Abstract
Objective
Cognitive impairments in type 1 diabetes may result from hyperglycemia‐associated cerebral microangiopathy. We aimed to identify cerebral microangiopathy and skin microvascular dysfunction—as a surrogate marker for generalized microvascular function—as predictors of cognitive performance over time.
Methods
In this prospective cohort study, 25 type 1 diabetes patients with proliferative retinopathy and 25 matched healthy controls underwent neurocognitive testing at baseline and after follow‐up (3.8 ± 0.8 years). At baseline, 1.5‐T cerebral magnetic resonance imaging was used to detect WML and cerebral microbleeds. Skin capillary perfusion was assessed by means of capillary microscopy.
Results
In type 1 diabetes patients, but not in healthy controls, the presence of WML (ß = −0.419; P = 0.037) as well as lower skin capillary perfusion (baseline: ß = 0.753; P < 0.001; peak hyperemia: ß = 0.743; P = 0.001; venous occlusion: ß = 0.675; P = 0.003; capillary recruitment: ß = 0.549; P = 0.022) at baseline was associated with lower cognitive performance over time, independent of age, sex, HbA1c, and severe hypoglycemia. The relationship between WML and lower cognitive performance was significantly reduced after adjusting for capillary perfusion.
Conclusions
These data fit the hypothesis that cerebral microangiopathy is a manifestation of generalized microvascular dysfunction, leading to lower cognitive performance.
Keywords: cerebral microbleeds, cognitive performance, skin perfusion, type 1 diabetes, white matter lesions
Abbreviations
- ACE
angiotensin‐converting enzyme
- Baseline
baseline capillary density
- BMI
body mass index
- cMB
cerebral microbleeds
- CR
capillary recruitment
- cSVD
cerebral small vessel disease
- DCCT
Diabetes Control and Complications Trial
- EDIC
Epidemiology of Diabetes Interventions and Complications
- FLAIR
fluid‐attenuated inversion recovery
- HbA1c
glycated hemoglobin
- IQ
intelligence quotient
- MRI
magnetic resonance imaging
- Peak
peak hyperemia capillary density
- RCI
reliable change index
- SD
standard deviation
- T1DM
type 1 diabetes
- VO
venous occlusion capillary density
- WML
white matter lesions
1. INTRODUCTION
Type 1 diabetes is a complex endocrine disease, which, in case of poor glycemic control, can lead to long‐term microvascular complications, affecting kidneys, peripheral and central nervous system, and eyes.1 The brain has also been recognized as a key organ that can be affected by long‐standing type 1 diabetes.2 Cross‐sectional and longitudinal studies have shown that type 1 diabetes is associated with mild‐to‐moderate impairments in multiple neurocognitive domains compared with healthy controls.3, 4
The pathophysiology underlying these cognitive decrements in type 1 diabetes has not yet been fully elucidated. Risk factors for cognitive impairment include longer duration and early onset of diabetes as well as poor glycemic control, which suggests that hyperglycemia‐induced damage is a pathophysiological determinant.5, 6 Additionally, the presence of diabetic microangiopathy—that is, retinopathy, nephropathy, and neuropathy—was cross‐sectionally associated with lower performance on several neuropsychological tests 3, 7, 8 and longitudinally with mild, but clinically relevant cognitive decline independent of lifetime HbA1c in the DCCT1 and its epidemiological follow‐up, the EDIC trial.6
In type 1 diabetes, diabetic microangiopathy has been proposed to reflect a state of generalized microvascular dysfunction, including in the brain, which may lead to cSVD. On brain MRI, cSVD presents with ischemic WML, lacunar infarcts, and non‐lobar cMB.9, 10 The link between cSVD and peripheral microvascular complications is supported by data from our own group, which show higher prevalence of cMB in type 1 diabetes patients with proliferative retinopathy compared with those without.11 In addition, diabetic retinopathy and neuropathy have been associated with the presence, volume, and severity of WML.8, 12 We therefore hypothesize that generalized microvascular dysfunction, manifest by impaired microvascular function of the skin, and cSVD may correlate with decline in cognitive function over time.
In this prospective cohort study, we aimed to identify cerebral microangiopathy and skin capillary perfusion—as a surrogate marker of generalized microvascular function—as independent predictors of cognitive performance, controlling for possible confounding factors including HbA1c, diabetes duration, and the number of severe hypoglycemic events.
2. MATERIALS AND METHODS
This prospective cohort study was part of a larger cross‐sectional study conducted at the VU University Medical Centre,13 in which we assessed the effects of type 1 diabetes and concomitant proliferative retinopathy on cognition, brain volume, and functional and structural connectivity. A randomly selected subgroup of 25 type 1 diabetes patients with proliferative retinopathy and 25 healthy controls—matched for age, gender, BMI, and IQ—were invited to return for follow‐up measurements between May 2007 and September 2009. Inclusion criteria at baseline were as follows: age 18‐56 years, right‐handedness, and, for the type 1 diabetes patients, a diabetes duration of at least 10 years. Exclusion criteria at both time points were psychiatric comorbidity, insufficient visual acuity (below 0.3) to perform neuropsychological tests, brain trauma, previous coma unrelated to hypoglycemia, alcohol consumption (men: >21 units a week; women: >14 units a week) and drug use, use of centrally acting medication, MRI contraindications, and, for the healthy controls, hypertension at baseline. At baseline and follow‐up, all participants were subjected to evaluation of cognitive function and brain MRI. Skin microvascular function was assessed at baseline by means of nail fold capillary microscopy. To be able to perform the neuropsychological tests, all participants were required to have normal or corrected to normal vision. Additionally, none of the patients had central proliferative retinopathy, thus not affecting the central focus area of vision. This study was conducted in accordance with the Declaration of Helsinki 201314 and approved by the medical ethics committee of the VU University Medical Centre. All participants signed written informed consent at baseline and during follow‐up.
2.1. Justification of sample size and group selection
This prospective cohort study is a randomly selected subsample of a larger cross‐sectional study, in which type 1 diabetes patients with and without retinopathy and healthy controls were included. Due to funding limitations, we were able to include 50 of the 153 participants that were included at baseline. We chose to include two groups of 25 participants, instead of three groups of approximately 16 participants to increase the power of the study. The groups were selected based on the largest expected differences, taking into consideration that these results cannot be translated to type 1 diabetes patients in general.
2.2. Cognitive functioning
All participants underwent a detailed neuropsychological assessment covering the domains of memory, information‐processing speed, executive functions, attention, motor, and psychomotor speed. The tests were described previously.15 General cognitive ability was constructed by averaging results with respect to the above‐mentioned six domains. Raw scores were transformed into z‐scores based on the mean and SD values from healthy controls of the larger cross‐sectional study and inversed if necessary so that higher z‐scores indicated better performance. To quantify change in cognitive performance over time, the RCI was computed from baseline and follow‐up data by the following formula: ((Xfollow‐up–Xbaseline)–(mean‐controls follow‐up–mean‐controls baseline))/standard deviation delta‐score controls. Thus, for each test the mean delta‐score of the controls was subtracted from the raw subject's delta‐score and divided by the standard deviation of the delta‐score of the controls.16 To rule out confounding as a result of hypo‐ or hyperglycemia, glucose levels had to be between 4 and 15 mmol/L before and during neuropsychological testing. When outside of this range, patients were instructed to eat or inject insulin and testing was postponed for 30 min until blood glucose levels were within the required range.17
2.3. Brain MRI
MRI scanning was performed on a 1.5‐T magnetic resonance system (Siemens‐Sonata, Erlangen, Germany). Further details on the magnetic resonance acquisition protocol have been described previously.13 Vascular WML were assessed using T2 FLAIR and cerebral microbleeds using T2* susceptibility weighted imaging. All images were rated by an experienced neuroradiologist, who was blinded to any clinical information. Both WML and cMB were scored as present or not present. The Fazekas scale is often used to indicate severity of WML18; however, in this study all but one subject had a Fazekas score of 1.
2.4. Skin microvascular function
Peripheral microvascular function was assessed by skin capillary microscopy as described previously.19 Briefly, nail fold capillaries in the dorsal skin of the third finger were visualized by a capillary microscope. Baseline capillary density (baseline) was defined as the number of continuously erythrocyte‐perfused capillaries per square millimeter. Capillary density during peak reactive hyperemia (peak) was counted after 4 min of arterial occlusion. Maximal capillary density was assessed during VO. CR was calculated as the absolute and relative increase in capillary density from baseline to capillary density during peak reactive hyperemia.
2.5. Statistical analyses
Data were analyzed with SPSS version 22.0 (IBM‐SPSS, Chicago, IL). Data are presented as mean ± SD, median[range] (not normally distributed), or raw numbers with percentages (categorical data). We assessed differences between patients and healthy controls using the unpaired t test or Mann–Whitney U test for continuous variables, depending on the data distribution, and the X² test for categorical data. Since the sample size of this study limits the number of variables allowed in our multiple regression analysis, we first performed several simple regression analysis with change in general cognition as dependent variable and the most evident confounding factors based on current literature (ie, BMI, smoking, hypertension, HbA1c, diabetes duration, and severe hypoglycemic events in the past) as independent variables.20, 21 Subsequently, multiple linear regression models were used to assess the significance of covariate‐adjusted associations between the change in general cognitive ability (dependent variable) and variables of cerebral microangiopathy and skin microvascular function (predictors/independent variables). Age and sex (model 2) and variables demonstrating associations in the simple regression analysis with a P‐value <0.1 (model 3) qualified as independent variables for inclusion into the multiple regression analyses. Next, we added capillary perfusion variables as independent variable to the regression models with WML as predictor and vice versa. Missing data were excluded pairwise. A two‐sided P‐value <0.05 was considered statistically significant. We performed a Cook's distance analysis to examine the influence of two apparent outliers based on the scatter plot. Finally, we carried out mediation analyses to examine whether the presence of WML was a mediator between skin perfusion and the change in general cognitive ability. This analysis was performed in STATA version 13SE (StatCorp LLC, College Station, TX) using the bootstrapping method according to Preacher and Hayes.22
3. RESULTS
3.1. Participants
Table 1 shows the characteristics of type 1 diabetes patients and healthy controls at baseline. There was no difference from baseline to follow‐up between type 1 diabetes patients and healthy controls (3.56 ± 0.65 and 3.94 ± 0.91 years, respectively; P = 0.098). Owing to the selection method, there also were no significant differences between the two groups in age (type 1 diabetes: 46.1 ± 6.3 years; controls: 44.3 ± 8.5 years; P = 0.410), sex (type 1 diabetes: 40% male; controls: 52% male; P = 0.395), IQ (type 1 diabetes: 112.3 ± 12.7; controls: 109.4 ± 13.1; P = 0.433), and BMI (type 1 diabetes: 26.2 ± 4.9 kg/m2; controls: 25.1 ± 2.9 kg/m2; P = 0.307). In addition, we also did not detect significant differences in diastolic blood pressure (type 1 diabetes: 75.4 ± 7.8 mmHg; controls: 78.9 ± 6.2 mmHg; P = 0.088), systolic blood pressure (type 1 diabetes: 133.0 [107.0‐151.5] mmHg; controls: 128.0 [101.0‐139.0] mmHg; P = 0.137), smoking (yes) (type 1 diabetes: 3 (12%); controls: 2 (8%); P = 0.637), and cholesterol levels (type 1 diabetes: 4.4 ± 0.8 mmol/L; controls: 4.8 ± 0.9 mmol/L; P = 0.120). By definition, HbA1c was higher in type 1 diabetes patients (7.9 ± 1.0%) than in healthy controls (5.3 ± 0.3%; P < 0.001).
Table 1.
Baseline characteristics
| Type 1 diabetes (n = 25) | Controls (n = 25) | P‐value | |
|---|---|---|---|
| Age (years) | 46.1 ± 6.3 | 44.3 ± 8.5 | 0.409 |
| Sex (m/f(%male)) | 10/15 (40%) | 13/12 (52%) | 0.395 |
| Estimated IQ | 112.3 ± 12.7 | 109.4 ± 13.1 | 0.433 |
| Education levela | 6 [2‐8] | 6 [4‐8] | 0.204 |
| BMI (kg/m²) | 26.2 ± 4.9 | 25.1 ± 2.9 | 0.307 |
| Diastolic blood pressure (mmHg) | 75.4 ± 7.8 | 78.9 ± 6.2 | 0.088 |
| Systolic blood pressure (mmHg) | 133.0 [107.0‐151.5] | 128.0 [101.0‐139.0] | 0.137 |
| Hypertensionb (n(%)) | 11 (44%) | ‐ | ‐ |
| Smoking (n(%)) | 3 (12%) | 2 (8%) | 0.637 |
| Total cholesterol (mmol/L) | 4.4 ± 0.8 | 4.8 ± 0.9 | 0.120 |
| HbA1c (mmol/mol) | 63 ± 10.9 | 34 ± 3.3 | <0.001 |
| HbA1c (%) | 7.9 ± 1.0 | 5.3 ± 0.3 | <0.001 |
| Diabetes duration (years) | 34.7 ± 8.1 | ‐ | ‐ |
| Diabetes early onsetc (n(%)) | 8 (32%) | ‐ | ‐ |
| Severe hypoglycemic eventsd | 1 [0‐25] | ‐ | ‐ |
| Albuminuria (n(%))e | 5 (20%) | ‐ | ‐ |
| Peripheral neuropathy (n(%))f | 10 (40%) | ‐ | ‐ |
Data are presented as mean ± SD, median[range] or absolute number(%). BMI, body mass index; HbA1c , glycated hemoglobin; IQ, intelligence quotient; m/f, male/female. Bold values are statistically significant results (P < 0.05).
Education level was based on a Dutch scoring system ranging from 1 to 8, One indicates unfinished primary school, and 8 indicates a completed university study at master's level.
Hypertension was defined as a systolic blood pressure of 140 mmHg or above, a diastolic blood pressure of 90 mmHg or above or the use of antihypertensive drugs.
Early onset of type 1 diabetes was defined as diabetes onset before the age of seven.
Severe hypoglycemic events were self‐reported.
Albuminuria was defined as an albumin: creatinine ratio >2.5 mg/mmol for men and >3.5 mg/mmol for women and assessed with 24‐hour urine sampling.
Peripheral neuropathy was based on medical records or, in case they were not available, based on self‐report.
3.2. Neurocognitive functioning
At baseline, type 1 diabetes patients had significantly lower general cognitive ability (z‐score) relative to healthy controls (type 1 diabetes: −0.3830 ± 0.45; controls: −0.0030 ± 0.36; P = 0.002), which was driven by significantly lower information‐processing speed (type 1 diabetes: −0.7622 ± 0.84; controls: −0.0002 ± 0.56; P < 0.001) and motor speed (type 1 diabetes: −0.4928 ± 0.91; controls: −0.0012 ± 0.81; P = 0.048). At follow‐up, general cognitive ability did not change significantly in both the patient and control group. The reliable change index of executive function was significantly lower in the type 1 diabetes patients (−0.337 ± 0.53) compared with the healthy controls (−0.003 ± 0.34; P = 0.010). These data are shown in Table 2 and were published previously 23). We have measured plasma glucose levels before neurocognitive testing. There were no significant differences between these measurements (baseline: 7.65 ± 3.75 versus follow‐up: 8.29 ± 3.44; P = 0.559).
Table 2.
Baseline and reliable change index of neurocognitive function
| Baseline (z‐scores) | Reliable change index | |||||
|---|---|---|---|---|---|---|
| Type 1 diabetes (n = 25) | Controls (n = 25) | P‐value | Type 1 diabetes (n = 25) | Controls (n = 25) | P‐value | |
| General cognitive ability | −0.383 ± 0.45 | −0.003 ± 0.36 | 0.002 | 0.045 ± 0.32 | 0.001 ± 0.19 | 0.550 |
| Memory | −0.353 ± 0.64 | −0.000 ± 0.62 | 0.052 | 0.160 ± 0.44 | 0.000 ± 0.50 | 0.233 |
| Information‐processing speed | −0.762 ± 0.84 | −0.000 ± 0.56 | <0.001 | 0.110 ± 0.66 | 0.000 ± 0.52 | 0.516 |
| Executive function | 0.056 ± 0.57 | −0.004 ± 0.45 | 0.684 | −0.337 ± 0.53 | −0.003 ± 0.34 | 0.010 |
| Attention | −0.272 ± 0.93 | 0.000 ± 0.75 | 0.267 | 0.024 ± 0.74 | 0.000 ± 0.79 | 0.915 |
| Motor speed | −0.493 ± 0.91 | 0.001 ± 0.81 | 0.048 | 0.144 ± 0.69 | 0.000 ± 0.61 | 0.439 |
| Psychomotor speed | −0.473 ± 0.80 | 0.000 ± 0.10 | 0.071 | 0.169 ± 0.77 | 0.000 ± 1.00 | 0.506 |
Data are presented as mean ± SD. Reliable change index = to quantify change in cognitive performance over time the reliable change index was computed from baseline and follow‐up data by the following formula: ((Xfollow‐up−Xbaseline)−(mean‐controls follow‐up−mean‐controls baseline))/standard deviation delta‐score controls. Bold values are statistically significant results (P < 0.05).
3.3. White matter lesions and cerebral microbleeds
At baseline, the presence of WML (type 1 diabetes: 6 (24%); controls: 6 (24%); P = 0.999) and cMB (type 1 diabetes: 5 (20%); controls: 4 (17.4%); P = 0.941) was not significantly different between type 1 diabetes patients and healthy controls in (Table 3).
Table 3.
Baseline parameters of cerebral microangiopathy and capillary perfusion
| Cerebral microangiopathy | Type 1 diabetes (n = 25) | Controls (n = 25) | P‐value |
|---|---|---|---|
| CMB (n(%))—present yes/no | 5 (20.0%) | 4 (17.4%) | 0.941 |
| WML (n(%))—present yes/no | 6 (24.0%) | 6 (24.0%) | 0.999 |
| Skin capillary densitya | Type 1 diabetes (n = 17) | Controls (n = 20) | |
|---|---|---|---|
| Baseline perfusion (n/mm²) | 45.0 ± 7.7 | 50.6 ± 11.1 | 0.087 |
| Peak perfusion (n/mm²) | 63.7 ± 13.6 | 70.4 ± 19.2 | 0.238 |
| Venous occlusion (n/mm²) | 65.2 ± 13.3 | 70.6 ± 18.9 | 0.334 |
| Capillary recruitment (absolute values) | 18.6 ± 7.9 | 19.7 ± 11.3 | 0.745 |
| Capillary recruitment (%) | 40.7 ± 16.1 | 38.4 ± 17.6 | 0.685 |
Data are presented as mean ± SD or number (percentage). CMB, cerebral microbleeds; CR, capillary recruitment (ie, increase of capillaries in n/mm² from baseline to peak perfusion); WML, white matter lesions.
13 out of 50 perfusion measurements were excluded based on improper quality of the video microscopy image.
3.4. Skin microvascular function
At baseline, we did not detect significant differences between type 1 diabetes patients and healthy controls in skin capillary perfusion: that is, baseline (type 1 diabetes 45.0 ± 7.7; controls: 50.6 ± 11.1; P = 0.087), peak (type 1 diabetes 63.7 ± 13.6; controls: 70.4 ± 19.2; P = 0.238), and VO (type 1 diabetes 65.2 ± 13.3; controls: 70.6 ± 18.9; P = 0.334) capillary density, as well as absolute CR (type 1 diabetes 18.6 ± 7.9; controls: 19.7 ± 11.3; P = 0.745) and CR percentage (type 1 diabetes 40.7 ± 16.1; controls: 38.4 ± 17.6; P = 0.685) (Table 3).
3.5. Simple regression analysis
A simple regression analysis with general cognitive ability (the mean of all tests) as dependent variable and BMI, smoking, hypertension, HbA1c, diabetes duration, early onset of diabetes, and severe hypoglycemic events in the past (self‐reported) as independent variables was performed to detect possible confounding factors. With a significance level of P < 0.100, HbA1c and the number of severe hypoglycemic events in the past were identified as possible confounding factors and were subsequently added to the multiple regression model (model 3).
3.6. Multiple regression analysis
In type 1 diabetes patients, a significant association was detected between poorer general cognitive ability over time and presence of WML (ß = −0.419; P = 0.037), as well as with lower baseline capillary density (ß = 0.753; P < 0.001), peak hyperemia capillary density (ß = 0.743; P = 0.001), venous occlusion capillary density (ß = 0.675; P = 0.003), and absolute capillary recruitment (ß = 0.549; P = 0.022) at baseline (Figure 1). These associations were independent of age, sex (model 2), and HbA1c and severe hypoglycemic events (model 3) and were not present in the control population (Table S4). There was no relationship between the presence of cMB and general cognitive ability in both type 1 diabetic patients and healthy controls. The presence of WML was not associated with one of the parameters of skin capillary perfusion (baseline: ß = −0.319; P = 0.213, peak: ß = −0.168; P = 0.519, VO: ß = −0.294; P = 0.252, CR absolute: ß = 0.020; P = 0.938, and CR percentage: ß = 0.117; P = 0.656). However, the relationship between WML and general cognitive ability decreased significantly (β change > 10%) and was no longer significant when combined with baseline (β = −0.184; P = 0.320), peak (β = −0.289; P = 0.100), and VO (β = −0.227; P = 0.269) capillary density. Adding WML to the regression analysis only slightly (<10%) changed the associations between capillary perfusion and general cognitive ability (baseline: β = 0.694; P = 0.002; peak: β = 0.695; P = 0.001; VO: β = 0.608; P = 0.008, and CR (abs) β = 0.558; P = 0.012). Cook's distance analysis for detecting influential cases showed no observations above 1. Further analysis of the six independent cognitive domains demonstrated in type 1 diabetes patients and controls that the presence of WML was significantly associated with decline in motor speed (type 1 diabetes: ß = −0.399; P = 0.048; controls: ß = −0.477; P = 0.025) (Table S5) (Figure 1), independent of age, sex, HbA1c, and (for type 1 diabetes patients only) severe hypoglycemic events. Furthermore, in type 1 diabetes patients, lower baseline capillary density (ß = 0.743; P = 0.001) and lower peak capillary density (ß = 0.703; P = 0.002) were associated with decline in attention (Table S6) (Figure 1), independent of sex, age, HbA1c, and severe hypoglycemia. The significant associations between changes in attention with VO (ß = 0.670; P = 0.003) and absolute CR (ß = 0.489; P = 0.046) disappeared after correcting for age and sex (VO: ß = 0.667; P = 0.052 and absolute CR: ß = 0.316; P = 0.279) (Table S6). Next, we performed mediation analyses, with the change in general cognitive ability as outcome variable, skin perfusion parameters as independent variables, and WML as mediator, to assess whether WML (partly) mediated the effect of skin perfusion on the change in general cognitive ability. In type 1 diabetic patients, the total effect of skin perfusion (baseline: B = 0.030; P < 0.001, peak: B = 0.017; P = 0.001, VO: B = 0.015; P = 0.003) was not mediated by WML, indicated by the non‐significant indirect coefficients (baseline: B = 0.011 P = 0.564; peak: B = 0.005 P = 0.806; VO: B = 0.008 P = 0.489).
Figure 1.
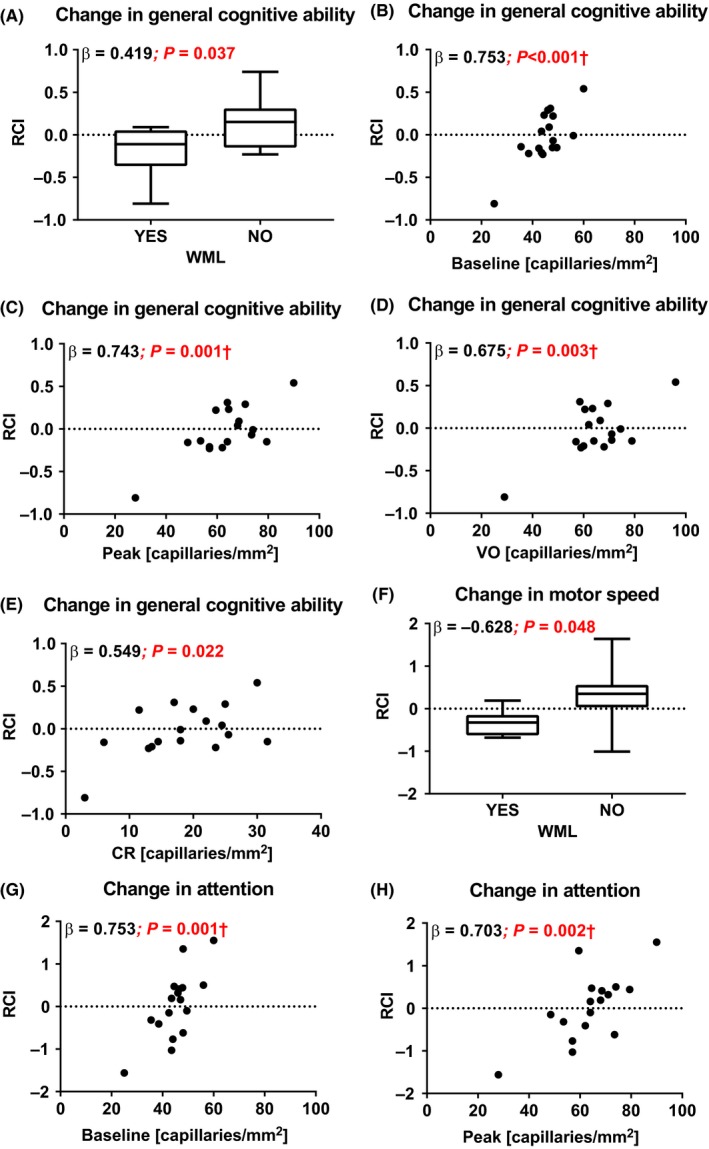
Correlations between RCI of general cognitive ability with (A) WML and nail fold capillary density during (B) baseline capillary density (baseline), (C) peak hyperemia (peak), (D) VO, and (E) absolute capillary recruitment (CR) in type 1 diabetic patients. And, correlations between RCI of motor speed with WML (F) and the RCI of attention with nail fold capillary density during baseline (G) and peak (H) in type 1 diabetic patients. †Cook's distance analysis below 1
4. DISCUSSION
In the present longitudinal study, we aimed to identify cerebral microangiopathy and skin capillary perfusion—as a surrogate marker for generalized microvascular function—as predictors of cognitive performance. We demonstrated that, in type 1 diabetes patients with proliferative retinopathy, the presence of WML and lower skin capillary perfusion at baseline was associated with lower performance in general cognitive ability over time, independent of age, sex, HbA1c, and severe hypoglycemic events. The association between WML and lower performance in general cognitive ability is driven by lower performance in motor speed, whereas lower capillary perfusion is related to lower performance in the attention domain. In contrast, in the healthy controls these associations were not found. These data suggest a possible influence of WML and impaired microvascular function on cognitive performance in type 1 diabetes patients. In addition, we showed that the relationship between WML and lower cognitive performance was significantly reduced when capillary perfusion variables were added to the regression model, suggesting that generalized microvascular dysfunction may underlie WML‐associated lower cognitive performance.
The etiology of cerebral WML is not yet fully understood. A multifactorial etiology is presumed, including but not limited to impairment of the blood‐brain barrier, ischemia, hypoxia, immune activation, and altered cell metabolic pathways.24 Previous cross‐sectional studies on the influence of WML on cognitive performance have shown correlations between WML severity (ie, Fazekas score) and location with (subjective) cognitive failures in non‐diabetic populations.25, 26 Furthermore, in type 1 diabetes patients, higher WML volume was associated with lower information‐processing speed.12 It has been shown previously that patients with childhood‐onset type 1 diabetes have more severe WML compared to healthy controls.12 In our study, the presence of WML was similar in type 1 diabetes patients and healthy controls at baseline. This was unexpected, however consistent with a previous study with a larger sample size.27 Study differences may be explained by variations in baseline characteristics such as age, diabetes duration, and the presence of early‐onset diabetes. Despite a similar prevalence in WML, we could only detect a relationship between the presence of WML at baseline and changes in general cognitive ability in the type 1 diabetes patients and not in the healthy controls. This suggests that WML do not have an effect on cognition in a healthy population with no other abnormalities and enough cognitive reserve capacity, whereas in a population with an underlying disease such as T1DM, WML may contribute to lower cognitive performance. Then again, when analyzing separate neurocognitive domains, the association between changes in motor speed and WML was found in both type 1 diabetes patients and healthy controls. The idea of a cognitive reserve capacity, which determines whether people experience cognitive decline or not has been postulated before.28 In this study, we included middle‐aged type 1 diabetes patients and control subjects, which we followed over a relatively short follow‐up period. In this period, we did not detect a mean difference in general cognitive ability. We speculate that in older subjects we would most likely find more WML, more cMB as well as more pronounced microvascular dysfunction in both control subjects and type 1 diabetic patients, since aging itself is known to be an important contributor to both (skin) microvascular dysfunction29 and cSVD.28 This may lead to more pronounced effects of general cognitive ability, and perhaps more pronounced differences between healthy subjects and type 1 diabetic patients.
Diabetes is known to accelerate microvascular aging.30 Mechanisms leading to the impairment of microcirculation in diabetes are extensive, including increased polyol pathway flux, enhanced formation of glycation end products (AGEs), abnormal activation of signaling cascades such as protein kinase C and increased hexosamine pathway flux.31, 32 These processes result from hyperglycemia‐induced overproduction of reactive oxygen species by the mitochondrial electron transport chain.32, 33, 34 These alterations in the vasculature lead to vascular leakage, a pro‐inflammatory, pro‐thrombotic and more vasoconstrictive state, and are involved in the development of both macro‐ and microvascular complications of diabetes. Skin microvascular function measurements are easy, non‐invasive, and fast and are considered a representative model for generalized microvascular (dys)function,19 including microvascular (dys)function in the brain. Several studies have demonstrated abnormalities in peripheral35, 36, 37 and cerebral38, 39, 40 microvascular function in type 1 diabetes patients. In the present study, we were unable to detect significant differences in baseline skin microvascular function, yet baseline and peak hyperemia capillary density are lower in the type 1 diabetes patients, indicating a possible power problem. Indeed, a previous analysis performed by us in a larger sample showed a trend across groups toward lower baseline capillary function in patients with type 1 diabetes compared to controls (type 1 diabetes with retinopathy: 45 ± 7 capillaries/mm2; type 1 diabetes patients without retinopathy: 46 ± 9 capillaries/mm2; healthy controls: 48 ± 10 n/mm2; P = 0.05). Similar results were shown for capillary density after arterial occlusion (peak reactive hyperemia).11 We therefore assume that the differences we detected in this study are real, but not reaching statistical significance due to our small sample size.
The treatment of type 1 diabetes is based on the balance between lowering HbA1c levels, without increasing (the risk of) hypoglycemic events. In our study, both HbA1c and severe hypoglycemic events were associated with cognitive performance over time, but neither influenced the relationship between WMLs and skin capillary perfusion with cognitive performance over time in the multiple regression model. The relationship between hypoglycemic episodes and cognitive dysfunction in middle‐aged type 1 diabetes is less evident than the relationship between hyperglycemia‐related damage on cognitive dysfunction. Retrospective studies in adult patients with type 1 diabetes have suggested an association between a history of recurrent severe hypoglycemia and a modest‐to‐severe degree of cognitive impairment.41 However, large prospective studies have failed to confirm this association.5, 41, 42 These contradictory results concerning the relationship between hypoglycemia and cognitive decline in middle‐aged patients with type 1 diabetes may be partially explained by the positive relationship between the frequency of hypoglycemic episodes and glycemic control (lower HbA1c), of which the latter improves cognitive function.
In this study, lower skin microvascular function at baseline was associated with lower cognitive performance over time in type 1 diabetes patients. Furthermore, the association between the presence of WML and lower performance in general cognitive ability over time was significantly reduced to non‐significant levels when adjusting for baseline capillary perfusion. Interestingly, we did not detect a correlation between skin microvascular function and cognition when analyzing our baseline data, even though the sample size of the cross‐sectional study was larger. Using longitudinal data has the great advantage of being less hampered by inter‐individual differences, which may have revealed these correlations. Nonetheless, we should be aware that, in this subsample, two data points have a large influence on the detected associations, despite the fact that they are not indicated as influential outliers by Cook's distance analyses. Theoretically, there is ground for a causal mechanism that links microvascular dysfunction to lower cognitive performance. Maintenance of adequate cerebral perfusion is vital for the preservation of normal brain function, since the brain has no buffer for nutrients and oxygen and relies exclusively on perfusion to meet neuronal metabolic demand.43 Cerebral autoregulation, including myogenic responses to changes in blood pressure, is an important mechanism for maintaining stable cerebral blood flow and to prevent hypoxia, hypo‐ and hypercapnia.43, 44, 45 In addition to myogenic responses, cerebral perfusion also depends on microvascular endothelial function. Endothelium‐dependent NO production is a key contributor of moment‐to‐moment adjustment of regional cerebral perfusion to changes in neuronal activity.43 Lower peak hyperemia and lower capillary recruitment of the skin could translate to both loss of this myogenic cerebral autoregulation and endothelium‐dependent microvascular function. Loss of these functions can potentially reduce oxygen delivery and alter neuronal activation and may therefore have detrimental effects on the brain. Indeed, in type 1 diabetes patient total gray matter cerebral blood flow is reduced 39, 46 and regional cerebral hemodynamic response to incremental exercise is blunted compared with control subjects.47 Furthermore, in animal models of diabetes, improvement of cerebral blood flow by chronic treatment with an angiotensin‐converting enzyme inhibitor (ACE‐inhibitor) was found to be associated with improvement of cognitive function.48
Limitations of our study design have to be considered, including the small sample size and observational character of the study. The small sample size made it impossible to correct for all possible confounding factors. In studies using MRI, this is often the case, since MRI techniques are expensive and time‐consuming. We therefore chose several confounding factors based on current literature 20, 21 and performed a simple regression analysis to identify variables for the multiple regression model. Furthermore, this was an observational study, which makes it impossible to draw conclusions on causality. Nevertheless, the prospective correlations we found fit the hypothesis that generalized impairment of microvascular function is involved in cognitive performance over time in subjects with type 1 diabetes. Other limitations include the follow‐up time, which was fairly brief (<4 years), and may explain why there were no significant changes in mean cognition in both groups. The use of a 1.5‐T magnetic resonance system was standard during the initiation of the baseline study in 2006, yet stronger 3T systems more sensitively detect cMB with a lower inter‐observer variability. To circumvent this problem as much as possible, we used susceptibility weighted imaging, which is highly sensitive to cMB. Nevertheless, our MRI field strength may have led to an underestimation of the amount of cMB and consequently a decreased chance of finding significant differences in cMB between the two study groups or correlations between cMBs and cognition over time. Third, in this study we selected T1DM patient with proliferative retinopathy, and therefore, the data cannot be translated to T1DM patients in general. T1DM patients with proliferative retinopathy have more deteriorated skin capillary perfusion compared with those without,11 which may have a more pronounced effect on cognitive decline, assuming it reflects a further deterioration of generalized microvascular dysfunction. Finally, the associations between general cognitive ability and baseline capillary density (Figure 1, panel B), VO capillary density (Figure 1, panel D), and capillary recruitment (Figure 1, panel E) are not significant when deleting the two most extreme data points from the analysis, although a positive non‐significant correlation is found (B = 0.090, P = 0.670; B = 0.391, P = 0.134; B = 0.325, P = 0.219, respectively). It is likely that correlations will diminish when the two most influential data points are deleted from a relatively small dataset. Since these data points are not statistical outliers, and the data points in these correlations are not derived from two specific individuals, we consider it correct to keep these data points in our analysis. In addition, the association between general cognitive ability and peak capillary density (Figure 1, panel C) remains statistically significant when deleting these two points (B = 0.533; P = 0.034).
In conclusion, this study demonstrates that in type 1 diabetes patients with proliferative retinopathy, the presence of WML and lower skin capillary perfusion at baseline is associated with lower performance in general cognitive ability over time. In addition, the relationship between WML and cognitive decline was significantly reduced when correcting for capillary perfusion measurements. We previously showed that type 1 diabetes patients with proliferative retinopathy have more cMB compared to those without retinopathy and that in patients with cMB capillary perfusion is impaired.11 Together, these data fit our hypothesis that cSVD is a manifestation of generalized microvascular dysfunction, leading to cognitive dysfunction. Future research with a larger sample size and longer follow‐up should confirm these observations. In addition, including type 1 diabetes patients without retinopathy helps to discriminate between hyperglycemia and microvascular damage as an underlying cause of lower cognitive performance.
PERSPECTIVE
These are the first prospective data that show a relationship between cognitive decline, cerebral small vessel disease, and microvascular dysfunction. These data fit our hypothesis that cerebral microangiopathy is a manifestation of generalized microvascular dysfunction, leading to cognitive dysfunction. Future research with a larger sample size and longer follow‐up should confirm these observations.
CONFLICT OF INTEREST
There is no conflict of interests to declare.
AUTHORS' CONTRIBUTIONS
A.L.E. performed the statistical analysis and wrote the manuscript. E.v.D. participated in the design of the study, collected all magnetic resonance imaging data and part of skin capillary data, and wrote the manuscript. M.P.W. rated the magnetic resonance imaging scans. M.K., F.B. and F.J.S., MD and RGI participated in the design of the study. E.C.E. and E.H.S. supervised the statistical analysis of the study. In addition, all authors were involved in drafting the manuscript and making revisions to the manuscript.
Supporting information
ACKNOWLEDGMENTS
This research is supported by grant 2005.00.006 of the Dutch Diabetes Research Foundation and the European Foundation for the Study of Diabetes.
Emanuel AL, van Duinkerken E, Wattjes MP, et al. The presence of cerebral white matter lesions and lower skin microvascular perfusion predicts lower cognitive performance in type 1 diabetes patients with retinopathy but not in healthy controls—a longitudinal study. Microcirculation. 2019;26:e12530 10.1111/micc.12530
Anna L. Emanuel and Eelco van Duinkerken contributed equally to the manuscript.
Deceased
REFERENCES
- 1. Diabetes C, Complications Trial Research G , Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long‐term complications in insulin‐dependent diabetes mellitus. N Engl J Med. 1993;329(14):977‐986. [DOI] [PubMed] [Google Scholar]
- 2. Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol. 2014;2(3):246‐255. [DOI] [PubMed] [Google Scholar]
- 3. Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta‐analysis. Diabetes Care. 2005;28(3):726‐735. [DOI] [PubMed] [Google Scholar]
- 4. McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379(9833):2291‐2299. [DOI] [PubMed] [Google Scholar]
- 5. Brismar T, Maurex L, Cooray G, et al. Predictors of cognitive impairment in type 1 diabetes. Psychoneuroendocrino. 2007;32(8‐10):1041‐1051. [DOI] [PubMed] [Google Scholar]
- 6. Jacobson AM, Ryan CM, Cleary PA, et al. Biomedical risk factors for decreased cognitive functioning in type 1 diabetes: an 18 year follow‐up of the Diabetes Control and Complications Trial (DCCT) cohort. Diabetologia. 2011;54(2):245‐255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ryan CM, Williams TM, Orchard TJ, Finegold DN. Psychomotor slowing is associated with distal symmetrical polyneuropathy in adults with diabetes mellitus. Diabetes. 1992;41(1):107‐113. [DOI] [PubMed] [Google Scholar]
- 8. Ferguson SC, Blane A, Perros P, et al. Cognitive ability and brain structure in type 1 diabetes: relation to microangiopathy and preceding severe hypoglycemia. Diabetes. 2003;52(1):149‐156. [DOI] [PubMed] [Google Scholar]
- 9. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821‐1828. [DOI] [PubMed] [Google Scholar]
- 10. Vernooij MW, van der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70(14):1208‐1214. [DOI] [PubMed] [Google Scholar]
- 11. Woerdeman J, van Duinkerken E, Wattjes MP, et al. Proliferative retinopathy in type 1 diabetes is associated with cerebral microbleeds, which is part of generalized microangiopathy. Diabetes Care. 2014;37(4):1165‐1168. [DOI] [PubMed] [Google Scholar]
- 12. Nunley KA, Ryan CM, Orchard TJ, et al. White matter hyperintensities in middle‐aged adults with childhood‐onset type 1 diabetes. Neurology. 2015;84(20):2062‐2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Duinkerken E, Schoonheim MM, Sanz‐Arigita EJ, et al. Resting‐state brain networks in type 1 diabetic patients with and without microangiopathy and their relation to cognitive functions and disease variables. Diabetes. 2012;61(7):1814‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Assoc WM. World medical association declaration of Helsinki ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191‐2194. [DOI] [PubMed] [Google Scholar]
- 15. van Duinkerken E, Ryan CM, Schoonheim MM, et al. Subgenual cingulate cortex functional connectivity in relation to depressive symptoms and cognitive functioning in type 1 diabetes mellitus patients. Psychosom Med. 2016;78(6):740‐749. [DOI] [PubMed] [Google Scholar]
- 16. Walker LA, Mendella PD, Stewart A, Freedman MS, Smith AM. Meaningful change in cognition in multiple sclerosis: method matters. Can J Neurol Sci. 2011;38(2):282‐288. [DOI] [PubMed] [Google Scholar]
- 17. van Duinkerken E, Klein M, Schoonenboom NS, et al. Functional brain connectivity and neurocognitive functioning in patients with long‐standing type 1 diabetes with and without microvascular complications: a magnetoencephalography study. Diabetes. 2009;58(10):2335‐2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scheltens P, Erkinjunti T, Leys D, et al. White matter changes on CT and MRI: an overview of visual rating scales. European Task Force on Age‐Related White Matter Changes. Eur Neurol. 1998;39(2):80‐89. [DOI] [PubMed] [Google Scholar]
- 19. Serne EH, Stehouwer CDA, ter Maaten JC, et al. Microvascular function relates to insulin sensitivity and blood pressure in normal subjects. Circulation. 1999;99(7):896‐902. [DOI] [PubMed] [Google Scholar]
- 20. Brands AM, Kessels RP, de Haan EH, Kappelle LJ, Biessels GJ. Cerebral dysfunction in type 1 diabetes: effects of insulin, vascular risk factors and blood‐glucose levels. Eur J Pharmacol. 2004;490(1‐3):159‐168. [DOI] [PubMed] [Google Scholar]
- 21. Zilliox LA, Chadrasekaran K, Kwan JY, Russell JW. Diabetes and cognitive impairment. Curr Diab Rep. 2016;16(9):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879‐891. [DOI] [PubMed] [Google Scholar]
- 23. van Duinkerken E, Steenwijk MD, Klein M, et al. Accelerated executive functions decline and gray matter structural changes in middle‐aged type 1 diabetes mellitus patients with proliferative retinopathy. J Diabetes. 2018;10(11):835‐846. [DOI] [PubMed] [Google Scholar]
- 24. Wardlaw JM, Hernandez MCV, Munoz‐Maniega S. What are white matter hyperintensities made of? Relevance to vascular cognitive impairment (vol 4, e001140, 2015) J Am Heart Assoc. 2015;4(6):001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Groot JC, de Leeuw FE, Oudkerk M, Hofman A, Jolles J, Breteler MM. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56(11):1539‐1545. [DOI] [PubMed] [Google Scholar]
- 26. Smith EE, Salat DH, Jeng J, et al. Correlations between MRI white matter lesion location and executive function and episodic memory. Neurology. 2011;76(17):1492‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Weinger K, Jacobson AM, Musen G, et al. The effects of type 1 diabetes on cerebral white matter. Diabetologia. 2008;51(3):417‐425. [DOI] [PubMed] [Google Scholar]
- 28. Pinter D, Enzinger C, Fazekas F. Cerebral small vessel disease, cognitive reserve and cognitive dysfunction. J Neurol. 2015;262(11):2411‐2419. [DOI] [PubMed] [Google Scholar]
- 29. Bentov I, Reed MJ. The effect of aging on the cutaneous microvasculature. Microvasc Res. 2015;100:25‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Madonna R, Balistreri CR, Geng YJ, De Caterina R. Diabetic microangiopathy: pathogenetic insights and novel therapeutic approaches. Vascul Pharmacol. 2017;90:1‐7. [DOI] [PubMed] [Google Scholar]
- 31. Bonnardel‐Phu E, Wautier JL, Schmidt AM, Avila C, Vicaut E. Acute modulation of albumin microvascular leakage by advanced glycation end products in microcirculation of diabetic rats in vivo. Diabetes. 1999;48(10):2052‐2058. [DOI] [PubMed] [Google Scholar]
- 32. Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA. 2002;288(20):2579‐2588. [DOI] [PubMed] [Google Scholar]
- 33. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615‐1625. [DOI] [PubMed] [Google Scholar]
- 34. Rask‐Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab. 2013;17(1):20‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tibirica E, Rodrigues E, Cobas RA, Gomes MB. Endothelial function in patients with type 1 diabetes evaluated by skin capillary recruitment. Microvasc Res. 2007;73(2):107‐112. [DOI] [PubMed] [Google Scholar]
- 36. Tibirica E, Rodrigues E, Cobas R, Gomes MB. Impairment of skin capillary recruitment precedes chronic complications in patients with type 1 diabetes. Rev Diabet Stud. 2007;4(2):85‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neubauer‐Geryk J, Kozera GM, Wolnik B, Szczyrba S, Nyka WM, Bieniaszewski L. Decreased reactivity of skin microcirculation in response to L‐arginine in later‐onset type 1 diabetes. Diabetes Care. 2013;36(4):950‐956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson PC, Brendel K, Meezan E. Thickened cerebral cortical capillary basement membranes in diabetics. Arch Pathol Lab Med. 1982;106(5):214‐217. [PubMed] [Google Scholar]
- 39. van Golen LW, Huisman MC, Ijzerman RG, et al. Cerebral blood flow and glucose metabolism measured with positron emission tomography are decreased in human type 1 diabetes. Diabetes. 2013;62(8):2898‐2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Siro P, Molnar C, Katona E, et al. Carotid intima‐media thickness and cerebrovascular reactivity in long‐term type 1 diabetes mellitus. J Clin Ultrasound. 2009;37(8):451‐456. [DOI] [PubMed] [Google Scholar]
- 41. Wessels AM, Scheltens P, Barkhof F, Heine RJ. Hyperglycaemia as a determinant of cognitive decline in patients with type 1 diabetes. Eur J Pharmacol. 2008;585(1):88‐96. [DOI] [PubMed] [Google Scholar]
- 42. Li W, Huang E, Gao S. Type 1 diabetes mellitus and cognitive impairments: a systematic review. J Alzheimers Dis. 2017;57(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 43. Toth P, Tarantini S, Csiszar A, Ungvari Z. Functional vascular contributions to cognitive impairment and dementia: mechanisms and consequences of cerebral autoregulatory dysfunction, endothelial impairment, and neurovascular uncoupling in aging. Am J Physiol Heart Circ Physiol. 2017;312(1):H1‐H20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng LZ, Gelb AW. Regulation of cerebral autoregulation by carbon dioxide. Anesthesiology. 2015;122(1):196‐205. [DOI] [PubMed] [Google Scholar]
- 45. Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1473‐R1495. [DOI] [PubMed] [Google Scholar]
- 46. van Golen LW, Kuijer JP, Huisman MC, et al. Quantification of cerebral blood flow in healthy volunteers and type 1 diabetic patients: comparison of MRI arterial spin labeling and [(15)O]H2O positron emission tomography (PET). J Magn Reson Imaging. 2014;40(6):1300‐1309. [DOI] [PubMed] [Google Scholar]
- 47. Tagougui S, Fontaine P, Leclair E, et al. Regional cerebral hemodynamic response to incremental exercise is blunted in poorly controlled patients with uncomplicated type 1 diabetes. Diabetes Care. 2015;38(5):858‐867. [DOI] [PubMed] [Google Scholar]
- 48. Manschot SM, Biessels GJ, Cameron NE, et al. Angiotensin converting enzyme inhibition partially prevents deficits in water maze performance, hippocampal synaptic plasticity and cerebral blood flow in streptozotocin‐diabetic rats. Brain Res. 2003;966(2):274‐282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


