Abstract
Chronic alcohol consumption renders the lung more susceptible to infections, in large part, by disrupting essential alveolar macrophage functions. Emerging evidence suggests that these functional deficits could be due to a suppression of GM-CSF signaling, which is believed to compromise monocyte growth and maturation in the lung. However, in addition to controlling monocyte behaviors, GM-CSF is also important for regulating surfactant homeostasis in the lung. For example, mice with targeted deletion of the gene for GM-CSF accumulate large amounts of phospholipids in their lung. Moreover, decreased GM-CSF signaling in humans has been mechanistically linked to the development of pulmonary alveolar proteinosis (PAP), a rare disorder in which surfactant lipids and proteins accumulate in alveolar macrophages and the lung exhibits enhanced susceptibility to respiratory infections. Consistent with parallel mechanisms in the PAP and alcoholic lung, we recently reported that levels of intrapulmonary lipids, albeit triglycerides and free fatty acids, were markedly increased in BAL fluid, whole lung digests and alveolar macrophages of chronically alcohol exposed rats. Additionally, we showed that uptake of saturated fatty acids alone was capable of inducing phenotypic and functional changes in alveolar macrophages that mimicked those in the alcohol-exposed rat and human lung. Herein, we discuss the role of GM-CSF in surfactant homeostasis and highlight the evidence that links decreased GM-CSF signaling to alveolar macrophage dysfunction in both the PAP and alcohol-exposed lung. Moreover, we propose a mechanism by which lipid accumulation itself can contribute to altering alveolar macrophage behaviors and propose how targeting this mechanism could potentially reduce the susceptibility to pulmonary infections in alcoholics.
Keywords: Alcohol use disorder, acute respiratory distress syndrome, pneumonia, metabolism, GM-CSF, pulmonary surfactant
Alveolar macrophages and their unique microenvironment:
Alveolar macrophages (AMs) are the professional phagocytic cells of the lung but also fulfill various other essential pulmonary functions, including the coordination of innate and adaptive immune responses, and the orchestration of repair and remodeling behaviors [1]. Like other macrophage populations, AMs are driven to perform their varied functions by continuously sensing, adapting and responding to different biological factors in their microenvironment. In most situations, this adaptation is driven by the production of cytokines and chemokines from neighboring immune and parenchymal cells, but other factors can also have a profound influence on AM behaviors, such as elevated levels of reactive oxygen species or marked reductions in local oxygen concentrations [1, 2]. The classic understanding is that these exposures drive AMs to adopt either an M1 pro-inflammatory or an M2 anti-inflammatory phenotype; however, it is now appreciated that AMs can also adopt a wide range of intermediate behaviors, making this M1/M2 paradigm more of a range than a dichotomy [3–5].
AMs reside in a microenvironment distinct from all other macrophage populations. Situated along the distal epithelial surfaces of the lung, these cells are exposed to essentially all of the toxic and non-toxic materials found in ambient air including innumerable organic and inorganic dusts, small and large size particulate matter, a multitude of infectious materials, and high levels of inspired and expired gases. Furthermore, AMs are also exposed to various other substances which originate from within the body like many of the proteins, lipids and metabolites found within the pulmonary circulation as well as the assortment of substances produced locally in the lung as part of pulmonary surfactant.
Pulmonary surfactant is a lipid-rich material produced by type II cells of the distal pulmonary epithelium and is best known for its role in reducing surface tension and preventing airspace collapse. However, substances contained within this material are also responsible for regulating pulmonary innate immunity and, in particular, for modulating AM behaviors [6–8]. By far, the most abundant substance in pulmonary surfactant is phospholipids, particularly phosphatidylcholine (PC), but other lipid species can also be found in smaller concentrations, such as triglycerides, cholesterol and free fatty acids [9]. Moreover, approximately 10% of surfactant mass is comprised of proteins, including the highly abundant surfactant proteins (SP)-A, B, C and D. Of these, it is the hydrophilic proteins SP-A and SP-D that have been shown to contribute most significantly to modulating AM behaviors [7, 8].
Although the majority of pulmonary surfactant is comprised of lipids, surprisingly little is known of their role in influencing AM behaviors. This is particularly remarkable when one considers the vast array of different lipid species present in surfactant and the significant numbers of modifications these lipids undergo in response to different enzymatic and non-enzymatic exposures. Perhaps even more remarkable is the fact that AMs are, at times, exposed to dramatically different levels of extracellular lipids, such as in the acute respiratory distress syndrome, where lipid levels are often low, or in the condition known as pulmonary alveolar proteinosis, where lipid levels are often markedly elevated. Moreover, lipids are also known to accumulate within AMs under numerous pathological states, including various fibrotic lung conditions, aspiration pneumonitis, amiodarone-induced toxicity and pulmonary alveolar proteinosis, to name a few [10–12]. Thus, the notion that quantitative or qualitative changes in surfactant lipids might have an influence on AM behaviors seems like a reasonable assumption given the ability of AMs to respond to so many other factors within their microenvironment.
GM-CSF in the regulation of surfactant homeostasis:
It has long been recognized that lipid-laden macrophages accumulate in the lung under various pathological states but the idea that AMs actually participate in homeostatic clearance of surfactant lipids is a concept that is relatively new to the pulmonary field. In fact, the first suggestion that AMs contribute to surfactant homeostasis dates back to only several decades ago when a surprising discovery was made in the lungs of mice that had targeted deletion of the gene encoding the cytokine granulocyte-monocyte colony stimulating factor (GM-CSF). In these mice, it was discovered that massive amounts of lipids, in particular phospholipids, accumulated in the distal pulmonary airspaces and in AMs, independent of any other lung provocation [13, 14]. Notably, this phenotype was highly unusual at the time because the only known function for GM-CSF was to promote the growth and differentiation of granulocytes and monocytes from bone marrow progenitors. However, GM-CSF KO mice did not display any hematopoietic abnormalities, other than mildly reduced blood eosinophil levels, prompting researchers to begin exploring the role of GM-CSF in surfactant homeostasis [13, 14].
After three decades of research, much is now known about the role of GM-CSF in surfactant homeostasis. For one, it has been established that GM-CSF deficiency has little to no effect on either the production or secretion of surfactant lipids or proteins from alveolar epithelial type II cells but has a markedly suppressive effect on the ability of AMs to clear surfactant materials (proteins and lipids) from the distal pulmonary airspaces [11, 15–17]. Further, it has been shown that this decrease in surfactant clearance by AMs is due, in large part, to the disruption of various downstream signaling events, in particular those mediated by the ETS transcription factor PU.1 [18]. In lymphoid tissues, PU.1 is known for its ability to regulate various genes involved in early and late stages of myeloid and lymphoid differentiation; however, in the context of lipid homeostasis, PU.1 controls the expression of several key lipid transporter genes, including the ATP-binding cassette (ABC) transports ABCA1 and ABCG1 [19–21]. In the cardiovascular field, these transporters have been studied extensively, playing a critical role in facilitating the export of cholesterol and other lipids from macrophages in atherosclerosis-prone tissues. Relevant to the lung, work by Thomassen et. al. showed that AMs isolated from GM-CSF deficient mice expressed lower levels of ABCG1 when compared to controls [20, 21]. Consistent with the notion that a decrease in the level of ABCG1 impairs surfactant clearance, targeted deletion of the ABCG1 gene in mice results in the accumulation of modest numbers of lipid-laden AMs, mimicking a mild form of the GM-CSF KO pulmonary phenotype [22]. Along with PU.1, another important gene regulated by GM-CSF is peroxisome proliferated-activated receptor γ (PPAR-γ), which is known to modulate the expression of multiple lipid transport proteins in cardiovascular tissues [19, 23, 24]. Like PU.1, levels of PPAR-γ are significantly reduced in AMs of GM-CSF KO mice. Moreover, genetic or pharmacological approaches overexpressing either PPAR-γ or PU.1 not only restore the expression of lipid transporters in the lung but also reduce the severity of lipid accumulation in AMs from mice with GM-CSF deficiency [24].
Pulmonary Alveolar Proteinosis and defective GM-CSF signaling.
While mouse models almost never fully recapitulate the features of any human disease, the pulmonary phenotype in GM-CSF knockout mice exhibited striking similarities to a rare lung disease called pulmonary alveolar proteinosis (PAP). PAP is condition that affects men, women and children of all ages, and occurs in all races and ethnic groups. Similar to the lungs of GM-CSF deficient mice, a hallmark feature of the PAP lung is the build-up of surfactant phospholipids and proteins in alveoli and alveolar macrophages [12]. Although the clinical course of PAP varies among different patient, the vast majority of individuals will develop some degree of respiratory insufficiency from surfactant accumulation. Moreover, patients with PAP are more prone to developing pulmonary infections, including those caused by common bacterial pathogens as well as atypical pathogens like Norcardia asteroids [11, 12], and this enhanced to infection is mostly linked to the immune suppressive effects that GM-CSF deficiency has on AM function [11].
Given the striking similarities between the lungs of GM-CSF deficient mice and patients with PAP, researchers soon began investigating the role of GM-CSF deficiency in the pathobiology of PAP. An important early clue suggesting that defective GM-CSF signaling might, in fact, contribute to the onset of disease came from a single case report showing that treatment with GM-CSF effectively ameliorated radiographic and physiological abnormalities of disease [25]. Ultimately, this case report served as the impetus for further investigations, and soon led to the discovery that most forms of acquired PAP resulted, not from a reduction in GM-CSF production, but rather from the synthesis of auto-antibodies against the GM-CSF cytokine [26–28]. As such, functional deficiency in GM-CSF due to autoantibodies is now believed to play a central role in the pathobiology of acquired forms of PAP. Although whole lung lavage remains the cornerstone to the treatment of all forms of PAP, GM-CSF replacement therapy and drugs that suppress antibody production (e.g. rituximab) have also demonstrated varying degrees of success in treatment of this condition [12, 29, 30].
Abnormal GM-CSF signaling in the alcohol exposed lung.
AMs in patients with PAP and chronic alcohol use disorder exhibit nearly identical functional deficits in phagocytosis, cytokine production, cellular adhesion and superoxide production [15, 24, 31–36]. While it seems unlikely that alcohol disrupts AM function through only one specific mechanism, there is now mounting evidence that decreased GM-CSF signaling plays an important contributory role in mediating lung immune dysfunction [37–40]. While GM-CSF levels in the lung or blood of alcoholics are not significantly different from that of controls, Joshi et al [39] showed that GMCSF receptor levels are markedly reduced in not just AMs but also in the alveolar epithelium after chronic alcohol exposure (Figure 1). Moreover, it has been determined that this decrease in GM-CSF receptor levels is mediated, at least in part, by a downregulation in the activity of PU.1 [38, 41]. Consistent with this mechanism, Joshi et al showed that that treatment with GM-CSF not only can enhance PU.1 activity in alcohol-exposed AMs, but also can restore many, if not all, essential immunological functions, including agonist-induced cytokine production and phagocytic activities. Although the mechanisms leading to reduced PU.1 activity in the alcoholic lung is mostly linked to a decrease in GM-CSF signaling, Mehta et al showed that this may also be related to a reduction in antioxidant defenses [42]. Chronic alcohol exposure reduces Nrf2 levels in the lung, which is the major transcription that regulates the expression of many, if not most, antioxidant genes in cells. Interestingly, Mehta et al showed that Nrf2 also regulates the expression of PU.1 in the lung, suggesting that disruption of antioxidant responses may be tied to both the reduction in GM-CSF activity and immune defects in the alcoholic lung.
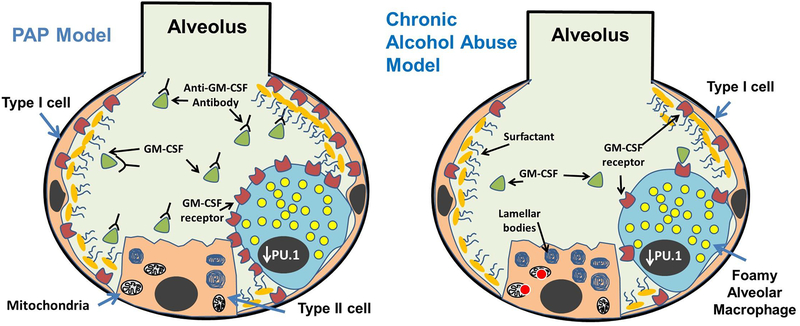
Figure 1:
Comparison of alveolar pathologies between pulmonary alveolar proteinosis (PAP) and chronically ethanol exposed lung. A hallmark of both conditions is the lipid-laden morphology of macrophages caused by the accumulation of lipids (yellow circles) in the cytosol, and the development of immunoregulatory dysfunction. Acquired forms of PAP develop because of autoantibodies preventing GM-CSF (green triangles) from binding to receptors in the plasma membranes of alveolar macrophages and type II alveolar epithelial cells. By contrast, levels of GM-CSF receptors (red color shapes) are reduced in the alveoli in response to chronic alcohol exposure. Lipid synthesis is also increased in alveolar epithelial type II cells in response to alcohol. In both the PAP and chronic alcohol exposed lung, decreased GM-CSF signaling leads to reduced PU.1 levels in the nucleus (charcoal gray oval) of AMs. Increased ROS levels have been observed in the mitochondria of ethanol metabolizing cells, which may also contribute to alterations in lipid homeostasis in the lung after chronic ethanol consumption.
As mentioned above, GM-CSF receptor levels are also decreased in the alveolar epithelium of rats after chronic alcohol exposure, indicating that alcohol can broadly disrupt GM-CSF signaling in the lung [38]. Importantly, this loss of GM-CSF signaling has been shown to contribute to reducing epithelial barrier function in the distal pulmonary epithelium of rats, perhaps explaining why alcoholics are more vulnerable to developing the “leaky” lung condition known as the acute respiratory distress syndrome (ARDS) [38, 39, 43–45]. Thus, based on these and other findings, it is now believed that GM-CSF deficiency plays a key role in inducing pulmonary dysfunction in response to alcohol exposure, and has led some experts in the field to propose GM-CSF replacement therapy as a means for boosting immune function and ameliorating infectious and non-infectious (ARDS) pulmonary complications in alcoholics [46].
Surfactant homeostasis is altered in the chronically alcohol exposed lung.
Despite the fact that chronic alcohol use is known to promote lipid accumulation in the liver, surprisingly little is known about the effects of alcohol on lipid homeostasis in the lung. Nearly four decades ago, Ryan et. al showed that the quantity of phosphatidylcholine, cholesterol, and triglycerides recovered from lung lavage of ethanol-fed rats was nearly two to three times that of controls [47]. Although Holguin et. al showed that surfactant lipids were not increased in response to a shorter course of alcohol exposure, we recently reported a nearly identical 2–3 fold increase in triglyceride and free fatty acid levels in the BAL fluid and whole lung tissues of rats exposed to 3 months of alcohol [35, 43]. Moreover, we found that extracellular lipid accumulation was associated with a dramatic increase in the number of lipid-laden AMs [35]. While the type (triglycerides vs phospholipids) and magnitude (2–3 fold vs 6 fold increase in lipids) of lipid-related changes appears to differ from that of GM-CSF deficient mice, our findings clearly illustrate that alcohol has a disruptive effect on pulmonary lipid homeostasis in the lung.
Although the mechanisms contributing to surfactant lipid accumulation in the alcohol-exposed lung have yet to be uncovered, one obvious explanation could be that surfactant clearance is reduced because of a deficiency in GM-CSF signaling. While we are unaware of any studies testing this hypothesis, Hunt et. al recently reported that fatty acid content in the liver of GMCSF KO mice was also increased by nearly 50% compared to controls [48]. Interestingly, this was associated with the development of mild hepatic steatosis and mild periportal fibrosis, suggesting that defective GM-CSF signaling could theoretically contribute to lipid dysregulation in multiple mouse and human tissues. That said, the mechanisms contributing to lipid accumulation in the livers of GM-CSF deficient mice were not determined in this study and we are unaware of any evidence to suggest that Kupffer cells participate in the export of lipids out of the liver. Furthermore, we found that levels of lipid transporters ABCA1 and ABCG1 were actually increased in rat AMs after chronic alcohol exposure [35], indicating, at the very least, that mechanisms contributing to the formation of lipid-laden AMs in the lungs of alcohol-exposed rats and GM-CSF KO mice do not fully overlap. A diagram illustrating some of the similarities and differences between the ways in which PAP and chronic alcohol consumption affect the alveolar environment is presented in Figure 1.
It also seems possible that factors other than GM-CSF deficiency might contribute to lipid accumulation in the alcohol-exposed lung. For example, it could be that alcohol disrupts pulmonary lipid homeostasis in ways similar to its effects in the liver. In the liver, lipid accumulation (a.k.a. hepatic steatosis), is attributable to a multitude of factors, including an increase in fatty acid production, a decrease in fatty acid breakdown (mitochondrial beta-oxidation), as well as a decrease in the ability of hepatocytes to export fatty acids out of the liver [49]. Consistent with these mechanisms, we recently reported that many key components of the lipid-synthesizing machinery were significantly upregulated in the rat lung after chronic alcohol exposure [35]. Furthermore, we found that alcohol readily induced de novo lipid synthesis in cultured alveolar epithelial type II cells and that this induction could be effectively inhibited by blocking the metabolism of alcohol with a CYP2E1 enzyme inhibitor. Finally, we found that transcript levels for carnitine palmitoyltransferase 1 were downregulated in the rat lung after chronic alcohol exposure, suggesting that increased lipid production and decreased fatty acid metabolism might both be contributing to lipid accumulation in our model system. The current state of research on the effects of ethanol consumption, PAP and GM-CSF deficiency on the alveolus and its components is summarized in Table 1.
Table 1:
| Condition | Effects on AElIs | Effects on Surfactant Homeostasis | Phenotypic Changes in Alveolar Macrophages | Functional Changes of Alveolar Macrophages |
|---|---|---|---|---|
| Chronic Alcohol Use Disorder | ↑Lipid Synthesis35 ↓junctional proteins46,55 ↓GM-CSF receptors |
↑Total and free cholesterol, ↑cholesteryl ester in lavage, PL, PC, and PG47 |
↑ M2 phenotype5,35,37 ↑ “foamy” cytopathology35 |
↓Phagocytosis34,37,39,45 ↓Chemotaxis and Cytokine production34,37,39,45 ↓ GM-CSF receptor, |
| Pulmonary Alveolar Proteinosis (PAP) | Normal lipid synthesis | ↑Free and esterified cholesterol51 | ↑ “foamy” cytopathology21,51 | ↓Phagocytosis56 ↓Chemotaxis, and bacterial killing33 ↓Cytokine production ↓Surfactant clearance ↓ GM-CSF signaling |
| GM-CSF KO mice | Normal lipid synthesis | ↑Surfactant proteins in alveolar space15,53
↑Sat PC57,58 |
↑ Mixed M1/M2 phenotype ↑ “foamy” cytopathology21 |
↓Phagocytosis17,41,59 ↓Bacterial Killing41,59 ↓Cytokine production17,18 ↓Surfactant clearance14,18,39 ↓ GM-CSF signaling |
Can lipid accumulation drive changes in AM behavior?
Because GM-CSF signaling is reduced in the chronically alcohol exposed lung, it has been assumed that phenotypic and functional changes within AMs are most likely explained by a decrease in monocyte maturation. However, this mechanism does not easily explain why AMs become polarized to an M2 phenotype in response to alcohol exposure nor does it explain why similar phenotypic and functional changes are not observed in macrophages from other tissues. This begs the question as to whether changes in AMs might be driven by indirect effects of alcohol on the local alveolar microenvironment, namely its effects on surfactant lipid homeostasis. In recent work, we tested this hypothesis by exposing AMs to different physiological concentrations of the saturated fatty acid palmitate and examining the effects on AM behaviors. Not surprising, we found that AMs exposed to elevated levels of palmitate readily adopted a lipid-laden appearance [35]. Moreover, we found that these cells polarize to an M2-like phenotype and exhibit functional changes similar to those in the alcohol exposed lung. This included a decrease in the ability to phagocytose heat-killed bacteria and a decrease in the induction of LPS-induced pro-inflammatory responses [35]. Importantly, these findings are consistent with emerging evidence in the pulmonary fibrosis field in which direct instillation of lipids into the airway has been shown to alter the behavior of AMs and promote pro-fibrotic responses. Interestingly, in this study, oxidized phospholipids rather than saturated fatty acids were instilled into the airway of mice, suggesting that different lipid species may be capable of triggering similar responses in immune cells.
To date, the mechanisms by which lipids induce changes in AMs remain to be determined; however, studies in the field of immunometabolism may provide some important clues. For example, studies have shown that the behavior of macrophages from different tissues can be readily influenced by either altering their exposure to different metabolic substrates or by directing cells to utilize different metabolic pathways [50]. In macrophage populations outside the lung, it has been shown that cells often rely on glycolysis for driving M1 behaviors whereas M2 responses are often dependent on the use of mitochondrial respiration [50, 51]. Furthermore, M2 macrophages appear to preferentially utilize fatty acids as a substrate, which requires the use of mitochondria for β-oxidation Interestingly, recent work has demonstrated that GM-CSF itself can direct cells to utilize glycolysis, suggesting that M2 polarization in the setting of chronic alcohol exposure could be a direct consequence to the loss of GM-CSF signaling [51]. Alternatively, it is possible that M2 polarization is just a byproduct of intracellular lipid accumulation and increased mitochondrial fatty acid β-oxidation. Importantly, because chronic ethanol exposure is known to adversely affect mitochondrial function in various tissues, it remains unclear how these effects might also impact cellular metabolism and, in turn, AM behaviors [52].
Novel therapeutic strategies to restore AM function in the alcoholic lung by targeting lipid homeostasis.
Clearly, the best approach to minimizing the devastating effects of chronic alcohol abuse on lung immune homeostasis would be to refrain from drinking. However, we know from many years of evaluating human behavior that this approach alone will probably never ever be fully effective. Thus, the goal of developing therapies to treat alcoholism would be to minimize organ complications. In the lung, we envision these approaches being applied to alcoholic patients at high risk for developing pulmonary complications, such as after a witnessed aspiration event, or during the early stages of pulmonary infections.
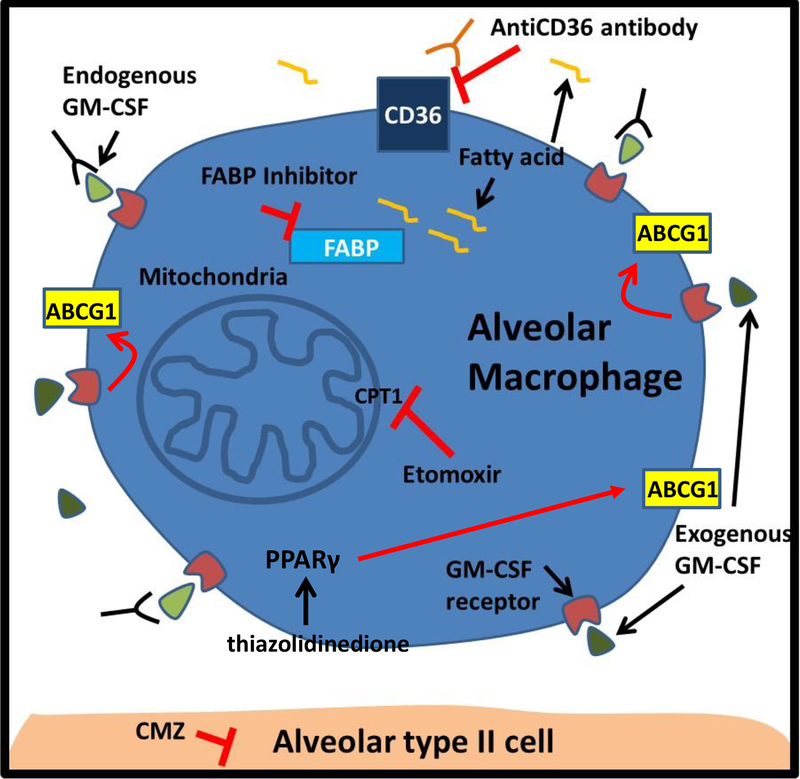
Borrowing concepts utilized for the treatment of PAP, one logical approach would be to restore immune function by administering GM-CSF (Figure 2). This could be done by intravenously administrating recombinant GM-CSF, which is a strategy already employed for boosting blood cell recovery in individuals after chemotherapy [25, 53]. Alternatively, GM-CSF could be aerosolized directly into the lungs [30], which would help to minimize side effects of systemic GM-CSF administration. The effectiveness of this method could theoretically be quantified by measuring the amounts of SP-B and sat-PC cleared from the surfactant and counting the number of lipid-laden macrophages in bronchoalveolar lavage fluid samples. Alternatively, GM-CSF signaling could be enhanced by augmenting activity of downstream mediators. For example, forced expression of PPAR-γ in AMs isolated from GM-CSF KO mice has been shown to restore PPAR-γ levels and rescue adhesion, morphology and surfactant catabolism [23, 24, 31]. To this end, one could reason that repurposing PPAR-γ agonists (thiazolidinedione) as short-term therapy could be utilized for augmenting the expression of lipid transporter proteins.
Figure 2:
Potential therapeutic approaches to blocking lipid-laden macrophage formation and restoring alveolar macrophage function in alcoholics. Exogenous GM-CSF replacement therapy (dark green) could be used to enhance GM-CSF signaling and upregulate the expression of lipid transporter proteins. This could also be achieved by augmenting the activity of molecules downstream of the GM-CSF receptor, like PPARγ (thiazolidinedione). Alternatively, the effects of fatty acids (yellow curved line) on macrophage behavior could be attenuated by blocking: 1) the uptake of fatty acids from scavenger receptors (CD36); 2) the binding of fatty acids to fatty acid binding proteins (FABP); and 3) the entry of fatty acids into mitochondria (CPT1 inhibitor etomoxir). Lastly, lipid-laden macrophage formation could be reduced by suppressing lipid synthesis in type II alveolar epithelial cells with drugs that block alcohol metabolism (bottom of figure).
Another theoretical approach for restoring immune function could be to supply alcoholics with nutritional supplements. Alcoholics are known to develop a wide range of nutritional deficiencies, and this is the basis behind administering multivitamins and thiamine to alcoholics in the hospital to prevent Wernicke-Korsakoff syndrome. In addition to vitamin deficiencies, dietary consumption of zinc is also low in many alcoholics and intracellular levels of zinc are indeed reduced in AMs after chronic alcohol exposure [34]. Importantly, zinc is essential for proper functioning of AMs through its ability to enhance the activity of growth factors, cytokines, and enzymes and other co-factors [42, 45]. Moreover, zinc supplementation has also been shown to restore phagocytic activity of AMs in alcohol-fed rats and is associated with both enhanced expression of GM-CSF receptors and increased PU.1 activity. Thus, it seems reasonable that zinc supplementation could be a low-cost and low-risk method for boosting immune function in alcoholics, although the effects of this therapy on lipid homeostasis in the lung has yet to be evaluated.
In addition to these approaches, it is also conceptually interesting to consider ways in which macrophage function could be restored by reducing lipid uptake or inhibiting intracellular lipid sensing by alveolar macrophages (Figure 2). This could be done through either blocking one or more of the scavenger receptors responsible for internalizing lipids or by inhibiting the interaction of lipids with one of the several cytoplasmic fatty acid binding proteins known to be present in alveolar macrophages, such as fatty acid binding proteins (FABP) 4 and 5 [54]. Additionally, it seems possible that macrophage function could be restored by targeting specific metabolic pathways, possibly through transiently blocking fatty acid entry into mitochondria (targeting CPT1). Lastly, one could consider transiently reducing the stimulus for surfactant lipid synthesis by inhibiting alcohol metabolism with a CYP2E1 inhibitor, which is a strategy already shown to be highly effective in blocking lipid production in alveolar epithelial cells in culture [35].
Conclusion and future directions:
Although the mechanisms by which alcohol renders the lung more susceptible to infection remains poorly understood, recent advances in the field suggest that defective GM-CSF signaling plays an important role. Because GM-CSF signaling is important for regulating surfactant homeostasis, it seems reasonable to assume that surfactant lipid levels may also be altered in the microenvironment of the distal lung of humans that chronically consume alcohol. In addition, it seems equally plausible that these extracellular changes in lipid concentrations could contribute to altering AM behaviors. In this context, therapeutic interventions which restore GM-CSF signaling in the lung or normalize surfactant lipid levels may help to limit the risk of developing pulmonary infections and their associated consequences (e.g. acute lung injury) in individuals with alcohol use disorder. However, additional preclinical studies and observational studies in humans will be needed before these types of therapeutic interventions can be applied in humans.
Highlights:
This review highlights the importance of the cytokine GM-CSF is surfactant lipid regulation and discusses parallel pathways in the lungs of patients’ with pulmonary alveolar proteinosis and alcohol use disorder.
This article introduces the novel concept that alterations in lung lipid homeostasis contribute to the development of macrophage dysfunction in the alcoholic lung.
This article proposes novel treatment approaches for alcohol-related lung diseases that target the machinery regulating pulmonary surfactant homeostasis.
Funding:
This works was funded by NIH AA023571 (RS)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Hussell T and Bell TJ, Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol, 2014. 14(2): p. 81–93. [DOI] [PubMed] [Google Scholar]
- 2.Lohmann-Matthes ML, Steinmuller C, and Franke-Ullmann G, Pulmonary macrophages. Eur Respir J, 1994. 7(9): p. 1678–89. [PubMed] [Google Scholar]
- 3.Murray PJ, Macrophage Polarization. Annu Rev Physiol, 2017. 79: p. 541–566. [DOI] [PubMed] [Google Scholar]
- 4.Sica A, et al. , Macrophage polarization in pathology. Cell Mol Life Sci, 2015. 72(21): p. 4111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thevenot P, et al. , Chronic alcohol induces M2 polarization enhancing pulmonary disease caused by exposure to particulate air pollution. Alcohol Clin Exp Res, 2013. 37(11): p. 1910–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fessler MB and Summer RS, Surfactant Lipids at the Host-Environment Interface. Metabolic Sensors, Suppressors, and Effectors of Inflammatory Lung Disease. Am J Respir Cell Mol Biol, 2016. 54(5): p. 624–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardai SJ, et al. , By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell, 2003. 115(1): p. 13–23. [DOI] [PubMed] [Google Scholar]
- 8.Lawson PR and Reid KB, The roles of surfactant proteins A and D in innate immunity. Immunol Rev, 2000. 173: p. 66–78. [DOI] [PubMed] [Google Scholar]
- 9.Olmeda B, Martinez-Calle M, and Perez-Gil J, Pulmonary surfactant metabolism in the alveolar airspace: Biogenesis, extracellular conversions, recycling. Ann Anat, 2017. 209: p. 78–92. [DOI] [PubMed] [Google Scholar]
- 10.Romero F, et al. , A pneumocyte-macrophage paracrine lipid axis drives the lung toward fibrosis. Am J Respir Cell Mol Biol, 2015. 53(1): p. 74–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trapnell BC, et al. , Pulmonary alveolar proteinosis, a primary immunodeficiency of impaired GM-CSF stimulation of macrophages. Curr Opin Immunol, 2009. 21(5): p. 514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trapnell BC, Whitsett JA, and Nakata K, Pulmonary alveolar proteinosis. N Engl J Med, 2003. 349(26): p. 2527–39. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida M, et al. , GM-CSF regulates protein and lipid catabolism by alveolar macrophages. Am J Physiol Lung Cell Mol Physiol, 2001. 280(3): p. L379–86. [DOI] [PubMed] [Google Scholar]
- 14.Ikegami M, et al. , Surfactant metabolism in transgenic mice after granulocyte macrophage-colony stimulating factor ablation. Am J Physiol, 1996. 270(4 Pt 1): p. L650–8. [DOI] [PubMed] [Google Scholar]
- 15.Dalrymple H, et al. , Alveolar macrophages of GM-CSF knockout mice exhibit mixed M1 and M2 phenotypes. BMC Immunol, 2013. 14: p. 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enzler T, et al. , Functional deficiencies of granulocyte-macrophage colony stimulating factor and interleukin-3 contribute to insulitis and destruction of beta cells. Blood, 2007. 110(3): p. 954–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paine R 3rd, et al. , Impaired functional activity of alveolar macrophages from GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol, 2001. 281(5): p. L1210–8. [DOI] [PubMed] [Google Scholar]
- 18.Berclaz PY, et al. , GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS. Am J Respir Cell Mol Biol, 2007. 36(1): p. 114–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker AD, et al. , PPARgamma regulates the expression of cholesterol metabolism genes in alveolar macrophages. Biochem Biophys Res Commun, 2010. 393(4): p. 682–7. [DOI] [PubMed] [Google Scholar]
- 20.Malur A, et al. , Lentivirus-ABCG1 instillation reduces lipid accumulation and improves lung compliance in GM-CSF knock-out mice. Biochem Biophys Res Commun, 2011. 415(2): p. 288–93. [DOI] [PubMed] [Google Scholar]
- 21.Thomassen MJ, et al. , ABCG1 is deficient in alveolar macrophages of GM-CSF knockout mice and patients with pulmonary alveolar proteinosis. J Lipid Res, 2007. 48(12): p. 2762–8. [DOI] [PubMed] [Google Scholar]
- 22.Bates SR, et al. , Pulmonary abnormalities due to ABCA1 deficiency in mice. Am J Physiol Lung Cell Mol Physiol, 2005. 289(6): p. L980–9. [DOI] [PubMed] [Google Scholar]
- 23.Baker AD, et al. , Targeted PPAR{gamma} deficiency in alveolar macrophages disrupts surfactant catabolism. J Lipid Res, 2010. 51(6): p. 1325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malur A, et al. , Restoration of PPARgamma reverses lipid accumulation in alveolar macrophages of GM-CSF knockout mice. Am J Physiol Lung Cell Mol Physiol, 2011. 300(1): p. L73–80. [DOI] [PubMed] [Google Scholar]
- 25.Kavuru MS, et al. , Exogenous granulocyte-macrophage colony-stimulating factor administration for pulmonary alveolar proteinosis. Am J Respir Crit Care Med, 2000. 161(4 Pt 1): p. 1143–8. [DOI] [PubMed] [Google Scholar]
- 26.Sakagami T, et al. , Patient-derived granulocyte/macrophage colony-stimulating factor autoantibodies reproduce pulmonary alveolar proteinosis in nonhuman primates. Am J Respir Crit Care Med, 2010. 182(1): p. 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakagami T, et al. , Human GM-CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med, 2009. 361(27): p. 2679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uchida K, et al. , GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis. N Engl J Med, 2007. 356(6): p. 567–79. [DOI] [PubMed] [Google Scholar]
- 29.Malur A, et al. , Rituximab therapy in pulmonary alveolar proteinosis improves alveolar macrophage lipid homeostasis. Respir Res, 2012. 13: p. 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reed JA, et al. , Aerosolized GM-CSF ameliorates pulmonary alveolar proteinosis in GM-CSF-deficient mice. Am J Physiol, 1999. 276(4 Pt 1): p. L556–63. [DOI] [PubMed] [Google Scholar]
- 31.Bonfield TL, et al. , Peroxisome proliferator-activated receptor-gamma is deficient in alveolar macrophages from patients with alveolar proteinosis. Am J Respir Cell Mol Biol, 2003. 29(6): p. 677–82. [DOI] [PubMed] [Google Scholar]
- 32.Charbeneau RP, et al. , Impaired synthesis of prostaglandin E2 by lung fibroblasts and alveolar epithelial cells from GM-CSF−/− mice: implications for fibroproliferation. Am J Physiol Lung Cell Mol Physiol, 2003. 284(6): p. L1103–11. [DOI] [PubMed] [Google Scholar]
- 33.Golde DW, et al. , Defective lung macrophages in pulmonary alveolar proteinosis. Ann Intern Med, 1976. 85(3): p. 304–9. [DOI] [PubMed] [Google Scholar]
- 34.Mehta AJ, et al. , Alcoholism causes alveolar macrophage zinc deficiency and immune dysfunction. Am J Respir Crit Care Med, 2013. 188(6): p. 716–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Romero F, et al. , Chronic alcohol ingestion in rats alters lung metabolism, promotes lipid accumulation, and impairs alveolar macrophage functions. Am J Respir Cell Mol Biol, 2014. 51(6): p. 840–9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Standiford TJ and Danforth JM, Ethanol feeding inhibits proinflammatory cytokine expression from murine alveolar macrophages ex vivo. Alcohol Clin Exp Res, 1997. 21(7): p. 1212–7. [PubMed] [Google Scholar]
- 37.Brown SD and Brown LA, Ethanol (EtOH)-induced TGF-beta1 and reactive oxygen species production are necessary for EtOH-induced alveolar macrophage dysfunction and induction of alternative activation. Alcohol Clin Exp Res, 2012. 36(11): p. 1952–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi PC, et al. , GM-CSF receptor expression and signaling is decreased in lungs of ethanol-fed rats. Am J Physiol Lung Cell Mol Physiol, 2006. 291(6): p. L1150–8. [DOI] [PubMed] [Google Scholar]
- 39.Joshi PC, et al. , Chronic ethanol ingestion in rats decreases granulocyte-macrophage colony-stimulating factor receptor expression and downstream signaling in the alveolar macrophage. J Immunol, 2005. 175(10): p. 6837–45. [DOI] [PubMed] [Google Scholar]
- 40.Liang Y, et al. , Alcohol induces mitochondrial redox imbalance in alveolar macrophages. Free Radic Biol Med, 2013. 65: p. 1427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shibata Y, et al. , GM-CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity, 2001. 15(4): p. 557–67. [DOI] [PubMed] [Google Scholar]
- 42.Mehta AJ, et al. , Zinc supplementation restores PU.1 and Nrf2 nuclear binding in alveolar macrophages and improves redox balance and bacterial clearance in the lungs of alcohol-fed rats. Alcohol Clin Exp Res, 2011. 35(8): p. 1519–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holguin F, et al. , Chronic ethanol ingestion impairs alveolar type II cell glutathione homeostasis and function and predisposes to endotoxin-mediated acute edematous lung injury in rats. J Clin Invest, 1998. 101(4): p. 761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moss M, et al. , The effect of chronic alcohol abuse on the incidence of ARDS and the severity of the multiple organ dysfunction syndrome in adults with septic shock: an interim and multivariate analysis. Chest, 1999. 116(1 Suppl): p. 97S–98S. [PubMed] [Google Scholar]
- 45.Joshi PC, et al. , Zinc deficiency mediates alcohol-induced alveolar epithelial and macrophage dysfunction in rats. Am J Respir Cell Mol Biol, 2009. 41(2): p. 207–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pelaez A, et al. , Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol, 2004. 286(1): p. L106–11. [DOI] [PubMed] [Google Scholar]
- 47.Liau DF, et al. , Alcohol-induced lipid change in th lung. J Lipid Res, 1981. 22(4): p. 680–6. [PubMed] [Google Scholar]
- 48.Hunt AN, et al. , Hepatic Steatosis Accompanies Pulmonary Alveolar Proteinosis. Am J Respir Cell Mol Biol, 2017. 57(4): p. 448–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson N and Borlak J, Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev, 2008. 60(3): p. 311–57. [DOI] [PubMed] [Google Scholar]
- 50.O’Neill LA, Kishton RJ, and Rathmell J, A guide to immunometabolism for immunologists. Nat Rev Immunol, 2016. 16(9): p. 553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Na YR, et al. , GM-CSF Induces Inflammatory Macrophages by Regulating Glycolysis and Lipid Metabolism. J Immunol, 2016. 197(10): p. 4101–4109. [DOI] [PubMed] [Google Scholar]
- 52.Hoek JB, Cahill A, and Pastorino JG, Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology, 2002. 122(7): p. 2049–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huffman JA, et al. , Pulmonary epithelial cell expression of GM-CSF corrects the alveolar proteinosis in GM-CSF-deficient mice. J Clin Invest, 1996. 97(3): p. 649–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Corsico B, et al. , Fatty acid transfer from intestinal fatty acid binding protein to membranes: electrostatic and hydrophobic interactions. J Lipid Res, 2005. 46(8): p. 1765–72. [DOI] [PubMed] [Google Scholar]
- 55.Curry-McCoy TV, et al. , Alcohol ingestion disrupts alveolar epithelial barrier function by activation of macrophage-derived transforming growth factor beta 1. Respir Res, 2013. 14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez-Rothi RJ and Harris JO, Pulmonary alveolar proteinosis: Further evaluation of abnormal alveolar macrophages. Chest, 1986. 90(5): p.656–661. [DOI] [PubMed] [Google Scholar]
- 57.Reed JA, et al. , Distinct changes in pulmonary surfactant homeostasis in common β-chain- and GM-CSF-deficient mice. Am J Physiol Lung Cell Mol Physiol, 2000. 278(6): L1164–1171. [DOI] [PubMed] [Google Scholar]
- 58.Ikegami M, et al. , SP-D and GM-CSF regulate surfactant homeostasis via distinct mechanisms. Am J Physiol Lung Cell Mol Physiol 2001. 281(3): L673–703. [DOI] [PubMed] [Google Scholar]
- 59.LeVine AM, et al. , GM-CSF-deficient mice are susceptible to pulmonary group B streptococcal infection. J Clin Inv 1999. 103(4): p. 563–569. [DOI] [PMC free article] [PubMed] [Google Scholar]