Aims
We aimed to assess and characterize sex differences in adverse drug reactions (ADRs) reported to the national pharmacovigilance centre in the Netherlands while considering differences in drug use.
Methods
ADRs spontaneously reported by healthcare professionals and patients to the Netherlands pharmacovigilance centre Lareb were used. Drug–ADR combinations reported at least 10 times between 2003–2016 for drugs used by ≥10,000 persons in that period were included. Data about the number of drug users was obtained from the National Health Care Institute. Sex‐specific ADRs, like gynaecological problems, were excluded. Sex differences in specific drug–ADR combinations were tested using bivariate logistic regression analyses in which the number of drug users per sex was taken into account.
Results
In total, 2483 drug–ADR combinations were analysed. Possibly relevant sex differences were shown in 363 combinations (15%). Most of these drug–ADR combinations were reported more often for women (322 combinations). Drugs with the highest number of ADRs that were more often reported for women included thyroid hormones (32 combinations) and antidepressants (16 combinations for the centrally acting sympathomimetics; 14 combinations for other antidepressants). Some ADRs were predominantly reported for women across a range of drugs such as headache and dizziness whereas other ADRs such as tendon ruptures and aggression were reported more often for men.
Conclusions
Identified sex differences in reported ADRs often referred to women. These differences may have various causes, including pharmacological and behavioural causes, which need to be further assessed. The results may ultimately lead to sex‐specific prescribing or monitoring recommendations.
What is already known about this subject
Women generally have a higher risk for adverse drug reactions (ADRs) being reported than men.
Information about possible sex differences is inconsistent and incomplete at the specific drug and ADR level.
More knowledge on sex differences in ADRs is needed to tailor drug treatment and management in clinical practice.
What this study adds
Drugs with a higher risk for ADRs being reported for women included thyroid hormones, tumour necrosis factor‐α inhibitors and several psychoanaleptics.
A higher risk of specific ADRs being reported was shown for both women and men for statins and selective serotonin reuptake inhibitors.
1. INTRODUCTION
Adverse drug reactions (ADRs) are common in clinical practice. A study using data from medical records showed that 12% of a randomly selected sample of adults from the general public in Sweden had an ADR in a 3‐month period of any of the drugs they used.1 Higher numbers of ADRs have been shown in studies using self‐reported data. For instance, around 25% of patients in primary care in Boston reported an ADR in a survey two weeks after receiving drug prescription by their physician.2 There may be various explanations for differences in ADR rates between studies including differences in data collection methods and ADR definitions. In general, however, the numbers indicate a point of concern since ADRs may be bothersome and may reduce treatment adherence, efficacy, quality of life and increase healthcare costs.3, 4, 5
Many factors may influence the occurrence of ADRs6 including sex. It has been shown that women have a 1.5–1.7 times higher risk for ADRs than men.7 However, information is inconsistent and incomplete at the specific drug and ADR level. For instance, 1 study showed that men reported more ADRs to antineoplastic drugs than women,8 whereas another study showed that women reported more ADRs to antineoplastic drugs than men.9 Recently, a study assessed sex differences in drug‐event combinations using the Food and Drug Administration Adverse Event Reporting System.10 The study showed significant differences between men and women in drug‐event combination frequency distributions in 307 of the 668 assessed drugs but did not take sex differences in the number of drug users into account. Previous studies showed that a higher proportion of women use drugs than men, that women use different drugs, and that women use more drugs than men.11, 12, 13, 14
More knowledge on sex differences in ADRs is needed to tailor drug treatment and management in clinical practice. The primary aim of this study was to assess the extent of possibly relevant sex differences in drug–ADR combinations reported to a pharmacovigilance centre, taking sex differences in the number of drug users into account. The secondary aims of this study were to assess for which drugs and for which ADRs sex differences were identified most often.
2. METHODS
2.1. Study design and data sources
We conducted an explorative observational study to identify possibly relevant sex differences in ADRs related to specific drugs. Data on these drug–ADR combinations for individual patients that are reported to the Netherlands pharmacovigilance centre Lareb from 1 January 2003 to 31 December 2016 were used. Healthcare professionals (HCPs) as well as patients are allowed to report ADRs to Lareb,15, 16 which is funded by the Ministry of Health, Welfare and Sport, and the Dutch Medicines Evaluation Board. All ADR reports related to drugs submitted to Lareb from patients, physicians and pharmacists, concerning patients aged between 5 and 99 years old were included (Figure 1).
Figure 1.
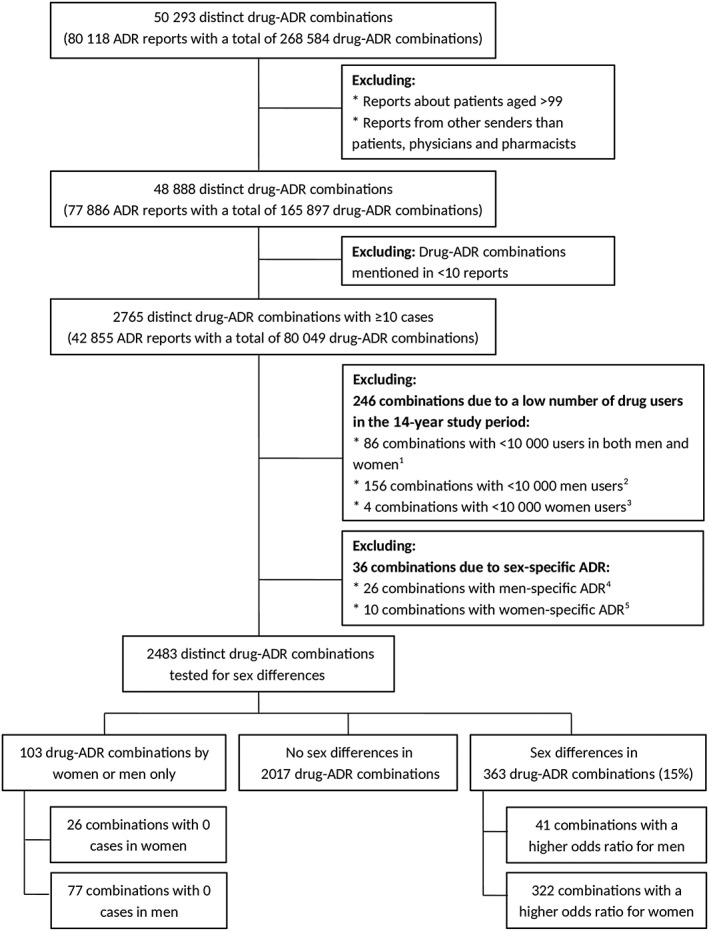
Flowchart of the number of drug–adverse drug reaction (ADR) combinations. 1 Anatomical therapeutic chemical (ATC) classification system codes (number of combinations): B02BD (1); B06AA (1); J01GB (1); J01XB (1); L01CD (8); L01XA (13); L01XC (12); L04 AC (1); N02AJ (6); N07BA (23); P01BB (16); R01AA (2); S01JA (1). 2 ATC codes (number of combinations): B03AC (16); G02BA (37); G02BB (10); G03AA (46); G03 AC (10); G03 AD (1); G03CA (10); G03HB (18); L02BG (8). 3 ATC codes (number of combinations): G04BE (2); G04CB (2) 4 medical dictionary for regulatory activities (MedDRA) preferred level terms (number of combinations): ejaculation disorder (2); ejaculation failure (1); erectile dysfunction (15); gynaecomastia (7); priapism (1) 5 MedDRA terms (number of combinations): amenorrhoea (1); female orgasmic disorder (1); menopausal symptoms (1); menstrual disorder (1); menstruation irregular (1); metrorrhagia (1); vaginal haemorrhage (1); vulvovaginal burning sensation (1); vulvovaginal candidiasis (1); vulvovaginal pruritus (1)
Data from the Drug Information System of the National Health Care Institute were used to retrieve the total number of women and men using the specific drugs in the study period.17 These data are based on reimbursement of drugs being used in an ambulatory setting, which are available for women and men aged between 5 and 99 years old.
2.2. Outcome variable
The outcome variables used in this study were specific drug–ADR combinations.
Drugs mentioned in the ADR report were classified according to the Anatomical Therapeutic Chemical (ATC) classification system.18 In this system, drugs are divided on five different levels based on the organ or system on which they act and their chemical, pharmacological, and therapeutic properties. For the specific drug assessment, drugs on the chemical subgroup, the fourth ATC level was used.
Reported ADRs were classified according to the Medical Dictionary for Regulatory Activities (MedDRA), version 20.0.19 The MedDRA is a medical dictionary that contains five levels ranging from very general (the System Organ Class level) to very specific terms (the Lowest Level Term level). The fourth, preferred term (PT) level was used for the ADR assessment which is the level most often used in safety reporting for analysis.20 The use of the PT level implies that a report containing several ADRs on the lowest level of detail but within the same PT were counted once.
2.3. Determinant
The determinant used in this study was the sex (i.e. women vs men) of the patients for which an ADR was reported. Reports indicating that sex was unknown were excluded.
2.4. Analyses
Descriptive statistics were used to analyse the reports. Sex differences in specific drug–ADR combinations were assessed for combinations mentioned in at least 10 reports (Figure 1). This number was arbitrarily chosen as being appropriate for detection of all possibly relevant sex differences in drug–ADR combinations. Duplicate reports (i.e. those reported by both patients and HCPs) were counted only once.
For both women and men, the number of users was calculated per drug resulting in the total number of users in the study period. Drug–ADR combinations for which the total number of men or the total number of women using the drug in the population over the 14‐year study period was <10 000 were excluded. Sex‐specific ADRs as labelled in the Gender Adverse Event Term Lists of the MedDRA21 were also excluded (Figure 1).
Sex differences in the remaining specific drug–ADR combinations were tested using bivariate logistic regression analyses in which the number of users of the specific drug in the study period was taken into account. In these analyses, our total population consisted of all users of the specific drug in the study period with the number of individuals experiencing the ADR being the number of reports received in the study period. Odds ratios and 95% confidence intervals (CIs) were calculated for each specific drug–ADR combination, and associations with P‐values <.05 are reported. Since the aim was to identify all possibly relevant sex differences, the results were not adjusted for multiple testing. A sensitivity analysis was conducted to assess the agreement of the findings between the patient and HCP reports. For this, the patient and HCP reports were analysed separately. All analyses were conducted using Stata version 13 (Stata Corp., College Station, TX, USA) and Microsoft Excel 2010 was used for a graphical presentation of the results.
3. RESULTS
In the study period, there were 80 118 ADR reports in which 268 584 drug–ADR combinations were reported. These concerned 50 293 distinct drug–ADR combinations (Figure 1). After exclusion of reports, there were 42 855 ADR reports who fulfilled the inclusion criteria. Two thirds of these reports (67%) concerned women. The mean ± standard deviation age in the reports concerning women was 51 ± 18 years and for men 56 ± 18 years. In total, 80 049 drug–ADR combinations were reported, which concerned 2765 distinct drug–ADR combinations (Figure 1).
3.1. Sex differences in specific drug–ADR combinations
Of the 2765 distinct drug–ADR combinations, 246 combinations were excluded because the number of drug users in the study period was <10 000. Another 36 combinations were excluded because of a sex‐specific ADR (Figure 1). Among the remaining 2483 combinations there were 103 drug–ADR combinations (4%) reported for 1 sex only. A possibly relevant sex difference was shown in 363 combinations (15%), which concerned 74 different drugs and 124 different ADRs. In most of these cases (89%), women had a higher odds ratio for a specific drug–ADR combination than men (322 vs 41 combinations). The results of all 2483 combinations are presented in Data S1.
For some drugs and ADRs, a multitude of sex differences were shown. Most common drugs with a multitude of ADRs for which women had higher odds were: thyroid hormones (ATC group H03AA; 32 combinations); centrally acting sympathomimetics (N06BA; 16 combinations); other antidepressants (N06AX; 14 combinations); and tumour necrosis factor‐α inhibitors (L04AB; 14 combinations). The most common ADRs with a multitude of differences with higher odds for women were: nausea (32 combinations); alopecia (28 combinations); headache (20 combinations); dizziness (18 combinations); and palpitations (18 combinations). Higher odds for men were particularly shown in combinations with the following ADRs: aggression; death; pyrexia; sexual dysfunction; tendon rupture; and tinnitus (all in 2 combinations).
Drugs for which a higher odds ratio was shown for a multitude of ADRs in both men and women, were β‐hydroxy β‐methylglutaryl (HMG)‐CoA reductase inhibitors (C10AA; 20 combinations with higher odds for women and 5 combinations with higher odds for men), and selective serotonin reuptake inhibitors (N06AB; 13 combinations with higher odds for women and 10 combinations with higher odds for men). The type of ADRs for HMG CoA reductase inhibitors (range for women: OR 7.23, 95% CI 1.62–32.29 for swollen tongue to OR 1.54, 95% CI 1.05–2.27 for palpitations; range for men: OR 0.05, 95% CI 0.01–0.34 for libido decreased to OR 0.44, 95% CI 0.19–0.98 for pancreatitis; Figure 2) and selective serotonin reuptake inhibitors (range for women: OR 6.19, 95% CI 1.91–20.06 for haematoma to OR 1.66, 95% CI 1.12–2.47 for nausea; range for men: OR 0.21, 95% CI 0.06–0.83 for micturition disorder to OR 0.62, 95% CI 0.45–0.85 for therapeutic response unexpected; Figure 3) with higher odds for men were different than the ADRs with higher odds for women.
Figure 2.
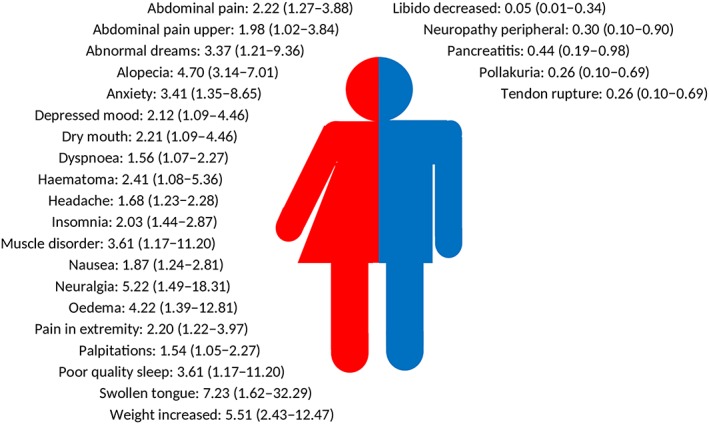
Odds ratios with 95% confidence intervals of adverse drug reactions with higher odds for women (left side) or for men (right side) of β‐hydroxy β‐methylglutaryl‐CoA reductase inhibitors
Figure 3.
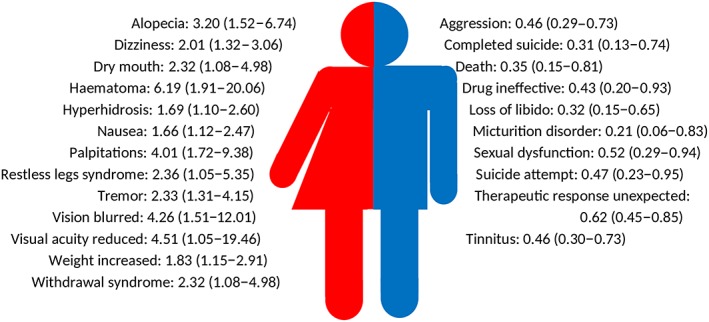
Odds ratios with 95% confidence intervals of adverse drug reactions with higher odds for women for (left side) or for men (right side) of selective serotonin reuptake inhibitors
Overall, the drug–ADR combinations with possibly relevant sex differences were shown in various drug classes (Figure 4) and ADR classes (Figure 5). For the sensitivity analysis, 537 drug–ADR combinations were tested using both the patient and HCP reports. Agreement in significance was shown in 397 drug–ADR combinations (74%; Figure S2).
Figure 4.
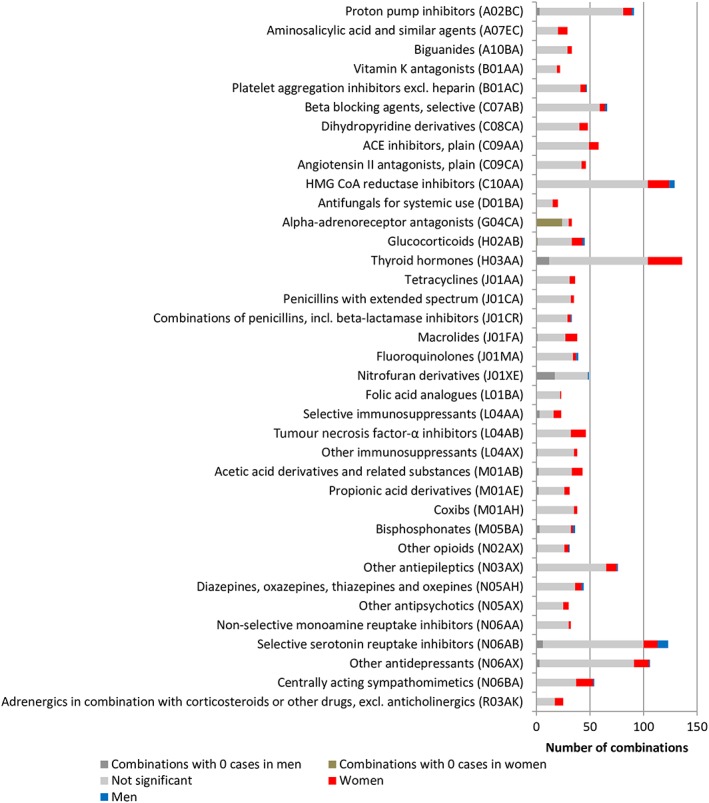
Overview of number of combinations for drug classes with ≥20 combinations ordered by anatomical therapeutic chemical (ATC) codes. The full list is presented in Figure S1
Figure 5.
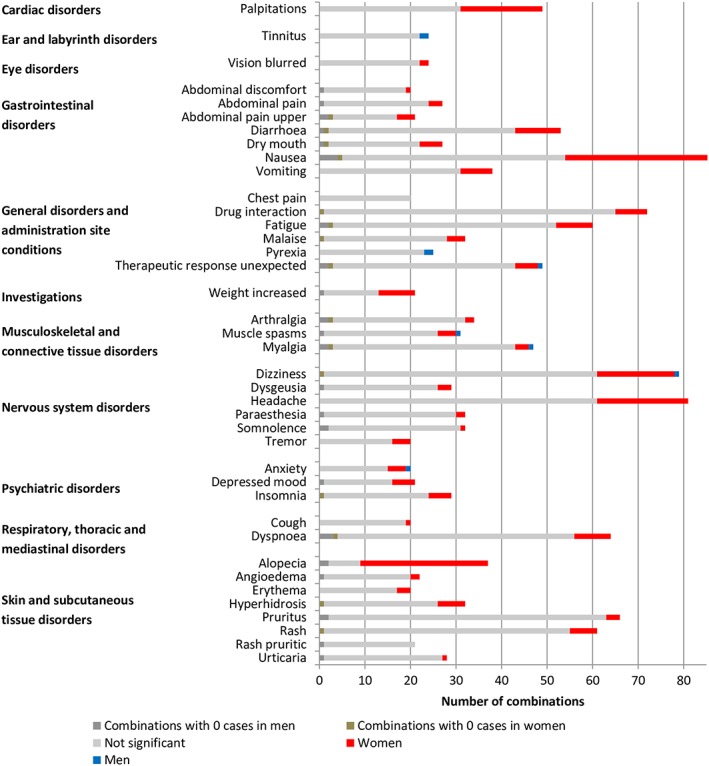
Overview of number of combinations per adverse drug reaction with ≥20 combinations ordered by system organ class. The full list is presented in Figure S3
4. DISCUSSION
We found that 15% of approximately 2500 drug–ADR combinations reported to the pharmacovigilance centre showed a possibly relevant difference in occurrence of ADRs between women and men after taking sex differences in drug use into account. In 89% of these cases, the risk was higher for women than for men. Drugs with a multitude of ADRs showing a higher risk for women included thyroid hormones, tumour necrosis factor‐α inhibitors and several psychoanaleptics. A higher risk of specific ADRs for both women and men was shown for HMG CoA reductase inhibitors (women: e.g. alopecia and headache; men: e.g. decreased libido and tendon rupture) and selective serotonin reuptake inhibitors (women: e.g. dizziness and nausea; men: e.g. aggression and sexual dysfunction). In general, several specific ADRs were more often reported for either women or men across a range of drugs, including nausea, alopecia and headache for women, and aggression, sexual dysfunction and tendon rupture for men.
Our study confirms findings from previous studies demonstrating that women have a higher risk for reporting ADRs than men (e.g.7, 8, 12, 22, 23, 24, 25, 26). It adds to this knowledge by presenting an overview of this higher risk for specific drug–ADR combinations. Moreover, it shows that men may have a higher risk for other specific drug–ADR combinations. Compared to other studies, our study assessed drugs and ADRs at a specific level and took sex differences in drug prescribing into account. Previous studies analysing ADRs at system organ class level showed for instance a higher risk for women in cardiac disorders26 and a higher risk for men in renal and urinary disorders.10 According to the current study, the higher risk for women seems to apply particularly to palpitations and the higher risk for men to haematuria, micturition disorder and pollakiuria (Figure S3).
Observed differences in the type and number of reported ADRs can be caused by sex‐ or gender‐related factors. Sex‐related factors refer to biological differences between women and men, whereas gender‐related factors refer to psychosocial, behavioural or cultural differences.27 Sex‐related differences that are relevant for the pharmacokinetic and pharmacodynamic behaviour of drugs include differences in physiology, genetic expression, immunological processes, and type and function of various hormones.28, 29, 30, 31, 32, 33 For instance, women generally have a lower body weight. Therefore, one would expect more so‐called type A ADRs in women. Type A reactions are dose dependent, occur frequently, have a low mortality, and can be explained based on the pharmacological properties of the drug.34 In clinical practice, some sex‐specific dose adjustments are recommended or used. For example, in the case of zolpidem, where women have a lower clearance than men, lower doses are recommended and prescribed for women.35, 36 In the current study, several of the common type A ADRs, such as nausea, headache and dizziness, were indeed predominantly present in women. However, no significant differences in reported ADRs for drugs with known differences in pharmacological properties, such as benzodiazepines or verapamil were observed.37, 38 For some drug classes such as β‐blocking agents, specific ADRs (e.g. palpitations) occurred more in women whereas other ADRs (e.g. coldness) occurred more in men.
ADRs are classified as type B reactions if these cannot be explained by pharmacological properties of the drug, which occur less frequently and are often more serious in nature. In the current study, some of the ADRs that were more present in men, may be type B reactions, such as aggression and tendon ruptures. However, these differences could also be explained by differences in background incidence of the phenomenon as tendon ruptures and aggression occur more frequently in men than in women without using drugs.
Besides sex‐related factors, differences in social roles, lifestyle factors, communication styles, health information‐seeking behaviour, and medication prescribing and adherence could also lead to gender‐specific differences in the occurrence, perception and reporting of ADRs. Some ADRs are more likely to be perceived or reported by either women or men, since the burden may depend on gender specific self‐image. An example is the occurrence of hair loss, which is more common in aging men than in aging women.39, 40, 41 Therefore, alopecia that occurs or is perceived as an ADR is likely to be considered as more disturbing in women than in men, which may explain more reports in women in the current study.
Reporting ADRs requires that the patient assigns signs or symptoms to a drug. Women and men may perceive these differently.42 Women appear to search more actively for health information than men.43, 44 Patients can either report by themselves or contact their healthcare professional who can decide to report. To our knowledge, possible sex differences in respect to ADR reporting behaviour have not been widely studied. It is known that there is underreporting of ADRs in spontaneous reporting45 but it seems that sex of the patient is not a factor for healthcare professionals in decision making whether or not to report an ADR to the pharmacovigilance centre.46, 47 Moreover, there seem to be no differences in reasons for and opinions about ADR reporting between female and male patients who report to a pharmacovigilance centre.48 Our study contained reports of both patients and HCPs. Separate analyses of the patient and HCP reports showed similar sex differences in drug–ADR combinations (Figure S2), suggesting that the observed differences are not due to sex differences in spontaneous reporting behaviour.
The occurrence of ADRs is clearly related to drug exposure. In the analyses, the number of drug users was taken into account, but individual drug exposure is dependent on medication prescribing and adherence behaviour. There can be differences in the dose or duration of drugs prescribed to women as compared to men. For example, women are more often prescribed a low‐dose of HMG CoA reductase inhibitors at treatment initiation than men who are more often prescribed the standard dose.49 Also, women may receive more co‐prescriptions, which could lead to a higher risk for ADRs.50 Previous studies are inconsistent about sex differences in adherence levels.51
To summarize, it is likely that both sex‐related and sex‐related factors may underlie the observed differences between women and men in drug–ADR combinations as reported to the pharmacovigilance centre. The distinction between sex‐ and sex‐related factors, however, is not straightforward since they are correlated and can influence each other.52, 53, 54 Further in‐depth studies are needed for the specific drug–ADR combinations with possibly relevant sex differences to assess the individual contribution of potential factors.
A strength of this study is the assessment of differences between women and men for all reported drug–ADR combinations while taking differences in the number of drug users into account. Trained assessors at the pharmacovigilance centre classified the ADRs using the MedDRA terms system. ADRs were analysed at the PT level to reduce possible inconsistent coding at the lowest level.
There are also some limitations that need to be acknowledged. We combined all ADR reports and the number of drug users per sex in the study period. Due to this aggregated level, we were not able to adjust for potential confounding factors at individual level, such as age. Therefore, we cannot make inferences at individual patient level. The number of drug users was based on data of reimbursed drugs prescribed in an ambulatory setting. This implies that drugs prescribed in another setting or used over‐the‐counter were not included. Furthermore, we analysed drugs at the chemical subgroup level of the ATC system but not all drugs that belong to a specific chemical subgroup can be considered as pharmacotherapeutically equivalent. Therefore, some relevant sex differences in ADRs may have been missed at the lowest level of the ATC system. However, a study assessing the heterogeneity of drugs within ATC classes showed that the mechanism of action and physiological effects of drugs in most classes were fairly homogeneous.55 Another limitation is the difference in number of cases for drug–ADR combinations (ranging from 10 to 1992), yielding differences in power. Moreover, some combinations were reported for either women or men only, but this mainly occurred for drugs with a higher number of users in women or men, respectively. Finally, the study was conducted using spontaneously reported ADRs to the pharmacovigilance centre in the Netherlands. Since cultural differences between men and women may influence the spontaneous reporting of ADRs, it is unknown to what extent the results are generalizable to other countries.
In conclusion, this study showed possibly relevant differences between women and men in 15% of the assessed drug–ADR combinations spontaneously reported to a pharmacovigilance centre. Both sex‐ and gender‐related factors may play a role in explaining the observed differences in ADRs.
COMPETING INTERESTS
The authors have no competing interests to declare.
CONTRIBUTORS
All authors contributed to the development and formulation of the research question. C.E. and E.P.v.P. extracted/collected the data. All authors were involved in the analyses plan. S.T.d.V. conducted the analyses. All authors contributed to the interpretation of the data. STdV drafted the manuscript. P.D., C.E., J.B., N.K., P.G.M.M., and E.P.v.P. reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Supporting information
Figure S1 Overview of number of combinations per drug class ordered at the first level of the Anatomical Therapeutic Chemical (ATC) code.
Figure S2 Comparison of drug‐adverse drug reaction (ADR) combinations tested in reports sent by patients and healthcare professionals.
Figure S3 Overview of number of combinations per adverse drug reaction by System Organ Class.
Data S1 Supporting information
ACKNOWLEDGEMENTS
We would like to thank Monique Schuurman for her assistance during the project and Phil Tregunno for his critical view on the manuscript. This study was funded by ZonMW – The Netherlands Organization for Health Research and Development (Project number 849100006).
de Vries ST, Denig P, Ekhart C, et al. Sex differences in adverse drug reactions reported to the National Pharmacovigilance Centre in the Netherlands: An explorative observational study. Br J Clin Pharmacol. 2019;85:1507‐1515. 10.1111/bcp.13923
The authors confirm that the PI for this paper is Prof. dr E.P. van Puijenbroek. This study was database‐based study; therefore, there was no direct clinical responsibility for patients.
REFERENCES
- 1. Hakkarainen KM, Gyllensten H, Jonsson AK, Andersson Sundell K, Petzold M, Hägg S. Prevalence, nature and potential preventability of adverse drug events ‐ a population‐based medical record study of 4970 adults. Br J Clin Pharmacol. 2014;78(1):170‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weingart SN, Gandhi TK, Seger AC, et al. Patient‐reported medication symptoms in primary care. Arch Intern Med. 2005;165(2):234‐240. [DOI] [PubMed] [Google Scholar]
- 3. Coleman JJ, Pontefract SK. Adverse drug reactions. Clin Med (Lond). 2016;16(5):481‐485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sultana J, Cutroneo P, Trifiro G. Clinical and economic burden of adverse drug reactions. J Pharmacol Pharmacother. 2013;4(Suppl 1):S73‐S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pollack MF, Purayidathil FW, Bolge SC, Williams SA. Patient‐reported tolerability issues with oral antidiabetic agents: associations with adherence; treatment satisfaction and health‐related quality of life. Diabetes Res Clin Pract. 2010;87(2):204‐210. [DOI] [PubMed] [Google Scholar]
- 6. Alomar MJ. Factors affecting the development of adverse drug reactions (review article). Saudi Pharm J. 2014;22(2):83‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Martin RM, Biswas PN, Freemantle SN, Pearce GL, Mann RD. Age and sex distribution of suspected adverse drug reactions to newly marketed drugs in general practice in England: analysis of 48 cohort studies. Br J Clin Pharmacol. 1998;46(5):505‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tran C, Knowles SR, Liu BA, Shear NH. Gender differences in adverse drug reactions. J Clin Pharmacol. 1998;38(11):1003‐1009. [DOI] [PubMed] [Google Scholar]
- 9. Montastruc JL, Lapeyre‐Mestre M, Bagheri H, Fooladi A. Gender differences in adverse drug reactions: analysis of spontaneous reports to a regional pharmacovigilance Centre in France. Fundam Clin Pharmacol. 2002;16(5):343‐346. [DOI] [PubMed] [Google Scholar]
- 10. Yu Y, Chen J, Li D, Wang L, Wang W, Liu H. Systematic analysis of adverse event reports for sex differences in adverse drug events. Sci Rep. 2016;6(1):24955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loikas D, Wettermark B, von Euler M, Bergman U, Schenck‐Gustafsson K. Differences in drug utilisation between men and women: a cross‐sectional analysis of all dispensed drugs in Sweden. BMJ Open. 2013;3(5):e002378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rademaker M. Do women have more adverse drug reactions? Am J Clin Dermatol. 2001;2(6):349‐351. [DOI] [PubMed] [Google Scholar]
- 13. Manteuffel M, Williams S, Chen W, Verbrugge RR, Pittman DG, Steinkellner A. Influence of patient sex and gender on medication use, adherence, and prescribing alignment with guidelines. J Womens Health (Larchmt). 2014;23(2):112‐119. [DOI] [PubMed] [Google Scholar]
- 14. Al‐Windi A, Elmfeldt D, Svardsudd K. The relationship between age, gender, well‐being and symptoms, and the use of pharmaceuticals, herbal medicines and self‐care products in a Swedish municipality. Eur J Clin Pharmacol. 2000;56(4):311‐317. [DOI] [PubMed] [Google Scholar]
- 15. de Langen J, van Hunsel F, Passier A, de Jong‐van den Berg L, van Grootheest K. Adverse drug reaction reporting by patients in the Netherlands: three years of experience. Drug Saf. 2008;31(6):515‐524. [DOI] [PubMed] [Google Scholar]
- 16. van Grootheest AC, Passier JL, van Puijenbroek EP. Direct reporting of side effects by the patient: favourable experience in the first year. Ned Tijdschr Geneeskd. 2005;149(10):529‐533. [PubMed] [Google Scholar]
- 17. National Healthcare Institute (Zorginstituut Nederland) . The drug information system of National Health Care Institute. https://www.gipdatabank.nl/infoPagina.asp?naam=English
- 18. World Health Organization (WHO). Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment 2015. Oslo, 2015.
- 19. Brown EG, Wood L, Wood S. The medical dictionary for regulatory activities (MedDRA). Drug Saf. 1999;20(2):109‐117. [DOI] [PubMed] [Google Scholar]
- 20. Morley G. Adverse event reporting: a brief overview of MedDRA. Medical Writing. 2014;23(2):113‐116. [Google Scholar]
- 21. Medical Dictionary for Regulatory Activities . Paediatric and gender adverse event term lists. https://www.meddra.org/paediatric‐and‐gender‐adverse‐event‐term‐lists. Accessed July 27, 2018.
- 22. Pistone G, Gurreri R, Alaimo R, Curiale S, Bongiorno MR. Gender differences in adverse drug reactions in dermatological patients in west Sicily: an epidemiological study. J Dermatolog Treat. 2014;25(6):510‐512. [DOI] [PubMed] [Google Scholar]
- 23. Holm L, Ekman E, Jorsater Blomgren K. Influence of age, sex and seriousness on reporting of adverse drug reactions in Sweden. Pharmacoepidemiol Drug Saf. 2017;26(3):335‐343. [DOI] [PubMed] [Google Scholar]
- 24. Kroenke K, Spitzer RL. Gender differences in the reporting of physical and somatoform symptoms. Psychosom Med. 1998;60(2):150‐155. [DOI] [PubMed] [Google Scholar]
- 25. Lucca J, Ramesh M, Ram D. Gender differences in the occurrences and pattern of adverse drug reactions in psychiatric patients: a prospective observational study. Trop J Med Res. 2017;20(1):84‐90. [Google Scholar]
- 26. Zopf Y, Rabe C, Neubert A, et al. Women encounter ADRs more often than do men. Eur J Clin Pharmacol. 2008;64(10):999‐1004. [DOI] [PubMed] [Google Scholar]
- 27. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA. 2016;316(18):1863‐1864. [DOI] [PubMed] [Google Scholar]
- 28. Soldin O, Mattison DR. Sex differences in pharmacokinetics and pharmacodynamics. Clin Pharmacokinet. 2009;48(3):143‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fadiran EO, Zhang L. Effects of sex differences in the pharmacokinetics of drugs and their impact on the safety of medicines in women In: Harrison‐Woolrych M, ed. Medicines For Women. Cham: Adis; 2015. [Google Scholar]
- 30. Franconi F, Campesi I. Pharmacogenomics, pharmacokinetics and pharmacodynamics: interaction with biological differences between men and women. Br J Pharmacol. 2014;171(3):580‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tannenbaum C, Day D, Matera Alliance . Age and sex in drug development and testing for adults. Pharmacol Res. 2017;121:83‐93. [DOI] [PubMed] [Google Scholar]
- 32. Kashuba ADM, Nafziger AN. Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet. 1998;34(3):203‐218. [DOI] [PubMed] [Google Scholar]
- 33. Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42(2):107‐121. [DOI] [PubMed] [Google Scholar]
- 34. Meyboom RHB, Gribnau FWJ, Hekster YA, de Koning GHP, Egberts ACG. Characteristics of topics in pharmacovigilance in the Netherlands. Clin Drug Investig. 1996;12(4):207‐219. [Google Scholar]
- 35. Farkas RH, Unger EF, Temple R. Zolpidem and driving impairment — identifying persons at risk. N Engl J Med. 2013;369(8):689‐691. [DOI] [PubMed] [Google Scholar]
- 36. Food and Drug Administration (FDA) . FDA drug safety communication: FDA approves new label changes and dosing for zolpidem products and a recommendation to avoid driving the day after using Ambien CR. https://www.fda.gov/Drugs/DrugSafety/ucm352085.htm. Accessed July 27, 2018.
- 37. Yonkers KA, Kando JC, Cole JO, Blumenthal S. Gender differences in pharmacokinetics and pharmacodynamics of psychotropic medication. Am J Psychiatry. 1992;149(5):587‐595. [DOI] [PubMed] [Google Scholar]
- 38. Krecic‐Shepard ME, Barnas CR, Slimko J, Jones MP, Schwartz JB. Gender‐specific effects on verapamil pharmacokinetics and pharmacodynamics in humans. J Clin Pharmacol. 2000;40(3):219‐230. [DOI] [PubMed] [Google Scholar]
- 39. Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341(7):491‐497. [DOI] [PubMed] [Google Scholar]
- 40. Turner GA, Bhogal RK. Hair and aging. Skinmed. 2016;14(5):338‐343. [PubMed] [Google Scholar]
- 41. Ji J, Ho BS, Qian G, Xie XM, Bigliardi PL, Bigliardi‐Qi M. Aging in hair follicle stem cells and niche microenvironment. J Dermatol. 2017;44(10):1097‐1104. [DOI] [PubMed] [Google Scholar]
- 42. Gustafson PE. Gender differences in risk perception: theoretical and methodological perspectives. Risk Anal. 1998;18(6):805‐811. [DOI] [PubMed] [Google Scholar]
- 43. Ek S. Gender differences in health information behaviour: a Finnish population‐based survey. Health Promot Int. 2015;30(3):736‐745. [DOI] [PubMed] [Google Scholar]
- 44. Hammar T, Nilsson A, Hovstadius B. Patients' views on electronic patient information leaflets. Pharm Pract. 2016;14(2):702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hazell L, Shakir SA. Under‐reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385‐396. [DOI] [PubMed] [Google Scholar]
- 46. Eland IA, Belton KJ, van Grootheest AC, Meiners AP, Rawlins MD, Stricker BH. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48(4):623‐627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ekman E, Bäckström M. Attitudes among hospital physicians to the reporting of adverse drug reactions in Sweden. Eur J Clin Pharmacol. 2009;65(1):43‐46. [DOI] [PubMed] [Google Scholar]
- 48. van Hunsel F, van der Welle C, Passier A, van Puijenbroek E, van Grootheest K. Motives for reporting adverse drug reactions by patient‐reporters in the Netherlands. Eur J Clin Pharmacol. 2010;66(11):1143‐1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. de Vries FM, Voorham J, Hak E, Denig P. Adherence to standard‐dose or low‐dose statin treatment and low‐density lipoprotein cholesterol response in type 2 diabetes patients. Curr Med Res Opin. 2015;31(12):2197‐2206. [DOI] [PubMed] [Google Scholar]
- 50. Venturini CD, Engroff P, Ely LS, et al. Gender differences, polypharmacy, and potential pharmacological interactions in the elderly. Clinics. 2011;66(11):1867‐1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kardas P, Lewek P, Matyjaszczyk M. Determinants of patient adherence: a review of systematic reviews. Front Pharmacol. 2013;4:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franconi F, Campesi I. Sex and gender influences on pharmacological response: an overview. Expert Rev Clin Pharmacol. 2014;7(4):469‐485. [DOI] [PubMed] [Google Scholar]
- 53. Kautzky‐Willer A, Harreiter J, Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr Rev. 2016;37(3):278‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Regitz‐Zagrosek V. Sex and gender differences in health: Science & Society Series on sex and science. EMBO Rep. 2012;13(7):596‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Winnenburg R, Bodenreider O. Exploring pharmacoepidemiologic groupings of drugs from a clinical perspective. Stud Health Technol Inform. 2013;192:827‐831. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Overview of number of combinations per drug class ordered at the first level of the Anatomical Therapeutic Chemical (ATC) code.
Figure S2 Comparison of drug‐adverse drug reaction (ADR) combinations tested in reports sent by patients and healthcare professionals.
Figure S3 Overview of number of combinations per adverse drug reaction by System Organ Class.
Data S1 Supporting information


