Summary
Chronic graft‐versus‐host disease (cGVHD) is a major complication affecting the long‐term survival of patients after allogeneic haematopoietic stem cell transplantation. The mechanism of cGVHD is unclear, and while previous studies have primarily focused on T cells, the role of B cells in the pathogenesis of cGVHD has been less reported. However, current studies on cGVHD are increasingly focused on the important role of B cells. In this review, we will introduce the newest studies and examine the role of B cells in cGVHD in detail with respect to the following aspects: altered B cell subpopulations, aberrant B cell signalling pathways, autoantibodies and T‐B cell interactions. Treatment strategies for the targeting of B cells during cGVHD will also be discussed.
Keywords: chronic graft‐versus‐host disease, B lymphocyte, mechanism, treatment
Allogeneic haematopoietic stem cell transplantation (allo‐HSCT) is one of the main approaches to cure malignant haematological disease. However, chronic graft‐versus‐host disease (cGVHD) is a major complication of allo‐HSCT, causing morbidity and mortality. It occurs in 30–70% of patients who survive more than 100 days after HSCT (Arai et al, 2015). Current cGVHD therapies are unsatisfactory. Traditional first‐line treatment is corticosteroids with or without calcineurin inhibitors with only a 50% response rate, and the therapeutic effects of second‐line treatments are debatable (Martin et al, 2015).
Unlike acute GVHD (aGVHD), which is mainly caused by abnormally activated donor T cells and cytokine storms with characteristics of donor lymphocyte infiltration and host target organ damage (Antin & Ferrara, 1992), the aetiology and pathogenesis of cGVHD remains unclear. Chronic GVHD is a complicated disease that is caused by many factors, such as thymus dysfunction (Wu et al, 2013), imbalanced T‐cell and B‐cell subsets (Xu et al, 2014), aberrant cytokines, microenvironment (Matsuoka et al, 2013), etc. Previous studies have predominantly focused on the pivotal role of T cells in this disease, such as T‐cell depletion (Koh et al, 2002; Pavletic et al, 2005), antithymocyte globulin (ATG) (Socié et al, 2011), post‐transplantation cyclophosphamide (PTCy) (Kanakry et al, 2014) and alemtuzumab (Nikiforow et al, 2013). Socié et al (2011) reported a randomized trial on GVHD prophylaxis with or without anti‐thymocyte globulin (ATG‐Fresenius) and found an exciting result on ATG treatment, the 3‐year cumulative incidence of extensive cGVHD, relapse and non‐relapse mortality were 12.2% vs. 45.0%, 19.4% vs. 33.5% and 55.2% vs. 43.3%, respectively. Another study, of PTCy (50 mg/kg/day on post‐transplantation days +3 and +4) as single‐agent GVHD prophylaxis after allogeneic bone marrow (BM) transplantation, also showed that cumulative incidence of cGVHD at 2 years was 14% (95% confidence interval, 7–21%) (Kanakry et al, 2014). Despite the remarkable effects of these treatments, further studies to explore cGVHD mechanisms are needed for better curative effects.
Increasing evidence suggested that B cells contribute to tissue damage characteristic in cGVHD. Immunoglobulin deposition was observed in liver and lung tissue from human biopsies and murine models of cGVHD (Srinivasan et al, 2012). Alloantibodies against the male tissue specific (H‐Y) antigen were found in a sex‐mismatched HSCT pattern from female‐to‐male (F→M) transplantation (Vogt et al, 2000a,b). Furthermore, transplanting donor cells from mice unable to secrete antibodies leads to less severe cGVHD (Jin et al, 2016). Use of the B cell‐depleting anti‐CD20 monoclonal antibody (mAb) rituximab for steroid‐refractory cGVHD has shown beneficial effects by alleviating autoimmune dysfunction (Sarantopoulos et al, 2011). Delayed B‐cell reconstitution and persistent high levels of B‐cell activating factor (BAFF, also termed TNFSF13B) are observed in cGVHD patients (Sarantopoulos & Ritz, 2015). Zhang et al (2006) demonstrated that donor CD4+ T and B cells in transplants induce cGVHD with autoimmune manifestations. Thus, we believe that exploring the mechanisms of B cells in the development of cGVHD can provide better approaches to prevent, predict and treat this disease. We discuss the relationship between B cells and cGVHD from the aspects of altered B‐cell subpopulations, aberrant B cell signalling pathways, autoantibodies and T‐B cell interactions.
Altered B‐cell subpopulations
In healthy individuals, precursor B cells in BM migrate to BM sinusoids and progress to the immature B‐cell stage. In this stage, immature B cells acquire B‐cell receptors (BCRs) on their surfaces and undergo negative selection to delete or edit self‐reactive B cells. Nonreactive immature B cells proceed through the circulation to the spleen and become transitional B cells, retaining high levels of immunoglobulin M (IgM) on their surfaces. In the spleen B‐cell follicle, transitional B cells change into mature B cells and enter into the peripheral blood. B cells that have not encountered antigens are called naive B cells. In blood circulation, mature B cells receive stimulation from exogenous antigens and migrate towards lymphoid follicles as a result of germinal centre (GC) formation. In the GC, B cells interact with antigens presented by follicular dendritic cells, and B cells with low affinity move towards apoptosis, while high‐affinity B cells proceed to plasma cells or memory B cells. This process is called positive selection (Chung et al, 2003) (Fig 1).
Figure 1.
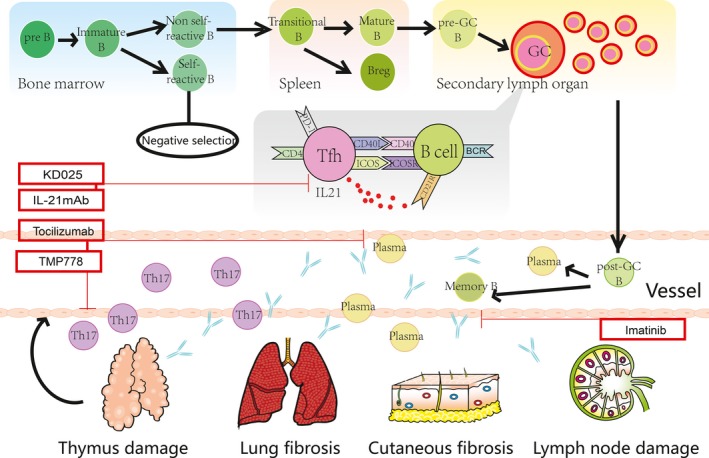
Overview of B‐cell differentiation and cellular and pathological processes in cGVHD patients. BCR, B‐cell receptor; cGVHD, chronic graft‐versus‐host disease; GC, germinal centre; mAb, monoclonal antibody; Tfh, T follicular helper cell; Th17, T‐helper cell type 17.
Immature B cells, transitional B cells and memory B cells
In transplant patients who do not develop cGVHD, supranormal numbers of naive and circulating transitional B cells are needed to sufficiently neutralize BAFF and promote the deletion of alloreactive and autoreactive B cells (Sarantopoulos & Ritz, 2015). Compared to patients without cGVHD, patients with cGVHD appear to have significant differences in their B‐cell subsets. Michonneau et al (2009) found that decreased B lineage‐specific haematopoietic progenitor cells (CD34+CD19+) are associated with GVHD. Recently Kolupaev et al (2018) found decreased numbers of common lymphoid progenitors (CLPs), pro‐, pre‐ and immature B cells in BM of the bronchiolitis obliterans syndrome (BOS) mouse model of cGVHD, and reported that B‐cell development is disrupted due to the aberrant B cell progenitors niche. Sarantopoulos et al (2009) observed a relative decrease in naive B cells (CD19+IgD CD38loCD27, which is consistent with previous observations that subsequent cGVHD is associated with the delayed reconstitution of naive B cells (Sarantopoulos & Ritz, 2015). Higher levels of BAFF are found in cGVHD patients, while circulating pre‐GC B cells (IgD+CD38hiCD27+) and post‐GC “plasmablast‐like” cells (IgDloCD38hiCD27+) increase along with elevated BAFF in a BAFF‐dependent way (Sarantopoulos et al, 2009). Expression of cell surface CD27 represents B cells that have encountered antigen. Scientists found that circulating CD27+ B cells (pre‐GC B cells and post‐GC “plasmablast‐like” cells) from cGVHD patients can produce antibodies without the help of BCR or secondary signal stimulation. Therefore, it is suspected that they might mediate anti‐host responses in a non‐antigen dependent fashion. Greinix et al (2008) observed increased immature/transitional CD21− B cells and decreased CD27+ B memory cells in active cGVHD patients, though they could not explain these changes. However, further studies have proven that CD19+CD21lo transitional B cells can serve as a biomarker for cGVHD diagnosis (Greinix et al, 2015) and for assessing cGVHD activities, such as cGVHD‐associated dysgammaglobulinaemia and cGVHD with BOS.(Kuzmina et al, 2011, 2013) (Table 1).
Table 1.
Specific predictors of cGVHD
| Predictor | Content | Reference |
|---|---|---|
| BAFF | Non‐relapse mortality | Liu and Davidson (2011) |
| CD19+CD21lo B cells | First diagnosis and later development of cGVHD | Greinix et al (2008) |
| H‐Y antibodies | cGVHD risk and non‐relapse mortality | Herrera et al (2014) |
| Bregs | Favourable prognosis |
Khoder et al (2014) Flores‐Borja et al (2013) van de Veen et al (2016) |
BAFF, B‐cell activating factor (also termed TNFSF13B); Bregs, B regulatory cells; cGVHD, chronic graft‐versus‐host disease.
Regulatory B cells (Bregs)
Bregs form specific regulatory B‐cell subsets that downregulate innate and adaptive immunity, inflammation and autoimmunity. The phenotypic definition of Breg cells has yet to be confirmed. Despite the different surface molecules between humans and mice, these B cells share the ability to secrete the anti‐inflammatory cytokine interleukin 10 (IL10) (Yazdanbakhsh, 2014). Deficiency in the function and reduction in the quantity of Bregs have been observed in many autoreactive diseases, such as systemic lupus erythematosus (SLE), arthritis and autoimmune diabetes (Yang et al, 2013). In recent years, Bregs have acquired more attention for their roles in the development of cGVHD. For example, in murine sclerodermatous cGVHD (Scl‐cGVHD) animals, Le Huu et al (2013) revealed that HSCT in CD19‐deficient donors appeared to induce more severe Scl‐cGVHD, while an early transfer of Bregs alleviated cGVHD symptoms, and donor‐derived Bregs suppressed Scl‐cGVHD. Blair et al (2010) identified a CD19+CD24hiCD38hi Breg subset that is enriched with CD19+IgM+CD27+ memory and CD19+CD24hiCD38hi transitional B‐cell subsets.
Multiple “Breg” cell subsets have been reported so far. Flores‐Borja et al (2013) demonstrated that CD19+CD24hiCD38hi Bregs can also play immunosuppressive roles by retaining Tregs as well as limiting T‐helper type 1 (Th1) and Th17 differentiation. More recently, van de Veen et al (2016) reported that CD73−CD25+CD71+ human B regulatory 1 could produce IL10. Iwata et al (2011) described other human Breg subsets enriched in the CD24hiCD27+ and CD27hiCD38hi plasmablast B‐cell compartments. These Bregs played roles in inhibiting monocyte activation and cytokine production from CD4+T cells (de Masson et al, 2015). Interestingly, the latter is in an IL10‐unrelated pathway. Some studies have shown that Bregs can serve as a predictor of favourable cGVHD prognosis (Table 1). Khoder et al (2014) observed that cGVHD patients have deficient Bregs, and demonstrated that the suppressive activities of the Bregs enriched within both the CD19+IgM+CD27+ memory and CD19+CD24hiCD38hi transitional B‐cell subsets, function by inhibiting the proliferation and γ‐interferon production of CD3/CD28‐stimulated autologous CD4+T cells that rely on IL10 and CD80/CD86 cell‐cell contact.
Treatment strategy
Effective treatments for cGVHD are aγssociated with quantitative changes in CD19+CD21lo transitional B cells and CD27+ B memory cells. Whittle and Taylor (2011) showed that there is a decrease in CD19+CD21lo B cell numbers after extracorporeal photopheresis in cGVHD patients. Mesenchymal stem cells (MSCs) are multipotent progenitors with a variety of functions in many fields, such as modulating the BM microenvironment and preventing GVHD, infections and relapse (Zhao & Liu, 2016). Gao et al (2016) observed a significantly increased number and frequency of CD27+ memory B cells after effective cGVHD prophylaxis with MSC infusion, indicating that MSC infusion control of cGVHD is associated with regulation of B‐cell subsets. Thus, we can imagine testing the efficacy of new drugs by monitoring specific B‐cell subsets that are evidently changed in cGVHD patients, such as naive B cells, immature/transitional CD21− B cells, pre‐GC B cells, post‐GC B cells, CD27+ B memory cells and Bregs (Table 2).
Table 2.
B‐cell subsets in cGVHD and their phenotypes
| B cell subsets in cGVHD | Phenotype | Change in level |
|---|---|---|
| Naive B cells | CD19+IgD CD38loCD27− | ↓ |
| Immature/Transitional B cells | CD21−, CD19+CD21lo | ↑ |
| Pre‐GC B cells | IgD+CD38hiCD27+ | ↑ |
| Post‐GC “plasmablast like” cells | IgDloCD38hiCD27+ | ↑ |
| CD27+ memory B cells | CD19+IgM+CD27+ | ↓ |
| Bregs | CD19+CD24hiCD38hi, CD19+IgM+CD27+ | ↓ |
↓, decrease; ↑, increase; Bregs, B regulatory cells; cGVHD, chronic graft‐versus‐host disease; GC, germinal centre.
Clinical trials have shown significant efficacies for B‐cell targeting by rituximab in cGVHD, but with various curative effects among patients (van Dorp et al, 2009; Johnston et al, 2014). Some patients with sustained naive lymphopenia or Breg deficiency are likely to have severe cGVHD after using rituximab. This indicates that the clinical efficacy depends on the practical immune state of cGVHD patients. B‐cell depletion by rituximab appears to eliminate effector B cells and Bregs simultaneously. Therefore, monitoring B‐cell subpopulations would guide clinicians using this anti‐CD20 therapy. Sarvaria et al (2016) demonstrated the function of Bregs in cord blood (CB). After CB transplantation, IL10+ Bregs within CB were present throughout recovery and protected against cGVHD by suppressing CD4+T cell activity, though these Bregs were deficient in cGVHD patients (Sarvaria et al, 2016). Hence, strategies that could enhance Bregs using grafts or support Breg reconstitution may be methods for treating cGVHD (e.g. donor‐derived Breg infusion).
Ofatumumab, a humanized anti‐CD20 mAb, is also promising for cGVHD therapy. Pidala et al (2015) reported its good efficacy and safety in combination with glucocorticoid for cGVHD patients, and their phase II study is currently ongoing. Additionally, IL6 plays an important role in long‐lived plasma cell generation and promotes collagen deposition and extracellular matrix production (Jourdan et al, 2014). Thus, blockade of IL6 may represent a new, effective approach for the treatment of cGVHD patients – especially those who present with fibrosis and joint stiffness. Tocilizumab is an anti‐IL6 mAb that has been widely used in some inflammatory and autoimmune diseases. Phase 2 clinical trial studies have been approved to test the efficacy of tocilizumab in the treatment of cGVHD in patients (NCT02701634). Promising results are expected; however, the mechanism by which anti‐IL6 regulation of plasma cell generation may influence cGVHD pathogenesis remains unaddressed.
Aberrant B cell pathways
Recent data have revealed aberrant B‐cell homeostasis in cGVHD, including characteristic autoreactive B cells and disordered signalling pathways. Studies have mainly concentrated on increased BAFF and abnormally activated BCR signals. Moreover, the NOTCH2 (Neurogenic locus notch homolog protein 2) signalling pathway has also come into focus as a promising therapeutic pathway for the treatment of cGVHD (Fig 2).
Figure 2.
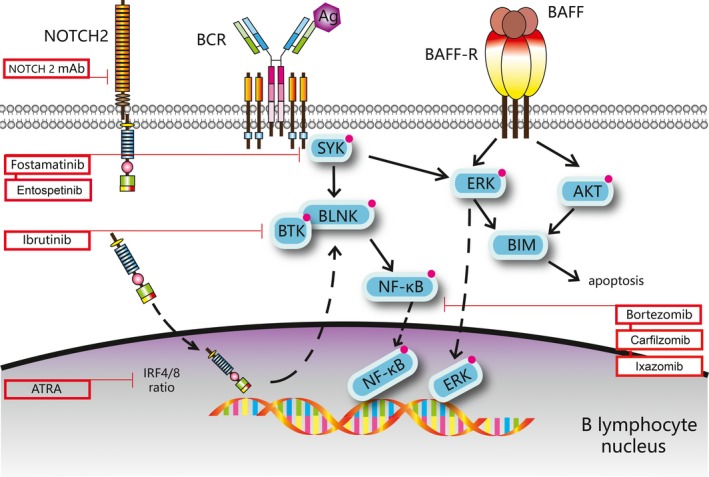
Three hyperactive signalling pathways in cGVHD patients and potential drugs for cGVHD therapy. Ag, antigen; ATRA, all‐trans retinoic acid; BAFF‐R, B‐cell activating factor receptor (also termed TNFRSF13C); BAFF, B‐cell activating factor (also termed TNFSF13B); BCR, B‐cell receptor; cGVHD, chronic graft‐versus‐host disease; mAb, monoclonal antibody.
BAFF signalling pathway
BAFF (TNFSF13B), a member of the tumour necrosis superfamily, is critical for mature B‐cell survival and differentiation (Gorelik et al, 2003). It is mainly expressed by mononuclear cells in peripheral blood, lymph nodes and the spleen (Rickert et al, 2011). BAFF combines with BAFF‐R (TNFRSF13C) on the surface of B cells and promotes B‐cell proliferation. High levels of BAFF have been found in many autoimmune diseases, such as SLE and rheumatoid arthritis (Woo et al, 2011; Chong et al, 2014), while low BAFF is often associated with lymphopenia (Liu & Davidson, 2011). This suggests that excessive BAFF levels are an inducible factor in B‐cell autoreactivity. Sarantopoulos et al (2009) performed detailed phenotypic and functional analyses of peripheral B cells and BAFF levels in 82 patients after HSCT, and observed delayed naive B‐cell reconstitution and persistent increase in BAFF concentrations in cGVHD patients (no cGVHD, 7.0 ng/ml vs. 3.0 ng/ml, P < 0.01). The authors explained that supranormal numbers of naive and circulating transitional B cells are ubiquitous in HSCT patients without cGVHD for neutralizing abundant BAFF and promoting the deletion of alloreactive and autoreactive B cells. BAFF levels have been shown to decrease following B‐cell recovery. However, in cGVHD patients, persistently rising and sustained levels of BAFF, as well as slow reconstitution of naive B cells leads to the formation of autoreactive B cells that ultimately result in a break of immune tolerance (Sarantopoulos & Ritz, 2015). They also found that excess BAFF in active cGVHD resulted in prolonged B‐cell survival and heightened metabolic states with enlarged proteins and higher protein levels per cell due to increases in the protein kinase B (PKB or AKT) and extracellular signal‐regulated kinase (ERK) signalling pathways (Allen et al, 2012). Increased AKT activation promotes B‐cell metabolism, while AKT and ERK activation leads to degradation of the pro‐apoptotic protein BIM (BCL2L11) and resistance to apoptotic death in B cells (Sarantopoulos & Ritz, 2015). Taken together, aberrant B‐cell homeostasis arises from the generation of activated autoreactive B cells. The latest research has demonstrated that BAFF plasma levels can serve as a predictor of non‐relapse mortality at the time of cGVHD diagnosis (Saliba et al, 2017) (Table 1).
BCR signalling pathway
In addition to abnormally excessive BAFF levels, increased BCR responsiveness to antigens is also observed in autoreactive B cells in the process of B‐cell recovery, as B cells isolated from cGVHD patients are likely to respond more rapidly to BCR stimulation. Allen et al (2014) demonstrated a significantly heightened proliferative response to BCR stimulation in cGVHD patients followed by elevated levels of the proximal BCR signalling molecules, spleen tyrosine kinase (SYK) and B cell linker protein (BLNK). After BCR activation, BLNK and SYK are phosphorylated by activating the NF‐κβ and ERK signalling pathways, ultimately promoting the survival and maturation of autoreactive B cells (Allen et al, 2014).
NOTCH2 signalling pathway
New studies have found that the NOTCH2 signalling pathway plays a vital role in cGVHD. NOTCH2 is a surface molecule and co‐stimulator of aberrant BCR responses in cGVHD. Poe et al (2017) revealed increased NOTCH2‐BCR signalling and a decreased IRF4/IRF8 expression ratio in cGVHD. Increased NOTCH2 activation heightens BCR responsiveness and promotes the expression of the proximal BCR protein BLNK in cGVHD patients. Therefore, targeting the NOTCH‐BCR signalling pathway is likely to be a new way to promote B‐cell hyperactivity in cGVHD patients. A striking pre‐clinical study on cGVHD in mice reported that NOTCH2 mAbs could inhibit the NOTCH2‐BCR axis while preserving early B cell reconstitution after HSCT, which indicates that NOTCH2 mAbs could simultaneously quell B‐cell dominant cGVHD and preserve humoral immunity. They also found that using all‐trans retinoic acid (ATRA) could increase IRF4 expression and eliminate BCR‐NOTCH2 hyperactivation as well as relieve cGVHD symptoms; this has also been shown to be effective in cGVHD mouse models (Poe et al, 2016).
Treatment strategy
The BAFF, BCR and NOTCH2 pathways are comprised of many signalling molecules. Hypotheses for targeting these molecules provide new inspiration for cGVHD therapy (Table 3). Preliminary studies have shown that the small‐molecule SYK inhibitor, fostamatinib, may be a promising drug to ameliorate BOS and scleroderma in murine cGVHD models by eliminating B cells, normalizing GC formation and decreasing activated CD80/86+ dendritic cells. It also increased apoptosis by human B cells purified from cGVHD patients (Flynn et al, 2015). Animal studies and clinical trials have shown the graft‐versus‐leukaemia (GVL) effects and anti‐tumour effects of fostamatinib against multiple haematological malignancies (Leonhardt et al, 2012; Krisenko & Geahlen, 2015), which reflect its powerful potential capacity in this field, but further studies and clinical trials are needed to evaluate its efficacy (NCT02611063). Another SYK inhibitor, entospetinib, is now in clinical trials in combination with systemic corticosteroids to test the efficacy and tolerability as first‐line therapy in adults with cGVHD (NCT02701634).
Table 3.
Clinical and preclinical drugs and strategies for cGVHD
| Strategies and drugs | Function | Reference/NCT identifier | Outome |
|---|---|---|---|
| Clinically used drugs | |||
| Ibrutinib | Inhibit BTK and IKT in B cells and T cells | Miklos et al (2017) | Sustained OR 71% (>20 weeks) |
| Bortezomib | Inhibit NF‐κβ in both BCR and BAFF pathways |
Pai et al (2014); Herrera et al (2014) |
CR: GI tract: 75%; Skin: 73% |
| Rituximab | Target CD20; reduce B cell frequency |
van Dorp et al (2009); Johnston et al (2014) |
OR 70% |
| Imatinib | Target PDGFR; inhibit fibrosis |
Olivieri et al (2009); Sánchez‐Ortega et al (2016) |
3‐year OS 72%, EFS 46% |
| Dasatinib | Inhibit leucocytes and TGFβ |
McFarland and Wetzstein (2009); Sánchez‐Ortega et al (2012) |
PR and/or CR 60% |
| Clinical trials | |||
| Ofatumumab | Target CD20; reduce B cell frequency | Pidala et al (2015) | 6‐month CR/PR: 8%/11% |
| Tocilizumab | Target IL6; inhibit plasma cells and Th17 | NCT02174263 | |
| Fostamatinib | Inhibit SYK and BCR pathways |
Flynn et al (2015); Leonhardt et al (2012) |
|
| Entospetinib | Inhibit SYK | NCT02701634 | |
| Carfilzomib | Second generation proteasome inhibitor | NCT02491359 | |
| Ixazomib | First oral proteasome inhibitor | NCT02513498 | |
| KD025 | Inhibit pSTAT3 and IL21 production | NCT02841995 | |
| Preclinical drugs and strategies | |||
| IL21 mAb | Targeting IL21 and depleting B cells remaining in target organs | Zeiser et al (2018) | |
| NOTCH2 mAb | Block NOTCH2‐BCR pathway | Poe et al (2016) | |
| ATRA | Increase IRF4 | Poe et al (2016) | |
| TMP778 | Inhibit RORγt and Th17 differentiation | Forcade et al (2017) | |
ATRA, all trans retinoic acid; BAFF, B‐cell activating factor (also termed TNFSF13B); BCR, B‐cell receptor; BTK, Bruton tyrosine kinase; cGVHD, chronic graft‐versus‐host disease; CR, complete response; EFS, event‐free survival; GI, gastrointestinal; IKT, IL2 inducible T‐cell kinase; IL, interleukin; mAb, monoclonal antibody; NCT, National Clinical Trial; NOTCH2, Neurogenic locus notch homolog protein 2; OR, overall response; OS, overall survival; PDGFR, platelet‐derived growth factor receptor; PR, partial response; RORγt, RAR‐orphan receptor gamma transcription factor; SYK, spleen tyrosine kinase; TGFβ, transforming growth factor β; Th17, T‐helper cell type 17.
Ibrutinib is a US Food and Drug Administration (FDA)‐approved potent inhibitor of both Bruton tyrosine kinase (BTK) and IL2 inducible T‐cell kinase (IKT) with inhibitory effects on pathogenic B cells and CD4+T cells (Schutt et al, 2015). BTK is a key signalling component in the downstream BCR pathway (Mohamed et al, 1999). IKT is involved in IL2 secretion and influences the balance between Tregs and Th17 (Gomez‐Rodriguez et al, 2014). Experiments have shown that ibrutinib has clinical and immunological functions in murine cGVHD models, demonstrating its significant roles in reducing B‐cell proliferation and autoantibody excretion (Dubovsky et al, 2014). Ibrutinib mainly affects B‐cell‐driven GC response and B‐cell functions and is likely to be applied for preventing severe cGVHD. Compared with B‐cell signalling, inhibiting IKT on T cells is less effective. Previous research showed that blocking IKT leads to CD8+T cell‐mediated GVL effects, but extensive inhibition of T‐cell activation prevents both GVHD and GVL effects (Cutler et al, 2017). A phase 2 study with ibrutinib in steroid refractory cGVHD has reported encouraging results, with a 67% overall response rate and a 71% sustained response rate >20 weeks using a daily dose of 420 mg. After a 13.9‐month follow‐up, 75% of responders had corticosteroid doses <0.15 mg/kg/day, and the median patient‐assessed overall cGVHD score decreased, from 7 (n = 42) to 4 (n = 14), at week 49. The most common adverse events were fatigue, diarrhoea, muscle spasms, nausea and bruising (Miklos et al, 2017). Further clinical studies are currently under investigation.
Bortezomib is a proteasome inhibitor with anti‐inflammatory and direct tumouricidal effects that is extensively used for treating myeloma and mantle cell lymphoma. Current studies and clinical trials found a B‐cell reduction in cGVHD of animals and patients after bortezomib therapy. Because of its indirect inhibitory effects on NF‐κB, bortezomib blocks both BAFF and BCR signatures, as NF‐κB is a prevalent molecule downstream in both pathways (Pellom et al, 2015). Pai et al (2014) demonstrated that bortezomib can successfully ameliorate cutaneous lesions in cGVHD mice and humans, as it decreases the number of GC B cells and BAFF expression. It also displays beneficial effects on T‐cell reconstitution and promotes differentiation towards Tregs while maintaining GVL effects. A phase 2 trial with bortezomib plus prednisone for initial cGVHD therapy demonstrated high response rates of 80% after 15 weeks of therapy (Herrera et al, 2014). The authors reported that the administration of bortezomib at 1.3 mg/m2 on days 1, 8, 15 and 22 during a 35‐day cycle for 3 cycles and prednisone at 0.5–1 mg/kg/day after the first cycle resulted in good organ specificity in the gastrointestinal tract (complete response [CR] 75%), skin (CR 73%) and liver (CR 53%). Only one patient manifested grade 3 sensory peripheral neuropathy as a side effect of bortezomib among the 22 patients enrolled (Herrera et al, 2014). However, Liang et al (2014) reported that delayed bortezomib administration may aggravate murine aGVHD in the IL1β and Toll‐like receptor 4 (TLR4) signalling pathways, and Sun et al (2005) demonstrated that delayed administration of bortezomib resulted in increased GVHD‐dependent gastrointestinal toxicity. Previous studies have reported complications, such as neurological, gastrointestinal and haematological toxicities in the early stages of long‐term bortezomib therapy (Sun et al, 2005; Herrera et al, 2014). Hence, physicians need to weigh the benefits and adverse factors and identify the optimal choice. A new generation of proteasome inhibitors are sought for cGVHD therapies. Carfilzomib is another FDA‐approved proteasome inhibitor used in multiple myeloma. The Fred Hutchinson Cancer Research Center recently completed a Phase II clinical trial using carfilzomib for the treatment of cGVHD (NCT02491359). Ixazomib, the first oral proteasome inhibitor, has been proven to be useful for cGVHD in murine experiments (Al‐Homsi et al, 2017; Chu & Gress, 2008), but its clinical efficacy for cGVHD patients is unknown. Four clinical trials (NCT02513498, NCT03225417, NCT0308267, NCT02250300) are currently underway and one phase II clinical trial (NCT02513498) has finished but the results have not yet been published.
NOTCH2‐related therapies require the hyperactivation of cGVHD B cells, and NOTCH2 mAbs and ATRA have been proven to be promising in ameliorating GVHD (Table 3). We hypothesize that the NOTCH‐BCR pathway represents a very promising direction for treatment of cGVHD in the near future. Blocking AKT and ERK molecules may be new strategies to relieve B‐cell metabolism and GVHD onset. A recent study found that targeting the PI3K/AKT/mTOR pathway may stop GVHD development in a T‐cell‐mediated fashion (Herrero‐Sánchez et al, 2016). The effect of these pathways in B cells is unknown at present and remains a vast field to explore, both experimentally and in the clinic.
In summary, despite these exciting new drug discoveries, expanding their applications will require more preclinical research, prospective randomized trials and evidence‐based studies.
Autoantibodies
Antibody deposition is common in cGVHD patients and animal models, but apart from some common antibodies such as the H‐Y and PDGFR antibodies mentioned above, the specific role of other deposited antibodies is unknown. Recently, Jin et al (2016) used transgenic IgHμγ1 DBA/2 mice (which possess B cells with normal antigen‐presentation as well as regulatory functions but without the ability to secrete IgM or IgG) as donors and found less severe cGVHD with less thymus damage, as well as reduced Th17 infiltration in peripheral and cutaneous lymph organs compared with wild‐type grafts. Moreover, injection of IgG‐containing sera from the cGVHD recipients with wild‐type grafts to recipients given IgHμγ1 grafts led to IgG deposition, fibrosis in target organs and Th17 infiltration (Jin et al, 2016). This result indicates that antibodies perpetuate cGVHD by increasing pathogenic Th17‐cell infiltration and promoting fibrogenesis (Jin et al, 2016) (Fig 1).
H‐Y autoantibodies
Tissue fibrosis and immunoglobulin deposition are common in cGVHD lesions in target organs, such as skin, lung and liver. Similarly, autoantibodies are also observed in many autoimmune diseases, such as SLE and scleroderma. Despite the existence of these antibodies, it is hard to identify the antigens for certain deposit antibodies. H‐Y antibodies generated from F→M HSCT patients with cGVHD have remarkably shown that antibodies are associated with cGVHD. Miklos et al (2005) found that H‐Y antibodies were not detectable in the early post‐transplantation period and not associated with aGVHD, confirming that H‐Y antibodies are needed at the onset of cGVHD. Nakasone et al (2015) considered a method to monitor H‐Y antibodies detected 3 months after HSCT to stratify cGVHD risk and pre‐emptively prevent cGVHD as well as predict incidence and non‐relapse mortality in F→M HSCT patients (Table 1).
PDGFR autoantibody
Svegliati et al (2007). identified stimulatory platelet‐derived growth factor receptor (PDGFR) autoantibodies in cGVHD patients, especially in those with extensive skin lesions and lung fibrosis. These antibodies recognize PDGFR, stimulate type I collagen gene expression and cause fibrosis via the Ha‐Ras‐extracellular‐signal‐regulated kinases 1‐ and 2‐reactive oxygen species (Ha‐Ras‐ERK 1/2) signalling pathway (Baroni et al, 2006). These findings expanded our understanding of autoantibodies in the development of cGVHD fibrosis and collagen deposition, as it was previously thought that these manifestations were mainly caused by Th2 cells (Shao et al, 2008). Moreover, we can speculate that targeting PDGFR or the Ha‐Ras‐ERK 1/2 pathway could overcome PDGFR‐antibody predominant sclerodermatous cGVHD.
Treatment strategies
The tyrosine kinase inhibitor (TKI), imatinib mesylate, has been used in steroid‐refractory cGVHD because it blocks the PDGFR pathway (Table 3). A phased clinical trial that included 39 steroid‐refractory cGVHD patients receiving 6 months of therapy with 100–400 mg/day imatinib showed an overall response rate of 49% (Olivieri et al, 2009). After 40 months of follow‐up, the 3‐year overall survival and event‐free survival were 72% and 46%, respectively. Common adverse events included fatigue, muscle pain and weakness. Moreover, the authors observed that the stimulating activities of anti‐PDGFR antibodies decreased in responding patients, as imatinib is likely to mediate this effect by reducing anti‐PDGFR antibody levels (Olivieri et al, 2009). Imatinib had potential effects on skin fibrosis, especially in sclerodermatous cGVHD. Imatinib is promising but its clinical effect is not as strong as expected, which can be explained by the fact that antibody spectrums vary in different cGVHD target organs. The second‐generation TKI, dasatinib, is also used as salvage therapy for steroid‐refractory and imatinib‐resistant cGVHD (Sánchez‐Ortega et al, 2016). Apart from the role of imatinib, dasatinib is a multi‐kinase inhibitor with antileukaemic effect and anti‐transforming growth factor‐β (TGF‐β) effect (McFarland & Wetzstein, 2009). Abnormally high levels of TGF‐β are found in many cGVHD patients with fibrotic manifestation. The TGF‐β signalling pathway promotes tissue regeneration and the maintenance of immune tolerance and also supports extracellular matrix protein formation and accumulation. Importantly, Sánchez‐Ortega et al (2012) demonstrated considerable efficacy of dasatinib for treatment of sclerotic cGVHD with 60% partial and/or complete responses.
Antibody deposition often synergizes with Th17 infiltration of target tissue, such as the skin, as IL17 produced by Th17 cells can augment collagen deposition; however, each can also function independently to augment tissue fibrosis and organ dysfunction. Thus, blockade of Th17 cell infiltration and function can be regarded as a new strategy to treat cGVHD (Forcade et al, 2017). It is reported that inhibition of IL6 in aGVHD models results in Treg expansion and subsequent decrease in Th17 differentiation (Chen et al, 2009). Kennedy et al (2014) designed a phase 1/2 clinical trial with long‐term tocilizumab for standard aGVHD, justified by tocilizumab's independent effect on Th17 differentiation. Tocilizumab for cGVHD therapy is currently being investigated in a phase II clinical trial (NCT02174263). Th17 cells are strictly defined by their secretion of IL17 and expression of RAR‐orphan receptor gamma transcription factor (RORγt) (Ivanov et al, 2006). In their cGVHD mouse model, Forcade et al (2017) revealed that targeting the Th17 pathway by using the RORγt inhibitor, TMP778, alleviated lung cGVHD severity. Thus, treatment strategies that target Th17 cell differentiation and function represent a new line of therapy for inhibiting Th17‐mediated tissue damage, as well as reducing Th17‐antibody synergistic tissue damage during cGVHD pathogenesis.(Table 3).
T‐B cell interactions
T cells and B cells are often considered as two arms in the immune system. There is no doubt that they have countless mutual effects and actions and coordinate with one another during immunoregulation.
T follicular helper (Tfh) cells
As naïve B cells migrate towards the GC in secondary lymphoid organs to encounter antigens and be activated to become plasma cells or memory B cells (Chung et al, 2003), GCs are important structures in the process of B‐cell activation. GCs are made up of proliferating B cells, T cells and follicular dendritic cells. T follicular helper cells (Tfhs) are identified by CD4, CXCR5 and PD‐1 (also termed PDCD1) on their surface (Sarantopoulos, 2016). They promote proliferation and differentiation of B cells through their surface molecules, such as inducible T‐cell co‐stimulator (ICOS) and CD40 ligand (CD40LG) (Choi et al, 2011, 2013). Moreover, the secretion of interleukin‐21 (IL21) helps B cells undergo class switching and enhances their immunoglobulin affinity (Zotos et al, 2010) (Fig 1). Flynn et al (2014) revealed an increased frequency of Tfhs and GC B cells in murine models of cGVHD and BOS, and explained that increased Tfhs are necessary for naïve B cells to differentiate into GC B cells and generate immune globulin deposition. However, new research in a mouse model from Deng et al (2017) found that cGVHD onset is associated with absence of GCs, and GC formation is not required for cGVHD induction. Their observation is inconsistent with the phenomenon that lymphopenia is often associated with cGVHD onset. Destruction or loss of GC formation may also result from damage to lymphoid niches during GVHD development. Unlike Tfhs in GCs, reduced frequency but increased function of circulating Tfhs (cTfhs) were observed during cGVHD (Forcade et al, 2016). cTfhs are identified by circulating CD4+CD45RA− in peripheral blood with concurrent expression of the surface molecule CXCR5. Activated cTfhs are differentiated towards Th2 and Th17 phenotypes, and increased Th2 and Th17 compartments are usually associated with severe cGVHD. Knorr et al (2016) also observed that cTfhs are markedly decreased in cGVHD patients and that treatment with steroid and calcineurin inhibitors decreases cTfhs. Therefore, depleting circulating Tfhs may represent a novel cGVHD therapy.
CD4+ T cells
Both donor CD4+ Tcells and B cells have been demonstrated to be crucial and indispensable in inducing cGVHD in murine models (Zhang et al, 2006). Inconsistent with the previous opinions that cGVHD is induced by thymus damage and de novo‐developed donor CD4+ T cells, Zhang et al (2006) found that mature CD4+ T cells in transplants are activated by host antigen‐presenting cells (APCs) and become autoreactive following this interaction. Subsequently, these activated CD4+ T cells promote mature donor B cells to differentiate and secrete antigens and finally cause autoimmune manifestations by recognizing host minor alloantigens presented by donor B cells. Another study demonstrated the function of donor‐derived B cells reacting with CD4+ T cells in autoimmune‐like cGVHD murine models (Young et al, 2012). As APCs, autoreactive donor B cells augment survival and clonal expression of pathogenic CD4+ T cells with both alloreactivity and autoreactivity, leading to their infiltration in target organs. As T‐cell depletion has been used in cGVHD prophylaxis, we can imagine that the depletion of B cells from donor grafts before HSCT may be a novel strategy to prevent cGVHD.
Recently, Deng et al (2017) found that GC formation and follicular CD4+T‐B interaction is dispensable for the induction of cGVHD. They described a type of pre‐Tfh‐like PSGL‐1loCXCR4hiCD4+ extrafollicular T cells, which were believed play a significant role in cGVHD pathogenesis. The extrafollicular T cells interact with B cells and these two types of cells are sufficient to induce cGVHD. They further demonstrated that these PSGL‐1loCXCR4hiCD4+ extrafollicular T cells can be blocked through ICOS/ICOSL interaction, BCL6 and signal transducer and activator of transcription 3 (Stat3) deficiency in donor CD4+T cells to suppress their proliferation and ameliorate cGVHD (Deng et al, 2017).
Tregs
Osteoblasts form a niche to support haematopoietic stem cells and early B‐cell progenitors. In GVHD patients and murine models of cGVHD, thte haematopoietic niche is targeted by CD4+T cells. It was recently found that, in a cGVHD model, Tregs are less related to cGVHD because of their protective effect on osteoblasts that form the BM niche for early B‐cell progenitors (Kolupaev et al, 2018). With a Treg‐expanded graft infusion, cGVHD in OBs mice is associated with reduced organ pathology and partially restored lung function. In addition, significantly higher frequency and total number of pro‐B, pre‐B and immature B cells in the BM of cGVHD mice were also observed, indicating that the presence of Tregs has a protective effects on osteoblasts, which form the BM niche for early B‐cell progenitors (Kolupaev et al, 2018). The specific mechanism is unknown and worth investigating.
Treatment strategies
Given that abundant Tfhs are observed in cGVHD patients, blocking their related signalling molecules such as IL21/IL21R, ICOS and CD40LG with mAbs may provide new approaches for cGVHD therapy. Experiments have shown the efficacy of targeting IL21 production to treat BOS, which cannot be treated by anti‐CD20 mAbs because anti‐CD20 mAbs only deplete peripheral B cells and not the B cells remaining in target organs (Zeiser et al, 2018). However, a recent study found that IL21 promotes thymopoiesis recovery and protects positive and negative T‐cell selection after HSCT; moreover, IL21 was observed to help B10‐cell expansion and prevent GVHD in animal models (Tormo et al, 2017). Zhang et al used ICOS−/− mice as donor and found reduced cGVHD manifestation in a mouse model compared with wild‐type donors, which was associated with decreased numbers of donor Tfhs, GCs and plasma cells (Zhang et al, 2018). KD025 is a Rho‐associated kinase 2 (ROCK2) inhibitor with great potential for cGVHD treatment. Previous studies found ROCK2 signalling is required for the generation of Tfhs (Flynn et al, 2016; Weiss et al, 2016). In a sclerodermatous cGVHD model, KD025 inhibited Tfhs generation as it blocks pStat3 and IL21 production (Flynn et al, 2016). KD025 is now in a phase 2 clinical trial for treatment of cGVHD (NCT02841995) (Table 3).
Conclusions
Chronic GVHD is a common yet complex disease that occurs in 30–70% of HSCT patients and influences nearly all tissues and organs by initiating complicated immunization routes. Due to its systemic manifestation, it significantly reduces patients’ quality of life and, in many cases, is life‐threatening. Thus, overcoming cGVHD is vital for better prognoses following HSCT. Therefore, accurately characterizing the aetiology and pathology of cGVHD is crucial for the development of cGVHD prophylaxis and treatments.
This review summarizes the latest preclinical and clinical research on aberrant B‐cell signalling pathways, autoreactive B cells and antibodies, altered B‐cell subpopulations and T‐B cell interactions during onset of cGHVD. The results from these studies provide us with new strategies for cGVHD therapy.
The latest opinions state that cGVHD onset is a consequence of altered immune reconstitution by lymphocytes (Bohmann et al, 2017). T‐lymphocyte subset monitoring is conventional after HSCT, while B‐cell subset monitoring is not. As B‐cell populations also reflect the immune state of patients to some degree, B‐cell subset monitoring requires more attention for determining the patients’ physical condition and guiding clinical treatment regimens. For example, high level of Bregs is a sign of a favourable prognosis following HSCT, and enhancing Breg numbers in grafts and promoting Breg recovery are promising ways to prevent cGVHD.
New strategies for cGVHD therapies are quite promising due to the preclinical success of blocking excessively activated B cells and related signalling pathways. For example, the SYK inhibitor, fostamatinib, is now in a preclinical period to determine its role in promoting B‐cell death, but only patients with an activated BCR pathway are potentially sensitive to this activity. The BTK inhibitor, ibrutinib, is also a promising and FDA‐approved cGVHD drug in phase 1 and 2 trials with significant clinical efficacy, but further animal studies and clinical randomized trials are needed for in‐depth exploration of its potential benefits and complications. The NF‐κβ inhibitor, bortezomib, is also a potential drug for cGVHD with promising efficacy, but has not yet been approved for cGVHD treatment. Other new drugs, such as the Rho‐associated kinase inhibitor KD025, the proteasome inhibitor carfizomib and the SYK inhibitor entospetinib are being currently examined.
Skewed B‐cell populations in cGVHD may cause some negative effects and even unexpected complications. B‐cell depletion in vivo or ex vivo may result in delayed B‐cell recovery and even severe cGVHD when Bregs are dominant among B‐cell subsets. Moreover, decreasing B‐cell function will also inhibit GVL effects. These issues rely heavily on B‐cell monitoring. New drugs also have unclear side effects, and more animal studies and preclinical trials are needed to evaluate the efficacy.
In addition to the new B cell‐related drugs for cGVHD mentioned above, there are increasing numbers of strategies with different pathways used in clinic or in preclinical trials. Ruxolitinib, also named Jakafi, is a novel and effective drug with anti‐inflammatory properties, demonstrated for treatment of both acute and chronic GVHD (Spoerl et al, 2014). Many clinical trials have proven their promising outcomes (Zeiser et al, 2015; Hurabielle et al, 2017). As a selective Janus kinase (JAK) 1/2 inhibitor, ruxolitinib blocks T cell activation and survival, while also influencing the differentiation and mutation of dendritic cells (Bendstrup et al, 2014). Pirfernidone is used in idiopathic pulmonary fibrosis (Du et al, 2017), and is also effective in murine models of sclerodermatous cGVHD, as it inhibits macrophage infiltration and TGF‐ production (Zhao et al, 2018), while macrophage reconstitution is very important to haematopoietic microenvironment and development of GVHD after transplantation. Low‐dose subcutaneous IL2 is another strategy for corticoid‐refractory cGVHD due to its crucial role in augmenting Treg expansion, survival and activity (Koreth et al, 2011).(Table 3).
With the new discovery of pivotal signalling molecules, surface proteins and biomarkers, an increasing number of monoclonal antibodies can be produced and applied in the clinic. Monoclonal antibodies promote considerable immunosuppressive effects but also reduce the ability to resist infections and delay immune reconstitution in patients in the meantime. Like ATG, an immunosuppressive product of T cells, mAbs have been proven to promote therapeutic effects in cGVHD prophylaxis and lower incidence, but the relapse rate, non‐relapse mortality and infection rate are even higher (Ofran et al, 2018). Therefore, mAbs should be applied with caution and more attention should be paid to the immune state of patients, particularly with regards to their B cells compartment, while also exploring B cell‐specific small molecule inhibitors for the treatment and prevention of cGVHD after transplantation.
Competing interests
None.
Author contributions
Xiaoping Li prepared the first draft of the paper, Xi Zhang designed the manuscript. All authors critiqued and approved the final manuscript.
Acknowledgement
This paper is supported by Army Key Foundation (No. AWS14C014), National key research and development plan (No. 2017YFA0105502), Chinese National Natural Science Foundation (No. 81570097), National Natural Youth Science Foundation of China (No. 81400081) and Clinical Foundation of Xinqiao Hospital (2015LYC02).
References
- Al‐Homsi, A.S. , Goodyke, A. , McLane, M. , Abdel‐Mageed, S. , Cole, K. , Muilenburg, M. & Feng, Y. (2017) Post‐transplantation cyclophosphamide and ixazomib combination rescues mice subjected to experimental graft‐versus‐host disease and is superior to either agent alone. Biology of Blood and Marrow Transplantation, 23, 255–261. [DOI] [PubMed] [Google Scholar]
- Allen, J.L. , Fore, M.S. , Wooten, J. , Roehrs, P.A. , Bhuiya, N.S. , Hoffert, T. , Sharf, A. , Deal, A.M. , Armistead, P. , Coghill, J. , Gabriel, D.A. , Irons, R. , Essenmacher, A. , Shea, T.C. , Richards, K. , Cutler, C. , Ritz, J. , Serody, J. , Baldwin, A.S. & Sarantopoulos, S. (2012) B cells from patients with chronic GVHD are activated and primed for survival via BAFF‐mediated pathways. Blood, 120, 2529–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen, J.L. , Tata, P.V. , Fore, M.S. , Wooten, J. , Rudra, S. , Deal, A.M. , Sharf, A. , Hoffert, T. , Roehrs, P.A. , Shea, T.C. , Serody, J.S. , Richards, K.L. , Jagasia, M. , Lee, S.J. , Rizzieri, D. , Horwitz, M.E. , Chao, N.J. & Sarantopoulos, S. (2014) Increased BCR responsiveness in B cells from patients with chronic GVHD. Blood, 123, 2108–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antin, J.H. & Ferrara, J.L. (1992) Cytokine dysregulation and acute graft‐versus‐host disease. Blood, 80, 2964–2968. [PubMed] [Google Scholar]
- Arai, S. , Arora, M. , Wang, T. , Spellman, S.R. , He, W. , Couriel, D.R. , Urbano‐Ispizua, A. , Cutler, C.S. , Bacigalupo, A.A. , Battiwalla, M. , Flowers, M.E. , Juckett, M.B. , Lee, S.J. , Loren, A.W. , Klumpp, T.R. , Prockup, S.E. , Ringdén, O.T.H. , Savani, B.N. , Socié, G. , Schultz, K.R. , Spitzer, T. , Teshima, T. , Bredeson, C.N. , Jacobsohn, D.A. , Hayashi, R.J. , Drobyski, W.R. , Frangoul, H.A. , Akpek, G. , Ho, V.T. , Lewis, V.A. , Gale, R.P. , Koreth, J. , Chao, N.J. , Aljurf, M.D. , Cooper, B.W. , Laughlin, M.J. , Hsu, J.W. , Hematti, P. , Verdonck, L.F. , Solh, M.M. , Norkin, M. , Reddy, V. , Martino, R. , Gadalla, S. , Goldberg, J.D. , McCarthy, P.L. , Pérez‐Simón, J.A. , Khera, N. , Lewis, I.D. , Atsuta, Y. , Olsson, R.F. , Saber, W. , Waller, E.K. , Blaise, D. , Pidala, J.A. , Martin, P.J. , Satwani, P. , Bornhäuser, M. , Inamoto, Y. , Weisdorf, D.J. , Horowitz, M.M. & Pavletic, S.Z. (2015) Increasing incidence of chronic graft‐versus‐host disease in allogeneic transplantation: a report from the center for international blood and marrow transplant research. Biology of Blood and Marrow Transplantation, 21, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baroni, S.S. , Santillo, M. , Bevilacqua, F. , Luchetti, M. , Spadoni, T. , Mancini, M. , Fraticelli, P. , Sambo, P. , Funaro, A. , Kazlauskas, A. , Avvedimento, E.V. & Gabrielli, A. (2006) Stimulatory autoantibodies to the PDGF receptor in systemic sclerosis. New England Journal of Medicine, 354, 2667–2676. [DOI] [PubMed] [Google Scholar]
- Bendstrup, E. , Hyldgaard, C. & Hilberg, O. (2014) Diagnostic criteria and possible treatment of idiopathic pulmonary fibrosis. Ugeskrift for Laeger, 176, 1060–1063. [PubMed] [Google Scholar]
- Blair, P.A. , Noreña, L.Y. , Flores‐Borja, F. , Rawlings, D.J. , Isenberg, D.A. , Ehrenstein, M.R. & Mauri, C. (2010) CD19+CD24hiCD38hi B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity, 32, 129–140. [DOI] [PubMed] [Google Scholar]
- Bohmann, E.M. , Fehn, U. , Holler, B. , Weber, D. , Holler, E. , Herr, W. , Hoffmann, P. , Edinger, M. & Wolff, D. (2017) Altered immune reconstitution of B and T cells precedes the onset of clinical symptoms of chronic graft‐versus‐host disease and is influenced by the type of onset. Annals of Hematology, 96, 299–310. [DOI] [PubMed] [Google Scholar]
- Chen, X. , Das, R. , Komorowski, R. , Beres, A. , Hessner, M.J. , Mihara, M. & Drobyski, W.R. (2009) Blockade of interleukin‐6 signaling augments regulatory T‐cell reconstitution and attenuates the severity of graft‐versus‐host disease. Blood, 114, 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y.S. , Kageyama, R. , Eto, D. , Escobar, T.C. , Johnston, R.J. , Monticelli, L. , Lao, C. & Crotty, S. (2011) ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity, 34, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi, Y.S. , Yang, J.A. & Crotty, S. (2013) Dynamic regulation of Bcl6 in follicular helper CD4 T (Tfh) cells. Current Opinion in Immunology, 25, 366–372. [PubMed: 23688737]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, B.F. , Tseng, L.C. , Kim, A. , Miller, R.T. , Yancey, K.B. & Hosler, G.A. (2014) Differential expression of BAFF and its receptors in discoid lupus erythematosus patients. Journal of Dermatological Science, 73, 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu, Y.W. & Gress, R.E. (2008) Murine models of chronic graft‐versus‐host disease: insights and unresolved issues. Biology of Blood and Marrow Transplantation, 14, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, J.B. , Silverman, M. & Monroe, J.G. (2003) Transitional B cells: step by step towards immune competence. Trends in Immunology, 24, 343–349. [DOI] [PubMed] [Google Scholar]
- Cutler, C.S. , Koreth, J. & Ritz, J. (2017) Mechanistic approaches for the prevention and treatment of chronic GVHD. Blood, 129, 22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, R. , Hurtz, C. , Song, Q. , Yue, C. , Xiao, G. , Yu, H. , Wu, X. , Muschen, M. , Forman, S. , Martin, P.J. & Zeng, D. (2017) Extra‐follicular CD4+T‐B interactioins are sufficient for inducing autoimmune‐like chronic graft‐versus‐host disease. Nature Communications, 8, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp, S. , Pietersma, F. , Wölfl, M. , Verdonck, L.F. , Petersen, E.J. , Lokhorst, H.M. , Martens, E. , Theobald, M. , van Baarle, D. , Meijer, E. & Kuball, J. (2009) Rituximab treatment before reduced‐intensity conditioning transplantation associates with a decreased incidence of extensive chronic GVHD. Biology of Blood and Marrow Transplantation, 15, 671–678. [DOI] [PubMed] [Google Scholar]
- Du, J. , Paz, K. , Flynn, R. , Vulic, A. , Robinson, T.M. , Lineburg, K.E. , Alexander, K.A. , Meng, J. , Roy, S. , Panoskaltsis‐Mortari, A. , Loschi, M. , Hill, G.R. , Serody, J.S. , Maillard, I. , Miklos, D. , Koreth, J. , Cutler, C.S. , Antin, J.H. , Ritz, J. , MacDonald, K.P. , Schacker, T.W. , Luznik, L. & Blazar, B.R. (2017) Pirfenidone ameliorates murine chronic GVHD through inhibition of macrophage infiltration and TGF‐β production. Blood, 129, 2570–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovsky, J.A. , Flynn, R. , Du, J. , Harrington, B.K. , Zhong, Y.M. , Kaffenberger, B. , Yang, C. , Towns, W.H. , Lehman, A. , Johnson, A.J. , Muthusamy, N. , Devine, S.M. , Jaglowski, S. , Serody, J.S. , Murphy, W.J. , Munn, D.H. , Luznik, L. , Hill, G.R. , Wong, H.K. , MacDonald, K. , Maillard, I. , Koreth, J. , Elias, L. , Cutler, C. , Soiffer, R.J. , Antin, J.H. , Ritz, J. , Panoskaltsis‐Mortari, A. , Byrd, J.C. & Blazar, B.R. (2014) Ibrutinib treatment ameliorates murine chronic graft‐versus‐host disease. The Journal of Clinical Investigation, 124, 4867–4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores‐Borja, F. , Bosma, A. , Ng, D. , Reddy, V. , Ehrenstein, M.R. , Isenberg, D.A. & Mauri, C. (2013) CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Science Translational Medicine, 5, 173ra123. [DOI] [PubMed] [Google Scholar]
- Flynn, R. , Du, J. , Veenstra, R.G. , Reichenbach, D.K. , Panoskaltsis‐Mortari, A. , Taylor, P.A. , Freeman, G.J. , Serody, J.S. , Murphy, W.J. , Munn, D.H. , Sarantopoulos, S. , Luznik, L. , Maillard, I. , Koreth, J. , Cutler, C. , Soiffer, R.J. , Antin, J.H. , Ritz, J. , Dubovsky, J.A. , Byrd, J.C. , MacDonald, K.P. , Hill, G.R. & Blazar, B.R. (2014) Increased T follicular helper cells and germinal center B cells are required for cGVHD and bronchiolitis obliterans. Blood, 123, 3988–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, R. , Allen, J.L. , Luznik, L. , MacDonald, K.P. , Paz, K. , Alexander, K.A. , Vulic, A. , Du, J. , Panoskaltsis‐Mortari, A. , Taylor, P.A. , Poe, J.C. , Serody, J.S. , Murphy, W.J. , Hill, G.R. , Maillard, I. , Koreth, J. , Cutler, C.S. , Soiffer, R.J. , Antin, J.H. , Ritz, J. , Chao, N.J. , Clynes, R.A. , Sarantopoulos, S. & Blazar, B.R. (2015) Targeting SYK‐activated B cells in murine and human chronic graft‐versus‐host disease. Blood, 125, 4085–4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, R. , Paz, K. , Du, J. , Reichenbach, D.K. , Taylor, P.A. , Panoskaltsis‐Mortari, A. , Vulic, A. , Luznik, L. , MacDonald, K.K. , Hill, G.R. , Nyuydzefe, M.S. , Weiss, J.M. , Chen, W. , Trzeciak, A. , Serody, J.S. , Aguilar, E.G. , Murphy, W.J. , Maillard, I. , Munn, D. , Koreth, J. , Cutler, C.S. , Antin, J.H. , Ritz, J. , Waksal, S.D. , Zanin‐Zhorov, A. & Blazar, B.R. (2016) Targeted Rho‐associated kinase 2 inhibition suppresses murine and human chronic GVHD through a Stat3‐dependent mechanism. Blood, 127, 2144–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcade, E. , Kim, H.T. , Cutler, C. , Wang, K. , Alho, A.C. , Nikiforow, S. , Ho, V.T. , Koreth, J. , Armand, P. , Alyea, E.P. , Blazar, B.R. , Soiffer, R.J. , Antin, J.H. & Ritz, J. (2016) Circulating T follicular helper cells with increased function during chronic graft‐versus‐host disease. Blood, 127, 2489–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forcade, E. , Paz, K. , Flynn, R. , Griesenauer, B. , Amet, T. , Li, W. , Liu, L.Y. , Bakoyannis, G. , Jiang, D. , Chu, H.W. , Lobera, M. , Yang, J.F. , Wilkes, D.S. , Du, J. , Gartlan, K. , Hill, G.R. , MacDonald, K.P. , Espada, E.L. , Blanco, P. , Serody, J.S. , Koreth, J. , Cutler, C.S. , Antin, J.H. , Soiffer, R.J. , Ritz, J. , Paczesny, S. & Blazar, B.R. (2017) An activated Th17‐prone T cell subset involved in chronic graft‐versushost disease sensitive to pharmacological inhibition. JCI Insight, 2, e92111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, L. , Zhang, Y. , Hu, B. , Liu, J. , Kong, P. , Lou, S. , Su, Y. , Yang, T. , Li, H. , Liu, Y. , Zhang, C. , Gao, L. , Zhu, L. , Wen, Q. , Wang, P. , Chen, X. , Zhong, J. & Zhang, X. (2016) Phase II multicenter, randomized, double‐blind controlled study of efficacy and safety of umbilical cord‐derived mesenchymal stromal cells in the prophylaxis of chronic graft‐versus‐host disease after HLA‐haploidentical stem‐cell transplantation. Journal of Clinical Oncology, 34, 2843–2850. [DOI] [PubMed] [Google Scholar]
- Gomez‐Rodriguez, J. , Wohlfert, E.A. , Handon, R. , Meylan, F. , Wu, J.Z. , Anderson, S.M. , Kirby, M.R. , Belkaid, Y. & Schwartzberg, P.L. (2014) IKT‐mediated integration of T cell receptor and cytokine signaling regulates the balance between Th17 and regulatory T cells. Journal of Experimental Medicine, 211, 529–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik, L. , Gilbride, K. , Dobles, M. , Kalled, S.L. , Zandman, D. & Scott, M.L. (2003) Normal B cell homeostasis requires B cell activation factor production by radiation‐resistant cells. Journal of Experimental Medicine, 198, 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greinix, H.T. , Pohlreich, D. , Kouba, M. , Körmöczi, U. , Lohmann, I. , Feldmann, K. , Zielinski, C. & Pickl, W.F. (2008) Elevated numbers of immature/transitional CD21‐ B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft‐versus‐host disease. Biology of Blood and Marrow Transplantation, 14, 208–219. [DOI] [PubMed] [Google Scholar]
- Greinix, H.T. , Kuzmina, Z. , Weigl, R. , Körmoczi, U. , Rottal, A. , Wolff, D. , Kralj, M. , Kalhs, P. , Mitterbauer, M. , Rabitsch, W. , Edinger, M. , Holler, E. & Pickl, W.F. (2015) CD19+CD21low B cells and CD4+CD45RA+CD31+ T cells correlate with first diagnosis of chronic graft‐versus‐host disease. Biology of Blood and Marrow Transplantation, 21, 250–258. [DOI] [PubMed] [Google Scholar]
- Herrera, A.F. , Kim, H.T. , Bindra, B. , Jones, K.T. , Alyea, E.P. , Armand, P. , Cutler, C.S. , Ho, V.T. , Nikiforow, S. , Blazar, B.R. , Ritz, J. , Antin, J.H. , Soiffer, R.J. & Koreth, J. (2014) A phase II study of bortezomib plus prednisone for initial therapy of chronic graft‐versus‐host disease. Biology of Blood and Marrow Transplantation, 20, 1737–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero‐Sánchez, M.C. , Rodríguez‐Serrano, C. , Almeida, J. , San Segundo, L. , Inogés, S. , Santos‐Briz, Á. , García‐Briñón, J. , Corchete, L.A. , San Miguel, J.F. , Del Cañizo, C. & Blanco, B. (2016) Targeting of PI3K/AKT/mTOR pathway to inhibit T cell activation and prevent graft‐versus‐host disease development. Journal of Hematology & Oncology, 9, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurabielle, C. , Sicre de Fontbrune, F. , Moins‐Teisserenc, H. , Robin, M. , Jachiet, M. , Coman, T. , Dhedin, N. , Cassius, C. , Chasset, F. , de Masson, A. , Michonneau, D. , Bagot, M. , Bergeron, A. , Socié, G. , Peffault de Latour, R. & Bouaziz, J.D. (2017) Efficacy and tolerance of ruxolitinib in refractory sclerodermatous chronic graft‐versus‐host disease. British Journal of Dermatology, 177, e206–e208. [DOI] [PubMed] [Google Scholar]
- Ivanov, I.I. , McKenzie, B.S. , Zhou, L. , Tadokoro, C.E. , Lepelley, A. , Lafaille, J.J. , Cua, A.J. & Littman, D.R. (2006) The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL‐17+ T helper cells. Cell, 126, 1121–1133. [DOI] [PubMed] [Google Scholar]
- Iwata, Y. , Matsushita, T. , Horikawa, M. , Dilillo, D.J. , Yanaba, K. , Venturi, G.M. , Szabolcs, P.M. , Bernstein, S.H. , Magro, C.M. , Williams, A.D. , Hall, R.P. , St Clair, E.W. & Tedder, T.F. (2011) Characterization of a rare IL‐10‐competent B‐cell subset in humans that parallels mouse regulatory B10 cells. Blood, 117, 530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin, H. , Ni, X. , Deng, R. , Song, Q. , Young, J. , Cassady, K. , Zhang, M. , Forman, S. , Martin, P.J. , Liu, Q. & Zeng, D. (2016) Antibodies from donor B cells perpetuate cutaneous chronic graft‐versus‐host disease in mice. Blood, 127, 2249–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston, H.F. , Xu, Y. , Racine, J.J. , Cassady, K. , Ni, X. , Wu, T. , Chan, A. , Forman, S. & Zeng, D. (2014) Administration of anti‐CD20 mAb is highly effective in preventing but ineffective in treating chronic graft‐versus‐host disease while preserving strong graft‐versus‐leukemia effects. Biology of Blood and Marrow Transplantation, 20, 1089–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdan, M. , Cren, M. , Robert, N. , Bolloré, K. , Fest, T. , Duperray, C. , Guilloton, F. , Hose, D. , Tarte, K. & Klein, B. (2014) IL‐6 supports the generation of human long‐lived plasma cells in combination with either APRIL or stromal cell‐soluble factors. Leukemia, 28, 1647–1656. [DOI] [PubMed] [Google Scholar]
- Kanakry, C.G. , O'Donnell, P.V. , Furlong, T. , de Lima, M.J. , Wei, W. , Medeot, M. , Mielcarek, M. , Champlin, R.E. , Jones, R.J. , Thall, P.F. , Andersson, B.S. & Luznik, L. (2014) Multi‐institutional study of post‐transplantation cyclophosphamide as single‐agent graft‐versus‐host disease prophylaxis after allogeneic bone marrow transplantation using myeloablative busulfan and fludarabine conditioning. Journal of Clinical Oncology, 32, 3497–3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy, G.A. , Varelias, A. , Vuckovic, S. , Texier, L.L. , Gartlan, K.H. , Zhang, P. , Thomas, G. , Anderson, L. , Boyle, G. , Cloonan, N. , Leach, J. , Sturgeon, E. , Avery, J. , Olver, S.D. , Lor, M. , Misra, A.K. , Hutchins, C. , Morton, A.J. , Durrant, S. , Subramoniapillai, E. , Butler, J.P. , Curley, C.I. , MacDonald, K. , Tey, S.K. & Hill, G.R. (2014) Addition of interleukin‐6 inhibition with tocilizumab to standard graft‐versus‐host disease prophylaxis after allogeneic stem‐cell transplantation: a phase 1/2 trial. The Lancet Oncology, 15, 1451–1459. [DOI] [PubMed] [Google Scholar]
- Khoder, A. , Sarvaria, A. , Alsuliman, A. , Chew, C. , Sekine, T. , Cooper, N. , Mielke, S. , de Lavallade, H. , Muftuoglu, M. , Fernandez Curbelo, I. , Liu, E. , Muraro, P.A. , Alousi, A. , Stringaris, K. , Parmar, S. , Shah, N. , Shaim, H. , Yvon, E. , Molldrem, J. , Rouce, R. , Champlin, R. , McNiece, I. , Mauri, C. , Shpall, E.J. & Rezvani, K. (2014) Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood, 124, 2034–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knorr, D.A. , Wang, H. , Aurora, M. , MacMillan, M.L. , Holtan, S.G. , Bergerson, R. , Cao, Q. , Weisdorf, D.J. , Cooley, S. , Brunstein, C. , Miller, J.S. , Wagner, J.E. , Blazar, B.R. & Verneris, M.R. (2016) Loss of T follicular helper cells in the peripheral blood of patients with chronic graft‐versus‐host disease. Biology of Blood and Marrow Transplantation, 22, 825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh, M.B. , Prentice, H.G. , Corbo, M. , Morgan, M. , Cotter, F.E. & Lowdell, M.W. (2002) Alloantigen‐specific T‐cell depletion in a major histocompatibility complex fully mismatched murine model provides effective graft‐versus‐host disease prophylaxis in the presence of lymphoid engraftment. British Journal of Haematology, 118, 108–116. [DOI] [PubMed] [Google Scholar]
- Kolupaev, O.V. , Dant, T.A. , Bommiasamy, H. , Bruce, D.W. , Fowler, K.A. , Tilley, S.L. , McKinnon, K.P. , Sarantopoulos, S. , Blazar, B.R. , Coghill, J.M. & Serody, J.S. (2018) Impaired bone marrow B‐cell development in mice with a bronchiolitis obliterans model of cGVHD. Blood Advances, 2, 2307–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koreth, J. , Matsuoka, K. , Kim, H.T. , McDonough, S.M. , Bindra, B. , Alyea, E.P. , Armand, P. , Cutler, C. , Ho, V.T. , Treister, N.S. , Bienfang, D.C. , Prasad, S. , Tzachanis, D. , Joyce, R.M. , Avigan, D.E. , Antin, J.H. , Ritz, J. & Soiffer, R.J. (2011) Interleukin‐2 and regulatory T cells in graft‐versus‐host disease. New England Journal of Medicine, 365, 2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisenko, M.O. & Geahlen, R.L. (2015) Calling in SYK: SYK's dual role as a tumor promoter and tumor suppressor in cancer. Biochimica et Biophysica Acta, 1853, 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmina, Z. , Greinix, H.T. , Weigl, R. , Kormoczi, U. , Rottal, A. , Frantal, S. , Eder, S. & Pickl, W.F. (2011) Significant differences in B‐cell subpopulations characterize patients with chronic graft‐versus‐host disease‐associated dysgammaglobulinemia. Blood, 117, 2265–2274. [DOI] [PubMed] [Google Scholar]
- Kuzmina, Z. , Krenn, K. , Petkov, V. , Kormoczi, U. , Weigl, R. , Rottal, A. , Kalhs, P. , Mitterbauer, M. , Ponhold, L. , Dekan, G. , Greinix, H.T. & Pickl, W.F. (2013) CD19(+)CD21(low) B cells and patients at risk for NIH‐defined chronic graft‐versus‐host disease with bronchiolitis obliterans syndrome. Blood, 121, 1886–1895. [DOI] [PubMed] [Google Scholar]
- Le Huu, D. , Matsushita, T. , Jin, G. , Hamaguchi, Y. , Hasegawa, M. , Takehara, K. , Tedder, T.F. & Fujimoto, M. (2013) Donor‐derived regulatory B cells are important for suppression of murine sclerodermatous chronic graft‐versus‐host disease. Blood, 121, 3274–3283. [DOI] [PubMed] [Google Scholar]
- Leonhardt, F. , Zirlik, K. , Buchner, M. , Prinz, G. , Hechinger, A.K. , Gerlach, U.V. , Fisch, P. , Schmitt‐Gräff, A. , Reichardt, W. & Zeiser, R. (2012) Spleen tyrosine kinase (SYK) is a potent target for GvHD prevention at different cellular levels. Leukemia, 26, 1617–1629. [DOI] [PubMed] [Google Scholar]
- Liang, Y. , Ma, S. , Zhang, Y. , Wang, Y. , Cheng, Q. , Wu, Y. , Jin, Y. , Zheng, D. , Wu, D. & Liu, H. (2014) IL‐1β and TLR4 signaling are involved in the aggravated murine acute graft‐versus‐host disease caused by delayed bortezomib administration. The Journal of Immunology, 192, 1277–1285. [DOI] [PubMed] [Google Scholar]
- Liu, Z. & Davidson, A. (2011) BAFF inhibition: a new class of drugs for the treatment of autoimmunity. Experimental Cell Research, 317, 1270–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, P.J. , Lee, S.J. , Przepiorka, D. , Horowitz, M.M. , Koreth, J. , Vogelsang, G.B. , Walker, I. , Carpenter, P.A. , Griffith, L.M. , Akpek, G. , Mohty, M. , Wolff, D. , Pavletic, S.Z. & Cutler, C.S. (2015) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft‐versus‐Host Disease: VI. The 2014 Clinical Trial Design Working Group Report. Biology of Blood and Marrow Transplantation, 21, 1343–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Masson, A. , Bouaziz, J.D. , Le Buanec, H. , Robin, M. , OMeara, A. , Parquet, N. , Rybojad, M. , Hau, E. , Monfort, J.B. , Branchtein, M. , Michonneau, D. , Dessirier, V. , Sicre de Fontbrune, F. , Bergeron, A. , Itzykson, R. , Dhédin, N. , Bengoufa, D. , Peffault de Latour, R. , Xhaard, A. , Bagot, M. , Bensussan, A. & Socié, G. (2015) CD24hiCD27+ and plasmablast‐like regulatory B cells in human chronic graft‐versus‐host disease. Blood, 125, 1830–1839. [DOI] [PubMed] [Google Scholar]
- Matsuoka, K. , Koreth, J. , Kim, H.T. , Bascug, G. , McDonough, S. , Kawano, Y. , Murase, K. , Cutler, C. , Ho, V.T. , Alyea, E.P. , Armand, P. , Blazar, B.R. , Antin, J.H. , Soiffer, R.J. & Ritz, J. (2013) Low‐dose interleukin‐2 therapy restores regulatory T cell homeostasis in patients with chronic graft‐versus‐host disease. Science Translational Medicine, 5, 179ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland, K.L. & Wetzstein, G.A. (2009) Chronic myeloid leukemia therapy: focus on second‐generation tyrosine kinase inhibitors. Cancer Control, 16, 132–140. [DOI] [PubMed] [Google Scholar]
- Michonneau, D. , Peffault de Latour, R. , Porcher, R. , Robin, M. , Benbunan, M. , Rocha, V. , Ribaud, P. , Ferry, C. , Devergie, A. , Vanneaux, V. , Gluckman, E. , Marolleau, J.P. , Socié, G. & Larghero, J. (2009) Influence of bone marrow graft B lymphocyte subsets on outcome after HLA‐identical sibling transplants. British Journal of Haematology, 145, 107–114. [DOI] [PubMed] [Google Scholar]
- Miklos, D. , Kim, H.T. , Miller, K.H. , Guo, L. , Zorn, M. , Lee, S.J. , Hochberg, E.P. , Wu, C.J. , Alyea, E.P. , Cutler, C.S. , Ho, V. , Soiffer, R.J. , Antin, J.H. & Ritz, J. (2005) Antibody responses to H‐Y minor histocompatibility antigens correlate with chronic graft‐versus‐host disease and disease remission. Blood, 105, 2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miklos, D. , Cutler, C.S. , Arora, M. , Waller, E.K. , Jagasia, M. , Pusic, I. , Flowers, M.E. , Logan, A.C. , Nakamura, R. , Blazar, B.R. , Li, Y.F. , Chang, S. , Lal, I. , Dubovsky, J. , James, D.F. , Styles, L. & Jaglowski, S. (2017) Ibrutinib for chronic graft‐versus‐host disease after failure of prior therapy. Blood, 130, 2243–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed, A.J. , Nore, B.F. , Christensson, B. & Smith, C.I. (1999) Signalling of Bruton's tyrosine kinase, Btk. Scandinavian Journal of Immunology, 49, 113–118. [DOI] [PubMed] [Google Scholar]
- Nakasone, H. , Tian, L. , Sahaf, B. , Kawase, T. , Schoenrock, K. , Perloff, S. , Ryan, C.E. , Paul, J. , Popli, R. , Wu, F. , Otani, J.M. , Coller, J. , Warren, E.H. & Miklos, D.B. (2015) Allogeneic HY antibodies detected 3 months after female‐to‐male HSCT predict chronic GVHD and nonrelapse mortality in humans. Blood, 125, 3193–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforow, S. , Kim, H.T. , Bindra, B. , McDonough, S. , Glotzbecker, B. & Armand, P.L. (2013) Phase I study of alemtuzumab for therapy of steroid‐refractory chronic graft‐versus‐host disease. Biology of Blood and Marrow Transplantation, 19, 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofran, Y. , Beohou, E. , Labopin, M. , Blaise, D. , Cornelissen, J. , de Groot, M.R. , Socié, G. , Huynh, A. , Maertens, J. , Baron, F. , Mohty, M. & Nagler, A. (2018) Anti‐thymocyte globulin for graft‐versus‐host disease prophylaxis in patients with intermediate‐ or high‐risk acute myeloid leukaemia undergoing reduced‐intensity conditioning allogeneic stem cell transplantation in first complete remission ‐ a survey on behalf of the Acute Leukaemia Working Party of the European Society for Blood and Marrow Transplantation. British Journal of Haematology. 10.1111/bjh.15131 [DOI] [PubMed] [Google Scholar]
- Olivieri, A. , Locatelli, F. , Zecca, M. , Sanna, A. , Cimminiello, M. , Raimondi, R. , Gini, G. , Mordini, N. , Balduzzi, A. , Leoni, P. , Gabrielli, A. & Bacigalupo, A. (2009) Imatinib for refractory chronic graft‐versus‐host disease with fibrotic features. Blood, 114, 709–718. [DOI] [PubMed] [Google Scholar]
- Pai, C.C. , Chen, M.Y. , Mirsoian, A. , Grossenbacher, S.K. , Tellez, J. , Ames, E. , Sun, K. , Jagdeo, J. , Blazar, B.R. , Murphy, W.J. & Abedi, M. (2014) Treatment of chronic graft‐versus‐host disease with bortezomib. Blood, 124, 1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavletic, S.Z. , Carter, S.L. , Kernan, N.A. , Henslee‐Downey, J. , Mendizabal, A.M. , Papadopoulos, E. , Gingrich, R. , Casper, J. & Yanovich, S. , Weisdorf, D. (2005) Influence of T‐cell depletion on chronic graft‐versus‐host disease: results of a multicenter randomized trial in unrelated marrow donor transplantation. Blood, 106, 3308–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellom, S.T. Jr , Dudimah, D.F. , Thounaojam, M.C. , Sayers, T. & Shanker, A. (2015) Modulatory effects of bortezomib on host immune cell functions. Immunotherapy, 7, 1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidala, J. , Kim, J. , Betts, B.C. , Alsina, M. , Ayala, E. , Fernandez, H.F. , Field, T. , Kharfan‐Dabaja, M.A. , Locke, F.L. , Mishra, A. , Nishihori, T. , Ochoa‐Bayona, L. , Perez, L. , Riches, M. & Anasetti, C. (2015) Ofatumumab in combination with glucocorticoids for primary therapy of chronic graft‐versus‐host disease: phase I trial results. Biology of Blood and Marrow Transplantation, 21, 1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe, J. , Jia, W. , Su, H. , Anand, S. , Rose, J.J. , Tata, P.V. , Sthers, A.N. , Jones, C.D. , Kuan, P.F. , Vincent, B.J. , Serody, J.S. , Horwitz, M.E. , Ho, V.T. , Pavletic, S.Z. , Hakim, F.T. , Owzar, K. , Zhang, D.D. , Blazar, B.R. , Siebe, C.W. , Chao, N.J. , Maillard, I. & Sarantopoulos, S. (2016) All‐Trans Retinoic Acid (ATRA) targets IRF4 deficient, NOTCH2‐activated B cells from chronic GVHD patients. Blood, 128, 669. [Google Scholar]
- Poe, J.C. , Jia, W. , Su, H. , Anand, S. , Rose, J.J. , Tata, P.V. , Suthers, A.N. , Jones, C.D. , Kuan, P.F. , Vincent, B.G. , Serody, J.S. , Horwitz, M.E. , Ho, V.T. , Pavletic, S.Z. , Hakim, F.T. , Owzar, K. , Zhang, D. , Blazar, B.R. , Siebel, C.W. , Chao, N.J. , Maillard, I. & Sarantopoulos, S. (2017) An aberrant NOTCH2‐BCR signaling axis in B cells from patients with chronic GVHD. Blood, 130, 2131–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickert, R.C. , Jellusova, J. & Miletic, A.V. (2011) Signaling by the tumor necrosis factor receptor superfamily in B‐cell biology and disease. Immunological Reviews, 244, 115–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba, R.M. , Sarantopoulos, S. , Kitko, C.L. , Pawarode, A. , Goldstein, S.C. , Magenau, J. , Alousi, A.M. , Churay, T. , Justman, H. , Paczesny, S. , Reddy, P. & Couriel, D.R. (2017) B‐cell activating factor (BAFF) plasma level at the time of chronic GvHD diagnosis is a potential predictor of non‐relapse mortality. Bone Marrow Transplantation, 52, 1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez‐Ortega, I. , Servitje, O. , Arnan, M. , Ortí, G. , Peralta, T. , Manresa, F. & Duarte, R.F. (2012) Dasatinib as salvage therapy for steroid refractory and imatinib resistant or intolerant sclerotic chronic graft‐versus‐host disease. Biology of Blood and Marrow Transplantation, 18, 318–323. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Ortega, I. , Parody, R. , Servitje, O. , Muniesa, C. , Arnan, M. , Patino, B. , Sureda, A. & Duarte, R.F. (2016) Imatinib and dasatinib as salvage therapy for sclerotic chronic graft‐vs‐host disease. Croatian Medical Journal, 57, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos, S. (2016) Antibodies are back for thymic attack in cGVHD. Blood, 127, 2170–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos, S. & Ritz, J. (2015) Aberrant B‐cell homeostasis in chronic GVHD. Blood, 125, 1703–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos, S. , Stevenson, K.E. , Kim, H.T. , Cutler, C.S. , Bhuiya, N.S. , Schowalter, M. , Ho, V.T. , Alyea, E.P. , Koreth, J. , Blazar, B.R. , Soiffer, R.J. , Antin, J.H. & Ritz, J. (2009) Altered B‐cell homeostasis and excess BAFF in human chronic graft‐versus‐host disease. Blood, 113, 3865–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarantopoulos, S. , Stevenson, K.E. , Kim, H.T. , Washel, W.S. , Bhuiya, N.S. , Cutler, C.S. , Alyea, E.P. , Ho, V.T. , Soiffer, R.J. , Antin, J.H. & Ritz, J. (2011) Recovery of B‐cell homeostasis after rituximab in chronic graft‐versus‐host disease. Blood, 117, 2275–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvaria, A. , Basar, R. , Mehta, R.S. , Shaim, H. , Muftuoglu, M. , Khoder, A. , Sekine, T. , Gokdemir, E. , Kondo, K. , Marin, D. , Daher, M. , Alousi, A.M. , Alsuliman, A. , Liu, E. , Oran, B. , Olson, A. , Jones, R.B. , Popat, U. , Hosing, C. , Champlin, R. , Shpall, E.J. & Rezvani, K. (2016) IL‐10+ regulatory B cells are enriched in cord blood and may protect against cGVHD after cord blood transplantation. Blood, 128, 1346–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt, S.D. , Fu, J. , Nguyen, H. , Bastian, D. , Heinrichs, J. , Wu, Y. , Liu, C. , McDonald, D.G. , Pidala, J. & Yu, X.‐Z. (2015) Inhibition of BTK and ITK with ibrutinib is effective in the prevention of chronic graft‐versus‐host disease in mice. PLoS ONE, 10, e0137641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao, D.D. , Suresh, R. , Vakil, V. , Gomer, R.H. & Pilling, D. (2008) Pivotal advance: Th‐1 cytokines inhibit, and Th‐2 cytokines promote fibrocyte differentiation. Journal of Leukocyte Biology, 83, 1323–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socié, G. , Schmoor, C. , Bethge, W.A. , Ottinger, H.D. , Stelljes, M. , Zander, A.R. , Volin, L. , Ruutu, T. , Heim, D.A. , Schwerdtfeger, R. , Kolbe, K. , Mayer, J. , Maertens, J.A. , Linkesch, W. , Holler, E. , Koza, V. , Bornhäuser, M. , Einsele, H. , Kolb, H.J. , Bertz, H. , Egger, M. , Grishina, O. & Finke, J. (2011) Chronic graft‐versus‐host disease: long‐term results from a randomized trial on graft‐versus‐host disease prophylaxis with or without anti‐T‐cell globulin ATG‐Fresenius. Blood, 117, 6375–6382. [DOI] [PubMed] [Google Scholar]
- Spoerl, S. , Mathew, N.R. , Bscheider, M. , Schmitt‐Graeff, A. , Chen, S. , Mueller, T. , Verbeek, M. , Fischer, J. , Otten, V. , Schmickl, M. , Maas‐Bauer, K. , Finke, J. , Peschel, C. , Duyster, J. , Poeck, H. , Zeiser, R. & von Bubnoff, N. (2014) Activity of therapeutic JAK 1/2 blockade in graft‐versus‐host disease. Blood, 123, 3832–3842. [DOI] [PubMed] [Google Scholar]
- Srinivasan, M. , Flynn, R. , Price, A. , Ranger, A. , Browning, J.L. , Taylor, P.A. , Ritz, J. , Antin, J.H. , Murphy, W.J. , Luznik, L. , Shlomchik, M.J. , Panoskaltsis‐Mortari, A. & Blazar, B.R. (2012) Donor B‐cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood, 119, 1570–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, K. , Wilkins, D.E. , Anver, M.R. , Sayers, T.J. , Panoskaltsis‐Mortari, A. , Blazar, B.R. , Welniak, L.A. & Murphy, W.J. (2005) Differential effects of proteasome inhibition by bortezomib on murine acute graft‐versus‐host disease (GVHD): delayed administration of bortezomib results in increased GVHD‐dependent gastrointestinal toxicity. Blood, 106, 3293–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svegliati, S. , Olivieri, A. , Campelli, N. , Luchetti, M. , Poloni, A. , Trappolini, S. , Moroncini, G. , Bacigalupo, A. , Leoni, P. , Avvedimento, E.V. & Gabrielli, A. (2007) Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft‐versus‐host disease. Blood, 110, 237–241. [DOI] [PubMed] [Google Scholar]
- Tormo, A. , Khodayarian, F. , Cui, Y. , Al‐Chami, E. , Kanjarawi, R. , Noé, B. , Wang, H. & Rafei, M. (2017) Interleukin‐21 promotes thymopoiesis recovery following hematopoietic stem cell transplantation. Journal of Hematology & Oncology, 10, 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veen, W. , Stanic, B. , Wirz, O.F. , Jansen, K. , Globinska, A. & Akdis, M. (2016) Role of regulatory B cells in immune tolerance to allergens and beyond. Journal of Allergy and Clinical Immunology, 138, 654–665. [DOI] [PubMed] [Google Scholar]
- Vogt, M.H. , de Paus, R.A. , Voogt, P.J. , Willemze, R. & Falkenburg, J.H. (2000a) DFFRY codes for a new human male‐specific minor transplantation antigen involved in bone marrow graft rejection. Blood, 95, 1100–1105. [PubMed] [Google Scholar]
- Vogt, M.H. , Goulmy, E. , Kloosterboer, F.M. , Blokland, E. , de Paus, R.A. , Willemze, R. & Falkenburg, J.H. (2000b) UTY gene codes for an HLA‐B60‐restricted human male‐specific minor histocompatibility antigen involved in stem cell graft rejection: characterization of the critical polymorphic amino acid residues for T‐cell recognition. Blood, 96, 3126–3132. [PubMed] [Google Scholar]
- Weiss, J.M. , Chen, W. , Nyuydzefe, M.S. , Trzeciak, A. , Flynn, R. , Tonra, J.R. , Marusic, S. , Blazar, B.R. , Waksal, S.D. & Zanin‐Zhorov, A. (2016) ROCK2 signaling is required to induce a subset of T follicular helper cells through opposing effects on STATs in autoimmune settings. Science Signalling, 9, ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle, R. & Taylor, P.C. (2011) Circulating B‐cell activating factor level predicts clinical response of chronic graft‐versus‐host disease to extracorporeal photopheresis. Blood, 118, 6446–6449. [DOI] [PubMed] [Google Scholar]
- Woo, Y.J. , Yoon, B.Y. , Jhun, J.Y. , Oh, H.J. , Min, S.W. , Cho, M.L. , Park, S.H. , Kim, H.Y. & Min, J.K. (2011) Regulation of B cell activating factor (BAFF) receptor expression by NF‐KappaB signaling in rheumatoid arthritis B cells. Experimental & Molecular Medicine, 43, 350–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, T. , Young, J.S. , Johnston, H. , Ni, X. , Deng, R. , Racine, J. , Wang, M. , Wang, A. , Todorov, I. , Wang, J. & Zeng, D. (2013) Thymic damage, impaired negative selection, and development of chronic graft‐versus‐host disease caused by donor CD4+ and CD8+ T cells. The Journal of Immunology, 191, 488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Wu, Y. , Wang, G. , Qin, Y. , Zhu, L. , Tang, G. & Shen, Q. (2014) Inducible costimulatory molecule deficiency induced imbalance of Treg and Th17/Th2 delays rejection reaction in mice undergoing allogeneic tracheal transplantation. American Journal of Translational Research, 6, 777–785. [PMC free article] [PubMed] [Google Scholar]
- Yang, M. , Rui, K. , Wang, S. & Lu, L. (2013) Regulatory B cells in autoimmune diseases. Cellular & Molecular Immunology, 10, 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdanbakhsh, K. (2014) cGVHD B(r)egs to differ. Blood, 124, 2005–2006. [DOI] [PubMed] [Google Scholar]
- Young, J.S. , Wu, T. , Chen, Y. , Zhao, D. , Liu, H. , Yi, T. , Johnston, H. , Racine, J. , Li, X. , Wang, A. , Todorov, I. & Zeng, D. (2012) Donor B cells in transplants augment clonal expansion and survival of pathogenic CD4+ T cells that mediate autoimmune‐like chronic graft‐versus‐host disease. The Journal of Immunology, 189, 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser, R. , Burchert, A. , Lengerke, C. , Verbeek, M. , Maas‐Bauer, K. , Metzelder, S.K. , Spoerl, S. , Ditschkowski, M. , Ecsedi, M. , Sockel, K. , Ayuk, F. , Ajib, S. , de Fontbrune, F.S. , Na, I.K. , Penter, L. , Holtick, U. , Wolf, D. , Schuler, E. , Meyer, E. , Apostolova, P. , Bertz, H. , Marks, R. , Lübbert, M. , Wäsch, R. , Scheid, C. , Stölzel, F. , Ordemann, R. , Bug, G. , Kobbe, G. , Negrin, R. , Brune, M. , Spyridonidis, A. , Schmitt‐Gräff, A. , van der Velden, W. , Huls, G. , Mielke, S. , Grigoleit, G.U. , Kuball, J. , Flynn, R. , Ihorst, G. , Du, J. , Blazar, B.R. , Arnold, R. , Kröger, N. , Passweg, J. , Halter, J. , Socié, G. , Beelen, D. , Peschel, C. , Neubauer, A. , Finke, J. , Duyster, J. & von Bubnoff, N. (2015) Ruxolitinib in corticosteroid‐refractory graft‐versus‐host disease after allogeneic stem cell transplantation: a multi‐center survey. Leukemia, 29, 2062–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser, R. , Sarantopoulos, S. & Blazar, B.R. (2018) B‐cell targeting in chronic graft‐versus‐host disease. Blood, 131, 1399–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Todorov, I. , Zhang, Z. , Liu, Y. , Kandeel, F. , Forman, S. , Strober, S. & Zeng, D. (2006) Donor CD4+ T and B cells in transplants induce chronic graft‐versus‐host disease with autoimmune manifestations. Blood, 107, 2993–3001. [DOI] [PubMed] [Google Scholar]
- Zhang, M.M. , Wu, Y.X. , Bastian, D. , Iamsawat, S. , Chang, J. , Daenthanasanmak, A. , Nguyen, H.D. , Schutt, S. , Dai, M. , Chen, F.P. , Suh, W. & Yu, X.Z. (2018) Inducible T‐cell co‐stimulator impacts chronic graft‐versus‐host disease by regulating both pathogenic and regulatory T cells. Frontiers in Immunology, 9, 1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, K. & Liu, Q. (2016) The clinical application of mesenchymal stromal cells in hematopoietic stem cell transplantation. Journal of Hematology & Oncology, 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H.Y. , Lyu, Z.S. , Duan, C.W. , Song, Y. , Han, T.T. , Mo, X.D. , Wang, Y. , Xu, L.P. , Zhang, X.H. , Huang, X.J. & Kong, Y. (2018) An unbalanced monocyte macrophage polarization in the bone marrow microenvironment of patients with poor graft function after allogeneic haematopoietic stem cell transplantation. British Journal of Haematology, 182, 679–692. [DOI] [PubMed] [Google Scholar]
- Zotos, D. , Coquet, J.M. , Zhang, Y. , Light, A. , D'Costa, K. , Kallies, A. , Corcoran, L.M. , Godfrey, D.I. , Toellner, K.M. , Smyth, M.J. , Nutt, S.L. & Tarlinton, D.M. (2010) IL‐21 regulates germinal center B cell differentiation and proliferation through a B cell‐intrinsic mechanism. Journal of Experimental Medicine, 207, 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]


