Abstract
Aim
To collate prevalence estimates of fetal alcohol spectrum disorder (FASD) among special subpopulations (defined by service use).
Design
Systematic literature review and meta‐analysis of original, quantitative studies published between 1 November 1973 and 1 December 2018. The PRISMAGATHER were adhered to. The review protocol [includes FASD prevalence in (a) general and (b) special populations] is available on PROSPERO (registration number: CRD42016033837). Prevalence estimates were collated for all included studies with country‐, disorder‐ [FASD and fetal alcohol syndrome (FAS)] and population‐specific random‐effects meta‐analyses conducted.
Setting and Participants
A number of service‐defined subpopulations globally (see Findings).
Measurements
The main outcome was the prevalence of FASD among special subpopulations. The critical appraisal of each study was conducted using the Joanna Briggs Institute tool.
Findings
We identified 69 studies, comprising 6177 individuals diagnosed with FASD from 17 countries: Australia (n = 5), Brazil (n = 2), Canada (n = 15), Chile (n = 4), eastern Europe (Moldova, Romania and Ukraine; n = 1), Germany (n = 1), Israel (n = 1), Lithuania (n = 1), the Netherlands (n = 1), Poland (n = 1), Russia (n = 9), South Korea (n = 1), Spain (n = 1), Sweden (n = 1) and United States (n = 25). FAS and FASD prevalence rates were collated for the following five subpopulations: children in care, correctional, special education, specialized clinical and Aboriginal populations. The estimated prevalence of FASD in these special subpopulations was 10–40 times higher compared with the 7.7 per 1000 (95% confidence interval = 4.9–11.7) global FASD prevalence in the general population.
Conclusions
Global subpopulations of children in care, correctional, special education, specialized clinical and Aboriginal populations have a significantly higher prevalence of fetal alcohol spectrum disorder compared with the general population, which poses a substantial global health problem.
Keywords: Fetal alcohol spectrum disorder, fetal alcohol syndrome, prenatal alcohol exposure, prevalence, special subpopulations, systematic literature review and meta‐analysis
Introduction
World‐wide, nearly one in 10 (9.8%) women in the general population consume alcohol during pregnancy 1. Prenatal alcohol exposure places these pregnancies at risk for many adverse outcomes, including fetal alcohol spectrum disorder (FASD), which is a life‐long disability that requires assistance from a wide range of service providers including health, community and remedial education, among many others 2. FASD has a very broad phenotype 3 and is further complicated by high rates of comorbidity—over 400 disease conditions have been reported to co‐occur in people with FASD 4, with the most prevalent conditions occurring within the congenital malformations, deformities and chromosomal abnormalities (43%) and mental and behavioural disorders (19%) chapters of the International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD‐10) 5. Some comorbid conditions (e.g. language, auditory, visual, developmental, cognitive, mental and behavioural problems) are highly prevalent, ranging from 50 to 91% 4. Further, it was recently estimated that approximately one in every 13 prenatally alcohol exposed infants will have FASD, which results in approximately 630 000 infants being born with FASD in the world each year 6. Given that FASD is a life‐long disability, it is estimated that more than 11 million individuals between 0 and 18 years of age, and 25 million individuals between 0–40 years of age, have FASD in the general population world‐wide 1.
Several studies have provided estimates of the cost of care for FASD among several populations or service providers 7, 8, 9, 10, 11. These cost estimates demonstrate that FASD poses a life‐time cost of approximately 1 million dollars 11 and, as such, the prevalence of FASD is a key factor in understanding the service demands and burden of FASD across different populations and various systems of care.
The prevalence of FASD in the general population as well as patterns of prenatal alcohol exposure during pregnancy (e.g. binge drinking, drinking throughout pregnancy or, most commonly reported, drinking during the first trimester of pregnancy) also appear to vary widely between countries and regions 1, 6, 12. Understandably, the prevalence of FASD varies not only between countries, but also between different subpopulations and service systems 6. However, no study consolidating all available data on the prevalence of FASD among all special subpopulations (e.g. children in care, psychiatric care populations, etc.) currently exists. Consolidating all existing evidence on the prevalence of FASD among special subpopulations will aid in the identification of knowledge gaps and areas of study for which evidence is limited or absent, with the intention of ultimately improving prevalence estimates. Improving estimates of FASD within special subpopulations and service‐defined populations would provide improved data to plan services and budgets to serve people affected by prenatal alcohol exposure.
This is the first study, to our knowledge, to collate prevalence estimates of FASD among special subpopulations (defined by service utilization), utilizing all published studies in the world literature. In addition, country‐, disorder‐ (FASD and Fetal Alcohol Syndrome (FAS; the dysmorphic subtype form of FASD)) and population‐specific random‐effects meta‐analyses were conducted for countries with available data. The meta‐analysed FASD prevalence estimates were compared with the global FAS/FASD prevalence 1, 6.
Methods
The systematic literature search and meta‐analyses were conducted and reported according to the standards set out in the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA), provided in the PRISMA Checklist in the Supporting information, Appendix S1 13. We have also adhered to the Guidelines for Accurate and Transparent Health Estimates Reporting guidelines 14.
Comprehensive systematic literature search
A comprehensive systematic literature search was performed to identify all studies that have reported the prevalence of FASD among a special sub‐population. The search was conducted in multiple electronic bibliographic databases, including (in alphabetical order): Cumulative Index to Nursing and Allied Health Literature, EMBASE, Education Resource Information Center, MEDLINE, MEDLINE in process, PsychINFO, Scopus and Web of Science. The search was conducted using multiple combinations of the following key words: (1) epidemiolog*, frequenc*, incidence*, morbidit*, occurren*, prevalence*, probability, rate* OR statistic*; AND (2) alcohol* embryopath*, alcohol* related* neurodevelopmental* disorder*, alcohol* related* birth defect*, arnd, arbd, fetal* alcohol* effect*, fae, fas, fasd, fetal alcohol syndrome*, fetal alcohol spectrum disorder*, foetal* alcohol* effect, foetal* alcohol syndrome*, foetal* alcohol spectrum disorder*, pfas, partial fetal alcohol syndrome, partial foetal alcohol syndrome, prenatal* alcohol expos* OR pre‐natal* alcohol expos*; AND (3) cohort stud*, cross* sectional stud*, prospective cohort stud* OR retrospective cohort stud*. The search was performed to identify studies published between 1 November 1973 and 1 December 2018, without language or geographical restrictions. Further, the content pages of the major epidemiological journals, as well as citations in the relevant articles, were manually screened. The full review protocol is available in PROSPERO [includes FASD prevalence in (a) general and (b) special sub‐populations; http://www.crd.york.ac.uk/PROSPERO/), registration number CRD42016033837].
Inclusion/exclusion criteria
Articles were retained if they: (a) consisted of original, quantitative research published in a peer‐reviewed journal or scholarly report; and (b) involved a measurement of the prevalence of FASD and/or FAS among a service‐defined population. Additionally, articles were retained if they: (a) provided a measure of uncertainty (confidence interval or standard error); or (b) provided the number of cases or sample size (information to derive a measure of uncertainty). Articles were excluded if they: (a) lacked FASD prevalence data; or (b) contained prevalence estimates not specific to special subpopulations (i.e. general populations only). For a detailed list of criteria assessed for each included study please refer to the Supporting information, Appendix S2.
Study selection and data extraction
Study selection began by screening titles and abstracts for inclusion. Then, full‐text articles of all studies screened as potentially relevant were considered. A data extraction form was developed to record relevant information, such as location of the study (country; province/territory or state), study year(s), sample size, setting, number of cases (by diagnostic category), prevalence (by diagnostic category), diagnostic guideline used, sex distribution of sample, age range of sample and method of ascertainment. Two investigators conducted each study selection step; any disagreements were reconciled by team discussion. All data were extracted by one investigator and then independently cross‐checked by a second investigator; all discrepancies were reconciled by team discussion. Non‐English‐language studies deemed to be potentially relevant were translated either by colleagues fluent in the respective language or using Google Translate (and subsequently cross‐checked by a native speaker).
Critical appraisal of included studies
The critical appraisal of each study was performed using the Joanna Briggs Institute tool, specifically designed for use in systematic reviews addressing questions of prevalence 15. The following seven criteria were used: (i) representativeness of the sample to the target population, (ii) appropriate recruitment of participants, (iii) adequate sample size, (iv) detailed description of participants and setting, (v) sufficient coverage of the identified sample, (vi) use of an objective, standard criteria for ascertaining FASD and (vii) appropriateness of statistical analysis. The explanation of every criterion included in this tool is available in the Supporting information, Appendix S2.
Two investigators independently appraised the quality of each study, and all discrepancies in quality ratings were reconciled by team discussion.
Meta‐analysis
Country‐, disorder‐ (FAS and FASD, inclusive of FAS) and population‐specific meta‐analyses were performed for those countries with two or more studies that used active case ascertainment (ACA; where cases are actively sought and diagnosed) and/or clinic‐based methods (prospectively conducted in prenatal clinics or hospitals) and specified the diagnostic criteria used to ascertain cases of FAS/FASD in the respective population. Although studies that utilized passive surveillance (PS) methods (the use of existing record collections, e.g. birth certificates, registries, medical charts, adoption records) were included in the current review, they were not used in the meta‐analyses, as they are known to produce underestimates of the prevalence 16. It is well known that the majority of the countries do not have the capacity and/or resources to use the ACA approach to identify FASD cases because FASD diagnosis requires a multi‐disciplinary team and specialized clinical skills. Due to these circumstances, PS is the only option for the majority of the countries.
For all analyses, logit‐transformed results were pooled using a Bayesian meta‐analysis and non‐informative (flat) prior distributions. The combined estimates were based on the mean of the posterior distributions and the 2.5th and 97.5th percentiles. The between‐study variances were quantified using the τ2 and I 2 statistics 17. All models assumed fixed effects, as between‐study heterogeneity is difficult to assess when there are only a small number of studies 17. Publication bias was tested by visually inspecting a funnel plot for skewed distribution, using a ranked correlation test proposed by Begg & Mazumdar 18 and by employing a weighted regression test proposed by Egger and colleagues 19 (see the Supporting information, Appendix S3). Publication bias was assessed, as studies which measure FAS and FASD may have been established in specific segments of subpopulations where the prevalence of FAS and/or FASD is high (compared to other segments of the same subpopulation). Analyses were performed using the statistical software R, version 3.3.2 20, and Stata statistical software, version 14.2 21.
Results
A total of 11 871 studies were identified in the search. Sixty‐nine studies, comprising 6177 individuals diagnosed with FASD in total, were retained for data extraction. These studies represented the following 17 countries: Australia (n = 5), Brazil (n = 2), Canada (n = 15), Chile (n = 4), eastern Europe (Moldova, Romania and Ukraine; n = 1), Germany (n = 1), Israel (n = 1), Lithuania (n = 1), the Netherlands (n = 1), Poland (n = 1), Russia (n = 9), South Korea (n = 1), Spain (n = 1), Sweden (n = 1) and the United States (n = 25). A schematic diagram depicting the search strategy employed is presented in Fig. 1.
Figure 1.
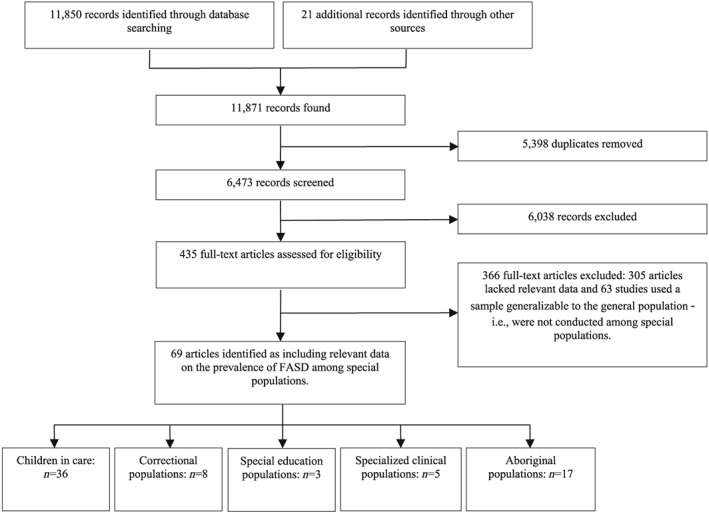
Schematic diagram depicting the search strategy employed
Following the identification of 69 studies, they were categorized into the following five special subpopulations: children in care (e.g. adoptees, foster children; n = 36), correctional (n = 8), special education (n = 3), specialized clinical (n = 5) and Aboriginal (n = 17).
The quality appraisals of the included studies indicated that 100% (n = 69) of studies were conducted on samples that were representative of the target population; 97.1% (n = 67) of studies appropriately recruited participants; 65.2% (n = 45) of studies had an adequate sample size; 84.1% (n = 58) of studies provided a detailed description of participants and setting; 95.7% (n = 66) of studies had sufficient coverage of the identified sample; 60.9% (n = 42) of studies used objective, standard criteria for ascertaining FASD; and 100% (n = 69) of studies used an appropriate statistical analysis. Overall, 29.0% (n = 20) of studies met all seven criteria. The quality appraisals of the included studies are presented in the Supporting information, Appendix S2.
Prevalence of FASD among children in care
The prevalence of FASD among children in care was available for the following countries: Brazil (n = 1), Canada (n = 4), Chile (n = 2), Germany (n = 1), Israel (n = 1), Lithuania (n = 1), the Netherlands (n = 1), Poland (n = 1), Russia (n = 9), Spain (n = 1), Sweden (n = 1) and the United States (n = 12); one study 22 reported the prevalence of FAS among children in care from eastern Europe (Moldova, Romania and Ukraine; n = 1). Twenty studies used ACA, two studies used clinic‐based methods, 10 studies used PS and four studies used mixed methods. Twenty (of 36) studies reported the diagnostic guideline/case definition used, with the majority (35.0%) using the four‐digit diagnostic code 23 (see Table 1).
Table 1.
Study characteristics and prevalence of FAS and FASD among children in care (n = 36) reported in the identified studies, by country.
| Reference | Country (State/Province/Territory) | Study year(s) | Type of institution(s)/Setting | Sample size | Number of cases of FAS | Prevalence of FAS (per 1000) | Number of cases of FASD | Prevalence of FASD (per 1000) | Diagnostic guidelines/Case definition | Sex (% male) | Age range (years) | Method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Children in care | ||||||||||||
| Strömland et al. 2015 24 | Brazil (Recife) | NA | Orphanage | 94 | 3 | 31.9 | 16 | 170.2 | Clarification of the IOM criteria (Hoyme et al. 2005 25) | 57.4 | 3m–14 | ACA |
| Burge, 2007 26 | Canada (Ontario) | 2003 | Permanent wards | 429 | NA | NA | 14 | 32.6 | NA | NA | 0–18 | PS |
| Fuchs et al. 2005 27 | Canada (Manitoba) | 2004–05 | Child welfare agencies | 5664 | NA | NA | 640 | 113.0 | NA | NA | 0–20 | PS |
| Fuchs & Burnside, 2014 28 | Canada (Alberta, Manitoba, Ontario) | 2010–14 | Child welfare agencies | 15 623 (Alberta: 6767; Manitoba: 8323; Ontario: 533) | NA | NA |
1776 (diagnosed and suspected; Alberta: 699; Manitoba: 1021; Ontario: 56) |
113.7 | NA | 51.3 (Alberta: 52.3; Manitoba: 50.1; Ontario: 57.8) | 0–21 | PS |
| Robert et al. 2009 29 | Canada (Quebec) | 2004–06 | Adoptees from eastern Europe | 29 | 1 | 34.5 | 7 | 241.4 | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | 59.0 | 4–8 | ACA |
| Mena et al. 1987 30 | Chile (VIII region) | 1984 | Foster care | 931 | 43 | 46.2 | 184 | 197.6 | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | 57.0 | NA | ACA |
| Mena et al. 1993 32 | Chile (Metropolitan region) | 1989–90 | Child welfare and homes for those with metal deficiencies | 291 | 18 | 61.9 | 178 | 611.7 | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | 31.0 | 1–20+ | ACA |
| Diamond et al. 2003 22 | Eastern Europe (Romania: 73%, Ukraine: 22%, Moldova: 5%) | 1999–2001 | Pre‐adoption: orphanage (84.1%) and foster care (15.9%) | 82 | 0 | 0.0 | NA | NA | NA | 51.0 | 2m–4 | ACA |
| Feldmann, 2012 33 | Germany | NA | Foster care | 267 | 62 | 232.2 | NA | NA | Fetal Alcohol Syndrome Questionnaire (developed by Feldmann) | NA | NA | PS |
| Tenenbaum et al. 2011 34 | Israel | NA | Pre‐adoption and foster care | 100 | 2 | 20.0 | 4 | 40.0 | IOM criteria (Stratton et al. 1996 35) | 42.0 | 0–2 | ACA |
| Kuzmenkoviene et al. 2012 36 | Lithuania | NA | Orphanages | 337 | 74 | 219.6 | 134 | 397.6 | Clarification of the IOM criteria (Hoyme et al. 2005 25) | NA | 3–5 | ACA |
| Knuiman et al. 2012 37 | Netherlands | 1999–2006 | Adoptees for Poland | 121 | 26 | 214.9 | 37 | 305.8 | NA | 52.1 | 5–17 | PS (questionnaire administered to adoptive parents) |
| Gyrczuk et al. 2014 38 | Poland (Otwock) | 2008–12 | Pre‐adoption intervention centre | 490 | 108 | 220.4 | NA | NA | NA | 46.3 | 0–1 | Clinic‐based |
| Aronson, 1997 39 | Russia | 1994–97 | Pre‐adoption: orphanages | 131 | 2 | 15.3 | NA | NA | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | NA | NA | Mixed methods (ACA and PS) |
| Konovalova et al. 2009 40 | Russia | NA | 41 institutions (boarding schools with special needs programmes for those with mental deficiencies, regular and special needs orphanages, and schools of the social welfare system) | 3675 | 320 | 87.1 | 557 | 151.6 | NA | 60.0 | 4–21 | ACA |
| Miller et al. 2006 41 | Russia | NA | Orphanages | 234 | 17 | 72.7 | NA | NA | 4‐digit diagnostic code (Astley & Clarren, 1999 23) and screening tool (Burd et al. 1999 42) | 52.0 | 1.5m–6 | ACA |
| Miller et al. 2007 43 | Russia | 2004–05 | Orphanages | 193 | 19 | 98.5 | NA | NA | 54.4 | 2m–6 | PS | |
| Riley et al. 2003 44 | Russia | 1999 | Boarding schools and orphanages for children with mental deficiencies | 2352 | 186 | 79.1 | NA | NA | Case definition provided | NA | NA | ACA |
| The St. Petersburg‐USA Orphanage Research Team, 2005 45 | Russia | 1997–2002 | Orphanages | 1167 | 112 | 96.0 | NA | NA | NA | 0–6 | PS | |
| Warren et al. 2001 46 | Russia | NA | Boarding schools and orphanages | 184 | 26 | 141.3 | NA | NA | IOM criteria (Stratton et al. 1996 35) | 67.0 | 8–17 | ACA |
| Bubnov, 2010 47 | Russia, Yekaterinburg | 2005–09 | Orphanages | 445 | 67 | 150.6 | 177 | 397.8 | Clarification of the IOM criteria (Hoyme et al. 2005 25) | NA | 2m–4 | ACA |
| Legonkova, 2011 48 | Russia, St Petersburg | 2004–10 | Orphanages for children with psychoneurological problems and orphanages for children with developmental abnormalities | NA | NA | 46.0–93.0 in orphanages for children with psycho‐neurological problems; and 464.0–680.0 in orphanages for children with developmental abnormalities | NA | NA | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | NA | 0–7 | ACA |
| Olivan‐Gonzalvo, 2011 49 | Spain | 2000–10 | Adoptees from eastern Europe (Russia: 92%) | 1062 | 117 | 110.2 | NA | NA | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | 60.0 | NA | ACA |
| Landgren et al. 2010 50 | Sweden | NA | Adoptees from eastern Europe (Estonia, Latvia, Poland Romania, Russia) | 71 | 21 | 295.8 | 37 | 521.1 | IOM criteria (Stratton et al. 1996 35) | 56.0 | 5–10 | ACA |
| Albers et al. 1997 51 | United States | 1991–95 | Adoptees from Europe | 56 | 1 | 17.9 | NA | NA | Smith's Recognizable Pattens of Human Malformation (Lyons, 1997 52) | 46.0 | 2.5m–9 | ACA |
| Astley et al. 2002 53 | United States (Washington) | 1999–2001 | Foster care | 600 | 6 | 10.0 | NA | NA | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | 52.0 | NA | ACA |
| Chasnoff et al. 2015 54 | United States (Illinois) | NA | Foster and adopted youth referred to a children's mental health centre | 547 | 93 | 170.0 | 156 | 285.2 | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | 63.8 | 4–18 | Clinic‐based |
| Farina et al. 2004 55 | United States | NA | Adoptees from Russia | 29 | 0 | 0.0 | 10 | 344.8 | NA | 48.0 | 1–7 | ACA |
| Johnson et al. 1996 56 | United States | NA | Adoptees from Eastern Europe (Belarus: 2%, Poland: 1%, Romania: 4%, Russia: 76%, Other: 17%) | 252 | 6 | 23.8 | NA | NA | NA | NA | 0–10 | PS |
| Loman et al. 2009 57 | United States | NA | Adoptees [post‐institutionalized and foster care for Eastern Europe (21%), South America (21.5%), Asia (57%) and Africa (0.5%)] | 200 | NA | NA | 8 | 40.0 | CDC diagnostic guidelines (Bertrand et al. 2004 58) | 46.5 | 8–11 | Mixed methods (ACA & PS) |
| McGuinness et al. 2000 59 | United States | 1997 | Adoptees from Eastern Europe | 105 | NA | NA | 7 | 66.7 | NA | 48.0 | 6–9 | PS |
| Miller & Hendrie, 2000 60 | United States | 1991–98 | Adoptees from China | 452 | 0 | 0.0 | NA | NA | NA | 2.0 | 2m–1 | ACA |
| Miller et al. 2005 61 | United States | 1988–2004 | Adoptees from Guatemala (orphanages, foster‐ and mixed‐care settings) | 103 | NA | NA | 19 | 184.5 | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | 53.0 | Mixed methods (ACA & PS) | |
| Miller et al. 2009 62 | United States | 2004–07 | Adoptees from eastern Europe | 138 | NA | NA | 10 | 72.5 | NA | 51.0 | 7m–5 | ACA |
| Miller et al. 2009 63 | United States | NA | Adoptees from eastern Europe (Bulgaria: 2%, Lithuania: 6%, Latvia: 2%, Moldova: 6%, Romania: 26%, Russia: 52%, Ukraine: 6%) | 50 | NA | NA | 2 | 40.0 | NA | 52.0 | 8–11 | Mixed methods (ACA & PS) |
| Ringeisen et al. 2008 64 | United States | 1999–2000 | Child welfare agencies | 5496 | 29 | 5.3 | NA | NA | NA | 50.0 | 0–14 | PS |
ACA = active case ascertainment; DSM = Diagnostic and Statistical Manual of Mental Disorders; FAS= fetal alcohol syndrome; FASD = fetal alcohol spectrum disorder; IOM = Institute of Medicine; m = months; NA = not available; ND–PAE: neurodevelopmental disorder associated with prenatal alcohol exposure; PS = passive surveillance; RSA = Research Society on Alcoholism.
The prevalence of FAS was reported to be the lowest among pre‐adoption children in orphanages and foster care in eastern Europe at 0.0 per 1000 (obtained via ACA) 22 and the highest among orphanages for children with developmental abnormalities in Russia at 680.0 per 1000 (obtained via ACA) 48, with median 79.1. The prevalence of FASD was reported to be the lowest among permanent wards in Canada at 32.6 per 1000 (obtained via PS) 26 and the highest among children in child welfare and homes for those with mental deficiencies in Chile at 611.7 per 1000 (obtained via ACA) 32, with median 177.3 per 1000.
A meta‐analysis on the prevalence of FAS/FASD among children in care was conducted for the following three countries: Chile, Russia and the United States. Based on two studies 30, 32, the pooled prevalence of FAS and FASD among children in care in Chile was estimated to be 51.9 per 1000 (95% CI = 40.3–64.9 per 1000) and 312.4 per 1000 (95% CI = 283.6–339.1 per 1000), respectively. In Russia, the pooled prevalence of FAS among children in care was estimated to be 95.5 per 1000 (95% CI = 85.3–105.4 per 1000) 39, 41, 44, 46, 47. The pooled prevalence of FAS and FASD among children in care in the United States was estimated to be 142.3 per 1000 (95% CI = 117.3–167.8 per 1000) 51, 53, 54 and 251.5 per 1000 (95% CI = 220.0–281.7 per 1000) 54, 57, 61, respectively (Table 2 and Figs 2 and 3).
Table 2.
Pooled prevalence of FAS and FASD among special subpopulations.
| Country | FAS/FASD | No. of studies | Prevalence per 1000 (%) | 95% confidence interval per 1000 | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Children in care | |||||
| Chile | FAS | 2 | 51.9 (5.2) | 40.3 | 64.9 |
| FASD | 2 | 312.4 (31.2) | 283.6 | 339.1 | |
| Russia | FAS | 5 | 95.5 (6.6) | 85.3 | 105.4 |
| United States | FAS | 3 | 142.3 (14.2) | 117.3 | 167.8 |
| FASD | 3 | 251.5 (25.2) | 220.0 | 281.7 | |
| Correctional populations | |||||
| Canada | FASD (adult) | 2 | 146.7 (14.7) | 98.2 | 204.9 |
| Special education populations | |||||
| Chile | FAS | 2 | 29.1 (2.9) | 19.2 | 42.0 |
| FASD | 2 | 84.2 (8.4) | 66.6 | 103.1 | |
| Aboriginal populations | |||||
| Australia | FAS | 2 | 2.3 (0.2) | 1.4 | 3.5 |
| FASD | 2 | 14.8 (1.5) | 11.4 | 18.6 | |
| Canada | FAS | 3 | 60.8 (6.1) | 42.1 | 83.4 |
| FASD | 3 | 43.6 (4.4) | 37.9 | 49.3 | |
| United States | FAS | 3 | 2.8 (0.3) | 2.2 | 3.5 |
| FASD | 2 | 4.4 (0.4) | 3.5 | 5.3 | |
Only studies that used active case ascertainment and/or clinic‐based methods and specified the diagnostic criteria used to ascertain cases of fetal alcohol syndrome/fetal alcohol spectrum disorder (FAS/FASD) in the respective population were included in the meta‐analyses. Studies that utilized passive surveillance methods were excluded from the meta‐analyses.
Figure 2.
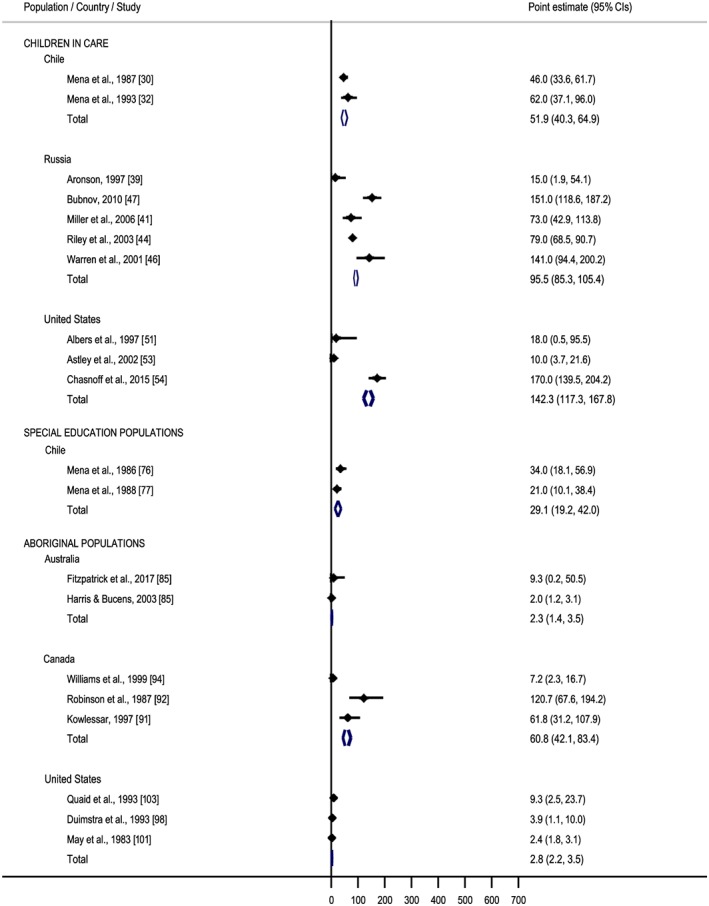
Forest plot of meta‐analysed fetal alcohol syndrome (FAS) prevalence studies. [Colour figure can be viewed at wileyonlinelibrary.com]
Figure 3.
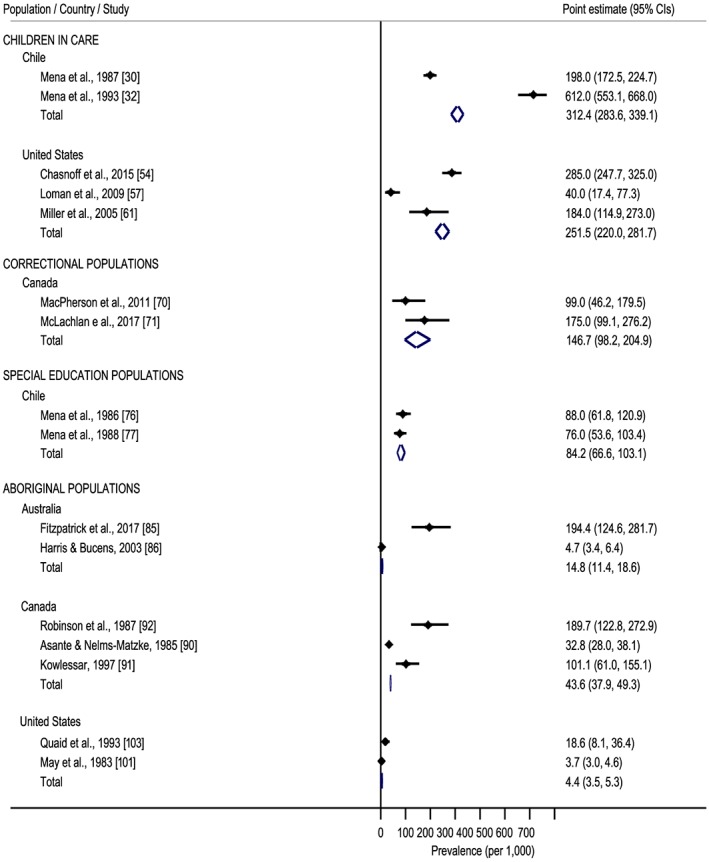
Forest plot of meta‐analysed fetal alcohol spectrum disorder (FASD) prevalence studies. [Colour figure can be viewed at wileyonlinelibrary.com]
Prevalence of FASD among correctional populations
The prevalence of FASD among correctional populations was available for three countries: Australia (n = 1), Canada (n = 6) and the United States (n = 1). Two studies used ACA, one study used clinic‐based methods, four studies used PS and one study used mixed methods. Five (of eight) studies reported the diagnostic guideline/case definition used; with the majority (28.6%) using the 2005 Canadian diagnostic guidelines 65 (see Table 3).
Table 3.
Study characteristics and prevalence of FAS and FASD among correctional populations (n = 8), special education (n = 3), specialized clinical populations (n = 5) and Aboriginal populations (17) reported in the identified studies, by country.
| Reference | Country (State/Province/Territory) | Study year(s) | Type of institution(s)/Setting | Sample size | Number of cases of FAS | Prevalence of FAS (per 1000) | Number of cases of FASD | Prevalence of FASD (per 1000) | Diagnostic guidelines/Case definition | Sex (% male) | Age range (years) | Method |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correctional populations | ||||||||||||
| Bower et al. 2018 66 | Australia (western Australia) | 2015–16 | Youth detention centre; 73.7% Aboriginal | 99 | – | – | 36 | 363.6 | Australian Guide to the Diagnosis of FASD 67 | 92.9 | 10–< 18 | ACA |
| Burd et al. 2003 68 | Canada | 2001–02 | Federal and provincial prisons | 148 797 | 13 | 0.1 | NA | NA | NA | 91.2 | NA | PS (survey) |
| Fast et al. 1999 69 | Canada (British Columbia and Yukon) | 1995–96 | In‐patient assessment unit of youth forensic psychiatric services | 287 | 3 | 10.5 | 67 | 233.5 | IOM criteria (Stratton et al. 1996 35) | NA | 12–18 | Clinic‐based |
| MacPherson et al. 2011 70 | Canada (Manitoba) | 2005–06 | Male‐only medium security penitentiary for adults | 91 | NA | NA | 9 | 98.9 | Canadian diagnostic guidelines (Chudley et al. 2005 65) | 100.0 | 19–30 | Mixed methods [ACA and PS (interview)] |
| McLachlan et al. 2017 71 | Canada (Yukon) | 2014–15 | Correctional centre, and offender and supervision Services | 80 | 0 | 0.0 | 14 | 175 | Canadian diagnostic guidelines (Chudley et al. 2005 65) | NA | 18–40 | ACA |
| Murphy et al. 2005 72 | Canada (British Columbia) | 2004 | Juvenile detention centres | 137 | NA | NA | 16 | 116.8 | NA | 89.8 | 14–19 | PS (survey) |
| Rojas & Gretton, 2007 73 | Canada (British Columbia) | 1985–2004 | Youth Sexual Offence Treatment Programme | 230 | NA | NA | 25 | 108.7 | Case definition provided (based on Boland et al. 2000 74) | 100.0 | 12–18 | PS |
| Burd et al. 2004 75 | United States | 2001–02 | Prison systems and community corrections facilities | 3 080 904 | 1 | 0.0003 | NA | NA | NA | 89.7 | NA | PS (survey) |
| Special educational populations | ||||||||||||
| Mena et al. 1986 76 | Chile (Concepción) | 1982 | Special schools for mentally handicapped children | 386 | 13 | 33.7 | 34 | 88.1 | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | NA | NA | ACA |
| Mena et al. 1988 77 | Chile (Cautin, Concepción, Linares, Ranco) | 1985–86 | Special schools for mentally handicapped children | 475 | 10 | 21.1 | 36 | 75.8 | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | NA | NA | ACA |
| Lee et al, 2016 78 | South Korea | NA | Institutions (children with mental retardation) | 307 | 13 | 42.3 | NA | NA | Case definition provided | NA | NA | ACA |
| Specialized clinical populations | ||||||||||||
| Grinfeld et al. 1999 79 | Brazil (São Paulo) | 1997 | Babies referred to genetic clinics | 16 640 | 17 | 1.0 | NA | NA | NA | NA | NA | PS |
| Bell & Chimata, 2015 80 | United States (Chicago) | 2013–14 | Psychiatric care population | 611 | NA | NA | 87 | 142.4 | DSM‐5 criteria of ND‐PAE (APA, 2013 81) | 43.0 | 4–78 | Clinic‐based |
| Cadle et al. 1996 82 | United States (Kentucky) | 1981–95 | Patients evaluated at genetic clinics | 4212 | 27 | 6.4 | NA | NA | NA | NA | NA | Clinic‐based |
| O'Connor et al. 2006 83 | United States | NA | Psychiatric care population | 122 | 10 | 82.0 | NA | NA | 4‐digit diagnostic code (Astley & Clarren, 1999 23) | 81.1 | NA | PS |
| Shanske & Kazi, 1980 84 | United States (New York) | NA | Developmentally disabled clinical population | 905 | 13 | 14.4 | 19 | 21.0 | NA | NA | 0–7 | Clinic‐based |
| Aboriginal populations | ||||||||||||
| Fitzpatrick et al. 2017 85 | Australia (northwestern) | 2010–11 | School‐aged children in very remote communities: community sites and local schools | 108 | 1 | 9.3 | 21 | 194.4 | Canadian diagnostic guidelines (Chudley et al. 2005 65) with adaptations to accommodate the cultural context | 52.8 | 7.5–9.6 | ACA |
| Harris & Bucens, 2003 86 | Australia (northern Territory) | 1990–2000 | Paediatric wing, hospital | 9077 | 18 | 2.0 | 43 | 4.7 | Adapted 4‐digit diagnostic code (Astley & Clarren, 1999 23) and the criteria by the AAP (2000 87) | NA | 0–10 | Mixed methods (PS & Clinic‐based) |
| Mutch et al. 2015 88 | Australia (western) | 1980–2010 | Children captured in the Western Australian Register of Developmental Anomalies | 45 078 | NA | NA | 188 | 4.1 | NA | NA | 0–15 | PS |
| Rothstein et al. 2007 89 | Australia (Queensland) | 2001–06 | Children for specialist paediatric follow‐up captured by the FNQ Paediatric Outreach Service | 2195 | NA | NA | 32 | 14.6 | NA | 55.0 | 0–18 | PS |
| Asante & Nelms‐Matzke, 1985 90 | Canada (northwest British Columbia and Yukon) | 1983–84 | Chronically handicapped children referred for assessment | 5065 | NA | NA | 166 | 32.8 | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | 63.0 | 0–16 | ACA |
| Kowlessar, 1997 91 | Canada (Manitoba) | 1981–90 | Local school in First Nations community | 178 | 11 | 61.8 | 19 | 101.1 | IOM criteria (Stratton et al. 1996 35) | NA | 5–15 | ACA |
| Robinson et al. 1987 92 | Canada (British Columbia) | 1984–85 | Community‐based: Native Indian community | 116 | 14 | 120.7 | 22 | 189.7 | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | 49.6 | 3–18 | ACA |
| Werk et al. 2013 93 | Canada | 2006 | Canadian census survey catered to Aboriginal children living off‐reserve | 11 868 | NA | NA | 83 | 7.0 | NA | NA | 0–5 | PS (survey) |
| Williams et al. 1999 94 | Canada (Manitoba) | 1994–96 | Live births occurring in Thompson General Hospital in 1994 | 696 | 5 | 7.2 | NA | NA | IOM criteria (Stratton et al. 1996 35) | NA | NA | Mixed methods (ACA & PS) |
| Chávez et al. 1988 95 | United States | 1981–86 | Birth Defects Monitoring Programme: hospitals with obstetric services | 19 412 | 58 | 3.0 | NA | NA | NA | NA | 0–1 (newborns) | PS |
| CDC, 1995 96 | United States (Iowa, Nebraska, North Dakota, South Dakota) | 1981–92 | Indian Health Service (IHS) and IHS contract facilities in tribal or American Indian communities | 22 222 | 60 | 2.7 | NA | NA | Criteria by Sokol & Clarren (1989 97) | NA | 0–31 | PS |
| Duimstra et al. 1993 98 | United States (Northern Plains) | 1987–90 | Indian Health Service facilities; IHS hospital out‐patient settings; home visits | 1022 | 4 | 3.9 | NA | NA | Guidelines established by the Fetal Alcohol Study Group of the RSA (Rosett, 1980 31) | NA | 5–18m | ACA |
| Egeland et al. 1998 99 | United States (Alaska) | 1977–92 | Paediatric practices that were referral centres for FAS; hospitals; regional native health corporations; state department of health and social services | 37 346 | 114 | 3.1 | NA | NA | Case definition provided | NA | 0–16 | PS |
| Fox et al. 2015 100 | United States (Arizona, Colorado, New York) | 2010 | Surveillance site using multiple data sources: genetic/developmental clinics; hospitals; health maintenance organizations; Medicaid; juvenile justice system | 13 938 | 28 | 2.0 | NA | NA | Case definition based on IOM criteria (Stratton et al. 1996 35) | NA | 7–9 | PS |
| May et al. 1983 101 | United States (southwestern USA: New Mexico, southern Colorado, southern Utah, northern Arizona) | 1980–82 | Children belonging to Navajo, Pueblo, and Plains culture tribes | 22 963 | 55 | 2.4 | 85 | 3.7 | Case definition provided | 55.6 | 0–14 | ACA |
| NBDPN, 2003 102 | United States (24 States) | 1996–2000 | State programmes providing surveillance data on birth defects | 77 630 | 32 | 0.4 | NA | NA | NA | NA | 0–1 (newborns) | PS |
| Quaid et al. 1993 103 | United States (central Oregon) | 1991 | Indian Health Service Clinic and assisting health/social services personnel; dysmorphology clinic | 429 | 4 | 9.3 | 8 | 18.7 | Criteria by Sokol & Clarren (1989 97) | NA | 0–3 | ACA |
ACA = active case ascertainment; DSM = Diagnostic and Statistical Manual of Mental Disorders; FAS = fetal alcohol syndrome; FASD = fetal alcohol spectrum disorder; IOM = Institute of Medicine; m: months; NA = not available; NBDPN = National Birth Defects Prevention Network; ND–PAE = neurodevelopmental disorder associated with prenatal alcohol exposure; PS = passive surveillance; RSA = Research Society on Alcoholism.
In Australia, the prevalence of FASD among a correctional population (73.7% were Aboriginal) was reported to be 363.6 per 1000 (obtained via ACA) 66. In Canada, the reported prevalence of FAS and FASD ranged from 0.0 per 1000 (obtained via ACA) 71 to 10.5 per 1000 (obtained via clinic‐based methods) 69 and 17.5 per 1000 (obtained via ACA) 66 to 233.5 per 1000 (obtained via clinic‐based methods) 69, with median 108.7. In the United States, the reported prevalence of FAS was 0.0003 per 1000 (obtained via PS) 75. The medians for FAS and FASD prevalence estimates in this special subpopulation (all countries) were 0.05 per 1000 and 112.8 per 1000, respectively. The pooled prevalence of FASD among adults in the correctional system in Canada was estimated to be 146.7 per 1000 (95% CI = 98.2–204.9 per 1000) 70, 71 (see Table 2 and Figs 2 and 3).
Prevalence of FASD among special education populations
The prevalence of FASD among special education populations was available for Chile (n = 2) and South Korea (n = 1). The reported prevalence of FAS and FASD among special education populations, obtained via ACA using the guidelines established by the Fetal Alcohol Study Group of the RSA 31, ranged from 21.1 per 1000 77 to 42.3 per 1000 78 with median 33.7 for FAS, and 75.8 per 1000 77 to 88.1 per 1000 76 with median 82.0 for FASD. The reported prevalence of FAS among a special education population in South Korea was 42.3 per 1000 (obtained via ACA using a study‐specific case definition) 78 (see Table 3).
The pooled prevalence of FAS and FASD among special education populations in Chile was estimated to be 29.1 per 1000 (95% CI = 19.2–42.0 per 1000) 76, 77 and 84.2 per 1000 (95% CI = 66.6–103.1 per 1000) 76, 77, respectively (see Table 2 and Figs 2 and 3).
Prevalence of FASD among specialized clinical populations
The prevalence of FASD among specialized clinical populations was available for two countries: Brazil (n = 1) and the United States (n = 4). Three studies used clinic‐based methods and two studies used PS. The reported prevalence of FAS among babies referred to genetic clinics in Brazil was 1.0 per 1000 (obtained via PS; diagnostic guideline/case definition used not specified) 79. The prevalence of FASD was reported for three specialized clinical populations in the United States: psychiatric care population (n = 2), patients evaluated at genetic clinics (n = 1) and a developmentally disabled clinical population (n = 1). One study 80 used the DSM‐5 criteria of ND‐PAE 81 and one study 83 used the four‐digit diagnostic code 23; the remaining two studies did not report the diagnostic guideline/case definition used. The lowest prevalence of FAS was reported among patients evaluated at genetic clinics at 6.4 per 1000 (obtained via clinic‐based methods) 82 and the highest prevalence was reported among a psychiatric care population at 82.0 per 1000 (obtained via PS) 83, with median 10.4. The lowest prevalence of FASD was reported among a developmentally disabled clinical population at 21.0 per 1000 (obtained via clinic‐based methods) 84 and the highest among a psychiatric care population at 142.4 per 1000 (obtained via clinic‐based methods) 80, with median 81.7 (see Table 3).
Based on inclusion criteria, it was not possible to conduct a meta‐analysis on the prevalence of FAS/FASD among specialized clinical populations for any country.
Prevalence of FASD among aboriginal populations
The prevalence of FASD among Aboriginal populations was available for three countries: Australia (n = 4), Canada (n = 5) and the United States (n = 8). Seven studies used ACA, eight studies used PS and two studies used mixed methods. Twelve (of 17) studies reported the diagnostic guideline/case definition used, with the majority (17.6%) using the guidelines established by the Fetal Alcohol Study Group of the Research Society on Alcoholism (RSA) 31 (see Table 3).
In Australia, the reported prevalence of FAS and FASD ranged from 2.0 per 1000 (obtained via PS and clinic‐based methods) 86 to 9.3 per 1000 (obtained via ACA) 85, with median 5.7 (FAS), and 4.1 per 1000 (obtained via PS) 88 to 194.4 per 1000 (obtained via ACA) 85, with median 9.7 (FASD), respectively. In Canada, the reported prevalence of FAS and FASD ranged from 7.2 per 1000 (obtained via ACA and PS) 94 to 120.7 per 1000 (obtained via ACA) 92, with median 61.8, and 7.0 per 1000 (obtained via PS) 93 to 189.7 per 1000 (obtained via ACA) 92, with median 66.9, respectively. In the United States, the reported prevalence of FAS and FASD ranged from 0.4 per 1000 (obtained via PS) 102 to 9.3 per 1000 (obtained via ACA) 103, with median 2.8 for FAS, and 3.7 per 1000 (obtained via ACA) 103 to 18.7 per 1000 (obtained via ACA) 103, with median 11.2, for FASD.
In Australia, the pooled prevalence of FAS and FASD among Aboriginal populations was estimated to be 2.3 per 1000 (95% CI = 1.4–3.5 per 1000) 85, 86 and 14.8 per 1000 (95% CI = 11.4–18.6 per 1000), respectively. In Canada, the pooled prevalence of FAS and FASD among Aboriginal populations was estimated to be 60.8 per 1000 (95% CI = 42.1–83.4 per 1000) 91, 92, 94 and 43.6 per 1000 (95% CI = 37.9–49.3 per 1000) 90, 91, 92, respectively. The pooled prevalence of FAS and FASD among Aboriginal populations in the United States was estimated to be 2.8 per 1000 (95% CI = 2.2–3.5 per 1000) 98, 101, 103 and 4.4 per 1000 (95% CI = 3.5–5.3 per 1000) 101, 103, respectively (see Table 2 and Figs 2 and 3).
The pooled prevalence and results of the tests of heterogeneity and publication bias for the meta‐analyses on the prevalence of FAS and FASD among subpopulations by country are presented in the Supporting information, Appendix S3.
Comparison of FASD prevalence in special subpopulations versus global FASD prevalence in general population
The meta‐analysed prevalence estimates of FASD among special subpopulations appear to far exceed those found among the general population. For example, compared to the recently estimated global prevalence of FASD in the general population (7.7 per 1000; 95% CI = 4.9–11.7) 6, the prevalence among children in care was 32 times higher in the United States (251.5 per 1000; 95% CI = 220.0–281.7) 54, 57, 61 and 40 times higher in Chile (312.4 per 1000; 95% CI = 283.6, 339.1) 30, 32; the prevalence among adults in the Canadian correctional system (146.7 per 1000; 95% CI = 98.2, 204.9) 70, 71 was 19 times higher; and the prevalence among special education populations in Chile (84.2 per 1000; 95% CI = 66.6–103.1) 76, 77 was over 10 times higher. Overall, the estimated prevalence of FASD in these special sub‐populations was 10‐40 times higher compared with the prevalence estimate for the global general population: 7.7 per 1000 (95% confidence interval: 4.9‐11.7).
Further, the prevalence reported in the individual studies is even more alarming. For instance, the prevalence of FASD among children in care with mental deficiencies in Chile was reported to be 620 per 1000 32, among adoptees from eastern Europe it was more than 520 per 1000 50 and among children residing in orphanages in Lithuania it was approximately 400 per 1000 36. The highest prevalence of FAS, between 460 and 680 per 1000, was reported in Russia in orphanages for children with developmental abnormalities 48. Additionally, the prevalence of FASD among youth in correctional services was reported to be more than 230 per 1000 in Canada 69 and more than 140 per 1000 among psychiatric care populations in the United States 80.
Discussion
This study demonstrates that the prevalence of FASD is highly variable, and disproportionately impacts some special subpopulations, and this is not unexpected given the context of the origin populations and the life‐course of individuals with FASD. In general, children are often placed in care due to a number of unfavourable circumstances, such as parental alcohol and/or other drug problems, abuse and/or neglect, abandonment and young maternal age. These circumstances are associated with an increased probability that a child had been exposed to alcohol in utero 104. If appropriate diagnosis, interventions and support services are not put in place early in life and maintained throughout their life, many youth and adults with FASD are at a high risk for becoming involved in the legal system, either as offenders or as victims. It was estimated that youth with FASD are 19 times more likely to be incarcerated than youth without FASD on any given day in a specific year 105. Lastly, individuals with FASD are likely to suffer from developmental delay, learning problems and mental health problems 4; therefore, a high prevalence among special education populations (e.g. in special schools for mentally handicapped children) and specialized clinical populations (e.g. in psychiatric care) is not surprising.
Several factors contribute to the prevalence of FASD in Aboriginal populations. For example, the prevalence of alcohol use during pregnancy in the Aboriginal populations of the United States and Canada were found to be approximately three to four times higher, respectively, compared to the general population 106. Even more alarmingly, approximately 20% of women who consume alcohol during pregnancy engage in binge drinking in the Aboriginal populations compared to 3% in the general population in both countries 106. The high prevalence of alcohol consumption and FASD in some Aboriginal populations must be understood within the historical and social context of colonization and the socio‐demographic realities. Intergenerational impacts of colonial history, including trauma, residential school experiences and economic and social marginalization, contribute to alcohol use in Aboriginal communities 107, 108.
While all these subpopulations share many risk markers, it is not clear whether FASD results in a common risk factor or impairment that increases risk for contact with certain service systems. It is also unclear whether the variation in the prevalence of FASD among the special subpopulations identified is due to differences in rates or patterns of prenatal alcohol exposure, dosimetry or increased susceptibility to alcohol exposure prenatally. Both missed diagnoses and underdiagnoses of FASD confound efforts to better understand these differences 54. What is clear, however, is that exposure to alcohol prenatally that leads to a diagnosis of FASD has predictive implications with respect to adversity. In the past, it could be argued that we had insufficient information on FASD to make public policy recommendations. We now have convincing evidence that FASD is a relatively prevalent alcohol‐related disorder that greatly increases the risk of long‐term adversity. As such, public policy and clinical care for people with FASD needs to change to respond to such predictable outcomes. The data presented in this study have important implications for health‐care providers, psychiatrists, psychologists, social workers, individuals working within the justice and child welfare systems, policymakers and, most importantly, for people affected with FASD and their families. These prevalence estimates are crucial for promoting early identification of FASD and provision of prevention and care interventions as well as for informing policymakers and service providers about the overall impact of FASD on population health. In addition, these prevalence estimates will help to generate policy and programme support for services required by people with FASD. Routine screening protocols should be established for identification of children, youth and adults in different settings such as child welfare, special education, justice system and others in order to provide them with appropriate support and early interventions. Service providers should be trained on FASD awareness, identification and interventions of people with higher risk for prenatal alcohol exposure and FASD.
There are several limitations in this study. First, FASD prevalence estimates were derived over an approximately 40‐year time‐span, so the prevalence of FASD, for example, in an American Indian community in the 1980s may not be relevant at all to current prevalence in that community, nor comparable to the prevalence in an aboriginal community in Australia captured 30 years later. Specifically, the majority of the studies reporting prevalence of FASD among Aboriginal populations in Canada are 2–3 decades old and suffer from many methodological limitations 90, 91, 92, 94, and thus those existing data are not applicable for decision‐making purposes and rigorous active case ascertainment studies are urgently needed in Canada. Further, outdated studies from Australia, which are based on PS, report an unrealistically low prevalence of FASD (lower or slightly above 1%) among Aboriginal populations 86, 89. However, a recent ACA study reported the prevalence of FASD among Aboriginal populations of Australia to be over 19% 85.
Further, existing studies suffer from variability in the quality and inconsistency in the methods used among them. Specifically, studies used 12 different diagnostic criteria to classify children or adults as FAS or FASD (all of which have substantial lack of overlap 109, not to mention that these studies had widely varying criteria for documenting quantity and frequency of alcohol consumption required. It is also possible that some prevalence studies were initiated due to the suspected high rate of FASD in these settings, demonstrated by an increased demand to service providers or increased health‐care cost, which may lead to overestimated results.
There are multiple other special subpopulations impacted by increased rates of FASD—two examples are children whose mothers are in treatment for substance use disorder(s) and infants requiring neonatal intensive care. However, there are no studies that examined the prevalence of FASD in these special subpopulations. Further, 45 years after discovering FAS, we found that it was not possible to conduct meta‐analyses among low socio‐economic populations and specialized clinical populations due to insufficient data; thus, rigorous research is urgently needed to appreciate those populations most impacted by FASD.
It appears that prenatal alcohol exposure defines a high‐risk population in need of long‐term monitoring 110. Our ability to develop enhanced care and monitoring of this high‐risk population (individuals with FASD) is limited by the very low rates of diagnosis for all age groups. For adults, diagnosis is often limited by difficulty determining prenatal alcohol exposure status (especially in cases where the biological mother is unknown) and uncertainty about the adult phenotype of FASD. This is even more problematic in elderly people. For correctional populations in particular, the setting may also result in a limited diagnostic capacity for FASD. Providing FASD diagnoses is further limited by a lack of resources, an impacted health‐care referral system and stigmatization of maternal alcohol consumption. In addition, current diagnostic guidelines have limited agreement 110, 111. Diagnostic screening and staff training on FASD in the respective systems/institutions are crucial in order to ensure that FASD‐affected individuals are receiving the appropriate care and treatment.
The results indicate that there is a critical need for ACA prevalence studies to be conducted among these populations/within these service systems in almost all countries throughout the world. Measuring and monitoring the prevalence of FASD and alcohol consumption during pregnancy over time in both the general population and population subgroups are crucial for understanding and identifying vulnerable populations, targeting prevention and treatment resources and establishing baselines to evaluate the effectiveness and cost‐effectiveness of prevention and treatment strategies. A comprehensive surveillance system could also allow for a better understanding of the associated morbidity and mortality rates, quality‐of‐life indicators and service utilization rates of affected individuals. This will reduce the risk of the development of other common adverse outcomes that often occur in individuals with FASD later in life, such as school failure and dropout, mental health problems, inappropriate sexual behaviour, alcohol and other drug problems, unemployment, dependent living and homelessness, as well as involvement with the law and incarceration 112.
Prenatal alcohol exposure is preventable through public health messaging and treatment of substance use disorder(s) in mothers. It is absolutely necessary to continue to improve prevention of alcohol consumption during pregnancy, screening strategies, targeted interventions for women of childbearing age with substance use problems, diagnosis‐informed care and the provision of support for people with FASD and their families, especially in these special sub‐populations.
Declaration of interests
None.
Supporting information
Appendix S1 PRISMA 2009 Checklist.
Appendix S2 Quality appraisal of the identified studies reporting on the prevalence of FASD among special sub‐populations and reference list.
Appendix S3 Measures of heterogeneity and potential publication bias.
Popova, S. , Lange, S. , Shield, K. , Burd, L. , and Rehm, J. (2019) Prevalence of fetal alcohol spectrum disorder among special subpopulations: a systematic review and meta‐analysis. Addiction, 114: 1150–1172. 10.1111/add.14598.
References
- 1. Popova S., Lange S., Probst C., Gmel G., Rehm J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta‐analysis. Lancet Glob Health 2017; 5: e290–e299. [DOI] [PubMed] [Google Scholar]
- 2. Stratton K., Howe C., Battaglia F., editors. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: Institute of Medicine; 1996, pp. 173–185. [Google Scholar]
- 3. National Institute on Alcohol Abuse and Alcoholism Consensus statement: Recognizing alcohol‐related neurodevelopmental disorder (ARND) in primary health care of children. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2011.
- 4. Popova S., Lange S., Shield K., Mihic A., Chudley A. E., Mukherjee R. A. et al Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta‐analysis. Lancet 2016; 387: 978–987. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO) . The International Classification of Diseases and Related Health Problems, 10th Revision (ICD‐10). Geneva: WHO; 2016. [Google Scholar]
- 6. Lange S., Probst C., Gmel G., Rehm J., Burd L., Popova S. Global prevalence of fetal alcohol spectrum disorder among children and youth: a systematic review and meta‐analysis. JAMA Pediatr 2017; 171: 948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Popova S., Lange S., Burd L., Rehm J. The economic burden of fetal alcohol spectrum disorder in Canada in 2013. Alcohol Alcohol 2016; 51: 367–375. [DOI] [PubMed] [Google Scholar]
- 8. Lupton C., Burd L., Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet 2004; 127c: 42–50. [DOI] [PubMed] [Google Scholar]
- 9. Stade B., Ali A., Bennett D., Campbell D., Johnston M., Lens C. et al The burden of prenatal exposure to alcohol: revised measurement of cost. Can J Clin Pharmacol 2009; 16: e91–e102. [PubMed] [Google Scholar]
- 10. Stade B., Ungar W., Stevens B., Beyene J., Koren G. The burden of prenatal exposure to alcohol: measurement of cost. J FAS Int 2006; 4: e5. [Google Scholar]
- 11. Thanh N. X., Jonsson E. Costs of fetal alcohol spectrum disorder in Alberta, Canada. Can J Clin Pharmacol 2009; 16: e80–e90. [PubMed] [Google Scholar]
- 12. Lange S., Probst C., Rehm J., Popova S. Prevalence of binge drinking during pregnancy by country and World Health Organization region: systematic review and meta‐analysis. Reprod Toxicol 2017; 73: 214–221. [DOI] [PubMed] [Google Scholar]
- 13. Liberati A., Altman D. G., Tetzlaff J., Mulrow C., Gotzche P., Ioannidis J. P. A. et al The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009; 6: e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stevens G. A., Alkema L., Black R. E., Boerma J. T., Collins G. S., Ezzati M. et al Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet 2016; 388: e19–e23. [DOI] [PubMed] [Google Scholar]
- 15. Munn Z., Moola S., Riitano D., Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag 2014; 3: 123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. May P. A., Gossage J. P. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health 2001; 25: 159–167. [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins J., Thompson S. G. Quantifying heterogeneity in a meta‐analysis. Stat Med 2002; 21: 1539–1558. [DOI] [PubMed] [Google Scholar]
- 18. Begg C. B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. [PubMed] [Google Scholar]
- 19. Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997; 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 21. StataCorp . Stata Statistical Software: Release 14. College Station, YX: StataCorp LP; 2015. [Google Scholar]
- 22. Diamond G. W., Senecky Y., Schurr D., Zuckerman J., Inbar D., Eidelman A. et al Pre‐placement screening in international adoption. Isr Med Assoc J 2003; 5: 763–766. [PubMed] [Google Scholar]
- 23. Astley S. J., Clarren S. K. Diagnostic Guide for Fetal Alcohol Syndrome and Related Conditions: the 4‐digit Diagnostic Code, second edn. Seattle, WA: University of Washington; 1999. [Google Scholar]
- 24. Stromland K., Ventura L. O., Mirzaei L., Fontes de Oliveira K., Bandim J. M., Ivo A. P. et al Fetal alcohol spectrum disorders among children in a Brazilian orphanage. Birth Defects Res A Clin Mol Teratol 2015; 103: 178–185. [DOI] [PubMed] [Google Scholar]
- 25. Hoyme H. E., May P. A., Kalberg W. O., Kodituwakku P., Gossaye J. P., Trujillo P. M. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics 2005; 115: 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Burge P. Prevalence of mental disorders and associated service variables among Ontario children who are permanent wards. Can J Psychiatry 2007; 52: 305–314. [DOI] [PubMed] [Google Scholar]
- 27. Fuchs D. B. L., Marchenski S., Murdy A. Children with Disabilities Receiving Services from Child Welfare Agencies in Manitoba. Ottawa, ON: Centre of Excellence for Child Welfare; 2005. [Google Scholar]
- 28. Fuchs D., Burnside L. A Tri‐Province Initiative to Expand Understanding of Costs, Services and Prevention of a Public Health Issue: Fetal Alcohol Spectrum Disorder and Children/Youth In Care (2010–2014): Study on the Prevalence of FASD in Canadian Child Welfare Settings: Final Report. Winnipeg, MB: University of Manitoba; 2014. [Google Scholar]
- 29. Robert M., Carceller A., Domken V., Ramos F., Dobrescu O., Simard M. N. et al Physical and neurodevelopmental evaluation of children adopted from Eastern Europe. Can J Clin Pharmacol 2009; 16: e432–e440. [PubMed] [Google Scholar]
- 30. Mena M., Nazal R., Fernandez E. V., Munoz M. B., Mora F., Olivo G. et al Prevalence of fetal alcohol syndrome in foster homes of the Servicio Nacional de Menores, VIII region. Chile. Rev Med Chile 1987; 115: 1218–1225. [PubMed] [Google Scholar]
- 31. Rosett H. L. A clinical perspective of the fetal alcohol syndrome. Alcohol Clin Exp Res 1980; 4: 19–22. [DOI] [PubMed] [Google Scholar]
- 32. Mena M., Navarrete P., Avila P., Bedregal P., Berrios X. Alcohol drinking in parents and its relation with intellectual score of their children. Rev Med Chile 1993; 121: 98–105. [PubMed] [Google Scholar]
- 33. Feldmann R. Prevalence of FAS in Germany. J Popul Ther Clin Pharmacol 2012; 19: e421. [Google Scholar]
- 34. Tenenbaum A., Hertz P., Dor T., Castiel Y., Sapir A., Wexler I. D. Fetal alcohol spectrum disorder in Israel: increased prevalence in an at‐risk population. Isr Med Assoc J 2011; 13: 725–729. [PubMed] [Google Scholar]
- 35. Stratton K., Howe C., Battaglia F. Fetal Alcohol Syndrome: Diagnosis, Epidemiology, Prevention, and Treatment. Washington, DC: Institute of Medicine; 1996. [Google Scholar]
- 36. Kuzmenkovienė E., Prasauskienė A., Endzinienė M. The prevalence of fetal alcohol spectrum disorders and concomitant disorders among orphanage children in Lithuania. J Popul Ther Clin Pharmacol 2012; 19: e423. [Google Scholar]
- 37. Knuiman S. R. C., Hoksbergen R., van Baar A. Fetal alcohol spectrum disorders in children adopted from Poland: neurobehavioral functioning and early detection. J Popul Ther Clin Pharmacol 2012; 19: e415. [Google Scholar]
- 38. Gyrczuk E. K. I., Topczewska‐Cabanek A., Kisza A., Nitsch‐Osuch A., Zycinska K., Wardyn K. A. Epidemiology of congenital malformations in children in the pre‐adoption intervention Centre in Otwock in 2008–2012. Fam Med Pri Care Rev 2014; 16: 231–232. [Google Scholar]
- 39. Aronson J. E. Prevalence of fetal alcohol syndrome and fetal alcohol effect in preadoptive evaluations of children in Russian orphanages. Evan B. Donald Institute Conference—Adoption and Prenatal Alcohol and Drug Exposure: The Research, Policy and Practice Challenges. Alexandria, VA, 1997.
- 40. Konovalova V.V. K. T., Marincheva G. S. Fetal'nyy alkogol'nyy sindrom u detey shkol'nogo vozrasta. [Fetal alcohol syndrome in schoolchildren]. Materiali IV Mezhdunarodnogo Kongressa ‘Molodoe pokolenie XXI veka: aktual'nye problemy sotsial'no‐psikhologicheskogo zdorov'ya’. [Materials from the IV International Congress;The Young Generation of the XXI Century: Actual Problems of Social and Mental Health’]. Kirov, Russia: Kirovskaya Gosudarstvennaya Medicinskaya Academiya [Kirov State Medical Academy]; 2009, pp. 106–7. [Google Scholar]
- 41. Miller L. C., Chan W., Litvinova A., Rubin A., Comfort K., Tirella L. et al Fetal alcohol spectrum disorders in children residing in Russian orphanages: a phenotypic survey. Alcohol Clin Exp Res 2006; 30: 531–538. [DOI] [PubMed] [Google Scholar]
- 42. Burd L., Cox C., Poitra B., Wentz T., Ebertowski M., Martsolf J. T. et al The FAS screen: a rapid screening tool for fetal alcohol syndrome. Addict Biol 1999; 4: 329–336. [DOI] [PubMed] [Google Scholar]
- 43. Miller L. C., Chan W., Litvinova A., Rubin A., Tirella L., Cermak S. Medical diagnoses and growth of children residing in Russian orphanages. Acta Paediatr 2007; 96: 1765–1769. [DOI] [PubMed] [Google Scholar]
- 44. Riley E. P., Mattson S. N., Li T. K., Jacobson S. W., Coles C. D., Kodituwakku P. et al Neurobehavioral consequences of prenatal alcohol exposure: an international perspective. Alcohol Clin Exp Res 2003; 27: 362–373. [DOI] [PubMed] [Google Scholar]
- 45. The St . Petersburg–USA Orphanage Research Team. Characteristics of children, and orphanages for young children in St Petersburg, Russian Federation. J Appl Dev Psychol 2005; 26: 477–506. [Google Scholar]
- 46. Warren K. R., Calhoun F. J., May P. A., Viljoen D. L., Li T. K., Tanaka H. et al Fetal alcohol syndrome: an international perspective. Alcohol Clin Exp Res 2001; 25: 202s–206s. [DOI] [PubMed] [Google Scholar]
- 47. Bubnov A. A. Morfo‐funktcionalnaia diagnostika posledstvii vnutriutrobnogo alkogolnogo vozdeistviia u detei rannego vozrasta [Morpho‐functional diagnostics of consequences of intrauterine alcohol exposure in infants]. Ural's State Medical Academy: Ekaterinburg, Russia; 2010. [Google Scholar]
- 48. Legonkova S. V. Kliniko‐funktcionalnaia kharakteristika fetalnogo alkogolnogo sindroma u detei rannego vozrasta [Clinical and functional characteristics of Fetal Alcohol Syndrome in early childhood]. St Petersburg's State Paediatric Medical Academy: St Petersburg, Russia; 2011. [Google Scholar]
- 49. Olivan‐Gonzalvo G. Frequency of fetal alcohol syndrome in institutionalized children of eastern European countries. Rev Neurol 2011; 53: 127–128. [PubMed] [Google Scholar]
- 50. Landgren M., Svensson L., Stromland K., Andersson Gronlund M. Prenatal alcohol exposure and neurodevelopmental disorders in children adopted from eastern Europe. Pediatrics 2010; 125: e1178–e1185. [DOI] [PubMed] [Google Scholar]
- 51. Albers L. H., Johnson D. E., Hostetter M. K., Iverson S., Miller L. C. Health of children adopted from the former Soviet Union and Eastern Europe. Comparison with preadoptive medical records. JAMA 1997; 278: 922–924. [PubMed] [Google Scholar]
- 52. Lyons K. L. Smith's Recognizable Patterns of Human Malformation, 5th edn. Philadelphia, PA: WB Saunders; 1997, pp. 555–558. [Google Scholar]
- 53. Astley S. J., Stachowiak J., Clarren S. K., Clausen C. Application of the fetal alcohol syndrome facial photographic screening tool in a foster care population. J Pediatr 2002; 141: 712–717. [DOI] [PubMed] [Google Scholar]
- 54. Chasnoff I. J., Wells A. M., King L. Misdiagnosis and missed diagnoses in foster and adopted children with prenatal alcohol exposure. Pediatrics 2015; 135: 264–270. [DOI] [PubMed] [Google Scholar]
- 55. Farina L. L. M., Chasnoff I. J. Attachment and behavioural difficulties in internationally adopted Russian children. Adopt Foster 2004; 28: 38–49. [Google Scholar]
- 56. Johnson D. E., Albers L. H., Iverson S., Mathers M., Dole K., Georgieff M. K. et al Health status of US adopted eastern European (EE) orphans. Pediatr Res 1996; 39: 134.8825398 [Google Scholar]
- 57. Loman M. M., Wiik K. L., Frenn K. A., Pollak S. D., Gunnar M. R. Postinstitutionalized children's development: growth, cognitive, and language outcomes. J Dev Behav Pediatr 2009; 30: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bertrand J. F. R., Weber M. K., O’Connor M., Riley E. P., Johnson K. A., Cohen D. E. National Task Force on FAS/FAE,. Fetal Alcohol Syndrome: Guidelines for Referral and Diagnosis. Atlanta, GA: Centers for Disease Control and Prevention; 2004. [Google Scholar]
- 59. McGuinness T. M., McGuinness J. P., Dyer J. G. Risk and protective factors in children adopted from the former Soviet Union. J Pediatr Health Care 2000; 14: 109–116. [PubMed] [Google Scholar]
- 60. Miller L. C., Hendrie N. W. Health of children adopted from China. Pediatrics 2000; 105: E76. [DOI] [PubMed] [Google Scholar]
- 61. Miller L., Chan W., Comfort K., Tirella L. Health of children adopted from Guatemala: comparison of orphanage and foster care. Pediatrics 2005; 115: e710–e717. [DOI] [PubMed] [Google Scholar]
- 62. Miller B. S., Kroupina M. G., Iverson S. L., Mason P., Narad C., Himes J. H. et al Auxological evaluation and determinants of growth failure at the time of adoption in eastern European adoptees. J Pediatr Endocrinol Metab 2009; 22: 31–39. [DOI] [PubMed] [Google Scholar]
- 63. Miller L. C. W., Tirella L., Perrin E. Outcomes of children adopted from Eastern Europe. Int J Behav Dev 2009; 33: 289–298. [Google Scholar]
- 64. Ringeisen H., Casanueva C., Urato M., Cross T. Special health care needs among children in the child welfare system. Pediatrics 2008; 122: e232–e241. [DOI] [PubMed] [Google Scholar]
- 65. Chudley A. E., Conry J., Cook J. L., Loock C., Rosales T., LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. Can Med Assoc J 2005; 172: S1–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bower C., Watkins R. E., Mutch R. C., Marriott R., Freeman J., Kippin N. R. et al Fetal alcohol spectrum disorder and youth justice: a prevalence study among young people sentenced to detention in Western Australia. BMJ Open 2018; 8: e019605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bower C., Elliott E. J., Zimmet M., Doorey J., Wilkins A., Russell V. et al Australian guide to the diagnosis of foetal alcohol spectrum disorder: a summary. J Paediatr Child Health 2017; 53: 1021–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Burd L., Selfridge R., Klug M., Juelson T. Fetal alcohol syndrome in the Canadian corrections system. J FAS Int 2003; 1: e14. [Google Scholar]
- 69. Fast D. K., Conry J., Loock C. A. Identifying fetal alcohol syndrome among youth in the criminal justice system. J Dev Behav Pediatr 1999; 20: 370–372. [DOI] [PubMed] [Google Scholar]
- 70. MacPherson P. H., Chudley A. E., Ba G. Fetal Alcohol Spectrum Disorder (FASD) in a correctional population: Prevalence, screening and characteristics. Correctional Service of Canada: Ottawa, ON; 2011. [Google Scholar]
- 71. McLachlan K. Final Report to Yukon Justice: Estimating the Prevalence of FASD, Mental Health, and Substance Use Problems in the Justice System. Yukon Department of Justice: Whitehorse, YT; 2017. [Google Scholar]
- 72. Murphy A., Chittenden M. The McCreary Centre Society. Time Out II: A Profile of BC Youth in Custody. The McCreary Centre Society: Vancouver, BC; 2005. [Google Scholar]
- 73. Rojas E. Y., Gretton H. M. Background, offence characteristics, and criminal outcomes of aboriginal youth who sexually offend: a closer look at Aboriginal youth intervention needs. Sex Abuse 2007; 19: 257–283. [DOI] [PubMed] [Google Scholar]
- 74. Boland F., Duwyn M., Serin R. Fetal alcohol syndrome: understanding its impact. Forum Correct Res 2000; 7: 34–47. [Google Scholar]
- 75. Burd L., Selfridge R., Klug M., Bakko S. Fetal alcohol syndrome in the United States corrections system. Addict Biol 2004; 9: 169–178. [DOI] [PubMed] [Google Scholar]
- 76. Mena M. C. V., Fernandez E., Carrasco R., Perez H. Fetal alcohol syndrome at schools for children mentally handicapped children in Concepcion. Chile Bull Pan Am Health Organ 1986; 20: 157–196. [PubMed] [Google Scholar]
- 77. Mena M., Nazal R., Albornoz C., Pettinelli H., Velasquez P., Soza G. Fetal alcohol syndrome: prevalence in 4 special education schools in Chile. Rev Med Chile 1988; 116: 1252–1256. [PubMed] [Google Scholar]
- 78. Lee H. S., Jones K. L., Lee H. K., Chambers C. D. Fetal alcohol spectrum disorders: Clinical phenotype among a high‐risk group of children and adolescents in Korea. Am J Med Genet A 2016; 170a: 19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Grinfeld H., Goldenberg S., Segre C. A., Chadi G. Fetal alcohol syndrome in Sao Paulo. Brazil. Paediatr Perinat Epidemiol 1999; 13: 496–497. [DOI] [PubMed] [Google Scholar]
- 80. Bell C. C., Chimata R. Prevalence of neurodevelopmental disorders among low‐income African Americans at a clinic on Chicago's south side. Psychiatr Serv 2015; 66: 539–542. [DOI] [PubMed] [Google Scholar]
- 81. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 5th edn (DSM‐5) edn. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 82. Cadle R. G., Dawson T., Hall B. D. The prevalence of genetic disorders, birth defects and syndromes in central and eastern Kentucky. J Ky Med Assoc 1996; 94: 237–241. [PubMed] [Google Scholar]
- 83. O'Connor M. J., Best A., McCracken J. T. Under recognition of prenatal alcohol exposure in a child inpatient psychiatric setting. Ment Health Aspects Dev Disabil 2006; 9: 105–109. [Google Scholar]
- 84. Shanske A. L., Kazi R. Prevalence of the fetal alcohol syndrome in a developmental clinic population. Am J Hum Genet 1980; 32: 128A. [Google Scholar]
- 85. Fitzpatrick J. P., Latimer J., Olson H. C., Carter M., Oscar J., Lucas B. R. et al Prevalence and profile of neurodevelopment and fetal alcohol Spectrum disorder (FASD) amongst Australian aboriginal children living in remote communities. Res Dev Disabil 2017; 65: 114–126. [DOI] [PubMed] [Google Scholar]
- 86. Harris K. R., Bucens I. K. Prevalence of fetal alcohol syndrome in the top end of the Northern Territory. J Paediatr Child Health 2003; 39: 528–533. [DOI] [PubMed] [Google Scholar]
- 87. American Academy of Pediatrics Fetal alcohol syndrome and alcohol‐related neurodevelopmental disorders. Pediatrics 2000; 106: 358–361. [PubMed] [Google Scholar]
- 88. Mutch R. C., Watkins R., Bower C. Fetal alcohol spectrum disorders: notifications to the Western Australian register of developmental anomalies. J Paediatr Child Health 2015; 51: 433–436. [DOI] [PubMed] [Google Scholar]
- 89. Rothstein J., Heazlewood R., Fraser M. Health of aboriginal and Torres Strait islander children in remote far North Queensland: findings of the Paediatric outreach service. Med J Aust 2007; 186: 519–521. [DOI] [PubMed] [Google Scholar]
- 90. Asante K. O., Nelmes‐Matzke J. Report on the Survey of Children with Chronic Handicaps and Fetal Alcohol Syndrome in the Yukon and Northwest British Columbia. Council for Yukon Indians: Whitehorse, YT; 1985. [Google Scholar]
- 91. Kowlessar D. L. A Examination of the Effects of Prenatal Alcohol Exposure on School‐age Children in a Manitoba First Nation Community. A Study of Fetal Alcohol Syndrome Prevalence and Dysmorphologies. Winnipeg, MN: University of Manitoba; 1997. [Google Scholar]
- 92. Robinson G. C., Conry J. L., Conry R. F. Clinical profile and prevalence of fetal alcohol syndrome in an isolated community in British Columbia. Can Med Assoc J 1987; 137: 203–207. [PMC free article] [PubMed] [Google Scholar]
- 93. Werk C. M., Cui X., Tough S. Fetal alcohol spectrum disorder among aboriginal children under six years of age and living off reserve. First Peoples Child Fam Rev 2013; 8: 7–16. [Google Scholar]
- 94. Williams R. J., Odaibo F. S., McGee J. M. Incidence of fetal alcohol syndrome in northeastern Manitoba. Can J Public Health 1999; 90: 192–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chavez G. F., Cordero J. F., Becerra J. E. Leading major congenital malformations among minority groups in the United States, 1981‐1986. Morb Mort Wkly Rep CDC Surveill Summ 1988; 37: 17–24. [PubMed] [Google Scholar]
- 96. Centers for Disease Control and Prevention (CDC) . Use of international classification of diseases coding to identify fetal alcohol syndrome—Indian Health Service facilities, 1981–1992. Morb Mortal Wkly Rep 1995; 44: 253–5,61. [PubMed] [Google Scholar]
- 97. Sokol R. J., Clarren S. K. Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring. Alcohol Clin Exp Res 1989; 13: 597–598. [DOI] [PubMed] [Google Scholar]
- 98. Duimstra C., Johnson D., Kutsch C., Wang B., Zentner M., Kellerman S. et al A fetal alcohol syndrome surveillance pilot project in American Indian communities in the Northern Plains. Public Health Rep 1993; 108: 225–229. [PMC free article] [PubMed] [Google Scholar]
- 99. Egeland G. M., Perham‐Hester K. A., Gessner B. D., Ingle D., Berner J. E., Middaugh J. P. Fetal alcohol syndrome in Alaska, 1977 through 1992: an administrative prevalence derived from multiple data sources. Am J Public Health 1998; 88: 781–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Fox D. J., Pettygrove S., Cunniff C., O'Leary L. A., Gilboa S. et al Fetal alcohol syndrome among children aged 7–9 years—Arizona, Colorado, and New York, 2010. Morb Mortal Wkly Rep 2015; 64: 54–57. [PMC free article] [PubMed] [Google Scholar]
- 101. May P. A., Hymbaugh K. J., Aase J. M., Samet J. M. Epidemiology of fetal alcohol syndrome among American Indians of the southwest. Soc Biol 1983; 30: 374–387. [DOI] [PubMed] [Google Scholar]
- 102. National Birth Defects Prevention Network . Birth defects surveillance data from selected states, 1996–2000. Birth Defects Res A Clin Mol Teratol 2003; 67: 729–818. [DOI] [PubMed] [Google Scholar]
- 103. Quaid J., Kirkpatrick J., Nakamura R., Aase J. M. Establishing the occurrence of FAS/FAE in a rural community. Provider 1993; 18: 71–75. [Google Scholar]
- 104. Burd L. C. C., Shaw R., Norris J. A court team model for care of young children in foster care: the role of prenatal alcohol exposure and fetal alcohol spectrum disorders. J Psychiatry Law 2011; 39: 179–191. [Google Scholar]
- 105. Popova S., Lange S., Bekmuradov D., Mihic A., Rehm J. Fetal alcohol spectrum disorder prevalence estimates in correctional systems: a systematic literature review. Can J Public Health 2011; 102: 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Popova S., Lange S., Probst C., Parunashvili N., Rehm J. Prevalence of alcohol consumption during pregnancy and fetal alcohol spectrum disorders among the general and aboriginal populations in Canada and the United States. Eur J Med Genet 2017; 60: 32–48. [DOI] [PubMed] [Google Scholar]
- 107. Szlemko W. J., Wood J. W., Thurman P. J. Native Americans and alcohol: past, present, and future. J Gen Psychol 2006; 133: 435–451. [DOI] [PubMed] [Google Scholar]
- 108. Sotero M. A conceptual model of historical trauma: implications for public health practice and research. J Health Dispar Res Pract 2006; 1: 93–108. [Google Scholar]
- 109. Coles C. D., Gailey A. R., Mulle J. G., Kable J. A., Lynch M. E., Jones K. L. A comparison among 5 methods for the clinical diagnosis of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2016; 40: 1000–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Burd L., Wilson H. Fetal, infant, and child mortality in a context of alcohol use. Am J Med Genet C Semin Med Genet 2004; 127c: 51–58. [DOI] [PubMed] [Google Scholar]
- 111. Burd L., Klug M. G., Li Q., Kerbeshian J., Martsolf J. T. Diagnosis of fetal alcohol spectrum disorders: a validity study of the fetal alcohol syndrome checklist. Alcohol 2010; 44: 605–614. [DOI] [PubMed] [Google Scholar]
- 112. Streissguth A. P., Barr H. M., Kogan J., Bookstein F. L. Understanding the occurrence of secondary disabilities in clients with fetal alcohol syndrome (FAS) and fetal alcohol effects (FAE). Seattle, Washington: University of Washington, Fetal Alcohol and Drug Unit; 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 PRISMA 2009 Checklist.
Appendix S2 Quality appraisal of the identified studies reporting on the prevalence of FASD among special sub‐populations and reference list.
Appendix S3 Measures of heterogeneity and potential publication bias.


