Abstract
Hypothalmic orexin/hypocretin (Orx) neurons in the lateral and dorsomedial perifornical region (LH-DMH/PeF) innervate broadly throughout the brain, and receive similar inputs. This wide distribution, as well as two Orx peptides (OrxA and OrxB) and two Orx receptors (Orx1 and Orx2) allow for functionally related but distinctive behavioral outcomes, that include arousal, sleep-wake regulation, food seeking, metabolism, feeding, reward, addiction, and learning. These are all motivational functions, and tie the orexin systems to anxiety and depression as well. We present evidence, that for affective behavior, Orx1 and Orx2 receptors appear to have opposing functions. The majority of research on anxiety- and depression-related outcomes has focused on Orx1 receptors, which appear to have primarily anxiogenic and pro-depressive actions. Although there is significant research suggesting contrary findings, the primary potential for pharmacotherapies linked to the Orx1 receptor is via antagonists to block anxious and depressive behavior. Dual orexin receptor antagonists have been approved for treatment of sleep disorders, and are likely candidates for adaptation for affect disorder treatments. However, we present evidence here that demonstrates the Orx2 receptors are anxiolytic and antidepressive. Using a new experimental pre-clinical model of anxious and depressive behavior stimulated by social stress and decision-making that produces two stable behavioral phenotypes, Escape/Resilient and Stay/Susceptible, we tested the effects of intracerebroventricular injections of Orx2 agonist and antagonist drugs. Over ten behavioral measures, we have demonstrated that Orx2 agonists promote resilience, as well as anxiolytic and antidepressive behavior. In contrast, Orx2 antagonists or knockdown kindle anxious and pro-depressive behavior plus increase susceptibility. The results suggest that the Orx2 receptor may be a useful target for pharmacotherapies to treat anxiety and depression.
Keywords: Anxiety, Depression, Fear conditioning, Orexin 2 receptor, Social defeat, Stress-Alternatives Model
1. Orexin / hypocretins’ regulatory role
The orexins (Orx; also called hypocretins) have an extremely broad distribution of projections and terminals from a tiny number of perikarya in the lateral-dorsomedial hypothalamus near the perifornical area (LH-DMH/PeF) (Baldo et al., 2003; Ch’ng and Lawrence, 2015; Chen et al., 1999; Cluderay et al., 2002; Marcus et al., 2001; Nambu et al., 1999; Peyron et al., 1998; Schmitt et al., 2012; Trivedi et al., 1998). The input to orexinergic perikarya is equally extensive (Yoshida et al., 2006), suggesting a broadly integrative behavioral regulatory role for the orexins (Bai et al., 2009; Bentzley and Aston-Jones, 2015; Blais et al., 2017; Borgland et al., 2009; Burgess, 2010; Choi et al., 2010; Sakurai, 2014; Scammell and Saper, 2005; Thompson and Borgland, 2011; Thorpe et al., 2005; Tsujino and Sakurai, 2013). As the list of orexin mediated behavioral outcomes includes arousal, emotional behavior, feeding and food seeking, locomotion, metabolism, reward and addictive behavior, sexual behavior, vigilance, and waking, it seems clear that orexins are involved in promoting motivational behavior associated with adaptive responses to stressful stimuli (Giardino and de Lecea, 2014; James et al., 2017b; Mahler et al., 2014; Sakurai, 2014). Importantly, brief and protracted exposure to stressors may differentially involve orexinergic systems, such that acute stress increases activation of Orx neurons (Furlong et al., 2009; Ida et al., 2000), and chronic social defeat stress reduces Orx levels in hypothalamus and elsewhere, which can be ameliorated by food restriction (Lutter et al., 2008; Nocjar et al., 2012). Motivational behavior is essential to affective condition, and the core suite of behaviors, such as arousal and reward, that orexin systems regulate. These are the fundamental stress-related elements that in dysfunctional states yield psychological disorders such as anxiety and depression.
2. Regulatory neurocircuitry for anxiety and depression
Recent studies of the efficacy of preclinical research using animal models to examine the predictive value of classical tests for clinical translation for patients with anxiety and depression, suggest that their effectiveness is low (Haller and Alicki, 2012; Haller et al., 2013). The misalignment between preclinical paradigms, animal models, and clinical translation suggests that the neurocircuitry involved in affective behavior must be carefully distinguished, and sorted to reflect specific neurochemical and molecular mechanisms. The mechanisms that generate anxiety and depression, comorbid as often as 63–81% (current vs lifetime) of the time (Lamers et al., 2011), are broadly associated with traumatically stressful events, and stress-related neurocircuitry, such that higher severity levels of depression and/or anxiety increase comorbidity (Schoevers et al., 2003). It is important to note, that while these affective states are frequently comorbid, they do not often occur simultaneously. That the neural mechanisms involved in fear and anxiety are similar in humans and other animals is substantiated by copious evidence (Davis et al., 2010; Tovote et al., 2015). As these systems and circuits are highly conserved over evolutionary time, and because non-human vertebrates also show distinctively anxious and depressive behavioral inhibition, it seems clear that the use of non-human vertebrate systems is not the problem: We have not been careful in the tests of affective behavior that we use, and therefore the specific neurocircuitry that we evaluate may be inappropriate (Blanchard et al., 2013; Keifer and Summers, 2016; Øverli et al., 2007; Vindas et al., 2016). It is time to carefully analyze anxious and depressive behavior experienced by other vertebrate animals that may be used as models, and be sure the tests designed to measure the species specific anxiety and depression are also species-appropriate (Blanchard et al., 2013; Pearson et al., 2017; Robertson et al., 2015).
Neurocircuitry elements that modulate chronic social stresses characteristic of stimuli that induce depression and anxiety include circuits involved in fear, decision-making, learning and memory, stress, and reward (Kent and Rauch, 2003; Shin and Liberzon, 2010). While the neural circuitries for stress, anxiety, and depression undoubtedly have unique attributes, much of the pathways (and diagnostic criteria) overlap, suggesting to some that these conditions are intrinsically related (Kalueff and Nutt, 2007; Roffman et al., 2005; Stein, 2009). We therefore, have not attempted to create separate descriptions of the neural circuitries for anxiety and depression here. Output from fear-related brain regions, such as the central amygdala (CeA) contribute potently to anxious and depressive behavior (Tasan et al., 2010; Walker and Davis, 2002a; Walker and Davis, 2002b; Zhang et al., 2011). Similarly, the hippocampus also appears to be involved in depression and anxiety (Erickson et al., 2012; Hong et al., 2015; Hritcu and Gorgan, 2014; Kajiyama et al., 2010; Maghsoudi et al., 2014; Mlyniec et al., 2015; Ye et al., 2011), and especially related to neurogenesis in the dentate gyrus (Aboul-Fotouh et al., 2018; Brymer et al., 2018; Gao et al., 2018; Hill et al., 2015; Jesulola et al., 2018; Zhang et al., 2016).
The development of the Social-Defeat Model along with the notion of stress-coping strategies clarified the existence of behavioral phenotypes that are susceptible or resistant to affective disorders (Berton et al., 2007; Iniguez et al., 2014; Keifer and Summers, 2016; Koolhaas et al., 1999; Koolhaas et al., 2007; Korzan and Summers, 2007; Vidal et al., 2011). Discovery of Susceptible and Resilient phenotypes led to the inclusion of reward circuitry, the ventral tegmental area (VTA) and its dopaminergic projections to the nucleus accumbens (NAc) and striatum, as structures that are critically involved in anxiety and depression, through its effects on susceptibility to stress (Krishnan et al., 2007; Muir et al., 2018). The NAc is often considered part of the extended amygdala, along with bed nucleus of the stria terminalis (BNST), which plays an important role in anxiety (Avery et al., 2016; Davis and Shi, 1999; Walker et al., 2003), as well as the amygdala per se (Davis, 1992; Gray et al., 2015; Liebsch et al., 1995; Shekhar et al., 2005). Specialized fear- and extinction-related neurons in the basolateral amygdala (BLA) project specifically to prelimbic (PrL) and infralimbic (IL) cortices respectively (Senn et al., 2014). Local inhibitory circuits associated with the intercalated cells and basolateral amygdala appear to play an important role regulating fear learning and extinction (Amano et al., 2010; Likhtik et al., 2008; McDonald and Mascagni, 2001; Trouche et al., 2013). Orexin-related signaling or inhibition has been demonstrated to have effects in these brain regions, influencing depressive (Arendt et al., 2013; Ito et al., 2009; Lutter et al., 2008; Nocjar et al., 2012; Nollet et al., 2011; Nollet et al., 2012; Ozsoy et al., 2017) and anxious behavior (Arendt et al., 2014; Azogu and Plamondon, 2017; Heydendael et al., 2014; Johnson et al., 2010; Johnson et al., 2012a; Johnson et al., 2012c; Khalil and Fendt, 2017; Lungwitz et al., 2012; Ozsoy et al., 2017). With interactive circuitries (Winsky-Sommerer et al., 2004; Winsky-Sommerer et al., 2005), Orx and corticotropin releasing factor (CRF or CRH) innervate stress-related brain regions (Achua et al., 2014; Ronan et al., 2016), which is important because CRF also directly modulates stress vulnerability along with anxious and depressive behaviors (Hauger et al., 2009; Holsboer and Ising, 2010; Litvin et al., 2007; Takahashi et al., 2001; Waters et al., 2015; Zorrilla et al., 2002).
3. Orx-CRF reciprocal interactions
Recent results suggest direct and indirect reciprocal interaction between brain CRF and Orx systems. Immunolabeling for CRF is abundant in the Orx field of the LH-DMH/PeF, along with Orx immunoreactivity (Achua et al., 2014; Ronan et al., 2016). In addition, Orx perikarya in this field also interact with each other. However, the CRF-Orx inter-reactivity is much more complex, partially due to the distributed nature of CRF synthesis, which contributes to the input to the LH-DMH/PeF (Sakurai et al., 2005; Yoshida et al., 2006) primarily via the ventral BNST (vBNST) and the paraventricular nucleus (PVN) (Achua et al., 2014; Ronan et al., 2016). The molecular mechanisms for these interactions are also significantly more complex than previously thought (Winsky-Sommerer et al., 2004; Winsky-Sommerer et al., 2005). Specifically, receptors for CRF are found on OrxA-immunopositive neurons in the LH-DMH/PeF and CRF positive neurons in the PVN and CeA express Orx receptors (Achua et al., 2014; Ronan et al., 2016). Direct CRF innervation of Orx-expressing neurons yields depolarization via CRF1 receptors, blocked by CRF1 antagonists or knockout (Winsky-Sommerer et al., 2004; Winsky-Sommerer et al., 2005). In addition, Orx neurons connect reciprocally to the CRF system, such that CRF1 receptors mediate stress-induced activation of orexinergic outputs that return activation in a positive feedback manner. New evidence suggests that both CRF1 and CRF2 receptors are involved in regulation of orexinergic neurons; both receptor types are found there (Winsky-Sommerer et al., 2004). While CRF1 receptors have been clearly demonstrated to influence activity of Orx neurons in the LH-DMH/PeF, our immunohistochemical results suggest this CRF1 effect may not be acting directly on Orx neurons. The CRF receptor type found on Orx cells appears to be distinctively CRF2 (Slater et al., 2016). The LH-DMH/PeF CRF1 receptor expression, which influences Orx cell firing rate, is localized to as yet unknown neuronal types (Winsky-Sommerer et al., 2004). Nevertheless, results clearly showing CRF2 expression on Orx cells does not dismiss the reality that CRF can affect orexin neurons through a CRF1-mediated mechanism. This was demonstrated by the ability of astressin, a CRF receptor antagonist, to block CRF-induced depolarization of orexin neurons ex-Wra in hypothalamic tissue slices (Winsky-Sommerer et al., 2004). Recent evidence suggests that more complex circuits are involved, with a wide limbic input of CRF to LH-DMH/PeF, additional regulatory influences by CRF2 and Orx2 receptors plus CRF binding protein (CRFBP), and even indirect CRF influences on the orexin field via the lateral septum (LS) (Achua et al., 2014; Ronan et al., 2016; Slater et al., 2016). The strongest CRF projections directly to the orexin field in the LH-DMH/PeF come from the vBNST, with few connections made directly from dorsal BNST (dBNST), CeA, or even nearby PVN (Achua et al., 2014; Ronan et al., 2016). Broad icv infusion of OrxA suggests that CRF systems are an important target, as 96% of CRFergic cells in the paraventricular nucleus of the hypothalamus (PVN; a major CRFergic area), and 45% in the central nucleus of the amygdala (CeA; also CRFergic), expressed fos activation (Sakamoto et al., 2004). Orexin receptors have been found in the PVN and application of OrxA in PVN has excitatory effects on CRF systems and the hypothalamo-pituitary-adrenal (HPA) axis (Hagan et al., 1999; Marcus et al., 2001). Microinjection of OrxA into the PVN results in large increases in plasma corticosterone levels (Samson et al., 2002). Importantly, BNST inputs, including CRF- and cholecystokinin (CCK)-expressing projections, modulate orexinergic tone in the lateral hypothalamus and condition emotional output (Giardino et al., 2018). Furthermore, physiological control of adrenocorticotropic hormone (ACTH) during acute stress is mediated by Orx2 receptors (Grafe et al., 2017). It seems likely that any regulatory role for Orx in stress-related initiation or inhibition of anxiety or depression is manifest in conjunction with CRF systems, and that interplay between these two systems must always be considered regarding basic mechanisms or therapeutic interventions.
4. Orx1 actions in anxious behavior and fear conditioning
While the actions of Orx1 receptors influence arousal and motivation (Mahler et al., 2014; Sakurai, 2014), they may also coincidently, or separately, act in emotion-related regions of the brain (sometimes via systemic delivery) to promote anxious behavior or anxiety (Alo et al., 2016; Azogu and Plamondon, 2017; Heydendael et al., 2014; Johnson et al., 2010; Johnson et al., 2012a; Johnson et al., 2012c; Khalil and Fendt, 2017; Lungwitz et al., 2012; Ozsoy et al., 2017). Interestingly, narcoleptic patients, while rarely fitting a diagnosis of major depression, exhibit heightened levels of anxiety, particularly related to social phobias (Fortuyn et al., 2010). Effects of OrxA treatments, appear to be primarily related to Orx1 receptor activity. Whole brain (icv) infusion of OrxA in mice increased anxiogenic behaviors in the Light-Dark Test (LDT) and Elevated Plus Maze (EPM) (Suzuki et al., 2005); while effective pharmacological reversal of the anxious behaviors stimulated by icv injection of OrxA or OrxB were highly dependent on the drugs’ specificity for monoaminergic receptors (Matsuzaki et al., 2002). Stress induced through corticosterone administration in mice promoted anxious responses in EPM, commensurate with increased OrxA (Jalewa et al., 2014). Activation of Orx cells using chemogenetic tools (DREADDs) during social defeat promotes a passive coping phenotype, expressing anxiogenic behaviors and reduced memory (Eacret et al., 2018). The functional efficacy of Orx1 antagonists as a pharmacotherapeutic pathway to limiting OrxA activity, and thereby reducing anxiety has merit, however, it also carries the inevitable side effect of modifying sleep-wake cycles, such that careful dosing would be necessary for their effective use.
Despite the uncertainty of its precise physiological role in modulating affect, the orexinergic system has a clear connection to depressive behaviors. Orexinergic cell function is reliably dysregulated by depression (Bowrey et al., 2017). In clinically depressed humans, the concentration of serum Orx was positively associated with incidences of physical or emotional neglect (Ozsoy et al., 2017). Similarly, chemogenetic inhibition (DREADDs) of Orx cells during social defeat increased social interaction, and decreased depressive behavior in vulnerable individuals (Grafe et al., 2018). In contrast, CSF concentrations of OrxA are significantly lower in patients with major depression (Brundin et al., 2007a; Brundin et al., 2007b) and exhibit blunted diurnal variation in this peptide (Salomon et al., 2003). Additionally, severity of the symptoms of depression, negatively correlate with CSF and plasma OrxA (Brundin et al., 2007b; Rotter et al., 2011). Stress induced through corticosterone administration in mice promoted depressive reactions in Tail Suspension Test (TST) trials, but further was associated with increased OrxA-containing cells in the hypothalamus (Jalewa et al., 2014). Wistar-Kyoto rats, a strain demonstrating depressive behaviors and disrupted sleep patterns, possess fewer OrxA-expressing neurons, and these cells have a reduced size in comparison to Wistar control rats (Allard et al., 2004). Similarly, social defeat-induced depressive behavior in rats was accompanied by a reduction of Orx in the VTA, medial prefrontal cortex (mPFC), and hypothalamus (Nocjar et al., 2012). In the ventral pallidum, with direct projections of Orx neurons, Orx activates GABAergic neurons and prevents depressive behaviors, promoting resilience (Ji et al., 2018).
Furthermore, Orx-deficient mice exhibit reduced reactivity to foot shocks, heightened anxious behavior in open field (OF) and LDT paradigms, and an increased fear response to predatory odors (Khalil and Fendt, 2017). In contrast, optogenetic excitation of Orx cells in rats increased anxiogenic aversion for a social target, increased exploratory behaviors, and resulted in the internalization of Orx1 receptors in the paraventricular thalamus (PVT) and locus ceruleus (LC) (Heydendael et al., 2014). These examples describe an influential role of Orx in affective symptomology. In addition to its broad implications to central dysfunction, Orx administered into distinct areas of the stress circuit, namely the BNST (Lungwitz et al., 2012), the CeA (Avolio et al., 2011), and the BLA (Kim et al., 2015), produced anxious and depressive behavior.
In addition, chronic social defeat epigenetically reduced prepro-Orx mRNA, but calorie restriction thereafter enhances activation of Orx cells, which results in an antidepressive response (Lutter et al., 2008). Similarly, early life stress dampened restraint-stimulated Orx cell activity, and produced a depressive behavioral phenotype, all of which were reversed by exercise in adolescent male rats (James et al., 2014). These reports together suggest that reduced Orx is critically associated with depression and preclinical depressive behavior (Bowrey et al., 2017; James et al., 2017a; Yeoh et al., 2014). What is more, in genetic models of depression, reduced Orx levels have also been measured (Allard et al., 2004; Taheri et al., 2001). Rodents bred with the Orx1 gene knocked out, exhibited increased anxious responses (Abbas et al., 2015), reduced depressive behaviors (Abbas et al., 2015; Scott et al., 2011), and impaired fear conditioning in reaction to cued and contextual stimuli (Flores et al., 2014; Soya et al., 2013). Collectively, these results suggest the potential importance of therapeutic antidepressive intervention by targeting Orx1 receptors.
Hyperactivity, a behavior characteristic of anxiety-related disorders, is heightened in prenatal pups exposed to alcohol, but can be alleviated using daily administration (ip) of SB-334867, a potent selective antagonist of Orx1 receptors (Stettner et al., 2011). Curiously, systemic (ip) delivery of SB-334867 does not appear to impact general anxiety in the EPM (Rodgers et al., 2013; Scott et al., 2011; Staples and Cornish, 2014); however, this drug can reverse decreased time in the open arms associated with acute nicotine-induced anxious behavior (Plaza-Zabala et al., 2010). Additionally, panic induced through exposure to hypercapnic air results in increased mean arterial pressure (MAP) and enhanced anxious behavior in the open field (OF), but these measures can be attenuated through injections (ip) of SB-334867 (Johnson et al., 2012c). Furthermore, panic-prone rats administered SB-334867 (ip) exhibited decreased freezing behavior (Johnson et al., 2010) and increased social interaction time (Johnson et al., 2015). Rats systemically-treated (ip) with SB-334867 more readily approached odors associated with naïve and familiar predators (Staples and Cornish, 2014). Administration (ip) of SB-334867 in mice heightened cellular activity in BLA extinction neurons (Flores et al., 2017), suggesting a direct pharmacological action on stress/fear neural circuits. Intracranial infusion of SB-334867 into the posterior PVT decreased time in closed arms (EPM) and reversed OrxA-promoted internalization of Orx1 receptors in the PVT (Heydendael et al., 2011). In a similar way, intra-BLA injections of SB-334867 facilitated fear extinction in mice (Flores et al., 2014). Collectively, these examples demonstrate therapeutic viability of SB-334867 in the treatment of anxiety disorders. In addition to SB-334867, other Orx antagonists have been used to control anxious behavioral output. An fMRI study in rats demonstrated GSK-1059865, a selective Orx1 antagonist, can attenuate abnormal activity in fronto-hippocampal and extended amygdalar regions after yohimbine-induced stress (Gozzi et al., 2013).
Injections of SB-334867 (ip) mimic the Orx1 null mice showing reduced depressive reactions in tests of behavioral despair (FST and TST) (Scott et al., 2011) and fear conditioning paradigms (Flores et al., 2014). In contrast, SB-334867 (ip) inhibited the reported antidepressive actions of icv OrxA in the Forced Swim Test (FST) in ddY mice, and prevented Orx-induced proliferation in the dentate gyrus (Ito et al., 2008).
5. Dual orexin receptor antagonists (DORAs)
Developed for use in treatment of insomnia, DORAs are antagonists of both Orx1 and Orx2 receptors (Preskorn, 2018), and while effectively yielding hypnotic results (Howland, 2014; Owen, 2016), have been suggested to be potentially useful in treatment of addiction as well (Khoo and Brown, 2014). These drugs, though not selective for the Orx1 receptor alone, have displayed promise as therapeutic tools for relieving signs of affect. Orally administered DORA-12 enhanced social interaction time in rats subjected to high levels of CO2 to promote a panic state (Johnson et al., 2015). Almorexant, a competitive Orx receptor antagonist designed to treat insomnia, lowered blood pressure (BP) in hypertensive rats, while having no impact on the resting BP of wildtype animals (Li et al., 2013). Further, almorexant, in a dose-dependent fashion, reduced fear potentiated startle in conditioned rats, while having no myorelaxant effects (Steiner et al., 2012). Using an unpredictable chronic mild stress (UCMS) model for depression, mice administered daily almorexant experienced decreased anxious and depressive behaviors that were comparable to fluoxetine-treated animals (Nollet et al., 2012). The effectiveness of dual orexin receptor antagonists suggests that the orexin receptors function in similar fashion; however, before this type of drug becomes the standard therapy, it seems judicious to clarify the functions of each receptor separately.
6. A case for opposite actions of Orx1 and Orx2 receptors
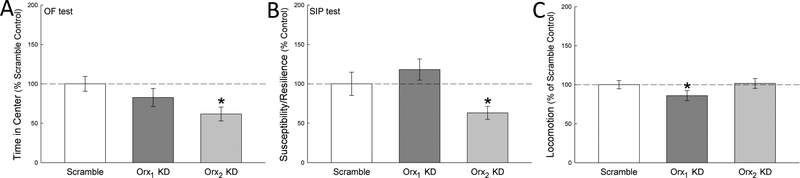
A study of depressive behavior using the forced swim test (FST) suggested a divergent pattern between hippocampal and amygdalar OrxA activity, and Orx1 gene expression associated with immobility (Arendt et al., 2013). The initial experimental results suggested to us that Orx1 activity may be depressive and/or anxiogenic, because the FST results showed a positive linear correlation between the duration of immobility and amygdalar Orx1 gene expression. In the following experiment, we saw that ten days of social defeat produced a similar increase of Orx1 mRNA in BLA of stress susceptible, but not resilient mice (Arendt et al., 2014). Interestingly, the results for Orx2 mRNA were opposite; mice susceptible to anxious and depressive behavior in the SIP test, and to increased anxious behavior in OF, exhibited decreased Orx2 gene expression, not measured in resilient mice. Regression analysis showed the same opposing trend, a strong linear, negative relationship between social preference (in the Social Interaction/Preference test, SIP) and Orx1 mRNA levels, similar to what we found for immobility (though in the opposite direction because the valence for SIP score and immobility is reversed), and strong positive linear relationship between Orx2 gene expression and social preference. Together the results suggest that Orx1 activity is anxiogenic, as has been demonstrated by other labs (Johnson et al., 2012b), but that Orx2 receptor activity is anxiolytic (Arendt et al., 2013; Arendt et al., 2014; Staton et al., 2018). Knockdown of BLA Orx1 or Orx2 activity, using short-hairpin RNA (shRNA), also stimulated opposing results (Fig. 1)(Arendt et al., 2014).
Fig. 1—
Intracranial injection of shRNA in the BLA produced knockdown (KD) of the Orx1 or Orx2 receptors, with scramble shRNA as control [from (Arendt et al., 2014)]. Intra-BLA KD of Orx1 receptors (dark gray bars) had no effect on measures of anxious behavior such as A) time spent in the center of the open field (OF; Orx1:t10 =1.0, p ≥ 0.33), B) Susceptibility / Resilience scores (time near container with a social target / time near a novel container lacking a social target × 100; such that a score < 100 indicates susceptibility, and a score > 100 indicates resilience) in the Social Interaction/Preference test (SIP Orx1:t10 = 0.9, p ≥ 0.39), or EPM (data not shown), but did C) reduce locomotion (Orx1:t12 = 2.5, p ≤ 0.033) compared to scramble controls (white bars). Conversely, Orx2 receptor KD in the BLA (light gray bars) was anxiogenic, reducing A) OF center time (Orx2: t11 = 2.7, p ≤ 0.021),B) Susceptibility / Resilience scores in SIP test (Orx2: t11 = 2.4, p ≤ 0.037), but C) did not reduce locomotion (Orx2: t12 =1.5, p ≥ 0.16) compared to scramble shRNA controls.
7. Knockout and knockdown of Orx receptors
Mice lacking prepro-orexin gene expression, Orx knockout (Orx KO), have been demonstrated to exhibit reduced feeding, as might be expected, but also obesity (Hara et al., 2001), which suggest that the metabolic effects of Orx are even more important that those associated with food seeking and feeding. These feeding-related orexinergic effects appear to be differentiated by sex, such that increased body weight in females is seen in Orx KO mice, but increased insulin resistance occurs (Tsuneki et al., 2008) in both sexes (Ramanathan and Siegel, 2014). In Orx KO mice abnormal sleep-wake patterns (Blumberg et al., 2007; Diniz Behn et al., 2010; Espana et al., 2007; Nakamura et al., 2007), elevated body temperature during sleep (Mochizuki et al., 2006), and narcolepsy are produced (Chemelli et al., 1999; Hara et al., 2001), and exacerbated when the Orx2 receptor is also deleted in the ventrolateral periaqueductal gray using a neurotoxin (Kaur et al., 2009). Combining feeding and narcoleptic effects, Orx KO mice further exhibit feeding elicited cataplexy (Clark et al., 2009). In addition, mice with Orx KO experience reduced cardiovascular and behavioral responses to social stress in the residentintruder test (Kayaba et al., 2003). This latter response is suggestive of a role for the orexin system in depressive behavior and social anxiety. Our model of social defeat, and species-specific anxious and depressive behavior (Carpenter and Summers, 2009; Smith et al., 2014; Summers et al., 2017), also suggests that Orx receptor activation promotes several kinds of learning (see below), and Orx KO impairs spatial working memory (Dang et al., 2018).
In Orx2 receptor KO mice, basal concentration of hypothalamic norepinephrine (NE), acetylcholine (ACh), and histamine (H) are significantly higher, but stimulated release of these transmitters was reduced (Ortega et al., 2012). By contrast, in prefrontal cortex (PFC), stimulated release of serotonin (5-HT), NE, and dopamine (DA) is significantly increased. A potential inhibitory Orx2 function in hypothalamus on basal concentrations of transmitters may be mirrored by Orx2 receptor down-regulation of evoked release in PFC. Therefore, genetic deletion of Orx2 receptors modulates neurotransmitter systems (Ortega et al., 2012) that are involved in depressive and anxious behavior in regions that are also involved. This idea is supported by the fact that Orx KO mice display anhedonia in the sucrose preference test (Matsuo et al., 2011). Additionally, Orx2 KO mice also display increased behavioral despair in FST and TST (Scott et al., 2011).
Following experiments in our lab that examined Orx1 and Orx2 gene expression in BLA and prelimbic prefrontal cortex, which suggested opposing functions for these two receptors in response to ten days of social defeat, we employed viral vectors designed to knock down the expression of the orexin receptors in unstressed mice, via bilateral injections targeting the BLA (Arendt et al., 2014). Separate plasmids with short hairpin RNA structures were designed to target specific regions of the Orx1 (CCAAAGGTCCCCACAGACATATTC) and Orx2 (AGAAACCCTTCAGTGGGACTTAAC) mRNAs (Scrambled control: CGGAATTTAGAAACCCGGCTCCAC). The unstressed Orx2 BLA-KD mice exhibited anxiogenic responses in the SIP (Orx2: t11 = 2.4, p ≤ 0.037) and open field (OF; Orx1:t12 = 1.0, p ≥ 0.33; Orx2: t11 = 2.7, p ≤ 0.021) tests (Fig. 1; from Arendt et al., 2014), without any change (Orx2: t12 = 1.5, p ≥ 0.16) in locomotion (Arendt et al., 2014). These results suggest an anxiolytic role for Orx2 receptors. After Orx1 KD in the BLA, mice had a significant reduction in locomotion (Orx1:t12 = 2.5, p ≤ 0.033), and an insignificant (Orx1:t10 = 0.9, p ≥ 0.39) rise in SIP score. The results suggested differential, if not strictly opposing, functional manifestations of unstressed Orx1 and Orx2 KD mice. The broader implication of the research is that Orx2 receptors may not play an anxiogenic role, as other research has suggested, but rather reduce anxious and depressive behavior, even in unstressed mice. The results also suggested the need for examining the effects of Orx2 receptors in mice in response to severe stressors.
8. The Stress-Alternatives Model
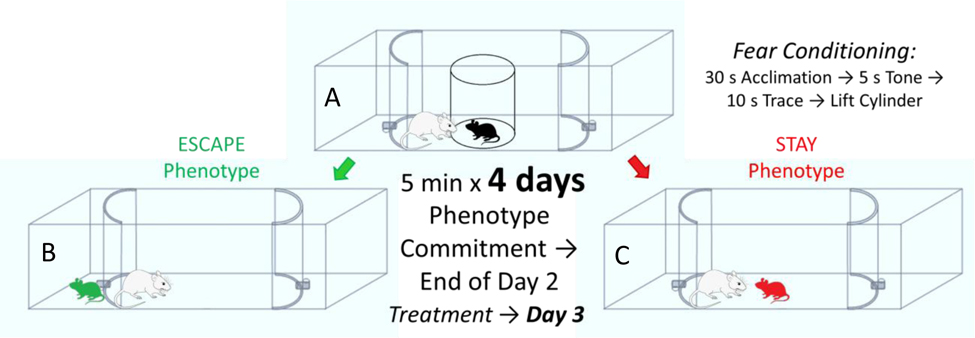
The Stress-Alternatives Model (SAM) is designed to examine the development of stress-related behaviors in response to aggressive social interaction (Fig. 2). We submit that these behaviors (See 9. Validation of the SAM) may reflect species-specific anxious and depressive behavior (Keifer and Summers, 2016). The SAM conceptual model and arena are designed to be ethologically relevant for the particular test species (Blanchard et al., 2013; Keifer and Summers, 2016; Pearson et al., 2017), while considering the necessity of social stress as a prerequisite for translational results (Haller and Alicki, 2012; Haller et al., 2013). The plan for the SAM apparatus devolved from two other highly successful models, the visible burrow system (Arakawa et al., 2007; Blanchard et al., 1995; Blanchard and Blanchard, 1989; Pobbe et al., 2010) and the social defeat model (Berton et al., 2007; Golden et al., 2011; Krishnan et al., 2007), such that the task involved was ethologically relevant, socially stressful, provided active behavior to measure, and included decision-making and learning (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Summers et al., 2017). We consider Escape and Stay behavioral outcomes to be the result of decision-making, because early responses are variable, and become stable with experience, and are modifiable by learning as well as anxiogenic or anxiolytic drugs (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018). The SAM apparatus has been adapted to accommodate studies on rodents (mice, rats, and slightly modified for hamsters) or fish (Rainbow trout) with results that indicate that stress-related behavior, and the neurocircuitry that mediates them, is highly conserved evolutionarily (Arendt et al., 2012; Carpenter and Summers, 2009; Keifer and Summers, 2016; Robertson et al., 2015; Smith et al., 2016; Summers et al., 2017). We propose that anxious and depressive behavior, considered in a species appropriate manner, may also be evolutionarily conserved, as it appears to also be primarily derived from stress-associated circuitry. The basic construct is a neutral oval open field arena in which a small test adult experiences social aggression from a novel and much larger conspecific each day for several days, but with escape routes available (Fig. 2). Surprisingly, in rodents and fish, on average over numerous experiments, only about half of the animals use the escape hole (Escape), while the other half remain submissively, and continue to be aggressively abused by the larger individual (Stay). While there is variability in the proportion of Escape and Stay animals with each cohort tested, and stressful animal care conditions can skew the ratio, Escape animals are reliably resilient to stress as measured by the social interaction or preference test (SIP: susceptibility/resilience scores are measured by time near container with a social target (at one edge of a 403 cm box) / time near a novel container lacking a social target × 100; such that a score < 100 indicates susceptibility, and a score > 100 indicates resilience) used in the social defeat model (Arendt et al., 2014; Golden et al., 2011; Krishnan et al., 2007; Staton et al., 2018; Yaeger et al., 2018). Similarly, Stay animals are reliably susceptible to stress and anti-social behavior measured by SIP; by which we mean that untreated Stay animals have SIP scores below 100, which indicates anxiety due to the lack of approach to potentially fearful animals. We suggest that these results may also indicate mouse-specific depressive tendencies, because this normally socially curious and gregarious test animal shuns social interaction after SAM-induced Stay phenotype development. This is an example of what we consider may be species-specific anxious and depressive responses. What is more, these Stay animals exhibit experience modified anxious behavior in the OF and EPM tests, as well as increased fear conditioned and conflict-stimulated freezing, elevated conflict-evoked startle, increased corticosterone concentrations, increased indecisiveness associated with anxiety (or CRF)-induced headshaking, and decreased novelty exploration (Carpenter and Summers, 2009; Robertson et al., 2015; Robertson et al., 2017; Smith et al., 2014; Smith et al., 2016; Staton et al., 2018; Summers et al., 2017; Yaeger et al., 2018); suggesting multiple stress-related, and potentially anxious and depressive, behavioral expressions, to validate equating the stay phenotype with susceptibility to these species-specific outcomes, which appear to be affective responses. These socially defeated animals (Stay) represent the end of an intensity gradient of anxious behavior produced by the SAM paradigm (Robertson et al., 2015; Smith et al., 2016). The gradient of anxiety that spans the contextual settings of escaping the open field, escaping from aggression, and submitting to aggression corresponds with increasing levels of plasma corticosterone and increasing levels of neuropeptide S (NPS) and brain-derived neurotrophic factor (BDNF) in the central amygdala. As behavioral responses escalate in concordance with physiological reactions associated with stress, anxiety and learning, and with each ameliorated differentially by endogenous neuropeptides, physical apparatus, and pharmaceutical interventions, it allows for parsing anxious SAM behavior into contextual niches along a species-specific gradient (Robertson et al., 2015; Smith et al., 2016). The addition of a conditioned stimulus (CS = tone) during the initial separation period (small test animal in an opaque cylindrical divider) prior to aggression (= unconditioned stimulus; US), with CS + US paired for 4 days of SAM interaction, results in a conditioned response (CR, freezing in mice, increased cortisol in trout) in the Stay phenotype only (Carpenter and Summers, 2009; Smith et al., 2014; Staton et al., 2018).
Fig. 2—
The Stress-Alternatives Model (SAM) social interaction regimen, begins with A) placement of a young adult male C57BL/6 N mouse in the center of the oval arena, but hidden by an opaque cylinder, while a novel and much larger CD1 mouse is also placed in the arena, but outside of the cylinder. After a 30s acclimation period (during which freezing is measured for comparison), a 5s tone is played as a conditioned stimulus (CS), which is followed by a 10s trace period (freezing is also measured during the tone and trace). The opaque cylinder is then removed and social interaction, including vigorous aggression, ensues for 5 min, repeated with a new CD1 aggressor each day for 4 days. During the first 2 days, mice choose a relatively stable behavioral phenotype, either B) Escape, which consists of making use of one of the available tunnels at the ends of the oval interaction arena and shortening the duration of their aggressive interaction, or C) Stay, in which mice remain in the SAM arena for the full 5 minutes and continues to receive attacks from the CD1. Importantly, both phenotypes receive significant aggression, and the phenotypes, while relatively stable can be reversed by anxiolytic or anxiogenic drugs. Drug treatments occur on day 3, after stable phenotypes have been formed.
While the Escape/Resilient and Stay/Susceptible phenotypes are relatively stable, they are not innate or immutable. Each phenotype, as well as the anxious or resilient behaviors expressed by Escape or Stay individuals, can be modified by environmental or neurochemical factors that are either anxiogenic (yohimbine - an α2-adrenoreceptor antagonist, social aggression) or anxiolytic (wheel running exercise, prior exposure to the escape hole, prior-experience escaping in the SAM, NPS, antalarmin (a CRF1 receptor antagonist) (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018).
The act of escape requires learning where the escape route is, at the distal ends of the oval OF for mice and in the upper corner of the 3-D OF aquarium for trout, which happens during the first moments of the initial social interaction in the SAM. The more complex aspect of learning has to do with the large aggressor, that must be avoided to affect the escape (Carpenter and Summers, 2009; Robertson et al., 2015; Summers et al., 2017). The SAM arena provided a unique test apparatus with multiple layers of behavioral evidence for our experiments on Orx2 receptor modulation of anxious and depressive behavior during socially stressful decision-making.
9. Validation of the SAM
The SAM is a recently devised preclinical behavioral model, designed to specifically address ecologically relevant, species-specific, anxious and depressive behavior (Blanchard et al., 2013) in mice, but using modified versions for rats and fish as well (Robertson et al., 2015). This model has only existed a few years, with its first published paper in 2009 (Carpenter and Summers, 2009), and the first data from a mouse version published in 2014 (Smith et al., 2014), and thus while we take great comfort in the results that suggest strong evolutionary conservation of stress-derived behavioral inhibition (Smith et al., 2016; Summers et al., 2017), which is similar to anxious and depressive behavior (Keifer and Summers, 2016), we understand that removing some caution regarding the results from this model awaits further validation. For this purpose, we consult an accepted set of criteria for assessing the validity of animal models, which has been used and refined for 5 decades (McKinney and Bunney, 1969; Willner, 1984; Willner, 1991; Willner and Mitchell, 2002). They are typically grouped into three categories:1) Construct Validity, relating to the model’s theoretical rationale 2) Predictive Validity, relating to the success of predictions made from the model, such as similarity in treatment effectiveness; and 3) Face Validity, relating to similarities between the symptomatology and behaviors resulting from the animal model (Willner, 1984). Validations for models of depression and anxiety are difficult due to largely or exclusively behavioral symptom profiles, as well as etiology and biological mechanisms involved that are imperfectly understood. As a result, none of the extant animal models are completely validated; nor are the predisposing factors, antecedent conditions, circuits, mechanisms of action, or even descriptive characteristics of common symptomology completely known for the disorders themselves.
Construct Validity - is usually treated as the most important of the three criteria (Sarter and Bruno, 2002) and may involve all elements that characterize the disorder, such as inclusion in the Diagnostic and Statistical Manual (DSM) or the International Classification of Diseases (ICD). It typically includes substantial attention to predisposing factors and antecedent conditions (and sometimes behavior), as components of the pattern of similarity of a model to factors contributing to the concept/definition of the target condition. These include the use of threatening or painful events to elicit behaviors or behavioral changes that are presumed to be related to anxiety, and chronic stressors used to induce changes that are treated as measures of depression. Models based on chronic social defeat, take advantage of the relationship between fear and anxiety (Allcoat et al., 2015; Barros and Tomaz, 2002; Haegler et al., 2010; Lang et al., 2000), but also social stress and depression (Grandjean et al., 2016; Heim and Binder, 2012), maintaining ecologically, ethologically, and species-specific relevance for the model (Blanchard et al., 2013; Keifer and Summers, 2016; Robertson et al., 2015) and relevance to the human disorder (Hendrie et al., 2013; Hunt et al., 2012). The development of the SAM, has been aimed at the observation that social stress produces the most significant elevation in stress hormones (Koolhaas et al., 1997) plus a high degree of metabolic cost in addition to the dimension of unpredictability (Koolhaas et al., 2011; Summers et al., 2005), and the fact that the incidence rates of adult affective disorders steeply rises during adolescence in parallel with a structural and functional reorganization of the neural circuitry underlying stress reactivity (Andersen, 2003; Bernstein et al., 1996; Coppens et al., 2011; Hankin et al., 1998; Romeo and McEwen, 2006). Since stress circuits and neuromodulatory factors are predisposing factors for human depression (Binder and Nemeroff, 2010; Swaab et al., 2005; Waters et al., 2015) and anxiety (Binder and Nemeroff, 2010; Swaab et al., 2005) as well as mouse depressive (Grandjean et al., 2016; Waters et al., 2015) and anxious behavior (Meng et al., 2015), the SAM fits the basic criteria for a preclinical model with substantial construct validity (Robertson et al., 2015; Smith et al., 2016; Summers et al., 2017).
Predictive Validity - The use of drugs, through both induction and reduction, as part of the definition of some pathological behavioral states remains a common approach, a predictive validity. For models of anxiety and depression, predictive validity involves the ability of established anxiolytics and antidepressants (respectively) to normalize behaviors that are induced or changed by some type of condition or manipulation (such as social defeat or social stress) that produces potentially anxiety- or depression-relevant patterns. (Iny et al., 1994) The problem of course, for anxiety and depression, is that “established” anxiolytics (like benzodiazepines) or antidepressants (such as serotonin or norepinephrine reuptake inhibitors), while being the primary drugs used for treatment, have not established a broad enough level of patient satisfaction (Young et al., 2001), and often include side-effects like addiction and withdrawal. Simplistic interpretations of the predictive validity concept tend to deflect attention from complex biochemical and behavioral etiologies and outcomes. As only one in three anxiety or depression patients receive effective treatment (Young et al., 2001), classical and established anxiolytic or antidepressant drugs have not been our priority for SAM validation, and we admit that this is a significant shortcoming. We have however, examined other drugs with known anxiety or depression related effects, including anxiolytics such as NPS, the anxiolytic and antidepressant antalarmin, as well as the anxiogenic drug yohimbine. For example, icv injection of the CRF1 receptor antagonist antalarmin on the 3rd day of a 4 day SAM protocol in mice, or on the 4th day of the 7 day protocol in trout, allows for Escape behavior in 40% of mice and 60% of trout that were previously Stay individuals. Anxiolytic factors also decrease the latency to escape, which is dramatically reduced by 2 days of familiarity with the task. Since the icv CRF1 antagonist treatment was after this 2 day period, escape latency reveals that Stay animals had also learned the behavioral strategies necessary to escape prior to treatment with the anxiolytic drug, but did not make use of these strategies until anxiolysis made it possible, at which time these CRF1 antagonist-treated animals escape with the same latency as those that had been escaping all along. These known anxiolytic, antidepressant, or anxiogenic drugs (NPS, antalarmin, yohimbine), provide some predictive validation for the relationship between the uniquely SAM behavioral measures and anxiety or depression (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017). An additional test, Social Interaction testing (including the SIP), that we often use along with the SAM has been validated against drugs commonly used therapeutically for anxiety (benzodiazepines) and depression (SSRIs), (File, 1980; Johnson et al., 2010; Lightowler et al., 1994; Molosh et al., 2018; Sanders and Shekhar, 1995). In addition, site-dependent positive and negative in vivo markers of depression such as BDNF, also are suggestive of a predictive validity for social defeat models, such as the SAM (Berton et al., 2007; Smith et al., 2014). What is more, similar to anxiety- and depression-related physiological dysfunction in humans, the SAM results depict elevated glucocorticoid concentrations in response to putatively anxious and depressive events and behaviors (Carpenter and Summers, 2009; Robertson et al., 2015; Smith et al., 2016; Staton et al., 2018).
Face Validity - Behavioral similarities, described as providing ‘face validity’, have typically been the least appreciated criteria for animal models. While normal behavioral actions of rodents or other nonhuman animals are substantially different from human actions having exactly the same function, motivational or incentive states may differ. We argue that adaptive functioning, based on the general evolutionary history of the animal, responsive to subject, stimulus, and situational characteristics, and characterized in terms of its immediate and longer-term outcomes, can constitute a highly efficient basis on which to evaluate parallels between behaviors in an animal model, and those of people (Blanchard et al., 2013; Keifer and Summers, 2016; Robertson et al., 2015). We argue that depressive behavioral inhibition, anhedonia, and despair, or enhanced anxious startle and an impending sense of danger that are commonly seen in human depression and/or anxiety patients, are also evident for nonhuman preclinical models. In our model, depressive behavioral inhibition is seen in absence of escape, delayed escape, enhanced fear conditioning, elevated conflict-induced freezing, and avoidance of the escape route. The lack of escape behavior or interest in the escape route are also indications of despair and anhedonia, because reducing stress levels promotes both behaviors. Anxious startle is evident by increased startle behavior during social interaction. An impending sense of danger, a classic symptom of anxiety, is evident in SAM fear conditioning. (Smith et al., 2016; Staton et al., 2018).
10. Evidence for potential pharmacotherapies targeting the Orx2 receptor
Our work, making use of the FST and social defeat model, clearly suggested that Orx2 receptors had a different behavioral profile than Orx1 receptors (Arendt et al., 2013; Arendt et al., 2014). Those results led us to hypothesize that Orx2 receptors were functionally anxiolytic, a deduction that was corroborated by experiments in which shRNA was used to knockdown the Orx1 and Orx2 receptors in the BLA (Fig. 1). While we did not see any anxiety- or depression-related behavioral effects from the shRNA Orx1 KD, aside from decreased locomotion, the Orx2 KD mice exhibited anxiogenic and pro-depressive effects in OF and SIP tests (Fig. 1).Neither KD had any effect on the elevated plus maze, however, since these were unstressed animals, we were not sure what the effect of Orx receptors would be after social defeat, although the gene expression data for defeated mice suggested the effect would be opposite for Orx1 and Orx2 (Arendt et al., 2014). Our next step was to apply Orx receptor treatments to stressed animals using the SAM apparatus.
We began by working with the Orx2 receptor drugs for two reasons. The first was that our work indicated the potential for anxiolytic and antidepressive effects from stimulation of the Orx2 receptor, and second, more was already known about Orx1. Additionally, it occurred to us that actual treatment for anxiety and depression via Orx2 pharmacotherapy would be necessarily systemic, and so our initial approach considered a need for understanding systemic effects, but at the same time we didn’t want to have central Orx2 actions be complicated by peripheral effects on metabolism or other physiological functions (Tsuneki et al., 2008; Zhang et al., 2005). We settled on an intracerebroventricular (icv) approach, targeting all or most of the brain, and used an Orx2 agonist ([Ala11-D-Leu15]-OrxB) and an antagonist (MK-1064), hypothesizing complementary but opposing results. In this set of SAM experiments, the results clearly suggest reduced anxious and depressive behavior associated with increased Orx2 activity.
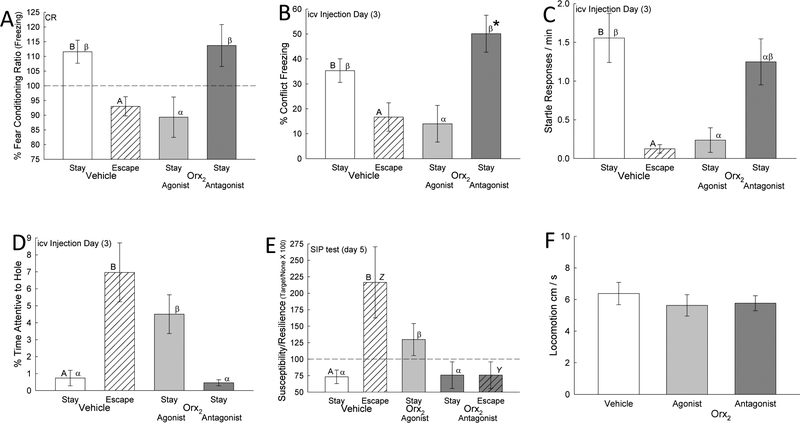
The SAM provides a variety of potentially anxiety- and depression-related measures, the first being whether mice that have demonstrated a reliable pattern of Escape or Stay behavior on days one and two, can be induced to switch the behavior from that of their established behavioral phenotype after Orx2 drug icv injection. First, icv injection of vehicle has no effect on behavioral phenotype. However, similar to what was seen in earlier experiments with the anxiogenic α2-adrenoreceptor antagonist yohimbine (Smith et al., 2016), in Escape mice (escaping 100% of the time on days 1 and 2) the Orx2 antagonist MK-1064 injected on day 3 of SAM interactions blocked 50% of those mice from escaping (Staton et al., 2018). It is important to remember that the Escape phenotype is stress resilient in the SIP test (Fig. 3E), and Stay animals are stress susceptible, so this result is the equivalent of taking resilient individuals, and making half of them susceptible to stress, and putatively anxious and/or depressive behavior. The other results from this set of experiments confirm this notion. When we started with Stay phenotype mice, those that are susceptible after the first 2 days of SAM interactions, and give an Orx2 agonist, there was a very small reversal to Escape in 11% of Stay mice, and this happened on day 4, one day after the day of injection (Staton et al., 2018). Clearly more work needs to be done to determine if there is an effect, but it is suggestive of a potential anxiolytic response.
Fig. 3—
Results from icv Orx2 agonist and antagonist experiments (Staton et al., 2018) that activation of Orx2 receptors is anxiolytic and anti-depressive. Behavioral phenotypes derived from social interaction in the Stress-Alternatives Model (SAM) differed, Stay (clear bar) vs Escape (hatched bar; statistical significance between behavioral phenotypes is represented by differing Roman letters atop the bar, such that A is different from B), with more anxious behavior evident in Stay mice by increased A) Mean (± SEM) freezing (t13 = 2.7, P ≤ 0.018) in response to fear conditioning ([Freezing after CS/Freezing before] × 100); B) Freezing during SAM social aggression (% time freezing; t16 = 2.4, P ≤ 0.03); and C) Startle responses (/min; t14 = 2.7, P ≤ 0.019); but enhanced anxiolytic responses in Escape phenotype mice by increased D) Mean (± SEM) % Time attentive to the hole (t16 = 4.67, P ≤ 0.001), and E) Resilience score in the Social Interaction Preference test (SIP; t18 = 3.82, P < 0.001), but F) did not affect locomotion. Activation of the Orx2 receptor by Ala15, D-Leu11-OrxB (light gray bars) reduces A) Freezing during fear conditioning in Stay mice (F2,31 = 4.86, P ≤ 0.015; statistical significance between Stay groups is represented by differing Greek letters atop the bar, such that α is different from β), B) Conflict induced freezing (F2,25 = 4.8, P < 0.017), and C) Startle responses (F2,21 = 3.89, P ≤ 0.036), but increases D) % Time attentive to the hole (F2,24 =12.37, P < 0.001), which may promote an 11% increase in escape behavior, and E) SIP resilience score “(time near container with a social target / time near a novel container lacking a social target × 100; such that a score < 100 indicates susceptibility, and a score > 100 indicates resilience) in Stay mice (F2,30 = 4.6, P ≤ 0.018; comparing Escape mice: t11 = 2.6, P ≤ 0.025). Inhibition of the Orx2 receptor by MK-1064 (dark gray bars) in Stay mice increases B) Conflict freezing on injection day compared to day 2, and plasma corticosterone (data not shown). In Escape phenotype mice the Orx2 antagonist (dark gray hatched bar) increases E) Susceptibility score in SIP test (statistical significance between Escape groups is represented by differing Italic letters atop the bar, such that Y is different from Z), and reduces the number of escaping mice by half (data not shown), but F) did not affect locomotion (F2,35 = 0.39, P ≥ 0.68).
We measured freezing in two ways in these experiments. The first is a part of a fear conditioning paradigm in which the conditioned stimulus (CS) is a tone played during the brief period of isolation for the test animal (in an opaque cylinder in the SAM apparatus) prior to aggressive interaction with a novel CD1 mouse. Social interaction with a CD1 (which was placed outside the cylinder) constitutes the unconditioned stimulus (US) that follows. The CS and US are paired for each of the 4 days of SAM social interaction. Freezing is measured during the trace period after the tone and prior to the divider being removed. On the 5th day, no larger CD1 mouse is present outside the cylinder, and the CS is presented alone, to test for a conditioned response (CR). We also measured conflict freezing during the SAM social interaction itself, something the small C57 black mouse did in response to, the presence of a larger CD1 mouse, or aggression from that animal. For both cued fear conditioning (Fig. 3A; t13 = 2.7, p ≤ 0.018) and conflict freezing (Fig. 3B; t16 = 2.4, p ≤ 0.03), vehicle-treated mice of the Stay phenotype did significantly more freezing (normalized for each mouse by the time spent in the SAM arena) than Escape mice did. Only Stay mice exhibit fear conditioning (CR = fear freezing ratio > 100%) in this measure on test day (Fig. 3A). The Orx2 agonist blocked the fear conditioned response in Stay phenotype mice on test day (Fig. 3A; F2,31 = 4.86, p ≤ 0.015), and significantly reduced conflict freezing (F2,25 = 4.8, p ≤ 0.017) during the period of actual social aggression on day 3 (Fig. 3B), when the drug was injected icv (Staton et al., 2018). That means that the Orx2 agonist had an immediate effect on conflict freezing, but also a prolonged effect, blocking the CR two days later. While the Orx2 antagonist did not affect the conditioned response, it did stimulate a continued increase in conflict freezing on injection day (day 3; Fig. 1B), which was not seen in vehicle-treated mice.
Fear enhanced startle is a common method for examining anxious behavior (Davis, 1992), and our model produces significant stress during the social interaction. Startle responses (whole-body flinching) measure an animal’s reaction to sudden environmental stimuli, often acoustic (Heesink et al., 2017; Sallinen et al., 1998). We surmised that as the intensity and amplitude of the acoustic-startle response are affected by stress-related events (Belzung et al., 2001; Risbrough et al., 2004), the frequency of socially-induced startle might also be enhanced by increasing stress during aggressive interactions. With this in mind, we pirated the concept of startle for use in the SAM, because we noticed that active approach of an aggressor mouse also elicits a similar startle response. Therefore, we made use of a stimulus that exists as a part of aggressive social interaction, the threat of attack, and measured the number of flinches or recoils that were induced by the approach of the larger CD1 mouse during each entire social interaction. We examined the number startle responses, rather than the amplitude of startle, stimulated by the approach of the aggressor mouse. The frequency of startle responses grew in vehicle-treated Stay phenotype mice, over each of the 4 days of the SAM social interactions (Staton et al., 2018), and was significantly greater (t14 = 2.7, p ≤ 0.019) in vehicle-treated Stay compared to Escape phenotype mice (Fig. 3C). In these Stay mice, the icv-injected Orx2 agonist on day 3 significantly (F2,21 = 3.89, p ≤ 0.036) reduced the frequency of startle responses.
As the SAM apparatus has escape routes at each end of the oval interaction arena, we developed a novel measure of exploration, which appears to be suggestive of intent to use the escape route. By examining the time that each mouse spent specifically attentive to the hole (inspecting, sniffing, partially entering, or in very close proximity [3 cm] to the escape tunnel) relative to the total time they were in the arena, it is possible to compare this set of behaviors with Escape or Stay phenotype and with other time sensitive behaviors that signal intention to escape (Staton et al., 2018). Not surprisingly, mice of the Escape phenotype (vehicle-treated in these experiments) are the most attentive (t16 = 4.67, p < 0.001) to the hole (Fig. 3D). What is somewhat surprising is that, although all mice rapidly notice and inspect, Stay mice spend so little time investigating the hole. The Orx2 antagonist actually reduces the amount of time, although not significantly as the amount of time spent is already below 1% of the total time in the SAM arena. Additionally, Escape mice spend proportionally more time investigating the hole as the time of egress approaches. In an experiment in which mice were pre-exposed to the escape tunnel, while 80% of mice escaped when SAM social interaction actually convened, 20% of the mice were irrevocably of the Stay phenotype. Although both groups had experienced the safety of the tunnel and the small chamber that it leads to, escaping mice spent more time attentive to the hole (Yaeger et al., 2018). However, the Orx2 agonist significantly (F2,24 =12.37, p < 0.001) increases the percentage of time examining the escape route on the day of icv injection (3) and on the next day as well (Fig. 3D). The day after icv Orx2 agonist injection (4) is significantly higher than on the day of injection, at which point the Stay animals, which are mostly still not escaping, are nearly as attentive to the hole as mice of the Escape Phenotype. What is more, the day 4 expression of greatest attentiveness to the hole coincides with the small 11% reversal of Stay to Escape phenotype described earlier. This conjunction of day 4 events suggests the possibility of neural plasticity relative to the prolonged effect. We see a similar prolonged effect in fear conditioning (measured on test day = 5) and the SIP test, also measured on the day after SAM interactions conclude.
Escape and Stay mice very clearly behaviorally self-select their phenotype, which corresponds later to Social Interaction/Preference (SIP; Fig. 3E). This apparent self-determination is evident, and Escape or Stay are not simply coping responses, because earlier studies clearly indicate that the magnitude or timing of aggression does not promote escape, even though early Escape-Stay responses are variable, and become stable with experience. Additionally, a stable phenotype is modifiable by anxiogenic and anxiolytic drugs, but also by learning (Carpenter and Summers, 2009; Prince et al., 2015; Robertson et al., 2015; Smith et al., 2014; Smith et al., 2016; Summers et al., 2017; Yaeger et al., 2018). Escape vehicle-treated mice spend significantly more time in close proximity to a container enclosing an unfamiliar aggressor mouse (CD1), than to that same novel container that is empty (Staton et al., 2018). This type of behavior (susceptibility/resilience scores = time near container with a social target / time near a novel container lacking a social target × 100; such that a score < 100 indicates susceptibility, and a score > 100 indicates resilience) has been interpreted to be resilient to stress, anxious and depressive behavior, because the mice exhibiting social preference also score low on independent measures of stress, and species specific anxiety and depression (Berton et al., 2007; Golden et al., 2011; Krishnan et al., 2007). Stay mice (vehicle-treated) avoid the social target in the container, and spend more time in proximity to the container without a social target. These results suggest that Escape and Stay (t18 = 3.82, p < 0.001), which are evoked as a reliable phenotypic response early (by day 2) in the SAM behavioral regimen, are essentially equivalent markers of Resilience and Susceptibility (Staton et al., 2018; Yaeger et al., 2018). Remarkably, the Orx2 agonist delivered icv on day 3 reverses Susceptibility in Stay mice making them Resilient (F2,30 = 4.6, p ≤ 0.018) and icv-administration of the Orx2 antagonist negates Resilience in Escape mice to render them Susceptible (t11 = 2.6, p ≤ 0.025; Fig. 3E). Importantly, none of the potentially anxiety- or depression-related behaviors induced by Orx2 receptor action or inhibition was attributable to changes in activity, as home cage locomotion was unchanged by the icv injection of the drugs (F2,35 = 0.39, p ≥ 0.68; Fig. 3F)
11. Clinical implications of DORA versus Orx1 versus Orx2 treatments
While Orx receptor antagonist drugs have been approved for treatment of insomnia (Citrome, 2014; Osborne, 2013), virtually no large-scale clinical examinations of their effectiveness in affective disorder treatment has been undertaken. The DORA hypnotic drug suvorexant (Belsomra) is clinically approved as a treatment to promote sleep by reducing arousal and wakefulness (Howland, 2014). While this drug effectively reduces sleep onset times and increases sleep duration, without rebound or withdrawal (Owen, 2016), it also lowered stress hormone levels cortisol and norepinephrine, and reduced the severity of anxiety and depression in psychiatric patients with insomnia (Nakamura and Nagamine, 2017). These results are consistent with the Orx1 antagonist activities of the drug, but contrasting with those expected for its Orx2 receptor antagonist actions. Alarmingly, these results, contrast sharply with a case study in which a renal dialysis patient with insomnia plus major depressive and generalized anxiety disorders, the latter two treated somewhat effectively with an antidepressant, found that the severity of depression worsened with suvorexant treatment (Petrous and Furmaga, 2017). Interestingly, in a preclinical trial of the DORA almorexant, a competitive Orx1 + Orx2 antagonist that had been in phase II clinical trials, anxious and depressive behavior were reduced to the same extent as with the antidepressant fluoxetine (Nollet et al., 2012). As chronic insomnia is highly comorbid with affective disorders, and is associated with up to a 4-fold increased risk of developing major depression (Baglioni and Riemann, 2012; Blake et al., 2018; Staner, 2010), it seems appropriate to test interactions between drugs, such as DORAs, taken for insomnia and antidepressants (Cruz et al., 2014). This study examined the drug interactions between desipramine and almorexant, in part because the enzyme primarily responsible for metabolism of the antidepressant is inhibited by almorexant. Not surprisingly, the DORA almorexant increased exposure to desipramine by nearly 4-fold, whereas the antidepressant had no relevant pharmacokinetic effects on almorexant. There was however, a slight increase in calmness in patients using almorexant. Ironically, this DORA with potential for reducing affective symptoms was removed from clinical trials on the basis of its safety profile. Another Orx receptor antagonist in phase II trials is Filorexant (MK-6096); these trials in patients with major depression were terminated early, without showing a significant difference in depression rating, but with one of the two most common adverse events being suicidal ideation, common in this type of patient (Connor et al., 2017).
The clinical implications for DORAs, as used clinically for treatment of insomnia, are not clear with respect to anxiety or depression. Interestingly, a preclinical optogenetic study found that although sleep disturbance impairs memory, dual and single Orx receptor antagonists used to promote sleep also improve cognitive processes (Li et al., 2018). However, comparisons may be difficult, since the dosages used, at least in relation to our own experiments, are on the order of ten-fold or more higher in hypnotic treatments or arousal studies (Staton et al., 2018)(Table 1). This difference in dosage is significant for several reasons, the first of which is that cross effects, and side effects, may be avoided, although significant further testing would be necessary to determine this. In contrast, anxious and panic behavior can be inhibited by doses of Orx1 antagonist (or DORA) similar to that used for sleep induction (Johnson et al., 2010; Johnson et al., 2012b; Johnson et al., 2012c; Johnson et al., 2015)(Table 1), suggesting that some Orx drugs may have a wide range of efficacy. The low doses necessary to produce anxiolytic or antidepressive actions from Orx2 agonist treatment, may prove to be a therapeutic advantage (Table 1). Thus, inhibition of Orx1 receptors, and stimulation of Orx2 receptors, both appear to have the potential for anxiolytic and antidepressive actions. While DORA treatment confounds the opposing actions of Orx1 and Orx2 receptors, it seem likely that it’s anti-panic effects derive mostly from antagonizing Orx1 actions (Johnson et al., 2015), given the evidence that blocking Orx2 receptors is anxiogenic and pro-depressive (Staton et al., 2018).
Table 1.
Orexin drug treatments for anxious and depressive behaviors
| Drug Description (animal) | Delivery Method | Dose (nmol) | Description of effect on anxious or depressive behavior | Citation |
|---|---|---|---|---|
| OrxA | ||||
| OrxA (mice) | icv | 1 nmol | ↑ Anxiogenic behaviors in LDT & EPM | Suzuki et al., 2005 |
| OrxA (rats) | icv | 10 nmol | ↑ Locomotion, face-washing, grooming, wet-dog shaking | Matsuzaki et al., 2002 |
| OrxA (rats) | iBNST | 3 nmol | ↑ Anxious behavior in SIP & EPM | Lungwitz et al., 2012 |
| OrxA (mice) | iBLA | 0.0001488 nmol (0.53 ng/0.5 μl) | ↑ Immobility in FST ↓ Social interaction in U-field test |
Kim et al., 2015 |
| OrxA (hamster) | iCeA | 0.02 nmol (20 nM) | ↑ Anxious and depressive behavior (LDT & EPM but not mentioned in review) | Avolio et al., 2011 |
| OrxA (rats) | i-pPVT | 28.08 nmol (0.1mg/250 nl) | ↑ Anxious behavior in EPM | Heydendael et al., 2011 |
| OrxA (rats) | iVP | 500 nmol (1 μM) | ↓ Anhedonia (Sucrose Preference) and behavioral despair (FST) after LV-shOrxlR knockdown | Ji et al., 2018 |
| OrxB | ||||
| OrxB (rats) | icv | 10 nmol | ↑ Locomotion, face-washing, grooming, wet-dog shaking | Matsuzaki et al., 2002 |
| OrxB (hamster) | iCeA | 0.06 nmol (60 nM) | ↑ Anxious and depressive behavior (LDT & EPM but not mentioned in review) | Avolio et al., 2011 |
| Orx1 antagonist | ||||
| SB-334867 (mice) | ip | 782.9 nmol (10 mg.kg) | ↓ Behavioral despair in FST & TST | Scott et al., 2011 |
| SB-334867 (mice) | ip | 234.9 nmol, 391.45 nmol, 782.9 nmol (3,5& 10 mg/kg) | ↓ Reaction to cued & contextual stimuli (freezing time to fear tests) | Flores et al., 2014 |
| SB-334867 (mice) | ip | 234.9–2,349 nmol1 (3–30 mg/kg) 782.9 nmol2 (10 mg/kg) | No general anxiety impact in EPM |
Rodgers et al, 20131 Scott et al., 20112 Staples & Cornish 20142 |
| SB-334867 (mice) | ip | 391.45 nmol & 782.9 nmol (5 & 10 mg/kg) | ↓ Anxiety induced by acute nicotine administration in EPM | Plaza-Zabala et al. 2010 |
| SB-334867 (rats) | ip | 1,879 nmol (20 mg/kg) | ↓ Hyperactivity in prenatal pups exposed to alcohol | Stettner et al., 2011 |
| SB-334867 (mice) | ip | 2,350 nmol (30 mg/kg) | ↓ Anxiety in OF induced by hypercapnic air | Johnson et al., 2012c |
| SB-334867 (rats) | ip | 30,530 nmol (30 mg/kg) | ↓ Freezing in Sprague-Dawley rats | Johnson et al., 2010 |
| SB-334867 (rats) | ip | 30,530 nmol (30 mg/kg) | ↓ Anxiety in SIP induced by hypercapnic air | Johnson et al., 2015 |
| SB-334867 (rats) | ip | 7,830 nmol (10 mg/kg) | ↓ Latency to approach novel & familiar predator odors | Staples & Cornish, 2014 |
| SB-334867 (ddY mice) | ip | 3,290 nmol (30 mg/kg) | ↓ Antidepressive action of icv OrxA inFST | Ito et al. 2008 |
| SB-334867 (rats) | i-pPVT | 313.2 nmol (0.1 mg/250 nl) | ↓ Anxious behavior in EPM | Heydenael et al. 2011 |
| SB-334867 (mice) | iBLA | 10 nmol | ↑ Contextually induced fear extinction | Flores et al. 2014 |
| GSK-1059865 (rats) | ip | 20,630 nmol (30 mg/kg) | ↓ Attenuate yohimbine induced stress fMRI | Gozzi et al. 2013 |
| Orx2 agonist | ||||
| Ala11-D-Leu15-OrxB (mice) | icv | 0.3 nmol | ↓ Anxious behaviors in stress-susceptible animals in the SAM | Staton et al. 2018 |
| Orx2 antagonist | ||||
| TCSOX229 (mice) | ip | 576.1 nmol & 864.1 nmol (10 & 15 mg/kg) | ↓ Freezing during contextual fear No effect w/ cued fear consolidation | Flores et al. 2014 |
| MK-1064 (mice) | icv | 0.3 nmol | ↑ Anxious behaviors in stress resilient animals in the SAM | Staton et al. 2018 |
| DORA | ||||
| DORA-12 (rats) | orally | 21,600 nmol (30 mg/kg) | ↓ Anxiety in SIP induced by hypercapnic air | Johnson et al. 2015 |
| Almorexant (rats) | orally | 14,600–146,000 nmol Dose dependent (30–300 mg/kg) | ↓ Fear potentiated startle in conditioned rats | Steiner et al 2012 |
| Almorexant (mice) | orally | 4,880 nmol per day (100 mg per day) | ↓ Anxious & depressive behaviors in UCMS model | Nollet et al. 2012 |
Drugs modifying orexin system and receptor activity used for preclinical and clinical treatments of anxious and depressive behaviors. Dosages presented as as converted to nmol based on either reported animal weights, or average weight for the development stage utilized in the study (and as published). A brief description of the drug’s effects on anxious and depressive behavior is included. Abbreviations: DORA = Dual Orexin Receptor Antagonist, EPM = Elevated Plus Maze, fMRI = functional Magnetic Resonance Imaging, FST = Forced Swim Test, iBLA = intra-Basolateral Amygdala, iBNST = intra-Bed Nucleus of the Stria Terminalis, iCeA= intra-Central Amygdala, icv = intracerebroventricular, ip = intraperitoneal, i-pPVT = intra-posterior Paraventricular Thalamus, iVP = intra-Ventral Pallidum, LDT = Light-Dark Test, OFT = Open Field Test, SAM = Stress-Alternatives Model, SIP = Social Interaction/Preference test, TST =Tail Suspension Test, UCMS = Unpredictable Chronic Mild Stress
12. Synopsis
Our results, as well as that of others, indicate that the orexinergic system contributes significantly to mediation of stress, as well as anxious and depressive behavior. These lines of evidence are reason enough to posit that pharmacotherapies involving the orexin/hypocretin neuromodulators and hormones may be beneficial in patients with anxiety and depression. While the orexinergic system has two active neuropeptides, OrxA and OrxB, it appears to be the receptors, Orx1 and Orx2, which primarily give rise to opposing functions relative to stress and affective behaviors. In keeping with the theme of this special issue, antagonists do have potential for limiting Orx1 receptor generated anxiety and panic (Johnson et al., 2010; Johnson et al., 2012b). Interestingly, in unstressed mice, Orx1 receptor KD had no effect on anxious behaviors, where Orx2 KD produced anxiogenic response in SIP and OF tests (Arendt et al., 2014). In socially stressed animals in the SAM apparatus, Orx2 receptors stimulated by icv injection of the agonist [Ala11, d-Leu15]-OrxB produced anxiolytic responses to fear conditioning, conflict stimulated freezing, startle, attentiveness to adaptive behavioral opportunities (attention to the escape hole), SIP Resilience/Susceptibility test, and potentially reversed the socially defeated Stay phenotype to Escape in a small percentage of animals (Staton et al., 2018). The Orx2 antagonist MK-1064 created anxiogenic responses by reversing Escape to Stay behavior, increasing conflict freezing, increasing Susceptibility responses in the SIP test, and increased plasma corticosterone in anxious stay mice. Taken together, these ten behavioral and physiological results, based on Orx2 receptor stimulation or inhibition, indicate a possible specific role for the Orx2 receptor in regulation of anxiety and depression. As only Orx2 receptor action among orexinergic systems, appears to regulate anxious or depressive behaviors in both unstressed and socially stressed animals, it may be that this receptor subtype plays an important role in the etiology of anxiety and depression (Arendt et al., 2014; Staton et al., 2018). These factors suggest that Orx2 receptor physiology may be an important target for anxiety and depression-related pharmacotherapeutic investigations.
Orexin/hypocretins innervate limbic regions associated with anxiety and depression
Orexins are reciprocally innervated with CRF
Orexin 1 receptors promote anxious and pro-depressive behavior
Orexin 2 receptor knockdown promotes anxious and depressive behaviors
Orexin 2 agonists are anxiolytic and anti-depressive
Acknowledgements
We would like to thank Kevin T. Krupp and Benard Onserio for compilation of data and assembling information necessary to support this report. Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH104485, and by NIH grant and P20 RR15567, a CBBRe Research Enhancement Pilot grant, and an anonymous donor to the Summers’ lab via the USD Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbas MG, Shoji H, Soya S, Hondo M, Miyakawa T, Sakurai T, 2015. Comprehensive Behavioral Analysis of Male Ox1r (−/−) Mice Showed Implication of Orexin Receptor-1 in Mood, Anxiety, and Social Behavior. Front Behav Neurosci. 9, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aboul-Fotouh S, Habib M, Asaad T, Kassim SK, Ghanem MH, 2018. Behavioral effects of toll-like receptor-4 antagonist ‘eritoran’ in an experimental model of depression: role of prefrontal and hippocampal neurogenesis and gamma-aminobutyric acid/glutamate balance. Behav Pharmacol. [DOI] [PubMed] [Google Scholar]
- Achua JK, Callahan LB, Brudvig JJ, Summers CH, Ronan PJ, 2014. Cross-talk between orexin/hypocretin and corticotropin releasing factor systems: a combined re trograde tracer immunohistochemical study Society for Neuroscience Abstracts. 40, 78.06. [Google Scholar]
- Allard JS, Tizabi Y, Shaffery JP, Trouth CO, Manaye K, 2004. Stereological analysis of the hypothalamic hypocretin/orexin neurons in an animal model of depression. Neuropeptides. 38, 311–5. [DOI] [PubMed] [Google Scholar]
- Allcoat D, Greville WJ, Newton PM, Dymond S, 2015. Frozen with fear: Conditioned suppression in a virtual reality model of human anxiety. Behav Processes. 118, 98–101. [DOI] [PubMed] [Google Scholar]
- Alo R, Avolio E, Mele M, Fazzari G, Carelli A, Facciolo RM, Canonaco M, 2016. Role of Leptin and Orexin-A Within the Suprachiasmatic Nucleus on Anxiety-Like Behaviors in Hamsters. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Amano T, Unal CT, Pare D, 2010. Synaptic correlates of fear extinction in the amygdala. Nat Neurosci.13, 489–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, 2003. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 27, 3–18. [DOI] [PubMed] [Google Scholar]
- Arakawa H, Blanchard DC, Blanchard RJ, 2007. Colony formation of C57BL/6J mice in visible burrow system: identification of eusocial behaviors in a background strain for genetic animal models of autism. Behav Brain Res. 176, 27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Smith JP, Bastida CC, Prasad MS, Oliver KD, Eyster KM, Summers TR, Delville Y, Summers CH, 2012. Contrasting hippocampal and amygdalar expression of genes related to neural plasticity during escape from social aggression. Physiol Behav. 107, 670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt DH, Ronan PJ, Oliver KD, Callahan LB, Summers TR, Summers CH, 2013. Depressive behavior and activation of the orexin/hypocretin system. Behav Neurosci. 127, 86–94. [DOI] [PubMed] [Google Scholar]
- Arendt DH, Hassell J, Li H, Achua JK, Guarnieri DJ, Dileone RJ, Ronan PJ, Summers CH, 2014. Anxiolytic function of the orexin 2/hypocretin A receptor in the basolateral amygdala. Psychoneuroendocrinology. 40,17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU, 2016. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology. 41,126–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avolio E, Alo R, Carelli A, Canonaco M, 2011. Amygdalar orexinergic-GABAergic interactions regulate anxiety behaviors of the Syrian golden hamster. Behav Brain Res. 218, 288–95. [DOI] [PubMed] [Google Scholar]
- Azogu I, Plamondon H, 2017. Inhibition of TrkB at the nucleus accumbens, using ANA-12, regulates basal and stress-induced orexin A expression within the mesolimbic system and affects anxiety, sociability and motivation. Neuropharmacology. 125, 129–145. [DOI] [PubMed] [Google Scholar]
- Baglioni C, Riemann D, 2012. Is chronic insomnia a precursor to major depression? Epidemiological and biological findings. Curr Psychiatry Rep.14, 511–8. [DOI] [PubMed] [Google Scholar]
- Bai YJ, Li YH, Zheng XG, Han J, Yang XY, Sui N, 2009. Orexin A attenuates unconditioned sexual motivation in male rats. Pharmacol Biochem Behav. 91,581–9. [DOI] [PubMed] [Google Scholar]
- Baldo BA, Daniel RA, Berridge CW, Kelley AE, 2003. Overlapping distributions of orexin/hypocretin- and dopamine-beta-hydroxylase immunoreactive fibers in rat brain regions mediating arousal, motivation, and stress. J Comp Neurol. 464, 220–37. [DOI] [PubMed] [Google Scholar]
- Barros M, Tomaz C, 2002. Non-human primate models for investigating fear and anxiety. Neurosci Biobehav Rev. 26,187–201. [DOI] [PubMed] [Google Scholar]
- Belzung C, El Hage W, Moindrot N, Griebel G, 2001. Behavioral and neurochemical changes following predatory stress in mice. Neuropharmacology. 41,400–8. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G, 2015. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 41,1149–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Cohen P, Skodol A, Bezirganian S, Brook JS, 1996. Childhood antecedents of adolescent personality disorders. Am J Psychiatry. 153, 907–13. [DOI] [PubMed] [Google Scholar]
- Berton O, Covington HE 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ, 2007. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 55, 289–300. [DOI] [PubMed] [Google Scholar]
- Binder EB, Nemeroff CB, 2010. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry. 15, 574–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blais A, Drouin G, Chaumontet C, Voisin T, Couvelard A, Even PC, Couvineau A, 2017. Impact of Orexin-A Treatment on Food Intake, Energy Metabolism and Body Weight in Mice. PLoS One.12, e0169908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake MJ, Trinder JA, Allen NB, 2018. Mechanisms underlying the association between insomnia, anxiety, and depression in adolescence: Implications for behavioral sleep interventions. Clin Psychol Rev. 63, 25–40. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR, 1995. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 20, 117–34. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Summers CH, Blanchard RJ, 2013. The role of behavior in translational models for psychopathology: functionality and dysfunctional behaviors. Neurosci Biobehav Rev. 37,1567–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, 1989. Antipredator defensive behaviors in a visible burrow system. J Comp Psychol. 103, 70–82. [DOI] [PubMed] [Google Scholar]
- Blumberg MS, Coleman CM, Johnson ED, Shaw C, 2007. Developmental divergence of sleep-wake patterns in orexin knockout and wild-type mice. Eur J Neurosci. 25, 512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A, 2009. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 29,11215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowrey HE, James MH, Aston-Jones G, 2017. New directions for the treatment of depression: Targeting the photic regulation of arousal and mood (PRAM) pathway. Depress Anxiety. 34, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin L, Bjorkqvist M, Petersen A, Traskman-Bendz L, 2007a. Reduced orexin levels in the cerebrospinal fluid of suicidal patients with major depressive disorder. Eur Neuropsychopharmacol. 17, 573–9. [DOI] [PubMed] [Google Scholar]
- Brundin L, Petersen A, Bjorkqvist M, Traskman-Bendz L, 2007b. Orexin and psychiatric symptoms in suicide attempters. J Affect Disord. 100, 259–63. [DOI] [PubMed] [Google Scholar]
- Brymer KJ, Fenton EY, Kalynchuk LE, Caruncho HJ, 2018. Peripheral Etanercept Administration Normalizes Behavior, Hippocampal Neurogenesis, and Hippocampal Reelin and GABAA Receptor Expression in a Preclinical Model of Depression. Front Pharmacol. 9,121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess CR, 2010. Histamine and orexin in the control of arousal, locomotion, and motivation. J Neurosci. 30, 2810–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter RE, Summers CH, 2009. Learning strategies during fear conditioning. Neurobiol Learn Mem. 91,415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ch’ng SS, Lawrence AJ, 2015. Distribution of the orexin-1 receptor (OX1R) in the mouse forebrain and rostral brainstem: A characterisation of OX1R-eGFP mice. J Chem Neuroanat. 66–67, 1–9. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M, 1999. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 98, 437–51. [DOI] [PubMed] [Google Scholar]
- Chen CT, Dun SL, Kwok EH, Dun NJ, Chang JK, 1999. Orexin A-like immunoreactivity in the rat brain. Neurosci Lett. 260, 161–4. [DOI] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC, 2010. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 167, 11–20. [DOI] [PubMed] [Google Scholar]
- Citrome L, 2014. Suvorexant for insomnia: a systematic review of the efficacy and safety profile for this newly approved hypnotic - what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 68,1429–41. [DOI] [PubMed] [Google Scholar]
- Clark EL, Baumann CR, Cano G, Scammell TE, Mochizuki T, 2009. Feeding-elicited cataplexy in orexin knockout mice. Neuroscience. 161, 970–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cluderay JE, Harrison DC, Hervieu GJ, 2002. Protein distribution of the orexin-2 receptor in the rat central nervous system. Regul Pept. 104, 131–44. [DOI] [PubMed] [Google Scholar]
- Connor KM, Ceesay P, Hutzelmann J, Snavely D, Krystal AD, Trivedi MH, Thase M, Lines C, Herring WJ, Michelson D, 2017. Phase II Proof-of-Concept Trial of the Orexin Receptor Antagonist Filorexant (MK-6096) in Patients with Major Depressive Disorder. Int J Neuropsychopharmacol. 20, 613–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens CM, Siripornmongcolchai T, Wibrand K, Alme MN, Buwalda B, de Boer SF, Koolhaas JM, Bramham CR, 2011. Social Defeat during Adolescence and Adulthood Differentially Induce BDNF-Regulated Immediate Early Genes. Front Behav Neurosci. 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz HG, Hay JL, Hoever P, Alessi F, te Beek ET, van Gerven JM, Dingemanse J, 2014. Pharmacokinetic and pharmacodynamic interactions between almorexant, a dual orexin receptor antagonist, and desipramine. Eur Neuropsychopharmacol. 24, 1257–68. [DOI] [PubMed] [Google Scholar]
- Dang R, Chen Q, Song J, He C, Zhang J, Xia J, Hu Z, 2018. Orexin knockout mice exhibit impaired spatial working memory. Neurosci Lett. 668, 92–97. [DOI] [PubMed] [Google Scholar]
- Davis M, 1992. The role of the amygdala in fear-potentiated startle: implications for animal models of anxiety. Trends Pharmacol Sci.13, 35–41. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C, 1999. The extended amygdala: are the central nucleus of the amygdala and the bed nucleus of the stria terminalis differentially involved in fear versus anxiety? Ann N Y Acad Sci. 877, 281–91. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Miles L, Grillon C, 2010. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 35,105–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz Behn CG, Klerman EB, Mochizuki T, Lin SC, Scammell TE, 2010. Abnormal sleep/wake dynamics in orexin knockout mice. Sleep. 33, 297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacret D, Grafe LA, Dobkin J, Gotter AL, Rengerb JJ, Winrow CJ, Bhatnagar S, 2018. Orexin signaling during social defeat stress influences subsequent social interaction behaviour and recognition memory. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Miller DL, Roecklein KA, 2012. The aging hippocampus: interactions between exercise, depression, and BDNF. Neuroscientist. 18, 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, McCormack SL, Mochizuki T, Scammell TE, 2007. Running promotes wakefulness and increases cataplexy in orexin knockout mice. Sleep. 30,1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- File SE, 1980. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 2, 219–38. [DOI] [PubMed] [Google Scholar]
- Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, Berrendero F, 2014. The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology. 39, 2732–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores A, Herry C, Maldonado R, Berrendero F, 2017. Facilitation of contextual fear extinction by Orexin-1 Receptor Antagonism Is associated with the Activation of Specific Amygdala Cell Subpopulations. Int J Neuropsychopharmacol. 20, 654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortuyn HA, Lappenschaar MA, Furer JW, Hodiamont PP, Rijnders CA, Renier WO, Buitelaar JK, Overeem S, 2010. Anxiety and mood disorders in narcolepsy: a case-control study. Gen Hosp Psychiatry. 32, 49–56. [DOI] [PubMed] [Google Scholar]
- Furlong TM, Vianna DM, Liu L, Carrive P, 2009. Hypocretin/orexin contributes to the expression of some but not all forms of stress and arousal. Eur J Neurosci. 30,1603–14. [DOI] [PubMed] [Google Scholar]
- Gao C, Du Q, Li W, Deng R, Wang Q, Xu A, Shen J, 2018. Baicalin Modulates APPL2/Glucocorticoid Receptor Signaling Cascade, Promotes Neurogenesis, and Attenuates Emotional and Olfactory Dysfunctions in Chronic Corticosterone-Induced Depression. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, de Lecea L, 2014. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol. 29,103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Eban-Rothschild A, Christoffel DJ, Li SB, Malenka RC, de Lecea L, 2018. Parallel circuits from the bed nuclei of stria terminalis to the lateral hypothalamus drive opposing emotional states. Nat Neurosci. 21,1084–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE 3rd, Berton O, Russo SJ, 2011A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 6,1183–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzi A, Lepore S, Vicentini E, Merlo-Pich E, Bifone A, 2013. Differential effect of orexin-1 and CRF-1 antagonism on stress circuits: a fMRI study in the rat with the pharmacological stressor Yohimbine. Neuropsychopharmacology. 38, 2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Luz S, Gotter AL, Renger JJ, Winrow CJ, Bhatnagar S, 2017. Orexin 2 receptor regulation of the hypothalamic-pituitary-adrenal (HPA) response to acute and repeated stress. Neuroscience. 348, 313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, Eacret D, Dobkin J, Bhatnagar S, 2018. Reduced Orexin System Function Contributes to Resilience to Repeated Social Stress. eNeuro. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean J, Azzinnari D, Seuwen A, Sigrist H, Seifritz E, Pryce CR, Rudin M, 2016. Chronic psychosocial stress in mice leads to changes in brain functional connectivity and metabolite levels comparable to human depression. Neuroimage. 142, 544–552. [DOI] [PubMed] [Google Scholar]
- Gray JM, Vecchiarelli HA, Morena M, Lee TT, Hermanson DJ, Kim AB, McLaughlin RJ, Hassan KI, Kühne C, Wotjak CT, Deussing JM, Patel S, Hill MN, 2015. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci. 35, 3879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haegler K, Zernecke R, Kleemann AM, Albrecht J, Pollatos O, Bruckmann H, Wiesmann M, 2010. No fear no risk! Human risk behavior is affected by chemosensory anxiety signals. Neuropsychologia. 48, 3901–8. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N, 1999. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 96,10911–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Alicki M, 2012. Current animal models of anxiety, anxiety disorders, and anxiolytic drugs. Curr Opin Psychiatry. 25, 59–64. [DOI] [PubMed] [Google Scholar]
- Haller J, Aliczki M, Gyimesine Pelczer K, 2013. Classical and novel approaches to the preclinical testing of anxiolytics: A critical evaluation. Neurosci Biobehav Rev. 37, 2318–30. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, Silva PA, McGee R, Angell KE, 1998. Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. J Abnorm Psychol. 107, 128–40. [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T, 2001. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 30, 345–54. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Risbrough V, Oakley RH, Olivares-Reyes JA, Dautzenberg FM, 2009. Role of CRF receptor signaling in stress vulnerability, anxiety, and depression. Ann N Y Acad Sci. 1179, 120–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesink L, Kleber R, Hafner M, van Bedaf L, Eekhout I, Geuze E, 2017. Anger and aggression problems in veterans are associated with an increased acoustic startle reflex. Biol Psychol. 123, 119–125. [DOI] [PubMed] [Google Scholar]
- Heim C, Binder EB, 2012. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 233, 102–11. [DOI] [PubMed] [Google Scholar]
- Hendrie C, Pickles A, Stanford SC, Robinson E, 2013. The failure of the antidepressant drug discovery process is systemic. J Psychopharmacol. 27, 407–13; discussion 413–6. [DOI] [PubMed] [Google Scholar]
- Heydendael W, Sharma K, Iyer V, Luz S, Piel D, Beck S, Bhatnagar S, 2011. Orexins/hypocretins act in the posterior paraventricular thalamic nucleus during repeated stress to regulate facilitation to novel stress. Endocrinology. 152, 4738–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heydendael W, Sengupta A, Beck S, Bhatnagar S, 2014. Optogenetic examination identifies a context-specific role for orexins/hypocretins in anxiety-related behavior. Physiol Behav. 130, 182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AS, Sahay A, Hen R, 2015. Increasing Adult Hippocampal Neurogenesis is Sufficient to Reduce Anxiety and Depression-Like Behaviors. Neuropsychopharmacology. 40, 2368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsboer F, Ising M, 2010. Stress hormone regulation: biological role and translation into therapy. Annu Rev Psychol. 61,81–109, C1–11. [DOI] [PubMed] [Google Scholar]
- Hong YP, Lee HC, Kim HT, 2015. Treadmill exercise after social isolation increases the levels of NGF, BDNF, and synapsin I to induce survival of neurons in the hippocampus, and improves depression-like behavior. J Exerc Nutrition Biochem.19,11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland RH, 2014. Suvorexant: a novel therapy for the treatment of insomnia. J Psychosoc Nurs Ment Health Serv. 52, 23–6. [DOI] [PubMed] [Google Scholar]
- Hritcu L, Gorgan LD, 2014. Intranigral lipopolysaccharide induced anxiety and depression by altered BDNF mRNA expression in rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 51,126–32. [DOI] [PubMed] [Google Scholar]
- Hunt C, Peters L, Rapee RM, 2012. Development of a measure of the experience of being bullied in youth. Psychol Assess. 24, 156–65. [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N, 2000. Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun. 270, 318–23. [DOI] [PubMed] [Google Scholar]
- Iniguez SD, Riggs LM, Nieto SJ, Dayrit G, Zamora NN, Shawhan KL, Cruz B, Warren BL, 2014. Social defeat stress induces a depression-like phenotype in adolescent male c57BL/6 mice. Stress.17, 247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iny LJ, Pecknold J, Suranyi-Cadotte BE, Bernier B, Luthe L, Nair NP, Meaney MJ, 1994. Studies of a neurochemical link between depression, anxiety, and stress from [3H]imipramine and [3H]paroxetine binding on human platelets. Biol Psychiatry. 36, 281–91. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Gamo Y, Nagai T, Oikawa T, Yamada H, Hanawa T, 2008. I.c.v. administration of orexin-A induces an antidepressive-like effect through hippocampal cell proliferation. Neuroscience. 157, 720–32. [DOI] [PubMed] [Google Scholar]
- Ito N, Yabe T, Nagai T, Oikawa T, Yamada H, Hanawa T, 2009. A possible mechanism underlying an antidepressive-like effect of Kososan, a Kampo medicine, via the hypothalamic orexinergic system in the stress-induced depression-like model mice. Biol Pharm Bull. 32,1716–22. [DOI] [PubMed] [Google Scholar]
- Jalewa J, Wong-Lin K, McGinnity TM, Prasad G, Holscher C, 2014. Increased number of orexin/hypocretin neurons with high and prolonged external stress-induced depression. Behav Brain Res. 272, 196–204. [DOI] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Walker FR, Smith DW, Richardson HN, Hodgson DM, Dayas CV, 2014. Exercise reverses the effects of early life stress on orexin cell reactivity in male but not female rats. Front Behav Neurosci. 8, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Campbell EJ, Dayas CV, 2017a. Role of the Orexin/Hypocretin System in Stress- Related Psychiatric Disorders. Curr Top Behav Neurosci. 33,197–219. [DOI] [PubMed] [Google Scholar]
- James MH, Mahler SV, Moorman DE, Aston-Jones G, 2017b. A Decade of Orexin/Hypocretin and Addiction: Where Are We Now? Curr Top Behav Neurosci. 33, 247–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesulola E, Micalos P, Baguley IJ, 2018. Understanding the pathophysiology of depression: From monoamines to the neurogenesis hypothesis model - are we there yet? Behav Brain Res. 341, 79–90. [DOI] [PubMed] [Google Scholar]
- Ji MJ, Zhang XY, Chen Z, Wang JJ, Zhu JN, 2018. Orexin prevents depressive-like behavior by promoting stress resilience. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A, 2010. A key role for orexin in panic anxiety. Nat Med.16,111–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A, 2012a. Orexin, stress, and anxiety/panic states. Prog Brain Res. 198, 133–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A, 2012b. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 107, 733–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Lightman SL, Lowry CA, Shekhar A, 2012c. Activation of the orexin 1 receptor is a critical component of CO2-mediated anxiety and hypertension but not bradycardia. Neuropsychopharmacology. 37,1911–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Federici LM, Fitz SD, Renger JJ, Shireman B, Winrow CJ, Bonaventure P, Shekhar A, 2015. Orexin 1 and 2 Receptor Involvement in Co2-Induced Panic-Associated Behavior and Autonomic Responses. Depress Anxiety. 32, 671–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiyama Y, Iijima Y, Chiba S, Furuta M, Ninomiya M, Izumi A, Shibata S, Kunugi H, 2010. Prednisolone causes anxiety- and depression-like behaviors and altered expression of apoptotic genes in mice hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 34, 159–65. [DOI] [PubMed] [Google Scholar]
- Kalueff AV, Nutt DJ, 2007. Role of GABA in anxiety and depression. Depress Anxiety. 24, 495–517. [DOI] [PubMed] [Google Scholar]
- Kaur S, Thankachan S, Begum S, Liu M, Blanco-Centurion C, Shiromani PJ, 2009. Hypocretin-2 saporin lesions of the ventrolateral periaquaductal gray (vlPAG) increase REM sleep in hypocretin knockout mice. PLoS One. 4, e6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayaba Y, Nakamura A, Kasuya Y, Ohuchi T, Yanagisawa M, Komuro I, Fukuda Y, Kuwaki T, 2003. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 285, R581–93. [DOI] [PubMed] [Google Scholar]
- Keifer J, Summers CH, 2016. Putting the “Biology” Back into “Neurobiology”: The Strength of Diversity in Animal Model Systems for Neuroscience Research. Front Syst Neurosci.10, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JM, Rauch SL, 2003. Neurocircuitry of anxiety disorders. Curr Psychiatry Rep. 5, 266–73. [DOI] [PubMed] [Google Scholar]
- Khalil R, Fendt M, 2017. Increased anxiety but normal fear and safety learning in orexin-deficient mice. Behav Brain Res. 320, 210–218. [DOI] [PubMed] [Google Scholar]
- Khoo SY, Brown RM, 2014. Orexin/hypocretin based pharmacotherapies for the treatment of addiction: DORA or SORA? CNS Drugs. 28, 713–30. [DOI] [PubMed] [Google Scholar]
- Kim TK, Kim JE, Park JY, Lee JE, Choi J, Kim H, Lee EH, Kim SW, Lee JK, Kang HS, Han PL, 2015. Antidepressant effects of exercise are produced via suppression of hypocretin/orexin and melanin-concentrating hormone in the basolateral amygdala. Neurobiol Dis. 79, 59–69. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, De Boer SF, De Rutter AJ, Meerlo P, Sgoifo A, 1997. Social stress in rats and mice. Acta Physiol Scand Suppl. 640, 69–72. [PubMed] [Google Scholar]
- Koolhaas JM, Korte SM, De Boer SF, Van Der Vegt BJ, Van Reenen CG, Hopster H, De Jong IC, Ruis MA, Blokhuis HJ, 1999. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 23, 925–35. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Buwalda B, van Reenen K, 2007. Individual variation in coping with stress: a multidimensional approach of ultimate and proximate mechanisms. Brain Behav Evol. 70, 218–26. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, Bartolomucci A, Buwalda B, de Boer SF, Flügge G, Korte SM, Meerlo P, Murison R, Olivier B, Palanza P, Richter-Levin G, Sgoifo A, Steimer T, Stiedl O, van Dijk G, Wohr M, Fuchs E, 2011. Stress revisited: a critical evaluation of the stress concept. Neurosci Biobehav Rev. 35,1291–301. [DOI] [PubMed] [Google Scholar]
- Korzan WJ, Summers CH, 2007. Behavioral diversity and neurochemical plasticity: selection of stress coping strategies that define social status. Brain Behav Evol. 70, 257–66. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ, 2007. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 131, 391–404. [DOI] [PubMed] [Google Scholar]
- Lamers F, van Oppen P, Comijs HC, Smit JH, Spinhoven P, van Balkom AJ, Nolen WA, Zitman FG, Beekman AT, Penninx BW, 2011. Comorbidity patterns of anxiety and depressive disorders in a large cohort study: the Netherlands Study of Depression and Anxiety (NESDA). J Clin Psychiatry. 72, 341–8. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A, 2000. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 61,137–59. [DOI] [PubMed] [Google Scholar]
- Li A, Hindmarch CC, Nattie EE, Paton JF, 2013. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 591, 4237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SB, Nevarez N, Giardino WJ, de Lecea L, 2018. Optical probing of orexin/hypocretin receptor antagonists. Sleep. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A, 1995. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 59, 229–39. [DOI] [PubMed] [Google Scholar]
- Lightowler S, Kennett GA, Williamson IJ, Blackburn TP, Tulloch IF, 1994. Anxiolytic-like effect of paroxetine in a rat social interaction test. Pharmacol Biochem Behav. 49, 281–5. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Pare D, 2008. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 454, 642–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvin Y, Pentkowski NS, Blanchard DC, Blanchard RJ, 2007. CRF type 1 receptors in the dorsal periaqueductal gray modulate anxiety-induced defensive behaviors. Horm Behav. 52, 244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungwitz EA, Molosh A, Johnson PL, Harvey BP, Dirks RC, Dietrich A, Minick P, Shekhar A, Truitt WA, 2012. Orexin-A induces anxiety-like behavior through interactions with glutamatergic receptors in the bed nucleus of the stria terminalis of rats. Physiol Behav. 107, 726–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter M, Krishnan V, Russo SJ, Jung S, McClung CA, Nestler EJ, 2008. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 28, 3071–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghsoudi N, Ghasemi R, Ghaempanah Z, Ardekani AM, Nooshinfar E, Tahzibi A, 2014. Effect of Chronic Restraint Stress on HPA Axis Activity and Expression of BDNF and Trkb in the Hippocampus of Pregnant Rats: Possible Contribution in Depression during Pregnancy and Postpartum Period. Basic Clin Neurosci. 5,131–7. [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, Aston-Jones G, 2014. Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci.17,1298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK, 2001. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 435, 6–25. [DOI] [PubMed] [Google Scholar]
- Matsuo E, Mochizuki A, Nakayama K, Nakamura S, Yamamoto T, Shioda S, Sakurai T, Yanagisawa M, Shiuchi T, Minokoshi Y, Inoue T, 2011. Decreased intake of sucrose solutions in orexin knockout mice. J Mol Neurosci. 43, 217–24. [DOI] [PubMed] [Google Scholar]
- Matsuzaki I, Sakurai T, Kunii K, Nakamura T, Yanagisawa M, Goto K, 2002. Involvement of the serotonergic system in orexin-induced behavioral alterations in rats. Regul Pept. 104, 119–23. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, 2001. Localization of the CB1 type cannabinoid receptor in the rat basolateral amygdala: high concentrations in a subpopulation of cholecystokinin-containing interneurons. Neuroscience. 107, 641–52. [DOI] [PubMed] [Google Scholar]
- McKinney WT Jr., Bunney WE Jr., 1969. Animal model of depression. I. Review of evidence: implications for research. Arch Gen Psychiatry. 21,240–8. [DOI] [PubMed] [Google Scholar]
- Meng FT, Zhao J, Fang H, Liu YJ, 2015. The influence of chronic stress on anxiety-like behavior and cognitive function in different human GFAP-ApoE transgenic adult male mice. Stress.18, 419–26. [DOI] [PubMed] [Google Scholar]
- Mlyniec K, Budziszewska B, Holst B, Ostachowicz B, Nowak G, 2015. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int J Neuropsychopharmacol.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki T, Klerman EB, Sakurai T, Scammell TE, 2006. Elevated body temperature during sleep in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 291, R533–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molosh AI, Dustrude ET, Lukkes JL, Fitz SD, Caliman IF, Abreu ARR, Dietrich AD, Truitt WA, Ver Donck L, Ceusters M, Kent JM, Johnson PL, Shekhar A, 2018. Panic results in unique molecular and network changes in the amygdala that facilitate fear responses. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir J, Lorsch ZS, Ramakrishnan C, Deisseroth K, Nestler EJ, Calipari ES, Bagot RC, 2018. In Vivo Fiber Photometry Reveals Signature of Future Stress Susceptibility in Nucleus Accumbens. Neuropsychopharmacology. 43, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T, 2007. Vigilance st ate-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol (1985). 102, 241–8. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Nagamine T, 2017. Neuroendocrine, Autonomic, and Metabolic Responses to an Orexin Antagonist, Suvorexant, in Psychiatric Patients with Insomnia. Innov Clin Neurosci. 14, 30–37. [PMC free article] [PubMed] [Google Scholar]
- Nambu T, Sakurai T, Mizukami K, Hosoya Y, Yanagisawa M, Goto K, 1999. Distribution of orexin neurons in the adult rat brain. Brain Res. 827, 243–60. [DOI] [PubMed] [Google Scholar]
- Nocjar C, Zhang J, Feng P, Panksepp J, 2012. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 218, 138–53. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Minier F, Tanti A, Belzung C, Leman S, 2011. Activation of orexin neurons in dorsomedial/perifornical hypothalamus and antidepressant reversal in a rodent model of depression. Neuropharmacology. 61,336–46. [DOI] [PubMed] [Google Scholar]
- Nollet M, Gaillard P, Tanti A, Girault V, Belzung C, Leman S, 2012. Neurogenesis-independent antidepressant-like effects on behavior and stress axis response of a dual orexin receptor antagonist in a rodent model of depression. Neuropsychopharmacology. 37, 2210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega JE, Katner J, Davis R, Wade M, Nisenbaum L, Nomikos GG, Svensson KA, Perry KW, 2012. Modulation of neurotransmitter release in orexin/hypocretin-2 receptor knockout mice: a microdialysis study. J Neurosci Res. 90, 588–96. [DOI] [PubMed] [Google Scholar]
- Osborne R, 2013. First-in-class insomnia drug on the brink of approval nod. Nat Rev Drug Discov.12, 492–3. [DOI] [PubMed] [Google Scholar]
- Øverli Ø, Sorensen C, Pulman KG, Pottinger TG, Korzan W, Summers CH, Nilsson GE, 2007. Evolutionary background for stress-coping styles: relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev. 31,396–412. [DOI] [PubMed] [Google Scholar]
- Owen RT, 2016. Suvorexant: efficacy and safety profile of a dual orexin receptor antagonist in treating insomnia. Drugs Today (Barc). 52, 29–40. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Olguner Eker O, Abdulrezzak U, Esel E, 2017. Relationship between orexin A and childhood maltreatment in female patients with depression and anxiety. Soc Neurosci. 12, 330–336. [DOI] [PubMed] [Google Scholar]
- Pearson BL, Crawley JN, Eilam D, Pentkowski NS, Summers CH, 2017. Curiosity as an approach to ethoexperimental analysis: Behavioral neuroscience as seen by students and colleagues of Bob Blanchard. Neurosci Biobehav Rev. 76, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrous J, Furmaga K, 2017. Adverse reaction with suvorexant for insomnia: acute worsening of depression with emergence of suicidal thoughts. BMJ Case Rep. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS, 1998. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 18, 9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plaza-Zabala A, Martin-Garcia E, de Lecea L, Maldonado R, Berrendero F, 2010. Hypocretins regulate the anxiogenic-like effects of nicotine and induce reinstatement of nicotine-seeking behavior. J Neurosci. 30, 2300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobbe RL, Pearson BL, Defensor EB, Bolivar VJ, Blanchard DC, Blanchard RJ, 2010. Expression of social behaviors of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behav Brain Res. 214, 443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preskorn SH, 2018. CNS Drug Development, Lessons Learned, Part 5: How Preclinical and Human Safety Studies Inform the Approval and Subsequent Use of a New Drug-Suvorexant as an Example. J Psychiatr Pract. 24,104–110. [DOI] [PubMed] [Google Scholar]
- Prince MA, Achua JK, Smith JP, Robertson JM, Ronan PJ, Summers TR, Summers CH, 2015. Decision-making during social interaction in mice is not driven by aggression, but is related to ventral dentate gyrus expression of orexin receptors. Society for Neuroscience Abstracts. 45, 433.19. [Google Scholar]
- Ramanathan L, Siegel JM, 2014. Gender differences between hypocretin/orexin knockout and wild type mice: age, body weight, body composition, metabolic markers, leptin and insulin resistance. J Neurochem. 131, 615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA, 2004. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 24, 6545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Prince MA, Achua JK, Carpenter RE, Arendt DH, Smith JP, Summers TL, Summers TR, Blanchard DC, Summers CH, 2015. Nuance and behavioral cogency: How the Visible Burrow System inspired the Stress-Alternatives Model and conceptualization of the continuum of anxiety. Physiol Behav. 146, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JM, Achua JK, Smith JP, Prince MA, Staton CD, Ronan PJ, Summers TR, Summers CH, 2017. Anxious behavior induces elevated hippocampal Cb2 receptor gene expression. Neuroscience. 352, 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers RJ, Wright FL, Snow NF, Taylor LJ, 2013. Orexin-1 receptor antagonism fails to reduce anxiety-like behaviour in either plus-maze-naive or plus-maze-experienced mice. Behav Brain Res. 243, 213–9. [DOI] [PubMed] [Google Scholar]
- Roffman JL, Marci CD, Glick DM, Dougherty DD, Rauch SL, 2005. Neuroimaging and the functional neuroanatomy of psychotherapy. Psychol Med. 35, 1385–98. [DOI] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS, 2006. Stress and the adolescent brain. Ann N Y Acad Sci. 1094, 202–14. [DOI] [PubMed] [Google Scholar]
- Ronan PJ, Skog TD, Brudvig JJ, Callahan LB, Summers CH, 2016. Topographical organization of perifornical orexin neurons; implications for stressw-induced disorders. Society for Neuroscience Abstracts. 42. [Google Scholar]
- Rotter A, Asemann R, Decker A, Kornhuber J, Biermann T, 2011. Orexin expression and promoter-methylation in peripheral blood of patients suffering from major depressive disorder. J Affect Disord. 131, 186–92. [DOI] [PubMed] [Google Scholar]
- Sakamoto F, Yamada S, Ueta Y, 2004. Centrally administered orexin-A activates corticotropin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus and central amygdaloid nucleus of rats: possible involvement of central orexins on stress-activated central CRF neurons. Regul Pept. 118, 183–91. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Nagata R, Yamanaka A, Kawamura H, Tsujino N, Muraki Y, Kageyama H, Kunita S, Takahashi S, Goto K, Koyama Y, Shioda S, Yanagisawa M, 2005. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 46, 297–308. [DOI] [PubMed] [Google Scholar]
- Sakurai T, 2014. The role of orexin in motivated behaviours. Nat Rev Neurosci.15, 719–31. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Haapalinna A, Viitamaa T, Kobilka BK, Scheinin M, 1998. Adrenergic alpha2C-receptors modulate the acoustic startle reflex, prepulse inhibition, and aggression in mice. J Neurosci.18, 3035–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, Nishino S, Mignot E, 2003. Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biol Psychiatry. 54, 96–104. [DOI] [PubMed] [Google Scholar]
- Samson WK, Taylor MM, Follwell M, Ferguson AV, 2002. Orexin actions in hypothalamic paraventricular nucleus: physiological consequences and cellular correlates. Regul Pept. 104, 97–103. [DOI] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A, 1995. Regulation of anxiety by GABAA receptors in the rat amygdala. Pharmacol Biochem Behav. 52, 701–6. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Saper CB, 2005. Orexin, drugs and motivated behaviors. Nat Neurosci. 8, 1286–8. [DOI] [PubMed] [Google Scholar]
- Schmitt O, Usunoff KG, Lazarov NE, Itzev DE, Eipert P, Rolfs A, Wree A, 2012. Orexinergic innervation of the extended amygdala and basal ganglia in the rat. Brain Struct Funct. 217, 233–56. [DOI] [PubMed] [Google Scholar]
- Schoevers RA, Beekman AT, Deeg DJ, Jonker C, van Tilburg W, 2003. Comorbidity and risk-patterns of depression, generalised anxiety disorder and mixed anxiety-depression in later life: results from the AMSTEL study. Int J Geriatr Psychiatry.18, 994–1001. [DOI] [PubMed] [Google Scholar]
- Scott MM, Marcus JN, Pettersen A, Birnbaum SG, Mochizuki T, Scammell TE, Nestler EJ, Elmquist JK, Lutter M, 2011. Hcrtr1 and 2 signaling differentially regulates depression-like behaviors. Behav Brain Res. 222, 289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn V, Wolff SB, Herry C, Grenier F, Ehrlich I, Grundemann J, Fadok JP, Muller C, Letzkus JJ, Luthi A, 2014. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron. 81,428–37. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Truitt W, Rainnie D, Sajdyk T, 2005. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress. 8, 209–19. [DOI] [PubMed] [Google Scholar]
- Shin LM, Liberzon I, 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 35, 169–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater PG, Noches V, Gysling K, 2016. Corticotropin-releasing factor type-2 receptor and corticotropin-releasing factor-binding protein coexist in rat ventral tegmental area nerve terminals originated in the lateral hypothalamic area. Eur J Neurosci. 43, 220–9. [DOI] [PubMed] [Google Scholar]
- Smith JP, Achua JK, Summers TR, Ronan PJ, Summers CH, 2014. Neuropeptide S and BDNF gene expression in the amygdala are influenced by social decision-making under stress. Frontiers in Behavioral Neuroscience. 8:121, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, Prince MA, Achua JK, Robertson JM, Anderson RT, Ronan PJ, Summers CH, 2016. Intensity of anxiety is modified via complex integrative stress circuitries. Psychoneuroendocrinology. 63, 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soya S, Shoji H, Hasegawa E, Hondo M, Miyakawa T, Yanagisawa M, Mieda M, Sakurai T, 2013. Orexin receptor-1 in the locus coeruleus plays an important role in cue-dependent fear memory consolidation. J Neurosci. 33,14549–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staner L, 2010. Comorbidity of insomnia and depression. Sleep Med Rev.14, 35–46. [DOI] [PubMed] [Google Scholar]
- Staples LG, Cornish JL, 2014. The orexin-1 receptor antagonist SB-334867 attenuates anxiety in rats exposed to cat odor but not the elevated plus maze: an investigation of Trial 1 and Trial 2 effects. Horm Behav. 65, 294–300. [DOI] [PubMed] [Google Scholar]
- Staton CD, Yaeger JDW, Khalid DD, Haroun F, Fernandez BS, Fernandez JS, Summers BK, Summers TR, Sathyanesan M, Newton SS, Summers CH, 2018. Orexin 2 receptor stimulation enhances resilience, while orexin 2 inhibition promotes susceptibility, to social stress, anxiety and depression. Neuropharmacology. 143, 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, 2009. Neurobiology of generalized anxiety disorder. J Clin Psychiatry. 70 Suppl 2, 15–9. [DOI] [PubMed] [Google Scholar]
- Steiner MA, Lecourt H, Jenck F, 2012. The brain orexin system and almorexant in fear-conditioned startle reactions in the rat. Psychopharmacology (Berl). 223, 465–75. [DOI] [PubMed] [Google Scholar]
- Stettner GM, Kubin L, Volgin DV, 2011. Antagonism of orexin 1 receptors eliminates motor hyperactivity and improves homing response acquisition in juvenile rats exposed to alcohol during early postnatal period. Behav Brain Res. 221, 324–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers CH, Forster GL, Korzan WJ, Watt MJ, Larson ET, Overli O, Hoglund E, Ronan PJ, Summers TR, Renner KJ, Greenberg N, 2005. Dynamics and mechanics of social rank reversal. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 191, 241–52. [DOI] [PubMed] [Google Scholar]
- Summers TR, Summers TL, Carpenter RE, Smith JP, Young SL, Meyerink B, Orr TZ, Arendt DH, Summers CH, 2017. Learning and CRF-Induced Indecision during Escape and Submission in Rainbow Trout during Socially Aggressive Interactions in the Stress-Alternatives Model. Front Neurosci.11,515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T, 2005. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 1044, 116–21. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ, 2005. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 4,141–94. [DOI] [PubMed] [Google Scholar]
- Taheri S, Gardiner J, Hafizi S, Murphy K, Dakin C, Seal L, Small C, Ghatei M, Bloom S, 2001. Orexin A immunoreactivity and preproorexin mRNA in the brain of Zucker and WKY rats. Neuroreport.12, 459–64. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Ho SP, Livanov V, Graciani N, Arneric SP, 2001. Antagonism of CRF(2) receptors produces anxiolytic behavior in animal models of anxiety. Brain Res. 902, 135–42. [DOI] [PubMed] [Google Scholar]
- Tasan RO, Nguyen NK, Weger S, Sartori SB, Singewald N, Heilbronn R, Herzog H, Sperk G, 2010. The central and basolateral amygdala are critical sites of neuropeptide Y/Y2 receptor-mediated regulation of anxiety and depression. J Neurosci. 30, 6282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JL, Borgland SL, 2011A role for hypocretin/orexin in motivation. Behav Brain Res. 217, 446–53. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM, 2005. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl). 182, 75–83. [DOI] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A, 2015. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience.16, 317–331. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, Guan XM, 1998. Distribution of orexin receptor mRNA in the rat brain. FEBS Lett. 438, 71–5. [DOI] [PubMed] [Google Scholar]
- Trouche S, Sasaki JM, Tu T, Reijmers LG, 2013. Fear extinction causes target-specific remodeling of perisomatic inhibitory synapses. Neuron. 80,1054–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujino N, Sakurai T, 2013. Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci. 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneki H, Murata S, Anzawa Y, Soeda Y, Tokai E, Wada T, Kimura I, Yanagisawa M, Sakurai T, Sasaoka T, 2008. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 51,657–67. [DOI] [PubMed] [Google Scholar]
- Vidal J, Buwalda B, Koolhaas JM, 2011. Male Wistar rats are more susceptible to lasting social anxiety than Wild-type Groningen rats following social defeat stress during adolescence. Behav Processes. 88, 76–80. [DOI] [PubMed] [Google Scholar]
- Vindas MA, Johansen IB, Folkedal O, Hoglund E, Gorissen M, Flik G, Kristiansen TS, Øverli Ø, 2016. Brain serotonergic activation in growth-stunted farmed salmon: adaption versus pathology. R Soc Open Sci. 3, 160030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M, 2002a. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 71, 379–92. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M, 2002b. Quantifying fear potentiated startle using absolute versus proportional increase scoring methods: implications for the neurocircuitry of fear and anxiety. Psychopharmacology (Berl). 164, 318–28. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M, 2003. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 463, 199–216. [DOI] [PubMed] [Google Scholar]
- Waters RP, Rivalan M, Bangasser DA, Deussing JM, Ising M, Wood SK, Holsboer F, Summers CH, 2015. Evidence for the role of corticotropin-releasing factor in major depressive disorder. Neurosci Biobehav Rev. 58, 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P, 1984. The validity of animal models of depression. Psychopharmacology (Berl). 83, 1–16. [DOI] [PubMed] [Google Scholar]
- Willner P, 1991. Animal models as simulations of depression. Trends Pharmacol Sci.12,131–6. [DOI] [PubMed] [Google Scholar]
- Willner P, Mitchell PJ, 2002. The validity of animal models of predisposition to depression. Behav Pharmacol.13,169–88. [DOI] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Yamanaka A, Diano S, Borok E, Roberts AJ, Sakurai T, Kilduff TS, Horvath TL, de Lecea L, 2004. Interaction between the corticotropin-releasing factor system and hypocretins (orexins): a novel circuit mediating stress response. J Neurosci. 24, 11439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Boutrel B, de Lecea L, 2005. Stress and arousal: the corticotrophin-releasing factor/hypocretin circuitry. Mol Neurobiol. 32, 285–94. [DOI] [PubMed] [Google Scholar]
- Yaeger JDW, Staton CD, Krupp KT, Summers TR, Summers CH, 2018. Anxious phenotypes: prior experience and orexin 2 (Orx2) receptor activation lead to social stress resilience Society for Neuroscience Abstracts. 44. [Google Scholar]
- Ye Y, Wang G, Wang H, Wang X, 2011. Brain-derived neurotrophic factor (BDNF) infusion restored astrocytic plasticity in the hippocampus of a rat model of depression. Neurosci Lett. 503, 15–9. [DOI] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, Dayas CV, 2014. Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci. 8, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE, 2006. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 494, 845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AS, Klap R, Sherbourne CD, Wells KB, 2001. The quality of care for depressive and anxiety disorders in the United States. Arch Gen Psychiatry. 58, 55–61. [DOI] [PubMed] [Google Scholar]
- Zhang LL, Wang JJ, Liu Y, Lu XB, Kuang Y, Wan YH, Chen Y, Yan HM, Fei J, Wang ZG, 2011. GPR26-deficient mice display increased anxiety- and depression-like behaviors accompanied by reduced phosphorylated cyclic AMP responsive element-binding protein level in central amygdala. Neuroscience. 196, 203–14. [DOI] [PubMed] [Google Scholar]
- Zhang S, Blache D, Vercoe PE, Adam CL, Blackberry MA, Findlay PA, Eidne KA, Martin GB, 2005. Expression of orexin receptors in the brain and peripheral tissues of the male sheep. Regul Pept. 124, 81–7. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hong J, Zhang S, Zhang T, Sha S, Yang R, Qian Y, Chen L, 2016. Postpartum estrogen withdrawal impairs hippocampal neurogenesis and causes depression- and anxiety-like behaviors in mice. Psychoneuroendocrinology. 66,138–49. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A, 2002. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 952,188–99. [DOI] [PubMed] [Google Scholar]