Abstract
The two isoforms of the nuclear estrogen receptor, ERα and ERβ are widely expressed in the central nervous system. Although they were first described as nuclear receptors, both isoforms have also been found at the cell membrane where they mediate cell signaling. Surface biotinylation studies using neuronal and glial primary cultures label an alternatively spliced form of ERα. The 52 kDa protein, ERαΔ4, is missing exon 4 and is highly expressed in membrane fractions derived from cultured cells. In vivo, both full-length (66 kDa) ERα and ERαΔ4 are present in membrane fractions. In response to estradiol, full-length ERα and ERαΔ4 are initially trafficked to the membrane, and then internalized in parallel. Previous studies determined that only the full-length ERα associates with metabotropic glutamate receptor-1a (mGluR1a), initiating cellular signaling. The role of ERαΔ4, remained to be elucidated. Here, we report ERαΔ4 trafficking, association with mGluR2/3, and downstream signaling in female rat arcuate nucleus (ARH). Caveolin (CAV) proteins are needed for ER transport to the cell membrane, and using co-immunoprecipitation CAV-3 was shown to associate with ERαΔ4. CAV-3 was necessary for ERαΔ4 trafficking to the membrane: in the ARH, microinjection of CAV-3 siRNA reduced CAV-3 and ERαΔ4a in membrane fractions by 50%, and 60%, respectively. Moreover, co-immunoprecipitation revealed that ERαΔ4 associated with inhibitory mGluRs, mGluR2/3. Estrogen benzoate (EB) treatment (5 µg; s.c.; every 4 days; 3 cycles) reduced levels of cAMP, an effect attenuated by antagonizing mGluR2/3. Following EB treatment, membrane levels of ERαΔ4 and mGluR2/3 were reduced implying ligand-induced internalization. These results implicate ERαΔ4 in an estradiol-induced inhibitory cell signaling in the ARH.
Keywords: estrogen receptor, alternative splicing, mGluR, caveolin, cAMP
INTRODUCTION
Over the years, several proteins have been suggested as putative estrogen receptors (ERs) in the central nervous system (1, 2). Direct nuclear action of estrogens modulating gene expression is mediated by ERα and ERβ [reviewed in (3)]. On the membrane, estrogens can act via the classical ERα and ERβ, G protein estrogen receptors (GPERs), or Gq membrane estrogen receptor (Gq-mER) to activate intracellular signaling pathways. These membrane ERs all share the ability to activate G protein-coupled cascades. In addition to the full-length ERα (66kDa), an ERα variant has also been found on the membranes of cells from the central nervous system (4–6). These studies revealed a 52 kDa-sized protein that is recognized by antibodies directed either at the amino or carboxyl terminals of ERα. Interestingly, 52 kDa is the predicted size of a protein coded for by a widely distributed alternatively spliced ERα mRNA, ERαΔ4, which was first identified in mammalian brains as an ERα isoform missing the fourth exon of the ESR1 gene (7).
Estradiol-initiated membrane signaling (EMS) involving ERα requires a transactivation of G protein-coupled metabotropic glutamate receptor 1a (mGluR1a) that activates phospholipase C (PLC), protein kinase C (PKC), and allows for the release of intracellular calcium. Activation of these signaling pathways requires the full-length, 66 kDa, ERα (5, 6, 8–11). Initially, treatment with estradiol transiently increases ERα and ERαΔ4 levels in the membrane, and then induces internalization and down-regulation of these receptors. In the membrane fraction of cultured neurons or astrocytes, ERαΔ4 is expressed in much higher levels compared with full-length ERα. However, in tissue from the arcuate nucleus of the hypothalamus (ARH), the relative amounts of these ERs are reversed; full-length ERα is the predominant isoform (12). Although we have previously observed ERαΔ4 on the membrane, we had not formally examined ERαΔ4 trafficking or signaling.
Initial experiments with cultured hippocampal neurons from males and females demonstrated that estradiol induces ERα trafficking to the membrane in association with caveolin (CAV) proteins (13). These observations have been confirmed in hypothalamic and striatal neurons and hypothalamic astrocytes and dorsal root ganglion cells from males and females (4, 5, 14–17). Knockdown of ARH caveolin-1 (CAV-1) prevented ERα trafficking to the membrane, but did not alter cytosolic levels of the receptor (12). Moreover, ERαΔ4 levels on the membrane remained stable, indicating that another caveolin was involved in ERαΔ4 trafficking. Caveolins also determine interactions with particular mGluRs [reviewed in (9)]. If another caveolin is associated with ERαΔ4, it is likely that ERαΔ4 would transactivate a different mGluR than full length ERα, leading to distinct effects on cell signaling. To study ERαΔ4 in vivo, we examined tissue from the female rat ARH, a region of particular interest because estradiol has facilitatory and inhibitory actions in this nucleus. Estradiol activates neurons in the ARH to influence sexual receptivity and food intake through an ERα mechanism (10, 18–20). On the other hand, one of the signature estradiol actions in the ARC is to dramatically downregulate the expression of kisspeptin (21–24).
Using co-immunoprecipitation and knockdown strategies, we examined ERαΔ4 trafficking, transactivation of mGluR and down-stream signaling in vivo and determined that ERαΔ4 association with mGluR2/3 requires CAV-3. Next, we examined whether ERαΔ4 activation induced an inhibitory response in ARH since mGluR2/3 couples with Gi/o proteins (25) and negatively regulates adenylyl cyclase (26, 27), which results a in a concomitant lowering of cAMP. We found that ERαΔ4, by transactivating mGluR2/3, reduced cAMP levels in the ARH, implicating ERαΔ4 in estradiol inhibition as previously hypothesized (28). Antagonizing mGluR2/3 in the ARH did not prevent the estradiol-induced down-regulation of kisspeptin expression, suggesting that this inhibition was not mediated by ERαΔ4. This was supported by immunohistochemical results where mGluR2/3 was not co-localized with neurokinin B (NKB), a marker for kisspeptin/neurokinin B/dynorphin expressing (KNDy) ARH neurons (29, 30).
METHODS
Ethics Statement
This study was carried out in accordance with the principles and procedures of the National Institute of Health Guide for the Care and Use of Laboratory Animals. The protocol was approved by the Chancellor’s Animal Research Committee at the University of California, Los Angeles (Protocol Number: ARC # 1999–020). All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering.
Animals
Ovariectomized (ovx) adult female Long-Evans rats (200 – 250 g) were purchased from Charles River (Wilmington, MA). Rats were shipped 1 week post-ovariectomy. Upon arrival, rats were housed two per cage in a climate-controlled room, with a 12-hr light, 12-hr dark cycle (lights on at 0600). Cannulae placement surgery and the hormone injections were started 2 weeks after the ovariectomies. Food and water were available ad libitum to the rats.
Bilateral ARH cannulae placement.
For the CAV-3 siRNA knockdown experiment, bilateral guide cannulae (22 gauge; Plastics One Inc., Roanoke, VA) directed at the ARH (coordinates from bregma; −2.8 mm, lateral 0.8 mm, ventral −7.4 mm from dura; tooth bar: −3.3 mm) were implanted using standard stereotaxic procedures while female rats were anesthetized with isoflurane (2–3% in equal parts oxygen and nitrous oxide). Cannulae were secured to the skull with dental acrylic and stainless steel bone screws. Stylets were placed in the guide cannulae, extending less than 0.5 mm beyond the opening of the guide cannulae. Animals were individually housed after surgery, received rimadyl (5 mg/kb s.c. injection every 12–24 hr; Zoetis, Kalamazoo, MI) and oral antibiotics (trimethoprim and sulfamethoxazole, 0.4 mg/ml; Hi-Tech Pharmacal, Amityville, NY) in the drinking water and were allowed to recover 6–7 days before steroid priming.
Steroid priming.
Estrogen injections began 2 weeks after ovariectomies. 17β-estradiol benzoate (EB) dissolved in safflower oil was injected (s.c.) in a volume of 0.1 ml per rat. Females received 5 μg EB every 4 days between 0800 and 0900 for three cycles to mimic the natural estrous cycle of female rats as previously described (31).
siRNA Microinjection.
For ARH site-specific microinjections, 2 μg/μl of CAV-3 siRNA (CAV-3 ON-TARGETplus SMARTpool siRNA; L-090855–02-XXXX; GE Dharmacon, Lafayette, CO) or non-targeting control siRNA (ON-TARGETplus Non-targeting Pool; D-001810–10-XX; GE Dharmacon) were dissolved in 1 µL of artificial cerebrospinal fluid (Tocris Bioscience, Minneapolis, MN). The ARH was microinfused bilaterally with 1 μl at a rate of 0.25 μl/min using an infusion pump (Harvard Apparatus, Holiston, MA) while animals were active and alert in their homecages. Microinjection needles (28 gauge, Plastics One Inc) protruded 1 mm or less beyond the opening of the cannula and remained in place for 1 min after infusion to allow for diffusion away from the injector. After microinjection, obturators were reinserted into guide cannulae and animals were returned to their home cage.
For CAV-3 knockdown, animals received four microinfusions of siRNA, one each day, beginning on the day of the second EB injection. The first and final microinfusions were given 30 min prior to EB injections. The timeline of the CAV-3 microinfusions can be seen in Supplemental Figure 1A.
Colchicine injections.
For immunohistochemical studies to identify NPY neurons, colchicine was necessary to visualize NPY in the cell bodies. Colchicine (100 μg), dissolved in 5 µL of artificial cerebrospinal fluid, was injected into the lateral ventricle with an infusion pump (Harvard Apparatus, Holliston, MA) at a rate of 0.5 μl/min while female rats were anesthetized with isoflurane (2–3% in equal parts oxygen and nitrous oxide). The infusion needle (28 gauge) protruded 1 mm beyond the opening of the cannula and was allowed to remain in place for 1 min after infusion to allow for diffusion of the drug.
In vivo inhibitor studies.
For studies on kisspeptin transcription, a single guide cannula (22 gauge; Plastics One Inc., Roanoke, VA) was directed toward the third ventricle (coordinates from bregma; −2.3 mm, lateral 0, ventral −8.5 mm from dura; tooth bar: −3.3 mm) while female rats were anesthetized with isoflurane (2–3% in equal parts oxygen and nitrous oxide). 1 µL ICI 182,780 (50 µg), LY341,495 (25 nmol), or vehicle (DMSO/aCSF for ICI, 182,780; NaOH/aCSF for LY341,495) were injected at a rate of 0.25 μl/min using an infusion pump (Harvard Apparatus). Microinjection needles (28 gauge) protruded 1 mm or less beyond the opening of the cannula and remained in place for 1 min after infusion to allow for diffusion away from the injector. 30 min after third ventricle infusion, 10 µg EB (s.c.) was injected followed by a second EB injection 24 hr later. Animals were anesthetized 24 h after the second EB injection and decapitated. ARH tissue was microdissected over ice, snap-frozen, and used for PCR analysis. The timeline for the ER and mGluR inhibitor studies on kisspeptin transcription is presented in Supplemental Figure 1B.
For cAMP studies, following cannula placement, 0.5 µL LY341,495 (25 nmol) or vehicle (NaOH/aCSF) were injected at a rate of 0.125 μl/min using an infusion pump. After 1 min to allow drugs to diffuse away from the microinjection needle, 0.5 µL E2 (10 nmol) or vehicle (DMSO/aCSF) were injected into the third ventricle. 30 min after the second infusion, animals were anesthetized and decapitated. ARH tissue was microdissected over ice, snap-frozen, and processed for cAMP ELISAs. The timeline for the mGluR inhibition on kisspeptin transcription is presented in Supplemental Figure 1C.
Membrane preparations
To verify CAV-3 knockdown and monitor membrane ERα levels in the ARH, animals that were microinjected with non-targeting control or CAV-3 siRNA were anesthetized 30 min after the last EB injection and decapitated (Supplemental Fig 1A). Brains were quickly isolated and ARH tissue was microdissected over ice. Membrane proteins were extracted from ARH tissue using the Plasma Membrane Protein Extraction Kit (ab65400; Abcam, Cambridge, MA) according to the manufacturer’s protocol. Briefly, tissues were homogenized in 2 volumes of 1X Homogenize Buffer with protease inhibitors until completely lysed (30 times). Samples were centrifuged at 700 X g for 10 min at 4°C to remove non membrane-associated proteins. Supernatants were collected in a microfuge tube and centrifuged at 10,000 xg for 30 min at 4° C to separate the cytosol fraction (supernatant) from total cellular membrane proteins (pellet). Membrane protein pellets were resuspended in RIPA Lysis Buffer containing protease inhibitors: 1 mM phenylmethylsulfonyl fluoride, 1 µg/ml peptstatin, 1 µg/ml leupeptin, 1µg/ml aprotinin, 1 mM sodium orthovanadate (Santa Cruz Biotechnology, Santa Cruz, CA) and used immediately for western blots.
For co-immunoprecipitation experiments, ARH membrane proteins were extracted 30 min after the third EB (or oil) injection using the Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher) according to manufacturer’s instructions. Briefly, ARH tissue was washed with Cell Wash Solution and transferred to a Dounce homogenizer. Tissue was homogenized in 1 mL Permeabilization Buffer (30 strokes) before mixing samples for 10 min at 4° C. Samples were centrifuged at 16,000 X g for 15 min at 4° C to separate cytosolic proteins (supernatant) from membrane proteins (pellet). Membrane protein pellets were resuspended in 0.5 mL Solubilization Buffer with 1% n-dodecyl-β-d-maltopyranoside (DDM; Thermo Fisher) to optimize the protein extraction, then samples were incubated for 30 min at 4°C with constant mixing before centrifuging at 16,000 X g for 15 min at 4° C. Solubilized membrane and membrane-associated proteins (supernatant) were used for co-immunoprecipitation experiments.
Co-immunoprecipitation
Concentrations of membrane proteins were determined by the bicinchoninic acid (BCA) assay (Thermo Fisher) (32). For immunoprecipitation, similar concentrations of membrane proteins (2.5 mg/ml; sample volume ∼100 μl) were used. For each reaction, 5 μl protein-A Magnetic Sepharose beads (GE Healthcare, Piscataway, NJ) were used according to manufacturer’s protocol with the MagRack6 magnetic rack (GE Healthcare). After equilibrating beads with Binding Buffer (TBS; 50 mM tris, 150 mM NaCl pH 7.5), beads were bound to an antibody directed towards CAV-3 or mGluR2/3 proteins (1:100; see below for antibody details) for at least 15 min at RT. After washing off unbound antibodies with Binding Buffer, membrane proteins diluted in Binding Buffer were added to the beads and incubated with slow end-over-end mixing for 1 hr at RT. After removing the flow through, beads were washed 3 times with buffer (TBS; 50 mM tris, 150 mM NaCl pH 7.5). Samples were eluted with 50 µL elution buffer (2.5% acetic acid) and used for western blots.
Antibodies
Antibodies used in these experiments are detailed in Table 1: Primary Antibodies and Table 2: Secondary Antibodies.
Table 1:
Primary antibodies
| Antibody Name | Peptide/Protein Target | Manufacturer | Catalog # | Application | Dilution |
|---|---|---|---|---|---|
| Estrogen Receptor α (ERα) | C-terminus of rat ERα | EMD Millipore | 06-935 | Western | 1:1000 |
| Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) | GAPDH from rat muscle | EMD Millipore | MAB376 | Western | 1:10,000 |
| Caveolin-3 (CAV-3) | mouse CAV-3 aa 1-19 | Abcam | ab2912 | Western | 1:1000 |
| Kisspeptin | Peptide from mouse kisspeptin 10 | EMD Millipore | AB9754 | Immunocytochemistry | 1:2000 |
| Metabotropic Glutamate Receptor 2 (mGluR2) | C-terminus of mGluR2 | Cell Signaling | 12056 | Western, Co-immunoprecipitation | 1:1000 |
| Metabotropic Glutamate Receptor 2/3 (mGluR2/3) | C-terminus of rat mGluR2 (NGREVVDSTTSSL) | EMD Millipore | AB1553 | Western, Co-immunoprecipitation | 1:500 |
| Flotillin 1 | resides 1-100 of human flotillin 1 | Abcam | ab41927 | Western | 1:1000 |
| Neurokinin B (NKB) | N-terminus of protachykinin B | Kind gift from Dr. Philippe Ciofi | Immunocytochemistry | 1:5000 | |
| Neuropeptide Y (NPY) | synthetic NPY peptide | EMD Millipore | AB1583 | Immunocytochemistry | 1:500 |
Table 2:
Secondary antibodies
| Antibody Name |
Protein Target | Manufacturer | Catalog # | Application | Dilution |
|---|---|---|---|---|---|
| Goat anti-Mouse IgG (H+L) Secondary Antibody, HRP conjugate | Mouse gamma immunoglobins heavy and light chains | Life Technologies | A16072 | Western | 1:10,000 |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, HRP conjugate | Rabbit gamma immunoglobins heavy and light chains | Life Technologies | A16104 | Western | 1:10,000 |
| Biotinylated donkey anti-sheep | sheep gamma immunoglobins heavy and light chains | Life Technologies | A16045 | Immunocytochemistry | 1:1000 |
| Alexa 594 Donkey anti-rabbit | Rabbit gamma immunoglobins heavy and light chains | Life Technologies | A21207 | Immunocytochemistry | 1:1000 |
| Cy2-Conjugated Streptavidin | Jackson Immunoresearch | 016-220-084 | Immunocytochemistry | 1:1000 |
Western Blots
Isolated membrane samples were loaded onto 8–16% SDS polyacrylamide gels (NuSep Inc, Bogart, GA) and transferred to polyvinylidene difluoride membranes (GE Healthcare, Pittsburgh, PA). Nonspecific binding sites were blocked with 5% nonfat milk in TBS-T (Tris buffered saline with 0.1% Tween 20) for 1 hr on an orbital shaker. Primary antibodies (Table 1) were diluted in 5% bovine serum album (for ERα) or 5% w/v nonfat milk (for mGluR2/3, Flotillin 1) in TBS-T and incubated overnight at 4° C. Membranes were washed three times with TBS-T prior to incubation with secondary antibody (Table 2) diluted in 5% nonfat milk in TBS-T for 1 hr. Membranes were washed three times with TBS-T and once with TBS prior to visualization on a Fluor ChemE imager (ProteinSimple, San Jose, CA) using enhanced chemiluminescene (ECL; Clarity, Bio-Rad).
Densitometric analysis.
Digitized images from the Fluor ChemE imager were analyzed using AlphaView 2.0 software (ProteinSimple). Total band intensity values for each sample were calculated by subtracting the background from each target signal. ERα, CAV-3, and mGluR2/3 loading were normalized with Flotillin 1 to determine the amount of each target. Signal ratios were normalized by dividing each set by the control ratio for each of the data sets and multiplied by 100 to obtain the relative change in signal intensity compared to the control group.
Immunohistochemistry
Free-floating sections were rinsed three times in TBS. To reduce autofluorescence, sections were incubated in 0.1% sodium borohydride (Sigma)/TBS for 10 min. After rinsing sections three times in TBS, sections were blocked for 1 hr in Block Buffer (1% H2O2, 10% normal donkey serum (NDS), 1% bovine serum albumin in TBS). Primary antibodies (Table 1) were diluted in TBS-Plus (2% NDS and 0.3% Triton X-100 in TBS) and applied to sections for a 48 hr incubation at 4°C. After washing unbound antibodies with TBS-Plus, sections were incubated with fluorphore-conjugated secondary antibodies or a biotinylated secondary antibody (Table 2) for 2 hr at room temperature. If a biotinylated secondary antibody was used, sections were exposed to a streptavidin-conjugated fluorophore after another set of TBS-Plus rinses. After a final set of TBS rinses, sections were mounted onto Superfrost Plus microscope slides (Thermo Fisher) and coverslipped using Fluoromount-G mounting medium (Southern Biotech; Birmingham, AL).
Quantitative PCR (qPCR)
Total RNA from microdissected ARH was isolated using TRIzol reagent (Life Technologies; Carlsbad, CA), according to a protocol recommended by the manufacturer. Briefly, 100 mg tissue was homogenized in 1 mL of TRIzol® (Thermo Fisher). Following the chloroform extraction of RNA and precipitation with 100% isopropanol, RNA pellet was washed with 75% ethanol/DEPC-treated water. Pellets were allowed to dry for 10 min at room temperature and were resuspended in DEPC-treated water. Concentration and quality of RNA was assessed using a spectrophotometer (NanoDrop™ 1000). 1–2 µg total RNA was used to synthesize cDNA using the SuperScript III Reverse Transcriptase kit (Invitrogen; Carlsbad, CA) with random hexamer primers. The RT reaction was performed at 25°C for 10 min, 50°C for 50 min, followed by a 5 min termination at 85°C. cDNA was used immediately for qPCR or stored at −20°C for ≤ 1 month.
The quantitative PCR primers used were: kisspeptin (NM_181692): (sense) 5’- TGGCACCTGTGGTGAACCCTGAAC −3’, (antisense) 5- ATCAGGCGACTGCGGGTGGC -ACAC −3’; GAPDH (NM_017008): (sense) 5’- CGGCAAGTTCAACGGCACAG −3’, (antisense) 5’- ACTCCACGACATACTCAGCAC −3’. The kisspeptin primers amplified a 192 amplicon corresponding to nucleotides 74–275 (33). The GAPDH primers amplified a 134 amplicon corresponding to nucleotides 228 to 361 (34). PCR reactions were run in duplicate, and at least 3 duplicates per condition were included in each PCR assay. At least 3 independent experiments were conducted and combined for each mRNA result. No template controls (NTC; cDNA reverse transcribed without RNA) were run alongside cDNA for each primer pair in every PCR experiment. No amplification was observed in NTC wells. PCR reactions were prepared using 2 µL of cDNA in a 20 µL reaction with SYBR GreenER qPCR SuperMix Universal (Invitrogen; Carlsbad, CA). Reactions were run on an Mx3000p thermal cycler (Agilent; Santa Clara, CA). Conditions for amplification were as follows: 2 min at 50°C (UDG incubation), 10 min at 95° C for UDG inactivation and DNA polymerase activation, then 50 cycles each consisting of 15 sec at 95° C and 60 sec at annealing temperature (see Table 2). Reactions were followed by melting curve analysis (55° C to 95° C).
cAMP ELISAs
For cAMP measurements in tissue, dissected ARH tissue was homogenized in 10 volumes of 0.1N HCl and analyzed with a Direct cAMP ELISA kit (Enzo Life Sciences) according to the manufacturer’s instructions. Interassay coefficients of variation were between 7.8% and 13.6%, and intra-assay coefficients of variation were between 7.6% and 8.4% for the acetylated version of the assay. For the non-acetylated version of the assay, interassay coefficients of variation were between 4.2% and 13.1%, and intra-assay coefficients of variation were between 4.3% and 8.9% for the acetylated version of the assay according to the manufacturer. ELISA data were normalized to protein concentrations of the lysates.
Statistics
Data are presented as means ± standard error (SEM) of a percent relative ratio. Statistical comparisons were performed using t-test or analysis of variance (ANOVA), with Student-Newman-Keuls post hoc test. Data were analyzed using SigmaPlot (Version 12.5; Systat Software Inc., San Jose, CA).
RESULTS
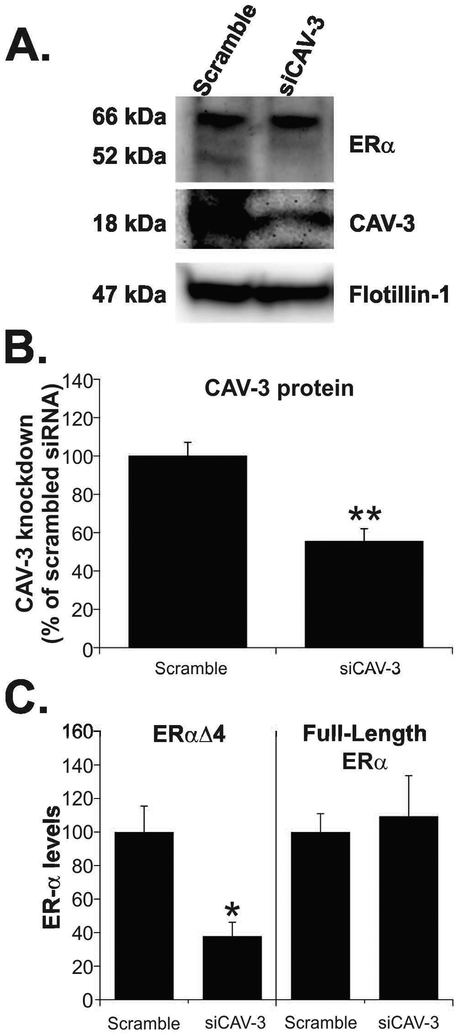
CAV-3 knockdown reduced levels of ERαΔ4 in ARH membrane fractions
Using siRNA, CAV-3 protein was targeted for knockdown in the ARH. Ovariectomized female rats primed with EB (5 µg every four days for 3 cycles) were bilaterally microinjected with scrambled siRNA or CAV-3 siRNA into the ARH between the second and third EB injections. Two days after the siRNA injection, CAV-3 protein levels were reduced 45% after CAV-3 siRNA compared with scrambled siRNA (Fig. 1A; siCAV-3: 55.5 ± 6.6; scrambled siRNA: 100.0 ± 7.1; t-test, P < 0.005; df = 9; t = 4.505). Membrane ERαΔ4 levels were reduced by 65% (Fig. 1B; siCAV-3: 34.5 ± 7.2 vs. scrambled siRNA: 100.0 ± 12.7; t-test, P < 0.005; df = 9; t = 4.226). Full-length ERα levels in the membrane fraction were unchanged (Fig. 1B; siCAV-3: 99.0 ± 21.3 vs. scrambled siRNA: 100.0 ± 9.0; t-test, P = 0.961; df = 9; t = 0.0442).
Figure 1. CAV-3 knockdown reduces ERαΔ4 trafficking to the membrane.
Ovx rats were primed with EB and microinjected with scrambled or CAV-3 siRNA into the ARH. 30 min after the last (third) EB treatment, rats were sacrificed and ARH membranes were isolated. (A) Representative western blots of membrane ERα levels in EB-treated rats treated with scrambled or CAV-3 siRNA. (B) Females microinjected with CAV-3 siRNA had a 55% reduction of CAV-3. (C) This resulted in a 60% decrease in ERαΔ4. Full-length ERα levels were unchanged by CAV-3 knockdown. Values are means ± SEM (n = 7-8 rats/group).
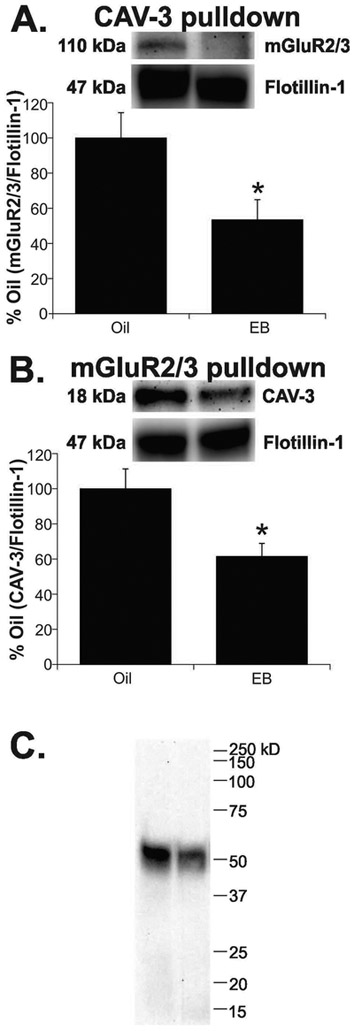
EB treatment decreased CAV-3/mGluR2/3 in ARH membrane fractions
Co-immunoprecipitation experiments using antibodies raised against CAV-3 or mGluR2/3 were used to study potential interactions between these two proteins in ARH membranes from ovx female rats primed with EB or oil. EB treatment resulted in a 45% decrease in mGluR2/3 protein pulled down using an antibody to CAV-3 (Fig. 2A; EB: 53.4 ± 11.5 vs. oil: 100.0 ± 14.4; t-test, P < 0.05; df = 12; t = 2.857). Using the reverse procedure, EB treatment caused a 40% decrease in CAV-3 protein pulled down by an antibody to mGluR2/3 (Fig. 2B; EB: 61.5 ± 7.4 vs. oil: 100.0 ±11.3; t-test, P < 0.05; df = 13; t = −2.480). In addition, to verify that ERαΔ4 was part of this complex, membranes were probed for ERα. We found only a single ERα immunoreactive band at 52 kDa, indicating that ERαΔ4 associates with mGluR2/3 (Fig. 2C).
Figure 2. EB treatment reduces CAV-3, mGluR2/3, and ERαΔ4 protein in ARH membranes.
(A) EB treatment resulted in a 45% decrease in mGluR2/3 protein pulled down using an antibody to CAV-3. (B) Using the reverse procedure, there was a 40% decrease in CAV-3 protein pulled down by an antibody to mGluR2/3. (C) In these same fractions, only ERαΔ4 (52 kDa) was detected with mGluR2/3. Values are means ± SEM (n = 7-8 rats/group).
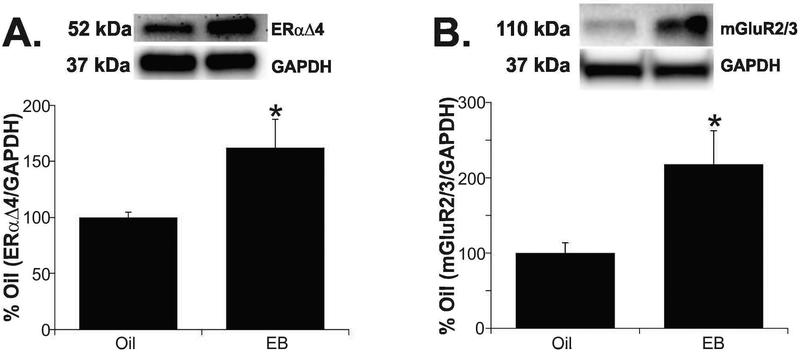
EB treatment increased ERαΔ4 and mGluR2/3 in the cytoplasm
We analyzed the cytosol fractions from samples used for membrane preparations to determine if the reduction of these proteins in the membrane fraction was coincident with an increase in the cytoplasm. Typically, membrane receptors are internalized following ligand binding (35, 36). Estradiol benzoate treatment increased cytosolic ERαΔ4 protein levels 60% (Fig 3A: EB: 162.3 ± 24.5 vs. oil: 100.0 ± 4.7; t-test, P < 0.05; df = 14; t = −2.458) and also increased mGluR2/3 protein more than 2-fold in the cytosolic fraction (Fig. 3B; EB: 217.9 ± 44.3 vs. oil: 100.0 ± 13.7; t-test, P < 0.05; df = 12; t = −2.543). CAV-3 protein was undetectable in the cytosolic fraction (Supplemental Figure 2).
Figure 3. ERαΔ4 and mGluR2 are internalized from ARH membranes following EB treatment.
(A) ERαΔ4 protein increased 60% in cytosol fractions of EB-treated animals. (B) Analysis of the cytosol fractions showed a 2-fold increase in mGluR2/3 protein in EB treated animals. Values are means ± SEM (n = 7-8 rats/group).
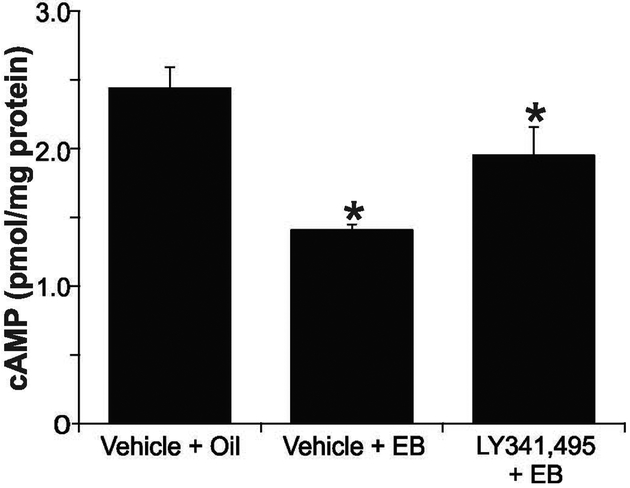
LY341,495 attenuated estradiol suppression of cAMP
We used LY341,495, a group II mGluR antagonist, to test whether inhibiting the mGluRs would block the EB-induced suppression of cAMP in rat ARH. Animals infused with LY341,495 (25 nmol) or vehicle (NaOH/aCSF) into the third ventricle were immediately by treated with EB (s.c.) or vehicle. A 30 min EB treatment suppressed cAMP levels relative to control. This suppression was attenuated with LY341,495 pretreatment (Fig. 4: aCSF+E2: 1.41 ± 0.4 pmol/mg; LY341,495+E2: 1.95 ± 0.21 pmol/mg vs. aCSF+vehicle: 2.44 ± 0.15 pmol/mg; one-way ANOVA, Student-Newman-Keuls; P < 0.001; df = 23; F = 10.19).
Figure 4. Antagonism of mGluR2/3 partially reverses estradiol suppression of cAMP in female rat ARH.
Third ventricle injection of E2 (10 nmol; 30 min) into ovx female rats resulted in a 40% decrease in cAMP levels in the ARH. LY341,495 (25 nmol) injected prior to E2 had prevented some E2-suppression of cAMP; however, cAMP levels were still lower than the control group. Values are means ± SEM (n = 7-8 rats/group).
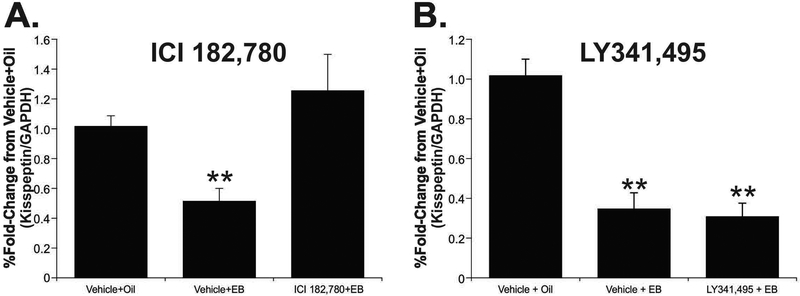
EB down-regulation of kisspeptin mRNA was not mediated by mGluR2/3
A potential inhibitory action of estradiol acting through ERαΔ4 and mGluR2/3 in the ARH is the estradiol-mediated suppression of kisspeptin expression in KNDy neurons (37–39). Treatment of female rats with EB decreased levels of kisspeptin mRNA by 50%. The EB-induced suppression of kisspeptin mRNA was blocked by the pan ER inhibitor, ICI 182,780 (50 µg), which maintained kisspeptin mRNA at control (no EB) levels. (Fig. 5A; vehicle + EB: 0.51 ± 0.08; ICI 182,780+EB: 1.26 ± 0.24 vs. vehicle + oil: 1.02 ± 0.07; one-way ANOVA, Student-Newman-Keuls; P < 0.05; df = 22; t = 6.258).
Figure 5. ER antagonism, but not mGluR2/3 blockade, inhibited estradiol-suppression of kisspeptin mRNA.
(A) EB suppressed ARH kisspeptin mRNA levels by 50%, which was inhibited by ICI 182,780 pretreatment (50 µg, icv). (B) Pretreatment LY341,495 (25 nmol, icv) had no effect on EB-suppression of kisspeptin of mRNA. Values are means ± SEM means ± SEM (n = 7-9 rats/group).
To test whether ERαΔ4-mGluR2/3 complex mediated the EB down-regulation of kisspeptin mRNA, we inhibited mGluR2/3 with LY341,495 (25 nmol, icv) 30 min prior to EB injection (10 µg, s.c.). Antagonism of mGluR2/3 did not affect EB-suppression of kisspeptin mRNA. This indicates that while EB-suppression of kisspeptin mRNA in the ARH is mediated by ERs, it is not mediated by the ERαΔ4-mGluR2/3 complex (Fig. 5B; vehicle + EB: 0.35 ± 0.08; LY341,495 + EB: 0.31 ± 0.07 vs. vehicle + oil: 1.01 ± 0.08; one-way ANOVA, Student-Newman-Keuls; P < 0.001; df = 18; t = 2.563).
mGluR2/3 was not localized in KNDy neurons
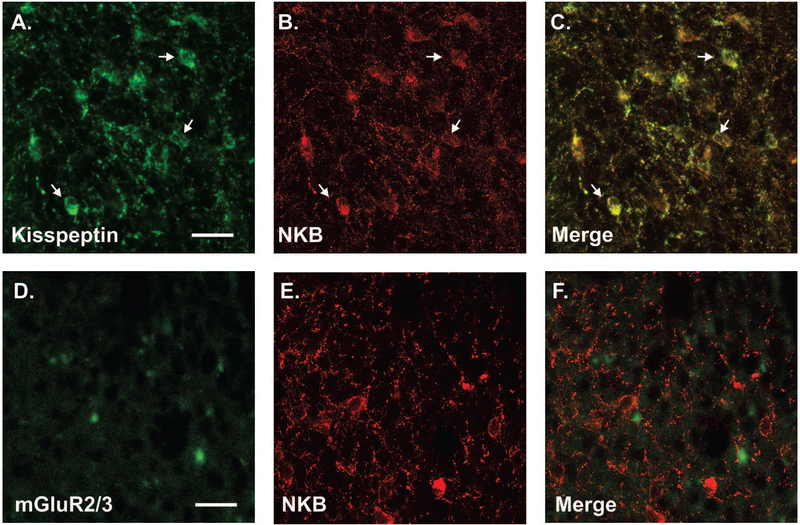
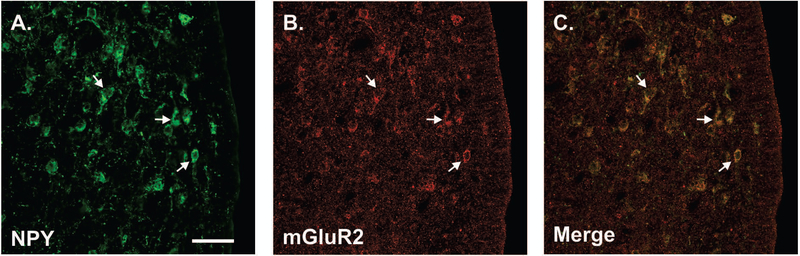
A parallel immunohistochemistry experiment was done to determine whether neurons that express mGluR2/3 in the ARH express kisspeptin, providing a morphological substrate for estradiol inhibition of kisspeptin expression. We used ARH tissue from colchicine-treated ovx Long-Evans rats. Since both antibodies to kisspeptin and mGluR2/3 are rabbit polyclonal antibodies, we first verified that ARH KNDy neurons could be detected with both kisspeptin and NKB antibodies using the rabbit polyclonal antibody to kisspeptin and a guinea pig polyclonal antibody to neurokinin B (NKB; gift from Dr. Philippe Ciofi, University of Bordeaux) and mGluR2/3. As previously reported, kisspeptin staining was co-localized in nearly all NKB cells [(29); Fig. 6A]. Next, ARH tissue was stained for NKB (KNDy marker) and mGluR2/3. No colocalization was observed for NKB and mGluR2/3 (Fig. 6B). These observations were consistent with the pharmacological results that estradiol signaling in KNDy neurons does not require mGluR2/3. Interestingly, NPY immunoreactive neurons of the ARH were mGluR2/3 immunoreactive (Fig. 7). These results provide support for the idea that ERαΔ4-mGluR2/3 signaling does not regulate kisspeptin expression by acting in KNDy neurons. It suggests that NPY neurons in the ARH may be modulated by ERαΔ4-mGluR2/3 mediated estradiol signaling.
Figure 6. KNDy neurons show little mGluR2 immunoreactivity.
Representative images of ARH KNDy neurons in ovx female rats stained with a rabbit polyclonal antibody to (A) kisspeptin (AB9754; EMD Millipore) and (B) a guinea pig polyclonal antibody to neurokinin B (NKB; kind gift from Dr. Philippe Ciofi, University of Bordeaux). (C) In the merged image, arrows show cells immunolabeled with both kisspeptin and NKB. To determine if mGluR2 co-localized with KNDy neurons, (D) a polyclonal rabbit to mGluR2/3 (AB1553; EMD Millipore) was used to label mGluR2/3 in (E) cells positive for NKB. (F) Immunoreactivity with NKB and mGluR2/3 were distinct with little overlap. Representative images of the distribution of kisspeptin, neurokinin B and mGluR2 in the ARH of 10 animals examined. Scale bar (25 µm) in (A & D).
Figure 7. NPY and mGluR2/3 colocalize in the female rat ARH.
30 min before EB & 48 h prior to perfusion rats were treated with 100 µg colchicine. (A) NPY was identified with antibody AB1583 (EMD Millipore) and (B) mGluR2/3 with antibody AB1553. (C) Arrows show cells immunolabled with both NPY and mGluR2/3. Scale bar (25 µm) in (A). Micrographs are representative images from 10 animals examined.
DISCUSSION
We report that a splice variant of ERα, ERαΔ4, localized to the plasma membrane, mediated inhibitory cell signaling. We demonstrated that ERαΔ4 associated with the scaffolding protein, CAV-3, and the membrane receptor, mGluR2/3. Knockdown of CAV-3 protein in female ARH tissue reduced membrane levels of ERαΔ4, indicating that trafficking of ERαΔ4 was dependent on this isoform of caveolin. Co-immunoprecipitation studies indicated that in addition to CAV-3, ERαΔ4 associates with mGluR2/3. Indeed, activation of ERαΔ4 decreased cAMP levels, a predicted inhibitory action of transactivating group II mGluRs (9). EB treatment reduced membrane levels of ERαΔ4 and mGluR2/3 and increased cytosolic levels, indicating a ligand-mediated internalization. While estradiol restrained the expression of kisspeptin in the ARH, this was not mediated via ERαΔ4.
As previously reported, ERαΔ4 is a prominent membrane ER in hypothalamic neurons and astrocytes (5, 6, 12, 40, 41). This receptor is an alternatively spliced variant of ERα that lacks exon 4 of ESR1. ERαΔ4 mRNA is present throughout the mammalian brain (7, 42). Exon 4, which is missing from ERαΔ4, codes for the ERα hinge region, nuclear translocation signal sequence and a part of the ligand binding domain (43, 44). Due to the absence of exon 4, ERαΔ4 would not be expected to be translocated into the nucleus because of the missing nuclear translocation signal coded by exon 4. Indeed, in COS7 cells transfected with cytomegalovirus (CMV) promoter-driven ERαΔ4 nuclear ERα staining is greatly reduced (45). While we have not done binding assays, ERαΔ4 behaves as if it is activated by estradiol (i.e., internalized following treatment with its ligand estradiol). The present study confirmed that estradiol treatment reduced membrane ERαΔ4 levels, and increased levels in the cytosolic fraction as expected for internalization in a ligand-dependent manner (3, 5, 41). This is a hallmark of membrane receptors that have been activated by their natural ligands ((46); reviewed in (36, 41, 47, 48)).
Caveolins, scaffolding proteins, are necessary for steroid nuclear receptor trafficking to the cell membrane (49–52). Additionally, CAV proteins appear to regulate the type of mGluR that associates with ERs (11). Previously, we found that knocking down CAV-1 protein levels reduced trafficking of full-length ERα, and mediated coupling with mGluR1a, which activates excitatory actions of estradiol, including: intracellular calcium, PKC, MAPK, and ultimately CREB (3, 5, 6, 12, 17, 20, 41, 53). In addition to the hypothalmus, the ER and mGluR interactions mediate estradiol suppression of GABAergic inhibition in female rats via an intracellular signaling pathway involving phospholipase C and inositol triphosphate (54, 55), inhibition of internal free calcium in DRG cells (56), and striatal function (15, 57, 58).
In the present study, we used co-immunoprecipitation to show that mGluR2/3 associates with the 52 kDa ERαΔ4, but not the full-length (66 kDa) ERα. This suggests that ERαΔ4 potentially mediates inhibitory estradiol actions. Activating ERαΔ4 decreased cAMP levels, most likely through the inhibition of adenylyl cyclase. While we did not measure adenylyl cyclase activity directly, our results are consistent with previous studies that have reported that high estrogen levels in hypothalamic tissue were associated with lower cAMP levels due to reduced adenylyl cyclase activity (59, 60), and that estradiol treatment in ovariectomized female rats reduced basal cAMP production in pituitary homogenates and pineal gland (61, 62). These results appear to contrast with other studies where estrogen activates cAMP signaling pathways in the ventromedial hypothalamus (63–65).
Altering cAMP levels ultimately affects intracellular calcium dynamics. PKA, which is activated by cAMP, activates L-type voltage gated calcium channels (VGCC). In neostriatal neurons, estradiol inhibited barium currents by targeting nifedipine-sensitive (L-type) calcium channels via a membrane receptor mediating Gαi activation (66). During aging, L-type VGCC current increases in the hippocampus due to dysregulation of calcium homeostasis, however estradiol reduces this age-related increase back to levels comparable to young animals (67). Moreover, estradiol inhibits ATP-induced intracellular calcium currents by attenuating L-type VGCC in dorsal root ganglion (DRG) neurons (56). This inhibition, which was tamoxifen- and ICI 182,780-sensitive, was mediated via mGluR2/3 (56, 68).
Palmitoylation, the addition of a 16 carbon fatty acid chain to cysteine residues, of ER and caveolins is required to localize ER/mGluR to the plasma membrane and for ER/mGluR signaling (49, 51, 69). Palmitoylation of ERα and CAV-1 are regulated by the palmitoyl acyltransferases DHHC7 and DHHC21 (51, 69). The palmitoylation sites on CAV-1 and CAV-3 are similar (70, 71), therefore, palmitoylation of CAV-3 may also be regulated by DHHC7 and DHHC21. Little is known about palmitoylation of mGluRs. Only mGluR4, but not mGluR1α, was shown to be palmitoylated (72, 73). While mGluR2 and mGluR3 have not been studied, it is expected that they abide by the same rules as established for other ER-mGluR interactions.
Previously, we demonstrated that the ERα-mGluR1a receptor complex was internalized as a unit (5, 41). Since ERαΔ4-mGluR2/3, like ERα-mGluR1a, is internalized after estradiol treatment, it is reasonable to assume a similar mechanism of internalized as a receptor complex. An interesting observation was that estradiol-induced internalization of ERαΔ4 was not associated with an increase in CAV-3 in cytosolic fractions. These results suggest that estradiol binding induces ERαΔ4-mGluR2/3 dissociation and internalization of ERαΔ4 without CAV-3. We had not measured CAV-1 in the cytoplasm, so we cannot compare the present results with CAV-3 to CAV-1 in our previous ERα-mGluR1a internalization studies. While a definitive conclusion will require further experiments, it appears that both the full-length ERα and the splice variant behave similarly after binding estadiol. Alternatively, another possibility is that the apparent disassociation of ERαΔ4 and mGluR2/3 was an artifact of an abundance of mGluR2/3 in the membrane that is not associated with ERαΔ4, thus, very little of the mGluR2/3 was internalized compared with membrane levels. Regardless of the exact explanation, the internalization of ERαΔ4, which requires ligand binding, is a strong indication that ERαΔ4 binds estradiol.
Estradiol in the ARH has both excitatory and inhibitory actions. Estradiol in the ARH activates female sexual receptivity, which depends on membrane-initiated actions mediated by the ERα-mGluR1a signaling complex (10, 20). Another powerful action of estradiol in the ARH is to down regulate kisspeptin expression (22, 38, 74–77). When it became apparent that estradiol signaling through the ERαΔ4-mGluR2/3 decreases cAMP, we sought to determine whether the estradiol inhibition of kisspeptin expression was due to signaling through the ERαΔ4-mGluR2/3 complex. To test this, we sequentially blocked ER and then mGluR2/3. As expected, ICI 182,780 prevented the estradiol-induced down regulation of kisspeptin mRNA in the ARH, which is consistent with previous studies showing that ERα mediates kisspeptin suppression (37). Antagonism of mGluR2/3, however, did not block the estradiol-induced down-regulation of kisspeptin expression. Immunohistochemical localization of a marker of KNDy neurons, NKB, and mGluR2/3 provided an explanation - KNDy neurons did express not mGluR2/3-immunoreactivity. Unexpectedly, mGluR2/3- and NPY-immunoreactivity were co-localized in ARH neurons, suggesting ERαΔ4 mediates estradiol-induced inhibition in a cell type different from KNDy neurons.
In the ARH, NPY has numerous important physiological actions that are influenced by estradiol, including regulation of energy balance and sexual receptivity (reviewed in (78–81)). However, the interactions between estradiol and NPY have been difficult to parse. An estradiol-induced increase in NPY mRNA and protein levels has been reported, but others report no change or even a decrease after estradiol treatment (82–88). Our finding of mGluR2/3 in NPY neurons suggests that estradiol’s action in NPY neurons may be both facilitatory and inhibitory, which may speak to the disparate findings in the literature. One hypothesis is that activation of ERα or another ER would increase NPY expression, but inhibition of NPY expression may be mediated via -ERαΔ4-mGluR2/3 signaling. Understanding the conflicting actions of estradiol in NPY neurons will require further investigation, but may relate to dose and/or timing of estradiol treatment.
In summary, ERαΔ4 is an estradiol-activated membrane receptor that is trafficked to the cell membrane in association with CAV-3. ERαΔ4 transactivates mGluR2/3 to negatively regulate adenylyl cyclase, resulting in reduced cAMP levels. Thus, ERαΔ4 may mediate some of the inhibitory actions ascribed to ERα. However, as we demonstrated, not all inhibitory actions can be explained by ERαΔ4 signaling. As we showed, estradiol-induced down-regulation of kisspeptin in the ARH is not dependent on ERαΔ4 and mGluR2/3.
Supplementary Material
Supplementary Figure 1. Timelines of animal experiments. (A) Bilateral cannulae directed toward the ARH for siRNA experiments were placed two weeks post ovx, 1 week after delivery. Following an additional week recovery, rats received a bolus injection of EB (5 µg s.c.). Four days later, CAV-3 siRNA or scrambled siRNA (2 µg) was infused into the ARH via the implanted cannulae and the rats received the second EB injection 30 mins later. Over the next four days CAV-3 siRNA or scrambled siRNA was infused three more times into the ARH (24 h, 72h, and 96 h after the initial siRNA infusion). Thirty minutes after the final siRNA infusion, the female rats received their last s.c. EB injection and there killed 30 later. ARH tissue was obtained and processed for western blotting. (B) For kisspeptin transcription studies, inhibitors of ER (ICI 182,780; 50 µg), mGluR2/3 (LY341,495; 25 nmol), or their respective vehicle (DMSO/aCSF for ICI 182,780 or NaOH/aCSF for LY341,495) were injected into the third ventricle 30 min prior to EB (10 µg; s.c.). Twenty-four hours later, rats received the second EB injection (10 µg; s.c.), and were killed 24 h later (48 h after the first EB injection). ARH tissue was fractionated into membrane and cytoplasmic samples and processed for kisspeptin qPCR. In (C) to examine cAMP levels, rats were treated with two doses of EB (5 µg; s.c.), the first 2 weeks after ovx, then 4 days later. After another 4 days, the third ventricle was infused with LY341,495 (25 nmol) or vehicle (NaOH/aCSF) and immediately followed by E2 (10 nmol) or vehicle. 30 min later, animals were killed and the ARH tissues collected, homogenized, and analyzed for cAMP levels with a commercial cAMP ELISA kit.
Supplementary Figure 2. Western blot demonstrating the localization of CAV-3 protein to the membrane fraction. CAV-3 protein was detected in the hypothalamic membrane fractions. No immunoreactive CAV-3 was localized in the cytoplasmic fraction even though 10x more protein (100 µg) was loaded compared with the membrane fraction. For the cytoplasmic fraction n=8.
ACKNOWLEDGEMENTS
The authors are grateful to Brennan Falcy for his insightful comments on the manuscript. This research was supported by the National Institutes of Health grants DA013185 and HD042645 for support of this research. The authors have no competing financial interests.
REFERENCES
- 1.Micevych P, Christensen A. Membrane-initiated estradiol signaling regulates the central nervous system. Frontiers in neuroendocrinology. 2012; 33(4): 329–30. [DOI] [PubMed] [Google Scholar]
- 2.Micevych PE, Meisel RL. Integrating Neural Circuits Controlling Female Sexual Behavior. Front Syst Neurosci. 2017; 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Frontiers in neuroendocrinology. 2009; 30(3): 315–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008; 154(4): 1173–7. [DOI] [PubMed] [Google Scholar]
- 5.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-alpha trafficking. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009; 29(48): 15323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dominguez R, Micevych P. Estradiol rapidly regulates membrane estrogen receptor alpha levels in hypothalamic neurons. The Journal of Neuroscience. 2010; 30(38): 12589–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skipper JK, Young LJ, Bergeron JM, Tetzlaff MT, Osborn CT, Crews D Identification of an isoform of the estrogen receptor messenger RNA lacking exon four and present in the brain. Proceedings of the National Academy of Sciences, USA. 1993; 90(August): 7172–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. The Journal of biological chemistry. 2003; 278(4): 2701–12. [DOI] [PubMed] [Google Scholar]
- 9.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Molecular neurobiology. 2008; 38(1): 66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007; 27(35): 9294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meitzen J, Mermelstein PG. Estrogen receptors stimulate brain region specific metabotropic glutamate receptors to rapidly initiate signal transduction pathways. J Chem Neuroanat. 2011; 42(4): 236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Christensen A, Micevych P. CAV1 siRNA reduces membrane estrogen receptor-alpha levels and attenuates sexual receptivity. Endocrinology. 2012; 153(8): 3872–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. Journal of Neuroscience. 2007; 27(37): 9941–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaban V, Li J, McDonald JS, Rapkin A, Micevych P. Estradiol attenuates the adenosine triphosphate-induced increase of intracellular calcium through group II metabotropic glutamate receptors in rat dorsal root ganglion neurons. Journal of neuroscience research. 2011; 89(11): 1707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross KS, Brandner DD, Martinez LA, Olive MF, Meisel RL, Mermelstein PG. Opposite Effects of mGluR1a and mGluR5 Activation on Nucleus Accumbens Medium Spiny Neuron Dendritic Spine Density. PLoS One. 2016; 11(9): e0162755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo J, Hamid N, Bondar G, Prossnitz ER, Micevych P. Membrane estrogen receptors stimulate intracellular calcium release and progesterone synthesis in hypothalamic astrocytes. Journal of Neuroscience. 2010; 30(39): 12950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane estrogen receptor-alpha interacts with metabotropic glutamate receptor type 1a to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2009; 150(3): 1369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibowitz SF, Akabayashi A, Alexander JT, Wang J. Gonadal steroids and hypothalamic galanin and neuropeptide Y: role in eating behavior and body weight control in female rats. Endocrinology. 1998; 139(4): 1771–80. [DOI] [PubMed] [Google Scholar]
- 19.Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011; 31(48): 17583–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewing P, Christensen A, Bondar G, Micevych P. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008; 149(12): 5934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dungan HM, Clifton DK, Steiner RA. Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006; 147(3): 1154–8. [DOI] [PubMed] [Google Scholar]
- 22.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006; 26(25): 6687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008; 149(4): 1951–9. [DOI] [PubMed] [Google Scholar]
- 24.Butera PC, Czaja JA. Intracranial estradiol in ovariectomized guinea pigs: effects on ingestive behaviors and body weight. Brain research. 1984; 322(1): 41–8. [DOI] [PubMed] [Google Scholar]
- 25.Kammermeier PJ, Davis MI, Ikeda SR. Specificity of metabotropic glutamate receptor 2 coupling to G proteins. Molecular pharmacology. 2003; 63(1): 183–91. [DOI] [PubMed] [Google Scholar]
- 26.Prezeau L, Manzoni O, Homburger V, Sladeczek F, Curry K, Bockaert J. Characterization of a metabotropic glutamate receptor: direct negative coupling to adenylyl cyclase and involvement of a pertussis toxin-sensitive G protein. Proceedings of the National Academy of Sciences of the United States of America. 1992; 89(17): 8040–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genazzani AA, Casabona G, L’Episcopo MR, Condorelli DF, Dell’Albani P, Shinozaki H, Nicoletti F. Characterization of metabotropic glutamate receptors negatively linked to adenylyl cyclase in brain slices. Brain research. 1993; 622(1–2): 132–8. [DOI] [PubMed] [Google Scholar]
- 28.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005; 25(20): 5066–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.True C, Kirigiti M, Ciofi P, Grove KL, Smith MS. Characterisation of arcuate nucleus kisspeptin/neurokinin B neuronal projections and regulation during lactation in the rat. Journal of neuroendocrinology. 2011; 23(1): 52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012; 153(6): 2800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Micevych PE, Abelson L, Fok H, Ulibarri C, Priest CA. Gonadal steroid control of preproenkephalin mRNA expression in the limbic-hypothalamic circuit: Comparison of adult with neonatal steroid treatments. J of Neurosci Res. 1994; 38386–98. [DOI] [PubMed] [Google Scholar]
- 32.Walker JM. The bicinchoninic acid (BCA) assay for protein quantitation. Methods in molecular biology (Clifton, NJ). 1994; 325–8. [DOI] [PubMed] [Google Scholar]
- 33.Kinsey-Jones JS, Li XF, Luckman SM, O’Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008; 149(3): 1004–8. [DOI] [PubMed] [Google Scholar]
- 34.Xu L, Qu Z, Guo F, Pang M, Gao S, Zhu H, Gu F, Sun X. Effects of ghrelin on gastric distention sensitive neurons in the arcuate nucleus of hypothalamus and gastric motility in diabetic rats. Peptides. 2013; 48137–46. [DOI] [PubMed] [Google Scholar]
- 35.Claing A, Laporte SA, Caron MG, Lefkowitz RJ. Endocytosis of G protein-coupled receptors: roles of G protein-coupled receptor kinases and beta-arrestin proteins. Prog Neurobiol. 2002; 66(2): 61–79. [DOI] [PubMed] [Google Scholar]
- 36.Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE. 2005; 2005(308): cm10. [DOI] [PubMed] [Google Scholar]
- 37.Dubois SL, Acosta-Martinez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, Urban JH, Levine JE. Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor alpha in kisspeptin neurons. Endocrinology. 2015; 156(3): 1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horii Y, Dalpatadu SL, Soga T, Ohta R, Watanabe G, Taya K, Parhar IS. Estrogenic regulation of Kiss1 mRNA variants in Hatano rats. General and comparative endocrinology. 2013; 181246–53. [DOI] [PubMed] [Google Scholar]
- 39.Yang JA, Yasrebi A, Snyder M, Roepke TA. The interaction of fasting, caloric restriction, and diet-induced obesity with 17beta-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Molecular and cellular endocrinology. 2016; 43735–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dominguez R, Dewing P, Kuo J, Micevych P. Membrane-initiated estradiol signaling in immortalized hypothalamic N-38 neurons. Steroids. 2013; 78(6): 607–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong AM, Abrams MC, Micevych PE. beta-arrestin regulates estradiol membrane-initiated signaling in hypothalamic neurons. PLoS One. 2015; 10(3): e0120530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pfeffer U, Fecarotta E, Castagnetta L, Vidali G. Estrogen receptor variant messenger RNA lacking exon 4 in estrogen-responsive human breast cancer cell lines. Cancer research. 1993; 53(4): 741–3. [PubMed] [Google Scholar]
- 43.Harris LF, Sullivan MR, Popken-Harris PD. Molecular dynamics simulation in solvent of the estrogen receptor protein DNA binding domain in complex with a non-consensus estrogen response element DNA sequence. Journal of biomolecular structure & dynamics. 1997; 15(3): 407–30. [DOI] [PubMed] [Google Scholar]
- 44.Pasqualini C, Guivarc’h D, Boxberg YV, Nothias F, Vincent JD, Vernier P. Stage- and region-specific expression of estrogen receptor alpha isoforms during ontogeny of the pituitary gland. Endocrinology. 1999; 140(6): 2781–9. [DOI] [PubMed] [Google Scholar]
- 45.Bollig A, Miksicek RJ. An estrogen receptor-alpha splicing variant mediates both positive and negative effects on gene transcription. Molecular endocrinology. 2000; 14(5): 634–49. [DOI] [PubMed] [Google Scholar]
- 46.Altier C, Khosravani H, Evans RM, Hameed S, Peloquin JB, Vartian BA, Chen L, Beedle AM, Ferguson SS, Mezghrani A, Dubel SJ, Bourinet E, McRory JE, Zamponi GW. ORL1 receptor-mediated internalization of N-type calcium channels. Nature neuroscience. 2006; 9(1): 31–40. [DOI] [PubMed] [Google Scholar]
- 47.Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009; 29(13): 4228–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y, Xia J, Liu S, Stein S, Ramon C, Xi H, Wang L, Xiong X, Zhang L, He D, Yang W, Zhao X, Cheng X, Yang X, Wang H. Endocytosis and membrane receptor internalization: implication of F-BAR protein Carom. Frontiers in bioscience (Landmark edition). 2017; 221439–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. The Journal of biological chemistry. 2007; 282(31): 22278–88. [DOI] [PubMed] [Google Scholar]
- 50.Deng Q, Wu Y, Zhang Z, Wang Y, Li M, Liang H, Gui Y. Androgen Receptor Localizes to Plasma Membrane by Binding to Caveolin-1 in Mouse Sertoli Cells. Int J Endocrinol. 2017; 20173985916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tonn Eisinger KR, Woolfrey KM, Swanson SP, Schnell SA, Meitzen J, Dell’Acqua M, Mermelstein PG. Palmitoylation of caveolin-1 is regulated by the same DHHC acyltransferases that modify steroid hormone receptors. The Journal of biological chemistry. 2018; 293(41): 15901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pastore MB, Landeros RV, Chen DB, Magness RR. Structural analysis of estrogen receptors: interaction between estrogen receptors and cav-1 within the caveolaedagger. Biology of reproduction. 2019; 100(2): 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boulware MI, Heisler JD, Frick KM. The memory-enhancing effects of hippocampal estrogen receptor activation involve metabotropic glutamate receptor signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013; 33(38): 15184–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012; 74(5): 801–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tabatadze N, Huang G, May RM, Jain A, Woolley CS. Sex Differences in Molecular Signaling at Inhibitory Synapses in the Hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015; 35(32): 11252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003; 118(4): 941–8. [DOI] [PubMed] [Google Scholar]
- 57.Peterson BM, Mermelstein PG, Meisel RL. Estradiol mediates dendritic spine plasticity in the nucleus accumbens core through activation of mGluR5. Brain Struct Funct. 2015; 220(4): 2415–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meitzen J, Meisel RL, Mermelstein PG. Sex Differences and the Effects of Estradiol on Striatal Function. Current opinion in behavioral sciences. 2018; 2342–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ani M, Butterworth PJ, Thomas PJ. Role of dopamine-sensitive adenylate cyclase in the sexual differentiation of the brain [proceedings]. The Journal of endocrinology. 1978; 79(2): 31p–2p. [PubMed] [Google Scholar]
- 60.Ani M, Butterworth PJ, Thomas PJ. Effect of estradiol on neurotransmitter sensitive adenylate cyclase. Its possible role in ‘sexual differentiation’. Brain research. 1980; 183(2): 341–53. [DOI] [PubMed] [Google Scholar]
- 61.Borgundvaag B, George SR. Estrogen regulation of rat anterior pituitary adenylate cyclase. Molecular and cellular endocrinology. 1988; 59(1–2): 35–45. [DOI] [PubMed] [Google Scholar]
- 62.Okatani Y, Hayashi K, Sagara Y. Effect of estrogen on melatonin synthesis in female peripubertal rats as related to adenylate cyclase activity. Journal of pineal research. 1998; 25(4): 245–50. [DOI] [PubMed] [Google Scholar]
- 63.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proceedings of the National Academy of Sciences of the United States of America. 1994; 91(18): 8517–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orihuela PA, Parada-Bustamante A, Cortes PP, Gatica C, Croxatto HB. Estrogen receptor, cyclic adenosine monophosphate, and protein kinase A are involved in the nongenomic pathway by which estradiol accelerates oviductal oocyte transport in cyclic rats. Biology of reproduction. 2003; 68(4): 1225–31. [DOI] [PubMed] [Google Scholar]
- 65.Chen C, Kuo J, Wong A, Micevych P. Estradiol Modulates Translocator Protein (TSPO) and Steroid Acute Regulatory Protein (StAR) via Protein Kinase A (PKA) signaling in Hypothalamic Astrocytes. Endocrinology. 2014; 155(8): 2976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum the female rat during paced copulatory behavior. Behav Neurosci. 1995; 109(2): 354–65. [DOI] [PubMed] [Google Scholar]
- 67.Brewer LD, Dowling AL, Curran-Rauhut MA, Landfield PW, Porter NM, Blalock EM. Estradiol reverses a calcium-related biomarker of brain aging in female rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009; 29(19): 6058–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca(2+) signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005; 81(1): 31–7. [DOI] [PubMed] [Google Scholar]
- 69.Meitzen J, Luoma JI, Boulware MI, Hedges VL, Peterson BM, Tuomela K, Britson KA, Mermelstein PG. Palmitoylation of estrogen receptors is essential for neuronal membrane signaling. Endocrinology. 2013; 154(11): 4293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song KS, Scherer PE, Tang Z, Okamoto T, Li S, Chafel M, Chu C, Kohtz DS, Lisanti MP. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. The Journal of biological chemistry. 1996; 271(25): 15160–5. [DOI] [PubMed] [Google Scholar]
- 71.Kim JH, Peng D, Schlebach JP, Hadziselimovic A, Sanders CR. Modest effects of lipid modifications on the structure of caveolin-3. Biochemistry. 2014; 53(27): 4320–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alaluf S, Mulvihill ER, McIlhinney RA. The metabotropic glutamate receptor mGluR4, but not mGluR1 alpha, is palmitoylated when expressed in BHK cells. Journal of neurochemistry. 1995; 64(4): 1548–55. [DOI] [PubMed] [Google Scholar]
- 73.Pickering DS, Taverna FA, Salter MW, Hampson DR. Palmitoylation of the GluR6 kainate receptor. Proceedings of the National Academy of Sciences of the United States of America. 1995; 92(26): 12090–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009; 29(29): 9390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004; 145(10): 4565–74. [DOI] [PubMed] [Google Scholar]
- 76.Bosch MA, Xue C, Ronnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: effects of 17beta-estradiol. The Journal of comparative neurology. 2012; 520(10): 2143–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takase K, Uenoyama Y, Inoue N, Matsui H, Yamada S, Shimizu M, Homma T, Tomikawa J, Kanda S, Matsumoto H, Oka Y, Tsukamura H, Maeda KI. Possible role of oestrogen in pubertal increase of Kiss1/kisspeptin expression in discrete hypothalamic areas of female rats. Journal of neuroendocrinology. 2009; 21(6): 527–37. [DOI] [PubMed] [Google Scholar]
- 78.Micevych PE, Mermelstein PG, Sinchak K. Estradiol Membrane-Initiated Signaling in the Brain Mediates Reproduction. Trends Neurosci. 2017; 40(11): 654–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kageyama H, Takenoya F, Hirako S, Wada N, Kintaka Y, Inoue S, Ota E, Ogawa T, Shioda S. Neuronal circuits involving neuropeptide Y in hypothalamic arcuate nucleus-mediated feeding regulation. Neuropeptides. 2012; 46(6): 285–9. [DOI] [PubMed] [Google Scholar]
- 80.Muroi Y, Ishii T. A novel neuropeptide Y neuronal pathway linking energy state and reproductive behavior. Neuropeptides. 2016; 591–8. [DOI] [PubMed] [Google Scholar]
- 81.Kohno D, Yada T. Arcuate NPY neurons sense and integrate peripheral metabolic signals to control feeding. Neuropeptides. 2012; 46(6): 315–9. [DOI] [PubMed] [Google Scholar]
- 82.Sahu A, Crowley WR, Kalra PS, Kalra SP. A selective sexually dimorphic response in the median eminence neuropeptide Y. Brain research. 1992; 573(2): 235–42. [DOI] [PubMed] [Google Scholar]
- 83.Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Withdrawal of [corrected] estrogen increases hypothalamic neuropeptide Y (NPY) mRNA expression in ovariectomized obese rat. Neuroscience letters. 1996; 204(1–2): 81–4. [DOI] [PubMed] [Google Scholar]
- 84.Nedungadi TP, Briski KP. Effects of estradiol on acute and recurrent insulin-induced hypoglycemia-associated patterns of arcuate neuropeptide Y, proopiomelanocortin, and cocaine- and amphetamine-related transcript gene expression in the ovariectomized rat. Neuroendocrinology. 2007; 86(4): 270–6. [DOI] [PubMed] [Google Scholar]
- 85.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. Journal of neuroendocrinology. 2007; 19(6): 426–31. [DOI] [PubMed] [Google Scholar]
- 86.Kalamatianos T, Grimshaw SE, Poorun R, Hahn JD, Coen CW. Fasting reduces KiSS-1 expression in the anteroventral periventricular nucleus (AVPV): effects of fasting on the expression of KiSS-1 and neuropeptide Y in the AVPV or arcuate nucleus of female rats. Journal of neuroendocrinology. 2008; 20(9): 1089–97. [DOI] [PubMed] [Google Scholar]
- 87.Silva LE, Castro M, Amaral FC, Antunes-Rodrigues J, Elias LL. Estradiol-induced hypophagia is associated with the differential mRNA expression of hypothalamic neuropeptides. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas. 2010; 43(8): 759–66. [DOI] [PubMed] [Google Scholar]
- 88.Roepke TA, Qiu J, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17beta-estradiol differentially modulate the M-current in neuropeptide Y neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011; 31(33): 11825–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Timelines of animal experiments. (A) Bilateral cannulae directed toward the ARH for siRNA experiments were placed two weeks post ovx, 1 week after delivery. Following an additional week recovery, rats received a bolus injection of EB (5 µg s.c.). Four days later, CAV-3 siRNA or scrambled siRNA (2 µg) was infused into the ARH via the implanted cannulae and the rats received the second EB injection 30 mins later. Over the next four days CAV-3 siRNA or scrambled siRNA was infused three more times into the ARH (24 h, 72h, and 96 h after the initial siRNA infusion). Thirty minutes after the final siRNA infusion, the female rats received their last s.c. EB injection and there killed 30 later. ARH tissue was obtained and processed for western blotting. (B) For kisspeptin transcription studies, inhibitors of ER (ICI 182,780; 50 µg), mGluR2/3 (LY341,495; 25 nmol), or their respective vehicle (DMSO/aCSF for ICI 182,780 or NaOH/aCSF for LY341,495) were injected into the third ventricle 30 min prior to EB (10 µg; s.c.). Twenty-four hours later, rats received the second EB injection (10 µg; s.c.), and were killed 24 h later (48 h after the first EB injection). ARH tissue was fractionated into membrane and cytoplasmic samples and processed for kisspeptin qPCR. In (C) to examine cAMP levels, rats were treated with two doses of EB (5 µg; s.c.), the first 2 weeks after ovx, then 4 days later. After another 4 days, the third ventricle was infused with LY341,495 (25 nmol) or vehicle (NaOH/aCSF) and immediately followed by E2 (10 nmol) or vehicle. 30 min later, animals were killed and the ARH tissues collected, homogenized, and analyzed for cAMP levels with a commercial cAMP ELISA kit.
Supplementary Figure 2. Western blot demonstrating the localization of CAV-3 protein to the membrane fraction. CAV-3 protein was detected in the hypothalamic membrane fractions. No immunoreactive CAV-3 was localized in the cytoplasmic fraction even though 10x more protein (100 µg) was loaded compared with the membrane fraction. For the cytoplasmic fraction n=8.