ABSTRACT
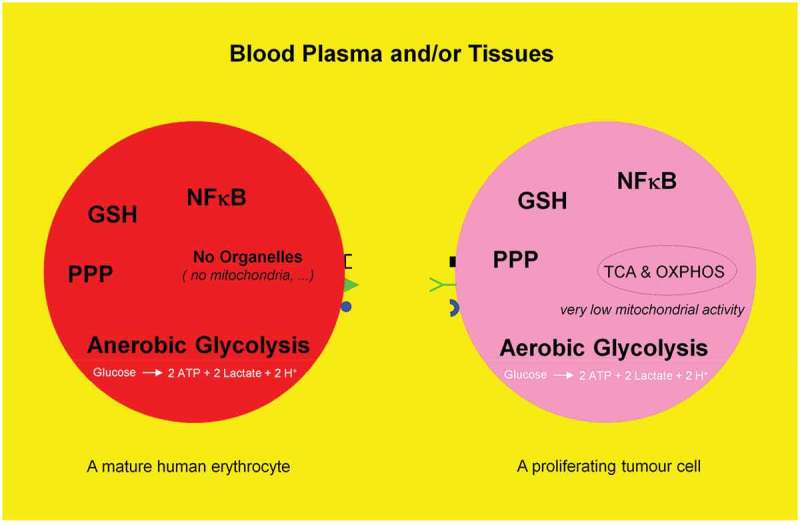
Mature human erythrocytes are dependent on anerobic glycolysis, i.e. catabolism (oxidation) of one glucose molecule to produce two ATP and two lactate molecules. Proliferating tumor cells mimick mature human erythrocytes to glycolytically generate two ATP molecules. They deliberately avoid or switch off their respiration, i.e. tricarboxylic acid (TCA) cycle and oxidative phosphorylation (OXPHOS) machinery and consequently dispense with the production of additional 36 ATP molecules from one glucose molecule. This phenomenon is named aerobic glycolysis or Warburg effect. The present review deals with the fate of a glucose molecule after entering a mature human erythrocyte or a proliferating tumor cell and describes why it is useful for a proliferating tumor cell to imitate a mature erythrocyte. Blood consisting of plasma and cellular components (99% of the cells are erythrocytes) may be regarded as a mobile organ, constantly exercising a direct interaction with other organs. Therefore, the use of drugs, which influences the biological activity of erythrocytes, has an immediate effect on the entire organism.
Abbreviations: TCA: tricarboxylic acid cycle; OXPHOS: oxidative phosphorylation; GSH: reduced state of glutathione; NFκB: Nuclear factor of kappa B; PKB (Akt): protein kinase B; NOS: nitric oxide synthase; IgG: immune globulin G; H2S: hydrogen sulfide; slanDCs: Human 6-sulfo LacNAc-expressing dendritic cells; IL-8: interleukin-8; LPS: lipopolysaccharide; ROS: reactive oxygen species; PPP: pentose phosphate pathway; NADPH: nicotinamide adenine dinucleotide phosphate hydrogen; R5P: ribose-5-phophate; NAD: nicotinamide adenine dinucleotide; FAD: flavin adenine dinucleotide; O2●−: superoxide anion; G6P: glucose 6-phosphate; HbO2: Oxyhemoglobin; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; GAP: glyceraldehyde-3-phosphate; 1,3-BPG: 1,3-bis-phosphoglycerate; 2,3-BPG: 2,3-bisphosphoglycerte; PGAM1: phosphoglycerate mutase 1; 3-PG: 3-phosphoglycerate; 2-PG: 2-phosphoglycerate; MIPP1: Multiple inositol polyphosphate phosphatase; mTORC1: mammalian target of rapamycin complex 1; Ru5P: ribulose 5-phosphate; ox-PPP: oxidative branch of pentose phosphate pathway; PGK: phosphoglycerate kinase; IFN-γ: interferon-γ; LDH: lactate dehydrogenase; STAT3: signal transducer and activator of transcription 3; Rheb: Ras homolog enriched in Brain; H2O2: hydrogen peroxide; ROOH: lipid peroxide; SOD: superoxide dismutase; MRC: mitochondrial respiratory chain; MbFe2+-O2: methmyoglobin; RNR: ribonucleotide reductase; PRPP: phosphoribosylpyrophosphate; PPi: pyrophosphate; GSSG: oxidized state of glutathione; non-ox-PPP: non-oxidative branch of pentose phosphate pathway; RPI: ribose-5-phosphate isomerase; RPE: ribulose 5-phosphate 3-epimerase; X5P: xylulose 5-phosphate; TK: transketolase; TA: transaldolase; F6P: fructose-6-phosphate; AR2: aldose reductase 2; SD: sorbitol dehydrogenase; HK: hexokinase; MG: mehtylglyoxal; DHAP: dihydroxyacetone phosphate; TILs: tumor-infiltrating lymphocytes; MCTs: monocarboxylate transporters; pHi: intracellular pH; Hif-1α: hypoxia-induced factor 1; NHE1: sodium/H+ (Na+/H+) antiporter 1; V-ATPase: vacuolar-type proton ATPase; CAIX: carbonic anhydrase; CO2: carbon dioxide; HCO3−: bicarbonate; NBC: sodium/bicarbonate (Na+/HCO3−) symporter; pHe: extracellular pH; GLUT-1: glucose transporter 1; PGK-1: phosphoglycerate kinase 1
KEYWORDS: Proliferating tumor cells, erythrocytes, glycolysis, pentose phosphate pathway, ion channels, tumor acidic microenvironment
Graphical Abstract
Background
For a long time, human erythrocytes have been mainly considered as a bioconcave container filled with hemoglobin and surrounded by a plasma membrane. This still predominant view limits their physiologic function to the transport of oxygen and CO2 and therefore ignores knowledge about similarities between erythrocytes and nucleated cells (e.g. immune and proliferating tumor cells), particularly regarding metabolic pathways. Proliferating tumor cells undergo an anabolic metabolism and synthesize abundance of DNA, proteins, and lipids for cell growth and proliferation. These capacities are lacking in organelles-free human erythrocytes. The incapability of erythrocytes regarding cell division is compensated by bone marrow, which permanently produces erythrocytes (two million per second). However, there are similarities in glucose metabolism and the thereof derived metabolic pathways (glycolysis, pentose phosphate and sorbitol pathways, glutathione biosynthesis, etc.). In order to shed a new light on our perception of erythrocytes, we summarize evidence for their multifunctional roles and show several relevant examples. We also describe how a differentiated cell turns into a tumor cell. Subsequently, a major focus will be on the surprising similarities between the glucose metabolism of erythrocytes and proliferating tumor cells.
Importance of human erythrocytes
a) Erythrocytes represent about 84% of the total cells in the human body [1]. They provide considerable amounts of the reduced form of glutathione (GSH) into the blood plasma, thereby contributing significantly to the dynamic interorgan-GSH-metabolism [2]. Compared to normal tissue or non-proliferating tumor cells, proliferating tumor cells take up a high amounts of GSH provided by erythrocytes. These large quantities of absorbed GSH, as well as the GSH produced by tumor cells themselves are subsequently used for processes, such as DNA synthesis, multi-drug resistance, and detoxification. Therefore, to treat cancer and other GSH-dependent diseases, it is crucial to decrease the systemic availability of GSH via pharmacological GSH-depletors, e.g. Bay 11–7082, parthenolide, or dimethyl fumarate [3] in vivo.
b) Mature human erythrocytes possess transcription factors, including all of the members of
the redox-sensitive NFκB-signaling pathways [4].
c) Impairment of nitrosylation induces eryptosis (programmed cell death of erythrocytes, similar to apoptosis) [5]. The terminology eryptosis was introduced by Lang et al. [6].
d) Protein kinase B (Akt) has physiological functions in human erythrocytes and a major role is activation of nitric oxide synthase (NOS), by which it can positively regulate the deformability of erythrocytes [7]. Among other functions, Akt can serve as a positive upstream-regulator/-activator of nuclear factor kappa B (NFκB) [8].
e) Denatured hemoglobin-induced clustering of band-3 (the major erythrocyte membrane-spanning protein) promotes autologous antibody (IgG) binding to senescent (aged) erythrocytes [9]. This process marks erythrocytes for macrophage-mediated clearance (for review see Ref. [10]). Erythrocyte senescence also induces the eryptosis machinery and contributes to removal of aged erythrocytes from the peripheral blood at high velocity. We were able to show this senescence-mediated eryptosis by age dependent fractionation of erythrocytes into fractions I to V [11], whereby fraction I constitutes the youngest and fraction V the oldest erythrocyte population. Interestingly, healthy and young erythrocytes prevent their premature clearance by mechanisms including vesicle formation, subsequent bundling with senescent cell antigens (e.g. modified band 3) and their release into the circulation [12].
f) An inverse correlation exists between NFκB-abundance and eryptosis. The fractionation of erythrocytes from healthy volunteers into five different fractions (fraction I to fraction V) demonstrated an anti-apoptotic function of NFκB in our experimental system: In comparison to fraction V (with the lowest NFκB abundance), the youngest erythrocyte population (fraction I with the highest NFκB abundance) required double the amount of NFκB inhibitors Bay 11–7082 or parthenolide in order to achieve the same eryptosis rate as compared to erythrocytes in fraction V. This phenomenon might be associated with an anti-apoptotic function of NFκBs [13].
g) Mature human erythrocytes possess a functional protein–degrading machinery, the 20S-proteasome [14]. Our recent data show that mature human erythrocytes seem to have an active protective mechanism selectively preventing the degradation of a part of their proteins like ß-actin during the entire life span. Other proteins, like NFκBs seem to be prone to degradation according to age [13]. These data provide evidence that mature human erythrocytes substitute their missing new induction of proteins by an effective, selective and yet unknown mechanism in order to protect some of their proteins from degradation processes.
h) Mammalian tissues are able to produce and degrade hydrogen sulfide (H2S) having various physiological effects on the cardiovascular, intestinal, and central nervous system. Human erythrocytes play a significant role in H2S turnover and in regulation of H2S levels in blood and in tissues [15].
i) Mature human erythrocytes do not circulate as isolated cells in the blood stream. They partially control the function of immune cells (including lymphocytes and immature dendritic cells (slanDCs)) in the peripheral blood [16–20]. The physical interaction of erythrocytes with slanDCs inhibits the slanDCs-mediated production of the inflammatory cytokine interleukin-12 [16]. In blood, mature human erythrocytes rapidly and reversibly bind interleukin-8 (IL-8), a leukocyte chemotaxin to prevent or control the stimulation of leukocytes [20]. Hemoglobin α and β chains bind LPS (lipopolysaccharide an endotoxin of Gram negative bacteria) and neutralize its activity [21]. Erythrocytes and platelets association with circulating bacteria regulates complement receptor mediated pathogen capture [22,23]. Finally, this process results in rapid clearance of complement-opsonized pathogens by phagocytes residing in liver and spleen (for review see Ref. [24]).
Mature human erythrocytes are multifunctional and their physiological functions in a healthy organism and also in diseases related to erythrocytes and other cell types must be looked at from a different perspective.
Proliferating tumor cells
Dynamic reciprocal interaction between tumor suppressor and oncoproteins is a prerequisite for a functioning cell. Mutations promoting activities of the oncogenes and or curbing activities of tumor suppressor genes enable the differentiated cell to turn into a tumor cell leading to a metabolic switch (Figure 1). Key component of such a metabolic switch is the aerobic glycolysis or Warburg effect, enhanced catabolism of glucose to lactate under normoxic conditions. A direct correlation between loss of function of the tumor suppressor p53 and uncontrolled activity of the oncogenic protein NFκB as positive physiological regulator of glycolysis was demonstrated [25–27]; for review see Ref. [28]. The rate of glycolysis and respiration (oxidative phosphorylation (OXPHOS)) are negatively correlated [29] (for review see [28]) and glycolysis is up to 100 times higher than respiration. A proliferating tumor cell is thus capable to downregulate its mitochondrial activities (~95%) known as truncated tricarboxylic acid cycle (TCA) and OXPHOS. Thereby, the growth inhibiting oxidative stress, i.e. the formation of reactive oxygen species (ROS) caused by mitochondria is drastically reduced (Figure 2). However, there is increasing evidence that some tumors (e.g. human and mouse malignant glioma cells) display glucose metabolism heterogeneity, i.e. OXPHOS- or glycolytic-dependent phenotypes for ATP supply and growth [30,31] (for review see Ref. [32]). Interestingly, these two metabolic phenotypes can even coexist in a tumor [33]. The positive and dynamic interplay between glycolysis (which is independent of intact mitochondrial function) and pentose phosphate pathway (PPP) provides the necessary ATP, NADPH, and ribose-5-phophate (R5P). The latter is an essential component of nucleotides (DNA and RNA) and co-factors NAD, NADP, and FAD (Figure 3). Apart from glycolysis and PPP a shift to an increased rate of glutamine metabolism [34] (for review see Refs. [35,36]) and fatty acid biosynthesis (for review see Refs. [37,38]) are additional hallmarks necessary for the upregulation of growth and proliferation of tumor cells. For further details see Figures 4 and 5. These events including angiogenesis [39] allow proliferating tumor cell to fulfill three metabolic requirements: (i) bioenergetics (ATP production), (ii) redox maintenance (NADPH and GSH regeneration), and (iii) macromolecules biosynthesis (e.g. DNA synthesis) to match its high proliferation rate.
Figure 1.
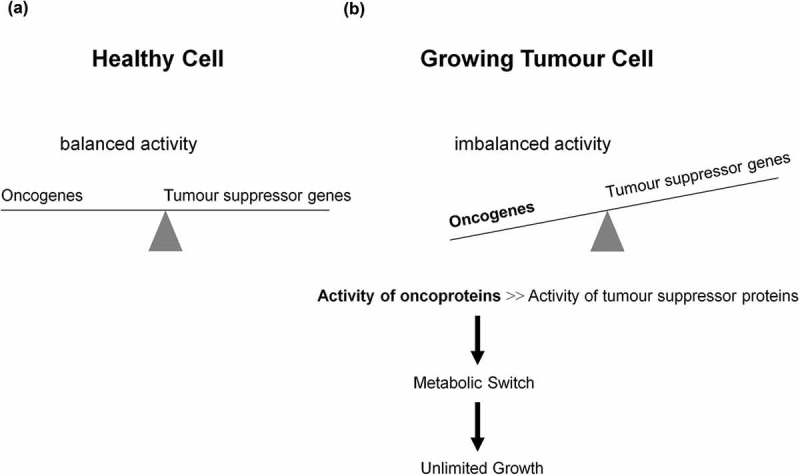
Role of oncogenes, tumor suppressor genes, and their corresponding proteins in tumorigenesis and metabolic switch. (a) A well-balanced expression of oncogenes and tumor suppressor genes, as well as a well-balanced activity of oncogenes and tumor suppressor proteins reciprocally controlling each other constitute the prerequisite of a normally functioning cell. Dependent on extracellular stimuli (e.g. glucose uptake) the synthesis and activity of these proteins are transiently activated or repressed. (b) However, mutations promoting synthesis and activities of oncoproteins or curbing synthesis and activities of tumor suppressor proteins enable the differentiated cell to turn into a undifferentiated tumor cell, thus introducing the metabolic switch, resulting in unlimited growth and proliferation.
Figure 2.

Schematic representation of anaerobic glycolysis, oxidative phosphorylation (OXPHOS/respiration), and aerobic glycolysis/Warburg effect. (a) Anaerobic glycolysis in anucleated and organelle-free human and mouse erythrocytes and conversion of glucose to lactate. Most differentiated cells convert glucose to pyruvate via glycolysis and subsequently to CO2 via TCA and OXPHOS, with marginal lactate production. (b) Proliferating cells prefer aerobic glycolysis, i.e. marginal amount of pyruvate is dispatched to mitochondrion and simultaneously high amounts of lactate are generated.
Figure 3.
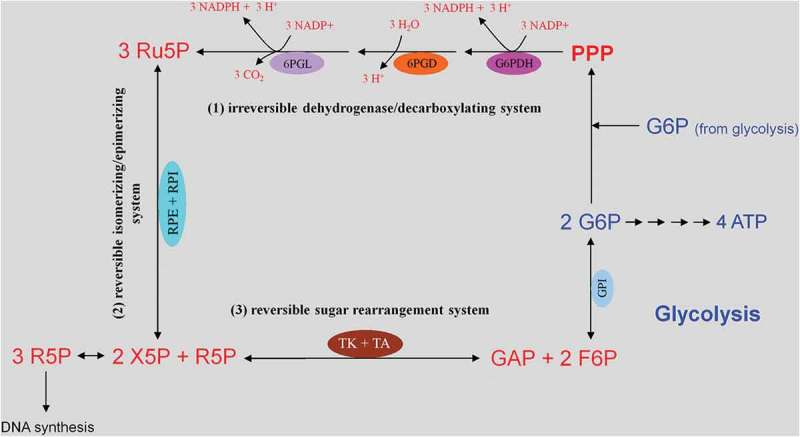
Glucose uptake and internalization by glucose transporters (GLUTs) and its phosphorylation at C6-position via the glycolytic enzyme hexokinase. Glycolysis implies conversion of glucose 6-phosphate (G6P) into lactate generating only two ATP molecules per one molecule of glucose. G6P can equally be entered into the pentose phosphate pathway (PPP), consisting of irreversible oxidative and reversible non-oxidative branches. The irreversible dehydrogenase/decarboxylase system of the oxidative branch of the PPP (ox-PPP) consisting of glucose 6-phosphate dehydrogenase (G6PDH), 6-phosphogluconate dehydrogenase (6PGD) and 6-phosphogluconolactonase (6PGL) break down G6P, yielding two NADPH and one ribulose-5-phosphate (Ru5P). Consequently, the two non-oxidative systems of PPP takes on the remaining work. The isomerizing/epimerizing system consisting of RPI and ribulose 5-phosphate 3-epimerase (RPE), interconverts Ru5P to xylulose 5-phosphate (X5P) and ribose 5-phosphate (R5P), whereas the sugar rearrangement system consisting of transketolase (TK) und transaldolase (TA), interconverts X5P and R5P to the glycolytic intermediates fructose 6-phosphate (F6P) und glyceraldehyde 3-phosphate (GAP). Thus, the PPP culminates in glycolysis. Furthermore, F6P and GAP can in turn be converted into R5P based on the reversible nature of the non-oxidative branch of the PPP (non-ox-PPP) .
Figure 4.
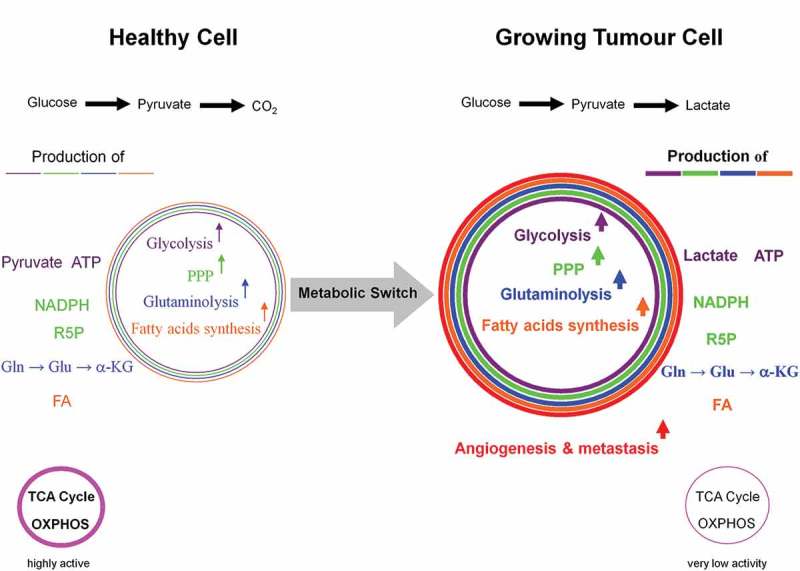
Dependency of growing tumor cells, activated T- and proliferating cells on glycolysis. The metabolic shift from respiration to glycolysis, known as the Warburg effect in proliferating tumor cells, is not solely restricted to them. For instance, activated T- and proliferating cells equally make use of the same mechanisms. The six hallmarks of a proliferating tumor cell are: high activities of glycolysis and pentose phosphate pathways, massive glutaminolysis and fatty acids biosynthesis, as well as angiogenesis and metastasis. However, the latter two features do not appear in healthy activated T- and proliferating cells.
Figure 5.
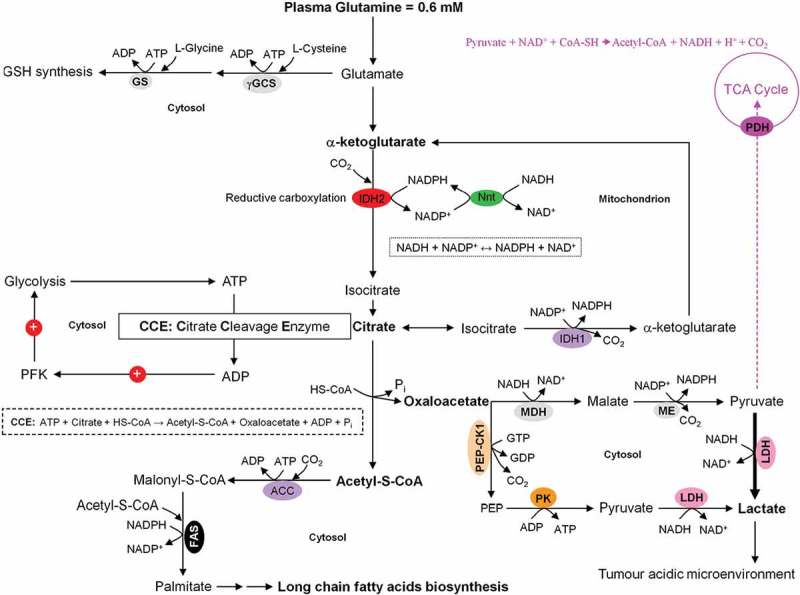
Coordinated interactions between glycolysis, citrate cleavage enzyme, and glutaminolysis for the fatty acids biosynthesis in growing tumor cells. Based on the very low activity of the TCA cycle in growing tumor cells, glucose is diverted away from mitochondrial acetyl-CoA and citrate production. Alternatively, glutamine-derived α-ketoglutarate (α-KG) and its subsequent reductive carboxylation by NADPH-dependent isocitrate dehydrogenase 2 (IDH2) results in citrate production. The latter is then converted to acetyl-CoA and oxaloacetate by the citrate cleavage enzyme (CCE). This extramitochondrial acetyl-CoA is used for long chain fatty acids biosynthesis. The enzymic decarboxylation of oxaloacetate by the cytosolic phosphoenolpyruvate carboxykinase 1 (PEP-CK1) results in phosphoenolpyruvate (PEP) formation which finally is primarily converted into lactate which leads to tumor acidic microenvironment. Marginal amount of pyruvate is also dispatched to mitochondrion. The oxaloacetate-to-malate conversion by NADH-dependent malate dehydrogenase (MDH) and the subsequent malic enzyme mediated decarboxylation of malate into pyruvate maintain the regeneration of NAD+ and a continual supply of NADPH, respectively. NAD+ regeneration ensures the perpetuation of the glycolysis pathway, whereas the generation of NADPH supports lipogenesis. The energy-linked nicotinamide nucleotide transhydrogenase (Nnt) acts as an effective buffer system and catalyze the direct transfer of a hydride ion between NADH and NADPH. The interconnection between the NADPH consuming reductive carboxylation and NADPH producing oxidation of α-KG is also demonstrated.
In the following important metabolic pathways, such as glycolysis, PPP, sorbitol, and glyoxylase pathway, as well as GSH synthesis machinery, all of which are actively present and play a vital role in eukaryotic and prokaryotic cells are discussed.
Metabolism
Metabolism is the sum of all chemical reactions in a cell or organism. Anabolism describes the processes by which precursors (e.g. amino acids, sugars, fatty acids, and nitrogenous bases) are converted into macromolecules under the consumption of energy (ATP) and reductive equivalents (NADPH and NADH). Catabolism signifies breakdown reactions of energy-bearing molecules (e.g. carbohydrates, lipids, and proteins) ultimately resulting in a gain of chemical energy (ATP) and reductive equivalents. These processes ensure the functioning of cells and organism and thus life itself (Figure 6).
Figure 6.
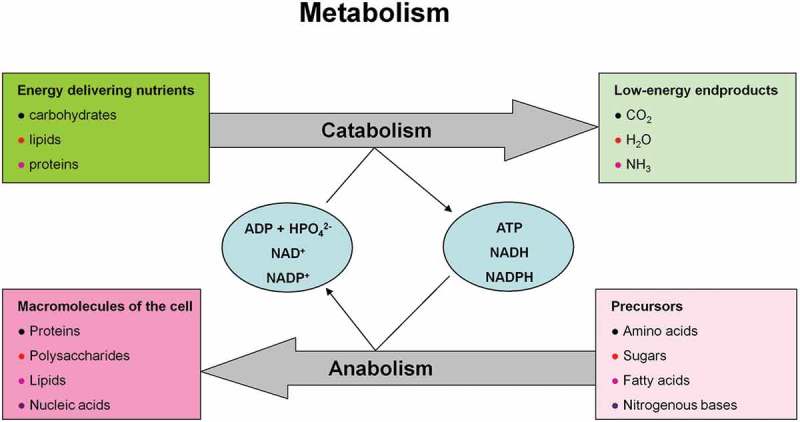
Metabolism consisting of catabolic and anabolic processes encompasses the sum of all chemical conversions in a cell or organism. (a) Anabolism and (b) catabolism.
Glycolysis and Rapoport–Luebering-pathway
This pathway (Glucose → Glucose 6-phosphate → → 2 ATP + 2 lactate generation) uncouples ATP generation from oxygen consumption and is the only source of metabolic energy for mature (anucleated) human erythrocytes and the major source of metabolic energy for proliferating cells, respectively (Figure 7(a)). Large amounts of ATP can be generated by glycolysis within a short time. Thus, a growing tumor cell largely tends to the slow-down oxygen-dependent ATP generation in mitochondria and can therefore be compared to organelle (mitochondria)-free human erythrocytes. Molecular oxygen (O2) becomes superfluous as the final electron acceptor for the ATP generation (aerobically ATP generation) and the formation of damaging superoxide anion (O2●−) and other ROS are avoided [40,41] (for reviews see Refs. [42,43]). For more details see Figure 2(b). This phenomenon is termed truncated TCA cycle and is regarded as ideal condition for the survival, growth, and proliferation of a tumor cell. Interestingly, glucose 6-phosphate (G6P) stimulates the transcription of several genes involved in glycolytic and lipogenic pathways [44,45]. The oxygen (O2) binding capacity of hemoglobin and the rate of human erythrocytes glycolysis are closely linked. Oxyhemoglobin (HbO2) stimulates the activity of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [46]. The latter oxidizes glyceraldehyde-3-phosphate (GAP) to 1,3-bis-phosphoglycerate (1,3-BPG). Subsequently, the multifunctional enzyme, 2,3-bisphosphoglycerte (2,3-BPG) synthase/2-phosphatase (BPGM), transfers a phosphoryl group from C1 to C2 of 1,3-BPG, synthesizing 2,3-BPG [47]. 2,3-BPG in turn facilitates oxygen release from oxyhemoglobin [48] improving tissue oxygenation; see Figure 7(b). 2,3-BPG also serves as phosphoglycerate mutase 1 (PGAM1) histidine phosphate donor and activator [49]. Subsequently, the activated PGAM1 converts 3-phosphoglycerate (3-PG) to 2-phosphoglycerate (2-PG), thereby accelerating the glycolysis pathway (Figure 7(a,c)). Multiple inositol polyphosphate phosphatase (MIPP1), a histidine phosphatase, directly converts 2,3-BPG to 2-PG [50], revealing the capability and maneuverability of glycolysis pathway to bypass 3-PG (Figure 7(c)), a precursor of serine biosynthesis. In response to insulin, the mammalian target of rapamycin complex 1 (mTORC1) activates the glycolytic enzyme phosphoglycerate kinase (PGK) [51], which catalyzes the formation of 3-PG from 1,3-BPG (Figure 7(a)).
Figure 7.
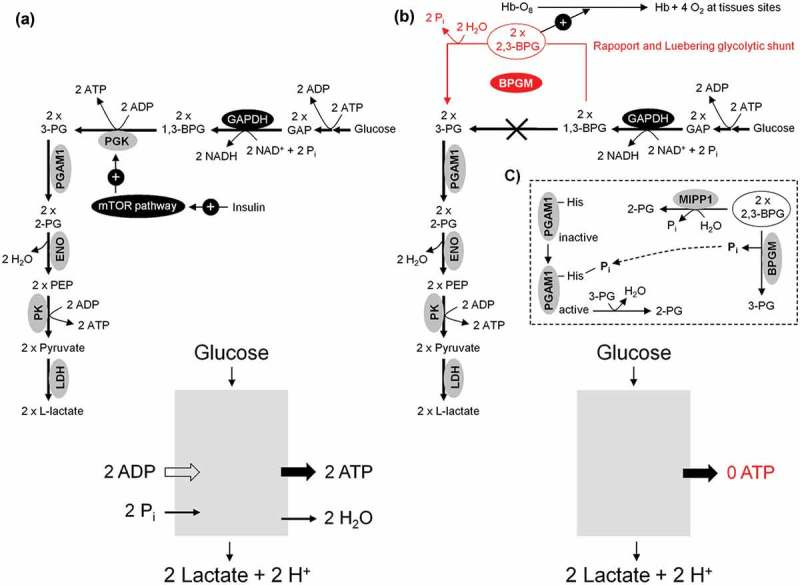
Anaerobic and aerobic glycolysis in mature human erythrocytes and growing tumor cells. (a) In both cases, the rate of the glycolytic ATP production is the same (Glucose + 2ADP → 2 Lactate + 2H+ + 2ATP). (b) For adequate supply of the organism with molecular oxygen, human erythrocytes divert 20% of the uptaken glucose to Rapoport and Luebering glycolytic shunt [102]. This carries an energetic cost based on bypassing the ATP generating phosphoglycerate kinase (PGK). (c) Besides 2,3-bisphosphoglycerte (2,3-BPG) synthase/2-phosphatase (BPGM), multiple inositol polyphosphate phosphatase (MIPP1) is also able to significantly decrease 2,3-BPG levels in vivo.
Role of the glycolytic enzymes in immune response, tumorigenesis, and biosynthesis
T cell effector function is a glucose-dependent process. There is a direct correlation between glycolysis and interferon-γ (IFN-γ) production in activated cytotoxic T cells. High glycolytic flux results in dissociation of GAPDH-IFN-γ mRNA complex, thus ending the inhibitory effect of GAPDH on IFN-γ translation and production (Glycolysis → IFN-γ production) [52]. This means the glycolytic switch is a prerequisite for a rapid IFN-γ production and for the effector function of cytotoxic T cells [53]. The glycolytic enzyme lactate dehydrogenase (LDH) acts as a positive regulator of glycolysis. LDH as downstream target of mTOR and signal transducer and activator of transcription 3 (STAT3) promotes tumorigenesis [54,55]. Under high glycolytic fluxes de novo serine and glycine biosynthesis (Glucose → → 3-PG → → Serine ↔ Glycine) promotes tumorigenesis (for review see Ref. [56]). Glycolysis and biosynthetic pathways are strongly interconnected. Under low glucose conditions GAPDH acts as negative regulator of mTORC1 pathway by its mere binding to mTORC1 activator Rheb (Ras homolog enriched in Brain). High glycolytic flux disrupts this physical interaction [57], leading to Rheb-mediated mTORC1 activation and mTORC1-dependent protein synthesis and cell growth (for review see Ref. [58]).
Pentose phosphate pathway
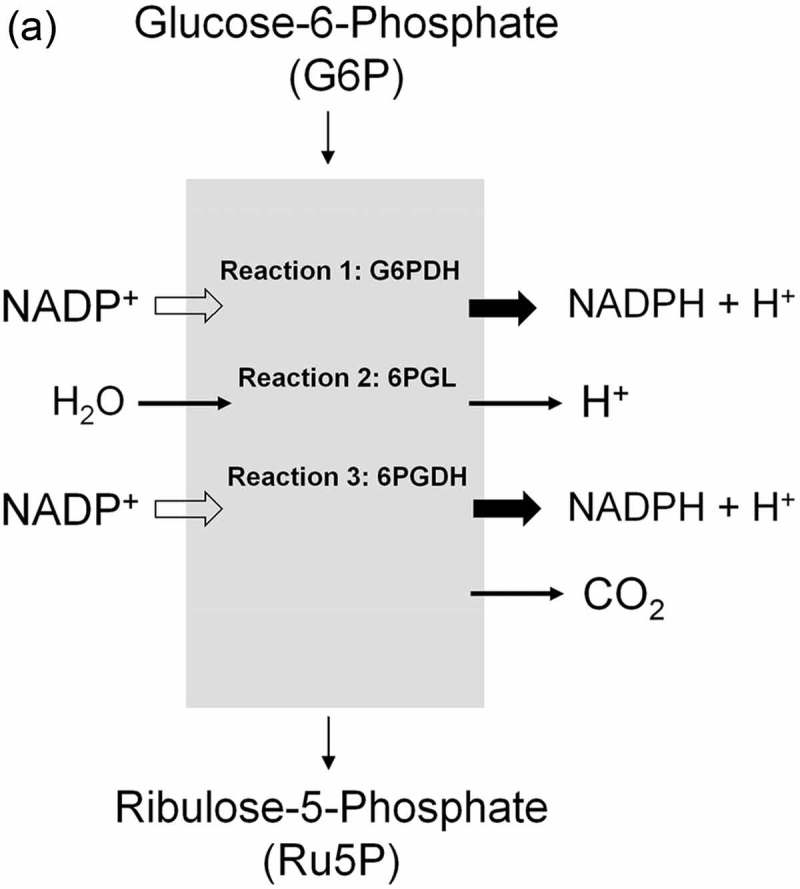
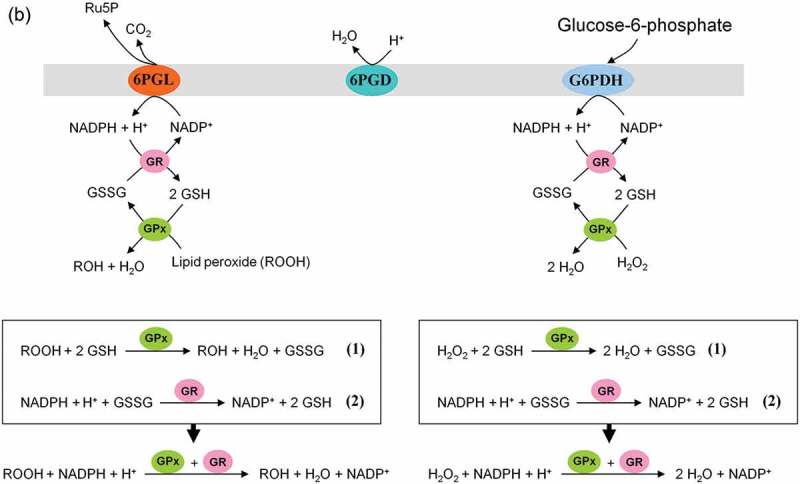
This pathway branches from glycolysis at the first committed step of glucose metabolism (Glucose-6-phosphate (G6P) → PPP). The PPP is divided into two branches, the oxidative (irreversible) and non-oxidative (reversible). A breakdown of the G6P molecule via the oxidative branch of PPP (ox-PPP) delivers 2 NADPH molecules and a pentose monophosphate termed ribulose 5-phosphate (Ru5P). The overall reaction of the ox-PPP is illustrated below: Glucose-6-P + 2NADP+ + H2O → Ribulose-5-P + 2NADPH + 2H+ + CO2 (Figures 3 and 8(a)). NADPH as electron donor for anabolic reduction reactions is needed for the biosynthesis of fatty acids and cholesterol. NADPH is required to neutralize ROS, such as hydrogen peroxide (H2O2) and lipid peroxides (ROOHs) preventing cellular damage (Figure 8(b)). Superoxide dismutases (SODs) convert superoxide anion (O2●−) into H2O2 and molecular oxygen (2 O2●− + 2 H+ → O2 + H2O2). O2●− itself is produced during the mitochondrial respiratory chain (MRC) by the electron univalent leak pathway. Through this process about 2% of molecular oxygen consumption is partially reduced to O2●− [59]. Furthermore, O2●− is produced during auto oxidation of about 3% of the total body oxyhemoglobin to methemoglobin (HbFe2+-O2 → HbFe3+ + O2●−) and oxymyoglobin to methmyoglobin (MbFe2+-O2 → MbFe3+ + O2●−) [60,61]. Submicromolar levels of H2O2 act as intra and intercellular signaling molecule promoting cell growth and proliferation, whereas its excess results in lipid peroxidation and cell death.
Figure 8.
(continued)
Figure 8.
(continued)
NADPH-dependent GSH-glutaredoxin and thioredoxin systems function as hydrogen donors for ribonucleotide reductase (RNR). For DNA synthesis ribonucleotides have to be reduced to deoxyribonucleotides by RNR. The de novo synthesis of phosphoribosylpyrophosphate (PRPP) as the backbone for ribonucleotides synthesis is controlled by the enzyme PRPP synthase which utilizes ATP and R5P as substrates (R5P + ATP → PRPP + AMP + PPi) [62,63]. For more details see Figure 8(c). It is to note that redox regulation in activation of NFκB and NFκB-mediated gene transcription needs a shift from high GSSG/low HS−Thioredoxin−SH conditions to low GSSG/high HS−Thioredoxin−SH levels [64–66].
Figure 8.

(continued)
ATP conservation is a major feature of metabolic regulation [67]. Oxidative stress impairs the energy balance of the cell and enhanced glycolytic enzyme activities are able to compensate for deficits in ATP supply and ATP-dependent GSH generation (Figure 5). Finally, in the non-oxidative branch of PPP (non-ox-PPP), the isomerizing/epimerizing system consisting of R5P isomerase (RPI) and ribulose 5-phosphate 3-epimerase (RPE) interconverts Ru5P to R5P and xylulose 5-phosphate (X5P), whereas the sugar rearrangement system consisting of transketolase (TK) und transaldolase (TA) interconverts X5P and R5P to the glycolytic intermediates fructose 6-phosphate (F6P) und GAP. F6P can either directly be used for glycolysis or be converted to G6P to replenish ox-PPP. The overall reaction of the non-ox-PPP is illustrated below:
DNA Synthesis ← 3 R5P ↔ 2 X5P + R5P ↔ 2 F6P + GAP → Glycolysis. For a correct presentation of stoichiometry, we assumed three G6P molecules here (Figures 3 and 8(d)). Independent of cell types and species, the conversion of glycolytic intermediates F6P and GAP into R5P (3 R5P ↔ 2 F6P + GAP) without production of NADPH is defined as riboneogenesis [68].
Figure 8.
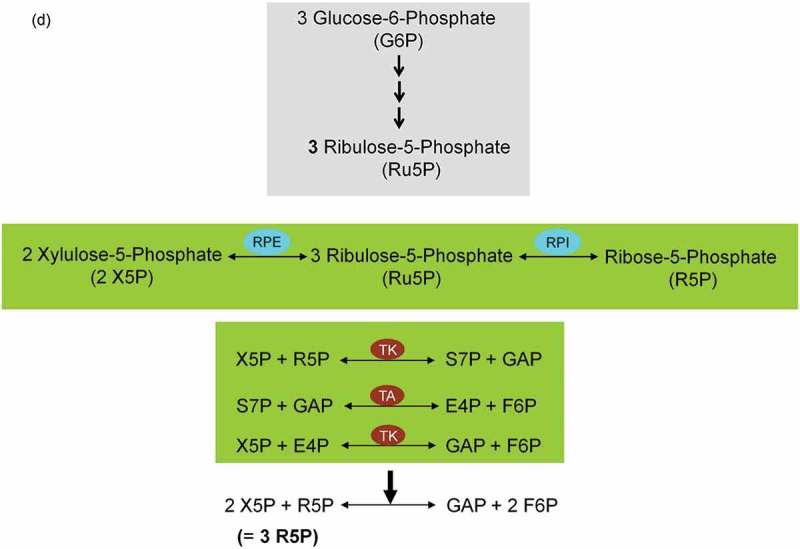
Role of the pentose phosphate pathway (PPP) in cellular defense and DNA synthesis. (a) In the oxidative branch of the PPP (ox-PPP), glucose 6-phosphate (G6P) is converted in three consecutive reactions into ribulose 5-phosphate (Ru5P), simultaneously yielding two NADPH molecules. (b) Linkage of glutathione (GSH) cycle to NADPH producing PPP provides cellular defense mechanisms against oxidative stress by glutathione reductase (GR) and -peroxidase dependent (GPx) detoxification of lipid peroxides (ROOHs) and hydrogen peroxide (H2O2). (c) In the non-oxidative branch of the PPP (non-ox-PPP), the isomerizing system interconverts Ru5P to xylulose-5-phosphate (X5P) and R5P. In the sugar rearrangement system transketolase (TK) which transfers two carbon units and transaldolase (TA), which transfers three carbon units, convert X5P and R5P into the glycolytic intermediates fructose-6-phosphate (F6P), and glyceraldehyde-3-phosphate (GAP). (d) The generated NADPH molecules are also required for and consumed during nucleotides biosynthesis. NADPH links PPP with the electron transmitting systems namely, GSH-glutaredoxin(Grx) and thioredoxin (Trx). The oxidation of NADPH channels its hydride ion (H −) to these hydrogen carrier systems which serve as electron (H −) donors. R5P conversion to phosphoribosyl pyrophosphate (PRPP), as well as purine serve as the backbone for ribonucleotides synthesis. Reduction of ribonucleotide diphosphate (rNDP) to deoxynucleotide diphosphate (dNDP) and its phosphorylation by nucleotide diphosphate kinase (NDK) is an absolute prerequisite for DNA synthesis.
Sorbitol (polyol) pathway
Glucose is a physiological substrate for the sorbitol/polyol pathway. Two enzymatic reactions are involved in this pathway. First, the NADPH-dependent enzyme aldose reductase 2 (AR2) reduces glucose to sorbitol (Glucose + NADPH → Sorbitol + NADP+). This NADPH originates from the oxidative branch of the PPP (ox-PPP). Subsequently, the NAD+-dependent enzyme sorbitol dehydrogenase (SD) oxidizes sorbitol to fructose (Sorbitol + NAD+ + Pi → Fructose + NADH) (Figure 9). The glycolytic enzyme hexokinase (HK), as well as the NADPH-dependent enzyme aldose reductase 2 (AR2) from the sorbitol pathway compete with each other for the utilization of glucose. The Michaelis-Menten constant (Km) of AR2 for glucose is in the range of 10–100 mM and of HK about 0.18 mM. Under physiological conditions, a minor amount of glucose (~3%) is utilized for sorbitol synthesis [69]. For more details see Figure 9. To react adequately to hyper- and hypotonic stress mammalian cells have developed the capacity to accumulate or release small organic solutes referred to as nonperturbing or compatible osmolytes namely sorbitol, myo-inositol, and taurine (for review see Ref. [70]). The sorbitol pathway is linked to both glycolysis and PPP via fructose conversion to F6P (PPP ← F6P → Glycolysis) (Figure 9).
Figure 9.
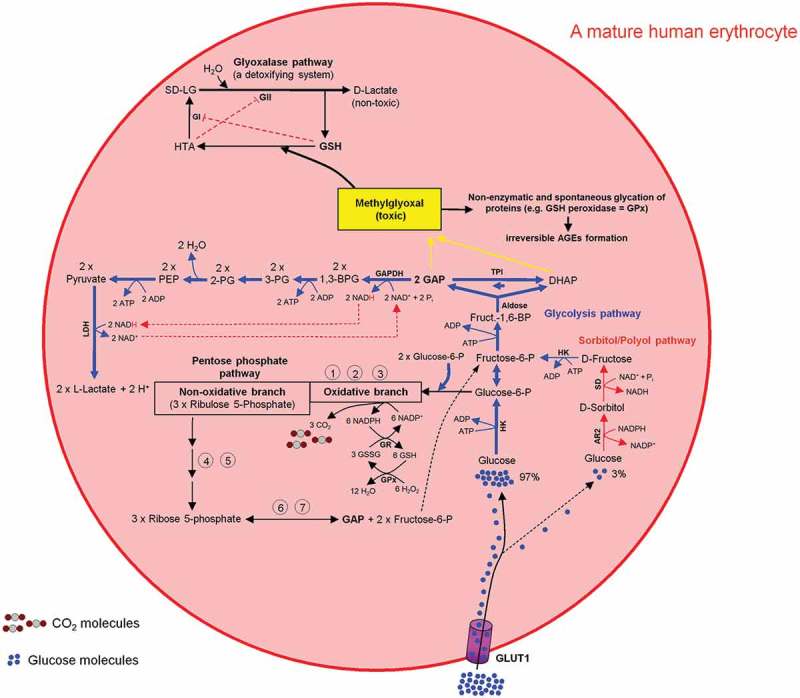
Intercross between glycolysis-, sorbitol-, pentosephosphate, and glyoxalase pathways in mature human erythrocytes. For more details see the main text.
Glyoxylate pathway-mediated detoxification of methylglyoxal
The toxic and mutagenic mehtylglyoxal (MG) is formed from the glycolytic intermediates dihydroxyacetone phosphate (DHAP) and GAP (DHAP ↔ GAP) [71,72]. MG contributes to inactivation of enzymes like GSH peroxidase and thus impairing the cell function. Very low concentration of DHAP, GAP, and MG will stop this negative chain of events. The glycolytic enzyme GAPDH oxidizes GAP into 1,3-bisphoglycerate, which is then used in further consecutive steps of the glycolysis pathway for L-lactate formation and ATP generation. The glyoxylate pathway utilizes GSH as an indispensable co-factor to convert MG to D-lactate. As a result, the intracellular MG is depleted during the L- and D-lactate building processes (Figure 9).
All metabolic pathways described in the preceding paragraphs are summarized in Figures 9 and 10.
Figure 10.
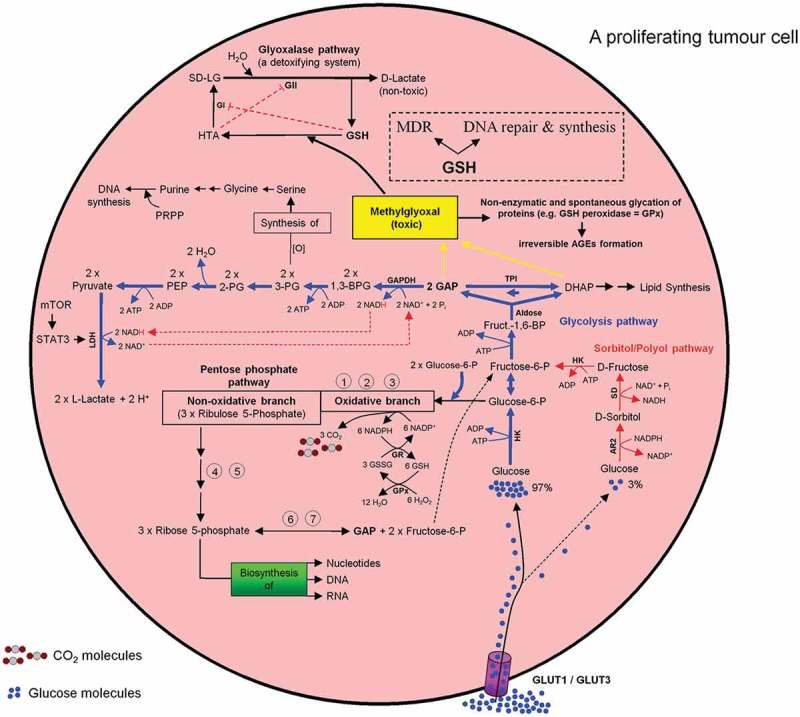
Intercross between glycolysis-, sorbitol-, pentosephosphate, and glyoxalase pathways in mammalian cells especially in a proliferating tumor cell. For more details see the main text.
Tumor microenvironment – a no-go-area for healthy neighboring and cytotoxic immune cells
The microenviornment of a growing tumor provides a hostile environment for healthy neighboring cells including tumor-infiltrating lymphocytes (TILs). Their growth, proliferation, and differentiation into effector cells is inhibited to a significant extent. As a result the anti-tumor effect of TILs is significantly decreased. A growing tumor cell can be compared to a black hole devouring all material necessary for its growth (e.g. glucose, vitamins, etc.) so that nothing can survive in its immediate surrounding. Normal and healthy cells such as activated human cytotoxic T lymphocytes exhibit an increased activity in glycolysis during their proliferation and/or activation phase [73]. Human erythrocytes are obligatory glucose consumers and exploiters. Hypoglycemia and reduced glycolysis causes neuronal death and endothelial cells gain more than 80% of their energy from glycolysis despite having direct access to molecular oxygen (O2). It exists a direct correlation between high glycolytic flux and unlimited proliferative potential in embryonic stem cells [74]. This positive selective pressure of evolution for gaining energy makes the phenomenon of glycolysis in mammalian cells an universal pathway with advantages from which healthy as well as tumor cells can profit. For the continuation of glycolysis lactic acid – as the glycolysis end product – is cotransported 1:1 with protons (H+) [75] and released into the extracellular environment. This process is performed by proton-linked monocarboxylate transporters (MCTs) [76]. Proliferating tumor cells show a high level of lactic acid production and tolerability and use lactic acid export as an effective measure to impair the metabolism and function of activated cytotoxic T cells [77]. Consequently, at a tumor microenvironment site T-cell responses against tumor-associated antigens are substantially inhibited. Under these conditions, tumor cells can escape immuno surveillance. Lactic acid functions as a protective shield for growing tumor cells. Processing glycolysis is directly associated with an increased intracellular lactate formation and a decrease of the intracellular pH (pHi). The latter impairs continuation of glycolysis, survival and proliferating tumor cell. To counteract this process, lactate-mediated hypoxia-induced factor 1 (Hif-1α) activation and Hif-1α-dependent gene transcription are necessary. The corresponding gene products erythrocyte-type MCT1 [76,78], MCT4 [79,80], sodium/H+ (Na+/H+) antiporter 1 (NHE1) [81] (for review see Ref. [82]), and vacuolar-type proton ATPase (V-ATPase) [83] (for review see Ref. [84]) act as pHi regulators and are responsible for the bulk of proton (H+) extrusion. The prosurvival ectopic enzyme carbonic anhydrase (CAIX) that catalyzes the hydration of carbon dioxide (CO2) into bicarbonate (HCO3−) and proton (H+) [85] (for review see Ref. [86]), as well as sodium/bicarbonate (Na+/HCO3−) symporter (NBC) [87,88] (for review see Ref. [89]), sodium (Na+)/H+ antiporter 1 (NHE-1) [90] (for reviews see Refs. [91,92]) all controlled by HIF-1α act as pH regulators. These processes result in maintenance of cytoplasmic alkalinization (pHi 7.2) and simultaneously contribute to acidification (pHe 6.8) of tumor microenvironment (Figure 11).
Figure 11.
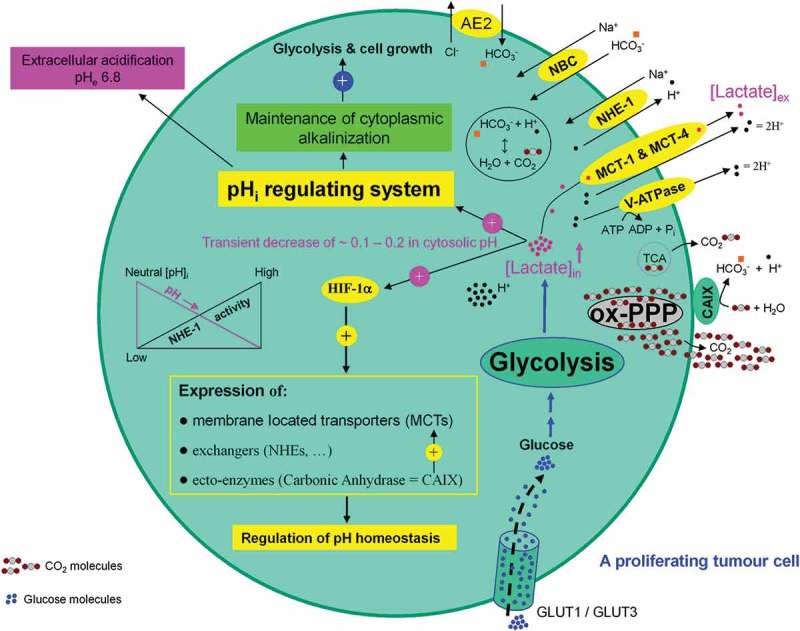
Association between high glycolytic flux and extracellular pH (pHe) gradient formation by carbonic anhydrase, as well as proton pump and exchangers. Glycolysis-associated lactate generation promotes hypoxia-inducible factor-1a (HIF-1α) activation and HIF-1α dependent transcription of several proton channels and exchangers. Extrusion of protons (H+) by these regulators contributes to a concomitant extracellular acidification (pHe: 6.8) and intracellular alkalinization (pHi: 7.2) in growing tumor cells. Additional source of tumor microenvironment acidity is the activity of carbonic anhydrase IX (CAIX). CAIX-dependent hydration of carbon dioxide (CO2) – predominantly generated by the oxidative branch of the pentose phosphate pathway (ox-PPP) and to a lesser extent by TCA cycle – delivers protons (H+) and hydrogen carbonate (HCO3−) ions. Subsequent uptake of HCO3− by Na+-dependent bicarbonate (NBC) transporter and anion exchanger 2 (AE2), replenishes the intracellular HCO3−. Titration of HCO3− by intracellular H+ ions, produced by glycolysis, results in the formation of CO2 which rapidly diffuses across the plasma membrane, where it meets CAIX. MCT: monocarboxylate transporter, V-ATPase: vacuolar-type proton ATPase, NHE-1: sodium/hydrogen exchanger 1, AE2: anion exchanger 2, NBC: sodium/bicarbonate (Na+/HCO3−) co-transporter, CAIX: carbonic anhydrase 9.
Hif-1α also stimulates the expression of metabolism related glucose transporters GLUT-1 [93] (for review see Ref. [94]) and glycolytic enzymes LDH and PGK-1 [95]. Hif-1α impairs mitochondrial respiration by preventing entry of pyruvate into the TCA cycle [96,97]. Intracellular lactate-mediated Hif-1α activation drives glycolysis and simultaneously downregulates mitochondrial respiratory function (for reviews see Refs. [98–101]). These processes build a solid basis for progression of the glycolysis machinery, PPP, growth, proliferation, and angiogenesis of tumor cells.
Conclusion
The very fast ATP generation along the glycolysis pathway and serine biosynthesis from the glycolytic intermediate 3-PG, as well as the permanent production of R5P and NADPH by the PPP promotes the autonomy of cancer cells in relation to their proliferative capacity. Mutations promoting activities of the oncogenes and or curbing activities of tumor suppressor genes and the resulted transition from mitochondrial ox phosphorylation to glycolysis force proliferating tumor cells to mimick organelle (mitochondria)-free human erythrocytes. The transferal of the mitochondrial ATP production to the cytosol avoids ATP associated mitochondrial formation of superoxide anion (O2●−) a precursor of most ROS a phenomenon known as truncated TCA cycle and OXPHOS (Figure 12). This is an elegant escape mechanism of proliferating tumor cells and should not be confused with a dysfunction of mitochondria. Comparing the biochemistry of mature human erythrocytes and proliferating tumor cells we are convinced that proliferating tumor cells gain the capacity to grow boundlessly by simply mimicking metabolic of mature human erythrocytes.
Figure 12.

(a) Inverse correlation between aerobic glycolysis and respiration in a proliferating tumor cell. (b) Positive and dynamic interaction between glycolysis and pentose phosphate pathway (PPP). (c) Mitochondrial respiratory chain (MRC) the main source of superoxide anion (O2●−) generation.
Funding Statement
This work was financed by Mehrdad Ghashghaeinia.
Authors’ contributions
M.G. designed the project and mainly wrote the manuscript. All figures were made by M.G. M.G. and I.B. wrote the section transporters. M.G. and U.M. wrote the section glycolysis. M.G. and M.K. wrote the sections polyol and glyoxylate pathways.
Key questions
Do mature human erythrocytes and proliferating tumor cells physically interact with each other?
What are the physiological and biological impacts of such interactions?
Can modulation of these interactions be useful for cancer therapy?
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Sender R, Fuchs S, Milo R.. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Giustarini D, Milzani A, Dalle-Donne I, et al. Red blood cells as a physiological source of glutathione for extracellular fluids. Blood Cells Mol Dis. 2008;40:174–179. [DOI] [PubMed] [Google Scholar]
- [3].Ghashghaeinia M, Giustarini D, Koralkova P, et al. Pharmacological targeting of glucose-6-phosphate dehydrogenase in human erythrocytes by Bay 11-7082, parthenolide and dimethyl fumarate. Sci Rep. 2016;6:28754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ghashghaeinia M, Toulany M, Saki M, et al. The NFkB pathway inhibitors Bay 11-7082 and parthenolide induce programmed cell death in anucleated erythrocytes. Cell Physiol Biochem. 2011;27:45–54. [DOI] [PubMed] [Google Scholar]
- [5].Ghashghaeinia M, Wesseling M, Ramos E, et al. trifluoperazine-induced suicidal erythrocyte death and s-nitrosylation inhibition, reversed by the nitric oxide donor sodium Nitroprusside. Cell Physiol Biochem. 2017;42:1985–1998. [DOI] [PubMed] [Google Scholar]
- [6].Lang KS, Lang P, Bauer C, et al. Mechanisms of suicidal erythrocyte death. Cell Physiol Biochem. 2005;15:195–202. [DOI] [PubMed] [Google Scholar]
- [7].Suhr F, Brenig J, Müller R, et al. Moderate exercise promotes human RBC-NOS activity, NO production and deformability through Akt kinase pathway. PLoS One. 2012;7:e45982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shant J, Cheng K, Marasa BS, et al. Akt-dependent NF-kappaB activation is required for bile acids to rescue colon cancer cells from stress-induced apoptosis. Exp Cell Res. 2009;315:432–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Low PS, Waugh SM, Zinke K, et al. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985;227:531–533. [DOI] [PubMed] [Google Scholar]
- [10].Qadri SM, Bissinger R, Solh Z, et al. Eryptosis in health and disease: A paradigm shift towards understanding the (patho)physiological implications of programmed cell death of erythrocytes. Blood Rev. 2017;31:349–361. [DOI] [PubMed] [Google Scholar]
- [11].Ghashghaeinia M, Cluitmans JCA, Akel A, et al. The impact of erythrocyte age on eryptosis. Br J Haematol. 2012;157:606–614. [DOI] [PubMed] [Google Scholar]
- [12].Willekens FL, Werre JM, Groenen-Döpp YAM, et al. Erythrocyte vesiculation: a self-protective mechanism? Br J Haematol. 2008;141:549–556. [DOI] [PubMed] [Google Scholar]
- [13].Ghashghaeinia M, Cluitmans JC, Toulany M, et al. Age sensitivity of NFkappaB abundance and programmed cell death in erythrocytes induced by NFkappaB inhibitors. Cell Physiol Biochem. 2013;32:801–813. [DOI] [PubMed] [Google Scholar]
- [14].Neelam S, Kakhniashvili DG, Wilkens S, et al. Functional 20S proteasomes in mature human red blood cells. Exp Biol Med (Maywood). 2011;236:580–591. [DOI] [PubMed] [Google Scholar]
- [15].Vitvitsky V, Yadav PK, Kurthen A, et al. Sulfide oxidation by a noncanonical pathway in red blood cells generates thiosulfate and polysulfides. J Biol Chem. 2015;290:8310–8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bippes CC, Feldmann A, Stamova S, et al. A novel modular antigen delivery system for immuno targeting of human 6-sulfo LacNAc-positive blood dendritic cells (SlanDCs. PLoS One. 2011;6:e16315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fonseca AM, Porto G, Uchida K, et al. Red blood cells inhibit activation-induced cell death and oxidative stress in human peripheral blood T lymphocytes. Blood. 2001;97:3152–3160. [DOI] [PubMed] [Google Scholar]
- [18].Melder RJ, Yuan J, Munn LL, et al. Erythrocytes enhance lymphocyte rolling and arrest in vivo. Microvasc Res. 2000;59:316–322. [DOI] [PubMed] [Google Scholar]
- [19].Profumo E, Buttari B, Petrone L, et al. Redox imbalance of red blood cells impacts T lymphocyte homeostasis: implication in carotid atherosclerosis. Thromb Haemost. 2011;106:1117–1126. [DOI] [PubMed] [Google Scholar]
- [20].Darbonne WC, Rice GC, Mohler MA, et al. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991;88:1362–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bahl N, Du R, Winarsih I, et al. Delineation of lipopolysaccharide (LPS)-binding sites on hemoglobin: from in silico predictions to biophysical characterization. J Biol Chem. 2011;286:37793–37803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nelson RA., Jr. The immune-adherence phenomenon: an immunologically specific reaction between microorganisms and erythrocytes leading to enhanced phagocytosis. Science. 1953;118:733–737. [DOI] [PubMed] [Google Scholar]
- [23].Manthei U, Nickells MW, Barnes SH, et al. Identification of a C3b/iC3 binding protein of rabbit platelets and leukocytes. A CR1-like candidate for the immune adherence receptor. J Immunol. 1988;140:1228–1235. [PubMed] [Google Scholar]
- [24].van Lookeren Campagne M, Verschoor A. Pathogen clearance and immune adherence “revisited”: immuno-regulatory roles for CRIg. Semin Immunol. 2018;37:4–11. [DOI] [PubMed] [Google Scholar]
- [25].Kawauchi K, Araki K, Tobiume K, et al. p53 regulates glucose metabolism through an IKK-NF-kappaB pathway and inhibits cell transformation. Nat Cell Biol. 2008;10:611–618. [DOI] [PubMed] [Google Scholar]
- [26].Johnson RF, Witzel II, Perkins ND. p53-dependent regulation of mitochondrial energy production by the RelA subunit of NF-kappaB. Cancer Res. 2011;71:5588–5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mauro C, Leow SC, Anso E, et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat Cell Biol. 2011;13:1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Moretti M, Bennett J, Tornatore L, et al. Cancer: NF-kappaB regulates energy metabolism. Int J Biochem Cell Biol. 2012;44:2238–2243. [DOI] [PubMed] [Google Scholar]
- [29].Parmeggiani A, Bowman RH. Regulation of phosphofructokinase activity by citrate in normal and diabetic muscle. Biochem Biophys Res Commun. 1963;12:268–273. [DOI] [PubMed] [Google Scholar]
- [30].Griguer CE, Oliva CR, Gillespie GY. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neurooncol. 2005;74:123–133. [DOI] [PubMed] [Google Scholar]
- [31].Duan K, Liu Z-J, Hu S-Q, et al. Lactic acid induces lactate transport and glycolysis/OXPHOS interconversion in glioblastoma. Biochem Biophys Res Commun. 2018;503:888–894. [DOI] [PubMed] [Google Scholar]
- [32].Moreno-Sanchez R, Rodriguez-Enriquez S, Marin-Hernandez A, et al. Energy metabolism in tumor cells. Febs J. 2007;274:1393–1418. [DOI] [PubMed] [Google Scholar]
- [33].Shibao S, Minami N, Koike N, et al. Metabolic heterogeneity and plasticity of glioma stem cells in a mouse glioblastoma model. Neuro Oncol. 2018;20:343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Metallo CM, Gameiro PA, Bell EL, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer. Curr Top Med Chem. 2018;18:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–208. [DOI] [PubMed] [Google Scholar]
- [38].Flavin R, Peluso S, Nguyen PL, et al. Fatty acid synthase as a potential therapeutic target in cancer. Future Oncol. 2010;6:551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. [DOI] [PubMed] [Google Scholar]
- [40].Treberg JR, Munro D, Jastroch M, et al. Comparing electron leak in vertebrate muscle mitochondria. Integr Comp Biol. 2018;58:495–505. [DOI] [PubMed] [Google Scholar]
- [41].Husen P, Solov’yov IA. Spontaneous binding of molecular oxygen at the Qo-Site of the bc1 complex could stimulate superoxide formation. J Am Chem Soc. 2016;138:12150–12158. [DOI] [PubMed] [Google Scholar]
- [42].Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015;4:381–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Taylor CT, Pouyssegur J. Oxygen, hypoxia, and stress. Ann N Y Acad Sci. 2007;1113:87–94. [DOI] [PubMed] [Google Scholar]
- [44].Mourrieras F, Foufelle F, Foretz M, et al. Induction of fatty acid synthase and S14 gene expression by glucose, xylitol and dihydroxyacetone in cultured rat hepatocytes is closely correlated with glucose 6-phosphate concentrations. Biochem J. 1997;326(Pt 2):345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Marie S, Diaz-Guerra MJ, Miquerol L, et al. The pyruvate kinase gene as a model for studies of glucose-dependent regulation of gene expression in the endocrine pancreatic beta-cell type. J Biol Chem. 1993;268:23881–23890. [PubMed] [Google Scholar]
- [46].Brookes PS, Land JM, Clark JB, et al. Stimulation of glyceraldehyde-3-phosphate dehydrogenase by oxyhemoglobin. FEBS Lett. 1997;416:90–92. [DOI] [PubMed] [Google Scholar]
- [47].Rapoport S, Luebering J. Glycerate-2,3-diphosphatase. J Biol Chem. 1951;189:683–694. [PubMed] [Google Scholar]
- [48].Benesch R, Benesch RE, Yu CI. Reciprocal binding of oxygen and diphosphoglycerate by human hemoglobin. Proc Natl Acad Sci U S A. 1968;59:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Oslund RC, Su X, Haugbro M, et al. Bisphosphoglycerate mutase controls serine pathway flux via 3-phosphoglycerate. Nat Chem Biol. 2017;13:1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cho J, King JS, Qian X, et al. Dephosphorylation of 2,3-bisphosphoglycerate by MIPP expands the regulatory capacity of the Rapoport-Luebering glycolytic shunt. Proc Natl Acad Sci U S A. 2008;105:5998–6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wang S, Jiang B, Zhang T, et al. Insulin and mTOR pathway regulate HDAC3-mediated deacetylation and activation of PGK1. PLoS Biol. 2015;13:e1002243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Chang CH, Curtis J, Maggi L, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gubser PM, Bantug GR, Razik L, et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat Immunol. 2013;14:1064–1072. [DOI] [PubMed] [Google Scholar]
- [54].Zha X, Wang F, Wang Y, et al. Lactate dehydrogenase B is critical for hyperactive mTOR-mediated tumorigenesis. Cancer Res. 2011;71:13–18. [DOI] [PubMed] [Google Scholar]
- [55].Tambe Y, Hasebe M, Kim CJ, et al. The drs tumor suppressor regulates glucose metabolism via lactate dehydrogenase-B. Mol Carcinog. 2016;55:52–63. [DOI] [PubMed] [Google Scholar]
- [56].Potente M, Carmeliet P. The link between angiogenesis and endothelial metabolism. Annu Rev Physiol. 2017;79:43–66. [DOI] [PubMed] [Google Scholar]
- [57].Lee MN, Ha SH, Kim J, et al. Glycolytic flux signals to mTOR through glyceraldehyde-3-phosphate dehydrogenase-mediated regulation of Rheb. Mol Cell Biol. 2009;29:3991–4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Babcock JT, Quilliam LA. Rheb/mTOR activation and regulation in cancer: novel treatment strategies beyond rapamycin. Curr Drug Targets. 2011;12:1223–1231. [DOI] [PubMed] [Google Scholar]
- [59].Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. [DOI] [PubMed] [Google Scholar]
- [60].Misra HP, Fridovich I. The generation of superoxide radical during the autoxidation of hemoglobin. J Biol Chem. 1972;247:6960–6962. [PubMed] [Google Scholar]
- [61].Gotoh T, Shikama K. Generation of the superoxide radical during autoxidation of oxymyoglobin. J Biochem. 1976;80:397–399. [DOI] [PubMed] [Google Scholar]
- [62].Fox IH, Kelley WN. Human phosphoribosylpyrophosphate synthetase. Distribution, purification, and properties. J Biol Chem. 1971;246:5739–5748. [PubMed] [Google Scholar]
- [63].Meyskens FL, Williams HE. Concentration and synthesis of phosphoribosylpyrophosphate in erythrocytes from normal, hyperuricemic, and gouty subjects. Metabolism. 1971;20:731–742. [DOI] [PubMed] [Google Scholar]
- [64].Galter D, Mihm S, Droge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem. 1994;221:639–648. [DOI] [PubMed] [Google Scholar]
- [65].Matthews JR, Wakasugi N, Virelizier JL, et al. Thioredoxin regulates the DNA binding activity of NF-kappa B by reduction of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Mihm S, Galter D, Droge W. Modulation of transcription factor NF kappa B activity by intracellular glutathione levels and by variations of the extracellular cysteine supply. Faseb J. 1995;9:246–252. [DOI] [PubMed] [Google Scholar]
- [67].Atkinson DE, Walton GM. Adenosine triphosphate conservation in metabolic regulation. Rat liver citrate cleavage enzyme. J Biol Chem. 1967;242:3239–3241. [PubMed] [Google Scholar]
- [68].Clasquin MF, Melamud E, Singer A, et al. Riboneogenesis in yeast. Cell. 2011;145:969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Morrison AD, Clements RS Jr., Travis SB, et al. Glucose utilization by the polyol pathway in human erythrocytes. Biochem Biophys Res Commun. 1970;40:199–205. [DOI] [PubMed] [Google Scholar]
- [70].Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S–623S. [DOI] [PubMed] [Google Scholar]
- [71].Putman SJ, Coulson AF, Farley IR, et al. Specificity and kinetics of triose phosphate isomerase from chicken muscle. Biochem J. 1972;129:301–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Brandt RB, Siegel SA. Methylglyoxal production in human blood. Ciba Found Symp. 1978;67:211–223. [DOI] [PubMed] [Google Scholar]
- [73].Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. [DOI] [PubMed] [Google Scholar]
- [74].Kondoh H, Lleonart ME, Nakashima Y. A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid Redox Signal. 2007;9:293–299. [DOI] [PubMed] [Google Scholar]
- [75].Spencer TL, Lehninger AL. L-lactate transport in Ehrlich ascites-tumour cells. Biochem J. 1976;154:405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Broer S, Rahman B, Pellegri G, et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J Biol Chem. 1997;272:30096–30102. [DOI] [PubMed] [Google Scholar]
- [77].Fischer K, Anensen N, Hovland R, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood. 2007;109:3812–3819. [DOI] [PubMed] [Google Scholar]
- [78].Broer S, Schneider H-P, Bröer A, et al. Characterization of the monocarboxylate transporter 1 expressed in Xenopus laevis oocytes by changes in cytosolic pH. Biochem J. 1998;333(Pt 1):167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Doyen J, Trastour C, Ettore F, et al. Expression of the hypoxia-inducible monocarboxylate transporter MCT4 is increased in triple negative breast cancer and correlates independently with clinical outcome. Biochem Biophys Res Commun. 2014;451:54–61. [DOI] [PubMed] [Google Scholar]
- [80].Dimmer KS, Friedrich B, Lang F, et al. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J. 2000;350(Pt 1):219–227. [PMC free article] [PubMed] [Google Scholar]
- [81].Bourguignon LY, Singleton PA, Diedrich F, et al. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem. 2004;279:26991–27007. [DOI] [PubMed] [Google Scholar]
- [82].Amith SR, Fong S, Baksh S, et al. Na (+)/H (+)exchange in the tumour microenvironment: does NHE1 drive breast cancer carcinogenesis? Int J Dev Biol. 2015;59:367–377. [DOI] [PubMed] [Google Scholar]
- [83].Martinez-Zaguilan R, Lynch RM, Martinez GM, et al. Vacuolar-type H(+)-ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol. 1993;265:C1015–1029. [DOI] [PubMed] [Google Scholar]
- [84].Nishi T, Forgac M. The vacuolar (H+)-ATPases–nature’s most versatile proton pumps. Nat Rev Mol Cell Biol. 2002;3:94–103. [DOI] [PubMed] [Google Scholar]
- [85].Chiche J, Ilc K, Laferrière J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 2009;69:358–368. [DOI] [PubMed] [Google Scholar]
- [86].Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. [DOI] [PubMed] [Google Scholar]
- [87].Lee S, Mele M, Vahl P, et al. Na+,HCO3- -cotransport is functionally upregulated during human breast carcinogenesis and required for the inverted pH gradient across the plasma membrane. Pflugers Arch. 2015;467:367–377. [DOI] [PubMed] [Google Scholar]
- [88].Lee S, Axelsen TV, Andersen AP, et al. Disrupting Na(+), HCO(3)(-)-cotransporter NBCn1 (Slc4a7) delays murine breast cancer development. Oncogene. 2016;35:2112–2122. [DOI] [PubMed] [Google Scholar]
- [89].Romero MF, Chen AP, Parker MD, et al. The SLC4 family of bicarbonate (HCO(3)(-)) transporters. Mol Aspects Med. 2013;34:159–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Busco G, Cardone RA, Greco MR, et al. NHE1 promotes invadopodial ECM proteolysis through acidification of the peri-invadopodial space. Faseb J. 2010;24:3903–3915. [DOI] [PubMed] [Google Scholar]
- [91].Fliegel L. The Na+/H+ exchanger isoform 1. Int J Biochem Cell Biol. 2005;37:33–37. [DOI] [PubMed] [Google Scholar]
- [92].Stock C, Schwab A. Protons make tumor cells move like clockwork. Pflugers Arch. 2009;458:981–992. [DOI] [PubMed] [Google Scholar]
- [93].Fan R, Hou W-J, Zhao Y-J, et al. Overexpression of HPV16 E6/E7 mediated HIF-1alpha upregulation of GLUT1 expression in lung cancer cells. Tumour Biol. 2016;37:4655–4663. [DOI] [PubMed] [Google Scholar]
- [94].Dgehne N, Brune B. HIF-1 in the inflammatory microenvironment. Exp Cell Res. 2009;315:1791–1797. [DOI] [PubMed] [Google Scholar]
- [95].Firth JD, Ebert BL, Pugh CW, et al. Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3ʹ enhancer. Proc Natl Acad Sci U S A. 1994;91:6496–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kim JW, Tchernyshyov I, Semenza GL, et al. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. [DOI] [PubMed] [Google Scholar]
- [97].Papandreou I, Cairns RA, Fontana L, et al. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. [DOI] [PubMed] [Google Scholar]
- [98].Weidemann A, Johnson RS. Biology of HIF-1alpha. Cell Death Differ. 2008;15:621–627. [DOI] [PubMed] [Google Scholar]
- [99].Payen VL, Porporato PE, Baselet B, et al. Metabolic changes associated with tumor metastasis, part 1: tumor pH, glycolysis and the pentose phosphate pathway. Cell Mol Life Sci. 2016;73:1333–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Shaw RJ. Glucose metabolism and cancer. Curr Opin Cell Biol. 2006;18:598–608. [DOI] [PubMed] [Google Scholar]
- [101].Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. [DOI] [PubMed] [Google Scholar]
- [102].Duhm J, Deuticke B, Gerlach E. Metabolism of 2,3-diphosphoglycerate and glycolysis in humna red blood cells under the infleucne of dipyridamole and inorganic sulfur compounds. Biochim Biophys Acta. 1968;170:452–454. [DOI] [PubMed] [Google Scholar]


