Abstract
Background:
Physical activity (PA) is inversely associated with adverse cardiovascular (CV) outcomes in healthy populations but the impact of physical activity in patients with heart failure with preserved ejection fraction (HFpEF) is less well characterized.
Methods:
The baseline self-reported PA of 1751 subjects enrolled in the Americas region of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial was categorized as poor, intermediate, or ideal PA using American Heart Association (AHA) criteria. PA was related to the primary composite outcome (heart failure [HF] hospitalization, CV mortality, or aborted cardiac arrest), its components, and all-cause mortality using multivariable Cox models.
Results:
The mean age at enrollment was 68.6 ± 9.6 years. Few patients met AHA criteria for ideal activity (11% ideal, 14% intermediate, 75% poor). Over a median follow-up of 2.4 years, the primary composite outcome occurred in 519 patients (397 HF hospitalizations, 222 CV deaths, and 6 aborted cardiac arrests). Compared to those with ideal baseline PA, poor and intermediate baseline PA were associated with a greater risk of the primary outcome (HR 2.05; 95% CI, 1.28–3.28; HR 1.95; CI, 1.15–3.33, respectively), HF hospitalization (HR 1.93; CI, 1.16–3.22; HR 1.84; CI, 1.02–3.31), CV mortality (HR 4.36; CI, 1.37–13.83; HR 4.05; CI 1.17–14.04), and all-cause mortality (HR 2.95; CI, 1.44–6.02; HR 2.05; CI 0.90, 4.67) after multivariable adjustment for potential confounders.
Conclusions:
In patients with HFpEF, both poor and intermediate self-reported PA were associated with higher risk of HF hospitalization and mortality.
Keywords: physical activity, heart failure, outcomes, preserved ejection fraction, clinical trial, spironolactone
INTRODUCTION
Higher levels of physical activity (PA) in healthy subjects have been associated with a lower risk of adverse cardiovascular (CV) outcomes, including lower incident heart failure, with a well-described dose-response relationship.1–6 The impact of exercise is less clear in those with heart failure with preserved ejection fraction (HFpEF). With exercise training, several studies have demonstrated improvement in measures of cardiorespiratory fitness (peak oxygen uptake) and quality of life but uncertain benefit in clinical outcomes such as heart failure (HF) hospitalization and mortality.7–15 Both pharmacologic trials and exercise training interventions have yet to demonstrate a mortality benefit in this population.16–19 Moreover, the impact of physical activity on adverse CV outcomes has not been examined in a large cohort of patients with HFpEF.20,21
We hypothesized that poor physical activity would be associated with subsequent adverse cardiovascular outcomes in HFpEF patients and tested this hypothesis in participants enrolled in the Treatment Of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. In this report, we describe the spectrum of baseline physical activity in this HFpEF cohort, their characteristics according to level of physical activity, and the relationship of physical activity with adverse cardiovascular outcomes in patients with HFpEF.
METHODS
Study Design and Population.
The study population is comprised of the 1751 subjects enrolled in the Americas region of the TOPCAT Trial (United States, Canada, Brazil, Argentina). We focused on patients enrolled in the Americas region because of significant differences in population characteristics and outcomes by region.22 The TOPCAT trial was designed to determine whether treatment with spironolactone would reduce morbidity and mortality in patients with HFpEF. Eligible subjects included those with symptomatic heart failure and left ventricular ejection fraction (LVEF) ≥ 45% who had either a hospitalization for heart failure within 12 months or elevated brain natriuretic peptide (BNP; BNP ≥ 100pg/mL or N-terminal pro-BNP ≥ 360pg/mL) within 60 days prior to randomization. Participants were randomized to spironolactone or placebo and stratified by entry criteria of hospitalization or natriuretic peptides. Additional details of the study design and exclusion criteria have been published, and the trial was conducted with the approval of local institutional review boards.19 All patients provided written informed consent. The primary outcome was defined as the composite of death from a cardiovascular cause, aborted cardiac arrest, or heart failure (HF) hospitalization. Secondary outcomes included the individual components in addition to myocardial infarction and stroke, all centrally adjudicated. All deaths and all hospitalizations were also defined as secondary outcomes of both the original TOPCAT trial and this analysis. An echocardiographic substudy was also conducted and represents a subset of baseline studies performed prior to randomization; the echocardiographic core laboratory at Brigham and Women’s Hospital performed analysis with methods previously described.23
Physical Activity.
At baseline, all participants were asked, “What has the subject’s usual pattern of exercise been during the past 2 weeks?” for 3 categories of activity: heavy (e.g. jogging, tennis, strenuous gardening, or housework), medium (brisk walking, moderate gardening, or housework), and light (slow walking). Subjects provided the number of times per week and number of minutes each time for each category of physical activity; the product of these values was calculated to provide the number of minutes per week of each category of activity. Heavy activity was classified as vigorous activity and medium activity was classified as moderate activity. Although not formally validated, the questions represent the American College of Sports Medicine descriptors of light, moderate, and vigorous activity and corresponding metabolic equivalents from the Compendium of Physical Activities.24,25 These questions also resemble those validated in other cohort studies such as the Women’s Health Initiative and the Behavioral Risk Factor Surveillance System Questionnaire.26,27
Minutes per week of moderate or vigorous activity were converted to intensity categories of PA (poor, intermediate, and ideal) using the American Heart Association (AHA) definition due to the skewness of the variable.2 Ideal PA was defined as ≥ 150 min/week of moderate activity, ≥ 75 min/week of vigorous activity, or ≥ 150 min/week of moderate + vigorous activity. Intermediate PA was defined as 1–149 min/week of moderate activity, 1–74 min/week of vigorous activity, or 1–149 min/week of moderate + vigorous activity. Poor PA was defined as 0 min/week of moderate + vigorous activity.
Finally, total physical activity per week was assessed in terms of metabolic equivalents in MET-min/week by equating heavy, medium, and light intensity activity into the low end of their MET equivalents as 6 METS, 3 METS, and 1 MET, respectively and multiplying by the duration of activity per week; the sum of MET-min/week of physical activity at each intensity level combined to give the total amount of physical activity per week.24,28
Statistical Analysis.
Descriptive statistics for the study population by PA category are presented as means ± standard deviation, proportions, or medians [interquartile range (IQR)] for skewed variables. Tests for trend were based on linear regression, chi-squared trend tests, and Cuzick’s non-parametric trend tests.29 A two-sided P-value of <0.05 was considered statistically significant.
We related AHA-assigned PA category at baseline to the primary outcome (heart failure hospitalization, cardiovascular mortality, or aborted cardiac arrest) using a Kaplan-Meier survival curve. We also related baseline PA category to the primary outcome, its components, all-cause mortality, and all-cause hospitalization using a multivariable Cox-proportional hazards model. Assessment of the proportional hazards assumption was performed using Schoenfeld residuals using linear, log-transformed, and rank-transformed time-scaling.30 In addition, analysis was repeated using a binary activity variable of ideal vs. non-ideal physical activity with results provided in the Supplemental material.
Variables in the Cox models included variables felt to be important confounders a priori [age, sex, race, treatment group, enrollment strata, previous myocardial infarction (MI), previous HF hospitalization, previous cerebrovascular accident (CVA), LVEF, smoking status, alcohol use, creatinine, hemoglobin, beta-blocker use].19,31–34 Model 1 adjusted for basic demographics: age, sex, white race, treatment group, and enrollment strata. Model 2 adjusted for additional confounders: previous MI, previous HF, previous CVA, LVEF, smoking status, alcohol use, creatinine, hemoglobin, and beta-blocker use. Model 3 considered the contribution of additional potential mediators: New York Heart Association (NYHA) class, diabetes mellitus (DM), systolic blood pressure (SBP), body mass index (BMI), chronic obstructive pulmonary disease (COPD), and heart rate.
We also related deciles of total physical activity in MET-min/week to the incidence rate (per 100 person years) of the primary outcome.
Those with missing physical activity data (n=18) were omitted from the analysis. Additional sensitivity analyses were performed to evaluate the validity of the results; results from repeated analyses excluding those with various comorbidities are available in the Supplemental material. Finally, evaluation included testing for interaction between PA and age, sex, BMI, and treatment, respectively.
Of the 935 echocardiographic studies performed, 643 studies remained for analysis after excluding subjects not in the Americas region and with missing physical activity data (n=11). Descriptive statistics for this subset are also presented.
All statistical analyses were performed using Stata Software (version 13, Stata Corp., College Station, TX, USA).
RESULTS
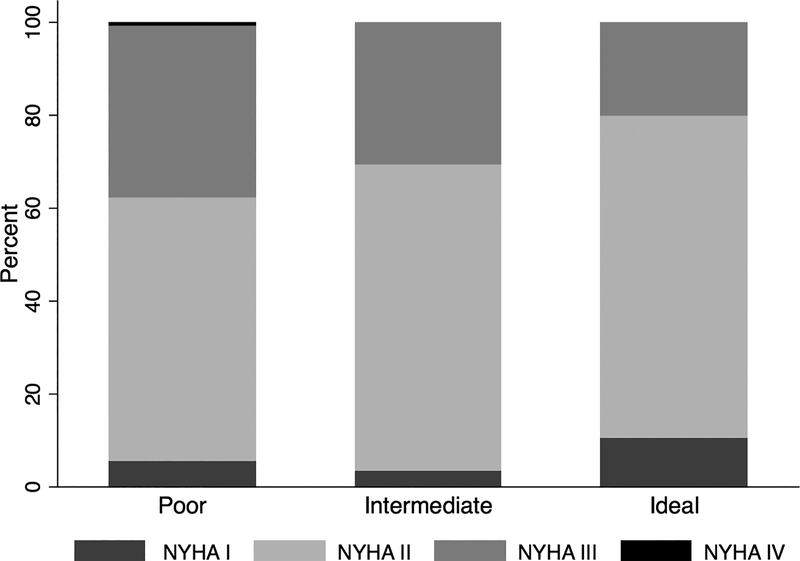
Baseline characteristics of participants by physical activity category are shown in Table 1. The mean age was 68.6 ± 9.6 years old. Eleven percent of participants met AHA criteria for ideal activity, 14% for intermediate activity, and 75% for poor activity (Table 1). Subjects primarily met criteria for ideal or intermediate activity by participating in moderate-intensity activity (median moderate intensity activity by group: ideal PA: 210 min/week [150, 360], intermediate PA: 60 min/week [35, 105], poor PA: 0 min/week [0,0]). Those with poor PA were more likely to be women and enrolled in the trial by the HF hospitalization entry criteria rather than BNP entry criteria. The majority of subjects were classified as NYHA Class II heart failure, including those with poor PA. Although those with NYHA Class III heart failure were more likely to have poor PA levels, approximately 20% demonstrated baseline activity levels consistent with ideal PA (Figure 1).
Table 1:
Baseline characteristics by AHA activity category and region (Americas)
| Poor Activity n=1312 | Intermediate Activity n=246 | Ideal Activity n=193 | P value (trend) | |
|---|---|---|---|---|
| Age | 71.6 ± 9.9 | 71.1 ± 9.4 | 72.2 ± 9.1 | 0.69 |
| Female | 671 (51.1) | 116 (47.2) | 84 (43.5) | 0.03 |
| Race | 0.27 | |||
| White | 1025 (78.1) | 188 (76.4) | 161 (83.4) | |
| Black | 229 (17.5) | 44 (17.9) | 23 (11.9) | |
| Inclusion Criteria | ||||
| HF Hospitalization | 756 (57.6) | 117 (47.6) | 93 (48.2) | 0.001 |
| BNP | 556 (42.4) | 129 (52.4) | 100 (51.8) | |
| Spironolactone | 655 (49.9) | 129 (52.4) | 96 (49.7) | 0.81 |
| LVEF | 58.2 ± 7.7 | 58.2 ± 7.4 | 57.6 ± 8.5 | 0.38 |
| NYHA Class | <0.001 | |||
| 1 | 70 (5.3) | 8 (3.3) | 20 (10.4) | |
| 2 | 742 (56.7) | 162 (65.9) | 134 (69.4) | |
| 3 | 487 (37.2) | 76 (30.9) | 39 (20.2) | |
| 4 | 10 (0.8) | - | - | |
| Overall KCCQ score (QOL) | 55.3 ±23.2 | 63.1 ±22.3 | 70.7 ±20.2 | <0.001 |
| Hypertension | 1186 (90.4) | 219 (89.0) | 169 (87.6) | 0.19 |
| Diabetes Mellitus | 616 (47.0) | 97 (39.4) | 68 (35.2) | <0.001 |
| Insulin use | 292 (47.4) | 51 (52.6) | 32 (47.1) | 0.72 |
| eGFR<60 ml/min/1.73 m2 | 661 (50.4) | 116 (47.2) | 73 (37.8) | <0.001 |
| Previous MI | 260 (19.8) | 54 (22.0) | 45 (23.3) | 0.2 |
| Previous HF hospitalization | 805 (61.4) | 128 (52.0) | 96 (49.7) | <0.001 |
| Previous CVA | 118 (9.0) | 23 (9.3) | 17 (8.8) | 1.00 |
| Atrial fibrillation | 547 (41.7) | 100 (40.7) | 93 (48.2) | 0.17 |
| Dyslipidemia | 912 (69.5) | 181 (73.6) | 148 (76.7) | 0.02 |
| COPD | 215 (16.4) | 39 (15.9) | 32 (16.6) | 0.98 |
| Smoking Status | 0.01 | |||
| Current | 82 (6.3) | 20 (8.1) | 14 (7.3) | |
| Past | 647 (49.3) | 136 (55.3) | 110 (57.0) | |
| Never | 583 (44.4) | 90 (36.6) | 69 (35.8) | |
| BMI, kg/m2 | 34.3 ± 8.6 | 32.9 ± 8.0 | 31.9 ± 6.9 | <0.001 |
| SBP, mmHg | 127.8 ± 16.2 | 127.1 ± 15.4 | 126.2 ± 14.1 | 0.18 |
| DBP, mmHg | 71.5 ± 11.7 | 71.6 ± 11.3 | 70.2 ± 10.3 | 0.24 |
| HR, beats/min | 69.4 ± 11.4 | 69.6 ± 10.8 | 65.9 ± 10.4 | <0.001 |
| BNP, pg/ml * | 260 [146, 462] n=490 | 234 [152, 415] n=127 | 280 [195, 410] n=69 | 0.73 |
| NT-proBNP, pg/ml * | 974 [585, 2101] n=284 | 976 [695, 1797] n=32 | 763 [479, 1720] n=43 | 0.35 |
| Hemoglobin, g/dl | 12.8 ± 1.7 | 13.0 ± 1.6 | 13.2 ± 1.5 | <0.001 |
| Potassium, mmol/l | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.06 |
| Creatinine, mg/dl | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.03 |
| Medication use | ||||
| Beta-blocker | 1023 (78.0) | 198 (80.5) | 156 (80.8) | 0.27 |
| Calcium channel blocker | 509 (38.8) | 102 (41.5) | 65 (33.7) | 0.38 |
| Diuretic | 1176 (89.7) | 219 (89.0) | 165 (85.5) | 0.10 |
| ACE-I/ARB | 1048 (79.9) | 192 (78.0) | 141 (73.1) | 0.03 |
| Aspirin | 770 (58.7) | 136 (55.3) | 114 (59.1) | 0.75 |
Data reported as n (%) or mean ± standard deviation or median [interquartile range].
Among those enrolling via BNP stratum. In the overall study, the study-qualifying BNP or NT-pro-BNP values were reported in 88.5% patients enrolled in this stratum; values were not collected for 11.5% who were enrolled before a change to the enrollment form was implemented in August 2007.
MI-myocardial infarction, HF-heart failure, BNP-brain natriuretic peptide, LVEF-left ventricular ejection fraction, NYHA-New York Heart Association, KCCQ (QOL)-Kansas City Cardiomyopathy Questionnaire (Quality of Life), eGFR- estimated glomerular filtration rate, CVA-cerebrovascular accident, COPD-chronic obstructive pulmonary disease. BMI-body mass index, SBP-systolic blood pressure, DBP-diastolic blood pressure, HR-heart rate, BNP-brain natriuretic peptide, NT-pro-BNP-N-terminal pro-BNP, ACE-I-angiotensin-converting enzyme inhibitor, ARB-angiotensin receptor blocker
Figure 1. Baseline distribution of NYHA class and physical activity category.
Proportion of participants in each NYHA class by physical activity category as defined by AHA criteria (poor, intermediate, or ideal), p>0.001 for global comparison. NYHA-New York Heart Association, AHA-American Heart Association.
Subjects with ideal PA were less likely to have diabetes mellitus, chronic kidney disease, and history of prior HF hospitalization and demonstrated lower BMI, resting heart rate, hemoglobin, and creatinine. There was no significant difference in LVEF or the prevalence of hypertension, insulin use, prior history of myocardial infarction, prior history of stroke, atrial fibrillation, or chronic obstructive pulmonary disease by PA category.
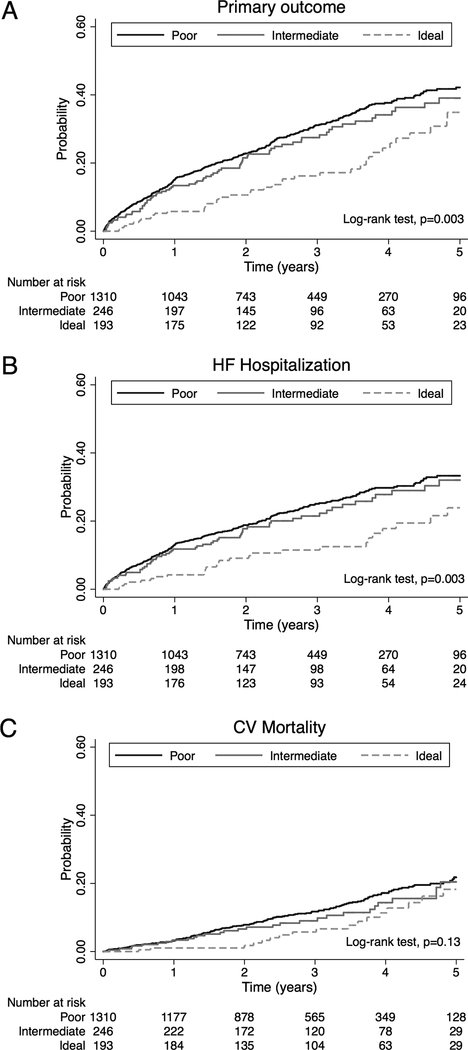
The median follow-up time was 2.4 years. The primary composite outcome occurred in 519 patients (397 HF hospitalizations, 222 CV deaths, and 6 aborted cardiac arrests). Time to primary outcome (log-rank test, p=0.003; Figure 2A) and time to first HF hospitalization (log-rank test, p=0.003; Figure 2B) were significantly different by PA category, whereas there was no significant difference in CV mortality by PA category (log-rank test, p=0.13; Figure 2C).
Figure 2. Kaplan-Meier plots of time to primary outcome and two major components by physical activity category.
A, Time to primary outcome (HF hospitalization, CV death, or aborted cardiac arrest); B, time to first confirmed HF hospitalization; and C, time to CV death. HF-heart failure, CV-cardiovascular.
Testing for proportional hazards demonstrated a significant interaction between baseline physical activity and time since randomization (Supplemental Table 1A), such that the associations between physical activity and outcomes were much stronger with closer proximity to randomization. As a result, all subsequent analyses were truncated at 2 years after randomization with no residual interaction detectable over this time interval (Supplemental Table 1B). Time to primary outcome, first HF hospitalization, and CV mortality are significantly different by PA category over the first 2 years (Supplemental Figure 1). In analyses beginning 2 years after randomization, no relationships were found between physical activity and subsequent outcomes. Cox-proportional hazards results for the full duration of the study are provided in the Supplemental material, including additional results accounting for the proportional hazards violation with baseline physical activity modeled using time-varying coefficients (Supplemental Table 2); again, the associations between physical activity and outcomes remained significant in the first two years with no association with outcomes in subsequent years.
Compared to those with ideal baseline PA, poor PA was associated with a greater risk of the primary outcome (HR 2.05; 95% CI, 1.28–3.28), HF hospitalization (HR 1.93; CI, 1.16–3.22), CV mortality (HR 4.36; CI, 1.37–13.83), and all-cause mortality (HR 2.95; CI, 1.44–6.02) after multivariable adjustment for potential confounders including age, sex, white race, treatment group, enrollment strata, previous MI, previous HF, LVEF, smoking status, alcohol use, creatinine, hemoglobin, and beta-blocker use (Model 2; Table 2). No significant association was present between PA category and all-cause hospitalization. Those with intermediate activity demonstrated a similar magnitude of increased risk of the primary outcome (HR 1.95; CI, 1.15–3.33), HF hospitalization (HR 1.84; CI, 1.02–3.31), and CV mortality (HR 4.05; CI, 1.17–14.04) as those with poor PA. Additional adjustment for potential mediators (Model 3 – NYHA class, DM, SBP, BMI, heart rate) attenuated the relationship between PA and the primary outcome (Table 2), yet the relationship between PA and the primary outcome remained significant. A linear trend was also present such that those with the least activity (poor PA) demonstrated the highest risk of the primary composite outcome, HF hospitalization, CV mortality, and all-cause mortality (p for linear trend, all ≤ 0.02); however, the magnitude of risk in those with intermediate and poor activity was similar. A pooled comparison of non-ideal vs. ideal activity is presented in Supplemental Table 3.
Table 2:
Summary of Trial Outcomes by AHA Activity Category (maximum 2 years follow-up)
| Participants with Event | Incidence Rate | Model 1* | Model 2† (+ Confounders) | Model 3‡ (+ Mediators) | ||||
|---|---|---|---|---|---|---|---|---|
| n (%) | n/100 person-yr | Hazard Ratio | p | Hazard Ratio | p | Hazard Ratio | p | |
| Primary Outcome | ||||||||
| Trend | 0.002 | 0.006 | 0.04 | |||||
| Ideal Activity | 19 (26.8%) | 5.6 (3.6–8.8) | Ref | - | Ref | - | Ref | - |
| Intermediate Activity | 49 (48.5%) | 12.2 (9.3–16.2) | 2.16 (1.27–3.68) | 0.004 | 1.95 (1.15–3.33) | 0.01 | 1.81 (1.06–3.09) | 0.03 |
| Poor Activity | 281 (49.4%) | 13.5 (12.0–15.2) | 2.25 (1.41–3.58) | 0.001 | 2.05 (1.28–3.28) | 0.003 | 1.80 (1.12–2.89) | 0.01 |
| HF Hospitalization | ||||||||
| Trend | 0.007 | 0.02 | 0.11 | |||||
| Ideal Activity | 16 (22.5%) | 4.7 (2.9–7.7) | Ref | - | Ref | - | Ref | - |
| Intermediate Activity | 40 (39.6%) | 9.9 (7.3–13.6) | 2.10 (1.17–3.75) | 0.01 | 1.84 (1.02–3.31) | 0.04 | 1.71 (0.95–3.08) | 0.07 |
| Poor Activity | 228 (40.1%) | 10.9 (9.6–12.5) | 2.17 (1.30–3.60) | 0.003 | 1.93 (1.16–3.22) | 0.01 | 1.66 (0.99–2.79) | 0.05 |
| CV Mortality | ||||||||
| Trend | 0.008 | 0.02 | 0.02 | |||||
| Ideal Activity | 3 (5.2%) | 0.8 (0.3–2.6) | Ref | - | Ref | - | Ref | - |
| Intermediate Activity | 15 (20.3%) | 3.4 (2.1–5.7) | 4.12 (1.19–14.23) | 0.03 | 4.05 (1.17–14.04) | 0.03 | 3.84 (1.10–13.34) | 0.03 |
| Poor Activity | 93 (21.4%) | 4.0 (3.3–4.9) | 4.72 (1.49–14.92) | 0.008 | 4.36 (1.37–13.83) | 0.01 | 4.19 (1.31–13.35) | 0.02 |
| All-cause Mortality | ||||||||
| Trend | 0.001 | 0.001 | 0.005 | |||||
| Ideal Activity | 8 (14.8%) | 2.2 (1.1–4.4) | Ref | - | Ref | - | Ref | - |
| Intermediate Activity | 20 (30.3%) | 4.5 (2.9–6.9) | 2.08 (0.92–4.72) | 0.08 | 2.05 (0.90–4.67) | 0.09 | 1.97 (0.86–4.49) | 0.11 |
| Poor Activity | 158 (40.0%) | 6.7 (5.7–7.8) | 3.07 (0.92–6.26) | 0.002 | 2.95 (1.44–6.02) | 0.003 | 2.64 (1.28–5.43) | 0.01 |
| All-cause Hospitalization | ||||||||
| Trend | 0.03 | 0.06 | 0.25 | |||||
| Ideal Activity | 86 (71.7%) | 32.9 (26.6–40.6) | Ref | - | Ref | - | Ref | - |
| Intermediate Activity | 111 (71.6%) | 35.0 (29.1–42.2) | 1.07 (0.81–1.42) | 0.64 | 1.00 (0.75–1.33) | 0.98 | 0.95 (0.71–1.27) | 0.73 |
| Poor Activity | 675 (78.7%) | 42.1 (39.0–45.4) | 1.24 (0.99–1.55) | 0.07 | 1.18 (0.94–1.48) | 0.15 | 1.09 (0.86–1.37) | 0.47 |
Adjusted hazard ratios for multiple trial outcomes calculated with the use of Cox proportional-hazards model. MI-myocardial infarction, HF-heart failure, CV-cardiovascular, CVA-cerebrovascular accident, LVEF-left ventricular ejection fraction, NYHA-New York Heart Association, DM-diabetes mellitus, SBP-systolic blood pressure, BMI-body mass index
Model 1 – adjusted for age, sex, white race, treatment group, enrollment strata
Model 2 – Model 1 + previous MI, previous HF hospitalization, previous CVA, LVEF, smoking status, alcohol use, creatinine, hemoglobin, beta-blocker use
Model 3 – Model 2 + NYHA class, DM, SBP, BMI, heart rate, COPD
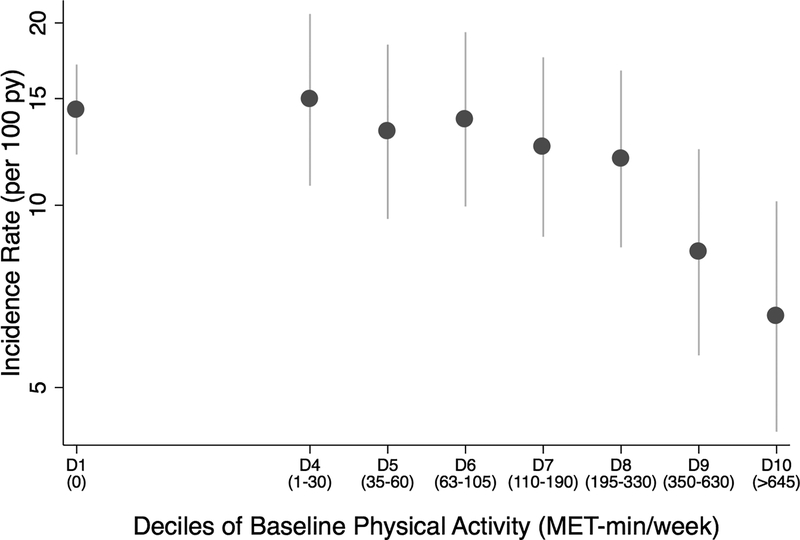
Evaluation of total physical activity per week (MET-min/week) as a continuous measure that also accounted for the amount of light intensity activity per week demonstrated a median total activity level of 60 MET-min/wk [0, 240] among all subjects. Total activity levels less than the AHA-recommended 500 MET-min/week of activity were associated with a higher risk of the primary outcome (HR 1.67; CI, 1.14–2.46) and HF hospitalization (HR 1.77; CI 1.14–2.74) but not CV mortality (HR: 1.97; CI, 0.95–4.07). Evaluation of incidence rates revealed a markedly lower risk of the primary outcome in those who achieved > 350 MET-min/week of activity (Figure 3).
Figure 3. Incidence rates of the primary outcome by amount of total physical activity.
Incidence rates of the primary outcome by decile of total physical activity (MET-min/week) through 2 years post-randomization. Total physical activity includes the amount of light activity in addition to AHA-recommended moderate and vigorous activity reported. Bars represent 95% confidence intervals. AHA-American Heart Association, D-decile.
Repeat analysis of the relationship of PA with the primary outcome yielded similar results after excluding those with previous MI and CVA as comorbidities that may be associated with a decline in activity; however, results are limited by fewer events in this subset (Supplemental Table 4). There was no significant interaction between PA and age, sex, or BMI, respectively. A marginally significant interaction (p=0.04) was present between PA category and treatment, such that poor activity may be associated with higher risk of the primary outcome and HF hospitalization in those who received placebo, however, this analysis is limited by the occurrence of fewer than 20 events in the ideal PA group (Supplemental Table 5).
Baseline characteristics of subjects who participated in the echocardiographic substudy represent a different population than the overall Americas sample with a significantly higher prevalence of MI and lower prevalence of atrial fibrillation in those with ideal activity (Supplemental Table 6). In this subset with echocardiographic parameters, including LVEF, diastolic function, E/e’, LV hypertrophy, LV mass index, and LA volume index were not significantly different between activity groups. Tricuspid regurgitation velocity was marginally significantly higher in those with poor activity.
DISCUSSION
This post hoc analysis demonstrates that in patients with HFpEF, poor and intermediate baseline PA compared to ideal PA were associated with a two-fold higher risk of HF hospitalization and mortality. Moreover, while risk remains high at lower levels of activity, near ideal baseline physical activity levels (> 350 MET-min/week) are associated with a lower risk of HF hospitalization and mortality in this population. These associations were strongest in the first two years following baseline activity assessment.
With a rising prevalence, HFpEF is responsible for substantial morbidity and mortality, accounting for approximately 50% of HF hospitalizations.35–39 Patients with HFpEF are typically older and burdened with multiple comorbidities, including obesity, hypertension, chronic kidney disease, and diabetes mellitus.37–40 Despite the heterogeneous phenotype among HFpEF patients, they commonly present with symptoms of exercise intolerance.15 The impact of physical activity, which may reflect a component of exercise tolerance and overall health status, on relevant clinical endpoints such as HF hospitalization and mortality in this population has not yet been demonstrated.8–13 In this study, we have extended previous findings by showing a favorable association between baseline physical activity and reduced HF hospitalizations and mortality risk in a large HFpEF cohort. Furthermore, this association was strongest within the first two years of baseline activity assessment, suggesting that the impact of physical activity changes over time.
Notably, there was no significant association between physical activity and all-cause hospitalization, suggesting a specific effect of PA on HF hospitalizations. With fewer hospitalizations for HF, ideal PA may play an important therapeutic role in reducing adverse CV outcomes. The echocardiographic substudy in TOPCAT represents a limited sample and thus limits conclusions related to the role of cardiac structure and function in this analysis. Still, the mechanism by which baseline PA may lower risk of adverse outcomes may be related to better indices of diastolic function associated with more active participants as demonstrated in cohort studies.41,42 Alternatively, peripheral mechanisms such as improvements in skeletal muscle function may play a role. In a study of 40 stable HFpEF outpatients, of which half participated in 4 months of endurance exercise training, peak oxygen consumption (VO2) was higher in the intervention group. Mediation analysis demonstrated that this was primarily attributed to increased peak arterial-venous oxygen difference, suggesting that either improvements in skeletal muscle function or microvascular function may be responsible.43
In this post hoc analysis, we focused on patients from the Americas region because this group represents a population more consistent with the diagnosis of HFpEF as demonstrated by the approximately 4 fold higher event rates compared to those in Russia and Georgia.22 Secondary analysis of the primary study demonstrated a reduction in the primary outcome, HF hospitalization, and CV mortality in those receiving spironolactone in the Americas region. After accounting for this treatment effect with multivariable adjustment, greater baseline physical activity remained significantly associated with better outcomes in this population, including fewer HF hospitalizations. Interaction testing suggests a slightly stronger treatment effect in less active patients; however, this analysis should be interpreted cautiously as it is limited by the occurrence of few events in those with ideal PA. Spironolactone has recently been associated with increased exercise tolerance (peak VO2) in patients with HFpEF and an abnormal diastolic response to exertion.44 Pooled data suggest that therapy with mineralocorticoid-receptor antagonists (MRA) is associated with an improvement with diastolic function rather than changes in LV structure or mass.45 This raises the question of whether spironolactone may reduce HF hospitalizations by improving diastolic function and exercise tolerance in those who are less active at baseline. Further studies are certainly needed to elucidate the mechanism.
While analysis of PA by AHA categories suggested a small dose-response relationship, those with poor and intermediate baseline PA appear to have a similar magnitude of increased risk of adverse CV outcomes compared to those with ideal PA. Notably, the dose-response relationship between increasing PA categories and risk of HF hospitalization dissipates in the most adjusted model that accounts for potential mediators. The absence of a significant dose-response relationship for HF hospitalization suggests that the dose should be evaluated more closely; perhaps there may be a threshold effect and/or greater than AHA-recommended doses may be required to achieve additional HF hospitalization risk reduction as noted in prospective cohort studies.3 Still, the risk of HF hospitalization remains significantly higher for those not achieving ideal levels of activity.
Additional analysis of total physical activity, including light intensity activity, demonstrated that levels less than the AHA-recommended 500 MET-min/week remained significantly associated with an increased risk of HF hospitalization and mortality. Further evaluation of incidence rates of the primary outcome in relation to increasing deciles of total physical activity suggest that risk remains high for those with low levels of activity; however, greater than 350 MET-min/week of activity was associated with lower risk. Prospective studies are needed to further examine this relationship and identify whether a threshold effect is indeed present.
As baseline physical activity in this post hoc analysis may reflect overall health status, attempts to exclude significant comorbidities and evaluate a population with similar overall health status in the sample showed a consistent magnitude and direction in the findings. Even after excluding those with a baseline history of MI and CVA from the analysis, a significantly increased risk of the primary outcome and HF hospitalizations was present in those with poor and intermediate baseline PA. Furthermore, approximately 20% of those that achieved ideal PA levels were classified as NYHA Class III, suggesting that many class III patients are able to achieve ideal levels of PA. Alternatively, the subjectivity inherent in NYHA classification may not fully account for the true functional limitations or activity patterns in HFpEF patients as reflected by PA assessment.
An effective therapy for patients with HFpEF remains elusive as large randomized trials have yet to show a mortality benefit with ACE-inhibitors, ARBs, or MRAs.16–19 Lifestyle modification with physical activity may be one way to modify the adverse CV outcomes associated with HFpEF. A number of studies have demonstrated the ability of exercise training to improve cardiorespiratory fitness and quality of life in these patients but have yet to show a clear benefit in reducing hospitalizations or mortality.8–13 The question remains whether exercise training would improve clinical outcomes in patients with HFpEF. This has been previously examined in patients with HFrEF in a large, randomized trial in which subjects were randomized to usual care ± exercise training, yet it demonstrated a non-significant reduction in the primary end point of all-cause mortality or hospitalization and only a borderline effect after adjustment for highly prognostic baseline characteristics 46 Subjects also had nearly perfect adherence to guideline-directed medical therapy, which may have impacted the lack of significant difference demonstrated between the two groups. Furthermore, adherence to exercise is notoriously difficult to maintain and ascertain. In contrast, guideline-directed therapy does not yet exist for those with HFpEF and the impact of exercise training on clinical outcomes in this population remains unknown. The heterogeneous population comprising those with HFpEF may limit the ability to identify those in which PA may carry a stronger association with outcomes or those who may benefit from exercise training. Identifying the appropriate exercise-training regimen and defining a specific population will be critical to understanding the impact of physical activity in this population. The findings from this post-hoc analysis support the need for randomized controlled studies to evaluate whether exercise training is an effective therapy in improving outcomes in patients with HFpEF.
The strengths of this study relative to other studies in HFpEF patients include its large size, long-term follow-up, and evaluation of cardiovascular outcomes associated with physical activity. Several limitations of this post hoc analysis should also be noted. Physical activity was evaluated only at baseline and self-reported measures of physical activity are subject to recall bias and may not be representative of cumulative activity or fitness levels; more validation studies are needed.47–49 The dissipation of the relationship between baseline PA and outcomes in the first two years may limit generalizability of outcomes beyond two years. Baseline PA may instead act as a surrogate for overall health status, which is subject to frequent changes with incident events; once an event or change in clinical status occurs, baseline PA may no longer be reflective of subsequent risk of adverse outcomes in a population with high morbidity and mortality. Despite adjusting for multiple confounders, we cannot rule out the possibility of unmeasured residual confounding. Moreover, conclusions about causality also cannot be made in this post hoc analysis. Additional analysis to exclude those with baseline co-morbidities revealed consistently increased risk of the primary outcome in those with poor baseline PA; however, effect estimates have large confidence intervals, likely due to the marked reduction in the number of events after excluding those with MI and CVA.
In summary, we found that in patients with HFpEF, both poor and intermediate baseline physical activity were associated with a two-fold increased risk of HF hospitalization and mortality. In particular, risk remains high for lower amounts of total physical activity until values near AHA-recommended levels are achieved. Prospective studies are needed to confirm the direction and magnitude of this association, determine if a threshold effect is present, and identify if and which type of exercise interventions are effective in reducing adverse cardiovascular outcomes in those with HFpEF.
Supplementary Material
CLINICAL PERSPECTIVE.
What is new?
Although it is well known that physical activity is inversely associated with adverse cardiovascular outcomes in healthy populations, the impact of physical activity in patients with HFpEF is less well characterized.
These findings demonstrate that low levels of physical activity among stable patients with HFpEF are associated with a higher risk of adverse outcomes, including HF hospitalization and CV mortality.
Subjects with American Heart Association-recommended poor and intermediate activity exhibit a similarly increased risk of adverse outcomes compared to those with ideal activity.
What are the clinical implications?
In patients with HFpEF, several studies have demonstrated the ability of exercise training to improve cardiorespiratory fitness and quality of life but have yet to show a clear benefit in reducing hospitalizations or mortality.
Lifestyle modification with physical activity and/or exercise training may be one way to modify the adverse cardiovascular outcomes and mortality associated with HFpEF.
Findings support the need for future prospective randomized studies to evaluate the role of physical activity, including the appropriate “dose” and type of exercise, on outcomes in patients with HFpEF.
Acknowledgments:
The authors thank the staff and participants of the TOPCAT trial for their important contribution.
Funding: This study was funded by the National Heart, Lung, and Blood Institute, NIH contract HHSN268200425207C. The content of this article does not necessarily represent the views of the National Heart, Lung, and Blood Institute, of the Department of Health and Human Services.
Disclosures: Dr. Hegde received grant support from the National Institutes of Health (T32 HL094301–06). Dr. Lewis has received research grants from the National Heart, Lung, and Blood Institute, Novartis, and Sanofi Aventis. Dr Pitt reports receiving consulting fees from Amorcyte, AstraZeneca, Aurasense, Bayer, BG Medicine, Gambro, Johnson & Johnson, Mesoblast, Novartis, Pfizer, Relypsa, and Takeda; receiving research grant support from Forest Laboratories; and holding stock in Aurasense, Relypsa, BG Medicine, and Aurasense. Dr. S. Shah has received consulting fees from Novartis, Bayer, DC Devices, Gilead, and Actelion. Dr Sweitzer has received research grants from the National Institutes of Health. Dr Pitt also reports a pending patent related to site-specific delivery of eplerenone to the myocardium. Dr. Pfeffer has received consulting fees from Amgen, AstraZeneca, Bayer, DalCor Pharma UK, Genzyme, Lilly, Medicines Company, MedImmune, Medtronic, Merck, Novartis, Novo Nordisk, Relypsa, Salix, Sanderling, Sanofi, Takeda, Teva, Thrasos and Vericel and having received research grant support from Amgen, Celladon, Novartis, Sanofi. The Brigham and Women’s Hospital has patents for the use of inhibitors of the renin–angiotensin system in selected survivors of myocardial infarction with Novartis Pharmaceuticals on which Dr. Pfeffer is a co-inventor. His share of the licensing agreement is irrevocably transferred to charity.
Footnotes
Clinical Trials Registration: URL: https://clinicaltrials.gov/ct2/show/NCT00094302; Unique Identifier: NCT00094302.
REFERENCES
- 1.Lee D-C, Pate RR, Lavie CJ, Sui X, Church TS, Blair SN. Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol. 2014;64:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee I-M, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC, Svetkey LP, Wadden TA, Yanovski SZ. 2013 AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S76–S99. [DOI] [PubMed] [Google Scholar]
- 3.Pandey A, Garg S, Khunger M, Darden D, Ayers C, Kumbhani DJ, Mayo HG, de Lemos JA, Berry JD. Dose-Response Relationship Between Physical Activity and Risk of Heart Failure: A Meta-Analysis. Circulation. 2015;132:1786–1794. [DOI] [PubMed] [Google Scholar]
- 4.Bell EJ, Lutsey PL, Windham BG, Folsom AR. Physical Activity and Cardiovascular Disease in African Americans in Atherosclerosis Risk in Communities: Med Sci Sports Exerc. 2013;45:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wen CP, Wai JPM, Tsai MK, Yang YC, Cheng TYD, Lee M-C, Chan HT, Tsao CK, Tsai SP, Wu X. Minimum amount of physical activity for reduced mortality and extended life expectancy: a prospective cohort study. Lancet. 2011;378:1244–1253. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Hu FB, Rich-Edwards JW, Colditz GA, Stampfer MJ, Willett WC, Speizer FE, Hennekens CH. A Prospective Study of Walking as Compared with Vigorous Exercise in the Prevention of Coronary Heart Disease in Women. N Engl J Med. 1999;341:650–658. [DOI] [PubMed] [Google Scholar]
- 7.Fujimoto N, Prasad A, Hastings JL, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. Am Heart J. 2012;164:869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzman DW, Brubaker PH, Herrington DM, Morgan TM, Stewart KP, Hundley WG, Abdelhamed A, Haykowsky MJ. Effect of Endurance Exercise Training on Endothelial Function and Arterial Stiffness in Older Patients With Heart Failure and Preserved Ejection Fraction: A Randomized, Controlled, Single-Blind Trial. J Am Coll Cardiol. 2013;62:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelmann F, Gelbrich G, Düngen H-D, Fröhling S, Wachter R, Stahrenberg R, Binder L, Töpper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Löffler M, Hasenfuss G, Halle M, Pieske B. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction: Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58:1780–1791. [DOI] [PubMed] [Google Scholar]
- 10.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise Training in Older Patients With Heart Failure and Preserved Ejection Fraction A Randomized, Controlled, Single-Blind Trial. Circ Heart Fail. 2010;3:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor RS, Davies EJ, Dalal HM, Davis R, Doherty P, Cooper C, Holland DJ, Jolly K, Smart NA. Effects of exercise training for heart failure with preserved ejection fraction: A systematic review and meta-analysis of comparative studies. Int J Cardiol. 2012;162:6–13. [DOI] [PubMed] [Google Scholar]
- 12.Smart NA, Haluska B, Jeffriess L, Leung D. Exercise Training in Heart Failure With Preserved Systolic Function: A Randomized Controlled Trial of the Effects on Cardiac Function and Functional Capacity. Congest Heart Fail. 2012;18:295–301. [DOI] [PubMed] [Google Scholar]
- 13.Pandey A, Parashar A, Kumbhani DJ, Agarwal S, Garg J, Kitzman D, Levine BD, Drazner M, Berry JD. Exercise Training in Patients With Heart Failure and Preserved Ejection Fraction Meta-Analysis of Randomized Control Trials. Circ Heart Fail. 2015;8:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, LeWinter MM, Rouleau JL, Bull DA, Mann DL, Deswal A, Stevenson LW, Givertz MM, Ofili EO, O’Connor CM, Felker GM, Goldsmith SR, Bart BA, McNulty SE, Ibarra JC, Lin G, Oh JK, Patel MR, Kim RJ, Tracy RP, Velazquez EJ, Anstrom KJ, Hernandez AF, Mascette AM, Braunwald E. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: A randomized clinical trial. JAMA. 2013;309:1268–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houstis NE, Lewis GD. Causes of Exercise Intolerance in Heart Failure With Preserved Ejection Fraction: Searching for Consensus. J Card Fail. 2014;20:762–778. [DOI] [PubMed] [Google Scholar]
- 16.Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, Michelson EL, Olofsson B, Östergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. The Lancet. 2003;362:777–781. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. [DOI] [PubMed] [Google Scholar]
- 18.Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, Anderson S, Donovan M, Iverson E, Staiger C, Ptaszynska A. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–2467. [DOI] [PubMed] [Google Scholar]
- 19.Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O’Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM. Spironolactone for Heart Failure with Preserved Ejection Fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 20.Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, Duvinage A, Stahrenberg R, Durstewitz K, Löffler M, Düngen HD, Tschöpe C, Herrmann-Lingen C, Halle M, Hasenfuss G, Gelbrich G, Pieske Bl. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: The aldo-dhf randomized controlled trial. JAMA. 2013;309:781–791. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Borlaug BA, Lewis GD, Mohammed SF, Semigran MJ, LeWinter MM, Deswal A, Hernandez AF, Lee KL, Braunwald E. PhosphdiesteRasE-5 Inhibition to Improve CLinical Status and EXercise Capacity in Diastolic Heart Failure (RELAX) Trial Rationale and Design. Circ Heart Fail. 2012;5:653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfeffer MA, Claggett B, Assmann SF, Boineau R, Anand IS, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Heitner JF, Lewis EF, O’Meara E, Rouleau J-L, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, McKinlay SM, Pitt B. Regional Variation in Patients and Outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circulation. 2015;131:34–42. [DOI] [PubMed] [Google Scholar]
- 23.Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, O’Meara E, Desai AS, Heitner JF, Li G, Fang J, Rouleau J, Zile MR, Markov V, Ryabov V, Reis G, Assmann SF, McKinlay SM, Pitt B, Pfeffer MA, Solomon SD. Cardiac Structure and Function and Prognosis in Heart Failure With Preserved Ejection Fraction: Findings From the Echocardiographic Study of the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) Trial. Circ Heart Fail. 2014;7:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. Exercise and Physical Activity for Older Adults: Med Sci Sports Exerc. 2009;41:1510–1530. [DOI] [PubMed] [Google Scholar]
- 25.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O’Brien WL, Bassett DR, Schmitz KH, Emplaincourt PO, Jacobs DR, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. [DOI] [PubMed] [Google Scholar]
- 26.Meyer A-M, Evenson KR, Morimoto L, Siscovick D, White E. Test-Retest Reliability of the Women’s Health Initiative Physical Activity Questionnaire: Med Sci Sports Exerc. 2009;41:530–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierannunzi C, Hu SS, Balluz L. A systematic review of publications assessing reliability and validity of the Behavioral Risk Factor Surveillance System (BRFSS), 2004–2011. BMC Med Res Methodol. 2013;13:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.USDHHS. 2008. Physical Activity Guidelines for Americans [Internet]. Washington DC: 2008 [cited 2015 Jan 7]. Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf. [Google Scholar]
- 29.Cuzick J A Wilcoxon-type test for trend. Stat Med. 1985;4:87–90. [DOI] [PubMed] [Google Scholar]
- 30.Schoenfeld D Partial residuals for the proportional hazards regression model. Biometrika. 1982;69:239–241. [Google Scholar]
- 31.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, DeMets D, Massie BM. Factors Associated With Outcome in Heart Failure With Preserved Ejection Fraction Findings From the Irbesartan in Heart Failure With Preserved Ejection Fraction Study (I-PRESERVE). Circ Heart Fail. 2011;4:27–35. [DOI] [PubMed] [Google Scholar]
- 32.Rahman I, Bellavia A, Wolk A. Relationship Between Physical Activity and Heart Failure Risk in Women. Circ Heart Fail. 2014;7:877–881. [DOI] [PubMed] [Google Scholar]
- 33.Agha G, Loucks EB, Tinker LF, Waring ME, Michaud DS, Foraker RE, Li W, Martin LW, Greenland P, Manson JE, Eaton CB. Healthy Lifestyle and Decreasing Risk of Heart Failure in Women: The Women’s Health Initiative Observational Study. J Am Coll Cardiol. 2014;64:1777–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saevereid HA, Schnohr P, Prescott E. Speed and Duration of Walking and Other Leisure Time Physical Activity and the Risk of Heart Failure: A Prospective Cohort Study from the Copenhagen City Heart Study. PLoS ONE. 2014;9:e89909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan MMY, Lam CSP. How do patients with heart failure with preserved ejection fraction die? Eur J Heart Fail. 2013;15:604–613. [DOI] [PubMed] [Google Scholar]
- 36.Hummel SL, Kitzman DW. Update on Heart Failure with Preserved Ejection Fraction. Curr Cardiovasc Risk Rep. 2013;7:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 38.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, Gong Y, Liu PP. Outcome of Heart Failure with Preserved Ejection Fraction in a Population-Based Study. N Engl J Med. 2006;355:260–269. [DOI] [PubMed] [Google Scholar]
- 39.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–2216. [DOI] [PubMed] [Google Scholar]
- 40.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC. Clinical Presentation, Management, and In-Hospital Outcomes of Patients Admitted With Acute Decompensated Heart Failure With Preserved Systolic Function: A Report From the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 41.Brinker SK, Pandey A, Ayers CR, Barlow CE, DeFina LF, Willis BL, Radford NB, Farzaneh-Far R, de Lemos JA, Drazner MH, Berry JD. Association of Cardiorespiratory Fitness With Left Ventricular Remodeling and Diastolic Function. JACC Heart Fail. 2014;2:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hegde SM, Gonçalves A, Claggett B, Evenson KR, Cheng S, Shah AM, Folsom AR, Solomon SD. Cardiac structure and function and leisure-time physical activity in the elderly: The Atherosclerosis Risk in Communities Study. Eur Heart J. 2016;37:2544–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of Endurance Training on the Determinants of Peak Exercise Oxygen Consumption in Elderly Patients With Stable Compensated Heart Failure and Preserved Ejection Fraction. J Am Coll Cardiol. 2012;60:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kosmala W, Rojek A, Przewlocka-Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68:1823–1834. [DOI] [PubMed] [Google Scholar]
- 45.Pandey A, Garg S, Matulevicius SA, Shah AM, Garg J, Drazner MH, Amin A, Berry JD, Marwick TH, Marso SP, de Lemos JA, Kumbhani DJ. Effect of Mineralocorticoid Receptor Antagonists on Cardiac Structure and Function in Patients With Diastolic Dysfunction and Heart Failure With Preserved Ejection Fraction: A Meta‐Analysis and Systematic Review. J Am Heart Assoc. 2015;4:e002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL, Investigators for the H-A. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. JAMA. 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shephard RJ. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med. 2003;37:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forsén L, Loland NW, Vuillemin A, Chinapaw MJM, van Poppel MNM, Mokkink LB, van Mechelen W, Terwee CB. Self-Administered Physical Activity Questionnaires for the Elderly. Sports Med. 2010;40:601–623. [DOI] [PubMed] [Google Scholar]
- 49.Silsbury Z, Goldsmith R, Rushton A. Systematic review of the measurement properties of self-report physical activity questionnaires in healthy adult populations. BMJ Open. 2015;5:e008430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.