Abstract
Objective
nformal caregivers provide substantial support for people living with cancer. Previous systematic reviews report on the efficacy of cancer caregiver interventions but not their potential to be implemented. The aim of this systematic review was to explore the potential for cancer caregiver interventions to be implemented into practice.
Methods
We searched three electronic databases to identify cancer caregiver interventions on 5 January 2018. We operationalised six implementation outcomes (acceptability, adoption, appropriateness, feasibility, fidelity, and costs) into a tool to guide data extraction.
Results
The search yielded 33 papers (27 papers from electronic databases and six papers from other sources) reporting on 26 studies that met review criteria. Fewer than half the studies (46%) contained evidence about the acceptability of interventions from caregivers' perspectives; only two studies (8%) included interventions developed with input from caregivers. Two studies (8%) addressed potential adoption of interventions, and no studies discussed intentions, agreement, or action to implement interventions into practice. All studies reported on intervention appropriateness by providing a rationale for the interventions. For feasibility, on average less than one‐third of caregivers who were eligible to be involved consented to participate. On fidelity, whether interventions were conducted as intended was reported in 62% of studies. Cost data were reported in terms of intervention delivery, requiring a median time commitment of staff of 180 minutes to be delivered.
Conclusions
Caregiver intervention studies lack components of study design and reporting that could bridge the gap between research and practice. There is enormous potential for improvements in cancer caregiver intervention study design to plan for future implementation.
Keywords: cancer, caregiver, carer, dissemination, framework, implementation, intervention, oncology, outcomes
1. BACKGROUND
Informal caregivers provide substantial practical and emotional support for people living with cancer, and in doing so, many receive minimal support themselves. Previous studies have outlined the negative impacts associated with being a caregiver, including depression,1 burden,2 social isolation,3 loss of self‐identity,4 sleep deprivation,5 financial burden,6 and significant changes to their lives.2 The role they take on in caring for the person with cancer is extensive, demanding, and often without training or resources.7
Many research papers focus on the development and evaluation of interventions aimed at improving the experience of caregivers, including several reviews of caregiver interventions.8, 9, 10, 11, 12, 13, 14, 15, 16 Of these, Northouse et al presented a meta‐analysis of the efficacy of caregiver intervention studies categorising interventions as psychoeducational, skills training, and therapeutic counselling. They concluded that interventions had beneficial small to medium effects on burden, coping, self‐efficacy, and quality of life.15 More recently, Ferrell and Wittenberg11 performed an updated review, identifying an increase in trials and the growing need to translate evidence into practice. Similarly, a review article drawing upon the literature and stakeholder perspectives from an in person meeting attended by more than 75 invited researchers, clinicians, advocates, and representatives recommended the implementation of successful interventions.17
Previous reviews have focused on the efficacy of caregiver interventions but not their potential to be implemented into practice. Implementation science frameworks contribute to reducing the evidence to practice gap18 by applying a theory to identify factors that may evaluate implementation success.19 Proctor et al26 developed a framework of implementation outcomes, defined as the “effects of deliberate and purposive actions to implement new treatments, practices and services” (p65). This framework has eight implementation outcomes: acceptability, adoption, appropriateness, (implementation) costs, feasibility, fidelity, penetration, and sustainability. Of these, the first six are relevant to the earlier stages of implementation, whereas penetration is relevant mid‐implementation and sustainability applies to longer‐term implementation. This framework has been applied to inform a variety of research topics including standardised multidisciplinary team meeting templates,20 shared decision‐making,21 cervical cancer prevention,22 and uptake of human papillomavirus (HPV) vaccines.23
Caregiver interventions show promise for potential implementation into practice.11, 15 However, we know little about whether interventions are designed and reported in a way that supports implementation.24, 25 There is a need to explore the implementation potential of existing cancer caregiver intervention studies to guide the development of future caregiver research. The aim of this review is to describe and appraise the cancer caregiving literature to explore the potential for caregiver interventions to be implemented into practice.
2. METHOD
2.1. Search strategy
This systematic review was registered on PROSPERO, number: CRD42018098838.
To identify studies for inclusion in this review, three electronic databases were searched, Cumulative Index to Nursing and Allied Health Literature (CINAHL) Complete, MEDLINE Complete, and PsycINFO Complete, representing the fields of nursing, medicine, and psychology. The terms used in the search were caregivers (as a subject heading) and cancer. No limitations were applied for language or publication date. The search was performed on 5 January 2018. The reference lists of papers meeting the inclusion criteria were scanned for additional papers for possible inclusion in the review.
We also searched reference lists of eight recent systematic reviews on caregivers of people with cancer.8, 10, 11, 12, 13, 14, 15, 16
2.2. Selection criteria
Studies were included in this review if they met the following criteria: (i) Participants were informal (unpaid) adult (18+ y) caregivers who had an active role in the provision of care for an adult with cancer; (ii) interventions were programmes, supports, sessions, or resources provided to, and directed towards supporting, caregivers to improve their own functioning or assist them in providing support for the patient (eg, programmes focusing on upskilling caregivers); (iii) study designs included at least two conditions (eg, randomised controlled trials and quasi‐experimental studies), one of which must have been a control condition (eg, active controls, waiting list controls, and treatment as usual [TAU] controls); and (iv) study outcomes focused on the caregiver. Pilot and feasibility studies were eligible for inclusion.
Studies were excluded if they met the following criteria: (i) They focused on spouses or other family without establishing that they had caregiving roles; (ii) 25% or more of patients had conditions other than cancer; (iii) the interventions focused on both patients and caregivers (interventions where minimal content was delivered to patients were eligible for inclusion, however); and (iv) the study design included two or more experimental conditions without a control condition. These exclusion criteria were established to ensure a focus on cancer caregiver interventions. Review papers were excluded from selection.
2.3. Study selection
Two authors (A.U. and C.J.G.) performed the eligibility assessment independently in an unbiased standardised manner. C.J.G. undertook an initial screening of papers, on the basis of title and then abstract. Both A.U. and C.J.G. then assessed papers on the basis of a full‐text review. Disagreements between reviewers were resolved through consensus. Deferring to a third reviewer was not necessary.
2.4. Data extraction
From each study meeting the selection criteria, data were extracted on study characteristics and the implementation outcomes of the interventions. Data extracted on study characteristics included (i) country of origin, (ii) aim, (iii) caregiver demographic characteristics (sample size, sex, and age), (iv) patient diagnosis, (v) relationship between caregiver and patient, (vi) study design, (vii) intervention details (format, content, setting, and who delivered the intervention), (viii) theory underpinning intervention (explicit statement required), (ix) evidence of prior pilot testing of intervention, (x) comparison condition, (xi) outcome measures, (xii) key findings, and (xiii) whether the conclusions were supported.
Operationalisation of the Proctor et al26 taxonomy of implementation outcomes (acceptability, adoption, appropriateness, feasibility, fidelity, and cost) guided the extraction of data on intervention implementation outcomes (see Table 1). This framework was selected because of the alignment between the implementation outcomes and the aims of the review. We selected this framework in preference to others in the implementation science discipline as it draws on a conceptual framework that addresses a range of outcomes. The outcomes are defined in a comprehensive manner that facilitates measurement for the purposes of a systematic review.
Table 1.
Operationalisation of Proctor's framework for implementation outcomes
| Implementation Outcome | Operationalisation in This Systematic Review | Response Options |
|---|---|---|
| Acceptability | Data collected on intervention acceptability from the perspectives of caregivers | Y/N/Partially/Possibly |
| Data collected on intervention acceptability from the perspectives of other stakeholders | Y/N/Partially/Possibly | |
| Caregiver input into intervention development | Had input into intervention development/Caregivers informed the development/Not involved | |
| Adoption | Evidence of intention, agreement, or action to try to employ intervention | Y/N; details |
| Appropriateness | Whether the intervention was a good fit | Y/N; details |
| Whether the intervention was targeted to high risk caregivers | Y/N; details | |
| Feasibility | Participation of caregivers screened: | Raw numbers, percentages, or not reported/not calculable |
| • People screened | ||
| • Eligible | ||
| • Consented | ||
| • Commenced study | ||
| Participation of caregivers in the intervention condition: | Raw numbers, percentages, or not reported/not calculable | |
| • Withdrawal rate (choosing to no longer participate) | ||
| • Unable to complete intervention (ceasing involvement due to change in circumstances) | ||
| • Percentage who completed intervention (ie, they did not withdraw or were unable to complete) | ||
| Participant time commitment required for full intervention delivery | Time (minutes) | |
| Fidelity | Whether the intervention ran as intended | Yes/No/Not reported; details |
| Dose delivered | Percentage | |
| Changes to the intervention during the study | None reported/details | |
| Costs | Staff time commitment required for delivery | Time in min/Not reported |
| Additional resources used | None reported/details | |
| Staff training and expertise required to deliver intervention | Not specified/details |
The framework was operationalised into a data collection tool by three authors (N.M.R., A.U., and C.J.G.). One author completed all data extraction (C.J.G.), with 20% of studies extracted by a second author (A.U.). Where necessary, two authors discussed ambiguities until consensus was achieved.
2.5. Data analysis
Descriptive statistics (frequencies, medians, and interquartile ranges [IQRs]) were used to summarise the data from the studies. Data were extracted to and analysed in Microsoft Excel.
3. RESULTS
The search of electronic databases yielded 7183 records (CINAHL Complete, n = 2306; MEDLINE Complete, n = 2757; and PsycINFO Complete, n = 2120), of which 2682 were duplicates (see Figure 1). Of the remaining 4501 entries, 103 were retained following screening the titles of papers. After reviewing the abstracts, 61 papers did not meet the selection criteria and were excluded. The full texts of the remaining 42 papers were reviewed, of which 27 papers were finally included in the review.
Figure 1.
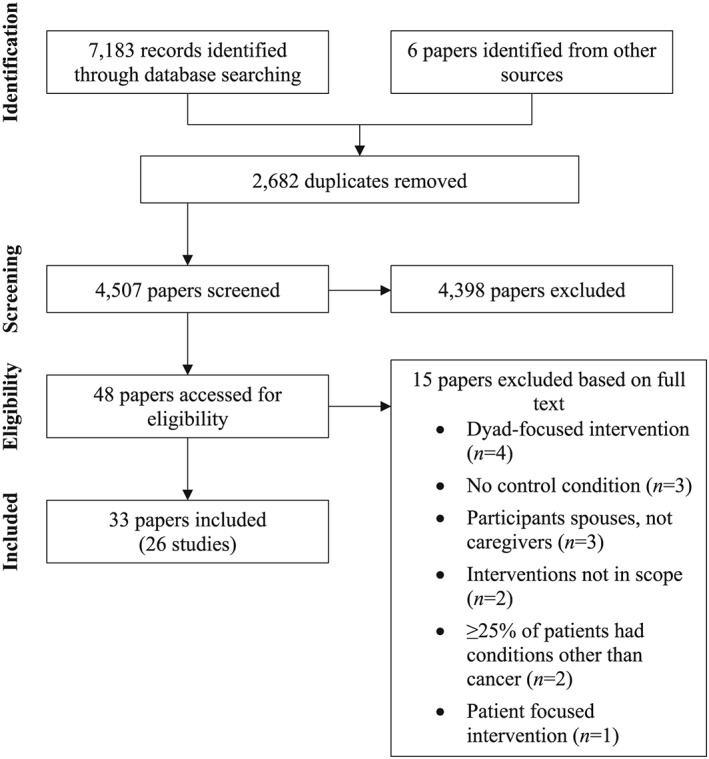
Identification and selection of studies for the systematic review
An additional six papers meeting the selection criteria came from other sources. Inspection of the reference lists of previous systematic reviews in the area8, 11, 12, 13, 14, 15, 16 enabled identification of a further five papers that met the selection criteria. One further paper was identified from a preliminary search that abandoned because the search was too narrow. Being relevant, this paper was included in the review. Checking the reference lists of the included papers resulted in no further papers being identified for inclusion. In total, 33 papers were included in the review, representing 26 studies (Table 2).
Table 2.
Study characteristics
| Study | Country | Primary Aim | Participants | Patient Diagnosis | Caregiver‐Patient Relationship | Design | Comparison Condition | Main Outcome Measures | Key Results |
|---|---|---|---|---|---|---|---|---|---|
| Bahrami and Farzi (2014)27 | Iran | To determine the effect of a supportive educational programme on the caring burden and quality of life in the family caregivers of women with breast cancer. | 64 caregivers (64% female; exp: mean age = 37, SD = 11; con: mean age = 39, SD = 10) | Breast cancer | Husband (27%), child (48%), sibling (22%), parents (3%) | RCT | TAU | Caregiver burden, QOL | ↓caregiver burden, ↑physical, mental, spiritual, and environmental domains and overall quality of life |
| Belgacem et al (2013)28 | France | To assess the effect of a caregiver educational on patients' and caregivers' QOL and caregivers' burden. | 67 caregivers (no descriptive statistics) | Haematological/other oncological cancer | Spouse (62%), offspring (17%), sibling (9%), parent (9%), friend (3%) | RCT | TAU | Caregiver burden, QOL | ↑QOL (caregivers and patients), ↓burden (caregivers) |
| Bultz et al (2000)30 | Canada | To trial of a brief psychoeducational group programme for partners of breast cancer patients. | 34 partners (100% male; mean age = 51, range 32‐67) | Breast cancer (stage I or II) | Spouse/partner (100%) | RCT | Wait list | Mood, marital satisfaction, social support | ↓mood disturbance (post‐intervention) |
| Carter (2006)31 | USA | To test the feasibility and effectiveness of a brief behavioural sleep intervention for family caregivers of persons with advanced stage cancer. | 30 caregivers (63% female; mean age = 53, SD = 17) | Advanced cancer | Spouse (57%), adult child (30%) | Repeated measures experimental design | Placebo | Sleep quality, depressive symptoms, QOL | ↑sleep quality, ↓depressive symptoms |
| DuBenske et al (2014)32; Namkoong et al (2012)51 | USA | To assess the impact of a web‐based lung cancer information, communication, and coaching system for caregivers versus the internet with a list of recommended lung cancer websites. | 285 caregivers (68% female; mean age = 56, range = 18‐84) | Advanced NSCLC lung cancer | Spouse/partner (72%) | RCT | Active | Caregiver burden, disruptiveness, mood | ↓burden, ↓negative mood; perceived bonding (i)positively affects caregivers' appraisal and problem‐focused coping strategies and (ii) mediates the effects of CHESS on coping strategies |
| Fegg et al (2013)33 | Germany | To test the effectiveness of existential behavioural therapy on mental stress and quality of life. | 133 caregivers (70% female; mean age = 55, SD = 13) | 83% cancer | Partner (62%), parent (26%), child (3%) | RCT | TAU | Somatisation, anxiety, depression, satisfaction with life, quality of life | Post treatment: ↓anxiety, ↑QOL. 12 mo: ↓depression, ↑QOL |
| Hendrix et al (2013)36 | USA | To investigate the effects of an individualised caregiver training programme on self‐efficacy in home care and symptom management. | 120 caregivers (83% female; 85% aged 46+) | Haematological | Spouse (77%), child (13%) | RCT | Placebo | Caregiver self‐efficacy for managing patient symptoms and psychological well‐being (depression, anxiety, quality of life); patient physical symptoms | ↑caregiver self‐efficacy |
| Hendrix et al (2016)35 | USA | To compare the effects of the enhanced caregiver training (enhanced‐CT) protocol to an education condition with respect to caregiver self‐efficacy in cancer symptom management and stress management and preparedness. | 138 caregivers (83% female; mean age = 55, SD = 13) | Cancer | Spouse (67%), child (15%), parent (10%) | RCT | Placebo | Caregiver self‐efficacy for managing patient symptoms, caregiver stress, preparedness for caregiving | ↑caregiver self‐efficacy, ↓caregiver stress, ↑preparedness for caregiving (at post‐training, but not at 2‐ and 4‐wk post‐discharge) |
| Holm et al (2016)37; Holm et al (2017)38 | Sweden | To evaluate the short‐term and long‐term effects of a psychoeducational group intervention designed to increase preparedness for family caregiving in specialised palliative home care. | 194 caregivers (66% female; exp: mean age = 63, SD = 13; con: mean age = 60, SD = 14) | 90% cancer | Spouse (48%), adult child (35%) | RCT | TAU | Preparedness for caregiving, competence for caregiving, rewards for caregiving, caregiver burden, health, anxiety, and depression | ↑preparedness for caregiving upon completion of intervention and at 2‐mo follow‐up, ↑competence for caregiving upon completion of intervention; caregivers who did not benefit: ↑preparedness and competence for caregiving and their health at baseline, ↓environmental burden at baseline |
| Hudson et al (2005)41 | Australia | To examine the effectiveness of a psychoeducational intervention to enhance the support and guidance offered to primary family caregivers of patients receiving home‐based palliative care for advanced cancer. | 106 caregivers (65% female; mean age = 61, SD = 14) | Advanced cancer | Spouse (67%), child (16%), parent (8%) | RCT | TAU | Caregiver preparedness, mastery, self‐efficacy, competence, rewards, anxiety | ↑caregiver rewards |
| Hudson et al (2013)39; Hudson et al (2015)40 | Australia | To test the effects of a psychoeducational intervention on caregiver distress, unmet needs, preparedness, competence, and emotions. | 298 caregivers (71% female; mean age = 59, SD = 14) | Advanced cancer | Not reported | RCT | TAU | Caregiver psychological distress, unmet needs, preparedness, competence, positive emotions | ↑caregiver preparedness and ↑competence (for those receiving two home visits) between baseline and 1‐wk post‐intervention, and ↓caregiver distress (for those receiving one home visit) between 1‐wk post‐intervention and 8‐wk post‐death |
| Kurtz et al (2005)42; Given et al (2006)34 | USA | To investigate whether a clinical nursing intervention focusing on teaching family caregivers and their cancer patients skills to better manage the patients' symptoms would reduce caregiver depressive symptomatology. | 237 caregivers (54% female; mean age = 55, SD = 14) | Cancer | Not reported | RCT | TAU | Depressive symptoms, reactions to assisting with chemotherapy symptoms, involvement in symptom management | ↓negative reactions to assisting with symptoms |
| Laudenslager et al (2015)43; Simoneau et al (2017)55 | USA | To test the effects of a psychosocial intervention (psychoeducation, paced respiration, and relaxation [PEPRR]) on the distress of caregivers of allogeneic haematopoietic stem cell transplant (allo‐HSCT) patients. | 148 caregivers (76% female; mean age = 54, CI = 52‐56) | Cancer, receiving allo‐HSCT | Spouse/partner (70%), parent (18%) | RCT | TAU | Caregiver distress, salivary cortisol awakening response | ↓caregiver distress |
| Lee et al (2016)44 | Taiwan | To test the ability of an integrative intervention programme for caregivers of advanced cancer patients to lower caregiving burden as death approaches. | 81 caregivers (76% female; exp: mean age = 51, SD = 16; con: mean age = 50, SD = 14) | Advanced cancer | Spouse (58%), child (27%) | Repeated measures, two‐group comparative design | TAU | Subjective and objective burden | ↓subjective burden, ↑caregiver self‐efficacy and objective burden |
| Leow et al (2015)45 | Singapore | To evaluate the effectiveness of a psychoeducational intervention, the caring for the caregiver programme. | 80 caregivers (68%; mean age = 47, SD = 12) | Advanced cancer (stage IV) | Child (58%), spouse (25%), sibling (4%), parent (3%), niece (1%), daughter‐in‐law (9%), grandchild (1%) | RCT | TAU | QOL, social support, stress, depression, self‐efficacy in self‐care, closeness with the patient, rewards of caregiving, knowledge | ↑QOL, ↑social support, ↓stress, ↓depression, ↑self‐efficacy in self‐care, ↑closeness with the patient, ↑rewards of caregiving, ↑knowledge |
| Manne et al (2004)47 | USA | To investigate the effects of a psychoeducational group intervention on the distress, coping, personal growth, and marital communication of wives of men diagnosed with prostate cancer. | 60 caregivers (100% female; mean age = 60, SD = 9) | Prostate cancer | Spouse/partner (100%) | RCT | TAU | Caregiver distress (general and cancer‐specific), coping, post‐traumatic growth, cancer‐specific marital interactions | ↑post‐traumatic growth |
| Mahendran et al (2017)46 | Singapore | To evaluate the pilot COPE (Caregivers of cancer Outpatients' Psycho‐Education support group therapy) intervention. | 97 caregivers (65% female; 52% aged 41‐60) | Cancer | Spouse (35%), child (33%) | Quasi‐experimental design without randomisation | Wait list | Depressive symptoms, anxiety, QOL | No significant effects |
| McMillan et al (2006)49; McMillan et al (2007)48 | USA | To determine whether hospice plus a coping skill training intervention improved family caregivers' quality of life, burden, coping, and mastery, compared with hospice plus emotional support, and usual hospice care. | 329 caregivers (85% female; exp: mean age = 63, SD = 14; con[TAU]: mean age = 60, SD = 15; con[support]: mean age = 62, SD = 15) | Advanced cancer | Not reported | RCT | Two comparison groups: TAU and placebo | Caregiver quality of life, caregiver burden due to patient symptoms, caregiver burden due to tasks, caregiver mastery | ↑caregiver quality of life, ↓caregiver burden due to patient symptoms, ↓caregiver burden due to tasks |
| Mitchell et al (2013)50 | Australia | To assess the efficacy of a general practitioner (GP)–based intervention incorporating a carer‐reported needs checklist and a supporting GP toolkit of resources, reduces the reported number and intensity of unmet carer needs. | 392 caregivers (67% female; exp: mean age = 57, SD = 13; con: mean age = 58, SD = 13) | Advanced cancer (locally invasive or metastatic disease) | Spouse/partner (68%), parent (9%), adult child (15%), sibling (2%) | RCT | TAU | Unmet needs, anxiety, depression, quality of life | ↑mental well‐being (at 6 mo, for those with baseline clinical anxiety), ↓anxiety (at 6 mo, for those with baseline clinical depression), ↑physical well‐being (at 1 mo, for those not anxious) |
| Pailler et al (2015)52 | USA | To assess the feasibility, acceptability, and efficacy of a supportive group‐based intervention for family caregivers. | 69 caregivers (57% female; mean age = 55, SD = 14) | Leukaemia | Spouse (64%), significant other (10%), parent (13%), other family (13%) | Pre‐post sequential cohort design | TAU | Distress (caregiver and patient), caregiver quality of life, satisfaction (with intervention) | ↓caregiver distress, ↑caregiver quality of life |
| Rexilius et al (2002)53 | USA | To examine the effect of massage therapy and healing touch on anxiety, depression, subjective caregiver burden, and fatigue experienced by caregivers of patients undergoing autologous haematopoietic stem cell transplant. | 36 caregivers (72% female; mean age = 52 y) | People undergoing autologous haematopoietic stem cell transplant | Spouse (69%), sister (17%), mother (11%), fiancé (3%) | Pre‐post design with randomisation of groups (not individuals) | Placebo | Anxiety, depression, subjective burden, fatigue | Massage therapy: ↓anxiety, ↓depression, ↓fatigue; healing touch: no effects |
| Shaw et al (2016)54 | Australia | To investigate the effectiveness of a structured telephone intervention for caregivers of people diagnosed with poor prognosis gastrointestinal cancer on psychosocial outcomes for both caregivers and patients. | 128 caregivers (39% female; exp: mean age = 60, SD = 14; con: mean age = 64, SD = 14) | Gastrointestinal cancer | Spouse/partner (70%), child (23%), parent (2%), sibling (2%), other family member (1%), friend (2%) | RCT | TAU | Caregivers' quality of life (QOL), caregiver burden, unmet supportive care needs, and distress. Patients' QOL, unmet supportive care needs, distress, and health service utilisation | 3 mo post hospital discharge: caregivers, ↑social support, ↓worry about finances; patients, ↓emergency department presentations, ↓unplanned hospital readmissions |
| Sun et al (2015)56 | USA | To test the effectiveness of an interdisciplinary palliative care intervention for family caregivers of patients diagnosed with stage I through IV non–small cell lung cancer. | 366 caregivers (62% female; exp: mean age = 58, SD = 14; con: mean age = 57, SD = 13) | Non–small cell lung cancer | Not reported | Prospective, sequential, quasi‐experimental design | TAU | Caregiver burden, caregiving skills preparedness, psychological distress, quality of life | ↓caregiver burden, ↓psychological distress, ↑social well‐being |
| Toseland et al (1995)57; Blanchard et al (1996)29 | USA | To assess the impact of a short‐term individual counselling programme for cancer caregivers. | 78 caregivers (50% male; exp: mean age = 56; con: mean age = 51) | Cancer | Spouse (100%) | RCT | TAU | Perceived health status, psychological well‐being (depressed mood, anxiety, burden, stress), social support, coping behaviour | 2 wk: no effects; 6 mo: ↓depressed mood |
| Tsianakas et al (2015)58 | England | To test the feasibility and acceptability of a complex intervention for carers of patients starting chemotherapy. | 43 caregivers (65% female; mean age = 53, range = 24‐76) | Breast, lung, or colorectal cancer | Spouse/partner (44%), child (26%), parent (5%), friend (7%), other relative (18%) | RCT and focus groups | TAU | Knowledge of chemotherapy and its side effects, experience of care, satisfaction with outpatient services, coping, emotional well‐being | ↑understanding of symptoms and side effects, ↑frequency of information needs being met |
| Walsh et al (2007)59 | England | To evaluate the effectiveness of increased support for distressed, informal carers of patients receiving palliative care. | 271 caregivers (79%; mean age = 56, SD = 14) | Cancer | Spouse/partner (64%), child (25%), other (12%) | RCT | TAU | Caregiver distress, strain, quality of life, satisfaction with care, bereavement outcome | No significant effects |
Abbreviations: RCT, randomised controlled trial; TAU, treatment as usual; QOL, quality of life.
3.1. Study characteristics
An overview of study characteristics is presented in Table 2. Intervention characteristics and implementation outcomes tables are attached as supporting information (Table S1).
3.1.1. Country of origin
Almost half of the studies (n = 12, 46%) were conducted in the United States.29, 31, 32, 34, 35, 36, 42, 43, 47, 48, 49, 51, 52, 53, 55, 56, 57 Australia was the second largest contributor of studies (n = 4, 15%).39, 40, 41, 50, 54
3.1.2. Participant characteristics
The median number of participants included in the studies was 113 (IQR = 68 to 226). The majority of participants were female in 22 of 26 studies (median = 67%, IQR = 63% to 76%). On average, two‐thirds of the caregivers were the patients' spouses/partners (median = 66%, IQR = 57% to 70%). In most studies (24 of 26), all patients had been diagnosed with cancer; of the remaining two studies33, 37, 38 83% and 90% of patients had cancer (the remaining patients had other chronic conditions).
3.1.3. Study design
Three quarters of studies (n = 20, 77%) were randomised controlled trials. The comparison condition in three quarters of studies (n = 19, 73%) was TAU, with placebo controls used in a further 19% (n = 5) of studies.31, 35, 36, 48, 49, 53
3.1.4. Intervention design
Two‐thirds of interventions were delivered face‐to‐face to individual caregivers (n = 18, 69%), with 27% (n = 7) delivered face‐to‐face to groups30, 33, 37, 38, 46, 47, 52, 58 and 4% (n = 1) requiring caregivers to access the intervention independently through the internet.32, 51 In addition, a quarter of interventions incorporated supplementary material, such as handouts and DVDs (n = 6, 23%).35, 36, 39, 41, 43, 52, 55, 58
Half the interventions included information provision (n = 14, 54%). Content also included skills development (n = 8, 31%),27, 28, 35, 36, 41, 43, 44, 45, 46, 48, 49, 55 social support (n = 6, 23%),32, 37, 38, 41, 45, 51, 52, 59 individual and group therapy (n = 5, 19%),29, 30, 31, 33, 46, 57 and self‐care (n = 4, 15%).31, 41, 43, 45, 53, 55 These percentages exceed 100% because of many interventions having multiple types of content.
The settings of two‐thirds of the interventions were health services (n = 18, 69%). Interventions also took place via telephone (n = 8, 31%),27, 34, 39, 40, 41, 42, 44, 45, 54, 59 in caregivers' homes (n = 5, 19%),32, 39, 40, 41, 45, 48, 49, 51 and at places convenient for caregivers (n = 2, 8%).31, 59 These percentages exceed 100% because of some interventions being delivered in multiple settings.
Staff most commonly delivering the interventions were nurses (n = 13, 50%), social workers (n = 5, 19%),37, 38, 43, 47, 55, 56, 57 and psychologists (n = 4, 15%).30, 46, 47, 54
Theoretical frameworks underpinned the interventions in under half of the studies (n = 12, 46%). Bandura's conceptualisation of self‐efficacy was the most commonly used theory (n = 5, 19%).34, 35, 36, 42, 44, 45
Interventions had been previously piloted in a third of studies (n = 8, 31%).33, 36, 39, 40, 41, 43, 44, 45, 46, 55, 59 In two studies (8%), aspects of the intervention had been piloted.35, 50 In a further two studies (8%), the investigations were pilot studies.45, 46 For the remaining studies, we found that pilots had not been conducted (n = 4, 15%) or were not reported (n = 10, 38%).
3.2. Implementation outcomes
The implementation outcomes across studies are presented in Table S2 (acceptability, adoption, and appropriateness), Table S3 (feasibility), and Table S4 (fidelity and costs). Findings are summarised below.
3.2.1. Acceptability
In almost half the studies (n = 12, 46%), there was no evidence to indicate the acceptability of interventions from caregivers' perspectives. For 11 studies (42%), acceptability data were reported, which were collected via surveys (n = 5),30, 36, 52, 53, 59 focus groups (n = 2),37, 38, 58 interviews (n = 2),46, 54 and engagement with the intervention or debriefing (n = 2).31, 43, 55 In one further study, acceptability data were collected on certain aspects of an intervention (ie, feedback was gathered on some aspects of the intervention but not others).35 For the two remaining studies, acceptability data may have been collected, but insufficient information was provided to enable firm judgements to be made.45, 50
Most studies (n = 21, 81%) did not report on the acceptability of interventions from the perspectives of other stakeholders (ie, stakeholders other than caregivers). These data were available for three studies and were collected via focus groups (n = 2)37, 38, 58 and surveys (n = 1).30 Stakeholder acceptability data were collected on some components of an intervention in one further study50 and may have been obtained in another study.45
Caregivers appeared to have limited input into intervention development. In most studies (n = 17, 65%), there was no evidence of caregiver involvement in the development of interventions. Caregivers were directly involved in the development of the intervention in one study (4%),58 and in eight studies (31%), caregivers were involved in separate studies, such as focus groups, that informed the interventions.39, 40, 41, 43, 45, 46, 50, 54, 55, 59
3.2.2. Adoption
No studies reported on intentions, agreement, or action to implement interventions into practice. However, two studies reported issues about the potential adoption of the interventions. In one, researchers reported that health care providers held reservations about possible implementation,28 and in the other paper, researchers noted that the intervention may not be sustainable in the longer term because of the costs involved in delivery.46
3.2.3. Appropriateness
In all studies, interventions were considered a good fit, with solid arguments presented as to why the interventions were appropriate for the cancer caregivers.
Few interventions were targeted towards specific population groups who may have high support needs or may benefit from interventions, such as caregivers experiencing high levels of distress or those from minority cultural backgrounds (n = 2, 8%).31, 59 One intervention targeted caregivers with high distress levels,59 and another one focused on caregivers who had experienced sleep difficulties for at least 1 month.31
3.2.4. Feasibility
Most caregivers screened were eligible for inclusion in the studies (median = 84%, IQR = 52% to 90%, data available from 65% of studies). On average, less than one‐third of eligible caregivers consented to participate (median = 28%, IQR = 17% to 55%, from 69% of studies). Most caregivers who consented to be involved commenced the interventions.
Most caregivers allocated to intervention conditions completed the interventions (median = 92%, IQR = 86% to 100%, from 65% of studies). On average, few caregivers withdrew (median = 6%, IQR = 0% to 13%, from 65% of studies). In only four studies were some caregivers unable to complete the interventions (because of circumstances such as the death of a patient) (non‐completion ranged from 3% to 23% across these studies).29, 32, 43, 54, 55, 57
The time commitment necessary for caregivers to complete interventions ranged from 79 minutes58 to 22 hours33 (median = 180 min, IQR = 120 to 360 min, from 65% of studies). Six studies had interventions that took 6 hours or more to deliver.29, 30, 33, 37, 38, 43, 47, 55, 57
3.2.5. Fidelity
In the majority of studies, interventions appeared to be conducted as intended (n = 19, 62%). In one study, researchers reported that caregivers did not engage with one aspect of the intervention (an online forum).45 For the remaining studies, no information about intervention fidelity was reported (n = 9, 35%).
On average, caregivers completed all aspects of the interventions, such as attending all sessions provided (median = 100%, IQR = 84% to 100%, from 54% of studies).
No changes to interventions during the studies were reported, and no changes to the dose, delivery, or strategies during the studies were reported.
3.2.6. Costs
The time commitment data were available for n = 19 (62%) of studies. Time required of staff ranged from 79 minutes58 to 22 hours33 (median = 180 min, IQR = 120 to 360 min).
The additional resources used in the interventions included written material (n = 7, 27%),35, 36, 41, 43, 48, 49, 50, 52, 55, 56, 58 audio material (n = 2, 8%),41, 52 DVDs (n = 2, 8%),45, 58 laptop computers with internet access for participants who required them (n = 1, 4%),32, 51 biofeedback devices (n = 1, 4%),43, 55 and home help aides (n = 1, 4%).48, 49 In over half of the studies (n = 15, 58%), no additional resources were reported.
For most studies (n = 22, 85%), aside from the occupations (and, in some cases, experience) of those who delivered the interventions, no information was provided on the training and expertise required to deliver the interventions. In two studies (8%), staff training was provided,33, 58 and in a further two studies (8%), the training and experience required was unspecified.27, 52
4. DISCUSSION
With recent calls for a need to focus on implementation of interventions,17, 60, 61 this review aimed to explore the implementation potential of cancer caregiver intervention studies. Although the reviewed studies focused on efficacy, there is a need to design, conduct, and report research that can be implemented into practice.24 The main finding from this review was that studies were not designed or reported in a way to maximise the potential for interventions to be successfully implemented. We also gained insights about the challenges of operationalising implementation outcomes from an established framework.
Results varied across the six implementation outcomes. These studies had limited evidence of acceptability, with few studies reporting on whether interventions were considered appropriate or involved consumers in the design of the interventions. There was little evidence for adoption. There was mixed support for interventions being appropriate: Although all interventions were reported to be a good fit through alignment with caregiver need and previous research, very few studies targeted groups specifically in need of support. There was limited support for feasibility, with data not reported for many studies, and low enrolment of caregivers in interventions. There was evidence for good fidelity of interventions. Costs were mostly reported in terms of staff time to deliver interventions and in some cases specified an investment required for staff time, training, or resources.
This review suggests that the reporting of cancer caregiver intervention studies requires improvement to support implementation into practice. There appears to be two key issues. Firstly, studies were not designed in ways that would maximise their potential to be successfully implemented. Secondly, in other instances, there is limited information reported relevant to implementation. Restrictions in reporting research in terms of journal requirements and required word counts may limit the opportunity to report evaluation data that includes outcomes of relevance to implementation.
There are other key findings from this review to highlight. The first is that consumer input into intervention development was notably low (acceptability outcome). In performing this review, we differentiated between studies that had active engagement with consumers as part of the project design, those studies that had developed interventions that were informed by the research team identifying a need, and those that had no consumer involvement. Consumer involvement into interventions is considered best practice,62 and it was surprising to find a paucity of studies utilising caregiver input. Future research should engage caregivers as team members and promote active roles in the development and refinement of caregiver interventions.
A further finding was the tendency for studies to recruit broadly rather than targeting groups more in need of support. This is in the context of consent rates that, while varied, had a median of less than a third of those approached across the studies, meaning that while many caregivers were eligible, this did not translate to enrolment. A recent systematic review and meta‐analysis exploring the efficacy of psychological interventions on anxiety in cancer patients found that low psychological distress at baseline was a key reason for low effectiveness, with authors advocating for screening and assessment of anxiety as an inclusion criterion before enrolment in psychological interventions.63 Caregivers not experiencing a problem may have low motivation to spend time in an intervention study they see as not relevant to their situation. Others have noted the need to increased research for vulnerable caregiving populations and risk stratification to target those most in need of support.17 Targeting groups in need of support is an important avenue for future caregiver intervention research.
This review also found that while most caregivers screened were eligible (feasibility outcome), this frequently was not well reported. Future studies should clearly report about the participants who were assessed for eligibility in accordance with CONSORT criteria and flow charts.64 There was also limited evidence available about intentions, agreements, or actions to implement interventions into practice (adoption outcome). There could be various reasons for this including that adoption is regarded as being outside the scope of conduct and reporting of studies, with adoption frequently reported at 6, 12, or 18 months after initial implementation. The lack of funding to test implementation processes has been acknowledged.65 Information about adoption agreements with service providers or potential would be a useful addition to papers reporting trials of interventions, even when the focus is on efficacy.
This review has operationalised Proctor's implementation outcomes framework. While there are other potential frameworks,19, 66 this framework was selected as the six domains resonated with the scope of the review. In practice, the operationalisation and data extraction allowed for key information to be assessed and findings support this framework as being appropriate for this review. Frameworks can be used to plan and design studies to strengthen the potential for implementation,24 and there may be potential to build on these results and use the Proctor framework in this context. This could strengthen the implementation potential of new studies. A recent literature review has outlined instruments to assess implementation outcomes, and addition of these measures could be considered in future trials.67 We did not include two implementation outcomes: penetration (the integration of an intervention within its setting) and sustainability (extent to which an intervention is maintained) given these are longer‐term outcomes.26 This review focused on cancer caregiver interventions, and issues of implementation potential may not be unique to this content.
4.1. Study limitations
This review has limitations to consider. Firstly, this review focused on implementation potential utilising a specific framework applied to the reporting of the original trial, but this may not mean that interventions were not implemented into practice. Studies may show limited implementation potential according this extracted data but may have been successfully implemented into practice. It appears that there are few published reports around implementation of cancer caregiver interventions; however, it was beyond the scope of our review to ascertain this. A further limitation is that it is important to acknowledge the diversity of cancer caregiving interventions in the literature. We screened abstracts broadly, and criteria focused on specific cancer caregiver interventions; for example, we omitted interventions directed at caregiver and patient dyads. This criterion was applied to ensure these interventions were specifically for caregivers. This review was conducted in the context of numerous caregiving reviews focusing on efficacy, and our aim was to complement these through exploring implementation potential.
4.2. Clinical and research implications
There are numerous implications for future research. Exploring any relationship between implementation outcomes and efficacy of interventions was outside the scope of this review, but this could be relevant for future research to inform optimal delivery on implementation outcomes. Exploring the potential of the implementation outcomes framework to plan and design studies may lead to stronger potential for implementation for cancer interventions. Additionally, given the findings of this review, the development and conduct of high‐quality cancer caregiver interventions that are able to be implemented into practice is essential.
5. CONCLUSIONS
Interventions must be cost‐effective and accessible; planning for implementation is important.24 Our findings suggest that the reporting of cancer caregiver interventions demonstrates limited capacity to be translated into practice. This is of significant concern as it may indicate limited public health or clinical benefit. This review has outlined the need for future caregiver studies to include caregivers in the design of interventions and focus resources and time commitments to those who need support. The demonstrated evidence for efficacy of caregiver interventions has limited relevance if interventions are not designed or conducted in a way to support implementation into practice. This review identifies the challenges involved in closing the evidence‐practice gap and contributes to the growing body of knowledge on which actions are required to ensure successful interventions actually reach targeted population groups.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Supporting Table 1: Overview of interventions.
Supporting Table 2: The acceptability, adoption, and appropriateness of interventions.
Supporting Table 3: The feasibility of interventions.
Supporting Table 4: The fidelity and costs of interventions.
ACKNOWLEDGEMENT
This work is funded by a Victorian Cancer Agency Early Career Seed Grant (Ugalde, ECSG14037).
Ugalde A, Gaskin CJ, Rankin NM, et al. A systematic review of cancer caregiver interventions: Appraising the potential for implementation of evidence into practice. Psycho‐Oncology. 2019;28:687–701. 10.1002/pon.5018
REFERENCES
- 1. Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: spouse caregivers. J Clin Oncol. 2007;25(30):4829‐4834. [DOI] [PubMed] [Google Scholar]
- 2. Grunfeld E, Coyle D, Whelan T, et al. Family caregiver burden: results of a longitudinal study of breast cancer patients and their principal caregivers. CMAJ. 2004;170(12):1795‐1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldstein NE, Concato J, Fried TR, Kasl SV. Factors associated with caregiver burden among caregivers of terminally ill patients with cancer. J Palliat Care. 2004;20:38. [PubMed] [Google Scholar]
- 4. Ugalde A, Krishnasamy M, Schofield P. Role recognition and changes to self‐identity in family caregivers of people with advanced cancer: a qualitative study. Support Care Cancer. 2012;20(6):1175‐1181. [DOI] [PubMed] [Google Scholar]
- 5. Carter PA. Caregivers' descriptions of sleep changes and depressive symptoms. Oncol Nurs Forum. 2002;29(9):1277‐1283. [DOI] [PubMed] [Google Scholar]
- 6. Carrera PM, Kantarjian HM, Blinder VS. The financial burden and distress of patients with cancer: understanding and stepping‐up action on the financial toxicity of cancer treatment. CA Cancer J Clin. 2018;68(2):153‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Ryn M, Sanders S, Kahn K, et al. Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology. 2011;20(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Applebaum AJ, Breitbart W. Care for the cancer caregiver: a systematic review. Palliat Support Care. 2013;11(03):231‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Candy B, Jones L, Williams R, et al. Interventions for supporting informal caregivers of patients in the terminal phase of a disease. Cochrane Database Syst Rev. 2009;15(6):CD007617. [DOI] [PubMed] [Google Scholar]
- 10. Chambers SK, Pinnock C, Lepore SJ, Hughes S, O'Connell DL. A systematic review of psychosocial interventions for men with prostate cancer and their partners. Patient Educ Couns. 2011;85(2):e75‐e88. [DOI] [PubMed] [Google Scholar]
- 11. Ferrell B, Wittenberg E. A review of family caregiving intervention trials in oncology. CA Cancer J Clin. 2017;67(4):318‐325. [DOI] [PubMed] [Google Scholar]
- 12. Harding R, Higginson IJ. What is the best way to help caregivers in cancer and palliative care? A systematic literature review of interventions and their effectiveness. Palliat Med. 2003;17(1):63‐74. [DOI] [PubMed] [Google Scholar]
- 13. Harding R, List S, Epiphaniou E, Jones H. How can informal caregivers in cancer and palliative care be supported? An updated systematic literature review of interventions and their effectiveness. Palliat Med. 2012;26(1):7‐22. [DOI] [PubMed] [Google Scholar]
- 14. Hudson PL, Remedios C, Thomas K. A systematic review of psychosocial interventions for family carers of palliative care patients. BMC Palliat Care. 2010;9(1):17‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta‐analysis of randomized trials. CA Cancer J Clin. 2010;60(5):317‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waldron EA, Janke EA, Bechtel CF, Ramirez M, Cohen A. A systematic review of psychosocial interventions to improve cancer caregiver quality of life. Psychooncology. 2013;22(6):1200‐1207. [DOI] [PubMed] [Google Scholar]
- 17. Kent EE, Rowland JH, Northouse L, et al. Caring for caregivers and patients: research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sales A, Smith J, Curran G, Kochevar L. Models, strategies, and tools: theory in implementing evidence‐based findings into health care practice. J Gen Intern Med. 2006;21:S43‐S49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci: IS. 2015;10(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rankin NM, Collett GK, Brown CM, et al. Implementation of a lung cancer multidisciplinary team standardised template for reporting to general practitioners: a mixed‐method study. BMJ Open. 2017;7(12):e018629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Scholl I, Hahlweg P, Lindig A, et al. Evaluation of a program for routine implementation of shared decision‐making in cancer care: study protocol of a stepped wedge cluster randomized trial. Implement Sci. 2018;13(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson LG, Armstrong A, Joyce CM, Teitelman AM, Buttenheim AM. Implementation strategies to improve cervical cancer prevention in sub‐Saharan Africa: a systematic review. Implemen Sci. 2018;13(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Selove R, Foster M, Mack R, Sanderson M, Hull PC. Using an implementation research framework to identify potential facilitators and barriers of an intervention to increase HPV vaccine uptake. J Public Health Manag Pract. 2017;23(3):e1‐e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Klesges LM, Estabrooks PA, Dzewaltowski DA, Bull SS, Glasgow RE. Beginning with the application in mind: designing and planning health behavior change interventions to enhance dissemination. Ann Behav Med. 2005;29(2):66‐75. [DOI] [PubMed] [Google Scholar]
- 25. Koorts H, Eakin E, Estabrooks P, Timperio A, Salmon J, Bauman A. Implementation and scale up of population physical activity interventions for clinical and community settings: the PRACTIS guide. Int J Behav Nutr Phys Act. 2018;15(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Proctor E, Silmere H, Raghavan R, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bahrami M, Farzi S. The effect of a supportive educational program based on COPE model on caring burden and quality of life in family caregivers of women with breast cancer. Iran J Nurs Midwifery Res. 2014;19(2):119‐126. [PMC free article] [PubMed] [Google Scholar]
- 28. Belgacem B, Auclair C, Fedor M‐C, et al. A caregiver educational program improves quality of life and burden for cancer patients and their caregivers: a randomised clinical trial. Eur J Oncol Nurs. 2013;17(6):870‐876. [DOI] [PubMed] [Google Scholar]
- 29. Blanchard CG, Toseland RW, McCallion P. The effects of a problem‐solving intervention with spouses of cancer patients. J Psychosoc Oncol. 1996;14(2):1‐21. [Google Scholar]
- 30. Bultz BD, Speca M, Brasher PM et al. A randomized controlled trial of a brief psychoeducational support group for partners of early stage breast cancer patients. Psycho‐Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 2000;9:303‐313. [DOI] [PubMed] [Google Scholar]
- 31. Carter PA. A brief behavioral sleep intervention for family caregivers of persons with cancer. Cancer Nurs. 2006;29(2):95‐103. [DOI] [PubMed] [Google Scholar]
- 32. DuBenske LL, Gustafson DH, Namkoong K, et al. CHESS improves cancer caregivers' burden and mood: results of an eHealth RCT. Health Psychol. 2014;33(10):1261‐1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fegg MJ, Brandstätter M, Kögler M, et al. Existential behavioural therapy for informal caregivers of palliative patients: a randomised controlled trial. Psychooncology. 2013;22(9):2079‐2086. [DOI] [PubMed] [Google Scholar]
- 34. Given B, Given CW, Sikorskii A, Jeon S, Sherwood P, Rahbar M. The impact of providing symptom management assistance on caregiver reaction: results of a randomized trial. J Pain Symptom Manage. 2006;32(5):433‐443. [DOI] [PubMed] [Google Scholar]
- 35. Hendrix CC, Bailey DE, Steinhauser KE, et al. Effects of enhanced caregiver training program on cancer caregiver's self‐efficacy, preparedness, and psychological well‐being. Support Care Cancer. 2016;24(1):327‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hendrix CC, Landerman R, Abernethy AP. Effects of an individualized caregiver training intervention on self‐efficacy of cancer caregivers. West J Nurs Res. 2013;35(5):590‐610. [DOI] [PubMed] [Google Scholar]
- 37. Holm M, Årestedt K, Carlander I, et al. Short‐term and long‐term effects of a psycho‐educational group intervention for family caregivers in palliative home care–results from a randomized control trial. Psychooncology. 2016;25(7):795‐802. [DOI] [PubMed] [Google Scholar]
- 38. Holm M, Årestedt K, Carlander I, Wengström Y, Öhlen J, Alvariza A. Characteristics of the family caregivers who did not benefit from a successful psychoeducational group intervention during palliative cancer care: a prospective correlational study. Cancer Nurs. 2017;40(1):76‐83. [DOI] [PubMed] [Google Scholar]
- 39. Hudson P, Trauer T, Kelly B, et al. Reducing the psychological distress of family caregivers of home‐based palliative care patients: short‐term effects from a randomised controlled trial. Psychooncology. 2013;22(9):1987‐1993. [DOI] [PubMed] [Google Scholar]
- 40. Hudson P, Trauer T, Kelly B, et al. Reducing the psychological distress of family caregivers of home based palliative care patients: longer term effects from a randomised controlled trial. Psychooncology. 2015;24(1):19‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hudson PL, Aranda S, Hayman‐White K. A psycho‐educational intervention for family caregivers of patients receiving palliative care: a randomized controlled trial. J Pain Symptom Manage. 2005;30(4):329‐341. [DOI] [PubMed] [Google Scholar]
- 42. Kurtz ME, Kurtz J, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30(2):112‐122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laudenslager ML, Simoneau TL, Kilbourn K, et al. A randomized control trial of a psychosocial intervention for caregivers of allogeneic hematopoietic stem cell transplant patients: effects on distress. Bone Marrow Transplant. 2015;50(8):1110‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lee K‐C, Yiin J‐J, Chao Y‐F. Effect of integrated caregiver support on caregiver burden for people taking care of people with cancer at the end of life: a cohort and quasi‐experimental clinical trial. Int J Nurs Stud. 2016;56:17‐26. [DOI] [PubMed] [Google Scholar]
- 45. Leow M, Chan S, Chan M. A pilot randomized, controlled trial of the effectiveness of a psychoeducational intervention on family caregivers of patients with advanced cancer. Oncol Nurs Forum. Oncology Nursing Society. 2015;42(2):E63‐E72. [DOI] [PubMed] [Google Scholar]
- 46. Mahendran R, Lim HA, Tan JY, et al. Evaluation of a brief pilot psychoeducational support group intervention for family caregivers of cancer patients: a quasi‐experimental mixed‐methods study. Health Qual Life Outcomes. 2017;15(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Manne S, Babb J, Pinover W et al. Psychoeducational group intervention for wives of men with prostate cancer. Psycho‐Oncology: Journal of the Psychological, Social and Behavioral Dimensions of Cancer 2004;13:37‐46. [DOI] [PubMed] [Google Scholar]
- 48. McMillan SC, Small BJ. Using the COPE intervention for family caregivers to improve symptoms of hospice homecare patients: a clinical trial. Oncol Nurs Forum. 2007;34(2):313‐321. [DOI] [PubMed] [Google Scholar]
- 49. McMillan SC, Small BJ, Weitzner M, et al. Impact of coping skills intervention with family caregivers of hospice patients with cancer: a randomized clinical trial. Cancer. 2006;106(1):214‐222. [DOI] [PubMed] [Google Scholar]
- 50. Mitchell GK, Girgis A, Jiwa M, Sibbritt D, Burridge LH, Senior HE. Providing general practice needs‐based care for carers of people with advanced cancer: a randomised controlled trial. Br J Gen Pract. 2013;63(615):e683‐e690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Namkoong K, DuBenske LL, Shaw BR, et al. Creating a bond between caregivers online: effect on caregivers' coping strategies. J Health Commun. 2012;17(2):125‐140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pailler ME, Johnson TM, Zevon MA, et al. Acceptability, feasibility, and efficacy of a supportive group intervention for caregivers of newly diagnosed leukemia patients. J Psychosoc Oncol. 2015;33(2):163‐177. [DOI] [PubMed] [Google Scholar]
- 53. Rexilius SJ, Mundt CA, Megel ME, Agrawal S. Therapeutic effects of massage therapy and healing touch on caregivers of patients undergoing autologous hematopoietic stem cell transplant. Oncol Nurs Forum. 2002;29(3):E35‐E44. [DOI] [PubMed] [Google Scholar]
- 54. Shaw JM, Young JM, Butow PN, et al. Improving psychosocial outcomes for caregivers of people with poor prognosis gastrointestinal cancers: a randomized controlled trial (family connect). Support Care Cancer. 2016;24(2):585‐595. [DOI] [PubMed] [Google Scholar]
- 55. Simoneau TL, Kilbourn K, Spradley J, Laudenslager ML. An evidence‐based stress management intervention for allogeneic hematopoietic stem cell transplant caregivers: development, feasibility and acceptability. Support Care Cancer. 2017;25(8):2515‐2523. [DOI] [PubMed] [Google Scholar]
- 56. Sun V, Grant M, Koczywas M, et al. Effectiveness of an interdisciplinary palliative care intervention for family caregivers in lung cancer. Cancer. 2015;121(20):3737‐3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Toseland RW, Blanchard CG, McCallion P. A problem solving intervention for caregivers of cancer patients. Soc Sci Med. 1995;40(4):517‐528. [DOI] [PubMed] [Google Scholar]
- 58. Tsianakas V, Robert G, Richardson A, et al. Enhancing the experience of carers in the chemotherapy outpatient setting: an exploratory randomised controlled trial to test impact, acceptability and feasibility of a complex intervention co‐designed by carers and staff. Support Care Cancer. 2015;23(10):3069‐3080. [DOI] [PubMed] [Google Scholar]
- 59. Walsh K, Jones L, Tookman A, et al. Reducing emotional distress in people caring for patients receiving specialist palliative care: randomised trial. Br J Psychiatry. 2007;190(02):142‐147. [DOI] [PubMed] [Google Scholar]
- 60. Longacre ML, Applebaum AJ, Buzaglo JS, et al. Reducing informal caregiver burden in cancer: evidence‐based programs in practice. Transl Behav Med. 2018;8(2):145‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rodin G. From evidence to implementation: the global challenge for psychosocial oncology. Psychooncology. 2018;27(10):2310‐2316. [DOI] [PubMed] [Google Scholar]
- 62. Goodare H, Lockwood S. Involving patients in clinical research: improves the quality of research. BMJ. 1999;319:724‐725. 10.1136/bmj.319.7212.724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanjida S, McPhail SM, Shaw J, et al. Are psychological interventions effective on anxiety in cancer patients? A systematic review and meta‐analyses. Psychooncology. 2018;27(9):2063‐2076. [DOI] [PubMed] [Google Scholar]
- 64. Moher D, Schulz KF, Altman DG, Group C . The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. In Elsevier 2001. [PubMed]
- 65. Kerner JF, Guirguis‐Blake J, Hennessy KD, et al. Translating research into improved outcomes in comprehensive cancer control. Cancer Causes Control. 2005;16(S1):27‐40. [DOI] [PubMed] [Google Scholar]
- 66. Tabak RG, Khoong EC, Chambers D, Brownson RC. Models in dissemination and implementation research: useful tools in public health services and systems research. Frontiers in Public Health Services and Syst Res 2013;2:8. [Google Scholar]
- 67. Lewis CC, Fischer S, Weiner BJ, Stanick C, Kim M, Martinez RG. Outcomes for implementation science: an enhanced systematic review of instruments using evidence‐based rating criteria. Implement Sci. 2015;10(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Table 1: Overview of interventions.
Supporting Table 2: The acceptability, adoption, and appropriateness of interventions.
Supporting Table 3: The feasibility of interventions.
Supporting Table 4: The fidelity and costs of interventions.


