Abstract
The glycom e describes the complete repertoire of glycoconjugates com posed of carbohydrate chains, or glycans, that are covalently linked to lipid or protein molecules. Glycoconjugates are formed through a process called glycosylation and can differ in their glycan sequences, the connections between them and their length. Glycoconjugate synthesis is a dynamic process that depends on the local milieu of enzymes, sugar precursors and organelle structures as well as the cell types involved and cellular signals. Studies of rare genetic disorders that affect glycosylation first highlighted the biological importance of the glycome, and technological advances have improved our understanding of its heterogeneity and complexity. Researchers can now routinely assess how the secreted and cell-surface glycom es reflect overall cellular status in health and disease. In fact, changes in glycosylation can modulate inflammatory responses, enable viral immune escape, promote cancer cell metastasis or regulate apoptosis; the com position of the glycom e also affects kidney function in health and disease. New insights into the structure and function of the glycom e can now be applied to therapy developm ent and could improve our ability tofine-tune im m unological responses and inflammation, optimize the performance of therapeutic antibodies and boost immune responses to cancer. These examples illustrate the potential of the emerging field of ‘glycomedicine’.
Glycobiology studies the structure, biosynthesis and biology of glycans, which are widely distributed in nature. Most glycans are found on the outermost surfaces of cellular and secreted macromolecules and are remark-ably diverse. Simple and highly dynamic protein-bound glycans are also abundant in the nucleus and cytoplasm of cells, where they exert regulatory effects. In fact, in addition to forming important structural features, the sugar components of glycoconju gates modulate or mediate a wide variety of functions in physiological and pathophysiological states1. Glycoproteins and polysaccharides also have important functions in bacterial cells, and glycoproteins have central roles in the biology of most viruses.
Glycoconjugates are formed by the addition of sugars to proteins and lipids; 17 monosaccharides commonly found in mammalian glycoconjugates are shown in Supplementary Table 1 (REF2). A vast number of naturally occurring sugars can be combined to create a variety of unique glycan structures on lipid and protein molecules that modulate their function. Multiple enzymatic site preferences, as well as the use of stereochemical α or β conjugations, create further diversity in where and how these sugars are linked to each other. In fact, altogether, these features imply the potential existence of ~10 12 different branched glycan structures3. Protein glycosylation includes the addition of N-linked glycans, O-linked glycans, phosphorylated glycans, glycosam inoglycans and glycosylphosphatidylinositol (GPI) anchors to peptide backbones as well as C-mannosylation of tryptophan residues (FIG. 1). Glycolipids are form ed through the addition of sugars to lipids; this type of glycoconjugate includes glycosphingolipids (GSLs)4,5 (Fig. 1). Glycosylation of proteins and lipids occurs in the endoplasm icreticulum (ER) and Golgi apparatus, with most of the term in al processing occurring in the cis-, medial- and trans-Golgi compartments. In these organelles, glycosyltransferases and glycosidases form carbohydrate structures in a series of steps that are controlled by substrate availability, enzyme activity, levels of gene transcription and enzyme location within the organelles (Fig. 2). In fact, the glycome of a particular cell reflects its unique gene-expression pattern, which controls the levels of the enzymes responsible for glycoconjugation. Unlike the genome, exome or proteome, the glycome is produced in a non-templated manner and is intricately controlled at multiple levels in the ER and Golgi apparatus.
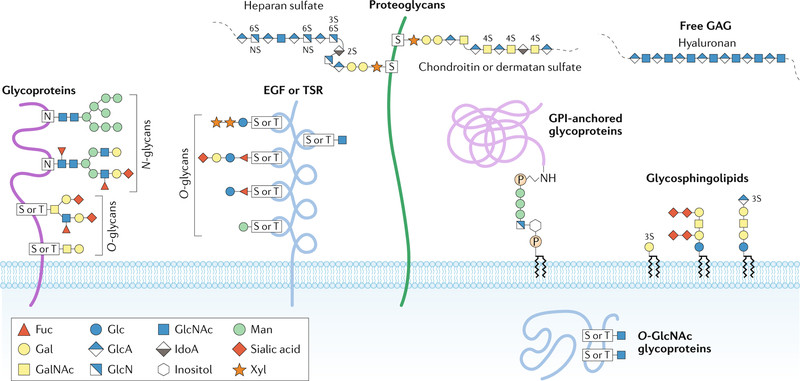
Fig. 1 |. Major types of glycosylation in humans.
Glycans can be covalently attached to proteins and lipids to form glycoconjugates; glycans in these compounds are classified according to the linkage to the lipid, glycan or protein moieties. Glycoproteins consist of glycans and glycan chains linked to nitrogen and oxygen atoms of amino acid residues and are thus termed N-glycans and O-glycans, respectively. N-glycans consist of N-acetylglucosamine (GlcNAc) attached by a β1-glycosidic linkage to the nitrogen atom of the amino group of Asn (N) at the consensus glycosylation motif Asn-X-Ser/Thr (in which X denotes any amino acid except for Pro). These branched and highly heterogeneous N-glycan structures consist of a core glycan containing two GlcNAc residues and three mannose (Man) residues. Perhaps the most diverse form of protein glycosylation is O-glycosylation, in which glycans attach to the oxygen atom of the hydroxyl groups of Ser (S) or Thr (T) residues. O-glycans can be further subclassified on the basis of the initial sugar attached to the protein and the additional sugar structures added to the initial glycan. For example, mucin-type O-glycosylation denotes that the initial glycan is N-acetylgalactosamine (GalNAc); mucin-type glycans can be further classified on the basis of the glycans attached to the initial GalNAc6. Other types of O-glycans, such as O-linked fucose (Fuc) and O-linked Man, often occur in specific proteins or protein domains, such as epidermal growth factor (EGF) repeats, thrombospondin type I repeats (TSR) or dystroglycan. N-glycans and O-glycans are often capped with negatively charged sialic acid. O-GlcNAc is a unique type of O-glycosylation that is synthesized by O-GlcNAc transferase; it occurs in the cytosol and nucleus. Proteoglycans represent a major class of glycoproteins that are defined by long glycosaminoglycan (GAG) chains attached to proteins through a tetrasaccharide core consisting of glucuronic acid (GlcA)–galactose (Gal)–Gal–xylose (Xyl); this carbohydrate core is attached to the hydroxyl group of Ser at Ser-Gly-X-Gly amino acid motifs. Proteoglycan GAGs can be further classified according to the number, composition and degree of sulfation of their repeating disaccharide units; common GAGs include heparan sulfate, chondroitin sulfate and dermatan sulfate. Glycosylphosphatidylinositol (GPI)-anchored glycoproteins represent another major class of glycoconjugates. These glycoproteins are linked at the carboxyl terminus through a phosphodiester linkage to phosphoethanolamine attached to a trimannosyl-nonacetylated glucosamine (Man3-GlcN) core; the GlcN residue is linked to phosphatidylinositol, which is embedded in the cell membrane. Glycosphingolipids are a class of glycoconjugate in which glycans, such as Gal or glucose (Glc), are attached to cellular membrane lipids. Another major class of glycans is represented by GAGs that are not attached to protein cores, such as hyaluronan, which is synthesized at the plasma membrane by sequential addition of GlcA and GlcNAc. IdoA, iduronic acid. Adapted with permission from ref.277, Springer Nature Limited and from ref.278, Stanley, P. Golgi glycosylation. Cold Spring Harb. Perspect. Biol. 0033, a005199 (2005), with permission from Cold Spring Harbor Laboratory Press.
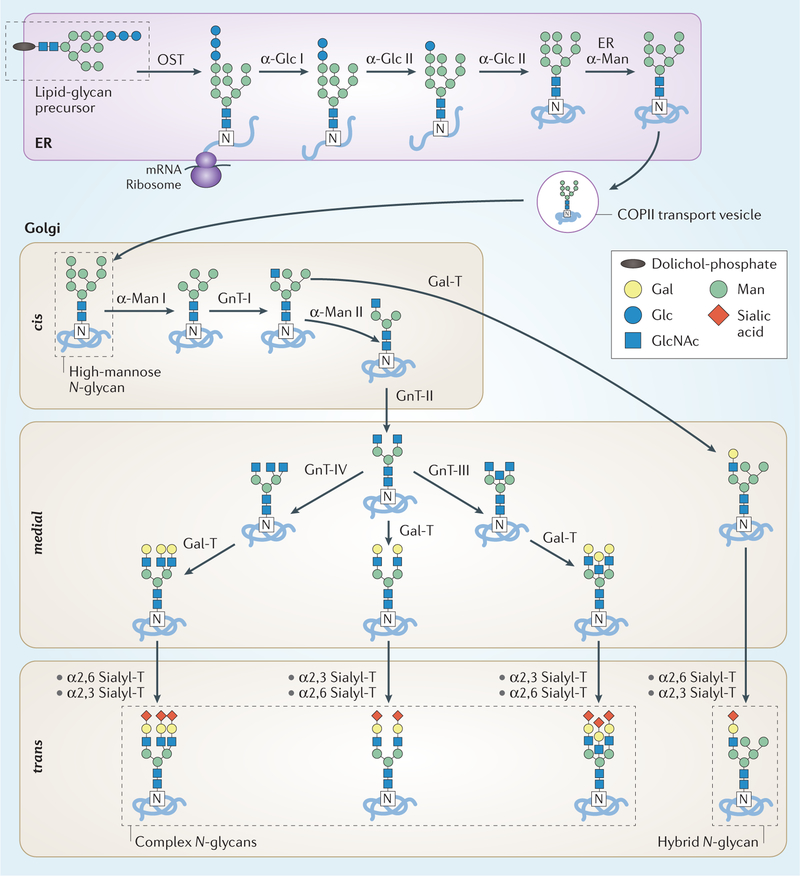
Fig. 2 |. N-glycan biosynthesis in the secretory pathway.
N-glycan synthesis is initiated in the endoplasmic reticulum (ER) by the en bloc transfer of a lipid-glycan precursor (that is, glucose (Glc)3 mannose (Man)9 N-acetylglucosamine (GlcNAc)2 bound to dolichol phosphate) to Asp by the multisubunit oligosaccharyltransferase (OST). The glucose residues are sequentially removed by two α-glucosidases (α-Glc I–II) and an initial Man residue is removed by the ER α-mannosidase (ER α-Man). After a quality-control checkpoint, the glycoprotein moves to the Golgi apparatus for additional trimming by α-mannosidase I and II (α-Man I–II) and further glycan modifications. A cis-to-trans distribution of glycosidases and transferases — GlcNAc-transferase I–IV (GnT-I–IV), β1,4 galactosyltransferases (Gal-T), α2,3 sialyltransferase (α2,3, Sialyl-T) and α2,6 sialyltransferase (α2,6 Sialyl-T) — facilitates further processing by these carbohydrate-modifying enzymes to create a plethora of N-glycoforms that often terminate with sialic acid moieties. The final site-specific N-glycan composition is affected by the expression levels of glycosyltransferases, the accessibility of the glycoprotein glycosylation sites and the length of time during which the glycoprotein remains in the ER and Golgi apparatus. Gal, galactose.
In this Review, we discuss fundamental concepts in glycobiology and integrate these with recent advances in understanding the key roles of the glycome in health and disease. We review how glycosylation patterns are altered in multiple hum an diseases, including congenital disorders of glycosylation (C D G s) as well as auto-immune, infectious and chronic inflammatory diseases, and cancer. We also provide specific examples of how an improved understanding of abnormal glycosylation in various diseases might offer new diagnostic options and/or targets for glycan-mediated therapeutic interventions.
Main types of glycosylation in humans
N - linked glycosylation.
Many proteins are modified by N-glycosylation, which refers to the attachment of N-acetylglucosamine (GlcNAc) to the nitrogen atom of an Asn side chain by a β−1N linkage. These Asn-linked glycoconjugates contain a G lcNAc2 mannose (Man)3 core, to which a variable number of other monosaccha-rides can be added or removed (FIGS 1,2). These additions include, for example, galactosylation, GlcNAclyation, sialylation and fucosylation, and they determine whether the final structure is classed as a high-mannose N-glycan, a hybrid N-glycan or a complex N-glycan (FIG. 2). N-glycans are found in most living organism s and have a crucial role in regulating m any intracellular and extracellular functions. N-glycosylation depends on the formation of a lipid precursor in which GlcNAc and Man form a branched carbohydrate structure that is attached to dolichol phosphate (Dol-P) on the cytoplasmic side of the ER6. This lipid precursor is then flipped to face the ER lumen, where M an and glucose units are added to form a 14-sugar structure — Glc3Man9GlcNAc2 (FIG. 2). Following completion of the Dol-P-carbohydrate structure, an oligosaccharyltransferase adds the carbohydrate chain to a protein at an Asn-X-Ser/Thr site (in which X denotes any amino acid except for Pro); the nascent carbohydrate-protein conjugate undergoes further processing in the ER, which usually involves removal of the glucose residues as p art of a quality-control process. The structure then moves to the Golgi apparatus (that is, the cis-Golgi), where the carbohydrate structures are trim med further by a series of specific m annosidases before being transferred to the medial- Golgi for maturation. It is within the medial- and trans-Golgi compartments that hybrid and complex N-glycans are produced through the addition of GlcNAc, galactose, sialic acid and fucose sugars6 (FIG. 2).
O - glycosylation.
Glycosylation can occur on amino acids with functional hydroxyl groups, which are most often Ser and Thr. In humans, the most com m on sugars linked to Ser or Thr are GlcN Ac and N-acetylgalactosamine (GalNAc)7 (FIG. 1). GalNAc-linked glycans, often called mucin-type O-glycans, are abundant on m any extracellular and secreted glycoproteins8–9, including mucins, which form a crucial interface between epithelial cells and the external mucosal surfaces of the body. Mucins are characterized by a variable number of tandem repeats with a high content of Pro, Ser and Thr, which creates m any sites for O-glycosylation. Moreover, these sites often have extended O-glycan cores that create a gel-like substance thought to protect both the glycoproteins and cellular surfaces from external stress, microbial infection and self-recognition by the immune system. This class of O-glycans contains six major basic core structures (that is, cores 1–4, terminal GalNAc (Tn) and sialyl-Tn antigens) and is integral, along with other O-linked glycoconjugates, to the classification of blood-group antigens (BOX 1). Mucin-type O-glycan synthesis is initiated by polypeptide GalNAc transferases (GA LN Ts)7. These GALNTs differ in their specificity for amino acid motifs, although they are often promiscuous, which adds a level of regulation to how and where O-glycans are attached. Non-templated sequential addition of glycans to the initial GalNAc gives rise to a diverse set of carbohydrate structures that are often highly clustered on certain glycoproteins, including mucins and human immunoglobulin A1 (IgA1). These sugars are added as the protein moves through the cis-, medial- and trans-Golgi compartments and, unlike N-glycans, pre-processing and post-processing that trim s existing sugar structures does not occur. Instead, the glycopeptide O-glycan chains are modified by distinct glycosyltransferases that can expand the existing structure with galactose, GlcNAc, sialic acid and, in some instances, fucose7.
Box 1 |. Blood-group sugars can be immunogenic.
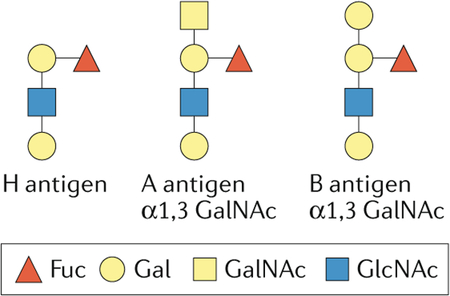
Many blood-group antigens on erythrocytes are glycans conjugated to lipids or proteins. The ABO(H) blood-group antigens were discovered in the early 20th century on the basis of the existence of antibodies in some individuals that agglutinated red blood cells from other individuals. Follow-up studies revealed that these antibodies recognized glycan epitopes on the cell surface of red blood cells that were also expressed in other cells of the same individual. These studies established for the first time that glycans can be antigenic. The ABO(H)-associated glycan epitopes are determined by the inheritance of glycosyltransferase genes in ABO, FUT1 and FUT2 loci. Group O individuals express the H antigen, the precursor to A and B antigens. Group A individuals have α1,3-N-acetylglucosamine (GalNAc) attached to the galactose residue of the H antigen, whereas Group B individuals have α1,3-Gal. Additional blood-group antigens have been described, and many contain glycan epitopes such as Lewis antigen. Compatibility of ABO(H) and other antigens between donor and recipient is necessary for a successful blood transfusion268,269. Some blood-group antigens affect the binding of bacteria and viruses to host cell surfaces and represent risk factors in chronic inflammatory diseases270–273. For example, the blood-group O phenotype is associated with disease severity in patients infected with Vibrio cholerae274. Human noroviruses, which cause sporadic and epidemic gastroenteritis, also selectively bind ABO(H) and Lewis blood-group antigens275; some viral strains recognize the A and/or B and H antigens, whereas others recognize only the Lewis and/or H antigens. Thus, susceptibility to these viruses is influenced by the host’s blood-group antigens and the sequence variations of the capsid protein276.
GlcNAc linked to Ser or Thr is typically found on intracellular glycoproteins present in nuclear, mitochondrial and cytoplasmic compartments (FIG. 1). Unlike the mucin-type O-glycans, which are GalN Ac-linked, addition of G lcN A c does not typically occur in the Golgi apparatus and is not extended; this synthesis is regulated through O-linked GlcNAc (O-GlcNAc) transferases (OGTs) and O-GlcNAcases (OGAs)10. Although different forms of these enzymes exist depending on the sub-cellular compartment, they all perform the same rapid cycle of addition and rem oval of GlcNAc from protein substrates. This dynamic process seem s to be unique to this glycosylation motif and is thought to regulate m any cellular functions, including cellular metabolism. Moreover, O-GlcNAc modification competes with protein phosphorylation at Ser and Thr residues, adding to the complexity of regulatory circuits11,12. This functional role of O-glycosylation can be controlled by the expression levels of the enzymes involved, as well as substrate concentrations. For the purposes of this Review, other glycoprotein conjugates will not be discussed in detail (reviewed in REFS4,13–15).
Glycosphingolipids.
GSLs comprise a sphingolipid to which a glycan is attached at the C1 hydroxyl position of a ceramide; they are one of the most abundant glycolipids in humans and are typically found in the lipid bilayers of cellular mem branes16 (FIG. 1). GSL glycosylation starts with the addition of glucose or galactose to the lipid moiety at the cytoplasmic side of the ER or the Golgi apparatus, but the structure is then flipped to the luminal side for further processing. The enzymes that initiate GSL glycosylation are specific for lipids, but further processing of the carbohydrate chain can be performed by more general glycosyltransferases. The distribution of different types of GSLs is controlled by functional competition between multiple glycosyltransferases16. GSLs perform critical cellular functions associated with the formation of lipid rafts, and their glycan com position imparts specific GSL properties16.
Proteoglycans and glycosaminoglycans.
Proteoglycans are glycoproteins in the extra cellular matrix that, in addition to containing canonical N-glycans and O-glycans, are characterized by the presence of long sugar repeats attached via O-linked glycosylation motifs17. These extended sugar chains are termed glycosaminoglycans and contribute to a substantial proportion of the proteoglycan’s molecular mass. Whereas N-glycans typically include 5–12 monosaccharides, a glycosaminoglycan motif can easily contain more than 80 sugars (for example, keratan sulfate is a poly-N-acetyllactosamine chain that contains up to 50 disaccharide units)16. These long chains are constructed through disaccharide repeats formed by GlcNAc or GalNAc, combined with an uronic acid (that is, glucuronic or iduronic acid) or galactose. Glycosaminoglycans are functionally diverse and include heparan sulfate, chondroitin sulfate, keratan sulfate and hyaluronan18. Glycosaminoglycans are crucial to the formation of the glycocalyx, an essential structure for the maintenance of the cell mem brane that also functions as a reservoir for sequestered growth factors18.
Extrinsic glycosylation events.
Extrinsic glycosylation is the process by which soluble glycan-modifying enzymes, such as glycosyltransferases that circulate in the blood, conjugate a monosaccharide to an existing sugar structure extracellularly19–21. Common acceptor structures for extrinsic glycosylation on mammalian glycoconjugates are galactose (G al)(β3)-GlcNAc, Gal(β3)-GalNAc, Gal(β4)-GlcNAc and GalNAc; β3 and β4 represent two different linkage positions on the conjugating sugar19–21. Circulating glycosyltransferases are generated by cleaving the soluble portion of the mem brane-associated enzyme and releasing it into the circulation; these soluble enzymes most often originate from liver hepatocytes and platelets. Platelets also contribute to extrinsic glycosylation processes by providing activated sugar intermediates, such as activated sialic acid, galactose and/or fucose19–21. Extrinsic glycosylation processes can generate Lewis and sialyl Lewis antigens, which are fucosylated carbohydrate moieties, as well as Tn and sialyl-Tn antigens, which are O-glycans with a terminal GalNAc or sialylated GalNAc, respectively. These glycosylation motifs have an important role in regulating leukocyte trafficking during inflammatory responses and in mediating the interactions between haematopoietic cells and their progenitors in the bone marrow22.
Congenital disorders of glycosylation
Genetic defects in glycosylation are often embryonic lethal, underlying the vital role of glycans23–32; C D G s are classified as type I and type II32. Type I C D G s are caused by abnormalities in the formation of the oligosaccharide structure on the glycolipid precursor before the attachment to the Asn residue of a protein (FIG. 2). Type II CDG s involve defects in the control of the N-linked branching structure on the nascent glycoprotein30,31.
CDGs are typically severe in their manifestations, as they affect m any muscular, developmental and neurological functions (TABLE 1). In fact, these disorders were originally discovered in children with previously unexplained multi-system disorders; detection of under-glycosylated serum transferrin in the affected children led to the identification of defective glycosylation as the cause for the disease33. The test used in the diagnosis of CDGs was origin ally devised to detect alcoholism on the basis of the hyposialylation of liver-derived serum transferrin in patients with alcoholism34. In some CDGs, the defect affects a single glycosylation step or pathway, whereas in other CDG s several pathways are affected. Depending on where the defect in glycosylation occurs, CDG phenotypes can result from altered activation, presentation or transport of sugar precursors; altered expression and/or activity of glycosidases or glycosyltransferases; and altered expression and/or activity of proteins that control the glycosylation machinery or maintain the Golgi apparatus.
Table 1 |.
Selected examples of congenital disorders of glycosylation
| CDGa or syndrome | Defective gene and protein | Affected pathway | Phenotypic outcome |
|---|---|---|---|
| ALG1-CDG | ALG1, β1,4-mannosyltransferase | N-glycan biosynthesis; ALG1 catalyses the first mannosylation step in the biosynthesis of the lipid-linked precursor oligosaccharide for N-glycosylation | Intellectual disability, hypotonia, seizures, microcephaly, infections and early death |
| B4GALT1-CDG | B4GALT1, β1,4-galactosyltrans-ferase 1 | Two alternative transcripts: one encodes a trans-Golgi-resident protein involved in glycoconjugate biosynthesis whereas the second transcript encodes a protein that forms soluble lactose synthase | Intellectual disability, developmental delay, hypotonia, macrocephaly, Dandy-Walker malformation, coagulopathy and myopathy |
| COG7-CDG | COG7, conserved oligomeric Golgi complex subunit 7 | Retrograde transport from the Golgi complex to the endoplasmic reticulum; involved in multiple glycosylation pathways | Hypotonia, microcephaly, growth retardation, adducted thumbs, failure to thrive, cardiac anomalies, wrinkled skin and early death |
| MPI-CDG | MPI, mannose-6-phosphate isomerase | Conversion of fructose-6-phosphate and mannose-6-phosphate; involved in multiple glycosylation pathways | Hepatic fibrosis, coagulopathy, hypoglycaemia, protein-losing enteropathy and vomiting; no neurological symptoms |
| NGLY1-CDG | NGLY1, PNGase | NGLY1 cleaves the β-aspartyl-glucosamine (GlcNAc) of the glycan and the amide side chain of Asn, converting Asn to Asp. This process assists in proteasome-mediated degradation of misfolded glycoproteins | Intellectual disability, developmental delay, seizures, abnormal liver function and hypolacrima or alacrima |
| PMM2-CDG | PMM2, phosphomannomutase 2 | Conversion of mannose-6-phosphate to mannose-1-phosphate; involved in multiple glycosylation pathways | Intellectual disability, hypotonia, seizures, strabismus, cerebellar hypoplasia, failure to thrive and cardiomyopathy; 20% lethality in the first 5 years of life |
| SRD5A3-CDG | SRD5A3, polyprenol reductase | SDR5A3 enzyme converts polyprenol into dolichol, a key step in the synthesis of dolichol-linked monosaccharide and the oligosaccharide precursors for N-glycosylation | Intellectual disability, hypotonia, eye and brain malformations, nystagmus, hepatic dysfunction, coagulopathy and ichthyosis |
| CHIME syndrome | PIGL,GlcNAc-PI de-N-acetylase | PIGL catalyses the second step of GPI biosynthesis, de-N-acetylation of GlcNAc-PI | Coloboma, congenital heart disease, ichthyosiform dermatosis, mental retardation and ear anomalies syndrome (CHIME) |
| GNE myopathy | GNE, bifunctional UDP-GlcNAc-2-epimerase/ManNAc kinase | Biosynthesis of sialic acid | Proximal and distal muscle weakness, wasting of the upper and lower limbs and selective sparing of the quadriceps |
| Walker-Warburg syndrome | Various genes, including POMT1 and POMT2 that encode two subunits of O-mannosyltransferase | Malfunction of various enzymes that modify α-dystroglycan | Defects in muscle, brain and eye development and elevated serum creatine kinase |
CDG, congenital disorder of glycosylation; GlcNAc, N-acetylglucosamine; GPI, glycosylphosphatidylinositol; ManNAc, N-acetylmannosamine; PI, phosphatidylinositol.
Current recommended nomenclature for CDGs is used, XX-CDG, in which XX defines the specific affected gene.
N - glycan - related CDGs.
PMM2-CDG is caused by mutations in PMM2, which encodes phosphoman - nomutase 2 (PMM2) and is the most common form of CDG; it can present with a neurological or multi-system phenotype29,35. PMM2 converts Man −6-phosphate (Man −6-P) to M an-1-P, a precursor for the synthesis of GDP-(Dol-P-Man). In turn, these com pounds are substrates for the mannosyltransferases involved in the synthesis of the lipid-bound precursor of N-glycans, Glc3Man9GlcNAc2-P-P-D ol29,35. The type of gene mutation affects the disease severity, which ranges from an embryonic lethal defect if the enzyme is completely inactive to mild cognitive impairment if the enzyme is still partially active29,35. A long-term follow-up of 75 patients with PMM2-CDG indicated that there were no significant changes in the overall clinical severity over time and that some bio chemical variables spontaneously improved35. Although there are currently no therapeutic options for patients with PMM2-CDG, new treatment strategies that involve the use of pharmacological chaperones to rescue PMM2 loss-of-function mutations are being explored36,37. This strategy is based on the rationale that increasing the stability of mutant enzymes, which often exhibit destabilizing and oligomerization properties, would improve the enzyme activity.
MPI-CDG, the second most common CDG, is caused by mutations in MPI, which encodes M an −6-P isomerase29. This enzyme is responsible for the interconversion of M an-6-P and fructose-6-phosphate. Man-6-P can also be generated directly by hexokinase-catalysed phosphorylation of M an, a pathway that is functional in patients with MPI-CDG. Thus, M an dietary supplementation is an effective treatment for MPI-CDG and is well tolerated27,38.
O-glycan-related CDGs.
Congenital muscular dystrophies, such as Walker-Warburg syndrome and muscle-eye-brain disease, involve abnormal Man O-glycosylation, primarily on α-dystroglycan39. O-Mannosylation of α-dystroglycan and similar proteins is initiated in the ER by the attachment of Man to a Ser or Thr residue, a reaction that is catalysed by the O-mannosyltransferase complex that contains POMT1 and POMT2 enzymes24–29. The Man linked to Ser or Thr is then further modified by multiple enzymes in the Golgi apparatus to generate an elongated glycan. The resultant polymeric glycan, matriglycan, is necessary for the binding of laminin and other extracellular matrix proteins to a-dystroglycan24,29. The disruption of this interaction alters the properties of cell mem branes and leads to the development of muscular dystrophy. Clinical manifestations of α-dystroglycanopathy frequently include alterations in the central nervous system and ocular disease manifestations, in addition to muscular dystrophy.
NGLY1 deficiency.
NGLY1 deficiency is an autosomal recessive disorder of the ER-associated protein degradation pathway. The level of protein loss (that is, loss of PNGase, encoded by NGLY1) correlates with neurological dysfunction, abnormal tear production and liver disease40; a nonsense mutation is associated with a particularly severe disease phenotype. PNGase is responsible for the translocation of misfolded proteins across the ER membrane into the cytosol for subsequent degradation by the proteasome41. A Drosophila model of PNGase deficiency led to the identification of various cellular processes associated with PNGase deficiency, including disruption of mitochondrial physiology, reduced cellular respiratory capacity and altered regulation of bone morphogenetic protein42–44. These new insights might lead to novel therapeutic approaches for NGLY1-CDG.
Current and future the rapies for CDG.
The design or improvement of a diagnostic test, or therapeutic option, for a CDG requires a clear understanding of its pathophysiological mechanism s. Dietary supplementation, such as M an therapy in MPI-CDG, can be very helpful as such approaches are generally inexpensive and broadly available. However, various dietary supplementation strategies have been tested in multiple CDG s and CDG disease models with varied results. Positive results were observed for dietary supplementation approaches in CAD-CDG, GNE-CDG, PGM1-CDG and SLC 35C1-CDG38. Other CDGs might benefit from a personalized medicine approach based on the identification of relevant genetic mutations in individual patients. Characterizing the underlying mechanism of a CDG at a molecular level should enable the development of new therapeutic approaches.
Glycans in immunity and inflammation
Cells of the immune system, similarly to all other cells, express cell surface-associated glycoproteins and glycolipids that, together with glycan-binding proteins and other molecules, sense environmental signals. Many immune receptors that are expressed on innate and adaptive immune cells recognize glycans found on the surface of microorganism s that are known as pathogen-associated molecular patterns. Examples of such glycan-containing molecules include bacterial lipopolysaccharides, peptidoglycans, teichoic acids, capsular polysaccharides and fungal mannans. The recognition of these glycosylated microbial patterns by the immune system has been exploited for the development of vaccines45,46; pneumococcal vaccines, for example, are formulated using a mixture of capsular polysaccharides47. The recent progress in H IV-1 vaccine development has also been driven by a better understanding of the HIV-1 envelope (Env) glycoprotein and the effects of its glycan composition on immune responses and immune evasion48–53. Moreover, the interactions between endothelial cells and leukocytes, which are crucial for leukocyte trafficking and recruitment to sites of tissue injury, are controlled by adhesion molecules that are in turn regulated by cellular glycosylation. Pro-inflammatory cytokines can also induce changes in cell-surface N-glycosylation of endothelial cells, suggesting that glycosylation might contribute to inflammatory vascular diseases54,55.
In the adaptive immune system, glycans also have crucial and multifaceted roles in B cell and T cell differentiation. These functions involve multiple cell-surface and secreted proteins (such as CD 43, CD 45, selectins, galectins and siglecs), different types of cell-cell interactions and the recognition of glycan-containing antigens56–58. The regulation of cellular glycosylation and its impact on the molecules that function as ligands and receptors during an inflammatory response is controlled through various mechanisms and is dependent on the inflammatory insult and its location. These mechanisms, which include ERK and p65 signalling, are critical to understanding the failure to control chronic inflammation in multiple disease states55. Immunoglobulins, for example, are crucial components of hum oral immunity, and altered glycosylation patterns of some immunoglobulin isotypes have been identified in chronic inflammatory, autoimmune and infectious diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and HIV infection59–68.
In fact, glycosylation patterns differentially affect the effector roles of immunoglobulins69–73. Below, we present several examples to illustrate various biological roles of glycans and glycan-recognizing molecules in B cell and T cell biology, including immunoglobulin effector functions.
CD43 and CD45.
The glycoproteins CD43 and CD45 are abundantly expressed on the surface of B cells and T cells and contain both O-glycans and N-glycans. Glycosylation of these proteins is modulated during cellular differentiation and activation, and regulates multiple T cell functions, including cellular migration, T cell receptor signaling, cell survival and apoptosis74,75. CD45 has an active receptor-like protein tyrosine phosphatase domain that interacts with Src family kinases in B cells and T cells to regulate the signalling threshold for the activation of B cell receptors (BCRs) and T cell receptors75–77. CD45 also has non-catalytic functions, for example, in modulating the function of the inhibitory co-receptor CD22 on B cells78. CD43 is involved in the multiple functions of lymphocytes and other cells of haematopoietic origin, including T cell adhesion and activation. CD43 has an elongated extracellular domain through which it interacts with multiple ligands, including ICAM1(CD54), major histocompatibility class I (MHCI), siglec1 (CD169), galectin 1 and E-selectin. Crosslinking of C D 43 with monoclonal antibodies can induce CD43 internalization, whereas phorbol ester-induced activation can lead to both protein internalization and proteolytic cleavage of the CD43 ectodomain; in T cells, CD43 shedding is linked to the regulation of apoptotic cell death79.
The glycans present in CD43 and CD45 (namely, their types, size and sites of attachment) are regulated by controlling the expression levels of specific glycosyltransferases and glycosidases. Alternative splicing of the gene that encodes CD45 creates further diversity as it enables the translation of several protein isoform s with different potential glycosylation sites74. The affinity of the interactions between glycosylated CD43 or CD45 ligands and their receptors is affected by the presence of core 2 O-glycans (that is, GlcNAcβ1 −6 (Galβ1–3) GalNAcαSer/Thr) versus core 1 O-glycans (that is, Galβ1–3GalNAcαSer/Thr); the presence of sialic acid is another important factor74.
Galectins.
Galectins are small soluble proteins that contain one or two carbohydrate-binding domains specific for galactose-containing glycans80. The members of this family of 15 carbohydrate-binding proteins are involved in m any processes in the immune system, including the regulation of T cell receptor signal strength as well as T cell and B cell death. For example, galectin 1 can induce apoptosis by binding to N-glycans or O-glycans present on C D 45 (REF81). Galectin 3 not only interacts with CD43 and CD45 but also binds to highly branched N-glycans on extracellular matrix glycoproteins such as laminin, fibronectin, vitronectin and integrin 1, thus affecting cellular adhesion80,82. In addition to binding to extracellular matrix proteins and cell-surface glycol-conjugates in a carbohydrate-dependent manner, galectins can also establish carbohydrate-independent interactions with cytosolic or nuclear targets82. Galectins interact with glycopeptides present on the cell surface through the formation of oligomers; oligomerization facilitates receptor clustering, lattice formation and cell-cell interactions. The binding affinity of galectins varies according to the type of glycan it interacts with, and changes in glycan characteristics can regulate the signals induced by galectin bin ding. For instance, the signal induced by the binding of galectin 1 to CD45 depends on several factors, including the CD45 isoform, which affects its glycosylation potential; the presence of core 2 O-glycans, which are high-affinity ligands for galectin 1; the com position of the N-glycan branching; and the extent of sialylation80. The addition of sialic acid is dependent on the relative expression of α2,6-sialyltransferases or α2,3-sialyltransferases, and the presence of α2,6-sialic acid on multi-antennary N-glycans prevents binding to galectin 1 (REF83). The multiple biological activities of galectins suggest these proteins are valuable potential therapeutic targets in inflammatory diseases and cancer; multiple galectin antagonists are currently under development84.
Siglecs.
Siglecs are sialic acid-binding proteins expressed on many cells of the immune system that perform various functions, including the regulation of antigen-specific immune responses and cell homing85. CD22 is one of 16 siglec proteins characterized in humans and is expressed on B cells, where it specifically binds a2,6-lin ked sialic acid-containing ligands; this interaction is crucial for the formation of nanoclusters in the cell mem brane that control BC R signalling following antigen binding86. Moreover, CD22 can act as a homing receptor, directing cells to tissues that express high amounts of α 2,6-linked sialic acids87. The CD22-CD22L interaction s are also essential for maintain in g self-tolerance, and CD 22-deficient m ice produce higher amounts of somatically mutated, high-affinity autoreactive IgG antibodies than wild-type controls88. Thus, it seems that CD22 is linked to the tight regulation of BCR signalling that maintains self-tolerance. Perturbations caused by CD22 deficiency might increase the likelihood of developing autoimmune diseases, and CD22 might be a therapeutic target for the treatment of autoimmune diseases such as SLE89. Importantly, the success of such a therapy would require a method for targeting CD22-mediated signalling on pathogenic autoreactive B cells without compromising the responses of pathogen-specific B cells.
Another important member of the siglec family is CD169 (also known as sialoadhesin or siglec 1), a macrophage adhesion molecule that binds sialic acid linked by an α2,3 bond to Gal on N-linked and O-linked glycans and glycolipids90–92. CD169 binds to sialic acid with low affinity; therefore, its ligands must be heavily sialylated and multimeric to enable an effective interaction93. This siglec mediates cell-cell interactions and the binding of immune cells to sialic acid-containing pathogens. CD169 is also used as a marker for a specific population of macrophages that not only have key roles in the initiation of antibacterial immune responses but are also involved in the transmission of some viruses and in the development of inflammation and several autoimmune diseases90,94–96.
Selectins.
The selectin family of proteins consists of E-selectin, P-selectin and L-selectin, which are mainly expressed on endothelial cells, platelets and leukocytes, respectively; these cell adhesion molecules are critical for leukocyte rolling on the endothelium before tissue extravasation97. Selectins recognize sialylated and fuco-sylated glycans but can also bind a subset of heparan sulfate glycosaminoglycans98. The finding that inhibition of selectin restored blood flow in a mouse model of sickle cell disease led to a trial of a small-molecule inhibitor of P-selectins and L-selectins, GMI 1070, in patients with sickle cell anaemia; the inhibitor reduced selectin-mediated cell adhesion and abrogated vascular occlusion and ‘sickle cell crisis’99. Another study demonstrated that inclacumab, a recombinant monoclonal antibody against P-selectin, reduces myocardial damage after a percutaneous coronary intervention in patients with non-ST-segment elevation myocardial infarction100. These examples demonstrate that targeting selectins may be beneficial in some inflammatory diseases.
Immunoglobulin glycosylation.
Immunoglobulin isotypes differ in the number of N-glycans present on their heavy chains62,64,66,73. Some immunoglobulins, such as IgA1 and IgD, also contain O-glycans, which are usually clustered in the hinge-region segments of these antibodies101,102. Immunoglobulin glycans impact the effector functions of antibodies depending on the b ranching of N-glycans and/or the term in al sugars of N-glycans or O-glycans, which include galactose and sialic acid. In fact, immunoglobulin glycosylation can determine whether an antibody glycoform is pro-inflammatory, such as IgG with galactose-deficient N-glycans, or anti-inflammatory, such as IgG with sialylated N-glycans.
All four human IgG subclasses have two variable biantennary glycans in their crystallizable fragment (Fc) region, attached at the conserved Asn297 site (FIG. 3a). Certain glycoforms of IgG, such as those deficient in sialic acid and galactose (Fig. 3b), are particularly abundant in some chronic diseases such as RA, SLE, inflammatory bowel disease (IBD), H IV and mycobacterial infections59–65; IgG1 galactose deficiency was also found in individuals with parasitic infection or asthma using population-wide immune activation studies66. Biochemical and immunological studies, as well as genome-wide association studies (GWAS), have identified specific genes, enzymes and pathways associated with the production of galactose-deficient IgG molecules; these include β-galactoside α2,6-sialyltransferase 1, cytokine-signalling adaptor gp 130 and T helper 17 (TH17) cell-dependent pathways67,68. IgG glycoengineering in vitro69 and in vivo70 confirmed the pathogenicity of IgGs with galactose-deficient (and therefore, also sialic acid-deficient) N-glycans. Conversely, sialylation of pathogenic IgG autoantibodies attenuated their pathogenic activity (Fig. 3c). In fact, N-glycosylation of the Fc region of IgG modulates its effector functions as it affects the binding efficiency of Fcγ recep tors (FcγR s)103–105. On the basis of these and other findings, glycoengineering of therapeutic antibodies and intravenous immunoglobulin (IVIG) has been used to produce therapeutic IgGs with tailored activity, such as sialylated IgG with anti-inflammatory properties71–73.
Fig. 3 |. Functional impact of variable Igg Fc glycan composition.
a | Immunoglobulin G (IgG) has two heavy and two light chains, and its crystallizable fragment (Fc) region can bind to Fcγ receptors and some proteins of the complement system. The IgG Fc region contains two N-glycans, one per heavy chain, attached at Asn297; these glycans contribute to the structural integrity of the Fc region and to its interactions with Fc receptors and complement. The Fc glycans in the IgG molecule are biantennary glycans with variable content of fucose (Fuc), bisecting N-acetylglucosamine (GlcNAc), galactose (Gal) and sialic acid; most IgG molecules are fucosylated. The glycan composition of IgG affects its biological activity; for example, Gal-deficient IgG glycoforms, which have been associated with chronic inflammatory diseases, can activate the lectin complement pathway. b | IgG glycoforms with Gal-deficient glycans are pro-inflammatory. c | IgG glycoforms with sialylated glycans are considered to be anti-inflammatory. Man, mannose.
Human IgA exists in two subclasses, IgA1 and IgA2. Both subclasses have several N-glycans in the Fc region, but IgA1 typically also has a cluster of 3–6 O-glycans in the hinge region (Fig. 4). IgA and IgM are found in the circulation and in mucosal secretions. Circulating IgA is predominantly monomeric IgA1 (~90%), whereas the remaining IgA is polymeric and consists of two or more monomers connected by a J chain. Secretory IgA is polymeric and includes the secretory component, a heavily N-glycosylated polypeptide that is derived from the polymeric immunoglobulin receptor and is added to IgA during transcytosis through mucosal epithelial cells106. IgA glycans have multiple biological roles, including glycan-mediated antigen-nonspecific binding to bacteria102,107. IgA1 has core 1 O-glycans (that is, GalNAc with β1,3-linked galactose), but in some diseases, including IgA nephropathy (IgAN), patients have elevated circulatory IgA1 with galactose-deficient O-glycans108.
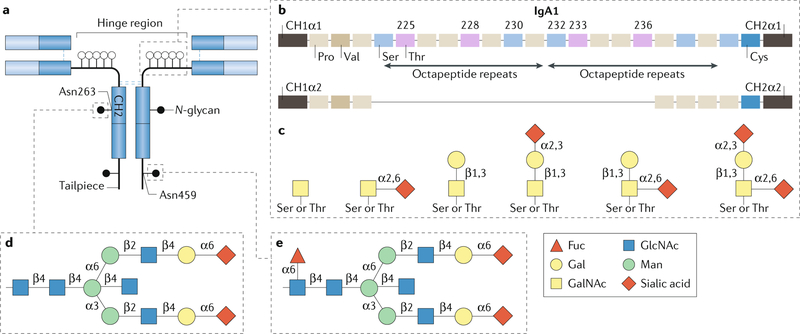
Fig. 4 |. Structure and glycosylation of human IgA.
Human immunoglobulin A (IgA) occurs in two subclasses, IgA1 and IgA2. a | The amino acid sequence is very similar for both subclasses, but the IgA1 heavy chain contains additional amino acids in the hinge region. Each heavy chain of IgA1 also contains two N-glycans, one in the CH2 domain (Asn263) and one in the tailpiece (Asn459). Although human IgA2 does not contain O-glycans, it can have more N-glycans per heavy chain than IgA1. b | The IgA1 hinge region is composed of two octapeptide repeats, which include nine Ser and Thr residues that are potential O-glycosylation sites; usually 3–6 of these residues are O-glycosylated and the most common IgA1 glycoforms have 4–5 O-glycans in the hinge region. c | The O-glycan composition of normal circulating human IgA1 is variable but usually consists of a core 1 disaccharide structure with N-acetylgalactosamine (GalNAc) in β1,3-linkage with galactose (Gal); each of these monosaccharides can be sialylated. d | The CH2 site of N-glycosylation contains digalactosylated biantennary glycans with or without a bisecting N-acetylglucosamine (GlcNAc), but it is not usually fucosylated. e | By contrast, the tailpiece N-glycosylation site contains fucosylated glycans. Fuc, fucose; Man, mannose.
Glycosylation in cancer
Mechanisms.
Tumour growth depends on the ability of cancer cells to bypass cellular division checkpoints, evade death signals and immune surveillance and migrate to metastatic sites; glycosylation has a role in all of these processes. For example, abnormal growth factor signalling is a critical component of cancer development that can be controlled by the specific glycosylation motifs on the relevant ligands, receptors and molecular scaffolding109. Glycosylation patterns were one of the first biomarkers of cancer and continue to be used to identify cells with stem-cell-like phenotypes, both within cancer and in healthy tissue110–112. Glycosylation changes that are often observed in cancer cells include an increase in sialyl Lewis structures, abnormal core fucosylation, increased N-glycan branching or exposure of the mucin-type O-glycan, Tn antigen. Many of these altered glycosylation patterns found in cancer have been termed ‘oncofetal’ as they resemble patterns often seen in early development113,114. As cancer cells evolve through multiple stages of disease, glycan composition can change in parallel with changes in cellular metabolism. This phenomenon includes incomplete synthesis, which refers to truncated glycosylation that produces the Tn antigen in O-glycans, and neo-synthesis, which produces abnormal glycosylation patterns such as sialyl Lewis X. These glyco-neoantigens, normally reserved for lymphocyte extravasation from the blood, are commonly found in cancer cells and facilitate metastatic spread115,116.
The number of studies concerning the functional implications of altered glycosylation in cancer has increased over the past decade, but the field still lacks good mechanistic studies of the processes that lead to cancer-associated changes in glycosylation. Proteins from the epidermal growth factor receptor family have both O-glycosylation and N-glycosylation sites that are modified in many types of cancer through altered expression of glycosyltransferase enzymes, such as polypeptide GALNT3 and Gal 3(4)-L-fucosyltransferase (FUT3). Receptor tyrosine-protein kinase erbB2 (also known as HER2) is overexpressed in a number of cancers, including prostate, breast and gastric cancers, and its functional properties are modulated through a range of complex post-translational modifications, including glycosylation117,118. Loss of GALNT3 can lead to increased production of pro-growth and pro-metastatic carbohydrate antigens, such as T and Tn antigens on HER2, whereas suppression of FUT3 can have an anti-metastatic and growth-suppressing effect by preventing Lewis Y antigen (LeY) production on HER2 (refs119,120). In addition, HER2 can also be regulated through glycoprotein GalNAc 3β-galactosyltransferase 1 (C1GalT1) activity, as increased mucin-type O-glycosylation enhances galectin 4 binding, which leads to HER2 activation118,121. Abnormal promoter region methylation in some glycosyltransferase genes, such as GALNT3, can also lead to aberrant enzyme expression122.
Currently, there is a dearth of studies in the cancer field that assess the intracellular mechanisms that drive changes in expression and activity of specific glycosyltransferases with respect to their targets. An exception is mucin 1 (MUC1), one of the most studied glycoconjugates in the cancer field, partly owing to its increased expression and altered glycan composition in many adenocarcinomas, and partly owing to its use as a cancer-vaccine antigen123–125. MUC1 contains a large extracellular domain (that is, the mucin domain) with five potential O-glycosylation sites in each of its multiple repeats that consist of ~20 amino acid residues. Studies of glycosyltransferase enzymes implicated the dysregulation of several enzymes in altering O-glycosylation of MUC1, including C1GalT1, β-galactoside α−2,3-sialyltransferase 1 (ST3GalI) and α-GalNAc α−2,6-sialyltransferase 1 (ST6GalNAcI); altered localization of GALNTs in the ER also has a role in changing MUC1 glycosylation126–129. GALNTs typically initiate O-glycosylation in the Golgi apparatus, but in cell-culture models these enzymes can translocate to the ER via a process that involves aberrant Src signalling, leading to an increased density of O-glycosylation of MUC1 repeats128,130; MUC1 produced by cancer cells also exhibits increased sialyl-Tn antigen129,131,132. In addition, altered glycosylation motifs on MUC1 can affect cancer immune surveillance, typically through co-opting cell-surface lectins such as CD169, which enhances macrophage activation after binding to sialylated MUC1 and promotes tumour growth133–135.
The glycosylation of specific proteins in serum and/or tumour tissues can be used as a diagnostic biomarker and to assess patient prognosis and responses to treatment; examples of such glycoproteins include CEA, MUC1, MUC16 and prostate-specific antigen (PSA)136–141. Carbohydrate antigen 19–9 (CA19–9) is detected with a specific mouse monoclonal antibody that recognizes the sialyl Lewis A (sLea) carbohydrate motif (that is, Neu5Acα2,3Galβ1,3(Fucα1,4)GlcNAcβ1-R) on a monosialoganglioside first identified in gastrointestinal cancer142. Synthesis of this sLea antigen is controlled by Lewis, a gene involved in ABO(H) blood-group determinants143 (Box 1). CA19–9 is often, but not always, elevated in the serum of patients with a variety of cancers, including pancreatic, gastric and colorectal cancers; the mechanisms that lead to this increase in serum levels in cancer are poorly understood, but it seems to be associated with dysregulated sialyltransferases, such as Gal-β−1,3-GalNAc-α−2,3-sialyltransferase 2 and 4 (ST3GalII and ST3GalIV, respectively)144. The presence of CA19–9 across multiple cancers highlights abnormal glycosylation as a fundamental feature of cancer pathobiology136,143.
Another important glycosylation pattern is the α2,6-linked sialic acid in N-glycans, which is commonly elevated in pancreatic and colon cancers and has pro-tumour effects; this sialic acid is added to the glycan backbone by β-Gal α−2,6-sialyltransferase 1 (ST6GalI), also upregulated in these cancers145–147 (Fig. 5). Increased expression of ST6GalI is associated with pro-survival pathways, and its sialylated products can inhibit tumour cell apoptosis and activate growth factor pathways110,111,148–150.
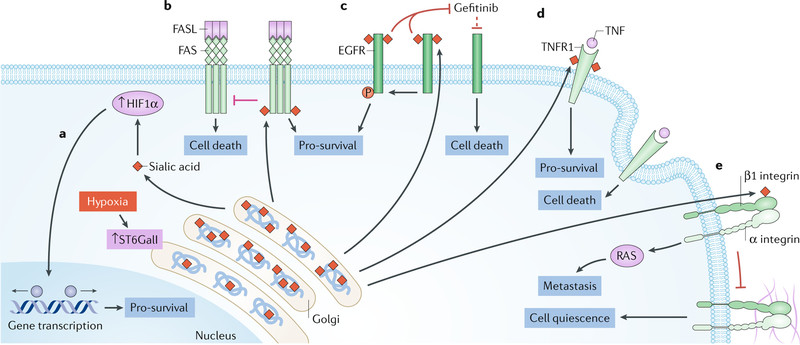
Fig. 5 |. ST6galI and abnormal sialylation in cancer.
N-glycans with terminal α2,6-sialylation are synthesized by the β-galactoside α−2,6-sialyltransferase 1 (ST6GalI). Both upregulation of ST6GalI and increase in α2,6-sialylation are observed in many types of cancers and are associated with negative patient outcomes. In fact, α2,6-sialylation is involved in the regulation of many key proteins that are known to contribute to cell survival and metastasis in cancer. a | Hypoxia increases the expression and activity of ST6GalI, leading to enhanced α2,6-sialylation; in turn, increased sialylation upregulates HIF1α and the expression of pro-survival HIF1α target genes, such as growth factors and glucose transporters. b | The death receptor FAS, also known as CD95, is a target of ST6GalI, and its sialylation inhibits the initiation of apoptotic signalling and subsequent receptor internalization. Increased expression of ST6GalI prevents FAS ligand (FASL)-induced apoptosis through FAS. c | Increased expression of ST6GalI in cancer cell lines enhances the α2,6-sialylation of epidermal growth factor receptor (EGFR), which increases its tyrosine kinase activity and the phosphorylation of its targets279. α2,6-Sialylation enhances the activity of EGFR, both at baseline and after cell activation, and leads to increased activation of pro-growth and survival genes. Moreover, cells in which ST6GalI is overexpressed, leading to enhanced α2,6-sialylation, are protected against cell death induced by the anticancer drug gefitinib, an EGFR inhibitor. d | In cells with low ST6GalI expression and reduced α2,6 sialylation, prolonged activation of tumour necrosis factor (TNF) receptor 1 (TNFR1) by TNF leads to receptor internalization, caspase activation and cell death. This apoptotic cell death pathway is prevented by enhanced α2,6-sialylation of TNFR. e | α2,6-Sialylation of β1 integrin in the Golgi apparatus by ST6GalI results in hypersialylation, which inhibits β1 integrin binding to matrix proteins such as type I collagen and fibronectin and prevents downstream signalling. These signals maintain cell quiescence, and their disruption due to enhanced sialylation leads to increased cell motility and invasion, which promotes cancer cell metastasis.
Glycome profiles.
Although the use of a single biomarker for the diagnosis and monitoring of disease progression is an attractive prospect, research shows that whole glycome profiles might be better than a single glycosylation pattern for the assessment of disease progression. In one study, glycome profiling of prostate cancer biopsy samples enabled the identification of indolent versus metastatic disease with 91% accuracy; conventional assessment was 72% accurate151. Matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) of hepatocellular carcinoma biopsy samples identified increases in fucosylation across a panel of N-glycosylation structures in 95% of patients; the specific glycome profiles correlated with median patient survival time152. Transcriptional analysis of melanoma biopsy samples indicated that expression of β1, 6-N-acetylglucosaminyltransferase (GCNT2) in these samples was decreased compared with those of healthy epidermal melanocytes. This decrease led to a decrease in Asn-linked I-branched glycans (that is, glycans that have additional GlcNAc-Gal branches, also termed ‘adult I’ blood-group antigen), a novel marker of metastatic melanoma progression153. Collectively, these studies highlight the advantages of whole glycome profiling over the detection of traditional single markers of glycosylation in cancer.
Xenotransplantation
The major bottleneck for organ transplantation continues to be the shortage of available viable organs. Xenotransplantation (for example, from pig donors) is a proposed solution for this problem154 but it has been hindered by several factors, including the presence of donor carbohydrate antigens that are not present in humans and can trigger immune rejection. Examples of such antigens include the Gal-α(1,3)-Gal epitope present on glycoproteins and glycolipids and N-glycolylneuraminic acid (Neu5Gc).
The Gal-α(1,3)-Gal epitope is synthesized by an α1,3-galactosyltransferase in New World primates and many non-primate mammals. However, the gene that encodes this enzyme, GGTA1, is inactivated in humans and Old World primates, which leads to the production of antibodies that recognize Gal-α(1,3)-Gal-containing glycoconjugates155. It is thought that these antibodies are induced by Gal-α(1,3)-Gal-containing compounds present in the microbiota and from dietary sources. The antibodies bind to vascular Gal-α(1,3)-Gal antigens of xenotransplants and induce complement-mediated endothelial cell cytotoxicity that results in graft rejection156. Importantly, deletion of GGTA1 from the pig genome revealed the presence of additional endothelial cell xenoantigens that could contribute to graft rejection156.
Neu5Gc is another relevant xenoantigen, and it is synthesized by CMP-N-acetylneuraminic acid hydroxylase. This enzyme is present in most mammals, including pigs, but it is inactive in humans, although Neu5Gc can be obtained from dietary sources and metabolically incorporated into many human glycoconjugates157,158. Neu5Gc, also termed Haganutzui–Deicher antigen, has been identified as the antigen in horse serum that causes serum sickness in humans159. A third example of an important xenoantigen found in pig tissues is terminal GalNAc, also known as the SDa blood-group antigen, which is synthesized by β1,4-N-acetylgalactosaminyltransferase 2, encoded by B4GALNT2. Although SDa is expressed in various human cells, anti-SDa IgM is commonly detected in human serum160.
It is hoped that modern approaches based on CRISPR–Cas-mediated gene editing might be used to remove these xenoantigens and aid the development of animal tissues and organs that are suitable for transplantation in humans155,157.
Autoimmunity and chronic inflammation
The immune system routinely recognizes and responds to foreign carbohydrate epitopes, such as lipopolysaccharides; however, loss of tolerance and autoimmunity might also occur, for example, if the glycosylation patterns of the host and the pathogen overlap, perhaps owing to abnormal enzymatic activity. Changes in the glycosylation patterns of proteins can also result in immune detection of these neo-glycan epitopes and lead to autoimmunity. In addition, IgG effector functions are controlled by N-glycosylation. Altered sialylation, galactosylation and/or fucosylation, as well as changes in glycan composition, can contribute to immune dysregulation and a range of autoimmune and chronic inflammatory diseases.
Rheumatoid arthritis.
RA is an autoimmune disease that results in chronic inflammation of joints and other associated tissues; it is characterized by the presence of serum rheumatoid factor and anti-cyclic citrullinated peptide antibodies161. Early studies of collagenous tissues from patients with RA found considerable immune aggregates consisting of IgG and IgA162. Moreover, IgG from patients with RA has overall and site-specific changes in glycosylation that affect the composition of Fc-associated and antigen-binding fragment (Fab)-associated glycans. In addition to an increased proportion of Gal-deficient Fc glycans, Fab-portion glycans contain high amounts of bisecting GlcNAc and core fucose163. Changes in the glycosylation of antigen-specific IgGs precede disease onset, and the degree of these changes correlates with disease severity164,165. Altogether, these findings suggest that changes in the glycosylation of IgG are a critical component of RA pathogenesis.
Inflammatory bowel disease.
IBD refers to chronic inflammatory diseases that affect the gastrointestinal tract and includes Crohn’s disease and ulcerative colitis. In IBD, both decreased secretion of mucins and structural changes to mucins themselves can occur; the mucins of the gastrointestinal tract form a physical barrier between the intestinal microbiota and the intestinal epithelium166. Chronic inflammation in the gastrointestinal tract is thought to involve gut microbiota-induced changes in the glycosylation patterns of the host that result in enhanced entry of bacteria or dietary lectins into the host tissue167–170. This process leads to increased inflammation that can lead to ulcer development and cancer171. In addition to potential changes in glycosylation associated with alterations in gut mucosa and the epithelium, changes in N-glycosylation of IgG are also observed. Patients with IBD have decreased galactosylation of IgG N-glycans, and the extent of under-galactosylation correlates with disease severity60. Specific changes in antibody glycosylation could be used to discriminate between patients with ulcerative colitis and those with Crohn’s disease172.
Systemic lupus erythematosus.
SLE is a systemic autoimmune disease characterized by the presence of polyreactive autoantibodies that bind to different host targets, including proteins, nucleic acids and their complexes. Similarly to other autoimmune diseases, reduced galactosylation and sialylation of IgG are associated with SLE63,173. Furthermore, a decrease in core fucosylation and an increase in bisecting GlcNAc of N-glycans is observed. Together, these findings show that alterations in the IgG glycome in SLE limit the negative feedback loops that rely on complete IgG glycosylation and promote an inflammatory glycan profile. It is unknown whether the changes in glycosylation of IgG associated with SLE correlate with a loss of B cell tolerance and the production of autoreactive antibodies. Interestingly, treatment with sialylated IgG protected against disease symptoms in a mouse model of SLE174.
Tn syndrome.
Originally called permanent mixed-field polyagglutinability, Tn syndrome is a rare blood disorder characterized by the presence of Tn antigens on all haematopoietic cell lineages175. In this syndrome, the Tn antigen-containing glycoconjugates are recognized by IgM antibodies, which leads to varying degrees of anaemia, leukopenia and thrombocytopenia in affected patients. Tn syndrome is a disorder of O-glycosylation in which Tn antigen is over-represented owing to a decrease in the galactosylation of terminal GalNAc by C1GalT1. This syndrome is an acquired and permanent disease that can occur in males and females at any age175 and results from a clonal somatic mutation in COSMC (also known as C1GALT1C1), which encodes a molecular chaperone specific for C1GalT1 (refs176,177). Mutations in COSMC reduce the amount of active C1GalT1, thus decreasing GalNAc galactosylation.
Granulomatosis with polyangiitis.
Patients with granulomatosis with polyangiitis (GPA; formerly called Wegener granulomatosis) often have circulating IgG anti-neutrophil cytoplasmic antibodies (ANCAs), typically specific for proteinase 3; these antibodies are thought to activate resident and/or infiltrating macrophages in the vasculature, leading to inflammation. GPA affects small-to-medium-sized blood vessels; therefore, the lung and kidney are particularly vulnerable to macrophage-mediated damage. Several studies reported that abnormal glycosylation of the IgG Fc region (namely, low galactosylation and sialylation) correlates with driving the enhanced macrophage activation. Anti-proteinase 3 IgG antibodies, which are significantly elevated in patients with GPA, are less sialylated than IgG from healthy individuals178–180. These hyposialylated autoantibodies lead to enhanced macrophage activation when compared with antibodies with normal sialylation, and increased levels of hyposialylated autoantibodies are associated with disease activity178–181.
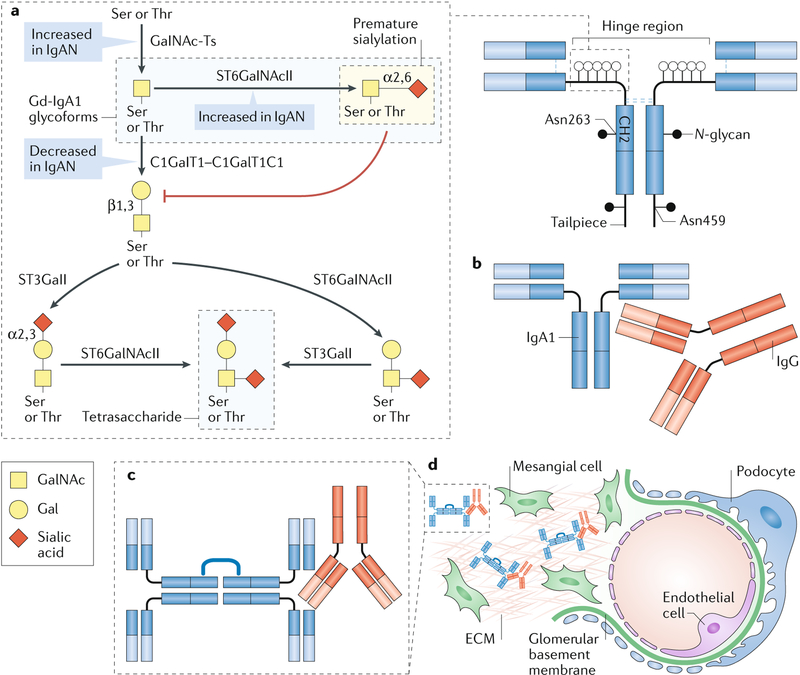
IgA nephropathy.
IgAN is a unique autoimmune disease in which the autoantigen is an antibody itself — specifically, IgA1 antibodies that contain galactose-deficient O-glycans and terminal GalNAc182 (Fig. 6). GWAS183,184 and biochemical studies185,186 identified an association between several glycosyltransferases, and related proteins, with galactose deficiency of IgA1 in IgAN. In fact, patients with IgAN have elevated levels of serum IgA1 with altered mucin-type O-glycans in the hinge region; serum levels of galactose-deficient IgA1 and the corresponding autoantibodies are predictive of disease progression182 (Figs 4,6). Disease pathogenesis is not completely understood but is thought to involve altered expression and activity of key enzymes in IgA1-secreting cells, which, in genetically susceptible individuals, lead to the production of IgG autoantibodies. Consequently, circulating immune complexes form in blood, some of which deposit in the mesangium and activate mesangial cells, leading to renal injury; approximately half of patients with IgAN progress to end-stage renal disease182 (Fig. 6).
Fig. 6 |. Aberrant O-glycosylation in IgA nephropathy.
Immunoglobulin A (IgA) nephropathy (IgAN) is an autoimmune disease that is thought to result from a four-hit process. a | Hit 1: increased production of circulating galactose (Gal)-deficient IgA1 (Gd-IgA1), in which some O-glycans do not contain Gal. The first step in the glycosylation of the hinge region of IgA1 is the addition of N-acetylgalactosamine (GalNAc) to Ser or Thr residues by a GalNAc-transferase (GalNAc-T) to form the terminal GalNAc (Tn) antigen. Premature sialylation of the terminal GalNAc by α-GalNAc α−2, 6-sialyltransferase 2 (ST6GalNAcII) can block subsequent glycosylation; however, most often GalNAc is galactosylated by the glycosyltransferase GalNAc 3β-galactosyltransferase 1 (C1GalT1). The chaperone C1GalT1C1 is required for appropriate expression and function of C1GalT1. After galactosylation, GalNAc, Gal or both sugars may be sialylated; ST6GalNAcII adds sialic acid to GalNAc and β-galactoside α−2,3-sialyltransferase 1 (ST3GalI) sialylates Gal; the largest glycan of circulatory IgA1 is a tetrasaccharide. In many patients with IgAN, the number of O-glycosylated residues in the hinge region of IgGA1 is also increased82,83. Increased initiation of glycosylation by GalNAc-Ts, premature sialylation of GalNAc by ST6GalNAcII and decreased galactosylation by C1GalT1 might each contribute to the formation of Gd-IgA1, the key autoantigen in IgAN. b | Hit 2: production of IgG autoantibodies that are specific for Gd-IgA1. c | Hit 3: formation of circulating Gd-IgA1–IgG immune complexes. For simplicity, IgA1 is shown as a dimer; IgA1 monomers are the main circulating molecular form, but polymeric IgA1 is the predominant molecular form of Gd-IgA1 in the circulation. d | Hit 4: glomerular deposition of immune complexes. Other serum proteins, such as complement, are likely to be involved in the formation of the pathogenic immune complexes that are deposited in the glomeruli, activate resident mesangial cells and cause renal injury. ECM, extracellular matrix.
In IgAN, several factors might contribute to increased levels of Tn and sialylated Tn (sTn) antigens on IgA1, including decreased expression of C1GalT1 or C1GalT1C1 as well as increased expression of ST6GalNAcII, leading to premature sialylation of GalNAc185 (Fig. 6). Alternatively, changes in the activity of GALNTs might alter the sites and/or densities of initial GalNAc attachment and thereby contribute to the formation of Tn and sTn antigens. Technological advances in glycoconjugate analysis have enabled researchers to recognize that there is restricted heterogeneity in the glycosylation pattern of the IgA1 hinge region, which not only varies between patients but can also vary within individuals187 (Box 2; Supplementary Table 2). Together, these mechanisms demonstrate the critical role of correct glycan composition during glycoprotein production and demonstrate that altered glycosylation can result in the presentation of glycan autoantigens and the onset of autoimmunity.
Box 2 |. Techniques for glycoconjugate analysis.
Over the past two decades, many different technologies have been developed for the analyses of glycans and glycoconjugates. Many of the initial glycan analyses were carried out using lectins that recognize a specific glycan or glycans, often combined with pretreatment with glycosidases to selectively remove monosaccharide residues or larger components of extended glycoconjugates. For more detailed analysis, mass spectrometric techniques are now commonly used for global and individual molecular analyses. The advent of chemo-enzymatic techniques for the synthesis of glycans has driven the more widespread use of glycan array technologies that enable the simultaneous screening of hundreds of individual glycan structures. Improved glycan synthesis has also enabled the development of glycan-specific antibodies, which have become more readily available. Standard ‘-omics’ technologies have been applied to the study of patterns of gene expression and levels of glycan synthetic enzymes to help identify novel clinical disorders that involve altered glycans. In terms of 3D structural analysis of glycans, NMR has been used heavily throughout the years as a means of understanding the shape of glycoconjugates in solution; molecular dynamics simulations have complemented these structural analyses by modelling glycans on protein structures. There are now many different techniques at the disposal of researchers for the analysis of glycoconjugates. Supplementary Table 2 provides a list of methodologies used for the analysis of glycoconjugates, examples of the techniques and reagents used, and referenced examples of how these methods have been applied in biomedical research.
Diabetes mellitus.
Diabetes mellitus is commonly characterized by excess glucose in the blood, which predictably leads to various glycosylation abnormalities in patients with both type 1 and type 2 diabetes. The most widely used marker for monitoring the long-term management of diabetes is glycated haemoglobin (HbA1c), which is a surrogate for the average blood glucose levels in the previous 3 months188–190. Non-enzymatic glycosylation (also known as glycation) based on reactions between haemoglobin and glucose can produce multiple glycoforms, but, in the case of HbA1c, it requires a multistep reaction between the amino-terminal Asp on the haemoglobin β-chain and a condensation reaction at the 1-hydroxyl of the glucose molecule followed by an Amadori rearrangement191–193. A similar non-enzymatic glycosylation process produces advanced glycation end products, which are glycated proteins and lipids that are elevated in diabetes and are linked to disease pathology194,195.
O-GlcNAc glycosylation has an important role in many cellular control mechanisms in general and in diabetes specifically, including the cellular response to insulin196; it is catalysed by OGT, which adds GlcNAc to Ser and/or Thr residues197–199. OGT uses UDP-GlcNAc as a substrate, which is a product of the hexosamine biosynthesis pathway. This pathway functions in part as a glucose sensor and regulates cellular responses to insulin by controlling the levels of UDP-GlcNAc-mediated glycosylation of targets related to insulin activity200,201. The hexosamine biosynthesis pathway is highly responsive to glucose levels, and its flux is significantly increased in some tissues of patients with diabetes, leading to increased levels of UDP-GlcNAc and, thus, elevated O-GlcNAc glycosylation202–204. For example, O-GlcNAc glycosylation of the transcription factor NeuroD1 is crucial for its nuclear translocation, which leads to insulin production by pancreatic β-cells in response to high glucose levels205. Long-term exposure to high glucose increases O-GlcNAc modifications and, thus, its biological effects; in the context of diabetes, increased O-GlcNAc glycosylation of proteins, such as AKT, can lead to enhanced β-cell death206,207.
Cardiomyocytes can be damaged by high blood glucose levels, leading to the high rates of systolic or diastolic dysfunction in patients with diabetes (~16.9%)208; in animal models of diabetes, contractility deficits in the heart are associated with O-GlcNAc glycosylation levels209. In a rat model of streptozotocin-induced diabetes, ventricular arrhythmias were associated with O-GlcNAc modification of the sodium channel protein type 5 subunit-α (HH1). Decreasing O-GlcNAc addition to HH1 during high-glucose stress improved the function of this sodium channel210. In addition, using an adenovirus injection to the heart to increase the expression of protein OGA, the enzyme that removes O-GlcNAc adducts, improved cardiomyocyte contractility and decreased O-GlcNAc-protein levels in the same model of diabetes211. However, in a model of myocardial ischaemia–reperfusion injury, decreased O-GlcNAc-protein levels were associated with increased infarct size in diabetic mice when compared with controls. Recovery of O-GlcNAc levels via miR-24 activation of OGT prevented the increase in infarct size in diabetic mice212. In the context of cardiac function, appropriate regulation of O-GlcNAc glycosylation is crucial as, depending on the cardiac insult, glycosylation that is either increased by OGT or reduced by OGA might be beneficial.
N-glycosylation defects can also cause diabetes, as demonstrated by a mouse model in which inactive N-acetylglucosaminyltransferase-IVa (GnT-IVa) led to impaired insulin release and hyperglycemia213. A mass spectrometric analysis of glycosylated proteins in the kidneys of two mouse models of diabetes, caused by leptin receptor-deficiency (db/db) or by administration of streptozotocin, showed significant differences in general protein N-glycosylation between diabetic mice and healthy controls, with some similarities between the two models of disease214. Another study of serum samples from 818 patients with diabetic kidney disease reported that HbA1c positively correlated with the degree of complex and branched N-glycan content on IgG215. In addition, hyposialylated IgG, which is often present in the serum of patients with type 2 diabetes216, activates endothelial FcγRIIb, which leads to insulin resistance in obese mice. Further studies are required to understand the mechanisms underlying N-glycosylation changes in diabetes.
Glycans and glomerular filtration
Glycans have many roles in physiological kidney function, including several major roles in glomerular filtration. The most relevant glycoconjugates are found in the glycocalyx of the fenestrated glomerular endothelial cells, in the glomerular basement membrane (GBM), formed by extracellular matrix proteins, and in the podocyte slit diaphragm (Fig. 7).
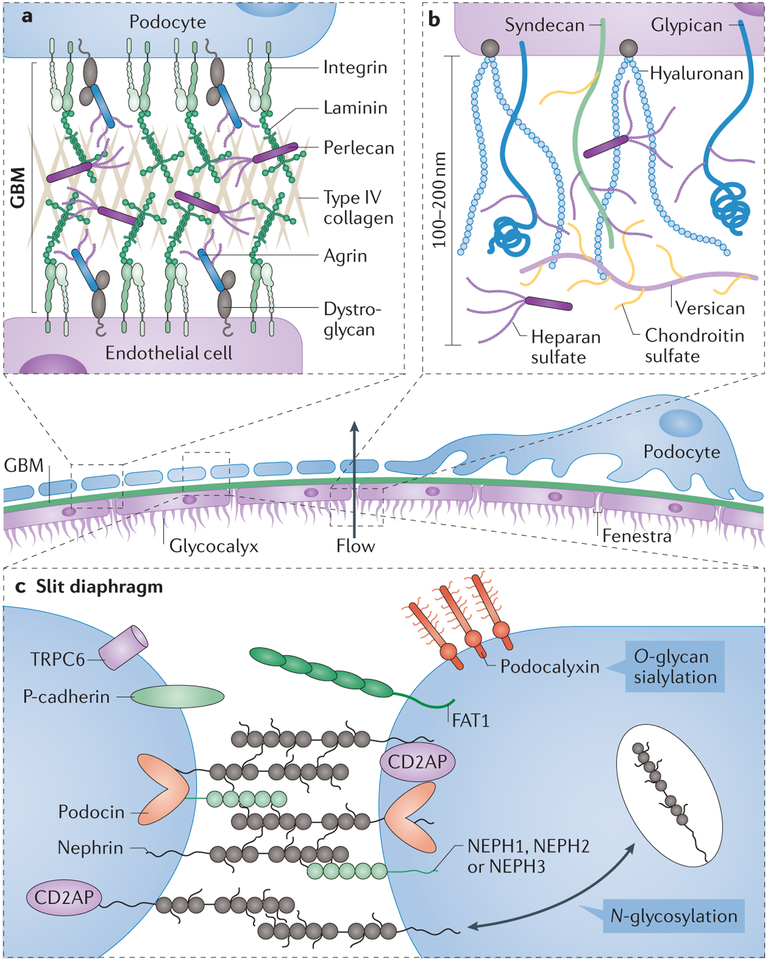
Fig. 7 |. Glycoconjugates and glomerular filtration.
Glomerular filtration occurs in the glomerulus, and endothelial cells and podocytes are the principal cells involved in this process. Mesangial cells have a key role in maintaining and supporting the endothelial and podocyte glomerular filtration barrier, in part through the production of extracellular matrix proteins, and in regulating blood pressure. a | The glomerular basement membrane (GBM) is found at the interface between endothelial cells and podocytes; the GBM contains a network of type IV collagen, negatively charged heparan sulfate proteoglycans, laminins and several other extracellular matrix proteins280. Loss of proteoglycans that contain heparan sulfate, such as agrin or perlecan, occurs in some glomerular diseases, including minimal change disease and membranous nephropathy. Glycosylation of membrane-associated α-dystroglycan has an important role in the interaction between the cells that line the GBM and the extracellular matrix through its interaction with laminin. b | The endothelial glycocalyx extends far from the plasma membrane of the glomerular endothelial cells, creating a physical barrier of glycoproteins, glycosaminoglycans and proteoglycans across the fenestrae. The glycan components of the glycocalyx have a key role in maintaining its mechanical and structural integrity and enabling its proper function as part of the glomerular filtration unit. Hyaluronan extends from the cell surface, whereas chondroitin sulfate and heparin sulfate are attached to extracellular matrix proteins, such as versican and perlecan, or membrane proteins such as syndecan and glypican. Together, these molecules make a dense negatively charged glycocalyx. c | The structure of the slit diaphragm, the key component of the glomerular molecular filter, relies heavily on the cell-surface adhesion protein nephrin; appropriate N-glycosylation of nephrin is critical for its surface expression and function. Sialylation of the cell-surface sialoglycoprotein podocalyxin also has a key role in podocyte morphogenesis and structural integrity. Many other cell-surface glycoproteins are involved in the formation of the slit diaphragm, including P-cadherin, podocin, nephrin-like proteins 1–3 (NEPH1–NEPH3), protocadherin fat 1 (FAT1) and transient receptor protein 6 (TRPC6). CD2-associated protein (CD2AP) is an adaptor molecule that can bind to the cytoplasmic domain of nephrin.
The first barrier that blood encounters in the glomeruli is the fenestrated endothelium, which determines the glomerular filtration rate217,218. The fenestrated endothelium also acts as a crucial barrier that prevents proteins from entering Bowman’s space; however, the pore size of the fenestrated endothelium cannot alone prevent albumin and other macromolecules from entering Bowman’s space217,218. Rather, the endothelial glycocalyx, which covers the endothelial fenestrae, probably contributes to the high permselectivity of glomeruli219. The glycocalyx is composed of negatively charged glycoproteins, glycosaminoglycans and membrane-associated and secreted proteoglycans that form an interlinking network of proteins and glycans.
One of the most evident roles of glycans in renal function relates to the formation and maintenance of the GBM, which connects the basal lamina of the fenestrated endothelial cells to the foot processes of podocytes. The GBM consists of three layers: the lamina rara interna, which is adjacent to the endothelial cells and is composed of negatively charged heparan sulfate proteoglycans; the lamina densa, a dark central zone composed of collagen IV and laminin; and the lamina rara externa, adjacent to podocyte foot processes and composed of heparan sulfate proteoglycans. The heavily sulfated glycans of proteoglycans, such as agrin and perlecan, are responsible for the negative charge of the GBM, which may have a role in regulating complement activation in the glomeruli220. Loss of heparan sulfate chains from agrin is observed in several glomerulopathies, including lupus nephritis, membranous nephropathy, minimal change disease and diabetic kidney disease, suggesting a prominent role for heparan sulfate in GBM function221. Although multiple mechanisms might account for this decrease in heparan sulfate, increased activity of heparanase, an endo-β(1–4)-D-glucuronidase that degrades heparan sulfate, has been observed in both rodent models and patients with diabetic kidney disease, minimal change disease, membranous nephropathy and IgAN222,223; heparanase treatment dramatically increases the permeability of the GBM224. In addition to heparan sulfate, O-mannosyl glycans of α-dystroglycan mediate the interface between podocyte foot processes and the GBM through direct interactions with agrin and laminin 11 (ref.225). Deglycosylated α-dystroglycan can no longer bind agrin, which might lead to the detachment of podocytes from the GBM and impaired glomerular filtration in vivo226.
Glycans also have important roles in the formation of the slit diaphragm, a specialized intracellular junction between adjacent podocyte foot processes that is formed by a complex network of proteins and mediates intracellular and extracellular interactions227. Nephrin is a membrane glycoprotein expressed on the surface of podocytes at the intersection of podocyte–podocyte foot processes228; it functions as an adhesion and scaffolding receptor and a signalling molecule. N-glycosylation of up to ten potential glycosylation sites is critical for the appropriate folding, transport, surface expression and function of nephrin229,230. In fact, N-glycan defects in nephrin result in poorly formed slit diaphragms and compromised kidney function231,232.
Mutations in a key enzyme involved in sialic acid biosynthesis, bifunctional UDP-N-GlcNAc 2-epimerase/N-acetylmannosamine (ManNAc) kinase, cause glomerular proteinuria in mice, which can be rescued with ManNAc administration. Analysis of renal tissue sections from these mice showed segmental splitting of the GBM and effacement of podocyte foot processes as well as decreased sialylation of O-glycans in the podocalyxin protein233. Podocalyxin is a sialoglycoprotein and a major constituent of the glycocalyx of podocytes in the glomerulus, where the negatively charged sialic acid is believed to separate adjacent foot processes and retain space for glomerular filtration234. In vivo, removal of α2,6-linked sialic acid from the glomerular filtration barrier results in proteinuria and renal failure, along with the apparent formation of irreversible tight junctions between adjacent podocytes235.
Glycomedicine: glycans in therapeutics
Developments in the field of glycobiology have enabled the development of a variety of glycan-based therapeutics (Table 2). For example, envelope glycoprotein gp120 is expressed on the surface of HIV-1, and its variable glycosylation facilitates viral escape from immune detection236,237. Adding new glycan-dependent epitopes to the recombinant gp120 used for vaccination increased the ability of broadly neutralizing monoclonal antibodies to recognize HIV-1, suggesting that this approach can be used to optimize vaccination protocols and antigens238,239. Moreover, HIV-1 envelope glycoproteins not only differentiate HIV-1 clades but can also be used to estimate the efficacy of vaccine regimens on the basis of antibody binding to a panel of gp120 glycan-dependent epitopes240.
Table 2 |.
glycan-based therapeutics currently available or under development
| Disease | Treatment | Outcome | Refs |
|---|---|---|---|
| Glycan-supplementation therapeutics | |||
| PGM1-CDG | Oral D-galactose supplementation | Improved liver function and normalized glycosylation markers and UDP-galactose levels | 38 |
| MPI-CDG | Oral mannose supplementation | Significant reduction in disease activity | 38 |
| CAD-CDG | Oral uridine supplementation | Significant reduction in disease activity | 38 |
| Vaccine therapeutics | |||
| Non-metastatic castration-resistant prostate cancer | Recombinant Tn-rmMUC1 peptide vaccine | PSA doubling time improved in phase l and phase II clinical trials | 281 |
| Breast cancer | MUC1 glycopeptidevaccine | Clinical trials in progress to evaluate immunogenicityand anticancer effects | 125 |
| HIV-1 | Increased high-mannose glycans of gp120 antigen | Enhanced binding of broadly neutralizing antibodies to HIV gp120 | 239 |
| Streptococcus pneumoniae | Bacterial strain-specific polysaccharide vaccine | Increased production and affinity of antigen-specific IgG in mouse model | 47 |
| Therapeutic antibodies | |||
| Adult T cell lymphoma | Defucosylated anti-CCR4 antibody | Overall response rate of 50% | 249 |
| Rheumatoid arthritis | Aglycosylated anti-CD4 antibody | Modest reduction in disease activity | 257 |
| Type 1 diabetes | Aglycosylated anti-CD3 antibody | Moderate efficacy to stabilize insulin secretion levels over time | 255 |
CCR4, CC-chemokine receptor 4; IgG, immunoglobulin G; PSA, prostate-specific antigen; rmMUC1, recombinant mucin 1; Tn, terminal N-acetylgalactosamine.
As outlined previously, glycosylation plays a critical role in regulating functional immune responses through complex receptor–glycan motif interactions. This aspect is now being exploited in immunoglobulin therapies241–243. One study found that IgG can create hexameric structures on the cell surface to increase Fc-mediated complement activation244. This information led to the creation of an hexamer composed of fused Fc segments from IgG; this multimer, termed Hexa-Fcs, enhanced Fc receptor avidity, which is co-dependent on IgG glycosylation. Furthermore, addition of new N-glycosylation sites to Hexa-Fcs increased the overall sialic acid content, which could potentially enhance its immunomodulatory function245. Specifically, enhanced sialylation of IgG Fc glycans decreases complement-mediated cytotoxicity by reducing C1q binding to IgG1 Fc246; these observations have important implications for the treatment of patients using IVIG therapy.
Glycan-dependent IgG effector functions are recognized as critical components of therapeutic antibodies in cancer247, which are now tailored through glycoengineering. For example, removal of fucose increases antibody-dependent cell-mediated cytotoxicity, which is very important for certain antibodies used to treat patients with cancer248,249. Increased galactosylation of the Fc in rituximab, an anti-CD20 antibody, enhances complement-dependent cytotoxicity250,251. Moreover, IgG with elevated content of α2,6-linked sialic acid has an anti-inflammatory effect, a property that could be exploited for the treatment of autoimmune diseases252,253. This anti-inflammatory activity is dependent on binding of CD209 (also known as DC-SIGN) on dendritic cells to sialylated IgG, which leads to the upregulation of FcγRIIb and regulatory T (Treg) cell expansion252,253. In addition to the modification of N-glycosylation motifs, the effector function of antibodies that lack glycosylation (that is, aglycosylated IgG) is also being investigated254. Some examples of aglycosylated biologics are mogamulizumab, an anti-CD194 for treatment of T cell lymphoma; otelixizumab, an anti-CD3 for the treatment of type 1 diabetes; and MTRX-1011 A, an anti-CD4 for the treatment of RA249,255–258.
The therapeutic use of sugars, or drugs that directly target glycosylation, has eluded clinical application, with the exception of the use of heparan to prevent blood clotting and the use of nutritional supplements to alleviate the symptoms of certain CDGs, as discussed earlier. Targeting the glycosylation pathways of galectin 1 with drugs such as anginex259 and OTX001 (ref.260) has also been tested for the reduction of angiogenesis in cancer; preliminary results showed a modest decrease in angiogenesis after treatment with anginex, whereas OTX001 had a better effect and is now in phase I clinical trials. Ex vivo treatment of T cells from patients with ulcerative colitis with addition of GlcNAc increased levels of N-glycosylation branches on the T cell receptor and led to a decrease in pro-inflammatory cytokines261. Thioglycosides, which are S-glycoside GlcNAc mimetics, have been studied for their anti-inflammatory effects along with O-glycosides and shown to significantly decrease sialyl Lewis X levels on endothelial cells and E-selectin-induced leukocyte rolling262. In vitro, thioglycosides also blocked SGLT1 and SGLT2 (also known as SLC5A1 and SLC5A2, respectively), which are targeted in diabetes to prevent the reabsorption of glucose in the kidney263.
An animal model of minimal change disease revealed that angiopoietin-related protein 4 (ANGPTL4) is hyposialylated and has a causative role in proteinuria. Oral treatment with the sialic acid precursor, ManNAc, rescued the hyposialylation of ANGPTL4 and prevented proteinuria264,265. In a mouse model of unilateral ureteral obstruction, glucosamine hydrochloride supplementation protected against renal fibrosis; glucosamine hydrochloride decreased N-glycosylation of transforming growth factor-β (TGFβ) receptor type 2 (TGFR2), the receptor for TGFβ1, thus inhibiting its translocation to the cell surface membrane and initiation of pro-fibrotic pathways266. Most therapeutics that target glycosylation pathways and/or synthetic saccharides for the treatment of non-CDG pathologies are still at an experimental stage, but they hold considerable promise given the key role of glycosylation in numerous pathobiologies.
Conclusions
Glycosylation is a common modification of proteins and lipids that involves non-templated dynamic and complex processes. Glycans have multiple crucial roles in cellular responses to environmental stimuli as well as cellular growth and differentiation; specific changes in glycan composition are directly linked to many diseases. Technological advances are beginning to overcome many of the challenges posed by the complexities of glycoconjugates, improving our understanding of the physiological and pathological processes that are regulated by glycans (Box 2). Such efforts are further supported by improvements in research tools as well as training in glycosciences1,267, both of which facilitate the advancement of glycomedicine, in which glycobiology is applied to the development of novel therapies.
Supplementary Material
Key points.
Glycosylation is critical for physiological and pathological cellular functions; advances in analytical techniques have driven progression in the field of glycobiology over the past decade.
Congenital disorders of glycosylation have provided considerable insight into basic mechanisms underlying the associations of specific glycoconjugates with disease phenotypes.
Interactions between immune cells that are mediated by cell surface molecules and drive cellular activation are regulated by the glycosylation motifs of membrane-bound glycoconjugates and their binding to sugar-specific receptors.
Cancers often exhibit oncofetal phenotypes that are reflected in the nature of their glycoconjugates; these changes in glycosylation drive metastatic properties, inhibition of apoptosis and resistance to chemotherapy.
The pathogenesis of many autoimmune diseases, such as immunoglobulin A (IgA) nephropathy, systemic lupus erythematosus and inflammatory bowel disease, involves abnormal glycosylation of one or more glycoproteins; diabetes involves abnormal O-linked N-acetylglucosamine-mediated signalling and enhanced glycation of multiple proteins.
Immunoglobulin glycosylation controls the effector functions of antibodies, which creates opportunities for the therapeutic application of glycoengineering.
Acknowledgements
The authors’ work was supported in part by grants from the US National Institutes of Health (DK106341, DK078244, GM098539, DK082753, DK109599, DK079337 and DK105124) and a gift from the IGA Nephropathy Foundation of America. The authors apologize to their colleagues in the field whose work is not adequately discussed or cited in this Review owing to space limitations.
Glossary
- Glycans
Saccharides or sugar chains that can be free or attached to proteins or lipids toform simple or complex glycoconjugates
- N-linked glycans
Branched protein glycans attached through a nitrogen atom of Asn residues at Asn-X-Ser/Thr motifs
- O-linked glycans
Diverse protein glycans typically attached to an oxygen atom of Ser or Thr residues
- Glycosaminoglycans
Long unbranched polysaccharides made of repeating disaccharide units often attached to proteins
- Glycosylphosphatidylinositol (GPI) anchors
Short glycolipids that link proteins to the cell membrane
- C-mannosylation
The attachment of an α-mannopyranosyl to the indole-C2 carbon of a Trp residue
- Glycosphingolipids
(GSLs). Sphingolipids with attached glycan moieties that exist in the cell membrane
- Glycosyltransferases
Enzymes that add glycans
- Glycosidases
Enzymes that remove glycans
- High-mannose N-glycan
A less-processed N-glycan with high mannose content
- Hybrid N-glycan
A partially processed N-glycan with mannose and one antenna containing N-acetylglucosamine
- Complex N-glycan
Processed N-glycan with two, three or four antennas and possibly with bisecting N-acetylglucosamine
- Basic core structures
For N-glycans, the basic core structure is a common pentasaccharide GlcNAc2Man3. For O-glycans, cores 1–4 are defined on the basis of the glycans attached to the initial N-acetylgalactosamine (GalNAc); for example, core 1 is GalNAc with p 1,3-linked galactose
- Glycocalyx
A cell-surface layer of glycosaminoglycans, proteoglycans and glycoproteins that extends far from the cell membrane
- Activated sugar intermediates
Monosaccharides with high-energy donors attached, such as UDP-sugar, GDP-sugar or, in the case of sialic acid, CMP-sialic acid
- Dandy-Walker malformation
A brain malformation that occurs during embryonic development of the cerebellum (linked to movement, coordination, cognition and behaviour) and the fourth ventricle (which channels fluid from inside to around the outside of the brain)
- Coloboma
An eye abnormality that occurs before birth and refers to missing pieces of tissue in structures that form the eye, such as the iris, retina, choroid or optic disc
- Multi-antennary N-glycans
N-linked glycans with multiple branching glycan structures (that is, three or more antennas)
- J chain
Joining chain that links monomers of immunoglobulin A (IgA) or IgM toform polymeric IgA or polymeric IgM
- Monosialoganglioside
A ganglioside, which is a ceramide with a carbohydrate motif, that contains a single sialic acid
- Rheumatoid factor
Autoantibodies specific for the crystallizable fragment (Fc) of immunoglobulin G (IgG) that are often present in the blood of patients with rheumatoid arthritis
- Condensation reaction
A reaction in which two molecules combine, releasing an H2o in the process
- Amadori rearrangement
A rearrangement reaction catalysed by a base or acid on the N-glycoside of an aldose to a 1-amino-1-deoxy-ketose
- Hexosamine biosynthesis
Nutrient-sensing pathway that converts fructose-6-phosphate to UDP-N-acetylgalactosamine.z
Footnotes
Competing interests
J.N. and M.B.R. are co-founders of Reliant Glycosciences, LLC. The other authors declare no competing interests.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewer information
Nature Reviews Nephrology thanks M. Wild and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Supplementary information
Supplementary information is available for this paper at https://doi.org/10.1038/s41581-019-0129-4.
References
- 1.Varki A Biological roles of glycans. Glycobiology 27, 3–49 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides a comprehensive overview of glycobiology and the various roles of glycans.
- 2.Varki A et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 25, 1323–1324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laine RA A calculation of all possible oligosaccharide isomers both branched and linear yields 1.05 × 10(12) structures for a reducing hexasaccharide: the Isomer Barrier to development of single-method saccharide sequencing or synthesis systems. Glycobiology 4, 759–767 (1994). [DOI] [PubMed] [Google Scholar]
- 4.Spiro RG Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology 12, 43R–56R (2002). [DOI] [PubMed] [Google Scholar]
- 5.Gagneux P & Varki A Evolutionary considerations in relating oligosaccharide diversity to biological function. Glycobiology 9, 747–755 (1999). [DOI] [PubMed] [Google Scholar]
- 6.Stanley P, Taniguchi N & Aebi M in Essentials of Glycobiology (ed. Varki A et al. ) 99–111 (2017). [Google Scholar]; This book chapter discusses the fundamentals of N-glycan synthesis, conjugation and location.
- 7.Brockhausen I & Stanley P in Essentials of Glycobiology (ed. Varki A et al. ) 113–123 (2017). [Google Scholar]
- 8.Bennett EP et al. Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family. Glycobiology 22, 736–756 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasudevan D & Haltiwanger RS Novel roles for O-linked glycans in protein folding. Glycoconj. J 31, 417–426 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachara N, Akimoto Y & Hart GW in Essentials of Glycobiology (ed. Varki A et al. ) 239–251 (2017). [Google Scholar]
- 11.van der Laarse SAM, Leney AC & Heck AJR Crosstalk between phosphorylation and O-GlcNAcylation: friend or foe. FEBS J. 285, 3152–3167 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Chiaradonna F, Ricciardiello F & Palorini R The nutrient-sensing hexosamine biosynthetic pathway as the hub of cancer metabolic rewiring. Cells 7, E53 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manya H & Endo T Glycosylation with ribitol-phosphate in mammals: new insights into the O-mannosyl glycan. Biochim. Biophys. Acta 1861, 2462–2472 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Lukose V, Walvoort MTC & Imperiali B Bacterial phosphoglycosyl transferases: initiators of glycan biosynthesis at the membrane interface. Glycobiology 27, 820–833 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferguson MA The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J. Cell Sci.112, 2799–2809 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Schnaar RL & Kinoshita T in Essentials of Glycobiology (ed. Varki A et al. ) 125–135 (2017). [Google Scholar]
- 17.Iozzo RV & Schaefer L Proteoglycan form and function: a comprehensive nomenclature of proteoglycans. Matrix Biol. 42, 11–55 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindahl U, Couchman J, Kimata K & Esko JD in Essentials of Glycobiology (ed. Varki A et al. ) 207–221 (2017). [Google Scholar]
- 19.Wandall HH et al. The origin and function of platelet glycosyltransferases. Blood 120, 626–635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MM et al. Platelets support extracellular sialylation by supplying the sugar donor substrate. J. Biol. Chem 289, 8742–8748 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manhardt CT, Punch PR, Dougher CWL & Lau JTY Extrinsic sialylation is dynamically regulated by systemic triggers in vivo. J. Biol. Chem 292, 13514–13520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee-Sundlov MM et al. Circulating blood and platelets supply glycosyltransferases that enable extrinsic extracellular glycosylation. Glycobiology 27, 188–198 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadat MA et al. Glycosylation, hypogammaglobulinemia, and resistance to viral infections. N. Engl. J. Med 370, 1615–1625 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen L et al. A glycogene mutation map for discovery of diseases of glycosylation. Glycobiology 25, 211–224 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeze HH, Eklund EA, Ng BG & Patterson MC Neurological aspects of human glycosylation disorders. Annu. Rev. Neurosci 38, 105–125 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monticelli M, Ferro T, Jaeken J, Dos Reis Ferreira V & Videira PA Immunological aspects of congenital disorders of glycosylation (CDG): a review. J. Inherit. Metab. Dis 39, 765–780 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Witters P, Cassiman D & Morava E Nutritional therapies in congenital disorders of glycosylation (CDG). Nutrients 9, E1222 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This review discusses the pathobiology of CDGs and the use of therapeutic sugars, including the mechanisms of nutraceutical intervention.
- 28.Ng BG & Freeze HH Perspectives on glycosylation and its congenital disorders. Trends Genet. 34, 466–476 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeze HH, Schachter H & Kinoshita T in Essentials of Glycobiology (ed. Varki A et al. ) 569–582 (2017). [Google Scholar]
- 30.Peanne R et al. Congenital disorders of glycosylation (CDG): quo vadis? Eur. J. Med. Genet 61, 643–663 (2017). [DOI] [PubMed] [Google Scholar]
- 31.Hennet T & Cabalzar J Congenital disorders of glycosylation: a concise chart of glycocalyx dysfunction. Trends Biochem. Sci 40, 377–384 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Al Teneiji A et al. Phenotypic and genotypic spectrum of congenital disorders of glycosylation type I and type II. Mol. Genet. Metab 120, 235–242 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Yamashita K et al. Sugar chains of serum transferrin from patients with carbohydrate deficient glycoprotein syndrome. Evidence of asparagine-N-linked oligosaccharide transfer deficiency. J. Biol. Chem 268, 5783–5789 (1993). [PubMed] [Google Scholar]
- 34.Stibler H, Borg S & Allgulander C Clinical significance of abnormal heterogeneity of transferrin in relation to alcohol consumption. Acta Med. Scand 206, 275–281 (1979). [DOI] [PubMed] [Google Scholar]; This publication demonstrates for the first time the relationship between alcohol abuse, clinical phenotype and abnormal glycosylation of transferrin.
- 35.Witters P et al. Long-term follow-up in PMM2-CDG: are we ready to start treatment trials? Genet. Med 10.1038/s41436-018-0301-4 (2018). [DOI] [PubMed] [Google Scholar]
- 36.Yuste-Checa P et al. Pharmacological chaperoning: a potential treatment for PMM2-CDG. Hum. Mutat 38, 160–168 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Gamez A et al. Protein misfolding diseases: prospects of pharmacological treatment. Clin. Genet 93, 450–458 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Brasil S et al. CDG therapies: from bench to bedside. Int. J. Mol. Sci 19, E1304 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beltran-Valero de Bernabe D et al. Mutations in the O-mannosyltransferase gene POMT1 give rise to the severe neuronal migration disorder Walker-Warburg syndrome. Am. J. Hum. Genet 71, 1033–1043 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article presents the original report in which a POMT1 gene mutation was identified as the cause for Walker–Warburg syndrome.
- 40.Enns GM et al. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulumassociated degradation pathway. Genet. Med 16, 751–758 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang L & Ten Hagen KG Enzymatic insights into an inherited genetic disorder. eLife 6, e31127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Owings KG, Lowry JB, Bi Y, Might M & Chow CY Transcriptome and functional analysis in a Drosophila model of NGLY1 deficiency provides insight into therapeutic approaches. Hum. Mol. Genet 27, 1055–1066 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong J et al. Mitochondrial function requires NGLY1. Mitochondrion 38, 6–16 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Galeone A et al. Tissue-specific regulation of BMP signaling by Drosophila N-glycanase 1. eLife 6, e27612 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsubara N et al. CD22-binding synthetic sialosides regulate B Lymphocyte proliferation through CD22 ligand-dependent and independent pathways, and enhance antibody production in mice. Front. Immunol 9, 820 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuai R et al. Dual TLR agonist nanodiscs as a strong adjuvant system for vaccines and immunotherapy. J. Control. Release 282, 131–139 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Polonskaya Z et al. T cells control the generation of nanomolar-affinity anti-glycan antibodies. J. Clin. Invest 127, 1491–1504 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwong PD & Mascola JR HIV-1 vaccines based on antibody identification, B cell ontogeny, and epitope structure. Immunity 48, 855–871 (2018). [DOI] [PubMed] [Google Scholar]; This review discusses how to overcome glycan epitope shielding to generate broadly neutralizing antibodies, one of the major hurdles in HIV vaccine development.
- 49.Crispin M, Ward AB & Wilson IA Structure and immune recognition of the HIV glycan shield. Annu. Rev. Biophys 47, 499–523 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore PL The neutralizing antibody response to the HIV-1 Env protein. Curr. HIV Res 16, 21–28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asbach B & Wagner R Particle-based delivery of the HIV envelope protein. Curr. Opin. HIV AIDS 12, 265–271 (2017). [DOI] [PubMed] [Google Scholar]
- 52.Medina-Ramirez M, Sanders RW & Sattentau QJ Stabilized HIV-1 envelope glycoprotein trimers for vaccine use. Curr. Opin. HIV AIDS 12, 241–249 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward AB & Wilson IA The HIV-1 envelope glycoprotein structure: nailing down a moving target. Immunol. Rev 275, 21–32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chacko BK, Scott DW, Chandler RT & Patel RP Endothelial surface N-glycans mediate monocyte adhesion and are targets for anti-inflammatory effects of peroxisome proliferator-activated receptor gamma ligands. J. Biol. Chem 286, 38738–38747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott DW, Vallejo MO & Patel RP Heterogenic endothelial responses to inflammation: role for differential N-glycosylation and vascular bed of origin. J. Am. Heart Assoc 2, e000263 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfert MA & Boons GJ Adaptive immune activation: glycosylation does matter. Nat. Chem. Biol 9, 776–784 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schnaar RL Glycobiology simplified: diverse roles of glycan recognition in inflammation. J. Leukoc. Biol 99, 825–838 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giovannone N et al. Galectin-9 suppresses B cell receptor signaling and is regulated by I-branching of N-glycans. Nat. Commun 9, 3287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Biermann MH et al. Sweet but dangerous - the role of immunoglobulin G glycosylation in autoimmunity and inflammation. Lupus 25, 934–942 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Go MF, Schrohenloher RE & Tomana M Deficient galactosylation of serum IgG in inflammatory bowel disease: correlation with disease activity. J. Clin. Gastroenterol 18, 86–87 (1994). [DOI] [PubMed] [Google Scholar]
- 61.Moore JS et al. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19, 381–389 (2005). [DOI] [PubMed] [Google Scholar]
- 62.Tomana M, Schrohenloher RE, Koopman WJ, Alarcon GS & Paul WA Abnormal glycosylation of serum IgG from patients with chronic inflammatory diseases. Arthritis Rheum. 31, 333–338 (1988). [DOI] [PubMed] [Google Scholar]
- 63.Tomana M, Schrohenloher RE, Reveille JD, Arnett FC & Koopman WJ Abnormal galactosylation of serum IgG in patients with systemic lupus erythematosus and members of families with high frequency of autoimmune diseases. Rheumatol. Int 12, 191–194 (1992). [DOI] [PubMed] [Google Scholar]
- 64.Rademacher TW, Williams P & Dwek RA Agalactosyl glycoforms of IgG autoantibodies are pathogenic. Proc. Natl Acad. Sci. USA 91, 6123–6127 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Zeben D et al. Early agalactosylation of IgG is associated with a more progressive disease course in patients with rheumatoid arthritis: results of a follow-up study. Br. J. Rheumatol 33, 36–43 (1994). [DOI] [PubMed] [Google Scholar]
- 66.de Jong SE et al. IgG1 Fc N-glycan galactosylation as a biomarker for immune activation. Sci. Rep 6, 28207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pfeifle R et al. Regulation of autoantibody activity by the IL-23-TH17 axis determines the onset of autoimmune disease. Nat. Immunol 18, 104–113 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lauc G et al. Loci associated with N-glycosylation of human immunoglobulin G show pleiotropy with autoimmune diseases and haematological cancers. PLOS Genet. 9, e1003225 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ohmi Y et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun 7, 11205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that sialylation of anti-citrullinated protein IgG autoantibodies in RA produced IgG with anti-pathogenic properties.
- 70.Pagan JD, Kitaoka M & Anthony RM Engineered sialylation of pathogenic antibodies in vivo attenuates autoimmune disease. Cell 172, 564–577 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Zhu Z, Chen W, Feng Y & Dimitrov DS Crystallizable fragment glycoengineering for therapeutic antibodies development. Front. Immunol 8, 1554 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bruckner C, Lehmann C, Dudziak D & Nimmerjahn F Sweet SIGNs: IgG glycosylation leads the way in IVIG-mediated resolution of inflammation. Int. Immunol 29, 499–509 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Lin CW et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl Acad. Sci. USA 112, 10611–10616 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that specific glycans of IgG affect binding kinetics to various Fc receptors to induce antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity.
- 74.Clark MC & Baum LG T cells modulate glycans on CD43 and CD45 during development and activation, signal regulation, and survival. Ann. NY Acad. Sci 1253, 58–67 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Justement LB The role of the protein tyrosine phosphatase CD45 in regulation of B lymphocyte activation. Int. Rev. Immunol 20, 713–738 (2001). [DOI] [PubMed] [Google Scholar]
- 76.Saunders AE & Johnson P Modulation of immune cell signalling by the leukocyte common tyrosine phosphatase, CD45. Cell Signal. 22, 339–348 (2010). [DOI] [PubMed] [Google Scholar]
- 77.Jackson SM et al. Key developmental transitions in human germinal center B cells are revealed by differential CD45RB expression. Blood 113, 3999–4007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coughlin S et al. An extracatalytic function of CD45 in B cells is mediated by CD22. Proc. Natl Acad. Sci. USA 112, E6515–E6524 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Modak M et al. Engagement of distinct epitopes on CD43 induces different co-stimulatory pathways in human T cells. Immunology 149, 280–296 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thiemann S & Baum LG Galectins and immune responses — just how do they do those things they do? Annu. Rev. Immunol 34, 243–264 (2016). [DOI] [PubMed] [Google Scholar]
- 81.Perillo NL, Pace KE, Seilhamer JJ & Baum LG Apoptosis of T cells mediated by galectin-1. Nature 378, 736–739 (1995). [DOI] [PubMed] [Google Scholar]
- 82.Johannes L, Jacob R & Leffler H Galectins at a glance. J. Cell Sci. 131, jcs208884 (2018). [DOI] [PubMed] [Google Scholar]
- 83.Earl LA, Bi S & Baum LG N-and O-glycans modulate galectin-1 binding, CD45 signaling, and T cell death. J. Biol. Chem 285, 2232–2244 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dings RPM, Miller MC, Griffin RJ & Mayo KH Galectins as molecular targets for therapeutic intervention. Int. J. Mol. Sci 19, E905 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Varki A, Schnaar RL & Crocker PR in Essentials of Glycobiology (ed. Varki A et al. ) 453–467 (2017). [Google Scholar]
- 86.Ereno-Orbea J et al. Molecular basis of human CD22 function and therapeutic targeting. Nat. Commun 8, 764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou JY, Oswald DM, Oliva KD, Kreisman LSC & Cobb BA The glycoscience of immunity. Trends Immunol. 39, 523–535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Keefe TL, Williams GT, Batista FD & Neuberger MS Deficiency in CD22, a B cell-specific inhibitory receptor, is sufficient to predispose to development of high affinity autoantibodies. J. Exp. Med 189, 1307–1313 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clark EA & Giltiay NV CD22: a regulator of innate and adaptive B cell responses and autoimmunity. Front. Immunol 9, 2235 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sewald X et al. Retroviruses use CD169-mediated trans-infection of permissive lymphocytes to establish infection. Science 350, 563–567 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that CD169 facilitates retroviral infection in macrophages via gangliosides on the viral surface.
- 91.Asano K et al. Intestinal CD169+ macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun 6, 7802 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Perez OA et al. CD169+ macrophages orchestrate innate immune responses by regulating bacterial localization in the spleen. Sci. Immunol 2, eaah5520 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fraschilla I & Pillai S Viewing Siglecs through the lens of tumor immunology. Immunol. Rev 276, 178–191 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hammonds JE et al. Siglec-1 initiates formation of the virus-containing compartment and enhances macrophage-to-T cell transmission of HIV-1. PLOS Pathog. 13, e1006181 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rose T et al. SIGLEC1 is a biomarker of disease activity and indicates extraglandular manifestation in primary Sjögren’s syndrome. RMD Open 2, e000292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Eakin AJ et al. Siglec-1 and −2 as potential biomarkers in autoimmune disease. Proteomics Clin. Appl 10, 635–644 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Lasky LA Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu. Rev. Biochem 64, 113–139 (1995). [DOI] [PubMed] [Google Scholar]
- 98.Norgard-Sumnicht K & Varki A Endothelial heparan sulfate proteoglycans that bind to L-selectin have glucosamine residues with unsubstituted amino groups. J. Biol. Chem 270, 12012–12024 (1995). [DOI] [PubMed] [Google Scholar]
- 99.Wun T et al. Phase 1 study of the E-selectin inhibitor GMI 1070 in patients with sickle cell anemia. PLOS ONE 9, e101301 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stahli BE et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention according to timing of infusion: insights from the SELECT-ACS trial. J. Am. Heart Assoc 5, e004255 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Woof JM & Mestecky J in Mucosal Immunology 4th edn (eds Mestecky J et al. ) (2015). [Google Scholar]
- 102.Royle L et al. Secretory IgA N− and O-glycans provide a link between the innate and adaptive immune systems. J. Biol. Chem 278, 20140–20153 (2003). [DOI] [PubMed] [Google Scholar]
- 103.Ha S et al. Isolation and characterization of IgG1 with asymmetrical Fc glycosylation. Glycobiology 21, 1087–1096 (2011). [DOI] [PubMed] [Google Scholar]
- 104.Okazaki A et al. Fucose depletion from human IgG1 oligosaccharide enhances binding enthalpy and association rate between IgG1 and FcgammaRIIIa. J. Mol. Biol 336, 1239–1249 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Shibata-Koyama M et al. The N-linked oligosaccharide at FcγRIIIa Asn-45: an inhibitory element for high FcγRIIIa binding affinity to IgG glycoforms lacking core fucosylation. Glycobiology 19, 126–134 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woof JM & Mestecky J Mucosal immunoglobulins. Immunol. Rev 206, 64–82 (2005). [DOI] [PubMed] [Google Scholar]
- 107.Nakajima A et al. IgA regulates the composition and metabolic function of gut microbiota by promoting symbiosis between bacteria. J. Exp. Med 215, 2019–2034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lai KN et al. IgA nephropathy. Nat. Rev. Dis. Primers 2, 16001 (2016). [DOI] [PubMed] [Google Scholar]
- 109.Ferreira IG et al. Glycosylation as a main regulator of growth and death factor receptors signaling. Int. J. Mol. Sci 19, E580 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schultz MJ et al. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 6, 25 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schultz MJ et al. The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 76, 3978–3988 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nairn AV et al. Regulation of glycan structures in murine embryonic stem cells: combined transcript profiling of glycan-related genes and glycan structural analysis. J. Biol. Chem 287, 37835–37856 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holmes EH, Ostrander GK, Clausen H & Graem N Oncofetal expression of Lex carbohydrate antigens in human colonic adenocarcinomas. Regulation through type 2 core chain synthesis rather than fucosylation. J. Biol. Chem 262, 11331–11338 (1987). [PubMed] [Google Scholar]
- 114.Pinho SS & Reis CA Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015). [DOI] [PubMed] [Google Scholar]; This paper provides a comprehensive review of major glycosylation motifs that contribute to cancer pathobiology and/or are used for detection and diagnosis.
- 115.Hakomori S Tumor-associated glycolipid antigens defined by monoclonal antibodies. Bull. Cancer 70, 118–126 (1983). [PubMed] [Google Scholar]
- 116.Oliveira-Ferrer L, Legler K & Milde-Langosch K Role of protein glycosylation in cancer metastasis. Semin. Cancer Biol 44, 141–152 (2017). [DOI] [PubMed] [Google Scholar]
- 117.Kunzke T et al. Native glycan fragments detected by MALDI-FT-ICR mass spectrometry imaging impact gastric cancer biology and patient outcome. Oncotarget 8, 68012–68025 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tsai CH et al. Metastatic progression of prostate cancer is mediated by autonomous binding of galectin-4-O-glycan to cancer cells. Cancer Res. 76, 5756–5767 (2016). [DOI] [PubMed] [Google Scholar]
- 119.Zhan L, Chen L & Chen Z Knockdown of FUT3 disrupts the proliferation, migration, tumorigenesis and TGFβ induced EMT in pancreatic cancer cells. Oncol. Lett 16, 924–930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chugh S, Meza J, Sheinin YM, Ponnusamy MP & Batra SK Loss of N-acetylgalactosaminyltransferase 3 in poorly differentiated pancreatic cancer: augmented aggressiveness and aberrant ErbB family glycosylation. Br. J. Cancer 114, 1376–1386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tzeng SF et al. O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 32, fj201800687 (2018). [DOI] [PubMed] [Google Scholar]
- 122.Vojta A, Samarzija I, Bockor L & Zoldos V Glyco-genes change expression in cancer through aberrant methylation. Biochim. Biophys. Acta 1860, 1776–1785 (2016). [DOI] [PubMed] [Google Scholar]; This study reported significant changes in the methylation of glycosylation genes in human biopsy samples that altered gene expression.
- 123.Muller S et al. High density O-glycosylation on tandem repeat peptide from secretory MUC1 of T47D breast cancer cells. J. Biol. Chem 274, 18165–18172 (1999). [DOI] [PubMed] [Google Scholar]
- 124.Hanson RL & Hollingsworth MA Functional consequences of differential O-glycosylation of MUC1, MUC4, and MUC16 (downstream effects on signaling). Biomolecules 6, E34 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Apostolopoulos V & McKenzie IFC Cellular mucins: targets for immunotherapy. Crit. Rev. Immunol 37, 421–437 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Chou CH et al. Up-regulation of C1GALT1 promotes breast cancer cell growth through MUC1-C signaling pathway. Oncotarget 6, 6123–6135 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sewell R et al. The ST6GalNAc-I sialyltransferase localizes throughout the Golgi and is responsible for the synthesis of the tumor-associated sialyl-Tn O-glycan in human breast cancer. J. Biol. Chem 281, 3586–3594 (2006). [DOI] [PubMed] [Google Scholar]
- 128.Gill DJ, Chia J, Senewiratne J & Bard F Regulation of O-glycosylation through Golgi-to-ER relocation of initiation enzymes. J. Cell Biol 189, 843–858 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dalziel M et al. The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J. Biol. Chem 276, 11007–11015 (2001). [DOI] [PubMed] [Google Scholar]
- 130.Gill DJ, Clausen H & Bard F Location, location, location: new insights into O-GalNAc protein glycosylation. Trends Cell Biol. 21, 149–158 (2011). [DOI] [PubMed] [Google Scholar]
- 131.Reis CA, David L, Seixas M, Burchell J & Sobrinho-Simoes M Expression of fully and under-glycosylated forms of MUC1 mucin in gastric carcinoma. Int. J. Cancer 79, 402–410 (1998). [DOI] [PubMed] [Google Scholar]
- 132.David L, Nesland JM, Clausen H, Carneiro F & Sobrinho-Simoes M Simple mucin-type carbohydrate antigens (Tn, sialosyl-Tn and T) in gastric mucosa, carcinomas and metastases. APMIS Suppl. 27, 162–172 (1992). [PubMed] [Google Scholar]
- 133.Saeland E et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol. Immunother 56, 1225–1236 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nath D et al. Macrophage-tumour cell interactions: identification of MUC1 on breast cancer cells as a potential counter-receptor for the macrophage-restricted receptor, sialoadhesin. Immunology 98, 213–219 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cascio S & Finn OJ Intra- and extra-cellular events related to altered glycosylation of MUC1 promote chronic inflammation, tumor progression, invasion, and metastasis. Biomolecules 6, E39 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Charpin C, Bhan AK, Zurawski VR Jr & Scully RE Carcinoembryonic antigen (CEA) and carbohydrate determinant 19–19 (CA 19–19) localization in 121 primary and metastatic ovarian tumors: an immunohistochemical study with the use of monoclonal antibodies. Int. J. Gynecol. Pathol 1, 231–245 (1982). [DOI] [PubMed] [Google Scholar]
- 137.Steele G Jr. et al. CEA monitoring among patients in multi-institutional adjuvant G. I. therapy protocols. Ann. Surg 196, 162–169 (1982). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frenette PS et al. The diagnostic value of CA 27–29, CA 15–13, mucin-like carcinoma antigen, carcinoembryonic antigen and CA 19–19 in breast and gastrointestinal malignancies. Tumour Biol. 15, 247–254 (1994). [DOI] [PubMed] [Google Scholar]
- 139.Cerwenka H et al. TUM2-PK (pyruvate kinase type tumor M2), CA19–9 and CEA in patients with benign, malignant and metastasizing pancreatic lesions. Anticancer Res. 19, 849–851 (1999). [PubMed] [Google Scholar]
- 140.Guadagni F et al. TAG-72 (CA 72–74 assay) as a complementary serum tumor antigen to carcinoembryonic antigen in monitoring patients with colorectal cancer. Cancer 72, 2098–2106 (1993). [DOI] [PubMed] [Google Scholar]
- 141.Marrelli D et al. Clinical utility of CEA, CA 19–19, and CA 72–74 in the follow-up of patients with resectable gastric cancer. Am. J. Surg 181, 16–19 (2001). [DOI] [PubMed] [Google Scholar]
- 142.Magnani JL et al. A monoclonal antibody-defined antigen associated with gastrointestinal cancer is a ganglioside containing sialylated lacto-N-fucopentaose II. J. Biol. Chem 257, 14365–14369 (1982). [PubMed] [Google Scholar]
- 143.Narimatsu H et al. Lewis and secretor gene dosages affect CA19–9 and DU-PAN-2 serum levels in normal individuals and colorectal cancer patients. Cancer Res. 58, 512–518 (1998). [PubMed] [Google Scholar]
- 144.Kudo T et al. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab. Invest 78, 797–811 (1998). [PubMed] [Google Scholar]
- 145.Wilson DF & Massey W Scanning electron microscopy of incinerated teeth. Am. J. Forensic Med. Pathol 8, 32–38 (1987). [DOI] [PubMed] [Google Scholar]
- 146.Lise M et al. Clinical correlations of α2, 6-sialyltransferase expression in colorectal cancer patients. Hybridoma 19, 281–286 (2000). [DOI] [PubMed] [Google Scholar]
- 147.Sata T, Roth J, Zuber C, Stamm B & Heitz PU Expression of α2,6-linked sialic acid residues in neoplastic but not in normal human colonic mucosa. A lectin-gold cytochemical study with Sambucus nigra and Maackia amurensis lectins. Am. J. Pathol 139, 1435–1448 (1991). [PMC free article] [PubMed] [Google Scholar]
- 148.Zhao Y et al. Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol. Cell. Proteomics 13, 520–536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 149.Park JJ & Lee M Increasing the α2, 6 sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver 7, 629–641 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chakraborty A et al. ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabinemediated DNA damage. J. Biol. Chem 293, 984–994 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Murphy K et al. Integrating biomarkers across omic platforms: an approach to improve stratification of patients with indolent and aggressive prostate cancer. Mol. Oncol 12, 1513–1525 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.West CA et al. N-linked glycan branching and fucosylation are increased directly in Hcc tissue as determined through in situ glycan imaging. J. Proteome Res 17, 3454–3462 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sweeney JG et al. Loss of GCNT2/I-branched glycans enhances melanoma growth and survival. Nat. Commun 9, 3368 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ekser B, Cooper DKC & Tector AJ The need for xenotransplantation as a source of organs and cells for clinical transplantation. Int. J. Surg 23, 199–204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Estrada JL et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 22, 194–202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Byrne GW, Stalboerger PG, Du Z, Davis TR & McGregor CG Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation 91, 287–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lutz AJ et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose α−1, 3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation 20, 27–35 (2013). [DOI] [PubMed] [Google Scholar]; This study shows that deletion of xenogeneic antigens in pig embryos reduces immune rejection.
- 158.Gao B et al. Anti-Neu5Gc and anti-non-Neu5Gc antibodies in healthy humans. PLOS ONE 12, e0180768 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Couvrat-Desvergnes G et al. Rabbit antithymocyte globulin-induced serum sickness disease and human kidney graft survival. J. Clin. Invest 125, 4655–4665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Byrne G, Ahmad-Villiers S, Du Z & McGregor C B4GALNT2 and xenotransplantation: a newly appreciated xenogeneic antigen. Xenotransplantation 25, e12394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kourilovitch M, Galarza-Maldonado C & Ortiz-Prado E Diagnosis and classification of rheumatoid arthritis. J. Autoimmun 48–49, 26–30 (2014). [DOI] [PubMed] [Google Scholar]
- 162.Ohno O & Cooke TD Electron microscopic morphology of immunoglobulin aggregates and their interactions in rheumatoid articular collagenous tissues. Arthritis Rheum. 21, 516–527 (1978). [DOI] [PubMed] [Google Scholar]
- 163.Youings A, Chang SC, Dwek RA & Scragg IG Site-specific glycosylation of human immunoglobulin G is altered in four rheumatoid arthritis patients. Biochem. J 314, 621–630 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rombouts Y et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann. Rheum. Dis 74, 234–241 (2015). [DOI] [PubMed] [Google Scholar]
- 165.Ercan A et al. Aberrant IgG galactosylation precedes disease onset, correlates with disease activity, and is prevalent in autoantibodies in rheumatoid arthritis. Arthritis Rheum. 62, 2239–2248 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boltin D, Perets TT, Vilkin A & Niv Y Mucin function in inflammatory bowel disease: an update. J. Clin. Gastroenterol 47, 106–111 (2013). [DOI] [PubMed] [Google Scholar]
- 167.Goto Y, Uematsu S & Kiyono H Epithelial glycosylation in gut homeostasis and inflammation. Nat. Immunol 17, 1244–1251 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Jostins L et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Xavier RJ & Podolsky DK Unravelling the pathogenesis of inflammatory bowel disease. Nature 448, 427–434 (2007). [DOI] [PubMed] [Google Scholar]
- 170.Theodoratou E et al. The role of glycosylation in IBD. Nat. Rev. Gastroenterol. Hepatol 11, 588–600 (2014). [DOI] [PubMed] [Google Scholar]
- 171.Campbell BJ, Yu LG & Rhodes JM Altered glycosylation in inflammatory bowel disease: a possible role in cancer development. Glycoconj. J 18, 851–858 (2001). [DOI] [PubMed] [Google Scholar]
- 172.Simurina M et al. Glycosylation of immunoglobulin G associates with clinical features of inflammatory bowel diseases. Gastroenterology 154, 1320–1333 e1310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vuckovic F et al. Association of systemic lupus erythematosus with decreased immunosuppressive potential of the IgG glycome. Arthritis Rheumatol. 67, 2978–2989 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Bartsch YC et al. Sialylated autoantigen-reactive IgG antibodies attenuate disease development in autoimmune mouse models of lupus nephritis and rheumatoid arthritis. Front. Immunol 9, 1183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Berger EG Tn-syndrome. Biochim. Biophys. Acta 1455, 255–268 (1999). [DOI] [PubMed] [Google Scholar]
- 176.Ju T & Cummings RD Protein glycosylation: chaperone mutation in Tn syndrome. Nature 437, 1252 (2005). [DOI] [PubMed] [Google Scholar]
- 177.Ju T & Cummings RD A unique molecular chaperone Cosmc required for activity of the mammalian core 1 β3-galactosyltransferase. Proc. Natl Acad. Sci. USA 99, 16613–16618 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kemna MJ et al. Galactosylation and sialylation levels of IgG predict relapse in patients with PR3-ANCA associated vasculitis. EBioMedicine 17, 108–118 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wuhrer M et al. Skewed Fc glycosylation profiles of anti-proteinase 3 immunoglobulin G1 autoantibodies from granulomatosis with polyangiitis patients show low levels of bisection, galactosylation, and sialylation. J. Proteome Res 14, 1657–1665 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Espy C et al. Sialylation levels of anti-proteinase 3 antibodies are associated with the activity of granulomatosis with polyangiitis (Wegener’s). Arthritis Rheum. 63, 2105–2115 (2011). [DOI] [PubMed] [Google Scholar]
- 181.Holland M et al. Hypogalactosylation of serum IgG in patients with ANCA-associated systemic vasculitis. Clin. Exp. Immunol 129, 183–190 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Knoppova B et al. The origin and activities of IgA1-containing immune complexes in IgA nephropathy. Front. Immunol 7, 117 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kiryluk K et al. GWAS for serum galactose-deficient IgA1 implicates critical genes of the O-glycosylation pathway. PLOS Genet. 13, e1006609 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gale DP et al. Galactosylation of IgA1 is associated with common variation in C1GALT1. J. Am. Soc. Nephrol 28, 2158–2166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Suzuki H et al. IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J. Clin. Invest 118, 629–639 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Suzuki H et al. Cytokines alter IgA1 O-glycosylation by dysregulating C1GalT1 and ST6GalNAc-II enzymes. J. Biol. Chem 289, 5330–5339 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study connects the altered glycosylation of IgA1 in IgAN, in response to cytokine exposure, to significant changes in the expression and activity of galactose and sialic acid transferases.
- 187.Renfrow MB et al. IgA nephropathy: an autoimmune kidney disease involving the clustered O-glycans of IgA1 as autoantigens. Glycobiology 27, 1177–1178 (2017). [Google Scholar]
- 188.Bunn HF, Haney DN, Kamin S, Gabbay KH & Gallop PM The biosynthesis of human hemoglobin A1c. Slow glycosylation of hemoglobin in vivo. J. Clin. Invest 57, 1652–1659 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koenig RJ & Cerami A Synthesis of hemoglobin AIc in normal and diabetic mice: potential model of basement membrane thickening. Proc. Natl Acad. Sci. USA 72, 3687–3691 (1975). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cerami A, Stevens VJ & Monnier VM Role of nonenzymatic glycosylation in the development of the sequelae of diabetes mellitus. Metabolism 28, 431–437 (1979). [DOI] [PubMed] [Google Scholar]
- 191.Lowrey CH, Lyness SJ & Soeldner JS The effect of hemoglobin ligands on the kinetics of human hemoglobin A1c formation. J. Biol. Chem 260, 11611–11618 (1985). [PubMed] [Google Scholar]
- 192.Shapiro R, McManus MJ, Zalut C & Bunn HF Sites of nonenzymatic glycosylation of human hemoglobin A. J. Biol. Chem 255, 3120–3127 (1980). [PubMed] [Google Scholar]
- 193.McDonald MJ, Shapiro R, Bleichman M, Solway J & Bunn HF Glycosylated minor components of human adult hemoglobin. Purification, identification, and partial structural analysis. J. Biol. Chem 253, 2327–2332 (1978). [PubMed] [Google Scholar]
- 194.Zhang W et al. Hyperglycemia-related advanced glycation end-products is associated with the altered phosphatidylcholine metabolism in osteoarthritis patients with diabetes. PLOS ONE 12, e0184105 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saremi A et al. Advanced glycation end products, oxidation products, and the extent of atherosclerosis during the VA diabetes trial and follow-up study. Diabetes Care 40, 591–598 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wright JN, Collins HE, Wende AR & Chatham JC O-GlcNAcylation and cardiovascular disease. Biochem. Soc. Trans 45, 545–553 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Holt GD & Hart GW The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J. Biol. Chem 261, 8049–8057 (1986). [PubMed] [Google Scholar]
- 198.Hart GW, Housley MP & Slawson C Cycling of O-linked β-N-acetylglucosamine on nucleocytoplasmic proteins. Nature 446, 1017–1022 (2007). [DOI] [PubMed] [Google Scholar]
- 199.Kreppel LK, Blomberg MA & Hart GW Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J. Biol. Chem 272, 9308–9315 (1997). [DOI] [PubMed] [Google Scholar]
- 200.Traxinger RR & Marshall S Coordinated regulation of glutamine:fructose-6-phosphate amidotransferase activity by insulin, glucose, and glutamine. Role of hexosamine biosynthesis in enzyme regulation. J. Biol. Chem 266, 10148–10154 (1991). [PubMed] [Google Scholar]
- 201.Marshall S, Garvey WT & Traxinger RR New insights into the metabolic regulation of insulin action and insulin resistance: role of glucose and amino acids. FASEB J. 5, 3031–3036 (1991). [DOI] [PubMed] [Google Scholar]
- 202.Wang J, Liu R, Hawkins M, Barzilai N & Rossetti L A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature 393, 684–688 (1998). [DOI] [PubMed] [Google Scholar]
- 203.Yki-Jarvinen H et al. Increased glutamine:fructose-6-phosphate amidotransferase activity in skeletal muscle of patients with NIDDM. Diabetes 45, 302–307 (1996). [DOI] [PubMed] [Google Scholar]
- 204.Clark RJ et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J. Biol. Chem 278, 44230–44237 (2003). [DOI] [PubMed] [Google Scholar]
- 205.Andrali SS, Qian Q & Ozcan S Glucose mediates the translocation of NeuroD1 by O-linked glycosylation. J. Biol. Chem 282, 15589–15596 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu K, Paterson AJ, Chin E & Kudlow JE Glucose stimulates protein modification by O-linked GlcNAc in pancreatic beta cells: linkage of O-linked GlcNAc to beta cell death. Proc. Natl Acad. Sci. USA 97, 2820–2825 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that the increased flux through the hexosamine biosynthetic pathway from glucose leads to toxic levels of O-GlcNAc modifications.
- 207.Kang ES et al. O-GlcNAc modulation at Akt1 Ser473 correlates with apoptosis of murine pancreatic beta cells. Exp. Cell Res. 314, 2238–2248 (2008). [DOI] [PubMed] [Google Scholar]
- 208.Dandamudi S et al. The prevalence of diabetic cardiomyopathy: a population-based study in Olmsted County, Minnesota. J. Card. Fail 20, 304–309 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fulop N et al. Impact of type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am. J. Physiol. Cell Physiol 292, C1370–C1378 (2007). [DOI] [PubMed] [Google Scholar]
- 210.Yu P et al. O-GlcNAcylation of cardiac Nav1.5 contributes to the development of arrhythmias in diabetic hearts. Int. J. Cardiol 260, 74–81 (2018). [DOI] [PubMed] [Google Scholar]
- 211.Hu Y et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ. Res 96, 1006–1013 (2005). [DOI] [PubMed] [Google Scholar]
- 212.Wang D et al. Diabetes exacerbates myocardial ischemia/reperfusion injury by down-regulation of MicroRNA and up-regulation of O-GlcNAcylation. JACC Basic Transl Sci. 3, 350–362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ohtsubo K Targeted genetic inactivation of N-acetylglucosaminyltransferase-IVa impairs insulin secretion from pancreatic beta cells and evokes type 2 diabetes. Methods Enzymol. 479, 205–222 (2010). [DOI] [PubMed] [Google Scholar]
- 214.Liljedahl L, Pedersen MH, Norlin J, McGuire JN & James P N-Glycosylation proteome enrichment analysis in kidney reveals differences between diabetic mouse models. Clin. Proteomics 13, 22 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Bermingham ML et al. N-glycan profile and kidney disease in type 1 diabetes. Diabetes Care 41, 79–87 (2018). [DOI] [PubMed] [Google Scholar]
- 216.Tanigaki K et al. Hyposialylated IgG activates endothelial IgG receptor FcγRIIB to promote obesity-induced insulin resistance. J. Clin. Invest 128, 309–322 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Luft JH Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc 25, 1773–1783 (1966). [PubMed] [Google Scholar]
- 218.Satchell SC & Braet F Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am. J. Physiol. Renal Physiol 296, F947–F956 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Haraldsson B, Nystrom J & Deen WM Properties of the glomerular barrier and mechanisms of proteinuria. Physiol. Rev 88, 451–487 (2008). [DOI] [PubMed] [Google Scholar]
- 220.Borza DB Glomerular basement membrane heparan sulfate in health and disease: a regulator of local complement activation. Matrix Biol. 57–58, 299–310 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.van den Born J et al. Distribution of GBM heparan sulfate proteoglycan core protein and side chains in human glomerular diseases. Kidney Int. 43, 454–463 (1993). [DOI] [PubMed] [Google Scholar]
- 222.van den Hoven MJ et al. Heparanase in glomerular diseases. Kidney Int. 72, 543–548 (2007). [DOI] [PubMed] [Google Scholar]
- 223.Rabelink TJ et al. Heparanase: roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat. Rev. Nephrol 13, 201–212 (2017). [DOI] [PubMed] [Google Scholar]
- 224.Kanwar YS, Linker A & Farquhar MG Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J. Cell Biol 86, 688–693 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that glycosaminoglycans are necessary to prevent increased permeability in the glomerulus, a common occurrence during kidney injury.
- 225.Kopp JB Dystroglycan in the molecular diagnosis of the podocytopathies. Clin. J. Am. Soc. Nephrol 4, 1696–1698 (2009). [DOI] [PubMed] [Google Scholar]
- 226.Vogtlander NP et al. Reactive oxygen species deglycosilate glomerular α-dystroglycan. Kidney Int 69, 1526–1534 (2006). [DOI] [PubMed] [Google Scholar]; This report demonstrates that free radicals in adriamycin-induced nephropathy cause significant insult by deglycosylating α-dystroglycan.
- 227.Grahammer F, Schell C & Huber TB The podocyte slit diaphragm — from a thin grey line to a complex signalling hub. Nat. Rev. Nephrol 9, 587–598 (2013). [DOI] [PubMed] [Google Scholar]
- 228.Li M, Armelloni S, Edefonti A, Messa P & Rastaldi MP Fifteen years of research on nephran: what we still need to know. Nephrol. Dial. Transplant 28, 767–770 (2013). [DOI] [PubMed] [Google Scholar]
- 229.Yan K, Khoshnoodi J, Ruotsalainen V & Tryggvason K N-Linked glycosylation is critical for the plasma membrane localization of nephrin. J. Am. Soc. Nephrol 13, 1385–1389 (2002). [DOI] [PubMed] [Google Scholar]
- 230.Khoshnoodi J, Hill S, Tryggvason K, Hudson B & Friedman DB Identification of N-linked glycosylation sites in human nephrin using mass spectrometry. J. Mass Spectrom 42, 370–379 (2007). [DOI] [PubMed] [Google Scholar]
- 231.Esposito T et al. Dysregulation of the expression of asparagine-linked glycosylation 13 short isoform 2 affects nephrin function by altering its N-linked glycosylation. Nephron 136, 143–150 (2017). [DOI] [PubMed] [Google Scholar]
- 232.Schoeb DS et al. Nineteen novel NPHS1 mutations in a worldwide cohort of patients with congenital nephrotic syndrome (CNS). Nephrol. Dial. Transplant 25, 2970–2976 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Galeano B et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J. Clin. Invest 117, 1585–1594 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Nielsen JS & McNagny KM Novel functions of the CD34 family. J. Cell Sci 121, 3683–3692 (2008). [DOI] [PubMed] [Google Scholar]
- 235.Gelberg H, Healy L, Whiteley H, Miller LA & Vimr E In vivo enzymatic removal of α2→6-linked sialic acid from the glomerular filtration barrier results in podocyte charge alteration and glomerular injury. Lab. Invest 74, 907–920 (1996). [PubMed] [Google Scholar]
- 236.Raska M et al. Differential glycosylation of envelope gp120 is associated with differential recognition of HIV-1 by virus-specific antibodies and cell infection. AIDS Res. Ther 11, 23 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Mikulak J, Di Vito C, Zaghi E & Mavilio D Host immune responses in HIV-1 infection: the emerging pathogenic role of siglecs and their clinical correlates. Front. Immunol 8, 314 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.O’Connell RJ, Kim JH & Excler JL The HIV-1 gp120 V1V2 loop: structure, function and importance for vaccine development. Expert Rev. Vaccines 13, 1489–1500 (2014). [DOI] [PubMed] [Google Scholar]
- 239.Doran RC et al. Glycan modifications to the gp120 immunogens used in the RV144 vaccine trial improve binding to broadly neutralizing antibodies. PLOS ONE 13, e0196370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yates NL et al. HIV-1 envelope glycoproteins from diverse clades differentiate antibody responses and durability among vaccinees. J. Virol 92, e01843–17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wahl A et al. Genome-wide association study on immunoglobulin G glycosylation patterns. Front. Immunol 9, 277 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Plomp R et al. Subclass-specific IgG glycosylation is associated with markers of inflammation and metabolic health. Sci. Rep 7, 12325 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.de Haan N, Reiding KR, Driessen G, van der Burg M & Wuhrer M Changes in healthy human IgG Fc-glycosylation after birth and during early childhood. J. Proteome Res 15, 1853–1861 (2016). [DOI] [PubMed] [Google Scholar]
- 244.Wang G et al. Molecular basis of assembly and activation of complement component C1 in complex with immunoglobulin G1 and antigen. Mol. Cell 63, 135–145 (2016). [DOI] [PubMed] [Google Scholar]
- 245.Blundell PA, Le NPL, Allen J, Watanabe Y & Pleass RJ Engineering the fragment crystallizable (Fc) region of human IgG1 multimers and monomers to fine-tune interactions with sialic acid-dependent receptors. J. Biol. Chem 292, 12994–13007 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Quast I et al. Sialylation of IgG Fc domain impairs complement-dependent cytotoxicity. J. Clin. Invest 125, 4160–4170 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Jefferis R Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 (2009). [DOI] [PubMed] [Google Scholar]
- 248.Yu X, Marshall MJE, Cragg MS & Crispin M Improving antibody-based cancer therapeutics through glycan engineering. BioDrugs 31, 151–166 (2017). [DOI] [PubMed] [Google Scholar]
- 249.Ishida T et al. Defucosylated anti-CCR4 monoclonal antibody (KW-0761) for relapsed adult T cell leukemia-lymphoma: a multicenter phase II study. J. Clin. Oncol 30, 837–842 (2012). [DOI] [PubMed] [Google Scholar]
- 250.Hodoniczky J, Zheng YZ & James DC Control of recombinant monoclonal antibody effector functions by Fc N-glycan remodeling in vitro. Biotechnol. Prog 21, 1644–1652 (2005). [DOI] [PubMed] [Google Scholar]
- 251.Peschke B, Keller CW, Weber P, Quast I & Lunemann JD Fc-galactosylation of human immunoglobulin gamma isotypes improves C1q binding and enhances complement-dependent cytotoxicity. Front. Immunol 8, 646 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Anthony RM, Kobayashi T, Wermeling F & Ravetch JV Intravenous gammaglobulin suppresses inflammation through a novel TH2 pathway. Nature 475, 110–113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Anthony RM & Ravetch JV A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J. Clin. Immunol 30 (Suppl. 1), S9–S14 (2010). [DOI] [PubMed] [Google Scholar]
- 254.Sazinsky SL et al. Aglycosylated immunoglobulin G1 variants productively engage activating Fc receptors. Proc. Natl Acad. Sci. USA 105, 20167–20172 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hale G et al. Pharmacokinetics and antibody responses to the CD3 antibody otelixizumab used in the treatment of type 1 diabetes. J. Clin. Pharmacol 50, 1238–1248 (2010). [DOI] [PubMed] [Google Scholar]
- 256.Ng CM, Stefanich E, Anand BS, Fielder PJ & Vaickus L Pharmacokinetics/pharmacodynamics of nondepleting anti-CD4 monoclonal antibody (TRX1) in healthy human volunteers. Pharm. Res 23, 95–103 (2006). [DOI] [PubMed] [Google Scholar]
- 257.Zheng Y et al. Translational pharmacokinetics and pharmacodynamics of an FcRn-variant anti-CD4 monoclonal antibody from preclinical model to phase I study. Clin. Pharmacol. Ther 89, 283–290 (2011). [DOI] [PubMed] [Google Scholar]
- 258.Yamamoto K et al. Phase I study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T cell leukemia-lymphoma and peripheral T cell lymphoma. J. Clin. Oncol 28, 1591–1598 (2010). [DOI] [PubMed] [Google Scholar]
- 259.Dong DF et al. Anti-angiogenesis and anti-tumor effects of AdNT4-anginex. Cancer Lett. 285, 218–224 (2009). [DOI] [PubMed] [Google Scholar]
- 260.Koonce NA, Griffin RJ & Dings RPM Galectin-1 inhibitor OTX008 induces tumor vessel normalization and tumor growth inhibition in human head and neck squamous cell carcinoma models. Int. J. Mol. Sci 18, E2671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dias AM et al. Metabolic control of T cell immune response through glycans in inflammatory bowel disease. Proc. Natl Acad. Sci. USA 115, E4651–E4660 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Wang SS et al. Thioglycosides are efficient metabolic decoys of glycosylation that reduce selectin dependent leukocyte adhesion. Cell Chem. Biol 25, 1519–1532 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Castaneda F, Burse A, Boland W & Kinne RK Thioglycosides as inhibitors of hSGLT1 and hSGLT2: potential therapeutic agents for the control of hyperglycemia in diabetes. Int. J. Med. Sci 4, 131–139 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Clement LC et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat. Med 17, 117–122 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Chugh SS, Clement LC & Mace C New insights into human minimal change disease: lessons from animal models. Am. J. Kidney Dis 59, 284–292 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Park J et al. Glucosamine hydrochloride exerts a protective effect against unilateral ureteral obstruction-induced renal fibrosis by attenuating TGFβ signaling. J. Mol. Med 91, 1273–1284 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Agre P et al. Training the next generation of biomedical investigators in glycosciences. J. Clin. Invest 126, 405–408 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Stanley P & Cummings RD in Essentials of Glycobiology (ed. Varki A et al. ) 161–178 (2017). [Google Scholar]
- 269.Yamamoto F Review: ABO blood group system — ABH oligosaccharide antigens, anti-A and anti-B, A and B glycosyltransferases, and ABO genes. Immunohematology 20, 3–22 (2004). [PubMed] [Google Scholar]
- 270.Heggelund JE, Varrot A, Imberty A & Krengel U Histo-blood group antigens as mediators of infections. Curr. Opin. Struct. Biol 44, 190–200 (2017). [DOI] [PubMed] [Google Scholar]
- 271.Ramani S, Hu L, Venkataram Prasad BV & Estes MK Diversity in rotavirus-host glycan interactions: a “sweet” spectrum. Cell. Mol. Gastroenterol. Hepatol 2, 263–273 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Dotz V & Wuhrer M Histo-blood group glycans in the context of personalized medicine. Biochim. Biophys. Acta 1860, 1596–1607 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Cooling L Blood groups in infection and host susceptibility. Clin. Microbiol. Rev 28, 801–870 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Barua D & Paguio AS ABO blood groups and cholera. Ann. Hum. Biol 4, 489–492 (1977). [DOI] [PubMed] [Google Scholar]
- 275.Huang P et al. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol 79, 6714–6722 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Shanker S et al. Structural basis for norovirus neutralization by an HBGA blocking human IgA antibody. Proc. Natl Acad. Sci. USA 113, E5830–E5837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Fuster MM & Esko JD The sweet and sour of cancer: glycans as novel therapeutic targets. Nat. Rev. Cancer 5, 526–542 (2005). [DOI] [PubMed] [Google Scholar]
- 278.Stanley P Golgi glycosylation. Cold Spring Harb. Perspect. Biol 3, a005199 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Britain CM, Holdbrooks AT, Anderson JC, Willey CD & Bellis SL Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res 11, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This report demonstrates that sialylation of epidermal growth factor receptor increases signalling activity and inhibits gefitinib-induced cell death.
- 280.Fogo AB & Kon V The glomerulus — a view from the inside — the endothelial cell. Int. J. Biochem. Cell Biol 42, 1388–1397 (2010). [DOI] [PubMed] [Google Scholar]
- 281.Scheid E et al. Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II trial in patients with nonmetastatic castrate-resistant prostate cancer. Cancer Immunol. Res 4, 881–892 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.