Mycobacterium abscessus is a biofilm-forming, multidrug-resistant nontuberculous mycobacterial (NTM) pathogen increasingly found in cystic fibrosis patients. Antibiotic treatment for these infections is often unsuccessful, partly due to M. abscessus’s high intrinsic antibiotic resistance.
KEYWORDS: artificial cystic fibrosis sputum, cystic fibrosis, Mycobacterium, Mycobacterium abscessus, antibiotic resistance, antibiotic tolerance, biofilms, glycolipids, multidrug resistance, nontuberculous mycobacterium
ABSTRACT
Mycobacterium abscessus is a biofilm-forming, multidrug-resistant nontuberculous mycobacterial (NTM) pathogen increasingly found in cystic fibrosis patients. Antibiotic treatment for these infections is often unsuccessful, partly due to M. abscessus’s high intrinsic antibiotic resistance. It is not clear whether antibiotic tolerance caused by biofilm formation also contributes to poor treatment outcomes. We studied the surface glycolipids and antibiotic tolerance of M. abscessus biofilms grown in artificial cystic fibrosis sputum (ACFS) medium to determine how they are affected by nutrient conditions that mimic infection. We found that M. abscessus displays more of the virulence lipid trehalose dimycolate when grown in ACFS than when grown in standard lab medium. In ACFS medium, biofilm-associated cells were more antibiotic tolerant than planktonic cells in the same well. This contrasts with standard lab media, where both biofilm and planktonic cells are highly antibiotic tolerant. These results indicate that M. abscessus cell physiology in biofilms depends on environmental factors and that nutrient conditions found within cystic fibrosis infections could contribute to both increased virulence and antibiotic tolerance.
INTRODUCTION
Infections caused by nontuberculous mycobacteria (NTM) are on the rise across the globe (1–3). Mycobacterium abscessus is a highly antibiotic-resistant NTM that causes soft tissue infections and is an increasingly common respiratory pathogen in cystic fibrosis (CF) patients (4–6). While most NTM infections are believed to be contracted through environmental exposure, M. abscessus has recently been shown to be transmitted between CF patients, probably by fomites (7). Unfortunately, M. abscessus infections in CF patients are very difficult to treat, and there are no standardized treatment regimens. Treatment usually involves some combination of clarithromycin, amikacin, imipenem, cefoxitin, and/or linezolid (8). Infection clearance after up to 3 years of antibiotic treatment is achieved in only 10% to 55% of patients (8). This level of treatment failure is likely partly due to intrinsic antibiotic resistance (9–11); however, antibiotic tolerance may also play a role (12), though the environmental conditions that induce tolerance in this organism are poorly understood.
The pathophysiology of M. abscessus infection in the CF lung has scarcely been studied, but one pathology study of explanted lungs from CF patients showed M. abscessus aggregates that appear to be forming a biofilm around the alveoli (13). Biofilms are bacterial communities that, in many species, are held together by exopolysaccharides (14). Mycobacterial biofilms appear to depend on surface glycolipids (15) and free mycolic acids (16, 17), though many questions remain about the composition of the matrix in mycobacterial biofilms. Because bacteria growing in biofilms are notoriously tolerant to antibiotics (18), this mode of growth during infection may contribute to the treatment recalcitrance of M. abscessus. While we currently lack a comprehensive model of the chemical and genetic basis of M. abscessus biofilm formation, it is clear that surface glycolipids are important. Glycopeptidolipids, which are found on the outer leaflet of the mycobacterial outer membrane, have been shown to affect biofilm structure (19) and are thought to modulate the course of infection (20, 21). Trehalose dimycolate (TDM), which is known to contribute to virulence in Mycobacterium tuberculosis (22), is also found on the surfaces of some M. abscessus strains, though its contribution to biofilm formation and virulence in M. abscessus has not been studied.
M. abscessus has two genetic isoforms: the wild-type “smooth” strains, which produce abundant glycopeptidolipids (GPLs) and minimal TDM, and “rough” mutants, which have genetic lesions in the GPL loci and display little or no GPLs but sometimes have higher levels of TDM (23, 24). The smooth strains form more robust biofilms, and it is thought that this form could contribute to colonization at infection sites (24), which is likely promoted by GPLs masking other cell surface molecules that activate innate immunity (21). The rough mutants cause more inflammation (21), are more virulent, and are thought to promote more tissue invasion (24). Rough mutants are more frequently isolated after a persistent infection has been established for a long period, in both CF and non-CF patients (25, 26).
The smooth and rough genetic variants represent two phenotypic extremes of M. abscessus physiology. However, the rough variants only form in some infections, and it is thought that most infections are established by smooth strains (24). Smooth strains are known to form biofilms in vitro (12, 19) and are thought to also form biofilms during infection (13). Antibiotic-recalcitrant M. abscessus infections are likely to involve mature biofilms growing in complex nutrient environments. However, in vitro M. abscessus biofilm studies have been conducted on immature biofilms in simple nutrient environments (12, 19): under these conditions, there are modest differences in antibiotic tolerance between biofilm-associated and planktonic cells (19). Thus, it is not clear to what extent biofilms contribute to antibiotic tolerance during infection.
In this work, we sought to determine how cell surface physiology, biofilm formation, and antibiotic tolerance are affected by medium conditions in M. abscessus ATCC 19977. We found that the cell surface, as measured by fluorescent staining, is different in M. abscessus in artificial cystic fibrosis sputum (ACFS) medium compared to that in either minimal Hartmans-de Bont (HdB) medium or the standard mycobacterial growth medium, 7H9. In addition, both smooth and rough strains displayed more TDM in the ACFS medium. When we compared biofilm and planktonic cells, we found that both populations were quite antibiotic tolerant in HdB and 7H9, while the planktonic cells were more susceptible to antibiotics in ACFS medium.
RESULTS
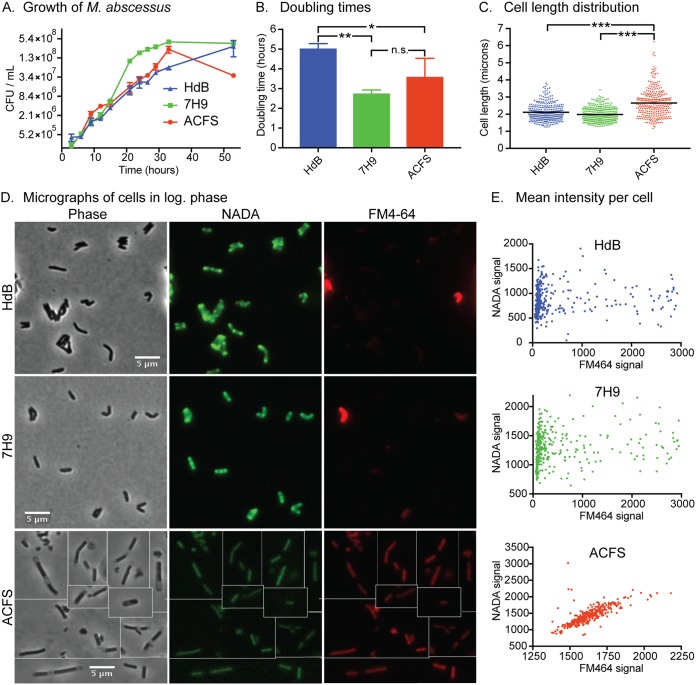
To determine how different nutrient conditions affect basic cell physiology, we measured growth rate and microscopically examined M. abscessus in three different media. M. abscessus grew fastest in 7H9 medium (Fig. 1A and B), even though the artificial cystic fibrosis sputum (ACFS) medium is richer (27). M. abscessus in ACFS medium died off more in stationary phase, implying that the nutrient richness may not prepare cells for certain stresses (Fig. 1A). We imaged cells by using phase microscopy and observed that cell lengths were longer in ACFS medium (Fig. 1C and D). We stained log-phase cells in each media with both 4-chloro-7-nitrobenzofurazan-conjugated d-alanine (NADA) (28), which is integrated into peptidoglycan, and FM4-64, which stains the inner membrane (29). Although growth rates were more similar in HdB and ACFS media, cell surface staining was more similar in HdB and 7H9. The NADA staining in HdB and 7H9 was consistent with studies performed in other mycobacterial cells: brighter staining at the septa and poles where new peptidoglycan is inserted (30). FM4-64 stained most cells in these medium types dimly, but a subset stained more brightly. Staining of cells in ACFS medium was more homogeneous (Fig. 1D and E). We observed in the phase images that cells growing in ACFS appear to be encased in translucent sheaths, which could alter access of the stains to the cell (Fig. 1D). These data show that M. abscessus is physiologically different in typical lab medium than in medium that mimics the nutrient conditions in the lungs of cystic fibrosis patients.
FIG 1.
The physiology of M. abscessus planktonic cells varies according to medium type. (A) CFU over time in the three growth media on a 2-log scale. (B) Maximum doubling times during log-phase growth, calculated from data in panel A. P values calculated by one-way ANOVA with Tukey’s correction. HdB versus 7H9, P = 0.0059; HdB versus ACFS, P = 0.0459. (C) Quantification of cell lengths of M. abscessus cells in log phase, in the three medium types. At least 300 cells from two biological replicates were imaged by phase microscopy, and cell lengths were calculated using MicrobeJ. ACFS versus HdB, P < 0.0001; ACFS versus 7H9, P < 0.0001. (D) Micrographs of log-phase cells stained with the fluorescent d-amino acid NADA and the membrane stain FM4-64. (E) Quantification of the average fluorescence intensity for at least 300 cells from two biological replicates, stained with NADA and FM4-64, as in panel D.
To study the physiology of biofilms, we sought to develop a method to separate biofilm and planktonic cells from the same culture well: this allows us to control internally for nutrient depletion in the medium, which also impacts cell physiology and antibiotic tolerance. We incubated standing cultures in tissue-culture-treated plates. Biofilms formed at the bottom of each well, while the planktonic cells remained in the medium in the upper half of the well. We pipetted off all the medium on the top of each well to isolate planktonic cells, and then resuspended the surface-associated cells on the bottom of each well in 7H9 with Tween 80, a nonionic detergent, which broke apart cell clumps. To determine whether 7H9 plus Tween 80 was sufficient to break apart clumps or whether sonication was necessary, we compared the CFU produced from biofilm resuspensions with and without sonication (see Fig. S1 in the supplemental material). Sonication did not change the CFU counts, and so we concluded that resuspension in 7H9 plus Tween 80 alone was sufficient to break apart smooth M. abscessus biofilms. Therefore, we used that method for biofilm enumeration in subsequent experiments.
We next analyzed how the three medium types affect biofilm development and morphology. We grew M. abscessus in the three different medium types over time and monitored biofilm development by CFU assay and photography. Biofilms growing in all medium types reach maximal cell density by day 6 (Fig. 2). Biofilms in ACFS formed three-dimensional plumes in standing culture, while those in HdB or 7H9 were flat and almost crystalline. We did not observe pellicles under the medium conditions we tested using smooth M. abscessus strains (Fig. 2). From these data, we conclude that biofilm morphology was affected by medium condition.
FIG 2.
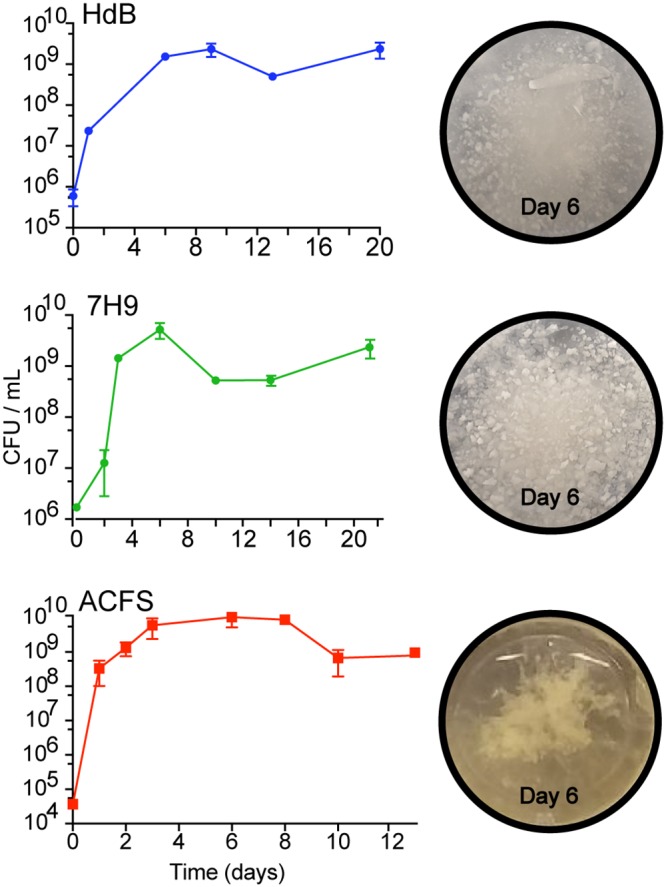
The development and macroscopic structure of M. abscessus biofilms in different media. CFU for biofilm-associated cells in standing culture over time. Images of corresponding wells taken at 6 days.
To probe the physiological differences between biofilm and planktonic cells in our assay, we tested their ability to transcriptionally and translationally respond to the induction of a reporter. We transformed M. abscessus with a plasmid that expresses Tag-red fluorescent protein (RFP) under the control of a constitutive PGroEL promoter and expresses enhanced green fluorescent protein (eGFP) under the control of an inducible Pnitrile promoter. We allowed biofilms of this culture to mature for 6 days, and then induced them with isovaleronitrile to induce the expression of eGFP. After 24 h of induction, we resuspended and fixed cells from the planktonic and biofilm populations in each medium type and visualized the cells by fluorescence microscopy (Fig. 3A). We found that under all medium conditions, cells in biofilms were less likely than planktonic cells to express significant eGFP upon induction, compared to the constitutive Tag-RFP (Fig. 3B). This shows that biofilm cells are either less permeable to the inducer, less transcriptionally and translationally active than planktonic cells in the same culture well, or both. These data also validate our method of separating planktonic cells from biofilm cells, by showing separated cell types are physiologically distinct in all three medium types.
FIG 3.
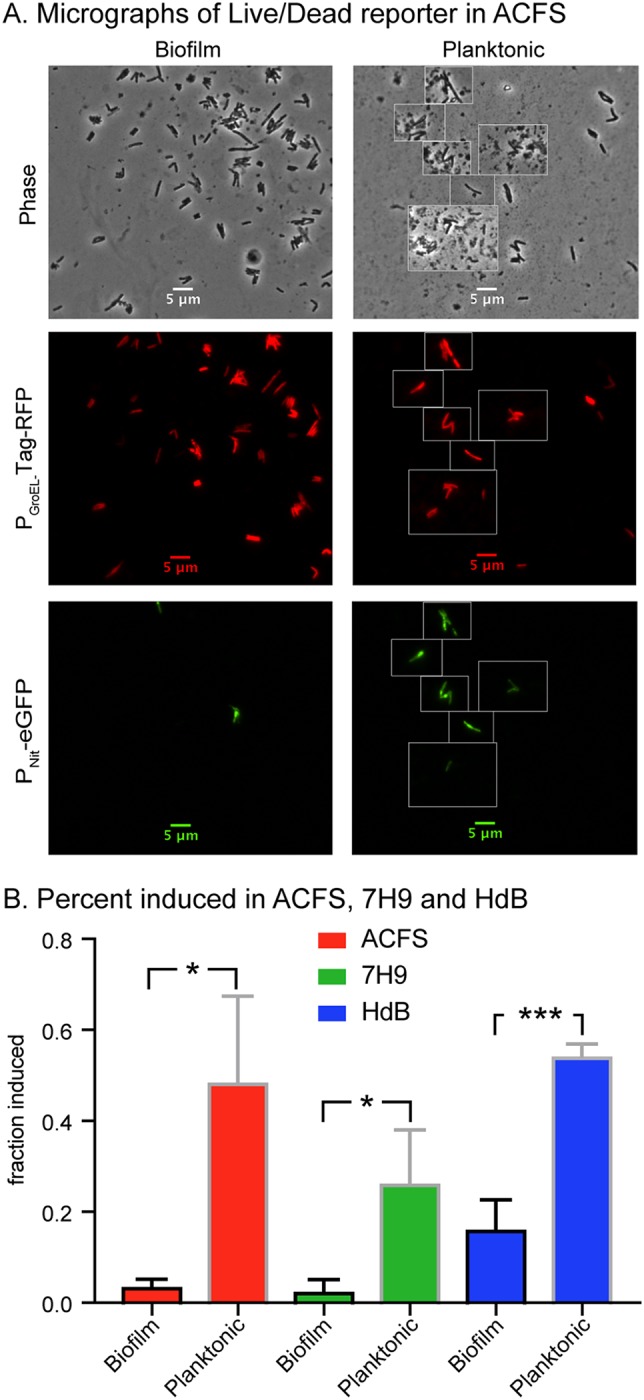
Cells in M. abscessus biofilms are less responsive to a transcriptional inducer. (A) Micrographs of M. abscessus cells carrying the pDE43-MEK-Nit-live/dead vector from biofilms grown for 6 days in ACFS and then induced with 10−6 isovaleronitrile for 1 day before fixation and imaging. Living cells that retain the vector show constitutive Tag-RFP signal, inducible cells also show eGFP signal. (B) Percentages of cells with average Tag-RFP signal intensities >400 that also have average eGFP signal intensities >100 under each condition. At least 100 cells each from three independent biological replicates were quantified for each condition. P values of biofilm versus planktonic for ACFS, 7H9, and HdB media are 0.0152, 0.0271, and 0.0007, respectively. P values were calculated using an unpaired t test.
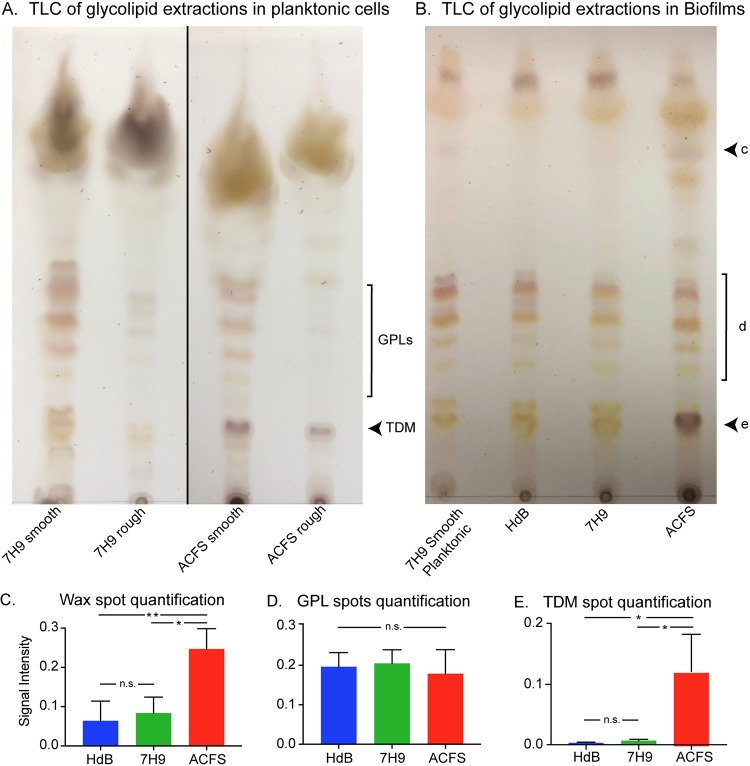
Because the biofilm morphologies varied so greatly between medium types (Fig. 2), we hypothesized that medium could affect glycolipid expression. We extracted surface glycolipids from planktonic smooth and rough strains grown in 7H9 and ACFS (Fig. 4A) as well as from mature biofilms grown under all three medium conditions (Fig. 4B). We then separated the glycolipids by thin-layer chromatography (TLC). TLC analysis showed that the smooth strain expressed similar levels and types of GPLs irrespective of medium condition, while the rough strain produced minimal GPLs (Fig. 4A). In biofilms, the smooth strain expressed GPLs under all conditions (Fig. 4B and D). Based on previous GPL analyses (24), the purple spot near the top (Fig. 4B, spot c) is an unidentified wax, while the dark purple spot at the bottom (Fig. 4B, spot d) is trehalose dimycolate (TDM) (31). Both smooth and rough strains have increased TDM in ACFS compared to that in lab medium, in both planktonic cultures (Fig. 4A) and biofilms (Fig. 4B). We observed a statistically significant increase in the wax (Fig. 4B and C) and TDM in biofilms in ACFS (Fig. 4B and E). The levels of GPLs were similar (Fig. 4B and D). These data show that medium conditions affect the surface glycolipid expression of M. abscessus and that M. abscessus grown in ACFS medium has higher levels of TDM, a glycolipid that is a virulence factor in M. tuberculosis infections (22).
FIG 4.
Glycolipid profiles are affected by medium condition. (A) Thin layer chromatography (TLC) of glycolipid extractions from planktonic cultures of rough and smooth M. abscessus strains grown in 7H9 and ACFS. GPLs, glycopeptidolipids; TDM, trehalose dimycolate. (B) Representative TLC of glycolipid extracts from mature biofilms of smooth M. abscessus in HdB, 7H9, and ACFS. Extraction from planktonic cells grown in 7H9 plus Tween 80 was used as a control sample. Black arrowheads indicate lipid species prominent in ACFS: c, unknown wax; d, GPLs; e, TDM. Quantification of the signal intensity for each spot in panel B by means of spot densitometry. Values shown are the means from three biological replicate experiments with error bars representing the standard deviations. (C) Wax, P values between ACFS versus HdB and ACFS versus 7H9 were 0.0079 and 0.0134, respectively. (D) GPLs, no significant differences. (E) TDM, P values between ACFS versus HdB and ACFS versus 7H9 were 0.0149 and 0.0171, respectively. P values were calculated using one-way analysis of variance (ANOVA) with Tukey’s correction for multiple comparisons. Signal intensities for panel D were quantified by using the red channel only of the TLC images to eliminate the signal from the yellow spots in the same area.
We sought to determine how biofilm maturity affects antibiotic tolerance. For these and all subsequent biofilm experiments, we compared the survival of biofilm and planktonic cells from the same well of a 24-well plate for each replicate. We incubated M. abscessus in ACFS medium for 3, 6, or 11 days, and then treated each well with two clinical drugs, clarithromycin and imipenem. We then measured survival over time and found that tolerance increased over time in standing culture, likely due to nutrient depletion of the medium (Fig. 5A and B). In 3-day-old and 6-day-old biofilms, planktonic cells died off substantially upon treatment, while at 11 days, both populations were quite tolerant (Fig. 5A). Six-day-old biofilms had higher tolerance than the planktonic cells in the same well at both 24 and 48 h of treatment (Fig. 5B). From this, we concluded that at 6 days, the biofilms in ACFS medium were mature enough to substantially protect cells against antibiotics. Based on these results, and the fact that biofilms in all medium types reached maximal cell density at 6 days (Fig. 2), we conducted all subsequent biofilm assays with 6-day-old biofilms.
FIG 5.
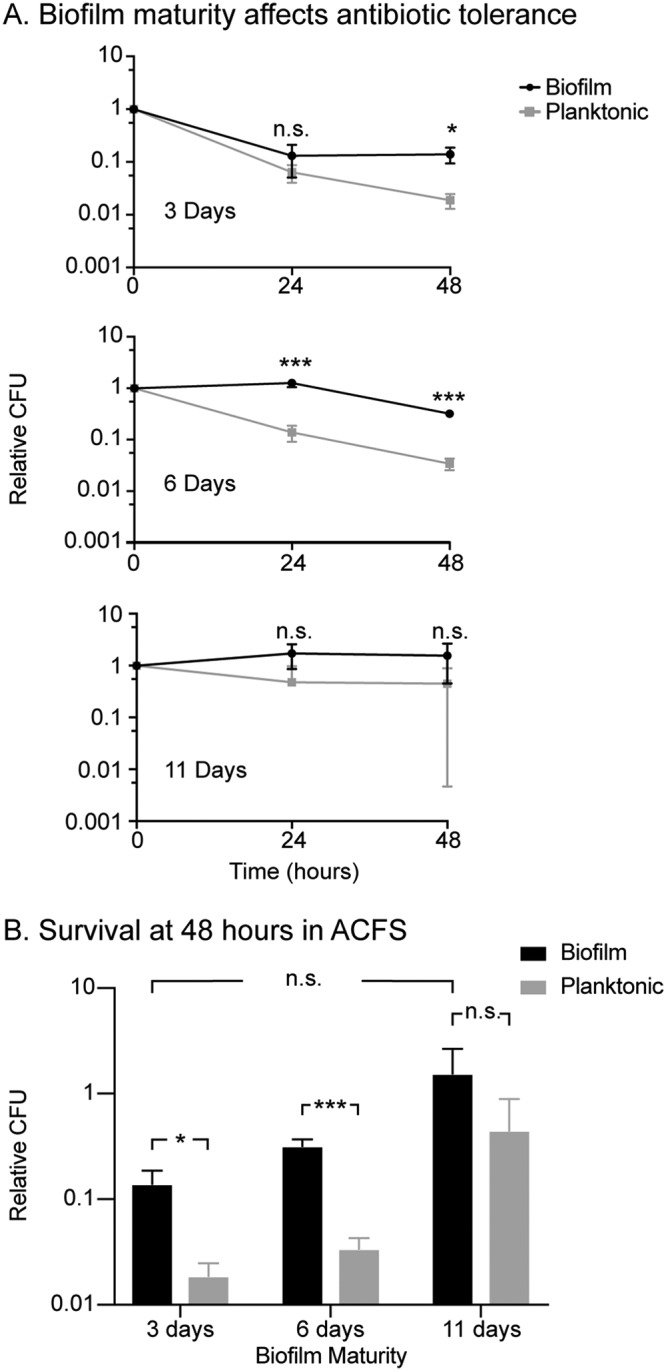
Antibiotic tolerance increases as ACFS biofilms mature. (A) Relative CFU (CFU at each time point divided by the CFU value before treatment, i.e., t = 0) from biofilms and planktonic cells from the same wells, grown for 3, 6, and 11 days in ACFS. Cultures were treated with clarithromycin (20 μg/ml) and imipenem (15 μg/ml) for 48 h, and CFU were measured at 0, 24, and 48 h. (B) Survival from 48-h CFU time points (triplicate wells). P values between biofilm and planktonic cells are 0.01099, 0.0006, and 0.1815 for 3, 6, and 11 days, respectively. P value for biofilms between 3 and 11 days is 0.09046. P values were calculated using the Student’s t test.
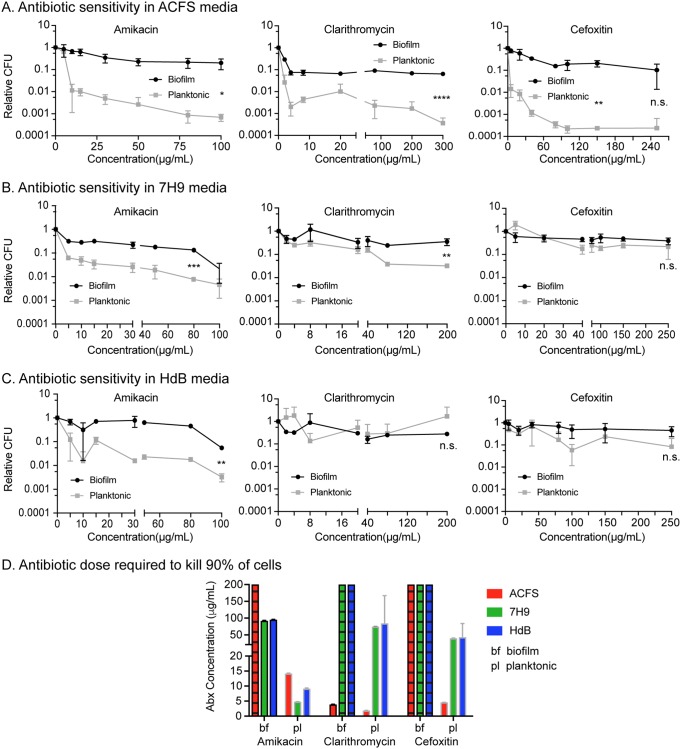
To determine how medium type affects the antibiotic tolerance of M. abscessus biofilms, we treated 6-day-old biofilms grown in different medium types with various concentrations of the clinically used antibiotics amikacin, clarithromycin, and cefoxitin and then plated both the biofilm and planktonic cells at 48 h after treatment. We found that amikacin consistently killed planktonic cells at nearly an order of magnitude more than the biofilm cells from the same well, across medium types. With clarithromycin and cefoxitin treatment, however, we saw much more variability. With both these antibiotics, both planktonic cells and biofilm-associated cells were highly antibiotic tolerant in both 7H9 and HdB. However, in ACFS, planktonic cells were much more sensitive to clarithromycin and cefoxitin than biofilm-associated cells. Similar results were observed in the rough strain (Fig. S3). Clarithromycin was the only drug that was able to kill biofilm-associated cells at concentrations <50 μg/ml, and this only occurred in ACFS medium (Fig. 6).
FIG 6.
Antibiotic sensitivity varies by antibiotic and medium type. (A, B, and C) Relative CFU of 6-day-old biofilms and planktonic cells from the same wells after 48 h of treatment with a range of antibiotic concentrations in 3 media. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; n.s., P > 0.05 by Student's t test. (D) LD90 (dose required to kill 90% of the cells) analysis of the data from panels A, B, and C. Bars with hatched lines indicate that we were unable to observe killing of 90% of the population at any concentration used, and so the LD90 is >200 μg/ml. The LD90 was calculated for each replicate, and the bars indicate means and standard deviations.
In our assays, the biofilm-associated cells likely had access to less oxygen than the planktonic cells. We sought to determine whether hypoxia could differentially affect cells depending on the medium. We made cultures of M. abscessus in tubes with minimal headspace, sealed the tubes with septum caps, and incubated them without shaking for 13 days. We then injected cefoxitin through the caps and measured survival after 48 h. The results show that hypoxic M. abscessus exhibited tolerance in both 7H9 and ACFS media (see Fig. S2), comparable to that seen in biofilms (Fig. 6A and B). Thus, as for M. tuberculosis (32), hypoxia promotes antibiotic tolerance in M. abscessus and is likely to be one factor in the antibiotic tolerance seen in biofilm-associated cells.
DISCUSSION
Bacterial physiology is enormously plastic. Bacterial cell size, shape, growth rates, surface properties, and gene expression all change profoundly, even between different lab medium conditions (33–35). This plasticity makes it very difficult to determine the physiological state of a pathogen during infection. In this study, we sought to physiologically profile the emerging pathogen M. abscessus in two standard lab growth media and one medium designed to mimic the nutrient conditions in the lungs of cystic fibrosis patients (ACFS). We find that, compared to standard lab media, ACFS medium induces profound physiological changes in M. abscessus, both in planktonic (Fig. 1 and 4) and biofilm (Fig. 4 and 6) states.
Bacterial biofilms are complex structures which, on the macro scale, vary physiologically according to developmental stage (36), surface type (37), and nutrient conditions (38). On the microscopic scale, there is also heterogeneity within a single biofilm: cells in different places within the biofilm express different surface factors and have different gene expression profiles (39–41). Thus, it is not meaningful to merely compare the phenotypes of “biofilm” and “planktonic” cells from a given species, as there is enormous phenotypic variety within each of these categories. In this work, we profiled some of the phenotypic diversity possible in M. abscessus biofilms in order to assess the extent to which in vitro biofilm experiments might be relevant to the physiology of M. abscessus biofilms within the lungs of CF patients. Our results show that M. abscessus biofilms in ACFS medium have important physiological differences from biofilms in the standard mycobacterial lab media HdB and 7H9 (Fig. 2, 4, and 6). The fact that such differences can be seen even between in vitro medium conditions shows that M. abscessus biofilm physiology is responsive to environmental conditions. We hypothesize that M. abscessus physiology in HdB and 7H9 media is unlikely to represent its physiology during infection. Our results do not establish ACFS medium as a proxy for infection, which of course includes many host immune factors that cannot be mimicked in vitro. However, these data show that nutrient conditions that might be encountered by M. abscessus in the host could have an important role in the pathogen’s expression of virulence glycolipids and responsiveness to antibiotic treatment.
Even in experiments with the smooth M. abscessus, which produces GPLs, we observed differences in the appearance of the biofilms that formed in the different medium types. The biofilms in both HdB and 7H9 appeared flat and flaky, while the biofilms that formed in the ACFS medium looked wet and formed tendrilly plumes (Fig. 2). These differences could be merely due to the chemical and viscosity differences between the medium types, or they could be due to changes in M. abscessus cell physiology in the different medium types. We profiled the surface glycolipids and antibiotic sensitivity of cells in these different biofilms and found that cells in ACFS biofilms are physiologically distinct: they display more trehalose dimycolate and a nonpolar wax species than biofilms in 7H9 and HdB (Fig. 4) and they exhibit more relative antibiotic tolerance than planktonic cells (Fig. 6).
Because nutrient availability affects the physiology of cells within a biofilm, we sought to develop methods that would allow us to disentangle responses to nutrients from responses to biofilm formation. Other groups solved this problem by moving biofilms to fresh medium each day (12, 19). We assumed that nutrients would not be unlimited during infection and so worked to assess biofilm physiology in nutrient-limited culture (Fig. 2, Fig. 5). To control for the well-known effects of nutrient limitation on antibiotic tolerance (42), we compared biofilm and planktonic cells from the same culture wells. These cells are sharing the same depleted nutrients, and so differences in physiology should be due to association with the biofilm or to differences in access to oxygen. While we observed considerable antibiotic tolerance in 7H9 and HdB against clarithromycin and cefoxitin, there was similar tolerance in planktonic and biofilm-associated cells (Fig. 6B and C), even though the planktonic cells presumably have more access to oxygen at the top of the well. This implies that antibiotic tolerance under these medium conditions is due to some other environmental factor, such as nutrient depletion, rather than to the protection of the biofilm or to hypoxia. In the more nutrient-rich ACFS medium, however, we see much higher sensitivity to cefoxitin and clarithromycin in the planktonic cells, while the biofilm-associated cells are tolerant. Therefore, the physiological difference between biofilm and planktonic cells is greater in ACFS medium than in typical lab media.
Our results imply that the modest degree of antibiotic tolerance in biofilms that has been observed before (19) may be a function of the culture conditions. Our data show that mature biofilms grown in ACFS medium afford considerable protection against three clinical antibiotics from different classes. While the ACFS medium may mimic the nutrient conditions of sputum, it does not replicate a complex host environment with active immune cells and tissue structures, and so the physiological state of M. abscessus in a true infection remains unknown. However, our results imply, during infection in CF patients, M. abscessus may express more of the virulence glycolipid TDM and be more antibiotic tolerant than experiments in regular lab media might indicate. Thus, treatments that help disrupt M. abscessus biofilms or prevent their establishment could help clear these infections more quickly. A deeper understanding of the chemistry and genetics of biofilm formation and physiological responses in M. abscessus will be needed in order to determine the role of biofilms in infection and treatment recalcitrance and to develop better treatment protocols.
MATERIALS AND METHODS
Media and culture conditions.
All M. abscessus ATCC 19977 cultures were started in 7H9 (Becton, Dickinson, Franklin Lakes, NJ) medium with 5 g/liter bovine serum albumin, 2 g/liter dextrose, 0.85 g/liter NaCl, 0.003 g/liter catalase, 0.2% glycerol, and 0.05% Tween 80 and shaken overnight at 37°C until log phase. All M. abscessus CFU counts were performed by plating serial dilutions on LB Lennox agar. Three medium types that varied in nutrient richness were used for all biofilm assays: Tween 80 was not added to any medium for biofilm assays. Hartmans-de Bont (HdB) minimal medium was made as described previously (43), and 7H9 for biofilms was made as described above, without the Tween 80.
Artificial cystic fibrosis sputum.
Two protocols were combined in order to make our ACFS medium (27, 44). In 500 ml of ultrapure water, the unique components that comprise CF sputum were added as described previously (44); 5 g of porcine mucin (NBS Biologicals), 4 g of DNA (Spectrum Chemicals), 5.9 mg diethylene triamine pentaacetic acid (DTPA), 5 g NaCl, 2.2 g KCl, and 1.81 g Tris base. Amino acid stocks were prepared as described previously (27) and stored at 4°C. Except for tryptophan, amino acids were added to the medium. The ACFS medium was adjusted to pH 7, increased to 1 liter, and autoclaved at 110°C for 15 min. The medium was cooled down at room temperature prior to adding tryptophan and 5 ml of egg yolk emulsion (Dalynn Biologicals). ACFS medium was stored at 4°C.
Biofilm assays.
Log-phase biological replication cultures of M. abscessus cultures in 7H9 were inoculated in tissue-culture-treated 24-well plates with 2 ml of the appropriate medium to a final optical density of 0.02. Sufficient wells were inoculated in order to have biological triplicates for each time point or concentration in the assay. Prior to incubation, each plate was covered with a Breathe-Easy sealing membrane (Electron Microscopy Sciences) to allow air exchange and reduce evaporation. Standing biofilm plates were incubated at 37°C. The Breathe-Easy membrane was removed after biofilm development in order to add antibiotics. The biofilm plates were then resealed and incubated again at 37°C for 24 or 48 h. For each data point, cells from biological replicate wells were used to plate for CFU calculations. To enumerate the planktonic cells, the supernatant was extracted from each well without disturbing the biofilm and plated. The remaining biofilm was then resuspended in 7H9 plus Tween 80, and serial dilutions were plated and incubated at 37°C for 4 days. Serial dilutions were conducted in 96-well plates in HdB plus Tween 80 and plated on plain LB agar plates. The relative CFU is the ratio between each CFU value and the initial CFU value at a t of 0 or concentration of 0.
Hypoxia assay.
M. abscessus was grown in 7H9 until log phase. To promote hypoxia, sterile glass tubes were filled near full (9 ml) with 7H9 or ACFS. All tubes were inoculated to an optical density of 0.02, capped with a sterile rubber septum, and wrapped with Parafilm to avoid uncapping. To establish growth cessation, standing tubes were incubated at 37°C, and CFU were enumerated every couple of days using biological replicates. Once we established that growth cessation occurs at 13 days, new tubes were inoculated and incubated for 13 days. To avoid oxygenation, replicates were treated by injecting 80 μg/ml of cefoxitin through the rubber septum. CFU were enumerated upon addition of antibiotic (t = 0) and 48 h posttreatment. Samples from all time points were plated on LB agar and incubated for 4 days.
Glycolipid extractions and thin-layer chromatography.
Mature biofilms and planktonic cultures were used for glycolipid extractions. GPLs were extracted as described previously (34) and spotted on TLC Silica Gel 60 plates (Millipore Sigma). In short, cells were isolated and centrifuged. Pellets were resuspended for lipid extraction in 10 ml chloroform-methanol (2:1). Extractions were performed twice at room temperature for 24 h and then centrifuged at 5,000 rpm for 30 min to collect organic supernatant. Organic samples (lipids) were evaporated with nitrogen, resuspended in 1 ml chloroform-methanol (2:1), and treated with equal volumes 0.2 M NaOH (in methanol). Lipids were incubated at 37°C for 30 min and then neutralized with a few drops of glacial acetic acid. Solvents were evaporated and the lipids were resuspended with 4 ml of chloroform, 2 ml of methanol, and 1 ml of water in each tube. Samples were mixed and then centrifuged at 5,000 rpm for 10 min to collect the organic layer. The solvent was evaporated, and the remaining lipids were resuspended in 100 μl of chloroform-methanol (2:1) and spotted. TLCs were developed in chloroform-methanol-water (100:14:0.8) as described previously (24). Plates were visualized with 10% sulfuric acid (in ethanol) and baked for 20 min at 120°C. The signal intensities of spots were analyzed and quantified using ImageJ software by means of spot densitometry. For spot d (TDM), the TLC image was split into single-color channels and quantified by measuring the signal intensity from the red channel only. Each spot intensity value was normalized against that for all the spots in a lane (entire lane; all in triplicates). We reran the TLCs, when necessary, so that total lane spot intensities were similar between samples.
Cell staining.
For experiments on log-phase cells, M. abscessus was stained with 3 μl/ml of 10 mM NADA and incubated at room temperature for 15 min in growth medium. Cells were then pelleted and resuspended in phosphate-buffered saline (PBS) plus Tween 80, and FM4-64 was added to a final concentration of 4 ng/ml and incubated at room temperature for 5 min. Cells were then fixed with 2% paraformaldehyde for 1 h at room temperature, washed, and resuspended in PBS plus Tween 80 for imaging.
Microscopy.
M. abscessus cells were resuspended as described for the plating of planktonic and biofilm cells and then fixed with 4% paraformaldehyde in PBS at room temperature for 2 h. Cells were immobilized on agarose pads and imaged using a Nikon Ti-2 widefield epifluorescence microscope with a Photometrics Prime 95B camera and a Plan Apo 100× 1.45 numerical aperture (NA) lens objective. Green fluorescence images were taken using a filter cube with a 470/40 nm excitation filter and a 525/50 nm emission filter. Red fluorescence images were taken using a filter cube with a 560/40 nm excitation filter and a 630/70 emission filter. Images were captured using NIS Elements software and analyzed using FIJI and MicrobeJ (45).
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by a grant from the Cystic Fibrosis Foundation (grant no. BOUTTE1810).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02488-18.
REFERENCES
- 1.World Health Organization. 2016. Global tuberculosis report. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Brode SK, Daley CL, Marras TK. 2014. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuber Lung Dis 18:1370–1377. doi: 10.5588/ijtld.14.0120. [DOI] [PubMed] [Google Scholar]
- 3.Stout JE, Koh W-J, Yew WW. 2016. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis 45:123–134. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation. 2017. 2016 Patient registry annual data report. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 5.Bar-On O, Mussaffi H, Mei-Zahav M, Prais D, Steuer G, Stafler P, Hananya S, Blau H. 2015. Increasing nontuberculous mycobacteria infection in cystic fibrosis. J Cyst Fibros 14:53–62. doi: 10.1016/j.jcf.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Esther CR Jr, Esserman DA, Gilligan P, Kerr A, Noone PG. 2010. Chronic Mycobacterium abscessus infection and lung function decline in cystic fibrosis. J Cyst Fibros 9:117–123. doi: 10.1016/j.jcf.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bryant JM, Grogono DM, Rodriguez-Rincon D, Everall I, Brown KP, Moreno P, Verma D, Hill E, Drijkoningen J, Gilligan P, Esther CR, Noone PG, Giddings O, Bell SC, Thomson R, Wainwright CE, Coulter C, Pandey S, Wood ME, Stockwell RE, Ramsay KA, Sherrard LJ, Kidd TJ, Jabbour N, Johnson GR, Knibbs LD, Morawska L, Sly PD, Jones A, Bilton D, Laurenson I, Ruddy M, Bourke S, Bowler I, Chapman SJ, Clayton A, Cullen M, Dempsey O, Denton M, Desai M, Drew RJ, Edenborough F, Evans J, Folb J, Daniels T, Humphrey H, Isalska B, Jensen-Fangel S, Jönsson B, Jones AM, et al. 2016. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science 354:751–757. doi: 10.1126/science.aaf8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DaCosta A, Jordan CL, Giddings O, Lin F-C, Gilligan P, Esther CR Jr. 2017. Outcomes associated with antibiotic regimens for treatment of Mycobacterium abscessus in cystic fibrosis patients. J Cyst Fibros 16:483–487. doi: 10.1016/j.jcf.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Rominski A, Selchow P, Becker K, Brülle JK, Dal Molin M, Sander P. 2017. Elucidation of Mycobacterium abscessus aminoglycoside and capreomycin resistance by targeted deletion of three putative resistance genes. J Antimicrob Chemother 72:2191–2200. doi: 10.1093/jac/dkx125. [DOI] [PubMed] [Google Scholar]
- 10.Rominski A, Roditscheff A, Selchow P, Böttger EC, Sander P. 2017. Intrinsic rifamycin resistance of Mycobacterium abscessus is mediated by ADP-ribosyltransferase MAB_0591. J Antimicrob Chemother 72:376–384. doi: 10.1093/jac/dkw466. [DOI] [PubMed] [Google Scholar]
- 11.Hurst-Hess K, Rudra P, Ghosh P. 2017. Mycobacterium abscessus WhiB7 regulates a species-specific repertoire of genes to confer extreme antibiotic resistance. Antimicrob Agents Chemother 61:e01347-17. doi: 10.1128/AAC.01347-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greendyke R, Byrd TF. 2008. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob Agents Chemother 52:2019–2026. doi: 10.1128/AAC.00986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qvist T, Eickhardt S, Kragh KN, Andersen CB, Iversen M, Høiby N, Bjarnsholt T. 2015. Chronic pulmonary disease with Mycobacterium abscessus complex is a biofilm infection. Eur Respir J 46:1823–1826. doi: 10.1183/13993003.01102-2015. [DOI] [PubMed] [Google Scholar]
- 14.Flemming HC, Neu TR, Wozniak DJ. 2007. The EPS matrix: the “house of biofilm cells. J Bacteriol 189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Recht J, Martínez A, Torello S, Kolter R. 2000. Genetic analysis of sliding motility in Mycobacterium smegmatis. J Bacteriol 182:4348–4351. doi: 10.1128/JB.182.15.4348-4351.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ojha A, Anand M, Bhatt A, Kremer L, Jacobs WR Jr, Hatfull GF. 2005. GroEL1: a dedicated chaperone involved in mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell 123:861–873. doi: 10.1016/j.cell.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 17.Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guérardel Y, Alahari A, Kremer L, Jacobs WR Jr, Hatfull GF. 2008. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol 69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart P. 2015. Antimicrobial tolerance in biofilms. Microbiol Spectr 3:MB-0010-2014. doi: 10.1128/microbiolspec.MB-0010-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clary G, Sasindran SJ, Nesbitt N, Mason L, Cole S, Azad A, McCoy K, Schlesinger LS, Hall-Stoodley L. 2018. Mycobacterium abscessus smooth and rough morphotypes form antimicrobial-tolerant biofilm phenotypes but are killed by acetic acid. Antimicrob Agents Chemother 62:e01782-17. doi: 10.1128/AAC.01782-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gutiérrez AV, Viljoen A, Ghigo E, Herrmann J-L, Kremer L. 2018. Glycopeptidolipids, a double-edged sword of the Mycobacterium abscessus complex. Front Microbiol 9:1145. doi: 10.3389/fmicb.2018.01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson LB, Nessar R, Kempaiah P, Perkins DJ, Byrd TF. 2011. Mycobacterium abscessus glycopeptidolipid prevents respiratory epithelial TLR2 signaling as measured by HβD2 gene expression and IL-8 release. PLoS One 6:e29148. doi: 10.1371/journal.pone.0029148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunter RL, Olsen MR, Jagannath C, Actor JK. 2006. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci 36:371–386. [PubMed] [Google Scholar]
- 23.Byrd TF, Lyons CR. 1999. Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect Immun 67:4700–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, Lyons CR, Byrd TF. 2006. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 152:1581–1590. doi: 10.1099/mic.0.28625-0. [DOI] [PubMed] [Google Scholar]
- 25.Kreutzfeldt KM, McAdam PR, Claxton P, Holmes A, Seagar AL, Laurenson IF, Fitzgerald JR. 2013. Molecular longitudinal tracking of Mycobacterium abscessus spp. during chronic infection of the human lung. PLoS One 8:e63237. doi: 10.1371/journal.pone.0063237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park IK, Hsu AP, Tettelin H, Shallom SJ, Drake SK, Ding L, Wu U-I, Adamo N, Prevots DR, Olivier KN, Holland SM, Sampaio EP, Zelazny AM. 2015. Clonal diversification and changes in lipid traits and colony morphology in Mycobacterium abscessus clinical isolates. J Clin Microbiol 53:3438–3447. doi: 10.1128/JCM.02015-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuru E, Hughes HV, Brown PJ, Hall E, Tekkam S, Cava F, de Pedro MA, Brun YV, VanNieuwenhze MS. 2012. In situ probing of newly synthesized peptidoglycan in live bacteria with fluorescent D-amino acids. Angew Chem Int Ed Engl 51:12519–12523. doi: 10.1002/anie.201206749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishov I, Woldringh CL. 1999. Visualization of membrane domains in Escherichia coli. Mol Microbiol 32:1166–1172. doi: 10.1046/j.1365-2958.1999.01425.x. [DOI] [PubMed] [Google Scholar]
- 30.Aldridge BB, Fernandez-Suarez M, Heller D, Ambravaneswaran V, Irimia D, Toner M, Fortune SM. 2012. Asymmetry and aging of mycobacterial cells lead to variable growth and antibiotic susceptibility. Science 335:100–104. doi: 10.1126/science.1216166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burbaud S, Laval F, Lemassu A, Daffé M, Guilhot C, Chalut C. 2016. Trehalose polyphleates are produced by a glycolipid biosynthetic pathway conserved across phylogenetically distant mycobacteria. Cell Chem Biol 23:278–289. doi: 10.1016/j.chembiol.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect Immun 64:2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schaechter M, MaalOe O, Kjeldgaard NO. 1958. Dependency on medium and temperature of cell size and chemical composition during balanced growth of Salmonella typhimurium. J Gen Microbiol 19:592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- 34.Ojha AK, Varma S, Chatterji D. 2002. Synthesis of an unusual polar glycopeptidolipid in glucose-limited culture of Mycobacterium smegmatis. Microbiology 148:3039–3048. doi: 10.1099/00221287-148-10-3039. [DOI] [PubMed] [Google Scholar]
- 35.Blair JMA, Richmond GE, Bailey AM, Ivens A, Piddock L. 2013. Choice of bacterial growth medium alters the transcriptome and phenotype of Salmonella enterica serovar Typhimurium. PLoS One 8:e63912. doi: 10.1371/journal.pone.0063912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostakioti M, Hadjifrangiskou M, Hultgren SJ. 2013. Bacterial biofilms: development, dispersal, and therapeutic strategies in the dawn of the postantibiotic era. Cold Spring Harb Perspect Med 3:a010306. doi: 10.1101/cshperspect.a010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landry RM, An D, Hupp JT, Singh PK, Parsek MR. 2006. Mucin-Pseudomonas aeruginosa interactions promote biofilm formation and antibiotic resistance. Mol Microbiol 59:142–151. doi: 10.1111/j.1365-2958.2005.04941.x. [DOI] [PubMed] [Google Scholar]
- 38.Jackson LMD, Kroukamp O, Wolfaardt GM. 2015. Effect of carbon on whole-biofilm metabolic response to high doses of streptomycin. Front Microbiol 6:953. doi: 10.3389/fmicb.2015.00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Serra DO, Richter AM, Klauck G, Mika F, Hengge R. 2013. Microanatomy at cellular resolution and spatial order of physiological differentiation in a bacterial biofilm. mBio 4:e00103-13. doi: 10.1128/mBio.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besharova O, Suchanek VM, Hartmann R, Drescher K, Sourjik V. 2016. Diversification of gene expression during formation of static submerged biofilms by Escherichia coli. Front Microbiol 7:1568. doi: 10.3389/fmicb.2016.01568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Williamson KS, Richards LA, Perez-Osorio AC, Pitts B, McInnerney K, Stewart PS, Franklin MJ. 2012. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J Bacteriol 194:2062–2073. doi: 10.1128/JB.00022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie Z, Siddiqi N, Rubin EJ. 2005. Differential antibiotic susceptibilities of starved Mycobacterium tuberculosis isolates. Antimicrob Agents Chemother 49:4778–4780. doi: 10.1128/AAC.49.11.4778-4780.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hartmans S, De Bont J. 1992. The genus Mycobacterium—nonmedical, 2nd ed Springer-Verlag, New York, NY. [Google Scholar]
- 44.Sriramulu DD, Lünsdorf H, Lam JS, Römling U. 2005. Microcolony formation: a novel biofilm model of Pseudomonas aeruginosa for the cystic fibrosis lung. J Med Microbiol 54:667–676. doi: 10.1099/jmm.0.45969-0. [DOI] [PubMed] [Google Scholar]
- 45.Ducret A, Quardokus EM, Brun YV. 2016. MicrobeJ, a tool for high throughput bacterial cell detection and quantitative analysis. Nat Microbiol 1:16077. doi: 10.1038/nmicrobiol.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.