Key Points
Question
What is the association of decision aids vs usual care with shared decision-making in men deciding whether to undergo prostate cancer screening?
Findings
This systematic review and meta-analysis of 19 randomized clinical trials comparing decision aids for prostate cancer screening (12 781 men) found that decision aids are probably associated with a small reduction in decisional conflict and are possibly associated with an increase in knowledge. Decision aids are possibly not associated with whether physicians and patients discuss prostate cancer screening and are possibly not associated with actual screening decisions.
Meaning
Randomized clinical trials have failed to provide compelling evidence for the use of decision aids for men contemplating prostate cancer screening that have, up to now, undergone rigorous testing to determine their outcome.
Abstract
Importance
US guidelines recommend that physicians engage in shared decision-making with men considering prostate cancer screening.
Objective
To estimate the association of decision aids with decisional outcomes in prostate cancer screening.
Data Sources
MEDLINE, Embase, PsycINFO, CINAHL, and Cochrane CENTRAL were searched from inception through June 19, 2018.
Study Selection
Randomized trials comparing decision aids for prostate cancer screening with usual care.
Data Extraction and Synthesis
Independent duplicate assessment of eligibility and risk of bias, rating of quality of the decision aids, random-effects meta-analysis, and Grading of Recommendations, Assessment, Development and Evaluations rating of the quality of evidence.
Main Outcomes and Measures
Knowledge, decisional conflict, screening discussion, and screening choice.
Results
Of 19 eligible trials (12 781 men), 9 adequately concealed allocation and 8 blinded outcome assessment. Of 12 decision aids with available information, only 4 reported the likelihood of a true-negative test result, and 3 presented the likelihood of false-negative test results or the next step if the screening test result was negative. Decision aids are possibly associated with improvement in knowledge (risk ratio, 1.38; 95% CI, 1.09-1.73; I2 = 67%; risk difference, 12.1; low quality), are probably associated with a small decrease in decisional conflict (mean difference on a 100-point scale, −4.19; 95% CI, −7.06 to −1.33; I2 = 75%; moderate quality), and are possibly not associated with whether physicians and patients discuss prostate cancer screening (risk ratio, 1.12; 95% CI, 0.90-1.39; I2 = 60%; low quality) or with men’s decision to undergo prostate cancer screening (risk ratio, 0.95; 95% CI, 0.88-1.03; I2 = 36%; low quality).
Conclusions and Relevance
The results of this study provide moderate-quality evidence that decision aids compared with usual care are associated with a small decrease in decisional conflict and low-quality evidence that they are associated with an increase in knowledge but not with whether physicians and patients discussed prostate cancer screening or with screening choice. Results suggest that further progress in facilitating effective shared decision-making may require decision aids that not only provide education to patients but are specifically targeted to promote shared decision-making in the patient-physician encounter.
This systematic review and meta-analysis of 19 randomized clinical trials estimates the association of decision aids with decisional outcomes in prostate cancer screening.
Introduction
Owing to increasing use of prostate-specific antigen (PSA) screening, the incidence of early-stage prostate cancer has increased during the last 25 years.1 Advocates of screening often cite the European Randomized study of Screening for Prostate Cancer (ERSPC)2—of the available trials, the one at lowest risk of bias3—that suggested that PSA screening reduces prostate cancer–specific mortality but not overall mortality.2 Opponents of screening often cite an earlier meta-analysis4 or other major trials5,6 that reported no association between PSA screening and prostate cancer–specific mortality and point out possible harms associated with surgery or radiotherapy.7
Men’s choice of whether to undergo prostate cancer screening is sensitive to their values and preferences: that is, fully informed men will make different choices depending on their experience and perspective. For such decisions, shared decision-making, characterized by cooperative communication between patient and clinician in which they share knowledge, values, and preferences, represents an ideal approach to decision-making.8 Major guidelines therefore acknowledge the importance of informing men about the risks and benefits of PSA screening.9,10,11,12 The US Preventive Services Task Force has recently recommended that the decision to undergo prostate cancer screening should be an individual one in which men should discuss potential benefits and harms with their clinician before screening and recommended that men who do not express a clear preference for screening should not be screened.11 Even more recently, a BMJ Rapid Recommendations’ panel made a weak recommendation against systematic PSA screening that acknowledged the need for shared decision-making.12
Shared decision-making is challenging because of time constraints and the specific skills that it requires.13 Well-designed decision aids may, at least in part, address these challenges by summarizing the current best evidence and by supporting conversations that address the issues that matter most to patients.14,15 The association of decision aids with the decision-making process remains, however, uncertain.8 We therefore undertook a systematic review and meta-analysis of the randomized clinical trials (RCTs)—many of which were conducted before major PSA trials,2,5,6 such as ERSPC,2 were published—that have addressed the effect of decision aids on the decision-making process in the context of prostate cancer screening.
Methods
We registered the protocol in the International Prospective Register of Systematic Reviews (PROSPERO CRD42016052816) and followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.16
Data Sources and Searches
We performed the search, developed in collaboration with an experienced research librarian (R.C.), on June 19, 2018, in MEDLINE, Embase, CINAHL, PsychINFO, and Cochrane Central Register of Controlled Trials (CENTRAL) without language limits (eAppendix 1 in the Supplement).
Eligibility Criteria
We included RCTs conducted among men who were potentially considering undergoing prostate cancer screening that compared decision aid interventions for prostate cancer screening with usual care. We evaluated decision aids and study protocols and judged interventions as either decision aids, information material, or usual care (not overlapping categories). We defined the interventions as decision aids if the material helped men making individual choices and included information regarding the association of screening with the following patient-important outcomes: risk of dying, risk of urinary or bowel symptoms, and risk of erectile dysfunction. We defined the intervention as usual care if clinicians provided no formal, structured presentation of information and informative material if interventions provided some structured information but did not meet our definition of a decision aid (eAppendix 2 in the Supplement).
We excluded studies comparing one decision aid with another and those that did not report on any of our specified outcomes (see the Outcomes subsection). We also excluded studies in which less than 50% of participants in intervention groups used a decision aid.
Outcomes
We evaluated the following outcomes: knowledge regarding prostate cancer screening, decisional conflict, discussions regarding screening between men and their physicians (screening discussion), decisions determining whether screening took place (actual screening decision), and satisfaction with screening decision.
Risk of Bias and the Quality of Decision Aids
We assessed the risk of bias using a modified version of the Cochrane Collaboration risk of bias tool addressing 5 criteria (eAppendix 3 in the Supplement). For each criterion, studies were judged to be at either high or low risk of bias. Studies with a high risk of bias for 3 or more criteria were classified as being at high risk of bias overall.
We identified decision aids used in the studies by following a multistep approach: (1) we first reviewed original articles to identify links or references to electronically available decision aids or those provided as appendices; (2) if unavailable, we conducted electronic searches for decision aids online; and (3) we contacted study authors by email, requesting access to the decision aid. We evaluated the available decision aids using a modified version of the International Patient Decision Aid Standards instrument (IPDASi), version 3 for screening17 by assessing 10 criteria (eAppendix 4 in the Supplement). We rated each criterion as met or unmet and summed the number of criteria met.
Study Selection and Data Extraction
We developed standardized forms with detailed instructions for screening of abstracts and full texts, risk of bias, quality of assessments of decision aids, and data extraction. Independently and in duplicate, 2 methodologically trained reviewers (J.M.R., T.P.K., S.C., A.A., P.J., N.P., P.O.R., J.R., H.S., and T.T.) applied the forms to screen study reports for eligibility and extracted data. Reviewers resolved disagreement through discussion and, if necessary, through consultation with an adjudicator (K.A.O.T.). We sent our consensus data extraction to the original authors for confirmation or correction and asked for clarification regarding missing or unclear information.
Statistical Analysis
For continuous outcomes in which investigators used different instruments to measure a construct, we standardized scores on a range from 0 to 10018,19 and summarized the data as means and SDs or, for skewed distributions, medians and interquartile ranges. For continuous variables, we expressed effects as mean differences and 95% CIs and for binary outcomes, as relative risks and 95% CIs. To obtain the absolute difference, we chose the percentage correct of the median of the control groups and applied the point estimate and 95% CIs of the pooled relative risk to that value. We categorized outcome effects as short-term (effect estimated ≤1 month after decision aid use) and long-term (>1 month after decision aid use) and focused on the last time point in either period in the primary analysis. All P values were from 2-sided tests, and results were deemed statistically significant at P < .05.
We conducted meta-analyses when data for a particular outcome were available from at least 3 trials. For studies with more than 1 intervention group, if we failed to reject the null hypothesis that the intervention groups did not differ (z test at 5% significance level), we pooled the groups within the study; if results differed, we used only the group with the largest effect. To study the potential differences in intervention effects on the outcomes by length of follow-up (short-term defined as ≤1 month and long-term as >1 month), we first conducted the repeated measure, random-effects, weighted mixed regression model analysis. The dependent variable was the outcome mean and the independent variables were the intervention, the follow-up term, the interaction of intervention and follow-up term, the random effects in study, and the baseline data. We reported the pooled analyses separately by length of follow-up if the interaction effect was significant; if not, we reported analyses using the longest follow-up. For analyses in which the I2 statistic was greater than 0%, we pooled the results using Hartung-Knapp-Sidik-Jonkman random-effects models. If the I2 statistic was 0%, we pooled results using fixed-effects models because, under these circumstances, the fixed-effects method is superior to the Hartung-Knapp-Sidik-Jonkman method in type I error.20 We examined the following variables as potential sources of heterogeneity using meta-regression: allocation concealment, blinding of data collectors, and missing data (low vs high risk of bias for all variables). We hypothesized that effects would be larger in high-risk-of-bias trials.
Quality of Evidence
To assess the quality of evidence, we used the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach that classifies evidence as high, moderate, low, or very low quality.21 We used published GRADE guidance for ratings of risk of bias,22 consistency,23 directness,24 precision,25 and publication bias.26 We made 1 major modification of GRADE: the GRADE quality of evidence ratings are intended to address causal inferences; because of journal policy, we applied the quality ratings to issues of association.
Results
Of 12 032 potentially relevant reports, 238 proved potentially eligible; after full-text screening, 19 articles27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45 proved eligible (Figure 1). Six of the 19 authors (32%) confirmed the accuracy of our data extraction28,33,37,39,42,43; none corrected errors or added additional information. Eleven of the 19 authors (58%)27,29,30,32,34,35,38,40,41,44,45 could not be contacted, and 2 authors (11%)31,36 were unable to assist. Trials were published between 1999 and 2017 (eFigure 1 in the Supplement) and randomized 12 781 men; the median of mean ages was 59 years (interquartile range, 57-62 years). Sixteen studies were performed in the United States, 2 in the United Kingdom, and 1 in Canada (Table 1).
Figure 1. Flowchart Outlining the Literature Search and Article Evaluation Process.
Table 1. General Characteristics, Overall Risk of Bias, and IPDASi Evaluation of Included Randomized Trials.
| Source | Country | Men Randomized, No. | Decision Aid Groups | Control Groups | Recruitment Years | Overall Risk of Bias | IPDASi Score | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Intervention | Men Randomized, No. | Age, y | Type of Control | Men Randomized, No. | Age, y | ||||||
| Stamm et al,27 2017 | United States | 329 | Group 1: printed leaflet; group 2: printed leaflet and shared decision-making | Group 1: 113; group 2: 110 | Group 1: 62; group 2: 61 | None | 106 | 63 | NR | High | NA |
| Landrey et al,28 2013 | United States | 303 | Printed leaflet | 145 | 62 | None | 158 | 62 | 2009-2010 | High | 2 |
| Taylor et al,29 2013 | United States | 1893 | Group 1: printed booklet; group 2: computer based | Group 1: 630; group 2: 631 | 57 | None | 632 | 57 | 2007-2010 | High | 9 |
| Lepore et al,30 2012 | United States | 490 | Telephone education and printed booklet | 244 | 55 | Food information | 246 | 55 | 2005-2006 | High | 2 |
| Sheridan et al,31 2012 | United States | 130 | Video, printed leaflet, and individual education | 60 | 57 | Highway safety video | 70 | 58 | 2005-2006 | Low | 4 |
| Chan et al,33 2011a | United States | 321 | Group education: video, printed booklet, script, and slides | 160 | NR | Group education: diabetes video and discussion | 157 | 61 | NR | Low | NA |
| Allen et al,32 2010a | United States | 2615 | Computer based | 1118 | NR | None | 1497 | NR | 2006-2007 | High | NA |
| Evans et al,34 2010 | United Kingdom | 514 | Group 1: computer based; group 2: printed booklet | Group 1: 129; group 2: 126 | NR | None | 259 | NR | 2008 | High | 10 |
| Rubel et al,35 2010 | United States | 200 | Printed booklet | 100 | 59 | None | 100 | 59 | 2005 | Low | NA |
| Frosch et al,36 2008 | United States | 611 | Group 1: computer-based chronic disease trajectory model; group 2: computer-based traditional model; group 3: computer-based combination of both 1 and 2 | Group 1: 153; group 2: 155; group 3: 152 | Group 1: 58; group 2: 59; group 3: 59 | Link to websites | 151 | 59 | 2005-2006 | High | NA |
| Husaini et al,37 2008a | United States | 430 | Group education: video, printed leaflet, and teaching session | 235 | 55 | None | 115 | 50 | NR | High | NA |
| Stephens et al,38 2008 | United States | 440 | Printed booklet | 200 | NR | None | 200 | NR | NR | Low | 7 |
| Krist et al,39 2007 | United States | 497 | Group 1: printed booklet; group 2: computer based | Group 1: 196; group 2: 226 | Group 1: 57; group 2: 56 | None | 75 | 57 | 2002-2004 | High | 9 |
| Taylor et al,40 2006 | United States | 294 | Group 1: video; group 2: printed booklet | Group 1: 95; group 2: 98 | Group 1: 56; group 2: 57 | None | 92 | 55 | 2001-2002 | High | 2 |
| Watson et al,41 2006 | United Kingdom | 1960 | Printed leaflet | 980 | 59 | None | 980 | 59 | 2004 | High | 7 |
| Partin et al,42 2004 | United States | 1152 | Group 1: printed booklet; group 2: video | Group 1: 384; group 2: 384 | 68 | None | 384 | 68 | 2001 | High | 4 (printed booklet); 6 (video) |
| Wilt et al,43 2001 | United States | 342 | Printed leaflet | 163 | 73 | None | 179 | 70 | 1998 | Low | 5 |
| Davison et al,44 1999 | Canada | 100 | Individual education: verbal and printed | 50 | 64 | General medical information | 50 | 61 | NR | High | NA |
| Volk et al,45 1999 | United States | 160 | Video and printed leaflet | 80 | 59 | None | 80 | 60 | 1997 | High | 6 |
Abbreviations: IPDASi, International Patient Decision Aid Standards instrument, version 3; NA, not applicable; NR, not reported.
Cluster randomized trial.
Risk of Bias
In all 19 studies, the allocation sequence was adequately generated; in 9 studies (47%), allocation was adequately concealed; and in 8 studies (42%), data collectors were blinded. Missing data were judged as high risk of bias in 7 of 13 studies (54%) for actual screening decision and in 11 of 19 studies (58%) for other outcomes (knowledge, screening discussion, decisional conflict, and satisfaction with decision) (Table 1; eFigure 2 in the Supplement).
Decision Aids
Investigators used several types of decision aids: 13 of 19 studies used printed material (8 used booklets of 8-28 pages29,30,34,35,38,39,40,42 and 5 used leaflets of 1-2 pages27,28,41,43,45), 5 studies used education (2 used group sessions33,37 and 3 used individual education30,31,44), 5 studies used computer-based tools,29,32,34,36,39 and 4 studies used videos.31,40,42,45 Two studies used the same video.42,45 One study used shared decision-making27 (eTable 1 in the Supplement).
We identified 12 decision aids: 5 by reviewing original articles,28,30,31,41,43 4 by electronic searches,29,34,38,40 and 3 from the authors.39,42,45 Two authors reported that the decision aid was no longer available (eTable 1 in the Supplement).35,44 Three decision aids scored well (8-10 points out of 10), 4 scored less well (5-7 points), and 5 scored poorly (≤4 points); the overall IPDASi mean (SD) score was 5.6 (2.9) (range, 2-10). All decision aids reported the screening aim; 11 of 12 decision aids (92%) reported the association of screening with overall or prostate cancer–specific mortality; and 10 of 12 decision aids (83%) reported the harms of the increase in surgery and radiotherapy that accompanies the increased diagnosis of prostate cancer consequent to screening (erectile dysfunction, urinary incontinence, and bowel problems). Four of 12 decision aids (33%) presented information regarding the probability of having a true-negative result; 3 of 12 decision aids (25%) presented the probability of a false-negative result or the next step if screening results were negative. Two of 12 decision aids (17%) presented the likelihood of detecting prostate cancer with and without the use of screening (eFigure 3 in the Supplement).
Outcomes
Knowledge
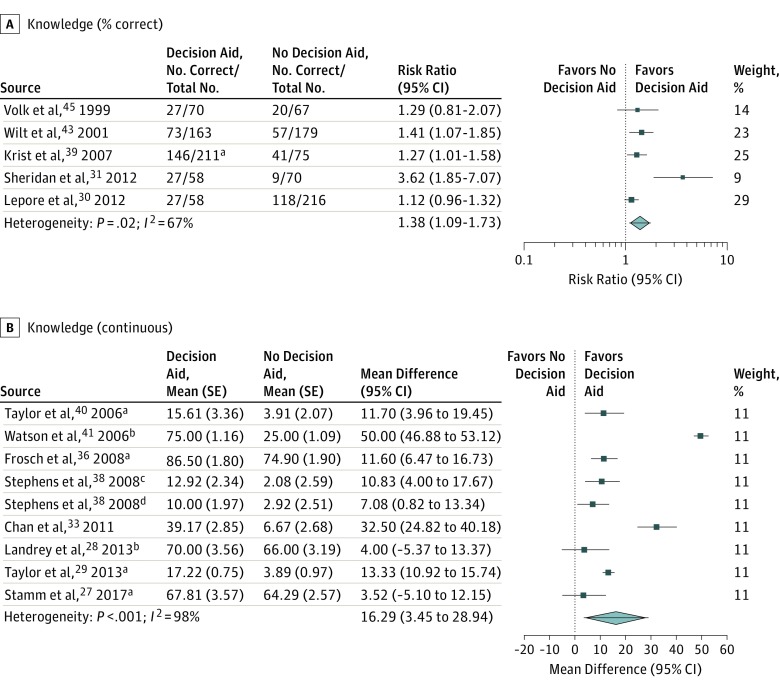
Of the 13 studies reporting short-term knowledge, 8 reported data as a continuous variable and 5 reported the proportion of correct items. Because the SDs of the latter are much smaller (owing to the nature of binomial distribution), they would dominate a pooled result of all 13 studies; therefore, we analyzed them separately. Pooled estimates from 8 studies reporting data as a continuous variable showed an increase in knowledge for decision aids (mean difference, 16.29; 95% CI, 3.45-28.94; low-quality evidence; Table 2 and Figure 2B). The proportion of correctness data from 5 studies demonstrated improved knowledge with decision aids, although the 95% CI includes a very small and likely unimportant difference (risk ratio, 1.38; 95% CI, 1.09-1.73; risk difference, 12.1; low-quality evidence; Table 2 and Figure 2A). Studies failed to demonstrate an association with knowledge in the long term (mean difference, 5.47; 95% CI, −0.52 to 11.45; low-quality evidence; eFigure 4 in the Supplement).
Table 2. GRADE Evidence Profile: Decision Aid vs Usual Care for Prostate Cancer Screening.
| Quality Assessment | Summary of Findings | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Patients With Data (No. of Studies) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Publication Bias | Relative Effect (95% CI) | Absolute Difference (95% CI) | Certainty in Estimates |
| Knowledge (short-term; percentage correct) | ||||||||
| 1167 (5) | Serious limitationsa | No serious limitations | No serious limitations | Serious limitations: CI includes a very small and likely unimportant difference | Undetected | Decision aid increased discussion about prostate cancer screening by 38% (from 9% to 73% increase) | Mean difference of 12.1 (from 2.9 increase to 24.5 increase) on percentage correct favoring decision aid | Lowb |
| Knowledge (short-term; continuous) | ||||||||
| 4272 (8) | Serious limitationsc | No serious limitations | No serious limitations | Serious limitations: CI includes a very small and likely unimportant difference | Undetected | NA | Mean difference of 16.3 (from 3.5 increase to 28.9 increase) on 100-point scale favoring decision aid | Lowb |
| Decisional Conflict | ||||||||
| 3700 (6) | Serious limitationsd | No serious limitations | No serious limitations | No serious limitations | Undetected | NA | Mean difference of 4.2 (from 1.3 to 7.1) on 100-point scale favoring decision aid | Moderatee |
| Screening Discussion | ||||||||
| 1927 (6) | Serious limitationsf | No serious limitations | No serious limitations | Serious limitations: CI crosses no difference | Undetected | Decision aid increased screening discussion by 12% (from 10% decrease to 39% increase) | No significant effect | Lowb |
| Actual Screening Decision | ||||||||
| 4286 (13) | Serious limitationsg | No serious limitations | No serious limitations | Serious limitations: CI crosses no difference | Undetected | Decision aid decreased screening by 5% (from 13% decrease to 4% increase) | No significant effect | Lowb |
Abbreviations: GRADE, Grading of Recommendations, Assessment, Development and Evaluations; NA, not applicable.
Of the 5 studies, 3 (60%) were at high risk of bias, and 2 (40%) were at low risk of bias (Table 1; eFigure 2 in the Supplement).
The low quality of the rating reflects concerns in 3 domains: risk of bias, inconsistency, and imprecision.
Of the 8 studies, 6 (75%) were at high risk of bias, and 2 (25%) were at low risk of bias (Table 1; eFigure 2 in the Supplement).
Of the 6 studies, 5 (83%) were at high risk of bias, and 1 (17%) was at low risk of bias (Table 1; eFigure 2 in the Supplement).
The moderate quality of rating reflects concerns in 2 domains: risk of bias and imprecision.
Of the 6 studies, 4 (67%) were at high risk of bias, and 2 (33%) were at low risk of bias (Table 1; eFigure 2 in the Supplement).
Of the 13 studies, 11 (85%) were at high risk of bias, and 2 (15%) were at low risk of bias (Table 1; eFigure 2 in the Supplement).
Figure 2. Forest Plots of Short-term Prostate Cancer Screening Knowledge.
aPooled result from multiple groups.
bUnadjusted from baseline.
cAfrican American study population.
dNon–African American study population.
Decisional Conflict
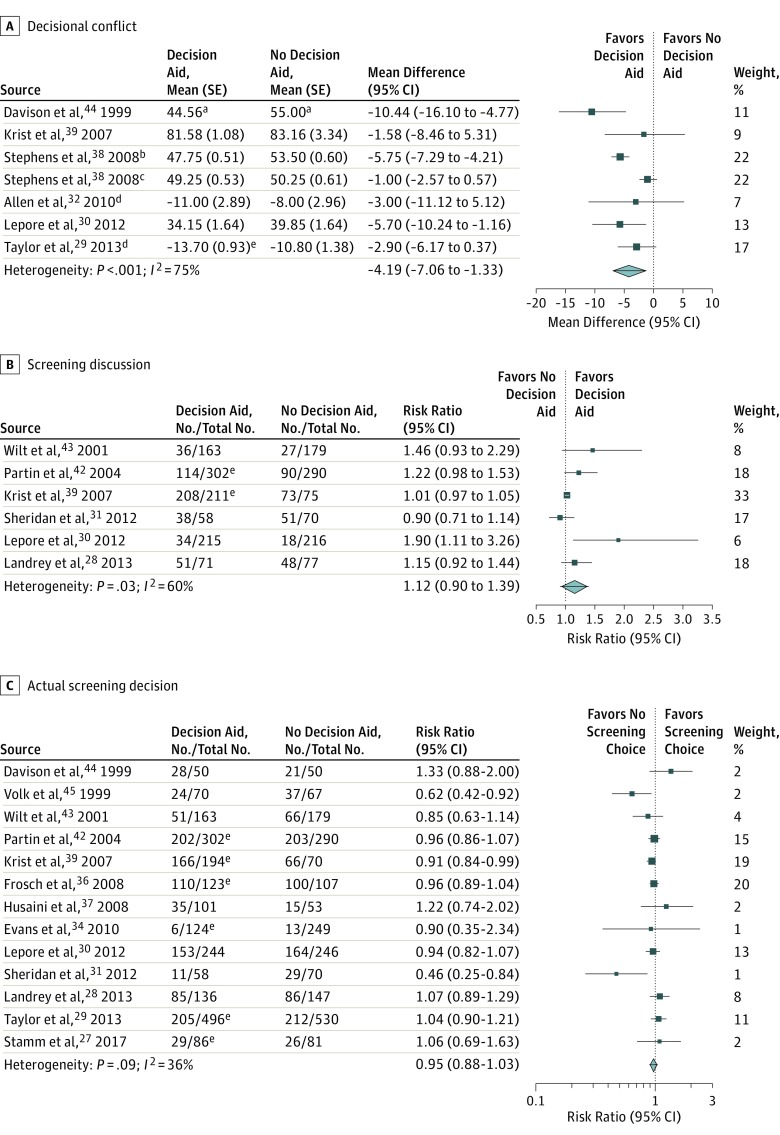
In the pooled analysis (6 studies), the decision aids were associated with a small but consistent and statistically significant decrease in decisional conflict (mean difference on a 100-point scale, −4.19; 95% CI, −7.06 to −1.33; moderate-quality evidence; Table 2 and Figure 3A).
Figure 3. Forest Plots of Prostate Cancer Screening Decisional Conflict, Screening Discussion, and Actual Screening Decision.
aScaled to 100.
bAfrican American study population.
cNon–African American study population.
dPooled result from multiple groups.
eUnadjusted from baseline.
Screening Discussion
The frequency with which a screening discussion with the clinician took place varied from 8% to 97% (median, 47%) in usual care groups and from 16% to 99% in decision aid groups (median, 52%). The pooled analysis from 6 studies failed to demonstrate an association with whether physicians and patients discussed prostate cancer screening (risk ratio, 1.12; 95% CI, 0.90-1.39; low-quality evidence; Table 2 and Figure 3B). In 4 studies,28,39,42,43 the decision aid was distributed 1 to 2 weeks before the visit or assessment; in 1 study,31 the decision aid was distributed 1 hour before the assessment; and in 1 study,30 the decision aid was distributed 8 months before the visit.
Actual Screening Decision
The frequency with which men choose to undergo prostate cancer screening ranged from 5% to 94% (median, 49%) in usual care groups and 5% to 90% in decision aid groups (median, 49%). The pooled analysis from 13 studies demonstrated no association in men’s decision to undergo or not undergo prostate cancer screening between the decision aid and usual care groups (risk ratio, 0.95; 95% CI, 0.88-1.03; low-quality evidence; Table 2 and Figure 3C).
Satisfaction With Decision
Three studies29,40,45 reported men’s satisfaction with their decision; 2 of these studies used the Satisfaction with Decision Scale,29,45,46 and 140 used a Likert scale. Two studies reported no difference in satisfaction between the intervention and control groups.40,46 One study29 reported that men in both the group that received a printed decision aid (odds ratio [OR], 1.79; 95% CI, 1.41-2.29) and the group that received a web-based decision aid (OR, 1.29; 95% CI, 1.02-1.66) were more likely to report high satisfaction at 1 month of follow-up compared with usual care (high satisfaction reported by 60.4% in the printed decision aid group and 52.2% in the web decision aid group compared with 45.5% in the control group). This difference persisted compared with the usual care group for the printed decision aid group (OR, 1.29; 95% CI, 1.01-1.66) but not for the web-based decision aid group (OR, 1.04; 95% CI, 0.81-1.34) at 13 months of follow-up. Furthermore, participants with printed material reported significantly greater satisfaction than with web material at 1 month (OR, 1.38; 95% CI, 1.07-1.77) but not at 13 months (OR, 1.24; 95% CI, 0.96-1.60). None of these studies examined whether satisfaction varied by whether the decision was to undergo prostate cancer screening or not to undergo screening. For no outcome did risk of bias explain the variability in results (eTable 2 in the Supplement).
Discussion
Main Findings
To examine the association of prostate cancer screening decision aids with decisional outcomes and screening decisions, we pooled data from 19 trials. Low-quality evidence suggests that decision aids are associated with an improvement in men’s knowledge regarding prostate cancer screening, and moderate-quality evidence suggests that decision aids are associated with a small decrease in decisional conflict. Overall, decision aids proved to not be statistically significantly associated with whether physicians and patients discussed prostate cancer screening, or with men’s decision to undergo or not undergo screening (low-quality evidence). The decision aids used in these studies provided most of the crucial information (benefits and harms of screening) but typically omitted test properties of the screening tests.
Strengths and Limitations of the Study
Strengths of our study include a comprehensive search, duplicate assessment of eligibility and data extraction, appraisal of risk of bias, use of outcomes that are important to patients, and evaluation of decision aids using the IPDASi instrument. To increase the precision of estimates, whenever possible, we conducted meta-analyses using appropriate statistical methods. The GRADE approach was applied to assess the quality of evidence for each outcome (Table 2).
Limitations of our review are largely those of the available literature. First, we were not able to use all studies: in 26 studies, there was no usual care control group, 5 studies did not report on any of our outcomes, and 1 study had very low adherence to the decision aid (eTable 3 in the Supplement). Second, we were able to conduct IPDASi evaluation in only 12 decision aids used in 13 studies. Third, most trials were performed before major PSA trials—ERSPC2; Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial5; and Cluster Randomized Trial of PSA Testing for Prostate Cancer6—provided data (eFigure 1 in the Supplement). Fourth, different instruments were used for assessment of knowledge. Fifth, we found only low-quality evidence for the association of decision aids with knowledge, whether a screening discussion was conducted, or patients’ decisions whether to undergo screening. Furthermore, many available decision aids have not undergone formal testing in randomized trials.
Association With Other Studies
Three previous systematic reviews have investigated decision aids for prostate cancer screening.47,48,49 One review published more than 10 years ago addressed different questions and did not include 14 studies included in our review.47
A systematic review published in 2015 concluded that decision aids increase patient knowledge and confidence in decision-making regarding prostate cancer testing.48 This review included 13 studies, of which we did not include 6 studies50,51,52,53,54,55 because of the lack of a standard care control group, but it failed to include 12 trials that proved to be eligible in our systematic review: 11 RCTs of decision aids that were reported before the publication of their review and apparently met their eligibility criteria28,35,36,37,38,39,40,41,43,44,45 and one study27 that was published after their review appeared. The authors failed to conduct a meta-analysis.48
Ivlev and colleagues49 have published the most recent systematic review on prostate cancer screening patient decision aids and concluded that integration of decision aids in clinical practice may result in a decrease in the number of men who elect to undergo PSA testing, which may in turn reduce screening uptake. Support for this statement came from an analysis of intent to screen (risk ratio, 0.88; 95% CI, 0.81-0.95). Their meta-analysis of 2 RCTs that addressed men’s actual decision found, however, no difference between the decision aid and usual care groups (risk ratio, 0.92; 95% CI, 0.62-1.36) and is consistent with our analysis of 13 RCTs (risk ratio, 0.95; 95% CI, 0.88-1.03).
The review by Ivlev et al49 included 13 RCTs and 5 observational studies; to avoid bias associated with prognostic imbalance, we restricted our eligible studies to RCTs. Of the RCTs that Ivlev and colleagues49 included, we did not include 3 studies54,55,56 because they did not have a standard care control group and 1 study57 because it lacked our prespecified outcomes. The review by Ivlev et al49 failed to include 10 of our 19 eligible trials: 3 trials28,31,33 were considered—contrary to our judgment—as not having a decision aid group, 3 trials29,35,38 were excluded because they did not meet their eligibility criteria of reporting immediate or deferred intention or utilization data, 1 trial44 was excluded without explanation, and 3 trials37,40,43 were either not identified by their search or were excluded during title and abstract screening (not possible to distinguish which reason). Other differences included our measuring of screening discussions and reporting a meta-analysis of decisional conflict, which were not in the review by Ivlev et al.49 Ivlev and colleagues49 stated in their methods (including PROSPERO CRD42017060606) that they used the GRADE approach21; however, they provided evidence quality for only 2 outcomes: intention to undergo PSA testing and knowledge. Our judgments applying the GRADE approach21 included all outcomes and differed from the review by Ivlev et al49 regarding knowledge because we considered the failure to use blinded assessments as a reason to rate the quality of evidence downward and they did not.
Implications for Clinicians and Policymakers, and Future Directions
Our results suggest modest and uncertain associations between existing decision aids and key outcomes: a possible increase in knowledge and likely a small decrease in decisional conflict but no apparent association with whether physicians and patients discussed prostate cancer screening or with men’s decision to undergo or not undergo prostate cancer screening. Many prostate cancer screening decision aids are available online, but only a few have undergone formal testing. All decisions aids included in our review provided education to patients, and all but 1 decision aid27 failed to show clear facilitation of screening discussions (ie, shared decision-making).14 The results demonstrate a lack of prostate cancer decision aids specifically geared toward or successful in facilitating shared decision-making.
The best available evidence suggests that PSA screening may have a small, although uncertain, benefit on prostate cancer mortality.3 Evidence shows, however, that PSA screening also harms men because of false-positive test results and overdiagnosis and overtreatment of prostate cancer.3 Before the major prostate cancer screening trials reported their results,2,5,6 there was insufficient evidence to recommend for or against screening. In our meta-analysis, only 2 trials27,28 began recruitment of patients after ERSPC and the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial had published their results (eFigure 1 in the Supplement). Although these 2 trials27,28 reported results similar to our pooled results, it is possible that decision aids with new, updated evidence summaries may have more benefit than earlier decision aids in which results were more uncertain. There is therefore a call for new trials with updated decision aids.12 In general, trustworthy decision aids require links to recent evidence-based summaries and clinical practice guidelines that carry out dynamic updating.12,14
Conclusions
Randomized clinical trials provide moderate-quality evidence that decision aids are associated with a small reduction in decisional conflict, while low-quality evidence suggests that they are associated with an increase in knowledge but not with whether physicians and patients discuss prostate cancer screening or with men’s decision to undergo or not undergo prostate cancer screening. The available evidence does not provide a compelling rationale for clinicians to use existing decision aids to facilitate shared decision-making in their discussions with men considering undergoing prostate cancer screening. Future decision aids should include provision for continuous updating and not only provide education to patients but also promote shared decision-making in the patient-physician encounter.
eAppendix 1. Search Strategies
eAppendix 2. Case Examples of Definitions Used to Characterize Interventions
eAppendix 3. Modified Version of Cochrane Risk of Bias Tool
eAppendix 4. Modified Version of International Patient Decision Aid Standards Instrument (IPDASi v3)
eFigure 1. Patient Recruitment Periods of the Included Decision Aid Trials in Comparison to the Publication of the Major Trials of Prostate Cancer Screening
eFigure 2. Risk of Bias Evaluation Summary
eTable 1. Summary of Decision Aids Used in Eligible Studies
eFigure 3. The International Patient Decision Aid Standards Instrument Rating for Screening (IPDASi v3) Evaluation of Decision Aids
eFigure 4. Pooled Analysis of Prostate Cancer Screening Long Term Knowledge
eTable 2. Subgroup Analysis of Pooled Outcomes
eTable 3. Excluded Studies in Alphabetical Order, With Reasons for Exclusion
References
- 1.Fitzmaurice C, Allen C, Barber RM, et al. ; Global Burden of Disease Cancer Collaboration . Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524-548. doi: 10.1001/jamaoncol.2016.5688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schröder FH, Hugosson J, Roobol MJ, et al. ; ERSPC Investigators . Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384(9959):2027-2035. doi: 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ilic D, Djulbegovic M, Jung JH, et al. . Prostate cancer screening with prostate-specific antigen (PSA) test: a systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ilic D, Neuberger MM, Djulbegovic M, Dahm P. Screening for prostate cancer. Cochrane Database Syst Rev. 2013;1(1):CD004720. doi: 10.1002/14651858.CD004720.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriole GL, Crawford ED, Grubb RL III, et al. ; PLCO Project Team . Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104(2):125-132. doi: 10.1093/jnci/djr500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RM, Donovan JL, Turner EL, et al. ; CAP Trial Group . Effect of a low-intensity PSA-based screening intervention on prostate cancer mortality: the CAP randomized clinical trial. JAMA. 2018;319(9):883-895. doi: 10.1001/jama.2018.0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen RC, Basak R, Meyer AM, et al. . Association between choice of radical prostatectomy, external beam radiotherapy, brachytherapy, or active surveillance and patient-reported quality of life among men with localized prostate cancer. JAMA. 2017;317(11):1141-1150. doi: 10.1001/jama.2017.1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stacey D, Bennett CL, Barry MJ, et al. . Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2011;10(10):CD001431. doi: 10.1002/14651858.CD001431.pub3 [DOI] [PubMed] [Google Scholar]
- 9.Mottet N, van den Bergh RCN, Briers E, et al. Prostate cancer. European Association of Urology website. http://uroweb.org/guideline/prostate-cancer/. Accessed May 20, 2019.
- 10.Carter HB, Albertsen PC, Barry MJ, et al. . Early detection of prostate cancer: AUA Guideline. J Urol. 2013;190(2):419-426. doi: 10.1016/j.juro.2013.04.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grossman DC, Curry SJ, Owens DK, et al. ; US Preventive Services Task Force . Screening for prostate cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;319(18):1901-1913. doi: 10.1001/jama.2018.3710 [DOI] [PubMed] [Google Scholar]
- 12.Tikkinen KAO, Dahm P, Lytvyn L, et al. . Prostate cancer screening with prostate-specific antigen (PSA) test: a clinical practice guideline. BMJ. 2018;362:k3581. doi: 10.1136/bmj.k3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stiggelbout AM, Van der Weijden T, De Wit MP, et al. . Shared decision making: really putting patients at the centre of healthcare. BMJ. 2012;344:e256. doi: 10.1136/bmj.e256 [DOI] [PubMed] [Google Scholar]
- 14.Agoritsas T, Heen AF, Brandt L, et al. . Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015;350:g7624. doi: 10.1136/bmj.g7624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elwyn G, Quinlan C, Mulley A, Agoritsas T, Vandvik PO, Guyatt G. Trustworthy guidelines—excellent; customized care tools—even better. BMC Med. 2015;13:199. doi: 10.1186/s12916-015-0436-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. . The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elwyn G, O’Connor AM, Bennett C, et al. . Assessing the quality of decision support technologies using the International Patient Decision Aid Standards instrument (IPDASi). PLoS One. 2009;4(3):e4705. doi: 10.1371/journal.pone.0004705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorlund K, Walter SD, Johnston BC, Furukawa TA, Guyatt GH. Pooling health-related quality of life outcomes in meta-analysis—a tutorial and review of methods for enhancing interpretability. Res Synth Methods. 2011;2(3):188-203. doi: 10.1002/jrsm.46 [DOI] [PubMed] [Google Scholar]
- 19.Guyatt GH, Thorlund K, Oxman AD, et al. . GRADE guidelines, 13: preparing summary of findings tables and evidence profiles—continuous outcomes. J Clin Epidemiol. 2013;66(2):173-183. doi: 10.1016/j.jclinepi.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 20.IntHout J, Ioannidis JP, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC Med Res Methodol. 2014;14:25. doi: 10.1186/1471-2288-14-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, et al. ; GRADE Working Group . GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926. doi: 10.1136/bmj.39489.470347.AD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyatt GH, Oxman AD, Vist G, et al. . GRADE guidelines, 4: rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64(4):407-415. doi: 10.1016/j.jclinepi.2010.07.017 [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . GRADE guidelines, 7: rating the quality of evidence–inconsistency. J Clin Epidemiol. 2011;64(12):1294-1302. doi: 10.1016/j.jclinepi.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 24.Guyatt GH, Oxman AD, Kunz R, et al. ; GRADE Working Group . GRADE guidelines, 8: rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64(12):1303-1310. doi: 10.1016/j.jclinepi.2011.04.014 [DOI] [PubMed] [Google Scholar]
- 25.Guyatt GH, Oxman AD, Kunz R, et al. . GRADE guidelines, 6: rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64(12):1283-1293. doi: 10.1016/j.jclinepi.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Oxman AD, Montori V, et al. . GRADE guidelines, 5: rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64(12):1277-1282. doi: 10.1016/j.jclinepi.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 27.Stamm AW, Banerji JS, Wolff EM, et al. . A decision aid versus shared decision making for prostate cancer screening: results of a randomized, controlled trial. Can J Urol. 2017;24(4):8910-8917. [PubMed] [Google Scholar]
- 28.Landrey AR, Matlock DD, Andrews L, Bronsert M, Denberg T. Shared decision making in prostate-specific antigen testing: the effect of a mailed patient flyer prior to an annual exam. J Prim Care Community Health. 2013;4(1):67-74. doi: 10.1177/2150131912447074 [DOI] [PubMed] [Google Scholar]
- 29.Taylor KL, Williams RM, Davis K, et al. . Decision making in prostate cancer screening using decision aids vs usual care: a randomized clinical trial. JAMA Intern Med. 2013;173(18):1704-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lepore SJ, Wolf RL, Basch CE, et al. . Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Ann Behav Med. 2012;44(3):320-330. doi: 10.1007/s12160-012-9392-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheridan SL, Golin C, Bunton A, et al. . Shared decision making for prostate cancer screening: the results of a combined analysis of two practice-based randomized controlled trials. BMC Med Inform Decis Mak. 2012;12:130. doi: 10.1186/1472-6947-12-130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen JD, Othus MK, Hart A Jr, et al. . A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: results from the Take the Wheel trial. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2172-2186. doi: 10.1158/1055-9965.EPI-09-0410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan EC, McFall SL, Byrd TL, et al. . A community-based intervention to promote informed decision making for prostate cancer screening among Hispanic American men changed knowledge and role preferences: a cluster RCT. Patient Educ Couns. 2011;84(2):e44-e51. doi: 10.1016/j.pec.2010.07.033 [DOI] [PubMed] [Google Scholar]
- 34.Evans R, Joseph-Williams N, Edwards A, et al. . Supporting informed decision making for prostate specific antigen (PSA) testing on the web: an online randomized controlled trial. J Med Internet Res. 2010;12(3):e27. doi: 10.2196/jmir.1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rubel SK, Miller JW, Stephens RL, et al. . Testing the effects of a decision aid for prostate cancer screening. J Health Commun. 2010;15(3):307-321. doi: 10.1080/10810731003686614 [DOI] [PubMed] [Google Scholar]
- 36.Frosch DL, Bhatnagar V, Tally S, Hamori CJ, Kaplan RM. Internet patient decision support: a randomized controlled trial comparing alternative approaches for men considering prostate cancer screening. Arch Intern Med. 2008;168(4):363-369. doi: 10.1001/archinternmed.2007.111 [DOI] [PubMed] [Google Scholar]
- 37.Husaini BA, Reece MC, Emerson JS, Scales S, Hull PC, Levine RS. A church-based program on prostate cancer screening for African American men: reducing health disparities. Ethn Dis. 2008;18(2)(suppl 2):S2-S179, 84. [PubMed] [Google Scholar]
- 38.Stephens RL, Xu Y, Volk RJ, et al. . Influence of a patient decision aid on decisional conflict related to PSA testing: a structural equation model. Health Psychol. 2008;27(6):711-721. doi: 10.1037/0278-6133.27.6.711 [DOI] [PubMed] [Google Scholar]
- 39.Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5(2):112-119. doi: 10.1370/afm.623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor KL, Davis JL III, Turner RO, et al. . Educating African American men about the prostate cancer screening dilemma: a randomized intervention. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2179-2188. doi: 10.1158/1055-9965.EPI-05-0417 [DOI] [PubMed] [Google Scholar]
- 41.Watson E, Hewitson P, Brett J, et al. . Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: a randomised controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Educ Couns. 2006;63(3):367-379. doi: 10.1016/j.pec.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 42.Partin MR, Nelson D, Radosevich D, et al. . Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. J Gen Intern Med. 2004;19(8):835-842. doi: 10.1111/j.1525-1497.2004.30047.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilt TJ, Paul J, Murdoch M, Nelson D, Nugent S, Rubins HB. Educating men about prostate cancer screening: a randomized trial of a mailed pamphlet. Eff Clin Pract. 2001;4(3):112-120. [PubMed] [Google Scholar]
- 44.Davison BJ, Kirk P, Degner LF, Hassard TH. Information and patient participation in screening for prostate cancer. Patient Educ Couns. 1999;37(3):255-263. doi: 10.1016/S0738-3991(98)00123-2 [DOI] [PubMed] [Google Scholar]
- 45.Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8(4):333-340. doi: 10.1001/archfami.8.4.333 [DOI] [PubMed] [Google Scholar]
- 46.Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1(1):22-28. doi: 10.1370/afm.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volk RJ, Hawley ST, Kneuper S, et al. . Trials of decision aids for prostate cancer screening: a systematic review. Am J Prev Med. 2007;33(5):428-434. doi: 10.1016/j.amepre.2007.07.030 [DOI] [PubMed] [Google Scholar]
- 48.Ilic D, Jammal W, Chiarelli P, et al. . Assessing the effectiveness of decision aids for decision making in prostate cancer testing: a systematic review. Psychooncology. 2015;24(10):1303-1315. doi: 10.1002/pon.3815 [DOI] [PubMed] [Google Scholar]
- 49.Ivlev I, Jerabkova S, Mishra M, Cook LA, Eden KB. Prostate cancer screening patient decision aids: a systematic review and meta-analysis. Am J Prev Med. 2018;55(6):896-907. doi: 10.1016/j.amepre.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams RM, Davis KM, Luta G, et al. . Fostering informed decisions: a randomized controlled trial assessing the impact of a decision aid among men registered to undergo mass screening for prostate cancer. Patient Educ Couns. 2013;91(3):329-336. doi: 10.1016/j.pec.2012.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watts KJ, Meiser B, Wakefield CE, et al. . Online prostate cancer screening decision aid for at-risk men: a randomized trial. Health Psychol. 2014;33(9):986-997. doi: 10.1037/a0034405 [DOI] [PubMed] [Google Scholar]
- 52.Myers RE, Daskalakis C, Kunkel EJ, et al. . Mediated decision support in prostate cancer screening: a randomized controlled trial of decision counseling. Patient Educ Couns. 2011;83(2):240-246. doi: 10.1016/j.pec.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 53.Volk RJ, Jibaja-Weiss ML, Hawley ST, et al. . Entertainment education for prostate cancer screening: a randomized trial among primary care patients with low health literacy. Patient Educ Couns. 2008;73(3):482-489. doi: 10.1016/j.pec.2008.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57(2):168-182. doi: 10.1016/j.pec.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 55.Gattellari M, Ward JE. Does evidence-based information about screening for prostate cancer enhance consumer decision-making? a randomised controlled trial. J Med Screen. 2003;10(1):27-39. doi: 10.1258/096914103321610789 [DOI] [PubMed] [Google Scholar]
- 56.Ilic D, Egberts K, McKenzie JE, Risbridger G, Green S. Informing men about prostate cancer screening: a randomized controlled trial of patient education materials. J Gen Intern Med. 2008;23(4):466-471. doi: 10.1007/s11606-007-0466-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tran VT, Kisseleva-Romanova E, Rigal L, Falcoff H. Impact of a printed decision aid on patients’ intention to undergo prostate cancer screening: a multicentre, pragmatic randomised controlled trial in primary care. Br J Gen Pract. 2015;65(634):e295-e304. doi: 10.3399/bjgp15X684817 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Search Strategies
eAppendix 2. Case Examples of Definitions Used to Characterize Interventions
eAppendix 3. Modified Version of Cochrane Risk of Bias Tool
eAppendix 4. Modified Version of International Patient Decision Aid Standards Instrument (IPDASi v3)
eFigure 1. Patient Recruitment Periods of the Included Decision Aid Trials in Comparison to the Publication of the Major Trials of Prostate Cancer Screening
eFigure 2. Risk of Bias Evaluation Summary
eTable 1. Summary of Decision Aids Used in Eligible Studies
eFigure 3. The International Patient Decision Aid Standards Instrument Rating for Screening (IPDASi v3) Evaluation of Decision Aids
eFigure 4. Pooled Analysis of Prostate Cancer Screening Long Term Knowledge
eTable 2. Subgroup Analysis of Pooled Outcomes
eTable 3. Excluded Studies in Alphabetical Order, With Reasons for Exclusion