Abstract
Aversive learning is thought to modulate perceptual thresholds, which can lead to overgeneralization. However, it remains undetermined whether this modulation is domain specific or a general effect. Moreover, despite the unique role of the visual modality in human perception, it is unclear whether this aspect of aversive learning exists in this modality. The current study was designed to examine the effect of visual aversive outcomes on the perception of basic visual and auditory features. We tested the ability of healthy participants, both males and females, to discriminate between neutral stimuli, before and after visual learning. In each experiment, neutral stimuli were associated with aversive images in an experimental group and with neutral images in a control group. Participants demonstrated a deterioration in discrimination (higher discrimination thresholds) only after aversive learning. This deterioration was measured for both auditory (tone frequency) and visual (orientation and contrast) features. The effect was replicated in five different experiments and lasted for at least 24 h. fMRI neural responses and pupil size were also measured during learning. We showed an increase in neural activations in the anterior cingulate cortex, insula, and amygdala during aversive compared with neutral learning. Interestingly, the early visual cortex showed increased brain activity during aversive compared with neutral context trials, with identical visual information. Our findings imply the existence of a central multimodal mechanism, which modulates early perceptual properties, following exposure to negative situations. Such a mechanism could contribute to abnormal responses that underlie anxiety states, even in new and safe environments.
SIGNIFICANCE STATEMENT Using a visual aversive-learning paradigm, we found deteriorated discrimination abilities for visual and auditory stimuli that were associated with visual aversive stimuli. We showed increased neural activations in the anterior cingulate cortex, insula, and amygdala during aversive learning, compared with neutral learning. Importantly, similar findings were also evident in the early visual cortex during trials with aversive/neutral context, but with identical visual information. The demonstration of this phenomenon in the visual modality is important, as it provides support to the notion that aversive learning can influence perception via a central mechanism, independent of input modality. Given the dominance of the visual system in human perception, our findings hold relevance to daily life, as well as imply a potential etiology for anxiety disorders.
Keywords: fear conditioning, fMRI, generalization, learning, visual system
Introduction
A major challenge facing organisms is distinguishing between alike stimuli (discrimination), while responding similarly, when stimuli are likely related (generalization). Both abilities are crucial for survival, enabling appropriate responses to diverse situations (Guttman and Kalish, 1956; Solomon and Moore, 1975; Rescorla, 1976; Shepard, 1987; McLaren and Mackintosh, 2002; Bouton, 2006). In the case of a conditioned stimulus (CS), which predicts an aversive unconditioned stimulus (US), theory and evidence suggest a response bias. While some studies report that aversive conditioning increases stimulus discrimination or detection (Li et al., 2008; Åhs et al., 2013), a large body of work indicates that aversive learning results in a wider generalization of the CS (Pavlov, 1927; Hearst, 1960; Watson and Rayner, 2000; Dunsmoor et al., 2009; Lissek, 2012; Vervliet et al., 2013; Dunsmoor and Paz, 2015). Wider generalization of a stimulus, paired with an aversive outcome, can facilitate a fast and efficient defensive behavior to similar stimuli. The decreased discrimination between these similar stimuli may occur already at the perceptual level (Schechtman et al., 2010; Resnik et al., 2011; Struyf et al., 2015; Zaman et al., 2015). Evidence supporting this mechanism arises from a recent series of studies, in which participants learned to associate neutral auditory tones (the CS) with aversive odors (Resnik et al., 2011), negative sounds (Resnik et al., 2011), or monetary loss (Laufer and Paz, 2012; Laufer et al., 2016). Following conditioning, participants exhibited increased auditory thresholds and failed to discriminate new tones from the original CS.
Changes in discrimination thresholds following aversive learning have been attributed to the activity of various brain regions, including the amygdala, insula, and anterior cingulate cortex (ACC; Laufer and Paz, 2012; Laufer et al., 2016). An alternate function of these brain regions, during aversive learning, may account for the inconsistent reports described above regarding discrimination. For example, insular activity was correlated with generalization (Laufer and Paz, 2012; Laufer et al., 2016), but also showed pattern similarity (less generalization) between the conditioned and aversive stimulus (Onat and Büchel, 2015). Furthermore, changes in the tuning properties of neurons in the primate amygdala may explain how stimulus generalization and better detection can exist side by side (Resnik and Paz, 2015).
Aversive stimuli may induce plasticity in early sensory regions via a central mechanism, independent of the specific input modality. Consistent with the perceptual hypothesis, studies in rodents (Aizenberg and Geffen, 2013) and humans (Laufer et al., 2016) provide evidence for the role of the auditory cortex in the underlying plasticity. However, despite the prominent role of the visual modality in human perception, the effect of visual aversive stimuli (as US) on discrimination, particularly of neutral basic features of visual perception (as CS), has been less studied (Dunsmoor and LaBar, 2013; Struyf et al., 2015). If indeed visual aversive learning also leads to alternations in visual discrimination, it would further imply the existence of a central mechanism. Furthermore, in humans, the visual system plays a role in the development of anxiety states. Thus, alternations in visual perception during aversive learning may underlie anxiety disorders, which are often characterized by overgeneralization (Jovanovic and Ressler, 2010; Lissek et al., 2010; Pitman et al., 2012; Lissek et al., 2014; Dunsmoor and Paz, 2015; Dymond et al., 2015; Laufer et al., 2016).
We tested the role of the visual system in overgeneralization with a set of five experiments. In these experiments, participants learned to associate auditory or visual neutral stimuli (CS) with aversive visual images (US). The effect of this pairing on auditory or visual discrimination thresholds was examined. In addition, we used functional magnetic resonance imaging (fMRI) to track brain activations during learning. We hypothesized that aversive images would induce an increase in discrimination thresholds for both auditory (frequency) and visual (contrast and orientation) features. We predicted that this increase will be accompanied by differences in brain activity measured during aversive visual learning, including in early visual brain regions.
Materials and Methods
Participants
A total of 315 healthy students (age range, 19–33 years; mean age, 24.12 years; SE, 0.11) from Ben-Gurion University of the Negev, Israel, were recruited for the study (Experiment 1: 99 participants, 61 females, 38 males; Experiment 2: 63 participants, 49 females, 14 males; Experiment 3: 58 participants, 36 females, 22 males; Experiment 4: 59 participants, 34 females, 25 males; Experiment 5: 36 participants, 30 females, 6 males). They received either a course credit or a monetary payment to compensate for their time. Experimental procedures for the behavioral studies were approved by the ethics committee of the Psychology Department at Ben-Gurion University of the Negev and by the Helsinki Committee of Soroka Medical Center, Beer-Sheva, Israel, for the fMRI experiments. All participants provided written informed consent. More information regarding participants included in each experiment is provided in the following relevant sections and in Table 1.
Table 1.
Composition of participants in each experiment (included and excluded)
| Exp no. | Group | Total | Included in total | Included females | Included males | Excluded due to unawareness to conditioning | Excluded due to image rating | Excluded due to low performance in memory tests | Excluded due to low performance in discrimination tasks |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Exp | 34 | 24 | 16 | 8 | 5 | 2 | 1 | 2 |
| Ctrl | 31 | 24 | 17 | 7 | 5 | 0 | 2 | 0 | |
| 24 h | 34 | 19 | 10 | 9 | 7 | 7 | 0 | 1 | |
| 2 | Exp | 38 | 29 | 24 | 5 | 6 | 0 | 2 | 1 |
| Ctrl | 25 | 19 | 15 | 4 | 4 | 0 | 1 | 1 | |
| 3 | Exp | 33 | 25 | 16 | 9 | 4 | 1 | 1 | 2 |
| Ctrl | 25 | 22 | 11 | 11 | 0 | 3 | 0 | 0 | |
| 4 | Exp | 30 | 30 | 15 | 15 | 0 | 0 | 0 | 0 |
| Ctrl | 29 | 29 | 19 | 10 | 0 | 0 | 0 | 0 | |
| 5 | Exp | 18 | 17 | 14 | 3 | 0 | 1 | 0 | 0 |
| Ctrl | 18 | 17 | 15 | 2 | 1 | 0 | 0 | 0 |
A total of 315 participants performed the experiment, and 255 participants were included in the analysis: 172 females, 83 males (mean age, 24.06 years; SE, 0.13). Sixty participants were excluded from analysis: 37 females, 23 males (mean age, 24.36 years; SE, 0.24). Exclusions were due to unawareness to conditioning during conditioning sessions, incoherent image rating, low performance in memory tests, or low performance in discrimination tasks (these criteria are detailed in the text). Exp, Experiment; Ctrl, control.
General experimental procedure
The framework for the experimental procedure was adapted from earlier studies (Resnik et al., 2011; Laufer and Paz, 2012). In all experiments, each participant first performed a two-alternative forced choice (2AFC) discrimination task to define his/her baseline perceptual thresholds [just noticeable difference (JND)] for the neutral stimuli, as follows: 1 or 2 kHz tones in the auditory discrimination experiment (Experiment 1), low- or high-contrast Gabors in the contrast discrimination experiment (Experiment 2), and black stripes in a vertical or horizontal orientation in the orientation discrimination experiments (Experiments 3, 4, and 5). Following the discrimination task, participants completed a conditioning (learning) session, where one of the neutral stimuli was paired with either aversive or neutral images (CS+), and the other stimulus with blank screens or scrambled images (CS−). Then, participants performed the discrimination task again for the two neutral stimuli. In a following stage, participants also performed a memory test to provide an indication for their attention to the stimuli during the conditioning session. Finally, participants performed a validation test (image rating) for the level of emotional arousal they experienced while watching images from both categories (aversive/neutral). Some of the participants in Experiments 1–3 were also recalled after 24 h and repeated the discrimination task, enabling us to estimate the stability of the effects over time. In Experiment 4, we conducted the conditioning session inside an MRI scanner and recorded brain activity during learning using functional imaging (fMRI). In Experiment 5, we measured participants' pupil sizes during the conditioning session and the validation test, as a physiological marker for the level of emotional arousal. A schematic representation of the experimental procedure is presented in Figure 1a. For a more detailed description of each task, see the relevant sections below and Table 2.
Figure 1.
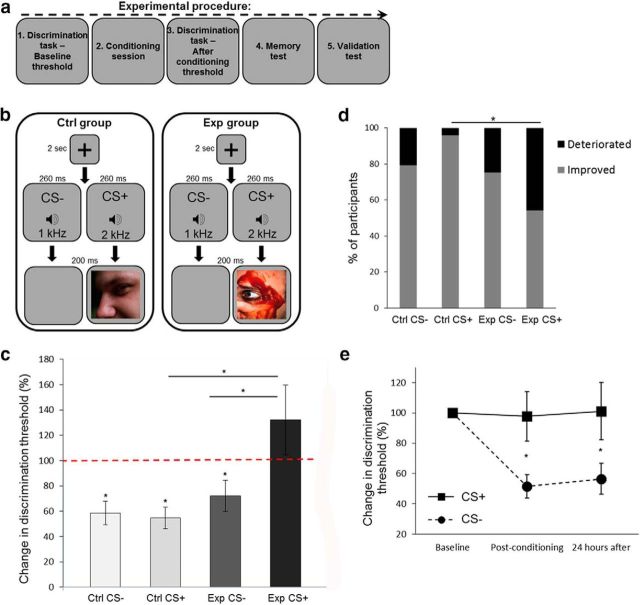
The effect of visual aversive conditioning on auditory discrimination thresholds (Experiment 1). a, A general description of the experimental protocol. b, A schematic representation of one trial from the conditioning session of the auditory experiment (Experiment 1). High (2 kHz) or low (1 kHz) pure tones were counterbalanced as CS+ (followed by images) or CS− (followed by blank screens). Images were aversive in the experimental group (Exp) and neutral in the control group (Ctrl). c, Discrimination thresholds for tone frequency were tested before and after conditioning (experimental group, n = 24; control group, n = 24). Discrimination thresholds before conditioning were normalized to 100% (red dashed line), which was the reference for postconditioning thresholds. A decrease in threshold (improvement) was measured in both groups for the CS− tone, and in the control group for the CS+ tone. The experimental group showed a deterioration in performance for the CS+ tone (paired with aversive images), compared with its results for the CS− tone, and to the CS+ tone in the control group, with a CS × group interaction effect. d, More experimental group participants deteriorated in discrimination thresholds for the CS+ tone compared with the control group. The gray bars show the percentage of participants for whom the postconditioning threshold was lower than the preconditioning threshold (improvement), and the black bars show the fraction of participants for whom the postconditioning threshold was higher (deterioration). e, Change in discrimination thresholds (compared with baseline) for the aversive CS+ (solid line) and the CS− (dashed line) of the experimental group (n = 19), immediately after conditioning and 24 h later. The stability of the thresholds persisted after 24 h. *p < 0.05, see text for details regarding specific significance values.
Table 2.
Experimental parameters
| Exp no. | CS type | CS duration in discrimination tasks | CS duration in conditioning session | Fixation duration in conditioning session | US duration in conditioning session | ITI duration in conditioning session | Stimulus type after CS− | Participants were informed of conditioning before experiment | Reinforcement rate | No. of trials per condition |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Sounds (frequency) | 260 ms | 260 ms | 2 s | 200 ms | 2–3 s | Blank screen | No | 100% | 30 CS+ |
| 30 CS− | ||||||||||
| 2 | Gabors (contrast) | 2 s | 1 s | 2 s | 200 ms | 2–3 s | Blank screen | No | 100% | 30 CS+ |
| 30 CS− | ||||||||||
| 3 | Stripes (orientation) | 0.3 s | 1 s | 2 s | 200 ms | 2–3 s | Blank screen | No | 100% | 30 CS+ |
| 30 CS− | ||||||||||
| 4 | Stripes (orientation) | 0.3 s | 2 s | 3 s | 400 ms | 5–6 s | Scrambled image | Yes | 50% | Reinforced: |
| 30 CS+ | ||||||||||
| 30 CS− | ||||||||||
| Nonreinforced: | ||||||||||
| 30 CS+ | ||||||||||
| 30 CS− | ||||||||||
| 5 | Stripes (orientation) | 0.3 s | 2 s | 3 s | 400 ms | 4–5 s | Scrambled image | No | 100% | 30 CS+ |
| 30 CS− |
Parameters of discrimination tasks and conditioning sessions are detailed for each experiment. Exp, Experiment.
Discrimination tasks
The task was a 2AFC. In each step, two neutral stimuli were presented to the participant in a random order, as follows: the original stimulus (the CS+ or CS−); and an additional stimulus with the same physical property. These two stimuli differed only in the quantity of their physical property (x and x + Δx, respectively, where x is the original magnitude of the physical property of the CS, and Δx is a small difference in this physical quantity). Participants had to decide (within a maximum duration of 10 s) which stimulus of these two options had the larger magnitude (x + Δx). In particular, in Experiment 1 two tones were presented (in a random order) in each trial of the discrimination task, f and f + Δf, where f is the tone frequency, and participants were asked: “Which tone had a higher pitch: first/second?” In Experiment 2, two Gabors were presented in each step, c and c + Δc, where c is the contrast of the Gabor, and we asked the participants: “Which stimuli had a higher contrast level: first/second?” In Experiments 3, 4, and 5, two black stripes were presented in different orientations, a and a + Δa, where a is the rotation angle of the stripe from a baseline orientation, and the question to the participants was as follows: “Which stripe was more rotated clockwise: first/second?” No feedback was provided (except for a short practicing session at the beginning of the task, where feedback was presented on the screen).
The task was an adaptive two-down one-up staircase converging procedure. The magnitude of Δx (difference between the two stimuli) was decreased after two correct answers and increased after one wrong answer. The task continued until six wrong answers were obtained. The procedure converged at the stimulus level (Δx), in which the probability of a “down” response (decrease in Δx) was equal to the probability of an “up” response (increase in Δx). If x was the original magnitude, Δx converged to the magnitude difference, at which a stimulus of x + Δx was correctly discriminated from x at the 70.7% level (Levitt, 1971). Discrimination thresholds (JND) for each participant are presented as a percentage of the original stimulus magnitude, that is, Δx/x. Specific task parameters for each experiment are detailed in the Experimental stimuli section below and in Table 2.
Conditioning session
Two neutral stimuli (described in the General experimental procedure section for each experiment) were presented in this stage. One of them was assigned as CS+ and the other as CS− (30 CS+ trials and 30 CS− trials randomly counterbalanced across participants in each experiment). Each trial began with the presentation of a fixation point on the screen, followed by the presentation of the CS+ or CS− (randomly). Immediately after this (zero delay and zero overlap), an image (for CS+ stimuli) or a blank screen (for CS− stimuli) was presented. In Experiments 4 and 5, a scrambled image (the original image cut into square blocks and shuffled) was presented after the CS−. The scrambled images were used instead of the blank screens, when measuring pupil size or brain activity, to eliminate the content of the images, while maintaining a constant luminance between the two conditions. Images were aversive in the experimental group and neutral in the control group. An example of one conditioning session trial for each group in each experiment is presented in Figures 1b (Experiment 1), 2a (Experiment 2), 3a (Experiment 3), and 4a (Experiment 4). Specific parameters for each experiment are detailed in the Experimental stimuli section below and in Table 2.
Figure 2.
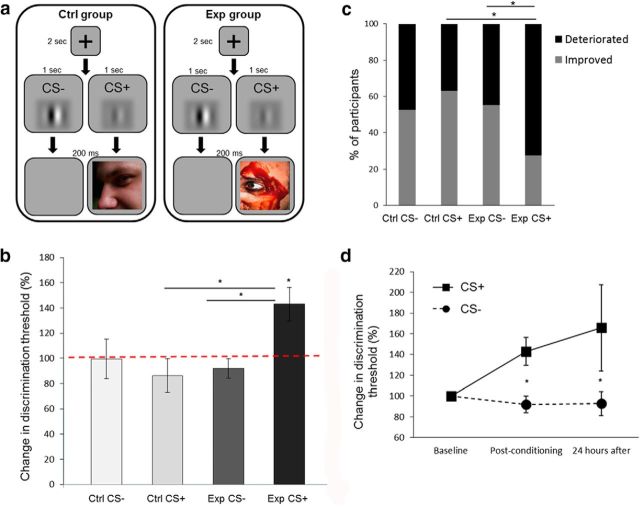
The effect of visual aversive conditioning on contrast discrimination thresholds (Experiment 2). a, A schematic representation of one trial from the conditioning session of the contrast discrimination experiment (Experiment 2). The paradigm was similar to the one described in Figure 1b, except for the presentation of a high- or low-contrast Gabor as the CS+ or CS−. b, Discrimination thresholds for contrast were tested before and after the conditioning session (experimental group. n = 29; control group, n = 19). No change in thresholds was measured in both groups for the CS− Gabors, or in control group participants for the CS+ Gabor. However, the increase in threshold (deterioration in performance) was measured in the experimental group for the CS+ Gabor (paired with aversive images), with a CS × group interaction effect. c, The majority of participants from the experimental group showed a deterioration in discrimination after conditioning to the CS+ Gabor. This percentage of participants was higher compared with the CS− Gabor in the same group and compared with participants in the control group for the CS+ Gabor. The gray bars show the percentage of participants for whom the postconditioning threshold was lower than the preconditioning threshold (improvement), and the black bars show the percentage of participants for whom their postconditioning threshold was higher (deterioration). d, Change in discrimination thresholds (compared with baseline) for the aversive CS+ (solid line) and the CS− (dashed line) stimuli of the experimental group (n = 20), immediately after and 24 h following conditioning. The stability of the thresholds persisted after 24 h. *p < 0.05, see text for details regarding specific significance values.
Figure 3.
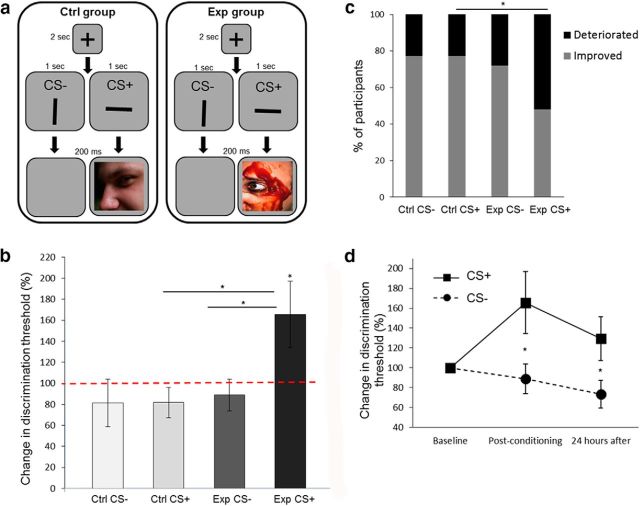
The effect of visual aversive conditioning on orientation discrimination thresholds (Experiment 3). a, A schematic representation of one trial from the conditioning session of the orientation discrimination experiment (Experiment 3). The paradigm was similar to the one described in Figure 1b, except for the presentation of a vertical or horizontal black stripe as the CS+ or CS−. b, Discrimination thresholds for orientation were tested before and after the conditioning session (experimental group, n = 25; control group, n = 22). No change in thresholds was measured in both groups for the CS− stripes, or in the control group for the CS+ stripe. However, experimental group participants deteriorated (increase in threshold) in the CS+ orientation (paired with aversive images). c, The majority of participants from the experimental group showed a deterioration in discrimination thresholds after conditioning to the CS+ stripe. This percentage of participants was higher compared with the control group for the same stripe. The gray bars show the percentage of the participants for whom the postconditioning threshold was lower than the preconditioning threshold (improvement), and the black bars show the percentage of participants for whom the postconditioning threshold was higher (deterioration). d, Change in discrimination thresholds (compared with baseline) for the aversive CS+ (solid line) and the CS− (dashed line) of the experimental group (n = 23), immediately after and 24 h after conditioning. The stability of the thresholds persisted after 24 h. *p < 0.05, see text for details regarding specific significance values.
Figure 4.
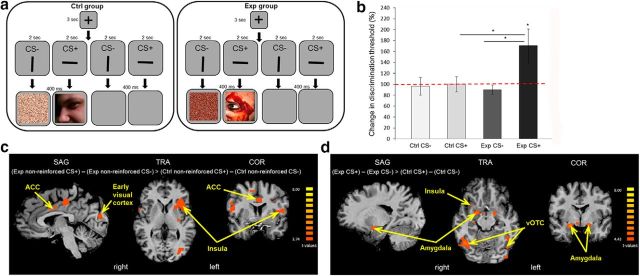
Brain activity is modulated by visual aversive learning (Experiment 4). a, A schematic representation of one trial from the conditioning session of the fMRI experiment (Experiment 4). The paradigm was similar to the one described in Figure 3a, except for a partial conditioning of 50%: half of the trials were reinforced trials (CS+ stripe paired with image or CS− stripe paired with scrambled image), and the other half were nonreinforced trials (CS+ or CS− stripes paired with blank screens). b, Discrimination thresholds for orientation were tested before and after the conditioning session (experimental group, n = 30; control group, n = 29). No change in thresholds was measured in both groups for the CS− stripe, or in the control group for the CS+ stripe. The experimental group deteriorated (increase in threshold) in the CS+ orientation (paired with aversive images), with a CS × group interaction effect. c, A contrast of (experimental group nonreinforced CS+ trials − experimental group nonreinforced CS− trials) > (control group nonreinforced CS+ trials − control group nonreinforced CS− trials) showed higher activations in the ACC, insula, and early visual cortex in the experimental group (Exp) compared with the control group (Ctrl). Activations are shown in statistical q(FDR) < 0.05 thresholds. d, A contrast of (experimental group CS+ trials − experimental group CS− trials) > (control group CS+ trials − control group CS− trials) showed higher activations in the amygdala, insula, and vOTC in the Exp compared with the Ctrl. Activations are shown in statistical q(FDR) < 0.05 thresholds. *p < 0.05, see text for details regarding specific significance values.
At the end of the conditioning session, participants were asked whether they noticed the association between the neutral stimuli and the presentation of the images (except for Experiment 4, in which we a priori instructed participants to be aware of the conditioning, without telling them which CS will be followed by the image). In the main analysis, we analyzed only the results of participants who could explicitly report that they had noticed the conditioning manipulation. This is consistent with previous studies, reporting that only participants that were aware of the conditioning [with International Affective Picture System (IAPS) images or electrical shocks as US] showed significant differences in conditioned reaction (e.g., skin conductance) to the CS (Tabbert et al., 2006; Dawson et al., 2007; Klucken et al., 2009). To strengthen the validity of our exclusion criteria, we also analyzed the results of Experiment 1 with the excluded participants. We calculated the differences between CS+ and CS− discrimination thresholds of all the participants in the experimental group (including participants from the 24 h group). Using bootstrap analysis, we sampled (randomly with replacement) from the population of participants, which we included in the main analyses, a number of samples that was equal to the number of excluded participants. Then, we calculated the mean of those samples. This calculation was iterated 100,000 times to create a histogram of the means. We compared the distribution of this histogram to the mean of the excluded participants. The mean value for participants, who were not aware of the conditioning, was out of the 99% range of the bootstrap distribution. Thus, we used this exclusion criterion in all experiments in an unbiased way. Table 1 summarizes data about excluded participants in each experiment.
Memory test
The memory test was conducted to provide an indication for participants' attention during the conditioning session. In this test, participants observed images, some of which were new and some of which were presented in the conditioning session. For each image, participants were required to decide whether it was new or old. Participants with a very low performance in the memory test (<55% success), which calls into question their attention to the stimuli during the conditioning session, were excluded from analysis (Table 1).
Validation test
This test was performed to validate the level of emotional arousal that participants experienced while watching the images during the conditioning session. Participants watched all the images that were presented in the conditioning session, and 10 more images selected randomly from the second category (aversive/neutral), to allow a balanced rating procedure. Participants were asked to rate each image by answering the following question: “How intensive is the emotion you feel while watching this image?” Rating was performed using an analog scale of 1 (not emotional at all) to 9 (very emotional). This allowed us to compare the ratings of each participant across the two categories of images.
In the main analysis, we only analyzed results of participants who had a difference of least 1.5 points between their ratings of the aversive and neutral categories, and who rated the aversive images with an average score of >4 (scale, 1–9). These values were chosen based on our cutoff criteria for image selection from the IAPS database (see the Experimental stimuli section below). We tested the validity of this exclusion criteria by also analyzing the results of Experiment 1 with the excluded participants. We calculated the differences between CS+ and CS− discrimination thresholds of all participants in the experimental group (including participants from the 24 h group). Then, we used bootstrap analysis, as described in the Conditioning session section above. The mean value for participants who did not rate the images by our criteria was out of the 99% range of the bootstrap distribution. Hence, we used this exclusion criterion in all experiments in an unbiased way. Table 1 summarizes the data about excluded participants in each experiment.
Experimental stimuli
Experiments were conducted in a dimly lit room. Participants sat in front of a PC screen, while their head was positioned in a chinrest and their eyes were located 60 cm from the screen. The conditioning session in Experiment 4 was conducted inside an MRI scanner and presented on an LCD screen located in the back of the scanner bore, behind the participant's head. Inside the scanner, participants viewed the stimuli through a tilted mirror mounted above their eyes on the head coil. Stimulus generation, presentation, and behavioral data acquisition and analysis were implemented in MATLAB (MathWorks). Specific experimental parameters for each experiment are described next and summarized in Table 2.
Experiment 1.
The auditory conditioned stimuli were pure tones of either 1 or 2 kHz (counterbalanced as CS+/CS− across participants) with a duration of 250 ms and onset/offset ramps of 5 ms, for a total of 260 ms. Tones were delivered through headphones (model SHL3000, Philips). In the discrimination tasks, Δf (change from original frequency) was 10% of the original tone at the beginning and was increased/decreased according to a participant's performance. In the conditioning session, each trial began with the presentation of a fixation screen for 2 s, then the tone was delivered. Following this, an image for CS+ trials or a blank screen for CS− trials was presented for 200 ms. Intertrial intervals randomly ranged between 2 and 3 s.
Experiment 2.
The conditioned stimuli were Gabors, presented in the middle of the screen (size, 50 × 50 mm; 128 × 128 pixels; visual angle, 4.77°) at two different and easy to distinguish contrast levels (based on pilot experiments). Contrast level (C) was calculated as (Cmax − Cmin)/(Cmax + Cmin), with Cmax and Cmin representing the highest and lowest luminance, respectively, of each Gabor. A Gabor with high contrast of 140 and a Gabor with low contrast of 60 were used as the CS+/CS− stimuli (counterbalanced across participants). In the discrimination task, each trial included the presentation of the first Gabor for 2 s, then a random pattern of white noise was presented (in the center of the screen, within a square at the same size of the Gabor) for 0.1 s, followed by the second Gabor for 2 s. Δc between the two Gabors in each trial started at 10% from the original contrast and was modified according to performance. In the conditioning session, each trial began with the presentation of a fixation screen for 2 s, then the Gabor was presented for 1 s. Following Gabor presentation, an image for CS+ trials or a blank screen for CS− trials was presented for 200 ms. Intertrial intervals randomly ranged between 2 and 3 s.
Experiment 3.
The conditioned stimuli were black stripes, presented in the center of the screen (size, 60 × 7 mm; 153 × 18 pixels; visual angle, 5.72°), in two different orientations (counterbalanced as CS+/CS− across participants). One of the stripes was at a 2° angle from the horizontal orientation (“horizontal stripe”), and the other, in a 2° angle from the vertical orientation (“vertical stripe”). We did not use straight horizontal/vertical orientations, because this could have made the orientation changes in the discrimination tasks too easy to distinguish. In the discrimination tasks, each trial included the presentation of the first black stripe for 0.3 s, then a white noise was presented (in the center of screen, within a square at the same size of the stimuli) for 1.3 s, followed by the second black stripe for 0.3 s. Δa between the stripes started at 5° and was modified according to performance. In the conditioning session, each trial began with the presentation of a fixation screen for 2 s, then the stripe was presented for 1 s. Following stripe presentation, an image for CS+ trials or a blank screen for CS− trials was presented for 200 ms. Intertrial intervals randomly ranged between 2 and 3 s.
Experiment 4.
The conditioned stimuli were black stripes counterbalanced as CS+/CS− across participants, as in Experiment 3. Discrimination tasks were the same as in Experiment 3. During the conditioning session of this experiment, brain activity was recorded using functional imaging (fMRI). Here, we used 50% partial conditioning: 30 nonreinforced CS+ trials and 30 nonreinforced CS− trials were added to the 30 CS+ and 30 CS− reinforced trials. The nonreinforced trials included the presentation of the CS+ or CS− (black stripes) without pairing them to the US. The nonreinforced trials were randomly interleaved between the reinforced trials, so participants could not predict which stripe would be associated with an image and which would not. In this manner, we could measure brain activity that resulted from learning and not from the presentation of the US (images). The conditioning session was divided into three separate event-related runs. Each run consisted of 10 trials of each kind (CS+, CS−, nonreinforced CS+, and nonreinforced CS−). Each trial of the conditioning session began with the presentation of a fixation screen for 3 s, then the stripe was presented for 2 s. Following stripe presentation, an image for CS+ trials, a scrambled image for CS− trials (with shuffled square blocks of 8 * 8 pixels), or blank screens for nonreinforced CS+/CS− trials were presented for 400 ms. Intertrial intervals randomly ranged between 5 and 6 s. We used longer stimulus presentation durations (relative to Experiments 1–3), because longer trials were required to enable brain activity to return to its baseline values before the beginning of each trial. Participants completed an MRI scanning session, which included a 3D anatomical scan, two resting-state runs, two amygdala localizer runs, and three event-related conditioning session runs. Only the anatomical scan and the conditioning session scans were used and analyzed in the current study.
Experiment 5.
The conditioned stimuli were black stripes counterbalanced as CS+/CS− across participants, as in Experiment 3. Discrimination tasks were the same as in Experiment 3. In this experiment, pupil size was recorded during the conditioning session and the validation test to obtain a physiological validation for the intensity of arousal that participants experienced during image watching. In the conditioning session, each trial began with the presentation of a fixation screen for 3 s, then the stripe was presented for 2 s. Following stripe presentation, an image for CS+ trials or a scrambled image for CS− trials (with shuffled square blocks of 8*8 pixels) was presented for 400 ms. Intertrial intervals randomly ranged between 4 and 5 s. We used longer stimulus presentation durations (relative to Experiments 1–3), because longer trials were needed to allow pupil size to return to its baseline value before the beginning of each trial. To measure pupil size during the validation test, we presented the scrambled version of each image for 3 s at the beginning of each trial and then the original image for 6 s (Bradley et al., 2008). After the presentation of the original image, participants were asked to rate it (see the Validation test section above). The intertrial intervals in the validation test were 4 s.
Images.
The visual US in the conditioning sessions were images from the IAPS database (Lang, 2008). Images were emotionally aversive for the experimental group or neutral for the control group. A total of 100 images were used in this study. Half of them were previously validated as emotionally aversive and got low scores at the valence index (<4 of 9; mean score, 1.94 ± 0.07) and high scores at the arousal index (>4 of 9; mean score, 6.37 ± 0.08). These images included, for example, scenes of crimes, accidents, and injured body parts. The other half of the images were previously validated as neutral and received average to high scores at the valence index (>5.5; mean score, 7.18 ± 0.11) and low scores at the arousal index (<4; mean score, 4.05 ± 0.11). These images included scenes of landscapes, people, or everyday objects (Lang, 2008). We validated that an equal number of faces and body parts appeared in each category of images. In an independent rating procedure completed at the end of the experimental session (validation test), we further verified that images were rated as aversive/neutral by our participants (see the Validation test section above). All images used in the experiment were presented at the center of gaze. The numbers of the selected images from the IAPS database are as follows: aversive images numbers, 2352.2, 3000, 3010, 3015, 3016, 3019, 3030, 3051, 3053, 3059, 3060, 3061, 3062, 3063, 3064, 3068, 3069, 3071, 3080, 3100, 3101, 3102, 3103, 3110, 3120, 3130, 3131, 3140, 3150, 3168, 3170, 3195, 3212, 3213, 3225, 3250, 3261, 3266, 3301, 3350, 3400, 3550, 6415, 8230, 9040, 9325, 9405, 9410, 9420, and 9433; neutral images numbers, 1450, 1605, 1900, 2026, 2039, 2102, 2151, 2156, 2191, 2217, 2222, 2235, 2273, 2299, 2308, 2314, 2332, 2339, 2342, 2347, 2358, 2359, 2370, 2377, 2382, 2384, 2388, 2390, 2393, 2411, 2488, 2530, 2594, 2980, 5210, 5390, 5831, 5836, 7001, 7009, 7026, 7041, 7052, 7493, 7505, 7507, 7509, 7512, 7513, and 9260.
MRI setup
Participants were scanned in a 3 T Ingenia scanner (Philips) equipped with a standard head coil, located at the Soroka Medical Center, Beer Sheva, Israel. fMRI BOLD contrast was acquired using the gradient-echo echoplanar imaging sequence with parallel acquisition (SENSE: factor 2.8). The specific scanning parameters were as follows: whole-brain coverage, 35 slices (3 × 3 × 3 mm3); transverse orientation; thickness, 3 mm; no gap; TR, 2000 ms; TE, 35 ms; flip angle, 90°; FOV, 256 × 256; matrix size, 96 × 96. High-resolution anatomical volumes were acquired with a T1-weighted 3D pulse sequence (1 × 1 × 1 mm3; 170 slices).
fMRI data analysis
The BrainVoyager QX software package (version 2.8, Brain Innovation) and MATLAB (MathWorks) were used for analysis. Preprocessing included 3D motion correction, slice time correction, filtering of low temporal frequencies (slow drift), and spatial smoothing with a Gaussian kernel of 6 mm FWHM. We analyzed each conditioning session run separately for each participant, using a whole-brain general linear model (GLM), conducted on a voxelwise level. Then we grouped together the data of all runs and all participants to create a random-effects group analysis GLM. The four conditioning session events were used as predictors (CS+ trials, CS− trials, nonreinforced CS+ trials, and nonreinforced CS− trials). To define brain activity differences in aversive compared with nonaversive learning, we used a contrast of (experimental group nonreinforced CS+ trials) − (experimental group nonreinforced CS− trials > control group nonreinforced CS+ trials − control group nonreinforced CS− trials). In addition, to validate the aversive nature of learning in the experimental group compared with the control group, we used a contrast of (experimental group CS+ trials − experimental group CS− trials) > (control group CS+ trials − control group CS− trials). We used the false discovery rate (FDR) procedure for correction of multiple comparisons. Significant activity was defined at q(FDR) < 0.05. Anatomical brain regions were identified based on known anatomical and functional landmarks according to the Talairach Brain Atlas and previous studies (Laufer and Paz, 2012; Laufer et al., 2016).
Eye tracker and pupil size analysis
We recorded participants' pupil size during the conditioning session and the validation test, using a video-based desktop mounted eye tracker, with a sampling rate of 1000 Hz (Eye Link 1000, SR Research, Ontario, Canada). At the beginning of each recording session (before the conditioning session and the validation test), the system was calibrated using a display of 9 points presented in a random order on the screen. The same display was also used for validation of the system. At the beginning of each trial, a fixation point appeared at the center of the screen, and participants had to fixate on this point and trigger the initiation of the trial by pressing a key on the keyboard (drift correction).
Baseline pupil size was calculated for each trial, as the average pupil size during the last 200 ms of fixation screen presentation (immediately before the presentation of the first stimulus). To normalize pupil size values, the average baseline was subtracted from all pupil size samples. Empty samples due to blinking were identified, and linear interpolation was used to estimate pupil size during these missing samples. In the conditioning sessions, average pupil size was calculated (for both scrambled and original images) as the mean pupil size value in a window of 2 to 6 s after image onset (to avoid the light reflex; Bradley et al., 2008). Images were no longer shown on the screen during that period, but the effect on pupil size carried on after the stimuli had disappeared. In the validation tests, average pupil size for the scrambled images was calculated as the mean pupil size in a window of 2 to 3 s following image onset (to avoid the light reflex), and the average size for the original images was calculated as the mean value in a window of 2 to 6 s after image onset. A technical problem that occurred while recording data during the conditioning sessions of 4 participants (1 in control group and 3 in experimental group), precluded data collection and so the available partial recordings for these participants were not used for our calculations.
Experimental design and statistical analysis
Statistical tests for the behavioral experiments were conducted using a two-way repeated-measures ANOVA test, with two levels of group: experimental/control and two levels of condition: CS+/CS−. Post hoc tests were used for examining specific contrasts with a priori hypotheses. Paired t tests were used to evaluate within-group JND changes from baseline thresholds. Imaging data in Experiment 4 were analyzed using a random-effects group analysis GLM, with a FDR procedure for correction of multiple comparisons (see fMRI Data Analysis section for more details). Pupil size changes in Experiment 5 were compared using an unpaired t test (between-group analysis) in the conditioning session or a two-way ANOVA test (with two levels of group: experimental/control and two levels of image valence: aversive/neutral) in the validation test. Numbers of participants used for analysis in each experiment are summarized in Table 1. Statistical analyses were conducted using Statistica (version 13, Dell), MATLAB (MathWorks), and the BrainVoyager QX software package (version 2.8, Brain Innovation). Results are presented as the mean ± SE.
Results
To examine the effect of visual aversive learning on neutral stimuli discrimination thresholds (JNDs), we conducted a classical conditioning session. In this session, participants learned to associate neutral stimuli (CSs) with the appearance (CS+) or absence (CS−) of an image (USs). The following two categories of images were selected from the IAPS database (Lang, 2008): aversive (for the experimental group); and neutral (for the control group). To validate participants' emotional responses, we asked them to rate the intensity of their emotion, while watching each of the conditioning session images (validation test). Rating was performed using an analog scale of 1 (not emotional at all) to 9 (very emotional). Across all participants in all experimental groups tested in the present study (n = 301, after the exclusion of 14 participants who did not perform well in the discrimination and memory tests; for details, see Materials and Methods and Table 1), aversive images were rated as significantly more emotional than neutral images (paired t test: t(300) = 30.17, p = 0.000; average score for aversive images, 6.85 ± 0.09; average score for neutral images, 3.09 ± 0.09). In a separate experiment (Experiment 5), we used pupil size as a physiological marker of fear to further validate the intensity of arousal that participants experienced while watching the neutral and aversive images (Steinhauer et al., 2004; Bradley et al., 2008; Tavakoli et al., 2014; R-Tavakoli et al., 2015). As expected, we found a larger change in pupil size for aversive images compared with neutral images (for more details, see Experiment 5 at the end of this section).
To detect changes in discrimination thresholds (JNDs), which result from aversive learning, we evaluated participants' JND values around each of the neutral stimuli. This was done using a 2AFC task (for more details, see Materials and Methods). This discrimination task was conducted before and immediately after the conditioning session. At the end of the conditioning session of each experiment, participants were asked whether they noticed the association between the CS and the presentation of the images. We analyzed only the results of participants who could explicitly report that they had noticed the conditioning manipulation, consistent with previous findings (Tabbert et al., 2006; Dawson et al., 2007; Klucken et al., 2009), and who rated the aversive images as more aversive than the neutral images (see Materials and Methods for more details regarding these exclusion criteria and their validation). Information regarding inclusion and exclusion of participants in each experiment is summarized in Table 1. A general description of the experimental protocol is presented in Figure 1a.
Effect of visual aversive conditioning on auditory discrimination (Experiment 1)
In this first experiment, auditory neutral sounds were used as the CS in the conditioning session. One tone of 1 or 2 kHz pure tones (CS+, counterbalanced) was paired with images, and the other tone (CS−) was paired with blank screens (Fig. 1b). There was no difference in the effect of aversive stimuli on JND values of the 1 kHz (n = 11) and 2 kHz (n = 13) tones as a CS+ (unpaired t test: t(22) = 0.27, p = 0.78). Analyses of JND values for each condition in each of the two groups (experimental group, n = 24; control group, n = 24) revealed that participants showed a significant decrease in threshold (improvement in discrimination) following conditioning to the CS− tone (unpaired with images), compared with the baseline threshold measured before conditioning. There was a decrease of −27.81 ± 12.26% from baseline threshold in the experimental group (paired t test: t(23) = 2.27, p = 0.03) and a decrease of −41.5 ± 9.37% in the control group (paired t test: t(23) = 4.43, p = 0.0002). For the CS+ tone in the control group (paired with neutral images), we also found an improvement in performance and a decrease of −45.39 ± 8.54% from baseline (paired t test: t(23) = 5.31, p = 0.00002). These findings are in accordance with those of previous studies demonstrating that mere repeated exposure to auditory stimuli can improve performance (Ahissar and Hochstein, 1996; Amitay et al., 2006; Ortiz and Wright, 2009). Further, there was no significant difference among the three cases (repeated-measures ANOVA: CS+ vs CS− for control group, F(1,46) = 0.046, p = 0.83; experimental versus control group for CS−, F(1,46) = 0.786, p = 0.38).
In contrast, experimental group participants did not improve in their performance for the CS+ tone (paired with aversive images). An increase of 32.1 ± 27.65% in their JND, compared with the preconditioning baseline, was observed. This was significantly higher from performance of the same participants around the CS− tone and from the performance of control participants around the CS+ tone, with an interaction effect between the CS+ and CS− across the experimental and control groups (repeated-measures ANOVA: CS × group interaction, F(1,46) = 6.17, p = 0.017, η2 = 0.12; CS+ vs CS− for experimental group, F(1,46) = 10.88, p = 0.002; experimental vs control group for CS+, F(1,46) = 7.17, p = 0.01). Thus, participants deteriorated in their performance around tone frequencies, which were conditioned to visual aversive stimuli, compared with tones, which were paired with neutral stimuli (Fig. 1c). The effect was observed for the CS+ tone in 45.83% of the participants in the experimental group compared with 4.17% of the participants in the control group (Fisher's exact test between groups, p = 0.0009; Fig. 1d).
We next asked whether the perceptual changes are maintained overnight, and can therefore point to perceptual learning rather than short-term adaptation. To do so, we measured perceptual thresholds in a new group of participants (n = 19). In this experiment, we used only one experimental group with an aversive conditioning session. Participants were recalled after 24 h to perform the threshold discrimination test one more time. The results of the original experiment were replicated with this new group (paired t test: t(18) = 2.65, p = 0.02) and remained stable for at least 24 h (paired t test: t(18) = 2.14, p = 0.046; Fig. 1e).
These results revealed that conditioning to aversive images increased the discrimination thresholds of auditory neutral stimuli. The finding is consistent with those of previous studies, showing the same effect using conditioning to aversive odors and sounds (Resnik et al., 2011). In the following experiments, we examined whether this effect could also be found in discrimination tests of visual neutral stimuli paired with unconditioned visual images.
Effect of visual aversive conditioning on contrast discrimination (Experiment 2)
In this experiment, we used Gabors with two different contrast levels (high and low), as conditioned visual stimuli (experimental group, n = 29; control group, n = 19; Fig. 2a). The experimental procedure was otherwise similar to the one described for Experiment 1. There was no difference in the effect of aversive stimuli on JND values of high-contrast (n = 13) or low-contrast (n = 16) contrast Gabors as the CS+ (unpaired t test, t(27) = 0.68, p = 0.49). In both groups, no change in contrast discrimination threshold was observed for the Gabors of the CS− condition, when we compared performance following conditioning to baseline performance (paired t test: experimental, t(28) = 1.03, p = 0.31; control, t(18) = 0.04, p = 0.97). No change was observed either for the CS+ Gabor of the control group (paired t test: t(18) = 1.02, p = 0.32). There was no significant difference between these three results (repeated-measures ANOVA: CS+ vs CS− for control group, F(1,46) = 0.52, p = 0.47; experimental vs control group for CS−, F(1,46) = 0.22, p = 0.64). This is in accordance with previous studies using perceptual learning tasks with discrimination of Gabor contrast, which did not find an improvement in performance under such conditions (Adini et al., 2004). Improved performance in visual perception tasks was reported in the literature only when a more intensive training was used compared with the procedure used in the present study (Karni and Sagi, 1993).
In contrast, for the CS+ Gabor of the experimental group (paired with aversive images) participants showed an increase of 42.9 ± 13.44% in discrimination threshold, compared with their baseline JND (paired t test: t(28) = 3.19, p = 0.003). This increase was significant also when compared directly to the CS− Gabor in the same group or to the CS+ Gabor in the control group, with an interaction effect between the CS+ and CS− across the experimental and control groups (repeated-measures ANOVA: CS × group interaction, F(1,46) = 7.52, p = 0.009, η2 = 0.14; CS+ vs CS− for experimental group, F(1,46) = 12.02, p = 0.001; experimental vs control group for CS+, F(1,46) = 8.14, p = 0.006; Fig. 2b). The majority of participants in the experimental group exhibited the increase for the CS+ (72.41%) compared with the CS− (44.83%; Fisher's exact test for experimental group between Gabors, p = 0.03), and compared with CS+ in the control group (36.84%; Fisher's exact test for CS+ Gabor between groups, p = 0.01; Fig. 2c). Here again, most of the participants in the experimental group (n = 20) were recalled the following day to perform the discrimination task again. The effect remained stable after these 24 h (paired t test: t(19) = 2.09, p = 0.049; Fig. 2d).
Effect of visual aversive conditioning on orientation discrimination (Experiment 3)
The goal of Experiment 3 was to examine whether the finding documented in Experiment 2 is restricted to the contrast feature or is robust to other basic features of visual perception. We therefore used the same paradigm as in Experiment 2, except for the use of black stripes in two different orientations, vertical and horizontal, as the CS+/CS− stimuli (experimental group, n = 25; control group, n = 22; Fig. 3a). There was no difference in the effect of aversive stimuli on JND values, when using the horizontal (n = 13) or vertical (n = 12) stripes as the CS+ (unpaired t test: t(23) = 0.31, p = 0.76). As in Experiment 2, there was no change in angle (orientation) discrimination thresholds compared with baseline thresholds for the CS− in both groups (paired t test: experimental, t(24) = 0.72, p = 0.48; control, t(21) = 0.82, p = 0.42). No change was found either for the CS+ in the control group (paired t test, t(21) = 1.26, p = 0.22). Additionally, there was no significant difference between these three cases (repeated-measures ANOVA: CS+ vs CS− for control group, F(1,46) = 0.0002, p = 0.99; experimental vs control group for CS−, F(1,46) = 0.08, p = 0.77).
In contrast, yet consistent with the previous experiments, participants showed an increase of 65.72 ± 31.47% in JND values for the CS+ that was paired with aversive images in the experimental group (paired t test: t(24) = 2.09, p = 0.047). This was significantly different when compared with the CS− condition in the same group or when compared with the CS+ condition in the control group (but here the CS × group interaction effect was not significant; repeated-measures ANOVA: CS × group interaction, F(1,45) = 7.52, p = 0.094, η2 = 0.061; CS+ vs CS− for experimental group, F(1,46) = 6.32, p = 0.02; experimental vs control group for CS+, F(1,46) = 5.37, p = 0.02; Fig. 3b). The effect occurred in the majority of experimental group participants (52%) for the CS+ condition compared with performance in the control group for the same condition (22.73%; Fisher's exact test between groups, p = 0.04; Fig. 3c). Experimental group participants (n = 23) performed the discrimination task again on the following day, and discrimination thresholds remained high (paired t test: t(22) = 2.26, p = 0.03; Fig. 3d).
Together, the described experiments show that the increased discrimination thresholds following conditioning to aversive visual stimuli are robust to the modality of the neutral stimulus (auditory/visual), and at least to some of its basic features.
Brain activity is modulated by visual aversive learning (Experiment 4)
To identify the underlying brain circuits that contribute to the observed changes in discrimination thresholds, neural activations during aversive visual learning were measured using fMRI. These activations were compared with activity during nonaversive visual learning (experimental group, n = 30; control group, n = 29). All conditioning sessions of this experiment were conducted in the course of a fMRI scan. The design of Experiment 4 was very similar to the one used in Experiment 3, except for the presentation of scrambled images instead of blank screens after the CS− stimuli, and the use of a random partial conditioning of 50%. This was accomplished by adding to the reinforced CS+ and CS− trials a similar number of nonreinforced trials (CS+ stripe or CS− stripe paired with a blank screen; Fig. 4a). This design allowed us to measure brain activity that is driven purely by the neutral CS, as it acquires value, without the additional response to the presentation of the US (images).
The behavioral results of the discrimination task were consistent with those obtained in the previous experiments. There was no difference in the effect of aversive stimuli on JND values of the horizontal (n = 16) or vertical (n = 14) stripes as on the CS+ (unpaired t test: t(28) = 0.22, p = 0.83). For the CS− condition in both groups, there was no change in angle discrimination thresholds compared with baseline (paired t test: experimental, t(29) = 1.14, p = 0.26; control, t(28) = 0.08, p = 0.94). Additionally, no change was found for the CS+ in the control group (paired t test: t(28) = 0.02, p = 0.98). There was no difference among these three cases (repeated-measures ANOVA: CS+ vs CS− for control group, F(1,58) = 0.02, p = 0.88; experimental vs control group for CS−, F(1,58) = 0.11, p = 0.74). For the CS+ of the experimental group (paired with aversive images), participants exhibited an increase of 70.6 ± 30.45% in threshold compared with baseline (paired t test: t(29) = 2.32, p = 0.03). This was significantly different when compared with the CS− condition in the same group or to the CS+ in the control group, with an interaction effect between the CS+ and CS− across the experimental and control groups (repeated-measures ANOVA: CS × group interaction, F(1,57) = 4.09, p = 0.048, η2 = 0.067; CS+ vs CS− for experimental group, F(1,57) = 8.67, p = 0.004; experimental vs control group for CS+, F(1,57) = 4.28, p = 0.04; Fig. 4b).
We conducted a whole-brain analysis of brain activity during the conditioning sessions, and compared the activation during aversive learning (experimental group) and nonaversive learning (control group). In a GLM group analysis, we first looked for differences in activity associated with nonreinforced CS+ and nonreinforced CS− trials (i.e., CS+ and CS− trials that were not followed by images). Notice that the visual information in those trials is essentially identical, because CS+ and CS− orientations were counterbalanced across participants. We compared the difference in activity between nonreinforced CS+ and nonreinforced CS− trials in the experimental and control groups. That is, we used a contrast of (experimental group nonreinforced CS+ trials − experimental group nonreinforced CS− trials) > (control group nonreinforced CS+ trials − control group nonreinforced CS− trials). This contrast revealed more activity in the ACC, insula, and, interestingly, the early visual cortex (Fig. 4c).
For the validation of the aversive nature of learning in the experimental group, we also compared differences in activity during the reinforced CS+ and CS− trials (i.e., CS+ followed by aversive or neutral images and CS− followed by scrambled images). We used a contrast of (experimental group CS+ trials − experimental group CS− trials) > (control group CS+ trials − control group CS− trials). This contrast revealed more activity in the amygdala, insula, and ventral occipital temporal cortex (vOTC; Fig. 4d). The results are in line with previous findings regarding the role of the amygdala, the insula, and the ACC in fear learning (Büchel et al., 1998; Pine et al., 2001; Carter et al., 2006; Nitschke et al., 2006; Dunsmoor et al., 2007; Schiller et al., 2008; Klucken et al., 2009; Sehlmeyer et al., 2009; Laufer and Paz, 2012; Resnik and Paz, 2015; Laufer et al., 2016). In addition, the results show that the ACC, and, interestingly, the early visual cortex, are involved in linking value to a previously neutral stimulus. Finally, we could not find any significant correlations between fMRI BOLD activity of the reported brain regions and JND values in the discrimination tasks.
Validation of emotional arousal using pupil size (Experiment 5):
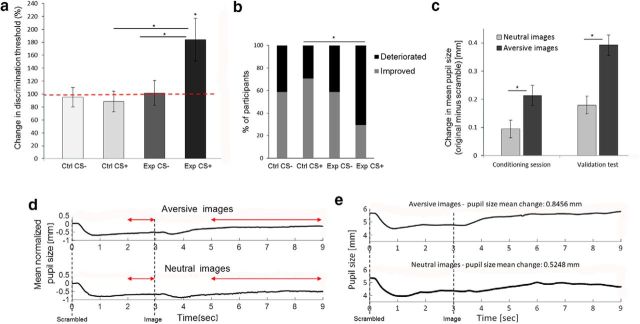
We monitored participants' pupil size to obtain a physiological validation for the intensity of the arousal that participants experienced while watching the images used in Experiments 1–4. Measurements were conducted, using an eye tracker, during the conditioning session and the validation test. Experiment 5 was very similar to Experiment 3, except for the presentation of scrambled images instead of blank screens after the CS− stimuli, as a reference for pupil size at similar luminance conditions. Importantly, the results of the discrimination task were replicated here (experimental group, n = 17; control group, n = 17). There was no difference in the effect of aversive stimuli on JND values of the horizontal (n = 9) or vertical (n = 8) stripes as on the CS+ (unpaired t test, t(15) = 1.32, p = 0.21). As in the previous experiments, for the CS− condition in both groups, there was no change in angle discrimination thresholds compared with baseline (paired t test: experimental, t(16) = 0.08, p = 0.93; control: t(16) = 0.33, p = 0.75). No change was found for the CS+ in the control group (paired t test: t(16) = 0.71, p = 0.49). There was no difference among these three cases (repeated-measures ANOVA: CS+ vs CS− for control group, F(1,46) = 0.068, p = 0.79; experimental vs control group for CS−, F(1,46) = 0.07, p = 0.79). For the CS+ in the experimental group (paired with aversive images), participants showed an increase of 83.84 ± 33.13% in threshold compared with baseline (paired t test: t(16) = 2.53, p = 0.02). This was significantly different when compared with the CS− condition in the same group or to the CS+ condition in the control group, with an interaction effect between the CS+ and CS− across the experimental and control groups (repeated-measures ANOVA: CS × group interaction, F(1,32) = 6.33, p = 0.02, η2 = 0.16; CS+ vs CS− for experimental group, F(1,32) = 10.87, p = 0.002; experimental vs control group for CS+, F(1,32) = 6.7, p = 0.01; Fig. 5a). The effect occurred in the majority of experimental group participants for the CS+ (70.59%), compared with the performance in the control group for the CS+ (29.41%; Fisher's exact test between groups, p = 0.02; Fig. 5b).
Figure 5.
Validation of emotional arousal using pupil size (Experiment 5). a, Discrimination thresholds for orientation were tested before and after the conditioning session (experimental group, n = 17; control group, n = 17). No change in thresholds was measured in both groups for the CS− stripe, or in the control group for the CS+ stripe. The experimental group deteriorated (increase in threshold) in the CS+ orientation (paired with aversive images), with a CS × group interaction effect. b, The majority of participants from the experimental group showed a deterioration in discrimination thresholds after conditioning to the CS+ stripe. This percentage of participants was higher compared with the control group for the same stripe. The gray bars show the percentage of participants for whom the postconditioning threshold was lower than the preconditioning threshold (improvement), and the black bars show the percentage of participants for whom their postconditioning threshold was higher (deterioration). c, The change in participants' pupil size was calculated as the difference between pupil sizes, while watching each image and its scrambled version (see panel d for more explanations). In the conditioning session (left, between-group comparison; experimental group, n = 14; control group, n = 16), as well as in the validation test (right, within-group comparison; experimental group, n = 17; control group, n = 17), changes were larger during aversive image watching compared with neutral image watching. d, Demonstration of the procedure used for calculating pupil size in c. The average pupil size time course (normalized by baseline subtraction) of the experimental group during the validation test is shown. The scrambled image was presented at time 0 s (followed by the light reflex of the pupil), and the original image at 3 s, as shown. Average pupil sizes for the scrambled and original images were calculated as the mean value in the range marked by the respective red arrows. e, An example for pupil size time course (without baseline normalization) of one experimental group participant during the validation test. *p < 0.05, see text for details regarding specific significance values.
We calculated the change in participants' pupil size (original image − scrambled image), while watching aversive and neutral images. This change in pupil size was used as an indicator for emotional arousal. In the conditioning session, each participant was exposed to only one category of images, aversive in the experimental group (full-length recordings of pupil size, n = 14) and neutral in the control group (full-length recordings of pupil size, n = 16). We found a larger change in pupil size for aversive images compared with neutral images (unpaired t test: t(28) = 2.06, p = 0.048). In the validation test, each participant was exposed to images from both categories, so we could compare changes in pupil size within participants (full-length recordings of pupil size, n = 17 in each group). We first verified that there was no effect for group type (repeated-measures ANOVA: F(1,32) = 0.017, p = 0.89). Consistent with the results of the conditioning session, changes in pupil size for aversive images were larger compared with changes for neutral images (repeated-measures ANOVA: F(1,32) = 32.68, p = 0.000002; Fig. 5c). The procedure used to calculate changes in pupil size is demonstrated in Figure 5d. This figure presents the pupil size average normalized time course of experimental group participants during the validation test. An example of a non-normalized time course of one experimental group participant is presented in Figure 5e.
Discussion
In the present study, we found that visual aversive conditioning increases discrimination thresholds for basic features of auditory and visual stimuli. Participants in the experimental groups deteriorated in their discrimination performance compared with their baseline abilities and compared with controls. Participants demonstrated the change in thresholds in a safe context during a postlearning session, and even 24 h later. This suggests that the change in thresholds is due to perceptual learning and circuit plasticity. Moreover, we observed differential brain activations during the learning process, even when the conditioned stimulus was not followed by the aversive stimulus. The modified activity was measured in the ACC, insula, amygdala, and the early visual cortex. Our findings demonstrate that changes in perceptual thresholds also occur in the visual domain, the main modality used by humans. The results further imply the existence of a central mechanism, which may be used during learning to modulate and alter early sensory neural representations. Below we discuss the implications of the results.
Visual aversive learning increases discrimination thresholds of auditory neutral stimuli
Previous studies have shown that an aversive outcome of different modalities can induce an increase in discrimination thresholds for tone frequencies (Resnik et al., 2011). This in turn can contribute to the wider generalization observed following aversive conditioning (Schechtman et al., 2010; Laufer and Paz, 2012). The first experiment we describe here provides evidence that complex visual images can induce a similar effect—an increase in thresholds of tone discrimination. The importance of this extension is double fold. First, combined with the aforementioned studies that used odors, sounds, or monetary loss, and with other studies using pain and sensation (Struyf et al., 2015), the replication to the visual domain reinforces the notion that the mechanism is not specific to any particular modality of the outcome. Rather, it supports the assumption that this is a fundamental learning principle related to negative valence. Second, given the dominance of the visual system in human perception, and the fact that many scenarios are experienced by vision, the finding holds relevance to daily life and suggests a potential mechanism for anxiety disorders (Lissek, 2012; Pitman et al., 2012; Dunsmoor and Paz, 2015; Laufer et al., 2016).
Visual aversive learning increases discrimination thresholds of visual neutral stimuli
Importantly, we show that aversive scenarios increase thresholds not only in the auditory domain, but also in the visual domain itself. This was shown with two different basic properties of visual perception—orientation and contrast (Sagi, 2011). One recent study demonstrated wider generalization to color (wavelength) following pairing with shocks (Dunsmoor and LaBar, 2013). Yet, in that study, it is harder to conclude whether there was a change in choice bias or in perceptual thresholds, because of the continuous reinforcement of the conditioned stimuli during the generalization test and the use of a decision-based task. In our study, the JNDs were measured with a “bias-free” 2AFC task (Green and Swets, 1989), and, in a safe context, when the conditioned stimuli were no longer reinforced. It remains an intriguing open question how the long-term change in simple feature perception affects the perception and generalization of complex scenarios (Dunsmoor and Paz, 2015). Studies have identified altered context generalization following fear learning (Maren et al., 2013). Therefore, it is reasonable to assume that changes in discrimination thresholds, as described here, can directly contribute to these processes, potentially via mechanisms such as pattern completion/separation (Donaldson and Hen, 2015). Future studies will need to address this matter. Additionally, the current study focuses on participants who were aware of the learning procedure, and who rated the aversive images as negative. An interesting direction for a future study could be to investigate how participants' awareness and image ratings affect discrimination.
Increased activity of the amygdala, insula, ACC, and early visual cortex during aversive learning
Since the deterioration in discrimination occurs for multiple outcome and input modalities, it is conceivable to assume that it is controlled by a central brain mechanism and network. Such a network should process the incoming valence and then exert its impact on sensory processing. We identified several brain regions that were more active during aversive learning compared with nonaversive learning. One of these regions is the ACC, whose activity was correlated with generalization and amygdala activity in previous studies (Laufer and Paz, 2012; Laufer et al., 2016). Another region is the insula, which was found to be involved in perceptual generalization (Laufer and Paz, 2012; Onat and Büchel, 2015).
Most interestingly, we found that the early visual cortex was more active when the same visual information predicted an aversive outcome compared with a neutral outcome. This could result from changes embedded in the level of processing of the conditioned stimuli or due to attentional effects related to the anticipation of aversive stimuli. Given the many feedback signals from higher cortical areas to the sensory cortex, it is possible that stimulus recognition involves the allocation of attentional resources to these areas. In other words, our results could indicate prioritized processing (especially as the effects require awareness). Therefore, the activations we observed may reflect an attentional state rather than low-level plasticity. Although this is a plausible interpretation, we believe that a stimulus-specific effect that lasts overnight (24 h), as we demonstrated here, is a good indication of perceptual plasticity. The current experiments and techniques (human imaging) do not allow dissociating local plasticity within early sensory regions from top-down attentional effects, which can also be a form of perceptual plasticity.
Previous studies have shown that the early visual cortex, which is thought to play a role in representing low-level stimulus features, can be modulated in various behavioral contexts and experiences (Ito and Gilbert, 1999; Gilbert et al., 2000; Sawtell et al., 2003; Salazar et al., 2004; Shuler and Bear, 2006; Serences, 2008). Accordingly, our results further challenge the classical view of the early visual cortex as a simple feature detector. Rather, they imply that aversive outcomes can modulate the low-level neural representations of basic features in early sensory regions. Consequently, stimuli that contain these basic features and might therefore entail the aversive outcome as well, could be processed faster, leading to a lower response time. In agreement with this interpretation, a previous study found that the primary auditory cortex was more active during fear conditioning and generalization in anxiety patients compared with healthy participants (Laufer et al., 2016).
A natural candidate region mediating this effect could be the amygdala. In addition to its traditional role in valence processing and anxiety, recent work revealed a correlation between its cellular or neural properties and behavioral generalization following aversive conditioning (Shaban et al., 2006; Ciocchi et al., 2010; Laufer and Paz, 2012; Ghosh and Chattarji, 2015). In the auditory domain, this might be due to the specific role and the anatomical projections that the amygdala has with the auditory system. Indeed, plasticity that correlates with auditory generalization was reported in the auditory thalamus and cortices (Han et al., 2008; Aizenberg and Geffen, 2013; Aizenberg et al., 2015; Laufer et al., 2016). It is possible that the amygdala may act during conditioning to shape representations in early visual cortices as well (Pessoa, 2010).
Overgeneralization in anxiety disorders
Finally, recent studies have provided evidence that overgeneralization plays a key role in anxiety disorders (Lissek, 2012; Pitman et al., 2012; Dunsmoor and Paz, 2015). Most of these studies focused on choice behavior in risky and unsafe environments. In such a scenario, it is indeed a rationale behavior to overgeneralize (i.e., have a bias). Recent findings in anxiety patients in the auditory domain (Laufer et al., 2016) and our current findings in the visual domain, imply that activity during aversive conditioning may modulate neural representations. These in turn can result in a later inability to discriminate between the stimuli, even in a safe context and even long after learning has ended. The results are also in line with the evidence that failures to process safety signals contribute to anxiety (Christianson et al., 2012; Jovanovic et al., 2012). Thus, the current study strengthens the notion that perception and overgeneralization play a role in anxiety. Specifically, it suggests that exposure to aversive outcome in a complex real-life scene can result in perceptual changes for basic features of multiple modalities experienced in the scene. The result can be a complex pattern of generalization, as abnormally exhibited in anxiety disorders.
Footnotes
The authors declare no competing financial interests.
References
- Adini Y, Wilkonsky A, Haspel R, Tsodyks M, Sagi D (2004) Perceptual learning in contrast discrimination: the effect of contrast uncertainty. J Vis 4(12):2, 993–1005. 10.1167/4.12.2 [DOI] [PubMed] [Google Scholar]
- Ahissar M, Hochstein S (1996) Learning pop-out detection: specificities to stimulus characteristics. Vision Res 36:3487–3500. 10.1016/0042-6989(96)00036-3 [DOI] [PubMed] [Google Scholar]
- Åhs F, Miller SS, Gordon AR, Lundström JN (2013) Aversive learning increases sensory detection sensitivity. Biol Psychol 92:135–141. 10.1016/j.biopsycho.2012.11.004 [DOI] [PubMed] [Google Scholar]
- Aizenberg M, Geffen MN (2013) Bidirectional effects of aversive learning on perceptual acuity are mediated by the sensory cortex. Nat Neurosci 16:994–996. 10.1038/nn.3443 [DOI] [PubMed] [Google Scholar]
- Aizenberg M, Mwilambwe-Tshilobo L, Briguglio JJ, Natan RG, Geffen MN (2015) Bidirectional regulation of innate and learned behaviors that rely on frequency discrimination by cortical inhibitory neurons. PLoS Biol 13:e1002308. 10.1371/journal.pbio.1002308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitay S, Irwin A, Moore DR (2006) Discrimination learning induced by training with identical stimuli. Nat Neurosci 9:1446–1448. 10.1038/nn1787 [DOI] [PubMed] [Google Scholar]
- Bouton M. (2006) Learning and behavior: a contemporary synthesis. Sunderland, MA: Sinauer Associates. [Google Scholar]
- Bradley MM, Miccoli L, Escrig MA, Lang PJ (2008) The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology 45:602–607. 10.1111/j.1469-8986.2008.00654.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Morris J, Dolan RJ, Friston KJ (1998) Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron 20:947–957. 10.1016/S0896-6273(00)80476-6 [DOI] [PubMed] [Google Scholar]
- Carter RM, O'Doherty JP, Seymour B, Koch C, Dolan RJ (2006) Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage 29:1007–1012. 10.1016/j.neuroimage.2005.09.011 [DOI] [PubMed] [Google Scholar]
- Christianson JP, Fernando AB, Kazama AM, Jovanovic T, Ostroff LE, Sangha S (2012) Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci 32:14118–14124. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Müller C, Lüthi A (2010) Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468:277–282. 10.1038/nature09559 [DOI] [PubMed] [Google Scholar]
- Dawson ME, Rissling AJ, Schell AM, Wilcox R (2007) Under what conditions can human affective conditioning occur without contingency awareness? Test of the evaluative conditioning paradigm. Emotion 7:755–766. 10.1037/1528-3542.7.4.755 [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Hen R (2015) From psychiatric disorders to animal models: a bidirectional and dimensional approach. Biol Psychiatry 77:15–21. 10.1016/j.biopsych.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, LaBar KS (2013) Effects of discrimination training on fear generalization gradients and perceptual classification in humans. Behav Neurosci 127:350–356. 10.1037/a0031933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunsmoor JE, Paz R (2015) Fear generalization and anxiety: behavioral and neural mechanisms. Biol Psychiatry 78:336–343. 10.1016/j.biopsych.2015.04.010 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC (2007) Impact of continuous versus intermittent CS−UCS pairing on human brain activation during pavlovian fear conditioning. Behav Neurosci 121:635–642. 10.1037/0735-7044.121.4.635 [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Mitroff SR, LaBar KS (2009) Generalization of conditioned fear along a dimension of increasing fear intensity. Learn Mem 16:460–469. 10.1101/lm.1431609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond S, Dunsmoor JE, Vervliet B, Roche B, Hermans D (2015) Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav Ther 46:561–582. 10.1016/j.beth.2014.10.001 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Chattarji S (2015) Neuronal encoding of the switch from specific to generalized fear. Nat Neurosci 18:112–120. 10.1038/nn.3888 [DOI] [PubMed] [Google Scholar]
- Gilbert C, Ito M, Kapadia M, Westheimer G (2000) Interactions between attention, context and learning in primary visual cortex. Vision Res 40:1217–1226. 10.1016/S0042-6989(99)00234-5 [DOI] [PubMed] [Google Scholar]
- Green D, Swets J (1989) Signal detection theory and psychophysics. Los Altos Hills, CA: Peninsula. [Google Scholar]
- Guttman N, Kalish HI (1956) Discriminability and stimulus generalization. J Exp Psychol 51:79–88. 10.1037/h0046219 [DOI] [PubMed] [Google Scholar]
- Han JH, Yiu AP, Cole CJ, Hsiang HL, Neve RL, Josselyn SA (2008) Increasing CREB in the auditory thalamus enhances memory and generalization of auditory conditioned fear. Learn Mem 15:443–453. 10.1101/lm.993608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearst E. (1960) Simultaneous generalization gradients for appetitive and aversive behavior. Science 132:1769–1770. 10.1126/science.132.3441.1769 [DOI] [PubMed] [Google Scholar]
- Ito M, Gilbert CD (1999) Attention modulates contextual influences in the primary visual cortex of alert monkeys. Neuron 22:593–604. 10.1016/S0896-6273(00)80713-8 [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ (2010) How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry 167:648–662. 10.1176/appi.ajp.2009.09071074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Kazama A, Bachevalier J, Davis M (2012) Impaired safety signal learning may be a biomarker of PTSD. Neuropharmacology 62:695–704. 10.1016/j.neuropharm.2011.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Sagi D (1993) The time course of learning a visual skill. Nature 365:250–252. 10.1038/365250a0 [DOI] [PubMed] [Google Scholar]
- Klucken T, Kagerer S, Schweckendiek J, Tabbert K, Vaitl D, Stark R (2009) Neural, electrodermal and behavioral response patterns in contingency aware and unaware subjects during a picture-picture conditioning paradigm. Neuroscience 158:721–731. 10.1016/j.neuroscience.2008.09.049 [DOI] [PubMed] [Google Scholar]
- Lang P. (2008) International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Gainesville, FL: University of Florida A-8. [Google Scholar]
- Laufer O, Paz R (2012) Monetary loss alters perceptual thresholds and compromises future decisions via amygdala and prefrontal networks. J Neurosci 32:6304–6311. 10.1523/JNEUROSCI.6281-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer O, Israeli D, Paz R (2016) Behavioral and neural mechanisms of overgeneralization in anxiety. Curr Biol 26:713–722. 10.1016/j.cub.2016.01.023 [DOI] [PubMed] [Google Scholar]
- Levitt H. (1971) Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49 [Suppl 2]:467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA (2008) Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science 319:1842–1845. 10.1126/science.1152837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S. (2012) Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of pavlovian fear-learning: the case for conditioned overgeneralization. Depress Anxiety 29:257–263. 10.1002/da.21922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, Lukenbaugh D, Geraci M, Pine DS, Grillon C (2010) Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am J Psychiatry 167:47–55. 10.1176/appi.ajp.2009.09030410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Kaczkurkin AN, Rabin S, Geraci M, Pine DS, Grillon C (2014) Generalized anxiety disorder is associated with overgeneralization of classically conditioned fear. Biol Psychiatry 75:909–915. 10.1016/j.biopsych.2013.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren IP, Mackintosh NJ (2002) Associative learning and elemental representation: II. Generalization and discrimination. Anim Learn Behav 30:177–200. 10.3758/BF03192828 [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ (2006) Functional neuroanatomy of aversion and its anticipation. Neuroimage 29:106–116. 10.1016/j.neuroimage.2005.06.068 [DOI] [PubMed] [Google Scholar]
- Onat S, Büchel C (2015) The neuronal basis of fear generalization in humans. Nat Neurosci 18:1811–1818. 10.1038/nn.4166 [DOI] [PubMed] [Google Scholar]
- Ortiz JA, Wright BA (2009) Contributions of procedure and stimulus learning to early, rapid perceptual improvements. J Exp Psychol Hum Percept Perform 35:188–194. 10.1037/a0013161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov I. (1927) Conditioned reflexes. London: Oxford UP. [Google Scholar]
- Pessoa L. (2010) Emotion and cognition and the amygdala: from “what is it?” to “what's to be done?” Neuropsychologia 48:3416–3429. 10.1016/j.neuropsychologia.2010.06.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine DS, Fyer A, Grun J, Phelps EA, Szeszko PR, Koda V, Li W, Ardekani B, Maguire EA, Burgess N, Bilder RM (2001) Methods for developmental studies of fear conditioning circuitry. Biol Psychiatry 50:225–228. 10.1016/S0006-3223(01)01159-3 [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I (2012) Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13:769–787. 10.1038/nrn3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. (1976) Stimulus generalization: some predictions from a model of pavlovian conditioning. J Exp Psychol Anim Behav Process 2:88–96. 10.1037/0097-7403.2.1.88 [DOI] [PubMed] [Google Scholar]
- Resnik J, Paz R (2015) Fear generalization in the primate amygdala. Nat Neurosci 18:188–190. 10.1038/nn.3900 [DOI] [PubMed] [Google Scholar]
- Resnik J, Sobel N, Paz R (2011) Auditory aversive learning increases discrimination thresholds. Nat Neurosci 14:791–796. 10.1038/nn.2802 [DOI] [PubMed] [Google Scholar]
- R-Tavakoli H, Atyabi A, Rantanen A, Laukka SJ, Nefti-Meziani S, Heikkilä J (2015) Predicting the valence of a scene from observers' eye movements. PLoS One 10:e0138198. 10.1371/journal.pone.0138198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi D. (2011) Perceptual learning in vision research. Vision Res 51:1552–1566. 10.1016/j.visres.2010.10.019 [DOI] [PubMed] [Google Scholar]
- Salazar RF, König P, Kayser C (2004) Directed interactions between visual areas and their role in processing image structure and expectancy. Eur J Neurosci 20:1391–1401. 10.1111/j.1460-9568.2004.03579.x [DOI] [PubMed] [Google Scholar]
- Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF (2003) NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron 38:977–985. 10.1016/S0896-6273(03)00323-4 [DOI] [PubMed] [Google Scholar]
- Schechtman E, Laufer O, Paz R (2010) Negative valence widens generalization of learning. J Neurosci 30:10460–10464. 10.1523/JNEUROSCI.2377-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA (2008) From fear to safety and back: reversal of fear in the human brain. J Neurosci 28:11517–11525. 10.1523/JNEUROSCI.2265-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C (2009) Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One 4:e5865. 10.1371/journal.pone.0005865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serences JT. (2008) Value-based modulations in human visual cortex. Neuron 60:1169–1181. 10.1016/j.neuron.2008.10.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaban H, Humeau Y, Herry C, Cassasus G, Shigemoto R, Ciocchi S, Barbieri S, van der Putten H, Kaupmann K, Bettler B, Lüthi A (2006) Generalization of amygdala LTP and conditioned fear in the absence of presynaptic inhibition. Nat Neurosci 9:1028–1035. 10.1038/nn1732 [DOI] [PubMed] [Google Scholar]
- Shepard RN. (1987) Toward a universal law of generalization for psychological science. Science 237:1317–1323. 10.1126/science.3629243 [DOI] [PubMed] [Google Scholar]
- Shuler MG, Bear MF (2006) Reward timing in the primary visual cortex. Science 311:1606–1609. 10.1126/science.1123513 [DOI] [PubMed] [Google Scholar]
- Solomon PR, Moore JW (1975) Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol 89:1192–1203. 10.1037/h0077183 [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, Pless M (2004) Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. Int J Psychophysiol 52:77–86. 10.1016/j.ijpsycho.2003.12.005 [DOI] [PubMed] [Google Scholar]
- Struyf D, Zaman J, Vervliet B, Van Diest I (2015) Perceptual discrimination in fear generalization: mechanistic and clinical implications. Neurosci Biobehav Rev 59:201–207. 10.1016/j.neubiorev.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D (2006) Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. Neuroimage 32:761–770. 10.1016/j.neuroimage.2006.03.038 [DOI] [PubMed] [Google Scholar]
- Tavakoli HR, Yanulevskaya V, Rahtu E, Heikkilä J, Sebe N (2014) Emotional valence recognition, analysis of salience and eye movements. Paper presented at the 22nd International Conference on Pattern Recognition, Stockholm, Sweden, August. [Google Scholar]
- Vervliet B, Baeyens F, Van den Bergh O, Hermans D (2013) Extinction, generalization, and return of fear: a critical review of renewal research in humans. Biol Psychol 92:51–58. 10.1016/j.biopsycho.2012.01.006 [DOI] [PubMed] [Google Scholar]
- Watson JB, Rayner R (2000) Conditioned emotional reactions. 1920. Am Psychol 55:313–317. 10.1037/0003-066X.55.3.313 [DOI] [PubMed] [Google Scholar]
- Zaman J, Vlaeyen JW, Van Oudenhove L, Wiech K, Van Diest I (2015) Associative fear learning and perceptual discrimination: a perceptual pathway in the development of chronic pain. Neurosci Biobehav Rev 51:118–125. 10.1016/j.neubiorev.2015.01.009 [DOI] [PubMed] [Google Scholar]