INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) represents a spectrum of liver disorders, including the initial stage of simple fat accumulation (hepatic steatosis); followed by inflammatory changes leading to nonalcoholic steatohepatitis (NASH); and, finally, fibrosis and scaring resulting in liver cirrhosis and its consequences.1
The current prevalence of NAFLD is estimated to be 20% to 30%,2,3 and it has become the second most common indication for liver transplantation in the United States, after chronic hepatitis C.4 It is projected that over the next 15 years, NASH will become the most common disease cause for liver transplantation.5 This exponential increase in the incidence and progression of NASH is attributed to the worldwide epidemic of obesity,6 which has also resulted in an increase in the prevalence of other obesity-related disorders, including metabolic syndrome, type 2 diabetes mellitus (DM), cardiovascular disease, and obstructive sleep apnea (OSA).7
OSA is caused by complete or partial obstruction of the upper airway. This results in repetitive episodes of shallow or paused breathing during sleep and causes a reduction in blood oxygen saturation. This nocturnal hypoxia, or chronic intermittent hypoxia (CIH), is the most important factor linking OSA and NAFLD.8–10 Recent studies have conclusively shown the role of OSA in the development and progression of NAFLD in terms of liver enzyme elevation and histologic alterations (steatosis, lobular inflammation, ballooning degeneration, and fibrosis).11–19 This article discusses the pathologic mechanisms associating OSA with NAFLD and the impact of OSA treatment on NAFLD outcomes.
OBSTRUCTIVE SLEEP APNEA
OSA is a common clinical condition in which the throat narrows or collapses repeatedly during sleep, resulting in episodes of intermittent oxygen desaturations and nocturnal awakenings.20,21 It is estimated to be present in 4% to 5% of the general population and is seen twice as commonly in men as in women. Advancing age, male gender, obesity, neck thickness, craniofacial changes, and upper airway soft tissue abnormalities are important risk factors for OSA.20–22
The direct consequences of airway collapse are snoring; increased respiratory efforts; hypercapnia; and, most importantly, CIH. This hypoxemia is sensed by carotid body receptors, leading to sympathetic activation; arousal; clearing of the airway; and, eventually, reoxygenation. The cycle of deoxygenation and reoxygenation is repeated several times every night and results in increased catecholamine release, reactive oxygen species (ROS) generation, oxidative stress, and a state of systemic inflammation. Lack of a restorative night sleep also results in excessive daytime sleepiness; morning headaches; concentration difficulties; anxiety; depression; road-traffic accidents; and, in general, a poor quality of life.20,21,23 OSA is also associated with hypertension, atherosclerosis, coronary artery disease, stroke, insulin resistance, and NAFLD.24–27
Polysomnography is the gold standard test for diagnosing OSA and involves recording of several physiologic parameters, including electroencephalogram, electrooculogram, and electromyogram, along with nasal and oral airflow measurements.28 An episode of apnea is defined by cessation of airflow for greater than 10 seconds despite ongoing inspiratory effort; whereas an episode of hypopnea is defined by greater than 50% reduction in airflow or moderate airflow reduction (<50%) along with either desaturation or electroencephalographic evidence of awakening. The severity of sleep apnea is characterized by the apnea-hypopnea index (AHI), which is simply calculated by dividing the number of events by number of hours of sleep. Accordingly, OSA can be classified as mild (AHI: 5–15), moderate (15–30), and severe (>30).29 Continuous positive airway pressure (CPAP) is the first-line treatment for OSA. It results in more restful sleep, reduced daytime symptoms, and improved quality of life.30 However, the effect of CPAP therapy on other chronic conditions, including metabolic syndrome and NAFLD, is less clear (see later discussion).
PATHOGENESIS OF NONALCOHOLIC STEATOHEPATITIS
Two-Hit Hypothesis
Berson and colleagues31 conducted a pivotal study, during which rat liver mitochondria and rat hepatocytes were exposed to a hepatotoxic drug 4,4′-diethylaminoethoxyhexestrol. This resulted in hepatic steatosis and inhibition of mitochondrial β-oxidation. Inhibition of mitochondrial respiration caused reduced adenosine triphosphate (ATP) levels and raised levels of ROS. The increased oxidative stress resulted in lipid peroxidation and subsequent cell death.
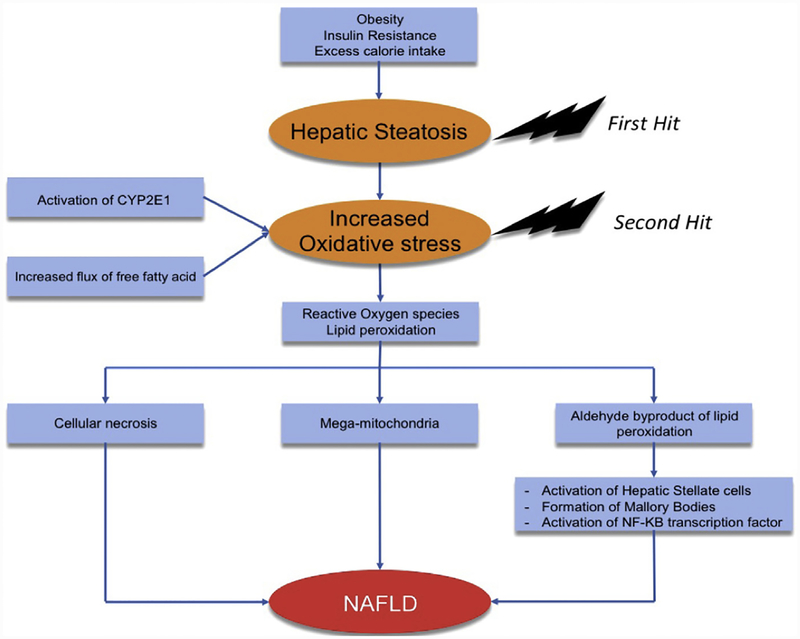
Day and James32 proposed the popular 2-hit hypothesis (Fig. 1) of steatohepatitis based on this study.31 The first hit represents hepatic steatosis, which could be related to factors such as excess caloric intake, obesity, or insulin resistance. A subsequent second hit, in the form of an oxidative stress, increased lipid peroxidation, and activation of inflammatory cascade, causes progression to steatohepatitis and fibrosis.
Fig. 1.
Two-hit hypothesis. CYP2E1, cytochrome P450 2E1; NF-κB, in nuclear factor-κβ.
Multiple-hit hypothesis
Although the 2-hit hypothesis remains extremely popular and is often cited, emerging data show that it is too simplistic to explain the complex interplay of the multiple factors involved in the development of NASH. An alternative a multiple-hit hypothesis has been proposed that attempts to take into account several of the underlying mechanisms that may contribute to the pathogenesis of NASH.33,34
Genetic predisposition, environmental factors, and dietary habits lead to development of obesity, metabolic syndrome, and insulin resistance. Insulin resistance is a key factor in the progression of NAFLD because it not only leads to increased peripheral lipolysis with increased flux of free fatty acids (FFAs) but also to hepatic de novo lipogenesis (DNL). It also causes adipose tissue dysfunction with altered secretion of adipokines and increased levels of inflammatory cytokines, interleukin (IL)-6, and tumor necrosis factor (TNF)-α.35 Alteration of gut microbiome causes increased gut permeability, systemic levels of lipopolysaccharides, and absorption of FFAs.17
All of these factors cause an increased flux of FFAs into the liver. This results in excess triglyceride (TG) deposition in the liver (hepatic steatosis) that parallels the generation of lipotoxic metabolites of FFAs. Further, these toxic metabolites cause mitochondrial dysfunction with increased oxidative stress, generation of ROS, and endoplasmic reticulum stress, which manifests in the form of hepatocyte injury and inflammation. Hence, TG accumulation in the hepatocyte is just an innocent bystander or an epiphenomenon in NAFLD pathogenesis, with the toxic metabolites of FFAs being the major mechanism for hepatotoxicity.33,34
LINK BETWEEN OBSTRUCTIVE SLEEP APNEA AND NONALCOHOLIC FATTY LIVER DISEASE
Several studies have firmly established the relationship between OSA and NAFLD in adult and pediatric populations (Table 1). The severity of sleep apnea and, particularly, its manifestation, CIH, is the most important trigger for increased oxidative stress, generation of ROS, and release of inflammatory cytokines, resulting systemic inflammation that drives the exacerbation of NAFLD and progression to liver fibrosis.10 CIH causes reduced oxygen tension in the liver, particularly in the hepatocytes surrounding the central vein (zone 3) and results in the expression of hypoxia inducible factors (HIFs), which are the key oxygen sensors that mediate the ability of the cell to respond to a hypoxic environment. HIFs are implicated in the development of dyslipidemia, hepatic steatosis, insulin resistance, and liver fibrosis, and are a key link in the association of OSA and NAFLD.10,50
Table 1.
Human studies assessing the correlation between obstructive sleep apnea and nonalcoholic fatty liver disease
| Reference | Type of Study (Subject Population [n]); Country | NAFLD Assessment | OSA Diagnosis | Important Findings |
|---|---|---|---|---|
| Tanne et al,36 2005 | PSG (163); France | LFT or liver biopsy | PSG | Severe OSA is associated with elevation of liver enzymes, insulin resistance, and steatohepatitis. |
| Kallwitz et al,37 2007 | Bariatric surgery (85); USA | Liver biopsy | PSG (AHI ≥15/h) | OSA was associated with elevated ALT levels and a trend toward histologic evidence of progressive liver disease (inflammation and fibrosis). |
| Polotsky et al,18 2009 | Bariatric surgery (90); USA | LFT or liver biopsy | PSG | Oxygen desaturation >4.6% was associated with 1.5-fold increase in insulin resistance. Significant desaturations may predispose to steatohepatitis. |
| Daltro et al,15 2010 | Bariatric surgery (40); Brazil | Liver biopsy | PSG | OSA (AHI ≥15/h) was associated with insulin resistance but not with the severity of NASH. |
| Aron-Wisnewsky et al,8 2012 | Bariatric surgery (101); France | Liver biopsy | ODI by nocturnal oximetry | CIH was independently associated with hepatic fibrosis, fibroinflammation, and NAFLD activity score. |
| Turkay et al,27 2012 | PSG (71); Turkey | LFT, ultrasound | PSG | AHI, ODI, lowest desaturation values, and percentage of sleep duration with SpO2 <90% were independent predictors of NAFLD. |
| Corey et al,38 2013 | Bariatric surgery (159); USA | Liver biopsy | Electronic medical record | Absence of OSA was associated with normal liver histology in subjects undergoing bariatric surgery. |
| Mir et al,39 2013 | Population-based study, NHANES database. (NAFLD cases = 1572, controls = 8969); USA | LFT | Sleep Disorders Questionnaire | NAFLD was associated with sleep apnea (OR = 1.39, 0.98–1.97) |
| Minville et al,40 2014 | PSG (226); France | SteatoTest, Nash Test, and FibroTest | PSG | On multivariate analysis, nocturnal cumulative time spent at <90% of oxygen saturation was associated with hepatic steatosis but not with NASH. |
| Sundaram et al,41 2014 | Obese children aged 10–18 y (25); USA | Liver biopsy or LFT | PSG | Severity of hypoxemia and OSA were associated with transaminitis and advanced liver histology. |
| Nobili et al,17 2014 | Children with NAFLD undergoing PSG (65); Italy | LFT or liver biopsy | PSG | Severity of OSA was independently associated with presence of NASH, significant fibrosis, and NAFLD activity score. |
| Lin et al,42 2015 | NAFLD subjects undergoing sleep apnea assessment (85); China | Ultrasound | PSG | ODI and average O2 saturation were independently associated with elevated ALT and AST, respectively. |
| Agrawal et al,43 2015 | Subjects from liver clinic with NAFLD and chest clinic with OSA (123); India | Ultrasound, Fibroscan |
PSG | OSA severity was an independent predictor of significant hepatic fibrosis in NAFLD subjects. |
| Corey et al,14 2015 | Bariatric surgery (213); USA | Liver biopsy | PSG | OSA was independently associated with transaminitis, NASH, and advanced liver histology. |
| Alkhouri et al,44 2015 | Obese children evaluated for OSA (58); USA | Circulating markers of hepatic apoptosis or inflammation | PSG | Circulating markers of apoptosis and macrophage activation were significantly increased in obese children with OSA. Treatment reduced markers of macrophage activation. |
| Petta et al,45 2015 | NAFLD subjects with elevated ALT, undergoing OSA assessment (126); Italy | LFT, liver biopsy | Cardiorespiratory polygraph |
Prevalence of OSA was higher in subjects with F2–F4 fibrosis. Significant fibrosis was associated with nocturnal oxygen saturation (SaO2) <95%. |
| Cakmak et al,46 2015 | NAFLD subject undergoing OSA assessment (137); Turkey | Ultrasound | PSG | AHI and ODI were significantly higher in subjects with moderate and severe NAFLD. A strong association was noted between reduction in lowest O2 saturation and increase in NAFLD severity. |
| Benotti et al,13 2016 | Bariatric surgery (362); USA | Liver biopsy | PSG | Severity of OSA was associated with NAFLD liver histology. |
| Qi et al,47 2016 | Nonobese subjects undergoing PSG and abdominal ultrasound (175); China | Ultrasound | PSG | In nonobese subjects, lowest oxygen saturation was independently associated with NAFLD. |
| Trzepizur et al,19 2016 | Multisite cross-sectional study (1285); France | Hepatic steatosis index, LFT, FibroMeter | PSG or home sleep test | Risk of hepatic steatosis increased with severity of OSA and sleep-related hypoxemia. |
| Asfari et al,48 2017 | Cross-sectional study using NIS database (OSA subjects = 1490150, non-OSA = 29,222 374); USA | ICD-9-CM | ICD-9-CM | OSA subjects were 3 times more likely to have NASH compared with subjects without OSA. |
| Ding et al,49 2018 | Subjects with suspected apnea undergoing OSA and NAFLD assessment (415); China | LFT and ultrasound | PSG | Percentage of total sleep time spent with oxygen saturation of <90%, lowest oxygen saturation, and insulin resistance were associated with NAFLD. |
Abbreviations: ICD-9-CM, international classification of diseases, ninth revision, clinical modification; LFT, liver function test; NHANES, National Health And Nutrition Examination Survey; NIS, national inpatient sample; ODI, oxygen desaturation index; PSG, polysomnography.
Obstructive Sleep Apnea and Dyslipidemia
Increased de novo lipogenesis
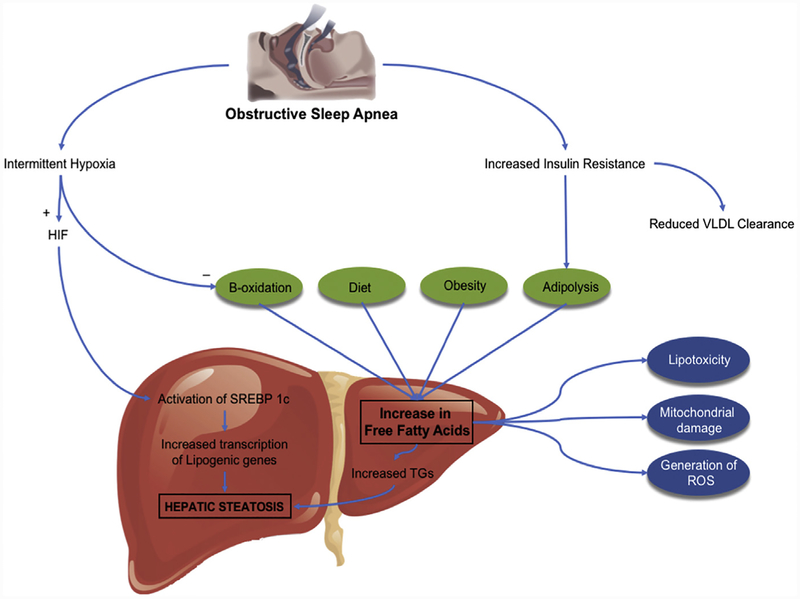
Entry of lipid substrates in the liver occurs through 1 of the following 3 mechanisms:(1) dietary intake of lipids and carbohydrates, (2) de novo lipogenesis, or (3) flux of FFAs from peripheral lipolysis, whereas liver disposes lipids through (1) storage as TGs, (2) oxidation of FAAs, or (3) export of TGs as very-low density lipoproteins (VLDLs) in the peripheral blood (Fig. 2).51 Studies have identified several regulators that control hepatic lipid metabolism. Sterol receptor element-binding protein (SREBP) is a transcription factor that plays a vital role in hepatic DNL.51,52 It consists of 3 isoforms, of which SREBP-1c is predominantly expressed in liver. It mediates the expression of lipogenic genes such as fatty acid synthase and acyl-CoA carboxylase and thereby promotes de novo FFAs and TG synthesis.55 It also increases stearoylcoenzyme-A desaturase (SCD)-1 activity that is responsible for converting polyun-saturated fatty acids into monounsaturated fatty acids (MUFAs). MUFAs are converted into cholesterol esters and TGs, which are incorporated into secreted particles.53
Fig. 2.
Increased de novo lipogenesis. SREBP, sterol receptor element-binding protein.
CIH, a major component of OSA, is independently associated with dyslipidemia in NAFLD.53,54 Studies in mice have shown that exposure to CIH results in enhanced expression of previously mentioned lipogenic genes, resulting in higher TG content in the liver.55 On the contrary, interruption of SREBP-1 signaling and depletion of SCD-1 prevents hyperlipidemia during CIH.56,57 In a study by Li and colleagues,58 under hypoxic conditions, protein levels of nuclear isoforms of SREBP-1 and SCD-1 were significantly lower in mice with partial deficiency of HIF-1α compared with wild-type mice. As a result, mice with partial deficiency of HIF-1α were protected against hypertriglyceridemia and hepatic fat accumulation during CIH. HIF-1α is a master regulator of metabolic responses to hypoxia and these data confirm that CIH increases lipogenesis through the mediation of HIF-1α.
Enhanced peripheral lipolysis and reduced lipoprotein clearance
CIH raises sympathetic activity and induces a state of insulin resistance. This promotes lipolysis in the adipose tissue and increased flux of FFAs in the liver. Under normoxia conditions, FFAs are metabolized by oxygen-dependent mitochondrial combustion through β-oxidation. Hence hypoxia creates a condition of excess FFAs and its reduced utilization through mitochondrial β-oxidation. More FFAs become available for TG and cholesterol synthesis, which eventually results in fatty liver, liver injury through oxidative stress, and NASH. CIH has also been shown to selectively inactivate the adipose tissue lipoprotein lipase and reduce the clearance of VLDL from circulation.59 In summary, CIH can cause dyslipidemia by upregulating DNL and lipoprotein secretion, and reducing lipoprotein clearance, along with enhanced peripheral lipolysis and influx of FFAs in the liver.
Obstructive Sleep Apnea and Insulin Resistance
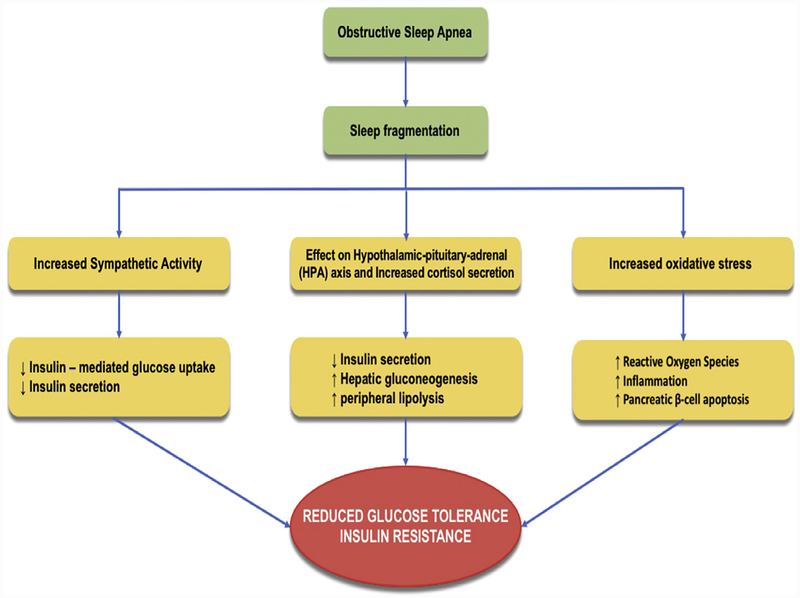
OSA is associated with CIH and sleep fragmentation, and an increasing pool of evidence now points toward an association between OSA, insulin resistance, and predisposition to type 2 DM (Fig. 3). In a study by Stamatakis and Punjabi,60 sleep was experimentally fragmented across all stages using auditory and mechanical stimuli in healthy normal volunteers. After 2 nights of sleep fragmentation, they were noted to have reduced insulin sensitivity and glucose effectiveness with an elevated morning cortisol level and increased sympathetic tone. Increase in cortisol levels61 and raised sympathetic tone62,63 are well known to promote insulin resistance by reduction of insulin secretion from the pancreas, inhibition of insulin-mediated glucose uptake, and increased hepatic gluconeogenesis. In another study, selective suppression of slow-wave sleep in young healthy adults showed similar results. Sensitivity to insulin was markedly reduced without adequate compensatory increase in insulin release, leading to reduced glucose tolerance and increased DM risk.64 In a study by Ip and colleagues,65 270 consecutive subjects referred for suspected sleep apnea and no underlying type 2 DM were included and tested for insulin resistance. OSA was associated with insulin resistance as measured by HOMA-IR, independent of body mass index, and progressive increase in insulin resistance was noted per each additional apnea or hypopnea per sleep hour. Mouse models of OSA have further implicated the role of ROS,66 pancreatic beta cell apoptosis,67 and inflammation68 in the development of insulin resistance and predisposition to type 2 DM.60 Hence, several human and animal studies establish a robust association between OSA and insulin resistance.
Fig. 3.
OSA and insulin resistance.
Insulin resistance is known to play a crucial role in the pathogenesis of NAFLD. Studies have shown increased adipose tissue and hepatic insulin resistance, and reduced whole-body sensitivity to insulin in NAFLD patients. These are manifested by increased peripheral lipolysis, impaired inhibition of hepatic gluconeogenesis, and reduced glucose disposal, respectively. Further, inability of the insulin to suppress peripheral lipolysis results in increased flux of FFAs to liver and contributes to hepatic DNL. Insulin resistance leads to a state of hyperinsulinemia, which stimulates lipogenic genes via SREBP-1c and further contributes to hepatic steatosis. Overall, hepatic DNL, increased flux of FFAs, and impaired mitochondrial oxidation of FFAs creates a perfect milieu for development and progression of NAFLD.
Obstructive Sleep Apnea and Adipose Tissue Dysfunction
Traditionally considered an inert tissue for pure energy storage, it now clear that adi-pose tissue is a major endocrine and a signaling organ. During obesity, hypertrophied adipocytes mediate inflammation and harbor an increased proportion of proinflammatory macrophages compared with the antiinflammatory type. Secretion of adiponectin, which mediates a protective role in NAFLD by improving insulin sensitivity and regulating fatty acid oxidation, is reduced. In contrast, the release of proinflammatory cytokines such as TNF-α and IL-6 is increased, which reduces hepatic insulin sensitivity. Peripheral lipolysis is also increased, along with the flux of FFAs to the liver, which further potentiates hepatic and muscle insulin resistance.69–71 It has been conclusively proven in animal models that during obesity adipose tissue is hypoxic and the local adipose tissue hypoxia is responsible for the dysregulated production of adipokines and metabolic syndrome.69
Because inflammation and hypoxia play crucial roles in obesity-mediated adipose tissue dysfunction, CIH, as it occurs in OSA, can be postulated to further intensify adipose tissue dysfunction. To this effect, various animal studies and models have conclusively established the role of intermittent hypoxia (IH) in inducing adipose tissue inflammation and dysfunction, even in the absence of obesity.70,72 In a recent study by Taylor and colleagues,73 human adipocytes exposed to IH showed increase in nuclear factor-κβ DNA-binding activity compared with controls. There was also a significant increase in the secretion of inflammatory cytokines such as IL-8, IL-6, and TNF-α with IH in adipocytes. Hence, it was concluded that human adipocytes are sensitive to IH, which enhances the expression of inflammatory genes and the release of inflammatory cytokines. Overall, these data provide evidence that IH can mediate adipose tissue dysfunction and the release of proinflammatory adipokines, which are known to be involved in pathogenesis of NAFLD. However, further studies are required in humans to understand the exact underlying mechanisms of IH, especially the impact of obesity.
Obstructive Sleep Apnea and Mitochondrial Dysfunction
Mitochondria are responsible for the production of 95% the cellular energy source, ATP. Under aerobic conditions, mitochondria produce ATP via 3 main biochemical pathways: the tricarboxylic acid cycle or Krebs cycle, oxidative phosphorylation, and fatty acid β-oxidation.74 In OSA, hypoxia, along with an increased oxidative stress and flux of FAAs, overwhelm these normal mechanisms and result in structural and functional alteration of the mitochondria. Structural alteration is characterized by depletion of mitochondrial DNA75 and upregulated transcriptional and replication machinery of mitochondrial biogenesis.76 Increased levels of TNF-α, ROS, and lipid peroxidation products alter the mitochondrial respiratory chain, block the flow of electrons in the respiratory chain, and increase the mitochondrial ROS formation. The resultant oxidative stress further activates inflammatory pathways, contributing to hepatocytes inflammation and the diverse hepatic lesions of NASH.77
Obstructive Sleep Apnea and Liver Fibrosis
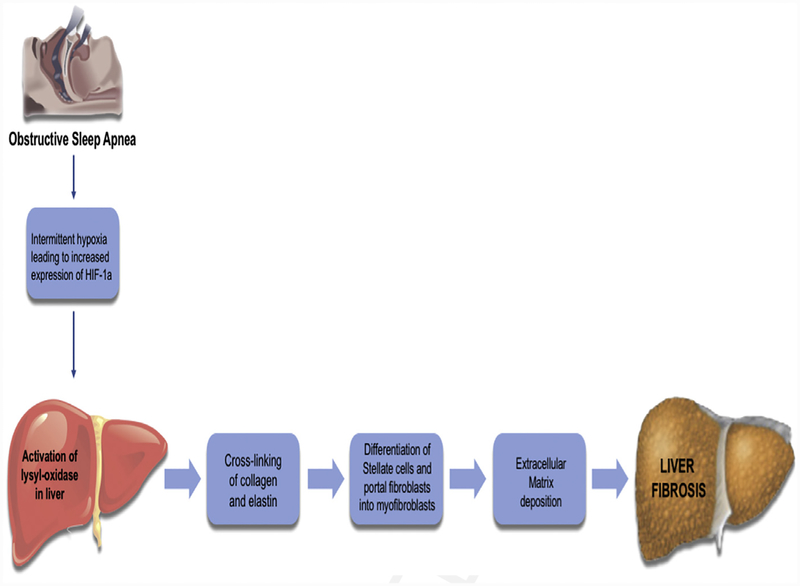
Stellate cells and portal fibroblasts are important sources of fibrillar collagen and lysyl oxidase (LOX) enzymes in the normal liver and after early hepatic injury (Fig. 4).78 Hypoxia is a potent stimulator of LOX activity, which in turn plays an important role in the covalent cross-linking of collagen and elastin, increasing liver stiffness.79 This increased stiffness causes increased mechanical tension that is crucial for the differentiation of hepatic stellate cell and portal fibroblasts into myofibroblasts, which are responsible for deposition of extracellular collagen and, eventually, the development of fibrosis.78 Mesarwi and colleagues80 have recently demonstrated that serum LOX is elevated in patients with NAFLD-associated hepatic fibrosis, relative to those without fibrosis. These same investigators also proposed the potential role of serum LOX as a biomarker of liver fibrosis in patients with severe obesity and OSA. HIF-1α has also been independently implicated in the development of liver fibrosis in a mouse model of NAFLD.81 Hence, it can be concluded that hypoxia induces HIF-1α, which in turn induces the expression of LOX enzyme and the subsequent development of fibrosis.
Fig. 4.
OSA and liver fibrosis.
Continuous positive airway pressure treatment and impact on nonalcoholic fatty liver disease
CPAP was originally described in 1983 by Sullivan and colleagues82 and is considered the gold standard therapy for moderate to severe OSA. CPAP therapy acts as a pneumatic support, causing the pharyngeal intraluminal pressure to exceed the surrounding pressure. It also stabilizes the upper airway by increasing the end-expiratory lung volume, thereby preventing the hypoxic events related to the upper airway collapse.83 Studies have conclusively established the benefits CPAP therapy in decreasing the hypoxic events and daytime sleepiness, lowering the risk of motor-vehicle accidents, and improving hypertension and a better quality of life in general.84 However, studies have yielded conflicting results about the efficacy of CPAP therapy on metabolic syndrome, including insulin resistance, lipid profile, and body fat composition.85,86
Given that CIH plays a vital role in mediation of NAFLD in OSA, treatment with CPAP would be expected to yield unequivocal benefits in NAFLD patients. However, the available studies have yielded mixed results and are listed in Table 2 and Table 3.80,87–102 In these studies, the impact of CPAP therapy on NAFLD was assessed by means of improvements in liver enzymes, hepatic adiposity, or fibrosis. Importantly, the observational studies that demonstrated the benefits of CPAP (see Table 2) were of longer duration than the randomized controlled trials that did not show CPAP to be beneficial (see Table 3). Progression of liver fibrosis from 1 stage to another takes an average of 7 years in patients with NASH and 14 years in those with NAFLD.103 Hence it is likely that studies of longer duration will be required to demonstrate the importance of OSA in the pathogenesis of NAFLD and the benefits of CPAP in its treatment. Increasing data indicate that CPAP should be considered as an integral component in the management off NAFLD patients with moderate to severe OSA and that more than 3 months of treatment with appropriate compliance is needed to notice any significant improvements in NAFLD parameters (see Table 2). As mentioned earlier, development of NAFLD requires multiple-hits and aberrations in multiple metabolic pathways. Hence, its effective management also needs a multi-modal approach with paramount emphasis on diet and lifestyle modification, and weight loss, with CPAP being an essential element in those with NAFLD and moderate to severe OSA.104
Table 2.
Studies showing beneficial impact of continuous positive airway pressure in subjects with obstructive sleep apnea and nonalcoholic fatty liver disease
| Reference | Study Design | Study Population; Country | Duration of CPAP Therapy | Findings |
|---|---|---|---|---|
| Impact on liver enzymes | ||||
| Chin et al,87 2003 | Prospective cohort study | 40 men; Japan | 6 mo | CPAP therapy had beneficial effects on serum aminotransferase abnormalities in obese OSA subjects. |
| Shpirer et al,88 2010 | Prospective cohort study | 11 subjects; Israel | 3 y | Significant reduction in liver enzyme levels and improvement in the mean liver attenuation index in CPAP subjects (n = 6) compared with control group (n = 5). |
| Hobzova et al,89 2015 | Prospective cohort study | 179 subjects; Czech Republic | 13 mo | Significant positive effect on liver enzymes in subjects with moderate to severe OSA. |
| Kim et al,90 2018a | Retrospective cohort study | 351 subjects; California | 6 mo | CPAP treatment was associated with significant biochemical improvement in liver enzymes. |
| Chen et al,93,101 2018 | Prospective cohort study | 160 subjects; China | 3 mo | CPAP therapy was significantly associated with improvement of ALT and AST levels. |
| Sundaram et al,100 2018 | Prospective cohort study | 9 subjects; USA | 3 mo | CPAP treatment reduces alanine aminotransferase, metabolic syndrome markers, and F (2)-isoprostanes. |
| Chen et al,93,101 2018 | Meta-analysis | 5 studies | — | CPAP was associated with a statistically significant decrease in the liver enzyme levels in OSA subjects. |
| Impact on liver steatosis | ||||
| Yoshiro et al,94 2014 | Retrospective cohort study | 61 male subjects; Japan | 31 mo | In male OSA subjects with abdominal obesity, significant decrease in liver fat content was observed after long-term CPAP therapy, only when fatty liver was present at baseline. |
| Buttacavoli et al,95 2016 | Observational study | 15 subjects (3 at 6 mo and 15 at 12 mo follow-up) | 6 and/or 12 mo | Long-term CPAP treatment may improve liver steatosis. |
| Impact on fibrosis | ||||
| Mesarwi et al,80 2015 | Prospective cohort study | 35 subjects; Brazil | 3 mo | A reduction in serum LOX (an enzyme that cross-links collagen and can serve as a biomarker of hepatic fibrosis) was seen in OSA subjects on CPAP. |
| Hang et al,99 2017 | Retrospective cohort study | Propensity-matched 5214 subjects; Taiwan | 2–10 y | CPAP plays an important role in the delay of the progression of liver disease in OSA subjects and decreases the incidence of liver disease among these groups of subjects. |
| Kim et al,90 2018a | Retrospective cohort study | 351 subjects; California | 6 mo | CPAP treatment was associated with reduction in NAFLD-related fibrosis. |
Represents same study with findings in 2 different headings.
Table 3.
Studies showing no significant impact of continuous positive airway pressure in subjects with obstructive sleep apnea and nonalcoholic fatty liver disease
| Reference | Study Design | Study Population; Country | Duration of CPAP | Findings |
|---|---|---|---|---|
| Impact on liver enzymes | ||||
| Kohler et al,91 2009 | Randomized controlled trial | 94 subjects; United Kingdom | 1 mo | CPAP therapy did not improve biochemical markers of potential NAFLD in OSA subjects. |
| Sivam et al,92 2012a | Randomized controlled trial | 27 subjects; Australia | 2 mo | No significant differences were observed in liver enzymes except ALP. |
| Impact on liver steatosis | ||||
| Sivam et al,92 2012a | Randomized controlled trial | 27 subjects; Australia | 2 mo | CPAP treatment did not change the adipose tissue distribution in the liver. |
| Hoyos et al,96 2012 | Randomized controlled trial | 65 men; Australia | 12 wk | No significant reduction in liver adiposity was observed with CPAP therapy. |
| Kritikou et al,97 2013 | Randomized controlled trial | 42 subjects; USA | 2 mo | Short-term CPAP treatment did not affect intrahepatic adiposity. |
| Impact on fibrosis | ||||
| Jullian-Desayes et al,98 2016 | Randomized controlled trial | 103 subjects; France | 6–12 wk | CPAP therapy did not demonstrate any significant impact on reduction of steatosis, NASH and liver fibrosis. |
| Labarca et al,102 2018 | Meta-analysis | 5 randomized controlled trials | — | CPAP treatment did not significantly contribute to the improvement in liver histology, liver steatosis, liver fibrosis, and aminotransferase levels. |
Represents same study with findings in 2 different headings.
SUMMARY
CIH is an important risk factor in the pathogenesis of NAFLD in patients with moderate to severe OSA. Reduced oxygen tension induces HIFs, which are implicated in the development of dyslipidemia, hepatic steatosis, insulin resistance, and liver fibrosis, and are a key link in the association of OSA and NAFLD. Given that several metabolic pathways are involved in its pathogenesis, a multipronged approach to NAFLD management is required, with emphasis on weight loss and lifestyle modification. The role of CPAP in the management of NAFLD is yet to be established firmly; however, adequate duration of CPAP therapy with appropriate compliance are important to notice any significant improvements in NAFLD parameters.
KEY POINTS.
Chronic intermittent hypoxia (CIH) is the most important factor linking obstructive sleep apnea (OSA) and nonalcoholic fatty liver disease (NAFLD).
CIH results in a state of systemic inflammation, increased oxidative stress, insulin resistance, and dyslipidemia, predisposing to various manifestations on NAFLD.
Even though the 2-hit theory has been a popular hypothesis to explain the pathogenesis of NAFLD, current evidence points toward a multiple-hit hypothesis involving complex interplay of environmental and dietary factors, role of insulin resistance, adipose tissue dysfunction, and altered gut microbiota in genetically predisposed subjects.
The role of continuous positive airway pressure (CPAP) in the management of NAFLD is yet to be established firmly.
In general, a multifaceted approach to NAFLD with emphasis on diet, life style modification, and weight loss is required, along with sufficiently longer duration of CPAP therapy and appropriate compliance in those with NAFLD and moderate to severe OSA.
Acknowledgments
Funded in part by NIH grant U01 DK061732.
Footnotes
Disclosure Statement: The authors have nothing to disclose.
REFERENCES
- 1.Ratziu V, Bellentani S, Cortez-Pinto H, et al. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010;53:372–84. [DOI] [PubMed] [Google Scholar]
- 2.Angulo P Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221–31. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64(1):73–84. [DOI] [PubMed] [Google Scholar]
- 4.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10: 330–44. [DOI] [PubMed] [Google Scholar]
- 5.Parikh ND, Marrero WJ, Wang J, et al. Projected increase in obesity and non-alcoholic-steatohepatitis-related liver transplantation waitlist additions in the United States. Hepatology 2017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 6.Ogden CL, Carroll MD, Flegal KM. Prevalence of obesity in the United States. JAMA 2014;312(2):189–90. [DOI] [PubMed] [Google Scholar]
- 7.Kopelman PG. Obesity as a medical problem. Nature 2000;404(6778):635–43. [DOI] [PubMed] [Google Scholar]
- 8.Aron-Wisnewsky J, Minville C, Tordjman J, et al. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol 2012;56(1):225–33. [DOI] [PubMed] [Google Scholar]
- 9.Dewan NA, Nieto FJ, Somers VK. Intermittent hypoxemia and OSA: implications for comorbidities. Chest 2015;147(1):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aron-Wisnewsky J, Clement K, Pépin JL. Nonalcoholic fatty liver disease and obstructive sleep apnea. Metabolism 2016;65(8):1124–35. [DOI] [PubMed] [Google Scholar]
- 11.Jin S, Jiang S, Hu A. Association between obstructive sleep apnea and non-alcoholic fatty liver disease: a systematic review and meta-analysis. Sleep Breath 2018;22(3):841–51. [DOI] [PubMed] [Google Scholar]
- 12.Sundaram SS, Halbower A, Pan Z, et al. Nocturnal hypoxia induced oxidative stress promotes progression of pediatric nonalcoholic fatty liver disease. J Hepatol 2016;65(3):560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benotti P, Wood GC, Argyropoulos G, et al. Impact of obstructive sleep apnea on nonalcoholic fatty liver disease in patients with severe obesity. Obesity (Silver Spring) 2016;24:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corey KE, Misdraji J, Gelrud L, et al. Obstructive sleep apnea is associated with nonalcoholic steatohepatitis and advanced liver histology. Dig Dis Sci 2015; 60(8):2523–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daltro C, Cotrim HP, Alves E, et al. Nonalcoholic fatty liver disease associated with obstructive sleep apnea: just a coincidence? Obes Surg 2010;20(11): 1536–43. [DOI] [PubMed] [Google Scholar]
- 16.Nobili V, Alisi A, Cutrera R, et al. Altered gut liver axis and hepatic adiponectin expression in OSAS: novel mediators of liver injury in pediatric non-alcoholic fatty liver. Thorax 2015;70(8):769–81. [DOI] [PubMed] [Google Scholar]
- 17.Nobili V, Cutrera R, Liccardo D, et al. Obstructive sleep apnea syndrome affects liver histology and inflammatory cell activation in pediatric nonalcoholic fatty liver disease, regardless of obesity/insulin resistance. Am J Respir Crit Care Med 2014;189(1):66–76. [DOI] [PubMed] [Google Scholar]
- 18.Polotsky VY, Patil SP, Savransky V, et al. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med 2009; 179(3):228–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trzepizur W, Boursier J, Mansour Y, et al. Association between severity of obstructive sleep apnea and blood markers of liver injury. Clin Gastroenterol Hepatol 2016;14(11):1657–61. [DOI] [PubMed] [Google Scholar]
- 20.Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. Eur J Intern Med 2012;23(7):586–93. [DOI] [PubMed] [Google Scholar]
- 21.Lévy P, Kohler M, McNicholas WT, et al. Obstructive sleep apnea syndrome. Nat Rev Dis Primers 2015;1:15015. [DOI] [PubMed] [Google Scholar]
- 22.Strollo PJ Jr, Rogers RM. Obstructive sleep apnea. N Engl J Med 1996;334: 99–104. [DOI] [PubMed] [Google Scholar]
- 23.Azagra-Calero E, Espinar-Escalona E, Barrera-Mora JM, et al. Obstructive sleep apnea syndrome (OSAS). Review of the literature. Med Oral Patol Oral Cir Bucal 2012;17(6):e925–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jean-Louis G, Zizi F, Clark LT, et al. Obstructive sleep apnea and cardiovascular disease: role of the metabolic syndrome and its components. J Clin Sleep Med 2008;4(3):261–72. [PMC free article] [PubMed] [Google Scholar]
- 25.Punjabi NM, Shahar E, Redline S, et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. Am J Epidemiol 2004;160:521. [DOI] [PubMed] [Google Scholar]
- 26.Togeiro SM, Carneiro G, Ribeiro Filho FF, et al. Consequences of obstructive sleep apnea on metabolic profile: a Population-Based Survey. Obesity (Silver Spring) 2013;21:847. [DOI] [PubMed] [Google Scholar]
- 27.Türkay C, Ozol D, Kasapoğlu B, et al. Influence of obstructive sleep apnea on fatty liver disease: role of chronic intermittent hypoxia. Respir Care 2012;57:244. [DOI] [PubMed] [Google Scholar]
- 28.American Academy of Sleep Medicine. International classification of sleep disorders. 3rd edition Darien (IL): American Academy of Sleep Medicine; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep 1999;22:667–89. [PubMed] [Google Scholar]
- 30.Giles TL, Lasserson TJ, Smith BJ, et al. Continuous positive airways pressure for obstructive sleep apnea in adults. Cochrane Database Syst Rev 2006;(1):CD001106. [DOI] [PubMed] [Google Scholar]
- 31.Berson A, De Beco V, Lettéron P, et al. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology 1998;114(4):764–74. [DOI] [PubMed] [Google Scholar]
- 32.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology 1998; 114(4):842–5. [DOI] [PubMed] [Google Scholar]
- 33.Buzzetti E, Pinzani M, Tsochatzis EA. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 2016;65(8):1038–48. [DOI] [PubMed] [Google Scholar]
- 34.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology 2010;52(2):774–88. [DOI] [PubMed] [Google Scholar]
- 35.Guilherme A, Virbasius JV, Puri V, et al. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanne F, Gagnadoux F, Chazouilleres O, et al. Chronic liver injury during obstructive sleep apnea. Hepatology 2005;41:1290–6. [DOI] [PubMed] [Google Scholar]
- 37.Kallwitz ER, Herdegen J, Madura J, et al. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol 2007;41(10):918–21. [DOI] [PubMed] [Google Scholar]
- 38.Corey KE, Misdraji J, Zheng H, et al. The absence of obstructive sleep apnea may protect against non-alcoholic fatty liver in patients undergoing bariatric surgery. PLoS One 2013;8(5):e62504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mir HM, Stepanova M, Afendy H, et al. Association of sleep disorders with nonalcoholic fatty liver disease (NAFLD): a population-based study. J Clin Exp Hepatol 2013;3(3):181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minville C, Hilleret MN, Tamisier R, et al. Nonalcoholic fatty liver disease, nocturnal hypoxia, and endothelial function in patients with sleep apnea. Chest 2014;145(3):525–33. [DOI] [PubMed] [Google Scholar]
- 41.Sundaram SS, Sokol RJ, Capocelli KE, et al. Obstructive sleep apnea and hypoxemia are associated with advanced liver histology in pediatric nonalcoholic fatty liver disease. J Pediatr 2014;164(4):699–706.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin QC, Chen LD, Chen GP, et al. Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath 2015; 19(1):273–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agrawal S, Duseja A, Aggarwal A, et al. Obstructive sleep apnea is an important predictor of hepatic fibrosis in patients with nonalcoholic fatty liver disease in a tertiary care center. Hepatol Int 2015;9(2):283–91. [DOI] [PubMed] [Google Scholar]
- 44.Alkhouri N, Kheirandish-Gozal L, Matloob A, et al. Evaluation of circulating markers of hepatic apoptosis and inflammation in obese children with and without obstructive sleep apnea. Sleep Med 2015;16(9):1031–5. [DOI] [PubMed] [Google Scholar]
- 45.Petta S, Marrone O, Torres D, et al. Obstructive sleep apnea is associated with liver damage and atherosclerosis in patients with non-alcoholic fatty liver disease. PLoS One 2015;10(12):e0142210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cakmak E, Duksal F, Altinkaya E, et al. Association between the severity of nocturnal hypoxia in obstructive sleep apnea and non-alcoholic fatty liver damage. Hepat Mon 2015;15(11):e32655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qi JC, Huang JC, Lin QC, et al. Relationship between obstructive sleep apnea and nonalcoholic fatty liver disease in nonobese adults. Sleep Breath 2016; 20(2):529–35. [DOI] [PubMed] [Google Scholar]
- 48.Asfari MM, Niyazi F, Lopez R, et al. The association of nonalcoholic steatohepatitis and obstructive sleep apnea. Eur J Gastroenterol Hepatol 2017;29(12): 1380–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding H, Huang JF, Xie HS, et al. The association between glycometabolism and nonalcoholic fatty liver disease in patients with obstructive sleep apnea. Sleep Breath 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shin MK, Drager LF, Yao Q, et al. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1α. PLoS One 2012;7(10):e46562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki T, Shinjo S, Arai T, et al. Hypoxia and fatty liver. World J Gastroenterol 2014;20(41):15087–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raghow R, Yellaturu C, Deng X, et al. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab 2008;19(2):65–73. [DOI] [PubMed] [Google Scholar]
- 53.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 2010;24(5):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adedayo AM, Olafiranye O, Smith D, et al. Obstructive sleep apnea and dyslipidemia: evidence and underlying mechanism. Sleep Breath 2012;18(1):13–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Thorne LN, Punjabi NM, et al. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res 2005;97(7):698–706. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Nanayakkara A, Jun J, et al. Effect of deficiency in SREBP cleavage activating protein on lipid metabolism during intermittent hypoxia. Physiol Genomics 2007;31(2):273–80. [DOI] [PubMed] [Google Scholar]
- 57.Savransky V, Jun J, Li J, et al. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res 2008;103(10):1173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, Bosch-Marce M, Nanayakkara A, et al. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia inducible factor-1alpha. Physiol Genomics 2006;25(3):450–7. [DOI] [PubMed] [Google Scholar]
- 59.Drager LF, Li J, Shin M-K, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnea. Eur Heart J 2012;33(6):783–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stamatakis KA, Punjabi NM. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest 2010;137(1):95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Andrews RC, Walker BR. Glucocorticoids and insulin resistance: old hormones, new targets. Clin Sci (Lond) 1999;96(5):513–23. [DOI] [PubMed] [Google Scholar]
- 62.Deibert DC, DeFronzo RA. Epinephrine-induced insulin resistance in man. J Clin Invest 1980;65(3):717–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Avogaro A, Toffolo G, Valerio A, et al. Epinephrine exerts opposite effects on peripheral glucose disposal and glucose-stimulated insulin secretion. A stable label intravenous glucose tolerance test minimal model study. Diabetes 1996; 45(10):1373–8. [DOI] [PubMed] [Google Scholar]
- 64.Tasali E, Leproult R, Ehrmann DA, et al. Slow-wave sleep and the risk of type 2 diabetes in humans. Proc Natl Acad Sci U S A 2008;105(3):1044–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med 2002;165(5):670–6. [DOI] [PubMed] [Google Scholar]
- 66.Peng YJ, Yuan G, Ramakrishnan D, et al. Heterozygous HIF-1αlpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 2006;577(Pt2):705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu J, Long YS, Gozal D, et al. Beta-cell death and proliferation after intermittent hypoxia: role of oxidative stress. Free Radic Biol Med 2009;46(6):783–90. [DOI] [PubMed] [Google Scholar]
- 68.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 2005;112(17):2660–7. [DOI] [PubMed] [Google Scholar]
- 69.Hosogai N, Fukuhara A, Oshima K, et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007;56(4):901–11. [DOI] [PubMed] [Google Scholar]
- 70.Ryan S Adipose tissue inflammation by intermittent hypoxia: mechanistic link between obstructive sleep apnea and metabolic dysfunction. J Physiol 2017; 595(8):2423–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Trayhurn P, Wang B, Wood IS. Hypoxia and the endocrine and signaling role of white adipose tissue. Arch Physiol Biochem 2008;114(4):267–76. [DOI] [PubMed] [Google Scholar]
- 72.Poulain L, Thomas A, Rieusset J, et al. Visceral white fat remodeling contributes to intermittent hypoxia-induced atherogenesis. Eur Respir J 2014;43(2):513–22. [DOI] [PubMed] [Google Scholar]
- 73.Taylor CT, Kent BD, Crinion SJ, et al. Human adipocytes are highly sensitive to intermittent hypoxia induced NF-kappaB activity and subsequent inflammatory gene expression. Biochem Biophys Res Commun 2014;447(4):660–5. [DOI] [PubMed] [Google Scholar]
- 74.Sharpe AJ, McKenzie M. Mitochondrial fatty acid oxidation disorders associated with short-chain Enoyl-CoA Hydratase (ECHS1) deficiency. Cells 2018;7(6):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim YS, Kwak JW, Lee KE, et al. Can mitochondrial dysfunction be a predictive factor for oxidative stress in patients with obstructive sleep apnea? Antioxid Redox Signal 2014;21(9):1285–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lacedonia D, Carpagnano GE, Crisetti E, et al. Mitochondrial DNA alteration in obstructive sleep apnea. Respir Res 2015;16(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol 2005;42: 928–40. [DOI] [PubMed] [Google Scholar]
- 78.Kagan HM. Lysyl oxidase: mechanism, regulation and relationship to liver fibrosis. Pathol Res Pract 1994;190(9–10):910–9. [DOI] [PubMed] [Google Scholar]
- 79.Liu SB, Ikenaga N, Peng ZW, et al. Lysyl oxidase activity contributes to collagen stabilization during liver fibrosis progression and limits spontaneous fibrosis reversal in mice. FASEB J 2016;30(4):1599–609. [DOI] [PubMed] [Google Scholar]
- 80.Mesarwi OA, Shin MK, Drager LF, et al. Lysyl oxidase as a serum biomarker of liver fibrosis in patients with severe obesity and obstructive sleep apnea. Sleep 2015;38(10):1583–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mesarwi OA, Shin MK, Bevans-Fonti S, et al. Hepatocyte hypoxia inducible factor-1 mediates the development of liver fibrosis in a mouse model of nonalcoholic fatty liver disease. PLoS One 2016;11(12):e0168572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sullivan C, Berthon-Jones M, Issa F. Nocturnal nasal-airway pressure for sleep apnea. N Engl J Med 1983;309:112. [DOI] [PubMed] [Google Scholar]
- 83.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnea. Lancet 2014;383:736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kryger MH, Malhotra A. Management of obstructive sleep apnea in adults In: Collop N, editor. UpToDate, Available at: https://www.uptodate.com/contents/management-of-obstructive-sleep-apnea-in-adults. Accessed November 24, 2018 [Google Scholar]
- 85.Spicuzza L, Caruso D, Di Maria G. Obstructive sleep apnea syndrome and its management. Ther Adv Chronic Dis 2015;6(5):273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Miao Y, Wu F, et al. Effect of CPAP therapy on liver disease in patients with OSA: a review. Sleep Breath 2018;22(4):963–72. [DOI] [PubMed] [Google Scholar]
- 87.Chin K, Nakamura T, Takahashi K, et al. Effects of obstructive sleep apnea syndrome on serum aminotransferase levels in obese patients. Am J Med 2003; 114(5):370–6. [DOI] [PubMed] [Google Scholar]
- 88.Shpirer I, Copel L, Broide E, et al. Continuous positive airway pressure improves sleep apnea associated fatty liver. Lung 2010;188(4):301–7. [DOI] [PubMed] [Google Scholar]
- 89.Hobzova M, Ludka O, Stepanova R, et al. Continuous positive airway pressure treatment and liver enzymes in sleep apnea patients. Sleep Med 2015;16:215–6. [Google Scholar]
- 90.Kim D, Ahmed A, Kushida C. Continuous positive airway pressure therapy on nonalcoholic fatty liver disease in patients with obstructive sleep apnea. J Clin Sleep Med 2018;14(8):1315–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kohler M, Pepperell JC, Davies RJ, et al. Continuous positive airway pressure and liver enzymes in obstructive sleep apnea: data from a randomized controlled trial. Respiration 2009;78(2):141–6. [DOI] [PubMed] [Google Scholar]
- 92.Sivam S, Phillips CL, Trenell MI, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnea. Eur Respir J 2012;40(4):913–8. [DOI] [PubMed] [Google Scholar]
- 93.Chen LD, Lin L, Zhang LJ, et al. Effect of continuous positive airway pressure on liver enzymes in obstructive sleep apnea: a meta-analysis. Clin Respir J 2018; 12(2):373–81. [DOI] [PubMed] [Google Scholar]
- 94.Yoshiro T, Kimihiko M, Masanori A, et al. Impacts of long-term CPAP therapy on fatty liver in male OSA patients with abdominal obesity. Eur Respir J 2014;44: S4661. [Google Scholar]
- 95.Buttacavoli M, Gruttad’Auria CI, Olivo M, et al. Liver steatosis and fibrosis in OSA patients after long-term CPAP treatment: a preliminary ultrasound study. Ultrasound Med Biol 2016;42(1):104–9. [DOI] [PubMed] [Google Scholar]
- 96.Hoyos CM, Killick R, Yee BJ, et al. Cardiometabolic changes after continuous positive airway pressure for obstructive sleep apnea: a randomized sham-controlled study. Thorax 2012;67(12):1081–9. [DOI] [PubMed] [Google Scholar]
- 97.Kritikou I, Basta M, Tappouni R, et al. Sleep apnea and visceral adiposity in middle-aged male and female subjects. Eur Respir J 2013;41(3):601–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jullian-Desayes I, Tamisier R, Zarski JP, et al. Impact of effective versus sham continuous positive airway pressure on liver injury in obstructive sleep apnea: data from randomized trials. Respirology 2016;21(2):378–85. [DOI] [PubMed] [Google Scholar]
- 99.Hang LW, Chen CF, Wang CB, et al. The association between continuous positive airway pressure therapy and liver disease development in obstructive sleep apnea/hypopnea syndrome patients: a nationwide population-based cohort study in Taiwan. Sleep Breath 2017;21(2):461–7. [DOI] [PubMed] [Google Scholar]
- 100.Sundaram SS, Halbower AC, Klawitter J, et al. Treating obstructive sleep apnea and chronic intermittent hypoxia improves the severity of nonalcoholic fatty liver disease in children. J Pediatr 2018;198:67–75.e1. [DOI] [PubMed] [Google Scholar]
- 101.Chen LD, Zhang LJ, Lin XJ, et al. Association between continuous positive airway pressure and serum aminotransferases in patients with obstructive sleep apnea. Eur Arch Otorhinolaryngol 2018;275(2):587–94. [DOI] [PubMed] [Google Scholar]
- 102.Labarca G, Cruz R, Jorquera J. Continuous positive airway pressure in patients with obstructive sleep apnea and non-alcoholic steatohepatitis: a systematic review and meta-analysis. J Clin Sleep Med 2018;14(1):133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2014;13(4):643–54.e1–9 [quiz: e39–40]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chirinos JA, Gurubhagavatula I, Teff K, et al. CPAP, weight loss, or both for obstructive sleep apnea. N Engl J Med 2014;370(24):2265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]