultured cell types may differ. It may only be worth considering this approach if the interaction between the co-cultured cell types is of particular significance for the disease, such as the interaction of fibroblasts and epithelial cells in fibrosis46,47 or the interaction between endothelial cells or immune cells and cancer cells in the context of tumor angiogenesis or cancer immunology.48–52 Additional possibilities to improve the relevance of cell models can be the incorporation of primary cells obtained from specific tissues.18 Primary patient tumor material can be used to generate organoids in vitro that can be used to evaluate therapies.53 The tumor material can be genetically characterized and the observed therapeutic response can lead to highly personalized treatment suggestions. While direct patient-derived organoids are therefore highly promising for personalized medicine, the source material is limited and the cost, logistics, and lack of prior characterization of patient tissues may limit their suitability for in vitro screening.42
Figure 2.
Nomenclature of 3D cell-based assays.
Induced pluripotent stem cells (iPSCs) are an attractive alternative to the direct use of primary cells in screening,54 since iPSCs can be generated from virtually any adult cell type reprogrammed with a combination of transcription factors (e.g., Oct4, Sox2, Klf4, and c-Myc55). The resulting pluripotent stem cells can be differentiated to generate a desired tissue type. As a result, iPSC-derived tissues have been used to model a variety of different diseases,56 such as cardiovascular, neurological,57 and hepatic58 disorders. Although the popularity of using iPSC-derived tissues in high-throughput screens is rapidly increasing, significant hurdles for routine use of iPSCs for this purpose are still posed by extensive differentiation procedures that are required and also the possibility of incomplete differentiation.59 In addition, slow growth60 and challenging culture conditions can complicate screening procedures.61 Interestingly, because 3D culturing of iPSC-derived tissues is known to facilitate rapid reprogramming,62 growing iPSC-derived tissues in 3D assays may overcome at least some of these hurdles. Although high-throughput screens with iPSCs can be performed,63,64 these screens are generally done in a 2D environment, and throughput may in general be lower than when these screens are done in a 3D environment due to the more demanding procedures of culturing iPSCs.
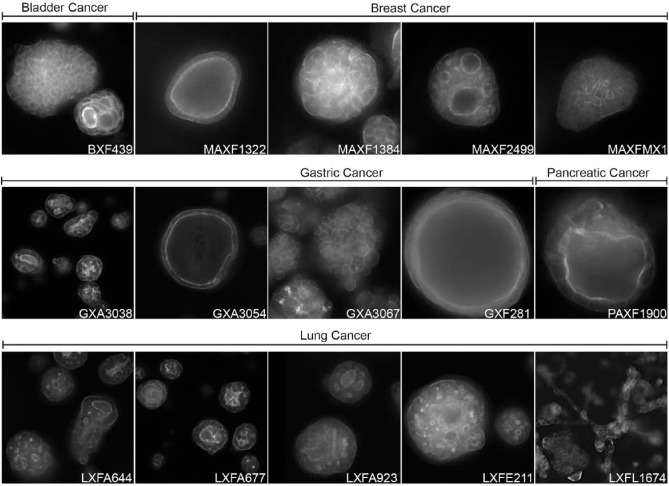
An alternative for the use of primary patient tumor material is the use of patient-derived xenograft (PDX) tumor material as a source of cells for 3D culture assays.65–67 These tumors are typically well characterized genetically and with respect to drug sensitivity in vivo, and the availability is not restricted as with patient tumor material. Practically, dissociated tumor cells can be allowed to reform as tumor spheroids in extracellular matrix (ECM) hydrogels for the screening of small molecules and biologics ( Fig. 3 ). The use of PDX-derived tumor material for in vitro tests also offers the possibility to subsequently test compounds in the autologous in vivo model. Such approaches are expected to improve the concordance between in vitro and in vivo data, although to what extent remains to be established. Recent advances in tissue culture technology have also enabled the generation of 3D organoid cultures of normal and diseased tissues from stem cells derived from tissue biopsies. Studies on panels of patient-derived organoids have shown that these can preserve the histology and genetic profile of the primary tissue and maintain an additional level of physiological relevance by forming more complex structures comprised of cells with different functions.15,16,68,69 While expansion of these tissue cultures is demanding compared with standard cell lines, they can still be used for compound screening.70,71 Factors that may limit the scale at which PDX tumor material can be used in a screening context are the in vivo tissue propagation, the in vitro growth rate, which differs between types of tissue, and the high costs of required cell culture media and growth factors. In addition, propagation of PDX tissue in mice may also have unwanted effects on the relevance to the original tissue.72
Figure 3.
3D cultures of PDX material. PDX material from different tumors can be cultured in 3D hydrogels to form complex microtissues that can be used for compound screening in a preclinically relevant context. Actin cytoskeleton visualized with rhodamine-phalloidin. PDX tumor material provided by Charles River Labs (Freiburg, Germany). Annotations refer to tumor type and PDX model number; BX = bladder; MAX = mammary; GX = gastric; PAX = pancreatic; LX = lung. 3D cultures and images generated by OcellO B.V.
Despite a number of successful studies showing the practical implementation of 3D cultures in routine screening,73–75 adoption of these model systems in routine drug discovery pipelines has been slow. Generally, high-reagent or cell culture expansion costs and low-throughput experimental procedures have long hampered the development of high-throughput screening platforms, and as a result, 3D cultures have mostly been used for small-scale experimentation and validation with single endpoint measurements, rather than for primary screens. Although several technical challenges remain, the appearance of a wide range of new reagents, technologies, and published methods has resulted in the increasing adoption of 3D cultures for compound screening and testing.
Matrix Composition and Automation
To provide a physiologically relevant context for 3D-cultured microtissues to develop and interrogate the effects of compounds, a microenvironment is required that provides cells with mechanical and physical interactions that normally occur in vivo.76 For this purpose, scaffolds have been used that can mimic the ECM.77–79 Since different cell types favor different interactions with their ECM, the matrix to select is highly dependent on the cell type. The most commonly used scaffolds include hydrogels, which can be natural, synthetic, or a combination.80 Natural hydrogels are animal-derived basement membrane (BM) extracts, which have fixed chemical and physical properties, but an undefined composition that varies between batches with unforeseen consequences. Examples of such natural hydrogels are collagen and the laminin-rich extracts produced from Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells (Matrigel). These gels contain many endogenous factors that can support viability of cell cultures.81 Synthetic hydrogels, in contrast, are well defined and can be readily modified and manufactured, thereby overcoming many problems associated with natural hydrogels. However, synthetic hydrogel matrices lack the properties to enable the remodeling required to support normal cell adhesion, growth, differentiation, and other cellular behaviors.82 Cells that grow under conditions where integrin-mediated interactions with the extracellular environment are compromised, such as in synthetic hydrogels, but also in hanging-drop, suspension media, or ultra-low-adhesion systems, typically require extended culture periods to enable the secretion of endogenous ECM proteins. The development of synthetic hydrogels with coupled functional peptides mimicking integrin attachment sites in natural ECM proteins will continue to advance the field considerably. Although predicting which functional peptides are required in a gel for a given cell type is highly challenging, covalent coupling of RGD and other peptides mimicking fibronectin, of peptides mimicking multiple laminin–integrin interaction sites, and of collagen-derived adhesive peptides, and integration of proteolytically degradable domains in the polymer backbone allow complex 3D cellular behavior, including morphogenesis, differentiation, and migration.83–87
Automation of liquid handling for 3D culturing techniques is a more technical challenge that can hamper the adoption of 3D microtissues in primary high-throughput screens. While liquid handling for suspension media and ultra-low-attachment microplates can be conveniently automated, this can be challenging for more viscous liquids such as collagen- and Matrigel-containing hydrogels.88 The polymerization of these gels is typically temperature sensitive, requiring extensive environment control and rapid liquid handling to avoid premature polymerization and blocked pipette tips. In addition, while automation of 3D culturing techniques can often be achieved for 96- or 384-well plates, further miniaturization may be problematic due to pipetting of smaller volumes.89
Sample Preparation
Additional challenges arise due to the environment in which cells are cultured. For example, for the detection of fluorescent signals, or for absorption measurements, the culture matrix often interferes with measurement, and this can be especially important for colorimetric measurements of cell viability or proliferation. Also, protein or RNA/DNA sample preparation techniques are often not compatible with the use of natural hydrogels that contain many endogenous factors, as the presence of matrix proteins can interfere with antibody labeling of protein or purification and detection of RNA and DNA.
Furthermore, the chemical and physical properties of the matrix can interfere with the free diffusion of certain compounds, especially large molecules, such as antibodies, or molecules that bind to ECM proteins. These properties of the gel can have an impact on the conditions that are tested (e.g., RNAi transfections, drug or antibody concentration at a target site) and may require longer treatment times and optimization of the protocols to determine the effects. For sample preparation, this means that standard procedures for immunofluorescent labeling have to be modified to allow sufficient time for diffusion of antibodies through the hydrogel. Also, washing steps need to be prolonged to allow excess antibody removal. On the other hand, these inconvenient properties, such as poor perfusion and adsorption, probably more faithfully recapitulate the in vivo situation. A development in the field of sample preparation has been optical clearing of 3D-cultured spheroids, which may alleviate some of the difficulties for sample preparation and imaging of these assays.90 Although it is unclear if these techniques can be used with gel-embedded 3D cultures, it is clear that this technique will improve image analysis of spheroids and enable analysis on a single-cell level.
Developments in 3D culture reagents and liquid handling technology will help to overcome many of these challenges, and the adoption of 3D cell cultures in high-throughput screening will inevitably continue to grow.
Quality Control and Standardization of Methods
There currently is a high need to standardize 3D cell culture methodology for use in medium- to high-throughput screens. In the previous sections, we aimed to illustrate the vast number of choices that can be made that precede the optimization of conditions for screening, and their influences on throughput and automation.
Compared with 2D cell cultures, 3D cultures are generally more challenging to automate due to the required reagents, and the cell type(s) may require extensive optimization for growth in miniaturized format. 3D cell cultures often suffer from lower reproducibility of results and higher assay variation even when simple readouts such as cell viability measurements are used.
In order to increase the assay quality and reproducibility, it is essential to control batch-to-batch variation—this is an especially important topic for the selection of natural hydrogels such as ECM, but equally relevant for the purchase of cell culture media and growth factors. By standardizing the protein content in and extensive testing of hydrogels before purchase, and purchasing large batches of assay reagents and antibodies for immunostaining at once, variation as a result of the reagent source can be minimized. Furthermore, assay plates can have differing qualities (e.g., ultra-low-attachment coatings) that may even differ between different batches, and it is essential to evaluate cell culture plates prior to starting a screen.
Additionally, in the context of co-cultures, but also in the context of organoids, seeding an accurate number of cells can be more of a challenge. Automatically counting cells with modern cell counters may therefore be preferable to manual counting. 3D cell culture assays often require more time than 2D cell cultures due to the time required for the cells to assemble into multicellular structures and the overall slower growth of (non-)immortalized cell lines in 3D. This also causes effects such as humidity and evaporation to have a larger impact on the assay outcome. Therefore, active humidification of incubators or using gas-permeable plate seals can help to counteract these negative effects.
As a more general remark, many different types of 3D cell-based assays now exist and are being developed. Assays that use patient-derived material may be especially challenging to reproduce. It is therefore essential that all procedures, reagents, and devices as well as image and data analysis scripts are properly documented and recorded. Due to the often enormous collections of images in high-content screens, it may be tempting to store these data in a compressed format or at lower resolution, but proper care must be taken that requantification of data remains possible.
Opportunities and Challenges in Phenotypic Profiling of 3D-Cultured Microtissues
High-throughput screens typically use single endpoint measurements for hit selection, such as cell viability, proliferation, or a reporter for a luciferase-based reporter assay for a single gene. This can compromise the quality of the selected hits, since only a narrow view of the cellular response to a treatment is reported. Automated microscopy enables capturing multiple features including real time to more adequately assess the full response to drug treatment. The greater morphological complexity of tissues cultured in 3D makes this type of high-content analysis particularly valuable, retrieving rich information that would be overlooked by single endpoint assays. Recent years have witnessed the development of (ultra-)high-content phenotypic screening and multiparametric analysis techniques that can fully exploit the complex cellular response patterns to classify compound effects.91–95 While currently used extensively for 2D-cultured cells, high-content screening of 3D cell-based assays presents challenges for imaging, image analysis, computation, and data storage, as well as data visualization.
Imaging of 3D-Cultured Microtissues
For imaging of 3D cell cultures and the selection of a plate type, the same basic rules apply as for 2D cell cultures. However, for anchorage-dependent 3D cultures, the optical properties of the plate bottom are generally not a limiting factor to obtain good-quality images, as this depends largely on the ECM scaffold used. Additionally, not all plate types may be equally suited for anchorage-dependent 3D cultures—glass plates often have desirable properties for confocal microscopy but often provide a smoother surface to which a gel can adhere less firmly. For anchorage-free 3D cultures, this is less of a problem, but due to the large variation in culture techniques, many custom plate types have been developed (e.g., hanging-drop spheroid plates, ultra-low-attachment plates), which may have implications for image capturing. For example, ultra-low-attachment 384-well plates are available as flat-bottom plates (where spheroids do not necessarily form in the center of the well) and round-bottom plates (spheroid centered). These different plate types are not always compatible with all microscopes.
To analyze cellular phenotypes, fixed and stained cultures are typically imaged using conventional wide-field or confocal fluorescence microscopy. While 2D cell cultures can generally be captured using a single xy image, a single xy image taken from gel-embedded microtissues captures only a fraction of the objects in a well, with the majority captured in a suboptimum plane. To retrieve sufficient information from a 3D culture, a series of xy images are captured at fixed steps in the vertical direction using automated microscopes,96 to obtain a z stack from each well ( Fig. 4A ). Although the entire well of a 384-well plate is typically captured with a 4× objective, stepping up to a 10× lens to capture more (sub-)cellular detail multiplies the number of xy fields and z planes required to capture the same number of objects—increasing the image capture time perhaps 10-fold. Because increasing the objective’s magnification will multiply the required imaging and analysis time, higher-magnification objectives (40–60×) are currently not suitable for imaging 3D cultures in a high-throughput setting. In addition, depending on the selected plate type and microscope, it may not be possible to image outer wells using higher-magnification objectives. Similar to increasing the objective’s magnification, using multiple fluorescent channels multiplies image capture time. Wide-field fluorescence imaging can speed up image capture time compared with confocal imaging, but requires postimaging deconvolution to reduce out-of-focus signal. An interesting idea that can help to maintain throughput while still obtaining high-magnification 3D object data is to perform on-the-fly phenotypic analysis on low-magnification image sections and reimage conditions of interest at a higher magnification.
Figure 4.
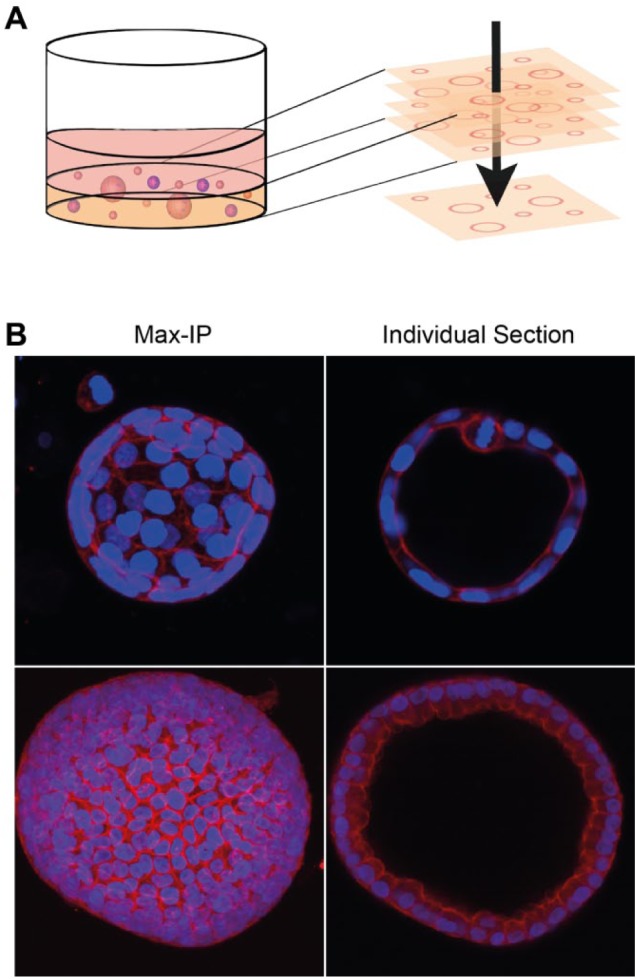
Maximum-intensity projections can cause loss of phenotypic information in 3D cultures. (A) Schematic representation of 2D maximum-intensity projections modified from Booij et al. (2016).96 Structures embedded in hydrogels are captured in xy and z directions using automated microscopy, and in-focus regions from all sections are projected into a 2D reconstruction. (B) 2D projections from 3D structures can cause loss of important phenotypic characteristics. These images display human kidney cyst-derived organoids, cultured in Matrigel and stained for F-actin (rhodamine-phalloidin, red) and nuclei (Hoechst 33258, blue) and imaged on a Nikon Ti Eclipse confocal microscope. Example 3D reconstructions from image sections obtained after imaging with a Nikon Ti Eclipse confocal microscope are included as Supplemental Video 1. Maximum-intensity projection performed with ImageJ software prevents lumen and cell shape detection.
The capturing of multiple xy images, often with multiple image channels, considerably increases data volumes compared with a 2D experiment. For example, a 384-well plate of 3D cultures imaged with a 4× lens can typically yield 50–100 GB of image data. Maximum focus or intensity projection algorithms are available in several software packages such as ImageJ97 and CellProfiler98 and convert 3D image stacks to 2D images, dramatically reducing data volume and the complexity of analysis ( Fig. 4A ). However, collapsing a 3D image stack to a single xy image results in a significant corruption of architecture, mismeasurement of objects blended from different z planes, and loss of the spatial association of objects between fluorescence channels, compromising co-localization measurements; analysis of intact 3D image stacks is necessary to retain this phenotypic information ( Fig. 4B ).99,100
2D cell cultures typically provide thousands of cells for phenotypic analysis as single-cell resolution in high-throughput screens can be achieved. 3D cultures, however, often only provide one object (in the case of spheroids generated using the hanging-drop technology or ultra-low-attachment plates26) or perhaps a hundred objects per well (spheroids or microtissues embedded in gel) for analysis because achieving single-cell resolution cannot be achieved with low-magnification objectives.
Low object numbers, coupled with heterogeneity of cell seeding and growth, can be potentially problematic when measuring single endpoints such as cell viability. Multiparametric high-content analysis can overcome these problems by allowing for normalization to object (spheroid or microtissue) number and can additionally exploit heterogeneity to study the effect of treatments on specific cellular subpopulations.99,100
While it is clear that adding a third dimension increases the image capture and computational demands, including live-cell 3D imaging in a multiwell screening format most certainly pushes the demands beyond the capacity of the available technology. However, such techniques could provide valuable information on tissue dynamics over time in more relevant biological systems.101 With advances in automated microscopy systems and image analysis software and the increases in computational power, live 3D image capture is expected to become accessible. Exciting new developments in this area include recent advances in automated brightfield imaging and light sheet fluorescence microscopy (LSFM) that overcome light penetration and bleaching issues associated with confocal microscopy.102–105 Ongoing efforts to implement LSFM in high-throughput applications would revolutionize the information that could be obtained from a 3D screening approach.
Image Analysis and Multiparametric Endpoints
Despite the availability of advanced image analysis tools through software such as ImageJ97 and CellProfiler,98 the true phenotypic complexity of 3D-cultured microtissues is often not exploited to its full extent.5 Software to apply true 3D phenotypic analysis and single-cell segmentation within 3D-cultured microtissues or organoids to high-throughput screening is not yet available off the shelf. For this purpose, in-house software has been developed at OcellO B.V. (Leiden, the Netherlands; L. Price). Traditionally, most screening microscopes have been developed for high-throughput 2D assays. As a result, the software provided with such systems is often not capable of analyzing the morphology of 3D-cultured microtissues. With the increased popularity of 3D cell culture techniques, microscope manufacturers have also increased the capabilities of their imagers and image analysis software. While several open-source and commercial software packages are available for 3D image processing and analysis, not all software may be able to handle screening data in an automated manner. An overview of available high-content screening systems as well as image analysis software is provided by Li et al. (2016).106
Although often requiring the use of high-magnification lenses and multiple z planes when imaging, it is relatively straightforward to capture single-cell-resolution images from cells cultured in a monolayer and apply this in an automated high-throughput format. However, it is not yet feasible to achieve this with 3D cultures—largely due to the inability of imager software to detect objects on the fly and home in for high-magnification image capture. However, this may be compensated by the additional features that can be measured from multicellular organotypic structures using lower-magnification lenses in a high-throughput format.
For many research questions, a simple parameter, such as spheroid size, may be adequate to discriminate a treatment response. As an advantage of using only a few parameters, readouts are generally simpler to interpret. But the use of a limited number of parameters ignores an abundance of the information that can be extracted from the 3D image stacks. Segmentation of 3D brightfield images followed by morphometric analysis enabled stratification of different organoid populations enriched with specific transcriptional signatures.102 Ultra-high-content analysis has also been applied to fluorescence images of 3D cultures, enabling the classification of drug treatments based on the morphological changes that are induced. However, additional information may be obtained from immunostaining or fluorescent markers, but this cannot be fully exploited if single-cell resolution images cannot be obtained.
We showed previously that the integration of multiple phenotypic descriptors can improve the classification of compounds according to phenotypic response.100 The analysis of high-dimensional data (often containing hundreds of different phenotypic measurements) requires the use of more advanced data processing and visualization software, such as KNIME, R, and Spotfire. As a result of using hundreds of phenotype-derived parameters, it can be difficult to extrapolate individual parameters to biological observations.107 To integrate high-dimensional data and generate meaningful visualizations, dimensionality reduction methods such as principal component analysis (PCA) can be useful. PCA linearly transforms high-dimensional data to a space of fewer dimensions, while retaining most of the variance of the data. Dimensionality reduction techniques have been used in a 3D invasive cancer model to differentiate between receptor tyrosine kinase inhibitors96 and also, more recently, to identify new potentially druggable targets for PKD and discrimination of compounds with efficacy and toxicity.108
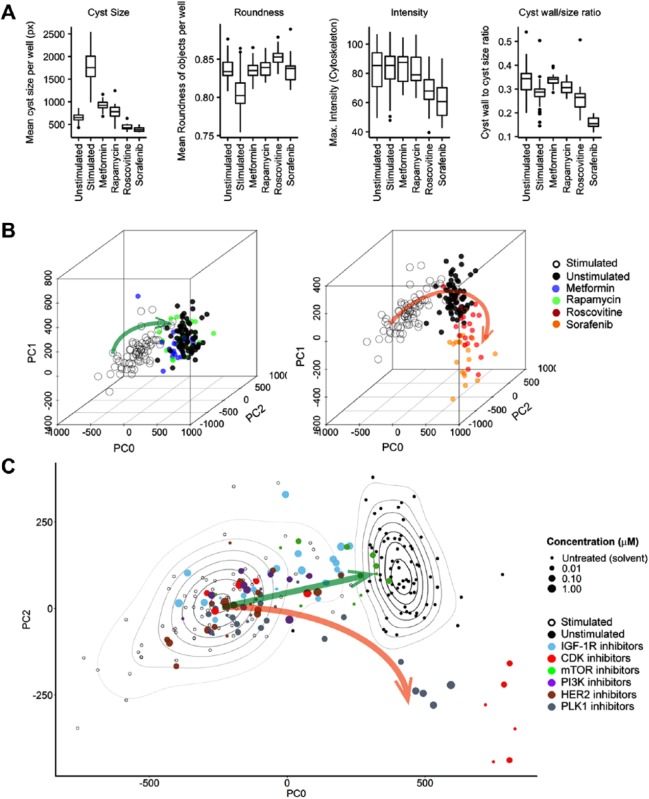
As an example of this approach, in Figure 5A we show the efficacy of four control molecules at inhibiting forskolin-induced cystogenesis. On the basis of single parameters such as cyst size and perimeter, all inhibitors show inhibition of cyst growth, with roscovitine and sorafenib being most potent. However, if a PCA-based visualization is used, such as shown in Figure 5B , the inhibitory effects of metformin and rapamycin can be discriminated from those of roscovitine and sorafenib, which induce a novel phenotype indicative of toxicity ( Fig. 5B ).108 This type of approach can also be useful in the classification of previously untested drugs ( Fig. 5C ).
Figure 5.
Exploiting multiparametric data to discriminate responses. (A) Metformin, rapamycin, roscovitine, and sorafenib inhibit forskolin-stimulated PKD cyst swelling, with roscovitine and sorafenib inducing the most potent response based on evaluation of individual parameters. Analysis was performed using Ominer software (OcellO B.V.). All displayed phenotypic parameters are derived from the rhodamine-phalloidin (f-actin) staining of 3D-cultured cysts. (B) Left panel: Three principal components summarizing 84% of variance in the data show a desirable phenotypic change (green arrow) in which 5 mM metformin (blue) and 10 nM rapamycin (green) revert a 2.5 µM forskolin-stimulated phenotype (swollen cyst, empty circles) to one indistinguishable from an unstimulated (solvent) phenotype (solid black circles). Right panel: 31.6 µM roscovitine and 10 µM sorafenib induce an aberrant phenotype (orange arrow); data points represent single wells. Figures adapted from Booij et al. (2017).108 (C) Two principal components from B showing multiple inhibitors targeting cyclin-dependent kinases (CDK), mammalian target of rapamycin (mTOR), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), human epidermal growth factor receptor 2 (HER2), and polo-like kinase (PLK1). Contour plots represent density estimations to emphasize the locations of forskolin-stimulated (empty circles) and unstimulated controls (solid black circles), respectively. Green arrow represents desirable compound efficacy, from forskolin-stimulated control (empty circles, swollen cysts) to unstimulated control (solid black circles, small cysts), and the orange arrow represents aberrant phenotypes that are observed after treatment with PLK1 inhibitors or high-dose CDK inhibitors, indicative of cytotoxicity.108
The use of multiparametric endpoints to profile compounds therefore represents an opportunity to extract more information from primary 3D screens and exploit this phenotypic information to better discriminate promising compounds at the earliest stage of the discovery process.
Conclusion and Perspectives
We propose that inclusion of biologically relevant in vitro model systems early in preclinical development will aid in selecting drugs that have a more desirable efficacy and safety profile, especially when these model systems are coupled to multiparametric phenotypic analysis strategies. It is likely that the current switch from immortalized cell lines to more challenging PDX, co-culture, and organoid models will also increase the demand for high-content analysis methods due to increased tissue complexity that cannot be exploited when using classical whole-well endpoint measurements. However, given the challenges that must be overcome and the substantial investments needed to do so, there is a strong need to validate these technologies and to demonstrate clearly that using biologically relevant in vitro systems actually improves the efficiency of early drug discovery. A direct comparison of the predictive value of 2D and 3D models for in vivo efficacy is required. Ideally, such an effort should include collections of molecules that have previously passed and failed in preclinical and clinical studies to determine the phenotypic footprint of successful medicines. Should the combination of complex 3D microtissues with high-content analysis score significantly better in this competition, investments in implementation of these technologies in the drug discovery pipeline may be warranted and ultimately lead to more effective discovery of more effective drugs.
Acknowledgments
The authors would like to thank personnel at OcellO for providing images and image analysis support.
Footnotes
Supplemental material is available online with this article.
Declaration of Conflicting Interests: The authors disclosed the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Leo S. Price is founder and shareholder of the compound testing CRO OcellO B.V.
Funding: The authors disclosed the following financial support for the research, authorship, and/or publication of this article: Tijmen H. Booij was supported by the Dutch Technology Foundation STW (Project 11823).
ORCID iDs: Tijmen H. Booij  https://orcid.org/0000-0002-7478-4704
https://orcid.org/0000-0002-7478-4704
Erik H. J. Danen  https://orcid.org/0000-0002-0491-6345
https://orcid.org/0000-0002-0491-6345
References
- 1. Langhans S. A. Three-Dimensional In Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng W., Thorne N., McKew J. C. Phenotypic Screens as a Renewed Approach for Drug Discovery. Drug Discov. Today 2013, 18, 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Swinney D. C., Anthony J. How Were New Medicines Discovered? Nat. Rev. Drug Discov. 2011, 10, 507–519. [DOI] [PubMed] [Google Scholar]
- 4. Swinney D. C., Phenotypic vs. Target-Based Drug Discovery for First-in-Class Medicines. Clin. Pharmacol. Ther. 2013, 93, 299–301. [DOI] [PubMed] [Google Scholar]
- 5. Singh S., Carpenter A. E., Genovesio A. Increasing the Content of High-Content Screening: An Overview. J. Biomol. Screen. 2014, 19, 640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Howes A. L., Richardson R. D., Finlay D., et al. 3-Dimensional Culture Systems for Anti-Cancer Compound Profiling and High-Throughput Screening Reveal Increases in EGFR Inhibitor-Mediated Cytotoxicity Compared to Monolayer Culture Systems. PLoS One 2014, 9, e108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reeves S. R., Barrow K. A., White M. P., et al. Stability of Gene Expression by Primary Bronchial Epithelial Cells over Increasing Passage Number. BMC Pulm. Med. 2018, 18, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee J. K., Bloom J., Zubeldia-Plazaola A., et al. Different Culture Media Modulate Growth, Heterogeneity, and Senescence in Human Mammary Epithelial Cell Cultures. PLoS One 2018, 13, e0204645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. van den Brand D., Massuger L. F., Brock R., et al. Mimicking Tumors: Toward More Predictive In Vitro Models for Peptide- and Protein-Conjugated Drugs. Bioconjug. Chem. 2017, 28, 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Snijder B., Vladimer G. I., Krall N., et al. Image-Based Ex-Vivo Drug Screening for Patients with Aggressive Haematological Malignancies: Interim Results from a Single-Arm, Open-Label, Pilot Study. Lancet Haematol. 2017, 4, e595–e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bickle M. The Beautiful Cell: High-Content Screening in Drug Discovery. Anal. Bioanal. Chem. 2010, 398, 219–226. [DOI] [PubMed] [Google Scholar]
- 12. Lee G. Y., Kenny P. A., Lee E. H., et al. Three-Dimensional Culture Models of Normal and Malignant Breast Epithelial Cells. Nat. Methods 2007, 4, 359–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Simian M., Bissell M. J. Organoids: A Historical Perspective of Thinking in Three Dimensions. J. Cell Biol. 2017, 216, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sakurai H., Nigam S. K. In Vitro Branching Tubulogenesis: Implications for Developmental and Cystic Disorders, Nephron Number, Renal Repair, and Nephron Engineering. Kidney Int. 1998, 54, 14–26. [DOI] [PubMed] [Google Scholar]
- 15. Clevers H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [DOI] [PubMed] [Google Scholar]
- 16. Takasato M., Er P. X., Chiu H. S., et al. Kidney Organoids from Human iPS Cells Contain Multiple Lineages and Model Human Nephrogenesis. Nature 2015, 526, 564–568. [DOI] [PubMed] [Google Scholar]
- 17. Fang Y., Eglen R. M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sokol E. S., Miller D. H., Breggia A., et al. Growth of Human Breast Tissues from Patient Cells in 3D Hydrogel Scaffolds. Breast Cancer Res. 2016, 18, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen O. W., Ronnov-Jessen L., Howlett A. R., et al. Interaction with Basement Membrane Serves to Rapidly Distinguish Growth and Differentiation Pattern of Normal and Malignant Human Breast Epithelial Cells. Proc. Natl. Acad. Sci. U.S.A. 1992, 89, 9064–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaw K. R., Wrobel C. N., Brugge J. S. Use of Three-Dimensional Basement Membrane Cultures to Model Oncogene-Induced Changes in Mammary Epithelial Morphogenesis. J. Mammary Gland Biol. Neoplasia 2004, 9, 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Debnath J., Brugge J. S. Modelling Glandular Epithelial Cancers in Three-Dimensional Cultures. Nat. Rev. Cancer 2005, 5, 675–688. [DOI] [PubMed] [Google Scholar]
- 22. Martin-Belmonte F., Yu W., Rodriguez-Fraticelli A. E., et al. Cell-Polarity Dynamics Controls the Mechanism of Lumen Formation in Epithelial Morphogenesis. Curr. Biol. 2008, 18, 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nguyen-Ngoc K. V., Cheung K. J., Brenot A., et al. ECM Microenvironment Regulates Collective Migration and Local Dissemination in Normal and Malignant Mammary Epithelium. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, E2595–E2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J. L., Streuli C. H. Integrins and Epithelial Cell Polarity. J. Cell Sci. 2014, 127, 3217–3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muschler J., Streuli C. H. Cell-Matrix Interactions in Mammary Gland Development and Breast Cancer. Cold Spring Harb. Perspect. Biol. 2010, 2, a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sirenko O., Mitlo T., Hesley J., et al. High-Content Assays for Characterizing the Viability and Morphology of 3D Cancer Spheroid Cultures. Assay Drug Dev. Technol. 2015, 13, 402–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hopkins A. M., DeSimone E., Chwalek K., et al. 3D In Vitro Modeling of the Central Nervous System. Prog. Neurobiol. 2015, 125, 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. D’Avanzo C., Aronson J., Kim Y. H., et al. Alzheimer’s in 3D Culture: Challenges and Perspectives. Bioessays 2015, 37, 1139–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Porras A. M., Hutson H. N., Berger A. J., et al. Engineering Approaches to Study Fibrosis in 3-D In Vitro Systems. Curr. Opin. Biotechnol. 2016, 40, 24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kimlin L. C., Casagrande G., Virador V. M. In Vitro Three-Dimensional (3D) Models in Cancer Research: An Update. Mol. Carcinog. 2013, 52, 167–182. [DOI] [PubMed] [Google Scholar]
- 31. Levinger I., Ventura Y., Vago R. Life Is Three Dimensional—As In Vitro Cancer Cultures Should Be. Adv. Cancer Res. 2014, 121, 383–414. [DOI] [PubMed] [Google Scholar]
- 32. Yamada K. M., Cukierman E. Modeling Tissue Morphogenesis and Cancer in 3D. Cell 2007, 130, 601–610. [DOI] [PubMed] [Google Scholar]
- 33. Smalley K. S., Lioni M., Herlyn M. Life Isn’t Flat: Taking Cancer Biology to the Next Dimension. In Vitro Cell. Dev. Biol. Anim. 2006, 42, 242–247. [DOI] [PubMed] [Google Scholar]
- 34. Desrochers T. M., Palma E., Kaplan D. L. Tissue-Engineered Kidney Disease Models. Adv. Drug Deliv. Rev. 2014, 69–70, 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Subramanian B., Rudym D., Cannizzaro C., et al. Tissue-Engineered Three-Dimensional In Vitro Models for Normal and Diseased Kidney. Tissue Eng. Part A 2010, 16, 2821–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Truong H. H., Xiong J., Ghotra V. P., et al. beta1 Integrin Inhibition Elicits a Prometastatic Switch through the TGFbeta-miR-200-ZEB Network in E-Cadherin-Positive Triple-Negative Breast Cancer. Sci. Signal. 2014, 7, ra15. [DOI] [PubMed] [Google Scholar]
- 37. Horvath P., Aulner N., Bickle M., et al. Screening Out Irrelevant Cell-Based Models of Disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [DOI] [PubMed] [Google Scholar]
- 38. Joshi P., Lee M. Y. High Content Imaging (HCI) on Miniaturized Three-Dimensional (3D) Cell Cultures. Biosensors 2015, 5, 768–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Edmondson R., Broglie J. J., Adcock A. F., et al. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kimlin L., Kassis J., Virador V. 3D In Vitro Tissue Models and Their Potential for Drug Screening. Expert. Opin. Drug Discov. 2013, 8, 1455–1466. [DOI] [PubMed] [Google Scholar]
- 41. Klinghammer K., Walther W., Hoffmann J. Choosing Wisely—Preclinical Test Models in the Era of Precision Medicine. Cancer Treat. Rev. 2017, 55, 36–45. [DOI] [PubMed] [Google Scholar]
- 42. Abbott R. D., Kaplan D. L. Strategies for Improving the Physiological Relevance of Human Engineered Tissues. Trends Biotechnol. 2015, 33, 401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang P. C., Takezawa T. Reconstruction of Renal Glomerular Tissue Using Collagen Vitrigel Scaffold. J. Biosci. Bioeng. 2005, 99, 529–540. [DOI] [PubMed] [Google Scholar]
- 44. Kang J. H., Gimble J. M., Kaplan D. L. In Vitro 3D Model for Human Vascularized Adipose Tissue. Tissue Eng. Part A 2009, 15, 2227–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hussain A., Collins G., Yip D., et al. Functional 3-D Cardiac Co-Culture Model Using Bioactive Chitosan Nanofiber Scaffolds. Biotechnol. Bioeng. 2013, 110, 637–647. [DOI] [PubMed] [Google Scholar]
- 46. Nowinski D., Lysheden A. S., Gardner H., et al. Analysis of Gene Expression in Fibroblasts in Response to Keratinocyte-Derived Factors In Vitro: Potential Implications for the Wound Healing Process. J. Invest. Dermatol. 2004, 122, 216–221. [DOI] [PubMed] [Google Scholar]
- 47. Nomoto Y., Kobayashi K., Tada Y., et al. Effect of Fibroblasts on Epithelial Regeneration on the Surface of a Bioengineered Trachea. Ann. Otol. Rhinol. Laryngol. 2008, 117, 59–64. [DOI] [PubMed] [Google Scholar]
- 48. Balcioglu H. E., van de Water B., Danen E. H. Tumor-Induced Remote ECM Network Orientation Steers Angiogenesis. Sci. Rep. 2016, 6, 22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Amann A., Zwierzina M., Koeck S., et al. Development of a 3D Angiogenesis Model to Study Tumour-Endothelial Cell Interactions and the Effects of Anti-Angiogenic Drugs. Sci. Rep. 2017, 7, 2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Maracle C. X., Jeucken K. C. M., Helder B., et al. Silencing NIK Potentiates Anti-VEGF Therapy in a Novel 3D Model of Colorectal Cancer Angiogenesis. Oncotarget 2018, 9, 28445–28455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sherman H., Gitschier H. J., Rossi A. E. A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity. Front. Immunol. 2018, 9, 857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Herter S., Morra L., Schlenker R., et al. A Novel Three-Dimensional Heterotypic Spheroid Model for the Assessment of the Activity of Cancer Immunotherapy Agents. Cancer Immunol. Immunother. 2017, 66, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vlachogiannis G., Hedayat S., Vatsiou A., et al. Patient-Derived Organoids Model Treatment Response of Metastatic Gastrointestinal Cancers. Science 2018, 359, 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ho B. X., Pek N. M. Q., Soh B. S. Disease Modeling Using 3D Organoids Derived from Human Induced Pluripotent Stem Cells. Int. J. Mol. Sci. 2018, 19, 936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 56. Park I. H., Arora N., Huo H., et al. Disease-Specific Induced Pluripotent Stem Cells. Cell 2008, 134, 877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dimos J. T., Rodolfa K. T., Niakan K. K., et al. Induced Pluripotent Stem Cells Generated from Patients with ALS Can Be Differentiated into Motor Neurons. Science 2008, 321, 1218–1221. [DOI] [PubMed] [Google Scholar]
- 58. Rashid S. T., Corbineau S., Hannan N., et al. Modeling Inherited Metabolic Disorders of the Liver Using Human Induced Pluripotent Stem Cells. J. Clin. Invest. 2010, 120, 3127–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Passier R., van Laake L. W., Mummery C. L. Stem-Cell-Based Therapy and Lessons from the Heart. Nature 2008, 453, 322–329. [DOI] [PubMed] [Google Scholar]
- 60. Narsinh K. H., Sun N., Sanchez-Freire V., et al. Single Cell Transcriptional Profiling Reveals Heterogeneity of Human Induced Pluripotent Stem Cells. J. Clin. Invest. 2011, 121, 1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ebert A. D., Liang P., Wu J. C. Induced Pluripotent Stem Cells as a Disease Modeling and Drug Screening Platform. J. Cardiovasc. Pharmacol. 2012, 60, 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caiazzo M., Okawa Y., Ranga A., et al. Defined Three-Dimensional Microenvironments Boost Induction of Pluripotency. Nat. Mater. 2016, 15, 344–352. [DOI] [PubMed] [Google Scholar]
- 63. Del Alamo J. C., Lemons D., Serrano R., et al. High Throughput Physiological Screening of iPSC-Derived Cardiomyocytes for Drug Development. Biochim. Biophys. Acta 2016, 1863, 1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kumari D., Swaroop M., Southall N., et al. High-Throughput Screening to Identify Compounds That Increase Fragile X Mental Retardation Protein Expression in Neural Stem Cells Differentiated from Fragile X Syndrome Patient-Derived Induced Pluripotent Stem Cells. Stem Cells Transl. Med. 2015, 4, 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xie B. Y., Wu A. W. Organoid Culture of Isolated Cells from Patient-Derived Tissues with Colorectal Cancer. Chin. Med. J. (Engl.) 2016, 129, 2469–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fong E. L., Wan X., Yang J., et al. A 3D In Vitro Model of Patient-Derived Prostate Cancer Xenograft for Controlled Interrogation of In Vivo Tumor-Stromal Interactions. Biomaterials 2016, 77, 164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fong E. L., Martinez M., Yang J., et al. Hydrogel-Based 3D Model of Patient-Derived Prostate Xenograft Tumors Suitable for Drug Screening. Mol. Pharm. 2014, 11, 2040–2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sachs N., Clevers H. Organoid Cultures for the Analysis of Cancer Phenotypes. Curr. Opin. Genet. Dev. 2014, 24, 68–73. [DOI] [PubMed] [Google Scholar]
- 69. Drost J., Clevers H. Organoids in Cancer Research. Nat. Rev. Cancer 2018, 18, 407–418. [DOI] [PubMed] [Google Scholar]
- 70. van de Wetering M., Francies H. E., Francis J. M., et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell 2015, 161, 933–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sachs N., de Ligt J., Kopper O., et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2018, 172, 373–386.e310. [DOI] [PubMed] [Google Scholar]
- 72. Ben-David U., Ha G., Tseng Y. Y., et al. Patient-Derived Xenografts Undergo Mouse-Specific Tumor Evolution. Nat. Genet. 2017, 49, 1567–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Krausz E., de Hoogt R., Gustin E., et al. Translation of a Tumor Microenvironment Mimicking 3D Tumor Growth Co-Culture Assay Platform to High-Content Screening. J. Biomol. Screen. 2013, 18, 54–66. [DOI] [PubMed] [Google Scholar]
- 74. Kunz-Schughart L. A., Freyer J. P., Hofstaedter F., et al. The Use of 3-D Cultures for High-Throughput Screening: The Multicellular Spheroid Model. J. Biomol. Screen. 2004, 9, 273–285. [DOI] [PubMed] [Google Scholar]
- 75. Kelm J. M., Timmins N. E., Brown C. J., et al. Method for Generation of Homogeneous Multicellular Tumor Spheroids Applicable to a Wide Variety of Cell Types. Biotechnol. Bioeng. 2003, 83, 173–180. [DOI] [PubMed] [Google Scholar]
- 76. Baker B. M., Chen C. S. Deconstructing the Third Dimension—How 3D Culture Microenvironments Alter Cellular Cues. J. Cell Sci. 2012, 125, 3015–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. DeVolder R., Kong H. J. Hydrogels for In Vivo-Like Three-Dimensional Cellular Studies. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 351–365. [DOI] [PubMed] [Google Scholar]
- 78. Tibbitt M. W., Anseth K. S. Hydrogels as Extracellular Matrix Mimics for 3D Cell Culture. Biotechnol. Bioeng. 2009, 103, 655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guan X., Avci-Adali M., Alarcin E., et al. Development of Hydrogels for Regenerative Engineering. Biotechnol. J. 2017, 12, 1600394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Barnes A. L., Genever P. G., Rimmer S., et al. Collagen–Poly(N-Isopropylacrylamide) Hydrogels with Tunable Properties. Biomacromolecules 2016, 17, 723–734. [DOI] [PubMed] [Google Scholar]
- 81. Kleinman H. K., McGarvey M. L., Hassell J. R., et al. Basement Membrane Complexes with Biological Activity. Biochemistry 1986, 25, 312–318. [DOI] [PubMed] [Google Scholar]
- 82. Cushing M. C., Anseth K. S. Materials Science. Hydrogel Cell Cultures. Science 2007, 316, 1133–1134. [DOI] [PubMed] [Google Scholar]
- 83. Yamada M., Sekiguchi K. Molecular Basis of Laminin-Integrin Interactions. Curr. Top. Membr. 2015, 76, 197–229. [DOI] [PubMed] [Google Scholar]
- 84. Streuli C. H., Bailey N., Bissell M. J. Control of Mammary Epithelial Differentiation: Basement Membrane Induces Tissue-Specific Gene Expression in the Absence of Cell-Cell Interaction and Morphological Polarity. J. Cell Biol. 1991, 115, 1383–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Legant W. R., Miller J. S., Blakely B. L., et al. Measurement of Mechanical Tractions Exerted by Cells in Three-Dimensional Matrices. Nat. Methods 2010, 7, 969–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gjorevski N., Sachs N., Manfrin A., et al. Designer Matrices for Intestinal Stem Cell and Organoid Culture. Nature 2016, 539, 560–564. [DOI] [PubMed] [Google Scholar]
- 87. Miller J. S., Shen C. J., Legant W. R., et al. Bioactive Hydrogels Made from Step-Growth Derived PEG-Peptide Macromers. Biomaterials 2010, 31, 3736–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ruel-Gariepy E., Leroux J. C. In Situ-Forming Hydrogels—Review of Temperature-Sensitive Systems. Eur. J. Pharm. Biopharm. 2004, 58, 409–426. [DOI] [PubMed] [Google Scholar]
- 89. Ryan S. L., Baird A. M., Vaz G., et al. Drug Discovery Approaches Utilizing Three-Dimensional Cell Culture. Assay Drug Dev. Technol. 2016, 14, 19–28. [DOI] [PubMed] [Google Scholar]
- 90. Boutin M. E., Voss T. C., Titus S. A., et al. A High-Throughput Imaging and Nuclear Segmentation Analysis Protocol for Cleared 3D Culture Models. Sci. Rep. 2018, 8, 11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bilgin C. C., Fontenay G., Cheng Q., et al. BioSig3D: High Content Screening of Three-Dimensional Cell Culture Models. PLoS One 2016, 11, e0148379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Paulose T., Montevil M., Speroni L., et al. SAMA: A Method for 3D Morphological Analysis. PLoS One 2016, 11, e0153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Abraham V. C., Towne D. L., Waring J. F., et al. Application of a High-Content Multiparameter Cytotoxicity Assay to Prioritize Compounds Based on Toxicity Potential in Humans. J. Biomol. Screen. 2008, 13, 527–537. [DOI] [PubMed] [Google Scholar]
- 94. Ljosa V., Caie P. D., Ter Horst R., et al. Comparison of Methods for Image-Based Profiling of Cellular Morphological Responses to Small-Molecule Treatment. J. Biomol. Screen. 2013, 18, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Horvath P., Aulner N., Bickle M., et al. Screening Out Irrelevant Cell-Based Models of Disease. Nat. Rev. Drug Discov. 2016, 15, 751–769. [DOI] [PubMed] [Google Scholar]
- 96. Booij T. H., Klop M. J., Yan K., et al. Development of a 3D Tissue Culture-Based High-Content Screening Platform That Uses Phenotypic Profiling to Discriminate Selective Inhibitors of Receptor Tyrosine Kinases. J. Biomol. Screen. 2016, 21, 912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Schneider C. A., Rasband W. S., Eliceiri K. W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Carpenter A. E., Jones T. R., Lamprecht M. R., et al. CellProfiler: Image Analysis Software for Identifying and Quantifying Cell Phenotypes. Genome. Biol. 2006, 7, R100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Sandercock A. M., Rust S., Guillard S., et al. Identification of Anti-Tumour Biologics Using Primary Tumour Models, 3-D Phenotypic Screening and Image-Based Multi-Parametric Profiling. Mol. Cancer 2015, 14, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Di Z., Klop M. J., Rogkoti V. M., et al. Ultra High Content Image Analysis and Phenotype Profiling of 3D Cultured Micro-Tissues. PLoS One 2014, 9, e109688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Sameni M., Anbalagan A., Olive M. B., et al. MAME Models for 4D Live-Cell Imaging of Tumor: Microenvironment Interactions That Impact Malignant Progression. J. Vis. Exp. 2012, 60, e3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Borten M. A., Bajikar S. S., Sasaki N., et al. Automated Brightfield Morphometry of 3D Organoid Populations by OrganoSeg. Sci. Rep. 2018, 8, 5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rios A. C., Clevers H. Imaging Organoids: A Bright Future Ahead. Nat. Methods 2018, 15, 24–26. [DOI] [PubMed] [Google Scholar]
- 104. Strobl F., Schmitz A., Stelzer E. H. K. Improving Your Four-Dimensional Image: Traveling through a Decade of Light-Sheet-Based Fluorescence Microscopy Research. Nat. Protoc. 2017, 12, 1103–1109. [DOI] [PubMed] [Google Scholar]
- 105. Andilla J., Jorand R., Olarte O. E., et al. Imaging Tissue-Mimic with Light Sheet Microscopy: A Comparative Guideline. Sci. Rep. 2017, 7, 44939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li L., Zhou Q., Voss T. C., et al. High-Throughput Imaging: Focusing in on Drug Discovery in 3D. Methods 2016, 96, 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bray M. A., Carpenter A.; Broad Institute of MIT and Harvard Imaging Platform; et al. Advanced Assay Development Guidelines for Image-Based High Content Screening and Analysis. In Assay Guidance Manual; Sittampalam G. S., Coussens N. P., Brimacombe K., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, 2004. [PubMed] [Google Scholar]
- 108. Booij T. H., Bange H., Leonhard W. N., et al. High-Throughput Phenotypic Screening of Kinase Inhibitors to Identify Drug Targets for Polycystic Kidney Disease. SLAS Discov. 2017, 22, 974–984. [DOI] [PMC free article] [PubMed] [Google Scholar]