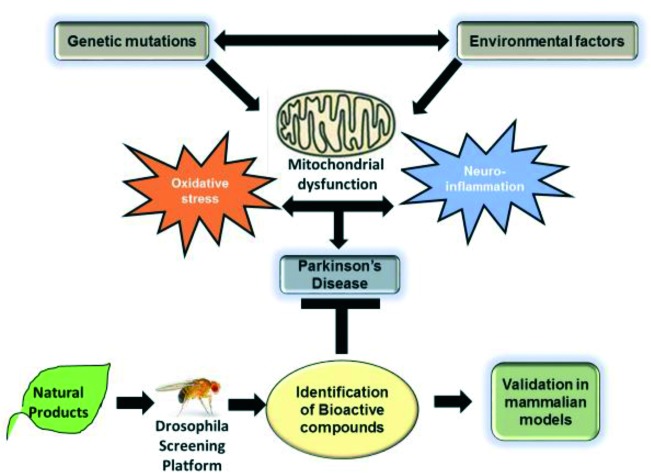
 The review provides an overview of discovery of new drug leads from natural extracts using Drosophila as a screening platform to evaluate the therapeutic potential of phytochemicals against Parkinson's disease.
The review provides an overview of discovery of new drug leads from natural extracts using Drosophila as a screening platform to evaluate the therapeutic potential of phytochemicals against Parkinson's disease.
Abstract
Parkinson's disease (PD) is a progressive neurodegenerative movement disorder with no cure. Despite intensive research, most of the currently available therapies are only effective in alleviating symptoms with no effect on disease progression. There is an urgent need for new therapeutics to impede disease progression. Natural products are valuable sources of bioactive compounds that can be exploited for novel therapeutic potential in PD pathogenesis. However, rapid screening of plant-derived natural products and characterization of bioactive compounds is costly and challenging. Drosophila melanogaster, commonly known as the fruit fly, has recently emerged as an excellent model for human neurodegenerative diseases, including PD. The high degree of conserved molecular pathways with mammalian models make Drosophila PD models an inexpensive solution to preliminary phases of target validation in the drug discovery pipeline. The present review provides an overview of drug discovery from natural extracts using Drosophila as a screening platform to evaluate the therapeutic potential of phytochemicals against PD.
Introduction
Parkinson's disease (PD) is the second most common age-associated neurodegenerative disorder affecting approximately 1% of the population over 50 years.1,2 Aging remains the most significant risk factor for developing idiopathic PD.3 Due to the increase in life expectancy, the number of people with PD is projected to exceed 12 million worldwide by 2040,4,5 thereby contributing to a significant healthcare burden.6 The prominent hallmark of PD is progressive loss of dopaminergic neurons (DA) from the substantia niagra parse compacta (SNpc) located in the midbrain that plays a significant role in controlling movement.7 Another clinical pathology of PD involves the accumulation of protein aggregates (inclusions) enriched in α-synuclein (α-syn) called Lewy bodies (LB) prominently in DA neurons.8 Loss of neurons that normally release the neurotransmitter dopamine results in motor dysfunction, rigidity, bradykinesia, tremor, postural instability, impaired balance and disturbance in sleep.9 This led to the development of the dopamine replacement drug levodopa (l-dopa), which crosses the blood–brain barrier and acts as a precursor to dopamine.10
Current PD therapies
Levodopa is most commonly prescribed as a dopamine replacement drug to treat PD symptoms. Currently, levodopa is used in conjunction with carbidopa that prevents the conversion of levodopa to dopamine before it enters the brain to improve drug effectiveness. However, long term usage of levodopa is associated with drug-induced toxicity and dyskinesia that leads to uncontrolled involuntary movement in PD patients.10,11 Other drug therapies include dopamine agonists like ropinirole (Requip), pramipexole (Mirapex), that mimic the effects of dopamine in the brain but they are associated with non-motor side effects like mood swing, sleepiness, hallucinations, fatigue and compulsive behaviors.12,13 COMT (catechol-O-methyltransferase) inhibitors are another class of medications that are used in combination with carbidopa–levodopa therapy to treat PD symptoms.14 COMT inhibitor slows the breakdown of levodopa, thereby increasing its availability in the brain. The other class of anti-PD drugs involve inhibitors of the enzyme, monoamine oxidase-B (MAO-B), that helps to break down and metabolize dopamine in the brain.15 Another treatment for PD involves surgical procedures like deep brain stimulation (DBS) in which electrodes are implanted to send electric pulses in specific areas of the brain that control movement.16 DBS has been shown to ameliorate motor dysfunction and improve tremor, rigidity and involuntary movements. Currently, gene therapy options are explored in preclinical models as a potential treatment regimen for PD.17,18 Most of the currently available drug therapies only provide symptomatic relief but does not slow or prevent the underlying neurodegeneration and becomes less effective with disease progression. Therefore, there is a dire need for new highly effective therapeutics suitable for early intervention to impede or halt disease progression.
Secondary plant metabolites in drug discovery – evolutionary perspective and cellular therapeutic targets
Epidemiological data suggest consumption of diet rich in fruits and vegetables confer protection against chronic diseases.19 Plant-derived natural products have been important drug leads used against multiple chronic disorders like cancer, cardiovascular disease, diabetes, and neurodegenerative disorders.20 It has been estimated that approximately 70% of currently FDA approved drugs are derived from natural products with plants being by far the most important source of pharmacologically active compounds.21 The following are just a few examples of natural products currently approved as drugs and used for the treatment of various diseases: artemisinin (malaria), galantamine (Alzheimer's disease), paclitaxel (cancer), morphine (chronic pain) and many others.22 For many years, it has been proclaimed that natural compounds exert most of the known biological effects by directly scavenging free radicals generated in cells.19 Direct free radical scavenging activity would require a high concentration of secondary metabolites (micromolar range) in every cell to get rid of highly reactive oxygen and nitrogen species produced during normal and pathological cell metabolism. This review discusses data favoring other hypotheses that have recently emerged in the field of drug discovery from natural products.
Samples of natural origin constitute an invaluable source of compounds with a diverse array of pharmacological activities. Despite the wealth of drug-like molecules produced by plants, the process of drug discovery from natural sources has diminished in the past two decades.23 One of the problems with currently used classical drug discovery approaches from natural samples is the fact that they often yield known substances and demand high resource commitment. The traditional approach involves isolation of individual compounds and subsequently testing them using in vitro cell-based assays or different model organisms.24 This approach often leads to the isolation of known and most abundant secondary metabolites with well-defined pharmacological activity. Early-stage testing of isolated chemicals in mammalian models is costly and not viable at the early stage of the drug discovery process. Unfortunately, high throughput screening techniques, which have been designed to speed up the process of drug discovery are not suitable for identification of active compounds present in complex matrices, e.g., plant extracts.23 This review presents the option of using Drosophila models of PD at the early stage of identification of plant-derived drug leads.
Pan-assay interference compounds (PAINS)
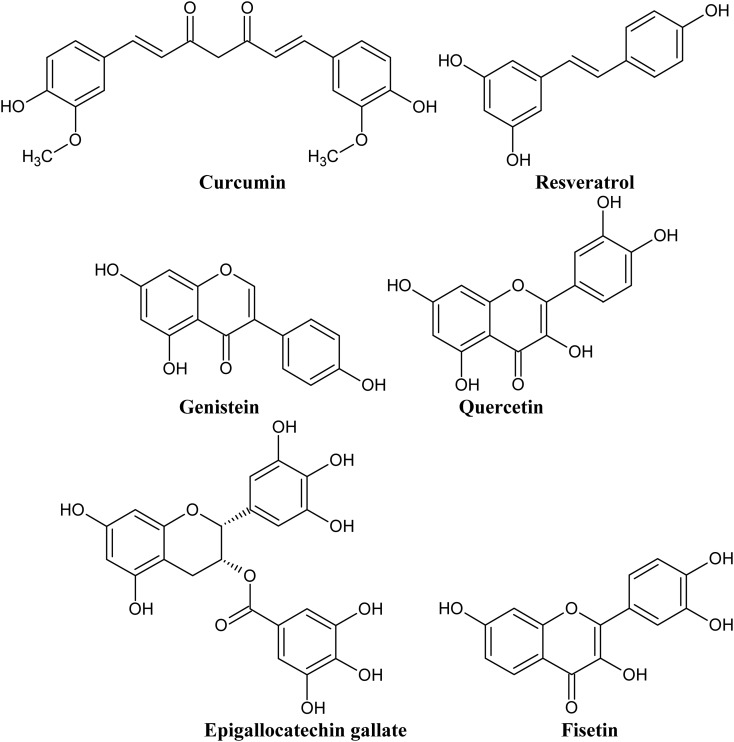
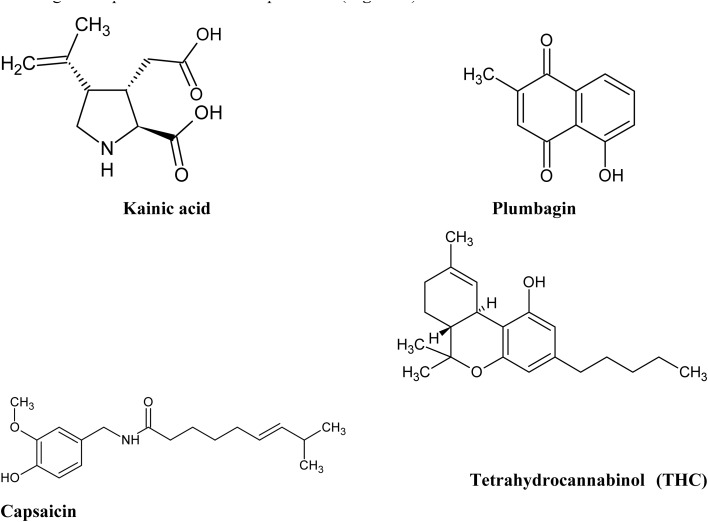
Another problem often raised by medicinal chemists in the context of drug discovery from plant samples is “dirtiness” of plant molecules.25 A molecule is considered dirty if it interacts with multiple endogenous protein targets increasing the risk of off-target detrimental side effects. Natural polyphenolic compounds are the classic example of “dirty” molecules, often referred in medicinal chemistry as PAINS.25 For example, curcumin has been found to interact with at least sixty molecular targets.26Fig. 1 presents examples of typical PAIN molecules, which have also been studied extensively and have shown to modulate key mammalian proteins implicated in the pathophysiology of numerous diseases, including neurodegenerative ailments. Medicinal chemistry “purists” strongly advise to exclude “dirty” plant molecules from any drug discovery programs, but there is another side to this coin.25–29
Fig. 1. Structures of selected polyphenolic compounds proven to bind to multiple protein targets and often considered in medicinal chemistry literature as pan-assay interference compounds (PAINS).
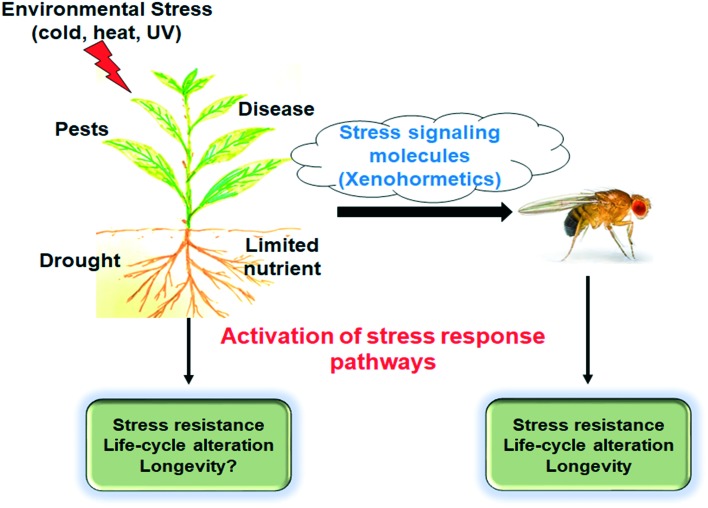
Most of these pan-assay interference compounds are remarkably safe despite their interaction with multiple protein targets.26,27 It has been postulated that these compounds may have evolved as signaling molecules used by herbivores and omnivores as chemical cues allowing heterotrophs to modify their metabolism and prepare for the deterioration of the environment, for example, shrinkage of food resources (Fig. 2).26,27,30 Sinclair and collaborators hypothesize common ancestor of plants and animals synthesized polyphenols.26,27,30,31 Currently, heterotrophs are able to detect environmental changes, for example, a possible decrease in food resources, as their enzymes and receptors evolved binding sites for chemical cues produced by autotrophs.26 By interacting with multiple protein targets, these signaling molecules activate stress response pathways increasing survival and life-span expansion of organisms feeding on the stressed plants. These beneficial effects are compared to the well-documented lifespan extension phenomenon observed in calorie-restricted animals.26
Fig. 2. The xenohormesis hypothesis. Adaptive stress response pathways in heterotrophs are activated by chemical cues produced by autotrophs and increase heterotrophs survival. Reproduced from ref. 30 with permission from John Wiley and Sons, Jul 16 2004.
Xenohormetics and geroneuroprotectors
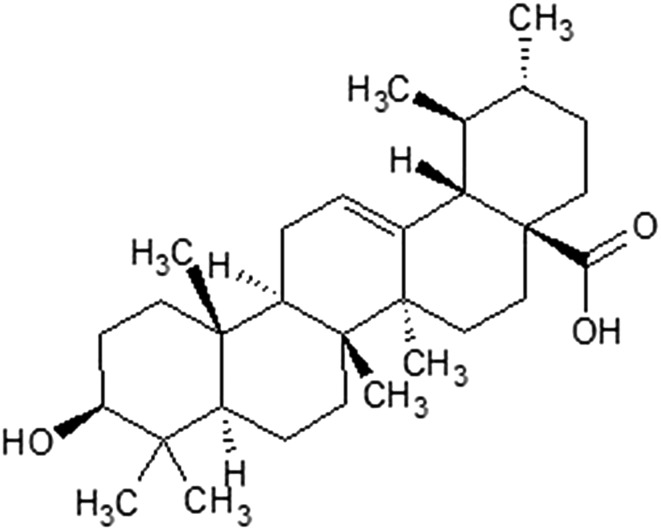
The compounds that potentially work as chemical cues allowing heterotrophs to detect approaching deterioration of food resources have been collectively named as xenohormetics.27 The majority of xenohormetics discussed to date are polyhydroxylated molecules. However, there is another class of compounds that may be a goldmine of potential drug-like chemical cues influencing the metabolism of heterotrophs – terpenoids. Terpenoids are synthesized by all living organisms and constitute natural candidates for potential chemical cues.32 Just recently a triterpenoid – ursolic acid (Fig. 3) was found to directly activate sirtuin 1 (NAD-dependent deacetylase sirtuin-1, SIRT1), an enzyme whose activation was found to increase the longevity of model organisms.33 Ursolic acid is commonly present in many plants, for example, fruit peels or commonly used herbs and spices.
Fig. 3. Structure of ursolic acid, a pentacyclic triterpenoid molecule, recently found to regulate age and longevity by influencing the activity of SIRT1.33.
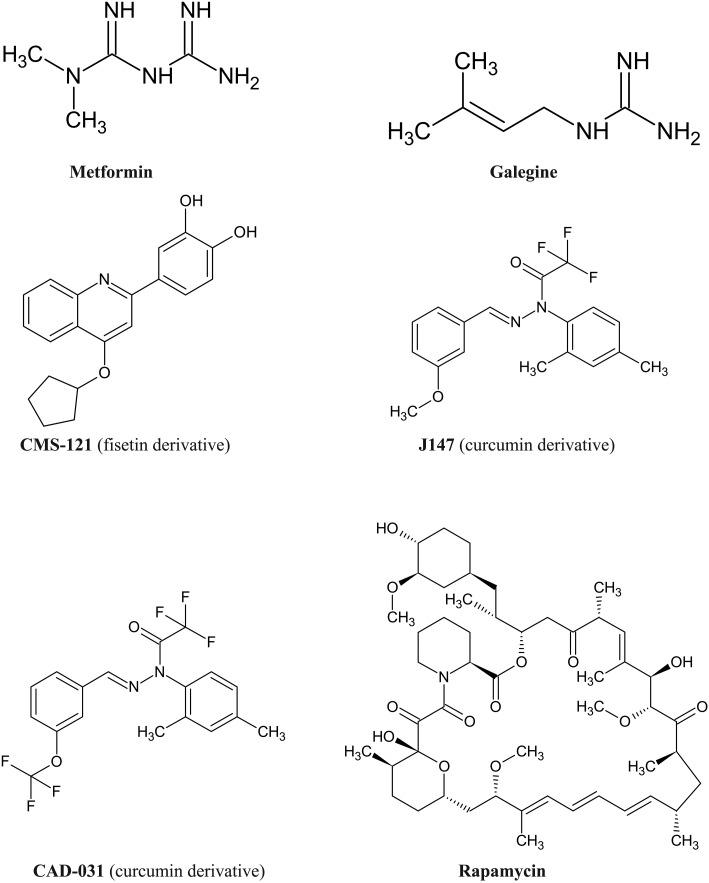
Recently several natural compounds, including polyhydroxylated molecules have been shown to slow down the rate of biological aging and proposed as candidates in the prevention of age-related diseases, including neurodegenerative ailments. Compounds expanding lifespan have been collectively named geroprotectors.34–43 The term geroneuroprotectors has been recently coined for natural compounds and their derivatives that meet the criteria for geroprotectors and reduce the risk of developing age-associated neurodegenerative ailments.34Fig. 4 presents structures of selected geroprotectors and geroneuroprotectors. Two well-studied geroprotectors are compounds derived from nature: rapamycin, a macrolide produced by Streptomyces hygroscopicus and metformin, a biguanide, derivative of noxious galegine identified in goat's rue (Galega officinalis). Derivatives of two polyhydroxylated natural compounds: curcumin and fisetin have been shown to share neuroprotective pathways with caloric restriction and well-studied geroprotectors (metformin and rapamycin).34
Fig. 4. Examples of selected natural compounds and their derivatives identified as geroprotectors and geroneuroprotectors.
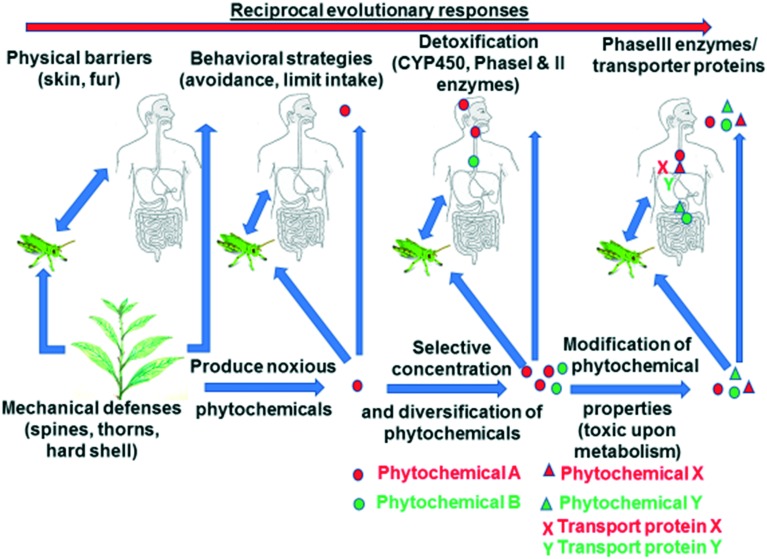
The story of secondary plant metabolites with beneficial effects for human health is more complex than only the story of xenohormetics and geroprotectors. Numerous phytochemicals have evolved as antifeedant and noxious chemicals to ward off herbivorous and omnivorous animals.19,44–48 It has been postulated that noxious phytochemicals are one of several environmental challenges that might have shaped the evolution of heterotrophs, including human evolution.49 Mattson et al. posit that humans evolved in an environment in which they were intermittently challenged with food scarcity, need to stay physically fit (aerobic exertion) and natural toxins ingested with plant-based food.49 This hypothesis postulates that cells recognize certain phytochemicals as noxious, which leads to the activation of stress response cellular signaling pathways that protect cells from different forms of stress.19Fig. 5 presents examples of noxious phytochemicals that have been identified to ward off herbivores and omnivores and activate stress response pathways. Many of these phytochemicals are concentrated in plant structures most exposed to environmental challenges and possible herbivorous predation (Fig. 6).
Fig. 5. Structures of selected natural noxious chemicals which elicit hormetic neurobiological response and play a role of feeding deterrents.
Fig. 6. Simplified model of evolutionary biochemical changes during co-evolution of plant and herbivores/omnivores. Reproduced from ref. 47 with permission from Elsevier, Oct 1 2015.
Small amounts of noxious phytochemicals switch on stress response cellular signaling pathways, while excessive amounts of the same compounds exert toxic effects. This phenomenon is known as hormesis, a biphasic dose response to a stressor, which at low dose provides beneficial effects while high dose leads to toxic effects.50 Mattson et al. observed that stressing cultured neurons with low doses of glutamate provides protective effects, while excessive amount led to neuronal death.19 Interestingly, the same group of researchers observed that while long-term activation of nuclear factor kappa B (NF-κB) in microglia and astrocytes leads to neuronal damage, short-term activation promotes cell survival and further provides protection from acute and chronic neurodegeneration.19 Intermittent switching of stress response pathways by noxious phytochemicals seems to be the key in providing cellular protection. Similarly, intermittent metabolic switching, during intermittent fasting provides neuroprotection by short-term activation of stress response pathways by ketones produced from adipose-derived fatty acids.51
To properly understand the possible effects of secondary plant metabolites on heterotroph's cells one needs to distinguish between phytochemicals role as xenohormetics and hormetics. Xenohormetics are defined as molecules produced by one organism (autotroph) that work as chemical signals recognized by another organism (heterotroph) that does not produce them.26 Sinclair hypothesizes that polyphenols were produced by a common ancestor of plants and animals and despite heterotrophs lost the ability to synthesize polyhydroxylated molecules during the evolution process, they possess polyphenol binding sites on numerous protein targets.26 The presence of these receptors allows heterotrophs to sense environmental changes and adjust the metabolism in preparation for food resources shrinkage. Hormetic effects are caused by small doses of noxious phytochemicals that are postulated to be a result of reciprocal evolutionary changes which occurred during co-evolution of autotrophs and heterotrophs (Fig. 6).19
It must be clearly stated that the theory of chemical cues (xenohormetics) and hormetic theory of noxious phytochemicals do not exclude each other. Plants may synthesize both classes of these compounds, and one compound may be a hormetic and a xenohormetic depending for example, on its concentration at a certain stage of plant development. Interestingly, numerous toxic secondary metabolites evolved in response to insect herbivores, making Drosophila a viable model for screening natural samples for pharmacologically active molecules. Noxious phytochemicals often influence evolutionary conserved cellular signaling pathways, allowing for extrapolation of these findings to mammalian models of diseases.
Molecular pathways as therapeutic targets
Both the previously discussed theory of xenohormetic chemical cues and hormetic theory of noxious phytochemicals postulate the existence of evolutionarily conserved pathways, whose activity can be modified by secondary plant metabolites. Here, we briefly discuss examples of adaptive cellular stress response pathways by which phytochemicals may provide neuroprotection against PD pathogenesis. An interested reader may find more information on the topic of adaptive cellular signaling pathways elsewhere.19
NF-E2-related factor-2/anti-oxidant response element (Nrf2/ARE) pathway is one of the critical defensive signaling pathways that regulate the expression of antioxidants and phase II detoxifying enzymes in response to phytochemicals. Several phytochemicals such as curcumin, genistein, epigallocatechin gallate (EGCG) have been shown to activate the Nrf2 pathway.44 Sulphorafane, a phytochemical found in cruciferous vegetables, is known to activate the Nrf2/ARE pathway and has been reported to provide neuroprotection in both mice and fly PD models.52,53 Activation of Nrf2 signaling pathway has been shown to be neuroprotective in a Drosophila PD model.54 In a mouse model, supplementation of curcumin induces the expression of detoxification enzymes like catalase, hemeoxygenase-1 (HO-1), glutathione-s-transferases, and glutathione reductase, in the liver and small intestine.55 EGCG has been shown to confer resistance against H2O2-induced cell death by upregulating the expression of HO-1 through activation of the Nrf2/ARE pathway.56
Another factor proposed to modulate the adaptive stress response pathway is the hypoxia-inducible factor (HIF-1) which plays a critical role in cellular adaptation to low oxygen levels. The flavonoid quercetin has been shown to activate HIF-1a, and green tea catechin EGCG upregulates the expression of genes downstream of HIF-1.57,58 A recent study reported that HIFα ortholog in Drosophila, similar (Sima) knockdown restores neuronal function in a Drosophila model of familial PD.59
NF-κB is a transcription factor that controls several genes involved in immunity, inflammation, stress response, cell survival, and proliferation.60 NF-κB dysregulation has been implicated in the pathogenesis of diverse diseases and appears to be a promising target for neurodegenerative disorders, including PD.61 Numerous phytochemicals, including curcumin, quercetin, and resveratrol have been identified to affect NF-κB activity.19
Peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily that controls glucose and lipid metabolism as well as growth and differentiation. Neuroprotective effects of PPAR agonists have been demonstrated in experimental models of PD.62,63 The PPAR agonist, pioglitazone was shown to protect against motor dysfunction and neuronal loss in both MPTP mouse and Drosophila model of amyotrophic lateral sclerosis (ALS).64,65
Sirtuins are NAD+-dependent protein deacetylases that regulate key transcription factors, including HIF-1, NF-κB, forkhead box subgroup O (FoxO).19 Several groups have reported the critical role of SIRT1 in calorie restriction-induced life span extension and confer protection in experimental models of neurodegenerative diseases.66 Numerous phytochemicals have been identified that can modulate SIRT1 activity, including resveratrol from red wine.19,31 Activation of SIRT1 and its homologs has been shown to be protective in cell culture, worm, fly and mouse models of PD.67 These studies highlight the shared molecular pathways between humans and fly models that can be used as therapeutic targets against neurodegenerative diseases.
Future studies should be directed towards the identification of bioactive compounds from natural products that can regulate the evolutionarily conserved pathways involved in PD pathogenesis. Plants and insects represent a classic case of coevolution where they rely on each other for survival. Some plants have evolved to produce secondary metabolites that target conserved pathways as a strategy to defend against herbivores and other pests. This makes Drosophila models excellent platform for screening natural products for identification of new drug target candidates that can be subsequently validated in a vertebrate system.
Use of Drosophila models as screening platforms in drug discovery
In the drug discovery pipeline, cell culture-based high-throughput screening is one of the most widely used approaches to identify bioactive compounds for therapeutic purposes. However, recent studies highlight the challenges of cell culture models in drug development since the influence of chemical microenvironments cannot be studied. In addition, the drug toxicity profile in cell culture models cannot represent tissue-specific responses and often poorly translate to in vivo testing. Therefore, most of the positive drug targets identified through in vitro screening platforms appear to be ineffective or toxic in subsequent validation experiments in animal models. This prompted the development of 3D based cell cultures that can recapitulate the in vivo responses to drug treatment.68 However, the application of 3D cell culture in high-throughput screening is costly and labor intensive. Alternatively, the use of rodent models for drug screening during the initial stages is relatively expensive, time-consuming and pose ethical concerns.
The fruit fly, Drosophila melanogaster, is one of the most common invertebrate model organisms in biomedical research.69–71 Drosophila is an optimal in vivo model for studying human diseases because of the highly conserved molecular pathways, and approximately 75% of genes linked with human diseases are conserved in Drosophila.72 In addition, the shorter life span and larger progeny number along with a fully annotated genome and the low cost of pathway-defining studies make Drosophila an excellent model to elucidate molecular pathways underlying human diseases.73 Furthermore, the fly model offers powerful genetics tools to easily and rapidly manipulate target gene expression in specified cell types and tissues.74 The Drosophila nervous system is much simpler compared to humans, which makes it an optimal model to elucidate the evolutionarily conserved signaling pathways underlying disease pathology. Therefore, Drosophila models offer an ideal solution for rapid and economical large-scale screening of phytochemicals for potential drug target against neurodegenerative diseases, including PD. In contrast, mouse and other rodent model systems require expensive and long-term experimentations, while cell culture models cannot reflect cell-specific interactions in the context of the whole organism.
One of the key features of the Drosophila model is the ability to manipulate the expression of specific genes using powerful molecular tools, including the FRT/FLP recombination approach, the GAL4/upstream activation sequence (UAS) system for targeted gene expression studies, and the RNAi-mediated gene knockdown in specific cells and tissues.75Drosophila holds great promise as a whole animal model for phenotypic screening of bioactive compounds due to its genetic amenability and low maintenance cost compared to rodent models.76 In adult flies, the therapeutic properties of natural products as drug candidate can be evaluated by several ways, including oral administration by mixing in the food or sucrose saturated filter paper or direct injection into specific sites. The toxicity levels can be assessed by survival assays which will allow to establish effective drug concentrations. Simple feeding assays can be employed to monitor the effect of the drug on food intake.77 Furthermore, the short life cycle along with low-cost maintenance enable screening a large number of drug candidates at a faster rate. Unlike in vitro cell culture systems, the Drosophila PD models also offer a wide variety of behavioral assays to monitor locomotion, climbing, circadian rhythms. For example, the Drosophila PD models can be used to evaluate the ability of candidate drugs to rescue mobility defects, one of the characteristics symptoms of PD. The negative geotaxis climbing assay is a fast and reliable way to monitor locomotion defects in flies.78 Therefore, the incorporation of Drosophila models as in vivo screening platform during the early phases of drug discovery will help to overcome the limitations of in vitro cell culture assays in terms of toxicity and behavioral assessment. Additionally, the early stage screening in the context of a whole organism will rapidly narrow down the pool of larger number of potential drug candidates to fewer higher quality positive hits that can be subsequently validated in mammalian models.79 Interestingly, Drosophila has been successfully used as an in vivo screening platform for anti-cancer drug discovery.80 Recently, Ali et al. reported the development of a high-throughput, functional screen in Drosophila to identify a novel antilithogenic compound for kidney stone disease.81 These studies underscore the usefulness of Drosophila as a rapid and cheaper solution to the early stages of screening and target validation in the drug discovery pipeline. In fact, commercial companies like Aktogen (; http://aktogen.com/) and Parkure Ltd. (; https://parkure.co.uk/solutions/) are offering drug screening services using Drosophila models. In our lab, we use Drosophila models to recognize potential mechanisms of action and possible uses of numerous phytochemicals as novel drug leads for the prevention and treatment of neurodegenerative diseases.
Like any other model systems, there are few disadvantages associated with Drosophila models which should be taken into consideration. Although there is a high degree of conservation of molecular signaling pathways underlying disease pathogenesis with mammalian models, there are marked physiological differences in terms of brain anatomy and blood–brain barrier permeability.82 It is also noteworthy that Drosophila has a simple immune system and lack a classical adaptive immune response, thereby limiting the study of neuroinflammation that is biologically relevant to humans.83 However, due to highly conserved innate immune pathways and responses with humans, the fly models allow to unravel the contribution of the innate immune response to disease pathogenesis without the interference of adaptive immune signaling. Unlike cell culture models, Drosophila strains cannot be frozen and require frequent passaging to maintain as living cultures. However, the low cost and shorter life cycle make it feasible to maintain large living stocks. Another potential drawback of using fly models is the presence of certain vertebrate-specific factors linked to disease pathology, thereby making it difficult to gain mechanistic insights using invertebrate models. Despite these shortcomings, the fly still remains a powerful model system that can be used not only as a screening platform for novel therapeutics during the initial stages of drug discovery pipeline but also to develop novel assays to elucidate the mechanistic effect of bioactive compounds on disease pathogenesis.
Drosophila models of PD
Despite intensive research in the field of neurodegenerative diseases, the etiology of PD remains unclear. Although several genes have been identified that are associated with PD, only 10–15% of overall PD cases are linked to genetic causes while most PD cases are sporadic.84 Several lines of evidence suggest that PD is a complex multifactorial disorder caused by a combination of genetic and environmental factors, which affect multiple key signaling pathways in different cell types leading to the progressive loss of DA neurons.85 Moreover, epidemiological data suggest that pesticide and other environmental toxins pose as a risk factor for developing PD.86,87 The Drosophila genome encodes orthologs of most of the genes that have been implicated in PD, and the fly model mimics PD associated disease pathology.69,70 The identification of several genes linked to the familial cases of PD such as SNCA (α-synuclein),88 Leucine-rich repeat kinase 2 (LRRK2),89 Parkin,90 PTEN-induced putative kinase 1 (PINK1)91 and DJ-192 highlighted the critical role of genetics in PD etiology.93 Moreover, genome-wide association studies suggest that some of these genes are also linked to sporadic forms of PD.1
Sporadic and genetic PD models
The availability of several transgenic PD models in Drosophila, including both genetic and sporadic, provides a flexible tool to screen natural products and elucidate the molecular mechanism underlying neurodegeneration in PD.94 The α-syn encoding gene, SNCA, is the first gene in which mutations such as A30P and A53T were reported to be associated with autosomal-dominant forms of familial cases of PD.88 The cellular function of α-syn is still unclear. Feany and Bender developed the first transgenic α-syn Drosophila PD models expressing either wild-type or familial mutants (A53T and A30P) of human α-syn.95 The transgenic α-syn flies were shown to mimic several symptoms of human PD, such as motor dysfunction, α-syn accumulation, and dopaminergic neuron loss. This model is widely used to decipher the molecular pathogenesis of PD and can also be employed for screening of drugs. In fact, this model was used to show the therapeutic effects of l-dopa and dopamine agonists against α-syn-induced toxicity.96 In addition, other therapeutic agents like SIRT2 and HDAC inhibitors were identified using the α-syn Drosophila PD model.97,98 This further demonstrates the utility of the Drosophila models as a drug screening platform. Mutations in LRRK2 are the most common cause of sporadic and familial forms of PD. LRRK2 transgenic fly models have been used to screen neuroprotective effects of anti-oxidants and phenolic compounds against PD pathogenesis.99,100 Parkin, which functions as an E3 ligase, was identified in families with autosomal recessive juvenile parkinsonism.101 Genomic knockout of parkin in Drosophila mutants exhibit mitochondrial damage and increased sensitivity to oxidative stress.102,103 Another gene linked to the autosomal recessive forms of PD encodes a serine/threonine kinase, PINK1. Recent work in Drosophila and mouse models suggest a specific role of PINK1 in controlling mitochondrial function,104 thereby highlighting the connection between mitochondrial dysfunction and PD pathogenesis. Due to the presence of functionally conserved genes linked to human PD, these Drosophila genetic models can serve as a powerful screening tool to identify new therapeutic targets.
It is now well recognized that the majority of PD cases result from an interaction between genetic and environmental risk factors.85Drosophila has been extensively used to unravel the mechanistic effects of environmental toxicants in PD pathogenesis. Several toxins and pesticides namely, MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), 6-hydroxydopamine, rotenone and paraquat (PQ), have been implicated to induce PD-like symptoms in both mammalian and fly models.105–109 These environmental toxin-induced models recapitulate the cardinal features of PD, including motor defects, dopaminergic neuron loss and the formation of Lewy bodies.110 MPTP has been shown to selectively damage dopaminergic neurons due to mitochondrial dysfunction and oxidative stress.111 The herbicide PQ-induced neurotoxicity is known to involve the generation of ROS, which leads to neuronal death.112 Furthermore, PQ has also been shown to promote α-syn aggregation in mouse models, thereby confirming a link between genetic and environmental toxins in PD pathogenesis.113 The availability of a wide selection of transgenic Drosophila PD models offers an excellent in vivo platform to screen potential therapeutic bioactive compounds from natural products.
Emerging natural products as therapeutics in Drosophila PD models
Although much progress has been made in identifying the genes associated with PD, the basic molecular mechanisms underlying neurodegeneration remain unanswered. Recent studies have demonstrated the involvement of mitochondrial dysfunction, neuroinflammation and environmental factors as the major contributing factors in PD pathogenesis.114,115 Moreover, oxidative stress is thought to play a critical role in cellular dysfunction in both familial and sporadic forms of PD.116,117 Reactive oxygen species (ROS) are produced in the cell as a normal component of cellular defense and homeostasis. The levels of ROS within the cell are regulated by antioxidant proteins like superoxide dismutase (SOD) and glutathione (GSH). However, oxidative stress occurs when there is an imbalance between ROS levels and antioxidants leading to cellular damage, and eventually cell death. Previous studies have reported the presence of oxidized lipids and proteins that cause alpha-synuclein aggregation in the brain tissues of PD patients.118 Therefore, significant efforts are directed towards the identification of novel antioxidants from natural sources as a potential treatment for PD.
Curcumin is the bioactive polyphenolic compound derived from the spice turmeric and is isolated from the plant Curcuma longa L.119 In recent years, curcumin has been shown to exhibit neuroprotective potential in both genetic and environmental toxin-induced Drosophila and mammalian PD models. Curcumin has been shown to extend both life and health span in transgenic Drosophila PD models by modulating the expression of genes associated with oxidative stress and aging.120–122 Furthermore, curcumin significantly inhibited both 6-OHDA and LPS-induced activation of NF-kB and the downstream expression of pro-inflammatory genes in mammalian cell culture models.123,124 Similarly, curcumin protected human DA SH-SY5Y cell line against 6-hydroxydopamine (6-OHDA) toxicity by suppressing ROS generation.125 In a Drosophila model of environmental toxin-induced PD, curcumin improved mobility defects against acute PQ toxicity.126 Curcumin has also been shown to exert its neuroprotective effect by activating the NF-E2-related factor-2/anti-oxidant response element (Nrf2/ARE) pathway.127 Nrf2 regulates the expression of antioxidant and phase II detoxifying enzymes in response to ROS. Additionally, curcumin has been reported to be protective against mitochondrial dysfunction and cell death in siRNA-mediated knockdown of PINK1 in SH-SY5Y neuroblastoma cells.128 These studies underscore the therapeutic potential of curcumin and validate the use of Drosophila as a screening platform due to similar effects on evolutionarily conserved pathways and molecular targets in both mammalian and fly models. Despite these beneficial effects in diverse disease models, the low oval bioavailability of curcumin hinders its clinical application. However, recent studies have reported different formulations of curcumin to improve bioavailability for therapeutic purposes.129
Several studies suggest a major involvement of neuroinflammation underlying PD pathogenesis and recent therapeutic intervention strategies have focused on identifying drug targets that can reduce the inflammatory responses.130,131 Therefore, Drosophila PD models can be employed to screen natural products that exert anti-inflammatory and antioxidant properties since both inflammation and oxidative stress are linked to PD pathogenesis. In Drosophila, the innate immune response is regulated by two major signaling cascades, the Toll and the immune deficiency (Imd) pathways, both of which activate members of the Nuclear Factor kappa B (NF-κB) family of transcription factors.132 Postmortem analysis of brains of PD patients revealed NF-κB activation, emphasizing the link between immune activation and neurodegenerative disease.133 Prolonged NF-κB hyperactivation in immune cells has been linked to neurodegenerative disorders. Therefore, there is an immense interest in identifying natural products and phytochemicals that can regulate NF-κB activity.134 For example, curcumin and luteolin have been shown to block LPS-induced NF-κB activation and pro-inflammatory responses in microglial cells.135,136 Another natural polyphenolic compound derived from red wine and grapes, resveratrol has been reported to attenuate LPS-mediated microglial inflammation by suppressing NF-κB signaling.137 NF-κB activation has also been linked to age-related neurodegeneration in Drosophila.138 The presence of highly conserved innate immune-related genes, and downstream signaling pathways makes Drosophila a versatile model to screen potential drug candidates against PD pathogenesis.
Several groups have reported the neuroprotective effects of plant-derived polyphenols in various Drosophila PD models.139,140 Grape skin extracts were shown to rescue mitochondrial defects, improve health-span and prolong lifespan in a Drosophila model of PD linked with PINK1 loss-of-function.141 The health-promoting effects of grape skin have been partially contributed to resveratrol, which has been proposed to be an anti-aging agent. Interestingly, resveratrol has been shown to have beneficial effects in a 6-OHDA-induced PD rat model.142 A previous study showed that grape extract could exert neuroprotection against mitochondrial damage and improve mobility defects in α-syn Drosophila PD model.143 A recent study reported protective effects of tangeritin, a flavonoid found in the peels of mandarin oranges (Citrus reticulata), in a Drosophila PD model by reducing oxidative stress markers and increasing dopamine levels.144 Recently, another isoflavone genistein was shown to prolong life span and delay mobility defects in a α-syn Drosophila PD model.145 Another research group reported the neuroprotective effects of saffron methanolic extract (SME) and its active constituent, crocin in rotenone-induced Drosophila PD model.146 Additionally, several other plant-derived bioactive compounds, including EGCG, apple polyphenols, and lutein, has been shown to extend life span and restore mobility defects in Drosophila PD models.140,147,148 The neuroprotective effects of some of the bioactive compounds have been linked to the activation of the Nrf2/ARE pathway in flies.149 In addition, a monoterpenoid, geraniol has been shown to improve climbing ability and reduce oxidative stress in transgenic α-syn Drosophila PD model.150 These studies underscore the importance of natural products as a valuable source of bioactive compounds with neuroprotective effects to combat PD.
Conclusions
The use of Drosophila models as an in vivo screening platform for the initial phases of drug discovery is still in its infancy. Unlike other traditional screening methods using in vitro cell culture and rodent models, Drosophila provides a cheaper, flexible and more rapid platform for the first stage screening of natural extracts due to rapid generation cycle, shorter life span, availability of genetic tools to manipulate specific gene expression and low maintenance cost. Therefore, the incorporation of Drosophila models into the therapeutic drug screening pipeline holds promise to accelerate the drug discovery process in a cost-effective manner. Considering the wealth of secondary metabolites produced by plants we predict to observe the increase of the number of natural compounds identified as neuroprotective. Numerous secondary metabolites are evolutionary optimized drug-like molecules and screening natural samples for pharmacologically active compounds using Drosophila as a model organism can be considered as a valid approach in drug discovery.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
This work was supported in part by American Society of Pharmacognosy Research Starter Grant and funds obtained from Regis Technologies and Rohto Pharmaceutical.
References
- Poewe W., Seppi K., Tanner C. M., Halliday G. M., Brundin P., Volkmann J., Schrag A. E., Lang A. E. Nat. Rev. Dis. Primers. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- Shulman J. M., De Jager P. L., Feany M. B. Annu. Rev. Phytopathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- Reeve A., Simcox E., Turnbull D. Ageing Res. Rev. 2014;14:19–30. doi: 10.1016/j.arr.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey E., Bloem B. JAMA Neurol. 2018;75:9–10. doi: 10.1001/jamaneurol.2017.3299. [DOI] [PubMed] [Google Scholar]
- Dorsey E., Constantinescu R., Thompson J., Biglan K., Holloway R., Kieburtz K., Marshall F., Ravina B., Schifitto G., Siderowf A., Tanner C. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Kowal S. L., Dall T. M., Chakrabarti R., Storm M. V., Jain A. Mov. Disord. 2013;28:311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- Galvan A., Wichmann T. Clin. Neurophysiol. 2008;119:1459–1474. doi: 10.1016/j.clinph.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie C. A. Br. Med. Bull. 2008;86:109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Cotzias G., Papavasiliou P., Gellene R. N. Engl. J. Med. 1969;280:337–345. doi: 10.1056/NEJM196902132800701. [DOI] [PubMed] [Google Scholar]
- Jankovic J. Mov. Disord. 2005;20:S1–S6. [Google Scholar]
- Jankovic J., Aguilar L. G. Neuropsychiatr. Dis. Treat. 2008;4:743–757. doi: 10.2147/ndt.s2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintner R., Jankovic J. Expert Opin. Invest. Drugs. 2003;12:1803–1820. doi: 10.1517/13543784.12.11.1803. [DOI] [PubMed] [Google Scholar]
- Muller T. Drugs. 2015;75:157–174. doi: 10.1007/s40265-014-0343-0. [DOI] [PubMed] [Google Scholar]
- Dezsi L., Vecsei L. CNS Neurol. Disord.: Drug Targets. 2017;16:425–439. doi: 10.2174/1871527316666170124165222. [DOI] [PubMed] [Google Scholar]
- Fang J. Y., Tolleson C. Neuropsychiatr. Dis. Treat. 2017;13:723–732. doi: 10.2147/NDT.S113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsen T. M., Woldbye D. P. D. J. Parkinson's Dis. 2018;8:195–215. doi: 10.3233/JPD-181331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Makela J., Di Liberto V., Mudo G., Belluardo N., Eriksson O., Saarma M. Cell. Mol. Life Sci. 2016;73:1365–1379. doi: 10.1007/s00018-015-2101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Jo D. G., Park D., Chung H. Y., Mattson M. P. Pharmacol. Rev. 2014;66:815–868. doi: 10.1124/pr.113.007757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D., Cragg G. J. Nat. Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman D. J., Cragg G. M. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- Shen B. Cell. 2015;163:1297–1300. doi: 10.1016/j.cell.2015.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. L., Edrada-Ebel R., Quinn R. J. Nat. Rev. Drug Discovery. 2015;14:111–129. doi: 10.1038/nrd4510. [DOI] [PubMed] [Google Scholar]
- Ciesla L., Moaddel R. Nat. Prod. Rep. 2016;33:1131–1145. doi: 10.1039/c6np00016a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baell J., Walters M. A. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- Howitz K. T., Sinclair D. A. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. A., Sinclair D. A. Am. J. Pharmacol. Toxicol. 2008;3:152–159. doi: 10.3844/ajptsp.2008.152.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper P. L., Hooper P. L., Tytell M., Vigh L. Cell Stress Chaperones. 2010;15:761–770. doi: 10.1007/s12192-010-0206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh Y. J. Ann. N. Y. Acad. Sci. 2011;1229:1–6. doi: 10.1111/j.1749-6632.2011.06097.x. [DOI] [PubMed] [Google Scholar]
- Lamming D. W., Wood J. G., Sinclair D. A. Mol. Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrewijn P., Oosten A. M. V. and Piron P. G. M., Natural terpenoids as messengers: a multidisciplinary study of their production, biological functions, and practical applications, Kluwer Academic Pub., Dordrecht, Boston, MA, 2001. [Google Scholar]
- Bakhtiari N., Mirzaie S., Hemmati R., Moslemee-Jalalvand E., Noori A. R., Kazemi J. Arch. Biochem. Biophys. 2018;650:39–48. doi: 10.1016/j.abb.2018.05.012. [DOI] [PubMed] [Google Scholar]
- Schubert D., Currais A., Goldberg J., Finley K., Petrascheck M., Maher P. Trends Pharmacol. Sci. 2018;39:1004–1007. doi: 10.1016/j.tips.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisimov V. N., Popovich I. G., Zabezhinski M. A., Anisimov S. V., Vesnushkin G. M., Vinogradova I. A. Biochim. Biophys. Acta. 2006;1757:573–589. doi: 10.1016/j.bbabio.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Khokhlov A. N., Prokhorov L., Klebanov A. A., Gorbacheva T. A., Esipov D. S. Adv. Gerontol. 2008;21:503–506. [PubMed] [Google Scholar]
- Boldyrev A. A., Stvolinsky S. L., Fedorova T. N., Suslina Z. A. Rejuvenation Res. 2010;13:156–158. doi: 10.1089/rej.2009.0923. [DOI] [PubMed] [Google Scholar]
- Korkushko O. V., Khavinson V., Shatilo V. B., Antonyk-Sheglova I. A. Bull. Exp. Biol. Med. 2011;151:366–369. doi: 10.1007/s10517-011-1332-x. [DOI] [PubMed] [Google Scholar]
- Bulterijs S. Rejuvenation Res. 2011;14:469–482. doi: 10.1089/rej.2011.1153. [DOI] [PubMed] [Google Scholar]
- Borts M. S., Nikolaeva E. G., Kozhemiakina N. V., Borzova I. V. Adv. Gerontol. 2011;24:701–706. [PubMed] [Google Scholar]
- Ryzhak A. P., Kuznik B. I., Putkovskaia V. N., Ryzhak G. A., Iu S. Titkov. Adv. Gerontol. 2012;25:356–359. [PubMed] [Google Scholar]
- Ryzhak A. P., Kuznik B. I., Putkovskaia V. N., Ryzhak G. A. Adv. Gerontol. 2012;25:139–142. [PubMed] [Google Scholar]
- Gomberg V. G., Ryzhak V. G., Liutov R. V. Adv. Gerontol. 2013;26:476–480. [PubMed] [Google Scholar]
- Son T. G., Camandola S., Mattson M. P. NeuroMol. Med. 2008;10:236–246. doi: 10.1007/s12017-008-8037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Cheng A. Trends Neurosci. 2006;29:632–639. doi: 10.1016/j.tins.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Son T. G., Camandola S. Dose–Response. 2007;5:174–186. doi: 10.2203/dose-response.07-004.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugaiyah V., Mattson M. P. Neurochem. Int. 2015;89:271–280. doi: 10.1016/j.neuint.2015.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Aragon S., Jimenez-Aliaga K. L., Benedi J., Bermejo-Bescos P. Phytomedicine. 2016;23:1285–1294. doi: 10.1016/j.phymed.2016.07.007. [DOI] [PubMed] [Google Scholar]
- Mattson M. P. Dose–Response. 2014;12:600–618. doi: 10.2203/dose-response.14-028.Mattson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P. Ageing Res. Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Moehl K., Ghena N., Schmaedick M., Cheng A. Nat. Rev. Neurosci. 2018;19:63–80. doi: 10.1038/nrn.2017.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh K., Moore K., Wes P. D., Muchowski P. J., Dey J., Andrews L., Pallanck L. J. J. Neurosci. 2008;28:465–472. doi: 10.1523/JNEUROSCI.4778-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chen B., Wang X., Wu L., Yang Y., Cheng X., Hu Z., Cai X., Yang J., Sun X., Lu W., Yan H., Chen J., Ye J., Shen J., Cao P. Sci. Rep. 2016;6:32206. doi: 10.1038/srep32206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone M. C., Sykiotis G. P., Bohmann D. Dis. Models Mech. 2011;4:701–707. doi: 10.1242/dmm.007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G., Xu C., Hu R., Jain M. R., Gopalkrishnan A., Nair S., Huang M. T., Chan J. Y., Kong A. N. Mol. Cancer Ther. 2006;5:39–51. doi: 10.1158/1535-7163.MCT-05-0293. [DOI] [PubMed] [Google Scholar]
- Wu C. C., Hsu M. C., Hsieh C. W., Lin J. B., Lai P. H., Wung B. S. Life Sci. 2006;78:2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Welford R. W., Schlemminger I., McNeill L. A., Hewitson K. S., Schofield C. J. J. Biol. Chem. 2003;278:10157–10161. doi: 10.1074/jbc.M211058200. [DOI] [PubMed] [Google Scholar]
- Zhou Y. D., Kim Y. P., Li X. C., Baerson S. R., Agarwal A. K., Hodges T. W., Ferreira D., Nagle D. G. J. Nat. Prod. 2004;67:2063–2069. doi: 10.1021/np040140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagin U., Duncan O. F., Gatt A. P., Dionne M. S., Sweeney S. T., Bateman J. M. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E6000–E6009. doi: 10.1073/pnas.1505036112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A., Ghosh S. Cold Spring Harbor Perspect. Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camandola S., Mattson M. P. Expert Opin. Ther. Targets. 2007;11:123–132. doi: 10.1517/14728222.11.2.123. [DOI] [PubMed] [Google Scholar]
- Agarwal S., Yadav A., Chaturvedi R. K. Biochem. Biophys. Res. Commun. 2017;483:1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R. K., Beal M. F. J. Neurochem. 2008;106:506–518. doi: 10.1111/j.1471-4159.2008.05388.x. [DOI] [PubMed] [Google Scholar]
- Laloux C., Petrault M., Lecointe C., Devos D., Bordet R. Pharmacol. Res. 2012;65:514–522. doi: 10.1016/j.phrs.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Joardar A., Menzl J., Podolsky T. C., Manzo E., Estes P. S., Ashford S., Zarnescu D. C. Hum. Mol. Genet. 2015;24:1741–1754. doi: 10.1093/hmg/ddu587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper R. H., Pirinen E., Auwerx J. Nat. Rev. Mol. Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovits A. Z., Guarente L. Cell Res. 2013;23:746–758. doi: 10.1038/cr.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans S. A. Front. Pharmacol. 2018;9:6. doi: 10.3389/fphar.2018.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen J., Bonini N. Annu. Rev. Genet. 2005;39:153–171. doi: 10.1146/annurev.genet.39.110304.095804. [DOI] [PubMed] [Google Scholar]
- Bonini N., Fortini M. Annu. Rev. Neurosci. 2003;26:627–656. doi: 10.1146/annurev.neuro.26.041002.131425. [DOI] [PubMed] [Google Scholar]
- Pandey U. B., Nichols C. D. Pharmacol. Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter L. T., Potocki L., Chien S., Gribskov M., Bier E. Genome Res. 2001;11:1114–1125. doi: 10.1101/gr.169101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGurk l., Berson A., Bonini N. Genetics. 2015;201:377–402. doi: 10.1534/genetics.115.179457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales K., Korey C., Larracuente A., Roberts D. Genetics. 2015;201:815–842. doi: 10.1534/genetics.115.183392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken K. J., Bellen H. J. Development. 2007;134:3571–3584. doi: 10.1242/dev.005686. [DOI] [PubMed] [Google Scholar]
- Giacomotto J., Segalat L. Br. J. Pharmacol. 2010;160:204–216. doi: 10.1111/j.1476-5381.2010.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja W., Carvalho G., Mak E., de la Rosa N., Fang A., Liong J., Brummel T., Benzer S. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargano J., Martin I., Bhandari P., Grotewiel M. Exp. Gerontol. 2005;40:386–395. doi: 10.1016/j.exger.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Fernandez-Hernandez I., Scheenaard E., Pollarolo G., Gonzalez C. EMBO Rep. 2016;17:471–472. doi: 10.15252/embr.201642080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav A. K., Srikrishna S., Gupta S. C. Trends Pharmacol. Sci. 2016;37:789–806. doi: 10.1016/j.tips.2016.05.010. [DOI] [PubMed] [Google Scholar]
- Ali S. N., Dayarathna T. K., Ali A. N., Osumah T., Ahmed M., Cooper T. T., Power N. E., Zhang D., Kim D., Kim R., St Amant A., Hou J., Tailly T., Yang J., Luyt L., Spagnuolo P. A., Burton J. P., Razvi H., Leong H. S. Dis. Models Mech. 2018;11(11):dmm035873. doi: 10.1242/dmm.035873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle S. J., Bainton R. J. Front. Neurosci. 2014;8:414. doi: 10.3389/fnins.2014.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchon N., Silverman N., Cherry S. Nat. Rev. Immunol. 2014;14:796–810. doi: 10.1038/nri3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton A. B., Farrer M. J., Bonifati V. Mov. Disord. 2013;28:14–23. doi: 10.1002/mds.25249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon J. R., Greenamyre J. T. Neurobiol. Dis. 2013;57:38–46. doi: 10.1016/j.nbd.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack A. L., Thiruchelvam M., Manning-Bog A. B., Thiffault C., Langston J. W., Cory-Slechta D. A., Di Monte D. A. Neurobiol. Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Uversky V. N. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- Polymeropoulos M., Lavedan C., Leroy E., Ide S., Dehejia A., Dutra A. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- Zimprich A., Biskup S., Leitner P., Lichtner P., Farrer M., Lincoln S., Kachergus J., Hulihan M., Uitti R. J., Calne D. B. Neuron. 2004;44:601–607. doi: 10.1016/j.neuron.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K. Nat. Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- Valente E., Abou-Sleiman P., Caputo V., Muqit M., Harvey K., Gispert S., Ali Z., Del Turco D., Bentivoglio A., Healy D. Science. 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- Bonifati V., Rizzu P., van Baren M., Schaap O., Breedveld G., Krieger E., Dekker M., Squitieri F., Ibanez P., Joosse M. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Farrer M. Nat. Rev. Genet. 2006;7:306–318. doi: 10.1038/nrg1831. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Yu J. Front. Neurol. 2018;9:228. doi: 10.3389/fneur.2018.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany M., Bender W. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- Pendleton R., Parvez F., Sayed M., Hillman R. J. Pharmacol. Exp. Ther. 2002;300:91–96. doi: 10.1124/jpet.300.1.91. [DOI] [PubMed] [Google Scholar]
- Kontopoulos E., Parvin J., Feany M. Hum. Mol. Genet. 2006;15:3012–3023. doi: 10.1093/hmg/ddl243. [DOI] [PubMed] [Google Scholar]
- Outeiro T., Kontopoulos E., Altmann S. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- Tan E. K., Skipper L. M. Hum. Mutat. 2007;28:641–653. doi: 10.1002/humu.20507. [DOI] [PubMed] [Google Scholar]
- Angeles D. C., Ho P., Dymock B. W., Lim K. L., Zhou Z. D., Tan E. K. Ann. Clin. Transl. Neurol. 2016;3:288–294. doi: 10.1002/acn3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Nature. 1998;392(6676):605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- Greene J., Whitworth A., Kuo I., Andrews L., Feany M., Pallanck L. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4078–4083. doi: 10.1073/pnas.0737556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesah Y., Pham T., Burgess H., Middlebrooks B., Verstreken P., Zhou Y., Harding M., Bellen H., Mardon G. Development. 2004;131:2183–2194. doi: 10.1242/dev.01095. [DOI] [PubMed] [Google Scholar]
- Morais V., Verstreken P., Roethig A., Smet J., Snellinx A., Vanbrabant M. EMBO Mol. Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston J., Ballard P., Tetrud J., Irwin I. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- McCormack A., Thiruchelvam M., Manning-Bog A., Thiffault C., Langston J., Cory-Slechta D., Di Monte D. Neurobiol. Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- Uversky V. Cell Tissue Res. 2004;318:225–241. doi: 10.1007/s00441-004-0937-z. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A., Bowling K., Funderburk C., Lawal H., Inamdar A., Wang Z., O'Donnell J. M. J. Neurosci. 2007;27:2457–2467. doi: 10.1523/JNEUROSCI.4239-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J., Prou D., Perier C., Przedborski S. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E. Int. J. Mol. Sci. 2018;19(11):3343. doi: 10.3390/ijms19113343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M., Wang X., Zhu X. Free Radical Biol. Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R., Li S., Rodriguez-Rocha H., Burns M., Panayiotidis M. I. Chem.-Biol. Interact. 2010;188:289–300. doi: 10.1016/j.cbi.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning-Bog A., McCormack A., Li J., Uversky V., Fink A., Di Monte D. J. Biol. Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Esposito E., Cuzzocrea S. Curr. Med. Chem. 2010;17:2764–2774. doi: 10.2174/092986710791859324. [DOI] [PubMed] [Google Scholar]
- Park J. S., Davis R. L., Sue C. M. Curr. Neurol. Neurosci. Rep. 2018;18:21. doi: 10.1007/s11910-018-0829-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias V., Junn E., Mouradian M. M. J. Parkinson's Dis. 2013;3:461–491. doi: 10.3233/JPD-130230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J., Trigo-Damas I., Quiroga-Varela A., Jackson-Lewis V. R. Front. Neuroanat. 2015;9:91. doi: 10.3389/fnana.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani R. J., Perry G., Siedlak S. L., Nunomura A., Shimohama S., Zhang J., Montine T., Sayre L. M., Smith M. A. Neurosci. Lett. 2002;319:25–28. doi: 10.1016/s0304-3940(01)02514-9. [DOI] [PubMed] [Google Scholar]
- Witkin J. M., Li X. CNS Neurol. Disord.: Drug Targets. 2013;12:487–497. doi: 10.2174/1871527311312040007. [DOI] [PubMed] [Google Scholar]
- Lee K., Lee B., Semnani S., Avanesian A., Um C., Jeon H., Seong K., Yu K., Min K., Jafari M. Rejuvenation Res. 2010;13:561–570. doi: 10.1089/rej.2010.1031. [DOI] [PubMed] [Google Scholar]
- Siddique Y. H., Naz F., Jyoti S. Biomed Res. Int. 2014;2014:606928. doi: 10.1155/2014/606928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. T., Vuu M. D., Huynh M. A., Yamaguchi M., Tran L. T., Dang T. P. T. Oxid. Med. Cell. Longevity. 2018;2018:2038267. doi: 10.1155/2018/2038267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Zhang D., Yang Z., Hu X., Qian S., Liu J., Wilson B., Block M., Hong J. S. Neurochem. Res. 2008;33:2044–2053. doi: 10.1007/s11064-008-9675-z. [DOI] [PubMed] [Google Scholar]
- Wang J., Du X. X., Jiang H., Xie J. X. Biochem. Pharmacol. 2009;78:178–183. doi: 10.1016/j.bcp.2009.03.031. [DOI] [PubMed] [Google Scholar]
- Jaisin Y., Thampithak A., Meesarapee B., Ratanachamnong P., Suksamrarn A., Phivthong-Ngam L., Phumala-Morales N., Chongthammakun S., Govitrapong P., Sanvarinda Y. Neurosci. Lett. 2011;489:192–196. doi: 10.1016/j.neulet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Park J., Jung J., Ahn Y., Kwon H. Pestic. Biochem. Physiol. 2012;104:118–122. [Google Scholar]
- Beal M. F. Parkinsonism Relat. Disord. 2009;15(Suppl 3):S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- van der Merwe C., van Dyk H. C., Engelbrecht L., van der Westhuizen F. H., Kinnear C., Loos B., Bardien S. Mol. Neurobiol. 2017;54:2752–2762. doi: 10.1007/s12035-016-9843-0. [DOI] [PubMed] [Google Scholar]
- Purpura M., Lowery R., Wilson J., Mannan H., Münch G., Razmovski-Naumovski V. Eur. J. Nutr. 2018;57:929–938. doi: 10.1007/s00394-016-1376-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch E. C., Vyas S., Hunot S. Parkinsonism Relat. Disord. 2012;18(Suppl 1):S210–S212. doi: 10.1016/S1353-8020(11)70065-7. [DOI] [PubMed] [Google Scholar]
- Amor S., Peferoen L. A., Vogel D. Y., Breur M., van der Valk P., Baker D., van Noort J. M. Immunology. 2014;142:151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E., Spellman P., Tzou P., Rubin G., Lemaitre B. EMBO J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S., Brugg B., Ricard D., Mitchel P., Muriel M.-P., Ruberg M., Faucheux B., Agid Y., Hirsch E. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner P., Heinrich M. J. Pharm. Pharmacol. 2002;54:453–472. doi: 10.1211/0022357021778637. [DOI] [PubMed] [Google Scholar]
- Yang S., Zhang D., Yang Z., Hu X., Qian S., Liu J., Wilson B., Block M., Hong J. Neurochem. Res. 2008;33:2044–2053. doi: 10.1007/s11064-008-9675-z. [DOI] [PubMed] [Google Scholar]
- Zhu L., Bi W., Qi R., Wang H., Lu D. J. Neurosci. 2011;121:329–336. doi: 10.3109/00207454.2011.569040. [DOI] [PubMed] [Google Scholar]
- Capiralla H., Vingtdeux V., Zhao H., Sankowski R., Al-Abed Y., Davies P., Marambaud P. J. Neurochem. 2012;120:461–472. doi: 10.1111/j.1471-4159.2011.07594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounatidis I., Chtarbanova S., Cao Y., Hayne M., Jayanth D., Ganetzky B., Ligoxygakis P. Cell Rep. 2017;19:836–848. doi: 10.1016/j.celrep.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Bang S. M., Lee J. W., Cho K. S. Evid. Based Complement Alternat. Med. 2014;2014:967462. doi: 10.1155/2014/967462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Del-Rio M., Guzman-Martinez C., Velez-Pardo C. Neurochem. Res. 2010;35:227–238. doi: 10.1007/s11064-009-0046-1. [DOI] [PubMed] [Google Scholar]
- Wu Z., Wu A., Dong J., Sigears A., Lu B. Exp. Gerontol. 2018;113:10–17. doi: 10.1016/j.exger.2018.09.014. [DOI] [PubMed] [Google Scholar]
- Khan M., Ahmad A., Ishrat T., Khan M., Hoda M., Khuwaja G., Raza S., Khan A., Javed H., Vaibhav K., Islam F. Brain Res. 2010;1328:139–151. doi: 10.1016/j.brainres.2010.02.031. [DOI] [PubMed] [Google Scholar]
- Long J., Gao H., Sun L., Liu J. Z.-W., Zhao-Wilson X. Rejuvenation Res. 2009;12:321–331. doi: 10.1089/rej.2009.0877. [DOI] [PubMed] [Google Scholar]
- Fatima A., Khanam S., Rahul R., Jyoti S., Naz F., Ali F., Siddique Y. Front. Biosci., Elite Ed. 2017;9:44–53. doi: 10.2741/e784. [DOI] [PubMed] [Google Scholar]
- Siddique Y., Naz F., Jyoti S., Ali F., Rahul J. Diet. Suppl. 2018;3:1–14. [Google Scholar]
- Rao S., Muralidhara, Yenisetti S., Rajini P. Neurotoxicology. 2016;52:230–242. doi: 10.1016/j.neuro.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Han S., Wang H., Wang T. Arch. Gerontol. Geriatr. 2014;58:153–159. doi: 10.1016/j.archger.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Peng C., Chan H. Y. E., Huang Y., Yu H., Chen Z. J. Agric. Food Chem. 2011;59:2097–2106. doi: 10.1021/jf1046267. [DOI] [PubMed] [Google Scholar]
- Sykiotis G., Bohmann D. Dev. Cell. 2008;14(1):76–85. doi: 10.1016/j.devcel.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddique Y. H., Naz F., Jyoti S., Ali F., Fatima A., Rahul, Khanam S. Environ. Toxicol. Pharmacol. 2016;43:225–231. doi: 10.1016/j.etap.2016.03.018. [DOI] [PubMed] [Google Scholar]