Abstract
Activating mutations in B-RAF and N-RAS occur in ~60 and ~15% of melanomas, respectively. The most common mutation in B-RAF is V600E, which activates B-RAF and the downstream MEK-ERK1/2 pathway. Thus, B-RAFV600E is a viable therapeutic target. PLX4720 is a selective inhibitor of mutant B-RAF and its analog, PLX4032, is currently undergoing clinical trials in melanoma. However, the effects of PLX4720 across the genotypic spectrum in melanoma remain unclear. Here, we describe that PLX4720 treatment rapidly induces hyperactivation of the MEK-ERK1/2 pathway in mutant N-RAS melanoma cells. Furthermore, we demonstrate that C-RAF is the major RAF isoform involved in this process. Importantly, PLX4720-induced hyperactivation of the MEK-ERK1/2 pathway promotes resistance to apoptosis in both non-invasive and invasive mutant N-RAS melanoma cells but does not enhance cell cycle properties. These findings underscore the need to genotypically stratify melanoma patients before enrollment on a mutant B-RAF inhibitor trial.
Keywords: apoptosis, B-RAF, N-RAS, PLX4720
RAF serine/threonine kinases initiate a signaling cascade downstream of the GTPase RAS to activate the MEK-ERK1/2 pathway. Three RAF isoforms exist: A-RAF, B-RAF and C-RAF. In melanoma, mutations in B-RAF occur in approximately 60% of melanomas (Davies et al., 2002), with valine to glutamic acid substitution at amino acid 600 (B-RAFV600E) being the most common. V600E is located within the active site of B-RAF and results in a constitutively active kinase (Davies et al., 2002; Wan et al., 2004). Activating mutations in N-RAS are detected in approximately 15% of melanomas but are mutually exclusive from B-RAFV600E mutations (Albino et al., 1989; Davies et al., 2002). Both oncogenic B-RAF and N-RAS enhance MEK-ERK1/2 activity, which in turn regulates transcriptional events and phosphorylation of proteins involved in proliferation, apoptosis and cell migration/ invasion (Hunger-Glaser et al., 2004; Boisvert-Adamo and Aplin, 2008; Pratilas et al., 2009).
Based on increasing evidence of oncogene addiction in melanoma (Solit et al., 2006), efforts are focused on targeted-based therapies. In this regard, PLX4720 was developed by a structure-guided approach to bind within the RAF selective-pocket and close to the ATP-binding site (Tsai et al., 2008). PLX4720 inhibits B-RAFV600E with tenfold lower IC50 than wild-type B-RAF and leads to growth arrest in mutant B-RAF melanoma cells (Tsai et al., 2008). In an early-stage clinical trial, a structural analog of PLX4720, PLX4032, has shown promising results in the majority of patients harboring B-RAFV600E mutations (Flaherty et al., 2009; Sondak and Smalley, 2009). However, effects of PLX4032/4720 in non-mutant B-RAF melanomas remain unclear. In this study, we demonstrate that treating non-mutant B-RAF melanoma cells with PLX4720 results in a rapid induction of the MEK-ERK1/2 pathway. This process is largely dependent on C-RAF and enhances resistance to apoptosis of mutant N-RAS, non-invasive and invasive melanomas.
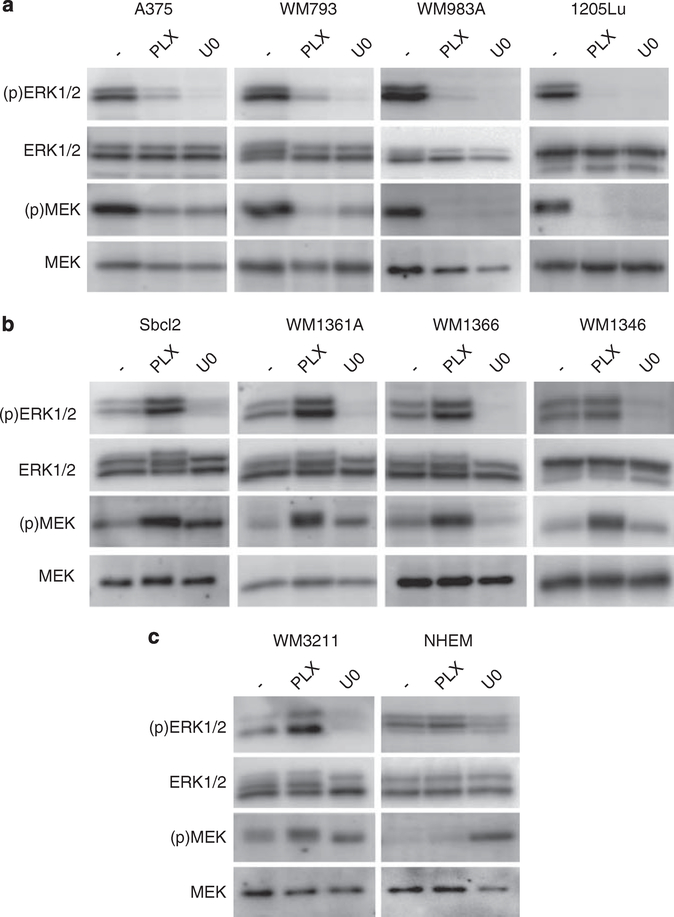
Consistent with previous reports (Conner et al., 2003; Tsai et al., 2008), the selective mutant B-RAF inhibitor, PLX4720 and the MEK inhibitor, U0126, effectively blocked MEK and ERK1/2 activation in mutant B-RAF harboring vertical growth and metastatic melanoma cell lines, A375, WM793, WM983A and 1205Lu (Figure 1a). By contrast, treatment of mutant N-RAS melanoma cell lines, Sbcl2, WM1361A, WM1366 and WM1346, resulted in a dramatic increase in MEK and ERK1/2 phosphorylation (Figure 1b). Similar effects were observed in mutant N-RAS SK-MEL-2 cells (data not shown). Paradoxical effects of RAF inhibitors on the ERK1/2 pathway have been previously described. ZM 336372, a potent in vitro C-RAF kinase inhibitor activated C-RAF in Swiss 3T3 fibroblasts (Hall-Jackson et al., 1999). Additionally, SB-590885, a B-RAF inhibitor activated ERK1/2 in human melanocytes (King et al., 2006). As structural analogs of PLX4720 are in phase II trials, our current findings in melanoma cells have added clinical importance. PLX4720 treatment also enhanced MEK and ERK1/2 phosphorylation in WM3211 cells, which are wild type for both B-RAF and N-RAS (Figure 1c). In these cells, the enhanced MEK and ERK1/2 phosphorylation may be promoted by autocrine growth factor activation of RAS (Satyamoorthy et al., 2003). Similar to mutant B-RAF melanomas, ERK1/2 phosphorylation was inhibited by the MEK inhibitor, U0126, in non-mutant B-RAF cells (Figures 1b and c). By contrast, treatment of primary human melanocytes with PLX4720 did not affect MEK–ERK1/2 signaling (Figure 1c).
Figure 1.
Treatment of mutant N-RAS melanoma cell lines induces MEK-ERK1/2 signaling. (a) Mutant B-RAF cell lines A375, WM793, WM983A and 1205Lu (all B-RAFV600E); (b) Mutant N-RAS cell lines Sbcl2, WM1361A, WM1366 and WM1346; and (c) wild-type B-RAF/N-RAS WM3211 cells and neonatal human melanocytes (NHEM) were cultured, as previously described (Satyamoorthy et al., 1997; Conner et al., 2003; Tsai et al., 2008). All cell lines were treated with DMSO (−), 1 μM PLX4720 (PLX) (Plexxikon) and 10 μM U0126 (U0) (Cell Signaling Technology, Danvers, MA, USA) for 16 h. Lysates were analyzed by western blotting as previously described using a Versadoc Imaging system (BioRad, Hercules, CA, USA) (Boisvert-Adamo and Aplin, 2006). Antibodies for ERK1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA); MEK1, phospho-ERK (Thr202/Tyr204) ((p)ERK1/2), phospho-MEK1/2 (Ser217/Ser221) ((p)MEK) (Cell Signaling Technology).
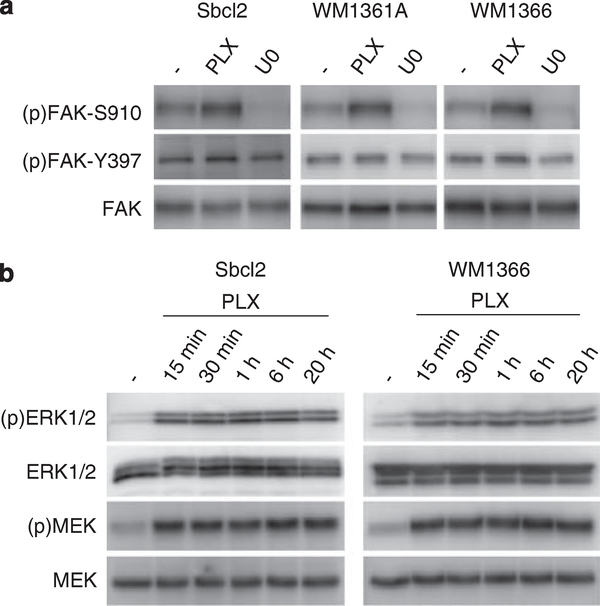
To determine if downstream targets of ERK1/2 were altered, we analyzed the serine 910 phosphorylation of focal adhesion kinase (FAK) (Hunger-Glaser et al., 2004). PLX4720 treatment enhanced FAK serine 910 phosphorylation in mutant N-RAS melanoma cells, whereas no effect on tyrosine 397 phosphorylation was detected (Figure 2a). Consistent with effects on ERK1/2, the MEK inhibitor, U0126, decreased FAK serine 910 phosphorylation. We next examined the kinetics of MEK-ERK1/2 hyperactivation in mutant N-RAS cells. In both Sbcl2 and WM1366, enhanced activation of MEK and ERK1/2 was detected within 15 min of PLX4720 treatment and the activation remained constant throughout the 20 h time course (Figure 2b). This rapid effect argues against transcriptional control of an ERK1/2 pathway component/regulator. We next tested if downstream events correlated with the kinetics of ERK1/2 activation. Within 15 min of PLX4720 treatment, increases in both FAK serine 910 and RSK serine 380 phosphorylation were observed in WM1366 time course lysates, which correlated with the activation of MEK and ERK1/2 (Supplementary Figure 1). Together, these data demonstrate that PLX4720 treatment of mutant N-RAS and wild-type N-RAS/B-RAF melanoma cell lines leads to enhanced activation of the MEK-ERK1/2 pathway.
Figure 2.
PLX4720-induced MEK-ERK1/2 hyperactivation occurs rapidly and regulates FAK serine 910 phosphorylation. (a) Sbcl2, WM1361A and WM1366 cell lines were treated with DMSO (−), 1 μM PLX4720 or 10 μM U0126 for 16 h. Lysates were analyzed by western blotting (Boisvert-Adamo and Aplin, 2006) using antibodies for (p)FAK-S910 (Biosource Int, Camarillo, CA, USA), (p)FAK-Y397 (Bd Biosciences, San Jose, CA, USA), and total FAK (Cell Signaling Technology). (b) Sbcl2 and WM1366 cells were treated with 1 μM PLX4720 for 15 min to 20 h. Lysates were analyzed by western blot analysis for (p)MEK, MEK, (p)ERK1/2 and ERK1/2.
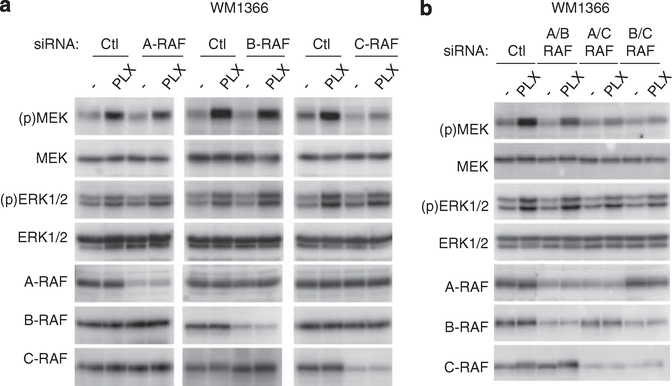
To determine the involvement of different RAF isoforms in PLX4720-induced hyperactivation of MEK-ERK1/2, we selectively depleted A-RAF, B-RAF or C-RAF from WM1366 cells using small interference RNAs. Individual C-RAF knockdown reduced the PLX4720-induced hyperphosphorylation of MEK and ERK1/2, whereas A-RAF or B-RAF knockdown elicited no effect (Figure 3a). RAF isoenzymes form both homodimers and heterodimers (Wan et al., 2004; Rushworth et al., 2006; Ritt et al., 2010). To test if multiple RAF isoenzymes contributed to PLX4720-induced MEK-ERK1/2 activation, WM1366 cells were transfected with different combinations of small interference RNAs targeting the RAF isoenzymes. Compared with the control small interference RNA, knockdown of A-RAF plus B-RAF did not affect the PLX4720-induced hyperphosphorylation of MEK and ERK1/2 (Figure 3b). Depletion of either A-RAF and C-RAF or B-RAF and C-RAF dramatically inhibited the hyperphosphorylation of MEK and ERK1/2 by PLX4720 (Figure 3b). These data indicate that the PLX4720-induced hyperactivation of MEK-ERK1/2 predominately occurs through C-RAF.
Figure 3.
Hyperactivation of MEK-ERK1/2 occurs predominately through C-RAF. (a) WM1366 cells were transfected using Oligofectamine (Invitrogen, Carlsbad, CA, USA) with 25 nM siRNAs for control, A-RAF, B-RAF and C-RAF (Dharmacon, Lafayette, CO, USA). The siRNA sequences (5′ to 3′) are: control-UAGCGACUAAACACAUCAAUU; A-RAF-ACCGAGAU CUCAAGUCUAA; B-RAF-ACAGAGACCUCAAGAGUAAUU; and C-RAF-GCACGGAGAUGUUGCAGUA. After 72 h, cells were treated with either DMSO (−) or 1 μM PLX4720 for 1 h. Lysates were analyzed by western blot analysis using antibodies for A-RAF, B-RAF, C-RAF (Santa Cruz Biotechnology), (p)MEK, MEK, (p)ERK1/2 and ERK1/2. (b) WM1366 cells were transfected with different combinations of siRNA and processed as in a.
The dependency of PLX4720-induced hyperactivation of MEK-ERK1/2 on C-RAF is consistent with mutant N-RAS melanomas primarily activating ERK1/2 through C-RAF (Dumaz et al., 2006). RAF isoforms heterodimerize (Wan et al., 2004; Rushworth et al., 2006; Ritt et al., 2010). C-RAF-B-RAF combinations have been shown to exhibit the highest level of RAF activity and activity was maintained when C-RAF dimerized with kinase-dead B-RAF (Rushworth et al., 2006). RAF inhibitors have been shown to induce low levels of C-RAF-B-RAF heterodimers leading to enhanced C-RAF activity (Heidorn et al., 2010).
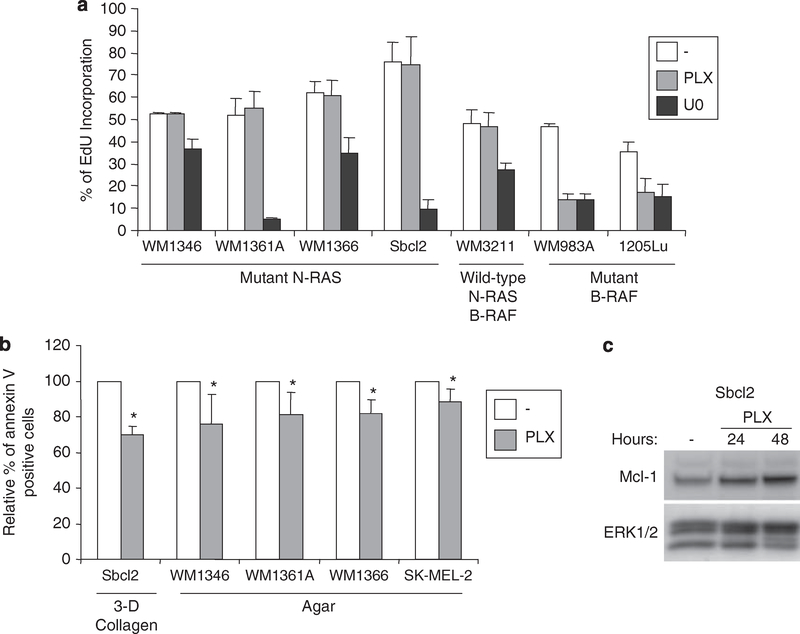
Activated MEK-ERK1/2 signaling in melanoma contributes to cell cycle progression and resistance to anoikis (Karasarides et al., 2004; Boisvert-Adamo and Aplin, 2006). Treatment of mutant N-RAS melanoma cells with PLX4720 did not alter the expression of the G1 to S regulator, cyclin D1, in mutant N-RAS melanoma cells, although PLX4720 efficiently inhibited cyclin D1 expression in a mutant B-RAF line (Supplementary Figure 2a). Furthermore, PLX4720 did not enhance incorporation of EdU, a thymidine analog in mutant N-RAS cells (Figure 4a). We next examined the kinetics of several key G1 to S cell cycle regulators in PLX4720-treated Sbcl2 and WM1366 cells. Treatment with PLX4720 did not alter the expression of cyclin D1, p21Cip1or p27Kip1 in either cell line throughout the experimental time course (Supplementary Figure 2b). Our data is consistent with previous reports showing PLX4720 does not alter cell proliferation of mutant N-RAS melanoma and thyroid carcinoma cells (Tsai et al., 2008; Salerno et al., 2010).
Figure 4.
PLX4720 promotes resistance to anoikis in mutant N-RAS cells. (a) Mutant N-RAS cells: WM1346, WM1361A, WM1366 and Sbcl2; wild type for both N-RAS and B-RAF cells, WM3211; and mutant B-RAF cells: WM983A and 1205Lu were treated with 1 μM PLX4720 or 10 μM U0126 for 16 h. 10 μM 5-ethynyl-2′-deoxyuridine (EdU) was added to the media for 8 hs and processed using the Click-iT™ EdU Flow Cytometry protocol (Invitrogen). The quantitation of mean −/+ s.d. from three independent experiments is shown. (b) Serum starved Sbcl2 cells were embedded in 0.8 mg/ml 3-D collagen gels (Organogenesis Inc, Canton, MA, USA) and cultured at 37 °C for 48 h in serum-free medium containing either DMSO (−) or 1 μM PLX4720. After 48 h, cells were released from gels with 1 mg/ml collagenase (Sigma-Aldrich, St Louis, MO, USA) and stained with annexin V-APC (BD Biosciences). Serum starved WM1346, WM1361A, wM1366 and SK-Mel-2 (ATCC) cells were seeded onto agar and cultured at 37 °C for 48 h in serum-free medium containing either DMSO (−) or 1 μM PLX4720 and cultured at 37 °C for 72 to 96 h. Cells were harvested and stained with annexin V-APC. Apoptosis was analyzed by flow cytometry on the FACSCalibur (BD Biosciences) and data analyzed with Flowjo software (Three Star, Inc, Ashland, OR, USA). The quantitation of mean −/+ s.d. from four independent experiments and statistical analysis of the data was performed using two sample, assuming unequal variances Student’s t-test. *P < 0.05 vs DMSO control for each cell lines tested. (c) Sbcl2 cells embedded in 3-D collagen were treated with 1 μM PLX4720 for 24 or 48 h. Cells were released from collagen, lysed, and cell lysates were analyzed by western blotting for levels of Mcl-1 (BD Biosciences) and total ERK1/2.
In apoptosis assays, early stage Sbcl2 cells were embedded in 3-D type I collagen to mimic the dermal microenvironment and treated with either DMSO or PLX4720 for 48 h. Compared with the DMSO control, treatment of Sbcl2 cells with PLX4720 resulted in a significant decrease in annexin V staining (Figure 4b). We have recently implicated Mcl-1 as a key pro-survival Bcl-2 family member in resistance to anoikis (Boisvert-Adamo et al., 2009). Upregulation of Mcl-1 was detected in PLX4720-treated SbCl2 cells embedded in 3-D type I collagen (Figure 4c). By contrast, invasive cells were highly resistant to apoptosis in 3-D collagen and treatment with PLX4720 led to a barely detectable decrease in annexin V staining (data not shown). To test invasive mutant N-RAS melanoma cell lines in conditions that mimic resistance to anoikis, WM1346, WM1361A, WM1366 and SK-MEL-2 cells were seeded in suspension (on agar) and treated with either DMSO or PLX4720 for 72–96 h. In these conditions, treatment with PLX4720 led to significant decreases in annexin V staining in all the invasive cell lines tested (Figure 4b). Together, these data indicate that PLX4720 treatment enhances resistance to apoptosis in mutant N-RAS melanomas.
During peer review of this manuscript, several reports were published demonstrating that RAF inhibitors, including PLX4720, activate ERK1/2 in mutant RAS cells (Halaban et al., 2010; Hatzivassiliou et al., 2010; Heidorn et al., 2010; Poulikakos et al., 2010). In these studies, a panel of RAF inhibitors promoted the formation of either B-RAF/C-RAF heterodimers or C-RAF/C-RAF homodimers. Within the dimer, C-RAF is transactivated by the interacting B-RAF/C-RAF partner that is bound by the RAF inhibitor. We have also shown that activation of MEK/ERK1/2 by PLX4720 occurs through C-RAF. Consistent with our data showing that phosphorylation of FAK and RSK is increased following PLX4720 treatment of mutant N-RAS cells, Halaban et al. (2010), showed that early response genes such as FOS and JUNB, which are also downstream ERK1/2 targets, are enhanced by PLX4032. Our data demonstrating that PLX4720 treatment enhances levels of the pro-survival protein, Mcl-1, and protects mutant N-RAS melanoma cells from anoikis indicates that PLX4720-treatment could promote tumor progression of mutant N-RAS cells. Notably, we did not observe an increase in G1 to S-phase progression or changes in cell cycle regulators with melanoma cells treated with PLX4720, although Halaban et al. 2010 demonstrated that PLX4032 enhances proliferation of growth-factor dependent, B-RAF wild-type melanoma cells (Halaban et al., 2010).
Taken together, our data suggest that PLX4720 may promote tumor progression in mutant N-RAS cells, at least in part, by inhibiting apoptosis. As mutant N-RAS and B-RAFV600E are mutually exclusive in melanoma, these findings highlight the need to genotypically stratify melanoma patients before starting a mutant B-RAF inhibitor regimen. Furthermore, they may represent a mechanistic basis for the prevalence of keratoacanthomas and squamous cell carcinomas in RAF-inhibitor-treated patients (Sondak and Smalley, 2009).
Supplementary Material
Acknowledgements
We thank Dr Gideon Bollag (Plexxikon) for providing PLX4720, Dr Meenhard Herlyn (Wistar Institute) for cell lines and Dr Michele Weiss for comments on the manuscript. This work was supported by National Institutes of Health Grants (R01-GM067893 and R01-CA125103), and Pennsylvania Department of Health (AF0301) to AE Aplin.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Albino AP, Nanus DM, Mentle IR, Cordon-Cardo C, McNutt NS, Bressler J et al. (1989). Analysis of ras oncogenes in malignant melanoma and precursor lesions: correlation of point mutations with differentiation phenotype. Oncogene 4: 1363–1374. [PubMed] [Google Scholar]
- Boisvert-Adamo K, Aplin AE . (2006). B-RAF and PI-3 kinase signaling protect melanoma cells from anoikis. Oncogene 25: 4848–4856. [DOI] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Aplin AE. (2008). Mutant B-RAF mediates resistance to anoikis via Bad and Bim. Oncogene 27: 3301–3312. [DOI] [PubMed] [Google Scholar]
- Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. (2009). Mcl-1 is required for melanoma resistance to anoikis. Mol Cancer Res 7: 549–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner SR, Scott G, Aplin AE. (2003). Adhesion-dependent activation of the ERK1/2 cascade is by-passed in melanoma cells. J Biol Chem 278: 34548–34554. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S et al. (2002). Mutations of the BRAF gene in human cancer. Nature 417: 949–954. [DOI] [PubMed] [Google Scholar]
- Dumaz N, Hayward R, Martin J, Ogilvie L, Hedley D, Curtin JA et al. (2006). In melanoma, RAS mutations are accompanied by switching signaling from BRAF to CRAF and disrupted cyclic AMP signaling. Cancer Res 66: 9483–9491. [DOI] [PubMed] [Google Scholar]
- Flaherty K, Puzanov I, Sosman J, Kim K, Ribas A, McArthur G et al. (2009). Phase I study of PLX4032: proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol 27: Abstract 9000. [Google Scholar]
- Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S et al. (2010). PLX4032, a selective BRAF V600E kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF WT melanoma cells. Pigment Cell & Melanoma Research 23: 190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Jackson CA, Eyers PA, Cohen P, Goedert M, Tom Boyle F, Hewitt N et al. (1999). Paradoxical activation of Raf by a novel Raf inhibitor. Chemistry & Biology 6: 559–568. [DOI] [PubMed] [Google Scholar]
- Hatzivassiliou G, Song K, Yen I, Brandhuber BJ, Anderson DJ, Alvarado R et al. (2010). RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 464: 431–435. [DOI] [PubMed] [Google Scholar]
- Heidorn SJ, Milagre C, Whittaker S, Nourry A, Niculescu-Duvas I, Dhomen N et al. (2010). Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 140: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunger-Glaser I, Fan RS, Perez-Salazar E, Rozengurt E. (2004). PDGF and FGF induce focal adhesion kinase (FAK) phosphorylation at Ser-910: dissociation from Tyr-397 phosphorylation and requirement for ERK activation. J Cell Physiol 200: 213–222. [DOI] [PubMed] [Google Scholar]
- Karasarides M, Chiloeches A, Hayward R, Niculescu-Duvaz D, Scanlon I, Friedlos F et al. (2004). B-RAF is a therapeutic target in melanoma. Oncogene 23: 6292–6298. [DOI] [PubMed] [Google Scholar]
- King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY et al. (2006). Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res 66: 11100–11105. [DOI] [PubMed] [Google Scholar]
- Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. (2010). RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 464: 427–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB et al. (2009). V600EBRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA 106: 4519–4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritt DA, Monson DM, Specht SI, Morrison DK. (2010). Impact of feedback phosphorylation and Raf heterodimerization on normal and mutant B-Raf signaling. Mol Cell Biol 30: 806–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth LK, Hindley AD, O’Neill E, Kolch W. (2006). Regulation and Role of Raf-1/B-Raf heterodimerization. Mol Cell Biol 26: 2262–2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE et al. (2010). Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab 95: 450–455. [DOI] [PubMed] [Google Scholar]
- Satyamoorthy K, DeJesus E, Linnenbach AJ, Kraj B, Kornreich DL, Rendle S et al. (1997). Melanoma cell lines from different stages of progression and their biological and molecular analyses. Melanoma Res 7: S35–S42. [PubMed] [Google Scholar]
- Satyamoorthy K, Li G, Gerrero MR, Brose MS, Volpe P, Weber BL et al. (2003). Constitutive mitogen-activated protein kinase activation in melanoma is mediated by both BRAF mutations and autocrine growth factor stimulation. Cancer Res 63: 756–759. [PubMed] [Google Scholar]
- Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A et al. (2006). BRAF mutation predicts sensitivity to MEK inhibition. Nature 439: 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondak VK, Smalley K. (2009). Targeting mutant BRAF and KIT in metastatic melanoma: ASCO 2009 meeting report. Pigment Cell Melanoma Res 22: 386–387. [DOI] [PubMed] [Google Scholar]
- Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S et al. (2008). Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 105: 3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan PT, Garnett MJ, Roe SM, Lee S, Niculescu-Duvaz D, Good VM et al. (2004). Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 116: 855–867. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.