Abstract
The diagnosis of neuropathic postural tachycardia syndrome (POTS) requires research techniques not available clinically. We hypothesized that these patients will have impaired vagal and sympathetic cardiovascular control that can be characterized with clinical autonomic tests. We included 12 POTS patients with possible neuropathic subtype because of normal plasma norepinephrine and no increase in upright blood pressure (BP). We compared them to 10 healthy subjects. We assessed hemodynamics, raw (rMSNA) and integrated neural activity (iMSNA) during autonomic testing, heart rate (HR) and BP variability, baroreflex sensitivity (BRS), and blood volume. In order to understand the vagal/sympathetic control we dissect the phase 2 of Valsalva maneuver (VM) into early (VM2e) and late (VM2l). POTS’ upright HR increased 43±3 bpm. Patients had normal plasma volume but reduced red blood cell volume (1.29 L vs. predicted normal values 1.58 L, p=0.02). Vagal indices of HR variability, HFRRI (430±130 vs.1680±900, p=0.04), PNN50 and RMSSD were lower in POTS. Patients showed a decrease in vagal BRS (VM2e, p=0.04). In POTS, iMSNA was lower at rest (12±1.5 vs. 20±2 burst/min, p=0.004) and rMSNA spike analysis showed blunted responses during VM2e, despite a greater drop in systolic blood pressure (34±5 in POTS and 14±6 mmHg in controls, p=0.01). This cohort of POTS patients enriched for possible neuropathic subtype had lower resting MSNA, impaired vagal cardiac control and exaggerated drop in BP in response to VM, and a delay in the sympathetic cardiovascular responsiveness during hypotensive challenge.
Keywords: Neuropathic POTS, Sympathetic, Vagal, Muscle sympathetic nerve activity, Valsalva Maneuver, Baroreflex control
Introduction
Postural tachycardia syndrome (POTS) is an increasingly recognized cause of functional disability in otherwise healthy young women. The prevalence of POTS is about 0.2%,1 it affects millions of people around the world, and is most common among otherwise healthy women of child-bearing age.2 The syndrome is characterized by frequent symptoms while upright that limit their ability to stand, and are relieved with lying down. Common symptoms include lightheadedness, mental clouding, blurred vision, irritation, palpitation, and chest discomfort. Symptoms should last for at least six months.3, 4 The hallmark of POTS is the remarkable increase in heart rate upon standing, greater than 30 bpm, in the absence of a significant drop in blood pressure.5
Multiple pathophysiologies have been described in order to explain the symptoms and findings in POTS, supporting the assumption that this syndrome is heterogeneous.6, 7 Despite the controversies of possible mechanistic causalities, altered autonomic cardiovascular regulation and disturbed plasma volume homeostasis are among the most intriguing mechanisms involved in this syndrome.8–11
We and others have reported that a subset of patients with POTS have distinct autonomic abnormalities, including reduced release of norepinephrine (NE) and abnormal NE clearance in lower limbs,12 reduced sympathetic cholinergic sudomotor activity,13, 14 increased systemic alpha1 and beta1-adrenoceptor sensitivity,12 and increased alpha-adrenoreceptor sensitivity in the superficial leg skin veins15. These finding are compatible with a partial sympathetic denervation of the lower extremities and have been used to defined “neuropathic POTS”.16
We hypothesized that patients with neuropathic POTS will have impaired vagal and sympathetic cardiovascular control. Because there are no standardized criteria to identify neuropathic POTS, we studied patients that fulfill the classical criteria for POTS17 but who did not have an exaggerated orthostatic increase in plasma catecholamine (>600 pg/ml) or the paradoxical orthostatic increase in BP characteristic of hyperadrenergic POTS.8 In this cohort of patients enriched for likely neuropathic POTS, we assessed vagal (spectral analysis of heart rate) and sympathetic (direct measurement of muscle sympathetic nerve activity, MSNA) regulation in response to the Valsalva’s maneuver, and compared their responses to healthy controls.
Our short-term goal was to determine if the response to Valsalva’s maneuver can be a simple useful tool for identifying patients with neuropathic POTS. Our long-term goal is to improve our understanding of the autonomic pathophysiology of neuropathic POTS, in order to further help to personalize their treatment.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Subjects:
Twelve female patients with POTS, referred to our tertiary Autonomic Dysfunction Center at Vanderbilt University, were selected after fulfilling the following criteria: chronic orthostatic symptoms such as dizziness, syncope, palpitation and chest tightness, fatigue, unable to stand for long time; an increase in HR more than 30 beats/ minute after an orthostatic challenge, either during head up tilt or standing still up to 10 minutes; upright plasma norepinephrine concentration not exceeding 600 pg/ml during an in-patient evaluation off medications; and no significant decrease or increase in systolic BP upon orthostatic challenge for at least 6 months. Patients were excluded if other medical condition explained their illness. Patients were compared to ten age- and sex-matched female healthy subjects that were recruited from our local pool of volunteers.
Protocol:
The study protocol was approved by the Vanderbilt University Institutional Review Board in Human Research. Patients were admitted to our center after discontinuing their medication for at least two weeks. After signing an informed consent, all subjects went through a medical history and physical examination, and completed an autonomic symptom questionnaire. Participants were on a control diet containing 150 mEq of sodium and 70 mEq of potassium for at least 3 days preceding the studies. They were not allowed to have any food or beverages containing caffeine, stimulants or other methylxantines. All participants were non-smokers. They refrained from exercise before each study. Each subject collected urine for 24 hours for catecholamine determination. The study was performed over two consecutive days. Following overnight fasting, blood and plasma volume (PV) were assessed for patients on the first study day. Thereafter, supine and upright plasma catecholamines, and autonomic function tests were assessed. On the second day, we recorded the neural sympathetic and hemodynamic response to the Valsalva’s maneuver (VM) in the supine posture, and the response to head up tilt. The tilt table protocol was a graded tilt table with increase of tilt angle by 15 degrees for each 5 minutes up to 75 degrees. The maximal tilt time was 30 minutes at maximal tilt angle or as tolerated. If the subject could not tolerate maximal tilt, then the last 5 minutes period (without a pre-syncopal event) was used for analysis.
Instrumentations and measurements
Autonomic Function Tests:
Orthostatic vital signs were determined by measuring heart rate (HR) and brachial BP using oscillometric brachial BP (Vital-Guard 450C, Ivy Biomedical Systems, Inc., Branford, CT) during upright posture after overnight supine period. In all following autonomic tests, continuous 3 lead ECG and continuous blood pressure waveform were measured on the middle finger of the non-dominant hand (Finapres, Ohmeda, Englewood, CO) and verified using brachial BP on the contralateral arm. Cardiovascular sympathetic indices were assessed as: 1) maximal decrease in systolic BP during one minute of hyperventilation; 2) maximal increase in systolic BP during hand grip (Model 76618, Lafayette Instrument Co., Indiana) at 30% of the maximal voluntary contraction for three minutes; 3) increase in systolic BP after immersion of the contra-lateral hand in iced water for one minute (Cold Pressor test); 4) maximal decrease in systolic BP during early phase 2 of the VM (VM2e, see details in the Online Supplement, Figure S2); and 5) pressure recovery time after the Valsalva maneuver (PRT, the amount of time required for the systolic BP to reach baseline from its nadir during phase 2I of VM, Figure S2). Cardio-vagal indices were assessed as: 1) sinus arrhythmia ratio, determined during 60 seconds of controlled breathing with 6 breaths per minutes and calculated as the ratio of the longest to the shortest R-R interval; 2) heart rate ratio during the VM, calculated from the maximal HR during straining divided by the lowest HR during the overshoot phase 4).18
Phase 2 of the VM was divided into two phases (VM2e, early and VM2l, late) as follows: the beginning of VM2e was considered as the first decrease in diastolic BP after phase I; the beginning of VM2l (also the end of VM2e) was defined as the first increase in diastolic BP after the nadir (see details in the Online Supplement Figure S2).
Blood Volume and Catecholamines:
Patients were on a diet containing 150 mEq of sodium and 70 mEq of potassium for at least three days prior to the study. After remaining supine overnight, subjects underwent assessment of PV and Hct. The blood volume analysis was performed by the radiopharmaceutical tracer dilution technique utilizing a calibrated dose of iodine (I-131) labeled human serum albumin (Volumex, DAXOR Corp, New York, NY) at 25 μCi. Predicted normal blood volumes and hematocrit were calculated as proposed by Feldschuh et al 1977 and others.19–21
Plasma and urine concentrations of norepinephrine and epinephrine were assayed by HPLC, in a method modified from Goldstein et al.22
Muscle Sympathetic Nerve activity:
Muscle sympathetic nerve activity (MSNA) was recorded from the peroneal nerve of the left leg as detailed in the Online Supplement and elsewhere.23 Satisfactory recordings of MSNA were defined by (1) heart pulse synchronicity; (2) activation during VM straining and suppression during the hypertensive overshoot phase after VM release; (3) increases in response to breath-holding; and (4) no change during tactile or auditory stimulation.24, 25 MSNA activity was assessed while supine, during controlled VM with exhaled strength up to 40 mmHg for 15 seconds, and during last 5 minutes of HUT.
Time and frequency domain analysis of HR variability (HRV), BP variability, and baroreflex sensitivity:
For HRV estimation in the time domain, root mean squared of successive differences of R-R intervals (RMSSD RRI) and percentage of interval changes greater than 50 milliseconds to normal sinus R-R intervals (PNN50) were calculated.26
For HRV estimation in the frequency domain, beat-to-beat values of detected RRI and systolic BP values were interpolated, low-pass filtered (cutoff 0.5 Hz) and re-sampled at 4 Hz. Data segments of 300 seconds were used for spectral analysis. Linear trends were removed and power spectral density was estimated with the FFT-based Welch algorithm using segments of 256 data points with 50% overlapping and Hanning window. The power in the frequency range of low frequencies (LF: 0.04 to 0.15 Hz) and high frequencies (HF: 0.15 to 0.40 Hz) was calculated following Task Force recommendations.27, 28
Spectral Baroreflex Gain (alpha-index) was computed as the root square of the ratios of the spectral powers of RRI to systolic BP in the LF and HF frequencies ranges, respectively.29.
Spontaneous Baroreflex Slope was calculated from the linear regression line between the systolic BP and the subsequent R-R intervals using sequences defined as episodes of systolic BP rise with RRI prolongation (BRSseq_up) or BP decrease with RRI shortening (BRSseq_down) for more than 0.5 mmHg per beat over at least 3 heart beats. Average values of all slopes with a correlation coefficient greater than 0.85 were calculated for BRSseq_up and BRSseq_down.30
Baroreflex Slope extrapolated from Valsalva’s maneuver. The cardio-vagal BRS was assessed during two phases. The first slope was extrapolated from plotting the changes in systolic BP against the changes in RRI during phase VM2e. The second slope was extrapolated from the same parameters during recovery phases, 3 and 4 of VM.31 The sympathetic BRS was calculated as the ratio between drop in BP, during phase 3 and the pressure recovery time (PRT).32, 33 , 34
Spike Detection in raw MSNA.
Action-potential spikes were detected in the raw neurogram recordings using two-stage kurtosis wavelet denoising and invariant stationary wavelet transform. Please see details in the Online Supplement. Action potentials were detected in the denoised MSNA signal and a resultant instantaneous spike rate series was constructed. Spike frequency parameters for each heart beat were estimated using mean, median, and maximum spike frequency for each beat-to-beat interval and smoothed with a sliding median filter over three heart beats.24, 35, 36.
Statistics:
Results are presented as mean±SD and statistical significance was set at 0.05. Parametric and non-parametric paired two-sided t-test or Wilcoxon test was used for comparison between measurements. A linear correlation was used to calculate the corresponding baroreflex slopes. Data was analyzed with Matlab (Mathworks, Natick, MA, USA), Microsoft Office Excel, and Prizm version 6.02 (GraphPad Software Inc., LA, Jolla, CA, USA).
Results
General data:
Participants’ general characteristics and symptoms are depicted in Table 1. The prevalence of syncope was high (75%) in this cohort of POTS patients, but the frequency of episodes was low, and had the clinical characteristics of vaso-vagal events. One control subject had a single episode of syncope during a febrile illness. Patients with POTS had a significant reduction in red blood cell volume (−18±1.4% of predicted normal values (p<0.05). Plasma and total blood volume were not significantly different as compared to the predicted normal values (0±0% and −7±1.3%, respectively).
Table 1:
General characteristics, blood and plasma volumes, urinary catecholamines, and symptoms of patients with postural tachycardia syndrome (POTS, N=12) and healthy controls (N=10).
| Parameter | POTS | Controls |
|---|---|---|
| General | ||
| Age, years | 30±1.8 | 32±3 |
| Body Mass Index, kg/m2 | 23±1 | 23±1 |
| Blood and Plasma Volume | ||
| Blood Volume, Liters | 4.08 ±0.05 | - |
| Plasma Volume, Liters | 2.8±0.03 | - |
| Δ BV% (from predicted normal values) | −7±1.3 | - |
| Δ PV% (from predicted normal values) | 0±0 | - |
| Δ RBC% (from predicted normal values) | −18±1.4 | - |
| 24 hours Urine | ||
| Norepinephrine μg/l | 3.2±5 | 3±5 |
| Epinephrine, μg/l | 5.1±1.2 | 5.1±1.1 |
| Symptoms | ||
| Duration of symptoms, years | 3.5±0.9 | - |
| Diagnosis POTS, years | 2.5±0.5 | - |
| Dizziness/lightheadedness | 11(95%) | 1 (10%) |
| Blurred vision | 3 (25%) | none |
| Sleep disorders | 5 (40%) | none |
| Headache | 6 (50%) | none |
| Syncope | 9 (75%) | 1 (10%) |
| Palpitation / Chest discomfort | 8 (67%) | |
| Fatigue | 8 (67%) | none |
| Bluish legs | 5 (40%) | none |
| Weight loss, cyclic | 3 (25%) | none |
| Irritable bowel syndrome | 4 (25%) | none |
| Fibromyalgia | 2 (16%) | none |
Hemodynamic, autonomic function tests and catecholamines:
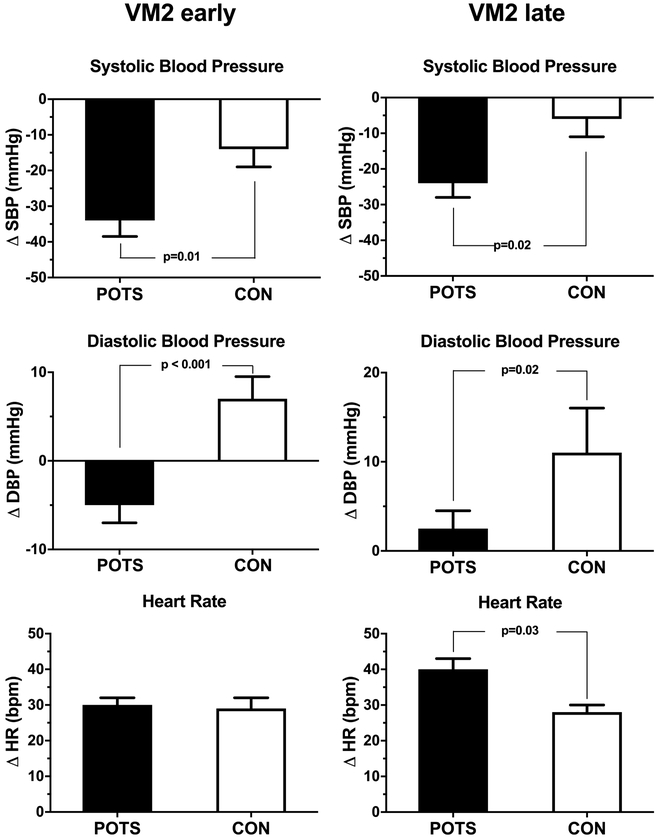
Blood pressure during supine and HUT was comparable in both groups (Table 2). In response to HUT, the HR increased 40±3 bpm in POTS and 16±3 bpm in controls, (p<0.001). Autonomic function tests showed that the sinus arrhythmia ratio was lower in patients compared to controls (p<0.001), but VM heart rate ratio was similar between groups. The POTS group had a significant decrease in systolic BP during phase 2 of the VM (p<0.05), whereas BP overshoot during phase 4 was not higher (p=0.40).
Table 2:
Autonomic function characteristics during supine and last 5 minutes of graded head up tilt (HUT), hyperventilation, handgrip, Valsalva maneuver, and deep breathing in patients with postural tachycardia syndrome (POTS, N=12) and healthy control (N=10).
| Parameter | POTS | Control | ||
|---|---|---|---|---|
| Supine | HUT | Supine | HUT | |
| Tilt Table Test | ||||
| HR, bpm | 78±3 | 122±5* | 66±3 | 85±3 |
| Systolic BP, mmHg | 103±2 | 107±3 | 107±2.5 | 110±2.5 |
| Diastolic BP, mmHg | 66±1.5 | 71±3 | 69±2.5 | 73±2 |
| iMSNA, burst/min | 12±1.5* | 38±3 | 20±2 | 42±5 |
| iMSNA, burst/100b | 14.3±2* | 29±3 | 31±3 | 41±4 |
| iMSNA, burst/100b | 2.5±0.4* | 12.5±2.5 | 5±0.5 | 12±2 |
| Norepinephrine, pg/ml | 164±14 | 503±55 | 209±32 | 480±50 |
| DHPG / NE ratio | 7.5±1 | 3.3±0.4 | 6.9±0.6 | 3.4±0.3 |
| Epinephrine, pg/ml | 22±3 | 80±10 | 14±3 | 110±20 |
|
Autonomic Function Test Sympathetic Response |
||||
| Δ BP Hyperventilation, mmHg | −8±3 | −9±3 | ||
| Δ BP Hand Grip, mmHg | 12±2.5 | 7.5±1.5 | ||
| Δ BP Cold Pressor Test, mmHg | 20±4 | 17±5 | ||
| Δ BP Valsalva Phase 2, mmHg | −32±5.8* | −14±4 | ||
| Δ BP Valsalva Phase 4, mmHg | 32±3 | 25±5 | ||
| Vagal Response | ||||
| Deep Breathing RSA Ratio | 1.3±0.01* | 1.6±0.05 | ||
| Valsalva Ratio | 2.0±0.1 | 1.9±0.1 | ||
HR, Heart Rate, BP, Blood Pressure, iMSNA, Integrated Muscle Sympathetic Activity, DHPG, Dihydroxyphenylglycol, NE, Norepinephrine, RSA, Respiratory Sinus Arrhythmia
significant group differences between POTS and controls, p<0.05
For the purpose of the present study, we dissected the hemodynamic profile of VM phase 2, comparing early phase (VM2e) to the late phase (VM2l). As shown in Figure 1 and 2, the decrease in systolic BP during VM2e was significantly greater in POTS, and did not recover to baseline level during VM2l (Figure 1, left; Figure 2, upper panel). Diastolic BP decreased only in POTS during VM2e and showed a blunted increase during VM2l (Figure 2, middle panel). Interestingly, both groups demonstrated similar HR increase during VM2e. But the HR increase during VM2l was significantly higher in patients (Figure 2, lower panel). A representative zoomed view during straining (Figure 1) is presented in the Online Supplement (Figure S3).
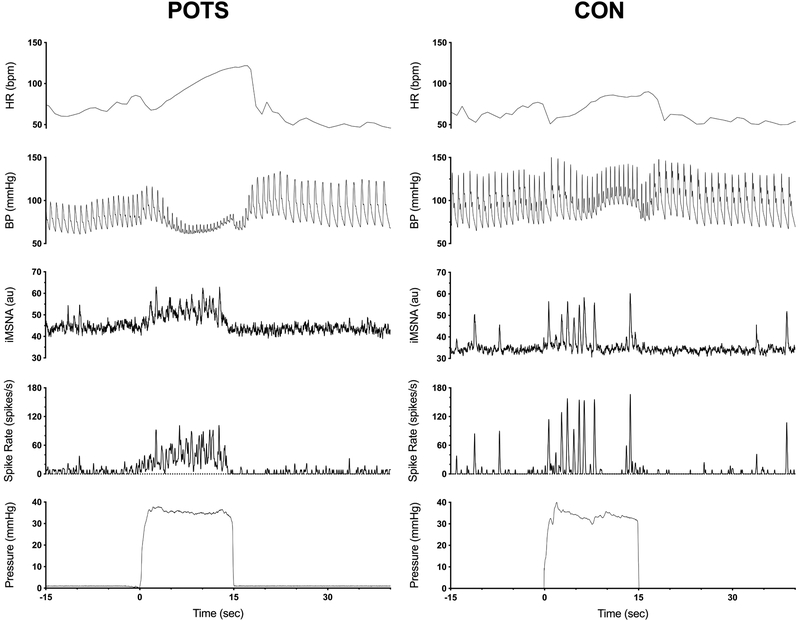
Figure 1.
Representative response of heart rate, blood pressure, integrated muscle sympathetic nerve activity (iMSNA), instantaneous spike frequency and straining pressure to Valsalva Maneuver in a patient with neuropathic postural tachycardia syndrome (POTS) and healthy volunteer. Spike frequency was derived from wavelet denoised raw MSNA and is expressed as number of spikes per seconds.
Figure 2.
Averaged changes of systolic (SBP) and diastolic (DBP) blood pressure and heart rate (HR) during early (VM2e) and late phase two (VM2l) of Valsalva in patients with postural tachycardia syndrome (POTS) and healthy controls (CON).
Plasma concentrations of norepinephrine (NE), epinephrine (EP), and dihydroxyphenylglycol to NE ratio (DHPG/NE) were similar in both groups during supine and upon standing up to 30 minutes. DHPG/NE ratio decreases similarly and significantly (p<0.002), in both groups. In addition, 24 hours urine catecholamines were similar (see Table 2).
Muscle sympathetic nerve activity:
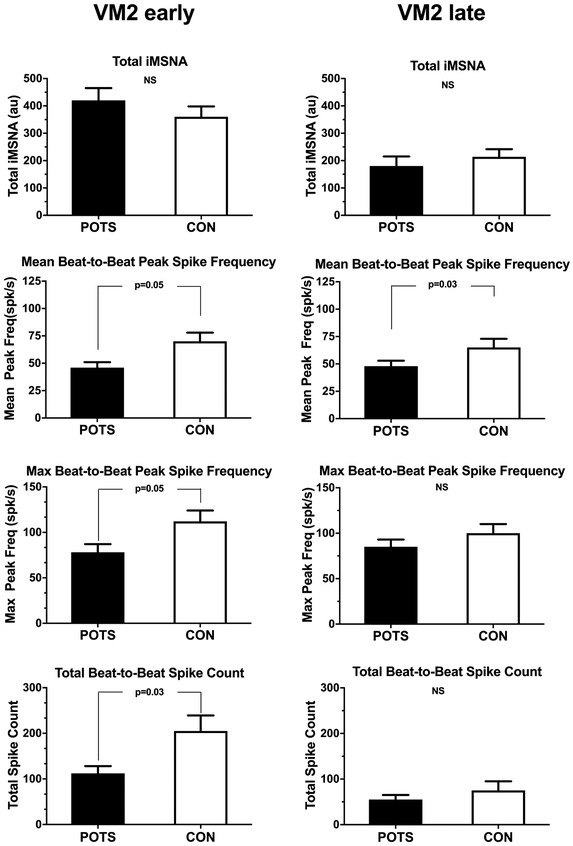
Integrated MSNA (iMSNA), expressed as burst/minute, burst/100b and area averaged over 5 minutes, measured during rest supine and during the last 5 minutes of HUT are shown in Table 2. Patients showed lower iMSNA during supine, but the increase was similar during HUT. Of note, iMSNA bursts/100b during HUT tended to be lower in POTS, but did not reach statistical significance. Also, total iMSNA was comparable during VM2e and VM2l, as shown in Figure 3 (upper panel).
Figure 3.
Averaged sympathetic response during early (VM2e, left panels) and late phase 2 (VM2l, right panel) of the Valsalva maneuver in patients with postural tachycardia syndrome (POTS) and healthy controls (CON). Response are derived from integrated muscle sympathetic activity (iMSNA) and action potential detection on the. raw neurogram (mean and peak spike frequency, and total spike count).
However, during VM2e, instantaneous averaged spike rate (POTS 19±3 vs. CON 30±4 spikes/sec, p=0.04) and maximal spike rate (75±10 vs. 114±14 spikes/s, p=0.03) were lower in patients as compared to controls (Online Supplement, Table S1). During VM2l, mean spike rate tend to be lower in patients (21±4 vs. 35±5 spikes/sec, p=0.06) but maximal spike rate was comparable in both groups (85±9 vs. 108±13 spikes/sec, p=0.16). Total beat-to-beat spike count was also significantly lower during VM2e in POTS, and comparable during VM2l between the groups, as shown in Figure 3 (second panel, for total data). Mean and maximal beat-to-beat peak spike frequency was lower in POTS during VM2e as shown in Figure 3 (bottom panels and Table S1). A representative expanded view during straining (Figure 1) is presented in the Online Supplement (Figure S3). This example shows that the organization of burst pattern changed to one with a weaker beat to beat synchronicity.
Spectral analysis of heart rate and blood pressure:
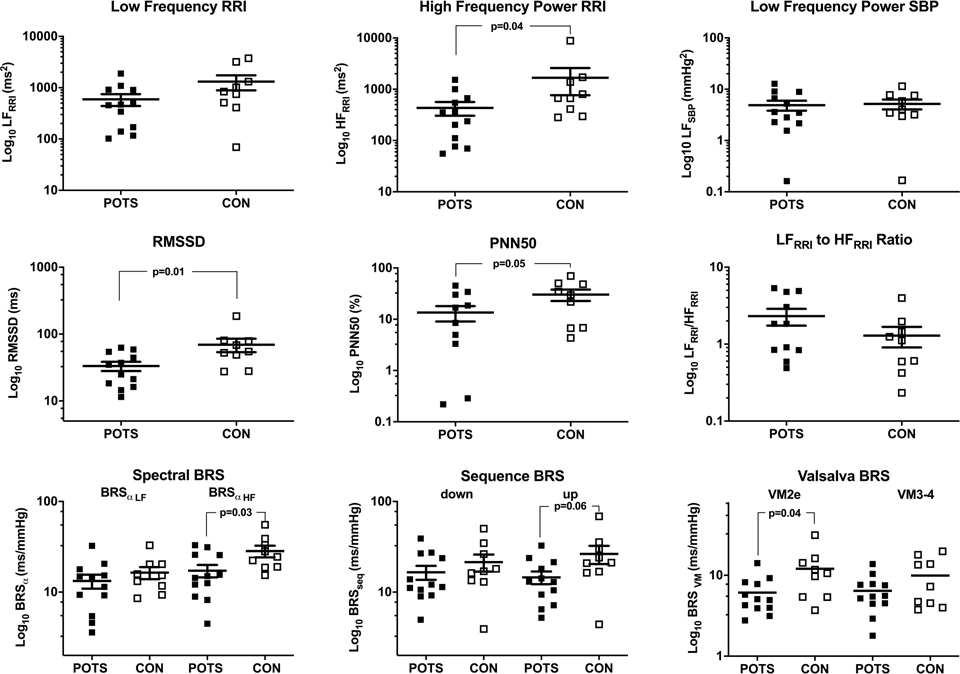
As shown in Figure 4, vagal indices of the frequency and time-domain analysis of the HR variability, HFRRI, PN50% and RMSSD, were significantly lower in patients compared to controls. But none of the sympathetic indices of HR and BP variability were different. However, LFRRI to HFRRI ratio tended to be higher in patients without reaching statistical significance.
Figure 4.
Spectral Characteristics of patients with postural tachycardia syndrome (POTS) and healthy controls (CON). RRI – R-R intervals. SBP – systolic blood pressure. LF – Low frequency power. HF- high frequency power. RMSSD – root of mean square of successive differences. PNN50 – percentage of normal heart beats with beat to beat differences less than 50 msec. BRS – baroreflex sensitivity by spectral analysis (Spectral BRS), or spontaneous sequence technique (Sequence BRS), or regression analysis of Valsalva response (Valsalva BRS).
Baroreflex sensitivity and rates:
As shown in Figure 4 (lower panel), both spectral and sequence methods showed significant change in the cardiac vagal BRS. The decrease in RR intervals in response to the decrease in BP (BRS seq_down) was preserved in patients compared to healthy controls. Interestingly, POTS patients showed a significant decrease in their vagal BRS as extrapolated from the response from the BP fall and the corresponding RR intervals shortening VM2e (p=0.04), but not from the VM4, where BP and RRI increase (p=0.35). The sympathetic BRS (Drop in SBP during phase 3 divided by PRT) was −16±1.2 in patients with POTS and −13.3±1.75 mmHg/sec in controls, p=0.23.
The rate of the rise in HR during VM2 was 4.3±0.3 beat/second in patients and 4.1±0.3 in controls (p=0.65). Pressure recovery time (PRT), the amount of time required for the systolic BP to reach baseline level from the nadir (phase 3 of VM) was similar in POTS compared to controls (2.25s±0.23 and 1.94s±0.17, respectively, p=0.35).
Discussion
The remarkable increase in HR upon standing, the nature of the symptoms, and the presence of high plasma norepinephrine in half of the patients with POTS would suggest that this syndrome is universally characterized by a “hyperadrenergic state”. However, we previously found that in some patients with POTS the high circulating plasma norepinephrine was due to a decrease in neuronal norepinephrine clearance rather than to an increase in systemic norepinephrine spillover.12, 37 Also, we found a defective norepinephrine spillover circumscribed to the lower extremities, whereas it was preserved in the upper extremities of these patients with POTS. This finding agrees with others showing a loss of sweating ability in the feet, as revealed by quantitative sudomotor axon reflex testing14 and hypersensitivity of alpha-1-adrenoreceptors in lower extremity veins in some patients with POTS.15 Therefore, this subgroup of patients was named “neuropathic POTS”.16
Even if the existence of a subset of patients with neuropathic POTS is generally recognized, the clinical criteria for identifying such patients has not been defined .8, 38 The disorder was originally recognized based on norepinephrine spillover methodology, but this is not available clinically.16, 39 Also, Gibbsons et al found that leg skin biopsy analysis of intradermal nerve fiber density is not universally helpful in distinguishing neuropathic from non-neuropathic POTS, because healthy controls could have similar findings.40 In the present study we excluded patients with POTS that had an exaggerated increase in blood pressure or plasma norepinephrine on standing, to enrich our cohort with those with “likely” neuropathic POTS. This cohort was characterized by an exaggerated decrease in SBP during early phase 2 of Valsalva and attenuated blood pressure recovery during late phase 2. Importantly, these findings were associated with lower sympathetic response to these blood pressure decreases, implying that indeed these patients likely correspond to neuropathic POTS.
MSNA is an important research tool but is not widely available. It is important, therefore, to highlight the autonomic phenotypic characteristics of the POTS patients described here as likely neuropathic. We found that these patients had: 1) a decrease in indices of vagal cardiac control (HFRRI), 2) reduced cardiovagal baroreflex gain, 3) exaggerated decrease in SBP during early phase 2 of Valsalva, and 4) attenuated blood pressure recovery during late phase 2 of Valsalva. Resting supine vagal indices of HR variability, both in the time and frequency domains (HFRRI, RMSSD and PNN50%) were significantly lower in our POTS patients compared to control. Likewise, all methods applied in this study to extrapolate the BRS showed impaired cardio-vagal baroreflex sensitivity during phase 2 of the VM. This allows us to clinically identify patients with likely neuropathic POTS, in whom impaired hemodynamic changes in the VM correlated with blunted responses of the sympathetic nervous system.
Hemodynamic responses to the VM have been suggested as helpful in the identification of POTS patient subsets. An exaggerated overshoot of BP during VM phase 4 was consider suggestive of hyperadrenergic POTS,8 whereas absence or attenuated increase in BP during VM2l was attributed as indicative of neuropathic POTS.14, 41 Our study, which included direct measurements of sympathetic nerve traffic, supports the latter.
Baroreflex sensitivity has an essential role in blood pressure and heart rate control and it reflects the functional integrity of the autonomic nervous system. In patients with widespread autonomic failure, the severity of the autonomic impairment correlates with the extent of the decrease in cardiac vagal baroreflex sensitivity, extrapolated from the VM.42 In neuropathic POTS the autonomic impairment is partial, as evidence by preserved orthostatic BP. However, there is no agreement from previous studies about the baroreflex control of HR in patients with POTS. Jacob et al12 and Stewart et la43 reported a decrease in the vagal arm of baroreflex, while Bonyhay et al44 and Muenter et al45 showed an increase in the sympathetic arm with preserved vagal arm of the baroreflex. Mustafa et al46 found only the sympathetic arm of baroreflex was compromised. However, Furlan et al47 and Jordan et al48 reported a normal function of the baroreflex. Similarly, confounding data exists on HR variability analysis, of both time- and frequency domains, in patients with POTS. Most reports showed high LFRRI/HFRRI ratio, and this has been interpreted as increased sympathetic control on HR, but the higher ratio was often due to very low HFRRI, rather than to a high LFRRI, which suggests for a compromised vagal cardiac control.43, 47, 49 We and others have suggested that these contradictory reports are the results of the heterogeneity of the syndrome and the inclusion of patients with neuropathic and hyperadrenergic subtypes.44 In our “neuropathic” cohort of POTS patients we found that the BRS extracted from VM2e (dominant cardiovagal inhibitory phase) is decreased, but the BRS extracted from VM3–4 (maximal sympathetic activation followed by cardiovagal activation) is preserved.
Direct sympathetic activity recording (MSNA) offers superior information on the central sympathetic control of the cardiovascular system. Previous reports about MSNA in POTS also suffer from inclusion of a heterogeneous group of patients, yielding contradictory results. Furlan et al, reported higher burst rate (bursts/min) of MSNA during rest and comparable with control during tilt.47 Muenter et al, showed normal MSNA (burst/min and burst/100beat) signal at rest and exaggerated MSNA response to baroreflex challenges, such as to VM2e.45 Lambert showed no differences in resting sympathetic activity but exaggerated response to tilt .50 Bonyhay and Freeman showed that POTS patients compared to healthy female control have comparable MSNA burst frequency (minute) and lower burst incidence (per 100 beats), during resting condition and increase upon baroreflex unloading.44 Our patient selection, enriched for patients with high probability of having neuropathic POTS, showed that incidence, burst frequency and area of integrated MSNA were lower as compared to controls while supine, but these indices were comparable to controls during HUT.
Traditional analysis relying on the integration of the neurogram and analysis of bursts has its limitations; it may not capture rapid changes in firing properties during the short Valsalva maneuver. We have developed a technique that allows direct analysis of action potentials in the raw neurogram, and applied this approach to study sympathetic activity.24, 35, 36, 51 Similar techniques have been also used by others to describe firing pattern during VM.52, 53 Whereas the analysis of the integrated neurogram iMSNA did not provide sufficient information during Valsalva maneuver, spike rate analysis confirmed changes in the sympathetic neural firing pattern especially in the early phase 2 of the Valsalva maneuver. It could be argued that beat-to-beat spike frequency results are compromised by the higher heart rates in POTS. However, averaged and maximal spike instantaneous rate, measured in spikes per seconds – which is independent of heart rate, is diminished in early phase 2 of VM in POTS. We found only marginal decrease of averaged beat-to beat spike count (p=0.05) and averaged beat-to-beat mean spike frequency (p=0.07) in late phase 2 of VM. This points out that the possible sympathetic dysfunction in POTS is mostly a problem of recruitment of neurons to counteract blood pressure fall during early phase VM with vasoconstriction efficiently. The reported increased overshoot in phase 4 of Valsalva maneuver might be the result of the delayed sympathetic activation.
Limitations
The initial selection of patients was arbitrary, mostly avoiding patients with clinical characteristics of hyperadrenergic POTS, by enriching the studied cohort with neuropathic POTS. We did not use norepinephrine spillover, the gold standard to define neuropathic POTS, because of purified isotopes for human use were not available. We cannot ensure, therefore that our patient cohort is exclusively comprised of neuropathic POTS. We did not add a group of “hyperadrenergic” POTS patients to the study because there is not a unique definition of “hyperadrenergic” POTS available. Existing definitions create a more diffuse cohort and make interrelation of data more difficult. Finally, our small sample size may result in type II errors in the differences that did not reach significance.
Summary:
This is a study on a subgroup of patients with POTS, that were selected due to their likely neuropathic type presentation. This group showed the following characteristics of hemodynamic and cardiovascular autonomic control: 1) remarkable decrease in systolic and diastolic BP during VM2e that did not recover to baseline during late phase VM2, 2) lower supine resting MSNA and lower MSNA during the orthostatic challenge, 3) changes in neural sympathetic firing pattern during baseline and in response to Valsalva characterized by diminished sympathetic control in the early phase VM2, and 4) reduced cardio-vagal baroreflex function.
Conclusion:
Based on the finding of the present study we may suggest that patients with suspected neuropathic POTS could be defined by applying the Valsalva’s maneuver hemodynamic characteristics and the cardio-vagal baroreflex sensitivity.
Perspectives:
We have shown that the engagement of vagal and sympathetic system to hypotensive stimuli is changed in a cohort with enriched neuropathic POTS. A more detailed analysis of sympathetic firing properties in POTS will be the next step in future research which might allow to identify subgroups of POTS for optimization of therapy.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
This study demonstrated that Valsalva’s maneuver carefully performed in patients with POST could help to select those patients having neuropathic etiology.
Hemodynamic dissection of the Valsalva’s’ maneuver in neuropathic POTS reveals an exaggerated decrease in blood pressure during phase 2 compared to healthy subjects.
Moreover, the vagal control of the heart in neuropathic POTS is compromised and the sympathetic activity neurovascular coupling may be impaired.
What Is Relevant?
A cohort of patients suffering from POTS, high heart rates but normal upright blood pressure, showed a peculiar and abnormal cardiac and vascular neuronal control.
Summary
A simple Valsalva’s maneuver performed in POTS patients, with adequate hemodynamic monitoring, is a useful tool to select patients with neuropathic etiology, and may help in tailoring an adequate treatment.
ACKNOWLEDGEMENTS
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Health under Award Number NIH 2P01HL056693–19, and in part by NIH 1R56HL142583–01 and CTSA UL1TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Bonnie Black, Emily Smith, and all nurses at the Vanderbilt Clinical Research Center for their support.
SOURCE OF FUNDINGS
NIH 2P01HL056693–19
NIH 1R56HL142583–01
CTSA UL1TR000445
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
No conflicts of interest. Nothing to disclose.
REFERENCES
- 1.Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127:2336–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart JM. Chronic orthostatic intolerance and the postural tachycardia syndrome (POTS). J Pediatr. 2004;145:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, Lennon VA, Shen WK and Low PA. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308–13. [DOI] [PubMed] [Google Scholar]
- 4.Sheldon RS, Grubb BP, 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK and Kanjwal K. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Low PA, Opfer-Gehrking TL, Textor SC, Benarroch EE, Shen WK, Schondorf R, Suarez GA and Rummans TA. Postural tachycardia syndrome (POTS). Neurology. 1995;45:S19–25. [PubMed] [Google Scholar]
- 6.Low PA, Sandroni P, Joyner M and Shen WK. Postural tachycardia syndrome (POTS). J Cardiovasc Electrophysiol. 2009;20:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mar PL and Raj SR. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front Physiol. 2014;5:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacob G, Robertson D, Mosqueda-Garcia R, Ertl AC, Robertson RM and Biaggioni I. Hypovolemia in syncope and orthostatic intolerance role of the renin-angiotensin system. Am J Med. 1997;103:128–33. [DOI] [PubMed] [Google Scholar]
- 10.Schutzman J, Jaeger F, Maloney J and Fouad-Tarazi F. Head-up tilt and hemodynamic changes during orthostatic hypotension in patients with supine hypertension. J Am Coll Cardiol. 1994;24:454–61. [DOI] [PubMed] [Google Scholar]
- 11.Stewart JM. Mechanisms of sympathetic regulation in orthostatic intolerance. J Appl Physiol (1985). 2012;113:1659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob G, Shannon JR, Costa F, Furlan R, Biaggioni I, Mosqueda-Garcia R, Robertson RM and Robertson D. Abnormal norepinephrine clearance and adrenergic receptor sensitivity in idiopathic orthostatic intolerance. Circulation. 1999;99:1706–1712. [DOI] [PubMed] [Google Scholar]
- 13.Peltier AC, Garland E, Raj SR, Sato K, Black B, Song Y, Wang L, Biaggioni I, Diedrich A and Robertson D. Distal sudomotor findings in postural tachycardia syndrome. Clin Auton Res. 2010;20:93–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schondorf R and Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993;43:132–7. [DOI] [PubMed] [Google Scholar]
- 15.Streeten DH and Scullard TF. Excessive gravitational blood pooling caused by impaired venous tone is the predominant non-cardiac mechanism of orthostatic intolerance. Clin Sci (Lond). 1996;90:277–285. [DOI] [PubMed] [Google Scholar]
- 16.Jacob G, Costa F, Shannon JR, Robertson RM, Wathen M, Stein M, Biaggioni I, Ertl A, Black B and Robertson D. The neuropathic postural tachycardia syndrome. N Engl J Med. 2000;343:1008–1014. [DOI] [PubMed] [Google Scholar]
- 17.Jacob G and Biaggioni I. Idiopathic orthostatic intolerance and postural tachycardia syndromes. Am J Med Sci. 1999;317:88–101. [DOI] [PubMed] [Google Scholar]
- 18.Gazit Y, Nahir AM, Grahame R and Jacob G. Dysautonomia in the joint hypermobility syndrome. Am J Med. 2003;115:33–40. [DOI] [PubMed] [Google Scholar]
- 19.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW and Robertson D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–82. [DOI] [PubMed] [Google Scholar]
- 20.Jacob G, Ertl AC, Shannon JR, Furlan R, Robertson RM and Robertson D. Effect of standing on neurohumoral responses and plasma volume in healthy subjects. J Appl Physiol (1985). 1998;84:914–21. [DOI] [PubMed] [Google Scholar]
- 21.Feldschuh J and Enson Y. Prediction of the normal blood volume. Relation of blood volume to body habitus. Circulation. 1977;56:605–12. [DOI] [PubMed] [Google Scholar]
- 22.Holmes C, Eisenhofer G and Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatogr B Biomed Appl. 1994;653:131–8. [DOI] [PubMed] [Google Scholar]
- 23.Wallin BG and Sundlof G. A quantitative study of muscle nerve sympathetic activity in resting normotensive and hypertensive subjects. Hypertension. 1979;1:67–77. [DOI] [PubMed] [Google Scholar]
- 24.Diedrich A, Charoensuk W, Brychta RJ, Ertl AC and Shiavi R. Analysis of raw microneurographic recordings based on wavelet de-noising technique and classification algorithm: wavelet analysis in microneurography. IEEE Trans Biomed Eng. 2003;50:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delius W, Hagbarth KE, Hongell A and Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand. 1972;84:65–81. [DOI] [PubMed] [Google Scholar]
- 26.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E and et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. [DOI] [PubMed] [Google Scholar]
- 27.Lucini D, Bertocchi F, Malliani A and Pagani M. A controlled study of the autonomic changes produced by habitual cigarette smoking in healthy subjects. Cardiovasc Res. 1996;31:633–9. [PubMed] [Google Scholar]
- 28.Malliani A, Pagani M and Lombardi F. Methodological aspects of noninvasive analysis of autonomic regulation of cardiovascular variability. Clin Sci (Lond). 1996;91 Suppl:68–71. [DOI] [PubMed] [Google Scholar]
- 29.Lucini D, Pagani M, Mela GS and Malliani A. Sympathetic restraint of baroreflex control of heart period in normotensive and hypertensive subjects. Clin Sci (Lond). 1994;86:547–56. [DOI] [PubMed] [Google Scholar]
- 30.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A and Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl. 1985;3:S79–81. [PubMed] [Google Scholar]
- 31.Cox JF, Tahvanainen KU, Kuusela TA, Levine BD, Cooke WH, Mano T, Iwase S, Saito M, Sugiyama Y, Ertl AC, Biaggioni I, Diedrich A, Robertson RM, Zuckerman JH, Lane LD, Ray CA, White RJ, Pawelczyk JA, Buckey JC Jr., Baisch FJ, Blomqvist CG, Robertson D and Eckberg DL. Influence of microgravity on astronauts’ sympathetic and vagal responses to Valsalva’s manoeuvre. J Physiol. 2002;538:309–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrezenmaier C, Singer W, Swift NM, Sletten D, Tanabe J and Low PA. Adrenergic and vagal baroreflex sensitivity in autonomic failure. Arch Neurol. 2007;64:381–6. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder C, Heusser K, Tank J, Diedrich A, Luft FC and Jordan J. The Valsalva maneuver: screening for drug-induced baroreflex dysfunction. Clin Auton Res. 2009;19:32–8. [DOI] [PubMed] [Google Scholar]
- 34.Mar PL, Shibao CA, Garland EM, Black BK, Biaggioni I, Diedrich A, Paranjape SY, Robertson D and Raj SR. Neurogenic hyperadrenergic orthostatic hypotension: a newly recognized variant of orthostatic hypotension in older adults with elevated norepinephrine (noradrenaline). Clin Sci (Lond). 2015;129:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brychta RJ, Shiavi R, Robertson D and Diedrich A. Spike detection in human muscle sympathetic nerve activity using the kurtosis of stationary wavelet transform coefficients. J Neurosci Methods. 2007;160:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tank J, Heusser K, Brinkmann J, Schmidt BM, Menne J, Bauersachs J, Haller H, Diedrich A and Jordan J. Spike rate of multi-unit muscle sympathetic nerve fibers after catheter-based renal nerve ablation. J Am Soc Hypertens. 2015;9:794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, Blakely RD and Robertson D. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. [DOI] [PubMed] [Google Scholar]
- 38.Joyner MJ and Masuki S. POTS versus deconditioning: the same or different? Clin Auton Res. 2008;18:300–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esler M Clinical application of noradrenaline spillover methodology: delineation of regional human sympathetic nervous responses. Pharmacol Toxicol. 1993;73:243–53. [DOI] [PubMed] [Google Scholar]
- 40.Gibbons CH, Bonyhay I, Benson A, Wang N and Freeman R. Structural and functional small fiber abnormalities in the neuropathic postural tachycardia syndrome. PLoS One. 2013;8:e84716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sandroni P, Novak V, Opfer-Gehrking TL, Huck CA and Low PA. Mechanisms of blood pressure alterations in response to the Valsalva maneuver in postural tachycardia syndrome. Clin Auton Res. 2000;10:1–5. [DOI] [PubMed] [Google Scholar]
- 42.Wada N, Singer W, Gehrking TL, Sletten DM, Schmelzer JD and Low PA. Comparison of baroreflex sensitivity with a fall and rise in blood pressure induced by the Valsalva manoeuvre. Clin Sci (Lond). 2014;127:307–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stewart JM. Autonomic nervous system dysfunction in adolescents with postural orthostatic tachycardia syndrome and chronic fatigue syndrome is characterized by attenuated vagal baroreflex and potentiated sympathetic vasomotion. Pediatr Res. 2000;48:218–26. [DOI] [PubMed] [Google Scholar]
- 44.Bonyhay I and Freeman R. Sympathetic nerve activity in response to hypotensive stress in the postural tachycardia syndrome. Circulation. 2004;110:3193–8. [DOI] [PubMed] [Google Scholar]
- 45.Muenter Swift N, Charkoudian N, Dotson RM, Suarez GA and Low PA. Baroreflex control of muscle sympathetic nerve activity in postural orthostatic tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2005;289:H1226–33. [DOI] [PubMed] [Google Scholar]
- 46.Mustafa HI, Raj SR, Diedrich A, Black BK, Paranjape SY, Dupont WD, Williams GH, Biaggioni I and Robertson D. Altered systemic hemodynamic and baroreflex response to angiotensin II in postural tachycardia syndrome. Circ Arrhythm Electrophysiol. 2012;5:173–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Furlan R, Jacob G, Snell M, Robertson D, Porta A, Harris P and Mosqueda-Garcia R. Chronic orthostatic intolerance: a disorder with discordant cardiac and vascular sympathetic control. Circulation. 1998;98:2154–2159. [DOI] [PubMed] [Google Scholar]
- 48.Jordan J, Shannon JR, Diedrich A, Black BK and Robertson D. Increased sympathetic activation in idiopathic orthostatic intolerance: role of systemic adrenoreceptor sensitivity. Hypertension. 2002;39:173–8. [DOI] [PubMed] [Google Scholar]
- 49.Okamoto LE, Raj SR, Gamboa A, Shibao CA, Arnold AC, Garland EM, Black BK, Farley G, Diedrich A and Biaggioni I. Sympathetic activation is associated with increased IL-6, but not CRP in the absence of obesity: lessons from postural tachycardia syndrome and obesity. Am J Physiol Heart Circ Physiol. 2015;309:H2098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambert E and Lambert GW. Sympathetic dysfunction in vasovagal syncope and the postural orthostatic tachycardia syndrome. Front Physiol. 2014;5:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Q, Liu Y, Brown L and Shoemaker JK. Challenges and opportunities in processing muscle sympathetic nerve activity with wavelet denoising techniques: detecting single action potentials in multiunit sympathetic nerve recordings in humans. Auton Neurosci. 2007;134:92–105. [DOI] [PubMed] [Google Scholar]
- 52.Salmanpour A, Frances MF, Goswami R and Shoemaker JK. Sympathetic neural recruitment patterns during the Valsalva maneuver. Conf Proc IEEE Eng Med Biol Soc. 2011;2011:6951–4. [DOI] [PubMed] [Google Scholar]
- 53.Zaydens E, Taylor JA, Cohen MA and Eden UT. Characterization and modeling of muscle sympathetic nerve spiking. IEEE Trans Biomed Eng. 2013;60:2914–24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.