Borrelia burgdorferi is the etiological agent of Lyme disease. In the current study, we used direct-detection PCR and electrospray ionization mass spectrometry to monitor and genotype B. burgdorferi isolates from serially collected whole-blood specimens from patients clinically diagnosed with early Lyme disease before and during 21 days of antibiotic therapy.
KEYWORDS: Borrelia burgdorferi, Lyme disease, antibiotic, genotype, time course
ABSTRACT
Borrelia burgdorferi is the etiological agent of Lyme disease. In the current study, we used direct-detection PCR and electrospray ionization mass spectrometry to monitor and genotype B. burgdorferi isolates from serially collected whole-blood specimens from patients clinically diagnosed with early Lyme disease before and during 21 days of antibiotic therapy. B. burgdorferi isolates were detected up to 3 weeks after the initiation of antibiotic treatment, with ratios of coinfecting B. burgdorferi genotypes changing over time.
TEXT
Lyme disease is caused by the bite of a tick infected with the spirochete Borrelia burgdorferi. The CDC estimates ∼300,000 new cases of Lyme disease in the United States per year (1). The near-pathognomonic skin rash erythema migrans (EM) is the only specific clinical sign that uniquely points to Lyme disease. However, the EM lesion is not seen in ∼30% of infected individuals (2), and even when present, it often does not have the hallmark bull’s-eye appearance characteristic of infection with a Borrelia species (3, 4). The current approved serological two-tier test for Lyme disease detects the presence of host antibodies for B. burgdorferi antigens but cannot distinguish active infection from prior exposure (5, 6), so there is no way to test for cure or measure response to treatment. We previously used broad-range PCR electrospray ionization mass spectrometry (PCR/ESI-MS) to directly detect and genotype B. burgdorferi isolates from ticks and whole blood from patients with early Lyme disease (7–9). This assay can also distinguish B. burgdorferi genotypes, even when present in mixtures (7, 8), which is important because coinfecting genotypes are generally common in wild hosts (10) and Ixodes scapularis ticks (7). Using our multilocus typing method, we have a much higher genotype resolution than ospC typing alone (7), and we now have identified nearly 90 unique B. burgdorferi genotypes (unpublished data). Our aim in this first-of-its-kind study was to use our direct molecular assay for B. burgdorferi to monitor microbiological response to treatment and to determine how long after initiating antibiotic therapy B. burgdorferi isolates can be detected in blood from patients with early Lyme disease. Furthermore, the present study was designed to identify genotype(s) of the infecting strain(s) of B. burgdorferi isolates in these serially collected specimens.
The study was approved by the Johns Hopkins Medicine Institutional Review Board, and written informed consent was obtained from all participants before enrollment. Four patients from an area in Maryland where Lyme disease is endemic were enrolled during the summer/fall of 2015 and 2016. All participants had a physician-documented and diagnosed EM and were excluded for the presence of an immunosuppressive illness or medication, pregnancy, or a history of receiving the Lyme vaccine. Of the four participants, two (patients A and D) self-reported a history of diagnosed and treated Lyme disease. All participants were antibiotic naive at the time of enrollment, and a day 0 specimen was collected before initiating a 21-day course of doxycycline. Three of the four participants said they missed no doses, and one of the four (patient A) missed one dose. A total of 20 ml of whole blood was collected at days 0, 1, 2, 4, 8, and 21 into two 10-ml EDTA purple-cap blood collection tubes and frozen for analysis by PCR/ESI-MS. Nucleic acids were extracted from four 5-ml aliquots of EDTA whole blood, and PCR/ESI-MS was performed as previously described (11). Three of the primer pairs (BCT6092, BCT6095, and BCT6101) were modified from our previous studies to improve their performance in the presence of a high background of human DNA without changing their targets (7, 8). The sequences of all primers used in this study are shown in Table 1; when a core six of eight primers produced a signature, we assigned a genotype to the detection as described previously (7).
TABLE 1.
PCR/ESI-MS primer sequences and gene targets
| Primer pair ID | Primer code | Gene target | Primer sequence (5′→3′) |
|---|---|---|---|
| BCT3511 | BCT8229F | gyrB | TGCATTTGAAAGCTTGGCATTGCC |
| BCT8230R | TCATTTTAGCACTTCCTCCAGCAGAATC | ||
| BCT6092 | BCT13037F | rplB | TCATCCACATGGTGGTGGTGAA |
| BCT13040R | TGCGAGTCTTATAGCCTTTAGTAGGC | ||
| BCT6095 | BCT13043F | rpoC | TACAAAGGAATGGGAATGTTATTGTGGT |
| BCT8236R | TGCGAGCTCTATATGCCCCAT | ||
| BCT6101 | BCT13044F | leuS | TCATGTTGGTCATCCGGAAGGATA |
| BCT13049R | TGTATTGCATAACTTTCAGCAGGAAG | ||
| BCT3517 | BCT8241F | flaB | TGCTGAAGAGCTTGGAATGCA |
| BCT8242R | TACAGCAATTGCTTCATCTTGATTTGC | ||
| BCT3518 | BCT8243F | ospC | TGACGGTATTTTTATTTATATCTTGTAATAATTCAGG |
| BCT8244R | TTTGCTTATTTCTGTAAGATTAGGCCCTTT | ||
| BCT3519 | BCT8245F | hbb | TCGAATAATGTTATTGAGTTTAGATCTTTTGGTAC |
| BCT8246R | TGGACGAAAATACGCAACATGATGATC | ||
| BCT3520 | BCT8247F | hbb | TGTCTTTTCCAAGAAGACCAAAGGTTACTAA |
| BCT8248R | TACCCTTAAGCTCTTCAAAAAAAGCATC |
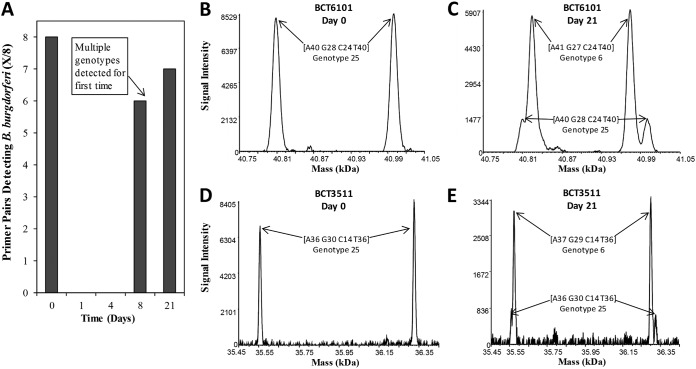
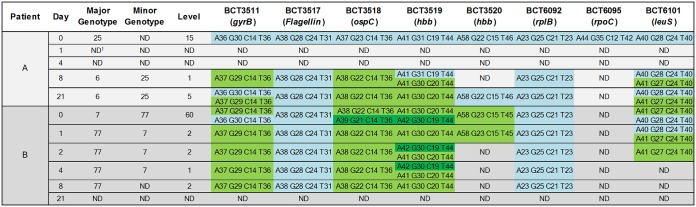
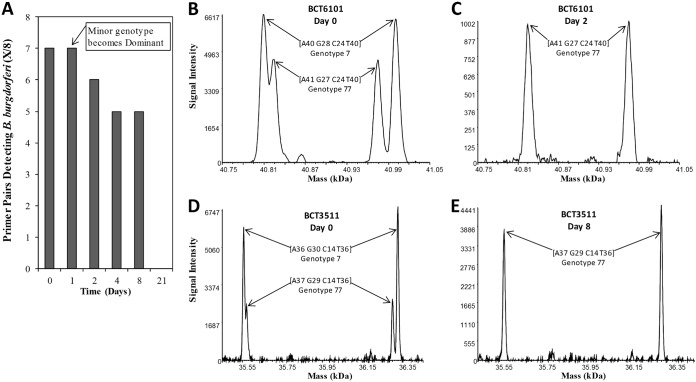
B. burgdorferi isolates were detected and genotyped at day 0 and subsequent time points by PCR/ESI-MS testing of whole blood collected from two of the four participants (patients A and B); patients C and D tested negative in all specimens. For patient A, eight of eight specific primer pairs detected our B. burgdorferi genotype 25 at day 0 (Fig. 1A, B, and D; Fig. 2). B. burgdorferi isolates were not detected at days 1 and 4 (blood was not collected at day 2 for patient A) (Fig. 1A). However, B. burgdorferi isolates were detected by six and seven of eight primer pairs at days 8 and 21, respectively (Fig. 1A), and at each of these time points, two genotypes (6 and 25) were detected. At these later visits, genotype 6 was determined as the major genotype and genotype 25 as the minor genotype (Fig. 1C and E; Fig. 2). For patient B, coinfection with two B. burgdorferi genotypes (major genotype 7 and minor genotype 77) was detected by PCR/ESI-MS from blood collected at day 0 (Fig. 2; Fig. 3B and D). After the start of antibiotic treatment, there was a genotypic shift, with genotype 77 becoming the major genotype detected at day 1 (Fig. 2). Evidence of both genotypes was observed through day 4, with genotype 7 becoming undetectable by day 8 (Fig. 2). Specifically, primer pair BCT6101 generated base-count signatures corresponding to genotypes 7 and 77 at days 0 and 1, but genotype 77 was the only genotype detected by primer pair BCT6101 at day 2 (Fig. 2; Fig. 3C). Base-count signatures corresponding to genotype 77 were last detected at day 8 (Fig. 2; Fig. 3E). B. burgdorferi isolates were not detected (0 of 8 primer pairs) at day 21 (Fig. 3A).
FIG 1.
Borrelia burgdorferi primer pair detections and deconvolved spectral data of PCR amplicons derived from patient A. PCR/ESI-MS was performed on serial whole blood from a patient with clinically diagnosed early Lyme disease with EM. Specific primer pairs detecting B. burgdorferi isolates are plotted over time (A), displayed as cumulative detections from four 5-ml aliquots of 20 ml blood. Primer pairs BCT6101 (leuS) (B) and BCT3511 (gyrB) (D) simultaneously detected a single genotype of Borrelia burgdorferi on day 0. (C and E) Detection of two genotypes of B. burgdorferi for these targets on day 21. Paired peaks correspond to the forward and reverse strands of the PCR amplicons, which separate under the conditions of electrospray ionization.
FIG 2.
Borrelia burgdorferi detections and genotypes with PCR amplicon base-count signatures from serial whole-blood collections. Base counts with the largest amplitude are shown on the bottom for cells with multiple base counts. Each base-count signature per column is differentiated by color. ND, no detection; level, relative intensity based on calibrant copies per reaction.
FIG 3.
Borrelia burgdorferi primer pair detections and deconvolved spectral data of PCR amplicons derived from patient B. PCR/ESI-MS was performed on serial whole blood from a patient with clinically diagnosed early Lyme disease with EM. Specific primer pairs detecting B. burgdorferi isolates are plotted over time (A), displayed as cumulative detections from the four 5-ml aliquots of 20 ml blood. Primer pairs BCT6101 (leuS) (B) and BCT3511 (gyrB) (D) simultaneously detected two genotypes of Borrelia burgdorferi on day 0. (C and E) The last time points, days 2 and 8, where one genotype of B. burgdorferi was detected for these targets. Paired peaks correspond to the forward and reverse strands of the PCR amplicons, which separate under the conditions of electrospray ionization.
In addition to PCR/ESI-MS analysis, two-tier antibody testing for B. burgdorferi infection was performed by Quest Diagnostics (Madison, NJ) from serum collected at days 0 and 21, and results were interpreted according to CDC recommendations (12) (Table 2). For all test specimens, the second-tier IgM/IgG Western blots were performed regardless of the enzyme-linked immunosorbent assay (ELISA) result. Patient B tested positive at day 0, whereas patients A and C seroconverted; however, the unusual pattern of reactivity shown by patient C suggested prior exposure to B. burgdorferi. Serological tests for patient D were negative at both day 0 and day 21 (Table 2).
TABLE 2.
B. burgdorferi two-tier serology results from patients with clinically diagnosed early Lyme disease with EM
| Patient | Day | 2-Tier resulta | ELISA | IgM bands | IgG bands |
|---|---|---|---|---|---|
| A | 0b | Neg | 0.94 | 0/3 | 1/10 |
| 21 | Pos | ≥5.00 | 2/3 | 3/10 | |
| B | 0 | Pos | ≥5.00 | 3/3 | 6/10 |
| 21 | Pos | ≥5.00 | 3/3 | 6/10 | |
| C | 0 | Neg | ≤0.90 | 0/3 | 5/10 |
| 21 | Pos | 1.18 | 0/3 | 5/10 | |
| D | 0 | Neg | ≤0.90 | 0/3 | 0/10 |
| 21 | Neg | 1.22 | 0/3 | 1/10 |
Neg, negative; Pos, positive. Results reported according to CDC recommendations (12).
Day 0, initial doctor's visit.
Unexpectedly, both patients who tested positive by PCR/ESI-MS were coinfected with more than one B. burgdorferi genotype. However, we have seen coinfecting genotypes in ticks (7, 13, 14). While unlikely, it cannot be ruled out that a second infection occurred during these two patients’ antibiotic treatments, because the study was carried out in an area where Lyme disease is endemic during tick-transmission season. Interestingly, the present work suggests that the ratios of coinfecting B. burgdorferi genotypes can change over time during antibiotic treatment. To date, few studies have focused on the ratio of genotypes in coinfections. Recently, Rynkiewicz et al. (15) showed that infection of mice with two strains of B. burgdorferi isolates resulted in similar fitness in single infections of each strain and asymmetric competition in coinfections. Moreover, two studies showed an apparent random founder effect, where some B. burgdorferi isolates dominated over others in murine models of B. burgdorferi infection (16, 17). As an alternative to the founder effect hypothesis, the host immune system or differential antibiotic susceptibility might have played a role in the observed genotypic shift (18).
Our study does have limitations, most obviously that we relied on a small convenience sample from four participants. However, our PCR/ESI-MS method used for detection of B. burgdorferi isolates has been utilized to great extent (11, 19–23), including genotyping B. burgdorferi and detecting other vector-borne pathogens from ticks and clinical specimens (7, 8, 13, 14, 24–26). An additional limitation is that we did not follow participants beyond the 21-day study period to systematically capture longer-term clinical or microbiological outcomes. This would have been of particular interest for patient A, who had detectible infection at the day 21 visit. Although not followed beyond the 21-day study period, none of the participants self-reported significant lingering subjective symptoms at the final study visit, nor did any of the participants present to us for further clinical evaluation.
Direct diagnostic tests have the advantage of being able to measure response to treatment by demonstrating clearance of the pathogen. Nucleic acids are an excellent analyte for direct diagnostic tests and are the basis of all molecular diagnostics because DNA/RNA is quickly cleared from the human body. A study measuring fetal DNA in the bloodstream of mothers carrying male fetuses before and after giving birth has shown that the mean half-life of circulating male fetal DNA in the bloodstream is 16.3 min (27). Moreover, a study where heat-killed B. burgdorferi isolates were injected under the skin of mice found that B. burgdorferi became virtually undetectable after 8 h (28). However, only culture and not the presence of B. burgdorferi nucleic acids can confirm the presence of viable organism (29). Other studies have shown that B. burgdorferi isolates are detectable by PCR in synovial fluid or synovial membranes after antibiotic treatment (30, 31), and one study found PCR positivity in plasma months after treatment (32). These findings and ours may suggest that the bacteria reside in parts of the body that are not readily cleared, and bacterial remnants may continue to leak into the circulatory system after antibiotic treatment (33).
The present work demonstrates the utility of a direct molecular test that can both detect and genotype B. burgdorferi isolates from serially collected specimens. Currently, there is no FDA-approved direct diagnostic test for Lyme disease, due to challenging low levels of B. burgdorferi isolates in clinical specimens (34). We previously demonstrated direct molecular detection of B. burgdorferi isolates in 1.25 ml of whole blood collected from patients with early Lyme disease by PCR/ESI-MS with a sensitivity of 62% (13/21) (8). To further increase sensitivity, we recently increased the blood volume to 20 ml, similar to the typical volume of blood used for bacterial culture (20 to 30 ml) (35). The continued development of a direct molecular test that can both detect and genotype B. burgdorferi isolates is paramount, not only to promptly diagnose early Lyme disease in patients, but to provide a tool for testing new antibiotics and to further our understanding of infection by B. burgdorferi genotype(s) and their impact on the human immune system and illness.
ACKNOWLEDGMENTS
This work was supported by the Bay Area Lyme Foundation and the Steven and Alexandra Cohen Foundation.
We thank Erica Mihm and Cheryl Novak for assistance with participant study visits.
M.R.M., H.E.C., S.C., C.M., D.J.E., and M.W.E. were all employees of Ibis Biosciences, an Abbott Company, which developed the PCR/ESI-MS assays and instrumentation used in these studies; assays described are for research use only. A.W.R., M.J.S., and J.N.A. have no conflicts of interest to declare.
REFERENCES
- 1.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed Lyme disease, United States, 2005–2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schutzer SE, Berger BW, Krueger JG, Eshoo MW, Ecker DJ, Aucott JN. 2013. Atypical erythema migrans in patients with PCR-positive Lyme disease. Emerg Infect Dis 19:815–817. doi: 10.3201/eid1905.120796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feder HM Jr, Whitaker DL. 1995. Misdiagnosis of erythema migrans. Am J Med 99:412–419. doi: 10.1016/S0002-9343(99)80190-9. [DOI] [PubMed] [Google Scholar]
- 4.Berger BW. 1993. Erythema migrans. Clin Dermatol 11:359–362. doi: 10.1016/0738-081X(93)90090-Y. [DOI] [PubMed] [Google Scholar]
- 5.Engstrom SM, Shoop E, Johnson RC. 1995. Immunoblot interpretation criteria for serodiagnosis of early Lyme disease. J Clin Microbiol 33:419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dressler F, Whalen JA, Reinhardt BN, Steere AC. 1993. Western blotting in the serodiagnosis of Lyme disease. J Infect Dis 167:392–400. doi: 10.1093/infdis/167.2.392. [DOI] [PubMed] [Google Scholar]
- 7.Crowder CD, Matthews HE, Schutzer S, Rounds MA, Luft BJ, Nolte O, Campbell SR, Phillipson CA, Li F, Sampath R, Ecker DJ, Eshoo MW. 2010. Genotypic variation and mixtures of Lyme Borrelia in Ixodes ticks from North America and Europe. PLoS One 5:e10650. doi: 10.1371/journal.pone.0010650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eshoo MW, Crowder CC, Rebman AW, Rounds MA, Matthews HE, Picuri JM, Soloski MJ, Ecker DJ, Schutzer SE, Aucott JN. 2012. Direct molecular detection and genotyping of Borrelia burgdorferi from whole blood of patients with early Lyme disease. PLoS One 7:e36825. doi: 10.1371/journal.pone.0036825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marques A, Telford SR 3rd, Turk SP, Chung E, Williams C, Dardick K, Krause PJ, Brandeburg C, Crowder CD, Carolan HE, Eshoo MW, Shaw PA, Hu LT. 2014. Xenodiagnosis to detect Borrelia burgdorferi infection: a first-in-human study. Clin Infect Dis 58:937–945. doi: 10.1093/cid/cit939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bose J, Kloesener MH, Schulte RD. 2016. Multiple-genotype infections and their complex effect on virulence. Zoology (Jena) 119:339–349. doi: 10.1016/j.zool.2016.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Metzgar D, Frinder MW, Rothman RE, Peterson S, Carroll KC, Zhang SX, Avornu GD, Rounds MA, Carolan HE, Toleno DM, Moore D, Hall TA, Massire C, Richmond GS, Gutierrez JR, Sampath R, Ecker DJ, Blyn LB. 2016. The IRIDICA BAC BSI assay: rapid, sensitive and culture-independent identification of bacteria and Candida in blood. PLoS One 11:e0158186. doi: 10.1371/journal.pone.0158186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). 1995. Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morb Mortal Wkly Rep 44:590–591. [PubMed] [Google Scholar]
- 13.Honig V, Carolan HE, Vavruskova Z, Massire C, Mosel MR, Crowder CD, Rounds MA, Ecker DJ, Ruzek D, Grubhoffer L, Luft BJ, Eshoo MW. 2017. Broad-range survey of vector-borne pathogens and tick host identification of Ixodes ricinus from Southern Czech Republic. FEMS Microbiol Ecol 93. doi: 10.1093/femsec/fix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eshoo MW, Carolan HE, Massire C, Chou DM, Crowder CD, Rounds MA, Phillipson CA, Schutzer SE, Ecker DJ. 2015. Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread Anaplasmataceae species. PLoS One 10:e0135828. doi: 10.1371/journal.pone.0135828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rynkiewicz EC, Brown J, Tufts DM, Huang CI, Kampen H, Bent SJ, Fish D, Diuk-Wasser MA. 2017. Closely-related Borrelia burgdorferi (sensu stricto) strains exhibit similar fitness in single infections and asymmetric competition in multiple infections. Parasit Vectors 10:64. doi: 10.1186/s13071-016-1964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Troy EB, Lin T, Gao L, Lazinski DW, Camilli A, Norris SJ, Hu LT. 2013. Understanding barriers to Borrelia burgdorferi dissemination during infection using massively parallel sequencing. Infect Immun 81:2347–2357. doi: 10.1128/IAI.00266-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rego RO, Bestor A, Stefka J, Rosa PA. 2014. Population bottlenecks during the infectious cycle of the Lyme disease spirochete Borrelia burgdorferi. PLoS One 9:e101009. doi: 10.1371/journal.pone.0101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sicklinger M, Wienecke R, Neubert U. 2003. In vitro susceptibility testing of four antibiotics against Borrelia burgdorferi: a comparison of results for the three genospecies Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto. J Clin Microbiol 41:1791–1793. doi: 10.1128/JCM.41.4.1791-1793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metzgar D, Frinder M, Lovari R, Toleno D, Massire C, Blyn LB, Ranken R, Carolan HE, Hall TA, Moore D, Hansen CJ, Sampath R, Ecker DJ. 2013. Broad-spectrum biosensor capable of detecting and identifying diverse bacterial and Candida species in blood. J Clin Microbiol 51:2670–2678. doi: 10.1128/JCM.00966-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bacconi A, Richmond GS, Baroldi MA, Laffler TG, Blyn LB, Carolan HE, Frinder MR, Toleno DM, Metzgar D, Gutierrez JR, Massire C, Rounds M, Kennel NJ, Rothman RE, Peterson S, Carroll KC, Wakefield T, Ecker DJ, Sampath R. 2014. Improved sensitivity for molecular detection of bacterial and Candida infections in blood. J Clin Microbiol 52:3164–3174. doi: 10.1128/JCM.00801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofstadler SA, Sannes-Lowery KA, Hannis JC. 2005. Analysis of nucleic acids by FTICR MS. Mass Spectrom Rev 24:265–285. doi: 10.1002/mas.20016. [DOI] [PubMed] [Google Scholar]
- 22.Ecker DJ, Sampath R, Li H, Massire C, Matthews HE, Toleno D, Hall TA, Blyn LB, Eshoo MW, Ranken R, Hofstadler SA, Tang YW. 2010. New technology for rapid molecular diagnosis of bloodstream infections. Expert Rev Mol Diagn 10:399–415. doi: 10.1586/erm.10.24. [DOI] [PubMed] [Google Scholar]
- 23.Deyde VM, Sampath R, Garten RJ, Blair PJ, Myers CA, Massire C, Matthews H, Svoboda P, Reed MS, Pohl J, Klimov AI, Gubareva LV. 2010. Genomic signature-based identification of influenza A viruses using RT-PCR/electro-spray ionization mass spectrometry (ESI-MS) technology. PLoS One 5:e13293. doi: 10.1371/journal.pone.0013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eshoo MW, Crowder CD, Carolan HE, Rounds MA, Ecker DJ, Haag H, Mothes B, Nolte O. 2014. Broad-range survey of tick-borne pathogens in southern Germany reveals a high prevalence of Babesia microti and a diversity of other tick-borne pathogens. Vector Borne Zoonotic Dis 14:584–591. doi: 10.1089/vbz.2013.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crowder CD, Matthews HE, Rounds MA, Li F, Schutzer SE, Sampath R, Sampath R, Hofstadler SA, Ecker DJ, Eshoo MW. 2012. Detection of heartworm infection in dogs via PCR amplification and electrospray ionization mass spectrometry of nucleic acid extracts from whole blood samples. Am J Vet Res 73:854–859. doi: 10.2460/ajvr.73.6.854. [DOI] [PubMed] [Google Scholar]
- 26.Eshoo MW, Crowder CD, Li H, Matthews HE, Meng S, Sefers SE, Sampath R, Stratton CW, Blyn LB, Ecker DJ, Tang YW. 2010. Detection and identification of Ehrlichia species in blood by use of PCR and electrospray ionization mass spectrometry. J Clin Microbiol 48:472–478. doi: 10.1128/JCM.01669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo YM, Zhang J, Leung TN, Lau TK, Chang AM, Hjelm NM. 1999. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 64:218–224. doi: 10.1086/302205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarus JJ, McCarter AL, Neifer-Sadhwani K, Wooten RM. 2012. ELISA-based measurement of antibody responses and PCR-based detection profiles can distinguish between active infection and early clearance of Borrelia burgdorferi. Clin Dev Immunol 2012:138069. doi: 10.1155/2012/138069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer R, Mukherjee P, Wang K, Simons J, Wormser GP, Schwartz I. 2013. Detection of Borrelia burgdorferi nucleic acids after antibiotic treatment does not confirm viability. J Clin Microbiol 51:857–862. doi: 10.1128/JCM.02785-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, McHugh G, Damle N, Sikand VK, Glickstein L, Steere AC. 2011. Burden and viability of Borrelia burgdorferi in skin or joints, of patients with erythema migrans or Lyme arthritis. Arthritis Rheum 63:2238–2247. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priem S, Burmester GR, Kamradt T, Wolbart K, Rittig MG, Krause A. 1998. Detection of Borrelia burgdorferi by polymerase chain reaction in synovial membrane, but not in synovial fluid from patients with persisting Lyme arthritis after antibiotic therapy. Ann Rheum Dis 57:118–121. doi: 10.1136/ard.57.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Picha D, Moravcova L, Vanousova D, Hercogova J, Blechova Z. 2014. DNA persistence after treatment of Lyme borreliosis. Folia Microbiol (Praha) 59:115–125. doi: 10.1007/s12223-013-0272-4. [DOI] [PubMed] [Google Scholar]
- 33.Wormser GP, Nadelman RB, Schwartz I. 2012. The amber theory of Lyme arthritis: initial description and clinical implications. Clin Rheumatol 31:989–994. doi: 10.1007/s10067-012-1964-x. [DOI] [PubMed] [Google Scholar]
- 34.Wilske B, Fingerle V, Schulte-Spechtel U. 2007. Microbiological and serological diagnosis of Lyme borreliosis. FEMS Immunol Med Microbiol 49:13–21. doi: 10.1111/j.1574-695X.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 35.Murray PR, Masur H. 2012. Current approaches to the diagnosis of bacterial and fungal bloodstream infections in the intensive care unit. Crit Care Med 40:3277–3282. doi: 10.1097/CCM.0b013e318270e771. [DOI] [PMC free article] [PubMed] [Google Scholar]