Abstract
Rationale & Objective:
While socioeconomic status has been associated with chronic kidney disease (CKD), little is known about its relationship to residential neighborhood context.
Study Design:
Secondary analysis of the Multi-Ethnic Study of Atherosclerosis (MESA), a prospective cohort study designed to investigate the development and progression of subclinical cardiovascular disease.
Setting & Participants:
6814 men and women who were between 45 and 84 years of age and free of CVD were recruited between 2000–2002 from: Baltimore, MD; Chicago, IL; Forsyth County, NC; Los Angeles, CA; New York, NY; and St. Paul, MN.
Exposures:
A composite neighborhood problem score (calculated based on seven participant-reported domains at study entry: adequacy of food sources; availability of parks/playground; noise; sidewalks; traffic; trash and litter; and violence) and a social cohesion score (calculated based on five participant-reported attributes of people in their neighborhood: close knit; get along; willing to help neighbors; trustworthy; and share values).
Outcomes:
eGFR (calculated using the CKD-EPI creatinine–cystatin C equation) and an indicator of eGFR decline >30% since study entry using follow-up eGFR quantified at four exams: 2000–2002, 2004–2005, 2005–2007, and 2010–2011.
Analytical Approach:
The associations between each neighborhood measure (in separate models) and eGFR decline >30% from baseline and annualized eGFR change were estimated using Cox proportional hazards and linear mixed regression models, respectively, adjusting for potential confounders.
Results:
While neighborhood social context differs by race/ethnicity, neither neighborhood problems nor social cohesion were independently associated with eGFR decline after adjustment for confounders.
Limitations:
Incomplete capture the early stages of eGFR decline, reliance on observational data, limited variation in the neighborhood measures, and the potential for residual confounding.
Conclusions:
While we showed no independent association between neighborhood context and eGFR decline, it is associated with many CKD risk factors and further work is needed to clarify if it has an independent role in CKD.
Keywords: chronic kidney diseases, neighborhood, socioeconomic status (SES), social cohesion, neighborhood problems, estimated glomerular filtration rate (eGFR), eGFR decline, kidney function, modifiable risk factor, health disparities, residential setting
INTRODUCTION
A growing literature indicates the importance of residential neighborhood context for disease risk,1 including the incidence and prevalence of many common risk factors for chronic kidney disease (CKD). Lower neighborhood socioeconomic status (SES) is associated with higher rates of hypertension, diabetes, and obesity.2–4 Furthermore, residence in neighborhoods where persons report access to healthy food2,5 and spaces for physical activity2 is associated with lower risk of incident hypertension and diabetes, compared to neighborhoods where these are less available. Yet, there is little known about the importance of neighborhood factors for CKD risk. While evidence suggests that neighborhood poverty is related to incidence of end stage renal disease (ESRD),6 it is not clear whether neighborhood context, most often captured by neighborhood SES, is related to the progression of earlier stages of CKD.7–12
It is possible that aspects of neighborhood context important at earlier stages of CKD are not captured by neighborhood SES indicators.7–12 Residential neighborhoods serve as a source of economic and material resources, but they also serve as a source of social resources, constraints, and stressors.1,13–15 Two markers of the neighborhood social environment that have been of particular interest are neighborhood problems and neighborhood social cohesion. Neighborhood problems are conceptualized as physical features such as litter, graffiti, and noise that represent an underlying social disorder.16,17 These problems, and the underlying social features they represent, may serve as a source of chronic stress that may result in a cascade of risks for health including poor sleep quality, poor nutritional diet, smoking, and heavy alcohol use.16 They may also represent barriers to engaging in behaviors such as regular outdoor physical activity, if the residents perceive these problems as markers of safety.18
Neighborhood social cohesion is conceptualized as the social ties among neighbors based on trust and norms of reciprocity.19,20 The close ties among neighbors represented by social cohesion may facilitate the dissemination of health-related information or reinforce healthy norms such as regular physical activity, nutritious diet, and preventive health care visits,21 and may also discourage unhealthy behaviors such as heavy alcohol use or smoking.18 However, depending on the sociodemographic composition of the neighborhood and other aspects of the socio-behavioral context (e.g., physical inactivity prevalence and acceptability), social cohesion may reinforce unhealthy norms or serve as source of stress if mutual obligations exceed the social or economic capacity of the residents.22
In this study, we use data from the Multi-Ethnic Study of Atherosclerosis to estimate the association between self-reported neighborhood problems and social cohesion and kidney function decline among persons without established cardiovascular disease (CVD). We hypothesize that those who live in neighborhoods with greater problems will show a greater decline in eGFR over the study period compared to those who live in neighborhoods with fewer problems. On the other hand, we hypothesize that those who live in neighborhoods with greater social cohesion will show a lesser decline in eGFR compared to their counterparts in neighborhoods with lower social cohesion. Clarifying the features of the neighborhood beyond SES may provide important information for both public health practitioners and clinicians as these sources of stress and resilience that may influence CKD-related health behaviors.
METHODS
Setting and Participants
We used data from the Multi-Ethnic Study of Atherosclerosis (MESA), a multiethnic prospective cohort study funded by the National Heart, Lung, and Blood Institute, designed to investigate the development and progression of subclinical cardiovascular disease (CVD). Detailed information on the recruitment and study design have been discussed elsewhere.23 Briefly, 6814 men and women who were between 45 and 84 years of age, free of CVD, and self-identified as White, Black, Hispanic, or Chinese were recruited between 2000–2002 from the following areas: Baltimore City and Baltimore County, MD; Chicago, IL; Forsyth County, NC; Los Angeles County, CA; Northern Manhattan and the Bronx, NY; and St. Paul, MN. There have been four follow up visits to date, 2002–2004 (Exam 2), 2004–2005 (Exam 3), 2005–2007 (Exam 4), and 2010–2011 (exam 5). Serum creatinine and cystatin C were collected at Exams 1, 3, 4, and 5. The institutional review boards at all participating centers approved the study, and all participants gave informed consent.
MESA participants were excluded from analysis if they were missing information on: (1) serum creatinine and cystatin C at baseline (n=65) or at follow up exams (n=218); or (2) either the neighborhood problems or social cohesion scores (n=639), yielding a final analytic sample size of 5892.
Primary Predictors
Neighborhood measures were collected by self-report of participants at baseline. Neighborhood problems were assessed by asking participants to evaluate the seriousness of specific problems using a four-point Likert-like scale in which greater values represented greater reported seriousness. The problems assessed were: (a) lack of adequate food shopping in neighborhood; (b) lack of parks/playground in neighborhood; (c) excessive noise in neighborhood; (d) poor sidewalks in neighborhood; (e) heavy traffic or speeding cars in neighborhood; (f) trash and litter problem in neighborhood; and (g) violence problem in neighborhood. A composite neighborhood problem score was calculated as the mean of the seven items with a greater score indicating greater reported seriousness of the problems. This measure has been validated and previously used.18,24–26 Neighborhood social cohesion was assessed by asking participants to evaluate their neighborhood on five characteristics using a five-point Likert scale in which greater values represent greater agreement with the statements. The characteristics were: (a) close knit neighborhood; (b) people in neighborhood don’t get along; (c) people willing to help their neighbors; (d) people in neighborhood can be trusted; and (e) people in neighborhood do not share the same values. A composite neighborhood social cohesion score was calculated, with items (b) and (e) reverse coded, as the mean of the five items, with greater values representing greater agreement that there is cohesion.2,5,18 This measure has been validated and previously used.18,24–26
Outcome
Kidney function was assessed by estimated glomerular filtration rate (eGFR) using the CKD-EPI creatinine–cystatin C equation.27 Serum assays were performed using frozen serum specimens that were stored at −70°C. Serum creatinine was measured by rate reflectance spectrophotometry using thin film adaptation of the creatine amidinohydrolase method on the Vitros analyzer (Johnson & Johnson Clinical Diagnostics, Inc., Rochester, NY) at the Collaborative Studies Clinical Laboratory at Fairview-University Medical Center (Minneapolis) and calibrated to the Cleveland Clinic. Cystatin C was measured by means of a particle-enhanced immunonephelometric assay (N Latex Cystatin C, Dade Behring) with a nephelometer (BNII, Dade Behring) and corrected for assay drift.
Our outcome of interest was eGFR decline, assessed using repeat assessments of eGFR in two ways. First, we created a dichotomous variable that indicated the presence of a decline in eGFR >30% from baseline to any of the follow up exams. Second, we estimated an annualized change in eGFR.
Analytic Approach
We examined baseline characteristics of participants across quartiles of neighborhood problems and social cohesion scores. We also examined the unadjusted cross-sectional associations between each neighborhood score and eGFR<60ml/min/1.73m2 at baseline and albuminuria, defined as urine albumin-creatinine ratio (UACR)>30mg/g, at baseline using logistic regression. To evaluate the association between neighborhood measures and a >30% decline in eGFR, the dichotomous outcome variable was modeled using Cox proportional hazards regression. To evaluate the association between neighborhood measures at baseline and annualized eGFR decline over time, eGFR from exams 1, 3, 4, and 5 were modeled using linear mixed models with random intercepts. This approach took into account the correlation of observations by participant.
We employed a nested model approach to the inclusion of covariates. Model 1 was unadjusted. Model 2 then included individual-level sociodemographic factors (age, gender, race, family income, and education) and MESA site as potential confounders. Finally, Model 3 additionally included potential mediators that may link neighborhood context to eGFR decline (diabetes, hypertension, smoking status, body mass index, low-density lipoproteins, high-density lipoproteins, C-reactive protein, and UACR). Neighborhood measures were evaluated as continuous and categorical (quartiles) variables. When modeled as categorical variables, the omitted category was the least “problematic” context (i.e., lowest problems and highest cohesion). To examine the possibility that the association between neighborhood measures and eGFR varied by baseline eGFR (< versus ≥60ml/min/1.73m2), we modeled the interaction between neighborhood measures and baseline eGFR. Models were further estimated stratified by race/ethnicity as neighborhood racial composition in MESA diverges by participant race/ethnicity, meaning that participants of different race/ethnicities are likely drawn from different neighborhood samples, as had been shown in the Atherosclerosis Risk in Communities (ARIC) study.10 We also modeled formal interactions between race/ethnicity and each of the two neighborhood measures.
Estimates with two-sided p-values<0.05 were considered statistically significant. Analyses were performed using Stata (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP.t and IBM SPSS statistical software (Version 24.0.0.1, IBM Corp. Released 2016, Armonk, NY: IBM Corp.).
RESULTS
Cohort Characteristics
This multi-ethnic sample was composed of 40% White, 27% Black, 22% Hispanic, and 12% Chinese adults with a mean age of 62 years at baseline (Table 1). The mean eGFR at baseline was 91 ml/min/1.73m2 and 8% (n=475) had an eGFR<60ml/min/1.73m2 (Table 1). At baseline, the median UACR was 5.1 mg/g, and 9% (n=501) had albuminuria as defined by UACR>=30mg/g (Table 1). A total of 716 (12%) participants experienced a >30% decline in eGFR from baseline, and this was most common among Black (16%), compared with 8% of Chinese, 11% of White, and 12% of Hispanic participants (Table S1). Those included in the analyses (i.e., those without missing information on key measures) were different from those excluded in certain ways (Table S1). Those excluded had slightly lower mean baseline eGFR and higher mean baseline UACR than those included. Consistent with this pattern, those excluded had a higher hypertension prevalence, higher diabetes prevalence, higher mean age at baseline, and lower levels of education and family income. The median follow up time was 9.22 (IQR, 8.41–9.59) years. eGFR was assessed at all four possible exams for 65% of the analytic sample (Table S2). eGFR was assessed at three of the possible exams for an additional 26%; the remaining participants had eGFRs from two of the four possible exams.
Table 1.
Participant characteristics by neighborhood problems and neighborhood social cohesion scores, MESA 2000–2002
| Total | Neighborhood problems score | Neighborhood social cohesion score | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Q1 (≤1.32; Lowest problems) | Q2 (1.33–1.47) | Q3 (1.48–1.63) | Q4 (≥1.64; highest problems) | Q4 (≥3.70; Highest cohesion) | Q3 (3.53–3.69) | Q2 (3.43–3.52) | Q1 (≤3.42; Lowest cohesion) | ||
| No. of patients | 1468 | 1477 | 1481 | 1466 | 1456 | 1492 | 1472 | 1472 | |
| ΔeGFR, ml/min/1.73m2 | −1.55 (4.21) | −1.19 (4.18) | −1.58 (3.99) | −1.58 (4.41) | −1.86 (4.21) | −1.35 (3.97) | −1.60 (4.22) | 1.22 (4.33) | −2.04 (4.25) |
| 30% decline eGFR (%) | 12 | 11 | 12 | 13 | 13 | 12 | 12 | 11 | 14 |
| eGFR, ml/min/1.73m2 | 91 (18) | 91 (18) | 90 (18) | 91 (17) | 92 (18) | 90 (18) | 91 (18) | 91 (17) | 92 (18) |
| Serum creatinine, mg/dL | 0.83 (0.24) | 0.82 (0.21) | 0.86 (0.31) | 0.83 (0.21) | 0.82 (0.20) | 0.87 (0.30) | 0.84 (0.21) | 0.81 (0.20) | 0.82 (0.22) |
| Serum cystatin C, mg/L | 0.89 (0.22) | 0.87 (0.20) | 0.90 (0.27) | 0.89 (0.19) | 0.89 (0.20) | 0.89 (0.27) | 0.89 (0.20) | 0.88 (0.20) | 0.88 (0.20) |
| Race (%) | |||||||||
| White | 40 | 34 | 43 | 52 | 30 | 63 | 40 | 40 | 15 |
| Black | 27 | 13 | 32 | 27 | 36 | 31 | 26 | 17 | 35 |
| Hispanic | 22 | 21 | 18 | 16 | 31 | 4 | 22 | 26 | 34 |
| Chinese | 12 | 31 | 8 | 4 | 4 | 2 | 12 | 17 | 16 |
| Age, years | 62 (10) | 62 (10) | 62 (10) | 62 (10) | 61 (10) | 62 (10) | 62 (10) | 61 (10) | 62 (10) |
| Male (%) | 48 | 50 | 50 | 45 | 45 | 49 | 50 | 47 | 44 |
| Income (%) | |||||||||
| <$20K | 21 | 25 | 19 | 18 | 26 | 9 | 19 | 27 | 33 |
| $20K - $39,999 | 27 | 24 | 26 | 25 | 32 | 22 | 26 | 28 | 31 |
| $40K - $74,999 | 27 | 25 | 30 | 28 | 27 | 34 | 27 | 26 | 23 |
| ≥ $75K | 24 | 27 | 25 | 29 | 15 | 36 | 29 | 19 | 13 |
| Education (%) | |||||||||
| <High school | 16 | 18 | 13 | 12 | 22 | 6 | 14 | 20 | 25 |
| = High school | 47 | 44 | 48 | 45 | 50 | 46 | 46 | 47 | 48 |
| > High school | 37 | 38 | 39 | 43 | 28 | 48 | 40 | 33 | 24 |
| BMI, kg/m2 | 28 (5) | 27 (5) | 29 (5) | 28 (6) | 29 (6) | 29 (5) | 28 (6) | 28 (5) | 28 (6) |
| Diabetes prevalence (%) | 12 | 12 | 12 | 10 | 13 | 9 | 12 | 12 | 14 |
| Hypertension prevalence (%) | 44 | 44 | 44 | 42 | 45 | 44 | 45 | 39 | 46 |
| SBP, mmHg | 126 (21) | 127 (22) | 127 (21) | 124 (20) | 125 (20) | 127 (20) | 126 (21) | 123 (21) | 126 (21) |
| DBP, mmHg | 72 (10) | 72 (10) | 72 (10) | 71 (10) | 72 (10) | 72 (10) | 72 (10) | 71 (10) | 73 (10) |
| Smoking (%) | |||||||||
| Never | 51 | 60 | 49 | 46 | 47 | 48 | 51 | 49 | 55 |
| Former | 37 | 31 | 39 | 41 | 36 | 41 | 37 | 37 | 32 |
| Current | 13 | 9 | 12 | 13 | 17 | 12 | 11 | 14 | 14 |
| UACR, mg/g | 5.1 (3.3, 10.2) | 5.1 (3.3, 9.4) | 5.2 (3.1, 10.3) | 5.1 (3.3, 10.6) | 5.2 (3.3, 10.6) | 4.6 (3.0, 8.4) | 5.1 (3.1, 10.3) | 5.4 (3.4, 10.4) | 5.7 (3.5, 12.0) |
N=5892. Continuous data expressed at mean +/− standard deviation or median [interquartile range].
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; SBP, systolic blood pressure; UACR, urine albumin-creatinine ratio; MESA, ___.
Sociodemographic characteristics and neighborhood context
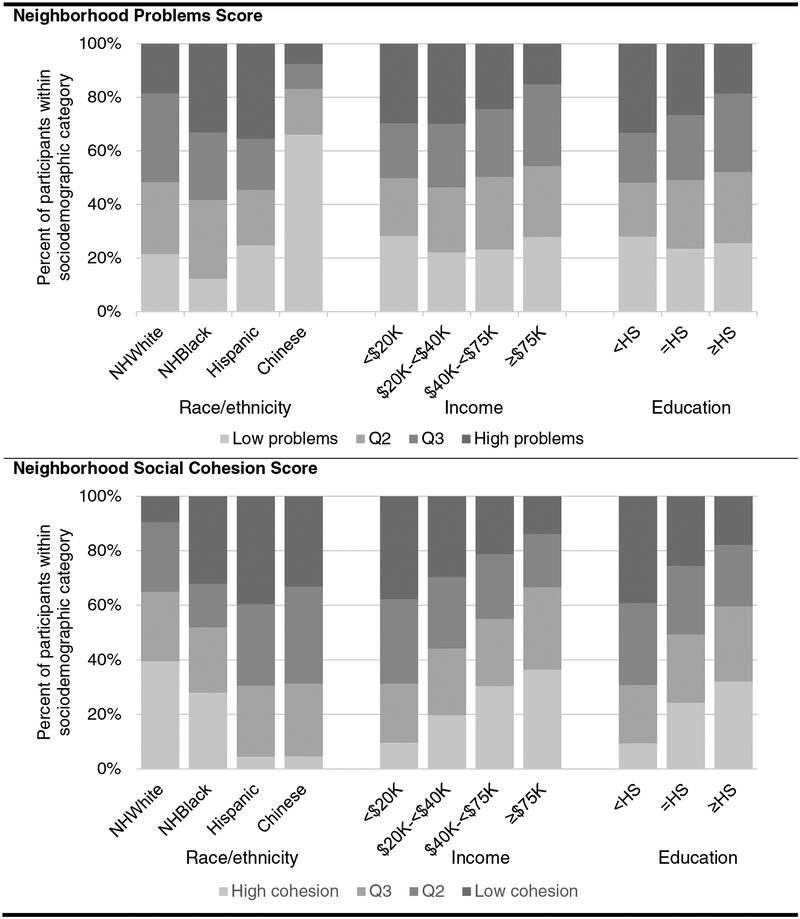
The neighborhood problems score could theoretically range from 1 (low problems) to 7 (high problems) and had a median of 1.47. The neighborhood social cohesion score could theoretically range from 1 (low cohesion) to 5 (high cohesion) and had a median of 3.52. The distribution of scores varied by race/ethnicity and SES characteristics. Specifically, Chinese participants were least likely to report residing in neighborhoods with high problem scores, compared with more than 1/3 of Hispanics and Blacks (Figure 1, top panel). White participants were most likely to report living in neighborhoods with high social cohesion (Figure 1, bottom panel). Regarding SES, participants with the highest levels of income and education also reported living in neighborhoods with low problem scores and high social cohesion (Figure 1, top panel).
Figure 1.
Proportion of participants in quartiles of neighborhoods problems and social cohesion scores by sociodemographic characteristics, Multi-Ethnic Study of Atherosclerosis 2000–2002 (n=5892)
Association between neighborhood context and low eGFR or albuminuria at baseline
There was no association between either of the neighborhood scores and eGFR<60ml/min/1.73m2 at baseline in the total sample or within any racial/ethnic group (Table S3). Furthermore, the associations between each of the neighborhood scores and albuminuria prevalence at baseline were not statistically significant after adjustment basic for sociodemographic characteristics (Table S4).
Association between neighborhood context and eGFR decline
Hazard of a decline in eGFR >30% from baseline varied only slightly by quartiles of each of the neighborhood scores in the total sample. For example, 11% of participants living in neighborhoods of the lowest quartile of problems experienced such an eGFR decline while 12%−13% of participants living in neighborhoods of the higher three quartiles experienced this decline (Table 2, top panel). On the other hand, 11%−12% of participants living in neighborhoods of the higher three quartiles of social cohesion experienced such an eGFR decline while 14% of their counterparts living in neighborhoods of the lowest quartile experienced this decline (Table 2, bottom panel).
Table 2.
Association of neighborhood problems and social cohesion scores with >30% decline in eGFR since baseline stratified by race/ethnicity, MESA 2000–2002
| N | % with decline | HR (95% CI) | |||
|---|---|---|---|---|---|
| Model 1c | Model 2d | Model 3e | |||
| Neighborhood Problems Scorea | |||||
| All | |||||
| Q1 | 1468 | 11 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 1477 | 12 | 1.11 (0.87, 1.38) | 0.94 (0.75, 1.19) | 0.93 (0.74, 1.17) |
| Q3 | 1481 | 13 | 1.18 (0.95, 1.47) | 1.02 (0.82, 1.29) | 0.97 (0.77, 1.22) |
| Q4 | 1466 | 13 | 1.21 (0.97, 1.51) | 1.03 (0.82, 1.30) | 0.98 (0.78, 1.24) |
| White | |||||
| Q1 | 504 | 10 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 628 | 11 | 1.09 (0.75, 1.58) | 1.12 (0.77, 1.63) | 1.07 (0.73, 1.56) |
| Q3 | 776 | 12 | 1.25 (0.88, 1.78) | 1.29 (0.91, 1.83) | 1.25 (0.87, 1.78) |
| Q4 | 434 | 10 | 0.88 (0.57, 1.35) | 0.99 (0.64, 1.53) | 0.99 (0.64, 1.54) |
| Black | |||||
| Q1 | 193 | 8 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 468 | 18 | 0.82 (0.53, 1.27) | 0.74 (0.48, 1.16) | 0.77 (0.49, 1.19) |
| Q3 | 400 | 14 | 0.95 (0.62, 1.48) | 0.85 (0.55, 1.32) | 0.81 (0.52, 1.27) |
| Q4 | 527 | 16 | 1.07 (0.71, 1.61) | 0.93 (0.61, 1.41) | 0.87 (0.57, 1.32) |
| Chinese | |||||
| Q1 | 457 | 7 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 118 | 10 | 1.38 (0.70, 2.73) | 1.32 (0.66, 2.65) | 1.21 (0.58, 2.53) |
| Q3 | 64 | 11 | 1.35 (0.57, 3.22) | 1.26 (0.52, 3.05) | 1.07 (0.43, 2.66) |
| Q4 | 53 | 4 | 0.53 (0.13, 2.20) | 0.39 (0.09, 1.65) | 0.39 (0.09, 1.65) |
| Hispanic | |||||
| Q1 | 341 | 13 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q2 | 263 | 11 | 0.82 (0.50, 1.35) | 0.80 (0.49, 1.31) | 0.80 (0.48, 1.33) |
| Q3 | 241 | 9 | 0.58 (0.33, 1.02) | 0.66 (0.38, 1.16) | 0.63 (0.35, 1.12) |
| Q4 | 452 | 12 | 0.91 (0.60, 1.38) | 1.04 (0.68, 1.59) | 1.08 (0.70, 1.66) |
| Neighborhood Social Cohesion Scoreb | |||||
| All | |||||
| Q4 | 1456 | 12 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q3 | 1492 | 12 | 0.99 (0.79, 1.23) | 0.98 (0.78, 1.22) | 0.94 (0.75, 1.17) |
| Q2 | 1472 | 11 | 0.89 (0.71, 1.11) | 0.96 (0.76, 1.21) | 0.92 (0.73, 1.16) |
| Q1 | 1472 | 14 | 1.14 (0.92, 1.41) | 1.07 (0.85, 1.35) | 1.01 (0.80, 1.27) |
| White | |||||
| Q4 | 923 | 11 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q3 | 597 | 11 | 1.06 (0.77, 1.45) | 1.01 (0.74, 1.39) | 0.99 (0.72, 1.36) |
| Q2 | 595 | 10 | 0.92 (0.66, 1.28) | 0.99 (0.71, 1.38) | 1.02 (0.73, 1.43) |
| Q1 | 227 | 13 | 1.22 (0.80, 1.86) | 1.14 (0.75, 1.74) | 1.18 (0.77, 1.81) |
| Black | |||||
| Q4 | 444 | 15 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q3 | 380 | 16 | 1.07 (0.73, 1.55) | 0.91 (0.63, 1.33) | 0.81 (0.56, 1.19) |
| Q2 | 253 | 17 | 1.10 (0.73, 1.65) | 0.95 (0.63, 1.43) | 0.79 (0.52, 1.20) |
| Q1 | 511 | 17 | 1.12 (0.80, 1.57) | 0.98 (0.69, 1.38) | 0.86 (0.61, 1.22) |
| Chinese | |||||
| Q4 | 32 | 3 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q3 | 184 | 7 | 2.21 (0.29, 16.97) | 1.95 (0.25, 15.03) | 1.93 (0.24, 15.38) |
| Q2 | 246 | 7 | 2.13 (0.28, 16.11) | 1.81 (0.23, 13.93) | 2.28 (0.29, 17.84) |
| Q1 | 230 | 11 | 3.70 (0.50, 27.34) | 2.65 (0.035, 19.92 | 2.75 (0.36, 21.19) |
| Hispanic | |||||
| Q4 | 57 | 12 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| Q3 | 331 | 12 | 0.88 (0.39, 1.98) | 0.92 (0.40, 2.07) | 1.00 (0.43, 2.32 |
| Q2 | 378 | 11 | 0.86 (0.39, 1.93) | 0.90 (0.40, 2.03) | 0.94 (0.41, 2.15) |
| Q1 | 504 | 12 | 0.92 (0.42, 2.02) | 0.95 (0.43, 2.12) | 1.01 (0.45, 2.30) |
N=5892.
The coefficients for the neighborhood problems score represent the risk of a >30% decrease in eGFR over the ten-year period comparing each quartile to quartile 1 (Q1). Quartiles of the neighborhood problems score are: Q1≤1.32, Q2=1.33–1.47, Q3=1.48–1.63, Q4≥1.64.
The coefficients for the neighborhood social cohesion score represents the risk of a >30% decrease in eGFR over the ten-year period comparing each quartile to Q1. Quartiles of the social cohesion score are: Q1≤3.42, Q2=3.43–3.52, Q3=3.53–3.69, Q4≥3.70.
Model 1 is unadjusted.
Model 2 includes adjustment for age, gender, race/ethnicity (in models with the total sample only), income, education, and MESA site.
Model 3 includes further adjustment for diabetes, hypertension, smoking, BMI, LDL, HDL, CRP, and UACR.
Abbreviations: CI, confidence interval; eGFR, ________; HR, hazard ratio; MESA, _____.
In unadjusted analyses, the hazard of an eGFR decline >30% from baseline was not statistically different among those living in neighborhoods with different levels of problems (HR for quartile 4 vs quartile 1 of 1.21 [95% CI, 0.97–1.51]; Table 2, top panel). However, those living in neighborhoods with greater problems show a greater annualized decline in eGFR compared to those living in neighborhoods with the lowest quartile of problems (beta of −0.17 [95% CI, −0.31 to −0.02] for quartile 2 vs quartile 1; Table 3, top panel). This association was attenuated slightly -- but to statistical non-significance -- after adjustment for sociodemographic characteristics. Social cohesion was not associated, either before or after adjustment for confounders, with either a hazard of a 30% eGFR decline from baseline or annualized eGFR change.
Table 3.
Association of neighborhood problems and social cohesion scores (quartiles) with annualized relative change in eGFR, stratified by race/ethnicity, MESA 2000–2002
| N | β (95% CI) | |||
|---|---|---|---|---|
| Model 1c | Model 2d | Model 3e | ||
| Neighborhood Problems Scorea | ||||
| ALL | ||||
| Q1 | 1468 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Q2 | 1477 | −0.17 (−0.31, −0.02) | −0.14 (−0.28, 0.01) | −0.10 (−0.24, 0.04) |
| Q3 | 1481 | −0.15 (−0.29, −0.01) | −0.13 (−0.27, 0.01) | −0.13 (−0.27, 0.01) |
| Q4 | 1466 | −0.07 (−0.22, 0.07) | −0.06 (−0.20, 0.08) | −0.04 (−0.17, 0.10) |
| White | ||||
| Q1 | 504 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Q2 | 628 | −0.03 (−0.18, 0.24) | 0.05 (−0.16, 0.25) | 0.08 (−0.12, 0.28) |
| Q3 | 776 | −0.05 (−0.25, 0.15) | −0.05 (−0.25, 0.14) | −0.04 (−0.24, 0.16) |
| Q4 | 434 | 0.20 (−0.03, 0.43) | 0.22 (−0.01, 0.45) | 0.27 (0.05, 0.50) |
| Black | ||||
| Q1 | 193 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Q2 | 468 | −0.34 (−0.69, 0.02) | −0.37 (−0.72, −0.01) | −0.35 (−0.70, −0.01) |
| Q3 | 400 | −0.33 (−0.70, 0.04) | −0.32 (−0.69, 0.04) | −0.33 (−0.69, 0.02) |
| Q4 | 527 | −0.29 (−0.64, 0.07) | −0.36 (−0.71, −0.01) | −0.33 (−0.67, 0.01) |
| Chinese | ||||
| Q1 | 457 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Q2 | 118 | −0.13 (−0.52, 0.27) | −0.15 (−0.54, 0.24) | −0.13 (−0.50, 0.24) |
| Q3 | 64 | −0.45 (−0.95, 0.04) | −0.46 (−0.94, 0.02) | −0.46 (−0.93, −0.002) |
| Q4 | 53 | −0.06 (−0.60, 0.47) | −0.07 (−0.60, 0.46) | −0.08 (−0.58, 0.43) |
| Hispanic | ||||
| Q1 | 314 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Q2 | 263 | 0.11 (−0.25, 0.47) | 0.14 (−0.20, 0.49) | 0.24 (−0.11, 0.58) |
| Q3 | 241 | 0.39 (0.03, 0.75) | 0.40 (0.05, 0.75) | 0.40 (0.05, 0.74) |
| Q4 | 452 | 0.38 (0.07, 0.68) | 0.41 90.11, 0.71) | 0.40 (0.11, 0.69) |
| Neighborhood Social Cohesion Scoreb | ||||
| ALL | −0.01 (−0.06, 0.04) | −0.01 (−0.06, 0.04) | −0.01 (−0.05, 0.04) | |
| Q1 | 1472 | −0.13 (−0.27, 0.02) | −0.12 (−0.24, 0.02) | −0.10 (−0.24, 0.03) |
| Q2 | 1472 | 0.09 (−0.06, 0.23) | 0.08 (−0.06, 0.22) | 0.09 (−0.05, 0.23) |
| Q3 | 1492 | −0.10 (−0.24, 0.05) | −0.11 (−0.25, 0.03) | −0.10 (−0.24, 0.04) |
| Q4 | 1456 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| White | ||||
| Q1 | 227 | 0.02 (−0.16, 0.21) | 0.03 (−0.15, 0.20) | 0.03 (−0.15, 0.20) |
| Q2 | 595 | 0.16 (−0.02, −0.35) | 0.15 (−0.03, 0.33) | 0.14 (−0.04, 0.32) |
| Q3 | 597 | −0.07, (−0.33, 0.19) | −0.08 (−0.33, 0.17) | −0.07 (−0.33, 0.18) |
| Q4 | 923 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Black | ||||
| Q1 | 511 | −0.51 (−0.82, −0.21) | −0.48 (−0.79, −0.18) | −0.44 (−0.74, −0.15) |
| Q2 | 253 | −0.26 (−0.59, 0.08) | −0.26 (−0.60, 0.08) | −0.25 (−0.58, 0.08) |
| Q3 | 380 | −0.16 (−0.44, 0.11) | −0.18 (−0.46, 0.10) | −0.15 (−0.42, 0.13) |
| Q4 | 444 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Chinese | ||||
| Q1 | 230 | −0.01 (−0.68, 0.68) | −0.04 (−0.70, 0.62) | −0.04 (−0.67, 0.60) |
| Q2 | 246 | 0.16 (−0.51, 0.82) | 0.17 (−0.48, 0.82) | 0.16 (−0.46, 0.79) |
| Q3 | 184 | −0.18 (−0.84, 0.49) | −0.20 (−0.85, 0.45) | −0.20 (−0.82, 0.43) |
| Q4 | 32 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
| Hispanic | ||||
| Q1 | 504 | −0.43 (−1.02, 0.17) | −0.42 (−0.99, 0.16) | −0.39 (−0.95, 0.17) |
| Q2 | 378 | −0.29 (−0.88, 0.29) | −0.29 (−0.85, 0.28) | −0.22 (−0.78, 0.33) |
| Q3 | 331 | −0.25 (−0.82, 0.33) | −0.25 (−0.81, 0.30) | −0.23 (−0.77, 0.31) |
| Q4 | 57 | 0.00 (reference) | 0.00 (reference) | 0.00 (reference) |
N=5892.
The coefficients for the neighborhood problems score represent the percent eGFR change per year comparing each quartile to quartile 4 (Q4). Quartiles of the neighborhood problems score are: Q1≤1.32, Q2=1.33–1.47, Q3=1.48–1.63, Q4≥1.64.
The coefficients for the neighborhood social cohesion score represent percent eGFR change per year comparing each quartile to Q4. Quartiles of the social cohesion score are: Q1≤3.42, Q2=3.43–3.52, Q3=3.53–3.69, Q4≥3.70.
Model 1 is unadjusted.
Model 2 includes adjustment for age, gender, race/ethnicity (in models with the total sample only), income, education, and MESA site.
Model 3 includes further adjustment for diabetes, hypertension, smoking, BMI, LDL, HDL, CRP, and UACR.
Abbreviations: β, standardized regression coefficient; eGFR, ________; CI, confidence interval; MESA, _____.
While the prevalence of eGFR decline >30% from baseline varied by race and neighborhood score quartile (Table 2), the association between either of the neighborhood scores and either eGFR decline >30% from baseline or annualized decline were generally null across racial/ethnic groups (Tables 2 and 3). However, there were exceptions when modeling annualized change in eGFR (Table 3). For example, Black participants living in neighborhoods with greater problems (i.e., quartiles 2, 3, or 4) showed a greater annualized eGFR decline compared to Black participants living in neighborhoods with the lowest quartile of problems, after adjusting for sociodemographic characteristics (beta of −0.37 [95% CI, −0.72 to −0.01] for quartile 2 vs quartile 1; Table 3, top panel). Nevertheless, interactions between race/ethnicity and either of the neighborhood measures were not statistically significant (results not shown).
Sensitivity analyses
To examine whether the association between neighborhood measures and annualized change in eGFR varied by baseline kidney function status, we modeled the interaction between neighborhood measures and baseline eGFR (< versus ≥60/min/min/1.73m2). None of the interactions were statistically significant at the p<0.05 level.
DISCUSSION
The literature on the association between neighborhood SES (captured either as poverty or as an index of multiple social and economic indicators) and earlier stages of CKD has yielded modest and mixed results; therefore, we examined two social features of the neighborhood context that may not be adequately captured by neighborhood SES – neighborhood problems and social cohesion. While our results show that neighborhood social context differs by race/ethnicity and that the prevalence of risk factors such as hypertension and diabetes differ slightly across neighborhood context, neither of these neighborhood measures was independently associated with kidney function decline after adjustment in this multi-ethnic cohort.
These null findings may be interpreted in several ways. It may be that neighborhood social context, as measured through SES, as in previous work, or in perceived social environment, as in this study, does not play an important role in the very early stages of CKD. Researchers have reported an association between neighborhood SES and more advanced stages of CKD.7,8,10–12 In other words, it may be that neighborhood social/socioeconomic context may be more important for disease progression, particularly at advanced disease stages or in older adults. However, it may also be that neighborhood social context is challenging to measure without error. Furthermore, social context may be tightly interrelated with neighborhood sociodemographic characteristics and it is difficult to disentangle these neighborhood features.
It may also be that individual-level factors, such as individual-level SES, are important for eGFR decline in a healthy cohort such as MESA. Yet, the literature on the independent association between individual-level SES and eGFR (either levels or change over time) is also unclear, with studies showing an independent role for SES with ESRD28 but not earlier CKD stages.7,8,11
Nevertheless, neighborhood SES is associated with many risk factors for CKD, including incident hypertension and markers of CVD prevalence,29–33 diabetes prevalence,34 and health behaviors such as smoking prevalence.35 Furthermore, with respect to the measures of neighborhood context examined in this study, previous work has shown that greater levels of neighborhood problems are associated with greater levels of inflammatory markers (i.e., fibrinogen, interleukin 6, C-reactive protein), depressive symptoms, smoking prevalence, and heavy alcohol use prevalence in the MESA sample.18,26 Therefore, future work is needed to clarify the interrelated associations among neighborhood context, CKD risk factors, and CKD itself.
With respect to neighborhood social cohesion specifically, our results of no association between this neighborhood score and eGFR is consistent with much (but not all32,36) of the literature linking this exposure to CKD risk factors.2,18,37 This may be because social cohesion can operate both as a resource and as a constraint, depending on other individual and community factors. Strong ties with one’s neighbors may provide a resource upon which to draw for social and material support. Furthermore, these ties may result in broader community norms of healthy behaviors. On the other hand, these ties may also promote unhealthy behaviors and may serve as a source of obligatory burdens. It may also be that the measure of social cohesion used in MESA does not adequately capture the salubrious nature of the underlying concept.
The results indicate that neighborhood problems and social cohesion are not related to eGFR decline for either Black or White adults, even though there are well-documented inequalities in both neighborhood environment and in CKD. Future work should examine the role of racial residential segregation, or the separation of different racial groups into neighborhoods of uneven quality through historical and contemporary policies and practices. While there are no studies linking segregation, specifically, to biomarkers of kidney disease, there are studies linking segregation to racial inequalities in kidney disease-relates outcomes. Black-White inequalities in hypertension, diabetes, and obesity are greater in areas where there is more segregation.4,38–41 Those living in neighborhoods with a greater proportion of Black residents are more likely to have kidney disease risk factors compared to residents in neighborhoods with a lower proportion of Black residents,42 independent of the individual’s race. Mortality for those on dialysis is higher in areas with greater segregation.43,44 Furthermore, the research suggests that the neighborhood racial composition rather than one’s own race is important, as the White residents of predominantly Black neighborhoods fare worse in terms of ESRD outcomes compared to the Black residents.44
While, to our knowledge, this is the first examination of the association between aspects of the neighborhood social environment and kidney function, it is not without limitations. We used markers of kidney function in a relatively healthy population and eGFR may not be sensitive enough to capture the impact of the social environment on early stages of function decline. We also used two measures of neighborhood social environment. These measures have limited variability in the MESA sample, although they have been related to other health outcomes in this sample.11,18 Future work should examine other measures including composite indices of the social and built environment2,5 (e.g., air pollution) or of racial residential segregation.45 These data are also observational in nature, meaning that we cannot absolutely determine the causal ordering or nature of these associations. We do examine neighborhood at baseline and relate that to change over time. However, we do not know whether there is residual confounding and changes in neighborhood (as in an intervention) would result in changes in eGFR decline.
That our results did not show a relation between two measures of the social environment and eGFR does not lessen the need to examine other aspects of the neighborhood environment in relation to CKD. A better understanding of the role of neighborhoods is vital because neighborhood characteristics are neither random nor naturally-occurring; they can be changed by altering policies or introducing interventions1 and thus may be an effective way to promote population health.46
Supplementary Material
Acknowledgements:
The authors thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Support: This work was supported by a career development award from the National Institute on Diabetes and Digestive and Kidney Diseases (5K01DK106322 to M.T.H.). This research was also supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the National Heart, Lung, and Blood Institute, and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from NCATS. The funders of this study had no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Table S1. Comparison of descriptive characteristics of those included and excluded from analyses.
Table S2. Number and pattern of visits with eGFR assessment.
Table S3. Association of neighborhood problems and social cohesion scores with baseline eGFR < 60.
Table S4. Association of neighborhood problems and social cohesion scores with baseline UACR > 30.
Supplementary Material Descriptive Text for Online Delivery
Supplementary Table S1 (PDF). Comparison of descriptive characteristics of those included and excluded from analyses.
Supplementary Table S2 (PDF). Number and pattern of visits with eGFR assessment.
Supplementary Table S3 (PDF). Association of neighborhood problems and social cohesion scores with baseline eGFR < 60.
Supplementary Table S4 (PDF). Association of neighborhood problems and social cohesion scores with baseline UACR > 30.
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review: Received June 12, 2018. Evaluated by 3 external peer reviewers, with editorial input from an Acting Editor-in-Chief (Steven M. Brunelli, MD, MSCE). Accepted in revised form October 31, 2018. The involvement of an Acting Editor-in-Chief to handle the peer-review and decision-making processes was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Contributor Information
Margaret T. Hicken, Institute for Social Research, University of Michigan.
Ronit Katz, Kidney Research Institute, University of Washington.
Carmen A. Peralta, The Kidney Health Research Collaborative at University of California San Francisco and San Francisco VA Medical Center.
Deidra C. Crews, Division of Nephrology, Department of Medicine, Johns Hopkins University School of Medicine; Johns Hopkins Center for Health Equity, Johns Hopkins Medical Institutions; Baltimore MD.
Holly J. Kramer, Department of Nephrology and Hypertension, Loyola University School of Medicine, Chicago, Illinois.
References
- 1.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. [DOI] [PubMed] [Google Scholar]
- 2.Christine PJ, Auchincloss AH, Bertoni AG, et al. Longitudinal Associations Between Neighborhood Physical and Social Environments and Incident Type 2 Diabetes Mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA internal medicine. 2015;175(8):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mujahid MS, Diez Roux AV, Cooper RC, Shea S, Williams DR. Neighborhood stressors and race/ethnic differences in hypertension prevalence (the Multi-Ethnic Study of Atherosclerosis). Am J Hypertens. 2011;24(2):187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kershaw KN, Albrecht SS, Carnethon MR. Racial and ethnic residential segregation, the neighborhood socioeconomic environment, and obesity among Blacks and Mexican Americans. Am J Epidemiol. 2013;177(4):299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser P, Diez Roux AV, Mujahid M, et al. Neighborhood Environments and Incident Hypertension in the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2016;183(11):988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Volkova N, McClellan W, Klein M, et al. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19(2):356–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoham DA, Vupputuri S, Diez Roux AV, et al. Kidney disease in life-course socioeconomic context: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2007;49(2):217–226. [DOI] [PubMed] [Google Scholar]
- 8.Shoham DA, Vupputuri S, Kaufman JS, et al. Kidney disease and the cumulative burden of life course socioeconomic conditions: the Atherosclerosis Risk in Communities (ARIC) study. Soc Sci Med. 2008;67(8):1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoham DA, Vupputuri S, Kshirsagar AV. Chronic kidney disease and life course socioeconomic status: a review. Adv Chronic Kidney Dis. 2005;12(1):56–63. [DOI] [PubMed] [Google Scholar]
- 10.Merkin SS, Coresh J, Diez Roux AV, Taylor HA, Powe NR. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2005;46(2):203–213. [DOI] [PubMed] [Google Scholar]
- 11.Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: The Cardiovascular Health Study. Soc Sci Med. 2007;65(4):809–821. [DOI] [PubMed] [Google Scholar]
- 12.McClellan WM, Newsome BB, McClure LA, et al. Poverty and racial disparities in kidney disease: the REGARDS study. Am J Nephrol. 2010;32(1):38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams DR, Collins C. Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Rep. 2001;116(5):404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acevedo-Garcia D, Osypuk TL, McArdle N, Williams DR. Toward a policy-relevant analysis of geographic and racial/ethnic disparities in child health. Health Aff (Millwood). 2008;27(2):321–333. [DOI] [PubMed] [Google Scholar]
- 15.Schulz AJ, Kannan S, Dvonch JT, et al. Social and physical environments and disparities in risk for cardiovascular disease: The Healthy Environments Partnership conceptual model. Environ Health Perspect. 2005;113(12):1817–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steptoe A, Feldman P. Neighborhood problems as sources of chronic stress: Development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med. 2001;23(3):177–185. [DOI] [PubMed] [Google Scholar]
- 17.Ross CE, Mirowsky J. Neighborhood disorder, subjective alienation, and distress. J Health Soc Behav. 2009;50(1):49–64. [DOI] [PubMed] [Google Scholar]
- 18.Echeverría S, Diez-Roux AV, Shea S, Borrell LN, Jackson S. Associations of neighborhood problems and neighborhood social cohesion with mental health and health behaviors: the Multi-Ethnic Study of Atherosclerosis. Health Place. 2008;14(4):853–865. [DOI] [PubMed] [Google Scholar]
- 19.Lochner K, Kawachi I, Kennedy BP. Social capital: a guide to its measurement. Health Place. 1999;5(4):259–270. [DOI] [PubMed] [Google Scholar]
- 20.Kawachi I, Berkman L. Social cohesion, social capital, and health. Social epidemiology. 2000;174:190. [Google Scholar]
- 21.Kim ES, Kawachi I. Perceived Neighborhood Social Cohesion and Preventive Healthcare Use. Am J Prev Med. 2017;53(2):e35–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muntaner C, Lynch J. Income inequality, social cohesion, and class relations: a critique of Wilkinson’s neo-Durkheimian research program. Int J Health Serv. 1999;29(1):59–81. [DOI] [PubMed] [Google Scholar]
- 23.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–881. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DA, Simonelli G, Moore K, et al. The Neighborhood Social Environment and Objective Measures of Sleep in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2017;40(1):zsw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mujahid MS, Diez Roux AV, Morenoff JD, Raghunathan T. Assessing the measurement properties of neighborhood scales: from psychometrics to ecometrics. Am J Epidemiol. 2007;165(8):858–867. [DOI] [PubMed] [Google Scholar]
- 26.Nazmi A, Diez Roux A, Ranjit N, Seeman TE, Jenny NS. Cross-sectional and longitudinal associations of neighborhood characteristics with inflammatory markers: Findings from the multi-ethnic study of atherosclerosis. Health Place. 2010;16(6):1104–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inker LA, Schmid CH, Tighiouart H, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367(1):20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crews DC, Gutierrez OM, Fedewa SA, et al. Low income, community poverty and risk of end stage renal disease. BMC Nephrol. 2014;15(1):192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cozier YC, Palmer JR, Horton NJ, Fredman L, Rosenberg L. Neighborhood socioeconomic status and the incidence of hypertension in the black women’s health study. Ann Epidemiol. 2004;14(8):599. [Google Scholar]
- 30.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. [DOI] [PubMed] [Google Scholar]
- 31.Nordstrom CK, Diez Roux AV, Jackson SA, Gardin JM. The association of personal and neighborhood socioeconomic indicators with subclinical cardiovascular disease in an elderly cohort. The cardiovascular health study. Soc Sci Med. 2004;59(10):2139–2147. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, Diez Roux AV, Kiefe CI, Kawachi I, Liu K. Do neighborhood socioeconomic deprivation and low social cohesion predict coronary calcification?: the CARDIA study. Am J Epidemiol. 2010;172(3):288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber S, Hickson DA, Wang X, Sims M, Nelson C, Diez-Roux AV. Neighborhood Disadvantage, Poor Social Conditions, and Cardiovascular Disease Incidence Among African American Adults in the Jackson Heart Study. Am J Public Health. 2016;106(12):2219–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig J, Sanbonmatsu L, Gennetian L, et al. Neighborhoods, Obesity, and Diabetes — A Randomized Social Experiment. N Engl J Med. 2011;365(16):1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diez Roux AV, Merkin SS, Hannan P, Jacobs DR, Kiefe CI. Area Characteristics, Individual-Level Socioeconomic Indicators, and Smoking in Young AdultsThe Coronary Artery Disease Risk Development in Young Adults Study. Am J Epidemiol. 2003;157(4):315–326. [DOI] [PubMed] [Google Scholar]
- 36.Kim ES, Park N, Peterson C. Perceived neighborhood social cohesion and stroke. Soc Sci Med. 2013;97:49–55. [DOI] [PubMed] [Google Scholar]
- 37.Mair C, Diez Roux AV, Shen M, et al. Cross-sectional and longitudinal associations of neighborhood cohesion and stressors with depressive symptoms in the multiethnic study of atherosclerosis. Ann Epidemiol. 2009;19(1):49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kershaw KN, Diez Roux AV, Burgard SA, Lisabeth LD, Mujahid MS, Schulz AJ. Metropolitan-level racial residential segregation and black-white disparities in hypertension. Am J Epidemiol. 2011;174(5):537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thorpe RJ Jr, Brandon DT, LaVeist TA. Social context as an explanation for race disparities in hypertension: Findings from the Exploring Health Disparities in Integrated Communities (EHDIC) Study. Soc Sci Med. 2008;67(10):1604–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Corral I, Landrine H, Hao Y, Zhao L, Mellerson JL, Cooper DL. Residential segregation, health behavior and overweight/obesity among a national sample of African American adults. Journal of health psychology. 2012;17(3):371–378. [DOI] [PubMed] [Google Scholar]
- 41.LaVeist T, Thorpe R Jr., Galarraga J, Bower K, Gary-Webb T. Environmental and Socio-Economic Factors as Contributors to Racial Disparities in Diabetes Prevalence. J Gen Intern Med. 2009;24(10):1144–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang VW, Hillier AE, Mehta NK. Neighborhood Racial Isolation, Disorder and Obesity. Soc Forces. 2009;87(4):2063–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kimmel PL, Fwu C-W, Eggers PW. Segregation, Income Disparities, and Survival in Hemodialysis Patients. J Am Soc Nephrol. 2013;24(2):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rodriguez RA, Sen S, Mehta K, Moody-Ayers S, Bacchetti P, O’Hare AM. Geography matters: relationships among urban residential segregation, dialysis facilities, and patient outcomes. Ann Intern Med. 2007;146(7):493–501. [DOI] [PubMed] [Google Scholar]
- 45.Kershaw KN, Osypuk TL, Do DP, De Chavez PJ, Diez Roux AV. Neighborhood-Level Racial/Ethnic Residential Segregation and Incident Cardiovascular Disease: The Multi-Ethnic Study of Atherosclerosis. Circulation. 2014;131(2):141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morenoff JD, diez Roux A, Osypuk T, Hansen B. Residential environments and obesity: How can observational studies inform policy interventions? In: Schoeni RF, House JS, Kaplan GA, Pollock H, eds. The National Poverty Center series on poverty and public policy. New York: Russell Sage Foundation; 2008:309–343. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.