Summary
A novel coloration named ‘mocha’ has been identified in the Burmese cat breed from Thailand. Tyrosinase (TYR) mutations are known to be associated with coat coloration in cats, such as the sable Burmese, the points of the Siamese and albino cats. Additionally, sable Burmese that produced mocha‐colored cats had unexpected genotypes for TYR. Therefore, TYR was considered a candidate gene for mocha in cats. Sanger sequencing for genomic DNA revealed NC_018732.3:chromosome D1:45 898 609_45 898 771dup in exon 2 and intron 2 of TYR. Transcription analysis using cDNA detected c.820_936delinsAATCTC (p.I274_L312delinsNL), which caused a 111‐bp (37 amino acid) deletion in the reading frame of TYR. The identified variant was concordant with the phenotype and segregated with TYR variants in a pedigree of 12 Burmese cats. This findings of this study suggest that TYR is associated with the mocha coloration in cats. The new color variant adds to the allelic series for TYR (C > c b = c s > c, c 2) and is recessive to full color (C); however, interactions with the c b and c s alleles are unclear due to the temperature‐sensitivity of the alleles.
Keywords: Burmese, feline, felis, Thailand, TYR
The color locus (C) in mammals is the gene tyrosinase (TYR), which codes for an enzyme in melanin synthesis. Cats have TYR variants causing the temperature‐sensitive colorations of the sable Burmese (c b c b) and the points of the Siamese (c s c s) (Lyons et al. 2005; Schmidt‐Küntzel et al. 2005; OMIA 000202‐9685). Both breeds originated in Thailand and are known as the Suphalak (Burmese) and Wichien‐maat (Siamese) (Clutterbuck 2004). Two complete albino variants (c, c 2) are also known in TYR for cats (Imes et al. 2006; Abitbol et al. 2017; OMIA 000202‐9685). A vast number of albinism variants are known for TYR in various species, including loss‐of‐function variants that cause oculocutaneous albinism Type 1A (OCA1A; OMIM: 203100) and variants that reduce enzymatic activity, causing oculocutaneous albinism Type 1B (OCA1B; OMIM: 606952) in humans. Similar temperature‐sensitive phenotypes have been noted in the Himalayan mouse (Kwon et al. 1989), rabbit (Aigner et al. 2000), gerbil (Petrij et al. 2001), mink (Benkel et al. 2009) and human (Giebel et al. 1991).
Recent breedings of Burmese cats have revealed an unusual color, termed ‘mocha’ (Fig. 1). Commercial genetic testing of mocha‐colored cats revealed wildtype alleles (CC) for all the TYR variants. Sable‐colored Burmese cats that have produced mocha kittens, which should be homozygous c b c b, have genotyped as Cc b. Thus, a novel allele in TYR, with the allelic designation c m, has been suspected to produce the new coloration. In the current study, TYR was investigated as a candidate gene for the novel mocha phenotype identified in Burmese cats that have recent Thai origins.
Figure 1.
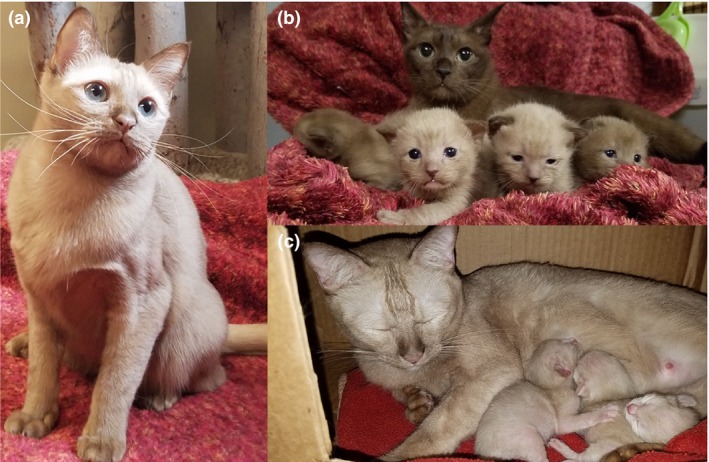
Mocha coloration in Burmese cats (a) Mocha coloration of an intact adult female (proband). This cat has the genotype aa,BB,CC,DD by commercial genetic testing, suggesting its coloration should be solid black. Mocha cats have aqua eye coloration and pink nose leather and paw pads. (b) A sable queen (c b c m) with two sable kittens (c b c b; left and right) and two mocha carrier kittens (middle). The two lighter kittens were identified as heterozygous carriers of the Burmese and the newly discovered TYR ‘mocha’ variant (c b c m), suggesting that the allele is temperature‐sensitive and co‐dominant with the Burmese (c b) allele. (c) The same mocha cat as in (a) and her kittens, whose sire is a seal point Siamese (aa, B‐, c s c s). These cats are suspected to have both the mocha and Siamese alleles (c m c s). Siamese kittens (c s c s) would be nearly white with only black ears, paws, face and paws (points). Although the DNA of these cats have not been directly examined, based on parentage, they are inferred to be compound heterozygotes for both the mocha and Siamese alleles (c m c s).
Detailed materials and methods are provided in Appendix S1; a list of primers is provided in Table S1. Five offspring and the proband mocha cat were ascertained for direct Sanger sequencing of the coding regions and the splice junctions of TYR (Figs. 1 & S1). A pedigree representing the breeding that produced a mocha colored cat is presented in Fig. S1. The parentage of the extended pedigree was confirmed using short tandem repeat polymorphisms (data not shown). All five TYR exons and the intron exon boundaries were amplified in 11 cats, including six cats in the pedigree (Fig. S1) and five unrelated non‐mocha Burmese cats from the US. Amplification of exon 2 (688 bp) produced a second amplicon of approximately 851 bp in five of 11 tested Burmese cats. One cat was homozygous for the larger amplicon (Fig. S2a), which was the mocha proband (Figs. 1a & S1). The approximate 851‐bp product was confirmed by Sanger sequencing as a tandem duplication containing 100 bp of the 3′ portion of exon 2 and 63 bp of the 5′ portion of intron 2 (NC_018732.3:45 898 609–45 898 771dup; Fig. 2). Sequence analyses also identified known single nucleotide variants within the TYR exons (Table S2). The known Burmese allelic variant, c.679G>T (p.Gly227Trp) in exon 1, was compound heterozygous with the mocha variant in tested cats (Fig. S1, Table S2). The mocha cat with the homozygous variant did not have the Burmese allele c.679G>T (p.Gly227Trp) nor any other known TYR variants causing alterations in coloration.
Figure 2.
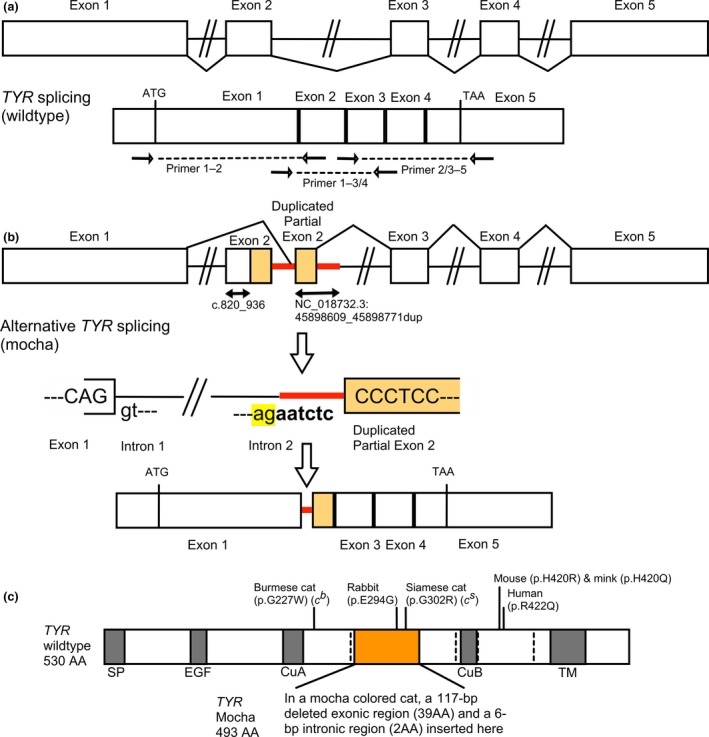
Tyrosinase (TYR) exon 2 tandem duplication in cats. (a) Schematic illustration of the TYR structure in genomic DNA and complementary DNA (cDNA) of a wildtype cat. Primer positions for RT‐PCR are indicated. (b) Splicing alteration in the mocha coated cat's cDNA is indicated. Duplicated areas of partial exon 2 are indicated by mocha‐colored blocks. Duplicated intronic regions are shown by solid red lines. Duplicated positions of partial exon 2 and the intronic region are indicated as NC_018732.3:45 898 609_45 898 771dup and by the bidirectional arrow. In the splicing process of TYR of the mocha‐coated cat, the normal exon 2 was skipped; however, the duplicated partial exon 2 and intronic 6 bp upstream (shown as red bold) are demonstrated to be transcribed due to the presence of the novel splice acceptor site (ag) (highlighted in yellow). The cDNA deletion region is shown by the bidirectional arrow and indicated as c.820_936. (c) Schematic illustration of cDNA TYR structure in deduced amino acids of a wildtype cat and a mocha‐coated cat. The domains are colored gray and annotated as SP (signal peptide), EGF (epidermal growth factor‐like domain), CuA (copper‐binding domain A), CuB (copper‐binding domain B) and TM (transmembrane domain). Several temperature‐sensitive TYR variants in mammals, including cats, are also annotated, and bars below each allelic designation is indicated by a black bar. The deleted region found in a mocha cat is colored orange. The dotted lines represent boundaries of each exon.
Six cats of the pedigree, which included four half‐siblings of the proband and their parents (Fig. S1), were genotyped for exon 2 by fragment analysis on an agarose gel. The parents and two of the kittens were heterozygous for mocha and the other two kittens were wildtype (Fig. 1b). The mocha variant was genotyped in 20 Khao Manee cats, five Thai cats and 28 random‐bred cats from Thailand (Table S3). All cats were wildtype except two random‐bred cats genotyped as heterozygous for the mocha variant, representing an allele frequency of 1.89% in cats from Thailand.
Complementary DNA (cDNA) analysis from a skin biopsy of the mocha proband was examined to determine the effect of the identified variant on the TYR transcript. The reverse transcription PCR (RT‐PCR) amplicon that included the duplicated region of a mocha cat was 875 bp, whereas the amplicon of the control cat was a 986 bp (Fig. S2b). Sequence analysis of the cDNA suggested that the mocha cat's TYR splicing pattern is altered, as presented in Fig. 2. In a mocha cat, the wildtype exon 2 is not transcribed. The exon 1 donor site (gt) is spliced to the novel splice acceptor site (ag), which is 7–8 bp upstream of the duplicated portion of the exon 2 sequence (Fig. 2), suggesting a greater splicing affinity than the normal exon 2 acceptor site (ag) and leading to exon 2 skipping. Six nucleotides of the intronic region are included within the altered transcript with the duplicated portion of exon 2, retaining the normal reading frame. The normal and intact splice‐donor site at the 3′ end of the duplicated portion of exon 2 is spliced to the splice‐acceptor of exon 3, staying in frame. Therefore, the variant is a 117‐bp deletion concurrent with a 6‐bp alternative transcription resulting in the altered transcript c.820_936delinsAATCTC (p.Ile274_Leu312delinsAsnLeu) (DDBJ accession no. LC424926). Comparison of known TYR variants in cats and other mammals and the nucleotide alignment are depicted in Figs. 2 & S3. [Please note, the sequence annotation of the latest feline genome assembly (Felis_catus_9.0; Felis catus Annotation Release 104) is incorrect for TYR and is clarified in Appendix S2.]
Burmese cats have low genetic diversity and high inbreeding, which has led to a prevalence of several diseases in the population, such as periodic hypokalemic polymyopathy, frontonasal dysplasia, diabetes and feline orofacial pain syndrome (Rand et al. 1997; Rusbridge et al. 2010; Gandolfi et al. 2012; Lyons et al. 2016). The novel mocha coat coloration was identified as a consequence of importation of street cats from Thailand into the Burmese breed to improve genetic diversity. ‘Mocha’ refers to a chocolate‐flavored variant of a caffè latte (Campbell & Smith 1993), and the term etymologically derives from Arabic, referring to Mocha, Yemen, a port on the Red Sea, which was a major marketplace for coffee during Ottoman rule. In keeping with Thai traditions, ‘Si Mai Thong’, which translates to ‘color of golden silk’ in Thai, is the suggested Thai name for the mocha coloration with the allelic designation c m. ‘Wila Krung Thep’ is suggested for the name of the Si Mai Thong (mocha) colored cats. Wila is an older word for cat in the Thai language and Krung Thep means Bangkok, which is the discovery location of the novel coloration. The current allelic series for TYR variants in cats is: C (full colour) > c b (Burmese) = c s (Siamese) > c, c 2 (albinos). The mocha allele is likely co‐dominant to the Burmese allele and temperature sensitive, as displayed in the genotyped kittens. But as adults, the Burmese temperature‐sensitive allele may appear more influential in coloration. The mocha allele is also likely dominant to the Siamese (c s) allele, as parentage‐inferred heterozygous kittens (c m c s) displayed the mocha coloration instead of the otherwise expected Siamese coloration (Fig. 1c). However, not all allelic combinations have been observed, particularly combinations with the full albino alleles.
A variety of loss‐of‐function variants that cause complete albinism in TYR have been identified in various species. Temperature‐sensitive variants have been identified in mouse, cat, mink and human (Kwon et al. 1989; Giebel et al. 1991; Aigner et al. 2000; Benkel et al. 2009). These amino acid changes apparently reduce enzymatic activity in warmer regions of the animal's body, such as the torso. Full coloration is displayed at cooler regions, such as the ears, face, paws and tail, leading to the term ‘points’ of the Siamese cat. The sable coloration of the Burmese cat is less thermally labile, producing a darker coloration for the cat with full black coloration at the points. The mocha variant appears to interact with the temperature‐sensitive alleles but also produces a more even, lighter coloration and is less thermally labile when homozygous. This variant introduces two novel amino acids and deletes 39 amino acids of exon 2, which causes a 37‐amino‐acid deletion and does not include major functional domains (Fig. 2).
In conclusion, a tandem duplication in exon/intron 2 in TYR, which causes alternative splicing and results in c.820_936delinsAATCTC (p.Ile274_Leu312delinsAsnLeu), is associated with a novel coloration termed ‘mocha’ in cats. Furthermore, genotyping of 53 cats derived from Thailand identified two cats heterozygous for this variant. Therefore, this allele is polymorphic among cats in Thailand.
Conflict of interest
The authors may receive supportive funds from a genetic testing laboratory that would offer this variant as a commercialized test in the future.
Supporting information
Figure S1 Pedigree depicting mocha coloration inheritance in Burmese cats.
Figure S2 Gel electrophoresis of TYR exon 2 for mocha coloration.
Figure S3 Partial nucleotide alignment of feline TYR and other species.
Table S1 PCR primers for the amplification of feline TYR.
Table S2 TYR exonic SNV genotypes in cats.
Table S3 Genotyping of Thailand cat populations for the TYR mocha variant.
Appendix S1 Materials and methods.
Appendix S2 Sequence context of the mocha variant (c m) and positions of the primers.
Acknowledgements
Funding provided by the University of Missouri Gilbreath McLorn Endowment for Comparative Medicine (Leslie A. Lyons). The authors thank the JSPS Overseas Challenge Program for Young Researchers for sponsoring the visiting scholarship of Yoshihiko Yu, at the College of Veterinary Medicine, University of Missouri – Columbia, Columbia, MO, US. We appreciate the data and funding support provided by the Mocha cat workgroup and interested cat breeders, especially Nolan Betterley, Cristy Bird, DVM, Jennifer Kelly and Janet Poulsen. We thank Stuart Durbridge, DVM, and veterinary technicians for the skin biopsy at Ashland Road Animal Clinic, Mansfield, OH, US. We also thank Reuben M. Buckley, Ph.D. and Thomas R. Juba, MSc for technical assistance.
References
- Abitbol M., Bosse P., Grimard B., Martignat L. & Tiret L. (2017) Allelic heterogeneity of albinism in the domestic cat. Animal Genetics 48, 127–8. [DOI] [PubMed] [Google Scholar]
- Aigner B., Besenfelder U., Muller M. & Brem G. (2000) Tyrosinase gene variants in different rabbit strains. Mammallian Genome 11, 700–2. [DOI] [PubMed] [Google Scholar]
- Benkel B.F., Rouvinen‐Watt K., Farid H. & Anistoroaei R. (2009) Molecular characterization of the Himalayan mink. Mammallian Genome 20, 256–9. [DOI] [PubMed] [Google Scholar]
- Campbell D. & Smith J. (1993). The Coffee Book. Pelican Publishing Company, Gretna, LA. [Google Scholar]
- Clutterbuck M.R. (2004) Siamese Cats: Legends and Reality, pp. 23–62. White Lotus, Bangkok, Thailand. [Google Scholar]
- Gandolfi B., Gruffydd‐Jones T.J., Malik R., Cortes A., Jones B.R., Helps C.R., Prinzenberg E.M., Erhardt G. & Lyons L.A. (2012) First WNK4‐hypokalemia animal model identified by genome‐wide association in Burmese cats. PLoS ONE 7, e53173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giebel L.B., Tripathi R.K., King R.A. & Spritz R.A. (1991) A tyrosinase gene missense mutation in temperature‐sensitive type I oculocutaneous albinism: a human homologue to the Siamese cat and the Himalayan mouse. Journal of Clinical Investigation 87, 1119–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D.L., Geary L.A., Grahn R.A. & Lyons L.A. (2006) Albinism in the domestic cat (Felis catus) is associated with a tyrosinase (TYR) mutation. Animal Genetics 37, 175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon B.S., Halaban R. & Chintamaneni C. (1989) Molecular basis of mouse Himalayan mutation. Biochemical and Biophysical Research Communications 161, 252–60. [DOI] [PubMed] [Google Scholar]
- Lyons L.A., Imes D.L., Rah H.C. & Grahn R.A. (2005) Tyrosinase mutations associated with Siamese and Burmese patterns in the domestic cat (Felis catus). Animal Genetics 36, 119–26. [DOI] [PubMed] [Google Scholar]
- Lyons L.A., Erdman C.A., Grahn R.A., Hamilton M.J., Carter M.J., Helps C.R., Alhaddad H. & Gandolfi B. (2016) Aristaless‐like homeobox protein 1 (ALX1) variant associated with craniofacial structure and frontonasal dysplasia in Burmese cats. Developmental Biology 409, 451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrij F., van Veen K., Mettler M. & Bruckmann V. (2001) A second acromelanistic allelomorph at the albino locus of the Mongolian gerbil (Meriones unguiculatus). Journal of Heredity 92, 74–8. [DOI] [PubMed] [Google Scholar]
- Rand J.S., Bobbermien L.M., Hendrikz J.K. & Copland M. (1997) Over representation of Burmese cats with diabetes mellitus. Australian Veterinary Journal 75, 402–5. [DOI] [PubMed] [Google Scholar]
- Rusbridge C., Heath S., Gunn‐Moore D.A., Knowler S.P., Johnston N. & McFadyen A.K. (2010) Feline orofacial pain syndrome (FOPS): a retrospective study of 113 cases. Journal of Feline Medicine and Surgery 12, 498–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt‐Küntzel A., Eizirik E., O'Brien S.J. & Menotti‐Raymond M. (2005) Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. Journal of Heredity 96, 289–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Pedigree depicting mocha coloration inheritance in Burmese cats.
Figure S2 Gel electrophoresis of TYR exon 2 for mocha coloration.
Figure S3 Partial nucleotide alignment of feline TYR and other species.
Table S1 PCR primers for the amplification of feline TYR.
Table S2 TYR exonic SNV genotypes in cats.
Table S3 Genotyping of Thailand cat populations for the TYR mocha variant.
Appendix S1 Materials and methods.
Appendix S2 Sequence context of the mocha variant (c m) and positions of the primers.


