This cohort study assesses association of the type and age of exposure to childhood maltreatment with hyperactive and hypoactive amygdala responses in young adults.
Key Points
Question
What is the association between exposure to childhood maltreatment at different ages and adult amygdala response to threatening faces?
Findings
In this cohort study of 202 young adults, self-reported exposure to maltreatment during early childhood was significantly associated with blunted amygdala response via functional magnetic resonance imaging, whereas early teen exposure was significantly associated with augmented amygdala response.
Meaning
The findings suggest that maltreatment may be a risk factor for disorders characterized by hyperactive amygdala response and disorders associated with hypoactive amygdala responses, which may be associated with differences in age at the time of exposure to specific types of adversity during childhood.
Abstract
Importance
Abnormalities in amygdala response to threatening faces have been observed in anxiety disorders, autism, bipolar disorder, depression, posttraumatic stress disorder, and schizophrenia. Abnormally hyperactive and hypoactive responses have typically been associated with anxiety and inhibition vs risk taking and inappropriate social behaviors. Maltreatment is a major risk factor for most of these disorders and is associated with abnormal amygdala function.
Objective
To identify the type and age of exposure to childhood maltreatment that are associated with hyperactive and hypoactive amygdala responses in young adulthood.
Design, Setting, and Participants
Data collection for this retrospective cohort study took place from November 8, 2010, to August 23, 2012. Data analyses were conducted from September 20, 2012, to June 27, 2018. Participants were recruited from the urban and suburban Boston vicinity without diagnostic restrictions based on exposure history.
Exposures
The Maltreatment and Abuse Chronology of Exposure (MACE) scale was used to retrospectively assess type and age of exposure to childhood maltreatment.
Main Outcomes and Measures
Activation and pattern information functional magnetic resonance imaging were used to assess bilateral amygdala response to angry and fearful faces vs neutral faces or shapes, and sensitive exposure periods were identified using cross-validated artificial intelligence predictive analytics (50 averaged randomized iterations with training on 63.3% and testing on 36.7% of the sample).
Results
Of the 202 participants (mean [SD] age, 23.2 [1.7] years; 118 [58.4%] female), 52 (25.7%) reported no exposure to maltreatment and 150 (74.3%) reported exposure to 1 or more maltreatment types. Eight participants (15.1%) with a MACE score of 0 and 51 (34.2%) with a MACE score of 1 or higher had a history of major depression (odds ratio, 2.40; 95% CI, 1.05-6.06; P = .03); 8 unexposed participants (15.1%) and 46 with MACE scores of 1 or higher (30.9%) had a history of 1 or more anxiety disorders (odds ratio, 2.45; 95% CI, 1.03-6.50; P = .03). Retrospective self-report of physical maltreatment between 3 and 6 years of age and peer emotional abuse at 13 and 15 years were associated with amygdala activation to emotional faces vs shapes. Early exposure was associated with blunted response (β = −0.17, P < .001), whereas later exposure was associated with augmented response (β = 0.16, P < .001). Prepubertal vs postpubertal maltreatment was associated with an opposite response on the voxelwise response pattern in clustering stimuli of the same type (eg, mean [SD] emotional ellipse areas for physical maltreatment at age 4 years vs nonverbal emotional abuse at 13 years: 1.41 [1.05] vs 0.25 [0.10], P < .001) and in distinguishing between stimuli of different types (eg, mean [SD] emotional vs neutral faces distance for peer emotional abuse at age 6 years vs 13 years: 1.89 [0.75] vs 0.80 [0.39], P < .001).
Conclusions and Relevance
The findings suggest that prepubertal vs postpubertal developmental differences in the association between maltreatment and amygdala response to threatening or salient stimuli exist. Understanding the role of adversity in different sensitive exposure periods and the potential adaptive significance of attenuated vs enhanced amygdala response may help explain why maltreatment may be a risk factor for many different disorders and foster creation of targeted interventions to preempt the emergence of psychopathology in at-risk youths.
Introduction
Maltreatment and household dysfunction account for 30% to 70% of the population-attributable risk for anxiety disorders, depression, addiction, and suicide attempts.1,2,3 Exposure to 5 or more forms of childhood adversity increases the risk of receiving anxiolytic prescriptions by 2-fold, antidepressant prescriptions by 3-fold, antipsychotic prescriptions by 10-fold, and mood stabilizer prescriptions by 17-fold.4 Maltreatment may increase the risk for psychopathologic findings through structural and functional brain changes.5,6,7,8 Two consistently reported abnormalities are increased amygdala activation to threat or salience9,10,11,12,13,14 and reduced functional connectivity of the amygdala with hippocampus, anterior cingulate, and prefrontal cortex.15,16 Understanding the effects of maltreatment on amygdala development is particularly important because amygdala abnormalities have been identified in studies of a wide array of psychiatric disorders, including posttraumatic stress disorder (PTSD),17 social and specific phobias,18 drug addiction,19 schizophrenia,20 unipolar and bipolar depression,21,22 autism,23 and borderline personality disorder.24
The apparent sensitivity of the amygdala to maltreatment is consistent with translational studies that revealed a high density of glucocorticoid receptors on stress-susceptible pyramidal cells25 and a postnatal developmental trajectory characterized by rapid initial growth followed by sustained growth to peek volumes between 9 and 11 years of age with gradual pruning thereafter.26 Stressors stimulate dendritic arborization and new spine formation on these pyramidal cells, leading to an enduring increase in volume.27,28 However, amygdala volume measures in individuals exposed to maltreatment are inconsistent, with several studies29,30 reporting a significant reduction but others8,31,32 reporting no difference or a significant increase. Enlarged amygdala findings were typically encountered in children or young adults exposed to early neglect, whereas reduced amygdala volume findings were most frequently observed in adults with serious psychiatric disorders who were probably exposed to abuse throughout childhood. Some of us have hypothesized that early maltreatment is associated with an increase in amygdala volume, but only in individuals with minimal exposure to subsequent stressors, and that early exposure sensitizes the amygdala so that subsequent stressors are associated with a graded decrease in volume.8 This hypothesis is consistent with the idea that there are sensitive periods when exposures to specific types of maltreatment are maximally associated with stress-susceptible brain regions.31,33,34,35
The overriding hypothesis is that stress affects the amygdala in a developmentally sensitive manner, working through ongoing processes. In general, brain regions overproduce synapses, receptors, and dendrites before puberty and then prune these processes during the transition between puberty and adulthood.36 Thus, early exposure may result in an increase in amygdala volume, whereas postpubertal exposure may result in a loss of volume.
This hypothesis, however, raises a question regarding the association between maltreatment and amygdala function. The literature, with one exception,37 indicates that maltreatment is associated with increased amygdala response to threatening faces.8 Alternatively, earlier and later childhood exposure may have an opposite association with the amygdala response. This consideration is critically important because heightened amygdala response has been associated with symptoms of anxiety and inhibition, as in PTSD17,38 and phobias,39,40 whereas blunted response may be associated with problems with disinhibition and impaired social judgment,41 such as in substance use42 and conduct disorders.43
To test this hypothesis, we assessed blood oxygenation level–dependent (BOLD) activation response to emotional (angry, fearful) faces, neutral faces, and shapes and used a novel tool called pattern-information functional magnetic resonance imaging (fMRI) to provide a detailed view of how the amygdala processes information. Traditional activation-based analysis has excelled in delineating brain regions where activity changes during a specific task. However, this approach fails to reflect neuronal population codes or to detect how the region processes and represents neuronal information in a fine-grained way.44,45 Pattern-information fMRI is an alternative analytical approach that provides a strategy for decoding activity pattern differences and inferring representational content. A previous study46 indicated in 10 heathy controls that it is possible to identify what an individual is observing based on this type of analysis in ventral temporal cortex. In this study, our aims were to assess whether there were prepubertal and postpubertal periods that are sensitive to exposure to childhood maltreatment and to test the hypothesis that exposure during these times is associated with opposite developmental differences in amygdala function.
Methods
Participants
Data collection for this retrospective cohort study took place from November 8, 2010, to August 23, 2012. Data analyses were conducted from September 20, 2012, to June 27, 2018. Participants were recruited from the urban and suburban Boston vicinity without diagnostic restrictions based on exposure history. Partners Healthcare Institutional Review Board approved this retrospective cohort study. After the study was explained, written informed consent was provided by all participants, who were evaluated following previously published methods.47,48 A transdiagnostic sample (ie, that was not specific to different diagnostic categories) (N = 515) was selected with the goal of recruiting an approximately equal number of participants with exposure to 0, 1, 2, and 3 or more types of maltreatment who met the same inclusion and exclusion criteria. The subsample selected for imaging consisted of 202 individuals who were all unmedicated except for birth control pills and occasional use of nonsedating antihistamines, nonsteroidal anti-inflammatory drugs, or an albuterol inhaler; this subsample has previously been studied.33,47,49 Exclusion criteria in this study included current or prior neurologic disease, experience of concussion or head trauma resulting in loss of consciousness for more than 5 minutes, and exposure to more than 3 unrelated forms of adversity. Participants were selected according to exposure history and not psychopathology except that substance abuse or high levels of drug or alcohol use (more than 15 days per month) were grounds for exclusion. Urine and breathalyzer tests were conducted before imaging and assessment. Each participant received $25 for the completion of online assessments, $100 per interview and assessment session, and $100 for a 1-hour MRI protocol. Additional participant recruitment is detailed in eMethods 1 in the Supplement.
Assessment Instruments
Severity of exposure to 10 types of maltreatment (each type of maltreatment has a severity score that ranges from 0 to 10) across each year of childhood was evaluated retrospectively using the Maltreatment and Abuse Chronology of Exposure (MACE) scale (eMethods 2 in the Supplement). This scale has excellent test-retest reliability49 and has been used to identify sensitive periods of maltreatment exposure associated with brain morphometry31,33 and risk of psychopathology.47,50,51 Structured Clinical Interviews for DSM-IV Axis I and II psychiatric disorders52 were conducted by mental health care professionals blind to the neuroimaging results.
Image Acquisition and Data Processing
Functional MRI scanning was performed on a TIM Trio Scanner (3T; Siemens AG, Siemens Medical Solutions) using a 32-element phased-array radiofrequency reception coil. Scan sequences and data processing strategies are presented in eMethods 3 in the Supplement.
fMRI Task
The implicit emotional face-matching paradigm53 consisted of 3 blocks of negative faces and 3 blocks of neutral faces interleaved with 7 blocks of sensorimotor control (geometric shapes). Details of the paradigm are presented in eMethods 4 in the Supplement.
Statistical Analysis
Random Forest Regression With Conditional Trees
A critical question is whether exposure to a particular type of maltreatment at a specific age is an important risk factor associated with amygdala response. Conventional analytic techniques are not suitable because there is substantial collinearity in the degree of exposure to specific types of maltreatment at adjacent ages. Instead, we identified the most important cross-validated risk factors associated with amygdala response using random forest regression with conditional inference trees (cforest in R package party54), a form of artificial intelligence analytics that has been reported to be resistant to collinearity, which we have used in prior sensitive period studies.31,33,47,50,51,55
For these analyses, the random forest was trained using data from 63.3% of the participants and evaluated on the withheld test set (36.7%). The variable importance (VI) of each regressor in the model was assessed by permuting the variable, refitting the forest, and calculating how much permutation of that variable increased the mean square error of the fit to the test set. Permuting important regressors produces a large increase in mean square error, whereas permuting unimportant regressors have negligible effect. This process was repeated 50 times to derive mean measures of VI for each variable. To gauge significance, the overall process was then repeated 2000 times using reshuffled amygdala values to calculate chance mean and SD importance levels for each variable. The significance of the z test difference between observed and chance VI measures for each variable was calculated and adjusted using Bonferroni correction to control for multiple comparisons. Details are presented in eMethods 5 in the Supplement.
Activation fMRI Approach
At the participant level, the time series of emotional faces was regressed to a canonic hemodynamic response function relative to neutral faces or shapes. We focused primarily on the emotional face to shape contrast because ambiguous neutral faces may induce responses similar to threatening faces56,57 and exposure to maltreatment may be associated with response to neutral faces,11,13 but we also report the emotional face to neutral face contrast as a more specific threat-related measure. These data were submitted to group-level analyses. The BOLD estimates were extracted from activated clusters within anatomically defined (via the Automated Anatomical Labeling Atlas) bilateral amygdala regions of interest, which exhibit significant main associations with the task at P < .05. Familywise error was corrected across the volumes of the entire brain (eTable 1 in the Supplement).
Pattern Information fMRI Approach
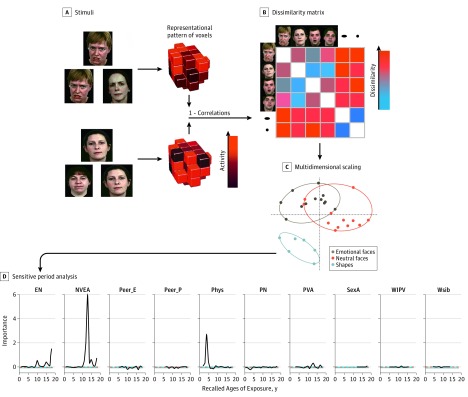
As shown in Figure 1, pattern-information fMRI and representational similarities analysis were used to assess the voxelwise pattern of response within the amygdala. Multidimensional scaling provides a means of visualizing differences in response pattern by portraying these differences in 2-dimensional euclidean space. We extracted 2 geometric features from these graphs. The ellipse area indicates how homogeneous the response patterns are between stimuli of the same class, with smaller ellipses indicating greater similarity. The d′ distance indicates how heterogeneous the response patterns are between stimuli of different classes, with a greater d′ indicative of larger differences. P < .05 was considered to be significant and was 2-tailed. P values from the random forest regressions were corrected for multiple comparisons. Further information is presented in eMethods 6 in the Supplement.
Figure 1. Representational Similarity Analysis.
A, The degree of similarity (ie, correlation) was calculated among every pair of stimuli (eg, emotional faces and shapes) in their pattern of voxel response, controlling for block effects by partialing out the degree of correlation observed among each pair of stimuli in cerebrospinal fluid and global white matter. B, A correlational matrix was derived for each research participant from the partial correlations among all pairs of stimuli and transformed into a dissimilarity matrix by subtracting the correlation coefficient from 1. C, The dissimilarity matrix was converted into its corresponding best-fitting euclidean 2-dimensional representation using multidimensional scaling with all representations fixed to have the same axis dimensions. On the basis of the 2-dimensional representation, we calculated the minimum spanning ellipses area for each stimulus category as well as the d′ value reflecting the discriminability among clusters as defined by the mean euclidean distance among ellipse centroids divided by the SD derived from the euclidean distances of each stimulus in a cluster from the category cluster centroid. The size of the minimum spanning ellipse is indicative of the degree of similarity among items within the same category. The smaller the ellipse, the more similar the pattern of voxel response among each stimulus within the category. The d′ distance indicates how different the pattern of voxelwise responses are between stimuli from different categories. D, Random forest regression with conditional inference trees was used to delineate the type and timing of exposure to maltreatment that was most closely associated with the ellipse area and d′ euclidean distance between 2 ellipses. EN indicates emotional neglect; NVEA, nonverbal emotional abuse; Peer_E, peer emotional bullying; Peer_P, peer physical bullying; Phys, parental physical abuse; PN, physical neglect; PVA, parental verbal abuse; SexA, sexual abuse; WIPV, witnessing interparental violence; and WSIB, witnessing violence toward siblings.
Results
A total of 515 individuals were recruited, and 202 young adults (mean [SD] age, 23.2 [1.7] years; 118 [58.4%] female) participated in this neuroimaging component of the study. Demographic data are presented in the Table. Overall, 52 participants (25.7%) reported no exposure to maltreatment; 47 (23.3%) reported exposure to 1 type of maltreatment, 38 (18.8%) to 2 types of maltreatment, and 65 (32.2%) to 3 or more types of maltreatment. A total of 8 participants (15.1%) with a MACE score of 0 and 51 (34.2%) with a MACE score of 1 or higher had a history of major depression (odds ratio, 2.40; 95% CI, 1.05-6.06; P = .03). Similarly, 8 unexposed participants (15.1%) and 46 participants with MACE scores of 1 or higher (30.9%) had a history of 1 or more anxiety disorders (odds ratio, 2.45; 95% CI, 1.03-6.50; P = .03).
Table. Characteristics of Research Participants.
| Characteristic | Finding (N = 202)a |
|---|---|
| Age, mean (SD), y | 23.2 (1.7) |
| Sex | |
| Male | 84 (42.6) |
| Female | 118 (58.4) |
| Pubertal age, mean (SD), y | 12.4 (1.4) |
| Participant’s educational level, mean (SD), y | 15.8 (1.8) |
| Father’s educational level, mean (SD), y | 15.6 (3.6) |
| Mother’s educational level, mean (SD), y | 15.4 (3.1) |
| Financial sufficiency during childhood | |
| Much less than enough money | 7 (3.5) |
| Less than enough money | 41 (20.3) |
| Enough money | 95 (47.0) |
| More than enough money | 54 (26.7) |
| Much more than enough money | 4 (2.0) |
| Race/ethnicity | |
| White | 140 (69.3) |
| Asian | 32 (15.8) |
| Black | 18 (8.9) |
| Hispanic | 26 (12.9) |
| Other | 12 (5.9) |
| Maltreatment, MACE score | |
| 0, No exposure | 52 (25.7) |
| 1, Exposure to 1 type | 47 (23.3) |
| 2, Exposure to 2 types | 38 (18.8) |
| ≥3, Exposure to ≥3 types | 65 (32.2) |
| ≥1, Exposure to ≥1 type | 150 (74.3) |
Abbreviation: MACE, Maltreatment and Abuse Chronology of Exposure.
Data are presented as number (percentage) of research participants unless otherwise indicated.
Sensitive Period Analysis of Maltreatment and Amygdala Activation
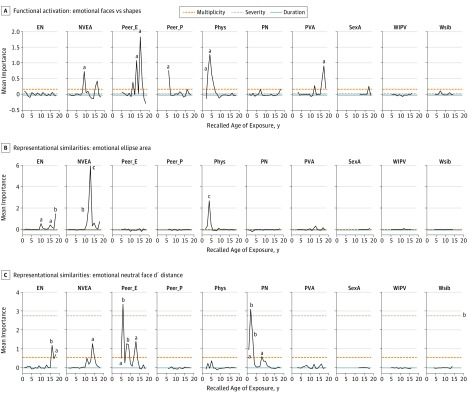
As seen in Figure 2A, the most important risk factors associated with bilateral amygdala response to emotional faces vs shapes were peer emotional abuse at 15 (VI, 1.83; P = .006) and 13 years of age (VI, 1.07; P = .03). Other important factors were parental physical abuse at 3 (VI, 0.74; P = .046) to 4 years of age (VI, 1.27; P = .02) and peer physical abuse at 6 years of age (VI, 0.76, P = .01) (eTable 2 in the Supplement).
Figure 2. Sensitive Period Analyses.
Random forest regression with conditional inference trees indicating the importance of 10 types of childhood maltreatment across ages on different aspects of bilateral amygdala response during an emotional face matching task. Mean importance is defined as the increase in mean square error of the fit following permutation of each variable. A, Blood oxygenation level–dependent functional magnetic resonance imaging activation to emotional faces vs shapes. B, Minimal spanning emotional ellipse area from representational similarities analysis and multidimensional scaling. C, Discriminability in pattern of voxel response between emotional and neutral faces based on d′ euclidean distance from representational similarities analysis and multidimensional scaling. EN indicates emotional neglect; NVEA, nonverbal emotional abuse; Peer_E, peer emotional bullying; Peer_P, peer physical bullying; Phys, parental physical abuse; PN, physical neglect; PVA, parental verbal abuse; SexA, sexual abuse; WIPV, witnessing interparental violence; and Wsib, witnessing violence toward siblings.
aP < .05.
bP < .01.
cP < .001.
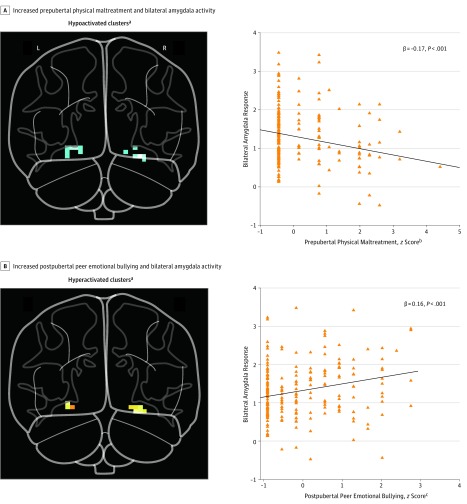
The stored random forest models were used to estimate the direction of change in amygdala activity resulting from varying the degree of exposure to the important risk factors. Increasing severity of peer emotional abuse at 13 and 15 years of age was associated with increased amygdala activation (eTable 2 in the Supplement). In contrast, severity of parental physical abuse at 3 and 4 years of age and peer physical abuse at 6 years of age were associated with decreased activation. As seen in Figure 3, combined exposure to peer emotional abuse at 13 and 15 years of age was associated with a substantially augmented response to emotional faces vs shapes (β = 0.16, P < .001), whereas earlier exposure was associated with a blunted response (β = −0.17, P < .001). Degree of BOLD activation to emotional faces vs shapes was positively associated with peer emotional abuse at 13 and 15 years of age (3.4% variance, P = .003) and number of activated voxels (13.0% variance, P < 10−7) and negatively associated with parental physical abuse at 3 to 4 years of age and peer physical abuse at 6 years of age (4.1% variance, P < .001) as well as amygdala gray matter volume (2.4% variance, P < .001).
Figure 3. Maltreatment at Peak Predictive Ages and Bilateral Amygdala Response to Emotional Faces vs Shapes.
A, Severity of exposure to prepubertal maltreatment defined as the sum of parental physical abuse at 3 to 4 years of age and peer physical bullying at 6 years of age. B, Severity of exposure to postpubertal emotional maltreatment defined as the sum of peer emotional abuse at 13 and 15 years of age.
aAll clusters in the glass brain are corrected for false display rate across the bilateral amygdala region of interest (P < .05).
bCombined severity of parental physical maltreatment at 3 and 4 years of age and peer physical bullying at age 6 years.
cCombined severity of peer emotional bullying at 13 and 15 years of age.
The most important factor associated with greater response to emotional faces vs neutral faces was peer physical abuse at 6 years of age (VI, 2.36; P < .001) (eFigure 1 in the Supplement). Other important factors were peer physical bullying at 11 years of age, witnessing interparental violence at 7 and 9 years of age, parental nonverbal emotional abuse at 9 years of age, parental verbal abuse at 17 to 18 years of age, physical neglect at 3 years of age, and emotional neglect at 14 years of age. As reported in eTable 2 in the Supplement, exposure to important types of maltreatment that occurred between 11 and 18 years of age was associated with increased amygdala activation. In contrast, exposure to important types of maltreatment between 3 and 9 years was associated with decreased response to emotional faces vs neutral faces (eFigure 2 in the Supplement). Sensitive period analysis of response to neutral faces vs shapes is presented in eResults 1 in the Supplement.
Sensitive Period Analysis of Maltreatment on Representational Geometry
As seen in Figure 2B, the most important risk factor associated with emotional face ellipse area was parental nonverbal emotional abuse at 12 (VI, 2.03; P = .006) and 13 years of age (VI, 5.94; P < .001) (eTable 3 in the Supplement). Other significant factors included emotional neglect at 10, 15, and 18 years of age and parental physical abuse at 4 years of age. The most important factor associated with d′ euclidean distance between emotional and neutral ellipses was peer emotional abuse at 6 years of age (VI, 3.35; P = .001) (Figure 2C). Other significant factors included physical neglect at 1 to 3 years of age, emotional neglect at 16 and 18 years of age, and nonverbal emotional abuse at 14 years of age.
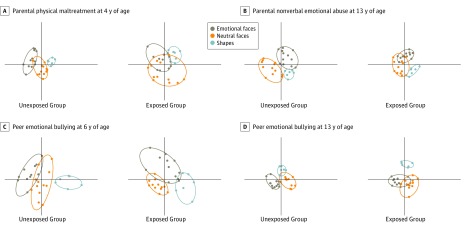
As seen in Figure 4A, participants reporting parental physical abuse at 4 years of age had larger emotional ellipse areas than participants reporting no exposure to physical abuse at this age (mean [SD], 1.41 [1.05] vs 0.29 [0.18]; P < .001). In contrast, participants reporting nonverbal emotional abuse at 13 years of age (Figure 4B) had smaller emotional ellipse areas compared with participants without this experience (mean [SD], 0.25 [0.15] vs 0.91 [0.59]; P < .001).
Figure 4. Multidimensional Scaling of Amygdala Response Based on Type and Timing of Maltreatment.
Comparison of multidimensional scaling patterns of voxelwise response to emotional faces, neutral faces, and shapes in groups of individuals with and without exposure to a particular type of maltreatment during a specific developmental phase. The ellipse area is indicative of the homogeneity of voxelwise response to stimuli within the same category, with smaller ellipses corresponding to more similar response patterns. Differences in d′ distance are indicative of heterogeneity of response to stimuli from different categories, with larger distances reflected in more separation and less overlap between category ellipses. A, Differences in the ellipse area associated with exposure to parental physical maltreatment at 4 years of age. B, Differences in the ellipse area associated with exposure to parental nonverbal emotional abuse at 13 years of age. C, Differences in d′ euclidean distance between emotional and neutral faces associated with exposure to peer emotional bullying at 6 years of age. D, Differences in d′ euclidean distance between emotional and neutral faces associated with exposure to peer emotional bullying at 13 years of age. The x-axis and y-axis are scaled the same for each plot and range from −0.9 to 1.5 for the x-axis and −1.0 to 1.2 for the y-axis.
The d′ euclidean distance between responses to emotional and neutral faces was greater in participants reporting peer emotional abuse at 6 years of age (Figure 4C) than in participants reporting no exposure at this age (mean [SD], 1.89 [0.75] vs 1.10 [0.77]; P < .001). Conversely, exposure to peer emotional abuse at 13 years of age (Figure 4D) was associated with decreased d′ distance (mean [SD], 0.80 [0.39] vs 1.94 [0.77]; P < .001). Additional findings are included in eTable 3 and eResults 2 in the Supplement.
Combined Prepubertal and Postpubertal Exposure
The association of combined exposure was assessed by comparing individuals with and without parental physical abuse at 4 years of age and nonverbal emotional abuse at 13 years of age. Prepubertal exposure alone was associated with a marked increase in emotional ellipse area (mean [SD], 3.59 [0.56]) vs neither type of exposure (mean [SD], 1.82 [1.23]) (P < .001), whereas postpubertal exposure was associated with a substantial decrease (mean [SD], 0.53 [0.27]) (P < .001). The ellipse area was intermediate in participants with combined exposure (mean [SD], 1.56 [0.67]) and similar in size to participants who experienced neither type. However, the d′ distance between emotional and neutral faces was significantly smaller in participants with combined exposure (mean [SD], 0.53 [0.35]) than in those who experienced neither type (mean [SD], 1.35 [1.01]) (P < .001). Further details are provided in eFigure 3 in the Supplement.
Discussion
Peer emotional abuse at 15 years of age and parental physical abuse at 4 years of age were primary risk factors associated with fMRI amygdala activity response to emotional faces vs shapes. Similarly, exposure to nonverbal emotional abuse at 13 years of age and parental physical abuse at 4 years of age were primary factors associated with emotional face ellipse area, whereas maltreatment exposures at ages 2, 4, and 13 to 14 years were associated with primary peaks in the distance between emotional and neutral ellipses areas. These findings suggest that some aspects of amygdala function are influenced in the prepubertal period at approximately 4 years of age and again postpubertally between 13 and 15 years of age. Results of the contrast between emotional and neutral faces were more complex and highlight the importance of maltreatment at other ages.
Increased amygdala response to emotional faces has been the most consistent functional imaging finding among individuals exposed to maltreatment.8 A noteworthy exception was noted by Taylor et al,37 who reported a hypoactive response to fearful and angry faces in adults raised in families characterized by harsh, chaotic, or conflict-ridden parenting. However, that study37 did not account for timing of exposure, which may obscure critical developmental differences. We believe that differences in age of exposure was the most important factor rather than type of maltreatment (ie, early physical vs later emotional) because peer emotional abuse at 6 to 9 years vs 13 years was associated with opposite effects on emotional to neutral face d′ distance. Similarly, peer physical abuse at 6 vs 11 years had an opposite association with emotional vs neutral face activation, whereas parental physical abuse and parental verbal abuse in adolescence had the same associations. To our knowledge, this was the first study to report that prepubertal and postpubertal maltreatment may be associated with opposite fMRI responses in brain function.
This was also the first study, to our knowledge, to assess the association of 10 different types of early adversity with amygdala response. Our findings underscore the importance of peer bullying, which prior studies have not considered to our knowledge. This oversight may be significant because an important longitudinal study58 found that peer bullying was the most important experiential factor associated with depression and that other types of childhood adversity were associated with only increased risk of depression indirectly by enhancing the risk of peer bullying. This same association may be seen with amygdala response, which in turn may mediate the association between peer bullying and depression59 (eDiscussion 1 in the Supplement).
Maltreatment-associated brain alterations have been proposed as phenotypic adaptations that enhance survival and reproductive success in our evolutionary past, although they may currently be counterproductive.8 This hypothesis leads to the question of whether prepubertal vs postpubertal maltreatment-associated differences in amygdala function make adaptive sense. During the early prepubertal period, children do not have the ability to control their exposure through fight-or-flight reactions and may need to remain strongly attached to parents who act abusively. Thus, their survival might depend on having a reduced amygdala response to threat. Furthermore, they may benefit from assessment of subtle differences in facial expression (as reflected in a large ellipse area) that might enable them to better judge how and when to approach individuals who are episodically abusive but also episodically nurturing.
In contrast, adolescents are better able to reduce their exposure to abuse through fight-or-flight reactions, and a strong amygdala response may be adaptive. Furthermore, it may be useful to view threatening emotional faces in a more homogeneous manner (smaller ellipse area) to facilitate rapid responses and to generalize their response to new individuals.
A hyperactive amygdala response to emotional faces can be disadvantageous in less hostile situations because individuals may misread neutral expressions as threatening, which may put them at risk for anxiety, depression, and unstable relationships. In contrast, individuals with a hypoactive amygdala response may fail to adequately respond to threats or salient signals and may repeatedly make the same kind of mistakes in life and relationships (eDiscussion 2 in the Supplement).
Developmental differences in amygdala responses fit with our proposed association between timing of exposure and amygdala volume. An inverse association between amygdala volume and activation has been reported in previous studies.60,61 Similarly, Chugani et al62 reported decreased amygdala activity in institutionalized children with early deprivation, who typically have enlarged amygdalas.32 We observed an inverse association between amygdala volume and BOLD response to emotional faces vs shapes, which was consistent with the previous findings.32,60,61,62 Our finding suggests that maltreatment during the amygdala growth phase is associated with enhanced amygdala volume and emotional ellipse area but with a diminished degree of activation to emotional faces vs shapes. In contrast, exposure during the pruning phase may be associated with reduced volume, smaller emotional ellipse areas, and increased activation.
The potential enduring association between maltreatment and amygdala function is important given the prominent role the amygdala plays in regulation of emotion, hypothalamic-pituitary-adrenal axis response, detection of salience, and risk of psychiatric disorders.17,22,59,63,64 These findings suggest that preschool children and early teens may be most susceptible to the association of adversity with some aspects of amygdala function but are affected in opposite ways. This finding may help explain why maltreatment is associated with such a diverse array of clinical outcomes. Furthermore, the opposite associations between prepubertal and postpubertal exposure and amygdala response may also explain why early adversity is associated in adulthood with reduced cortisol levels and blunted adrenocortical response,65,66,67 whereas later exposure (particularly to sexual abuse68) is associated with increased adrenocortical response.65,69
Limitations
Some limitations in this study should be acknowledged. First, type and timing of maltreatment were assessed using retrospective self-report of maltreatment that occurred years previously. Self-report could be affected by memory impairment associated with psychiatric symptoms and mood-congruent memory biases. However, Brewin et al,70 in a comprehensive review, found little evidence to support such criticisms. Studies show that retrospective reports of abuse are verifiable71 and contemporary instruments for assessing maltreatment have impressive test-retest reliability.49,72 Less is known regarding the validity of retrospective self-report in capturing timing of exposure. Evidence that supports the validity of self-reported timing, even for early childhood events, is presented in eDiscussion 3 in the Supplement. Nevertheless, these findings need to be verified longitudinally.
A second limitation is that the sample was too small for sex-specific sensitive period analyses, and some factors may apply to one sex more than the other, such as the peer emotional abuse peeks at 13 and 15 years of age in amygdala activation. However, this needs to be evaluated in a larger sample.
The main limitation of using random forest regression with conditional inference trees, besides lengthy processing times, is that it indicates the importance of the regressor variables but does not provide a clear understanding of the relationship between the regressor and the outcome. Thus, we needed to use the stored random forest models to estimate the direction of the association between maltreatment and amygdala response by varying the level of exposure to one specific risk factor while holding the other variables constant at their modal value. This provides one view of the association but does not reveal potential interactive effects between regressors that may be contained within the model.
In addition, the retrospective nature of this observational study design does not permit indication of causal relationships between the various exposures of maltreatment and the fMRI amygdala activity outcomes.
Conclusions
The present findings provide new insights into the complex role of childhood maltreatment in the development of psychiatric disorders. The findings suggest that prepubertal vs postpubertal developmental differences in the association between maltreatment and amygdala response to threatening or salient stimuli exist. A key question is whether the presence of multiple sensitive periods provides windows of opportunity when treatments can most effectively correct the consequences of exposure during earlier sensitive periods.
eMethods 1. Participant Recruitment
eMethods 2. Maltreatment and Abuse Chronology of Exposure scale (MACE)
eMethods 3. Scan Parameters and Image Preprocessing
eMethods 4. Emotional Face Matching Paradigm
eMethods 5. Sensitive Period Analysis – Random Forest Regression
eMethods 6. Statistical Analysis of pi-fMRI Representational Similarity Results
eResults 1. Sensitive Period Analysis of Amygdala Activation to Emotional>Neutral Faces and Neutral Faces>Shapes
eResults 2. Sensitive Period Analysis of Maltreatment on Representational Geometry
eDiscussion 1. Types of Maltreatment and Amygdala Response
eDiscussion 2. Prepubertal vs Postpubertal Exposure and d′ Prime Distance.
eDiscussion 3. Retrospective Assessment of Timing of Exposure to Maltreatment
eTable 1. Main Effects of Amygdala Activation in Emotional Face Matching Task
eTable 2. Important Predictors of Differential Amygdala Activation to Emotional Faces, Neutral Faces and Shapes
eTable 3. Identification of Important Predictor Variables
eFigure 1. Sensitive Periods
eFigure 2. Prepubertal Exposure and BOLD fMRI
eFigure 3. Graphs for Combined Prepubertal and Postpubertal Exposure
References
- 1.Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med. 2003;37(3):268-277. doi: 10.1016/S0091-7435(03)00123-3 [DOI] [PubMed] [Google Scholar]
- 2.Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111(3):564-572. doi: 10.1542/peds.111.3.564 [DOI] [PubMed] [Google Scholar]
- 3.Green JG, McLaughlin KA, Berglund PA, et al. Childhood adversities and adult psychiatric disorders in the National Comorbidity Survey Replication I: associations with first onset of DSM-IV disorders. Arch Gen Psychiatry. 2010;67(2):113-123. doi: 10.1001/archgenpsychiatry.2009.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anda RF, Brown DW, Felitti VJ, Bremner JD, Dube SR, Giles WH. Adverse childhood experiences and prescribed psychotropic medications in adults. Am J Prev Med. 2007;32(5):389-394. doi: 10.1016/j.amepre.2007.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52(11):1066-1078. doi: 10.1016/S0006-3223(02)01459-2 [DOI] [PubMed] [Google Scholar]
- 6.Jensen SK, Dickie EW, Schwartz DH, et al. Effect of early adversity and childhood internalizing symptoms on brain structure in young men. JAMA Pediatr. 2015;169(10):938-946. doi: 10.1001/jamapediatrics.2015.1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671-690. doi: 10.1002/dev.20494 [DOI] [PubMed] [Google Scholar]
- 8.Teicher MH, Samson JA, Anderson CM, Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat Rev Neurosci. 2016;17(10):652-666. doi: 10.1038/nrn.2016.111 [DOI] [PubMed] [Google Scholar]
- 9.Tottenham N, Hare TA, Millner A, Gilhooly T, Zevin JD, Casey BJ. Elevated amygdala response to faces following early deprivation. Dev Sci. 2011;14(2):190-204. doi: 10.1111/j.1467-7687.2010.00971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrory EJ, De Brito SA, Sebastian CL, et al. Heightened neural reactivity to threat in child victims of family violence. Curr Biol. 2011;21(23):R947-R948. doi: 10.1016/j.cub.2011.10.015 [DOI] [PubMed] [Google Scholar]
- 11.Garrett AS, Carrion V, Kletter H, Karchemskiy A, Weems CF, Reiss A. Brain activation to facial expressions in youth with PTSD symptoms. Depress Anxiety. 2012;29(5):449-459. doi: 10.1002/da.21892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry. 2012;71(4):286-293. doi: 10.1016/j.biopsych.2011.10.021 [DOI] [PubMed] [Google Scholar]
- 13.Van Harmelen AL, van Tol MJ, Demenescu LR, et al. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci. 2013;8(4):362-369. doi: 10.1093/scan/nss007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdan R, Williamson DE, Hariri AR. Mineralocorticoid receptor Iso/Val (rs5522) genotype moderates the association between previous childhood emotional neglect and amygdala reactivity. Am J Psychiatry. 2012;169(5):515-522. doi: 10.1176/appi.ajp.2011.11060855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jedd K, Hunt RH, Cicchetti D, et al. Long-term consequences of childhood maltreatment: altered amygdala functional connectivity. Dev Psychopathol. 2015;27(4, pt 2):1577-1589. doi: 10.1017/S0954579415000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herringa RJ, Birn RM, Ruttle PL, et al. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proc Natl Acad Sci U S A. 2013;110(47):19119-19124. doi: 10.1073/pnas.1310766110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens JS, Kim YJ, Galatzer-Levy IR, et al. Amygdala reactivity and anterior cingulate habituation predict posttraumatic stress disorder symptom maintenance after acute civilian trauma. Biol Psychiatry. 2017;81(12):1023-1029. doi: 10.1016/j.biopsych.2016.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169-191. doi: 10.1038/npp.2009.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217-238. doi: 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suslow T, Lindner C, Dannlowski U, et al. Automatic amygdala response to facial expression in schizophrenia: initial hyperresponsivity followed by hyporesponsivity. BMC Neurosci. 2013;14:140. doi: 10.1186/1471-2202-14-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grotegerd D, Stuhrmann A, Kugel H, et al. Amygdala excitability to subliminally presented emotional faces distinguishes unipolar and bipolar depression: an fMRI and pattern classification study. Hum Brain Mapp. 2014;35(7):2995-3007. doi: 10.1002/hbm.22380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein-Piekarski AN, Korgaonkar MS, Green E, et al. Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proc Natl Acad Sci U S A. 2016;113(42):11955-11960. doi: 10.1073/pnas.1606671113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tottenham N, Hertzig ME, Gillespie-Lynch K, Gilhooly T, Millner AJ, Casey BJ. Elevated amygdala response to faces and gaze aversion in autism spectrum disorder. Soc Cogn Affect Neurosci. 2014;9(1):106-117. doi: 10.1093/scan/nst050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krause-Utz A, Winter D, Niedtfeld I, Schmahl C. The latest neuroimaging findings in borderline personality disorder. Curr Psychiatry Rep. 2014;16(3):438. doi: 10.1007/s11920-014-0438-z [DOI] [PubMed] [Google Scholar]
- 25.Sarrieau A, Dussaillant M, Agid F, Philibert D, Agid Y, Rostene W. Autoradiographic localization of glucocorticosteroid and progesterone binding sites in the human post-mortem brain. J Steroid Biochem. 1986;25(5B):717-721. doi: 10.1016/0022-4731(86)90300-6 [DOI] [PubMed] [Google Scholar]
- 26.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PloS One. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102(26):9371-9376. doi: 10.1073/pnas.0504011102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667-673. doi: 10.1016/j.neuroscience.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 29.Schmahl CG, Vermetten E, Elzinga BM, Douglas Bremner J. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Res. 2003;122(3):193-198. doi: 10.1016/S0925-4927(03)00023-4 [DOI] [PubMed] [Google Scholar]
- 30.Hanson JL, Nacewicz BM, Sutterer MJ, et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77(4):314-323. doi: 10.1016/j.biopsych.2014.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236-244. doi: 10.1016/j.neuroimage.2014.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tottenham N, Hare TA, Quinn BT, et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci. 2010;13(1):46-61. doi: 10.1111/j.1467-7687.2009.00852.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teicher MH, Anderson CM, Ohashi K, et al. Differential effects of childhood neglect and abuse during sensitive exposure periods on male and female hippocampus. Neuroimage. 2018;169:443-452. doi: 10.1016/j.neuroimage.2017.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen SL, Tomada A, Vincow ES, Valente E, Polcari A, Teicher MH. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J Neuropsychiatry Clin Neurosci. 2008;20(3):292-301. doi: 10.1176/jnp.2008.20.3.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyons-Ruth K, Pechtel P, Yoon SA, Anderson CM, Teicher MH. Disorganized attachment in infancy predicts greater amygdala volume in adulthood. Behav Brain Res. 2016;308:83-93. doi: 10.1016/j.bbr.2016.03.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci Biobehav Rev. 2003;27(1-2):3-18. doi: 10.1016/S0149-7634(03)00005-8 [DOI] [PubMed] [Google Scholar]
- 37.Taylor SE, Eisenberger NI, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biol Psychiatry. 2006;60(3):296-301. doi: 10.1016/j.biopsych.2005.09.027 [DOI] [PubMed] [Google Scholar]
- 38.Badura-Brack A, McDermott TJ, Heinrichs-Graham E, et al. Veterans with PTSD demonstrate amygdala hyperactivity while viewing threatening faces: a MEG study. Biol Psychol. 2018;132:228-232. doi: 10.1016/j.biopsycho.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klumpp H, Angstadt M, Nathan PJ, Phan KL. Amygdala reactivity to faces at varying intensities of threat in generalized social phobia: an event-related functional MRI study. Psychiatry Res. 2010;183(2):167-169. doi: 10.1016/j.pscychresns.2010.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Arch Gen Psychiatry. 2002;59(11):1027-1034. doi: 10.1001/archpsyc.59.11.1027 [DOI] [PubMed] [Google Scholar]
- 41.Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia. 2011;49(4):745-759. doi: 10.1016/j.neuropsychologia.2010.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61(11):1306-1309. doi: 10.1016/j.biopsych.2006.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiatry. 2014;71(6):627-636. doi: 10.1001/jamapsychiatry.2013.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kriegeskorte N, Bandettini P. Analyzing for information, not activation, to exploit high-resolution fMRI. Neuroimage. 2007;38(4):649-662. doi: 10.1016/j.neuroimage.2007.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mur M, Bandettini PA, Kriegeskorte N. Revealing representational content with pattern-information fMRI: an introductory guide. Soc Cogn Affect Neurosci. 2009;4(1):101-109. doi: 10.1093/scan/nsn044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy L, Tsuchiya N, Serre T. Reading the mind’s eye: decoding category information during mental imagery. Neuroimage. 2010;50(2):818-825. doi: 10.1016/j.neuroimage.2009.11.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan A, McCormack HC, Bolger EA, et al. Childhood maltreatment, depression, and suicidal ideation: critical importance of parental and peer emotional abuse during developmental sensitive periods in males and females. Front Psychiatry. 2015;6:42. doi: 10.3389/fpsyt.2015.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teicher MH, Anderson CM, Ohashi K, Polcari A. Childhood maltreatment: altered network centrality of cingulate, precuneus, temporal pole and insula. Biol Psychiatry. 2014;76(4):297-305. doi: 10.1016/j.biopsych.2013.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Teicher MH, Parigger A. The ‘Maltreatment and Abuse Chronology of Exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PloS One. 2015;10(2):e0117423. doi: 10.1371/journal.pone.0117423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schalinski I, Breinlinger S, Hirt V, Teicher MH, Odenwald M, Rockstroh B. Environmental adversities and psychotic symptoms: the impact of timing of trauma, abuse, and neglect. Schizophr Res. 2019;205:4-9. doi: 10.1016/j.schres.2017.10.034 [DOI] [PubMed] [Google Scholar]
- 51.Schalinski I, Teicher MH, Nischk D, Hinderer E, Müller O, Rockstroh B. Type and timing of adverse childhood experiences differentially affect severity of PTSD, dissociative and depressive symptoms in adult inpatients. BMC Psychiatry. 2016;16:295. doi: 10.1186/s12888-016-1004-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders–Clinician Version (SCID-CV). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 53.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17(1):317-323. doi: 10.1006/nimg.2002.1179 [DOI] [PubMed] [Google Scholar]
- 54.Strobl C, Boulesteix A-L, Zeileis A, Hothorn T. Bias in random forest variable importance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8(1):25. doi: 10.1186/1471-2105-8-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomoda A, Polcari A, Anderson CM, Teicher MH. Reduced visual cortex gray matter volume and thickness in young adults who witnessed domestic violence during childhood. PloS One. 2012;7(12):e52528. doi: 10.1371/journal.pone.0052528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marusak HA, Zundel C, Brown S, Rabinak CA, Thomason ME Is neutral really neutral? converging evidence from behavior and corticolimbic connectivity in children and adolescents [published online December 21, 2019]. Soc Cogn Affect Neurosci. doi: 10.1093/scan/nsw182 [DOI] [PMC free article] [PubMed]
- 57.White MG, Bogdan R, Fisher PM, Muñoz KE, Williamson DE, Hariri AR. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 2012;11(7):869-878. doi: 10.1111/j.1601-183X.2012.00837.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banny AM, Cicchetti D, Rogosch FA, Oshri A, Crick NR. Vulnerability to depression: a moderated mediation model of the roles of child maltreatment, peer victimization, and serotonin transporter linked polymorphic region genetic variation among children from low socioeconomic status backgrounds. Dev Psychopathol. 2013;25(3):599-614. doi: 10.1017/S0954579413000047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85(3):505-511. doi: 10.1016/j.neuron.2014.12.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kalmar JH, Wang F, Chepenik LG, et al. Relation between amygdala structure and function in adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2009;48(6):636-642. doi: 10.1097/CHI.0b013e31819f6fbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siegle GJ, Konecky RO, Thase ME, Carter CS. Relationships between amygdala volume and activity during emotional information processing tasks in depressed and never-depressed individuals: an fMRI investigation. Ann N Y Acad Sci. 2003;985(1):481-484. doi: 10.1111/j.1749-6632.2003.tb07105.x [DOI] [PubMed] [Google Scholar]
- 62.Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of postinstitutionalized Romanian orphans. Neuroimage. 2001;14(6):1290-1301. doi: 10.1006/nimg.2001.0917 [DOI] [PubMed] [Google Scholar]
- 63.Heim C, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33(6):693-710. doi: 10.1016/j.psyneuen.2008.03.008 [DOI] [PubMed] [Google Scholar]
- 64.Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37(1):27-38. doi: 10.1016/j.psyneuen.2011.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Bellis MD, Zisk A. The biological effects of childhood trauma. Child Adolesc Psychiatr Clin N Am. 2014;23(2):185-222, vii. doi: 10.1016/j.chc.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology (Berl). 2011;214(1):367-375. doi: 10.1007/s00213-010-2007-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ouellet-Morin I, Odgers CL, Danese A, et al. Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol Psychiatry. 2011;70(11):1016-1023. doi: 10.1016/j.biopsych.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284(5):592-597. doi: 10.1001/jama.284.5.592 [DOI] [PubMed] [Google Scholar]
- 69.Linares LO, Shrout PE, Nucci-Sack A, Diaz A. Child maltreatment, dating perpetration of physical assault, and cortisol reactivity among disadvantaged female adolescents. Neuroendocrinology. 2013;97(3):252-259. doi: 10.1159/000342958 [DOI] [PubMed] [Google Scholar]
- 70.Brewin CR, Andrews B, Gotlib IH. Psychopathology and early experience: a reappraisal of retrospective reports. Psychol Bull. 1993;113(1):82-98. doi: 10.1037/0033-2909.113.1.82 [DOI] [PubMed] [Google Scholar]
- 71.Chu JA, Frey LM, Ganzel BL, Matthews JA. Memories of childhood abuse: dissociation, amnesia, and corroboration. Am J Psychiatry. 1999;156(5):749-755. [DOI] [PubMed] [Google Scholar]
- 72.Bernstein DP, Fink L. Childhood Trauma Questionnaire Manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Participant Recruitment
eMethods 2. Maltreatment and Abuse Chronology of Exposure scale (MACE)
eMethods 3. Scan Parameters and Image Preprocessing
eMethods 4. Emotional Face Matching Paradigm
eMethods 5. Sensitive Period Analysis – Random Forest Regression
eMethods 6. Statistical Analysis of pi-fMRI Representational Similarity Results
eResults 1. Sensitive Period Analysis of Amygdala Activation to Emotional>Neutral Faces and Neutral Faces>Shapes
eResults 2. Sensitive Period Analysis of Maltreatment on Representational Geometry
eDiscussion 1. Types of Maltreatment and Amygdala Response
eDiscussion 2. Prepubertal vs Postpubertal Exposure and d′ Prime Distance.
eDiscussion 3. Retrospective Assessment of Timing of Exposure to Maltreatment
eTable 1. Main Effects of Amygdala Activation in Emotional Face Matching Task
eTable 2. Important Predictors of Differential Amygdala Activation to Emotional Faces, Neutral Faces and Shapes
eTable 3. Identification of Important Predictor Variables
eFigure 1. Sensitive Periods
eFigure 2. Prepubertal Exposure and BOLD fMRI
eFigure 3. Graphs for Combined Prepubertal and Postpubertal Exposure