Abstract
While efficient glucose transport is essential for all cells, in the case of the human placenta, glucose transport requirements are two-fold; provision of glucose for the growing fetus in addition to the supply of glucose required the changing metabolic needs of the placenta itself. The rapidly evolving environment of placental cells over gestation has significant consequences for the development of glucose transport systems. The two-fold transport requirement of the placenta means also that changes in expression will have effects not only for the placenta but also for fetal growth and metabolism. This review will examine the localization, function and evolution of placental glucose transport systems as they are altered with fetal development and the transport and metabolic changes observed in pregnancy pathologies.
Keywords: Glucose transporters; Placenta, human; Pregnancy; Diabetes; Preeclampsia; IUGR
1. Introduction
The key role played by glucose transporters in fetal nutrition and growth has long been recognized and the primary components performing transplacental glucose transfer have been identified [1]. Beyond this, the response of placental glucose transporting systems to major pregnancy pathologies has also been investigated, albeit slowly. However, the amalgamation of this information into a more integrated picture of transport and metabolism is still lacking. In this review we hope, in part, to address this deficiency, examining glucose transport across the placenta and its role in fetoplacental growth and metabolism, as well as its role in pregnancy pathologies. Before that discussion however, it is essential to understand the characteristics of the multiple glucose transporters that have been described in the placenta, including localization and development, as this information is important in decoding their physiological function.
In this review, we will first delineate the current knowledge on types of glucose transporter expressed in the placenta, followed by discussion of transporter localization and the functional effects of their distribution. Next, we will address what is known of the regulatory factors shown to modulate glucose transporter expression. These sections provide the foundation for exploring the mechanisms by which the placental glucose transport system participates in the greater network of metabolic functions controlling fetoplacental growth. Finally, we will examine the changes in glucose transporter expression and function in pregnancy pathologies such as diabetes and preeclampsia as well as the way in which these changes impact the pathology.
We have restricted ourselves to a discussion of the human placental system for two reasons. First because the nature of human pregnancy is different substantially from many of the common animal models in reproductive type, that is K-selected species (low number of offspring, high survival, high parental investment), vs r-selected species (high number of offspring, high growth rate, minimal parental care). Human pregnancy also differs more specifically in placental type (e.g. hemochorial vs. epitheliochorial). In addition, while we are capable of simulating some aspects of human pregnancy pathologies in animal models, few simulations encompass all the facets of the “experiments of nature” presented by pregnancy pathologies. As the transfer of glucose from the maternal circulation into the placenta and fetus forms the primary task for glucose transporters, most of the published information is concerned with glucose transporters in the epithelial barrier layer of the placenta, the multinucleate syncytiotrophoblast. Some information exists also on transporters in the mononuclear, syncytial precursor cells, the cytotrophoblast, and there is a limited amount of data on transporters in other cells of the placenta, including the fetal microvascular endothelium. Most of the reports on glucose transporters in pregnancy pathologies however involve investigations of the syncytiotrophoblast microvillous (apical) or basal membrane transporters as the key components of transplacental transfer.
2. Glucose transporter types
Three types of protein solute carriers have been identified for glucose transport, the facilitated diffusion transporters (GLUT, SLC2), the sodium-coupled symporters (SGLT, SLC5) and the more recently identified SWEET transporters (SLC50) [2–5]. The latter two types are not present in the human placenta and thus this discussion is confined to the GLUT family. The GLUT family consists of 14 proteins (GLUT1–14) of ~500 amino acids, divided into three sub-classes [2,4]. Class I contains the classical transporters, GLUT1–4, plus the gene duplication of GLUT3, assigned as GLUT14. Class II transporters (a.k.a. the “odd transporters”) comprise the GLUT5, 7, 9 and 11 isoforms. The final group, Class III, covers the GLUT6, 8, 10 and 13 isoforms, although GLUT13 is also known as the proton-driven myoinositol transporter, HMIT. The GLUT proteins possess 12 transmembrane segments, with both the N and C termini in the cytoplasm. They have a single site of N-linked glycosylation, which resides in the first exofacial linker domain of the Class 1 and 2 GLUTs and in the fifth exofacial linker domain of the Class 3 proteins. The GLUT family are generally seen as electroneutral facilitative hexose transporters with the exception of GLUT13 (proton-coupled), GLUT9 and possibly, GLUT12. GLUT9 is primarily an urate transporter which operates via an electrogenic mechanism; two isoforms (GLUT9a, GLUT9b) are distinguishable by the manner in which urate transport is modified in the presence of hexoses [6]. GLUT12 has been reported to be voltage dependent and also transports alpha-methyl-D-glucopyranoside, similar to the SGLT family [7], suggesting a different, as yet to be discovered, physiological role. Of interest also is the reported role of 4F2hc (CD98) in GLUT1 function. CD98, known as the heavy chain subunit of the LAT1 amino acid transporter, is highly expressed in the human placenta and it has been suggested that it has a role in stabilizing GLUT1 perhaps, by limiting GLUT1 degradation [8].
3. Glucose transporters in the human placenta
The human placenta has been reported to express seven isoforms of the glucose transporter, GLUT1, 3, 4, 8, 9, 10 and 12 (Table 1) in a variety of locations and also showing changes in expression over the course of gestation.
Table 1.
Glucose transporters in the human placenta.
| Isoform | Primary substrate | Primary Km (mM) | Other substrates |
|---|---|---|---|
| GLUT1 | Glucose | 3 | Galactose, mannose, glucosamine, DHA* |
| GLUT3 | Glucose | 1.5 | Galactose, fructose mannose, xylose, DHA |
| GLUT4 | Glucose | 5 | Glucosamine, DHA |
| GLUT8 | Glucose | 2 | Fructose, galactose |
| GLUT9a | Urate | 0.9 | Glucose, fructose |
| GLUT9b | Urate | 0.9 | Glucose, fructose |
| GLUT10 | Glucose | 0.3 | Galactose |
| GLUT12 | Glucose | ND | Galactose, fructose |
3.1. GLUT1
The GLUT1 isoform is expressed ubiquitously in the placenta, in the syncytiotrophoblast, cytotrophoblast and endothelial cells as well as villous stromal elements (Fig. 1) [9–13]. The highest degree of expression by far is in the syncytiotrophoblast. Western blotting of the separate, purified syncytial maternal-facing microvillous and fetal-facing basal membrane fractions has revealed that there is significantly higher expression of GLUT1 on the microvillous membrane of the syncytiotrophoblast, suggesting an approximately 3-fold greater density of GLUT1 on that membrane compared to the basal membrane [9,11]. An important aspect to remember however is that the microvillous nature of the syncytial apical membrane expands the membrane surface area of the maternal-facing face of the syncytiotrophoblast. The extent of the microvillous surface enlargement factor varies with gestational age, such that from 25 to 36 weeks of gestation, it ranges between 9.5 (25 weeks) and 7.5 (36 weeks) [14,15]. While it is not clear whether there are differences in GLUT1 protein expression between the microvillous tips and crypts, it is likely that the microvillous:basal GLUT1 ratio is substantially > 3 and could be ≥20. In addition, there are gestational changes in GLUT1 expression. While microvillous membrane GLUT1 expression remains unaltered, basal membrane GLUT1 expression increases ~2-fold between the 16–22 and 27–30 week periods, and remains at that level until term [9,16,17].
Fig. 1.
Distribution of placental glucose transporters in first and third trimester human placenta. This figure shows the distribution of glucose transporters in first and third trimester human placenta as described in Section 3. The localization of GLUT8, 9a and 9b in the first trimester is unknown. No information is available on GLUT10 localization in either first or third trimester. MVM, microvillous membrane; BM, basal membrane; STB, syncytiotrophoblast; CTB, cytotrophoblast; EC, endothelial cell; VS, villous stromal cell.
3.2. GLUT3
Initially there was some uncertainty about the localization of GLUT3 [10,18–20], however more recently the microvillous membrane of the syncytiotrophoblast has been identified as the primary location for GLUT3 expression in the placenta [12,21,22]. This isoform has also been identified in the fetal endothelium [18,20], however, it is not thought that the endothelial localization has any role in transplacental transport [23–25] and that GLUT3 in this location is important primarily for endothelial metabolic uptake. This in itself is unusual in that endothelial cells are not usually identified as a site for GLUT3 expression [26]. Aside from the microvillous localization of GLUT3, another aspect, the change in gestational expression of placental GLUT3 is notable. The microvillous membrane expression of GLUT3 decreases to 48% of the first trimester value in the second trimester and to 34% of the first trimester value in the third trimester [21], suggesting that its role in the first trimester is more important than later in gestation.
The question arises as to the functional reason for the localization and gestational profile of GLUT3 in the placenta. The syncytial and endothelial localization and the gestational changes in syncytial GLUT3 are all consistent with the theory that the GLUT3 transporter functions to enable rapid, high volume glucose transport, especially under conditions where extracellular glucose is lower than the normal plasma glucose concentrations [21,26]. GLUT3 has a Km for 2-deoxyglucose of 1.4 mM as opposed to the 6.9 and 4.6 mM for GLUT1 and GLUT4 respectively. Therefore, early in placental development, during the first trimester, in the absence of a fully formed maternal circulation, glucose concentrations in the poorly-exchanging extracellular fluids which bathe the placenta are likely to be well below those normally observed in the circulation [27]. The presence of the high affinity GLUT3 isoform would enable sufficient placental glucose uptake in these otherwise adverse conditions. This theory is supported, indirectly, by the hypoxic nature of the placental environment in the first trimester, since GLUT3 expression is up-regulated by hypoxia [22,28], possibly via HIF-1 mediation [29,30]. This would account for the loss of GLUT3 expression over gestation, as the oxygenation status of the placenta improves with the onset of a functional intervillous circulation and placental hypoxia is diminished. Similarly, GLUT3 expression in fetal endothelial cells may also be a response to lowered plasma glucose, since the fetal arterial glucose concentration of blood entering the placenta is lower than umbilical venous concentration and significantly lower than normal adult plasma glucose concentration as a result of fetal metabolism [31]. In view of the reduced oxygenation in the fetal arterial circulation, it is also possible that GLUT3 may be up-regulated in response.
3.3. GLUT4
The placenta is not known as an insulin-regulatable tissue to the same extent as muscle, for example, although there is data suggesting a response to insulin, especially in the first trimester [12]. GLUT4 has been detected in the placenta, primarily in the syncytiotrophoblast. There is agreement over the cytosolic expression of GLUT4 in syncytial cells in the first trimester [12,13]. The cytosolic localization is consistent with an already synthesized and membrane-incorporated GLUT isoform which can be recruited rapidly to the cell surface. The extent of expression in the third trimester is less clear but is significantly reduced. Other studies have reported GLUT4 in the villous stroma [32] and in placental homogenates [22] at term. Ericsson et al. demonstrated that insulin increased glucose uptake in first trimester, but not term placental fragments [12]. The absence of the insulin-regulatable GLUT4 at term fits with the corresponding observations regarding the presence of the insulin receptor. While the insulin receptor is expressed on the microvillous surface of the syncytiotrophoblast in the first trimester, by term, syncytial expression is down-regulated and instead the primary localization of the receptor is to the fetal endothelium [33–35].
3.4. GLUT8
GLUT8 was reported in both syncytio- and cytotrophoblast cells at term [36]. In the same report the authors also described the presence of GLUT8 in extravillous trophoblast cells, in cultured trophoblast cells and in the HTR8/SVneo cell line (an extravillous trophoblast model cell line). Those investigations suggested localization to the cytoplasmic fraction of the cells, consistent with the observations in neuronal cells, adipocytes and others [37,38]. In all the cell types in which GLUT8 has been identified, there appears to be no translocation of GLUT8 to the cell surface. An exception is the blastocyst, where GLUT8 appears to be responsible for insulin-stimulated glucose uptake [39]. While the localization in syncytio- and cytotrophoblast cells in tissue is ambiguous, GLUT8 protein expression in cultured primary trophoblast and HRT8/SVneo cells is clearly cytoplasmic. Another study has localized GLUT8 to both endothelial cells and to the syncytiotrophoblast cytoplasm [40]; the same study also noted up-regulation of GLUT8 in BeWo choricarcinoma cells following transfection with IGF-I. Its role is not apparent, although it has been suggested that it functions to deliver glucose to intracellular organelles [36]. Against this is the argument that there is likely to be very little intracellular free glucose as a result of the action of high-affinity hexokinases; an alternative is that like GLUT9, it acts in vivo as a transporter for another, as yet unidentified, substrate.
3.5. GLUT9
This GLUT isoform has been identified unambiguously as an urate transporter [41], although it is capable of transporting hexoses, having a particularly high affinity for fructose [6,42]. GLUT9 has two primary sub-isoforms. GLUT9a and GLUT9b which differ in their N-terminal sequence and are differentially expressed in polarized cells, such that GLUT9b is found localized apically, while GLUT9a is found on basolateral membranes [41,43]. This localization was confirmed for the human placenta [44]. In addition, GLUT9a also appears to be expressed in fetal endothelial cells and, possibly, other villous stromal locations [13,44].
Both isoforms act functionally as symmetric electrogenic uniporters of urate, with the cell membrane potential driving efflux from the cell. For the syncytium, this implies that these transporters are less functional as a means of transplacental transport of urate than as a means of clearing the syncytial epithelium. There are differences however in the responses of these two isoforms to the presence of hexoses, such that the D-glucose trans-stimulation of urate efflux is abrogated in GLUT9b (lacking 28 amino acids in the N-terminus) but not GLUT 9a, suggesting that the basal efflux might be favored. It is also probable, given the presence of a PKA phosphorylation site on the “extra” amino acid region in GLUT9a, there may be regulatory changes in hexose effects, quite possibly cell-specific [6]; more information is necessary to determine the role of GLUT9 in placental function and transplacental transfer.
3.6. GLUT10
There are few reports regarding the Class III isoform, GLUT10, in the human placenta. Its presence was determined in the early tissue surveys [45,46], and more recently it has been shown to demonstrate a significant degree of promoter methylation, increasing over gestation [47]. Placental localization and function are unknown, however studies in other species/tissues have suggested that GLUT10 has an intracellular localization, possibly to the endoplasmic reticulum [48], or the mitochondrion [49]. One interesting possibility is suggested by the fact that arterial tortuosity syndrome, a heritable connective tissue disorder characterized by elongation and generalized tortuosity of major arteries, is associated with mutations in GLUT10 [50]. At the same time, GLUT10 has been identified as a transporter of dehydroascorbic acid, similar to GLUT1 [48,51], and it is theorized that GLUT10 is responsible for dehydroascorbic acid into the endoplasmic reticulum or mitochondrion and its loss contributes to defects in protein folding and/or increased oxidative stress [48,49]. Whether GLUT10 plays this role in the placenta has yet to be determined.
3.7. GLUT12
The GLUT12 isoform was originally described as an analogue for the insulin-regulatable GLUT4 transporter. It has the di-leucine motifs at the N- and C-termini that characterize GLUT4 and are seen potentially as regions which cause intracellular retention of the transporter in the absence of a stimulus [52]. In addition, it is recruited to the cell surface in response to insulin [53], mitogen signaling through the mTOR pathway [54], estradiol, dihydrotestosterone and epidermal growth factor (EGF) [55,56]. However, the transport mechanism differs from GLUT4 in that GLUT12 glucose transport appears to be coupled to ion movement [56]. Its physiological role is therefore seen less as a backup to GLUT4 and more as complementary to GLUT4. Finally, GLUT12 has been classed as one of the main transporters, along with GLUT1, for the increased glycolytic metabolism characteristic of cancer cells (see Section 6.2) and that activation of p53 will suppress its expression [57].
Given these interesting characteristics, what is its localization and role in the placenta? GLUT12 was found in normal term placenta and in extracts from the JAR, JEG3 and HTR8/SVneo cell lines [58]. Immunohistochemistry revealed the presence of GLUT12 in first trimester syncytiotrophoblast, localized primarily to the cytoplasm. It was also noted in villous cytotrophoblast and extravillous trophoblast. By term, expression was mainly seen in villous vascular smooth muscle cells and villous stromal cells with no apparent syncytial expression [58]. The temporal changes, like those for GLUT4, follow the alterations in insulin receptor expression, leading to the suggestion that GLUT12 may function early in gestation in an insulin-regulatable manner, similar to GLUT4, however the varied nature of its regulation and the ion-coupled nature of the transport mechanism leave open the possibility of other physiological functions.
3.8. Other GLUT mechanisms in the placenta
In addition to the role played in hexose transport, the GLUT family serves another important substrate, the oxidized form of the anti-oxidant vitamin C, dehydroascorbic acid. As humans do not synthesize vitamin C, the recycling of externally-sourced vitamin C is important for multiple redox purposes. The GLUT family of transporters provide for the essential role of dehydroascorbic acid uptake. They do not use the reduced form, ascorbic acid, as a substrate, but rather dehydroascorbic acid; transport via the GLUT family is competitive with glucose and is inhibited by cytochalasin B, a common GLUT family inhibitor of hexose transport [59]. In addition to GLUT1, several other placental GLUT isoforms also transport dehydroascorbic acid, including GLUT3, GLUT4, and GLUT 10; transport via GLUT1 and GLUT3 overshadows the more limited degree of transport observed for GLUT4 and GLUT10 [48,51,59]. The potential localization of the latter to the mitochondrion however may be of particular importance given the organelle’s possible role in the regeneration of the oxidized form, and the anti-oxidant role of ascorbic acid [60,61].
4. Functional consequences of glucose transporter distribution
The current models for the transport of glucose across the placenta identify GLUT1 as the primary transporter. Multiple studies have concluded that, as a result of the asymmetrical distribution of GLUT1 between the maternal-facing (microvillous) and fetal-facing (basal) membranes of the syncytium, the basal membrane is the rate-limiting step in transsyncytial transport [62–64], especially if augmented by GLUT3 on the microvillous membrane. Under these circumstances, changes in the expression of basal membrane GLUT1, as observed in some placental pathologies (see Section 7), can potentially result in significant changes in transplacental glucose transport. One of these studies, examining the effect of basal or microvillous glucose transporter inhibition on transsyncytial glucose transport [62], also verified that there are no separate pools of glucose for transport and metabolism; transsyncytial glucose transport was correlated with cellular glucose consumption.
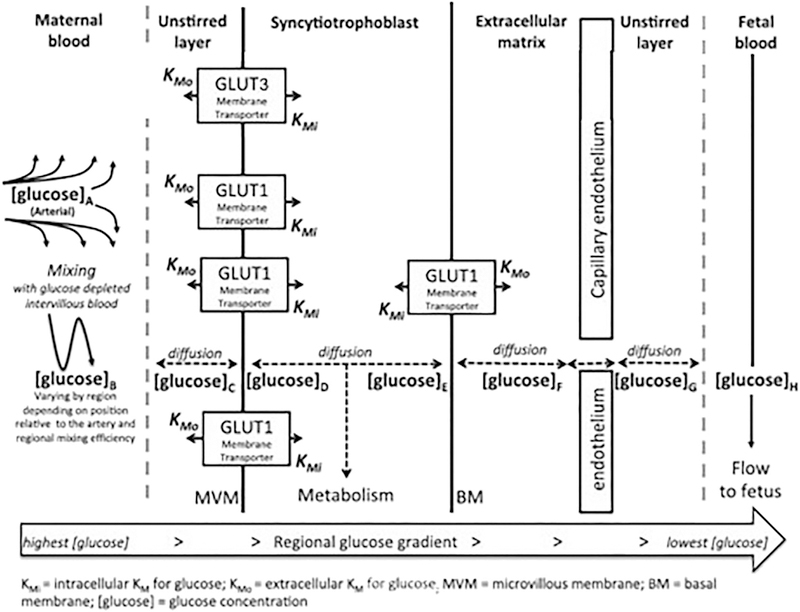
There are other elements which may also have a role to play in glucose transport. Normally, glucose transport is classified as flow-limited, that is that transplacental transport is sufficiently rapid that transfer from mother to fetus is regulated by the rate at which glucose is delivered to the transport site (maternal blood flow) and removed from the site of transfer (fetal blood flow). Using the perfused human placental model, we have shown that maternal flow reduction from 100% (equivalent to 600 mL/min) to 60% reduces glucose transport by ~20%, however a further 40% reduction in flow (from 60% to 20% of the original) reduces glucose transport by a total of 60% [65]. Glucose transport is therefore relatively resistant to initial reductions in maternal flow however, it becomes more susceptible at lower flow rates. On the fetal side, there appears to be little reduction in transfer with reduction in fetal flow rate from the maximal rate (equivalent) of 300 mL/min until it reaches ~100 mL/min, when transfer drops off markedly. Overall, in the absence of substantial reductions in maternal and/or fetal blood flow, other factors such as circulatory mixing in the intervillous space, transporter density, membrane surface area and unstirred layers will have significant effects. Some of these are shown in Fig. 2, adapted from the model of Day et al. [64], which depicts the path of mediated glucose transfer across the placenta.
Fig. 2.
Factors affecting glucose transfer kinetics across the human placenta.
Glucose will diffuse, or be transported by facilitative diffusion transporters from regions of high to low concentration. Maternal diet and hepatic glucose release keep maternal glucose high while fetal consumption reduces fetal levels. Glucose concentrations decrease progressively from the maternal artery to vein and from [glucose]A > [glucose]B > [glucose]C ≫ [glucose]H (note that [glucose]B may vary in different regions of the placenta). The glucose concentration in any region will be determined by the rate at which glucose diffuses out in the fetal direction and the rate at which new glucose diffuses in from the maternal side. Glucose metabolism within the syncytiotrophoblast and uptake by other placental cells will also affect glucose concentrations in specific regions. It should be noted that there is no fixed relationship between the direction of maternal blood flow and blood flow within the villi.
(Adapted, with permission, from Day, PE et al., Placenta 34 (2013), 953–958)
5. Placental glucose transporter regulation
By contrast with the increasingly reliable data on isoform identification and localization, information on the regulation of glucose transporter expression in the placenta is relatively sparse. As might be expected, most of the regulatory data is focused on GLUT1, with some information on GLUT3 and GLUT4. Most studies are focused on the effect of regulatory agents on a single, or perhaps two isoforms. In this section we will deal with data derived from in vitro experimentation using cell lines, primary cells and tissue fragments. In the subsequent sections we will address information derived from in vivo, pathological conditions, where, frequently, there are multiple, overlapping regulatory influences at work. We will also address physiological effectors such as hypoxia and oxidative stress, because while we can mimic them in vitro, they are essentially in vivo conditions, which need to be considered in context.
5.1. Substrates and endocrine effectors
The effects of glucose concentration on GLUT1 transporter expression have been tested in JAR and JEG3 cells. Our data showed that, compared to a normoglycemic concentration of 4 mM, incubation for 24 h. in 1 or 12 mM glucose had no effects on expression or activity [66]. Hahn et al. showed no effects of increased glucose on GLUT1 and GLUT3 in JEG3, however there was an increased expression of GLUT1 and a decreased expression of GLUT3 mRNA in JAR cells incubated in hyperglycemic conditions for 48 or 96 h. [67]; Western blotting showed increases in both GLUT1 and GLUT3. In cultured syncytial cells by contrast both GLUT1 expression and activity were inversely related to the concentration of glucose in which they had been incubated, while fragments showed suppression of GLUT1 expression after incubation with 20 mM glucose, but no effects of 0 or 1 mM glucose [66]. Suppression of GLUT1 expression by incubation in 25 mM glucose was also observed by Li et al. [68]. No information is available on the effects of other substrates such as fructose on placental GLUT expression.
Several reports discuss the effects of circulating factors on the expression of the GLUT family in the human placenta. Those factors include insulin [12,69,70], insulin-like growth factor I (IGF-I) [71], adiponectin [72], resistin [73], leptin, TNF-α [74], hepatocyte growth factor (HGF) [75], glucocorticoids [76,77] and CRH [78].
The first of these agents, insulin, IGF-I, adiponectin, resistin, are generally considered to be factors involved in fetoplacental metabolism and growth, showing changes which are associated with alterations in fetal and placental weight. The two reports on insulin up-regulation of glucose transport at term [69,70] are at odds with the distribution of the insulin receptor, described above, however the third report, showing an increase in glucose transport in first trimester villous fragments [12] is consistent with the presence of both GLUT4 and the insulin receptor in the syncytiotrophoblast. The increased transport of glucose as a result of IGF-I stimulation is matched by increased GLUT1 expression [71]. The correlation between IGF-I and fetal and/or placental weight [79–81] corresponds with the alterations in placental GLUT1 [82–85]. While the response of GLUT3 in term placenta is unknown, expression in BeWo choriocarcinoma cells was upregulated following transfection with IGF-I [40], as was expression of GLUT1, GLUT8 and GLUT9.
The effects of adiponectin on placental function are mediated, in part, through a down-regulation of nutrient transporters [72,86], consistent with the inverse relationship between maternal circulating adiponectin and birth weight [87,88]. Resistin, another adipokine, is released, in the human, by peripheral blood cells, macrophages and others, and is involved not only in insulin resistance (for which it was named), but also in cardiovascular disease, inflammatory diseases and others [89]. It has a modulatory effect on GLUT1 expression, showing a biphasic concentration dependence (stimulatory, followed by inhibitory) in both BeWo cells and primary cytotrophoblast [73]. Leptin and TNF-α have also been tested in BeWo cells, where they demonstrated a concentration-dependent up-regulation of GLUT1 [74]. Hepatocyte growth factor (HGF) can be added to these metabolic factors in view of its effects on glucose uptake and metabolism in pancreatic β-cells, intestine epithelial cells, adipocytes and skeletal muscle cells [90]. In placental explants, HGF stimulates glucose uptake via a cytochalasin B-inhibitable mechanism, correlated with up-regulation of GLUT1 [75]. Like resistin, HGF appears to act to regulate glucose transporter expression via an ERK1/2 mediated pathway [73,75]. It appears therefore that glucose transporters, and thus glucose uptake, are regulated by a broad range of circulating factors which answer to changes in body habitus.
The effects of glucocorticoids on glucose transporter expression, tested in cultured term primary trophoblast cells, showed a dose-dependent suppression of both GLUT1 and GLUT3 mRNA and protein [76]. By contrast, human placental endothelial cells treated with glucocorticoid demonstrated up-regulation of both GLUT1 and GLUT3 mRNA and protein [77].
5.2. Epigenetic effects
Data on the epigenetic regulation of glucose transporters in the placenta is very limited. The extent of promoter methylation of the GLUT family was found to be quite variable, with GLUT1, 4, 6, 8, 11, 12 and 13 showing a minimal degree of methylation (< 10%) [91]. By contrast, GLUT2, 3, 5, 7, 9 and 14 showed greater levels of methylation, ranging from 0.4 to 0.9. The gestational age pattern of methylation was notable for an absence of changes in GLUT1 and GLUT4, suggesting that promoter methylation changes are not involved in the increasing expression of GLUT1 or the decreasing expression of GLUT4 over gestation. However GLUT3 demonstrated an increasing degree of promoter methylation over gestation, consistent with the loss of expression observed over the time course of pregnancy [21]. Two other transporter isoforms, GLUT9 and GLUT10, also showed increased promoter methylation over gestation however the expression time course has not been determined. Analysis of the methylation differences between preeclamptic placenta and age-matched controls revealed a decreased degree of methylation at CpG sites associated with GLUT1 in early onset preeclampsia (i.e. SLC2A1) and a negative correlation between expression and methylation [92]. It is not clear how the decreased methylation observed in preeclampsia and associated with increased GLUT1 expression is compatible with the decreased GLUT1 protein expression demonstrated in syncytial microvillous membranes from preeclamptic pregnancies [84].
5.3. Transporter distribution
Another means by which glucose transport function can be controlled is the distribution of transporters. Glucose transported to the fetus first crosses the microvillous of the syncytial layer then the basal membrane. Much of this transport is via GLUT1, which is asymmetrically distributed between the two syncytial surfaces. This arrangement makes basal membrane transport the rate-limiting step in transsyncytial glucose transfer. The other primary glucose transporter in the syncytium is GLUT3, which is localized exclusively to the microvillous membrane, increasing the asymmetry. This is consistent with other reports describing GLUT3 in polarized epithelia [93], and with the finding by Inukai et al., that GLUT3 targeting to the apical membrane is regulated by a unique cytosolic sorting motif [94]. They also suggested that GLUT1 not only lacks recognized basolateral sorting signals but also appears not to have a basolateral targeting domain, at least in the cytosolic C-terminal region.
In vivo, basal membrane transport controls the extent of transsyncytial transport and thus basal membrane GLUT1 content becomes a crucial regulator of glucose transfer to the fetus. This issue raises the question of how GLUT1 protein is distributed to the microvillous and basal membranes; what mechanism is responsible for the uneven distribution? Membrane protein sorting in the trans-Golgi network involves the hierarchical interaction between different determinants of targeting. Thus basolateral insertion is frequently dependent on a cytoplasmic-domain targeting signal, whereas apical transport occurs in the absence of the basolateral signal and is commonly dependent on glycosylation and/or signals in the membrane-anchoring domain of the protein [95]. Much of the research investigating sorting of membrane proteins in polarized epithelial cells has been concerned with proteins that are targeted exclusively to either the apical or basolateral domain. There is much less known concerning the regulated targeting of the same protein to both apical and basolateral domains, where the levels of protein expression in the respective membranes differ significantly. To explain this type of distribution, it is necessary to invoke something akin to the apical saturation effect postulated by Marmorstein et al. [96], in which target proteins interact with some sort of apical sorting receptor structure prior to incorporation into lipid rafts for transport and insertion into the apical membrane. They predicted that this type of mechanism, having a finite number of sorting receptors, would display a saturation effect, that is, following saturation of the apical transport receptors, the remaining protein would be targeted to the basolateral membrane. With respect to the placenta, in this scenario GLUT1 protein is targeted for transport to the microvillous membrane until some crucial threshold or saturation limit is reached, at which point GLUT1 protein is directed to its secondary target, the basal membrane. The consequence of such a mechanism is that changes in cellular GLUT1 expression manifest primarily as alteration in basal membrane expression, consistent with the in vivo effects observed in diabetes and in chronic hypoxia (see below).
6. Placental glucose transporters in fetoplacental growth and metabolism
6.1. Developmental profiles
Developmentally, the contrasting trajectories of basal membrane GLUT1 and microvillous membrane GLUT3 point to the differing roles of these transporters. Early in gestation, a high-affinity glucose transporter which is highly regulated by hypoxia and less sensitive to other influences (GLUT3) is important in ensuring placental uptake under conditions where glucose delivery to the placental may be more limited. With the development of the maternal intervillous circulation, there is less need for this type of transporter, and more for a high-volume conduit necessary for supplying the growing fetus and responsive to the nutritional environment (GLUT1). The role of GLUT4 (and GLUT12?) early in gestation is not clear, although its recruitment to the microvillous membrane may enhance placental glucose uptake at times when insulin signals a surfeit of maternal nutrient, maximizing growth.
6.2. Metabolic reprogramming in the placenta
Another regulatory aspect connecting glucose transport to fetoplacental growth and metabolism intersects with the pathological responses described below. The exploration of oxygen transfer in high-altitude pregnancies led to some interesting conclusions regarding glucose metabolism. Rather than reducing oxygen delivery to the fetus, the chronic hypoxia of the high-altitude pregnancies resulted in the maintenance of fetal oxygen delivery to the fetus [97], at the cost of reduced fetal glucose transfer [31]. Chronic hypoxia led to a decrease in placental oxidative metabolism and a significant increase in placental glucose metabolism, substituting energy generation from glycolysis for oxidative metabolism [98]. This “aerobic glycolysis”, also known as metabolic reprogramming and widely recognized in cancer, is a substantial metabolic re-orientation of the cell, mediated by hypoxia-inducible factor-1 (HIF-1), which includes multiple alterations in oxidative and glycolytic systems [99,100]. HIF-1-mediated up-regulation of glucose transporters is normally part of the metabolic reprogramming response, intended to maximize glucose uptake to compensate for the increased glycolytic rate. However, in the placenta, we confirmed our earlier observations, that in conditions of chronic hypoxia, basal membrane GLUT1 expression is actually decreased and fetal delivery of glucose drops, leading to reduced fetal insulin secretion [31]. This suggests that the overriding drive, under conditions of inadequate oxygenation, is to reduce fetal growth to match nutrient supply, possibly via changes in insulin and the insulin-like growth factors. We speculate that under conditions of chronic hypoxia, microvillous GLUT1 expression may be saturated and glucose transport could only be increased further by additional GLUT3 expression, a possibility which remains to be explored.
7. Glucose transporters in pregnancy pathologies
As should be apparent from the preceding sections, the patterns of expression of glucose transporters in the human placenta reveal a complex series of processes, regulated not only via epigenetic changes but also by nutritional, endocrine and metabolic inputs. Under these circumstances, it is not surprising that alteration of placental glucose transporter expression has been reported in pregnancy complications such as diabetes, intrauterine growth restriction (IUGR) or preeclampsia.
7.1. Diabetes
In pregnancies affected by insulin-dependent diabetes mellitus, GLUT1 expression in syncytial basal membrane (BM) fractions correlates positively with fetal birth weight, whereas GLUT1 expression in microvillous membranes (MVM) is not altered [82,83]. Whereas both groups reporting this effect showed changes in basal membrane GLUT1 expression in the pre-gestational diabetics, they differed with respect to the gestational diabetics. A second report from Jansson confirmed their findings with pregestational diabetics, although their population combined subjects controlled by diet alone with those requiring insulin for glycemic control [101]. Nevertheless, there is still some question regarding pregestational diabetics. Taricco et al. showed a clear increase in placental weight in their study of gestational diabetic pregnancies [102], whereas those in the GLUT1 studies described above [101] were not increased. Another study which differentiated between diet- and diet plus insulin-controlled gestational diabetics, showed an increase in GLUT1 only in the latter [103].
What seems likely from these studies is that conditions earlier in pregnancy, prior to the accepted early third-trimester point for diagnosis of gestational diabetes, may have a lasting influence on parameters such as GLUT1 expression and placental weight in the third trimester, despite tight glycemic control in the last 8–10 weeks of pregnancy. The role of glucose transporters other than GLUT1 in diabetic pregnancies is unclear. In pregnancies complicated by gestational and pregestational diabetes, increases in placental GLUT1, GLUT4, and GLUT9 were reported from immunohistochemical measurements, indicating that in diabetic pregnancies these transporters may be involved in the modulation of fetal growth [13]. These findings contrast with the study by Colomiere et al. [103], who showed a decrease in both protein and mRNA expression of the GLUT4 transporter in insulin-controlled GDM compared with normal controls. Other investigations have previously shown an absence of changes in GLUT3 and GLUT4 in both macrosomia and diabetes [104].
The functional effect of the increase in GLUT1 on the basal membrane of the syncytiotrophoblast will be to increase the rate of transsyncytial transfer. In the presence of maternal hyperglycemia, this will generate an increase in fetal glucose levels, potentially stimulating a response from the fetal pancreatic β-cells earlier than normal and contributing to fetal (over)growth. Even in the absence of maternal hyperglycemia, the increased expression of basal membrane GLUT1 may continue to produce greater levels of glucose transfer than would be observed in the normoglycemic controls. The roles of GLUT4, and possibly, GLUT12 are more difficult to assess. Early in gestation, prior to diagnosis of gestational diabetes, it is possible that maternally-induced insulin stimulation may produce recruitment of glucose transporters to the syncytiotrophoblast, however there is no information on the targeting of GLUT4 in polarized cells. It is possible that these isoforms are recruited to the microvillous membrane by insulin, however it is probable that there will be little effect, in light of the substantial expression of GLUT1 on the microvillous membrane [9]. If GLUT4 is recruited to the basal membrane, as implied by some of the early GLUT4 targeting investigations [105,106], then early maternal hyperinsulinemia may be responsible for increased transporter expression at the rate-limiting step, a stimulatory process which fades with the relocalization of the insulin receptor away from the microvillous membrane, but consistent with the early gestational changes proposed for gestational diabetes. This also raises the question of mechanism for the effects of pre-gestational diabetes on GLUT expression. As the subjects are being treated for diabetes before, and presumably, during early pregnancy, it is not clear why these cases, presumably with better glycemic control than the as-yet undiagnosed gestational diabetics, should show such clear stimulation of syncytial basal membrane GLUT1. Perhaps maternal hyperinsulinemia, in the metabolically-changing environment of early pregnancy, stimulates glucose transporter expression directly, leading to fetal hyperglycemia and the subsequent placental response.
7.2. Intrauterine growth restriction
Intrauterine growth restriction (IUGR) is a complex pathology, not the least because of the problems with both diagnosis and co-morbidity. The definition as “a fetus which does not meet its growth potential” is straightforward and this is best determined by serial measurements of fetal growth, where sustained and increasing deviation from the gestational growth trajectory, whether at the 5th or 50th centile, is the appropriate measure [107]. However, it is not always possible to make such measurements and researchers must often instead rely on a single cross-sectional measurement of growth centile, defining IUGR as fetuses whose birth weight is less than 3rd, 5th or 10th centile for gestational age. Inherent in such a definition is the problem that there will be fetuses with birth weights at or under these cut-offs which are physiologically normal and should be regarded as small-for-gestational-age (SGA) rather than pathologically defined as IUGR. The problem is distinguishing between these two alternatives; in addition, co-morbidities such as preeclampsia or diabetes, or the presence of chronic hypoxia complicate the stratification. Classification schemes such as that suggested by the Milan group [107], or other additional measures (e.g. Doppler ultrasound measures of umbilical blood flow) go some way towards ameliorating this challenge.
Previously, we reported that expression of GLUT1 protein in syncytial membrane fractions from term and preterm IUGR placentas were unaltered when compared to normal placenta [9]. This is consistent with another study demonstrating that IUGR is not associated with changes in activity or expression of GLUT1 in syncytial membranes [108]. This suggests that the fetal hypoglycemia frequently observed in IUGR [109,110], is not due to a decrease in placental glucose transporter expression or altered glucose transport capacity but may be related to reduced blood flow and/or increased placental glucose consumption [31]. In other studies, while GLUT1 and GLUT4 have been unchanged, GLUT3 mRNA and protein have been shown to be increased in IUGR, although it was suggested that the increased GLUT3 was localized to cytotrophoblast, rather than syncytiotrophoblast cells [22]. The authors of this study suggested that this increase within late-term IUGR cytotrophoblasts leads to an increase of glucose consumption by the placenta itself, leading to a decreased transplacental glucose transport capacity to the fetus. It is also possible that hypoxia-stimulated increases in glucose metabolism throughout the placenta might produce the same effects, including the up-regulation of GLUT3. The same authors also noted an increase in GLUT8 in the cytoplasmic compartment of extravillous trophoblast from IUGR pregnancies.
It is important to note that there are several etiologies associated with IUGR. Beyond blood flow reduction, IUGR is associated with hypertension (especially preeclampsia), hypoxia, deficient supply of nutrients, smoking, malnutrition, anemia, drug abuse etc. While the first two are discussed below, little is known of the effects of the other etiologies on glucose transporter expression and function. Placental malaria is also associated with intervillositis and IUGR. Chandrasiri et al. reported that GLUT1 expression in basal membrane fractions correlated negatively with monocyte infiltrate density and positively with birth weight suggesting that the reduction of GLUT1 density in basal membrane is responsible for impaired fetal growth in this condition [111].
7.3. Hypoxia
It is difficult to separate out hypoxia as a pathology because, in vivo, it is almost always part of another pathology. Nevertheless, it is important, if possible, to isolate and understand the effects of hypoxia as a specific entity precisely because it is usually overlaid with other influences. There is strong in vitro evidence to show that hypoxia is capable of modulating placental glucose transporter expression. We have shown up-regulation of both GLUT1 and GLUT3 in hypoxia-exposed model trophoblast cells, the BeWo choriocarcinoma line, and shown also that this is mediated by HIF-1 [29]. In the same report while hypoxia-treated villous explants (6 h, 3% O2) demonstrated no changes in GLUT1 protein, treatment with the proteasome inhibitor MG-132 (inhibiting HIF-1alpha degradation) did reveal a degree of GLUT1 upregulation. These results confirm and expand on other reports demonstrating up-regulation of GLUT1 and GLUT3 [28], mediated by HIF-1 [30].
As it is essentially impossible to study hypoxia in pathological conditions in vivo without interference from other effectors, researchers have taken advantage of an experiment of nature, human residence at high altitude to investigate the effects of hypoxia in pregnancy. High altitude residence is correlated with a reduction in birth weight, modified by time of residence at altitude and ethnicity [112,113]. At the same time as demonstrating fetal and placental hypoxia in high altitude pregnancies, we showed a reduction in the expression of syncytial basal membrane GLUT1 of 40% at 3100 m altitude [85], confirmed by a 46% reduction in a later study at 3600 m [31]. This raises the very obvious question of the conflicting in vitro and in vivo results [28–31,85]. There seems to be no question that hypoxia alone leads to elevation of GLUT1 and GLUT3 expression via the HIF-1 mediated mechanism, which makes it clear that there are other factors operating in vivo, opposing the hypoxia-stimulated increase in expression. These will include those factors which restrain fetal growth in the face of nutrient challenge. Thus, the decreased level of fetal insulin [31], which will have the effect of decreasing fetal IGF-I secretion, and the increased production of maternal circulating insulin-like growth factor binding protein 1 (IGFBP1) [114], which will reduce maternal circulating IGF-I, are both factors which will act to oppose the effects of hypoxia. This confluence of effectors is, of course, a probable circumstance for most pathologies, making it exceedingly difficult to recreate in vitro.
7.4. Preeclampsia
Published recently, the only report to address glucose transporters in this pathology showed that, compared to controls, GLUT1 protein expression is down-regulated on the microvillous membrane of the syncytiotrophoblast in preeclampsia. This corresponds with a decrease in glucose transport capacity across the microvillous membrane of ~40% [84]. In contrast, basal membrane GLUT1 protein expression was unchanged and glucose uptake into basal membrane microvesicles showed no differences between the preeclampsia and control groups. The functional consequence of this change is unknown. The maternal blood glucose levels reported in these subjects were not different from control, although intervillous glucose concentration may not be the same. It is possible that this large a reduction in transporter density on the microvillous membrane might result in the equivalent of a decreased intrasyncytial glucose level, reducing the gradient across the basal membrane and reducing transsyncytial transport. In the absence of knowledge about the complex situation with respect to the phosphorylation and dephosphorylation of glucose transiting the syncytiotrophoblast, it is difficult to model these effects. It seems possible however, judging from the data reported by Vardhana and Illsley, using the BeWo model [62], that a decrease in microvillous glucose transporter activity of this magnitude might lead to reduced transsyncytial transport. The GLUT1 changes reported in preeclampsia are at variance with the microvillous/basal targeting and distribution idea postulated in 5.3, which would have predicted almost complete loss of basal membrane GLUT1 prior to loss at the microvillous membrane. As an alternative, it is possible to see that the “receptors” targeting GLUT1 to the microvillous membrane might be reduced or impaired, leading to a decreased microvillous complement of GLUT1 at saturation, followed by diversion of the remaining GLUT1 to the basal membrane.
Another facet of preeclampsia is the increased level of oxidative stress observed both in the placenta and in circulating agents such as lipid peroxides [115,116]. Oxidative stress has been shown to down-regulate glucose transporters, however there may be multiple mechanisms for these effects. Incubation in high glucose concentrations, which is associated with increased lipid peroxide production, produced a decrease in GLUT1 mRNA in primary cultured trophoblast [68.]. In experiments where an oxidative stress-inducing agent was incubated with BeWo cells, cytochalasin B-inhibitable uptake of glucose was reduced but GLUT1 mRNA was not altered [117]. Using a hypoxanthine/xanthine oxidase system, which generates reactive oxygen species, Lappas and colleagues showed a down-regulation of GLUT1 mRNA and protein with no effects on GLUT3 (mRNA and protein) or GLUT4 mRNA [118]. Other studies have suggested that reactive oxygen species will stabilize HIF-1alpha [119,120], however as the trophoblast response to HIF-1 stimulation is up-regulation of glucose transporter expression [29,30], this is at odds with the results of oxidative stress cited above and suggests that this is not the mechanism of oxidative stress. The differential effects of antioxidant agents in these cases suggest different mechanisms, both of action and inhibition.
Whether the preeclampsia-related changes in glucose transporter expression play a role in the development of IUGR, a severe complication in preeclampsia, remains to be elucidated by further studies. It should be noted however that hypoxia and oxidative stress are important components of preeclampsia and each, as well as the effects of altered growth factor secretion, may have a role to play in the modulation of glucose transporter expression and distribution in preeclampsia.
7.5. Functional effects in pregnancy pathologies
While not the only argument in favor of fetoplacental adaptation to environment, the up-regulation of basal membrane GLUT1 in diabetic pregnancy [82,83], a condition of higher nutrient sufficiency, suggests that increased nutrient availability is a stimulus to growth. Conversely, the down-regulation of basal membrane GLUT1 observed under hypoxic conditions [31,85], implies that a fetoplacental system which limits growth in the face of an inadequate environment. The absence of changes in IUGR is puzzling [9,108], however one of the constant problems with IUGR is determination of cause, and the studies investigating GLUT expression in IUGR, including our own, did not determine etiology, but rather applied birthweight criteria. Thus, the data from these studies may have resulted from more than one etiology; hypertensive disease is frequently mentioned as a co-morbidity. It would be interesting to measure transporter expression in IUGR cases where a non-hypertensive reduction in maternal blood flow was identified as causative. It is notable in this regard, that the study measuring GLUT1 in preeclampsia did so in cases where the fetuses in the pathological group, while born early (31 weeks gestational age), were not IUGR [84]. In this report the preeclamptics showed a diminished microvillous GLUT1 which may have been sufficient to reduce transport across the microvillous membrane and decrease the glucose gradient across the basal membrane.
8. Conclusions
Although we have been aware of protein-mediated transport of glucose in the placenta for many years, our knowledge of transporter localization and function is thin. For example, the first detailed examination of glucose transporters in preeclampsia was only published in 2017 [84]. Coupling multiple regulators to transporter responses in vitro will continue to pose problems, although the advent of models such as “placenta-on-a-chip” [121], may significantly improve our investigative abilities. Meanwhile, dissection of in vivo mechanisms will, as previously, continue to be deviled by questions of definition and comorbidity as well as the ever-present problem of human variability. Nevertheless, we are beginning to see the response of glucose transporters to adverse conditions less as isolated events, and more as part of a network of elements which adjust metabolism and fetoplacental growth to those conditions in order to maximize intact fetal survival.
Acknowledgments
This work was part of qualification for doctoral candidacy, thus, I would like to especially thank my qualifying committee, Drs. Pantin, Perrino, and Prado, for their encouragement and support.
Footnotes
This article is part of a Special Issue entitled: Membrane Transporters and Receptors in Pregnancy Metabolic Complications edited by Luis Sobrevia.
Transparency document
The Transparency document associated with this article can be found, in online version.
References
- [1].Illsley NP, Glucose transporters in the human placenta, Placenta 21 (2000) 14–22. [DOI] [PubMed] [Google Scholar]
- [2].Augustin R, The protein family of glucose transport facilitators: It’s not only about glucose after all, IUBMB Life 62 (2010) 315–333. [DOI] [PubMed] [Google Scholar]
- [3].Thorens B, Mueckler M, Glucose transporters in the 21st century, Am. J. Physiol. Endocrinol. Metab 298 (2010) E141–E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mueckler M, Thorens B, The SLC2 (GLUT) family of membrane transporters, Mol. Asp. Med 34 (2013) 121–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Deng D, Yan N, GLUT, SGLT, and SWEET: structural and mechanistic investigations of the glucose transporters, Protein Sci. 25 (2016) 546–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Witkowska K, Smith KM, Yao SY, Ng AM, O’Neill D, Karpinski E, Young JD, Cheeseman CI, Human SLC2A9a and SLC2A9b isoforms mediate electrogenic transport of urate with different characteristics in the presence of hexoses, Am. J. Physiol. Ren. Physiol 303 (2012) F527–F539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pujol-Gimenez J, Barrenetxe J, Gonzalez-Muniesa P, Lostao MP, The facilitative glucose transporter GLUT12: what do we know and what would we like to know? J. Physiol. Biochem 69 (2013) 325–333. [DOI] [PubMed] [Google Scholar]
- [8].Ohno H, Nakatsu Y, Sakoda H, Kushiyama A, Ono H, Fujishiro M, Otani Y, Okubo H, Yoneda M, Fukushima T, Tsuchiya Y, Kamata H, Nishimura F, Kurihara H, Katagiri H, Oka Y, Asano T, 4F2hc stabilizes GLUT1 protein and increases glucose transport activity, Am. J. Phys 300 (2011) C1047–C1054. [DOI] [PubMed] [Google Scholar]
- [9].Jansson T, Wennergren M, Illsley NP, Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation, J. Clin. Endocrinol. Metab 77 (1993) 1554–1562. [DOI] [PubMed] [Google Scholar]
- [10].Jansson T, Cowley EA, Illsley NP, Cellular localization of glucose transporter messenger RNA in human placenta, Reprod. Fertil. Dev 7 (1995) 1425–1430. [DOI] [PubMed] [Google Scholar]
- [11].Barros LF, Yudilevich DL, Jarvis SM, Beaumont N, Baldwin SA, Quantitation and immunolocalization of glucose transporters in the human placenta, Placenta 16 (1995) 623–633. [DOI] [PubMed] [Google Scholar]
- [12].Ericsson A, Hamark B, Powell TL, Jansson T, Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta, Hum. Reprod 20 (2005) 521–530. [DOI] [PubMed] [Google Scholar]
- [13].Stanirowski PJ, Szukiewicz D, Pyzlak M, Abdalla N, Sawicki W, Cendrowski K, Impact of pre-gestational and gestational diabetes mellitus on the expression of glucose transporters GLUT-1, GLUT-4 and GLUT-9 in human term placenta, Endocrine 55 (2017) 799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Teasdale F, Jean-Jacques G, Intrauterine growth retardation: morphmetry of the microvillous membrane of the human placenta, Placenta 9 (1988) 47–55. [DOI] [PubMed] [Google Scholar]
- [15].Teasdale F, Jean-Jacques G, Morphometric evaluation of the microvillous surface enlargement factor in the human placenta from mid-gestation to term, Placenta 6 (1985) 25–31. [DOI] [PubMed] [Google Scholar]
- [16].Sakata M, Kurachi H, Imai T, Tadokoro C, Yamaguchi M, Yoshimoto Y, Oka Y, Miyake A, Increase in human placental glucose transporter-1 during pregnancy, Eur. J. Endocrinol 132 (1995) 206–212. [DOI] [PubMed] [Google Scholar]
- [17].Hauguel-de Mouzon S, Leturque A, Alsat E, Loizeau M, Evain-Brion D, Girard J, Developmental expression of Glut1 glucose transporter and c-fos genes in human placental cells, Placenta 15 (1994) 35–46. [DOI] [PubMed] [Google Scholar]
- [18].Hauguel-de Mouzon S, Challier JC, Kacemi A, Cauzac M, Malek A, Girard J, The GLUT3 glucose transporter isoform is differentially expressed within human placental cell types, J. Clin. Endocrinol. Metab 82 (1997) 2689–2694. [DOI] [PubMed] [Google Scholar]
- [19].Arnott G, Coghill G, McArdle HJ, Hundal HS, Immunolocalization of GLUT1 and GLUT3 glucose transporters in human placenta, Biochem. Soc. Trans 22 (1994) 272S. [DOI] [PubMed] [Google Scholar]
- [20].Clarson LH, Glazier JD, Sides MK, Sibley CP, Expression of the facilitated glucose transporters (GLUT1 and GLUT3) by a choriocarcinoma cell line (JAr) and cytotrophoblast cells in culture, Placenta 18 (1997) 333–340. [DOI] [PubMed] [Google Scholar]
- [21].Brown K, Heller DS, Zamudio S, Illsley NP, Glucose transporter 3 (GLUT3) protein expression in human placenta across gestation, Placenta 32 (2011) 1041–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU, Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction, Placenta 34 (2013) 1072–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Eaton BM, Leach L, Firth JA, Permeability of the fetal villous microvasculature in the isolated perfused term human placenta, J. Physiol 463 (1993) 141–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Leach L, Firth JA, Advances in understanding permeability in fetal capillaries of the human placenta: a review of organization of the endothelial paracellular clefts and their junctional complexes, Reprod. Fertil. Dev 7 (1995) 1451–1456. [DOI] [PubMed] [Google Scholar]
- [25].Leach L, Firth JA, Structure and permeability of human placental microvasculature, Microsc. Res. Tech 38 (1997) 137–144. [DOI] [PubMed] [Google Scholar]
- [26].Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ, The facilitative glucose transporter GLUT3: 20 years of distinction, Am. J. Physiol. Endocrinol. Metab 295 (2008) E242–E253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Burton GJ, Hempstock J, Jauniaux E, Nutrition of the human fetus during the first trimester—a review, Placenta 22 (Suppl A) (2001) S70–S77. [DOI] [PubMed] [Google Scholar]
- [28].Esterman A, Greco MA, Mitani Y, Finlay TH, Ismail-Beigi F, Dancis J, The effect of hypoxia on human trophoblast in culture: morphology, glucose transport and metabolism, Placenta 18 (1997) 129–136. [DOI] [PubMed] [Google Scholar]
- [29].Baumann MU, Zamudio S, Illsley NP, Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1, Am. J. Phys 293 (2007) C477–C485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hayashi M, Sakata M, Takeda T, Yamamoto T, Okamoto Y, Sawada K, Kimura A, Minekawa R, Tahara M, Tasaka K, Murata Y, Induction of glucose transporter 1 expression through hypoxia-inducible factor 1alpha under hypoxic conditions in trophoblast-derived cells, J. Endocrinol 183 (2004) 145–154. [DOI] [PubMed] [Google Scholar]
- [31].Zamudio S, Torricos T, Fik E, Oyala M, Echalar L, Pullockaran J, Tutino E, Martin B, Belliappa S, Balanza E, Illsley NP, Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth, PLoS One 5 (2010) e8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Xing AY, Challier JC, Leperq L, Caüzac M, Charron MJ, Girard J, Hauguel-de Mouzon S, Unexpected expression of glucose transporter 4 in villous stromal cells of human placenta, J. Clin. Endocrinol. Metab 83 (1998) 4097–4101. [DOI] [PubMed] [Google Scholar]
- [33].Desoye G, Hartmann M, Jones CJ, Wolf HJ, Kohnen G, Kosanke G, Kaufmann P, Location of insulin receptors in the placenta and its progenitor tissues, Microsc. Res. Tech 38 (1997) 63–75. [DOI] [PubMed] [Google Scholar]
- [34].Desoye G, Hartmann M, Blaschitz A, Dohr G, Hahn T, Kohnen G, Kaufmann P, Insulin receptors in syncytiotrophoblast and fetal endothelium of human placenta. Immunohistochemical evidence for developmental changes in distribution pattern, Histochemistry 101 (1994) 277–285. [DOI] [PubMed] [Google Scholar]
- [35].Hiden U, Glitzner E, Hartmann M, Desoye G, Insulin and the IGF system in the human placenta of normal and diabetic pregnancies, J. Anat 215 (2009) 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Janzen C, Lei MYY, Jeong ISD, Ganguly A, Sullivan P, Paharkova V, Capodanno G, Nakamura H, Perry A, Shin BC, Lee KW, Devaskar SU, Humanin (HN) and glucose transporter 8 (GLUT8) in pregnancies complicated by intrauterine growth restriction, PLoS One 13 (2018) e0193583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Membrez M, Hummler E, Beermann F, Haefliger JA, Savioz R, Pedrazzini T, Thorens B, GLUT8 is dispensable for embryonic development but influences hippocampal neurogenesis and heart function, Mol. Cell. Biol 26 (2006) 4268–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Widmer M, Uldry M, Thorens B, GLUT8 subcellular localization and absence of translocation to the plasma membrane in PC12 cells and hippocampal neurons, Endocrinology 146 (2005) 4727–4736. [DOI] [PubMed] [Google Scholar]
- [39].Carayannopoulos MO, Chi MM, Cui Y, Pingsterhaus JM, McKnight RA, Mueckler M, Devaskar SU, Moley KH, GLUT8 is a glucose transporter responsible for insulin-stimulated glucose uptake in the blastocyst, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 7313–7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jones HN, Crombleholme T, Habli M, Adenoviral-mediated placental gene transfer of IGF-1 corrects placental insufficiency via enhanced placental glucose transport mechanisms, PLoS One 8 (2013) e74632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Caulfield MJ, Munroe PB, O’Neill D, Witkowska K, Charchar FJ, Doblado M, Evans S, Eyheramendy S, Onipinla A, Howard P, Shaw-Hawkins S, Dobson RJ, Wallace C, Newhouse SJ, Brown M, Connell JM, Dominiczak A, Farrall M, Lathrop GM, Samani NJ, Kumari M, Marmot M, Brunner E, Chambers J, Elliott P, Kooner J, Laan M, Org E, Veldre G, Viigimaa M, Cappuccio FP, Ji C, Iacone R, Strazzullo P, Moley KH, Cheeseman C, SLC2A9 is a high-capacity urate transporter in humans, PLoS Med. 5 (2008) e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Manolescu AR, Augustin R, Moley K, Cheeseman C, A highly conserved hydrophobic motif in the exofacial vestibule of fructose transporting SLC2A proteins acts as a critical determinant of their substrate selectivity, Mol. Membr. Biol 24 (2007) 455–463. [DOI] [PubMed] [Google Scholar]
- [43].Augustin R, Carayannopoulos MO, Dowd LO, Phay JE, Moley JF, Moley KH, Identification and characterization of human glucose transporter-like protein-9 (GLUT9): alternative splicing alters trafficking, J. Biol. Chem 279 (2004) 16229–16236. [DOI] [PubMed] [Google Scholar]
- [44].Bibee K, Illsley NP, Moley KH, Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies, Reprod. Sci 18 (2011) 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW, Sequence and functional analysis of GLUT10: a glucose transporter in the type 2 diabetes-linked region of chromosome 20q12–13.1, Mol. Genet. Metab 74 (2001) 186–199. [DOI] [PubMed] [Google Scholar]
- [46].McVie-Wylie AJ, Lamson DR, Chen YT, Molecular cloning of a novel member of the GLUT family of transporters, SLC2a10 (GLUT10), localized on chromosome 20q13.1: a candidate gene for NIDDM susceptibility, Genomics 72 (2001) 113–117. [DOI] [PubMed] [Google Scholar]
- [47].Novakovic B, Gordon L, Robinson WP, Desoye G, Saffery R, Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation, J. Nutr. Biochem. 24 (2013) 282–288. [DOI] [PubMed] [Google Scholar]
- [48].Nemeth CE, Marcolongo P, Gamberucci A, Fulceri R, Benedetti A, Zoppi N, Ritelli M, Chiarelli N, Colombi M, Willaert A, Callewaert BL, Coucke PJ, Grof P, Nagy SK, Meszaros T, Banhegyi G, Margittai E, Glucose transporter type 10-lacking in arterial tortuosity syndrome-facilitates dehydroascorbic acid transport, FEBS Lett 590 (2016) 1630–1640. [DOI] [PubMed] [Google Scholar]
- [49].Syu YW, Lai HW, Jiang CL, Tsai HY, Lin CC, Lee YC, GLUT10 maintains the integrity of major arteries through regulation of redox homeostasis and mitochondrial function, Hum. Mol. Genet 27 (2018) 307–321. [DOI] [PubMed] [Google Scholar]
- [50].Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GM, Kambouris M, Gardella R, Facchetti F, Willems PJ, Forsyth R, Dietz HC, Barlati S, Colombi M, Loeys B, De Paepe A, Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome, Nat. Genet 38 (2006) 452–457. [DOI] [PubMed] [Google Scholar]
- [51].Segade F, Glucose transporter 10 and arterial tortuosity syndrome: the vitamin C connection, FEBS Lett 584 (2010) 2990–2994. [DOI] [PubMed] [Google Scholar]
- [52].Rogers S, Macheda ML, Docherty SE, Carty MD, Henderson MA, Soeller WC, Gibbs EM, James DE, Best JD, Identification of a novel glucose transporter-like protein-GLUT-12, Am. J. Physiol. Endocrinol. Metab 282 (2002) E733–E738. [DOI] [PubMed] [Google Scholar]
- [53].Stuart CA, Howell ME, Zhang Y, Yin D, Insulin-stimulated translocation of glucose transporter (GLUT) 12 parallels that of GLUT4 in normal muscle, J. Clin. Endocrinol. Metab 94 (2009) 3535–3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wilson-O’Brien AL, Dehaan CL, Rogers S, Mitogen-stimulated and rapamycin-sensitive glucose transporter 12 targeting and functional glucose transport in renal epithelial cells, Endocrinology 149 (2008) 917–924. [DOI] [PubMed] [Google Scholar]
- [55].Macheda ML, Rogers S, Best JD, Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer, J. Cell. Physiol 202 (2005) 654–662. [DOI] [PubMed] [Google Scholar]
- [56].Pujol-Gimenez J, Perez A, Reyes AM, Loo DD, Lostao MP, Functional characterization of the human facilitative glucose transporter 12 (GLUT12) by electrophysiological methods, Am. J. Phys 308 (2015) C1008–C1022. [DOI] [PubMed] [Google Scholar]
- [57].Zawacka-Pankau J, Grinkevich VV, Hunten S, Nikulenkov F, Gluch A, Li H, Enge M, Kel A, Selivanova G, Inhibition of glycolytic enzymes mediated by pharmacologically activated p53: targeting Warburg effect to fight cancer, J. Biol. Chem 286 (2011) 41600–41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, Erwich JJHM, Timmer A, King RG, GLUT12 expression in human placenta in first trimester and term, Placenta 24 (2003) 566–570. [DOI] [PubMed] [Google Scholar]
- [59].Rivas CI, Zuniga FA, Salas-Burgos A, Mardones L, Ormazabal V, Vera JC, Vitamin C transporters, J. Physiol. Biochem 64 (2008) 357–375. [DOI] [PubMed] [Google Scholar]
- [60].Li X, Cobb CE, Hill KE, Burk RF, May JM, Mitochondrial uptake and recycling of ascorbic acid, Arch. Biochem. Biophys 387 (2001) 143–153. [DOI] [PubMed] [Google Scholar]
- [61].May JM, Li L, Qu ZC, Cobb CE, Mitochondrial recycling of ascorbic acid as a mechanism for regenerating cellular ascorbate, Biofactors 30 (2007) 35–48. [DOI] [PubMed] [Google Scholar]
- [62].Vardhana PA, Illsley NP, Transepithelial glucose transport and metabolism in BeWo choriocarcinoma cells, Placenta 23 (2002) 653–660. [DOI] [PubMed] [Google Scholar]
- [63].Barta E, Drugan A, Glucose transport from mother to fetus—a theoretical study, J. Theor. Biol 263 (2010) 295–302. [DOI] [PubMed] [Google Scholar]
- [64].Day PE, Cleal JK, Lofthouse EM, Hanson MA, Lewis RM, What factors determine placental glucose transfer kinetics? Placenta 34 (2013) 953–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Illsley NP, Hall S, Stacey TE, The modulation of glucose transfer across the human placenta by intervillous flow rates: an invitro perfusion study, Trophoblast Res. 2 (1987) 535–544. [Google Scholar]
- [66].Illsley NP, Sellers MC, Wright RL, Glycaemic regulation of glucose transporter expression and activity in the human placenta, Placenta 19 (1998) 517–524. [DOI] [PubMed] [Google Scholar]
- [67].Hahn T, Barth S, Hofman W, Reich O, Lang I, Desoye G, Hyperglycemia regulates the glucose-transport system of clonal choriocarcinoma cells in vitro. A potential molecular mechanism contributing to the adjunct effect of glucose in tumor therapy, Int. J. Cancer 78 (1998) 353–360. [DOI] [PubMed] [Google Scholar]
- [68].Li H, Gu Y, Zhang Y, Lucas MJ, Wang Y, High glucose levels down-regulate glucose transporter expression that correlates with increased oxidative stress in placental trophoblast cells in vitro, J. Soc. Gynecol. Investig 11 (2004) 75–81. [DOI] [PubMed] [Google Scholar]
- [69].Brunette MG, Lajeunesse D, Leclerc M, Lafond J, Effect of insulin on D-glucose transport by human placental brush border membranes, Mol. Cell. Endocrinol 69 (1990) 59–68. [DOI] [PubMed] [Google Scholar]
- [70].Acevedo CG, Marquez JL, Rojas S, Bravo I, Insulin and nitric oxide stimulates glucose transport in human placenta, Life Sci 76 (2005) 2643–2653. [DOI] [PubMed] [Google Scholar]
- [71].Baumann MU, Schneider H, Malek A, Palta V, Surbek DV, Sager R, Zamudio S, Illsley NP, Regulation of human trophoblast GLUT1 glucose transporter by insulin-like growth factor I (IGF-I), PLoS One 9 (2014) e106037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Duval F, Dos Santos E, Maury B, Serazin V, Fathallah K, Vialard F, Dieudonne MN, Adiponectin regulates glycogen metabolism at the human fetal-maternal interface, J. Mol. Endocrinol 61 (2018) 139–152. [DOI] [PubMed] [Google Scholar]
- [73].Di Simone N, Di Nicuolo F, Marzioni D, Castellucci M, Sanguinetti M, D’Lppolito S, Caruso A, Resistin modulates glucose uptake and glucose transporter-1 (GLUT-1) expression in trophoblast cells, J. Cell. Mol. Med 13 (2009) 388–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Orlando MJ Jr, Adipokine Regulation of Human Placental GLUT-1 Transporter, BS Honors Thesis University of Florida, 2018, http://ufdc.ufl.edu/AA00063220/00001. [Google Scholar]
- [75].Visiedo F, Bugatto F, Carrasco-Fernandez C, Saez-Benito A, Mateos RM, Cozar-Castellano I, Bartha JL, Perdomo G, Hepatocyte growth factor is elevated in amniotic fluid from obese women and regulates placental glucose and fatty acid metabolism, Placenta 36 (2015) 381–388. [DOI] [PubMed] [Google Scholar]
- [76].Hahn T, Barth S, Graf R, Engelman M, Beslagic D, Reul J, H. F, G. Dohr, G. Desoye, placental glucose transporter expression is regulated by glucocorticoids, J. Clin. Endocrinol. Metab 84 (1999) 1445–1452. [DOI] [PubMed] [Google Scholar]
- [77].Kipmen-Korgun D, Ozmen A, Unek G, Simsek M, Demir R, Korgun ET, Triamcinolone up-regulates GLUT 1 and GLUT 3 expression in cultured human placental endothelial cells, Cell Biochem. Funct 30 (2012) 47–53. [DOI] [PubMed] [Google Scholar]
- [78].Gao L, Lv C, Xu C, Li Y, Cui X, Gu H, Ni X, Differential regulation of glucose transporters mediated by CRH receptor type 1 and type 2 in human placental trophoblasts, Endocrinology 153 (2012) 1464–1471. [DOI] [PubMed] [Google Scholar]
- [79].Giudice LC, De Zegher F, Gargosky SE, Dsupin BA, De Las Fuentes L, Crystal RA, Hintz RL, Rosenfeld RG, Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremesof intrauterine growth, J. Clin. Endocrinol. Metab 80 (1995) 1548–1555. [DOI] [PubMed] [Google Scholar]
- [80].Klauwer D, Blum WF, Hanitsch S, Rascher W, Lee PD, Kiess W, IGF-I IGF-II, free IGF-I and IGFBP-1, −2 and −3 levels in venous cord blood: relationship to birthweight, length and gestational age in healthy newborns, Acta Paediatr. 86 (1997) 826–833. [DOI] [PubMed] [Google Scholar]
- [81].Clapp JF, Schmidt S 3rd, Paranjape A, Lopez B, Maternal insulin-like growth factor-I levels (IGF-I) reflect placental mass and neonatal fat mass, Am. J. Obstet. Gynecol 190 (2004) 730–736. [DOI] [PubMed] [Google Scholar]
- [82].Gaither K, Quraishi AN, Illsley NP, Diabetes alters the expression and activity of the human placental GLUT1 glucose transporter, J. Clin. Endocrinol. Metab 84 (1999) 695–701. [DOI] [PubMed] [Google Scholar]
- [83].Jansson T, Wennergren M, Powell TL, Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes, Am. J. Obstet. Gynecol 180 (1999) 163–168. [DOI] [PubMed] [Google Scholar]
- [84].Luscher BP, Marini C, Joerger-Messerli MS, Huang X, Hediger MA, Albrecht C, Baumann MU, Surbek DV, Placental glucose transporter (GLUT)-1 is down-regulated in preeclampsia, Placenta 55 (2017) 94–99. [DOI] [PubMed] [Google Scholar]
- [85].Zamudio S, Baumann MU, Illsley NP, Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters, Placenta 27 (2006) 49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Aye IL, Powell TL, Jansson T, Review: adiponectin—the missing link between maternal adiposity, placental transport and fetal growth? Placenta 34 (Suppl) (2013) S40–S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Jansson N, Nilsfelt A, Gellerstedt M, Wennergren M, Rossander-Hulthen L, Powell TL, Jansson T, Maternal hormones linking maternal body mass index and dietary intake to birth weight, Am. J. Clin. Nutr 87 (2008) 1743–1749. [DOI] [PubMed] [Google Scholar]
- [88].Lowe LP, Metzger BE, Lowe WL Jr., A.R. Dyer, T.W. McDade, H.D. McIntyre, H.S.C.R. Group, Inflammatory mediators and glucose in pregnancy: results from a subset of the hyperglycemia and adverse pregnancy outcome (HAPO) study, J. Clin. Endocrinol. Metab 95 (2010) 5427–5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Jamaluddin MS, Weakley SM, Yao Q, Chen C, Resistin: functional roles and therapeutic considerations for cardiovascular disease, Br. J. Pharmacol 165 (2012) 622–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Perdomo G, Martinez-Brocca MA, Bhatt BA, Brown NF, O’Doherty RM, Garcia-Ocana A, Hepatocyte growth factor is a novel stimulator of glucose uptake and metabolism in skeletal muscle cells, J. Biol. Chem 283 (2008) 13700–13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Novakovic B, Gordon L, Robinson WP, Desoye G, Saffery R, Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation, J. Nutr. Biochem 24 (2013) 282–288. [DOI] [PubMed] [Google Scholar]
- [92].Blair JD, Yuen RK, Lim BK, McFadden DE, von Dadelszen P, Robinson WP, Widespread DNA hypomethylation at gene enhancer regions in placentas associated with early-onset pre-eclampsia, Mol. Hum. Reprod 19 (2013) 697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Pascoe WS, Inukai K, Oka Y, Slot JW, James DE, Differential targeting of facilitative glucose transporters in polarized epithelial cells, Am. J. Phys 271 (1996) C547–C554. [DOI] [PubMed] [Google Scholar]
- [94].Inukai K, Shewan AM, Pascoe WS, Katayama S, James DE, Oka Y, Carboxy terminus of glucose transporter 3 contains an apical membrane targeting domain, Mol. Endocrinol 18 (2004) 339–349. [DOI] [PubMed] [Google Scholar]
- [95].Folsch H, Ohno H, Bonifacino JS, Mellman I, A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells, Cell 99 (1999) 189–198. [DOI] [PubMed] [Google Scholar]
- [96].Marmorstein AD, Csaky KG, Baffi J, Lam L, Rahaal F, Rodriguez-Boulan E, Saturation of, and competition for entry into, the apical secretory pathway, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 3248–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Postigo L, Heredia G, Illsley NP, Torricos T, Dolan C, Echalar L, Tellez W, Maldonado I, Brimacombe M, Balanza E, Vargas E, Zamudio S, Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude, J. Physiol 587 (2009) 693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Illsley NP, Caniggia I, Zamudio S, Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int. J. Dev. Biol 54 (2010) 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Semenza GL, Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1, Biochem. J 405 (2007) 1–9. [DOI] [PubMed] [Google Scholar]
- [100].Aragones J, Fraisl P, Baes M, Carmeliet P, Oxygen sensors at the crossroad of metabolism, Cell Metab. 9 (2009) 11–22. [DOI] [PubMed] [Google Scholar]
- [101].Jansson T, Ekstrand Y, Wennergren M, Powell TL, Placental glucose transport in gestational diabetes mellitus, Am. J. Obstet. Gynecol 184 (2001) 111–116. [DOI] [PubMed] [Google Scholar]
- [102].Taricco E, Radaelli T, Nobile de Santis MS, Cetin I, Foetal and placental weights in relation to maternal characteristics in gestational diabetes, Placenta 24 (2003) 343–347. [DOI] [PubMed] [Google Scholar]
- [103].Colomiere M, Permezel M, Riley C, Desoye G, Lappas M, Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus, Eur. J. Endocrinol 160 (2009) 567–578. [DOI] [PubMed] [Google Scholar]
- [104].Kainulainen H, Jarvinen T, Heinonen PK, Placental glucose transporters in fetal intrauterine growth retardation and macrosomia, Gynecol. Obstet. Investig 44 (1997) 89–92. [DOI] [PubMed] [Google Scholar]
- [105].Kanzaki M, Pessin JE, Insulin signaling: GLUT4 vesicles exit via the exocyst, Curr. Biol 13 (2003) R574–R576. [DOI] [PubMed] [Google Scholar]
- [106].Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ, Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells, Cell 93 (1998) 731–740. [DOI] [PubMed] [Google Scholar]
- [107].Pardi G, Marconi AM, Cetin I, Placental-fetal interrelationship in IUGR fetuses—a review, Placenta 23 (Suppl A) (2002) S136–S141. [DOI] [PubMed] [Google Scholar]
- [108].Jansson T, Ylven K, Wennergren M, Powell TL, Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction, Placenta 23 (2002) 392–399. [DOI] [PubMed] [Google Scholar]
- [109].Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G, The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference, Obstet. Gynecol 87 (1996) 937–942. [DOI] [PubMed] [Google Scholar]
- [110].Economides DL, Nicolaides KH, Campbell S, Relation between maternal-to-fetal blood glucose gradient and uterine and umbilical Doppler blood flow measurements, Br. J. Obstet. Gynaecol 97 (1990) 543–544. [DOI] [PubMed] [Google Scholar]
- [111].Chandrasiri UP, Chua CL, Umbers AJ, Chaluluka E, Glazier JD, Rogerson SJ, Boeuf P, Insight into the pathogenesis of fetal growth restriction in placental malaria: decreased placental glucose transporter isoform 1 expression, J. Infect. Dis 209 (2014) 1663–1667. [DOI] [PubMed] [Google Scholar]
- [112].Zamudio S, Moore LG, Altitude and fetal growth: current knowledge and future directions, Ultrasound Obstet. Gynecol 16 (2000) 6–8. [DOI] [PubMed] [Google Scholar]
- [113].Moore LG, Niermeyer S, Zamudio S, Human adaptation to high altitude: regional and life-cycle perspectives, Am. J. Phys. Anthropol (Suppl) (1998) 25–64. [DOI] [PubMed] [Google Scholar]
- [114].Krampl E, Kametas NA, McAuliffe F, Cacho-Zegarra AM, Nicolaides KH, Maternal serum insulin-like growth factor binding protein-1 in pregnancy at high altitude, Obstet. Gynecol 99 (2002) 594–598. [DOI] [PubMed] [Google Scholar]
- [115].Hubel CA, Oxidative stress in the pathogenesis of preeclampsia, Proc. Soc. Exp. Biol. Med 222 (1999) 222–235. [DOI] [PubMed] [Google Scholar]
- [116].Roberts JM, Hubel CA, Oxidative stress in preeclampsia, Am. J. Obstet. Gynecol 190 (2004) 1177–1178. [DOI] [PubMed] [Google Scholar]
- [117].Araujo JR, Pereira AC, Correia-Branco A, Keating E, Martel F, Oxidative stress induced by tert-butylhydroperoxide interferes with the placental transport of glucose: in vitro studies with BeWo cells, Eur. J. Pharmacol 720 (2013) 218–226. [PubMed] [Google Scholar]
- [118].Lappas M, Andrikopoulos S, Permezel M, Hypoxanthine-xanthine oxidase down-regulates GLUT1 transcription via SIRT1 resulting in decreased glucose uptake in human placenta, J. Endocrinol 213 (2012) 49–57. [DOI] [PubMed] [Google Scholar]
- [119].Majmundar AJ, Wong WJ, Simon MC, Hypoxia-inducible factors and the response to hypoxic stress, Mol. Cell 40 (2010) 294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Myatt L, Muralimanoharan S, Maloyan A, Effect of preeclampsia on placental function: influence of sexual dimorphism, microRNA’s and mitochondria, Adv. Exp. Med. Biol 814 (2014) 133–146. [DOI] [PubMed] [Google Scholar]
- [121].Blundell C, Tess ER, Schanzer AS, Coutifaris C, Su EJ, Parry S, Huh D, A microphysiological model of the human placental barrier, Lab Chip 16 (2016) 3065–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Uldry M, Thorens B, The SLC2 family of facilitated hexose and polyol transporters, Pflugers Arch. 447 (2004) 480–489. [DOI] [PubMed] [Google Scholar]