Abstract
Sarcopenia is a potentially modifiable risk factor for falls and fractures in older adults, but the strength of the association between sarcopenia, falls, and fractures is unclear. This study aims to systematically assess the literature and perform a meta‐analysis of the association between sarcopenia with falls and fractures among older adults. A literature search was performed using MEDLINE, EMBASE, Cochrane, and CINAHL from inception to May 2018. Inclusion criteria were the following: published in English, mean/median age ≥ 65 years, sarcopenia diagnosis (based on definitions used by the original studies' authors), falls and/or fractures outcomes, and any study population. Pooled analyses were conducted of the associations of sarcopenia with falls and fractures, expressed in odds ratios (OR) and 95% confidence intervals (CIs). Subgroup analyses were performed by study design, population, sex, sarcopenia definition, continent, and study quality. Heterogeneity was assessed using the I 2 statistics. The search identified 2771 studies. Thirty‐six studies (52 838 individuals, 48.8% females, and mean age of the study populations ranging from 65.0 to 86.7 years) were included in the systematic review. Four studies reported on both falls and fractures. Ten out of 22 studies reported a significantly higher risk of falls in sarcopenic compared with non‐sarcopenic individuals; 11 out of 19 studies showed a significant positive association with fractures. Thirty‐three studies (45 926 individuals) were included in the meta‐analysis. Sarcopenic individuals had a significant higher risk of falls (cross‐sectional studies: OR 1.60; 95% CI 1.37–1.86, P < 0.001, I 2 = 34%; prospective studies: OR 1.89; 95% CI 1.33–2.68, P < 0.001, I 2 = 37%) and fractures (cross‐sectional studies: OR 1.84; 95% CI 1.30–2.62, P = 0.001, I 2 = 91%; prospective studies: OR 1.71; 95% CI 1.44–2.03, P = 0.011, I 2 = 0%) compared with non‐sarcopenic individuals. This was independent of study design, population, sex, sarcopenia definition, continent, and study quality. The positive association between sarcopenia with falls and fractures in older adults strengthens the need to invest in sarcopenia prevention and interventions to evaluate its effect on falls and fractures.
Keywords: Sarcopenia, Falls, Fractures, Meta‐analysis
Introduction
Approximately one‐third of older adults fall at least once a year1 and a median of 4.1% of falls results in fractures.2 Falls are associated with physical disability, functional impairment, dependency in activities of daily living, institutionalization, increased morbidity, and mortality.3, 4 A number of risk factors have been found to predispose older adults to falls. These include old age, female sex, fear of falling, impaired cognition, mobility, and gait.5, 6, 7, 8 One of the potentially modifiable risk factors is sarcopenia, that is, age‐related low skeletal muscle mass, strength, and physical performance.9
Sarcopenia is prevalent between 2% and 37% in community‐dwelling older adults, depending on the sarcopenia definition applied10, 11, 12 and associated with decreased mobility, impaired standing balance, functional decline, hospitalization, and mortality.13, 14, 15 Interventions to prevent and treat sarcopenia have been shown to be effective in increasing muscle mass, strength, and physical performance,9, 16 although it is not proven yet that this leads to a decrease of falls and fractures.
The aim of this systematic review and meta‐analysis was to evaluate whether sarcopenic individuals have a higher risk of falls and fractures compared with non‐sarcopenic individuals and whether this association is influenced by study design, population, sex, sarcopenia definition, continent, or study quality.
Methods
Data sources and searches
The protocol of the systematic review was registered at PROSPERO International prospective register of systematic reviews: CRD42017068485. The systematic review was conducted according to the PRISMA standards.17 A systematic search was performed by a librarian in four electronic databases, that is, MEDLINE, EMBASE, Cochrane Central, and CINAHL from date of inception to 1 May 2018 (Online Resource S1). The search included the keywords ‘sarcopenia’, ‘falls’, ‘fractures’, and synonyms. The reference section of each included article was also used to identify additional related research studies.
Study selection
The studies obtained using the search strategy were assessed for eligibility independently by two authors (S. S. Y. Y. and V. K. P.) by screening titles and abstracts. Subsequently, the full‐text articles of potentially relevant studies were screened independently by two reviewers (S. S. Y. Y. and V. K. P.). A third reviewer (E. M. R.) resolved any disagreements between the authors regarding the eligibility by discussion and reaching a consensus. Studies were included in the systematic review when the following inclusion criteria were met: published in English; mean or median age of ≥65 years or with subgroup analysis in those aged ≥65 years; diagnosis of sarcopenia using any definition used by the original studies' authors; and at least one of the following outcomes: falls and/or fractures. No restriction regarding study population was applied. Studies were excluded if they did not contain primary data (conference abstracts, reviews, letters to the editor, and case reports with <5 cases). Studies were excluded if no comparison group was included; that is, all individuals suffered from falls, fractures, or sarcopenia. If studies used data from the same cohort,18, 19 the studies with the largest sample size were included.18
Data extraction and quality assessment
The following variables were extracted independently by two reviewers (S. S. Y. Y. and V. K. P.) from the included studies: author, year of publication, total number of individuals included in the study, mean/median age of individuals, percentage of females, population, continent, prevalence of falls, study design of falls outcome, prevalence of fractures, study design of fractures outcome, applied definition(s) of sarcopenia, prevalence of sarcopenia, assessment method of muscle mass, cut‐off point of muscle mass, assessment method of muscle strength, cut‐off point of muscle strength, assessment method of physical performance, and cut‐off point of physical performance.
Risk of bias of the included studies was assessed independently by two reviewers (S. S. Y. Y. and V. K. P.) using the Newcastle Ottawa Scale (NOS)20, 21 for case–control and cohort studies and a modified version of the NOS for cross‐sectional studies. A system of points was given to the eligible categories: (i) selection of the study population, (ii) comparability, and (iii) description of the outcome (Online Resource S2). A study was given a maximum of one point in each item within the Selection and Outcome categories and a maximum of two points was given for the Comparability category. The scale scores varied depending on the study design. For case–control and cohort studies, it ranged from 0 to 9 points with ≥7 points classified as high quality.20 For cross‐sectional studies, it ranged from 0 to 7 points. Because a modified version of NOS was used and there was no cut‐off available from the literature, a median of ≥4 points was considered as high quality for cross‐sectional studies.22, 23
Data synthesis and analysis
A meta‐analysis was performed stratified for falls and fractures, using a random‐effects model because of assumed heterogeneity between the studies. Studies were excluded from the meta‐analysis if an odds ratio (OR) could not be calculated because of insufficient data or confidence intervals (CIs) were not given. When both crude and adjusted ORs were reported, adjusted ORs were used. When the studies only reported ORs stratified by sex, the overall OR was calculated from a two‐by‐two table including the total number of sarcopenic and non‐sarcopenic individuals with falls/fractures. Sarcopenia definitions differ in their composition including muscle mass, muscle strength, and physical performance, and applying different definitions has an impact on the prevalence of sarcopenia.11, 12 Some definitions are based on low muscle mass alone: Baumgartner et al.,24, 25, 26, 27, 28 Delmonico et al.,24, 27 Newman et al.,25 Cheng et al.,29 Scott et al.,28 Sanada et al.,30, 31 Levine and Crimmins,28 and Bouchard et al..28 Other definitions are based on both low muscle mass and low muscle strength/physical performance: European Working Group on Sarcopenia in Older People (EWGSOP),24, 25, 28, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52 Asian Working Group for Sarcopenia (AWGS),18, 51, 53, 54 Foundation for the National Institutes of Health (FNIH),24, 25, 27, 35, 44, 46, 55 International Working Group on Sarcopenia (IWGS),24, 25, 27, 35 Society for Sarcopenia, Cachexia, and Wasting Disorders (SCWD),24, 27 and ESPEN Special Interest Group on ‘cachexia‐anorexia in chronic wasting diseases’ and ‘nutrition in geriatrics’.24 In cases where studies applied multiple sarcopenia definitions, results based on the EWGSOP definition52 were prioritized over the Baumgartner definition56 and other definitions.57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68
Forest plots were used to visualize the results. Heterogeneity between the studies in effect measures were assessed using the I 2 statistic. I 2 values greater than 25% were considered to reflect low heterogeneity, 50% moderate, and 75% high heterogeneity.69 Subgroup analyses were performed regarding study design, population, sex, sarcopenia definition, continent, and study quality. We contacted 17 authors of studies to obtain the data needed to compute ORs when the study did not report ORs stratified by sex. Ten authors responded, which allowed us to include these studies in the subgroup analysis.27, 28, 32, 33, 40, 41, 42, 43, 49, 54 Funnel plots of log OR against its standard error were plotted to visually evaluate publication bias, while Egger's regression test70 and Begg's test71 were used to statistically evaluate publication bias. Comprehensive Meta‐Analysis (CMA version 2.0; Biostat Inc., Engle‐wood, NJ) was used to produce pooled estimates and forest plots. P‐values < 0.05 were considered statistically significant (two‐sided).
Results
Search results
Online Resource S3 shows the flow chart of the study selection. A total of 4129 studies were retrieved through electronic database searches. After removal of duplicates, 2771 studies were identified for title and abstract screening. Review of the titles and abstracts yielded 241 relevant studies for full‐text screening. Thirty‐six studies met all inclusion criteria and were included in this review.18, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 54, 55, 72, 73, 74, 75 A total of 33 studies were included in the meta‐analysis; four of them presented data for both falls and fractures, leaving 20 studies included in the meta‐analysis for falls24, 26, 28, 32, 33, 34, 35, 36, 40, 41, 42, 43, 44, 48, 49, 50, 72, 73, 74, 75 and 17 studies for fractures.18, 27, 29, 30, 31, 34, 35, 38, 39, 42, 46, 47, 49, 51, 54, 55, 73
Study characteristics
Table 1 shows the study characteristics of the included studies. A total of 52 838 individuals (48.8% females) with a mean age of the study populations ranging from 65.0 to 86.7 years were included, and sample sizes ranged from 58 to 6,658 individuals. Study populations included community‐dwelling individuals (22 studies),18, 24, 25, 26, 27, 28, 34, 35, 36, 40, 41, 42, 44, 45, 46, 48, 49, 50, 51, 72, 73, 75 hospitalized patients (3 studies),43, 47, 54 outpatients (4 studies),32, 38, 39, 55 and nursing home residents (3 studies).33, 37, 74 Four studies included a combined group of hospitalized patients with fractures and community‐dwelling individuals without fractures.29, 30, 31, 51 Two studies reported retrospective data,31, 55 20 studies were cross‐sectional,26, 29, 30, 32, 35, 36, 38, 39, 41, 42, 43, 44, 48, 49, 50, 51, 54, 72, 73, 75 13 studies were prospective,18, 25, 27, 28, 33, 34, 37, 40, 45, 46, 47, 53, 74 and 1 study was a randomized controlled trial examining the effect of nutritional supplementation on bone mineral density and risk of falls.24 Most of the studies were performed in Europe (12 studies),26, 27, 33, 35, 40, 42, 47, 49, 55, 73, 74 and Asia (12 studies),18, 29, 30, 31, 44, 48, 50, 51, 53, 54, 72, 75 followed by Australia (5 studies),28, 37, 38, 39, 46 South America (4 studies),32, 36, 41, 43 and North America (3 studies).24, 25, 34 The prevalence of falls ranged from 4.2% to 63.8%, and the prevalence of fractures ranged from 3.5% to 63.6% in the studies. Follow‐up periods varied from 1 to 3 years for falls and 2 to 11 years for fractures.
Table 1.
Study characteristics and falls and fractures outcomes
| Author | Year | N | Mean age ± SD (years) | Female, n (%) | Population | Continent | Falls | Fractures | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence/incidencea, n (%) | Study design | Prevalence/incidencea, n (%) | Study design | |||||||
| Bae | 2017 | 3901 | ≥65 | 2259 (57.9) | Community | Asia | 109 (2.5) | Cross‐sectional | NA | NA |
| Benjumea | 2018 | 534 | 74.4 ± 8.2 | 403 (75.5) | Outpatient | South America | 309 (60.4) | Cross‐sectional | NA | NA |
| Bischoff‐Ferrari | 2015 | 445 | 71.0 ± 4.61 | 246 (55.3) | Community | North America | 231 (51.9) | RCT | NA | NA |
| Buckinx | 2018 | 565 | 82.8 ± 9.0 | 413 (73.1) | Nursing home | Europe | 211 (37.3) | Prospective | NA | NA |
| Cawthon | 2015 | 5934 | 73.6 ± 6.0 | 0 | Community | North America | NA | NA | 207 (3.5) | Prospective |
| Chalhoub | 2015 | 6658 | 74.34 ± 5.0 | 1114 (16.7) | Community | North America | 1518 (22.8) | Retrospective | 1142 (17.2) | Prospective |
| Clynes | 2015 | 298 | 76.1 ± 2.57 | 142 (47.7) | Community | Europe | 190 (63.8) | Cross‐sectional | 70 (23.5) | Cross‐sectional |
| Dietzel | 2015 | 288 | 71.9 ± 7.5 | 142 (49.3) | Community | Europe | 47 (16.0) | Cross‐sectional | NA | NA |
| Gadelha | 2018 | 196 | 68.6 ± 6.45 | 196 (100) | Community | South America | 65 (33.2) | Cross‐sectional | NA | NA |
| Hars | 2016 | 913 | 65.0 ± 1.4 | 729 (79.9) | Community | Europe | NA | NA | 40 (4.4) | Prospective |
| Henwood | 2017 | 58 | 84.5 ± 8.2 | 41 (70.7) | Nursing home | Australia | 24 (41.4) | Prospective | NA | NA |
| Hida | 2013 | 2868 | 71.3 ± 10.4 | 2197 (76.6) | Hospital and outpatients | Asia | NA | NA | 357 (12.4) | Cross‐sectional |
| Hida | 2016 | 1824 | 70.4 ± 9.5 | 1824 (100) | Hospital and outpatients | Asia | NA | NA | 216 (11.8) | Retrospective |
| Hong | 2015 | 3077 | 78.0 ± 6.6 | 1492 (48.5) | Hospital and community | Asia | NA | NA | 757 (24.6) | Cross‐sectional |
| Huo | 2015 | 680 | 79.0 ± 7.1 | 455 (66.9) | Outpatient | Australia | NA | NA | 242 (35.6) | Cross‐sectional |
| Huo | 2016 | 680 | 79.0 ± 9.0 | 418 (61.5) | Outpatient | Australia | NA | NA | 293 (43.1) | Cross‐sectional |
| Iolascon | 2015 | 121 | 67.2 ± 8.47 | 121 (100) | Outpatient | Europe | NA | NA | 77 (63.6) | Retrospective |
| Landi | 2012 | 260 | 86.7 ± 5.4 | 177 (68.1) | Community | Europe | 37 (14.2) | Prospective | NA | NA |
| Lera | 2017 | 1006 | 67.6 ± 5.9 | 687 (68.3) | Community | South America | 332 (33.0) | Cross‐sectional | NA | NA |
| Locquet | 2018 | 288 | 74.7 ± 5.7 | 170 (59.0) | Community | Europe | NA | NA | 134 (46.5) | Cross‐sectional |
| Martinez | 2015 | 110 | 71.0 ± 8.2 | 46 (41.8) | Hospital | South America | 28 (25.5) | Cross‐sectional | NA | NA |
| Matsumoto | 2017 | 162 | 74.2 ± 7.1 | 103 (63.6) | Community | Asia | 50 (30.9) | Prospective | NA | NA |
| Menant | 2017 | 419 | 81.2 ± 4.5 | 207 (49.4) | Community | Australia | 194 (46.3) | Prospective | NA | NA |
| Meng | 2015 | 771 | 73.0 ± 5.7 | 359 (46.6) | Community | Asia | 173 (22.4) | Cross‐sectional | NA | NA |
| Schaap | 2018 | 496 | 75.2 ± 6.4 | 250 (50.4) | Community | Europe | 130 (26.6) | Prospective | 60 (12.1) | Prospective |
| Scott | 2017 | 861 | 76.6 ± 5.5 | 0 | Community | Australia | 371 (30.0) | Prospective | 152 (17.7) | Prospective |
| Sjöblom | 2013 | 590 | 67.9 ± 1.9 | 590 (100) | Community | Europe | 119 (21.7) | Cross‐sectional | 85 (14.9) | Cross‐sectional |
| Steihaug | 2018 | 201b | 79.4 ± 8.2 | 151 (75.1) | Hospital | Europe | NA | NA |
14 (7.0) 15 (7.9) |
Cross‐sectional Prospective |
| Tanimoto | 2014 | 1110 | 73.4 ± 6.0 | 738 (66.5) | Community | Asia | 220 (19.8) | Cross‐sectional | NA | NA |
| Trajanoska | 2018 | 5911 | 69.2 ± 9.1 | 3361 (56.8) | Community | Europe | 1097 (18.6) | Cross‐sectional | 939 (15.9) | Cross‐sectional |
| Van Puyenbroeck | 2012 | 276 | 83.4 | 193 (69.9) | Nursing home | Europe | 69 (25.0) | Prospective | NA | NA |
| Woo | 2014 | 2848 | 73.17 (SE 0.14) | 1675 (58.8) | Community | Asia | 120 (4.2) | Cross‐sectional | NA | NA |
| Yamada | 2013 | 1882 | 74.9 ± 5.5 | 1314 (69.8) | Community | Asia | 470 (25.0) | Cross‐sectional | NA | NA |
| Yoo | 2016 | 1970 | 66.3 ± 9.1 | 1221 (62) | Hospital and community | Asia | NA | NA | 359 (18.2) | Case–control |
| Yoshimura | 2018 | 637 | 74 ± 13 | 366 (57.5) | Hospital | Asia | NA | NA | 131 (20.6) | Cross‐sectional |
| Yu | 2014 | 4000 | 72.5 ± 5.2 | 2000 (50) | Community | Asia | NA | NA | 565 (14.1) | Prospective |
N, sample size; NA, not applicable; RCT, randomised controlled trial; SD, standard deviation.
Prevalence is reported for cross‐sectional study design; incidence is reported for prospective study design.
n = 191 for complete follow‐up.
Table 2 shows the prevalence and applied diagnostic criteria of sarcopenia. The prevalence of sarcopenia varied from 0.3% to 73.0%, depending on the sarcopenia definition applied and the study population. Sarcopenia was diagnosed using one definition18, 26, 29, 30, 31, 32, 33, 34, 36, 37, 38, 39, 40, 41, 42, 43, 47, 48, 49, 50, 53, 54, 72, 73, 75 or more than one definition.24, 25, 27, 28, 35, 44, 45, 46, 51, 55, 74 Out of the 36 included studies, EWGSOP (23 studies) was the most commonly used definition,24, 25, 28, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 51 followed by FNIH (7 studies),24, 25, 27, 35, 45, 55, 56 Baumgartner definition (5 studies),24, 25, 26, 27, 28 AWGS (4 studies),18, 51, 53, 54 and IWGS (4 studies).24, 25, 27, 35
Table 2.
Prevalence and diagnostic criteria of sarcopenia of the included studies
| Author | Year | N | Sarcopenia | Diagnostic criteria | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Definition | Prevalence, n (%) | Muscle mass | Muscle strength | Physical performance | ||||||
| Measure | Cut‐off | Measure | Cut‐off | Measure | Cut‐off | |||||
| Bae | 2017 | 3827 | Cho et al. | 1619 (42.3) | DXA | ASM (as % body weight): M: <30.3%; F: <23.8% | NA | NA | NA | NA |
| Benjumea | 2018 | 534 | EWGSOP | 380 (71.2) | Lee equation | ASM/ht2: M: ≤6.37 kg/m2; F: ≤8.90 kg/m2 | HGS | M: <30 kg; F: <20 kg | 4‐m GS | ≤0.8 m/s |
| Bischoff‐Ferrari | 2015 | 443 | Baumgartner | 49 (11.0) | DXA | ALM/ht2: M: ≤7.26 kg/m2; F: ≤5.45 kg/m2 | NA | NA | NA | NA |
| 443 | Delmonico 1 | 75 (16.9) | DXA | ALM/ht2: M: ≤7.25 kg/m2; F: ≤5.67 kg/m2 | NA | NA | NA | NA | ||
| 443 | Delmonico 2 | 95 (21.4) | DXA | Observed ALM—predicted ALM: <20th percentile of the sex‐specific distribution | NA | NA | NA | NA | ||
| 445 | EWGSOP | 31 (7.0) | DXA | ALM/ht2: M: ≤7.26 kg/m2; F: ≤5.54 kg/m2 | HGS | M: <30 kg; F: <20 kg | 15‐ft GS | <0.8 m/s | ||
| 440 | IWGS | 22 (4.9) | DXA | ALM/ht2: M: ≤7.23 kg/m2; F: ≤5.67 kg/m2 | NA | NA | 15‐ft GS | <1.0 m/s | ||
| 445 | SCWD | 12 (2.7) | DXA | ALM/ht2: M: ≤6.81 kg/m2; F: ≤5.18 kg/m2 | NA | NA | 15‐ft GS | <1.0 m/s | ||
| 445 | Muscaritoli | 104 (23.6) | DXA | SM/body mass: M: ≤37%; F: ≤28% | NA | NA | 15‐ft GS | <0.8 m/s | ||
| 443 | FNIH 1 | 52 (11.7) | DXA | ALMBMI: M: <0.789; F: <0.512 | NA | NA | NA | NA | ||
| 445 | FNIH 2 | 14 (3.1) | DXA | ALMBMI: M: <0.789; F: <0.512 | HGS | M: <26 kg; F: <16 kg | NA | NA | ||
| Buckinx | 2018 | 247 | EWGSOP | 166 (67.2) | BIA | Not specified | HGS | Not specified | SPPB | ≤8 points |
| Cawthon | 2015 | 5934 | Baumgartner | 1301 (21.9) | DXA | ALM/ht2: M: ≤7.23 kg/m2 | NA | NA | NA | NA |
| 5934 | EWGSOP | 257 (4.3) | DXA | ALM/ht2: M: ≤7.23 kg/m2 | HGS | M: <30 kg | 6‐m GS | ≤0.8 m/s | ||
| 5934 | IWGS | 277 (4.7) | DXA | ALM/ht2: M: ≤7.23 kg/m2 | NA | NA | 6‐m GS | <1.0 m/s | ||
| 5934 | FNIH 1 | 88 (1.5) | DXA | ALMBMI: M: <0.789 | NA | NA | 6‐m GS | ≤0.8 m/s | ||
| 5934 | FNIH 2 | 18 (0.3) | DXA | ALMBMI: M: <0.789 | HGS | M: <26 kg | 6‐m GS | ≤0.8 m/s | ||
| 5934 | Newman | 1186 (20.0) | DXA | Residual of actual ALM minus predicted ALM: ≤−0.204 kg/m2 | NA | NA | NA | NA | ||
| Chalhoub | 2015 | 6658 | EWGSOP | 371 (5.6) | DXA | ALM adjusted for height and fat mass: 20th percentile of the distribution of residuals | HGS | M: <30 kg; F: <20 kg | 6‐m GS | <0.8 m/s |
| Clynes | 2015 | 298 | IWGS | 25 (8.4) | DXA | ALM/ht2: M: ≤7.23 kg/m2; F: ≤5.67 kg/m2 | NA | NA | 3‐m GS | <1.0 m/s |
| 298 | EWGSOP | 10 (3.4) | DXA | SMI: M: ≤7.26 kg/m2; F: ≤5.5 kg/m2 | HGS | M: <30 kg; F: <20 kg | 3‐m GS | ≤0.8 m/s | ||
| 298 | FNIH | 6 (2.0) | DXA | ALMBMI: M: <0.789; F: <0.512 | HGS | M: <26 kg; F: <16 kg | NA | NA | ||
| Dietzel | 2015 | 288 | Baumgartner | 34 (11.8) | DXA | ASM/ht2: M: <7.26 kg/m2; F: <5.5 kg/m2 | NA | NA | NA | NA |
| Gadelha | 2018 | 196 | EWGSOP | 36 (18.4) | DXA | SMM (as % body mass): not specified | Isokinetic muscle torque | Not specified | TUG | Not specified |
| Hars | 2016 | 913 | Baumgartner | 102 (11.2) | DXA | ALM/ht2: M: <7.26 kg/m2; F: <5.45 kg/m2 | NA | NA | NA | NA |
| 913 | Delmonico 1 | 157 (17.2) | DXA | ALM/ht2: M: <7.25 kg/m2; F: <5.67 kg/m2 | NA | NA | NA | NA | ||
| 913 | Delmonico 2 | 184 (20.2) | DXA | Observed ALM minus predicted ALM: <20th percentile of the sex‐specific distribution | NA | NA | NA | NA | ||
| 913 | IWGS | 156 (17.1) | DXA | ALM/ht2: M: ≤7.23 kg/m2; F: ≤5.67 kg/m2 | NA | NA | NA | NA | ||
| 913 | SCWD | 42 (4.6) | DXA | ALM/ht2: M: ≤6.81 kg/m2; F: ≤5.18 kg/m2 | NA | NA | NA | NA | ||
| 913 | FNIH | 32 (3.5) | DXA | ALMBMI: M: <0.789; F: <0.512 | NA | NA | NA | NA | ||
| Henwood | 2017 | 58 | EWGSOP | 23 (40.2) | BIA | SMM/ht2: M: <8.87 kg/m2; F: <6.42 kg/m2 | HGS | M: <30 kg; F: <20 kg | 2.4‐m GS | <0.8 m/s |
| Hida | 2013 | 2868 | Sanada | 1019 (35.5) | DXA | ALM/ht2: M: <6.87 kg/m2; F: <5.46 kg/m2 | NA | NA | NA | NA |
| Hida | 2016 | 1824 | Sanada | 493 (27.0) | DXA | ALM/ht2: F: <5.46 kg/m2 | NA | NA | NA | NA |
| Hong | 2015 | 3077 | Cheng | 966 (31.4) | DXA | SMI: M: <7.01 kg/m2; F: <5.42 kg/m2 | NA | NA | NA | NA |
| Huo | 2015 | 680 | EWGSOP | 345 (50.7) | DXA | ALM/ht2: M: <7.26 kg/m2; F: <5.5 kg/m2 | HGS | M: <30 kg; F: <20 kg | GS | <0.8 m/s |
| Huo | 2016 | 680 | EWGSOP | 380 (55.9) | DXA | ALM/ht2: M: <7.26 kg/m2; F: <5.5 kg/m2 | HGS | M: <30 kg; F: <20 kg | GS | <0.8 m/s |
| Iolascon | 2015 | 121 | FNIH 1 | 10 (8.3) | DXA | ALMBMI: F: <0.512 | HGS | F: ≥16 | 4‐m GS | ≤0.8 m/s |
| FNIH 2 | 13 (10.7) | DXA | ALMBMI: F: <0.512 | HGS | F: <16 | 4‐m GS | ≤0.8 m/s | |||
| Landi | 2012 | 260 | EWGSOP | 66 (25.4) | MAMC | M: <21.1 cm; F: <19.2 cm | HGS | M: <30 kg; F: <20 kg | 4‐m GS | <0.8 m/s |
| Lera | 2017 | 1006 | EWGSOP | 192 (19.1) | DXA | ASM/ht2: M: <7.19 kg/m2; F: <5.77 kg/m2 | HGS | M: ≤27 kg; F: ≤15 kg | 3‐m GS | <0.8 m/s |
| Locquet | 2018 | 288 | EWGSOP | 43 (14.9) | DXA | AMM/ht2: M: <7.26 kg/m2; F: <5.50 kg/m2 | HGS | M: <30 kg; F: <20 kg | SPPB | <8 points |
| Martinez | 2015 | 110 | EWGSOP | 24 (21.8) | Lee equation | SMM/ht2: M: ≤8.90 kg/m2; F: ≤6.37 kg/m2 | HGS | M: <30 kg; F: <20 kg | 6‐m GS | ≤0.8 m/s |
| Matsumoto | 2017 | 162 | AWGS | 9 (5.6) | BIA | M: <7.0 kg/m2; F: <5.7 kg/m2 | HGS | M: <26 kg; F: <18 kg | 5‐m GS | ≤0.8 m/s |
| Menant | 2017 | 410 | EWGSOP | 88 (21.5) | DXA | ASM/ht2: M: <7.2 kg/m2; F: <5.5 kg/m2 | HGS | M: <30 kg; F: <20 kg | 6‐m GS | ≤0.8 m/s |
| 419 | Baumgartner | 97 (23.2) | DXA | ASM/ht2: M: <7.26 kg/m2; F: <5.45 kg/m2 | NA | NA | NA | NA | ||
| 419 | Scott | 139 (33.2) | DXA | Bottom tertile of the residuals from the regression of ALM (g) on height (m) and fat mass (g): M: <326.4; F: <2217.8 | NA | NA | NA | NA | ||
| 419 | Levine & Crimmins | 57 (13.6) | DXA | ALM (as % body mass): M: <25.72%; F: <19.43% | NA | NA | NA | NA | ||
| Menant | 2017 | 419 | Bouchard | 306 (73.0) | DXA | ASM/ht2: M: <8.51 kg/m2; F: <6.29 kg/m2 | NA | NA | NA | NA |
| 314 | HGS‐based | 127 (40.4) | NA | NA | HGS | M: <30 kg; F: <20 kg | NA | NA | ||
| 419 | KES‐based | 84 (20.0) | NA | NA | KES | M: <23.64 kg; F: <15.24 kg | NA | NA | ||
| Meng | 2015 | 771 | EWGSOP 1 | 44 (5.7) | DXA | ALM/ht2: M: <6.39 kg/m2; F: <4.84 kg/m2 | HGS | M: <30 kg; F: <20 kg | 5‐m GS | <0.8 m/s |
| EWGSOP 2 | 75 (9.7) | DXA | ALM (as % body mass): M: <27.1%; F: <22.3% | HGS | M: <30 kg; F: <20 kg | 5‐m GS | <0.8 m/s | |||
| Schaap | 2018 | 496 | EWGSOP | 158 (31.9) | DXA | ASM/ht2: M: ≤7.26 kg/m2; F: ≤ 5.45 kg/m2 | HGS | M: <30 kg; F: <20 kg | GS (walk 3 m, a turn of 180° and walk the 3 m) | ≤0.8 m/s |
| FNIH 1 | 39 (7.9) | DEXA | M: <19.75 kg; F: <15.02 kg | HGS | M: <26 kg; F: <16 kg | NA | NA | |||
| FNIH 2 | 31 (6.3) | DEXA | M: <19.75 kg; F: <15.02 kg | HGS | M: <26 kg; F: <16 kg | GS (walk 3 m, a turn of 180° and walk the 3 m) | ≤0.8 m/s | |||
| Scott | 2017 | 1486 | EWGSOP | 237 (15.9) | DXA | ALM/ht2: M: <7.25 kg/m2 | HGS | M: <30 kg | 6‐m GS | ≤0.8 m/s |
| 1486 | FNIH | 119 (8.0) | DXA | ALMBMI: M: <0.789 | HGS | M: <26 kg | NA | NA | ||
| Steihaug | 2018 | 201 | EWGSOP | 77 (38.3) | Heymsfield formula using anthropometry to estimate ALM (Kim et al. formula) | ALM/ht2: M: ≤7.25 kg/m2; F: ≤5.67 kg/m2 | HGS | M: ≤30 kg; F: ≤20 kg | Questionnaire (new mobility score) | <5 points |
| Sjöblom | 2013 | 590 | NG | 69 (11.7) | DXA | Relative SMI: F: <6.3 kg/m2 | HGS | F: <22.3 kPA | 10‐m GS | F: >7 s |
| Tanimoto | 2014 | 1110 | EWGSOP | 160 (14.4) | BIA | AMM/ht2: M: <7.0 kg/m2; F: <5.8 kg/m2 | HGS | Lowest HGS quartile | 5‐m GS | Slowest GS quartile |
| Trajanoska | 2018 | 5911 | EWGSOP | 260 (4.4) | DXA | ALM/ht2: M: ≤7.25 kg/m2; F: ≤5.67 kg/m2 | HGS | M: ≤29 kg (if BMI ≤ 24); ≤30 kg (if BMI ≤ 24.1–28); ≤32 kg (if BMI > 28); F: ≤17 kg (if BMI ≤ 23); ≤17.3 kg (if BMI ≤ 23.1–26), ≤18 kg (BMI ≤ 26.1–29), ≤21 kg (if BMI > 29) | 5.79‐m GS | M: <0.65 m/s (if height ≤ 173 cm) or <0.76 m/s (if height > 173 cm); F: <0.65 m/s (if height ≤ 159 cm) or <0.76 m/s (if height > 159 cm) |
| Van Puyenbroeck | 2012 | 276 | NG | 67 (24.3) | BIA | SM/ht2: M: 8.058 kg/m2; F: 6.154 kg/m2 | NA | NA | NA | NA |
| 276 | NG | 225 (81.5) | BIA | SM/weight × 100: M: <33.94; F: <24.76 | NA | NA | NA | NA | ||
| 276 | NG | 178 (64.5) | BIA | SM: M: <25.99 kg; F: <16.15 kg | NA | NA | NA | NA | ||
| Woo | 2014 | 2848 | Kim | 1404 (49.3) | DXA | ASM/weight: M: <29.9%; F: <25.1% | NA | NA | NA | NA |
| Yamada | 2013 | 1882 | EWGSOP | 414 (22.0) | BIA | Appendicular SMM/ht2: M: <6.75 kg/m2; F: <5.07 kg/m2 | HGS | M: <30 kg; F: <20 kg | 10‐m GS | <0.8 m/s |
| Yoo | 2016 | 1970 | AWGS | 352 (17.8) | DXA | SMM/ht2: M: <7.0 kg/m2; F: <5.4 kg/m2 | NA | NA | NA | NA |
| 1970 | EWGSOP | 439 (22.3) | DXA | SMM/ht2: M: <7.26 kg/m2; F: <5.5 kg/m2 | NA | NA | NA | NA | ||
| Yoshimura | 2018 | 637 | AWGS | 343 (53.0) | BIA | SM/ht2: M: <7.0 kg/m2; F: <5.7 kg/m2 | HGS | M: <26 kg; F: <18 kg | NA | NA |
| Yu | 2014 | 4000 | AWGS | 293 (7.3) | DXA | ASM/ht2: M: <7.0 kg/m2; F: <5.4 kg/m2 | HGS | M: <26 kg; F: <18 kg | 6‐m GS | <0.8 m/s |
ALM, appendicular lean mass; AMM, appendicular muscle mass; ASM, appendicular skeletal muscle mass; AWGS, Asia Working Group for Sarcopenia; BIA, bioelectrical impedance analysis; BMI, body mass index; DXA, dual energy X‐ray absorptiometry; EWGSOP, European Working Group on Sarcopenia in Older People; F, females; FNIH, Foundation for the National Institutes of Health; GS, gait speed; HGS, handgrip strength; ht, height; IWGS, International Working Group on Sarcopenia; KES, knee extension strength; M, males; MAMC, mid‐arm muscle circumference; N, sample size; NA, not applicable; NG, not given; SCWD, Society for Sarcopenia, Cachexia, and Wasting Disorders; SM, skeletal muscle; SMM, skeletal muscle mass; SMI, skeletal muscle index; SPPB, short physical performance battery; TUG, Timed Up & Go.
Study quality
Online Resource S4 shows the results of the NOS quality assessment of the included studies. The quality of 12 falls studies24, 26, 33, 35, 37, 41, 45, 48, 53, 72, 73, 75 and 14 fracture studies18, 25, 27, 29, 30, 31, 34, 35, 45, 49, 51, 54, 55, 73 was rated high. Ten studies for falls were rated as low quality.28, 32, 34, 36, 40, 43, 44, 49, 50, 74 Five studies for fractures were rated as low quality.38, 39, 42, 46, 47
Association of sarcopenia with falls
Twenty‐two studies investigated the association of sarcopenia and falls, of which 10 studies (45%) reported higher risks of falls among sarcopenic individuals compared with non‐sarcopenic individuals.28, 34, 40, 41, 48, 50, 53, 72, 73, 75 Non‐significant associations between sarcopenia and falls were found in the remaining 12 studies.24, 26, 32, 33, 35, 36, 37, 43, 44, 45, 49, 74
Among the 20 studies included in the meta‐analysis, a pooled OR of 1.60 for cross‐sectional studies (95% CI 1.37–1.86, P < 0.001, I 2 = 34%) and a pooled OR of 1.89 for prospective studies (95% CI 1.33–2.68, P < 0.001, I 2 = 37%) indicated a significantly higher risk of falls for sarcopenic compared with non‐sarcopenic individuals (Figure 1 A). The results of the subgroup analyses are presented in Figure 1 A–F. The significant association between sarcopenia and falls was independent of study design (Figure 1 A), study population (Figure 1 B), and sex (Figure 1 C). When stratified by sarcopenia definition, sarcopenia diagnosed by use of EWGSOP (OR 1.62, 95% CI 1.38–1.90, P < 0.001), Baumgartner (OR 1.50, 95% CI 1.07–2.12, P = 0.020), and IWGS (OR 2.02, 95% CI 1.09–3.74, P = 0.025) definitions was significantly associated with falls, but the association was insignificant for the FNIH definition (two studies) (OR 0.67, 95% CI 0.26–1.77, P = 0.422) (Figure 1 D). The significant association between sarcopenia and falls was independent of continent (Figure 1 E) and study quality (Figure 1 F).
Figure 1.
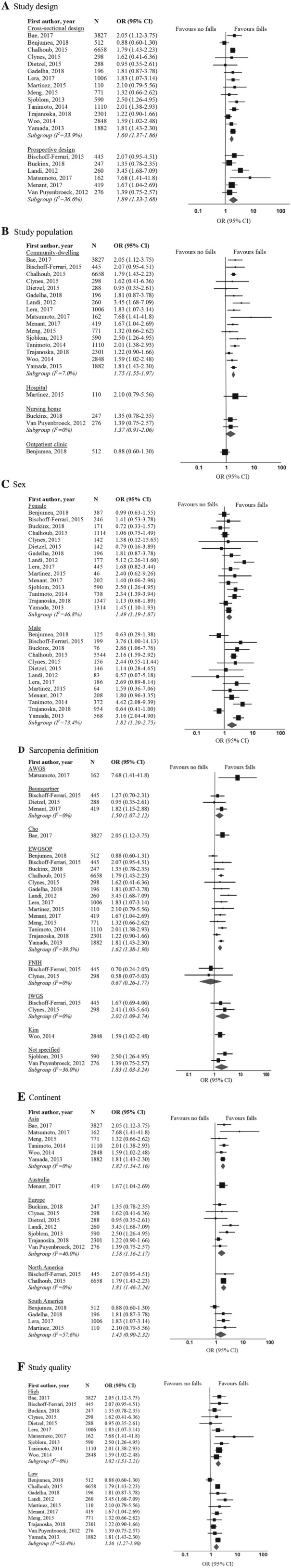
Forest plots of odds ratio for falls in sarcopenic individuals vs. non‐sarcopenic individuals, stratified by (A) study design; (B) study population; (C) sex; (D) sarcopenia definition; (E) continent; and (F) study quality. AWGS, Asia Working Group for Sarcopenia; CI, confidence interval; EWGSOP, European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; IWGS, International Working Group on Sarcopenia; OR, odds ratio.
Association of sarcopenia with fractures
Nineteen studies investigated the association of sarcopenia and fractures. Higher risks of fractures were reported in 11 studies (58%) among sarcopenic individuals compared with non‐sarcopenic individuals.18, 27, 29, 30, 31, 34, 39, 46, 49, 51, 73 Non‐significant associations between sarcopenia and fractures were found in eight studies.25, 35, 38, 42, 45, 47, 54, 55
Among the 17 studies included in the meta‐analysis, a significantly higher risk of fractures was found for sarcopenic compared with non‐sarcopenic individuals (cross‐sectional studies: pooled OR 1.84, 95% CI 1.30–2.62, P = 0.001, I 2 = 91%; prospective studies: pooled OR 1.71, 95% CI 1.44–2.03, P = 0.011, I 2 = 0%) (Figure 2 A). The association between sarcopenia and fractures remained significant when excluding one particular study with large CIs,51 and heterogeneity decreased from 91% to 10%. The results of the subgroup analysis are presented in Figure 2 A–F. The significant association between sarcopenia and fractures was independent of study design (Figure 2 A), study population (Figure 2 B), and sex (Figure 2 C). Sarcopenia diagnosed by use of EWGSOP (OR 1.93, 95% CI 1.19–3.13, P = 0.008) and Sanada et al. (OR 1.66, 95% CI 1.26–2.18, P < 0.001) definitions was associated with fractures, while the association between sarcopenia and fractures was not significant for sarcopenia diagnosed with AWGS (3 studies), FNIH (3 studies), and IWGS (2 studies) definitions (Figure 2 D). The significant association between sarcopenia and fractures was independent of continent (Figure 2 E) and study quality (Figure 2 F).
Figure 2.
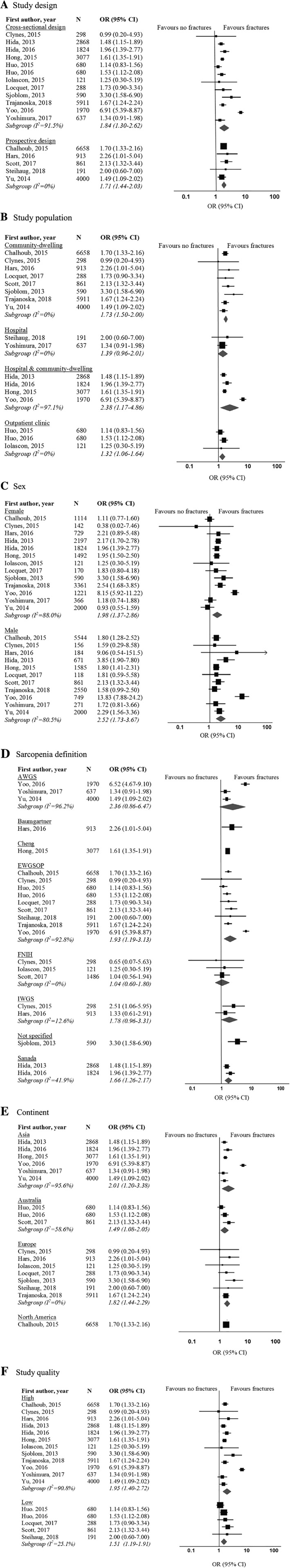
Forest plots of odds ratio for fractures in sarcopenic individuals vs. non‐sarcopenic individuals, stratified by (A) study design; (B) study population; (C) sex; (D) sarcopenia definition; (E) continent; and (F) study quality. AWGS, Asia Working Group for Sarcopenia; CI, confidence interval; EWGSOP, European Working Group on Sarcopenia in Older People; FNIH, Foundation for the National Institutes of Health; IWGS, International Working Group on Sarcopenia; OR, odds ratio.
Publication bias
Asymmetry was observed by visual inspection of funnel plots (Online Resource S5). However, Egger's regression test (P = 0.463 for falls and P = 0.928 for fractures) and Begg's test (P = 0.627 for falls and P = 0.232 for fractures) indicated no statistically significant publication bias among the studies in this meta‐analysis.
Discussion
This systematic review and meta‐analysis highlights the positive association between sarcopenia, falls, and fractures; this was independent of study design, population, sex, sarcopenia definition, continent, and study quality.
This is the first meta‐analysis examining the association between sarcopenia, falls, and fractures among older adults including various definitions of sarcopenia. A meta‐analysis76 published in 2004 showed a positive association between muscle strength and falls; since then, the literature has expanded substantially. A previous systematic review assessing various health outcomes of sarcopenia showed positive associations but was based on the EWGSOP definition only.14 A recently published meta‐analysis (9 studies)77 has found a significant association between sarcopenia and fractures with a smaller pooled effect size (risk ratio 1.34) compared with the subgroup analysis for community‐dwelling older adults (OR: 1.73, 95% CI: 1.50–2.00) in our meta‐analysis. The previous study included only prospective studies in community‐dwelling older adults aged 60 years, which contrasts our review addressing both prospective studies and cross‐sectional studies in adults aged 65 years and older.
Evidence was found for both cross‐sectional and prospective studies, implying the existence of different directions of causal pathways, that is, sarcopenia as a cause for falls and fractures, and falls and fractures as a cause for sarcopenia. Falls and fractures can result in loss of mobility, fear of falling, and hospital admissions.78 Physical inactivity associated with these consequences accelerates loss of muscle mass and muscle strength.79 This may explain the results from cross‐sectional studies in which sarcopenic individuals had higher risk of retrospective falls and fractures compared with non‐sarcopenic individuals. On the other hand, impaired standing balance is a strong risk factor for falls.80 The ability to maintain balance requires interaction of motor (muscle), nervous, and sensory systems.81 Muscle strength and muscle mass have been shown to be positively associated with the ability to maintain standing balance in older adults,15, 82 which may explain the positive associations between sarcopenia and falls/fractures in the prospective studies.
Most of the studies included in this systematic review and meta‐analysis were conducted among community‐dwelling individuals. Three included studies examined the association between sarcopenia and falls among nursing home residents33, 37, 74 and one study among hospitalized patients,43 but no associations were found. In these specific populations, sarcopenia as a risk for falls may be overshadowed by other high prevalent risk factors such as the number of diseases, urinary incontinence, polypharmacy, and antidepressant use.83
Sarcopenia is mainly prevalent in older adults compared with younger ages, where disease pathology is likely to be different. Muscle mass loss is multifactorial. Lifestyle behaviours such as physical inactivity and poor diet are important contributors to the loss of muscle mass and strength at any age, and also, genetic contributions have been described.84 With the aging process, other contributing factors include state of chronic inflammation,85 functional and structural decline of the neuromuscular systems, lower muscle turnover and repair capacity due to decreased muscle protein synthesis, and altered endocrine function.86, 87, 88, 89, 90
Our study showed that the positive association between sarcopenia with falls and fractures was independent of most of the applied sarcopenia definitions. However, using the EWGSOP and IWGS definitions, which include low physical performance and/or grip strength in addition to low muscle mass in their diagnostic algorithm,24 higher risks of falls and fractures among sarcopenic individuals compared with non‐sarcopenic individuals were shown. This indicates that low muscle function has an additional role in the association with falls and fractures compared with muscle mass alone. Cross‐sectional analysis among 3493 non‐institutionalized older adults found that low muscle mass and low muscle function are independent risk factors for losing physical independence in later life. However, individuals with both low muscle mass and low muscle function presented the highest risk for losing physical independence.91 In addition, a prospective study suggested that muscle strength rather than muscle mass at baseline was associated with increased falls risk score and fracture incidence at 10 years follow‐up in community‐dwelling older adults.92
This highlights the importance of muscle strength or physical performance in the sarcopenia definition, in line with current definitions.58, 59, 61, 62, 68, 93 However, literatures also showed the value of including muscle mass in sarcopenia definitions. Muscle mass but not muscle strength or physical performance was associated with bone mineral density94 and insulin resistance.95 This reflects the complex role of muscle as not only a strength generator but also an important organ performing protein storage, glucose regulation, hormone production, and other cellular mechanisms.96 A discussion on the use of a single diagnostic criterion or a combination of diagnostic criteria for sarcopenia should take into account which criterion has the strongest predictive value on clinical outcomes.
High heterogeneity was found for the association between sarcopenia and fractures. This heterogeneity can largely be attributed to one specific study, which included a combination of 359 hospitalized patients with fracture and 1614 community‐dwelling older individuals as control group in the same study population.51 In that study, the hospitalized patients were older than the control group. Because the prevalence of sarcopenia is higher with age,97 the association between sarcopenia and fractures may be overestimated, which is further underpinned by a high crude OR of the association between sarcopenia and fractures. Note that the association between sarcopenia and fractures remained significant after excluding aforementioned study from the meta‐analysis.
Clinical implications
The robust outcome from our meta‐analysis that sarcopenic individuals have a significantly higher risk of falls and fractures compared with non‐sarcopenic individuals stresses the urgency for timely diagnosis and treatment of sarcopenia as a modifiable risk factor for falls and fractures. Interventions aimed at slowing down the decline of muscle mass and muscle strength and at treating sarcopenia should be considered. Current evidence suggests that progressive resistance training improves risk factors for falls and fractures such as muscle function, balance, and functional mobility.16 However, it is unclear if the effect of progressive resistance training translates directly into a reduction in incidence of falls and fractures.98 Further randomized controlled trials examining the effect of progressive resistance training on falls and fractures outcomes are warranted.
Strengths and limitations
In the absence of an international consensus definition of sarcopenia, we included studies with different diagnostic criteria of sarcopenia. In cases of missing data, we contacted authors of studies to obtain the data needed to compute ORs.
A limitation of the present review was that results of the included studies were expressed as crude as well as adjusted ORs with varying adjustments. The inconsistency in reporting effect size might have either overestimated or underestimated the overall association of interest. In addition, most of the studies included in the systematic review and meta‐analysis were conducted among community‐dwelling individuals and a limited number of institutionalized individuals. Subgroup analysis by continent was conducted instead of ethnicity because data stratified by ethnicity was not available.
Conclusions
This systematic review and meta‐analysis highlights the positive association between sarcopenia, falls, and fractures. These findings are independent of study design, population, sex, sarcopenia definition, continent, and study quality. This strengthens the need to invest in studies evaluating sarcopenia prevention and intervention programmes on its effect on falls and fractures.
Conflict of interest
S.S.Y.Y., E.M.R., V.K.P., M.C.T., W.K.L., C.G.M.M., and A.B.M. declare that they have no conflict of interest.
Funding
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie‐Sklodowska‐Curie grant agreement no. 675003 (PANINI programme) and no. 689238 (PreventIT). The funders had no role in the design and conduct of the study, data collection and analysis, interpretation of data, or preparation of the manuscript.
Ethical approval
Ethical approval not required.
Supporting information
Online Resource S1: Search strategy.
Online Resource S2: Newcastle‐Ottawa Scale quality assessment explanation.
Online Resource S3: Flow chart of study selection.
Online Resource S4: Results of the Newcastle‐Ottawa Scale quality assessment for (a) falls and (b) fractures.
Online Resource S5: Funnel plots showing the association between sarcopenia with (a) falls and (b) fractures.
Acknowledgements
The authors certify that they comply with the ethical guidelines for authorship and publishing of the Journal of Cachexia, Sarcopenia and Muscle.99
Yeung S. S. Y., Reijnierse E. M., Pham V. K., Trappenburg M. C., Lim W. K., Meskers C. G. M., and Maier A. B. (2019) Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta‐analysis, Journal of Cachexia, Sarcopenia and Muscle, 10: 485–500. 10.1002/jcsm.12411.
References
- 1. Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged >/=65 years—United States, 2014. MMWR Morb Mortal Wkly Rep 2016;65:993–998. [DOI] [PubMed] [Google Scholar]
- 2. Morrison A, Fan T, Sen SS, Weisenfluh L. Epidemiology of falls and osteoporotic fractures: a systematic review. Clinicoecon Outcomes Res 2013;5:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Padron‐Monedero A, Damian J, Pilar Martin M, Fernandez‐Cuenca R. Mortality trends for accidental falls in older people in Spain, 2000–2015. BMC Geriatr 2017;17:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Terroso M, Rosa N, Torres Marques A, Simoes R. Physical consequences of falls in the elderly: a literature review from 1995 to 2010. Eur Rev Aging Phys Act 2014;11:51–59. [Google Scholar]
- 5. Welmer AK, Rizzuto D, Laukka EJ, Johnell K, Fratiglioni L. Cognitive and physical function in relation to the risk of injurious falls in older adults: a population‐based study. J Gerontol A Biol Sci Med Sci 2017;72:669–675. [DOI] [PubMed] [Google Scholar]
- 6. Gale CR, Westbury LD, Cooper C, Dennison EM. Risk factors for incident falls in older men and women: the English longitudinal study of ageing. BMC Geriatr 2018;18:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community‐dwelling older people: a systematic review and meta‐analysis. Epidemiology 2010;21:658–668. [DOI] [PubMed] [Google Scholar]
- 8. Guirguis‐Blake JM, Michael YL, Perdue LA, Coppola EL, Beil TL. Interventions to prevent falls in older adults: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2018;319:1705–1716. [DOI] [PubMed] [Google Scholar]
- 9. Beaudart C, Dawson A, Shaw SC, Harvey NC, Kanis JA, Binkley N, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int 2017;28:1817–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shafiee G, Keshtkar A, Soltani A, Ahadi Z, Larijani B, Heshmat R. Prevalence of sarcopenia in the world: a systematic review and meta‐analysis of general population studies. J Diabetes Metab Disord 2017;16:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reijnierse EM, Trappenburg MC, Leter MJ, Blauw GJ, Sipila S, Sillanpaa E, et al. The impact of different diagnostic criteria on the prevalence of sarcopenia in healthy elderly participants and geriatric outpatients. Gerontology 2015;61:491–496. [DOI] [PubMed] [Google Scholar]
- 12. Bijlsma AY, Meskers CGM, Ling CHY, Narici M, Kurrle SE, Cameron ID, et al. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woo J, Leung J, Morley JE. Defining sarcopenia in terms of incident adverse outcomes. J Am Med Dir Assoc 2015;16:247–252. [DOI] [PubMed] [Google Scholar]
- 14. Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health outcomes of sarcopenia: a systematic review and meta‐analysis. PLoS ONE 2017;12:e0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bijlsma AY, Pasma JH, Lambers D, Stijntjes M, Blauw GJ, Meskers CGM, et al. Muscle strength rather than muscle mass is associated with standing balance in elderly outpatients. J Am Med Dir Assoc 2013;14:493–498. [DOI] [PubMed] [Google Scholar]
- 16. Papa EV, Dong X, Hassan M. Resistance training for activity limitations in older adults with skeletal muscle function deficits: a systematic review. Clin Interv Aging 2017;12:955–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med 2009;151:264–269, W64. [DOI] [PubMed] [Google Scholar]
- 18. Yu R, Leung J, Woo J. Sarcopenia combined with FRAX probabilities improves fracture risk prediction in older Chinese men. J Am Med Dir Assoc 2014;15:918–923. [DOI] [PubMed] [Google Scholar]
- 19. Yu R, Leung J, Woo J. Incremental predictive value of sarcopenia for incident fracture in an elderly Chinese cohort: results from the Osteoporotic Fractures in Men (MrOs) study. J Am Med Dir Assoc 2014;15:551–558. [DOI] [PubMed] [Google Scholar]
- 20. Lo CK, Mertz D, Loeb M. Newcastle‐Ottawa Scale: comparing reviewers' to authors' assessments. BMC Med Res Methodol 2014;14:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐analyses. Ottawa Hospital Research Institue: University of Ottawa, Ottawa, Ontario, Canada; 2001. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 15 Aug 2018. [Google Scholar]
- 22. Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282:1054–1060. [DOI] [PubMed] [Google Scholar]
- 23. Hermont AP, Oliveira PAD, Martins CC, Paiva SM, Pordeus IA, Auad SM. Tooth erosion and eating disorders: a systematic review and meta‐analysis. PLoS ONE 2014;9:e111123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bischoff‐Ferrari HA, Orav JE, Kanis JA, Rizzoli R, Schlogl M, Staehelin HB, et al. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community‐dwelling seniors age 65 and older. Osteoporos Int 2015;26:2793–2802. [DOI] [PubMed] [Google Scholar]
- 25. Cawthon PM, Blackwell TL, Cauley J, Kado DM, Barrett‐Connor E, Lee CG, et al. Evaluation of the usefulness of consensus definitions of sarcopenia in older men: results from the Observational Osteoporotic Fractures in Men cohort study. J Am Geriatr Soc 2015;63:2247–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dietzel R, Felsenberg D, Armbrecht G. Mechanography performance tests and their association with sarcopenia, falls and impairment in the activities of daily living—a pilot cross‐sectional study in 293 older adults. J Musculoskelet Neuronal Interact 2015;15:249–256. [PMC free article] [PubMed] [Google Scholar]
- 27. Hars M, Biver E, Chevalley T, Herrmann F, Rizzoli R, Ferrari S, et al. Low lean mass predicts incident fractures independently from FRAX: a prospective cohort study of recent retirees. J Bone Miner Res 2016;31:2048–2056. [DOI] [PubMed] [Google Scholar]
- 28. Menant JC, Weber F, Lo J, Sturnieks DL, Close JC, Sachdev PS, et al. Strength measures are better than muscle mass measures in predicting health‐related outcomes in older people: time to abandon the term sarcopenia? Osteoporos Int 2017;28:59–70. [DOI] [PubMed] [Google Scholar]
- 29. Hong W, Cheng Q, Zhu X, Zhu H, Li H, Zhang X, et al. Prevalence of sarcopenia and its relationship with sites of fragility fractures in elderly Chinese men and women. PLoS ONE 2015;10:e0138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hida T, Ishiguro N, Shimokata H, Sakai Y, Matsui Y, Takemura M, et al. High prevalence of sarcopenia and reduced leg muscle mass in Japanese patients immediately after a hip fracture. Geriatr Gerontol Int 2013;13:413–420. [DOI] [PubMed] [Google Scholar]
- 31. Hida T, Shimokata H, Sakai Y, Ito S, Matsui Y, Takemura M, et al. Sarcopenia and sarcopenic leg as potential risk factors for acute osteoporotic vertebral fracture among older women. Eur Spine J 2016;25:3424–3431. [DOI] [PubMed] [Google Scholar]
- 32. Benjumea AM, Curcio CL, Duque G, Gomez F. Dynapenia and sarcopenia as a risk factor for disability in a falls and fractures clinic in older persons. Open Access Maced J Med Sci 2018;6:344–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buckinx F, Croisier JL, Reginster JY, Lenaerts C, Brunois T, Rygaert X, et al. Prediction of the incidence of falls and deaths among elderly nursing home residents: the SENIOR study. J Am Med Dir Assoc 2018;19:18–24. [DOI] [PubMed] [Google Scholar]
- 34. Chalhoub D, Cawthon PM, Ensrud KE, Stefanick ML, Kado DM, Boudreau R, et al. Risk of nonspine fractures in older adults with sarcopenia, low bone mass, or both. J Am Geriatr Soc 2015;63:1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clynes MA, Edwards MH, Buehring B, Dennison EM, Binkley N, Cooper C. Definitions of sarcopenia: associations with previous falls and fracture in a population sample. Calcif Tissue Int 2015;97:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gadelha AB, Neri SGR, de Oliveira RJ, Bottaro M, de David AC, Vainshelboim B, et al. Severity of sarcopenia is associated with postural balance and risk of falls in community‐dwelling older women. Exp Aging Res 2018;44:258–269. [DOI] [PubMed] [Google Scholar]
- 37. Henwood T, Hassan B, Swinton P, Senior H, Keogh J. Consequences of sarcopenia among nursing home residents at long‐term follow‐up. Geriatr Nurs 2017;38:406–411. [DOI] [PubMed] [Google Scholar]
- 38. Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Muir SW, et al. Phenotype of osteosarcopenia in older individuals with a history of falling. J Am Med Dir Assoc 2015;16:290–295. [DOI] [PubMed] [Google Scholar]
- 39. Huo YR, Suriyaarachchi P, Gomez F, Curcio CL, Boersma D, Gunawardene P, et al. Phenotype of sarcopenic obesity in older individuals with a history of falling. Arch Gerontol Geriatr 2016;65:255–259. [DOI] [PubMed] [Google Scholar]
- 40. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr 2012;31:652–658. [DOI] [PubMed] [Google Scholar]
- 41. Lera L, Albala C, Sanchez H, Angel B, Hormazabal MJ, Marquez C, et al. Prevalence of sarcopenia in community‐dwelling Chilean elders according to an adapted version of the European Working Group on Sarcopenia in Older People (EWGSOP) criteria. J Frailty Aging 2017;6:12–17. [DOI] [PubMed] [Google Scholar]
- 42. Locquet M, Beaudart C, Bruyere O, Kanis JA, Delandsheere L, Reginster JY. Bone health assessment in older people with or without muscle health impairment. Osteoporos Int 2018;29:1057–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martinez BP, Batista AK, Gomes IB, Olivieri FM, Camelier FW, Camelier AA. Frequency of sarcopenia and associated factors among hospitalized elderly patients. BMC Musculoskelet Disord 2015;16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Meng NH, Li CI, Liu CS, Lin CH, Lin WY, Chang CK, et al. Comparison of height‐ and weight‐adjusted sarcopenia in a Taiwanese metropolitan older population. Geriatr Gerontol Int 2015;15:45–53. [DOI] [PubMed] [Google Scholar]
- 45. Schaap LA, van Schoor NM, Lips P, Visser M. Associations of sarcopenia definitions, and their components, with the incidence of recurrent falling and fractures; the Longitudinal Aging Study Amsterdam. J Gerontol A Biol Sci Med Sci 2018;73:1199–1204. [DOI] [PubMed] [Google Scholar]
- 46. Scott D, Seibel M, Cumming R, Naganathan V, Blyth F, Le Couteur DG, et al. Sarcopenic obesity and its temporal associations with changes in bone mineral density, incident falls, and fractures in older men: the Concord Health and Ageing in Men Project. J Bone Miner Res 2017;32:575–583. [DOI] [PubMed] [Google Scholar]
- 47. Steihaug OM, Gjesdal CG, Bogen B, Kristoffersen MH, Lien G, Hufthammer KO, et al. Does sarcopenia predict change in mobility after hip fracture? A multicenter observational study with one‐year follow‐up. BMC Geriatr 2018;18:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tanimoto Y, Watanabe M, Sun W, Sugiura Y, Hayashida I, Kusabiraki T, et al. Sarcopenia and falls in community‐dwelling elderly subjects in Japan: Defining sarcopenia according to criteria of the European Working Group on Sarcopenia in Older People. Arch Gerontol Geriatr 2014;59:295–299. [DOI] [PubMed] [Google Scholar]
- 49. Trajanoska K, Schoufour JD, Darweesh SK, Benz E, Medina‐Gomez C, Alferink LJ, et al. Sarcopenia and its clinical correlates in the general population: the Rotterdam study. J Bone Miner Res 2018;33:1209–1218. [DOI] [PubMed] [Google Scholar]
- 50. Yamada M, Nishiguchi S, Fukutani N, Tanigawa T, Yukutake T, Kayama H, et al. Prevalence of sarcopenia in community‐dwelling Japanese older adults. J Am Med Dir Assoc 2013;14:911–915. [DOI] [PubMed] [Google Scholar]
- 51. Yoo JI, Ha YC, Kwon HB, Lee YK, Koo KH, Yoo MJ. High prevalence of sarcopenia in Korean patients after hip fracture: a case–control study. J Korean Med Sci 2016;31:1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010;39:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Matsumoto H, Tanimura C, Tanishima S, Osaki M, Noma H, Hagino H. Sarcopenia is a risk factor for falling in independently living Japanese older adults: a 2‐year prospective cohort study of the GAINA study. Geriatr Gerontol Int 2017;17:2124–2130. [DOI] [PubMed] [Google Scholar]
- 54. Yoshimura Y, Wakabayashi H, Bise T, Tanoue M. Prevalence of sarcopenia and its association with activities of daily living and dysphagia in convalescent rehabilitation ward inpatients. Clin Nutr 2018;37:2022–2028. [DOI] [PubMed] [Google Scholar]
- 55. Iolascon G, Moretti A, Giamattei MT, Migliaccio S, Gimigliano F. Prevalent fragility fractures as risk factor for skeletal muscle function deficit and dysmobility syndrome in post‐menopausal women. Aging Clin Exp Res 2015;27:S11–S16. [DOI] [PubMed] [Google Scholar]
- 56. Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 1998;147:755–763. [DOI] [PubMed] [Google Scholar]
- 57. Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc 2003;51:1602–1609. [DOI] [PubMed] [Google Scholar]
- 58. Studenski SA, Peters KW, Alley DE, Cawthon PM, McLean RR, Harris TB, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci 2014;69:547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc 2011;12:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 2007;55:769–774. [DOI] [PubMed] [Google Scholar]
- 61. Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, et al. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc 2011;12:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Muscaritoli M, Anker SD, Argiles J, Aversa Z, Bauer JM, Biolo G, et al. Consensus definition of sarcopenia, cachexia and pre‐cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia‐anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin Nutr 2010;29:154–159. [DOI] [PubMed] [Google Scholar]
- 63. Cheng Q, Zhu X, Zhang X, Li H, Du Y, Hong W, et al. A cross‐sectional study of loss of muscle mass corresponding to sarcopenia in healthy Chinese men and women: reference values, prevalence, and association with bone mass. J Bone Miner Metab 2014;32:78–88. [DOI] [PubMed] [Google Scholar]
- 64. Scott D, Sanders KM, Aitken D, Hayes A, Ebeling PR, Jones G. Sarcopenic obesity and dynapenic obesity: 5‐year associations with falls risk in middle‐aged and older adults. Obesity (Silver Spring) 2014;22:1568–1574. [DOI] [PubMed] [Google Scholar]
- 65. Levine ME, Crimmins EM. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring) 2012;20:2101–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bouchard DR, Dionne IJ, Brochu M. Sarcopenic/obesity and physical capacity in older men and women: data from the Nutrition as a Determinant of Successful Aging (NuAge)—the Quebec longitudinal study. Obesity (Silver Spring) 2009;17:2082–2088. [DOI] [PubMed] [Google Scholar]
- 67. Sanada K, Miyachi M, Tanimoto M, Yamamoto K, Murakami H, Okumura S, et al. A cross‐sectional study of sarcopenia in Japanese men and women: reference values and association with cardiovascular risk factors. Eur J Appl Physiol 2010;110:57–65. [DOI] [PubMed] [Google Scholar]
- 68. Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc 2014;15:95–101. [DOI] [PubMed] [Google Scholar]
- 69. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–1101. [PubMed] [Google Scholar]
- 72. Bae EJ, Kim YH. Factors affecting sarcopenia in Korean adults by age groups. Osong Public Health Res Perspect 2017;8:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sjoblom S, Suuronen J, Rikkonen T, Honkanen R, Kroger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 2013;75:175–180. [DOI] [PubMed] [Google Scholar]
- 74. Van Puyenbroeck K, Roelandts L, Van Deun T, Van Royen P, Verhoeven V. The additional value of bioelectrical impedance analysis‐derived muscle mass as a screening tool in geriatric assessment for fall prevention. Gerontology 2012;58:407–412. [DOI] [PubMed] [Google Scholar]
- 75. Woo N, Kim SH. Sarcopenia influences fall‐related injuries in community‐dwelling older adults. Geriatr Nurs 2014;35:279–282. [DOI] [PubMed] [Google Scholar]
- 76. Moreland JD, Richardson JA, Goldsmith CH, Clase CM. Muscle weakness and falls in older adults: a systematic review and meta‐analysis. J Am Geriatr Soc 2004;52:1121–1129. [DOI] [PubMed] [Google Scholar]
- 77. Zhang Y, Hao Q, Ge M, Dong B. Association of sarcopenia and fractures in community‐dwelling older adults: a systematic review and meta‐analysis of cohort studies. Osteoporos Int 2018;29:1253–1262. [DOI] [PubMed] [Google Scholar]
- 78. Araujo AHN, Patricio A, Ferreira MAM, Rodrigues BFL, Santos TDD, Rodrigues TDB, et al. Falls in institutionalized older adults: risks, consequences and antecedents. Rev Bras Enferm 2017;70:719–725. [DOI] [PubMed] [Google Scholar]
- 79. English KL, Paddon‐Jones D. Protecting muscle mass and function in older adults during bed rest. Curr Opin Clin Nutr Metab Care 2010;13:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Muir SW, Berg K, Chesworth B, Klar N, Speechley M. Quantifying the magnitude of risk for balance impairment on falls in community‐dwelling older adults: a systematic review and meta‐analysis. J Clin Epidemiol 2010;63:389–406. [DOI] [PubMed] [Google Scholar]
- 81. Pasma JH, Engelhart D, Schouten AC, van der Kooij H, Maier AB, Meskers CGM. Impaired standing balance: the clinical need for closing the loop. Neuroscience 2014;267:157–165. [DOI] [PubMed] [Google Scholar]
- 82. Ochi M, Tabara Y, Kido T, Uetani E, Ochi N, Igase M, et al. Quadriceps sarcopenia and visceral obesity are risk factors for postural instability in the middle‐aged to elderly population. Geriatr Gerontol Int 2010;10:233–243. [DOI] [PubMed] [Google Scholar]
- 83. Damian J, Pastor‐Barriuso R, Valderrama‐Gama E, de Pedro‐Cuesta J. Factors associated with falls among older adults living in institutions. BMC Geriatr 2013;13:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Beenakker KGM, Koopman JJE, van Bodegom D, Kuningas M, Slagboom PE, Meij JJ, et al. Variants of the IL‐10 gene associate with muscle strength in elderly from rural Africa: a candidate gene study. Aging Cell 2014;13:862–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beenakker KGM, Ling CH, Meskers CGM, de Craen AJM, Stijnen T, Westendorp RGJ, et al. Patterns of muscle strength loss with age in the general population and patients with a chronic inflammatory state. Ageing Res Rev 2010;9:431–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Malafarina V, Uriz‐Otano F, Iniesta R, Gil‐Guerrero L. Sarcopenia in the elderly: diagnosis, physiopathology and treatment. Maturitas 2012;71:109–114. [DOI] [PubMed] [Google Scholar]
- 87. Siparsky PN, Kirkendall DT, Garrett WE Jr. Muscle changes in aging. Sports Health 2013;6:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ogawa S, Yakabe M, Akishita M. Age‐related sarcopenia and its pathophysiological bases. Inflamm Regen 2016;36:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Beenakker KG, Duijnisveld BJ, Van Der Linden HM, Visser CP, Westendorp RG, Butler‐Brown G, et al. Muscle characteristics in patients with chronic systemic inflammation. Muscle Nerve 2012;46:204–209. [DOI] [PubMed] [Google Scholar]
- 90. Beenakker KG, Westendorp RG, de Craen AJ, Slagboom PE, van Heemst D, Maier AB. Pro‐inflammatory capacity of classically activated monocytes relates positively to muscle mass and strength. Aging Cell 2013;12:682–689. [DOI] [PubMed] [Google Scholar]
- 91. Dos Santos L, Cyrino ES, Antunes M, Santos DA, Sardinha LB. Sarcopenia and physical independence in older adults: the independent and synergic role of muscle mass and muscle function. J Cachexia Sarcopenia Muscle 2017;8:245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Balogun S, Winzenberg T, Wills K, Scott D, Jones G, Aitken D, et al. Prospective associations of low muscle mass and function with 10‐year falls risk, incident fracture and mortality in community‐dwelling older adults. J Nutr Health Aging 2017;21:843–848. [DOI] [PubMed] [Google Scholar]
- 93. Cruz‐Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2018; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bijlsma AY, Meskers MC, Molendijk M, Westendorp RG, Sipila S, Stenroth L, et al. Diagnostic measures for sarcopenia and bone mineral density. Osteoporos Int 2013;24:2681–2691. [DOI] [PubMed] [Google Scholar]
- 95. Bijlsma AY, Meskers CG, van Heemst D, Westendorp RG, de Craen AJ, Maier AB. Diagnostic criteria for sarcopenia relate differently to insulin resistance. Age (Dordr) 2013;35:2367–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab 2013;20:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Cruz‐Jentoft AJ, Landi F, Schneider SM, Zuniga C, Arai H, Boirie Y, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014;43:748–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Hopewell S, Adedire O, Copsey BJ, Boniface GJ, Sherrington C, Clemson L, et al. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2018;7:CD012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2017. J Cachexia Sarcopenia Muscle 2017;8:1081–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource S1: Search strategy.
Online Resource S2: Newcastle‐Ottawa Scale quality assessment explanation.
Online Resource S3: Flow chart of study selection.
Online Resource S4: Results of the Newcastle‐Ottawa Scale quality assessment for (a) falls and (b) fractures.
Online Resource S5: Funnel plots showing the association between sarcopenia with (a) falls and (b) fractures.


