Abstract
Objective
To evaluate the efficacy and safety of standard term (12 months) or long term (>12 months) dual antiplatelet therapy (DAPT) versus short term (<6 months) DAPT after percutaneous coronary intervention (PCI) with drug-eluting stent (DES).
Design
Systematic review and network meta-analysis.
Data sources
Relevant studies published between June 1983 and April 2018 from Medline, Embase, Cochrane Library for clinical trials, PubMed, Web of Science, ClinicalTrials.gov, and Clinicaltrialsregister.eu.
Review methods
Randomised controlled trials comparing two of the three durations of DAPT (short term, standard term, and long term) after PCI with DES were included. The primary study outcomes were cardiac or non-cardiac death, all cause mortality, myocardial infarction, stent thrombosis, and all bleeding events.
Results
17 studies (n=46 864) were included. Compared with short term DAPT, network meta-analysis showed that long term DAPT resulted in higher rates of major bleeding (odds ratio 1.78, 95% confidence interval 1.27 to 2.49) and non-cardiac death (1.63, 1.03 to 2.59); standard term DAPT was associated with higher rates of any bleeding (1.39, 1.01 to 1.92). No noticeable difference was observed in other primary endpoints. The sensitivity analysis revealed that the risks of non-cardiac death and bleeding were further increased for ≥18 months of DAPT compared with short term or standard term DAPT. In the subgroup analysis, long term DAPT led to higher all cause mortality than short term DAPT in patients implanted with newer-generation DES (1.99, 1.04 to 3.81); short term DAPT presented similar efficacy and safety to standard term DAPT with acute coronary syndrome (ACS) presentation and newer-generation DES placement. The heterogeneity of pooled trials was low, providing more confidence in the interpretation of results.
Conclusions
In patients with all clinical presentations, compared with short term DAPT (clopidogrel), long term DAPT led to higher rates of major bleeding and non-cardiac death, and standard term DAPT was associated with an increased risk of any bleeding. For patients with ACS, short term DAPT presented similar efficacy and safety with standard term DAPT. For patients implanted with newer-generation DES, long term DAPT resulted in more all cause mortality than short term DAPT. Although the optimal duration of DAPT should take personal ischaemic and bleeding risks into account, this study suggested short term DAPT could be considered for most patients after PCI with DES, combining evidence from both direct and indirect comparisons.
Systematic review registration
PROSPERO CRD42018099519.
Introduction
Dual antiplatelet therapy (DAPT), with aspirin and a P2Y12-receptor inhibitor, is a basis for the care of patients after percutaneous coronary intervention (PCI).1 2 3 The recommended duration of DAPT for patients after drug-eluting stent (DES) implantation is ≥12 months for patients with acute coronary syndrome (ACS), and six months for patients with stable coronary artery disease.2 3 Despite these recommendations, the optimal timing of switching from DAPT to a single antiplatelet therapy continues to be a matter of debate, owing to refinements in DES technologies and the advent of potent P2Y12 receptor inhibitors.4
The recommendation for ≥12 months of DAPT after PCI with DES has received scrutiny by several randomised controlled trials, which proved non-superiority compared with three to six months of DAPT.5 6 7 8 9 Furthermore, shorter durations, as opposed to longer durations of DAPT, were associated with lower rates of all cause mortality as a result of lower rates of bleeding-related deaths.10 Nevertheless, the wide non-inferiority margins of up to six months of DAPT from single randomised controlled trials have prevented researchers from concluding that short term DAPT could replace the conventional standard duration. Additionally, a recent individual patient data meta-analysis of six randomised controlled trials suggested that three months of DAPT was associated with an increased risk of ischemia in patients with ACS.11
Coronary artery disease is a leading cause of reduced health globally, as well as in each world region.12 A cost effectiveness analysis of different durations of DAPT after PCI with DES showed that three to six months of DAPT was better than ≥12 months of DAPT.13 Moreover, DAPT disruption owing to non-compliance or bleeding, which is more frequent with longer durations of DAPT, increases the risk of adverse events.14 Thus, shortening the recommended duration of DAPT might relieve the global health burden. However, previous studies have focused on comparing two arms representing longer or shorter durations of DAPT when investigating the efficacy and safety of the discontinuation of DAPT after PCI with DES.15 16 17 18 Without more quantified criteria for various durations, it would be unlikely to make a strong inference regarding rationality of up to six months of DAPT based on the current evidence. Additionally, the limited head-to-head trials might weaken the conclusiveness of pairwise meta-analysis and network meta-analysis results with small sample sizes or unsuitable arms.
Therefore, we performed this network meta-analysis to better quantify durations of DAPT and make full use of direct and indirect evidence to provide a more comprehensive evaluation with more precise results.19 Here, we concentrated on both the general population of coronary artery disease and subgroups (eg, patients with ACS) to increase the universality of the conclusions.
Methods
The detailed protocol, which followed the template of a Cochrane review for multiple interventions is available in the PROSPERO registry (CRD42018099519).20 This systematic review was prepared according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) and the PRISMA extension statement for network meta-analysis.21 22
Search strategy and selection criteria
We conducted a systematic search of the literature in April 2018. The databases included Medline, Embase, Cochrane Library for clinical trials, PubMed, and Web of Science. We also searched ClinicalTrials.gov and Clinicaltrialsregister.eu. The MeSH search terms included the following: drug eluting stents, percutaneous coronary intervention, platelet aggregation inhibitors, antiplatelet therapy, and aspirin. Our search strategy was tailored to each database (appendix 1).
We included randomised controlled trials that met the following criteria: participants were adults (aged ≥18) who received DAPT after PCI with DES; the interventions were candidate durations of DAPT (that is, short term (≤6 months), standard term (12 months), and long term (>12months) DAPT); comparisons with another candidate duration were made; or the outcomes included death, myocardial infarction, stroke, and bleeding.
We excluded studies that met the following criteria: ≤1 month of DAPT, analyses of non-randomised trials, cross-sectional studies, case reports or case series, ongoing trials, or insufficient data from original studies.
The prespecified efficacy endpoints included all cause mortality, cardiac death, non-cardiac death, myocardial infarction, stroke, definite or probable stent thrombosis, and net adverse clinical events. The safety endpoint included major bleeding and any bleeding. The endpoint definitions applied in each trial (table B in appendix 3) were incorporated.
Data extraction and risk of bias assessment
For each eligible randomised controlled trial, we extracted the study characteristics (eg, trial registration number, year of publication, first author, arms and treatment regimens, follow-up time, number of intention-to-treat patients, region), patient characteristics (eg, proportions of patients with ACS or diabetes, mean age), and outcome measures (table B in appendix 3). The reviewers independently screened the titles and abstracts of the retrieved studies in pairs (SHLY, PX, BW, HY) to exclude any that did not research the question of interest. Pairs of reviewers (SHLY, PX, BW, HY) then independently screened full texts of the remaining articles to identify studies that met all of the criteria for inclusion in the quantitative synthesis. We manually checked the reference list of each acquired article for relevant studies. For qualified trials, the data were extracted independently by pairs of reviewers (SHLY, PX, BW, HY), and the discrepancies were resolved by a third reviewer.
The quality of the included studies was assessed according to the Cochrane Collaboration’s tool for assessing the risk of bias.23 Any discrepancies were resolved by consensus, referring to the original articles and consulting with a third reviewer.
Data synthesis and statistical analysis
We applied odds ratios and 95% confidence intervals to summary statistics to quantify the effects of different durations. Odds ratios greater than one represented an efficacy or safety benefit favouring the control duration. Two sided P<0.05 was considered significant.
We used a frequentist approach to conduct network meta-analyses, because of the complete graphical tools that depict the network geometry. We assumed a common heterogeneity variance across all pairwise comparisons and used the between studies variance τ2 to present heterogeneity across the network. Estimates of τ2 of approximately 0.04, 0.16, and 0.36 are considered to represent a low, moderate, and high degree of heterogeneity, respectively.24 We statistically evaluated inconsistency between direct and indirect sources of evidence globally (by fitting the inconsistency model) and locally (by calculating differences between direct and indirect estimates in closed loops),25 and provided P values in table C of appendix 3. We used forest plots to present the results of odds ratios and 95% confidence intervals. We presented the treatment hierarchy (fig C in appendix 2) of all endpoints according to cumulative rank probabilities.26 We assessed small study effects and potential publication bias with comparison-adjusted funnel plot symmetry.25 We also conducted a pairwise meta-analysis with both a random-effects model of DerSimonian and Laird’s method and a fixed effect model of Mantel and Haenszel’s method, providing the direct estimates (fig A in appendix 2). Analyses were performed in STATA version 14.0 (StataCorp).
To validate the robustness of the findings, we performed a sensitivity analysis by restricting long term DAPT to ≥18 months of DAPT, as well as applying a random effects Bayesian network meta-analysis to account for methodological and clinical heterogeneity across studies.27 We used Markov chain Monte Carlo methods with the GeMTC package (version 0.8-2) in R (version 3.4.4) to calculate odds ratios and 95% credible intervals. Three Markov chains were run simultaneously with 100 000 simulated draws after a burn-in of 50 000 iterations. Trace plots and the Brooks-Gelman-Rubin statistic were assessed to ensure convergence.28 We evaluated consistency with a node-splitting technique that compares the direct and indirect estimates for each comparison.29 Model fit was evaluated with the total residual deviance, which indicated good fit, if it approximated the number of data points (table D in appendix 3).
To further consider the effects of clinical presentations and stent technologies, we conducted subgroup analyses in the frequentist framework for patients with ACS and patients implanted with newer generation DES, by using published subpopulation data of the included trials.
Quality of evidence
Additionally, we assessed the quality of evidence using the GRADE framework with GRADEpro GDT,30 which characterises the quality of a body of evidence based on the study design, risk of bias, inconsistency, indirectness, imprecision, and other considerations, for each outcome.31 32 The GRADE approach rates the evidence as high, moderate, low, and very low quality. We also calculated the absolute effects in each comparison for all endpoints.33
Patient and public involvement
No patients or the public were involved in setting the research question or designing the study, nor were they involved in the outcome measures or implementation of the study. No patients were asked to advise on the interpretation or writing of the results. There were no plans to disseminate the results of the research to the study participants or relevant patient communities. It was not evaluated whether the studies included in the review had any patient involvement. It was not evaluated whether the studies included in the review had any patient involvement.
Results
Characteristics of included studies and bias assessment
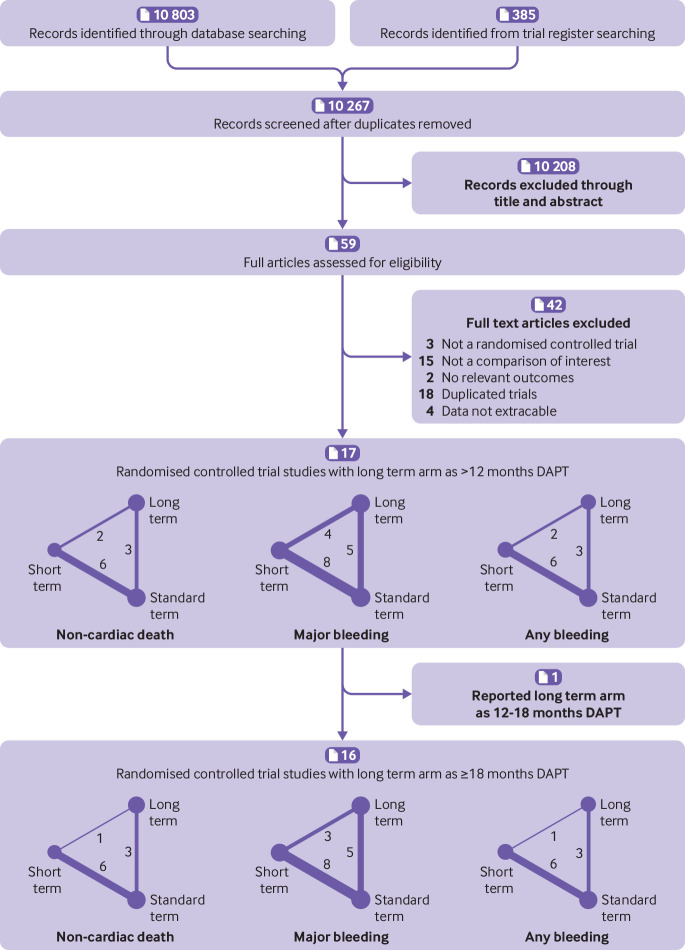
Figure 1 shows that overall, 10 803 citations met the search criteria, and the full text of 59 potentially eligible articles was scrutinized. All available studies from trial registries were included in the database search, resulting in 17 studies of 18 parallel randomised controlled trials from 2010 to 2018 and including 46 864 participants (range 1259-9961 in each study).5 6 7 8 9 34 35 36 37 38 39 40 41 42 43 44 45 The shortest duration of DAPT was three months and the longest duration was 48 months. Overall, 13 234 participants were randomly assigned to short term DAPT, 18 473 to standard term DAPT, and 15 157 to long term DAPT. All randomised controlled trials reported full clinical and demographic characteristics (table 1 and table A in appendix 3).
Fig 1.
Flowchart and network showing the procedure for identifying the relevant publications. Circular nodes show each treatment with the circle size indicating the total number of patients. The weight of the line and number on the line indicate the number of direct treatment comparisons within the same study
Table1.
Effects of treatment on outcomes in 17 studies
| Months of DAPT | Participants | All cause mortality | Cardiac death | Non-cardiac death | Myocardial infarction | Definite or probable stent thrombosis | Stroke | Net adverse clinical events | Major bleeding | Any bleeding |
|---|---|---|---|---|---|---|---|---|---|---|
| OPTIMA-C (Lee, 2018) | ||||||||||
| 6 | 683 | 2 | 1 | 1 | 1 | 0 | 0 | NA | 1 | NA |
| 12 | 684 | 3 | 2 | 1 | 1 | 1 | 2 | NA | 1 | NA |
| I-LOVE-IT 2 (Han, 2016) | ||||||||||
| 6 | 909 | 11 | 6 | 5 | 41 | 5 | 11 | 66 | 11 | 50 |
| 12 | 920 | 14 | 7 | 7 | 36 | 2 | 13 | 60 | 6 | 52 |
| IVUS-XPL study (Hong, 2016) | ||||||||||
| 6 | 699 | 5 | 3 | 2 | 1 | 2 | 6 | 15 | 5 | NA |
| 12 | 701 | 10 | 5 | 5 | 0 | 2 | 3 | 14 | 7 | NA |
| ISAR-SAFE (Schulz-Schupke, 2015) | ||||||||||
| 6 | 1997 | 8 | NA | NA | 13 | 5 | 7 | 29 | 2 | 27 |
| 12 | 2003 | 12 | NA | NA | 14 | 4 | 5 | 32 | 8 | 55 |
| SECURITY (Colombo, 2014) | ||||||||||
| 6 | 682 | 8 | 5 | 3 | 16 | 2 | 6 | 31 | 4 | 5 |
| 12 | 717 | 8 | 3 | 5 | 15 | 3 | 2 | 27 | 8 | 8 |
| OPTIMIZE (Feres, 2013) | ||||||||||
| 3 | 1563 | 43 | 29 | 14 | 49 | 13 | 5 | 93 | 10 | 35 |
| 12 | 1556 | 45 | 32 | 13 | 42 | 12 | 5 | 90 | 14 | 45 |
| EXCELLENT (Gwon, 2012) | ||||||||||
| 6 | 722 | 4 | 2 | 2 | 13 | 6 | 3 | 24 | 2 | 4 |
| 12 | 721 | 7 | 3 | 4 | 7 | 1 | 5 | 21 | 4 | 10 |
| RESET (Kim, 2012) | ||||||||||
| 3 | 1059 | 5 | NA | NA | 2 | 2 | 6 | NA | 2 | 5 |
| 12 | 1058 | 8 | NA | NA | 4 | 3 | 6 | NA | 6 | 10 |
| SMART-DATE (Hahn, 2018) | ||||||||||
| 6 | 1357 | 35 | 18 | 17 | 24 | 15 | 11 | NA | 6 | 35 |
| 12.6 to 18 | 1355 | 39 | 24 | 15 | 10 | 10 | 12 | NA | 10 | 51 |
| NIPPON (Nakamura, 2017) | ||||||||||
| 6 | 1654 | 16 | NA | NA | 4 | 2 | 7 | 34 | 11 | NA |
| 18 | 1653 | 7 | NA | NA | 1 | 1 | 6 | 24 | 12 | NA |
| ITALIC (Didier, 2017) | ||||||||||
| 6 | 926 | 11 | 5 | 6 | 12 | 6 | 6 | NA | 0 | NA |
| 24 | 924 | 20 | 5 | 15 | 9 | 3 | 7 | NA | 4 | NA |
| PRODIGY (Valgimigli, 2012) | ||||||||||
| 6 | 983 | 65 | NA | NA | 41 | 15 | 14 | NA | 6 | 34 |
| 24 | 987 | 65 | NA | NA | 39 | 13 | 21 | NA | 16 | 73 |
| OPTIDUAL (Helft, 2015) | ||||||||||
| 12 | 690 | 24 | NA | NA | 16 | 1 | 7 | 52 | 4 | 20 |
| 48 | 695 | 16 | NA | NA | 11 | 3 | 5 | 40 | 4 | 18 |
| DAPT Study (Mauri, 2014) | ||||||||||
| 12 | 4941 | 74 | 47 | 27 | 198 | 65 | 43 | NA | 26 | 137 |
| 30 | 5020 | 98 | 45 | 53 | 99 | 19 | 37 | NA | 38 | 263 |
| DES LATE (Lee, 2014) | ||||||||||
| 12 | 2514 | 32 | 19 | 13 | 27 | 11 | 21 | 74 | 24 | NA |
| 36 | 2531 | 46 | 28 | 18 | 19 | 7 | 21 | 89 | 34 | NA |
| ARCTIC-Interruption (Collet, 2014) | ||||||||||
| 12 | 624 | 9 | NA | NA | 9 | 3 | 4 | NA | 1 | 3 |
| 18 to 30 | 635 | 7 | NA | NA | 9 | 0 | 6 | NA | 7 | 12 |
| REAL-ZEST LATE (Park, 2010) | ||||||||||
| 12 | 1344 | 13 | 8 | 5 | 7 | 4 | 4 | NA | 1 | NA |
| 36 | 1357 | 20 | 13 | 7 | 10 | 5 | 9 | NA | 3 | NA |
DAPT=dual antiplatelet therapy; NA=not available
The risk of bias assessment was performed for each randomised controlled trial and summarised (table A in appendix 3). Most of the studies were in the lowest categories for risk of bias, random sequence generation (16/17, 94%), selective reporting (16/17, 94%), incomplete outcome data (15/17, 88%), and allocation concealment (13/17, 76%). A few studies were in the highest categories for risk of bias, blinding of participants and personnel (2/17, 12%), blinding of outcome assessment (4/17, 24%), and other bias (6/17, 35%). The category of unclear risk contained the most studies for other bias (10/17, 59%), blinding of participants and personnel (9/17, 53%), as well as blinding of outcome assessment (8/17, 47%).
Outcomes of network meta-analysis
All cause mortality, cardiac death, and non-cardiac death
We evaluated all studies reporting all cause mortality and 11 studies with a total of 32 826 participants reporting cardiac death. Table 1 shows that we also deduced non-cardiac death from all cause mortality and cardiac death.
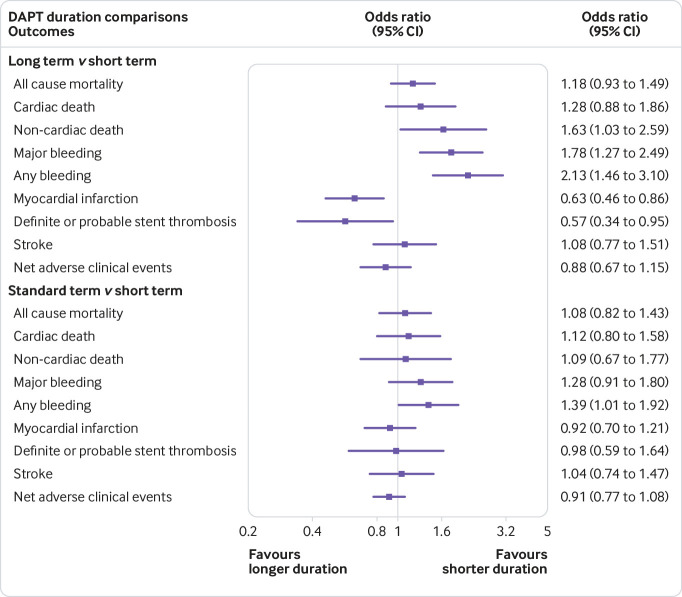
Although long term DAPT (>12 months) resulted in more non-cardiac death than short term (≤6 months) DAPT (odds ratio 1.63, 95% confidence interval 1.03 to 2.59, τ2=0.02), all cause mortality and cardiac death showed no significant differences (1.18, 0.93 to 1.49, 0; 1.28, 0.88 to 1.86, 0). Figure 2 shows that standard term DAPT showed rates similar to those of short term DAPT for the three endpoints.
Fig 2.
Network meta-analysis results of all endpoints between two pairs of duration of DAPT
Ischaemic and haemorrhagic endpoints
Table 1 shows that all studies reported myocardial infarction, definite or probable stent thrombosis, and major bleeding, and 11 studies with 31 194 participants reported any bleeding.
Compared with short term DAPT, long term DAPT decreased the risk of ischemia, myocardial infarction, and definite or probable stent thrombosis. Simultaneously, the risk of major bleeding and any bleeding (odds ratio 0.63, 95% confidence interval 0.46 to 0.86, τ2=0.17; 0.57, 0.34 to 0.95, 0.27; 1.78, 1.27 to 2.49, 0; 2.13, 1.46 to 3.10, 0.29) was increased. Standard term DAPT resulted in higher any bleeding than short term DAPT (1.39, 1.01 to 1.92, 0.29). Figure 2 shows that similar rates of myocardial infarction and definite or probable stent thrombosis were noted between standard term and short term DAPT.
Stroke and net adverse clinical events
Table 1 shows that all studies reported stroke and nine studies, with a total of 22 927 participants, reported net adverse clinical events. We noted that the three durations presented similar rates of these two outcomes.
Sensitivity analysis
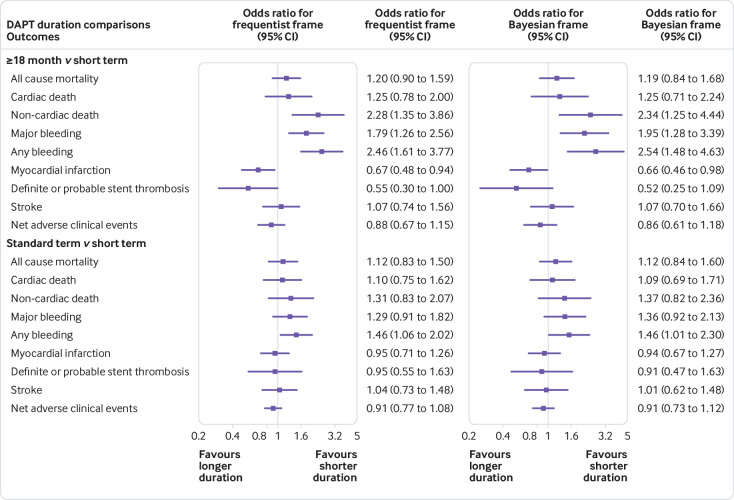
The SMART-DATE trial compared short term versus long term DAPT, which might weaken the discrimination among three arms.44 Thus, we excluded it to restrict the long term arm to ≥18 months of DAPT and generated a group of 16 studies with 44 152 patients. Figure 3 shows the results with more obvious differences, under both frequentist and Bayesian frameworks. Compared with short term DAPT, ≥18 months of DAPT resulted in higher rates of non-cardiac death (frequentist odds ratio 2.28, 95% confidence interval 1.35 to 3.86; Bayesian 2.34, 1.25 to 4.44), major bleeding (frequentist 1.79, 1.26 to 2.56; Bayesian 1.95, 1.28 to 3.39), and any bleeding (frequentist 2.46, 1.61 to 3.77; Bayesian 2.54, 1.48 to 4.63).
Fig 3.
Network meta-analysis results of all endpoints between two pairs of duration of DAPT
According to fig B2 in appendix 2, differences also presented between ≥18 months versus standard term DAPT in non-cardiac death (frequentist odds ratio 1.74, 95% confidence interval 1.23 to 2.47; Bayesian 1.71, 1.05 to 2.76), any bleeding (frequentist 1.68, 1.15 to 2.47; Bayesian 1.74, 1.02 to 2.82), and major bleeding (frequentist 1.39, 1.03 to 1.88; Bayesian 1.43, 0.99 to 2.27). These results indicated that the risk of non-cardiac death and bleeding increased synchronously when the duration of DAPT was increased.
Other endpoints presented similar efficacy and heterogeneity as the 17 studies group (fig 3, table C2 in appendix 3).
Subgroup analyses based on stent type and patient health
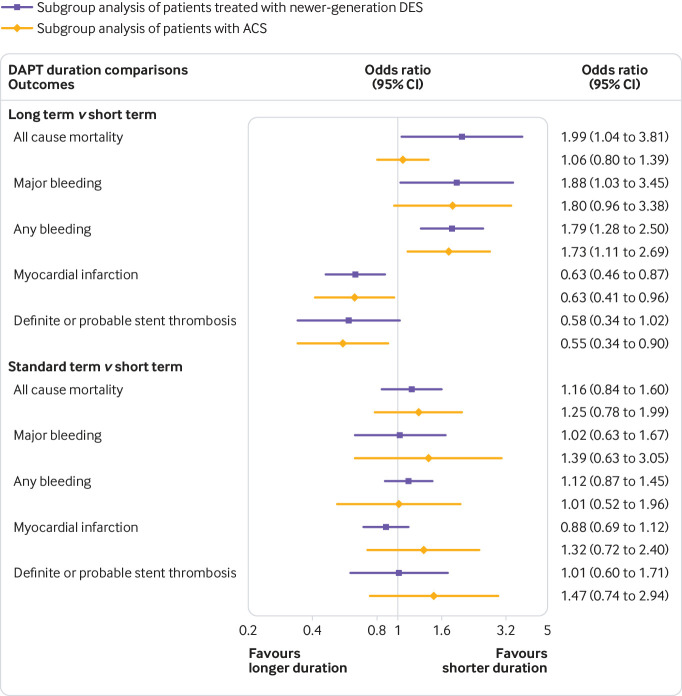
Newer-generation DES improve mortality and ischaemic outcomes compared with first-generation DES,46 47 and patients with ACS have higher ischaemic risks than patients with stable coronary artery disease.48 49 Thus, we regarded the patients with the two conditions derived from subgroup or pooled analyses of pertinent randomised controlled trials respectively.
Table 2 shows that 11 trials reported endpoints of newer-generation DES subgroup with 23 753 participants, and eight trials reported endpoints of ACS subgroup with 12 376 participants. In long term DAPT, higher risks of all cause mortality (odds ratio 1.99, 95% confidence interval 1.04 to 3.81), major bleeding (1.88, 1.03 to 3.45), and any bleeding (1.79, 1.28 to 2.50) were observed when compared with short term DAPT in the newer-generation DES subgroup, and merely an increased risk of any bleeding (1.73, 1.11 to 2.69) was noted in the ACS subgroup. Figure 4 shows that no significant difference was obtained for all endpoints between standard term and short term DAPT, in both subgroups.
Table 2.
Effects of treatments on outcomes in subgroups of patients
| Original study | Subgroup characteristic | Subgroup reference | Months of DAPT treatment | Total | All cause mortality | Myocardial infarction | Definite or probable stent thrombosis | Major bleeding | Any bleeding |
|---|---|---|---|---|---|---|---|---|---|
| Newer-generation DES | |||||||||
| OPTIMA-C (Lee, 2018) | BES/ZES | Lee, 20187 | 6 | 683 | 2 | 1 | 0 | 1 | NA |
| 12 | 684 | 3 | 1 | 1 | 1 | NA | |||
| I-LOVE-IT 2 (Han, 2016) | BP-SES | Han, 20168 | 6 | 909 | 11 | 41 | 5 | 11 | 50 |
| 12 | 920 | 14 | 36 | 2 | 6 | 52 | |||
| IVUS-XPL study (Hong, 2016) | EES | Hong, 201642 | 6 | 699 | 5 | 1 | 2 | 5 | NA |
| 12 | 701 | 10 | 0 | 2 | 7 | NA | |||
| SECURITY (Colombo, 2014) | ZES/BES/EES | Colombo, 201437 | 6 | 682 | 8 | 16 | 2 | 4 | 5 |
| 12 | 717 | 8 | 15 | 3 | 8 | 8 | |||
| OPTIMIZE (Feres, 2013) | ZES | Feres, 201335 | 3 | 1563 | 43 | 49 | 13 | 10 | 35 |
| 12 | 1556 | 45 | 42 | 12 | 14 | 45 | |||
| EXCELLENT (Gwon, 2012) | EES | Gwon, 20125 | 6 | 540 | 3 | 9 | 3 | 2 | 3 |
| 12 | 539 | 4 | 6 | 1 | 3 | 9 | |||
| SMART-DATE (Hahn, 2018) | EES/ZES/BES | Hahn, 201844 | 6 | 1357 | 35 | 24 | 15 | 6 | 35 |
| 12.6 to 18 | 1355 | 39 | 10 | 10 | 10 | 51 | |||
| NIPPON (Nakamura, 2017) | BES | Nakamura, 20179 | 6 | 1654 | 16 | 4 | 2 | 11 | NA |
| 18 | 1653 | 7 | 1 | 1 | 12 | NA | |||
| ITALIC (Didier, 2017) | EES | Didier, 201743 | 6 | 926 | 11 | 12 | 6 | 0 | NA |
| 24 | 924 | 20 | 9 | 3 | 4 | NA | |||
| PRODIGY (Valgimigli, 2012) | ZES/EES | Valgimigli, 201350 | 6 | 492 | 25 | 12 | 1 | 25 | NA |
| 24 | 496 | 30 | 16 | 4 | 30 | NA | |||
| DAPT Study (Mauri, 2014) | EES | Hermiller, 201651 | 12 | 2358 | 26 | 72 | 16 | 7 | 30 |
| 30 | 2345 | 49 | 48 | 6 | 21 | 57 | |||
| Patient health | |||||||||
| ISAR-SAFE (Schulz-Schupke, 2015) | Acute coronary syndrome | Lohaus, 201652 | 6 | 794 | 5 | 6 | 2 | 1 | 3 |
| 12 | 807 | 7 | 8 | 2 | 2 | 5 | |||
| IVUS-XPL study (Hong, 2016) | Acute coronary syndrome with 2nd generation stent | Jang, 201853 | 3 to 6 | 1119 | 9 | 7 | 6 | 4 | 9 |
| EXCELLENT (Gwon, 2012) | |||||||||
| 12 | 1097 | 11 | 9 | 4 | 6 | 14 | |||
| RESET (Kim, 2012) | |||||||||
| SMART-DATE (Hahn, 2018) | Acute coronary syndrome | Hahn, 201844 | 6 | 1357 | 15 | 24 | 15 | 6 | 35 |
| 12.6 to 18 months | 1355 | 10 | 10 | 10 | 10 | 51 | |||
| ITALIC (Didier, 2017) | Acute coronary syndrome | Didier, 201743 | 6 | 400 | 4 | 7 | 5 | 0 | NA |
| 24 | 406 | 9 | 6 | 3 | 2 | NA | |||
| PRODIGY (Valgimigli, 2012) | Acute coronary syndrome | Costa, 201554 | 6 | 733 | 56 | 39 | 5 | 6 | 3 |
| 24 | 732 | 52 | 33 | 10 | 9 | 18 | |||
| DAPT Study (Mauri, 2014) | Acute myocardial infarction | Yeh, 201555 | 12 | 1711 | 27 | 88 | 32 | 9 | 35 |
| 30 | 1805 | 24 | 39 | 9 | 13 | 76 |
DAPT=dual antiplatelet therapy; NA=not available
Fig 4.
Network meta-analysis results of subgroups based on patients treated with newer generation drug-eluting stent (DES) and patients with acute coronary syndrome (ACS) between two pairs of duration of DAPT
Therefore, long term DAPT might be associated with increased all cause mortality in patients implanted with newer-generation DES, and short term DAPT might be non-inferior to standard term DAPT independent of DES generation and clinical presentation.
Network coherence and quality of evidence
There was no noticeable difference between direct and indirect estimates in closed loops that allowed the assessment of network coherence in all endpoints (table C in appendix 3). The total residual deviance for the outcomes of all endpoints (table D in appendix 3) suggested a good model fit in the sensitivity analysis under the Bayesian framework. We verified the convergence of chains visually in the trace plots and by inspecting the Brooks-Gelman-Rubin diagnostic statistic with values of approximately one.28
A summary of the quality assessment of endpoints by the GRADE criteria was presented in table E in appendix 3. The quality of endpoints was determined to be moderate and high for most of the comparisons. Non-cardiac death and major bleeding were rated high.
Discussion
Principal findings
In our meta-analysis, which included 17 studies and 46 864 patients, we analysed the comparative efficacy and safety of three durations of DAPT after PCI with DES. We applied frequentist and Bayesian frameworks in intention-to-treat populations to increase confidence in our findings.
In patients with all clinical presentations, firstly, long term DAPT led to a higher risk of non-cardiac death and major bleeding than short term DAPT in patients, and the discrimination was more noticeable when restricting long term DAPT to ≥18 months. Secondly, myocardial infarction and stent thrombosis showed no obvious difference between short term and standard term DAPT, and standard term DAPT increased the risk of any bleeding. Thirdly, the risk of non-cardiac death and bleeding increased synchronously with increasing durations of DAPT. Fourthly, all cause mortality, cardiac death, stroke, and net adverse clinical events presented similar risks for the three durations.
In both subgroups of newer-generation DES and patients with ACS, long term DAPT was associated with higher bleeding events than short term DAPT, and short term DAPT showed similar efficacy and safety to standard term DAPT. Long term DAPT resulted in increased all cause mortality compared with short term DAPT in the newer-generation DES subgroup.
Comparison with other studies
Previous trials and pairwise meta-analyses, which were limited to two durations of DAPT as extended term or short term, failed to find disparate risks of mortality for different durations.56 57 Palmerini and colleagues conducted an individual patient data study showing variant ischaemic risks of three months of DAPT between patients with ACS and stable coronary artery diseases.11 However, the population of patients with ACS was 4758, which might affect the confidence in the conclusion. Another network meta-analysis studying the impacts of stent types and duration of DAPT, might be limited in clinical practice, owing to too many arms introduced.58
In our pooled analysis, we studied short term, standard term, and long term DAPT in both the general population of coronary artery disease and subgroups of patients with newer-generation DES or ACS to evaluate durations of DAPT in a succinct way.
Short term versus long term DAPT
NIPPON investigators reported that 18 months of DAPT seemed to incur less all cause mortality than six months of DAPT (7/1653 v 16/1654).9 However, a meta-analysis based on 10 randomised controlled trials concluded that a DAPT duration of more than one year was associated with increased mortality because of an increased risk of non-cardiovascular mortality.59 Another systematic review of 11 randomised controlled trials concluded that 18 to 48 months of DAPT showed no difference in all cause mortality compared with six to 12 months of DAPT.60
Our results support that non-cardiac death, instead of non-cardiovascular death, occurred less frequently with short term DAPT than with long term DAPT, and this effect was more apparent when long term DAPT was restricted to ≥18 months. This finding indicates that vascular death (such as death caused by cerebrovascular disease, dissecting aneurysm, or other vascular diseases)61 might play a role in long term DAPT. We also found that the risks of non-cardiac death and bleeding increased synchronously with prolonged duration of DAPT; this finding is supported by a study that reported shorter durations of DAPT were associated with a lower risk of bleeding-related death than longer durations of DAPT.10 Additionally, long term DAPT was related to a higher risk of all cause mortality in patients implanted with newer-generation DES, compared with short term DAPT.
Short term versus standard term DAPT
Randomised controlled trials have always concluded that short term DAPT was non-inferior to standard term DAPT.5 6 7 8 35 37 40 42 44 62 Piccolo’s analysis of 38 919 patients reported that long term DAPT exposure showed increased major adverse cardiac and cerebrovascular events (MACCE) through 90 days after DAPT continuation which was not observed in <12 months of DAPT.63 However, several studies showed that long term DAPT was associated with similar major adverse cardiac events and a higher risk of bleeding after PCI with DES compared with short term DAPT, regardless of diabetes diagnosis or sex.18 57 64
We extracted data regarding net adverse clinical events, a pooled outcome including MACCE and major bleeding, and found no difference among the three durations, which is supported by Palmerini’s individual patient data meta-analysis.11 In our analysis, the risk of bleeding was higher in standard term DAPT than in short term DAPT, and other endpoints (including ischemia-related and death-related endpoints) were noted with similar rates. Thus, compared with standard term DAPT, short term DAPT might present superiority with higher safety and similar efficacy in the general population of coronary artery disease. However, in subgroups of patients with newer-generation DES or ACS we did not observe a noticeable difference between short term and standard term DAPT for all endpoints.
Strengths and limitations of this study
The main strength of our study is that we divided the durations of DAPT into three categories with short term (≤6 months) DAPT as a control. With a combination of direct and indirect comparisons, network meta-analysis often leads to substantially more precise summary results.19 The frequentist results were confirmed in in Bayesian framework. Thus, these findings have robust statistical consistency. Furthermore, although the reviewed randomised controlled trials covered the past years of research, heterogeneity was low across trials. We tried our best to extract original data form each study and performed post hoc subgroup analyses according to these data.
This study has several limitations. Firstly, we primarily evaluated durations of DAPT based on clopidogrel, so the conclusion might vary when applying other P2Y12 inhibitors such as prasugrel and ticagrelor. Secondly, we performed analyses of outcomes from different trials with pooled definitions. Thirdly, several endpoints were not reported by a few trials, like cardiac death (RESET, OPTIDUAL, and NIPPON trial reported cardiovascular death only), which might partly explain the slight divergence between results under the frequentist and Bayesian frameworks.
Policy implications
Standard term (12 months) DAPT was the recommended duration for most patients in guidelines published between 2011 and 2014. However, some factors are important in determining the duration of DAPT, such as whether a patient has ACS, type of DES, and bleeding and ischaemic risks.3 65 Therefore, the current American College of Cardiology/American Heart Association guideline presented critical questions to choose among three to six months, 12 months, and more than 12 months of DAPT according to variant factors.3
According to our analysis, three to six months of DAPT with clopidogrel presented similar efficacy and safety to 12 months of DAPT in patients treated with newer-generation DES and patients with ACS, without considering personal haemorrhagic profiles. Though the guidelines recommended six months of DAPT to patients with ACS only when high bleeding risks were considered.2 3 Moreover, in a broader spectrum including patients with ACS and stable coronary artery disease, three to six months of DAPT was associated with higher safety than 12 months of DAPT. The present findings suggest additional benefit of three to six months of DAPT.
Additionally, compared with three to six months, long term DAPT was associated with higher all cause mortality in patients implanted with newer-generation DES, as well as higher non-cardiac death in the general population of patients with coronary artery disease. Therefore, it might be reasonable to apply long term DAPT to a narrower spectrum of patients.
Conclusion
Our comprehensive network meta-analysis provides evidence that short term DAPT (with clopidogrel) could be considered for most patients after PCI with DES. Long term DAPT resulted in more death and bleeding-related events, and standard term DAPT presented similar efficacy and safety. Further studies, such as prespecified randomised controlled trials of patients with newer-generation DES and ACS are required to validate the rationality of short term DAPT after PCI with DES.
What is already known on this topic
A longer duration of dual antiplatelet therapy (DAPT) for patients receiving DAPT after drug-eluting stent (DES) implantation is associated with an increased risk of bleeding, and a shorter duration of DAPT is associated with an increased risk of ischemia
Pairwise meta-analyses are limited to two durations of DAPT (short term and long term) and network meta-analyses are inclined to evaluate inappropriate arms
What this study adds
Short term DAPT (<6 months) is recommended for patients implanted with DES and treated with clopidogrel, in both the general population of patients with coronary artery disease and subgroups of patients with newer-generation DES or acute coronary syndrome
Long term DAPT (>12 months) resulted in more death and bleeding-related events
Standard term DAPT (12 months) showed similar efficacy and safety compared with short term DAPT
Acknowledgments
We thank Qing-Bo Xu, from King’s College London British Heart Foundation Centre, for his helpful comments on this study.
Web Extra.
Extra material supplied by the author
Supplementary materials: Search strategy
Supplementary materials: Supplementary figures
Supplementary materials: Supplementary tables
Contributors: JJC, XS, and HY contributed equally to this project and are joint first authors. SHLY and HY conceived and designed the project. HY and JJC supervised the project. HY, XS, and JJC performed the review and approval of the manuscript. SHLY, PX, BW, and HY contributed to the design of the study, writing the protocol, screening trials, data extraction, analysis and interpretation, and writing and final approval of the report. PX and BW contributed equally to this work. SHLY and HY generated the tables and figures. PX, SHLY, and BW assessed the quality of included trials. BW performed the literature search. SHLY, HY, and XS drafted the manuscript. SHLY, JJC, YL, QYW, MLZ, and JRW participated in revising the manuscript before submission. QYW, MLZ, and JRW contributed equally to the work. SHLY, HY, JJC, and XS participated in the formal revision, including data processing, statistical analysis, generating figures and tables, and text modification. All authors had full access to the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis. HY is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work was supported by grants from the Major Science and Technology Project of Hunan Province (No 2016SK1001), The National Natural Science Foundation of China (No 81770403 and No 81470535) and National Key Research and Development Projects (No 2016YFC0900802). The sponsors or funders had no involvements in any parts of this study. All authors confirm the independence of researchers from funding sources.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Major Science and Technology Project of Hunan Province, The National Natural Science Foundation of China, and National Key Research and Development Projects for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Not required.
Data sharing: No additional data are available.
The manuscript’s guarantor (HY) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned and registered have been explained.
References
- 1. Rollini F, Franchi F, Angiolillo DJ. Switching P2Y12-receptor inhibitors in patients with coronary artery disease. Nat Rev Cardiol 2016;13:11-27. 10.1038/nrcardio.2015.113. [DOI] [PubMed] [Google Scholar]
- 2. Valgimigli M, Bueno H, Byrne RA, et al. ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;2017. 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 3. Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016;68:1082-115. 10.1016/j.jacc.2016.03.513. [DOI] [PubMed] [Google Scholar]
- 4. Miyazaki Y, Suwannasom P, Sotomi Y, et al. Single or dual antiplatelet therapy after PCI. Nat Rev Cardiol 2017;14:294-303. 10.1038/nrcardio.2017.12. [DOI] [PubMed] [Google Scholar]
- 5. Gwon HC, Hahn JY, Park KW, et al. Six-month versus 12-month dual antiplatelet therapy after implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss After Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012;125:505-13. 10.1161/CIRCULATIONAHA.111.059022. [DOI] [PubMed] [Google Scholar]
- 6. Kim BK, Hong MK, Shin DH, et al. RESET Investigators A new strategy for discontinuation of dual antiplatelet therapy: the RESET Trial (REal Safety and Efficacy of 3-month dual antiplatelet Therapy following Endeavor zotarolimus-eluting stent implantation). J Am Coll Cardiol 2012;60:1340-8. http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/809/CN-00878809/frame.html. 10.1016/j.jacc.2012.06.043 [DOI] [PubMed] [Google Scholar]
- 7. Lee BK, Kim JS, Lee OH, et al. Safety of six-month dual antiplatelet therapy after second-generation drug-eluting stent implantation: OPTIMA-C Randomised Clinical Trial and OCT Substudy. EuroIntervention 2018;13:1923-30. 10.4244/EIJ-D-17-00792. [DOI] [PubMed] [Google Scholar]
- 8. Han Y, Xu B, Xu K, et al. Six Versus 12 Months of Dual Antiplatelet Therapy After Implantation of Biodegradable Polymer Sirolimus-Eluting Stent: Randomized Substudy of the I-LOVE-IT 2 Trial. Circ Cardiovasc Interv 2016;9:e003145. 10.1161/CIRCINTERVENTIONS.115.003145. [DOI] [PubMed] [Google Scholar]
- 9. Nakamura M, Iijima R, Ako J, et al. NIPPON Investigators Dual Antiplatelet Therapy for 6 Versus 18 Months After Biodegradable Polymer Drug-Eluting Stent Implantation. JACC Cardiovasc Interv 2017;10:1189-98. 10.1016/j.jcin.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 10. Palmerini T, Bacchi Reggiani L, Della Riva D, et al. Bleeding-Related Deaths in Relation to the Duration of Dual-Antiplatelet Therapy After Coronary Stenting. J Am Coll Cardiol 2017;69:2011-22. 10.1016/j.jacc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 11. Palmerini T, Della Riva D, Benedetto U, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur Heart J 2017;38:1034-43. 10.1093/eurheartj/ehw627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roth GA, Johnson C, Abajobir A, et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1-25. 10.1016/j.jacc.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arbel Y, Bennell MC, Goodman SG, Wijeysundera HC. Cost-Effectiveness of Different Durations of Dual-Antiplatelet Use After Percutaneous Coronary Intervention. Can J Cardiol 2018;34:31-7. 10.1016/j.cjca.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 14. Mehran R, Baber U, Steg PG, et al. Cessation of dual antiplatelet treatment and cardiac events after percutaneous coronary intervention (PARIS): 2 year results from a prospective observational study. Lancet 2013;382:1714-22. 10.1016/S0140-6736(13)61720-1. [DOI] [PubMed] [Google Scholar]
- 15. Palmerini T, Stone GW. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: conceptual evolution based on emerging evidence. Eur Heart J 2016;37:353-64. 10.1093/eurheartj/ehv712. [DOI] [PubMed] [Google Scholar]
- 16. Wang W, Liu J, Fang J, et al. The optimal discontinuation of dual antiplatelet therapy in patients undergoing percutaneous coronary intervention with drug-eluting stents: A meta-analysis of randomized trials. Int J Cardiol 2017;235:73-86. 10.1016/j.ijcard.2017.02.091. [DOI] [PubMed] [Google Scholar]
- 17. Spencer FA, Prasad M, Vandvik PO, Chetan D, Zhou Q, Guyatt G. Longer- Versus Shorter-Duration Dual-Antiplatelet Therapy After Drug-Eluting Stent Placement: A Systematic Review and Meta-analysis. Ann Intern Med 2015;163:118-26. 10.7326/M15-0083. [DOI] [PubMed] [Google Scholar]
- 18. Palmerini T, Sangiorgi D, Valgimigli M, et al. Short- versus long-term dual antiplatelet therapy after drug-eluting stent implantation: an individual patient data pairwise and network meta-analysis. J Am Coll Cardiol 2015;65:1092-102. 10.1016/j.jacc.2014.12.046. [DOI] [PubMed] [Google Scholar]
- 19. Riley RD, Jackson D, Salanti G, et al. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ 2017;358:j3932. 10.1136/bmj.j3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin S, Xu P, Wang B, et al. Should we shorten the duration of dual antiplatelet therapy following implanting drug-eluting stents in general population? A systematic review and network meta-analysis: PROSPERO; 2018 https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=99519.
- 21. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. [DOI] [PubMed] [Google Scholar]
- 22. Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations [published Online First: 2015/06/02]. Ann Intern Med 2015;162:777-84. 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J, Altman D, Sterne, et al. Chapter 8: Assessing risk of bias in included studies. In: Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 ed. The Cochrane Collaboration. https://handbook-5-1.cochrane.org/ 2011.
- 24. da Costa BR, Juni P. Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J 2014;35:3336-45. 10.1093/eurheartj/ehu424. [DOI] [PubMed] [Google Scholar]
- 25. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. 10.1371/journal.pone.0076654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu Y, Wang W, Zhang AB, Bai X, Zhang S. Epley and Semont maneuvers for posterior canal benign paroxysmal positional vertigo: A network meta-analysis. Laryngoscope 2016;126:951-5. 10.1002/lary.25688. [DOI] [PubMed] [Google Scholar]
- 27. Mills EJ, Thorlund K, Ioannidis JP. Demystifying trial networks and network meta-analysis. BMJ 2013;346:f2914. 10.1136/bmj.f2914. [DOI] [PubMed] [Google Scholar]
- 28. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55. [Google Scholar]
- 29. van Valkenhoef G, Dias S, Ades AE, Welton NJ. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis. Res Synth Methods 2016;7:80-93. 10.1002/jrsm.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]: McMaster University; 2015 (developed by Evidence Prime, Inc.) https://gradepro.org/
- 31. Puhan MA, Schünemann HJ, Murad MH, et al. GRADE Working Group A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 32. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9:e99682. 10.1371/journal.pone.0099682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schünemann H, Brożek J, Guyatt G, et al. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group Updated October 2013. https://gradepro.org/handbook
- 34. Valgimigli M, Campo G, Monti M, et al. Prolonging Dual Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia Study (PRODIGY) Investigators Short- versus long-term duration of dual-antiplatelet therapy after coronary stenting: a randomized multicenter trial. Circulation 2012;125:2015-26. 10.1161/CIRCULATIONAHA.111.071589. [DOI] [PubMed] [Google Scholar]
- 35. Feres F, Costa RA, Abizaid A, et al. OPTIMIZE Trial Investigators Three vs twelve months of dual antiplatelet therapy after zotarolimus-eluting stents: the OPTIMIZE randomized trial. JAMA 2013;310:2510-22. 10.1001/jama.2013.282183. [DOI] [PubMed] [Google Scholar]
- 36. Collet J, Silvain J, Barthélémy O, et al. Dual-antiplatelet treatment beyond 1 year after drug-eluting stent implantation (ARCTIC-Interruption): a randomised trial. Lancet 2014;384:1577-85. 10.1016/S0140-6736(14)60612-7 [DOI] [PubMed] [Google Scholar]
- 37. Colombo A, Chieffo A, Frasheri A, et al. Second-generation drug-eluting stent implantation followed by 6- versus 12-month dual antiplatelet therapy: the SECURITY randomized clinical trial. J Am Coll Cardiol 2014;64:2086-97. 10.1016/j.jacc.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 38. Lee CW, Ahn JM, Park DW, et al. Optimal duration of dual antiplatelet therapy after drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014;129:304-12. 10.1161/CIRCULATIONAHA.113.003303. [DOI] [PubMed] [Google Scholar]
- 39. Mauri L, Kereiakes DJ, Yeh RW, et al. DAPT Study Investigators Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 2014;371:2155-66. 10.1056/NEJMoa1409312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulz-Schüpke S, Byrne RA, Ten Berg JM, et al. Intracoronary Stenting and Antithrombotic Regimen: Safety And EFficacy of 6 Months Dual Antiplatelet Therapy After Drug-Eluting Stenting (ISAR-SAFE) Trial Investigators ISAR-SAFE: a randomized, double-blind, placebo-controlled trial of 6 vs. 12 months of clopidogrel therapy after drug-eluting stenting. Eur Heart J 2015;36:1252-63. 10.1093/eurheartj/ehu523. [DOI] [PubMed] [Google Scholar]
- 41. Helft G, Steg PG, Le Feuvre C, et al. OPTImal DUAL Antiplatelet Therapy Trial Investigators Stopping or continuing clopidogrel 12 months after drug-eluting stent placement: the OPTIDUAL randomized trial. Eur Heart J 2016;37:365-74. 10.1093/eurheartj/ehv481. [DOI] [PubMed] [Google Scholar]
- 42. Hong SJ, Shin DH, Kim JS, et al. IVUS-XPL Investigators 6-Month Versus 12-Month Dual-Antiplatelet Therapy Following Long Everolimus-Eluting Stent Implantation: The IVUS-XPL Randomized Clinical Trial. JACC Cardiovasc Interv 2016;9:1438-46. 10.1016/j.jcin.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 43. Didier R, Morice MC, Barragan P, et al. 6- Versus 24-Month Dual Antiplatelet Therapy After Implantation of Drug-Eluting Stents in Patients Nonresistant to Aspirin: Final Results of the ITALIC Trial (Is There a Life for DES After Discontinuation of Clopidogrel). JACC Cardiovasc Interv 2017;10:1202-10. 10.1016/j.jcin.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 44. Hahn JY, Song YB, Oh JH, et al. SMART-DATE investigators 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non--inferiority trial. Lancet 2018;391:1274-84. 10.1016/S0140-6736(18)30493-8. [DOI] [PubMed] [Google Scholar]
- 45. Park SJ, Park DW, Kim YH, et al. Duration of dual antiplatelet therapy after implantation of drug-eluting stents. N Engl J Med 2010;362:1374-82. 10.1056/NEJMoa1001266. [DOI] [PubMed] [Google Scholar]
- 46. Palmerini T, Benedetto U, Biondi-Zoccai G, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents: Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol 2015;65:2496-507. 10.1016/j.jacc.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 47. Kang SH, Chae IH, Park JJ, et al. Stent Thrombosis With Drug-Eluting Stents and Bioresorbable Scaffolds: Evidence From a Network Meta-Analysis of 147 Trials. JACC Cardiovasc Interv 2016;9:1203-12. 10.1016/j.jcin.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 48. Kukreja N, Onuma Y, Garcia-Garcia HM, Daemen J, van Domburg R, Serruys PW, Interventional Cardiologists of the Thoraxcenter (2000 to 2005) The risk of stent thrombosis in patients with acute coronary syndromes treated with bare-metal and drug-eluting stents. JACC Cardiovasc Interv 2009;2:534-41. 10.1016/j.jcin.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 49. Loh JP, Pendyala LK, Kitabata H, et al. Comparison of outcomes after percutaneous coronary intervention among different coronary subsets (stable and unstable angina pectoris and ST-segment and non--ST-segment myocardial infarction). Am J Cardiol 2014;113:1794-801. 10.1016/j.amjcard.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 50. Valgimigli M, Borghesi M, Tebaldi M, Vranckx P, Parrinello G, Ferrari R, PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY Investigators Should duration of dual antiplatelet therapy depend on the type and/or potency of implanted stent? A pre-specified analysis from the PROlonging Dual antiplatelet treatment after Grading stent-induced Intimal hyperplasia studY (PRODIGY). Eur Heart J 2013;34:909-19. 10.1093/eurheartj/ehs460. [DOI] [PubMed] [Google Scholar]
- 51. Hermiller JB, Krucoff MW, Kereiakes DJ, et al. DAPT Study Investigators Benefits and Risks of Extended Dual Antiplatelet Therapy After Everolimus-Eluting Stents. JACC Cardiovasc Interv 2016;9:138-47. 10.1016/j.jcin.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 52. Lohaus R, Michel J, MayMayer K, et al. Six Versus Twelve Months Clopidogrel Therapy After Drug-Eluting Stenting in Patients With Acute Coronary Syndrome: An ISAR-SAFE Study Subgroup Analysis. Sci Rep 2016;6:33054. 10.1038/srep33054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jang JY, Shin DH, Kim JS, et al. Optimal duration of DAPT after second-generation drug-eluting stent in acute coronary syndrome. PLoS One 2018;13:e0207386. 10.1371/journal.pone.0207386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Costa F, Vranckx P, Leonardi S, et al. Impact of clinical presentation on ischaemic and bleeding outcomes in patients receiving 6- or 24-month duration of dual-antiplatelet therapy after stent implantation: a pre-specified analysis from the PRODIGY (Prolonging Dual-Antiplatelet Treatment After Grading Stent-Induced Intimal Hyperplasia) trial. Eur Heart J 2015;36:1242-51. 10.1093/eurheartj/ehv038. [DOI] [PubMed] [Google Scholar]
- 55. Yeh RW, Kereiakes DJ, Steg PG, et al. DAPT Study Investigators Benefits and Risks of Extended Duration Dual Antiplatelet Therapy After PCI in Patients With and Without Acute Myocardial Infarction. J Am Coll Cardiol 2015;65:2211-21. 10.1016/j.jacc.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Elmariah S, Mauri L, Doros G, et al. Extended duration dual antiplatelet therapy and mortality: a systematic review and meta-analysis. Lancet 2015;385:792-8. 10.1016/S0140-6736(14)62052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gargiulo G, Windecker S, da Costa BR, et al. Short term versus long term dual antiplatelet therapy after implantation of drug eluting stent in patients with or without diabetes: systematic review and meta-analysis of individual participant data from randomised trials. BMJ 2016;355:i5483. 10.1136/bmj.i5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. D’Ascenzo F, Iannaccone M, Saint-Hilary G, et al. Impact of design of coronary stents and length of dual antiplatelet therapies on ischaemic and bleeding events: a network meta-analysis of 64 randomized controlled trials and 102 735 patients. Eur Heart J 2017;38:3160-72. 10.1093/eurheartj/ehx437. [DOI] [PubMed] [Google Scholar]
- 59. Palmerini T, Benedetto U, Bacchi-Reggiani L, et al. Mortality in patients treated with extended duration dual antiplatelet therapy after drug-eluting stent implantation: a pairwise and Bayesian network meta-analysis of randomised trials. Lancet 2015;385:2371-82. 10.1016/S0140-6736(15)60263-X. [DOI] [PubMed] [Google Scholar]
- 60. Bittl JA, Baber U, Bradley SM, Wijeysundera DN, Evidence Review Committee Members Duration of Dual Antiplatelet Therapy: A Systematic Review for the 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2016;134:e156-78. 10.1161/cir.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 61. Cutlip DE, Windecker S, Mehran R, et al. Academic Research Consortium Clinical end points in coronary stent trials: a case for standardized definitions. Circulation 2007;115:2344-51. 10.1161/CIRCULATIONAHA.106.685313. [DOI] [PubMed] [Google Scholar]
- 62. Kedhi E, Fabris E, van der Ent M, et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non--inferiority trial. BMJ 2018;363:k3793. 10.1136/bmj.k3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Piccolo R, Feres F, Abizaid A, et al. Risk of Early Adverse Events After Clopidogrel Discontinuation in Patients Undergoing Short-Term Dual Antiplatelet Therapy: An Individual Participant Data Analysis. JACC Cardiovasc Interv 2017;10:1621-30. 10.1016/j.jcin.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 64. Sawaya FJ, Morice MC, Spaziano M, et al. Short-versus long-term Dual Antiplatelet therapy after drug-eluting stent implantation in women versus men: A sex-specific patient-level pooled-analysis of six randomized trials. Catheterization and cardiovascular interventions: official journal of the Society for Cardiac Angiography & Interventions 2017;89:178-89. 10.1002/ccd.26653 [DOI] [PubMed] [Google Scholar]
- 65. Costa F, van Klaveren D, James S, et al. PRECISE-DAPT Study Investigators Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet 2017;389:1025-34. 10.1016/S0140-6736(17)30397-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials: Search strategy
Supplementary materials: Supplementary figures
Supplementary materials: Supplementary tables