Previous studies on drug efficacy showed low protection against abortion and vertical transmission of Toxoplasma gondii in pregnant sheep. Bumped kinase inhibitors (BKIs), which are ATP-competitive inhibitors of calcium-dependent protein kinase 1 (CDPK1), were shown to be highly efficacious against several apicomplexan parasites in vitro and in laboratory animal models.
KEYWORDS: BKI-1294, Toxoplasma gondii, abortion, protein kinase inhibitor, safety, sheep, treatment, vertical transmission
ABSTRACT
Previous studies on drug efficacy showed low protection against abortion and vertical transmission of Toxoplasma gondii in pregnant sheep. Bumped kinase inhibitors (BKIs), which are ATP-competitive inhibitors of calcium-dependent protein kinase 1 (CDPK1), were shown to be highly efficacious against several apicomplexan parasites in vitro and in laboratory animal models. Here, we present the safety and efficacy of BKI-1294 treatment (dosed orally at 100 mg/kg of body weight 5 times every 48 h) initiated 48 h after oral infection of sheep at midpregnancy with 1,000 TgShSp1 oocysts. BKI-1294 demonstrated systemic exposure in pregnant ewes, with maximum plasma concentrations of 2 to 3 μM and trough concentrations of 0.4 μM at 48 h after each dose. Oral administration of BKI-1294 in uninfected sheep at midpregnancy was deemed safe, since there were no changes in behavior, fecal consistency, rectal temperatures, hematological and biochemical parameters, or fetal mortality/morbidity. In ewes infected with a T. gondii oocyst dose lethal for fetuses, BKI-1294 treatment led to a minor rectal temperature increase after infection and a decrease in fetal/lamb mortality of 71%. None of the lambs born alive in the treated group exhibited congenital encephalitis lesions, and vertical transmission was prevented in 53% of them. BKI-1294 treatment during infection led to strong interferon gamma production after cell stimulation in vitro and a low humoral immune response to soluble tachyzoite antigens but high levels of anti-SAG1 antibodies. The results demonstrate a proof of concept for the therapeutic use of BKI-1294 to protect ovine fetuses from T. gondii infection during pregnancy.
INTRODUCTION
Toxoplasma gondii is an apicomplexan parasite that causes significant economic losses due to abortions after primary infection of pregnant sheep (1). Congenital transmission of T. gondii mainly occurs through ingestion of oocysts during pregnancy (2). Infection during early and midpregnancy is usually associated with abortion or vertical transmission of the parasite, while infection in late pregnancy produces a congenitally infected but generally viable lamb, sometimes harboring toxoplasmic lesions (3). Once the infection occurs, there is generally a delay of 4 weeks until abortion occurs (1). However, earlier abortions (during the second week postinfection [p.i.]) have been described in several experimental inoculations of sheep with sporulated oocysts (4–7).
For the control of ovine toxoplasmosis, several measures have been proposed (8). Minimizing the burden of T. gondii oocysts in the environment is essential to reducing horizontal transmission. However, these farm biosecurity measures are not enough to control the disease, and therefore vaccines and drugs are needed (9). For this purpose, a live attenuated vaccine (Toxovax; MSD) that confers protection against abortions and decreases tissue cyst development (2) is commercially available in some European Union countries and in New Zealand (10, 11). Although a set of drugs showed efficacy in vitro and in laboratory animal models (9), only monensin (12, 13), folate inhibitors (14), and decoquinate (15) have been evaluated against T. gondii in pregnant sheep. In these studies, protection against abortion was found in 20 to 40% of infected ewes (13), and there was limited or no protection against vertical transmission (12–15). Thus, presently there is no efficacious drug for the treatment or prevention of ovine toxoplasmosis.
Current treatment options for human toxoplasmosis are limited. Clinical cases in humans with encephalitis or ocular disorders due to toxoplasmosis are often treated with pyrimethamine in combination with a sulfonamide, which are often toxic to the host and cause serious adverse side effects (16). Antiparasitic drug development based on targeting protein kinase enzymes is a well-established approach (17). Calcium-dependent protein kinase 1 (CDPK1) represents a promising drug target, as CDPK1 is likely descended from the plant lineage of T. gondii and thus is absent from mammalian hosts (18–21). CDPK1 activity is essential for microneme secretion, host cell invasion, and egress of T. gondii (18, 22, 23) and can be selectively targeted by a class of ATP-competitive compounds, collectively named bumped kinase inhibitors (BKIs). BKIs have broad-spectrum activity that affects many apicomplexan parasites (24). BKI-1294 is effective against T. gondii in vitro (25) and in vivo against acute (26, 27) and chronic (26) toxoplasmosis in mice, as well as against vertical transmission in a pregnant mouse model of toxoplasmosis (28).
Contrary to the case for mice, in sheep and humans there is a lack of T. gondii profilin-mediated activation of Toll-like-receptors (TLR) 11 and 12, which primes interferon gamma (IFN-γ) production by T cells and consequently upregulates the immunity-related GTPases (IRGs). Other TLRs present in humans and sheep, such as TLR7 and TLR9, are activated by parasite DNA and RNA and help to tackle the parasite (29). These similarities in sheep and human innate immunity suggest that the pregnant sheep model of T. gondii infection is a good model for the evaluation of new vaccine and drug candidates for the prevention and treatment of human pregnancy toxoplasmosis. We report here on the safety and efficacy of BKI-1294 treatment in pregnant sheep experimentally infected with T. gondii oocysts at midgestation.
RESULTS
To summarize the experimental design, in group 1 (G1; infected/treated), 48 h after oral administration of 1,000 TgShSp1 oocysts to sheep at midpregnancy, BKI-1294 was orally applied 5 times at 100 mg/kg of body weight every 48 h. Sheep in group 2 (G2; infected/untreated) were infected with the same oocyst dose but did not receive the treatment. Efficacy of BKI-1294 was assessed by monitoring fetal/lamb mortality and vertical transmission in live lambs (seropositivity and parasite detection/lesion in brain or lungs). In addition, rectal temperatures and cellular and humoral immune responses to T. gondii infection were assessed. To evaluate safety of BKI-1294, pregnant sheep in group 3 (G3) were not infected but received the compound. Rectal temperatures, gastrointestinal and behavioral changes, fetal viability, and hematological and biochemical parameters were monitored. Finally, group 4 (G4), uninfected but receiving the vehicle of the drug, was used as a sentinel control. In treated groups (G1 and G3), drug levels of BKI-1294 were determined (see Materials and Methods).
Pharmacokinetics.
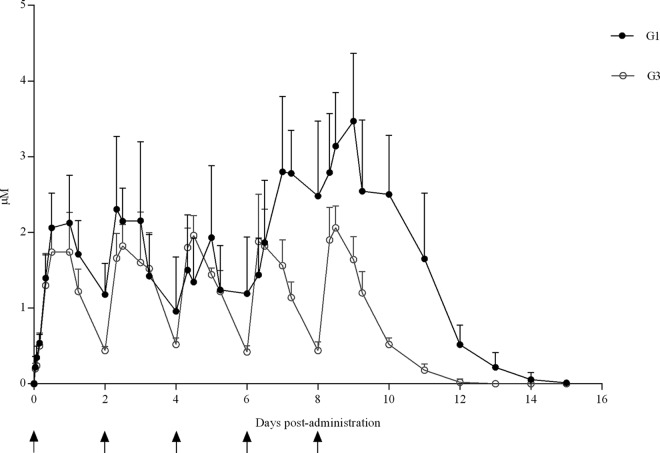
In G1 (T. gondii infected and BKI-1294 treated), maximum concentrations (Cmax) of 2.2 ± 0.8 μM after the 1st, 2nd, and 3rd doses and of 3.5 ± 0.7 μM after the 4th and 5th doses were reached at 8 to 24 h after BKI-1294 administration. Likewise, trough plasma concentrations of 1.1 ± 0.6 μM 48 h after the 1st, 2nd, and 3rd doses, 2.4 ± 0.8 μM 48 h after the 4th and 5th doses, and detectable drug levels until 6 to 7 days after the last dose were found (Fig. 1). In G3 (uninfected and BKI-1294-treated group), a Cmax of 2 ± 0.3 μM was reached 8 to 24 h after each dose, with trough plasma concentrations of 0.4 ± 0.1 μM 48 h after the 1st, 2nd, 3rd, 4th, and 5th doses and detectable drug levels until 3 to 4 days after the last dose (Fig. 1).
FIG 1.
BKI-1294 plasma concentrations in infected and uninfected ewes. Values from infected ewes (G1) and uninfected ewes (G3) dosed with BKI-1294 are shown. Each point represents the means ± SD at the different sampling times for each group. Arrows indicate the time points of drug administrations.
There were significant differences between sheep in G1 (infected/treated) and sheep in G3 (uninfected/treated) in the area under the curve (AUC) (P < 0.05) and Cmax at the 4th (P < 0.05) and 5th (P < 0.05) doses. No significant differences were observed for the Cmax or AUCs between the ewes that suffered from fetal/lamb mortality and those that gave birth to healthy lambs in G1 (infected/treated). There were also no significant differences for Cmax or AUC for the ewes from G1 (infected/treated), with at least one T. gondii-positive lamb compared to those ewes with no T. gondii-positive offspring.
Hematology and biochemistry.
Means and standard deviations for hematological and biochemical parameters in G3 (uninfected/treated) and G4 (uninfected/untreated) before and after treatment (4 days after the 5th BKI-1294 dose) and reference values are shown in Table 1. Mean values for hematological and biochemical parameters were in the physiological range at initial and final time points. The only exception concerned the creatine kinase (CK) in G4 (uninfected/untreated) at the final time point, which showed a mean value above the reference.
TABLE 1.
Hematological and biochemical parameters before treatment and 4 days after the 5th BKI-1294 dose
| Parameterb (units) | Reference value | Level fora
: |
|||
|---|---|---|---|---|---|
| G3 (uninfected/treated) |
G4 (uninfected/vehicle alone) |
||||
| Initial | Final | Initial | Final | ||
| Erythrocytes (×106) | 9–14 | 10.68 ± 0.67 | 9.26 ± 0.57 | 10.74 ± 0.55 | 9.04 ± 0.66 |
| Hemoglobin (g/dl) | 8–15 | 11.78 ± 0.40 | 10.36 ± 0.78 | 12.24 ± 1.04 | 10.38 ± 0.86 |
| Packed cell volume (%) | 28–40 | 32.74 ± 1.96 | 28.52 ± 1.49 | 32.94 ± 2.01 | 27.94 ± 1.89 |
| Platelets (×103) | 250–750 | 394.20 ± 97.85 | 533.80 ± 121.61 | 583.4 ± 73.67 | 615 ± 114.58 |
| Leukocytes (×103) | 4–12 | 4.85 ± 1.12 | 4.84 ± 0.63 | 7,21 ± 1.42 | 6.53 ± 1.98 |
| Segment neutrophils (%) | 10–50 | 38.72 ± 4.86 | 41.50 ± 12.58 | 45.3 ± 5.13 | 38.24 ± 9.39 |
| Lymphocytes (%) | 40–75 | 51.44 ± 4.04 | 46.84 ± 13.13 | 45 ± 5.77 | 51.48 ± 11 |
| Monocytes (%) | 1–6 | 3.70 ± 1.04 | 5.28 ± 1.99 | 4.12 ± 1.14 | 4.46 ± 1.47 |
| Eosinophils (%) | 0–15 | 3.62 ± 1.47 | 4.04 ± 2.43 | 1.88 ± 0.94 | 2.38 ± 1.73 |
| Proteins (g/dl) | 6–8 | 6.42 ± 0.34 | 6.56 ± 0.26 | 6.8 ± 0.36 | 6.96 ± 0.32 |
| AST (UI/liter) | 70–210 | 97.80 ± 27.34 | 82.60 ± 27.27 | 97.4 ± 15.24 | 119 ± 39.05 |
| GGT (UI/liter) | 36–93 | 56.60 ± 9.65 | 61.80 ± 12.07 | 66 ± 11.95 | 64 ± 7.71 |
| ALP (UI/liter) | 44–355 | 191.40 ± 62.79 | 221.80 ± 64.61 | 304.2 ± 60.60 | 266.4 ± 77.18 |
| CK (UI/liter) | 50–180 | 91.75 ± 4.78 | 143.75 ± 60.11 | 170.66 ± 47.81 | 301 ± 151.70 |
| Urea (mg/dl) | 8.4–30.8 | 12.08 ± 1.60 | 15.36 ± 3.46 | 15.46 ± 1.16 | 12.02 ± 4.18 |
| Creatinine (mg/dl) | 0.9–1.7 | 0.98 ± 0.04 | 0.9 ± 0.04 | 1 ± 0.07 | 1.22 ± 0.08 |
| Calcium (mg/dl) | 7.1–9.8 | 9.80 ± 0.68 | 10.24 ± 0.75 | 10.22 ± 0.50 | 9.76 ± 0.50 |
| Phosphorus (mg/dl) | 3.5–7.3 | 4.98 ± 1.03 | 7.18 ± 0.95 | 6.06 ± 0.35 | 5.9 ± 0.38 |
| Sodium (mEq/liter) | 139–152 | 147.20 ± 0.83 | 150 ± 2 | 148.8 ± 1.30 | 146 ± 1.22 |
| Potassium (mEq/liter) | 3.9–5.2 | 4.64 ± 0.24 | 4.94 ± 0.33 | 4.7 ± 0.46 | 4.92 ± 0.18 |
Values are represented as means ± SD.
AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; ALP, alkaline phosphatase; CK, creatine kinase.
Clinical observations.
No significant increase in rectal temperature was found throughout 12 days following the first BKI-1294 treatment in sheep that remained uninfected but received BKI-1294 treatment (G3) compared to those that received vehicle alone (G4) (Fig. 2). In addition, no alterations in the fecal consistency or any other gastrointestinal or behavioral changes were observed in the BKI-1294-treated groups. Likewise, no fetal/lamb mortality was detected in the uninfected, BKI-1294-treated group (G3), and ewes gave birth to healthy lambs between 146 and 150 days of pregnancy. Dams from the uninfected group dosed with vehicle alone (G4) also gave birth to healthy lambs between days 146 and 150 of pregnancy (Fig. 3).
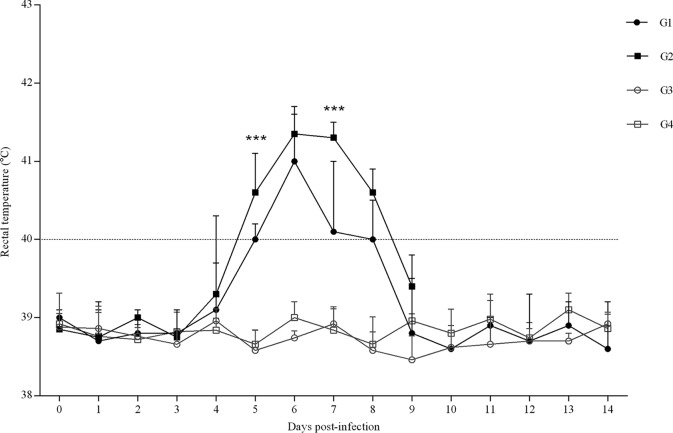
FIG 2.
Rectal temperatures of infected (G1 and G2) and uninfected groups (G3 and G4). In infected groups, G1 received BKI-1294 treatment and G2 did not. In uninfected groups, G3 was dosed with BKI-1294 while G4 received vehicle alone. Each point represents the means ± SD for each group. Rectal temperatures were analyzed using one-way ANOVA followed by Tukey’s multiple test. For significant differences between infected groups, P < 0.001 (***).
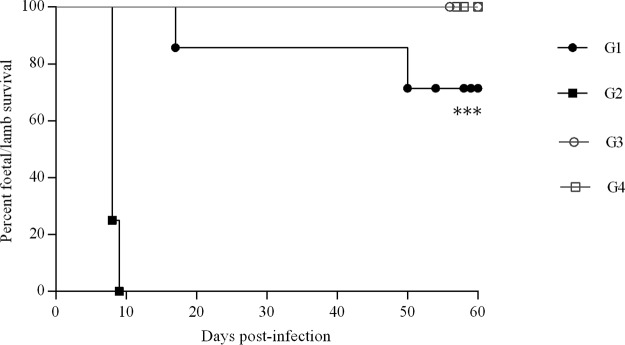
FIG 3.
Kaplan-Meier survival curves for fetuses in the infected groups (G1 and G2) and uninfected groups (G3 and G4). In infected groups, G1 received BKI-1294 treatment and G2 did not. In uninfected groups, G3 was dosed with BKI-1294 while G4 received vehicle alone. Each point represents the percentage of surviving animals on that day, and downward steps correspond to observed deaths. Fetal survival curves were compared by the log-rank (Mantel-Cox) test. For significant differences between fetal survival curves of infected groups, P < 0.001 (***).
Concerning the infected groups, significantly increased rectal temperatures were found between days 4 (P < 0.05) and 8 (P < 0.001) p.i. in the T. gondii-infected, untreated G2 compared to the uninfected control G4. However, compared to those of G4 (uninfected/untreated), increased rectal temperatures were found in G1 (infected/treated) between days 5 (P < 0.0001) and 8 (P < 0.05) p.i. Comparing both infected groups, a significant decrease in rectal temperature was observed in G1 (infected/treated) on days 5 (P < 0.001) and 7 (P < 0.001) p.i. (days 3 and 5 from the start of treatment) (Fig. 2). In G1 (infected/treated), the rectal temperatures in the two ewes with fetal/lamb mortality were higher on days 6 (P < 0.05) and 9 p.i. (P < 0.0001) than in ewes that gave birth to healthy lambs. Severely reduced voluntary food intake was found in T. gondii-infected animals from 6 to 11 days p.i. From day 14 p.i. until the end of the experiment, no changes were found in rectal temperatures or voluntary food intake.
Fetal/lamb mortality was detected in 2 out of 7 ewes from the infected and BKI-1294-treated group (G1). One ewe aborted three fetuses on day 17 p.i., one ewe suffered premature stillbirth on day 140 of pregnancy (50 days p.i.), and both ewes were euthanized. Therefore, fetal/lamb mortality was found in 4 out 17 fetuses/lambs. However, in the infected, untreated group (G2), 100% (8 out of 8) of the pregnant ewes suffered early abortions (on days 8 and 9 p.i.) during the acute phase of the disease and were euthanized. Significant differences were found in the fetal/lamb survival curve between G1 (infected/treated) and G2 (infected/untreated) (P < 0.001) (Fig. 3) and also in the number of lambs born in G1 (infected/treated) and G2 (infected/untreated) (P < 0.0001). The remaining dams from the infected, BKI-1294-treated group (G1) gave birth to 13 healthy lambs on days 144 and 150 of pregnancy.
The birth weight of the lambs born from sole pregnancies was 4,632 ± 348 g in G3 (uninfected/treated) and 4,505 ± 585 g in G4 (uninfected/untreated). In twin pregnancies, the birth weight of the lambs was 3,217 ± 341 g in G1 (infected/treated), 3,868 ± 682 g in G3 (uninfected/treated), and 3,962 ± 487 g in G4 (uninfected/untreated). Finally, in triplet and quadruplet pregnancies, the lambs weighed 2,919 ± 398 g in G1 (infected/treated) and 3,228 ± 155 g in G3 (uninfected/treated). The only significant difference was the lower birth weight of lambs born from twin pregnancies in G1 (infected/treated) compared to birth weight of lambs born from twin pregnancies in G4 (uninfected/untreated) (P < 0.05). However, no significant differences in the birth weight were found in G1 (infected/treated) between PCR-positive lambs (T. gondii congenitally infected) and PCR-negative (uninfected) lambs.
Cellular and humoral immune responses.
IFN-γ levels in supernatant of blood cell cultures were significantly increased in samples from G1 (infected/treated) isolated on day 7 p.i. (P < 0.01) compared to G2 (infected/untreated) and G4 (uninfected/untreated), with maximum IFN-γ levels on day 10 p.i. Furthermore, IFN-γ levels in G1 (infected/treated) maintained a 100-fold increase compared to G4 (uninfected/untreated) at the end of the sampling period. In G2 (infected/untreated), no significant increase was found until the end of the sampling period (7 days p.i.) compared to G4 (uninfected/untreated); however, a 10-fold increase was found on the IFN-γ levels on days 5 and 7 p.i. Blood cell cultures from uninfected, untreated animals (G4) showed IFN-γ levels throughout the experimental study that corresponded to basal levels recorded prior to inoculation (Fig. 4).
FIG 4.
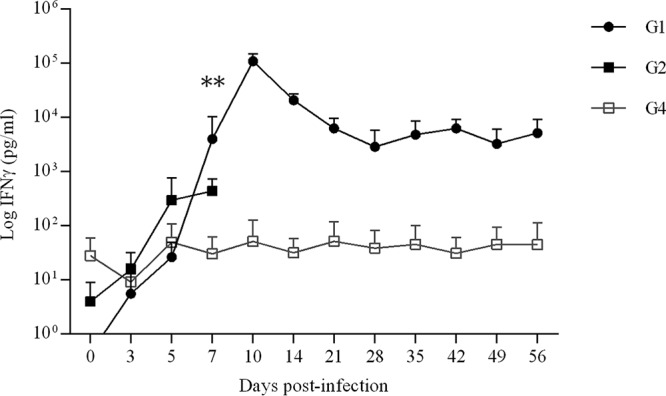
IFN-γ in supernatants of peripheral blood cell cultures. Values from infected groups (G1 and G2) and the group receiving vehicle alone (G4) are shown. Each point represents the means ± SD at the different sampling times for each group. Concentrations of IFN-γ are expressed in pg/ml. Cellular immune responses were analyzed using two-way ANOVA of repeated measures until day 7 p.i. For significant differences between infected groups, P < 0.01 (**).
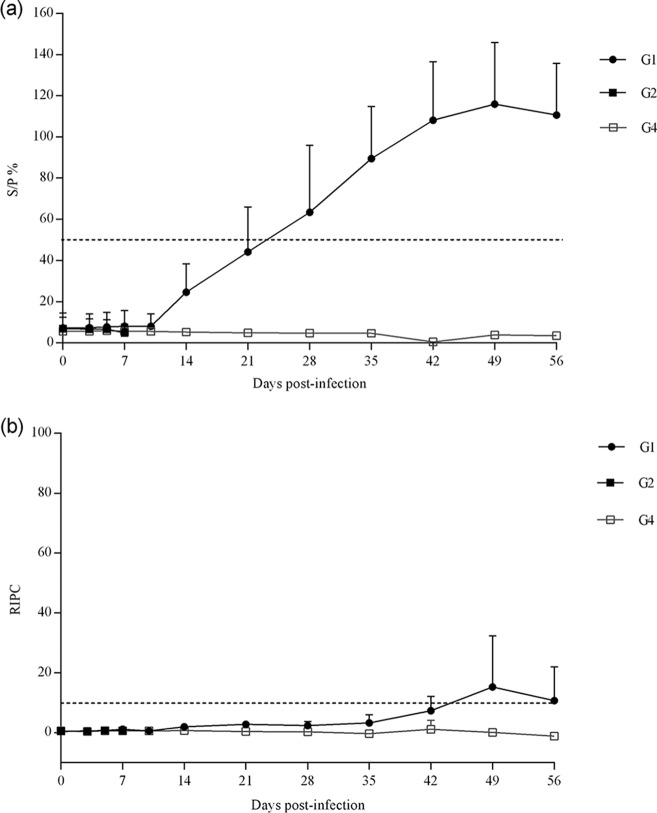
The Toxoplasma-specific IgG responses in dams were measured by enzyme-linked immunosorbent assay (ELISA) and are shown in Fig. 5. In G1 (infected/treated), the ewe that aborted on day 17 p.i. was seronegative by both ELISAs at all time points tested. The remaining ewes were seropositive by the SAG1 commercial ELISA (2 dams were seropositive from day 21 p.i., 1 dam was seropositive from day 28 p.i., and the remaining 3 dams in this group were seropositive from day 35 p.i.) (Fig. 5A). Only the ewe that gave birth to a stillborn lamb on day 140 of pregnancy and 2 of the ewes that gave birth to healthy but congenitally infected lambs were seropositive on days 42 to 49 p.i. by ELISA based on T. gondii soluble antigens (Fig. 5B). All animals from G2 (infected/untreated) and G4 (uninfected/untreated) were seronegative by both ELISAs throughout the experimental study; however, ewes in G2 (infected/untreated) were euthanized before ELISA responses were observed in G1 (infected/treated), presumably explaining the negative ELISA values.
FIG 5.
IgG responses in sera by ELISA based on T. gondii SAG1 protein (A) or soluble antigens (B). Values from infected groups (G1 and G2) and the group receiving vehicle alone (G4) are shown. Each point represents the means ± SD at the different sampling times for each group.
In G1 (infected/treated), anti-T. gondii SAG1 antibodies were detected by Western blotting (WB) in the serum samples of the 4 ewes that gave birth to healthy lambs obtained on days 49 and 56 p.i. and in the serum sample from the ewe that gave birth to a stillborn obtained on day 49 p.i. (on day 56 p.i. this ewe had already been euthanized). Likewise, antibodies directed against recombinant T. gondii BAG1 were detected by WB in all samples obtained on days 49 and 56 p.i. except in those from one ewe that gave birth to healthy lambs (see Fig. S1 in the supplemental material).
Aborted fetuses in G1 (infected/treated) and G2 (infected/untreated), as well as lambs born alive in G1 (infected/treated), were all seronegative, with the exception of the stillborn detected in G1 (infected/treated) with an indirect fluorescent antibody test (IFAT) titer of 1:256 (Table 2). Specific IgG responses against parasite antigens were not detected in fetuses/lambs from the uninfected group receiving vehicle alone (G4).
TABLE 2.
Parasite detection and serology of fetuses/lambs from ewes infected with T. gondii and treated with BKI-1294
| Fetus/lamb viabilitya | No. of ewes with T. gondii-positive offspringb | No. fetuses/lambs positive by: |
||
|---|---|---|---|---|
| IFATc | PCRd
|
|||
| Brain | Lung | |||
| Abortion (17) | 1/1 | 0/3 | 1/3 (2/9) | 0/3 (0/9) |
| Stillborn lambs (50) | 1/1 | 1/1 (1:256) | 1/1 (3/3) | 0/1 (0/3) |
| Live lambs | 3/5 | 0/13 | 6/13 (7/39) | 0/13 (0/39) |
The value in parentheses is the day postchallenge when aborted fetuses or stillborn lambs were detected.
Number of ewes with at least one fetus/lamb positive by serology or PCR/total number of ewes.
Number of fetuses/lambs being T. gondii seropositive by IFAT/total number of fetuses or lambs. Shown in parentheses are the positive IFAT titers.
Number of fetuses/lambs with at least one positive sample by PCR/total number of fetuses or lambs. Parasite detection is shown in parentheses (PCR-positive samples/total number of samples analyzed).
Histopathological examination.
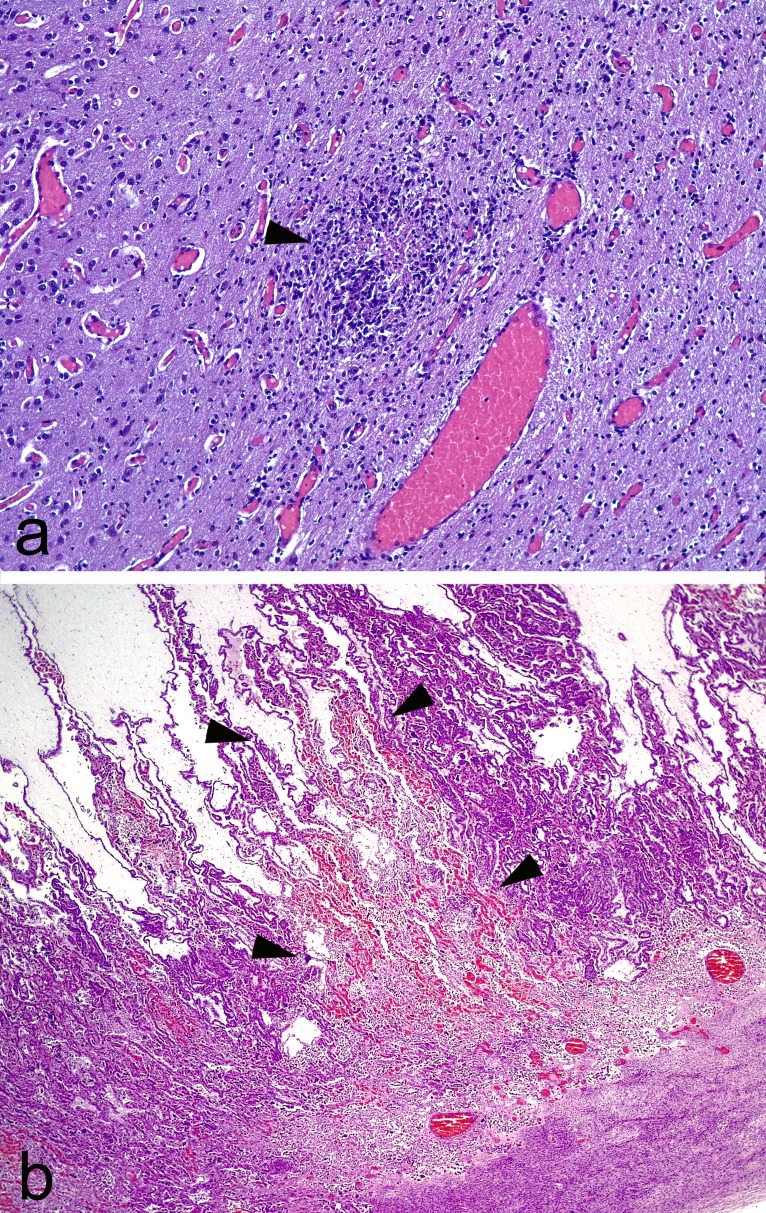
Multifocal nonpurulent encephalitis (Fig. 6A) and several necrotic foci in the lung samples were found in the stillborn lamb in G1 (infected/treated). The brains from fetuses aborted on day 17 p.i. were too autolytic to allow proper histological evaluation; however, placentome samples from this ewe showed multifocal areas of coagulative necrosis affecting the whole thickness of the interdigitated area. These areas of necrosis were well demarcated by congestion in their outer limits. The lumen of a scarce number of vessels in relation, or not, to these necrotic areas was occupied by fibrin thrombi. No significant lesion was found in the brains of the lambs born healthy in G1 (infected/treated). Likewise, no significant lesions were found in the lungs from the aborted fetuses and the lambs born healthy in G1 (infected/treated).
FIG 6.
Hematoxylin and eosin staining. (a) Foci of gliosis with a central area of necrosis (arrowhead). Note the diffuse congestion in the sample. Brain, ×4 magnification. (b) Area of necrosis in the interdigitate area of the placentome, denoted by loss of histological architecture and congestion. Note the sharply demarcated borders (arrowheads) between the affected area and nonaffected area. Placenta, ×1.6 magnification.
In G2 (infected/untreated), the brains from fetuses were too autolytic to allow proper histological evaluation. No significant lesions were found in the lungs from these fetuses. However, all the placentas from this group showed thrombosis in at least one of the placentomes examined, while most of the remaining placentomes were autolytic. These lesions were characterized by areas of necrosis sharply demarcated from nonaffected tissue by bands of congestion (Fig. 6B). These lesions usually affected the full thickness of the placentome. Finally, no histopathological findings were found in the uninfected group (G4).
Parasite detection in placental and fetal tissues.
In placental tissues from G1 (infected/treated), T. gondii DNA was detected in all placentomes from the ewe that gave birth to a stillborn on day 140 of pregnancy, whereas placentomes from the ewe that aborted on day 17 p.i. and the cotyledons from the remaining ewes that gave birth were PCR negative. In G2 (infected/untreated), in which ewes aborted at 8 to 9 dpi, T. gondii DNA was detected in 1 out of 48 samples from placentomes of aborted ewes.
In fetal tissues from G1 (infected/treated), T. gondii DNA was detected in all brain samples from the stillborn and in 66% of the brain samples from one of the three fetuses aborted on day 17 p.i. T. gondii DNA was not detected in the lung samples from the aborted fetus, the stillbirth, or the lambs born healthy. In seven out of thirteen lambs born healthy in G1 (infected/treated) (four of them belonging to two different dams), T. gondii DNA was not detected in the brain samples. However, in the lambs born alive from the other three dams in G1 (infected/treated), brain samples positive for T. gondii DNA were detected (33 to 66% of brain samples were T. gondii positive) (Table 2). In G2 (infected/untreated), T. gondii DNA was not detected in any of the fetal brain samples and only in 2/51 (4%) of fetal lung samples from two different fetuses. As expected, all samples from G4 (uninfected/untreated) were negative.
DISCUSSION
Previous studies on drugs against T. gondii in pregnant sheep showed low efficacy against abortion and/or vertical transmission (12–15). In addition, current options for the treatment of human toxoplasmosis are scarce and often prone to adverse effects (16). Therefore, studies on the efficacy of drug candidates against T. gondii are needed (30). This study reports on BKI-1294 drug levels in the plasma of pregnant sheep and the safety and efficacy of BKI-1294 in a pregnant sheep model of toxoplasmosis. The efficacy was assessed with respect to the clinical course of disease, cellular and humoral immune responses, and parasite detection and lesions in placental and fetal tissues.
Compared to other BKIs, such as BKI-1553, BKI-1294 produces lower plasma concentrations in mice (31, 32). In calves, plasma levels of 1 μM until 24 h after treatment were demonstrated after a single administration of BKI-1294 at 10 mg/kg (31). In this experiment, Phosal vehicle (60% Phosal 53 MCT, 30% polyethylene glycol 400 [PEG400], 10% ethanol 96°) was used, since it gave reproducible and higher exposures than our typical vehicle, 70% Tween 80–30% ethanol 96° (unpublished data). Administration of 100 mg/kg of BKI-1294 in pregnant sheep led to maximum concentrations of 2 to 3 μM, with trough plasma concentrations of 0.4 to 1 μM at 48 h after treatment. Detectable drug levels were found until 13 to 17 days after the first dose. Studies on the in vitro efficacy of BKI-1294 against T. gondii ME49 reported a half-maximal inhibitory concentration (IC50) of 0.22 ± 0.06 μM (25). Thus, plasma concentrations, including troughs, of BKI-1294 in pregnant ewes were higher than the IC50 for T. gondii. This perhaps could translate into good efficacy in the pregnant sheep model of toxoplasmosis, although different factors, such as protein binding and penetration of BKI-1294 through the placental and blood-brain barriers, could influence it.
BKI-1294 at 100 mg/kg twice daily for 5 days in mice did not exhibit toxicity, since no changes in weight, histology, enzymes, or blood cell counts were found (33). Concerning side effects on pregnancy, a 50% decrease in fetus viability was found in pregnant CD1 mice treated with BKI-1294 at 50 mg/kg for 5 days (28); however, no detrimental effect on fetus viability was found using BALB/c mice (25). In calves, BKI-1294 was safe, with no toxicity observed after 5 mg/kg twice daily oral administration given over 5 days (31). In this study, BKI-1294 treatment did not result in an increase in rectal temperature or in alterations of hematological and biochemical parameters, behavior, or fecal consistency. The only exception was an increase in CK values in G4 (uninfected/untreated) at the final time point, although increased CK values can typically appear in late pregnancy (34). In spite of the potential fetal toxicity in mice, no abortions were found in pregnant sheep treated with BKI-1294 and all lambs were born healthy (with no decrease in birth weight), although long-term effects of the drug were not assessed in the lambs. Therefore, administration in sheep at midpregnancy of 5 oral doses of BKI-1294 at 100 mg/kg (formulated in 60% Phosal 53 MCT, 30% PEG400, and 10% ethanol 96°) given every other day was safe.
Previous drugs tested against T. gondii in pregnant sheep were dosed starting 10 days prior to infection until parturition (12, 13, 15). However, in this study the beginning of BKI-1294 treatment was planned 48 h after infection, in the same way as previous studies of BKI therapy of toxoplasmosis in mice (27, 28, 35). At this time point, the infection has already been established and the parasites have been widely distributed in the animals. In this experiment, pregnant ewes that were infected but not treated showed 100% fetal mortality as previously described (36). Clinical observations in the BKI-1294-treated group revealed high protection against fetal/lamb mortality, as 5 out of 7 (71%) of the ewes gave birth to healthy lambs (13 out of 17 fetuses/lambs were born healthy), with statistically improved fetal/lamb survival rates compared to those of the infected, untreated group. Although previous drugs tested orally against T. gondii in pregnant sheep were dosed starting 10 days prior to infection until parturition, allowing a better chance to control T. gondii infection, our treatment with 5 doses of BKI-1294 initiated 48 h after infection resulted in higher protection against fetal/lamb mortality (71%) than studies using monensin (38% of increase in live lambs) (13) and decoquinate (22% increase in live lambs) (15). Likewise, contrary to this study, in which 100% fetal/lamb mortality was found in infected but untreated pregnant ewes, only 46 to 55% of fetal/lamb mortality was detected in infected but untreated pregnant ewes from studies testing monensin and decoquinate (13, 15), indicating less aggressive infections. In previous studies, a decreased birth weight has been described in lambs born from T. gondii-infected ewes (15). In this study, lambs born from twin pregnancies in the infected and treated group exhibited slightly lower birth weights than the uninfected group receiving vehicle alone, but since no significant differences were found between lambs that were PCR positive and those that were PCR negative, it is likely that this lower growth of the twin fetuses is a consequence of decreased food intake in the dams from the infected, treated group for 6 days (from 96 to 101 days of pregnancy, associated with marked fever due to T. gondii infection), as previously described (37). Likewise, all lambs born in the infected and treated group were healthy, while in a previous study using the TgShSp1 isolate in pregnant sheep, weak lambs were often observed (36). The rectal temperatures from infected and treated pregnant ewes were lower than those of infected, untreated pregnant ewes on days 5 and 7 p.i. (days 3 and 5 of BKI-1294 dosing), suggesting that BKI-1294 had an impact on parasite replication. Decreased rectal temperatures and/or a delay in rectal temperature increase have also been described in experiments testing monensin (13) or decoquinate (15) against toxoplasmosis in pregnant ewes.
The proinflammatory cytokine IFN-γ plays an important role by inhibiting the intracellular multiplication of T. gondii and creates a Th-1 type immune response (38). Analysis of the peripheral immune responses in pregnant ewes at different time points demonstrated a significant increase in IFN-γ release in stimulated peripheral blood cultures from the infected, treated group on day 7 p.i. compared to those of the infected, untreated group and uninfected group receiving vehicle alone. The infected, treated group showed a peak of IFN-γ release on day 10 p.i. as well as 100-fold increased levels until delivery, while there are no samples from infected and untreated ewes beyond day 7 p.i. In vitro studies showed that BKI-1294 triggered the development of intracellular multinucleated complexes, with parasites unable to complete the lytic cycle. These multinucleated complexes have increased expression of some stage-specific proteins such as SAG1 and BAG1 (25). In vivo, although no cell infection was demonstrated in this study, these multinucleated complexes could stimulate the host immune responses, resulting in higher IFN-γ-specific levels in treated ewes, as previously found (39) after treatment with BKI-1553 of Neospora caninum experimentally infected pregnant ewes. The SAG1 molecule is an immunodominant surface protein found on tachyzoites and is one of the most extensively studied antigens, as it is capable of inducing a T cell response with parasiticidal activity for extracellular T. gondii tachyzoites (40). Therefore, the effects of BKI-1294 to stimulate SAG1 and BAG1 production may act synergistically with the IFN-γ response, leading to enhanced immune protection against infection. The increased levels of IFN-γ in the infected, treated group might have led to greater initial control of parasitemia, diminishing the numbers of parasites reaching and invading the placenta (41).
Concerning humoral immune responses, all infected and treated animals (G1) were positive for the presence of anti-T. gondii SAG1 antibodies, except for one ewe that aborted on day 17 p.i. However, using the ELISA based on T. gondii soluble antigens, only one ewe that gave birth to a stillborn lamb and two dams that gave healthy lambs seroconverted, while the remaining ewes were seronegative at the end of the sampling period. The findings that soluble antigens are exposed to the immune system during replication (42) and that much lower humoral immune responses to soluble antigens were found compared to those of ewes infected with lower oocyst doses (36) suggest that there was only low replication of the parasite throughout the experiment. In contrast, SAG1 and BAG1 antibodies in the infected and treated group may be derived from their enhanced expression in vivo, as predicted by the multinucleated complexes found in vitro after BKI-1294 treatment of T. gondii-infected cultures (25). Likewise, it is known that the SAG1 antigen triggers an antibody response with an inhibitory effect on invasion (43).
In the present study, we evaluated transplacental transmission of the parasite through fetal serology, parasite detection, and histological lesions in fetal tissues. In previous assessments of drugs against congenital toxoplasmosis in pregnant sheep, vertical transmission was evaluated through techniques that are less sensitive than the PCR used in this study; specifically, previous studies primarily used fetal serology and/or microscopic observation of lesions in fetal brains and/or placental tissues. Using these techniques, there was a reduction of 50% in the animals with vertical transmission after treatment with monensin (13). In the study using decoquinate (15), only placental tissues were evaluated for T. gondii-induced lesions, and in this case, there was a 20 to 40% reduction of placentas showing lesions in the treated group. However, 12 to 25% of infected and untreated sheep did not show placental lesions (13, 15) or seropositive offspring (13). The study evaluating sulfamezathine/pyrimethamine showed 100% vertical transmission in untreated animals, and treated animals exhibited a 67% reduction in the placentas with lesions but no differences in fetal serology (14). In all the lambs born after infection with a 100-fold lower TgShSp1 oocyst dose than that used in the present study, T. gondii was detected in all their brains and lungs and lesions were found in most of the brains. However, in abortions during the acute phase of the disease caused by the infection of pregnant ewes with TgShSp1 oocysts (days 7 to 10 p.i.), T. gondii was scarcely detected in fetal tissues and placental thrombosis was often found (36). In lungs, which are commonly infected by T. gondii (44), no lesions or parasites were detected in lambs born in the BKI-1294-treated group. Likewise, parasites were not detected in the brain tissues of 7 of the 13 lambs born healthy (53% protection against vertical transmission in lambs born). There were no lesions found in the brains of any of the 13 healthy lambs. There was slight dissemination of the parasite in the lambs with no antibodies detected by IFAT, although this technique may suffer from low sensitivity (7). These results are in line with those from a pregnant mouse model of toxoplasmosis, in which 100% protection against pup mortality and 93% protection against vertical transmission in the surviving offspring was accomplished with BKI-1294 (28). The increased protection of BKI-1294 in pregnant mice compared to pregnant sheep could be explained by the lower offspring mortality and vertical transmission of T. gondii in mice compared to that in sheep (36).
In conclusion, BKI-1294 treatment in T. gondii-infected dams resulted in a decrease in rectal temperatures upon infection, strong IFN-γ production after cell stimulation in vitro, a low humoral immune response to soluble antigens but high levels of SAG1 antibodies, and a decrease of 71% in the fetal/lamb mortality. In the offspring, BKI-1294 prevented vertical transmission in 53% of lambs born alive. Results of protection against abortion and vertical transmission of BKI-1294 are substantially better than those of previous drugs tested against ovine toxoplasmosis. In light of these findings, BKI-1294 exhibits a therapeutic systemic exposure in pregnant ewes, is safe, and confers high protection against abortion and vertical transmission of the parasite in a pregnant sheep model of toxoplasmosis. Further studies are necessary to improve efficacy of BKI-1294 by applying alternative formulations, drug dosages, and dosing regimens. In addition, other members of the BKI class of compounds under development could be tested in the near future against ruminant toxoplasmosis.
MATERIALS AND METHODS
Ethics statement.
All protocols involving animals were approved by the Animal Welfare Committee of the Community of Madrid, Spain (PROEX 166/14), by following proceedings described in Spanish and EU legislation (Law 32/2007, R.D. 53/2013, and Council Directive 2010/63/EU). Good clinical practices were used to minimize suffering.
Animals and experimental design.
Thirty-five pure Rasa Aragonesa breed female ewes aged 12 months were selected from a commercial flock. All animals were seronegative for T. gondii, N. caninum, Border disease virus (BDV), Schmallenberg virus (SBV), Coxiella burnetii, and Chlamydia abortus as determined by ELISA. They were estrus synchronized and mated with pure-breed Rasa Aragonesa pups for 2 days. Pregnancy and fetal viability were confirmed by ultrasound scanning (US) on day 40 postmating, and twenty-five pregnant sheep were selected for the experiment. Pregnant ewes (n = 25) were randomly distributed into four experimental groups (Table 3) and housed at the animal facilities of the Animal Health Department in the Faculty of Veterinary Sciences (Complutense University of Madrid, Spain). Fifteen ewes were allocated into groups 1 (G1; n = 7) and 2 (G2; n = 8), which were dosed orally with 1,000 T. gondii sporulated oocysts of the T. gondii isolate TgShSp1 (PCR-RFLP genotype 3) (36) at day 90 of gestation (dg). Oocysts were shed by cats after oral infection with brains from infected mice (36). The ten remaining pregnant ewes were allocated to groups 3 (G3; n = 5) and 4 (G4; n = 5), which received 50 ml of phosphate-buffered saline (PBS) at 90 dg.
TABLE 3.
Experimental design
| Group | No. of pregnant ewes | No. of fetuses/lambs | Challenge (p.o.a ) | Treatment (p.o.a ) |
|---|---|---|---|---|
| G1 | 7 | 17 | 1,000 TgShSp1 sporulated oocysts | BKI-1294, 5 doses at 100 mg/kg every other day |
| G2 | 8 | 17 | 1,000 TgShSp1 sporulated oocysts | None |
| G3 | 5 | 8 | PBS | BKI-1294, 5 doses at 100 mg/kg every other day |
| G4 | 5 | 7 | PBS | 60% Phosal 53 MCT, 30% PEG 400, 10% ethanol 96° (vehicle), 5 doses every other day |
p.o., per os (orally).
BKI-1294 was synthesized by WuXi AppTec Inc. (Shanghai, China) and further purified in the Department of Chemistry of the University of Washington. The drug formulation was prepared by dissolving the compound in 60% Phosal 53 MCT (Lipoid GmbH, Ludwigshafen, Germany), 30% PEG400 (Sigma-Aldrich, Madrid, Spain), and 10% ethanol 96° (Panreac, Barcelona, Spain) by heating at 37°C and shaking for 3 h at a final concentration of 45 mg/ml. Starting at 48 h p.i., BKI-1294 was administered orally through an ororuminal probe to G1 (infected/treated) and G3 (uninfected/untreated), both groups at 100 mg/kg of body weight, 5 doses every other day. Each ewe from G1 (infected/treated) and G3 (uninfected/treated) received 146 ± 20 ml for each dose. In addition, ewes from G4 (uninfected/untreated) received 5 doses (140 ± 8 ml), every other day, of vehicle alone. The safety of BKI-1294 was evaluated by monitoring of rectal temperatures, gastrointestinal (fecal consistency) and behavioral changes, fetal viability, and hematological and biochemical parameters. The efficacy of this compound against congenital toxoplasmosis in sheep was assessed by monitoring fetal/lamb mortality and vertical transmission in live lambs (seropositivity and parasite detection/lesion in brain or lungs) (36). In addition, rectal temperatures and humoral and cellular immune responses to T. gondii infection were assessed.
Clinical monitoring.
Pregnant ewes were observed daily during the experimental period. Fetal viability was assessed postinfection by US monitoring of fetal heartbeat and movements once a week. Rectal temperatures were examined daily from day 0 until 14 days p.i. and then weekly. Animals were considered febrile when rectal temperatures were over 40°C (45).
When fetal death or birth of stillborn lambs occurred, or 48 h after delivery of live lambs, dams and lambs were sedated with xylazine (Rompun; Bayer, Mannhein, Germany) and euthanized with an intravenous overdose of embutramide and mebezonium iodide (T61; Intervet, Salamanca, Spain). Deliveries up to day 140 of pregnancy were considered premature. Lambs born from day 141 onwards were weighed immediately after birth. Live lambs were clinically inspected for 48 h after birth.
Collection of blood samples.
To determine BKI-1294 exposure, blood samples from G1 (infected/treated) and G3 (uninfected/treated) were collected at multiple time points by jugular venipuncture into 1-ml tubes (Aquisel, Barcelona, Spain) containing lithium heparin. Blood was collected prior to BKI-1294 administration; 1, 2, 4, 8, 12, 24, 30, and 48 h after the 1st dose; 8, 12, 24, 30, and 48 h after the 2nd, 3rd, 4th, and 5th doses; and finally, daily until 7 days after the 5th dose. Heparinized blood samples were centrifuged at 805 × g for 30 min at 4°C, and plasma samples were stored at −20°C until analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS).
For evaluation of hematological and biochemical parameters, blood samples were collected into 5-ml vacutainer tubes (Becton, Dickinson and Company, Plymouth, UK), with ethylenediaminetetraacetic acid (EDTA) as anticoagulant, and into 5-ml vacutainer tubes (Becton, Dickinson and Company, Plymouth, UK) without anticoagulant before and after treatment (4 days after the 5th BKI-1294 dose) from G3 (uninfected/treated) and G4 (uninfected/untreated). Tubes without anticoagulant were allowed to clot and were centrifuged to obtain serum samples that were stored at −80°C until analysis.
Blood samples to evaluate peripheral immune responses were collected from G1 and G2 (both infected) and G4 (uninfected/untreated) prior to infection, at 3, 5, 7, and 10 days p.i., and then weekly by jugular venipuncture into 5-ml vacutainer tubes (Becton, Dickinson and Company, Plymouth, UK) with and without lithium heparin as anticoagulant. Precolostral serum was collected from lambs born in G1 (infected/treated), G2 (infected/untreated), and G4 (uninfected/untreated) and maintained at −80°C for subsequent serological analysis. Udders were covered with a piece of cloth 1 week before the expected date of delivery to avoid any accidental suckling from lambs born overnight.
Postmortem collection of tissue and body fluid samples.
To evaluate vertical transmission of the parasite, pieces from brains and lungs from fetuses in G1 (infected/treated), G2 (infected/untreated), and G4 (uninfected/untreated) were stored at −80°C for DNA extraction and fixed in 10% formalin for histopathological examination. In addition, six randomly selected placentomes were recovered from each placenta of aborted dams in G1 (infected/treated), G2 (infected/untreated), and G4 (uninfected/untreated). For histopathological examinations the placentomes were transversally cut into slices 2 to 3 mm thick and were stored in 10% formalin. The rest of the placentomes were stored at −80°C for parasite DNA detection. In the remaining dams that gave birth in G1 (infected/treated), G2 (infected/untreated), and G4 (uninfected/untreated), six randomly selected cotyledons were recovered after delivery of the placenta and stored at −80°C for parasite DNA detection. Fetal thoracic and abdominal fluids were also collected from fetuses in G1 (infected/treated), G2 (infected/untreated), and G4 (uninfected/untreated) and maintained at −80°C for serology.
BKI-1294 pharmacokinetics.
BKI-1294 was extracted from the plasma samples taken from G1 (infected/treated) and G3 (uninfected/treated) using acetonitrile–0.1% formic acid with an internal standard. A matrix-matched standard curve was prepared for comparison and quantification. BKI-1294 was measured with an Acquity ultra-performance liquid chromatography (UPLC) system in tandem with a Xevo TQ-S mass spectrometer (Waters, Milford, MA, USA). Calculations of Cmax for each dose and AUC were determined using Prism (GraphPad, San Diego, CA).
Hematological and biochemical analyses.
As previously described (39), complete blood counts (CBCs) were determined in whole blood using the automated laser-based hematology analyzer Advia 120 (Siemens Healthcare Diagnostics GmbH, Eschborn, Germany). Biochemical parameters were measured in serum using the sequential automatic autoanalyzer Konelab 30 (Thermo Fisher Scientific, Waltham, USA). Ions were assessed in serum using a Microlyte 3 (Beckman Coulter, Brea, CA). Reference values were obtained from previous studies (46).
Peripheral blood cell stimulation assay and assessment of IFN-γ production.
Peripheral blood stimulation assay and assessment of IFN-γ production were carried out as previously described (39), using T. gondii soluble antigens for stimulation. Briefly, heparinized blood was cultured in 24-well flat-bottom plates in the presence of either soluble T. gondii antigens or concanavalin A (ConA; Sigma-Aldrich, Madrid, Spain), both at a final concentration of 5 μg/ml. Plates were incubated in a 5% CO2, 37°C, 100% humidity atmosphere for 24 h. They were then centrifuged at 1,000 × g for 10 min at 4°C, and IFN-γ was detected in culture supernatants using a commercial bovine enzyme immunoassay that shows cross-reactivity with ovine IFN-γ (Mabtech AB, Sweden).
Serological analyses.
T. gondii-specific IgG antibody levels in ewes were measured using an in-house indirect ELISA (6) and a commercial test of anti-T. gondii SAG1 (p30) antibodies, ID Screen toxoplasmosis indirect multispecies (IDvet, Grabels, France). In-house ELISA was carried out in 96-well microtiter plates (Thermo Fisher Scientific, Waltham, MA) that were coated with 100 μl of soluble T. gondii antigens (1.5 μg/ml in 100 mM carbonate buffer, pH 9.6) overnight at 4°C. Plates were blocked using 3% bovine serum albumin diluted in PBS containing 0.05% Tween 20 (PBS-T), and serum samples were diluted 1:100 in the blocking solution. Subsequently, horseradish peroxidase-conjugated protein G (Sigma-Aldrich, Madrid, Spain) diluted 1:6,000 in PBS-T was added, followed by 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS; Roche, Basel, Switzerland), used as the substrate. The reaction was stopped by adding 100 μl of 0.3 M oxalic acid, and the optical density was read at 405 nm (OD405). For each plate, values of the OD were transformed into a relative index percent (RIPC) using the following formula: RIPC = (OD405 sample – OD405 negative control)/(OD405 positive control – OD405 negative control) × 100. An RIPC value of ≥10 indicates a positive result. Commercial ELISA was performed by following the manufacturer’s instructions, and values of the OD were converted into S/P percent using the following formula: S/P % = (OD450 sample – OD450 negative control)/(OD450 positive control – OD450 negative control) × 100. An S/P % of ≥50 indicates a positive result.
Anti-T. gondii SAG1 antibodies were evaluated by WB in sera from ewes in G1 (infected/treated). For WB, samples containing 2 × 107 cell culture-derived T. gondii ME49 strain tachyzoites were sonicated in an ultrasonic bath at 15°C for 15 min and then heated for 5 min at 95°C prior to use. Electrophoresis was performed in 15% polyacrylamide-N,N′-diallyltartardiamide (DATD) minigels, followed by transfer to a nitrocellulose membrane (Mini Trans-Blot cell; Bio-Rad Laboratories, CA, USA). The presence of anti-T. gondii BAG1 antibodies was also assessed by WB. Coated membranes and immunoblotting were performed similarly to a previously described method (47) but under reducing conditions using dithiothreitol (DTT). Fifteen micrograms of T. gondii BAG1 recombinant protein expressed in Escherichia coli (GenScript, Nanjing, China) was electrophoresed in 15% polyacrylamide-DATD minigels and transferred to nitrocellulose membranes (Mini Trans-Blot cell; Bio-Rad Laboratories, CA, USA). WB was carried out similarly to the method described for N. caninum (48), and serum samples were used at a 1:20 dilution. Serum samples of 4 ewes from G1 (infected/treated) that gave birth to healthy lambs and the ewe that gave birth to a stillborn in G1 (infected/treated) were assayed on days 0, 49, and 56 p.i. Serum on day 49 p.i. from one ewe infected with 50 TgShSp1 oocysts that gave birth in a previous study (36) was used as a positive control of infection. Serum from the uninfected group was used as a negative control. Horseradish peroxidase-conjugated protein G (Sigma-Aldrich, Madrid, Spain) was used at a 1:400 dilution.
IFAT was used to detect specific IgG anti-Toxoplasma antibodies in fetal fluids and precolostral sera (49), using an anti-sheep IgG (Sigma-Aldrich, Madrid, Spain) diluted 1:200 in Evans Blue (Sigma-Aldrich, Madrid, Spain). Fetal fluids and precolostral sera were diluted at 2-fold serial dilutions in PBS starting at 1:8 (for fetal fluids) and 1:50 (for precolostral sera) up to the endpoint titer. Continuous tachyzoite membrane fluorescence at a titer of ≥8 for fetal fluids or ≥50 for precolostral sera was considered a positive reaction.
Histopathology.
After fixation in 10% neutral buffered formalin for 5 days, placentomes, brains, and lungs from fetuses were cut coronally, embedded in paraffin wax, and processed by standard procedures for hematoxylin and eosin staining. Conventional histological evaluation was conducted in all sections.
DNA extraction and PCR for parasite detection.
Genomic DNA was extracted from 50 to 100 mg of six samples from placental tissues and three samples from fetal brain and fetal lung tissues using the commercial Maxwell 16 mouse tail DNA purification kit, developed for the automated Maxwell 16 system (Promega, WI, USA), by following the manufacturer’s recommendations. The concentration of DNA for all samples was determined by spectrophotometry and adjusted to 50 to 100 ng/μl. T. gondii DNA detection was carried out by an ITS-1 PCR adapted to a single tube as previously described (6). Each reaction mixture had a final volume of 25 μl with 5 μl of sample DNA. Samples from G4 (uninfected/untreated) were included in each round of DNA extraction and PCR as negative controls.
Statistical analysis.
Occurrence of fetal/lamb mortality was analyzed by the Kaplan-Meier survival method, and fetal/lamb survival curves were then compared by the log-rank (Mantel-Cox) test. Comparison of the number of fetuses/lambs suffering mortality was done using the χ2 or Fisher’s exact F-test. Rectal temperatures were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s multiple test until 14 days p.i. Cellular immune responses were analyzed using two-way ANOVA of repeated measures until day 7 p.i. Weights of the lambs were compared using one-way ANOVA followed by t test for pairwise comparisons. BKI-1294 Cmax and AUC comparisons were evaluated using the Mann-Whitney test. Statistical significance for all analyses was defined at a P value of <0.05. All statistical analyses were performed using GraphPad Prism 6.01 software (San Diego, CA, USA).
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the SALUVET group (Complutense University of Madrid, Spain), Luis Miguel Ferrer, Jose Ventura, José Calasanz Jiménez, Francisco Saura, Teresa Navarro, and José María González from the University of Zaragoza (Spain), and Victor Herrero and Javier Blanco from Clinical Veterinary Hospital (Complutense University of Madrid, Spain) for their excellent technical assistance, Dale Kempf and Kennan Marsh, from AbbVie, Inc., for their suggestion of the Phosal vehicle used in the experiments reported here, Rama S. R. Vidadala and Dustin J. Maly, Department of Chemistry, University of Washington, for characterization and further purification of BKI-1294, and advice from Robert Choy and Eugenio L. de Hostos from the PATH Drug Development Program. The Animal Experimentation Service (SEA) at the University of Zaragoza is acknowledged for providing their facilities to carry out the reproduction program. R.S.-S. is supported by a fellowship from the Spanish Ministry of Education, Culture and Sports (MECD), as a part of the Program of Training of University Teaching Staff (FPU; grant number FPU13/03438). A.H. is supported by a Swiss National Science Foundation grant (no. 310030-165782). This work was supported by the Public Health Service, National Institutes of Health, Bethesda, MD (grants R01 AI 111341 and R01 HD 080670), the U.S. Department of Agriculture (grant 2014-67015-22106), and the Community of Madrid, Spain (PLATESA2, P2018/BAA-4370). I.F., A.H., K.K.O., W.C.V.V., and L.M.O.-M. conceived the study and participated in its design. R.S.-S. wrote the manuscript, with results interpretation and discussion inputs from I.F., J.R.-C., A.H., M.A.H., L.K.B., W.C.V.V., and L.M.O.-M. J.J.R. selected the animals and executed the reproductive program. R.S.-S., I.F., and M.R. carried out oocyst infection and drug administration. R.S.-S., I.F., M.R., M.P.D., M.G.-H., E.T., and J.B. participated in clinical examination and sampling of animals and performed necropsies and hematological, biochemical, and histopathological analyses. M.H., L.K.B., R.C., G.R.W., and K.K.O. determined the pharmacokinetics of the compound. R.S.-S. performed peripheral blood stimulation assay, serological assays, PCR analyses, statistical analysis, and interpretation of the results. All authors read and approved the final manuscript. W.C.V.V. is the president and co-owner of ParaTheraTech, Inc., a company that is developing BKIs for animal health. W.C.V.V. did not perform the experiments or interpret the results of the experiments, but he did edit the paper and helped plan the experiments. The other authors have no competing interests to declare.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.02527-18.
REFERENCES
- 1.Dubey J. 2010. Toxoplasmosis of animals and humans. CRC Press, Boca Raton, FL. [Google Scholar]
- 2.Innes EA, Bartley PM, Buxton D, Katzer F. 2009. Ovine toxoplasmosis. Parasitology 136:1887–1894. doi: 10.1017/S0031182009991636. [DOI] [PubMed] [Google Scholar]
- 3.Buxton D, Maley SW, Wright SE, Rodger S, Bartley P, Innes EA. 2007. Toxoplasma gondii and ovine toxoplasmosis: new aspects of an old story. Vet Parasitol 149:25–28. doi: 10.1016/j.vetpar.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Trees AJ, Crozier SJ, Buxton D, Blewett DA. 1989. Serodiagnosis of ovine toxoplasmosis: an assessment of the latex agglutination test and the value of IgM specific titers after experimental oocyst-induced infections. Res Vet Sci 46:67–72. doi: 10.1016/S0034-5288(18)31120-2. [DOI] [PubMed] [Google Scholar]
- 5.Owen MR, Clarkson MJ, Trees AJ. 1998. Diagnosis of Toxoplasma abortion in ewes by polymerase chain reaction. Vet Rec 25:445–448. doi: 10.1136/vr.142.17.445. [DOI] [PubMed] [Google Scholar]
- 6.Castaño P, Fuertes M, Ferre I, Fernández M, del Carmen Ferreras M, Moreno-Gonzalo J, González-Lanza C, Katzer F, Regidor-Cerrillo J, Ortega-Mora LM. 2014. Placental thrombosis in acute phase abortions during experimental Toxoplasma gondii infection in sheep. Vet Res 45:9. doi: 10.1186/1297-9716-45-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castaño P, Fuertes M, Regidor-Cerrillo J, Ferre I, Fernández M, Ferreras MC, Moreno-Gonzalo J, González-Lanza C, Pereira-Bueno J, Katzer F. 2016. Experimental ovine toxoplasmosis: influence of the gestational stage on the clinical course, lesion development and parasite distribution. Vet Res 47:43. doi: 10.1186/s13567-016-0327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubey J. 2009. Toxoplasmosis in sheep–the last 20 years. Vet Parasitol 163:1–14. doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Sanchez R, Vazquez P, Ferre I, Ortega-Mora LM. 2018. Treatment of toxoplasmosis and neosporosis in farm ruminants: state of knowledge and future trends. Curr Top Med Chem 18:1304–1323. doi: 10.2174/1568026618666181002113617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buxton D, Thomson K, Maley S, Wright S, Bos HJ. 1991. Vaccination of sheep with a live incomplete strain (s48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet Rec 129:89–93. doi: 10.1136/vr.129.5.89. [DOI] [PubMed] [Google Scholar]
- 11.Buxton D, Thomson KM, Maley S, Wright S, Bos HJ. 1993. Experimental challenge of sheep 18 months after vaccination with a live (S48) Toxoplasma gondii vaccine. Vet Rec 133:310–312. doi: 10.1136/vr.133.13.310. [DOI] [PubMed] [Google Scholar]
- 12.Buxton D, Donald KM, Finlayson J. 1987. Monensin and the control of experimental ovine toxoplasmosis: a systemic effect. Vet Rec 120:618–619. doi: 10.1136/vr.120.26.618. [DOI] [PubMed] [Google Scholar]
- 13.Buxton D, Blewett D, Trees A, McColgan C, Finlayson J. 1988. Further studies in the use of monensin in the control of experimental ovine toxoplasmosis. J Comp Pathol 98:225–236. doi: 10.1016/0021-9975(88)90021-7. [DOI] [PubMed] [Google Scholar]
- 14.Buxton D, Thomson KM, Maley S. 1993. Treatment of ovine toxoplasmosis with a combination of sulphamezathine and pyrimethamine. Vet Rec 132:409–411. doi: 10.1136/vr.132.16.409. [DOI] [PubMed] [Google Scholar]
- 15.Buxton D, Brebner J, Wright S, Maley SW, Thomson KM, Millard K. 1996. Decoquinate and the control of experimental ovine toxoplasmosis. Vet Rec 138:434–436. doi: 10.1136/vr.138.18.434. [DOI] [PubMed] [Google Scholar]
- 16.Fung HB, Kirschenbaum HL. 1996. Treatment regimens for patients with toxoplasmic encephalitis. Clin Ther 18:1037–1056. doi: 10.1016/S0149-2918(96)80059-2. [DOI] [PubMed] [Google Scholar]
- 17.Rotella DP. 2012. Recent results in protein kinase inhibition for tropical diseases. Bioorg Med Chem Lett 22:6788–6793. doi: 10.1016/j.bmcl.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 18.Lourido S, Shuman J, Zhang C, Shokat KM, Hui R, Sibley LD. 2010. Calcium-dependent protein kinase 1 is an essential regulator of exocytosis in Toxoplasma. Nature 465:359–362. doi: 10.1038/nature09022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murphy RC, Ojo KK, Larson ET, Castellanos-Gonzalez A, Perera BGK, Keyloun KR, Kim JE, Bhandari JG, Muller NR, Verlinde CLMJ, White AC, Merritt EA, Van Voorhis WC, Maly DJ. 2010. Discovery of potent and selective inhibitors of CDPK1 from C. parvum and T. gondii. ACS Med Chem Lett 1:331–335. doi: 10.1021/ml100096t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojo KK, Larson ET, Keyloun KR, Castaneda LJ, Derocher AE, Inampudi KK, Kim JE, Arakaki TL, Murphy RC, Zhang L, Napuli AJ, Maly DJ, Verlinde CLMJ, Buckner FS, Parsons M, Hol WGJ, Merritt EA, Van Voorhis WC. 2010. Toxoplasma gondii calcium-dependent protein kinase 1 is a target for selective kinase inhibitors. Nat Struct Mol Biol 17:602–607. doi: 10.1038/nsmb.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cardew EM, Verlinde CL, Pohl E. 2018. The calcium-dependent protein kinase 1 from Toxoplasma gondii as target for structure-based drug design. Parasitology 145:210–218. doi: 10.1017/S0031182017001901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kieschnick H, Wakefield T, Narducci CA, Beckers C. 2001. Toxoplasma gondii attachment to host cells is regulated by a calmodulin-like domain protein kinase. J Biol Chem 276:12369–12377. doi: 10.1074/jbc.M011045200. [DOI] [PubMed] [Google Scholar]
- 23.Lourido S, Tang K, Sibley LD. 2012. Distinct signaling pathways control Toxoplasma egress and host-cell invasion. EMBO J 31:4524–4534. doi: 10.1038/emboj.2012.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Voorhis WC, Doggett JS, Parsons M, Hulverson MA, Choi R, Arnold SLM, Riggs MW, Hemphill A, Howe DK, Mealey RH, Lau AOT, Merritt EA, Maly DJ, Fan E, Ojo KK. 2017. Extended-spectrum antiprotozoal bumped kinase inhibitors: a review. Exp Parasitol 180:71–83. doi: 10.1016/j.exppara.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winzer P, Muller J, Aguado-Martinez A, Rahman M, Balmer V, Manser V, Ortega-Mora LM, Ojo KK, Fan E, Maly DJ, Van Voorhis WC, Hemphill A. 2015. In vitro and in vivo effects of the bumped kinase inhibitor 1294 in the related cyst-forming apicomplexans Toxoplasma gondii and Neospora caninum. Antimicrob Agents Chemother 59:6361–6374. doi: 10.1128/AAC.01236-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lourido S, Jeschke GR, Turk BE, Sibley LD. 2013. Exploiting the unique ATP-binding pocket of Toxoplasma calcium-dependent protein kinase 1 to identify its substrates. ACS Chem Biol 8:1155–1162. doi: 10.1021/cb400115y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doggett JS, Ojo KK, Fan E, Maly DJ, Van Voorhis WC. 2014. Bumped kinase inhibitor 1294 treats established Toxoplasma gondii infection. Antimicrob Agents Chemother 58:3547–3549. doi: 10.1128/AAC.01823-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Müller J, Aguado-Martínez A, Ortega-Mora L-M, Moreno-Gonzalo J, Ferre I, Hulverson MA, Choi R, McCloskey MC, Barrett LK, Maly DJ, Ojo KK, Van Voorhis W, Hemphill A. 2017. Development of a murine vertical transmission model for Toxoplasma gondii oocyst infection and studies on the efficacy of bumped kinase inhibitor (BKI)-1294 and the naphthoquinone buparvaquone against congenital toxoplasmosis. J Antimicrob Chemother 72:2334–2341. doi: 10.1093/jac/dkx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gazzinelli RT, Mendonca-Neto R, Lilue J, Howard J, Sher A. 2014. Innate resistance against Toxoplasma gondii: an evolutionary tale of mice, cats, and men. Cell Host Microbe 15:132–138. doi: 10.1016/j.chom.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller J, Hemphill A. 2013. New approaches for the identification of drug targets in protozoan parasites. Int Rev Cell Mol Biol 301:359–401. doi: 10.1016/B978-0-12-407704-1.00007-5. [DOI] [PubMed] [Google Scholar]
- 31.Schaefer DA, Betzer DP, Smith KD, Millman ZG, Michalski HC, Menchaca SE, Zambriski JA, Ojo KK, Hulverson MA, Arnold SLM, Rivas KL, Vidadala RSR, Huang W, Barrett LK, Maly DJ, Fan E, Van Voorhis WC, Riggs MW. 2016. Novel bumped kinase inhibitors are safe and effective therapeutics in the calf clinical model for cryptosporidiosis. J Infect Dis 214:1856–1864. doi: 10.1093/infdis/jiw488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vidadala RSR, Rivas KL, Ojo KK, Hulverson MA, Zambriski JA, Bruzual I, Schultz TL, Huang W, Zhang Z, Scheele S, DeRocher AE, Choi R, Barrett LK, Siddaramaiah LK, Hol WGJ, Fan E, Merritt EA, Parsons M, Freiberg G, Marsh K, Kempf DJ, Carruthers VB, Isoherranen N, Doggett JS, Van Voorhis WC, Maly DJ. 2016. Development of an orally available and central nervous system (CNS) penetrant Toxoplasma gondii calcium-dependent protein kinase 1 (Tg CDPK1) inhibitor with minimal human ether-a-go-go-related gene (hERG) activity for the treatment of toxoplasmosis. J Med Chem 59:6531–6546. doi: 10.1021/acs.jmedchem.6b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojo KK, Eastman RT, Vidadala R, Zhang Z, Rivas KL, Choi R, Lutz JD, Reid MC, Fox AMW, Hulverson MA, Kennedy M, Isoherranen N, Kim LM, Comess KM, Kempf DJ, Verlinde CLMJ, Su X-Z, Kappe SHI, Maly DJ, Fan E, Van Voorhis WC. 2014. A specific inhibitor of Pf CDPK4 blocks malaria transmission: chemical-genetic validation. J Infect Dis 209:275–284. doi: 10.1093/infdis/jit522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yokus B, Cakir D, Kanay Z, Gulten T, Uysal E. 2006. Effects of seasonal and physiological variations on the serum chemistry, vitamins and thyroid hormone concentrations in sheep. J Vet Med Ser A 53:271–276. doi: 10.1111/j.1439-0442.2006.00831.x. [DOI] [PubMed] [Google Scholar]
- 35.Huang W, Ojo KK, Zhang Z, Rivas K, Vidadala RSR, Scheele S, DeRocher AE, Choi R, Hulverson MA, Barrett LK, Bruzual I, Siddaramaiah LK, Kerchner KM, Kurnick MD, Freiberg GM, Kempf D, Hol WGJ, Merritt EA, Neckermann G, de Hostos EL, Isoherranen N, Maly DJ, Parsons M, Doggett JS, Van Voorhis WC, Fan E. 2015. SAR studies of 5-aminopyrazole-4-carboxamide analogs as potent and selective inhibitors of Toxoplasma gondii CDPK1. ACS Med Chem Lett 6:1184–1189. doi: 10.1021/acsmedchemlett.5b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Sánchez R, Ferre I, Regidor-Cerrillo J, Gutiérrez-Expósito D, Ferrer LM, Arteche-Villasol N, Moreno-Gonzalo J, Müller J, Aguado-Martínez A, Pérez V. 2019. Virulence in mice of a Toxoplasma gondii type II isolate does not correlate with the outcome of experimental infection in pregnant sheep. Front Cell Infect Microbiol 8:436. doi: 10.3389/fcimb.2018.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gardner DS, Buttery PJ, Daniel Z, Symonds ME. 2007. Factors affecting birth weight in sheep: maternal environment. Reproduction 133:297–307. doi: 10.1530/REP-06-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Innes EA, Vermeulen AN. 2006. Vaccination as a control strategy against the coccidial parasites Eimeria, Toxoplasma and Neospora. Parasitology 133(Suppl):S145–S168. doi: 10.1017/S0031182006001855. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez-Sánchez R, Ferre I, Re M, Vázquez P, Ferrer LM, Blanco-Murcia J, Regidor-Cerrillo J, Pizarro Díaz M, González-Huecas M, Tabanera E, García-Lunar P, Benavides J, Castaño P, Hemphill A, Hulverson MA, Whitman GR, Rivas KL, Choi R, Ojo KK, Barrett LK, Van Voorhis WC, Ortega-Mora LM. 2018. Safety and efficacy of the bumped kinase inhibitor BKI-1553 in pregnant sheep experimentally infected with Neospora caninum tachyzoites. Int J Parasitol Drugs Drug Resist 8:112–124. doi: 10.1016/j.ijpddr.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan IA, Smith KA, Kasper LH. 1988. Induction of antigen-specific parasiticidal cytotoxic T cell splenocytes by a major membrane protein (P30) of Toxoplasma gondii. J Immunol 141:3600–3605. [PubMed] [Google Scholar]
- 41.Entrican G. 2002. Immune regulation during pregnancy and host-pathogen interactions in infectious abortion. J Comp Pathol 126:79–94. doi: 10.1053/jcpa.2001.0539. [DOI] [PubMed] [Google Scholar]
- 42.Joiner KA, Roos DS. 2002. Secretory traffic in the eukaryotic parasite Toxoplasma gondii: less is more. J Cell Biol 157:557–563. doi: 10.1083/jcb.200112144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mineo JR, McLeod R, Mack D, Smith J, Khan IA, Ely KH, Kasper LH. 1993. Antibodies to Toxoplasma gondii major surface protein (SAG-1, P30) inhibit infection of host cells and are produced in murine intestine after peroral infection. J Immunol 150:3951–3964. [PubMed] [Google Scholar]
- 44.Gutierrez J, O’Donovan J, Williams E, Proctor A, Brady C, Marques PX, Worrall S, Nally JE, McElroy M, Bassett H, Sammin D, Buxton D, Maley S, Markey BK. 2010. Detection and quantification of Toxoplasma gondii in ovine maternal and fetal tissues from experimentally infected pregnant ewes using real-time PCR. Vet Parasitol 172:8–15. doi: 10.1016/j.vetpar.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 45.Diffay BC, McKenzie D, Wolf C, Pugh DG. 2002. Handling and examination of sheep and goats, p 5–7. In Pugh DG. (ed), Sheep and goat medicine. WB Saunders Company, Philadelphia, PA. [Google Scholar]
- 46.Ramos-Antón JJ, Ferrer-Mayayo LM. 2007. La exploración clínica del ganado ovino y su entorno. Servet, Zaragoza, Spain. [Google Scholar]
- 47.Aguado-Martínez A, Álvarez-García G, Fernández-García A, Risco-Castillo V, Arnaiz-Seco I, Rebordosa-Trigueros X, Navarro-Lozano V, Ortega-Mora LM. 2008. Usefulness of rNcGRA7- and rNcSAG4-based ELISA tests for distinguishing primo-infection, recrudescence, and chronic bovine neosporosis. Vet Parasitol 157:182–195. doi: 10.1016/j.vetpar.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Álvarez-García G, Pereira-Bueno J, Gómez-Bautista M, Ortega-Mora LM. 2002. Pattern of recognition of Neospora caninum tachyzoite antigens by naturally infected pregnant cattle and aborted fetuses. Vet Parasitol 107:15–27. doi: 10.1016/S0304-4017(02)00091-2. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez-García G, Collantes-Fernández E, Costas E, Rebordosa X, Ortega-Mora LM. 2003. Influence of age and purpose for testing on the cutoff selection of serological methods in bovine neosporosis. Vet Res 34:341–352. doi: 10.1051/vetres:2003009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.