Abstract
The pedicle screw placement procedure is the most commonly used technique for spinal fixation and can provide reliable three‐column stabilization. Accurate screw placement is necessary in clinical practice. To avoid screw malposition, which may decrease the stiffness of the screw‐rod construct or increase the likelihood of neural and vascular injuries, the surgeons must fully understand the regional anatomy. Deformities, such as scoliosis, kyphosis or congenital anomalies, may complicate the application of the pedicle screw placement technique and increase the chance of screw encroachments. Incidences of pedicle screw malposition vary in different districts and hospitals and with surgeons and techniques. Today, the minimally invasive spinal surgery is well developed. However, the narrow corridors and limited views for surgeons increase the difficulty of pedicle screw placement and the possibility of screw encroachment. Evidenced by previous studies, robotic surgery can provide accurate screw placement, especially in settings of spinal deformities, anatomical anomalies, and minimally invasive procedures. Based on the consensus of consultant specialists, the literature review and our local experiences, this guideline introduces the robotic system and describes the workflow of robot‐assisted procedures and the precautions to take during procedures. This guideline aims to outline a standardized method for robotic surgery for thoracolumbar pedicle screw placement.
Keywords: Accuracy, Pedicle screw, Robot‐assisted surgery, Thoracolumbar spine
Introduction
First reported in 19591, pedicle screw instrumentation is commonly used in the clinic and remains one of the most popular and fundamental procedures for treatment of thoracolumbar disease. Pedicle screw instrumentation can effectively stabilize the segments and restore the three‐column stabilization of the spine2, 3. However, the distinct anatomy of thoracolumbar vertebrae poses a great challenge to the accurate and safe placement of the pedicle screws in this area. Thoracolumbar vertebrae are located deep inside the human body, with the spinal cord, the conus medullaris, the cauda equina, and nerve roots travelling inside the spinal canal, thoracic and abdominal organs, major blood vessels and sympathetic chain lying anteriorly, and muscles and ligamentous tissue lying posteriorly. Freehand placement of thoracolumbar pedicle screw, which is still the most widely adopted procedure, has great risk of adjacent tissue injury and complications, including adjacent neurologic and vascular injury, cerebrospinal fluid leakage, adjacent abdominal or thoracic organ injury, and even compromised stability of the operated segment4, 5. The accuracy of the traditional freehand technique for thoracolumbar pedicle screw placement is approximately 25.0%–86.6% according to several systematic reviews6, 7, 8.
Computer‐assisted navigation techniques started be applied in spine surgery in 1995, with the purpose of improving the accuracy of instrumentation and the safety of surgery9. Studies have shown the increased accuracy and precision of pedicle screw placement6, 7, 8, 10, 11, 12, 13, 14, 15, 16, the decreased intraoperative radiation exposure10, 11, 17, 18, and the improved safety of minimally invasive spinal surgery19, 20, 21, 22 under the assistance of navigation techniques. Driven by the developments in technology, combining computer‐assisted navigation and surgical robot techniques, orthopaedic surgical robots are now being to be applied in the clinic. The advantages of orthopaedic surgical robots over navigation techniques includes, theoretically, eliminating tremor and the unstable factor of human hand manipulation and reducing the time of navigation image matching and registration. Thus, guiding information and improved stability are benefits of using surgical robots, and, eventually, better clinical outcomes, can be achieved.
There are several orthopaedic surgical robots that have been reported to be applicable for thoracolumbar pedicle screw instrumented fixation, including the TianJi Robot, SpineAssist, and Renaissance. They can be used in the surgical manipulation for the treatment of spinal trauma, spinal tumors, spinal deformity, intervertebral disease, spondylolisthesis, spinal canal stenosis, and spinal instability13. Particularly for minimally invasive or revision surgeries with limited or unsatisfied exposure, the assistance of the robot has shown greater advantages compared with traditional methods23. The TianJi Robot system is an orthopaedic surgical robot developed in China with completely independent intellectual property. It is the first multi‐indication orthopaedic navigation robot worldwide, and the only robot that is indicated for the whole spine. The following content will focus on the technical key points of orthopaedic surgical robot‐assisted thoracolumbar pedicle screw instrumentation, using the TianJi Robot as an example, to standardize its clinical application and, hopefully, to benefit more patients with precision medicine.
Orthopaedic Surgical Robot
The orthopaedic surgical robotic system mainly uses preoperative or intraoperative images for surgical planning, providing accurate positioning of surgical tools or implants through robotic arm movement and rigid guidance, assisting the surgeon to complete surgical operations. The work processes mainly include four steps: (i) surgical planning, with the surgeon carrying out the surgical planning and selecting suitable implants based on the patient images using the device software; (ii) spatial registration, obtaining the spatial coordinates of the surgical trajectories via patent algorithm and tools; (iii) trajectory positioning, with the robotic arm providing automatous movement by holding the surgical instruments to the desired position according to the spatial coordinates of the surgical trajectory; and (iv) assisted surgery, with the surgeon performing the surgical operation under the guidance of the robotic arm.
The orthopaedic surgical robotic system is composed of multiple sets of equipment, and its work steps involve images and optical data acquisition, spatial registration and image fusion, surgical planning, and mechanical positioning. To avoid ambiguity and standardize the work steps, this study will define the hardware equipment, operation steps, and concepts related to the orthopaedic surgical robotic system. The principles and equipment were illustrated in Fig. 1.
Figure 1.
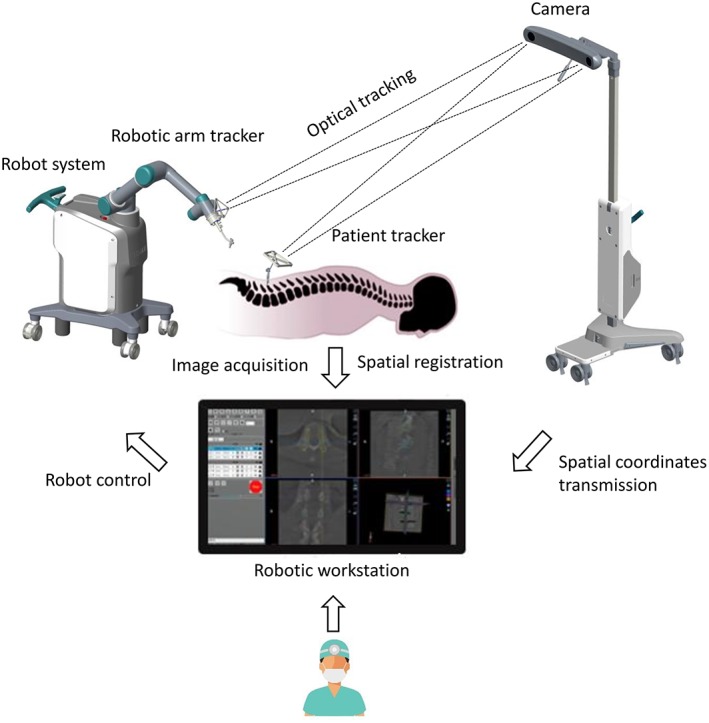
The working principles of TianJi Robot system.
Computer‐Assisted Navigation Technique
A surgical assisted technique that combines modern computer, stereotactic, and medical imaging technologies to guide surgeons for precise surgical planning and operation.
Orthopaedic Surgical Robotic Technique
A surgical assisted technique that combines computer‐assisted navigation and surgical robotics to guide the surgeons and enable precise surgical planning and operation.
Patient Tracker
A tracker connected to the patient’s anatomy during surgery to reflect or emit infrared light to the optical tracking camera.
Robotic Arm
A mechanism having two or more degrees of freedom, a certain degree of autonomy, and that is capable of automatically performing a predetermined task according to human instructions.
Robotic Arm Tracker
A tracker connected to the robotic arm during surgery to reflect or emit infrared light to the optical tracking camera.
Camera
The main component of the optical tracking system, a mechanical device for spatial positioning and tracking.
Guider
A navigation surgical tool that is attached to the end of the robot arm for positioning the surgical trajectory and that has a quick connection interface at the base.
Registration
A mechanical device for spatial coordinate mapping and calibration.
Robotic Workstation
The workstation holds the navigation computer system, the surgical planning software, and the robot operation software.
Positioning
The process of moving the robotic arm and guider to the planned trajectory position.
Target Audience
All personnel who participate in orthopaedic surgical robot‐assisted thoracic or lumbar pedicle screw instrumentation, including spine surgeons, nurses and engineers, are the target audience for this clinical practice guideline. Manipulation of surgical procedure and relative equipment or mechanical devices should follow the standardized operative process recommended by the guideline. Proceed with caution and vigilance to the factors that could influence robotic navigation accuracy or endanger the safety of the patient.
Indications and Contraindications
Indications
Orthopaedic surgical robot‐assisted thoracolumbar pedicle screw instrumentation is indicated for most thoracic and lumbar diseases, including thoracolumbar spinal trauma, degenerative disease, tumors, thoracolumbar deformity, and iatrogenic thoracolumbar spinal instability. Minimally invasive surgery, revision surgery, and deformity correction of the thoracolumbar spine are considered to benefit most from orthopaedic surgical roboticassistance24.
Spinal trauma: Thoracic and lumbar burst fracture, osteoporotic vertebral compressive fracture.
Degenerative spinal disease: Lumbar spondylolisthesis, intervertebral disc herniation, thoracic spinal canal stenosis, and lumbar spinal canal stenosis
Spinal deformity: Congenital spondyloptosis, scoliosis16, kyphotic deformity.
Spinal tumor: Primary vertebral tumor, intracanal tumor.
Spinal infection: Tuberculosis of the spine18.
Contraindications
Systematic disease too severe to tolerate anesthesia or surgery, including severe hemorrhagic disease, circulatory or respiratory function failure.
Impossible or unsatisfied patient position for surgery.
Patient unable to tolerate radiation exposure.
Impossible or unsatisfied tracker placement.
Impossible or unsatisfied navigation images acquirement.
Robot‐Assisted Procedures
Patient Preparation
After general anesthesia, patient positioning is the same as the requirements for conventional procedures. Any obstruction to fluoroscopy should be removed preoperatively. During prepping and draping, the anchoring site for the patient tracker should be prepped and exposed. In open surgery, exposure is extended for anchoring the patient tracker. In percutaneous minimally invasive surgery, the patient tracker should be anchored at first and subsequently the stab wounds are created under robotic guidance.
Robotic Equipment Preparation
The TianJi Robot system consists of robotic arm, an optical tracking system, a robotic workstation, and a navigation toolkit. The TianJi Robot system and the components around the robotic arm are shown in Figs 2 and 3. All the relevant equipment is recommended to be arranged as follows in the operating room (Fig. 4).
Figure 2.
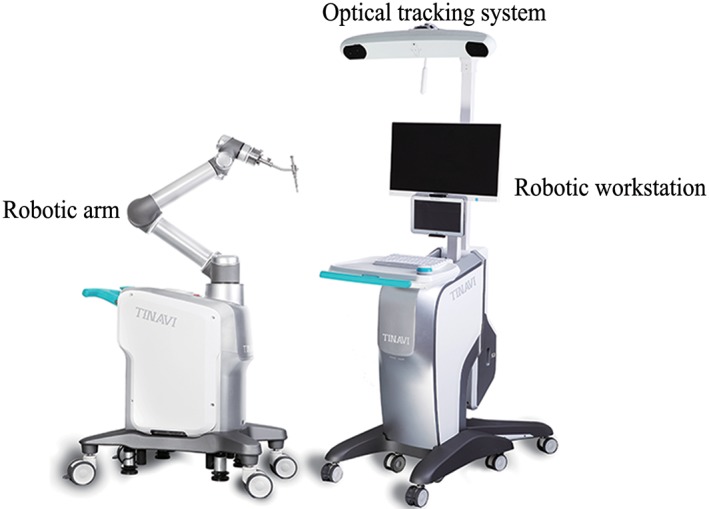
TianJi Robot system.
Figure 3.

TianJi Robot navigation and positioning tool installation, including fixed ring, tracker, tool guider, lock screw, and holder base.
Figure 4.
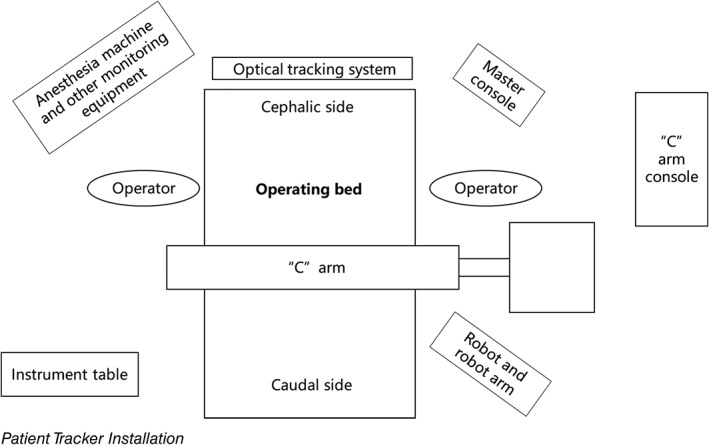
Schematic diagram of operation room. When performing robotic surgery, it is recommended that the operation room be arranged as shown.
Patient Tracker Installation
The patient tracker is commonly anchored on adjacent vertebral spinous process by a clamp. The clamp connecting the patient tracker to spinous process should be tightened and then the patient tracker is powered on. There are severe precautions that should be taken in patient tracker installation: (i) avoid interfering with the surgeon or assistant; (ii) do not block the optical tracking camera; and (iii) avoid the soft tissue stretching the tracker.
Navigation and Positioning Tool Installation
Cover the robot with a sterile cover. Then install the registration and place it into the operation area so that the registration is within the fluoroscopic field (Fig. 2).
Image Acquisition
Robot systems commonly use intraoperative real‐time 3D image navigation. After the image acquisition is completed, the system will complete the registration automatically and display the registration accuracy.
Operation Planning
Planning and design are performed in the robotic workstation. Select the spinal segment, the screw diameter, the screw length, and the trajectory.
Path Positioning
After the screw guider is installed, move the guider to the surgical field. The positioning accuracy will be displayed in the software interface in real time during the movement of the robot arm.
Note: (i) if you find that the robot arm may touch the patient or surrounding obstacles, immediately press the emergency stop button; and (ii) the guider should be as close as possible to the operating area.
Sleeve Installation
Place the sleeve into the screw guider. For percutaneous minimally invasive surgery, incision can be made according to the position of the sleeve in contact with the skin. For open surgery, the sleeve is brought to the cortical bone surface after the bone surface is exposed.
Screw Placement
K‐wire was drilled into the vertebrae, then the optimal position is confirmed by fluoroscopy. If it is a cannulated screw, it can be instrumented directly along the K‐wire; if it is a normal screw, use the cannulated tap to prepare the placement path first, then implant the screw.
Image Verification
After the instrumentation is completed, verify the screw position by fluoroscopy. If the position is satisfactory, the robot system can be withdrawn; if it is not satisfactory, the path should be positioned again after fine tuning of the robot, or positioning image acquisition should be restarted.
Remaining Procedures
Robotic planning can be used to perform minimally invasive surgery with aberrant anatomical landmarks or deformity with variation, and it can complete the decompression and lesion removal accurately. The work flowchart is shown in Fig. 5.
Figure 5.
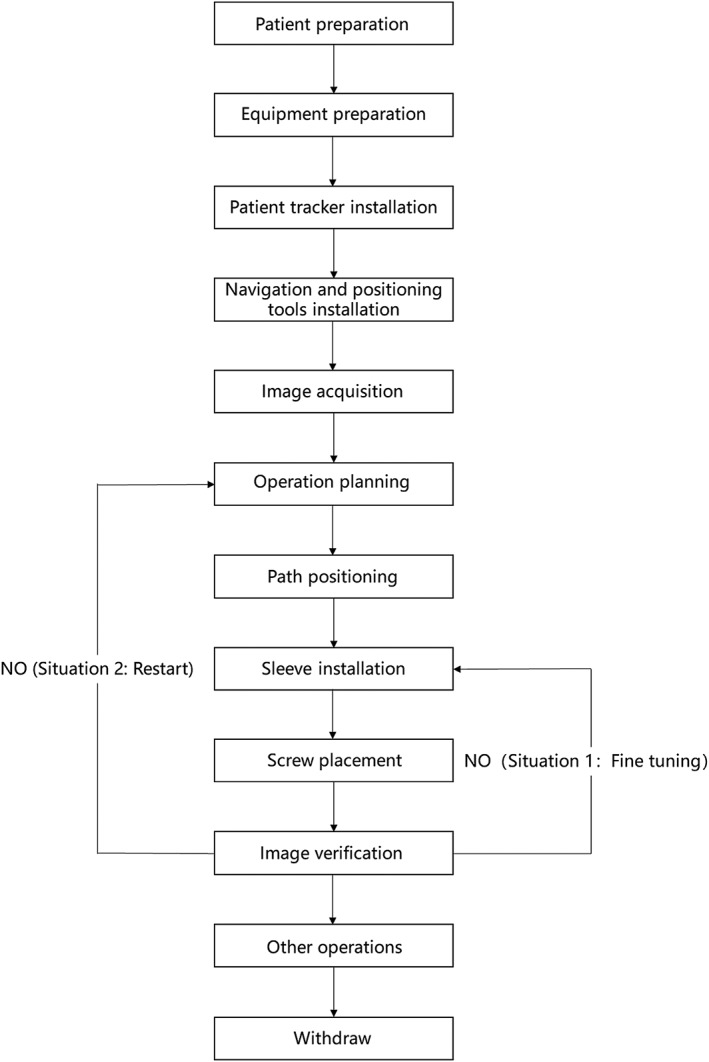
Workflow of robot‐assisted procedures.
Precautions
Robot‐assisted orthopaedic technology is a complex system based on advanced technologies such as image registration and fusion, robotics and automation technology, and precise equipment manufacturing. Its accuracy depends on these components of systems working properly; thus, it is affected by many factors. Common factors causing errors and strategies dealing with common errors are listed below.
Personnel Requirements
Doctors should have conventional surgical experience, relevant anatomical knowledge to determine whether the navigation system is accurate or not, and the ability to switch to conventional surgery when the robotic system fails to operate.
Environmental and Equipment Requirements
The operating room should have an appropriate area, with a good grounding system and power supply conditions. The operating table should be radiolucent, avoiding metal artifacts that may affect the fluoroscopic process. The operating table base should not obstruct the intraoperative fluoroscopic machine obtaining intraoperative images. The operating environment should meet the normal working requirements according to the robot manual (including ambient temperature, humidity, air pressure, and voltage).
The preoperative navigation kit should be sterilized and placed on the operating table. The camera is placed on one side of the operating bed, above and facing towards the field, and should not be blocked by the tray or the head frame. The C‐arm machine is moved from one side of the operating bed when it is used and is it suggested that there is a ground mark to guide the position properly. The main control trolley and the C‐arm should be far from the operating area to facilitate the technician’s operation.
Image Acquisition Requirements
The fluoroscopic images should demonstrate all the bony structures of key anatomical regions. The registration is clearly shown in the fluoroscopic field and the camera could simultaneously recognize and capture the spatial position information of the patient tracker and the robot arm tracker.
Navigational Deviations: Image Drift
The spatial position of the anatomy at the surgical site is required to be relatively fixed after the images are acquired. Any factors causing the image deviations between the guided position and real position are regarded as image drift. The doctor should have the ability to determine if the navigation image is drifting. When image drift is suspected, select obvious anatomical landmarks, such as the apex of the spinous process, facet joints, or transverse roots for verification. If the positioning is accurate, continue the surgery; however, if it is uncorrectable drift, rescanning is necessary. Common reasons for image drift include the following items, which need to be noted:
Relative displacement of the anatomical structures relative to the patient tracker.
When performing surgery on a region with a high degree of motion, if the surgeon excessively pulls the soft tissue, it will cause a large relative displacement between the bony structures. Therefore, the intraoperative manipulation needs to be gentle, and the positioning accuracy should be noted during the manipulation.
Decompression or osteotomy will destroy the stability of the spine, resulting in relative displacement between the anatomical structures. If intraoperative conditions permit, the procedure of fixation is advised to be performed first, followed by remaining procedures to avoid image drift. If the accuracy is still uncertain, the doctor should select an anatomical landmark for verification.
When the long segmental fixation is performed, the distal vertebral body operation will cause relatively large displacement between the anatomical structures. It is recommended that the intraoperative operation should be performed away from the patient tracker position towards the patient tracker.
If the patient’s position changes, it may cause a change in the spatial position of the patient’s anatomical structures, and image acquisition and surgical planning should be re‐executed.
During the image acquisition, the amount of respiratory tidal volume could be reduced if the anesthesia conditions permit, to reduce the impact of respiratory motion on image accuracy.
Patient Tracker Loosening
The patient tracker needs to be firmly anchored to the patient’s anatomy. If the intraoperative tool or the surgeon accidentally moves or touches the tracker, or the tracker is pulled by the skin during minimally invasive surgery, the position of trackers is changed. That will lead to a decrease in positioning accuracy or a failure in positioning. In this situation, image acquisition and surgical planning should be repeated.
Misalignment Caused by Lighting Problems
The robot system must maintain good reflection and reception of infrared light. If the angle or distance exceeds the receiving range, or other light interference, misalignment may occur. The camera should be adjusted so that the surgical field is in the center of its detection range. The strong light or blood should be avoided from the tracker.
Regular Maintenance of Robot Equipment
Data cable: Check whether the transmission data line interface is loosening or disconnected. If the data cable is aging, it needs to be replaced.
Robot tool: Before the operation, the robot tool should be carefully checked for metal fatigue to prevent the tool from breaking during the operation.
System accuracy: Accuracy calibration should be performed periodically.
Other
If the robotic arm is found to be unable to reach the designated position due to the surrounding environment during surgery, the surgical path should be adjusted in time. If there is a sudden power failure of the robot system, the system should be restarted: If only the mechanical system of the mechanical arm is powered off, it is generally unnecessary to re‐acquire images and surgical planning, but if the overall system of the robot is powered off, the image acquisition and surgical planning should be repeated.
List of Names of Consultant Specialists
Lin Cai, Xiang‐yang Cao, Huan‐sheng Fang, Chun‐zheng Gao, Ding‐jun Hao, Yong‐cheng Hu, Ming Li, Chun‐xia Li, Chun‐de Li, Kai‐nan Li, Yi Liao, Hao Liu, Bao‐ge Liu, Jun Liu, Shi‐dong Liu, Xin‐yu Liu, Ya‐jun Liu, Xin‐long Ma, Cai‐liang Shen, Hong‐xing Shen, Yue‐ming Song, Tian‐sheng Sun, Wei Tian, Yue Wang, Ya‐yi Xia, Jian‐zhong Xu, Hui‐lin Yang, Xue‐jun Yang, Guo‐yong Yin, Shu‐dong Zhang, Wei‐guo Zhang, Yu Zhao, Yue Zhu.
Acknowledgments
This guideline was created and revised by multiple consultant specialists in several associations, including the Technical Committee on Medical Robot Engineering of the Chinese Society of Biomedical Engineering, the Medical Robotics Branch of the Chinese Medical Doctor Association, and the Chinese Orthopaedic Association of the Chinese Medical Association. We are grateful for their support and contributions.
Grant Sources: This work was supported by the National Key R&D Program of China (2016YFC0105800), the National Natural Science Foundation of China (CN) (U1713221); Beijing Natural Science Foundation (Z170001), and Beijing Municipal Science and Technology Program (CN) (Z171100000417019).
References
- 1. Boucher HH. A method of spinal fusion. J Bone Joint Surg Br, 1959, 41: 248–259. [DOI] [PubMed] [Google Scholar]
- 2. Barnes AH, Eguizabal JA, Acosta FL Jr, Lotz JC, Buckley JM, Ames CP. Biomechanical pullout strength and stability of the cervical artificial pedicle screw. Spine (Phila Pa 1976), 2009, 34: E16–E20. [DOI] [PubMed] [Google Scholar]
- 3. Verma K, Boniello A, Rihn J. Emerging techniques for posterior fixation of the lumbar spine. J Am Acad Orthop Surg, 2016, 24: 357–364. [DOI] [PubMed] [Google Scholar]
- 4. Katonis P, Christoforakis J, Kontakis G, et al Complications and problems related to pedicle screw fixation of the spine. Clin Orthop Relat Res, 2003, 414: 86–94. [DOI] [PubMed] [Google Scholar]
- 5. Jutte PC, Castelein RM. Complications of pedicle screws in lumbar and lumbosacral fusions in 105 consecutive primary operations. Eur Spine J, 2002, 11: 594–598. [DOI] [PubMed] [Google Scholar]
- 6. Gelalis ID, Paschos NK, Pakos EE, et al Accuracy of pedicle screw placement: a systematic review of prospective in vivo studies comparing free hand, fluoroscopy guidance and navigation techniques. Eur Spine J, 2012, 21: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Verma R, Krishan S, Haendlmayer K, Mohsen A. Functional outcome of computer‐assisted spinal pedicle screw placement: a systematic review and meta‐analysis of 23 studies including 5,992 pedicle screws. Eur Spine J, 2010, 19: 370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shin BJ, James AR, Njoku IU, Härtl R. Pedicle screw navigation: a systematic review and meta‐analysis of perforation risk for computer‐navigated versus freehand insertion. J Neurosurg Spine, 2012, 17: 113–122. [DOI] [PubMed] [Google Scholar]
- 9. Amiot LP, Labelle H, DeGuise JA, Sati M, Brodeur P, Rivard CH. Computer‐assisted pedicle screw fixation. A feasibility study. Spine (Phila Pa 1976), 1995, 20: 1208–1212. [DOI] [PubMed] [Google Scholar]
- 10. Fan Y, Du JP, Liu JJ, Zhang JN, Liu SC, Hao DJ. Radiological and clinical differences among three assisted technologies in pedicle screw fixation of adult degenerative scoliosis. Sci Rep, 2018, 8: 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kantelhardt SR, Martinez R, Baerwinkel S, Burger R, Giese A, Rohde V. Perioperative course and accuracy of screw positioning in conventional, open robotic‐guided and percutaneous robotic‐guided, pedicle screw placement. Eur Spine J, 2011, 20: 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan Y, Du J, Zhang J, et al Comparison of accuracy of pedicle screw insertion among 4 guided technologies in spine surgery. Med Sci Monit, 2017, 23: 5960–5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devito DP, Kaplan L, Dietl R, et al Clinical acceptance and accuracy assessment of spinal implants guided with SpineAssist surgical robot: retrospective study. Spine (Phila Pa 1976), 2010, 35: 2109–2115. [DOI] [PubMed] [Google Scholar]
- 14. Molliqaj G, Schatlo B, Alaid A, et al Accuracy of robot‐guided versus freehand fluoroscopy‐assisted pedicle screw insertion in thoracolumbar spinal surgery. Neurosurg Focus, 2017, 42: E14. [DOI] [PubMed] [Google Scholar]
- 15. Tian W, Liu Y, Zheng S, Lv Y. Accuracy of lower cervical pedicle screw placement with assistance of distinct navigation systems: a human cadaveric study. Eur Spine J, 2013, 22: 148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macke JJ, Woo R, Varich L. Accuracy of robot‐assisted pedicle screw placement for adolescent idiopathic scoliosis in the pediatric population. J Robot Surg, 2016, 10: 145–150. [DOI] [PubMed] [Google Scholar]
- 17. Gao S, Lv Z, Fang H. Robot‐assisted and conventional freehand pedicle screw placement: a systematic review and meta‐analysis of randomized controlled trials. Eur Spine J, 2018, 27: 921–930. [DOI] [PubMed] [Google Scholar]
- 18. Alaid A, von EK, Smoll NR, et al Robot guidance for percutaneous minimally invasive placement of pedicle screws for pyogenic spondylodiscitis is associated with lower rates of wound breakdown compared to conventional fluoroscopy‐guided instrumentation. Neurosurg Rev, 2018, 41: 489–496. [DOI] [PubMed] [Google Scholar]
- 19. Tian W, Xu YF, Liu B, et al Computer‐assisted minimally invasive transforaminal lumbar interbody fusion may be better than open surgery for treating degenerative lumbar disease. Clin Spine Surg, 2017, 30: 237–242. [DOI] [PubMed] [Google Scholar]
- 20. Kim CW, Lee YP, Taylor W, Oygar A, Kim WK. Use of navigation‐assisted fluoroscopy to decrease radiation exposure during minimally invasive spine surgery. Spine J, 2008, 8: 584–590. [DOI] [PubMed] [Google Scholar]
- 21. Spetzger U, Von Schilling A, Winkler G, Wahrburg J, König A. The past, present and future of minimally invasive spine surgery: a review and speculative outlook. Minim Invasive Ther Allied Technol, 2013, 22: 227–241. [DOI] [PubMed] [Google Scholar]
- 22. Lang Z, Tian W, Liu Y, Liu B, Yuan Q, Sun Y. Minimally invasive pedicle screw fixation using intraoperative 3‐dimensional fluoroscopy‐based navigation (CAMISS Technique) for Hangman fracture. Spine (Phila Pa 1976), 2016, 41: 39–45. [DOI] [PubMed] [Google Scholar]
- 23. Lieberman IH, Hardenbrook MA, Wang JC, Guyer RD. Assessment of pedicle screw placement accuracy, procedure time, and radiation exposure using a miniature robotic guidance system. J Spinal Disord Tech, 2012, 25: 241–248. [DOI] [PubMed] [Google Scholar]
- 24. Härtl R, Lam KS, Wang J, Korge A, Kandziora F, Audigé L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg, 2013, 79: 162–172. [DOI] [PubMed] [Google Scholar]


