Abstract
During hypoxia, a cellular adaptive response activates hypoxia-inducible factors (HIFs; HIF-1 and HIF-2) that respond to low tissue-oxygen levels and induce the expression of a number of genes that promote angiogenesis, energy metabolism, and cell survival. HIF-1 and HIF-2 regulate endothelial cell (EC) adaptation by activating gene-signaling cascades that promote endothelial migration, growth, and differentiation. An HIF-1 to HIF-2 transition or switch governs this process from acute to prolonged hypoxia. In the present study, we evaluated the mechanisms governing the HIF switch in 10 different primary human ECs from different vascular beds during the early stages of hypoxia. The studies demonstrate that the switch from HIF-1 to HIF-2 constitutes a universal mechanism of cellular adaptation to hypoxic stress and that HIF1A and HIF2A mRNA stability differences contribute to HIF switch. Furthermore, using 4 genome-wide mRNA expression arrays of HUVECs during normoxia and after 2, 8, and 16 h of hypoxia, we show using bioinformatics analyses that, although a number of genes appeared to be regulated exclusively by HIF-1 or HIF-2, the largest number of genes appeared to be regulated by both.—Bartoszewski, R., Moszyńska, A., Serocki, M., Cabaj, A., Polten, A., Ochocka, R., Dell’Italia, L., Bartoszewska, S., Króliczewski, J., Dąbrowski, M., Collawn, J. F. Primary endothelial cell–specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia.
Keywords: human endothelial cells, HIF1A, EPAS1
Maintaining oxygen homeostasis is crucial for multicellular organisms, and the imbalance between oxygen availability and demand leads to activation of hypoxia-inducible adaptive responses that facilitate cellular survival. Hypoxia-inducible factors (HIFs; HIF-1 and HIF-2) are essential transcription factors that drive gene expression to resolve this physiologic stress. HIFs recognize and bind to hypoxia-response elements (HREs) in the promoters or enhancers of numerous target genes that regulate cell metabolism, survival, and proliferation (1).
HIF-1 and HIF-2 are heterodimers composed of inducible oxygen-sensitive α subunits and constitutively expressed β subunits, and they belong to the basic helix-loop-helix–Per/ARNT/Sim transcription factor family (2, 3). Under normal oxygen tension, HIF-α subunits are rapidly post-translationally hydroxylated by specific oxygen-dependent HIF prolyl-hydroxylases (PHDs) (4–11). The prolyl-hydroxylation of HIF-α subunits leads to their recognition by von Hippel–Lindau tumor suppressor protein, a component of an E3 ubiquitin ligase complex (12), and results in HIF-α polyubiquitination and rapid degradation (12, 13). Furthermore, another hydroxylase, factor-inhibiting HIF-1 (FIH-1), which also requires molecular oxygen, prevents HIF transcriptional activity by hydroxylation of a single asparagine residue in transactivation domains of the HIF-α subunits (14, 15). Hypoxia leads to inactivation of PHDs and FIH-1 activity and allows for HIF-α stability and HIF complex activity (16). During hypoxia, HIF-1 and HIF-2 have both unique and overlapping target genes. The common targets include VEGFA and glucose transporter 1 (17). HIF-1 also induces the expression of glycolytic genes (18), some proangiogenic genes, and genes involved in pH regulation (19). HIF-2 stimulates matrix metalloproteinases and erythropoietin gene expression (20). Although HIF-1 initiates angiogenesis, the maturation of the vascular network is governed by HIF-2 (1).
HIF-1 governs the acute adaptation to hypoxia, whereas HIF-2 activity begins later (1), and this creates a transitional switch between the 2 HIF proteins. The inability to reduce the HIF-1 levels during prolonged hypoxia leads to cell death (21). Numerous protein factors have been proposed to modulate the HIF transitional switch (22–24) [reviewed in Serocki et al. (21)], and the mechanisms underlying the HIF switch remain poorly understood.
In the present study, we evaluated the mechanisms governing the HIF switch in different human endothelial cells (ECs) from different vascular beds. Our studies demonstrate that the switch from HIF-1 to HIF-2 constitutes a universal mechanism of human endothelium adaptation to prolonged hypoxia, and furthermore, HIF-1 and HIF-2α mRNA stability differences contribute to the HIF-1/HIF-2 transitional switch. We also demonstrate that the majority of genes responsible for HIF-dependent cellular responses to hypoxia can be regulated by both HIF-1 and HIF-2.
MATERIALS AND METHODS
Cell culture
Primary HUVECs were purchased from Cellworks (San Jose, CA, USA) and cultured in Endothelial Growth Medium (EGM)-2 BulletKit (Lonza, Basel, Switzerland). Primary human aortic ECs (HAECs), primary human cardiac artery ECs (HCAECs), primary human iliac ECs, and primary human pulmonary artery ECs were purchased from Lonza and cultured in EGM-2 medium. Primary human cardiac microvascular ECs (HMVEC-Cs), primary human dermal microvascular ECs (HMVEC-Ds), primary human lung microvascular ECs (HMVEC-Ls), and primary human uterine microvascular ECs (UtMVECs) were purchased from Lonza and cultured in microvascular EGM-2 (EGM-2MV) medium (Lonza). Primary human pulmonary artery smooth muscle cells (HPASMCs) were purchased from Thermo Fisher Scientific (Waltham, MA, USA) and cultured in Medium 231 supplemented with smooth muscle growth supplement (Thermo Fisher Scientific). Except for HUVECs that were pooled from 10 individual donors, all other primary human ECs used were obtained from single donors. All experiments were conducted between passage 2 and 6 at a confluence of 70–80%.
Induction of hypoxia
Hypoxia was induced in a CO2/O2 incubator/chamber specific for hypoxia research (InvivO2; Baker Ruskin, Sanford, ME, USA). Briefly, cells were cultured in 35- or 60-mm dishes (for RNA isolation and protein isolation, respectively) at 0.9% O2 for the time periods specified (PO2 was 10–12 mmHg). Control cells were maintained in normoxia in a CO2/O2 incubator (Binder, Tuttlingen, Germany).
Isolation of RNA
Total RNA [containing both mRNA and microRNA (miRNA)] was isolated using an miRNeasy Kit (Qiagen, Germantown, MD, USA). RNA concentrations were calculated based on the absorbance at 260 nm. RNA samples were stored at −70°C until use.
Measurement of mRNA quantitative real-time PCR
We used TaqMan One-Step Real-Time PCR Master Mix Reagents (Thermo Fisher Scientific) as previously described (25, 26) using the manufacturer’s protocol (retrotranscription: 15 min, 48°C). The relative expressions were calculated using the 2−ΔΔCt method (27) with the TATA-binding protein (TBP) and 18S rRNA genes as reference genes for the mRNA. TaqMan probes identifiers used were: HIF1A (Hs00153153_m1); HIF-2α [alias endothelial PAS domain protein 1 (EPAS1)] (Hs01026149_m1); 18S (Hs99999901_s1); and TBP (Hs00427620_m1).
Measurement of mRNA half-life
HIF1A and EPAS1 mRNA half-lives were measured as described in (28, 29) with the following modifications. Cells were grown on 35-mm plastic dishes to ∼80% confluency. Parallel experiments were performed in cells exposed to hypoxia and control (normoxic). Actinomycin D (5 μg/ml; MilliporeSigma, Burlington, MA, USA) was added to stop transcription at the start of the experiment, and the RNA was isolated at the indicated time intervals using Qiagen mRNeasy. Actinomycin D was maintained throughout the experiments. Total HIF1A and EPAS1 mRNA levels were measured by real-time PCR at each time point using TaqMan-based assays and normalized to endogenous 18S rRNA levels (amplified using a standard primer set). mRNA values for each time point were calculated from 3 individual samples generated in at least 2 independent experiments. Relative HIF1A and EPAS1 mRNA levels were plotted as percent differences from HIF1A and EPAS1 mRNA levels at the initial time point (t = 0). The mRNA half-lives were calculated from the exponential decay using the trend line equation C/C0 = e–kdt, where C and C0 are mRNA amounts at time t and t0, respectively, and kd is the mRNA decay constant as previously described (28, 29).
Western blots
Cells were lysed in SDS lysis buffer (4% SDS, 20% glycerol, 125 mM Tris-HCl pH = 6.8) supplemented with protease inhibitors (cOmplete Mini; Roche, Basel, Switzerland). The insoluble material was removed by centrifugation at 15,000 g for 15 min. Protein concentrations were determined by Bio-Rad Detergent Compatible (DC) Protein Assay (Bio-Rad, Hercules, CA, USA) using bovine serum albumin (BSA) as standard. Following the normalization of protein concentrations, the lysates were mixed with an equal volume of 2× Laemmli sample buffer and incubated for 5 min at 95°C prior to separation by SDS-PAGE on Criterion Tris-Glycine eXtended stain-free 4–15% gradient gels (Bio-Rad). Following SDS-PAGE, the proteins were transferred to polyvinylidene fluoride membranes (30 V overnight at 4°C). The membranes were then blocked with BSA (MilliporeSigma) dissolved in PBS/Tween-20 (3% BSA, 0.5% Tween-20 for 1–2 h), followed by immunoblotting with the primary antibody: mouse anti–HIF-1α (1:2000, ab16066; Abcam, Cambridge, MA, USA); rabbit anti–HIF-2α (1:1000, ab199; Abcam); rabbit anti–β-Actin (1:1000, ab1801; Abcam). After the washing steps, the membranes were incubated with goat anti-rabbit IgG (heavy and light chains) or with goat anti-mouse IgG (heavy and light chains) horseradish peroxidase–conjugated secondary antibodies (Bio-Rad) and detected using SuperSignal West Pico ECL (Thermo Fisher Scientific). Densitometry was performed using Image Lab software v.4.1 (Bio-Rad).
Gene expression microarray SurePrint G3 processing and hybridization
The Agilent Technologies human transcriptome array (SurePrint G3 Gene Expression Microarray v.3 G4851C) and dedicated Agilent Technologies reagent set were used to analyze the mRNA expression pattern of primary HUVECs. This microarray features unique oligonucleotide probes that encompass transcripts and variants, and it represents 26,083 human genes. Preparation of labeled cRNA, hybridization, and scanning of microarrays were performed according to the manufacturer’s protocol (Agilent Technologies, Santa Clara, CA, USA). Internal control transcripts have been added to the samples (RNA Spike-In Kit; Agilent Technologies).
Briefly, 100 ng of total RNA was converted into double-stranded cDNA by reverse transcription. Cyanine-3–labeled cRNA was generated by converting the cDNA sample using the Low Input One Color Labeling Kit (Agilent Technologies). Labeled cRNA was purified using RNeasy Mini Kit (Qiagen) and hybridized on the SurePrint G3 Gene Expression Microarray while rotating at 60 rpm for 17 h at 65°C. After hybridization, the microarray was washed with Gene Expression Wash Buffer 1 (Agilent Technologies) supplemented with 0.005% Triton X-102 and prewarmed Gene Expression Wash Buffer 2 (Agilent Technologies). The slides were scanned at Agilent G4900DA SureScan Microarray Scanner System and analyzed in Agilent Feature Extraction software. This software extracts the data and performs all subsequent data transformation and normalization processes. This includes mainly background subtraction and Lowess spatial normalization of the obtained intensities. Further data analysis was performed using GeneSpring GX 14.8 software (Agilent Technologies). Log2 fold-change values for genes were calculated as the ratio of the signal values of the experimental groups compared with the control group. Log2 fold changes in gene expression were calculated based on 3 independent biologic replicates, and P < 0.005 was considered significant (Supplemental Table S1A, B).
HIF response element analysis
The promoters of gene transcripts that were affected by hypoxia in the microarray experiments were analyzed for HIF-1 or HIF-2 binding sites. In each gene promoter sequence that was defined as a 20-kb window around the transcription start site, we examined only the open chromatin regions that were established in the HUVEC cell line by the Encyclopedia of DNA Elements (ENCODE) (30) project. We merged both DNase I hypersensitive sites sequencing HUVEC datasets found in Ensembl (31) (v.79). We focused on 2 distinct HRE motifs annotated to HIF-1 (M00139, alias HIF1A) and HIF-2 (M00074, alias EPAS1) from the Homo sapiens Comprehensive Model Collection (HOCOMOCO) v.9 database (32). We used the Nencki Genomics Database (33) (v.79_1) to obtain genomic coordinates of these motif instances. For each gene, we calculated the number of instances found in the open chromatin regions for both HIF-1 and HIF-2 and then computed the cumulative distribution function for each motif in the compared groups (time points) of genes. Statistical testing was done using the 1-sided Kolmogorov-Smirnov (K-S) test from the statistic package in R (v.3.2.4) (34).
Bioinformatics analysis of potential HIF effects
The GeneAnalytics webserver (geneanalytics.genecards.org) (35) was used to place HIF-1 and HIF-2 target prediction results into a physiologic context and predict potential HIF effects (22). The scoring algorithm in the pathways category is based on the algorithm used by the GeneDecks Set Distiller tool (36). Briefly, all genes in each SuperPath are given a similar weight in the analysis, and the matching score is based on the cumulative binomial distribution. This is used to test the null hypothesis that the queried genes are not overrepresented within any SuperPath pathway. Furthermore, the analyses were limited to experimentally verified interactions, and no extended gene enrichment set analyses were performed. The Adenylate-Uridylate (AU)-Rich Element (ARE)site1 database (http://nibiru.tbi.univie.ac.at/cgi-bin/AREsite/AREsite.cgi) was used to perform ARE analysis. Heat maps were generated with Morpheus software (https://software.broadinstitute.org/morpheus).
Statistical analyses
Results were expressed as means ± sd. Statistical significance was determined using the Student’s t test and ANOVA on ranks, with P < 0.005 considered significant. The correlation was accessed via the Pearson product-moment correlation method.
RESULTS
The HIF-1α and HIF-2α switch is a universal adaptation of human ECs to prolonged hypoxia
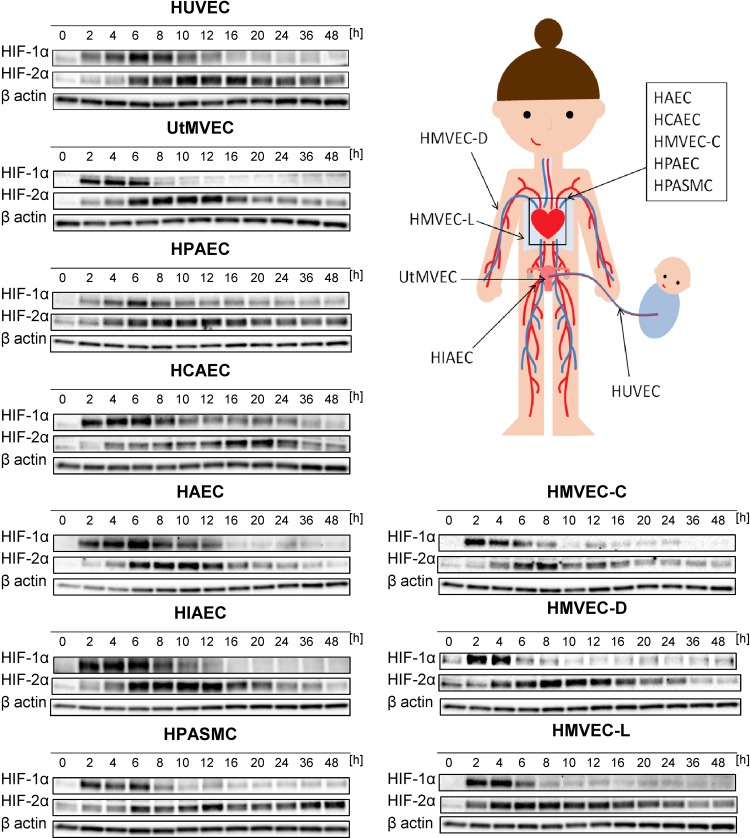
Although previous studies have reported that the transcriptional signaling is passed from HIF-1 to HIF-2 during chronic hypoxia in human ECs (37–39), our goal here was to determine whether this switch occurs in ECs of different vascular beds and to understand the kinetics of hypoxia-induced changes in HIF-1α and HIF-2α levels. To accomplish this, as shown in Fig. 1, we performed parallel time-course studies during hypoxia in 10 human primary EC lines of different vascular beds.
Figure 1.
Hypoxia results in accumulation of HIF-1α and HIF-2α in human ECs. Cells were exposed to hypoxia for the time periods specified, and total RNA and protein lysates were collected. The changes in HIF-1α and HIF-2α protein levels were evaluated by Western blot normalized to β-actin and total protein levels and related to the normoxic control. The densitometry analyses are provided in Supplemental Fig. S1. HIAEC, human iliac EC; HPAEC, human pulmonary artery EC.
This analysis indicated that HIF-1α rapidly accumulated during acute hypoxia in all tested ECs and reached maximal levels from 2 to 6 h of hypoxia. Afterwards, HIF-1α abundance was gradually reduced in response to chronic hypoxia as shown in Fig. 1 and Supplemental Fig. S1. Notably, HIF-1α was more rapidly induced and present for a shorter time period in microvascular ECs (UtMVEC, HMVEC-C, HMVEC-D, and HMVEC-L) and in HPASMCs than in the other vascular ECs.
HIF-2α gradually accumulated during the hypoxia time course in all tested ECs and reached maximal levels at 8–20 h (Fig. 1). Then, HIF-2α slowly decreased and remained slightly elevated even up to 48 h in HUVECs, human pulmonary artery ECs, and HPASMCs. HIF-2α appeared less stable in UtMVECs, HCAECs, HMVEC-Ls, HMVEC-Ds, HAECs, and HMVEC-Cs. Notably, in terms of HIF-2α expression changes, there did not appear to be any general differences or trends between vascular and microvascular ECs. Taken together, this analysis confirmed that, in all human ECs tested, acute hypoxia results in accumulation of HIF-1α that switches to HIF-2α upon prolonged hypoxia in a time frame that was remarkably similar.
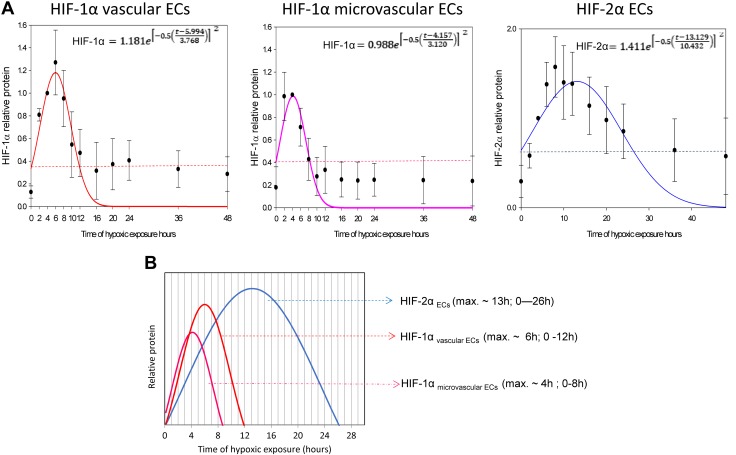
Based on these results, we propose a general model of the HIF-1α to HIF-2α switch in human ECs, in which we calculated the maximum protein expression levels for HIF-1α (for both vascular and microvascular ECs) and HIF-2α (for all ECs) using the logarithmic normal 3 parameter function (Fig. 2A).
Figure 2.
The hypoxic switch between HIF-1α and HIF-2α constitutes a universal mechanism of human endothelium adaptation to prolonged hypoxia. A) The mathematic representation of HIF-1α/HIF-2α switch in human ECs. The changes in HIF-1α (obtained from vascular or microvascular ECs) and HIF-2α levels (from all ECs tested) during hypoxia time course were analyzed using the logarithmic normal 3 parameter function (using 200 iterations, P < 0.005). The dashed lines represent predicted normoxic HIF levels. The error bars represent sd. B) The general model of endothelial HIF-1α/HIF-2α proposed based on the mathematical modeling. Max., maximum.
The maximal levels of HIF-1α are observed at 6 ± 0.5 h (P = 0.00123) and 4 ± 0.3 h (P = 0.00165) of hypoxia in vascular and microvascular ECs, respectively (Fig. 2B). The maximum for HIF-2α is observed at 13 ± 1 h (P = 0.00103) (Fig. 2B). Furthermore, elevation of the HIF-1α protein expression above normoxic levels lasts up to 8 and 12 h in vascular and microvascular ECs, respectively. In contrast, the elevation of HIF-2α lasts up to 26 h (Fig. 2B). Because both HIF-1α and HIF-2α rapidly accumulate during acute hypoxia (Fig. 2), the switch from HIF-1 to HIF-2 signaling results from the rapid reduction of HIF-1 levels. Importantly, both HIF-1α and HIF-2α are significantly accumulated between 6 and 8 h of hypoxia and thus either coregulate or compete for transcriptional targets.
The hypoxic HIF1A and EPAS1 (HIF-2α) mRNA profiles
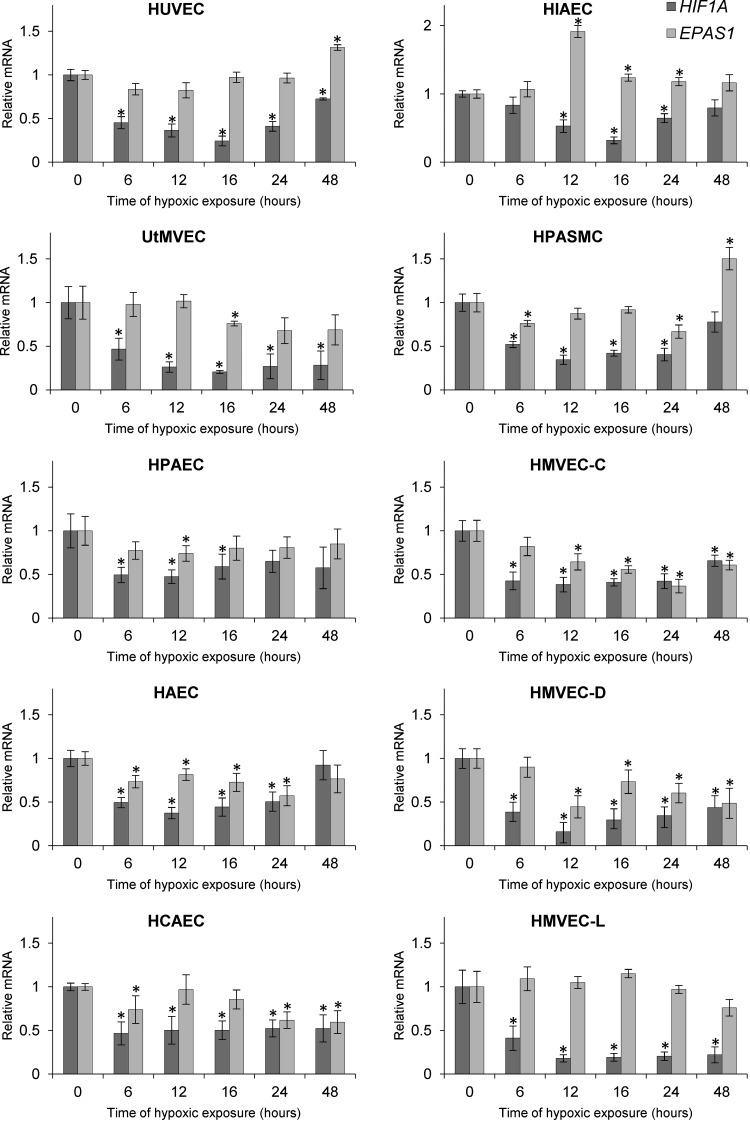
Although previous studies have reported that HIF1A and EPAS1 mRNA levels are reduced during hypoxia in human ECs (37–39), our goal was to understand the kinetics of hypoxia-induced changes in HIF mRNA levels. To accomplish this, we performed time-course studies during hypoxia in the same set of human primary ECs and found that HIF1A mRNA is rapidly reduced at 6 h in all ECs (Fig. 3) and declines to about 20–40% of its initial level at 12–16 h. Interestingly, hypoxia has a limited effect on the EPAS1 mRNA and reduced its levels only in a subset of tested cell lines (HMVEC-Cs, HMVEC-Ds, HAECs, and HCAECs) (Fig. 3). In contrast, the EPAS1 mRNA was induced in human iliac ECs and HPASMCs at 12 and 48 h, respectively (Fig. 3).
Figure 3.
Hypoxia reduces HIF1A and EPAS1 mRNA levels in human ECs. Cells were exposed to hypoxia for the time periods specified, and total RNA lysates were collected. HIF1A and EPAS1 mRNA levels were quantified by quantitative real-time PCR and normalized to TBP and 18S rRNA levels and expressed as a fold change over normoxic samples. Data represent the mean ± sd of 2 independent experiments (3 replicates each). *P < 0.05 was considered significant. HIAEC, human iliac EC; HPAEC, human pulmonary artery EC.
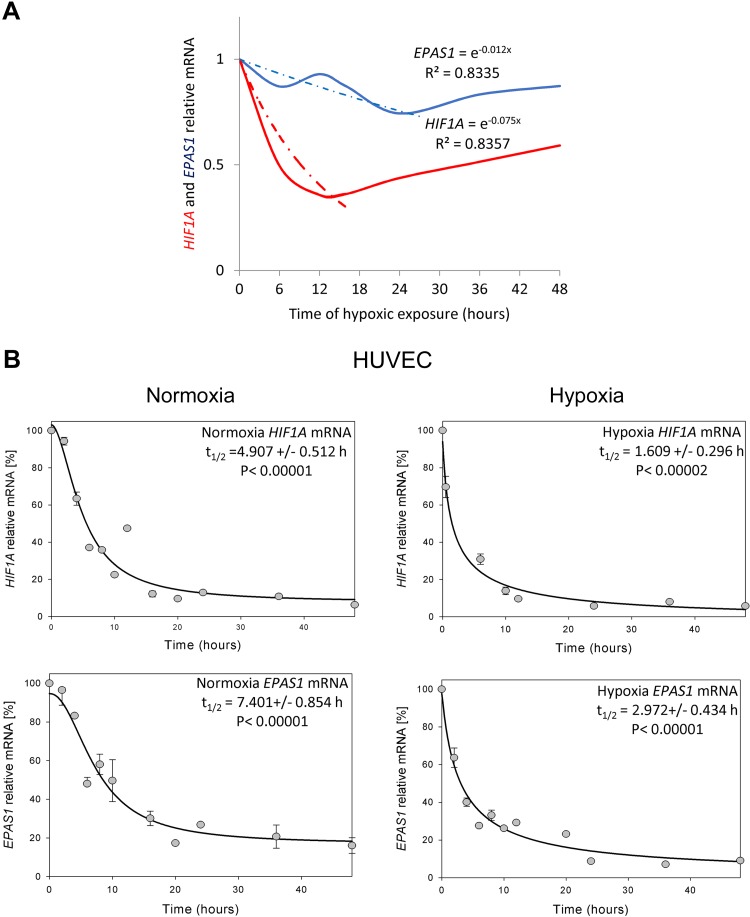
Based on the HIF1A and EPAS1 mRNA hypoxic expression profiles, we calculated the differences in these transcripts during hypoxia as shown in Fig. 4A. During hypoxia, HIF1A mRNA is reduced to lower levels 6 times faster than the EPAS1 transcript (kdHIF1A = 0.075; kdEPAS1 = 0.012). We conclude that although both the HIF-1α and HIF-2α subunits rapidly accumulate during hypoxia, the HIF1A mRNA is dramatically and rapidly reduced in the ECs. To follow this process, we next monitored the HIF1A and EPAS1 mRNA half-lives in HUVECs cultured in normoxia and during hypoxia (Fig. 4B). In normoxia, HUVEC HIF1A mRNA t1/2 is ∼5 h, whereas EPAS1 mRNA t1/2 is ∼7.5 h. During hypoxia, HIF1A t1/2 is ∼1.5 h, whereas EPAS1 t1/2 is ∼3 h, indicating that the EPAS1 mRNA is more stable under both conditions, and this provides some explanation for the HIF-1–to–HIF-2 transition. Furthermore, as shown in Supplemental Fig. S2A, destabilizing HIF1A and EPAS1 mRNAs during hypoxia via inducing mRNA cleavage with specific small-interfering RNAs (40) results in significantly lower HIF-1α and HIF-2α hypoxic levels. This observation is consistent with the hypothesis that HIF1A and EPAS1 mRNA stability differences contribute to the HIF switch.
Figure 4.
Hypoxia reduces mRNA levels and half-lives of HIF1A and EPAS1. A) The mathematic representation of HIF1A and EPAS1 mRNA levels during hypoxia in human ECs. The changes in mRNA levels obtained from all ECs tested during hypoxia time course were analyzed using the natural exponential function. The dashed lines represent mathematically predicted reduction curves, whereas solid lines represent experimental data. B) HIF1A and EPAS1 mRNA half-life measurements were taken in HUVECs exposed to hypoxia and cultured in normoxia. Actinomycin D was added to stop transcription, after which the RNA was isolated, and total HIF1A and EPAS1 mRNA levels at each time point were measured by real-time PCR and normalized to endogenous 18S rRNA levels. mRNA values for each time point were calculated from 2 individual samples generated in at least 2 independent experiments. Relative HIF1A and EPAS1 mRNA levels at the time points indicated were plotted as percent differences from HIF1A and EPAS1 mRNA levels at the initial time point (t = 0). The mRNA half-lives were calculated from the exponential decay using the trend line equation C/C0 = e–kdt (where C and C0 are mRNA amounts at time t and at the t0, respectively, and kd is the mRNA decay constant). The error bars represent sd. P < 0.05 was considered significant.
Effects of the HIF switch on the HUVEC transcriptome
HIF-1 and HIF-2 are considered the crucial transcriptional regulators of cellular adaptation to hypoxia and are believed to mediate different aspects of this process. Hence, the switch between these 2 transcription factors in human ECs should be best illustrated by the hypoxic transcriptome. In order to test for the effects of the HIF switch on global gene expression, we utilized HUVECs as our primary model and, in this case, utilized a 10-donor pool. We isolated total RNA from cells in normoxia and from cells exposed to hypoxia for 2 h (mostly HIF-1 expression), 8 h (both activities), and 16 h (mostly HIF-2 expression). Next, the samples were subjected to genome-wide mRNA expression arrays followed by bioinformatics analyses. We only selected mRNAs that were significantly affected by more than a 2-fold change in all 3 biologic replicates.
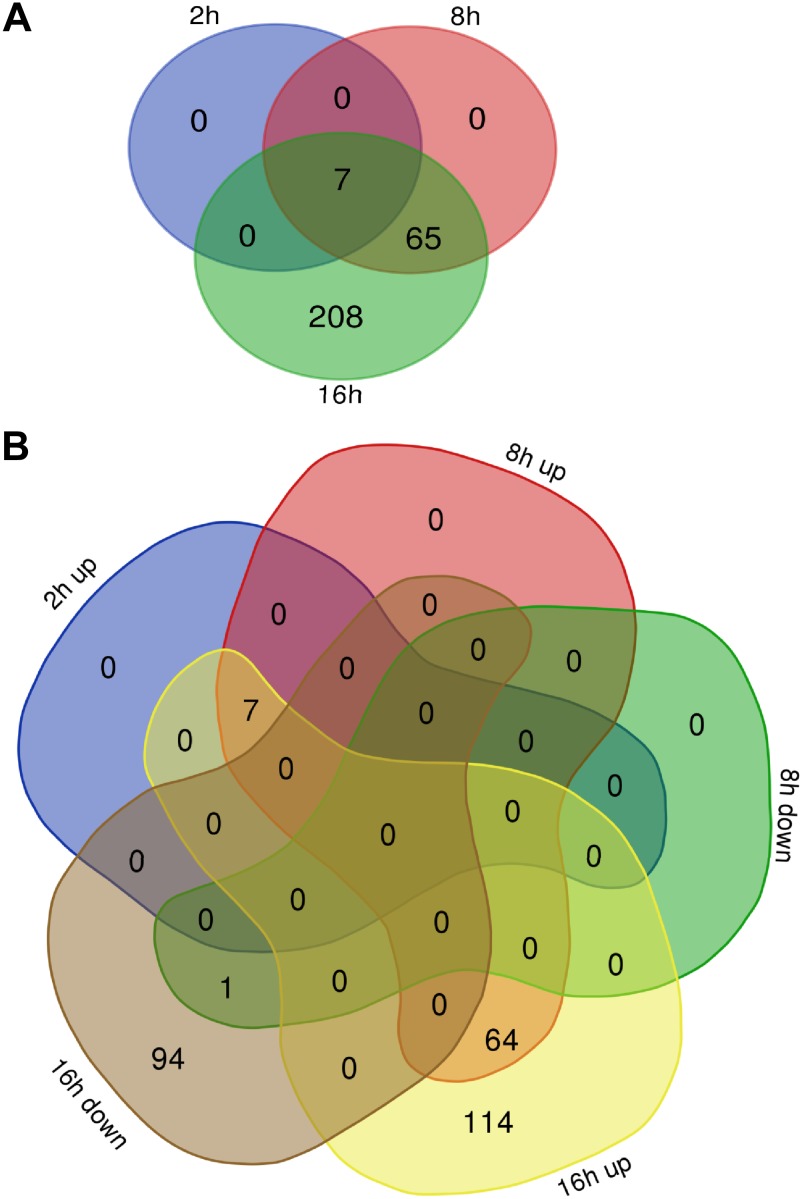
As shown in Fig. 5A (Supplemental Table S1A), only 7 genes were affected after 2 h of hypoxia, whereas this number rapidly increased to 72 and 280 genes affected after 8 and 16 h of hypoxia, respectively. Notably, as shown in Fig. 5B during acute hypoxia, the vast majority of genes were induced (7 after 2 h, and 71 after 8 h), whereas prolonged hypoxic exposure (16 h) resulted in a significant reduction of 95 transcripts. Gene ontology analyses of these gene sets indicate that after 2 h of exposure to hypoxia, significant proangiogenic signals appear, whereas after 8 h, there are glycolytic and antiapoptotic responses. After 16 h of exposure, the previous signaling processes are maintained, whereas the down-regulated genes are mainly responsible for a general reduction in protein translation (Supplemental Table S2). Taken together, our analysis indicates that 8 h of hypoxia resulted in the classic response to hypoxia, whereas after 16 h, activation of numerous secondary signaling networks masked the HIF-specific effects.
Figure 5.
Acute hypoxia has a different effect on the HUVECs’ genome-wide mRNA profiles compared with prolonged hypoxia. A) Venn diagram (41) represents the general distribution of unique transcripts significantly affected during 2, 8, and 16 h of hypoxia. B) The distribution of up-regulated and down-regulated mRNAs at each time point. The mRNA levels were expressed as log2 fold change relative to normoxic control, and the groups were compared with ANOVA to select transcripts significantly different between the normoxia and hypoxia groups (P < 0.005 was considered significant).
A greater number of HRE motifs is associated with an earlier response to hypoxia and to a specific HIF at its time of maximum activity
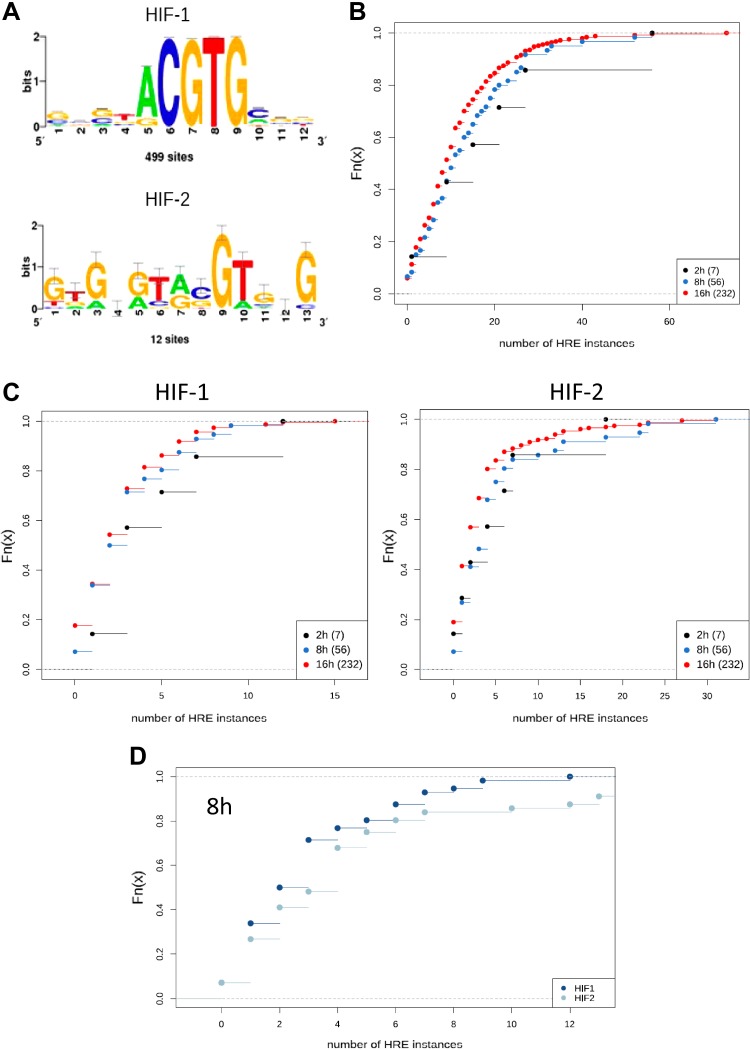
Although HIF-2 is recognized as an important regulator, its role is underestimated in gene ontology databases, and many targets have been attributed to HIF-1 function. To address this issue, we analyzed our gene sets for the presence of specific HIF-1 and HIF-2 HRE motifs in their target gene promoter regions. In each gene promoter sequence, we looked only at the open chromatin regions established in the HUVEC cell line by the ENCODE project and focused on 2 distinct HRE motifs annotated to HIF-1 and HIF-2 (Fig. 6A). For each gene identified, we calculated the counts of HREs found in the open chromatin regions, either jointly (summed) or separately for HIF-1 and HIF-2, and then computed the cumulative distribution function for the counts of those motifs in the 3 time-point groups of genes.
Figure 6.
A higher HRE binding motif number is associated with an earlier response to hypoxia and to a specific HIF at the time of its maximum activity. A) Logos of the HIF-1 and HIF-2 HRE binding motifs (HOCOMOCO v.9) used in this analysis. B) Cumulative distribution functions of counts of HREs (HIF-1–specific and HIF-2–specific summed) per gene. Numbers of genes forming each group are given in brackets. C) Cumulative distribution functions of the numbers of HIF-1 and HIF-2 motif instances considered separately. The number of HIF-2 instances was significantly (K-S test, P = 0.00241) higher in the 8-h group than in the 16-h group. D) Cumulative distribution functions of the counts of HIF-1 and HIF-2 instances in the 8-h group. The number of HIF-2 instances was significantly (K-S test, P = 0.04891) higher than that of HIF-1 instances.
Nearly all (230 of 232) of the genes affected during hypoxia contained HRE motifs. Generally, genes that were affected earlier had more HRE binding regions than those affected later (Fig. 6B). The number of HIF-1 HREs was highest in the 2-h group, and the number of HIF-2 HREs was significantly (P = 0.00241) higher in the 8-h group than in the 16-h group (Fig. 6C). Additionally, we found that in the 8-h group, the number of HIF-2 HREs was significantly (P = 0.04891) higher than the number of HIF-1 HREs (Fig. 6D).
Furthermore, we observed that HRE-containing genes affected by acute hypoxia remained active during prolonged exposure, in spite of the fact that the genes’ promoter regions were enriched with HIF-1 motifs and that the genes affected during more prolonged hypoxia had more HIF-2 motifs in their promoter regions. These findings support the hypothesis of separating the transcriptional signaling by the HIF switch. Moreover, our results of HRE motif count analysis for genes induced with different kinetics after hypoxia suggests that there is a preference of HIF-1 and HIF-2 to their respective annotated motifs. This is more important than one would suspect because a careful comparison of the results of 2 previous studies (42, 43) reveal that both HIFs can bind to both motifs. For example, 1 study suggested that despite differences in the distribution patterns of the HIF-1 and HIF-2 binding motifs, determining the specificity of HIF-1 or HIF-2 binding to the 2 motifs was not possible (42). That being said, our in silico analyses are supported by chromatin immunoprecipitation sequencing (ChIP-seq) results from the recent work of Smythies et al. (44), which implies comparable results in the cells lines evaluated in this study.
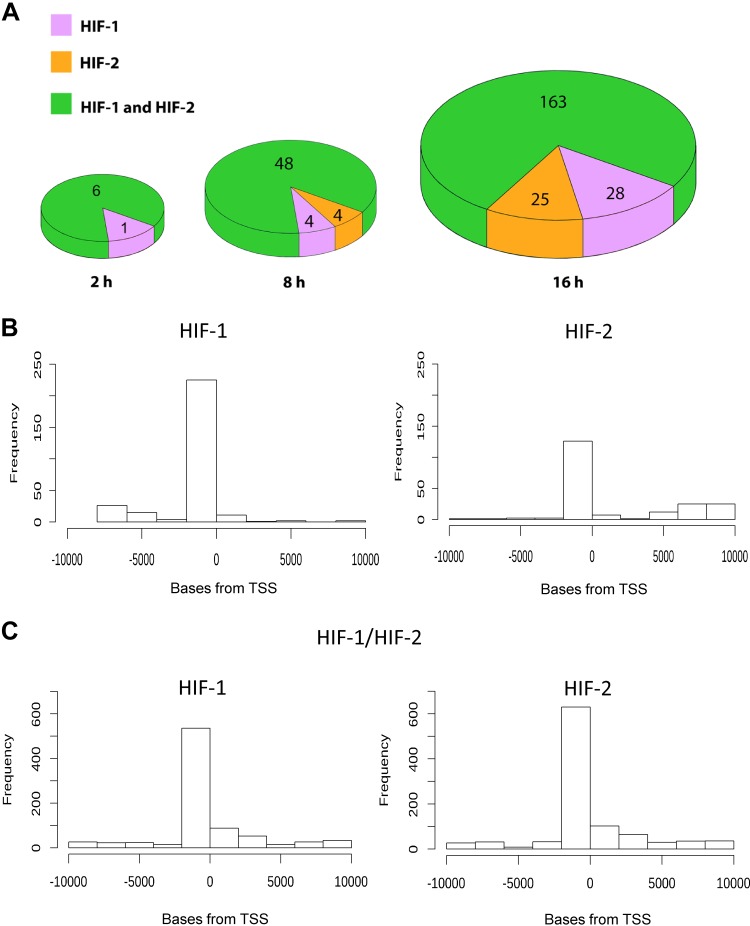
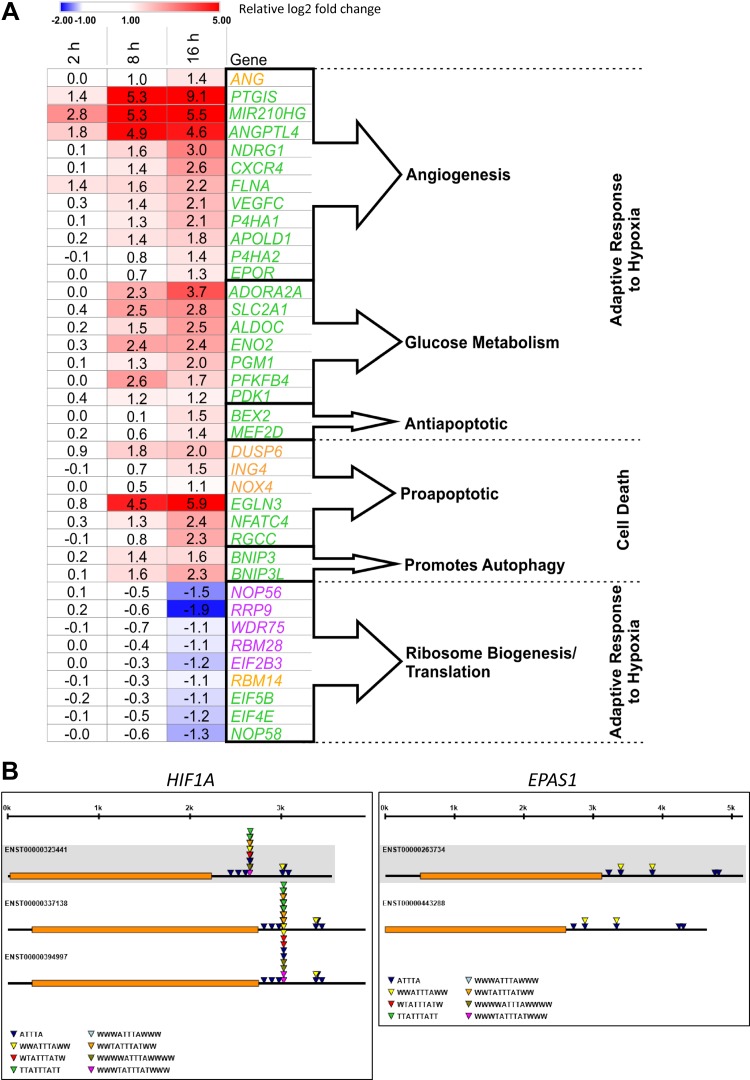
Although we observed a number of genes that appeared to be regulated exclusively by HIF-1 or HIF-2, the largest increase was observed in the number of genes that could be regulated by both (Fig. 7 and Supplemental Table S1C). To further address the transcriptomic consequences of the HIF-1/HIF-2 switch, we analyzed the biologic role of unique (HIF-1 or HIF-2) and common (HIF-1 and HIF-2) HIF targets using the gene ontology database. As shown in Fig. 8A and Supplemental Table S2, surprisingly, the gene set analysis at 16 h only identified genes regulated by HIF-1 (Fig. 8A, violet text) that could reduce ribosome biogenesis (nucleolar protein 56, nucleolar protein 58, rRNA processing 9, WD repeat domain 75, RNA binding motif protein 28, and eukaryotic translation initiation factor 2B subunit γ), supporting previous findings that hypoxia restricts ribosomal biogenesis and translation to preserve energy equilibrium and viability (45). Whereas the HIF-2–specific set (Fig. 8A, orange text) could not be assigned to any pathway [although some of them were proapoptotic (dual-specificity phosphatase 6, inhibitor of growth family member 4, NADPH oxidase 4)]. Interestingly, the genes predicted to be regulated by both HIF-1 and HIF-2 at 16 h were the ones responsible for the specific regulation of cellular response to hypoxia (Fig. 8A, green text). Furthermore, we show that the majority of genes responsible for HIF-dependent cellular responses to hypoxia can be regulated by both HIF-1 and HIF-2, and thus the switch from HIF-1 to HIF-2 in ECs ensures the continuous activity of the crucial hypoxia adaptive pathways and thus the prolonged hypoxia survival. Thus, the data suggest that the switch from HIF-1 to HIF-2 has transcriptional and physiologic consequences for ECs and that both HIFs are required to fully adapt ECs to prolonged hypoxia.
Figure 7.
A) Majority of HRE motifs containing genes affected during hypoxia are mainly transcriptional targets of both HIF-1 and HIF-2. The genome-wide distribution of genes containing HRE sequences specific for HIF-1 (violet), HIF-2 (orange), or both (green) at different times of hypoxic exposure in HUVECs. B) Distributions of motif instances directed distances from the transcription start site (TSS) between genes containing only motifs for HIF-1 or only motifs for HIF-2 differ significantly (K-S test, P = 1.123 × 10−9). C) Distributions of distances of motifs for HIF-1 and of motifs for HIF-2 in genes containing both types of motifs do not differ significantly (K-S test, P = 0.7257).
Figure 8.
A) Genes that mediate cellular responses to hypoxia are mainly transcriptional targets of both HIF-1 and HIF-2. Heat maps show the significant (P < 0.005) expression changes of unique HIF-1 (violet), HIF-2 (orange), and HIF-1/HIF-2 (green) regulated transcripts during 2, 8, and 16 h of hypoxia, and they were assigned with the GeneAnalytics database. These analysis results were verified with the literature (Supplemental Table S2). Heat maps were generated with Morpheus software. ADORA2A, adenosine A2a receptor; ALDOC, aldolase, fructose-bisphosphate C; ANG, angiogenin; ANGPTL4, angiopoietin-like 4; APOLD1, apolipoprotein L domain containing 1; BEX2, brain expressed X-linked 2; BNIP3, BCL2 interacting protein 3; BNIP3L, BCL2 interacting protein 3 like; CXCR4, C-X-C motif chemokine receptor 4; DUSP6, dual specificity phosphatase 6; EGLN3, Egl-9 family hypoxia inducible factor 3; EIF2B3, eukaryotic translation initiation factor 2B subunit γ; EIF4E, eukaryotic translation initiation factor 4E; EIF5B, eukaryotic translation initiation factor 5B; ENO2, enolase 2; EPOR, erythropoietin receptor; FLNA, filamin A; ING4, inhibitor of growth family member 4; MEF2D, myocyte enhancer factor 2D; MIR210HG, MIR210 host gene; NDRG1, N-Myc downstream regulated 1; NFATC4, nuclear factor of activated T-cell 4; NOP56, nucleolar protein 56; NOP58, nucleolar protein 58; NOX4, NADPH oxidase 4; P4HA2, prolyl 4-hydroxylase subunit alpha 2; PDK1, pyruvate dehydrogenase kinase 1; PFKFB4, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4; PGM1, phosphoglucomutase 1; P4HA1, prolyl 4-hydroxylase subunit α1; PTGIS, prostaglandin I2 synthase; RBM14, RNA binding motif protein 14; RBM28, RNA binding motif protein 28; RGCC, regulator of cell cycle; RRP9, ribosomal RNA processing 9; SLC2A1, solute carrier family 2 member 1; VEGFC, vascular endothelial growth factor C; WDR75, WD repeat domain 75.
DISCUSSION
To take advantage of regulating endothelial adaptation to hypoxia as a possible therapeutic intervention, it is important to understand the molecular pathways governing this process and especially the separate and common roles of HIF-1 and HIF-2 in mediating cell recovery. Although numerous interactions have been proposed to explain hypoxic HIF-1α destabilization [reviewed in Serocki et al. (21)], the majority of these studies were performed in cancer cells, and few were validated in normal ECs. Furthermore, most of the current studies followed the effects on HIF-1 and HIF-2 levels in a single type of EC.
The results of our analysis indicate that the HIF switch constitutes a universal mechanism of cellular adaptation to hypoxia in human ECs. We also show that HIF-1α expression during hypoxia generally differs between vascular and microvascular ECs. HIF-1α was more rapidly induced and present for shorter time periods in microvascular ECs and in HPASMC cells than in the other vascular ECs. Interestingly, the analysis of HIF-2α changes during hypoxia were similar in both vascular and microvascular ECs.
To understand the mechanism responsible for the transition between HIF-1α and HIF-2α, we performed mRNA time-course studies during hypoxia in the same set of EC lines. As it was also previously reported for some ECs (37–39), we observed that the hypoxic reduction of HIF1A mRNA was more dramatic than that of EPAS1 mRNA and that this was attributed to the changes in mRNA half-lives. Our data provide the first comparison of HIF1A and EPAS1 mRNA stability in multiple human ECs and are in good agreement with the previously reported human 3′UTR HIF1A mRNA stability measured in vitro with luciferase reporter vectors (46). The differences in HIF1A mRNA and EPAS1 mRNA stabilities can result from their reduction by different miRNA’s regulation [as reviewed in (21)] as well as by different distributions of mRNA’s destabilizing AREs in their 3′UTRs. Notably, although regulation of HIF1A mRNA through its 3′UTR ARE sites has been reported (46–49), its presence in 3′UTRs of EPAS1 mRNA has not been tested. Using the ARE1 web server (50), we analyzed ARE distribution in both HIF1A and EPAS1 mRNA. As shown in Fig. 8B, the EPAS1 3′UTR is much less prone to ARE-dependent destabilization than HIF1A mRNA. Although, to date, numerous miRNAs were shown to directly destabilize HIF1A mRNA in ECs, no such miRNA has been identified for EPAS1 mRNA (21). Nevertheless, chronic hypoxia impairs dicer (DICER1) activity in a von Hippel-Lindau–dependent manner, and thus miRNA biogenesis affecting both HIF-α isoforms and, notably, EPAS1 mRNA is regulated by the dicer-dependent miRNA 185, which is down-regulated by hypoxia (51). However, whether miRNA activity is repressing gene activity by blocking translation of their mRNA targets (52, 53) or by promoting mRNA degradation (54, 55) remains open to debate. Hence, the miRNA’s contribution to the switch from HIF-1 to HIF-2 seems plausible. Their impact on mRNA stability during hypoxia requires further study.
Although the differences in mRNA half-life of HIF1A and EPAS1 mRNAs could explain the faster reduction of HIF1A mRNA during hypoxia, these differences are too small to be entirely responsible for the HIF-1 to HIF-2 switch. Furthermore, the faster degradation of HIF-1α that we observed in microvascular ECs was neither reflected in their HIF1A mRNA levels nor their mRNA half-lives (Supplemental Fig. S2B).
Other post-translational mechanisms besides mRNA stability may be required to facilitate the HIF switch. Indeed, numerous factors that selectively modulate HIF-1α and HIF-2α stability have been reported that include spliceosome associated factor 1 (the human homolog of murine hypoxia-associated factor) (56), carboxyl terminus of Hsc70-interacting protein complex (57), the receptor for activated kinase C1, the Kruppel-like factor 2 (22, 57), and the heat shock protein 90 (59, 60). Furthermore, the HIF-1–dependent induction of PHD3 can also lead to HIF-2α degradation (61). Clearly, further studies are needed to clarify whether these above mechanisms contribute to the selection between HIF-1 and HIF-2 in ECs.
The final point addressed in these studies was to examine the relative contributions of HIF-1 and HIF-2 to the hypoxia-induced transcriptome. To date, only a few studies have evaluated the genome-wide effects of HIFs in ECs after 24 h of hypoxia (62, 63), and there are no reports that focus on acute hypoxia (<12 h) (64, 65). There is, however, 1 study that followed the effects of prolonged hypoxia (14 d) in human microvascular ECs (66).
To segregate the effects of HIF-1 and HIF-2, we analyzed points that we predicted would be HIF-1 specific (2 h) or HIF-2 specific (16 h) or both (8 h) and showed that the number of genes affected by hypoxia increased over the time course. Furthermore, the 8 h of hypoxic exposure resulted in the classic responses to hypoxia, whereas after 16 h of hypoxia, changes in a large number of up- and down-regulated genes in a variety of signaling networks made it more difficult to determine the HIF-specific effects (Supplemental Table S1A).
To focus on the HIF-1– vs. the HIF-2–specific effects, we screened for the presence of known HIF-1– and HIF-2–specific binding motifs (HREs) in the promoter regions of the genes affected during hypoxia. Furthermore, we have validated our HIF HREs analysis with the newest experimental (ChIP-seq based) identification of HIF-1 and HIF-2 binding by Smythies et al. (44). As shown in Supplemental Fig. S3 (color marked genes) and Supplemental Table S1C, despite different cell lines used (cancer vs. primary endothelial) and different analysis settings between this work and our approach, our data are in very good agreement with their results.
HIF-1 and HIF-2 were shown to bind different but overlapping sets of sites in chromatin and induce only partially overlapping patterns of gene expression (18, 42, 44, 67). We observed that HRE-containing genes affected by acute hypoxia remained active during prolonged exposure in spite of the fact that these genes’ promoter regions were enriched with HIF-1 motifs. As expected, those genes that were only affected during more prolonged hypoxia had more HIF-2 motifs, and this observation is consistent with the model for the transitional switch between the 2 HIFs. Furthermore, our analysis [similar to that shown in Smythies, et al. (44)] clearly shows the different distribution patterns of HIF-1 and HIF-2 sites in their target genes (Fig. 7B), supporting the hypothesis that HIF-1 and HIF-2 do not compete for binding sites.
Importantly, however, the largest changes during hypoxia we noted were for genes that could be potentially regulated by both HIFs (containing both HIF-1– and HIF-2–specific motifs). Notably, within these genes, there was no significant difference in distribution patterns of HIF sites between of HIF-1 and HIF-2 as shown in Fig. 7C, supporting the previous observation that, for these genes (HIF-1/HIF-2), binding must occur in a noncompetitive and noncompensatory manner at the same regions (18, 42, 44, 67). The majority of HIF-1/HIF-2 common genes were induced after 8 and 16 h of hypoxia, at a time when HIF-1 was dramatically reduced. Surprisingly, these genes predicted to be regulated by both HIF-1 and HIF-2 (HIF-1/HIF-2) were mainly responsible for the specific regulation of cellular response to hypoxia. This suggests that, whereas HIF-1–specific signaling is time limited by the presence of HIF-1α, the switch from HIF-1 to HIF-2 in ECs ensures the continuous activity of the adaptive pathways and prolonged hypoxia survival. This also suggests that the switch from HIF-1 to HIF-2 has transcriptional and physiologic consequences for ECs and that both HIFs are required to effectively adapt the cells to hypoxia.
Taken together, our study demonstrates that the switch from HIF-1 to HIF-2 constitutes a universal mechanism of human endothelium adaptation to prolonged hypoxia and partially results from different stabilities of the HIF1A and EPAS1 mRNAs. Furthermore, we show that the majority of genes responsible for HIF-dependent cellular responses to hypoxia can be regulated by both HIF-1 and HIF-2, and thus the switch from HIF-1 to HIF-2 in ECs ensures the continuous activity of the hypoxia adaptive pathways and thus prolonged hypoxia survival.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Perlan Technologies, Polska Spółka z ograniczoną odpowiedzialnością (Sp. z o.o.) for performing microarray experiments and analysis. This research was funded by National Science Center SONATA BIS Program under contract UMO-2015/18/E/NZ3/00687 (to R.B.). J.F.C. was funded by the U.S. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK072482, and a Research Development Program grant from the Cystic Fibrosis Foundation. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The authors declare no conflicts of interest.
Glossary
- ARE
adenylate-uridylate-rich element
- AU
adenylate-uridylate-rich
- BSA
bovine serum albumin
- ChIP-seq
chromatin immunoprecipitation sequencing
- EC
endothelial cell
- EGM
endothelial growth medium
- EPAS1
endothelial PAS domain protein 1
- FIH-1
factor-inhibiting HIF-1
- HAEC
human aortic EC
- HCAEC
human cardiac artery EC
- HIF
hypoxia-inducible factor
- HMVEC-C
human cardiac microvascular EC
- HMVEC-D
human dermal microvascular EC
- HMVEC-L
human lung microvascular EC
- HPASMC
human pulmonary artery smooth muscle cell
- HRE
hypoxia response element
- K-S
Kolmogorov-Smirnov
- miRNA
microRNA
- PHD
prolyl-hydroxylase
- TBP
TATA-binding protein
- UtMVEC
human uterine microvascular EC
- WDR75
WD repeat domain 75
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
R. Bartoszewski and M. Dąbrowski conceptualized and supervised the study and contributed methodology; R. Bartoszewski, A. Moszyńska, M. Serocki, A. Cabaj, and A. Polten contributed to the investigation; R. Ochocka, R. Bartoszewski, and M. Dąbrowski contributed resources; A. Moszyńska, A. Cabaj, S. Bartoszewska, and J. Króliczewski contributed to the study visualization; R. Bartoszewski, L. Dell’Italia, S. Bartoszewska, M. Dąbrowski, and J. F. Collawn wrote the original draft of the manuscript; R. Bartoszewski, A. Moszyńska, R. Ochocka, S. Bartoszewska, J. Króliczewski, M. Dąbrowski, and J. F. Collawn reviewed and edited the final manuscript; and R. Bartoszewski acquired funding.
REFERENCES
- 1.Koh M. Y., Powis G. (2012) Passing the baton: the HIF switch. Trends Biochem. Sci. 37, 364–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loboda A., Jozkowicz A., Dulak J. (2012) HIF-1 versus HIF-2--is one more important than the other? Vascul. Pharmacol. 56, 245–251 [DOI] [PubMed] [Google Scholar]
- 4.Safran M., Kaelin W. G., Jr (2003) HIF hydroxylation and the mammalian oxygen-sensing pathway. J. Clin. Invest. 111, 779–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaelin W. G., Jr., Ratcliffe P. J. (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 [DOI] [PubMed] [Google Scholar]
- 6.Kaelin W. G. (2005) Proline hydroxylation and gene expression. Annu. Rev. Biochem. 74, 115–128 [DOI] [PubMed] [Google Scholar]
- 7.Bruick R. K., McKnight S. L. (2001) A conserved family of prolyl-4-hydroxylases that modify HIF. Science 294, 1337–1340 [DOI] [PubMed] [Google Scholar]
- 8.Epstein A. C. R., Gleadle J. M., McNeill L. A., Hewitson K. S., O’Rourke J., Mole D. R., Mukherji M., Metzen E., Wilson M. I., Dhanda A., Tian Y. M., Masson N., Hamilton D. L., Jaakkola P., Barstead R., Hodgkin J., Maxwell P. H., Pugh C. W., Schofield C. J., Ratcliffe P. J. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 [DOI] [PubMed] [Google Scholar]
- 9.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., Salic A., Asara J. M., Lane W. S., Kaelin W. G., Jr (2001) HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292, 464–468 [DOI] [PubMed] [Google Scholar]
- 10.Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., von Kriegsheim A., Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 11.Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 20, 5197–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 13.Tanimoto K., Makino Y., Pereira T., Poellinger L. (2000) Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 19, 4298–4309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahon P. C., Hirota K., Semenza G. L. (2001) FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lando D., Peet D. J., Whelan D. A., Gorman J. J., Whitelaw M. L. (2002) Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 295, 858–861 [DOI] [PubMed] [Google Scholar]
- 16.Wang G. L., Semenza G. L. (1993) General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 90, 4304–4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loboda A., Jozkowicz A., Dulak J. (2010) HIF-1 and HIF-2 transcription factors--similar but not identical. Mol. Cells 29, 435–442 [DOI] [PubMed] [Google Scholar]
- 18.Hu C. J., Wang L. Y., Chodosh L. A., Keith B., Simon M. C. (2003) Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol. Cell. Biol. 23, 9361–9374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kalinowski L., Janaszak-Jasiecka A., Siekierzycka A., Bartoszewska S., Woźniak M., Lejnowski D., Collawn J. F., Bartoszewski R. (2016) Posttranscriptional and transcriptional regulation of endothelial nitric-oxide synthase during hypoxia: the role of microRNAs. Cell. Mol. Biol. Lett. 21, 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keith B., Johnson R. S., Simon M. C. (2011) HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat. Rev. Cancer 12, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serocki M., Bartoszewska S., Janaszak-Jasiecka A., Ochocka R. J., Collawn J. F., Bartoszewski R. (2018) miRNAs regulate the HIF switch during hypoxia: a novel therapeutic target. Angiogenesis 21, 183–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartoszewski R., Serocki M., Janaszak-Jasiecka A., Bartoszewska S., Kochan-Jamrozy K., Piotrowski A., Króliczewski J., Collawn J. F. (2017) miR-200b downregulates Kruppel like factor 2 (KLF2) during acute hypoxia in human endothelial cells. Eur. J. Cell Biol. 96, 758–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janaszak-Jasiecka A., Bartoszewska S., Kochan K., Piotrowski A., Kalinowski L., Kamysz W., Ochocka R. J., Bartoszewski R., Collawn J. F. (2016) miR-429 regulates the transition between Hypoxia-Inducible Factor (HIF)1A and HIF3A expression in human endothelial cells. Sci. Rep. 6, 22775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartoszewska S., Kochan K., Piotrowski A., Kamysz W., Ochocka R. J., Collawn J. F., Bartoszewski R. (2015) The hypoxia-inducible miR-429 regulates hypoxia-inducible factor-1α expression in human endothelial cells through a negative feedback loop. FASEB J. 29, 1467–1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janaszak-Jasiecka A., Siekierzycka A., Bartoszewska S., Serocki M., Dobrucki L. W., Collawn J. F., Kalinowski L., Bartoszewski R. (2018) eNOS expression and NO release during hypoxia is inhibited by miR-200b in human endothelial cells. Angiogenesis 21, 711–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bartoszewska S., Kamysz W., Jakiela B., Sanak M., Króliczewski J., Bebok Z., Bartoszewski R., Collawn J. F. (2017) miR-200b downregulates CFTR during hypoxia in human lung epithelial cells. Cell. Mol. Biol. Lett. 22, 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 28.Bartoszewski R., Rab A., Fu L., Bartoszewska S., Collawn J., Bebok Z. (2011) CFTR expression regulation by the unfolded protein response. Methods Enzymol. 491, 3–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartoszewski R., Rab A., Twitty G., Stevenson L., Fortenberry J., Piotrowski A., Dumanski J. P., Bebok Z. (2008) The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J. Biol. Chem. 283, 12154–12165 [DOI] [PubMed] [Google Scholar]
- 30.ENCODE Project Consortium (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zerbino D. R., Achuthan P., Akanni W., Amode M. R., Barrell D., Bhai J., Billis K., Cummins C., Gall A., Girón C. G., Gil L., Gordon L., Haggerty L., Haskell E., Hourlier T., Izuogu O. G., Janacek S. H., Juettemann T., To J. K., Laird M. R., Lavidas I., Liu Z., Loveland J. E., Maurel T., McLaren W., Moore B., Mudge J., Murphy D. N., Newman V., Nuhn M., Ogeh D., Ong C. K., Parker A., Patricio M., Riat H. S., Schuilenburg H., Sheppard D., Sparrow H., Taylor K., Thormann A., Vullo A., Walts B., Zadissa A., Frankish A., Hunt S. E., Kostadima M., Langridge N., Martin F. J., Muffato M., Perry E., Ruffier M., Staines D. M., Trevanion S. J., Aken B. L., Cunningham F., Yates A., Flicek P. (2018) Ensembl 2018. Nucleic Acids Res. 46, D754–D761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kulakovskiy I. V., Vorontsov I. E., Yevshin I. S., Sharipov R. N., Fedorova A. D., Rumynskiy E. I., Medvedeva Y. A., Magana-Mora A., Bajic V. B., Papatsenko D. A., Kolpakov F. A., Makeev V. J. (2018) HOCOMOCO: towards a complete collection of transcription factor binding models for human and mouse via large-scale ChIP-Seq analysis. Nucleic Acids Res. 46, D252–D259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krystkowiak I., Lenart J., Debski K., Kuterba P., Petas M., Kaminska B., Dabrowski M. (2013) Nencki genomics database–Ensembl funcgen enhanced with intersections, user data and genome-wide TFBS motifs. Database (Oxford) 2013, bat069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RDevelopmentCoreTeam (2008) R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 35.Ben-Ari Fuchs S., Lieder I., Stelzer G., Mazor Y., Buzhor E., Kaplan S., Bogoch Y., Plaschkes I., Shitrit A., Rappaport N., Kohn A., Edgar R., Shenhav L., Safran M., Lancet D., Guan-Golan Y., Warshawsky D., Shtrichman R. (2016) GeneAnalytics: an integrative gene set analysis tool for next generation sequencing, RNAseq and microarray data. OMICS 20, 139–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stelzer G., Inger A., Olender T., Iny-Stein T., Dalah I., Harel A., Safran M., Lancet D. (2009) GeneDecks: paralog hunting and gene-set distillation with GeneCards annotation. OMICS 13, 477–487 [DOI] [PubMed] [Google Scholar]
- 37.McQuillan L. P., Leung G. K., Marsden P. A., Kostyk S. K., Kourembanas S. (1994) Hypoxia inhibits expression of eNOS via transcriptional and posttranscriptional mechanisms. Am. J. Physiol. 267, H1921–H1927 [DOI] [PubMed] [Google Scholar]
- 38.Fish J. E., Matouk C. C., Yeboah E., Bevan S. C., Khan M., Patil K., Ohh M., Marsden P. A. (2007) Hypoxia-inducible expression of a natural cis-antisense transcript inhibits endothelial nitric-oxide synthase. J. Biol. Chem. 282, 15652–15666 [DOI] [PubMed] [Google Scholar]
- 39.Robb G. B., Carson A. R., Tai S. C., Fish J. E., Singh S., Yamada T., Scherer S. W., Nakabayashi K., Marsden P. A. (2004) Post-transcriptional regulation of endothelial nitric-oxide synthase by an overlapping antisense mRNA transcript. J. Biol. Chem. 279, 37982–37996 [DOI] [PubMed] [Google Scholar]
- 40.Elbashir S. M., Harborth J., Lendeckel W., Yalcin A., Weber K., Tuschl T. (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411, 494–498 [DOI] [PubMed] [Google Scholar]
- 41.Heberle H., Meirelles G. V., da Silva F. R., Telles G. P., Minghim R. (2015) InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16, 169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schödel J., Oikonomopoulos S., Ragoussis J., Pugh C. W., Ratcliffe P. J., Mole D. R. (2011) High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117, e207–e217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee M. C., Huang H. J., Chang T. H., Huang H. C., Hsieh S. Y., Chen Y. S., Chou W. Y., Chiang C. H., Lai C. H., Shiau C. Y. (2016) Genome-wide analysis of HIF-2α chromatin binding sites under normoxia in human bronchial epithelial cells (BEAS-2B) suggests its diverse functions. Sci. Rep. 6, 29311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smythies J. A., Sun M., Masson N., Salama R., Simpson P. D., Murray E., Neumann V., Cockman M. E., Choudhry H., Ratcliffe P. J., Mole D. R. (2019) Inherent DNA-binding specificities of the HIF-1α and HIF-2α transcription factors in chromatin. EMBO Rep. 20, e46401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fähling M. (2009) Surviving hypoxia by modulation of mRNA translation rate. J. Cell. Mol. Med. 13, 2770–2779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chamboredon S., Ciais D., Desroches-Castan A., Savi P., Bono F., Feige J.-J., Cherradi N. (2011) Hypoxia-inducible factor-1α mRNA: a new target for destabilization by tristetraprolin in endothelial cells. Mol. Biol. Cell 22, 3366–3378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim T. W., Yim S., Choi B. J., Jang Y., Lee J. J., Sohn B. H., Yoo H. S., Yeom Y. I., Park K. C. (2010) Tristetraprolin regulates the stability of HIF-1alpha mRNA during prolonged hypoxia. Biochem. Biophys. Res. Commun. 391, 963–968 [DOI] [PubMed] [Google Scholar]
- 48.Galbán S., Kuwano Y., Pullmann R., Jr., Martindale J. L., Kim H. H., Lal A., Abdelmohsen K., Yang X., Dang Y., Liu J. O., Lewis S. M., Holcik M., Gorospe M. (2008) RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 28, 93–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.López de Silanes I., Zhan M., Lal A., Yang X., Gorospe M. (2004) Identification of a target RNA motif for RNA-binding protein HuR. Proc. Natl. Acad. Sci. USA 101, 2987–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fallmann J., Sedlyarov V., Tanzer A., Kovarik P., Hofacker I. L. (2016) AREsite2: an enhanced database for the comprehensive investigation of AU/GU/U-rich elements. Nucleic Acids Res. 44, D90–D95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho J. J., Metcalf J. L., Yan M. S., Turgeon P. J., Wang J. J., Chalsev M., Petruzziello-Pellegrini T. N., Tsui A. K., He J. Z., Dhamko H., Man H. S., Robb G. B., Teh B. T., Ohh M., Marsden P. A. (2012) Functional importance of Dicer protein in the adaptive cellular response to hypoxia. J. Biol. Chem. 287, 29003–29020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bazzini A. A., Lee M. T., Giraldez A. J. (2012) Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336, 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Djuranovic S., Nahvi A., Green R. (2012) miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336, 237–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hendrickson D. G., Hogan D. J., McCullough H. L., Myers J. W., Herschlag D., Ferrell J. E., Brown P. O. (2009) Concordant regulation of translation and mRNA abundance for hundreds of targets of a human microRNA. PLoS Biol. 7, e1000238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guo H., Ingolia N. T., Weissman J. S., Bartel D. P. (2010) Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466, 835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koh M. Y., Powis G. (2006) SART1/HAF, a protein widely over expressed in human cancer, decreases HIF-1α levels and inhibits tumor growth. Cancer Res. 66, 409 [Google Scholar]
- 57.Luo W., Zhong J., Chang R., Hu H., Pandey A., Semenza G. L. (2010) Hsp70 and CHIP selectively mediate ubiquitination and degradation of hypoxia-inducible factor (HIF)-1alpha but Not HIF-2alpha. J. Biol. Chem. 285, 3651–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kawanami D., Mahabeleshwar G. H., Lin Z., Atkins G. B., Hamik A., Haldar S. M., Maemura K., Lamanna J. C., Jain M. K. (2009) Kruppel-like factor 2 inhibits hypoxia-inducible factor 1alpha expression and function in the endothelium. J. Biol. Chem. 284, 20522–20530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y. V., Semenza G. L. (2007) RACK1 vs. HSP90: competition for HIF-1 alpha degradation vs. stabilization. Cell Cycle 6, 656–659 [DOI] [PubMed] [Google Scholar]
- 60.Liu Y. V., Baek J. H., Zhang H., Diez R., Cole R. N., Semenza G. L. (2007) RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cell 25, 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Appelhoff R. J., Tian Y. M., Raval R. R., Turley H., Harris A. L., Pugh C. W., Ratcliffe P. J., Gleadle J. M. (2004) Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia-inducible factor. J. Biol. Chem. 279, 38458–38465 [DOI] [PubMed] [Google Scholar]
- 62.Manalo D. J., Rowan A., Lavoie T., Natarajan L., Kelly B. D., Ye S. Q., Garcia J. G., Semenza G. L. (2005) Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105, 659–669 [DOI] [PubMed] [Google Scholar]
- 63.Weigand J. E., Boeckel J. N., Gellert P., Dimmeler S. (2012) Hypoxia-induced alternative splicing in endothelial cells. PLoS One 7, e42697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alizadeh E., Mammadzada P., André H. (2018) The different facades of retinal and choroidal endothelial cells in response to hypoxia. Int. J. Mol. Sci. 19, E3846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mammadzada P., Gudmundsson J., Kvanta A., André H. (2016) Differential hypoxic response of human choroidal and retinal endothelial cells proposes tissue heterogeneity of ocular angiogenesis. Acta Ophthalmol. 94, 805–814 [DOI] [PubMed] [Google Scholar]
- 66.Nauta T. D., van den Broek M., Gibbs S., van der Pouw-Kraan T. C., Oudejans C. B., van Hinsbergh V. W., Koolwijk P. (2017) Identification of HIF-2α-regulated genes that play a role in human microvascular endothelial sprouting during prolonged hypoxia in vitro. Angiogenesis 20, 39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choudhry H., Schödel J., Oikonomopoulos S., Camps C., Grampp S., Harris A. L., Ratcliffe P. J., Ragoussis J., Mole D. R. (2014) Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep. 15, 70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.