Abstract
Marine organisms comprising animals and plants are wealthiest sources of bioactive compounds possessing various pharmacological properties specifically: free radical scavenging, antitumor, antimicrobial, analgesic, neuroprotective and immunomodulatory. Marine drugs provide an alternative source to meet the demand of effective, safe and low-cost drugs that are rising with the continuously growing world population. Cancer is one of the leading reasons of mortality in western nations in contrast to communicable diseases of developing nations. In spite of outstanding developments in cancer therapy in past three decades, there is still an insistent necessity for innovative drugs in the area of cancer biology, especially in the unexplored area of marine anticancer compounds. However, recent technological innovations in structure revelation, synthetic creation of new compounds and biological assays have made possible the isolation and clinical assessment of innumerable unique anticancer compounds from marine environment. This review provides an insight into the anticancer research so far conducted in the area of the marine natural products/synthetic derivatives, their possible molecular targets and the current challenges in the drug development.
Graphical abstract
Keywords: Marine bioactive compounds, Antiproliferation, Cell death, Cytotoxic, Apoptosis, Autophagy
Introduction
Marine environment is considered as a foremost reservoir of the millions of bioactive compounds having possibility to be applied in different disciplines like medicine, technology and food. During past 50 years, key compounds substantiating their potential for industrial development as functional foods, nutritional supplements, pharmaceuticals, enzymes and therapeutic agents have been provided by marine sources [1, 2]. A wide range of bioactive compounds of plant origin are presently in clinical/preclinical trials or in the advanced stages of exploration. Though marine compounds are under-explored at present but predictably in the future era, marine milieu would be the treasured reserve for the future unique compounds as it embodies 90% of the biosphere [3, 4].
Interestingly, reports have shown that natural products sequestered from marine invertebrates are often of bacterial or cyanobacterial derivation. Nevertheless, these microbes, usually cannot be cultivated alone as their symbiotic association with higher organisms is imperious for their growth. Many of the genes responsible for the biosynthetic ability remain unexpressed in vitro, if they are cultivated under axenic conditions [5]. Invertebrates covers majority of the animals living in the marine milieu in terms of variety and dispersal as well. This makes obvious reason for focusing utmost of the scientific studies related to marine drug invention on these organisms. In retort to the harsh, severe and fluctuating habitats of ocean these organisms generate a broad range of unique secondary metabolites that are not present in terrestrial ones. Compiled data from scientific studies [6] have showed that approximately 80% of the existing Marine Natural Products (MNPs) were belonged to the phyla Porifera and Cnidaria with 47.1% and 33.5% respectively. The remaining of them were belonged to the phyla Echinodermata, Chordata, and Mollusca with 7.4%, 6.0% and 5.0% correspondingly (Fig. 1). Marine environment also represents a huge ecological reserve encompassing a range of aquatic plants and animals that are endowed with vivid antimicrobial, immunomodulatory, anti-inflammatory, anticancer, neuroprotective, analgesic, and antimalarial activities [7].
Fig. 1.
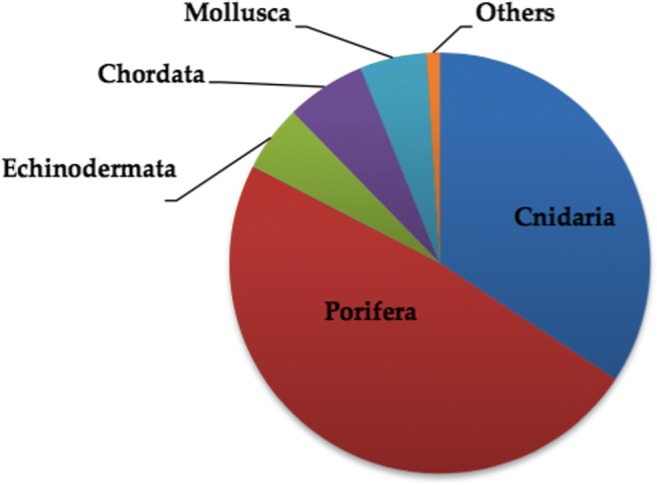
Representation of the different phyla according to the % of the existing Marine Natural Products (MNP) as per Leal et al. [6]
Cancer comprise the group of diseases in which some of the body’s cells begin to divide continuously and have potential to invade or migrate to other body parts. The last two decades have seen outstanding progress in cancer research. However, despite striking innovations in understanding the depth of the cancer & its prevention and treatment, the disease continues to affect millions of people worldwide dreadfully. Numerous factors are associated with the disease that makes its therapy as one of the challenging tasks in medical science. Moreover, another face of the cancer’s complexity is compounded with financial, policy and regulatory blockades that has slowed the rate of development against cancer. Despite many breakthroughs, the treatment for most cancers is still a long way from reality.
Marine milieu encompasses a treasure of strong biological composites for e.g. alkaloids, steroids, terpenes, peptides, and polyketides [8]. Remarkably, marine-derived compounds and oncotherapy are interrelated since very beginning as evident by one of the first marine-derived clinically used drug, Spongothymidine discovered in 1945 [9–11]. To date, there are variety of marine based compounds in distinctive clinical studies phases and number of constituents already has been scanned for laboratory and animal studies. Nevertheless, evolvement of marine derivatives as therapeutic agents is still in its emergent phase. A primary obstruction is the uninterrupted and continuous supply of the profuse quantity of required creatures and their derived compounds without disturbing the marine milieu at the same time. Moreover, unlike earthly habitations there is lack of written medical scripts of marine-derived compounds. Since last few decades, noteworthy efforts have been made to sequester and categorize novel marine-derived products. If we could be able to resolve these obstacles in a financial and an ecologically safe manner, then only marine drugs development can rise.
This publication is envisioned to showcase the details of several marine natural compounds, approved drugs, derivatives and some other products of medicinal value which are advancing through anticancer clinical trials with the details of their molecular targets and current challenges of the drug development process so far.
Marine milieu: At a glance
The Ocean encompasses one of the wealthiest ecosystems on earth that is a natural habitat for an extensive diversity of living organisms. It is imperative to note that surviving circumstances in the oceans vary drastically from terrestrial ones. Moreover, the biodiversity in the oceans vary in different zones and places. Factors that accounts for this variation includes huge ranges in temperature (from ice sea to deep hydrothermal systems), pressure, light and nutritional conditions. Such taxonomic diversity also makes the basis of chemical diversity, a factor imperative for new drug development. Interestingly out of 36 animal phyla reported, a total of 32 phyla are represented in the marine environment out of which 15 varieties are absolutely present in the marine environment [7].
Marine environment comprises of vast range of fauna (fishes, sponges, tunicates, soft corals, nudibranchs, sea hares, sea slugs, mollusks, echinoderms, bryozoans, prawns and shells) and flora (various types of microorganisms including bacteria, cyano- & actinobacteria, fungi, micro/macroalgae, halophytes [salt-resistant or salt-tolerant plants] and mangroves [tropical coastal vegetation]) [12]. One striking characteristic of marine life is the intimate associations between different groups of organisms so as to adapt themselves to grow in the harsh and challenging ocean conditions that are fundamentally different from those in terrestrial region. One crucial consequence of this adaption results in the production of secondary metabolites that functions in intra- and interspecies signaling, dissuasion of herbivores, competition with neighbors, inhibition of microbial invasion and protection against radiation and predators. Such properties of these chemicals account for their enormous potential to serve as bioactive compounds or future candidate drugs [5].
Marine pharmacology
Marine pharmacology is a relatively new branch of pharmacological sciences that explores and study the substances having active pharmacological properties present in marine species of plants and animals. Marine natural products are usually secondary metabolites having no primary function associated with the development, growth or propagation of a species. A comparative study has already confirmed the superiority of marine based natural products over earthly natural products in relation to chemical distinctiveness [13].
Marine anticancer agents (faunal derivatives)
Cancer is a global deadliest disease whose projected worldwide frequency is about 6 million cases per year. Many innovations have already been made or continued in the development of all already existing measures; nevertheless, some of the problems are unresolved yet for e.g. Heterogeneity of the cancer cell subpopulation, development of resistant phenotypes and its recurrence. These obstacles demand the elaboration of new tactics and molecules for drug discovery. Our interest in marine ecosystems has been accelerated in last two decades although in the chronicle of pharmacology, references to marine-based remedies are rare. Nonetheless, a number of marine compounds with diversified chemical structures has been screened [14] and reported for variety of activities for e.g. antimicrobial, antiparasitic, antitumor and anti-inflammatory [15].
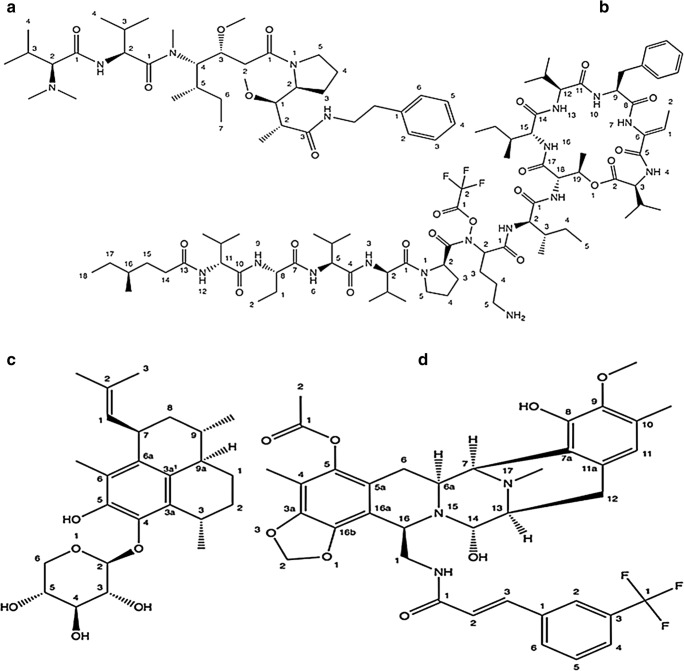
Interestingly, National Cancer Institute had reported in preclinical cytotoxicity testing that the ratio of the terrestrial versus marine samples in exhibiting anti-tumor profile is approximately 1:10 [16]. At present, 08 drugs sequestered from sea milieu have already been accepted for distinctive tenacities (cytarabine, ziconotide, vidarabine, acid ethyl esters, eribulin mesylate, trabectedin, iota-carrageenan and brentuximab vedotin) [17]. Out of these, five have been permitted as pharmaceutical medicines in cancer therapy namely, cytarabine (first marine-derived clinically used drug), trabectedin, eribulin mesylate, ziconotide and brentuximab vedotin [17]. The list of some marine faunal anticancer compounds and derivatives is shown in Table 1.
Approved marine anticancer drugs
Current Anticancer drugs of marine origin approved for human use worldwide are as follows:
Cytarabine
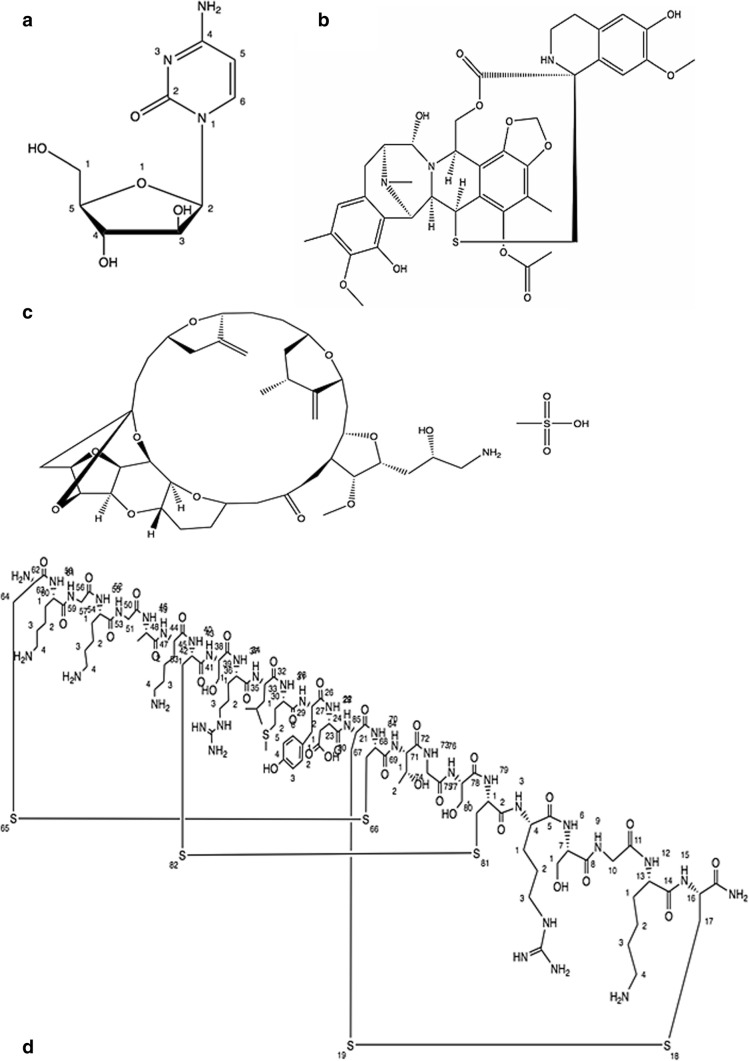
The breakthrough came in the discovery of marine-based cytostatic agents with the unearthing of the nucleosides spongothymidine and spongouridine derived by caribbean sponge Tethya crypta, that were discovered serendipitously. Grounded on this invention, the synthetic referend cytosine arabinoside cytarabine (Fig. 2) has been established as an effective antileukemic agent [18, 19] that was subsequently permitted by Food and drug administration, (FDA) U.S. in 1969. Side effects include gastrointestinal toxicity, diarrhoea, intestinal ulceration, oral mucositis and septicemia. Temperature rise and increase of hepatic enzymes are fairly common, that however do not signify therapeutic troubles [20].
Fig. 2.
Approved marine anticancer drugs. (a) Cytarabine, (b) Trabectedin, (c) Eribulin mesylate and (Dd) Ziconotide
Mechanism of action
Pyrimidine nucleoside is a chemical nature of cytarabine that acts as a prodrug. Inside the cells it is transformed to cytosine arabinoside triphosphate, which damage DNA by competing with the biological substrate deoxycytidine leading to the inhibition of DNA polymerase and eventually synthesis of DNA [4, 5, 18, 19].
Trabectedin
It is isolated from the ascidian Ecteinascidia turbinate an inhabitant of Mediterranean and Caribbean Sea. The animals live on corals and generate the compound as a safeguard against microorganisms. Trabectedin molecule is an alkaloid of tetrahydroisoquinoline class (Fig. 2). In 2007, Trabectedin was the first anticancer molecule of marine source that got approval in European Union (EU) to treat reverted incidents of platinum-sensitive ovarian cancer and sarcoma (soft-tissue) [21]. It was approved by FDA in 2015. This drug has been reported to show hepatoxicity and hematologic side effects in various studies. It was reported that patients with alcohol abuse, underlying liver disease/elevated bilirubin or hematologic abnormalities may not be the best candidates for this drug [22].
Mechanism of action
It is noteworthy to state that Trabectedin is an anti-neoplastic compound that acts on unwanted proliferative cells and also on the tumor micromilieu as well. Cytostatic action of Trabectedin is contributed by its binding ability to the DNA minor groove and consequently preventing transcription by inhibition of the DNA binding of transcription factors. Moreover, it cooperates straight by the RNA polymerase II. It is known to affect the micromilieu of tumor by controlling the quantity of tumor-linked macrophages and thus in turn regulating the production of angiogenic factors and cytokines [4, 5, 21].
Eribulin mesylate
It is a synthetic analogue of marine natural product halichondrin B, extracted from sponges for e.g. Halichondria okadai and Lyssodendoryx sp. It comes under polyketides nontaxane derivatives (Fig. 2). The medication was permitted by the FDA in 2010 and subsequently by the EU in 2011 as an anticancer drug for patients with locally advanced or metastatic breast cancer. Some related side effects of this drug include neutropenia, peripheral neuropathy, mild alopecia, cough and fatigue [23].
Mechanism of action
It acts by influencing the microtubule dynamics. It inhibits the progression phase of microtubules and further segregates the tubulin into nonproductive masses thus results arrest in G2/M phase of the cell cycle and apoptosis [24].
Brentuximab vedotin
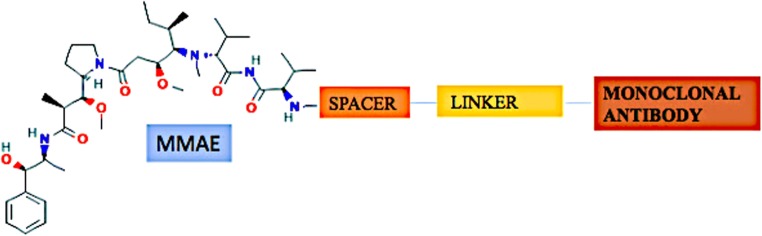
Brentuximab vedotin combines an effective cytotoxic agent together with a tumor targeted specific monoclonal antibody (Fig. 3). FDA U.S. in 2011 gave approval to the Brentuximab vedotin for the management of patients of systemic ALCL (anaplastic large cell lymphoma) and reverted as well as refractory Hodgkin lymphoma. It comprises cytotoxic compound (antimitotic agent) monomethyl auristatin E (MMAE) and an antibody targeted against CD30 (cell-membrane protein) belong to the surface of tumor cells but found rarely on normal cells (Fig. 3). The antibody and cytotoxic components are linked through an enzyme-cleavable peptide-based linker bonds (valine-citrulline dipeptide) [25, 26]. Auristatin, synthetic referend of the marine-based natural invention Dolastatin 10, is a linear depsipeptide. Dolastatins embodies a series of cytotoxic peptides formerly isolated from the Indian Ocean mollusk Dolabella auricularia but in very small quantities. At present dolastatins are obtained by cyanobacteria of the genus Symploca [27]. Side effects reported with this drug are fatigue, low platelet count, diarrhea and hepatotoxicity.
Fig. 3.
Schematic representation of the structure of Monomethyl Auristatin E -Monoclonal Antibody (MMAE-MAB)-conjugate. Basic structure of the MMAE-conjugate is same except the difference of monoclonal antibody which is targeted against specific factor as described below: Tisotumab vedotin = MMAE + Spacer- Linker +Tissue Factor (TF) targeting antibody. Pinatuzumab vedotin = MMAE + Spacer- Linker+ CD22 targeting antibody. Glembatumumab vedotin = MMAE + Spacer- Linker +glycoprotein NMB (gpNMB) targeting antibody. Brentuximab vedotin = MMAE + Spacer- Linker +CD30 targeting antibody
Mechanism of action
Its high toxicity makes it unfeasible for its use alone but by aiding with conjugated antibody it can be focused specifically for tumor cells. Brentuximab vedotin interacts with the extracellular domain of CD30 receptor present on tumor cells that subsequently gets internalized by the clathrin-assisted endocytosis. Ultimately as it comes to lysosome, lysosomal proteases cleave the linker peptide and MMAE gets released into the cytosol [28] where it binds tubulin and prevents polymerization leading to G2/M cell cycle arrest and finally to Apoptosis (programmed cell death). Interestingly, it has been studied that interaction of MMAE into the micromilieu of the CD30 expressing cells can impact CD30 lacking lymphoma cells too [29].
Ziconotide
It was the first drug of marine origin to obtain approval in 2004 from the FDA (U.S.) to ameliorate severe and chronic pain. It was originally extracted from the venom of sea snail named as magical cone (Conus magus). It is the mock derivative of a ω-conotoxin peptide (Fig. 2).
Mechanism of action
Ziconotide exploits a selective N-type voltage-gated Ca2+ (calcium) channel as an inhibitor. Eventually it impedes the release of neurochemicals like glutamate, substance P in the brain and spinal cord and calcitonin gene-related peptide that accounts for pain relief [30]. Ziconotide is administrated intrathecally for management of varied neuropathic/nociceptive pain in cancer and non-cancer patients too [31]. Although it was reported that use of Ziconotide induces some adverse effects like nausea, rise in creatine kinase levels and rhabdomyolysis but it doesn’t work out to make the possibility of refusing the drug as it must be decided on an individual basis [32]. Mild side effects reported are nausea, dizziness, vomiting etc.
Marine anticancer compounds in clinical phase III trial
Plitidepsin
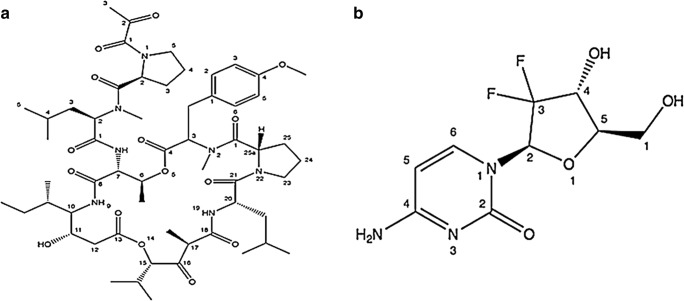
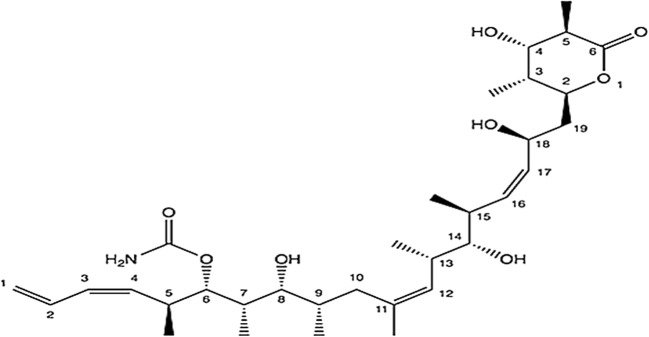
It is a natural marine cyclic depsipeptide (features a peptide chemical structure) but now accessible via chemical synthesis (Fig. 4). It was primarily sequestered from a Mediterranean tunicate Aplidium albicans found in the Mediterranean Sea. For the management of numerous neoplasias comprising breast and lung cancers (non-small-cell), it is currently in phase III stage of clinical studies [17]. It has been reported to elicit apoptosis at low nanomolar (nM) range of IC50 values. Moreover, clinical trial (Phase I and II) studies of plitidepsin showed brilliant antitumor property in patients with multiple myeloma, advanced medullary thyroid carcinoma, non-Hodgkin’s lymphoma, advanced melanoma and urothelium carcinoma [33].
Fig. 4.
Marine anticancer compounds in clinical phase III trial. (a) Plitidepsin and (b) Gemcitabine
Mechanism of action
It provokes cell cycle arrest dose-dependently and leads to apoptosis in cultured cells obtained from solid tumors [16]. These consequences are linked to the provocation of early oxidative stress via stimulation of Rac1 GTPase and the inhibition of protein phosphatases which in concurrence cause the prolonged activation of c-Jun N-terminal kinase (JNK) and p38 MAPK [34].
Gemcitabine (dFdC)
It is an anticancer nucleoside (a fluorinated derivative of cytarabine) metabolic inhibitor. It is a synthetic version of pyrimidine nucleoside analog acting as a prodrug in which the hydrogen atoms on the 2′ carbon of deoxycytidine are substituted by fluorine atoms (Fig. 4). It is currently in phase II & III clinical studies [35, 36] being used for the management of numerous carcinomas of pancreas, bladder, breast and lung cancer (non-small cell) [37]. Gemcitabine is used as a prime treatment for pancreatic cancer alone and also in conjunction with cisplatin for the management of late-stage or metastatic bladder and lung cancer (non-small cell).
Mechanism of action
Once transported into the cell, gemcitabine is transformed into the functional form difluorodeoxycytidine diphosphate (dFdCDP) and subsequently into difluorodeoxycytidine triphosphate (dFdCTP) by deoxycytidine kinase. Both forms restrain the progression of DNA synthesis. dFdCTP contends with deoxycytidine triphosphate (dCTP) and is incorporated into DNA, a most possible mechanism of gemcitabine induced cell death. Consequent to the assimilation of gemcitabine nucleotide on the end of the elongating DNA strand during replication, one more deoxynucleotide is added and afterwards the DNA polymerases becomes incapable to proceed further. This phenomenon apparently screws the drug into DNA because of the fact that proofreading enzymes becomes incapable to remove gemcitabine from its location. Additionally, dFdCDP impedes ribonucleotide reductase, thereby reducing the deoxynucleotide pool accessible for DNA synthesis. The decline in the intracellular concentration of dCTP potentiates the incorporation of dFdCTP into DNA [38]. To sum up, Gemcitabine metabolites serve to augment the overall inhibitory actions on cell growth.
Marine anticancer compounds in clinical phase II trial
Soblidotin
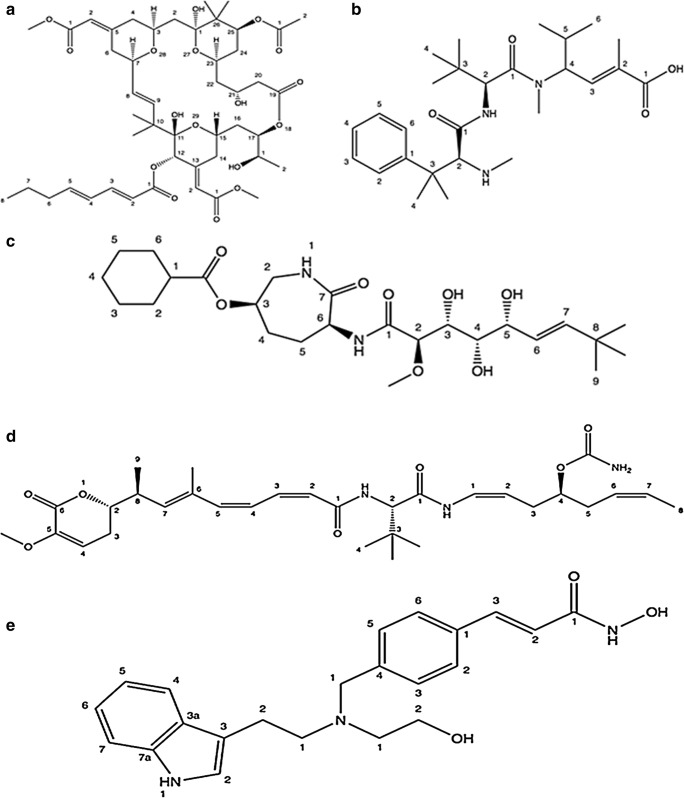
It is isolated from sea hare Dolabella auricularia of the Indian Ocean. It is a synthetic derivative of the dolastatin backbone from dolastatin 10 (Fig. 5). Presently it is undergoing phase II clinical tests.
Fig. 5.
Marine anticancer compounds in phase II clinical trial. (a) Soblidotin (b) Elisidepsin (c) Pseudopterosins and (Dd) PM1004
Mechanism of action
Soblidotin is a vascular disrupting agent that specifically causes collapse to tumor vasculatures along with its tubulin inhibitory activity and cytotoxic effect [39]. It inhibits microtubule polymerization and interferes with microtubule assembly/disassembly equilibria through interaction with tubulin.
Elisidepsin (PM02734)
It is a marine based mock cyclic depsipeptide that belongs to the compounds of Kahalalide family (Fig. 5) [40]. It is isolated by a sacoglossan (sap-sucking sea slugs) slug, Elysia rufescens (herbivorous marine Indopacific mollusk) and belongs to the Kahalalide family (dehydro aminobutyric acid-containing peptides) of compounds. It is undergoing Phase II clinical studies for its antitumor property and promising therapeutic index [21]. It has been reported to have effective in vitro cytotoxicity against diverse human tumor cell lines, which may be because of oncolytic cell death induction instead of apoptosis [21].
Mechanism of action
Although its mechanism of action is still debated, it has been reported that it provokes caspase-free/independent demise of cell linked with characteristics of autophagy (or autophagocytosis) via reticence of the Akt/mTOR based signaling and stimulation of death-associated protein kinase (DAPK, a family of serine/ threonine kinases) [41].
Glembatumumab vedotin
It is one of the fully-human monoclonal antibody-drug conjugate (ADC) obtained from mollusks and cyanobacteria. At present, it is in phase II clinical trial, used for the management of breast cancer and melanoma [42].
Mechanism of action
It is targeted against a glycoprotein NMB (gpNMB) (Fig. 3), that is reported to be overexpressed in various cancers. gpNMB has been shown to be linked with the metastasis. CR011, a gpNMB-targeting antibody is allied to an effective cytotoxic monomethyl auristatin E (MMAE) thus forming an antibody-drug conjugate (ADC). Interestingly, Glembatumumab vedotin is stable in the bloodstream and linker is broken to liberate monomethyl auristatin E once internalize into gpNMB-expressing tumor cells eventually causing cytotoxicity. It is currently in progress for the management of local advanced or metastatic breast cancer [43].
Pseudopterosins
It belongs to diterpene based glycosides (Fig. 5) acquired by the coral (soft coral) Pseudopterogorgia elisabethae. It had been reported to exhibit effective wound-healing and anti-inflammatory property in phase II clinical studies [44].
Mechanism of action
Pseudopterosin A is a strong phorbol myristate acetate inhibitor. It has been reported to cause topical inflammation in a mouse model with degranulation of human polymorphonuclear leukocytes and prevents phagosome creation in tetrahymena cells. In a double-blind Phase II clinical trial pseudopterosins were found to enhance re-epithelialization process in the early wound repair process [21]. Recently it has been shown that Pseudopterosin impedes the NF-κB signaling thus blocks the pro-inflammatory cytokine liberation in breast cancer cells and the corresponding interaction with accompanying immune cells [45].
PM1004
It has been reported as a synthetic analogue of tetrahydroisoquinolone alkaloid (Fig. 5). Structurally it is similar to a natural alkaloid, jorumycin sequestered from the Pacific nudibranch Jorunna funebris [46, 47] and additionally it is also reported to be found in sponges and tunicates [47]. It has been reported as an effective chemotherapeutic compound in the management of solid type human tumors and blood malignancies. At present it is in phase II clinical studies [47, 48].
Mechanism of action
It is a novel alkaloid with DNA-binding capability leading to the cell cycle arrest and impairment of transcription.
Marine anticancer compounds in clinical phase I/II trial
Discodermolide
It is a polyketide (Fig. 6) isolated from the deep-water marine sponge Discodermia dissoluta by Gunasekera and co-workers and currently undergoing phase I/II clinical trials [48]. Discodermolide has been reported as an effective inhibitor of tumor cell growth in several MDR (Multidrug resistant) cancer cell lines and shows immunosuppressive properties both in vitro and in vivo [49, 50].
Fig. 6.
Marine anticancer compound (Discodermolide) in phase I/II clinical trial
Mechanism of action
It acts as a microtubule-targeted drug. It stabilizes microtubules by preventing the depolymerization of microtubules [51]. Consequently, it arrests mitosis because of microtubule bundling and impediment of the separation of sister chromatid pairs thus induces G2-M cell cycle arrest [52].
Marine anticancer compounds in clinical phase I trial
Bryostatin 1
It belongs to macrocyclic lactones (Fig. 7) sequestered from the marine based bryozoan Bugula neritina (Bugulidae). Interestingly, it has been reported to possess both antineoplastic and immunopotentiating properties. Bryostatin has been reported to possess various properties for e.g. T cell activation, immunomodulatory action and activation of haematopoietic progenitor cells.
Fig. 7.
Marine anticancer compounds in phase I clinical trial. (a) Bryostatin, (b) Taltobulin, (c) LAF389, (d) PM060184 and (e) NVP-LAQ824
Mechanism of action
Bryostatin 1 possess high binding affinity to cysteine rich domains of protein kinase C (PKC) results in its triggering and further translocation to the cell membrane. Subsequent to the phosphorylation of specific protein substrates, PKC gets ubiquitinated and finally directed for proteolysis. Bryostatin 1 acts synergistically with other anti-cancer agents by down-regulating PKC thus enhancing their cytotoxicity. Bryostatin 1 has been reported to the stimulate tumor cell differentiation and inducing apoptosis in tumor cell [53, 54]. Moreover, it also shows immunomodulation, cytokine release stimulation and expansion of tumour specific lymphocyte populations [55, 56]. These may be the possible base for both immunostimulating and anticancer properties.
Pinatuzumab vedotin
It is an antibody-drug conjugate, isolated from mollusks and cyanobacteria. It comprises of the potent antimicrotubule agent monomethyl auristatin E (MMAE) conjugated to an antibody (CD22) (Fig. 3). CD22 is a B lymphocyte-specific adhesion molecule of the Ig superfamily found on the surface of most B cells. It regulates signaling related to antigen receptor and regulates B cell function, survival and apoptosis. Pinatuzumab vedotin is targeted to manage malignancies of B cell including many leukemias and lymphomas.
Mechanism of action
Upon binding to CD22 expressing cells, pinatuzumab vedotin is internalized consequently releasing the cytotoxic agent that eventually leads to microtubule disruptions and cell death. Pinatuzumab vedotin has been reported to show inhibitory action against Non-Hodgkin Lymphoma (NHL) and Chronic Lymphocytic Leukemia (CLL) [57]. It is imperative to note that CD22 receptor expression is limited to B-cells and also exist on NHL and CLL.
Tisotumab Vedotin
It is one of the antibody-drug based conjugate (Fig. 3) isolated from mollusks and cyanobacteria. It is directed against Tissue Factor (TF), a protein reportedly involved in tumor signaling and angiogenesis [58, 59]. Tissue Factor (TF) is considered as a potent target for antibody-drug conjugates since it is found to be vastly expressed on many solid types of tumors.
Mechanism of action
Tisotumab vedotin includes an antibody targeting Tissue Factor (TF) conjugated with monomethyl auristatin E (MMAE) via a cleavable linker. TF is aberrantly expressed in a broad type of solid tumors including cervical cancer and is associated with poor prognosis [58, 59]. Upon binding to TF expressing tumor cells, Tisotumab vedotin is internalized after which the cytotoxic agent is released resulting in microtubule disruptions and further cell death. Release of MMAE in tumor microenvironment also induces bystander killing of neighboring cancer cells.
HTI286 (Taltobulin)
It is a synthetic derivative (Fig. 7) belong to a small family of naturally occurring cytotoxic peptides, Hemiasterlins. These were formerly originated from extracts of marine sponges (Cymbastela sp., Hemiasterella minor, Siphonochalina sp., and Auletta sp.). Hemiasterlins are tripeptides containing three sterically congested amino acids [60–62]. Hemiasterlin (E7974) is a compound that is currently in phase I clinical studies. It is highly efficient in inhibiting the growth of paclitaxel and vincristine resistant tumors associated with P-glycoprotein overexpression.
Mechanism of action
Hemiasterlin is an effective cell growth inhibitor that leads to microtubules depolymerisation and cell cycle arrest in the G2-M phase [63].
LAF389
It is a synthetic derivative (Fig. 7) of a class of marine natural products, bengamides. Bengamides are biological products originally sequestered from Jaspidae sponges [64]. Bengamide B is one of the most effective members of the family. LAF389 has antiproliferative, antitumor & antiangiogenetic properties [65].
Mechanism of action
Bengamides acts by inhibiting the action of methionine amino peptidases (MetAps) directly or indirectly [66]. MetAP is involved in the functioning of numerous factors involved in DNA repair mechanism, signal transduction, cell transformation, secretory vesicle trafficking and infection. Its inhibition leads to cell cycle arrest.
PM060184
It comes under the group of tubulin binding agents that was initially sequestered from the Lithoplocamia lithistoides, a marine sponge but at present obtained by synthetic production (Fig. 7). Interestingly, it is under clinical trials in patients with advanced stages of cancers. PM060184 has been documented to have the highest known affinities among tubulin binding agents and targets tubulin dimers at new binding site by reducing microtubule dynamicity [67].
Mechanism of action
It covalently fixes to the residues resides in the minor groove of DNA. Consequently, it results in suspended progression through S phase of the cell-cycle, arrest in the G2/M phase and subsequent apoptosis.
NVP-LAQ824
It is an indolic cinnamyl hydroxamate (Fig. 7 and Table 1) currently in clinical (phase I) studies on the patients having tumors and leukemia. It is a synthetic compound derived from marine natural product Psammaplin A, a symmetrical bromotyrosine disulfide possessing oxime groups. NVP-LAQ824 has been reported to show potent in vitro antitumor activity. It is interesting to mention that Psammaplins have been sequestered from a variety of sponges and the brominated aromatic amino acid byproducts are shared by marine bacteria. This suggests that these metabolites may truly derive from biosynthetic pathways of microorganisms living in association with such sponges.
Table 1.
Some representatives of the marine faunal derived anticancer compounds and derivatives
| Marine Organism | Compound | Activity | Mechanism of action | References |
|---|---|---|---|---|
|
Reniera sarai, renamed Haliclona (Rhizoneira) sarai (Marine sponge) |
3-alkylpyridinium polymers | Cytotoxic against non-small cell lung cancer | Upregulation of pro-apoptotic and down-regulation of anti-apoptotic proteins, mitochondrial depolarization. | [68] |
|
Sinularia flexibilis (Soft coral) |
Sinulariolide | Antiproliferative on A375 melanoma cells | Induce apoptosis by mitochondria-mediated & caspase-dependent pathways. | [69] |
| Pseudoceratina sp. (Marine sponge) | Dibromotyrosine Derivative | Cytotoxic activity against K562 cells. | IKK/NFκB signaling pathway, caspases-3 and − 9 activation, PARP cleavage. | [70] |
|
Dicathais orbita (Marine gastropod) |
tyrindoleninone and 6-bromoisatin | Anticancer on leukemia, lymphoma and colorectal (HCT-116) cell lines. | Cell-cycle arrest and apoptosis inducer. | [71] |
|
Fascaplysinopsis bergquist sp. (Marine sponge) |
Fascaplysin | Anticancer against small cell lung cancer cell lines. | DNA-intercalating capability, cyclin-dependent kinase 4 (CDK4) inhibitory activity. | [72] |
|
Xestospongia sp. (Marine sponge) |
Araguspongine | Cytotoxic | Autophagic cell death, inhibitor to receptor tyrosine kinases, suppression of PI3K/Akt/mTOR signaling. | [73] |
|
Holothurioidea, Echinodermata (Sea cucumber) |
Triterpene glycosides | Antitumor, cytotoxic | Cell-cycle arrest, regulation of extracellular signal-regulated kinase (ERK), Focal adhesion kinase (FAK). | [74] |
|
Sphyrna tiburo (Bonnethead sharks) |
Epigonal conditioned medium (ECM) | Cytotoxic | Caspase activation, PARP cleavage, activation of apoptotic proteins. | [75] |
| Marthasterias glacialis (Star fish) | Ergosta-7,22-dien-3-ol and Palmitic Acid | Cytotoxic | Cell-cycle arrest, apoptosis by C/EBP homologous protein (CHOP)-mediated pathway of ER-stress. | [76] |
| Smenospongia aurea | Smenamides A and B | Pro-apoptotic, cytotoxic | Cytotoxic activity at nanomolar quantities on lung cancer (Calu-1) cells. | [77] |
| Ianthella sp. (Australian marine sponge) | Lamellarin O | Cytotoxic against colon cancer cells | Inhibit ABC transporters that induce MDR. | [78] |
| Callyspongia siphonella | Sipholane triterpenoids (sipholenol A, sipholenone E, sipholenol L, and siphonellinol D) | Cytotoxicity enhancer of other cancer drugs, reverse chemoresistance. | Specific inhibitors of Pgp (P-Glycoprotein), increase the intracellular accumulation of drugs. | [79] |
| Haliclona (Soestella) mucosa | Panicein A | Cytotoxic against human melanoma cell lines | Enhance the cytotoxic and proapoptotic action of doxorubicin | [80] |
| Kirckpatrickia variolosa (Antarctic sponge) | Variolin B | Anti-tumor and antiviral compound | DNA intercalator | [81] |
| Mycale hentscheli | Peloruside A | Cytotoxic | Effect microtubule dynamics, abnormal spindle formation. | [15] |
| Agelas dendromorpha and Cymbastella sp. | Agelastatin A | Antineoplastic | p53-independent Apoptosis inducer, Wnt signalling inhibitor | [82] |
Mechanism of action
NVP-LAQ824 inhibits histone deacetylase enzymatic activities. NVP-LAQ824-induced apoptotic signaling involves up-regulation in the expression of p21, activation of caspases and cleavage of poly adenosine diphosphate (ADP) ribose (PARP) [83].
Marine anticancer compounds (floral derivatives)
Marine milieu floras comprise micro flora, micro/macro algae and various flowering plants. Since prehistoric time’s marine based floras have been used traditionally for medicinal tenacities especially in Asia (India & China) and Europe. The list of marine floral anticancer compounds and derivatives is shown in Table 2.
Table 2.
Some representatives of the marine anticancer floral derivatives/compounds
| Marine flora | Chemical constituent | Biological Activity | Mechanism of action | References |
|---|---|---|---|---|
| Bacteria | ||||
| Salinispora tropica and Salinispora arenicola | Salinosporamide A (Marizomib) | Anticancer on multiple myeloma, lymphoma, glioblastoma, can cross blood-brain barrier. | Proteasome inhibitor. | [84] |
| Streptomyces variabilis | Ammosamides | Anticancer agaist neuroblastoma, colon carcinoma. | Cell cycle modulators, inhibitors of quinone reductase 2, Tubulin polymerisation inhibitors. | [85] |
| Halomonas sulfitobacter (Brine-seawater interface of the Red Sea) | Crude extract | Anticancer | – | [86] |
| Halophilic sp., chromohalobacter salexigens, Halomonas meridian, Idiomarina loihiensis, Chromohalobacter israelensis (Brine pools of the Red sea and from sediment). | Crude extract | Anticancer | – | [87] |
| Serinicoccus sp. | Seriniquinone | Anticancer against melanoma. | Autophagy inducer, targets small protein dermicidin. | [88] |
| Streptomyces sp. | Chlorizidines A and B | Cytotoxic against colon cancer cells. | Target enolase & Glyceraldehyde-3-phosphate dehydrogenase. | [89] |
| Micromonospora marina | Thiocoraline | Cytotoxic profile for melanoma and cancer cell lines (lung and colon). | Inhibiting DNA polymerase α, induces cell-cycle arrest. | [90] |
| Fungus | ||||
| Aspergillus sp. | Synthetic analogue of metabolite Halimide | Non-small cell lung cancer, Neutropenia, Glioblastoma, Brain cancer. | Guanine nucleotide exchange factor stimulants; Mitosis inhibitors; Tubulin polymerisation inhibitors; Vascular disrupting agents. | [91] |
| Halorosellinia sp., Guignardia sp. | Anthracenedione | Anticancer | Type II topoisomerase inhibitor; it disrupts DNA synthesis and DNA repair. | [92] |
| Myrothecium roridum | Verrucarin A | Antitumor | Inhibition of the activation of the mitogen activated kinases c-JUN and p38. | [93] |
| Penicillium sp. | Chromanone A44 | Antitumor | Alteration of carcinogen metabolizing enzymes and protection from DNA damage. | [94] |
| Microsporum sp. | Neoechinulin A | Cytotoxic effect on human cervical carcinoma HeLa cells | Upregulates p53, p21, Bax, Caspase 3, and Caspase 9 proteins and downregulates Bcl-2. | [95] |
| Aspergillus fumigatus | Fumigaclavine C | Antiproliferative in MCF-7 cells. | DNA fragmentation, cell-cycle arrest, modulate the apoptotic protein expressions. | [96] |
| Neosartorya pseufofischeri | Gliotoxin | Antiproliferative in colon cancer cells. | Apoptosis inducer, Wnt signalling inhibitor | [97] |
| Algae | ||||
| Stigonema sp | Scytonemin | Antiproliferative and anti-inflammatory actions, Cytotoxic against human fibroblasts and endothelial cells. | Protein kinase inhibitor. | [98] |
| Symploca sp. | Largazole | Antiproliferative activity in colon cancer. | Histone deacetylase inhibitor. | [99] |
| Nostoc sp. | Cryptophycins | Cytotoxicity against human solid tumors. | Microtubule inhibition. | [100] |
| Caulerpa taxifolia | Caulerpenyne | Cytotoxic, antiproliferating and anticancerous profile. | Inhibition of microtubule polymerization. | [101] |
| Lyngbya boulloni, Lyngbya majuscula | Apratoxin E, Apratoxin A | Cytotoxic profile against adenocarcinoma. | Inhibits Janus kinase (JAK)/STAT signaling, cell cycle arrest. | [102] |
| Ascophyllum nodosum, Sargassum thunbergii | Fucoidan | Anticancer and fibrinolytic. | Cell cycle arrest, decrease in anti-apoptotic proteins expression, activation of JNK and p38. | [103, 104] |
| Undaria pinnatifida | Fucoidan | Cytotoxic against human hepatocellular carcinoma SMMC-7721. | Cell death by ROS mediated-mitochondrial signaling pathway. | [105, 106] |
| Acanthophora spicifera | Ketoseroids | Antitumor, cytotoxic against human cancer cell lines. | Regress viable cell count & volume of tumor. | [107] |
| Lyngbya majuscula | Curacin A | Antiproliferative in cancer cell lines. | Colchicine Site Antimitotic Agent. | [108] |
| Sargassum fusiforme, Sargassum trichophyllum | Laminarin | Apoptosis of LoVo human metastatic colon cell | Caspase-3, caspase-9 induction and cell- cycle arrest. | [109] |
| Laminaria japonica | Fucoxanthin | Antitumor, anti-lung cancer. | Cell cycle arrest, modulates p21, p53, PUMA, Fas, Bcl-2 and caspase-3/8 expression. | [110] |
| Rhodomela confervoides | Bis (2,3-dibromo-4,5-dihydroxy-phenyl) -methane (BDDPM) | Cytotoxic | Inhibit integrin-FAK signaling. | [111] |
| Caribbean tunicate Trididemnum solidum (symbiotic cyanobacterium living in association with the tunicate) | Didemnin B | Antitumor | Inhibits the synthesis of DNA, RNA and proteins | [112] |
| Monanchora pulchra | Sesterterpenoid and cholestane-type steroids | Antineoplastic | Inhibit Wnt/-Catenin Signaling | [113] |
| Hippospongia metachromia | Ilimaquinone and ethylsmenoquinone | Antineoplastic | Inhibit Wnt/-Catenin Signaling, p53 activation, apoptosis inducer | [114] |
| Mangroves and other coastal plants | ||||
| Ceriops decandra | Mangrove tea, triterpenes | Inhibit DMBA Induced Hamster Buccal Pouch Carcinoma | Antimicrobial against cancer causing bacteria, Free radical scavenging. | [115] |
| Acanthus ilicifolius | acanthicifoline and benzoxazinium compounds | Antineoplastic | Apoptosis induer, Free radical scavenging. | [116] |
| Conocarpus erectus | trimethoxyellagic acid glucuronide | Antineoplastic in MCF-7 snd Hep G2 cells | Cytotoxic, Free radical scavenging. | [117] |
| Avicienna alba | Naphthoquinones. | Anticancer | Free radical scavenging. | [118] |
| Calophylum inophylum | Phenylcoumarins, xanthone, biflavonoids, Neoflavonoids. | Anticancer | Cell cycle arrest, decreased Bcl-2 expression, increase expression of Bax, cytochrome C and p53. | [119] |
| Excoecaria agallocha | Diterpenes, tannin, alkaloids, Exoecarin, Mycertin. | Anticancer | Free radical scavenging. | [120] |
MNPS cancer molecular targets: An updated knowledge
In the year 2000 two researchers Hanahan and Weinberg proposed [121] in their report, the characteristic and harmonizing proficiencies which allow cancer development and metastatic propagation in the form of six major hallmark: “self-sufficiency in growth signaling, insensitivity to antigrowth signals, evading apoptosis, limitless replicative potential, sustained angiogenesis, and tissue invasion and metastasis”. A decade later in the way of outstanding progress in cancer study consequent to this publication, an updated review [122] and revisited concepts added 2 emergent hallmarks: “reprogramming of energy metabolism and evading immune response and two enabling traits: instability of genome and mutation and inflammation that promotes tumor”. Fouad and Aanei [123] in 2017, attempted to present a more systematized and revised depiction of the cancer in the form of seven hallmarks: “selective growth and proliferative advantage, altered stress response favoring overall survival, vascularization, invasion and metastasis, metabolic rewiring, an abetting microenvironment, and immune modulation”. In this section, we will focus upon the cellular targets, modulated by the MNPs and their contribution to their anticancer profile.
Anti-tubulin agents
Microtubules are filamentous cytoskeletal proteins that play a crucial role in intracellular migration and transport of vesicles, cell shape maintenance, polarity, signaling and mitosis thus serves as an imperative therapeutic target in tumor cells [124]. Microtubules constitutes the mitotic spindle in dividing cells and are extremely dynamic and sensitive to number of therapeutic inhibitors thus supports the fact, why such compounds that alters microtubule action have established to be extremely functional in cancer patients. In the field of cancer before the conception of targeted therapy, microtubules were the solitary alternative to DNA as a therapeutic target. Microtubules encompasses head-to-tail arrangement of heteropolymers having α-, β and γ-tubulin forming dimers that further arranges itself into 13 protofilaments (arranged in parallel manner) forming a rigid hollow structure.
A variety of chemically distinct compounds deriving from natural (terrestrial or marine) or synthetic sources interacts with tubulin and/or microtubules, thus alters its polymerization and dynamics in diverse ways. Such compounds are said to be antimitotic representatives that impede cell proliferation by binding and destroying microtubule dynamics, either growing (polymerization) or shortening (depolymerisation) at susceptible mitotic stage.
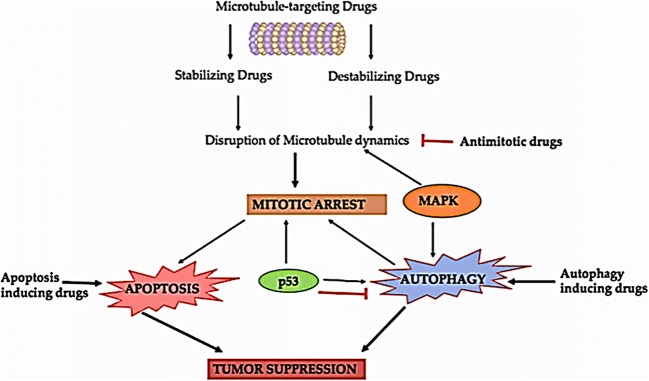
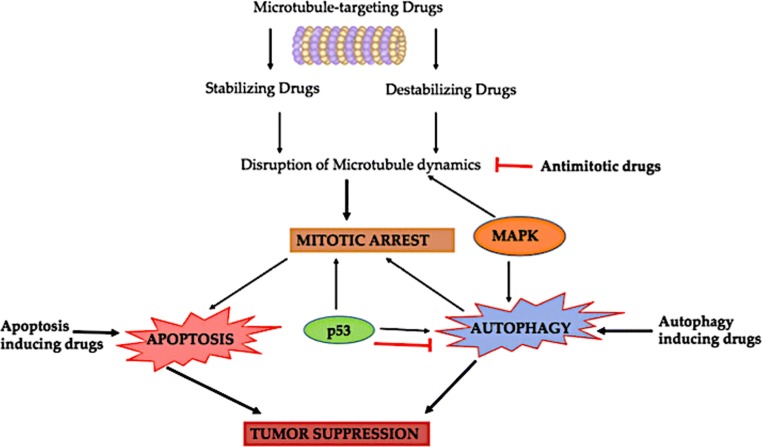
Antimitotic drugs that targets microtubule is usually classified into two chief groups: the microtubule-destabilizing and stabilizing agents. As suggested by the name itself, “Destabilizing” antimitotic agents obstruct polymerization of the microtubule at the high drug concentrations. Most of compounds that belongs to this group interacts in one of the two domains present on tubulin, namely the “Vinca” domain & the other i.e. “Colchicine” domain. Compounds that binds on Vinca site include the cryptophycins, vinca alkaloids, eribulin, rhizoxin, dolastatins, spongistatin, tasidotin and maytansinoids. Colchicine-site binders include colchicine and its analogs. Another class i.e. “microtubule-stabilizing” antimitotic agents augment the polymerization of microtubule at high concentrations of the drug. Some of the marine compounds as Anti-tubulin agents are listed in Table 3 and their mechanism of action is depicted in Fig. 8.
Table 3.
Some marine anti-cancerous compounds and their molecular targets
| S. No. | Molecular targets | Name of the Drugs | References | |
|---|---|---|---|---|
| Microtubules | Dolastatin-10 (linear pentapeptide), diazonamide A (peptide), scleritodermin A (peptide), Vitilevuamide (bicyclic 13 amino acid peptide), Spongistatins (macrocyclic lactones), Taltobulin (HTI-286, tripeptide), Peloruside A (macrolide polyketide), Zampanolide (macrolide), Dictyostatin (macrolide polyketide), Discodermolide (polyketide), Laulimalide (macrolide). | [15, 124] | ||
| Apoptosis & Autophagy | Nortopsentins A, B & C (imidazolediylbis [indole] alkaloids), Ilimaquinone (sesquiterpene quinone), Frondoside A (glycosylated triterpene), Stelletin A (triterpenoid), Echinoside A (sulfonylated holostane tetrasaccharide) and ds-echinoside A (a non-sulfated triterpene glycoside), makaluvamines (pyrroloiminoquinone alkaloids), Tyrindoleninone & 6-bromoisatin (indole derivatives), Monanchocidin A (guanidine alkaloid), didemnin B, CS5931(Polypeptide), Dolastatin 10, 15 and bryostatin 1 (Polyketides), Jasplakinolide (depsipeptide). | [15, 121–131] | ||
| 1.1.1.1. | Kinase inhibitors | a. PKC (Protein Kinase-C) | 11-hydroxystaurosporine, bryostatin-1, xestocyclamine A, (Z)-Axinohydantoin and debromo-Z-axinohydantoin, hymenialdisine and debromohymenialdisine, frondosins, BRS1, nakijiquinones, spongianolides, lasonolide A, penazetidine A, corallidictyals A and B. | [132–141] |
| b. Cyclin Dependent Kinases |
(a) Cyclin Dependent Kinase-1 inhibiting marine compounds: Hymenialdisine, Microxine, Variolin B. (b) Cyclin Dependent Kinase-4 inhibiting marine compounds: fascaplysin, Konbu’acidin A, halistanol sulfate. |
[133] | ||
| c. Tyrosine Protein Kinase (TPK) | Penta-, hexa- and hepta-prenylhydroquinone 4-sulfates, halenaquinone, halenaquinol, halenaquinol sulfate and xestoquinone, Melemeleone, 14-methoxyhalenaquinone and xestoquinolide. | [133] | ||
| d. Epidermal Growth Factor Receptor | Tauroacidins A and B, Ma’edamine A, Spongiacidins A and B, (+)-aeroplysinin-1, 3,9-dimethyldibenzo [b,d]furan-1,7-diol, 3-(hydroxymethyl)-9-methyldibenzo[b,d]furan-1,7-diol, 1,7-dihydroxy-9-methyldibenzo [b,d] furan-3-carboxylic acid, 3′-norspongiolactone, gracilins, tetrahydroaplysulphurin-1. | [133] | ||
| e. Mitogen-Activated Protein Kinase | Hymenialdisine and debromohymenialdisine, Liphagal 1, Dolastatin, Kahalalide F, Elisidepsin trifluoroacetate, Aplidine (dehydrodidemnin B, DDB, Aplidin), onnamide A and theopederin B. | [133] | ||
| Angiogenesis, Invasion or Metastasis Inhibitors | Geodiamolide H, Stylissamide X, Suberreamoline A, Cortistatin A, Aeroplysinin-1, Motuporamines A, B and C, Bastadin 6, 9 & 16, Phidianidine A, Sceptrin, Latrunculins A and B, Laulimalide, ds-echinoside A, holothurin A and 24-dehydroechinoside A, frondoside A, perhydrobenzoxepine (A & B) and bicyclodecane (C & D), frondoside A, sipholenone A and sipholenol A & E and sipholenone A, Globostellatic acid X, Heteronemin, pachycladins A and D, Sarcophines, inulodurins A and B, Strongylophorine-26, Smenospongine, WA-25, Psammaplin A, Microsporins A and B, Azumamides A–E, Largazole. | [15, 142–149] | ||
| Hypoxia Inducible Factor (HIF) Inhibitors | Laurenditerpenol, Furospongolide, Benzo[g]chromen-4-one and Benzo[h]chromen-4-one, Manassantin B, 7-hydroxyneolamellarin A. | [150–152] | ||
| Topoisomerase Inhibitor | Makaluvamine A, Ascididemin, Lamellarin D. | [153, 154] | ||
| Wnt Inhibitor | Gliotoxin, Fucoidan, cholestane-type steroids, ilimaquinone and ethylsmenoquinone | [97, 106, 113, 114] | ||
Fig. 8.
Pictorial representation of the interlinking of various molecular targets of marine anticancer compounds
Inducers of apoptosis and autophagy
Defective apoptosis signifies as a chief contributing element in the growth and progression of cancer. The capability of tumor cells to escape engagement of apoptosis play a noteworthy role. Cancer cells do this by deregulating the signals that leads to apoptosis in normal manner and thus become masters of their own destinies. By understanding the intricacies of apoptosis and the machineries developed by tumor cells to resist commitment of cell death, strategies can be developed to selectively induce apoptosis in cancer cells.
Apoptotic implicates the genetically programmed abolition of cells involved in development, differentiation, normal cell turnover and most important, in the removal of damaged/stressed or harmful cells that is otherwise likely to induce the tissue hurt and local inflammation [125]. A variety of pathological ailments including cancer can results from the dysregulation of the apoptotic program alteration by imbalance of pro- & anti-apoptotic signals [126]. It occurs by two core pathways i.e. extrinsic death receptor pathway [127] or intrinsic pathway [128] which are initiated either by extracellular death receptors such as FAS, TNF-α, and TRAIL or by intracellular stimuli such as irreparable genetic damage, hypoxia, severe oxidative stress, and nutrient deprivation. Both the pathways allied to the expression of ligands as a signal for phagocytic cell receptors, caspase-3 cleavage, fragmentation of DNA (DNA laddering), mortification of the nuclear and cytoskeletal proteins, creation of vesicles called apoptotic bodies and ultimately uptake by phagocytic cells [129]. The proteins that belong to Bcl-2 family [130] regulates the intrinsic pathway that results in the release of cytochrome c (Cyt c) from the mitochondria to the cytosol. Cytoplasmic Cyt c together with Apaf-1 and pro-caspase 9 triggers initiator caspase 9 via the creation of a complex known as apoptosome that initiates a protease cascade which consequently activates caspases 3 and 7.
Autophagy, an indispensable and regulated conserved catabolic process principally facilitates the salvaging and turnover of numerous cytoplasmic eukaryotic cell constituents. It is strictly regulated by autophagy-related genes (ATGs) [131]. Autophagy performs a twin part in the regulation of pro-survival under certain circumstances and pro-death signaling pathways in a range of diseases including cancer [155]. Autophagy is frequently stimulated by conditions of hypoxia, accumulation of Reactive Oxygen Species (ROS), nutrient deficit situations, triggering by drugs or under the influence of endoplasmic reticulum stress (ERS). The mTORC1 (mammalian target of Rapamycin Complex 1), PI3K (Phosphatidylinositol 3 kinase), AKT or Protein kinase B, Beclin-1 (mammalian homologue of Atg6) and p53 are chief proteins that play imperative role in autophagy [156]. The regulatory mechanisms of autophagy are still controversial. To figure out the function of autophagy in cancer cell fate, out of numerous theories one proposes that its role fluctuates depending on the stage of tumor development. On one hand, autophagy confines tumor formation in early stages but on other hand it favors tumor cell survival, invasion, and metastasis after tumors formation [157, 158]. Some of the marine compounds as inducer of Apoptosis and autophagy are listed in Table 3 and their interlinking with other molecular targets are depicted in Fig. 8.
Kinase inhibitors
The enzyme, Protein kinase plays a diverse and dynamic character in cell regulation by regulating signaling pathways involved in numerous processes like cell differentiation, propagation, biotransformation, DNA damage repair, cell-motility and apoptosis [132]. Their deregulation has been reported to cause a number of diseases including cancer, central nervous system disorders, autoimmune and metabolic diseases. Thus, understanding the machinery and directive of several kinases may definitely have potential as a novel medicinal goal. The protein kinase family comprises all enzymes catalyzing the chemical relocation of phosphate (PO43−) group (termed as phosphorylation) via a comparatively high energy compound for example ATP (Adenine Triphosphate) to a precise acceptor (substrate). Kinase inhibitors derived from marine sponges were reported in a review [133]. Different types of kinases can be summarized as:
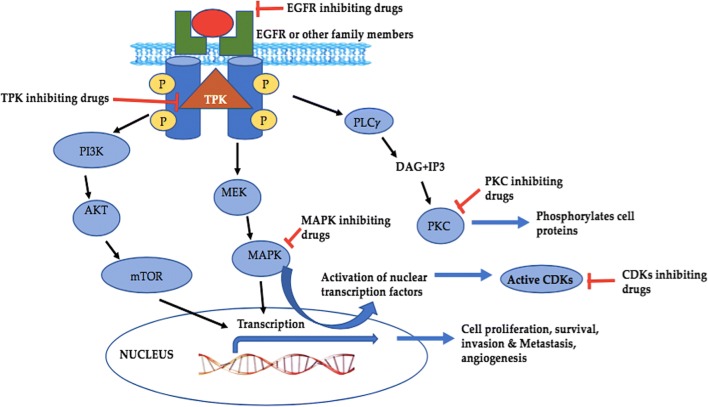
PKC (protein kinase-C)
PKC encompass eleven isozymes and involved in monitoring the task of other proteins through the phosphorylation of hydroxyl groups of amino acids (serine and threonine) residues. Protein kinase-C enzymes are stimulated by signals such as upsurges in the concentration of DAG (diacylglycerol), phosphatidyl serine (PS), inositol triphosphate (IP3) and calcium ions (Ca2+). They are potential therapeutic targets for cancer as they play a fundamental role in signal transduction pathways.
Cyclin dependent kinases
Cyclin Dependent kinases (CDKs) comes under the family of serine/threonine kinases, comprising nearly 25 different cyclin families which perform an imperative role in cell-cycle regulation [134]. Over the past three decades it has been well-defined that the hyperactivity of Cyclin Dependent Kinases (CDKs) is one of the mechanisms involved in the cancer proliferation. As evident by name, their enzymatic activation requires the binding of the regulatory subunit known as cyclin [135]. The variable concentrations of distinctively stimulated CDK/cyclin complexes determine the cell cycle phases whose mechanism of action implicates the phosphorylation of several discrete proteins specifically at threonine or serine residues.
Tyrosine protein kinase (TPK)
Tyrosine protein kinase or Tyrosine kinase includes the enzymes that can catalyze the addition of phosphate group (phosphorylation) especially at tyrosine residues and functions as an “on” or “off” switch in many cellular roles. They are of two types namely; Receptor tyrosine kinase (having an intracellular tyrosine kinase domain, a transmembrane domain and an extracellular ligand-binding domain) & Non-receptor (cytoplasmic) tyrosine kinases. Tyrosine protein kinase are reported to be linked with proliferative ailments for example cancer, leukemia (cancer of the bone marrow), psoriasis owed to their function in controlling key cell tasks like cell growth, delineation and signaling to prevent apoptosis [136].
Epidermal growth factor receptor
The epidermal growth factor receptor (EGFR) is a transmembrane receptor for members of the epidermal growth factor family (EGF family) of extracellular protein ligands. EGFR subfamily comprises erbB-1, −2, −3 and − 4 [137]. It has been reported that cancer could be the outcome of the mutations that disturbs EGFR expression or activity. Overexpression of EGFR is reported to be associated very frequently in cancers, particularly breast cancer [138].
Mitogen-activated protein kinase
A MAPK or MAP kinase (Mitogen-activated protein kinases) comprises a family of proteins that particularly add phosphate group (phosphorylation) at tyrosine, threonine and serine amino acids and intently linked to several cellular assignments of cancer for example cellular division, propagation, cycle, apoptosis, metastasis and angiogenesis [139]. They are stimulated by exterior influences and encourages the transmission of internal (intracellular) signals via the phosphorylation and triggering of related directs that conclusively reach the nucleus and produce a cellular reaction [140]. The three main subclasses that belong to this family includes Extracellular Signal-Regulated Kinases (ERKs), p38 MAPKs and c-Jun N-terminal Kinase (JNK)/Stress-Activated Protein Kinase (SAPKs) [141]. Mitogen-activated protein kinases are often overexpressed or upregulated in cancer cells. It is obvious and reported too that discerning compounds that inhibit these kinases affect cellular proceedings in an extreme specific way and thus they are significantly important in anticancer pharmaceuticals. Some of the marine compounds as kinase inhibitors are listed in Table 1 and interlinking of various subtypes are shown in Fig. 9.
Fig. 9.
The relation between different kinases and their cross-talk capable to provoke cell responses involved in the cancer. Abbreviations: EGFR; Epidermal growth factor receptor, PLCγ: Phospholipase C, DAG; Diacylglycerol, IP3; Inositol 1,4,5-triphosphate, CDKs; cyclin-dependent kinases, MAPKs; Mitogen Activated Protein Kinases, MEK; Mitogen-activated protein kinase kinase, TPK; Tyrosine Protein Kinases, mTOR; mammalian target of Rapamycin, PKC; Protein kinase C
Angiogenesis, invasion or metastasis inhibitors
Angiogenesis besides playing an imperative role in normal physiological activities, also plays a grave part in the development and spread of cancer. As a part of normal physiological process, it occurs during menstruation, placenta formation, pregnancy or organogenesis particularly in a damaged organ and in wound-healing action. Angiogenesis is regulated by an equilibrium of angiogenic inhibitors and stimulators. Moreover, in cancer angiogenesis nourishes tumor progression and spreads diseased cells through the blood to further organs thus provoking the development of secondary malignant growths (metastasis) [142]. Being symbol of cancer [122], angiogenesis is measured as a one of the commanding objective to overpower tumor progression and secondary malignant growths. Numerous factors imperative for targeting the phenomenon of angiogenesis are as follows, i.e., vascular endothelial growth factor-related kinases [143], Matrixins or MMPs (Matrix Metalloproteinases) [144], Methionine Aminopeptidase (a metalloenzyme) [145], Actin, Microtubule [146] and Histone Deacetylases (HDACs) [147]. Hence compounds that impede angiogenesis-linked factors has become an effective target for therapy of cancer. Among them Histone deacetylases (HDAC) are currently being investigated for their use in anticancer therapy. They are group of enzymes involving in the removal of acetyl groups thus playing pivotal role in the regulation of chromatin structure and gene expression. It has been reported that marine sponges and their associations yielded the Psammaplin family of compounds (bromotyrosine derivatives) as a potent histone deacetylase and DNA methyltransferase inhibitors [159]. Moreover, Microsporins A and B derived from marine- fungus Microsporum cf. gypseum has been documented as potent inhibitors of histone deacetylase and exhibit cytotoxicity against human colon adenocarcinoma (HCT-116) and the panel of 60 cancer cells of National Cancer Institute [160]. Azumamides A–E (cyclic tetrapeptides) isolated from the marine sponge Mycale izuensis are another group of histone deacetylase inhibitors [161] but the marine cyanobacterial metabolite largazole is among the most potent HDAC inhibitors found to date [162]. Interestingly, largazole thiol has been reported to be more active than any other marine-derived HDAC inhibitor such as psammaplin [159] and azumamides [161]. The studies have shown that the involvement of HDACs plays a crucial role in angiogenesis by alterating numerous pro- and anti-angiogenic genes [163].
Metastasis, the extreme feature of cancer determines the clinical stages and prognosis [148]. MMPs, belongs to the zinc-reliant/dependent group of endopeptidases, performing imperative functions in mortification of the Extracellular Matrix (ECM) & also in penetration & the process of metastasis. ICAM-1, Integrins (heterodimeric transmembrane receptors), MMPs and ECM are some of the foremost elements involved in metastasis. Investigations on the formation of new blood vessels (angiogenesis) and metastatic progressions is primarily concentrated on composites that can disturb or regulate these processes thus serving as prospective medications for cancer therapy [149]. Some representative angiogenesis inhibitors are listed in Table 3.
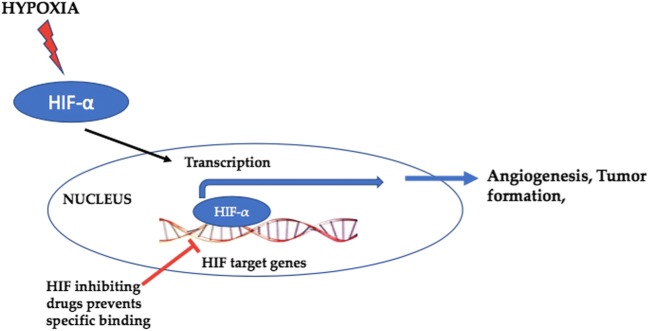
Hypoxia inducible factor (HIF) inhibitors
Hypoxia-inducible factors (HIFs) are the transcription factors that act to decline available oxygen in the cellular milieu or hypoxia. Transcriptional retort to hypoxia is facilitated by hypoxia inducible factors (HIF1–3). The oxygen-sensitive signal being produced by a sequence of protein hydroxylases catalyze prolyl and asparaginyl hydroxylation on specific residues in the regulatory HIF-α subunits (HIF1α, HIF-2α, and HIF-3α). Tumor progression is associated with intratumoral hypoxia [150] that promotes the growth/formation of blood vessels important for developing a vascular system in embryos and tumors. Additionally, loss of HIF-1 action has been reported to be related to the massive deleterious effects on tumor growth and vascularization [151, 152]. Therefore, numerous compounds that inhibit HIF needs to be targeted with the purpose of discovering a novel way to tumor treatment. Some of the marine compounds as HIF inhibitors [153] are listed in Table 3 and depicted by Fig. 10.
Fig. 10.
HIF as a molecular target for cancer treatment
Topoisomerase inhibitors
Topoisomerases are enzymes that participate in the over winding or under winding of DNA thus show a foremost function in supporting the integrity of the DNA helix throughout the vital processes of transcription, replication and mitosis [154]. There are compounds known as Topoisomerase inhibitors that hinders the act of topoisomerase (topoisomerase I and II). Latter is an enzyme that regulates the alterations in DNA organization by accelerating the breaking and retorting of the phosphodiester back of the DNA. They impede DNA replication process and transcription thus results in the demise of cells trying to undertake these progressions. Therefore, they are now being directed for anticancer treatment. Some of the marine compounds as Topoisomerase inhibitors are listed in Table 3 and depicted by Fig. 11.
Fig. 11.
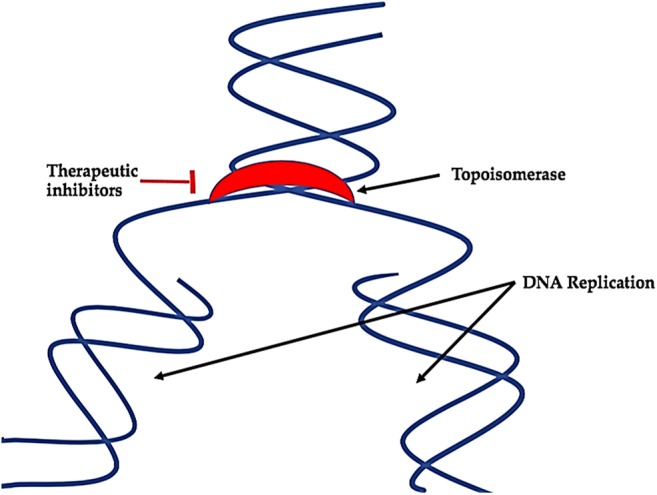
Role of topoisomerase as a molecular target for cancer treatment
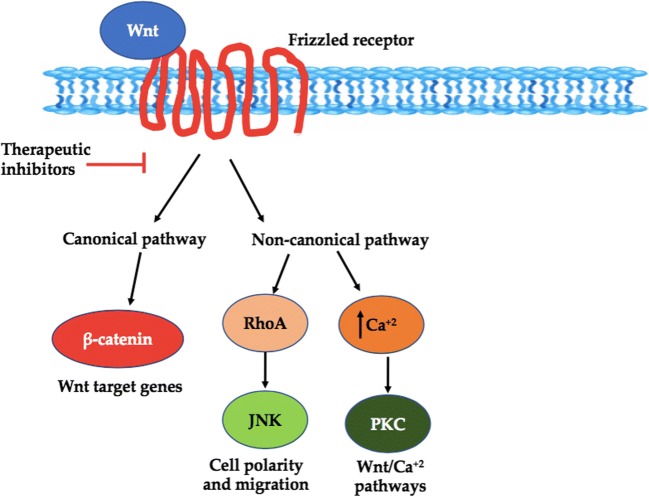
Wnt signaling inhibitors
Wnt signalling has been recognized as a regulator of cancer development and progression for more than 3 decades while these factors are active in numerous other contexts as well, for e.g. tissue homeostasis and embryogenesis [164]. Abnormal activation or aberrations in this pathway is involved in virtually all stages of oncogenesis eventually resulting in deteriorating anticancer immunity, a crucial contributor to tumor progression and eventual resistance to treatment [165, 166]. Recently inappropriate WNT signalling has been reported to destabilize the process of cancer immunosurveillance thus unfortunately facilitating resistance to immunotherapeutics and immunoevasion by directly altering plethora of regulators responsible for the antitumor activities of T cells, especially helper, regulatory and effector T cells [167, 168]. The distinctive feature of this signalling is the activation of β-catenin, the multitasking pivotal nuclear effector that acts both as a transcriptional co-regulator and as an adaptor protein for intracellular adhesion.
Wnt, a chief regulator of β-catenin belonging to a family of 19 glycoproteins. Interaction of Wnt ligands with frizzled receptor complexes can elicit numerous intracellular signalling cascades; which can be broadly divided into two types based on the involvement of transcriptional co-regulator β-catenin: In the first type of signalling i.e. β-catenin dependent (canonical) Wnt cascade, β-catenin is the core molecule that transduce the signal to the nucleus and triggers transcription of Wnt-target genes. Such genes are responsible for regulating cell fate decisions and plethora of other biological processes linked with progression of cancer for e.g. cell death, senescence & differentiation, tumor formation, proliferation its metastasis and tumor metabolism [169]. Interestingly, it has been documented that the canonical Wnt pathway facilitate cancer cells with a metabolically flexible environment by altering their metabolic activities as per their microenvironment and metabolic status. However, this pathway is itself regulated by the nutrients, enzymes and by-products of the metabolic pathway. Therefore, it signifies that Wnt/−catenin signalling represents a crucial target in the regulation of tumours metabolism. The second type of Wnt signalling known as independent (non-canonical) signalling is a collective term for numerous β-catenin independent pathways and comprises of Wnt/PCP (Planar Cell Polarity), WNT/Ca2+, Wnt/PKC (protein kinase C) and various other pathways. Out of these reported noncanonical pathways, the Wnt/Ca2+signaling pathway has been reported to be linked with tumor formation and progression [169]. Therefore, Wnt signalling could provide a brilliant target for the development of better cancer therapeutics. Some of the marine compounds as Wnt inhibitors are listed in Table 3 and depicted by Fig. 12.
Fig. 12.
Wnt pathway as a target for cancer treatment
Restraints of marine inhabiting invertebrates as Foundation for Anti-Cancerous Agents
The usage of biologically active composites of unexplored oceanic world as an alternative natural source will be increasing in the upcoming years for the invention of new drugs. Nonetheless, some restrictions in the form of challenges cannot be overlooked:
Supply problem and target identification.
Scarcity of adequate quantity of natural product.
Complications in retrieving the foundation of the samples, isolation and purification procedures.
Complications linked with ingathering of the product.
Difficulties in manufacturing the required quantities of the compound.
Excessive toxicity of the effective compound.
Unavoidable Inorganic salts resultant from the creatures or milieu.
Comprehensive variety of chemical composites formed by a creature.
Presence of indistinct pharmacological focusses.
Ecological concerns.
Government policies.
Shortage of infrastructure and inadequate principal investment.
Inspite of drastic progress in the field of biomedicine and marine science, still there is scarcity of marine products that have paved their way to the marketplace. Variety of marine microorganisms are challenging to culture because of the unfeasibility in mimicking the drastic oceanic conditions. Moreover, scarcity of text on the sequestration techniques and standardized culture circumstances makes the condition even worse. Still it is of concern that very less numbers of academics are experts and undertaking such analyses. Cancer has been a disease since ages but till date lack of understanding of cancer biomarkers, the complexity of cancer genomics & proteomics pose a pronounced confront for marine anticancer drug investigation. There is an essential prerequisite for integrative and cooperative efforts so that innovated ideas can find their way to research organizations and being translated into new treatments. Lack of ample funding and infrastructure reserves to conduct the advanced clinical testing needed for regulatory approval of a drug, pose an impediment to the marine antitumor medication research. The vast structural diversity of marine natural compounds makes it challenging for the sequestration and refinement of these compounds and the progress of innovative anticancer drugs from these natural bases in contrast to synthetic compounds. Furthermore, prevailing extortions to worldwide marine bio-diversity comprising habitat thrashing, overfishing, pollutants, rising water temperatures due to global warming and ocean acidification are building marine anticancer drug investigation increasingly difficult. These issues demand to be resolved for effective drug development of these drugs.
Contending downsides
Advances in technologies such as high-throughput screening approaches, usage of computational biology and bioinformatics, sampling approaches, nanoscale NMR for structure determination, development of synthetic or semisynthetic analogues derivatives and genetic engineering are altogether central to journey of marine natural products to become drug leads. As using these technologies we can produce compounds that are more efficient and have lower toxic, customized or undesired effects [170–174].
The issue of supply problem can be overwhelmed using an amalgamation of new methods in chemical synthesis and refining yield, aquaculture or isolation procedures [175]. Additional regulating aspect in the usage of marine creatures is the probable occurrence of toxic contaminants. Analytical procedures are presently available for the segregation, categorization and separation of effective compounds from marine extracts [176, 177]. Using controlled aquaculture techniques, we could escape the setback of fatiguing the marine assets and can generate the necessitated biomass for the high scale manufacture. To resolve the obstacle of identifying the molecular targets of the newly discovered MNPs, high-throughput screening procedures in conjunction with Omics (proteomics, genomics & metabolomics) and computer-generated screening have huge influence on the documentation of novel antitumor targets. The comparatively emerging fields of pharmacogenomics and pharmacogenetics should be integrated to overcome the limitations.
Conclusion
Increasing environmental abuses continue to intensify the frequencies of cancer with a challenge to find a cure with no or low side effects. Natural derivatives play an important role to check the cancer occurrences as an alternative source with low side effects and additional benefits. Drugs derived from marine biologicals have an enormous influence on antitumor drug discovery regimen of the existing day, nonetheless immense majority of oceanic habitation is underexplored in anticancer drug discovery due to numerous issues. This publication reviews the most newly sequestered, synthetically achieved or derived compounds/chemical constituents from oceanic environment (fauna & flora) that have displayed potential as cancer therapies with their mechanism of action as updated so far.
Acknowledgments
Authors are very thankful to all the authors whose work has been cited in this paper. Figures of the compounds has been drawn using ChemDraw software.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical issues
This study does not involve any animal study or clinical trial itself, hence no ethical issues.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Manisha Nigam, Email: m.nigam@hnbgu.ac.in.
Hafiz Ansar Rasul Suleria, Email: hafiz.suleria@uqconnect.edu.au.
Mohammad Hosein Farzaei, Phone: +989183393351, Email: mh.farzaei@gmail.com.
Abhay Prakash Mishra, Phone: +91-9452002557, Email: abhaypharmachemhnbgu@gmail.com.
References
- 1.Suleria HAR, Gobe G, Masci P, Osborne SA. Marine bioactive compounds and health promoting perspectives; innovation pathways for drug discovery. Trends Food Sci Technol. 2016;50:44–55. [Google Scholar]
- 2.Suleria HAR, Osborne S, Masci P, Gobe G. Marine-based nutraceuticals: an innovative trend in the food and supplement industries. Mar Drugs. 2015;13:6336–6351. doi: 10.3390/md13106336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kathiresan K, Duraisamy A. Current issues of marine microbiology. ENVIS is Envoirnmental information system. 2005;4:3–5. [Google Scholar]
- 4.Jimeno J, Faircloth G, Sousa-Faro JMF, Scheuer P, Rinehart K. New marine derived anticancer therapeutics ─ a journey from the sea to clinical trials. Mar Drugs. 2004;2:14–29. [Google Scholar]
- 5.Lindequist U. Marine derived pharmaceuticals - challenges and opportunities. Biomol Ther. 2016;24:561–571. doi: 10.4062/biomolther.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leal MC, Madeira C, Brandão CA, Puga J, Calado R. Bioprospecting of marine invertebrates for new natural products — a chemical and zoogeographical perspective. Molecules. 2012;17:9842–9854. doi: 10.3390/molecules17089842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malve H. Exploring the ocean for new drug developments: marine pharmacology. J Pharm Bioallied Sci. 2016;8:83–91. doi: 10.4103/0975-7406.171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dyshlovoy SA, Honecker F. Marine compounds and cancer: where do we stand? Mar Drugs. 2015;13:5657–5665. doi: 10.3390/md13095657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergmann W, Feeney RJ. Contributions to the study of marine products. XXXII. The nucleosides of sponges. I. 1. J Org Chem. 1951;16:981–987. [Google Scholar]
- 10.Bergmann W, Stempien MF. Contributions to the study of marine products. XLIII. The nucleosides of sponges. V. the synthesis of spongosine 1. J Org Chem. 1957;22:1575–1577. [Google Scholar]
- 11.Bergmann W, Burke DC. Contributions to the study of marine products. XL. The nucleosides of sponges.1 IV. Spongosine 2. J Org Chem. 1956;21:226–228. [Google Scholar]
- 12.Suleria HAR, Masci P, Gobe G, Osborne S. Current and potential uses of bioactive molecules from marine processing waste. J Sci Food Agric. 2016;96:1064–1067. doi: 10.1002/jsfa.7444. [DOI] [PubMed] [Google Scholar]
- 13.Kong D-X, Jiang Y-Y, Zhang H-Y. Marine natural products as sources of novel scaffolds: achievement and concern. Drug Discov Today. 2010;15:884–886. doi: 10.1016/j.drudis.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat Rev Drug Discov. 2009;8:69–85. doi: 10.1038/nrd2487. [DOI] [PubMed] [Google Scholar]
- 15.Ruiz-Torres V, Encinar JA, Herranz-López M, Pérez-Sánchez A, Galiano V, Barrajón-Catalán E, et al. An updated review on marine anticancer compounds: the use of virtual screening for the discovery of small-molecule cancer drugs. Molecules. 2017;22:E1037. doi: 10.3390/molecules22071037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munro MH, Blunt JW, Dumdei EJ, Hickford SJ, Lill RE, Li S, et al. The discovery and development of marine compounds with pharmaceutical potential. J Biotechnol. 1999;70(1–3):15–25. doi: 10.1016/s0168-1656(99)00052-8. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz-Alonso MJ, González-Santiago L, Zarich N, Martínez T, Alvarez E, Rojas JM, Muñoz A. Plitidepsin has a dual effect inhibiting cell cycle and inducing apoptosis via Rac1/c-Jun NH2-terminal kinase activation in human melanoma cells. J Pharmacol Exp Ther. 2008;324:1093–1101. doi: 10.1124/jpet.107.132662. [DOI] [PubMed] [Google Scholar]
- 18.Landowne RA, Bergmann W. Contributions to the study of marine products. L. Phospholipids of sponges1,2. J Org Chem. 1961;26:1257–1261. [Google Scholar]
- 19.Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87:1710–1717. [PubMed] [Google Scholar]
- 20.Stentoft J. The toxicity of cytarabine. Drug Saf. 1990;5:7–27. doi: 10.2165/00002018-199005010-00003. [DOI] [PubMed] [Google Scholar]
- 21.Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, Little RD, et al. The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci. 2010;31:255–265. doi: 10.1016/j.tips.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 22.Gajdos C, Elias A. Trabectedin: safety and efficacy in the treatment of advanced sarcoma. Clin Med Insights Oncol. 2011;5:35–43. doi: 10.4137/CMO.S4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shetty N, Gupta S. Eribulin drug review. South Asian J Cancer. 2014;3:57–59. doi: 10.4103/2278-330X.126527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JA, Wilson L, Azarenko O, Zhu X, Lewis BM, Littlefield BA, et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry. 2010;49:1331–1337. doi: 10.1021/bi901810u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doronina SO, Mendelsohn BA, Bovee TD, Cerveny CG, Alley SC, Meyer DL, et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 2006;17:114–124. doi: 10.1021/bc0502917. [DOI] [PubMed] [Google Scholar]
- 26.Ansell SM. Brentuximab vedotin: delivering an antimitotic drug to activated lymphoma cells. Expert Opin Investig Drugs. 2011;20:99–105. doi: 10.1517/13543784.2011.542147. [DOI] [PubMed] [Google Scholar]
- 27.Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH. Isolation of dolastatin 10 from the marine cyanobacterium Symploca species VP642 and total stereochemistry and biological evaluation of its analogue symplostatin 1. J Nat Prod. 2001;64:907–910. doi: 10.1021/np010049y. [DOI] [PubMed] [Google Scholar]
- 28.Sutherland MSK, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, et al. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281:10540–10547. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- 29.Fromm JR, McEarchern JA, Kennedy D, Thomas A, Shustov AR, Gopal AK. Clinical binding properties, internalization kinetics, and clinicopathologic activity of brentuximab vedotin: an antibody-drug conjugate for CD30-positive lymphoid neoplasms. Clin Lymphoma Myeloma Leuk. 2012;12:280–283. doi: 10.1016/j.clml.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 30.McGivern JG. Ziconotide: a review of its pharmacology and use in the treatment of pain. Neuropsychiatr Dis Treat. 2007;3:69–85. doi: 10.2147/nedt.2007.3.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. 2004;291:63–70. doi: 10.1001/jama.291.1.63. [DOI] [PubMed] [Google Scholar]
- 32.De NP, Tirri T, Mameli S, Papa A. Intrathecal ziconotide for cancer pain relief: when, how and why. J Anesth Clin Res. 2012;3:227. [Google Scholar]
- 33.Cheung RCF, Ng TB, Wong JH. Marine peptides: bioactivities and applications. Mar Drugs. 2015;13:4006–4043. doi: 10.3390/md13074006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñoz-Alonso MJ, González-Santiago L, Martínez T, Losada A, Galmarini CM, Muñoz A. The mechanism of action of plitidepsin. Curr Opin Investig Drugs. 2009;10:536–542. [PubMed] [Google Scholar]
- 35.Krege S, Rexer H, vom Dorp F, de Geeter P, Klotz T, Retz M, et al. Prospective randomized double-blind multicentre phase II study comparing gemcitabine and cisplatin plus sorafenib chemotherapy with gemcitabine and cisplatin plus placebo in locally advanced and/or metastasized urothelial cancer: SUSE (AUO-AB 31/05) BJU Int. 2014;113:429–436. doi: 10.1111/bju.12437. [DOI] [PubMed] [Google Scholar]
- 36.Akashi Y, Oda T, Ohara Y, Miyamoto R, Kurokawa T, Hashimoto S, et al. Anticancer effects of gemcitabine are enhanced by co-administered iRGD peptide in murine pancreatic cancer models that overexpressed neuropilin-1. Br J Cancer. 2014;110:1481–1487. doi: 10.1038/bjc.2014.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuo W-T, Huang J-Y, Chen M-H, Chen C-Y, Shyong Y-J, Yen K-C, et al. Development of gelatin nanoparticles conjugated with phytohemagglutinin erythroagglutinating loaded with gemcitabine for inducing apoptosis in non-small cell lung cancer cells. J Mater Chem B. 2016;4:2444–2454. doi: 10.1039/c5tb02598b. [DOI] [PubMed] [Google Scholar]
- 38.Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22:3–10. [PubMed] [Google Scholar]
- 39.Natsume T, Watanabe J, Ogawa K, Yasumura K, Kobayashi M. Tumor-specific antivascular effect of TZT-1027 (Soblidotin) elucidated by magnetic resonance imaging and confocal laser scanning microscopy. Cancer Sci. 2007;98:598–604. doi: 10.1111/j.1349-7006.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamann MT, Otto CS, Scheuer PJ, Dunbar DC. Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. (1) J Org Chem. 1996;61:6594–6600. doi: 10.1021/jo960877+. [DOI] [PubMed] [Google Scholar]
- 41.Ling Y-H, Aracil M, Zou Y, Yuan Z, Lu B, Jimeno J, et al. PM02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death-associated protein kinase. Clin Cancer Res. 2011;17:5353–5366. doi: 10.1158/1078-0432.CCR-10-1948. [DOI] [PubMed] [Google Scholar]
- 42.Ott PA, Pavlick AC, Johnson DB, Hart LL, Infante JR, Luke JJ, et al. A phase II study of glembatumumab vedotin (GV), an antibody-drug conjugate (ADC) targeting gpNMB, in advanced melanoma. J Clin Oncol. 2017;35:109. [Google Scholar]
- 43.Keir CH, Vahdat LT. The use of an antibody drug conjugate, glembatumumab vedotin (CDX-011), for the treatment of breast cancer. Expert Opin Biol Ther. 2012;12:259–263. doi: 10.1517/14712598.2012.642357. [DOI] [PubMed] [Google Scholar]
- 44.Caplan S, Zheng B, Dawson-Scully K, White C, West L. Pseudopterosin a: protection of synaptic function and potential as a neuromodulatory agent. Mar Drugs. 2016;14:55. doi: 10.3390/md14030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sperlich J, Kerr R, Teusch N. The marine natural product Pseudopterosin blocks cytokine release of triple-negative breast cancer and monocytic leukemia cells by inhibiting NF-κB signaling. Mar Drugs. 2017;15:E262. doi: 10.3390/md15090262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fontana A, Cavaliere P, Wahidulla S, Naik CG, Cimino G. A new antitumor isoquinoline alkaloid from the marine nudibranch Jorunna funebris. Tetrahedron. 2000;56:7305–7308. [Google Scholar]
- 47.Oku N, Matsunaga S, van Soest RWM, Fusetani N. Renieramycin J, a highly cytotoxic tetrahydroisoquinoline alkaloid, from a marine sponge Neopetrosia sp. J Nat Prod. 2003;66:1136–1139. doi: 10.1021/np030092g. [DOI] [PubMed] [Google Scholar]
- 48.Petek BJ, Jones RL. PM00104 (Zalypsis®): a marine derived alkylating agent. Molecules. 2014;19:12328–12335. doi: 10.3390/molecules190812328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Longley RE, Caddigan D, Harmody D, Gunasekera M, Gunasekera SP. Discodermolide--a new, marine-derived immunosuppressive compound. II. In-vivo studies. Transplantation. 1991;52:656–661. doi: 10.1097/00007890-199110000-00015. [DOI] [PubMed] [Google Scholar]
- 50.Longley RE, Caddigan D, Harmody D, Gunasekera M, Gunasekera SP. Discodermolide--a new, marine-derived immunosuppressive compound. I. In-vitro studies. Transplantation. 1991;52:650–656. doi: 10.1097/00007890-199110000-00014. [DOI] [PubMed] [Google Scholar]
- 51.ter Haar E, Rosenkranz HS, Hamel E, Day BW. Computational and molecular modeling evaluation of the structural basis for tubulin polymerization inhibition by colchicine site agents. Bioorganic Med Chem. 1996;4:1659–1671. doi: 10.1016/0968-0896(96)00158-7. [DOI] [PubMed] [Google Scholar]
- 52.Honore S, Kamath K, Braguer D, Wilson L, Briand C, Jordan MA. Suppression of microtubule dynamics by discodermolide by a novel mechanism is associated with mitotic arrest and inhibition of tumor cell proliferation. Mol Cancer Ther. 2003;2:1303–1311. [PubMed] [Google Scholar]
- 53.Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J. Isolation and structure of bryostatin 1. J Am Chem Soc. 1982;104:6846–6848. [Google Scholar]
- 54.Trenn G, Pettit GR, Takayama H, Hu-Li J, Sitkovsky MV. Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins. J Immunol. 1988;140:433–439. [PubMed] [Google Scholar]
- 55.Kobayashi M, Natsume T, Tamaoki S, Watanabe J, Asano H, Mikami T, et al. Antitumor activity of TZT-1027, a novel dolastatin 10 derivative. Jpn J Cancer Res. 1997;88:316–327. doi: 10.1111/j.1349-7006.1997.tb00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hornung RL, Pearson JW, Beckwith M, Longo DL. Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in-vitro versus in-vivo activity. Cancer Res. 1992;52:101–107. [PubMed] [Google Scholar]
- 57.Advani RH, Lebovic D, Chen A, Brunvand M, Goy A, Chang JE, et al. Phase I study of the anti-CD22 antibody-drug conjugate Pinatuzumab Vedotin with/without rituximab in patients with relapsed/refractory B-cell non-hodgkin lymphoma. Clin Cancer Res. 2017;23:1167–1176. doi: 10.1158/1078-0432.CCR-16-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Förster Y, Meye A, Albrecht S, Schwenzer B. Tissue factor and tumor: clinical and laboratory aspects. Clin Chim Acta. 2006;364:12–21. doi: 10.1016/j.cca.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 59.Cocco E, Varughese J, Buza N, Bellone S, Glasgow M, Bellone M, et al. Expression of tissue factor in adenocarcinoma and squamous cell carcinoma of the uterine cervix: implications for immunotherapy with hI-con1, a factor VII-IgGFc chimeric protein targeting tissue factor. BMC Cancer. 2011;11:263. doi: 10.1186/1471-2407-11-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coleman JE, Dilip de Silva E, Kong F, Andersen RJ, Allen TM. Cytotoxic peptides from the marine sponge Cymbastela sp. Tetrahedron. 1995;51:10653–10662. [Google Scholar]
- 61.Talpir R, Benayahu Y, Kashman Y, Pannell L, Schleyer M. Hemiasterlin and geodiamolide TA; two new cytotoxic peptides from the marine sponge Hemiasterella minor (Kirkpatrick) Tetrahedron Lett. 1994;35:4453–4456. [Google Scholar]
- 62.Gamble WR, Durso NA, Fuller RW, Westergaard CK, Johnson TR, Sackett DL, et al. Cytotoxic and tubulin-interactive hemiasterlins from Auletta sp. and Siphonochalina spp. sponges. Bioorganic Med Chem. 1999;7:1611–1615. doi: 10.1016/s0968-0896(99)00089-9. [DOI] [PubMed] [Google Scholar]
- 63.Anderson HJ, Coleman JE, Andersen RJ, Roberge M. Cytotoxic peptides hemiasterlin, hemiasterlin a and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother Pharmacol. 1997;39:223–226. doi: 10.1007/s002800050564. [DOI] [PubMed] [Google Scholar]
- 64.Quinoa E, Adamczeski M, Crews P, Bakus GJ. Bengamides, heterocyclic anthelmintics from a Jaspidae marine sponge. J Org Chem. 1986;51:4494–4497. [Google Scholar]
- 65.Dumez H, Gall H, Capdeville R, Dutreix C, van Oosterom AT, Giaccone G. A phase I and pharmacokinetic study of LAF389 administered to patients with advanced cancer. Anti-Cancer Drugs. 2007;18:219–225. doi: 10.1097/CAD.0b013e328010ef5b. [DOI] [PubMed] [Google Scholar]
- 66.Towbin H, Bair KW, DeCaprio JA, Eck MJ, Kim S, Kinder FR, et al. Proteomics-based target identification: bengamides as a new class of methionine aminopeptidase inhibitors. J Biol Chem. 2003;278:52964–52971. doi: 10.1074/jbc.M309039200. [DOI] [PubMed] [Google Scholar]
- 67.Martínez-Díez M, Guillén-Navarro MJ, Pera B, Bouchet BP, Martínez-Leal JF, Barasoain I, et al. PM060184, a new tubulin binding agent with potent antitumor activity including P-glycoprotein over-expressing tumors. Biochem Pharmacol. 2014;88:291–302. doi: 10.1016/j.bcp.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 68.Zovko A, Viktorsson K, Lewensohn R, Kološa K, Filipič M, Xing H, et al. APS8, a polymeric alkylpyridinium salt blocks α7 nAChR and induces apoptosis in non-small cell lung carcinoma. Mar Drugs. 2013;11:2574–2594. doi: 10.3390/md11072574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li H-H, Su J-H, Chiu C-C, Lin J-J, Yang Z-Y, Hwang W-I, et al. Proteomic investigation of the sinulariolide-treated melanoma cells A375: effects on the cell apoptosis through mitochondrial-related pathway and activation of caspase cascade. Mar Drugs. 2013;11:2625–2642. doi: 10.3390/md11072625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su J-H, Chen Y-C, El-Shazly M, Du Y-C, Su C-W, Tsao C-W, et al. Towards the small and the beautiful: a small dibromotyrosine derivative from Pseudoceratina sp. sponge exhibits potent apoptotic effect through targeting IKK/NFκB signaling pathway. Mar Drugs. 2013;11:3168–3185. doi: 10.3390/md11093168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Esmaeelian B, Benkendorff K, Johnston MR, Abbott CA. Purified brominated indole derivatives from Dicathais orbita induce apoptosis and cell cycle arrest in colorectal cancer cell lines. Mar Drugs. 2013;11:3802–3822. doi: 10.3390/md11103802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hamilton G. Cytotoxic effects of fascaplysin against small cell lung cancer cell lines. Mar Drugs. 2014;12:1377–1389. doi: 10.3390/md12031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akl MR, Ayoub NM, Ebrahim HY, Mohyeldin MM, Orabi KY, Foudah AI, et al. Araguspongine C induces autophagic death in breast cancer cells through suppression of c-Met and HER2 receptor tyrosine kinase signaling. Marine Drugs. 2015;13:288–311. doi: 10.3390/md13010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aminin DL, Menchinskaya ES, Pisliagin EA, Silchenko AS, Avilov SA, Kalinin VI. Anticancer activity of sea cucumber triterpene glycosides. Mar Drugs. 2015;13:1202–1223. doi: 10.3390/md13031202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Walsh CJ, Luer CA, Yordy JE, Cantu T, Miedema J, Leggett SR, et al. Epigonal conditioned media from bonnethead shark, Sphyrna tiburo, induces apoptosis in a T-cell leukemia cell line, Jurkat E6-1. Mar Drugs. 2013;11:3224–3257. doi: 10.3390/md11093224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pereira DM, Correia-da-Silva G, Valentão P, Teixeira N, Andrade PB. Palmitic acid and ergosta-7,22-dien-3-ol contribute to the apoptotic effect and cell cycle arrest of an extract from Marthasterias glacialis L. in neuroblastoma cells. Mar Drugs. 2013;12:54–68. doi: 10.3390/md12010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teta R, Irollo E, Della Sala G, Pirozzi G, Mangoni A, Costantino V. Smenamides a and B, chlorinated peptide/polyketide hybrids containing a dolapyrrolidinone unit from the Caribbean sponge Smenospongia aurea. Evaluation of their role as leads in antitumor drug research. Mar Drugs. 2013;11:4451–4463. doi: 10.3390/md11114451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang X-C, Xiao X, Zhang Y-K, Talele TT, Salim AA, Chen Z-S, et al. Lamellarin O, a pyrrole alkaloid from an Australian marine sponge, Ianthella sp., reverses BCRP mediated drug resistance in cancer cells. Mar Drugs. 2014;12:3818–3837. doi: 10.3390/md12073818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Abraham I, Jain S, Wu C-P, Khanfar MA, Kuang Y, Dai C-L, et al. Marine sponge-derived sipholane triterpenoids reverse P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Biochem Pharmacol. 2010;80:1497–1506. doi: 10.1016/j.bcp.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fiorini L, Tribalat M-A, Sauvard L, Cazareth J, Lalli E, Broutin I, et al. Natural paniceins from mediterranean sponge inhibit the multidrug resistance activity of patched and increase chemotherapy efficiency on melanoma cells. Oncotarget. 2015;6:22282–22297. doi: 10.18632/oncotarget.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canals A, Arribas-Bosacoma R, Albericio F, Álvarez M, Aymamí J, Coll M. Intercalative DNA binding of the marine anticancer drug variolin B. Sci Rep. 2017;7:39680. doi: 10.1038/srep39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Choi MY, Cardenas JM, Lu D, Yu J, Stout EP, Wu RP, et al. Agelastatin A (AgA), a marine sponge derived alkaloid, inhibits Wnt/Beta-catenin signaling and selectively induces apoptosis in chronic lymphocytic leukemia independently of p53. Blood. 2011;118:1786. [Google Scholar]
- 83.Catley L, Weisberg E, Tai Y-T, Atadja P, Remiszewski S, Hideshima T, et al. NVP-LAQ824 is a potent novel histone deacetylase inhibitor with significant activity against multiple myeloma. Blood. 2003;102:2615–2622. doi: 10.1182/blood-2003-01-0233. [DOI] [PubMed] [Google Scholar]
- 84.Potts BC, Albitar MX, Anderson KC, Baritaki S, Berkers C, Bonavida B, et al. Marizomib, a proteasome inhibitor for all seasons: preclinical profile and a framework for clinical trials. Curr Cancer Drug Targets. 2011;11:254–284. doi: 10.2174/156800911794519716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hughes CC, MacMillan JB, Gaudêncio SP, Jensen PR, Fenical W. The ammosamides: structures of cell cycle modulators from a marine-derived Streptomyces species. Angew Chem Int Ed Engl. 2009;48:725–727. doi: 10.1002/anie.200804890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sagar S, Esau L, Holtermann K, Hikmawan T, Zhang G, Stingl U, et al. Induction of apoptosis in cancer cell lines by the Red Sea brine pool bacterial extracts. BMC Complement Altern Med. 2013;13:344. doi: 10.1186/1472-6882-13-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sagar S, Esau L, Hikmawan T, Antunes A, Holtermann K, Stingl U, et al. Cytotoxic and apoptotic evaluations of marine bacteria isolated from brine-seawater interface of the Red Sea. BMC Complement Altern Med. 2013;13:29. doi: 10.1186/1472-6882-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trzoss L, Fukuda T, Costa-Lotufo LV, Jimenez P, La Clair JJ, Fenical W. Seriniquinone, a selective anticancer agent, induces cell death by autophagocytosis, targeting the cancer-protective protein dermcidin. Proc Natl Acad Sci U S A. 2014;111:14687–14692. doi: 10.1073/pnas.1410932111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alvarez-Mico X, Jensen PR, Fenical W, Hughes CC. Chlorizidine, a cytotoxic 5H-pyrrolo[2,1-a]isoindol-5-one-containing alkaloid from a marine Streptomyces sp. Org Lett. 2013;15:988–991. doi: 10.1021/ol303374e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Erba E, Bergamaschi D, Ronzoni S, Faretta M, Taverna S, Bonfanti M, et al. Mode of action of thiocoraline, a natural marine compound with anti-tumour activity. Br J Cancer. 1999;80:971–980. doi: 10.1038/sj.bjc.6690451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh AV, Bandi M, Raje N, Richardson P, Palladino MA, Chauhan D, et al. A novel vascular disrupting agent plinabulin triggers JNK-mediated apoptosis and inhibits angiogenesis in multiple myeloma cells. Blood. 2011;117:5692–5700. doi: 10.1182/blood-2010-12-323857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Tao L, Liang Y, Chen L, Mi Y, Zheng L, et al. Anthracenedione derivatives as anticancer agents isolated from secondary metabolites of the mangrove endophytic fungi. Mar Drugs. 2010;8:1469–1481. doi: 10.3390/md8041469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oda T, Namikoshi M, Akano K, Kobayashi H, Honma Y, Kasahara T. Verrucarin a inhibition of MAP kinase activation in a PMA-stimulated promyelocytic leukemia cell line. Mar Drugs. 2005;3:64–73. [Google Scholar]
- 94.Gamal-Eldeen AM, Abdel-Lateff A, Okino T. Modulation of carcinogen metabolizing enzymes by chromanone a; a new chromone derivative from algicolous marine fungus Penicillium sp. Environ Toxicol Pharmacol. 2009;28:317–322. doi: 10.1016/j.etap.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 95.Wijesekara I, Zhang C, Van Ta Q, Vo T-S, Li Y-X, Kim S-K. Physcion from marine-derived fungus Microsporum sp. induces apoptosis in human cervical carcinoma HeLa cells. Microbiol Res. 2014;169:255–261. doi: 10.1016/j.micres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 96.Li Y-X, Himaya SWA, Dewapriya P, Zhang C, Kim S-K. Fumigaclavine C from a marine-derived fungus Aspergillus fumigatus induces apoptosis in MCF-7 breast cancer cells. Mar Drugs. 2013;11:5063–5086. doi: 10.3390/md11125063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen J, Wang C, Lan W, Huang C, Lin M, Wang Z, et al. Gliotoxin inhibits proliferation and induces apoptosis in colorectal cancer cells. Mar Drugs. 2015;13:6259–6273. doi: 10.3390/md13106259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stevenson CS, Capper EA, Roshak AK, Marquez B, Eichman C, Jackson JR, et al. The identification and characterization of the marine natural product scytonemin as a novel antiproliferative pharmacophore. J Pharmacol Exp Ther. 2002;303:858–866. doi: 10.1124/jpet.102.036350. [DOI] [PubMed] [Google Scholar]
- 99.Taori K, Paul VJ, Luesch H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J Am Chem Soc. 2008;130:1806–1807. doi: 10.1021/ja7110064. [DOI] [PubMed] [Google Scholar]
- 100.Moore RE. Cyclic peptides and depsipeptides from cyanobacteria: a review. J Ind Microbiol. 1996;16:134–143. doi: 10.1007/BF01570074. [DOI] [PubMed] [Google Scholar]
- 101.Fischel JL, Lemee R, Formento P, Caldani C, Moll JL, Pesando D, et al. Cell growth inhibitory effects of caulerpenyne, a sesquiterpenoid from the marine algae Caulerpa taxifolia. Anticancer Res. 1995;15:2155–2160. [PubMed] [Google Scholar]
- 102.Magarvey NA, Beck ZQ, Golakoti T, Ding Y, Huber U, Hemscheidt TK, et al. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from cyanobionts. ACS Chem Biol. 2006;1:766–779. doi: 10.1021/cb6004307. [DOI] [PubMed] [Google Scholar]
- 103.Atashrazm F, Lowenthal RM, Woods GM, Holloway AF, Dickinson JL. Fucoidan and cancer: a multifunctional molecule with anti-tumor potential. Mar Drugs. 2015;13:2327–2346. doi: 10.3390/md13042327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yende SR, Harle UN, Chaugule BB. Therapeutic potential and health benefits of Sargassum species. Pharmacogn Rev. 2014;8:1–7. doi: 10.4103/0973-7847.125514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang L, Wang P, Wang H, Li Q, Teng H, Liu Z, et al. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar Drugs. 2013;11:1961–1976. doi: 10.3390/md11061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boo H-J, Hong J-Y, Kim S-C, Kang J-I, Kim M-K, Kim E-J, et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar Drugs. 2013;11:2982–2999. doi: 10.3390/md11082982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi D, Guo S, Fan X. A new ketosteroid from red alga Acanthophora spicifera. Chin J Ocean Limnol. 2011;29:674–678. [Google Scholar]
- 108.Verdier-Pinard P, Lai JY, Yoo HD, Yu J, Marquez B, Nagle DG, et al. Structure-activity analysis of the interaction of curacin a, the potent colchicine site antimitotic agent, with tubulin and effects of analogs on the growth of MCF-7 breast cancer cells. Mol Pharmacol. 1998;53:62–76. doi: 10.1124/mol.53.1.62. [DOI] [PubMed] [Google Scholar]
- 109.Kuda T, Yano T, Matsuda N, Nishizawa M. Inhibitory effects of laminaran and low molecular alginate against the putrefactive compounds produced by intestinal microflora in-vitro and in rats. Food Chem. 2005;91:745–749. [Google Scholar]
- 110.Mei C, Zhou S, Zhu L, Ming J, Zeng F, Xu R. Antitumor effects of Laminaria extract fucoxanthin on lung cancer. Mar Drugs. 2017;15:39. doi: 10.3390/md15020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu N, Luo J, Jiang B, Wang L, Wang S, Wang C, et al. Marine bromophenol bis (2,3-Dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-integrin/FAK signaling. Mar Drugs. 2015;13:1010–1025. doi: 10.3390/md13021010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu Y, Kersten RD, Nam S-J, Lu L, Al-Suwailem AM, Zheng H, et al. Bacterial biosynthesis and maturation of the didemnin anti-cancer agents. J Am Chem Soc. 2012;134:8625–8632. doi: 10.1021/ja301735a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Park H, Tuan N, Oh J, Son Y, Hamann M, Stone R, et al. Sesterterpenoid and steroid metabolites from a deep-water Alaska sponge inhibit Wnt/β-catenin signaling in colon cancer cells. Mar Drugs. 2018;16:297. doi: 10.3390/md16090297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Park S, Yun E, Hwang I, Yoon S, Kim D-E, Kim J, et al. Ilimaquinone and ethylsmenoquinone, marine sponge metabolites, suppress the proliferation of multiple myeloma cells by down-regulating the level of β-catenin. Mar Drugs. 2014;12:3231–3244. doi: 10.3390/md12063231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sithranga Boopathy N, Kandasamy K, Subramanian M, You-Jin J. Effect of mangrove tea extract from Ceriops decandra (Griff.) Ding Hou. On salivary bacterial flora of DMBA induced hamster buccal pouch carcinoma. Indian J Microbiol. 2011;51:338–344. doi: 10.1007/s12088-011-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Firdaus M, Prihanto AA, Nurdiani R. Antioxidant and cytotoxic activity of Acanthus ilicifolius flower. Asian Pac J Trop Biomed. 2013;3:17–21. doi: 10.1016/S2221-1691(13)60017-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hameed E. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif, Saudi Arabia. European J Med Plants. 2012;2:93–112. [Google Scholar]
- 118.Thatoi H, Samantaray D, Das SK. The genus Avicennia, a pioneer group of dominant mangrove plant species with potential medicinal values: a review. Front Life Sci. 2016;9:267–291. [Google Scholar]
- 119.Jaikumar K, Md. Sheik NM, Anand D, Saravanan P. anticancer activity of Calophyllum inophyllum L., ethanolic leaf extract in MCF human breast cell lines. Int J Pharm Sci Res 2016;7:3330–3335.
- 120.Masuda T, Yonemori S, Oyama Y, Takeda Y, Tanaka T, Andoh T, et al. Evaluation of the antioxidant activity of environmental plants: activity of the leaf extracts from Seashore plants. J Agric Food Chem. 1999;47:1749–1754. doi: 10.1021/jf980864s. [DOI] [PubMed] [Google Scholar]
- 121.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 122.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 123.Fouad YA, Aanei C. Revisiting the hallmarks of cancer. Am J Cancer Res. 2017;7:1016–1036. [PMC free article] [PubMed] [Google Scholar]
- 124.Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 125.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta. 2010;1805:53–71. doi: 10.1016/j.bbcan.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 126.Fulda S, Pervaiz S. Apoptosis signaling in cancer stem cells. Int J Biochem Cell Biol. 2010;42:31–38. doi: 10.1016/j.biocel.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 127.Oliver L, Vallette FM. The role of caspases in cell death and differentiation. Drug Resist Updat. 2005;8:163–170. doi: 10.1016/j.drup.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 128.Kroemer G. Mitochondrial control of apoptosis: an introduction. Biochem Biophys Res Commun. 2003;304:433–435. doi: 10.1016/s0006-291x(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 129.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 131.Pyo JO, Nah J, Jung YK. Molecules and their functions in autophagy. Exp Mol Med. 2012;44:73–80. doi: 10.3858/emm.2012.44.2.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheetham GM. Novel protein kinases and molecular mechanisms of autoinhibition. Curr Opin Struct Biol. 2004;14:700–705. doi: 10.1016/j.sbi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 133.Skropeta D, Pastro N, Zivanovic A. Kinase inhibitors from marine sponges. Mar Drugs. 2011;9:2131–2154. doi: 10.3390/md9102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sharma PS, Sharma R, Tyagi R. Inhibitors of cyclin dependent kinases: useful targets for cancer treatment. Curr Cancer Drug Targets. 2008;8:53–75. doi: 10.2174/156800908783497131. [DOI] [PubMed] [Google Scholar]
- 135.Morgan D, Morgan DO. The cell cycle: principles of control: OUP/New Science Press; 2007.
- 136.Levitzki A, Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu Rev Biochem. 2006;75:93–109. doi: 10.1146/annurev.biochem.75.103004.142657. [DOI] [PubMed] [Google Scholar]
- 137.Carpenter G, Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- 138.Nakao Y, Fusetani N. Enzyme inhibitors from marine invertebrates. J Nat Prod. 2007;70:689–710. doi: 10.1021/np060600x. [DOI] [PubMed] [Google Scholar]
- 139.Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 140.Brown MD, Sacks DB. Protein scaffolds in MAP kinase signalling. Cell Signal. 2009;21:462–469. doi: 10.1016/j.cellsig.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kannan-Thulasiraman P, Katsoulidis E, Tallman MS, Arthur JS, Platanias LC. Activation of the mitogen- and stress-activated kinase 1 by arsenic trioxide. J Biol Chem. 2006;281:22446–22452. doi: 10.1074/jbc.M603111200. [DOI] [PubMed] [Google Scholar]
- 142.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 143.Rapisarda A, Melillo G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res. 2012;114:237–267. doi: 10.1016/B978-0-12-386503-8.00006-5. [DOI] [PubMed] [Google Scholar]
- 144.Siefert SA, Sarkar R. Matrix metalloproteinases in vascular physiology and disease. Vascular. 2012;20:210–216. doi: 10.1258/vasc.2011.201202. [DOI] [PubMed] [Google Scholar]
- 145.Yin SQ, Wang JJ, Zhang CM, Liu ZP. The development of MetAP-2 inhibitors in cancer treatment. Curr Med Chem. 2012;19:1021–1035. doi: 10.2174/092986712799320709. [DOI] [PubMed] [Google Scholar]
- 146.Bayless KJ, Johnson GA. Role of the cytoskeleton in formation and maintenance of angiogenic sprouts. J Vasc Res. 2011;48:369–385. doi: 10.1159/000324751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mottet D, Castronovo V. Histone deacetylases: anti-angiogenic targets in cancer therapy. Curr Cancer Drug Targets. 2010;10:898–913. doi: 10.2174/156800910793358014. [DOI] [PubMed] [Google Scholar]
- 148.Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–424. doi: 10.1146/annurev.med.49.1.407. [DOI] [PubMed] [Google Scholar]
- 149.Kubota Y. Tumor angiogenesis and anti-angiogenic therapy. Keio J Med. 2012;61:47–56. doi: 10.2302/kjm.61.47. [DOI] [PubMed] [Google Scholar]
- 150.Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–350. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- 151.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 152.Kung AL, Wang S, Klco JM, Kaelin WG, Livingston DM. Suppression of tumor growth through disruption of hypoxia-inducible transcription. Nat Med. 2000;6:1335–1340. doi: 10.1038/82146. [DOI] [PubMed] [Google Scholar]
- 153.Bhatnagar I, Kim S-K. Marine antitumor drugs: status, shortfalls and strategies. Mar Drugs. 2010;8:2702–2720. doi: 10.3390/md8102702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Champoux JJ. DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem. 2001;70:369–413. doi: 10.1146/annurev.biochem.70.1.369. [DOI] [PubMed] [Google Scholar]
- 155.Green DR, Levine B. To be or not to be? How selective autophagy and cell death govern cell fate. Cell. 2014;157:65–75. doi: 10.1016/j.cell.2014.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mukhtar E, Adhami VM, Khan N, Mukhtar H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr Drug Targets. 2012;13:1831–1841. doi: 10.2174/138945012804545489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morselli E, Galluzzi L, Kepp O, Vicencio JM, Criollo A, Maiuri MC, et al. Anti- and pro-tumor functions of autophagy. Biochim Biophys Acta. 2009;1793:1524–1532. doi: 10.1016/j.bbamcr.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 158.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 159.Pina IC, Gautschi JT, Wang GY, Sanders ML, Schmitz FJ, France D, et al. Psammaplins from the sponge Pseudoceratina purpurea: inhibition of both histone deacetylase and DNA methyltransferase. J Org Chem. 2003;68(10):3866–3873. doi: 10.1021/jo034248t. [DOI] [PubMed] [Google Scholar]
- 160.Gu W, Cueto M, Jensen PR, Fenical W, Silverman RB. Microsporins a and B: new histone deacetylase inhibitors from the marine-derived fungus Microsporum cf. gypseum and the solid-phase synthesis of microsporin a. Tetrahedron. 2007;63(28):6535–6541. [Google Scholar]
- 161.Nakao Y, Yoshida S, Matsunaga S, Shindoh N, Terada Y, Nagai K, et al. Azumamides A–E: histone deacetylase inhibitory cyclic tetrapeptides from the marine sponge Mycale izuensis. Angew Chem Int Ed. 2006;45(45):7553–7557. doi: 10.1002/anie.200602047. [DOI] [PubMed] [Google Scholar]
- 162.Liu Y, Salvador LA, Byeon S, Ying Y, Kwan JC, Law BK, et al. Anticolon cancer activity of largazole, a marine-derived tunable histone deacetylase inhibitor. J Pharmacol Exp Ther. 2010;335(2):351–361. doi: 10.1124/jpet.110.172387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu T, Kuljaca S, Tee A, Marshall GM. Histone deacetylase inhibitors: multifunctional anticancer agents. Cancer Treat Rev. 2006;32(3):157–165. doi: 10.1016/j.ctrv.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 164.Tabatabai R, Linhares Y, Bolos D, Mita M, Mita A. Targeting the Wnt pathway in cancer: a review of novel therapeutics. Target Oncol. 2017;12:623–641. doi: 10.1007/s11523-017-0507-4. [DOI] [PubMed] [Google Scholar]
- 165.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 166.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 167.Galluzzi L, Spranger S, Fuchs E, López-Soto A. WNT signaling in cancer immunosurveillance. Trends Cell Biol. 2018;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang B, Tian T, Kalland KH, Ke X, Qu Y. Targeting Wnt/beta-catenin signaling for cancer immunotherapy. Trends Pharmacol Sci. 2018;39:648–658. doi: 10.1016/j.tips.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 169.Sherwood V. WNT signaling: an emerging mediator of cancer cell metabolism? Mol Cell Biol. 2015;35:2–10. doi: 10.1128/MCB.00992-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Nigam M, Ranjan V, Srivastava S, Sharma R, Balapure AK. Centchroman induces G0/G1 arrest and caspase-dependent apoptosis involving mitochondrial membrane depolarization in MCF-7 and MDA MB-231 human breast cancer cells. Life Sci. 2008;82:577–590. doi: 10.1016/j.lfs.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 171.Nigam M, Singh N, Ranjan V, Zaidi D, Sharma R, Nigam D, et al. Centchroman mediated apoptosis involves cross-talk between extrinsic/intrinsic pathways and oxidative regulation. Life Sci. 2010;87:750–758. doi: 10.1016/j.lfs.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 172.Singh N, Nigam M, Ranjan V, Sharma R, Balapure AK, Rath SK. Caspase mediated enhanced apoptotic action of cyclophosphamide- and resveratrol-treated MCF-7 cells. J Pharmacol Sci. 2009;109:473–485. doi: 10.1254/jphs.08173fp. [DOI] [PubMed] [Google Scholar]
- 173.Singh N, Nigam M, Ranjan V, Zaidi D, Garg VK, Sharma S, et al. Resveratrol as an adjunct therapy in cyclophosphamide-treated MCF-7 cells and breast tumor explants. Cancer Sci. 2011;102:1059–1067. doi: 10.1111/j.1349-7006.2011.01893.x. [DOI] [PubMed] [Google Scholar]
- 174.Mishra AP, Salehi B, Sharifi-Rad M, Pezzani R, Kobarfard F, Sharifi-Rad J, et al. Programmed cell death, from a cancer perspective: an overview. Mol Diagn Ther. 2018;22:281–295. doi: 10.1007/s40291-018-0329-9. [DOI] [PubMed] [Google Scholar]
- 175.Kong D, Yamori T, Kobayashi M, Duan H. Antiproliferative and antiangiogenic activities of Smenospongine, a marine sponge sesquiterpene aminoquinone. Mar Drugs. 2011;9:154–161. doi: 10.3390/md9020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Grienke U, Silke J, Tasdemir D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014;142:48–60. doi: 10.1016/j.foodchem.2013.07.027. [DOI] [PubMed] [Google Scholar]
- 177.Tornero V, Hanke G. Chemical contaminants entering the marine environment from sea-based sources: a review with a focus on European seas. Mar Pollut Bull. 2016;112:17–38. doi: 10.1016/j.marpolbul.2016.06.091. [DOI] [PubMed] [Google Scholar]