Abstract
A newly proposed form of brain structural plasticity consists of non-newly generated, “immature” neurons of the adult cerebral cortex. Similar to newly generated neurons, these cells express the cytoskeletal protein Doublecortin (DCX), yet they are generated prenatally and then remain in a state of immaturity for long periods. In rodents, the immature neurons are restricted to the paleocortex, whereas in other mammals, they are also found in neocortex. Here, we analyzed the DCX-expressing cells in the whole sheep brain of both sexes to search for an indicator of structural plasticity at a cellular level in a relatively large-brained, long-living mammal. Brains from adult and newborn sheep (injected with BrdU and analyzed at different survival times) were processed for DCX, cell proliferation markers (Ki-67, BrdU), pallial/subpallial developmental origin (Tbr1, Sp8), and neuronal/glial antigens for phenotype characterization. We found immature-like neurons in the whole sheep cortex and in large populations of DCX-expressing cells within the external capsule and the surrounding gray matter (claustrum and amygdala). BrdU and Ki-67 detection at neonatal and adult ages showed that all of these DCX+ cells were generated during embryogenesis, not after birth. These results show that the adult sheep, unlike rodents, is largely endowed with non-newly generated neurons retaining immature features, suggesting that such plasticity might be particularly important in large-brained, long-living mammals.
SIGNIFICANCE STATEMENT Brain plasticity is important in adaptation and brain repair. Structural changes span from synaptic plasticity to adult neurogenesis, the latter being highly reduced in large-brained, long-living mammals (e.g., humans). The cerebral cortex contains “immature” neurons, which are generated prenatally and then remain in an undifferentiated state for long periods, being detectable with markers of immaturity. We studied the distribution and developmental origin of these cells in the whole brain of sheep, relatively large-brained, long-living mammals. In addition to the expected cortical location, we also found populations of non-newly generated neurons in several subcortical regions (external capsule, claustrum, and amygdala). These results suggests that non-neurogenic, parenchymal structural plasticity might be more important in large mammals with respect to adult neurogenesis.
Keywords: cerebral cortex, doublecortin, immature neurons, plasticity, postnatal development
Introduction
The mammalian CNS is made up mostly of nonrenewable (perennial) neurons with cell processes that are connected physically and functionally in a largely invariant way. Although anatomical invariability is a necessary requisite to ensure stability of connections in the neural circuits (Frotscher, 1992), exceptions do exist in the form of cellular modifications referred to as “structural plasticity,” which affect the brain anatomy at different levels and degrees (Bonfanti, 2006; Theodosis et al., 2008). These exceptions span from formation/elimination of synapses in preexisting neurons (synaptic plasticity; Bonfanti-and Theodosis, 2009; Bailey et al., 2015) to addition/replacement of new neurons (adult neurogenesis; Bonfanti-and Peretto, 2011; Aimone et al., 2014). The occurrence, amount, type, and location of neural structural plasticity, as well as its reparative capacity, vary greatly in the animal world (Bonfanti-and Peretto, 2011; Grandel and Brand, 2013) and, to a lesser extent, among mammals (Feliciano et al., 2015; Lipp and Bonfanti, 2016). The cytoskeletal protein doublecortin (DCX) is an excellent marker for cells that retain high potential for structural plasticity in the CNS (Gleeson et al., 1999; Nacher et al., 2001). Due to its heavy expression in newly generated neuroblasts and during the early phases of their migration/differentiation, DCX is commonly used as a marker for adult neurogenesis (Brown et al., 2003). Nevertheless, it is now clear that at least one type of the neurons located in layer II of the adult mammalian cerebral cortex that are not newly generated (“immature neurons”; Gomez-Climent et al., 2008) express DCX during adulthood (for review, see Bonfanti-and Nacher, 2012; Nacher and Bonfanti, 2015). Current data indicate that the occurrence and distribution of DCX+ cells can vary substantially in brain regions of different mammals (Feliciano et al., 2015; Lipp and Bonfanti, 2016), suggesting species-specific heterogeneity in their capability to undergo both neurogenic and non-neurogenic structural plasticity. For instance, the rate of adult neurogenesis decreases in mammals with extended life expectance (e.g., humans, dolphins, and sheep; Sanai et al., 2011; Brus et al., 2013a; Parolisi et al., 2015, 2017) compared with the relatively short-living laboratory rodents (for review, see Lipp and Bonfanti, 2016; Paredes et al., 2016). In contrast, the occurrence of cortical layer II immature neurons is higher in rabbits, guinea pigs, and cats compared with rats and mice, extending into neocortical regions in the former and being restricted to paleocortex in the latter (Xiong et al., 2008; Cai et al., 2009; Luzzati et al., 2009). Here, the occurrence of DCX+ cells was investigated in sheep (Ovis aries) with the idea of analyzing the distribution of this indicator of structural plasticity in a relatively long-living mammal endowed with a large-sized, gyrencephalic brain. Sheep possess a brain as large as a macaque monkey and have a similar life span (10/30 years in wild/captivity). In addition, experimental procedures such as BrdU injection for subsequent immunocytochemical detection of newly generated cells in the brain tissue can be performed in these animals. Neonatal and adult animals treated with BrdU (injected in pregnant ewes in the case of neonates) were studied to assess the time of genesis (prenatal vs postnatal) of the DCX+ cells. We show that, in addition to cortical immature neurons, different populations of non-newly generated DCX+ cells are present consistently in the external capsule and adjacent regions of the adult sheep brain. Interestingly, quantification of these latter cells in neonatal, prepubertal, and adult animals showed that they are not depleted by aging.
Materials and Methods
Animals, BrdU injections, and tissue preparation
Neonatal, prepubertal, and adult animals (breed: Ile de France) were raised at the Institut National de la Recherche Agronomique (INRA) research center (Nouzilly, Indre et Loire, France). Experiments were conducted on 9 adult (females, 2 year old), 3 prepubertal (males, 4 month old), and 7 neonatal (4 males, 3 females, 1 week old) sheep (Table 1). Adult ewes were housed in an individual pen (2 × 1 m) and received four intravenous injections of BrdU during pregnancy (1 injection/d, 20 mg/kg in 0.9% saline; Sigma-Aldrich), a thymidine analog incorporated into the DNA during the S-phase of the mitotic division. Two days after lambing, ewes were anesthetized with thiopental and decapitated by a licensed butcher in an official slaughterhouse (ethical permissions are reported in Brus et al., 2013b). Three different survival times were analyzed in these adult animals: 1, 2, and 4 months (Brus et al., 2013b). Because all of the ewes were pregnant, the intravenous injections of BrdU could allow the molecule to pass to the fetuses and be incorporated into their brains. All lambs used in this study were collected from mothers being injected 3 months before parturition (i.e., at 2 month gestational days). Brains were perfused through both carotid arteries with 2 L of 1% sodium nitrite in phosphate buffer saline, followed by 4 L of ice-cold 4% paraformaldehyde solution in 0,1 m phosphate buffer, pH 7.4. The brains were then dissected out, cut into blocks, and postfixed in the same fixative for 48 h. The tissues were then stored in 30% sucrose. Each hemisphere was cut into 4 coronal slices (∼1.5 cm thick), embedded in optimum cutting temperature medium (Bio-Optica), frozen in isopentane, and stored at −80°C. Cryostat coronal sections (40 μm thick) were cut to be used in free-floating immunohistochemistry and immunofluorescence procedures. We then obtained the outlines of four levels of interest, representing the whole sheep brain (L1–L4) by combining the analysis of our cryostat sections and photographs from the atlas of Brain Biodiversity Bank of Michigan State University (Fig. 1).
Table 1.
Animals used in the present study
| Source | Sex | Breed | Age | BrdU treatment |
|---|---|---|---|---|
| INRA | 4 M, 3 F | Ile-de-France | Neonate (7 d) | Survival: 97 d (injection: 90 d prepartum) |
| 3 M | Prepubertal (4 mo) | — | ||
| 9 F | Adult (2 y) | Survival: 120 d | ||
| Survival: 60 d | ||||
| Survival: 30 d |
Figure 1.
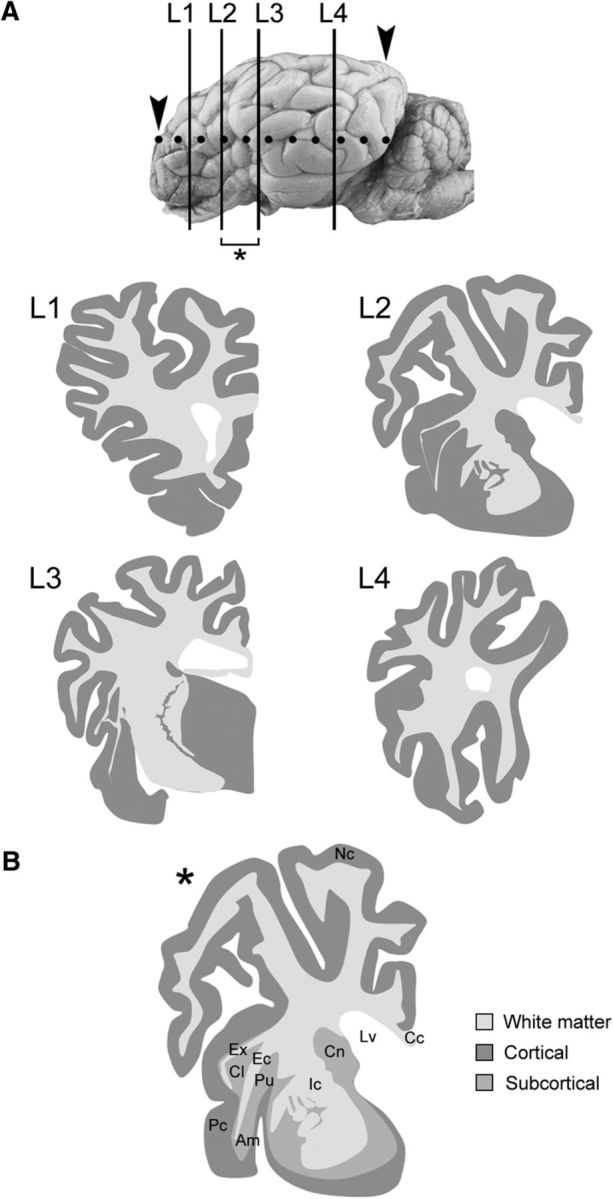
A, Four levels of interest (L1–L4) in the whole sheep brain, obtained by redrawing the Atlas of Brain Biodiversity Bank (Michigan State University; https://msu.edu/∼brains/brains/sheep/index.html) and adapted to our cryostat sections; the whole extension of the brain analyzed is comprised between the arrowheads (dotted line). B, By combining levels L2 and L3, an additional, “ideal” level containing the most important neuroanatomical structures analyzed here was obtained (asterisk); this ideal level was used to represent Results. This representation refers to adult animals, yet no significant morphological/neuroanatomical differences were observed in younger animals. Nc, Neocortex; Pc, paleocortex; Ic, internal capsule; Ec, external capsule; Ex, capsula extrema; Cl, claustrum; Am, amygdala; Pu, putamen; Cn, caudate nucleus; Lv, lateral ventricle; Cc, corpus callosum.
Immunohistochemistry
Immunohistochemical reactions were performed on free-floating sections; when necessary, antigen retrieval was performed using citric acid at 90°C for 5–10 min. The section were incubated in blocking buffer (2% normal serum, 0.5–1% Triton X-100 in 0.01 m PBS, pH 7.4) for 2 h at room temperature, then incubated for 24–48 h at 4°C in a solution of 0.01 m PBS, pH 7.4, containing 0,5–1% Triton X-100, 2% normal serum, and the primary antibodies (Table 2). Sections were then incubated with appropriate solutions of secondary antibody: Alexa Fluor 488-conjugated goat anti-mouse (1:400; Invitrogen), Alexa Fluor 488-conjugated goat anti-rabbit (1:400; Invitrogen), Alexa Fluor 488-conjugated donkey anti-rat (1:400 Jackson ImmunoResearch), Alexa Fluor-555-conjugated goat anti-mouse (1:800; Invitrogen), Alexa Fluor-555-conjugated goat anti-rabbit (1:800; Invitrogen), Alexa Fluor-555-conjugated goat anti-guinea pig (1:800; Invitrogen), cyanine 3 (cy3)-conjugated goat anti-mouse (1:800; Jackson ImmunoResearch), cyanine 3 (cy3)-conjugated goat anti-rabbit (1:800; Jackson ImmunoResearch), cyanine 3 (cy3)-conjugated donkey anti-goat (1:800,Jackson ImmunoResearch), Alexa Fluor-647conjugated donkey anti-mouse (1:800; Jackson ImmunoResearch), and Alexa Fluor-647-conjugated donkey anti-rabbit, for 3 h at room temperature (RT). Sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; KPL), coverslipped with MOWIOL 4–88 (Calbiochem), and examined. For 3,3′-diaminobenzidine (DAB) immunohistochemistry, sections were incubated in a solution of 0,3% H2O2 in 0,01 m PBS, pH 7.4, for 15 min to inhibit the endogenous peroxidase before incubation with blocking buffer. After primary antibody incubation, sections were incubated with goat anti-rabbit IgG biotinylated secondary antibody (1:350; Vector Laboratories) or horse anti-goat IgG biotinylated secondary antibody (1:250; Vector Laboratories) for 2 h at RT. Sections were washed and incubated in avidin–biotin–peroxidase complex (Vectastain ABC kit; Vector Laboratories) for 2 h at RT. The reaction was developed with the DAB Peroxidase Substrate Kit (Vector Laboratories) for 3–5 min. Sections were mounted on 3-aminopropyl-triethoxysilan-treated slides and then counterstained with 1% cresyl violet acetate solution (1–2 min exposure) and coverslipped with Neo-Mont 109016 (Merck).
Table 2.
Primary antibodies used in the present study
| Antigen | Host | Dilution | RRID | Source |
|---|---|---|---|---|
| BrdU | Rat | 1:300 | AB_609566 | AbDSerotec |
| DCX | Rabbit | 1:3000 | AB_732011 | Abcam |
| Goat | 1:2000 | AB_2088494 | Santa Cruz Biotechnology | |
| Guinea pig | 1:1000 | AB_2230227 | Abcam | |
| GFAP | Rabbit | 1:800 | AB_10013382 | Agilent Technologies |
| Mouse | 1:200 | AB_94844 | Millipore | |
| Ki-67 | Mouse | 1:500 | AB_393778 | Agilent Technologies |
| NeuN | Mouse | 1:1000 | AB_177621 | Millipore |
| Sp8 | Rabbit | 1:10000 | AB_877304 | Millipore |
| Tbr1 | Rabbit | 1:1000 | AB_10806888 | Chemicon |
| PSA-NCAM | Mouse | 1:1400 | AB_95211 | Millipore |
| HuC/D | Mouse | 1:800 | AB_221448 | Invitrogen |
| CNGA3 | Rabbit | 1:250 | AB_2039822 | Alomone Labs |
Quantitative analyses
Cell counting was performed using Neurolucida software (MicroBrightfield).
Diameters of DCX+ objects in the external capsule and pericapsular regions.
The average object diameter (orthogonal to main axis) was measured for all clusters and groups of scattered cells in the external capsule, claustrum, and amygdala of three adult animals using the straight line tool of ImageJ program after a proper calibration and then reporting the minimum and maximum length to obtain a size range. (For more information, see the Results section.)
Density of DCX+ objects in the external capsule at neonatal and adult ages.
The DCX+ cell clusters and groups were counted in the whole extension of the external capsule in three adult and three newborn animals using a serial step of 12 cryostat sections (480 μm; 11 sections for the adult and 10 for the newborn). The density was calculated as the total number of objects/area (mm2). A 3D reconstruction was made to further characterize the DCX+ clusters in the adult in the posterior part of the external capsule (24 serial cryostat sections, 40 μm thick, corresponding to 0.96 mm of white matter tissue).
Cell density in the amygdala and claustrum at neonatal and adult ages.
The total DCX+ cells present within the two pericapsular regions was counted in three adult and three newborn animals (three slices corresponding to the anterior, middle, and posterior part of each anatomical structure were considered). The density was calculated as the total number of cells/area (in square millimeters).
Linear density of DCX+ neurons in the cerebral cortex at neonatal and adult ages.
The DCX+ cells present in the cortical layer II were counted within two brain levels (L2 and L3) and three regions (the cingulate cortex, the medial margin of the suprasylvian gyrus in the neocortex, and the piriform cortex in the paleocortex; see Fig. 6) using three cryostat sections/level in three adult and three newborn animals. The linear density was calculated as the total number of cells/the cortical tract length (in millimeters).
Figure 6.
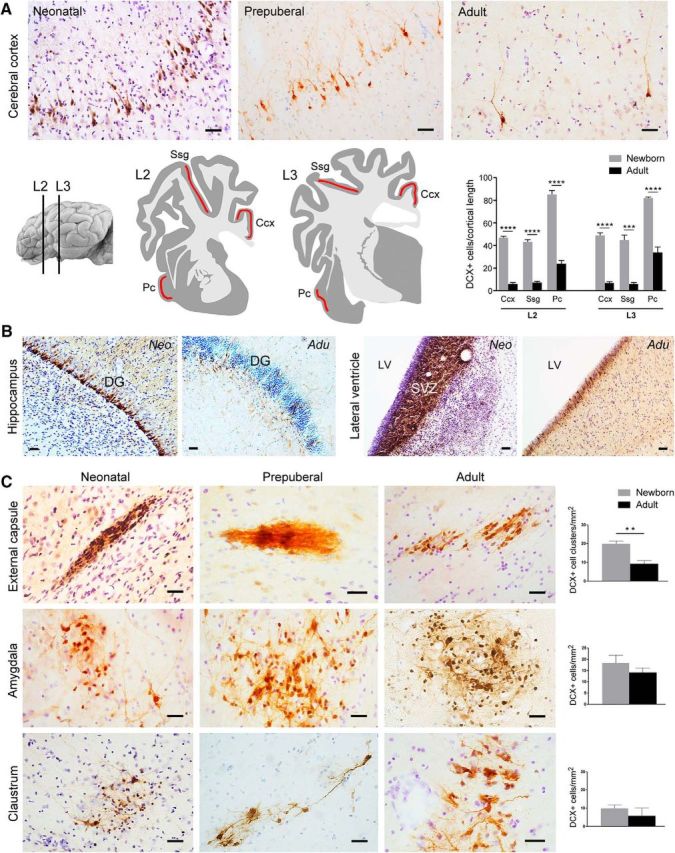
DCX+ cells in the sheep brain at different ages. A, Evident reduction of the amount of DCX+ neurons in the cortical layer II with increasing age is clearly visible after qualitative analysis (top). Quantitative evaluation of DCX+ cell linear density (number of DCX+ neurons in layer II/cortical tract length; bottom) in three cortical regions (red areas) at two brain levels of the newborn and adult sheep. Pc, Piriform cortex; Ssg, suprasylvian gyrus; Ccx, cingulate cortex (***p = 0,0001, ****p < 0,0001). B, Striking reduction of DCX+ cell populations is clearly evident in the dentate gyrus (DG; note the dilution of the DCX+ cell layer) and SVZ (note the reduction in thickness of the DCX+ germinal layer) neurogenic sites of neonatal and adult sheep. C, Occurrence, morphology, distribution, and amount (quantifications on the right; see also Tables 3, 4, 5) of DCX+ cells in the sheep capsular/pericapsular regions do not vary significantly at different ages (apart from a slight reduction observed in the external capsule; see text). Scale bars, 30 μm (**p= 0,001).
Cell soma diameters of DCX+ cells.
The average cell soma diameter (orthogonal to main axis) was measured for 200 cells in the cortex, claustrum, and amygdala of three adult animals using the straight line tool of ImageJ after a proper calibration and then reporting the minimum and maximum length to obtain a size range.
DCX+/BrdU+ double staining.
The percentage of double-stained cells was calculated after analyzing 200 cells in the cortex, claustrum, external capsule, and amygdala of 3 newborn animals (number of DCX+/BrdU+ cell of DCX+, single-stained cells) and 3 adult animals (for each survival time: 1, 2, and 4 months).
DCX+/NeuN+ double staining.
The percentage of double-stained cells was calculated after analyzing 200 cells in the cortex, claustrum, and amygdala of three adult animals (number of DCX+/NeuN+ cells of DCX+, single-stained cells).
DCX+/Tbr1+ and DCX+/Sp8+ double staining.
The percentage of double-stained cells was calculated after analyzing 200 cells in the cortex, external capsule, claustrum, and amygdala of three newborn animals (number of DCX+/Tbr1+ or DCX+/Sp8+ cells of DCX+, single-stained cells).
Statistical analysis.
All graphics and relative statistical analysis were performed using GraphPad Prism 5 Software and included unpaired (two-tailed) Student's t test (comparing only two groups) and two-way ANOVAs. p < 0.05 was considered as statistically significant. Data are expressed as averages ± SD.
Image acquisition and processing
Images from immunofluorescence specimens were collected with a Leica TCS SP5 confocal microscope. Images from DAB immunohistochemistry were collected with a Nikon Eclipse 80i microscope connected to a color CCD Camera. Images were processed using Photoshop CS4 (Adobe Systems) and ImageJ. Only general adjustments to color, contrast, and brightness were made. The 3D reconstruction in the external capsule was performed using Neurolucida software (MicroBrightfield) by aligning 24 consecutive coronal sections starting from the onset of the thalamus. The sections were previously immunoreacted for DCX using DAB peroxidase staining and counterstained with 1% cresyl violet acetate solution.
Results
Distribution of DCX+ cells in the adult sheep brain
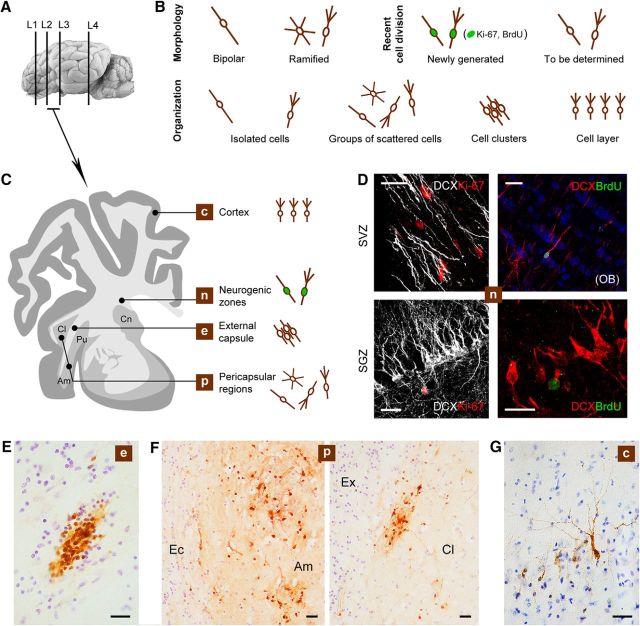
After systematic screening of the whole adult sheep brain, several populations of DCX+ cells were detected at different locations (Fig. 2): (1) newly generated neuroblasts/neuronal-like cells within the SVZ and hippocampal neural stem cell niches, (2) neuronal-like cells in the superficial layers of the cerebral cortex, and (3) groups of neuroblasts/neuronal-like cells in the external capsule and surrounding gray matter (claustrum and amygdaloid nuclei). No DCX+ cells were detected in the striatum/putamen. In addition to their different location, these DCX+ cells appeared to vary in their morphology and spatial organization (Fig. 2B,C). Cells in the neurogenic zones typically appeared as described previously (Brus et al., 2010, 2013b): single, elongated, bipolar neuroblasts in the SVZ adjacent to the lateral ventricle wall and at the lower aspect of the hippocampal dentate gyrus (Fig. 2D); these neurogenic regions were used as an internal control for detection of local cell division markers (Ki-67 antigen) and newly generated neurons identifiable through injection of exogenous markers (BrdU) and subsequent detection at different survival times.
Figure 2.
DCX+ cells in the adult sheep brain. A, C, Representative levels of the brain showing different locations of the DCX+ cells. B, Main types of DCX+ cells encountered in our analysis (DCX+ “objects”) classified according to their morphology, spatial organization, and cell division history (newly born vs non-newly generated). D, Newly generated neuroblasts in the SVZ and dentate gyrus (SGZ); in both neurogenic sites, DCX+ cells are intermingled with several nuclei immunoreactive for Ki-67 antigen (rarely double stained in the SVZ due to different expression time course of the markers); BrdU injected 60 d before the animals were killed is detectable in DCX+ neuroblasts of the olfactory bulb (OB) and in hippocampal granule cells. E–G, Representative photographs of the DCX+ cells/cell populations at the different locations showed in C. E, Clusters of DCX+ cells in the external capsule (Ec). F, Scattered DCX+ cells in the amygdala (Amy) and claustrum (Cl); Ex, capsula extrema. G, Layer II cortical neurons. Scale bars: 30 μm; D (bottom right), 20 μm.
DCX+ cells in the cerebral cortex
As described previously in some cortical areas of other mammals (for review, see Bonfanti-and Nacher, 2012), DCX+ neuronal-like cells were detected in the cerebral cortex of the adult sheep (Fig. 3). These cells were present in the superficial layers of most paleocortex and neocortex (Fig. 3A,B). They were further characterized to use them as a population of DCX+ cortical neurons that was described previously in other species and studied here in sheep. In the cortical cytoarchitecture, the DCX+ neuronal-like cells were localized in the upper part of layer II (at the limit with layer I), being present along the entire anteroposterior and dorsoventral axis of the brain. They appeared to be more abundant in the piriform cortex (paleocortex) with respect to neocortex (Fig. 2B; see below for quantitative analysis). Two main morphological types falling into two cell body size categories were identified: small cells with a diameter of 4–7 μm (type 1 cells) and large cells with a diameter of 9–12 μm (type 2 cells) (Fig. 3C,D). The large-sized cells frequently appeared as pyramidal-like neurons (similar to the “principal cells” previously described in rats by Gomez-Climent et al., 2008), whereas the small cells mostly showed a simpler, bipolar morphology (Fig. 3C,D). The principal cell type can be further split into two main patterns linked to soma shape and dendritic complexity: those with oval-shaped cell body and poorly ramified apical dendrites (here referred to as type 2a) and others with more developed apical dendrite arborization and basal dendrites (here referred to as type 2b) (Fig. 3C,D). Type 2 cells were far less abundant with respect to type 1 cells (∼7%; Fig. 3D).
Figure 3.
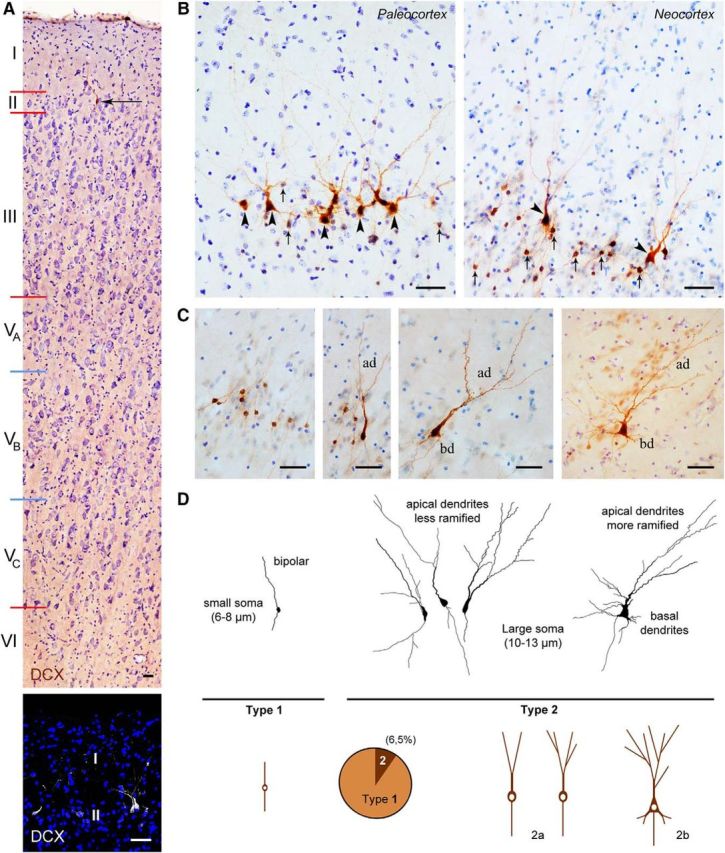
DCX+ cells in the cerebral cortex of adult sheep. A, Location of DCX+ neurons in the cortical layer II. Top, DCX (brown) and cresyl violet staining. Coronal section cut at the level of the frontal lobe layer IV (inner granular layer) is absent in the agranular isocortex of sheep (Beul and Hilgetag, 2014). WM, White matter. Bottom, Confocal image of the first two cortical layers (DCX, white; DAPI, blue). B, DCX+ neurons are present both in paleocortex (piriform cortex) and neocortex. Arrows indicate type 1 neurons; arrowheads, type 2 neurons (see D). C, D, Main morphological types of the DCX+ neurons (neocortex); type 1: small cell body and simpler apical dendritic arborization (ad); type 2: large cell body and more elaborated apical dendritic arborization (type 2a), also including basal dendrites (bd; type 2b); type 2 cells represent ∼7% of total DCX+ cells. Scale bars, 30 μm.
DCX+ cells in the external capsule and surrounding regions
Additional DCX+ cell populations were detectable in the external capsule and surrounding regions (claustrum and amygdala). Most of these cells were grouped into discrete bulks to form either tightly packed cell clusters (mainly in the white matter; Fig. 2E) or groups of scattered cells (more frequently observed in gray matter; Fig. 2G). Due to their heterogeneity and to perform a quantitative analysis, they were considered as “DCX+ objects” divided into compact cell clusters (within the external capsule) and groups of scattered cells (in the surrounding regions: amygdala and claustrum).
External capsule
Most DCX+ cells in capsular white matter appeared as bipolar, elongated elements, often endowed with very long cell processes, which were assembled to form tightly packed cell clusters (Fig. 4C). These clusters showed remarkable diameter variability (between 50 and 380 μm). When observed in single coronal sections, some of them were reminiscent of the thick chains of neuroblasts described in the SVZ of rodents (Lois et al., 1996) and in the surrounding regions of rabbits (Luzzati et al., 2003; Ponti et al., 2006). Therefore, a 3D reconstruction was performed through an anterior–posterior portion of the external capsule (Fig. 4B; see Materials and Methods) to assess their relationship with the SVZ neurogenic site. The analysis revealed that all clusters appeared as discrete bulks of cells not forming long streams and never contacting the SVZ neurogenic site. To investigate the relationship with surrounding cells (e.g., the occurrence of specific astrocytic arrangements), immunocytochemistry for glial fibrillary astrocytic protein (GFAP) was performed. GFAP staining did not revealed any special density or niche-like organization adjacent to the DCX+ cells (Fig. 4D); however, when the DCX+ cells were organized to form tightly packed clusters, they occupied glial-empty spaces, the astrocytes being segregated outside of them (Fig. 4D). Quantification of DCX+ cell clusters (see below and Tables 3, 4, and 5) revealed that virtually all of them were contained within the external capsule, mostly residing in its posterior part (Fig. 4B). This analysis further excluded any direct contact between the clusters and the SVZ neurogenic site. Groups of less tightly packed cells and scattered/isolated cells were also observed (Fig. 4C).
Figure 4.
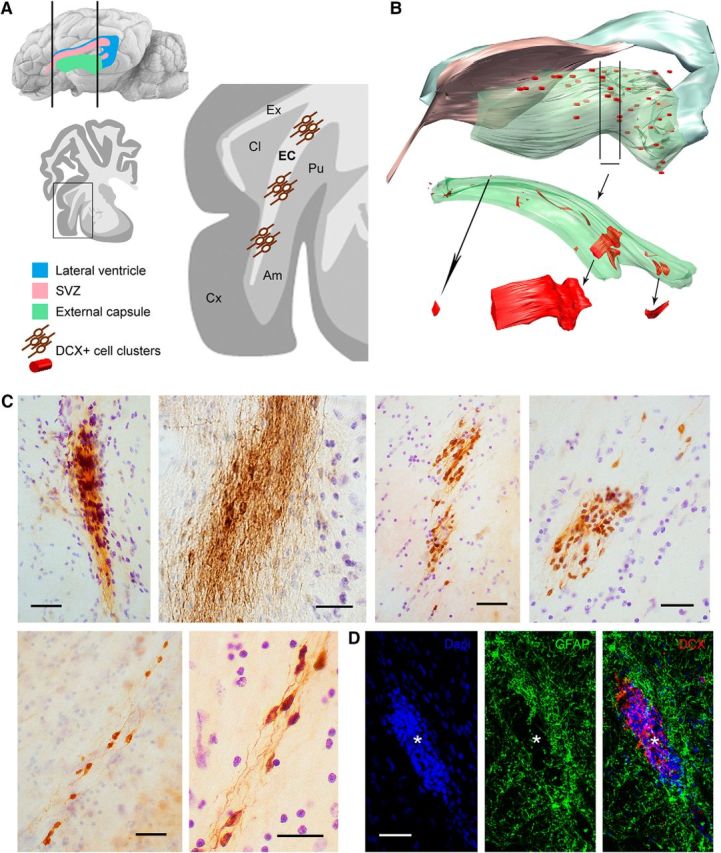
DCX+ cells in the external capsule of the adult sheep brain. A, C, Numerous clusters of tightly packed, DCX+ cells are present in most of the external capsule (EC). B, Serial reconstruction showing their distribution and size. Ex, Capsula extrema; Am, amygdala; Pu, putamen; Cx, cerebral cortex. C, Examples of DCX+ cell clusters showing different types of organization spanning from large, tightly packed cell masses to small groups of dispersed cells. D, Cell clusters (red, asterisk) occupy empty spaces within white matter areas devoid of astrocytes (green). Scale bars, 30 μm.
Table 3.
Quantification of DCX+ clusters/cells in the capsular/pericapsular and cortical regions of neonatal and adult sheep brains
| Newborn |
Adult |
|||||
|---|---|---|---|---|---|---|
| Area (mm2) | No. of cells | Density (DCX+ objects/mm2) | Area (mm2) | No. of cells | Density (DCX+ objects/mm2) | |
| External capsule | 1.72 ± 0.3 | 34 ± 4a | 19.97 ± 1.4 | 4.28 ± 0.3 | 39 ± 7a | 9.21 ± 1.6 |
| Claustrum | 7.52 ± 0.7 | 75 ± 22 | 9.78 ± 2.1 | 12.56 ± 1.2 | 77 ± 62 | 5.86 ± 4.3 |
| Amygdala | 11.44 ± 1.0 | 209 ± 75 | 18.44 ± 6.9 | 18.33 ± 3.5 | 252 ± 18 | 14.02 ± 2.1 |
| Length (mm) | No. of cells | Linear density (cells/mm) | Length (mm) | No. of cells | Linear density (cells/mm) | |
|---|---|---|---|---|---|---|
| Neocortex | 7.20 ± 0.4 | 329 ± 4 | 45.69 ± 2.6 | 9.47 ± 2.4 | 62 ± 15 | 6.54 ± 0.5 |
| Paleocortex | 4.46 ± 0.3 | 371 ± 11 | 83.41 ± 2.3 | 5.53 ± 0.8 | 164 ± 68 | 29.65 ± 7.2 |
aDCX+ objects.
Table 4.
Two-way ANOVA for all brain regions and t test for each region comparing newborn and adult values of perimeter/area and number and density of DCX+ cells
| Newborns vs adults |
|||
|---|---|---|---|
| Area (mm2) | No. of cells | Density | |
| Two-way ANOVA | F = 5.389; p = 0.003* | F = 136.551; p < 0.0001* | F = 84.258; p < 0.0001* |
| External capsule | <0.0001* | 0.300** | 0.001* |
| Claustrum | 0.003* | 0.961** | 0.231** |
| Amygdala | 0.030* | 0.422** | 0.347** |
| No. of cells | Length (mm) | Linear density (cells/mm) | |
|---|---|---|---|
| Neocortex | <0.0001* | <0.0001* | <0.0001* |
| Paleocortex | 0.164** | 0.001* | <0.0001* |
Numbers indicate p values.
*p < 0.05;
**p > 0.05.
Table 5.
Pairwise two-way ANOVA analyses of the density and number of DCX+ cells/objects in different brain regions between newborn and adult animals
| Amygdala | Claustrum | External capsulea | Paleocortex | Neocortex | |
|---|---|---|---|---|---|
| No. of cells/objects | |||||
| Amygdala | 0.535** | 0.463** | <0.0001* | <0.0001* | |
| Claustrum | 0.535** | 0.933** | <0.0001* | <0.0001* | |
| External capsulea | 0.463** | 0.933** | <0.0001* | <0.0001* | |
| Paleocortex | <0.0001* | <0.0001* | <0.0001* | <0.0001* | |
| Neocortex | <0.0001* | <0.0001* | <0.0001* | <0.0001* | |
| Density | |||||
| Amygdala | 0.923** | 0.181** | <0.0001* | <0.0001* | |
| Claustrum | 0.923** | 0.055*** | <0.0001* | <0.0001* | |
| External capsulea | 0.181** | 0.055*** | <0.0001* | <0.0001* | |
| Paleocortex | <0.0001* | <0.0001* | <0.0001* | <0.0001* | |
| Neocortex | <0.0001* | <0.0001* | <0.0001* | <0.0001* |
This analysis compares age-related changes between pairs of brain regions indicated in the first row and column. Numbers indicate p-values for the interaction between age and brain region.
*Significant interaction;
**nonsignificant interaction;
***close to critical value of p = 0.05.
aDCX+ objects.
Amygdala and claustrum
Compact cell clusters were rare in the pericapsular gray matter, whereas populations of scattered DCX+ cells were prevalent (Fig. 5). These cell groups were larger in the amygdala (diameter ranging between 125 and 385 μm) than in claustrum (40–200 μm). Within both regions, large multipolar DCX+ neurons endowed with long, dendritic-like processes were mixed with smaller, bipolar cells (Fig. 5B,C). Bipolar cells represented by far the most frequent cell morphology (∼98–99%; Fig. 5D). Three main cell morphologies were present in the amygdala (Fig. 5B): type 1 cells (small, bipolar, soma diameter 3–6 μm), type 2 cells (medium-sized, similar to type 2a cells in cortex, soma diameter 8–9 μm), and type 3 cells (large, multipolar, soma diameter 9–11 μm). Two main cell types were detectable in the claustrum (Fig. 5C): type 1 cells (small, unipolar or bipolar, soma diameter 3–6 μm) and type 2 cells (soma diameter 7–9 μm).
Figure 5.
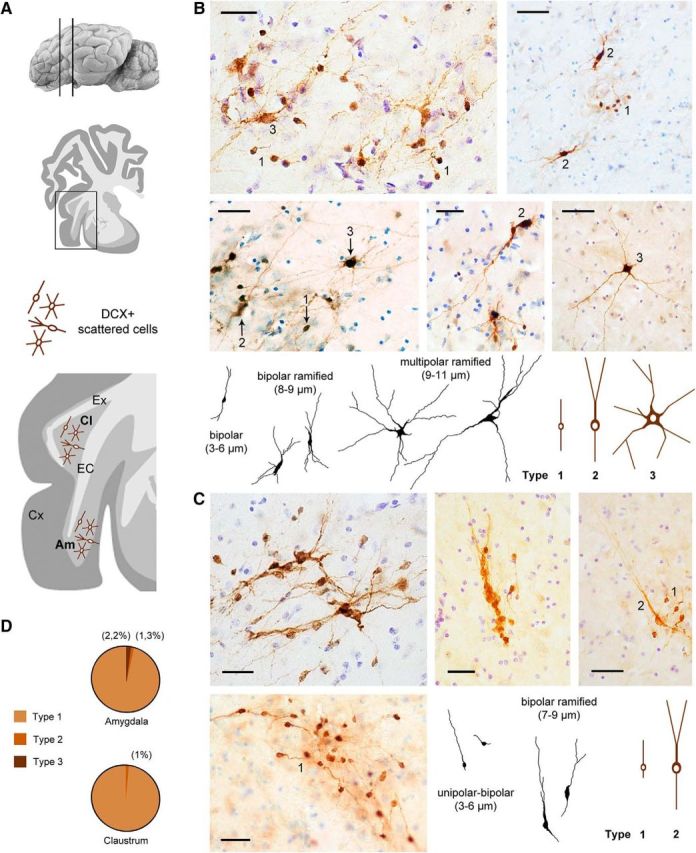
DCX+ cells in the pericapsular regions of the adult sheep brain. EC, External capsule; Ex, capsula extrema; Cx, cerebral cortex. A, Groups of scattered, DCX+ cells are present within the amygdala (Am; images in B) and claustrum (Cl, images in C). The morphology of the DCX+ spans from small bipolar to large multipolar in the amygdala (B, bottom; in black, real drawing of some cells; in brown, main cell types); it appears simpler in the claustrum, in which most cells are small unipolar/bipolar and some show simple ramifications (C, bottom right). D, Quantification of the relative amount of different cell types. Scale bars, 30 μm.
DCX+ cell populations at different ages
The same types of analyses performed on the DCX+ cells in the cortex and capsular and pericapsular regions were performed on early postnatal brains (7 d after birth). Under a qualitative profile, by comparing the occurrence and distribution of DCX+ cell populations at different ages, the only substantial difference concerned the presence of cell clusters within the putamen of the newborn and within the corpus callosum of neonatal and prepubertal animals (data not shown), both cell populations then disappearing in adults (La Rosa C., Bonfanti L., unpublished data). All other DCX+ cell populations, including cortical immature neurons, neuroblasts of the neurogenic sites, and DCX+ cells in capsular/pericapsular regions were present at both ages. However, quantitative evaluations performed in neonates and adults revealed differences among these regions (Fig. 6). Compared with newborns, the cerebral cortex of adult animals showed an evident and generalized decrease in the amount of DCX+ immature neurons (Fig. 6A, top). To quantify this reduction, we calculated the linear density of layer II DCX+ cells in three cortical segments, including both neocortex and paleocortex of neonatal and adult sheep (see Materials and Methods and Fig. 6A, bottom). In all analyzed regions, both the number and density of DCX+ cells underwent an approximately fourfold reduction with age (see Tables 3, 4, and 5 for statistical comparisons). Such a trend, suggesting an age-related reduction in cortical plasticity of the layer II (as shown previously in several species; Abrous et al., 1997; Varea et al., 2009), parallels the remarkable drop described for the DCX+ cell populations in the neurogenic sites of different mammals (Sanai et al., 2011; Lipp and Bonfanti, 2016), which is also strikingly evident in both SVZ and hippocampus of sheep (Fig. 6B).
Interestingly enough, in contrast to their neocortical counterparts, both the number and the density of DCX+ cell populations in the capsular and pericapsular regions were stable at both ages (for statistics, see Fig. 6C and Tables 3, 4, and 5). The slight reduction in the density of DCX+ cell clusters detectable in the external capsule is related to the relative increase in the area of this region with respect to others (increasing volume of the capsule itself; see Tables 3, 4, and 5). Two-way ANOVA analyses confirmed the presence of a significant interaction between brain region and age for both number (F = 136,551; p < 0.0001) and density of (F = 84,258; p < 0.0001) DCX+ cells/objects. Pairwise comparisons clearly showed that subcortical regions had a similar age related trend that differed from that of both paleo and neocortex (Tables 3, 4, and 5). As to the topographical localization of the DCX+ objects within each brain region, these structures were located more posteriorly in the adult external capsule, whereas in the newborn, they were distributed homogeneously along the entire anteroposterior axis. In claustrum and amygdala, the distribution was generally homogeneous (mainly located in the basolateral nucleus in the latter).
Cell proliferation analysis
The heavy occurrence of DCX+ cells in the cerebral cortex, external capsule, and surrounding regions of the sheep brain raises the question of whether they are newly generated. Analysis with Ki-67 antigen and BrdU in adults consistently revealed immunopositive nuclei in both SVZ and hippocampal neurogenic sites, here used as internal controls for the detection of cell proliferation markers and neurogenesis (Fig. 2). In contrast, no Ki-67/DCX colocalization was detectable in any of the cortical or capsular/pericapsular regions analyzed (Fig. 7A) and no BrdU staining was found in association with parenchymal DCX+ cells in any of the adult animals injected with the exogenous cell proliferation marker and subsequently analyzed at 30, 60, and 120 d survival (Fig. 7A). The results obtained by joining local cell proliferation marker and BrdU pulse-labeling analyses strongly indicate that the external capsule/pericapsular DCX+ cells do not divide at any of the adult ages considered, thus excluding the occurrence of parenchymal neurogenesis.
Figure 7.
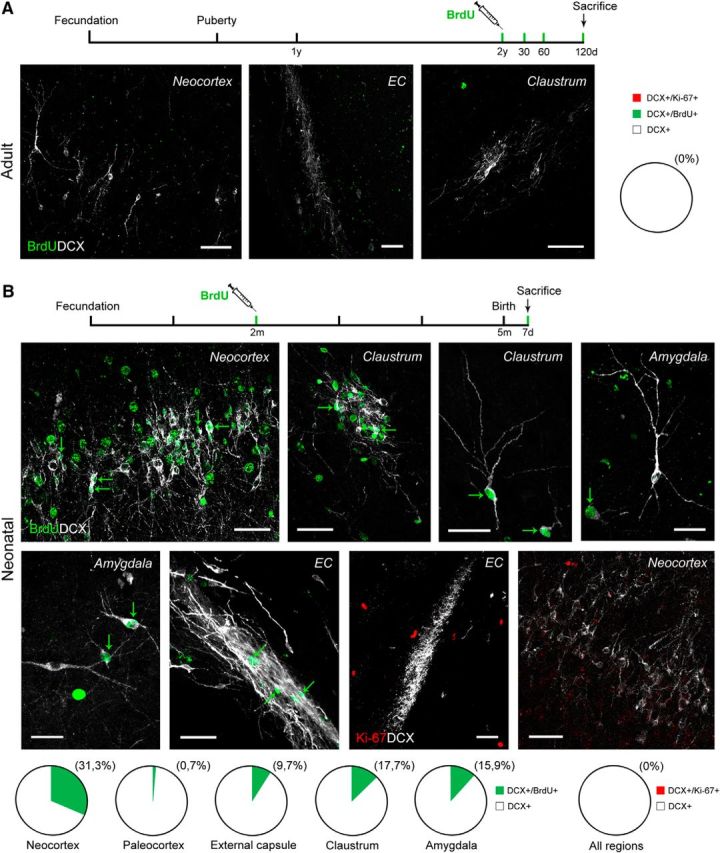
DCX+ cells in cortical layer II, external capsule, and pericapsular regions of the adult sheep are non-newly generated. A, Double staining with cell proliferation markers and DCX in the brain parenchyma of the adult sheep: no double-stained cells were found in any of the regions investigated (same results with Ki-67 antigen; images not shown). B, BrdU and Ki-67 antigen double staining with DCX in different brain regions of the neonatal brain (after BrdU treatment of the ewes at the second month of pregnancy). Quantification of DCX+/BrdU+ cells in neonatal lambs are represented in pie charts: populations of embryonically generated cells are detectable both in cortex and in capsular/pericapsular regions. Scale bars, 30 μm.
To confirm that these cell populations were generated earlier, during embryogenesis, DCX/BrdU double staining was performed in lambs born from mothers injected with BrdU 3 months before parturition (Fig. 7B). Numerous BrdU+ nuclei were present consistently in all areas analyzed. In the external capsule of neonates, some of them were detectable in DCX+ cells of the tightly packed clusters as well as in isolated cells (Fig. 7B). Some BrdU+ cells were DCX−, likely corresponding to postmitotic (mature) neurons that had been generated during embryogenesis and had already lost their DCX staining (see below). DCX+ cells not immunoreactive for BrdU were also present, indicating immature cells generated at previous or later developmental stages. After analysis with Ki-67 antigen in neonates, some scattered immunopositive nuclei were detectable at different locations of the brain parenchyma, yet never after double staining with DCX+ cells (Fig. 7B), thus indicating that these latter are no more proliferating after birth. A similar pattern was observed in the cortex, where no Ki-67+/DCX+ or BrdU/DCX+ cells were detectable in the adult. Whereas BrdU+/DCX+ cells were detected systematically in cortical layer II of neonates, these cells were far more abundant in the neocortex with respect to the paleocortex (see pie charts in Fig. 7B). Similarly to what was observed in capsular/pericapsular regions, some Ki-67+ nuclei were present in the cortex of neonates but never after double staining with DCX.
Cell maturity/immaturity and pallial/subpallial origin markers
To get some insight concerning the degree of maturation of the DCX+ cell populations described here, we used markers commonly used to assess their neuronal maturational stage. We analyzed NeuN, an RNA-binding protein expressed by most postmitotic neurons that start differentiation (Mullen et al., 1992; Fig. 8A). Only a small percentage of the DCX+ cells (∼10%) coexpressed NeuN in different regions (11.7% in cortical layer II; 9.1% in claustrum; 8.3% in amygdala; Fig. 8B). In the cortex, all the DCX+/NeuN+ neurons fell in the type 2 cell morphology with ramified dendrites. In the external capsule, NeuN was not detectable within the tightly packed cell clusters, whereas some isolated cells detached from the clusters were double stained (Fig. 8A). This gives support to the hypothesis that larger DCX+ cells in layer II are slightly more mature than small cells, showing increases in NeuN expression as described during neuronal differentiation in the adult dentate gyrus (Kempermann et al., 2004; Marqués-Marí et al., 2007). Similarly, another marker of mature neuronal cells (HuC/D RNA-binding protein; Barami et al., 1995) was detected only in some of the cortical DCX+ neurons (again, mostly large, type 2 cells; Fig. 8C). In parallel, subpopulations of DCX+ cells in all regions investigated were immunoreactive for PSA-NCAM, a marker of immaturity expressed by cells retaining plasticity (Bonfanti, 2006). Unlike newly generated neuroblasts of the classic neurogenic sites (SVZ and dentate gyrus), which are mostly PSA-NCAM immunoreactive in cortex, amygdala, claustrum, and external capsule, the staining was detectable only in some DCX+ cells, being restricted to parts of their membrane (Fig. 8D). A similar pattern was observed with the A3 subunit of the cyclic nucleotide-gated ion channel (CNGA3; Fig. 8E), which was shown previously in immature cortical neurons (Varea et al., 2011) and is considered to be involved in brain plasticity (Michalakis et al., 2011). The results obtained with the above-mentioned markers were substantially similar in all regions and ages considered. Many DCX+ cells (of both types) also expressed markers of immaturity, whereas only small subpopulations (NeuN, mainly type 2 cells) or a few of them (HUC/D) expressed markers of differentiation/maturity (summarized in Fig. 8F).
Figure 8.
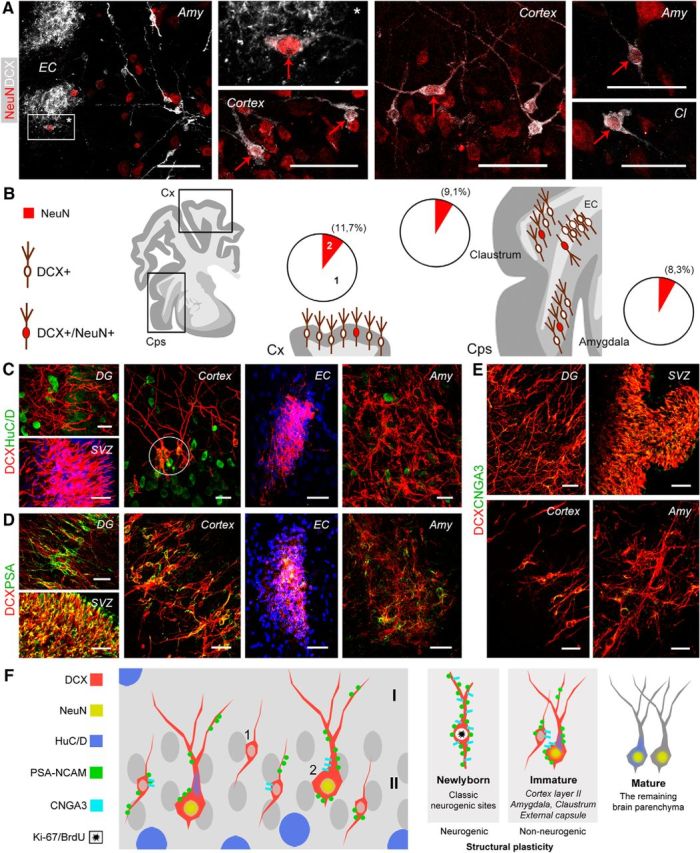
Markers of neuronal maturity/immaturity in DCX+ cells of the adult (A, B, D) and young (C, E) sheep brain. A, Double staining for DCX and NeuN (red arrows) in different brain regions. EC, External capsule; Amy, amygdala; Cl, claustrum. B, Both in cortical (Cx) and in capsular/pericapsular regions (Cps), the DCX+/NeuN+ cells represent a small subpopulation of all DCX+ cells (red areas in pie charts); 1 and 2 in cortex pie chart refer to type 1/type 2 cells (Fig. 2). C, Marker of initial differentiation and maturity HuC/D is not detectable in the neurogenic sites (DG, dentate gyrus; SVZ, subventricular zone) and mostly absent in DCX+ immature neurons apart from a weak expression in some type 2 cells of the cortical layer II (circle). D, Double staining with the marker of immaturity PSA-NCAM reveals all DCX+ cells largely decorated in the neurogenic zones (DG and SVZ), whereas only subpopulations of DCX+ cells are partially stained in the cortical and subcortical regions. E, Similarly to PSA-NCAM, the A3 subunit of the cyclic nucleotide-gated ion channel (CNGA3) is detectable in most DCX+ cells of the neurogenic sites and in subpopulations of DCX+ cells in other brain regions. F, Schematic summary of maturity/immaturity features in DCX+ cells of the sheep as revealed by different cellular markers (showed for the cortex but representative of all regions investigated). Scale bars, 30 μm.
After the prenatal origin of the parenchymal DCX+ cells was assessed, the embryologic divisions (neural progenitor domains) of their origin were investigated by using two markers of pallial (T-box transcription factor, Tbr1) and subpallial (zinc-finger protein, Sp8; experiments performed on neonates) origin. The presence of these two proteins was analyzed in the various areas investigated. As reported previously in other mammals, Tbr1 was present in pallial derivatives such as the hippocampus, claustrum, amygdala, and piriform cortex, being frequently associated with the DCX+ neurons (Fig. 9). In the neocortex, Tbr1 was strongly expressed in deeper layers with respect to upper layers (where it was mainly found in type 1 cells of the layer II; Fig. 9A). Interestingly, in cortical upper layers, this transcription factor is downregulated during neuronal maturation, at least in mice (Toma et al., 2014). Only rare cells were positive for Sp8 in these two regions (2.2% in cortex and 1.4% in claustrum). In contrast, the situation was more heterogeneous in the external capsule and amygdala: two intermixed but distinct cell populations were immunopositive for each one of the two markers, with a prevalence of Tbr1+ cells (Fig. 9B). These results strongly support the view that the DCX+ immature cells in subcortical regions are generated from both subpallial and pallial regions (∼25% and 75%, respectively) of the embryonic SVZ.
Figure 9.
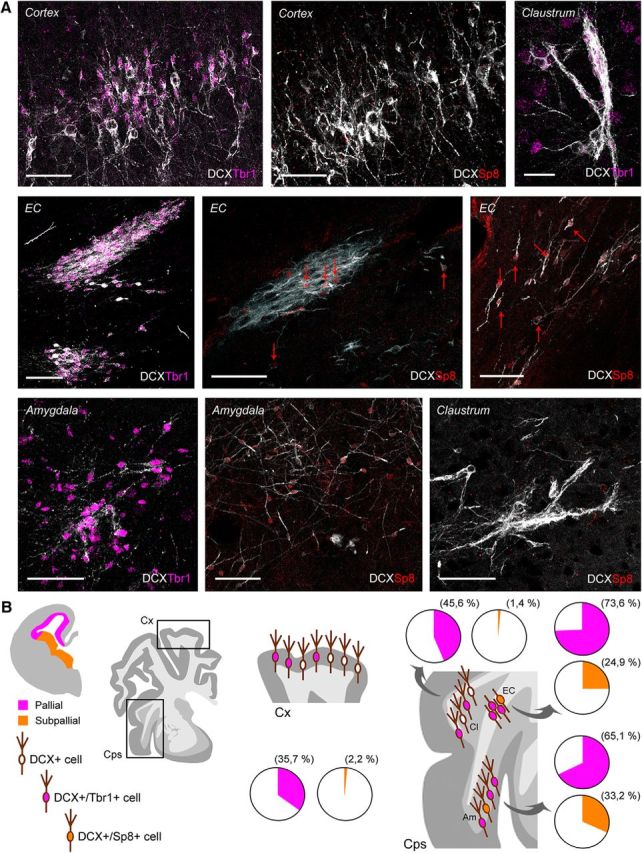
Origin of DCX+ cells by detection of pallial/subpallial markers in the neonatal sheep brain. A, Distribution of Tbr1 and Sp8 proteins in different DCX+ cell populations of the cerebral cortex, claustrum, external capsule, and amygdala; DCX, white; Tbr1, purple; Sp8, red. Scale bars, 30 μm. B, Schematic summary of pallial (purple; SVZ counterpart of dorsal, ventral, lateral, and medial pallium) and subpallial (yellow; lateral and medial ganglionic eminences) origin of the DCX+ cells in cortical (Cx) and capsular/pericapsular structures (Cps). Quantification results are reported in pie charts; most of the DCX+ cells in cortex and claustrum are only Tbr1+, whereas a mixture of Tbr1+ and Sp8+ cell populations is detectable in the external capsule and amygdaloid nuclei.
Discussion
The cytoskeletal protein DCX is associated with neuronal maturation and cell shape global remodeling and thus is involved in structural plasticity (Nacher et al., 2001; Brown et al., 2003). For decades, much attention was drawn to adult neurogenesis as a striking process of plasticity involving the production of new neurons that affect learning and memory, also opening possibilities for brain repair (Martino et al., 2011; Peretto and Bonfanti, 2014; Berninger and Jessberger, 2016). In mammals, the functional integration of newborn neurons is highly restricted to olfactory bulb and hippocampus (Bonfanti-and Peretto, 2011), their stem cell niches being less active in humans than in rodents (Sanai et al., 2011; Lipp and Bonfanti, 2016). An emerging form of plasticity consists of cells retaining features of immaturity through adulthood, including the persistent expression of DCX, although they are not generated de novo postnatally (“immature neurons”; Gomez-Climent et al., 2008). Originally, these cells were described in the paleocortex of rodents (Seki and Arai, 1991; Bonfanti-et al., 1992). Their distribution and role remain largely object of investigation, with systematic studies being scarce (Bonfanti-and Nacher, 2012; König et al., 2016). In some mammals, similar cells are also present in neocortex (Xiong et al., 2008; Cai et al., 2009; Luzzati et al., 2009; Zhang et al., 2009), leading to speculate that non-neurogenic structural plasticity might be prominent in non-rodent species (Bonfanti, 2016 and present work).
We screened the occurrence, location, distribution, and developmental origin of DCX+ cells as an indicator of non-neurogenic plasticity in sheep: long-living mammals endowed with relatively large-sized, gyrencephalic brains. Using markers of cell division (Ki-67) and pulse labeling of BrdU, we revealed the presence of abundant DCX+ cell populations born prenatally and not generated after birth. These cells were not restricted to cerebral cortex; they also occurred in white and gray matter of the following pallial subcortical regions: external capsule, claustrum, and amygdala. In contrast to the substantial decrease in number of DCX+ cortical neurons at increasing ages (Abrous et al., 1997; Xiong et al., 2008; Cai et al., 2009; Varea et al., 2009), here confirmed in sheep, the subcortical DCX+ cells appear steadily maintained through time, at least in young adults (Figs. 6, 10). Groups of DCX+ cells were previously found close to the external capsule in rabbits (Luzzati et al., 2003) and in the amygdala of nonhuman primates (Zhang et al., 2009; deCampo et al., 2012). A small portion of them were considered as newly generated in the amygdala of rabbits (Luzzati et al., 2003), mice (Jhaveri et al., 2017), and primates (Bernier et al., 2002). Our experiments in the sheep excluded the occurrence of adult newly born neurons in any of the parenchymal regions containing DCX+ cells, thus revealing species-specific heterogeneity in their regional distribution.
Figure 10.
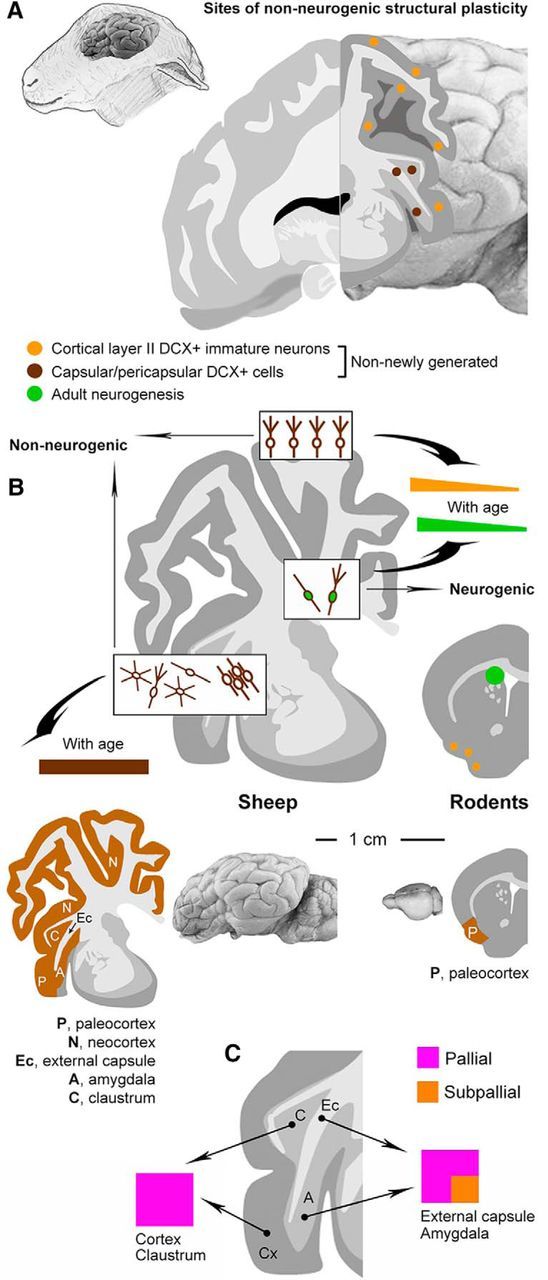
Summary and comparative aspects. A, Two main populations of non-newly generated, “immature” DCX+ cells are present in the cerebral cortex and capsular/pericapsular regions of the sheep brain. B, Unlike newly generated neuroblasts of the main neurogenic sites and immature neurons of the cortical layer II, which are consistently reduced with age (Fig. 5), the number of DCX+ cells in the sheep capsular/pericapsular regions does not vary from neonatal to adult age. Compared with results reported for laboratory rodents, our findings in sheep strongly suggests that parenchymal, non-neurogenic structural plasticity (brown areas) can be maintained/increased in large-brained, long -living mammals, thus following an opposite trend with respect to adult neurogenesis (green). C, Pallial and mixed (pallial and subpallial) origin of the DCX+ cells in different brain regions.
As to the maturational stage of the cells, many of them expressed the markers of immaturity/plasticity PSA-NCAM and CNGA3 in addition to DCX. Also, Tbr1 expression in DCX+ cortical cells (mainly of type 1) in layer II (wherein the transcription factor is usually downregulated with maturation; see Toma et al., 2014) further supports their immature state. Conversely, the vast majority (∼90%) did not express NeuN, a soluble nuclear protein with immunoreactivity that becomes obvious as neurons are initiating cellular differentiation (Mullen et al., 1992), and only a few of them did express HuC/D. The small percentage of DCX+ cells that express markers of differentiation/maturity (mainly those with complex morphology) are likely in a state ready for further differentiation. In cortex and amygdala, their morphology is reminiscent of the principal cell type (Washburn and Moises, 1992). Therefore, most of the DCX+ cells appear to be in an intermediate state of immaturity (Fig. 8F). Theoretically, they may either be adult “immature neurons” in standby mode or adult neurons undergoing structural plasticity (i.e., a “dematuration and rematuration” process akin to dedifferentiation and redifferentiation). Due to obvious difficulties in performing functional experimental tests in sheep, these latter options remain hypothetical. However, these results, including the fact that immature cell populations in subcortical regions appear more stable over time with respect to their cortical counterpart, open new possibilities for the existence of unusual/unknown forms of plasticity in multiple brain regions of nonrodent mammals. The fact that “immature” cell populations in the sheep brain are not regionally restricted like they are in rodents confirms that non-neurogenic structural plasticity might be higher in nonrodent species (Bonfanti-and Nacher, 2012; Fig. 10). Because adult neurogenesis is well preserved in rodents and highly reduced in species evolutionarily and structurally closer to humans (Sanai et al., 2011; Parolisi et al., 2015, 2017; Paredes et al., 2016; Lipp and Bonfanti, 2016), we suggest that non-neurogenic plasticity might have been preserved better in long-living, large-brained mammals (though only studies through mammalian orders might identify putative phylogenetic trends).
Markers of pallial/subpallial origin were used to gain insight into the embryologic origin and, to a lesser extent, the possible fate of the DCX+ cells described here. According to the tetrapartite model of pallial subdivision in vertebrate brain, four main territories of progenitor domains can be recognized in pallial germinative regions: medial, dorsal, lateral, and ventral pallium (Holmgren, 1925; Puelles et al., 2000). Tbr1 is a neuron-specific, postmitotic transcription factor mostly found in pallium-derived neurons committed to differentiate into excitatory glutamatergic neurons (Puelles et al., 2000; Bedogni et al., 2010; McKenna et al., 2011). Most of the DCX+ cells described here in cortical and subcortical regions expressed Tbr1, so they belong to the glutamatergic principal cortical cells of pallial origin (Hevner et al., 2001). Whereas, in rodents, the DCX+ immature neurons are confined in the ventral pallial derivative (paleocortex), we show that immature neurons in sheep extend into other ventral (amygdala), dorsal (neocortex), and lateral (clastrum) pallial derivatives. In the external capsule and amygdala, part of the DCX+ cells expressed Sp8, a transcription factor marking specific populations of olfactory bulb interneurons that is strongly expressed in the dorsal lateral ganglionic eminence (a subpallium domain; Waclaw et al., 2006) and in one-fifth of adult cortical interneurons (Ma et al., 2012). We show that the vast majority of DCX+ cortical and claustrum neurons in sheep are of pallial origin, whereas capsular and amygdalar DCX+ cells are of mixed origin (pallial and nonpallial sources). Therefore, it is very likely that capsular and pericapsular immature neurons derive from different populations during embryogenesis, although genetic lineage tracing would be required to confirm this. Because the anlage of the external capsule is a migration route for Tbr1+ and Sp8+ cells directed to claustrum and amygdala (Waclaw et al., 2006; Puelles et al., 2017), the DCX+ cells in subcortical regions might represent a remnant of immature cells remaining in the white matter during postnatal brain growth.
As to the possible function of the DCX+ “immature” neurons, no substantial insights have been obtained until now, even in rodents. In the rat paleocortex, they are considered as a reservoir of undifferentiated elements somehow kept in a “stand-by” mode (Gomez-Climent et al., 2008). The current hypothesis (utterly theoretical) is that they might lose immaturity at a certain point of life, possibly integrating in the neural circuits by accomplishing their differentiation (Bonfanti-and Nacher, 2012). In brain regions hosting adult neurogenesis (olfactory bulb, hippocampus), young neurons are endowed with special plastic properties enabling them to affect neural functions substantially and independently from their long-term integration (Stone et al., 2011; Ishikawa et al., 2014). Therefore, an intriguing possibility is that, in some mammals, other pallial regions may foster related types of plasticity by extending the immature phase of specific neuronal subpopulations without the need for increasing their number. In the cortex, immature Tbr1/DCX+ neurons gradually fade off during postnatal life (Abrous et al., 1997; Xiong et al., 2008; Cai et al., 2009; this study), suggesting that they could be related to cortical maturation. The reason that the number of DCX+ cells in claustrum and amygdala remain strikingly constant during postnatal stages is puzzling. These cells may represent a specific subpopulation that express DCX constitutively throughout life and support a form of plasticity that is independent from the general trend of maturation-related structural plasticity. All brain structures hosting the DCX+ immature neurons mediate high cognitive functions, including learning, memory, and emotional activities. The amygdala has an essential role in the formation of emotion-related memories (LaBar and Phelps, 1998), whereas the claustrum is considered important in consciousness (Crick and Koch, 2005). Finally, an important subject for future research will be to determine the evolutionary relationships of the DCX+ cells. Tbr1+/DCX+ cells are present in pallial derivatives of reptiles (including the dorsal ventricular ridge, a ventral pallial derivative homologous of the amygdala; Puelles et al., 2017), leading to the proposal that they could represent a conserved pallial cell type (Luzzati, 2015). Collectively, these observations support the possibility that a population of slowly maturing DCX+ cells might be shared by multiple pallial domains being conserved during evolution despite the profound functional/anatomical changes. Independently from any specific function, “immature” neurons raise interest in the general context of mammalian structural plasticity, representing an endogenous reserve of potentially plastic cells.
Footnotes
This work was supported by MIUR-PRIN2015 (Grant 2015Y5W9YP), the University of Turin (doctoral program in veterinary sciences), Fondazione CRT (Bando Ricerca e Istruzione 2014), and the French Agence Nationale pour la Recherche (PLASTMATBEHAV ANR12-BSV7-0017). We thank the Istituto Zooprofilattico del Piemonte, Liguria e Valle D'Aosta, and Dr. Alessandra Pecora for technical help in the first phases of the study.
References
- Abrous DN, Montaron MF, Petry KG, Rougon G, Darnaudéry M, Le Moal M, Mayo W (1997) Decrease in highly polysialylated neuronal cell adhesion molecules and in spatial learning during ageing are not correlated. Brain Res 744:285–292. 10.1016/S0006-8993(96)01115-8 [DOI] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH (2014) Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev 94:991–1026. 10.1152/physrev.00004.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey CH, Kandel ER, Harris KM (2015) Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol 7:a021758. 10.1101/cshperspect.a021758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA (1995) Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol 28:82–101. 10.1002/neu.480280108 [DOI] [PubMed] [Google Scholar]
- Bedogni F, Hodge RD, Elsen GE, Nelson BR, Daza RA, Beyer RP, Bammler TK, Rubenstein JL, Hevner RF (2010) Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci U S A 107:13129–13134. 10.1073/pnas.1002285107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A (2002) Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A 99:11464–11469. 10.1073/pnas.172403999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berninger B, Jessberger S (2016) Engineering of adult neurogenesis and gliogenesis. Cold Spring Harb Perspect Biol 8: pii: a018861. 10.1101/cshperspect.a018861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beul SF, Hilgetag CC (2014) Towards a “canonical” agranular cortical microcircuit. Front Neuroanat 8:165. 10.3389/fnana.2014.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L. (2006) PSA-NCAM in mammalian structural plasticity and neurogenesis. Prog Neurobiol 80:129–164. 10.1016/j.pneurobio.2006.08.003 [DOI] [PubMed] [Google Scholar]
- Bonfanti L. (2016) Adult neurogenesis 50 years later: Limits and opportunities in mammals. Front Neurosci 10:44. 10.3389/fnins.2016.00044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Nacher J (2012) New scenarios for neuronal structural plasticity in non-neurogenic brain parenchyma: the case of cortical layer II immature neurons. Prog Neurobiol 98:1–15. 10.1016/j.pneurobio.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P (2011) Adult neurogenesis in mammals: a theme with many variations. Eur J Neurosci 34:930–950. 10.1111/j.1460-9568.2011.07832.x [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Theodosis DT (2009) Polysialic acid and activity-dependent synapse remodeling. Cell Adh Migr 3:43–50. 10.4161/cam.3.1.7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfanti L, Olive S, Poulain DA, Theodosis DT (1992) Mapping of the distribution of polysialylated neural cell adhesion molecule throughout the central nervous system of the adult rat: an immunohistochemical study. Neuroscience 49:419–436. 10.1016/0306-4522(92)90107-D [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG (2003) Transient expression of doublecortin during adult neurogenesis. J Comp Neurol 467:1–10. 10.1002/cne.10874 [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Franceschini I, Keller M, Lévy F (2010) Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Horm Behav 58:737–746. 10.1016/j.yhbeh.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Brus M, Keller M, Lévy F (2013a) Temporal features of adult neurogenesis: differences and similarities across mammalian species. Front Neurosci 7:135–144. 10.3389/fnins.2013.00135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Gheusi G, Keller M, Lledo PM, Lévy F (2013b) Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J Comp Neurol 521:169–188. 10.1002/cne.23169 [DOI] [PubMed] [Google Scholar]
- Cai Y, Xiong K, Chu Y, Luo DW, Luo XG, Yuan XY, Struble RG, Clough RW, Spencer DD, Williamson A, Kordower JH, Patrylo PR, Yan XX (2009) Doublecortin expression in adult cat and primate cerebral cortex relates to immature neurons that develop into GABAergic subgroups. Exp Neurol 216:342–356. 10.1016/j.expneurol.2008.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FC, Koch C (2005) What is the function of the claustrum? Philos Trans R Soc Lond B Biol Sci 360:1271–1279. 10.1098/rstb.2005.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- deCampo DM, Fudge JL (2012) Where and what is the paralaminar nucleus? A review on a unique and frequently overlooked area of the primate amygdala. Neurosci Biobehav Rev 36:520–535. 10.1016/j.neubiorev.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano DM, Bordey A, Bonfanti L (2015) Noncanonical Sites of Adult Neurogenesis in the Mammalian Brain. Cold Spring Harb Perspect Biol 7:a018846. 10.1101/cshperspect.a018846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frotscher M. (1992) Specificity of interneuronal connections. Ann Anat 174:377–382. 10.1016/S0940-9602(11)80254-2 [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA (1999) Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23:257–271. 10.1016/S0896-6273(00)80778-3 [DOI] [PubMed] [Google Scholar]
- Gómez-Climent MA, Castillo-Gómez E, Varea E, Guirado R, Blasco-Ibáñez JM, Crespo C, Martínez-Guijarro FJ, Nácher J (2008) A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb Cortex 18:2229–2240. 10.1093/cercor/bhm255 [DOI] [PubMed] [Google Scholar]
- Grandel H, Brand M (2013) Comparative aspects of adult neural stem cell activity in vertebrates. Dev Genes Evol 223:131–147. 10.1007/s00427-012-0425-5 [DOI] [PubMed] [Google Scholar]
- Hevner RF, Shi L, Justice N, Hsueh Y, Sheng M, Smiga S, Bulfone A, Goffinet AM, Campagnoni AT, Rubenstein JL (2001) Tbr1 regulates differentiation of the preplate and layer 6. Neuron 29:353–366. 10.1016/S0896-6273(01)00211-2 [DOI] [PubMed] [Google Scholar]
- Holmgren N. (1925) Points of view concerning forebrain morphology in higher vertebrates. Acta Zool Stockh 6:413–477. 10.1111/j.1463-6395.1925.tb00271.x [DOI] [Google Scholar]
- Ishikawa R, Kim R, Namba T, Kohsaka S, Uchino S, Kida S (2014) Time-dependent enhancement of hippocampus-dependent memory after treatment with memantine: Implications for enhanced hippocampal adult neurogenesis. Hippocampus 24:784–793. 10.1002/hipo.22270 [DOI] [PubMed] [Google Scholar]
- Jhaveri DJ, Tedoldi A, Hunt S, Sullivan R, Watts NR, Power JM, Bartlett PF, Sah P (2017) Evidence for newly generated interneurons in the basolateral amygdala of adult mice. Mol Psychiatry. In press. 10.1038/mp.2017.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Jessberger S, Steiner B, Kronenberg G (2004) Milestones of neuronal development in the adult hippocampus. Trends Neurosci 27:447–452. 10.1016/j.tins.2004.05.013 [DOI] [PubMed] [Google Scholar]
- König R, Benedetti B, Rotheneichner P, O'Sullivan A, Kreutzer C, Belles M, Nacher J, Weiger TM, Aigner L, Couillard-Després S (2016) Distribution and fate of DCX/PSA-NCAM expressing cells in the adult mammalian cortex: a local reservoir for adult cortical neuroplasticity? Front Biol 11:193–213. 10.1007/s11515-016-1403-5 [DOI] [Google Scholar]
- LaBar KS, Phelps EA (1998) Role of the human amygdala in arousal mediated memory consolidation. Psychol Sci 9:490–493. 10.1111/1467-9280.00090 [DOI] [Google Scholar]
- Lipp HP, Bonfanti L (2016) Adult neurogenesis in mammals: variations and confusions. Brain Behav Evol 87:205–221. 10.1159/000446905 [DOI] [PubMed] [Google Scholar]
- Lois C, García-Verdugo JM, Alvarez-Buylla A (1996) Chain migration of neuronal precursors. Science 271:978–981. 10.1126/science.271.5251.978 [DOI] [PubMed] [Google Scholar]
- Luzzati F. (2015) A hypothesis for the evolution of the upper layers of the neocortex through co-option of the olfactory cortex developmental program. Front Neurosci 9:162. 10.3389/fnins.2015.00162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F, Peretto P, Aimar P, Ponti G, Fasolo A, Bonfanti L (2003) Glia-independent chains of neuroblasts through the subcortical parenchyma of the adult rabbit brain. Proc Natl Acad Sci U S A 100:13036–13041. 10.1073/pnas.1735482100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F, Bonfanti L, Fasolo A, Peretto P (2009) DCX and PSA-NCAM expression identifies a population of neurons preferentially distributed in associative areas of different pallial derivatives and vertebrate species. Cereb Cortex 19:1028–1041. 10.1093/cercor/bhn145 [DOI] [PubMed] [Google Scholar]
- Ma T, Zhang Q, Cai Y, You Y, Rubenstein JL, Yang Z (2012) A subpopulation of dorsal lateral/caudal ganglionic eminence-derived neocortical interneurons expresses the transcription factor Sp8. Cereb Cortex 22:2120–2130. 10.1093/cercor/bhr296 [DOI] [PubMed] [Google Scholar]
- Marqués-Marí AI, Nacher J, Crespo C, Gutièrrez-Mecinas M, Martínez-Guijarro FJ, Blasco-Ibáñez JM (2007) Loss of input from the mossy cells blocks maturation of newly generated granule cells. Hippocampus 17:510–524. 10.1002/hipo.20290 [DOI] [PubMed] [Google Scholar]
- Martino G, Pluchino S, Bonfanti L, Schwartz M (2011) Brain regeneration in physiology and pathology: the immune signature driving therapeutic plasticity of neural stem cells. Physiol Rev 91:1281–1304. 10.1152/physrev.00032.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna WL, Betancourt J, Larkin KA, Abrams B, Guo C, Rubenstein JL, Chen B (2011) Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci 31:549–564. 10.1523/JNEUROSCI.4131-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalakis S, Kleppisch T, Polta SA, Wotjak CT, Koch S, Rammes G, Matt L, Becirovic E, Biel M (2011) Altered synaptic plasticity and behavioral abnormalities in CNGA3-deficient mice. Genes Brain Behav 10:137–148. 10.1111/j.1601-183X.2010.00646.x [DOI] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM (1992) NeuN, a neuronal specific nuclear protein in vertebrates. Development 116:201–211. [DOI] [PubMed] [Google Scholar]
- Nacher J, Bonfanti L (2015) New neurons from old beliefs in the adult piriform cortex? A commentary on: “Occurrence of new neurons in the piriform cortex”. Front Neuroanat 9:62. 10.3389/fnana.2015.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS (2001) Doublecortin expression in the adult rat telencephalon. Eur J Neurosci 14:629–644. 10.1046/j.0953-816x.2001.01683.x [DOI] [PubMed] [Google Scholar]
- Paredes MF, Sorrells SF, Garcia-Verdugo JM, Alvarez-Buylla A (2016) Brain size and limits to adult neurogenesis. J Comp Neurol 524:646–664. 10.1002/cne.23896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolisi R, Peruffo A, Messina S, Panin M, Montelli S, Giurisato M, Cozzi B, Bonfanti L (2015) Forebrain neuroanatomy of the neonatal and juvenile dolphin (T. truncatus and S. coeruloalba). Front Neuroanat 9:140. 10.3389/fnana.2015.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolisi R, Cozzi B, Bonfanti L (2017) Non-neurogenic SVZ-like niche in dolphins, mammals devoid of olfaction. Brain Struct Funct 222:2625–2639. 10.1007/s00429-016-1361-3 [DOI] [PubMed] [Google Scholar]
- Peretto P, Bonfanti L (2014) Major unsolved points in adult neurogenesis: doors open on a translational future? Front Neurosci 8:154. 10.3389/fnins.2014.00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Aimar P, Bonfanti L (2006) Cellular composition and cytoarchitecture of the rabbit subventricular zone (SVZ) and its extensions in the forebrain. J Comp Neurol 498:491–507. 10.1002/cne.21043 [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubenstein JL (2000) Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol 424:409–438. [DOI] [PubMed] [Google Scholar]
- Puelles L, Sandoval JE, Ayad A, del Corral R, Alonso A, Ferran JL, Martınez-de-la-Torre M (2017) The pallium in reptiles and birds in the light of the updated tetrapartite pallium model. In: Evolution of nervous systems, Ed 2, Vol 1 (Kaas JH, editor). New York, NY: Elsevier. [Google Scholar]
- Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai HH, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo JM, Rowitch DH, Alvarez-Buylla A (2011) Corridors of migrating neurons in the human brain and their decline during infancy. Nature 478:382–386. 10.1038/nature10487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki T, Arai Y (1991) Expression of highly polysialylated NCAM in the neocortex and piriform cortex of the developing and the adult rat. Anat Embryol (Berl) 184:395–401. 10.1007/BF00957900 [DOI] [PubMed] [Google Scholar]
- Stone SS, Teixeira CM, Zaslavsky K, Wheeler AL, Martinez-Canabal A, Wang AH, Sakaguchi M, Lozano AM, Frankland PW (2011) Functional convergence of developmentally and adult-generated granule cells in dentate gyrus circuits supporting hippocampus-dependent memory. Hippocampus 21:1348–1362. 10.1002/hipo.20845 [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH (2008) Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev 88:983–1008. 10.1152/physrev.00036.2007 [DOI] [PubMed] [Google Scholar]
- Toma K, Kumamoto T, Hanashima C (2014) The timing of upper-layer neurogenesis is conferred by sequential derepression and negative feed back from deep-layer neurons. J Neurosci 34:13259–13276. 10.1523/JNEUROSCI.2334-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea E, Castillo-Gomez E, Gomez-Climent MA, Guirado R, Blasco-Ibanez JM, Crespo C, Martinez-Guijarro FJ, Nacher J (2009) Differential evolution of PSANCAM expression during aging of the rat telencephalon. Neurobiol Aging 5:808–818. 10.1016/j.neurobiolaging.2007.08.016 [DOI] [PubMed] [Google Scholar]
- Varea E, Belles M, Vidueira S, Blasco-Ibáñez JM, Crespo C, Pastor AM, Nacher J (2011) PSA-NCAM is expressed in immature, but not recently generated, neurons in the adult cat cerebral cortex layer II. Front Neurosci 5:17. 10.3389/fnins.2011.00017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waclaw RR, Allen ZJ 2nd, Bell SM, Erdélyi F, Szabó G, Potter SS, Campbell K (2006) The zinc finger transcription factor Sp8 regulates the generation and diversity of olfactory bulb interneurons. Neuron 49:503–516. 10.1016/j.neuron.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Washburn MS, Moises HC (1992) Electrophysiological and morphological properties of rat basolateral amygdaloid neurons in vitro. J Neurosci 12:4066–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong K, Luo DW, Patrylo PR, Luo XG, Struble RG, Clough RW, Yan XX (2008) Doublecortin-expressing cells are present in layer II across the adult guinea pig cerebral cortex: Partial colocalization with mature interneuron markers. Exp Neurol 211:271–282. 10.1016/j.expneurol.2008.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Cai Y, Chu Y, Chen EY, Feng JC, Luo XG, Xiong K, Struble RG, Clough RW, Patrylo PR, Kordower JH, Yan XX (2009) Doublecortin-expressing cells persist in the associative cerebral cortex and amygdala in aged nonhuman primates. Front Neuroanat 3:17. 10.3389/neuro.05.017.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]