Abstract
Objectives/Hypothesis:
The purpose of this study was to determine the effects of octanoic acid on acoustic, perceptual and functional aspects of essential voice tremor (EVT).
Study Design:
Prospective, double-blind, placebo-controlled, cross-over study.
Methods:
Sixteen participants with a diagnosis of EVT were randomized to a 3-week dosing condition of octanoic acid or placebo, followed by a 2-week washout period and crossover to the other condition for an additional 3 weeks. Baseline and post-testing sessions were completed before and at the completion of each condition. Primary outcome measures were the magnitude of amplitude and frequency tremor, measured from the acoustic signal. Secondary outcomes were auditory-perceptual ratings of tremor severity and self-ratings of voice handicap.
Results:
Magnitude of amplitude and frequency tremor were significantly lower after 3 weeks of octanoic acid dosing as compared to the placebo condition. Auditory-perceptual ratings of tremor severity did not show significant differences between conditions. A trend toward better voice was seen for the sustained vowel ratings, but not the sentence-level ratings. No significant differences between conditions was seen on self-reported voice disability as assessed on the Voice Handicap Index-10.
Conclusions:
The results of this controlled investigation support the potential utility of OA for reducing the magnitude of tremor in people with EVT. Further research is needed to determine whether different dosing or treatment combinations can improve functional communication in EVT.
Keywords: Tremor, voice, essential tremor, octanoic acid, cross-over, placebo-controlled
INTRODUCTION
Essential tremor (ET) is a common neurological movement disorder that particularly affects people over the age of 65. The prevalence of ET is likely to increase considerably in upcoming years based on a projected increase of 9% in this age demographic by 2060, when nearly one quarter of the population will be ages 65 and older1. The voice is affected in 20–30% of people with ET2,3, producing involuntary oscillations of the respiratory4, laryngeal5,6, and oropharyngeal7,8 muscles during speech production. People with essential voice tremor experience unstable, shaky, and hoarse voice quality, increased effort associated with speaking, decreased speech intelligibility, and pronounced voice-related disability7.
Treatment options are inadequate for improving voice in people with essential voice tremor (EVT)9,10. The source of the oscillations in ET is thought to be within the olivocerebellar neural circuits11,12 of the central nervous system, indicating that pharmacologic treatments which have central versus solely peripheral effects are more likely to impact tremor severity. Treatment-related research in EVT and clinical practice has primarily focused on botulinum toxin A (BTA), a treatment that can be helpful for approximately one-third of people with ETV8,13,14, but is considered less than optimal due to its variable efficacy9,14, invasiveness, and negative side effects. The long-chain alcohol 1-octanol and its derivative octanoic acid (OA) have been studied in ET of the limbs as a potential treatment for tremor which may have similar mechanisms of action as ethanol, one of the most effective tremor-reducing agents15,16. Studies to date have shown that 1-octanol or OA can reduce the severity of tremor in the hands by as much as 41%, with minimal/mild side effects17–20. Treatment of EVT with OA may be advantageous to BTA due to its effect on the central and peripheral nervous system (CNS) regions21,22, its potential diffuse effects on muscles across multiple speech sub-systems which are affected by EVT, and the reported positive effect of its related parent compound ethanol for improving voice tremor7,23.
Controlled investigations of alternative pharmacologic interventions for EVT are critical to improved clinical care and level of evidence for treatment decisions. The purpose of this study was to perform a randomized, double-blind, placebo-controlled investigation of the effects of OA on acoustic, perceptual and functional aspects of voice. It was hypothesized that the following changes in tremor variables would occur after the OA condition when compared to the placebo condition: 1) the magnitude of amplitude tremor (Matr) would be lower, 2) the magnitude of frequency tremor (Mftr) would be lower, 3) auditory-perceptual ratings of perceived tremor severity would be better, and 4) participants would report less voice disorder disability/handicap.
METHODS
Participants
Seventeen participants with EVT were enrolled in the study after providing informed consent, with procedures completed for 16 participants (see Figure 1 for study enrollment flow chart). An Investigational New Drug (IND) application was approved through the Federal Drug Administration (FDA) for the use of OA as a potential drug treatment for essential voice tremor. Participants were recruited from regional otolaryngologists, neurologists, and primary care physicians. Octanoic acid had not been previously tested for people with EVT, but an initial power analysis was performed using an estimated effect size from a study that included similar acoustic outcome measures24. With an 80% power requirement, this analysis produced a required sample size of 16 participants; initial study targets included 6 additional participants (22 total) to adjust for possible attrition rates. Due to reaching the end of the funded study period, data collection was ended after enrollment of 17 participants. The analyzed sample size of 16 in this study generally exceeds that of other published, prospective studies addressing treatment of people with EVT6,14,24–26.
Figure 1:
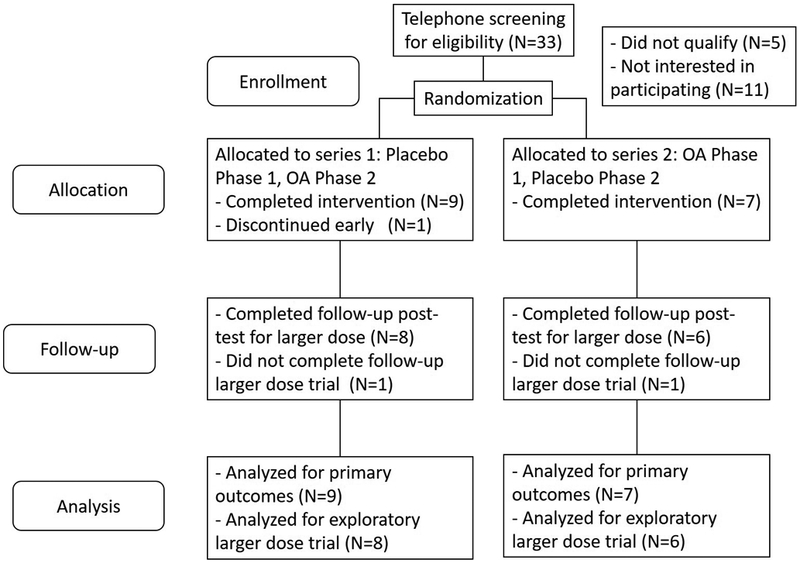
Study enrollment and allocation flow chart
Inclusion and exclusion criteria were determined through an initial naso-endoscopy evaluation performed by a laryngologist and a voice evaluation with acoustic recordings performed by a speech-language pathologist (SLP), as well as through patient report. All participants were diagnosed with EVT by an otolaryngologist, with confirmation of essential tremor by a neurologist. Stringent inclusion and exclusion criteria were implemented (see Appendix A).
A randomized, double-blind, cross-over design was used to compare effects of OA to a placebo control. Total study duration was approximately 11 weeks for each participant, which included Phases 1 and 2 for the active or placebo drugs (see Table 1 for study procedures and timeline). Each phase included baseline testing, 3 weeks of consecutive drug dosing, and post-testing at the end of each 3-week drug period with a 2-week intervening wash-out. With an established elimination half-life of OA at 150 minutes20, this wash-out period well exceeds any potential for residual effects from Phase 1 to Phase 2. To control for possible order effects, a randomized, counterbalanced list (generated by an independent person) was used to assign drug order for each participant upon enrollment. Subjects were randomized to one of two sequences (placebo-Phase 1 then OA-Phase 2 or OA-Phase 1 then placebo-Phase 2). Participants and researchers were blinded to drug status (OA or placebo) throughout all study procedures, including data analysis and statistical testing.
Table 1:
Timeline and study procedures.
| Time | Week 1 | Weeks 2-4 | Weeks 5-6 | Week 7 | Weeks 8-10 | Week 11 |
|---|---|---|---|---|---|---|
| Procedures | Qualifying assessments | 3 weeks at full drug dosing | Drug dosing lowered, then withdrawn | 3 weeks at full drug dosing | Drug dosing lowered, then withdrawn | |
| Initial questionnaires | 2 week drug washout period | |||||
| Pre-intervention nurse visit (#1/4) | Nurse visit in first week of full dosing, drug 1 (#2/4) | Nurse visit in first week of full dosing, drug 2 (#3/4) | Post-intervention nurse visit (#4/4) | |||
| Baseline testing sessions 1, 2, & 3 for drug 1 | Post-testing sessions 1 & 2 at 16 mg/kg dose | Baseline testing sessions 1, 2, & 3 for drug 2 | Post-testing sessions 1 & 2 at 16 mg/kg dose | |||
| Drug step-up | Post-testing session 3 at 32 mg/kg dose | Drug step-up, then full dosing drug 2 | Post-testing session 3 at 32 mg/kg dose | |||
| Phase | Drug 1 at 16 mg/kg dose | Drug 1 | Cross-over to drug 2 at 16 mg/kg dose | Drug 2 | ||
| (Placebo or octanoic acid) | (Placebo or octanoic acid) | (Placebo or octanoic acid) | (Placebo or octanoic acid) |
Baseline and Post-Testing Assessment Procedures
Baseline and post-testing data were collected over three consecutive testing days at the start and end of each study phase, resulting in six data collection time points for each study phase. Post-testing (post-treatment) occurred on the final 3 days of each 3-week drug intake phase. Two acoustic measures of tremor magnitude served as the primary outcome measures, and two perceptual measures (listener ratings and patient self-impact ratings) served as the secondary outcome measures. During the initial testing of Phase 1, additional procedures were performed to descriptively assess the extent of oropharyngeal and laryngeal tremor and the extent of body tremor. A standardized naso-endoscopy examination was performed which included all tasks required for the Vocal Tremor Scoring System (VTSS), a validated scale for the visual-perceptual rating of voice tremor affecting the laryngeal and oropharyngeal regions8. A digital video recording of the participant performing a range of functional tasks was obtained during initial testing, and later scored by a neurologist using a validated measure (TETRAS)27 to determine the presence and severity of tremor across all body regions. As a self-assessment, the participant completed a rating scale (QUEST)28 to assess life impact of overall body tremor. The VTSS, TETRAS and QUEST were included for descriptive purposes only to delineate tremor characteristics of this participant group.
Additional data were collected at each of the three baseline and post-testing sessions. Voice recordings were performed in a sound-attenuated booth with a head-mounted condenser microphone (AKG C550, Harman International Industries, Stamford, CT) positioned 9 cm from the mouth. The Computerized Speech Lab (CSL 4500, KayPENTAX, Montvale, NJ) was used for audio recordings, with a sampling rate of 50 kHz. Speaking tasks included three repetitions of sustained /ɑ/ vowels, standardized sentences29, and an all-voiced sentence30, all at a comfortable pitch and loudness. Participants also completed several questionnaires at each visit, including the Voice Handicap Index-1031 (VHI-10) and a suicide risk screening32,33 per FDA requirements for clinical trials. For all baseline and post-testing, participants were instructed to avoid alcohol (48 hours prior) and caffeine (testing day), and to fast the morning of post-testing. Testing was performed 3 hours after drug intake for all post-testing visits, based on prior OA studies addressing timing of effects17,19.
Drug Trials
Octanoic acid was formulated in softgel capsules with dosing sizes of 400 and 200 mg (Best Formulations, City of Industry, CA). Dosing of 16 mg/kg was chosen for the main drug dosing period (20 days), based on prior studies showing that dosing at 64 mg/kg dosing of 1-octanol produced significant reduction in dominant hand tremor in a 90 minute period18, with few and mild side adverse effects up to 128 mg/kg18,20, whereas 4 mg/kg dosing of OA did not significantly improve hand tremor until 300 minutes post intake17. A higher dosing of 32 mg/kg was implemented on the final day of the drug phase (day 21) to obtain preliminary data on possible dose-dependent effects. Soybean oil was formulated in identical softgel capsules and sizes for the placebo dosing, with identical procedures and dosing used for the placebo phase. Participants were instructed to take each dose in the morning with a meal, with intake time logged daily.
Safety Monitoring
Four independent nurse monitoring visits occurred over the course of the study, and results were reviewed regularly by the study physician. Visits occurred at baseline, within week 1 of each drug phase, and upon study completion. Vital signs and a patient questionnaire that addressed adverse effects and their severity were assessed at each visit. The questionnaire included 30 symptoms assessed/reported in prior OA studies17,18 (see Appendix B). An electrocardiogram (EKG) and hepatic function panel (HFP) were collected at baseline (prior to any drug intake) and upon study end.
Outcome Measures and Data Analysis
All data analysis for the dependent variables was performed by a researcher who was not involved with data collection, and was blind to subject identity, drug condition, and time point. The objective acoustic measures of magnitude of amplitude (Matr) and frequency tremor (Mftr) reflected the extent of tremor in the acoustic signal and served as the primary outcome measures. Auditory-perceptual tremor severity ratings and self-reported voice disability (VHI-10) served as secondary outcome measures. The sustained vowel /ɑ/ provided the necessary steady-state context for extracting Matr and Mftr measures, which were computed for a 4-sec interval of each vowel token, 1.0 sec after onset to avoid any onset effects. Matr and Mftr were computed from the amplitude and frequency contours respectively using higher-order perturbation functions over an analysis window, and expressed as a percent of the mean overall signal to normalize each measure34 (Motor Speech Profile, KayPENTAX). Perturbation measures follow guidelines established by the National Center for Voice and Speech35. Mean values of the three /ɑ/ tokens per testing session were computed for each tremor magnitude variable36.
Three raters with experience in voice disorders performed paired-sample ratings of tremor severity for the first sustained /ɑ/ utterance and the all-voiced sentence for each respective baseline to post-test session. The sustained vowel provided a context in which tremor is known to be more perceptually salient37, while the all-voiced sentence provided a context more representative of everyday speaking. Paired samples were counterbalanced for baseline/post-test order, and then randomized for blinded analysis. Sound-pressure level was balanced for each token of the paired sample to avoid listener bias based on SPL disparities. Listeners rated samples independently in a quiet environment using the audio signal only, with immediate repetition of each sample as needed.
Statistical Analyses
An independent statistician who was blinded to drug condition performed all statistical analyses using SAS and R- codes. Statistical testing was performed on each of the primary outcome measures (Matr and Mftr) as well as the secondary outcome measures (auditory-perceptual ratings and VHI-10). Mixed effect statistical modeling was used to determine post-treatment drug differences, with testing session as a repeated factor and baseline averages as a covariate. An additional statistical model was run to assess post-treatment differences without the inclusion of baseline covariance, based on statistical recommendations for two-period crossover treatment studies38. Analysis of outcome measures for the 3-week, daily 16 mg/kg was determined from mean values for each variable from baseline 1 and 2 and post-test 1 and 2, and exploratory analyses of the 1-day dosing effects at 32 mg/kg were determined from the comparison of baseline 3 to post-test 3. Intra-rater reliability for the auditory-perceptual ratings was assessed with Cohen’s kappa for 16% of the samples for each speaking context, which were included as repeated samples in the overall randomized samples that were rated. Inter-rater reliability across the three raters was assessed using Fleiss’ kappa for all samples within each speaking context.
RESULTS
Average age for the 16 participants was 70.3 (SD=8.7), with 2 males and 14 females, consistent with prior reported demographics7,24,36 (see Table 2 for full participant characteristics). Group means, standard deviations, and statistical results for the primary and secondary outcome measures are summarized in Tables 3, 4 and 5. Period and sequence effects were not significant for any of the primary outcome measures, indicating that there was no influence of drug order. Kappa reliability for the auditory-perceptual ratings of the sustained vowel ranged from 0.604 to 0.636, whereas reliability for the sentence-level speaking context ranged from 0.497 to 0.515 (Table 6). These levels are considered substantial and moderate respectively, as described by Landis and Koch39.
Table 2:
Demographics of the 16 participants who completed the study. Mean (standard deviation), EVT = essential voice tremor, VHI-10 = Voice Handicap Index-10, VTSS = Vocal Tremor Scoring System, TETRAS = Tremor Research Group Essential Tremor Rating Assessment Scale, QUEST = Quality of Life in Essential Tremor Questionnaire
| Value | Additional Information | |
| Mean Age (SD) | 70.3 years (8.7) | |
| Sex | 14 F, 2 M | |
| Affected body areas | ||
| Presence of other body tremor | Yes: 10 No: 6 |
Head: 4 Jaw: 1 Face (lips): 1 Upper extremities: 8 Lower extremities: 1 |
| Positive family history for tremor | Yes: 10 No: 6 |
|
| Self-reported voice improvement with alcohol | Unknown: 6 Yes: 3 No: 7 |
|
| Auditory-perceptual tremor severity (sustained vowel) | Mild: 1 Mild-moderate: 4 Moderate: 5 Moderate-severe: 2 Severe: 4 |
|
| Mean (SD) | Range and % of Points Possible | |
| Years post EVT onset | 9.34 (7.12) | 1.00 - 25.00 |
| Initial VHI-10 Score, Baseline 1, Phase 1 (10 items, 40 points possible) | 19.38 (8.50) | Range = 2.00 - 34.00 % of Points Possible = 48.45% |
| VTSS (6 items, 18 points possible) | 10.80 (3.99) | 4.00 - 17.00 % of Points Possible = 60.00% |
| TETRAS Total Score (21 items, 112 points possible) | 17.22 (14.12) | 7.00 - 62.00 % of Points Possible = 15.38% |
| • Activities of Daily Living subscale (12 items, 48 points possible) | 10.00 (7.03) | 4.00 - 34.00 % of Points Possible = 20.83% |
| • Performance subscale (9 items, 64 points possible) | 7.22 (7.47) | 1.00 - 28.00 % of Points Possible = 11.28% |
| QUEST Total Score (30 items, 120 points possible | 29.75 (20.11) | 7.00 - 74.00 % of Points Possible = 24.79% |
| • QUEST, Voice Impact Sub-analysis (4 questions, 16 points possible) Questions #3, #19, #20 & #21 relate to tremor interfering with telephone use, communication, & speech intelligibility | 8.06 (4.30) | 0.00 - 15.00 % of Points Possible = 50.38% |
Table 3:
Magnitude (Magn) of amplitude tremor (as percent modulation) and magnitude of frequency tremor (as percent modulation): group means, standard deviations (SD), F-statistics and P values with and without baseline (BL) covariance adjustment (alpha <0.05). Means include data from Baseline 1 and 2 and Post-test 1 and 2 for each drug phase.
|
Magn
of Amplitude Tremor |
SD |
F value
(with BL covariance) |
P value
(with BL covariance) |
F value
(without BL covariance) |
P value
(without BL covariance) |
|
|---|---|---|---|---|---|---|
| Octanoic acid | ||||||
| Baseline | 13.49 | 6.48 | ||||
| Post-test | 9.35 | 5.42 | ||||
| Placebo | ||||||
| Baseline | 12.91 | 6.46 | ||||
| Post-test | 12.43 | 7.37 | ||||
| Statistical Results | 4.69 | 0.0499* | 6.68 | 0.0216* | ||
|
Magn of
Frequency Tremor |
SD |
F value
(with BL covariance) |
P value
(with BL covariance) |
F value
(without BL covariance) |
P value
(without BL covariance) |
|
| Octanoic acid | ||||||
| Baseline | 5.19 | 3.58 | ||||
| Post-test | 3.98 | 2.94 | ||||
| Placebo | ||||||
| Baseline | 5.57 | 4.29 | ||||
| Post-test | 5.35 | 3.76 | ||||
| Statistical Results | 4.85 | 0.0450* | 5.53 | 0.0339* | ||
Table 4:
Paired comparison baseline to post-treatment (BL-PT) auditory-perceptual ratings where 1 = post-test “better” than baseline, points possible = 0-3 (sum of three raters’ scores): group means for summed scores, standard deviations (SD), F-test statistics, and P values (alpha <0.05).
| Auditory- Perceptual Ratings (BL-PT) |
SD |
F value (Main effect, drug) |
P value (Main effect, drug) |
F value (Main effect, task) |
P value (Main effect, task) |
F value (Interaction effect, drug*task) |
P value (Interaction effect, drug*task) |
|
|---|---|---|---|---|---|---|---|---|
| Octanoic acid | ||||||||
| Sustained vowel | 1.53 | 1.13 | ||||||
| All-voiced sentence | 1.19 | 0.87 | ||||||
| Placebo | ||||||||
| Sustained vowel | 1.25 | 1.13 | ||||||
| All-voiced sentence | 1.63 | 1.12 | ||||||
| Statistical Results | 0.14 | 0.7172 | 0.00 | 0.9602 | 2.02 | 0.1699 |
Table 5:
Voice Handicap Index-10 (VHI-10): group means, standard deviations (SD), F-statistics and P values with and without baseline (BL) covariance (alpha <0.05). Means include data from Baseline 1 and 2 and Post-test 1 and 2 for each drug phase.
| VHI-10 Mean | SD |
F value (with BL covariance) |
P value (with BL covariance) |
F value (without BL covariance) |
P value (without BL covariance) |
|
|---|---|---|---|---|---|---|
| Octanoic acid | ||||||
| Baseline | 16.75 | 6.88 | ||||
| Post-test | 15.16 | 7.51 | ||||
| Placebo | ||||||
| Baseline | 18.38 | 8.59 | ||||
| Post-test | 16.56 | 7.62 | ||||
| Statistical Results | 0.00 | 0.9831 | 2.05 | 0.1739 |
Table 6:
Intra-rater and inter-rater reliability (kappa coefficients) for auditory-perceptual ratings of tremor severity.
| Task | Intra-rater reliability (Computed with Cohen’s Kappa) |
Inter-rater reliability (Computed with Fleiss’ Kappa) |
||
|---|---|---|---|---|
| Mean | Range | Mean | Range | |
| Sustained vowel | 0.604 | 0.402-0.778 | 0.636 | N/A |
| All-voiced sentence | 0.515 | 0.359-0.664 | 0.497 | N/A |
A significantly lower Matr was seen after the OA condition as compared to the placebo condition, supporting the first hypothesis (Table 3). Mean values for the OA condition showed a baseline to post-test reduction of 4.14 in Matr, as compared with a 0.48 reduction for the placebo condition, with a Cohen’s d effect size of 0.645. Eleven of the 16 participants (69%) showed a reduction of >10% of their baseline mean in Matr upon post-testing. Likewise, Mftr was significantly lower after the OA condition as compared to the placebo condition, supporting the second hypothesis. Mean values for the OA condition showed a baseline to post-test reduction of 1.21 in Mftr, as compared with a 0.22 reduction for the placebo condition, with a Cohen’s d effect size of 0.588. Ten of the 16 participants (63%) showed a reduction of >10% of their baseline mean in Mftr upon post-testing.
Auditory-perceptual paired comparison ratings of differences in tremor severity between baseline and post-treatment voice recordings were not significantly different after the OA condition as compared to the placebo condition, not supporting the third hypothesis (Table 4). Ratings for the sustained vowel showed trends toward “better” for the OA condition, but not for the all-voiced sentence ratings. Self-perceived voice handicap as measured by the VHI-10 was also not significantly different after the OA condition as compared to the placebo condition, not supporting the fourth hypothesis (Table 5).
Due to participant logistics and traveling distance, the data for the exploratory analysis for the higher, 32 mg/kg dosing were completed for only 14 participants. None of these comparisons showed significant differences for the primary or secondary outcome measures, although trends toward lower tremor magnitude on the acoustic measures and lower ratings of tremor severity on the sustained vowel were evidenced after the OA condition (see Table 7).
Table 7:
Exploratory higher dose (32 mg/kg) results for Magnitude (Magn) of amplitude and frequency tremor, Voice Handicap Index (VHI-10) and auditory-perceptual (A-P) tremor severity ratings: group means, standard deviations (SD), F-statistics and P values with and without baseline (BL) covariance adjustment (alpha <0.05). Means are for data from Baseline 3 and Post-test 3 for each drug phase.
| Magn of Amplitude Tremor Mean | SD | F value (with BL covariance) | P value (with BL covariance) | F value (without BL covariance) | P value (without BL covariance) | |
| Octanoic acid | ||||||
| Baseline | 12.73 | 6.74 | ||||
| Post-test | 9.79 | 5.28 | ||||
| Placebo | ||||||
| Baseline | 13.07 | 6.75 | ||||
| Post-test | 12.98 | 8.24 | ||||
| Statistical Results | 1.90 | 0.1934 | 3.69 | 0.0787 | ||
| Magn of Frequency Tremor Mean | SD | F value (with BL covariance) | P value (with BL covariance) | F value (without BL covariance) | P value (without BL covariance) | |
| Octanoic acid | ||||||
| Baseline | 5.68 | 3.90 | ||||
| Post-test | 5.14 | 3.19 | ||||
| Placebo | ||||||
| Baseline | 5.74 | 4.24 | ||||
| Post-test | 5.64 | 3.73 | ||||
| Statistical Results | 0.37 | 0.5530 | 0.28 | 0.6094 | ||
| VHI-10 Mean | SD | F value (with BL covariance) | P value (with BL covariance) | F value (without BL covariance) | P value (without BL covariance) | |
| Octanoic acid | ||||||
| Baseline | 16.33 | 7.44 | ||||
| Post-test | 14.29 | 8.13 | ||||
| Placebo | ||||||
| Baseline | 19.21 | 8.60 | ||||
| Post-test | 15.79 | 7.64 | ||||
| Statistical Results | 0.00 | 0.9831 | 0.01 | .9323 | ||
| A-P Tremor Severity Mean | SD | F value | P value | |||
| Octanoic acid | ||||||
| Sustained vowel | 1.79 | 1.31 | ||||
| All-voiced sentence | 1.29 | 1.33 | ||||
| Placebo | ||||||
| Sustained vowel | 1.57 | 1.45 | ||||
| All-voiced sentence | 1.36 | 1.01 | ||||
| Statistical Results | ||||||
| Drug (Main effect) | 0.03 | 0.8670 | ||||
| Task (Main effect) | 1.84 | 0.1921 | ||||
| Drug*Task | 0.20 | 0.6587 | ||||
Drug safety and side effects were assessed in the nurse visits throughout the study for each participant. No participants showed any indication of adverse medical effects when comparing baseline to end-of-study EKG and HFP results. One adverse event (non-serious) resulted in early study termination for a participant. Deblinding and follow-up by the study physician indicated that the participant’s symptoms were related to a pre-existing medical condition, with end-of-study deblinding showing the event occurred during the placebo (Phase 1). Mean number of reported adverse effects was similar for the placebo (3.4) and OA conditions (3.7), and were similar to the baseline symptom frequency (before any intervention) for both conditions. Mean severity of symptoms was between the levels of “minimal” to “mild” for both the placebo (1.35) and OA (1.53) conditions (see Appendix B for symptom summary information).
DISCUSSION
Whereas several pharmacologic options with Level A or B evidence are available for the treatment of ET that affects the limbs, few such options are available for treating EVT. Efficacy studies for EVT primarily address BTA treatment14,40, or have implemented open-label designs or retrospective analyses to study pharmacological alternatives such as propranolol26 or primidone41. Prospective studies that include strong experimental controls and objective measures of voice tremor are needed to determine the potential benefit of drug alternatives in EVT. Well controlled open-label18,42 and double-blind trials17 of 1-octanol and OA have provided preliminary support for its ability to reduce magnitude of tremor in the limbs, but voice tremor was not addressed. The current study investigated the effects of OA on acoustic, perceptual and functional aspects of EVT. To our knowledge, this is the first double-blind, placebo-controlled pharmacological trial for people with EVT. In contrast to prior OA studies for ET which limited the inclusion of participants to those who showed alcohol-responsive tremor reduction, participants meeting stringent criteria for EVT were included regardless of whether their voice tremor was alcohol responsive.
Significantly lower magnitudes of amplitude and frequency tremor were measured after the OA condition as compared to the placebo condition, with moderate to large effect sizes43 for each of the primary outcome variables. In limb tremor, tremor amplitude rather than tremor frequency typically responds to pharmacological treatment44, and is the primary outcome measure in many treatment efficacy studies as assessed with accelerometry17,18,42,44–46. Essential voice tremor is characterized by modulations in the amplitude and frequency of the acoustic signal36,47, and changes to pitch and loudness can differentially affect these two parameters36. The results of the current study are consistent with prior studies showing that pharmacologic intervention (BTA) can improve the extent of frequency or amplitude tremor14,24, and that differential levels of change for each variable may occur after treatment14.
These objective Matr and Mftr measures are derived from sustained vowel utterances which are characterized by normally invariant frequency and amplitude characteristics that enable the reliable measurement of signal modulation, and this task is important in differential diagnosis and characterization of voice tremor48. Tremor is perceived to be significantly more severe during sustained vowels as compared to connected speech37, a context in which frequency and amplitude modulation cannot be appropriately measured because high modulation is expected. Other acoustic measures also may not reflect tremor characteristics well in conversational (connected) speech48. The mixture of voiced and unvoiced sounds, rapid speaking rate, and natural variations in pitch and loudness can mask tremor in connected speech, making tremor less perceptible.
In the current study, auditory-perceptual ratings of tremor severity in sustained vowels showed trends toward lower severity for the OA condition relative to the placebo. It is likely that this speaking context allowed the greatest sensitivity to small changes in tremor severity. In contrast, the ratings of the connected speech sentence did not show concordant trends and may have been more difficult for listeners to consistently rate. Reliability of listener ratings supports this; although intra-rater and inter-rater kappa reliability levels for both speaking contexts fell in the range of “moderate” agreement39, only the kappa values for the sustained vowel reached ≥60%, with values up to 14% higher for the vowel versus the connected speech sentence (Table 6). A recent open-label study26 included auditory-perceptual ratings of tremor severity to assess the efficacy of BTA and propranolol, and also found low inter-rater kappa reliability (0.25) for sentence-level ratings among experienced listeners. Auditory-perceptual tremor severity did not show significant change after either form of treatment, leading the authors to suggest that objective tremor measures may be important to determine EVT treatment outcome26. In the current study, the lack of self-perceived voice disability difference on the VHI-10 for the OA condition is consistent with the auditory-perceptual findings. Because perceptual saliency of tremor is lower in connected speech, larger amounts of change may be necessary in EVT to functionally improve communication. Additionally, although baseline means for the VHI-10 in this study were two and a half to three standard deviations greater than means previously reported for a non voice-disordered control group31, it is possible that higher initial scores on this impact measure would have allowed for a greater degree of post-treatment change.
Although the objective reduction in tremor magnitude from the acoustic analysis of sustained vowels in this study provides some support for the efficacy of OA, future studies are needed to determine whether differences in the amount and timing of dosing, combined modality treatment, or other factors could produce functionally meaningful voice improvement in people with EVT. Our exploratory analysis of a higher OA dose did not support a dose-dependent effect, as these results showed trends but no statistically significant benefit of OA. However, these data had far fewer data points than the regular dosing, and dosing at 32 mg/kg occurred on only one day as compared to 20 days for the 16 mg/kg dose. It is possible that higher dosing for a longer time would be needed for better OA efficacy. The significant reductions in tremor magnitude after 3 weeks of OA intake are promising due to their potential applicability to the overall population of people with EVT (not just those who are alcohol responsive), as well as the high percentages of participants who showed at least some acoustically measurable reduction in tremor. However, further investigation of multiple OA variables in larger study samples is needed to determine its potential for meaningful improvement in communication for people with EVT.
Supplementary Material
Appendix A: Inclusion and exclusion criteria for participants
Appendix B: Symptom questionnaire for adverse effects
ACKNOWLEDGMENTS
We would like to gratefully acknowledge all of the research participants who dedicated substantial amounts of time and energy to this study with the desire to help others who are affected. We would also like to gratefully acknowledge Best Formulations, who made the production of the placebo and active drug feasible for this study in their effort to support clinical trials.
Funding for this study was provided by the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, R03DC012429.
Footnotes
All authors have approved the manuscript and have agreed to its contents. All authors certify that they have no financial disclosures related to this work. All authors certify that they have no conflict of interest to report in relation to this work.
REFERENCES
- 1.Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the united states: population estimates and projections. US Census Bureau website. Available at: https://www.census.gov/data/tables/2014/demo/popproj/2014-summary-tables.html Accessed May 2018. [Google Scholar]
- 2.Lou JS, Jankovic J. Essential tremor: clinical correlates in 350 patients. Neurology 1991; 41:234–238. [DOI] [PubMed] [Google Scholar]
- 3.Diaz NL, Louis ED. Survey of medication usage patterns among essential tremor patients: movement disorder specialists vs. general neurologists. Parkinsonism Relat Disord 2010; 16:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tomoda H, Shibasaki H, Kuroda Y, Shin T. Voice tremor: dysregulation of voluntary expiratory muscles. Neurology 1987; 37:117–122. [DOI] [PubMed] [Google Scholar]
- 5.Koda J, Ludlow CL. An evaluation of laryngeal muscle activation in patients with voice tremor. Otolaryngol Head Neck Surg 1992; 107:684–696. [DOI] [PubMed] [Google Scholar]
- 6.Hertegard S, Granqvist S, Lindestad PA. Botulinum toxin injections for essential voice tremor. Ann Otol Rhinol Laryngol 2000; 109:204–209. [DOI] [PubMed] [Google Scholar]
- 7.Sulica L, Louis ED. Clinical characteristics of essential voice tremor: a study of 34 cases. Laryngoscope 2010; 120:516–528. [DOI] [PubMed] [Google Scholar]
- 8.Bove M, Daamen N, Rosen C, Wang CC, Sulica L, Gartner-Schmidt J. Development and validation of the vocal tremor scoring system. Laryngoscope 2006; 116:1662–1667. [DOI] [PubMed] [Google Scholar]
- 9.Ludlow CL, Mann EA. Management of the spasmodic dysophonias. In: Rubin JS, Sataloff RT, Korovin GS, eds. Diagnosis and Treatment of Voice Disorders. Clifton Park: Thompson Delmar Learning, 2003:457–478. [Google Scholar]
- 10.Boone DR, McFarlane SC, Von Berg SL, Zraick RI. The Voice and Voice Therapy. Boston, MA: Allyn & Bacon, 2010. [Google Scholar]
- 11.Deuschl G, Raethjen J, Lindemann M, Krack P. The pathophysiology of tremor. Muscle Nerve 2001; 24:716–735. [DOI] [PubMed] [Google Scholar]
- 12.Rincon F, Louis ED. Benefits and risks of pharmacological and surgical treatments for essential tremor: disease mechanisms and current management. Expert Opin Drug Saf 2005; 4:899–913. [DOI] [PubMed] [Google Scholar]
- 13.Blitzer A, Brin MF, Fahn S, Lovelace RE. Clinical and laboratory characteristics of focal laryngeal dystonia: study of 110 cases. Laryngoscope 1988; 98:636–640. [DOI] [PubMed] [Google Scholar]
- 14.Warrick P, Dromey C, Irish JC, Durkin L, Pakiam A, Lang A. Botulinum toxin for essential tremor of the voice with multiple anatomical sites of tremor: a crossover design study of unilateral versus bilateral injection. Laryngoscope 2000; 110:1366–1374. [DOI] [PubMed] [Google Scholar]
- 15.Growdon JH, Shahani BT, Young RR. The effect of alcohol on essential tremor. Neurology 1975; 25:259–262. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic J Treatment of hyperkinetic movement disorders. Lancet Neurol 2009; 8:844–856. [DOI] [PubMed] [Google Scholar]
- 17.Haubenberger D, McCrossin G, Lungu C et al. Octanoic acid in alcohol-responsive essential tremor: a randomized controlled study. Neurology 2013; 80:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nahab FB, Wittevrongel L, Ippolito D et al. An Open-Label, Single-Dose, Crossover Study of the Pharmacokinetics and Metabolism of Two Oral Formulations of 1-Octanol in Patients with Essential Tremor. Neurotherapeutics 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shill HA, Bushara KO, Mari Z, Reich M, Hallett M. Open-label dose-escalation study of oral 1-octanol in patients with essential tremor. Neurology 2004; 62:2320–2322. [DOI] [PubMed] [Google Scholar]
- 20.Voller B, Lines E, McCrossin Get al. Dose-escalation study of octanoic acid in patients with essential tremor. J Clin Invest 2016; 126:1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao H, Thompson-Westra J, Hallett M, Haubenberger D. The response of the central and peripheral tremor component to octanoic acid in patients with essential tremor. Clin Neurophysiol 2018; 129:1467–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinton CM, Krosser BI, Walton KD, Llinas RR. The effectiveness of different isomers of octanol as blockers of harmaline-induced tremor. Pflugers Arch 1989; 414:31–36. [DOI] [PubMed] [Google Scholar]
- 23.Andrews ML. Manual of Voice Treatment: Pediatrics Through Geriatrics. Clifton Park: Thomson/Delmar Learning, 2006. [Google Scholar]
- 24.Adler CH, Bansberg SF, Hentz JGet al. Botulinum toxin type A for treating voice tremor. Arch Neurol 2004; 61:1416–1420. [DOI] [PubMed] [Google Scholar]
- 25.Busenbark K, Ramig L, Dromey C, Koller WC. Methazolamide for essential voice tremor. Neurology 1996; 47:1331–1332. [DOI] [PubMed] [Google Scholar]
- 26.Justicz N, Hapner ER, Josephs JS, Boone BC, Jinnah HA, Johns MM, 3rd. Comparative effectiveness of propranolol and botulinum for the treatment of essential voice tremor. Laryngoscope 2016; 126:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elble R, Comella C, Fahn Set al. Reliability of a new scale for essential tremor. Mov Disord 2012; 27:1567–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Troster AI, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in Essential Tremor Questionnaire (QUEST): development and initial validation. Parkinsonism Relat Disord 2005; 11:367–373. [DOI] [PubMed] [Google Scholar]
- 29.Kempster GB, Gerratt BR, Verdolini Abbott K, Barkmeier-Kraemer J, Hillman RE. Consensus auditory-perceptual evaluation of voice: development of a standardized clinical protocol. Am J Speech Lang Pathol 2009; 18:124–132. [DOI] [PubMed] [Google Scholar]
- 30.Roy N, Whitchurch M, Merrill RM, Houtz D, Smith ME. Differential diagnosis of adductor spasmodic dysphonia and muscle tension dysphonia using phonatory break analysis. Laryngoscope 2008; 118:2245–2253. [DOI] [PubMed] [Google Scholar]
- 31.Rosen CA, Lee AS, Osborne J, Zullo T, Murry T. Development and validation of the voice handicap index-10. Laryngoscope 2004; 114:1549–1556. [DOI] [PubMed] [Google Scholar]
- 32.Posner K, Brown GK, Stanley B et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011; 168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chappell P, Feltner DE, Makumi C, Stewart M. Initial validity and reliability data on the Columbia-Suicide Severity Rating Scale. Am J Psychiatry 2012; 169:662–663; author reply 663. [DOI] [PubMed] [Google Scholar]
- 34.Deliyski DD, DeLassus Gress C. Characteristics of Motor Speech Performance: Normative Data American Speech-Language-Hearing Conference. Boston, MA: American Speech-Language-Hearing Association, 1997. [Google Scholar]
- 35.Titze IR. Workshop on Acoustic Voice Analysis: Summary Statement. Workshop on Acoustic Voice Analysis. University of Iowa: National Center for Voice and Speech, 1994. [Google Scholar]
- 36.Dromey C, Warrick P, Irish J. The influence of pitch and loudness changes on the acoustics of vocal tremor. J Speech Lang Hear Res 2002; 45:879–890. [DOI] [PubMed] [Google Scholar]
- 37.Lederle A, Barkmeier-Kraemer J, Finnegan E. Perception of vocal tremor during sustained phonation compared with sentence context. J Voice 2012; 26:668 e661–669. [DOI] [PubMed] [Google Scholar]
- 38.Fleiss JL, Wallenstein S, Rosenfeld R. Adjusting for baseline measurements in the two-period crossover study: a cautionary note. Control Clin Trials 1985; 6:192–197. [DOI] [PubMed] [Google Scholar]
- 39.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174. [PubMed] [Google Scholar]
- 40.Adler CH, Shill HA, Beach TG. Essential tremor and Parkinson’s disease: Lack of a link. Mov Disord 2011; 26:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nida A, Alston J, Schweinfurth J. Primidone Therapy for Essential Vocal Tremor. JAMA Otolaryngol Head Neck Surg 2016; 142:117–121. [DOI] [PubMed] [Google Scholar]
- 42.Bushara KO, Goldstein SR, Grimes GJ Jr., Burstein AH, Hallett M. Pilot trial of 1-octanol in essential tremor. Neurology 2004; 62:122–124. [DOI] [PubMed] [Google Scholar]
- 43.Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates, 1988. [Google Scholar]
- 44.Winkler GF, Young RR. Efficacy of chronic propranolol therapy in action tremors of the familial, senile or essential varieties. N Engl J Med 1974; 290:984–988. [DOI] [PubMed] [Google Scholar]
- 45.Zesiewicz TA, Elble R, Louis EDet al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2005; 64:2008–2020. [DOI] [PubMed] [Google Scholar]
- 46.Calzetti S, Findley LJ, Perucca E, Richens A. Controlled study of metoprolol and propranolol during prolonged administration in patients with essential tremor. J Neurol Neurosurg Psychiatry 1982; 45:893–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ludlow CL, Bassich CJ, Connor NP, Coulter DC. Phonatory characteristics of vocal fold tremor. J Phonetics 1986; 14:509–515. [Google Scholar]
- 48.Barkmeier JM, Case JL. Differential diagnosis of adductor-type spasmodic dysphonia, vocal tremor, and muscle tension dysphonia. Current Opinion in Otolaryngology & Head and Neck Surgery 2000; 8:174–179. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A: Inclusion and exclusion criteria for participants
Appendix B: Symptom questionnaire for adverse effects


